Abstract
SAM pointed domain-containing Ets transcription factor (SPDEF), a member of the ETS transcription factor family, has been associated with prostate cancer development; however, its role in tumour development and progression is controversial. In the present study, SPDEF expression was analysed on a tissue microarray with >12,000 prostate cancer samples. SPDEF expression levels were higher in most prostate cancer samples than in normal prostate epithelium, suggesting SPDEF was upregulated in cancer. Nuclear SPDEF expression was identified in 80% of prostate cancer samples, and considered weak in 26.4%, moderate in 40.1% and strong in 13.5% of cases. SPDEF positivity was significantly associated with tumour stage, Gleason grade, lymph node metastasis and PSA recurrence (all P<0.0001). SPDEF overexpression was more common in ERG positive (94%) than in ERG negative cancer (69%; P<0.0001). Elevated SPDEF expression predicted poor prognosis independent from established prognostic parameters, including Gleason grade, pT, pN, serum PSA level and nodal status (P<0.01). In summary, SPDEF overexpression was associated with aggressive behaviour, particularly in ERG negative prostate cancer, and may have potential for clinical application.
Keywords: SAM pointed domain-containing Ets transcription factor, ETS-related gene, deletion, prostate cancer, tissue microarray
Introduction
Prostate cancer is the most prevalent cancer in men in Western societies (1), but only a small subset is highly aggressive and needs extensive treatment (2,3). Predictive preoperative prognostic parameters are limited to Gleason score and tumour extent on biopsies, prostate-specific antigen (PSA) serum level and clinical stage. Thus it is hoped that additional biomarkers can be identified which will improve the prediction of an aggressive tumour course.
The E26 transformation-specific (ETS) family of transcription factors has been named after its evolutionarily conserved DNA-binding domain (4). ETS factors play important roles in many human tumour types. In prostate cancer, the ETS family member ERG is fused to the TMPRSS2 serine protease in approximately 50% of cases (5–7). Another ETS factor with relevance in prostate cancer is SAM pointed domain-containing Ets transcription factor (SPDEF). SPDEF is physiologically expressed in normal tissues of the prostate (8), breast (9), ovary (10), lung (10), brain (10) and gastrointestinal tract (11). Studies on breast (9,12–14), prostate (13,15), ovarian (16) and colon cancers (17) have described frequent dysregulation of SPDEF with conflicting results. In prostate cancer some authors see SPDEF as an oncogenic driver (8,13) while others claim a tumour metastasis suppressor role for SPDEF (15,18–22).
To better understand the role of SPDEF in prostate cancer, we took advantage of our large prostate cancer tissue microarray (TMA) to study expression of SPDEF with the monoclonal antibody MAB9916 clone 4A5 by immunohistochemistry in more than 12,000 prostate cancer samples, and then compared SPDEF expression with relevant clinical and pathological parameters in a patient cohort which was castration-sensitive or naïve to androgenic deprivation therapy.
Materials and methods
Patients
Radical prostatectomy specimens were available from 12,427 patients, undergoing surgery between 1992 and 2012 at the Department of Urology and the Prostate Cancer Center Martini Clinic at the University Medical Center Hamburg-Eppendorf. All prostate specimens were analysed according to a standard procedure, including a complete embedding of the entire prostate for histological analysis (23). Histopathological data was retrieved from the patient files, including tumour stage, Gleason grade, nodal stage and status of the resection margin. In addition to the classical Gleason categories, ‘quantitative’ Gleason grading was performed by estimating the percentage of Gleason 4 patterns as previously described (24). Follow-up data were available for a total of 11,152 patients with a median follow-up of 60 months (range: 1 to 241 months; Table I). Prostate specific antigen (PSA) serum values were measured following surgery and PSA recurrence was defined as a postoperative serum PSA of at least 0.2 ng/ml and increasing at subsequent measurements. The TMA manufacturing process was described earlier in detail (25). In short, for each patient, a 0.6 mm diameter core was taken from a representative tissue block. The tissues were distributed among 27 TMA blocks, each containing 144 to 522 tumour samples. For internal controls, each TMA block also contained various control tissues, including normal prostate tissue. The use of leftover archived diagnostic tissues for manufacturing of tissue microarrays and their analysis for research purposes, as well as patient data analysis, has been approved by local laws (HmbKHG, §12a) and by the local ethics committee (Ethics Commission Hamburg, WF-049/09). The present study has been carried out in compliance with the Helsinki Declaration.
Table I.
Pathological and clinical data of the arrayed prostate cancer samples.
| Variables | Study cohort on TMAa, n | Biochemical relapse, n (%) |
|---|---|---|
| Follow-up | 11,152 | 2,769 (24.8) |
| Mean/median (months) | 64.4/60.0 | – |
| Age (years) | ||
| ≤50 | 323 | 81 (25.1) |
| 51–59 | 2,696 | 705 (26.1) |
| 60–69 | 6,528 | 1,610 (24.7) |
| ≥70 | 1,498 | 370 (24.7) |
| Pretreatment PSA (ng/ml) | ||
| <4 | 1,585 | 242 (15.3) |
| 4–10 | 7,480 | 1,355 (18.1) |
| >10–20 | 2,412 | 737 (30.6) |
| >20 | 812 | 397 (48.9) |
| pT stage (AJCC 2002) | ||
| pT2 | 8,187 | 1,095 (13.4) |
| pT3a | 2,660 | 817 (30.7) |
| pT3b | 1,465 | 796 (54.3) |
| pT4 | 63 | 51 (81.0) |
| Gleason grade | ||
| ≤3+3 | 2,297 | 230 (10.0) |
| 3+4 | 6,679 | 1,240 (18.6) |
| 3+4 Tertiary 5 | 433 | 115 (26.6) |
| 4+3 | 1,210 | 576 (47.6) |
| 4+3 Tertiary 5 | 646 | 317 (49.1) |
| ≥4+4 | 416 | 348 (83.7) |
| pN stage | ||
| pN0 | 6,970 | 1,636 (23.5) |
| pN+ | 693 | 393 (56.7) |
| Surgical margin | ||
| Negative | 9,990 | 1,848 (18.5) |
| Positive | 2,211 | 853 (38.6) |
Numbers do not always add up to 12,432 in different categories because of cases with missing data. AJCC, American Joint Committee on Cancer; PSA, prostate-specific antigen; TMA, tissue microarray.
Immunohistochemistry
The freshly cut TMA sections were collectively immunostained in a single run. Slides were deparaffinized and exposed to heat-induced antigen retrieval (121°C, 5 min, pH 7,8 Tris-EDTA-citrate buffer). Primary SPDEF antibody (mouse monoclonal antibody, MAB9916, clone 4A5; Abnova Germany, dilution 1:4050) was applied (37°C, 60 min) and bound antibody was then visualized using the EnVision kit (Dako, Glostrup, Denmark) according to the manufacturer's directions. The SPDEF staining was typically nuclear and slightly cytoplasmic in prostate cancer and usually negative in benign prostate tissue. The staining intensity (0, 1+, 2+, and 3+) and the fraction of positive tumour cells were separately recorded for each tissue spot. A final score was assigned as described (26): Negative scores had a complete absence of staining; weak scores had a staining intensity of 1+ in ≤70 % of the tumour cells or a staining intensity of 2+ in ≤30% of the tumour cells; moderate scores had a staining intensity of 1+ in >70% of tumour cells, a staining intensity of 2+ in >30% but in ≤70% of the tumour cells, or a staining intensity of 3+ in ≤30% of the tumour cells; and strong scores had a staining intensity of 2+ in >70% of the tumour cells or a staining intensity of 3+ in >30% of the tumour cells. Ki-67 labelling index (Ki67-LI) data were taken from a previous publication (27).
