Abstract
Human renal membrane transporters play key roles in the disposition of renally cleared drugs and endogenous substrates, but their ontogeny is largely unknown. Using 184 human postmortem frozen renal cortical tissues (preterm newborns to adults) and a subset of 62 tissue samples, we measured the mRNA levels of 11 renal transporters and the transcription factor pregnane X receptor (PXR) with quantitative real‐time polymerase chain reaction, and protein abundance of nine transporters using liquid chromatography tandem mass spectrometry selective reaction monitoring, respectively. Expression levels of p‐glycoprotein, urate transporter 1, organic anion transporter 1, organic anion transporter 3, and organic cation transporter 2 increased with age. Protein levels of multidrug and toxin extrusion transporter 2‐K and breast cancer resistance protein showed no difference from newborns to adults, despite age‐related changes in mRNA expression. Multidrug and toxin extrusion transporter 1, glucose transporter 2, multidrug resistance‐associated protein 2, multidrug resistance‐associated protein 4 (MRP4), and PXR expression levels were stable. Using immunohistochemistry, we found that MRP4 localization in pediatric samples was similar to that in adult samples. Collectively, our study revealed that renal drug transporters exhibited different rates and patterns of maturation, suggesting that renal handling of substrates may change with age.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ Membrane transporters in the human kidney play major roles in the disposition of many drugs and endogenous substances. Pediatric patients often exhibit age‐related differences in drug disposition. This may be related to ontogenic changes in the expression levels of renal transporters, but knowledge is lacking.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ How do gene expression and protein abundance of 11 renal membrane transporters change with age?
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ By analyzing 184 human postmortem kidney samples from newborns to adults, our study showed that 11 renal drug transporters exhibited different patterns of maturation, with most changes occurring in children <2 years of age.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
☑ These data, which suggest a mechanism for age‐related changes in drug disposition, may be used in modeling and simulation to predict drug dosing in children. Our findings set the stage for future research in the mechanisms underlying ontogenic changes in the expression of renal drug transporters.
Renal membrane transporters, which are located on the apical and basolateral sides of the tubular epithelium, are key players in tubular secretion and reabsorption of a plethora of endogenous and exogenous compounds in the kidneys.1, 2 Because of their role in renal elimination, many transporters in the kidneys play critical roles in the disposition, efficacy, and toxicity of drugs. Notably, renal drug transporters have received increasing regulatory attention in recent years, highlighting their significance in drug disposition.3, 4, 5, 6
Interindividual variation in expression levels and functional activities of membrane transporters can affect the homeostasis of endogenous substrates, as well as the pharmacokinetics and pharmacodynamics of drugs.1 As a result of developmental changes in key transporters and enzymes, levels of endogenous substrates, such as metabolites, nutrients, antioxidants, and hormones, change as children grow.7 Reduced hepatic clearance of the opioid morphine in newborns and young infants was reported.8 This was suggested due to significantly lower hepatic levels of both the drug‐metabolizing enzyme uridine 5‐diphosphoglucuronic acid glucuronyl transferase 2B7 and the organic cation transporter (OCT) 1 in young pediatric populations compared with adults.9, 10 In contrast to the liver, less is known about the maturation and ontogeny pattern of renal membrane transporters. This knowledge gap limits the ability to predict the pharmacokinetics of renally eliminated drugs in children, which may be critical for rational dosing and drug efficacy and safety. Thus, there is an urgent need to understand the ontogeny of human drug transporters in the kidneys.
The current study aimed to identify age‐related differences in gene expression and protein abundance of renal transporters. We chose to focus on renal transporters with demonstrated clinical relevance in drug disposition, and those that handle various endogenous and exogenous substances important for developing children11, 12 (i.e., breast cancer resistance protein (gene name/protein name ABCG2/BCRP), multidrug and toxin extrusion protein (SLC47A/MATE) 1 and 2‐K, multidrug resistance protein 1 (ABCB1/MDR1/p‐glycoprotein (P‐gp)), multidrug resistance‐associated protein (ABCC/MRP) 2 and 4, and urate transporter 1 (SLC22A12/URAT1) on the apical site of the membrane and glucose transporter 2 (SLC2A2/GLUT2), organic anion transporter 1 (SLC22A6/OAT1) and 3 (SLC22A8/OAT3), and SLC22A2/OCT2 located on the basolateral site. In an effort to explore a regulatory mechanism for maturation of transporter expression, we also studied renal gene expression of the nuclear pregnane X receptor (PXR) in relation to the transporter expression levels.13
In addition, altered localization of a transporter may introduce variation in pharmacokinetics of transporter substrates. However, little is known about the localization of transporters during development of the kidneys. MRP4 is an apical efflux transporter involved in transport of a range of endogenous molecules, including cyclic nucleotides, urate and conjugated steroid hormones, and drugs that are used in children, including antivirals and diuretics.14 We performed immunohistochemistry as a proof‐of‐concept to visualize the location of MRP4 in our pediatric kidney tissues.
Results
Two sample sets, which provided a total of 184 postmortem renal cortical tissues, were analyzed in this study (Figure 1 and Table 1 ). Sample set 1 represented 62 samples from individuals of different ages ranging from preterm newborns (gestational age (GA) > 24 weeks, postnatal age (PNA) 1 day) to adult donors (oldest 75 years). The 122 tissues in sample set 2 were from African American and white term newborns to adults. No statistical difference was observed in gene expression or protein abundance levels for any of the transporters between boys and girls and between African Americans and whites (Tables [Link], [Link], [Link]); hence, subsequent analyses were performed by combining both sexes and all ethnic groups.
Figure 1.
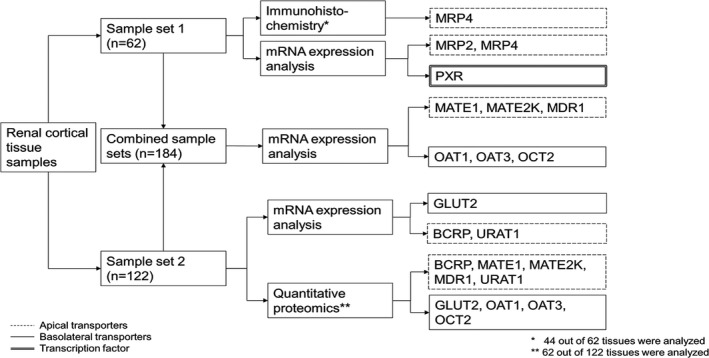
Sample analysis scheme. The subset of 62 samples from sample set 2 for quantitative proteomics consisted of the 57 African American samples and 5 adult white samples (see Table 1 ). BCRP, breast cancer resistance protein; GLUT, glucose transporter; MATE, multidrug and toxin extrusion protein; MDR, multidrug resistance protein; MRP, multidrug resistance‐associated protein; OAT, organic anion transporter; OCT, organic cation transporter; PXR, pregnane X receptor; URAT, urate transporter.
