ABSTRACT
The commensal species Candida parapsilosis is an emerging human pathogen that has the ability to form biofilms. In this study, we explored the impact of the divalent cations cobalt (Co2+), copper (Cu2+), iron (Fe3+), manganese (Mn2+), nickel (Ni2+) and zinc (Zn2+) on biofilm formation of clinical isolates of C. parapsilosis with no, low and high biofilm forming abilities at 30 and 37°C. All strains besides one isolate showed a concentration-dependent enhancement of biofilm formation at 30°C in the presence of Mn2+ with a maximum at 2 mM. The biofilm forming ability of no and low biofilm forming isolates was >2-fold enhanced in the presence of 2 mM Mn2+, while the effect in high biofilm forming isolate was significantly less pronounced. Of note, cells in the biofilms of no and low biofilm forming strains differentiated into yeast and pseudohyphal cells similar in morphology to high biofilm formers. The biofilm transcriptional activator BCR1 has a dual developmental role in the absence and presence of 2 mM Mn2+ as it promoted biofilm formation of no biofilm forming strains, and, surprisingly, suppressed cells of no biofilm forming strains to develop into pseudohyphae and/or hyphae. Thus, environmental conditions can significantly affect the amount of biofilm formation and cell morphology of C. parapsilosis with Mn2+ to overcome developmental blocks to trigger biofilm formation and to partially relieve BCR1 suppressed cell differentiation.
Keywords: Candida parapsilosis, biofilm formation, metal ions, manganese, BCR1, yeast cells, pseudohyphae, hyphae, cell morphology
Impact of manganese on biofilm formation in Candida parapsilosis.
INTRODUCTION
A primary cause of morbidity and mortality in hospitals is nosocomial fungal infections due to Candida species. Nosocomial bloodstream infections have Candida species as the fourth most common causative agent with Candida albicans as the most common species isolated from these infections (Wisplinghoff et al. 2004). The frequency of invasive infections by non-albicans Candida species especially Candida parapsilosis has increased over the past few decades (Tóth et al. 2019), with C. parapsilosis often being the second most commonly isolated species from bloodstream infections (Pfaller et al. 2011). However, C. parapsilosis can sometimes even become the leading cause of Candida infections in hospitals around the globe (Ng et al. 2001; Pfaller et al. 2001; Medrano et al. 2006; Tóth et al. 2019). In one-fourth of fungal infections in newborns with low weight in the United Kingdom, C. parapsilosis is the causative agent of mortality in neonatal intensive care units (Roilides et al. 2004; Clerihew et al. 2007; Tóth et al. 2019).
Typically, C. parapsilosis is not an obligate human pathogen, but a harmless commensal of the human skin (Weems 1992). However, the hands of healthcare workers colonized by C. parapsilosis are the most relevant source of transmission and outbreaks in hospitals, when infecting susceptible individuals (Kuhn et al. 2004). Candida parapsilosis, though, is more widespread and has also been isolated in nature from sources such as animals, soil and insects (Trofa, Gácser and Nosanchuk 2008). This species is notorious for its capacity to form biofilms on catheters and other implanted devices (Trofa, Gácser and Nosanchuk 2008; van Asbeck, Clemons and Stevens 2009). Therefore, patients with prolonged use of a central venous catheter or other indwelling devices are at increased risk for infection with C. parapsilosis (Trofa, Gácser and Nosanchuk 2008). The ability to form biofilms has been considered to be an important, if not the major, virulence factor of C. parapsilosis.
In C. albicans, systematic analyses identified the gene network regulating biofilm formation. Especially, a panel of transcription factors has been demonstrated to play a predominant role in the regulation of biofilm formation in C. albicans (Finkel and Mitchell 2011; Nobile et al. 2012; Araújo, Henriques and Silva 2017). Bcr1, a major biofilm transcriptional regulator of C. albicans, was shown to have a differential role in biofilm formation of C. parapsilosis (Ding and Butler 2007; Ding et al. 2011). While Bcr1 promotes biofilm formation in low biofilm forming isolates of C. parapsilosis (Pannanusorn et al. 2014), it does not trigger biofilm formation in high biofilm forming isolates of C. parapsilosis (Pannanusorn et al. 2014). Also, other transcriptional factors can have distinct roles in C. parapsilosis. For example, Ume6 does not have a major effect on biofilm formation in C. albicans, but significantly promotes biofilm formation in C. parapsilosis (Holland et al. 2014; Araújo, Henriques and Silva 2017).
Metal ions are vital for microorganisms, as they are required to perform fundamental biological processes such as DNA replication, transcription, biosynthesis of precursors, respiration and the oxidative stress response (Allison et al. 2016). Nevertheless, the full spectrum of biological functions of metal ions has not been explored (Cvetkovic et al. 2010). The availability and biological functionality of metal ions vary widely (Mackie et al. 2016; Rodríguez and Mandalunis 2018). Metal ions also play a role in biofilm formation and cell morphology. In C. albicans and Candida tropicalis, various metal ions affected cell differentiation during biofilm growth (Harrison et al. 2007). Candida albicans biofilms are composed of yeast and hyphal cells (Odds 1988). Hyphal formation in biofilms of C. albicans was inhibited by arsenic, cadmium, chromium, cobalt, copper, selenium, silver and zinc, while enhanced by lead (Harrison et al. 2007). Similarly, in C. tropicalis hyphal formation was enhanced by chromium and zinc, but inhibited by arsenic, cadmium, cobalt, copper, lead, selenium and silver (Odds 1988). The morphological transition from yeast to a pseudohyphal/hyphal form has an important role in the virulence of C. albicans and is affected by numerous environmental conditions (Odds 1988; Lackey et al. 2013). Candida parapsilosis, however, has not been considered to form true hyphae like C. albicans (Trofa, Gácser and Nosanchuk 2008). High biofilm forming strains of C. parapsilosis showed a morphologically distinct biofilm composed of yeast and pseudohyphae, while biofilms of low biofilm forming strains were composed of yeast cells (Pannanusorn, Fernandez and Römling 2013; Pannanusorn et al. 2014). Candida parapsilosis yeast cells have an oval, round or cylindrical shape suggesting different, noncategorized cell types (Trofa, Gácser and Nosanchuk 2008).
Previously, we have categorized clinical strains of C. parapsilosis from bloodstream infection into no, low and high biofilm formers (Pannanusorn et al. 2014). The concentration of divalent cations can vary widely in environmental settings and in the human body. Here, we explored the impact of enhanced concentrations of different essential divalent metal ions on the biofilm forming ability of selected clinical isolates of C. parapsilosis. Interestingly, we demonstrate that in the presence of 2 mM of the divalent manganese cation (Mn2+) at 30°C, biofilm formation in no and low biofilm formers was consistently >2-fold enhanced and cells differentiated into yeasts and pseudohyphae. This enhanced biofilm forming ability of no biofilm formers in the presence of Mn2+ was dependent on Bcr1 with cell differentiation suppressed by Bcr1. Moreover, we identified the presence of hyphae, long thin filamentous cells with minimal constriction at the septal junction, mainly in the bcr1∆ mutant. In summary, Bcr1 has opposite effects on the amount of biofilm formation and differential cell morphology. Thus, Bcr1 is a transcriptional regulator of biofilm formation versus cell differentiation in C. parapsilosis pronounced under elevated Mn2+ concentration.
