Abstract
High-altitude natives have evolved to overcome environmental hypoxia and provide a compelling system to understand physiological function during reductions in oxygen availability. The sympathoadrenal system plays a key role in responses to acute hypoxia, but prolonged activation of this system in chronic hypoxia may be maladaptive. Here, we examined how chronic hypoxia exposure alters adrenal catecholamine secretion and how adrenal function is altered further in high-altitude natives. Populations of deer mice (Peromyscus maniculatus) native to low and high altitudes were each born and raised in captivity at sea level, and adults from each population were exposed to normoxia or hypobaric hypoxia for 5 mo. Using carbon fiber amperometry on adrenal slices, catecholamine secretion evoked by low doses of nicotine (10 µM) or acute hypoxia (Po2 ∼15–20 mmHg) was reduced in lowlanders exposed to hypobaric hypoxia, which was attributable mainly to a decrease in quantal charge rather than event frequency. However, secretion evoked by high doses of nicotine (50 µM) was unaffected. Hypobaric hypoxia also reduced plasma epinephrine and protein expression of 3,4-dihydroxyphenylalanine (DOPA) decarboxylase in the adrenal medulla of lowlanders. In contrast, highlanders were unresponsive to hypobaric hypoxia, exhibiting typically low adrenal catecholamine secretion, plasma epinephrine, and DOPA decarboxylase. Highlanders also had consistently lower catecholamine secretion evoked by high nicotine, smaller adrenal medullae with fewer chromaffin cells, and a larger adrenal cortex compared with lowlanders across both acclimation environments. Our results suggest that plastic responses to chronic hypoxia along with evolved changes in adrenal function attenuate catecholamine release in deer mice at high altitude.
Keywords: catecholamines, chromaffin cells, evolutionary physiology, oxygen chemosensitivity
INTRODUCTION
Reductions in environmental oxygen availability (hypoxia) pose a serious threat to homeostasis in most species. The coordinated efforts of the sympathetic nervous system (SNS) and the adrenal medulla, the two limbs of the sympathoadrenal system, provide an essential line of defense during acute exposure to low-oxygen conditions. As a key effector in the hypoxic chemoreflex, the system works to increase cardiac output and modify blood flow distribution to safeguard the delivery of oxygen to vital tissues, particularly the heart and brain, at the expense of other less sensitive tissues (20). The preganglionic splanchnic nerve of the SNS supplies cholinergic innervation to the adrenal medulla and stimulates the adrenal medullary chromaffin cells (AMCs) to release catecholamines [e.g., noepinephrine (NE) and epinephrine (E)] into the circulation in response to stressors such as hypoxia (6, 35). This system is highly regulated and designed to ready the organism for coping with acute stress. Indeed, interference of catecholamine (CAT) release from AMCs reduces survival during acute exposure to extreme hypoxia (41). Although the benefits of sympathoadrenal activation during short-term exposures to hypoxia are well established, chronic activation of this system is maladaptive and can lead to long-term cardiovascular complications such as increased arterial stiffness, systemic hypertension, and compromised exercise performance (12).
The regulation of CAT biosynthesis and secretion is tightly controlled by sympathetic nerve activity and relatively plastic in response to different stressors (reviewed in Ref. 43). Early investigation of sympathoadrenal responses to hypoxia showed that activation is highly dependent upon both the severity and duration of exposure (24). However, unlike the responses to acute hypoxia, chronic exposure to either moderate or severe hypoxia over days or weeks is accompanied by high levels of both SNS activity and catecholamine (CAT) release from the adrenal medulla into the blood (5, 24). Relatively little is known about the mechanisms underlying changes in sympathoadrenal activity during chronic exposures in vivo, but studies based on exposure of isolated AMCs to short-term (≤48 h) chronic hypoxia in vitro suggest that stabilization of hypoxia-inducible transcription factors enhances low-threshold CAT secretion via increased T-type calcium channel expression (7), and enhances neurotrophin (BDNF)-induced CAT secretion via increased neurotrophin receptor (TrkB) expression (38). Elevated levels of tyrosine hydroxylase and dopamine β-hydroxylase, two enzymes involved in CAT production, have also been noted after chronic long-term hypoxia (19).
Honed by generations of natural selection, many high-altitude natives have evolved exquisite mechanisms for coping with chronic hypoxia and the rigors of everyday life at high altitude. High-altitude natives can thus provide insight into mechanisms that are truly adaptive and contribute to fitness at high altitude. Deer mice (Peromyscus maniculatus) are a valuable model organism for studying high-altitude adaptation, as they occupy the greatest altitudinal range of all North American mammals, from sea level to ∼4,300 m (33). They are exposed to extreme hypoxia at the summit of their altitudinal range, where O2 partial pressures are just above half of those at sea level. Highland populations are genetically distinct from lowland populations, based on comparisons of α- and β-globins as well as neutral autosomal loci (32, 45). They have also evolved several key physiological specializations that improve hypoxia resistance and aerobic performance (31, 44). Therefore, contrasting the phenotypes of high- and low-altitude populations is a powerful approach for elucidating adaptive strategies for coping with hypoxia stress. In this study, we examine possible adaptive modifications to the sympathoadrenal system at the level of the adrenal medulla. Specifically, we compared the cellular physiology and molecular profiles of chromaffin cells, as well as the plasma catecholamine levels and structural features of the medulla, in both high- and low-altitude populations of deer mice. Comparisons between each of these populations were made after chronic exposure to both normoxic and hypoxic conditions to distinguish the effects of high-altitude ancestry from acclimation environment. Our results suggest that the adrenal medulla is a site of considerable plasticity during exposure to chronic hypoxia in low-altitude mice and is also a target tissue for adaptive evolutionary change in high-altitude natives.
MATERIALS AND METHODS
Ethical approval.
All procedures for animal handling and tissue isolation followed guidelines established by the Canadian Council on Animal Care and were approved by the Animal Research Ethics Board at McMaster University.
Animal Procedures.
Wild deer mice were live trapped using Sherman traps at each of two locations: 1) on the Great Plains in Nine Mile Prairie, Lancaster County, Nebraska (40°52′12”N, 96°48′20.3”W; 430 m above sea level) and 2) on the summit of Mount Evans in Clear Creek County, CO (39°35′18”N, 105°38′38”W; 4,350 m above sea level). Mice were shipped to McMaster University by a commercial animal transporter (World Courier) in conventional rodent shipping containers with dividers for individual housing. These mice were then housed and bred within each population in common laboratory conditions to produce first generation (G1) progeny of either low-altitude or high-altitude ancestry (often referred to here as either “lowland” or “highland” populations). These G1 mice were maintained in normoxic laboratory conditions until ∼6 mo of age, after which mice from both populations were acclimated for 18–20 wk to one of two different acclimation conditions: standard normobaric normoxia or hypobaric hypoxia. The hypobaric conditions recapitulated environmental pressures and low levels of oxygen experienced at the native altitude of our high-altitude population (barometric pressure of 60 kPa; O2 partial pressure of 12.5 kPa) using specialized hypobaric chambers (described previously in Refs. 22 and 26). Mice in hypobaric conditions were temporarily (∼20 min/wk) returned to normobaric conditions for cage cleaning.
Carbon Fiber Amperometry.
Mice were placed into a small jar (∼500 ml) containing high concentrations of volatile isoflurane (administered on a lightly soaked cotton ball), and as soon as they lost consciousness and reached a surgical plane of anesthesia (typically <15 s) they were euthanized by cervical dislocation. The intact adrenal glands were then isolated, dissected, and placed in cold L-15 plating medium (Gibco, Grand Island, NY) and then promptly transferred to a glass petri dish with oxygenated, chilled Tyrode solution (115 mM NaCl, 10 mM glucose, 10 mM HEPES, 2 mM KCl, and 3 mM MgCl2, pH 7.4). The glands were secured to the base stage of the vibrotome (VT1000; Leica Biosystems) by embedding in 3% agarose and were then cut into 200-μm thick sections and incubated in bicarbonate buffer (24 mM sodium bicarbonate, 115 mM NaCl, 10 mM glucose, 12 mM sucrose, 5 mM KCl, 2 mM CaCl2, and 1 mM MgCl2) that was bubbled with 95% O2 and 5% CO2 and maintained at 37°C. Both adrenal glands were isolated from each mouse, and one to three slices were obtained for amperometric measurements from each gland.
