ABSTRACT
Telomere maintenance is a critical requirement for enabling replicative immortality and tumour development. Here, telomerase expression and activity, telomere length (TL) and potential regulatory factors that can underlie telomerase machinery alterations in small intestinal neuroendocrine tumours (SI-NETs) were analyzed. Telomerase activity assessed by TRAP assay was increased in SI-NETs compared to normal ileum (P < 0.001). The telomerase reverse transcriptase gene (TERT) was over-expressed in SI-NETs vs. normal ileal samples (P = 0.01). Furthermore, relative TL assessed by qPCR was found shorter in tumours compared with normal ileum (P = 0.02) and in distant metastasis samples compared to primary tumours and local metastases (P= 0.02). TERT promoter hotspot mutations were not present and TERT copy number gain was only observed in 3/70 tumour samples. TERT or chromosome 18 copy number alterations were not associated with telomerase expression and activity or TL. However, hypermethylation of TERT promoter in Region B – in the proximity of the transcription start site – was inversely correlated with TERT expression and telomerase activity and positively correlated with TL. Global LINE1 methylation was positively correlated with TERT promoter Region B methylation and was inversely correlated with telomerase activity, TERT expression and the upstream Region A methylation. The results show that telomerase activation, TERT expression and shorter telomeres are commonly found in SI-NETs. Aberrant DNA methylation of TERT promoter and of LINE1 can be implicated in abnormal regulation of TERT in SI-NETs.
KEYWORDS: Telomere, telomerase, TERT, SI-NET, DNA methylation
Introduction
Small intestinal neuroendocrine tumours (SI-NETs) are slow-growing endocrine malignancies that are often metastasized at the initial diagnosis, thus often causing lifelong symptoms [1]. Due to the long-lasting nature of these tumours, enabling replicative immortality could potentially be considered as a major driver in tumour development of SI-NETs.
Telomere erosion and aberrant telomerase activity are considered as important factors in the development of many tumours including endocrine tumours [2]. The Hayflick limit leads to senescence after multiple cell divisions unless the emerging malignant cells bypass telomere shortening through aberrant activation of telomere extension mechanisms [3]. While normally absent in non-immortalized cells, telomerase activity has been detected in around 90% of human cancer cells [4].
Telomerase activation has been linked to many endocrine malignancies [2]. Telomerase activity and TERT over-expression have been associated with TERT promoter mutation and DNA copy number gains in follicular cell-derived thyroid carcinoma and with TERT DNA methylation at an upstream promoter region in medullary thyroid carcinoma [5]. While increased methylation at an upstream region (Region A at positions −578 to −541) was associated with poor outcome [6,7], low methylation of a downstream region (region B at −162 to −100 bp) close to the transcription start site (TSS) was associated with TERT expression in vitro [8] as well as in adrenocortical carcinomas [9]. Furthermore, in pancreatic neuroendocrine tumours the ATRX and DAXX genes are recurrently mutated [10], a phenomenon that is strongly associated with the ‘alternative lengthening of telomeres’ (ALT) pathway [11], however, these genes have not been found recurrently mutated in SI-NETs [12].
Still, our knowledge on telomeres and telomerase activity in SI-NETs is limited. In this study, we detected telomerase activity, TERT expression and telomere length alteration in SI-NETs and investigated the possible underlying mechanisms, including TERT copy number alteration, as well as TERT promoter methylation density and mutational status.
Materials and methods
Tissue samples
Frozen tissue samples of SI-NETs and normal adjacent ileum were obtained from the Karolinska University Hospital Biobank. Samples were collected with informed patient consent and an ethical committee approved the study. The collection of tissue samples had been done according to a standardized procedure whereby samples were snap-frozen in liquid nitrogen and stored at −86°C for up to 33 y until use. A total of 70 tumour samples from 50 patients were studied including 37 primary tumours, 23 regional metastases and 10 distant metastases (Supplementary Table S1). Tumour characteristics of 44 tumours from 32 patients were reported in our previous study [13]. An extended number of 26 samples from 18 patients were also included in this study. Fifteen ileal adjacent tissue samples were used for comparison to a non-tumour tissue.
Assessment of telomerase activity
Telomerase activity was assessed for 49 SI-NETs and 3 normal ileal samples in duplicate using TeloTAGGG Telomerase PCR ELISA kit (Roche Diagnostics GmbH) following the manufacturer’s instructions. HEK-293 lysate provided with the kit served as a positive control and its heat-inactivated counterpart as a negative control. The absorbance was read at OD450-OD670. OD values were subtracted from lysis buffer background absorbance and were normalized to positive control. Values >0.2 were considered as positive for telomerase activity.
DNA and RNA extraction
DNA and RNA were extracted from fresh frozen tissues using Qiagen using DNeasy Blood and tissue and RNeasy Plus Mini kit, following the manufacturer’s instructions (Qiagen).
