Abstract
Unrelated genes establish head-to-tail polarity in embryos of different fly species, raising the question of how they evolve this function. We show that in moth flies (Clogmia, Lutzomyia), a maternal transcript isoform of odd-paired (Zic) is localized in the anterior egg and adopted the role of anterior determinant without essential protein change. Additionally, Clogmia lost maternal germ plasm, which contributes to embryo polarity in fruit flies (Drosophila). In culicine (Culex, Aedes) and anopheline mosquitoes (Anopheles), embryo polarity rests on a previously unnamed zinc finger gene (cucoid), or pangolin (dTcf), respectively. These genes also localize an alternative transcript isoform at the anterior egg pole. Basal-branching crane flies (Nephrotoma) also enrich maternal pangolin transcript at the anterior egg pole, suggesting that pangolin functioned as ancestral axis determinant in flies. In conclusion, flies evolved an unexpected diversity of anterior determinants, and alternative transcript isoforms with distinct expression can adopt fundamentally distinct developmental roles.
Research organism: D. melanogaster, Other
eLife digest
With very few exceptions, animals have ‘head’ and ‘tail’ ends that develop when they are an embryo. The genes involved in specifying these ends vary between species and even closely-related animals may use different genes for the same roles. For example, the products of two unrelated genes called bicoid in fruit flies and panish in common midges accumulate at one end of their respective eggs to distinguish head from tail ends. It remained unclear how other fly species, which have neither a bicoid nor a panish gene, distinguish the head from the tail end, or how genes can evolve the specific function of bicoid and panish.
Cells express genes by producing gene templates called messenger ribonucleic acids (or mRNAs for short). The central portions of mRNAs, known as protein-coding sequences, are then used to produce the protein. Proteins can play several distinct roles, which they acquire through evolution. This can happen in different ways, for example, genetic mutations in the part of a gene that codes for protein may alter the resulting protein, giving it a new activity. Alternatively, sequences at the beginning and the end of an mRNA molecule that do not code for protein, but regulate when and where proteins are made, can influence a protein’s role by changing its environment. Many genes produce mRNAs with alternative sequences at the beginning or the end, a process known as alternative transcription.
Here, Yoon et al. identified three unrelated genes that perform similar roles to bicoid and panish in the embryos of several different moth flies and mosquitoes. These genes appear to have acquired their activity because one of their alternative transcripts accumulated at the future head end, rather than through mutations in the protein-coding sequences. Studying multiple species also made it clear that panish inherited its function from a localized alternative transcript of an old gene that duplicated and diverged.
These findings suggest that alternative transcription may provide opportunities for genes to evolve new roles in fundamental processes in flies. Most animal genes use alternative start and stop sites for transcription, but the reasons for this remain largely obscure. This is especially the case in the human brain. The findings of Yoon et al., therefore, raise the question of whether alternative transcription has played an important role in the evolution of the human brain.
Introduction
The specification of the primary axis (head-to-tail) in embryos of flies (Diptera) offers important advantages for studying how new essential gene functions evolve in early development. This process rests on lineage-specific maternal mRNAs that are localized at the anterior egg pole (‘anterior determinants’), which, surprisingly, have changed during the evolution of flies. While the anterior determinants of most flies remain unknown, they can be identified by comparing the transcriptomes of anterior and posterior egg halves (Klomp et al., 2015). Furthermore, their function can be analyzed in the syncytial early embryos of a broad range of species via microinjection, considering timing and subcellular localization. It is therefore possible to conduct phylogenetic comparisons at the functional level. Finally, when the function of anterior determinants is suppressed, embryos develop into an unambiguous, predictable phenotype: these embryos lack all anterior structures and develop as two outward facing tail ends (‘double abdomen’).
Anterior determinants can be encoded by new genes with a dedicated function in establishing embryonic polarity. One example is bicoid in the fruit fly Drosophila melanogaster. Maternal mRNA of bicoid is localized in the anterior pole of the egg and Bicoid protein is expressed in a gradient in the early embryo (Berleth et al., 1988). Bicoid-deficient embryos fail to develop anterior structures and instead form a second tail end, or a symmetrical double abdomen when the maternal activity gradient of another gene, hunchback, is disrupted simultaneously (Driever, 1993). The bicoid gene originated in the lineage of cyclorrhaphan flies more than 140 million years ago by duplication of zerknüllt (zen; aka Hox3), which, in insects, plays an important role in extraembryonic tissue development (Schmidt-Ott et al., 2010). The expression and function of cyclorrhaphan bicoid orthologs are conserved but bicoid has not been found outside this group, and has been lost in some lineages within the Cyclorrhapha.
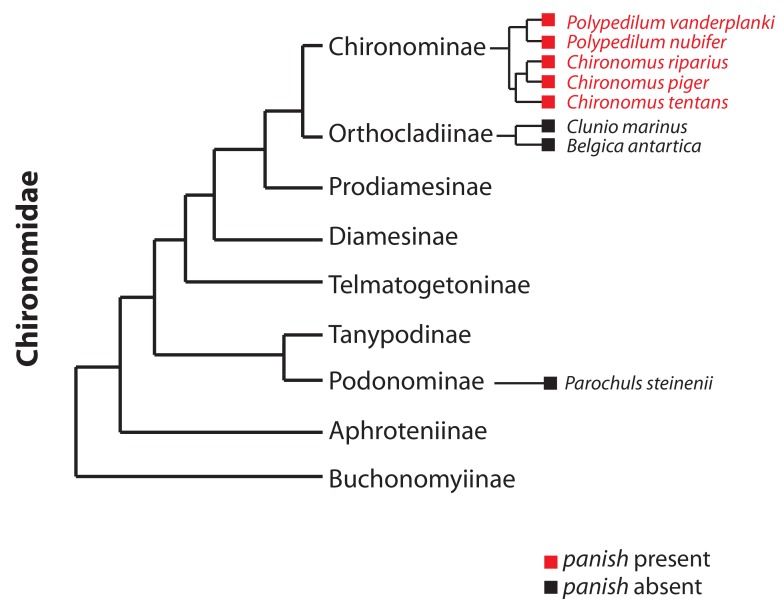
Another example is panish, which encodes the anterior determinant of a midge, Chironomus riparius. This gene evolved by gene duplication of the Tcf homolog pangolin (pan) and capture of the maternal promoter of a nucleoside kinase gene, and has been called panish (for pan“ish’) (Klomp et al., 2015). Pangolin functions as the effector of ß-catenin-dependent Wnt signaling pathway (‘canonical’ Wnt signaling) but Panish lacks the ß-catenin domain of Pangolin, and sequence similarity between Pangolin and Panish is limited to the cysteine-clamp domain (30 amino acids). panish has not been found outside the family Chironomidae, suggesting that lower dipterans use different anterior determinants.
Here, we have used embryos of a wider range of dipteran species that lack bicoid and panish to address the question of how anterior determinants evolve. We started our analysis with moth flies (Psychodidae: Clogmia albipunctata, Lutzomyia longipalpis) and subsequently extended it to mosquitoes (Culicidae: Culex quinquefasciatus, Aedes aegypti, Anopheles gambiae, Anopheles coluzzii), and to crane flies (Tipulidae: Nephrotoma suturalis) (Figure 1A). Our results reveal three distinct old genes that evolved anterior determinants by localizing an alternative maternal transcript isoform at the anterior egg pole of the respective species. Therefore, alternative transcription might have played an important role in the evolution of this gene function and gene regulatory networks in fly embryos.
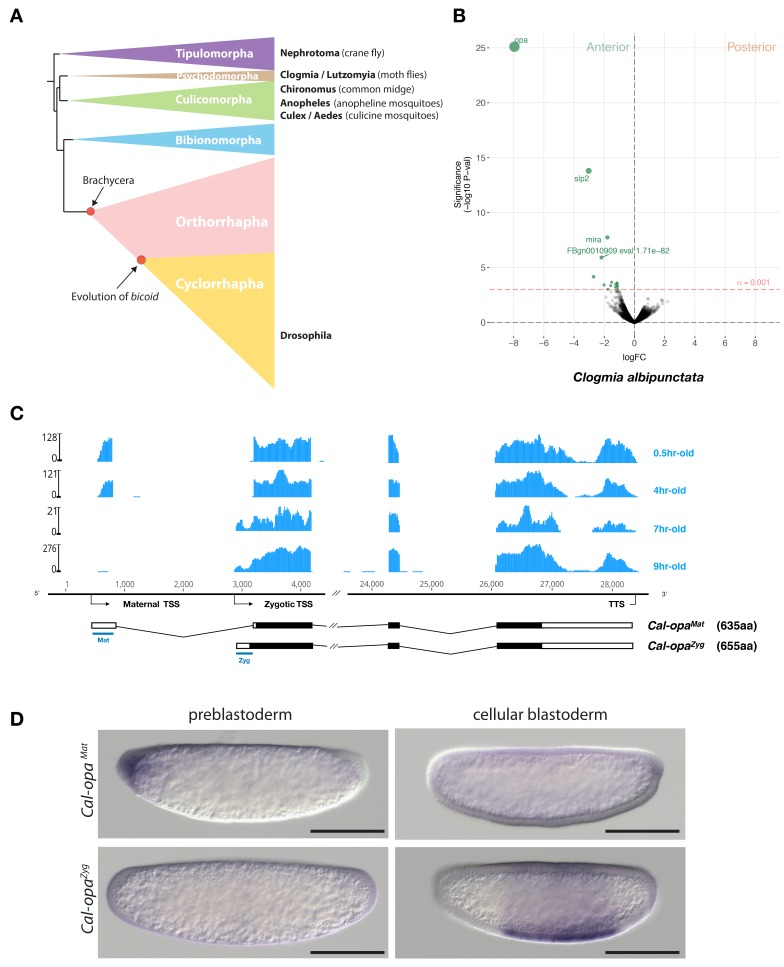
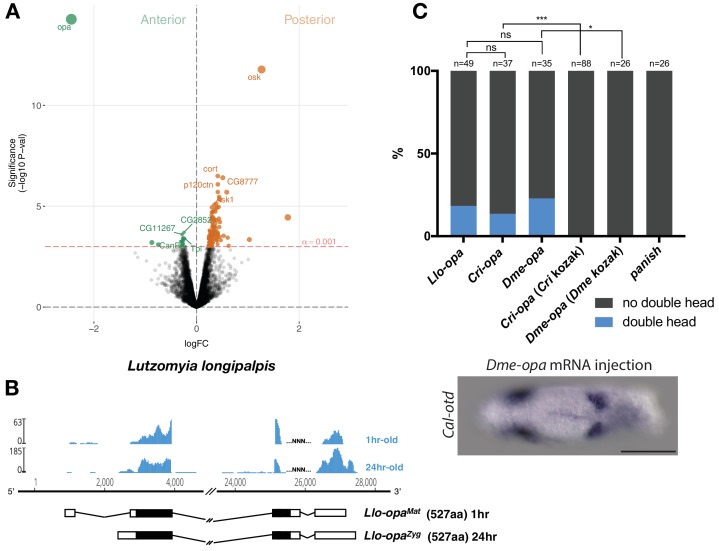
Figure 1. Expression of alternative Cal-opa transcripts in Clogmia embryos.
(A) Phylogenetic relationship of fly species referred to in the text (Wiegmann et al., 2011). (B) Differential expression analysis of maternal transcripts between anterior and posterior halves of 1 hr-old Clogmia embryos. logFC: log fold-change. (C) Stage-specific RNA-seq read coverage of Cal-opa locus. Transcription start sites (TSS) and transcription termination sites (TTS) are indicated on the genomic scaffold (solid line with 1000 bp intervals marked) and were confirmed by RACE. Exon-intron sketches of Cal-opaMat and Cal-opaZyg transcript variants are shown with the open reading frame in black and the position of in situ hybridization probes and dsRNA underlined in blue. (D) RNA in situ hybridization of Cal-opaMat and Cal-opaZyg transcripts in 1 hr-old preblastoderm and 7 hr-old cellular blastoderm embryos. Anterior is left and dorsal up. Scale bar: 100μm.
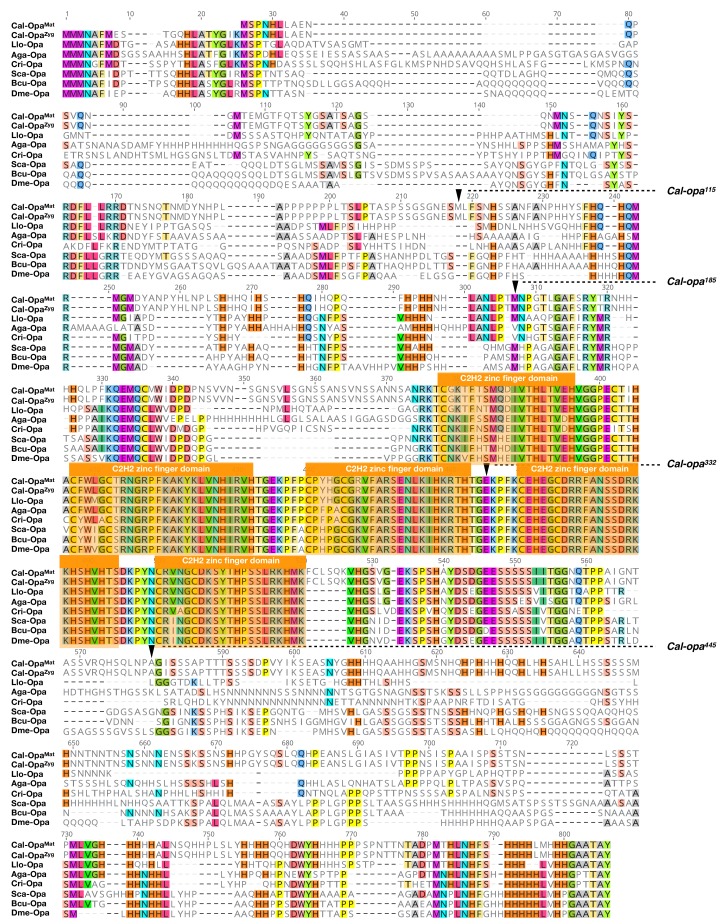
Figure 1—figure supplement 1. Protein alignment of predicted dipteran Odd-paired orthologs.
Results
An alternative maternal transcript of the conserved segmentation gene odd-paired functions as anterior determinant in Clogmia
We annotated 5602 transcripts from the anterior and posterior transcriptomes of 1 hr-old bisected Clogmia embryos and ranked them according to the magnitude of their differential expression scores and P values (Figure 1B). In the anterior embryo, the most enriched transcript was homologous to odd-paired, the Drosophila homolog of mammalian Zic (zinc finger of the cerebellum) genes. ZIC proteins are known to function as transcription factors or co-factors (Houtmeyers et al., 2013). odd-paired was discovered in a screen for early Drosophila segmentation genes and subsequently classified as a ‘pair-rule’ gene, since odd-paired mutants fail to develop alternating segments (Jürgens et al., 1984). During the Drosophila segmentation process, odd-paired is expressed in a single broad domain and controls the 'frequency-doubling' of other pair-rule genes (Clark and Akam, 2016).
The Clogmia genome contains a single odd-paired locus (Cal-opa) (Vicoso and Bachtrog, 2015). Using RNA-seq data from preblastoderm and blastoderm embryos and Rapid Amplication of cDNA Ends (RACE), we identified maternal and zygotic Cal-opa transcripts with alternative first exons that we mapped onto a 54 kb genomic scaffold (Figure 1C). The maternal transcript (Cal-opaMat) was detected in preblastoderm embryos (0.5 hr-old) and syncytial blastoderm embryos (4 hr-old). The zygotic transcript (Cal-opaZyg) was found in cellularized blastoderm embryos (7 hr-old) and gastrulating embryos (9 hr-old). Protein alignments with homologs from other flies suggest that Cal-opaZyg encodes the full-length Cal-Opa protein (655 amino acids), while Cal-opaMat encodes a truncated protein variant (635 amino acids), lacking the N-terminal 20 amino acids of Cal-OpaZyg (Figure 1—figure supplement 1).
To confirm the alternative Cal-opaMat and Cal-opaZyg transcripts and their non-overlapping expression patterns, we performed whole mount RNA in situ hybridization experiments with transcript-specific probes. The Cal-opaMat transcript was anteriorly localized in preblastoderm embryos but absent at the cellular blastoderm stage. Conversely, the Cal-opaZyg transcript was absent in preblastoderm embryos but expressed broadly in the trunk region of 7 hr-old blastoderm embryos (Figure 1D), like odd-paired in Drosophila. These observations suggest that Cal-opa produces transcript isoforms with spatially and temporally distinct expression patterns.
