Abstract
Soluble HLA‐G (sHLA‐G) levels in human seminal plasma (SP) can be diverse and may affect the establishment of maternal‐fetal tolerance and thereby the outcome of pregnancy. We investigated whether sHLA‐G levels in SP are associated with polymorphisms in the 3′‐untranslated region (UTR) and UTR haplotypes of the HLA‐G gene. Furthermore, we compared the HLA‐G genotype distribution and sHLA‐G levels between men, whose partner experienced unexplained recurrent miscarriage (RM), and controls. Soluble HLA‐G levels (n = 156) and HLA‐G genotyping (n = 176) were determined in SP samples. The concentration of sHLA‐G was significantly associated with several single‐nucleotide polymorphisms (SNPs): the 14 base pair (bp) insertion/deletion (indel), +3010, +3142, +3187, +3196, and + 3509. High levels of sHLA‐G were associated with UTR‐1 and low levels with UTR‐2, UTR‐4, and UTR‐7 (P < .0001). HLA‐G genotype distribution and sHLA‐G levels in SP were not significantly different between the RM group (n = 44) and controls (n = 31). In conclusion, seminal sHLA‐G levels are associated with both singular SNPs and 3UTR haplotypes. HLA‐G genotype and sHLA‐G levels in SP are not different between men whose partner experienced RM and controls, indicating that miscarriages are not solely the result of low sHLA‐G levels in SP. Instead, it is more likely that these miscarriages are the result of a multifactorial immunologic mechanism, whereby the HLA‐G 3′UTR 14 bp ins/ins genotype plays a role in a proportion of the cases. Future studies should look into the functions of sHLA‐G in SP and the consequences of low or high levels on the chance to conceive.
Keywords: immunology, pregnancy, recurrent miscarriage, seminal plasma, soluble HLA‐G
Abbreviations
- 14 bp indel
14 base pair insertion/deletion
- 3′UTR
3′‐untranslated region
- ELISA
enzyme‐linked immunosorbent assay
- Ins/del
insertion/deletion
- IVF
in vitro fertilization
- miRNA
microRNA
- RM
recurrent miscarriage
- sHLA
soluble HLA
- SNP
single‐nucleotide polymorphisms
- SP
seminal plasma
- UTR
untranslated region
- UTR‐N
untranslated region new
1. INTRODUCTION
Semen contains various immunomodulatory factors, such as chemokines and cytokines,1 but also soluble human leukocyte antigens (sHLA), which together can induce a local immune response in an immune regulatory environment.2 The presence of seminal plasma (SP) in the female reproductive tract after coitus can lead to an influx of immune cells, for example, the number of CD14+ macrophages and CD1a + dendritic cells were shown to be approximately 2‐fold increased upon semen exposure.3 Immune recognition of paternal antigens may play a role in pregnancy complications: change of partner is a risk factor for intrauterine growth restriction, preterm birth, low birth weight and infant mortality, and it counteracts the protective effect of multiparity against preeclampsia.4, 5, 6 Additionally, the length of unprotected sexual cohabitation affects the incidence of pregnancy‐induced hypertensive disorders,7, 8 and oral exposure to semen is correlated with a diminished occurrence of preeclampsia.2 Furthermore, preeclampsia occurs more frequently in pregnancies induced by artificial insemination with donor semen.9 Combined, these findings indicate that exposure to paternal antigens prior to gestation has a beneficial effect on pregnancy outcome. Besides the classical HLA antigens, SP contains soluble HLA‐G (sHLA‐G).10 Compared to classical HLA, HLA‐G shows a low level of polymorphism, and does not have a major role in antigen presentation. The primary function of HLA‐G lies most probably in regulating immune functions through interaction with receptors on various immune cell subsets.11 Whereas HLA‐G can inhibit the cytotoxic function of both natural killer (NK) cells and CD8+ T cells, it is also involved in the induction of immunoregulatory antigen presenting cells and CD4+ T‐cells.12, 13, 14 Furthermore, the presence of sHLA‐G has been shown to be beneficial for the success rate of assisted reproduction techniques.15
The level of sHLA‐G in body fluids appears to be related to specific polymorphisms in the 3′‐untranslated region (3′UTR) of the HLA‐G gene. The 14 base pair (bp) insertion/deletion (indel) polymorphism has shown to be associated with sHLA‐G levels in blood and semen.16, 17 Furthermore, the G/C at position +3142 and the G/A at position +3187, which are involved in microRNA (miRNA)‐mediated post‐transcriptional regulation, seem to influence sHLA‐G levels in blood.18, 19 Other SNPs, such as +3003 T/C, +3010 G/C, +3027 C/A, and + 3035 C/T have been proposed as potential miRNA binding sites,20 but they have not been studied extensively in relation to sHLA‐G levels. At least eight polymorphisms together make up UTR haplotypes.21 UTR haplotypes containing the 14 bp deletion (ie, UTR‐1) are associated with high sHLA‐G levels in blood plasma, whereas those with the 14 bp insertion (ie, UTR‐7) are associated with low sHLA‐G levels.22
Although several studies have demonstrated associations between HLA‐G 3′UTR polymorphic sites and sHLA‐G concentration, these were solely focused on sHLA‐G concentrations in blood plasma.22 The association between the 14 bp indel polymorphic site and sHLA‐G levels was previously evaluated in SP,17 but the full 3′UTR region was not included. Here, we assess for the first time the correlation between sHLA‐G levels in semen samples with the sequence of multiple HLA‐G 3′UTR variation sites determining extensive haplotypes. Low levels of HLA‐G in women have been associated with recurrent miscarriage (RM),23, 24 but the effect of sHLA‐G in semen on RM has not been studied. Additionally, we studied sHLA‐G levels in SP of couples with a history of RM, with the aim to determine whether aberrant sHLA‐G levels in SP could be an explanation for these couples experiencing RM.
