Abstract
Objectives:
To determine the functional network organization of the brain in infants born very preterm at term equivalent age and to relate network alterations to known clinical risk factors for poor neurologic outcomes in prematurity.
Study design:
Resting state functional MRI data from 66 VPT infants (gestational age < 32wks and birth weight <1500g) and 66 healthy full term neonates, acquired as part of a prospective, cross-sectional study, were compared at term age using graph theory. Features of resting state networks, including integration, segregation and modularity, were derived from correlated hemodynamic activity arising from 93 cortical and subcortical regions of interest and compared between groups.
Results:
Despite preserved small world topology and modular organization, resting state networks of VPT infants at TEA were less segregated and less integrated than those of full term infants. Chronic respiratory illness (ie, bronchopulmonary dysplasia and the length of oxygen support) was associated with decreased global efficiency and increased path lengths (p-value < 0.05). In both cohorts, four functional modules with similar composition were observed (parietal/temporal, frontal, subcortical/limbic and occipital). The density of connections in three out of the four modules was decreased in the VPT network (P value < .01), however in the occipital/visual cortex module, connectivity was increased in VPT relative to controls (p-value < 0.0001).
Conclusions:
Early exposure to the ex utero environment is associated with altered resting state network functional organization in VPT infants at TEA likely reflecting disrupted brain maturational processes.
Resting state functional MRI (rs-fMRI) is an imaging technique that reveals, without any explicit external stimulation, consistent and reproducible patterns of interacting brain regions called resting state networks that correspond to known functionally relevant brain systems (i.e., visual, sensorimotor, and auditory, among others) [1,2]. In preterm infants, rs-fMRI has allowed researchers to begin to characterize the effects of early exposure to the extrauterine environment on the neurodevelopment of preterm infants [3–6]. Resting state fMRI estimates neuronal activity by measuring spontaneous, low-frequency fluctuations in blood oxygen level dependent signals (BOLD). Because rs-fMRI is non-invasive, rapidly acquired, requires no input from the participant, and investigates multiple brain systems simultaneously [2], it has emerged as a promising tool for evaluating pediatric brain function. In preterm infants, rs-fMRI has shown intact resting state network patterns but with reduced complexity and altered connectivity strengths in some neural networks [4,6–8]. Connectivity changes in preterm brains have also been shown to correlate with long-term neurologic outcomes [9,10].
Studies of preterm resting state networks using a mathematical approach known as graph theory [11–13] showed that the organization of preterm brains for specialized and distributed processing of information differed from healthy, full term controls. Building on these network studies, we compared the functional organization of resting state neural networks in a sample of unsedated preterm infants without or with mild brain injury and healthy full term newborns. We investigated the effects of clinical risk factors on network connectivity and hypothesized that the effect of prematurity would be associated with measurable alterations in strength and organization of the resting state networks.
Methods
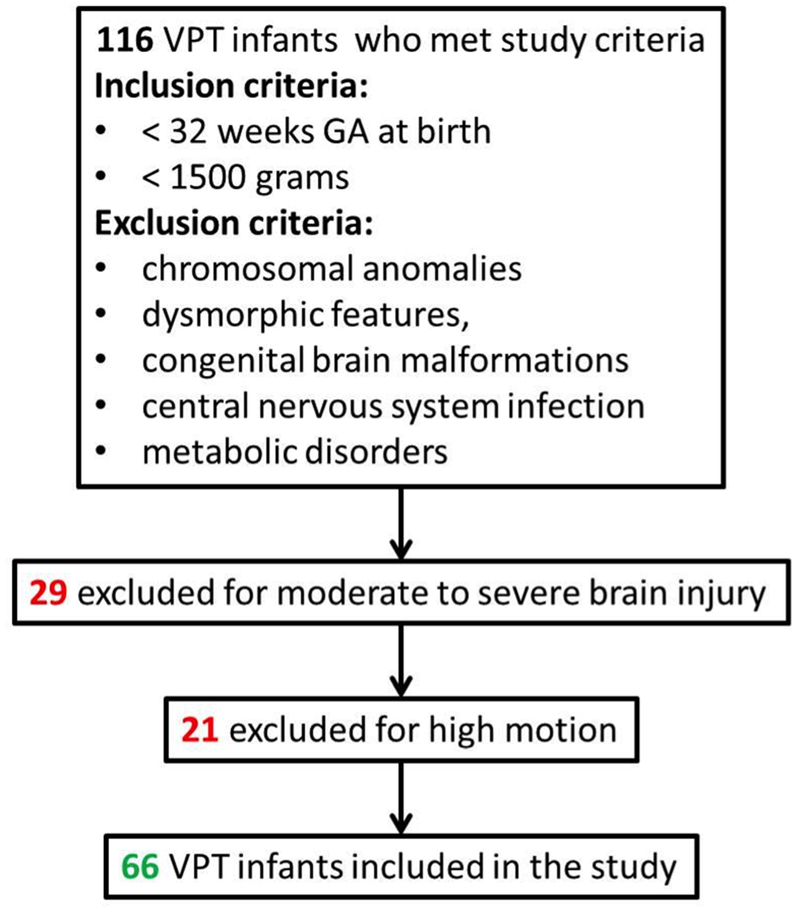
VPT infants and healthy full term controls were enrolled in two prospective observational studies [14]. For the VPT cohort, inclusion criteria were: gestational age (GA) at birth <32 weeks and birth weight < 1500 grams. Exclusion criteria included: chromosomal anomalies, dysmorphic features, congenital brain malformations, central nervous system infection, and metabolic disorders. Only VPT infants without brain injury or with mild brain injury (e.g., low grade intraventricular hemorrhage, or mild white matter injury; Figure 1 [available at www.jpeds.com]) based on Kidokoro’s brain MRI scoring system were included [15]. All healthy full-term control infants were born without delivery complications and had normal brain MRI scans. Exclusion criteria for the control group were: maternal drug use and disease (e.g., gestational diabetes, psychiatric disorders), congenital infections, chromosomal abnormalities, and dysmorphic features.
Figure 1 Online. Preterm inclusion and exclusion criteria.
