Abstract
Background:
Expression of hypoxia-inducible factors (HIFs) has been observed, but their prognostic role in advanced cancers remains uncertain. We conducted a meta-analysis to establish the prognostic effect of HIFs and to better guide treatment planning for advanced cancers.
Methods:
Pooled hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated. Trial sequential analysis (TSA) was also performed. The clinical outcomes included overall survival (OS), disease-free survival (DFS), progression-free survival (PFS), cancer-specific survival (CSS), relapse/recurrence-free survival (RFS), and metastasis-free survival (MFS) in patients with advanced tumors according to multivariate analysis.
Results:
A total of 31 studies including 3453 cases who received chemotherapy, radiotherapy, or chemoradiotherapy were identified. Pooled analyses revealed that HIF-1α expression was correlated with worse OS (HR = 1.61, p < 0.001), DFS (HR = 1.61, p < 0.001), PFS (HR = 1.49, p = 0.01), CSS (HR = 1.65, p = 0.056), RFS (HR = 2.10, p = 0.015), or MFS (HR = 2.36, p = 0.002) in advanced cancers. HIF-1α expression was linked to shorter OS in the digestive tract, epithelial ovarian, breast, non-small cell lung, and clear cell renal cell carcinomas. Subgroup analysis by study region showed that HIF-1α expression was correlated with poor OS in Europeans and Asians, while an analysis by histologic subtypes found that HIF-1α expression was not associated with OS in squamous cell carcinoma. No relationship was found between HIF-2α expression and OS, DFS, PFS, or CSS.
Conclusions:
Targeting HIF-1α may be a useful therapeutic approach to improve survival for advanced cancer patients. Based on TSA, more randomized controlled trials are strongly suggested.
Keywords: advanced cancer, HIF-1α, HIF-2α, multivariate analysis, prognosis, therapies
Introduction
Cancer is still a major public health problem throughout the world; cancer is a leading cause of death and has high morbidity rates. According to GLOBOCAN estimates, approximately 14.1 million new cases and 8.2 million deaths occurred due to cancer in 2012 worldwide.1 Although surgical techniques, chemotherapy/radiotherapy, targeted molecular therapy, and immunotherapy regimens have greatly improved advanced disease management in recent years, the 5-year survival rate of most advanced cancers is still low.1–3 Combination treatments are commonly used to improve treatment outcomes for most advanced cancers.4,5 In clinics, current management of cancer patients still relies mainly on clinical staging assessments to guide treatment and determine prognosis, which cannot always be accurately used to classify disease prognosis to target cancers.6 Thus, an effective indicator needs to be developed to better predict the behavior of advanced cancer patients, enhance the selection of appropriate treatment and management strategies, and guide necessary clinical trial implementation.
Meta-analysis suggests that the development of strategies against biomarkers may be a cost-effective therapeutic approach in solid tumors.7 Solid tumors generally exhibit hypoxia, which plays a central role in tumor angiogenesis and cancer metastasis.8,9 Moreover, hypoxia is associated with metabolism, differentiation, necrosis, rapid tumor growth, and other malignant biological behaviors, leading to resistance to radiotherapy and chemotherapy.10,11 Two common hypoxia-inducible factors (HIFs) (HIF-1α and HIF-2α) have been identified as key regulators of the response to hypoxic stress.12 HIFs are involved in the regulation of angiogenesis through vascular endothelial growth factor (VEGF: a potent angiogenic protein) and platelet-derived growth factor, enhancing the transcriptional activity of Notch signaling, mediating cancer metabolic pathways (glucose, lipid, and amino acid metabolism), and exerting a tumor-promoting effect by immunosuppression.13–15 HIFs also may induce epithelial-to-mesenchymal transition (EMT) via the PI3K/AKT/mTOR pathway, regulate proto-oncogene c-Myc activity, and activate stem cell factors such as Oct4 and Nanog.16–18
Expression of HIFs in cancer cells contributes to metastasis, but inactivation of HIFs decreases metastasis of cancer cells.19 HIF-1α and HIF-2α are most frequently reported. Their expression is detected in various human cancers and may be associated with a worse prognosis of many tumors, such as gastric cancer, breast cancer, and non-small cell lung cancer.13,20 However, the clinical outcomes of HIF-1α and HIF-2α expression according to multivariate analysis are still controversial in advanced cancer. For example, HIF-1α expression was not linked to OS in colorectal cancer,21,22 but was associated with shorter OS in colorectal cancer by Wilson and colleagues.23 Additionally, the prognostic impact of HIFs expression in advanced cancers is still unclear when investigated via meta-analysis.
Therefore, the purpose of the current meta-analysis is to investigate the relationship between HIF-1α and HIF-2α expression and survival outcomes for advanced/metastatic tumor patients treated with chemotherapy, radiotherapy, or chemoradiotherapy, thereby allowing more effective and rational development of combination therapy strategies to optimize treatment.
Materials and methods
Search strategy
The electronic databases PubMed, Embase, EBSCO, and the Cochrane Library were systematically searched to identify eligible papers published before 23 February 2018. We used the following search terms and text words: ‘hypoxia inducible factor OR hypoxia-inducible factors OR HIF OR hypoxia-inducible factor 1 OR hypoxia-inducible factor 2 OR endothelial PAS domain-containing protein 1 OR EPAS1’, ‘metastatic OR advanced OR metastasized OR recurrent’, ‘cancer OR tumor OR carcinoma OR neoplasm’, ‘survival OR outcome OR prognosis OR mortality’ (Table S1). We also hand-searched the reference lists of the eligible studies to identify other potential articles. Three authors (S.H., T.H., and F.H.) independently evaluated the publications, and discrepancies were discussed by consensus. The present meta-analysis was conducted based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.24
Study selection
Studies that fulfilled the following selection criteria were included: studies recording patients with advanced/metastatic cancer, stage III cancer, or stage IV cancer; studies published in English reporting patients treated with or without surgery and chemotherapy, radiotherapy, or chemoradiotherapy, etc.; studies reporting the prognostic information of HIF-1α, HIF-2α, and HIF-3α expression regarding the hazard ratio (HR) with the corresponding 95% confidence interval (CI) for overall survival (OS), disease-free survival (DFS), progression-free survival (PFS), cancer-specific survival (CSS), relapse/recurrence-free survival (RFS), or metastasis-free survival (MFS) using multivariate analysis; in the case of insufficient information, such as only HR or 95% CI, HR and 95% CI were calculated to evaluate the prognostic data based on the described methods, if possible,25,26 or the corresponding author was contacted by sending an email to request useful information. If authors published multiple papers using overlapping sample data, only the most recent publication or the study with the largest study population was included. Those with no relevant studies, case reports, animal studies, reviews, and no prognostic value of HIFs in advanced cancer for multivariate analysis were mainly excluded.
Data extraction and study reporting quality
The quality of the included studies was assessed using the Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK) criteria.27 The REMARK criteria reported 20 items for each eligible study (Introduction: 1 item, Materials and Methods: 10 items, Results: 7 items, and Discussion: 2 items), and each item consists of three possible values (0, 1, and 2), allowing for evaluation of the study objective, method, data analysis, and relevant discussion, with a maximal score of 40. The classifications were as follows; an item was not defined or applicable at all (0 score); an item clearly stated all aspects (2 scores); and an item was incompletely described (1 score). According to the overall scores, studies were divided into two groups: studies with a score of ⩾24 (60% of the maximum score) were considered high quality, and the study with a REMARK score of <24 was low quality (Table S2). The following data were extracted from the full texts of the eligible studies, including the first author’s surname, year of publication, case number, study source, mean or median age, tumor type, testing method, therapy regime, study design, sample type, cut-off value, survival status, adjusted variables, and clinical outcomes. Any disagreements were resolved by consensus.
Statistical analysis
The pooled HR and 95% CI were calculated to estimate the effect of HIF-1α and HIF-2α expression status on advanced cancer survival (OS, DFS, PFS, CSS, RFS, or MFS of multivariate analysis). An observed HR >1 implied a worse prognosis, whereas a HR <1 indicated a favorable prognosis. The between-study heterogeneity was determined using Cochran’s Q statistic.28 The random-effects model (DerSimonian-Laird) was applied in the current meta-analysis.29,30 For substantial heterogeneity (p < 0.1) in ⩾10 of the included studies, we conducted subgroup analyses based on some of the baseline features of the eligible studies, such as the study region, tumor location, and survival rate, to determine the potential source of heterogeneity and the difference between subgroups. Publication bias was examined using Egger’s regression model and Begg’s test for the results with more than 10 studies.31,32
A meta-analysis included a small number of participants, the associated random errors may cause spurious results.33,34 Trial sequential analysis (TSA) was performed to avoid type I error rate (α) and estimate the required sample information.35 A type I error of 5% and type II error (β) of 10% (1–β = 90% power) were set. We used a relative risk reduction (RRR) of 20% and the optimal a priori anticipated information size (APIS) method. A sequential monitoring boundary was constructed to determine whether a trial could be terminated early. A cumulative Z-curve that crossed the trial sequential monitoring boundary suggested that the statistical evidence was conclusive. In other cases, additional studies were needed to achieve sufficient evidence. Data were analyzed using Stata software, version 12.0 (Stata Corp., College Station, TX, USA) and R software, version 3.4.2 (The R Foundation for Statistical Computing; Vienna, Austria).
