Abstract
Few population-based studies have examined bleeding associated with clopidogrel drug-drug interactions (DDIs). We sought to identify precipitant drugs taken concomitantly with clopidogrel (an object drug) that increased serious bleeding rates. We screened 2000–2015 Optum commercial health insurance claims to identify DDI signals. We performed self-controlled case series studies for clopidogrel + precipitant pairs, examining associations with gastrointestinal bleeding or intracranial hemorrhage. To distinguish native bleeding effects of a precipitant, we reexamined associations using pravastatin as a negative control object drug. Among 431 analyses, 28 clopidogrel + precipitant pairs were statistically significantly positively associated with serious bleeding. Ratios of rate ratios ranged from 1.13–3.94. Among these pairs, 13 were expected given precipitant drugs alone increased and/or were harbingers of serious bleeding. The remaining 15 pairs constituted new DDI signals, none of which are currently listed in two major DDI knowledge bases.
Keywords: Clopidogrel, drug interactions, pharmacoepidemiology, pharmacovigilance
INTRODUCTION
Clopidogrel is a widely-used antiplatelet drug, prescribed or continued during ~19 million ambulatory care office visits in the United States in 2015.1 Serious bleeding is the most prominent adverse effect of clopidogrel, occurring at an incidence of 5.6–15.7% per patient-year.2 While several studies have examined drug-drug interactions (DDIs) that may interfere with clopidogrel’s effectiveness in preventing thrombosis,3 few studies have examined drugs that may potentiate clopidogrel’s bleeding risk, which may be of great clinical and public health importance. Because potentiation of clopidogrel’s bleeding risk might occur through many known or unknown actions of concomitant medications and thus be difficult to predict, and because spontaneous reporting databases may be ineffective in identifying an increased risk of an expected adverse event (e.g., bleeding in persons receiving an antiplatelet drug),4 we aimed to conduct high-throughput pharmacoepidemiologic screening to identify signals of potentially clinically-significant DDIs involving clopidogrel that increase the rate of serious bleeding so that these potential DDIs can be confirmed and elucidated in future studies.
RESULTS
Characteristics of persons constituting the study cohorts are summarized in Table 1. The clopidogrel cohort (all of whom experienced serious bleeding while on clopidogrel) consisted of 21,577 persons. The plurality (26.7%) were female Caucasian older adults. The pravastatin cohort consisted of 5,785 persons experiencing serious bleeding while on pravastatin. The plurality (28.5%) were also female Caucasian older adults. In both cohorts, substantial proportions of individuals had diagnosed cerebrovascular and/or ischemic heart disease, consistent with indications for clopidogrel and pravastatin. More than half of individuals had a prior serious bleed. Very few observation days included concomitant exposure to anticoagulants, other antiplatelet agents, or nonsteroidal anti-inflammatory drugs (NSAIDs). In contrast, approximately one-quarter of observation days included concomitant exposure to a gastro-protective agent such as a histamine-2 receptor antagonist or proton pump inhibitor.
Table 1.
Descriptors of the object drug cohorts.
| Object drug cohort | |||
|---|---|---|---|
| Clopidogrel | Pravastatin | ||
| Persons | 21,577 | 5,785 | |
| Days of observation, sum | 16,285,312 | 5,112,014 | |
| Days of observation, median (quartile 1–quartile 3) per person | 490 (183–1,089) | 758 (331–1,427) | |
| Gastrointestinal bleed/intracranial hemorrhage outcomes, sum | 32,839 | 8,575 | |
| Demographics | |||
| Age in years, median (quartile 1–quartile 3) | 73.7 (65.5–78.7) | 72.9 (65.1–78.8) | |
| Sex, sum (%) female | 10,125 (46.9) | 2,999 (51.8) | |
| Race, sum (%) | African American | 2,709 (12.6) | 788 (13.6) |
| Asian | 409 (1.9) | 117 (2.0) | |
| Caucasian | 15,075 (69.9) | 4,025 (69.6) | |
| Hispanic | 1,771 (8.2) | 440 (7.6) | |
| Unknown | 1,613 (7.5) | 415 (7.2) | |
| Dose covariate, time-varying | |||
| Object drug average daily dose (quartile 1–quartile 3), median, in milligrams | 75.0 (75.0–75.0) | 40.0 (20.0–40.0) | |
| Drug covariates, time-varying* | |||
| Anticoagulant, person-days (%) | 677,620 (4.2) | 514,442 (10.1) | |
| Aspirin, person-days (%) | 45,043 (0.3) | 32,489 (0.6) | |
| Cilostazol, person-days (%) | 138,014 (0.8) | 28,817 (0.6) | |
| Dipyridamole, person-days (%) | 21,138 (0.1) | 28,845 (0.6) | |
| Gastroprotective agent, person-days (%) | 4,129,210 (25.4) | 1,203,886 (23.6) | |
| Nonsteroidal anti-inflammatory drug, person-days (%) | 596,580 (3.7) | 209,489 (4.1) | |
| Disease covariates, time varying | |||
| Cerebrovascular disease†, person-days (%) | 9,856,287 (60.5) | 2,331,869 (45.6) | |
| Gastrointestinal bleed/intracranial hemorrhage‡, person-days (%) | 8,502,055 (52.2) | 2,766,370 (54.1) | |
| Ischemic heart disease†, person-days (%) | 13,541,836 (83.2) | 2,994,267 (58.6) | |
dispensed (pursuant to a prescription) on the day of observation or within the prior 30 days
diagnosis (any position, any claim type) on the day of observation or ever prior
diagnosis (any position, any claim type) ever prior to the day of observation
We identified 536 and 436 precipitant drugs frequently co-prescribed with clopidogrel and with pravastatin, respectively. We conducted a self-controlled case series study for each of these object-precipitant pairs. The object drug cohorts had 431 precipitant drugs in common. Summary data on unadjusted, adjusted, and semi-Bayes adjusted rate ratios for gastrointestinal bleeding/intracranial hemorrhage are presented in Table 2. See Figure S1 for a heat map depicting overall and risk period-specific semi-Bayes adjusted rate ratios and ratios of rate ratios. The secondary analysis permitting a wider range of true rate ratios during semi-Bayes shrinkage had similar findings (Figure S2).