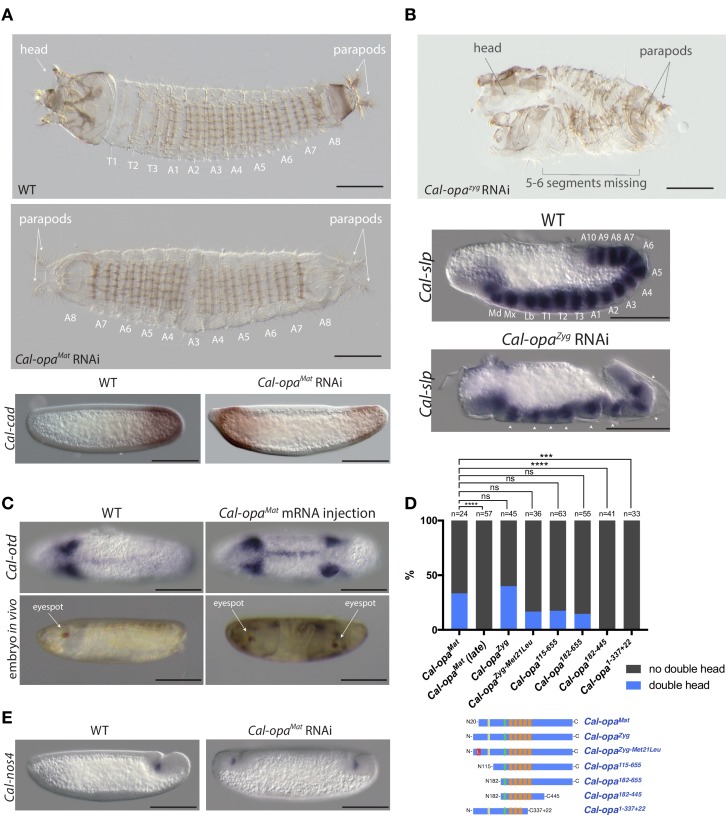
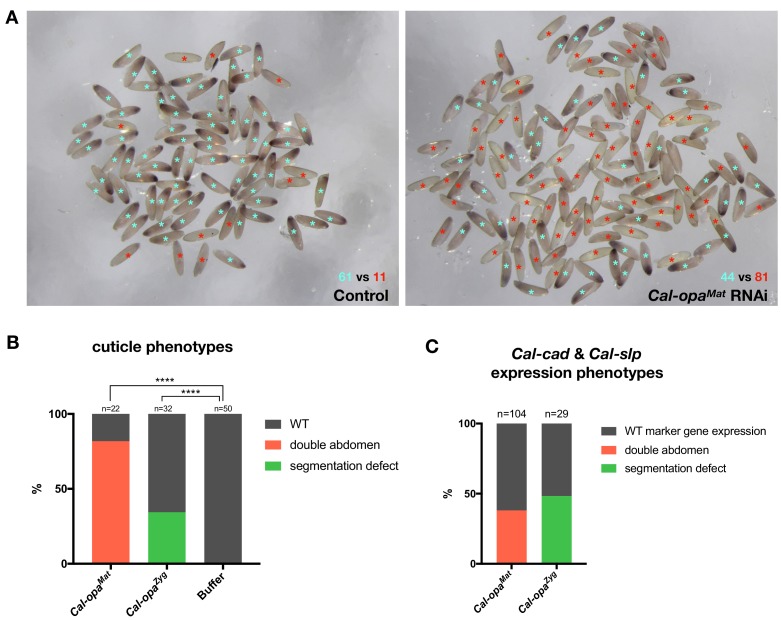
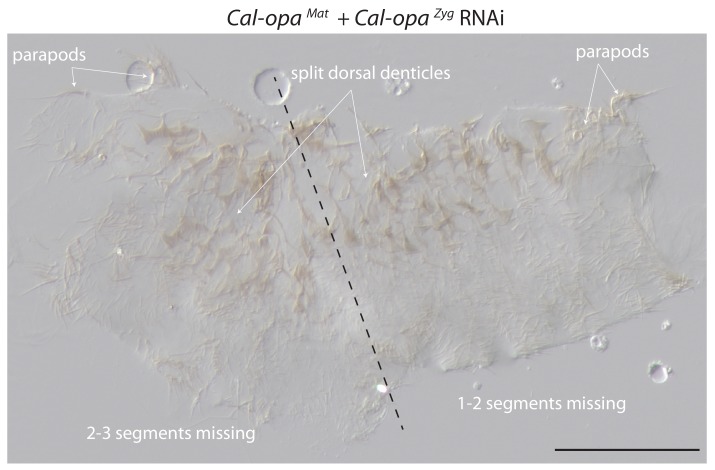
To determine the function of Cal-opaMat and Cal-opaZyg, we established a protocol for microinjecting early Clogmia embryos and conducted transcript-specific RNA interference (RNAi) experiments. Injection of Cal-opaMat double-stranded RNA (dsRNA) led to mirror-image duplications of the tail end (double abdomen; Figure 2A and Figure 2—figure supplement 1). In contrast, injection of dsRNA targeting Cal-opaZyg resulted in half the number of segmental expression domains of Cal-slp (the ortholog of pair-rule gene sloppy-paired) and caused defects in segmentation, dorsal closure, and head development but did not alter embryo polarity (Figure 2B). Finally, injection of dsRNA targeting both Cal-opaMat and Cal-opaZyg resulted in double abdomens with missing segments (Figure 2—figure supplement 2). These observations indicate distinct roles of Cal-opaMat and Cal-opaZyg in specifying embryo polarity and in segmentation, respectively.
Figure 2. Function of alternative Cal-opa transcripts in early Clogmia embryo.
(A) 1 st instar larval cuticle of wild type (top) and following Cal-opaMat RNAi (middle). RNA in situ hybridization of Cal-cad in a wild-type preblastoderm embryo (bottom left) and stage-matched Cal-opaMat RNAi embryos (bottom right). Anterior is left and dorsal up. T: thoracic segment; A: abdominal segment. Segment numbers in Cal-opaMat RNAi larval cuticle were assigned based on the assumption of polarity reversal. Scale bar: 100μm. (B) 1 st instar larval cuticle phenotype following Cal-opaZyg RNAi (top) and RNA in situ hybridization of Cal-slp in extending wild-type germband (middle) and stage-matched Cal-opaZyg RNAi embryo (bottom). Anterior is left and dorsal up. Md: mandibular segment; Mx: maxillary segment; Lb: labial segment; T: thoracic segment; A: abdominal segment. Scale bar: 100μm. (C) RNA in situ hybridizations of Cal-otd in wild-type gastrula (top left) and stage-matched embryo following posterior Cal-opaMat mRNA injection (top right) are shown in ventral view. A live wild-type embryo (bottom left) and a stage-matched embryo following posterior Cal-opaMat mRNA injection (bottom right) in lateral view. Anterior is left. Scale bar: 100μm. (D) Posterior injection of Cal-opa mRNA and mutated variants. Complete, symmetrical duplication of the bilateral Cal-otd expression domain in gastrulating embryos was counted as double head (blue, see Figure 2C). All other phenotypes, including incomplete duplications and wild type, were conservatively counted as ‘no double head’ (black). Sketches of predicted Cal-Opa proteins are shown with ZIC/Opa conserved motif (ZOC) in yellow, the ZIC family protein N-terminal conserved domain (ZFNC) in green, and zinc finger domains in orange. The Met21Leu mutation in Cal-OpaZyg-Met21Leu is marked in red. Cal-opaMat (late): Cal-opaMat was injected during the syncytial blastoderm stage (4 hr). ns: p>0.05; ***: p<0.001; ****: p<0.0001, Fisher’s exact test. (E) RNA in situ hybridization of Cal-nos4 in a gastrulating embryo (left) and stage-matched Cal-opaMat RNAi embryos (right).
Figure 2—figure supplement 1. Frequencies of strong Cal-opaMat and Cal-opaZyg RNAi phenotypes.
Figure 2—figure supplement 2. Cuticle phenotype of a 1 st instar larva following Cal-opaMat and Cal-opaZyg double RNAi.
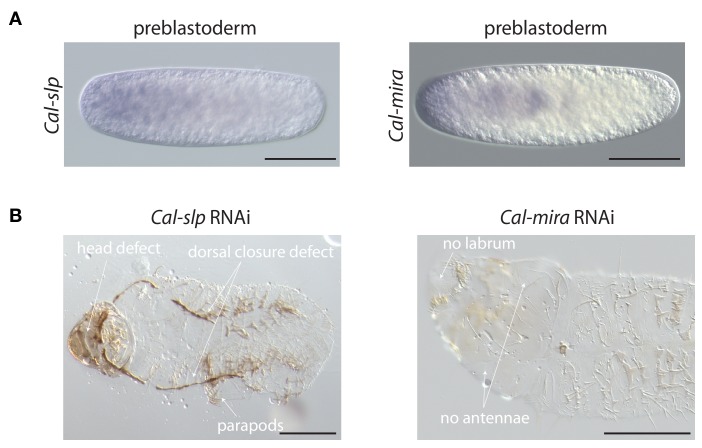
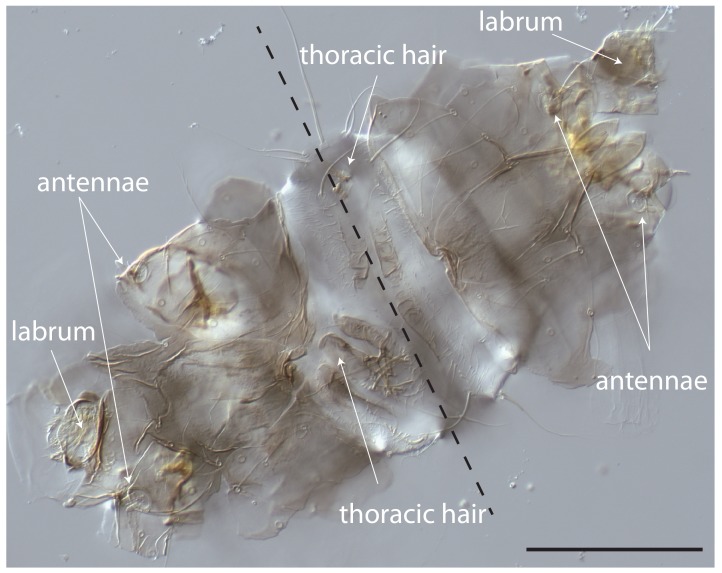
Figure 2—figure supplement 3. Maternal transcript localization and RNAi phenotypes of Cal-slp and Cal-mira in Clogmia.
Figure 2—figure supplement 4. Cuticle phenotype of a 1 st instar Clogmia larva following Cal-opaMat mRNA injection.
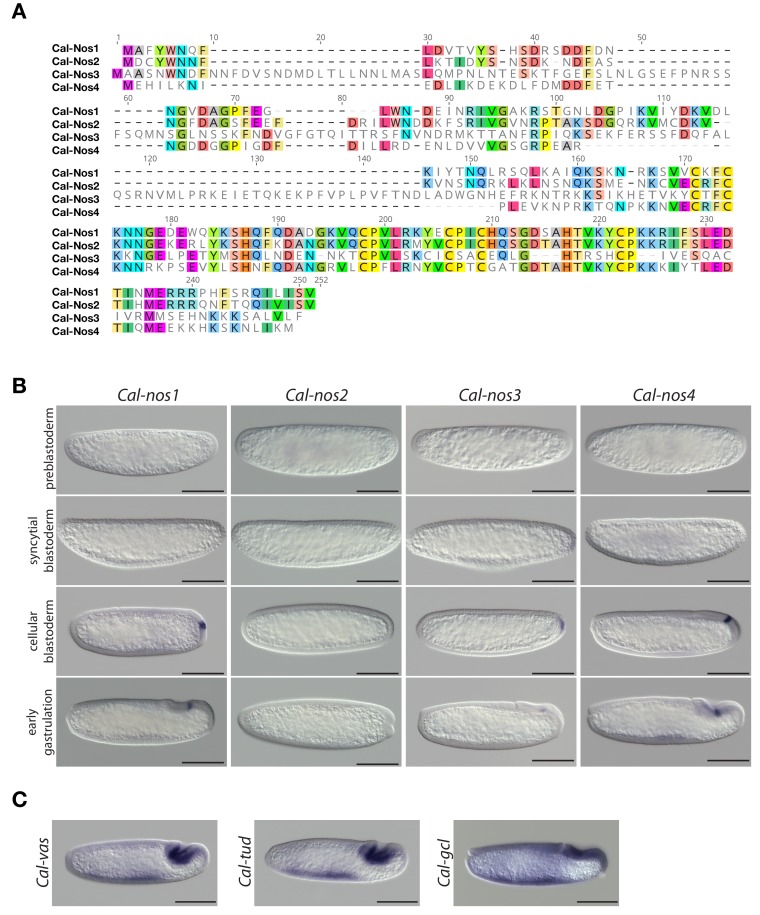
Figure 2—figure supplement 5. Clogmia homologs of nanos, vasa, tudor, and germ cell-less.
We noticed that maternal transcripts of Cal-slp and Cal-mira, a homolog of miranda, which encodes an adaptor protein for cell fate determinants in Drosophila (Ikeshima-Kataoka et al., 1997; Adams et al., 2000), were also slightly enriched in the anterior portion of embryo (Figure 1B). This observation was confirmed by RNA in situ hybridizations (Figure 2—figure supplement 3). Injection of Cal-slp dsRNA resulted in head and dorsal closure defects while Cal-mira dsRNA caused labrum and antennal defects, but in both cases embryo polarity was retained (Figure 2—figure supplement 3).
To test whether Cal-opaMat can induce head development ectopically, we injected Cal-opaMat mRNA into the posterior pole of 1 hr-old embryos. These embryos expressed a head marker, Cal-otd (ortholog of ocelliless/orthodenticle), on both ends of the embryo and developed a symmetrical double head, including some duplicated thoracic elements (Figure 2C and Figure 2—figure supplement 4 and Video 1). These observations suggest that anterior enrichment of maternal transcripts other than Cal-opaMat mRNA is not essential for head development, and that Cal-opaMat localization is sufficient for establishing embryo polarity.
Video 1. Live double head embryo in ventrolateral view.
The larval cuticle of this embryo is shown in Figure 3—figure supplement 1.
The anterior determinant function of Cal-opa is sensitive to expression timing but insensitive to 5’ truncation of the open reading frame
Next, we asked whether the early timing of odd-paired expression is critical for its function as anterior determinant in moth flies. To test this hypothesis, we conducted posterior injections of Cal-opaMat mRNA during the syncytial blastoderm stage (4 hr) and examined Cal-otd expression after gastrulation. These embryos developed with normal head-to-tail polarity (Figure 2D). This result places the requirement of odd-paired for axis specification prior to the syncytial blastoderm stage and suggests that early timing of odd-paired activity is essential for its function as anterior determinant.
Cal-opaMat and Cal-opaZyg mRNAs not only differ in the timing of expression, but also differ in their 5’UTRs and predicted N-terminal protein sequences, as mentioned above (Figure 1C and Figure 1—figure supplement 1). To test whether the open reading frame difference is required for the anterior determinant function, we injected Cal-opaZyg mRNA at the posterior pole of preblastoderm embryos. Embryos from this experiment also developed as double heads (Figure 2D). Because the translation start site of Cal-OpaMat is located downstream of the Cal-OpaZyg translation start site, we also tested Cal-opaZygmRNA in which the putative start codon for Cal-OpaMat was mutated to encode leucine (Cal-opaZyg-Met21Leu). Posterior injection of Cal-opaZyg-Met21LeumRNA also resulted in double heads (Figure 2D). These findings indicate that the protein difference between Cal-OpaMat and Cal-OpaZyg is not essential for the anterior determinant function of odd-paired in Clogmia.
To test whether only a small portion of the Cal-opa open reading frame is required for its function as anterior determinant, we examined the ability of various truncated variants of Cal-Opa mRNAs (Figure 1—figure supplement 1) to induce head development at the posterior egg pole (Figure 2D). mRNAs of protein variants with large N-terminal truncation (Cal-Opa115-655 and Cal-Opa182-655) retained the ability to induce double heads. However, mRNAs of protein variants with C-terminal truncation (Cal-Opa182-445 and Cal-Opa1-337+22, a hypothetical splice variant) failed to induce double heads. These results indicate that the ability of Cal-Opa to specify embryo polarity requires the C-terminal portion of the protein but is largely insensitive to N-terminal truncation, corroborating our above conclusion that N-terminal differences between Cal-OpaMat and Cal-OpaZyg were not essential for evolving the anterior determinant function of Cal-opa.
Cal-opaMat suppresses zygotic germ cell specification at the anterior pole and Clogmia lacks maternal germ plasm
In Drosophila and other dipterans, maternal germ plasm in the posterior embryo not only specifies primordial germ cells but also contributes to and stabilizes embryo polarity via nanos, which suppresses the translation of anterior determinants in the posterior embryo (Tautz, 1988; Gavis and Lehmann, 1992; Struhl et al., 1992; Lemke and Schmidt-Ott, 2009). The activity of nanos in the posterior preblastoderm is dependent on oskar (Lehmann, 2016), which is conserved in many insects (Ewen-Campen et al., 2010). However, in Clogmia, expression profiling of anterior and posterior egg halves did not reveal any posteriorly localized maternal transcripts (Figure 1B, alpha = 0.001, unadj.), and no oskar homolog was found in our Clogmia transcriptomes or the Clogmia genome (Vicoso and Bachtrog, 2015). To test whether Clogmia lacks maternal germ plasm, we examined the expression of candidate germ cell markers, including Clogmia homologs of nanos (Cal-nos1, Cal-nos2, Cal-nos3, and Cal-nos4), vasa (Cal-vas), tudor (Cal-tud), and germ cell-less (Cal-gcl). Cal-nos1, Cal-nos3, and Cal-nos4 were not localized in the posterior of preblastoderm embryos but were expressed in a small set of cells at the posterior pole of cellular blastoderm and gastrulating embryos along with Cal-vas, Cal-tud, and Cal-gcl that were expressed more broadly (Figure 2—figure supplement 5). These observations suggest that Clogmia lacks maternal germ plasm and that Clogmia may induce the germ cell fate zygotically. To test this hypothesis, we examined Cal-nos expression in Cal-opaMat RNAi embryos. Cal-nos positive cells were duplicated in double abdomens (Figure 2E), indicating that Clogmia uses an inductive mechanism for germ cell specification, which is repressed in the anterior embryo by Cal-opaMat. Therefore, axis specification in the Clogmia embryo is independent from germ cell specification. To our knowledge, Clogmia is also the first example of inductive germ cell specification in flies.