Demographic and clinical information was collected from medical record reviews. In the preterm cohort, several risk factors were chosen a priori based on previous literature for their potential adverse effect on brain development, these included: the presence of mild brain injury (yes/no), moderate to severe bronchopulmonary dysplasia (BPD), the length of supplemental oxygen requirement, postnatal steroid treatment, necrotizing enterocolitis diagnosis, need for cardiac vasopressor support, and need for patent ductus arteriosus surgical ligation [16–25].
Image acquisition and processing
All infants were scanned without sedation using the same 3T GE scanner. Acquisition parameters are detailed in Appendix 1 (available at www.jpeds.com). Resting state data were preprocessed using a previously validated pipeline that employed a series of temporal and spatial denoising strategies to minimize the influence of noise from head motion, scanner drift, and subject physiology (i.e., respiration), among others, on the measured BOLD signal [26]. BOLD signals were measured from 93 regions of interest (ROIs): 90 cortical and subcortical ROIs defined using an automated anatomical labelling atlas [27] mapped to a neonatal brain [28] and 3 infratentorial ROIs derived from DrawEM segmentation [29]. Correlation (Pearson r) between BOLD signals from all possible ROI pairs were then computed and used for network analyses. Appendix 1 includes additional information.
Network analyses
We compared network functional organization between the 2 groups using the following graph metrics: 1) clustering coefficient (Cc), 2) local efficiency (lE), 3) characteristic path length (L), 4) global efficiency (GE), 5) small world architecture, and 6) modular organization [30]. Clustering coefficient and local efficiency are segregation parameters. Segregation reflects the extent to which densely connected regions of the brain (modules or clusters) perform specialized functions [31]. Segregated networks have high Cc and high lE. Network integration refers to the ability of resting state networks to share information globally and is captured by the metrics characteristic path length and global efficiency. Integrated networks have short L and high GE. Small-world topology, quantified with the small world index (σ) that relates Cc and L, refers to the balance between network specialization and integration observed in complex networks like the brain [11,32–36]; if σ > 1, meaning Cc is high and L is low, then the network is considered small-world. Modularity is related to segregation and reflects the tendency of networks to subdivide into functionally meaningful clusters. A module refers to a group of nodes that are highly connected to each other but are sparsely connected to others outside of their group [37]. This relationship is reflected by the modularity index (Q; [38]). For a mathematical description of these measures, please refer to the work of Rubinov and Sporns [30].
In the preterm cohort, we studied the relationship between clinical risk factors and global properties of the resting state networks. We computed the averaged global network metrics (Cc, L, gE, lE) over the range of correlation thresholds and assessed the relationship between risk factors associated with prematurity and global resting state networks properties using ANCOVA.
Statistical Analyses
Statistical analyses were performed using Matlab 2017a and SAS 9.3. Between the preterm cohort and healthy full-term group, we compared segregation and integration metrics from individual network using permutation testing (100,000 iterations) on the residuals adjusted for postmenstrual age at MRI, sex, and motion. Modularity was evaluated on group-averaged connectomes at a density of 15% (sparsest density with the least probability of spurious connections). We then identified modular communities using 10,000 iterations of the Louvain algorithm [40]. Between the preterm cohort and healthy full-term group, we compared the modularity index and the intra-modular functional connectivity using two-sided two samples t-test, and the intra-modular density using Chi-square test. Finally, the relationship between risk factors associated with prematurity and global resting state networks properties was assessed using ANCOVA analysis controlling for sex, GA at birth, day of life at MRI, and motion.
Results
We studied 132 infants: 66 VPT infants at TEA and 66 healthy full-term control infants. Of the 66 VPT infants, 21 had mild brain injury: seven with low grade intraventricular hemorrhage (grade I-II), 13 with mild white matter injury, and one with small cerebellar punctate hemorrhage. Postmenstrual age at MRI was higher in the full-term group; this was used as a covariate in the rest of the analysis. Table 1 summarizes the demographic and clinical characteristics of the cohorts.
Table 1.
Clinical characteristics of preterm infants and full-term control infants.
| Preterm Infants, n=66 | Healthy Full-term Infants, n=66 | P-value | |