TERT expression analyses
TERT expression was assessed for 63 SI-NETs and 12 normal ileal samples using TaqMan assay Hs00972656_m1 (Thermo Fisher Scientific) based on the previously described delta-delta Ct method [14]. cDNA synthesis was performed using High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific) and qPCR was performed in an 7900HT Applied Biosystems instrument. Ct values were normalized to ACTB Hs99999903_m1 (Thermo Fisher Scientific).
TERT mutation analyses
A quantity of 50 ng genomic DNA for 70 SI-NETs was amplified using AmpliTaq Gold® 360 Master Mix and PCR primers for Sanger sequencing analysis of the C228T and C250T hotspot mutation region in the TERT promoter, using primers F: 5ʹ-CACCCGTCCTGCCCCTTCACCTT-3ʹ and R: 5ʹ-GGCTTCCCACGTGCGCAGCAGGA-3ʹ.
DNA bisulfite conversion and pyrosequencing
One hundred to 500 ng DNA from 70 SI-NETs was bisulfite converted using EpiTect Bisulfit kit (Qiagen). Twenty ng bisulfite converted DNA was amplified in two different regions of the TERT promoter. The upstream methylation Region A, encompassing positions −578 to −541 was amplified using biotinylated primer-F: 5ʹ- GGGTTTGTGTTAAGGAGTTTAAGT-3ʹ and primer-R: 5ʹ- AAACCCAAAACTACCTCCA-3ʹ. The downstream methylation Region B, encompassing positions −162 to −100 bp was targeted using primer-F: 5ʹ- GGTGGTAGGGGTTAGGGTTTTTTA-3ʹ and biotinylated primer-R: 5ʹ- TACCCCTTCACCTTCCAACTC-3ʹ. The following sequence was analyzed for Region A: 5ʹ-CGGCATTCGTGGTGCCCGGAGCCCGACGCCCCGCGTCCGG-3ʹ (CpG sites in bold). The sequence analyzed for Region B was: 5ʹ-CCCCGTCCCGACCCCTCCCGGGTCCCCGGCCCAGCCCCCTCCGGGCCCTCC CAGCCCCTCCCC-3ʹ (where CpGs are specified in bold, C250T and C228T mutation hotspots indicated in simple and double underline, respectively).
Pyrosequencing was performed with previously described methodology [14] using sequencing primer-S: 5ʹ- CCAAAACTACCTCCAAAT-3ʹ for Region A and primer-S: 5ʹ- GGGGTAGAGGAAAGGAA-3ʹ for Region B. LINE1 methylation was assessed for 70 SI-NETs and 15 normal ileal samples by Pyrosequencing methodology described before [14]. For LINE1 results have been previously published for a subset of cases (Supplementary Table S1 [14];). We identified Methylation Index (MetI) as the mean of methylation density for all representative CpG sites in one examined region.
TERT and CDH19 copy number analysis
DNA copy numbers of TERT were determined in 70 SI-NETs using TaqMan Copy Number Assay (Thermo Fisher Scientific, Hs01237576_cn) and an endogenous control assay, RNaseP (Thermo Fisher Scientific, part number 4403326). The values were calibrated to a commercially available pool of 10 healthy individual blood DNA samples (Promega). The reactions were run in quadruplicate in a Step One Plus qRT-PCR machine following the manufacturer’s instructions (Thermo Fisher Scientific) [15]. We also assessed chromosome 18q copy numbers using CDH19 TaqMan copy number assay (Thermo Fisher Scientific, Hs02826809_cn), and RNase P as a reference gene, as explained in detail before [13]. For a subset of cases results for CDH19 have been previously published [13].
Assessment of telomere length
Relative telomere length was determined using previously described methodology [16,17]. Briefly, 5 ng DNA was amplified for 70 SI-NETs and 13 normal ileal samples in duplicate in a CYBR green qPCR reaction (Thermo Fisher Scientific) using primers against telomeres: (Tel 1b 5ʹ-CGGTTTGTTTGGGTTTGGGTTTGGGTTTGGGTTTGGGTT-3ʹ and Tel 2b 5ʹ-GGCTTGCCTTACCCTTACCCTTACCCTTACCCTTACCCT-3ʹ). The mean Ct values were normalized (dCt) to the endogenous control HGB, F: 5ʹ-TGTGCTGGCCCATCACTTTG-3ʹ and R: 5ʹ-ACCAGCCACCACTTTCTGATAGG-3ʹ. Pooled healthy blood DNA samples (Promega) were run as a reference to calculate ddCt = dCt- mean reference Ct. Telomere length was then calculated as 2^-ddCt.
Statistical analysis
Categorical and continuous variables were analyzed using Fisher Exact test and Mann Whitney U test, respectively. For correlation analysis, Spearman rank order correlation was used. IBM SPSS Statistics 22 was used for all statistical analyses. P-values <0.05 were defined as statistically significant.