Table Table 1.
Overview of sample size and age range of sample sets 1 and 2
| Age group | Number of samples | Age range | ||||
|---|---|---|---|---|---|---|
| Sample set 1 | Sample set 2 | Total | Gestational age | Postnatal age | ||
| Race unknown | White | African American | ||||
| Preterm newborns | 9 | − | − | 9 | 34.00 (24.00–36.71) weeks | 1.29 (0.14–4.00) weeks |
| Term newborns | 8 | 10 | 1 | 19 | NA | 1.29 (0.14–3.86) weeks |
| Infants | 21 | 30 | 30 | 81 | NA | 17.86 (4.14–103.00) weeks |
| Children | 7 | 15 | 16 | 38 | NA | 4.74 (2.00–11.56) years |
| Adolescents | − | 5 | 5 | 10 | NA | 13.38 (12.48–15.26) years |
| Adults | 17 | 5 | 5 | 27 | NA | 45.00 (16.75–75.00) years |
| Total | 62 | 65 | 57 | 184 | ||
| 122 | ||||||
NA, not available.
Relative mRNA quantitation
All 184 tissues were processed for mRNA quantitation (Figure 2 and Table S4 ). The mRNA levels of the selected transporters were detected successfully in all samples, with the exception of MATE1 in two samples. Glyceraldehyde 3‐phosphate dehydrogenase mRNA expression did not change with age (r s = −0.12; P = 0.119).
Figure 2.
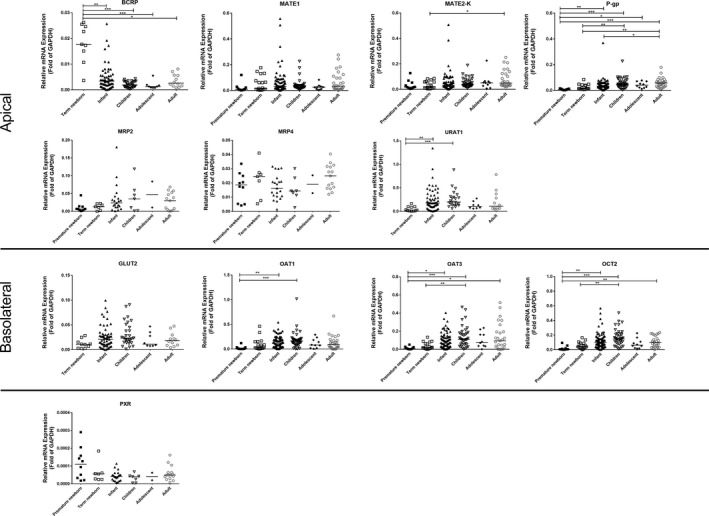
Relative mRNA expression of 11 renal membrane transporters and pregnane X receptor in different age groups. Transporters are grouped according to their primary localization in the kidney (basolateral or apical). The bar represents the median for each age group. *P < 0.05, **P < 0.01, ***P < 0.001. BCRP, breast cancer resistance protein; GAPDH, glyceraldehyde 3‐phosphate dehydrogenase; GLUT, glucose transporter; MATE, multidrug and toxin extrusion protein; MDR, multidrug resistance protein; MRP, multidrug resistance‐associated protein; OAT, organic anion transporter; OCT, organic cation transporter; PXR, pregnane X receptor; URAT, urate transporter.
Overall, a large variability in the developmental changes in transporter mRNA levels was observed (Figure 2 and Table S4 ). MATE2‐K, P‐gp, URAT1, OAT1, OAT3, and OCT2 levels in premature and/or term newborns were significantly lower than in the older age groups. In contrast, term newborns showed significantly higher BCRP mRNA levels than children and adolescents. MATE1, MRP2, MRP4, GLUT2, and PXR levels were not different between all age groups (preterm newborn, term newborn, infants, children, adolescents, and adults).
Proteomics
Sixty‐two samples were assessed for transporter protein levels (Figure 3 and Table S5 ). The median total membrane protein yield for all samples was 49.5 mg/g (range 41.7–62.1 mg/g) renal cortical tissue. All nine transporters were detected and quantified in our samples. P‐gp was found to be the most abundant transporter, whereas MATE2‐K was the least abundant (Table S5 ).
Figure 3.
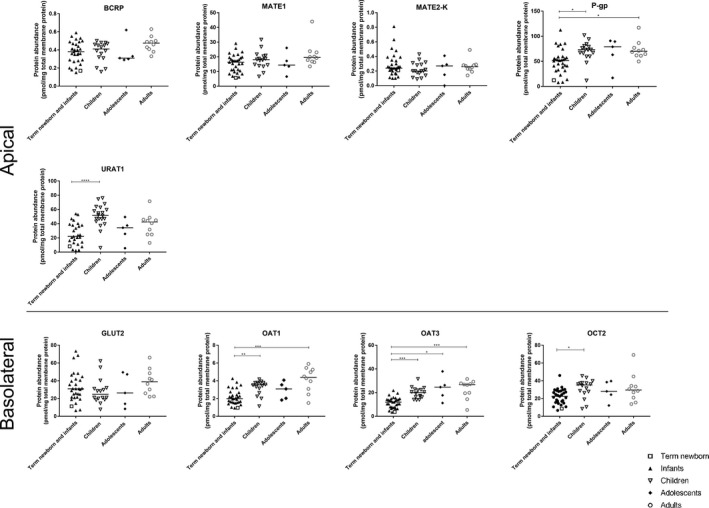
Protein abundance levels of nine renal membrane transporters in different age groups. The bar represents the median for each age group. Term newborn and infants were combined here for analysis because there was only one term newborn included for this part of the study. *P < 0.05, **P < 0.01, ***P < 0.001. GLUT, glucose transporter; MATE, multidrug and toxin extrusion protein; MDR, multidrug resistance protein; MRP, multidrug resistance‐associated protein; OAT, organic anion transporter; OCT, organic cation transporter; PXR, pregnane X receptor; URAT, urate transporter.