Table 2.
Summary data on rate ratios for serious bleeding, by object drug cohort.
| Object drug cohort | |||||
|---|---|---|---|---|---|
| Clopidogrel | Pravastatin | Ratio of Clopidogrel to Pravastatin | |||
| Before semi-Bayes adjustment | Unadjusted analyses | ||||
| Candidate interacting precipitant drugs examined, number | 536 | 436 | 433 | ||
| RR | DDI signals, number (%) | 189 (35.3) | 141 (32.3) | 98 (22.6) | |
| Increased rate* | 124 (23.1) | 94 (21.6) | 48 (11.1) | ||
| Decreased rate† | 65 (12.1) | 47 (10.8) | 50 (11.5) | ||
| Geometric mean ± standard deviation | 1.14 ± 1.97 | 1.14 ± 2.30 | 0.99 ± 2.23 | ||
| Range, minimum to maximum | 0.08 – 24.67 | 0.07 – 28.19 | 0.05 – 14.21 | ||
| Confounder-adjusted analyses | |||||
| Candidate interacting precipitant drugs examined, number | 536 | 434 | 431 | ||
| RR | DDI signals, number (%) | 174 (32.5) | 125 (28.8) | 97 (22.5) | |
| Increased rate* | 107 (20.0) | 81 (18.7) | 51 (11.8) | ||
| Decreased rate† | 67 (12.5) | 44 (10.1) | 46 (10.7) | ||
| Geometric mean ± standard deviation | 1.14 ± 1.98 | 1.12 ± 2.26 | 0.99 ± 2.26 | ||
| Range, minimum to maximum | 0.06 – 24.43 | 0.05 – 42.22 | 0.04 – 16.92 | ||
| After semi-Bayes adjustment | Unadjusted analyses | ||||
| Candidate interacting precipitant drugs examined, number | 536 | 436 | 433 | ||
| RR | DDI signals, number (%) | 150 (28.0) | 109 (25.0) | 72 (16.6) | |
| Increased rate* | 101 (18.8) | 78 (17.9) | 33 (7.6) | ||
| Decreased rate† | 49 (9.1) | 31 (7.1) | 39 (9.0) | ||
| Geometric mean ± standard deviation | 1.12 ± 1.43 | 1.14 ± 1.47 | 0.98 ± 1.52 | ||
| Range, minimum to maximum | 0.36 – 4.53 | 0.45 – 6.95 | 0.20 – 3.81 | ||
| Confounder-adjusted analyses | |||||
| Candidate interacting precipitant drugs examined, number | 536 | 434 | 431 | ||
| RR | DDI signals, number (%) | 139 (25.9) | 90 (20.7) | 73 (16.9) | |
| Increased rate* | 90 (16.8) | 63 (14.5) | 37 (8.6) | ||
| Decreased rate† | 49 (9.1) | 27 (6.2) | 36 (8.4) | ||
| Geometric mean ± standard deviation | 1.11 ± 1.42 | 1.12 ± 1.48 | 0.99 ± 1.54 | ||
| Range, minimum to maximum | 0.35 – 4.15 | 0.40 – 8.11 | 0.17 – 3.95 | ||
DDI = drug-drug interaction; RR = rate ratio
lower bound of the 95% confidence interval for the RR of interest was greater than the null value
upper bound of the 95% confidence interval for the RR of interest was less than the null value
When using pravastatin as the quantitative negative control object drug, 37 of the 431 candidate DDIs had statistically significantly elevated ratios of rate ratios for serious bleeding when examined over the entire observation period. We excluded nine candidates solely driven by protective findings among pravastatin users, i.e., the rate ratio for the precipitant drug was not statistically significantly elevated in users of clopidogrel but was statistically significantly protective in users of pravastatin, as these unlikely indicated a clopidogrel DDI. Twenty-eight remained (6.5% of all candidate DDIs) and were deemed potential signals of DDIs involving clopidogrel that lead to serious bleeding. Interacting precipitants included central nervous system agents (N = 12, including four NSAIDs and two opioids), cardiovascular agents (N = 3), endocrine/metabolic agents (N = 3), renal/genitourinary agents (N = 3, including two phosphate binders), nutritional agents (N = 3, including two vitamins), hematologic agents (N = 2, both anticoagulants), an anti-infective (N = 1), and a gastrointestinal agent (N = 1). Rate ratios ranged from 1.13 (95% confidence interval [CI]: 1.01–1.26) for potassium chloride to 3.94 (1.69–9.20) for cholecalciferol. Six (21.4%) of these 28 potential DDI signals are each listed in clopidogrel’s label, Micromedex, and Lexicomp as potentially increasing bleeding risk.