MATERIALS AND METHODS
Strains and growth conditions
Candida parapsilosis clinical isolates SMI 416, SMI 501, SMI 630, SMI 661, SMI 681, SMI 694, SMI 706, SMI 798 and SMI 828 from bloodstream infection (Pannanusorn, Fernandez and Römling 2013; Pannanusorn et al. 2014) and the reference strain ATCC 22019 from the American Type Culture Collection (Manassas, VA, USA) were used in this study (Table S1, Supporting Information). YPD agar plates (2% peptone, 1% yeast extract, 2% glucose and 1.5% agar) were used to grow C. parapsilosis for 48 h at 37°C. Single colonies were suspended in YNB medium (0.67% yeast nitrogen base, Sigma) supplemented with 2% glucose (YNB/2% glucose) and incubated overnight at 30 or 37°C depending on the incubation temperature in the next step. Divalent transition metal ions were added as a salt of nitrate (Co(NO3)2, Cu(NO3)2, Fe(NO3)3, Mn(NO3)2, Ni(NO3)2 and Zn(NO3)2) at different concentrations as indicated. In YNB medium, Mn2+ concentration of 2.367 µM (manufacturer information) was set as 0 mM.
bcr1∆ mutant construction
A bcr1∆ mutant was constructed in the clinical isolate SMI 416 and the reference strain ATCC 22019. Construction of the BCR1 deletion and complementation of the bcr1∆ mutant was performed by using the SAT1 flipping strategy (Reuss et al. 2004; Sasse and Morschhäuser 2012). The bcr1 deletion mutant plasmid pBCR1CpM2 constructed previously (Pannanusorn et al. 2014) was used for two sequential rounds of transformation and recycling of the cassette to delete bcr1 on both chromosomes. Two independent bcr1∆ mutants were constructed for each strain. For complementation of the ATCC 22019 bcr1∆ mutant, pBCR1CpMK1 (Pannanusorn et al. 2014) was integrated into one of the bcr1∆ alleles of the homozygous mutant, followed by recycling of SAT1 flipper cassette. All deletion events and the complementation were verified by Southern blotting using the BCR1 upstream fragment as the hybridization probe.
Biofilm formation
Biofilms were allowed to form in presterilized polystyrene flat-bottom 96-well microtiter plates in the presence of different concentrations of metal ions in YNB/2% glucose. Wells of the microtiter plate were inoculated with 200 µl cell suspension (OD600 = 0.1) in YNB/2% glucose medium without or with the desired concentration of the divalent metal ion. Cobalt (Co2+), copper (Cu2+), iron (Fe3+), manganese (Mn2+), nickel (Ni2+) and zinc (Zn2+) were used at 0.1, 0.2, 0.5, 1, 2 and 10 mM concentration as a salt of nitrate. To allow yeast cells to adhere to the polystyrene surface of the well, the plates were incubated at 30 or 37°C for 3 h. After the adhesion phase, the cell suspension was removed and each well was washed three times with 250 µl fresh medium (without added glucose and metal ion). Fresh YNB/2% glucose medium (200 µl) without or with the desired concentration of metal ion was added in each well. The plates were incubated at 30 or 37°C for 48 h.
Crystal violet assay
Biofilm formation was quantified by the crystal violet assay (O’Toole 2011). For the crystal violet assay, wells with developed biofilms were washed three times with 250 µl PBS (1×). Then, wells were stained with 200 µl 0.2% crystal violet (Sigma) for 10 min. Stained wells were washed three times with 250 µl sterilized distilled water. Wells were destained with 200 µl 30% acetic acid. The dissolved crystal violet was quantified by measuring the absorbance at 600 nm.
Assessment of cell morphology in biofilms
To assess cell morphology in surface attached biofilms, C. parapsilosis was grown in presterilized polystyrene flat-bottom 24-well microtiter plates to produce biofilms in the presence of YNB/2% glucose medium with and without 2 mM Mn2+ at 30°C. Each well of the microtiter plate was inoculated with 1 ml cell suspension (OD600 = 0.1) in YNB/2% glucose medium without or with 2 mM Mn2+. Yeast cells were allowed to adhere for 3 h. After the adhesion phase, the cell suspension was removed and each well was washed three times with 2 ml fresh medium without Mn2+. Fresh YNB/2% glucose medium (1 ml) without or with 2 mM Mn2+ was added in each well. The plates were incubated at 30°C for 48 h. After 48 h, biofilm wells were washed three times with 2 ml PBS (1×) and the cell morphology was investigated under the light microscope (Leica) with 20× magnification.
For confocal microscopy, C. parapsilosis was grown in presterilized polystyrene flat-bottom 24-well microtiter plates to produce biofilms in the presence of Mn2+ as described earlier. After 48 h, biofilms were stained with 0.05% Calcofluor solution. To assess viability, biofilms were stained with the LIVE/DEAD BacLight Viability Kit according to the manufacturer’s instruction. Biofilms were observed from the bottom of the microtiter plate under an Olympus FV1000 confocal microscope. ImageJ was used for analyzing images (Schneider, Rasband and Eliceiri 2012).
Bioinformatic analysis
Bcr1 from Candida orthopsilosis (XP_003869584.1), Candida viswanathii (XP_026591228.1), C. tropicalis (XP_002545827.1), Candida dubliniensis (XP_002422159.1), C. albicans (KHC28444.1) and Lodderomyces elongisporus (XP_001525195.1) were identified by a Blast search (National Center for Bioinformatic Information) with Bcr1 (CCE42957.1) from C. parapsilosis. The proteins were aligned by Clustal using standard parameters. The alignment was visualized with the sequence alignment editor BioEdit (http://www.mbio.ncsu.edu/BioEdit/bioedit.html).
RESULTS
Impact of different transition metal ions on biofilm forming ability of C. parapsilosis
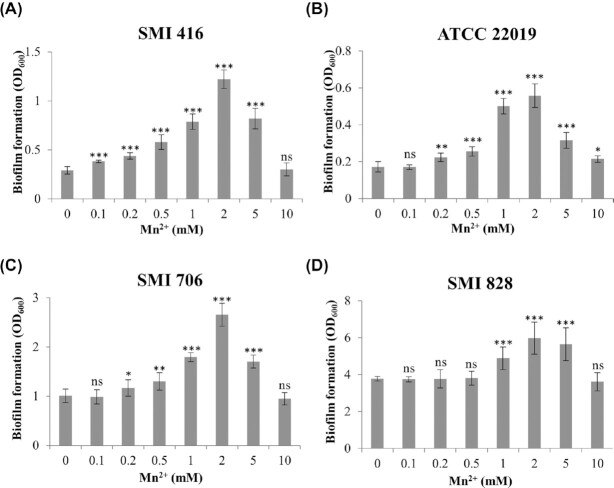
Thirty-three epidemiologically unrelated strains of C. parapsilosis from bloodstream infection had been categorized into no, low and high biofilm forming phenotypes according to their performance on different surfaces such as a silicone elastomer and a polystyrene surface (Pannanusorn, Fernandez and Römling 2013; Pannanusorn et al. 2014). The categorization of these strains was initially based on quantification of biofilms by the XTT reduction assay (Pannanusorn, Fernandez and Römling 2013). Microscopic assessment of no biofilm formers showed occasional adherence of single yeast cells to the surface in contrast to low biofilm formers, which formed clusters of yeast cell. Most high biofilm formers built a differentiated biofilm composed of dense cell clusters consisting of yeast and pseudohyphae, so-called spider-like biofilm, after 48 h of growth (Pannanusorn, Fernandez and Römling 2013; Pannanusorn et al. 2014). To study the impact of transition metal ions on the biofilm forming ability of C. parapsilosis, we selected isolates with no (SMI 416), low (SMI 706) and high (SMI 828) biofilm forming abilities to investigate the impact of Co2+, Cu2+, Fe3+, Mn2+, Ni2+ and Zn2+ on biofilm formation at 30 and 37°C. Strains were grown in polystyrene 96-well microtiter plates in YNB/2% glucose medium and crystal violet staining was used to assess biofilm formation. Increasing concentrations of Co2+, Cu2+, Fe3+, Ni2+ and Zn2+ from 0.1 to 10 mM in the growth medium led to a decrease in the biofilm forming ability of SMI 416, SMI 706 and SMI 828 at 30 and 37°C after 48 h (Fig. S1, Supporting Information). A similar decrease was observed in the case of Mn2+ at 37°C after 48 h (Fig. S2A, C and D, Supporting Information). However, after 48 h at 30°C, we consistently observed a concentration-dependent increase in the ability to form a biofilm, statistically significant already at 0.1–0.2 mM Mn2+ in no and low biofilm forming strains with a maximum at 2 mM Mn2+ (Fig. 1A, C and D). Interestingly, the effect was most pronounced in no and low biofilm forming strains, with a 4-fold increase in the biofilm forming ability of the no biofilm forming strain SMI 416 at 2 mM Mn2+ (Fig. 1A). To further elucidate the role of Mn2+ in the enhancement of biofilm formation of no and low biofilm formers, we investigated the impact of Mn2+ on the biofilm forming ability of the C. parapsilosis reference strain ATCC 22019, a poor biofilm former. No change in the biofilm forming ability of ATCC 22019 was observed at 37°C upon addition of Mn2+ (Fig. S2B, Supporting Information). However, an ∼3-fold increase in the biofilm forming ability of ATCC 22019 was observed with 2 mM Mn2+ at 30°C (Fig. 1B). These data indicated a putative positive role of Mn2+ in biofilm formation of C. parapsilosis at 30°C especially in the case of the no and low biofilm forming strains SMI 416, ATCC 22019 and SMI 706.
