Abstract
Two TTX-resistant sodium channels, SNS and NaN, are preferentially expressed in c-type dorsal root ganglion (DRG) neurons and have been shown recently to have distinct electrophysiological signatures, SNS producing a slowly inactivating and NaN producing a persistent sodium current with a relatively hyperpolarized voltage-dependence. An attenuation of SNS and NaN transcripts has been demonstrated in small DRG neurons after transection of the sciatic nerve. However, it is not known whether changes in the currents associated with SNS and NaN or in the expression of SNS and NaN channel protein occur after axotomy of the peripheral projections of DRG neurons or whether similar changes occur after transection of the central (dorsal root) projections of DRG neurons.
Peripheral and central projections of L4/5 DRG neurons in adult rats were axotomized by transection of the sciatic nerve and the L4 and L5 dorsal roots, respectively. DRG neurons were examined using immunocytochemical and patch-clamp methods 9–12 d after sciatic nerve or dorsal root lesion. Levels of SNS and NaN protein in the two types of injuries were paralleled by their respective TTX-resistant currents. There was a significant decrease in SNS and NaN signal intensity in small DRG neurons after peripheral, but not central, axotomy compared with control neurons. Likewise, there was a significant reduction in slowly inactivating and persistent TTX-resistant currents in these neurons after peripheral, but not central, axotomy compared with control neurons. These results indicate that peripheral, but not central, axotomy results in a reduction in expression of functional SNS and NaN channels in c-type DRG neurons and suggest a basis for the altered electrical properties that are observed after peripheral nerve injury.
Keywords: axotomy, dorsal root ganglion, NaN, SNS, sodium channel, tetrodotoxin-resistant
Dorsal root ganglion (DRG) neurons are unusual in expressing tetrodotoxin (TTX)-resistant sodium currents in addition to TTX-sensitive currents (Kostyuk et al., 1981; McLean et al., 1988; Caffrey et al., 1992; Roy and Narahashi, 1992; Elliott and Elliott, 1993; Rizzo et al., 1994). Because of their preferential expression in nociceptive neurons, these TTX-resistant sodium currents and the channels producing them are of considerable interest. Recently, two TTX-resistant sodium channels, one termed SNS (Akopian et al., 1996) or PN3 (Sangameswaran et al., 1996) and a second termed NaN (Dib-Hajj et al., 1998b) or SNS2 (Tate et al., 1998), have been cloned and have been shown to be expressed preferentially in c-type DRG neurons. Studies in wild-type and SNS-null transgenic mice have demonstrated that these two channels have distinct electrophysiological signatures, SNS producing a slowly inactivating TTX-resistant sodium current with relatively depolarized voltage-dependence of activation and inactivation, and NaN producing a persistent sodium current with a large overlap between activation and steady-state inactivation and a relatively hyperpolarized voltage-dependence (Cummins et al., 1999;Dib-Hajj et al., 1999a). These studies demonstrate, moreover, that it is possible to separate these two TTX-resistant currents in DRG neurons using prepulse conditioning protocols that take advantage of the ultraslow inactivation of NaN current (Cummins et al., 1999). To date, these two TTX-resistant currents have not been individually studied in injured DRG neurons.
It is now well established that, after axotomy of their peripherally directed axons within the sciatic nerve, DRG neurons display lower levels of SNS (Dib-Hajj et al., 1996; Okuse et al., 1997) and NaN (Dib-Hajj et al., 1998b; Tate et al., 1998) mRNA. However, translational regulation and post-translational modulation, as well as transcriptional regulation, contribute to the control of ion channel expression within excitable cells so that changes in mRNA are not necessarily accompanied by alterations in deployment of functional channel protein (Sharma et al., 1993; Sucher et al., 1993; Hales and Tyndale, 1994; Black et al., 1998). To address this issue, we have used subtype-specific antibodies to ask whether levels of SNS and NaN protein change within DRG neurons after peripheral axotomy (sciatic nerve ligation). We also examined axotomy of centrally directed axons (dorsal rhizotomy) of the same cell types and compared these results with peripheral axotomy, because it is known that central axotomy can have different effects, compared with peripheral axotomy, on the excitability of primary sensory neurons (Czeh et al., 1977; Gallego et al., 1987; Gurtu and Smith, 1988) and on the expression of TTX-sensitive sodium channels in these neurons (Rizzo et al., 1995;Black et al., 1999a). In parallel experiments, we used patch-clamp methods to study the sodium currents produced by these two channels and show that, together with changes in channel protein expression, there are changes in the magnitudes of the two different TTX-resistant sodium currents that they produce.
MATERIALS AND METHODS
Peripheral projections of L4/5 DRG neurons in adult rats were axotomized by transection of the sciatic nerve at the mid-thigh level (Dib-Hajj et al., 1996), or central projections of L4/5 DRG neurons were axotomized by transection of L4 and L5 dorsal roots (dorsal rhizotomy) (Kenney and Kocsis, 1997). Immunocytochemical and patch-clamp methods were used 9–12 d after transection of either peripheral or central projections of DRG neurons to study the expression of sodium channels SNS and NaN and their TTX-resistant currents within these cells. SNS and NaN transcript levels in control and rhizotomized DRG were also examined by in situhybridization and quantitative reverse transcription-PCR. DRG neurons in vitro were studied after short-term (<24 hr) culture; this protocol was chosen to provide as close a match as possible to earlier studies (Cummins and Waxman, 1997), which provided quantitative patch-clamp data on the TTX-sensitive sodium currents in axotomized DRG neurons.
Surgery
For transection of the peripheral projections of the DRG neurons, adult female Sprague Dawley rats were anesthetized with ketamine (80 mg/kg, i.p.) and xylazine (5 mg/kg, i.p.), right sciatic nerves were exposed at the mid-thigh level, ligated with 4–0 silk sutures and transected, and the proximal stumps were placed in silicon cuffs to prevent regeneration (Waxman et al., 1994). Hydroxystilbamine methanesulfonate (4% w/v; Molecular Probes, Eugene, OR), the active component of Fluorogold and a retrogradely transported fluorescent label, was placed in all cuffs before insertion of the nerve stump. The fluorescent label identified neurons in which their axons were transected. The contralateral DRG served as controls.
For transection of the central projections of the DRG neurons, adult female Sprague Dawley rats were anesthetized with sodium pentobarbital (60 mg/kg, i.p.) and an L3 laminectomy was performed. An incision was made in the dura, and the L4 and L5 dorsal roots were identified and transected (dorsal rhizotomy) with iridectomy scissors. The lesion was packed with Gel-foam and the overlying muscles and skin were closed in layers with 4–0 silk sutures.
Nine to 12 d after surgery, rats were killed with an overdose of ketamine–xylazine and decapitated, and the DRG were harvested for cell culture or the rats were anesthetized with ketamine–xylazine and perfused with 4% paraformaldehyde in 0.14m Sorensen's phosphate buffer, and tissue was obtained forin situ hybridization or immunocytochemical studies.
Cell culture
Cultures of DRG neurons were established as described previously (Rizzo et al., 1994). Briefly, peripherally or centrally axotomized and control (uninjured) lumbar ganglia (L4, L5) were excised, freed from their connective tissue sheaths, and incubated sequentially in enzyme solutions containing collagenase and then papain. The tissue was triturated in culture medium containing 1:1 DMEM and Hank's F12 medium and 10% fetal calf serum, 1.5 mg/ml trypsin inhibitor, 1.5 mg/ml bovine serum albumin, 100 U/ml penicillin, and 0.1 mg/ml streptomycin and plated on polyornithine–laminin-coated coverslips. The cells were maintained at 37°C in a humidified 95% air–5% CO2 incubator overnight and then used for patch-clamp investigation or processed for immunocytochemical studies as described previously (Cummins and Waxman, 1997; Black et al., 1998).
In situ hybridization
Ten micrometer cryosections of intact control and peripherally or centrally axotomized DRG from perfused rats were processed for in situ hybridization cytochemistry with riboprobes specific for SNS and NaN as described previously (Black et al., 1996; Dib-Hajj et al., 1998b).
Quantitative PCR
RNA preparation and cDNA synthesis. Total cellular RNA was isolated from control and dorsal rhizotomized DRG (7 d after axotomy) by the single-step guanidinum isothiocyanate-acid phenol procedure (Chomczynski and Sacchi, 1987). The extraction buffer was used at 25 μl per 1 mg of tissue. The purified RNA was treated with RNase-free DNase-I (Roche Molecular Biochemicals, Indianapolis, IN) and repurified over Qiagen (Valencia, CA) RNeasy mini-column; RNA was eluted in 70 μl volume. First-strand cDNA was reverse transcribed in a final volume of 10 μl using 1 μl of purified DNA-free total RNA, 1 mm random hexamer (Roche), 40 U of SuperScript II reverse transcriptase (Life Technologies, Gaithersburg, MD), and 40 U of RNase Inhibitor (Roche Products). The buffer consisted of (in mm): 50 Tris-HCl, pH 8.3, 75 KCl, 3 MgCl2, 10 DTT, and 5 mm dNTP. The reaction was allowed to proceed at 37°C for 90 min and 42°C for 30 min and then terminated by heating to 65°C for 10 min. A similar reaction mixture lacking the reverse transcriptase enzyme was prepared and used as a template to demonstrate absence of contaminating genomic DNA (data not shown).
