Abstract
Immunoglobulin E (IgE) is the key immunoglobulin in the pathogenesis of IgE associated allergic diseases affecting 30% of the world population. Recent data suggest that allergen-specific IgE levels in serum of allergic patients are sustained by two different mechanisms: inducible IgE production through allergen exposure, and continuous IgE production occurring even in the absence of allergen stimulus that maintains IgE levels. This assumption is supported by two observations. First, allergen exposure induces transient increases of systemic IgE production. Second, reduction in IgE levels upon depletion of IgE from the blood of allergic patients using immunoapheresis is only temporary and IgE levels quickly return to pre-treatment levels even in the absence of allergen exposure. Though IgE production has been observed in the peripheral blood and locally in various human tissues (e.g., nose, lung, spleen, bone marrow), the origin and main sites of IgE production in humans remain unknown. Furthermore, IgE-producing cells in humans have yet to be fully characterized. Capturing IgE-producing cells is challenging not only because current staining technologies are inadequate, but also because the cells are rare, they are difficult to discriminate from cells bearing IgE bound to IgE-receptors, and plasma cells express little IgE on their surface. However, due to the central role in mediating both the early and late phases of allergy, free IgE, IgE-bearing effector cells and IgE-producing cells are important therapeutic targets. Here, we discuss current knowledge and unanswered questions regarding IgE production in allergic patients as well as possible therapeutic approaches targeting IgE.
Keywords: allergy, IgE, human, mouse, CD23, FcεRI, B cell, T cell, tracing, targeting, therapy
1. Introduction
Immunoglobulin E (IgE) associated allergic diseases in their various forms affect approximately 30% of the world population. Symptoms may range from relatively mild, such as rhinoconjunctivitis, to potentially life-threatening, such as asthma or anaphylaxis. Development of allergic disease is associated both with environmental and individual genetic factors [1]. The latter includes a genetic predisposition towards allergen-specific immune responses and factors promoting Th2 responses as well as IgE production [1,2,3]. IgE is a key player in development and maintenance of allergic disease. Symptoms of the early phase of allergic inflammation are driven by mediators released from basophils and mast cells upon allergen-induced crosslinking of IgE bound to its high affinity surface receptor (FcεRI) [4,5]. Furthermore, IgE also contributes to the enhancement of the late phase response. IgE is present on the surface of antigen presenting cells (APCs) bound to FcεRI or the low affinity IgE receptor CD23. Allergen-IgE complexes are internalized by these receptor on APCs and presented via major histocompatibility complex II (MHCII), thus augmenting allergen-specific T cell responses [6,7,8,9]. IgE may also bind to soluble IgE receptors and IgE-binding proteins, e.g., soluble CD23 or epsilon binding protein [10].
Among all immunoglobulin subclasses, IgE stands out with respect to function, half-life, and low serum concentration. With a serum concentration of 5 × 10−5 mg/mL [11] it represents only 0.0005% of total free serum Igs in non-atopic adults [12]. Its half-life of 2 days within the serum is rather short compared to the half-life of, for example, IgG1, lasting 21 days [11]. In contrast to many other subclasses, IgE does not activate complement but exerts its role through binding to its cognate receptors [11]. It has been shown that long-lasting removal of IgE from the circulation is difficult to achieve. After extracorporeal immunoadsorption, IgE levels return to baseline within a week after treatment, even in the absence of exogenous allergen stimulus [13]. This shows that despite its low abundance, IgE is continuously produced to maintain constant IgE levels in blood.
The central role of IgE in mediating allergic diseases makes it an important and attractive target for development of novel therapeutic approaches [14,15,16]. So far, only the anti-IgE antibody omalizumab has been marketed and successfully reduces the burden of severe and otherwise uncontrollable asthma [17,18,19]. Several new approaches such as depleting IgE through extracorporal IgE immunoabsorption [13,20] as well as specifically targeting effector [21,22] or IgE+ B cells [23] are being explored and will be discussed in this review.
2. IgE Production
The pathway of B cell differentiation with respect to the nature and location of potential IgE+ memory cells as well as long-lived IgE producing plasma cells in allergy is still not completely understood. Investigation of human IgE responses are impeded by the fact that IgE-producing cells and B cells are rare in human tissues that are easy to access, such as blood, nasal mucosa, or tonsils [24,25,26,27]. Therefore, most of our current knowledge on the mechanisms underlying IgE production is based on data from murine models. Advances in this field have been made in the past decade due to the generation of fluorescent protein reporter IgE mice [28]. Experiments in these mice led to the perception that IgE expressing B cells only transiently contribute to the germinal center reaction and are rather biased towards a plasma cell fate [29,30].
2.1. Murine Models to Investigate IgE-Production and Allergic Disease
Murine models are very valuable for our understanding of general mechanisms of allergy in humans as well as for testing novel therapeutic approaches, but it is necessary to bear several differences in mind (Table 1). The cytokine milieu required for induction of class switch to IgE differs between mice and humans. While IL-4 alone directs class switching to IgE and IgG1 in mice [31], both IL-4 and IL-13 contribute to IgE synthesis in humans [32]. In addition, mice do not mount IgG4 subclass responses like allergic patients. With regard to allergen epitope specific Ig responses, the two species differ. Recent studies have indicated that the allergen-specific IgE responses in mice develop by switching from IgG1 to IgE [29]. In this respect, it has been shown that IgG raised in mice towards the major grass pollen allergens Phl p 1, 2, 5, or dog albumin were able to block IgE binding, which indicates that, at least for these selected allergens, IgG and IgE of sensitized mice recognize the same epitope [33]. However, direct class switching from IgM to IgE has also been observed in mice [34]. In contrast, IgE and IgG in humans have been shown to recognize distinct epitopes of the allergen [35,36]. This is also supported by the fact that only IgE but no other immunoglobulin subclass is boosted upon seasonal allergen exposure [37,38].
Table 1.
Comparison of allergy in humans and murine models.
| Mice | Humans | |
|---|---|---|
| Genetic background | Inbred | Outbred |
| Percentage lymphocytes of total leukocytes | 75–90% [39] | 30–50% [40] |
| IgG subclasses | IgG1, IgG2, and IgG3 | IgG1, IgG2, IgG3, and IgG4 |
| IgE receptors on eosinophils | No FcεRI [41] | FcεRI [42] |
| Access to tissue for analysis | All tissues available | Limited access—mainly blood |
| Asthma development | Induced by sensitization, sometimes Th1-like | Induced by natural allergen exposure, mostly Th2-like |
| Allergy | Induced by sensitization [43,44,45] | Spontaneous by natural allergen exposure |
| IgE epitopes of respiratory allergens | Mainly sequential | Mainly conformational |
| T cell epitopes of respiratory allergens | Dominating T cell epitopes | High diversity |
| Cytokines required for IgE class switch | IL-4 [31] | IL-4, IL-13 [32] |
| Mechanisms of class-switch to IgE | Mainly sequential [33] | Evidence for sequential [46,47,48,49,50,51,52] and non-sequential [53] |
| Rise in Ig subtype in response to allergen challenge | IgE, IgG1 | IgE, IgG4 |
With respect to the composition of the immune system, there are further differences to consider. While lymphocytes are the predominant mononuclear cells present in the peripheral blood of mice (representing 75–90% of total leukocytes [39]), neutrophils (50–70%) are predominant in humans with lymphocytes comprising only 30–50% of all leukocytes [40].
Another caveat is that mouse models mimicking allergic rhinitis, a key feature of human disease, are rare and are hampered by technical difficulties such as measuring isolated nasal obstruction. Additionally, the nasal anatomy of mice differs from human anatomy [54,55]. In allergic asthma, murine models have provided important insights into immunologic mechanisms. However, several shortcomings are clearly visible: Mice are inbred and live in specific pathogen-free facilities, whereas humans bear individual genetic backgrounds and are continuously exposed to a myriad of environmental agents, many of them known to influence and modulate airway inflammation [56,57]. Human airways are, for instance, richer in submucosal glands and show a greater variety of airway structure [56]. In terms of pathophysiological mechanisms, there are marked differences in eosinophil degranulation and infiltration as well as in the effects of mast cell degranulation on bronchoconstriction between mice and humans [57]. In fact, murine models of asthma sometimes resemble features of Type IV hypersensitivity rather than Type I allergy [3,58,59]. To summarize, there are some important differences between murine and human allergic responses that need to be taken into account when interpreting murine data.
2.2. Human IgE Production
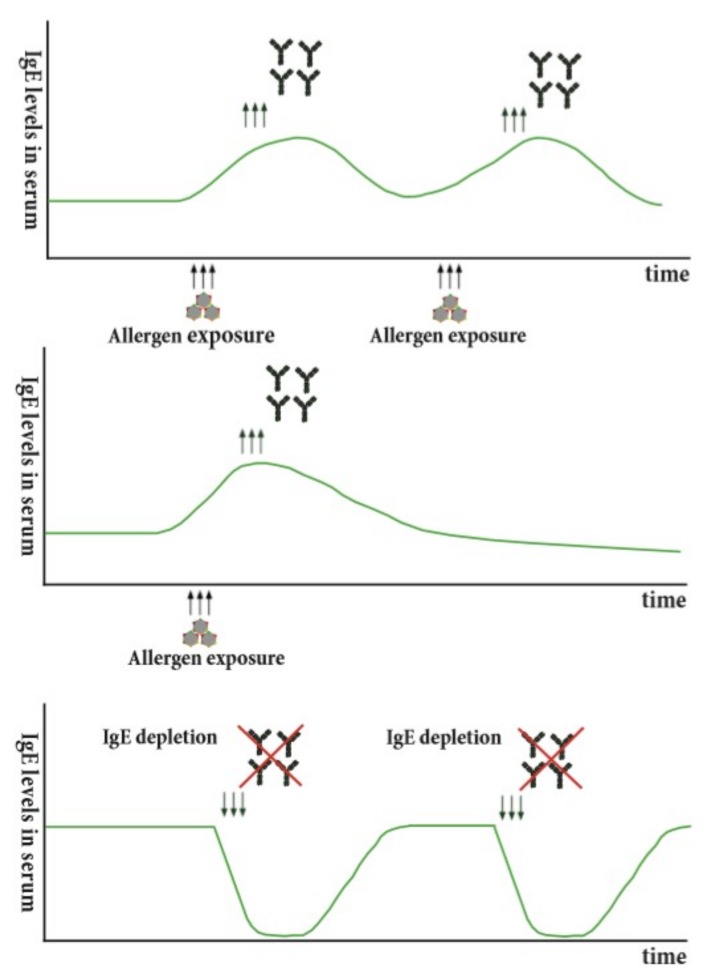
In humans, allergen-specific IgE memory persists over years or even decades, even in the absence of antigenic stimulation [60]. At the same time, seasonal allergen exposure can induce a rapid increase of allergen-specific IgE levels and boosts IgE levels [37,61]. These findings indicate that there may be two different processes governing IgE production: one that continuously replenishes the IgE pool—perhaps long-lived plasma cells [62,63]—and another that is inducible upon allergen contact. In fact, there are various studies in favor of this hypothesis. Firstly, the observation that the reduction in IgE levels after depletion of IgE from human blood using immune apheresis is only temporary and returns to pre-treatment levels within a week in the absence of antigenic stimulus [13,20] (Figure 1, Bottom) indicates the presence of continuous IgE production. The mechanism how this IgE production is maintained is not fully understood. It is conceivable that repeated boosts of allergen-specific IgE production are required to keep this IgE production ongoing (Figure 1, Top). Activation could either be achieved by repeated allergen-contact (e.g., seasonal exposure) or in the absence of this also by polyclonal activation (e.g., microbial stimulation, bystander T-help) as has been described for the maintenance of antigen-specific IgG memory [64]. If repeated activation sustains IgE levels and the specificities of IgE, a lack of antigenic exposure should lead to a slow decline in IgE production (Figure 1, Middle). This could be investigated in the context of allergy by relocation of an allergic patient to an allergen-free area for seasonal allergens or dietary restriction for food derived allergens. In support of this, it has been observed that lack of antigen exposure in the context of egg or cow-milk allergy leads to a decline in allergen-specific IgE levels [65,66,67].
Figure 1.
Potential mechanisms for maintenance of continuous IgE production. (Top) Repeated allergen exposure maintains IgE production. (Middle) Lack of allergenic stimulation leads to a slow steady decline in IgE production. (Bottom) Depletion of IgE, e.g., using IgE immune adsorption, leads only to a temporary decline of IgE levels, which subsequently return to baseline levels.
Aside from continuous IgE production there appears to be a second mechanism where IgE production is boosted upon allergen contact as seasonal allergen exposure via the respiratory mucosa has been shown to induce a strong increase of allergen-specific IgE levels [37,61]. A rise in allergen-specific T cell proliferation has also been observed during the season [68], but within individual patients B and T cell responses are poorly associated [69]. In humans the origin of IgE-switched B cells is not yet entirely clear [52] with studies both in favor of direct and sequential class switching. The observation that upon seasonal allergen exposure only established allergen-specific memory is boosted [37] supports the hypothesis of direct class switching and suggests the existence of IgE+ memory B cells that can be stimulated upon allergen contact. Furthermore, no de novo sensitizations [70] or changes in allergen-specific IgM, IgG, or IgA production [37,38] have been described upon allergen challenge in humans.