Evolution of the anterior determinant function of moth fly odd-paired
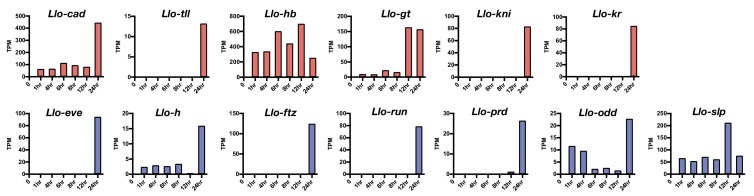
Maternal odd-paired transcript is absent in freshly deposited eggs of chironomids (Klomp et al., 2015) and mosquitoes (Akbari et al., 2013), both of which belong to the Culicomorpha lineage (Figure 1A). To test whether localized maternal odd-paired transcript is broadly conserved in the Psychodomorpha lineage, we examined maternal transcript localization in the eggs of the sand fly Lutzomyia longipalpis, a moth fly species of public health concern due to its role in the transmission of visceral leishmaniasis. Of 5392 annotated transcripts, the most enriched maternal transcript in the anterior half of 1–2 hr old embryos was homologous to odd-paired and was therefore named Llo-opaMat (Figure 3A). In the posterior Lutzomyia embryo, the most enriched transcript was homologous to oskar, indicating that Lutzomyia eggs contain maternal germ plasm at the posterior pole, unlike the Clogmia eggs. These findings suggest that a broad range of moth flies use odd-paired transcript as anterior determinant, and that maternal germ plasm was lost only in the Clogmia lineage. Close examination of Lutzomyia transcriptomes from 1 hr-old and 24 hr-old embryos also revealed zygotic odd-paired transcript (Llo-opaZyg) (Figure 3B). Llo-opaMat and Llo-opaZyg share the same open reading frame but differ at their untranslated 5’ and 3’ ends. Since the N-terminal ends of Llo-OpaMat/Llo-OpaZyg and Cal-OpaZyg proteins are homologous (Figure 1—figure supplement 1), we infer that the N-terminal truncation of Cal-OpaMat occurred after the transcript had evolved maternal expression and anterior localization. The detection of Llo-opaZyg transcript in 24 hr-old embryos coincided with that of gap and pair-rule segmentation gene homologs (Figure 3—figure supplement 1), indicating that Llo-opaZyg functions during segmentation.
Figure 3. Alternative odd-paired transcript isoforms in Lutzomyia and ectopic head induction by mRNAs derived from Cal-opa orthologs in Clogmia.
(A) Differential expression analysis of maternal transcripts between anterior and posterior halves of 1–2 hr-old Lutzomyia embryos. logFC: log fold-change. (B) Stage-specific RNA-seq read coverage of Llo-opa genomic locus and sketches of Llo-opaMat and Llo-opaZyg transcripts (see also legend to Figure 1A). (C) Posterior injection of odd-paired mRNAs from Lutzomyia, Chironomus, and Drosophila in Clogmia embryo. Phenotypes were counted as in (Figure 2D) and double head from Dme-opa mRNA injection is shown as example. Cri-opa and Dme-opa mRNAs include Kozak sequence of Cal-opaMat (TAAG upstream of the predicted translation start site). Cri-kozak and Dme-kozak refer to odd-paired sequences with donor-specific Kozak-sequences from Chironomus (AAAA) and Drosophila (GACC), respectively. ns.: p>0.05; *: p<0.05. ***: p<0.001, Fisher’s exact test.
Figure 3—figure supplement 1. Expression level of select Lutzomyia gap gene (red) and pair-rule gene (blue) homologs.
The odd-paired gene of ancestral moth flies could have evolved the ability to establish the embryo polarity via specific amino acid substitutions. In this case, odd-paired homologs from species with a different anterior determinant, such as Drosophila or Chironomus, should not induce ectopic head development in Clogmia embryos. Alternatively, odd-paired could have evolved its role as axis determinant in moth flies independent of any amino acid substitution via co-option. In this case, odd-paired homologs from Drosophila or Chironomus could have the ability to induce head development in Clogmia embryos when appropriately expressed. To test this possibility, we injected odd-paired mRNA from Lutzomyia, Chironomus, or Drosophila into the posterior pole of early Clogima embryos. All of these odd-paired homologs induced double heads in Clogmia, provided that the endogenous kozak sequence of Cal-opaMat was used for optimal translation efficiency (Figure 3C). Since neither Drosophila nor Chironomus uses odd-paired for specifying embryo polarity, these results suggest that amino acid substitutions were not essential for the evolution of the anterior determinant function of odd-paired in moth flies. We therefore propose that this gene function evolved via co-option when alternative maternal transcript of moth fly odd-paired became enriched at the anterior egg pole.
A previously uncharacterized C2H2 zinc finger gene, named cucoid, functions as anterior determinant in culicine mosquitoes
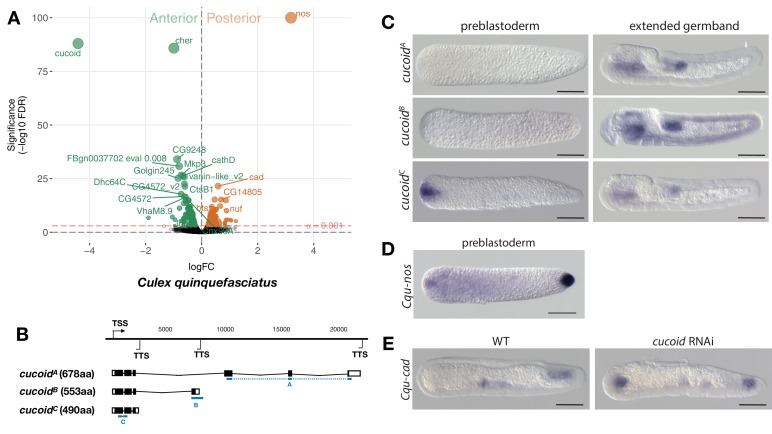
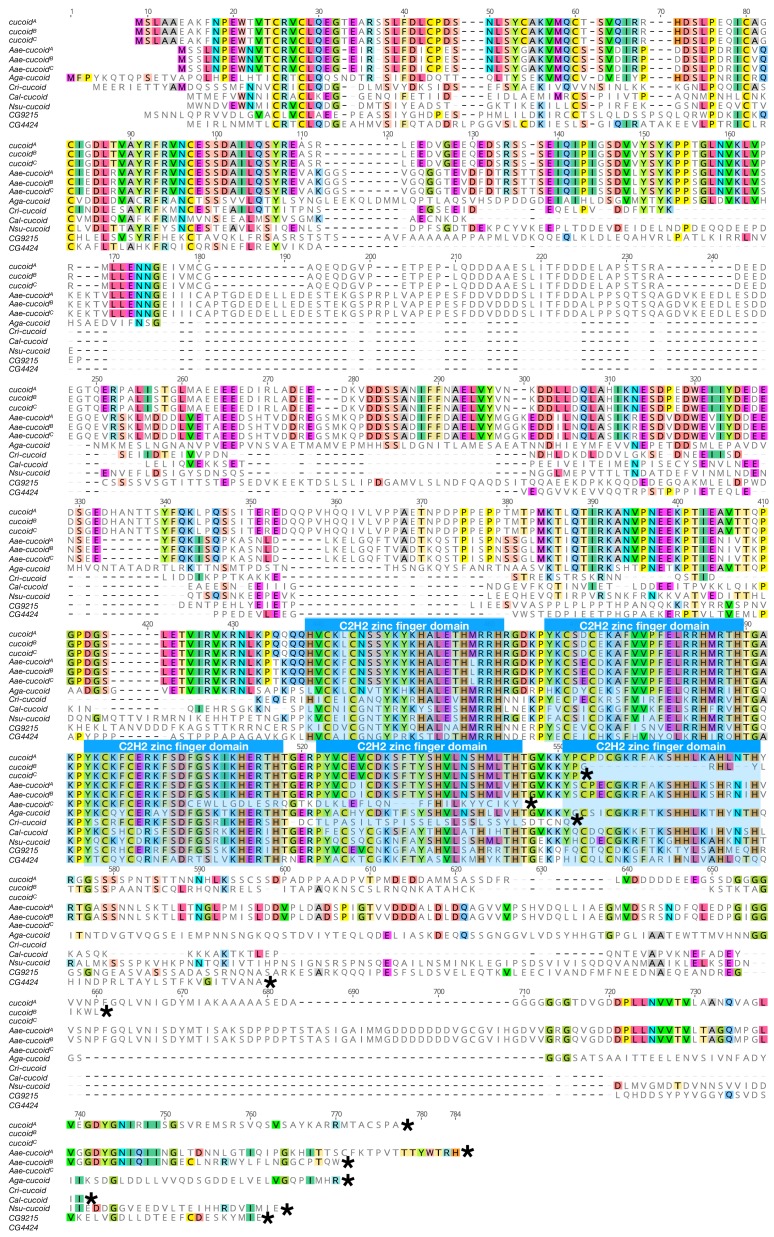
Given that freshly deposited mosquito eggs lack maternal odd-paired transcript orthologs (Akbari et al., 2013), we extended our search for anterior determinants to mosquitoes. Initially, we focused on the Southern House Mosquito Culex quinquefasciatus, a vector of West Nile virus (the leading cause of mosquito-borne disease in the continental United States) and of Wuchereria bancrofti (the major cause of lymphatic filariasis). This species was chosen because their eggs are large and have clearly distinguishable anterior and posterior egg poles. We annotated 8239 Culex transcripts from the pooled anterior and posterior transcriptomes of 1 hr-old preblastoderm embryos and ranked them according to the magnitude of their differential expression scores and P values (Figure 4A). In the posterior embryo, the most enriched transcript was related to nanos, consistent with the presence of maternal germ plasm in this species (Figure 4D) (Juhn et al., 2008). The most enriched transcript in the anterior embryo was closely related to an uncharacterized gene of Drosophila (CG9215). However, reciprocal BLAST searches suggest that CG9215 belongs to a poorly defined larger gene family in Drosophila that might be represented by a single gene in mosquitoes. We named this gene cucoid to reflect its bicoid-like function in a culicine mosquito. cucoid encodes a protein with five C2H2 zinc finger domains (Figure 4—figure supplement 1). RACE experiments with cDNA from 0 to 7 hr-old embryos revealed three alternative cucoid transcripts with distinct 3’ ends (cucoidA, cucoidB, and cucoidC) (Figure 4B), but only cucoidB and cucoidC were recovered from cDNA of 0–2 hr-old preblastoderm embryos, suggesting that one or both these transcripts might be maternally localized at the anterior pole. To test this hypothesis, we performed RNA in situ hybridizations with specific probes for cucoidA (probe A) or cucoidB (probe B) and, due to the very short sequence unique to cucoidC (121 nucleotides), a probe against all three isoforms (probe C). cucoidA and cucoidB expression was detected in the fore and hind gut of extended germbands but not in 1 hr-old preblastoderm embryos. In contrast, the probe against all three isoforms detected maternally localized cucoid transcript at the anterior pole in addition to the zygotic expression pattern (Figure 4C). Taken together, these results suggest that only the cucoidC isoform is maternally localized at the anterior pole and could function as anterior determinant. To test this hypothesis, we injected cucoid dsRNA from the shared 5’ region and examined the expression of a posterior marker (Cqu-cad) in gastrulating embryos. Many of these embryos expressed Cqu-cad in the anterior and underwent ectopic gastrulation at the anterior pole, suggesting that normal head-to-tail polarity was lost (Figure 4E). Taken together, our results suggest that cucoid acquired the anterior determinant function via the localization of a maternal transcript isoform with an alternative 3’ end.
Figure 4. Expression and function of alternative cucoid isoforms in Culex.
(A) Differential expression analysis of maternal transcripts between anterior and posterior halves of 1 hr-old Culex embryos. logFC: log fold-change. (B) Sketches of cucoid transcript isoforms based on RNA-seq and RACE experiments (see also legend to Figure 1A). Blue lines the position of in situ hybridization probes. (C) RNA in situ hybridization of cucoidA, cucoidB, and cucoidC transcripts in 1 hr-old preblastoderm and germband extending embryos. Anterior is left and dorsal up. Scale bar: 100μm. (D) RNA in situ hybridization of Cqu-nos in 1 hr-old preblastoderm embryo. Anterior is left. Scale bar: 100μm (E) RNA in situ hybridization of Cqu-cad in a wild-type gastrulating embryo (left) and in a stage-matched cucoid RNAi embryos (right; 16/48 with cucoid dsRNA versus 0/25 with lacZ control dsRNA; p<0.0005). Anterior is left and dorsal up. Scale bar: 100μm.
Figure 4—figure supplement 1. Protein alignment of dipteran cucoid orthologs and a Drosophila paralog CG4424.
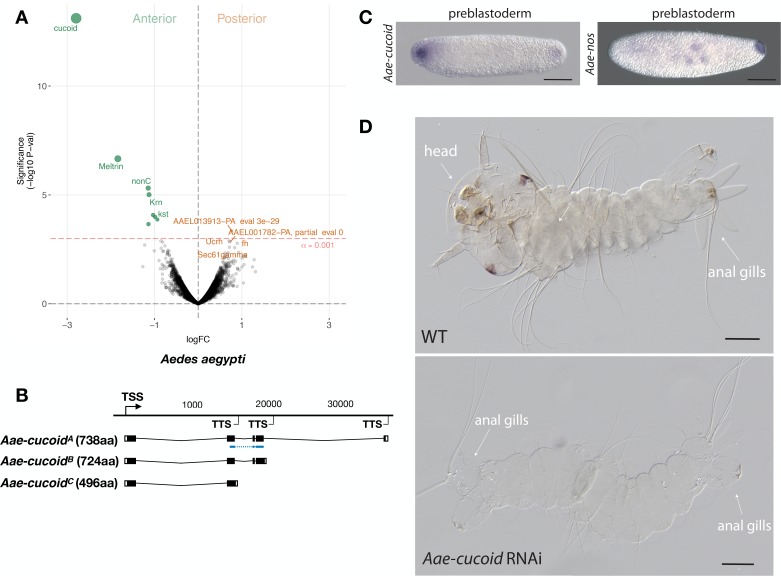
We obtained similar results in another culicine mosquito, the Yellow Fever Mosquito Aedes aegypti, which transmits Dengue, Chikungunya, and Zika viruses. In this species, expression profiling of 5802 transcripts from the anterior and posterior transcriptomes of 1 hr-old preblastoderm embryos also identified cucoid (Aae-cucoid) as the gene with the most significantly enriched transcript in the anterior embryo (Figure 5A). RACE experiments with cDNA from 1 hr to 6 hr-old embryos revealed three similar Aae-cucoid transcripts with alternative 3’ ends (Aae-cucoidA, Aae-cucoidB, and Aae-cucoidC) (Figure 5B), and RNA in situ hybridization experiments with a probe against all three isoforms confirmed the anterior localization of Aae-cucoid transcript in preblastoderm embryos (Figure 5C). Aae-cucoid expression in Aedes germbands could not be examined for technical reasons.
Figure 5. Expression and function cucoid in Aedes.
(A) Differential expression analysis of maternal transcripts between anterior and posterior halves of 1 hr-old Aedes embryos. logFC: log fold-change. (B) Sketches of Aae-cucoid transcript isoforms based on RNA-seq and RACE experiments with the position of the in situ hybridization probe and dsRNA underlined in blue (see also legend to Figure 1A). (C) RNA in situ hybridization Aae-cucoid (left) and Aae-nos (right) transcript in 1 hr-old preblastoderm embryo. Anterior is left and dorsal up. Scale bar: 100μm. (D) 1 st instar Aedes larval cuticle of wild type (top) and following Aae-cucoid RNAi (bottom; 9/26 versus 0/22 with control dsRNA; p<0.005). Scale bar: 100μm.
In the posterior embryo, no highly enriched transcripts were observed. This was unexpected given that whole mount in situ hybridizations revealed posterior localized transcript of Aedes nanos in 1 hr-old embryos (Figure 5C) and that maternal transcript of Aedes oskar is also localized at the posterior pole (Juhn and James, 2006). Low statistical power of our differential expression analysis in Aedes might explain this discrepancy, since we could have confounded the anterior and posterior pole in some of the bisected Aedes eggs (see Materials and methods). Alternatively, only a small portion of these transcripts might be localized at the posterior pole. Injection of Aae-cucoid dsRNA against the shared region of all transcripts resulted in double abdomens (Figure 5D), suggesting that cucoid evolved its function as anterior determinant prior to the divergence of the Culex and Aedes lineages.
pangolin/Tcf functions as anterior determinant in anopheline mosquitoes
The Anopheles gambiae species complex constitutes an outgroup to the Culex-Aedes clade. It includes eight or more sub-Saharan species that are difficult to distinguish due to widespread genealogical heterogeneity across the genome, incomplete lineage sorting and introgression (Thawornwattana et al., 2018). We interchangeably used A. gambiae and A. coluzzii, two sibling species within this species complex that are responsible for the majority of malaria transmission in Africa, to identify the anterior determinant of this mosquito lineage. Whole mount RNA in situ hybridizations with a probe against the Anopheles gambiae ortholog of cucoid did not detect any anterior localized transcript in 1 hr-old embryos, suggesting that Anopheles uses a different anterior determinant than Culex and Aedes.
