Abstract
Replication protein A (RPA) is the major eukaryotic ssDNA-binding protein and has essential roles in genome maintenance. RPA binds to ssDNA through multiple modes, and recent studies have suggested that the RPA–ssDNA interaction is dynamic. However, how RPA alternates between different binding modes and modifies ssDNA structures in this dynamic interaction remains unknown. Here, we used single-molecule FRET to systematically investigate the interaction between human RPA and ssDNA. We show that RPA can adopt different types of binding complexes with ssDNAs of different lengths, leading to the straightening or bending of the ssDNAs, depending on both the length and structure of the ssDNA substrate and the RPA concentration. Importantly, we noted that some of the complexes are highly dynamic, whereas others appear relatively static. On the basis of the above observations, we propose a model explaining how RPA dynamically engages with ssDNA. Of note, fluorescence anisotropy indicated that RPA can also associate with RNA but with a lower binding affinity than with ssDNA. At the single-molecule level, we observed that RPA is undergoing rapid and repetitive associations with and dissociation from the RNA. This study may provide new insights into the rich dynamics of RPA binding to ssDNA and RNA.
Keywords: DNA binding protein, DNA-protein interaction, DNA structure, single-molecule biophysics, enzyme mechanism, molecular dynamics, fluorescence resonance energy transfer (FRET), DNA recombination, DNA replication, replication protein A
Introduction
Replication protein A (RPA)3 is a highly conserved single-stranded DNA (ssDNA)-binding protein in eukaryotes that plays important roles in genome maintenance (1). RPA binds to the ssDNA formed during DNA replication, recombination, and repair with high affinity, transiently protecting the ssDNA from nucleases and preventing hairpin formation that would interfere with DNA processing (2, 3). In addition, RPA participates in the targeting of other proteins to the corresponding activation sites on DNA through direct protein–protein interactions (4, 5). RPA also functions in activation of the DNA damage checkpoint and fork restart (6–8). As a stable heterotrimer, RPA is composed of a 70-kDa subunit (RPA1), a 32-kDa subunit (RPA2), and a 14-kDa subunit (RPA3), and a trimerization core is formed through the interaction between each subunit (Fig. 1A) (9). In addition, RPA has six structurally related oligonucleotide-binding (OB) folds that interact with DNA and a winged helix domain that primarily interacts with protein partners. The OB folds are connected by flexible linkers that make RPA a very flexible complex (10, 11).
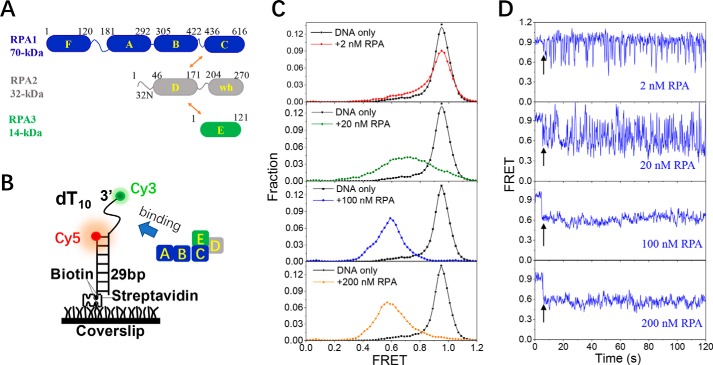
Figure 1.
RPA undergoes dynamic association and dissociation with 10-nt ssDNA. A, molecular structure of RPA. wh in RPA2 represents the winged helix domain. Arrows indicate the intersubunit associations. B, schematic representation of the smFRET experimental setup. C, FRET histograms of the DNA substrate dT10 alone and with various concentrations of RPA. Each FRET histogram was constructed from more than 300 traces. D, representative FRET traces of dT10 in the presence of RPA. Black arrows indicate the addition of RPA.
The interactions between RPA and ssDNA have been extensively studied (1–3, 9). It is generally agreed that RPA binds tightly to ssDNA with a defined 5′–3′ polarity (12, 13). A study using small-angle X-ray scattering showed that RPA engages ssDNA in two different modes either with the tandem high-affinity 70A-B domains if only 10 nt of ssDNA is available or with both 70A-B and the trimerization core on the 20-nt or longer ssDNA because of the C and D domains of RPA (2). Moreover, the stability of the interaction between RPA and ssDNA depends on the length of ssDNA available. Similarly, several other studies revealed that RPA associates with ssDNA in a low-affinity mode in which 70A-B of RPA1 binds ∼10-nt ssDNA and a high-affinity mode in which 70A-B and the trimerization core cover ∼30 nt of ssDNA (14–21). An intermediate mode involving 70A-C may also exist (9).
RPA is essential for the homologous recombination (HR) process that is indispensable for the repair of DNA double-strand breaks (DSBs) (22, 23). During HR, DSB formation is followed by end-processing reactions in which long 3′ ends of ssDNA are produced. These ends are quickly bound by RPA in eukaryotes to protect the ssDNA from degradation and to remove any secondary structures. Therefore, RPA must be capable of binding very tightly to ssDNA. However, RPA must also be displaced from ssDNA so that the ssDNA can be accessed by other downstream proteins. Recently, several studies have proposed dynamic models for the RPA function in DNA-processing pathways (2, 3, 9, 24–32) that include the rapid exchange between the bound and free states, microscopic rearrangement of the DNA-binding domains, and rapid diffusion. However, more work is still required to fully understand the rich dynamics of RPA in complex with DNA; in particular, the mechanisms by which RPA navigates between its different DNA-binding states and remodels the structure of ssDNA in the dynamic process remain unknown.
RPA functions as a key sensor of ssDNA at stalled replication forks and DNA damage sites. Therefore, in addition to the 3′ partial duplex in the HR process as described above, RPA binding to ssDNA may also occur at a variety of structures such as the 5′ partial duplex and the DNA fork, which mimics the stalled replication fork structure. How the identity of the DNA substrates influences the binding of RPA also needs to be determined.
Recently, RPA has been found downstream of the promoter in transcribed regions of genes (33). Genome-wide ChIP analysis shows that RPA localization strongly correlates with RNA polymerase II throughout the genome. In addition, RPA has also been reported to be involved in the suppression of R-loops (34, 35), which are transcription intermediates containing RNA:DNA hybrids and displaced ssDNA, and has emerged as a major source of genomic instability. Nguyen et al. (35) discovered that RPA colocalizes with RNaseH1 and R-loops in cells, and RPA enhances the association of RNaseH1 with RNA:DNA hybrids, stimulating the activity of RNaseH1 on R-loops. This study revealed that RPA is a sensor of R-loops, extending the versatile role of RPA in suppression of genomic instability. The aforementioned tantalizing links among transcription, R-loops, and RPA raise the interesting question of whether RPA can associate with single-stranded RNA similarly to its interaction with ssDNA.
In this study, we investigated the dynamic binding of human RPA on ssDNA or RNA by single-molecule FRET (smFRET), fluorescence anisotropy, and gel filtration. First, we demonstrated that RPA can adopt different types of binding complexes with different lengths of ssDNA, leading to the straightening or bending of ssDNA. Importantly, some of these complexes are highly dynamic, and others appear relatively static. Second, we discovered that the properties of the complexes vary with the identity of the substrate (i.e. 3′ versus 5′ overhang, DNA fork, etc.), suggesting that RPA may bind differently to ssDNA in different DNA-processing pathways. Third, using the fluorescence anisotropy assay, we found that RPA can associate with RNA and that the binding affinity is lower with RNA than with ssDNA. At the single-molecule level, we observed that RPA is undergoing rapid and repetitive association and dissociation on RNA. This study is expected to help us to understand how RPA navigates between different binding modes and remodels the structure of ssDNA in different DNA-processing pathways, including those of replication and homologous recombination. In addition, the newly identified RNA-binding activity may provide important clues in understanding how RPA senses R-loops and associates with RNA polymerase in cells.
Results
Human RPA is composed of a 70-kDa subunit, a 32-kDa subunit, and a 14-kDa subunit (Fig. 1A). The result of the gel filtration analysis presented in Fig. S1A confirms that, in free solution, RPA exists in a single form in which the three subunits are tightly associated. The DNA-binding properties of RPA were also characterized by fluorescence anisotropy measurements under equilibrium conditions. The apparent dissociation constant, KD, was obtained by fitting the binding curve with the Hill equation (Fig. S1B). Fig. S1C shows that the KD value increases only slightly from 0 to 100 mm KCl; however, in 200 mm KCl, the binding affinity is reduced, possibly due to increased electrostatic screening. Therefore, 100 mm KCl was adopted in all of the experiments. In addition, the KD value for the 200 mm KCl experiment was 4.83 ± 0.2 nm, consistent with that previously reported, 5.8 ± 0.4 nm, in similar buffer (2).