Real-time PCR. The concept and validation of real-time quantitative PCR have been described previously (Gibson et al., 1996;Heid et al., 1996; Winer et al., 1999). We have used the relative standard curve method to determine the SNS and NaN transcript levels in control and dorsal rhizotomized DRG. rRNA (18 S) was used as an endogenous control to normalize the expression level of the transcripts. Standard curves for 18 S rRNA and SNS and NaN were constructed using serial dilutions of cDNA from P0 DRG. Standards and experimental conditions were amplified in quadruplet. The standard curves for the sodium channel targets and 18 S rRNA endogenous control (standards) were constructed from the respective meanCt value, and the linear equation was derived using the Sequence Detection System (SDS) software (PE Biosystems, Norwalk, CT). The amount of template in the cDNA pool of the respective experimental conditions was then determined by applying the mean Ct value of that reaction in the equation of the standard curve. The level of expression of the sodium channel target was normalized to the respective 18 S rRNA value. The normalized values of control and dorsal rhizotomized samples were compared.
Primers and probes of the sodium channel targets were designed using Primer Express software (PE Biosystems) according to the specification of the TaqMan protocols (Winer et al., 1999). Primers and probes for the respective SNS and NaN are listed in Table1 (primers synthesized and purified by Life Technologies; probes synthesized and purified by PE Biosystems). Primers and probes for 18 S rRNA were obtained from PE Biosystems. Primers for the sodium channels and 18 S rRNA were used at a final concentration of 900 and 50 nm, respectively, whereas the probes were used at a final concentration of 200 nm. The primer–probe combinations are not limiting at these concentrations (data not shown). Amplification was done in a 50 μl final volume, under the following cycling conditions: 10 min at 50° and then 40 cycles of 95°, 15 sec, followed by 60°C, 1 min. Sodium channels and 18 S rRNA templates were amplified in separate wells (Gibson et al., 1996; Heid et al., 1996).
Table 1.
Sequences of sodium channel primer–probe TaqMan set
| Gene | Forward primer | Reverse primer | Probe |
|---|---|---|---|
| NaN | TGCCCTACCCACCTCACAAC | CCGGGCTAGTGAGCTGCTT | FAM-TTCAGGCCGGTGACCTCCCTCC-TAMRA |
| SNS | TGGTCAACTGCGTGTGCAT | AATCAGAGCCTCGAAGGTGTAAA | FAM-CCGAACTGATCTTCCAGAGAAAGTCGAGTACGT-TAMRA |
To determine levels of SNS and NaN transcripts, the relative standard curve method (Gibson et al., 1996; Heid et al., 1996) was used. Standard curves for endogenous control (18 S rRNA) and SNS and NaN targets were constructed using appropriate primers–probes (Table 1), and serial dilutions of cDNAs from P0 DRG. Linear equations for the two standard curves for NaN quantitation were as follows: NaN,y = 31.66 − 3.339x(R2 = 0.998); and 18 S rRNA,y = 19.86 − 2.946x(R2 = 0.993). The equations for SNS quantitation were as follows: SNS, y = 34.447 − 3.617x (R2 = 0.987); and 18 S rRNA, y = 20.774 − 2.940x (R2 = 0.994). The control and rhizotomized DRG samples were also amplified using the respective primers–probes (in separate reactions). The relative amounts of SNS and NaN targets were quantitated by linear extrapolation of the Ct values using the equation of the line obtained from the standard curve. These values were then normalized by the relative amounts of the endogenous control 18 S rRNA determined by the linear extrapolation of the respectiveCt values and line formula.
Immunocytochemistry
Antibodies. Isoform-specific polyclonal antibodies generated against unique sequences of sodium channels NaN and SNS were used in these experiments. The generation and characterization of anti-SNS (Black et al., 1999b) and anti-NaN (Fjell et al., 2000) sodium channel antibodies have been described previously.
Immunostaining. Coverslips with neurons derived from control or peripherally or centrally axotomized L4/5 DRG were maintainedin vitro for <24 hr and were processed for immunocytochemistry as follows: (1) complete saline solution, two times for 1 min each; (2) 4% paraformaldehyde in 0.14m Sorensen's phosphate buffer, 10 min; (3) PBS, three times for 3 min each; (4) PBS containing 5% normal goat serum, 2% bovine serum albumin, and 0.1% Triton X-100, 15 min; (5) primary antibody (NaN, 1:500, in blocking solution; SNS, 1:100, in blocking solution), overnight at 4°C; (6) PBS, six times for 5 min each; (7) secondary antibody (goat anti-rabbit IgG-Cy3, 1: 3000; Amersham Pharmacia Biotech, Arlington Heights, IL); and (8) PBS, six times for 5 min each.
Ten micrometer cryosections of intact control and peripherally or centrally axotomized DRG from perfused rats were also examined for SNS and NaN protein levels. The sections were mounted on poly-l-lysine-coated glass slides and processed for immunocytochemistry as described above with the following minor modifications: (1) the slides were incubated in 50 mmNH4Cl2 (20 min, room temperature) to reduce autofluorescence; (2) the slides were not incubated in 4% paraformaldehyde; and (3) the slides were incubated in blocking solution for 30–45 min. Following the immunocytochemical procedure, the slides were mounted with Aqua-poly-mount (Polysciences, Warrington, PA).
Control experiments included incubation without primary antibody and preadsorption of the antibody with 100–500 m excess of immunizing peptide. Only background levels of fluorescence were detected in the control experiments (data not shown).
Quantitative analysis
A Leitz (Wetzlar, Germany) Aristoplan light microscope equipped with bright-field, Nomarski, and epifluorescence optics was used for sample observation. IPLab Spectrum software (Scanalytics, Fairfax, VA) was used for image capture and analysis. Control and experimental conditions were evaluated in identical manners. Three separate cultures for peripheral axotomy and two separate cultures for central axotomy were examined in this study; only DRG neurons <25 μm in diameter were included in the data analysis. Coverslips were scanned from the upper left quadrant using bright-field optics with a nonoverlapping pattern, and the first 10–15 fields containing at least three identifiable small DRG neurons were captured. After capture of the bright-field image, fluorescent images were captured with Leica (Nussloch, Germany) filter blocks N2.1 (Cy3) and D (hydroxystilbamine methanesulfonate). Statistical comparisons of control and experimental groups were performed with a two-sample t test using Microsoft Excel software.
Electrophysiology
Sodium currents in small (18–27 μm in diameter) DRG neurons were studied after short-term culture (12–24 hr). Whole-cell patch-clamp recordings were conducted at room temperature (∼21° C) using an EPC-9 amplifier and the Pulse program (version 7.89). Fire-polished electrodes (0.8–1.5 MΩ) were fabricated from 1.7 mm capillary glass (VWR Scientific, Piscataway, NJ) using a Sutter Instruments (Novato, CA) P-97 puller. The average access resistance was 1.6 ± 0.7 MΩ (mean ± SD; n = 106). Voltage errors were minimized using 80–85% series resistance compensation, and the capacitance artifact was canceled using the computer-controlled circuitry of the patch-clamp amplifier. Linear leak subtraction, based on resistance estimates from four to five hyperpolarizing pulses applied before the depolarizing test potential, was used for all voltage-clamp recordings. Membrane currents were usually filtered at 2.5 kHz and sampled at 10 kHz. The pipette solution contained (in mm): 140 CsF, 1 EGTA, 10 NaCl, and 10 HEPES, pH 7.3. The standard bathing solution was (in mm): 140 NaCl, 3 KCl, 1 MgCl2, 1 CaCl2, 0.1 CdCl2, and 10 HEPES, pH 7.3. Cadmium was included to block calcium currents. The osmolarity of all solutions was adjusted to 310 mOsm.
RESULTS
SNS and NaN expression are reduced in peripherally axotomized DRG neurons
Because only ∼70% of the neurons in L4/5 DRG ganglia are axotomized by a mid-thigh transection of the sciatic nerve (Himes and Tessler, 1989) and thus are directly affected by this lesion, we have reexamined the downregulation of NaN and SNS transcripts in small DRG neurons that we identified as axotomized via backfilling with retrogradely transported fluorescent label. In addition, we have examined the levels of NaN and SNS protein in identified peripherally injured small DRG neurons and have contrasted these results with those obtained after central axotomy (dorsal rhizotomy) of the DRG neurons.
As described previously (Black et al., 1996; Sangameswaran et al., 1996; Dib-Hajj et al., 1998b; Tate et al., 1998; Fjell et al., 1999c), SNS and NaN transcripts are preferentially expressed in small (<30 μm in diameter) DRG neurons (Fig.1a,e). SNS mRNA is also observed in some larger (30–50 μm in diameter) DRG neurons, consistent with the recent demonstration that SNS channels produce a slowly inactivating TTX-resistant current in large cutaneous afferent neurons (Renganathan et al., 2000). Mid-thigh transection of the sciatic nerve results in a downregulation of transcripts for both SNS and NaN in neurons within ipsilateral L4/5 DRG (Fig.1b,f). Axotomized neurons, identified by the inclusion of the retrograde tracer hydroxystilbamine methanesulfonate, exhibit a gradient of fluorescent label intensity, ranging from intense to more moderate (Fig.1c,g). In our analyses, only those neurons that fluoresced substantially above background levels were deemed to be transected. This high threshold may exclude some neurons that were in fact transected from being included in the axotomized neuron category, but it ensures that all neurons in this category were transected. Superimposition of images for SNS or NaN hybridization signal and retrograde labeling demonstrates no overlap of these two signals (Fig.1d,h), indicating that neurons that have been transected downregulate SNS or NaN transcripts. These results also demonstrate that SNS and NaN mRNA are not downregulated in nonaxotomized neurons, consistent with a direct (i.e., axotomy), and not indirect (i.e., paracrine–autocrine) (Mantyh et al., 1994; Acheson and Lindsay 1996), effect on their transcript levels.
Fig. 1.