FISH
Details of the method were previously published for ERG (26), 5q21 (CHD1) (28), 6q15 (MAP3K7) (29), PTEN (10q23) (30), and 3p13 (FOXP1) (31).
Cell culture and Western blotting
DU-145 (prostate cancer) cells were obtained from the Leibniz-Institute DSMZ-German collection of microorganisms and cell cultures (no.: ACC 261, Human) and grown in DMEM (Dulbecco's Modified Eagles Medium), enriched with 10% heat-inactivated and filtered foetal bovine serum, 1% penicillin-streptomycin, 1% NEAA, 1% pyruvate. The cells were incubated in 75 cm2 flasks at 37°C and 5% CO2. For electrophoresis and blotting about 10×106 cells were harvested after being washed once with 1× PBS in 150 µl lysis buffer. The cells were scratched with a cell scraper and transferred into a precooled 1.5 ml Eppendorf tube, rested 30 min on ice, and were centrifuged at 14,000 rpm for 5 min at 4°C. The amount of protein was measured with the Quibit fluorometer at 550 nm. Four-times Laemmli-buffer (BioRad) and β-mercaptoethanol (1:10 dilution) were added to the DU-145 lysate sample and water control, heated at 95°C for 5 min, and loaded together with the size marker Precision Plus Protein™ dual colour standard (BioRad) to a 4–15% polyacrylamide gel. The gel was run for 30 min at 180 V in a mini-protean Tetra cell system in 1× Tris-glycin-SDS buffer (BioRAD). The proteins were transferred at 2A in 7 min to a polyvinylidene fluoride membrane, washed with PBS-Tween 20× (PBS + 0.5% Tween 20), and incubated for 1 h with blocking buffer (5% milk powder in PBS-Tween 20, filtered and boiled). MAB9916 clone 4A5 (1:1,400 in blocking buffer) was added overnight at 4°C, washed 3× 15 min with PBS-Tween and incubated with secondary antibody (peroxidase goat anti mouse, diluted 1:1,000, Dianova) for 40 min at room temperature on a shaker. The membrane was developed with enhanced chemiluminescence substrate (Bio Rad) and recorded on a ChemiDoc™ imaging system.
Statistical analysis
Contingency tables and the χ2-test were performed to search for associations between molecular parameters and tumour phenotype. Ki67 labelling data were tested by ANOVA. Kaplan-Meier curves were tested by log-rank for differences between groups and Cox proportional hazards regression analysis was performed to test for independence and significance between pathological, molecular and clinical variables. Separate models were calculated with different sets of parameters, according to their availability before or after the prostatectomy. Statistical calculations were done with JMP 10 (SAS Institute Inc., NC, USA).
Results
SPDEF-staining
A total of 9403 (76%) of tumour samples were interpretable in our TMA analysis. Reasons for non-informative cases (3024, 24%) included lack of tissue samples or absence of unequivocal cancer tissue in the TMA spot.
SPDEF expression in normal and cancerous prostate tissues
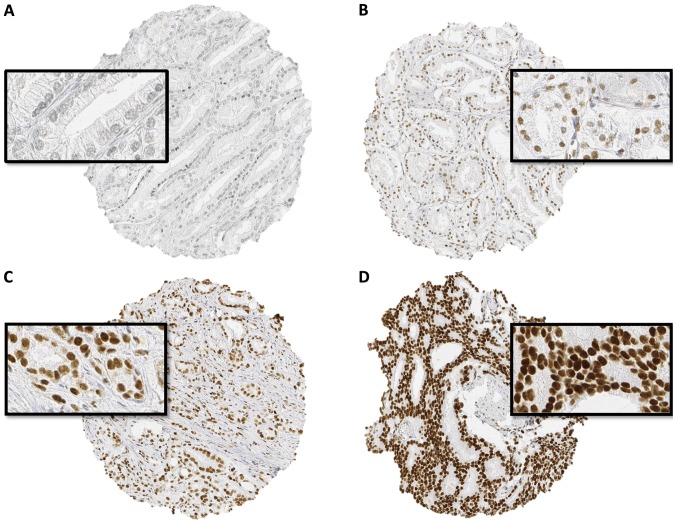
Normal prostate tissue only rarely showed weak nuclear staining, which was limited to epithelial cells. In cancer, nuclear SPDEF immunostaining was found in the majority (7522 of 9403; 80%) of interpretable prostate cancers and was considered weak in 26%, moderate in 40% and strong in 14% of cases. Representative images of SPDEF staining are given in Fig. 1.
Figure 1.
Representative images of 600-µm tissue spots. (A) negative, (B) weak, (C) moderate and (D) strong SAM pointed domain-containing Ets transcription factor nuclear staining in prostate cancer. Magnification, ×100 and ×400.
Association with TMPRSS2:ERG fusion status and ERG protein expression
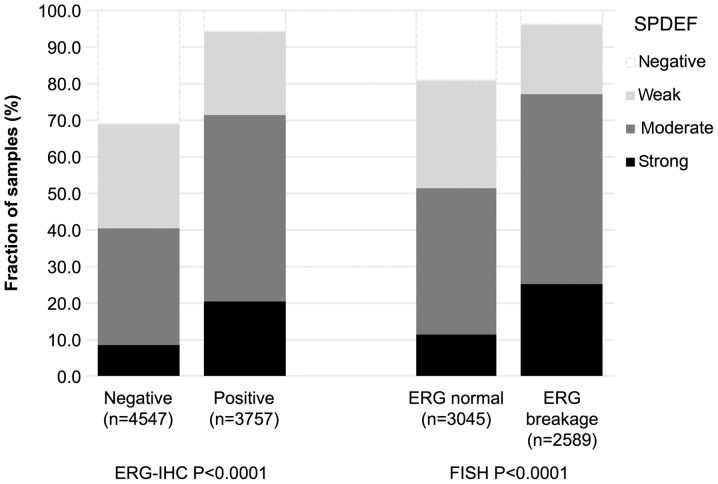
Data on TMPRSS2:ERG fusion status previously obtained by FISH were available from 5634 and by immunohistochemistry from 8304 tumours with evaluable SPDEF immunostaining [18,19]. Data on both ERG FISH and IHC were available from 5421 cancers, and concordant results (ERG IHC positive and break by FISH or ERG IHC negative and missing break by FISH) were found in 5171 of 5421 (95.4%) cancers. The SPDEF staining score was associated with TMPRSS2:ERG rearrangement and ERG positivity. For example, SPDEF immunostaining was seen in 94 and 96% of cancers with TMPRSS2:ERG fusion detected by IHC and FISH. In contrast, only 69% of cancers without ERG staining and 81% of cancers without ERG rearrangements were SPDEF positive (P<0.0001 each; Fig. 2).