RPA rapidly associates and dissociates with 10-nt ssDNA
After examining the purity and binding properties of RPA, we next characterized the dynamic binding process between RPA and ssDNA at the single-molecule level using smFRET. The substrate contained ssDNA at the 3′ end of a 29-bp duplex that mimics the DNA structure after the end resection process in the early stage of DNA recombination, and it was anchored on a coverslip by a biotin–streptavidin link (Fig. 1B). The 3′ end of the ssDNA and the 5′ end of the stem strand were labeled with Cy3 and Cy5, respectively.
As RPA may bind to ssDNA by different modes in a length-dependent manner (2, 9), we systematically investigated the binding of RPA on a series of ssDNAs with various lengths. We first tested a short 10-nt ssDNA, dT10. In 2 nm RPA (Fig. 1C, panel 1), the population at E0.95 (EFRET represents the state with the subscript FRET value), which corresponds to the free-ssDNA state, decreased slightly, whereas the populations at lower EFRET values, which reflect the RPA-bound state, simultaneously increased. The individual FRET trajectories showed that, in 2 nm RPA, the FRET value fluctuated frequently between ∼E0.95 and ∼E0.6 in abrupt steps (Fig. 1D, panel 1), reflecting the rapid association and dissociation of RPA on dT10. The above results also indicate that RPA binding can induce the straightening of dT10, as the RPA-bound ssDNA has a lower EFRET value than the free ssDNA. With increases in RPA concentrations, the FRET distribution shifted significantly from E0.95 to E0.6 (Fig. 1C), suggesting the likelihood that the dT10 association with RPA increased. Furthermore, the stable FRET level at ∼E0.6 indicated that almost all DNA molecules were extended in 100–200 nm RPA (Fig. 1D, panels 3 and 4). Fig. S2, A and B, display the fluorescence signals of dT10 in the presence of 20 nm RPA for a substrate labeled with both Cy3 and Cy5 or in control reactions using dT10 substrates labeled with only Cy5 or only Cy3. These controls verify that the RPA-dependent changes in fluorescence emission we observed were due to distance-dependent differences and not due to changes in fluorophore emission properties such as protein-induced enhancing or quenching. After the free RPA is flushed from the fluidic chamber with buffer (Fig. S2C), the FRET level of the dT10 returned to the original value of free DNA (E0.95), suggesting the dissociation of RPA from the 10-nt ssDNA.
The gel filtration results suggest that RPA forms one type of complex with dT10 in a broad range of protein concentrations (Fig. S3A), most likely due to the reported 10-nt mode in which only the 70A-B domains in RPA1 make contact with the ssDNA (2, 18). Occasionally, a subtle intermediate state could be observed by smFRET (Fig. S2D), and it may represent the sequential engagement of the two domains. Taken together, these findings indicate that the binding of RPA to dT10 is highly dynamic with very rapid association and dissociation from the ssDNA, consistent with the low ssDNA affinity in the 10-nt mode (2, 18).
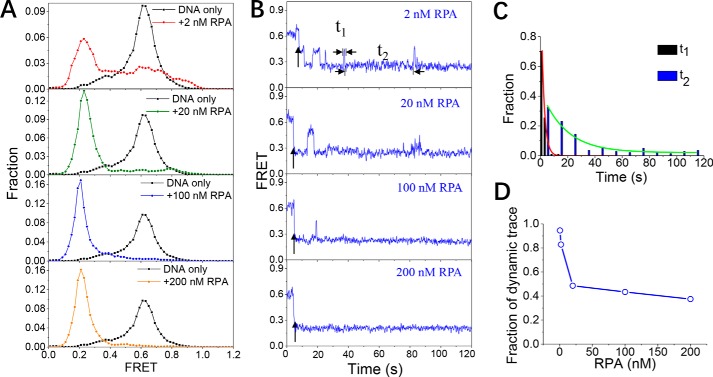
RPA induces the straightening of a 20-nt ssDNA at two different states
As RPA binds to ssDNA with different modes depending on the length of the ssDNA that it contacts (2, 9), we next examined the association of RPA with a 20-nt ssDNA, dT20. In 2 nm RPA, the FRET histogram of dT20 shows a shift from ∼E0.6 to a lower band at ∼E0.25, indicating the straightening of the ssDNA (Fig. 2A, panel 1). In addition, from the individual trace (Fig. 2B, panel 1), two different FRET states, one at ∼E0.45 and one at ∼E0.25, can be observed. The distributions of dwell time at E0.45 and E0.25 followed a single-exponential decay with time constants of 2.1 and 18.1 s, respectively (Fig. 2C), suggesting that the E0.25 state is much more stable than the E0.45 state. More importantly, dT20 dynamically switched between those two states, instead of remaining static, in the presence of 2 nm RPA.
Figure 2.
RPA binding induces the straightening of a 20-nt ssDNA. A, FRET histograms of the DNA substrate dT20 alone and with various concentrations of RPA. Each FRET histogram was constructed from more than 300 traces. B, representative FRET traces of dT20 in the presence of RPA. Black arrows indicate the addition of RPA. In 2 nm RPA, the dwell times at ∼E0.45 and ∼E0.25 (t1 and t2) were measured to characterize the different states of ssDNA in complex with RPA. C, both t1 and t2 follow a single-exponential decay with time constants of 2.1 and 18.1 s, respectively. D, after DNA association with RPA in the beginning, the fraction of FRET traces showing dynamic properties (RPA dissociation from ssDNA or switching to other binding modes) decreased significantly with increasing RPA concentration.
With the increase in RPA concentration, the FRET histogram of dT20 shifts significantly from ∼E0.6 to ∼E0.25, indicating that almost all the DNA molecules are straightened (Fig. 2, A and B, panels 2–4). The gel filtration results suggest that RPA may form two different types of complexes with dT20 with different molecular weights, depending on the protein concentration (Fig. S3B). Therefore, it is likely that, at high protein concentrations, two RPAs may bind side-by-side to the dT20.
We also determined the fractions of the FRET traces that show dynamic properties after association with RPA within the 2-min recording time due to RPA dissociation or navigation between different binding modes (Fig. 2D). In 2 nm RPA, almost each trace is highly dynamic; however, with increases in protein concentration, the majority of these traces become static, possibly indicating the stable association of dT20 by two RPAs. Taken together, the above results suggest that the 20-nt ssDNA may adopt different types of binding complexes with RPA; additionally, some of the complexes are highly dynamic, whereas some are static.
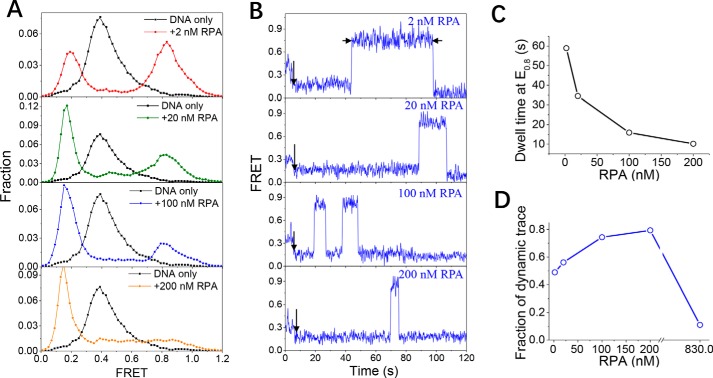
RPA induces the bending of a 30- or 40-nt ssDNA
To further characterize the binding of RPA to the longer ssDNA, we designed a DNA substrate, dT30, with a 30-nt ssDNA strand. When 2 nm RPA was used, we detected both a decrease in FRET values from E0.4 to E0.2 and an increase in FRET values from E0.4 to E0.85 (Fig. 3, A and B, panel 1), reflecting straightening as well as bending of the ssDNA. As the FRET increase was not observed for dT10 or dT20 at the same protein concentrations, this increase may reflect the appearance of the 30-nt-binding mode of RPA in which RPA is fully engaged to 24–30 nt of the ssDNA, and the complex is curved (2, 36). When the length of the ssDNA is further increased to 40 nt, the majority of molecules show increased FRET values (Fig. S4A, panel 1), indicating bending of the ssDNA. In addition, the individual FRET trace is similar to the corresponding dT30 trace (Fig. S4B, panel 1).