However, there is also evidence indicating that sequential class switching occurs in humans [46,47,48,49,50,51,52]. In this respect, the presence of sγ switch circles was observed in supernatant of nasal mucosa explants [47]. Furthermore, IgH repertoire analysis in blood in allergic patients showed that IgE cells may also derive from secondary isotype switching of mutated IgG1 expressing cells [46,52]. In this line, treatment with the human monoclonal antibody dupilumab, which blocks IL-4 and IL-13, reduced total IgE levels in clinical trials, thus also indicating that de novo class switching may occur in humans [71,72,73,74]. However, when interpreting sequencing data several aspects need to be considered carefully. Firstly, next generation sequencing only analyzes heavy and not light chain sequences. Furthermore, so far it is not possible to investigate the allergen-specificity through the use of this technique. Although IgE+ memory cells have been observed in the blood of allergic subjects [46], their switch origin and their contribution to IgE production has not been elucidated. Additionally, the relative contribution of direct versus sequential class switching to the IgE pool is not yet fully understood either.
In summary, continuous IgE production, most likely by long-lived plasma cells as well as by memory cells rapidly responding to allergen by IgE production, seem to contribute to the maintenance of allergen-specific IgE levels in allergic patients despite the short half-life of IgE.
3. Tracing IgE Producing Cells
Another open question is where the major sites of IgE production are in humans. The majority of studies investigating mechanisms of human IgE production have used peripheral blood mononuclear cells (PBMCs) [24,75,76,77] or tonsil derived B cells [78,79]. Though in vitro cultures using isolated PBMCs have provided important insights on the role of specific T cell help and IL-4 in IgE production [77,80,81,82,83], these studies are not helpful for understanding allergen-specific IgE production because IL-4 stimulation induces class-switching in any IgM+ B cells but does not influence allergen-specific IgE production [77]. Since the demonstration that one can isolate allergen-specific IgE Fabs from the peripheral blood of allergic patients by combinatorial cloning [84], it is clear that cells producing allergen-specific IgE occur in the blood of allergic patients, but the nature of these cells needs to be characterized in more detail [25]. In order to claim that one has identified a major site of IgE production in allergic patients, one would need to demonstrate that the IgE-producing cells in blood are derived from this site and/or that the majority of IgE is synthesized at this particular site [25,85].
3.1. Identification of IgE Producing Cells by IgE Staining Techniques
The assumption that IgE-producing cells are mainly localized in the bone marrow and other human lymphoid compartments is difficult to prove because these tissues are quite difficult to access in humans. It seems to be more convenient to work with human peripheral blood; however, the amount of IgE-producing cells in human blood is minimal and there are numerous cell types that carry IgE on their surface but do not produce it. In this section, we summarize current approaches for the identification of IgE-producing cells.
3.1.1. ELISpot
One possible method for detecting IgE-producing cells in the blood is the ELISpot assay. This method is more effective for plasmablasts than for the determination of memory B cells, as the latter do not secrete antibodies. In order to obtain antibody-secreting cells from B cells, stimulation with CD40L and IL-4 or allergen is usually performed [86,87]. However, as pointed out, this stimulation does not amplify existing allergen-specific IgE production but leads to de novo class switching of IgM+ BCR bearing cells of unknown specificity into IgE producing cells [77,86,87].
3.1.2. Flow Cytometry
The most commonly used method for detecting and isolating putative IgE-producing B-lymphocytes is flow cytometry, which is based on the determination of surface markers of target cells. There are several approaches for IgE+ B lymphocyte detection. For the step-by-step determination of the total subpopulation of IgE+ B lymphocytes from the PBMC pool, first, non-relevant cells (e.g., T cells, monocytes) are excluded by cells expressing the following markers: CD3, CD14, CD16, CD235a, and CD123. Consequently, IgE+ B lymphocytes are identified by positive staining for CD19 as well IgE. However, this approach does not exclusively identify IgE+ memory cells as IgE can be present on the B cell surface not only in the form of the IgE B cell receptor (BCR) but also bound to its low affinity receptor CD23 [88]. This fact needs to be considered carefully, as it may lead to overestimation of the number of IgE+ B cells.
In addition to the above-stated panel, antibodies to CD23 can be added, which should help to exclude false-positive events from the gate of IgE+ cells. However, by excluding all CD23+ lymphocytes, one may potentially remove the subset of IgE-producing cells that express CD23 at the same time.
Compared to the previous approach a more successful strategy may be enrichment of B lymphocytes by using magnetic separation or RosetteSep prior to antibody staining [89,90]. This has two main advantages: Firstly, the number of cells required for measurement is strongly decreased. Secondly, antibodies targeting further B cell-related surface markers may be implemented as no markers for exclusion of irrelevant cells are necessary.
Antigen-specific labeling of B lymphocytes is in general a successful method for isolating B cells responding to a particular antigen [91]. A crucial determinant in this approach is the production of antigen with a high level of fluorescence. A high level of fluorescence can be achieved using tetrameric technology, by which a complex is preformed from fluorescently labeled avidin bound to four biotinylated antigen molecules [92].
Taking into consideration the low percentage of target cells compared to background levels, it may be preferable to use several antigen conjugates labeled with different fluorescent labels in order to show reproducible target cell isolation.
For a more reliable isolation of IgE+ B lymphocytes, this strategy can be combined with additional staining for other surface Igs (IgM, IgD, IgG, IgA) [93,94]. Jiménez-Saiz et al. [86] applied this strategy for the isolation of IgE+ memory cells from human blood. They stained B cells enriched from PBMCs by magnetic separation with anti-IgM, anti-IgD, anti-IgG, anti-IgA, and anti-IgE antibodies. Cells were analyzed and IgE+ subpopulations were isolated by using flow cytometry. The purity of the IgE+ subpopulation was then confirmed by single cell sequencing and revealed that only 0.0019% of total B cells were putative IgE+ memory B cells [86] and that these cells were rarer than previously reported [46,89]. This very low percentage is rather challenging since it is even less than the frequency of residual tumor populations, which is observed in minimal residual disease [95].
Furthermore, circulating IgE+ B lymphocytes can be divided into plasmablasts and memory cells based on antibodies against CD27, CD38, and CD138 [25]. Using this approach, Heeringa et al. identified the following IgE+ subsets from donor blood samples: IgE+CD27− memory B cells (CD19+CD21+CD38dimIgD-IgM-IgE+CD27-), IgE+CD27+ memory B cells (CD19+CD21+CD38dimIgD-IgM-IgE+CD27+), and IgE+ plasmablasts (CD19+CD38highCD27+IgM-IgD-IgE+) [89]. Alternatively, plasmablasts can also be identified using cellular affinity matrix technology in negatively enriched B cells [24].
To summarize, the major obstacles in any attempt to characterize IgE-producing plasma cells and potentially IgE memory B cells in the blood of allergic patients are the small numbers of allergen-specific lymphocytes [24,86] and the difficulty in clearly identifying them by flow cytometry [28].
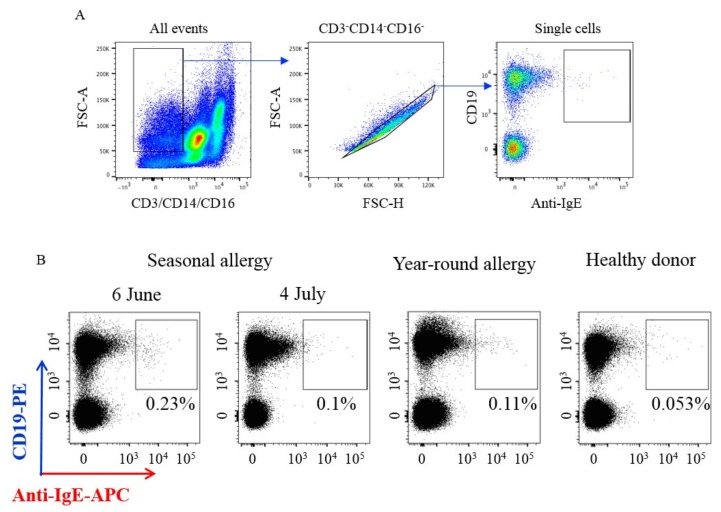
In an attempt to overcome this obstacle, we have recently developed an approach where cells were stained for CD19 in combination with a fluorescently labelled monoclonal anti-IgE antibody, which discriminates membrane-bound IgE from receptor-bound IgE in the blood of birch pollen allergic patients (Figure 2).
Figure 2.
Isolation of IgE+ B lymphocytes from PBMC of donors with different type of allergy. (A) Gating strategy for detection of IgE+ B lymphocyte. B lymphocyte subset were gated as CD3-CD14-CD16- (FSC area/CD3, CD14, CD16), single cells (FSC area/FSC height), CD19+, and then detection of IgE+ B cells was based on surface IgE expression. (B) Example of different percentage of IgE+ B cells in donor samples with different types of allergy. The highest percentage of IgE+ cells were detected in a donor sample with seasonal allergy shortly after the pollen season (6th June) and they disappeared a month later (4th July). As a control, we used blood samples from healthy donors, as well as donors with year-round allergies.
To detect IgE-producing B lymphocytes of patients with seasonal birch pollen allergy, we isolated PBMCs and stained them with a cocktail of antibodies CD19-PE, CD3-FITC, CD14-FITC, CD16-FITC, and anti-IgE-APC. Figure 2 shows that IgE-producing B-lymphocytes accounted for 0.23% of subpopulations of CD19+ cells after the birch pollen season (Figure 2, 6th June) and after one more month (Figure 2, 4th July) this subpopulation disappeared. This would suggest that IgE-producing cells appear in the peripheral blood after allergen exposure. In fact, they seem to be present in allergic patients with a perennial allergy (Figure 2, Year-around allergy) and are absent in a healthy non-allergic donor blood (Figure 2, Healthy donor).
3.2. Sites of IgE Production
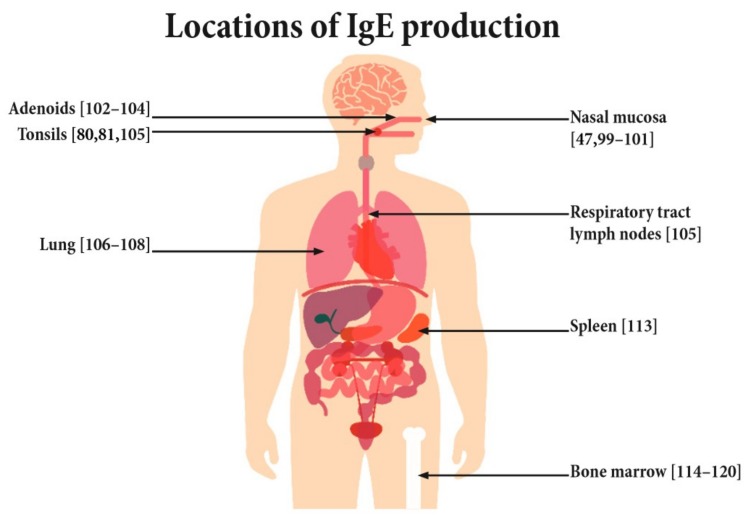
Though both IgE-producing plasma cells and IgE+ memory cells have been observed in the blood of allergic patients [24,46,86,89], they are scarce and produce only around 0.2% of the IgE present in the serum [25]. Thus, the majority of IgE producing cells and IgE memory are thought to reside elsewhere [85]. Human B memory cells, for example, in response to vaccinations, have been shown to reside not only in the bone marrow but also in other different lymphatic organs such as the spleen and tonsils [91,96,97,98]. Likewise, IgE production has been suggested to occur locally at several different sites throughout the body (Figure 3). For example, at the nasal mucosa, the site of first contact for airborne allergens, increased numbers of IgE-positive B and plasma cells have been observed [99]. The presence of local IgE synthesis by the detection of ε germline and ε circle transcripts have been shown in nasal mucosa biopsies from allergic patients after ex vivo challenge with the respective allergen [47,100,101,102]. In addition, there are various other lymphatic tissues of the upper respiratory tract such as adenoids and tonsils that are potential sites of antigen encounter after uptake of the antigen by the nasal mucosa. These lymphatic tissues have also been shown to harbor IgE+ cells [102,103] and the production of IgE has been shown [78,79,104,105]. Similarly, IgE transcripts and antibodies have been detected in the sputum and lungs of allergic and asthmatic patients [106,107,108]. However, it should be noted that blood-derived cells (which includes IgE producing cells) also contribute to the cellular population of these lymphatic and respiratory tissues, and therefore it is difficult to say if these tissues really are predominant sites for IgE production.
Figure 3.
Potential sites of IgE production.
As sites known to house large number of memory cells, both the spleen and bone marrow are potential sites of interest [91,97,109,110,111]. However, the contribution of the spleen in maintaining even the IgG repertoire and memory is still discussed. Although vaccinia virus-specific memory B cells are present in the spleen of patient’s even decades after smallpox has been eradicated [112], splenectomized individuals mount comparable anti-tetanus toxoid IgG levels upon revaccination to normal healthy patients [91]. Even less is known about the presence of IgE production in the spleen. Only one report observed the presence of IgE production in the spleen of an asthmatic patient who tragically died from an asthma attack [113]. With regard to the presence of IgE production in bone marrow there are several observations from patients receiving allogenic donations, which show the transmission of allergy after bone marrow transplantation [114,115,116,117,118,119,120]. However, results are somewhat ambiguous. Some reports observe matching sensitization profiles between donors and recipients [114,115,116,117] whilst others show that recipients may not acquire all the allergies of the donor [118,120] or may even acquire additional de novo sensitizations [119].
The presence of IgE production has so far been shown at various lymphatic sites; however, the relative contribution of each of these areas to the continuous and de novo production in allergic responses remains unanswered. One may speculate that lymphatic tissues at the sites of allergen exposure (e.g., nasal mucosa, gut mucosa) contain IgE+ memory cells, which can be activated, whereas plasma cells in bone marrow may be responsible for the continuous long-lived IgE production. Nevertheless, further investigations are needed to identify the sites of IgE production in allergic patients.