Results
Telomerase activation and increased TERT expression in SI-NETs
Telomerase activity was detected in 27/49 of tumour samples (55%, range 0.08–0.88 normalized to the positive control, HEK293 cell lysate), but in none of three normal ileal tissue samples used as non-tumour comparison. There was a significantly higher telomerase activity in tumours compared to non-tumour samples (P< 0.001, Figure 1(a)).
Figure 1.
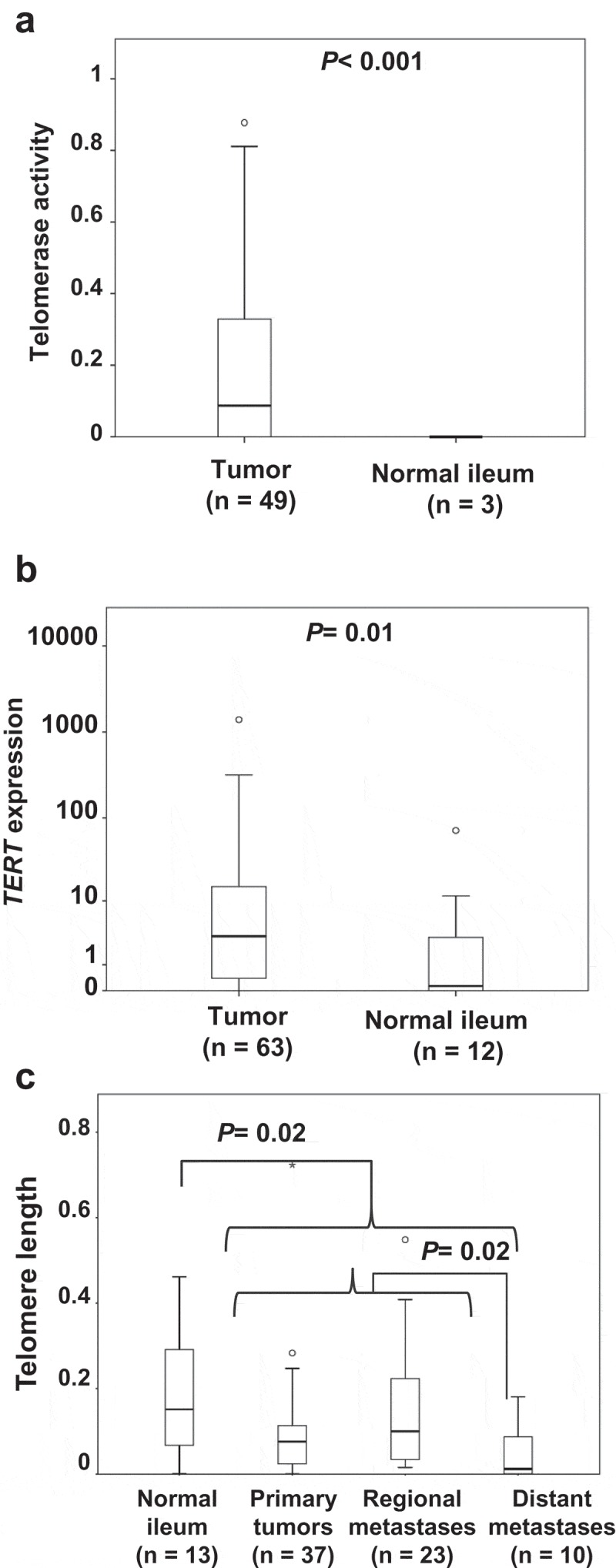
Aberrant telomerase activity, TERT expression and telomere length in SI-NETs. (a) Telomerase activity in normal ileum compared with SI-NETs. (b) TERT expression in normal ileum compared with SI-NETs. (c) Telomere length in normal ileum and different subgroups of SI-NETs.
TERT expression was determined by quantitative PCR and found detectable in 60/63 (95%) SI-NETs and in 8/12 (67%) normal ileum samples. Lower levels were observed in normal ileum (median 0.14) as compared to SI-NETs (median 3.29) (P= 0.01, Figure 1(b) Supplementary Table S1).
Shorter telomere lengths in SI-NETs
Relative telomere length was assessed by a qPCR-based method. Telomeres were found shorter in tumours compared with normal ileal samples (P= 0.02, Figure 1(c)). Furthermore, comparison of tumour subgroups revealed shorter telomeres in distant metastasis as compared to primary tumours and regional metastases (P= 0.02, Figure 1(c)).
Low frequency of TERT promoter mutations and TERT copy number gain in SI-NETs
The TERT promoter region spanning the two reported hotspot mutations known as C228T and C250T was sequenced using Sanger sequencing. No mutation was detected in any of the 70 tumour samples analyzed, suggesting that TERT promoter mutations are rare in the SI-NET entity.
