Abstract
Despite the success of renin-angiotensin system (RAS) blockade by angiotensin-converting enzyme (ACE) inhibitors and angiotensin II type 1 receptor (AT1R) blockers, current therapies for hypertension and related cardiovascular diseases are still inadequate. Identification of additional components of the RAS and associated vasoactive pathways, as well as new structural and functional insights into established targets, have led to novel therapeutic approaches with the potential to provide improved cardiovascular protection and better blood pressure control and/or reduced adverse side effects. The simultaneous modulation of several neurohumoral mediators in key interconnected blood pressure–regulating pathways has been an attractive approach to improve treatment efficacy, and several novel approaches involve combination therapy or dual-acting agents. In addition, increased understanding of the complexity of the RAS has led to novel approaches aimed at upregulating the ACE2/angiotensin-(1-7)/Mas axis to counter-regulate the harmful effects of the ACE/angiotensin II/angiotensin III/AT1R axis. These advances have opened new avenues for the development of novel drugs targeting the RAS to better treat hypertension and heart failure. Here we focus on new therapies in preclinical and early clinical stages of development, including novel small molecule inhibitors and receptor agonists/antagonists, less conventional strategies such as gene therapy to suppress angiotensinogen at the RNA level, recombinant ACE2 protein, and novel bispecific designer peptides.
I. Introduction
Cardiovascular disease is responsible for more than 30% of all deaths worldwide, most of which occur in developing countries (Benjamin et al., 2017). Hypertension is the main risk factor for cardiovascular disease; despite the availability of more than 100 commercial drugs and drug combinations for treating hypertension, a substantial proportion of the hypertensive population has uncontrolled or suboptimally controlled hypertension (Oparil and Schmieder, 2015). This contributes to the growing global burden of cardiovascular disease (Oparil et al., 2018). In addition, patients receiving treatment may suffer from significant side effects such as angiotensin-converting enzyme (ACE) inhibitor–induced persistent cough and, more rarely, life-threatening angioedema (Simon et al., 1992; Agah et al., 1997; Bas, 2017; Stone and Brown, 2017). Suboptimal control of hypertension is associated with target organ damage leading to heart failure, ischemic heart disease, stroke, kidney dysfunction, retinopathy, and vascular dementia, all of which are major causes of disability and premature death. Hence, there is a growing need for novel antihypertensive and cardiovascular drugs that are effective, affordable, and safe with no adverse side effects and that reduce the need for the administration of multiple drugs.
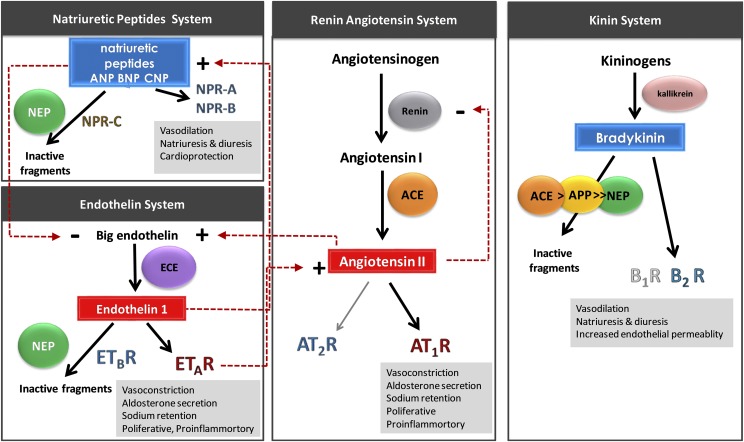
Blood pressure and cardiovascular function are regulated by multiple interacting systems, including in large part the enzyme-catalyzed formation and degradation of vasoactive peptides and hormones in overlapping regulatory systems (Fig. 1). Peptidases and receptors within these systems are important drug targets for the treatment of various cardiovascular diseases, including hypertension, heart failure, and coronary artery syndrome.
Fig. 1.
Outline of the systems involved in blood pressure regulation. Vasoconstrictor and vasodilator peptides are shown in red and blue rectangles, respectively. Vasopeptidases responsible for the production or degradation of vasoactive peptides are shown in colored spheres (ACE, APP, ECE, and NEP). Production of the vasoconstrictor peptides Ang II and ET-1 (red rectangles) in the RAS and endothelin system, respectively, lead to vasoconstriction, aldosterone secretion, and sodium retention. Bradykinin and NPs (ANP, BNP, and CNP) are potent vasodilatory peptides that counter-regulate the effects of Ang II and ET-1. The vasoactive peptides mediate their physiologic effect via a range of receptors (AT1R, AT2R, B1R, B2R, ETAR, ETBR, NPR-A, NPR-B, and NPR-C).
II. Vasoactive Systems Controlling Blood Pressure and Cardiovascular Function
A. The Renin-Angiotensin System
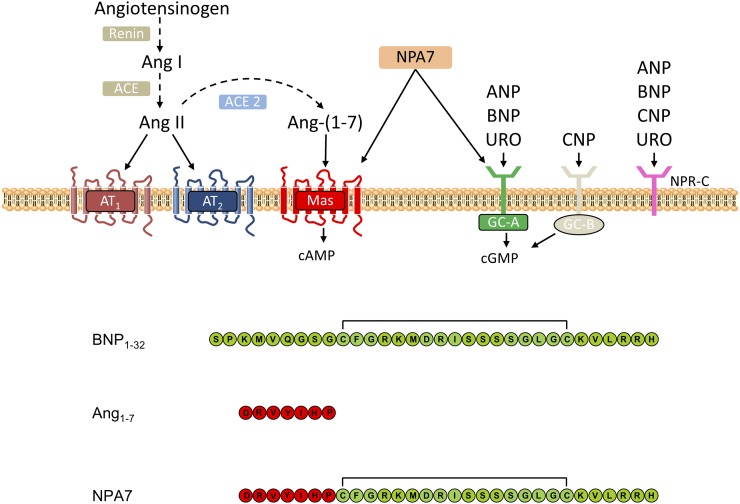
The systemic renin-angiotensin system (RAS) plays a central role in regulating extracellular fluid volume and arterial vasoconstriction (Fig. 1). A reduction in renal blood flow or blood sodium levels leads to the release of renin into the circulation, mostly from renal juxtaglomerular cells in the walls of the afferent arterioles of the kidney (Davis and Freeman, 1976). Renin, an aspartyl protease, is responsible for hydrolyzing the serum globulin, angiotensinogen, releasing the peptide angiotensin I (Ang I; Ang 1-10) (Page and Helmer, 1940). Ang I is then converted to the potent vasoconstrictor, angiotensin II (Ang II; Ang 1-8), by the zinc metalloprotease, ACE, which is highly expressed by endothelial and epithelial cells in the vasculature, kidneys, and lungs (Skeggs et al., 1956) and shed into the circulation by unknown proteases (Ehlers et al., 1996; Woodman et al., 2000). Ang II, the main vasoactive peptide of angiotensin metabolites in the systemic RAS, elicits its downstream physiologic and pathophysiological effects predominantly via the angiotensin II type 1 receptor (AT1R), which is ubiquitously expressed in the cardiovascular system. Binding to AT1R results in vasoconstriction and aldosterone secretion, leading to salt and water retention and ultimately increasing arterial blood pressure. The RAS is regulated by a negative feedback loop whereby Ang II reduces renin gene transcription and renal renin secretion by interacting directly with the juxtaglomerular cells (Naftilan and Oparil, 1978), decreasing the flux through the pathway. Ang II can also mediate vasodilatory effects by binding to the angiotensin II type 2 receptor (AT2R). However, this receptor is only expressed at very low levels in the cardiovascular system of healthy adults. The Ang II receptors are reviewed in de Gasparo et al. (2000).
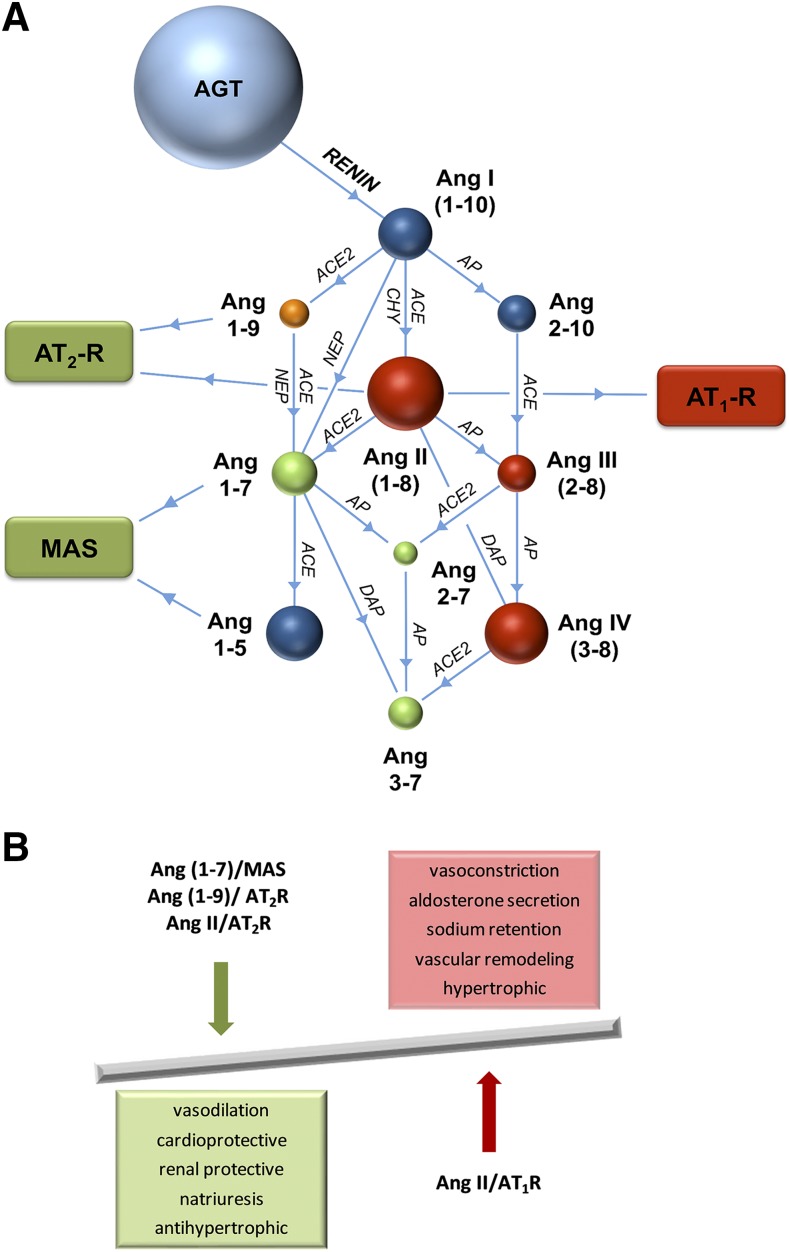
Drugs targeting various components of the systemic RAS, including renin inhibitors, ACE inhibitors, and angiotensin II type 1 receptor blockers (ARBs), are used to treat cardiovascular diseases (Atlas, 2007; Mentz et al., 2013). All of these drugs are primarily designed to block and/or reduce the detrimental effects of Ang II. There is, however, increasing evidence that in addition to Ang II, many other angiotensin peptides including Ang III (Ang 2-8), Ang 1-7, Ang 1-9, Ang 3-7, and Ang 3-8 have important physiologic effects. Multiple amino-, endo-, and carboxypeptidases are involved in producing a range of angiotensin metabolites (Fig. 2A), responsible for the activation and/or inhibition of numerous receptors that lead to downstream physiologic effects.
Fig. 2.
(A) Angiotensin metabolism. Angiotensin peptides are shown as colored spheres (AGT and Ang metabolites). Peptidases responsible for peptide cleavage are indicated (ACE, ACE2, AP, CHY, DAP, and NEP). Receptors for vasoactive peptides responsible for mediating vasoconstrictive and counteractive vasodilatory responses are indicated in colored rectangles (AT1R, AT2R, and Mas). (B) Schematic showing the counter-regulatory effects of the Ang 1-7/Mas, Ang 1-9/AT2R, and Ang II/AT2R pathways on the Ang II/AT1R pathway. AGT, angiotensinogen; AP, aminopeptidase; CHY, chymase; DAP, dipeptidyl aminopeptidase.
The cardiovascular protective peptide Ang 1-7 leads to vasodilatory, antiproliferative, and anti-inflammatory effects mediated via the G protein–coupled receptor (GPCR) Mas (Santos et al., 2018). Ang 1-7 is produced via the peptidase-mediated cleavage of Ang I, Ang 1-9, or Ang II (Fig. 2A). Several peptidases can form Ang 1-7, including neprilysin (NEP), ACE homolog ACE2, vascular endothelium prolyl endopeptidase, and smooth muscle thimet oligopeptidase (Welches et al., 1993; Chappell et al., 1995; Vickers et al., 2002). Activation of the ACE2/Ang 1-7/Mas axis leads to cardiovascular and renal-protective actions that counter-regulate the harmful actions of the ACE/Ang II/AT1R pathway (reviewed in Santos et al., 2013; Jiang et al., 2014; and Patel et al., 2016) (Fig. 2B). There is also accumulating evidence that additional receptors, including AT2R (Walters et al., 2005; Ohshima et al., 2014; Shimada et al., 2015) and Mas-related GPCR member D (Gembardt et al., 2008; Lautner et al., 2013; Tetzner et al., 2016), can function as Ang 1-7 receptors and that receptors for Ang II and Ang 1-7 constitute an intricate crossregulated signaling network (reviewed in Karnik et al., 2017). A recent study suggested that Ang 1-7 acts as a biased agonist of AT1R, promoting β-arrestin activation while behaving as a competitive antagonist for detrimental AT1R pathways initiated by Ang II (Galandrin et al., 2016). In addition, Yu et al. (2016) showed that the metabolite Ang 1-5 displays cardioprotective properties, stimulating the release of the cardioprotective atrial natriuretic peptide (ANP) via the Mas axis similarly to its parent peptide Ang 1-7. Ang 1-7 is currently in clinical trials to treat diabetic foot ulcers and cancer based on its ability to stimulate wound healing and hematopoietic progenitor cells, respectively (Rodgers et al., 2015; Savage et al., 2016; Pinter et al., 2018), further exemplifying the diverse functions of this peptide and the RAS.
ACE2 also plays a role in the conversion of Ang I to Ang 1-9 (albeit with much lower efficiency than conversion of Ang II to Ang 1-7), an additional counter-regulatory peptide that reduces adverse cardiovascular remodeling, cardiomyocyte hypertrophy, and cardiac fibrosis in various animal models of hypertension and myocardial infarction after subcutaneous administration (Ocaranza et al., 2010, 2014; Flores-Muñoz et al., 2011, 2012). These beneficial effects were blocked by coadministration of an AT2R antagonist but not a Mas antagonist, suggesting that these counter-regulatory effects, independent of the ACE2/Ang 1-7/Mas axis, are mediated through AT2R (Flores-Muñoz et al., 2011, 2012). In addition, Fattah et al. (2016) showed that gene therapy with Ang 1-9 is cardioprotective in a murine model of myocardial infarction. Ang 1-9 is also a competitive inhibitor of ACE, thereby decreasing Ang II levels and, like Ang 1-7, has been shown to potentiate bradykinin effects via the B2 receptor (B2R) (Jackman et al., 2002). Angiotensin metabolites, including Ang 1-7, Ang 3-7, and Ang 3-8, also display unique pharmacological effects in biologic processes beyond blood pressure regulation and cardiovascular function, including brain function, dopamine regulation, and insulin secretion (Wright et al. 1993; Stragier et al., 2005; Ferreira et al., 2007).
ARBs and ACE inhibitors alter the peptide fluxes through the systemic RAS by elevating renin secretion, although ACE inhibitors in particular lead to a prominent increase in plasma levels of Ang 1-7, potentiating their antihypertensive and cardioprotective effects (Table 1). ACE is the primary enzyme responsible for the degradation of Ang 1-7 (Chappell et al., 1998), which further explains the increase in Ang 1-7 and Ang 1-9 plasma levels associated with ACE inhibitors. An undesirable effect of ACE inhibition is the increase in renin secretion and consequently the flux through the RAS due to suppression of the Ang II–mediated negative feedback loop. These processes lead to decreased pharmacologic efficacy of ACE inhibitors during long-term treatment caused by incomplete inhibition of Ang II formation while Ang I is abundantly present as a substrate for ACE due to a high plasma renin activity. This results in a new steady state where Ang II levels are no longer suppressed, whereas Ang 1-7 levels are elevated (Table 1). ACE inhibitors are very effective in many cardiovascular diseases and are the first-line treatment of heart failure, myocardial infarction, and nephropathy unless ACE inhibitors are poorly tolerated. ARBs were not found to be superior to ACE inhibitors for these conditions and are recommended when ACE inhibitors are not tolerated (Pitt et al., 2000; Dahlöf et al., 2002; Granger et al., 2003; Yusuf et al., 2008). More recently, ACE2 activators, AT2R agonists, and Mas agonists have been investigated in preclinical models as antihypertensive agents to oppose harmful effects of the RAS (Tamargo et al., 2015).
TABLE 1.
Effects of antihypertensive drug classes on plasma vasoactive peptide levels and renin activity
| Drug Class | Ang I | Ang II | Ang 1-7 | BK 1-9 | ANP, BNP | PRC | PRA |
|---|---|---|---|---|---|---|---|
| ARB | ↑ | ↑↑ | ↑ | = | = | ↑↑ | ↑↑ |
| ACEi | ↑↑ | ↓ | ↑↑ | ↑ | = | ↑↑ | ↑↑ |
| C-ACEi | ↑↑ | ↓ | ↑ | = | = | ↑↑ | ↑↑ |
| NEPi | ↑ | ↑ | ↓ | ↑ | ↑ | = | = |
| ACEi/NEPi | ↑↑ | ↓ | ↑ | ↑↑ | ↑ | ↑↑ | ↑↑ |
| ARNI | ↑ | ↑↑ | = | ↑ | ↑ | ↑↑ | ↑↑ |
| AGT-siRNA | ↓↓ | ↓↓ | ↓↓ | = | = | ↑↑ | ↓↓ |
| DRI | ↓↓ | ↓↓ | ↓↓ | = | = | ↑↑ | ↓↓ |
| APAi | = | = | = | = | = | = | = |
| rhACE2 | ↑ | ↓↓ | ↑↑↑ | = | = | ↑↑ | ↑↑ |
Upward arrows indicate upregulation, downward arrows indicate downregulation, and equal signs indicate no change. ACEi, ACE inhibitor; ACEi/NEPi, dual ACE and NEP inhibitor; AGT-siRNA, angiotensinogen siRNA; APAi, amino peptidase A inhibitor; C-ACEi, C-domain–selective ACE inhibitor; DRI, direct renin inhibitor; NEPi, NEP inhibitor; PRA, plasma renin activity; PRC, plasma renin concentration.
B. The Kinin System
The kinin system is a key hormonal pathway that counter-regulates an overactive RAS. Kinin peptides, of which the best-known member is bradykinin (BK 1-9), are potent vasodilators and important inflammatory mediators generated from kininogen precursors by the serine protease, kallikrein (Fig. 1) (Regoli and Barabé, 1980; Kakoki and Smithies, 2009). Bradykinin causes vasodilation, induces prostaglandin production, and increases vascular permeability and fluid extravasation. Two kinin receptors have been identified: B1R and B2R (Leeb-Lundberg et al., 2005). The vasodilatory effects of bradykinin are predominantly mediated through B2R, which is constitutively expressed in most tissues and is abundant in vascular endothelial cells. B1R is minimally expressed in healthy tissue but is induced by tissue injury and plays a role in chronic pain and inflammation.
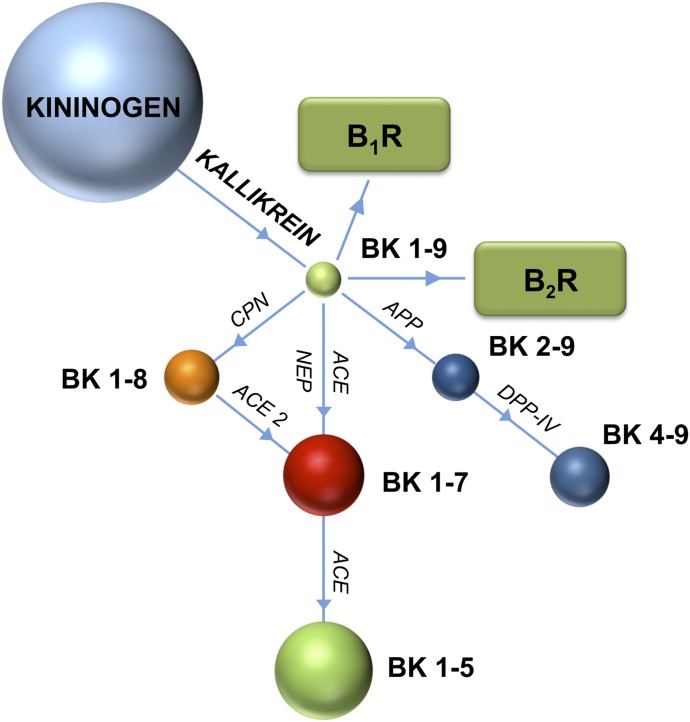
Bradykinin is cleaved into inactive fragments by ACE and several other peptidases, including aminopeptidase P (APP), NEP, endothelin-converting enzyme (ECE)-1, ACE2, carboxypeptidase N, and dipeptidyl peptidase IV (Skidgel et al., 1984; Hoang and Turner, 1997; Kuoppala et al., 2000; Fryer et al., 2008) (Fig. 3). ACE is the major bradykinin-metabolizing enzyme in human blood plasma (Kuoppala et al., 2000) and accordingly, treatment with ACE inhibitors results in a substantial increase in bradykinin levels, potentiating their vasodilatory and antihypertensive effects. There is also crosstalk between the RAS and the kinin system, and the benefits of ACE inhibition can be partially attributed to an intracellular inhibitor-induced ACE-mediated signaling cascade that leads to changes in gene expression and potentiation of the bradykinin response by inhibiting the desensitization of B2R (Benzing et al., 1999; Marcic et al., 1999; Tom et al., 2001; Guimarães et al., 2011). Bradykinin potentiation is, however, a double-edged sword: although the potent vasodilatory effects of ACE inhibitors can be attributed in part to increased levels of bradykinin, excessive bradykinin potentiation seems to be associated with the principle side effects caused by ACE inhibitors. The major side effect is persistent cough. It was also thought that ACE inhibitor–associated angioedema is due to increased bradykinin (Israili and Hall, 1992; Fox et al., 1996) but recent clinical studies do not support this (Straka et al., 2017). Considering the functional interactions between the RAS and kinin systems, there is growing interest in developing new drugs that target both systems, which would have greater efficacy than targeting only one system.
Fig. 3.
Bradykinin metabolism. Bradykinin peptides are shown as colored spheres. Peptidases responsible for peptide cleavage are indicated (ACE, ACE2, APP, CPN, DPPIV, and NEP). Bradykinin receptors B1R and B2R are indicated in green rectangles. CPN, carboxypeptidase N; DPP-IV, dipeptidyl peptidase IV.
C. The Natriuretic Peptide System
Natriuretic peptides (NPs) are a family of structurally related signaling molecules that signal through activation of guanylyl cyclases. They have natriuretic and vascular smooth muscle–relaxing activity and regulate cardiovascular, skeletal, and kidney function. In general, NPs are cardiovascular protective and lower blood pressure, maintain fluid volume homeostasis, and reduce cardiovascular fibrosis (reviewed in Pandey, 2005). There are three forms of NPs, ANP, B-type natriuretic peptide (BNP), and C-type natriuretic peptide (CNP), all of which are processed from preprohormones to mature forms that contain a C-terminal disulphide ring structure. ANP is expressed and stored primarily in granules in the atria, but it is expressed at lower levels in other tissues, including the ventricles and kidney. ANP release is primarily stimulated by atrial wall stretching (de Bold et al., 1986; Edwards et al., 1988) but can also be stimulated by cardiac transmural pressure as well as various hormone stimuli (Lachance et al., 1986; Stasch et al., 1989; Soualmia et al., 1997), such as endothelin, Ang 1-9, and Ang 1-7. Although BNP was originally isolated from the brain and is commonly referred to as brain NP, it is predominantly expressed in the ventricles of the heart, where it is transcriptionally regulated by cardiac wall stretching. Both ANP and BNP plasma levels can be elevated up to 100-fold in patients with heart failure (Cody et al., 1986; Raine et al., 1986; Mukoyama et al., 1991; Maisel et al., 2002; Abassi et al., 2004). In contrast, CNP is found at low levels in the heart, and its plasma levels are generally unchanged during heart failure (Wei et al., 1993). Rather, CNP is expressed at high concentrations in chondrocytes, where it regulates bone growth (Hagiwara et al., 1994). In addition, CNP is believed to be an endothelium-derived hyperpolarizing factor, mediating relaxation in the vascular wall (Villar et al., 2007).
The complexity of the NP system is further increased by the presence of three types of natriuretic peptide receptors (NPRs). The classification and specific roles of these receptors have been extensively reviewed (Pandey, 2005). Briefly, NPR-A [particulate guanylyl cyclase A (pGC-A)] and NPR-B are transmembrane guanylate cyclases and are primarily responsible for the physiologic effects of NPs. ANP and BNP activate NPR-A and CNP activates NPR-B, leading to the production of second-messenger cGMP. NPR-C serves as a clearance receptor for all three peptides indiscriminately, mediating NP internalization followed by lysosomal degradation. In addition to receptor-mediated clearance, all three NPs are cleared rapidly from the extracellular matrix by NEP (Potter, 2011), a glycosylated neutral zinc endopeptidase expressed at high levels in the proximal tubule cells of the kidney.
Because of the counter-regulatory actions of the NPs on detrimental Ang II/AT1R effects, augmentation of the NP system has been explored as an additional therapeutic strategy for the treatment of hypertension and cardiovascular disease. Intravenous administration of recombinant forms of ANP and BNP can improve the clinical status of patients with heart failure (Colucci et al., 2000; Suwa et al., 2005; Hata et al., 2008; O’Connor et al., 2011), but increased rates of hypotension and short half-lives have restricted their routine clinical use. NP analogs (M-ANP, cenderitide-NP, and PL-3994 (Hept-cyclo(Cys-His-Phe-d-Ala-Gly-Arg-d-Nle-Asp-Arg-Ile-Ser-Cys)-Tyr-[Arg mimetic]-NH(2)), which are more resistant to enzymatic degradation and act as NPR agonists, are currently undergoing clinical testing. The other approach investigated extensively to increase circulating NP levels is NEP inhibition. Under normal conditions, NPR-C and NEP make similar contributions to NP clearance (Okolicany et al., 1992; Charles et al., 1996); however, in pathologic conditions, in which NP levels are elevated and clearance receptors may be saturated, NEP plays a more significant role and inhibition of NEP is sufficient to elevate NP levels (Hashimoto et al., 1994). Nevertheless, despite the successful development of potent NEP inhibitors effective at increasing NP levels, NEP inhibition has only proved useful for blood pressure control and cardiovascular function when combined with inhibition of the RAS (reviewed in Campbell, 2017) and is discussed in more detail below.
D. The Endothelin System
The endothelin system (Fig. 1) functions together with the RAS to maintain blood pressure and vascular tone. Preproendothelin-1 is a precursor of proendothelin-1, produced largely by endothelial cells, and is processed by furan convertase to the 38-amino-acid peptide big endothelin-1 (Itoh et al., 1988; Denault et al., 1995). Big endothelin-1 is then cleaved by another zinc metallopeptidase, ECE-1 (Takahashi et al., 1993), to produce endothelin-1 (ET-1), a 21-amino-acid vasoactive peptide (Yanagisawa et al., 1988; Kimura et al., 1989) that mediates its potent vasoconstrictor actions via the GPCRs endothelin receptors A and B (ETAR and ETBR, respectively) (Jandeleit-Dahm, 2006; Motte et al., 2006), which, much like the angiotensin receptors AT1R and AT2R, exert opposing effects on vasoregulation and cell growth. ETAR mediates the predominant responses associated with pathologic conditions, including potent prolonged vasoconstriction as a result of irreversible ET-1 binding (Rubanyi and Polokoff, 1994; Kedzierski and Yanagisawa, 2001). In contrast, endothelial ETBR mediates nitric oxide–mediated vasorelaxation and functions as a clearance receptor, facilitating removal of ET-1 from the circulation for lysosomal degradation (Bremnes et al., 2000). There are multiple complex interactions between the RAS and the endothelin systems, including a positive dual-feedback system: Ang II increases expression of preproendothelin-1 mRNA and functional ECE-1 activity, leading to an increase in ET-1 levels (Imai et al., 1992; Barton et al., 1997; Rossi et al., 1999). However, in some clinical conditions such as preeclampsia, ET-1 activation was associated with reduced levels of renin and aldosterone and increased mean arterial pressure (Verdonk et al., 2015). The natriuretic and endothelin systems are also linked by a feedback mechanism whereby ET-1 stimulates the release of NPs, which in turn suppress the actions of the endothelin system (Stasch et al., 1989). ET-1 is degraded by NEP (Vijayaraghavan et al., 1990); thus, the vasodilatory effect of elevated NP levels caused by NEP inhibitors may be counter-regulated by increased levels of ET-1.