P‐gp, URAT1, OAT1, OAT3, and OCT2 protein abundance levels were significantly lower in term newborn and infants than in the older age groups (Figure 3 ). Sigmoidal maximum effect (Emax) models were used to fit the protein abundance levels of these five transporters, and all but URAT1 expression data conformed to the model (Figure 4 a). OCT2 and P‐gp expressions increased at a faster rate than OAT1 and OAT3, as evidenced by the younger age at which half of the adult expression was reached (TM50). Moreover, the transporters OCT2 and P‐gp shared a similar maturation pattern, as well as the transporters OAT1 and OAT3 (Figure 4 b). No difference in protein abundance levels was found among age groups for BCRP, MATE1, MATE2‐K, and GLUT2.
Figure 4.
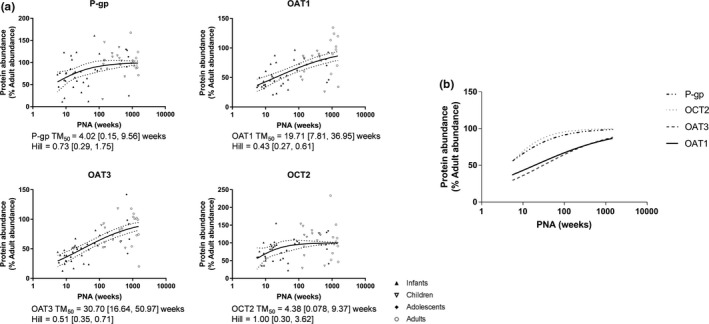
Ontogeny of protein abundance of P‐gp, OAT1, OAT3, and OCT2 as described by sigmoidal maximum effect (Emax) model. Individual sigmoidal curves are presented in (a) (solid lines). Dashed lines represent the 95% confidence bands. Superimposing the sigmoidal curves as presented in (b) showed that the pair transporters, P‐gp/OCT2 and OAT1/OAT3, shared similar maturation patterns. OAT, organic anion transporter; OCT, organic cation transporter; P‐gp, p‐glycoprotein; PNA, postnatal age; TM50, half of the adult expression was reached.
Correlation between mRNA expression and protein abundance levels
Potential correlation between mRNA expression and protein abundance levels of the transporters was investigated (Figure S1 ). Significant correlation was found for MATE1, P‐gp, URAT1, OAT3, and OCT2.
Intertransporter correlation
To assess the potential shared expression regulation, we studied the correlation of mRNA expression and protein abundance levels between transporters (Table 2 ). Levels of OAT1 and OAT3 were the most significantly correlated.
Table Table 2.
Intertransporter Spearman correlations
| Apical | Basolateral | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BCRP | MATE1 | MATE2‐K | MDR1 | MRP2 | MRP4 | URAT1 | GLUT2 | OAT1 | OAT3 | OCT2 | |
| Apical | |||||||||||
| BCRP | − | 0.69 | 0.44 | NA | NA | 0.59 | 0.43 | ||||
| MATE1 | − | 0.63 |
0.36 0.57 |
0.74 |
0.50 0.57 |
0.44 |
0.53 0.61 |
0.54 0.54 |
0.62 0.57 |
||
| MATE2‐K | − | 0.39 | 0.57 | 0.51 | 0.47 | 0.59 | 0.56 | 0.49 | |||
| MDR1 | − | 0.47 |
0.32 0.56 |
0.37 |
0.52 0.72 |
0.49 0.60 |
0.57 0.67 |
||||
| MRP2 | − | NA | NA | 0.79 | 0.83 | 0.67 | |||||
| MRP4 | − | NA | NA | ||||||||
| URAT1 | − | 0.42 |
0.54 0.49 |
0.50 0.49 |
0.46 0.52 |
||||||
| Basolateral | |||||||||||
| GLUT2 | − | 0.64 | 0.64 | 0.49 | |||||||
| OAT1 | − |
0.85 0.83 |
0.74 0.70 |
||||||||
| OAT3 | − |
0.73 0.72 |
|||||||||
| OCT2 | − | ||||||||||
All figures in italics are the mRNA expressions.
All figures in boldface are protein expressions.
All reached P < 0.0001. Data not presented if P > 0.001.
GLUT, glucose transporter; MATE, multidrug and toxin extrusion protein; MDR, multidrug resistance protein; MRP, multidrug resistance‐associated protein; NA, not available; OAT, organic anion transporter; OCT, organic cation transporter; PXR, pregnane X receptor; URAT, urate transporter.
Correlation between PXR and transporter mRNA expression
Weak negative correlations with PXR were found for MATE1 (r s = −0.27; P = 0.035), MRP2 (r s = −0.29; P = 0.021), and OCT2 (r s = −0.26; P = 0.043), whereas no correlation was found for MATE2‐K, P‐gp, MRP4, OAT1, and OAT3.
Localization of MRP4 in pediatric kidney tissue
As a proof‐of‐concept, postmortem kidney tissues of 43 pediatric patients (GA > 24 weeks, PNA 2 days to 14 years old) and 1 adult were analyzed. Positive MRP4 immunostaining was detected as early as 27 weeks of gestation (PNA 9 days), despite negative staining found in three tissues from one child and two adolescents. For all the positive stained samples, MRP4 was found to be located at the apical side of the proximal tubule (Figure 5 a,b). See Figure 5 c for the negative control. Although the examples showed lower staining at 3.3 weeks (Figure 5 a) than 3.1 years old (Figure 5 b), no statistically significant age‐related changes were detected in the semiquantification of the staining in the whole sample set (Figure 5 d).
Figure 5.
