Abstract
Alcohol has both acute and chronic effects on neuroimmune signaling, including triggering pro-inflammatory cytokine release by microglia. Minocycline, a second-generation tetracycline antibiotic, inhibits microglial activation and reduces neuroinflammation in preclinical studies. In mice, minocycline also reduces ethanol intake, attenuates ethanol-induced conditioned place preference, and inhibits ethanol-induced microglial activation and pro-inflammatory cytokine release. Here, for the first time, we tested the effects of minocycline on subjective response to ethanol and acute ethanol-induced inflammation in humans. Forty-eight heavy drinkers participated in a double-blind, placebo-controlled trial in which they were randomized to receive placebo, 100mg, or 200mg of minocycline for 10 days. Each subject then underwent two experimental sessions in which they were given a fixed dose of intravenous ethanol using a “clamp” procedure (100mg%) or placebo (normal saline) on days 8 and 10 of treatment. Minocycline was well tolerated, but there was no effect of either dose of minocycline on subjective response to ethanol or ethanol-induced craving; minocycline effects on cognitive function seem to interact with age. Minocycline treatment did not alter serum cytokine levels at baseline or during ethanol-exposure, although certain baseline cytokine levels predict sedative response to ethanol. These findings indicate that a short-term treatment with minocycline may not alter ethanol-related inflammation or subjective response to ethanol in humans. Further research is needed to identify pharmacological agents with robust effects on ethanol-induced inflammation to determine whether neuroimmune modulation represents a viable treatment strategy for alcohol use disorder.
Keywords: Alcoholism, Craving, Cytokines, Ethanol, Inflammation, Minocycline
Introduction
Alcohol Use Disorder (AUD) is one of the most prevalent and costly psychiatric disorders, with an estimated economic burden of $220 billion per year (Bouchery et al. 2011). Recent evidence suggests that the prevalence of AUD, alcohol use and high risk drinking has increased since 2001 (Grant et al. 2017). Available pharmacotherapies are limited by modest efficacy, emphasizing the need for more effective treatments. It is of high clinical importance to develop more effective pharmacotherapies with novel neuropharmacological targets.
A growing body of evidence has highlighted the importance of the interplay between ethanol and neuroimmune pathways (Coleman and Crews 2018). Exposure to ethanol acutely increases the level of cytokines (small proteins or peptides used for cell signaling), chemokines (small cytokines that attract other immune cells), and other neuroimmune factors (Coleman et al. 2017; Lippai et al. 2013). Prolonged exposure to ethanol leads to a pro- inflammatory state in the CNS (Rubio-Araiz et al. 2017). Cumulating evidence from preclinical studies led to a hypothesis that activation of microglia, the intrinsic immune cells of the brain, plays a key role in heavy drinking, withdrawal, and the transition to dependence (Coller and Hutchinson 2012; Crews et al. 2011; Kelley and Dantzer 2011). More recently, evidence from neuroimaging studies has suggested that there may be lower microglial levels in individuals with AUD compared to healthy controls (Hillmer et al. 2017; Kalk et al. 2017), although medications to treat alcohol withdrawal may have confounded the results (Kalk et al. 2017). It has also been hypothesized that these results may reflect the result of chronic microglial activation due to repeated alcohol consumption which eventually “burns out” microglia (Hillmer et al. 2017).
Medications targeting inflammation may have utility as potential treatments for AUD. One such medication is minocycline, a tetracycline derivative antibiotic which also inhibits microglia activation and release of pro-inflammatory cytokines, chemokines, and nitric oxide (NO) production (Dean et al. 2012). Previous animal studies suggest that minocycline may attenuate the reinforcing effects of ethanol. In one study, minocycline treatment attenuated the acute ethanol-induced sedative effects (loss of righting reflex, sleep time test), but enhanced the motor impairment (assessed with rotarod test) in mice (Wu et al. 2011). In another study, 4 days of treatment with 50mg/kg minocycline significantly reduced ethanol intake in mice using a free choice voluntary drinking model (Agrawal et al. 2011). Further, a similar tetracycline antibiotic, doxycycline reduced ethanol intake in mice (McIver et al. 2012). In a study with healthy controls, we have observed that 200 mg/day of minocycline treatment for 4 days attenuated dextroamphetamine-induced subjective rewarding effects in humans. In addition, minocycline treatment also decreased reaction time on a Go No-Go task, and reduced plasma cortisol levels (Sofuoglu et al. 2011a). These findings suggested that minocycline may be a potential treatment for addictive disorders. However, minocycline’s potential use for the treatment of AUD has not been investigated in humans
To follow-up on these promising findings, we conducted a double-blind, randomized, placebo-controlled human laboratory study to determine whether minocycline changes the acute effects of ethanol in heavy drinking subjects. To produce stable levels of ethanol, we used an intravenous clamp procedure (O’Connor et al. 1998). The main outcomes were subjective ethanol effects as well as the effect of ethanol on cognitive and motor dexterity functions. The subjective effects of ethanol are characterized as biphasic, having both stimulatory and sedative effects-both of which contribute to the rewarding effect of ethanol and are important in understanding drinking behavior (Morean and Corbin 2010). Based on the previous preclinical studies, we hypothesized that minocycline treatment would attenuate subjective stimulation, cognition and motor impairment induced by ethanol.