TERT copy number was assessed using TaqMan copy number assay in all 70 SI-NETs and 13 normal ileum samples. Copy number gain was observed in 3 SI-NETs, including two with gain from 2 to 3 copies and one case with amplification to 10 copies (Supplementary Table S1). All normal ileal samples were found diploid. TERT copy number alterations were not found to be associated with telomerase activation, TERT expression or telomere length.
Aberrant TERT promoter methylation in SI-NETs
We quantified DNA methylation at two different regions on TERT promoter, a downstream TERT promoter region (Region B) spanning the C228T and C250T hotspot mutation sites, including 5 CpG sites and an upstream TERT promoter region (Region A) including 8 CpG sites (Figure 2(a)). For Region B the methylation index (MetI) ranged from 2% to 32% in the 68 SI-NET samples and from 4% to 17% in the 11 ileal samples. In Region A, the 70 SI-NETs showed MetI from 9% to 89%, and in the 11 normal ileum samples from 9% to 47%. Some variation in methylation density between CpG sites assessed was noted (Figure 2(a)). For Region B, the MetI was inversely correlated with telomerase activity (P= 0.055, R = −0.279; Table 1 and Figure 2(b)) and inversely correlated with TERT expression (P= 0.001, R = −0.414; Table 1 and Figure 2(c)). Telomere length was correlated with MetI at region B (P = 0.015, R = 0.295, Table 1 and Figure 2(d)).
Figure 2.
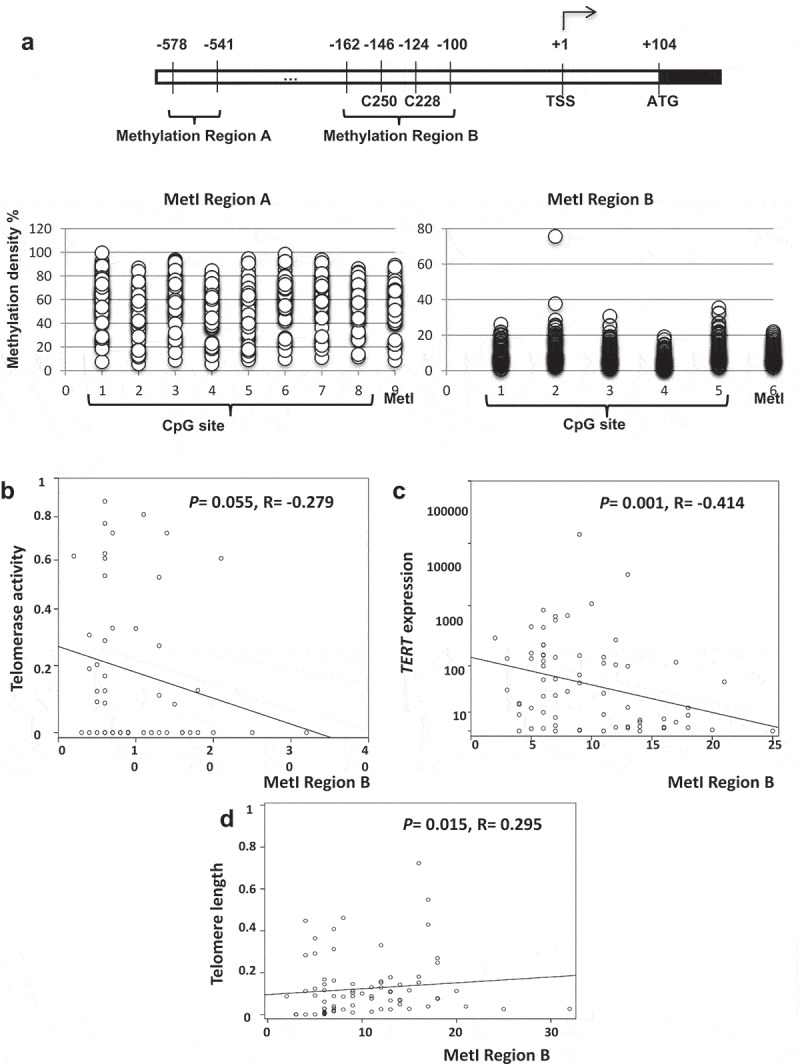
DNA methylation Index (MetI) at TERT promoter methylationRegion B. (a) Schematic illustration of the TERT promoter with indication of methylation Region A and B, the location of hotspot TERT mutations at C250 and C228, and the transcriptional start site (TSS). Below is shown the methylation density determined at 8 CpG sites in Region A and 5 CpG sites in Region B and their mean values (MetI). (b) Inverse correlation between Region B MetI and telomerase activity in SI-NETs. (c) Inverse correlation between Region B MetI and TERT expression in SI-NETs. (d) Correlation between Region B MetI and telomere length in SI-NETs.
Table 1.