SNS and NaN mRNA expression in control and peripherally axotomized DRG neurons. Sections of control and peripherally axotomized DRG were processed for nonisotopic in situ hybridization detection of SNS and NaN transcripts. SNS (a) and NaN (e) transcripts are preferentially expressed in small-diameter DRG neurons, although SNS mRNA is also expressed in some larger neurons. Sciatic nerve transection attenuates the number of DRG neurons expressing hybridization signal for SNS (b) or NaN (f). DRG neurons that are transected and retrogradely transport the fluorescent label hydroxystilbamine methanesulfonate fluoresce with a green hue (c, g). Overlay of the images for SNS and NaN hybridization (red) and backfill signals (green) demonstrates that backfilled neurons do not express detectable levels of either SNS (d) or NaN (h) transcripts. Scale bar, 50 μm.
To determine whether SNS and NaN protein levels in transected DRG neurons parallel the transcript levels in these cells, we combined retrograde fluorescent labeling with immunofluorescent labeling of SNS and NaN sodium channels using isoform-specific antibodies. We have examined SNS and NaN protein levels in DRG neurons after transection of their peripheral projections first in cryosections of intact DRG and subsequently in neurons maintained in culture for <24 hr, a time chosen so that we could compare immunocytochemical results with patch-clamp studies.
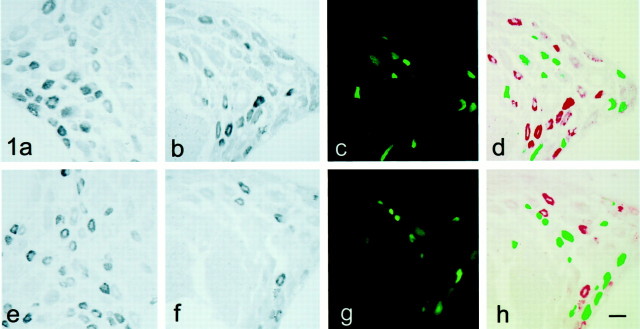
Immunolabeling of control L4/5 DRG sections with SNS- and NaN-specific antibodies shows a high level of intensity in small neurons (Fig.2a,e), confirming the presence of SNS and NaN protein in these cells. SNS immunofluorescence is also observed in some larger DRG neurons, in agreement with previous descriptions of SNS protein (Novakovic et al., 1998; Tate et al., 1998) and the slowly inactivating TTX-resistant current ascribed to this channel (Renganathan et al., 2000). As with transcript levels of SNS and NaN, sciatic nerve transection is accompanied by an attenuation in the number of neurons immunolabeled with SNS or NaN antibodies within ipsilateral L4/5 DRG (Fig.2b,f). In ganglia from the axotomized side, a substantial number of the neurons are labeled with the retrograde tracer (Fig. 2c,g). Overlay of backfilled (green) and SNS- or NaN-positive (red) images demonstrates that SNS and NaN protein are not detectable in most peripherally axotomized neurons. However, unlike a lack of detectable SNS or NaN transcripts in backfilled cells, there are a few (<15%) peripherally axotomized neurons that are SNS or NaN immunolabeled (yellow) (Fig.2d,h). The differences observed between transcript and protein signals in axotomized neurons may result from the retrograde tracer inhibiting hybridization signal (e.g., modifying hybridization) or, alternatively, the hybridization signal (a precipitate) may quench the backfilled fluorescent signal, thus leading to a lack of any neurons displaying both hybridization signal and retrograde label. It is also possible that sodium channel protein is more stable (Waechter et al., 1983; Ritchie, 1988) than its mRNA, so that residual amounts would be detectable in neurons 9–12 d after axotomy.
Fig. 2.
SNS and NaN protein expression in control and peripherally axotomized DRG neurons. Sections of control and peripherally axotomized DRG were processed for SNS and NaN protein localization using isoform-specific antibodies. SNS (a) and NaN (e) protein are present in small DRG neurons, and SNS is also observed in some larger neurons. Sciatic nerve transection results in a decrease in the number of DRG neurons with detectable levels of SNS (b) or NaN (f) protein. Transected neurons that are backfilled with the retrograde label fluoresce green towhite (c, g). Overlay of images for SNS and NaN localization and backfilled neurons indicates that most backfilled (green) neurons do not possess SNS (d) or NaN (h) immunoreactivity. SNS- and NaN-immunopositive neurons (red) are typically not backfilled, although a small subpopulation (<15%) of backfilled neurons exhibit SNS or NaN (yellow). Scale bar, 50 μm.
To facilitate quantification of SNS and NaN immunolabeling signals and colocalization of retrograde tracer and to more directly compare with patch-clamp results obtained from neurons treated in the same manner, control (noninjured) and peripherally axotomized L4/5 DRG were dissociated and maintained in culture for <24 hr (Black et al., 1999a). Representative examples of SNS and NaN immunolabeling of control and axotomized DRG neurons in vitro are shown in Figure 3, a, b,e, and f, and superimposition of SNS or NaN labeling and fluorescent backfill are shown in Figure 3, dand h. Similar to observations with DRG sections, >50% axotomized (green) DRG neurons do not exhibit detectable SNS or NaN immunostaining, although ∼20–30% of retrogradely labeled neurons are immunolabeled for SNS, and a similar number are labeled for NaN protein (yellow). There are also small neurons within these cultures derived from ipsilateral L4/l5 DRG that are not backfilled and that exhibit SNS or NaN immunostaining (red). The somewhat greater percentage of axotomized DRG neurons in vitro exhibiting SNS or NaN protein signal compared with neurons within DRG sections may reflect differences in summing the signal from the entire neuron (15–25 μm in diameter) in cultured cells versus only a slice (10 μm thickness) in tissue sections.
Fig. 3.
SNS and NaN protein expression in control and peripherally axotomized DRG neurons maintained in culture <24 hr. SNS (a) and NaN (e) protein are present in control DRG neurons. There is an attenuation of SNS (b) and NaN (f) protein in neurons derived from DRG that were peripherally transected 9–12 d before plating. Transected and backfilled neurons are intenselygreen-to-white fluorescent (c, g). Overlay of images for SNS (d) or NaN (h) immunostaining (red) and backfilling demonstrate that most backfilled neurons (green) are not SNS- or NaN-immunopositive. SNS and NaN immunolabeling is clearly present in neurons that are not backfilled. A few neurons are backfilled and maintain SNS or NaN labeling (yellow). There are some neurons that are neither backfilled nor immunolabeled with SNS or NaN (gray neurons). Scale bar, 50 μm.
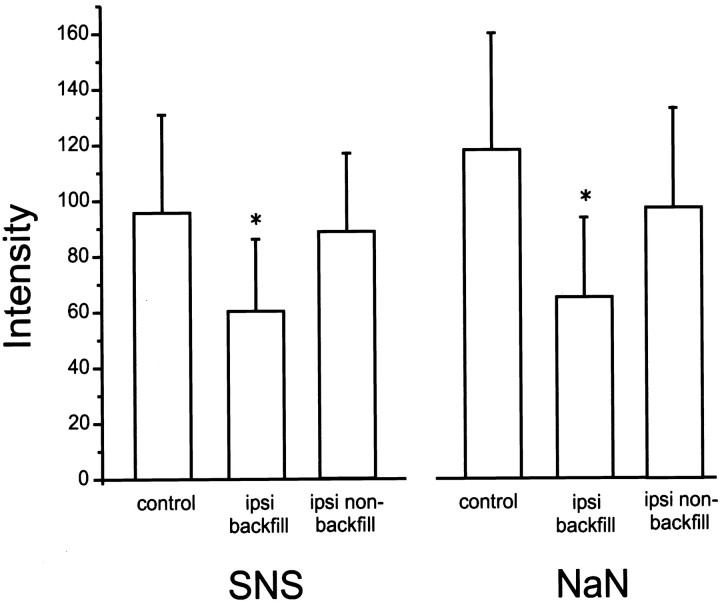
Quantification of the SNS and NaN signal intensities in control and peripherally axotomized small DRG neurons is shown in Figure4. For both SNS and NaN, there is a significant (p < 0.05) reduction in mean signal intensity in axotomized (backfilled) (SNS, 60.4 ± 25.8,n = 162; NaN, 65.0 ± 28.6, n = 105) compared with control (SNS, 95.7 ± 35.3, n = 205; NaN, 117.9 ± 42, n = 288) neurons. Mean signal intensities for SNS and NaN in nonbackfilled neurons within cultures obtained from ipsilateral (sciatic nerve transected) DRG are also shown in Figure 4. The mean signal intensities for SNS and NaN in ipsilateral, backfilled neurons is significantly (p < 0.05) reduced compared with ipsilateral, nonbackfilled neurons (SNS, 91.1 ± 32.4, n = 96; NaN, 91.3 ± 41.3, n = 160) within these cultures. Although there is a small decrease in the signal intensities for both SNS and NaN in ipsilateral, nonbackfilled neurons compared with control neurons, this change does not reach the level of statistical significance (p < 0.05). Despite the possibility that axotomized but not backfilled neurons were included in the ipsilateral nonbackfilled group of neurons, we cannot exclude a possible small change in SNS and NaN expression in nonaxotomized neurons induced by sciatic nerve transection.
Fig. 4.
Quantification of SNS and NaN signal intensities in control, ipsilateral backfilled (axotomized) and ipsilateral nonbackfilled small DRG neurons. Mean ± SD SNS and NaN fluorescent signals above background level are shown. For both SNS and NaN, there is a significant (p < 0.05) decrease in intensity in ipsilateral backfilled neurons compared with control neurons and also with ipsilateral nonbackfilled neurons. There is a small but not significant decrease in both SNS and NaN intensities in ipsilateral nonbackfilled neurons compared with control neurons.