Methods:
Subjects:
A total of 49 heavy drinking subjects were recruited from the New Haven area by advertisement and compensated for their participation. The study was powered (at least 80% power) to detect large effects (f=0.45) with N=17 per group assuming alpha=0.05 (total N=51) thus we largely met our recruitment target. In order to be included, subjects had to be heavy drinkers (≥14 standard alcoholic drinks per week for males and ≥7 for females) with ≥1 weekly binge drinking day in the past month (NIAAA 2004) and be between the ages of 21 and 50. Subjects also needed to be medically healthy on the basis of history, physical examination, and laboratory screening. Female subjects were required to be using acceptable birth control methods, have a negative pregnancy test at the time of screening, and not be actively breast-feeding. Of the total sample, 32/47 (data was not available for 2 participants) subjects (68%) met criteria for an alcohol use disorder but subjects were excluded if they had physiological dependence on alcohol, were actively treatment seeking in the past 6 months, had substance use disorder of other drugs of abuse (except tobacco), had positive urine toxicology on test days (except for cannabis), had current major psychiatric illnesses including mood, psychotic, or anxiety disorders, as determined by the Structured Clinical Interview for DSM-5 (SCID) (First 2015; NIAAA 2004) or had liver function tests (ALT or AST) greater than 3 times normal limits. Alcohol consumption data was collected using the Time Line Follow Back method (TLFB), a calendar based method to assess drinking over the past 90 days(Sobell et al. 1988). Subjects were also excluded if they had an allergy to minocycline or other tetracyclines or if they were unwilling to remain abstinent from alcohol for 48 hours prior to each test day. Institutional review boards of the VA Connecticut Healthcare System and Yale University School of Medicine approved this study. The trial was also registered (ClinicalTrials.gov Identifier: NCT02187211).
Procedures
This study was a randomized, double-blind, placebo-controlled, outpatient study with a between-group design. After signing informed consent, subjects underwent baseline screening, including structured interview, physical examination, and laboratory testing, including urine toxicology. After screening, subjects were randomly assigned to one of 2 doses of minocycline (100 or 200 mg/day) or placebo for 10 days; subjects remained on the same dose of the study medication for all 10 days. The doses were chosen based on the typical daily regime used in clinical trials for the treatment of infections, as well as previous studies with minocycline (Sofuoglu et al. 2011a; Sofuoglu et al. 2009; Zhang et al. 2018). Medication was taken once per day. All participants were randomized to the three conditions using urn randomization stratified by gender and race. Randomization produced 17 subjects on high-dose minocycline, 12 subjects on low-dose minocycline, and 20 subjects on placebo (Supplemental Figure 1).
Subjects had 3 outpatient visits (day 1, 3 and 5) for medication administration, dispensing of take-home doses and monitoring of any adverse effects from study medications. On each of days 8 and 10 of treatment, subjects were scheduled for a laboratory session where ethanol or placebo was administered using an intravenous “clamp” procedure (O’Connor et al. 1998). Intravenous ethanol or saline was administered in a randomized fashion under double-blind procedures. Each participant was randomized to an order of placebo (0mg%) or ethanol (100mg%) challenge on day 8 and 10, using a crossover design (i.e., each participant received both treatments).
Test Sessions
Prior to each test session, subjects were instructed not to consume alcohol or caffeine for 48 hours. They presented to the Biological Studies Unit of the VA Connecticut Healthcare System, West Haven campus, at approximately 9:00 AM. All subjects were given a standardized breakfast at the beginning of each test day and crackers during the test day, as requested. Prior to testing, subjects underwent urine drug screening for toxicology and breathalyzer screening. Provided that these tests were negative, an intravenous line was placed. Subjects received an infusion of placebo or ethanol (target BrAC=100mg%) intravenously for approximately 20 minutes, until the target BrAC was achieved. Once the BrAC was achieved it was maintained using the clamp procedure for 60 minutes. This was achieved using a Computer-Assisted Infusion Method (CAIS) in which real-time pharmacokinetic modeling automatically made calculations and corrected the infusion rate based on real-time BrAC data entry by staff as well as the pharmacokinetic profile of each subject. The procedure used to administer ethanol has been used previously by our group and others and is described in detail elsewhere.(Ray et al. 2011; Ray and Hutchison 2004; Subramanian et al. 2002). BrAC data was entered, analyzed and plotted to ensure that target ethanol levels were reached and were the same between groups (See Supplemental Figure 2) Outcome measures
Subjective measures:
Subjective intoxication ratings were obtained at baseline, 10 minutes after starting the infusion, immediately upon reaching the target ethanol level, and at minute 30, 80, 110, 140, and 200 after reaching the target ethanol level (using Biphasic Alcohol Effects Scale (BAES) (the stimulating and sedating effects associated with ethanol intoxication), Visual Analogue Scale (VAS) for ‘buzzed’, and ‘drowsy ‘(0 = not at all, 7 = extremely), and the Number of Drinks Scale (NDS) (subjects report the number of drinks they felt they had consumed). Subjects also reported on similarity to alcohol as measured by the Visual Analogue Scales of Similarity to Drugs of Abuse (VASSDA) (0=not at all, 7= extremely) measuring the perceived similarity to ethanol and other drugs. These have been used by our laboratory in alcohol administration studies (Kerfoot et al. 2013; Petrakis et al. 2010; Ralevski et al. 2017) and in other similar studies administering agents used as “probes” for alcohol (Dickerson et al. 2010; Petrakis et al. 2004).
The timing of assessments/procedures of the test days is outlined in Supplemental Figure 3.
Craving for alcohol:
Subjective craving for alcohol was measured by the Yale Craving Scale (YCS), a single item scale (Rojewski et al. 2015).
Motor coordination and dexterity:
Changes in hand-to-eye coordination were assessed using the Grooved Pegboard Test (Lafayette Instrument Company) (Ruff and Parker 1993). This is a manipulative dexterity test, consisting of a board with randomly positioned slots into which the subject inserts pegs under timed conditions and are tested for both the dominant and non-dominant hand.