Figure 1.
Impact of increasing concentration of Mn2+ on the biofilm forming ability of no (SMI 416 and ATCC 22019), low (SMI 706) and high (SMI 828) biofilm formers of C. parapsilosis on a polystyrene surface in YNB/2% glucose medium after 48 h at 30°C. (A) SMI 416, (B) ATCC 22019, (C) SMI 706 and (D) SMI 828. Values are the mean of three independent experiments conducted in three biological replicates. Error bars indicate standard deviation. Statistical significance of the differences in biofilm formation in the presence of Mn2+ compared to 0 mM Mn2+ was determined by one-way ANOVA (ns—not significant, *P < 0.05, **P < 0.01 and ***P < 0.001).
To investigate whether the effect of Mn2+ on biofilm formation extended to other no biofilm forming strains, we tested the impact of 2 mM Mn2+ on the biofilm forming capacity of additional six clinical isolates of C. parapsilosis, SMI 501, SMI 630, SMI 661, SMI 681, SMI694 and SMI 798, at 30°C. Consistent with previous observations, the presence of 2 mM Mn2+ in the medium led to a variable, but statistically significant increase in the biofilm forming capacity of five C. parapsilosis clinical isolates (Fig. S3, Supporting Information). No change in the biofilm forming ability of SMI 798 in the presence of 2 mM Mn2+ was observed.
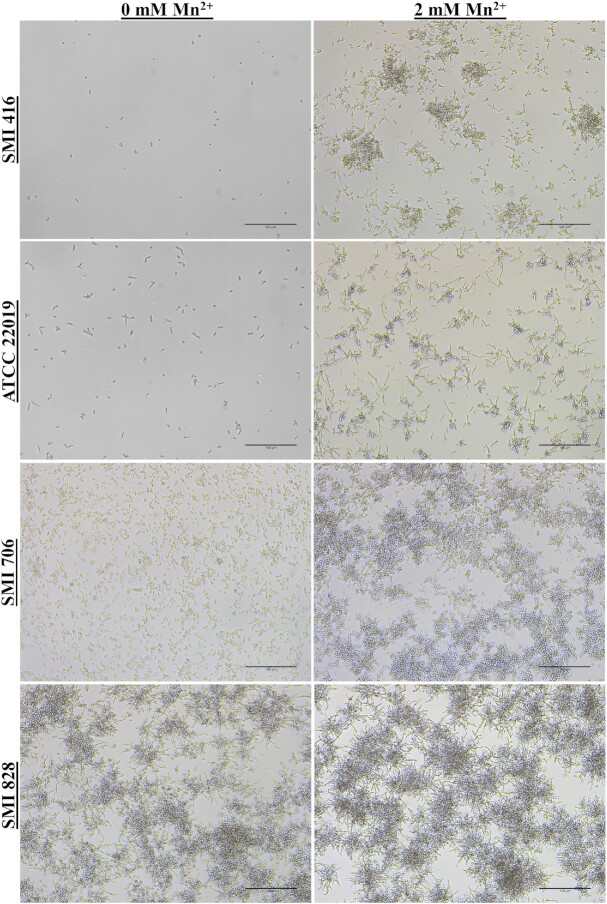
Impact of Mn2+ on the cell morphology of C. parapsilosis
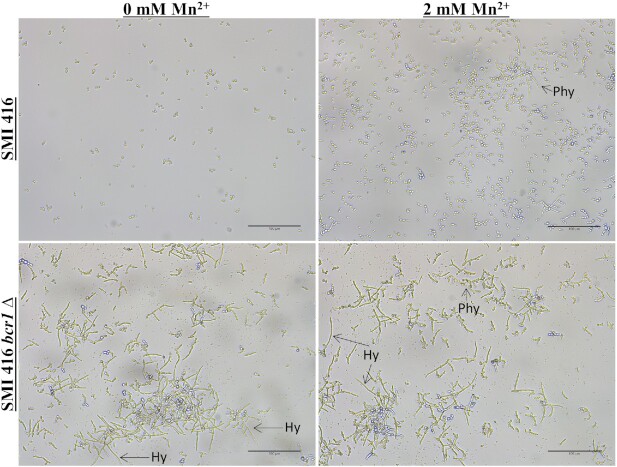
Enhanced biofilm formation in C. parapsilosis is associated with a change in cell clustering and differentiation (Pannanusorn et al. 2014). Subsequently, biofilm formation was assessed with light microscopy to investigate biofilm morphology and cell differentiation. Biofilms of no (ATCC 22019 and SMI 416), low (SMI 706) and high (SMI 828) biofilm formers of C. parapsilosis were grown without and with 2 mM Mn2+ to achieve a maximum increase in biofilm formation at 30°C. Consistent with previously published observations (Pannanusorn et al. 2014), without Mn2+, no biofilm forming strains did not show formation of microcolonies, but SMI 416 occasionally attached to the surface as a single yeast cell, while ATCC 22019 formed elongated cells and clusters of two to three cells (Fig. 2). Supplementation of the medium with 2 mM Mn2+ led to a dramatic morphological change in the biofilm of SMI 416 and ATCC 22019 strains; increased biofilm formation was accompanied by cells to attach as smaller and extended cell clusters to the polystyrene surface and to morphologically differentiate into yeast and pseudohyphal cells (Fig. 2). Similarly, the biofilm of the low biofilm forming strain SMI 706 consisted of individually attached yeast cells, smaller and larger, mainly yeast cell clusters with the occasional formation of pseudohyphae (Pannanusorn et al. 2014). Upon addition of 2 mM Mn2+ to the YNB/2% glucose medium, SMI 706 developed a biofilm consisting mainly of extended cell clusters comprised of yeast and pseudohyphal cells (Fig. 2). The high biofilm forming strain SMI 828 developed a differentiated biofilm comprised of yeast and pseudohyphae under all investigated conditions with no major change in biofilm morphology observed upon addition of 2 mM Mn2+ (Fig. 2). These data suggest that the presence of enhanced concentrations of Mn2+ in the medium promotes biofilm formation and the development of pseudohyphae especially in no and low biofilm forming strains.