|---|---|---|---|
| Perinatal Characteristics | |||
| Birth gestational age, wk, mean ± SD [range] | 27.36 ± 2.68 [22.4 - 32] | 39.48 ± 0.99 [37.3-41.3] | <0.0001 |
| Birthweight, g, mean ± SD [range] | 931 ± 305 [480-1500] | 3373 ± 356 [2590-4011] | <0.0001 |
| Small for gestational age1, n (%) | 9 (14) | 6 (9) | 0.41 |
| Male, n (%) | 29 (44) | 36 (55) | 0.76 |
| Native American; Hispanic; White Asian; Black; Multiethnic, n (%) | 0; 15 (23); 9 (14) 0; 39 (59); 3 (4) |
2 (3); 6 (9); 16 (24) 5 (8); 24 (51); 3 (5) |
0.021 |
| Vaginal delivery, n (%) | 27 (40) | 45 (68) | 0.0017 |
| Apgar score at 5 min, median [range] | 8 [1-9] | 9 [8-10] | <0.001 |
| Maternal age, y, mean ± SD | 27.91 ± 5.9 | 29.2 ± 7.18 | 0.26 |
| MRI Characteristics | |||
| Postmenstrual age at MRI, wk, mean ± SD [range] | 40.21 ± 1.56 [37.4-44.4] | 41.1 ± 1.1 [38.4-44.3] | 0.0002 |
| Day of life at MRI, d, mean ± SD [range] | 89.97 ± 21.4 [41-137] | 11.21 ± 4.88 [4-20] | <0.0001 |
| Head circumference at MRI, cm, mean ± SD [range] | 33.26 ± 2 [27-37] | 35.71 ± 1.09 [32-39] | <0.0001 |
| Weight at MRI, g, mean ± SD [range] | 2818 ± 681 [1870-5400] | 3596 ± 422 [2475-4470] | <0.0001 |
SGA if BW less than the 10% percentile for sex and GA, based on the Fenton growth chart
Network segregation, integration, and small-world architecture
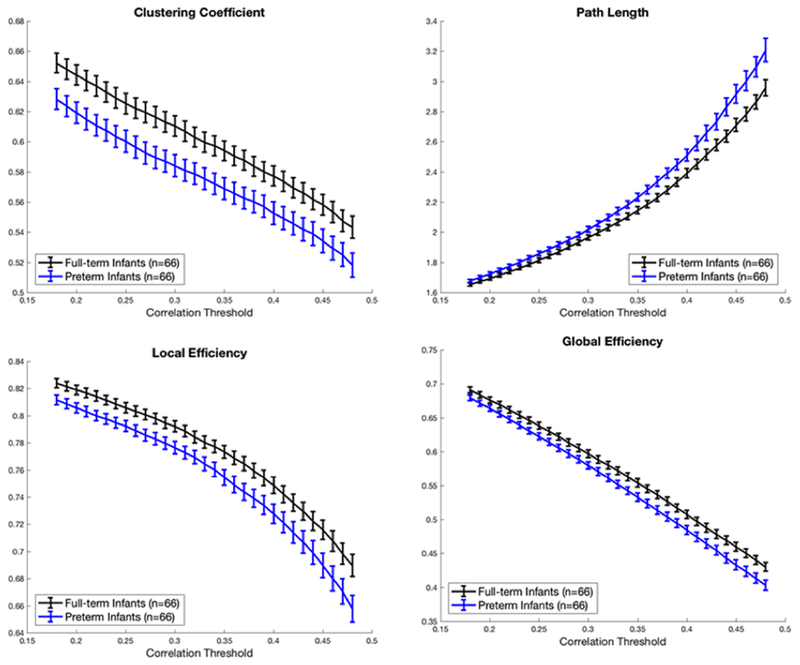
Resting state networks of VPT and full-term infants exhibited small-world topology (σ > 1). This balance between network integration and segregation suggested that even in the VPT infants, efficient global processing of information was preserved. However, segregation and integration measures per se were reduced in VPT infants compared with healthy newborns (Figure 2). Segregation parameters clustering coefficient (Cc) and local efficiency (lE) and integration metrics path length (L) and global efficiency (gE) were all significantly decreased in VPT infants.
Figure 2. Clustering coefficient, path length, global and local efficiency in VPT (blue) and full term infants (black).
Metrics were significantly different between groups at all tested thresholds using permutation testing (p-value < 0.05); tests controlled for sex, postmenstrual age at MRI, and motion.
Modular organization
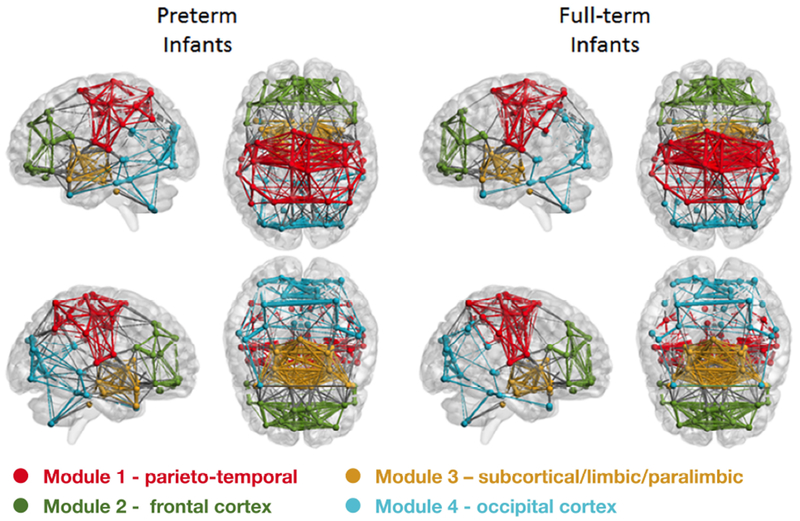
Preterm and full-term infant resting state networks showed modular organization (Figure 3). The modularity index, however, was significantly lower in the VPT infants compared with the full-term infants suggesting reduced density of connections within modules in VPT. Although modules in VPT resting state networks tend to be less connected, their composition was highly similar to full-term newborns (Figure 3). In both cohorts, resting state networks were divided into four modules (Table 2 and Figure 3), namely: (1) Module 1 - primary somatosensory, motor, and auditory cortices and association areas within the parietal/temporal cortices; (2) Module 2 - regions in the frontal cortex; (3) Module 3 - subcortical, limbic, and paralimbic brain regions; and (4) Module 4 - primary and association visual cortices/occipital cortex.
Figure 3. Modular brain organization in preterm and full-term infants.
Spheres are nodes, lines are edges; color represent module membership. Note reduced number of connection between nodes in modules 1-3 (1 – red, 2 – green, and 3 – yellow) and increased density in module 4 (blue) in VPT compared with full-term infants.
Table 2.
Modular brain organization differences between preterm and full-term infants
| Preterm Infants, n=66 | Full-term Control Infants, n=66 | P-value | ||
|---|---|---|---|---|
| Modularity (Q) | 0.49 ± 0.005 | 0.51 ± 0.002 | <.001 | |
| Module 1 -Parietal/Temporal | Node | 26 | 24 | |
| Density | 42.2% | 52.5% | 0.01 | |
| Connectivity strength | 0.49 ± 0.2 | 0.54 ± 0.22 | 0.0086 | |
| Module 2 -Frontal | Node | 22 | 22 | |
| Density | 36.4% | 49.4% | 0.0048 | |
| Connectivity strength | 0.55 ± 0.25 | 0.55 ± 0.25 | 0.68 | |
| Module 3 -Subcortical/Limbic | Node | 22 | 21 | |
| Density | 69.7% | 80.5% | 0.0092 | |
| Connectivity strength | 0.51 ± 0.21 | 0.56 ± 0.2 | 0.0023 | |
| Module 4 - Occipital | Node | 23 | 26 | |
| Density | 41.5% | 23.7% | <0.0001 | |
| Connectivity strength | 0.51 ± 0.25 | 0.535 ± 0.22 | 0.239 | |
| Between Module Connections | Density | 4.8% | 4.2 % | 0.44 |
| Connectivity strength | 0.39 ± 0.11 | 0.42 ± 0.13 | 0.0256 |
When we compared the number of connections within each module at a density threshold of 15 % (i.e., same total number of connections in VPT and full-term group-averaged functional connectome), density and connectivity strength within each module were perturbed in VPT infants (Table 2). VPT infants had significant lower density in all modules except Module 4 (i.e., occipital areas) where connection density was higher. Connectivity strength was significantly reduced in VPT infants in the parieto-temporal and subcortical/limbic modules (Table 2).