The endothelin system has been a target for therapeutic intervention due to its pathophysiological role in hypertension, pulmonary arterial hypertension, heart failure, renal disease, and diabetes. ETAR/ETBR and selective ETAR antagonists, such as bosentan, macitentan, and ambrisentan, are used clinically for the treatment of pulmonary arterial hypertension, but their use is associated with side effects, including edema, anemia, increased risk of heart failure, and hepatic transaminitis (Wei et al., 2016; Packer et al., 2017). Based on the dual-feedback system linking the endothelin system and the RAS, dual AT1R/ETAR antagonists have been developed and tested in the clinic for pulmonary arterial hypertension, essential hypertension, and chronic kidney disease (Murugesan et al., 2002, 2005; Neutel et al., 2008; Komers and Plotkin, 2016; Komers et al., 2017); however, it is still to be established whether the protective benefits outweigh the risk of adverse reactions. ECE-1 inhibitors as well as dual ECE-1/NEP inhibitors have also been developed but showed poor efficacy in humans (Dickstein et al., 2004).
E. General Biochemical Features of Vasoactive Peptide Hormone Cascades
Biochemical processes involved in the generation of vasoactive hormones and peptides are complex and involve the mechanisms of hormone formation and secretion. For the peptide cascades addressed in this article, two distinct mechanisms that can be described in terms of stoichiometric relations and localization of the corresponding precursor hormones. Whereas endothelins and NPs are typically generated by intracellular processing or membrane-bound enzymes locally in tissues (Russell and Davenport, 1999), angiotensins and bradykinins are derived from primarily liver-secreted precursor peptides that are abundantly present in plasma, serving as a virtually inexhaustible source for the formation of active hormone molecules throughout the body. Angiotensinogen levels in human plasma range between 50 and 150 µg/ml (1–3 µM); women have much higher plasma angiotensinogen concentrations, especially during pregnancy (Verdonk et al., 2015). Plasma concentrations for the bradykinin precursors, low molecular weight and high molecular weight kininogen, were reported to be in the low micromolar range (Kleniewski, 1979; Lalmanach et al., 2010). Therefore, plasma concentrations of both the bradykinin and the angiotensin precursor are more than 100,000-fold higher than plasma concentration for BK 1-9 and Ang I, which are reported to be in the low picomolar range. This allows for virtually unlimited hormone synthesis within the plasma compartment. However, formation of bradykinin and angiotensin in the circulation is determined by the tightly regulated concentration and activity of the enzymes kallikrein and renin. Ang I and BK 1-9 are continuously produced by plasma renin and kallikrein throughout the body while being simultaneously converted to other downstream metabolites by a variety of soluble proteases including ACE and aminopeptidases, representing the major metabolic pathways in human plasma. Although the enzymatic composition of plasma is similar throughout the body, local peptide hormone levels can be different due to tissue expression of enzymes and receptors producing, converting, or binding certain peptide metabolites and thereby modifying the baseline peptide hormone profile that is established by intrinsic peptide formation within the plasma compartment.
Drugs interfering with proteases involved in hormone metabolism directly affect formation and degradation rates of peptide products and substrates. Depending on the site of target expression, pharmacodynamic effects might be seen in plasma or limited to tissue sites, which requires careful selection of analytic approaches when aiming to establish relationships between pharmacodynamics and physiologic effects.
III. Therapeutic Targets of the Renin-Angiotensin System and Associated Pathways
ACE inhibitors, the first drugs targeting the RAS, have been used effectively for the treatment of a wide range of indications related to hypertension, cardiovascular disease, and renal disease for over 30 years. Since the discovery of the first ACE inhibitors and later the development of ARBs, there has been growing interest in the development of inhibitors that target other structurally related vasopeptidases as well as other receptors for vasoactive peptides. Driven largely by the success of RAS blockade but also the observation that suppression of the RAS does not, in many cases, lead to an adequate reduction in blood pressure, extensive effort has gone into developing therapies that target multiple vasoactive pathways controlling blood pressure and cardiovascular function.
A. Angiotensin-Converting Enzyme
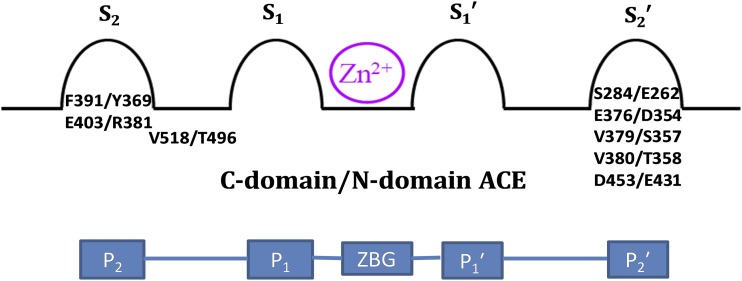
ACE (also known as peptidyl dipeptidase; EC 3.4.15.1) is a type I membrane-anchored zinc dipeptidyl carboxypeptidase responsible for the cleavage of a diverse set of substrates, including angiotensin peptides, bradykinin, substance P, and gonadotropin-releasing hormone or luteinizing hormone-releasing hormone. ACE exists as two isoforms, somatic ACE and testes ACE, that are transcribed from the same gene in a tissue-specific manner. Somatic ACE is a 1227-amino-acid protein that is expressed widely, particularly in endothelial and epithelial cells. Testes ACE is a smaller form consisting of 701 amino acids expressed only in sperm cells. Both isoforms consist of a heavily glycosylated ectodomain that can be shed from the membrane. Plasma ACE is derived from endothelial ACE by shedding a 1203-amino-acid isoform of 150–180 kDa. The concentration of ACE in human plasma is reported to range between 36 and 288 ng/ml (260–2076 pM), which is an almost 200-fold molar excess compared with Ang I, its major substrate within the RAS (Fagyas et al., 2014). Despite this excess of enzyme over its substrate, soluble ACE likely has limited impact on tissue Ang II levels, which might be more dependent on the local conversion of Ang I to Ang II by endothelial ACE in direct proximity to AT1R (Danser et al., 2007). The ectodomain of somatic ACE consists of two homologous catalytically active domains, the N and C domains, whereas the ectodomain of testes ACE consists of only the C domain (Soubrier et al., 1988). Crystal structures of individual N and C domains reveal that they are ellipsoid in shape and mostly α-helical (Natesh et al., 2003; Corradi et al., 2006). The catalytic zinc ion is buried deep in the active-site cavity and a chloride ion is typically observed at each of the two chloride binding sites. The active site contains the conserved HEXXH zinc binding motif, containing the two histidine residues that coordinate the zinc ion together with a conserved glutamate residue and a water molecule that is displaced upon ligand coordination (Williams et al., 1994). The N and C domains display distinct but overlapping substrate specificity and physiologic functions, differences in chloride dependence, and distinct glycosylation patterns (Wei et al., 1992; O’Neill et al., 2008). Both domains catalyze the degradation of bradykinin with similar efficiency and both N-domain and C-domain knockout mice show similar bradykinin plasma levels as wild-type mice (van Esch et al., 2005; Bernstein et al., 2011), suggesting that bradykinin cleavage by one domain can effectively compensate for the absence of the other domain. In contrast, the C domain is the primary site for Ang II formation and is essential and sufficient for controlling blood pressure in vivo (Junot et al., 2001; van Esch et al., 2005; Fuchs et al., 2008; Bernstein et al., 2011; Burger et al., 2014). The N domain is the primary site for the clearance of the tetrapeptide N-acetyl-Ser–Asp–Lys–Pro (Ac-SDKP) (Azizi et al., 1999; Junot et al., 2001; Fuchs et al., 2004), a potent anti-inflammatory and antifibrotic peptide. Consequently, it may be desirable to selectively target individual domains, and several domain-specific residues within the active site have been identified that are important for conferring domain selectivity (Fig. 4) (Watermeyer et al., 2008, 2010; Kröger et al., 2009). To date, there are more than 30 structures of the individual domains in complex with a variety of peptides and inhibitors.
Fig. 4.
A schematic diagram of ACE active sites [Schechter and Berger nomenclature (Schechter and Berger, 1967)] showing the subsite binding pockets accommodating the residues on either side of the ZBG of peptide substrates. ACE domain-specific amino acid residues important for conferring domain selectivity are shown within the relevant subsites of the ACE active site.
B. Angiotensin-Converting Enzyme 2
Another important peptidase in the RAS is ACE2 (also known as ACE-related carboxypeptidase; EC 3.4.17.23), a type I membrane-anchored zinc carboxypeptidase (Donoghue et al., 2000; Tipnis et al., 2000). ACE2 cleaves multiple substrates including vasoactive peptides involved in the pathology of cardiovascular disease. ACE2 converts Ang II to Ang 1-7 by removing the C-terminal phenylalanine residue (Tipnis et al., 2000; Vickers et al., 2002) and is thus a major component of the counter-regulatory axis of the RAS (Santos et al., 2013, 2018). ACE2 also acts on Ang I to produce Ang 1-9, albeit with lower efficiency. This ACE2-dependent formation of Ang 1-9 is particularly relevant during ACE inhibition, where Ang I is abundantly present as a substrate in humans and ACE2 treatment might result in a profound increase of Ang 1-9 (Basu et al., 2017). In addition to these angiotensin peptide substrates within the RAS, it is very likely that Ang III and Ang IV, sharing their C terminus with the preferred ACE2 substrate Ang II, serve as additional ACE2 substrates, but this still needs to be demonstrated in vivo. Other ACE2 substrates include des-Arg9-bradykinin, apelin-13, and dynorphin A-(1-13) (Vickers et al., 2002); in addition to its catalytic functions, ACE2 also has noncatalytic functions, acting as a functional receptor for the coronavirus that causes severe acute respiratory syndrome (Li et al., 2003, 2005) and playing a role in amino acid transport (Kowalczuk et al., 2008).
ACE2 is predominantly localized on endothelial cells and is widely expressed within tissues, including the heart, kidneys, testes, brain, intestine, and lungs (Tipnis et al., 2000). ACE2 is an 805-amino-acid protein with a single catalytic domain that shares ∼60% and ∼40% sequence identity with the N and C domains of somatic ACE, respectively. The transmembrane region and cytoplasmic tail of ACE2 is distinct from ACE, sharing close homology with collectrin, a molecular chaperone of a renal amino acid transporter B0AT1 (Danilczyk et al., 2006) and ACE2 indeed acts as a chaperone for the same amino acid transporter in the small intestine where collectrin is not expressed (Kowalczuk et al., 2008). Like ACE, the glycosylated ectodomain of ACE2 is shed from the membrane and released into circulation: ACE2 is shed by the disintegrin and metalloprotease ADAM 17 (Lambert et al., 2005), although the enzymes responsible for ACE shedding have not yet been identified.
In humans, circulating ACE2 is not detectable in healthy individuals and its presence is highly associated with cardiovascular risk factors. In a study involving 534 healthy subjects, ACE2 activity was detectable in 40 subjects only, whereas soluble ACE2 levels were below the assay detection limit of 2.7 pM in the remaining 494 subjects. The 40 subjects having mean ACE2 levels of 33.0 pM showed a stronger exposure to cardiovascular risk factors including abdominal adiposity, hypertension, and elevated fasting glucose and lipid levels (Rice et al., 2006). Serum ACE2 activity is increased in patients with heart failure while correlating with the severity of heart failure (Epelman et al., 2008) and was reported to predict the combined clinical endpoint of all-cause mortality, heart failure hospitalization, and heart transplantation in a cohort of 113 stable patients with chronic systolic heart failure (Epelman et al., 2009).
The catalytic domain of ACE2 consists of two subdomains linked together by a hinge region. Comparison of inhibitor-bound and free X-ray structures reveals that a hinge-bending motion, resulting in changes to the relative position of the subdomains, is important for catalysis (Towler et al., 2004). These structures have also revealed insights into the observed selectivity of ACE2 relative to ACE, showing that a single amino acid substitution in ACE2 hinders the S2′ subsite. This explains why ACE2 acts as a carboxypeptidase rather than a peptidyl dipeptidase like ACE and why conventional ACE inhibitors such as lisinopril and captopril do not inhibit ACE2. Structure-based methods have been used to develop allosteric ACE2 activators by exploiting conformational differences observed in ACE2 crystal structures (Hernández Prada et al., 2008; Gjymishka et al., 2010). These activators bind to surface-binding pockets in the hinge region, locking the protein in an active conformation. ACE2 activators have shown antihypertensive and cardioprotective effects in a range of rodent models (Santos et al., 2018). Other approaches to increase ACE2 activity, with the aim of activating the ACE2/Ang 1-7/Mas axis, have included viral overexpression of ACE2 (Grobe et al., 2007) and oral or intravenous administration of recombinant ACE2 (Shenoy et al., 2014) (discussed in more detail in section VII). In addition to its effect on alternative RAS activation, ACE2 efficiently degrades Ang II, which in turn reduces the detrimental effects of Ang II/AT1R signaling, explaining why recombinant ACE2 has shown efficacy in many Ang II infusion models. Importantly, understanding the dynamics of the RAS in response to ACE2 administration or activation in vivo as well as the crosstalk of ACE2 with other pharmacologic treatments targeting the RAS may be of major importance to achieve therapeutic efficacy in complex pathologic settings in vivo.
C. Neprilysin
NEP (also known as neutral endopeptidase 24.11, enkephalinase, or CD10; EC 3.4.24.11) is a type II membrane-anchored zinc-dependent endopeptidase originally purified from the brush borders of rabbit kidneys (Kerr and Kenny, 1974). This widely expressed enzyme is tethered to the cell surface and has a large C-terminal extracellular catalytic domain responsible for the cleavage of a variety of physiologically active peptides including NPs, Ang I, Ang II, bradykinin, ET-1, adrenomedullin, enkephalins, substance P, insulin, gastrin, and amyloid-β peptide (Malfroy et al., 1978; Roques et al., 1980, 1993; Erdös and Skidgel, 1989; Turner and Tanzawa, 1997; Iwata et al., 2001; Shirotani et al., 2001).
Several crystal structures of the soluble ectodomain in complex with various inhibitors have provided insight into the structure and specificity of NEP (Oefner et al., 2000, 2004, 2007; Sahli et al., 2005; Glossop et al., 2011; Schiering et al., 2016). The ectodomain consists of two α-helical lobes linked by interlacing polypeptide chains. The large lobe is structurally similar to zinc-dependent bacterial endopeptidases such as thermolysin, and it contains the catalytic zinc binding motif HEXXH and other conserved motifs and residues involved in zinc coordination, catalysis, and ligand binding (Oefner et al., 2000). The smaller lobe, absent in related bacterial enzymes, acts as a molecular sieve, limiting the size of ligands to about 3000 Da (Oefner et al., 2000). NEP has a large flexible active site with broader substrate specificity than ACE. The prime side of the binding pocket is primarily responsible for substrate potency and selectivity. The S1′ pocket displays the most stringent specificity and preferentially binds aromatic or other large hydrophobic groups (Llorens et al., 1980; Roques et al., 1980). The large S2′ subsite, extending into the solvent region, has broader specificity. There is fluidity between the S1′ and S2′ subsites, with the side chains of residues dividing the two pockets shifting to accommodate large groups at either site. Consequently, however, the simultaneous binding of large groups at both subsites is unfavorable and would require a substantial induced fit requiring backbone motion (Oefner et al., 2004).
D. Endothelin-Converting Enzyme-1
ECE-1 (EC 3.4.24.71), named for its role in the hydrolysis of endothelins, is widely distributed in mammalian tissue, with particularly high levels of expression in the cardiovascular, reproductive, and endocrine systems (Korth et al., 1999). ECE-1 belongs to the same family of proteins as NEP and their ectodomains have overlapping specificity and a high degree of structural similarity, with an overall sequence identity of 40% (Bur et al., 2001). There is one crystal structure of ECE-1 available, which shows phosphoramidon bound within the active site (Schulz et al., 2009), revealing that the NEP and ECE-1 active sites share a high degree of conservation. Structure–activity relationship (SAR) studies on a series of phosphinic inhibitors by Jullien et al. (2010) revealed the following differences in ECE-1 and NEP specificity: 1) ECE-1 can tolerate a bulky group at the S1′ and S2′ sites, whereas NEP can only tolerate a bulky group at one of these sites; and 2) ECE-1 can tolerate a stereocenter in the S or R configuration at Cα in the P1′ position, whereas NEP can only tolerate a stereocenter in the S configuration at this position.
E. Aminopeptidase A
Aminopeptidase A (APA; EC 3.4.11.7) is a 160-kDa homodimeric type II membrane-bound monozinc aminopeptidase. APA hydrolyzes the N-terminal glutamate or aspartate residue from peptidic substrates such as Ang II or cholecystokinin-8 in vitro (Nagatsu et al., 1970; Healy and Wilk, 1993) and in vivo in the brain (Migaud et al., 1996; Zini et al., 1996) and its activity is enhanced by Ca2+ (Glenner et al., 1962). APA is expressed in various tissues such as the intestinal and renal brush border epithelial cells and vascular endothelium and within the brain (Lojda and Gossrau, 1980). This enzyme has also been identified in several brain nuclei involved in the control of body fluid homeostasis and cardiovascular functions (Zini et al., 1997). Using the crystal structure of leukotriene-A4 hydrolase (EC 3.3.2.6) (Thunnissen et al., 2001) as a template and functional information collected from site-directed mutagenesis studies on APA, a three-dimensional (3D) model of the mouse APA ectodomain from residues 79 to 559, including the active site of the enzyme, was built (Rozenfeld et al., 2002). In this model, the zinc atom is coordinated by the two histidine residues (His 385 and His 389) of the consensus sequence HEXXH, Glu 408, and a water molecule (Wang and Cooper, 1993; Vazeux et al., 1996). Analysis of the APA 3D model complexed with an APA inhibitor, 4-amino-4-phosphobutyric acid (GluPO3H2) (Lejczak et al., 1993) showed that Tyr 471 is involved in transition state stabilization (Vazeux et al., 1997). The model also demonstrated an interaction between the N-terminal amine of GluPO3H2 and two glutamate residues of APA: Glu 352 in the GAMEN motif conserved among monozinc aminopeptidases and Glu 215, which is responsible for APA exopeptidase specificity (Vazeux et al., 1998; Rozenfeld et al., 2003). Ca2+ was then introduced into the 3D model of APA and was localized at the bottom of the S1 subsite where it interacts with the acidic side chains of Asp 213 and Asp 218, ensuring acidic APA substrate specificity (Goto et al., 2007; Claperon et al., 2008). The crystal structure of human APA (residues 76–956) was recently resolved (Yang et al., 2013) and a comparison of this structure with the 3D homology mouse APA model showed a perfect overlap for the APA active site and the same structural organization of the S1 subsite. The S1 subsite of APA displays the most stringent specificity and was optimally blocked by an acidic amino-acid residue such a glutamate, leading to the development of the first specific and selective APA inhibitor, EC33 [(3S)-3-amino-4-sulfanyl-butane-1-sulfonic acid] (Chauvel et al., 1994). The S1′ subsite is hydrophobic, whereas the S2′ subsite preferentially recognizes negatively charged residues derived from aspartic acid, leading to the design of APA inhibitors with subnanomolar inhibitory potency (David et al., 1999).
F. Angiotensin II Receptors
AT1R and AT2R are members of the seven-transmembrane domain superfamily of GPCRs and have a 34% nucleic acid sequence homology. The single AT1R gene in humans is located on chromosome 3 and encodes a 359-amino-acid protein. In rodents, however, there are two subtypes, AT1R a and AT1R b (located on chromosomes 17 and 2, respectively), which are highly conserved in the coding region (Sandberg et al., 1992). AT1R is widely expressed and well conserved between species (de Gasparo et al., 2000). Ang II activates a number of signaling pathways, such as G protein–mediated (Gq and Gi), Janus kinase/signal transducers and activators of transcription, and mitogen-activated protein kinase or extracellular signal-regulated kinase pathways, causing hypertension, endothelial dysfunction, vascular remodeling, and end organ damage. In addition, there is G protein–independent signaling through the adapter proteins β-arrestin 1 and β-arrestin 2 that can have distinct functional and physiologic consequences (Rajagopal et al., 2010). AT1R conformations stabilized by β-arrestin–biased peptide agonists differ from Ang II–induced conformations. These agonists have had a significant impact on AT1R pharmacology and alter the intracellular trafficking of the receptor in addition to the activation of the β-arrestin–mediated signaling pathway (Namkung et al., 2016).
AT1R forms homo- and heterodimers with other GPCRs and many of these dimers have been linked to altered ability to activate G protein and/or β-arrestin (AbdAlla et al., 2000; Hansen et al., 2004; Tóth et al., 2018). Ang II and Ang III have a similar binding affinity for AT1R and AT2R, and thus the expression of these receptors regulates which receptor subtype mediates responses to Ang II and Ang III (Rabey et al., 2010; Bosnyak et al., 2011). Moreover, crosstalk between AT1R and AT2R results in stimulation of one receptor modulating the expression of the other (AbdAlla et al., 2001).
The high-resolution crystal structure of human AT1R in complex with its selective antagonist ZD7155 [5,7-Diethyl-3,4-dihydro-1-[[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl]-1,6-naphthyridin-2(1H)-one] (precursor to the antihypertensive candesartan) has provided fundamental insights into the structure–function relationship of the receptor (Zhang et al., 2015). Surprisingly, three residues (Arg 167, Tyr 35, and Trp 84) that have not previously been shown to make interactions with ligands made important contacts with the antagonist. In addition, mutagenesis and docking studies revealed residues that were critical for peptide and nonpeptide binding. Exploitation of these interactions paves the way for new mechanistic studies and therapeutic strategies.
The AT2R gene, located on human chromosome X, encodes the 363-amino-acid GPCR with a molecular mass of 41 kDa (Kambayashi et al., 1993). In contrast to AT1R, the activation of phosphatases such as mitogen-activated protein kinase phosphatase 1 and protein phosphatase 2 is an important signaling mechanism for AT2R (Kang et al., 1995; Yamada et al., 1996). Furthermore, AT2R couples to Gi proteins and stimulates ion channel currents (Kang et al., 1994). The crystal structures of the AT2R in complex with AT2R and AT1R/AT2R ligands showed helix 8 in a noncanonical position that stabilizes the active state of the GPCR. Moreover, interaction of H8 with H5 and H6 prevented binding of G proteins and β-arrestins, providing a molecular basis for its alternative G-protein signaling (Zhang et al., 2017). Although the signaling mechanism of AT2R is not fully understood, there are similarities between the AT2R and Mas signaling, such as the involvement of SH2-containing protein tyrosine phosphotases SHP-1/SHP-2 and phosphoinositide 3-kinase/AKT/endothelial nitric oxide synthase (Seguin et al., 2012). In addition, AT2R and Mas can form heterodimers and Ang 1-7 effects are blocked by AT2R antagonists. Physiologically, the levels of AT2R expression are low; however, expression levels have been found to be higher during development, disappearing in adult rats except in the brain, ovary and uterus (Cook et al., 1991; Millan et al., 1991; Pucell et al., 1991; Song et al., 1992; Lenkei et al., 1997), and liver and kidney of rats compared with those in neonates (Yu et al., 2010). Gene expression of AT2R is regulated by numerous factors, including intracellular calcium and protein kinase C (Kijima et al., 1996), interleukin-1β and insulin (Kambayashi et al., 1996), and multiple growth factors (Ichiki et al., 1995). AT2R mediates a variety of protective actions such as immune modulation and antifibrotic, anti-inflammatory, neuroprotective, neuroregeneration, antihypertensive, and antiapoptotic actions (Namsolleck et al., 2014; Steckelings et al., 2017). Thus, a number of AT2R agonists have been developed for therapeutic intervention and will provide important information about the future prospect of drugs targeting the AT2R (Santos et al., 2019).
G. Mas Receptor
Mas (also called MAS1 proto-oncogene) was the first identified member of the Mas-related GPCR subfamily of proteins and consists of seven transmembrane domains typical of GPCRs (reviewed in Bader et al., 2014, 2018). It was first described as an oncogene, based on a human oncogene assay in which the human MAS gene was purified from a tumor that was induced in a nude mouse by injecting the animal with NIH 3T3 cells cotransfected with DNA purified from a human tumor (Young et al., 1986). However, further studies showed that Mas can only transform cells when artificially overexpressed (Rabin et al., 1987; van’t Veer et al., 1993), suggesting that it is not an oncogene as initially reported. Mas was originally proposed to be the functional receptor for Ang II (Jackson et al., 1988), but this was also later disproved by subsequent signaling experiments (Ambroz et al., 1991), cloning of AT1R (Murphy et al., 1991; Sasaki et al., 1991), and the discovery that Mas and AT1R interact directly, explaining the indirect involvement of Mas in Ang II signaling (Kostenis et al., 2005). In 2003, Mas was identified as the functional receptor of Ang 1-7 responsible for the beneficial physiologic effects of Ang 1-7 (Santos et al., 2003), making it a key component of the counter-regulatory axis of the RAS and a potential target for therapeutic intervention. Subsequent studies provided additional evidence supporting this, showing that the specific labeling of tissues/cell lines by labeled Ang 1-7 is lost in Mas-deficient animals/cells (Tallant et al., 2005; Fraga-Silva et al., 2008; Leal et al., 2009). However, recent extensive biochemical studies were unable to support the direct interaction between Ang 1-7 and Mas (Gaidarov et al., 2018), bringing into question whether Ang 1-7 is indeed the endogenous agonist of this receptor. In addition to Ang 1-7, several putative Mas agonists including AVE 0991 [3-ethyl-1-[3-[4-[(5-formyl-4-methoxy-2-phenylimidazol-1-yl)methyl]phenyl]-5-(2-methylpropyl)thiophen-2-yl]sulfonylurea] (Wiemer et al., 2002) and CGEN-856S [amino acid sequence: FLGYSIYLNRKRRGDPAFKRRLRD] (Pinheiro et al., 2004; Savergnini et al., 2010) and antagonists A-779 (D-Ala7 Ang 1-7) (Santos et al., 1994) and D-Pro7 Ang 1-7 (Santos et al., 2003) have been used to study the actions of Mas, although a rigorous analysis of their Mas binding affinity is lacking. These Mas agonists have shown a range of cardioprotective effects in animal models (reviewed by Bader et al., 2014, 2018). Other endogenous peptides able to act as Mas agonists have also been reported (Jankowski et al., 2011; Tirupula et al., 2014; Yu et al., 2016) and like many other GPCRs, Mas displays biased agonism with different ligands activating different downstream pathways (Bader et al., 2014; Karnik et al., 2015). Potential downstream signaling pathways of Mas stimulated by Ang 1-7 and related analogs include the phospholipase A2 pathway to generate arachidonic acid (Santos et al., 2003) and the phosphoinositide 3-kinase/AKT pathway leading to the activation of endothelial nitric oxide synthase (Sampaio et al., 2007; Lopez Verrilli et al., 2012; Savergnini et al., 2013; Than et al., 2013). Ang 1-7 activation of Mas in glomerular mesangial cells is cAMP dependent and is thought to mediate a protective action in experimental models of renal injury (Liu et al., 2012). Mas-mediated activation of the phospholipase C/Ca2+ signaling pathway has been reported for other agonists, including the endogenous ligand neuropeptide FF, but not for Ang 1-7 (Shemesh et al., 2008; Zhang et al., 2012; Tirupula et al., 2014). Mas is expressed at the highest levels in the brain and testis and has been found at low levels in a wide range of other organs; the functions of Mas and other GPCRs in various tissue was reviewed in Bader et al. (2014). There are currently no high-resolution structures of any of the Mas-related GPCRs.
IV. Targeting Angiotensin-Converting Enzyme, Neprilysin, and Endothelin-Converting Enzyme-1 with Vasopeptidase Inhibitors
The structural similarity between ACE, NEP, and ECE-1 and overlapping substrate specificity has enabled the development of single molecules that target two or even three of these enzymes. Remarkably, the design of current-generation ACE inhibitors as well as vasopeptidase inhibitors that have entered clinical trials to date has been achieved with limited knowledge of the sequences and 3D structures of the enzymes. Rather, the first ACE inhibitors were designed based on the expected functional homology of ACE with carboxypeptidase A (Cushman et al., 1977). Despite this misconception, ACE inhibitors are a successful class of drugs in cardiovascular disease, although failure to appreciate the two-domain structure of ACE has contributed, at least in part, to the adverse event profile of these drugs. Development of NEP inhibitors dates to the 1980s and was largely based on the homology between NEP and the better characterized bacterial metalloendopeptidase thermolysin. Several selective NEP inhibitors have been described, including thiorphan (Roques et al., 1980), ecadotril, candoxatril, and sacubitril, but these inhibitors showed poor efficacy in the clinic (Ando et al., 1995; Cleland and Swedberg, 1998). Although it had been ascertained that NEP inhibition leads to elevated NP, adrenomedullin, and bradykinin levels, which have vasorelaxant, natriuretic, and cardioprotective actions, clinical studies also confirmed that NEP inhibition increased Ang II and ET-1 levels, which possibly counteract the therapeutic effects (Ferro et al., 1998; Weber, 2001; Roksnoer et al., 2015). Given that Ang I is a better NEP substrate than Ang II (Rice et al., 2004), Ang II is increased in the presence of NEP inhibitors primarily by increasing Ang I levels (allowing more Ang I to II conversion by ACE) and secondly by blocking NEP-mediated Ang II degradation. The next progression from this was to establish whether the additional suppression of Ang II production (and later ET-1) would be effective. This was supported by a study demonstrating that combining an NEP inhibitor with an ACE inhibitor reduced blood pressure in hypertensive rats to a greater extent than either inhibitor administered alone (Seymour et al., 1991). A similar result was later reported in humans (Favrat et al., 1995), setting the stage for the development of dual ACE/NEP vasopeptidase inhibitors, a new class of drugs for the treatment of hypertension.