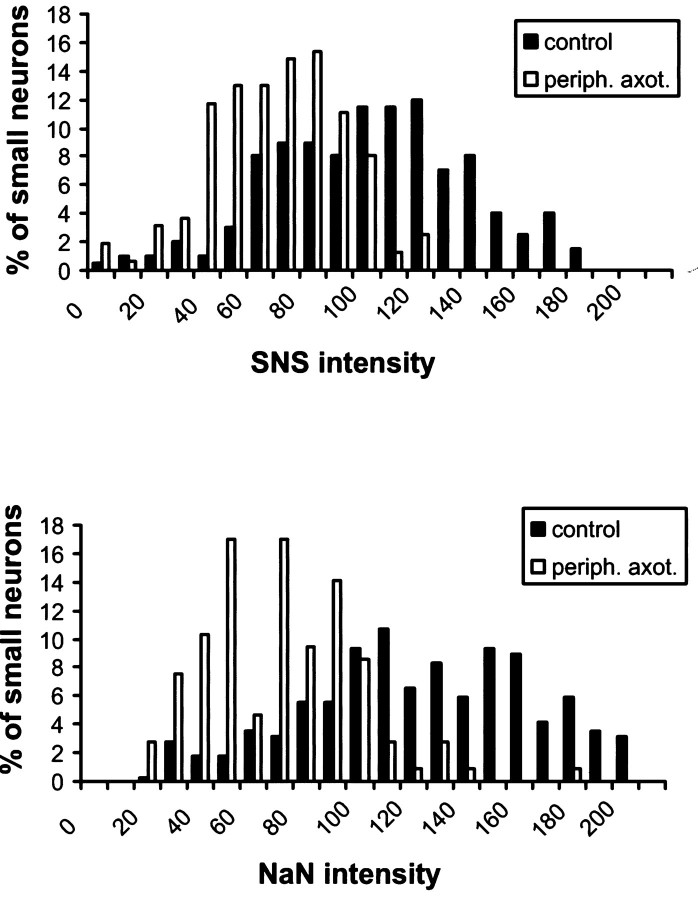
Histograms of intensities of SNS or NaN signal versus percentage of cells are shown in Figure 5; axotomy clearly shifts the histograms for axotomized (backfilled) neurons to the left (more cells with less intensity) compared with histograms for control neurons. For instance, only 5.5 and 2.5% of peripherally axotomized neurons express high levels (intensity of ≥20) of SNS and NaN, respectively, compared with 39.0 and 55.0% of control neurons.
Fig. 5.
Histogram showing distribution of SNS or NaN intensities in small DRG neurons. Data for both control (filled bars) and backfilled, peripherally axotomized (open bars) neurons are shown. Percentage of DRG neurons versus SNS or NaN signal intensity (bin size, 10) is plotted, with results from control and axotomized neurons juxtaposed to comparison. Both SNS and NaN show a shift to lower intensities (to theleft) in peripherally axotomized neurons compared with control neurons. SNS control, n = 205; SNS backfill, n = 162; NaN control,n = 288; NaN backfilled, n = 105.
SNS and NaN expression are unaffected in centrally axotomized DRG neurons
We also asked whether transection of the central projections (dorsal roots) of small DRG neurons evoked a similar attenuation of SNS and NaN mRNA and protein as peripheral axotomy. Unlike peripheral axotomy, dorsal rhizotomy is not accompanied by a downregulation of either SNS or NaN mRNA (Figs. 6,7). Sections of L4/5 DRG obtained from control and dorsal rhizotomized and processed for in situhybridization cytochemistry show no qualitative differences in the respective SNS and NaN hybridization signals between the two conditions (Fig. 6). Transcript levels of SNS and NaN in control and dorsal rhizotomized DRG were also assessed by quantitative PCR (see Materials and Methods). As shown in Figure 7, similar levels of SNS mRNA are detected in control and rhizotomized DRG; likewise, levels of NaN transcripts are similar in control and rhizotomized DRG.
Fig. 6.

In situ hybridization for SNS and NaN transcripts in control and dorsal rhizotomized DRG neurons. Hybridizations signals for SNS are similar between control (a) and rhizotomized (b) DRG. NaN hybridization signal in control (c) and rhizotomized (d) DRG exhibit similar levels. Scale bar, 25 μm.
Fig. 7.
Normalized transcript levels of NaN and SNS from DRG after rhizotomy of L4/5 dorsal roots. Transcript levels of NaN and SNS from control and rhizotomized DRG were normalized to the endogenous control 18 S rRNA. Each measurement was done in quadruplet, and the relative amount of target was quantitated by the relative standard curve method. The slight difference in the respective transcript levels of NaN and SNS between control and rhizotomized DRG was not statistically significant. For NaN, normalized transcript levels in control and rhizotomized DRG are 0.35 ± 0.009 and 0.33 ± 0.024, respectively, whereas for SNS, these values are 0.29 ± 0.011 and 0.27 ± 0.008, respectively.
Consistent with the levels of SNS and NaN transcripts in control and rhizotomized DRG, similar levels of SNS and NaN immunoreactivity were observed for the respective sodium channels in control and rhizotomized DRG neurons in situ (Fig. 8) and in vitro (Fig. 9). In these experiments, the transected neurons were not backfilled with a fluorescent label; all of the neurons in the L4 and L5 ganglia are likely to have been axotomized by dorsal rhizotomy because there is no branching of the axons before the root entry zone (complete transection of L4 and L5 dorsal roots was verified at the time of perfusion or culture). Quantification of the fluorescent signals in cultured small DRG neurons revealed no significant differences in mean intensities between control and dorsal rhizotomized neurons for either SNS or NaN (Fig. 10).
Fig. 8.
SNS and NaN protein expression in control and centrally axotomized DRG neurons. Sections of control and centrally axotomized DRG were processed for SNS and NaN protein immunostaining using isoform-specific antibodies. Control (a,c) and centrally axotomized (b,d) DRG sections exhibit similar levels of SNS (a, b) and NaN (c,d) immunolabeling. Scale bar, 50 μm.
Fig. 9.
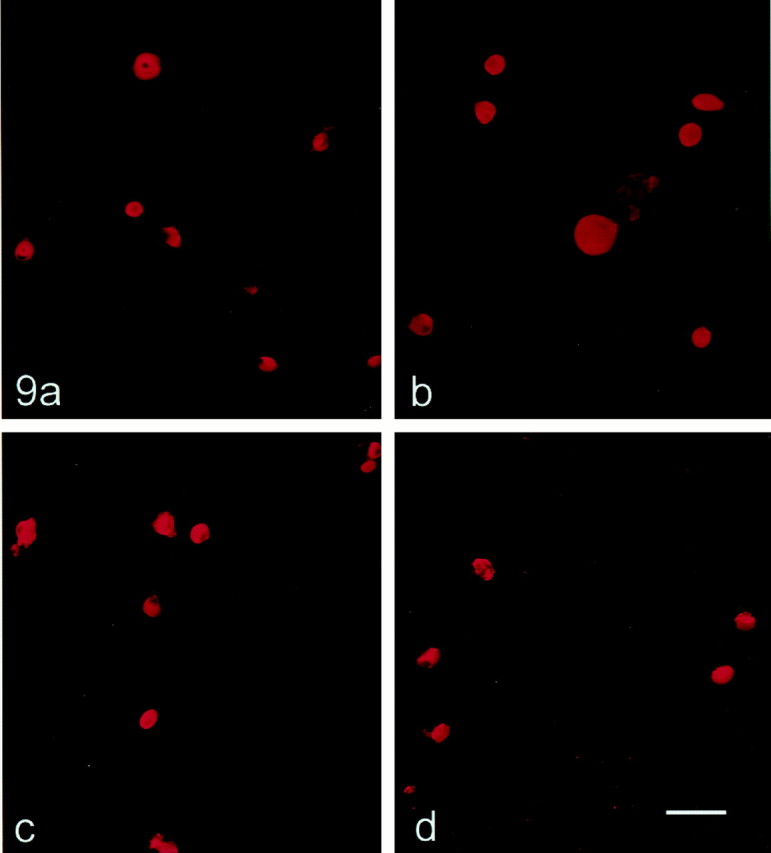
SNS and NaN protein expression in control and centrally axotomized DRG neurons maintained in culture <24 hr. Control (a, c) and centrally axotomized (b, d) DRG neurons show similar levels of SNS (a, b) and NaN (c,d) immunolabeling. Scale bar, 50 μm.
Fig. 10.
Quantification of SNS and NaN signal intensities in control and centrally axotomized (rhizotomy) small DRG neurons. Mean ± SD SNS and NaN fluorescent signals above background level are plotted for control and centrally axotomized neurons. Control and rhizotomized DRG neurons exhibit similar mean intensities for SNS and NaN, respectively.
SNS and NaN sodium currents are reduced in peripherally but not centrally axotomized DRG neurons
Previous studies have demonstrated an attenuation of overall TTX-resistant sodium current in small (<25 μm in diameter) DRG neurons after peripheral transection of their axons (Cummins and Waxman, 1997). Recently, it has been shown that two separable TTX-resistant sodium currents with distinct voltage-dependence and kinetic properties, which appear to be produced by SNS and NaN channels, are present in small DRG neurons (Cummins et al., 1999). However, the effect of nerve injury on these two TTX-resistant sodium currents has not been studied.