Measurements of vesicular catecholamine release from adrenal medullary cells were made for each population in each acclimation condition, using carbon fiber amperometry approaches that have been described elsewhere (38). Release was measured from freshly sectioned adrenal gland slices that were perfused with bicarbonate buffer and exposed to the following series of conditions: 1) bicarbonate buffer alone (5–10 min), 2) nitrogen-purged bicarbonate buffer (hypoxia treatment, 1 min), 3) 10 μM nicotine (low dose, 1 min), 4) 50 μM nicotine (high dose, 1 min), and finally, 5) 30 mM KCl (positive control, 30 s). The slice was reperfused with bicarbonate buffer alone between each treatment for a minimum of 5 min or the required time for the recording to return to the original baseline. For the hypoxia treatment, a high-purity nitrogen (95%) and carbon dioxide (5%) gas mixture was bubbled into buffer for ≥30 min before use, as done in previous studies (3, 13, 50, 51), and has been shown to elicit O2 tensions of 15–20 Torr in the buffer solution (4). Measurements were made using a polarized (+800 mV) carbon fiber electrode (Dagan, Minneapolis, MN) that was attached to a CV203BU headstage and was gently lowered to the surface of the slice near the center of the adrenal medullary region. The signal was amplified with Axopatch 200B (Molecular Devices, Sunnyvale, CA), recorded at 10 kHz using Digidata 1322A Software, and analyzed with Clampex version 9.2. Any events recorded that measured greater than two standard deviations above baseline noise and >0.5 ms in duration were included in the analysis.
For each recording, the integrated area of secretory events (in units fC; indicative of the no. of oxidizable catecholamines) was measured to determine total secretion across the entire duration of the response. The end of the response was deemed to be the point at which the last significant event (2× standard deviations greater than baseline) occurred. Total secretion (fC), response duration (s), quantal frequency (events/min), secretion rate (fC/min), and quantal charge (integrated area of each event, fC) were determined for each recording in which there was a significant response to KCl. All of the recorded measurements obtained from the three to four slices isolated from each mouse were averaged together to reflect the phenotype of that individual animal (n). In total, adrenal glands were isolated from n = 10 animals of each population for the normoxia acclimation groups (which included 16 slices from lowlanders and 17 slices from highlanders) and n = 9 animals of each population for the hypoxia acclimation groups (11 slices from lowlanders, 15 slices from highlanders).
Immunohistochemistry.
Adrenal glands isolated from adult deer mice as above were immediately placed and incubated overnight at 4°C in fixative [4% paraformaldehyde in phosphate-buffered saline (PBS): 150 mM NaCl and 15 mM NaH2PO4, pH 7.4] and then cryoprotected for 24 h at 4°C in PBS containing 30% sucrose. The glands were then coated in embedding medium (Cryomatrix embedding resin; ThermoFisher Scientific, Mississauga, ON, Canada), flash frozen in liquid nitrogen, and stored at −80°C. Serial cryosections (10 μm) of the adrenal glands were obtained in a cryostat at −20°C, mounted onto glass slides, air-dried, and returned to storage at −80°C. Sections were later thawed and washed in 0.1 M PBS (10 min) and then incubated for 1 h in blocking medium (1% BSA in PBS). Sections were then incubated overnight in PBS containing 10% BSA, 0.5% Triton-X, and the following primary antibodies: anti-neurofilament (NF; 1:100, host rabbit; Millipore cat. no. AB1989, RRID: AB_91202) (34), anti-growth-associated protein 43 (GAP-43; 1:2,000, host rabbit; Millipore cat. no. AB5220, RRID: AB_2107282) (47), and anti-tyrosine hydroxylase (TH; 1:2,500, host mouse; Millipore cat. no. 657010-100UG, RRID: AB_212601) (54). The next day, sections were washed three times in PBS (15 min each) and then incubated for 2 h in PBS containing secondary antibodies conjugated to either Alexa 488 or 594 (both used at 1:400; Molecular Probes cat. no. A-11094, RRID:AB_221544; I-21413, RRID: AB_2313921). Sections were again washed in PBS as before and then incubated in PBS containing 0.2 μM DAPI for 25 min. Several droplets of Vectasheild (Vector Laboratories, Burlington, ON, Canada) were applied to each slide for coverslipping.
Specimen epifluorescence was examined using an Olympus BX60 (×20 air objective), and images of the entire adrenal gland cross-section were created by stitching together individual images using ImageJ and the stitching plugin [developed by Preibisch et al. (36)]. For each adrenal gland, eight cross-sections (60 μm apart) were included in the analysis. Several measurements were made on the stitched images using ImageJ (version 1.46r), including the maximal sectional area of the entire adrenal gland (determined by the outer edge of the Zona Glomerulosa) and the adrenal medulla alone (measured by the outer edge of TH+ cells), the number of TH+/DAPI cells in the medulla, and the integrated neuronal density in the adrenal medulla (densiometric analysis of NF florescence in a defined area). Adrenal medulla and adrenal cortex volumes are expressed here as a percentage of the entire gland volume. Volumetric values of the medullary and cortical regions were estimated from the relative areas of each layer across evenly spaced sections throughout the gland (10-μm sections). For TH+ cell counts and neurofilament densitometry, the images were first set to a threshold (range 30–255) to eliminate background florescence. Automated cell counts within the section medullary boundary were determined in individual images via Analyze Particle function (settings: >50 pixel diameter and circularity of 0.5–1.00) and are expressed here as the number of cells within the medullary volume.
ELISA measurements of plasma catecholamines.
Plasma catecholamine levels were measured during routine activity. Mice (n = 8 normoxic lowlanders, n = 6 hypoxic lowlanders, n = 14 normoxic highlanders, and n = 8 hypoxic highlanders) were first anesthetized deeply by quietly and carefully dropping an isoflurane-soaked cotton ball into their cage, and mice were then immediately decapitated for blood collection. Blood samples were collected in heparinized tubes and were centrifuged for 6 min in a hematocrit centrifuge. Plasma samples were then flash-frozen in liquid nitrogen and stored at −80°C. At the time of the catecholamine measurements, samples were thawed and kept cold on ice. Epineprine, norepineprine, and dopamine concentrations were measured using 3-CAT Research ELISA (Rocky Mountain Diagnostics, Colorado Springs, CO) as per the manufacturer’s protocol.
Western Blot analysis.
Both adrenal glands were dissected from each mouse and placed in ice-cold L-15 Medium (Gibco, Grand Island, NY). The glands were then further dissected to isolate the inner adrenal medulla relatively free from the surrounding cortical tissue. The dissected adrenal medullae were flash-frozen in liquid nitrogen and stored at −80°C. Because of the required protein levels in each sample for this assay, each sample (n) contained the adrenal medulla from four adrenal glands pooled from two mice. Samples were later mechanically dissociated in 50 μl of ice-cold RIPA buffer [150 mM NaCl, 1% NP-40, 0.5% deoxycholic acid, 0.1% SDS, and 50 mM Tris (pH 8.0); Roche ULTRA protease inhibitor tablet, Roche PhosSTOP phosphatase inhibitor tablet]. Homogenates were then incubated on ice for 1 h and centrifuged at 16,000 g for 15 min at 4°C. The total protein content within each sample was measured by a DC protein assay (Bio-Rad, Mississauga, ON, Canada).
For each sample, 10 μg of protein was combined with 2× Laemmli sample buffer (Bio-Rad), incubated in 95°C for 5 min, centrifuged briefly, and then loaded onto a gradient precast polyacrylamide gel (4–15%; Bio-Rad) for electrophoretic separation. Separated proteins were transferred onto a polyvinyl-difluoride membrane using the Trans-Blot Turbo Transfer System (Bio-Rad). Membranes were washed and incubated for 1 h at room temperature in Tris-buffered saline (TBS) containing 5% nonfat milk powder and 1% Tween-20. The membranes were then incubated overnight at 4°C in TBS with Tween-20 alone (TBS-T) containing one of the following primary antibodies: anti-tyrosine hydroxylase (TH; 1:1,000 dilution, host rabbit; Millipore cat. no. AB59866, RRID: AB_92190) (8), anti-3,4-dihydroxyphenylalanine (DOPA) decarboxylase (DDC; 1:1,000, host rabbit; Abcam cat. no. 3905, RRID: AB_304145) (52), anti-dopamine β hydroxylase (DβH; 1:1,000, host sheep; Abcam cat. no. 19353, RRID: AB_731851) (28), or anti-phenylethanolamine N-methyltransferase (PMNT; 1:1,000, host rabbit; Abcam cat. no. ab167427) (28). After the incubation in primary antibody, membranes were washed three times (3 × 10 min) in TBS-T at room temperature and then incubated in TBS-T containing horseradish peroxidase-conjugated secondary antibody against primary antibodies from either rabbit (1:5,000) or sheep (1:1,500) (GE Healthcare Life Sciences, Mississauga, ON, Canada) for 2 h at room temperature. Membranes were then washed again and developed using enhanced chemiluminescence developer (5 min; Bio-Rad) and imaged used a Chemidoc Imaging System (Bio-Rad). Densitometry measurements of band intensity were conducted using Image Laboratory Software 5.2 (Bio-Rad).