Summary of results from correlation analyses in SI-NETs.
| Telomerase |
TERT |
TERT MetI |
TERT MetI |
LINE1 |
Telomere |
||
|---|---|---|---|---|---|---|---|
| Parameter | activity | expression | Region B | Region A | MetI | length | |
| Telomerase activity | Correlation coefficient | 1.000 | .000 | −.279 | .090 | −.411 | −.205 |
| Sig. (2-tailed) | .999 | .055 | .537 | .003 | .157 | ||
| N | 49 | 45 | 48 | 49 | 49 | 49 | |
| TERT expression | Correlation coefficient | .000 | 1.000 | −.414 | .189 | −.372 | −.124 |
| Sig. (2-tailed) | .999 | .001 | .137 | .003 | .334 | ||
| N | 45 | 63 | 61 | 63 | 63 | 63 | |
| TERT MetI Region B | Correlation coefficient | −.279 | −.414 | 1.000 | .108 | .566 | .295 |
| Sig. (2-tailed) | .055 | .001 | .380 | .000 | .015 | ||
| N | 48 | 61 | 68 | 68 | 68 | 68 | |
| TERT MetI Region A | Correlation coefficient | .090 | .189 | .108 | 1.000 | −.357 | −.116 |
| Sig. (2-tailed) | .537 | .137 | .380 | .002 | .338 | ||
| N | 49 | 63 | 68 | 70 | 70 | 70 | |
| LINE1 MetI | Correlation coefficient | −.411 | −.372 | .566 | −.357 | 1.000 | .199 |
| Sig. (2-tailed) | .003 | .003 | .000 | .002 | .098 | ||
| N | 49 | 63 | 68 | 70 | 70 | 70 | |
| Telomere length | Correlation coefficient | −.205 | −.124 | .295 | −.116 | .199 | 1.000 |
| Sig. (2-tailed) | .157 | .334 | .015 | .338 | .098 | ||
| N | 49 | 63 | 68 | 70 | 70 | 70 |
LINE1 methylation
We also analyzed LINE1 methylation as an indicator of global methylation density and possible regulator of TERT. LINE1 was found hypomethylated in SI-NETs (range 44–76%) compared to normal ileum (range 71–78%) (P< 0.001) and in distant metastases compared to primary tumours and regional metastases (P= 0.04, Supplementary Figure S1). LINE1 MetI was inversely correlated with telomerase activity (P = 0.003, R = −0.411, Figure 3(a)) and with TERT expression (P= 0.003, R = −0.372, Figure 3(b)). LINE1 MetI was correlated with MetI at Region B (P< 0.001, R = 0.566, Figure 3(c)), and inversely correlated with MetI at Region A (P= 0.002, R = −0.357, Figure 3(d)).
Figure 3.
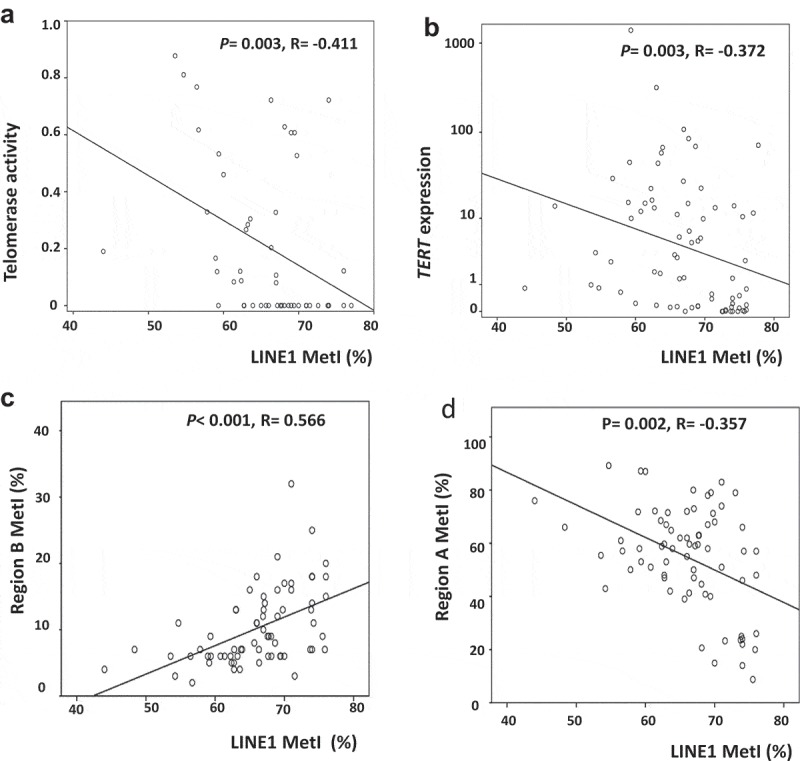
Aberrant LINE1 methylation in SI-NETs. (a) Inverse correlation between LINE1 MetI and telomerase activity. (b) Inverse correlation between LINE1 MetI and TERT expression. (c) Correlation between LINE1 MetI and Region B MetI. D. Inverse correlation between LINE1 MetI and Region A MetI.