Figure 2.
Impact of Mn2+ on biofilm formation and cell morphology on a polystyrene surface of no (SMI 416 and ATCC 22019), low (SMI 706) and high (SMI 828) biofilm formers of C. parapsilosis at 30°C. Scale bar represents 100 μm.
Having established the association between enhanced crystal violet staining and the amount of biofilm, we were subsequently wondering at which stage in the biofilm development, upon initial attachment and/or development of a mature biofilm, Mn2+ exerts its effect. To study the impact of Mn2+ on initial attachment, we allowed the adherence of C. parapsilosis SMI 416 and ATCC 22019 cells to the polystyrene surface of 96-well microtiter plates in YNB/2% glucose without and with 2 mM Mn2+ at 30°C for 3 h. Crystal violet staining indicated no difference in attachment of SMI 416 and ATCC 22019 cells to the polystyrene surface without and with 2 mM Mn2+ (Fig. S4, Supporting Information). These data indicate that Mn2+ must play a role at a later stage of biofilm formation in the no biofilm forming strains SMI 416 and ATCC 22019.
In addition, we investigated the impact of Mn2+ on the growth of SMI 416. The OD600 of SMI 416 after 48 h of growth in the presence of 2 mM Mn2+ was 50% lower (data not shown). Microscopic observation of SMI 416 in liquid culture indicated only yeast cells, while in the presence of 2 mM Mn2+ formation of small aggregates of <10 cells with hardly any single cell was observed (data not shown). We further tested cell viability of the biofilm formed by SMI 416 in the presence of 2 mM Mn2+ at 30°C after 48 h. Most cells in the Mn2+-triggered biofilm formed by SMI 416 were viable (Fig. S5, Supporting Information).
Role of BCR1 in Mn2+-dependent biofilms of no biofilm forming C. parapsilosis
Bcr1 is a major regulator of biofilm formation in low biofilm forming strains of C. parapsilosis (Ding and Butler 2007; Pannanusorn et al. 2014). To investigate a potential impact of BCR1 deletion on Mn2+-dependent biofilm formation in the no biofilm forming strains SMI 416 and ATCC 22019, we compared development of wild-type biofilms with the respective bcr1∆ mutants on a polystyrene surface in 96-well microtiter plates in the presence of different concentrations of Mn2+ (0, 1, 2 and 10 mM) at 30 and 37°C. No substantial change in biofilm formation between the wild-types and bcr1∆ mutants was observed at 37°C at any Mn2+ concentration (Fig. S6, Supporting Information). However, Mn2+-dependent biofilm formation of no biofilm forming strains SMI 416 and ATCC 22019 was dramatically decreased in the absence of BCR1 at 30°C at all investigated Mn2+ concentrations (Fig. 3), while complementation of BCR1 in the ATCC 22019 strain restored Mn2+-dependent biofilm formation (Fig. 3B). These data indicate that Mn2+-dependent biofilm formation in the no biofilm forming strains ATCC 22019 and SMI 416 is dependent on BCR1.
Figure 3.
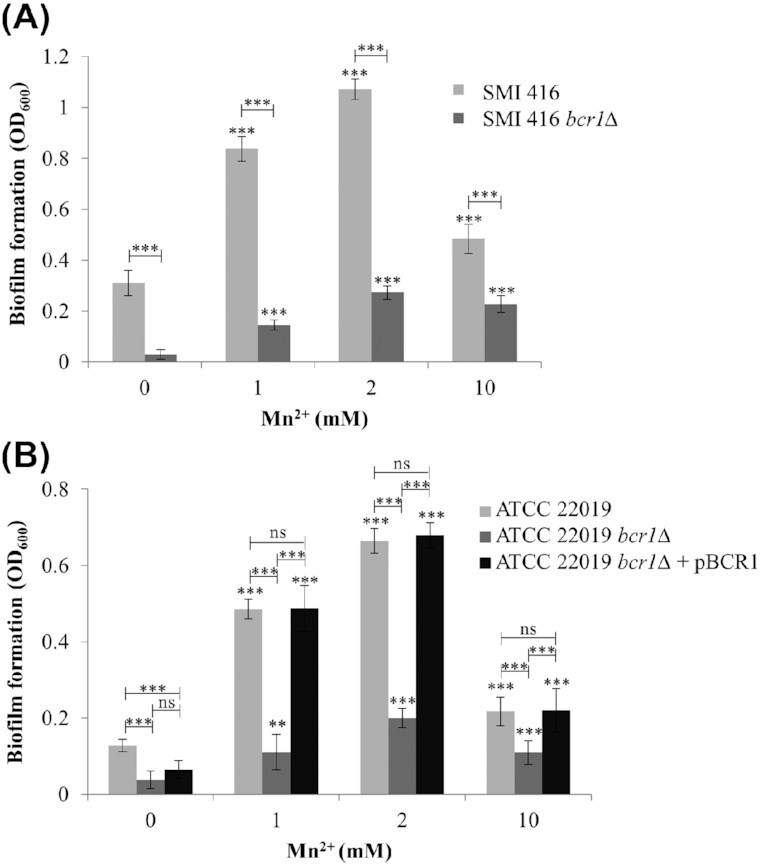
Impact of different concentrations of Mn2+ on the biofilm forming ability of wild-types SMI 416 and ATCC 22019, bcr1Δ mutants SMI 416 bcr1Δ and ATCC 22019 bcr1Δ, and BCR1 complementation ATCC 22019 bcr1Δ + pBCR1 on a polystyrene surface in YNB/2% glucose medium at 30°C after 48 h. Values are the mean of three independent experiments conducted in three biological replicates. Error bars indicate standard deviation. Statistical significance of the differences in biofilm formation in the presence of Mn2+ compared to the absence of Mn2+ and wild-type compared to bcr1Δ mutant/BCR1 complementation was determined by one-way ANOVA (ns—not significant, **P < 0.01 and ***P < 0.001).
Biofilm formation of SMI 416 bcr1∆ increased in the presence of 2 mM Mn2+ around 9-fold compared to YNB/2% glucose medium without added Mn2+ (Fig. 3A), while the relative increase in biofilm formation of the wild-type SMI 416 was around 5-fold. Similar results were observed for the reference strain ATCC 22019 (Fig. 3B). These data indicate that enhanced biofilm formation of no biofilm formers associated with elevated Mn2+ concentrations is suppressed by BCR1.
