Abstract
In this review, we discuss the often overlooked tissue-resident fetal macrophages, Hofbauer cells, which are found within the chorionic villi of the human placenta. Hofbauer cells have been shown to have a phenotype associated with regulatory and anti-inflammatory functions. They are thought to play a crucial role in the regulation of pregnancy and in the maintenance of a homeostatic environment that is crucial for fetal development. Even though the numbers of these macrophages are some of the most abundant immune cells in the human placenta, which are sustained throughout pregnancy, there are very few studies that have identified their origin, their phenotype, and functions and why they are maintained throughout gestation. It is not yet understood how Hofbauer cells may change in function throughout normal pregnancy, and especially in those complicated by maternal gestational diabetes, preeclampsia, and viral infections, such as Zika, cytomegalovirus, and human immunodeficiency virus. We review what is known about the origin of these macrophages and explore how common complications of pregnancy dysregulate these cells leading to adverse birth outcomes in humans. Our synthesis sheds light on areas for human studies that can further define these innate regulatory cells.
Keywords: Placenta, Macrophages, Hofbauer cells, Human immunodeficiency virus, ZIKA
Introduction
Macrophages are tissue-resident immune cells in the same way as fibroblasts, MAIT cells, and Langerhans cells and perform crucial immunological functions such as antigen presentation, phagocytosis, cytokine secretion, and coordination of innate and adaptive immune responses [1]. Macrophages play a role in virtually every aspect of an organism's biology, from development and homeostasis to tissue repair, as well as immune responses to pathogens. They are a heterogeneous population of immune cells that constantly change their functional state in response to changes in tissue physiology or environmental challenges [2]. The plasticity of these cells allows them to respond to various environmental signals and change their phenotype and physiology in response to cytokines and microbial signals [3]. Historically, tissue-resident macrophages were believed to be continuously replenished by blood-circulating monocytes that originate from progenitors in adult bone marrow. This concept was central in defining the “mononuclear phagocyte system” that grouped together precursors of monocytes in the bone marrow, monocytes in peripheral blood, and macrophages in the tissues [4, 5]. However, advanced techniques for studying cellular ontogeny showed that the homeostatic contribution of peripheral blood monocytes to tissue-resident macrophage populations may be confined to specific tissues such as gut, dermis, and the heart with tissue-specific turnover rates. Alternatively, most tissue-resident macrophages arise from embryonic precursors prior to birth and maintain themselves locally throughout adulthood, independent of circulating monocytes [6, 7].
The chorionic villi of placental mammals has conspicuous macrophages known as the Hofbauer cells, which are considered fetal in origin [8]. Three-dimensional studies and histological analyses of human placental villous tissue show that Hofbauer cells are large (10–30 µm), pleomorphic, highly vacuolated with granular cytoplasm [9, 10, 11]. They are found within the fetal villi of the placenta from the first trimester of pregnancy until birth [12], and histological analyses show their location to be close to fetal vessels and trophoblasts, making them likely candidates for placental development and homeostasis [13]. These macrophages were named after an American gynecologist, J. Isfred Isidore Hofbauer (1879–1961); however, little is known about his research or work. (online suppl. Fig. 1 shows the only known portrait of J. Isfred Isidore Hofbauer, courtesy of Rubenstein Rare Book and Manuscript Library of Duke University, for all online suppl. material, see www.karger.com/doi/10.1159/000/497416). Although their origin has yet to be fully established, some studies have proposed that during the first trimester of pregnancy, they originate from mesenchymal progenitor cells [14, 15, 16], while the existence of transitional forms between monocytes and macrophages in the second and third trimester suggests that they differentiate from circulating monocytes of the fetus [17, 18]. Takahashi and colleagues reported that primitive macrophages appear as early as day 10 of gestation in the blood vessels of the chorionic villi of the mouse placenta, and that these macrophages enter the chorionic villous mesenchymal stroma and ingest fluid-like stromal materials to transform into Hofbauer cells [19]. Using chromogenic in situ hybridization for Y-chromosome (DYZ1), Kim and colleagues showed that placental villous tissues from male neonates had chromogenic in situ hybridization+ signals from Y chromosome in most macrophages, but not in lymphocytes, suggesting that most macrophages were of fetal origin [20].
Hofbauer cells are targets of a number of viruses and other pathogens at the maternal–fetal interface [21, 22, 23]. Better characterized are decidual macrophages, which are the second most abundant immune cell type in the uterine decidua after decidual natural killer cells [24, 25]. No studies appear to exist that directly compare the numbers and function of HCs and decidual macrophages. In fact, characterizing the role of Hofbauer cells in protecting the fetus and maintaining tolerance has somehow been neglected, especially when there is an adverse pregnancy outcome.
Results
Classical and Alternative Polarization of Hofbauer Cells
The functional maturation of macrophages was described in a manner similar to the well-characterized concept of T helper type 1and type 2 polarization of effector T cells [26, 27]. They have been classified as M1 (classically activated) and M2 (alternatively activated) macrophages [28, 29], with M1-like macrophages secreting proinflammatory cytokines and mediating resistance to pathogens, but also contributing to tissue destruction; while M2 macrophages secrete anti-inflammatory cytokines and promote tissue repair and remodeling [29, 30]. However, this paradigm of macrophage activation is simplistic, especially in humans [2]. This definition only considered the effects of particular stimuli on macrophage polarization, neglecting complex mechanisms that leads to macrophage activation in different tissues. A great variety of intermediates have been described based on their plasticity and adaptability [3] so that this concept may need to be revised. However, a comprehensive classification that will consider in vitro stimulus activation, cell origin, tissue microenvironment, pathology, and time is still required [2]. One M1-like phenotype has been described and compared to several M2 phenotypes, such as M2a, M2b, M2c, and M2d [31, 32], where M2b macrophages share characteristics with M1-like macrophages [33]. Each of the M2 macrophage subtypes differs in a number of aspects such as expression of certain surface molecules, cytokine secretion, and function [3]. Although oversimplified, the M1/M2 paradigm provides a useful framework, especially for specific immune responses. Defining the phenotype of HCs within this spectrum of macrophage polarity will illuminate the functional role of these cells in the placenta. Do HCs express M1-like markers, as they protect the fetus from maternally transmitted infections, or do they express an M2-like phenotype, to promote tolerance? Or, do these cells coexpress M1/M2 markers depending on the role being played at any given time?
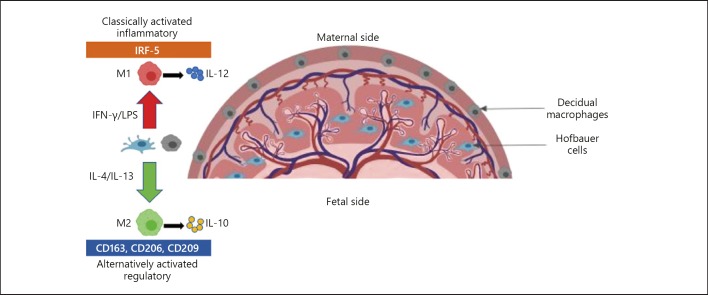
Figure 1 shows the location of Hofbauer cells and decidual macrophages in the chorionic villi and the decidua of the placenta, respectively. Based on the M1/M2 macrophage polarization paradigm, IL-4 or IL-13 would polarize these macrophages toward a regulatory, M2 phenotype that is characterized by the expression of the scavenger receptor, CD163; mannose receptor, CD206; DC-SIGN, CD209; and secretion of IL-10. Whereas, IFN-γ or LPS would polarize these macrophages toward a proinflammatory, M1 phenotype that is characterized by the lack of expression of markers associated with the M2 phenotype, IRF-5 expression and high secretion of proinflammatory cytokines, such as IL-12 [34, 35, 36, 37]. Apart from placental-tissue localization, there are no specific-markers to distinguish decidual macrophages from HCs and all other human tissue macrophages. To understand the developmental nature of macrophages in the placenta, and hence how they contribute to maternal–fetal tolerance, there is a need to distinguish between these macrophage populations.