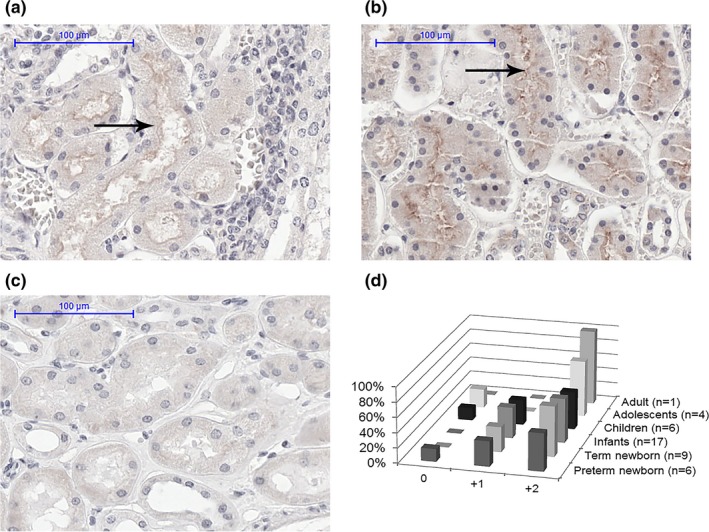
Apical proximal tubule localization of multidrug resistance‐associated protein (MRP4) (arrow) by immunohistochemically staining in postmortem tissue of samples with (a) gestational age (GA) of 27.7 weeks; postnatal age (PNA) age 3.3 weeks, and (b) GA of 40.0 weeks; PNA 3.1 years (c) represents the negative control, and (d) the semiquantification of MRP4 staining in various age groups: negative (0), low staining (+1), or high staining (+2).
Discussion
This study, to our best knowledge, is the first to comprehensively describe the ontogeny of human renal membrane transporters via mRNA expression analysis and quantitative proteomics in tissues representing a large span of ages. Albeit data on developmental changes in transporter mRNA expression in animals were reported previously,15, 16, 17, 18, 19 cross‐species differences limit extrapolation, especially concerning the rates of maturation.20
Our study revealed two major findings with respect to the developmental maturation of renal transporters: (i) the expression of most of the transporters characterized in this study increased with age during the earliest developmental periods (<2 years old), and (ii) maturation pattern was transporter‐dependent. Additionally, we observed that: (i) there were maturational differences between mRNA expression and protein abundance, (ii) there were correlations between the expression levels of various transporters, (iii) PXR seems to play a minimal role in regulating mRNA expression of transporters in the kidney, and (iv) stable MRP4 mRNA expression was accompanied by proper apical localization during development.
Transporter‐dependent maturation patterns during the earliest developmental periods
The findings that most of the studied transporters showed a transporter‐dependent age‐related increase in their expression levels, especially during the earliest years of life, were expected. Renal membrane transporters play critical roles in elimination and detoxification pathways in the body. They work in concert with enzymes in the kidneys, as well as enzymes and transporters in other organs, such as the intestine and liver, to mediate the removal of ingested potential harmful compounds, such as toxins derived from food, environmental toxins, drugs, and their metabolites.7 During infancy, dietary exposure to potential toxins is limited and begins to increase as infants are switched from an exclusively milk diet to foods that may contain more toxins.21, 22 Thus, detoxification pathways are increasingly needed as the diet of infants expands and diversifies into childhood.
Furthermore, besides changes in dietary intake and nutritional requirement, ontogeny of renal transporters can alter the disposition of endogenous compounds, suggesting important developmental roles for these renal transporters. Both BCRP and URAT1 are thought to play a clear role in uric acid (UA) homeostasis.23, 24 It was previously reported that the fractional excretion of UA (FEUA; the % of filtered UA not reabsorbed by the tubules), was 30–40% in term newborns < 5 days old, which then decreased to 8–10% in children of 3 years old.25, 26, 27 Our transporter maturation data, in addition to age‐related physiological changes (e.g., urinary acidification and concentration ability), may explain this observation: the decreasing BCRP mRNA expression from birth is accompanied by an increased expression of URAT1, a reabsorptive transporter, from birth until childhood, resulting in a net decrease in UA excretion.25, 27 Interestingly, sex‐related differences in FEUA especially during adolescence were reported, which could be due to differences in proximal tubular secretion of UA.27 Yet, no significant sex‐related differences were found in our study consisting mainly of pediatric samples. As the influence of sex seems to be transporter‐specific in adults,28 follow‐up studies with more samples and also with other transporters that handle UA, such as GLUT9, would be needed to fully understand the changes in FEUA.
In vivo pharmacokinetic data of drugs that are transporter substrates may be used to support our expression data. However, renal elimination of these drugs is accomplished not only by active tubular secretion facilitated by various transporters but also by glomerular filtration, which is also subjected to age‐dependent changes. As children grow and develop, the glomerular filtration rate (GFR) matures and is predicted to reach 50% of adult values by 2 months PNA and 90% of adult values by 1 year of age.29 Although the full complement of nephrons in each kidney is complete around GA 36 weeks,30 GFR continues to develop as a result of increases in kidney blood flow, improvements in filtration coefficients, and maturation of the tubules. Our findings that there are age‐related changes in transporter expression early in life (<2 years) support the notion that active tubular secretion matures in parallel with GFR maturation. Thus, observed age‐related changes in pharmacokinetics of transporter substrates are likely due to a combination of both maturation in transporter expression and GFR. For instance, after hepatic metabolism, the antiviral drug valacyclovir undergoes renal elimination via glomerular filtration and active tubular secretion likely by OAT1/3.31 Apparent clearance of valacyclovir in infants < 3 months old is 50% lower than that in young children.31 The GFR in infants < 3 months old is expected to be >50%,29 and, therefore, this discrepancy may be explained by our findings that the TM50 of OAT1 and OAT3 was ~ 4 and 8 months (Figure 4 a). Famotidine, an OAT3 substrate used in the treatment of gastritis, provides another example of maturational changes in both GFR and secretory transporters.32 Given the complex developmental changes in renal elimination processes, our data could be integrated into physiologically‐based pharmacokinetic models to improve prediction of pediatric drug clearance. When doing so, scaling factors should be used to correct for membrane protein yield and total organ weight.
Other observations from data analysis
The abundance pattern of the transporters in our adult samples was assessed. P‐gp has the highest abundance, followed by URAT1, GLUT2, OCT2, OAT3, MATE1, OAT1, BCRP, and MATE2‐K. This abundance pattern is different from that reported in Prasad et al.,33 where OCT2 was the most abundant transporter, followed by OAT1, MATE1, OAT3, and P‐gp. This discrepancy could be due to actual intersample variation in expression levels of the transporters, or to differences in the inclusion criteria and quality of the tissue samples used in the studies. In addition, our study also showed much higher absolute abundance for most proteins in our adult samples compared with other studies.34 As Li et al.35 suggested, such interlaboratory difference may, in part, be explained by different instrumental performance and varying tissue handling techniques.
