Abstract
Objectives
To evaluate the risk of cancers of digestive system with incretin-based therapies among patients with type 2 diabetes mellitus.
Research design and methods
Medline, Embase, Cochrane Library and ClinicalTrials.gov databases were searched for randomized controlled clinical trials that compared incretin-based drugs with placebo or other antidiabetic drugs. Paired reviewers independently screened citations, extracted data and assessed risk of bias of included studies. Network meta-analysis was performed, followed by subgroup analysis. The Grading of Recommendations Assessment, Development and Evaluation system was used to assess the quality of evidence.
Results
A total of 84 studies (n=101 595) involving cancers of digestive system were identified (a median follow-up of 30 weeks). The risk of cancers of digestive system with incretin-based therapies was comparable with insulin (OR: 0.86, 95% CI 0.27 to 2.69), metformin (OR: 0.32, 95% CI 0.07 to 1.38), sodium-glucose co-transporter 2 (OR: 5.26, 95% CI 0.58 to 47.41), sulfonylureas (OR: 1.27, 95% CI 0.68 to 2.39), thiazolidinediones (OR: 0.42, 95% CI 0.13 to 1.42), alpha-glucosidase inhibitors (OR: 2.98, 95% CI 0.12 to 73.80), and placebo (OR: 0.87, 95% CI 0.71 to 1.05). The results of subgroup analysis based on the type of digestive system cancers indicated that incretin-based therapies did not increase the risk of gastrointestinal cancers, respectively. The results of subgroup analysis based on age, duration, mean HbA1c, trial duration, and sample size did not indicate the risk of digestive system cancers.
Conclusions
Moderate to high Grading of Recommendations Assessment, Development and Evaluation evidence suggests that incretin-based therapies were not associated with an increased risk of cancer of digestive system in patients with type 2 diabetes mellitus.
Keywords: incretin, digestive system, cancer, meta-analysis, type 2 diabetes
Significance of this study.
What is already known about this subject?
It is well known that incretin-based therapies are widely used in patients with type 2 diabetes. Incretin-based drugs have been shown to effectively lower blood glucose with minimal hypoglycemia.
What are the new findings?
There are three new findings in the study. First, the risk of cancers of digestive system with incretin-based therapies was comparable with insulin, metformin, sodium-glucose co-transporter 2, sulfonylureas, thiazolidinediones, alpha-glucosidase inhibitors, and placebo. Second, the results of subgroup analysis based on the type of digestive system cancers indicated that incretin-based therapies did not increase the risk of digestive system cancers, respectively. Third, the results of subgroup analysis based on age, duration, mean HbA1c, trial duration, and sample size did not indicate the risk of digestive system cancers.
How might these results change the focus of research or clinical practice?
Our results offer the best available evidence based on real-world patterns of incretin-based therapies and thus should help clinicians make decisions about the relative benefit harm balance of these treatments.
Introduction
Incretins are gut peptides which can augment nutrient-stimulated insulin secretion after dietary intake. Incretin-based therapies include incretin mimetics of glucagon-like peptide-1 receptor agonists (GLP-1 RA) and incretin enhancers of dipeptidyl peptidase-4 (DPP-4) inhibitors.1 Because of their moderate efficiency in glucose lowering and little side effects of hypoglycemia and weight gain, incretin-based therapies are widely used in patients with type 2 diabetes.2–4
In 2006, the US Food and Drug Administration (FDA) approved sitagliptin as the first DPP-4 inhibitor for the treatment of type 2 diabetes mellitus (T2DM) either in monotherapy or in combination with other hypoglycemic agents.5 Then vildagliptin was the second in class, followed by saxagliptin, linagliptin, and alogliptin.5 GLP-1 RAs were first introduced in the USA in 2005. Exenatide was the first approved GLP-1 RA, followed by liraglutide in 2010 and albiglutide and dulaglutide in 2015.6 Recently great concerns about cancers of digestive system have been raised.7–11 GLP-1-based therapies as a potential stimulus to β-cell regeneration have constituted the possibility that exocrine pancreatic cells might be affected simultaneously.7 Debate about long-term safety of GLP-1 RA therapies follows conflicting reports from preclinical and epidemiological studies regarding risk for pancreatitis and pancreatic cancer.12 A cohort study based on real-world drug utilization patterns investigating the risk of colorectal cancer for DPP-4 inhibitors versus other antidiabetic drugs, and GLP-1 RAs versus long-acting insulin indicated that incretin-based therapies did not promote colorectal cancer.13 Garg et al14 found no evidence of an increased risk of pancreatitis with exenatide in a retrospective cohort study. Gallwitz5 reported that the administration of exenatide and sitagliptin may have increased risk of pancreatitis in patients with T2DM. There is a signal for cancer of the pancreas for exenatide in both the US FDA and German regulatory databases and for sitagliptin in the FDA database.15 16 Clinical studies completed thus far are insufficient to confirm or exclude an increased long-term risk of pancreatitis, or pancreatic cancer with incretin-based therapies.17 18
Therefore, we conducted a meta-analysis by investigating all randomized controlled clinical trials (RCT) to evaluate cancer risk of digestive system related with incretin-based therapies in patients with T2DM.