Results
Study characteristics
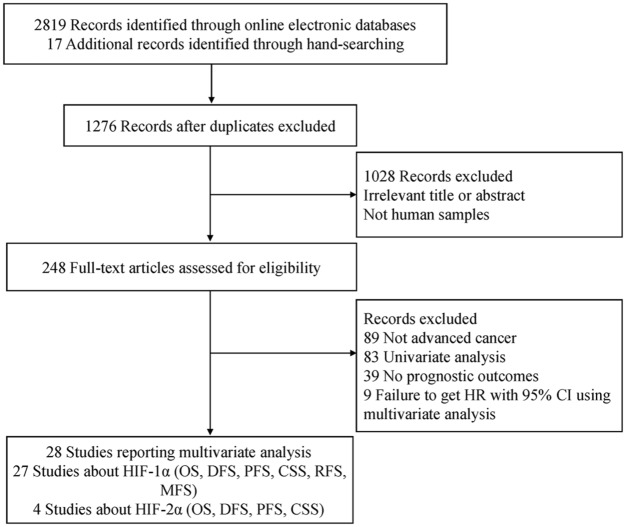
Figure 1 describes the detailed steps for the literature search, and a total of 28 articles met the eligibility criteria of this meta-analysis. All studies using multivariate analysis were published from 2002 to 2017. Of these, 27 studies21–23,36–59 evaluated the prognostic effect of HIF-1α expression and included 3056 individuals. Four studies36,41,55,60 including 397 individuals assessed the prognostic role of HIF-2α expression. Most studies reported the 5-year survival outcome, and HIF-1α and HIF-2α expression were mainly detected using an immunohistochemistry (IHC) method. The antibodies and staining procedure used for the IHC method are listed in Table S3. A total of 13 studies had quality scores ⩾24, and 15 studies had a score of <24. The main characteristics of the included studies are listed in Table 1.
Figure 1.
Flow diagram of the study identification process.
95% CI, 95% confidence interval; CSS, cancer-specific survival; DFS, disease-free survival; HIF-1α, hypoxia-inducible factor-1α; HIF-2α, hypoxia-inducible factor-2α; HR, hazard ratio; MFS, metastasis-free survival; OS, overall survival; PFS, progression-free survival; RFS, relapse/recurrence-free survival.
Table 1.
Baseline characteristics of the included studies investigating the prognosis.
| Gene | First author | Country | Age | Method | Histology | Study design | Specimen type | Cases | Therapy | Method patterns | Cut off | Survival status | Adjusted variables | Prognosis reported | Scores |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HIF-1α | Schindl59 | Austria | 52.3 | IHC | Advanced breast cancer | Prospective, multicenter | Paraffin-embedded tumor specimens | 206 | Surgery and combined chemotherapy with tamoxifen | Clone monoclonal antibody H1 67, NB 100–105; Novus Biologicals, Littleton, CO; Dilution: 1:60 | Nuclear 10% | 5 years | HER-2 staining intensity, patient’s age at time of diagnosis, menopausal status, histological grading, estrogen receptor density, and tumor stage | OS, DFS | 29 |
| HIF-1α | Bachtiar57 | Austria | NA | IHC, blind | Advanced cervical cancer | Retrospective, single-center | Paraffin-embedded tumor specimens | 67 | Radiotherapy | No. H72320; BD Transduction Laboratories, Franklin Lakes; Dilution: 1:25 | Nuclear 10% | 3 years | Tumor size, patients’ age, nodal status, FIGO stage, and histological grading | CSS, PFS | 29 |
| HIF-1α | Burri58 | Switzerland | 64 | IHC, blind | Advanced cervical cancer | Retrospective, NA | Paraffin-embedded tumor specimens |
78 | Radiotherapy and chemotherapy | H1α67, Novus Biologicals, Littleton, CO; Dilution: 1:5000 |
Nuclear 0% | 5 years | Tumor stage, nodal status, histology, anemia, and median total dose | OS | 28 |
| HIF-1α | Theodoropoulos56 | Greece | 68 | IHC, blind | Advanced rectal cancer | Retrospective, multicenter | Tissue | 92 | Surgery, chemotherapy and radiotherapy | Mab H1α67, IgG2b isotype; StressGene, Victoria, British Columbia, Canada; Dilution: 1:1200 |
Nuclear 10% | 3 years | Tumor grade, pattern of tumor growth, vascular invasion, and lymph node status | OS, DFS | 24 |
| HIF1α | Winter55 | UK | NA | IHC | Advanced head and neck squamous cell carcinoma | Retrospective, single-center | Paraffin-embedded tumor specimens | 140 | Surgery and radiotherapy | ESEE122; Dilution: 1:30 | Nuclear 10% | 5 years | Advanced disease, anemia, gender, age, smoking history, lymph node status, tumor subsite, and tumor grade | CSS, OS, DFS | 22 |
| HIF-1α | Generali54 | UK | NA | IHC, blind | Advanced breast cancer | Prospective randomized clinical trial, single-center | Paraffin-embedded tumor specimens | 187 | Surgery and chemoendocrine therapy | ESEE 122, IgG1 monoclonal antibody; Dilution: 1:40 | Weak-strong | 5 years | T stage, N status, steroid hormone receptor status, c-erb2, bcl2, p53, and Ki67 | DFS | 29 |
| HIF-1α | Klatte53 | USA | NA | IHC, blind | Metastatic clear cell RCC | Retrospective, single-center | Paraffin-embedded tumor specimens | 141 | Immunotherapy | IgG2b, cloneH1α67-sup, final concentration, 6 Ag/mL; Novus Biologicals; NA | Nuclear 35% | 5 years | ECOG PS, T stage, concomitant lymph node metastases, Fuhrman grade, and number of metastatic sites | CSS | 23 |
| HIF-1α | Dellas52 | Germany | 58.4 | IHC | Advanced cervical cancer | Retrospective, NA | Paraffin-embedded tumor specimens | 44 | Radiotherapy | Ab463; Abcam, UK; NA | Nuclear, weak-intensive | 5 years | Tumor stage | CSS | 15 |
| HIF-1α | Koo and Kim51 | Korea | 53.2 | IHC | Metastatic squamous cell carcinoma | Retrospective, single-center | Paraffin-embedded tumor specimens | 17 | Chemo/radiation therapy | EP1215Y, Biocare, CA, USA; Dilution: 1:100 | Nuclear or cytoplasmic (or both) 10% | 3 years | NDR | OS | 11 |
| HIF-1α | Shioya48 | Japan | 59 | IHC, blind | Advanced rectal cancer | Retrospective, NA | Paraffin-embedded tumor specimens | 50 | Surgery and hyperthermo-chemoradiotherapy | Neomarkers, Fremont, CA; Dilution: 1:20000 | Nuclear 40% | 3 years | Radiation dose, chemotherapy course, treatment time of hyperthermia, age, gender, and stage | RFS, MFS | 21 |
| HIF-1α | Xiang50 | China | 50 | IHC, blind | Hepatocellular carcinoma with abdominal LN metastases | Retrospective, single-center | Paraffin-embedded tumor specimens | 69 | Radiotherapy | Santa Cruz Biotechnology, Santa Cruz, CA; NA |
Nuclear or cytoplasmic (or both) 10% | 3 years | Hb, intrahepatic tumor number, vascular invasion, child-Pugh score, cumber of metastatic LN, and intrahepatic tumor control etc. | OS, RFS | 25 |
| HIF-1α | Wan47 | China | 43.1 | IHC, blind | Advanced nasopharyngeal carcinoma | Randomized controlled trial | Tissue | 144 | Chemotherapy and radiotherapy | Millipore, Billerica, MA, USA; Dilution: 1:200 | Nuclear or cytoplasmic (or both) 5 scores | 5 years | Age, gender, histological style, TNM stage, and Aurora-A | OS, MFS, PFS | 25 |
| HIF-1α | Fraga49 | Brazil | NA | IHC | Upper aerodigestive tract cancer with cervical lymph nodes | Retrospective, single-center | Paraffin-embedded tumor specimens |
26 | Surgery and radiotherapy | Clone HIF-1α 67, Sigma-Aldrich, St. Louis, USA; NA | NA | 3 years | NDR | OS | 17 |
| HIF-1α | Shim46 | Korea | 62 | IHC, blind | Advanced rectal cancer | Retrospective, single-center | Paraffin-embedded tumor specimens | 104 | Surgery and chemoradiotherapy | Novus Biologicals, Littleton, CO; Dilution: 1:50 | 2 scores | 3 years | Age and stage | RFS | 22 |
| HIF-1α | Shimomura45 | Japan | 62 | IHC | Colorectal liver metastasis | Retrospective, single-center | Paraffin-embedded tumor specimens | 64 | Surgery and chemotherapy | Novus Biologicals, Littleton, CO; Dilution: 1:50 | Cytoplasm 5 scores | 5 years | N stage, no. of liver tumors, and CEA level etc. | DFS | 22 |
| HIF-1α | Wu44 | China | NA | IHC | Advanced non-small cell lung cancer | Retrospective, single-center | Frozen tissues | 162 | Chemotherapy | Millipore Corporation®, USA; Dilution: 1:150 | 6 scores | NA | Age, sex, smoking status, histology, stage, chemotherapy regimens, and response status | OS | 21 |