Differences in the patterns and rates of change among mRNA expression and protein abundance levels of various transporters were noticed. For example, age‐related changes were found in BCRP, GLUT2, and MATE2‐K mRNA levels but not in protein abundance levels. This may suggest maturational differences in the regulation of gene transcription and post‐translational processing. For gene transcription, alternative splicing is suggested to occur due to developmental signals.36 Some of the alternatively spliced mRNA transcripts may not be translated into the protein of interest but will be quantified by quantitative real‐time polymerase chain reaction as the total mRNA expression could be derived from a mixture of different transcripts of the targeted gene.37 Quantitative proteomic overcomes this challenge by measuring the actual expression of the protein of interest. This process could explain the lack of correlation between mRNA and protein expression.
Our data showed that transporter expression is correlated among various transporters. The strong correlation between expression of OAT1 and OAT3 is not surprising as they are located in adjacent regions on chromosome 11.38 Moreover, they are both regulated by the transcription factors hepatocyte nuclear factor 1α and 1β, which increase their transcription.39 Our study of transcription factors was confined to PXR, and in agreement with previously reported findings; PXR mRNA levels in kidneys were low in all age groups compared with the mRNA levels of the studied transporters.28, 40, 41 Thus, PXR seems to have a minor role in regulating transporter gene expression in the kidneys than in other organs. This is supported by findings in mice, where the potent rodent PXR activator pregnenolone‐16α‐carbonitrile induced transporter expression in the liver and intestine, but not in the kidneys.40 More research is needed to identify the developmental triggers by which transcription of transporters increase and decrease. Moreover, the relationship between transcription factors maintaining basal expression level, like the hepatocyte nuclear factor family, and renal transporter expression should be studied.
Detoxification involves the interplay between enzymes and transporters that are ubiquitously expressed in tissues throughout the human body. P‐gp expression levels in the liver only reached 50% of adult expression levels at 2.9 years of age,9 whereas our results suggested that full adult levels were achieved in the kidneys by that age. For BCRP, our results for mRNA expression in the kidneys were consistent with that in the liver, which showed a decline from newborns to adults.42 As more transporter ontogenetic data in different organs become available, more reliable prediction in transporter‐mediated substrate disposition on the whole‐body level during development will be achieved.
Potential limitations
Certain limitations are present in this study. In addition to age, there are other potential factors, such as the use of comedications and inflammation that can influence transporter expression and thereby contribute to the expression variability.43 The impact of acute and chronic inflammation on transporter expression and activity is related to the activity of multiple proinflammatory cytokines.44 The exact mechanism remains unknown and may be related to various nuclear receptors and transcription factors. Similarly, certain medications and environmental toxins could lead to activation of nuclear receptor pathway, and could, therefore, influence the transporter expression.44, 45 The underlying reason for death of our tissue donors is heterogeneous and so are the exposure of drugs and environmental toxins. Yet, despite all these inevitable differences, significant changes in the expression levels by age were still observed. However, due to the lack of detailed clinical data available for our samples, these factors could not be explored. Although protein and mRNA levels in the postmortem samples used in our study were excellent, the amount of degradation in these levels from death to freezing is not known. Degradation may vary among samples, and may result in reduced absolute levels and increased variability in expression level measurements. Moreover, with the exception of PXR, we did not study the ontogeny of other transcription factors and proteins involved in gene and protein regulation; therefore, the mechanisms underlying the ontogeny of transporters observed in this study are not known. Finally, mRNA expression, protein abundance and transporter activity ex vivo and in vivo studies are needed to confirm the implications of our results to drug disposition in the kidneys.
Conclusions
These results showed that the ontogeny of certain renal membrane transporters displayed an age‐dependent pattern, suggesting that the clearance of exogenous and endogenous substrates for these kidney transporters are subject to transporter‐specific age‐related changes. Although future work is clearly needed in refining predictive models for pediatric drug disposition, leveraging our expression data in modeling and simulation strategies may improve predictability of pediatric drug disposition and exposure models. Importantly, our findings set the stage for future research in understanding the mechanisms of developmental changes in renal drug transporters.
Methods
Tissue procurement and sample characteristics
Two sample sets were analyzed, and the demographic information of donors is reported in Table 1 . Age groups were predefined based on the International Council for Harmonisation guidelines: preterm newborns (PNA 0–28 days; GA < 37 weeks), newborns (PNA 0–28 days), infants (1–24 months old), children (2–12 years old), adolescents (12–16 years old), and adults (>16 years old).4 Sample set 1 consisted of postmortem autopsy kidney samples and surgical adult kidney samples from the Erasmus MC Tissue Bank, Rotterdam, The Netherlands. Sample set 2 consisted of 122 human postmortem frozen renal cortical tissues (donors aged 1 day to 30 years old), which were obtained from National Institutes of Health NeuroBioBank at the University of Maryland, Baltimore, MD. Tissues, which were selected for having no renal abnormalities in pathology and primary diagnosis, were procured at the time of autopsy within 48 hours after death and were stored at −196°C (sample set 1) and −80°C (sample set 2) for later use. The quantitative proteomic analysis was done completely in the United States on the subset of samples from sample set 2 and the immunohistochemistry was performed entirely in the Netherlands on sample set 1. Gene expression analysis was conducted in both laboratories, and the data from the two sources were first analyzed separately, followed by a combined analysis. Combined analysis was deemed appropriate as no significant differences were observed between the expression levels of six transporter genes (MATE1, MATE2, P‐gp, OAT1, OAT3, and OCT2) in adult samples obtained in the United States and in the Netherlands. Furthermore, developmental patterns in expression of the transporters in the two sample sets showed comparable results.
mRNA expression
Figure 1 illustrates the sample analysis scheme. For sample set 1, the protocol on quantitative real‐time polymerase chain reaction is described in Material S1 and Table S6 . For sample set 2, the protocol described in Chen et al.46 was followed with slight modifications (Material S1 ).
Quantitative proteomics using liquid chromatography tandem mass spectrometry with selective reaction monitoring
Quantitative proteomics was only performed in sample set 2 (Figure 1 ). Unless otherwise stated, reagents from MyOmicsDx (Towson, MD) were used. Details of the liquid chromatography and mass spectrometry method and parameters are described in the supplemental documents (Material S2 ). Briefly, membrane proteins were extracted from the renal cortical tissues using MyPro‐MembraneEx buffer. The total extracted membrane protein concentration was determined using the Bicinchoninic acid assay protein assay kit. The membrane protein samples were then processed by MyOmicsDx using the filter‐aided sample preparation method.47
Five peptides were chosen for each transporter as selective reaction monitoring (SRM) quantifying targets and six best transitions per peptide precursors were selected for SRM quantification (Table S7 ). Peptide samples that were previously reconstituted in MyPro‐Buffer 3 were spiked with MyPro‐SRM Internal Control Mixture and were subjected to SRM analysis. The peptide samples were eluted through an online Agilent 1290 HPLC system into the Jet Stream ESI source of an Agilent 6495 Triple Quadrupole Mass Spectrometer (Agilent, Santa Clara, CA).