Role of BCR1 in Mn2+-dependent cell morphology of no biofilm forming C. parapsilosis
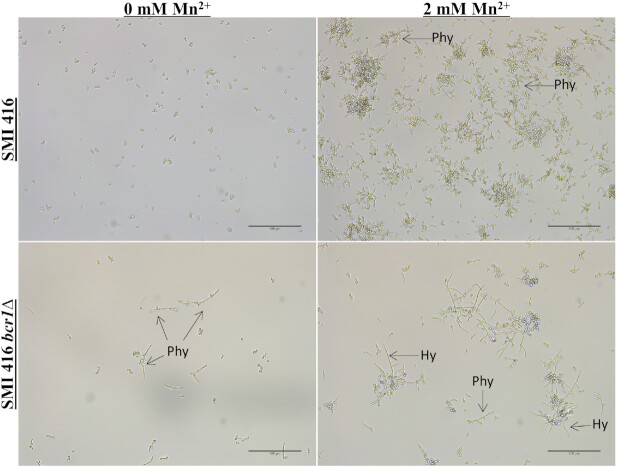
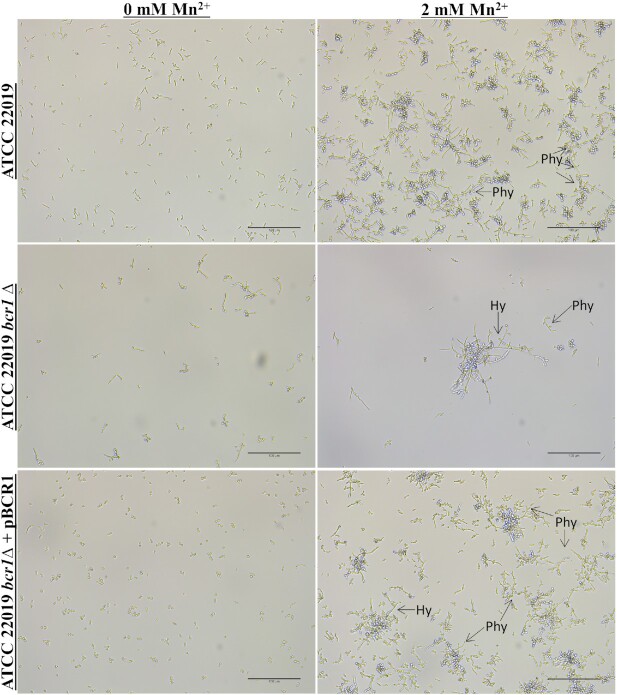
Subsequently, we explored the impact of the BCR1 deletion on cell morphology of no biofilm forming strains SMI 416 and ATCC 22019 without and with 2 mM of Mn2+ at 30°C. Biofilm formation was observed under the light microscope after 48 h of development. While the effect of BCR1 on cell differentiation was moderate without Mn2+, in the presence of 2 mM Mn2+, the bcr1∆ mutants of no biofilm forming strains SMI 416 (Fig. 4) and ATCC 22019 (Fig. 5), although significantly reduced in amount, showed biofilms comprised of yeast and pseudohyphal cells. In addition, we observed for the first time hyphae-like cells in C. parapsilosis biofilms. These hyphal-like cells are extensively elongated compared to pseudohyphal cells and have a reduced and uniform width (Figs 6 and 7; Fig. S7, Supporting Information). Upon complementation with BCR1, the bcr1∆ mutant of ATCC 22019 developed again a wild-type-like biofilm comprised of yeast and pseudohyphal cells in the presence of 2 mM Mn2+ (Fig. 5). These results indicate a role of BCR1 in hyphal development in C. parapsilosis in the presence of Mn2+.
Figure 4.
Impact of the BCR1 deletion on biofilm formation and cell morphology of wild-type C. parapsilosis SMI 416 and its bcr1Δ mutant at 30°C on a polystyrene surface in YNB/2% glucose medium with 0 and 2 mM of Mn2+. Arrows indicate pseudohyphae (Phy) and hyphae (Hy). Scale bar represents 100 μm.
Figure 5.
Impact of BCR1 deletion on biofilm formation and cell morphology of C. parapsilosis ATCC 22019 wild-type, its bcr1Δ mutant and BCR1 complementation at 30°C on a polystyrene surface in YNB/2% glucose medium with 0 and 2 mM of Mn2+. Arrows indicates the presence of pseudohyphae (Phy) and hyphae (Hy). Scale bar represents 100 μm.
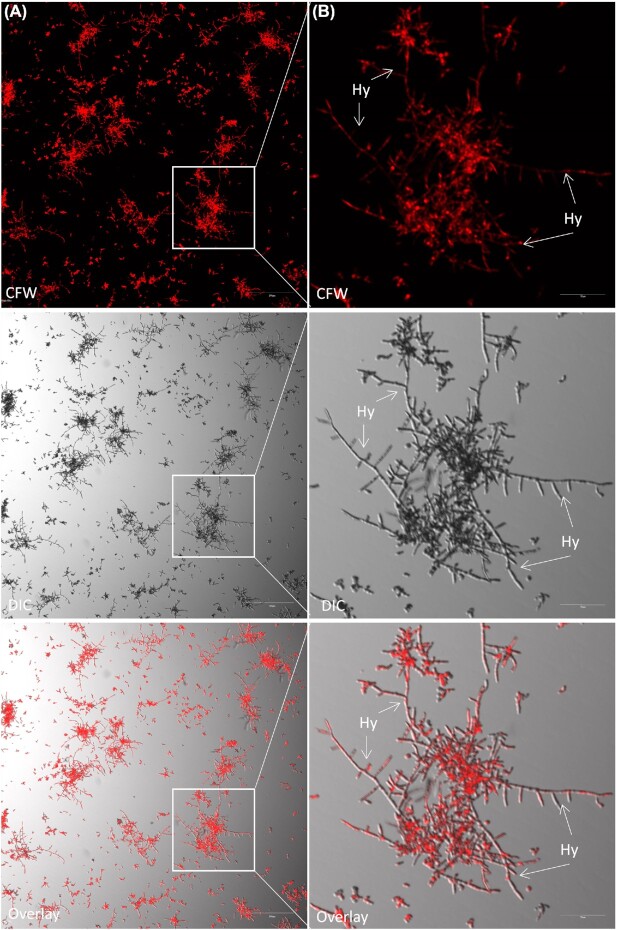
Figure 6.
Visualization of hyphal development in the bcr1Δ mutant of C. parapsilosis SMI 416 at 30°C on a polystyrene surface in YNB/2% glucose medium with 2 mM Mn2+. Arrows indicate the presence of hyphae (Hy). Scale bars: (A) 200 μm and (B) 50 μm.
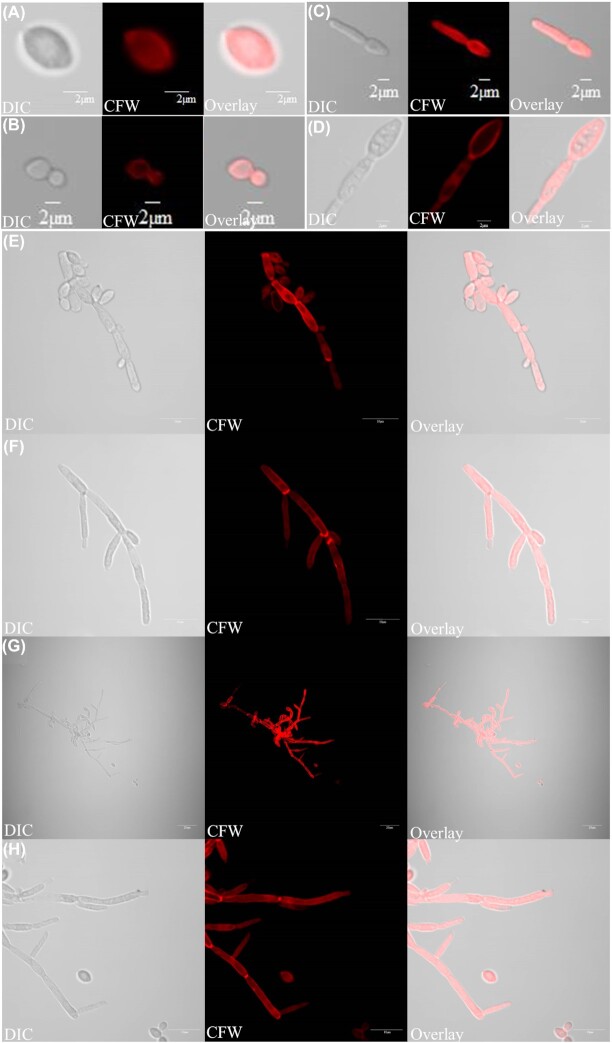
Figure 7.
Different cell morphologies observed in this study: (A) blastospore, (B) budding yeast, (C, D) germ tube, (E) pseudohyphae and (F–H) hyphae. Light (left) and fluorescent (Calcofluor staining; middle) microscopy images of different cell types were overlaid (right). Scale bars: (A–D) 2 μm, (E, F, H) 10 μm and (G) 20 μm.