2. MATERIALS AND METHODS
2.1. Study samples
Semen samples were obtained from 156 men visiting the reproductive medicine clinic at the Leiden University Medical Center (LUMC). Of these, 101 semen samples were obtained from men visiting the in vitro fertilization (IVF) clinic. SP samples were collected through masturbation and samples containing leukocytes, as a marker for infection, were excluded from this study. Forty‐four samples were collected from men enrolled in a study of couples with a history of RM. These couples had experienced at least three miscarriages, for which the cause remained unknown after a full clinical work‐up at the reproductive medicine clinic at the LUMC. Blood collected from men of RM couples was used for HLA‐G genotyping. As a control group, we collected blood and semen samples from men, who fathered at least one live birth and did not have a history of RM. We obtained 31 unique blood samples from these controls and 11 unique semen samples. Within 4 hours after collection, semen samples were centrifuged at 2000 rpm for 10 minutes, sperm cells were discarded and aliquots of SP were stored at −80°C.
2.2. HLA‐G genotype determination
HLA‐G genotype determination has previously been described.25 In short, genomic DNA was isolated from blood or from SP, when blood was not available. The 699/713‐bp fragment covering the 3′UTR of exon 8 was sequenced, starting just before the 14 bp insertion/deletion and ending 591 bp downstream of the insertion/deletion. To sequence the haplotype on each of the two alleles, amplification reactions were performed using the generic 3′‐primer that was tailed with a M13 sequence to cover the 3′UTR region of HLA‐G. The following polymorphisms were identified: the 14 bp insertion/deletion (rs371194629), +3003C > T (rs1707), +3010G > C (rs1710), +3027C > A (rs17179101), +3035C > T (rs17179108), +3142C > G (rs1063320), +3187A > G (rs9380142), +3196C > G (rs1610696), +3422C > T (rs17875408), +3496A > G (rs1233330), and + 3509G > T (rs1611139).
UTR haplotypes were composed based on the combination of eight SNPs. Nomenclature was used according to Castelli et al.21 In case the combination of SNPs could not fit any of the established UTR haplotypes, these samples were categorized as UTR‐N. Conversion of sequencing data to UTR haplotypes was carried out by using a specialized HLA interpretation software tool (SBT Engine, GenDX, Utrecht, The Netherlands). The forward primer (GTGATGGGCTGTTTAAAGTGTCACC), the reverse primer (GACGTTGTAAAACGACGGCCAGTAGGGGAAGAGGTGTAGGGGTCTG), and the M13 universal primer (GACGTTGTAAAACGACGGCCAGT) were ordered from Sigma (St. Louis, Missouri). The underlining represents the M13 sequence.
2.3. Soluble HLA‐G determination
For sHLA‐G determination, samples were thawed at room temperature and centrifuged at 14 000 rpm for 4 minutes. The level of soluble HLA‐G1/HLA‐G5 molecules in the plasma samples was determined by a commercially available sandwich enzyme‐linked immunosorbent assay (ELISA) (EXBIO, Praha, Czech Republic) according to the manufacturer's instructions. This ELISA specifically detects soluble HLA‐G1 and HLA‐G5 in a β2‐microglobulin‐associated form. The limit of detection was 0.6 units/mL. The standard curve ranged from 3.9 to 125 units/mL. Samples were tested in the assay at 1:5 and 1:10 dilution, using dilution buffer 1 of the kit. Subsequently, samples were measured at different dilutions to remain in the linear part of the standard curve (ranging from 1:2 to 1:100).
Samples were run in duplicate and mean absorbance was measured at 450 nm wavelength using a BIO‐RAD Microplate Reader and Microplate Manager 6 software (Hercules, California). Calculations were performed according to the manufacturer's guidelines. Standard curves based on the absorbance of calibrators of known concentrations were used for the determination of sHLA‐G concentration in the samples of interest. Results were expressed as units/mL.