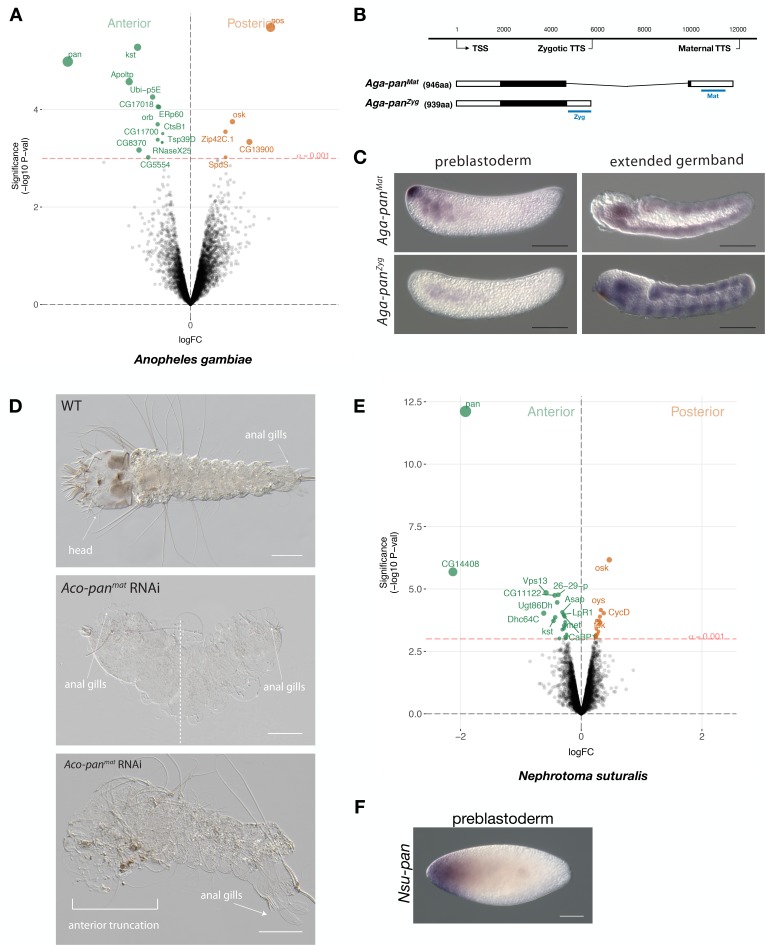
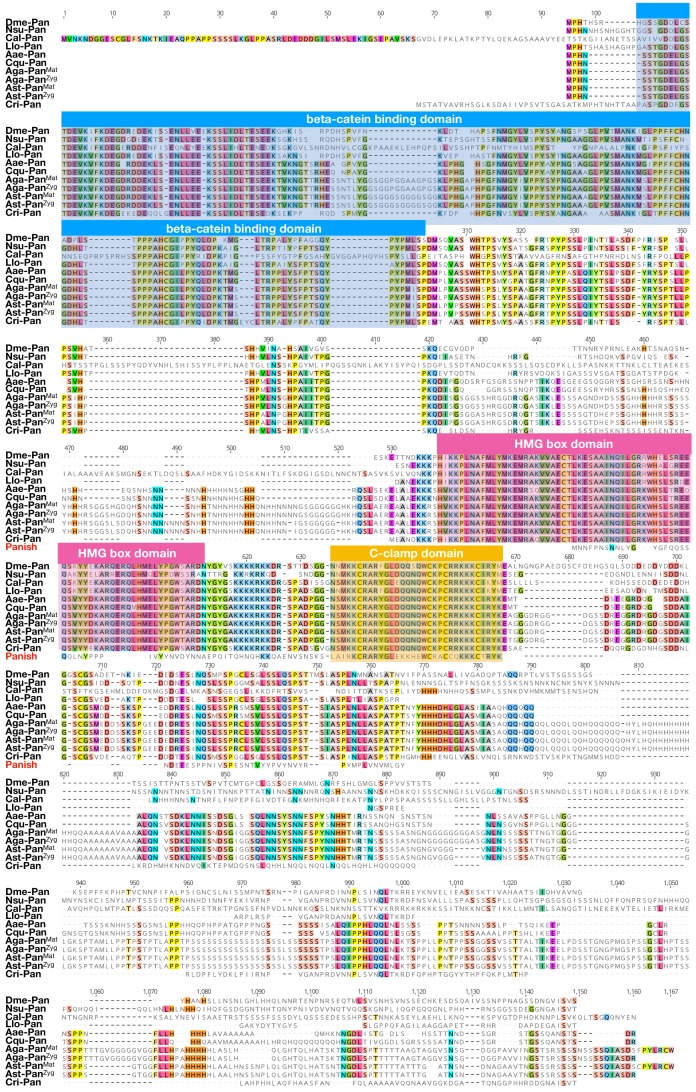
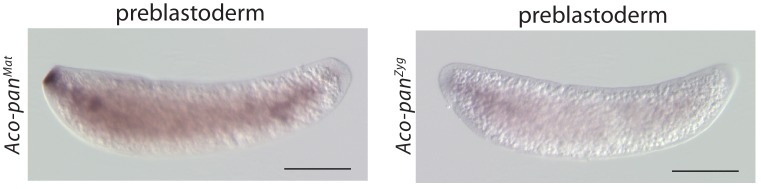
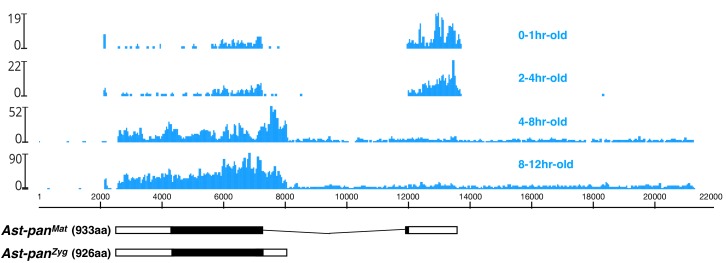
To further test this possibility, we sequenced the anterior and posterior transcriptomes 1 hr-old preblastoderm embryos of A. gambiae and ranked 9353 transcripts according to the magnitude of their differential expression scores and P values. In the posterior embryo, the most enriched transcript was homologous to nanos. In the anterior embryo, the most enriched transcript was homologous to pangolin (also known as Tcf) (Figure 6A). To test for potential alternative maternal and zygotic isoforms of pangolin in Anopheles, we mapped the assembled transcripts and 5’ and 3’ RACE products from 1 to 6 hr-old embryos onto an available A. gambiae genome assembly (AgamP4). We identified two alternative transcript variants with non-overlapping 3’UTRs but nearly identical open reading frames that we named Aga-panMat and Aga-panZyg, respectively (Figure 6B and Figure 6—figure supplement 1). Aga-panMat was tightly localized at the anterior pole of 1–2 hr-old preblastoderm embryos and only weakly expressed in elongated germbands, whereas Aga-panZyg was expressed segmentally in elongated germbands but not in embryos younger than 2 hr-old preblastoderm embryos (Figure 6C). Both pangolin isoforms were conserved in Anopheles coluzzii with sequence identity above 99% and the maternal isoform (Aco-panMat) was localized at the anterior pole (Figure 6—figure supplement 2). The stage-specific expression of both pangolin isoforms was also conserved outside the Anopheles gambiae species complex in Anopheles stephensi (Figure 6—figure supplement 3). Alignments of dipteran Pangolin proteins suggest that, in Anopheles, the maternal variant includes an additional seven amino acids at C-terminal end due to alternative polyadenylation and splicing (Figure 6—figure supplement 1).
Figure 6. Expression and function of alternative pangolin isoforms in Anopheles.
(A) Differential expression analysis of maternal transcripts between anterior and posterior halves of 1 hr-old Anopheles embryos. logFC: log fold-change. (B) Sketches of Aga-panMat and Aga-panZyg transcripts based on RNA-seq and RACE experiments (see also legend to Figure 1A). Blue lines the position of in situ hybridization probes and dsRNA. (C) RNA in situ hybridization of Aga-panMat and Aga-panZyg transcripts in 1 hr-old preblastoderm and germband extending embryos of A.gambiae. Anterior is left and dorsal up. Scale bar: 100μm. (D) 1 st instar A.coluzzii larval cuticle of wild type (top). Cuticle phenotypes of double abdomen (middle; 3/37) and intermediate, anterior truncation phenotype (bottom; 8/37) following Aco-panMat RNAi. Scale bar: 100μm. (E) Differential expression analysis of maternal transcripts between anterior and posterior halves of 1 hr-old Nephrotoma (Tipulidae) embryos. logFC: log fold-change. (F) RNA in situ hybridization of Nsu-panMat.
Figure 6—figure supplement 1. Protein alignment of dipteran Pangolin orthologs and Panish.
Figure 6—figure supplement 2. RNA in situ hybridization of Aco-panMat and Aco-panZyg transcripts in 1 hr-old A.coluzzii preblastoderm embryos.
Figure 6—figure supplement 3. Stage-specific RNA-seq read coverage of Ast-pan genomic locus and sketches of Ast-panMat and Ast-panZyg transcripts.
Figure 6—figure supplement 4. Cuticle phenotype of a A.coluzzii 1 st instar larva following Aco-panZyg RNAi (11/23).
Figure 6—figure supplement 5. panish RNAi rescue experiments with Cri-pan mRNA.
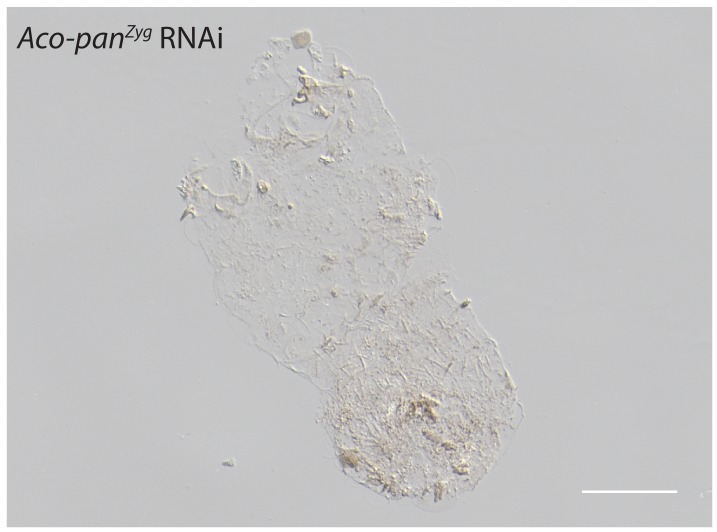
To investigate the function of localized maternal pangolin expression in Anopheles, we specifically targeted this isoform in Anopheles coluzzii. Injection of Aco-panMat-specific dsRNA into several hundred 1 hr-old A. coluzzii embryos resulted in only 37 cuticles with variable phenotypes, including anterior truncations and, in extreme cases, double abdomens (Figure 6D). We noticed perturbed segmentation boundaries in the double abdomens, suggesting that Aga-panMat may also function in segmentation, as suggested by its weak zygotic expression pattern. Injection of Aco-panZyg-specific dsRNA into 1 hr-old A. coluzzii embryos resulted in severe segmentation defects that were difficult to characterize, but no double abdomens or anterior-specific truncation defects were found (Figure 6—figure supplement 4). Taken together with the isoform-specific transcript localization data presented above, these RNAi results support the hypothesis that pangolin acquired the anterior determinant function via the localization of a maternal transcript isoform with an alternative 3’ end.
Localization of maternal pangolin transcript in crane flies suggests that pangolin functioned as anterior determinant in ancestral flies
Anterior-localized maternal pangolin (Tc-pan) transcript has also been observed in the eggs of a beetle (Tribolium castaneum) (Bucher et al., 2005), but the function of this transcript remains unknown. Previous Tc-pan RNAi experiments targeted both maternal and zygotic transcripts and only revealed a function in posterior development, due to the role of zygotic Tc-pan in canonical Wnt signaling in the posterior growth zone (Bolognesi et al., 2008; Fu et al., 2012; Prühs et al., 2017; Ansari et al., 2018). To test whether ancestral dipterans localized maternal pangolin transcript at the anterior pole of the egg, we established a culture of the crane fly Nephrotoma suturalis (Tipulidae), which belongs to the Tipulomorpha, one of the oldest branches of dipterans (Grimaldi and Engel, 2005; Wiegmann et al., 2011). We sequenced the anterior and posterior transcriptomes of freshly deposited Nephrotoma egg halves and ranked 5371 transcripts according to the magnitude of their differential expression scores and P values (Figure 6E). The most enriched transcript in the posterior embryo was related to oskar, suggesting that crane fly eggs contain maternal germ plasm at the posterior pole. The most enriched transcript in the anterior embryo was homologous to pangolin and therefore named Nsu-pan. The anterior localization of this transcript was confirmed by RNA in situ hybridization (Figure 6F). RACE experiments with cDNA from 1 hr-old embryos identified multiple isoforms with slightly variable 5’ ends but the same open reading frame. An alignment of the predicted Nsu-Pan protein from this open reading frame with other dipteran Pangolin homologs revealed conserved N-terminal and C-terminal ends in Nsu-Pan. Taken together with our Anopheles data, our results in Nephrotoma suggest that ancestral dipteran insects localized maternal pangolin transcript in the anterior egg pole, where this transcript may have functioned as anterior determinant.
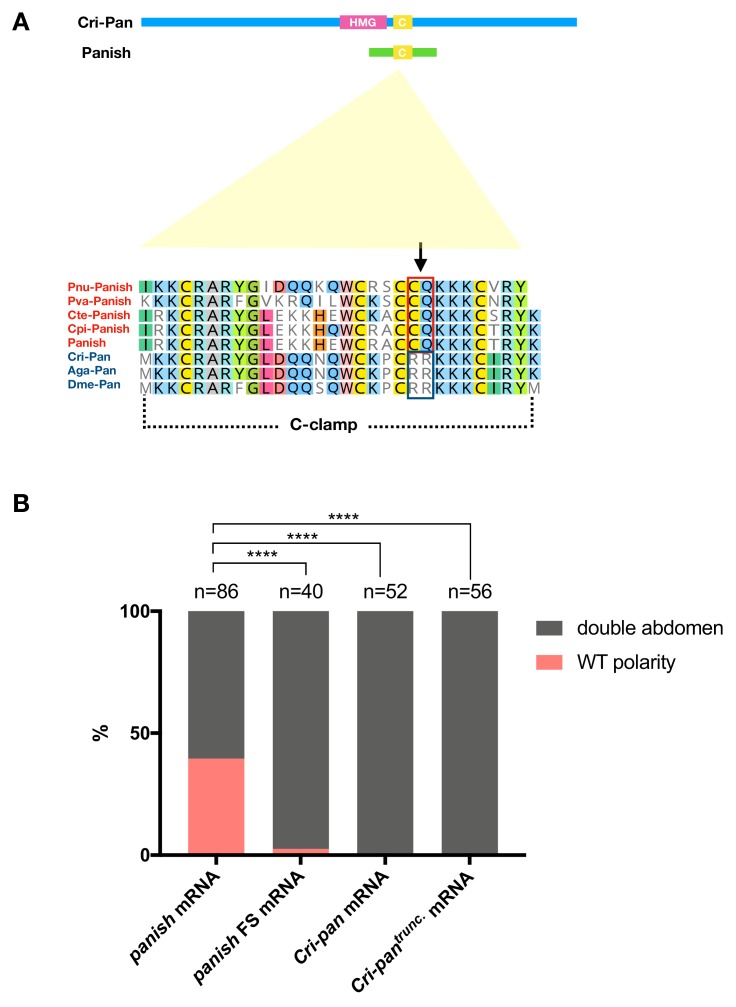
Pangolin cannot substitute for Panish in Chironomnus
In the midge Chironomus, the ortholog of pangolin (Cri-pan) is not expressed maternally but its diverged paralog panish functions maternally as anterior determinant (Klomp et al., 2015). Given that panish evolved from pangolin via gene duplication, panish probably inherited its role from pangolin. Therefore, it is possible that Cri-pan and panish are still functionally equivalent when expressed at the anterior pole of preblastoderm Chironomus embryos. Alternatively, Panish may have co-evolved with the targets required for anterior patterning and Pangolin can no longer interact with those targets. In this case, Cri-pan should no longer be able to fulfill the function of panish. To distinguish between these possibilities, we examined the ability of panish and Cri-pan mRNAs to rescue the RNAi phenotype of panish. We have previously shown that dsRNA of the panish 3’UTR can induce the double abdomen phenotype with a penetrance of nearly 100%, and that this phenotype can be rescued in roughly half of the embryos by injecting panish mRNA with heterologous UTRs at the anterior pole, shortly after the injection of dsRNA (Klomp et al., 2015). We used this assay to compare the functions of panish mRNA (positive control), frame shifted panish mRNA (negative control), Cri-pan mRNA, and a modified Cri-pan mRNA designed to better resemble panish mRNA (Cri-pantrunc. mRNA). Cri-pantrunc. mRNA encodes a N-terminal truncated Cri-Pan variant, lacking the ß-Catenin binding and HMG box domains, with two mutations in the cysteine-clamp domain to mimic conserved changes of the Panish cysteine-clamp (Figure 6—figure supplement 5A). Only panish mRNA rescued panish RNAi embryos (Figure 6—figure supplement 5B), suggesting that panish co-evolved with its targets and functionally diverged after its origin via gene duplication from pangolin.
Discussion
Role of alternative transcription in the evolution of embryonic axis determinants from old genes
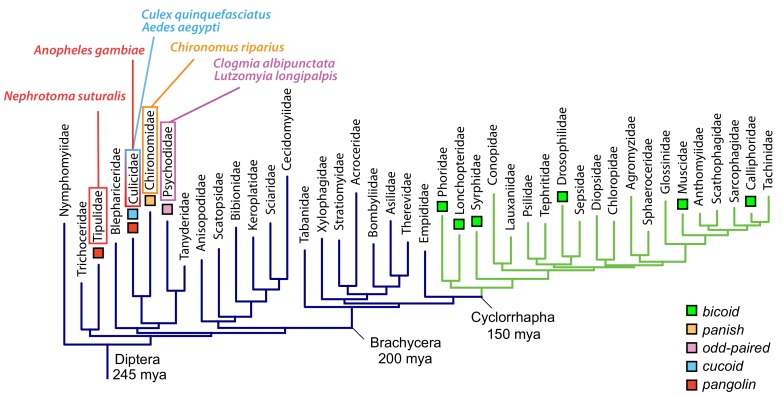
In this study, we have identified three unrelated old genes that encode the anterior determinant in moth flies, culicine mosquitoes, and anopheline mosquitoes, respectively (Figure 7). All three genes not only localize their maternal transcript at the anterior egg pole; they also are subject to alternative transcription, which allows a single gene to generate multiple transcript isoforms with distinct 5’ and 3’ ends through the use of alternative promoters (alternative transcription initiation) and polyadenylation signals (alternative transcription termination).