Figure 2.
Association of SPDEF expression with ERG fusion probed by IHC and FISH. ERG, ETS-related gene; FISH, fluorescence in situ hybridization; IHC, immunohistochemistry; SPDEF, SAM pointed domain-containing Ets transcription factor.
Associations with tumour phenotype
High-level SPDEF immunostaining was associated with advanced pT stage, higher Gleason grade, lymph node metastasis (P<0.0001 each) and positive surgical margin (P=0.0105; Table II). The associations with pT stage and high Gleason grade held true for both the ERG negative and ERG positive cancer cohorts (P<0.0001 each; Tables III and IV).
Table II.
Association between SPDEF expression and prostate cancer phenotype.
| SPDEF (%) | ||||||
|---|---|---|---|---|---|---|
| Parameters | Number, n | Negative | Weak | Moderate | Strong | P-value |
| All cancer samples | 9,403 | 20.0 | 26.4 | 40.1 | 13.5 | |
| Tumour stagea | <0.0001 | |||||
| pT2 | 5,987 | 22.9 | 27.9 | 37.7 | 11.5 | |
| pT3a | 2,148 | 16.2 | 24.2 | 42.1 | 17.6 | |
| pT3b | 1,179 | 12.6 | 23.7 | 48.1 | 15.6 | |
| pT4 | 53 | 13.2 | 9.4 | 50.9 | 26.4 | |
| Gleason gradea | <0.0001 | |||||
| ≤3+3 | 2,126 | 28.9 | 32.1 | 31.7 | 7.2 | |
| 3+4 | 5,302 | 18.8 | 26.0 | 41.5 | 13.8 | |
| 4+3 | 1,468 | 13.8 | 20.9 | 45.5 | 19.8 | |
| ≥4+4 | 460 | 12.4 | 23.0 | 45.4 | 19.1 | |
| Lymph node metastasisa | <0.0001 | |||||
| N0 | 5,384 | 17.6 | 24.6 | 42.6 | 15.2 | |
| N+ | 560 | 13.0 | 18.6 | 48.6 | 19.8 | |
| Preoperative PSA level (ng/ml)a | 0.0394 | |||||
| <4 | 1,132 | 16.5 | 27.6 | 43.2 | 12.7 | |
| 4–10 | 5,602 | 20.1 | 26.6 | 39.8 | 13.4 | |
| >10–20 | 1,885 | 21.3 | 25.7 | 38.6 | 14.5 | |
| >20 | 676 | 21.0 | 24.1 | 41.6 | 13.3 | |
| Surgical margina | 0.0105 | |||||
| Negative | 7,419 | 20.4 | 26.9 | 39.6 | 13.1 | |
| Positive | 1,811 | 18.2 | 25.1 | 41.9 | 14.9 | |
Category with some missing data. The χ2-test was used to calculate P-values. PSA, prostate-specific antigen; SPDEF, SAM pointed domain-containing Ets transcription factor.
Table III.
Association between SPDEF expression and prostate cancer phenotype in the ERG negative subset.
| SPDEF (%) | ||||||
|---|---|---|---|---|---|---|
| Parameters | Number, n | Negative | Weak | Moderate | Strong | P-value |
| All cancer samples | 4,547 | 31.0 | 28.5 | 32.2 | 8.4 | |
| Tumour stage | <0.0001 | |||||
| pT2 | 3,015 | 33.8 | 29.5 | 30.0 | 6.6 | |
| pT3a | 928 | 28.8 | 27.4 | 33.0 | 10.9 | |
| pT3b | 567 | 19.2 | 26.3 | 40.9 | 13.6 | |
| pT4 | 24 | 25.0 | 12.5 | 54.2 | 8.3 | |
| Gleason grade | <0.0001 | |||||
| ≤3+3 | 953 | 44.5 | 31.2 | 21.5 | 2.8 | |
| 3+4 | 2,552 | 30.2 | 29.2 | 32.6 | 8.1 | |
| 4+3 | 757 | 20.9 | 24.4 | 40.6 | 14.1 | |
| ≥4+4 | 267 | 18.4 | 25.8 | 41.2 | 14.6 | |
| Lymph node metastasis | 0.0002 | |||||
| N0 | 2,659 | 27.6 | 28.8 | 34.0 | 9.6 | |
| N+ | 262 | 20.2 | 22.1 | 43.1 | 14.5 | |
| Preoperative PSA level (ng/ml) | 0.6675 | |||||
| <4 | 466 | 27.5 | 28.8 | 35.4 | 8.4 | |
| 4–10 | 2,671 | 31.3 | 28.4 | 32.3 | 8.0 | |
| >10–20 | 998 | 31.5 | 29.0 | 30.4 | 9.2 | |
| >20 | 373 | 30.6 | 27.3 | 32.7 | 9.4 | |
| Surgical margin | 0.4234 | |||||
| Negative | 3,597 | 31.3 | 28.7 | 31.9 | 8.1 | |
| Positive | 870 | 29.2 | 28.5 | 32.9 | 9.4 | |
The χ2-test was used to calculate P-values. ERG, ETS-related gene; PSA, prostate-specific antigen; SPDEF, SAM pointed domain-containing Ets transcription factor.
Table IV.
Association between SPDEF expression and prostate cancer phenotype in the ERG positive subset.
| SPDEF (%) | ||||||
|---|---|---|---|---|---|---|
| Parameters | Number, n | Negative | Weak | Moderate | Strong | P-value |
| All cancer samples | 3,757 | 5.8 | 23.0 | 50.7 | 20.5 | |
| Tumor stage | <0.0001 | |||||
| pT2 | 2,200 | 6.8 | 24.7 | 49.4 | 19.2 | |
| pT3a | 1,019 | 4.6 | 20.9 | 50.7 | 23.7 | |
| pT3b | 499 | 4.2 | 21.0 | 56.7 | 18.0 | |
| pT4 | 22 | 4.5 | 4.5 | 40.9 | 50.0 | |
| Gleason grade | <0.0001 | |||||
| ≤3+3 | 803 | 9.5 | 31.9 | 45.7 | 13.0 | |
| 3+4 | 2,211 | 5.2 | 22.2 | 51.8 | 20.9 | |
| 4+3 | 578 | 4.2 | 15.6 | 52.4 | 27.9 | |
| ≥4+4 | 143 | 2.8 | 16.8 | 53.8 | 26.6 | |
| Lymph node metastasis | 0.4186 | |||||
| N0 | 2,179 | 4.7 | 19.7 | 52.8 | 22.8 | |
| N+ | 241 | 5.0 | 15.4 | 56.0 | 23.7 | |
| Preoperative PSA level (ng/ml) | 0.1717 | |||||
| <4 | 502 | 5.4 | 24.1 | 52.8 | 17.7 | |
| 4–10 | 2,278 | 5.7 | 23.8 | 49.9 | 20.5 | |
| >10–20 | 688 | 6.4 | 20.6 | 49.7 | 23.3 | |
| >20 | 237 | 5.9 | 18.1 | 56.5 | 19.4 | |
| Surgical margin | 0.1312 | |||||
| Negative | 2,918 | 6.2 | 23.7 | 50.1 | 20.0 | |
| Positive | 767 | 4.7 | 21.1 | 52.7 | 21.5 | |
The χ2-test was used to calculate P-values. ERG, ETS-related gene; PSA, prostate-specific antigen; SPDEF, SAM pointed domain-containing Ets transcription factor.