A. Dual Angiotensin-Converting Enzyme/Neprilysin Inhibitors
The dual ACE/NEP inhibitors were the first vasopeptidase inhibitors to enter clinical trials. They were developed to simultaneously block the ACE-mediated formation of the vasoconstrictor Ang II and the NEP-mediated degradation of NP vasodilators. Eleven dual ACE/NEP inhibitors have been tested to varying extents in the clinic (Dimitropoulos et al., 2010). Of these, omapatrilat progressed the furthest but eventually failed to obtain U.S. Food and Drug Administration (FDA) approval after large phase III clinical trials, due to a reported increased risk of angioedema.
The early dual inhibitors were designed rationally based on specific ACE and NEP inhibitors. Combining a P1′ benzyl group, known to be important for NEP inhibition, with a P2′ proline group as seen in the first ACE inhibitors (e.g., captopril) led to a series of potent mercaptoacyl dipeptides with dual inhibitory activity (Robl et al., 1994; Turcaud et al., 1995). Further SAR studies to optimize for in vivo activity led to conformationally restricted dipeptide mimetics and, eventually, omapatrilat, a 7,6-fused bicyclic thiazepinone (Robl et al., 1997). Omapatrilat displayed potent inhibition in the low nanomolar range against both ACE and NEP in vitro, as well as chronic potent antihypertensive and cardioprotective effects in experimental models of hypertension and heart failure (Robl et al., 1997, 1999; Trippodo et al., 1998; Intengan and Schiffrin, 2000; Pu et al., 2002).
Early preliminary clinical data were also promising: tested doses of omapatrilat showed more potent antihypertensive effects than any other drug class tested and appeared to be effective in improving cardiac function in patients with heart failure. However, there were concerns about omapatrilat-associated angioedema. To further study the efficacy and safety of omapatrilat, large randomized clinical trials were undertaken to assess the efficacy and safety profile of omapatrilat in patients with hypertension and heart failure, compared with the conventional ACE inhibitor enalapril (Coats, 2002). The OCTAVE (Omapatrilat Cardiovascular Treatment Assessment Versus Enalapril) trial, including more than 25,000 hypertensive patients, showed antihypertensive efficacy of omapatrilat but, disappointingly, the rate of angioedema was 3-fold higher than observed for enalapril (2.17% vs. 0.68%) and cases of angioedema tended to occur earlier and be more severe in the omapatrilat group (Kostis et al., 2004). OVERTURE (Omapatrilat Versus Enalapril Randomized Trial of Utility in Reducing Events) was carried out in 5770 patients and showed that omapatrilat was as good as, but no better than, enalapril at reducing primary endpoint events in patients with heart failure (Packer et al., 2002). Although the incidence of angioedema was lower in OVERTURE than OCTAVE, the rate of angioedema was still higher in the omapatrilat group (0.8% vs. 0.5%). Based on the OVERTURE and OCTAVE trials, the FDA concluded that the benefits of treating patients with hypertension or heart failure with omapatrilat did not outweigh the risks (Zanchi et al., 2003).
Since both ACE and NEP contribute to the breakdown of bradykinin, and bradykinin accumulation is associated with angioedema (Fox et al., 1996; Molinaro et al., 2002), it was thought that the increased risk of this serious, potentially life-threatening complication would also affect other dual ACE/NEP inhibitors, halting the development of this once-promising class of drugs. Although it is conceivable that changing the relative levels of ACE and NEP inhibition could improve the efficacy and safety profiles of these dual inhibitors, and some promising early-stage clinical trials of other inhibitors were reported in the years after the omapatrilat studies (Azizi et al., 2006; Johnson et al., 2006), large-scale trials of other inhibitors have not been conducted. It is also worth noting that omapatrilat had off-target effects that may have contributed to its poor side effect profile, which could differ from off-target effects of other inhibitors in this class. After omapatrilat’s failure in the clinic, it was discovered that omapatrilat inhibits a third zinc metallopeptidase involved in bradykinin inactivation, APP (Ki of 0.25 µM) (Sulpizio et al., 2005; Fryer et al., 2008). Fryer et al. (2008) showed that bradykinin is degraded in rats with an enzyme rank efficacy of ACE > APP >> NEP, suggesting that APP inhibition may contribute significantly to the increase in kinin-mediated side effects observed for omapatrilat. More recent enzyme and structural data has confirmed that omapatrilat is a nonselective potent inhibitor of both ACE domains, interacting with conserved residues within the N and C domain active sites (Cozier et al., 2018). Dual ACE/NEP inhibitors also result in the elevation of other peptides such as ET-1 which, like bradykinin, increase endothelial nitric oxide levels, which may also contribute to angioedema and other adverse effects, including flushing.
B. Dual Neprilysin/Endothelin-Converting Enzyme-1 Inhibitors
Dual NEP/ECE-1 inhibitors were also explored to increase the efficacy of NEP inhibition: the inhibition of ECE-1 prevents the formation of ET-1, thereby avoiding the accumulation of ET-1 observed during the inhibition of NEP, the primary enzyme responsible for ET-1 degradation (Fig. 1). The discovery that phosphoramidon inhibited ECE-1 in addition to NEP initiated the development of other NEP/ECE-1 dual inhibitors (Xu et al., 1994; Kukkola et al., 1995). However, the most advanced dual ECE-1/NEP inhibitor daglutril (SLV 306), despite effectively elevating plasma NP and big ET-1 levels in a dose-dependent manner, was ineffective at lowering systemic blood pressure in clinical studies (Dickstein et al., 2004), suggesting that effective antihypertensive treatment must incorporate blockade of the RAS.
C. Triple Angiotensin-Converting Enzyme/Neprilysin/Endothelin-Converting Enzyme-1 Inhibitors
Another strategy is to simultaneously block the RAS, NP degradation, and ET-1 formation with triple ACE/NEP/ECE-1 inhibitors. These inhibitors are expected to show improved efficacy over dual inhibitors, reducing the need for polypharmacy, but once again safety issues are a concern. The most extensively studied triple vasopeptidase inhibitor, CGS-35601 [L-tryptophan, N-[[1-[[(2S)-2-mercapto-4-methyl-1-oxopentyl]amino]-cyclopentyl]carbonyl]], is an α-mercaptodipeptide with a central cyclic non-natural amino acid and a P2′ tryptophan that is accommodated in the S2′ site of all three enzymes. CGS-35601 showed good efficacy in various rat models of hypertension; in addition, although treatment resulted in significant accumulation of bradykinin, nitric oxide levels were substantially reduced compared with treatment with omapatrilat (Daull et al., 2005, 2006b). It is yet to be shown whether the decrease in plasma ET-1 concentration and associated reduction in nitric oxide release can compensate for elevated bradykinin levels. Even though preclinical testing of CGS-35601 in rats showed no toxic effects (Daull et al., 2006a), no triple vasopeptidase inhibitors have yet been tested in humans. The effect of these broad-spectrum inhibitors on vasopeptide levels will need to be carefully evaluated due to the complexity of these interconnected pathways. Off-target effects will also need to be minimized to ensure that the activity of additional enzymes involved in kinin inactivation, such as APP and carboxypeptidase N, is not affected.
Adverse reactions associated with ACE inhibitors and vasopeptidase inhibitors are likely due to undesired effects on peptide levels besides Ang II, particularly bradykinin, but other peptides such as ET-1, substance P, and so forth may also contribute. Adverse effects occur in up to 28% of patients (Steckelings et al., 2001; Weber and Messerli, 2008), which is astounding considering that ACE inhibitors have been routinely used to treat large numbers of patients for decades. Although current evidence suggests that vasopeptidase inhibitors are potentially more effective than conventional ACE inhibitors, the safety profile remains a concern.
ACE inhibitors and vasopeptidase inhibitors that target both the N and C domains of ACE were tested clinically prior to knowledge of the different roles of these ACE domains. Now that it is well established that the C domain is predominantly responsible for Ang II formation in vivo and that both domains inactivate bradykinin at a similar rate, selectively inhibiting the C domain has the potential to reduce the accumulation of bradykinin levels and other peptides cleaved by the N domain during ACE inhibitor treatment.
Multiple crystal structures of inhibitors in complex with the individual ACE N and C domains, NEP, and ECE-1 have provided molecular insights into enzyme specificity and function. This, together with increased knowledge of the integrated network between the RAS, NPS, kallikrein-kinin system, and endothelin system and several decades of SAR studies on these enzymes, provides a strong foundation for the design of next-generation inhibitors.
V. Angiotensin-Converting Enzyme C-Domain–Selective Vasopeptidase Inhibitors
A. C-Domain–Selective Angiotensin-Converting Enzyme Inhibitors
Knowledge of the ACE sequence and 3D structures of the individual domains has facilitated the development of both N- and C-domain–selective ACE inhibitors. N-domain–selective inhibitors may prove useful for indications such as fibrosis, where it would be beneficial to inhibit N-domain–specific Ac-SDKP formation without affecting blood pressure (Dive et al., 1999; Douglas et al., 2014; Fienberg et al., 2018). In vitro mutagenesis studies, in which C-domain–specific residues are systematically mutated to their N-domain counterparts (or vice versa), have provided valuable information on residue–inhibitor interactions important for conferring domain selectivity (Watermeyer et al., 2008; Kröger, et al., 2009; Watermeyer et al., 2010) (Fig. 4).
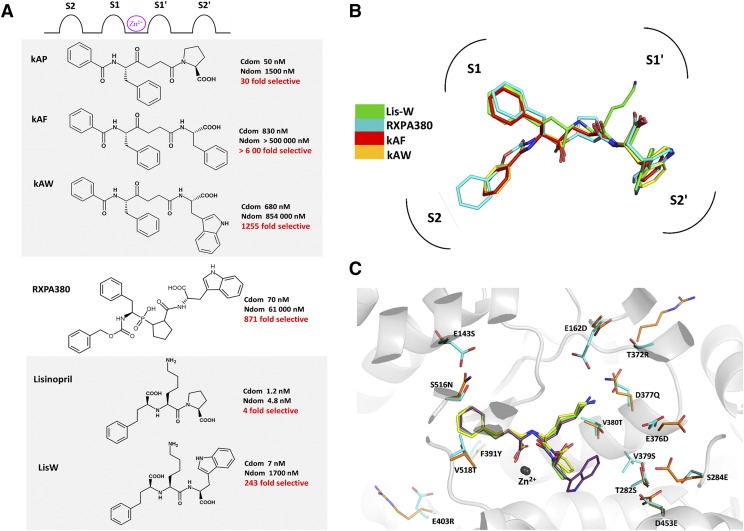
Several inhibitors, typically derivatives of nonselective ACE inhibitors, have been developed that show greater than two orders of magnitude selectivity for the C domain in vitro. These compounds include the ketomethylene inhibitors kAW and kAF (Nchinda et al., 2006b; Watermeyer et al., 2008) derived from the moderately C-selective compound kAP (Almquist et al., 1980; Deddish et al., 1998), phosphinic inhibitor RXPA380 (Georgiadis et al., 2003; Kröger, et al., 2009), and carboxylic inhibitor LisW (Nchinda et al., 2006a; Watermeyer et al., 2010), derived from the conventional inhibitor lisinopril (Fig. 5). Crystal structures of these inhibitors in complex with the C domain reveal that a bulky hydrophobic residue that binds to the S2′ pocket is a common feature of these inhibitors, typically conferring ∼30- to 70-fold of the observed C-domain selectivity (Corradi et al., 2007; Watermeyer et al., 2008, 2010). Mutational data suggest that cooperative effects of a number of C-domain–specific residues within the S2′ subsite contribute to the selectivity of these compounds, but additional residues in other subsites also play a role. Several bradykinin-potentiating peptides, the first compounds identified for their antihypertensive properties, also display C-domain selectivity (Cotton et al., 2002). The structures of the most selective bradykinin-potentiating peptide, BPPb, in complex with the C domain (Masuyer et al., 2012) and N domain, together with mutagenesis studies, have provided a structural basis for the selectivity of these peptides (Sturrock et al., 2019), providing additional insights for the design of selective inhibitors.
Fig. 5.
(A) Chemical structures of C-domain–selective inhibitors and the corresponding in vitro inhibition constants for the N and C domains. (B) Overlay of C-domain–selective inhibitors bound to the active site of the C domain from crystal structures [PDB codes 3BKK (kAF), 3BKL (kAW), 2OC2 (RXPA380), and 3L3N (LisW)]. (C) Overlay of crystal structures of ACE N and C domains in complex with lisinopril is shown in yellow (N domain) and green (C domain) (PDB codes 2C6N and 1O86 respectively) and the ACE C domain in complex with LisW in purple (PDB code 3L3N). C-domain unique residues are shown in cyan with corresponding N-domain residues in orange. Cdom, C domain; Ndom, N domain; PDB, Protein Data Bank.
Ex vivo and in vivo studies with LisW, the most extensively studied C-domain–selective inhibitor, have further confirmed that C-domain–selective inhibition is pharmacologically relevant, resulting in unique vasopeptide metabolism profiles compared with nonselective ACE inhibitors. A study in hypertensive mice that express active human renin showed that LisW reduced blood pressure and Ang II levels similarly to lisinopril without increasing bradykinin levels (Burger et al., 2014). Another study in rat myocardial infarction determined the pharmacodynamic effects of LisW on angiotensin metabolites and Ac-SDKP levels (Sharp et al., 2015). Lisinopril, but not LisW, decreased Ang 1-5/Ang 1-7 ratios and Ac-SDKP levels. This confirms that LisW inhibits the C domain selectively, since Ang 1-7 and Ac-SDKP are N-domain–selective substrates (Deddish et al., 1998).
Based on the in vivo data for LisW, C-domain–selective ACE inhibitors offer hope for a new generation of ACE inhibitors with improved safety but are unlikely to offer improved efficacy unless combined with other drugs. This class of inhibitors is yet to be tested in the clinic.
B. Dual Angiotensin-Converting Enzyme C-Domain–Selective/Endothelin-Converting Enzyme-1 Inhibitors
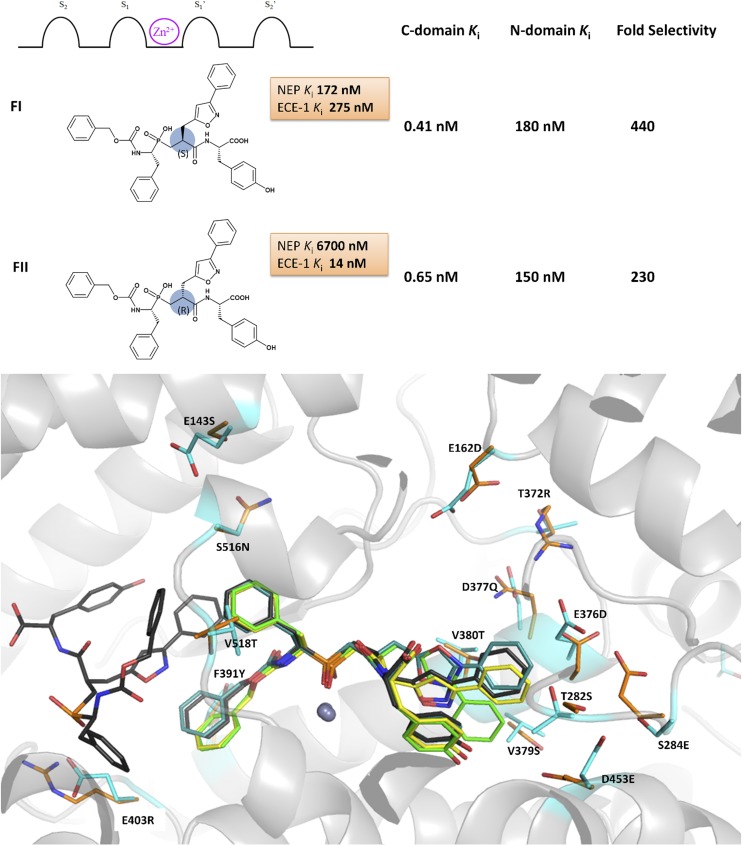
Jullien et al. (2010) have taken this concept one step further, developing dual ACE C-domain–selective/ECE-1 inhibitors. These inhibitors are designed to inhibit the formation of vasoconstrictors Ang II and ET-1, while leaving the ACE N domain and NEP free to degrade bradykinin (Jullien et al., 2010). Structure-based design, initially based on the structures of the C-domain–selective inhibitor RXPA380 and dual NEP/ECE-1 inhibitor phosphoramidon, led to a series of phosphinic tripeptides. The stereochemistry of the bulky bicyclic P1′ residue proved to be important for discriminating between ECE-1 and NEP, as illustrated by the differences in inhibition constants observed for compounds FI and FII (Fig. 6). The unusual R configuration of the P1′ residue in FII was highly selective for ECE-1 over NEP and maintained potent ACE C-domain activity.
Fig. 6.
(A) Chemical structures of C-domain–selective phosphinic tripeptides FI and FII, showing residue positions relative to the zinc binding group together with the in vitro inhibition data for NEP, ECE-1, and ACE N and C domains. (B) FI and FII bound to the active sites of the ACE N and C domains: FI bound to the N domain and C domain in green and cyan, respectively; FII bound to the N domain and C domain shown in yellow and black, respectively. C-domain unique residues within the active site are shown in cyan with corresponding N-domain residues in orange.
The crystal structures of both the ACE C domain and N domain in complex with these compounds unexpectedly reveal that the bulky P1′ group is accommodated by the S2′ pocket in all four structures, highlighting the fluidity between the S1′ and S2′ pockets and the S2′ pocket’s ability to accommodate conformationally diverse, bulky hydrophobic groups (Fig. 6) (Akif et al., 2011; Masuyer et al., 2014). Another surprising finding was that an additional FII inhibitor molecule occupied the C-domain active site, binding on the nonprime side of the first molecule. FII, the most promising dual ACE C-domain–selective/ECE-1 inhibitor, displayed 230-fold C-domain selectivity and 480-fold selectivity for ECE-1 over NEP. Administration of FII to hypertensive rats resulted in antihypertensive effects (Jullien et al., 2010), but the effect of FII on bradykinin levels and metabolism of other vasoactive peptides was not reported. This compound displays high selectivity for the C domains over the N domain, but it still inhibits the N domain in the nanomolar range, and there is only a 10-fold difference between the Ki for the N domain and ECE-1. Confirmation that this inhibitor results in a distinct peptide metabolism profile compared with conventional dual ACE/ECE-1 inhibitors in vivo is also still to be reported.
C. Dual Angiotensin-Converting Enzyme C-Domain–Selective/Neprilysin Inhibitors
It may be unnecessary to leave both the ACE N domain and NEP free to degrade bradykinin: since ACE is the primary bradykinin-metabolizing enzyme (Fryer et al., 2008), the N domain may compensate sufficiently for the C domain in preventing the buildup of dangerous levels of bradykinin. Consequently, dual ACE C-domain–selective/NEP inhibitors could offer a promising alternative for the treatment of hypertension and cardiovascular disease by potentiating NP levels in addition to blocking Ang II formation.
VI. Angiotensin Receptor–Neprilysin Inhibitors: A Current Perspective
There are parallels between dual ACE C-domain–selective/NEP inhibitors and the dual-acting angiotensin-receptor/NEP inhibitor (LCZ696 or sacubitril/valsartan, called Entresto; Novartis, East Hanover, NJ), a novel drug formulation containing equimolar amounts of the ARB valsartan and the NEP inhibitor sacubitril, which is a prodrug. Sacubitril/valsartan is the first in a new class of drugs that combines NEP inhibition together with Ang II receptor blockade (Gu et al., 2010; Ruilope et al., 2010; McMurray et al., 2013; Vardeny et al., 2014). Similar to dual ACE C-domain–selective/NEP inhibitors, this drug combination serves to enhance NEP activity while inhibiting the detrimental effects of the RAS, with no effect on bradykinin and other NEP-derived vasoprotective factors. Sacubitril/valsartan has been evaluated in the management of hypertension, heart failure with reduced ejection fraction (HFrEF), and heart failure with preserved ejection fraction (HFpEF) and has demonstrated clinical efficacy in the reduction of blood pressure in patients with essential hypertension and without HFpEF and a reduction in hospitalizations and mortality for patients with HFrEF. The landmark clinical trial, PARADIGM-HF (Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure), showed that sacubitril/valsartan was significantly more effective for the treatment of heart failure with HFrEF compared with enalapril (McMurray et al., 2014; Mogensen et al., 2018). In 2015, sacubitril/valsartan was approved by the FDA for the treatment of HFrEF and the drug is now included in American (Yancy et al., 2016) and European (Ponikowski et al., 2016) clinical guidelines for the treatment of heart failure.
Despite the robust evidence of clinical benefit seen in the PARADIGM-HF trial, as well as inclusion of the drug in clinical guidelines, this medication is approved in the United States for the treatment of heart failure only and prescribing of this new therapeutic has been slow. This has been ascribed to the phenomenon of “clinical inertia” (Jarcho, 2019), which is driven by clinician unfamiliarity, reluctance to switch stable patients, safety concerns, and payer-reimbursement issues (Sauer et al., 2019). A recent study estimated that ∼28,484 deaths could be prevented each year in the United States with optimal implementation of sacubitril/valsartan therapy (Fonarow et al., 2016); thus, because the potential reduction in mortality could be substantial, there have been calls that a paradigm shift is warranted in clinical practice (Sauer et al., 2019). The PIONEER-HF study (comparison of sacubitril/valsartan versus enalapril on effect on nt-pro-bnp in patients stabilized from an acute heart failure episode), which showed that treatment with sacubitril/valsartan produced a significantly greater reduction in N-terminal pro–B-type natriuretic peptide (NT-proBNP) levels than enalapril without increasing the rates of major adverse events in patients hospitalized with acute decompensated heart failure (Velazquez et al., 2019), may help in overcoming the clinical inertia (Jarcho, 2019). The angiotensin receptor–neprilysin inhibitor (ARNI) has shown considerable cardiovascular benefit and absolute risk reduction compared with the standard-of-care treatment in the PARADIGM (Yandrapalli et al., 2018) and PIONEER-HF (Morrow et al., 2019; Velazquez et al., 2019) trials. The composite endpoint, which was explored as part of a prespecified exploratory analysis, consisted of death, rehospitalization for heart failure (hospital stay >24 hours), requirement for a left ventricular assist device insertion, or listing for a cardiac transplantation. Furthermore, a recent meta-analysis of data from the PARADIGM-HF trial (Srivastava et al., 2018) showed that the 5-year estimated number needed to treat for the primary outcome of cardiovascular death or heart failure hospitalization with ARNI therapy incremental to ACE inhibitor therapy in the overall cohort was 14. This value is considered clinically meaningful and supports guideline recommendations for use of ARNI therapy among eligible patients with HFrEF. The therapeutic role of ARNI in HFpEF is still unclear and currently under investigation.
However, the effects of long-term NEP inhibition are yet to be established. NEP is responsible for the metabolism of many peptides; thus, chronic inhibition may have a range of physiologic effects. Indeed, too much NEP inhibition over and above angiotensin receptor blockade may increase ET-1 chronically, thereby diminishing the blood pressure–lowering potential of this combination, most likely because ET-1 upregulates sodium-hydrogen exchanger 3 in the kidney and constrictor ETBR in the vascular wall (Roksnoer et al., 2015). Chronic NEP inhibition may additionally influence Alzheimer disease progression due to NEP’s role in the degradation of amyloid-β peptides. To date, β-amyloid concentration has not been shown to be increased in cerebrospinal fluid in healthy volunteers treated with sacubitril/valsartan (Langenickel et al., 2016), and there was no increase in cognitive defects versus enalapril in the PARADIGM-HF trial (Cannon et al., 2017). The PERSPECTIVE trial evaluates the efficacy and safety of LCZ696 compared to valsartan on cognitive function in patients with chronic heart failure and preserved ejection fraction. It will collect data on long-term cognitive effects in patients with chronic heart failure treated with sacubitril/valsartan or valsartan (Sauer et al., 2019). Furthermore, sacubitril/valsartan may have an effect on inflammation, polyneuropathy, bronchial reactivity, and cancer, as recently reviewed in detail (Campbell, 2017). Long-term clinical data from treatment with sacubitril/valsartan will provide information on both the beneficial and adverse effects of chronic inhibition, which will be important for the development of new vasopeptidase inhibitors.
Potential future indications of sacubitril/valsartan include myocardial infarction, HFpEF, (diabetic) nephropathy, and stroke. ARNI attenuated adverse cardiac remodeling and dysfunction after myocardial infarction in rats compared with the ACE inhibitor perindopril (Kompa et al., 2018), and it preserved left ventricular ejection fraction after myocardial infarction in rabbits, whereas valsartan did not (Torrado et al., 2018). Studies in patients with HFpEF showed that after 12 weeks of treatment, ARNI lowered NT-proBNP more strongly than valsartan (Solomon et al., 2012). HFpEF accounts for a large percentage of patients with heart failure and is associated with significant morbidity and mortality. Current medications are suboptimal and new therapies are being sought including sacubitril/valsartan. The PARAGON-HF (Prospective Comparison of ARNI with ARB Global Outcomes in HF With Preserved Ejection Fraction) trial is designed to determine the efficacy and safety of the sacubitril/valsartan combination compared with valsartan (Solomon et al., 2017). PARAGON-HF is an event-driven trial and all randomized patients will be followed up until at least 1847 total heart failure hospitalizations and cardiovascular deaths occur. The study, which is ongoing, will provide important information on the potential therapeutic use of sacubitril/valsartan in HFpEF.
ARNI may also be effective in cardiovascular disease associated with diabetes. In rats made diabetic with streptozotocin, the NEP inhibitor thiorphan combined with an ARB prevented functional renal decline, improving glomerulosclerosis, fibrosis, and inflammation versus ARB alone (Roksnoer et al., 2016b; Malek et al., 2019). Secondary analysis of patients with heart failure and type 2 diabetes in the PARADIGM-HF trial also revealed that ARNI attenuated the effect of diabetes to accelerate renal deterioration (Damman et al., 2018; Packer et al., 2018). Yet ARNI displayed similar effects on kidney function and albuminuria as irbesartan in patients with chronic kidney diseases, although it did display superior blood pressure effects (Haynes et al., 2018). Remarkably, complete prevention of stroke was obtained with ARNI, but not valsartan, in stroke-prone spontaneously hypertensive rats (SHRs) (Rubattu et al., 2018), although Bai et al. (2015) observed that ARNI prevented ischemic brain damage after middle cerebral artery occlusion in a much more marked manner than valsartan.
Given these promising effects, a full understanding of the mechanisms of action of ARNI is urgently needed. Studies often report an increase in BNP and a decrease in NT-proBNP. The latter is the inactive side-product yielded upon cleavage of proBNP into biologically active BNP. Elevated serum NT-proBNP is a well known marker for heart failure severity, as it is associated with increased risk of mortality and hospitalization. NT-proBNP is not degraded by NEP and thus has a longer half-life than BNP. A decrease in NT-proBNP levels suggests reduced pro-BNP production due to reduced cardiac wall tension (e.g., due to blood pressure lowering), whereas an increase in BNP either suggests the opposite or might be due to NEP inhibition. Hence, a combination of blood pressure lowering and NEP inhibition may even result in no change in BNP levels. Furthermore, the decreases in NT-proBNP and rises in BNP that have been reported might also be an assay artifact, due to the fact that ARNI promotes peptide glycosylation, thereby affecting the assays of NT-proBNP and BNP: NT-proBNP would become invisible, while proBNP would additionally be detected in BNP assays (Røsjø et al., 2015). Another complicating factor is that BNP of all NPs is the least susceptible to degradation by NEP and thus acts as an endogenous inhibitor of NEP. If so, patients with elevated BNP levels already undergo NEP inhibition, and accordingly may be less responsive to ARNI (Vodovar et al., 2015).