Method
This study was registered on the International Prospective Register of Systematic Reviews (registration number CRD42019123457) (online supplementary appendix 1). The study was conducted according to the Preferred Reporting Items for Systematic Reviews and Network Meta-Analyses (PRISMA-NMA) checklist.
bmjdrc-2019-000728supp001.pdf (48.6KB, pdf)
Search strategy
Medline, Embase, ClinicalTrials.gov and the Cochrane Library were searched from inception to 23 June 2017. We used ‘Glucagon-Like Peptide-1 Receptor’ and ‘Dipeptidyl-Peptidase IV Inhibitors’ as keywords or MeSH terms, accompanied with relevant free words to search these databases; details of search strategies were provided in online supplementary appendix 2.
bmjdrc-2019-000728supp002.pdf (122.5KB, pdf)
Study selection
Only RCTs involving DPP-4 inhibitors or GLP-1 RAs compared with placebo or other antidiabetic agents (metformin (Met), insulin, sulfonylureas (SU), thiazolidinediones (TZD), alpha-glucosidase inhibitors (AGI), sodium-glucose co-transporter 2 (SGLT-2)) in patients with T2DM were included in the analysis (online supplementary appendix 3). The eligibility of studies was assessed independently by three reviewers (SY, SBC and FS), with any disagreement resolved by consensus.
bmjdrc-2019-000728supp003.pdf (164.8KB, pdf)
Data extraction and quality evaluation
Data were extracted using ADDIS software including trial information (author, publication year, sample size, trial duration, types of intervention and control), population characteristics (background therapy, diabetes duration, age, baseline level of HbA1c), reported outcomes about cancers of digestive system (includes stomach, colon, rectum, liver, pancreas) and information on methodology. Three investigators (SY, SBC and FS) extracted data independently in duplicate. Any disagreement was resolved by consensus.
Quality of studies was assessed according to Cochrane risk of bias tool,19 including adequate method for random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and company funding. The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) system was used to rate the quality of evidence for each outcome as high, moderate, low or very low.20 The study was reported according to the PRISMA-NMA checklist.21
Data analysis
Methods for direct treatment comparisons
Traditional pairwise meta-analysis was performed using DerSimonian-Laird random effects model.22 OR and 95% CI for cancers of digestive system were calculated. I2 was used to assess the heterogeneity of direct treatment.
Methods for indirect and mixed comparisons
We used the frequentist framework to perform a random effect network meta-analysis. OR for cancers of digestive system with 95% CI was summarized. We conducted subgroup analysis based on mean age, duration, mean HbA1c, trial duration, and sample size of the trials. Node-splitting model and23 loop-specific approach24 were used to assess the inconsistency between direct and indirect treatment effects. Predictive interval plot that incorporates the extent of heterogeneity was used to evaluate the extent of uncertainty in the estimated effect size for the network meta-analysis. Uncertainty affected by heterogeneity was defined as disagreement between the CIs of relative treatment effects and their predictive intervals.
Publication bias, subgroup analyses and meta-regression
The difference between the observed effect size and comparison-specific summary effect for each study was calculated. Then this variable was regressed on SE, thus a simple linear regression line was added in the funnel plot, which could help us explore visually if there is a publication bias in the results between small and large studies.