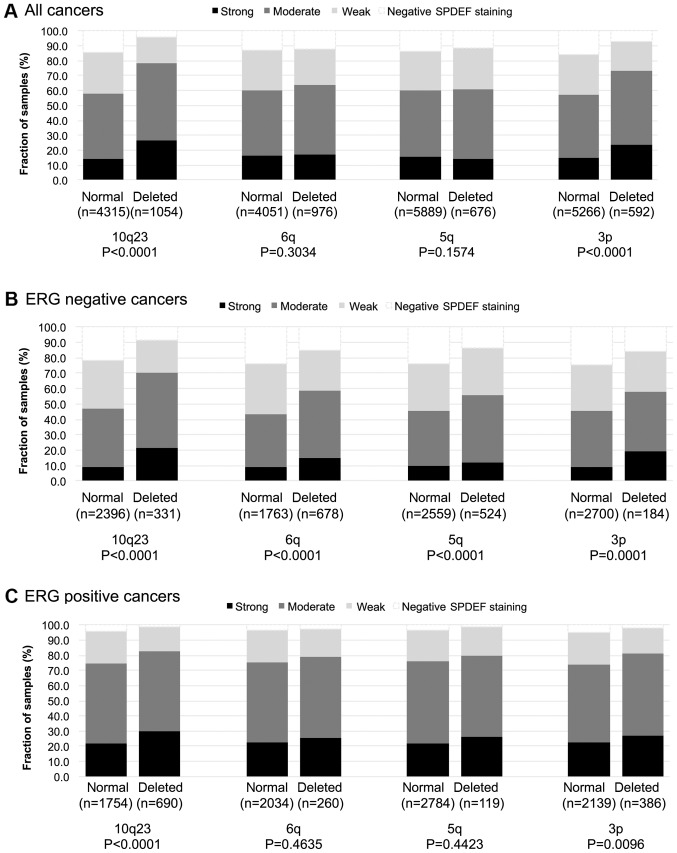
Association to key genomic deletions
To examine whether SPDEF expression might be associated with one or several of the most common genomic deletions, SPDEF data were compared to pre-existing data on PTEN (10q23), 3p13 (FOXP1), 6q15 (MAP3K7) and 5q21 (CHD1) deletions (Fig. 3A-C). Strong SPDEF expression was associated with all analysed deletions in ERG negative cancers (P≤0.0008). In contrast, SPDEF expression was only linked to PTEN deletions (P<0.0001) and 3p13 deletions (P=0.0096) but not to the other genomic deletions in ERG positive samples.
Figure 3.
SPDEF expression vs. 10q23. 6q15. 5q21 and 3p13 deletion probed by fluorescence in situ hybridization analysis in (A) all cancer samples, (B) ERG negative subset and (C) ERG positive subset. ERG, ETS-related gene; SPDEF, SAM pointed domain-containing Ets transcription factor.
Association with Ki67-labelling index (LI)
High levels of SPDEF immunostaining were significantly associated with accelerated cellular proliferation as measured by Ki67-LI (Table V; P<0.0001). This also applied for all analysed subgroups of tumours with identical Gleason scores (P<0.0001 for Gleason 3+3, 3+4, 4+3 and ≥4+4).
Table V.
Association between SPDEF expression and Ki67LI.
| Gleason | SPDEF IHC | Number, n | Ki67LI, mean ± SEM | P-value |
|---|---|---|---|---|
| Total | Negative | 1,247 | 1.44±0.07 | <0.0001 |
| Weak | 1,502 | 2.75±0.07 | ||
| Moderate | 2,163 | 3.24±0.06 | ||
| Strong | 689 | 3.80±0.10 | ||
| ≤3+3 | Negative | 369 | 1.25±0.10 | <0.0001 |
| Weak | 386 | 2.46±0.10 | ||
| Moderate | 374 | 2.65±0.10 | ||
| Strong | 80 | 3.16±0.22 | ||
| 3+4 | Negative | 647 | 1.43±0.09 | <0.0001 |
| Weak | 801 | 2.50±0.08 | ||
| Moderate | 1,240 | 3.10±0.06 | ||
| Strong | 409 | 3.43±0.11 | ||
| 4+3 | Negative | 97 | 1.41±0.33 | <0.0001 |
| Weak | 134 | 4.01±0.28 | ||
| Moderate | 218 | 3.47±0.22 | ||
| Strong | 98 | 4.23±0.33 | ||
| ≥4+4 | Negative | 39 | 2.05±0.74 | <0.0001 |
| Weak | 60 | 3.85±0.60 | ||
| Moderate | 98 | 4.95±0.47 | ||
| Strong | 41 | 7.73±0.72 |
ANOVA was used to calculate P-values. LI, labeling index; SPDEF, SAM pointed domain-containing Ets transcription factor.