Cognitive function:
Assessment for changes in cognitive function included use of the Hopkins Verbal Learning Test (HVLT) (Brandt 1991). The HVLT is word list learning test of verbal memory and has the advantage of six different versions that permit multiple episodes of testing. The procedures associated with the test allow some degree of distinction between immediate recall, delayed recall, and recognition. In addition, the Rapid Information Processing Task (RVIP) which is a widely used task to assess sustained attention, with a working memory component was also used (Wesnes et al. 1983). In this task, a series of single digits is presented on a computer screen and targets are defined as three consecutive odd digits (e.g., 7–9-3) or three consecutive even digits (e.g., 2–8-6); the proportion of correct responses to targets relative to the percentage of commission errors to non-targets will be the main outcome measure. Finally, the Go No-Go task, which has been used in similar laboratory studies (Sofuoglu et al. 2008),assessed the ability to withhold responses to an infrequently occurring target. (No-Go trials). A series of digits are presented and participants are instructed to press a spacebar to every digit except the number “3”, and to give equal importance to speed and accuracy. The primary outcome is the number of commission errors.
Serum cytokines:
Serum levels of pro-inflammatory cytokines were assayed using electrochemiluminescence multi-array technology (Meso Scale Discovery, Gaithersburg, MD) (DellaGioia et al. 2013). These include Interleukin-1 beta (IL-1β), Interleukin-2 (IL-2), Interleukin-6 (IL-6), Interleukin-10 (IL-10), Interleukin12 p70 (IL12p70), Interferon gamma (IFNg), Tumor Necrosis Factor alpha (TNFα), and Granulocyte-Macrophage Colony Stimulating Factor (GM-CSF). Serum samples were obtained in each laboratory session, before and following ethanol or saline administration.
Data Analysis:
Mixed effects models were the primary analysis method using all available data on all individuals. Categorical variables were analyzed using the chi square test. In the mixed models medication with three levels (placebo, 100 mg minocycline, 200 mg minocycline) was a between-subject factor, IV ethanol condition with two levels (ethanol vs. saline) and time as a categorical factor with nine levels (1 pre-infusion, 5 during infusion, and 3 post-infusion) were within-subject factors. The models assessed all interactions as well as main effects of the fixed factors. We were primarily focused on interactions involving medication especially between medication and IV condition with smaller differences between the ethanol and saline conditions on active drug (either dose of minocycline) vs. placebo expected to be supportive of our hypothesis. In order to eliminate the confounding effect of individual differences post-infusion, the subjective response to alcohol data (BAES and VAS) were reanalyzed by using the same model but including time with only six levels and excluding the post infusion time-points. There was a separate analysis performed using the same model including age as a covariate to account for possible age differences in relationship to inflammatory processes. Results were unchanged except for the cognitive results, so those are the only ones presented in the manuscript. Finally, to examine the relationship between cytokines and subjective effects of ethanol we performed mixed model analyses testing whether certain selected cytokine levels at baseline (before laboratory testing but after 7 days of medication) were related to stimulant or sedative effects of ethanol regardless of treatment condition but comparing ethanol administration and placebo over time.
Results:
Demographics:
The mean age of the sample was 34.4 (SD = 10.3). The majority of participants were male (71.4%), and greater than half of the sample identified as African American (59.2%). Participants began drinking at mean age of 19.1 (SD = 3.6) and began drinking heavily at a mean age of 23.8 (SD = 6.9). Subject consumed alcohol on 50% of the past 90 days, binged on 27.6% of the past 90 days, and had mean Alcohol Use Disorder Identification Test (AUDIT) scores of 10.6 (SD = 4.7). Those receiving placebo were slightly younger (30.3) than the group receiving low dose of minocycline (37.8) or high dose of minocycline (36.9). There were no significant demographic differences between the groups (Table 1). Participants received 3 doses of minocycline administered by study personnel, including a dose on the test days. The majority (37/45 or 82.2%) of the participants (data missing on 4 participants) reported complying with all (100%) of their medication regiment.
Table 1.
Demographic and Drinking Characteristics
| Variables | Placebo | Minocycline 100mg | Minocycline 200mg | Statistics | |||
|---|---|---|---|---|---|---|---|
| n=20 | n=12 | n=17 | |||||
| n | % | n | % | n | % | χ2, p | |
| Sex (Female) | 6 | 30.00 | 4 | 33.33 | 4 | 23.52 | 0.37, 0.83 |
| Caucasian | 7 | 35.00 | 4 | 33.33 | 3 | 17.64 | |
| African-American | 9 | 45.00 | 8 | 66.66 | 12 | 70.58 | 4.71, 0.32 |
| Other Race | 4 | 20.00 | 0 | 0.00 | 2 | 11.76 | |
| Single | 15 | 75.00 | 10 | 83.33 | 10 | 58.82 | |
| Divorced | 1 | 5.00 | 0 | 0.00 | 2 | 11.76 | 2.91, 0.57 |
| Partner / Co-habitating | 4 | 20.00 | 2 | 16.66 | 5 | 29.41 | |