| HIF-1α | Wilson23 | USA | 62 | qRT-PCR | Metastatic colorectal cancer | Retrospective, multicenter | Paraffin-embedded tumor specimens | 42 | FOLFOX4 chemotherapy plus the VEGFR inhibitor PTK787/ZK 222584 (vatalanib) | Applied Biosystems, Foster City, CA, USA; NA | mRNA 1.84 ratio | <3 years | Performance status and serum LDH level | OS | 25 |
| HIF-1α | Zhang43 | China | NA | IHC, blind | Metastastic esophageal squamous cell carcinoma | Retrospective, single-center | Paraffin-embedded tumor specimens | 69 | Surgery and radiotherapy/chemotherapy | Clone H1α67; Novus Biologicals, Inc., Littleton, CO; NA | Nucleus and cytoplasm 44% | 5 years | NDR | OS, DFS | 16 |
| HIF-1α | Braicu42 | Germany, Belgium, Austria | 58 | ELISA | Advanced epithelial ovarian cancer | Prospective, multicenter | Tissue | 275 | Surgery and platinum-based chemotherapy | ELISA kit (R&D Systems, Inc., Minneapolis, MN, USA; NA | 80 pg/mg protein | 5 years | Age at first diagnosis, FIGO stage, histological subtype, histological grade, presence and volume of ascites, residual tumor mass after surgery, peritoneal dissemination, and responses to platinum-based chemotherapy | OS | 26 |
| HIF-1α | Xie 201541 | China | 63 | ELISA, blind | Metastatic renal cell carcinoma | Prospective, open-label, single-arm, multicenter, phase II trial | Serum | 86 | Second-line treatment with pazopanib after failure of first-line sunitinib treatment | (ELISA) kit (R&D Systems, Minneapolis, MN; NA | 80.2 pg/mg protein | <3 years | Previous nephrectomy, six IMDC (International Metastatic RCC Database Consortium) risk factors (anaemia, neutrophilia, Karnofski performance status (KPS) <80%, thrombocytosis, hypercalcaemia, and time from diagnosis to treatment interval <1 year), number of organs involved, and best response to prior sunitinib therapy | OS, PFS | 25 |
| HIF-1α | Berk21 | Turkey | 55 | IHC | Metastatic colorectal cancer | Retrospective, single-center | Paraffin-embedded tumor specimens | 53 | Chemotherapy combinations with Bevacizumab | Thermo scientificAb-4, (Clone H1α67, U.K; Dilution: 1:50 | Cytoplasm 5 scores | <3 years | Age, gender, K-ras status, dose reduction, dose delay, ECOG PS, metastases, and chemotherapy regimen | OS, PFS | 21 |
| HIF-1α | Goos22 | The Netherlands | NA | IHC | Colorectal cancer liver metastasis |
Retrospective, multicenter | Paraffin-embedded tumor specimens | 328 | Surgery and systemic therapy | BD Transduction Laboratories, Breda, The Netherlands; Dilution: 1:500 | Nuclear 25% | 5 years | Primary tumor-to-CRCLM interval of less than 12 months, lymph node positivity at the time of diagnosis of the primary tumor, maximal CRCLM diameter of greater than 5.0 cm, number of CRCLM greater than 1, and serum CEA level greater than 200 ng/mL | OS | 28 |
| HIF-1α | Shultz40 | USA | NA | Proximity ligation assay/quantitative PCR | Advanced or metastatic pancreatic cancer | Randomized control trial, multicenter | Plasma | 229 | Gemcitabine and erlotinib | Model 7500, Applied Biosystems; NA | Protein | <3 years | Age, sex, race, ECOG performance status, pain intensity, and disease stage | OS | 26 |
| HIF-1α | Chen39 | China | 53 | IHC, blind | Advanced pharyngeal cancer | Retrospective, single-center | Paraffin-embedded tumor specimens | 57 | Chemoradiotherapy/radiotherapy | Not clear; NA | Nuclear 80% | <3 years | TNM classification, volumetric parameters, texture indices, and primary tumor origin | CSS, RFS | 22 |
| HIF-1α | Nyström38 | Sweden | 71 | IHC | Advanced soft tissue sarcoma of extremities and trunk wall | Retrospective, single-center | Tissue | 73 | Surgery and radiotherapy/chemotherapy | Clone 54, BD Biosciences, Sweden; Dilution: 1:50 | Nuclear 10% | 5 years | Size, vascular invasion, necrosis, and tumor depth | OS, MFS | 22 |
| HIF-1α | Moreno-Acosta37 | Colombia | 46.3 | IHC | Advanced squamous cell cervical carcinoma | Prospective, National Cancer Institute | Fresh tissue | 149 | Chemo-radiotherapy and brachytherapy | ESEE122, ab8366, abcam; Dilution: 1:400 | 10% | 5 years | FIGO, differentiation degree, treatment type, and anemia | OS, PFS | 21 |
| HIF-1α | Beuselinck36 | France, Belgium | 59 | qRT-PCR | Metastatic clear cell renal cell carcinoma | Prospective, multicenter | Tissue | 104 | Sunitinib | Fluidigm, South San Francisco, CA; NA | mRNA | 5 years | Arcomatoid dedifferentiation >25% of tumor volume, neutrophil count, bone metastasis, liver metastasis, and Karnofsky performance status | OS | 23 |
| HIF-2α | Winter55 | United Kingdom | NA | IHC | Advanced head and neck squamous cell carcinoma | Retrospective, single-center | Paraffin-embedded tumor specimens | 140 | Surgery and radiotherapy | ESEE122; Dilution: 1:30 | Nuclear 10% | 5 years | Advanced disease, anemia, gender, age, smoking history, lymph node status, tumor subsite, and tumor grade | CSS, OS, DFS | 22 |
| HIF-2α | Garcia-Donas60 | Spain | 66 | IHC | Advanced clear cell renal cell carcinoma | Prospective, multicenter | Paraffin-embedded tumor specimens | 67 | Sunitinib | Polyclonal Novus Biologicals NB100–122; Dilution: 1:200 | 5% | <3 years | MSKCC prognostic classification and gender | OS, PFS | 26 |
| HIF-2α | Xie 201541 | China | 63 | ELISA, blind | Metastatic renal cell carcinoma | Prospective, open-label, single-arm, multicenter, phase II trial |
Serum | 86 | Second-line treatment with pazopanib after failure of first-line sunitinib treatment | (ELISA) kit (R&D Systems, Minneapolis, MN): NA | 80.2 pg/mg protein | <3 years | Previous nephrectomy, six IMDC (International Metastatic RCC Database Consortium) risk factors (anaemia, neutrophilia, Karnofski performance status (KPS) <80%, thrombocytosis, hypercalcaemia, and time from diagnosis to treatment interval <1 year), number of organs involved, and best response to prior sunitinib therapy | OS, PFS | 25 |
| HIF-2α | Beuselinck36 | France | 59 | qRT-PCR | Metastatic clear cell renal cell carcinoma | Prospective, multicenter | Tissue | 104 | Sunitinib | Fluidigm, South San Francisco, CA; NA | mRNA | 5 years | Arcomatoid dedifferentiation > 25% of tumor volume, neutrophil count, bone metastasis, liver metastasis, and Karnofsky performance status | OS, PFS | 23 |
CEA, carcino embryonic antigen; CRCLM, colorectal cancer liver metastasis; CSS, cancer-specific survival; DFS, disease-free survival; ECOG PS, Eastern Cooperative Oncology Group performance status; FIGO, International Federation of Gynecology and Obstetrics; Hb, hemoglobin; HIF-1α, hypoxia inducible factor-1α, HIF-2α, hypoxia inducible factor-2α; IHC, immunohistochemistry; LDH, lactate dehydrogenase; LN, lymph nodes; MFS, metastasis-free survival; MSKCC, Memorial Sloan-Kettering Cancer Center; NA, not applicable; NDR, not detailed report; OS, overall survival; PFS, progression-free survival; qRT-PCR, quantitative reverse transcription polymerase chain reaction; enzyme-linked immunosorbent assay; RCC, renal cell carcinoma; RFS, relapse/recurrence-free survival; TNM, tumor node metastasis.