Association with PSA recurrence
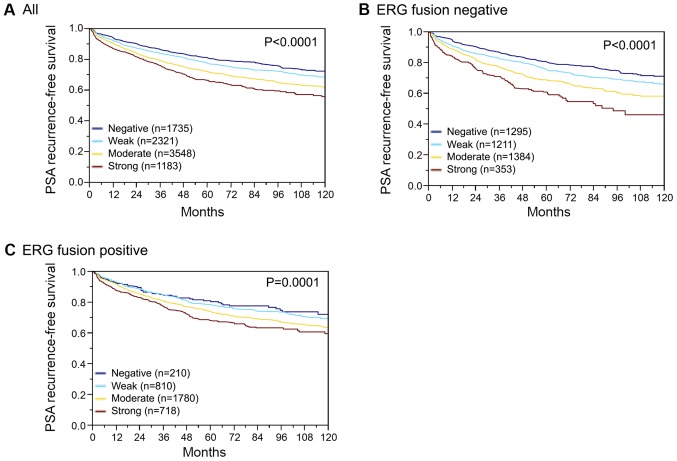
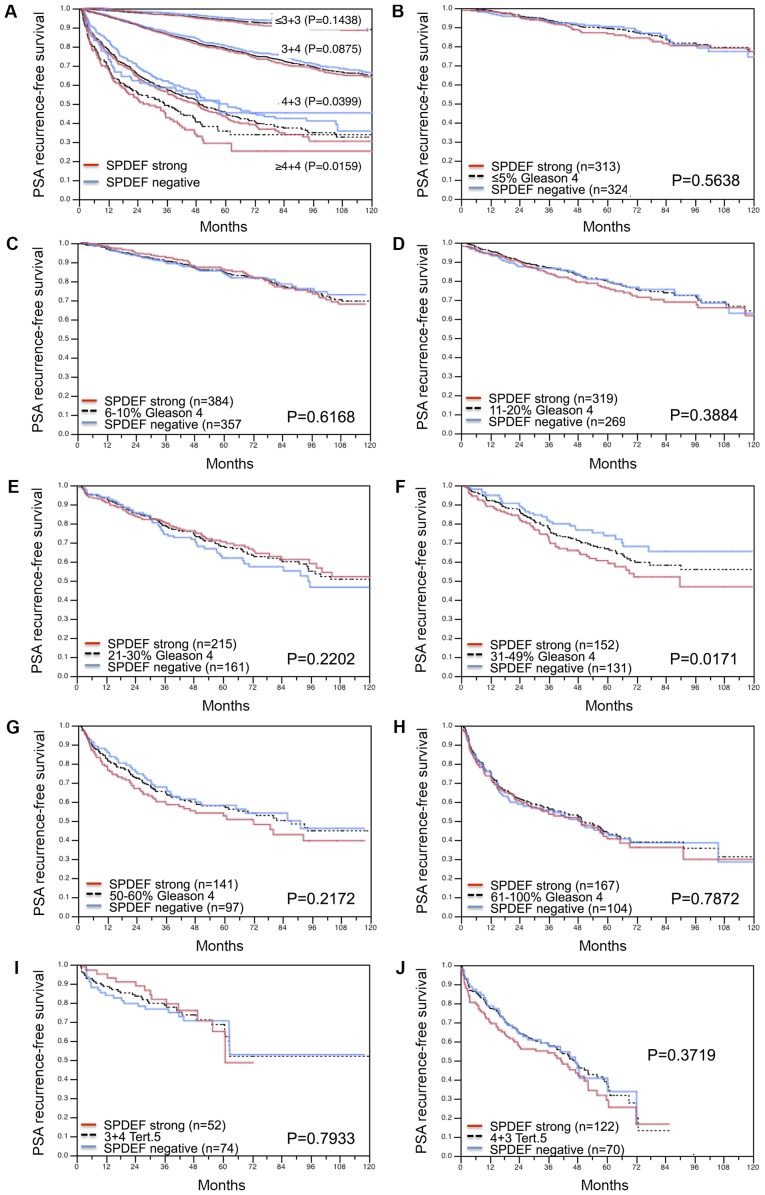
Follow-up data were available for 8787 patients with interpretable SPDEF immunostaining on the TMA. A highly significant association was seen between PSA recurrence and elevated SPDEF expression (P<0.0001; Fig. 4A). Subset analysis further revealed that the prognostic impact was stronger in the subgroup of ERG negative (P<0.0001; Fig. 4B) than in ERG positive cancers (P=0.0001; Fig. 4C). Further subset analyses of cancers with identical classical and quantitative Gleason grade revealed a prognostic impact of SPDEF immunostaining beyond classical Gleason grade for the 4+3 and ≥4+4 score groups (Fig. 5A) and the quantitative Gleason grade group 31–49% Gleason 4 (Fig. 5B-J).
Figure 4.
Kaplan-Meier analysis of PSA recurrence-free survival and negative, weak, moderate or strong SAM pointed domain-containing Ets transcription factor expression in (A) all cancer samples, (B) ERG fusion negative and (C) ERG fusion positive prostate cancer. Overall P-values are presented for all groups (log-rank test). ERG, ETS-related gene; PSA, prostate-specific antigen.
Figure 5.
Prognostic impact of SPDEF expression in a subsets of cancer defined by (A) classical Gleason score categories, the quantitative Gleason score categories defined by the percentage of (B) ≤5%, (C) 6–10%, (D) 11–20%, (E) 21–30%, (F) 31–49%, (G) 50–60% and (H) 61–100% Gleason 4 patterns, and with a tertiary Gleason 5 pattern of (I) 3+4 tertiary grade 5 and (J) 4+3 tertiary grade 5. Overall P-values are presented for all groups (log-rank test). SPDEF, SAM pointed domain-containing Ets transcription factor; Tert. 5, tertiary grade 5.
Multivariate analysis
To evaluate the clinical relevance of SPDEF expression in different scenarios, four different types of multivariable analyses were performed, as previously described (32). In brief, scenario 1 included postoperative parameters (pathological tumour stage, pathological lymph node status (pN), surgical margin status, preoperative PSA value, pathological Gleason grade). In scenario 2, all postoperatively available parameters with exception of nodal status were included. This takes into account that the indication and extent of lymph node dissection is not standardized in the surgical therapy of prostate cancer. Two additional scenarios had the purpose to model the preoperative situation: Scenario 3 included preoperative PSA, clinical tumour stage (cT stage) and Gleason grade obtained on the prostatectomy specimen. Since postoperative determination of a tumour's Gleason grade is of higher quality than the preoperatively determined Gleason grade [subject to sampling errors and consequently under-grading in more than one third of the cases (33)], another multivariable analysis was added. In scenario 4, the preoperative Gleason grade obtained on the original biopsy was combined with preoperative PSA, cT stage and SPDEF expression. In all four scenarios, the multivariate analysis demonstrated that SPDEF expression provides independent prognostic information in the subset of ERG negative cancers and also in the non-stratified cohort of all cancers. In the ERG positive subset, only the preoperative model 4 showed a significant prognostic effect (P=0.0024, Table VI). The overall univariate Cox proportional hazard ratio for strong versus negative SPDEF expression was weak (1.99, 95% CI 1.72–2.20, Table SI).
Table VI.
Multivariate analysis of Cox proportional hazards for biochemical RPE in various models and ERG subsets.
| P-value of χ2 test | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ERG subset | Model | Number, n | Preoperative PSA-Level | pT stage | cT stage | RPE Gleason | Gleason Biopsy | Nodal stage | R status | SPDEF expression |
| Total | 1 | 5,203 | <0.0001 | <0.0001 | – | <0.0001 | – | <0.0001 | 0.0006 | 0.0074 |
| 2 | 8,265 | <0.0001 | <0.0001 | – | <0.0001 | – | – | <0.0001 | 0.0020 | |
| 3 | 8,131 | <0.0001 | – | <0.0001 | <0.0001 | – | – | – | <0.0001 | |
| 4 | 8,021 | <0.0001 | – | <0.0001 | – | <0.0001 | – | – | <0.0001 | |
| Negative | 1 | 2,564 | 0.0002 | <0.0001 | – | <0.0001 | – | <0.0001 | 0.1299 | 0.0013 |
| 2 | 3,986 | <0.0001 | <0.0001 | – | <0.0001 | – | – | 0.0014 | <0.0001 | |
| 3 | 3,942 | <0.0001 | – | <0.0001 | <0.0001 | – | – | – | <0.0001 | |
| 4 | 3,891 | <0.0001 | – | <0.0001 | – | <0.0001 | – | – | <0.0001 | |
| Positive | 1 | 2,116 | 0.0013 | <0.0001 | – | <0.0001 | – | 0.0041 | 0.0113 | 0.4037 |
| 2 | 3,307 | <0.0001 | <0.0001 | – | <0.0001 | – | – | 0.0002 | 0.6837 | |
| 3 | 3,230 | <0.0001 | – | <0.0001 | <0.0001 | – | – | – | 0.4474 | |
| 4 | 3,182 | <0.0001 | – | 0.0031 | – | <0.0001 | – | – | 0.0024 | |
Model 1 and 2 included postoperatively available parameters. Model 3 and 4 are mixed models with post- and preoperatively available parameters. ERG, ETS-related gene; PSA, prostate-specific antigen; RPE, relapse after prostatectomy; SPDEF, SAM pointed domain-containing Ets transcription factor.