The membrane was then stripped using Blot Restore Solution (Millipore, Temecula, CA) as per the manufacturer’s instructions. The membrane was incubated at room temperature with solution A for 10 min, transferred to solution B for 15 min, and then rinsed well with TBS-T. Membranes were then incubated overnight at 4°C in TBS-T containing a primary antibody against β-actin (1:2500, host mouse; Sigma-Aldrich cat. no. A1978, RRID: AB_476692), which was used as a loading control. As described above, the membrane was then washed and placed in TBS-T containing horseradish peroxidase-conjugated secondary antibody against mouse primary antibody (1:2,500; GE Healthcare Life Sciences) for 2 h. Imaging and densitometry measurements of band intensity for β-actin were then performed as above.
Normalized protein abundance of each protein of interest was determined as follows. We first carried out a within-gel normalization by dividing the band intensity of the protein of interest to the band intensity of the load control (β-actin) for each sample to control for variation in the amount of protein loaded into each lane. All samples for each protein of interest were processed at the same time, but they had to be run across multiple (2, 3) gels, so we ran a cross gel control sample (a mixed collection of all other samples) on each gel. We then carried out a cross-gel normalization by dividing the value for each sample from the within gel normalization to the value of the cross-gel control on each corresponding gel. These normalized values are all expressed as a percentage of the mean normalized value for normoxic lowland mice.
Statistics.
Statistical analyses were performed using Prism (version 5, RRID: SCR_002798; GraphPad Software) and data compared using two-way ANOVA, and Bonferroni multiple comparisons were used to determine the effects of population or acclimation environment for each parameter. The tests used and P values are specified in the results and figure captions for each figure presented. P < 0.05 was considered to be statistically significant.
RESULTS
Catecholamine secretion via nicotinic acetylcholine receptor activation is blunted in highland deer mice.
Application of a potent nAChR agonist, nicotine, was used to simulate sympathetic activation of chromaffin cells within the adrenal medulla slice preparation. High-resolution amperometric detection of real-time catecholamine secretion demonstrated that chromaffin cells from normoxic lowland mice had robust responses to both low (10 μM) and high (50 μM) concentrations of nicotine (Fig. 1, A and C). This high concentration of nicotine is approximately twofold the recorded EC50 for nicotine-mediated activation of human and rat chromaffin cells (4, 18) and is the lowest dose that produces maximal catecholamine release in bovine chromaffin cells (40). The low concentration is ∼50% of the EC50 (21) but is great enough (>5 μM) to produce measurable responses without inducing desensitization of nicotinic receptors (18). Hypoxia acclimation reduced the secretory response of lowlanders to 10 μM nicotine, leading to a significant main effect of acclimation in two-way ANOVA (P = 0.037). However, secretion in response to 50 μM of nicotine was unaffected by hypoxia acclimation (Fig. 1C). By contrast, CAT secretion was very low in highland mice during stimulation with both low (Fig. 1, B and C, left) and high (Fig. 1, B and C, right) concentrations of nicotine (main effects of population: P = 0.039 at 10 μM, P = 0.012 at 50 μM) and was unaffected by hypoxia acclimation.
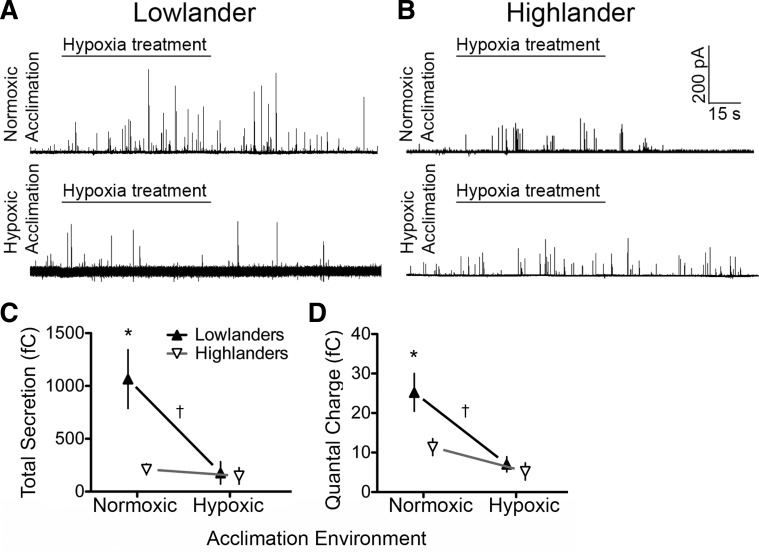
Fig. 1.
Nicotine-induced catecholamine release measured in situ from the adrenal medulla of deer mice native to low altitude and high altitude and acclimated to normoxic or hypoxic conditions. A and B: sample amperometric recordings from chromaffin cells in adrenal slices from a lowland mouse (A) and a highland mouse (B), each acclimated to normoxia, in which the tissue was bathed in low (10 μM) and then high (50 μM) concentrations of nicotine for 1 min, followed by 30 s of exposure to 30 μM K+ (positive control). C: cumulative catecholamine secretion in response to 10 μM nicotine was higher in lowlanders (▲; n = 8) than in highlanders (∇; n = 10) among mice acclimated to normoxia, but both highlanders (n = 8) and lowlanders (n = 9) had low levels of catecholamine secretion after hypoxia acclimation (left). However, for lowlanders, total secretion in response to 50 μM nicotine was high in both acclimation groups, similar to secretion in response to 10 μM nicotine in normoxia-acclimated lowlanders, and higher than the consistently low levels of secretion in highlanders (C, right). D: patterns of variation for the quantal charge of secretory events was very similar to the pattern of variation for total catecholamine secretion. *Pairwise differences between populations within an acclimation environment; †significant main effect of population in 2-way ANOVA. Error bars are ± SE.
Variation in the quantal charge per secretion event, a measurement that reflects vesicular loading and/or the concentration of CAT released per vesicle, was very similar to the variation in total CAT secretion. For 10 μM nicotine, quantal charge was significantly (P = 0.034) greater for lowlanders than for highlanders in comparisons of normoxic mice (Fig. 1D). As was the case for total secretion, hypoxia acclimation reduced quantal secretion induced by 10 μM nicotine in lowlanders (Fig. 1D, left), leading to a significant main effect of hypoxia acclimation (P = 0.009). However, there was not a significant effect of hypoxia acclimation on the response to higher nicotine concentrations (50 μM), as secretion in hypoxia-acclimated lowlanders was similar to normoxia-acclimated lowlanders (Fig. 1D, right). Highlanders maintained consistently low levels of quantal charge for both low and high concentrations of nicotine. Interestingly, other amperometric measurements suggested that the population differences in total CAT secretion appeared to be largely if not exclusively due to the variation in quantal charge described above. There were no differences in the frequency of nicotine-induced quantal events between populations in mice acclimated to either normoxia or hypoxia (Table 1). As a result, the quantal secretion rate, measured as the total quanta released per minute (i.e., the product of quantal charge and event frequency), exhibited a similar pattern of variation to that for quantal charge (Table 1). The duration of nicotine-induced release of CAT from chromaffin cells was significantly lower (<50%) in highlanders acclimated to hypoxia compared with lowlanders, but there were no differences between lowlanders and highlanders when acclimated to normoxia (Table 1). Taken together, variations in total catecholamine secretion between highlanders and lowlanders or in lowlanders in response to hypoxia acclimation were due primarily to the differences in quantal charge rather than the duration of the nicotinic response or the frequency of vesicular release.
Table 1.
Adrenal catecholamine secretion in response to nicotine treatment
| Secretion Variants | Nonacclimated, μM |
Acclimated, μM |
||
|---|---|---|---|---|
| 10 | 50 | 10 | 50 | |
| Lowlanders | ||||
| Duration, s | 150.5 ± 23.4 | 174.4 ± 42.4 | 173.9 ± 48.6 | 226.8 ± 52.3 |
| Frequency, Hz | 6.9 ± 2.7 | 8.3 ± 1.7 | 6.8 ± 2.7 | 14.7 ± 8.6 |
| Secretion rate, fC/min | 238.6 ± 59.2* | 198.7 ± 48.7* | 55.9 ± 14.6 | 175.8 ± 129.2* |
| Highlanders | ||||
| Duration, s | 186.8 ± 23.5 | 203.9 ± 48.3 | 57.0 ± 14.2* | 109.5 ± 17.5* |
| Frequency, Hz | 6.1 ± 0.8 | 5.9 ± 1.6 | 13.9 ± 4.1 | 10.1 ± 1.7 |
| Secretion rate, fC/min | 74.6 ± 28.1 | 82.3 ± 29.1 | 64.8 ± 22.9 | 68.9 ± 21.5 |
Values are means ± SE.