Overall survival of HIF-1α expression
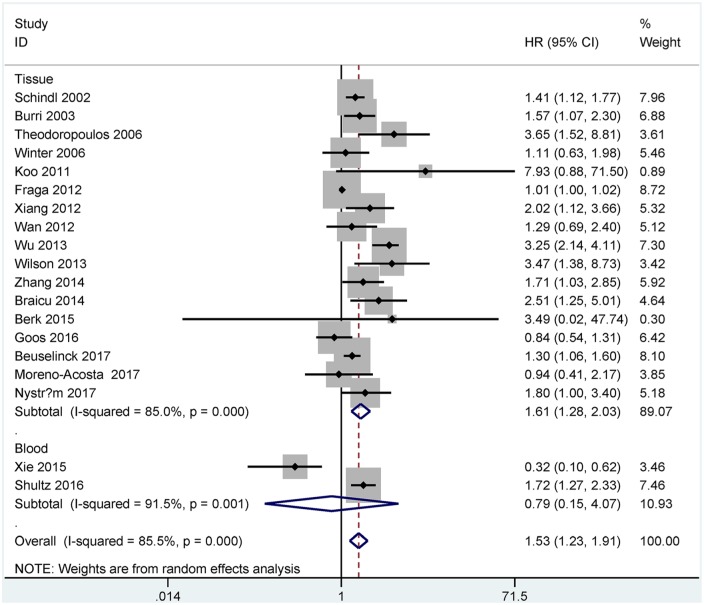
A total of 19 studies including 2342 cases were identified in the analysis of HIF-1α expression and OS. Multivariate analysis showed that HIF-1α expression was associated with worse OS in tissue samples (n = 17 studies with 2027 cases, HR = 1.61, 95% CI = 1.28–2.03, p < 0.001), but was not correlated with OS in blood samples (n = two studies with 315 cases, HR = 0.79, 95% CI = 0.15–4.07, p = 0.774) (Figure 2).
Figure 2.
Forest plot for the relationship between HIF-1α expression and OS.
HIF-1α, hypoxia-inducible factor-1α; OS, overall survival
Subgroup analyses were performed based on the available information in tissue samples, and Table 2 lists the results of the subgroup analyses to explain potential sources of heterogeneity for OS. However, all p values for heterogeneity per subgroup were not more than 0.1, suggesting that the subgroup analyses failed to explore the heterogeneity sources.
Table 2.
Subgroup analyses of HIF-1α expression with OS in tissue samples.
| Variables | HR with 95% CI | Heterogeneity (p) | p value | Studies | Cases | TSA |
|---|---|---|---|---|---|---|
| Study region | ||||||
| Asian | 2.14 (1.40–3.28) | 0.036 | <0.001 | 5 | 461 | More studies |
| European | 1.39 (1.13–1.71) | <0.001 | 0.002 | 12 | 1566 | More studies |
| Tumor location | ||||||
| Metastatic colorectal cancer | 1.70 (0.50–5.84) | 0.021 | 0.398 | 3 | 423 | More studies |
| Advanced cervical cancer | 1.40 (0.91–2.13) | 0.273 | 0.122 | 2 | 227 | More studies |
| Others | 1.71 (1.30–2.24) | <0.001 | <0.001 | 12 | 1377 | More studies per cancer type |
| Histologic subtype | ||||||
| Squamous cell carcinoma | 1.38 (0.87–2.17) | 0.214 | 0.171 | 4 | 375 | More studies |
| Others | 1.67 (1.29–2.16) | <0.001 | <0.001 | 13 | 1652 | No need |
| Survival status | ||||||
| 5 years | 1.36 (1.18–1.57) | 0.264 | <0.001 | 10 | 1566 | No need |
| 3 years | 2.05 (0.96–4.39) | 0.001 | 0.064 | 4 | 204 | More studies |
| <3 years | 3.47 (1.41–8.52) | 0.998 | 0.007 | 2 | 95 | More studies |
| Study design | ||||||
| Randomized controlled trial | 1.29 (0.69–2.40) | NA | 0.422 | 1 | 144 | More studies |
| Prospective | 1.39 (1.14–1.68) | 0.263 | 0.001 | 4 | 734 | More studies |
| Retrospective | 1.79 (1.25–2.56) | <0.001 | 0.001 | 12 | 1149 | More studies |
| Age (years) | ||||||
| >60 | 2.10 (1.40–3.15) | 0.192 | <0.001 | 4 | 285 | More studies |
| ⩽60 | 1.44 (1.21–1.71) | 0.315 | <0.001 | 8 | 1017 | No need |
| Not clear | 1.40 (0.83–2.34) | <0.001 | 0.204 | 5 | 725 | More studies |
| Study quality | ||||||
| ⩾24 | 1.66 (1.24–2.21) | 0.015 | 0.001 | 8 | 1234 | More studies |
| <24 | 1.54 (1.09–2.18) | <0.001 | 0.014 | 9 | 793 | More studies |
| Center design | ||||||
| Multicenter | 1.50 (1.12–2.02) | 0.008 | 0.007 | 7 | 1196 | More studies |
| Single-center | 1.80 (1.10–2.95) | <0.001 | 0.019 | 8 | 609 | More studies |
| Not clear | 1.49 (1.07–2.06) | 0.598 | 0.017 | 2 | 222 | More studies |
| Sample size | ||||||
| >100 | 1.45 (1.06–1.99) | <0.001 | 0.019 | 8 | 1508 | More studies |
| ⩽100 | 1.89 (1.28–2.77) | <0.001 | 0.001 | 9 | 519 | More studies |
| Treatment regimen | ||||||
| Surgery and nonsurgical treatment | 1.40 (1.08–1.82) | <0.001 | 0.012 | 8 | 1209 | More studies |
| Nonsurgical treatment | 1.85 (1.29–2.64) | <0.001 | 0.001 | 9 | 818 | More studies |
Nonsurgical treatment such as chemotherapy, radiotherapy, or chemoradiotherapy etc. was used.
95% CI, 95% confidence interval; HIF-1α, hypoxia inducible factor-1α; HR, hazard ratio; OS, overall survival; TSA, trial sequential analysis.
Stratified analysis by study region showed a poor OS for 12 studies with European subjects (n = 1566 cases, HR = 1.39, p = 0.002) and for five studies with Asian subjects (n = 461 cases, HR = 2.14, p < 0.001). Stratified analysis by tumor location indicated that a poor OS was found for 12 studies with other cancer types (n = 1377 cases, HR = 1.71, p < 0.001), but not colorectal cancer (n = three studies with 423 cases, p = 0.398) and cervical cancer (n = two studies with 227 cases, p = 0.122). Stratified analysis by histologic subtypes demonstrated that no correlation was found between HIF-1α expression and OS in squamous cell carcinoma (n = four studies with 375 cases), but HIF-1α expression was linked to worse OS in other histotypes (n = 1652 cases, HR = 1.67, p < 0.001). Subgroup analysis by survival status showed that HIF-1α expression was significantly associated with worse prognosis for 5-year OS (n = 10 studies with 1566 cases, HR = 1.36, p < 0.001) and <3-year OS (n = two studies with 95 cases, HR = 3.47, p = 0.007) subgroups, but no relationship was found between the HR of the 3-year OS (n = four studies with 204 cases, p = 0.064).
Stratified analysis by the study design determined that HIF-1α expression had a negative prognostic impact on patient OS in prospective and retrospective studies (HR = 1.39, 95% CI = 1.14–1.68, p = 0.001, four studies, 734 patients; HR = 1.79, 95% CI = 1.25–2.56, p = 0.001, 12 studies, 1149 patients; respectively), but no significant association was noted among a randomized controlled trial (p = 0.422, one study, 144 patients). Stratified analysis by age (years) showed that patients aged less than 60 years had worse OS (HR = 1.44, 95% CI = 1.21–1.71, p < 0.001, eight studies, 1017 patients), and patients older than 60 years had a prognostic impact on OS (HR = 2.10, 95% CI = 1.40–3.15, p < 0.001, four studies, 285 patients). Subgroup analysis by treatment regimen showed that HIF-1α expression was associated with worse OS in patients receiving surgery and nonsurgical treatment (HR = 1.40, 95% CI = 1.08–1.82, p = 0.012) and patients receiving the nonsurgical treatment such as chemotherapy, radiotherapy, or chemoradiotherapy (HR = 1.85, 95% CI = 1.29–2.64, p = 0.001). We also noted a negative prognostic impact of HIF-1α expression on patient OS in the other three features (center design, sample size, and study reporting quality) (Table 2).