2.4. Statistical analysis
Statistical analyses were performed using GraphPad Prism version 7.02 for Windows (GraphPad Software, California) and SPSS Statistics 23 (IBM SPSS Software, New York). Normality of distribution was examined with D'Agostino & Pearson normality test. Differences between groups were tested by Mann‐Whitney U tests or χ² tests. P‐values of <.05 were considered to indicate statistical significance. Spearman's correlation coefficient (r) was used to demonstrate the relationship between the volume of the ejaculate and the sHLA‐G concentration. Distribution of genotype frequencies among groups was tested by a Kruskal‐Wallis test. The association between the presence of specific HLA‐G genotypes in RM or healthy controls semen samples was studied with binary logistic regression. For each HLA‐G genotype, the highest prevalence was defined as the reference group. If percentages in a group were below 5%, no calculations were performed. For the calculations on the HLA‐G genotypes, Dunn's post hoc test was used to correct for multiple comparisons. Observed heterozygosity in both groups was computed by the direct counting method. Adherences of genotypic proportions to expectations under Hardy‐Weinberg equilibrium were tested separately for each SNP using PyPop 0.7.0 software (California, USA).26
3. RESULTS
3.1. HLA‐G genotype and distributions
We analyzed multiple SNPs to distinguish HLA‐G 3′UTR haplotypes. All genotyped SNPs fitted the Hardy‐Weinberg (HWE) expected proportions, except for +3003 and + 3010 (Table S1). When these HWE analyses were performed for the three groups separately, only the IVF group did not fit the HWE analysis for the +3010, whereas the other two groups did (Table S2).
3.2. sHLA‐G levels in seminal plasma are associated with HLA‐G 3′UTR haplotype and HLA‐G 3′UTR polymorphisms
Soluble HLA‐G levels did not fit a Gaussian distribution (P < .0001) and therefore, we used non‐parametric statistical tests. Median sHLA‐G levels for all HLA‐G 3′UTR haplotypes can be found in Table 1. For some controls, only the HLA‐G 3′UTR genotype was determined, but we did not have SP samples to determine sHLA‐G concentrations (“missing” in Table 1). The level of sHLA‐G was not influenced by the volume of the ejaculate (Figure S2). Since with heterozygous 3′UTR haplotypes combinations (diplotypes), it is unclear which haplotypes has the most dominant influence on sHLA‐G levels, we analyzed homozygous samples. Homozygous haplotypes showed significant differences between UTR‐1, UTR‐2, UTR‐3, UTR‐4, and UTR‐7 (P < .0001) (Figure 1). Dunn's post hoc test showed that sHLA‐G levels between UTR‐1 (median: 639.4 units/mL) and UTR‐2 (median: 102.5 units/mL; P < .0001) and between UTR‐1 and UTR‐4 (median: 132.4 units/mL; P = .0377) were significantly different after correction for multiple comparisons.
Table 1.
Soluble HLA‐G in seminal plasma per haplotype
| n | Missing | % | Median | Minimum | Maximum | |
|---|---|---|---|---|---|---|
| UTR‐1 | 93 | 13 | 26.4 | 639.4 | 93.0 | 4988.0 |
| UTR‐2 | 92 | 10 | 26.1 | 102.5 | 23.6 | 1799.1 |
| UTR‐3 | 43 | 7 | 12.2 | 255.9 | 62.6 | 2408.0 |
| UTR‐4 | 64 | 7 | 18.8 | 132.4 | 23.0 | 3917.3 |
| UTR‐5 | 11 | 0 | 3.1 | 256.8 | 108.0 | 1838.8 |
| UTR‐6 | 2 | 0 | 0.6 | 1582.6 | 909.6 | 2255.6 |
| UTR‐7 | 23 | 2 | 6.5 | 159.0 | 58.2 | 1769.5 |
| UTR‐8 | 1 | 0 | 0.3 | 206.5 | 206.5 | 206.5 |
| UTR‐10 | 2 | 0 | 0.6 | 202.8 | 108.0 | 297.7 |
| UTR‐18 | 4 | 0 | 1.1 | 2578.6 | 1848.0 | 4642.7 |
| UTR‐N | 17 | 1 | 4.8 | 425.8 | 23.0 | 4642.7 |
| Total | 312 | 40 | 100% |
Abbreviation: UTR, untranslated region; UTR‐N, untranslated region new. The 3'UTR haplotype nomenclature is consistent with publication by Castelli et al.
Figure 1.