Fig. 1.
Anatomical location of decidual macrophages and Hofbauer cells in the placenta. M1 macrophages are distinguished by the lack of expression of M2 markers. M1 macrophages secrete high levels of IL-12 while M2 macrophages secrete high levels of the IL-10 cytokine upon polarization.
The Role of Hofbauer Cells in Pregnancy
During pregnancy, the fetus with both maternal and paternal alleles develops within an active maternal immune system without succumbing to immunological rejection [38, 39]. Medawar [40] proposed the presence of immunological tolerance toward the semi-allogeneic fetus that facilitates pregnancy; however, this mechanism is yet to be fully understood. Macrophages have been shown to play a role in regulating pregnancy [41], promote tolerance toward the semi-allogeneic fetus [42], and maintain a homeostatic environment crucial for normal fetal development [38, 43]. In a study aimed at investigating the antigenic phenotype of Hofbauer cells from first- and third-trimester placentas, Goldstein and colleagues showed that these villous stromal macrophages were numerically higher than other villous stromal mononuclear cells and that their phenotype was different from that of macrophages from other tissues. They also showed that the Hofbauer cells possessed surface expression of CD4 and suggested that these cells may serve as a portal of entry, or a reservoir, of human immunodeficiency virus (HIV) in placentas from viremic mothers [44], as will be discussed more in depth.
Hofbauer cells, like other macrophages, express the 3 IgG Fcγ receptors (FcγR): FcγRI (CD64), FcγRII (CD32), and FcγRIII (CD16) [45] and also the pan-macrophage marker, CD68 [46]. They are thought to have an immunoregulatory phenotype consistent with that of M2 anti-inflammatory macrophages, and several studies have shown that Hofbauer cells can be stimulated by glucocorticoids [47] and IL-10 [43] to express CD163, CD206, and CD209 [43], while secreting IL-10 and TGF-β [48]. Hofbauer cells have also been reported to constitute a mixture of M2a, M2b, and M2c macrophages that differ in marker surface expression, cytokine secretion and functions [49], reinforcing the concept of a regulatory rather than inflammatory role of these cells. There is limited information on the role of these cells in placental physiology; however, there have been suggestions that they may play a role in transport of nutrients within the villous stroma and in transmission of antibodies from the mother to fetus through the surface expression of Fc receptors [50, 51, 52]. Hofbauer cells have also been associated with numerous complications of pregnancy such as chorioamnionitis (CA), miscarriage, and preterm birth [53]. The potential role of HCs in the pathophysiology of complications of pregnancy such as villitis of unknown etiology and histological CA, has been discussed by Tang et al. [53]; however, there is very little information on the effect of other common complications of pregnancy and maternal infections on HC phenotypic polarity and gene expression. The polarity and function of maternal decidual macrophages have been reviewed by Brown et al. [54] and others [55] and will not be featured in this review. Rather, we will focus on the impact of common complications of pregnancy; CA, preeclampsia (PE), and gestational diabetes mellitus (GDM) on the phenotype and function of Hofbauer cells and their role in the vertical transmission of congenital viral infections such as Zika virus (ZIKV), HIV, and cytomegalovirus (CMV). We also highlight gaps in knowledge about Hofbauer cells and contradictory findings of studies on their dysregulation in pathological pregnancies.
Impact of Common Complications of Pregnancy on Hofbauer Cells
Chorioamnionitis
CA is an acute inflammation of placental membranes and the chorion due to microbial infections such as Escherichia coli and Group B Streptococcus [56, 57, 58]. Elevated levels of proinflammatory cytokines during CA-specific immune responses are associated with disease in the fetal pulmonary system and brain damage, making CA a risk factor for neonatal morbidity and mortality [59]. Although the pathophysiology of CA is yet to be elucidated, it is believed to be a consequence of the disturbed immune homeostasis in the placenta associated with Hofbauer cells.
There is a contradictory evidence for the effect of CA on the polarization of Hofbauer cells. Joerink et al. [60], suggested that maternal allergen sensitization and the presence of CA have no effect on the phenotype of Hofbauer cells; however, phenotypic and genetic studies have reported impaired function of Hofbauer cells during CA infection [61]. In line with this study, Vinnars et al. [62] had earlier reported that there is a drastic decrease in the number of CD68+ (pan-macrophage marker) Hofbauer cells in the presence of CA compared to healthy controls. Conversely, Hung et al. [63] and Toti et al. [64] showed that the number of CD68+ Hofbauer cells of placentas complicated by CA is increased. We suggest that the discrepancies may be due to differences in quantification and propose that a stringent standardized method should be used to quantify immunohistochemistry and immunofluorescence data. There is also very little information on the impact of altered numbers and/or regulation of selected macrophage markers by CA.
Gestational Diabetes Mellitus
GDM is maternal hyperglycemia due to insulin resistance that develops during pregnancy to create a glucose gradient necessary for the supply of energy and nutrients to the developing fetus [65, 66]. GDM prevalence ranges from 3 to 20% in pregnant women [67] and is associated with chronic low-grade inflammation of the placenta [68, 69]. Sisino et al. [70] reported that in pregnant rats receiving streptozotocin during pregnancy, diabetes alters the normal phenotype of HCs from an M2 anti-inflammatory regulatory phenotype to a proinflammatory phenotype. A recent pilot study by Schliefsteiner et al. [71] contradicts these findings as they reported that HCs maintain an anti-inflammatory M2 phenotype despite the presence of GDM in humans. During inflammatory diseases of the placenta, such as villitis of unknown etiology, there is increased infiltration of the chorionic villi by Hofbauer cells [20, 72], a phenomenon similarly reported in pregnancies complicated by GDM [73]. However, the role and function that Hofbauer cells play or are involved in these conditions are unclear.
Preeclampsia
PE is a placental disorder of unknown etiology, and it is likely that aberrant immune activation plays a role in its pathogenesis [74]. It is characterized by hypertension (> 140/90 mm Hg) after 20 weeks of gestation and proteinuria (> 300 mg/L per day) [75]. Its prevalence ranges from 5 to 10% globally, making it one of the leading causes of morbidity and mortality in mother and child [76]. Przybyl et al. [77] reported that CD74, a human leukocyte antigen class II histocompatibility antigen-γ chain, is downregulated on Hofbauer cells in the presence of PE. They suggested that this downregulation alters macrophage polarization from an immunoregulatory M2 phenotype toward a proinflammatory signature affecting essential macrophage-trophoblast crosstalk [77]. It has been reported that the number of Hofbauer cells is significantly decreased in the presence of PE and that the CD209+ HCs from pregnancies with PE secrete less IL-10 than those from normal pregnancies [78]. These data suggest that PE affects an essential immune regulatory role of Hofbauer cells in maternal–fetal tolerance. More studies are needed to investigate the bidirectional effects of PE and Hofbauer cells, and the role of these cells in regulating pregnancies is complicated by PE.