4. Role of IgE in Mediating Immediate Allergic Symptoms and T Cell-Mediated Allergic Inflammation
IgE plays an important role in propagating allergic responses both in the early and late phases of allergic immune responses. In the early phase, IgE is central for mast and basophil degranulation upon allergen contact; however, the timely interplay between increases in IgE levels in the blood upon allergen challenge and potential rises in effector cell sensitivity are not yet fully understood. In the late phase, IgE-bearing B cells contribute to increased allergen-specific T cell response via internalization and presentation of IgE-allergen complexes via CD23 in a process called IgE-facilitated allergen presentation.
4.1. Connecting IgE Production to Clinical Effect—How Circulating IgE Influences Mast Cell and Basophil Sensitivity
The major players propagating the early symptoms experienced by allergic individuals in immediate hypersensitivity reactions are basophils and mast cells. Both of these cells utilize IgE to mediate their allergic responses, and thus understanding the kinetics of how circulating IgE influences the reactivity profile of these cells is crucial to treating the disease. Basophils and mast cells are similar in that they are both granulocytes, both are derived from hematopoietic stem cells, and both contain pre-formed intracellular granules that are rapidly exocytosed from the cell membrane upon FcεRI cross linking in response to IgE allergen recognition [121]. Despite their similarities there are some key differences to consider. Firstly, mast cells are long-lived tissue-resident cells, surviving for months. Basophils on the other hand circulate in the blood and survive only for a few days. Basophils terminally differentiate in the bone marrow whereas mast cells do so in the tissues. Additionally, basophils do not usually proliferate after maturation whereas mast cells can in order to self-renew [122].
Due to the high affinity of FcεRI for IgE, receptor bound IgE—in contrast to free IgE—has a relatively long half-life of 2–3 weeks [123]. Additionally, circulating IgE has been shown to have a positive effect on the stability and expression of FcεRI expression on mast cells and basophils by preventing receptor internalization and degradation [124]. These two mechanisms of slow off rate and increased FcεRI expression and stability mean that once IgE is bound to its receptor it remains bound most likely for the lifespan of the host cell. Thus, it would be reasonable to presume that the specificity of IgE in the circulation will reflect the reactivity profile of mast cells and basophils. This has indeed been shown to be the case in that specific serum IgE levels correlate quite well with skin prick test results in adults [125,126].
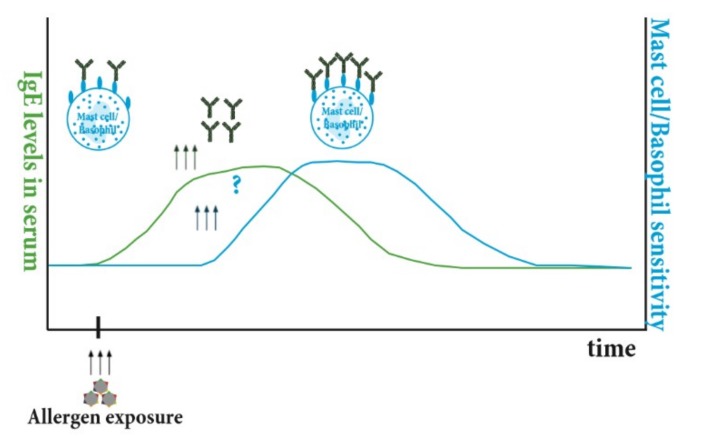
Nasal allergen exposure in pre-sensitized individuals leads to a rise in allergen specific IgE with peak serum levels occurring 4–6 weeks after exposure [37,127,128]. Taking into consideration the differences in life span of basophils and mast cells it would be logical to assume that basophils would be the first to reflect this change in the IgE repertoire followed later by mast cells (Figure 4). So far there is no study directly addressing this question; however, conclusions can be drawn from allergen challenge or therapeutic studies removing IgE from the circulation. Upon intranasal allergen challenge, no change in basophil sensitivity was observed within the observation period of 3 weeks after peak allergen-specific IgE increase [128]. However, in an extracorporeal IgE-specific immuneapheresis study by Lupinek et al., a reduction in basophil sensitivity was observed 8 weeks after start of the treatment [13]. Similarly, basophil sensitivity continuously dropped after neutralization of IgE with the anti-IgE antibody omalizumab [129,130,131,132]. IgE surface levels on basophils were undetectable 4 weeks after omalizumab treatment but rose significantly 8 weeks after cessation of treatment [133]. Thus, these data indicate that there is a time window of at least 4–8 weeks for changes in IgE levels to be reflected in changes of basophil sensitivity. With regards to changes in skin prick tests (SPT), which act as a surrogate of mast cell sensitivity, the seasonal increase in SPT sensitivity observed several weeks after the start of the study in birch allergic control patients was absent in patients undergoing IgE immunoapheresis [13]. Furthermore, a reduction in SPT reactivity was also observed in omalizumab-treated patients within 3 months of treatment [131,134]. This indicates that it takes longer for mast cells than basophils to adapt to changes in IgE levels, as expected, due to their longer life-span. However, a detailed study addressing the timely interplay has not been performed yet and would be needed for a better understanding of how the kinetics of the IgE response transform into clinical sensitivity.
Figure 4.
Timely interplay of rises in IgE levels and mast cell and basophil sensitivity. Upon allergen exposure there is a rise in allergen-specific IgE levels (green line) followed by an increase in basophil and mast cell sensitivity (blue line).
4.2. Importance of IgE-Facilitatated Allergen Presentation Mediated by CD23 and Rules Guiding this Process
The activation of allergen-specific T cells by APCs plays an important role in the development of an allergic reaction, especially in mediating late phase reactions [135]. In fact, T cell activation increases the levels of Th2 cytokines such as IL-4, IL-13, and IL-5, which are important for eosinophil recruitment into the target tissues of allergic inflammation and leads to subsequent tissue damage and remodeling. The uptake of IgE-allergen complexes by CD23 was firstly described nearly 30 years ago [136] and the process was termed IgE-facilitated allergen presentation (IgE-FAP). CD23 is mainly expressed on the surface of resting naïve IgD+ B cells [88]. Upon binding of IgE-allergen complexes to CD23, these complexes are endocytosed and processed, leading to the loading of allergen-derived peptides on MHC II, which can be recognized by specific T cells [137]. Alternatively, CD23 bearing primary B cells may also transfer IgE-allergen complexes to dendritic cells for processing of the allergen and presentation of allergen-derived peptides to T cells [138,139]. CD23 may also play an important role in the transcytosis of IgE and IgE-antigen complexes across human intestinal [140] and respiratory epithelial cells [141], as well as in transporting IgE-antigen complexes to B cell follicles in mice [142]. IgE-FAP is a very efficient process of inducing T cell activation, as 100–1000 fold lower amounts of allergen complexed with specific IgE than allergen alone were needed to trigger T cell activation in vitro [6,143]. Moreover, allergen-specific T cell activation by CD23-mediated FAP is accompanied by the release of pro-inflammatory cytokines [6]. The blocking of CD23 with a specific anti-CD23 antibody, lumiliximab, in allergen-stimulated PBMCs reduced allergen-specific T cell activation by 50%, highlighting the role of IgE-FAP in allergen presentation [144]. Its importance is underlined by the fact that IgG blocking antibodies, which are induced upon immunotherapy, inhibit IgE-FAP, thus reducing specific T cell proliferation and the release of pro-inflammatory cytokines [145,146,147,148,149,150]. Besides its role in mediating IgE-FAP, CD23 is also known to regulate serum IgE levels in murine models by capturing IgE by CD23-expressing cells [151,152].
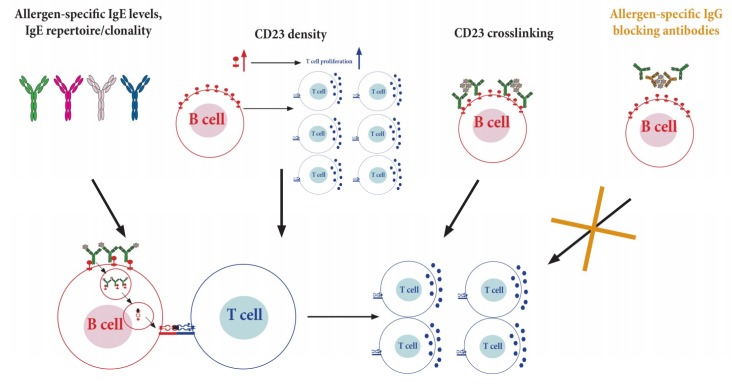
It is therefore important to understand the modes of how IgE-allergen complexes bind to CD23 [153] and the factors controlling the extent of CD23-mediated IgE-FAP (Figure 5). Allergen-specific IgE levels and the complexity of the IgE repertoire, with regards to their clonality and affinity for an allergen, determine the formation of IgE-allergen complexes, and therefore affect the activation of allergen-specific T cells [154]. Recently, CD23 density on B cells has been described to correlate with IgE levels in the serum and is associated with the extent of allergen-specific T cell activation [88]. Additionally, other factors might be involved in controlling IgE-FAP such as the extent of CD23 crosslinking. Further studies analyzing the afore mentioned components of CD23 would help to shed light on the different elements affecting IgE-FAP.
Figure 5.
Factors affecting the extent of CD23-mediated facilitated allergen presentation (FAP) and subsequent T cell activation. Binding of IgE-allergen-complexes to CD23 present on B cells (bottom section, red cell) leads to endocytosis of these complexes followed by processing and loading of allergen-derived peptides on MHCII, which can be recognized by specific T cells (blue cells). This process, called IgE FAP, enhances activation and proliferation of T cells. Factors involved in controlling IgE-FAP include allergen-specific IgE levels, IgE repertoire/clonality, CD23 density, the extent of IgE crosslinking, and allergen-specific blocking IgG antibodies.
5. Targeting of IgE and IgE+ Cells
As IgE is central to mediating symptoms of allergic diseases, it represents an important and attractive target for developing novel therapeutics (Appendix A Table A1). There are currently two different approaches: targeting of the antibody itself or interfering with the activation of IgE receptor-bearing effector cells. The second is the elimination of IgE-producing cells to inhibit IgE production at its origin.
5.1. Targeting IgE and Interference with Activation of Effector Cells
The heavy chain of IgE is composed of four constant Cε domains. The binding site for IgE to its high and low affinity receptors has been mapped to the Cε3 domain [155,156]. Thus, the first generation of therapeutic anti-IgE antibodies were designed to bind selectively to this domain to reduce free IgE levels and to inhibit binding of IgE to its receptor thereby leading to strong reduction in activation of mast and basophils upon allergen contact [157,158,159]. Administration of omalizumab, the first licensed humanized monoclonal IgG1 antibody directed towards IgE, has been shown to reduce free IgE levels by 99% within 2 h of administration, and reduces human basophil responsiveness within 3 months [129,160]. It may also inhibit IgE synthesis in B cells in vitro [161]. It is successfully used for treatment of severe asthma as well as urticaria [17,162]. Recently, a biosimilar antibody to omalizumab was developed by Shanghai Biomabs Pharmaceutical Co., Ltd. [163] and is currently being tested in 400 asthmatic patients in a multicenter phase III trial (NCT03468790), which is expected to be finished by December 2019. Ligelizumab (QGE031) is also targeting the Cε3 region but with a 50-fold greater affinity than omalizumab in vitro. However, in a clinical study, asthmatic patients receiving ligelizumab performed only slightly better than omalizumab-treated patients [164]. Phase III clinical trials with ligelizumab in chronic spontaneous urticaria are still ongoing (NCT03580369, NCT03580356). MEDI4121 is another humanized IgG1 antibody for neutralization of free IgE domain and binds selectively the Cε3 and Cε4 region of IgE [165]. It bears a 106-fold greater affinity for IgE as compared to omalizumab. Consequently, administration in a pharmacokinetic study of MEDI4121 in humans led to a rapid decrease of IgE [166]. However, MEDI4121 is eliminated quickly in vivo resulting in the quick recovery of free IgE levels back to baseline. Thus, despite having a higher affinity, the antibody would need to be administered at short intervals to maintain IgE suppression and currently no further studies have been performed. In addition, macromolecular inhibitors, so called designed ankyrin repeat proteins (DARPins), have been developed that do not interact with free IgE but actively promote the dissociation of receptor-bound IgE from FcεRI [167]. DARPins have been shown to efficiently prevent passive cutaneous sensitization in mice [168]. Recently, the research group of Alexander Eggel has developed a bispecific DARPin co-ligating the inhibitory FcγRIIB with FcεRI bound IgE on effector cells. This specific targeting strategy resulted in reduced allergen-induced effector cell degranulation as well as inhibition of systemic anaphylaxis in vivo [169]. The approach of inducing auto-antibodies to human IgE receptor-binding sites by peptide vaccination has only been tried in rodent models [170,171] and so far has not been brought forward to clinical testing. As an alternative approach, especially in patients who are not suited for omalizumab therapy due to excessive IgE levels, IgE may also be removed from the blood using extracorporal immunoapheresis. Here, two studies showed that immunoadorption using anti-IgE antibodies reduced peripheral IgE levels by around 90% in patients with IgE levels up to 10,000 kU/L [13,20]. Immunoadsorption led to improvement of clinical symptoms both in atopic dermatitis and in allergic asthma. Thus, it may additionally be a valuable pre-treatment for patients with very high IgE levels to enable them to start omalizumab therapy.
Another strategy aims at reducing the number of effector cells. In mouse models it has been shown that amelioration of allergic disease can be achieved by targeting mast cells and basophils with anti-FcεRIa Fab coated micelles loaded with celastrol [21]. The latter is a quinone methide triterpene derived from Tripterygium wilfordii and capable of potentiating TNF-induced apoptosis [172]. CTLA4Fcε, a Th2 modulatory component, has so far only been tested in vitro [22]. CTLA4Fcε is a recombinant fusion protein of the ectodomain of the immunoregulatory molecules cytotoxic T lymphocyte antigen 4 (CTLA-4) with a fragment of IgE heavy chain constant region and thus binds to IgE receptors as well as CD80 and CD86. Due to these properties it is thought to reduce both IgE production via soluble CD23 as well as lymphocyte proliferation.