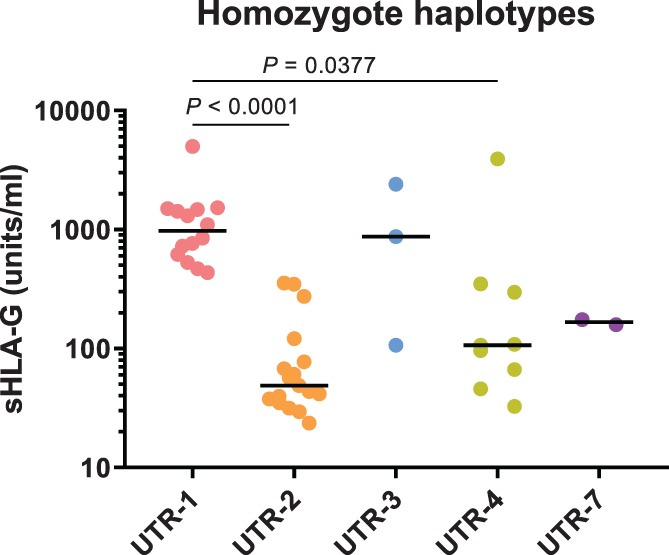
sHLA‐G levels in SP for separate homozygous haplotypes. sHLA‐G levels in SP are significantly different for the homozygous HLA‐G 3′UTR haplotypes UTR‐1, UTR‐2, UTR‐3, UTR‐4 and UTR‐7 (P < .0001). Dunn's post hoc test showed that sHLA‐G levels in UTR‐1 and UTR‐2 (P < .0001) and UTR‐1 and UTR‐4 (P = .0377) were significantly different after correcting for multiple comparisons
To evaluate whether specific SNPs are involved in differences in sHLA‐G levels per haplotype, we analyzed sHLA‐G levels for SNPs separately. The concentration of sHLA‐G in SP samples was significantly associated with the 14 bp ins/del, +3003 C/T, +3010 C/G, +3142 C/G, +3187 A/G, +3196 C/G, +3496 A/G and + 3509 G/T polymorphic sites in the 3′UTR part of the HLA‐G gene (Figure 2A‐F and Table S3).
Figure 2.
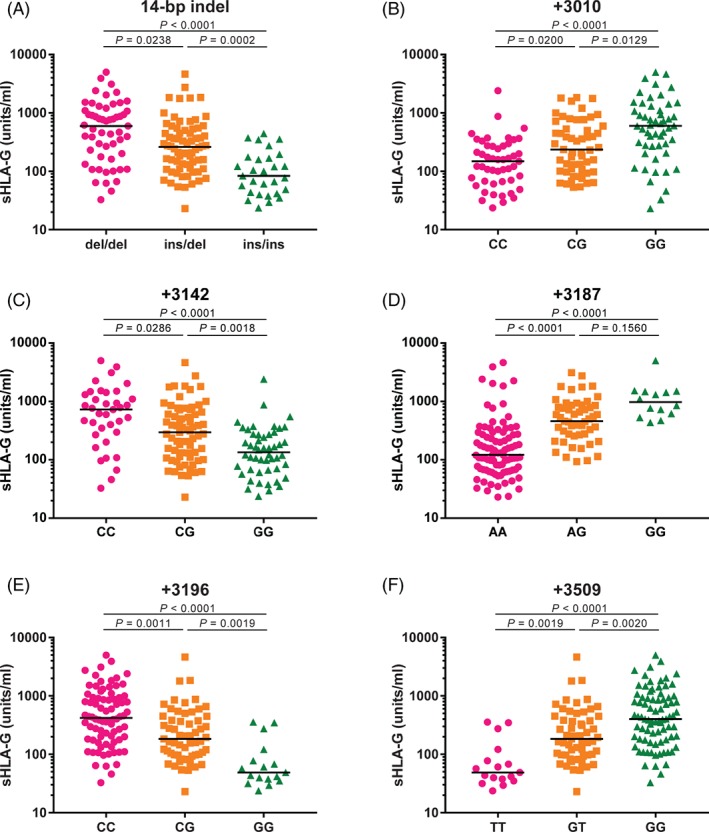
The soluble HLA‐G (sHLA‐G) concentration in seminal plasma (SP) samples is associated with several SNPs of the 3′UTR part HLA‐G gene. The concentration of sHLA‐G in SP samples is significantly associated with (A) 14 bp ins/del, (B) +3010 C/G, (C) +3142 C/G, (D) +3187 A/G, (E) +3196 C/G and (F) +3509 G/T polymorphic sites in the HLA‐G 3′UTR after correction for multiple comparisons
The 14 bp del/del genotype showed the highest level of sHLA‐G, and the 14 bp ins/ins genotype showed the lowest sHLA‐G level (P < .0001). Furthermore, individuals with +3142 CC (median: 776.7 units/mL), +3196 CC (median: 443.0 units/mL), +3010 GG (median: 619.5 units/mL), +3187 GG (median: 973.1 units/mL), +3496 GG (median: 359.7 units/mL) and + 3509 GG (median: 436 units/mL) showed higher sHLA‐G levels than individuals with +3142 GG (median: 153.5 units/mL, P < .0001), +3196 GG (median: 56.74 units/mL, P < .0001), +3010 CC (median: 182.9 units/mL, P = .0013), +3187 AA (median: 121.6 units/mL, P < .0001), +3496 AA (median: 81.39 units/mL, P = .0095), and + 3509 TT genotypes (median: 56.74 units/mL, P < .0001), respectively. Dunn's post hoc test for multiple comparisons showed significant differences for all these polymorphisms, except for +3003 and + 3496 (Figure 2A‐F).