OS of HIF-1α expression in various cancer systems
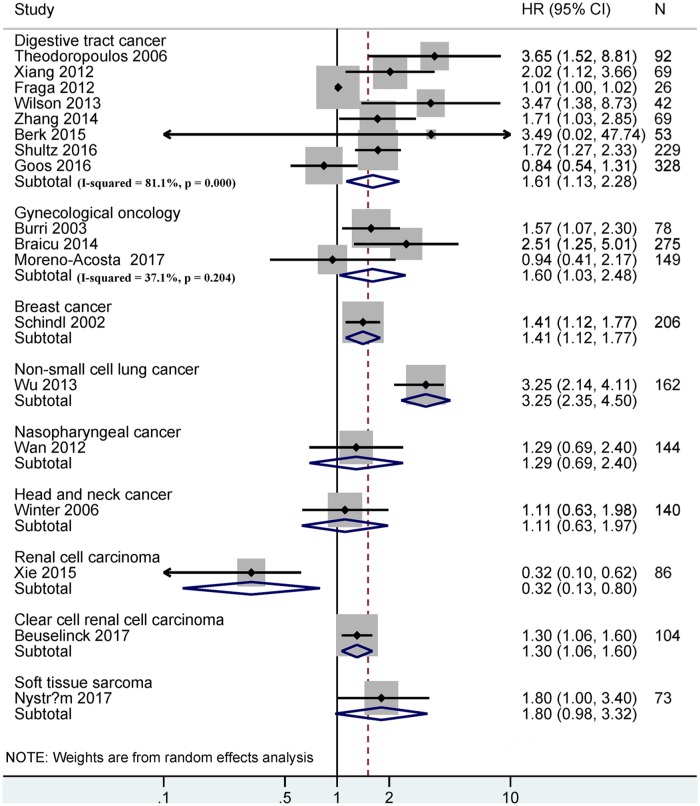
Among various cancer systems, HIF-1α expression was associated with shorter OS in the digestive tract (n = eight studies with 908 cases, HR = 1.61, 95% CI = 1.13–2.28, p = 0.008), gynecological (n = three studies with 502 cases, HR = 1.60, 95% CI = 1.03–2.48, p = 0.035), breast (n = one study with 206 cases, HR = 1.41, 95% CI = 1.12–1.77, p = 0.003), non-small cell lung (n = one study with 162 cases, HR = 3.25, 95% CI = 2.35–4.50, p < 0.001), and clear cell renal cell carcinomas (n = one study with 104 cases, HR = 1.30, 95% CI = 1.06–1.60, p = 0.011), but there was no association in nasopharyngeal (n = 144 cases, p = 0.422) and head and neck cancers (n = 140 cases, p = 0.721) (Figure 3).
Figure 3.
Forest plot for the relationship between HIF-1α expression and OS in different cancer systems.
HIF-1α, hypoxia-inducible factor-1α; OS, overall survival
DFS, PFS, CSS, RFS, and MFS of HIF-1α expression
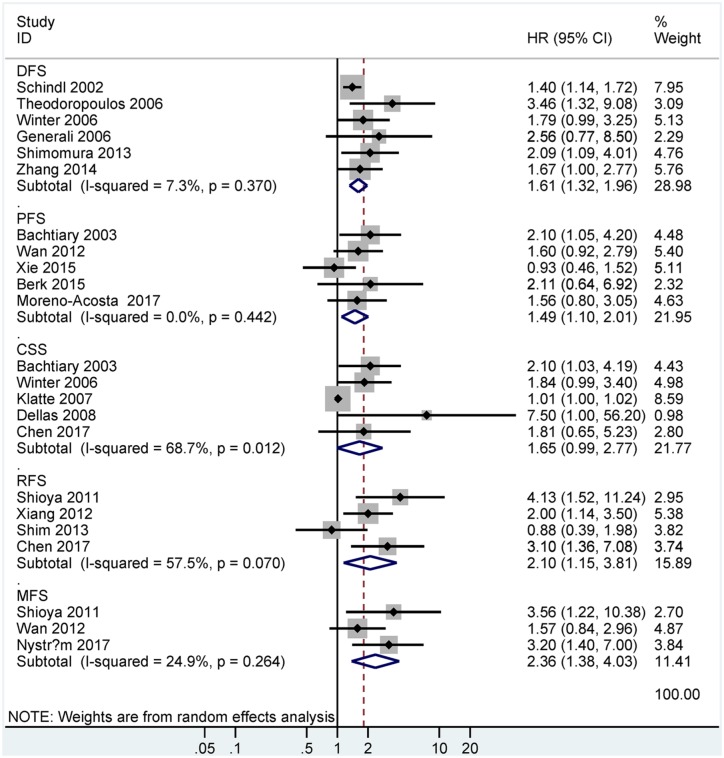
Data suggested that HIF-1α expression was also correlated with worse survival in DFS (HR = 1.61, 95% CI = 1.32–1.96, p < 0.001, six studies, 758 patients), PFS (HR = 1.49, 95% CI = 1.10–2.01, p = 0.01, five studies, 499 patients), CSS (HR = 1.65, 95% CI = 0.99–2.77, p = 0.056, five studies, 449 patients), RFS (HR = 2.10, 95% CI = 1.15–3.81, p = 0.015, four studies, 280 patients), and MFS (HR = 2.36, 95% CI = 1.38–4.03, p = 0.002, three studies, 267 patients) (Figure 4).
Figure 4.
Forest plot for the relationship between HIF-1α expression and prognosis in DFS, PFS, CSS, RFS, or MFS.
DFS, disease-free survival; PFS, progression-free survival; CSS, cancer-specific survival; RFS, relapse/recurrence-free survival; MFS metastasis-free survival.
The prognostic role of HIF-1α expression was also performed based on sample collection (Table 3), the results showed that HIF-1α expression was associated with worse OS (HR = 1.70, 95% CI = 1.31–2.20, p < 0.001) and DFS (HR = 1.47, 95% CI = 1.22–1.76, p < 0.001) in patients without previously received therapy prior to testing.
Table 3.
The prognostic role of HIF-1α expression based on sample collection.
| Sample collection | HR with 95% CI | Heterogeneity (p) | p value | Studies | Cases | TSA |
|---|---|---|---|---|---|---|
| OS | ||||||
| Samples without previously received therapy | 1.70 (1.31–2.20) | 0.003 | <0.001 | 9 | 1447 | No need |
| Samples with previously received therapy | 0.69 (0.18–2.73) | 0.003 | 0.602 | 2 | 190 | More studies |
| DFS | ||||||
| Samples without previously received therapy | 1.47 (1.22–1.76) | 0.645 | <0.001 | 3 | 415 | More studies |
| Samples with previously received therapy | NA | NA | NA | NA | NA | NA |
95% CI, 95% confidence interval; DFS, disease-free survival; HIF-1α, hypoxia inducible factor-1α; HR, hazard ratio; NA, not applicable; OS, overall survival; TSA, trial sequential analysis.
Publication bias
Egger’s and Begg’s tests were used to detect the potential publication bias for OS of HIF-1α expression (Figure S1). No evidence of publication bias was found using Begg’s test (p = 0.484), while there was obvious evidence of publication bias based on Egger’s test (p = 0.002). When we removed this study by Fraga and colleagues,49 the recalculated result from the remaining 18 studies remained significant (HR = 1.59, 95% CI = 1.28–1.98, p < 0.001), with no evidence of publication bias (p = 0.582).
Prognosis of HIF-2α expression
No relationship was found between HIF-2α expression and prognosis in OS (HR = 0.75, 95% CI = 0.38–1.47, p = 0.399, four studies, 396 patients), DFS (HR = 1.57, 95% CI = 0.82–3.01, one study, 139 patients), PFS (HR = 0.64, 95% CI = 0.27–1.53, three studies, 257 patients), and CSS (HR = 1.39, 95% CI = 0.66–2.89, one study, 139 patients) (Figure S2).
Trial sequential analysis
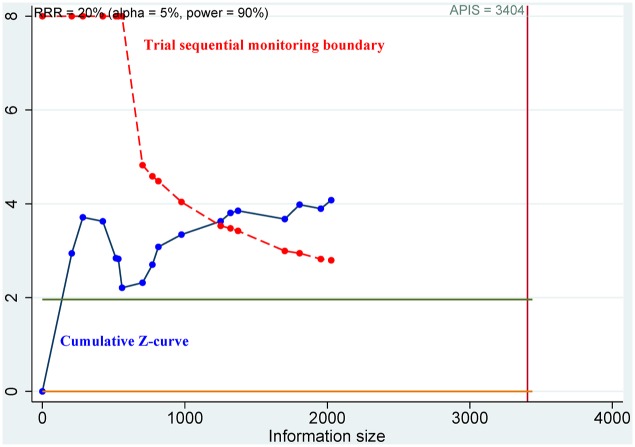
The required sample information was quantified by TSA. The cumulative Z-curve significantly crossed the trial sequential monitoring boundary for OS of HIF-1α expression in tissue samples (Figure 5) and its subgroups such as 5-year OS and patients aged less than 60 years, and thus, additional studies were not required (Table 2). The cumulative Z-curve did not obviously cross the trial sequential monitoring boundary for DFS, PFS, CSS, RFS, or MFS of HIF-1α expression (Table S4); the remaining subgroups of HIF-1α expression in OS (Table 2); and the clinical outcomes of HIF-2α expression (Table S4), which indicated that further studies were needed.
Figure 5.
Trial sequential analysis between HIF-1α expression and OS.
HIF-1α, hypoxia-inducible factor-1α; OS, overall survival
Discussion
Traditional chemoradiotherapeutic regimens generally cannot eradicate cancer cells. Drug resistance and cancer recurrence are common obstacles for improving the long-term survival of cancer patients.61,62 HIF-1α and HIF-2α are two of the most significant transcription factors regulating cellular adaptation to hypoxia, have been found in the etiology of a number of human cancers, and have an adverse impact on the efficacy of radiotherapy and chemotherapy.19,63 The expression of HIF-1α and HIF-2α in human cancers has been reported and detected.13 HIF-1α and HIF-2α expression may be associated with poor prognoses in many cancers.13,19,64 However, the prognostic significance of HIF-1α and HIF-2α expression in advanced cancer patients remains unclear based on a meta-analysis.