Viral Permissiveness of Hofbauer Cells to ZIKV
Vertical transmission of a number of viruses such as ZIKV, rubella, CMV, herpes simplex virus, and HIV-1, from an infected mother to her developing fetus, suggests viral tropism for placental cells [79]. ZIKV is a mosquito-borne flavivirus, which can be vertically transmitted from an infected mother to her fetus [80, 81], causing adverse birth outcomes such as fetal brain abnormalities and microcephaly [82]. ZIKV-specific antigen was detected in Hofbauer cells and histiocytes in the intervillous space of a placenta from a mother with ZIKA [83]. During pregnancy, increased levels of proinflammatory cytokines such as IL-6, CXCL8 (IL-8), and TNF have been reported in the amniotic fluid, decidua, fetal membranes, and maternal serum [84, 85]. The mechanism by which ZIKV breaches the placental barrier is not clear, and ZIKV RNA has been detected in amniotic fluid and in fetal and newborn brain tissue [83, 86, 87]. Vertical transmission of the ZIKV to the human fetus is mediated through productive infection of Hofbauer cells [88, 89], leading to cell proliferation and hyperplasia of these cells in the second and third trimester [90]. Thus, placental inflammatory abnormalities are not a component of vertical transmission of the ZIKV per se.
Quicke et al. [88] demonstrated that human placental Hofbauer cells are much more susceptible to productive ZIKV infection than autologous cytotrophoblasts. They showed that, upon infection, Hofbauer cells induce secretion of type I interferon, other proinflammatory cytokines, and upregulation of antiviral gene expression [88]. Their findings corresponded to those of Jurado et al. [89], Simoni et al. [91], and others [92]. However, these studies did not demonstrate the phenotypic changes or shift in activation status of Hofbauer cells that may be associated with abnormal functions of Hofbauer cells upon ZIKV infection. ZIKV infection has been shown to be enhanced by preexisting anti-flavivirus immunity through IgG engagement of the FcγR [93]. This antibody-dependent enhancement of infection has also been reported in other flaviviridae, such as dengue virus [94, 95]. Although there is evidence of in vitro Hofbauer cells infection by ZIKV, the mechanisms by which the virus reaches these cells in the villi and how it is further transmitted to the fetal circulation needs further investigation. Phenotypic and functional changes of Hofbauer cells upon ZIKV infection have not yet been fully elucidated [90, 91, 96].
Human Immunodeficiency Virus-1
The large burden of HIV infection in sub-saharan Africa, where adolescent girls and young women between the ages of 15 and 24 years are bearing the brunt of the epidemic [97], has resulted in most HIV-1-infected pregnant women being placed on combination antiretroviral therapy (ART). These women show a high prevalence of aggravated PE [98] and adverse birth outcomes, including fetal death, preterm births [99, 100], and small-for-gestational age infants [100, 101, 102]. Whether Hofbauer cells are determinants of these adverse birth outcomes is not understood. The phenotype of Hofbauer cells favors productive infection with HIV-1 [52] and the 15–30% [103] vertical transmission rate, prior to prevention of mother-to-child transmission with ART, is testimony to this. In vivo [104] and in vitro [48] studies confirmed that Hofbauer cells are indeed targets of HIV-1.
During maternal HIV-1 infection, antibody- and cell-associated virions, cell-free virions, and maternal broadly neutralizing antibodies cross the placental barrier to interact with Hofbauer cells prior to entering the fetal circulation [105]. However, whether Hofbauer cells are involved in facilitating in utero HIV infection is unclear as these cells have proved to be invariably infected in vitro [106], where it has been shown that they can limit HIV-1 replication by the induction of immunoregulatory cytokines [48]. These cells also possess intrinsic adaptations that facilitate the sequestration of HIV-1 that may serve as a protective viral reservoir allowing for antiretroviral (ARV) drug entry in utero and potentially the inactivation of the virus [106]. Therefore, how Hofbauer cells play a role in utero in protecting or mediating HIV-1 transmission is unclear. Various studies have demonstrated the permissiveness of Hofbauer cells to HIV-1 infection [107]; however, there are few data on the effect of HIV-1 and/or ARV drugs on the phenotype and function of these cells. Further investigation of the involvement of Hofbauer cells in HIV infection may shed light on the ability of these macrophages to inhibit or facilitate viral transmission, which can be relevant to understand other maternally acquired viruses.
Now with successful prevention of mother-to-child transmission programs, where viral transmission has been reduced to 1–2% [108, 109], there is concern that maternal–fetal tolerance may be disrupted by ARV drugs per se. As discussed above, widespread use of ARV drugs has been associated with adverse birth outcomes prevalent among HIV-1-infected women on ART [99, 100]. Whether ARV drugs have teratogenic effects via Hofbauer cells dysfunction remains unknown.
Human Cytomegalovirus
Human CMV (HCMV) is among the leading causes of congenital infections globally [110]. It is a prevalent species-specific herpes virus that causes asymptomatic infections in healthy individuals but leads to sensorineural hearing and neurodevelopmental delay in infants [111] and increased morbidity and mortality in immunocompromised individuals [112]. HCMV can be transmitted from an infected mother to her developing fetus through the placenta [113, 114] and is a major cause of intrauterine growth restriction [115]. The mechanisms involved in transplacental HCMV transmission are poorly understood. Maternal infections with HCMV during pregnancy are associated with high risk of viral transmission to the fetus [111], where primary HCMV transmission from mother to child during gestation is 35–40% [116]. El Costa et al. [117] proposed that transmission of viruses is facilitated by either the hemotogenous spread to the placenta or by cellular transfer from the maternal decidua to the anchoring placental villi in early pregnancy.
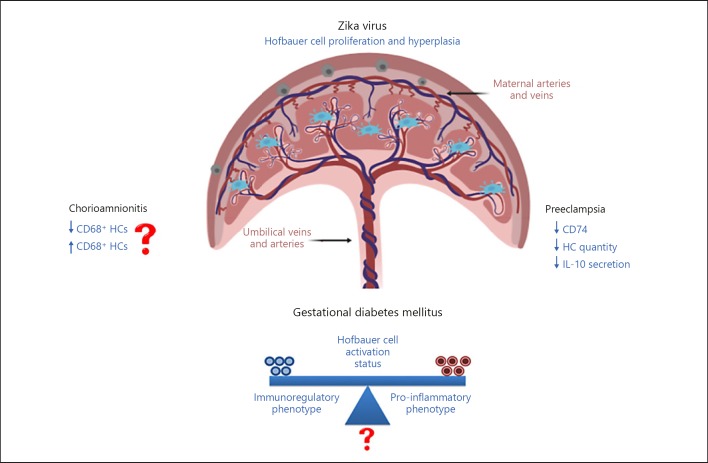
A number of studies have reported the ability of HCMV to infect monocytes, macrophages, and their progenitors [118, 119], suggesting that the macrophage lineage provides long-lived reservoirs for HCMV latency [120, 121]. At the maternal–fetal interface, HCMV has been shown to induce a distinct decidual tissue innate immune response and to dysregulate tolerance [122]. A better understanding of innate immune mechanisms at the maternal–fetal interface that modulates transplacental transmission of viruses is important to define, so that adverse birth outcomes that are associated with these infections can be prevented. Figure 2 highlights the lack of consensus and gaps in knowledge (denoted by “?”) from studies showing the impact of pregnancy complications and viral diseases on Hofbauer cell quantity, phenotype, and function. The lack of consensus in the literature is summarized in Table 1.