Clinical risk factors
In the preterm cohort, we showed that both moderate to severe BPD and prolonged need for supplemental oxygen were associated with increased path length (β>0) and decreased global efficiency (β<0) (Table 3), suggesting reduced integrative abilities in VPT infants. In addition, longer period of oxygen support was also associated with decreased local efficiency (β<0) suggesting decreased specialization in VPT resting state networks (Table 3).
Table 3.
Relationship between clinical risk factors and global network properties in the preterm cohort.
| Risk Factors Studied | Preterm infants (n=66) | Global network metric | Model p-value | Association with global network metrics |
|---|---|---|---|---|
| Mild brain injury | 21 (32%) | cc | 0.002 | β=0.001; p=0.94 |
| L | 0.002 | β=0.01; p=0.85 | ||
| gE | 0.011 | β=−0.001; p=0.86 | ||
| lE | 0.0008 | β=0.002; p=0.85 | ||
| Moderate to severe bronchopulmonary dysplasia* | 24 (37%) | cc | 0.0005 | β=−0.02 ;p=0.15 |
| L | <0.0001 | β=+0.17; p=0.01* | ||
| gE | <0.0001 | β=−0.03; p= 0.02* | ||
| lE | 0.0001 | β=−0.02; p=0.05 | ||
| Length of oxygen support (days) | 67 ± 41 [0-172] | cc | 0.0006 | β=−0.0004 ; p=0.36 |
| L | 0.0001 | L: β=+0.0027; p=0.03* | ||
| gE | 0.0001 | gE: β=−0.004; p=0.01* | ||
| lE | <0.0001 | lE: β =−0.0004; p=0.026* | ||
| Postnatal steroid treatment | 21 (32%) | cc | 0.0013 | β=−0.001; p=0.92 |
| L | 0.0012 | β=−0.038; p=0.58 | ||
| gE | 0.0006 | β=2.2×10”6; p=0.99 | ||
| lE | 0.0006 | β=0.005; p=0.69 | ||
| Necrotizing enterocolitis | 19 (29%) | cc | 0.0019 | β=−0.004; p=0.78 |
| L | 0.0017 | β=−0.05; p=0.42 | ||
| gE | 0.0011 | β=0.002; p=0.84 | ||
| lE | 0.0007 | β=0.006; p=0.63 | ||
| Cardiac vasopressor treatment | 19 (30%) | cc | 0.0006 | β=0.021; p=0.18 |
| L | 0.0014 | β=−0.02; p=0.76 | ||
| gE | 0.0006 | β=−9.9 ×10−6; p=0.99 | ||
| lE | 0.0003 | β=0.016; p=0.18 | ||
| Surgical ligation of patent ductus arteriosus | 12 (18%) | cc | 0.002 | β=0.0018; p=0.92 |
| L | 0.0023 | β=3.6χ10−4 ; p=0.99 | ||
| gE | 0.0011 | β=−0.002 ; p=0.86 | ||
| lE | 0.0008 | β=7χ10−4 ; p=0.96 |
Severity of BPD in VPT infants: treatment with oxygen for at least 28 days and need for more than 21% oxygen support (moderate: less than 30% oxygen; severe: more than 30% oxygen) at discharge or at 36 weeks postmenstrual age (PMA) whichever comes first [30]
Discussion
Our study revealed aberrant functional network organization in a large sample of very preterm infants scanned at term equivalent age. Compared with healthy term newborn infants, VPT infants with no or mild brain injury had reduced resting state network segregation and integration measures and altered modular connectivity. Among the risk factors analyzed, only the clinical indicators of severity of respiratory illness (i.e., moderate to severe BPD and prolonged supplemental oxygen support) were associated with network alterations in VPT infants. These findings underscore the potential detrimental link between respiratory illness severity and functional brain development in VPT infants.
We showed small world organization in VPT and full-term infants consistent with previous studies using rs-fMRI [3,11–13,39]. This organization, reflective of efficient, specialized and integrated neuronal communication [41], likely emerges before the 31st week of gestation and continues to mature as gestation progresses [13]. Although small world architecture was preserved, resting state networks of VPT infants were less segregated (i.e., lower clustering coefficients and reduced local efficiency) and integrated (i.e., longer path lengths and reduced global efficiency). Reduced clustering was previously reported by Scheinost et al in a smaller cohort (n = 12 preterm infants) [12]; they, however, did not observe differences in path lengths between premature and term infants, possibly due to the smaller sample size. Our results suggest that preterm birth somehow reconfigures connections among brain regions. In preterm infants, longitudinal studies have reported age-dependent increases in functional connectivity strength [4,6,7], network complexity and magnitude [7], integration [11,13], and modularity [11,13] during the third trimester suggesting a vulnerability window. Thus, it is not surprising that in some networks at TEA, preterm infants exhibit reductions in connectivity strength [7], decreased interhemispheric connectivity [5,6], altered lateralization of language areas [42], altered thalamocortical connectivity [8,43], and impaired basal ganglia – frontal cortex connectivity [44]; and it provides additional support to the likely effects of preterm birth on the organization of emerging functional networks. Our understanding of how functional connectivity in VPT infants evolves over time remains incomplete, but available follow-up studies already suggest that functional connectivity remains compromised in adults born very preterm [45–49].
Interestingly, only the clinical risk factors linked to respiratory disturbances showed a negative association with resting state network organization: moderate to severe BPD and length of oxygen support were linked to reduced global and/or local efficiency of the resting state networks. Respiratory issues such as BPD and prolonged need for oxygen support have been associated with impaired brain development and adverse cognitive functioning [19,20,50,51]. Hypoxic episodes resulting from respiratory disturbances are thought to underlie the pathophysiological mechanisms responsible for these cerebral alterations [52–56]. Hypoxic-ischemic events are a major cause of prematurity related brain injury [57–59], and disturbances in cerebral oxygenation have also been associated with subtler delays in structural brain maturation in high-risk neonatal population [60,61]. Taken together, these findings emphasize the crucial role of adequate oxygen supply in the early establishment of the neural circuitry.