VII. Recombinant Angiotensin-Converting Enzyme 2 as a Therapeutic Intervention
The enzymatic conversion of the proinflammatory, profibrotic vasoconstrictor Ang II into the anti-inflammatory antifibrotic and cardioprotective vasodilator Ang 1-7 appears to be a reasonable therapeutic approach for treating conditions in which Ang II has been shown to be involved in the pathologic mechanism and Ang 1-7 could mediate protective effects. Therefore, recombinant human angiotensin-converting enzyme 2 (rhACE2) is currently considered for treating acute respiratory distress syndrome and pulmonary arterial hypertension. In a safety and tolerability study in healthy volunteers, single doses between 100 and 1200 µg/kg rhACE2 were administered intravenously, revealing a plasma half-life of the enzyme in the range of 10 hours with peak plasma concentrations up to 20 µg/ml (223 nM) for the highest-dose cohort. Plasma levels stayed in the range of 1 µg/ml (11.2 nM) until 24 hours after administration of a single dose of 400 µg/kg (Haschke et al., 2013). Compared with the undetectable levels of ACE2 present in healthy volunteers (<2.7 pM) (Rice et al., 2006), a more that 4000-fold increase of circulating ACE2 levels is achieved until at least 24 hours after administration of a moderate intravenous dose of the recombinant enzyme. Although no significant decrease in blood pressure could be detected in healthy volunteers, the treatment showed the expected biochemical in vivo effects—that is, a profound suppression of circulating Ang II, while Ang 1-7 and Ang 1-5 levels were increased (Haschke et al., 2013).
In a recent pilot trial conducted in patients with acute respiratory distress syndrome (ClinicalTrials.gov identifier NCT01597635), ACE2-mediated conversion of Ang II to Ang 1-7 and Ang 1-5 could be confirmed and the compound was well tolerated. Although a trend for reduced interleukin-6 levels was reported for rhACE2-treated subjects, no significant changes were observed in the ratio of partial pressure of arterial oxygen to fraction of inspired oxygen, oxygenation index, or sequential organ failure assessment score. The study was terminated after including 39 of 60 patients, as an interim analysis revealed the unlikeliness to reach a significant outcome. Primary outcomes including safety and tolerability were successfully reached. A rapid modulation of angiotensin metabolite levels was also observed in individual patients with circulating Ang II levels up to 600 pM, indicating that pharmacologic efficacy can be achieved at a state of high RAS activity. One reason for the lack of effect might be the large heterogeneity in baseline RAS activity in this population, although this does reflect reality (Khan et al., 2017). Given that ACE2-mediated Ang 1-7 formation critically depends on the availability of Ang II as its primary substrate, patient stratification on the basis of renin or Ang II might be a reasonable approach to enhance the therapeutic efficacy observed for a treatment aiming to reduce Ang II levels while promoting the alternative RAS via increased Ang 1-7 formation.
Two studies investigating the intravenous administration of rhACE2 are currently recruiting patients. The first is conducted in human healthy volunteers under acute hypoxia and exercise (NCT03000686) in a placebo-controlled crossover design, in which modification of pulmonary artery systolic pressure by rhACE2 serves as the primary outcome. The second study is an open-label dose escalation study for rhACE2 (NCT03177603) in patients with pulmonary arterial hypertension, in which changes in pulmonary vascular resistance, cardiac output, and mean pulmonary artery pressure in response to rhACE2 serve as primary outcome measures.
Endogenous ACE2 is predominantly expressed on endothelial surfaces and in a variety of tissues, including the heart, kidney, and lung (Tipnis et al., 2000). Angiotensin metabolites are continuously generated in blood plasma, which is a process mainly driven by kidney-derived renin, hepatic angiotensinogen, and membrane-bound ACE and aminopeptidases. Indeed, endothelial surfaces play a key role in generating a local RAS by modifying the angiotensin mix supplied by the blood. In its natural environment, ACE2 is likely to compete with AT1R for local Ang II. To what extent this balanced system is affected by an excess of rhACE2 in the circulation at different states of RAS activity (e.g., how such high amounts of rhACE2 affect the plasma angiotensin substrate supply for endothelial surfaces in the absence and presence of compensatory mechanisms) is obviously critical for the clinical success of rhACE2 and requires further investigation.
VIII. Regulation of Hypertension by Central-Acting Aminopeptidase A Inhibitors
Several decades of investigation have provided evidence for the existence of a brain RAS and its involvement in the control of cardiovascular functions (Veerasingham and Raizada, 2003; Sakai and Sigmund, 2005). All of the components of the systemic RAS—the precursor, angiotensinogen; the enzymes, renin, ACE, ACE2, APA, and aminopeptidase N (APN); the peptides, Ang I, Ang II, Ang III, and Ang 1-7; and the receptors, AT1R and AT2R as well as Mas—are present within the brain (reviewed in Lenkei et al., 1997; Wright and Harding, 1997; and Santos et al., 2018). Whether they are of local origin or derived from plasma remains a matter of debate (Sigmund et al., 2017; van Thiel et al., 2017).
Brain RAS hyperactivity has been implicated in the development and maintenance of hypertension in several experimental and genetic animal models of hypertension, such as SHRs, deoxycorticosterone acetate (DOCA)-salt rats, and transgenic mice overexpressing both angiotensinogen and renin human genes (Basso et al., 1981; Ganten et al., 1983; Davisson et al., 1998). Among the bioactive peptides of the RAS, Ang II and Ang III display similar affinities for AT1R (Wright and Harding, 1995). When injected into the brain, these peptides similarly increase blood pressure and arginine-vasopressin release (Phillips, 1987; Zini et al., 1996; Reaux et al., 1999). However, because Ang II is converted into Ang III in vivo, the nature of the effector peptide of the brain RAS remains to be defined.
Using radiolabeled angiotensins in the presence or absence of specific and selective APA and APN inhibitors, EC33 (Chauvel et al., 1994) and PC18 [(2S)-2-amino-4-methylsulfanyl butane thiol] (Fournié-Zaluski et al., 1992), respectively, administered by the intracerebroventricular route, brain APA was shown to generate Ang III from Ang II by removing the N-terminal aspartate residue, whereas APN (EC 3.4.11.2), another membrane-bound zinc metalloprotease, metabolized Ang III into Ang IV (Zini et al., 1996). The use of EC33 and PC18 injected alone by the central route showed that endogenous Ang III, rather than Ang II, is one of the main effector peptides of the brain RAS in the control of blood pressure and arginine-vasopressin release (Zini et al., 1996; Reaux et al., 1999; Wright et al., 2003; Fournie-Zaluski et al., 2004). Brain Ang III exerts a tonic stimulatory control over blood pressure in two experimental models of hypertension: the SHR (Reaux et al., 1999; Marc et al., 2012) and the DOCA-salt rat (Fournie-Zaluski et al., 2004), with both models exhibiting hyperactivity of the brain RAS. The activity of the systemic RAS is normal in the SHR model and depressed in DOCA-salt rats (characterized by low plasma renin levels and high plasma arginine-vasopressin levels), accounting for the resistance of hypertensive DOCA-salt rats to treatment by systemic RAS blockers.
Brain APA, the enzyme responsible for generating brain Ang III, therefore constitutes a promising target for hypertension treatment, justifying the development of potent and selective APA inhibitors as central-acting antihypertensive agents. A prodrug of EC33, RB150 (4,4-dithio-{bis[(3S)-3-aminobutyl sulfonic acid]}), renamed firibastat, was developed for clinical use (Fournie-Zaluski et al., 2004). This compound is composed of two molecules of EC33 linked by a disulfide bridge. Orally administered RB150 crosses the intestinal, hepatic, and blood–brain barriers. Upon brain entry, the disulfide bridge is rapidly cleaved by brain reductases to generate two active molecules of EC33, which inhibit brain APA activity, block the formation of brain Ang III (Fournie-Zaluski et al., 2004), and decrease blood pressure and arginine-vasopressin release in alert hypertensive rats (Bodineau et al., 2008; Marc et al., 2018). The RB150/firibastat-induced blood pressure decrease is due to the following: 1) decreases in sympathetic tone and, consequently, vascular resistance; 2) a decrease in arginine-vasopressin release into the bloodstream from the posterior pituitary, reducing extracellular volume; and 3) an improvement in baroreflex function (Fig. 7) (Bodineau et al., 2008; Marc et al., 2012; Huang et al., 2013). No blood pressure effect was noted in normotensive rats, showing that RB150/firibastat is an antihypertensive agent and not a hypotensive agent. Moreover, the blood pressure decrease was greater in hypertensive DOCA-salt rats than in SHRs, suggesting that RB150/firibastat may be especially effective in salt-dependent hypertension.
Fig. 7.
Mode of action of the APA inhibitor prodrug RB150/firibastat on the control of blood pressure in hypertensive rats. After oral administration, the disulfide bridge enables RB150 to cross the blood–brain barrier and to enter the brain. At the opposite, EC33 is not able to enter the brain. In the brain, the disulfide bridge of RB150 is cleaved by brain reductases generating two active molecules of EC33. EC33 subsequently inhibits brain APA activity and blocks the formation of brain Ang III, known to exert, in brain structures (PVN, SON, PPit, NTS, and RVLM), a stimulatory action on the control of blood pressure in hypertensive rats. This results in a blood pressure decrease via a decrease in arginine-vasopressin release and sympathetic neuron activity and an improvement of the baroreflex function. The red dashed lines represent the neuronal angiotensinergic pathways in the adult rat brain. MnPO, median preoptic nucleus; NTS, nucleus of the solitary tract; OVLT, organum vasculosum of the lamina terminalis; PPit, posterior pituitary; PVN, paraventricular nucleus; RVLM, rostral ventrolateral medulla; SFO, subfornical organ; SON, supraoptic nucleus.
Together, these data led to the first evaluation of RB150/firibastat in humans. Clinical studies in healthy volunteers, in single ascending oral doses (phase Ia) and multiple oral doses (phase Ib), have shown that RB150/firibastat is well tolerated (Balavoine et al., 2014) up to 750 mg twice daily for 7 days. Two phase II clinical trials were then conducted. The first, a phase IIa trial carried out in 34 hypertensive patients (grade I and II), was a randomized double-blind study comparing the effect of RB150 (250 mg twice daily for 1 week, then 500 mg twice daily for 3 weeks) to placebo (Azizi et al., 2017). In the intention-to-treat population, daytime ambulatory systolic blood pressure (SBP) and office SBP decreased by 2.7 and 4.7 mm Hg, respectively, after 4 weeks of firibastat treatment versus placebo, but the difference between the groups was not statistically significant (P = 0.157 and P = 0.151, respectively). In the per-protocol population (n = 29 patients), firibastat treatment induced a larger decrease in daytime ambulatory SBP (median, −9.4 mm Hg; interquartile range, −12.5 to −3.0) in patients with a basal value of daytime ambulatory SBP between 154 and 172 mm Hg, whereas placebo treatment did not induce any change (median, 0.75 mm Hg; interquartile range, −5.5 to −1.9). In the multiple linear regression analysis for the per-protocol population, only treatment with firibastat (P = 0.06) and baseline daytime ambulatory SBP (P = 0.01) were associated with changes in daytime ambulatory SBP. This suggests that the more the basal daytime ambulatory SBP is elevated, the more the firibastat-induced SBP decrease is apparent. This is in agreement with the observation that, in experimental models of hypertension, firibastat acted as an antihypertensive agent and not as a hypotensive agent. This study showed that RB150/firibastat treatment was safe and tended to decrease daytime ambulatory SBP, but not significantly (Azizi et al., 2017), possibly due to the small number of patients and the short duration of treatment. These data were used to guide the design of a large phase IIb clinical trial, NEW-HOPE (Novel Evaluation with QGC001 in Hypertensive Overweight Patients of Multiple Ethnic Origins), carried out in 250 overweight hypertensive patients (SBP 145–170 mm Hg), 50% of whom were self-identified African Americans or Hispanics. In patients receiving firibastat for 2 months (250 mg twice daily orally for 2 weeks, then 500 mg twice daily; 25 mg hydrochlorothiazide daily could be added after 1 month if SBP ≥ 160 mm Hg and/or DBP ≥ 100 mm Hg), a significant blood pressure–lowering efficacy and a safe tolerability profile were observed (NCT03198793) (Ferdinand et al., 2018).
If the proof of concept of firibastat efficacy is confirmed in pivotal phase III trials, RB150/firibastat could constitute the first of a new class of centrally acting antihypertensive agents. Firibastat may be especially effective in African Americans who are poorly responsive to blockers of the systemic RAS and who are salt sensitive with high plasma arginine-vasopressin levels and low plasma renin activity.
IX. Targeting Angiotensinogen: Antisense Oligonucleotides and Small Interfering RNA
Since all angiotensins stem from angiotensinogen, deleting angiotensinogen will diminish the stimulation of all angiotensin receptors, including AT2R and Mas. Circulating angiotensinogen is derived from the liver, and it is generally believed that additional angiotensinogen production occurs in the brain, kidney, and adipose tissue (Campbell and Habener, 1986; Thomas and Sernia, 1988; Matsusaka et al., 2012). Although it is attractive to speculate that this angiotensinogen contributes to “local” angiotensin production, direct evidence that tissue angiotensin generation occurs independently of liver angiotensinogen is still lacking. Indeed, deleting renal angiotensinogen unexpectedly did not affect renal angiotensin levels at baseline or under pathophysiological conditions (Matsusaka et al., 2012, 2014). Future studies should address brain and adipose tissue angiotensin levels in the absence of hepatic angiotensinogen to solve this issue (Uijl et al., 2018). Such studies require highly sensitive angiotensin assays, since brain angiotensin levels in particular are extremely low (van Thiel et al., 2017; Lombard-Banek et al., 2019).
In humans, circulating angiotensinogen levels are in the micromolar range (i.e., close to the Km of its reaction with renin) and 5 to 6 orders of magnitude above the levels of circulating Ang II. Circulating, liver-derived angiotensinogen diffuses slowly into the interstitial space and hence contributes to tissue angiotensin production (e.g., in the heart and vascular wall) (de Lannoy et al., 1997). Upregulation of angiotensinogen levels, such as in subjects carrying the T235 allele of the angiotensinogen gene or in pregnant women (due to the fact that estrogen stimulates angiotensinogen synthesis) (Schunkert et al., 1997; Danser et al., 1998), results in renin downregulation, thereby normalizing angiotensin generation. In contrast, renin upregulation, as occurs in patients with heart failure, particularly during treatment with diuretics and RAS blockers, diminishes angiotensinogen and, if excessive, may result in near-complete angiotensinogen depletion (Danser et al., 1997; Klotz et al., 2009). Such depletion tends to occur earlier at the tissue level rather than in the circulation (Klotz et al., 2009). Nevertheless, the inverse relationship between renin and angiotensinogen allows angiotensin levels to remain intact over a wide range of angiotensinogen levels, even in patients with angiotensinogen levels that are <25% of normal (Danser et al., 1997; Klotz et al., 2009). Of interest, mice display angiotensinogen levels that are at most a few percent of those in humans, and they still display similar angiotensin levels to humans. This is due to the fact that their renin levels are several orders of magnitude higher than those in humans (van Thiel et al., 2017). Here it is important to realize that humans are also capable of upregulating renin levels several hundredfold (Balcarek et al., 2014), thereby compensating for significant angiotensinogen depletion. Taken together, this implies that to suppress angiotensin levels in blood plasma, angiotensinogen depletion should be substantial (most likely >90%), whereas suppression of angiotensins at the tissue level may already occur at less impressive angiotensinogen reductions.
Currently, suppression of angiotensinogen can be achieved by interference at the RNA level with oligonucleotides (Fig. 8). Antisense oligonucleotides (ASOs) are single-stranded synthetic nucleic acids that are complementary to a specific mRNA region (Watts and Corey, 2012). Once hybridized, mRNA degradation occurs, thereby abrogating protein (angiotensinogen) synthesis. ASOs generally consisting of 15–30 nucleotides and are highly unstable; thus, chemical modifications are required to increase nuclease resistance. Such modifications may also help to increase RNA affinity and selectivity. Furthermore, conjugation to a hepatocyte-targeting ligand [triantennary N-acetylgalactosamine (GalNAc)] facilitates selective accumulation in the liver, thereby increasing potency 10- to 30-fold (Mullick et al., 2017; Ren et al., 2018). GalNAc binds to the asialoglycoprotein receptor on hepatocytes, allowing transport and release of the ASO into the intracellular compartment (Levin, 2019). Without GalNAc, the ASOs may also accumulate outside the liver, for example, in the above-mentioned putative angiotensinogen-synthesizing organs. Small interfering RNA (siRNA) shares with ASOs the principle of oligonucleotide binding to a target RNA through Watson-Crick base pairing. Yet siRNA is double stranded (increasing stability) and, once in the cell, one strand (the “passenger” strand) is lost, whereas the other strand (the “guide” strand) is loaded into the RNA-induced silencing complex (RISC). RISC is a protein complex that allows the guide strand to bind to a complementary RNA region, after which an enzyme (Argonaute) that is part of RISC cleaves the mRNA. GalNAc conjugation can be applied to siRNA as well, which in the case of siRNA for proprotein convertase subtilisin/kexin type 9 (PCSK9)–enabled biannual dosing to suppress PCSK9 (Ray et al., 2017). If true for angiotensinogen as well, this might revolutionize hypertension pharmacotherapy, particularly in patients who are nonadherent.
Fig. 8.
Overview of AGT suppression using siRNAs. siRNAs enter the cell and are incorporated into the RISC in the cytoplasm. The RISC complex with the active guide strand binds the complementary sequence within the target mRNA, resulting in Argonaut 2–mediated cleavage and subsequent AGT mRNA degradation. AGT, angiotensinogen.
Early studies with angiotensinogen ASOs administered by intracerebroventricular injection in the brain of SHRs showed a modest reduction of angiotensinogen levels in the hypothalamus (from ≈60 to ≈40 pmol/g), but not in the brainstem, cortex, midbrain, or cerebellum (Wielbo et al., 1995). Remarkably, baseline brain angiotensinogen levels were comparable in all brain regions and corresponded to <5% of blood plasma angiotensinogen levels. These data argue against angiotensinogen expression in selected brain nuclei, and they favor the presence of trapped plasma angiotensinogen in the brain. Furthermore, brainstem Ang II levels decreased marginally from 70 to 60 pg/g after angiotensinogen ASO administration (Gyurko et al., 1993), yet blood pressure decreased by >35 mm Hg. It remains difficult to link this large hypotensive effect to the inconsistent or even absent changes in brain angiotensinogen and Ang II; thus, before concluding that this reflects exclusive interference with brain angiotensinogen expression, these studies should now be repeated with the current, more selective, potent and stable angiotensinogen ASOs. Recent studies evaluated such ASOs and siRNAs in various rat models (Mullick et al., 2017) and mice (Ye et al., 2019) and also compared the GalNAc-conjugated and nonconjugated variants.
In mice, hepatocyte-specific angiotensinogen deficiency abolished angiotensinogen accumulation in proximal tubules and greatly diminished renal Ang II levels, supporting the concept that renal angiotensin generation depends on liver-derived angiotensinogen (Roksnoer et al., 2016a). In rats, both GalNAc-conjugated and unconjugated angiotensinogen ASO suppressed circulating angiotensinogen, although only the unconjugated ASO additionally suppressed renal and adipose angiotensinogen mRNA. Nevertheless, both the GalNAc-conjugated ASO and siRNA effectively lowered blood pressure in SHRs (Mullick et al., 2017; Uijl et al., 2019), suggesting that interference with renal or adipose angiotensinogen is not required for this effect. This was also true for lipid nanoparticle-encapsulated angiotensinogen siRNA delivered to the liver in SHRs (Olearczyk et al., 2014). Unexpectedly, GalNAc-conjugated angiotensinogen ASO also lowered blood pressure in SHRs fed 8% salt, whereas classic RAS blockers are ineffective in this model (Mullick et al., 2017). Moreover, GalNAc-conjugated angiotensinogen ASO did not induce renal dysfunction (reflected by reduced creatinine clearance) in rats with 5/6 nephrectomy, unlike both nonconjugated angiotensinogen ASO and captopril (Mullick et al., 2017). The authors speculated that the preservation of renal angiotensinogen in the 5/6 nephrectomy model with GalNAc-conjugated angiotensinogen ASO might have prevented kidney function deterioration, but failed to support this concept by determining renal angiotensin levels under the various conditions. Renal angiotensinogen production is unlikely to underlie the effectiveness of GalNAc-conjugated angiotensinogen ASO in SHRs fed 8% salt. Finally, unconjugated angiotensinogen ASO slowed polycystic kidney disease in various polycystic kidney disease mouse models (Ravichandran et al., 2015; Fitzgibbon et al., 2018). Since this approach lowered both renal and hepatic angiotensinogen expression, as well as angiotensinogen in serum, it cannot be concluded to what degree this was due to interference with renal angiotensinogen, although it is likely to be due to suppression of renal Ang II. Clearly, future studies combining GalNAc-conjugated and unconjugated angiotensinogen ASO/siRNA together with renal Ang II measurements in various models are needed to finally settle the issue of renal angiotensinogen versus hepatic angiotensinogen contributing to renal angiotensin generation.
In summary, angiotensinogen ASO and siRNA show promising results in rodent models for hypertension and kidney failure. Their long-lasting effects are particularly exciting, and if translated to a clinical application of at most a few administrations per year, may help to eliminate nonadherence. Yet major hurdles remain with regard to both safety (e.g., immune responses, liver toxicity, nonspecific effects, what to do in situations where RAS activity is acutely needed, etc.) and efficacy, particularly in the context of common comorbidities such as heart failure and chronic kidney disease, and in combination with other RAS blockers.
X. Dual Receptor Activation of Particulate Guanylyl Cyclase A and Mas
In contemporary drug discovery, an emerging strategy is the design and development of bispecific therapeutics. A bispecific drug, as either a small molecule or peptide, targets two independent signaling pathways. Importantly, the goal of bispecific drugs is to achieve therapeutic synergy that transcends the effects of single-pathway activation. As an example, this concept has been supported by the approval of the small molecule sacubitril/valsartan for heart failure, which has also demonstrated efficacy in hypertension (Ruilope et al., 2010; McMurray et al., 2013).
Most recently, advances in peptide engineering have been employed to design and develop novel designer peptides that target pGC-A (Meems and Burnett, 2016; Chen et al., 2018). This molecular target is well recognized to mediate cardiorenal protection in cardiovascular disease, via its second-messenger cGMP, for which the cardiac hormones ANP and BNP are its endogenous ligands. Indeed, stimulation of the pGC-A/cGMP pathway results in a number of biologic properties, including natriuresis, diuresis, blood pressure lowering, inhibition of cardiomyocyte hypertrophy and fibroblast proliferation, browning of white adipocytes with enhanced energy utilization, suppression of inflammatory cytokines and T cells, and inhibition of aldosterone (Bordicchia et al., 2012; Ma et al., 2013; Kuhn, 2016). Cataliotti et al. (2011) also reported that chronic activation of pGC-A by adenoviral BNP gene delivery in SHRs reduced blood pressure and attenuated cardiac hypertrophy and diastolic dysfunction. In human heart failure, chronic pGC-A augmentation with daily subcutaneous BNP injections improved cardiorenal function and clinical symptoms (Chen et al., 2012).
A second but separate molecular target in cardiorenal therapeutics is the Mas. Mas activation mediates antiapoptotic, anti-inflammatory, vasodilatory, antithrombotic, and AT1R antagonizing actions by activation via its ligand Ang 1-7 and its second-messenger cAMP (Santos et al., 2003; Trask and Ferrario, 2007). In addition to cAMP activation, other downstream pathways activated by Ang 1-7/Mas include the phospholipase A2 pathway (Santos et al., 2003) and the phosphoinositide 3-kinase/AKT pathway (Sampaio et al., 2007; Lopez Verrilli et al., 2012; Savergnini et al., 2013; Than et al., 2013). This Ang 1-7/Mas axis also has cardiorenal protective actions in models of hypertension and heart failure (Mori et al., 2014; van Twist et al., 2014). The therapeutic development of Mas has been limited, however, by the rapid in vivo degradation of Ang 1-7 (Iusuf et al., 2008).
A first-in-class bispecific designer peptide that cotargets Mas and pGC-A in one peptide entity was recently engineered (Meems et al., 2019). This designer peptide NPA7 replaces the 9-amino-acid N terminus of BNP1-32 with the Mas agonist Ang 1-7 (Fig. 9). The goal was to create a bispecific drug (i.e., NPA7) that would possess greater systemic and renal vasodilating, natriuretic, diuretic, and cardiac unloading properties compared with either Ang 1-7 or BNP alone and which potentially would have beneficial efficacy for the treatment of cardiovascular disease such as hypertension and heart failure.
Fig. 9.
NPA7 is a single peptide entity that coactivates the Mas and pGC-A receptors and their second messengers cAMP and cGMP, respectively. NPA7 incorporates key amino acids from BNP1-32 (a pGC-A activator) and Ang 1-7 (a Mas activator), resulting in a novel bispecific first-in-class bispecific peptide. GC, guanylyl cyclase; URO, urodilatin.
In a recent report, pGC-A and Mas activation by NPA7 was validated and it was shown that NPA7 is biologically active in vivo with potent and more sustained cardiorenal actions that go beyond Mas or pGC-A alone (Meems et al., 2019). Further validation of stimulation of both receptors in vitro was shown and increases in the second messengers of pGC-A and Mas in HEK293 cells with increases in cGMP and cAMP, respectively, were demonstrated. Importantly, blockade of Mas attenuated the hemodynamic, natriuretic, and diuretic responses to NPA7 in vivo, underscoring the important activation of Mas by NPA7. These findings are the first studies of a novel and unique Mas activator that possesses Ang 1-7 properties, hence generating in vitro and in vivo actions that represent alternative RAS activation together with pGC-A targeting.
NPA7 as a therapeutic has implications for the treatment of cardiovascular disease, especially for those disease states in which RAS activation plays a pivotal role (i.e., hypertension and heart failure). Hypertension and heart failure have neurohumoral imbalance, which is characterized by a relative NP deficiency with excessive RAS activation (Hawkridge et al., 2005; Macheret et al., 2012). Treatment with drugs that target both the pGC-A receptor system and Mas have the potential to restore this imbalance. Indeed, studies have reported that chronic pGC-A receptor activation in human heart failure is associated with improved cardiorenal function, left ventricular function and/or structure, and overall clinical outcomes (Chen et al., 2012). Long-term treatment with Ang 1-7 in experimental models of cardiovascular disease improves cardiac function mice and prevents cardiomyocyte hypertrophy, apoptosis, and fibrosis (Mori et al., 2014; Papinska et al., 2016). Therefore, NPA7 may not only improve hemodynamic function, diuresis and natriuresis in the short term but may have wide potential application in cardiovascular, renal, and metabolic diseases when chronically used. Thus, future studies are needed to address the full therapeutic potential of this first-in-class peptide and the concept of optimizing therapy with bivalency.
XI. Conclusion
Blockers of the RAS have profoundly influenced clinical medicine. In particular, ACE inhibitors have had a major impact on cardiovascular medicine, especially in the treatment of heart failure, hypertension, and ischemic heart disease (Ferrario and Mullick, 2017; Oparil et al., 2018). Many major evidence-based cardiovascular guidelines recommend ACE inhibitors as first-line therapy, at least for heart failure and hypertension (Rosendorff et al., 2015; Yancy et al., 2017; Whelton et al., 2018; Wright et al., 2018). However, despite enormous therapeutic advances in the management of these conditions, patients treated with ACE inhibitors are still at increased risk for cardiovascular morbidity and premature death (Moukarbel and Solomon, 2008). Reasons for this are multifactorial, including the fact that the “ideal ACE inhibitor” has yet to be developed. Clinically, this was addressed, in part, with the development of vasopeptidase inhibitors such as omapatrilat, a dual inhibitor of ACE and NEP (Nawarskas et al., 2001; Tabrizchi, 2001; Lapointe and Rouleau, 2002).
These drugs had the promise of being highly effective in the treatment of endothelial dysfunction, atherosclerosis, hypertension, and heart failure and were termed by some as the “super ACE inhibitors.” However, large clinical trials did not live up to the expectations and omapatrilat failed to obtain FDA approval as a result of the high incidence of angioedema, which has also been associated with ACE inhibitors and to a lesser extent with ARBs and ARB/NEP inhibitors (Owens and Oliphant, 2017; Kostis et al., 2018), especially in African Americans, smokers, women, older individuals, and patients with previous drug rash or reaction, seasonal allergies, or use of immunosuppressive drugs.
Although the exact causes of vasopeptidase inhibitor– and ACE inhibitor–induced angioedema remain elusive, evidence suggests that this may be due to the excess bradykinin formation owing to inhibition of both the domains of ACE (Baş et al., 2015; Bas, 2017; Stone and Brown, 2017; Straka et al., 2017). Accordingly, the potential benefits of selective inhibition of the ACE C domain that primarily inhibits production of Ang II from Ang I, while at the same time reducing side effects by preventing bradykinin buildup via continued bradykinin degradation by an intact N domain, seem attractive. The ACE N domain also regulates the breakdown of other peptides, including amyloid-β peptide, tetrapeptide Ac-SDKP, and GnRH, hence maintaining a functional N domain to prevent accumulation of amyloid-β peptide, and other peptides may have additional cardiovascular protective and health benefits (Bernstein et al., 2012).