5.2. Therapeutic Targeting of IgE-Producing Cells
Several approaches have been made to eliminate IgE-producing cells. For example, quilizumab is directed towards the M1 domain of the membrane-bound IgE B cell receptor and was shown to reduce IgE levels as well as numbers of IgE-producing plasma cells in a murine model [173]. Despite a reduction of serum IgE in humans by 25–40% [23,174], a large clinical trial with more than 500 patients suffering from allergic asthma uncontrolled by standard therapy showed no reduction of asthma exacerbations within the 36 weeks of treatment [23]. However, the IgE levels, especially in the treatment group receiving the highest dose of quilizumab, showed a continuous decline during the 48 weeks of safety follow up. Similar results were obtained in a study where chronic spontaneous urticaria was treated with quilizumab [175]. Taking into account the longevity of plasma cells that cannot be destroyed by this approach due to the absence of a B cell receptor, it is conceivable that the treatment effect may have been underestimated due to the relative shortness of the treatment and observation period. Alternatively, a bispecific IgE-CD3 antibody has been developed with the aim of destroying IgE-bearing B cells by directing the cytotoxic activity of T cells to them [176]. It is directed towards epitopes of IgE, which are inaccessible when IgE is bound to its Fc receptors [177]. Initial in vitro experiments have shown that this non-anaphylactogenic antibody is capable of inducing lysis of IgE+ membrane B cells by cytotoxic T cells [176]; however, whether it will also be successful in treatment of allergic patients has not been studied. Other approaches aim at targeting or co-targeting of Fcγ receptors for the elimination of IgE+ B cells. In this respect, mutations of the above mentioned anti-IgE antibody MEDI4121 initially developed for binding to free serum IgE have been selected with improved binding to FcγRIIIa, a receptor involved in antibody-dependent cellular toxicity [178]. Another Fcγ receptor, namely FcγRIIb, is also involved in down regulation of BCR signaling and is thus targeted by XmAb8915, an antibody that co-engages FcγRIIb and the IgE B cell receptor [179]. It was shown to successfully reduce IgE production by PBMCs in vitro and to specifically reduce IgE production in SCID mice engrafted with human PBMCs. In a very recent approach, off-springs of mice vaccinated with anti-IgE during pregnancy showed suppressed IgE levels in response to antigen challenge [180]. This suggests that treatment with anti-IgE antibody during pregnancy could prevent allergic sensitization.
6. Conclusions
Though IgE is the least abundant class of immunoglobulins with an extremely short half-life it plays a central role in allergic disease. Allergen-specific IgE production in allergic patients seems to consist of two modes: a continuous mode, which maintains IgE levels even in the absence of an allergen stimulus, and a reactionary mode, where increases of IgE production occur after exogenous allergen stimulus. Neither the precise sites nor the nature of the IgE-producing cells involved in these two modes of IgE production in allergic patients are known. Another as yet unanswered matter is the timely interplay between rises in IgE production occurring in response to allergen exposure and the loading of effector cells capable of binding IgE via their high and low affinity receptors. Due to fundamental differences between allergic patients and experimental animal models for allergy, research in patients will be required to address the open research questions regarding IgE-producing cells, mechanisms, and sites of IgE production, as well as the loading of IgE to effectors cells, which is responsible for allergic inflammation. Clinical experience with therapeutic strategies depleting IgE and preventing IgE binding to its receptors indicates that the inactivation IgE-mediated effector cell activation is not harmful. The elimination of IgE-producing cells may therefore represent a safe therapeutic strategy, which may lead to a cure of allergy but will require the identification and characterization of IgE-producing cells in allergic patients.
Acknowledgments
Open Access Funding by the Austrian Science Fund (FWF).
Abbreviations
| APC | Antigen Presenting Cell |
| BCR | B Cell Receptor |
| CD23 | Low Affinity Receptor for IgE |
| FcεRI | High Affinity Receptor for IgE |
| IgE | Immunoglobulin E |
| MHC | Major Histocompatibility Complex |
| PBMC | Peripheral Blood Mononuclear Cell |
Appendix A
Table A1.
(A) Therapeutic approaches targeting effector IgE+ and IgE producing cells. Randomised Control Trial (RCT), Adverse Event (AE). (B) Therapeutic approaches targeting effector IgE+ and IgE producing cells. Randomised Control Trial (RCT), Adverse Event (AE).
| A | Approach | Biological Effect | Study Design | Disease Target | Subjects | Length of Observation | Outcome | Current Phase | References |
| Strategies targeting IgE | Immuno adsorption | Removal of circulating IgE or total Ig through Plasmapheresis | RCT | Allergic Asthma | N = 15 | 16 weeks | ↓Total IgE ↓ basophil activation |
Pre-Clinical | [13] |
| RCT | Atopic Dermatitis | N = 50 | 8 weeks | Less AE in IgE group vs pan Ig | Pre-Clinical | [20] | |||
| Omalizumab | Monoclonal antibody against Fc portion of IgE—prevents receptor binding | RCT | Allergic Asthma | N = 317 | 20 weeks | ↓IgE ↓Steroid use |
Marketed | [17] | |
| CMAB007 | Biosimilar to Omalizumab developed by China | RCT | Allergic Asthma | N = 400 | 24 weeks | Not yet completed | Phase III NCT03468790 |
[163] | |
| DARPins | Ankyrin repeat domains that affect stability and function of target protein | In vitro study | Allergy | Isolated basophils | N/A | Removal of IgE from basophils + ↓ basophil activation | Pre-Clinical | [168] | |
| MEDI4212 | Monoclonal antibody against Fc portion of IgE—prevents receptor binding | RCT | Allergy/Atopy | N = 86 | 12 weeks | Greater ↓total IgE vs Omalizumab worse half life | Phase I NCT01544348 |
[166] | |
| MEDI4212 Variant | Monoclonal antibody against Fc portion of IgE and Fc potion of monoclonal antibody binds to inhibitory receptor FcγRIIIa on B-cells | In vitro study | Allergy | Cell lines and human B cells | N/A | Elimination of IgE expressing B cells | Pre-Clinical | [178] | |
| IgE Peptide Vaccine | Induction of autoantibodies against Fc region of IgE | In vitro study | Allergy | FcεRI–ELISA | N/A | Autoantibodies block IgE binding to FcεRI | Pre-Clinical | [171] | |
| QGE031 (Ligelizumab) | Monoclonal antibody against Fc portion of IgE—prevents receptor binding | RCT | Allergic Asthma | N = 37 | 10 weeks | QGE031 > Omalizumab | Phase II NCT01703312 |
[164] | |
| B | Approaches | Biological Effect | Study Design | Disease Target | Subjects | Length of Observation | Outcome | Current Trail Phase | References |
| Strategies targeting IgE production or effector cells | Quilizumab | Monoclonal antibody targeting M1-prime segment of membrane bound IgE expressed on IgE switched B cells leading to cell depletion | RCT | Allergic Asthma | N = 578 | 36 weeks with 48 week safety follow-up | Acceptable safety and reduced serum IgE but no clinically meaningful benefit in clinical outcome parameters | Phase II NCT01582503 |
[23] |
| DARPins | Ankyrin repeat domains that affect stability and function of target protein | In vitro study | Allergy | Human basophils | N/A | Targets FcγIIB and inhibits basophil degranulation | Pre-Clinical | [169] | |
| Bsc-IgE/CD3 Construct | Monoclonal antibody binding to cells with membrane bound IgE and targets T-cell cytotoxic activity towards them | In vitro study | Allergy | Cells isolated from allergic human donors | N/A | Bsc-IgE/CD3 effective at eliminating IgE+ B cells without inducing degranulation of mast cells | Pre-Clinical | [176] | |
| Anti-FcεRI Fab-conjugated celastrol-loaded micelles | Fusion with cell membrane and induction of apoptosis of FcεRI expressing cells | In vitro study | Allergy | Mast cell line | N/A | Efficient induction of apoptosis of mast cells and reduction of allergic inflammation in mouse model | Pre-Clinical | [21] | |
| CTLA4Fcε Fusion Protein | Binds FcεRI and CD23, prevents CD23 cleavage, and blocks CD80/CD86 costimulation | In vitro study | Allergy | Cell line and human PBMC samples | N/A | Reduces sCD23 and lymphocyte proliferation | Pre-Clinical | [22] | |
| Maternal Anti-IgE Vaccination | IgG anti IgE antibodies transferred from mother to fetus and prevent onset of allergy by targeting IgE memory B cells | In vivo mouse study | Allergy | N/A | 9 weeks after birth | Reduced IgE levels in mouse offspring | Pre-Clinical | [180] |
Author Contributions
J.E.D., S.V.M., N.J.C., M.B., A.F., and R.V. wrote the manuscript. J.E.D., S.V.M., N.J.C., A.F., M.B., and R.V. designed the figures and tables. A.K. and K.R. helped revising the manuscript, read and approved the final version. J.E.D., S.V.M., N.J.C., M.B., A.F., D.K., A.K., K.R., M.K., A.K., V.N. and R.V. critically read and revised the manuscript.
Funding
Supported by grants F4605, F4613, and DK1248-B13 from the Austrian Science Fund (FWF), by the Russian Academic Excellence Project 5-100, and by a Megagrant of the Government of the Russian Federation, grant No 14.W03.31.0024. AF is supported by grant from the Russian Science Foundation (project 19-15-00331).
Conflicts of Interest
RV has received research grants from Biomay AG and Viravaxx, Vienna, Austria and serves as a consultant for Viravaxx. The other authors declare that they have no conflicts of interest.
References
- 1.Valenta R., Karaulov A., Niederberger V., Gattinger P., van Hage M., Flicker S., Linhart B., Campana R., Focke-Tejkl M., Curin M., et al. Molecular aspects of allergens and allergy. Adv. Immunol. 2018;138:195–256. doi: 10.1016/bs.ai.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Bønnelykke K., Sparks R., Waage J., Milner J.D. Genetics of allergy and allergic sensitization: Common variants, rare mutations. Curr. Opin. Immunol. 2015;36:115–126. doi: 10.1016/j.coi.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neunkirchner A., Kratzer B., Köhler C., Smole U., Mager L.F., Schmetterer K.G., Trapin D., Leb-Reichl V., Rosloniec E., Naumann R., et al. Genetic restriction of antigen-presentation dictates allergic sensitization and disease in humanized mice. EBioMedicine. 2018;31:66–78. doi: 10.1016/j.ebiom.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bischoff S.C. Role of mast cells in allergic and non-allergic immune responses: Comparison of human and murine data. Nat. Rev. Immunol. 2007;7:93–104. doi: 10.1038/nri2018. [DOI] [PubMed] [Google Scholar]
- 5.Bruhns P., Jönsson F. Mouse and human FcR effector functions. Immunol. Rev. 2015;268:25–51. doi: 10.1111/imr.12350. [DOI] [PubMed] [Google Scholar]
- 6.Van Der Heijden F.L., Van Neerven R.J.J., Van Katwijk M., Bos J.D., Kapsenberg M.L. Serum-IgE-facilitated allergen presentation in atopic disease. J. Immunol. 1993;150:3643–3650. [PubMed] [Google Scholar]
- 7.Mudde G.C., Bheekha R., Bruijnzeel-Koomen C.A. IgE-mediated antigen presentation. Allergy. 1995;50:193–199. doi: 10.1111/j.1398-9995.1995.tb01132.x. [DOI] [PubMed] [Google Scholar]
- 8.Maurer D., Ebner C., Reininger B., Fiebiger E., Kraft D., Kinet J.P., Stingl G. The high affinity IgE receptor (FcεRI) mediates IgE-dependent allergen presentation. J. Immunol. 1995;154:6285–6290. [PubMed] [Google Scholar]