Fig. 2.
The impact of common complications (Preeclampsia, Gestational Diabetes Mellitus [GDM] and Chorioamnionitis [CA]) of pregnancy and viral infections (Zika) on the quantity, phenotype and function of Hofbauer cells. “?” denotes unknown or poorly defined and arrows represent either up- or down-regulated.
Table 1.
Lack of consensus in the literature on the impact of complications of pregnancy and maternal viral infection on HC
| Pregnancy complication | Effect on HCs | References |
|---|---|---|
| Chorioamnionitis | Decrease in numbers | [62] |
| Chorioamnionitis | Increase in numbers | [63, 64] |
| Chorioamnionitis | No effect on polarization status | [60] |
| Gestational diabetes mellitus | Acquisition of a proinflammatory phenotype | [70] |
| Gestational diabetes mellitus | Maintain an anti-inflammatory phenotype | [71] |
| Preeclampsia | Downregulation of CD74 and acquisition of a proinflammatory phenotype | [77] |
| Preeclampsia | Decrease in numbers | [78] |
| ZIKV infection | Proliferation and hyperplasia | [90] |
| ZIKV infection | IFN & antiviral gene induction | [88] |
| HIV infection | Induction of immune-modulatory cytokines | [48] |
Conclusion
The origin and role of Hofbauer cells at the maternal–fetal interface and in the promotion of tolerance and regulation of pregnancy are not clear, and their function during gestation warrants further investigation. What contributes to the elusive nature of these cells is their apparent ability to adapt to their microenvironment, including the placenta – and likely shape the link between mother and fetus. The selective role of Hofbauer cells to different viruses highlights their pleiotropic nature and a possible gatekeeping role in determining which viral infections are congenital in the newborn infant. How Hofbauer cells appear to be relatively resistant to HIV, as opposed to Zika, remains an evolutionary and developmental enigma. We propose that identifying a biomarker of Hofbauer cell dysfunction associated with various maternal infections during pregnancy will allow for the development of placental interventions to mitigate adverse birth outcomes and improve the health of both mother and her newborn infant.
Statement of Ethics
The authors have no ethical conflicts to disclose.
Disclosure Statement
The authors have no conflicts of interest to declare.
Funding Source
M.Z.Z. was a recipient of the Newton Fund Biomedical Sciences Exchange PhD Studentship, a South African National Research Foundation (NRF) scholarship and a Canadian Africa Prevention Trials (CAPT) network scholarship.
Author Contributions
M.Z.Z. conceived the review; M.Z.Z., F.O.M., S.G., and C.M.G. wrote the review.
Supplementary Material
Supplementary data
References
- 1.Fujiwara T, Fukushi J, Yamamoto S, Matsumoto Y, Setsu N, Oda Y, et al. Macrophage infiltration predicts a poor prognosis for human ewing sarcoma. Am J Pathol. 2011 Sep;179((3)):1157–70. doi: 10.1016/j.ajpath.2011.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014 Mar;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008 Dec;8((12)):958–69. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Furth R, Cohn ZA, Hirsch JG, Humphrey JH, Spector WG, Langevoort HL. The mononuclear phagocyte system: a new classification of macrophages, monocytes, and their precursor cells. Bull World Health Organ. 1972;46((6)):845–52. [PMC free article] [PubMed] [Google Scholar]
- 5.Yona S, Gordon S. From the reticuloendothelial to mononuclear phagocyte system - the unaccounted years. Front Immunol. 2015 Jul;6:328. doi: 10.3389/fimmu.2015.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoeffel G, Ginhoux F. Ontogeny of Tissue-Resident Macrophages. Front Immunol. 2015 Sep;6:486. doi: 10.3389/fimmu.2015.00486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ginhoux F, Guilliams M. Tissue-Resident Macrophage Ontogeny and Homeostasis. Immunity. 2016 Mar;44((3)):439–49. doi: 10.1016/j.immuni.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 8.Reyes L, Wolfe B, Golos T. Hofbauer Cells: Placental Macrophages of Fetal Origin. Results Probl Cell Differ. 2017;62:45–60. doi: 10.1007/978-3-319-54090-0_3. [DOI] [PubMed] [Google Scholar]
- 9.Castellucci M, Zaccheo D, Pescetto G. A three-dimensional study of the normal human placental villous core. I. The Hofbauer cells. Cell Tissue Res. 1980;210((2)):235–47. doi: 10.1007/BF00237612. [DOI] [PubMed] [Google Scholar]
- 10.Castellucci M, Kosanke G, Verdenelli F, Huppertz B, Kaufmann P. Villous sprouting: fundamental mechanisms of human placental development. Hum Reprod Update. 2000 Sep-Oct;6((5)):485–94. doi: 10.1093/humupd/6.5.485. [DOI] [PubMed] [Google Scholar]
- 11.Enders AC, King BF. The cytology of Hofbauer cells. Anat Rec. 1970 Jun;167((2)):231–6. doi: 10.1002/ar.1091670211. [DOI] [PubMed] [Google Scholar]
- 12.Wetzka B, Clark DE, Charnock-Jones DS, Zahradnik HP, Smith SK. Isolation of macrophages (Hofbauer cells) from human term placenta and their prostaglandin E2 and thromboxane production. Hum Reprod. 1997 Apr;12((4)):847–52. doi: 10.1093/humrep/12.4.847. [DOI] [PubMed] [Google Scholar]
- 13.Katabuchi H. THE MYSTERY OF HOFBAUER CELLS. Placenta. 2014;35((10)):A2–2. [Google Scholar]
- 14.Fox H. The incidence and significance of Hofbauer cells in the mature human placenta. J Pathol Bacteriol. 1967 Apr;93((2)):710–7. doi: 10.1002/path.1700930239. [DOI] [PubMed] [Google Scholar]
- 15.Vacek Z. Derivation and ultrastructure of the stroma cells of the human chorionic villus. Folia Morphol (Praha) 1970;18((1)):1–13. [PubMed] [Google Scholar]
- 16.Kaufmann P, Stark J, Stegner HE. The villous stroma of the human placenta. I. The ultrastructure of fixed connective tissue cells. Cell Tissue Res. 1977 Feb;177((1)):105–21. doi: 10.1007/BF00221122. [DOI] [PubMed] [Google Scholar]
- 17.Selkov SA, Selutin AV, Pavlova OM, Khromov-Borisov NN, Pavlov OV. Comparative phenotypic characterization of human cord blood monocytes and placental macrophages at term. Placenta. 2013 Sep;34((9)):836–9. doi: 10.1016/j.placenta.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Moskalewski S, Czarnik Z, Ptak W. Demonstration of cells with igg receptor in human placenta. Biol Neonate. 1975;26((3-4)):268–73. doi: 10.1159/000240738. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi K, Naito M, Katabuchi H, Higashi K. Development, differentiation, and maturation of macrophages in the chorionic villi of mouse placenta with special reference to the origin of Hofbauer cells. J Leukoc Biol. 1991 Jul;50((1)):57–68. doi: 10.1002/jlb.50.1.57. [DOI] [PubMed] [Google Scholar]