Nevertheless, even having an ideal ACE inhibitor without side effects may not be sufficient to treat all patients, given the multiple counter-regulatory mechanisms within the RAS that allow Ang II levels to return to their original status even in the presence of ACE inhibition. Hence, we need alternative RAS blockers like angiotensinogen ASO and siRNA, which are capable of significantly suppressing/eliminating RAS activity, even when renin is upregulated, simply because it removes the substrate from which all angiotensins stem. This approach (siRNA, in particular) additionally has the advantage of an exceptional long half-life, allowing application to be limited to a few times per year, thus offering the possibility to simultaneously combat nonadherence. Of course, a matter of debate remains how far one should suppress the RAS, since we cannot live without a functional RAS—too much RAS blockade will yield the well known side effects observed in trials applying multiple RAS blockers at the same time, such as hypotension, renal dysfunction, and hyperkalemia. The optimal degree of RAS suppression is unlikely to be identical in all patients and undoubtedly requires individualization of therapy. In other words, there is a need for significant or even “complete” RAS blockade in some patients, but a modest degree of RAS blockade might be sufficient in others. Alternative options would be to either combine classic RAS blockers with drugs that interfere with other hormonal systems that are known to be involved in hypertension, to upregulate the so-called protective arm of the RAS, or to aim at RAS blockade at one specific location (e.g., in the brain with firibastat), normalizing brain RAS hyperactivity and consequently regulating sympathetic tone, baroreflex function, and arginine-vasopressin release. Here, exciting new developments are currently taking place, such as the combination of an ACE C-domain–selective inhibitor with an NEP inhibitor, which increases NP levels by inhibiting breakdown to inactive fragments. Theoretically, this constitutes a second opportunity to create a “super ACE inhibitor” that is now safe for widespread use in the clinic. The combination of an ARB with an NEP inhibitor is already a clinical reality with proven superior effectiveness versus single RAS blockade. Recombinant ACE2 and brain-selective APA inhibition are currently being tested clinically in pulmonary arterial hypertension and overweight patients with hypertension, respectively, based on the concept that ACE2 degrades Ang II and upregulates the protective Ang 1-7, whereas APA blockade prevents brain AT1R stimulation by Ang III. Finally, dual Mas/pGC-A activation has shown promising results in animal studies and should now be taken to the next step. Taken together, our possibilities to improve and extend classic RAS blockade are rapidly expanding and should eventually result in novel treatment modalities with superior efficacy, diminished side effects, reduced dosing frequency, enhanced brain specificity, and/or the capacity to upregulate protective mechanisms.
Acknowledgments
We gratefully acknowledge Vinasha Ramasamy for assistance with figure preparation.
Abbreviations
- 3D
three-dimensional
- Ac-SDKP
N-acetyl-Ser–Asp–Lys–Pro
- ACE
angiotensin-converting enzyme
- Ang
angiotensin
- ANP
atrial natriuretic peptide
- APA
aminopeptidase A
- APN
aminopeptidase N
- APP
aminopeptidase P
- ARB
angiotensin II receptor blocker
- ARNI
angiotensin receptor–neprilysin inhibitor
- ASO
antisense oligonucleotide
- AT1R
angiotensin II type 1 receptor
- AT2R
angiotensin II type 2 receptor
- B1R
B1 receptor
- B2R
B2 receptor
- BK
bradykinin
- BNP
B-type natriuretic peptide
- CNP
C-type natriuretic peptide
- DOCA
deoxycorticosterone acetate
- EC33
(3S)-3-amino-4-sulfanyl-butane-1-sulfonic acid
- ECE
endothelin-converting enzyme
- ET
endothelin
- ETAR
endothelin receptor A
- ETBR
endothelin receptor B
- FDA
U.S. Food and Drug Administration
- GalNAc
triantennary N-acetylgalactosamine
- GPCR
G protein–coupled receptor
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- NEP
neprilysin (neutral endopeptidase)
- NP
natriuretic peptide
- NPR
natriuretic peptide receptor
- NT-proBNP
N-terminal pro–B-type natriuretic peptide
- PC18
(2S)-2-amino-4-methylsulfanyl butane thiol
- pGC-A
particulate guanylyl cyclase A
- RAS
renin-angiotensin system
- RB150
4,4-dithio-{bis[(3S)-3-aminobutyl sulfonic acid]}
- rhACE2
recombinant human angiotensin-converting enzyme 2
- RISC
RNA-induced silencing complex
- SAR
structure–activity relationship
- SBP
systolic blood pressure
- SHR
spontaneously hypertensive rat
- siRNA
small interfering RNA
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Arendse, Danser, Poglitsch, Touyz, Burnett, Llorens-Cortes, Ehlers, Sturrock.
Footnotes
This work was supported by the British Heart Foundation [Grants RG/13/7/30099, RE/13/5/30177, and CH/4/29762 (to R.M.T.)], the National Institutes of Health National Heart, Lung and Blood Institute [Grants R01-HL36634 and R01-HL134668 (to J.C.B.)], and the South African National Research Foundation [Grants CPRR160331161352 and EQ160511164723 (to E.D.S.)].
References
- Abassi Z, Karram T, Ellaham S, Winaver J, Hoffman A. (2004) Implications of the natriuretic peptide system in the pathogenesis of heart failure: diagnostic and therapeutic importance. Pharmacol Ther 102:223–241. [DOI] [PubMed] [Google Scholar]
- AbdAlla S, Lother H, Abdel-tawab AM, Quitterer U. (2001) The angiotensin II AT2 receptor is an AT1 receptor antagonist. J Biol Chem 276:39721–39726. [DOI] [PubMed] [Google Scholar]
- AbdAlla S, Lother H, Quitterer U. (2000) AT1-receptor heterodimers show enhanced G-protein activation and altered receptor sequestration. Nature 407:94–98. [DOI] [PubMed] [Google Scholar]
- Agah R, Bandi V, Guntupalli KK. (1997) Angioedema: the role of ACE inhibitors and factors associated with poor clinical outcome. Intensive Care Med 23:793–796. [DOI] [PubMed] [Google Scholar]
- Akif M, Schwager SL, Anthony CS, Czarny B, Beau F, Dive V, Sturrock ED, Acharya KR. (2011) Novel mechanism of inhibition of human angiotensin-I-converting enzyme (ACE) by a highly specific phosphinic tripeptide. Biochem J 436:53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almquist RG, Chao WR, Ellis ME, Johnson HL. (1980) Synthesis and biological activity of a ketomethylene analogue of a tripeptide inhibitor of angiotensin converting enzyme. J Med Chem 23:1392–1398. [DOI] [PubMed] [Google Scholar]
- Ambroz C, Clark AJ, Catt KJ. (1991) The mas oncogene enhances angiotensin-induced [Ca2+]i responses in cells with pre-existing angiotensin II receptors. Biochim Biophys Acta 1133:107–111. [DOI] [PubMed] [Google Scholar]
- Ando S, Rahman MA, Butler GC, Senn BL, Floras JS. (1995) Comparison of candoxatril and atrial natriuretic factor in healthy men. Effects on hemodynamics, sympathetic activity, heart rate variability, and endothelin. Hypertension 26:1160–1166. [DOI] [PubMed] [Google Scholar]
- Atlas SA. (2007) The renin-angiotensin aldosterone system: pathophysiological role and pharmacologic inhibition. J Manag Care Pharm 13 (Suppl B):9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizi M, Bissery A, Peyrard S, Guyene TT, Ozoux ML, Floch A, Ménard J. (2006) Pharmacokinetics and pharmacodynamics of the vasopeptidase inhibitor AVE7688 in humans. Clin Pharmacol Ther 79:49–61. [DOI] [PubMed] [Google Scholar]
- Azizi M, Courand P, Denolle T, Zhygalina V, Delsart P, Amar L, Lantelme P, Deplanque D, Mounier-Vehier C, Balavoine F. (2017) [OP. 4A. 08] A randomized double-blind placebo controlled crossover study to compare QGC001, a brain aminopeptidase A inhibitor, with placebo in patients with grade I/II essential hypertension (Abstract). J Hypertens 35:e36. [DOI] [PubMed] [Google Scholar]
- Azizi M, Ezan E, Reny JL, Wdzieczak-Bakala J, Gerineau V, Ménard J. (1999) Renal and metabolic clearance of N-acetyl-seryl-aspartyl-lysyl-proline (AcSDKP) during angiotensin-converting enzyme inhibition in humans. Hypertension 33:879–886. [DOI] [PubMed] [Google Scholar]
- Bader M, Alenina N, Andrade-Navarro MA, Santos RA. (2014) MAS and its related G protein-coupled receptors, Mrgprs. Pharmacol Rev 66:1080–1105. [DOI] [PubMed] [Google Scholar]
- Bader M, Alenina N, Young D, Santos RAS, Touyz RM. (2018) The meaning of Mas. Hypertension 72:1072–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai HY, Mogi M, Nakaoka H, Kan-No H, Tsukuda K, Chisaka T, Wang XL, Kukida M, Shan BS, Yamauchi T, et al. (2015) Pre-treatment with LCZ696, an orally active angiotensin receptor neprilysin inhibitor, prevents ischemic brain damage. Eur J Pharmacol 762:293–298. [DOI] [PubMed] [Google Scholar]
- Balavoine F, Azizi M, Bergerot D, De Mota N, Patouret R, Roques BP, Llorens-Cortes C. (2014) Randomised, double-blind, placebo-controlled, dose-escalating phase I study of QGC001, a centrally acting aminopeptidase A inhibitor prodrug. Clin Pharmacokinet 53:385–395. [DOI] [PubMed] [Google Scholar]
- Balcarek J, Sevá Pessôa B, Bryson C, Azizi M, Ménard J, Garrelds IM, McGeehan G, Reeves RA, Griffith SG, Danser AHJ, et al. (2014) Multiple ascending dose study with the new renin inhibitor VTP-27999: nephrocentric consequences of too much renin inhibition. Hypertension 63:942–950. [DOI] [PubMed] [Google Scholar]
- Barton M, Shaw S, d’Uscio LV, Moreau P, Lüscher TF. (1997) Angiotensin II increases vascular and renal endothelin-1 and functional endothelin converting enzyme activity in vivo: role of ETA receptors for endothelin regulation. Biochem Biophys Res Commun 238:861–865. [DOI] [PubMed] [Google Scholar]
- Bas M. (2017) The angiotensin-converting-enzyme-induced angioedema. Immunol Allergy Clin North Am 37:183–200. [DOI] [PubMed] [Google Scholar]
- Baş M, Greve J, Stelter K, Havel M, Strassen U, Rotter N, Veit J, Schossow B, Hapfelmeier A, Kehl V, et al. (2015) A randomized trial of icatibant in ACE-inhibitor-induced angioedema. N Engl J Med 372:418–425. [DOI] [PubMed] [Google Scholar]
- Basso N, Ruiz P, Mangiarua E, Taquini AC. (1981) Renin-like activity in the rat brain during the development of DOC-salt hypertension. Hypertension 3:II-14-II-17. [DOI] [PubMed] [Google Scholar]
- Basu R, Poglitsch M, Yogasundaram H, Thomas J, Rowe BH, Oudit GY. (2017) Roles of angiotensin peptides and recombinant human ACE2 in heart failure. J Am Coll Cardiol 69:805–819. [DOI] [PubMed] [Google Scholar]
- Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee (2017) Heart disease and stroke statistics-2017 update: a report from the American Heart Association [published correction appears in Circulation (2017) 135:e646 and 136:e196]. Circulation 135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzing T, Fleming I, Blaukat A, Müller-Esterl W, Busse R. (1999) Angiotensin-converting enzyme inhibitor ramiprilat interferes with the sequestration of the B2 kinin receptor within the plasma membrane of native endothelial cells. Circulation 99:2034–2040. [DOI] [PubMed] [Google Scholar]
- Bernstein KE, Ong FS, Blackwell WL, Shah KH, Giani JF, Gonzalez-Villalobos RA, Shen XZ, Fuchs S, Touyz RM. (2012) A modern understanding of the traditional and nontraditional biological functions of angiotensin-converting enzyme. Pharmacol Rev 65:1–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein KE, Shen XZ, Gonzalez-Villalobos RA, Billet S, Okwan-Duodu D, Ong FS, Fuchs S. (2011) Different in vivo functions of the two catalytic domains of angiotensin-converting enzyme (ACE). Curr Opin Pharmacol 11:105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodineau L, Frugière A, Marc Y, Inguimbert N, Fassot C, Balavoine F, Roques B, Llorens-Cortes C. (2008) Orally active aminopeptidase A inhibitors reduce blood pressure: a new strategy for treating hypertension. Hypertension 51:1318–1325. [DOI] [PubMed] [Google Scholar]
- Bordicchia M, Liu D, Amri EZ, Ailhaud G, Dessì-Fulgheri P, Zhang C, Takahashi N, Sarzani R, Collins S. (2012) Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest 122:1022–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosnyak S, Jones ES, Christopoulos A, Aguilar MI, Thomas WG, Widdop RE. (2011) Relative affinity of angiotensin peptides and novel ligands at AT1 and AT2 receptors. Clin Sci (Lond) 121:297–303. [DOI] [PubMed] [Google Scholar]
- Bremnes T, Paasche JD, Mehlum A, Sandberg C, Bremnes B, Attramadal H. (2000) Regulation and intracellular trafficking pathways of the endothelin receptors. J Biol Chem 275:17596–17604. [DOI] [PubMed] [Google Scholar]
- Bur D, Dale GE, Oefner C. (2001) A three-dimensional model of endothelin-converting enzyme (ECE) based on the X-ray structure of neutral endopeptidase 24.11 (NEP). Protein Eng 14:337–341. [DOI] [PubMed] [Google Scholar]
- Burger D, Reudelhuber TL, Mahajan A, Chibale K, Sturrock ED, Touyz RM. (2014) Effects of a domain-selective ACE inhibitor in a mouse model of chronic angiotensin II-dependent hypertension. Clin Sci (Lond) 127:57–63. [DOI] [PubMed] [Google Scholar]
- Campbell DJ. (2017) Long-term neprilysin inhibition—implications for ARNIs. Nat Rev Cardiol 14:171–186. [DOI] [PubMed] [Google Scholar]
- Campbell DJ, Habener JF. (1986) Angiotensinogen gene is expressed and differentially regulated in multiple tissues of the rat. J Clin Invest 78:31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon JA, Shen L, Jhund PS, Kristensen SL, Køber L, Chen F, Gong J, Lefkowitz MP, Rouleau JL, Shi VC, et al. PARADIGM-HF Investigators and Committees (2017) Dementia-related adverse events in PARADIGM-HF and other trials in heart failure with reduced ejection fraction. Eur J Heart Fail 19:129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataliotti A, Tonne JM, Bellavia D, Martin FL, Oehler EA, Harders GE, Campbell JM, Peng KW, Russell SJ, Malatino LS, et al. (2011) Long-term cardiac pro-B-type natriuretic peptide gene delivery prevents the development of hypertensive heart disease in spontaneously hypertensive rats. Circulation 123:1297–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell MC, Pirro NT, Sykes A, Ferrario CM. (1998) Metabolism of angiotensin-(1-7) by angiotensin-converting enzyme. Hypertension 31:362–367. [DOI] [PubMed] [Google Scholar]
- Chappell MC, Tallant EA, Brosnihan KB, Ferrario CM. (1995) Conversion of angiotensin I to angiotensin-(1-7) by thimet oligopeptidase (EC 3.4. 24.15) in vascular smooth muscle cells. J Vasc Med Biol 5:129–137. [Google Scholar]
- Charles CJ, Espiner EA, Nicholls MG, Richards AM, Yandle TG, Protter A, Kosoglou T. (1996) Clearance receptors and endopeptidase 24.11: equal role in natriuretic peptide metabolism in conscious sheep. Am J Physiol 271:R373–R380. [DOI] [PubMed] [Google Scholar]
- Chauvel EN, Llorens-Cortès C, Coric P, Wilk S, Roques BP, Fournié-Zaluski MC. (1994) Differential inhibition of aminopeptidase A and aminopeptidase N by new beta-amino thiols. J Med Chem 37:2950–2957. [DOI] [PubMed] [Google Scholar]
- Chen HH, Glockner JF, Schirger JA, Cataliotti A, Redfield MM, Burnett JC., Jr (2012) Novel protein therapeutics for systolic heart failure: chronic subcutaneous B-type natriuretic peptide. J Am Coll Cardiol 60:2305–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Harty GJ, Huntley BK, Iyer SR, Heublein DM, Harders GE, Meems L, Pan S, Sangaralingham SJ, Ichiki T, et al. (2018) CRRL269: a novel designer and renal-enhancing pGC-A peptide activator. Am J Physiol Regul Integr Comp Physiol 314:R407–R414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claperon C, Rozenfeld R, Iturrioz X, Inguimbert N, Okada M, Roques B, Maigret B, Llorens-Cortes C. (2008) Asp218 participates with Asp213 to bind a Ca2+ atom into the S1 subsite of aminopeptidase A: a key element for substrate specificity. Biochem J 416:37–46. [DOI] [PubMed] [Google Scholar]
- Cleland JG, Swedberg K, The International Ecadotril Multi-centre Dose-ranging Study Investigators (1998) Lack of efficacy of neutral endopeptidase inhibitor ecadotril in heart failure. Lancet 351:1657–1658. [DOI] [PubMed] [Google Scholar]
- Coats AJ. (2002) Omapatrilat--the story of Overture and Octave. Int J Cardiol 86:1–4. [DOI] [PubMed] [Google Scholar]
- Cody RJ, Atlas SA, Laragh JH, Kubo SH, Covit AB, Ryman KS, Shaknovich A, Pondolfino K, Clark M, Camargo MJ, et al. (1986) Atrial natriuretic factor in normal subjects and heart failure patients. Plasma levels and renal, hormonal, and hemodynamic responses to peptide infusion. J Clin Invest 78:1362–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colucci WS, Elkayam U, Horton DP, Abraham WT, Bourge RC, Johnson AD, Wagoner LE, Givertz MM, Liang CS, Neibaur M, et al. Nesiritide Study Group (2000) Intravenous nesiritide, a natriuretic peptide, in the treatment of decompensated congestive heart failure. N Engl J Med 343:246–253. [DOI] [PubMed] [Google Scholar]
- Cook VI, Grove KL, McMenamin KM, Carter MR, Harding JW, Speth RC. (1991) The AT2 angiotensin receptor subtype predominates in the 18 day gestation fetal rat brain. Brain Res 560:334–336. [DOI] [PubMed] [Google Scholar]
- Corradi HR, Chitapi I, Sewell BT, Georgiadis D, Dive V, Sturrock ED, Acharya KR. (2007) The structure of testis angiotensin-converting enzyme in complex with the C domain-specific inhibitor RXPA380. Biochemistry 46:5473–5478. [DOI] [PubMed] [Google Scholar]
- Corradi HR, Schwager SL, Nchinda AT, Sturrock ED, Acharya KR. (2006) Crystal structure of the N domain of human somatic angiotensin I-converting enzyme provides a structural basis for domain-specific inhibitor design. J Mol Biol 357:964–974. [DOI] [PubMed] [Google Scholar]
- Cotton J, Hayashi MA, Cuniasse P, Vazeux G, Ianzer D, De Camargo AC, Dive V. (2002) Selective inhibition of the C-domain of angiotensin I converting enzyme by bradykinin potentiating peptides. Biochemistry 41:6065–6071. [DOI] [PubMed] [Google Scholar]
- Cozier GE, Arendse LB, Schwager SL, Sturrock ED, Acharya KR. (2018) Molecular Basis for Multiple Omapatrilat Binding Sites within the ACE C-Domain. J Med Chem 61 (22):10141–10154. [DOI] [PubMed] [Google Scholar]
- Cushman DW, Cheung HS, Sabo EF, Ondetti MA. (1977) Design of potent competitive inhibitors of angiotensin-converting enzyme. Carboxyalkanoyl and mercaptoalkanoyl amino acids. Biochemistry 16:5484–5491. [DOI] [PubMed] [Google Scholar]
- Dahlöf B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, Fyhrquist F, Ibsen H, Kristiansson K, Lederballe-Pedersen O, et al. LIFE Study Group (2002) Cardiovascular morbidity and mortality in the Losartan Intervention for Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 359:995–1003. [DOI] [PubMed] [Google Scholar]
- Damman K, Gori M, Claggett B, Jhund PS, Senni M, Lefkowitz MP, Prescott MF, Shi VC, Rouleau JL, Swedberg K, et al. (2018) Renal effects and associated outcomes during angiotensin-neprilysin inhibition in heart failure. JACC Heart Fail 6:489–498. [DOI] [PubMed] [Google Scholar]
- Danilczyk U, Sarao R, Remy C, Benabbas C, Stange G, Richter A, Arya S, Pospisilik JA, Singer D, Camargo SM, et al. (2006) Essential role for collectrin in renal amino acid transport. Nature 444:1088–1091. [DOI] [PubMed] [Google Scholar]
- Danser AHJ, Batenburg WW, van den Meiracker AH, Danilov SM. (2007) ACE phenotyping as a first step toward personalized medicine for ACE inhibitors. Why does ACE genotyping not predict the therapeutic efficacy of ACE inhibition? Pharmacol Ther 113:607–618. [DOI] [PubMed] [Google Scholar]
- Danser AHJ, Derkx FH, Hense HW, Jeunemaître X, Riegger GA, Schunkert H. (1998) Angiotensinogen (M235T) and angiotensin-converting enzyme (I/D) polymorphisms in association with plasma renin and prorenin levels. J Hypertens 16:1879–1883. [DOI] [PubMed] [Google Scholar]
- Danser AHJ, van Kesteren CA, Bax WA, Tavenier M, Derkx FH, Saxena PR, Schalekamp MA. (1997) Prorenin, renin, angiotensinogen, and angiotensin-converting enzyme in normal and failing human hearts. Evidence for renin binding. Circulation 96:220–226. [DOI] [PubMed] [Google Scholar]
- Daull P, Benrezzak O, Arsenault D, Pheng LH, Blouin A, Cayer J, Beaudoin M, Belleville K, Sirois P, Nantel F, et al. (2005) Triple vasopeptidase inhibition normalizes blood pressure in conscious, unrestrained, and spontaneously hypertensive rats. Am J Hypertens 18:1606–1613. [DOI] [PubMed] [Google Scholar]
- Daull P, Blouin A, Belleville K, Beaudoin M, Arsenault D, Leonard H, Sirois P, Nantel F, Jeng AY, Battistini B. (2006b) Triple VPI CGS 35601 reduces high blood pressure in low-renin, high-salt Dahl salt-sensitive rats. Exp Biol Med (Maywood) 231:830–833. [PubMed] [Google Scholar]
- Daull P, Lepage R, Benrezzak O, Cayer J, Beaudoin M, Belleville K, Blouin A, Sirois P, Nantel F, Jeng AY, et al. (2006a) The first preclinical pharmacotoxicological safety assessment of CGS 35601, a triple vasopeptidase inhibitor, in chronically instrumented, conscious, and unrestrained spontaneously hypertensive rats. Drug Chem Toxicol 29:183–202. [DOI] [PubMed] [Google Scholar]
- David C, Bischoff L, Meudal H, Mothé A, De Mota N, DaNascimento S, Llorens-Cortes C, Fournié-Zaluski MC, Roques BP. (1999) Investigation of subsite preferences in aminopeptidase A (EC 3.4.11.7) led to the design of the first highly potent and selective inhibitors of this enzyme. J Med Chem 42:5197–5211. [DOI] [PubMed] [Google Scholar]
- Davis JO, Freeman RH. (1976) Mechanisms regulating renin release. Physiol Rev 56:1–56. [DOI] [PubMed] [Google Scholar]
- Davisson RL, Yang G, Beltz TG, Cassell MD, Johnson AK, Sigmund CD. (1998) The brain renin-angiotensin system contributes to the hypertension in mice containing both the human renin and human angiotensinogen transgenes. Circ Res 83:1047–1058. [DOI] [PubMed] [Google Scholar]
- de Bold AJ, de Bold ML, Sarda IR. (1986) Functional-morphological studies on in vitro cardionatrin release. J Hypertens Suppl 4:S3–S7. [PubMed] [Google Scholar]
- de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. (2000) International Union of Pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev 52:415–472. [PubMed] [Google Scholar]
- de Lannoy LM, Danser AHJ, van Kats JP, Schoemaker RG, Saxena PR, Schalekamp MA. (1997) Renin-angiotensin system components in the interstitial fluid of the isolated perfused rat heart. Local production of angiotensin I. Hypertension 29:1240–1251. [DOI] [PubMed] [Google Scholar]
- Deddish PA, Marcic B, Jackman HL, Wang HZ, Skidgel RA, Erdös EG. (1998) N-domain-specific substrate and C-domain inhibitors of angiotensin-converting enzyme: angiotensin-(1-7) and keto-ACE. Hypertension 31:912–917. [DOI] [PubMed] [Google Scholar]
- Denault JB, Claing A, D’Orléans-Juste P, Sawamura T, Kido T, Masaki T, Leduc R. (1995) Processing of proendothelin-1 by human furin convertase. FEBS Lett 362:276–280. [DOI] [PubMed] [Google Scholar]
- Dickstein K, De Voogd HJ, Miric MP, Willenbrock R, Mitrovic V, Pacher R, Koopman PA. (2004) Effect of single doses of SLV306, an inhibitor of both neutral endopeptidase and endothelin-converting enzyme, on pulmonary pressures in congestive heart failure. Am J Cardiol 94:237–239. [DOI] [PubMed] [Google Scholar]
- Dimitropoulos N, Papakyriakou A, Dalkas GA, Sturrock ED, Spyroulias GA. (2010) A computational approach to the study of the binding mode of dual ACE/NEP inhibitors. J Chem Inf Model 50:388–396. [DOI] [PubMed] [Google Scholar]
- Dive V, Cotton J, Yiotakis A, Michaud A, Vassiliou S, Jiracek J, Vazeux G, Chauvet MT, Cuniasse P, Corvol P. (1999) RXP 407, a phosphinic peptide, is a potent inhibitor of angiotensin I converting enzyme able to differentiate between its two active sites. Proc Natl Acad Sci USA 96:4330–4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R, et al. (2000) A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res 87:E1–E9. [DOI] [PubMed] [Google Scholar]
- Douglas RG, Sharma RK, Masuyer G, Lubbe L, Zamora I, Acharya KR, Chibale K, Sturrock ED. (2014) Fragment-based design for the development of N-domain-selective angiotensin-1-converting enzyme inhibitors. Clin Sci (Lond) 126:305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards BS, Zimmerman RS, Schwab TR, Heublein DM, Burnett JC., Jr (1988) Atrial stretch, not pressure, is the principal determinant controlling the acute release of atrial natriuretic factor. Circ Res 62:191–195. [DOI] [PubMed] [Google Scholar]
- Ehlers MR, Schwager SL, Scholle RR, Manji GA, Brandt WF, Riordan JF. (1996) Proteolytic release of membrane-bound angiotensin-converting enzyme: role of the juxtamembrane stalk sequence. Biochemistry 35:9549–9559. [DOI] [PubMed] [Google Scholar]
- Epelman S, Shrestha K, Troughton RW, Francis GS, Sen S, Klein AL, Tang WH. (2009) Soluble angiotensin-converting enzyme 2 in human heart failure: relation with myocardial function and clinical outcomes. J Card Fail 15:565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epelman S, Tang WH, Chen SY, Van Lente F, Francis GS, Sen S. (2008) Detection of soluble angiotensin-converting enzyme 2 in heart failure: insights into the endogenous counter-regulatory pathway of the renin-angiotensin-aldosterone system. J Am Coll Cardiol 52:750–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdös EG, Skidgel RA. (1989) Neutral endopeptidase 24.11 (enkephalinase) and related regulators of peptide hormones. FASEB J 3:145–151. [PubMed] [Google Scholar]
- Fagyas M, Úri K, Siket IM, Fülöp G, Csató V, Daragó A, Boczán J, Bányai E, Szentkirályi IE, Maros TM, et al. (2014) New perspectives in the renin-angiotensin-aldosterone system (RAAS) II: albumin suppresses angiotensin converting enzyme (ACE) activity in human. PLoS One 9:e87844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattah C, Nather K, McCarroll CS, Hortigon-Vinagre MP, Zamora V, Flores-Munoz M, McArthur L, Zentilin L, Giacca M, Touyz RM, et al. (2016) Gene therapy with angiotensin-(1-9) preserves left ventricular systolic function after myocardial infarction. J Am Coll Cardiol 68:2652–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favrat B, Burnier M, Nussberger J, Lecomte JM, Brouard R, Waeber B, Brunner HR. (1995) Neutral endopeptidase versus angiotensin converting enzyme inhibition in essential hypertension. J Hypertens 13:797–804. [PubMed] [Google Scholar]