Analysis of the IVF group and the RM group separately showed similar associations between HLA‐G genotype and sHLA‐G levels, although significance for several SNPs was lost after multiple comparisons due to small samples sizes (data not shown). The group with fertile controls was too small for separate analysis.
3.3. sHLA‐G levels in seminal plasma of RM group and controls
To evaluate whether differences in HLA‐G genotype and sHLA‐G levels could be found for semen samples of men, whose partner experienced RM, we analyzed groups separately. No differences in frequency for individual SNPs (Table S4) or in haplotype distribution (Table 2) were found between semen samples from the RM group and semen samples from controls. However, although not significant, the frequency of the 14 bp ins/ins genotype, which was associated with low levels of sHLA‐G, was three times higher in the RM group than in controls (18% vs 6%, P = .137). The median concentration of sHLA‐G was 269.7 units/mL in all SP samples compared to 233.8 units/mL in SP samples from men with a history of RM and 297.3 units/mL in SP samples of healthy controls (Table 3). The levels of sHLA‐G were not significantly different between the RM group and controls (Figure S1).
Table 2.
HLA‐G 3′UTR haplotype frequencies in determined (RM) semen samples and samples of fertile controls
| RM (2n = 88) | % | Fertile controls (2n = 62) | % | OR | Lower (95% CI) | Upper (95% CI) | P‐value | |
|---|---|---|---|---|---|---|---|---|
| UTR‐1 | 23 | 26.1 | 17 | 27.4 | 0.937 | 0.450 | 1.950 | .861 |
| UTR‐2 | 25 | 28.4 | 17 | 27.4 | 1.050 | 0.509 | 2.169 | .894 |
| UTR‐3 | 12 | 13.6 | 8 | 12.9 | 1.066 | 0.408 | 2.784 | .897 |
| UTR‐4 | 18 | 20.5 | 15 | 24.2 | 0.806 | 0.370 | 1.755 | .587 |
| UTR‐5 | 4 | 4.5 | 0 | 0 | Inf. | 0.000 | Inf. | .999 |
| UTR‐7 | 3 | 3.4 | 3 | 4.8 | 0.694 | 0.135 | 3.558 | .662 |
| UTR‐N | 3 | 3.4 | 2 | 3.2 | 1.059 | 0.172 | 6.531 | .951 |
Note: All univariate logistic regression analyses.
Abbreviations: CI, confidence interval; OR, odds ratio; UTR, untranslated region; UTR‐N, untranslated region new.
The 3′UTR haplotype nomenclature is consistent with publication by Castelli et al.
Table 3.
Soluble HLA‐G levels in semen samples
| All (n = 176) | IVF (n = 101) | RM (n = 44) | Fertile controls (n = 11) | |
|---|---|---|---|---|
| Missing | 20 | 0 | 0 | 20 |
| Median | 269.67 | 271.38 | 233.77 | 297.26 |
| Mean | 546.93 | 600.59 | 477.67 | 331.18 |
| SD | 794.02 | 872.29 | 684.92 | 211.53 |
| Minimum | 23.03 | 23.03 | 27.43 | 100.50 |
| Maximum | 4988.79 | 4988.79 | 3917.29 | 851.32 |
Abbreviations: IVF, in vitro fertilization; RM, recurrent miscarriage.
4. DISCUSSION
In this study, we showed an association of sHLA‐G levels with HLA‐G 3′UTR haplotypes, as well as with singular SNPs. Furthermore, there was no significant difference in HLA‐G genotype and sHLA‐G levels in semen between men whose partner had a history of RM and controls.
When comparing genotype frequencies to expected HWE frequencies for each group, we observed that the IVF group deviates from HWE for the +3010 polymorphism, whereas the other two groups fit. Since the samples in this study group are not from healthy controls, this could indicate that this SNP may play a role in conception, but additional research is required to draw any conclusions.