To assess whether the results were impacted by study characteristics (effect modifiers), subgroup network meta-analysis was conducted according to age group, body mass index (BMI), HbA1c% level and duration of T2DM. Univariable and multivariable network meta-regression in the context was further conducted to examine the potential modification effects of age, BMI, HbA1c% level and duration of T2DM.
All analyses were conducted using STATA V.13.1 (pairwise meta-analysis, network meta-analysis, estimation of inconsistency and heterogeneity, and funnel plot) and R V.3.5.0.
Results
Study characteristics
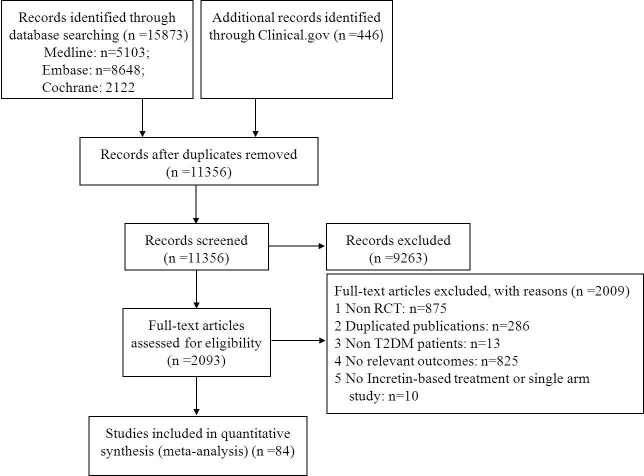
The flow chart of the literature search is shown in figure 1. Overall, 84 RCTs (n=101 595 patients) met the eligibility criteria and were included in this network meta-analysis. Publication year varied from 2006 to 2017. Trial duration ranged from 12 to 262 weeks with a median follow-up of 30 weeks (IQR: 24–104 weeks). The average age of included patients was 60.66 years (SD: 4.68). The mean diabetes duration at baseline was 9.35 years (SD: 2.94), and the mean baseline HbA1c level was 8.03% (SD: 0.54%).
Figure 1.
Flow chart of studies considered for inclusion. RCT, randomized controlled trial; T2DM, type 2 diabetes mellitus.
Evidence network
Eight classes of treatments were analyzed, including DPP-4i (consisting of any drug among sitagliptin, vildagliptin, saxagliptin, linagliptin, alogliptin, and teneligliptin), GLP-1 RAs (consisting of any drug among albiglutide, exenatide, lixisenatide, liraglutide, and semaglutide), six other classes of active antidiabetic agents (Met, insulin, SU, TZDs, AGIs, SGLT-2) and placebo. Seventy-eight trials (92.9%) were two-arm studies and the remaining six trials were three-arm studies (figure 2).
Figure 2.
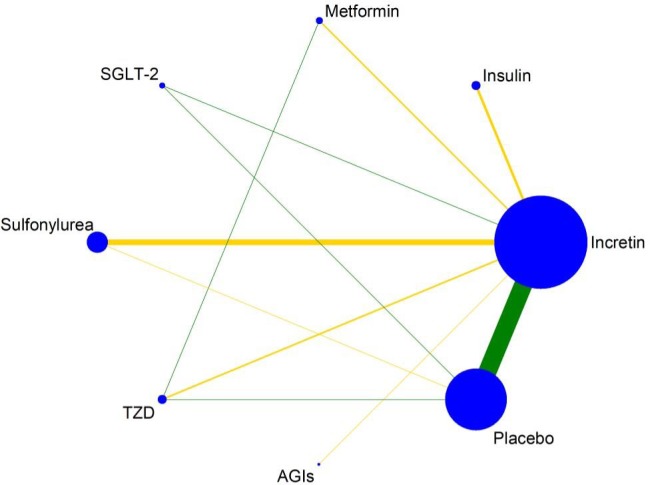
Evidence structure of incretin-based therapies on cancers of digestive system. The numbers along the link lines indicate the number of trials or pairs of trial arms. Lines connect the interventions that have been studied in head-to-head (direct) comparisons in the eligible randomized controlled trials. The width of the lines represents the cumulative number of randomized controlled trials for each pairwise comparison, and the size of every node is proportional to the number of randomized participants (sample size). AGI, alpha-glucosidase inhibitor; SGLT-2, sodium-glucose co-transporter 2; TZD, thiazolidinedione.