Figure 7. Anterior axis determinants in Diptera.
The phylogenetic tree of dipteran families is based on published data and shows Cylorrhapha in green (Wiegmann et al., 2011). Mya, million years ago.
Figure 7—figure supplement 1. Occurrence of panish in Chironomidae genomes.
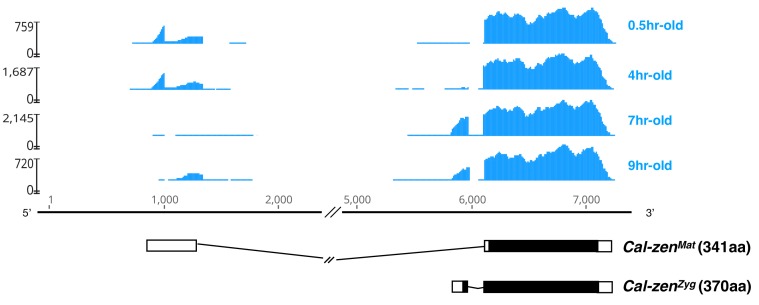
Figure 7—figure supplement 2. Maternal and zygotic transcript variant of Clogmia zerknüllt (Cal-zen).
In moth flies, the localized maternal odd-paired transcript that functions as anterior determinant has an alternative first exon compared to the canonical isoform (Figure 1B–D, Figure 2, and Figure 3A–B). In the case of mosquitoes, maternal transcript isoforms of cucoid (in culicine mosquitoes) or pangolin (in anopheline mosquitoes) with alternative last exons are localized at the anterior pole of the egg and function as anterior determinant (Figure 4, Figure 5, and Figure 6). Since anterior determinants are localized in the anterior egg, and signals for the subcellular localization of transcripts are typically found in UTRs (Holt and Bullock, 2009), it is possible that alternative transcription facilitates the evolution of anterior determinants by providing the UTR sequence for isoform-specific localization signals that do not interfere with other gene functions. For example, it has been shown that alternative last exons of transcript isoforms confer isoform-specific localization in neurons (Taliaferro et al., 2016; Ciolli Mattioli et al., 2019). Additional experiments will be needed to test whether the unique UTR sequences of anterior determinants are essential for their localization at the anterior egg pole.
In addition to changes in UTR sequences, alternative transcription also can result in the truncation or elongation of the open reading frame. For example, the anterior determinant of Clogmia (Cal-OpaMat) lacks the N-terminal 20 amino acids (Figure 1—figure supplement 1), and the anterior determinant of Anopheles (Aga-panMat) encodes protein that includes additional seven amino acids at the C-terminal end (Figure 6—figure supplement 1). However, these changes to the protein may not have been important for adopting a function as anterior determinant. The truncation in the maternal Odd-paired protein that we observed in Clogmia is not conserved in Lutzomyia, in which Llo-opaMat and Llo-opaZyg encode the same protein, and full-length Odd-paired homologs from these and other species can function as anterior determinant in Clogmia (Figure 3B–C). Also, the elongation of Aga-PanMat protein is not conserved in Nephrotoma, in which the localized Nsu-panMat transcript encodes a Pangolin protein with a conserved C-terminal end (Figure 6—figure supplement 1). Therefore, modifications in the open reading frame of these genes may reflect secondary changes.
Evolution of new genes that encode embryonic axis determinants
Unlike the anterior determinants identified in this study, the previously described anterior determinants of Drosophila and Chironomus are encoded by newly evolved genes, bicoid and panish. These genes seem to be dispensable outside the context of axis specification (Driever, 1993; Klomp et al., 2015), suggesting that they evolved specifically for this function. They could have acquired their function de novo via protein evolution or via inheritance from the progenitor gene. Our findings suggest that the role of pangolin in axis specification was already present in ancestral dipterans (Figure 7). We therefore propose that panish, which evolved from pangolin via gene duplication in the Chironominae lineage (Figure 7—figure supplement 1), inherited its function from pangolin. Future examinations of pangolin isoforms and their expression in the eggs of chironomids that lack panish orthologs (species representing basal chironomid lineages) could reveal intermediate steps in this process, such as a localized truncated pangolin isoform.
Similarly, bicoid could have acquired its function de novo via protein evolution or via inheritance from its progenitor gene, zerknüllt. Several previous studies have hypothesized that Bicoid replaced Orthodenticle, a conserved homeodomain protein with similar DNA-binding affinity that functions in animal head development (Wimmer et al., 2000; Schröder, 2003; Lynch et al., 2006; Datta et al., 2018). Ancestrally reconstructed homeodomains confirmed that a single amino acid change in the homeodomain of Bicoid (Q50K), which is shared by Bicoid and Orthodenticle, caused a dramatic shift of Bicoid’s DNA-binding affinity in vitro and target recognition in vivo (Liu et al., 2018).
We cannot rule out that orthodenticle functioned as anterior determinant in ancestral brachyceran flies. However, in analogy with our findings, it is also possible that a maternal zerknüllt isoform became localized at the anterior pole of the egg and acquired the role of anterior determinant via co-option, prior to the origin of bicoid via gene duplication (Figure 7—figure supplement 2). Maternal zerknüllt expression is common in lower Diptera but was lost in Cyclorrhapha (Stauber et al., 2002). If bicoid inherited its function from zerknüllt, the Q50K mutation in the homeodomain of Bicoid must have been a secondary, potentially maladaptive change. In this case, it may have been fixed in the cyclorrhaphan stem lineage via a compensatory or balancing mechanism and would have driven co-evolution of its targets.
It may be objected that, in Drosophila, reverting the K50 residue of the Bicoid homeodomain to Q50 is lethal and results in a bicoid null phenotype (Liu et al., 2018). However, how the ancestral gene network responded to the Q50K mutation of Bicoid cannot be inferred from observations in Drosophila. Moreover, published biochemical data suggest that the Q50K mutation increases interaction with the consensus Bicoid binding DNA motif much stronger than it reduces interaction with the consensus Zerknüllt binding DNA motif. It is therefore conceivable that the Q50K mutation had a less dramatic effect in ancestral flies, in which the target genes of the anterior determinant were activated via Zerknüllt binding sites, than in Drosophila, in which the target genes of the anterior determinant are activated via Bicoid binding sites. Examination of the mechanisms that determine embryo polarity in non-cyclorrhaphan Brachycera flies might help to test this hypothesis. If panish and bicoid inherited their functions, their evolutionary origin and divergence could have served the purpose of reducing the pleiotropy of their progenitor genes, pangolin and zerknüllt, rather than allowing them to take on an entirely new function in development.
Role of alternative transcription in the evolution
Recent genome-wide analyses have shown that alternative transcription is a widespread phenomenon. For example, there are on average four alternative transcription start sites per gene in humans (Forrest et al., 2014) and at least 50–70% of mammalian genes are subject to alternative polyadenylation (Shepard et al., 2011; Adams et al., 2000; Derti et al., 2012). Alternative transcript isoforms can be tightly regulated in a cell or tissue specific manner and can affect transcription and translation efficiency as well as splicing (An et al., 2008; Davuluri et al., 2008; Lau et al., 2010; Pinto et al., 2011; Smibert et al., 2012; Anvar et al., 2018; Tushev et al., 2018; Taliaferro et al., 2016; Ciolli Mattioli et al., 2019; Shabalina et al., 2010). Functional studies in model organisms have shown that alternative transcription can generate dominant negative and alternatively localized protein isoforms (Davuluri et al., 2008; Bharti et al., 2008; Vacik et al., 2011; Berkovits and Mayr, 2015; Schrankel et al., 2016), while misregulation of alternative transcript isoforms has been associated with human diseases including cancer (Mayr and Bartel, 2009; Wiesner et al., 2015; Pal et al., 2012; Shapiro et al., 2011). However, the contribution of alternative promoters (alternative transcription initiation) and polyadenylation signals (alternative transcription termination) to the evolution of new gene functions and regulatory networks remains poorly understood (Carroll et al., 2005; Peter and Davidson, 2011; Wittkopp and Kalay, 2011; de Klerk and 't Hoen, 2015).
The results of several previous studies suggest that alternative transcription may underlie the evolutionary diversification of gene functions. For example, a large fraction of alternative promoter sequences is conserved between human and mice, but those with cell or tissue restricted expression have frequently changed during mammalian evolution (Baek et al., 2007; Forrest et al., 2014), suggesting that alternative promoters may have played a significant role in cell type evolution. Another likely substrate for evolutionary diversification are the protein terminal ends generated by alternative transcription in conjunction with alternative splicing. These terminal ends commonly contain intrinsically disordered regions which are enriched in sites that mediate protein-protein interactions (Buljan et al., 2013; Shabalina et al., 2014). A recent case study found that a light fur color variant of beach mice evolved repeatedly via selection for an alternative agouti isoform with increased translation efficiency (Linnen et al., 2013; Mallarino et al., 2017).
Our in vivo study revealed three old genes that evolved the anterior determinant function by localizing an alternative transcript isoform at the anterior pole of the egg. Therefore, we propose that differential expression of alternative transcript isoforms can result in the evolution of new gene functions, independent of, and prior to gene duplication and sub-functionalization. Given that alternative transcription is a widespread phenomenon, it could play an important role in the evolution of gene regulatory networks.
Materials and methods
Key resources table.
| Reagent type (species) or resource |
Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (Nephrotoma suturalis) | Nsu | https://doi.org/10.1083/jcb.25.1.95 | NA | |
| Strain, strain background (Chironomus riparius) | Cri | https://doi.org/10.1126/science.aaa7105; https://doi.org/10.1111/mec.1411 | CRIP_Laufer | |
| Strain, strain background (Clogmia albipunctata) | Cal | https://doi.org/10.1007/s004270050238 | NA | |
| Strain, strain background (Culex quinquefasciatus) | Cqu | NIAID | NA | |
| Strain, strain background (Anopheles gambiae) | A.gambiae | NIAID | G-3 strain | |
| Strain, strain background (Anopheles coluzzii) | A.coluzzii | Insect transformation facility, University of Maryland | NA | |
| Strain, strain background (Aedes aegypti) | Aae | NIAID | Liverpool ‘Black eye’ | |
| Gene (Clogmia albipunctata) | Cal-opaMat | This paper | MN122104 | See Materials and methods - Cloning procedures and mRNA/dsRNA synthesis; RNA in situ hybridization;Rapid Amplification of cDNA Ends (RACE) |
| Gene (Clogmia albipunctata) | Cal-opaZyg | This paper | MN122105 | See Materials and methods - Cloning procedures and mRNA/dsRNA synthesis; RNA in situ hybridization; Rapid Amplification of cDNA Ends (RACE) |
| Gene (Clogmia albipunctata) | Cal-slp | This paper | MN122106 | See Materials and methods - Cloning procedures and mRNA/dsRNA synthesis; RNA in situ hybridization |
| Gene (Clogmia albipunctata) | Cal-mira | This paper | MN122107 | See Materials and methods - Cloning procedures and mRNA/dsRNA synthesis; RNA in situ hybridization |
| Gene (Clogmia albipunctata) | Cal-otd | This paper | MN122108 | See Materials and methods - RNA in situ hybridization |
| Gene (Clogmia albipunctata) | Cal-nos1 | This paper | MN122109 | See Materials and methods - RNA in situ hybridization |
| Gene (Clogmia albipunctata) | Cal-nos2 | This paper | MN122110 | See Materials and methods - RNA in situ hybridization |
| Gene (Clogmia albipunctata) | Cal-nos3 | This paper | MN122111 | See Materials and methods - RNA in situ hybridization |
| Gene (Clogmia albipunctata) | Cal-nos4 | This paper | MN122112 | See Materials and methods - RNA in situ hybridization |
| Gene (Clogmia albipunctata) | Cal-vas | This paper | MN122113 | See Materials and methods - RNA in situ hybridization |
| Gene (Clogmia albipunctata) | Cal-tud | This paper | MN122114 | See Materials and methods - RNA in situ hybridization |
| Gene (Clogmia albipunctata) | Cal-gcl | This paper | MN122115 | See Materials and methods - RNA in situ hybridization |
| Gene (Lutzomyia longipalpis) | Llo-opaMat | This paper | MN122116 | See Materials and methods - Rapid Amplification of cDNA Ends (RACE) |
| Gene (Lutzomyia longipalpis) | Llo-opaZyg | This paper | MN122117 | See Materials and methods - Rapid Amplification of cDNA Ends (RACE) |
| Gene (Lutzomyia longipalpis) | Llo-osk | This paper | MN122118 | See Materials and methods - RNA in situ hybridization |
| Gene (Chironomus riparius) | Cri-opa | This paper | MN122119 | See Materials and methods - RNA in situ hybridization |
| Gene (Culex quinquefasciatus) | cucoidA | This paper | MN122120 | See Materials and methods - RNA in situ hybridization |
| Gene (Culex quinquefasciatus) | cucoidB | This paper | MN122121 | See Materials and methods - RNA in situ hybridization |
| Gene (Culex quinquefasciatus) | cucoidC | This paper | MN122122 | See Materials and methods - Cloning procedures and mRNA/dsRNA synthesis; RNA in situ hybridization |
| Gene (Culex quinquefasciatus) | Cqu-nos | This paper | MN122123 | See Materials and methods - RNA in situ hybridization |
| Gene (Aedes aegypti) | Aae-cucoidA | This paper | MN122124 | See Materials and methods - Rapid Amplification of cDNA Ends (RACE) |
| Gene (Aedes aegypti) | Aae-cucoidB | This paper | MN122125 | See Materials and methods - Rapid Amplification of cDNA Ends (RACE) |
| Gene (Aedes aegypti) | Aae-cucoidC | vectorbase.org | AAEL013321 | See Materials and methods - Rapid Amplification of cDNA Ends (RACE) |
| Gene (Aedes aegypti) | Aae-nos | vectorbase.org | AAEL012107 | See Materials and methods - RNA in situ hybridization |
| Gene (Anopheles gambiae) | Aga-panMat | This paper | MN122126 | See Materials and methods - Cloning procedures and mRNA/dsRNA synthesis; RNA in situ hybridization; Rapid Amplification of cDNA Ends (RACE) |
| Gene (Anopheles gambiae) | Aga-panZyg | This paper | MN122127 | See Materials and methods - Cloning procedures and mRNA/dsRNA synthesis; RNA in situ hybridization; Rapid Amplification of cDNA Ends (RACE) |
| Gene (Anopheles gambiae) | Aga-cad | This paper | MN122128 | See Materials and methods - RNA in situ hybridization |
| Gene (Nephrotoma suturalis) | Nsu-pan | This paper | MN122129 | See Materials and methods - RNA in situ hybridization |
| Sequence-based reagent | dsRNAs | This paper | see Materials and methods - Cloning procedures and mRNA/dsRNA | |
| Sequence-based reagent | RNA probes | This paper | See Materials and methods - RNA in situ hybridization | |
| Commercial assay or kit | SMARTer RACE 5'/3' Kit | Takara | 634858 | |
| Commercial assay or kit | mMESSAGE mMACHINE SP6 | Thermo Fisher | AM1340 | |
| Commercial assay or kit | QuikChange Lightning Site-Directed Mutagenesis Kit | Agilient | 210515 | |
| Software, algorithm | Geneious | https://www.geneious.com | RRID:SCR_010519 | version 11.1.5 |
| Software, algorithm | GraphPad Prism | https://graphpad.com | RRID:SCR_015807 | version 1.4 |
Cloning procedures and mRNA/dsRNA synthesis
Coding sequences from Clogmia, Lutzomyia, Chironomus, Anopheles, Nephrotoma, Culex, and Aedes were amplified from embryonic cDNA with primers constructed from RNA-seq data. Coding sequence of odd-paired was amplified from cDNA (FI01113) that was obtained from the BDGP Gold collection of the Drosophila Genomics Resource Center. Amplified cDNA was cloned into the expression vector pSP35T (Amaya et al., 1991), using In-Fusion HD Cloning Kit (Clontech), and PstI- or EcoRI-linearized vector was used for mRNA synthesis using mMESSAGE mMACHINE SP6. panish, Cri-pan, and Cri-pantrunc mRNAs were synthesized from PCR template (containing T7 polymerase binding site) using mMESSAGE mMACHINE T7ultra. Mutations in the open reading frame (Cal-opaZyg-Met21Leu, panish FS, Cri-pantrunc) and in the kozak sequences of Dme-opa and Cri-opa were generated using QuikChange Lightning Site-Directed Mutagenesis Kit (Agilent). Double-stranded RNA (dsRNA) was generated from PCR-amplified templates using embryonic cDNA and primers containing T7 polymerase binding sites as described (Klomp et al., 2015).