DISCUSSION
This pharmacoepidemiologic screening study of potential DDIs involving clopidogrel leading to serious bleeding yielded expected results for oral anticoagulants and NSAIDs, although not all of the semi-Bayes adjusted association measures were statistically significant. For anticoagulants, concomitant use of clopidogrel (vs. pravastatin) with warfarin or dabigatran was associated with statistically significant 1.3-fold and 1.2-fold rates of serious bleeding respectively, while the 1.3- and 1.5-fold rates for apixaban and rivaroxaban were not statistically significant. DDIs between clopidogrel and oral anticoagulants would be expected mechanistically given the independent cumulative effects of antiplatelet agents and anticoagulants on hemostasis,5,6 and the magnitude of these associations were generally consistent with prior epidemiologic studies.2,7,8 Concomitant use of clopidogrel (vs. pravastatin) with the NSAIDs meloxicam, piroxicam, nabumetone, and etodolac was associated with statistically significant 1.6-, 2.5-, 2.8-, and 3.2-fold rates (respectively) of serious bleeding, while the 1.1-, 1.8-, 1.9-, 2.8-, 2.8-, and 4.4-fold rates associated with naproxen, diclofenac, ibuprofen, oxaprozin, indomethacin, and sulindac (respectively) were not statistically significant. These findings are mechanistically plausible given independent effects of clopidogrel and NSAIDs on bleeding risk,9 and also generally consistent with prior epidemiologic studies.9–11 We believe that the consistency of the results for oral anticoagulants and NSAIDs with both mechanistic expectations and prior epidemiologic findings support the validity of our screening approach, and that the lack of statistical significance for some expected pairs may suggest that the assumptions we employed in our semi-Bayes adjustment were appropriately conservative for use in a hypothesis-generating screening context.
We also identified increased rates of serious bleeding upon concomitant use of clopidogrel with several drugs that are commonly used in persons with chronic kidney disease (CKD): bumetanide; cholecalciferol; cinacalcet; lanthanum; paricalcitol; sevelamer; and torsemide. Use of these precipitants may portend worsening renal function and/or dialysis. Advanced CKD and end stage kidney disease increase one’s risk of bleeding due to platelet dysfunction12 and heightened risk of angioectasias (i.e., thin-walled, dilated, ectatic blood vessels),13 which may be exacerbated in the setting of clopidogrel treatment.5,14,15 Cinacalcet, lanthanum, paricalcitol, and sevelamer are used almost exclusively in the treatment of abhorrent bone and mineral metabolism in persons with advanced CKD and end stage kidney disease. These disorders of bone and mineral metabolism can lead to gastrointestinal mucosal calcinosis and calciphylaxis,16 which may increase the risk of bleeding. Several case reports also describe deposition of sevelamer crystals in the gastrointestinal mucosa, directly resulting in ulceration and necrosis.17,18 It is unclear whether findings for these precipitants likely reflect confounding by indication or important DDIs that may place these patients at greater than expected risk of bleeding. Thus, the risk of clopidogrel-associated DDIs in advanced renal disease is an important area for future investigation.
The remaining 15 pairs associated with serious bleeding were deemed potentially clinically-relevant DDI signals (Table 3). These have neither been described in published case reports nor examined in population-based studies. It is therefore understandable that none are listed as an interaction (leading to a serious bleed) in clopidogrel’s label, Micromedex, or Lexicomp. Our automated screening approach did not consider preexisting mechanistic knowledge, which may be a poor predictor of clinically-important DDIs because of incomplete knowledge of off-target drug effects, failure to identify complex multi-pathway interactions,19 and traditional over-reliance on commonly considered mechanisms, most notably cytochrome P450 (CYP) inhibition. This is exemplified by the fact that some of our DDI signals may have identifiable putative mechanisms (e.g., primidone induces CYP2C19 and other hepatic isozymes20), while others do not. Future work should seek to elucidate mechanisms underlying these signals.
Table 3.
Clopidogrel DDI signals* of potential clinical concern given statistically significantly increased rates of serious bleeding, by therapeutic class of precipitant drug.
| Precipitant used concomitantly with clopidogrel | Ratio of rate ratios for clopidogrel to pravastatin | 95% confidence interval | Putative mechanism(s) of interaction with clopidogrel** |
|---|---|---|---|
| Anti-infective | |||
| demeclocycline | 3.06 | 1.10–8.49 | unknown |
| Cardiovascular | |||
| bisoprolol | 1.75 | 1.09–2.81 | unknown |
| gemfibrozil | 1.64 | 1.12–2.42 | induction of CYP3A4 |
| Central nervous system | |||
| diazepam | 1.85 | 1.26–2.73 | unknown |
| eszopiclone | 1.99 | 1.12–3.55 | unknown |
| hydromorphone | 2.00 | 1.06–3.77 | unknown |
| pramipexole | 2.57 | 1.54–4.29 | unknown |
| primidone | 2.08 | 1.14–3.78 | induction of CYP2C19, 2C9, 3A4, 1A2, 2B6 |
| prochlorperazine | 2.27 | 1.23–4.18 | unknown |
| quetiapine | 1.47 | 1.06–2.05 | unknown |
| tramadol | 1.26 | 1.05–1.51 | unknown |
| Endocrine and metabolic | |||
| megestrol | 1.46 | 1.02–2.09 | unknown |
| pioglitazone | 1.47 | 1.05–2.05 | induction of CYP3A4 |
| Gastrointestinal | |||
| ursodiol | 2.82 | 1.24–6.43 | unknown |
| Nutritional | |||
| potassium chloride | 1.13 | 1.01–1.26 | unknown |
CYP = cytochrome P450; DDI = drug-drug interaction
operational definition of a signal: statistically significantly elevated ratio of rate ratios for serious bleeding, excluding instances in which the rate ratio for the precipitant drug was not statistically significantly elevated in users of clopidogrel but statistically significantly protective in users of pravastatin
per DrugBank version 5.1.1 (The Metabolomics Innovation Centre: Edmonton, Alberta, Canada) enzyme, carrier, and transporter pathways
Our study has notable strengths. First, it utilized a self-controlled case series design, ideal for DDI screening,21 to minimize confounding. Second, we used a bi-directional implementation of the design to minimize exposure trend bias.22 Third, we utilized pravastatin as a negative control object drug to which clopidogrel findings were quantitatively compared. Fourth, we studied a clinically meaningful outcome identified by algorithms with excellent performance metrics. Finally, we minimized false positive findings by using semi-Bayes shrinkage to account for multiple estimation.