Copy number loss at chromosome 18q
We also examined a link between chromosome 18q copy number loss, a hallmark of SI-NETs, and telomere-associated features. Totally 39 tumour samples exhibited copy number loss at 18q while 27 tumours were diploid, and another 3 tumours showed gain with 3 or 4 copies of chromosome 18q. We did not observe any link between chromosome 18 copy number loss at the CDH19 locus and telomerase activity, TERT expression or telomere length (data not shown). However, higher MetI in Region B was observed in SI-NETs with copy number loss of chromosome 18q as compared to tumours without this abnormality (P= 0.03, Supplementary Figure S2).
Discussion
Biological mechanisms underlying development and progression of SI-NETs are still to a large extent unknown. Although telomerase machinery abnormalities have been implicated in many tumours, comprehensive and informative telomere-related studies of SI-NETs are missing. The telomerase complex is now considered as a therapeutic target in various tumours, through different strategies including small molecule inhibitors, antisense oligonucleotides, immunotherapy and gene therapy, some having entered clinical trials phase I or II [18].
In this study, we analyzed alterations in telomerase activity, mRNA expression of its catalytic component TERT and telomere length in SI-NETs and sought for possible mechanisms that can underlie those alterations. We found telomerase activity in 55% of analysed tumours but none in normal adjacent ileal controls. TERT was over-expressed in tumours as compared to normal ileal samples. A large number of studies indicate the implication of TERT and telomerase in tumorigeneses. The observed telomerase activity and TERT over-expression in SI-NETs demonstrate the involvement of telomerase also in this tumour entity. In this study we used adjacent ileum for comparison of SI-NETs to a non-tumorous tissue, however, the low proportion of enterochromaffin cells from which SI-NETs originate makes this an imperfect reference which is a known dilemma for this disease. With the development of TERT antibodies, a direct comparison of SI-NET cells to normal enterochromaffin cells could be performed by double TERT/Chromogranin A immunohistochemistry, directly on the slides. However, at present, available TERT antibodies are a poor predictor of TERT activation in other tumour types [19].
Telomerase activity is considered a risk factor in many different tumours including pulmonary NETs [20]. However, a previous report on NETs did not find any association between telomerase activity and clinicopathological characteristics [21]. We detected shorter telomeres in distant metastases vs. primary tumours and local metastases, as well as normal ileal samples. Telomere erosion and shorter telomeres in the tumour cells with higher ratio of proliferation especially in metastasized ones are expected [2]. In SI-NETs telomerase activity is enhanced from normal ileum to primary tumours and metastases. Concomitantly, telomeres become shorter, probably due to multiple cell divisions and possibly higher proliferation rate. This could explain a high ratio of chromosomal instabilities; a hallmark of SI-NETs [22], caused by gain of telomerase activity in abnormally eroded chromosomal telomeres.
We also studied the possible mechanisms underlying TERT overexpression and the telomerase machinery alterations in SI-NETs. We did not detect any TERT promoter mutation in the two hotspots C228T and C250T that can be implicated in many tumours [17]. This is consistent with a recent report indicating rare incidence of these mutations in pancreatic NETs [23]. Moreover, significant copy number alterations of the TERT locus on chromosome 5p were also lacking. However, we did see an inverse correlation between higher MetI in Region B and telomerase activation and TERT expression and an inverse correlation to shorter telomere length.
The TERT promoter methylation analysis illustrated its implication in gene expression and consequently telomerase activity and telomere shortening. The TERT promoter is regulated by a variety of transcription factors and regulators [24]. Therefore, aberrant DNA methylation on different promoter loci leads to different effects on TERT expression. DNA methylation of the two regions of TERT promoter that were analysed in this study has already been reported to have opposite effects on TERT expression, as methylation of the transcription start site proximal locus, Region B suppresses TERT transcription, while methylation of the distal locus, Region A can pose a transcriptional stimulatory effect [6,25]. We found that Region B MetI was inversely correlated with TERT expression and telomerase activity, indicating a typical inhibitory effect posed by promoter CpG methylation on transcriptional machinery [26]. Methylation of this region was also correlated with telomere length.
The loss of chromosome 18q with regards to telomerase machinery alterations was further studied and an association between Region B MetI and chromosome 18q loss was identified; however, we did not find further association between telomere machinery alterations and chromosome 18 loss. Interestingly, Karpathakis and colleagues described clusters with molecular subgroups according to, e.g., CpG hypermethylator phenotype and CNA at chromosome 18 [27].