Risk of bias
For the total 84 studies included in our analysis, the large majority of studies have reported the implementation of random sequence generation (100%), allocation concealment (92.86%), blinding of participants and personnel (86.91%), blinding of outcome assessment (84.53%), complete outcome data (95.24%), and selective reporting (97.62%). 98.81% of the studies were funded by enterprises. Overall, the risk of bias across evidence network was relatively low (online supplementary appendix 4).
bmjdrc-2019-000728supp004.pdf (106.3KB, pdf)
Direct meta-analysis of incretin-based therapies on cancers of digestive system and subgroup analysis
Figure 3 shows the comparative effect of incretin-based therapies (DPP-4 inhibitors and GLP-1 RAs combined), other antidiabetic agents and placebo on cancers of digestive system from pairwise meta-analyses.
Figure 3.
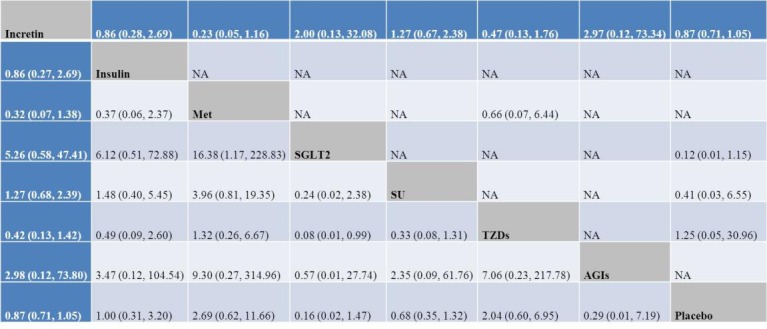
OR with 95% CI of network meta-analysis for cancers of digestive system. Results of direct comparisons were listed in the upper triangle, and the estimation was calculated as the row-defining treatment compared with the column-defining treatment. Results of network meta-analysis were listed in the lower triangle, and the estimation was calculated as the column-defining treatment compared with the row-defining treatment. AGI, alpha-glucosidase inhibitor; Met, metformin; NA, not available; SGLT-2, sodium-glucose co-transporter-2; SU, sulfonylurea; TZD, thiazolidinedione.
Incretin-based therapies did not increase the incidence about cancers of digestive system compared with insulin (OR: 0.86, 95% CI 0.28 to 2.69; n=17, N=4937, I2=0.0%), Met (OR: 0.23, 95% CI 0.05 to 1.16; n=8, N=1555, I2=0.0%), SGLT-2 (OR: 2.00, 95% CI 0.13 to 32.08; n=2, N=1775, I2=0.0%), SU (OR: 1.27, 95% CI 0.67 to 2.38; n=55, N=14 346, I2=0.0%), TZDs (OR: 0.47, 95% CI 0.13 to 1.76; n=12, N=2262, I2=0.0%), AGIs (OR: 2.97, 95% CI 0.12 to 73.34; n=3, N=380, I2=0.0%) and placebo (OR: 0.87, 95% CI 0.71 to 1.05; n=450, N=79 081, I2=0.0%), respectively.
In addition to a general analysis of the incidence of digestive system cancers, subgroup analysis of the incidence of cancers was performed based on stomach, intestine, kidney, liver and pancreas. The results of subgroup analysis based on the type of digestive system cancers (including stomach, colon, rectum, liver and pancreas) indicated that incretin-based therapies did not increase the risk of digestive system cancers, respectively (online supplementary appendix 5).
bmjdrc-2019-000728supp005.pdf (401.8KB, pdf)
The results of subgroup analysis based on age, duration, mean HbA1c, trial duration, and sample size did not indicate the risk of digestive system cancers (online supplementary appendix 6).
bmjdrc-2019-000728supp006.pdf (46KB, pdf)
Network meta-analysis of incretin-based therapies on cancers of digestive system
As shown in figure 3, the risk of incretin on cancers of digestive system was comparable with insulin (OR: 0.86, 95% CI 0.27 to 2.69), Met (OR: 0.32, 95% CI 0.07 to 1.38), SGLT-2 (OR: 5.26, 95% CI 0.58 to 47.41), SU (OR: 1.27, 95% CI 0.68 to 2.39), TZDs (OR: 0.42, 95% CI 0.13 to 1.42), AGIs (OR: 2.98, 95% CI 0.12 to 73.80), and placebo (OR: 0.87, 95% CI 0.71 to 1.05). Also, treatment with Met (OR: 16.38, 95% CI 1.17 to 228.83) was associated with an increased risk about cancers of digestive system than SGLT-2. However, the widened CI indicated a small sample size and the presence of rare events in the included trials.