Figure 3.
RPA induces the bending of a 30-nt ssDNA. A, FRET histograms of the DNA substrate dT30 alone and with various concentrations of RPA. Each FRET histogram was constructed from more than 300 traces. B, representative FRET traces of dT30 in the presence of RPA. Black arrows indicate the addition of RPA. The dwell time at ∼E0.85 was measured to characterize the RPA-binding state with a high FRET value. C, with increasing RPA concentration, the dwell time decreased significantly. D, after DNA association with RPA in the beginning, the fraction of FRET traces showing dynamic properties increased with increases in the RPA concentration. However, when the RPA concentration reached 830 nm, only ∼10% of FRET traces showed dynamic properties.
The dwell times that dT30 was in the E0.85 and E0.2 states were also measured for more than 300 traces, and the average values were 58.9 and 53.5 s, respectively. The above results indicate that the 30-nt ssDNA underwent a slow dynamic transition (dwell time of ∼1 min) between the extended state (E0.2) and the bent state (E0.85), possibly due to the different binding modes of RPA.
With increasing protein concentrations, the fractions of dT30 at ∼E0.85 gradually decreased, whereas the population at ∼E0.2 increasingly dominated (Fig. 3A), indicating that the majority of DNA molecules became straight. In addition, the FRET value dynamically switched between E0.85 and E0.2 (Fig. 3B), and the dwell time at E0.85 (bending of ssDNA) decreased significantly from 58.9 s in 2 nm RPA to 10.2 s in 200 nm RPA (Fig. 3C). Therefore, with increases in protein concentration, the competition for the ssDNA between the free RPA and DNA-bound RPA may have eventually led to a reduced duration of DNA in the bent state. The changes in FRET distributions and the individual traces of dT40 with increases in RPA concentration were consistent with those of dT30 (Fig. S4).
The gel filtration results further demonstrated that RPA can form three types of complexes with dT30 with different molecular weights in a broad range of protein concentrations (Fig. S3C). Therefore, we speculate that a 30-nt ssDNA can accommodate one, two, or three RPAs in a concentration-dependent manner. The fractions of the dynamic traces within the 2-min recording time were determined (Fig. 3D). In 2 nm RPA, ∼50% of traces were static after the initial association with RPA; however, with increases in the RPA concentration, the majority of the traces showed increasing dynamic changes, suggesting that the DNA may be occupied by two or three RPAs in an unstable manner. When 830 nm RPA was used, ∼90% of ssDNA molecules remained in a constant state at ∼E0.2 after the association with RPA, suggesting that dT30 can be bound by multiple RPAs (most likely three) very stably once the protein concentration is extremely high. These findings suggest that, depending on the protein concentration, the 30-nt ssDNA can adopt different types of binding complexes with RPA and that they show different dynamic properties.
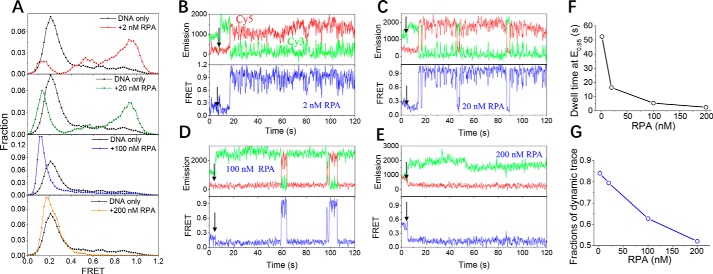
RPA induces the bending of 60-nt ssDNA and quickly diffuses on it
Because the length of the ssDNA overhang in DSBs may be much longer than 30 nt, we designed a substrate, named dT60, to mimic the interaction between RPA and a long ssDNA. In 2 nm RPA, the FRET distribution of dT60 shifted from ∼E0.2 (free DNA) to higher EFRET values, with two peaks showing at ∼E0.95 and ∼E0.55, indicating the bending of the ssDNA (Fig. 4A, panel 1). There is also a minor peak at E0.15, reflecting the straightening of dT60. The individual FRET trace indicates that RPA binding to dT60 occurs immediately after protein addition as indicated by the subtle decrease in FRET value from E0.2 to E0.15; after a short duration, the FRET value rose to a high level from E0.15 to E0.95, possibly caused by the wrapping of dT60 around RPA in the 30-nt mode (2, 36). However, in striking contrast with the stable FRET level of the dT30 associated with RPA in the 30-nt mode (Fig. 3B, panel 1), the FRET value of dT60 showed rapid fluctuations between E0.95 and E0.55 (Fig. 4B). Because this type of FRET fluctuation has never been observed for short ssDNA, it may be caused by the quick diffusion of RPA forward and backward on ssDNA that is much longer than 30 nt.
Figure 4.
RPA induces the bending of a 60-nt ssDNA and quickly diffuses on the ssDNA. A, FRET histograms of the DNA substrate dT60 alone and with various concentrations of RPA. Each FRET histogram was constructed from more than 300 traces. B–E, representative fluorescence emission and FRET traces of dT60 with 2–200 nm RPA. Black arrows indicate the addition of RPA. The dwell time at ∼E0.95 was measured. F, with increasing RPA concentration, the dwell time decreased significantly. G, after association with RPA in the beginning, the fraction of FRET traces showing dynamic properties decreased with increases in the RPA concentration.
With increases in the protein concentration, the fractions of DNA at E0.95 gradually decreased, whereas the population at E0.15 became more significant (Fig. 4A). The individual FRET traces reveal two major properties. First, the FRET values switch between E0.95 and E0.15 (Fig. 4, C and D). This switching might be caused by the competition between the DNA-bound RPA in the 30-nt mode and the free RPA in the solution, leading to the unwrapping of dT60 from RPA, or additional RPA molecules may occupy the ssDNA. Fig. 4F displays the dwell time in the bent state at E0.95: with increases in protein concentration, the dwell time significantly decreased from 52.4 s in 2 nm RPA to 2.5 s in 200 nm RPA, further suggesting that the protein–protein competition may be disrupting the continuous wrapping of the ssDNA around a single RPA. Second, the FRET data show quick fluctuations between E0.95 and E0.55 when the DNA is wrapping around RPA (Fig. 4, C and D), suggesting the diffusion of RPA. However, in 200 nm RPA, the DNA is in a relatively static state without quick fluctuations after the association with RPA (Fig. 4E). The fractions of the dynamic trace (Fig. 4G) indicate that the DNA became increasingly static with increasing protein concentration, suggesting that dT60 may form a relatively stable complex with multiple RPA molecules.
The gel filtration results shown in Fig. S3D further suggest that RPA is able to form four different types of complexes with 60-nt ssDNA with different molecular weights in a wide range of protein concentrations and that there is no excess protein. We speculate that, with further increases in protein concentration, new types of complexes may be formed. Once again, these observations indicate that the 60-nt ssDNA can adopt different types of binding complexes with RPA, depending on the protein concentration.
RPA binds differently to ssDNA at the 5′ end of a duplex
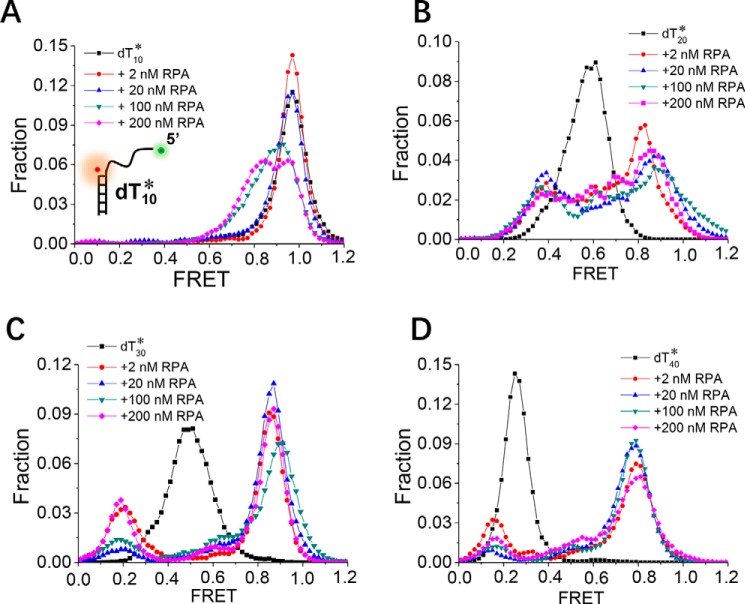
DNA processing involves not only RPA binding to ssDNA at the 3′ end of a duplex, as presented above, but also partial duplexes with a variety of structures. Previous studies have suggested a junction-binding mode in which the RPA trimerization core is alone sufficient for binding a partial duplex with a 5′ ssDNA overhang (37–39). To further examine whether RPA binds differently to the 5′ partial duplex, which mimics the exposed ssDNA in lagging strand DNA replication, we used smFRET to monitor the interaction between RPA and the 5′ partial duplex with ssDNA lengths from 10 to 40 nt (referred to as dT10*, dT20*, dT30*, and dT40*).