Significant differences between the populations within each group.
Catecholamine secretion in response to acute hypoxia is altered in highland deer mice.
A subpopulation of chromaffin cells in adrenal slices from adult deer mice retained direct O2 sensitivity (Fig. 2), similar to other rodents (15, 41, 51). Hypoxia-induced CAT secretion in such cells was particularly robust in AMCs from normoxic lowlanders (Fig. 2, A and C), but hypoxia acclimation reduced this response (Fig. 2, B and C), and there was a corresponding, significant main effect of hypoxia acclimation in two-way ANOVA (P = 0.006). However, hypoxia-induced CAT secretion was lower in highlanders than in lowlanders in normoxia (significant main effect of population in two-way ANOVA, P = 0.010; Fig. 2, B and C). Furthermore, hypoxia acclimation did not change acute responsiveness of highlander AMCs to low oxygen treatment, which remained low in both acclimation groups (Fig. 2C). The response duration and frequency of vesicular release were not different between any of the groups (Table 2). Variation in quantal charge was the primary contributor to differences in total secretion, as was the case for nicotine-induced CAT release, and quantal charge was highest in normoxic lowlanders (Fig. 2D). We noted that the secretion of CAT in response to acute low oxygen was still appreciably lower than the secretion response to nicotine (e.g., ∼threefold lower than the response to 10 μM nicotine in normoxia-acclimated lowland mice).
Fig. 2.
Hypoxia-induced catecholamine release measured in tissue slices from the adrenal medulla of deer mice native to low altitude and high altitude and acclimated to normoxic or hypoxic conditions. A and B: examples of amperometric recordings from adrenal medullae isolated from lowland mice (A) and highland mice (B) acclimated to either normoxia (top) or hypoxia (bottom) following application of a physiological bath with low oxygen levels (Po2 ∼15–20 mmHg for 1 min). C: cumulative catecholamine secretion in response to low oxygen treatment was significantly higher in lowlanders (▲; n = 10) than in highlanders (∇; n = 10) when acclimated to similar normoxic conditions (P < 0.01), but both populations had low levels of catecholamine secretion after hypoxia acclimation. D: average quantal charge in response to low oxygen treatment was higher in normoxia-acclimated lowlanders compared with all other groups. *Pairwise differences between populations within an acclimation environment; †significant main effect of population in 2-way ANOVA. Error bars are ± SE.
Table 2.
Adrenal catecholamine secretion in response to hypoxia treatment
| Secretion Variants | Lowlanders |
Highlanders |
||
|---|---|---|---|---|
| Nonacclimated | Acclimated | Nonacclimated | Acclimated | |
| Duration, s | 174.4 ± 42.4 | 198.5 ± 53.4 | 203.9 ± 48.2 | 109.5 ± 17.6 |
| Frequency, Hz | 14.4 ± 2.6 | 6.5 ± 1.5 | 8.7 ± 1.3 | 10.9 ± 3.0 |
| Secretion, fC/min | 1,065.1 ± 278.1* | 176.0 ± 106.0 | 211.0 ± 51.7 | 148.6 ± 78.4 |
Values are means ± SE.
Significant differences between populations within the group.
Structural comparison between adrenal glands of highland and lowland deer mice.
The adrenal gland contains two distinct regions, the outer cortex and the inner medulla, and the proportion of adrenal gland volume that was composed of medullary tissue was much smaller in highland mice than in lowland mice (Fig. 3C). This difference was evident in mice that were acclimated to either normoxic or hypoxic conditions, and there was a significant main effect of population in two-way ANOVA (P = 0.0002). The opposite relationship was observed for the cortical tissue (cortical layers designated in Fig. 3A), with highland mice having significantly higher proportional volume of cortical tissue within the adrenal gland than lowland mice (P < 0.0001; Fig. 3D).
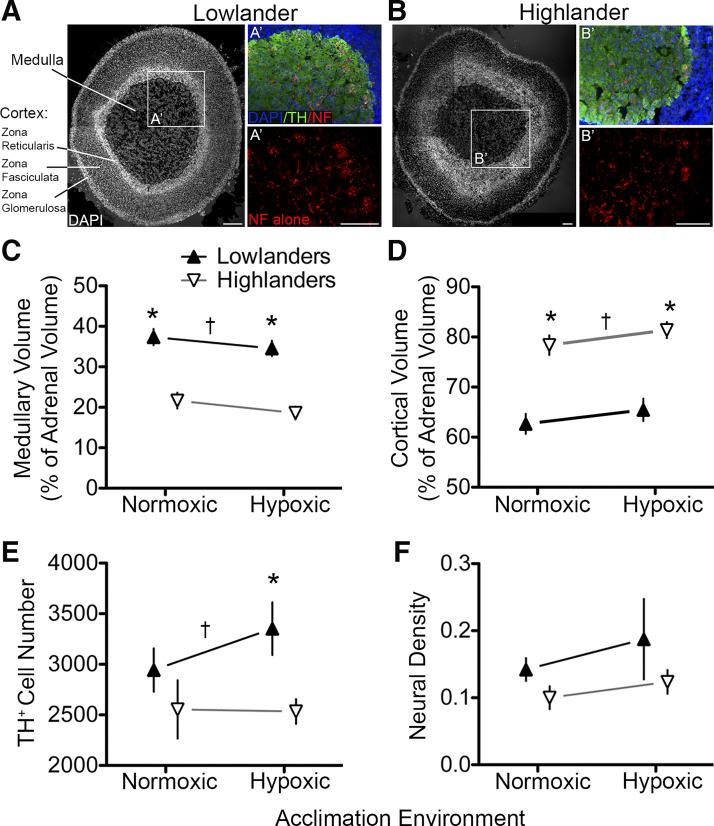
Fig. 3.
Anatomy of the adrenal gland from deer mice native to low altitude and high altitude following acclimation to normoxia or hypoxia. A and B: immunohistochemical sections labeled with a nuclear marker (DAPI) to illustrate the adrenal medulla and cortex of the glands in lowlanders (A) and highlanders (B). Inset images A' and B' illustrate staining for tyrosine hydroxylase to identify chromaffin cells (TH+ cells) and neurofilament (NF) and growth-associated protein 43 (GAP-43) to identify neural elements (nerve fibers). C: tissue volume of the adrenal medulla relative to the overall gland size was greater in lowlanders acclimated to either normoxia (n = 4) or hypoxia (n = 7) (▲) compared with normoxic (n = 10) or hypoxic (n = 8) highland mice (∇; P < 0.001). D: correspondingly, the relative cortical tissue volume within the adrenal gland of lowlanders was lower than that of highlanders (P < 0.001). E: differences in the relative no. of TH+ cells (P = 0.028) contribute to the population differences in adrenal medulla volume. F: there were no significant differences in neural density within the medulla based on densitometric analysis of NF/GAP-43 (bottom images A' and B'). *Pairwise differences between populations within an acclimation environment; †significant main effect of population in 2-way ANOVA. Scale bars, 50 μm. Error bars are ± SE.
To determine whether the smaller medullary volumes in the highland population were due to differences in chromaffin cell number or size, we performed tyrosine hydroxylase-immunoreactive (TH+) cell counts by quantifying the number of DAPI-stained nuclei that colocalized with TH+ staining. There was a main effect of population on TH+ cell counts, with highland mice having fewer chromaffin cells than lowland mice (P = 0.028), and pairwise differences were most prominent in the hypoxia acclimation groups (Fig. 3E; P < 0.05). In addition, we examined possible differences in the level of sympathetic innervation of the adrenal medulla, given the observed differences in the responsiveness of chromaffin cells to nicotine. We found that the density of neuronal processes, labeled with neurofilament and GAP-43, within a fixed area of medulla tissue sections was not significantly different between the populations in either acclimation condition (P = 0.219; Fig. 3F). Taken together, these findings suggest that there has been an evolved reduction in AMC number and in the volume of medullary tissue within the adrenal gland of highland mice, but they have retained sympathetic innervation.
Circulating plasma epinephrine levels are relatively low in highlanders and are reduced by hypoxia acclimation in lowlanders.