Activation of HIF transcription leads to the upregulation of many HIF-targeted genes, and HIFs regulate these targeted genes, which encode proteins such as Oct4 and Nanog in cancer stem cells.65,66 HIFs also play roles in therapy resistance by activating the multidrug resistance 1 (MDR1) gene and ATP-binding cassette sub-family G member 2 (ABCG2)13,67 and inflammation and immunity by activating the expression of ligands such as programmed death ligand 1 (PD-L1) and increasing cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) expression on CD8+ T cells,68,69 which are involved in decreasing the effectiveness of anticancer therapies, such as radiotherapy, chemotherapy, and immunotherapy.
To the best of the authors’ knowledge, our study is the first comprehensive meta-analysis of 27 studies including a total of 3056 cases (HIF-1α) and four studies including a total of 397 cases (HIF-2α). We assessed the prognostic significance of HIF-1α and HIF-2α expression in advanced cancer patients receiving chemotherapy, radiotherapy, chemoradiotherapy, or immunotherapy. Our analyses did not find data associated with hypoxia-targeting agents and inhibitors of HIF activity for advanced cancer using multivariate analysis in preclinical and clinical studies.
The expression of HIF-2α was not linked to prognosis according to OS, DFS, PFS, or CSS. The pooled data indicated that the expression of HIF-1α was associated with reduced OS (HR = 1.61, p < 0.001), DFS (HR = 1.61, p < 0.001), PFS (HR = 1.49, p = 0.01), CSS (HR = 1.65, p = 0.056), RFS (HR = 2.10, p = 0.015), or MFS (HR = 2.36, p = 0.002). Moreover, evidence from some of the previous studies published is consistent with the current results, where HIF-1α expression was reported to be correlated with poor OS,23,36,38,40,42–44,49,50,56,58,59 DFS,43,45,56,59 PFS,57 CSS,52,53,57 RFS,39,48,50 and MFS38,48 in advanced cancers. These results were further confirmed using TSA, and TSA suggested that additional trials were necessary to validate these conclusions, including the association between HIF-1α expression and inferior DFS, PFS, CSS, RFS, and MFS and that there was no association between HIF-2α expression and survival. Additionally, based on different cancer systems, we found that HIF-1α expression was linked to shorter OS in digestive tract (HR = 1.61, p = 0.008), gynecological (HR = 1.60, P = 0.035), breast (HR = 1.41, p = 0.003), non-small cell lung (HR = 3.25, p < 0.001), and clear cell renal cell carcinomas (HR = 1.30, p = 0.011), but no correlation was observed in nasopharyngeal and head and neck cancers. Recent research has highlighted that chemotherapeutic treatments such as paclitaxel can induce the expression of HIF-1α.66 We demonstrated that HIF-1α expression was correlated with poor OS and DFS in patients without previously received therapy.
Stratification by study region showed a worse OS for European and Asian subjects; stratification by tumor location indicated no correlation between HIF-1α expression and OS in colorectal cancer and cervical cancer, but was significantly linked to reduced OS in pancreatic cancer (HR = 1.72, 95% CI = 1.27–2.33) and epithelial ovarian cancer (HR = 2.505, 95% CI = 1.252–5.013). Additionally, evidence from some previously published studies on these specific tumor types is consistent with our analyses, such as colorectal and cervical cancer. 21,22,37 When classified by survival status, HIF-1α expression was linked to worse prognosis for 5-year OS (HR = 1.36, p < 0.001) and <3-year OS (HR = 3.47, p = 0.007); classification by study design, HIF-1α expression showed a negative prognostic impact on OS in four prospective studies (HR = 1.39, p = 0.001)36,37,42,59 and 12 retrospective studies (HR = 1.79, p = 0.001). Classification by age subgroup showed that HIF-1α expression was related to worse OS in patients aged less than 60 years (HR = 1.44, p < 0.001) and older than 60 years (HR = 2.10, p < 0.001). Finally, we further applied TSA to obtain more meaningful results. TSA showed that there was sufficient data to draw reliable conclusions regarding the 5-year OS and patients less than 60 years of age subgroups (Table 2). Additional well-designed multicenter randomized controlled trials (RCTs) are needed to provide more accurate and conclusive evidence.
Interestingly, according to histologic subtypes, we found that HIF-1α expression was not associated with OS in squamous cell carcinoma, whereas the remaining studies with unclear or mixed histotypes showed a significant association. Other histotypes, such as adenocarcinoma, were unclear and lacking; it is possible that HIF-1α expression in other histotypes might affect the prognosis. Additionally, Furukawa and colleagues reported that HIF-1α-regulated glucose transporter (GLUT) 1 in lung adenocarcinoma may promote tumor aggressiveness and serve as a prognostic indicator of worse prognosis, but not in lung squamous cell carcinoma.70 Additional clinical studies are needed among other histologic subtypes of advanced cancer.
Our study has some important implications. First, HIF-1α expression is associated with worse outcomes, which suggests that HIF-1α may be a key druggable therapeutic target. This is important for advanced cancer patients who are treated with common chemotherapy, radiotherapy, or chemoradiotherapy. Second, a number of subgroup analyses have been conducted. Third, HIF-1α expression is linked to poor OS in European and Asian subjects, which suggests that HIF-1α may play important roles in different ethnic populations. Fourth, HIF-1α expression is related to an unfavorable OS in younger and older cancer patients, which indicates that HIF-1α may be a potential therapeutic target for younger or older cancer stratification. Finally, HIF-1α expression was not related to OS in squamous cell carcinoma, suggesting that additional prospective studies are essential to further validate whether HIF-1α expression has therapeutic implications in other histotypes, such as adenocarcinoma, due to different histological features.
This meta-analysis had several limitations. First, publication bias is present in the current meta-analysis, as indicated using Egger’s test because predominantly positive results were published. Articles with other styles, such as papers in other languages, unpublished papers, and conference abstracts, were excluded due to insufficient information, which may lead to potential bias. In addition, sensitivity analysis by omitting an individual study demonstrated a similar trend for the OS of HIF-1α expression results. Second, the number of some eligible studies had small sample sizes between HIF-1α expression and DFS, PFS, CSS, RFS, and MFS, and some subgroups on OS. The number of the included studies and sample sizes was relatively small between HIF-2α and the prognosis. Although all eligible studies were well performed, these results should be interpreted with caution based on TSA. Third, the cut-off values of HIF-1α and HIF-2α expression from the included studies may differ, and, in the future, HIF-1α and HIF-2α expression should be defined as positive or negative based on a standard, such as within a single cancer; for example, for lung cancer, the American Society of Clinical Oncology (ASCO), the College of American Pathologists (CAP), and the Association for Molecular Pathology (AMP) had to come together to standardize results for epidermal growth factor receptor (EGFR) detection.71 Fourth, the molecular features of various cancer types might differ to some extent and thus represent a complicated network. Biomarkers may also be affected by patient baseline characteristics. Thus, our study only included data adjusted by multivariate survival, and multivariable survival analysis adjusted factors are more valuable than the study that used univariable survival analysis. Fifth, even within a single cancer (colorectal), different treatment regimens were also found because the data are not an individual patient data analysis. In addition, only two RCTs evaluated the prognostic significance of HIF-1α expression in advanced cancer. We lacked sufficient RCTs to further prove our findings, and more trials that include subgroup analyses are warranted. Finally, the different sample types employed in these studies, including paraffin-embedded tumor tissue specimens, fresh tissue, serum, and plasma may be a potential source of heterogeneity. A detailed investigation of the best sample processing was not possibly performed in this meta-analysis. Therefore, the development of a stable high-performance assay with good sensitivity can be a good method for HIF-1α and HIF-2α detection and may help overcome this issue in the future.
In conclusion, the current study showed that HIF-1α expression was associated with a worse prognosis for advanced cancer patients treated with chemotherapy, radiotherapy, or chemoradiotherapy, which suggested that targeting HIF-1α may be a useful therapeutic approach to improve survival in advanced cancer patients. Based on the REMARK criteria, further large-scale prospective clinical trials including training and validation sets are strongly suggested to confirm our findings and help stratify the clinical treatment of patients into specific cancer types.
Supplemental Material
Supplemental material, Supplementary_file for The prognostic value of hypoxia-inducible factor-1α in advanced cancer survivors: a meta-analysis with trial sequential analysis by Susu Han, Tao Huang, Fenggang Hou, Liting Yao, Xiyu Wang and Xing Wu in Therapeutic Advances in Medical Oncology
Footnotes
Author contributions: Susu Han and Tao Huang contributed to the conception and design of this research. Susu Han, Xing Wu, Xiyu Wang, Liting Yao, and Tao Huang contributed to the drafting of the article and final approval of the submitted version. Susu Han, Tao Huang, Xing Wu, Xiyu Wang, Liting Yao, and Fenggang Hou contributed to data analyses and the interpretation and completion of the figures and tables. All authors read and approved the final manuscript.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Ethical review from patients: Our study was not primary research involving human samples, but rather a secondary analysis of human subject data published in public databases.