| Mean | SD | Mean | SD | Mean | SD | F, p | |
| Age | 30.30 | 7.91 | 37.75 | 10.83 | 36.94 | 11.27 | 2.95, .06 |
| Age drinking regularly | 19.70 | 4.02 | 18.67 | 2.01 | 18.82 | 4.14 | .391, .68 |
| Age heaviest drinking | 23.65 | 6.70 | 24.25 | 8.39 | 23.59 | 6.34 | .037, .96 |
| Age difficult to stop drinking | 28.94 | 19.65 | 22.44 | 5.76 | 22.57 | 7.22 | 1.03, .37 |
| Drinking days* | 46.30 | 24.90 | 48.41 | 17.87 | 42.88 | 25.38 | .207, .81 |
| Binge drinking days* | 25.75 | 26.64 | 25.08 | 20.80 | 23.88 | 22.30 | .028, .97 |
| AUDIT Score | 11.45 | 4.31 | 8.42 | 2.19 | 11.06 | 6.12 | 1.71, .19 |
Data was collected for the 90 days prior to baseline
AUDIT: Alcohol Use Disorders Identification Test
Subjective effects:
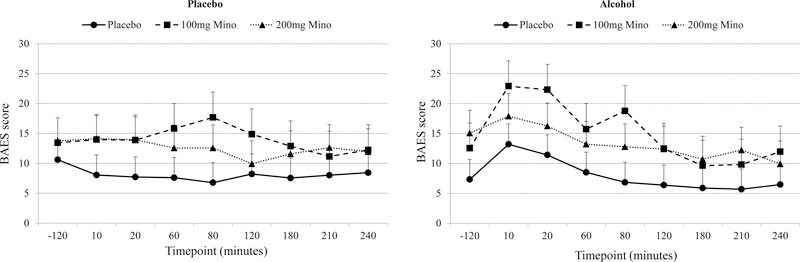
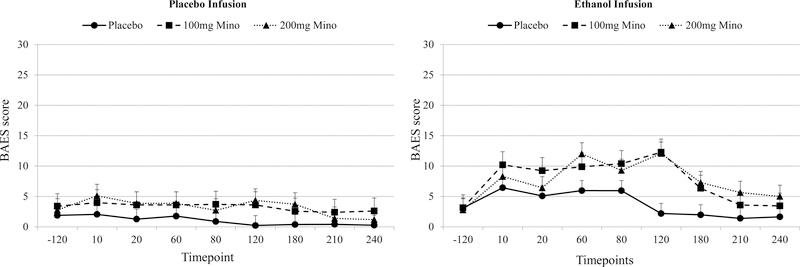
There was a significant ethanol condition by time interaction for the stimulant and sedative BAES subscales, the number of drinks scale, and one of the VAS items (“buzzed”). For all measures, there was a greater change in subjective effects over time in the ethanol condition versus the placebo condition. However, for all subjective ethanol effects, there were no significant medication by time interactions, medication by ethanol condition interactions, or medication by time by ethanol condition interactions, indicating that while there was an expected effect of ethanol on subjective effects, minocycline did not alter the subjective response to ethanol (Tables 2). Secondary analyses removing the post-infusion data revealed no change on the BAES or VAS scales.
Table 2.
Results of Analyses of Main Outcome Measures
| Main Effects | Two- Way Interactions | Three Way Interaction | ||||||
|---|---|---|---|---|---|---|---|---|
| Mino | ETOH | Time | Mino X ETOH | Mino X Time | ETOH X Time | Mino X ETOH X Time | ||
| F, p | F, p | F, p | F, p | F, p | F, p | F, p | ||
| Subje ctive Effects | BAES Stimula tion | 0.99, .38 | 0.68, .41 | 5.75, .0001 | 0.24, .78 | 1.68, .06 | 7.18, .0001 | 1.17, .30 |
| BAES Sedatio n | 2.31, .11 | 16.52, .0001 | 6.42, .0001 | 0.38, .68 | 1.63, .07 | 3.24, .003 | 0.93, .54 | |
| VAS: Drowsy | 1.08, .35 | 2.94, .10 | 2.58, .01 | 0.06, .93 | 1.21, .26 | 1.20, .30 | 1.65, .06 | |
| VAS:Buzzed | 0.64, .53 | 66.19, .0001 | 28.39, .0001 | 0.31, .73 | 1.38, .16 | 17.45, .0001 | 0.61, .87 | |
| NDS | 0.04, .95 | 101.69, .0001 | 26.77, .0001 | 0.13, .87 | 1.98, .02 | 19.37, .0001 | 0.96, .50 | |
| Alcohol Craving | YCS | 0.44, .64 | 12.87, .001 | 8.67, .0001 | 2.63, .08 | 1.80, .04 | 5.10, .0001 | 0.95, .51 |
| Cerebellar Effects | Grooved Pegboard (DH) (seconds) | 0.88, .42 | 41.70, .0001 | 16.83, .0001 | 0.82, .44 | 1.76, .11 | 10.15, .0001 | 0.45, .84 |
| Grooved Pegboard (NDH) (seconds) | 2.99, .05 | 7.75, .006 | 4.43, .005 | 2.25, .11 | 1.30, .26 | 2.45, .06 | 0.70, .65 | |
| Cognitive Effects | Go/No Go RT (ms) | 0.29, .74 | 19.72, .0001 | 6.83, .0001 | 2.55, .09 | 0.50, .80 | 2.60, .06 | 2.87, .012 |
| RVIP Proportion Correct (%) | 0.16, .85 | 8.53, .005 | 7.82, .0001 | 0.45, .64 | 0.54, .78 | 3.13, .03 | 0.51, .80 | |
| HVLT Trial 1 | 0.09, .91 | 2.29, .14 | 11.29, .0001 | 4.26, .02 | 0.51, .73 | 1.90, .16 | 2.57, .04 | |
| Correct HVLT Trial 2 Correct | 0.79, .46 | 10.49, .002 | 43.37, .0001 | 3.59, .03 | 0.95, .436 | 3.12, .05 | 1.12, .35 | |
| HVLT Trial 3 Correct | 0.61, .55 | 13.71, .0001 | 26.01, .0001 | 0.36, .70 | 2.57, .05 | 2.19, .12 | 2.42, .06 | |
BAES: Biphasic Alcohol Effects Scale; DH: Dominant Hand; HVLT: Hopkin’s Verbal Learning Task; ms: milliseconds; NDH: Non-dominant Hand; RT: Reaction Time; RVIP: Rapid Visual Information Processing Task; VAS: Visual Analog Scale; YCS: Yale Craving Scale
Craving:
There was a significant ethanol condition by time interaction and medication by time interaction on the YCS, but no significant medication by ethanol condition interaction, or medication by time by ethanol condition interaction. While the medication by time was statistically significant, its clinical interpretation is not easily understood as it seems that low dose is associated with lower craving over time while high dose is not clearly associated with difference in either direction. (See Supplemental Figure 4)
Cognitive effects:
For the cognitive effects (Go/no-go, RVIP and HVLT) there were significant main effects for ethanol and time but no significant main effects for medication and no significant two or three-way interactions. When co-varying for age, there was an age by ethanol condition by time interaction (p=0.02) on the Go-no-Go task indicating that older adults had overall slower reaction time that was more affected by the ethanol then younger adults. The results for the RVIP were similar with a significant age by ethanol condition interaction (p=0.01) suggesting that older adults were more susceptible to ethanol effects then younger adults. Age was not a factor in recognition on the HVLT test. The HVLT Delayed recall results showed a significant medication by ethanol condition by time interaction (p=0.004). Results suggest that older adults had fewer correct responses overall except those that received 200 mg whose recall was better on the placebo day. There were also medication by ethanol by time interactions for Trials1 (=0.05) and 3 (p=0.03). The results for the number of correct responses on Trial 1 were the in the same direction with a medication by ethanol condition by time interaction (p=0.05). Interestingly, on Trial 3 the significant medication by ethanol condition by time interaction (p=0.03) suggested that while younger adults had poorer performance as minocycline dose increased on both ethanol and placebo day the older adults performed better as minocycline dose increased on both placebo and ethanol dose day.