Mild brain injury was not related to alterations in global properties of the resting state networks in our study. Because white matter injury has been associated with altered functional connectivity in preterm infants [5,43,62], we hypothesize that the degree of brain injury severity plays a crucial role thus the potential functional connectivity disturbances following mild brain injury still remains to be elucidated.
Small-world topology of networks relate closely to its modular organization [32,63]. Modular systems with functionally specialized subsets of brain regions that are sparsely connected to other modules, such as what we observed in resting state networks of VPTs at TEA and full-term newborns, tend to be small world (i.e., high segregation balanced with high integration; [64]). Modular organization of the developing brain has been described in third trimester fetuses [65], preterm infants [11,13], and healthy neonates [11,39]. We also reported modular organization of resting state networks in VPT infants at TEA, but with reduced modularity compared with full-term infants. Four functional modules with similar composition to full-term infants were identified. Consistent with previous work, modules in infants were mostly anatomically constrained [3,39,66]; adjacent areas/regions belonging to the same lobe tend to belong to the same module. In contrast, adult modules are composed of neighboring (i.e. subcortical module), as well as spatially distant, but functionally associated regions such as the default-mode and attention modules (fronto-parietal) [37,64,67,68]. Cognitive networks are immature at birth [69] and the default-mode network only starts to exhibit adult-like properties during the first years of life [69–71], thus it is not surprising that the modular organization at term age does not include cognitive module yet. Due to the postnatal development of cognitive processes and establishment of long-range connections during early childhood, regional organization of the resting state networks evolve from a local organization during childhood to a distributed organization in adulthood [63,72,73].
Preterm networks, while still modular, were less segregated (i.e. lower modularity index) compared with full term resting state networks. Previous work has shown that networks become more segregated with increasing age in the first two years of life [74]. This reduced specialization in VPT infants may suggest less mature brain networks compared with their full term counterparts. Examining the density of connections within each module, however, suggests a more nuanced picture where, depending on the neural network, both delayed and accelerated maturation seem to occur. In preterm infants, connection density was reduced in three out of the four modules – parieto-temporal, frontal, and subcortical/limbic. Interestingly, VPT infants had an increased number of connections in the occipital/visual module. This finding converges with the results of Padilla et al that demonstrated increased volumes of areas involved in visual processing in extremely preterm infants at TEA compared with healthy controls.[75] This increased connection density in the visual cortex is likely due to experience-dependent processes. Early extrauterine exposure is likely associated with increased visual stimulation during a critical period of development for the visual system [76–78]. Synaptic density of the visual cortex dramatically increases from mid-gestation to the first months of postnatal life [76,77,79,80]. Increased visual inputs during this critical period might have lasting consequences on visual processing [78,81,82]. Additional studies are needed to elucidate the relationship between early visual experiences, functional organization of the visual cortex, and impairments in cortical visual processing. It would be intriguing to explore if other sensory areas of the brain (i.e., auditory cortex) that are prematurely exposed to ex utero stimulation would also present with increased connection density. The modular analysis performed was not suited to elucidate the possible link between prematurity and the functional development of other sensory areas sensitive to exogenous stimulation during the third trimester.
Our study limitations deserve mention. First, our understanding of the biological mechanisms underlying BOLD responses remains incomplete [83], even more so in newborn infants than in adults [84]. As such, interpreting and comparing findings must be done carefully. Having said that, there is a high degree of consistency between our findings and other published reports in newborn infants. In addition, observed emergence of resting state networks coincide with the timing of established developmental processes (i.e. development of somatosensory and motor before higher cognitive/association networks). Second, recent work has shown that the disruption of resting state networks in preterm infants may begin in utero [85] with an additive negative effect of fetal growth restriction [86]. Thus, prenatal, in addition to postnatal risk factors associated with preterm birth may also impact the early development of the resting state networks. Next, similar to other studies [34], only positive correlations were included in our network analyses because most existing graph methods were optimized for networks with positive correlations [87–89]. To evaluate negatively correlated brain activity, newer approaches accounting for anti-correlated activity need to be used [30,89,90]. Last, newborn imaging poses numerous technical challenges one of which is motion correction. To minimize the influence of motion, we removed (‘scrubbed’) high motion volumes, and used motion parameters and their derivatives as regressors in our statistical analyses. Correcting geometric distortion in EPI images is another technical issue. We did not perform distortion correction in our study, however, rigorous visual evaluation of our EPI images show minimal shape distortion. We have now included field map acquisition in our newborn MRI sequences so we can systematically evaluate distortion effects on connectivity measures.
To summarize, we report reduced segregation and integration in resting state networks of VPT infants at TEA and their association with prematurity related respiratory illness severity. Notably, these network alterations were present despite intact small-world topology and modularity. Longitudinal studies covering the prenatal (i.e., healthy fetal controls) and early school age periods would be necessary to provide additional insights into the role of premature birth on the development of functional brain networks, including possibly identifying the onset of these alterations, and to understand its long-term impact on neurobehavior.
Supplementary Material
Acknowledgments
Funded by NHLBI R01 HL116585-01. The authors declare no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Portions of this study were presented as a poster at the Pediatric Academic Societies annual meeting, May 6-9, 2017, San Francisco, California.