Discussion
The results of our study identify high SPDEF expression as an independent predictor of poor prognosis in prostate cancers. The increased SPDEF staining in the analysed prostate cancers, compared to the negative to weak SPDEF expression in normal prostate tissue, suggests for role of SPDEF in prostate cancer development. These observations fit well with a recent study by Situ et al (34) describing low or absent SPDEF staining in normal prostate tissue but strong up regulation in cancer. Sood et al (13) also described a slightly increasing rate of SPDEF positivity from normal (27%) to high-grade prostatic intraepithelial neoplasia (33%) and invasive prostate cancer (40%). Within our 9,403 interpretable prostate carcinomas, elevated SPDEF expression levels were strikingly linked to advanced tumour stage, high Gleason grade, rapid cell proliferation and early PSA recurrence. These findings fit well with a role for SPDEF as a potential oncogenic driver in prostate cancer. Other authors had also reported increasing SPDEF expression from intermediate to high Gleason grade in prostate cancer (13,34). A link between high SPDEF expression and cancer development and progression was also found in breast (12,35,36) and ovarian (16,37) cancers. It is of note that others have reported loss of SPDEF expression during tumour progression, and found reduced levels of SPDEF in prostate cancer as compared to normal epithelium in cohorts of 40 and 73 patients (15,20). Similar findings were also reported for breast (14), bladder (38) and colon (17) cancer. Such discrepancies are most likely due to differences in the reagents used, and possibly also due to patient cohort selection issues. We are confident in our reagents used. The monoclonal antibody (MAB9916 clone 4A5 from Abnova) used in this study was tested by Western blot, where it showed a single band of the predicted molecular mass of 37.5 kDa (Fig. S1) (8).
The prognostic effect of SPDEF expression was seen in both ERG positive and ERG negative cancers, but was stronger in the latter group. It is well known that aberrant expression of the ERG transcription factor leads to dysregulation of at least 1,600 genes in affected prostate cancer cells, and that these changes may impact the role of various prognostic molecular features. In earlier studies on the same set of tumours, prognostic factors that were restricted to either ERG positive (39,40) or ERG negative cancers (29,41) were often found. Given the different average SPDEF expression levels between ERG positive and ERG negative cancers, it is possible that our immunohistochemistry protocol was better suited to distinguish expression differences in cancers with somewhat lower expression levels, such as in ERG negative cancers, than in tumours with higher expression, such as in ERG positive cancers. Most chromosomal deletions are decidedly more frequent in either ERG positive (3p, PTEN) or ERG negative (5q, 6q) cancers (29,31,39,42). Because SPDEF is also associated with ERG status, a positive association with 3p and PTEN deletions and an inverse association with 5q and 6q deletions was expected in unselected prostate cancer cohorts. The association of high SPDEF expression with the cell proliferation marker Ki-67 fits well with the role of SPDEF as an oncogenic activator and with previous reports (8,13).
Our data are restricted to naïve prostate cancer patients, who had not received androgen deprivation therapy (ADT). Under ADT and in patients with castration resistant prostate cancer after ADT as well as in transgenic mouse models and prostate cancer cell lines it has been shown that SPDEF suppresses tumour metastasis (15,18–20,22). While ADT reduces SPDEF expression and cell proliferation, it relieves repression of TGFB1, CCL2, and MMP9 key drivers of metastasis. This provides an example of how a therapy which blocks growth of the primary tumour, may paradoxically promote metastasis (43).
The data of this study suggest that the SPDEF protein level may constitute a clinically useful marker in prostate cancer. SPDEF expression exerted a prognostic impact that was independent of established prognostic parameters. It is of note that the most critical clinical need in prostate cancer is not finding prognostic markers that are independent of established parameters. Most of all, parameters are needed that are more reproducible and reliable than the established ones. The Gleason grade, the strongest established prognostic parameter, suffers from very substantial interobserver variability reaching up to 40% in individual biopsies (44). Based on the data from this study, we assume that SPDEF expression measurement has potential to become part of a future multiparametric prognostic test for prostate cancer prognosis assessment.
In summary, these data show that SPDEF is a weak to moderate prognostic parameter in prostate cancer, especially in the ERG negative subset. Our data are consistent with a particularly strong up regulation of SPDEF in response to accelerated cell proliferation in the subset of PTEN deficient and genetically instable prostate cancer.
Supplementary Material
Acknowledgements
The authors would like to thank Dr Wilfried Fehrle (Department of Pathology, University Medical Center Hamburg-Eppendorf, Hamburg, Germany) for help in revision of the manuscript, and are grateful to Mrs. Sünje Seekamp and Mrs. Inge Brandt (Department of Pathology, University Medical Center Hamburg-Eppendorf, Hamburg, Germany) for excellent technical assistance.
Glossary
Abbreviations
- ERG
ETS-related gene
- ETS
E26 transformation-specific
- SPDEF
SAM pointed domain-containing Ets transcription factor
- TMA
tissue microarray
- TMPRSS2
transmembrane protease, serine 2
- UICC
International Union Against Cancer
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present study are included in this published article.