In contrast to the frequent binding of RPA to dT10 (Fig. 1), RPA seldom binds to dT10* at concentrations of 2–20 nm, as the FRET distribution and FRET traces show little change (Figs. 5A and S5A). Even in 200 nm protein, only ∼50% of dT10* molecules are bound by RPA, as suggested by the left shift in the FRET distribution. This result is most likely because the respective proximities of the 10-nt ssDNA to the duplex are different in dT10 and dT10*. As RPA binds in a 5′ to 3′ orientation on the ssDNA, there is a significant differential steric factor caused by the presence of the duplex. The binding of RPA to dT20* also differs significantly from the binding to dT20. At either low or high protein concentrations, RPA binding mainly results in straightening of the 20-nt ssDNA at the 3′ end of the duplex (Fig. 2); however, the FRET value decrease for dT20* from E0.6 to E0.35 and the increase from E0.6 to E0.85 indicate that dT20* not only can be straightened but also can be bent by RPA (Fig. 5B). The FRET traces further indicate that dT20* is undergoing dynamic transitions between these two bound states (Fig. S5B). The binding of RPA to dT30* or dT40* is also different from the binding to dT30 or dT40 (Figs. 5, C and D, and S5, C and D).
Figure 5.
RPA binds to ssDNA at the 5′ end of the duplex differently. FRET histograms of the DNA substrates dT10* (A), dT20* (B), dT30* (C), and dT40* (D) alone and with various concentrations of RPA are shown. Each FRET histogram was constructed from more than 300 traces.
Notably, the binding of RPA to dT20*, dT30*, or dT40* is not very sensitive to the protein concentration, as the FRET distributions show only slight changes (Fig. 5, B–D). Therefore, even at very high protein concentrations, the likelihood that these substrates can accommodate multiple RPA molecules is low, possibly because of the steric factor from the presence of the duplex. All these observations suggest that RPA binds to ssDNA at the 5′ end of the duplex differently than it binds to the 3′ ssDNA overhang.
The binding of RPA to the DNA fork structure is highly dynamic
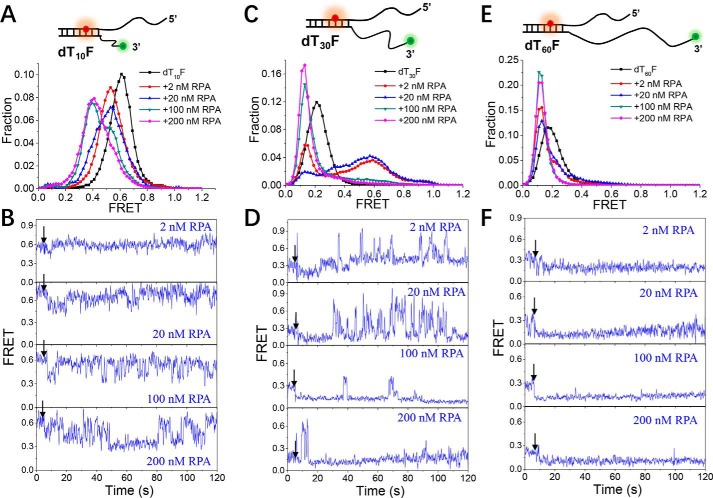
During DNA replication, the uncoupling of the helicase and polymerase may generate large stretches of ssDNA on the leading strand of the replication fork (40). As a highly abundant and high-affinity ssDNA-binding protein, RPA is expected to be the first responder to ssDNA, whether it is formed during normal DNA replication or in response to the replication stress (41). Therefore, we designed three DNA fork substrates (dT10F, dT30F, and dT60F) to study the interaction between RPA and the replication fork. The 5′ ssDNA strand of each substrate is 26 nt, and the inside of the duplex DNA rather than the junction was labeled with Cy5.
In general, the existence of the 5′ ssDNA strand in the fork has a significant influence on the binding of RPA. For instance, RPA seldom bound to the 3′ ssDNA of dT10F at low concentrations (2–20 nm), as shown by the FRET traces that barely changed (Fig. 6B, panels 1 and 2); at higher RPA concentrations, the FRET traces show frequently fluctuations (Fig. 6B, panels 3 and 4) instead of a stable level throughout (compare with Fig. 1, C and D), reflecting the repetitive association and dissociation of RPA. Therefore, the fork structure can significantly weaken the binding affinity of RPA. For the 3′ ssDNA that is increased to 30 nt, at low protein concentrations (2–20 nm), both a FRET value decrease from E0.25 to E0.15 and an increase from E0.25 to E0.6 were found, reflecting the straightening and bending of the DNA (Fig. 6, C and D). However, in striking contrast with dT30, the bending state in the fork structure was very unstable: the FRET traces display a large number of quick bursts (Fig. 6D, panels 1 and 2) instead of a stable level within a dwell time of ∼1 min (Fig. 3B). At high protein concentrations, although the majority of DNA molecules show a decreased FRET value, similar FRET bursts can also be observed (Fig. S6). It is worth noting that this kind of quick burst has never been observed in the binding of RPA to a partial duplex; therefore, these findings likely reflect highly dynamic binding between RPA and ssDNA in the fork structure. When the length of the 3′ ssDNA is further increased to 60 nt, at both low and high protein concentrations, the FRET values of most molecules decrease and remain at a stable level (Fig. 6, E and F). Therefore, in the presence of a long ssDNA strand, the inhibition effect from the fork structure may be avoided.
Figure 6.
The binding of RPA to the DNA fork structure is highly dynamic. A, C, and E, FRET histograms of the DNA substrates dT10F, dT30F, and dT60F alone and with various concentrations of RPA. Each FRET histogram was constructed from more than 300 traces. B, D, and F, representative FRET traces of dT10F, dT30F, and dT60F in the presence of 2–200 nm RPA. Black arrows indicate the addition of RPA.
Taken together, the above results suggest that RPA binds to the DNA fork differently than it binds to the partial duplex. In particular, RPA binding to the fork structure is highly dynamic when the 3′ ssDNA is between 10 and 30 nt. This might be because another RPA molecule can be recruited by the 26-nt 5′ ssDNA such that the binding of RPA to the 3′ ssDNA is impeded due to steric hindrance. In addition, there might be an interaction between the 5′ ssDNA and the RPA associated with the 3′ ssDNA. Interestingly, with increased ssDNA length, RPA may circumvent the fork and bind to other regions of the 3′ ssDNA, thereby avoiding the inhibitory effect of the fork and entering a stable binding state.
RPA is able to associate with single-stranded RNA
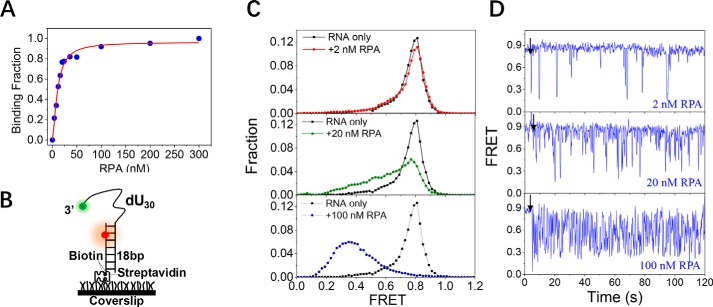
As RPA has been found downstream of the promoter in transcribed regions of genes (33) and is also involved in the suppression of R-loops that contain RNA:DNA hybrids and displaced ssDNA (34, 35), we examined whether RPA is also able to bind to single-stranded RNA similarly to the way it binds ssDNA. The RNA-binding properties of RPA were first characterized by fluorescence anisotropy measurements under equilibrium conditions. The apparent dissociation constant, KD, was 15.2 ± 1.8 nm (Fig. 7A), indicating that RPA can indeed bind to single-stranded RNA; however, the binding affinity was weaker than it was for ssDNA (KD ∼ 4 nm in 100 mm KCl). We next characterized the dynamic binding process between RPA and RNA at the single-molecule level using smFRET (Fig. 7B). In stark contrast with the binding to the 10- and 20-nt ssDNAs, RPA was not able to bind to RNA of similar length, as shown in Fig. S7, A and B. However, when the length of RNA reached 30 nt, the binding of RPA was clearly observed (Fig. 7, C and D). Although in 2 nm RPA the FRET histogram of dU30 did not show much change (Fig. 7C, panel 1), the individual FRET traces showed occasional fluctuations between ∼E0.85 and ∼E0.3 in abrupt steps (Fig. 7D, panel 1), reflecting the rapid association and dissociation of RPA. With increases in RPA concentration, the FRET distribution shifted significantly from E0.85 to E0.3 (Fig. 7C, panels 2 and 3), and the FRET traces showed frequent fluctuations between ∼E0.85 and ∼E0.3 (Fig. 7D, panels 2 and 3), indicating that dU30 underwent frequent association and dissociation with RPA instead of staying in a stable bound state.