- Ferdinand K, Balavoine F, Besse B, Black H, Desbrandes S, Dittrich H, Nesbitt S. (2018) Efficacy and safety of a novel antihypertensive pharmacotherapy approach in a high-risk diverse population (Abstract). Circulation 138:e766–e767. [Google Scholar]
- Ferrario CM, Mullick AE. (2017) Renin angiotensin aldosterone inhibition in the treatment of cardiovascular disease. Pharmacol Res 125:57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira PM, Souza Dos Santos RA, Campagnole-Santos MJ. (2007) Angiotensin-(3-7) pressor effect at the rostral ventrolateral medulla. Regul Pept 141:168–174. [DOI] [PubMed] [Google Scholar]
- Ferro CJ, Spratt JC, Haynes WG, Webb DJ. (1998) Inhibition of neutral endopeptidase causes vasoconstriction of human resistance vessels in vivo. Circulation 97:2323–2330. [DOI] [PubMed] [Google Scholar]
- Fienberg S, Cozier GE, Acharya KR, Chibale K, Sturrock ED. (2018) The design and development of a potent and selective novel diprolyl derivative that binds to the N-domain of angiotensin-I converting enzyme. J Med Chem 61:344–359. [DOI] [PubMed] [Google Scholar]
- Fitzgibbon WR, Dang Y, Bunni MA, Baicu CF, Zile MR, Mullick AE, Saigusa T. (2018) Attenuation of accelerated renal cystogenesis in Pkd1 mice by renin-angiotensin system blockade. Am J Physiol Renal Physiol 314:F210–F218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Muñoz M, Smith NJ, Haggerty C, Milligan G, Nicklin SA. (2011) Angiotensin1-9 antagonises pro-hypertrophic signalling in cardiomyocytes via the angiotensin type 2 receptor. J Physiol 589:939–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Muñoz M, Work LM, Douglas K, Denby L, Dominiczak AF, Graham D, Nicklin SA. (2012) Angiotensin-(1-9) attenuates cardiac fibrosis in the stroke-prone spontaneously hypertensive rat via the angiotensin type 2 receptor. Hypertension 59:300–307. [DOI] [PubMed] [Google Scholar]
- Fonarow GC, Hernandez AF, Solomon SD, Yancy CW. (2016) Potential mortality reduction with optimal implementation of angiotensin receptor neprilysin inhibitor therapy in heart failure. JAMA Cardiol 1:714–717. [DOI] [PubMed] [Google Scholar]
- Fournié-Zaluski MC, Coric P, Turcaud S, Bruetschy L, Lucas E, Noble F, Roques BP. (1992) Potent and systemically active aminopeptidase N inhibitors designed from active-site investigation. J Med Chem 35:1259–1266. [DOI] [PubMed] [Google Scholar]
- Fournie-Zaluski MC, Fassot C, Valentin B, Djordjijevic D, Reaux-Le Goazigo A, Corvol P, Roques BP, Llorens-Cortes C. (2004) Brain renin-angiotensin system blockade by systemically active aminopeptidase A inhibitors: a potential treatment of salt-dependent hypertension. Proc Natl Acad Sci USA 101:7775–7780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AJ, Lalloo UG, Belvisi MG, Bernareggi M, Chung KF, Barnes PJ. (1996) Bradykinin-evoked sensitization of airway sensory nerves: a mechanism for ACE-inhibitor cough. Nat Med 2:814–817. [DOI] [PubMed] [Google Scholar]
- Fraga-Silva RA, Pinheiro SVB, Gonçalves ACC, Alenina N, Bader M, Santos RAS. (2008) The antithrombotic effect of angiotensin-(1-7) involves mas-mediated NO release from platelets. Mol Med 14:28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer RM, Segreti J, Banfor PN, Widomski DL, Backes BJ, Lin CW, Ballaron SJ, Cox BF, Trevillyan JM, Reinhart GA, et al. (2008) Effect of bradykinin metabolism inhibitors on evoked hypotension in rats: rank efficacy of enzymes associated with bradykinin-mediated angioedema. Br J Pharmacol 153:947–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs S, Xiao HD, Cole JM, Adams JW, Frenzel K, Michaud A, Zhao H, Keshelava G, Capecchi MR, Corvol P, et al. (2004) Role of the N-terminal catalytic domain of angiotensin-converting enzyme investigated by targeted inactivation in mice. J Biol Chem 279:15946–15953. [DOI] [PubMed] [Google Scholar]
- Fuchs S, Xiao HD, Hubert C, Michaud A, Campbell DJ, Adams JW, Capecchi MR, Corvol P, Bernstein KE. (2008) Angiotensin-converting enzyme C-terminal catalytic domain is the main site of angiotensin I cleavage in vivo. Hypertension 51:267–274. [DOI] [PubMed] [Google Scholar]
- Gaidarov I, Adams J, Frazer J, Anthony T, Chen X, Gatlin J, Semple G, Unett DJ. (2018) Angiotensin (1-7) does not interact directly with MAS1, but can potently antagonize signaling from the AT1 receptor. Cell Signal 50:9–24. [DOI] [PubMed] [Google Scholar]
- Galandrin S, Denis C, Boularan C, Marie J, M’Kadmi C, Pilette C, Dubroca C, Nicaise Y, Seguelas MH, N’Guyen D, et al. (2016) Cardioprotective angiotensin-(1-7) peptide acts as a natural-biased ligand at the angiotensin II type 1 receptor. Hypertension 68:1365–1374. [DOI] [PubMed] [Google Scholar]
- Ganten D, Hermann K, Bayer C, Unger T, Lang RE. (1983) Angiotensin synthesis in the brain and increased turnover in hypertensive rats. Science 221:869–871. [DOI] [PubMed] [Google Scholar]
- Gembardt F, Grajewski S, Vahl M, Schultheiss HP, Walther T. (2008) Angiotensin metabolites can stimulate receptors of the Mas-related genes family. Mol Cell Biochem 319:115–123. [DOI] [PubMed] [Google Scholar]
- Georgiadis D, Beau F, Czarny B, Cotton J, Yiotakis A, Dive V. (2003) Roles of the two active sites of somatic angiotensin-converting enzyme in the cleavage of angiotensin I and bradykinin: insights from selective inhibitors. Circ Res 93:148–154. [DOI] [PubMed] [Google Scholar]
- Gjymishka A, Kulemina LV, Shenoy V, Katovich MJ, Ostrov DA, Raizada MK. (2010) Diminazene aceturate is an ACE2 activator and a novel antihypertensive drug. FASEB J 24:1032.3. [Google Scholar]
- Glenner GG, McMILLAN PJ, Folk JE. (1962) A mammalian peptidase specific for the hydrolysis of N-terminal α-L-glutamyl and aspartyl residues. Nature 194:867. [DOI] [PubMed] [Google Scholar]
- Glossop MS, Bazin RJ, Dack KN, Fox DN, MacDonald GA, Mills M, Owen DR, Phillips C, Reeves KA, Ringer TJ, et al. (2011) Synthesis and evaluation of heteroarylalanine diacids as potent and selective neutral endopeptidase inhibitors. Bioorg Med Chem Lett 21:3404–3406. [DOI] [PubMed] [Google Scholar]
- Goto Y, Hattori A, Mizutani S, Tsujimoto M. (2007) Asparatic acid 221 is critical in the calcium-induced modulation of the enzymatic activity of human aminopeptidase A. J Biol Chem 282:37074–37081. [DOI] [PubMed] [Google Scholar]
- Granger CB, McMurray JJ, Yusuf S, Held P, Michelson EL, Olofsson B, Östergren J, Pfeffer MA, Swedberg K, CHARM Investigators and Committees (2003) Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet 362:772–776. [DOI] [PubMed] [Google Scholar]
- Grobe JL, Der Sarkissian S, Stewart JM, Meszaros JG, Raizada MK, Katovich MJ. (2007) ACE2 overexpression inhibits hypoxia-induced collagen production by cardiac fibroblasts. Clin Sci (Lond) 113:357–364. [DOI] [PubMed] [Google Scholar]
- Gu J, Noe A, Chandra P, Al-Fayoumi S, Ligueros-Saylan M, Sarangapani R, Maahs S, Ksander G, Rigel DF, Jeng AY, et al. (2010) Pharmacokinetics and pharmacodynamics of LCZ696, a novel dual-acting angiotensin receptor-neprilysin inhibitor (ARNi). J Clin Pharmacol 50:401–414. [DOI] [PubMed] [Google Scholar]
- Guimarães PB, Alvarenga EC, Siqueira PD, Paredes-Gamero EJ, Sabatini RA, Morais RL, Reis RI, Santos EL, Teixeira LG, Casarini DE, et al. (2011) Angiotensin II binding to angiotensin I-converting enzyme triggers calcium signaling. Hypertension 57:965–972. [DOI] [PubMed] [Google Scholar]
- Gyurko R, Wielbo D, Phillips MI. (1993) Antisense inhibition of AT1 receptor mRNA and angiotensinogen mRNA in the brain of spontaneously hypertensive rats reduces hypertension of neurogenic origin. Regul Pept 49:167–174. [DOI] [PubMed] [Google Scholar]
- Hagiwara H, Sakaguchi H, Itakura M, Yoshimoto T, Furuya M, Tanaka S, Hirose S. (1994) Autocrine regulation of rat chondrocyte proliferation by natriuretic peptide C and its receptor, natriuretic peptide receptor-B. J Biol Chem 269:10729–10733. [PubMed] [Google Scholar]
- Hansen JL, Theilade J, Haunsø S, Sheikh SP. (2004) Oligomerization of wild type and nonfunctional mutant angiotensin II type I receptors inhibits galphaq protein signaling but not ERK activation. J Biol Chem 279:24108–24115. [DOI] [PubMed] [Google Scholar]
- Haschke M, Schuster M, Poglitsch M, Loibner H, Salzberg M, Bruggisser M, Penninger J, Krähenbühl S. (2013) Pharmacokinetics and pharmacodynamics of recombinant human angiotensin-converting enzyme 2 in healthy human subjects. Clin Pharmacokinet 52:783–792. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Nakao K, Hama N, Imura H, Mori S, Yamaguchi M, Yasuhara M, Hori R. (1994) Clearance mechanisms of atrial and brain natriuretic peptides in rats. Pharm Res 11:60–64. [DOI] [PubMed] [Google Scholar]
- Hata N, Seino Y, Tsutamoto T, Hiramitsu S, Kaneko N, Yoshikawa T, Yokoyama H, Tanaka K, Mizuno K, Nejima J, et al. (2008) Effects of carperitide on the long-term prognosis of patients with acute decompensated chronic heart failure: the PROTECT multicenter randomized controlled study. Circ J 72:1787–1793. [DOI] [PubMed] [Google Scholar]
- Hawkridge AM, Heublein DM, Bergen HR, 3rd, Cataliotti A, Burnett JC, Jr, Muddiman DC. (2005) Quantitative mass spectral evidence for the absence of circulating brain natriuretic peptide (BNP-32) in severe human heart failure. Proc Natl Acad Sci USA 102:17442–17447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes R, Judge PK, Staplin N, Herrington WG, Storey BC, Bethel A, Bowman L, Brunskill N, Cockwell P, Hill M, et al. (2018) Effects of sacubitril/valsartan versus irbesartan in patients with chronic kidney disease. Circulation 138:1505–1514. [DOI] [PubMed] [Google Scholar]
- Healy DP, Wilk S. (1993) Localization of immunoreactive glutamyl aminopeptidase in rat brain. II. Distribution and correlation with angiotensin II. Brain Res 606:295–303. [DOI] [PubMed] [Google Scholar]
- Hernández Prada JA, Ferreira AJ, Katovich MJ, Shenoy V, Qi Y, Santos RA, Castellano RK, Lampkins AJ, Gubala V, Ostrov DA, et al. (2008) Structure-based identification of small-molecule angiotensin-converting enzyme 2 activators as novel antihypertensive agents. Hypertension 51:1312–1317. [DOI] [PubMed] [Google Scholar]
- Hoang MV, Turner AJ. (1997) Novel activity of endothelin-converting enzyme: hydrolysis of bradykinin. Biochem J 327:23–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang BS, Ahmad M, White RA, Marc Y, Llorens-Cortes C, Leenen FH. (2013) Inhibition of brain angiotensin III attenuates sympathetic hyperactivity and cardiac dysfunction in rats post-myocardial infarction. Cardiovasc Res 97:424–431. [DOI] [PubMed] [Google Scholar]
- Ichiki T, Kambayashi Y, Inagami T. (1995) Multiple growth factors modulate mRNA expression of angiotensin II type-2 receptor in R3T3 cells. Circ Res 77:1070–1076. [DOI] [PubMed] [Google Scholar]
- Imai T, Hirata Y, Emori T, Yanagisawa M, Masaki T, Marumo F. (1992) Induction of endothelin-1 gene by angiotensin and vasopressin in endothelial cells. Hypertension 19:753–757. [DOI] [PubMed] [Google Scholar]
- Intengan HD, Schiffrin EL. (2000) Vasopeptidase inhibition has potent effects on blood pressure and resistance arteries in stroke-prone spontaneously hypertensive rats. Hypertension 35:1221–1225. [DOI] [PubMed] [Google Scholar]
- Israili ZH, Hall WD. (1992) Cough and angioneurotic edema associated with angiotensin-converting enzyme inhibitor therapy. A review of the literature and pathophysiology. Ann Intern Med 117:234–242. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Yanagisawa M, Ohkubo S, Kimura C, Kosaka T, Inoue A, Ishida N, Mitsui Y, Onda H, Fujino M, et al. (1988) Cloning and sequence analysis of cDNA encoding the precursor of a human endothelium-derived vasoconstrictor peptide, endothelin: identity of human and porcine endothelin. FEBS Lett 231:440–444. [DOI] [PubMed] [Google Scholar]
- Iusuf D, Henning RH, van Gilst WH, Roks AJ. (2008) Angiotensin-(1-7): pharmacological properties and pharmacotherapeutic perspectives. Eur J Pharmacol 585:303–312. [DOI] [PubMed] [Google Scholar]
- Iwata N, Tsubuki S, Takaki Y, Shirotani K, Lu B, Gerard NP, Gerard C, Hama E, Lee HJ, Saido TC. (2001) Metabolic regulation of brain Abeta by neprilysin. Science 292:1550–1552. [DOI] [PubMed] [Google Scholar]
- Jackman HL, Massad MG, Sekosan M, Tan F, Brovkovych V, Marcic BM, Erdös EG. (2002) Angiotensin 1-9 and 1-7 release in human heart: role of cathepsin A. Hypertension 39:976–981. [DOI] [PubMed] [Google Scholar]
- Jackson TR, Blair LA, Marshall J, Goedert M, Hanley MR. (1988) The mas oncogene encodes an angiotensin receptor. Nature 335:437–440. [DOI] [PubMed] [Google Scholar]
- Jandeleit-Dahm KA. (2006) Dual ACE/NEP inhibitors—more than playing the ACE card. J Hum Hypertens 20:478–481. [DOI] [PubMed] [Google Scholar]
- Jankowski V, Tölle M, Santos RA, Günthner T, Krause E, Beyermann M, Welker P, Bader M, Pinheiro SVB, Sampaio WO, et al. (2011) Angioprotectin: an angiotensin II-like peptide causing vasodilatory effects. FASEB J 25:2987–2995. [DOI] [PubMed] [Google Scholar]
- Jarcho J. (2019) PIONEERing the in-hospital initiation of sacubitril-valsartan. N Engl J Med 380:590–591. [DOI] [PubMed] [Google Scholar]
- Jiang F, Yang J, Zhang Y, Dong M, Wang S, Zhang Q, Liu FF, Zhang K, Zhang C. (2014) Angiotensin-converting enzyme 2 and angiotensin 1-7: novel therapeutic targets. Nat Rev Cardiol 11:413–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AG, Pearce GL, Danoff TM. (2006) A randomized, double-blind, placebo-controlled, parallel-group study to assess the efficacy and safety of dual ACE/NEP inhibitor GW660511X in mild-to-moderate hypertensive patients. J Hum Hypertens 20:496–503. [DOI] [PubMed] [Google Scholar]
- Jullien N, Makritis A, Georgiadis D, Beau F, Yiotakis A, Dive V. (2010) Phosphinic tripeptides as dual angiotensin-converting enzyme C-domain and endothelin-converting enzyme-1 inhibitors. J Med Chem 53:208–220. [DOI] [PubMed] [Google Scholar]
- Junot C, Gonzales MF, Ezan E, Cotton J, Vazeux G, Michaud A, Azizi M, Vassiliou S, Yiotakis A, Corvol P, et al. (2001) RXP 407, a selective inhibitor of the N-domain of angiotensin I-converting enzyme, blocks in vivo the degradation of hemoregulatory peptide acetyl-Ser-Asp-Lys-Pro with no effect on angiotensin I hydrolysis. J Pharmacol Exp Ther 297:606–611. [PubMed] [Google Scholar]
- Kakoki M, Smithies O. (2009) The kallikrein-kinin system in health and in diseases of the kidney. Kidney Int 75:1019–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambayashi Y, Bardhan S, Takahashi K, Tsuzuki S, Inui H, Hamakubo T, Inagami T. (1993) Molecular cloning of a novel angiotensin II receptor isoform involved in phosphotyrosine phosphatase inhibition. J Biol Chem 268:24543–24546. [PubMed] [Google Scholar]
- Kambayashi Y, Nagata K, Ichiki T, Inagami T. (1996) Insulin and insulin-like growth factors induce expression of angiotensin type-2 receptor in vascular-smooth-muscle cells. Eur J Biochem 239:558–565. [DOI] [PubMed] [Google Scholar]
- Kang J, Posner P, Sumners C. (1994) Angiotensin II type 2 receptor stimulation of neuronal K+ currents involves an inhibitory GTP binding protein. Am J Physiol 267:C1389–C1397. [DOI] [PubMed] [Google Scholar]
- Kang J, Richards EM, Posner P, Sumners C. (1995) Modulation of the delayed rectifier K+ current in neurons by an angiotensin II type 2 receptor fragment. Am J Physiol 268:C278–C282. [DOI] [PubMed] [Google Scholar]
- Karnik SS, Singh KD, Tirupula K, Unal H. (2017) Significance of angiotensin 1-7 coupling with MAS1 receptor and other GPCRs to the renin-angiotensin system: IUPHAR Review 22. Br J Pharmacol 174:737–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnik SS, Unal H, Kemp JR, Tirupula KC, Eguchi S, Vanderheyden PM, Thomas WG. (2015) International Union of Basic and Clinical Pharmacology. XCIX. Angiotensin receptors: interpreters of pathophysiological angiotensinergic stimuli [published correction appears in Pharmacol Rev (2015) 67:820]. Pharmacol Rev 67:754–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedzierski RM, Yanagisawa M. (2001) Endothelin system: the double-edged sword in health and disease. Annu Rev Pharmacol Toxicol 41:851–876. [DOI] [PubMed] [Google Scholar]
- Kerr MA, Kenny AJ. (1974) The purification and specificity of a neutral endopeptidase from rabbit kidney brush border. Biochem J 137:477–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan BA, Perkins AJ, Gao S, Hui SL, Campbell NL, Farber MO, Chlan LL, Boustani MA. (2017) The confusion assessment method for the ICU-7 delirium severity scale: a novel delirium severity instrument for use in the ICU. Crit Care Med 45:851–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kijima K, Matsubara H, Murasawa S, Maruyama K, Ohkubo N, Mori Y, Inada M. (1996) Regulation of angiotensin II type 2 receptor gene by the protein kinase C-calcium pathway. Hypertension 27:529–534. [DOI] [PubMed] [Google Scholar]
- Kimura S, Kasuya Y, Sawamura T, Shinimi O, Sugita Y, Yanagisawa M, Goto K, Masaki T. (1989) Conversion of big endothelin-1 to 21-residue endothelin-1 is essential for expression of full vasoconstrictor activity: structure-activity relationships of big endothelin-1. J Cardiovasc Pharmacol 13 (Suppl 5):S5–S7, discussion S18. [DOI] [PubMed] [Google Scholar]
- Kleniewski J. (1979) Plasma high molecular weight kininogen concentration in health and in chosen impairments of haemostasis. Evidence that plasmin uncovers a new antigenic site in high molecular weight kininogen. Thromb Haemost 42:1046–1055. [PubMed] [Google Scholar]
- Klotz S, Burkhoff D, Garrelds IM, Boomsma F, Danser AHJ. (2009) The impact of left ventricular assist device-induced left ventricular unloading on the myocardial renin-angiotensin-aldosterone system: therapeutic consequences? Eur Heart J 30:805–812. [DOI] [PubMed] [Google Scholar]
- Komers R, Gipson DS, Nelson P, Adler S, Srivastava T, Derebail VK, Meyers KE, Pergola P, MacNally ME, Hunt JL, et al. (2017) Efficacy and safety of sparsentan compared with irbesartan in patients with primary focal segmental glomerulosclerosis: randomized, controlled trial design (DUET). Kidney Int Rep 2:654–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komers R, Plotkin H. (2016) Dual inhibition of renin-angiotensin-aldosterone system and endothelin-1 in treatment of chronic kidney disease. Am J Physiol Regul Integr Comp Physiol 310:R877–R884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kompa AR, Lu J, Weller TJ, Kelly DJ, Krum H, von Lueder TG, Wang BH. (2018) Angiotensin receptor neprilysin inhibition provides superior cardioprotection compared to angiotensin converting enzyme inhibition after experimental myocardial infarction. Int J Cardiol 258:192–198. [DOI] [PubMed] [Google Scholar]
- Korth P, Bohle RM, Corvol P, Pinet F. (1999) Cellular distribution of endothelin-converting enzyme-1 in human tissues. J Histochem Cytochem 47:447–462. [DOI] [PubMed] [Google Scholar]
- Kostenis E, Milligan G, Christopoulos A, Sanchez-Ferrer CF, Heringer-Walther S, Sexton PM, Gembardt F, Kellett E, Martini L, Vanderheyden P, et al. (2005) G-protein-coupled receptor Mas is a physiological antagonist of the angiotensin II type 1 receptor. Circulation 111:1806–1813. [DOI] [PubMed] [Google Scholar]
- Kostis JB, Packer M, Black HR, Schmieder R, Henry D, Levy E. (2004) Omapatrilat and enalapril in patients with hypertension: the Omapatrilat Cardiovascular Treatment vs. Enalapril (OCTAVE) trial. Am J Hypertens 17:103–111. [DOI] [PubMed] [Google Scholar]
- Kostis WJ, Shetty M, Chowdhury YS, Kostis JB. (2018) ACE inhibitor-induced angioedema: a review. Curr Hypertens Rep 20:55. [DOI] [PubMed] [Google Scholar]
- Kowalczuk S, Bröer A, Tietze N, Vanslambrouck JM, Rasko JE, Bröer S. (2008) A protein complex in the brush-border membrane explains a Hartnup disorder allele. FASEB J 22:2880–2887. [DOI] [PubMed] [Google Scholar]
- Kröger WL, Douglas RG, O’Neill HG, Dive V, Sturrock ED. (2009) Investigating the domain specificity of phosphinic inhibitors RXPA380 and RXP407 in angiotensin-converting enzyme. Biochemistry 48:8405–8412. [DOI] [PubMed] [Google Scholar]
- Kuhn M. (2016) Molecular physiology of membrane guanylyl cyclase receptors. Physiol Rev 96:751–804. [DOI] [PubMed] [Google Scholar]
- Kukkola PJ, Savage P, Sakane Y, Berry JC, Bilci NA, Ghai RD, Jeng AY. (1995) Differential structure-activity relationships of phosphoramidon analogues for inhibition of three metalloproteases: endothelin-converting enzyme, neutral endopeptidase, and angiotensin-converting enzyme. J Cardiovasc Pharmacol 26 (Suppl 3):S65–S68. [PubMed] [Google Scholar]
- Kuoppala A, Lindstedt KA, Saarinen J, Kovanen PT, Kokkonen JO. (2000) Inactivation of bradykinin by angiotensin-converting enzyme and by carboxypeptidase N in human plasma. Am J Physiol Heart Circ Physiol 278:H1069–H1074. [DOI] [PubMed] [Google Scholar]
- Lachance D, Garcia R, Gutkowska J, Cantin M, Thibault G. (1986) Mechanisms of release of atrial natriuretic factor. I. Effect of several agonists and steroids on its release by atrial minces. Biochem Biophys Res Commun 135:1090–1098. [DOI] [PubMed] [Google Scholar]
- Lalmanach G, Naudin C, Lecaille F, Fritz H. (2010) Kininogens: more than cysteine protease inhibitors and kinin precursors. Biochimie 92:1568–1579. [DOI] [PubMed] [Google Scholar]
- Lambert DW, Yarski M, Warner FJ, Thornhill P, Parkin ET, Smith AI, Hooper NM, Turner AJ. (2005) Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2). J Biol Chem 280:30113–30119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenickel TH, Tsubouchi C, Ayalasomayajula S, Pal P, Valentin MA, Hinder M, Jhee S, Gevorkyan H, Rajman I. (2016) The effect of LCZ696 (sacubitril/valsartan) on amyloid-β concentrations in cerebrospinal fluid in healthy subjects. Br J Clin Pharmacol 81:878–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapointe N, Rouleau JL. (2002) Cardioprotective effects of vasopeptidase inhibitors. Can J Cardiol 18:415–420. [PubMed] [Google Scholar]
- Lautner RQ, Villela DC, Fraga-Silva RA, Silva N, Verano-Braga T, Costa-Fraga F, Jankowski J, Jankowski V, Sousa F, Alzamora A, et al. (2013) Discovery and characterization of alamandine: a novel component of the renin-angiotensin system [published correction appears in Circ Res (2013) 112:e156]. Circ Res 112:1104–1111. [DOI] [PubMed] [Google Scholar]
- Leal MC, Pinheiro SV, Ferreira AJ, Santos RA, Bordoni LS, Alenina N, Bader M, França LR. (2009) The role of angiotensin-(1-7) receptor Mas in spermatogenesis in mice and rats. J Anat 214:736–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeb-Lundberg LM, Marceau F, Müller-Esterl W, Pettibone DJ, Zuraw BL. (2005) International Union of Pharmacology. XLV. Classification of the kinin receptor family: from molecular mechanisms to pathophysiological consequences. Pharmacol Rev 57:27–77. [DOI] [PubMed] [Google Scholar]
- Lejczak B, De Choszczak MP, Kafarski P. (1993) Inhibition of aminopeptidases by phosphonic acid and phosphinic acid analogues of aspartic and glutamic acids. J Enzyme Inhib 7:97–103. [DOI] [PubMed] [Google Scholar]
- Lenkei Z, Palkovits M, Corvol P, Llorens-Cortès C. (1997) Expression of angiotensin type-1 (AT1) and type-2 (AT2) receptor mRNAs in the adult rat brain: a functional neuroanatomical review. Front Neuroendocrinol 18:383–439. [DOI] [PubMed] [Google Scholar]
- Levin AA. (2019) Treating disease at the RNA level with oligonucleotides. N Engl J Med 380:57–70. [DOI] [PubMed] [Google Scholar]
- Li F, Li W, Farzan M, Harrison SC. (2005) Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science 309:1864–1868. [DOI] [PubMed] [Google Scholar]
- Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, et al. (2003) Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 426:450–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu GC, Oudit GY, Fang F, Zhou J, Scholey JW. (2012) Angiotensin-(1-7)-induced activation of ERK1/2 is cAMP/protein kinase A-dependent in glomerular mesangial cells. Am J Physiol Renal Physiol 302:F784–F790. [DOI] [PubMed] [Google Scholar]
- Llorens C, Gacel G, Swerts JP, Perdrisot R, Fournie-Zaluski MC, Schwartz JC, Roques BP. (1980) Rational design of enkephalinase inhibitors: substrate specificity of enkephalinase studied from inhibitory potency of various dipeptides. Biochem Biophys Res Commun 96:1710–1716. [DOI] [PubMed] [Google Scholar]
- Lojda Z, Gossrau R. (1980) Study on aminopeptidase A. Histochemistry 67:267–290. [DOI] [PubMed] [Google Scholar]
- Lombard-Banek C, Yu Z, Swiercz AP, Marvar PJ, Nemes P. (2019) A microanalytical capillary electrophoresis mass spectrometry assay for quantifying angiotensin peptides in the brain. Anal Bioanal Chem 411:4661–4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez Verrilli MA, Rodriguez Fermepín M, Longo Carbajosa N, Landa S, Cerrato BD, García S, Fernandez BE, Gironacci MM. (2012) Angiotensin-(1-7) through Mas receptor up-regulates neuronal norepinephrine transporter via Akt and Erk1/2-dependent pathways. J Neurochem 120:46–55. [DOI] [PubMed] [Google Scholar]
- Ma L, Li J, Wang G, Gong S, Zhang L, Li K, Ji X, Liu Y, Chen P, Xiang X. (2013) Atrial natriuretic peptide suppresses Th17 development through regulation of cGMP-dependent protein kinase and PI3K-Akt signaling pathways. Regul Pept 181:9–16. [DOI] [PubMed] [Google Scholar]
- Macheret F, Heublein D, Costello-Boerrigter LC, Boerrigter G, McKie P, Bellavia D, Mangiafico S, Ikeda Y, Bailey K, Scott CG, et al. (2012) Human hypertension is characterized by a lack of activation of the antihypertensive cardiac hormones ANP and BNP. J Am Coll Cardiol 60:1558–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisel AS, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, Omland T, Storrow AB, Abraham WT, Wu AH, et al. Breathing Not Properly Multinational Study Investigators (2002) Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med 347:161–167. [DOI] [PubMed] [Google Scholar]