We further investigated the impact of the BCR1 deletion on attachment of no biofilm forming strains SMI 416 and ATCC 22019 without and with 2 mM Mn2+ at 30°C after 3 h. Crystal violet staining showed no difference in attachment of the bcr1∆ mutants of SMI 416 and ATCC 22019 without and with 2 mM Mn2+, as observed for the wild-type. Thus, without Mn2+ equally as in the presence of 2 mM Mn2+, a >3-fold decrease in attachment of the bcr1∆ mutants compared to the wild-type of SMI 416 and ATCC 22019 was observed (Fig. S4, Supporting Information). Thus, initial reduction in attachment contributes to reduced biofilm formation of the bcr1∆ mutants of SMI 416 and ATCC 22019 compared to their respective wild-types in Mn2+-triggered biofilm formation.
Impact of media composition on BCR1-dependent cell morphology of no biofilm forming C. parapsilosis in the presence of Mn2+
To elucidate the role of different media in the cell morphology in biofilm formation on a polystyrene surface in the presence of 2 mM Mn2+, wild-type SMI 416 and its bcr1∆ mutant were grown in commonly used media (Spider, YPD and RPMI) supplemented with 2% glucose without and with 2 mM Mn2+ at 30°C. In YPD/2% glucose medium, SMI 416 was composed of yeast cells, while addition of 2 mM Mn2+ caused induction of pseudohyphae (Fig. 8). Interestingly, SMI 416 bcr1∆ showed extended hyphal development in the absence and presence of Mn2+ in the same medium (Fig. 8). In Spider and RPMI media, no change in cell morphology between the wild-type SMI 416 and its bcr1∆ mutant was observed in the presence or absence of 2 mM Mn2+ (data not shown). These data indicate medium-specific hyphal development in the absence of BCR1 in the no biofilm forming clinical isolate SMI 416.
Figure 8.
YPD/2% glucose medium induces filamentous growth of C. parapsilosis SMI 416 in a polystyrene surface. Cell morphology of C. parapsilosis SMI 416 wild-type and bcr1Δ mutant in YPD/2% glucose medium with and without 2 mM Mn2+ at 30°C. Scale bar represents 100 μm.
DISCUSSION
In a previous study, 33 clinical isolates of C. parapsilosis from bloodstream infection were categorized into no, low and high based on their biofilm forming ability (Pannanusorn, Fernandez and Römling 2013; Pannanusorn et al. 2014). Here, we show that Mn2+ positively affects biofilm formation of clinical isolates with no and low biofilm forming ability at 30°C. Furthermore, Mn2+ has a pronounced effect on cell morphology, especially in the absence of BCR1, triggering pseudohyphae and hyphae formation in no biofilm formers.
All forms of life require various metal ions as cofactors playing a structural and catalytic role in macromolecules involved in various fundamental biological processes. The transition metal ions Co2+, Cu2+, Fe3+, Mn2+, Ni2+ and Zn2+ are required by most organisms, as they are involved in essential physiological processes, but are toxic at higher concentrations. We tested the impact of these transition metal ions on biofilm formation of C. parapsilosis isolates with different biofilm forming ability at 30 and 37°C. Most of the metal ions reduced biofilm formation in C. parapsilosis clinical isolates independent of temperature and the biofilm forming capacity of the strains (Supporting Information). Surprisingly, in case of Mn2+ at 30°C, we observed enhanced biofilm formation of strains previously categorized to make no biofilm in YNB/2% glucose medium. The amount of biofilm formed by no biofilm forming strains in the presence of 2 mM Mn2+ corresponded to the amount of biofilm formed by the low biofilm forming strain SMI 706. In the presence of 2 mM Mn2+, we, however, observed enhanced development of pseudohyphae in no biofilm formers of C. parapsilosis. The role of metal ions in biofilm formation of C. albicans and C. tropicalis was previously investigated (Harrison et al. 2006, 2007). Metal ions can alter the structure of the biofilm by either blocking or enhancing the transition between yeast and hyphal cell types (Harrison et al. 2007). For example, Pb2+ in C. albicans and Zn2+ and CrO42− in C. tropicalis were observed to trigger hyphal formation (Harrison et al. 2007).
Biofilm formation is a major virulence factor of Candida species that is regulated by a network of transcription factors, which affects adhesion, hyphal formation and the production of extracellular polymeric substances (Cavalheiro and Teixeira 2018). Screening a mutant library of 165 transcriptional regulators identified six transcription factors BCR1, BRG1, EFG1, NDT80, ROB1 and TEC1 to affect biofilm formation of C. albicans on rat denture and in catheter models (Nobile et al. 2012). This highly complex regulatory network of different transcriptional regulators promotes the expression of a high number of target genes with overlapping target promoters for several transcriptional regulators (Nobile et al. 2012). In C. parapsilosis, overlapping transcription factor networks for ACE2, BCR1, EFG1 and UME6 to regulate biofilm formation in C. parapsilosis have been established (Ding and Butler 2007; Connolly et al. 2013; Holland et al. 2014). We demonstrated previously that BCR1 promotes biofilm formation of all low, and one of two high filamentous, biofilm forming C. parapsilosis clinical isolates, but does not play a role in, or even enhances, biofilm formation of high biofilm forming clinical isolates with spider-like biofilm morphology (Pannanusorn et al. 2014). Thus, BCR1 can have different roles in biofilm formation in the same species. Here, we show that BCR1 activates biofilm formation also in the no biofilm forming isolates SMI 416 and ATCC 22019 upon Mn2+-dependent enhancement of biofilm formation. This Mn2+-dependent effect on biofilm formation has a BCR1-dependent and -independent component indicating the importance of environmental conditions that alter regulation of biofilm formation by various transcription factors.
To colonize and form biofilm at a surface, C. parapsilosis initially adheres as single cells and subsequently builds up microcolonies (Pannanusorn et al. 2014). The initial adherence of Candida cells is an important factor for dissemination of the Candida infection and regulated by different environmental conditions, the host and the physiological status of the Candida cell (Silva et al. 2011; Araújo, Henriques and Silva 2017). Candida possesses different cell wall proteins for this adherence process (Silva et al. 2011; Araújo, Henriques and Silva 2017). BCR1 is involved in cell adhesion of low and high biofilm forming isolates (Bastidas, Heitman and Cardenas 2009; Pannanusorn et al. 2014; Araújo, Henriques and Silva 2017). The presence of Mn2+, though, has no effect on the initial BCR1-dependent adhesion indicating that this stage of biofilm formation is not affected by Mn2+.
One of the important factors for virulence of C. albicans is the ability to switch between the different cell morphologies: yeast, pseudohyphae and hyphae (Sudbery, Gow and Berman 2004). This morphology switch is important for colonization, tissue penetration and invasion (Gow, Brown and Odds 2002; Trevijano-Contador, Rueda and Zaragoza 2016). Pseudohyphae and hyphae are invasive, but pseudohyphae may have different role and properties compared to hyphae during infection (Sudbery, Gow and Berman 2004). Candida parapsilosis is believed to not form true hyphae, but to exist in either a yeast or a pseudohyphal form. We show in this work that no biofilm forming strains, which otherwise adhere as single cells, develop biofilms comprised of yeasts and pseudohyphae in the presence of Mn2+. Deletion of BCR1 in these no biofilm formers led to development of hyphae on the polystyrene surface, predominantly Mn2+-triggered biofilm formation. Thus, BCR1 is a repressor of hyphal development in initially no biofilm forming strains, while BCR1 contributes to extended pseudohyphal formation in high spider-like biofilm forming strains (Pannanusorn et al. 2014).