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
ORCID iDs: Susu Han  https://orcid.org/0000-0002-3999-0078
https://orcid.org/0000-0002-3999-0078
Tao Huang  https://orcid.org/0000-0002-9198-2868
https://orcid.org/0000-0002-9198-2868
Availability of data and materials: All data supporting our findings are listed in this manuscript.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Susu Han, Shanghai Municipal Hospital of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, 274 Zhijiang Road, 200071, People’s Republic of China.
Tao Huang, The Affiliated Hospital of Ningbo University, Ningbo, Zhejiang, People’s Republic of China.
Fenggang Hou, Shanghai Municipal Hospital of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, People’s Republic of China.
Liting Yao, Shanghai Municipal Hospital of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, People’s Republic of China.
Xiyu Wang, Shanghai Municipal Hospital of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, People’s Republic of China.
Xing Wu, Shanghai Municipal Hospital of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, People’s Republic of China.
References
- 1. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87–108. [DOI] [PubMed] [Google Scholar]
- 2. McQuade JL, Daniel CR, Hess KR, et al. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. Lancet Oncol 2018; 19: 310–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cousins SE, Tempest E, Feuer DJ. Surgery for the resolution of symptoms in malignant bowel obstruction in advanced gynaecological and gastrointestinal cancer. Cochrane Database Syst Rev 2016; 1: CD002764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Strigari L, Pinnaro P, Carlini P, et al. Efficacy and mucosal toxicity of concomitant chemo-radiotherapy in patients with locally-advanced squamous cell carcinoma of the head-and-neck in the light of a novel mathematical model. Crit Rev Oncol Hematol 2016; 102: 101–110. [DOI] [PubMed] [Google Scholar]
- 5. Hind D, Tappenden P, Tumur I, et al. The use of irinotecan, oxaliplatin and raltitrexed for the treatment of advanced colorectal cancer: systematic review and economic evaluation. Health Technol Assess 2008; 12: iii–ix, xi–162. [DOI] [PubMed] [Google Scholar]
- 6. Dienstmann R, Mason MJ, Sinicrope FA, et al. Prediction of overall survival in stage II and III colon cancer beyond TNM system: a retrospective, pooled biomarker study. Ann Oncol 2017; 28: 1023–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ocana A, Vera-Badillo F, Seruga B, et al. HER3 overexpression and survival in solid tumors: a meta-analysis. J Natl Cancer Inst 2013; 105: 266–273. [DOI] [PubMed] [Google Scholar]
- 8. Lohse I, Lourenco C, Ibrahimov E, et al. Assessment of hypoxia in the stroma of patient-derived pancreatic tumor xenografts. Cancers (Basel) 2014; 6: 459–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harris AL. Hypoxia–a key regulatory factor in tumour growth. Nat Rev Cancer 2002; 2: 38–47. [DOI] [PubMed] [Google Scholar]
- 10. Jochmanova I, Yang C, Zhuang Z, et al. Hypoxia-inducible factor signaling in pheochromocytoma: turning the rudder in the right direction. J Natl Cancer Inst 2013; 105: 1270–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marignol L, Rivera-Figueroa K, Lynch T, et al. Hypoxia, notch signalling, and prostate cancer. Nat Rev Urol 2013; 10: 405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang P, Yao Q, Lu L, et al. Hypoxia-inducible factor 3 is an oxygen-dependent transcription activator and regulates a distinct transcriptional response to hypoxia. Cell Rep 2014; 6: 1110–1121. [DOI] [PubMed] [Google Scholar]
- 13. Wigerup C, Pahlman S, Bexell D. Therapeutic targeting of hypoxia and hypoxia-inducible factors in cancer. Pharmacol Ther 2016; 164: 152–169. [DOI] [PubMed] [Google Scholar]
- 14. Maes C, Carmeliet G, Schipani E. Hypoxia-driven pathways in bone development, regeneration and disease. Nat Rev Rheumatol 2012; 8: 358–366. [DOI] [PubMed] [Google Scholar]
- 15. Gustafsson MV, Zheng X, Pereira T, et al. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev Cell 2005; 9: 617–628. [DOI] [PubMed] [Google Scholar]
- 16. Xie J, Gao H, Peng J, et al. Hispidulin prevents hypoxia-induced epithelial-mesenchymal transition in human colon carcinoma cells. Am J Cancer Res 2015; 5: 1047–1061. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17. Agani F, Jiang BH. Oxygen-independent regulation of HIF-1: novel involvement of PI3K/AKT/mTOR pathway in cancer. Curr Cancer Drug Targets 2013; 13: 245–251. [DOI] [PubMed] [Google Scholar]
- 18. Gordan JD, Thompson CB, Simon MC. HIF and c-Myc: sibling rivals for control of cancer cell metabolism and proliferation. Cancer Cell 2007; 12: 108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rankin EB, Giaccia AJ. Hypoxic control of metastasis. Science 2016; 352: 175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Giatromanolaki A, Koukourakis MI, Sivridis E, et al. Relation of hypoxia inducible factor 1 alpha and 2 alpha in operable non-small cell lung cancer to angiogenic/molecular profile of tumours and survival. Br J Cancer 2001; 85: 881–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Berk V, Deniz K, Bozkurt O, et al. Predictive significance of VEGF and HIF-1alpha expression in patients with metastatic colorectal cancer receiving chemotherapy combinations with bevacizumab. Asian Pac J Cancer Prev 2015; 16: 6149–6154. [DOI] [PubMed] [Google Scholar]
- 22. Goos JA, de Cuba EM, Coupe VM, et al. Glucose transporter 1 (SLC2A1) and vascular endothelial growth factor A (VEGFA) predict survival after resection of colorectal cancer liver metastasis. Ann Surg 2016; 263: 138–145. [DOI] [PubMed] [Google Scholar]
- 23. Wilson PM, Yang D, Azuma M, et al. Intratumoral expression profiling of genes involved in angiogenesis in colorectal cancer patients treated with chemotherapy plus the VEGFR inhibitor PTK787/ZK 222584 (vatalanib). Pharmacogenomics J 2013; 13: 410–416. [DOI] [PubMed] [Google Scholar]
- 24. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Altman DG, Bland JM. How to obtain the confidence interval from a P value. BMJ 2011; 343: d2090. [DOI] [PubMed] [Google Scholar]
- 26. Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007; 8: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McShane LM, Altman DG, Sauerbrei W, et al. Reporting recommendations for tumor marker prognostic studies (REMARK). J Natl Cancer Inst 2005; 97: 1180–1184. [DOI] [PubMed] [Google Scholar]
- 28. Zintzaras E, Ioannidis JP. HEGESMA: genome search meta-analysis and heterogeneity testing. Bioinformatics 2005; 21: 3672–3673. [DOI] [PubMed] [Google Scholar]
- 29. Evangelou E, Ioannidis JP. Meta-analysis methods for genome-wide association studies and beyond. Nat Rev Genet 2013; 14: 379–389. [DOI] [PubMed] [Google Scholar]
- 30. DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials 2007; 28: 105–114. [DOI] [PubMed] [Google Scholar]
- 31. Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50: 1088–1101. [PubMed] [Google Scholar]
- 33. Miladinovic B, Mhaskar R, Hozo I, et al. Optimal information size in trial sequential analysis of time-to-event outcomes reveals potentially inconclusive results because of the risk of random error. J Clin Epidemiol 2013; 66: 654–659. [DOI] [PubMed] [Google Scholar]
- 34. Thorlund K, Devereaux PJ, Wetterslev J, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta-analyses? Int J Epidemiol 2009; 38: 276–286. [DOI] [PubMed] [Google Scholar]
- 35. Brok J, Thorlund K, Gluud C, et al. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta-analyses. J Clin Epidemiol 2008; 61: 763–769. [DOI] [PubMed] [Google Scholar]
- 36. Beuselinck B, Verbiest A, Couchy G, et al. Pro-angiogenic gene expression is associated with better outcome on sunitinib in metastatic clear-cell renal cell carcinoma. Acta Oncol 2018; 57: 498–508. [DOI] [PubMed] [Google Scholar]
- 37. Moreno-Acosta P, Vallard A, Carrillo S, et al. Biomarkers of resistance to radiation therapy: a prospective study in cervical carcinoma. Radiat Oncol 2017; 12: 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nyström H, Jonsson M, Werner-Hartman L, et al. Hypoxia-inducible factor 1alpha predicts recurrence in high-grade soft tissue sarcoma of extremities and trunk wall. J Clin Pathol 2017; 70: 879–885. [DOI] [PubMed] [Google Scholar]