Sodium currents were recorded in the presence of 250 nm TTX to isolate TTX-resistant currents. Figure11A shows representative currents for each of the three groups of small (<25 μm in diameter) neurons: control, sciatic axotomy, and dorsal rhizotomy. In control neurons, the amplitude (mean ± SE) of the total TTX-resistant current was 24.7 ± 3.8 nA (n= 43). Similar to our previous work (Cummins and Waxman, 1997), peripherally axotomized neurons (containing retrograde label) had significantly smaller total TTX-resistant currents (5.3 ± 1.8 nA,n = 33) than control neurons (p< 0.001) (Fig. 11B). Unlike peripheral axotomy, however, the total TTX-resistant current amplitude in centrally axotomized neurons was similar to that of control neurons (26.2 ± 4.8 nA, n = 28). As an additional measure of TTX-resistant current expression in the DRG neurons, we also determined the percentage of cells expressing total TTX-resistant current densities >100 pA/pF. Whereas 84% of control and 82% of dorsal rhizotomized neurons displayed TTX-resistant current densities >100 pA/pF, only 27% of peripherally axotomized neurons (identified by fluorescent backfill) did.
Fig. 11.
TTX-resistant sodium currents in small DRG neurons are reduced after peripheral axotomy but not after central rhizotomy. A, TTX-resistant currents recorded from representative control, rhizotomized, and peripheral axotomized neurons with a holding potential of −120 mV. The capacitance of the cells was 23 (control), 25 (rhizotomy), and 28 (peripheral axotomy) pF. The series resistance values were 1.1, 1.3, and 1.5 MΩ, respectively. Calcium currents were blocked with 100 μm cadmium in the bath solution, and TTX (250 nm) blocked the fast-inactivating currents. B, The peak TTX-resistant current amplitude is plotted for the control, rhizotomy, and axotomy groups. Neurons were held at −120 mV and depolarized to voltages ranging from −80 to 40 mV to measure the peak current amplitude.C, The cell capacitance is slightly larger for axotomized neurons. *p < 0.005.
It is now established that small DRG neurons can express two distinct TTX-resistant sodium currents, a slowly inactivating current and a low voltage-activated persistent current. Akopian et al. (1999)demonstrated that in mice the SNS sodium channel α-subunit encodes the slowly inactivating component. Cummins et al. (1999) confirmed this observation and also demonstrated that a distinct, large persistent TTX-resistant current can be elicited by depolarizing stimuli when DRG neurons from SNS-null mutant mice are held at negative potentials (i.e., −120 mV). These studies conclusively demonstrated that the slowly inactivating and persistent TTX-resistant currents are generated by different genes in mice DRG neurons. Moreover, additional evidence has been presented to support the proposal that the NaN sodium channel underlies the persistent TTX-resistant sodium current (Cummins et al., 1999). Although pharmacological blockers have not yet been identified that can distinguish between the slowly inactivating and the persistent TTX-resistant sodium currents, it has been shown that, in wild-type mice and human DRG neurons, these two TTX-resistant currents can be separated using preconditioning protocols that vary the holding potential (Cummins et al., 1999; Dib-Hajj et al., 1999a).
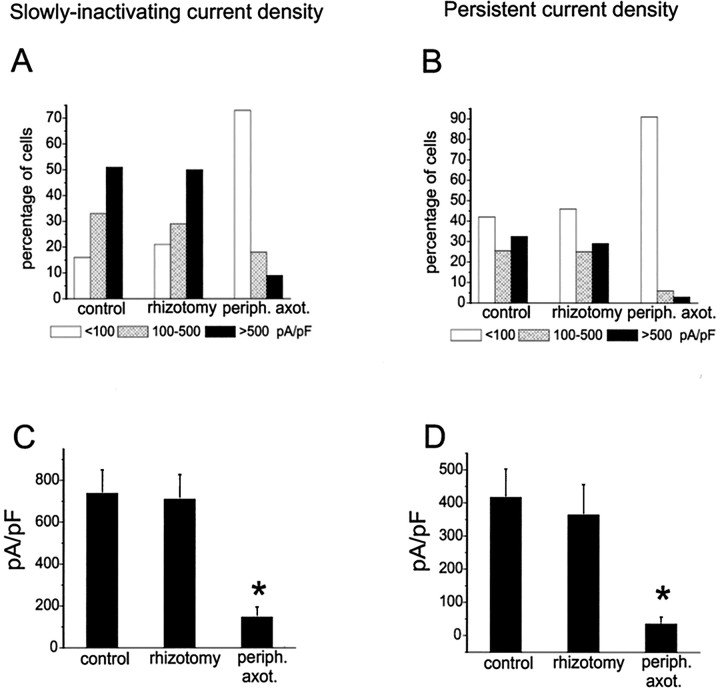
As illustrated in Figure 11A, which shows recordings from a control DRG neuron, both slowly inactivating and persistent TTX-resistant currents can be elicited with depolarizing steps when small DRG neurons are held at −120 mV. When the cells are depolarized from a holding potential of −60 mV, the slowly inactivating current predominates (Fig.12A). The persistent TTX-resistant current can be obtained in relative isolation by digitally subtracting the current obtained withVhold of −60 mV from that obtained with Vhold of −120 mV (Fig.12B). Thus, the two TTX-resistant currents can be separated in rat DRG neurons by varying the holding or prepulse potential (Cummins et al., 1999). Using these protocols, a cell can be classified as expressing slowly inactivating TTX-resistant current, persistent TTX-resistant current, both currents, or neither current. For quantification of the number of cells expressing these currents, a cell was considered to express slowly inactivating current if the current density obtained with Vholdof − 60 mV was >100 pA/pF and, similarly, a cell was considered to express persistent current if the current density obtained with the digital subtraction was >100 pA/pF. For the control cells, 58% expressed both slowly inactivating and persistent TTX-resistant currents, 26% expressed only slowly inactivating TTX-resistant current, and 16% expressed neither. Similar results were obtained with neurons from centrally axotomized DRG, with 50% of the cells expressing both currents, 28% expressing only slowly inactivating, 4% expressing solely persistent TTX-resistant current, and 18% expressing neither. In contrast, only 9% of the backfilled peripherally axotomized DRG neurons expressed both currents, 18% expressed only slowly inactivating TTX-resistant current, and 73% expressed neither type of TTX-resistant sodium current. After peripheral axotomy, the mean current density decreased by 80% for the slowly inactivating current and by 85% for the persistent current. Figure13, A and B, shows that the slowly inactivating and persistent TTX-resistant current density distributions were similar for control and rhizotomized, but not peripherally axotomized, neurons. Figure 13, C andD shows that the mean slowly inactivating TTX-resistant current was significantly (p < 0.005) lower in backfilled, peripherally axotomized DRG neurons (146.6 ± 48.3 pA/pF) compared with control (738.5 ± 112.4 pA/pF) or rhizotomized (709.8 ± 119.2 pA/pF) neurons. Similarly, the mean persistent TTX-resistant current was significantly reduced (p < 0.005) in backfilled, peripherally axotomized neurons (121.3 ± 35.3 pA/pF) compared with control (417.0 ± 86.1 pA/pF) or rhizotomized (364.4 ± 91.1 pA/pF) neurons. The voltage-dependent properties of the TTX-resistant currents were similar for control and rhizotomized neurons. The midpoint of activation was −21.2 ± 1.2 and −22.4 ± 2.2 mV for the slowly inactivating currents and −54.2 ± 1.3 and −52.6 ± 2.8 mV for the persistent currents in control (n = 27) and rhizotomized (n = 15) cells, respectively. Thus, both the slowly inactivating (SNS-type) and the persistent (NaN-type) TTX-resistant sodium currents in rat primary sensory neurons are attenuated by peripheral, but not central, axotomy.
Fig. 12.
Rat small DRG neurons express multiple TTX-resistant currents. Slowly inactivating (A) and persistent (B) TTX-resistant currents recorded from representative control, rhizotomized, and peripherally axotomized DRG neurons. Currents were recorded from the same neurons as in Figure 9A. A, Predominantly slowly inactivating currents were recorded if the neurons were held at −60 mV, and a 500 msec step to −120 mV preceded the test pulses. Holding the cells at −60 mV for more than 10 sec induces ultra-slow inactivation of the persistent current (Cummins et al., 1999). The 500 msec prepulse to −120 mV is not long enough to allow recovery of the persistent current from ultra-slow inactivation but is used to allow recovery of slowly inactivating current that inactivated at −60 mV.B, Subtraction of the slowly inactivating component (Fig. 10A) from the total TTX-resistant current (Fig. 9A) reveals the persistent TTX-resistant current.
Fig. 13.
Peripheral axotomy, but not dorsal rhizotomy, decreases both slowly inactivating (A) and persistent (B) TTX-resistant sodium current densities in small DRG neurons. The slowly inactivating and persistent TTX-resistant currents were isolated as described in Figure 10. Current densities were estimated by dividing the peak current amplitude by the cell capacitance. Cells were assigned to one of three groups (100, 100–500, or >500 pA/pF) based on current density. The density distribution for the slowly inactivating and the persistent TTX-resistant sodium current are not altered by rhizotomy, but both are dramatically changed, with a reduction in percentage of backfilled cells showing medium or high density, after peripheral axotomy.C, The average peak slowly inactivating TTX-resistant sodium current density is plotted for control, rhizotomy, and peripheral-axotomy groups. D, The average peak persistent TTX-resistant sodium current density is plotted for control, rhizotomy, and peripheral-axotomy groups. *p < 0.005.