Cancers of digestive system between incretin-based therapies versus incretin-based therapy non-users
The risk of incretin-based therapies on cancers of digestive system was comparable with incretin-based therapy non-users (all other antidiabetic treatments and placebo) (OR: 0.89, 95% CI 0.75 to 1.05). Fixed effects model was used and I2 was 0.0%.
Inconsistency and heterogeneity check
The result of local inconsistency about cancers of digestive system showed that most loops were consistent according to the CI (online supplementary appendix 7a). The test for inconsistency using node-splitting model revealed no significant difference about cancers of digestive system between direct and indirect comparisons (global inconsistency, p=0.9050) (online supplementary appendix 7b). There was little heterogeneity in this study (online supplementary appendix 8) and global I2 was 0%. The summary estimations of network meta-analysis were relatively robust.
bmjdrc-2019-000728supp007.pdf (116.1KB, pdf)
bmjdrc-2019-000728supp008.pdf (157.6KB, pdf)
Publication bias, subgroup analyses and meta-regression analyses
Funnel plot was shown in online supplementary appendix 9. Scatters in the funnel plot were almost symmetrical visually, indicating the publication bias in the results about cancers of digestive system between small and large studies was relatively low.
bmjdrc-2019-000728supp009.pdf (93.4KB, pdf)
Multivariable meta-regression indicated that for incretin versus placebo, the pooled OR would increase by 0.28 per 1-year change of DM duration.
The result suggested that there may be heterogeneity (online supplementary appendix 10).
bmjdrc-2019-000728supp010.pdf (46.7KB, pdf)
Quality of evidence
The GRADE process was completed using the CINeMA software (http://cinema.ispm.ch/). The quality of most studies was moderate to high. The quality of evidence in each outcome was listed in online supplementary appendix 11.
bmjdrc-2019-000728supp011.pdf (20.5KB, pdf)
Discussion
Overall, we analyzed 84 eligible RCTs including 101 595 patients. Our network meta-analysis showed that: (1) there was no evidence to indicate increased cancer risk of digestive system associated with incretin-based therapies in patients with T2DM compared with other antidiabetic agents or placebo; (2) subgroup analysis in patients treated for ≥48 weeks, based on mean age, duration, mean HbA1c, and sample size, suggested that incretin-based therapies did not increase cancer risk of digestive system.
In agreement with our findings, Wang et al25 reported that treatment with incretins was not associated with an increased risk of pancreatic cancer in patients with T2DM (n=79 971) in a meta-analysis of RCT (OR: 0.67, 95% CI 0.44 to 1.02). Moreover, their subgroup analysis, followed up for ≥104 weeks, showed a lower risk of pancreatic cancer was associated with incretin-based therapies (OR: 0.62, 95% CI 0.41 to 0.95). An RCT on the sitagliptin versus placebo revealed the 0.3% colon cancer risk among sitagliptin initiators versus 0.5% risk among placebo over 3-year follow-up of treatment.26 Htoo and colleagues13 found no effect of DPP-4 inhibitors (follow-up 0.7–0.9 years) and GLP-1 RA (follow-up 0.8 and 1.2 years) on colorectal cancer incidence, respectively. An observational study of liraglutide also did not find an association between GLP-1 RA use and pancreatic cancer.27 In agreement with observational findings, recent cardiovascular outcome trials found no increased risk of pancreatic cancer among alogliptin users28 and saxagliptin users,29 compared with the placebo group. A retrospective cohort analysis of patients with T2DM (n=209 306) found no association between exenatide use and pancreatic cancer.30 Another cohort study found that the hazard of pancreatic cancer with DPP-4 inhibitors was lower relative to SU (HR=0.6, 95% CI 0.4 to 0.9) and similar to TZD (HR=1.0, 95% CI 