Motor coordination and dexterity:
There was a significant ethanol condition-by-time interaction on the Grooved Pegboard test using the dominant hand, consistent with ethanol’s effects on motor function, but no medication by time and no three-way interaction, suggesting minocycline did not affect ethanol-induced motor effects. There were no significant two-way or three-way interactions for test performance using the non-dominant hand. Age did not significantly influence motor coordination and dexterity function.
Cytokines:
There was no significant main effect of minocycline or ethanol and no significant two or three-way interactions for any of the cytokines tested suggesting no effect of either minocycline or ethanol on serum cytokine levels. The additional analyses examining whether certain cytokine levels predicted subjective effects of ethanol focused on cytokines (IL1b, IL2, IFNg, IL12p70) that have been previously shown to change with minocycline and to be associated with change in negative symptoms in schizophrenia (Zhang et al. 2018). Results show that baseline cytokines (IL1b, IL2, IFNg, IL12p70) produced significant baseline cytokine by alcohol condition interactions (p=0.04; p=0.01; p<0.01; p=0.03 respectively) indicating that individuals with higher cytokine levels experienced the sedative effects of ethanol more intensely than those with lower cytokine levels. Cytokine levels were not significant predictors of the stimulant effects of ethanol.
Adverse effects:
There were 2 medication-associated discontinuations from this study. They include one subject (minocycline 200mg) who was discharged from the study for dizziness and lightheadedness after taking medication for 3 days. Another subject (minocycline 100 mg) was discharged from the study for difficulty urinating following the active ethanol infusion and required urinary catheterization; this was thought to be related to the ethanol rather than minocycline.
Discussion:
This trial explored the effects of two doses of minocycline (100mg and 200mg) on human psychopharmacological responses to ethanol in heavy drinkers using a laboratory paradigm. Although ethanol administration led to expected changes in subjective response, craving, and cognition, there was no effect of pretreatment with minocycline on any of these main outcomes. Minocycline was well tolerated and was not found to enhance ethanol effects. Minocycline effects on cognitive function may interact with age in a way that warrants further exploration. Analysis of cytokine levels indicates that minocycline did not reduce baseline inflammation or alter cytokine levels during the infusion as measured by serum cytokines. This provides preliminary evidence that minocycline, at the current doses, may not be an effective intervention for heavy alcohol users. However, exploratory analyses suggest a role of level of inflammation, as measured by cytokines, in the subjective effects of ethanol, particularly the sedative effects
To our knowledge, only one previous trial has tested a neuroimmune modulating agent in heavy drinkers. In that randomized placebo-controlled crossover study, ibudilast, a drug which inhibits pro-inflammatory signaling, had no significant effect on ethanol-induced subjective response and craving in individuals with alcohol use disorder (Ray et al. 2017). Ibudilast was only found to affect subjective response in a post-hoc exploratory analyses, particularly when factoring in depressive symptomatology. It is unclear whether ibudilast altered ethanol-induced inflammatory response as this was not tested.
Three other clinical trials have examined whether minocycline alters the pharmacological effects of nicotine, dextroamphetamine, and opioids (Arout et al. 2018) (Arout C. A. et al. in review; Sofuoglu et al. 2011a; Sofuoglu et al. 2009). In these studies, minocycline at doses comparable to those in the current study, was found to modestly reduce subjective response to stimulants, but not nicotine or opioids. In the present study, results suggest that minocycline may attenuate ethanol-effects in some cognitive domains but that these results interact with age. .Although preliminary, these findings are consistent with previous studies which reported improved performance in a response inhibition task in healthy humans (Sofuoglu et al. 2011b) and opioid-dependent individuals maintained on agonist treatment as well as improved performance in social decision making tasks (Kato et al. 2012; Watabe et al. 2012). Given the poor decision making and impulsivity associated with alcohol use disorder (Galandra et al. 2018), cognitive enhancement has been suggested to be a potential treatment approach (Bates et al. 2013; Sofuoglu et al. 2013). Overall, these results are consistent in suggesting that minocycline may not have a role in treating the primary addictive process. Its effect in the cognitive effects of addictive disorders would warrant further study.