Our study also has limitations. First, it did not examine higher order (i.e., beyond pairwise) DDIs. Such findings may be of future interest given bleeding rates with dual antiplatelet and triple antithrombotic therapies reported in ISAR-TRIPLE, PIONEER, REDUAL, and WOEST and under investigation in AUGUSTUS and ENTRUST, as examples. Second, because clopidogrel + precipitant pairs and pravastatin + precipitant pairs were required for the parameter of interest, candidate DDI signals were identified among the intersection of concomitantly used drugs identified for both objects. This prohibited us from examining ratios of rate ratios for ~19% of precipitant drugs concomitantly prescribed with clopidogrel, but not pravastatin. Third, we did not examine time-invariant covariates as potential effect modifiers. Fourth, the bi-directional self-controlled case series design may be susceptible to reverse causality, especially for suspected DDIs. If a clinician posited that a precipitant induced a serious bleed in an object drug user (even if it had no effect on the bleeding rate), the precipitant may be subsequently discontinued. This may result in a spuriously elevated rate ratio for that precipitant. However, it seems unlikely to us that reverse causality is responsible for associations with newly-identified DDI signals because: a) DDIs are often overlooked in clinical practice and therefore clinicians would unlikely attribute a serious bleed to an interaction and discontinue the precipitant to reduce future risk; b) such precipitant discontinuation would only have the potential to cause bias if differential among users of clopidogrel and pravastatin; and c) a post hoc analysis employing a right-censored uni-directional self-controlled case series design (resistant to reverse causality, but vulnerable to exposure trend bias) replicated the signals described herein (Table S1). Fifth, our reliance on a prescription dispensing as a surrogate for drug consumption and inability to assess adherence raise concerns of exposure misclassification. Sixth, residual confounding may be present; we did not adjust for precipitant drug dose, severity of chronic diseases, frailty, or socioeconomic status—factors not always static throughout an individual’s observation. Finally, our findings may not be generalizable beyond a commercially-insured, ambulatory care population.
We used longitudinal health insurance data to identify 15 previously undescribed and/or unappreciated clopidogrel DDIs associated with serious bleeding. Vigilance during clopidogrel prescribing is warranted, since these potentially clinically-relevant interactions are not documented in two major DDI knowledge bases.
METHODS
Overview
We conducted automated, high-throughput pharmacoepidemiologic screening of commercial health insurance claims to identify signals of DDIs with clopidogrel. First, we identified drugs that were frequently co-prescribed with clopidogrel as candidate interacting precipitants. Second, we identified DDI signals by performing confounder-adjusted self-controlled case series studies for clopidogrel + precipitant (i.e., interacting drug) pairs, with hospital presentation for serious bleeding as the study outcome. To help distinguish native bleeding effects of a precipitant drug from a DDI involving clopidogrel, we repeated these steps for pravastatin, which served as a quantitative comparator (i.e., negative control object drug).23 Pravastatin was selected because it is a widely-used cardiovascular drug that does not affect the risk of serious bleeding,24 minimally inhibits human carboxylesterase 1,25 and lacks substantive CYP-based effects26 that could affect other drugs’ bleeding risk.
Data source
We used 2000–2015 data from the Optum Clinformatics Data Mart (OptumInsight: Eden Prairie, MN, United States).27 Optum includes enrollment and healthcare billing data from >71 million commercially-insured and Medicare Advantage beneficiaries of a large United States-based insurer. Data elements include: demographics (e.g., age, sex, race); enrollment periods; medical encounters (e.g., ambulatory care visits, emergency department visits, inpatient hospitalizations) and their accompanying diagnoses and procedures; pharmacy dispensings; and laboratory orders and results. We selected Optum as our data source because of its generalizability to the United States population, as ~65% of Americans receive healthcare coverage via commercial health plans or Medicare.28 The University of Pennsylvania’s Office of Regulatory Affairs determined that research using Optum was exempted from institutional review board review.
Identifying candidate interacting precipitant drugs
We used pharmacy claim dates and days’ supply values to identify all pharmacy dispensings of clopidogrel (the object drug of interest) and all oral drugs concomitantly used with clopidogrel (precipitant drugs of interest). This step was repeated for pravastatin, as it served as the negative control object drug.23 The intersection of drugs identified for clopidogrel and pravastatin served as candidate precipitant drugs. We selected the intersection rather than union of drugs because measures of association for clopidogrel + precipitant pairs and pravastatin + precipitant pairs were required for the parameter of interest (described later).
Identifying DDI signals via automated pharmacoepidemiologic screening
For each object + candidate precipitant pair, we conducted a bi-directional self-controlled case series study to examine the rate of serious bleeding for an individual receiving the object drug of interest after initiating vs. not receiving a precipitant. Although the “case series” phrase within self-controlled case series may seem to imply the absence of a comparator, the approach is a rigorous, controlled epidemiologic study design that is the cohort analogue of the case-crossover design.22 The self-controlled case series design has the following advantages that make it ideal for DDI screening: 1) it is highly computationally-efficient,29 since it includes only persons who experienced the outcome of interest; 2) the causal contrast is made within individual and thus inherently controls for confounding by both measured and unmeasured factors that remain constant within an individual over the observation period (e.g., sex, genetics, chronic diseases, frailty, socioeconomic status); 3) the underlying statistical model can accommodate time-varying factors;30 and 4) a high-throughput approach has been developed and used previously.21
Creating study cohorts of new users of object drugs
Separate cohorts were constructed for clopidogrel and pravastatin. For persons 18–90 years of age, we utilized pharmacy claim dates and days’ supply values to build object drug exposure episodes consisting of ≥1 object drug dispensing(s). We permitted a 7-day grace period between contiguous dispensings and at the end of the terminal dispensing to account for imperfect adherence; this approximated 80% adherence for each 30-day dispensing. We then selected new users of the object drug by requiring a baseline period (defined below) that was devoid of a dispensing for that object or a therapeutic alternative (i.e., anagrelide, cangrelor, cilostazol, dipyridamole, prasugrel, ticagrelor, ticlopidine, or vorapaxar for clopidogrel, and other 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors for pravastatin). Since the self-controlled case series design is a “case-only” approach, we also required new users to experience a serious bleeding event (defined below) during their observation period.