0.7 to 1.4).31 Elashoff et al32 reported cases of pancreatic cancer were 2.9-fold greater with exenatide and 2.7-fold greater with sitagliptin in patients, respectively, compared with other therapies. It is of note that those data, derived from the FDA Adverse Event Reporting System (FDA-AERS), have some limitations. First, the FDA-AERS database depends on immediate reporting and may be influenced by reporting bias. Second, the AERS database does not provide information about smoking habits, alcohol consumption, obesity or chronic pancreatitis, which are all well-known risk factors in pancreatic cancer.33
Our understanding of incretin biology is still poor, so it is valuable to obtain more data from in vitro studies regarding the influence and mechanisms of action of GLP-1 RAs and DPP-4 inhibitors on tumor formation. There were also in vitro experiments about incretin’s effect on cancers of digestive system. Kissow et al34 reported that treatment with DPP-4 inhibitors does not show any tumor-promoting effects in the colon. Animal experiments on rats with a dosage similar to human therapeutic dose indicated that sitagliptin decreased the incidence of colon carcinogenesis.35 GLP-1 has been reported to inhibit cell growth and increase apoptosis in colon cancer cells, suggesting a protective effect against cancer.36 Kissow et al34 investigated the intestinal growth effect of the GLP-1 RAs (liraglutide and exenatide) and sitagliptin in healthy mice. The results showed both liraglutide and sitagliptin did not promote dysplasia of the colon. Some studies about animals reported that both sitagliptin and vildagliptin had the effect of inhibiting colon carcinogenesis.37 38
As we know the formation of malignancies is a process that takes years, we still need information concerning long-term side effects. Therefore, we hope in the future more and more large-scale prospective randomized clinical trials with appropriate duration of time to ascertain whether these drugs are safe for T2DM.
A major strength of our study is the comprehensive search and analysis of the risk of cancers of digestive system with incretin-based therapies compared with placebo and other antidiabetic agents. Moreover, we conducted subgroup analyses and meta-regression according to study characteristics (age, BMI, HbA1c%, years of T2DM) to test the heterogeneity of studies. Additionally, we assessed the quality of evidence and incorporate it into explaining the results by the GRADE framework.
The present study has some limitations. First, cancers of digestive system were not the primary outcome of the included trials. Therefore, there were some limitations in the occurrence of cancer events and the timing of research. Second, no specific weight was given for digestive cancers. Follow-up studies hope that clinical experts will give weight scores to different digestive cancers. Third, the primary outcome of the included trials was drug efficacy and safety, not digestive cancers; and non-cancer patients did not perform exclusion test.
Tumor formation is a hidden, chronic and complex process. At present, these studies are far from clear about the risk of pancreatic cancer. Expect more RCT studies associated with long-term use of incretin drugs, providing more evidence-based medical evidence for the long-term safety of such drugs.
In conclusion, our meta-analysis suggests that incretin-based therapies are not associated with an increased risk of cancer of digestive system in patients with T2DM.
Footnotes
Contributors: FS and LJ designed the study. SZ and HW gave guidance to this work. SBC and SY retrieved the document. SBC, SY, ZY, SW, LG, and YZ built databases and extracted data. SY input data and conducted statistical analysis. SBC wrote the manuscript.
Funding: This work was supported by the National Natural Science Foundation of China (81302508, 71673003).