- 39. Chen SW, Shen WC, Lin YC, et al. Correlation of pretreatment (18)F-FDG PET tumor textural features with gene expression in pharyngeal cancer and implications for radiotherapy-based treatment outcomes. Eur J Nucl Med Mol Imaging 2017; 44: 567–580. [DOI] [PubMed] [Google Scholar]
- 40. Shultz DB, Pai J, Chiu W, et al. A novel biomarker panel examining response to gemcitabine with or without erlotinib for pancreatic cancer therapy in NCIC clinical trials group PA.3. PLoS One 2016; 11: e0147995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Xie M, He CS, Huang JK, et al. Phase II study of pazopanib as second-line treatment after sunitinib in patients with metastatic renal cell carcinoma: a Southern China urology cancer consortium trial. Eur J Cancer 2015; 51: 595–603. [DOI] [PubMed] [Google Scholar]
- 42. Braicu EI, Luketina H, Richter R, et al. HIF1alpha is an independent prognostic factor for overall survival in advanced primary epithelial ovarian cancer - a study of the OVCAD Consortium. Onco Targets Ther 2014; 7: 1563–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang L, Ye SB, Li ZL, et al. Increased HIF-1alpha expression in tumor cells and lymphocytes of tumor microenvironments predicts unfavorable survival in esophageal squamous cell carcinoma patients. Int J Clin Exp Pathol 2014; 7: 3887–3897. [PMC free article] [PubMed] [Google Scholar]
- 44. Wu F, Zhang J, Liu Y, et al. HIF1alpha genetic variants and protein expressions determine the response to platinum based chemotherapy and clinical outcome in patients with advanced NSCLC. Cell Physiol Biochem 2013; 32: 1566–1576. [DOI] [PubMed] [Google Scholar]
- 45. Shimomura M, Hinoi T, Kuroda S, et al. Overexpression of hypoxia inducible factor-1 alpha is an independent risk factor for recurrence after curative resection of colorectal liver metastases. Ann Surg Oncol 2013; 20(Suppl. 3): S527–S536. [DOI] [PubMed] [Google Scholar]
- 46. Shim BY, Jung JH, Lee KM, et al. Glucose transporter 1 (GLUT1) of anaerobic glycolysis as predictive and prognostic values in neoadjuvant chemoradiotherapy and laparoscopic surgery for locally advanced rectal cancer. Int J Colorectal Dis 2013; 28: 375–383. [DOI] [PubMed] [Google Scholar]
- 47. Wan XB, Fan XJ, Huang PY, et al. Aurora-A activation, correlated with hypoxia-inducible factor-1alpha, promotes radiochemoresistance and predicts poor outcome for nasopharyngeal carcinoma. Cancer Sci 2012; 103: 1586–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shioya M, Takahashi T, Ishikawa H, et al. Expression of hypoxia-inducible factor 1alpha predicts clinical outcome after preoperative hyperthermo-chemoradiotherapy for locally advanced rectal cancer. J Radiat Res 2011; 52: 821–827. [DOI] [PubMed] [Google Scholar]
- 49. Fraga CA, de Oliveira MV, de Oliveira ES, et al. A high HIF-1alpha expression genotype is associated with poor prognosis of upper aerodigestive tract carcinoma patients. Oral Oncol 2012; 48: 130–135. [DOI] [PubMed] [Google Scholar]
- 50. Xiang ZL, Zeng ZC, Fan J, et al. The expression of HIF-1alpha in primary hepatocellular carcinoma and its correlation with radiotherapy response and clinical outcome. Mol Biol Rep 2012; 39: 2021–2029. [DOI] [PubMed] [Google Scholar]
- 51. Koo JS, Kim H. Hypoxia-related protein expression and its clinicopathologic implication in carcinoma of unknown primary. Tumour Biol 2011; 32: 893–904. [DOI] [PubMed] [Google Scholar]
- 52. Dellas K, Bache M, Pigorsch SU, et al. Prognostic impact of HIF-1alpha expression in patients with definitive radiotherapy for cervical cancer. Strahlenther Onkol 2008; 184: 169–174. [DOI] [PubMed] [Google Scholar]
- 53. Klatte T, Seligson DB, Riggs SB, et al. Hypoxia-inducible factor 1 alpha in clear cell renal cell carcinoma. Clin Cancer Res 2007; 13: 7388–7393. [DOI] [PubMed] [Google Scholar]
- 54. Generali D, Berruti A, Brizzi MP, et al. Hypoxia-inducible factor-1alpha expression predicts a poor response to primary chemoendocrine therapy and disease-free survival in primary human breast cancer. Clin Cancer Res 2006; 12: 4562–4568. [DOI] [PubMed] [Google Scholar]
- 55. Winter SC, Shah KA, Han C, et al. The relation between hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha expression with anemia and outcome in surgically treated head and neck cancer. Cancer 2006; 107: 757–766. [DOI] [PubMed] [Google Scholar]
- 56. Theodoropoulos GE, Lazaris AC, Theodoropoulos VE, et al. Hypoxia, angiogenesis and apoptosis markers in locally advanced rectal cancer. Int J Colorectal Dis 2006; 21: 248–257. [DOI] [PubMed] [Google Scholar]
- 57. Bachtiary B, Schindl M, Potter R, et al. Overexpression of hypoxia-inducible factor 1alpha indicates diminished response to radiotherapy and unfavorable prognosis in patients receiving radical radiotherapy for cervical cancer. Clin Cancer Res 2003; 9: 2234–2240. [PubMed] [Google Scholar]
- 58. Burri P, Djonov V, Aebersold DM, et al. Significant correlation of hypoxia-inducible factor-1alpha with treatment outcome in cervical cancer treated with radical radiotherapy. Int J Radiat Oncol Biol Phys 2003; 56: 494–501. [DOI] [PubMed] [Google Scholar]
- 59. Schindl M, Schoppmann SF, Samonigg H, et al. Overexpression of hypoxia-inducible factor 1alpha is associated with an unfavorable prognosis in lymph node-positive breast cancer. Clin Cancer Res 2002; 8: 1831–1837. [PubMed] [Google Scholar]
- 60. Garcia-Donas J, Leandro-Garcia LJ, Gonzalez Del Alba A, et al. Prospective study assessing hypoxia-related proteins as markers for the outcome of treatment with sunitinib in advanced clear-cell renal cell carcinoma. Ann Oncol 2013; 24: 2409–2414. [DOI] [PubMed] [Google Scholar]
- 61. Willers H, Azzoli CG, Santivasi WL, et al. Basic mechanisms of therapeutic resistance to radiation and chemotherapy in lung cancer. Cancer J 2013; 19: 200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Begg AC, Stewart FA, Vens C. Strategies to improve radiotherapy with targeted drugs. Nat Rev Cancer 2011; 11: 239–253. [DOI] [PubMed] [Google Scholar]
- 63. Beasley NJ, Leek R, Alam M, et al. Hypoxia-inducible factors HIF-1alpha and HIF-2alpha in head and neck cancer: relationship to tumor biology and treatment outcome in surgically resected patients. Cancer Res 2002; 62: 2493–2497. [PubMed] [Google Scholar]
- 64. Amelio I, Melino G. The p53 family and the hypoxia-inducible factors (HIFs): determinants of cancer progression. Trends Biochem Sci 2015; 40: 425–434. [DOI] [PubMed] [Google Scholar]
- 65. Lu H, Samanta D, Xiang L, et al. Chemotherapy triggers HIF-1-dependent glutathione synthesis and copper chelation that induces the breast cancer stem cell phenotype. Proc Natl Acad Sci U S A 2015; 112: E4600–E4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Samanta D, Gilkes DM, Chaturvedi P, et al. Hypoxia-inducible factors are required for chemotherapy resistance of breast cancer stem cells. Proc Natl Acad Sci U S A 2014; 111: E5429–E5438. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 67. Rohwer N, Cramer T. Hypoxia-mediated drug resistance: novel insights on the functional interaction of HIFs and cell death pathways. Drug Resist Updat 2011; 14: 191–201. [DOI] [PubMed] [Google Scholar]
- 68. Balamurugan K. HIF-1 at the crossroads of hypoxia, inflammation, and cancer. Int J Cancer 2016; 138: 1058–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Palazon A, Goldrath AW, Nizet V, et al. HIF transcription factors, inflammation, and immunity. Immunity 2014; 41: 518–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Furukawa T, Miyata Y, Kushitani K, et al. Association between [18F]-fluoro-2-deoxyglucose uptake and expressions of hypoxia-induced factor 1alpha and glucose transporter 1 in non-small cell lung cancer. Jpn J Clin Oncol 2015; 45: 1154–1161. [DOI] [PubMed] [Google Scholar]
- 71. Kalemkerian GP, Narula N, Kennedy EB, et al. Molecular testing guideline for the selection of patients with lung cancer for treatment with targeted tyrosine kinase inhibitors: American society of clinical oncology endorsement of the college of American pathologists/international association for the study of lung cancer/association for molecular pathology clinical practice guideline update. J Clin Oncol 2018; 36: 911–919. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_file for The prognostic value of hypoxia-inducible factor-1α in advanced cancer survivors: a meta-analysis with trial sequential analysis by Susu Han, Tao Huang, Fenggang Hou, Liting Yao, Xiyu Wang and Xing Wu in Therapeutic Advances in Medical Oncology