Defining observation and baseline periods
For each new user meeting inclusion criteria, the observation period began upon object drug initiation and was censored upon the earliest of: a) lapsed exposure to the object (accounting for the 7-day grace period); b) a switch from the object to a therapeutic alternative; c) health plan disenrollment; or d) the end of the study dataset. We did not censor observation time upon outcome occurrence since this would violate an assumption underlying the self-controlled case series design.29,31
The baseline period was defined as the six months immediately before the start of the observation period. It was required to be devoid of: a) a dispensing for the object drug of interest or a therapeutic alternative; and b) an interruption in health plan coverage.
Categorizing observation period time based on precipitant drug exposure
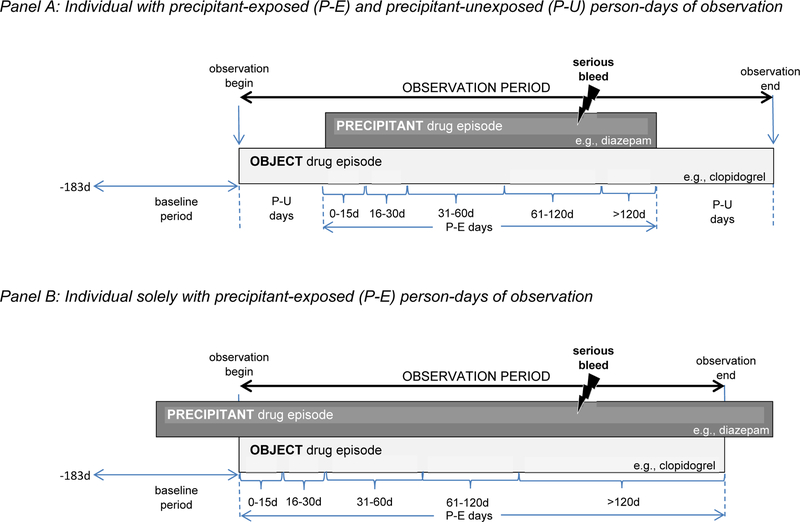
Each person-day of the observation period was dichotomized as a precipitant-exposed or precipitant-unexposed day. Precipitant-exposed days were defined by concomitant exposure to the candidate interacting precipitant drug using days’ supply values without a grace period. Precipitant-unexposed days consisted of all other person-days of observation time. Consistent with a bi-directional self-controlled case series design, precipitant-unexposed days were permitted before and/or after precipitant-exposed days; this approach helped to minimize exposure trend bias.22 Although observation periods were not required to have both precipitant-exposed and precipitant-unexposed person-days, only observation periods with such discordance contributed to the estimation of the parameter of interest. See Figure 1 for a graphical representation of the design.
Figure 1. Examples of object drug exposure episodes potentially eligible for inclusion.
Panel A depicts an individual with P-E and precipitant-unexposed P-U person-days of observation. Panel B depicts an individual solely with P-E person-days of observation. P-E = precipitant-exposed; P-U = precipitant-unexposed.
Several studies have shown that the risk of an adverse event due to a pharmacokinetic DDI often peaks shortly after initiating concomitant therapy and declines thereafter.32–34 Therefore, we examined a duration-response relationship for each object–precipitant pair. Precipitant-exposed observation time was divided into the following risk periods following the initiation of concomitance: 0–15, 16–30, 31–60, 61–120, and >120 days.
Defining the exposure of interest and covariates
The exposure of interest was use of the candidate interacting precipitant drug. Time-invariant covariates (e.g., genetic polymorphisms, race, ethnicity) are inherently controlled for by the self-controlled case series design.31 In each regression model, we included the following time-varying covariates putatively associated with serious bleeding risk among antiplatelet drug users, as adapted from S2TOP-BLEED35 and its predecessors36: a) prior history of or current ischemic heart disease and/or cerebrovascular disease; b) prior gastrointestinal bleeding and/or intracranial hemorrhage; c) ongoing concomitant therapy with an anticoagulant (e.g., warfarin), a non-clopidogrel antiplatelet drug (e.g., aspirin), a gastroprotective agent (e.g., lansoprazole), and/or an NSAID (e.g., ibuprofen); and d) average daily dispensed object drug dose. Because some of these drugs are available without a prescription, reliance on claims data may lead to under-ascertainment. Table S2 includes additional detail on covariates.
Identifying outcomes
The composite outcome of interest was serious bleeding, defined as hospital presentation for gastrointestinal bleeding or intracranial hemorrhage identified by International Classification of Diseases 9th Revision Clinical Modification discharge diagnosis codes. Operational definitions, including quantitative measures of algorithm performance,37–40 are presented in Table 4. Gastrointestinal bleeding and intracranial hemorrhage are the most common types of serious bleeding in persons taking antiplatelet drugs, and can be fatal.41 This was the rationale for studying a composite outcome of serious bleeding from these sites.
Table 4.