We examined whether the variation in CAT secretion and structure of the adrenal medulla was associated with variation in plasma levels of dopamine (DA), norepineprine (NE), and epinephrine (E), under routine conditions. These CATs are synthesized from l-tyrosine within the chromaffin cells by an intracellular biochemical pathway that is illustrated in Fig. 4A. The secreted products dopamine (DA; ∼5%), norepinephrine (NE; ∼15%), and epinephrine (E; ∼80%) are released via vesicular exocytosis in response to nicotinic receptor activation (25). Chromaffin cells are the main source of epinephrine in the blood stream, in contrast to dopamine and norepinephrine, which can also enter the blood by spillover from peripheral SNS synapses. Hypoxia acclimation reduced plasma epinephrine in lowlanders, which led to a significant main effect of hypoxia acclimation (P = 0.0479; Fig. 4D). However, highlanders had three times lower plasma epinephrine levels than lowlanders among normoxia-acclimated mice, and highlanders did not respond to hypoxia acclimation, such that there was a significant difference in the hypoxia acclimation response between populations (i.e., significant population × acclimation interaction, P = 0.0159). In contrast, there were no significant population differences in plasma levels of dopamine (lowland versus highland, P = 0.5896; Fig. 4B) or norepinephrine (P = 0.5265; Fig. 4C) and no significant main effects of hypoxia acclimation for these two catecholamines (norepinephrine, P = 0.4349; and dopamine, P = 0.8299). Therefore, the patterns of variation in circulating epinephrine levels are consistent with the patterns of variation in CAT release evoked by low doses of nicotine or acute hypoxia.
Fig. 4.
Plasma catecholamine levels in deer mice native to low altitude and high altitude following acclimation to normoxia or hypoxia. A: schematic shows the catecholamine biosynthesis pathway within chromaffin cells of the adrenal medulla leading up to vesicular release into the circulation (14). B–D: in mice acclimated to normoxic conditions, the plasma concentrations of dopamine (B) and norephinephrine (C) were similar in lowland (▲) and highland (∇) populations, but levels of epinephrine (D) were higher in lowlanders (n = 8) compared with highlanders (n = 14). All differences in catecholamine levels between populations were abolished after mice were acclimated to hypoxic conditions (hypoxic lowlanders, n = 6; hypoxic highlanders, n = 8). *Pairwise differences between populations within an acclimation environment; †significant interaction between population and acclimation environment in 2-way ANOVA. Error bars are ± SE.
Variation in expression of catecholamine biosynthetic enzymes.
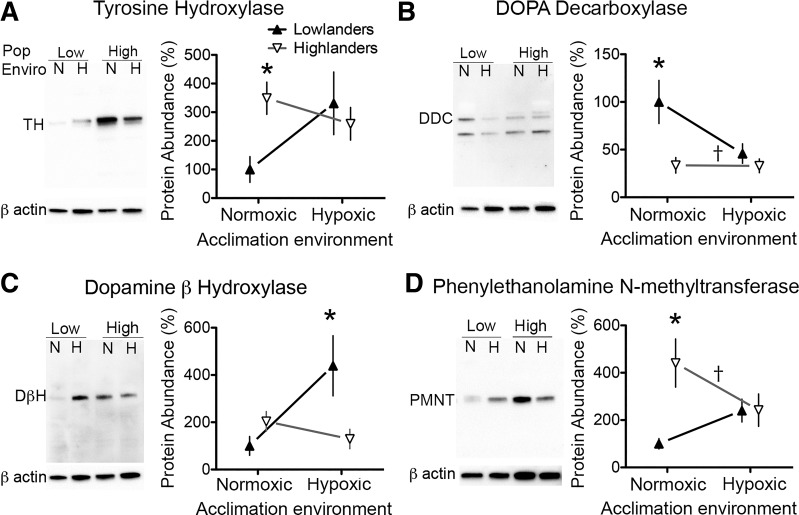
Hypoxia is known to alter the expression of enzymes involved in CAT synthesis (see Fig. 4A) (1), so we next sought to determine whether variation in CAT secretion could be explained by lowered enzyme expression. This was not the case for tyrosine hydroxylase (TH), which was more highly expressed in highlanders than in lowlanders among normoxia acclimation groups (P < 0.05), but there were no differences between the populations after hypoxia acclimation (Fig. 5A). There was also a significant population × acclimation interaction for this enzyme in two-way ANOVA (P = 0.0263).
Fig. 5.
Relative protein abundance of catecholamine biosynthetic enzymes in the adrenal medulla of deer mice native to low altitude (low; ▲) and high altitude (high; ∇) following acclimation to normoxia (N) or hypoxia (H). A: tyrosine hydroxylase (TH; single band at ∼60 kDa) levels were lower in lowlanders than in highlanders when compared in normoxic conditions (P < 0.05) but were equivalent between lowland (n = 5) and highland (n = 8) populations after hypoxia acclimation. B: relatively low levels of DOPA decarboxylase (DDC) were found in the highlanders in both acclimation conditions, and levels were lower than those in lowlanders among normoxia-acclimated mice exposures (P < 0.01). The DDC antibody detected bands at ∼55 and 27 kDa, and the 55 kDa band was the expected size of the enzyme and the band that was quantified. C: dopamine β-hydroxylase (DβH; single band at ∼70 kDa) levels did not change with hypoxia acclimation in highland mice, but levels were elevated in lowlanders after hypoxia acclimation to higher levels than in highlanders (P < 0.01). D: phenylethanolamine N-methyltransferase (PNMT; single band at ∼28 kDa) levels were highest in normoxic highlanders, but populations were equivalent after hypoxia acclimation. β-Actin (∼42 kDa) was used as a loading control, and the normalized abundance of each protein of interest is expressed as a percentage of the mean normalized value for normoxic lowland mice (see materials and methods). *Pairwise differences between populations within an acclimation environment; †significant main effect of population in 2-way ANOVA. Error bars are ± SE.
In contrast, expression of DOPA decarboxylase (DDC) mirrored the variation in CAT release evoked by 10 μM nicotine or acute hypoxia (Fig. 5B). The antibody to DDC detected the enzyme dimer as a single or double band at the expected size of ∼55 kDa, which we have quantified here, as well as a known reduced form at ∼27 kDa. DDC expression was reduced after hypoxia acclimation in lowlanders (hypoxia; n = 5, P = 0.0252). DDC expression changed little with hypoxia acclimation in highlanders, in which expression was lower than lowlanders among normoxia-acclimated mice (P = 0.0282), and there was a significant main effect of population in two-way ANOVA (P = 0.0023).
The expression of dopamine β hydroxylase (DβH) exhibited a different pattern of variation (Fig. 5C). Hypoxia acclimation appeared to increase DβH in lowlanders, leading to a marginally significant acclimation effect in two-way ANOVA (P = 0.050). However, hypoxia acclimation had no effect on DβH expression in highlanders, such that DβH was lower in highlanders than in lowlanders after hypoxia acclimation (P < 0.010), and there was a significant population effect in two-way ANOVA (P = 0.0037).
The variation in expression of phenylethanolamine N-methyltransferase (PNMT) was similar to that for TH (Fig. 5D). There was no significant effect of acclimation environment on PMNT expression, but expression was greater in highlanders among normoxia-acclimated mice, and the main effect of population in two-way ANOVA was significant (P = 0.037).
DISCUSSION
High-altitude natives of several species demonstrate various physiological adaptations to the challenges of living in hypoxic environments. Deer mice living in the highlands of North America are no exception. Previous work has demonstrated that highland populations of this species have evolved higher aerobic capacity in hypoxia in association with evolved changes in control of breathing, hemoglobin-O2 affinity, cardiac function, muscle capillarity and metabolic phenotype, and tissue gene expression (10, 22, 23, 26, 27, 39, 42, 46, 49, 53). In this study, we demonstrate that the function of the sympathoadrenal system has also evolved in high-altitude deer mice. Phenotypic plasticity of the adrenal medulla was evident in response to chronic hypoxia exposure in lowlanders, yet in highlanders there was a relative lack of plasticity, with adrenal function staying virtually fixed at low levels of catecholamine secretion across varying environmental conditions. This was reflected by the relatively low levels of circulating epinephrine in the plasma of highlanders when examined during routine activity in normoxia or after hypoxia acclimation. Our results suggest that genetically based modifications in adrenal medulla function may play an important role in high-altitude adaptation in deer mice.
Chronic hypoxia alters sympathoadrenal function in lowlanders.