- 20.Kim JS, Romero R, Kim MR, Kim YM, Friel L, Espinoza J, et al. Involvement of Hofbauer cells and maternal T cells in villitis of unknown aetiology. Histopathology. 2008 Mar;52((4)):457–64. doi: 10.1111/j.1365-2559.2008.02964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arora N, Sadovsky Y, Dermody TS, Coyne CB. Microbial Vertical Transmission during Human Pregnancy. Cell Host Microbe. 2017 May;21((5)):561–7. doi: 10.1016/j.chom.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cao B, Diamond MS, Mysorekar IU. Maternal-Fetal Transmission of Zika Virus: Routes and Signals for Infection. J Interferon Cytokine Res. 2017 Jul;37((7)):287–94. doi: 10.1089/jir.2017.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDonagh S, Maidji E, Ma W, Chang HT, Fisher S, Pereira L. Viral and bacterial pathogens at the maternal-fetal interface. J Infect Dis. 2004 Aug;190((4)):826–34. doi: 10.1086/422330. [DOI] [PubMed] [Google Scholar]
- 24.Nagamatsu T, Schust DJ. The immunomodulatory roles of macrophages at the maternal-fetal interface. Reprod Sci. 2010 Mar;17((3)):209–18. doi: 10.1177/1933719109349962. [DOI] [PubMed] [Google Scholar]
- 25.Svensson-Arvelund J, Ernerudh J. The Role of Macrophages in Promoting and Maintaining Homeostasis at the Fetal-Maternal Interface. Am J Reprod Immunol. 2015 Aug;74((2)):100–9. doi: 10.1111/aji.12357. [DOI] [PubMed] [Google Scholar]
- 26.Romagnani P, Annunziato F, Piccinni MP, Maggi E, Romagnani S. Th1/Th2 cells, their associated molecules and role in pathophysiology. Eur Cytokine Netw. 2000 Sep;11((3)):510–1. [PubMed] [Google Scholar]
- 27.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000 Jun;164((12)):6166–73. doi: 10.4049/jimmunol.164.12.6166. [DOI] [PubMed] [Google Scholar]
- 28.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010 Oct;11((10)):889–96. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 29.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003 Jan;3((1)):23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 30.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008 Jan;13((13)):453–61. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 31.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004 Dec;25((12)):677–86. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 32.Ferrante CJ, Pinhal-Enfield G, Elson G, Cronstein BN, Hasko G, Outram S, et al. The adenosine-dependent angiogenic switch of macrophages to an M2-like phenotype is independent of interleukin-4 receptor alpha (IL-4Rα) signaling. Inflammation. 2013 Aug;36((4)):921–31. doi: 10.1007/s10753-013-9621-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sironi M, Martinez FO, D'Ambrosio D, Gattorno M, Polentarutti N, Locati M, et al. Differential regulation of chemokine production by Fcgamma receptor engagement in human monocytes: association of CCL1 with a distinct form of M2 monocyte activation (M2b, Type 2) J Leukoc Biol. 2006 Aug;80((2)):342–9. doi: 10.1189/jlb.1005586. [DOI] [PubMed] [Google Scholar]
- 34.Barros MH, Hauck F, Dreyer JH, Kempkes B, Niedobitek G. Macrophage polarisation: an immunohistochemical approach for identifying M1 and M2 macrophages. PLoS One. 2013 Nov;8((11)):e80908. doi: 10.1371/journal.pone.0080908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krausgruber T, Saliba D, Blazek K, Lockstone H, Sahgal N, Alzabin S, et al. IRF5 as a defining factor of M1 macrophage polarization. Cytokine. 2010;52((1-2)):44–44. [Google Scholar]
- 36.Buechler C, Ritter M, Orsó E, Langmann T, Klucken J, Schmitz G. Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro- and antiinflammatory stimuli. J Leukoc Biol. 2000 Jan;67((1)):97–103. [PubMed] [Google Scholar]
- 37.Tarique AA, Logan J, Thomas E, Holt PG, Sly PD, Fantino E. Phenotypic, functional, and plasticity features of classical and alternatively activated human macrophages. Am J Respir Cell Mol Biol. 2015 Nov;53((5)):676–88. doi: 10.1165/rcmb.2015-0012OC. [DOI] [PubMed] [Google Scholar]
- 38.Erlebacher A. Immunology of the Maternal-Fetal Interface. In: Littman DR, Yokoyama WM, editors. Annual Review of Immunology. Volume 31. 2013. pp. pp. 387–411. [DOI] [PubMed] [Google Scholar]
- 39.Gomez-Lopez N, StLouis D, Lehr MA, Sanchez-Rodriguez EN, Arenas-Hernandez M. Immune cells in term and preterm labor. Cell Mol Immunol. 2014 Nov;11((6)):571–81. doi: 10.1038/cmi.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Medawar PB. Some Immunological and Endocrinological Problems Raised by the Evolution of Viviparity in Vertebrates. Symp Soc Exp Biol. 1953;7:320–38. [Google Scholar]
- 41.Mor G, Abrahams VM. Potential role of macrophages as immunoregulators of pregnancy. Reprod Biol Endocrinol. 2003 Dec;1((1)):119–119. doi: 10.1186/1477-7827-1-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Svensson-Arvelund J, Mehta RB, Lindau R, Mirrasekhian E, Rodriguez-Martinez H, Berg G, et al. The human fetal placenta promotes tolerance against the semiallogeneic fetus by inducing regulatory T cells and homeostatic M2 macrophages. J Immunol. 2015 Feb;194((4)):1534–44. doi: 10.4049/jimmunol.1401536. [DOI] [PubMed] [Google Scholar]
- 43.Svensson J, Jenmalm MC, Matussek A, Geffers R, Berg G, Ernerudh J. Macrophages at the fetal-maternal interface express markers of alternative activation and are induced by M-CSF and IL-10. J Immunol. 2011 Oct;187((7)):3671–82. doi: 10.4049/jimmunol.1100130. [DOI] [PubMed] [Google Scholar]
- 44.Goldstein J, Braverman M, Salafia C, Buckley P. The phenotype of human placental macrophages and its variation with gestational age. Am J Pathol. 1988 Dec;133((3)):648–59. [PMC free article] [PubMed] [Google Scholar]
- 45.Bright NA, Ockleford CD, Anwar M. Ontogeny and distribution of Fc gamma receptors in the human placenta. Transport or immune surveillance? J Anat. 1994 Apr;184((Pt 2)):297–308. [PMC free article] [PubMed] [Google Scholar]
- 46.Vinnars MT, et al. The number of CD68+(Hofbauer) cells is decreased in placentas with chorioamnionitis and with advancing gestational age. Placenta. 2008;29((8)):A48–48. doi: 10.2350/09-03-0632-OA.1. [DOI] [PubMed] [Google Scholar]