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American diabetes association and the European association for the study of diabetes. Diabetes Care 2015;38:140–9. 10.2337/dc14-2441 [DOI] [PubMed] [Google Scholar]
- 2.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006;368:1696–705. 10.1016/S0140-6736(06)69705-5 [DOI] [PubMed] [Google Scholar]
- 3.Ismail-Beigi F. Clinical practice. glycemic management of type 2 diabetes mellitus. N Engl J Med 2012;366:1319–27. 10.1056/NEJMcp1013127 [DOI] [PubMed] [Google Scholar]
- 4.Cefalu WT, Buse JB, Del Prato S, et al. Beyond metformin: safety considerations in the decision-making process for selecting a second medication for type 2 diabetes management: reflections from a diabetes care editors' expert forum. Diabetes Care 2014;37:2647 10.2337/dc14-1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gallwitz B. Emerging DPP-4 inhibitors: focus on linagliptin for type 2 diabetes. Diabetes Metab Syndr Obes 2013;6:1–9. 10.2147/DMSO.S23166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trujillo JM, Nuffer W, Ellis SL. Glp-1 receptor agonists: a review of head-to-head clinical studies. Ther Adv Endocrinol Metab 2015;6:19–28. 10.1177/2042018814559725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butler PC, Elashoff M, Elashoff R, et al. A critical analysis of the clinical use of incretin-based therapies: are the GLP-1 therapies safe? Diabetes Care 2013;36:2118–25. 10.2337/dc12-2713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagel AK, Ahmed-Sarwar N, Werner PM, et al. Dipeptidyl peptidase-4 inhibitor-associated pancreatic carcinoma: a review of the FAERS database. Ann Pharmacother 2016;50:27–31. 10.1177/1060028015610123 [DOI] [PubMed] [Google Scholar]
- 9.Butler AE, Campbell-Thompson M, Gurlo T, et al. Marked expansion of exocrine and endocrine pancreas with incretin therapy in humans with increased exocrine pancreas dysplasia and the potential for glucagon-producing neuroendocrine tumors. Diabetes 2013;62:2595–604. 10.2337/db12-1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Itou M, Kawaguchi T, Taniguchi E, et al. Dipeptidyl peptidase-4: a key player in chronic liver disease. World J Gastroenterol 2013;19:2298–306. 10.3748/wjg.v19.i15.2298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Labuzek K, Kozłowski M, Szkudłapski D, et al. Incretin-based therapies in the treatment of type 2 diabetes--more than meets the eye? Eur J Intern Med 2013;24:207–12. 10.1016/j.ejim.2013.01.009 [DOI] [PubMed] [Google Scholar]
- 12.Cefalu WT, Rosenstock J, Henry RR, et al. Signals and noise in drug safety analyses: the incretin therapy debate provides the rationale for revamping epidemiologic pharmacovigilance. Diabetes Care 2013;36:1804–6. 10.2337/dc13-0895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Htoo PT, Buse JB, Gokhale M, et al. Effect of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors on colorectal cancer incidence and its precursors. Eur J Clin Pharmacol 2016;72:1013–23. 10.1007/s00228-016-2068-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garg R, Chen W, Pendergrass M. Acute pancreatitis in type 2 diabetes treated with exenatide or sitagliptin: a retrospective observational pharmacy claims analysis. Diabetes Care 2010;33:2349–54. 10.2337/dc10-0482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elashoff M, Matveyenko AV, Gier B, et al. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology 2011;141:150–6. 10.1053/j.gastro.2011.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spranger J, Gundert-Remy U, Stammschulte T. GLP-1-based therapies: the dilemma of uncertainty. Gastroenterology 2011;141:20–3. 10.1053/j.gastro.2011.05.019 [DOI] [PubMed] [Google Scholar]
- 17.Monami M, Nreu B, Scatena A, et al. Safety issues with glucagon-like peptide-1 receptor agonists (pancreatitis, pancreatic cancer and cholelithiasis): data from randomized controlled trials. Diabetes Obes Metab 2017;19:1233–41. 10.1111/dom.12926 [DOI] [PubMed] [Google Scholar]
- 18.Drab SR. Glucagon-Like peptide-1 receptor agonists for type 2 diabetes: a clinical update of safety and efficacy. Curr Diabetes Rev 2016;12:403–13. 10.2174/1573399812666151223093841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins JPT GS, Cochrane handbook for interventions version 5.1.0. The Cochrane Collaboration, 2011, 2008updated March 2011. [Google Scholar]