References
- [1].Damoiseaux JS, Rombouts SARB, Barkhof F, Scheltens P, Stam CJ, Smith SM, et al. Consistent resting-state networks across healthy subjects. Proceedings of the National Academy of Sciences 2006;103:13848–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 1995;34:537–41. [DOI] [PubMed] [Google Scholar]
- [3].Fransson P, Aden U, Blennow M, Lagercrantz H. The functional architecture of the infant brain as revealed by resting-state fMRI. Cereb Cortex 2011;21:145–54. [DOI] [PubMed] [Google Scholar]
- [4].Doria V, Beckmann CF, Arichi T, Merchant N, Groppo M, Turkheimer FE, et al. Emergence of resting state networks in the preterm human brain. Proc Natl Acad Sci U S A 2010;107:20015–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Smyser CD, Snyder AZ, Shimony JS, Blazey TM, Inder TE, Neil JJ. Effects of white matter injury on resting state fMRI measures in prematurely born infants. PLoS One 2013;8:e68098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Smyser CD, Inder TE, Shimony JS, Hill JE, Degnan AJ, Snyder AZ, et al. Longitudinal analysis of neural network development in preterm infants. Cereb Cortex 2010;20:2852–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Smyser CD, Snyder AZ, Shimony JS, Mitra A, Inder TE, Neil JJ. Resting-State Network Complexity and Magnitude Are Reduced in Prematurely Born Infants. Cereb Cortex 2014. doi: 10.1093/cercor/bhu251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Toulmin H, Beckmann CF, O’Muircheartaigh J, Ball G, Nongena P, Makropoulos A, et al. Specialization and integration of functional thalamocortical connectivity in the human infant. Proc Natl Acad Sci U S A 2015;112:6485–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Schafer RJ, Lacadie C, Vohr B, Kesler SR, Katz KH, Schneider KC, et al. Alterations in functional connectivity for language in prematurely born adolescents. Brain 2009;132:661–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gozzo Y, Vohr B, Lacadie C, Hampson M, Katz KH, Maller-Kesselman J, et al. Alterations in neural connectivity in preterm children at school age. Neuroimage 2009;48:458–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].van den Heuvel MP, Kersbergen KJ, de Reus MA, Keunen K, Kahn RS, Groenendaal F, et al. The Neonatal Connectome During Preterm Brain Development. Cereb Cortex 2014. doi: 10.1093/cercor/bhu095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Scheinost D, Kwon SH, Shen X, Lacadie C, Schneider KC, Dai F, et al. Preterm birth alters neonatal, functional rich club organization. Brain Struct Funct 2016;221:3211–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cao M, He Y, Dai Z, Liao X, Jeon T, Ouyang M, et al. Early Development of Functional Network Segregation Revealed by Connectomic Analysis of the Preterm Human Brain. Cereb Cortex 2016. doi: 10.1093/cercor/bhw038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bouyssi-Kobar M, Murnick J, Brossard-Racine M, Chang T, Mahdi E, Jacobs M, et al. Altered Cerebral Perfusion in Infants Born Preterm Compared with Infants Born Full Term. J Pediatr 2018;193:54–61.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kidokoro H, Neil JJ, Inder TE. New MR imaging assessment tool to define brain abnormalities in very preterm infants at term. AJNR Am J Neuroradiol 2013;34:2208–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hack M, Wilson-Costello D, Friedman H, Taylor GH, Schluchter M, Fanaroff AA. Neurodevelopment and predictors of outcomes of children with birth weights of less than 1000 g: 1992-1995. Arch Pediatr Adolesc Med 2000;154:725–31. [DOI] [PubMed] [Google Scholar]
- [17].Barrington KJ. The adverse neuro-developmental effects of postnatal steroids in the preterm infant: a systematic review of RCTs. BMC Pediatr 2001;1:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Vermeulen GM, Bruinse HW, de Vries LS. Perinatal risk factors for adverse neurodevelopmental outcome after spontaneous preterm birth. Eur J Obstet Gynecol Reprod Biol 2001;99:207–12. [DOI] [PubMed] [Google Scholar]
- [19].Short EJ, Klein NK, Lewis BA, Fulton S, Eisengart S, Kercsmar C, et al. Cognitive and academic consequences of bronchopulmonary dysplasia and very low birth weight: 8-year-old outcomes. Pediatrics 2003;112:e359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Thompson DK, Warfield SK, Carlin JB, Pavlovic M, Wang HX, Bear M, et al. Perinatal risk factors altering regional brain structure in the preterm infant. Brain 2007;130:667–77. [DOI] [PubMed] [Google Scholar]
- [21].Bennet L, Van Den Heuij L, Dean JM, Drury P, Wassink G, Gunn AJ. Neural plasticity and the Kennard principle: Does it work for the preterm brain? Clin Exp Pharmacol Physiol 2013;40:774–84. [DOI] [PubMed] [Google Scholar]
- [22].Batton B, Li L, Newman NS, Das A, Watterberg KL, Yoder BA, et al. Early blood pressure, antihypotensive therapy and outcomes at 18-22 months’ corrected age in extremely preterm infants. Arch Dis Child Fetal Neonatal Ed 2016;101:F201–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Cassiano RGM, Gaspardo CM, Linhares MBM. Prematurity, neonatal health status, and later child behavioral/emotional problems: a systematic review. Infant Ment Health J 2016;37:274–88. [DOI] [PubMed] [Google Scholar]
- [24].Kersbergen KJ, Makropoulos A, Aljabar P, Groenendaal F, de Vries LS, Counsell SJ, et al. Longitudinal Regional Brain Development and Clinical Risk Factors in Extremely Preterm Infants. J Pediatr 2016;178:93–100.e6. [DOI] [PubMed] [Google Scholar]
- [25].Weisz DE, Mirea L, Rosenberg E, Jang M, Ly L, Church PT, et al. Association of Patent Ductus Arteriosus Ligation With Death or Neurodevelopmental Impairment Among Extremely Preterm Infants. JAMA Pediatr 2017;171:443–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].De Asis-Cruz J, Donofrio MT, Vezina G, Limperopoulos C. Aberrant brain functional connectivity in newborns with congenital heart disease before cardiac surgery. Neuroimage Clin 2018;17:31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002;15:273–89. [DOI] [PubMed] [Google Scholar]
- [28].Shi F, Yap P-T, Wu G, Jia H, Gilmore JH, Lin W, et al. Infant brain atlases from neonates to 1- and 2-year-olds. PLoS One 2011;6:e18746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Makropoulos A, Gousias IS, Ledig C, Aljabar P, Serag A, Hajnal JV, et al. Automatic whole brain MRI segmentation of the developing neonatal brain. IEEE Trans Med Imaging 2014;33:1818–31. [DOI] [PubMed] [Google Scholar]