- Malek V, Sharma N, Sankrityayan H, Gaikwad AB. (2019) Concurrent neprilysin inhibition and renin-angiotensin system modulations prevented diabetic nephropathy. Life Sci 221:159–167. [DOI] [PubMed] [Google Scholar]
- Malfroy B, Swerts JP, Guyon A, Roques BP, Schwartz JC. (1978) High-affinity enkephalin-degrading peptidase in brain is increased after morphine. Nature 276:523–526. [DOI] [PubMed] [Google Scholar]
- Marc Y, Gao J, Balavoine F, Michaud A, Roques BP, Llorens-Cortes C. (2012) Central antihypertensive effects of orally active aminopeptidase A inhibitors in spontaneously hypertensive rats. Hypertension 60:411–418. [DOI] [PubMed] [Google Scholar]
- Marc Y, Hmazzou R, Balavoine F, Flahault A, Llorens-Cortes C. (2018) Central antihypertensive effects of chronic treatment with RB150: an orally active aminopeptidase A inhibitor in deoxycorticosterone acetate-salt rats. J Hypertens 36:641–650. [DOI] [PubMed] [Google Scholar]
- Marcic B, Deddish PA, Jackman HL, Erdös EG. (1999) Enhancement of bradykinin and resensitization of its B2 receptor. Hypertension 33:835–843. [DOI] [PubMed] [Google Scholar]
- Masuyer G, Akif M, Czary B, Beau F, Schwager SL, Sturrock ED, Isaac RE, Dive V, Acharya KR. (2014) Crystal structures of highly specific phosphinic tripeptide enantiomers in complex with the angiotensin-I converting enzyme. FEBS J 281 (3):943–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuyer G, Schwager SL, Sturrock ED, Isaac RE, Acharya KR. (2012) Molecular recognition and regulation of human angiotensin-I converting enzyme (ACE) activity by natural inhibitory peptides. Sci Rep 2:717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsusaka T, Niimura F, Pastan I, Shintani A, Nishiyama A, Ichikawa I. (2014) Podocyte injury enhances filtration of liver-derived angiotensinogen and renal angiotensin II generation. Kidney Int 85:1068–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsusaka T, Niimura F, Shimizu A, Pastan I, Saito A, Kobori H, Nishiyama A, Ichikawa I. (2012) Liver angiotensinogen is the primary source of renal angiotensin II. J Am Soc Nephrol 23:1181–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau J, Shi VC, Solomon SD, Swedberg K, et al. PARADIGM-HF Committees and Investigators (2013) Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin-converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure trial (PARADIGM-HF). Eur J Heart Fail 15:1062–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, et al. PARADIGM-HF Investigators and Committees (2014) Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 371:993–1004. [DOI] [PubMed] [Google Scholar]
- Meems LMG, Andersen IA, Pan S, Harty G, Chen Y, Zheng Y, Harders GE, Ichiki T, Heublein DM, Iyer SR, et al. (2019) Design, synthesis, and actions of an innovative bispecific designer peptide. Hypertension 73:900–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meems LMG, Burnett JC., Jr (2016) Innovative therapeutics: designer natriuretic peptides. JACC Basic Transl Sci 1:557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentz RJ, Bakris GL, Waeber B, McMurray JJ, Gheorghiade M, Ruilope LM, Maggioni AP, Swedberg K, Piña IL, Fiuzat M, et al. (2013) The past, present and future of renin-angiotensin aldosterone system inhibition. Int J Cardiol 167:1677–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migaud M, Durieux C, Viereck J, Soroca-Lucas E, Fournié-Zaluski MC, Roques BP. (1996) The in vivo metabolism of cholecystokinin (CCK-8) is essentially ensured by aminopeptidase A. Peptides 17:601–607. [DOI] [PubMed] [Google Scholar]
- Millan MA, Jacobowitz DM, Aguilera G, Catt KJ. (1991) Differential distribution of AT1 and AT2 angiotensin II receptor subtypes in the rat brain during development. Proc Natl Acad Sci USA 88:11440–11444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen UM, Gong J, Jhund PS, Shen L, Køber L, Desai AS, Lefkowitz MP, Packer M, Rouleau JL, Solomon SD, et al. (2018) Effect of sacubitril/valsartan on recurrent events in the Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure trial (PARADIGM-HF). Eur J Heart Fail 20:760–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinaro G, Cugno M, Perez M, Lepage Y, Gervais N, Agostoni A, Adam A. (2002) Angiotensin-converting enzyme inhibitor-associated angioedema is characterized by a slower degradation of des-arginine(9)-bradykinin. J Pharmacol Exp Ther 303:232–237. [DOI] [PubMed] [Google Scholar]
- Mori J, Patel VB, Abo Alrob O, Basu R, Altamimi T, Desaulniers J, Wagg CS, Kassiri Z, Lopaschuk GD, Oudit GY. (2014) Angiotensin 1-7 ameliorates diabetic cardiomyopathy and diastolic dysfunction in db/db mice by reducing lipotoxicity and inflammation. Circ Heart Fail 7:327–339. [DOI] [PubMed] [Google Scholar]
- Morrow DA, Velazquez EJ, DeVore AD, Prescott MF, Duffy CI, Gurmu Y, McCague K, Rocha R, Braunwald E. (2019) Cardiovascular biomarkers in patients with acute decompensated heart failure randomized to sacubitril-valsartan or enalapril in the PIONEER-HF trial. Eur Heart J [published ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motte S, McEntee K, Naeije R. (2006) Endothelin receptor antagonists. Pharmacol Ther 110:386–414. [DOI] [PubMed] [Google Scholar]
- Moukarbel GV, Solomon SD. (2008) Early use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers: evidence from clinical trials. Curr Heart Fail Rep 5:197–203. [DOI] [PubMed] [Google Scholar]
- Mukoyama M, Nakao K, Hosoda K, Suga S, Saito Y, Ogawa Y, Shirakami G, Jougasaki M, Obata K, Yasue H, et al. (1991) Brain natriuretic peptide as a novel cardiac hormone in humans. Evidence for an exquisite dual natriuretic peptide system, atrial natriuretic peptide and brain natriuretic peptide. J Clin Invest 87:1402–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullick AE, Yeh ST, Graham MJ, Engelhardt JA, Prakash TP, Crooke RM. (2017) Blood pressure lowering and safety improvements with liver angiotensinogen inhibition in models of hypertension and kidney injury. Hypertension 70:566–576. [DOI] [PubMed] [Google Scholar]
- Murphy TJ, Alexander RW, Griendling KK, Runge MS, Bernstein KE. (1991) Isolation of a cDNA encoding the vascular type-1 angiotensin II receptor. Nature 351:233–236. [DOI] [PubMed] [Google Scholar]
- Murugesan N, Gu Z, Fadnis L, Tellew JE, Baska RAF, Yang Y, Beyer SM, Monshizadegan H, Dickinson KE, Valentine MT, et al. (2005) Dual angiotensin II and endothelin A receptor antagonists: synthesis of 2′-substituted N-3-isoxazolyl biphenylsulfonamides with improved potency and pharmacokinetics. J Med Chem 48:171–179. [DOI] [PubMed] [Google Scholar]
- Murugesan N, Tellew JE, Gu Z, Kunst BL, Fadnis L, Cornelius LA, Baska RAF, Yang Y, Beyer SM, Monshizadegan H, et al. (2002) Discovery of N-isoxazolyl biphenylsulfonamides as potent dual angiotensin II and endothelin A receptor antagonists. J Med Chem 45:3829–3835. [DOI] [PubMed] [Google Scholar]
- Naftilan AJ, Oparil S. (1978) Inhibition of renin release from rat kidney slices by the angiotensins. Am J Physiol 235:F62–F68. [DOI] [PubMed] [Google Scholar]
- Nagatsu I, Nagatsu T, Yamamoto T, Glenner GG, Mehl JW. (1970) Purification of aminopeptidase A in human serum and degradation of angiotensin II by the purified enzyme. Biochim Biophys Acta 198:255–270. [DOI] [PubMed] [Google Scholar]
- Namkung Y, Le Gouill C, Lukashova V, Kobayashi H, Hogue M, Khoury E, Song M, Bouvier M, Laporte SA. (2016) Monitoring G protein-coupled receptor and β-arrestin trafficking in live cells using enhanced bystander BRET. Nat Commun 7:12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namsolleck P, Recarti C, Foulquier S, Steckelings UM, Unger T. (2014) AT(2) receptor and tissue injury: therapeutic implications. Curr Hypertens Rep 16:416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natesh R, Schwager SL, Sturrock ED, Acharya KR. (2003) Crystal structure of the human angiotensin-converting enzyme-lisinopril complex. Nature 421:551–554. [DOI] [PubMed] [Google Scholar]
- Nawarskas J, Rajan V, Frishman WH. (2001) Vasopeptidase inhibitors, neutral endopeptidase inhibitors, and dual inhibitors of angiotensin-converting enzyme and neutral endopeptidase. Heart Dis 3:378–385. [DOI] [PubMed] [Google Scholar]
- Nchinda AT, Chibale K, Redelinghuys P, Sturrock ED. (2006a) Synthesis and molecular modeling of a lisinopril-tryptophan analogue inhibitor of angiotensin I-converting enzyme. Bioorg Med Chem Lett 16:4616–4619. [DOI] [PubMed] [Google Scholar]
- Nchinda AT, Chibale K, Redelinghuys P, Sturrock ED. (2006b) Synthesis of novel keto-ACE analogues as domain-selective angiotensin I-converting enzyme inhibitors. Bioorg Med Chem Lett 16:4612–4615. [DOI] [PubMed] [Google Scholar]
- Neutel JM, Germino WF, Punzi H, McBride M, Bryson CC, Belder R. (2008) Results of a double blind placebo controlled study to evaluate the efficacy and safety of PS433540 in human subjects with hypertension (Abstract). Circulation 118 (Suppl 18):S886. [Google Scholar]
- Ocaranza MP, Lavandero S, Jalil JE, Moya J, Pinto M, Novoa U, Apablaza F, González L, Hernández C, Varas M, et al. (2010) Angiotensin-(1-9) regulates cardiac hypertrophy in vivo and in vitro. J Hypertens 28:1054–1064. [DOI] [PubMed] [Google Scholar]
- Ocaranza MP, Moya J, Barrientos V, Alzamora R, Hevia D, Morales C, Pinto M, Escudero N, García L, Novoa U, et al. (2014) Angiotensin-(1-9) reverses experimental hypertension and cardiovascular damage by inhibition of the angiotensin converting enzyme/Ang II axis. J Hypertens 32:771–783. [DOI] [PubMed] [Google Scholar]
- O’Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, Heizer GM, Komajda M, Massie BM, McMurray JJ, et al. (2011) Effect of nesiritide in patients with acute decompensated heart failure [published correction appears in N Engl J Med (2011) 365:773]. N Engl J Med 365:32–43. [DOI] [PubMed] [Google Scholar]
- Oefner C, D’Arcy A, Hennig M, Winkler FK, Dale GE. (2000) Structure of human neutral endopeptidase (neprilysin) complexed with phosphoramidon. J Mol Biol 296:341–349. [DOI] [PubMed] [Google Scholar]
- Oefner C, Pierau S, Schulz H, Dale GE. (2007) Structural studies of a bifunctional inhibitor of neprilysin and DPP-IV. Acta Crystallogr D Biol Crystallogr 63:975–981. [DOI] [PubMed] [Google Scholar]
- Oefner C, Roques BP, Fournie-Zaluski MC, Dale GE. (2004) Structural analysis of neprilysin with various specific and potent inhibitors. Acta Crystallogr D Biol Crystallogr 60:392–396. [DOI] [PubMed] [Google Scholar]
- Ohshima K, Mogi M, Nakaoka H, Iwanami J, Min LJ, Kanno H, Tsukuda K, Chisaka T, Bai HY, Wang XL, et al. (2014) Possible role of angiotensin-converting enzyme 2 and activation of angiotensin II type 2 receptor by angiotensin-(1-7) in improvement of vascular remodeling by angiotensin II type 1 receptor blockade. Hypertension 63:e53–e59. [DOI] [PubMed] [Google Scholar]
- Okolicany J, McEnroe GA, Koh GY, Lewicki JA, Maack T. (1992) Clearance receptor and neutral endopeptidase-mediated metabolism of atrial natriuretic factor. Am J Physiol 263:F546–F553. [DOI] [PubMed] [Google Scholar]
- Olearczyk J, Gao S, Eybye M, Yendluri S, Andrews L, Bartz S, Cully D, Tadin-Strapps M. (2014) Targeting of hepatic angiotensinogen using chemically modified siRNAs results in significant and sustained blood pressure lowering in a rat model of hypertension. Hypertens Res 37:405–412. [DOI] [PubMed] [Google Scholar]
- O’Neill HG, Redelinghuys P, Schwager SL, Sturrock ED. (2008) The role of glycosylation and domain interactions in the thermal stability of human angiotensin-converting enzyme. Biol Chem 389:1153–1161. [DOI] [PubMed] [Google Scholar]
- Oparil S, Acelajado MC, Bakris GL, Berlowitz DR, Cífková R, Dominiczak AF, Grassi G, Jordan J, Poulter NR, Rodgers A, et al. (2018) Hypertension. Nat Rev Dis Primers 4:18014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oparil S, Schmieder RE. (2015) New approaches in the treatment of hypertension. Circ Res 116:1074–1095. [DOI] [PubMed] [Google Scholar]
- Owens RE, Oliphant CS. (2017) Angioedema spotlight: a closer examination of sacubitril/valsartan safety results. J Am Board Fam Med 30:556–557. [DOI] [PubMed] [Google Scholar]
- Packer M, Califf RM, Konstam MA, Krum H, McMurray JJ, Rouleau JL, Swedberg K. (2002) Comparison of omapatrilat and enalapril in patients with chronic heart failure: the Omapatrilat Versus Enalapril Randomized Trial of Utility in Reducing Events (OVERTURE). Circulation 106:920–926. [DOI] [PubMed] [Google Scholar]
- Packer M, Claggett B, Lefkowitz MP, McMurray JJV, Rouleau JL, Solomon SD, Zile MR. (2018) Effect of neprilysin inhibition on renal function in patients with type 2 diabetes and chronic heart failure who are receiving target doses of inhibitors of the renin-angiotensin system: a secondary analysis of the PARADIGM-HF trial. Lancet Diabetes Endocrinol 6:547–554. [DOI] [PubMed] [Google Scholar]
- Packer M, McMurray JJV, Krum H, Kiowski W, Massie BM, Caspi A, Pratt CM, Petrie MC, DeMets D, Kobrin I, et al. ENABLE Investigators and Committees (2017) Long-term effect of endothelin receptor antagonism with bosentan on the morbidity and mortality of patients with severe chronic heart failure: primary results of the ENABLE trials. JACC Heart Fail 5:317–326. [DOI] [PubMed] [Google Scholar]
- Page IH, Helmer OM. (1940) A crystalline pressor substance (angiotonin) resulting from the reaction between renin and renin-activator. J Exp Med 71:29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey KN. (2005) Biology of natriuretic peptides and their receptors. Peptides 26:901–932. [DOI] [PubMed] [Google Scholar]
- Papinska AM, Soto M, Meeks CJ, Rodgers KE. (2016) Long-term administration of angiotensin (1-7) prevents heart and lung dysfunction in a mouse model of type 2 diabetes (db/db) by reducing oxidative stress, inflammation and pathological remodeling. Pharmacol Res 107:372–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel VB, Zhong JC, Grant MB, Oudit GY. (2016) Role of the ACE2/angiotensin 1-7 axis of the renin-angiotensin system in heart failure. Circ Res 118:1313–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips MI. (1987) Functions of angiotensin in the central nervous system. Annu Rev Physiol 49:413–435. [DOI] [PubMed] [Google Scholar]
- Pinheiro SVB, Simões e Silva AC, Sampaio WO, de Paula RD, Mendes EP, Bontempo ED, Pesquero JB, Walther T, Alenina N, Bader M, et al. (2004) Nonpeptide AVE 0991 is an angiotensin-(1-7) receptor Mas agonist in the mouse kidney. Hypertension 44:490–496. [DOI] [PubMed] [Google Scholar]
- Pinter M, Kwanten WJ, Jain RK. (2018) Renin-angiotensin system inhibitors to mitigate cancer treatment-related adverse events. Clin Cancer Res 24:3803–3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt B, Poole-Wilson PA, Segal R, Martinez FA, Dickstein K, Camm AJ, Konstam MA, Riegger G, Klinger GH, Neaton J, et al. (2000) Effect of losartan compared with captopril on mortality in patients with symptomatic heart failure: randomised trial--the Losartan Heart Failure Survival Study ELITE II. Lancet 355:1582–1587. [DOI] [PubMed] [Google Scholar]
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, et al. ESC Scientific Document Group (2016) 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC [published corrections appear in Eur Heart J (2018) 39:860, 1206]. Eur Heart J 37:2129–2200. [DOI] [PubMed] [Google Scholar]
- Potter LR. (2011) Natriuretic peptide metabolism, clearance and degradation. FEBS J 278:1808–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu Q, Touyz RM, Schiffrin EL. (2002) Comparison of angiotensin-converting enzyme (ACE), neutral endopeptidase (NEP) and dual ACE/NEP inhibition on blood pressure and resistance arteries of deoxycorticosterone acetate-salt hypertensive rats. J Hypertens 20:899–907. [DOI] [PubMed] [Google Scholar]
- Pucell AG, Hodges JC, Sen I, Bumpus FM, Husain A. (1991) Biochemical properties of the ovarian granulosa cell type 2-angiotensin II receptor. Endocrinology 128:1947–1959. [DOI] [PubMed] [Google Scholar]
- Rabey FM, Karamyan VT, Speth RC. (2010) Distribution of a novel binding site for angiotensins II and III in mouse tissues. Regul Pept 162:5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin M, Birnbaum D, Young D, Birchmeier C, Wigler M, Ruddle FH. (1987) Human ros1 and mas1 oncogenes located in regions of chromosome 6 associated with tumor-specific rearrangements. Oncogene Res 1:169–178. [PubMed] [Google Scholar]
- Raine AE, Erne P, Bürgisser E, Müller FB, Bolli P, Burkart F, Bühler FR. (1986) Atrial natriuretic peptide and atrial pressure in patients with congestive heart failure. N Engl J Med 315:533–537. [DOI] [PubMed] [Google Scholar]
- Rajagopal S, Rajagopal K, Lefkowitz RJ. (2010) Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat Rev Drug Discov 9:373–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravichandran K, Ozkok A, Wang Q, Mullick AE, Edelstein CL. (2015) Antisense-mediated angiotensinogen inhibition slows polycystic kidney disease in mice with a targeted mutation in Pkd2. Am J Physiol Renal Physiol 308:F349–F357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray KK, Landmesser U, Leiter LA, Kallend D, Dufour R, Karakas M, Hall T, Troquay RP, Turner T, Visseren FL, et al. (2017) Inclisiran in patients at high cardiovascular risk with elevated LDL cholesterol. N Engl J Med 376:1430–1440. [DOI] [PubMed] [Google Scholar]
- Reaux A, Fournie-Zaluski MC, David C, Zini S, Roques BP, Corvol P, Llorens-Cortes C. (1999) Aminopeptidase A inhibitors as potential central antihypertensive agents. Proc Natl Acad Sci USA 96:13415–13420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regoli D, Barabé J. (1980) Pharmacology of bradykinin and related kinins. Pharmacol Rev 32:1–46. [PubMed] [Google Scholar]
- Ren L, Sun Y, Lu H, Ye D, Han L, Wang N, Daugherty A, Li F, Wang M, Su F. (2018) (Pro)renin receptor inhibition reprograms hepatic lipid metabolism and protects mice from diet-induced obesity and hepatosteatosis. Circ Res 122:730–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice GI, Jones AL, Grant PJ, Carter AM, Turner AJ, Hooper NM. (2006) Circulating activities of angiotensin-converting enzyme, its homolog, angiotensin-converting enzyme 2, and neprilysin in a family study. Hypertension 48:914–920. [DOI] [PubMed] [Google Scholar]
- Rice GI, Thomas DA, Grant PJ, Turner AJ, Hooper NM. (2004) Evaluation of angiotensin-converting enzyme (ACE), its homologue ACE2 and neprilysin in angiotensin peptide metabolism. Biochem J 383:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robl JA, Sieber-McMaster E, Asaad MM, Bird JE, Delaney NG, Barrish JC, Neubeck R, Natarajan S, Cohen M, Rovnyak GC. (1994) Mercaptoacyl dipeptides as dual inhibitors of angiotensin converting enzyme and neutral peptidase. Preliminary structure-activity studies. Bioorg Med Chem Lett 4:1783–1789. [Google Scholar]
- Robl JA, Sulsky R, Sieber-McMaster E, Ryono DE, Cimarusti MP, Simpkins LM, Karanewsky DS, Chao S, Asaad MM, Seymour AA, et al. (1999) Vasopeptidase inhibitors: incorporation of geminal and spirocyclic substituted azepinones in mercaptoacyl dipeptides. J Med Chem 42:305–311. [DOI] [PubMed] [Google Scholar]
- Robl JA, Sun CQ, Stevenson J, Ryono DE, Simpkins LM, Cimarusti MP, Dejneka T, Slusarchyk WA, Chao S, Stratton L, et al. (1997) Dual metalloprotease inhibitors: mercaptoacetyl-based fused heterocyclic dipeptide mimetics as inhibitors of angiotensin-converting enzyme and neutral endopeptidase. J Med Chem 40:1570–1577. [DOI] [PubMed] [Google Scholar]
- Rodgers KE, Bolton LL, Verco S, diZerega GS. (2015) NorLeu3-angiotensin (1-7) [DSC127] as a therapy for the healing of diabetic foot ulcers. Adv Wound Care (New Rochelle) 4:339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roksnoer LC, Heijnen BF, Nakano D, Peti-Peterdi J, Walsh SB, Garrelds IM, van Gool JM, Zietse R, Struijker-Boudier HA, Hoorn EJ, et al. (2016a) On the origin of urinary renin: a translational approach. Hypertension 67:927–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roksnoer LC, van Veghel R, de Vries R, Garrelds IM, Bhaggoe UM, Friesema EC, Leijten FP, Poglitsch M, Domenig O, Clahsen-van Groningen MC, et al. (2015) Optimum AT1 receptor-neprilysin inhibition has superior cardioprotective effects compared with AT1 receptor blockade alone in hypertensive rats. Kidney Int 88:109–120. [DOI] [PubMed] [Google Scholar]
- Roksnoer LC, van Veghel R, van Groningen MC, de Vries R, Garrelds IM, Bhaggoe UM, van Gool JM, Friesema EC, Leijten FP, Hoorn EJ, et al. (2016b) Blood pressure-independent renoprotection in diabetic rats treated with AT1 receptor-neprilysin inhibition compared with AT1 receptor blockade alone. Clin Sci (Lond) 130:1209–1220. [DOI] [PubMed] [Google Scholar]
- Roques BP, Fournié-Zaluski MC, Soroca E, Lecomte JM, Malfroy B, Llorens C, Schwartz JC. (1980) The enkephalinase inhibitor thiorphan shows antinociceptive activity in mice. Nature 288:286–288. [DOI] [PubMed] [Google Scholar]
- Roques BP, Noble F, Daugé V, Fournié-Zaluski MC, Beaumont A. (1993) Neutral endopeptidase 24.11: structure, inhibition, and experimental and clinical pharmacology. Pharmacol Rev 45:87–146. [PubMed] [Google Scholar]
- Rosendorff C, Lackland DT, Allison M, Aronow WS, Black HR, Blumenthal RS, Cannon CP, de Lemos JA, Elliott WJ, Findeiss L, et al. American Heart Association; American College of Cardiology; American Society of Hypertension (2015) Treatment of hypertension in patients with coronary artery disease: a scientific statement from the American Heart Association, American College of Cardiology, and American Society of Hypertension. J Am Coll Cardiol 65:1998–2038. [DOI] [PubMed] [Google Scholar]
- Røsjø H, Dahl MB, Jørgensen M, Røysland R, Brynildsen J, Cataliotti A, Christensen G, Høiseth AD, Hagve TA, Omland T. (2015) Influence of glycosylation on diagnostic and prognostic accuracy of N-terminal pro-B-type natriuretic peptide in acute dyspnea: data from the Akershus Cardiac Examination 2 Study. Clin Chem 61:1087–1097. [DOI] [PubMed] [Google Scholar]
- Rossi GP, Sacchetto A, Cesari M, Pessina AC. (1999) Interactions between endothelin-1 and the renin-angiotensin-aldosterone system. Cardiovasc Res 43:300–307. [DOI] [PubMed] [Google Scholar]
- Rozenfeld R, Iturrioz X, Maigret B, Llorens-Cortes C. (2002) Contribution of molecular modeling and site-directed mutagenesis to the identification of two structural residues, Arg-220 and Asp-227, in aminopeptidase A. J Biol Chem 277:29242–29252. [DOI] [PubMed] [Google Scholar]
- Rozenfeld R, Iturrioz X, Okada M, Maigret B, Llorens-Cortes C. (2003) Contribution of molecular modeling and site-directed mutagenesis to the identification of a new residue, glutamate 215, involved in the exopeptidase specificity of aminopeptidase A. Biochemistry 42:14785–14793. [DOI] [PubMed] [Google Scholar]
- Rubanyi GM, Polokoff MA. (1994) Endothelins: molecular biology, biochemistry, pharmacology, physiology, and pathophysiology. Pharmacol Rev 46:325–415. [PubMed] [Google Scholar]
- Rubattu S, Cotugno M, Forte M, Stanzione R, Bianchi F, Madonna M, Marchitti S, Volpe M. (2018) Effects of dual angiotensin type 1 receptor/neprilysin inhibition vs. angiotensin type 1 receptor inhibition on target organ injury in the stroke-prone spontaneously hypertensive rat. J Hypertens 36:1902–1914. [DOI] [PubMed] [Google Scholar]
- Ruilope LM, Dukat A, Böhm M, Lacourcière Y, Gong J, Lefkowitz MP. (2010) Blood-pressure reduction with LCZ696, a novel dual-acting inhibitor of the angiotensin II receptor and neprilysin: a randomised, double-blind, placebo-controlled, active comparator study. Lancet 375:1255–1266. [DOI] [PubMed] [Google Scholar]
- Russell FD, Davenport AP. (1999) Secretory pathways in endothelin synthesis. Br J Pharmacol 126:391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahli S, Frank B, Schweizer WB, Diederich F, Blum‐Kaelin D, Aebi JD, Böhm H, Oefner C, Dale GE. (2005) Second‐generation inhibitors for the metalloprotease neprilysin based on bicyclic heteroaromatic scaffolds: synthesis, biological activity, and X‐ray crystal‐structure analysis. Helv Chim Acta 88:731–750. [Google Scholar]
- Sakai K, Sigmund CD. (2005) Molecular evidence of tissue renin-angiotensin systems: a focus on the brain. Curr Hypertens Rep 7:135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaio WO, Souza dos Santos RA, Faria-Silva R, da Mata Machado LT, Schiffrin EL, Touyz RM. (2007) Angiotensin-(1-7) through receptor Mas mediates endothelial nitric oxide synthase activation via Akt-dependent pathways. Hypertension 49:185–192. [DOI] [PubMed] [Google Scholar]
- Sandberg K, Ji H, Clark AJ, Shapira H, Catt KJ. (1992) Cloning and expression of a novel angiotensin II receptor subtype. J Biol Chem 267:9455–9458. [PubMed] [Google Scholar]
- Santos RA, Campagnole-Santos MJ, Baracho NC, Fontes MA, Silva LC, Neves LA, Oliveira DR, Caligiorne SM, Rodrigues AR, Gropen Júnior C, et al. (1994) Characterization of a new angiotensin antagonist selective for angiotensin-(1-7): evidence that the actions of angiotensin-(1-7) are mediated by specific angiotensin receptors. Brain Res Bull 35:293–298. [DOI] [PubMed] [Google Scholar]
- Santos RA, Ferreira AJ, Verano-Braga T, Bader M. (2013) Angiotensin-converting enzyme 2, angiotensin-(1-7) and Mas: new players of the renin-angiotensin system. J Endocrinol 216:R1–R17. [DOI] [PubMed] [Google Scholar]
- Santos RA, Simoes e Silva AC, Maric C, Silva DM, Machado RP, de Buhr I, Heringer-Walther S, Pinheiro SV, Lopes MT, Bader M, et al. (2003) Angiotensin-(1-7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci USA 100:8258–8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos RAS, Oudit GY, Verano-Braga T, Canta G, Steckelings UM, Bader M. (2019) The renin-angiotensin system: going beyond the classical paradigms. Am J Physiol Heart Circ Physiol 316:H958–H970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos RAS, Sampaio WO, Alzamora AC, Motta-Santos D, Alenina N, Bader M, Campagnole-Santos MJ. (2018) The ACE2/angiotensin-(1–7)/MAS axis of the renin-angiotensin system: focus on angiotensin-(1–7). Physiol Rev 98:505–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K, Yamano Y, Bardhan S, Iwai N, Murray JJ, Hasegawa M, Matsuda Y, Inagami T. (1991) Cloning and expression of a complementary DNA encoding a bovine adrenal angiotensin II type-1 receptor. Nature 351:230–233. [DOI] [PubMed] [Google Scholar]