- 47.Tang Z, Niven-Fairchild T, Tadesse S, Norwitz ER, Buhimschi CS, Buhimschi IA, et al. Glucocorticoids enhance CD163 expression in placental Hofbauer cells. Endocrinology. 2013 Jan;154((1)):471–82. doi: 10.1210/en.2012-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johnson EL, Chakraborty R. Placental Hofbauer cells limit HIV-1 replication and potentially offset mother to child transmission (MTCT) by induction of immunoregulatory cytokines. Retrovirology. 2012 Dec;9((1)):101. doi: 10.1186/1742-4690-9-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Loegl J, Hiden U, Nussbaumer E, Schliefsteiner C, Cvitic S, Lang I, et al. Hofbauer cells of M2a, M2b and M2c polarization may regulate feto-placental angiogenesis. Reproduction. 2016 Nov;152((5)):447–55. doi: 10.1530/REP-16-0159. [DOI] [PubMed] [Google Scholar]
- 50.Jensen TS, Matre R. Fc gamma-receptor activity in the developing human placenta. APMIS. 1995 Jun;103((6)):433–8. [PubMed] [Google Scholar]
- 51.Saji F, Koyama M, Matsuzaki N. Current topic: human placental Fc receptors. Placenta. 1994 Jul;15((5)):453–66. doi: 10.1016/s0143-4004(05)80415-1. [DOI] [PubMed] [Google Scholar]
- 52.Simister NE. Human placental Fc receptors and the trapping of immune complexes. Vaccine. 1998 Aug-Sep;16((14-15)):1451–5. doi: 10.1016/s0264-410x(98)00107-8. [DOI] [PubMed] [Google Scholar]
- 53.Tang Z, Abrahams VM, Mor G, Guller S. Placental Hofbauer cells and complications of pregnancy. Ann N Y Acad Sci. 2011 Mar;1221((1)):103–8. doi: 10.1111/j.1749-6632.2010.05932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brown MB, von Chamier M, Allam AB, Reyes L. M1/M2 macrophage polarity in normal and complicated pregnancy. Front Immunol. 2014 Nov;5:606. doi: 10.3389/fimmu.2014.00606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ning F, Liu H, Lash GE. The Role of Decidual Macrophages During Normal and Pathological Pregnancy. Am J Reprod Immunol. 2016 Mar;75((3)):298–309. doi: 10.1111/aji.12477. [DOI] [PubMed] [Google Scholar]
- 56.Czikk MJ, McCarthy FP, Murphy KE. Chorioamnionitis: from pathogenesis to treatment. Clin Microbiol Infect. 2011 Sep;17((9)):1304–11. doi: 10.1111/j.1469-0691.2011.03574.x. [DOI] [PubMed] [Google Scholar]
- 57.Redline RW. Inflammatory response in acute chorioamnionitis. Semin Fetal Neonatal Med. 2012 Feb;17((1)):20–5. doi: 10.1016/j.siny.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 58.Kawamura H, Takeuchi M, Sasahara J, Ishii K, Mitsuda N. Inflammatory Response in Acute Chorioamnionitis and Outcome of Very Low Birth Weight Infants. Placenta. 2015;36((10)):A10–1. [Google Scholar]
- 59.Bracci R, Buonocore G. Chorioamnionitis: a risk factor for fetal and neonatal morbidity. Biol Neonate. 2003;83((2)):85–96. doi: 10.1159/000067956. [DOI] [PubMed] [Google Scholar]
- 60.Joerink M, Rindsjö E, van Riel B, Alm J, Papadogiannakis N. Placental macrophage (Hofbauer cell) polarization is independent of maternal allergen-sensitization and presence of chorioamnionitis. Placenta. 2011 May;32((5)):380–5. doi: 10.1016/j.placenta.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 61.Ben Amara A, Gorvel L, Baulan K, Derain-Court J, Buffat C, Vérollet C, et al. Placental macrophages are impaired in chorioamnionitis, an infectious pathology of the placenta. J Immunol. 2013 Dec;191((11)):5501–14. doi: 10.4049/jimmunol.1300988. [DOI] [PubMed] [Google Scholar]
- 62.Vinnars MT, Rindsjö E, Ghazi S, Sundberg A, Papadogiannakis N. The number of CD68(+) (Hofbauer) cells is decreased in placentas with chorioamnionitis and with advancing gestational age. Pediatr Dev Pathol. 2010 Jul-Aug;13((4)):300–4. doi: 10.2350/09-03-0632-OA.1. [DOI] [PubMed] [Google Scholar]
- 63.Hung TH, Chen SF, Hsu JJ, Hsieh CC, Hsueh S, Hsieh TT. Tumour necrosis factor-alpha converting enzyme in human gestational tissues from pregnancies complicated by chorioamnionitis. Placenta. 2006 Sep-Oct;27((9-10)):996–1006. doi: 10.1016/j.placenta.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 64.Toti P, Arcuri F, Tang Z, Schatz F, Zambrano E, Mor G, et al. Focal increases of fetal macrophages in placentas from pregnancies with histological chorioamnionitis: potential role of fibroblast monocyte chemotactic protein-1. Am J Reprod Immunol. 2011 May;65((5)):470–9. doi: 10.1111/j.1600-0897.2010.00927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Araújo JR, Keating E, Martel F. Impact of gestational diabetes mellitus in the maternal-to-fetal transport of nutrients. Curr Diab Rep. 2015 Feb;15((2)):569. doi: 10.1007/s11892-014-0569-y. [DOI] [PubMed] [Google Scholar]
- 66.Hiden U, Maier A, Bilban M, Ghaffari-Tabrizi N, Wadsack C, Lang I, et al. Insulin control of placental gene expression shifts from mother to foetus over the course of pregnancy. Diabetologia. 2006 Jan;49((1)):123–31. doi: 10.1007/s00125-005-0054-x. [DOI] [PubMed] [Google Scholar]
- 67.Zhu Y, Zhang C. Prevalence of Gestational Diabetes and Risk of Progression to Type 2 Diabetes: a Global Perspective. Curr Diab Rep. 2016 Jan;16((1)):7. doi: 10.1007/s11892-015-0699-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jawerbaum A, González E. Diabetic pregnancies: the challenge of developing in a pro-inflammatory environment. Curr Med Chem. 2006;13((18)):2127–38. doi: 10.2174/092986706777935302. [DOI] [PubMed] [Google Scholar]
- 69.Radaelli T, Varastehpour A, Catalano P, Hauguel-de Mouzon S. Gestational diabetes induces placental genes for chronic stress and inflammatory pathways. Diabetes. 2003 Dec;52((12)):2951–8. doi: 10.2337/diabetes.52.12.2951. [DOI] [PubMed] [Google Scholar]
- 70.Sisino G, Bouckenooghe T, Aurientis S, Fontaine P, Storme L, Vambergue A. Diabetes during pregnancy influences Hofbauer cells, a subtype of placental macrophages, to acquire a pro-inflammatory phenotype. Biochim Biophys Acta. 2013 Dec;1832((12)):1959–68. doi: 10.1016/j.bbadis.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 71.Schliefsteiner C, Peinhaupt M, Kopp S, Lögl J, Lang-Olip I, Hiden U, et al. Human Placental Hofbauer Cells Maintain an Anti-inflammatory M2 Phenotype despite the Presence of Gestational Diabetes Mellitus. Front Immunol. 2017 Jul;8:888. doi: 10.3389/fimmu.2017.00888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Russell P. Inflammatory lesions of the human placenta. III. The histopathology of villitis of unknown aetiology. Placenta. 1980 Jul-Sep;1((3)):227–44. doi: 10.1016/s0143-4004(80)80005-1. [DOI] [PubMed] [Google Scholar]