Operational definition of serious bleeding.
| Outcome component | Discharge diagnosis descriptor | Discharge diagnosis ICD-9-CM code(s) | Discharge diagnosis position and claim type | Positive predictive value |
|---|---|---|---|---|
| Gastrointestinal bleeding | esophageal ulcer, with hemorrhage | 530.21 | Any-position discharge diagnosis on an inpatient hospitalization claim | ~81% |
| gastric ulcer, with hemorrhage | 531.0X, 531.2X, 531.4X, 531.6X | |||
| duodenal ulcer, with hemorrhage | 532.0X, 532.2X, 532.4X, 532.6X | |||
| peptic ulcer, with hemorrhage | 533.0X, 533.2X, 533.4X, 533.6X | |||
| gastrojejunal ulcer, with hemorrhage | 534.0X, 534.2X, 534.4X, 534.6X | |||
| gastritis and duodenitis, with hemorrhage | 535.01, 535.11, 535.21, 535.31, 535.41, 535.51, 535.61, 535.71 | |||
| other specified disorder of stomach and duodenum, with hemorrhage | 537.83, 537.84 | |||
| diverticula of intestine, with hemorrhage | 562.02, 562.03, 562.12, 562.13 | |||
| other disorders of intestine, with hemorrhage | 569.85, 569.86 | |||
| gastrointestinal hemorrhage | 578.X | |||
| Intracranial hemorrhage | subarachnoid hemorrhage | 430 | Any-position discharge diagnosis on an emergency department or inpatient hospitalization claim | ~77–94% |
| intracerebral hemorrhage | 431 |
ICD-9-CM = international classification of diseases 9th revision clinical modification
Statistical analysis
We constructed an analytic file for clopidogrel and one for pravastatin in which the unit of observation was the person-day of time covered by an active prescription to that object drug. The binary dependent variable was whether serious bleeding occurred on that day. Independent variables included a unique subject identifier, the subject’s observation period, the observation day (categorized as precipitant-exposed vs. precipitant-unexposed), and time-varying covariates discussed above. The parameter of interest was the outcome occurrence rate during precipitant-exposed vs. precipitant-unexposed days, i.e., rateobject+precipitant / rateobject. In a secondary analysis, we examined outcome occurrence separately for the five risk periods discussed above. We used conditional Poisson regression models (xtpoisson, Stata v.15: College Station, TX, United States) to estimate rate ratios and 95% CIs.29,31,42 To avoid statistically unstable estimates, we did not estimate rate ratios when there were fewer than five precipitant-exposed patients or no events during precipitant-exposed time. Further, we did not report rate ratios from nonconverged conditional Poisson regression models or if the variance of the beta estimate for the parameter of interest was >10.
To account for the multiple estimation inherent in calculating hundreds of rate ratios and CIs, we used a semi-Bayes shrinkage method. This increases the validity of effect estimates and preserves the nominal type-1 error rate.43,44 Operationally, we prespecified a variance (σ2 = 0.25) to assume that 95% of true rate ratios would be within an unspecified 7-fold range, then shrunk outlying effect estimates toward their geometric mean. In a secondary analysis, we increased the variance (σ2 = 0.67) to assume that 95% of true rate ratios would be within an unspecified 25-fold range.
As discussed above, we used pravastatin as the negative control object drug to which clopidogrel findings were quantitatively compared. Therefore, we divided the semi-Bayes adjusted rate ratio for each clopidogrel-precipitant pair by the semi-Bayes adjusted rate ratio for the corresponding pravastatin-precipitant pair, and calculated 95% CIs using the delta method.45 To contextualize findings, we compared DDI signals generated by our automated approach to putative interactions described in: clopidogrel’s label; and Micromedex (IBM Watson Health: Cambridge, MA, United States) and Lexicomp (Wolters Kluwer: Alphen aan den Rijn, South Holland, Netherlands) DDI knowledge bases.
Supplementary Material
Figure S1. Heatmap depicting semi-Bayes adjusted rate ratios and ratios of semi-Bayes adjusted rate ratios for serious bleeding, overall and during prespecified risk periods: semi-Bayes adjustment assuming that 95% of true rate ratios are within a 7-fold range.
Figure S2. Heatmap depicting semi-Bayes adjusted rate ratios and ratios of semi-Bayes adjusted rate ratios for serious bleeding, overall and during prespecified risk periods: semi-Bayes adjustment assuming that 95% of true rate ratios are within a 25-fold range.
Table S1. Ratios of rate ratios, prior to semi-Bayes shrinkage, for serious bleeding for object-precipitant pairs suggestive of DDI signals (N = 28).
Table S2. Time-varying covariates included in conditional Poisson regression models.
Table S3. International Classification of Diseases, 9th Revision, Clinical Modification codes used to define ischemic heart disease.
Table S4. International Classification of Diseases, 9th Revision, Clinical Modification codes used to define cerebrovascular disease.
Table S5. International Classification of Diseases, 9th Revision, Clinical Modification codes used to define gastrointestinal bleeding / intracranial hemorrhage.
STUDY HIGHLIGHTS.
What is the current knowledge on the topic?
While several studies have examined drug-drug interactions that may interfere with clopidogrel’s effectiveness in preventing thrombosis, few studies have examined drugs that may potentiate clopidogrel’s bleeding risk. Both are of great clinical and public health importance.
What question did this study address?
Which precipitant drugs taken concomitantly with clopidogrel can increase the rate of hospital presentation for gastrointestinal bleeding or intracranial hemorrhage?
What does this study add to our knowledge?
Our high-throughput pharmacoepidemiologic screening of longitudinal healthcare data identified fifteen previously undescribed and/or unappreciated clopidogrel drug interactions that may be associated with serious bleeding. These potentially clinically-important interactions deserve further investigation.
How might this change clinical pharmacology or translational science?
The goals of drug-drug interaction research include screening for previously unanticipated interactions, elucidating their potential pharmacokinetic and/or pharmacodynamic mechanisms, predicting and examining their effects on pharmacokinetic and clinical outcomes, and developing and evaluating approaches to manage their risks in clinical settings. Future research on clopidogrel drug-drug interaction signals identified herein should contribute to broader pharmacologic knowledge of the drugs involved and the biological pathways involved in their kinetics and dynamics, thus yielding generalizable biologic knowledge.
ACKNOWLEDGEMENTS
The authors thank Min Du and Qing Liu from the University of Pennsylvania for their computer programming support.