Sympathetic activation of chromaffin cells from the adrenal medulla results in fast secretion of catecholamines into the bloodstream. The medulla is innervated by sympathetic fibers of the splanchnic nerve, which release acetylcholine to activate neuronal-type nicotinic acetylcholine receptors (nAChRs) expressed by chromaffin cells. In this study, we found that the responsiveness of AMCs to mild nicotinic ACh receptor stimulation was significantly diminished following chronic exposure (18–20 wk) of lowland mice to hypoxia in association with a decrease in circulating epinephrine levels in the plasma. After hypoxia acclimation, higher concentrations (50 μM) of nicotine were needed for CAT release from chromaffin cells to match that seen in normoxic lowlanders. Therefore, chromaffin cells maintained the capacity to respond to nicotinic stimulation, but their activation threshold was higher. These adjustments mimic the changes in chromaffin cell sensitivity that have been observed in rats exposed to hypoxemia or nicotine in early development. For example, adrenal CAT depletion and reduced chromaffin cell activation were noted in neonates born to mothers exposed to chronic hypoxemia (11). Similarly, prenatal exposure to chronic nicotine (14 days) blunted the CAT secretory responses to hypoxic stimulation of adrenal chromaffin cells from neonatal rat pups (3). Considering the potentially maladaptive consequences of having chronically elevated circulating CAT levels, decreasing the responsiveness of AMCs may be a beneficial cellular modification to long-term hypoxia in adults. However, in the current study, lowlanders were exposed to hypoxia for 5 mo. Although we did not determine the amount of time needed for these adjustments of AMCs to long-term hypoxia to arise, lowlanders colonizing high altitude may be at a disadvantage until they have had sufficient time to fully acclimatize.
We also confirmed that the adrenal medulla of adult lowland mice retained some capacity for nonneurogenic CAT release in response to acute hypoxia, but this response was significantly reduced following long-term acclimation of the animal to chronic hypoxia. Hypoxia sensing and CAT release via chromaffin cells are required in the neonate for the essential priming of the cardiovascular system for birth. Although much of this sensitivity is diminished in postnatal life following innervation of the adrenal medulla (50, 51), subsets of chromaffin cells seem to preserve this function into adulthood in certain species (15, 21, 48). The chronic hypoxia-induced reductions in this nonneurogenic stimulation of CAT secretion by low oxygen, as well as the sensitivity to nicotinic stimulation, were both due primarily to a decrease in quantal charge rather than the frequency of quantal events. These findings suggest that total vesicular CAT storage or the fractional release of vesicular CAT stores was reduced after hypoxia acclimation.
Regulation of catecholamine biosynthesis in lowlanders exposed to long-term hypoxia.
One of the primary determinants of vesicular CAT content is the cascade of enzymes that regulate CAT production within chromaffin cells. Previous studies have shown that expression of CAT biosynthetic enzymes is altered by chronic hypoxia, with much of the focus on tyrosine hydroxylase (TH) and dopamine β-hydroxylase (DβH) due to their reliance on molecular oxygen for catalytic activity (9, 37). Similar to our findings here (Fig. 5), expression of both TH and DβH in the adrenal gland was elevated in rats after days or weeks of sustained hypoxia (10% O2) (19). However, in the present study, these increases were not associated with increased CAT secretion by adrenal tissue slices or with increased plasma CAT levels.
In contrast to TH and DβH, the expression of DOPA decarboxylase (DDC; or aromatic l-amino acid decarboxylase) in the adrenal gland was significantly reduced following hypoxia acclimation in lowlanders. Because DDC expression was correlated with adrenal CAT secretion evoked by mild nicotine and with plasma epinephrine following hypoxia acclimation, this enzyme may have acted as a critical regulator of CAT biosynthesis. DDC expression is developmentally regulated by hypoxia-inducible factor-2α, a transcription factor that is elevated in chromaffin cells during hypoxia exposure (1). DDC is not normally considered to be a rate-limiting factor in neurotransmitter biosynthesis, but it can limit the production of CAT in situations when its substrates are in abundance. For example, production of dopamine and serotonin is directly determined by DDC activity in patients treated with either exogenous L-DOPA for Parkinson’s disease (2) or 5-HTP for mild depression (30), respectively. Although not directly tested, it is likely that CAT synthesis was not limited by L-DOPA production, particularly after exposure to chronic hypoxia when TH expression in chromaffin cells had increased in lowland mice, but instead that the reduced levels of DDC limited downstream CAT synthesis.
Evolutionary adaptations to high altitude in the sympathoadrenal system.
In amperometric studies on adrenal slices, we show that catecholamine secretion evoked by high doses of nicotine is dramatically reduced in highland deer mice when compared with their lowland counterparts. Furthermore, CAT secretion evoked by low doses of nicotine or by acute hypoxia was not altered by acclimation to long-term hypoxia in highland mice and was fixed at levels that were similar to hypoxia-acclimated lowlanders. This lack of plasticity within the adrenal medulla of highland deer mice is reminiscent of that observed in other highland species in the carotid body, a key peripheral chemoreceptor that initiates the hypoxic chemoreflex and regulates sympathetic activity in adult mammals. For example, in guinea pigs, a species that originates from the Andes, stimulus-evoked CAT secretion by the carotid body is unaffected by chronic hypoxia due to two factors: 1) dampened chemosensitivity, as demonstrated by reduced activation of chemoreceptor (glomus) cells in response to hypoxic stimuli; and 2) diminished CAT content within the glomus cells (16). Similarly, diminished CAT content appears to contribute to the attenuation of CAT secretion from adrenal chromaffin cells of highland deer mice because of the significant reduction of the quantal charge, yet there is no change in the frequency or duration of vesicle release in response to nicotine or acute hypoxia. It is plausible that the low cellular CAT content results from a decline in CAT synthesis, given the correspondingly low expression of either DDC or DβH in the adrenal medullae of highlanders acclimated to normoxia or hypoxia, respectively.
Highland deer mice also had a much smaller volume of medullary tissue within the adrenal gland and had fewer TH+ chromaffin cells compared with their lowland counterparts. Interestingly, reduced numbers of TH+ cells in the adrenal medulla have also been reported in adult rats that had previously been exposed to chronic prenatal hypoxia (15 days; E5–E20) (29), suggesting that developmental plasticity of the tissue can occur and have persistent effects. In addition, recent studies on high-altitude species or populations have shown that the characteristic hypertrophy of the carotid body that occurs in many low-altitude natives (including mice and rats) exposed to chronic hypoxia is not observed in guinea pigs (16) or high-altitude deer mice (22). The evolved structural differences in the adrenal medulla likely contributed to the lower levels of plasma CAT found in highlanders compared with lowlanders in normoxia.
Surprisingly, very little is known about how evolutionary adaptation has shaped sympathoadrenal function in high-altitude populations and species. This study is one of the first to suggest that genetically based variation in adrenal medulla physiology can contribute to high-altitude adaptation. The unique differences in highlanders that persist after exposure to chronic hypoxia, namely, the differences in adrenal gland structure and the reduced catecholamine secretion in response to strong nicotinic stimulation, may be particularly important for providing highlanders with an advantage in their native high-altitude environment. The attenuation of the adrenal medulla’s role in CAT secretion may help avoid some potentially maladaptive effects of chronic hypoxia in adulthood, such as systemic hypertension and/or increases in vascular resistance in many tissues (12). It may also improve reproductive success by helping offset intrauterine growth restriction and impairments in placental blood flow that are associated with chronically elevated CAT in pregnant females (32). However, given that the sympathoadrenal system is critical in lowlanders for responses involved in surviving acute stressors (e.g., the “fight or flight” response involved in escaping predation, attaining food, and coping with extreme weather) and for regulating blood pressure after blood loss, there may be tradeoffs associated with evolved changes in the sympathoadrenal system in high-altitude populations. These tradeoffs could foreseeably lead to compensatory changes in other systems to maintain these important homeostatic responses. The greater volume of the adrenal cortex in highland deer mice may also have important consequences. This difference was analogous to the cortical hyperplasia observed in rats following sustained hypobaric hypoxia (17) and suggest that corticosteroid, aldosterone, and/or androgen signaling pathways may play an altered role in high-altitude environments.
Perspectives and Significance
Because high-altitude natives have evolved exquisite mechanisms for coping with chronic hypoxia, comparisons between highland and lowland natives can provide unique and valuable insight into mechanisms of hypoxia resistance that have been favored by natural selection. Our results suggest that genetically based modifications in adrenal medulla function play an important role in high-altitude adaptation in deer mice. High-altitude mice had small adrenal medullae due to an evolved reduction in chromaffin cell number, and their chromaffin cells generally exhibited low rates of catecholamine secretion in response to nicotinic stimulation. The mechanisms underlying these evolved changes are not fully understood, but our findings suggest that reductions in catecholamine synthesis and/or vesicle loading play an important role and have thus provided insight into the molecular underpinnings governing chromaffin cell physiology. This apparent outcome of natural selection in high-altitude deer mice provides significant insight into aspects of adrenal medulla physiology that may become a liability during chronic hypoxia, as well as how these liabilities can be avoided. This and future work on high-altitude natives will continue to provide valuable insight into the genetic, molecular, cellular, and physiological bases of adaptive mechanisms for coping with chronic hypoxia in mammals.