- 73.Yu J, Zhou Y, Gui J, Li AZ, Su XL, Feng L. Assessment of the number and function of macrophages in the placenta of gestational diabetes mellitus patients. J Huazhong Univ Sci Technolog Med Sci. 2013 Oct;33((5)):725–9. doi: 10.1007/s11596-013-1187-7. [DOI] [PubMed] [Google Scholar]
- 74.Mattar R, Amed AM, Lindsey PC, Sass N, Daher S. Preeclampsia and HIV infection. Eur J Obstet Gynecol Reprod Biol. 2004 Dec;117((2)):240–1. doi: 10.1016/j.ejogrb.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 75.Roberts JM, et al. American College of Obstetricians and Gynecologists. Task Force on Hypertension in Pregnancy Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013 Nov;122((5)):1122–31. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 76.Fayyad AM, Harrington KF. Prediction and prevention of preeclampsia and IUGR. Early Hum Dev. 2005 Nov;81((11)):865–76. doi: 10.1016/j.earlhumdev.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 77.Przybyl L, Haase N, Golic M, Rugor J, Solano ME, Arck PC, et al. CD74-Downregulation of Placental Macrophage-Trophoblastic Interactions in Preeclampsia. Circ Res. 2016 Jun;119((1)):55–68. doi: 10.1161/CIRCRESAHA.116.308304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang SW, Cho EH, Choi SY, Lee YK, Park JH, Kim MK, et al. DC-SIGN expression in Hofbauer cells may play an important role in immune tolerance in fetal chorionic villi during the development of preeclampsia. J Reprod Immunol. 2017 Nov;124:30–7. doi: 10.1016/j.jri.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 79.Koi H, Zhang J, Parry S. The mechanisms of placental viral infection. Ann N Y Acad Sci. 2001 Sep;943((1)):148–56. doi: 10.1111/j.1749-6632.2001.tb03798.x. [DOI] [PubMed] [Google Scholar]
- 80.Musso D, Gubler DJ. Zika Virus. Clin Microbiol Rev. 2016 Jul;29((3)):487–524. doi: 10.1128/CMR.00072-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lissauer D, Smit E, Kilby MD. Zika virus and pregnancy. BJOG. 2016 Jul;123((8)):1258–63. doi: 10.1111/1471-0528.14071. [DOI] [PubMed] [Google Scholar]
- 82.Moshfeghi DM, de Miranda HA, 2nd, Costa MC. Zika Virus, Microcephaly, and Ocular Findings. JAMA Ophthalmol. 2016 Aug;134((8)):945–945. doi: 10.1001/jamaophthalmol.2016.1303. [DOI] [PubMed] [Google Scholar]
- 83.Noronha L, Zanluca C, Azevedo ML, Luz KG, Santos CN. Zika virus damages the human placental barrier and presents marked fetal neurotropism. Mem Inst Oswaldo Cruz. 2016 May;111((5)):287–93. doi: 10.1590/0074-02760160085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Young A, Thomson AJ, Ledingham M, Jordan F, Greer IA, Norman JE. Immunolocalization of proinflammatory cytokines in myometrium, cervix, and fetal membranes during human parturition at term. Biol Reprod. 2002 Feb;66((2)):445–9. doi: 10.1095/biolreprod66.2.445. [DOI] [PubMed] [Google Scholar]
- 85.Ornelas AM, Pezzuto P, Silveira PP, Melo FO, Ferreira TA, Oliveira-Szejnfeld PS, et al. Immune activation in amniotic fluid from Zika virus-associated microcephaly. Ann Neurol. 2017 Jan;81((1)):152–6. doi: 10.1002/ana.24839. [DOI] [PubMed] [Google Scholar]
- 86.Calvet G, Aguiar RS, Melo AS, Sampaio SA, de Filippis I, Fabri A, et al. Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: a case study. Lancet Infect Dis. 2016 Jun;16((6)):653–60. doi: 10.1016/S1473-3099(16)00095-5. [DOI] [PubMed] [Google Scholar]
- 87.Lum FM, Low DK, Fan Y, Tan JJ, Lee B, Chan JK, et al. Zika Virus Infects Human Fetal Brain Microglia and Induces Inflammation. Clin Infect Dis. 2017 Apr;64((7)):914–20. doi: 10.1093/cid/ciw878. [DOI] [PubMed] [Google Scholar]
- 88.Quicke KM, Bowen JR, Johnson EL, McDonald CE, Ma H, O'Neal JT, et al. Zika Virus Infects Human Placental Macrophages. Cell Host Microbe. 2016 Jul;20((1)):83–90. doi: 10.1016/j.chom.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jurado KA, Simoni MK, Tang Z, Uraki R, Hwang J, Householder S, et al. Zika virus productively infects primary human placenta-specific macrophages. JCI Insight. 2016 Aug;1((13)):e88461. doi: 10.1172/jci.insight.88461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schwartz DA. Viral infection, proliferation, and hyperplasia of Hofbauer cells and absence of inflammation characterize the placental pathology of fetuses with congenital Zika virus infection. Arch Gynecol Obstet. 2017 Jun;295((6)):1361–8. doi: 10.1007/s00404-017-4361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Simoni MK, Jurado KA, Abrahams VM, Fikrig E, Guller S. Zika virus infection of Hofbauer cells. Am J Reprod Immunol. 2017 Feb;77((2)):e12613. doi: 10.1111/aji.12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rosenberg AZ, Yu W, Hill DA, Reyes CA, Schwartz DA. Placental Pathology of Zika Virus: Viral Infection of the Placenta Induces Villous Stromal Macrophage (Hofbauer Cell) Proliferation and Hyperplasia. Arch Pathol Lab Med. 2017 Jan;141((1)):43–8. doi: 10.5858/arpa.2016-0401-OA. [DOI] [PubMed] [Google Scholar]
- 93.Bardina SV, Bunduc P, Tripathi S, Duehr J, Frere JJ, Brown JA, et al. Enhancement of Zika virus pathogenesis by preexisting antiflavivirus immunity. Science. 2017 Apr;356((6334)):175–80. doi: 10.1126/science.aal4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Balsitis SJ, Williams KL, Lachica R, Flores D, Kyle JL, Mehlhop E, et al. Lethal antibody enhancement of dengue disease in mice is prevented by Fc modification. PLoS Pathog. 2010 Feb;6((2)):e1000790. doi: 10.1371/journal.ppat.1000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kliks S. Antibody-enhanced infection of monocytes as the pathogenetic mechanism for severe dengue illness. AIDS Res Hum Retroviruses. 1990 Aug;6((8)):993–8. doi: 10.1089/aid.1990.6.993. [DOI] [PubMed] [Google Scholar]
- 96.Zimmerman MG, Quicke KM, O'Neal JT, Arora N, Machiah D, Priyamvada L, et al. Cross-Reactive Dengue Virus Antibodies Augment Zika Virus Infection of Human Placental Macrophages. Cell Host Microbe. 2018 Nov;24((5)):731–742.e6. doi: 10.1016/j.chom.2018.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kharsany AB, Karim QA. HIV Infection and AIDS in Sub-Saharan Africa: Current Status, Challenges and Opportunities. Open AIDS J. 2016 Apr;10((1)):34–48. doi: 10.2174/1874613601610010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tooke L, Riemer L, Matjila M, Harrison M. Antiretrovirals causing severe pre-eclampsia. Pregnancy Hypertens. 2016 Oct;6((4)):266–8. doi: 10.1016/j.preghy.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 99.Naidoo M, Sartorius B, Tshimanga-Tshikala G. Maternal HIV infection and preterm delivery outcomes at an urban district hospital in KwaZulu-Natal 2011. S Afr J Infect Dis. 2016;31((1)):25–8. [Google Scholar]