Funding
The work herein was supported by grants (R01AG025152, R01AG060975) from the United States Department of Health and Human Services’ National Institute on Aging.
Conflict of Interest Statement
Dr. Leonard serves on the Executive Committee of and Dr. Hennessy directs the University of Pennsylvania’s Center for Pharmacoepidemiology Research and Training. The Center receives unrestricted funding from Pfizer and Sanofi. Dr. Leonard’s spouse is employed by a health technology company that receives funding from AbbVie, Adamas, Celgene, Lilly, Lundbeck, Novartis, and Sunovion. All other authors declared no competing interests for this work.
ABBREVIATIONS LIST
- CI
confidence interval
- CKD
chronic kidney disease
- CYP
cytochrome P450
- DDI
drug-drug interaction
- NSAID
nonsteroidal anti-inflammatory drug
Footnotes
Statement of Integrity
Dr. Leonard had full access to study data and takes responsibility for its integrity and that of the data analysis.
REFERENCES
- (1).National Ambulatory Medical Care Survey, 2015. Public-use data file and documentation (2018).
- (2).Hansen ML, et al. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch. Intern. Med 170, 1433–1441 (2010). [DOI] [PubMed] [Google Scholar]
- (3).Wang ZY, et al. Pharmacokinetic drug interactions with clopidogrel: updated review and risk management in combination therapy. Ther. Clin. Risk Manag 11, 449–467 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Dal Pan GJ, Lindquist M & Gelperin K Postmarketing spontaneous pharmacovigilance reporting systems. In Pharmacoepidemiology (eds. Strom BL, Kimmel SE & Hennessy S) 137–157 (Wiley-Blackwell, West Sussex, 2012). [Google Scholar]
- (5).Jiang X, Samant S, Lesko LJ & Schmidt S Clinical pharmacokinetics and pharmacodynamics of clopidogrel. Clinical pharmacokinetics 54, 147–166 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Kalyanasundaram A & Lincoff AM Managing adverse effects and drug-drug interactions of antiplatelet agents. Nature reviews cardiology 8, 592–600 (2011). [DOI] [PubMed] [Google Scholar]
- (7).Vitry AI, et al. Major bleeding risk associated with warfarin and co-medications in the elderly population. Pharmacoepidemiol. Drug Saf 20, 1057–1063 (2011). [DOI] [PubMed] [Google Scholar]
- (8).Jobski K, Behr S & Garbe E Drug interactions with phenprocoumon and the risk of serious haemorrhage: a nested case-control study in a large population-based German database. European journal of clinical pharmacology 67, 941–951 (2011). [DOI] [PubMed] [Google Scholar]
- (9).Schjerning Olsen AM, et al. Association of NSAID use with risk of bleeding and cardiovascular events in patients receiving antithrombotic therapy after myocardial infarction. JAMA 313, 805–814 (2015). [DOI] [PubMed] [Google Scholar]
- (10).Delaney JA, Opatrny L, Brophy JM & Suissa S Drug drug interactions between antithrombotic medications and the risk of gastrointestinal bleeding. Canadian Medical Association journal 177, 347–351 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Nam YH, et al. Nonsteroidal anti-inflammatory drug choice and adverse outcomes in clopidogrel users: A retrospective cohort study. PLoS One 13, e0193800 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Lutz J, Menke J, Sollinger D, Schinzel H & Thurmel K Haemostasis in chronic kidney disease. Nephrol. Dial. Transplant 29, 29–40 (2014). [DOI] [PubMed] [Google Scholar]
- (13).Kalman RS & Pedrosa MC Evidence-based review of gastrointestinal bleeding in the chronic kidney disease patient. Semin. Dial 28, 68–74 (2015). [DOI] [PubMed] [Google Scholar]
- (14).Fischer MJ, Ho PM, McDermott K, Lowy E & Parikh CR Chronic kidney disease is associated with adverse outcomes among elderly patients taking clopidogrel after hospitalization for acute coronary syndrome. BMC Nephrol 14, 107–2369-14–107 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Tanios BY, Itani HS & Zimmerman DL Clopidogrel use in end-stage kidney disease. Semin. Dial 28, 276–281 (2015). [DOI] [PubMed] [Google Scholar]
- (16).Nigwekar SU, Thadhani R & Brandenburg VM Calciphylaxis. N. Engl. J. Med 378, 1704–1714 (2018). [DOI] [PubMed] [Google Scholar]
- (17).Swanson BJ, et al. Sevelamer crystals in the gastrointestinal tract (GIT): a new entity associated with mucosal injury. Am. J. Surg. Pathol 37, 1686–1693 (2013). [DOI] [PubMed] [Google Scholar]
- (18).Yuste C, et al. Gastrointestinal complications induced by sevelamer crystals. Clin. Kidney J 10, 539–544 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Noor A, Assiri A, Ayvaz S, Clark C & Dumontier M Drug-drug interaction discovery and demystification using Semantic Web technologies. J. Am. Med. Inform. Assoc 24, 556–564 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Perucca E Clinically relevant drug interactions with antiepileptic drugs. Br. J. Clin. Pharmacol 61, 246–255 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Han X, Chiang CW, Leonard CE, Bilker WB, Brensinger CM & Hennessy S Biomedical informatics approaches to identifying drug-drug interactions: application to insulin secretagogues. Epidemiology 28, 459–468 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Maclure M, et al. When should case-only designs be used for safety monitoring of medical products? Pharmacoepidemiol. Drug Saf 21 Suppl 1, 50–61 (2012). [DOI] [PubMed] [Google Scholar]
- (23).Hennessy S, et al. Pharmacoepidemiologic Methods for Studying the Health Effects of Drug-Drug Interactions. Clin Pharmacol Ther 99, 92–100 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Gulmez SE, et al. Do statins protect against upper gastrointestinal bleeding? Br. J. Clin. Pharmacol 67, 460–465 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Fukami T, Takahashi S, Nakagawa N, Maruichi T, Nakajima M & Yokoi T In vitro evaluation of inhibitory effects of antidiabetic and antihyperlipidemic drugs on human carboxylesterase activities. Drug Metab. Dispos 38, 2173–2178 (2010). [DOI] [PubMed] [Google Scholar]
- (26).Gottlieb RA Cytochrome P450: major player in reperfusion injury. Arch. Biochem. Biophys 420, 262–267 (2003). [DOI] [PubMed] [Google Scholar]
- (27).Optum Inc. Clinformatics Data Mart. OPTPRJ5232, https://www.optum.com/content/dam/optum/.../Clinformatics_for_Data_Mart.pdf (2014).