Authors' contributions
JM, FB, RS and GS designed the study, and drafted the manuscript. TS, MG, JRI, HHe and HHu participated in study design. KS, CB, CG, AH, VR and SW performed IHC analysis and scoring. FJ, CMK, TM, NCB, FL, FV, ML, CF and SM participated in pathology data analysis. CH-M, NCB and RS performed statistical analysis. SB, KM, and DH participated in data interpretation, and helped to draft the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The Ethics Committee of the Ärztekammer Hamburg approved the study protocol (approval no. WF-049/09). According to local laws (HmbKHG §12a), patient informed consent was not required. Patient records/information were anonymized and de-identified prior to analysis. All procedures were performed in compliance with the principles outlined in the Helsinki Declaration.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Thompson IM, Jr, Tangen CM. Prostate cancer-uncertainty and a way forward. N Engl J Med. 2012;367:270–271. doi: 10.1056/NEJMe1205012. [DOI] [PubMed] [Google Scholar]
- 3.Wilt TJ, Brawer MK, Jones KM, Barry MJ, Aronson WJ, Fox S, Gingrich JR, Wei JT, Gilhooly P, Grob BM, et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012;367:203–213. doi: 10.1056/NEJMoa1113162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharrocks AD. The ETS-domain transcription factor family. Nat Rev Mol Cell Biol. 2001;2:827–837. doi: 10.1038/35099076. [DOI] [PubMed] [Google Scholar]
- 5.Clark JP, Cooper CS. ETS gene fusions in prostate cancer. Nat Rev Urol. 2009;6:429–439. doi: 10.1038/nrurol.2009.127. [DOI] [PubMed] [Google Scholar]
- 6.Kumar-Sinha C, Tomlins SA, Chinnaiyan AM. Recurrent gene fusions in prostate cancer. Nat Rev Cancer. 2008;8:497–511. doi: 10.1038/nrc2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tomlins SA, Bjartell A, Chinnaiyan AM, Jenster G, Nam RK, Rubin MA, Schalken JA. ETS gene fusions in prostate cancer: From discovery to daily clinical practice. Eur Urol. 2009;56:275–286. doi: 10.1016/j.eururo.2009.04.036. [DOI] [PubMed] [Google Scholar]
- 8.Oettgen P, Finger E, Sun Z, Akbarali Y, Thamrongsak U, Boltax J, Grall F, Dube A, Weiss A, Brown L, et al. PDEF, a novel prostate epithelium-specific ets transcription factor, interacts with the androgen receptor and activates prostate-specific antigen gene expression. J Biol Chem. 2000;275:1216–1225. doi: 10.1074/jbc.275.2.1216. [DOI] [PubMed] [Google Scholar]
- 9.Ghadersohi A, Sood AK. Prostate epithelium-derived Ets transcription factor mRNA is overexpressed in human breast tumors and is a candidate breast tumor marker and a breast tumor antigen. Clin Cancer Res. 2001;7:2731–2738. [PubMed] [Google Scholar]
- 10.Ghadersohi A, Odunsi K, Lele S, Collins Y, Greco WR, Winston J, Liang P, Sood AK. Prostate derived Ets transcription factor shows better tumor-association than other cancer-associated molecules. Oncol Rep. 2004;11:453–458. [PubMed] [Google Scholar]
- 11.Horst D, Gu X, Bhasin M, Yang Q, Verzi M, Lin D, Joseph M, Zhang X, Chen W, Li YP, et al. Requirement of the epithelium-specific Ets transcription factor Spdef for mucous gland cell function in the gastric antrum. J Biol Chem. 2010;285:35047–35055. doi: 10.1074/jbc.M110.164541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gunawardane RN, Sgroi DC, Wrobel CN, Koh E, Daley GQ, Brugge JS. Novel role for PDEF in epithelial cell migration and invasion. Cancer Res. 2005;65:11572–11580. doi: 10.1158/0008-5472.CAN-05-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sood AK, Saxena R, Groth J, Desouki MM, Cheewakriangkrai C, Rodabaugh KJ, Kasyapa CS, Geradts J. Expression characteristics of prostate-derived Ets factor support a role in breast and prostate cancer progression. Hum Pathol. 2007;38:1628–1638. doi: 10.1016/j.humpath.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feldman RJ, Sementchenko VI, Gayed M, Fraig MM, Watson DK. Pdef expression in human breast cancer is correlated with invasive potential and altered gene expression. Cancer Res. 2003;63:4626–4631. [PubMed] [Google Scholar]
- 15.Johnson TR, Koul S, Kumar B, Khandrika L, Venezia S, Maroni PD, Meacham RB, Koul HK. Loss of PDEF, a prostate-derived Ets factor is associated with aggressive phenotype of prostate cancer: Regulation of MMP 9 by PDEF. Mol Cancer. 2010;9:148. doi: 10.1186/1476-4598-9-148. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Rodabaugh KJ, Mhawech-Fauceglia P, Groth J, Lele S, Sood AK. Prostate-derived Ets factor is overexpressed in serous epithelial ovarian tumors. Int J Gynecol Pathol. 2007;26:10–15. doi: 10.1097/01.pgp.0000225386.41244.bd. [DOI] [PubMed] [Google Scholar]
- 17.Moussa O, Turner DP, Feldman RJ, Sementchenko VI, McCarragher BD, Desouki MM, Fraig M, Watson DK. PDEF is a negative regulator of colon cancer cell growth and migration. J Cell Biochem. 2009;108:1389–1398. doi: 10.1002/jcb.22371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu X, Zerbini LF, Out HH, Bhasin M, Yang Q, Joseph MG, Grall F, Onatunde T, Correa RG, Libermann TA. Reduced PDEF expression increases invasion and expression of mesenchymal genes in prostate cancer cells. Cancer Res. 2007;67:4219–4226. doi: 10.1158/0008-5472.CAN-06-3689. [DOI] [PubMed] [Google Scholar]
- 19.Turner DP, Findlay VJ, Moussa O, Semenchenko VI, Watson PM, LaRue AC, Desouki MM, Fraig M, Watson DK. Mechanisms and functional consequences of PDEF protein expression loss during prostate cancer progression. Prostate. 2011;71:1723–1735. doi: 10.1002/pros.21389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghadersohi A, Sharma S, Zhang S, Azrak RG, Wilding GE, Manjili MH, Li F. Prostate-derived Ets transcription factor (PDEF) is a potential prognostic marker in patients with prostate cancer. Prostate. 2011;71:1178–1188. doi: 10.1002/pros.21333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steffan JJ, Koul S, Meacham RB, Koul HK. The transcription factor SPDEF suppresses prostate tumor metastasis. J Biol Chem. 2012;287:29968–29978. doi: 10.1074/jbc.M112.379396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng XH, Black M, Ustiyan V, Le T, Fulford L, Sridharan A, Medvedovic M, Kalinichenko VV, Whitsett JA, Kalin TV. SPDEF inhibits prostate carcinogenesis by disrupting a positive feedback loop in regulation of the Foxm1 oncogene. PLoS Genet. 2014;10:e1004656. doi: 10.1371/journal.pgen.1004656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erbersdobler A, Fritz H, Schnöger S, Graefen M, Hammerer P, Huland H, Henke RP. Tumour grade, proliferation, apoptosis, microvessel density, p53, and bcl-2 in prostate cancers: Differences between tumours located in the transition zone and in the peripheral zone. Eur Urol. 2002;41:40–46. doi: 10.1016/S0302-2838(01)00021-5. [DOI] [PubMed] [Google Scholar]