Figure 7.
The binding of RPA to the single-stranded RNA is highly dynamic. A, binding fractions of RPA on the single-stranded RNA in 100 mm KCl were determined by fluorescence polarization assay. The binding curve was fitted by the Hill equation: y = [RPA]n/(KDn + [RPA]n) where y is the binding fraction, n is the Hill coefficient, and KD is the apparent dissociation constant. B, schematic representation of the smFRET experimental setup. C, FRET histograms of the RNA substrate dU30 alone and with various concentrations of RPA. Each FRET histogram was constructed from more than 300 traces. D, representative FRET traces of dU30 in the presence of RPA. Black arrows indicate the addition of RPA.
The above results strongly suggest that RPA is able to associate with single-stranded RNA. However, the interplay between RPA and RNA is quite different from that between RPA and DNA. First, the binding affinity of RPA for RNA was weaker than it was for DNA, as suggested by both the higher KD value and the lack of RPA binding to 10–20-nt RNA. Second, RPA induced both bending and straightening of the 30-nt ssDNA, and the complex underwent slow dynamic change (∼1 min for each state). In contrast, RPA bound to 30-nt RNA in a highly dynamic manner and induced only the straightening of the RNA. This evidence may suggest that, in its participation in DNA transcription (33) and processing of R-loops (34, 35), RPA could dynamically associate with linear RNA strands.
Discussion
RPA is an important ssDNA-binding protein in eukaryotes through its participation in numerous aspects of nucleic acid metabolism. However, the structural change in ssDNA induced by RPA in different binding modes was unknown. In addition, the modulation of RPA binding by protein concentration, DNA length, and DNA structure was also undetermined. To address these issues, we first investigated the dynamic binding of RPA on ssDNA with a series of lengths at the single-molecule level. The ssDNA can form different types of complexes with RPA such that different dynamic properties are presented, and the DNA can be either straightened or bent. Importantly, with increasing protein concentration, ssDNA is densely coated by RPA, resulting in a relatively static DNA filament. All these findings suggest that RPA is a highly adaptive protein that can effectively bind to ssDNA by adjusting its binding mode within a broad range of protein concentrations.
RPA may undergo a dynamic switch between different binding modes on ssDNA
During DNA processing, RPA tightly bound to ssDNA must be displaced efficiently to enable completion of the processing pathway. Therefore, RPA binding and release are expected to be highly dynamic such that the DNA can rapidly be made accessible to subsequent processing machinery.
Recently, several studies have shown the dynamic binding of RPA on ssDNA (2, 3, 9, 24–32). For instance, Gibb et al. (30) reported that RPA remains bound to ssDNA for long periods of time when free protein is absent from solution; however, RPA rapidly dissociates from ssDNA when free RPA is present in solution, enabling rapid transitions between the free and bound states. Another study by Chen et al. (28) demonstrated that RPA binding has at least two kinetic states characterized by different dissociation kinetics, and it is likely that the two binding modes are in equilibrium, reflecting the dynamics of the RPA–DNA complex. Kemmerich et al. (29) characterized the force-dependent binding dynamics of human and yeast RPA and discovered that RPA uses a “toehold-like” mechanism to trap small transient openings in the DNA helix with a microdomain; these openings are then expanded as the full protein is wedged into the helix for binding. In addition, Nguyen et al. (24) analyzed individual molecules of RPA bound to ssDNA and showed that human RPA rapidly diffuses along ssDNA without dissociating. Furthermore, this study indicated that this rapid diffusion was productive and promoted the destabilization of adjacent DNA hairpins. However, more work is required to fully understand the rich dynamics of RPA in complex with DNA; in particular, how RPA navigates between its different DNA-binding modes is not clear.
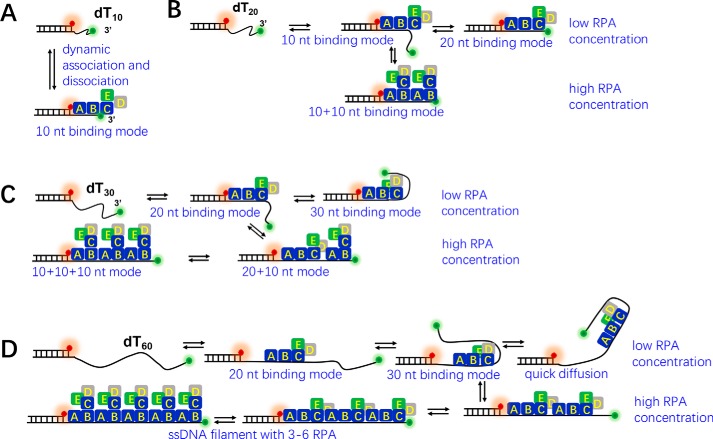
In the present study, we combined smFRET and ensemble approaches to examine the binding of RPA to ssDNA in a variety of situations. Based on the dynamic change of ssDNA structures (Figs. 1–4) and the previously established RPA-binding modes on different lengths of ssDNA (2), we propose a model to show the dynamic engagement of RPA on ssDNA (Fig. 8). In general, at very low protein concentrations, RPA undergoes dynamic binding on ssDNA of different lengths. First, RPA rapidly associates and dissociates with a short ssDNA of 10 nt through the previously reported 10-nt-binding mode with 70A-B domains in RPA1 (2) (Fig. 8A). Second, RPA binds to a 20-nt ssDNA with two different modes (10-nt mode and 20-nt mode, which involves both the 70A-B and trimerization core and is more stable) (2), and there is a frequent transition between those two binding modes (Fig. 8B). Third, RPA binds to a 30-nt ssDNA with two different modes (20- and 30-nt modes) (2), and there is a slow transition between those two modes with a dwell time of ∼1 min (Fig. 8C). According to Brosey et al. (2), RPA uses the 70A-B domains and the trimerization core to bind ssDNA longer than 20 nt, and the stability of the interaction between RPA and ssDNA depends on the length of the ssDNA available. In that study, RPA binding to a 20-nt ssDNA was weaker than binding to the 24–30-nt ssDNA because RPA was more comfortably and stably engaged in the latter condition (2). This finding may explain the longer dwell time of dT30 in the bent state (58.9 s, E0.85) than in the straight state (53.5 s, E0.2) in 2 nm RPA. Finally, RPA binds to a 60-nt ssDNA dominantly with the 30-nt mode and rapidly diffuses forward and backward on the ssDNA (Fig. 8D). However, at very high protein concentrations, all 10–60-nt ssDNA molecules may exist as stable filaments densely coated by RPAs side-by-side in the 10-nt mode, showing low FRET dynamics (Fig. 8, A–D). Therefore, although Nguyen et al. (24) showed that human RPA rapidly diffuses along ssDNA without dissociating, our results further demonstrate that diffusion of RPA occurs when the DNA strand is much longer than 30 nt and at protein concentrations too low for a compact filament to form.
Figure 8.
The proposed model of the dynamic binding between RPA and ssDNA. A, RPA undergoes rapid association and dissociation with dT10 using the 10-nt-binding mode. B, RPA switches dynamically between the 10- and 20-nt-binding modes on a 20-nt ssDNA. With increasing protein concentrations, two RPAs may contact the DNA side-by-side in the 10 + 10–nt mode. C, RPA switches dynamically between the 20- and 30-nt-binding modes on a 30-nt ssDNA. With increasing protein concentrations, two or three RPAs may contact the DNA in the 20 + 10–nt mode and the 10 + 10 + 10–nt mode. D, RPA switches dynamically between the 20- and 30-nt-binding modes on a 60-nt ssDNA and quickly diffuses on the ssDNA. With increasing protein concentrations, two to six RPAs may contact the DNA in the 30 + 30–nt mode, the 20 + 20 + 20–nt mode, the 20 + 20 + 10 + 10–nt mode, the 20 + 10 + 10 + 10 + 10–nt mode, and the 10 + 10 + 10 + 10 + 10 + 10–nt mode.