In the present study, cytokine levels were associated with increased sedative response to ethanol; there was no effect on stimulant response. Preclinical studies have suggested that cytokine levels are associated with the level of alcohol dependence, as they may be related to bouts of stress and abuse.(Crews et al. 2017) It should be noted that in this study, minocycline did not have an effect on cytokine levels. This is in contract to a recent study evaluating minocycline effect on negative symptoms of schizophrenia. (Zhang et al. 2018) In that study, using comparable doses to this study (100mg, 200mg) the high dose was associated with improvement in negative symptoms of schizophrenia and in decreases in cytokine levels (IL1b, IL2, IFNg, IL12p70). The studies differ in the duration of minocycline treatment; in the present study subject were taking minocycline for 10 days compared to 3 months.
There were several important limitations to the current study. We measured the effects of minocycline on ethanol-induced response but not on ethanol self-administration (Gowin et al. 2017), therefore it remains unknown whether minocycline alters alcohol consumption. However, medications that affect drug self-administration in human laboratory studies typically attenuate craving (McKee et al. 2012; O’Malley et al. 2002), which suggests that minocycline is unlikely to reduce consumption behaviors. We tested two doses of minocycline over ten days, so it remains possible that higher doses of minocycline or longer duration of treatment could lead to different outcomes. Other factors that may have influenced subjective effects such as nicotine consumption were not systematically collected and as such cannot be ruled out. Additionally, our measurement of inflammatory response used circulating cytokines, which may or may not accurately reflect central inflammatory response. In this study, we were unable to detect ethanol-induced changes in inflammation. Future studies should consider using positron emission tomography to measure the effect of treatment on central neuroinflammation, although finding effective tracers remains an active area of research (Kim et al. 2018). It is also possible that a personalized medicine approach would be more effective for anti-inflammatory agents. We did not measure baseline cytokine levels, but individuals with elevated baseline inflammatory markers (e.g. cytokines, erythrocyte sedimentation rate, c-reactive protein) may selectively benefit from immune modulating agents, and this hypothesis should be considered in subsequent clinical trials.
The current findings suggest that minocycline does not alter ethanol-induced subjective response or inflammation. Determining which pharmacological agents effectively reduce inflammation in heavy drinkers is of critical importance to elucidate whether targeting neuroimmune responses can alter alcohol craving and consumption. Future trials should seek to identify and test novel neuroimmune modulating agents and determine which biomarkers provide adequate measures of central inflammation.
Supplementary Material
Fig1.
BAES Stimulant Effects for Medication Conditions (Placebo Saline, 100mg of Minocycline and 200mg of Minocycline) During Placebo and Ethanol Infusion
BAES: Biphasic Alcohol Effects Scale
Mino: Minocycline
BAES Stimulant scores range from 0–70
Fig2.
BAES Sedative Effects for Medication Conditions (Placebo Saline, 100mg of Minocycline and 200mg of Minocycline) During Placebo and Ethanol Infusion
BAES: Biphasic Alcohol Effects Scale
Mino: Minocycline
BAES Sedative scores range from 0–70
Acknowledgments:
Support was provided by NIAAA R21 AA023150 (PI Petrakis) and the VISN I Mental Illness Research Education and Clinical Center (MIRECC). We wished to thank the contributions of the Biostudies staff (Jane Weiner RN; Elizabeth O’Donnell RN; Angelina Genovese RNC, BSN, MBA; Margaret Dion-Marovitz MS, RN; Karen E. Prema RN, BSN)
Footnotes
Dr Ismene L. Petrakis has served as a consultant for Alkermes over the past 3 years. Drs Elizabeth Ralevski, Ralitza Gueorguieva, Matthew E. Sloan, Lesley Devine, PhD, Gihyun Yoon, Albert J. Arias, and Mehmet Sofuoglu have no conflict of interest.
References
- Agrawal RG, Hewetson A, George CM, Syapin PJ, Bergeson SE (2011) Minocycline reduces ethanol drinking. Brain Behav Immun 25 Suppl 1: S165–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arout CA, Waters AJ, MacLean RR, Compton P, Sofuoglu M (in review) Minocycline does not affect experimental pain or addiction-related outcomes in opioid maintained patients [DOI] [PMC free article] [PubMed]
- Arout CA, Waters AJ, MacLean RR, Compton P, Sofuoglu M (2018) Minocycline does not affect experimental pain or addiction-related outcomes in opioid maintained patients. Psychopharmacology: 1–10. [DOI] [PMC free article] [PubMed]
- Bates ME, Buckman JF, Nguyen TT (2013) A role for cognitive rehabilitation in increasing the effectiveness of treatment for alcohol use disorders. Neuropsychology review 23: 27–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, Brewer RD (2011) Economic costs of excessive alcohol consumption in the U.S., 2006. Am J Prev Med 41: 516–24. [DOI] [PubMed] [Google Scholar]
- Brandt J (1991) The Hopkins Verbal Learning Test: development of a new memory test with six equivalent forms. The Clinical Neuropsychologist 5: 125–142. [Google Scholar]
- Coleman LG Jr., Crews FT (2018) Innate Immune Signaling and Alcohol Use Disorders. Handb Exp Pharmacol [DOI] [PMC free article] [PubMed]