DISCUSSION
SNS and NaN are two distinct TTX-resistant sodium channels encoded by genes located on the same chromosome (mouse chromosome 9; human chromosome 3) (Dib-Hajj et al., 1999b) but with different amino acid sequences and distinct physiological signatures (Cummins et al., 1999). We have used subtype-specific antibodies to examine SNS and NaN channel expression, and also their TTX-resistant currents, in DRG neuron cell bodies after peripheral and central axotomy. We observed parallel changes in SNS and NaN protein levels and in the currents ascribed to these channels. The results presented here demonstrate an attenuation in the expression of SNS and NaN protein and of the currents associated with activity in SNS and NaN channels after peripheral, but not central, axotomy in small DRG neurons. These observations extend previous work that reports a downregulation of SNS and NaN transcripts (Dib-Hajj et al., 1996,1998b; Okuse et al., 1997; Tate et al., 1998) and total TTX-resistant sodium current (Cummins et al., 1997) in c-type DRG neurons after transection of the sciatic nerve. The results also demonstrate that some peripherally axotomized DRG neurons maintain expression of SNS and NaN sodium channels and their currents despite the lesion.
Peripheral but not central axotomy effects SNS and NaN expression
DRG neurons are pseudo-unipolar cells whose processes bifurcate, sending projections to peripheral and central targets. It has become clear, however, that although the peripherally and centrally projecting axons of these cells arise from a common axon trunk, peripheral and central axotomy of these cells do not evoke universally similar neuronal responses. For instance, whereas peripheral axotomy of DRG neurons upregulates some proteins [e.g., GAP-43 (Bisbee, 1988); β-tubulin (Oblinger and Lasek, 1988; Schreyer and Skene, 1993)] and neuropeptides [e.g., neuropeptide Y and vasoactive intestinal peptide (Reimer and Kanje, 1999)] and downregulates other molecules [e.g., substance P (Bisbee and Keen, 1986); neurofilament proteins (Hoffman et al., 1987)], central axotomy has little effect on most of these molecules (Oblinger and Lasek, 1988, Schreyer and Skene, 1993; Reimer and Kanje, 1999). In contrast, both peripheral and central axotomy upregulate BDNF mRNA and protein levels and increase anterograde transport in DRG neurons (Tonra et al., 1998). Peripheral, but not central, axotomy has also been reported to alter electrophysiological properties of sensory neurons in the petrosal ganglion (Gallego et al., 1987); peripheral lesion increases action potential duration and decreases the amplitude and duration of the spike afterhyperpolarization, whereas central transection does not effect electrophysiological properties. Moreover, it has been demonstrated recently that peripheral axotomy of DRG neurons upregulates type III sodium channel mRNA and protein, which is not detectable in normal adult rat DRG, with a concomitant appearance of a rapidly repriming TTX-sensitive sodium current, whereas central axotomy is without effect on these properties (Black et al., 1999a). Because both peripheral and central axotomies prevent retrograde transport from target tissues, these observations suggest that the nature of the target is an important determinant in events that modulate molecular synthesis in the cell body.
The differential effect of peripheral versus central axotomy may reflect differences in the availability of target-derived factors and/or transport mechanisms. SNS and NaN expression are modulated by the neurotrophic factors NGF and glial-derived neurotrophic factor (GDNF). SNS is upregulated in DRG neurons in vivo by conditions in which the NGF levels in their terminal fields are elevated (Tanaka et al., 1998; Fjell et al., 1999c) and is downregulated after reduced levels of circulating NGF (Fjell et al., 1999a). Moreover, the reduction in SNS transcript levels in peripherally axotomized DRG neurons is partially reversed by delivery of exogenous NGF to the transected nerve stump (Dib-Hajj et al., 1998a). NGF, in contrast, appears to have little effect on the expression of NaN. In an in vitro model of axotomy, NaN transcript levels in small DRG neurons were rescued by exogenously administered GDNF but not NGF (Fjell et al., 1999b). GDNF has also been shown to have electrophysiological effects on DRG neurons in vivo. Intrathecal administration of GDNF ameliorates the reduction in conduction velocity of c-type fibers that follows peripheral axotomy (Munson and McMahon, 1997; Bennett et al., 1998). These observations support the idea that NGF and GDNF modulate the expression of the TTX-resistant sodium channels SNS and NaN and also suggest that there may be differences in the sources of target-derived neurotrophic factors and/or transport mechanisms that underlie the differential effects of peripheral versus central axotomy.
SNS and NaN TTX-resistant sodium currents after axotomy
Previous work has demonstrated two separable TTX-resistant sodium currents in small c-type DRG neurons (Cummins et al., 1999). The results presented here demonstrate that there is an attenuation of both TTX-resistant sodium currents in small DRG neurons after peripheral, but not central, axotomy. On the basis of their amino acid sequences, it has been proposed that SNS and NaN are both TTX-resistant (Akopian et al., 1996; Dib-Hajj et al., 1998b). Studies on SNS knock-out mice indicate that SNS underlies a slowly inactivating TTX-resistant sodium current, whereas NaN is responsible for a persistent TTX-resistant current in c-type DRG neurons (Cummins et al., 1999). This interpretation is consistent with the downregulation of SNS and NaN transcripts (Dib-Hajj et al., 1996,1998b; Okuse et al., 1997; Tate et al., 1998) and protein (Novakovic et al., 1998; this report) that occur in small DRG neurons after peripheral axotomy. Similarly, SNS underlies the slowly inactivating TTX-resistant current in large cutaneous DRG neurons, which do not express either NaN or the persistent TTX-resistant current (Renganathan et al., 2000). The selective expression of SNS and NaN in specific subpopulations of DRG neurons (Amaya et al., 2000), which appears to correlate with specific patterns of electrogenesis in these cells (Honmou et al., 1994), is not surprising, because SNS and NaN are not detectable within the brain or spinal cord (Dib-Hajj et al., 1996, 1998).
It is interesting that a subpopulation of peripherally axotomized small DRG neurons (identified by fluorescent backfill label) continue to exhibit >100 pA/pF SNS and NaN current 9–12 d after peripheral axotomy. Consistent with this observation, moderate-to-high levels of SNS and NaN immunoreactivity are maintained in 5.5 and 2.5% of DRG neurons, respectively. These results suggest that the expression of SNS or NaN in these neurons is unaffected, or only partially affected, by axotomy or that these neurons initially possessed higher levels of current that have yet to attenuate below the threshold value. In support of the first alternative, SNS and NaN immunostaining persist in some peripherally axotomized neurons for at least 40 d after sciatic nerve transection (J. A. Black, unpublished observations), and patch-clamp recordings show that TTX-resistant currents, although reduced in amplitude, are not totally abolished in DRG neurons at 22–60 d after axotomy (Cummins and Waxman 1997).
Functional implications of axotomy-induced changes
An increase in excitability and a tendency to fire repetitively have been observed in primary sensory neurons after peripheral, but not central, axotomy (Gallego et al., 1987). Increased excitability has been reported after peripheral axotomy of c-type DRG neurons (Zhang et al., 1997) and this may, at least in part, be attributable to the increased density of TTX-sensitive sodium channels (Rizzo et al., 1995; Zhang et al., 1997; Black et al., 1999a) and the emergence of rapidly repriming TTX-sensitive sodium currents (Cummins and Waxman, 1997) in these axotomized neurons. However, reductions in the expression of slowly inactivating and persistent TTX-resistant currents may also contribute to hyperexcitability. Because of their different voltage-dependence and kinetics, these currents appear to interact with other TTX-sensitive sodium conductances, and with potassium conductances, in a complex manner (Schild and Kunze, 1997). Computer simulations (Elliott, 1997; Schild and Kunze, 1997) provide evidence that a loss of slowly inactivating TTX-resistant sodium currents can lead to a lower action potential threshold and to a tendency to fire repetitively and, in some cases, in a spontaneous manner in the absence of stimulation. It has also been proposed that the loss of persistent TTX-resistant currents can result in hyperexcitability. The physiological properties of the TTX-resistant persistent current associated with NaN, which include a broad overlap between activation and steady-state inactivation centered close to resting potential, suggest that NaN channels contribute a depolarizing influence to resting potential (Cummins et al., 1999). Consistent with this proposal, a persistent sodium conductance is known to contribute to resting potential in sensory axons within the optic nerve, and blockade of this sodium conductance produces a hyperpolarization (Stys et al., 1993). In the skeletal muscle disease hyperkalemic periodic paralysis, it is believed that muscle weakness is caused by an abnormal persistent sodium current that depolarizes the muscle membrane resting potential by an additional 5–10 mV, resulting in reduced availability of the TTX-resistant sodium channel SkM1 that underlies action potential generation in this tissue (Lehmann-Horn et al., 1987). In an analogous manner, we hypothesize that attenuation of the persistent TTX-resistant sodium current would produce a hyperpolarizing shift in resting potential which, by relieving inactivation of TTX-sensitive channels (which are known to be primarily inactivated close to resting potential; Caffrey et al., 1992; Schild and Kunze, 1997), result in increased excitability of peripherally axotomized DRG neurons. This hyperexcitability could contribute to neuropathic pain and/or paraesthesia.
Footnotes
This work was supported in part by grants from the National Multiple Sclerosis Society and from the Medical Research and Rehabilitation Research Service, Department of Veterans Affairs. We also thank the Eastern Paralyzed Veterans Association and the Paralyzed Veterans of America for support. We thank Bart Tofness for excellent technical support and Dr. S. Tate, Glaxo-Wellcome Research and Development, for the gift of the SNS antibody.
Correspondence should be addressed to Dr. Joel A. Black, Neuroscience Research (127A), Veterans Affairs of Connecticut, 950 Campbell Avenue, West Haven, CT 06516. E-mail: joel.black@yale.edu.