The different cell morphologies observed in surface biofilms of C. parapsilosis, yeast (blastospore and budding yeast), pseudohyphae and hyphae (germ tube and hyphae), are shown in Fig. 7. Although hyphae and pseudohyphae are both filamentous in morphology (Thompson, Carlisle and Kadosh 2011), clear morphological differences exist between hyphae and pseudohyphae. Hyphae are uniform in width with no constriction at the neck of mother cell (Fig. 7F–H; Fig. S7B–D, Supporting Information), while pseudohyphae are wider and have a clear constriction at the neck of the mother cell (Fig. 7E; Fig. S7A, Supporting Information) (Sudbery, Gow and Berman 2004). In C. albicans, the width of hyphal cells is around 2 µm, while pseudohyphal cells are around 2.8 µm (Sudbery, Gow and Berman 2004). Regulation of hyphal development in C. parapsilosis requires further investigations, but key genes involved in hyphal formation are present in the C. parapsilosis genome (Fig. 9).
Figure 9.
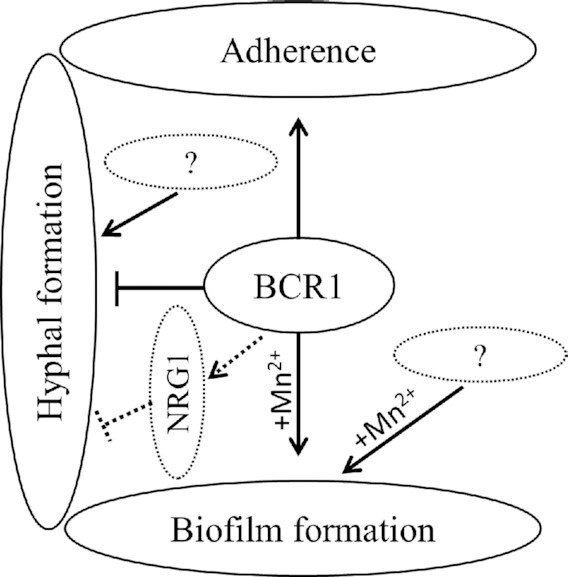
A tentative model of the role of Bcr1 in no biofilm forming isolates of C. parapsilosis.
Major transcriptional regulators of biofilm formation can direct other physiological processes, including hyphal differentiation (Cavalheiro and Teixeira 2018). Bcr1 is involved in regulation of opaque cell filamentation in C. albicans (Guan et al. 2013). The role of the transcription factor Nrg1 has not been defined in C. parapsilosis (Pais et al. 2016). Nrg1 represses the filamentous growth in C. albicans and C. tropicalis (Braun, Kadosh and Johnson 2001; Zhang et al. 2016). Bcr1, which directly represses the NRG1 promoter (Nobile et al. 2012; Guan et al. 2013), does not play a role in regulation of hyphal development. However, BCR1 activates genes encoding hyphal surface proteins during biofilm formation (Nobile and Mitchell 2005). Here, we observed a high number of pseudohyphae and hyphae (filamentous forms) in biofilms formed by the bcr1∆ mutants in Mn2+-triggered biofilm formation of no biofilm forming strains of C. parapsilosis. Possibly, deletion of BCR1 abolishes expression of NRG1, which, in combination with rewiring of the regulatory circuit, relieves repression on filamentous growth in surface-attached cells of Mn2+-triggered biofilm formation of no biofilm formers of C. parapsilosis (Fig. 9). The role of Nrg1, its regulation by Bcr1 and the Bcr1 network require further investigation in these clinical isolates of C. parapsilosis.
In summary, Bcr1 has at least three different functionalities in C. parapsilosis in no, low and high biofilm forming strains with respect to biofilm formation and plasticity in cell morphology leading to pseudohyphae and hyphae development. Bcr1 also represses protease activity and contributes to antimicrobial peptide resistance and altered colony and cell morphology (Pannanusorn et al. 2014). But what can be the molecular basis of the differential activities of Bcr1 in the different categories of biofilm forming strains of C. parapsilosis and C. albicans? In C. albicans, network circuit diversification leading Bcr1 to differentially control the transcription factor Brg1 is partially responsible for differential regulation of biofilm and cell morphology (Huang et al. 2019). Nucleotide sequence variation in promoter regions of target genes and Bcr1 as a target for extensive post-translational modification can be the cause of circuit diversification. Bcr1 is a complex 724 amino acid long invariant transcription factor in C. parapsilosis with tandem highly conserved C2H2 zinc finger repeats (188–218 and 219–247) flanked by lysine-rich sequences (Fig. S8, Supporting Information). Otherwise, Bcr1 is predicted to consist of 84% disordered sequences with compositional bias toward serine, glutamine and proline, which make up over 40% of the amino acids of the protein. Besides C. parapsilosis, Bcr1 homologues are found in C. orthopsilosis, C. viswanathii, C. tropicalis, C. dubliniensis, C. albicans and L. elongisporus. Homology outside the C2H2 zinc finger motifs is below 45% with gaps with extended amino acid stretches.
Most of Bcr1 functionality is known in C. albicans where Bcr1 expression and activity is highly regulated. For example, the hyphal regulator Tec1 activates and Tup1 represses BCR1 expression (Gutiérrez-Escribano et al. 2012). Furthermore, phosphorylation of Bcr1 of C. albicans at threonine 191 and serine 556 by the serine/threonine protein kinase Cbk1 is required for transcriptional activity (Gutiérrez-Escribano et al. 2012). A Cbk1 homologue with 77% homology is also present in C. parapsilosis (data not shown). Threonine 191 present in C2H2 zinc finger repeats is highly conserved in Bcr1 of all species (Fig. S8, Supporting Information). However, absence of serine 556 in C. orthopsilosis and C. parapsilosis can contribute to different activities of Bcr1 between C. parapsilosis and C. albicans. In summary, Bcr1 is a major stimulator of biofilm formation and a repressor of cell morphology in no biofilm forming strains dependent on environmental conditions that requires its differential role to be further investigated in C. parapsilosis.
Supplementary Material
FUNDING
This work was supported by grant from the EU (European Commission) under grant agreement no. 634588 through NOMORFILM project (Horizon 2020).
Conflict of interest . None declared.