Quantitative data were imported into Skyline 3.1.48 The abundance of a target peptide was represented by the area under the curve (AUC) of all its transitions normalized to the total AUC of all transitions from the most nearby (sharing a similar hydrophobicity) heavy isotope‐labeled peptide from MyPro‐SRM Internal Control Mixture spiked in before the SRM analysis. Absolute quantification of each protein is performed through applying AQUA Peptides (Sigma‐Aldrich, St. Louis, MO).
Immunohistochemistry
Localization of MRP4 was explored in a representative subpopulation of sample set 1. Immunohistochemistry was performed using an immunoperoxidase staining method for amplified antigen detection. Sections of 4‐μm thick cortex were gained from formalin‐fixed, paraffin‐embedded, postmortem kidney tissue blocks and were mounted on glass slides. They were heated at 60°C for 30 minutes, deparaffinized in xylene, and rehydrated with a series of graded ethanol. Enhanced antigen retrieval was performed by treating slides in TRIS‐EDTA (10 mM Tris base; 1 mM EDTA solution; 0.05% Tween 20; and pH 9.0) for 15 minutes at 98°C. Endogenous peroxidase activity was quenched by incubating slides in 3% H2O2 for 30 minutes at room temperature. The sections were blocked with Avidin/Biotin blocking solution (Vector Laboratories, Burlingame, CA) for 15 minutes each.
Primary antibodies rat anti‐MRP4 (ab15598 Abcam, Cambridge, UK) at dilution of 1:20 were incubated over night at 4°C in 1% bovine serum albumin. A biotinylated secondary rabbit anti‐rat serum (R1371B, Acris Antibodies GmbH, Herford, DE) at dilution of 1:1,000 was then applied for 30 minutes. Immunoreactive sides were detected using the ABC kit (Vector Laboratories) for 30 minutes and 3,3 diaminobenzidine tetrahydrochloride (Sigma‐Aldrich) solution staining for 15 minutes. The nuclei were counterstained with Mayers Hematoxylin Solution (Sigma‐Aldrich). One negative‐control staining lacking the primary antibody was performed for every age group.
Data analysis and statistics
Data were expressed as median (range). Kruskal–Wallis tests with Dunn's post hoc test were used for multiple comparisons of expression levels between age groups, using the P values adjusted for multiple testing. If no difference in expression was found between age groups, the ontogeny would be referred to as “stable.” Sigmoidal Emax models are used often for maturational processes as it allows gradual maturation of clearance in early life and a “mature” clearance to be achieved at a later age.49 Therefore, Emax models were used to fit the protein abundance data on a continuous scale of age for those transporters that showed between‐group differences. The data point from the term newborn in this set of data was excluded prior to fitting to eliminate bias, as it was the only sample quantified for that age group. We first set the median of adult data to be 100% and then normalized the data points from pediatric samples toward the median of adult data. Potential outliers were assessed and excluded using the Robust Regression followed by Outlier Identification method during the model fit process.50 The age at which TM50 was reached was determined from the Emax model. Visual inspection and 95% confidence interval of the Emax parameter estimates were used to assess the goodness‐of‐fit of the sigmoidal model. Spearman's correlation analysis was used to evaluate the relationship between mRNA and protein abundances within the same and among other transporters.
For the analysis of staining intensity after immunohistochemistry, a semiquantitative scoring system was used, graded by two observers (B.D.vG. and M.D.vB.) who independently confirmed cell staining intensity as negative (0), low staining (+1), or high staining (+2). Simultaneously, the localization of MRP4 in the kidney tissue was determined for each sample by the same observers.
Statistical analysis was performed using IBM SPSS Statistics software version 21.0 (Armonk, NY), and a significance level of P < 0.05 was used throughout the study. Graphical exploration was performed using GraphPad Prism software version 5.00 (La Jolla, CA).
Funding
This work was supported, in part, by the National Institute of Health grants R01GM117163 and R01DK103729 (to K.M.G), by the US Food and Drug Administration (FDA) through Medical Countermeasures Initiative, grant U01FD004979, which supports the UCSF‐Stanford Center of Excellence in Regulatory Science and Innovation, and by the National Center for Advancing Translational Sciences, National Institute of Health, through UCSF‐CTSI Grant UL1TR001872.
Dr Kit Wun Kathy Cheung was supported, in part, by the appointments to the Research Participation Program at the Center for Drug Evaluation and Research, administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the US Department of Energy and the FDA.
Conflict of Interest
The authors declared no competing interests for this work. As an Associate Editor for Clinical Pharmacology & Therapeutics, Shiew‐Mei Huang was not involved in the review or decision process for this paper.
Author Contributions
K.W.K.C., B.D.vG., E.S., M.D.vB., D.T., J.N.S., R.M.V., B.S., L.Z., S.‐M.H., K.M.G., and S.N.dW. wrote the manuscript. K.W.K.C., B.D.vG., E.S., M.D.vB., Y.S‐O., D.T., J.N.S., R.M.V., B.S., L.Z., S.‐M.H., K.M.G., and S.N.dW. designed the research. K.W.K.C., B.D.vG., E.S., M.D.vB., A.C.J.M.dB., Y.S.‐O., and R.M.V. performed research. K.W.K.C., B.D.vG., E.S., M.D.vB., L.Z., S.‐M.H., K.M.G., and S.N.dW. analyzed the data.
Disclaimer
Views expressed in this paper are those of authors and do not necessarily reflect the official views or policies of the FDA; nor does any mention of trade names, commercial practices, or organization imply endorsement by the US Government.
Supporting information
Figure S1. Correlations between mRNA expressions and protein expressions (Spearman's correlation coefficient, rs).
Table S1. Mann–Whitney U‐test to examine the differences in mRNA and protein expression between girls and boys.
Table S2. Kruskal–Wallis followed by multiple comparisons to examine the differences in mRNA expression between African American and white subjects in different age groups.