REFERENCES
- 1.Acheson A, Lindsay RM. Non target-derived roles of the neurotrophins. Philos Trans R Soc Lond B Biol Sci. 1996;351:417–422. doi: 10.1098/rstb.1996.0037. [DOI] [PubMed] [Google Scholar]
- 2.Akopian AN, Sivilotti L, Wood JN. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Science. 1996;379:257–262. doi: 10.1038/379257a0. [DOI] [PubMed] [Google Scholar]
- 3.Akopian AN, Soulova V, England S, Okuse K, Ogata N, Ure J, Smith A, Kerr BJ, McMahon SB, Boyce S, Hill R, Stanfa LC, Dickenson AH, Wood JN. The tetrodotoxin-resistant sodium channel SNS has a specialized function in pain pathways. Nat Neurosci. 1999;2:541–548. doi: 10.1038/9195. [DOI] [PubMed] [Google Scholar]
- 4.Amaya F, Decosterd I, Samad TA, Plumpton C, Tate S, Mannion RJ, Costigan M, Woolf CJ. Diversity of expression of the sensory neuron-specific TTX-resistant voltage-gated sodium ion channels SNS and SNS2. Mol Cell Neurosci. 2000;15:331–342. doi: 10.1006/mcne.1999.0828. [DOI] [PubMed] [Google Scholar]
- 5.Bennett DL, Michael GJ, Ramacharndran N, Munson JB, Averill S, Yan Q, McMahon SB, Priestley JV. A distinct subgroup of small DRG cells express GDNF receptor components and GDNF is protective for these neurons after nerve injury. J Neurosci. 1998;18:3059–3072. doi: 10.1523/JNEUROSCI.18-08-03059.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bisbee MA. Dependence of GAP-43 (B50, F1) transport on axonal regeneration in rat dorsal root ganglia. Brain Res. 1988;458:157–161. doi: 10.1016/0006-8993(88)90509-4. [DOI] [PubMed] [Google Scholar]
- 7.Bisbee MA, Keen P. Regeneration of primary afferent neurons containing substance P-like immunoreactivity. Brain Res. 1986;365:85–95. doi: 10.1016/0006-8993(86)90725-0. [DOI] [PubMed] [Google Scholar]
- 8.Black JA, Dib-Hajj S, McNabola K, Jeste S, Rizzo MA, Kocsis JD, Waxman SG. Spinal sensory neurons express multiple sodium channel α-subunit mRNAs. Mol Brain Res. 1996;43:117–131. doi: 10.1016/s0169-328x(96)00163-5. [DOI] [PubMed] [Google Scholar]
- 9.Black JA, Dib-Hajj S, Cohen S, Hinson AW, Waxman SG. Glial cells have heart: rH1 Na+ channel mRNA and protein in spinal cord astrocytes. Glia. 1998;23:200–208. [PubMed] [Google Scholar]
- 10.Black JA, Cummins TR, Plumpton C, Chen YH, Hormuzdiar W, Clare JJ, Waxman SG. Upregulation of a silent sodium channel after peripheral, but not central, nerve injury in DRG neurons. J Neurophysiol. 1999a;82:2776–2785. doi: 10.1152/jn.1999.82.5.2776. [DOI] [PubMed] [Google Scholar]
- 11.Black JA, Fjell J, Dib-Hajj SD, Duncan ID, O'Connor LT, Fried K, Gladwell Z, Tate S, Waxman SG. Abnormal expression of SNS/PN3 in cerebellar Purkinje cells following loss of myelin in the taiep rat. NeuroReport. 1999b;10:913–918. doi: 10.1097/00001756-199904060-00004. [DOI] [PubMed] [Google Scholar]
- 12.Caffrey JM, Eng DL, Black JA, Waxman SG, Kocsis JD. Three types of sodium channels in adult rat dorsal root ganglion neurons. Brain Res. 1992;592:283–297. doi: 10.1016/0006-8993(92)91687-a. [DOI] [PubMed] [Google Scholar]
- 13.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 14.Cummins TR, Waxman SG. Downregulation of tetrodotoxin-resistant sodium currents and upregulation of a rapidly repriming tetrodotoxin-sensitive sodium current in small spinal sensory neurons after nerve injury. J Neurosci. 1997;17:3503–3514. doi: 10.1523/JNEUROSCI.17-10-03503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cummins TR, Dib-Hajj SD, Black JA, Akopian AN, Wood JN, Waxman SG. A novel persistent tetrodotoxin-resistant sodium current in SNS-null and wild-type small primary sensory neurons. J Neurosci 19 1999. RC43(1–6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Czeh G, Kudo N, Kuno M. Membrane properties and conduction velocity in sensory neurons following central or peripheral axotomy. J Physiol (Lond) 1977;270:165–180. doi: 10.1113/jphysiol.1977.sp011944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dib-Hajj S, Black JA, Felts P, Waxman SG. Down-regulation of transcripts for Na channel α-SNS in spinal sensory neurons following axotomy. Proc Natl Acad Sci USA. 1996;93:14950–14954. doi: 10.1073/pnas.93.25.14950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dib-Hajj SD, Black JA, Cummins TR, Kenney AM, Kocsis JD, Waxman SG. Rescue of α-SNS sodium channel expression in small dorsal root ganglion neurons after axotomy by nerve growth factor in vivo. J Neurophysiol. 1998a;79:2668–2676. doi: 10.1152/jn.1998.79.5.2668. [DOI] [PubMed] [Google Scholar]
- 19.Dib-Hajj S, Tyrrell L, Black JA, Waxman SG. NaN, a novel voltage-gated Na channel, is expressed preferentially in peripheral sensory neurons and down-regulated after axotomy. Proc Natl Acad Sci USA. 1998b;95:8963–8968. doi: 10.1073/pnas.95.15.8963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dib-Hajj S, Tyrrell L, Cummins TR, Black JA, Wood PM, Waxman SG. Two tetrodotoxin-resistant sodium channels in human dorsal root ganglion neurons. FEBS Lett. 1999a;462:117–120. doi: 10.1016/s0014-5793(99)01519-7. [DOI] [PubMed] [Google Scholar]
- 21.Dib-Hajj S, Tyrrell L, Escayg A, Wood PM, Meisler MH, Waxman SG. Coding sequence, genomic organization, and conserved chromosomal localization of the mouse gene Scn11a encoding the sodium channel NaN. Genomics. 1999b;59:309–318. doi: 10.1006/geno.1999.5890. [DOI] [PubMed] [Google Scholar]
- 22.Elliott JR. Slow Na+ channel inactivation and bursting discharge in a simple model axon: implications for neuropathic pain. Brain Res. 1997;754:221–226. doi: 10.1016/s0006-8993(97)00072-3. [DOI] [PubMed] [Google Scholar]
- 23.Elliott AA, Elliott JR. Characterization of TTX-sensitive and TTX-resistant sodium currents in small cells from adult rat dorsal root ganglia. J Physiol (Lond) 1993;463:39–56. doi: 10.1113/jphysiol.1993.sp019583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fjell J, Cummins TR, Davis BM, Albers K, Fried K, Waxman SG, Black JA. Sodium channel expression in NGF-overexpressing transgenic mice. J Neurosci Res. 1999a;57:39–47. doi: 10.1002/(SICI)1097-4547(19990701)57:1<39::AID-JNR5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 25.Fjell J, Cummins TR, Dib-Hajj SD, Fried K, Black JA, Waxman SG. In vivo NGF deprivation reduces SNS expression and TTX-R sodium currents in IB4-negative DRG neurons. J Neurophysiol. 1999b;81:803–810. doi: 10.1152/jn.1999.81.2.803. [DOI] [PubMed] [Google Scholar]
- 26.Fjell J, Cummins TR, Dib-Hajj SD, Fried K, Black JA, Waxman SG. Differential role of GDNF and NGF in the maintenance of two TTX-resistant sodium channels in adult DRG neurons. Mol Brain Res. 1999c;67:267–282. doi: 10.1016/s0169-328x(99)00070-4. [DOI] [PubMed] [Google Scholar]
- 27.Fjell J, Hjelmström P, Hormuzdiar W, Milenkovic M, Aglieco F, Tyrrell L, Dib-Hajj S, Waxman SG, Black JA. Localization of the tetrodotoxin-resistant sodium channel NaN in nociceptors. NeuroReport. 2000;11:199–202. doi: 10.1097/00001756-200001170-00039. [DOI] [PubMed] [Google Scholar]
- 28.Gallego R, Ivorra I, Morales A. Effects of central or peripheral axotomy on membrane properties of sensory neurones in the petrosal ganglion of the cat. J Physiol (Lond) 1987;391:39–56. doi: 10.1113/jphysiol.1987.sp016724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gibson UE, Heid CA, Williams PM. A novel method for real time quantitative RT-PCR. Genome Res. 1996;6:995–1001. doi: 10.1101/gr.6.10.995. [DOI] [PubMed] [Google Scholar]
- 30.Gurtu S, Smith PA. Electrophysiological characteristics of hamster dorsal root ganglion cells and their response to axotomy. J Neurophysiol. 1988;59:408–423. doi: 10.1152/jn.1988.59.2.408. [DOI] [PubMed] [Google Scholar]
- 31.Hales TG, Tyndale RG. Few cell lines with GABAA mRNA have functional receptors. J Neurosci. 1994;14:5429–5436. doi: 10.1523/JNEUROSCI.14-09-05429.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heid CA, Steven J, Livak KJ, Williams PM. Real time quantitative PCR. Genome Res. 1996;6:986–994. doi: 10.1101/gr.6.10.986. [DOI] [PubMed] [Google Scholar]
- 33.Himes BT, Tessler A. Death of some dorsal root ganglion neurons and plasticity of others following sciatic nerve section in adult and neonatal rats. J Comp Neurol. 1989;284:215–230. doi: 10.1002/cne.902840206. [DOI] [PubMed] [Google Scholar]