Forward and reverse primer sequences for generating templates for mRNA synthesis were:
Cal-opaMat
5’-TAAGATGAGTCCGAATCACTTACTGGCC
5’-TTAATAGGCCGTCGCTGCACC
Cal-opazyg
5’-CAACATGATGATGAACGCTTTTATGGAA
5’-TTAATAGGCCGTCGCTGCACC
Cal-opa115-655
5’-ATGCTCTTCTCAAATCACTCTTCAGC
5’-TTAATAGGCCGTCGCTGCACC
Cal-opa182-655
5’-ATGAACCCGGGAACCTTGGG
5’-TTAATAGGCCGTCGCTGCACC
Cal-opa182-445
5’-ATGAACCCGGGAACCTTGGG
5’-TTACGCGGGATTCAGCTGACTATG
Cal-opa1-337+22
5’-CAACATGATGATGAACGCTTTTATGGAA
5’-TCACAAAATTTCACTGAATTCCGTCAAAATATCACTAGA
Llo-opa
5’-AAAGATGATGATGAATGCATTTATGGACACAG
5’-TCAGTACGCCGTGGCGGCG
Dme-opaDme kozak
5’-GACCATGATGATGAACGCCTTCA
5’-GTCAATACGCCGTCGCTGCGCCGGG
Cri-opaCri kozak
5’-AAAAATGATGATGAATGGTTTTATGGACACA
5’-TCAATAAGCTGTCGTTGGACCGTGAT
Cri-pantrunc
5’- AAAAATGTATCCAGATTGGAGCTCGC
5’- TTACGTCACACTAATAGCATTTCCATCATCCC
Forward and reverse primer sequences for dsRNA (lengths of dsRNAs in brackets; gene specific sequence of primers underlined):
Cal-opaMat (222 bp):
5’-CAGAGATGCATAATACGACTCACTATAGGGAGAAAACAATTGTGAAGTGCGACA
5’-CAGAGATGCATAATACGACTCACTATAGGGAGACAAATTTCCAAACGATGACAGA
Cal-opazyg (315 bp):
5’- CAGAGATGCATAATACGACTCACTATAGGGAGAACTACCGCCGCGAACACACG
5’-CAGAGATGCATAATACGACTCACTATAGGGAGAGTCCAGTCGATTCCATAAAAGC
Cal-slp (927 bp):
5’- CAGAGATGCATAATACGACTCACTATAGGGAGATCGATCAGCTCCCTTTTGCC
5’-CAGAGATGCATAATACGACTCACTATAGGGAGATGAGATCGTTCCCGTTGGAC
Cal-mira (993 bp):
5’- CAGAGATGCATAATACGACTCACTATAGGGAGAACAGCAAAAAGGAAGCGAAA
5’-CAGAGATGCATAATACGACTCACTATAGGGAGAGGGATTCAATTTGCCTTTGA
Aga-panMat (976 bp):
5’- CAGAGATGCATAATACGACTCACTATAGGGAGACACACAGGGCACAATAATCG
5’- CAGAGATGCATAATACGACTCACTATAGGGAGAGACTGCATGTCCGTCGTCTA
Aga-panZyg (843 bp):
5’- CAGAGATGCATAATACGACTCACTATAGGGAGAACATCACACACCCCACACAC
5’- CAGAGATGCATAATACGACTCACTATAGGGAGATTGGTCCGTTCGTGATTGTA
cucoid (926 bp):
5’ - CAGAGATGCATAATACGACTCACTATAGGGAGACGAGGATGTTGCTGGAGAAT
5’- CAGAGATGCATAATACGACTCACTATAGGGAGAACTCCCGAAATCGGAAAACT
Aae-cucoid (857 bp):
5’-CAGAGATGCATAATACGACTCACTATAGGGAGACGACAAGCCCTACAAATGCT
5’-CAGAGATGCATAATACGACTCACTATAGGGAGATGATCTGGATGTTGCCGTAG
Microinjection of embryos
Chironomus embryo injection was done as previously described (Klomp et al., 2015). Clogmia eggs were dissected from ovaries and activated under water. Eggs of Aedes, Culex, and Anopheles were collected in a dark chamber on a moist filter paper for about 30 min (Fisher Scientific, Cat. No 09–795C). These eggs were transferred to another filter paper cut into 4 cm x 2 cm pieces and aligned perpendicularly to the edge of a cover glass (Fisher Scientific, Cat. No 12-648-5C) with the prospective injection side pointing towards the glass edge. We noticed that injecting eggs near the anterior or posterior pole was critical for survival of the procedure. During the alignment procedure, water was applied to the filter paper as needed to prevent eggs from desiccation. After aligning the eggs, the cover glass was removed and excess water on the filter paper was absorbed using filter paper. A second cover glass with a layer of double-sided tape (Scotch 3M) was slightly pressed against the aligned eggs to transfer the eggs to the double-sided tape. The embryos were then immediately covered with halocarbon oil to prevent desiccation. For cuticle preparations, the embryos were injected under halocarbon oil 27 (Sigma, MKBZ7202V). The oil was washed off under a gentle stream of water immediately after injection. In the case of Clogmia, Aedes, and Culex the cover glass was transferred to a moist chamber (petri dish with wet kimwipe paper) and kept at 28 °C, and water was added every day to prevent desiccation. In the case of Anopheles, the eggs were allowed to develop under water. Removal of the halocarbon oil was critical to ensure embryo survival until late developmental stages and hatching. For eggs to be fixed within a day following injection, we used a 1:1 mixture of halocarbon oil 27 and halocarbon oil 700 (Sigma, MKCB5817) and left the injected eggs immersed in the oil until fixation. Embryos were injected with quartz needles using a Narishige IM-300 microinjector. Quartz capillaries (Sutter Instruments Q100-70-10) were pulled with a Sutter instrument P-2000 laser-based micropipette puller. Our settings for the needle puller were: Heat 645, Fil 4, Vel 40, Del 125, Pul 130. Needles were back-filled and the tip was broken open at the time of injection by slightly touching the first egg.
Embryo fixation
Clogmia Embryos were dechorionated using a 10% dilution of commercial bleach (8.25% sodium hypochloride) for 3 min. For Nephrotoma embryos, a 25% dilution was used for 3 min until the chorion became slightly transparent. Embryos of Aedes, Culex, and Anopheles were dechorionated as described (Juhn and James, 2012). Dechorionated embryos were fixed in a 50 mL falcon tube, using 20 mL of boiling salt/detergent-solution (100 μL 10% triton-X, 500 μL 28% NaCl, up to 20 mL of water). After 10 s, water was applied to the tube to cool down the embryos. If needed, the embryos were devitellinized in a 1:1 mixture of n-heptane and methanol by gentle shaking. Embryos with vitelline membrane attached were further devitellinized using sharp tungsten needles in an agar plate covered with methanol. Devitellized embryos were stored in 100% methanol at −20°C.
RNA in situ hybridization
RNA in situ hybridizations were conducted as described (Klomp et al., 2015), using digoxigenin (DIG)-labeled probes and Fab fragments from anti-DIG antibodies conjugated with alkaline phosphatase (AP) (Roche, IN, USA). Probes were prepared from PCR templates, using sequence-specific forward primers and reverse primers with T7 promoter sequence (see above for Cal-opaMat, Cal-opaZyg, Cal-cad, Cal-slp, Cal-mira, Aga-panMat, Aga-panZyg, and Aae-cucoid; gene specific sequence underlined).
Cal-nos1 (450 bp):
5’-AGCACTTTTCCCCCAAGAGT
5’-CAGAGATGCATAATACGACTCACTATAGGGAGAGGCATTCATATTTCCTCAGCA
Cal-nos2 (475 bp):
5’-AATTATTCTGTTCCAAAGTTGAGATT
5’-CAGAGATGCATAATACGACTCACTATAGGGAGACCCCAGACTGGTGACAAAT
Cal-nos3 (548 bp):
5’-TGAGTTAAATAGAGTGAAAACAGCAAA
5’-CAGAGATGCATAATACGACTCACTATAGGGAGATACCGTCTCGTGCTTAATCG
Cal-nos4 (440 bp):
5’-GGCAAAATTTTCCAAGTGAA
5’-CAGAGATGCATAATACGACTCACTATAGGGAGACGTGTCCTCAAGCGTGTAGAT
Cal-vas (938 bp):
5’-CTGAGGCGAACTTGTGTGAA
5’-CAGAGATGCATAATACGACTCACTATAGGGAGAATTGGCAATGTCCAGTCCTC
Cal-tud (921 bp):
5’-ATTCTGCAAGTCGTCGAGGT
5’-CAGAGATGCATAATACGACTCACTATAGGGAGACCTGTACCAGCCATTGTCCT
Cal-gcl (450 bp):
5’-GCAGAACCCCTTGGACATTA
5’-CAGAGATGCATAATACGACTCACTATAGGGAGAGTAACGCCCACAATTCGTCT
cucoidA (939 bp):
5’-ACGATGAGGAGGAGGGTTCT
5’- CAGAGATGCATAATACGACTCACTATAGGGAGACGCACTTCACCGTGTGTAAC
cucoidB (717 bp):
5’-GGGGCGACATCTATATCTCACT
5’-CAGAGATGCATAATACGACTCACTATAGGGAGAACAGTGAGAAAAATTCCCAACTTTAGT
cucoidC (926 bp):
5’-CGAGGATGTTGCTGGAGAAT
5’-CAGAGATGCATAATACGACTCACTATAGGGAGAACTCCCGAAATCGGAAAACT
Cqu-cad (956 bp):
5’-CACGTGTTCCATCAGTCCAG
5’-CAGAGATGCATAATACGACTCACTATAGGGAGAATGAGGCTTAACGAGGATGG
Cqu-nos (927 bp):
5’-AAGTGCCGTGAATTTTGTCC
5’-CAGAGATGCATAATACGACTCACTATAGGGAGAGCGAAACCAATTCGACAGTT
Nsu-pan (966 bp):
5’-TCGCGGCAAGATCATAGTCC
5’-CAGAGATGCATAATACGACTCACTATAGGGAGACCTGCAGGGTTTACACCACT
Aae-nos (914 bp):
5’- CAAACGTGAAGCGGAAGATT
5’- CAGAGATGCATAATACGACTCACTATAGGGAGAATTACGTCCGGAAGTGTTCG
Rapid Amplification of cDNA Ends (RACE)
Total RNA was phenol/chloroform extracted from Clogmia (1 hr-old and 9 hr-old embryos), Anopheles (1–6 hr-old embryos), Culex (0–7 hr old embryos), and Nephrotoma (1–29 hr-old embryos) fixed in TRIzol Reagent (Invitrogen) and precipitated with isopropanol. 5’/3’ RACE was performed using SMARTer RACE 5'/3' Kit (Clontech) with the custom-made primers (including at the 5’ end 15 nucleotides of pRACE vector sequence). Gene specific sequences are underlined.
Cal-opa 5’RACE primer: 5’-GATTACGCCAAGCTTCTGGGTGACGCCGTGGGCAAGGACGTCA
Cal-opa 3’RACE primer: 5’-GATTACGCCAAGCTTCGCGTCGATCGTCACGCCCCCAAATTCG
Aga-pan 5’RACE primer: 5’-GATTACGCCAAGCTTCGAATCTCCGGCCGCGGAATTGAGACTT
Aga-pan 3’RACE primer: 5’-GATTACGCCAAGCTTAGCTTCACGCGACCAGCAAAACCAACGG
cucoid 5’RACE primer: 5’- GATTACGCCAAGCTTCGTGACGGCTTCGATGGTTGGTTTTTCC
cucoid 3’RACE primer: 5’- GATTACGCCAAGCTTCGCACGTGTTGAACAGTCACATGTTGAC
Aae-cucoid 5’RACE primer: 5’- GATTACGCCAAGCTTGATCCGGTGGATCGGACTTGGCCGAGAT
Aae-cucoid 3’RACE primer: 5’- GATTACGCCAAGCTTAACCTCCCTCGGGGTTGAACGTGAAGCT
Aae-cucoid 5’RACE primer: 5’- GATTACGCCAAGCTTGATCCGGTGGATCGGACTTGGCCGAGAT
Aae-cucoid 3’RACE primer: 5’- GATTACGCCAAGCTTAACCTCCCTCGGGGTTGAACGTGAAGCT
Nsu-pan 5’RACE primer: 5’-GATTACGCCAAGCTTTCTGGTCGTGCGACGTTCTTCCAAATCG
Nsu-pan 3’RACE primer: 5’-GATTACGCCAAGCTTTCCCGTTGGTGCAAATCCACGAGATGTG
Cuticle preparations
Cuticles were prepped four to five days after injection. Eggshells was removed with tungsten needles and the embryos were transferred to a glass block dish with a drop of 1:4 glycerol/acetic acid. Following incubation in 1:4 glycerol/acetic acid overnight at room temperature, the cuticles were transferred onto a glass slide, oriented, mounted in 1:1 Hoyer’s medium/lactic acid (Stern and Sucena, 2000), covered with a cover glass, and dried overnight at 65 °C.
RNA-seq sample preparation and sequencing
Bisection of anterior and posterior embryo halves, RNA extraction, and sequencing were conducted as described (Klomp et al., 2015). In the case of Clogmia, anterior or posterior embryo halves from three 1 hr-old embryos were pooled and RNA-seq data were obtained from two replicates. In case of Lutzomyia, embryo halves from ten 1–2 hr-old embryos were pooled four replicates were generated. In case of Anopheles (G-3 strain), embryo halves from five 1 hr-old embryos were pooled and three replicates were generated, In the case of Culex, embryo halves from seven 1 hr-old embryos were pooled and three replicates were generated. In case of Aedes (Liverpool ‘black eye’ strain), embryo halves from five 1 hr-old embryos were pooled and four replicates were generated. In case of Nephrotoma, embryo halves from nine 1 hr-old embryos were pooled and three replicates were generated. Stage-specific Clogmia transcriptomes were generated from the offspring of a single mother and total RNA from five embryos was used for each stage. In the case of Lutzomyia, about 100 staged embryos were pooled for RNA extraction, and two independent RNA extractions from each time point were combined and submitted for sequencing.
Prior to library construction, RNA integrity, purity, and concentration were assessed using an Agilent 2100 Bioanalyzer with an RNA 6000 Nano Chip (Agilent Technologies, USA). Purification of messenger RNA (mRNA) was performed using the oligo-dT beads provided in the Illumina TruSEQ mRNA RNA-SEQ kit (Illumina, USA). Complementary DNA (cDNA) libraries for Illumina sequencing were constructed using the Illumina TruSEQ mRNA RNA-SEQ kit (Illumina, USA), using the manufacturer-specified protocol. Briefly, the mRNA was chemically fragmented and primed with random oligos for first strand cDNA synthesis. Second strand cDNA synthesis was then carried out with dUTPs to preserve strand orientation information. The double-stranded cDNA was then purified, end repaired, and ‘a-tailed’ for adaptor ligation. Following ligation, the samples were selected a final library size (adapters included) of 400–550 bp using sequential AMPure XP bead isolation (Beckman Coulter, USA). The libraries were sequenced in an Illumina HiSeq 4000 DNA sequencer, utilizing a pair end sequencing flow cell with a HiSeq Reagent Kit v4 (Illumina, USA).
RNA-seq data preprocessing
The TrimGalore (Krueger, 2012) wrapper for Cutadapt (Martin, 2011) and FastQC (Andrews, 2010) was used to remove adapters and low quality sequences from raw fastq files. Overlapping reads were combined with Flash (Magoč and Salzberg, 2011) prior to assembly.
Transcriptome assembly and annotation
Trinity 2.4.0 (Grabherr et al., 2011) on the Indiana University Karst high-performance computing cluster was used for assembling contiguous sequences (contigs) from the paired end (PE) sequence data of Clogmia, Lutzomyia, Anopheles, and Nephrotoma. ABySS 2.0 (Jackman et al., 2017) was used for assembling contigs from Culex and Aedes data. Only contigs of 200 nucleotides or greater were retained. BLAST+ tools (Camacho et al., 2009) were used to annotate contigs by conducting best-reciprocal-blast first against the Drosophila melanogaster transcriptome (BDGP6) peptide sequences (blastx/tblastn) and then the coding sequence (tblastx) with a maximum threshold evalue of 1e-10. Biomart and AnnotationDbi packages were used for gene ids and names. The longest open reading frames (ORFs) of unannotated transcripts were compared to the RefSeq invertebrate protein database (downloaded 4-1-2017) using blastp (max evalue 1e-10) followed by a similar comparison to remove transcripts with ORFs matching RefSeq plant, protozoan, archaea, bacteria, fungi, plasmid, or viral sequences (downloaded 6-1-2017). Remaining transcripts were designated by the top BLAST hit in D. melanogaster.
Alignment and differential expression analysis
Cleaned paired-end read data was aligned and analyzed using R base (Ihaka and Gentleman, 1996) and Bioconductor (Gentleman et al., 2004) software packages. Sequence alignment was conducted with the seed-and-vote aligner, Subread, as implemented in the Rsubread package (Liao et al., 2013) with up to five multi-mapping locations, six mismatches, and 20 subreads/seeds per read. Sequence file manipulation, including sorting and indexing of ‘.bam’ files, was done using Rsamtools (Morgan et al., 2013).
To avoid potential biases in transcript localization unrelated to anterior-posterior axis formation, transcripts annotated with mitochondrial, ribosomal, or ambiguous status (e.g., predicted, hypothetical, or uncharacterized) were filtered out prior to the differential expression comparisons. Transcripts with 20 or fewer counts in any of the A-P pairs were also excluded from the analysis prior to library normalization. Lower scoring, potentially related transcripts matching a given gene from the D. melanogaster transcriptome were retained for initial differential expression comparisons but removed for clarity of presentation in subsequent analyses and volcano plots. Trimmed mean of M-values (TMM) (Robinson and Oshlack, 2010) was used for normalization and EdgeR (Robinson et al., 2010) was used to perform quasi-likelihood F-tests between A-P samples, corrected for multiple testing using FDR (Benjamini-Hochberg). Following filtering based on annotation and detection of >20 counts per paired samples, we used the following number of transcripts for differential expression comparisons: 5602 for Clogmia; 5392 for Lutzomyia; 8239 for Culex; 5802 for Aedes; 9353 for Anopheles; 5371 for Nephrotoma.
Mapping RNA-seq reads to genomic loci
RNA-seq reads from stage-specific transcriptomes were mapped to genomic scaffolds containing a gene of interest using TopHat RNA-seq aligner (Trapnell et al., 2009). Publicly available Anopheles stephensi transcriptomes used in this paper were: SRR515316, SRR515341, SRR514863, and SRR515304.