- 9.Eckl-Dorna J., Villazala-Merino S., Linhart B., Karaulov A.V., Zhernov Y., Khaitov M., Niederberger-Leppin V., Valenta R. Allergen-Specific Antibodies Regulate Secondary Allergen-Specific Immune Responses. Front. Immunol. 2019;9:3131. doi: 10.3389/fimmu.2018.03131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Platzer B., Ruiter F., Van Der Mee J., Fiebiger E. Soluble IgE receptors—Elements of the IgE network. Immunol. Lett. 2011;141:36–44. doi: 10.1016/j.imlet.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dullaers M., De Bruyne R., Ramadani F., Gould H.J., Gevaert P., Lambrecht B.N. The who, where, and when of IgE in allergic airway disease. J. Allergy Clin. Immunol. 2012;129:635–645. doi: 10.1016/j.jaci.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 12.Hamilton R.G., Adkinson N.F., Jr. 23. Clinical laboratory assessment of IgE-dependent hypersensitivity. J. Allergy Clin. Immunol. 2003;111:S687–S701. doi: 10.1067/mai.2003.123. [DOI] [PubMed] [Google Scholar]
- 13.Lupinek C., Derfler K., Lee S., Prikoszovich T., Movadat O., Wollmann E., Cornelius C., Weber M., Fröschl R., Selb R., et al. Extracorporeal IgE Immunoadsorption in Allergic Asthma: Safety and Efficacy. EBioMedicine. 2017;17:119–133. doi: 10.1016/j.ebiom.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu J., Chen J., Ye L., Cai Z., Sun J., Ji K. Anti-IgE therapy for IgE-mediated allergic diseases: From neutralizing IgE antibodies to eliminating IgE+ B cells. Clin. Transl. Allergy. 2018;8:27. doi: 10.1186/s13601-018-0213-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu L.C., Scheerens H. Targeting IgE production in mice and humans. Curr. Opin. Immunol. 2014;31:8–15. doi: 10.1016/j.coi.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Balbino B., Conde E., Marichal T., Starkl P., Reber L.L. Aproaches to target IgE antibodies in allergic disease. Pharmacol. Ther. 2018;191:50–64. doi: 10.1016/j.pharmthera.2018.05.015. [DOI] [PubMed] [Google Scholar]
- 17.Milgrom H., Fick R.B., Jr., Su J.Q., Reimann J.D., Bush R.K., Watrous M.L., Metzger W.J. Treatment of allergic asthma with monoclonal anti-IgE antibody. Rhumab-e25 study group. N. Engl. J. Med. 1999;341:1966–1973. doi: 10.1056/NEJM199912233412603. [DOI] [PubMed] [Google Scholar]
- 18.Busse W., Corren J., Lanier B.Q., McAlary M., Fowler-Taylor A., Della Cioppa G., Van As A., Gupta N. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J. Allergy Clin. Immunol. 2001;108:184–190. doi: 10.1067/mai.2001.117880. [DOI] [PubMed] [Google Scholar]
- 19.Humbert M., Busse W., Hanania N.A., Lowe P.J., Canvin J., Erpenbeck V.J., Holgate S. Omalizumab in Asthma: An Update on Recent Developments. J. Allergy Clin. Immunol. Pract. 2014;2:525–536. doi: 10.1016/j.jaip.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Reich K., Deinzer J., Fiege A.-K., Von Gruben V., Sack A.-L., Thraen A., Weisenseel P., Breuer K., Jäckle S., Meier M. Panimmunoglobulin and IgE-selective extracorporeal immunoadsorption in patients with severe atopic dermatitis. J. Allergy Clin. Immunol. 2016;137:1882–1884. doi: 10.1016/j.jaci.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 21.Peng X., Wang J., Li X., Lin L., Xie G., Cui Z., Li J., Wang Y., Li L. Targeting Mast Cells and Basophils with Anti-FcεRIα Fab-Conjugated Celastrol-Loaded Micelles Suppresses Allergic Inflammation. J. Biomed. Nanotechnol. 2015;11:2286–2299. doi: 10.1166/jbn.2015.2163. [DOI] [PubMed] [Google Scholar]
- 22.Perez-Witzke D., Miranda-García M.A., Suárez N., Becerra R., Duque K., Porras V., Fuenmayor J., Montano R.F. CTLA4Fcε, a novel soluble fusion protein that binds B7 molecules and the IgE receptors, and reduces human in vitro soluble CD23 production and lymphocyte proliferation. Immunology. 2016;148:40–55. doi: 10.1111/imm.12586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris J.M., Maciuca R., Bradley M.S., Cabanski C.R., Scheerens H., Lim J., Cai F., Kishnani M., Liao X.C., Samineni D., et al. A randomized trial of the efficacy and safety of quilizumab in adults with inadequately controlled allergic asthma. Respir. Res. 2016;17:29. doi: 10.1186/s12931-016-0347-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horst A., Hunzelmann N., Arce S., Herber M., Manz R.A., Radbruch A., Nischt R., Schmitz J., Assenmacher M. Detection and characterization of plasma cells in peripheral blood: Correlation of IgE+ plasma cell frequency with IgE serum titre. Clin. Exp. Immunol. 2002;130:370–378. doi: 10.1046/j.1365-2249.2002.02025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pree I., Reisinger J., Marth K., Chen K.-W., Vrtala S., Spitzauer S., Valenta R., Niederberger V., Eckl-Dorna J., Eckl-Dorna J. The majority of allergen-specific IgE in the blood of allergic patients does not originate from blood-derived B cells or plasma cells. Clin. Exp. Allergy. 2012;42:1347–1355. doi: 10.1111/j.1365-2222.2012.04030.x. [DOI] [PubMed] [Google Scholar]
- 26.Østergaard P.A. Tonsillar IgE plasma cells predict atopic disease. Clin. Exp. Immunol. 1982;49:163–166. [PMC free article] [PubMed] [Google Scholar]
- 27.Smurthwaite L., Walker S.N., Wilson D.R., Birch D.S., Merrett T.G., Durham S.R., Gould H.J. Persistent IgE synthesis in the nasal mucosa of hay fever patients. Eur. J. Immunol. 2001;31:3422–3431. doi: 10.1002/1521-4141(200112)31:12<3422::AID-IMMU3422>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 28.Wu L.C., Zarrin A.A. The production and regulation of IgE by the immune system. Nat. Rev. Immunol. 2014;14:247–259. doi: 10.1038/nri3632. [DOI] [PubMed] [Google Scholar]
- 29.He J.S., Meyer-Hermann M., Xiangying D., Zuan L.Y., Jones L.A., Ramakrishna L., de Vries V.C., Dolpady J., Aina H., Joseph S., et al. The distinctive germinal center phase of IgE+ B lymphocytes limits their contribution to the classical memory response. J. Exp. Med. 2013;210:2755–2771. doi: 10.1084/jem.20131539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Erazo A., Kutchukhidze N., Leung M., Christ A.P.G., Urban J.F., De Lafaille M.A.C., Lafaille J.J. Unique maturation program of the IgE response in vivo. Immunity. 2007;26:191–203. doi: 10.1016/j.immuni.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kühn R., Rajewsky K., Müller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991;254:707–710. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- 32.Punnonen J., Yssel H., de Vries J.E. The relative contribution of IL-4 and IL-13 to human IgE synthesis induced by activated CD4+ or CD8+ T cells. J. Allergy Clin. Immunol. 1997;100:792–801. doi: 10.1016/S0091-6749(97)70276-8. [DOI] [PubMed] [Google Scholar]
- 33.Vrtala S., Ball T., Spitzauer S., Pandjaitan B., Suphioglu C., Knox B., Sperr W.R., Valent P., Kraft D., Valenta R. Immunization with purified natural and recombinant allergens induces mouse IgG1 antibodies that recognize similar epitopes as human IgE and inhibit the human IgE-allergen interaction and allergen-induced basophil degranulation. J. Immunol. 1998;160:6137–6144. [PubMed] [Google Scholar]
- 34.Xiong H., Dolpady J., Wabl M., De Lafaille M.A.C., Lafaille J.J. Sequential class switching is required for the generation of high affinity IgE antibodies. J. Exp. Med. 2012;209:353–364. doi: 10.1084/jem.20111941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pree I., Reisinger J., Focke M., Vrtala S., Pauli G., Van Hage M., Cromwell O., Gadermaier E., Egger C., Reider N., et al. Analysis of Epitope-Specific Immune Responses Induced by Vaccination with Structurally Folded and Unfolded Recombinant Bet v 1 Allergen Derivatives in Man. J. Immunol. 2007;179:5309–5316. doi: 10.4049/jimmunol.179.8.5309. [DOI] [PubMed] [Google Scholar]
- 36.Curin M., Swoboda I., Wollmann E., Lupinek C., Spitzauer S., Van Hage M., Valenta R. Microarrayed dog, cat, and horse allergens show weak correlation between allergen-specific IgE and IgG responses. J. Allergy Clin. Immunol. 2014;133:918–921. doi: 10.1016/j.jaci.2013.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niederberger V., Ring J., Rakoski J., Jager S., Spitzauer S., Valent P., Horak F., Kundi M., Valenta R. Antigens drive memory IgE responses in human allergy via the nasal mucosa. Int. Arch. Allergy Immunol. 2007;142:133–144. doi: 10.1159/000096439. [DOI] [PubMed] [Google Scholar]
- 38.Egger C., Lupinek C., Ristl R., Lemell P., Horak F., Zieglmayer P., Spitzauer S., Valenta R., Niederberger V. Effects of Nasal Corticosteroids on Boosts of Systemic Allergen-Specific IgE Production Induced by Nasal Allergen Exposure. PLoS ONE. 2015;10:e0114991. doi: 10.1371/journal.pone.0114991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doeing D.C., Borowicz J.L., Crockett E.T. Gender dimorphism in differential peripheral blood leukocyte counts in mice using cardiac, tail, foot, and saphenous vein puncture methods. BMC Clin. Pathol. 2003;3:3. doi: 10.1186/1472-6890-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mestas J., Hughes C.C.W. Of Mice and Not Men: Differences between Mouse and Human Immunology. J. Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 41.Kita H., Gleich G.J. Eosinophils and IgE receptors: A continuing controversy. Blood. 1997;89:3497–3501. [PubMed] [Google Scholar]
- 42.Gounni A.S., Lamkhioued B., Ochiai K., Tanaka Y., Delaporte E., Capron A., Kinet J.-P., Capron M. High-affinity IgE receptor on eosinophils is involved in defence against parasites. Nature. 1994;367:183–186. doi: 10.1038/367183a0. [DOI] [PubMed] [Google Scholar]
- 43.Noti M., Kim B.S., Siracusa M.C., Rak G.D., Kubo M., Moghaddam A.E., Sattentau Q.A., Comeau M.R., Spergel J.M., Artis D. Exposure to food allergens through inflamed skin promotes intestinal food allergy through the thymic stromal lymphopoietin–basophil axis. J. Allergy Clin. Immunol. 2014;133:1390–1399. doi: 10.1016/j.jaci.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takeda K., Gelfand E.W. Mouse Models of allergic diseases. Curr Opin Immunol. 2009;21:660–665. doi: 10.1016/j.coi.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 45.Spergel J. Atopic dermatitis and the atopic march. J. Allergy Clin. Immunol. 2003;112:S118–S127. doi: 10.1016/j.jaci.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 46.Berkowska M.A., Heeringa J.J., Hajdarbegovic E., van der Burg M., Thio H.B., van Hagen P.M., Boon L., Orfao A., van Dongen J.J., van Zelm M.C. Human IgE+ B cells are derived from T cell-dependent and T cell-independent pathways. J. Allergy Clin. Immunol. 2014;134:688–697. doi: 10.1016/j.jaci.2014.03.036. [DOI] [PubMed] [Google Scholar]
- 47.Cameron L., Gounni A.S., Frenkiel S., Lavigne F., Vercelli D., Hamid Q. Sε, sµ and sγ switch circles in human nasal mucosa following ex vivo allergen challenge: Evidence for direct as well as sequential class switch recombination. J. Immunol. 2003;171:3816–3822. doi: 10.4049/jimmunol.171.7.3816. [DOI] [PubMed] [Google Scholar]
- 48.Ramadani F., Bowen H., Upton N., Hobson P.S., Chan Y.C., Chen J.B., Chang T.W., McDonell J.M., Sutton B.J., Fear D.J., et al. Ontogeny of human IgE-expressing B cells and plasma cells. Allergy. 2017;72:66–76. doi: 10.1111/all.12911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takhar P., Smurthwaite L., Coker H.A., Fear D.J., Banfield G.K., Carr V.A., Durham S.R., Gould H.J. Allergen Drives Class Switching to IgE in the Nasal Mucosa in Allergic Rhinitis. J. Immunol. 2005;174:5024–5032. doi: 10.4049/jimmunol.174.8.5024. [DOI] [PubMed] [Google Scholar]
- 50.Mills F.C., Mitchell M.P., Harindranath N., Max E.E. Human Ig S gamma regions and their participation in sequential switching to IgE. J. Immunol. 1995;155:3021–3026. [PubMed] [Google Scholar]
- 51.Looney T.J., Lee J.Y., Roskin K.M., Hoh R.A., King J., Glanville J., Liu Y., Pham T.D., Dekker C.L., Davis M.M., et al. Human B-cell isotype switching origins of IgE. J. Allergy Clin. Immunol. 2016;137:579–586. doi: 10.1016/j.jaci.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saunders S.P., Ma E.G.M., Aranda C.J., De Lafaille M.A.C. Non-classical B Cell Memory of Allergic IgE Responses. Front. Immunol. 2019;10:710–715. doi: 10.3389/fimmu.2019.00715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Niederberger V., Niggemann B., Kraft D., Spitzauer S., Valenta R. Evolution of IgM, IgE and IgG1–4 antibody responses in early childhood monitored with recombinant allergen components: Implications for class switch mechanisms. Eur. J. Immunol. 2002;32:576–584. doi: 10.1002/1521-4141(200202)32:2<576::AID-IMMU576>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 54.Wagner J.G., Harkema J.R. Rodent models of allergic rhinitis: Relevance to human pathophysiology. Curr. Allergy Asthma Rep. 2007;7:134–140. doi: 10.1007/s11882-007-0011-5. [DOI] [PubMed] [Google Scholar]