Regarding HLA‐G 3′UTR haplotypes we found five haplotypes exhibiting frequencies higher than 5% (UTR‐1, UTR‐2, UTR‐3, UTR‐4, UTR‐7) and five others with lower frequencies (UTR‐5, UTR‐6, UTR‐8, UTR‐10, UTR‐18). Some combinations of SNPs did not fit any of the established UTR haplotypes and were therefore categorized as UTR‐N. In line with previous data,27 UTR‐1 and UTR‐2 were the most frequently observed haplotypes. We reported the frequencies of 11 polymorphic sites: 14 bp ins/del, +3003C/T, +3010C/G, +3027A/C, +3035C/T, +3142C/G, +3187A/G, +3196C/G, +3422C/T, +3496A/G and + 3509G/T. The most studied polymorphism of the 3′UTR of the HLA‐G gene is the 14 bp indel polymorphism, which has been associated with altered HLA‐G expression. We observed that individuals exhibiting the 14 bp del/del genotype indeed exhibited higher sHLA‐G levels in SP compared to the 14 bp ins/ins genotype. This is in line with other studies, describing the association between the 14 bp insertion allele and decreased levels of sHLA‐G in blood plasma and serum.16, 28, 29 It is suggested that the insertion of 14 bases leads to removal of 92 bases from the start of exon 8, affecting mRNA stability and degradation rate.30
Other SNPs at the 3′UTR, which are associated with HLA‐G expression levels, are represented by the presence of guanine in the position +3142, which increases the affinity of specific miRNA for HLA‐G mRNA, leading to decreased HLA‐G expression.19 Another SNP is represented by the presence of an adenine at position +3187, decreasing the stability of HLA‐G mRNA.18 Indeed, we did observe lower sHLA‐G levels in the semen of individuals with +3142 GG or + 3187 AA genotype. Of all haplotypes found in this study, the only haplotype presenting a guanine at position +3187 is UTR‐1. Moreover, taking the possible effect of each of the known variation sites that may influence HLA‐G production together, UTR‐1 is theoretically the most suitable to produce high HLA‐G amounts, because it is the only UTR that harbors the +3187 G allele, as well as the +3142 C and the 14 bp del. Indeed, in the present study UTR‐1 was clearly associated with higher levels of sHLA‐G. UTR‐2 and UTR‐7 both harbor the +3187 A allele, as well as the +3142 G and the 14 bp ins. In line with our expectations, these UTR haplotypes were associated with low HLA‐G levels. Remarkably, the UTR‐4 was generally associated with low sHLA‐G levels, even though this haplotype harbors the 14 bp del and the +3142 C. It appears that the influence of adenine at position +3187 on sHLA‐G levels is very strong or that another yet unknown factor influences the level of sHLA‐G in these cases.
We found a higher incidence of the 14 bp ins/ins in men whose partner experienced RM (18%) compared to controls (6%), although this difference was not significant. Taking into account that this genotype is associated with lower sHLA‐G levels, this may underline the concept that rather a multifactorial process accounts for miscarriage.
We were restricted to collecting one semen sample per man. Therefore, we were not able to analyze sHLA‐G concentrations over time and we cannot exclude the possibility that sHLA‐G levels in SP fluctuate over time.
In summary, we provided data on the impact of the most frequent HLA‐G 3′UTR variation sites on sHLA‐G levels in SP, and conclude that sHLA‐G levels in SP are influenced by HLA‐G haplotypes and separate SNPs. On the population level, we did not find differences in sHLA‐G levels between SP samples from RM and controls, indicating that miscarriages cannot solely be explained by HLA‐G genes and low sHLA‐G levels in SP. Instead, it is more likely that these miscarriages are the result of a multifactorial immunologic mechanism, in which the HLA‐G 3′UTR 14 bp ins/ins genotype plays a role in a proportion of the cases. Future studies should look into the functions of sHLA‐G in SP and the consequences of low or high levels on the chance to conceive.
Supporting information
FIGURE S1 Soluble HLA‐G (sHLA‐G) concentrations in seminal plasma (SP) samples from recurrent miscarriage (RM) cases and controls. sHLA‐G concentrations in SP samples were not significantly different between men whose partner experienced RM (median 233.8 units/mL) and controls (median 297.3 units/mL).
FIGURE S2 Soluble HLA‐G (sHLA‐G) concentrations in relation to semen volumes (n = 177). sHLA‐G concentrations are not associated with semen volume (Spearman's r = −0.0533).