GRANTS
G. R. Scott, A. Scott, and C. Nurse are funded by Natural Sciences and Engineering Research Council operating grants.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.L.S. and G.R.S. conceived and designed research; A.L.S. and N.A.P. performed experiments; A.L.S. and N.A.P. analyzed data; A.L.S., N.A.P., C.A.N., and G.R.S. interpreted results of experiments; A.L.S. prepared figures; A.L.S. drafted manuscript; A.L.S., C.A.N., and G.R.S. edited and revised manuscript; A.L.S., N.A.P., C.A.N., and G.R.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We sincerely thank Paras Patal for excellent technical assistance.
REFERENCES
- 1.Brown ST, Kelly KF, Daniel JM, Nurse CA. Hypoxia inducible factor (HIF)-2 alpha is required for the development of the catecholaminergic phenotype of sympathoadrenal cells. J Neurochem 110: 622–630, 2009. doi: 10.1111/j.1471-4159.2009.06153.x. [DOI] [PubMed] [Google Scholar]
- 2.Burkhard P, Dominici P, Borri-Voltattorni C, Jansonius JN, Malashkevich VN. Structural insight into Parkinson’s disease treatment from drug-inhibited DOPA decarboxylase. Nat Struct Biol 8: 963–967, 2001. doi: 10.1038/nsb1101-963. [DOI] [PubMed] [Google Scholar]
- 3.Buttigieg J, Brown S, Holloway AC, Nurse CA. Chronic nicotine blunts hypoxic sensitivity in perinatal rat adrenal chromaffin cells via upregulation of KATP channels: role of alpha7 nicotinic acetylcholine receptor and hypoxia-inducible factor-2alpha. J Neurosci 29: 7137–7147, 2009. doi: 10.1523/JNEUROSCI.0544-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buttigieg J, Brown S, Zhang M, Lowe M, Holloway AC, Nurse CA. Chronic nicotine in utero selectively suppresses hypoxic sensitivity in neonatal rat adrenal chromaffin cells. FASEB J 22: 1317–1326, 2008. doi: 10.1096/fj.07-9194com. [DOI] [PubMed] [Google Scholar]
- 5.Calbet JA. Chronic hypoxia increases blood pressure and noradrenaline spillover in healthy humans. J Physiol 551: 379–386, 2003. doi: 10.1113/jphysiol.2003.045112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cannon WB, Hoskins RG. The effects of asphyxia, hyperncea, and sensory stimulation on adrenal secretion. Am J Physiol 29: 274–279, 1911. doi: 10.1152/ajplegacy.1911.29.2.274. [DOI] [Google Scholar]
- 7.Carabelli V, Marcantoni A, Comunanza V, de Luca A, Díaz J, Borges R, Carbone E. Chronic hypoxia up-regulates alpha1H T-type channels and low-threshold catecholamine secretion in rat chromaffin cells. J Physiol 584: 149–165, 2007. doi: 10.1113/jphysiol.2007.132274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Close JL, Yao Z, Levi BP, Miller JA, Bakken TE, Menon V, Ting JT, Wall A, Krostag AR, Thomsen ER, Nelson AM, Mich JK, Hodge RD, Shehata SI, Glass IA, Bort S, Shapovalova NV, Ngo NK, Grimley JS, Phillips JW, Thompson CL, Ramanathan S, Lein E. Single-cell profiling of an in vitro model of human interneuron development reveals temporal dynamics of cell type production and maturation. Neuron 93: 1035–1048.e5, 2017. [Erratum in: Neuron 96: 949, 2017.] 10.1016/j.neuron.2017.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daubner SC, Le T, Wang S. Tyrosine hydroxylase and regulation of dopamine synthesis. Arch Biochem Biophys 508: 1–12, 2011. doi: 10.1016/j.abb.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dawson NJ, Lyons SA, Henry DA, Scott GR. Effects of chronic hypoxia on diaphragm function in deer mice native to high altitude. Acta Physiol (Oxf) 223: e13030, 2018. doi: 10.1111/apha.13030. [DOI] [PubMed] [Google Scholar]
- 11.DeCristofaro JD, LaGamma EF. Neonatal stress: effects of hypoglycemia and hypoxia on adrenal tyrosine hydroxylase gene expression. Pediatr Res 36: 719–723, 1994. doi: 10.1203/00006450-199412000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Dempsey JA, Morgan BJ. humans in hypoxia: a conspiracy of maladaptation?! Physiology (Bethesda) 30: 304–316, 2015. doi: 10.1152/physiol.00007.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fearon IM, Thompson RJ, Samjoo I, Vollmer C, Doering LC, Nurse CA. O2-sensitive K+ channels in immortalised rat chromaffin-cell-derived MAH cells. J Physiol 545: 807–818, 2002. doi: 10.1113/jphysiol.2002.028415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flatmark T. Catecholamine biosynthesis and physiological regulation in neuroendocrine cells. Acta Physiol Scand 168: 1–17, 2000. doi: 10.1046/j.1365-201x.2000.00596.x. [DOI] [PubMed] [Google Scholar]
- 15.García-Fernández M, Mejías R, López-Barneo J. Developmental changes of chromaffin cell secretory response to hypoxia studied in thin adrenal slices. Pflugers Arch 454: 93–100, 2007. doi: 10.1007/s00424-006-0186-y. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Obeso E, Docio I, Olea E, Cogolludo A, Obeso A, Rocher A, Gomez-Niño A. Guinea pig oxygen-sensing and carotid body functional properties. Front Physiol 8: 285, 2017. doi: 10.3389/fphys.2017.00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gosney JR. Adrenal corticomedullary hyperplasia in hypobaric hypoxia. J Pathol 146: 59–64, 1985. doi: 10.1002/path.1711460107. [DOI] [PubMed] [Google Scholar]
- 18.Hone AJ, Michael McIntosh J, Rueda-Ruzafa L, Passas J, de Castro-Guerín C, Blázquez J, González-Enguita C, Albillos A. Therapeutic concentrations of varenicline in the presence of nicotine increase action potential firing in human adrenal chromaffin cells. J Neurochem 140: 37–52, 2017. doi: 10.1111/jnc.13883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hui AS, Striet JB, Gudelsky G, Soukhova GK, Gozal E, Beitner-Johnson D, Guo SZ, Sachleben LR Jr, Haycock JW, Gozal D, Czyzyk-Krzeska MF. Regulation of catecholamines by sustained and intermittent hypoxia in neuroendocrine cells and sympathetic neurons. Hypertension 42: 1130–1136, 2003. doi: 10.1161/01.HYP.0000101691.12358.26. [DOI] [PubMed] [Google Scholar]
- 20.Hussain A, Suleiman MS, George SJ, Loubani M, Morice A. Hypoxic pulmonary vasoconstriction in humans: tale or myth. Open Cardiovasc Med J 11: 1–13, 2017. doi: 10.2174/1874192401711010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inoue M, lin H, Imanaga I, Ogawa K, Warashina A. InsP3 receptor type 2 and oscillatory and monophasic Ca2+ transients in rat adrenal chromaffin cells. Cell Calcium 35: 59–70, 2004. doi: 10.1016/S0143-4160(03)00172-6. [DOI] [PubMed] [Google Scholar]
- 22.Ivy CM, Scott GR. Control of breathing and ventilatory acclimatization to hypoxia in deer mice native to high altitudes. Acta Physiol (Oxf) 221: 266–282, 2017. doi: 10.1111/apha.12912. [DOI] [PubMed] [Google Scholar]
- 23.Ivy CM, Scott GR. Evolved changes in breathing and CO2 sensitivity in deer mice native to high altitudes. Am J Physiol Regul Integr Comp Physiol 315: R1027–R1037, 2018. doi: 10.1152/ajpregu.00220.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson TS, Young JB, Landsberg L. Sympathoadrenal responses to acute and chronic hypoxia in the rat. J Clin Invest 71: 1263–1272, 1983. doi: 10.1172/JCI110876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leszczyszyn DJ, Jankowski JA, Viveros OH, Diliberto EJ Jr, Near JA, Wightman RM. Nicotinic receptor-mediated catecholamine secretion from individual chromaffin cells. Chemical evidence for exocytosis. J Biol Chem 265: 14736–14737, 1990. [PubMed] [Google Scholar]