- 55.Harkema J.R., Carey S.A., Wagner J.G. The Nose Revisited: A Brief Review of the Comparative Structure, Function, and Toxicologic Pathology of the Nasal Epithelium. Toxicol. Pathol. 2006;34:252–269. doi: 10.1080/01926230600713475. [DOI] [PubMed] [Google Scholar]
- 56.Wenzel S., Holgate S.T. The mouse trap: It still yields few answers in asthma. Am. J. Respir. Crit. Care Med. 2006;174:1173–1176. doi: 10.1164/rccm.2609002. [DOI] [PubMed] [Google Scholar]
- 57.Gordon C.J., Grafton G., Wood P.M., Larché M., Armitage R.J. Modelling the human immune response: Can mice be trusted? Commentary. Curr. Opin. Pharmacol. 2001;1:431–435. doi: 10.1016/S1471-4892(01)00074-1. [DOI] [PubMed] [Google Scholar]
- 58.Hessel E.M., Van Oosterhout A.J., Van Ark I., Van Esch B., Hofman G., Van Loveren H., Savelkoul H.F., Nijkamp F.P. Development of airway hyperresponsiveness is dependent on interferon-gamma and independent of eosinophil infiltration. Am. J. Respir. Cell Mol. Boil. 1997;16:325–334. doi: 10.1165/ajrcmb.16.3.9070618. [DOI] [PubMed] [Google Scholar]
- 59.Spergel J.M., Mizoguchi E., Brewer J.P., Martin T.R., Bhan A.K., Geha R.S. Epicutaneous sensitization with protein antigen induces localized allergic dermatitis and hyperresponsiveness to methacholine after single exposure to aerosolized antigen in mice. J. Clin. Investig. 1998;101:1614–1622. doi: 10.1172/JCI1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mitre E., Nutman T.B. IgE memory: Persistence of antigen-specific IgE responses years after treatment of human filarial infections. J. Allergy Clin. Immunol. 2006;117:939–945. doi: 10.1016/j.jaci.2005.12.1341. [DOI] [PubMed] [Google Scholar]
- 61.Henderson L., Larson J., Gleich G. Maximal rise in IgE antibody following ragweed pollination season. J. Allergy Clin. Immunol. 1975;55:10–15. doi: 10.1016/S0091-6749(75)80003-0. [DOI] [PubMed] [Google Scholar]
- 62.Manz R.A., Thiel A., Radbruch A. Lifetime of plasma cells in the bone marrow. Nature. 1997;388:133–134. doi: 10.1038/40540. [DOI] [PubMed] [Google Scholar]
- 63.Manz R.A., Arce S., Cassese G., Hauser A.E., Hiepe F., Radbruch A. Humoral immunity and long-lived plasma cells. Curr. Opin. Immunol. 2002;14:517–521. doi: 10.1016/S0952-7915(02)00356-4. [DOI] [PubMed] [Google Scholar]
- 64.Traggiai E., Bernasconi N.L., Lanzavecchia A. Maintenance of Serological Memory by Polyclonal Activation of Human Memory B Cells. Science. 2002;298:2199–2202. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- 65.Hochwallner H., Schulmeister U., Swoboda I., Balic N., Geller B., Nystrand M., Harlin A., Thalhamer J., Scheiblhofer S., Niggemann B., et al. Microarray and allergenic activity assessment of milk allergens. Clin. Exp. Allergy. 2010;40:1809–1818. doi: 10.1111/j.1365-2222.2010.03602.x. [DOI] [PubMed] [Google Scholar]
- 66.Hochwallner H., Schulmeister U., Swoboda I., Focke-Tejkl M., Civaj V., Balic N., Nystrand M., Harlin A., Thalhamer J., Scheiblhofer S., et al. Visualization of clustered IgE epitopes on alpha-lactalbumin. J. Allergy Clin. Immunol. 2010;125:1279–1285. doi: 10.1016/j.jaci.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 67.Reininger R., Exner H., Kuderna C., Rumpold H., Balic N., Valenta R., Spitzauer S. Possible Modes of Allergen-Specific Sensitization and Boosting in an Atopic Child. Int. Arch. Allergy Immunol. 2003;130:275–279. doi: 10.1159/000070214. [DOI] [PubMed] [Google Scholar]
- 68.Bradleyeisenbrey A., Agarwal M., Offord K., Adolphson C., Yunginger J., Gleich G. Seasonal variation of in vitro lymphocyte proliferative response to ragweed antigen E. J. Allergy Clin. Immunol. 1985;75:84–90. doi: 10.1016/0091-6749(85)90017-X. [DOI] [PubMed] [Google Scholar]
- 69.Eckl-Dorna J., Campana R., Valenta R., Niederberger V. Poor association of allergen-specific antibody, T- and B-cell responses revealed with recombinant allergens and a CFSE dilution-based assay. Allergy. 2015;70:1222–1229. doi: 10.1111/all.12661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lupinek C., Marth K., Niederberger V., Valenta R. Analysis of serum IgE reactivity profiles with microarrayed allergens indicates absence of de novo IgE sensitizations in adults. J. Allergy Clin. Immunol. 2012;130:1418–1420. doi: 10.1016/j.jaci.2012.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Blauvelt A., Simpson E.L., Tyring S.K., Purcell L.A., Shumel B., Petro C.D., Akinlade B., Gadkari A., Eckert L., Graham N.M., et al. Dupilumab does not affect correlates of vaccine-induced immunity: A randomized, placebo-controlled trial in adults with moderate-to-severe atopic dermatitis. J. Am. Acad. Dermatol. 2019;80:158–167. doi: 10.1016/j.jaad.2018.07.048. [DOI] [PubMed] [Google Scholar]
- 72.Bachert C., Mannent L., Naclerio R.M., Mullol J., Ferguson B.J., Gevaert P., Hellings P., Jiao L., Wang L., Evans P.R., et al. Effect of subcutaneous Dupilumab on nasal polyp burden in patients with chronic sinusitis and nasal polyposis: A randomized clinical trial. JAMA. 2016;315:469–479. doi: 10.1001/jama.2015.19330. [DOI] [PubMed] [Google Scholar]
- 73.Beck L.A., Thaci D., Hamilton J.D., Graham N.M., Bieber T., Rocklin R., Ming J.E., Ren H., Kao R., Simpson E., et al. Dupilumab Treatment in Adults with Moderate-to-Severe Atopic Dermatitis. N. Engl. J. Med. 2014;371:130–139. doi: 10.1056/NEJMoa1314768. [DOI] [PubMed] [Google Scholar]
- 74.Wenzel S., Ford L., Pearlman D., Spector S., Sher L., Skobieranda F., Wang L., Kirkesseli S., Rocklin R., Bock B., et al. Dupilumab in Persistent Asthma with Elevated Eosinophil Levels. N. Engl. J. Med. 2013;368:2455–2466. doi: 10.1056/NEJMoa1304048. [DOI] [PubMed] [Google Scholar]
- 75.Fiser P.M., Buckley R.H. Human IgE biosynthesis in vitro: Studies with atopic and normal blood mononuclear cells and subpopulations. J. Immunol. 1979;123:1788–1794. [PubMed] [Google Scholar]
- 76.Romagnani S., Maggi E., Del Prete G.F., Troncone R., Ricci M. In vitro production of IgE by human peripheral blood mononuclear cells. I. Rate of IgE biosynthesis. Clin. Exp. Immunol. 1980;42:167–174. [PMC free article] [PubMed] [Google Scholar]
- 77.Steinberger P., Bohle B., DiPadova F., Wrann M., Liehl E., Scheiner O., Kraft D., Valenta R. Allergen-specific IgE production of committed B cells from allergic patients in vitro. J. Allergy Clin. Immunol. 1995;96:209–218. doi: 10.1016/S0091-6749(95)70010-2. [DOI] [PubMed] [Google Scholar]
- 78.Ohta K., Manzara T., Harbeck R.J., Kirkpatrick C.H. Human tonsillar IgE biosynthesis in vitro. I. Enhancement of IgE and IgG synthesis in the presence of pokeweed mitogen by T-cell irradiation. J. Allergy Clin. Immunol. 1983;71:212–223. doi: 10.1016/0091-6749(83)90102-1. [DOI] [PubMed] [Google Scholar]
- 79.Takenaka H., Kusumi T., Mizukoshi O. In vitro synthesis of IgE antibody by human tonsil mononuclear cells. Preliminary report. Acta Oto-Laryngol. 1988;105:133–137. doi: 10.3109/00016488809125016. [DOI] [PubMed] [Google Scholar]
- 80.Jabara H.H. CD40 and IgE: Synergism between anti-CD40 monoclonal antibody and interleukin 4 in the induction of IgE synthesis by highly purified human B cells. J. Exp. Med. 1990;172:1861–1864. doi: 10.1084/jem.172.6.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Maggi E., Del Prete G.F., Parronchi P., Tiri A., Macchia D., Biswas P., Simonelli C., Ricci M., Romagnani S. Role for T cells, IL-2 and IL-6 in the IL-4-dependent in vitro human IgE synthesis. Immunology. 1989;68:300–306. [PMC free article] [PubMed] [Google Scholar]
- 82.Pene J., Rousset F., Briere F., Chretien I., Bonnefoy J.Y., Spits H., Yokota T., Arai N., Arai K., Banchereau J., et al. IgE production by normal human lymphocytes is induced by interleukin 4 and suppressed by interferons gamma and alpha and prostaglandin e2. Proc. Natl. Acad. Sci. USA. 1988;85:6880–6884. doi: 10.1073/pnas.85.18.6880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gascan H., Gauchat J.F., Aversa G., Van Vlasselaer P., de Vries J.E. Anti-CD40 monoclonal antibodies or CD4+ T cell clones and IL-4 induce IgG4 and IgE switching in purified human B cells via different signaling pathways. J. Immunol. 1991;147:8–13. [PubMed] [Google Scholar]
- 84.Steinberger P., Kraft D., Valenta R. Construction of a combinatorial IgE library from an allergic patient. Isolation and characterization of human IgE Fabs with specificity for the major timothy grass pollen allergen, Phl p 5. J. Biol. Chem. 1996;271:10967–10972. doi: 10.1074/jbc.271.18.10967. [DOI] [PubMed] [Google Scholar]
- 85.Eckl-Dorna J., Niederberger V. What Is the Source of Serum Allergen-Specific IgE? Curr. Allergy Asthma Rep. 2013;13:281–287. doi: 10.1007/s11882-013-0348-x. [DOI] [PubMed] [Google Scholar]
- 86.Jiménez-Saiz R., Ellenbogen Y., Bruton K., Spill P., Sommer D.D., Lima H., Waserman S., Patil S.U., Shreffler W.G., Jordana M. Human BCR analysis of single-sorted, putative IgE. J. Allergy Clin. Immunol. 2019;144:336–339. doi: 10.1016/j.jaci.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wong K.J., Timbrell V., Xi Y., Upham J.W., Collins A.M., Davies J.M. IgE+ B cells are scarce, but allergen-specific B cells with a memory phenotype circulate in patients with allergic rhinitis. Allergy. 2015;70:420–428. doi: 10.1111/all.12563. [DOI] [PubMed] [Google Scholar]
- 88.Selb R., Eckl-Dorna J., Neunkirchner A., Schmetterer K., Marth K., Gamper J., Jahn-Schmid B., Pickl W.F., Valenta R., Niederberger V. CD23 surface density on B cells is associated with IgE levels and determines IgE-facilitated allergen uptake, as well as activation of allergen-specific T cells. J. Allergy Clin. Immunol. 2017;139:290–299. doi: 10.1016/j.jaci.2016.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Heeringa J.J., Rijvers L., Arends N.J., Driessen G.J., Pasmans S.G., Van Dongen J.J.M., De Jongste J.C., Van Zelm M.C. IgE-expressing memory B cells and plasmablasts are increased in blood of children with asthma, food allergy, and atopic dermatitis. Allergy. 2018;73:1331–1336. doi: 10.1111/all.13421. [DOI] [PubMed] [Google Scholar]
- 90.Croote D., Darmanis S., Nadeau K.C., Quake S.R. High-affinity allergen-specific human antibodies cloned from single IgE B cell transcriptomes. Science. 2018;362:1306–1309. doi: 10.1126/science.aau2599. [DOI] [PubMed] [Google Scholar]
- 91.Giesecke C., Frölich D., Reiter K., Mei H.E., Wirries I., Kuhly R., Killig M., Glatzer T., Stölzel K., Perka C., et al. Tissue Distribution and Dependence of Responsiveness of Human Antigen-Specific Memory B Cells. J. Immunol. 2014;192:3091–3100. doi: 10.4049/jimmunol.1302783. [DOI] [PubMed] [Google Scholar]
- 92.Franz B., May K.F., Dranoff G., Wucherpfennig K. Ex vivo characterization and isolation of rare memory B cells with antigen tetramers. Blood. 2011;118:348–357. doi: 10.1182/blood-2011-03-341917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Blanco E., Pérez-Andrés M., Arriba-Mendez S., Contreras-SanFeliciano T., Criado I., Pelák O., Serra-Caetano A., Romero A., Puig N., Remesal A., et al. Age-associated distribution of normal B-cell and plasma cell subsets in peripheral blood. J. Allergy Clin. Immunol. 2018;141:2208–2219. doi: 10.1016/j.jaci.2018.02.017. [DOI] [PubMed] [Google Scholar]
- 94.Oliveria J.P., Salter B.M., MacLean J., Kotwal S., Smith A., Harris J.M., Scheerens H., Sehmi R., Gauvreau G.M. Increased IgE+ B cells in sputum, but not blood, bone marrow, or tonsils, after inhaled allergen challenge in subjects with asthma. Am. J. Respir. Crit. Care Med. 2017;196:107–109. doi: 10.1164/rccm.201611-2274LE. [DOI] [PubMed] [Google Scholar]
- 95.Hedley B.D., Keeney M. Technical issues: Flow cytometry and rare event analysis. Int. J. Lab. Hematol. 2013;35:344–350. doi: 10.1111/ijlh.12068. [DOI] [PubMed] [Google Scholar]