TABLE S1 Hardy‐Weinberg analyses for HLA‐G 3′UTR genotypes in all semen samples
TABLE S2 Hardy‐Weinberg analyses for HLA‐G 3′UTR genotypes in separate groups of semen samples
TABLE S3 Comparisons of seminal plasma soluble HLA‐G levels (units/ml) in the whole group, stratified according to the SNPs in the HLA‐G 3′UTR
TABLE S4 HLA‐G 3′UTR SNPs in RM samples and fertile controls
ACKNOWLEDGMENTS
We thank the fertility clinic of the Leiden University Medical Center for providing the SP samples. This research was not supported by any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
Craenmehr MHC, Haasnoot GW, Drabbels JJM, et al. Soluble HLA‐G levels in seminal plasma are associated with HLA‐G 3′UTR genotypes and haplotypes. HLA. 2019;94:339–346. 10.1111/tan.13628
Funding information Leiden University Medical Center
DATA ACCESSIBILITY
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Politch JA, Tucker L, Bowman FP, Anderson DJ. Concentrations and significance of cytokines and other immunologic factors in semen of healthy fertile men. Hum Reprod. 2007;22(11):2928‐2935. [DOI] [PubMed] [Google Scholar]
- 2. Koelman CA, Coumans AB, Nijman HW, Doxiadis II, Dekker GA, Claas FH. Correlation between oral sex and a low incidence of preeclampsia: a role for soluble HLA in seminal fluid? J Reprod Immunol. 2000;46(2):155‐166. [DOI] [PubMed] [Google Scholar]
- 3. Sharkey DJ, Tremellen KP, Jasper MJ, Gemzell‐Danielsson K, Robertson SA. Seminal fluid induces leukocyte recruitment and cytokine and chemokine mRNA expression in the human cervix after coitus. J Immunol. 2012;188(5):2445‐2454. [DOI] [PubMed] [Google Scholar]
- 4. Vatten LJ, Skjaerven R. Effects on pregnancy outcome of changing partner between first two births: prospective population study. BMJ. 2003;327(7424):1130‐1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kleijer ME, Dekker GA, Heard AR. Risk factors for intrauterine growth restriction in a socio‐economically disadvantaged region. J Matern Fetal Neonatal Med. 2005;18(1):23‐30. [DOI] [PubMed] [Google Scholar]
- 6. Tubbergen P, Lachmeijer AM, Althuisius SM, Vlak ME, van Geijn HP, Dekker GA. Change in paternity: a risk factor for preeclampsia in multiparous women? J Reprod Immunol. 1999;45(1):81‐88. [DOI] [PubMed] [Google Scholar]
- 7. Verwoerd GR, Hall DR, Grove D, Maritz JS, Odendaal HJ. Primipaternity and duration of exposure to sperm antigens as risk factors for pre‐eclampsia. Int J Gynaecol Obstet. 2002;78(2):121‐126. [DOI] [PubMed] [Google Scholar]
- 8. Robillard PY, Hulsey TC, Perianin J, Janky E, Miri EH, Papiernik E. Association of pregnancy‐induced hypertension with duration of sexual cohabitation before conception. Lancet. 1994;344(8928):973‐975. [DOI] [PubMed] [Google Scholar]
- 9. Salha O, Sharma V, Dada T, et al. The influence of donated gametes on the incidence of hypertensive disorders of pregnancy. Hum Reprod. 1999;14(9):2268‐2273. [DOI] [PubMed] [Google Scholar]
- 10. Larsen MH, Bzorek M, Pass MB, et al. Human leukocyte antigen‐G in the male reproductive system and in seminal plasma. Mol Hum Reprod. 2011;17(12):727‐738. [DOI] [PubMed] [Google Scholar]
- 11. Persson G, Melsted WN, Nilsson LL, Hviid TVF. HLA class Ib in pregnancy and pregnancy‐related disorders. Immunogenetics. 2017;69(8–9):581‐595. [DOI] [PubMed] [Google Scholar]
- 12. Rouas‐Freiss N, Goncalves RM, Menier C, Dausset J, Carosella ED. Direct evidence to support the role of HLA‐G in protecting the fetus from maternal uterine natural killer cytolysis. Proc Natl Acad Sci U S A. 1997;94(21):11520‐11525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ristich V, Liang S, Zhang W, Wu J, Horuzsko A. Tolerization of dendritic cells by HLA‐G. Eur J Immunol. 2005;35(4):1133‐1142. [DOI] [PubMed] [Google Scholar]
- 14. Selmani Z, Naji A, Zidi I, et al. Human leukocyte antigen‐G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. 2008;26(1):212‐222. [DOI] [PubMed] [Google Scholar]
- 15. Rebmann V, Switala M, Eue I, Schwahn E, Merzenich M, Grosse‐Wilde H. Rapid evaluation of soluble HLA‐G levels in supernatants of in vitro fertilized embryos. Hum Immunol. 2007;68(4):251‐258. [DOI] [PubMed] [Google Scholar]
- 16. Chen XY, Yan WH, Lin A, Xu HH, Zhang JG, Wang XX. The 14 bp deletion polymorphisms in HLA‐G gene play an important role in the expression of soluble HLA‐G in plasma. Tissue Antigens. 2008;72(4):335‐341. [DOI] [PubMed] [Google Scholar]