- [30].Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage 2010;52:1059–69. [DOI] [PubMed] [Google Scholar]
- [31].Sporns O. Structure and function of complex brain networks. Dialogues Clin Neurosci 2013;15:247–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 2009;10:186–98. [DOI] [PubMed] [Google Scholar]
- [33].Watts DJ, Strogatz SH. Collective dynamics of small world networks. Nature 1998;393:440–2. [DOI] [PubMed] [Google Scholar]
- [34].van den Heuvel MP, Stam CJ, Boersma M, Hulshoff Pol HE. Small-world and scale-free organization of voxel-based resting-state functional connectivity in the human brain. Neuroimage 2008;43:528–39. [DOI] [PubMed] [Google Scholar]
- [35].Mathias N, Gopal V. Small worlds: how and why. Phys Rev E Stat Nonlin Soft Matter Phys 2001;63:021117. [DOI] [PubMed] [Google Scholar]
- [36].Humphries MD, Gurney K, Prescott TJ. The brainstem reticular formation is a small-world, not scale-free, network. Proc Biol Sci 2006;273:503–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sporns O, Betzel RF. Modular Brain Networks. Annu Rev Psychol 2016;67:613–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Newman MEJ. Modularity and community structure in networks. Proc Natl Acad Sci U S A 2006;103:8577–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].De Asis-Cruz J, Bouyssi-Kobar M, Evangelou I, Vezina G, Limperopoulos C. Functional properties of resting state networks in healthy full-term newborns. Sci Rep 2015;5:17755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Blondel VD, Guillaume J-L, Lambiotte R, Lefebvre E. Fast unfolding of communities in large networks. J Stat Mech 2008;2008:P10008. [Google Scholar]
- [41].Bassett DS, Bullmore ET. Human brain networks in health and disease. Curr Opin Neurol 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kwon SH, Scheinost D, Lacadie C, Sze G, Schneider KC, Dai F, et al. Adaptive mechanisms of developing brain: cerebral lateralization in the prematurely-born. Neuroimage 2015;108:144–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Cai Y, Wu X, Su Z, Shi Y, Gao J-H. Functional thalamocortical connectivity development and alterations in preterm infants during the neonatal period. Neuroscience 2017;356:22–34. [DOI] [PubMed] [Google Scholar]
- [44].Ball G, Aljabar P, Arichi T, Tusor N, Cox D, Merchant N, et al. Machine learning to characterise neonatal functional connectivity in the preterm brain. Neuroimage 2015. doi: 10.1016/j.neuroimage.2015.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Papini C, White TP, Montagna A, Brittain PJ, Froudist-Walsh S, Kroll J, et al. Altered resting-state functional connectivity in emotion-processing brain regions in adults who were born very preterm. Psychol Med 2016;46:3025–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].White TP, Symington I, Castellanos NP, Brittain PJ, Froudist Walsh S, Nam K-W, et al. Dysconnectivity of neurocognitive networks at rest in very-preterm born adults. Neuroimage Clin 2014;4:352–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Bäuml JG, Daamen M, Meng C, Neitzel J, Scheef L, Jaekel J, et al. Correspondence Between Aberrant Intrinsic Network Connectivity and Gray-Matter Volume in the Ventral Brain of Preterm Born Adults. Cereb Cortex 2015;25:4135–45. [DOI] [PubMed] [Google Scholar]
- [48].Bäuml JG, Meng C, Daamen M, Baumann N, Busch B, Bartmann P, et al. The association of children’s mathematic abilities with both adults’ cognitive abilities and intrinsic fronto-parietal networks is altered in preterm-born individuals. Brain Struct Funct 2017;222:799–812. [DOI] [PubMed] [Google Scholar]
- [49].Lawrence EJ, McGuire PK, Allin M, Walshe M, Giampietro V, Murray RM, et al. The very preterm brain in young adulthood: the neural correlates of verbal paired associate learning. J Pediatr 2010;156:889–95. [DOI] [PubMed] [Google Scholar]
- [50].Boardman JP, Counsell SJ, Rueckert D, Hajnal JV, Bhatia KK, Srinivasan L, et al. Early growth in brain volume is preserved in the majority of preterm infants. Ann Neurol 2007;62:185–92. [DOI] [PubMed] [Google Scholar]
- [51].Anjari M, Counsell SJ, Srinivasan L, Allsop JM, Hajnal JV, Rutherford MA, et al. The association of lung disease with cerebral white matter abnormalities in preterm infants. Pediatrics 2009;124:268–76. [DOI] [PubMed] [Google Scholar]
- [52].Garg M, Kurzner SI, Bautista DB, Keens TG. Clinically unsuspected hypoxia during sleep and feeding in infants with bronchopulmonary dysplasia. Pediatrics 1988;81:635–42. [PubMed] [Google Scholar]
- [53].Chahboune H, Ment LR, Stewart WB, Rothman DL, Vaccarino FM, Hyder F, et al. Hypoxic injury during neonatal development in murine brain: correlation between in vivo DTI findings and behavioral assessment. Cereb Cortex 2009;19:2891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Martin RJ, Di Fiore JM, Walsh MC. Hypoxic Episodes in Bronchopulmonary Dysplasia. Clin Perinatol 2015;42:825–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Martin RJ, Wang K, Koroglu O, Di Fiore J, Kc P. Intermittent hypoxic episodes in preterm infants: do they matter? Neonatology 2011;100:303–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Dassios T, Curley A, Krokidis M, Morley C, Ross-Russell R. Correlation of radiographic thoracic area and oxygenation impairment in bronchopulmonary dysplasia. Respir Physiol Neurobiol 2016;220:40–5. [DOI] [PubMed] [Google Scholar]
- [57].Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol 2009;8:110–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Back SA. White matter injury in the preterm infant: pathology and mechanisms. Acta Neuropathol 2017;134:331–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Galinsky R, Lear CA, Dean JM, Wassink G, Dhillon SK, Fraser M, et al. Complex interactions between hypoxia-ischemia and inflammation in preterm brain injury. Developmental Medicine & Child Neurology 2018;60:126–33. [DOI] [PubMed] [Google Scholar]
- [60].Neubauer V, Junker D, Griesmaier E, Schocke M, Kiechl-Kohlendorfer U. Bronchopulmonary dysplasia is associated with delayed structural brain maturation in preterm infants. Neonatology 2015;107:179–84. [DOI] [PubMed] [Google Scholar]