It is worth noting that, even if there is rapid exchange of RPA on the ssDNA and in solution as reported previously (30), this exchange may not be observed by smFRET, as the FRET level is already very low in densely coated DNA (E0.1–E0.2) and may not be sensitive to subtle changes in distance (42). All our findings suggest that the behavior of RPA is highly adaptive and very sensitive to ssDNA length and protein concentration.
RPA binding induces either straightening or bending of ssDNA
Although RPA binds to ssDNA with a high affinity, the flexibility of the RPA structure and its dynamic binding to ssDNA have made it difficult to study the structure of the RPA–DNA complex. However, several earlier studies have focused on the structural changes in RPA in the presence of ssDNA (14–16, 36, 43–45). According to Brosey et al. (2), RPA occupies an ensemble of architectural forms in solution that can be viewed as in an equilibrium with a range of extended, intermediate, and compact states. More importantly, binding to ssDNA drives the equilibrium toward more compact conformations. The same group also reported that DNA binding dramatically reorients and couples the interdomain motion of 70A and 70B in RPA1 (43). Therefore, ssDNA binding can significantly change the structure of the RPA trimer. However, how RPA binding can modulate the structure of ssDNA is still not well-understood. In 2012, the structure of Ustilago maydis RPA stably bound to ssDNA was determined (36). This structure has a compact quaternary structure held together by a four-way interface among DBD-B, DBD-C, the intervening linker (BC linker), and ssDNA, which is significantly bent in the complex. However, whether human RPA binds to the 30-nt ssDNA in the same way remains to be determined.
In the present study, we demonstrated that ssDNA can be either straightened or bent in different situations and that these changes are modulated by RPA concentration, DNA length, and DNA structure. For instance, both the 10- and 20-nt ssDNAs at the 3′ end of duplex DNA were extended at all protein concentrations. However, the 30-, 40-, and 60-nt ssDNA molecules were all bent by a single RPA protein, resulting in a high FRET value. With increases in protein concentration, the accommodation for multiple RPAs ultimately led to straightened ssDNA. Therefore, the ssDNA generated in the DNA recombination repair process at the 3′ end of the duplex might become a straight filament densely coated by RPA proteins in cells.
Salas et al. (46) previously proposed a model of RPA binding with a 31-nt ssDNA. In a 1:1 complex, only one molecule of RPA binds ssDNA in the 30-nt mode (elongated extended form). RPA1 is localized at the 5′ side of ssDNA, and the RPA2 subunit is then bound to the ssDNA. RPA3 is positioned near the 3′ end of the ssDNA. However, in our study, we clearly demonstrated that, in the 30-nt mode, ssDNA is in a bent form rather than the extended form. Salas et al. (46) further proposed that two or three RPA molecules might form complexes with 31-nt ssDNA. In our study, based on the smFRET observations, gel filtration results, and the previously established RPA-binding modes with different lengths of ssDNA (2), we speculated (Fig. 8) that two RPAs may bind to the 20-nt ssDNA in the 10 + 10–nt mode; two or three RPAs may bind to the 30-nt ssDNA in the 20 + 10–nt or 10 + 10 + 10–nt modes; and more than four RPAs may bind to the 60-nt ssDNA in a variety of modes such as the 20 + 20 + 10 + 10–nt, 20 + 10 + 10 + 10 + 10–nt, or 10 + 10 + 10 + 10 + 10 + 10–nt modes. In the future, other methods such as small-angle X-ray scattering or electron microscopy (EM) may be used to precisely determine the structures of these complexes.
Potential biological significance of the dynamic binding of RPA on ssDNA or RNA
Fig. S8 illustrates several possible biological contexts in which the dynamic binding behavior of RPA may play important roles, including homologous recombination, DNA replication, processing of R-loops, and DNA transcription. Our smFRET study indeed confirms that RPA is able to bind to ssDNA at the 3′ and 5′ overhangs and fork DNA structures, consistent with the in vivo functional sites of RPA in HR and DNA replication (Fig. S8, A and B). Our study further suggests that RPA has high adaptability when binding ssDNA; i.e. RPA is able to efficiently associate with ssDNA by switching between different binding modes with variation of protein concentrations and ssDNA length. Therefore, depending on the local concentrations of RPA at the repair foci or replication foci, the RPA filaments on long ssDNA strands may have different protein densities, with each RPA covering 10-, 20-, and even 30-nt ssDNA.
RPA acts as a versatile platform in DNA processing, which means RPA must bind very tightly to ssDNA and must also be easily displaced to enable the association of other downstream proteins. Several previous studies have demonstrated that the interaction between RPA and ssDNA is dynamic (9, 24, 26–30). Our study is consistent with those findings as, in the range of low protein concentrations, the RPA–ssDNA complex was highly dynamic (Figs. 2D, 3D, and 4G). In addition, we further demonstrated that, at very high protein concentrations, the RPA–ssDNA complexes appear to be relatively static. Therefore, it is possible that, when RPA is replaced by the downstream proteins, ssDNA may not be extensively coated by RPA, and the RPA–ssDNA complex thus undergoes dynamic change; alternatively, the displacement of RPA may require the participation of other proteins such as BRCA2 in the HR process (47). In the stalled replication fork, the hybridization of the dsDNA may push RPA away, leading to the dissociation of RPA from the DNA fork as reported previously (29).
We also discovered at both the bulk and single-molecule levels that RPA is able to associate with single-stranded RNA, and this interaction may play important roles in the cellular function of RPA in R-loop sensing and in transcribed regions of the transcription process (Fig. S8, C and D). Nguyen et al. (35) reported that RPA interacts with RNaseH1 and colocalizes with both RNaseH1 and R-loops in cells. RPA directly enhances the association of RNaseH1 with the RNA:DNA hybrids and stimulates the activity of RNaseH1 on R-loops. However, how RPA recognizes the R-loop structures remains unclear. The authors speculated that RPA may directly recognize the displaced ssDNA in R-loops, but this hypothesis has not been proven by experiments. In addition, the reasons that RPA can be detected in the transcribed regions of active genes is also unclear (33). Our findings provide one possible explanation: RPA sensing of R-loops in cells and its participation in transcription may be caused by activity induced by binding to RNA. Providing evidence in vivo to support our findings in vitro may be an essential future project.
Experimental procedures
Buffers
The RPA reaction buffer contained 100 mm KCl in 20 mm Tris-HCl (pH 8.0). For single-molecule measurements, 0.8% d-glucose, 1 mg/ml glucose oxidase (266, 600 units/g, Sigma), 0.4 mg/ml catalase (2000–5000 units/mg, Sigma), and 4 mm Trolox were added to the reaction buffer. In all RNA-related experiments, RNase inhibitor (Promega) was added with the final concentration of 0.1 unit/μl.
DNA and RNA constructs
All oligonucleotides were purchased from Sangon Biotech (Shanghai, China). The sequences and labeling positions of all the oligonucleotides are listed in Table S1. For the DNA or RNA constructs used in single-molecule measurements, 5 nm DNA or RNA was annealed with a 1:2 mixture of the stem and ssDNA or RNA strands by incubating the mixture at 95 °C for 5 min in 20 mm Tris-HCl (pH 8.0) containing 100 mm KCl and then slowly cooling the mixture to room temperature over ∼7 h. Strands without biotin were used in excess to reduce the possibility of nonannealed strands anchoring to the coverslip surface.
Protein purification
The expression and purification of RPA were carried out essentially according to Henricksen et al. (48). Escherichia coli strain BL21 (DE3) was transformed with plasmid p11d-tRPA for recombinant human RPA that permits the coexpression of RPA1, RPA2, and RPA3. Then, RPA was purified with Affi-Gel blue, hydroxyapatite (Bio-Rad), and Q-Sepharose chromatography columns (GE Healthcare). The purified protein was finally eluted in phosphate buffer containing ∼300 mm KCl (pH 7.5) and stored at −80 °C.