REFERENCES
- Allison DL, Willems HM, Jayatilake JAet al.. Candida–bacteria interactions: their impact on human disease. Microbiol Spectr. 2016;4:VMBF-0030-2016, DOI: 10.1128/microbiolspec.VMBF-0030-2016. [DOI] [PubMed] [Google Scholar]
- Araújo D, Henriques M, Silva S. Portrait of Candida species biofilm regulatory network genes. Trends Microbiol. 2017;25:62–75. [DOI] [PubMed] [Google Scholar]
- Bastidas RJ, Heitman J, Cardenas ME. The protein kinase Tor1 regulates adhesin gene expression in Candida albicans. PLoS Pathog. 2009;5:e1000294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun BR, Kadosh D, Johnson AD. NRG1, a repressor of filamentous growth in C. albicans, is down-regulated during filament induction. EMBO J. 2001;20:4753–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalheiro M, Teixeira MC. Candida biofilms: threats, challenges, and promising strategies. Front Med. 2018;5:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerihew L, Lamagni TL, Brocklehurst Pet al.. Candida parapsilosis infection in very low birthweight infants. Arch Dis Child Fetal Neonatal Ed. 2007;92:F127–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly LA, Riccombeni A, Grózer Zet al.. The APSES transcription factor Efg1 is a global regulator that controls morphogenesis and biofilm formation in Candida parapsilosis. Mol Microbiol. 2013;90:36–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvetkovic A, Menon AL, Thorgersen MPet al.. Microbial metalloproteomes are largely uncharacterized. Nature. 2010;466:779–82. [DOI] [PubMed] [Google Scholar]
- Ding C, Butler G. Development of a gene knockout system in Candida parapsilosis reveals a conserved role for BCR1 in biofilm formation. Eukaryot Cell. 2007;6:1310–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding C, Vidanes GM, Maguire SLet al.. Conserved and divergent roles of Bcr1 and CFEM proteins in Candida parapsilosis and Candida albicans. PLoS One. 2011;6:e28151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel JS, Mitchell AP. Genetic control of Candida albicans biofilm development. Nat Rev Microbiol. 2011;9:109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gow NAR, Brown AJP, Odds FC. Fungal morphogenesis and host invasion. Curr Opin Microbiol. 2002;5:366–71. [DOI] [PubMed] [Google Scholar]
- Guan G, Xie J, Tao Let al.. Bcr1 plays a central role in the regulation of opaque cell filamentation in Candida albicans. Mol Microbiol. 2013;89:732–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez-Escribano P, Zeidler U, Suárez MBet al.. The NDR/LATS kinase Cbk1 controls the activity of the transcriptional regulator Bcr1 during biofilm formation in Candida albicans. PLoS Pathog. 2012;8:e1002683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison JJ, Ceri H, Yerly Jet al.. Metal ions may suppress or enhance cellular differentiation in Candida albicans and Candida tropicalis biofilms. Appl Environ Microbiol. 2007;73:4940–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison JJ, Rabiei M, Turner RJet al.. Metal resistance in Candida biofilms. FEMS Microbiol Ecol. 2006;55:479–91. [DOI] [PubMed] [Google Scholar]
- Holland LM, Schröder MS, Turner SAet al.. Comparative phenotypic analysis of the major fungal pathogens Candida parapsilosis and Candida albicans. PLoS Pathog. 2014;10:e1004365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang MY, Woolford CA, May Get al.. Circuit diversification in a biofilm regulatory network. PLoS Pathog. 2019;15:e1007787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn DM, Mikherjee PK, Clark TAet al.. Candida parapsilosis characterization in an outbreak setting. Emerg Infect Dis. 2004;10:1074–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackey E, Vipulanandan G, Childers DSet al.. Comparative evolution of morphological regulatory functions in Candida species. Eukaryot Cell. 2013;12:1356–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie J, Szabo EK, Urgast DSet al.. Host-imposed copper poisoning impacts fungal micronutrient acquisition during systemic Candida albicans infections. PLoS One. 2016;11:e0158683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medrano DJ, Brilhante RS, Cordeiro RAet al.. Candidemia in a Brazilian hospital: the importance of Candida parapsilosis. Rev Inst Med Trop Sao Paulo. 2006;48:17–20. [DOI] [PubMed] [Google Scholar]
- Ng KP, Saw TL, Na SLet al.. Systemic Candida infection in University hospital 1997–1999: the distribution of Candida biotypes and antifungal susceptibility patterns. Mycopathologia. 2001;149:141–6. [DOI] [PubMed] [Google Scholar]
- Nobile CJ, Fox EP, Nett JEet al.. A recently evolved transcriptional network controls biofilm development in Candida albicans. Cell. 2012;148:126–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobile CJ, Mitchell AP. Regulation of cell-surface genes and biofilm formation by the C. albicans transcription factor Bcr1p. Curr Biol. 2005;15:1150–5. [DOI] [PubMed] [Google Scholar]
- Odds FC. Candida and Candidosis. London: Tindall, 1988. [Google Scholar]
- O’Toole GA. Microtiter dish biofilm formation assay. J Vis Exp. 2011;47:2437, DOI: 10.3791/2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pais P, Costa C, Cavalheiro Met al.. Transcriptional control of drug resistance, virulence and immune system evasion in pathogenic fungi: a cross-species comparison. Front Cell Infect Microbiol. 2016;6:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannanusorn S, Fernandez V, Römling U. Prevalence of biofilm formation in clinical isolates of Candida species causing bloodstream infection. Mycoses. 2013;56:264–72. [DOI] [PubMed] [Google Scholar]
- Pannanusorn S, Ramírez-Zavala B, Lünsdorf Het al.. Characterization of biofilm formation and the role of BCR1 in clinical isolates of Candida parapsilosis. Eukaryot Cell. 2014;13:438–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller MA, Diekema DJ, Jones RNet al.. International surveillance of bloodstream infections due to Candida species: frequency of occurrence and in vitro susceptibilities to fluconazole, ravuconazole, and voriconazole of isolates collected from 1997 through 1999 in the SENTRY Antimicrobial Surveillance Program. J Clin Microbiol. 2001;39:3254–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller MA, Moet GJ, Messer SAet al.. Candida bloodstream infections: comparison of species distributions and antifungal resistance patterns in community-onset and nosocomial isolates in the SENTRY Antimicrobial Surveillance Program, 2008–2009. Antimicrob Agents Chemother. 2011;55:561–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuss O, Vik A, Kolter Ret al.. The SAT1 flipper, an optimized tool for gene disruption in Candida albicans. Gene. 2004;341:119–27. [DOI] [PubMed] [Google Scholar]
- Rodríguez J, Mandalunis PM. A review of metal exposure and its effects on bone health. J Toxicol. 2018;2018:4854152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roilides E, Farmaki E, Evdoridou Jet al.. Neonatal candidiasis: analysis of epidemiology, drug susceptibility, and molecular typing of causative isolates. Eur J Clin Microbiol Infect Dis. 2004;23:745–50. [DOI] [PubMed] [Google Scholar]
- Sasse C, Morschhäuser J. Gene deletion in Candida albicans wild-type strains using the SAT1-flipping strategy. Methods Mol Biol. 2012;845:3–17. [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva S, Negri M, Henriques Met al.. Adherence and biofilm formation of non-Candida albicansCandida species. Trends Microbiol. 2011;19:241–7. [DOI] [PubMed] [Google Scholar]
- Sudbery P, Gow N, Berman J. The distinct morphogenic states of Candida albicans. Trends Microbiol. 2004;12:317–24. [DOI] [PubMed] [Google Scholar]
- Thompson DS, Carlisle PL, Kadosh D. Coevolution of morphology and virulence in Candida species. Eukaryot Cell. 2011;10:1173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tóth R, Nosek J, Mora-Montes HMet al.. Candida parapsilosis: from genes to the bedside. Clin Microbiol Rev. 2019;32:e00111-18, DOI: 10.1128/CMR.00111-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevijano-Contador N, Rueda C, Zaragoza O. Fungal morphogenetic changes inside the mammalian host. Semin Cell Dev Biol. 2016;57:100–9. [DOI] [PubMed] [Google Scholar]
- Trofa D, Gácser A, Nosanchuk JD. Candida parapsilosis, an emerging fungal pathogen. Clin Microbiol Rev. 2008;21:606–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Asbeck EC, Clemons KV, Stevens DA. Candida parapsilosis: a review of its epidemiology, pathogenesis, clinical aspects, typing and antimicrobial susceptibility. Crit Rev Microbiol. 2009;35:283–309. [DOI] [PubMed] [Google Scholar]
- Weems JJ. Candida parapsilosis: epidemiology, pathogenicity, clinical manifestations, and antimicrobial susceptibility. Clin Infect Dis. 1992;14:756–66. [DOI] [PubMed] [Google Scholar]
- Wisplinghoff H, Bischoff T, Tallent SMet al.. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–17. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Tao L, Guan Get al.. Regulation of filamentation in the human fungal pathogen Candida tropicalis. Mol Microbiol. 2016;99:528–45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.