- Coleman LG Jr., Zou J, Crews FT (2017) Microglial-derived miRNA let-7 and HMGB1 contribute to ethanol-induced neurotoxicity via TLR7. J Neuroinflammation 14: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller JK, Hutchinson MR (2012) Implications of central immune signaling caused by drugs of abuse: mechanisms, mediators and new therapeutic approaches for prediction and treatment of drug dependence. Pharmacol Ther 134: 219–45. [DOI] [PubMed] [Google Scholar]
- Crews FT, Lawrimore CJ, Walter TJ, Coleman LG Jr. (2017) The role of neuroimmune signaling in alcoholism. Neuropharmacology 122: 56–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Zou J, Qin L (2011) Induction of innate immune genes in brain create the neurobiology of addiction. Brain Behav Immun 25 Suppl 1: S4–S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean OM, Data-Franco J, Giorlando F, Berk M (2012) Minocycline: therapeutic potential in psychiatry. CNS Drugs 26: 391–401. [DOI] [PubMed] [Google Scholar]
- DellaGioia N, Devine L, Pittman B, Hannestad J (2013) Bupropion pre-treatment of endotoxin-induced depressive symptoms. Brain Behav Immun 31: 197–204. [DOI] [PubMed] [Google Scholar]
- Dickerson D, Pittman B, Ralevski E, Perrino A, Limoncelli D, Edgecombe J, Acampora G, Krystal JH, Petrakis I (2010) Ethanol-like effects of thiopental and ketamine in healthy humans. J Psychopharmacol 24: 203–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Williams JBW, Karg RS, & Spitzer RL (2015) StructuredClinical Interview for DSM‐5: Clinician Version AmericanPsychiatric Association, Arlington, VA [Google Scholar]
- Galandra C, Basso G, Cappa S, Canessa N (2018) The alcoholic brain: neural bases of impaired reward-based decision-making in alcohol use disorders. Neurological Sciences 39: 423–435. [DOI] [PubMed] [Google Scholar]
- Gowin JL, Sloan ME, Stangl BL, Vatsalya V, Ramchandani VA (2017) Vulnerability for Alcohol Use Disorder and Rate of Alcohol Consumption. Am J Psychiatry: appiajp201716101180. [DOI] [PMC free article] [PubMed]
- Grant BF, Chou SP, Saha TD, Pickering RP, Kerridge BT, Ruan WJ, Huang B, Jung J, Zhang H, Fan A, Hasin DS (2017) Prevalence of 12-Month Alcohol Use, High-Risk Drinking, and DSM-IV Alcohol Use Disorder in the United States, 2001–2002 to 2012–2013: Results From the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA psychiatry [DOI] [PMC free article] [PubMed]
- Hillmer AT, Sandiego CM, Hannestad J, Angarita GA, Kumar A, McGovern EM, Huang Y, O’Connor KC, Carson RE, O’Malley SS, Cosgrove KP (2017) In vivo imaging of translocator protein, a marker of activated microglia, in alcohol dependence. Mol Psychiatry 22: 1759–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalk NJ, Guo Q, Owen D, Cherian R, Erritzoe D, Gilmour A, Ribeiro AS, McGonigle J, Waldman A, Matthews P, Cavanagh J, McInnes I, Dar K, Gunn R, Rabiner EA, Lingford-Hughes AR (2017) Decreased hippocampal translocator protein (18 kDa) expression in alcohol dependence: a [(11)C]PBR28 PET study. Translational psychiatry 7: e996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato TA, Watabe M, Tsuboi S, Ishikawa K, Hashiya K, Monji A, Utsumi H, Kanba S (2012) Minocycline modulates human social decision-making: possible impact of microglia on personality-oriented social behaviors. PloS one 7: e40461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley KW, Dantzer R (2011) Alcoholism and inflammation: neuroimmunology of behavioral and mood disorders. Brain Behav Immun 25 Suppl 1: S13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerfoot K, Pittman B, Ralevski E, Limoncelli D, Koretski J, Newcomb J, Arias AJ, Petrakis IL (2013) Effects of family history of alcohol dependence on the subjective response to alcohol using the intravenous alcohol clamp. Alcohol Clin Exp Res 37: 2011–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SW, Wiers CE, Tyler R, Shokri-Kojori E, Jang YJ, Zehra A, Freeman C, Ramirez V, Lindgren E, Miller G, Cabrera EA, Stodden T, Guo M, Demiral SB, Diazgranados N, Park L, Liow JS, Pike V, Morse C, Vendruscolo LF, Innis RB, Koob GF, Tomasi D, Wang GJ, Volkow ND (2018) Influence of alcoholism and cholesterol on TSPO binding in brain: PET [(11)C]PBR28 studies in humans and rodents. Neuropsychopharmacology 43: 1832–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippai D, Bala S, Petrasek J, Csak T, Levin I, Kurt-Jones EA, Szabo G (2013) Alcohol-induced IL-1beta in the brain is mediated by NLRP3/ASC inflammasome activation that amplifies neuroinflammation. J Leukoc Biol 94: 171–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIver SR, Muccigrosso MM, Haydon PG (2012) The effect of doxycycline on alcohol consumption and sensitivity: consideration for inducible transgenic mouse models. Exp Biol Med (Maywood) 237: 1129–33. [DOI] [PubMed] [Google Scholar]
- McKee SA, Weinberger AH, Shi J, Tetrault J, Coppola S (2012) Developing and validating a human laboratory model to screen medications for smoking cessation. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco 14: 1362–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morean ME, Corbin WR (2010) Subjective response to alcohol: a critical review of the literature. Alcohol Clin Exp Res 34: 385–95. [DOI] [PubMed] [Google Scholar]
- NIAAA NACoAAaA (2004) NIAAA Council Approves Definition of Binge Drinking NIAAA Newsletter, Bethesda, MD [Google Scholar]