Equilibrium DNA-binding assay with RPA
The binding of RPA to ssDNA was analyzed by a fluorescence polarization assay (49) using Infinite F200 PRO (Tecan Group, Switzerland) at 25 °C. Various amounts of protein were added to a 150-μl aliquot of binding buffer (20 mm Tris-HCl (pH 8.0) for 0–200 mm KCl) containing 5 nm ssDNA. Each sample was allowed to equilibrate in the solution for 5 min, after which the fluorescence polarization was measured. The binding curve was fitted by the Hill equation: y = [RPA]n/(KDn + [RPA]n) where y is the binding fraction, n is the Hill coefficient, and KD is the apparent dissociation constant.
Single-molecule fluorescence data acquisition
Single-molecule fluorescence experiments were performed as described previously (50). Streptavidin (10 μg/ml) in a buffer containing 100 mm KCl and 20 mm Tris-HCl (pH 8.0) was added to the microfluidic chamber made of the PEG-coated coverslip and incubated for 10 min. After washing, 50 pm DNA or RNA was added to the chamber where it was immobilized for 10 min. Then, the free DNA or RNA was removed by washing with the reaction buffer. Afterward, the chamber was filled with the reaction buffer with an oxygen-scavenging system (0.8% d-glucose, 1 mg/ml glucose oxidase, 0.4 mg/ml catalase, and 4 mm Trolox). Imaging was initiated before the protein was flowed into the chamber. We used an exposure time of 100 ms for all single-molecule measurements at a constant temperature of 22 °C.
FRET data analysis
The FRET efficiency was calculated using IA/(ID + IA) where ID and IA represent the donor and acceptor intensities, respectively. Basic data analysis was carried out by scripts written in MATLAB, and all data fitting was performed by Origin 8.0. All fluorescent spots in each movie were selected unless the trace showed a poor signal:noise ratio or the intensity changes of the donor and acceptor did not match well. Each FRET histogram was constructed from more than 300 traces.
Gel filtration
DNA substrates were annealed in 20 mm Tris-HCl (pH 8.0) and 100 mm KCl with a 1:1 mixture of the stem and ssDNA strands (Table S1) by incubating the mixture at 95 °C for 5 min and then slowly cooling the mixture to room temperature over ∼7 h. Afterward, 2 μm DNA was incubated with varied amounts of RPA for 5 min. The gel filtration was performed at 25 °C using an FPLC system (GE Healthcare) with a Superdex 200 column (analytical grade) equilibrated with binding buffer (20 mm Tris-HCl (pH 8.0) and 100 mm KCl). A 100-μl aliquot of RPA–DNA complex was loaded onto the column and eluted at a flow rate of 0.3 ml/min with the buffer. The absorbances were monitored at 280 and 260 nm UV rays.
Author contributions
Q.-M. W. and X.-M. H. conceptualization; Q.-M. W. and X.-M. H. data curation; Q.-M. W., Y.-T. Y., Y.-R. W., B. G., and X. X. investigation; X.-M. H. funding acquisition; X.-M. H. writing-original draft; X.-M. H. project administration; X.-M. H. writing-review and editing.
Supplementary Material
This work was supported by Chinese Universities Scientific Fund Grant Z109021718 and National Natural Science Foundation of China Grant 11574252. The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Figs. S1–S8 and Table S1.
- RPA
- replication protein A
- ssDNA
- single-stranded DNA
- smFRET
- single-molecule fluorescence resonance energy transfer
- nt
- nucleotide(s)
- OB
- oligonucleotide-binding
- HR
- homologous recombination
- DSB
- double-strand break
- DBD
- DNA-binding domain.
References
- 1. Wold M. S. (1997) Replication protein A: a heterotrimeric, single-stranded DNA-binding protein required for eukaryotic DNA metabolism. Annu. Rev. Biochem. 66, 61–92 10.1146/annurev.biochem.66.1.61 [DOI] [PubMed] [Google Scholar]
- 2. Brosey C. A., Yan C., Tsutakawa S. E., Heller W. T., Rambo R. P., Tainer J. A., Ivanov I., and Chazin W. J. (2013) A new structural framework for integrating replication protein A into DNA processing machinery. Nucleic Acids Res. 41, 2313–2327 10.1093/nar/gks1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen R., and Wold M. S. (2014) Replication protein A: single-stranded DNA's first responder: dynamic DNA-interactions allow replication protein A to direct single-strand DNA intermediates into different pathways for synthesis or repair. BioEssays 36, 1156–1161 10.1002/bies.201400107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sugitani N., and Chazin W. J. (2015) Characteristics and concepts of dynamic hub proteins in DNA processing machinery from studies of RPA. Prog. Biophys. Mol. Biol. 117, 206–211 10.1016/j.pbiomolbio.2014.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Oakley G. G., and Patrick S. M. (2010) Replication protein A: directing traffic at the intersection of replication and repair. Front. Biosci. 15, 883–900 10.2741/3652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zou L., and Elledge S. J. (2003) Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science 300, 1542–1548 10.1126/science.1083430 [DOI] [PubMed] [Google Scholar]
- 7. Liu Y., Vaithiyalingam S., Shi Q., Chazin W. J., and Zinkel S. S. (2011) BID binds to replication protein A and stimulates ATR function following replicative stress. Mol. Cell. Biol. 31, 4298–4309 10.1128/MCB.05737-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xu X., Vaithiyalingam S., Glick G. G., Mordes D. A., Chazin W. J., and Cortez D. (2008) The basic cleft of RPA70N binds multiple checkpoint proteins, including RAD9, to regulate ATR signaling. Mol. Cell. Biol. 28, 7345–7353 10.1128/MCB.01079-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fanning E., Klimovich V., and Nager A. R. (2006) A dynamic model for replication protein A (RPA) function in DNA processing pathways. Nucleic Acids Res. 34, 4126–4137 10.1093/nar/gkl550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brosey C. A., Chagot M. E., Ehrhardt M., Pretto D. I., Weiner B. E., and Chazin W. J. (2009) NMR analysis of the architecture and functional remodeling of a modular multidomain protein, RPA. J. Am. Chem. Soc. 131, 6346–6347 10.1021/ja9013634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bochkarev A., and Bochkareva E. (2004) From RPA to BRCA2: lessons from single-stranded DNA binding by the OB-fold. Curr. Opin. Struct. Biol. 14, 36–42 10.1016/j.sbi.2004.01.001 [DOI] [PubMed] [Google Scholar]
- 12. Iftode C., and Borowiec J. A. (2000) 5′ → 3′ molecular polarity of human replication protein A (hRPA) binding to pseudo-origin DNA substrates. Biochemistry 39, 11970–11981 10.1021/bi0005761 [DOI] [PubMed] [Google Scholar]
- 13. Kolpashchikov D. M., Khodyreva S. N., Khlimankov D. Y., Wold M. S., Favre A., and Lavrik O. I. (2001) Polarity of human replication protein A binding to DNA. Nucleic Acids Res. 29, 373–379 10.1093/nar/29.2.373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bochkareva E., Belegu V., Korolev S., and Bochkarev A. (2001) Structure of the major single-stranded DNA-binding domain of replication protein A suggests a dynamic mechanism for DNA binding. EMBO J. 20, 612–618 10.1093/emboj/20.3.612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bochkareva E., Korolev S., Lees-Miller S. P., and Bochkarev A. (2002) Structure of the RPA trimerization core and its role in the multistep DNA-binding mechanism of RPA. EMBO J. 21, 1855–1863 10.1093/emboj/21.7.1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bastin-Shanower S. A., and Brill S. J. (2001) Functional analysis of the four DNA binding domains of replication protein A. The role of RPA2 in ssDNA binding. J. Biol. Chem. 276, 36446–36453 10.1074/jbc.M104386200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Blackwell L. J., and Borowiec J. A. (1994) Human replication protein A binds single-stranded DNA in two distinct complexes. Mol. Cell. Biol. 14, 3993–4001 10.1128/MCB.14.6.3993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bochkarev A., Pfuetzner R. A., Edwards A. M., and Frappier L. (1997) Structure of the single-stranded-DNA-binding domain of replication protein A bound to DNA. Nature 385, 176–181 10.1038/385176a0 [DOI] [PubMed] [Google Scholar]
- 19. Kim C., Paulus B. F., and Wold M. S. (1994) Interactions of human replication protein A with oligonucleotides. Biochemistry 33, 14197–14206 10.1021/bi00251a031 [DOI] [PubMed] [Google Scholar]