- 96.Tangye S.G., Liu Y.-J., Aversa G., Phillips J.H., De Vries J.E. Identification of Functional Human Splenic Memory B Cells by Expression of CD148 and CD27. J. Exp. Med. 1998;188:1691–1703. doi: 10.1084/jem.188.9.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ellyard J.I., Avery D.T., Phan T.G., Hare N.J., Hodgkin P.D., Tangye S.G. Antigen-selected, immunoglobulin-secreting cells persist in human spleen and bone marrow. Blood. 2004;103:3805–3812. doi: 10.1182/blood-2003-09-3109. [DOI] [PubMed] [Google Scholar]
- 98.Martínez-Gamboa L., Mei H., Loddenkemper C., Ballmer B., Hansen A., Lipsky P.E., Emmerich F., Radbruch A., Salama A., Dorner T. Role of the spleen in peripheral memory B-cell homeostasis in patients with autoimmune thrombocytopenia purpura. Clin. Immunol. 2009;130:199–212. doi: 10.1016/j.clim.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 99.Kleinjan A., Vinke J., Severijnen L., Fokkens W. Local production and detection of (specific) IgE in nasal B-cells and plasma cells of allergic rhinitis patients. Eur. Respir. J. 2000;15:491–497. doi: 10.1034/j.1399-3003.2000.15.11.x. [DOI] [PubMed] [Google Scholar]
- 100.Cameron L., Hamid Q., Wright E., Nakamura Y., Christodoulopoulos P., Muro S., Frenkiel S., Lavigne F., Durham S., Gould H. Local synthesis of epsilon germline gene transcripts, IL-4, and IL-13 in allergic nasal mucosa after ex vivo allergen exposure. J. Allergy Clin. Immunol. 2000;106:46–52. doi: 10.1067/mai.2000.107398. [DOI] [PubMed] [Google Scholar]
- 101.Durham S.R., Gould H.J., Thienes C.P., Jacobson M.R., Masuyama K., Rak S., Lowhagen O., Schotman E., Cameron L., Hamid Q.A. Expression of ε germ-line gene transcripts and mrna for the ε heavy chain of IgE in nasal B cells and the effects of topical corticosteroid. Eur. J. Immunol. 1997;27:2899–2906. doi: 10.1002/eji.1830271123. [DOI] [PubMed] [Google Scholar]
- 102.Papatziamos G., van der Ploeg I., Hemlin C., Patwardhan A., Scheynius A. Increased occurrence of IgE+ and FcεRI+ cells in adenoids from atopic children. Allergy. 1999;54:916–925. doi: 10.1034/j.1398-9995.1999.00033.x. [DOI] [PubMed] [Google Scholar]
- 103.Papatziamos G., Van Hage-Hamsten M., Lundahl J., Hemlin C. IgE-positive plasma cells are present in adenoids of atopic children. Acta Oto-Laryngol. 2006;126:180–185. doi: 10.1080/00016480500265976. [DOI] [PubMed] [Google Scholar]
- 104.Shin S.-Y., Choi S.-J., Hur G.-Y., Lee K.-H., Kim S.-W., Cho J.-S., Park H.-S. Local production of total IgE and specific antibodies to the house dust mite in adenoid tissue. Pediatr. Allergy Immunol. 2009;20:134–141. doi: 10.1111/j.1399-3038.2008.00756.x. [DOI] [PubMed] [Google Scholar]
- 105.Ganzer U., Bachert C. Localization of IgE synthesis in immediate-type allergy of the upper respiratory tract. ORL. 1988;50:257–264. doi: 10.1159/000276000. [DOI] [PubMed] [Google Scholar]
- 106.Snow R.E., Djukanovic R., Stevenson F.K. Analysis of immunoglobulin E VH transcripts in a bronchial biopsy of an asthmatic patient confirms bias towards VH5, and indicates local clonal expansion, somatic mutation and isotype switch events. Immunology. 1999;98:646–651. doi: 10.1046/j.1365-2567.1999.00910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nahm D.H., Park H.-S. Analysis of induced sputum for studying allergen-specific IgE antibodies in airway secretion from asthmatic patients. Clin. Exp. Allergy. 1998;28:686–693. doi: 10.1046/j.1365-2222.1998.00291.x. [DOI] [PubMed] [Google Scholar]
- 108.Takhar P., Corrigan C.J., Smurthwaite L., O’Connor B.J., Durham S.R., Lee T.H., Gould H.J. Class switch recombination to IgE in the bronchial mucosa of atopic and nonatopic patients with asthma. J. Allergy Clin. Immunol. 2007;119:213–218. doi: 10.1016/j.jaci.2006.09.045. [DOI] [PubMed] [Google Scholar]
- 109.Chang H.-D., Tokoyoda K., Radbruch A. Immunological memories of the bone marrow. Immunol. Rev. 2018;283:86–98. doi: 10.1111/imr.12656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Paramithiotis E., Cooper M.D. Memory B lymphocytes migrate to bone marrow in humans. Proc. Natl. Acad. Sci. USA. 1997;94:208–212. doi: 10.1073/pnas.94.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Slifka M.K., Antia R., Whitmire J.K., Ahmed R. Humoral Immunity Due to Long-Lived Plasma Cells. Immunity. 1998;8:363–372. doi: 10.1016/S1074-7613(00)80541-5. [DOI] [PubMed] [Google Scholar]
- 112.Mamani-Matsuda M., Cosma A., Weller S., Faili A., Staib C., Garçon L., Hermine O., Beyne-Rauzy O., Fieschi C., Pers J.-O., et al. The human spleen is a major reservoir for long-lived vaccinia virus-specific memory B cells. Blood. 2008;111:4653–4659. doi: 10.1182/blood-2007-11-123844. [DOI] [PubMed] [Google Scholar]
- 113.Snow R.E., Chapman C.J., Frew A.J., Holgate S.T., Stevenson F.K. Analysis of Ig VH region genes encoding IgE antibodies in splenic B lymphocytes of a patient with asthma. J. Immunol. 1995;154:5576–5581. [PubMed] [Google Scholar]
- 114.Bellou A., Kanny G., Fremont S., Moneret-Vautrin D. Transfer of Atopy Following Bone Marrow Transplantation. Ann. Allergy Asthma Immunol. 1997;78:513–516. doi: 10.1016/S1081-1206(10)63240-1. [DOI] [PubMed] [Google Scholar]
- 115.Walker S.A., Riches P.G., Wild G., Ward A.M., Shaw P.J., Desai S., Hobbs J.R. Total and allergen-specific IgE in relation to allergic response pattern following bone marrow transplantation. Clin. Exp. Immunol. 1986;66:633–639. [PMC free article] [PubMed] [Google Scholar]
- 116.Desai S., Walker S., Shaw P., Riches P., Hobbs J., Wild G., Harper J. Expression of Donor Allergic Response Patterns by Bone Marrow Transplant Recipients. Lancet. 1984;324:1148. doi: 10.1016/S0140-6736(84)91571-X. [DOI] [PubMed] [Google Scholar]
- 117.Saarinen U.M. Transfer of latent atopy by bone marrow transplantation? A case report. J. Allergy Clin. Immunol. 1984;74:196–200. doi: 10.1016/0091-6749(84)90286-0. [DOI] [PubMed] [Google Scholar]
- 118.Agosti J.M., Sprenger J.D., Lum L.G., Witherspoon R.P., Fisher L.D., Storb R., Henderson W.R. Transfer of Allergen-Specific IgE-Mediated Hypersensitivity with Allogeneic Bone Marrow Transplantation. N. Engl. J. Med. 1988;319:1623–1628. doi: 10.1056/NEJM198812223192502. [DOI] [PubMed] [Google Scholar]
- 119.Hallstrand T.S., Sprenger J.D., Agosti J.M., Longton G.M., Witherspoon R.P., Henderson W.R. Long-term acquisition of allergen-specific IgE and asthma following allogeneic bone marrow transplantation from allergic donors. Blood. 2004;104:3086–3090. doi: 10.1182/blood-2004-05-1775. [DOI] [PubMed] [Google Scholar]
- 120.Storek J., Vliagoftis H., Grizel A., Lyon A.W., Daly A., Khan F., Bowen T., Game M., Larratt L., Turner R., et al. Allergy transfer with hematopoietic cell transplantation from an unrelated donor. Bone Marrow Transpl. 2011;46:605–606. doi: 10.1038/bmt.2010.150. [DOI] [PubMed] [Google Scholar]
- 121.Kubo M. Mast cells and basophils in allergic inflammation. Curr. Opin. Immunol. 2018;54:74–79. doi: 10.1016/j.coi.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 122.Varricchi G., Raap U., Rivellese F., Marone G., Gibbs B.F. Human mast cells and basophils-How are they similar how are they different? Immunol. Rev. 2018;282:8–34. doi: 10.1111/imr.12627. [DOI] [PubMed] [Google Scholar]
- 123.Geha R.S., Helm B., Gould H. Inhibition of the Prausnitz–Küstner reaction by an immunoglobulin ε-chain fragment synthesized in E. coli. Nature. 1985;315:577–578. doi: 10.1038/315577a0. [DOI] [PubMed] [Google Scholar]
- 124.Borkowski T.A., Jouvin M.-H., Lin S.-Y., Kinet J.-P. Minimal Requirements for IgE-Mediated Regulation of Surface FcεRI. J. Immunol. 2001;167:1290–1296. doi: 10.4049/jimmunol.167.3.1290. [DOI] [PubMed] [Google Scholar]
- 125.Kim J.K., Hong S.-C., Lee H.-M., Cho J.H., Suh J.D., Park I.-H. Correlation between Skin-prick Testing, Individual Specific IgE Tests, and a Multiallergen IgE Assay for Allergy Detection in Patients with Chronic Rhinitis. Am. J. Rhinol. Allergy. 2014;28:388–391. doi: 10.2500/ajra.2014.28.4074. [DOI] [PubMed] [Google Scholar]
- 126.Finnerty J., Summerell S., Holgate S. Relationship between skin-prick tests, the multiple allergosorbent test and symptoms of allergic disease. Clin. Exp. Allergy. 1989;19:51–56. doi: 10.1111/j.1365-2222.1989.tb02343.x. [DOI] [PubMed] [Google Scholar]
- 127.Naclerio R.M., Adkinson N.F., Moylan B., Baroody F.M., Proud D., Kagey-Sobotka A., Lichtenstein L.M., Hamilton R. Nasal provocation with allergen induces a secondary serum IgE antibody response. J. Allergy Clin. Immunol. 1997;100:505–510. doi: 10.1016/S0091-6749(97)70143-X. [DOI] [PubMed] [Google Scholar]
- 128.Eckl-Dorna J., Froschl R., Lupinek C., Kiss R., Gattinger P., Marth K., Campana R., Mittermann I., Blatt K., Valent P., et al. Intranasal administration of allergen increases specific IgE whereas intranasal omalizumab does not increase serum IgE levels-a pilot study. Allergy. 2018;73:1003–1012. doi: 10.1111/all.13343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lin H., Boesel K.M., Griffith D.T., Prussin C., Foster B., Romero F., Townley R., Casale T.B. Omalizumab rapidly decreases nasal allergic response and FcεRI on basophils. J. Allergy Clin. Immunol. 2004;113:297–302. doi: 10.1016/j.jaci.2003.11.044. [DOI] [PubMed] [Google Scholar]
- 130.Holgate S., Casale T., Wenzel S., Bousquet J., Deniz Y., Reisner C. The anti-inflammatory effects of omalizumab confirm the central role of IgE in allergic inflammation. J. Allergy Clin. Immunol. 2005;115:459–465. doi: 10.1016/j.jaci.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 131.MacGlashan D.W., Jr., Bochner B.S., Adelman D.C., Jardieu P.M., Togias A., McKenzie-White J., Sterbinsky S.A., Hamilton R.G., Lichtenstein L.M. Down-regulation of FcεRI expression on human basophils during in vivo treatment of atopic patients with anti-IgE antibody. J. Immunol. 1997;158:1438–1445. [PubMed] [Google Scholar]
- 132.Noga O., Hanf G., Kunkel G., Kleine-Tebbe J. Basophil histamine release decreases during omalizumab therapy in allergic asthmatics. Int. Arch. Allergy Immunol. 2008;146:66–70. doi: 10.1159/000112504. [DOI] [PubMed] [Google Scholar]
- 133.Saini S.S., MacGlashan D.W., Jr., Sterbinsky S.A., Togias A., Adelman D.C., Lichtenstein L.M., Bochner B.S. Down-regulation of human basophil IgE and FcεRIα surface densities and mediator release by anti-IgE-infusions is reversible in vitro and in vivo. J. Immunol. 1999;162:5624–5630. [PubMed] [Google Scholar]
- 134.Noga O., Hanf G., Kunkel G. Immunological and Clinical Changes in Allergic Asthmatics following Treatment with Omalizumab. Int. Arch. Allergy Immunol. 2003;131:46–52. doi: 10.1159/000070434. [DOI] [PubMed] [Google Scholar]
- 135.Campana R., Moritz K., Marth K., Neubauer A., Huber H., Henning R., Blatt K., Hoermann G., Brodie T.M., Kaider A., et al. Frequent occurrence of T cell-mediated late reactions revealed by atopy patch testing with hypoallergenic rBet v 1 fragments. J. Allergy Clin. Immunol. 2016;137:601–609. doi: 10.1016/j.jaci.2015.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pirron U., Schlunck T., Prinz J.C., Rieber E.P. IgE-dependent antigen focusing by human B lymphocytes is mediated by the low-affinity receptor for IgE. Eur. J. Immunol. 1990;20:1547–1551. doi: 10.1002/eji.1830200721. [DOI] [PubMed] [Google Scholar]
- 137.Wilcock L.K., Francis J.N., Durham S.R. IgE-facilitated antigen presentation: Role in allergy and the influence of allergen immunotherapy. Immunol. Allergy Clin. N. Am. 2006;26:333–347. doi: 10.1016/j.iac.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 138.Ding Z., Dahlin J.S., Xu H., Heyman B. IgE-mediated enhancement of CD4+ T cell responses requires antigen presentation by CD8α− conventional dendritic cells. Sci. Rep. 2016;6:28290. doi: 10.1038/srep28290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Engeroff P., Fellmann M., Yerly D., Bachmann M.F., Vogel M. A novel recycling mechanism of native IgE-antigen complexes in human B cells facilitates the transfer of antigen to dendritic cells for antigen presentation. J. Allergy Clin. Immunol. 2018;142:557–568. doi: 10.1016/j.jaci.2017.09.024. [DOI] [PubMed] [Google Scholar]