We analyzed LINE1 methylation in an extended cohort to our previous study [14] and observed LINE1 hypomethylation in distant metastases compared to primary tumours and regional metastases. These observations are in agreement with findings based on global DNA methylation profiling showing global hypomethylation in SI-NETs, which was also more pronounced in liver metastases as compared to primary tumours [28], as well as in regional metastases as compared to primary tumours [29]. LINE1 MetI was inversely correlated with TERT expression and telomerase activity. Taking into consideration that LINE1 methylation can inhibit LINE1 expression, this observation is in line with reports that LINE1 induces TERT expression and function [30]. Positive correlation between Region B MetI and LINE1 MetI and on the other hand negative correlation between Region A MetI and LINE1 are consistent with the previously reported opposite regulatory functions of these regions [25].
In conclusion, this is the first study that couples aberrant TERT promoter methylation to TERT induction in SI-NETs. Our study showed that telomerase activation and TERT expression are common in SI-NETs. The C228T and C250T hotspot TERT promoter mutations were not present in SI-NETs. However, TERT promoter methylation of this mutation-hotspot region may regulate TERT expression.
Funding Statement
The study was supported by grants from the Swedish Cancer Society, the Cancer Society in Stockholm, the Gustav V Jubilee Foundation, Stockholm county council and Karolinska Institutet. This work was supported by the Cancerfonden [CAN 2017/571]; Cancerföreningen i Stockholm [161143]; Karolinska Institutet [There is no number.]; Stiftelsen Konung Gustaf V:s Jubileumsfond [174203]; Stockholms Läns Landsting [Dnr LS 1311-1460].
Acknowledgments
Ms Lisa Ånfalk at KI is acknowledged for excellent tissue handling.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
References
- [1].Modlin IM, Oberg K, Chung DC, et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008. January;9(1):61–72. PubMed PMID: 18177818. [DOI] [PubMed] [Google Scholar]
- [2].Pacini F, Cantara S, Capezzone M, et al. Telomerase and the endocrine system. Nat Rev Endocrinol. 2011. March 29;7(7):420–430. PubMed PMID: 21448143. [DOI] [PubMed] [Google Scholar]
- [3].Shay JW, Wright WE.. Hayflick, his limit, and cellular ageing. Nat Rev Mol Cell Biol. 2000. October;1(1):72–76. PubMed PMID: 11413492. [DOI] [PubMed] [Google Scholar]
- [4].Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011. March 04;144(5):646–674. PubMed PMID: 21376230. [DOI] [PubMed] [Google Scholar]
- [5].Wang N, Liu T, Sofiadis A, et al. TERT promoter mutation as an early genetic event activating telomerase in follicular thyroid adenoma (FTA) and atypical FTA. Cancer. 2014. October 1;120(19):2965–2979. PubMed PMID: 24898513. [DOI] [PubMed] [Google Scholar]
- [6].Wang N, Kjellin H, Sofiadis A, et al. Genetic and epigenetic background and protein expression profiles in relation to telomerase activation in medullary thyroid carcinoma. Oncotarget. 2016. April 19;7(16):21332–21346. PubMed PMID: 26870890; PubMed Central PMCID: PMCPMC5008288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Paulsson JO, Mu N, Shabo I, et al. TERT aberrancies: a screening tool for malignancy in follicular thyroid tumours. Endocr Relat Cancer. 2018. July;25(7):723–733. PubMed PMID: 29692346. [DOI] [PubMed] [Google Scholar]
- [8].Renaud S, Loukinov D, Abdullaev Z, et al. Dual role of DNA methylation inside and outside of CTCF-binding regions in the transcriptional regulation of the telomerase hTERT gene. Nucleic Acids Res. 2007;35(4):1245–1256. PubMed PMID: 17267411; PubMed Central PMCID: PMCPMC1851636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Svahn F, Paulsson JO, Stenman A, et al. TERT promoter hypermethylation is associated with poor prognosis in adrenocortical carcinoma. Int J Mol Med. 2018. September;42(3):1675–1683. PubMed PMID: 29956721. [DOI] [PubMed] [Google Scholar]