- [61].Kelly CJ, Makropoulos A, Cordero-Grande L, Hutter J, Price A, Hughes E, et al. Impaired development of the cerebral cortex in infants with congenital heart disease is correlated to reduced cerebral oxygen delivery. Sci Rep 2017;7:15088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].He L, Parikh NA. Brain functional network connectivity development in very preterm infants: The first six months. Early Hum Dev 2016;98:29–35. [DOI] [PubMed] [Google Scholar]
- [63].Meunier D, Achard S, Morcom A, Bullmore E. Age-related changes in modular organization of human brain functional networks. Neuroimage 2009;44:715–23. [DOI] [PubMed] [Google Scholar]
- [64].Meunier D, Lambiotte R, Bullmore ET. Modular and hierarchically modular organization of brain networks. Front Neurosci 2010;4:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Thomason ME, Brown JA, Dassanayake MT, Shastri R, Marusak HA, Hernandez-Andrade E, et al. Intrinsic functional brain architecture derived from graph theoretical analysis in the human fetus. PLoS One 2014;9:e94423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Gao W, Zhou H, Giovanello K, Smith JK, Shen D, Gilmore J, et al. Brain Functional Networks in the Developing Brain Using Resting BOLD In: Papageorgiou TD, Christopoulos GI, Smirnakis SM, editors. Advanced Brain Neuroimaging Topics in Health and Disease - Methods and Applications, InTech; 2014. [Google Scholar]
- [67].He Y, Wang J, Wang L, Chen ZJ, Yan C, Yang H, et al. Uncovering intrinsic modular organization of spontaneous brain activity in humans. PLoS One 2009;4:e5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Bullmore E, Sporns O. The economy of brain network organization. Nat Rev Neurosci 2012;13:336–49. [DOI] [PubMed] [Google Scholar]
- [69].Gao W, Lin W, Grewen K, Gilmore JH. Functional Connectivity of the Infant Human Brain: Plastic and Modifiable. Neuroscientist 2016. doi: 10.1177/1073858416635986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Gao W, Zhu H, Giovanello KS, Smith JK, Shen D, Gilmore JH, et al. Evidence on the emergence of the brain’s default network from 2-week-old to 2-year-old healthy pediatric subjects. Proc Natl Acad Sci U S A 2009;106:6790–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Alcauter S, Lin W, Smith JK, Goldman BD, Reznick JS, Gilmore JH, et al. Frequency of spontaneous BOLD signal shifts during infancy and correlates with cognitive performance. Dev Cogn Neurosci 2014;12C:40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Meunier D, Lambiotte R, Fornito A, Ersche KD, Bullmore ET. Hierarchical modularity in human brain functional networks. Front Neuroinform 2009;3:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Supekar K, Menon V, Rubin D, Musen M, Greicius MD. Network analysis of intrinsic functional brain connectivity in Alzheimer’s disease. PLoS Comput Biol 2008;4:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Gao W, Gilmore JH, Giovanello KS, Smith JK, Shen D, Zhu H, et al. Temporal and spatial evolution of brain network topology during the first two years of life. PLoS One 2011;6:e25278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Padilla N, Alexandrou G, Blennow M, Lagercrantz H, Aden U. Brain Growth Gains and Losses in Extremely Preterm Infants at Term. Cereb Cortex 2015;25:1897–905. [DOI] [PubMed] [Google Scholar]
- [76].Rice D, Barone S Jr. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect 2000;108 Suppl 3:511–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Madan A, Jan JE, Good WV. Visual development in preterm infants. Dev Med Child Neurol 2005;47:276–80. [DOI] [PubMed] [Google Scholar]
- [78].Lickliter R The Integrated Development of Sensory Organization. Clin Perinatol 2011;38:591–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Bourgeois JP, Jastreboff PJ, Rakic P. Synaptogenesis in visual cortex of normal and preterm monkeys: evidence for intrinsic regulation of synaptic overproduction. Proc Natl Acad Sci U S A 1989;86:4297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Hevner RF. Development of connections in the human visual system during fetal mid-gestation: a Dil-tracing study. J Neuropathol Exp Neurol 2000;59:385–92. [DOI] [PubMed] [Google Scholar]
- [81].Graven SN. Early visual development: implications for the neonatal intensive care unit and care. Clin Perinatol 2011;38:671–83. [DOI] [PubMed] [Google Scholar]
- [82].Jandó G, Mikó-Baráth E, Markó K, Hollódy K, Török B, Kovacs I. Early-onset binocularity in preterm infants reveals experience-dependent visual development in humans. Proc Natl Acad Sci U S A 2012;109:11049–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Logothetis NK. The underpinnings of the BOLD functional magnetic resonance imaging signal. J Neurosci 2003;23:3963–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Colonnese M, Khazipov R. Spontaneous activity in developing sensory circuits: Implications for resting state fMRI. Neuroimage 2012;62:2212–21. [DOI] [PubMed] [Google Scholar]
- [85].Thomason ME, Scheinost D, Manning JH, Grove LE, Hect J, Marshall N, et al. Weak functional connectivity in the human fetal brain prior to preterm birth. Sci Rep 2017;7:39286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Padilla N, Fransson P, Donaire A, Figueras F, Arranz A, Sanz-Cortes M, et al. Intrinsic Functional Connectivity in Preterm Infants with Fetal Growth Restriction Evaluated at 12 Months Corrected Age. Cereb Cortex 2017;27:4750–8. [DOI] [PubMed] [Google Scholar]
- [87].Butts CT. Network inference, error, and informant (in)accuracy: a Bayesian approach. Soc Networks 2003;25:103–40. [Google Scholar]
- [88].Fortunato S, Barthelemy M. Resolution limit in community detection. Proc Natl Acad Sci U S A 2007;104:36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Zhan L, Jenkins LM, Wolfson OE, GadElkarim JJ, Nocito K, Thompson PM, et al. The significance of negative correlations in brain connectivity. J Comp Neurol 2017;525:3251–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Schwarz AJ, McGonigle J. Negative edges and soft thresholding in complex network analysis of resting state functional connectivity data. Neuroimage 2011;55:1132–46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