Data availability
This project was deposited at the National Center for Biotechnology Information under Bioproject ID PRJNA454000 and the reads were deposited in the Short Reads Archives under accessions SRR7132661, SRR7132662, SRR7132659, SRR7132660, SRR7132665, SRR7132666, SRR7132663 and SRR7132664 for Clogmia, SRR7134470, SRR7134469, SRR7134472, SRR7134471, SRR7134468, and SRR7134467 for Lutzomiya, SRR8729860, SRR8729859, SRR8729858, SRR8729857, SRR8729856 and SRR8729855 for Anopheles, SRR8729854, SRR8729853, SRR8729852 and SRR8729851 for Aedes, SRR8729868, SRR8729867, SRR8729870, SRR8729869, SRR8729864 and SRR8729863 for Culex and SRR8729866, SRR8729865, SRR8729872, SRR8729871, SRR8729861 and SRR8729862 for Nephrotoma. Transcript sequences are listed on the Key Resources Table.
Acknowledgements
We thank Chun Wai Kwan, Claudia Vacca, and Nicole Horio for technical assistance, Arthur Forer (York University, Canada) for the Nephrotoma culture, Robert Harrell and Channa Aluvihare (Insect Transformation Facility, University of Maryland, MD) for shipping blood-fed mosquitoes, Vanessa Macias and Anthony James (University of California at Irvine, CA) for Aedes and Culex reagents, Molly Duman Scheel (Indiana University, IN) for logistic support, and Edwin L Ferguson (University of Chicago, IL) and M Feder (University of Chicago, IL) for laboratory equipment. EL Ferguson provided detailed comments on a manuscript draft. This work was supported by funds from the National Science Foundation (IOS-1355057), the National Institute of General Medical Science (5R01GM127366-02), the University of Chicago, and the National Center for Advancing Translational Sciences of the National Institutes of Health (UL1 TR000430) to U S-O, and the Intramural Program of the National Institute of Allergy and Infectious Diseases to J R YY was the recipient of an award of University of Chicago Henry Hinds Funds for Graduate Student Research in Evolutionary Biology. Transcriptomic data are available at the National Center for Biotechnology Information Sequence Read Archive (Bioproject ID PRJNA454000). This work utilized the computational resources of the NIH HPC Biowulf cluster.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Contributor Information
Urs Schmidt-Ott, Email: uschmid@uchicago.edu.
Patricia J Wittkopp, University of Michigan, United States.
Patricia J Wittkopp, University of Michigan, United States.
Funding Information
This paper was supported by the following grants:
National Science Foundation IOS-1355057 to Urs Schmidt-Ott.
National Institute of General Medical Sciences R01 GM127366-01A1 to Urs Schmidt-Ott.
National Center for Advancing Translational Sciences UL1 TR000430 to Urs Schmidt-Ott.
University of Chicago Institutional fund to Urs Schmidt-Ott.
National Institute of Allergy and Infectious Diseases Intramural Program to Jose Ribeiro.
University of Chicago Henry Hinds Funds for Graduate Student Research in Evolutionary Biology to Yoseop Yoon.
Additional information
Competing interests
No competing interests declared.
Author contributions
Conceptualization, Formal analysis, Funding acquisition, Investigation, Visualization, Writing—original draft, Writing—review and editing.
Conceptualization, Formal analysis, Investigation, Visualization, Writing—original draft, Writing—review and editing.
Formal analysis, Investigation, Visualization.
Investigation.
Supervision.
Resources, Formal analysis, Supervision, Funding acquisition.
Conceptualization, Supervision, Funding acquisition, Writing—original draft, Project administration, Writing—review and editing.
Additional files
Gene names are those indicated as best-reciprocal-blast hits (see Methods). Contig names correspond to transcriptome assembly. Read counts are given based on non-unique mapping of pre-processed RNA-seq data to annotated transcriptome.
Gene names are those indicated as best-reciprocal-blast hits (see Methods). Contig names correspond to transcriptome assembly. Read counts are given based on non-unique mapping of pre-processed RNA-seq data to annotated transcriptome.
Gene names are those indicated as best-reciprocal-blast hits (see Methods). Contig names correspond to transcriptome assembly. Read counts are given based on non-unique mapping of pre-processed RNA-seq data to annotated transcriptome.
Gene names are those indicated as best-reciprocal-blast hits (see Methods). Contig names correspond to transcriptome assembly. Read counts are given based on non-unique mapping of pre-processed RNA-seq data to annotated transcriptome.
Gene names are those indicated as best-reciprocal-blast hits (see Methods). Contig names correspond to transcriptome assembly. Read counts are given based on non-unique mapping of pre-processed RNA-seq data to annotated transcriptome.
Gene names are those indicated as best-reciprocal-blast hits (see Methods). Contig names correspond to transcriptome assembly. Read counts are given based on non-unique mapping of pre-processed RNA-seq data to annotated transcriptome.
Data availability
Sequencing data have been deposited at the National Center for Biotechnology Information Sequence Read Archive (Bioproject ID PRJNA454000).
The following dataset was generated:
Yoon Y, Klomp J, Martin-Martin I, Criscione F, Calvo E, Ribeiro J, Schmidt-Ott U. 2018. Evolution of an Embryonic Axis Determinant via Alternative Transcription. NCBI Bioproject. PRJNA454000
The following previously published dataset was used:
Xiaofang Jiang. 2012. Anopheles stephensi strain:Indian Wild Type (Walter Reid) Transcriptome or Gene expression. NCBI Bioproject. PRJNA168517
References
- Adams MD, Celniker SE, Holt RA, Evans CA, Gocayne JD, Amanatides PG, Scherer SE, Li PW, Hoskins RA, Galle RF, George RA, Lewis SE, Richards S, Ashburner M, Henderson SN, Sutton GG, Wortman JR, Yandell MD, Zhang Q, Chen LX, Brandon RC, Rogers YH, Blazej RG, Champe M, Pfeiffer BD, Wan KH, Doyle C, Baxter EG, Helt G, Nelson CR, Gabor GL, Abril JF, Agbayani A, An HJ, Andrews-Pfannkoch C, Baldwin D, Ballew RM, Basu A, Baxendale J, Bayraktaroglu L, Beasley EM, Beeson KY, Benos PV, Berman BP, Bhandari D, Bolshakov S, Borkova D, Botchan MR, Bouck J, Brokstein P, Brottier P, Burtis KC, Busam DA, Butler H, Cadieu E, Center A, Chandra I, Cherry JM, Cawley S, Dahlke C, Davenport LB, Davies P, de Pablos B, Delcher A, Deng Z, Mays AD, Dew I, Dietz SM, Dodson K, Doup LE, Downes M, Dugan-Rocha S, Dunkov BC, Dunn P, Durbin KJ, Evangelista CC, Ferraz C, Ferriera S, Fleischmann W, Fosler C, Gabrielian AE, Garg NS, Gelbart WM, Glasser K, Glodek A, Gong F, Gorrell JH, Gu Z, Guan P, Harris M, Harris NL, Harvey D, Heiman TJ, Hernandez JR, Houck J, Hostin D, Houston KA, Howland TJ, Wei MH, Ibegwam C, Jalali M, Kalush F, Karpen GH, Ke Z, Kennison JA, Ketchum KA, Kimmel BE, Kodira CD, Kraft C, Kravitz S, Kulp D, Lai Z, Lasko P, Lei Y, Levitsky AA, Li J, Li Z, Liang Y, Lin X, Liu X, Mattei B, McIntosh TC, McLeod MP, McPherson D, Merkulov G, Milshina NV, Mobarry C, Morris J, Moshrefi A, Mount SM, Moy M, Murphy B, Murphy L, Muzny DM, Nelson DL, Nelson DR, Nelson KA, Nixon K, Nusskern DR, Pacleb JM, Palazzolo M, Pittman GS, Pan S, Pollard J, Puri V, Reese MG, Reinert K, Remington K, Saunders RD, Scheeler F, Shen H, Shue BC, Sidén-Kiamos I, Simpson M, Skupski MP, Smith T, Spier E, Spradling AC, Stapleton M, Strong R, Sun E, Svirskas R, Tector C, Turner R, Venter E, Wang AH, Wang X, Wang ZY, Wassarman DA, Weinstock GM, Weissenbach J, Williams SM, Woodage T, Worley KC, Wu D, Yang S, Yao QA, Ye J, Yeh RF, Zaveri JS, Zhan M, Zhang G, Zhao Q, Zheng L, Zheng XH, Zhong FN, Zhong W, Zhou X, Zhu S, Zhu X, Smith HO, Gibbs RA, Myers EW, Rubin GM, Venter JC. The genome sequence of Drosophila melanogaster. Science. 2000;287:2185–2195. doi: 10.1126/science.287.5461.2185. [DOI] [PubMed] [Google Scholar]
- Akbari OS, Antoshechkin I, Amrhein H, Williams B, Diloreto R, Sandler J, Hay BA. The developmental transcriptome of the mosquito Aedes aegypti, an invasive species and major arbovirus vector. G3: Genes|Genomes|Genetics. 2013;3:1493–1509. doi: 10.1534/g3.113.006742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaya E, Musci TJ, Kirschner MW. Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in xenopus embryos. Cell. 1991;66:257–270. doi: 10.1016/0092-8674(91)90616-7. [DOI] [PubMed] [Google Scholar]
- An JJ, Gharami K, Liao GY, Woo NH, Lau AG, Vanevski F, Torre ER, Jones KR, Feng Y, Lu B, Xu B. Distinct role of long 3' UTR BDNF mRNA in spine morphology and synaptic plasticity in hippocampal neurons. Cell. 2008;134:175–187. doi: 10.1016/j.cell.2008.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews S. FastQC. Babraham Bioinformatics 2010
- Ansari S, Troelenberg N, Dao VA, Richter T, Bucher G, Klingler M. Double abdomen in a short-germ insect: zygotic control of Axis formation revealed in the beetle Tribolium castaneum. PNAS. 2018;115:1819–1824. doi: 10.1073/pnas.1716512115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anvar SY, Allard G, Tseng E, Sheynkman GM, de Klerk E, Vermaat M, Yin RH, Johansson HE, Ariyurek Y, den Dunnen JT, Turner SW, 't Hoen PAC. Full-length mRNA sequencing uncovers a widespread coupling between transcription initiation and mRNA processing. Genome Biology. 2018;19:46. doi: 10.1186/s13059-018-1418-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek D, Davis C, Ewing B, Gordon D, Green P. Characterization and predictive discovery of evolutionarily conserved mammalian alternative promoters. Genome Research. 2007;17:145–155. doi: 10.1101/gr.5872707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkovits BD, Mayr C. Alternative 3' UTRs act as scaffolds to regulate membrane protein localization. Nature. 2015;522:363–367. doi: 10.1038/nature14321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berleth T, Burri M, Thoma G, Bopp D, Richstein S, Frigerio G, Noll M, Nüsslein-Volhard C. The role of localization of bicoid RNA in organizing the anterior pattern of the Drosophila embryo. The EMBO Journal. 1988;7:1749–1756. doi: 10.1002/j.1460-2075.1988.tb03004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharti K, Liu W, Csermely T, Bertuzzi S, Arnheiter H. Alternative promoter use in eye development: the complex role and regulation of the transcription factor MITF. Development. 2008;135:1169–1178. doi: 10.1242/dev.014142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolognesi R, Farzana L, Fischer TD, Brown SJ. Multiple wnt genes are required for segmentation in the short-germ embryo of tribolium castaneum. Current Biology. 2008;18:1624–1629. doi: 10.1016/j.cub.2008.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher G, Farzana L, Brown SJ, Klingler M. Anterior localization of maternal mRNAs in a short germ insect lacking bicoid. Evolution and Development. 2005;7:142–149. doi: 10.1111/j.1525-142X.2005.05016.x. [DOI] [PubMed] [Google Scholar]
- Buljan M, Chalancon G, Dunker AK, Bateman A, Balaji S, Fuxreiter M, Babu MM. Alternative splicing of intrinsically disordered regions and rewiring of protein interactions. Current Opinion in Structural Biology. 2013;23:443–450. doi: 10.1016/j.sbi.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Bullock SL, Ish-Horowicz D. Conserved signals and machinery for RNA transport in Drosophila oogenesis and embryogenesis. Nature. 2001;414:611–616. doi: 10.1038/414611a. [DOI] [PubMed] [Google Scholar]
- Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TL. BLAST+: architecture and applications. BMC Bioinformatics. 2009;10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll SB, Grenier JK, Weatherbee SD. Molecular Genetics and the Evolution of Animal Design. Blackwell: Oxford Press; 2005. From DNA to diversity: . [Google Scholar]
- Ciolli Mattioli C, Rom A, Franke V, Imami K, Arrey G, Terne M, Woehler A, Akalin A, Ulitsky I, Chekulaeva M. Alternative 3' UTRs direct localization of functionally diverse protein isoforms in neuronal compartments. Nucleic Acids Research. 2019;47:2560–2573. doi: 10.1093/nar/gky1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark E, Akam M. Odd-paired controls frequency doubling in Drosophila segmentation by altering the pair-rule gene regulatory network. eLife. 2016;5:e18215. doi: 10.7554/eLife.18215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cranston PS, Hardy NB, Morse GE. A dated molecular phylogeny for the Chironomidae (Diptera) Systematic Entomology. 2012;37:172–188. doi: 10.1111/j.1365-3113.2011.00603.x. [DOI] [Google Scholar]
- Datta RR, Ling J, Kurland J, Ren X, Xu Z, Yucel G, Moore J, Shokri L, Baker I, Bishop T, Struffi P, Levina R, Bulyk ML, Johnston RJ, Small S. A feed-forward relay integrates the regulatory activities of bicoid and orthodenticle via sequential binding to suboptimal sites. Genes & Development. 2018;32:723–736. doi: 10.1101/gad.311985.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davuluri RV, Suzuki Y, Sugano S, Plass C, Huang TH. The functional consequences of alternative promoter use in mammalian genomes. Trends in Genetics. 2008;24:167–177. doi: 10.1016/j.tig.2008.01.008. [DOI] [PubMed] [Google Scholar]
- de Klerk E, 't Hoen PA. Alternative mRNA transcription, processing, and translation: insights from RNA sequencing. Trends in Genetics. 2015;31:128–139. doi: 10.1016/j.tig.2015.01.001. [DOI] [PubMed] [Google Scholar]
- Derti A, Garrett-Engele P, Macisaac KD, Stevens RC, Sriram S, Chen R, Rohl CA, Johnson JM, Babak T. A quantitative atlas of polyadenylation in five mammals. Genome Research. 2012;22:1173–1183. doi: 10.1101/gr.132563.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driever W. The Development of Drosophila Melanogaster. Laboratory Press: Cold Spring Harbor; 1993. 'Maternal control of anterior development in the Drosophila embryo.'. [Google Scholar]
- Ewen-Campen B, Schwager EE, Extavour CG. The molecular machinery of germ line specification. Molecular Reproduction and Development. 2010;77:3–18. doi: 10.1002/mrd.21091. [DOI] [PubMed] [Google Scholar]
- Forrest AR, Kawaji H, Rehli M, Baillie JK, de Hoon MJ, Haberle V, Lassmann T, Kulakovskiy IV, Lizio M, Itoh M, Andersson R, Mungall CJ, Meehan TF, Schmeier S, Bertin N, Jørgensen M, Dimont E, Arner E, Schmidl C, Schaefer U, Medvedeva YA, Plessy C, Vitezic M, Severin J, Semple C, Ishizu Y, Young RS, Francescatto M, Alam I, Albanese D, Altschuler GM, Arakawa T, Archer JA, Arner P, Babina M, Rennie S, Balwierz PJ, Beckhouse AG, Pradhan-Bhatt S, Blake JA, Blumenthal A, Bodega B, Bonetti A, Briggs J, Brombacher F, Burroughs AM, Califano A, Cannistraci CV, Carbajo D, Chen Y, Chierici M, Ciani Y, Clevers HC, Dalla E, Davis CA, Detmar M, Diehl AD, Dohi T, Drabløs F, Edge AS, Edinger M, Ekwall K, Endoh M, Enomoto H, Fagiolini M, Fairbairn L, Fang H, Farach-Carson MC, Faulkner GJ, Favorov AV, Fisher ME, Frith MC, Fujita R, Fukuda S, Furlanello C, Furino M, Furusawa J, Geijtenbeek TB, Gibson AP, Gingeras T, Goldowitz D, Gough J, Guhl S, Guler R, Gustincich S, Ha TJ, Hamaguchi M, Hara M, Harbers M, Harshbarger J, Hasegawa A, Hasegawa Y, Hashimoto T, Herlyn M, Hitchens KJ, Ho Sui SJ, Hofmann OM, Hoof I, Hori F, Huminiecki L, Iida K, Ikawa T, Jankovic BR, Jia H, Joshi A, Jurman G, Kaczkowski B, Kai C, Kaida K, Kaiho A, Kajiyama K, Kanamori-Katayama M, Kasianov AS, Kasukawa T, Katayama S, Kato S, Kawaguchi S, Kawamoto H, Kawamura YI, Kawashima T, Kempfle JS, Kenna TJ, Kere J, Khachigian LM, Kitamura T, Klinken SP, Knox AJ, Kojima M, Kojima S, Kondo N, Koseki H, Koyasu S, Krampitz S, Kubosaki A, Kwon AT, Laros JF, Lee W, Lennartsson A, Li K, Lilje B, Lipovich L, Mackay-Sim A, Manabe R, Mar JC, Marchand B, Mathelier A, Mejhert N, Meynert A, Mizuno Y, de Lima Morais DA, Morikawa H, Morimoto M, Moro K, Motakis E, Motohashi H, Mummery CL, Murata M, Nagao-Sato S, Nakachi Y, Nakahara F, Nakamura T, Nakamura Y, Nakazato K, van Nimwegen E, Ninomiya N, Nishiyori H, Noma S, Noma S, Noazaki T, Ogishima S, Ohkura N, Ohimiya H, Ohno H, Ohshima M, Okada-Hatakeyama M, Okazaki Y, Orlando V, Ovchinnikov DA, Pain A, Passier R, Patrikakis M, Persson H, Piazza S, Prendergast JG, Rackham OJ, Ramilowski JA, Rashid M, Ravasi T, Rizzu P, Roncador M, Roy S, Rye MB, Saijyo E, Sajantila A, Saka A, Sakaguchi S, Sakai M, Sato H, Savvi S, Saxena A, Schneider C, Schultes EA, Schulze-Tanzil GG, Schwegmann A, Sengstag T, Sheng G, Shimoji H, Shimoni Y, Shin JW, Simon C, Sugiyama D, Sugiyama T, Suzuki M, Suzuki N, Swoboda RK, 't Hoen PA, Tagami M, Takahashi N, Takai J, Tanaka H, Tatsukawa H, Tatum Z, Thompson M, Toyodo H, Toyoda T, Valen E, van de Wetering M, van den Berg LM, Verado R, Vijayan D, Vorontsov IE, Wasserman WW, Watanabe S, Wells CA, Winteringham LN, Wolvetang E, Wood EJ, Yamaguchi Y, Yamamoto M, Yoneda M, Yonekura Y, Yoshida S, Zabierowski SE, Zhang PG, Zhao X, Zucchelli S, Summers KM, Suzuki H, Daub CO, Kawai J, Heutink P, Hide W, Freeman TC, Lenhard B, Bajic VB, Taylor MS, Makeev VJ, Sandelin A, Hume DA, Carninci P, Hayashizaki Y, FANTOM Consortium and the RIKEN PMI and CLST (DGT) A promoter-level mammalian expression atlas. Nature. 2014;507:462–470. doi: 10.1038/nature13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Posnien N, Bolognesi R, Fischer TD, Rayl P, Oberhofer G, Kitzmann P, Brown SJ, Bucher G. Asymmetrically expressed axin required for anterior development in tribolium. PNAS. 2012;109:7782–7786. doi: 10.1073/pnas.1116641109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavis ER, Lehmann R. Localization of Nanos RNA controls embryonic polarity. Cell. 1992;71:301–313. doi: 10.1016/0092-8674(92)90358-J. [DOI] [PubMed] [Google Scholar]
- Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, Hornik K, Hothorn T, Huber W, Iacus S, Irizarry R, Leisch F, Li C, Maechler M, Rossini AJ, Sawitzki G, Smith C, Smyth G, Tierney L, Yang JY, Zhang J. Bioconductor: open software development for computational biology and bioinformatics. Genome Biology. 2004;5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, Adiconis X, Fan L, Raychowdhury R, Zeng Q, Chen Z, Mauceli E, Hacohen N, Gnirke A, Rhind N, di Palma F, Birren BW, Nusbaum C, Lindblad-Toh K, Friedman N, Regev A. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nature Biotechnology. 2011;29:644–652. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi D, Engel MS. Evolution of Insects. Cambridge: Cambridge University Press; 2005. [Google Scholar]
- Gusev O, Suetsugu Y, Cornette R, Kawashima T, Logacheva MD, Kondrashov AS, Penin AA, Hatanaka R, Kikuta S, Shimura S, Kanamori H, Katayose Y, Matsumoto T, Shagimardanova E, Alexeev D, Govorun V, Wisecaver J, Mikheyev A, Koyanagi R, Fujie M, Nishiyama T, Shigenobu S, Shibata TF, Golygina V, Hasebe M, Okuda T, Satoh N, Kikawada T. Comparative genome sequencing reveals genomic signature of extreme desiccation tolerance in the anhydrobiotic midge. Nature Communications. 2014;5:4784. doi: 10.1038/ncomms5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt CE, Bullock SL. Subcellular mRNA localization in animal cells and why it matters. Science. 2009;326:1212–1216. doi: 10.1126/science.1176488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtmeyers R, Souopgui J, Tejpar S, Arkell R. The ZIC gene family encodes multi-functional proteins essential for patterning and morphogenesis. Cellular and Molecular Life Sciences. 2013;70:3791–3811. doi: 10.1007/s00018-013-1285-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihaka R, Gentleman R. R: a language for data analysis and graphics. Journal of Computational and Graphical Statistics. 1996;5:299–314. doi: 10.2307/1390807. [DOI] [Google Scholar]
- Ikeshima-Kataoka H, Skeath JB, Nabeshima Y, Doe CQ, Matsuzaki F. Miranda directs Prospero to a daughter cell during Drosophila asymmetric divisions. Nature. 1997;390:625–629. doi: 10.1038/37641. [DOI] [PubMed] [Google Scholar]
- Jackman SD, Vandervalk BP, Mohamadi H, Chu J, Yeo S, Hammond SA, Jahesh G, Khan H, Coombe L, Warren RL, Birol I. ABySS 2.0: resource-efficient assembly of large genomes using a bloom filter. Genome Research. 2017;27:768–777. doi: 10.1101/gr.214346.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhn J, Marinotti O, Calvo E, James AA. Gene structure and expression of Nanos (nos) and Oskar (osk) orthologues of the vector mosquito, culex quinquefasciatus. Insect Molecular Biology. 2008;17:545–552. doi: 10.1111/j.1365-2583.2008.00823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhn J, James AA. Oskar gene expression in the vector mosquitoes, anopheles gambiae and aedes aegypti. Insect Molecular Biology. 2006;15:363–372. doi: 10.1111/j.1365-2583.2006.00655.x. [DOI] [PubMed] [Google Scholar]
- Juhn J, James AA. Hybridization in situ of salivary glands, ovaries, and embryos of vector mosquitoes. Journal of Visualized Experiments. 2012:3709. doi: 10.3791/3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jürgens G, Wieschaus E, Nüsslein-Volhard C, Kluding H. Mutations affecting the pattern of the larval cuticle in Drosophila melanogaster: II. Zygotic loci on the third chromosome. Wilhelm Roux's Archives of Developmental Biology. 1984;193:283–295. doi: 10.1007/BF00848157. [DOI] [PubMed] [Google Scholar]
- Kaiser TS, Poehn B, Szkiba D, Preussner M, Sedlazeck FJ, Zrim A, Neumann T, Nguyen LT, Betancourt AJ, Hummel T, Vogel H, Dorner S, Heyd F, von Haeseler A, Tessmar-Raible K. The genomic basis of circadian and circalunar timing adaptations in a midge. Nature. 2016;540:69–73. doi: 10.1038/nature20151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley JL, Peyton JT, Fiston-Lavier AS, Teets NM, Yee MC, Johnston JS, Bustamante CD, Lee RE, Denlinger DL. Compact genome of the antarctic midge is likely an adaptation to an extreme environment. Nature Communications. 2014;5:4611. doi: 10.1038/ncomms5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Oh M, Jung W, Park J, Choi H-G, Shin SC. Genome sequencing of the winged midge, Parochlus steinenii, from the antarctic peninsula. GigaScience. 2017;6 doi: 10.1093/gigascience/giw009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klomp J, Athy D, Kwan CW, Bloch NI, Sandmann T, Lemke S, Schmidt-Ott U. Embryo development. A cysteine-clamp gene drives embryo polarity in the midge chironomus. Science. 2015;348:1040–1042. doi: 10.1126/science.aaa7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger F. Babraham Bioinformatics; 2012. [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. Clustal W and clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Lau AG, Irier HA, Gu J, Tian D, Ku L, Liu G, Xia M, Fritsch B, Zheng JQ, Dingledine R, Xu B, Lu B, Feng Y. Distinct 3'UTRs differentially regulate activity-dependent translation of brain-derived neurotrophic factor (BDNF) PNAS. 2010;107:15945–15950. doi: 10.1073/pnas.1002929107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann R. Germ plasm biogenesis--an Oskar-Centric perspective. Current Topics in Developmental Biology. 2016;116:679–707. doi: 10.1016/bs.ctdb.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke S, Schmidt-Ott U. Evidence for a composite anterior determinant in the hover fly Episyrphus balteatus (Syrphidae), a cyclorrhaphan fly with an anterodorsal serosa anlage. Development. 2009;136:117–127. doi: 10.1242/dev.030270. [DOI] [PubMed] [Google Scholar]
- Liao Y, Smyth GK, Shi W. The subread aligner: fast, accurate and scalable read mapping by seed-and-vote. Nucleic Acids Research. 2013;41:e108. doi: 10.1093/nar/gkt214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnen CR, Poh YP, Peterson BK, Barrett RD, Larson JG, Jensen JD, Hoekstra HE. Adaptive evolution of multiple traits through multiple mutations at a single gene. Science. 2013;339:1312–1316. doi: 10.1126/science.1233213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Onal P, Datta RR, Rogers JM, Schmidt-Ott U, Bulyk ML, Small S, Thornton JW. Ancient mechanisms for the evolution of the bicoid homeodomain's function in fly development. eLife. 2018;7:e34594. doi: 10.7554/eLife.34594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JA, Brent AE, Leaf DS, Pultz MA, Desplan C. Localized maternal orthodenticle patterns anterior and posterior in the long germ wasp Nasonia. Nature. 2006;439:728–732. doi: 10.1038/nature04445. [DOI] [PubMed] [Google Scholar]
- Magoč T, Salzberg SL. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27:2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallarino R, Linden TA, Linnen CR, Hoekstra HE. The role of isoforms in the evolution of cryptic coloration in Peromyscus mice. Molecular Ecology. 2017;26:245–258. doi: 10.1111/mec.13663. [DOI] [PubMed] [Google Scholar]
- Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.journal. 2011;17:10–12. doi: 10.14806/ej.17.1.200. [DOI] [Google Scholar]
- Mayr C, Bartel DP. Widespread shortening of 3'UTRs by alternative cleavage and polyadenylation activates oncogenes in cancer cells. Cell. 2009;138:673–684. doi: 10.1016/j.cell.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan M, Pages H, Obenchain V, Hayden N. Rsamtools: Binary alignment (BAM), FASTA, variant call (BCF), and tabix file import. Bioconductor 2013
- Pal S, Gupta R, Davuluri RV. Alternative transcription and alternative splicing in Cancer. Pharmacology & Therapeutics. 2012;136:283–294. doi: 10.1016/j.pharmthera.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Peter IS, Davidson EH. Evolution of gene regulatory networks controlling body plan development. Cell. 2011;144:970–985. doi: 10.1016/j.cell.2011.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto PA, Henriques T, Freitas MO, Martins T, Domingues RG, Wyrzykowska PS, Coelho PA, Carmo AM, Sunkel CE, Proudfoot NJ, Moreira A. RNA polymerase II kinetics in polo polyadenylation signal selection. The EMBO Journal. 2011;30:2431–2444. doi: 10.1038/emboj.2011.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prühs R, Beermann A, Schröder R. The roles of the Wnt-Antagonists axin and Lrp4 during embryogenesis of the red flour beetle tribolium castaneum. Journal of Developmental Biology. 2017;5:10. doi: 10.3390/jdb5040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, Oshlack A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biology. 2010;11:R25. doi: 10.1186/gb-2010-11-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Ott U, Rafiqi AM, Lemke S. Hox3/zen and the evolution of extraembryonic epithelia in insects. Advances in Experimental Medicine and Biology. 2010;689:133–144. doi: 10.1007/978-1-4419-6673-5_10. [DOI] [PubMed] [Google Scholar]
- Schrankel CS, Solek CM, Buckley KM, Anderson MK, Rast JP. A conserved alternative form of the purple sea urchin HEB/E2-2/E2A transcription factor mediates a switch in E-protein regulatory state in differentiating immune cells. Developmental Biology. 2016;416:149–161. doi: 10.1016/j.ydbio.2016.05.034. [DOI] [PubMed] [Google Scholar]
- Schröder R. The genes orthodenticle and hunchback substitute for bicoid in the beetle tribolium. Nature. 2003;422:621–625. doi: 10.1038/nature01536. [DOI] [PubMed] [Google Scholar]
- Shabalina SA, Spiridonov AN, Spiridonov NA, Koonin EV. Connections between alternative transcription and alternative splicing in mammals. Genome Biology and Evolution. 2010;2:791–799. doi: 10.1093/gbe/evq058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabalina SA, Ogurtsov AY, Spiridonov NA, Koonin EV. Evolution at Protein ends: major contribution of alternative transcription initiation and termination to the transcriptome and proteome diversity in mammals. Nucleic Acids Research. 2014;42:7132–7144. doi: 10.1093/nar/gku342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro IM, Cheng AW, Flytzanis NC, Balsamo M, Condeelis JS, Oktay MH, Burge CB, Gertler FB. An EMT-driven alternative splicing program occurs in human breast Cancer and modulates cellular phenotype. PLOS Genetics. 2011;7:e1002218. doi: 10.1371/journal.pgen.1002218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard PJ, Choi EA, Lu J, Flanagan LA, Hertel KJ, Shi Y. Complex and dynamic landscape of RNA polyadenylation revealed by PAS-Seq. RNA. 2011;17:761–772. doi: 10.1261/rna.2581711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smibert P, Miura P, Westholm JO, Shenker S, May G, Duff MO, Zhang D, Eads BD, Carlson J, Brown JB, Eisman RC, Andrews J, Kaufman T, Cherbas P, Celniker SE, Graveley BR, Lai EC. Global patterns of tissue-specific alternative polyadenylation in Drosophila. Cell Reports. 2012;1:277–289. doi: 10.1016/j.celrep.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauber M, Jäckle H, Schmidt-Ott U. The anterior determinant bicoid of Drosophila is a derived hox class 3 gene. PNAS. 1999;96:3786–3789. doi: 10.1073/pnas.96.7.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauber M, Prell A, Schmidt-Ott U. A single Hox3 gene with composite bicoid and zerknullt expression characteristics in non-Cyclorrhaphan flies. PNAS. 2002;99:274–279. doi: 10.1073/pnas.012292899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern DL, Sucena E. Preparation of larval and adult cuticles for light microscopy. In: Sullivan W, Ashburner M, Hawley RS, editors. Drosophila Protocols. Cold Spring Harbor: CSHL Press; 2000. [Google Scholar]
- Struhl G, Johnston P, Lawrence PA. Control of Drosophila body pattern by the hunchback morphogen gradient. Cell. 1992;69:237–249. doi: 10.1016/0092-8674(92)90405-2. [DOI] [PubMed] [Google Scholar]
- Taliaferro JM, Vidaki M, Oliveira R, Olson S, Zhan L, Saxena T, Wang ET, Graveley BR, Gertler FB, Swanson MS, Burge CB. Distal alternative last exons localize mRNAs to neural projections. Molecular Cell. 2016;61:821–833. doi: 10.1016/j.molcel.2016.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tautz D. Regulation of the Drosophila segmentation gene hunchback by two maternal morphogenetic centres. Nature. 1988;332:281–284. doi: 10.1038/332281a0. [DOI] [PubMed] [Google Scholar]
- Thawornwattana Y, Dalquen D, Yang Z. Coalescent analysis of phylogenomic data confidently resolves the species relationships in the anopheles gambiae species complex. Molecular Biology and Evolution. 2018;35:2512–2527. doi: 10.1093/molbev/msy158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25:1105–1111. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tushev G, Glock C, Heumüller M, Biever A, Jovanovic M, Schuman EM. Alternative 3' UTRs modify the localization, regulatory potential, stability, and plasticity of mRNAs in neuronal compartments. Neuron. 2018;98:495–511. doi: 10.1016/j.neuron.2018.03.030. [DOI] [PubMed] [Google Scholar]
- Vacik T, Stubbs JL, Lemke G. A novel mechanism for the transcriptional regulation of wnt signaling in development. Genes & Development. 2011;25:1783–1795. doi: 10.1101/gad.17227011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicoso B, Bachtrog D. Numerous transitions of sex chromosomes in diptera. PLOS Biology. 2015;13:e1002078. doi: 10.1371/journal.pbio.1002078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegmann BM, Trautwein MD, Winkler IS, Barr NB, Kim JW, Lambkin C, Bertone MA, Cassel BK, Bayless KM, Heimberg AM, Wheeler BM, Peterson KJ, Pape T, Sinclair BJ, Skevington JH, Blagoderov V, Caravas J, Kutty SN, Schmidt-Ott U, Kampmeier GE, Thompson FC, Grimaldi DA, Beckenbach AT, Courtney GW, Friedrich M, Meier R, Yeates DK. Episodic radiations in the fly tree of life. PNAS. 2011;108:5690–5695. doi: 10.1073/pnas.1012675108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesner T, Lee W, Obenauf AC, Ran L, Murali R, Zhang QF, Wong EW, Hu W, Scott SN, Shah RH, Landa I, Button J, Lailler N, Sboner A, Gao D, Murphy DA, Cao Z, Shukla S, Hollmann TJ, Wang L, Borsu L, Merghoub T, Schwartz GK, Postow MA, Ariyan CE, Fagin JA, Zheng D, Ladanyi M, Busam KJ, Berger MF, Chen Y, Chi P. Alternative transcription initiation leads to expression of a novel ALK isoform in Cancer. Nature. 2015;526:453–457. doi: 10.1038/nature15258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer EA, Carleton A, Harjes P, Turner T, Desplan C. Bicoid-independent formation of thoracic segments in Drosophila. Science. 2000;287:2476–2479. doi: 10.1126/science.287.5462.2476. [DOI] [PubMed] [Google Scholar]
- Wittkopp PJ, Kalay G. Cis-regulatory elements: molecular mechanisms and evolutionary processes underlying divergence. Nature Reviews Genetics. 2011;13:59–69. doi: 10.1038/nrg3095. [DOI] [PubMed] [Google Scholar]