- 34.Hoffman PN, Cleveland DW, Griffin JW, Landes PW, Cowan NJ, Price DL. Neurofilament gene expression: a major determinant of axonal caliber. Proc Natl Acad Sci USA. 1987;84:3472–3476. doi: 10.1073/pnas.84.10.3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Honmou O, Utzschneider DA, Rizzo MA, Bowe CM, Waxman SG, Kocsis JD. Delayed depolarization and slow sodium currents in cutaneous afferents. J Neurophysiol. 1994;71:1627–1637. doi: 10.1152/jn.1994.71.5.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kenney AM, Kocsis JD. Temporal variability of jun family transcription factor levels in peripherally or centrally transected adult rat dorsal root ganglia. Mol Brain Res. 1997;52:53–61. doi: 10.1016/s0169-328x(97)00211-8. [DOI] [PubMed] [Google Scholar]
- 37.Kostyuk PG, Veselovsky NS, Tsyandryenko AY. Ionic currents in the somatic membrane of rat dorsal root ganglion neurons. I. Sodium currents. Neuroscience. 1981;6:2423–2430. doi: 10.1016/0306-4522(81)90088-9. [DOI] [PubMed] [Google Scholar]
- 38.Lehmann-Horn F, Kuther G, Ricker K, Grafe P, Ballanyi K, Rudel R. Adynamia episodica hereditaria with myotonia: a non-inactivating sodium current and the effect of extracellular pH. Muscle Nerve. 1987;10:363–374. doi: 10.1002/mus.880100414. [DOI] [PubMed] [Google Scholar]
- 39.Mantyh PW, Allen CJ, Rogers S, DeMaster E, Ghilardi JR, Mosconi T, Kruger L, Mannon PJ, Taylor IL, Vigna SR. Some sensory neurons express neuropeptide Y receptors: potential paracrine inhibition of primary afferent nociceptors following peripheral injury. J Neurosci. 1994;14:3958–3968. doi: 10.1523/JNEUROSCI.14-06-03958.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McLean MJ, Bennett PB, Thomas RM. Subtypes of dorsal root ganglion neurons based on different inward currents as measured by whole-cell voltage clamp. Mol Cell Biochem. 1988;80:95–107. doi: 10.1007/BF00231008. [DOI] [PubMed] [Google Scholar]
- 41.Munson JB, McMahon SB. Effects of GDNF on axotomized sensory and motor neurons in adult rats. Eur J Neurosci. 1997;9:1126–1129. doi: 10.1111/j.1460-9568.1997.tb01465.x. [DOI] [PubMed] [Google Scholar]
- 42.Novakovic SD, Tzoumaka E, McGivern JG, Haraguchi M, Sangameswaran L, Gogas KR, Eglen RM, Hunter JC. Distribution of the tetrodotoxin-resistant sodium channel PN3 in rat sensory neurons in normal and neuropathic conditions. J Neurosci. 1998;18:2174–2187. doi: 10.1523/JNEUROSCI.18-06-02174.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oblinger MM, Lasek RJ. Axotomy-induced alterations in the synthesis and transport of neurofilaments and microtubules in dorsal root ganglion cells. J Neurosci. 1988;8:1747–1758. doi: 10.1523/JNEUROSCI.08-05-01747.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okuse K, Chaplan SR, McMahon SB, Luo ZD, Calcutt NA, Scott BP, Akopian AN, Wood JN. Regulation of expression of the sensory neuron-specific sodium channel SNS in inflammatory and neuropathic pain. Mol Cell Neurosci. 1997;10:196–207. doi: 10.1006/mcne.1997.0657. [DOI] [PubMed] [Google Scholar]
- 45.Reimer M, Kanje M. Peripheral but not central axotomy promotes axonal outgrowth and induces alterations in neuropeptide synthesis in the nodose ganglion of the rat. Eur J Neurosci. 1999;11:3415–3423. doi: 10.1046/j.1460-9568.1999.00757.x. [DOI] [PubMed] [Google Scholar]
- 46.Renganathan M, Cummins TR, Hormuzdiar WN, Waxman SG. a-SNS produces the slow TTX-resistant sodium current in large cutaneous afferent DRG neurons. J Neurophysiol. 2000;82:2431–2442. doi: 10.1152/jn.2000.84.2.710. [DOI] [PubMed] [Google Scholar]
- 47.Ritchie JM. Sodium channel turnover in rabbit cultured Schwann cells. Proc R Soc Lond B Biol Sci. 1988;233:423–430. doi: 10.1098/rspb.1988.0031. [DOI] [PubMed] [Google Scholar]
- 48.Rizzo MA, Kocsis JD, Waxman SG. Slow sodium conductances of dorsal root ganglion neurons: intraneuronal homogeneity and interneuronal heterogeneity. J Neurophysiol. 1994;72:2796–2815. doi: 10.1152/jn.1994.72.6.2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rizzo MA, Kocsis JD, Waxman SG. Selective loss of slow and enhancement of fast Na+ currents in cutaneous afferent dorsal root ganglion neurons following axotomy. Neurobiol Dis. 1995;2:87–96. doi: 10.1006/nbdi.1995.0009. [DOI] [PubMed] [Google Scholar]
- 50.Roy ML, Narahashi T. Differential properties of tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels in rat dorsal root ganglion neurons. J Neurosci. 1992;12:2104–2111. doi: 10.1523/JNEUROSCI.12-06-02104.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sangameswaran L, Delgado SG, Fish LM, Koch BD, Jakeman LB, Stewart GR, Sze P, Hunter JC, Eglen RM, Herman RC. Structure and function of a novel voltage-gated tetrodotoxin-resistant sodium channel specific to sensory neurons. J Biol Chem. 1996;271:5953–5956. doi: 10.1074/jbc.271.11.5953. [DOI] [PubMed] [Google Scholar]
- 52.Schild JH, Kunze DL. Experimental and modeling study of Na+ current heterogeneity in rat nodose neurons and its impact on neuronal discharge. J Neurophysiol. 1997;78:3198–3209. doi: 10.1152/jn.1997.78.6.3198. [DOI] [PubMed] [Google Scholar]
- 53.Schreyer DJ, Skene JHP. Injury-associated induction of GAP-43 expression displays axon branch specificity in rat dorsal root ganglions. J Neurobiol. 1993;24:959–970. doi: 10.1002/neu.480240709. [DOI] [PubMed] [Google Scholar]
- 54.Sharma N, D'Arcangelo G, Kleinhous A, Halegaus S, Trimmer JS. Nerve growth factor regulates the abundance and distribution of K+ channels in PC12 cells. J Cell Biol. 1993;123:1835–1845. doi: 10.1083/jcb.123.6.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stys PK, Sontheimer H, Ransom BR, Waxman SG. Noninactivating, tetrodotoxin sensitive Na+ conductance in rat optic nerve axons. Proc Natl Acad Sci USA. 1993;90:6976–6980. doi: 10.1073/pnas.90.15.6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sucher NJ, Brose N, Dietcher DL, Awobuluyi M, Gasic GP, Bading H, Cepko CL, Greenberg ME, Jahn R, Heinemann SF. Expression of endogenous NMDAR1 transcripts without receptor protein suggests post-transcriptional control in PC 12 cells. J Biol Chem. 1993;268:22299–22304. [PubMed] [Google Scholar]
- 57.Tanaka M, Cummins TR, Ishikawa K, Dib-Hajj SD, Black JA, Waxman SG. SNS Na+ channel expression increases in dorsal root ganglion neurons in the carrageenan inflammatory pain model. NeuroReport. 1998;9:967–972. doi: 10.1097/00001756-199804200-00003. [DOI] [PubMed] [Google Scholar]
- 58.Tate S, Benn S, Hick C, Trezise D, John V, Mannion RJ, Costigan M, Plumpton C, Grose D, Gladwell Z, Kendall G, Dale K, Bountra C, Woolf CJ. Two sodium channels contribute to the TTX-R sodium current in primary sensory neurons. Nat Neurosci. 1998;1:653–655. doi: 10.1038/3652. [DOI] [PubMed] [Google Scholar]
- 59.Tonra JR, Curtis R, Wong V, Cliffer KD, Park JS, Timmes A, Nguyen T, Lindsay RM, Aceson A, DiStefan PS. Axotomy upregulates the anterograde transport and expression of brain-derived neurotrophic factor by sensory neurons. J Neurosci. 1998;18:4374–4383. doi: 10.1523/JNEUROSCI.18-11-04374.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Waechter CJ, Schmidt JW, Catterall WA. Glycosylation is required for maintenance of functional sodium channels in neuroblastoma cells. J Biol Chem. 1983;258:5117–5123. [PubMed] [Google Scholar]
- 61.Waxman SG, Kocsis JD, Black JA. Type III sodium channel mRNA is expressed in embryonic but not adult spinal sensory neurons, and is reexpressed following axotomy. J Neurophysiol. 1994;72:466–472. doi: 10.1152/jn.1994.72.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Winer J, Jung CK, Shackel I, Williams PM. Development and validation of real-time quantitative reverse transcription-polymerase chain reaction for monitoring gene expression in cardiac myocytes in vitro. Anal Biochem. 1999;270:41–49. doi: 10.1006/abio.1999.4085. [DOI] [PubMed] [Google Scholar]
- 63.Zhang J-M, Donnelly DF, Song X-J, LaMotte RH. Axotomy increases the excitability of dorsal root ganglion cells with unmyelinated axons. J Neurophysiol. 1997;78:2790–2794. doi: 10.1152/jn.1997.78.5.2790. [DOI] [PubMed] [Google Scholar]