- [10].Jiao Y, Shi C, Edil BH, et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science. 2011. March 04;331(6021):1199–1203. PubMed PMID: 21252315; PubMed Central PMCID: PMCPMC3144496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Heaphy CM, Subhawong AP, Hong SM, et al. Prevalence of the alternative lengthening of telomeres telomere maintenance mechanism in human cancer subtypes. Am J Pathol. 2011. October;179(4):1608–1615. PubMed PMID: 21888887; PubMed Central PMCID: PMCPMC3181356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Francis JM, Kiezun A, Ramos AH, et al. Somatic mutation of CDKN1B in small intestine neuroendocrine tumors. Nat Genet. 2013. December;45(12):1483–1486. PubMed PMID: 24185511; PubMed Central PMCID: PMCPMC4239432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hashemi J, Fotouhi O, Sulaiman L, et al. Copy number alterations in small intestinal neuroendocrine tumors determined by array comparative genomic hybridization. BMC Cancer. 2013. October 29;13(1):505 PubMed PMID: 24165089; PubMed Central PMCID: PMC3819709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Fotouhi O, Adel Fahmideh M, Kjellman M, et al. Global hypomethylation and promoter methylation in small intestinal neuroendocrine tumors: an in vivo and in vitro study. Epigenetics. 2014. April 24;9(7). PubMed PMID: 24762809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sulaiman L, Juhlin CC, Nilsson IL, et al. Global and gene-specific promoter methylation analysis in primary hyperparathyroidism. Epigenetics. 2013. June;8(6):646–655. PubMed PMID: 23764768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002. May 15;30(10):e47 PubMed PMID: 12000852; PubMed Central PMCID: PMCPMC115301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Liu T, Wang N, Cao J, et al. The age- and shorter telomere-dependent TERT promoter mutation in follicular thyroid cell-derived carcinomas. Oncogene. 2014. October 16;33(42):4978–4984. PubMed PMID: 24141777. [DOI] [PubMed] [Google Scholar]
- [18].Ruden M, Puri N. Novel anticancer therapeutics targeting telomerase. Cancer Treat Rev. 2013. August;39(5):444–456. PubMed PMID: 22841437. [DOI] [PubMed] [Google Scholar]
- [19].Paulsson JO, Olander A, Haglund F, et al. TERT Immunohistochemistry Is a Poor Predictor of TERT Promoter Mutations and Gene Expression in Follicular Thyroid Carcinoma. Endocr Pathol. 2018. December;29(4):380–383. PubMed PMID: 30306386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zaffaroni N, De Polo D, Villa R, et al. Differential expression of telomerase activity in neuroendocrine lung tumours: correlation with gene product immunophenotyping. J Pathol. 2003. September;201(1):127–133. PubMed PMID: 12950025. [DOI] [PubMed] [Google Scholar]
- [21].Bockhorn M, Frilling A, Clauer U, et al. Telomerase activity in neuroendocrine tumors. Int J Surg Investig. 2000;2(3):219–225. PubMed PMID: 12678522. [PubMed] [Google Scholar]
- [22].Cunningham JL, Diaz de Stahl T, Sjoblom T, et al. Common pathogenetic mechanism involving human chromosome 18 in familial and sporadic ileal carcinoid tumors. Genes Chromosomes Cancer. 2011. February;50(2):82–94. PubMed PMID: 21104784. [DOI] [PubMed] [Google Scholar]
- [23].Vinagre J, Nabais J, Pinheiro J, et al. TERT promoter mutations in pancreatic endocrine tumours are rare and mainly found in tumours from patients with hereditary syndromes. Sci Rep. 2016. July 14;6:29714 PubMed PMID: 27411289; PubMed Central PMCID: PMCPMC4944231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Cifuentes-Rojas C, Shippen DE. Telomerase regulation. Mutat Res. 2012. February 1;730(1–2):20–27. PubMed PMID: 22032831; PubMed Central PMCID: PMCPMC3256259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zinn RL, Pruitt K, Eguchi S, et al. hTERT is expressed in cancer cell lines despite promoter DNA methylation by preservation of unmethylated DNA and active chromatin around the transcription start site. Cancer Res. 2007. January 1;67(1):194–201. PubMed PMID: 17210699. [DOI] [PubMed] [Google Scholar]
- [26].Butler JE, Kadonaga JT. The RNA polymerase II core promoter: a key component in the regulation of gene expression. Genes Dev. 2002. October 15;16(20):2583–2592. PubMed PMID: 12381658. [DOI] [PubMed] [Google Scholar]
- [27].Karpathakis A, Dibra H, Pipinikas C, et al. Prognostic Impact of Novel Molecular Subtypes of Small Intestinal Neuroendocrine Tumor. Clin Cancer Res. 2016. January 1;22(1):250–258. PubMed PMID: 26169971. [DOI] [PubMed] [Google Scholar]
- [28].Karpathakis A, Dibra H, Pipinikas C, et al. Progressive epigenetic dysregulation in neuroendocrine tumour liver metastases. Endocr Relat Cancer. 2017. February;24(2):L21–L25. PubMed PMID: 28049633. [DOI] [PubMed] [Google Scholar]
- [29].Verdugo AD, Crona J, Starker L, et al. Global DNA methylation patterns through an array-based approach in small intestinal neuroendocrine tumors. Endocr Relat Cancer. 2014. January 21;21(1):L5–7. PubMed PMID: 24192231. [DOI] [PubMed] [Google Scholar]
- [30].Aschacher T, Wolf B, Enzmann F, et al. LINE-1 induces hTERT and ensures telomere maintenance in tumour cell lines. Oncogene. 2016. January 7;35(1):94–104. PubMed PMID: 25798839. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