- 100.Chen JY, Ribaudo HJ, Souda S, Parekh N, Ogwu A, Lockman S, et al. Highly active antiretroviral therapy and adverse birth outcomes among HIV-infected women in Botswana. J Infect Dis. 2012 Dec;206((11)):1695–705. doi: 10.1093/infdis/jis553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ndirangu J, Newell ML, Bland RM, Thorne C. Maternal HIV infection associated with small-for-gestational age infants but not preterm births: evidence from rural South Africa. Hum Reprod. 2012 Jun;27((6)):1846–56. doi: 10.1093/humrep/des090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wedi CO, Kirtley S, Hopewell S, Corrigan R, Kennedy SH, Hemelaar J. Perinatal outcomes associated with maternal HIV infection: a systematic review and meta-analysis. Lancet HIV. 2016 Jan;3((1)):e33–48. doi: 10.1016/S2352-3018(15)00207-6. [DOI] [PubMed] [Google Scholar]
- 103.Teasdale C.A., Marais B.J., Abrams E.J. HIV: prevention of mother-to-child transmission. BMJ Clin Evid, 2011. 2011 [PMC free article] [PubMed] [Google Scholar]
- 104.Lewis SH, Reynolds-Kohler C, Fox HE, Nelson JA. HIV-1 in trophoblastic and villous Hofbauer cells, and haematological precursors in eight-week fetuses. Lancet. 1990 Mar;335((8689)):565–8. doi: 10.1016/0140-6736(90)90349-a. [DOI] [PubMed] [Google Scholar]
- 105.Palmeira P, Quinello C, Silveira-Lessa AL, Zago CA, Carneiro-Sampaio M. IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol. 2012;2012:985646. doi: 10.1155/2012/985646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Johnson EL, Chu H, Byrareddy SN, Spearman P, Chakraborty R. Placental Hofbauer cells assemble and sequester HIV-1 in tetraspanin-positive compartments that are accessible to broadly neutralizing antibodies. J Int AIDS Soc. 2015 Jan;18((1)):19385. doi: 10.7448/IAS.18.1.19385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Al-Husaini AM. Role of placenta in the vertical transmission of human immunodeficiency virus. J Perinatol. 2009 May;29((5)):331–6. doi: 10.1038/jp.2008.187. [DOI] [PubMed] [Google Scholar]
- 108.Newell ML. Mechanisms and timing of mother-to-child transmission of HIV-1. AIDS. 1998 May;12((8)):831–7. doi: 10.1097/00002030-199808000-00004. [DOI] [PubMed] [Google Scholar]
- 109.De Cock KM, Fowler MG, Mercier E, de Vincenzi I, Saba J, Hoff E, et al. Prevention of mother-to-child HIV transmission in resource-poor countries: translating research into policy and practice. JAMA. 2000 Mar;283((9)):1175–82. doi: 10.1001/jama.283.9.1175. [DOI] [PubMed] [Google Scholar]
- 110.Manicklal S, Emery VC, Lazzarotto T, Boppana SB, Gupta RK. The “silent” global burden of congenital cytomegalovirus. Clin Microbiol Rev. 2013 Jan;26((1)):86–102. doi: 10.1128/CMR.00062-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lilleri D, Kabanova A, Revello MG, Percivalle E, Sarasini A, Genini E, et al. Fetal human cytomegalovirus transmission correlates with delayed maternal antibodies to gH/gL/pUL128-130-131 complex during primary infection. PLoS One. 2013;8((3)):e59863. doi: 10.1371/journal.pone.0059863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Emery VC. Investigation of CMV disease in immunocompromised patients. J Clin Pathol. 2001 Feb;54((2)):84–8. doi: 10.1136/jcp.54.2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pereira L, Maidji E, McDonagh S, Tabata T. Insights into viral transmission at the uterine-placental interface. Trends Microbiol. 2005 Apr;13((4)):164–74. doi: 10.1016/j.tim.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 114.Weisblum Y, Panet A, Haimov-Kochman R, Wolf DG. Models of vertical cytomegalovirus (CMV) transmission and pathogenesis. Semin Immunopathol. 2014 Nov;36((6)):615–25. doi: 10.1007/s00281-014-0449-1. [DOI] [PubMed] [Google Scholar]
- 115.Weisblum Y, Oiknine-Djian E, Zakay-Rones Z, Vorontsov O, Haimov-Kochman R, Nevo Y, et al. APOBEC3A Is Upregulated by Human Cytomegalovirus (HCMV) in the Maternal-Fetal Interface, Acting as an Innate Anti-HCMV Effector. J Virol. 2017 Nov;91((23)):e01296-17. doi: 10.1128/JVI.01296-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Stagno S, Pass RF, Dworsky ME, Alford CA., Jr Maternal cytomegalovirus infection and perinatal transmission. Clin Obstet Gynecol. 1982 Sep;25((3)):563–76. doi: 10.1097/00003081-198209000-00014. [DOI] [PubMed] [Google Scholar]
- 117.El Costa H, Gouilly J, Mansuy JM, Chen Q, Levy C, Cartron G, et al. ZIKA virus reveals broad tissue and cell tropism during the first trimester of pregnancy. Sci Rep. 2016 Oct;6((1)):35296. doi: 10.1038/srep35296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ibanez CE, Schrier R, Ghazal P, Wiley C, Nelson JA. Human cytomegalovirus productively infects primary differentiated macrophages. J Virol. 1991 Dec;65((12)):6581–8. doi: 10.1128/jvi.65.12.6581-6588.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Maciejewski JP, Bruening EE, Donahue RE, Sellers SE, Carter C, Young NS, et al. Infection of mononucleated phagocytes with human cytomegalovirus. Virology. 1993 Aug;195((2)):327–36. doi: 10.1006/viro.1993.1383. [DOI] [PubMed] [Google Scholar]
- 120.Taylor-Wiedeman J, Sissons JG, Borysiewicz LK, Sinclair JH. Monocytes are a major site of persistence of human cytomegalovirus in peripheral blood mononuclear cells. J Gen Virol. 1991 Sep;72((Pt 9)):2059–64. doi: 10.1099/0022-1317-72-9-2059. [DOI] [PubMed] [Google Scholar]
- 121.Mendelson M, Monard S, Sissons P, Sinclair J. Detection of endogenous human cytomegalovirus in CD34+ bone marrow progenitors. J Gen Virol. 1996 Dec;77((Pt 12)):3099–102. doi: 10.1099/0022-1317-77-12-3099. [DOI] [PubMed] [Google Scholar]
- 122.Weisblum Y, Panet A, Zakay-Rones Z, Vitenshtein A, Haimov-Kochman R, Goldman-Wohl D, et al. Human cytomegalovirus induces a distinct innate immune response in the maternal-fetal interface. Virology. 2015 Nov;485:289–96. doi: 10.1016/j.virol.2015.06.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data