- 17. Dahl M, Perin TL, Djurisic S, et al. Soluble human leukocyte antigen‐G in seminal plasma is associated with HLA‐G genotype: possible implications for fertility success. Am J Reprod Immunol. 2014;72(1):89‐105. [DOI] [PubMed] [Google Scholar]
- 18. Yie SM, Li LH, Xiao R, Librach CL. A single base‐pair mutation in the 3′‐untranslated region of HLA‐G mRNA is associated with pre‐eclampsia. Mol Hum Reprod. 2008;14(11):649‐653. [DOI] [PubMed] [Google Scholar]
- 19. Tan Z, Randall G, Fan J, et al. Allele‐specific targeting of microRNAs to HLA‐G and risk of asthma. Am J Hum Genet. 2007;81(4):829‐834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Castelli EC, Moreau P, Oya e Chiromatzo A, et al. In silico analysis of microRNAS targeting the HLA‐G 3′ untranslated region alleles and haplotypes. Hum Immunol. 2009;70(12):1020‐1025. [DOI] [PubMed] [Google Scholar]
- 21. Castelli EC, Mendes‐Junior CT, Deghaide NH, et al. The genetic structure of 3'untranslated region of the HLA‐G gene: polymorphisms and haplotypes. Genes Immun. 2010;11(2):134‐141. [DOI] [PubMed] [Google Scholar]
- 22. Martelli‐Palomino G, Pancotto JA, Muniz YC, et al. Polymorphic sites at the 3′ untranslated region of the HLA‐G gene are associated with differential hla‐g soluble levels in the Brazilian and French population. PLoS One. 2013;8(10):e71742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Akhter A, Faridi RM, Das V, Pandey A, Naik S, Agrawal S. In vitro up‐regulation of HLA‐G using dexamethasone and hydrocortisone in first‐trimester trophoblast cells of women experiencing recurrent miscarriage. Tissue Antigens. 2012;80(2):126‐135. [DOI] [PubMed] [Google Scholar]
- 24. Jassem RM, Shani WS, Loisel DA, Sharief M, Billstrand C, Ober C. HLA‐G polymorphisms and soluble HLA‐G protein levels in women with recurrent pregnancy loss from Basrah province in Iraq. Hum Immunol. 2012;73(8):811‐817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Meuleman T, Drabbels J, van Lith JMM, et al. Lower frequency of the HLA‐G UTR‐4 haplotype in women with unexplained recurrent miscarriage. J Reprod Immunol. 2018;126:46‐52. [DOI] [PubMed] [Google Scholar]
- 26. Lancaster AK, Single RM, Solberg OD, Nelson MP, Thomson G. PyPop update‐‐a software pipeline for large‐scale multilocus population genomics. Tissue Antigens. 2007;69(Suppl 1):192‐197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Castelli EC, Ramalho J, Porto IO, et al. Insights into HLA‐G genetics provided by worldwide haplotype diversity. Front Immunol. 2014;5:476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hviid TV, Rizzo R, Christiansen OB, Melchiorri L, Lindhard A, Baricordi OR. HLA‐G and IL‐10 in serum in relation to HLA‐G genotype and polymorphisms. Immunogenetics. 2004;56(3):135‐141. [DOI] [PubMed] [Google Scholar]
- 29. Twito T, Joseph J, Mociornita A, Rao V, Ross H, Delgado DH. The 14‐bp deletion in the HLA‐G gene indicates a low risk for acute cellular rejection in heart transplant recipients. J Heart Lung Transplant. 2011;30(7):778‐782. [DOI] [PubMed] [Google Scholar]
- 30. Rousseau P, Le Discorde M, Mouillot G, Marcou C, Carosella ED, Moreau P. The 14 bp deletion‐insertion polymorphism in the 3' UT region of the HLA‐G gene influences HLA‐G mRNA stability. Hum Immunol. 2003;64(11):1005‐1010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1 Soluble HLA‐G (sHLA‐G) concentrations in seminal plasma (SP) samples from recurrent miscarriage (RM) cases and controls. sHLA‐G concentrations in SP samples were not significantly different between men whose partner experienced RM (median 233.8 units/mL) and controls (median 297.3 units/mL).
FIGURE S2 Soluble HLA‐G (sHLA‐G) concentrations in relation to semen volumes (n = 177). sHLA‐G concentrations are not associated with semen volume (Spearman's r = −0.0533).
TABLE S1 Hardy‐Weinberg analyses for HLA‐G 3′UTR genotypes in all semen samples
TABLE S2 Hardy‐Weinberg analyses for HLA‐G 3′UTR genotypes in separate groups of semen samples
TABLE S3 Comparisons of seminal plasma soluble HLA‐G levels (units/ml) in the whole group, stratified according to the SNPs in the HLA‐G 3′UTR
TABLE S4 HLA‐G 3′UTR SNPs in RM samples and fertile controls
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