- 24.Sauter G, Steurer S, Clauditz TS, Krech T, Wittmer C, Lutz F, Lennartz M, Janssen T, Hakimi N, Simon R, et al. Clinical utility of quantitative gleason grading in prostate biopsies and prostatectomy specimens. Eur Urol. 2016;69:592–598. doi: 10.1016/j.eururo.2015.10.029. [DOI] [PubMed] [Google Scholar]
- 25.Kononen J, Bubendorf L, Kallioniemi A, Bärlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4:844–847. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- 26.Minner S, Wittmer C, Graefen M, Salomon G, Steuber T, Haese A, Huland H, Bokemeyer C, Yekebas E, Dierlamm J, et al. High level PSMA expression is associated with early PSA recurrence in surgically treated prostate cancer. Prostate. 2011;71:281–288. doi: 10.1002/pros.21241. [DOI] [PubMed] [Google Scholar]
- 27.Minner S, Jessen B, Stiedenroth L, Burandt E, Köllermann J, Mirlacher M, Erbersdobler A, Eichelberg C, Fisch M, Brümmendorf TH, et al. Low level HER2 overexpression is associated with rapid tumor cell proliferation and poor prognosis in prostate cancer. Clin Cancer Res. 2010;16:1553–1560. doi: 10.1158/1078-0432.CCR-09-2546. [DOI] [PubMed] [Google Scholar]
- 28.Burkhardt L, Fuchs S, Krohn A, Masser S, Mader M, Kluth M, Bachmann F, Huland H, Steuber T, Graefen M, et al. CHD1 is a 5q21 tumor suppressor required for ERG rearrangement in prostate cancer. Cancer Res. 2013;73:2795–2805. doi: 10.1158/0008-5472.CAN-12-1342. [DOI] [PubMed] [Google Scholar]
- 29.Kluth M, Hesse J, Heinl A, Krohn A, Steurer S, Sirma H, Simon R, Mayer PS, Schumacher U, Grupp K, et al. Genomic deletion of MAP3K7 at 6q12-22 is associated with early PSA recurrence in prostate cancer and absence of TMPRSS2:ERG fusions. Mod Pathol. 2013;26:975–983. doi: 10.1038/modpathol.2012.236. [DOI] [PubMed] [Google Scholar]
- 30.Krohn A, Diedler T, Burkhardt L, Mayer PS, De Silva C, Meyer-Kornblum M, Kötschau D, Tennstedt P, Huang J, Gerhäuser C, et al. Genomic deletion of PTEN is associated with tumor progression and early PSA recurrence in ERG fusion-positive and fusion-negative prostate cancer. Am J Pathol. 2012;181:401–412. doi: 10.1016/j.ajpath.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 31.Krohn A, Seidel A, Burkhardt L, Bachmann F, Mader M, Grupp K, Eichenauer T, Becker A, Adam M, Graefen M, et al. Recurrent deletion of 3p13 targets multiple tumour suppressor genes and defines a distinct subgroup of aggressive ERG fusion-positive prostate cancers. J Pathol. 2013;231:130–141. doi: 10.1002/path.4223. [DOI] [PubMed] [Google Scholar]
- 32.Burdelski C, Reiswich V, Hube-Magg C, Kluth M, Minner S, Koop C, Graefen M, Heinzer H, Tsourlakis MC, Wittmer C, et al. Cytoplasmic accumulation of sequestosome 1 (p62) is a predictor of biochemical recurrence, rapid tumor cell proliferation, and genomic instability in prostate cancer. Clin Cancer Res. 2015;21:3471–3479. doi: 10.1158/1078-0432.CCR-14-0620. [DOI] [PubMed] [Google Scholar]
- 33.Epstein JI, Feng Z, Trock BJ, Pierorazio PM. Upgrading and downgrading of prostate cancer from biopsy to radical prostatectomy: Incidence and predictive factors using the modified Gleason grading system and factoring in tertiary grades. Eur Urol. 2012;61:1019–1024. doi: 10.1016/j.eururo.2012.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Situ J, Zhang H, Lu L, Li K, Hu C, Wang DJ. Clinical significance of PSMA, TERT and PDEF in malignant tumors of the prostate. Eur Rev Med Pharmacol Sci. 2017;21:3347–3352. [PubMed] [Google Scholar]
- 35.Mukhopadhyay A, Khoury T, Stein L, Shrikant P, Sood AK. Prostate derived Ets transcription factor and Carcinoembryonic antigen related cell adhesion molecule 6 constitute a highly active oncogenic axis in breast cancer. Oncotarget. 2013;4:610–621. doi: 10.18632/oncotarget.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sood AK, Geradts J, Young J. Prostate-derived Ets factor, an oncogenic driver in breast cancer. Tumour Biol. 2017;39:1010428317691688. doi: 10.1177/1010428317691688. [DOI] [PubMed] [Google Scholar]
- 37.Ghadersohi A, Odunsi K, Zhang S, Azrak RG, Bundy BN, Manjili MH, Li F. Prostate-derived Ets transcription factor as a favorable prognostic marker in ovarian cancer patients. Int J Cancer. 2008;123:1376–1384. doi: 10.1002/ijc.23667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsui KH, Lin YH, Chung LC, Chuang ST, Feng TH, Chiang KC, Chang PL, Yeh CJ, Juang HH. Prostate-derived ets factor represses tumorigenesis and modulates epithelial-to-mesenchymal transition in bladder carcinoma cells. Cancer Lett. 2016;375:142–151. doi: 10.1016/j.canlet.2016.02.056. [DOI] [PubMed] [Google Scholar]
- 39.Krohn A, Freudenthaler F, Harasimowicz S, Kluth M, Fuchs S, Burkhardt L, Stahl P, C Tsourlakis M, Bauer M, Tennstedt P, et al. Heterogeneity and chronology of PTEN deletion and ERG fusion in prostate cancer. Mod Pathol. 2014;27:1612–1620. doi: 10.1038/modpathol.2014.70. [DOI] [PubMed] [Google Scholar]
- 40.Kluth M, Runte F, Barow P, Omari J, Abdelaziz ZM, Paustian L, Steurer S, Christina Tsourlakis M, Fisch M, Graefen M, et al. Concurrent deletion of 16q23 and PTEN is an independent prognostic feature in prostate cancer. Int J Cancer. 2015;137:2354–2363. doi: 10.1002/ijc.29613. [DOI] [PubMed] [Google Scholar]
- 41.Kluth M, Scherzai S, Büschek F, Fraune C, Möller K, Höflmayer D, Minner S, Göbel C, Möller-Koop C, Hinsch A, et al. 13q deletion is linked to an adverse phenotype and poor prognosis in prostate cancer. Genes Chromosomes Cancer. 2018;57:504–512. doi: 10.1002/gcc.22645. [DOI] [PubMed] [Google Scholar]
- 42.Grupp K, Diebel F, Sirma H, Simon R, Breitmeyer K, Steurer S, Hube-Magg C, Prien K, Pham T, Weigand PU, et al. SPINK1 expression is tightly linked to 6q15- and 5q21-deleted ERG-fusion negative prostate cancers but unrelated to PSA recurrence. Prostate. 2013;73:1690–1698. doi: 10.1002/pros.22707. [DOI] [PubMed] [Google Scholar]
- 43.Luk IY, Reehorst CM, Mariadason JM. ELF3, ELF5, EHF and SPDEF transcription factors in tissue homeostasis and cancer. Molecules. 2018;23(pii):E2191. doi: 10.3390/molecules23092191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Egevad L, Ahmad AS, Algaba F, Berney DM, Boccon-Gibod L, Compérat E, Evans AJ, Griffiths D, Grobholz R, Kristiansen G, et al. Standardization of Gleason grading among 337 European pathologists. Histopathology. 2013;62:247–256. doi: 10.1111/his.12008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during the present study are included in this published article.