- (28).State Health Facts: Health insurance coverage of the total population (2016) (2017). [Google Scholar]
- (29).Whitaker HJ, Hocine MN & Farrington CP The methodology of self-controlled case series studies. Stat. Methods Med. Res 18, 7–26 (2009). [DOI] [PubMed] [Google Scholar]
- (30).Lee KJ & Carlin JB Fractional polynomial adjustment for time-varying covariates in a self-controlled case series analysis. Stat. Med 33, 105–116 (2014). [DOI] [PubMed] [Google Scholar]
- (31).Whitaker HJ, Farrington CP, Spiessens B & Musonda P Tutorial in biostatistics: the self-controlled case series method. Stat. Med 25, 1768–1797 (2006). [DOI] [PubMed] [Google Scholar]
- (32).Juurlink DN, Mamdani M, Kopp A, Laupacis A & Redelmeier DA Drug-drug interactions among elderly patients hospitalized for drug toxicity. JAMA 289, 1652–1658 (2003). [DOI] [PubMed] [Google Scholar]
- (33).Schelleman H, Bilker WB, Brensinger CM, Wan F & Hennessy S Anti-infectives and the risk of severe hypoglycemia in users of glipizide or glyburide. Clin. Pharmacol. Ther 88, 214–222 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Douketis JD, Melo M, Bell CM & Mamdani MM Does statin therapy decrease the risk for bleeding in patients who are receiving warfarin? Am. J. Med 120, 369.e9–369.e14 (2007). [DOI] [PubMed] [Google Scholar]
- (35).Hilkens NA, et al. Predicting major bleeding in patients with noncardioembolic stroke on antiplatelets: S2TOP-BLEED. Neurology 89, 936–943 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Hilkens NA, Algra A & Greving JP Prediction models for intracranial hemorrhage or major bleeding in patients on antiplatelet therapy: a systematic review and external validation study. J. Thromb. Haemost 14, 167–174 (2016). [DOI] [PubMed] [Google Scholar]
- (37).Schelleman H, Bilker WB, Brensinger CM, Wan F, Yang YX & Hennessy S Fibrate/Statin initiation in warfarin users and gastrointestinal bleeding risk. Am. J. Med 123, 151–157 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Arnason T, Wells PS, van Walraven C & Forster AJ Accuracy of coding for possible warfarin complications in hospital discharge abstracts. Thromb. Res 118, 253–262 (2006). [DOI] [PubMed] [Google Scholar]
- (39).Birman-Deych E, Waterman AD, Yan Y, Nilasena DS, Radford MJ & Gage BF Accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Med. Care 43, 480–485 (2005). [DOI] [PubMed] [Google Scholar]
- (40).Andrade SE, et al. A systematic review of validated methods for identifying cerebrovascular accident or transient ischemic attack using administrative data. Pharmacoepidemiol. Drug Saf 21 Suppl 1, 100–128 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Becker RC, et al. Bleeding complications with the P2Y12 receptor antagonists clopidogrel and ticagrelor in the PLATelet inhibition and patient Outcomes (PLATO) trial. Eur. Heart J 32, 2933–2944 (2011). [DOI] [PubMed] [Google Scholar]
- (42).Whitaker HW Using Stata for the self-controlled case series method (2005). [Google Scholar]
- (43).Greenland S & Poole C Empirical-Bayes and semi-Bayes approaches to occupational and environmental hazard surveillance. Arch. Environ. Health 49, 9–16 (1994). [DOI] [PubMed] [Google Scholar]
- (44).Steenland K, Bray I, Greenland S & Boffetta P Empirical Bayes adjustments for multiple results in hypothesis-generating or surveillance studies. Cancer Epidemiol. Biomarkers Prev 9, 895–903 (2000). [PubMed] [Google Scholar]
- (45).Bieler GS & Williams RL Ratio estimates, the delta method, and quantal response tests for increased carcinogenicity. Biometrics 49, 793–801 (1993). [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Heatmap depicting semi-Bayes adjusted rate ratios and ratios of semi-Bayes adjusted rate ratios for serious bleeding, overall and during prespecified risk periods: semi-Bayes adjustment assuming that 95% of true rate ratios are within a 7-fold range.
Figure S2. Heatmap depicting semi-Bayes adjusted rate ratios and ratios of semi-Bayes adjusted rate ratios for serious bleeding, overall and during prespecified risk periods: semi-Bayes adjustment assuming that 95% of true rate ratios are within a 25-fold range.
Table S1. Ratios of rate ratios, prior to semi-Bayes shrinkage, for serious bleeding for object-precipitant pairs suggestive of DDI signals (N = 28).
Table S2. Time-varying covariates included in conditional Poisson regression models.
Table S3. International Classification of Diseases, 9th Revision, Clinical Modification codes used to define ischemic heart disease.
Table S4. International Classification of Diseases, 9th Revision, Clinical Modification codes used to define cerebrovascular disease.
Table S5. International Classification of Diseases, 9th Revision, Clinical Modification codes used to define gastrointestinal bleeding / intracranial hemorrhage.