- O’Connor S, Morzorati S, Christian J, Li TK (1998) Clamping breath alcohol concentration reduces experimental variance: application to the study of acute tolerance to alcohol and alcohol elimination rate. Alcoholism: Clinical & Experimental Research 22: 202–10. [PubMed] [Google Scholar]
- O’Malley S, Krishnan-Sarin S, Farren C, Sinha R, Kreek M (2002) Naltrexone decreases craving and alcohol self administration in alcohol dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology 160: 19–29. [DOI] [PubMed] [Google Scholar]
- Petrakis I, Pittman B, Limoncelli D, Koretski J, Newcomb J, Ralevski E, Trevisan L, Krystal J (2010) Subjective Response to Iv Ethanol Using the Clamp in Young Healthy Adults Both with and without Family History of Alcoholism. Alcoholism 34: 137a–137a. [Google Scholar]
- Petrakis IL, Limoncelli D, Gueorguieva R, Jatlow P, Boutros NN, Trevisan L, Gelernter J, Krystal JH (2004) Altered NMDA glutamate receptor antagonist response in individuals with a family vulnerability to alcoholism. Am J Psychiatry 161: 1776–82. [DOI] [PubMed] [Google Scholar]
- Ralevski E, Horvath TL, Shanabrough M, Hayden R, Newcomb J, Petrakis I (2017) Ghrelin is Supressed by Intravenous Alcohol and is Related to Stimulant and Sedative Effects of Alcohol. Alcohol Alcohol 52: 431–438. [DOI] [PubMed] [Google Scholar]
- Ray JM, Pyne JM, Gevirtz RN (2017) Alcohol Use Disorder Moderates the Effect of Age on Heart Rate Variability in Veterans With Posttraumatic Stress Disorder. J Nerv Ment Dis 205: 793–800. [DOI] [PubMed] [Google Scholar]
- Ray LA, Chin PF, Heydari A, Miotto K (2011) A human laboratory study of the effects of quetiapine on subjective intoxication and alcohol craving. Psychopharmacology (Berl) 217: 341–51. [DOI] [PubMed] [Google Scholar]
- Ray LA, Hutchison KE (2004) A polymorphism of the mu-opioid receptor gene (OPRM1) and sensitivity to the effects of alcohol in humans. Alcohol Clin Exp Res 28: 1789–95. [DOI] [PubMed] [Google Scholar]
- Rojewski AM, Morean ME, Toll BA, McKee SA, Krishnan-Sarin S, Green BG, Bartoshuk LM, O’Malley SS (2015) The Yale Craving Scale: Development and psychometric properties. Drug Alcohol Depend 154: 158–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio-Araiz A, Porcu F, Perez-Hernandez M, Garcia-Gutierrez MS, Aracil-Fernandez MA, Gutierrez-Lopez MD, Guerri C, Manzanares J, O’Shea E, Colado MI (2017) Disruption of blood-brain barrier integrity in postmortem alcoholic brain: preclinical evidence of TLR4 involvement from a binge-like drinking model. Addict Biol 22: 1103–1116. [DOI] [PubMed] [Google Scholar]
- Ruff RM, Parker SB (1993) Gender- and age-specific changes in motor speed and eye-hand coordination in adults: normative values for the Finger Tapping and Grooved Pegboard Tests. Percept Mot Skills 76: 1219–30. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, Leo GI, Cancilla A (1988) Reliability of a timeline method: assessing normal drinkers’ reports of recent drinking and a comparative evaluation across several populations. British Journal of Addiction 83: 393–402. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, DeVito EE, Waters AJ, Carroll KM (2013) Cognitive enhancement as a treatment for drug addictions. Neuropharmacology 64: 452–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Mooney M, Kosten T, Waters A, Hashimoto K (2011a) Minocycline attenuates subjective rewarding effects of dextroamphetamine in humans. Psychopharmacology (Berl) 213: 61–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Mooney M, Kosten T, Waters A, Hashimoto K (2011b) Minocycline attenuates subjective rewarding effects of dextroamphetamine in humans. Psychopharmacology 213: 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Waters AJ, Mooney M, Kosten T (2008) Riluzole and D-amphetamine interactions in humans. Prog Neuropsychopharmacol Biol Psychiatry 32: 16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Waters AJ, Mooney M, O’Malley SS (2009) Minocycline reduced craving for cigarettes but did not affect smoking or intravenous nicotine responses in humans. Pharmacol Biochem Behav 92: 135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian M, Heil S, Kruger M, Collins K, Buck P, Zawacki T, Abbey A, Sokol R, Diamond M (2002) A three-stage alcohol clamp procedure in human subjects. Alcoholism: Clinical and Experimental Research 26: 1479–83. [DOI] [PubMed] [Google Scholar]
- Watabe M, Kato TA, Monji A, Horikawa H, Kanba S (2012) Does minocycline, an antibiotic with inhibitory effects on microglial activation, sharpen a sense of trust in social interaction? Psychopharmacology 220: 551–557. [DOI] [PubMed] [Google Scholar]
- Wesnes K, Warburton DM, Matz B (1983) Effects of nicotine on stimulus sensitivity and response bias in a visual vigilance task. Neuropsychobiology 9: 41–4. [DOI] [PubMed] [Google Scholar]
- Wu Y, Lousberg EL, Moldenhauer LM, Hayball JD, Robertson SA, Coller JK, Watkins LR, Somogyi AA, Hutchinson MR (2011) Attenuation of microglial and IL-1 signaling protects mice from acute alcohol-induced sedation and/or motor impairment. Brain Behav Immun 25 Suppl 1: S155–64. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zheng H, Wu R, Zhu F, Kosten TR, Zhang XY, Zhao J (2018) Minocycline adjunctive treatment to risperidone for negative symptoms in schizophrenia: Association with pro-inflammatory cytokine levels. Prog Neuropsychopharmacol Biol Psychiatry 85: 69–76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.