Table S3. Mann–Whitney U‐test to examine the differences in protein expression levels between African American adults and white adults.
Table S4. Relative mRNA expression of 11 transporters and PXR in different age groups.
Table S5. Absolute abundance of nine selected renal membrane transporters.
Table S6. Primer sequences used for sample set 1.
Table S7. Surrogate peptides for each of the renal membrane transporters studied and their corresponding MS/MS parameters.
Material S1. RT‐qPCR protocol sample set 1 and sample set 2.
Material S2. Detail description of LC‐MS sample preparation and method parameters for quantitative proteomics.
Acknowledgments
The authors would like to thank Jeanne Pertijs for excellent help with immunohistochemistry, and Dr Gilbert Burckart, Dr Sook Wah Yee, Dr Huan‐Chieh Chien, Dr John Witte, and Qi Luo for valuable and insightful discussions. Human tissue was, in part, obtained from the National Institutes of Health NeuroBioBank at the University of Maryland, Baltimore, MD, and from the Erasmus MC Tissue Bank, Rotterdam, The Netherlands. Part of this work was previously presented at the Annual Meeting of the American Society for Clinical Pharmacology & Therapeutics (ASCPT) held March 22–24, 2018, in Orlando, FL.
References
- 1. Morrissey, K.M. , Stocker, S.L. , Wittwer, M.B. , Xu, L. & Giacomini, K.M. Renal transporters in drug development. Annu. Rev. Pharmacol. Toxicol. 53, 503–529 (2013). [DOI] [PubMed] [Google Scholar]
- 2. Motohashi, H. et al Precise comparison of protein localization among OCT, OAT, and MATE in human kidney. J. Pharm. Sci. 102, 3302–3308 (2013). [DOI] [PubMed] [Google Scholar]
- 3. US Food and Drug Administration . In vitro metabolism‐ and transporter‐mediated drug‐drug interaction studies < https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm064982.htm>. Accessed September 24, 2018.
- 4. International Council for Harmonisation . Guidance for Industry. E11 Clinical investigation of medicinal products in the pediatric population. (2000). [PubMed]
- 5. European Medicines Agency . Guideline on the investigation of drug interactions. Committee for Human Medicinal Products <http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/07/WC500129606.pdf>. Accessed April 3, 2018.
- 6. Pharmaceuticals Medical Devices Agency . Guideline on drug‐drug interactions <http://www.pmda.go.jp/files/000225191.pdf> (2018). Accessed September 24, 2018.
- 7. Nigam, S.K. What do drug transporters really do? Nat. Rev. Drug Discov. 14, 29–44 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Knibbe, C.A. et al Morphine glucuronidation in preterm neonates, infants and children younger than 3 years. Clin. Pharmacokinet. 48, 371–385 (2009). [DOI] [PubMed] [Google Scholar]
- 9. Prasad, B. et al Ontogeny of hepatic drug transporters as quantified by LC‐MS/MS proteomics. Clin. Pharmacol. Ther. 100, 362–370 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lu, H. & Rosenbaum, S. Developmental pharmacokinetics in pediatric populations. J. Pediatr. Pharmacol. Ther. 19, 262–276 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Giacomini, K.M. et al Membrane transporters in drug development. Nat. Rev. Drug Discov. 9, 215–236 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brouwer, K.L. et al Human ontogeny of drug transporters: review and recommendations of the pediatric transporter working group. Clin. Pharmacol. Ther. 98, 266–287 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang, B. , Xie, W. & Krasowski, M.D. PXR: a xenobiotic receptor of diverse function implicated in pharmacogenetics. Pharmacogenomics 9, 1695–1709 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ritter, C.A. , Jedlitschky, G. , Meyer zu Schwabedissen, H. , Grube, M. , Kock, K. & Kroemer, H.K. Cellular export of drugs and signaling molecules by the ATP‐binding cassette transporters MRP4 (ABCC4) and MRP5 (ABCC5). Drug Metab. Rev. 37, 253–278 (2005). [DOI] [PubMed] [Google Scholar]
- 15. Sweeney, D.E. , Vallon, V. , Rieg, T. , Wu, W. , Gallegos, T.F. & Nigam, S.K. Functional maturation of drug transporters in the developing, neonatal, and postnatal kidney. Mol. Pharmacol. 80, 147–154 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pinto, N. , Halachmi, N. , Verjee, Z. , Woodland, C. , Klein, J. & Koren, G. Ontogeny of renal P‐glycoprotein expression in mice: correlation with digoxin renal clearance. Pediatr. Res. 58, 1284–1289 (2005). [DOI] [PubMed] [Google Scholar]
- 17. Maher, J.M. , Slitt, A.L. , Cherrington, N.J. , Cheng, X. & Klaassen, C.D. Tissue distribution and hepatic and renal ontogeny of the multidrug resistance‐associated protein (MRP) family in mice. Drug Metab. Dispos. 33, 947–955 (2005). [DOI] [PubMed] [Google Scholar]
- 18. Nakajima, N. et al Developmental changes in multispecific organic anion transporter 1 expression in the rat kidney. Kidney Int. 57, 1608–1616 (2000). [DOI] [PubMed] [Google Scholar]
- 19. Slitt, A.L. , Cherrington, N.J. , Hartley, D.P. , Leazer, T.M. & Klaassen, C.D. Tissue distribution and renal developmental changes in rat organic cation transporter mRNA levels. Drug Metab. Dispos. 30, 212–219 (2002). [DOI] [PubMed] [Google Scholar]
- 20. Chu, X. , Bleasby, K. & Evers, R. Species differences in drug transporters and implications for translating preclinical findings to humans. Expert Opin. Drug Metab. Toxicol. 9, 237–252 (2013). [DOI] [PubMed] [Google Scholar]
- 21. Nicklaus, S. The role of dietary experience in the development of eating behavior during the first years of life. Ann. Nutr. Metab. 70, 241–245 (2017). [DOI] [PubMed] [Google Scholar]
- 22. Bearer, C.F. Environmental health hazards: how children are different from adults. Future Child. 5, 11–26 (1995). [PubMed] [Google Scholar]
- 23. Brackman, D.J. & Giacomini, K.M. Reverse translational research of ABCG2 (BCRP) in human disease and drug response. Clin. Pharmacol. Ther. 103, 233–242 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xu, L. , Shi, Y. , Zhuang, S. & Liu, N. Recent advances on uric acid transporters. Oncotarget 8, 100852–100862 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Passwell, J.H. , Modan, M. , Brish, M. , Orda, S. & Boichis, H. Fractional excretion of uric acid in infancy and childhood. Index of tubular maturation. Arch. Dis. Child. 49, 878–882 (1974). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Baldree, L.A. & Stapleton, F.B. Uric acid metabolism in children. Pediatr. Clin. North Am. 37, 391–418 (1990). [DOI] [PubMed] [Google Scholar]