- 26.Lui MA, Mahalingam S, Patel P, Connaty AD, Ivy CM, Cheviron ZA, Storz JF, McClelland GB, Scott GR. High-altitude ancestry and hypoxia acclimation have distinct effects on exercise capacity and muscle phenotype in deer mice. Am J Physiol Regul Integr Comp Physiol 308: R779–R791, 2015. doi: 10.1152/ajpregu.00362.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahalingam S, McClelland GB, Scott GR. Evolved changes in the intracellular distribution and physiology of muscle mitochondria in high-altitude native deer mice. J Physiol 595: 4785–4801, 2017. doi: 10.1113/JP274130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahata SK, Zheng H, Mahata S, Liu X, Patel KP. Effect of heart failure on catecholamine granule morphology and storage in chromaffin cells. J Endocrinol 230: 309–323, 2016. doi: 10.1530/JOE-16-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mamet J, Peyronnet J, Roux JC, Perrin D, Cottet-Emard JM, Pequignot JM, Lagercrantz H, Dalmaz Y. Long-term prenatal hypoxia alters maturation of adrenal medulla in rat. Pediatr Res 51: 207–214, 2002. doi: 10.1203/00006450-200202000-00014. [DOI] [PubMed] [Google Scholar]
- 30.Matussek N, Angst J, Benkert O, Gmür M, Papousek M, Rüther E, Woggon B. The effect of L-5-hydroxytryptophan alone and in combination with a decarboxylase inhibitor (Ro-4-4602) in depressive patients. Adv Biochem Psychopharmacol 11: 399–404, 1974. [PubMed] [Google Scholar]
- 31.McClelland GB, Scott GR. Evolved mechanisms of aerobic performance and hypoxia resistance in high-altitude natives. Annu Rev Physiol 81: 561–583, 2019. doi: 10.1146/annurev-physiol-021317-121527. [DOI] [PubMed] [Google Scholar]
- 32.Moore LG, Young D, McCullough RE, Droma T, Zamudio S. Tibetan protection from intrauterine growth restriction (IUGR) and reproductive loss at high altitude. Am J Hum Biol 13: 635–644, 2001. doi: 10.1002/ajhb.1102. [DOI] [PubMed] [Google Scholar]
- 33.Natarajan C, Hoffmann FG, Lanier HC, Wolf CJ, Cheviron ZA, Spangler ML, Weber RE, Fago A, Storz JF. Intraspecific polymorphism, interspecific divergence, and the origins of function-altering mutations in deer mouse hemoglobin. Mol Biol Evol 32: 978–997, 2015. doi: 10.1093/molbev/msu403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ojino K, Shimazawa M, Izawa H, Nakano Y, Tsuruma K, Hara H. Involvement of endoplasmic reticulum stress in optic nerve degeneration after chronic high intraocular pressure in DBA/2J mice. J Neurosci Res 93: 1675–1683, 2015. doi: 10.1002/jnr.23630. [DOI] [PubMed] [Google Scholar]
- 35.Podvin S, Bundey R, Toneff T, Ziegler M, Hook V. Profiles of secreted neuropeptides and catecholamines illustrate similarities and differences in response to stimulation by distinct secretagogues. Mol Cell Neurosci 68: 177–185, 2015. doi: 10.1016/j.mcn.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Preibisch S, Saalfeld S, Tomancak P. Globally optimal stitching of tiled 3D microscopic image acquisitions. Bioinformatics 25: 1463–1465, 2009. doi: 10.1093/bioinformatics/btp184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rahman MK, Rahman F, Rahman T, Kato T. Dopamine-β-hydroxylase (DBH), its cofactors and other biochemical parameters in the serum of neurological patients in bangladesh. Int J Biomed Sci 137: S28, 2009. doi: 10.1016/j.ijcard.2009.09.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scott AL, Zhang M, Nurse CA. Enhanced BDNF signalling following chronic hypoxia potentiates catecholamine release from cultured rat adrenal chromaffin cells. J Physiol 593: 3281–3299, 2015. doi: 10.1113/JP270725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scott GR, Elogio TS, Lui MA, Storz JF, Cheviron ZA. Adaptive modifications of muscle phenotype in high-altitude deer mice are associated with evolved changes in gene regulation. Mol Biol Evol 32: 1962–1976, 2015. doi: 10.1093/molbev/msv076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shirvan MH, Pollard HB, Heldman E. Mixed nicotinic and muscarinic features of cholinergic receptor coupled to secretion in bovine chromaffin cells. Proc Natl Acad Sci USA 88: 4860–4864, 1991. doi: 10.1073/pnas.88.11.4860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Slotkin TA, Seidler FJ, Haim K, Cameron AM, Antolick L, Lau C. Neonatal central catecholaminergic lesions with intracisternal 6-hydroxydopamine: effects on development of presynaptic and postsynaptic components of peripheral sympathetic pathways and on the ornithine decarboxylase/polyamine system in heart, lung and kidney. J Pharmacol Exp Ther 247: 975–982, 1988. [PubMed] [Google Scholar]
- 42.Snyder GK, Black CP, Birchard GF. Development and metabolism during hypoxia in embryos of high-altitude anser indicus versus sea-level branta-canadensis geese. Physiol Zool 55: 113–123, 1982. doi: 10.1086/physzool.55.2.30155845. [DOI] [Google Scholar]
- 43.Souvatzoglou A. The sympathoadrenal system: integrative regulation of the cortical and the medullary adrenal functions. In: Adrenal Glands: Diagnostic Aspects and Surgical Therapy, edited by Linos D and van Heerden JA. Berlin: Springer-Verlag, 2005, p. 33–39. [Google Scholar]
- 44.Storz JF, Cheviron ZA. Functional genomic insights into regulatory mechanisms of high-altitude adaptation. Adv Exp Med Biol 903: 113–128, 2016. doi: 10.1007/978-1-4899-7678-9_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Storz JF, Natarajan C, Cheviron ZA, Hoffmann FG, Kelly JK. Altitudinal variation at duplicated β-globin genes in deer mice: effects of selection, recombination, and gene conversion. Genetics 190: 203–216, 2012. doi: 10.1534/genetics.111.134494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Storz JF, Runck AM, Sabatino SJ, Kelly JK, Ferrand N, Moriyama H, Weber RE, Fago A. Evolutionary and functional insights into the mechanism underlying high-altitude adaptation of deer mouse hemoglobin. Proc Natl Acad Sci USA 106: 14450–14455, 2009. doi: 10.1073/pnas.0905224106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Su J, Gao T, Shi T, Xiang Q, Xu X, Wiesenfeld-Hallin Z, Hökfelt T, Svensson CI. Phenotypic changes in dorsal root ganglion and spinal cord in the collagen antibody-induced arthritis mouse model. J Comp Neurol 523: 1505–1528, 2015. doi: 10.1002/cne.23749. [DOI] [PubMed] [Google Scholar]
- 48.Takeuchi Y, Mochizuki-Oda N, Yamada H, Kurokawa K, Watanabe Y. Nonneurogenic hypoxia sensitivity in rat adrenal slices. Biochem Biophys Res Commun 289: 51–56, 2001. doi: 10.1006/bbrc.2001.5913. [DOI] [PubMed] [Google Scholar]
- 49.Tate KB, Ivy CM, Velotta JP, Storz JF, McClelland GB, Cheviron ZA, Scott GR. Circulatory mechanisms underlying adaptive increases in thermogenic capacity in high-altitude deer mice. J Exp Biol 220: 3616–3620, 2017. doi: 10.1242/jeb.164491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thompson RJ, Farragher SM, Cutz E, Nurse CA. Developmental regulation of O(2) sensing in neonatal adrenal chromaffin cells from wild-type and NADPH-oxidase-deficient mice. Pflugers Arch 444: 539–548, 2002. doi: 10.1007/s00424-002-0853-6. [DOI] [PubMed] [Google Scholar]
- 51.Thompson RJ, Jackson A, Nurse CA. Developmental loss of hypoxic chemosensitivity in rat adrenomedullary chromaffin cells. J Physiol 498: 503–510, 1997. doi: 10.1113/jphysiol.1997.sp021876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Torres-Rosas R, Yehia G, Peña G, Mishra P, del Rocio Thompson-Bonilla M, Moreno-Eutimio MA, Arriaga-Pizano LA, Isibasi A, Ulloa L. Dopamine mediates vagal modulation of the immune system by electroacupuncture. Nat Med 20: 291–295, 2014. doi: 10.1038/nm.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Velotta JP, Senner NR, Wolf CJ, Schweizer RM, Cheviron ZA. Convergent evolution of physiological and genomic responses to hypoxia in peromyscus mice. Integr Comp Biol 58: E242, 2018. [Google Scholar]
- 54.Verstegen AMJ, Vanderhorst V, Gray PA, Zeidel ML, Geerling JC. Barrington’s nucleus: neuroanatomic landscape of the mouse “pontine micturition center”. J Comp Neurol 525: 2287–2309, 2017. doi: 10.1002/cne.24215. [DOI] [PMC free article] [PubMed] [Google Scholar]