- 20.University of Bern IoSaPM Cinema: confidence in network meta-analysis, 2017. Available: cinema.ispm.ch
- 21.Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 2015;162:777–84. 10.7326/M14-2385 [DOI] [PubMed] [Google Scholar]
- 22.DerSimonian R, Laird N. Meta-Analysis in clinical trials. Control Clin Trials 1986;7:177–88. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 23.Dias S, Welton NJ, Caldwell DM, et al. Checking consistency in mixed treatment comparison meta-analysis. Stat Med 2010;29:932–44. 10.1002/sim.3767 [DOI] [PubMed] [Google Scholar]
- 24.Higgins JPT, Jackson D, Barrett JK, et al. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods 2012;3:98–110. 10.1002/jrsm.1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang H, Liu Y, Tian Q, et al. Incretin-Based therapies and risk of pancreatic cancer in patients with type 2 diabetes: a meta-analysis of randomized controlled trials. Diabetes Obes Metab 2018;20:910–20. 10.1111/dom.13177 [DOI] [PubMed] [Google Scholar]
- 26.Green JB, Bethel MA, Armstrong PW, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;373:232–42. 10.1056/NEJMoa1501352 [DOI] [PubMed] [Google Scholar]
- 27.Funch D, Gydesen H, Tornøe K, et al. A prospective, claims-based assessment of the risk of pancreatitis and pancreatic cancer with liraglutide compared to other antidiabetic drugs. Diabetes Obes Metab 2014;16:273–5. 10.1111/dom.12230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013;369:1327–35. 10.1056/NEJMoa1305889 [DOI] [PubMed] [Google Scholar]
- 29.Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013;369:1317–26. 10.1056/NEJMoa1307684 [DOI] [PubMed] [Google Scholar]
- 30.Romley JA, Goldman DP, Solomon M, et al. Exenatide therapy and the risk of pancreatitis and pancreatic cancer in a privately insured population. Diabetes Technol Ther 2012;14:904–11. 10.1089/dia.2012.0075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gokhale M, Buse JB, Gray CL, et al. Dipeptidyl-peptidase-4 inhibitors and pancreatic cancer: a cohort study. Diabetes Obes Metab 2014;16:1247–56. 10.1111/dom.12379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elashoff M, Matveyenko AV, Gier B, et al. Increased incidence of pancreatitis and cancer among patients given glucagon like peptide-1 based therapy. Gastroenterology 2011;141:150–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monami M, Dicembrini I, Martelli D, et al. Safety of dipeptidyl peptidase-4 inhibitors: a meta-analysis of randomized clinical trials. Curr Med Res Opin 2011;27 Suppl 3:57–64. 10.1185/03007995.2011.602964 [DOI] [PubMed] [Google Scholar]
- 34.Kissow H, Hartmann B, Holst JJ, et al. Glucagon-Like peptide-1 (GLP-1) receptor agonism or DPP-4 inhibition does not accelerate neoplasia in carcinogen treated mice. Regul Pept 2012;179:91–100. 10.1016/j.regpep.2012.08.016 [DOI] [PubMed] [Google Scholar]
- 35.Femia AP, Raimondi L, Maglieri G, et al. Long-Term treatment with sitagliptin, a dipeptidyl peptidase-4 inhibitor, reduces colon carcinogenesis and reactive oxygen species in 1,2-dimethylhydrazine-induced rats. Int J Cancer 2013;133:2498–503. 10.1002/ijc.28260 [DOI] [PubMed] [Google Scholar]
- 36.Koehler JA, Kain T, Drucker DJ. Glucagon-Like peptide-1 receptor activation inhibits growth and augments apoptosis in murine CT26 colon cancer cells. Endocrinology 2011;152:3362–72. 10.1210/en.2011-1201 [DOI] [PubMed] [Google Scholar]
- 37.Amritha CA, Kumaravelu P, Chellathai DD. Evaluation of anti cancer effects of DPP-4 inhibitors in colon Cancer- an invitro study. J Clin Diagn Res 2015;9:FC14–16. 10.7860/JCDR/2015/16015.6979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yorifuji N, Inoue T, Iguchi M, et al. The dipeptidyl peptidase-4 inhibitor sitagliptin suppresses mouse colon tumorigenesis in type 2 diabetic mice. Oncol Rep 2016;35:676–82. 10.3892/or.2015.4429 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjdrc-2019-000728supp001.pdf (48.6KB, pdf)
bmjdrc-2019-000728supp002.pdf (122.5KB, pdf)
bmjdrc-2019-000728supp003.pdf (164.8KB, pdf)
bmjdrc-2019-000728supp004.pdf (106.3KB, pdf)
bmjdrc-2019-000728supp005.pdf (401.8KB, pdf)
bmjdrc-2019-000728supp006.pdf (46KB, pdf)
bmjdrc-2019-000728supp007.pdf (116.1KB, pdf)
bmjdrc-2019-000728supp008.pdf (157.6KB, pdf)
bmjdrc-2019-000728supp009.pdf (93.4KB, pdf)
bmjdrc-2019-000728supp010.pdf (46.7KB, pdf)
bmjdrc-2019-000728supp011.pdf (20.5KB, pdf)