- 27. Stiburkova, B. & Bleyer, A.J. Changes in serum urate and urate excretion with age. Adv. Chronic Kidney Dis. 19, 372–376 (2012). [DOI] [PubMed] [Google Scholar]
- 28. Benson, E.A. et al Rifampin regulation of drug transporters gene expression and the association of microRNAs in human hepatocytes. Front. Pharmacol. 7, 111 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rhodin, M.M. et al Human renal function maturation: a quantitative description using weight and postmenstrual age. Pediatr. Nephrol. 24, 67–76 (2009). [DOI] [PubMed] [Google Scholar]
- 30. Faa, G. et al Morphogenesis and molecular mechanisms involved in human kidney development. J. Cell. Physiol. 227, 1257–1268 (2012). [DOI] [PubMed] [Google Scholar]
- 31. Mooij, M.G. et al Development of human membrane transporters: drug disposition and pharmacogenetics. Clin. Pharmacokinet. 55, 507–524 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Motohashi, H. , Uwai, Y. , Hiramoto, K. , Okuda, M. & Inui, K. Different transport properties between famotidine and cimetidine by human renal organic ion transporters (SLC22A). Eur. J. Pharmacol. 503, 25–30 (2004). [DOI] [PubMed] [Google Scholar]
- 33. Prasad, B. et al Abundance of drug transporters in the human kidney cortex as quantified by quantitative targeted proteomics. Drug Metab. Dispos. 44, 1920–1924 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fallon, J.K. , Smith, P.C. , Xia, C.Q. & Kim, M.S. Quantification of four efflux drug transporters in liver and kidney across species using targeted quantitative proteomics by isotope dilution NanoLC‐MS/MS. Pharm. Res. 33, 2280–2288 (2016). [DOI] [PubMed] [Google Scholar]
- 35. Li, H. , Han, J. , Pan, J. , Liu, T. , Parker, C.E. & Borchers, C.H. Current trends in quantitative proteomics – an update. J. Mass Spectrom. 52, 319–341 (2017). [DOI] [PubMed] [Google Scholar]
- 36. Baralle, F.E. & Giudice, J. Alternative splicing as a regulator of development and tissue identity. Nat. Rev. Mol. Cell Biol. 18, 437–451 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang, M. et al Quantification of gene expression while taking into account RNA alternative splicing. Genomics (2018). 10.1016/j.ygeno.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 38. Kent, W.J. et al The human genome browser at UCSC. Genome Res. 12, 996–1006 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang, L. & Sweet, D.H. Renal organic anion transporters (SLC22 family): expression, regulation, roles in toxicity, and impact on injury and disease. AAPS J. 15, 53–69 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cheng, X. & Klaassen, C.D. Regulation of mRNA expression of xenobiotic transporters by the pregnane x receptor in mouse liver, kidney, and intestine. Drug Metab. Dispos. 34, 1863–1867 (2006). [DOI] [PubMed] [Google Scholar]
- 41. Miki, Y. , Suzuki, T. , Tazawa, C. , Blumberg, B. & Sasano, H. Steroid and xenobiotic receptor (SXR), cytochrome P450 3A4 and multidrug resistance gene 1 in human adult and fetal tissues. Mol. Cell. Endocrinol. 231, 75–85 (2005). [DOI] [PubMed] [Google Scholar]
- 42. Mooij, M.G. et al Proteomic analysis of the developmental trajectory of human hepatic membrane transporter proteins in the first three months of life. Drug Metab. Dispos. 44, 1005–1013 (2016). [DOI] [PubMed] [Google Scholar]
- 43. Vet, N.J. et al Inflammation and organ failure severely affect midazolam clearance in critically ill children. Am. J. Respir. Crit. Care Med. 194, 58–66 (2016). [DOI] [PubMed] [Google Scholar]
- 44. Evers, R. et al Disease‐associated changes in drug transporters may impact the pharmacokinetics and/or toxicity of drugs: a white paper from the International Transporter Consortium. Clin. Pharmacol. Ther. 104, 900–915 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Prakash, C. et al Nuclear receptors in drug metabolism, drug response and drug interactions. Nucl. Receptor Res. 2, 101178 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chen, E.C. et al Targeted disruption of organic cation transporter 3 attenuates the pharmacologic response to metformin. Mol. Pharmacol. 88, 75–83 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wisniewski, J.R. , Zougman, A. , Nagaraj, N. & Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 6, 359–362 (2009). [DOI] [PubMed] [Google Scholar]
- 48. MacLean, B. et al Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics 26, 966–968 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Anderson, B.J. & Holford, N.H. Mechanism‐based concepts of size and maturity in pharmacokinetics. Annu. Rev. Pharmacol. Toxicol. 48, 303–332 (2008). [DOI] [PubMed] [Google Scholar]
- 50. Motulsky, H.J. & Brown, R.E. Detecting outliers when fitting data with nonlinear regression ‐ a new method based on robust nonlinear regression and the false discovery rate. BMC Bioinformatics 7, 123 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Correlations between mRNA expressions and protein expressions (Spearman's correlation coefficient, rs).
Table S1. Mann–Whitney U‐test to examine the differences in mRNA and protein expression between girls and boys.
Table S2. Kruskal–Wallis followed by multiple comparisons to examine the differences in mRNA expression between African American and white subjects in different age groups.
Table S3. Mann–Whitney U‐test to examine the differences in protein expression levels between African American adults and white adults.
Table S4. Relative mRNA expression of 11 transporters and PXR in different age groups.
Table S5. Absolute abundance of nine selected renal membrane transporters.
Table S6. Primer sequences used for sample set 1.
Table S7. Surrogate peptides for each of the renal membrane transporters studied and their corresponding MS/MS parameters.
Material S1. RT‐qPCR protocol sample set 1 and sample set 2.
Material S2. Detail description of LC‐MS sample preparation and method parameters for quantitative proteomics.