- Sauer AJ, Cole R, Jensen BC, Pal J, Sharma N, Yehya A, Vader J. (2019) Practical guidance on the use of sacubitril/valsartan for heart failure. Heart Fail Rev 24:167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage PD, Lovato J, Brosnihan KB, Miller AA, Petty WJ. (2016) Phase II trial of angiotensin-(1-7) for the treatment of patients with metastatic sarcoma. Sarcoma 2016:4592768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savergnini SQ, Beiman M, Lautner RQ, de Paula-Carvalho V, Allahdadi K, Pessoa DC, Costa-Fraga FP, Fraga-Silva RA, Cojocaru G, Cohen Y, et al. (2010) Vascular relaxation, antihypertensive effect, and cardioprotection of a novel peptide agonist of the MAS receptor. Hypertension 56:112–120. [DOI] [PubMed] [Google Scholar]
- Savergnini SQ, Ianzer D, Carvalho MB, Ferreira AJ, Silva GA, Marques FD, Peluso AAB, Beiman M, Cojocaru G, Cohen Y, et al. (2013) The novel Mas agonist, CGEN-856S, attenuates isoproterenol-induced cardiac remodeling and myocardial infarction injury in rats. PLoS One 8:e57757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter I, Berger A. (1967) On the size of active proteases. I. Papain. Biochem Biophys Res Commun 27:157–162. [DOI] [PubMed] [Google Scholar]
- Schiering N, D’Arcy A, Villard F, Ramage P, Logel C, Cumin F, Ksander GM, Wiesmann C, Karki RG, Mogi M. (2016) Structure of neprilysin in complex with the active metabolite of sacubitril. Sci Rep 6:27909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz H, Dale GE, Karimi-Nejad Y, Oefner C. (2009) Structure of human endothelin-converting enzyme I complexed with phosphoramidon. J Mol Biol 385:178–187. [DOI] [PubMed] [Google Scholar]
- Schunkert H, Danser AH, Hense HW, Derkx FH, Kürzinger S, Riegger GA. (1997) Effects of estrogen replacement therapy on the renin-angiotensin system in postmenopausal women. Circulation 95:39–45. [DOI] [PubMed] [Google Scholar]
- Seguin LR, Villarreal RS, Ciuffo GM. (2012) AT2 receptors recruit c-Src, SHP-1 and FAK upon activation by Ang II in PND15 rat hindbrain. Neurochem Int 60:199–207. [DOI] [PubMed] [Google Scholar]
- Seymour AA, Swerdel JN, Abboa-Offei B. (1991) Antihypertensive activity during inhibition of neutral endopeptidase and angiotensin converting enzyme. J Cardiovasc Pharmacol 17:456–465. [DOI] [PubMed] [Google Scholar]
- Sharp S, Poglitsch M, Zilla P, Davies NH, Sturrock ED. (2015) Pharmacodynamic effects of C-domain-specific ACE inhibitors on the renin-angiotensin system in myocardial infarcted rats. J Renin Angiotensin Aldosterone Syst 16:1149–1158. [DOI] [PubMed] [Google Scholar]
- Shemesh R, Toporik A, Levine Z, Hecht I, Rotman G, Wool A, Dahary D, Gofer E, Kliger Y, Soffer MA, et al. (2008) Discovery and validation of novel peptide agonists for G-protein-coupled receptors. J Biol Chem 283:34643–34649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy V, Kwon KC, Rathinasabapathy A, Lin S, Jin G, Song C, Shil P, Nair A, Qi Y, Li Q, et al. (2014) Oral delivery of angiotensin-converting enzyme 2 and angiotensin-(1-7) bioencapsulated in plant cells attenuates pulmonary hypertension. Hypertension 64:1248–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K, Furukawa H, Wada K, Wei Y, Tada Y, Kuwabara A, Shikata F, Kanematsu Y, Lawton MT, Kitazato KT, et al. (2015) Angiotensin-(1-7) protects against the development of aneurysmal subarachnoid hemorrhage in mice. J Cereb Blood Flow Metab 35:1163–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirotani K, Tsubuki S, Iwata N, Takaki Y, Harigaya W, Maruyama K, Kiryu-Seo S, Kiyama H, Iwata H, Tomita T, et al. (2001) Neprilysin degrades both amyloid beta peptides 1-40 and 1-42 most rapidly and efficiently among thiorphan- and phosphoramidon-sensitive endopeptidases. J Biol Chem 276:21895–21901. [DOI] [PubMed] [Google Scholar]
- Sigmund CD, Diz DI, Chappell MC. (2017) No brain renin-angiotensin system: déjà vu all over again? Hypertension 69:1007–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon SR, Black HR, Moser M, Berland WE. (1992) Cough and ACE inhibitors. Arch Intern Med 152:1698–1700. [PubMed] [Google Scholar]
- Skeggs LT, Jr, Kahn JR, Shumway NP. (1956) The preparation and function of the hypertensin-converting enzyme. J Exp Med 103:295–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skidgel RA, Engelbrecht S, Johnson AR, Erdös EG. (1984) Hydrolysis of substance P and neurotensin by converting enzyme and neutral endopeptidase. Peptides 5:769–776. [DOI] [PubMed] [Google Scholar]
- Solomon SD, Rizkala AR, Gong J, Wang W, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, et al. (2017) Angiotensin receptor neprilysin inhibition in heart failure with preserved ejection fraction: rationale and design of the PARAGON-HF trial. JACC Heart Fail 5:471–482. [DOI] [PubMed] [Google Scholar]
- Solomon SD, Zile M, Pieske B, Voors A, Shah A, Kraigher-Krainer E, Shi V, Bransford T, Takeuchi M, Gong J, et al. Prospective Comparison of ARNI with ARB on Management Of Heart Failure with Preserved Ejection Fraction (PARAMOUNT) Investigators (2012) The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet 380:1387–1395. [DOI] [PubMed] [Google Scholar]
- Song K, Allen AM, Paxinos G, Mendelsohn FA. (1992) Mapping of angiotensin II receptor subtype heterogeneity in rat brain. J Comp Neurol 316:467–484. [DOI] [PubMed] [Google Scholar]
- Soualmia H, Barthélemy C, Masson F, Maistre G, Eurin J, Carayon A. (1997) Angiotensin II-induced phosphoinositide production and atrial natriuretic peptide release in rat atrial tissue. J Cardiovasc Pharmacol 29:605–611. [DOI] [PubMed] [Google Scholar]
- Soubrier F, Alhenc-Gelas F, Hubert C, Allegrini J, John M, Tregear G, Corvol P. (1988) Two putative active centers in human angiotensin I-converting enzyme revealed by molecular cloning. Proc Natl Acad Sci USA 85:9386–9390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava PK, Claggett BL, Solomon SD, McMurray JJV, Packer M, Zile MR, Desai AS, Rouleau JL, Swedberg K, Fonarow GC. (2018) Estimated 5-year number needed to treat to prevent cardiovascular death or heart failure hospitalization with angiotensin receptor-neprilysin inhibition vs standard therapy for patients with heart failure with reduced ejection fraction: an analysis of data from the PARADIGM-HF trial. JAMA Cardiol 3:1226–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasch JP, Hirth-Dietrich C, Kazda S, Neuser D. (1989) Endothelin stimulates release of atrial natriuretic peptides in vitro and in vivo. Life Sci 45:869–875. [DOI] [PubMed] [Google Scholar]
- Steckelings UM, Artuc M, Wollschläger T, Wiehstutz S, Henz BM. (2001) Angiotensin-converting enzyme inhibitors as inducers of adverse cutaneous reactions. Acta Derm Venereol 81:321–325. [DOI] [PubMed] [Google Scholar]
- Steckelings UM, Kloet A, Sumners C. (2017) Centrally mediated cardiovascular actions of the angiotensin II type 2 receptor. Trends Endocrinol Metab 28:684–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone C, Jr, Brown NJ. (2017) Angiotensin-converting enzyme inhibitor and other drug-associated angioedema. Immunol Allergy Clin North Am 37:483–495. [DOI] [PubMed] [Google Scholar]
- Stragier B, Hristova I, Sarre S, Ebinger G, Michotte Y. (2005) In vivo characterization of the angiotensin-(1-7)-induced dopamine and γ-aminobutyric acid release in the striatum of the rat. Eur J Neurosci 22:658–664. [DOI] [PubMed] [Google Scholar]
- Straka BT, Ramirez CE, Byrd JB, Stone E, Woodard-Grice A, Nian H, Yu C, Banerji A, Brown NJ. (2017) Effect of bradykinin receptor antagonism on ACE inhibitor-associated angioedema. J Allergy Clin Immunol 140:242–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturrock ED, Lubbe L, Cozier GE, Schwager SLU, Arowolo AT, Arendse LB, Belcher E, Acharya KR. (2019) Structural basis for the C-domain-selective angiotensin-converting enzyme inhibition by bradykinin-potentiating peptide b (BPPb). Biochem J 476 (10):1553–1570. [DOI] [PubMed] [Google Scholar]
- Sulpizio AC, Pullen MA, Edwards RM, Louttit JB, West R, Brooks DP. (2005) Mechanism of vasopeptidase inhibitor-induced plasma extravasation: comparison of omapatrilat and the novel neutral endopeptidase 24.11/angiotensin-converting enzyme inhibitor GW796406. J Pharmacol Exp Ther 315:1306–1313. [DOI] [PubMed] [Google Scholar]
- Suwa M, Seino Y, Nomachi Y, Matsuki S, Funahashi K. (2005) Multicenter prospective investigation on efficacy and safety of carperitide for acute heart failure in the ‘real world’ of therapy. Circ J 69:283–290. [DOI] [PubMed] [Google Scholar]
- Tabrizchi R. (2001) Omapatrilat. Bristol-Myers Squibb. Curr Opin Investig Drugs 2:1414–1422. [PubMed] [Google Scholar]
- Takahashi M, Matsushita Y, Iijima Y, Tanzawa K. (1993) Purification and characterization of endothelin-converting enzyme from rat lung. J Biol Chem 268:21394–21398. [PubMed] [Google Scholar]
- Tallant EA, Ferrario CM, Gallagher PE. (2005) Angiotensin-(1-7) inhibits growth of cardiac myocytes through activation of the mas receptor. Am J Physiol Heart Circ Physiol 289:H1560–H1566. [DOI] [PubMed] [Google Scholar]
- Tamargo J, Duarte J, Ruilope LM. (2015) New antihypertensive drugs under development. Curr Med Chem 22:305–342. [DOI] [PubMed] [Google Scholar]
- Tetzner A, Gebolys K, Meinert C, Klein S, Uhlich A, Trebicka J, Villacañas Ó, Walther T. (2016) G-protein-coupled receptor MrgD is a receptor for angiotensin-(1-7) involving adenylyl cyclase, cAMP, and phosphokinase A. Hypertension 68:185–194. [DOI] [PubMed] [Google Scholar]
- Than A, Leow MK, Chen P. (2013) Control of adipogenesis by the autocrine interplays between angiotensin 1-7/Mas receptor and angiotensin II/AT1 receptor signaling pathways. J Biol Chem 288:15520–15531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas WG, Sernia C. (1988) Immunocytochemical localization of angiotensinogen in the rat brain. Neuroscience 25:319–341. [DOI] [PubMed] [Google Scholar]
- Thunnissen MM, Nordlund P, Haeggström JZ. (2001) Crystal structure of human leukotriene A(4) hydrolase, a bifunctional enzyme in inflammation. Nat Struct Biol 8:131–135. [DOI] [PubMed] [Google Scholar]
- Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. (2000) A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem 275:33238–33243. [DOI] [PubMed] [Google Scholar]
- Tirupula KC, Desnoyer R, Speth RC, Karnik SS. (2014) Atypical signaling and functional desensitization response of MAS receptor to peptide ligands. PLoS One 9:e103520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tom B, de Vries R, Saxena PR, Danser AHJ. (2001) Bradykinin potentiation by angiotensin-(1-7) and ACE inhibitors correlates with ACE C- and N-domain blockade. Hypertension 38:95–99. [DOI] [PubMed] [Google Scholar]
- Torrado J, Cain C, Mauro AG, Romeo F, Ockaili R, Chau VQ, Nestler JA, Devarakonda T, Ghosh S, Das A, et al. (2018) Sacubitril/valsartan averts adverse post-infarction ventricular remodeling and preserves systolic function in rabbits. J Am Coll Cardiol 72:2342–2356. [DOI] [PubMed] [Google Scholar]
- Tóth AD, Turu G, Hunyady L, Balla A. (2018) Novel mechanisms of G-protein-coupled receptors functions: AT1 angiotensin receptor acts as a signaling hub and focal point of receptor cross-talk. Best Pract Res Clin Endocrinol Metab 32:69–82. [DOI] [PubMed] [Google Scholar]
- Towler P, Staker B, Prasad SG, Menon S, Tang J, Parsons T, Ryan D, Fisher M, Williams D, Dales NA, et al. (2004) ACE2 X-ray structures reveal a large hinge-bending motion important for inhibitor binding and catalysis. J Biol Chem 279:17996–18007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trask AJ, Ferrario CM. (2007) Angiotensin-(1-7): pharmacology and new perspectives in cardiovascular treatments. Cardiovasc Drug Rev 25:162–174. [DOI] [PubMed] [Google Scholar]
- Trippodo NC, Robl JA, Asaad MM, Fox M, Panchal BC, Schaeffer TR. (1998) Effects of omapatrilat in low, normal, and high renin experimental hypertension. Am J Hypertens 11:363–372. [DOI] [PubMed] [Google Scholar]
- Turcaud S, Gonzalez W, Michel J, Roques BP, Fournie-Zaluski M. (1995) Diastereoselective synthesis of mixanpril, an orally active dual inhibitor of neutral endopeptidase and angiotensin converting enzyme. Bioorg Med Chem Lett 5:1893–1898. [Google Scholar]
- Turner AJ, Tanzawa K. (1997) Mammalian membrane metallopeptidases: NEP, ECE, KELL, and PEX. FASEB J 11:355–364. [DOI] [PubMed] [Google Scholar]
- Uijl E, Mirabito Colafella KM, Sun Y, Ren L, van Veghel R, Garrelds IM, de Vries R, Poglitsch M, Zlatev I, Kim JB, et al. (2019) Strong and sustained antihypertensive effect of small interfering RNA targeting liver angiotensinogen. Hypertension 73:1249–1257. [DOI] [PubMed] [Google Scholar]
- Uijl E, Ren L, Danser AHJ. (2018) Angiotensin generation in the brain: a re-evaluation. Clin Sci (Lond) 132:839–850. [DOI] [PubMed] [Google Scholar]
- van Esch JH, Tom B, Dive V, Batenburg WW, Georgiadis D, Yiotakis A, van Gool JM, de Bruijn RJ, de Vries R, Danser AH. (2005) Selective angiotensin-converting enzyme C-domain inhibition is sufficient to prevent angiotensin I-induced vasoconstriction. Hypertension 45:120–125. [DOI] [PubMed] [Google Scholar]
- van Thiel BS, Góes Martini A, Te Riet L, Severs D, Uijl E, Garrelds IM, Leijten FPJ, van der Pluijm I, Essers J, Qadri F, et al. (2017) Brain renin–angiotensin system: does it exist? Hypertension 69:1136–1144. [DOI] [PubMed] [Google Scholar]
- van Twist DJ, Kroon AA, de Leeuw PW. (2014) Angiotensin-(1-7) as a strategy in the treatment of hypertension? Curr Opin Nephrol Hypertens 23:480–486. [DOI] [PubMed] [Google Scholar]
- van’t Veer LJ, van der Feltz MJ, van den Berg-Bakker CA, Cheng NC, Hermens RP, van Oorschot DA, Kievits T, Schrier PI. (1993) Activation of the mas oncogene involves coupling to human alphoid sequences. Oncogene 8:2673–2681. [PubMed] [Google Scholar]
- Vardeny O, Miller R, Solomon SD. (2014) Combined neprilysin and renin-angiotensin system inhibition for the treatment of heart failure. JACC Heart Fail 2:663–670. [DOI] [PubMed] [Google Scholar]
- Vazeux G, Iturrioz X, Corvol P, Llorens-Cortès C. (1997) A tyrosine residue essential for catalytic activity in aminopeptidase A. Biochem J 327:883–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazeux G, Iturrioz X, Corvol P, Llorens-Cortes C. (1998) A glutamate residue contributes to the exopeptidase specificity in aminopeptidase A. Biochem J 334:407–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazeux G, Wang J, Corvol P, Llorens-Cortès C. (1996) Identification of glutamate residues essential for catalytic activity and zinc coordination in aminopeptidase A. J Biol Chem 271:9069–9074. [DOI] [PubMed] [Google Scholar]
- Veerasingham SJ, Raizada MK. (2003) Brain renin-angiotensin system dysfunction in hypertension: recent advances and perspectives. Br J Pharmacol 139:191–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velazquez EJ, Morrow DA, DeVore AD, Duffy CI, Ambrosy AP, McCague K, Rocha R, Braunwald E, PIONEER-HF Investigators (2019) Angiotensin–neprilysin inhibition in acute decompensated heart failure. N Engl J Med 380:539–548. [DOI] [PubMed] [Google Scholar]
- Verdonk K, Saleh L, Lankhorst S, Smilde JE, van Ingen MM, Garrelds IM, Friesema EC, Russcher H, van den Meiracker AH, Visser W, et al. (2015) Association studies suggest a key role for endothelin-1 in the pathogenesis of preeclampsia and the accompanying renin-angiotensin-aldosterone system suppression. Hypertension 65:1316–1323. [DOI] [PubMed] [Google Scholar]
- Vickers C, Hales P, Kaushik V, Dick L, Gavin J, Tang J, Godbout K, Parsons T, Baronas E, Hsieh F, et al. (2002) Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem 277:14838–14843. [DOI] [PubMed] [Google Scholar]
- Vijayaraghavan J, Scicli AG, Carretero OA, Slaughter C, Moomaw C, Hersh LB. (1990) The hydrolysis of endothelins by neutral endopeptidase 24.11 (enkephalinase). J Biol Chem 265:14150–14155. [PubMed] [Google Scholar]
- Villar IC, Panayiotou CM, Sheraz A, Madhani M, Scotland RS, Nobles M, Kemp-Harper B, Ahluwalia A, Hobbs AJ. (2007) Definitive role for natriuretic peptide receptor-C in mediating the vasorelaxant activity of C-type natriuretic peptide and endothelium-derived hyperpolarising factor. Cardiovasc Res 74:515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodovar N, Séronde MF, Laribi S, Gayat E, Lassus J, Januzzi JL, Jr, Boukef R, Nouira S, Manivet P, Samuel JL, et al. GREAT Network (2015) Elevated plasma B-type natriuretic peptide concentrations directly inhibit circulating neprilysin activity in heart failure. JACC Heart Fail 3:629–636. [DOI] [PubMed] [Google Scholar]
- Walters PE, Gaspari TA, Widdop RE. (2005) Angiotensin-(1-7) acts as a vasodepressor agent via angiotensin II type 2 receptors in conscious rats. Hypertension 45:960–966. [DOI] [PubMed] [Google Scholar]
- Wang J, Cooper MD. (1993) Histidine residue in the zinc-binding motif of aminopeptidase A is critical for enzymatic activity. Proc Natl Acad Sci USA 90:1222–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watermeyer JM, Kröger WL, O’Neill HG, Sewell BT, Sturrock ED. (2008) Probing the basis of domain-dependent inhibition using novel ketone inhibitors of angiotensin-converting enzyme. Biochemistry 47:5942–5950. [DOI] [PubMed] [Google Scholar]
- Watermeyer JM, Kröger WL, O’Neill HG, Sewell BT, Sturrock ED. (2010) Characterization of domain-selective inhibitor binding in angiotensin-converting enzyme using a novel derivative of lisinopril. Biochem J 428:67–74. [DOI] [PubMed] [Google Scholar]
- Watts JK, Corey DR. (2012) Silencing disease genes in the laboratory and the clinic. J Pathol 226:365–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber MA. (2001) Vasopeptidase inhibitors. Lancet 358:1525–1532. [DOI] [PubMed] [Google Scholar]
- Weber MA, Messerli FH. (2008) Angiotensin-converting enzyme inhibitors and angioedema: estimating the risk. Hypertension 51:1465–1467. [DOI] [PubMed] [Google Scholar]
- Wei A, Gu Z, Li J, Liu X, Wu X, Han Y, Pu J. (2016) Clinical adverse effects of endothelin receptor antagonists: insights from the meta-analysis of 4894 patients from 24 randomized double-blind placebo-controlled clinical trials. J Am Heart Assoc 5:e003896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei CM, Heublein DM, Perrella MA, Lerman A, Rodeheffer RJ, McGregor CG, Edwards WD, Schaff HV, Burnett JC., Jr (1993) Natriuretic peptide system in human heart failure. Circulation 88:1004–1009. [DOI] [PubMed] [Google Scholar]
- Wei L, Clauser E, Alhenc-Gelas F, Corvol P. (1992) The two homologous domains of human angiotensin I-converting enzyme interact differently with competitive inhibitors. J Biol Chem 267:13398–13405. [PubMed] [Google Scholar]
- Welches WR, Brosnihan KB, Ferrario CM. (1993) A comparison of the properties and enzymatic activities of three angiotensin processing enzymes: angiotensin converting enzyme, prolyl endopeptidase and neutral endopeptidase 24.11. Life Sci 52:1461–1480. [DOI] [PubMed] [Google Scholar]
- Whelton PK, Carey RM, Aronow WS, Casey DE, Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. (2018) 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines [published correction appears in J Am Coll Cardiol (2018) 71:2275–2279]. J Am Coll Cardiol 71:e127–e248. [DOI] [PubMed] [Google Scholar]
- Wielbo D, Sernia C, Gyurko R, Phillips MI. (1995) Antisense inhibition of hypertension in the spontaneously hypertensive rat. Hypertension 25:314–319. [DOI] [PubMed] [Google Scholar]
- Wiemer G, Dobrucki LW, Louka FR, Malinski T, Heitsch H. (2002) AVE 0991, a nonpeptide mimic of the effects of angiotensin-(1-7) on the endothelium. Hypertension 40:847–852. [DOI] [PubMed] [Google Scholar]
- Williams TA, Corvol P, Soubrier F. (1994) Identification of two active site residues in human angiotensin I-converting enzyme. J Biol Chem 269:29430–29434. [PubMed] [Google Scholar]
- Woodman ZL, Oppong SY, Cook S, Hooper NM, Schwager SL, Brandt WF, Ehlers MR, Sturrock ED. (2000) Shedding of somatic angiotensin-converting enzyme (ACE) is inefficient compared with testis ACE despite cleavage at identical stalk sites. Biochem J 347:711–718. [PMC free article] [PubMed] [Google Scholar]
- Wright JM, Musini VM, Gill R. (2018) First-line drugs for hypertension. Cochrane Database Syst Rev 4:CD001841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JW, Harding JW. (1995) Brain angiotensin receptor subtypes AT1, AT2, and AT4 and their functions. Regul Pept 59:269–295. [DOI] [PubMed] [Google Scholar]
- Wright JW, Harding JW. (1997) Important role for angiotensin III and IV in the brain renin-angiotensin system. Brain Res Brain Res Rev 25:96–124. [DOI] [PubMed] [Google Scholar]
- Wright JW, Miller-Wing AV, Shaffer MJ, Higginson C, Wright DE, Hanesworth JM, Harding JW. (1993) Angiotensin II(3-8) (ANG IV) hippocampal binding: potential role in the facilitation of memory. Brain Res Bull 32:497–502. [DOI] [PubMed] [Google Scholar]
- Wright JW, Tamura-Myers E, Wilson WL, Roques BP, Llorens-Cortes C, Speth RC, Harding JW. (2003) Conversion of brain angiotensin II to angiotensin III is critical for pressor response in rats. Am J Physiol Regul Integr Comp Physiol 284:R725–R733. [DOI] [PubMed] [Google Scholar]
- Xu D, Emoto N, Giaid A, Slaughter C, Kaw S, deWit D, Yanagisawa M. (1994) ECE-1: a membrane-bound metalloprotease that catalyzes the proteolytic activation of big endothelin-1. Cell 78:473–485. [DOI] [PubMed] [Google Scholar]
- Yamada T, Horiuchi M, Dzau VJ. (1996) Angiotensin II type 2 receptor mediates programmed cell death. Proc Natl Acad Sci USA 93:156–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa M, Inoue A, Ishikawa T, Kasuya Y, Kimura S, Kumagaye S, Nakajima K, Watanabe TX, Sakakibara S, Goto K, et al. (1988) Primary structure, synthesis, and biological activity of rat endothelin, an endothelium-derived vasoconstrictor peptide. Proc Natl Acad Sci USA 85:6964–6967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Colvin MM, Drazner MH, Filippatos G, Fonarow GC, Givertz MM, et al. (2016) 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America [published correction appears in J Am Coll Cardiol (2016) 68:1495]. J Am Coll Cardiol 68:1476–1488. [DOI] [PubMed] [Google Scholar]
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, et al. (2017) 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 70:776–803. [DOI] [PubMed] [Google Scholar]
- Yandrapalli S, Khan MH, Rochlani Y, Aronow WS. (2018) Sacubitril/valsartan in cardiovascular disease: evidence to date and place in therapy. Ther Adv Cardiovasc Dis 12:217–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Liu C, Lin YL, Li F. (2013) Structural insights into central hypertension regulation by human aminopeptidase A. J Biol Chem 288:25638–25645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye F, Wang Y, Wu C, Howatt DA, Wu CH, Balakrishnan A, Mullick AE, Graham MJ, Danser AHJ, Wang J, et al. (2019) Angiotensinogen and megalin interactions contribute to atherosclerosis—brief report. Arterioscler Thromb Vasc Biol 39:150–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D, Waitches G, Birchmeier C, Fasano O, Wigler M. (1986) Isolation and characterization of a new cellular oncogene encoding a protein with multiple potential transmembrane domains. Cell 45:711–719. [DOI] [PubMed] [Google Scholar]
- Yu L, Yuan K, Phuong HTA, Park BM, Kim SH. (2016) Angiotensin-(1-5), an active mediator of renin-angiotensin system, stimulates ANP secretion via Mas receptor. Peptides 86:33–41. [DOI] [PubMed] [Google Scholar]
- Yu L, Zheng M, Wang W, Rozanski GJ, Zucker IH, Gao L. (2010) Developmental changes in AT1 and AT2 receptor-protein expression in rats. J Renin Angiotensin Aldosterone Syst 11:214–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, Dagenais G, Sleight P, Anderson C, ONTARGET Investigators (2008) Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 358:1547–1559. [DOI] [PubMed] [Google Scholar]
- Zanchi A, Maillard M, Burnier M. (2003) Recent clinical trials with omapatrilat: new developments. Curr Hypertens Rep 5:346–352. [DOI] [PubMed] [Google Scholar]
- Zhang H, Han GW, Batyuk A, Ishchenko A, White KL, Patel N, Sadybekov A, Zamlynny B, Rudd MT, Hollenstein K, et al. (2017) Structural basis for selectivity and diversity in angiotensin II receptors. Nature 544:327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Unal H, Gati C, Han GW, Liu W, Zatsepin NA, James D, Wang D, Nelson G, Weierstall U, et al. (2015) Structure of the angiotensin receptor revealed by serial femtosecond crystallography. Cell 161:833–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Li Z, Dang H, Chen R, Liaw C, Tran TA, Boatman PD, Connolly DT, Adams JW. (2012) Inhibition of Mas G-protein signaling improves coronary blood flow, reduces myocardial infarct size, and provides long-term cardioprotection. Am J Physiol Heart Circ Physiol 302:H299–H311. [DOI] [PubMed] [Google Scholar]
- Zini S, Fournie-Zaluski MC, Chauvel E, Roques BP, Corvol P, Llorens-Cortes C. (1996) Identification of metabolic pathways of brain angiotensin II and III using specific aminopeptidase inhibitors: predominant role of angiotensin III in the control of vasopressin release. Proc Natl Acad Sci USA 93:11968–11973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zini S, Masdehors P, Lenkei Z, Fournie-Zaluski MC, Roques BP, Corvol P, Llorens-Cortes C. (1997) Aminopeptidase A: distribution in rat brain nuclei and increased activity in spontaneously hypertensive rats. Neuroscience 78:1187–1193. [DOI] [PubMed] [Google Scholar]