- 140.Tu Y., Salim S., Bourgeois J., Di Leo V., Irvine E.J., Marshall J.K., Perdue M.H. CD23-Mediated IgE Transport Across Human Intestinal Epithelium: Inhibition by Blocking Sites of Translation or Binding. Gastroenterology. 2005;129:928–940. doi: 10.1053/j.gastro.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 141.Palaniyandi S., Tomei E., Li Z., Conrad D.H., Zhu X., Wang S., Zhang K., Stepkowski S.M., Li J., Chen G., et al. CD23-Dependent Transcytosis of IgE and Immune Complex across the Polarized Human Respiratory Epithelial Cells. J. Immunol. 2011;186:3484–3496. doi: 10.4049/jimmunol.1002146. [DOI] [PubMed] [Google Scholar]
- 142.Hjelm F., Karlsson M.C.I., Heyman B. A novel B cell-mediated transport of IgE-immune complexes to the follicle of the spleen. J. Immunol. 2008;180:6604–6610. doi: 10.4049/jimmunol.180.10.6604. [DOI] [PubMed] [Google Scholar]
- 143.Van Der Heijden F.L., Van Neerven R.J., Kapsenberg M.L. Relationship between facilitated allergen presentation and the presence of allergen-specific IgE in serum of atopic patients. Clin. Exp. Immunol. 1995;99:289–293. doi: 10.1111/j.1365-2249.1995.tb05547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Poole J.A., Meng J., Reff M., Spellman M.C., Rosenwasser L.J. Anti-CD23 monoclonal antibody, lumiliximab, inhibited allergen-induced responses in antigen-presenting cells and T cells from atopic subjects. J. Allergy Clin. Immunol. 2005;116:780–788. doi: 10.1016/j.jaci.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 145.Van Neerven R.J., Wikborg T., Lund G., Jacobsen B., Brinch-Nielsen A., Arnved J., Ipsen H. Blocking antibodies induced by specific allergy vaccination prevent the activation of CD4+ T cells by inhibiting serum-IgE-facilitated allergen presentation. J. Immunol. 1999;163:2944–2952. [PubMed] [Google Scholar]
- 146.Wachholz P.A., Soni N.K., Till S.J., Durham S.R. Inhibition of allergen-IgE binding to B cells by IgG antibodies after grass pollen immunotherapy. J. Allergy Clin. Immunol. 2003;112:915–922. doi: 10.1016/S0091-6749(03)02022-0. [DOI] [PubMed] [Google Scholar]
- 147.Van Neerven R.J.J., Arvidsson M., Ipsen H., Sparholt S.H., Rak S., Würtzen P. A double-blind, placebo-controlled birch allergy vaccination study: Inhibition of CD23-mediated serum-immunoglobulin E-facilitated allergen presentation. Clin. Exp. Allergy. 2004;34:420–428. doi: 10.1111/j.1365-2222.2004.01899.x. [DOI] [PubMed] [Google Scholar]
- 148.Shamji M.H., Wilcock L.K., Wachholz P.A., Dearman R.J., Kimber I., Würtzen P.A., Larché M., Durham S.R., Francis J.N. The IgE-facilitated allergen binding (FAB) assay: Validation of a novel flow-cytometric based method for the detection of inhibitory antibody responses. J. Immunol. Methods. 2006;317:71–79. doi: 10.1016/j.jim.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Würtzen P.A., Lund G., Lund K., Arvidsson M., Rak S., Ipsen H. A double-blind placebo-controlled birch allergy vaccination study II: Correlation between inhibition of IgE binding, histamine release and facilitated allergen presentation. Clin. Exp. Allergy. 2008;38:1290–1301. doi: 10.1111/j.1365-2222.2008.03020.x. [DOI] [PubMed] [Google Scholar]
- 150.Pree I., Shamji M.H., Kimber I., Valenta R., Durham S.R., Niederberger V. Inhibition of CD23-dependent facilitated allergen binding to B cells following vaccination with genetically modified hypoallergenic Bet v 1 molecules. Clin. Exp. Allergy. 2010;40:1346–1352. doi: 10.1111/j.1365-2222.2010.03548.x. [DOI] [PubMed] [Google Scholar]
- 151.Yu P., Kosco-Vilbois M., Richards M., Köhler G., Lamers M.C. Negative feedback regulation of IgE synthesis by murine CD23. Nature. 1994;369:753–756. doi: 10.1038/369753a0. [DOI] [PubMed] [Google Scholar]
- 152.Cheng L.E., Wang Z., Locksley R.M. Murine B cells regulate serum IgE levels in a CD23-dependent manner1. J. Immunol. 2010;185:5040–5047. doi: 10.4049/jimmunol.1001900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Reginald K., Eckl-Dorna J., Zafred D., Focke-Tejkl M., Lupinek C., Niederberger V., Keller W., Valenta R. Different modes of IgE binding to CD23 revealed with major birch allergen, Bet v 1-specific monoclonal IgE. Immunol. Cell Biol. 2013;91:167–172. doi: 10.1038/icb.2012.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Holm J., Willumsen N., Würtzen P.A., Christensen L.H., Lund K. Facilitated antigen presentation and its inhibition by blocking IgG antibodies depends on IgE repertoire complexity. J. Allergy Clin. Immunol. 2011;127:1029–1037. doi: 10.1016/j.jaci.2011.01.062. [DOI] [PubMed] [Google Scholar]
- 155.Vangelista L., Laffer S., Turek R., Gronlund H., Sperr W.R., Valent P., Pastore A., Valenta R. The immunoglobulin-like modules Cε3 and α2 are the minimal units necessary for human IgE-FcεRI interaction. J. Clin. Investig. 1999;103:1571–1578. doi: 10.1172/JCI6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Garman S.C., Wurzburg B.A., Tarchevskaya S.S., Kinet J.-P., Jardetzky T.S. Structure of the Fc fragment of human IgE bound to its high-affinity receptor FcεRIα. Nature. 2000;406:259–266. doi: 10.1038/35018500. [DOI] [PubMed] [Google Scholar]
- 157.Jardieu P. Anti-IgE therapy. Curr. Opin. Immunol. 1995;7:779–782. doi: 10.1016/0952-7915(95)80047-6. [DOI] [PubMed] [Google Scholar]
- 158.Heusser C. Therapeutic potential of anti-IgE antibodies. Curr. Opin. Immunol. 1997;9:805–813. doi: 10.1016/S0952-7915(97)80182-3. [DOI] [PubMed] [Google Scholar]
- 159.Stadler B.M., Rudolf M.P., Zurcher A.W., Miescher S., Vogel M. Anti-IgE in allergic sensitization. Immunol. Cell Biol. 1996;74:195–200. doi: 10.1038/icb.1996.27. [DOI] [PubMed] [Google Scholar]
- 160.Segal M., Stokes J.R., Casale T.B. Anti-immunoglobulin E therapy. World Allergy Organ. J. 2008;1:174–183. doi: 10.1097/WOX.0b013e318187a310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chan M.A., Gigliotti N.M., Dotson A.L., Rosenwasser L.J. Omalizumab may decrease IgE synthesis by targeting membrane IgE+ human B cells. Clin. Transl. Allergy. 2013;3:29. doi: 10.1186/2045-7022-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Güzelbey O., Ardelean E., Magerl M., Zuberbier T., Maurer M., Metz M. Successful treatment of solar urticaria with anti-immunoglobulin E therapy. Allergy. 2008;63:1563–1565. doi: 10.1111/j.1398-9995.2008.01879.x. [DOI] [PubMed] [Google Scholar]
- 163.Zhou B., Lin B., Li J., Qian W., Hou S., Zhang D., Kou G., Li B., Wang H., Chen Y., et al. Tolerability, pharmacokinetics and pharmacodynamics of CMAB007, a humanized anti-immunoglobulin E monoclonal antibody, in healthy chinese subjects. mAbs. 2012;4:110–119. doi: 10.4161/mabs.4.1.18349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gauvreau G.M., Arm J.P., Boulet L.-P., Leigh R., Cockcroft D.W., Davis B.E., Mayers I., Fitzgerald J.M., Dahlen B., Killian K.J., et al. Efficacy and safety of multiple doses of QGE031 (ligelizumab) versus omalizumab and placebo in inhibiting allergen-induced early asthmatic responses. J. Allergy Clin. Immunol. 2016;138:1051–1059. doi: 10.1016/j.jaci.2016.02.027. [DOI] [PubMed] [Google Scholar]
- 165.Cohen E.S., Dobson C.L., Käck H., Wang B., Sims D.A., Lloyd C.O., England E., Rees D.G., Guo H., Karagiannis S.N., et al. A novel IgE-neutralizing antibody for the treatment of severe uncontrolled asthma. mAbs. 2014;6:755–763. doi: 10.4161/mabs.28394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sheldon E., Schwickart M., Li J., Kim K., Crouch S., Parveen S., Kell C., Birrell C. Pharmacokinetics, Pharmacodynamics, and Safety of MEDI4212, an Anti-IgE Monoclonal Antibody, in Subjects with Atopy: A Phase I Study. Adv. Ther. 2016;33:225–251. doi: 10.1007/s12325-016-0287-8. [DOI] [PubMed] [Google Scholar]
- 167.Eggel A., Baumann M.J., Amstutz P., Stadler B.M., Vogel M. DARPins as Bispecific Receptor Antagonists Analyzed for Immunoglobulin E Receptor Blockage. J. Mol. Boil. 2009;393:598–607. doi: 10.1016/j.jmb.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 168.Eggel A., Baravalle G., Hobi G., Kim B., Buschor P., Forrer P., Shin J.-S., Vogel M., Stadler B.M., Dahinden C.A., et al. Accelerated dissociation of IgE-FcεRI complexes by disruptive inhibitors actively desensitizes allergic effector cells. J. Allergy Clin. Immunol. 2014;133:1709–1719. doi: 10.1016/j.jaci.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zellweger F., Gasser P., Brigger D., Buschor P., Vogel M., Eggel A. A novel bispecific darpin targeting FcγRIIB and FcεRI-bound IgE inhibits allergic responses. Allergy. 2017;72:1174–1183. doi: 10.1111/all.13109. [DOI] [PubMed] [Google Scholar]
- 170.Hellman L. Profound reduction in allergen sensitivity following treatment with a novel allergy vaccine. Eur. J. Immunol. 1994;24:415–420. doi: 10.1002/eji.1830240222. [DOI] [PubMed] [Google Scholar]
- 171.Peng Z., Liu Q., Wang Q., Rector E., Ma Y., Warrington R. Novel IgE peptide-based vaccine prevents the increase of IgE and down-regulates elevated IgE in rodents. Clin. Exp. Allergy. 2007;37:1040–1048. doi: 10.1111/j.1365-2222.2007.02741.x. [DOI] [PubMed] [Google Scholar]
- 172.Sethi G., Ahn K.S., Pandey M.K., Aggarwal B.B. Celastrol, a novel triterpene, potentiates TNF-induced apoptosis and suppresses invasion of tumor cells by inhibiting NF-κB regulated gene products and tak1-mediated NF-κB activation. Blood. 2007;109:2727–2735. doi: 10.1182/blood-2006-10-050807. [DOI] [PubMed] [Google Scholar]
- 173.Brightbill H.D., Jeet S., Lin Z., Yan D., Zhou M., Tan M., Nguyen A., Yeh S., Delarosa D., Leong S.R., et al. Antibodies specific for a segment of human membrane IgE deplete IgE-producing B cells in humanized mice. J. Clin. Investig. 2010;120:2218–2229. doi: 10.1172/JCI40141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gauvreau G.M., Harris J.M., Boulet L.P., Scheerens H., Fitzgerald J.M., Putnam W.S., Cockcroft D.W., Davis B.E., Leigh R., Zheng Y., et al. Targeting membrane-expressed IgE B cell receptor with an antibody to the M1 prime epitope reduces IgE production. Sci. Transl. Med. 2014;6:243ra85. doi: 10.1126/scitranslmed.3008961. [DOI] [PubMed] [Google Scholar]
- 175.Scheerens H., Samineni D., Cochran C., Staubach P., Metz M., Sussman G., Maurer M., Harris J.M., Cabanski C.R., Bradley M.S. A randomized trial of quilizumab in adults with refractory chronic spontaneous urticaria. J. Allergy Clin. Immunol. 2016;138:1730–1732. doi: 10.1016/j.jaci.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 176.Kirak O., Riethmuller G. A novel, nonanaphylactogenic, bispecific IgE-CD3 antibody eliminates IgE+ B cells. J. Allergy Clin. Immunol. 2015;136:800–802. doi: 10.1016/j.jaci.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 177.Rudolf M.P., Zuercher A.W., Nechansky A., Ruf C., Vogel M., Miescher S.M., Stadler B.M., Kricek F. Molecular Basis for Nonanaphylactogenicity of a Monoclonal Anti-IgE Antibody. J. Immunol. 2000;165:813–819. doi: 10.4049/jimmunol.165.2.813. [DOI] [PubMed] [Google Scholar]
- 178.Nyborg A.C., Zacco A., Ettinger R., Jack Borrok M., Zhu J., Martin T., Woods R., Kiefer C., Bowen M.A., Suzanne Cohen E., et al. Development of an antibody that neutralizes soluble IgE and eliminates IgE expressing B cells. Cell. Mol. Immunol. 2016;13:391–400. doi: 10.1038/cmi.2015.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Chu S.Y., Horton H.M., Pong E., Leung I.W., Chen H., Nguyen D.-H., Bautista C., Muchhal U.S., Bernett M.J., Moore G.L., et al. Reduction of total IgE by targeted coengagement of IgE B-cell receptor and FcγRIIb with Fc-engineered antibody. J. Allergy Clin. Immunol. 2012;129:1102–1115. doi: 10.1016/j.jaci.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 180.Morita H., Tamari M., Motomura K., Koezuka Y., Ichien G., Matsumoto K., Ishizaka K., Saito H. IgE-class-specific immunosuppression in offspring by administraton of anti-IgE to pregnant mice. J. Allergy Clin. Immunol. 2019;143:1261–1264. doi: 10.1016/j.jaci.2018.11.008. [DOI] [PubMed] [Google Scholar]