- 20. Arunkumar A. I., Stauffer M. E., Bochkareva E., Bochkarev A., and Chazin W. J. (2003) Independent and coordinated functions of replication protein A tandem high affinity single-stranded DNA binding domains. J. Biol. Chem. 278, 41077–41082 10.1074/jbc.M305871200 [DOI] [PubMed] [Google Scholar]
- 21. Blackwell L. J., Borowiec J. A., and Mastrangelo I. A. (1996) Single-stranded-DNA binding alters human replication protein A structure and facilitates interaction with DNA-dependent protein kinase. Mol. Cell. Biol. 16, 4798–4807 10.1128/MCB.16.9.4798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kowalczykowski S. C. (2015) An overview of the molecular mechanisms of recombinational DNA repair. Cold Spring Harb. Perspect. Biol. 7, a016410 10.1101/cshperspect.a016410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kaniecki K., De Tullio L., and Greene E. C. (2018) A change of view: homologous recombination at single-molecule resolution. Nat. Rev. Genet. 19, 191–207 10.1038/nrg.2017.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nguyen B., Sokoloski J., Galletto R., Elson E. L., Wold M. S., and Lohman T. M. (2014) Diffusion of human replication protein A along single-stranded DNA. J. Mol. Biol. 426, 3246–3261 10.1016/j.jmb.2014.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bain F. E., Fischer L. A., Chen R., and Wold M. S. (2018) Single-molecule analysis of replication protein A-DNA interactions. Methods Enzymol. 600, 439–461 10.1016/bs.mie.2017.11.016 [DOI] [PubMed] [Google Scholar]
- 26. Ma C. J., Gibb B., Kwon Y., Sung P., and Greene E. C. (2017) Protein dynamics of human RPA and RAD51 on ssDNA during assembly and disassembly of the RAD51 filament. Nucleic Acids Res. 45, 749–761 10.1093/nar/gkw1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pokhrel N., Origanti S., Davenport E. P., Gandhi D., Kaniecki K., Mehl R. A., Greene E. C., Dockendorff C., and Antony E. (2017) Monitoring replication protein A (RPA) dynamics in homologous recombination through site-specific incorporation of non-canonical amino acids. Nucleic Acids Res. 45, 9413–9426 10.1093/nar/gkx598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen R., Subramanyam S., Elcock A. H., Spies M., and Wold M. S. (2016) Dynamic binding of replication protein a is required for DNA repair. Nucleic Acids Res. 44, 5758–5772 10.1093/nar/gkw339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kemmerich F. E., Daldrop P., Pinto C., Levikova M., Cejka P., and Seidel R. (2016) Force regulated dynamics of RPA on a DNA fork. Nucleic Acids Res. 44, 5837–5848 10.1093/nar/gkw187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gibb B., Ye L. F., Gergoudis S. C., Kwon Y. H., Niu H., Sung P., and Greene E. C. (2014) Concentration-dependent exchange of replication protein A on single-stranded DNA revealed by single-molecule imaging. PLoS One 9, e87922 10.1371/journal.pone.0087922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen J., Le S., Basu A., Chazin W. J., and Yan J. (2015) Mechanochemical regulations of RPA's binding to ssDNA. Sci. Rep. 5, 9296 10.1038/srep09296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yates L. A., Aramayo R. J., Pokhrel N., Caldwell C. C., Kaplan J. A., Perera R. L., Spies M., Antony E., and Zhang X. (2018) A structural and dynamic model for the assembly of replication protein A on single-stranded DNA. Nat. Commun. 9, 5447 10.1038/s41467-018-07883-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sikorski T. W., Ficarro S. B., Holik J., Kim T., Rando O. J., Marto J. A., and Buratowski S. (2011) Sub1 and RPA associate with RNA polymerase II at different stages of transcription. Mol. Cell 44, 397–409 10.1016/j.molcel.2011.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ohle C., Tesorero R., Schermann G., Dobrev N., Sinning I., and Fischer T. (2016) Transient RNA-DNA hybrids are required for efficient double-strand break repair. Cell 167, 1001–1013.e7 10.1016/j.cell.2016.10.001 [DOI] [PubMed] [Google Scholar]
- 35. Nguyen H. D., Yadav T., Giri S., Saez B., Graubert T. A., and Zou L. (2017) Functions of replication protein A as a sensor of R loops and a regulator of RNaseH1. Mol. Cell 65, 832–847.e4 10.1016/j.molcel.2017.01.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fan J., and Pavletich N. P. (2012) Structure and conformational change of a replication protein A heterotrimer bound to ssDNA. Genes Dev. 26, 2337–2347 10.1101/gad.194787.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pestryakov P. E., Khlimankov D. Y., Bochkareva E., Bochkarev A., and Lavrik O. I. (2004) Human replication protein A (RPA) binds a primer-template junction in the absence of its major ssDNA-binding domains. Nucleic Acids Res. 32, 1894–1903 10.1093/nar/gkh346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pestryakov P. E., Weisshart K., Schlott B., Khodyreva S. N., Kremmer E., Grosse F., Lavrik O. I., and Nasheuer H. P. (2003) Human replication protein A. The C-terminal RPA70 and the central RPA32 domains are involved in the interactions with the 3 ′-end of a primer-template DNA. J. Biol. Chem. 278, 17515–17524 10.1074/jbc.M301265200 [DOI] [PubMed] [Google Scholar]
- 39. Lavrik O. I., Kolpashchikov D. M., Weisshart K., Nasheuer H. P., Khodyreva S. N., and Favre A. (1999) RPA subunit arrangement near the 3′-end of the primer is modulated by the length of the template strand and cooperative protein interactions. Nucleic Acids Res. 27, 4235–4240 10.1093/nar/27.21.4235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Byun T. S., Pacek M., Yee M. C., Walter J. C., and Cimprich K. A. (2005) Functional uncoupling of MCM helicase and DNA polymerase activities activates the ATR-dependent checkpoint. Genes Dev. 19, 1040–1052 10.1101/gad.1301205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bhat K. P., and Cortez D. (2018) RPA and RAD51: fork reversal, fork protection, and genome stability. Nat. Struct. Mol. Biol. 25, 446–453 10.1038/s41594-018-0075-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Roy R., Hohng S., and Ha T. (2008) A practical guide to single-molecule FRET. Nat. Methods 5, 507–516 10.1038/nmeth.1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pretto D. I., Tsutakawa S., Brosey C. A., Castillo A., Chagot M. E., Smith J. A., Tainer J. A., and Chazin W. J. (2010) Structural dynamics and single-stranded DNA binding activity of the three N-terminal domains of the large subunit of replication protein A from small angle X-ray scattering. Biochemistry 49, 2880–2889 10.1021/bi9019934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Witosch J., Wolf E., and Mizuno N. (2014) Architecture and ssDNA interaction of the Timeless-Tipin-RPA complex. Nucleic Acids Res. 42, 12912–12927 10.1093/nar/gku960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Brosey C. A., Soss S. E., Brooks S., Yan C., Ivanov I., Dorai K., and Chazin W. J. (2015) Functional dynamics in replication protein A DNA binding and protein recruitment domains. Structure 23, 1028–1038 10.1016/j.str.2015.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Salas T. R., Petruseva I., Lavrik O., and Saintomé C. (2009) Evidence for direct contact between the RPA3 subunit of the human replication protein A and single-stranded DNA. Nucleic Acids Res. 37, 38–46 10.1093/nar/gkn895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jensen R. B., Carreira A., and Kowalczykowski S. C. (2010) Purified human BRCA2 stimulates RAD51-mediated recombination. Nature 467, 678–683 10.1038/nature09399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Henricksen L. A., Umbricht C. B., and Wold M. S. (1994) Recombinant replication protein A. Expression, complex formation, and functional characterization. J. Biol. Chem. 269, 11121–11132 [PubMed] [Google Scholar]
- 49. Dou S. X., Wang P. Y., Xu H. Q., and Xi X. G. (2004) The DNA binding properties of the Escherichia coli RecQ helicase. J. Biol. Chem. 279, 6354–6363 10.1074/jbc.M311272200 [DOI] [PubMed] [Google Scholar]
- 50. Hou X. M., Wu W. Q., Duan X. L., Liu N. N., Li H. H., Fu J., Dou S. X., Li M., and Xi X. G. (2015) Molecular mechanism of G-quadruplex unwinding helicase: sequential and repetitive unfolding of G-quadruplex by Pif1 helicase. Biochem. J. 466, 189–199 10.1042/BJ20140997 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.