Abstract
Inflammasomes are supramolecular organizing centers that operate to drive interleukin-1 (IL-1) dependent inflammation. Depending on context, inflammatory caspases act upstream or downstream of inflammasome assembly, serving as the principal enzymes that control activities of these organelles. In this Review, we discuss mechanisms of inflammasome assembly and signaling. We posit that upstream regulatory proteins, classically known as pattern recognition receptors, operate to assess infectious and non-infectious threats to the host. Threat assessment is achieved through two general strategies: (1) direct binding of receptors to microbial or host-derived ligands in the cytosol or (2) indirect detection of changes in cellular homeostasis. Upon activation, these upstream regulatory factors seed the assembly of inflammasomes, leading to IL-1 family cytokine release from living (hyperactive) or dead (pyroptotic) cells. The molecular and physiological consequences of these distinct cell fate decisions are discussed.
eTOC
Proteins that drive inflammasome activities are responsive to diverse threats to the host, including infection, tissue damage (or both). Upon threat identification, specific regulatory proteins seed the assembly of inflammasomes, which drive inflammatory responses that influence local and systemic immunity. Evavold and Kagan discuss mechanisms and consequences of inflammasome activities.
The innate immune system as a threat assessment computer
All organisms must interact with their environment in order to survive. Fundamental to these interactions is the ability of the host to discern its surroundings, such that adaptations to any environmental changes can be made. The specific changes that occur in the environment are countless, but they can be grouped into two broad categories. The first category encompasses beneficial changes, such as friendly (commensal) encounters or exposure to favorable temperatures, salinity, or nutrients. The second category includes detrimental changes, such as dangerous (pathogenic) encounters or conditions that cause susceptibility to infection and tissue damage. Determination of potential threat to the host is a task that is crucial to adapt to any new environment.
At the cellular level, research over the last several years has revealed a variety of responses to environmental changes, which range from neuronal circuits that detect pain and temperature alterations to immune pathways that detect microbial encounters and tissue injury (Iwasaki and Medzhitov, 2010; Koch et al., 2018). While these discoveries have provided a clearer view of the cells and genes important for host adaptation to the environment, there is a lack of broad-based hypotheses to explain the logic underlying cell sensory networks.
Herein, we discuss recent developments into how mammalian cells detect environmental change, with a specific focus on detrimental changes to the host. We discuss how these threats can be grouped into two major classes of molecular signals, which are defined by classical terms: pathogen associated molecular patterns (PAMPs) and damage associated molecular patterns (DAMPs) (Janeway, 1989; Matzinger, 1994). The former class, when detected by the host, indicates encounters with microbes and their products. The latter class indicates encounters with environmental conditions that caused a homeostatic disruption, perhaps resulting in cellular or subcellular injury. Thus, the fundamental distinction between DAMPs and PAMPs is the source of the molecule, not their chemical structures.
A growing body of evidence supports the idea that a common sensory system is used to detect DAMPs and PAMPs. This sensory system consists of a diverse family of receptors, defined by Charles Janeway Jr, as pattern recognition receptors (PRRs) (Janeway, 1989). PRRs have been viewed as sentinels of the microbial world around us. However, the ability of PRRs to serve as exclusive indicators of microbial encounters through detection of PAMPs is seemingly compromised by the increasing acceptance that these receptors also detect DAMPs. Thus, PRRs can no longer be considered to have evolved for the exclusive purpose of detecting microbes. An alternative view of the innate immune system is that PAMP detection is but one function of PRRs, and that the detection of DAMPs is as important to the host as the detection of PAMPs in terms of adapting to environmental changes. Consistent with this idea are the activities of tissue resident macrophages. These cells have been historically viewed as antimicrobial cells that use PRRs to detect infection, but recent work has extended their functions to include regulation of tissue homeostasis and detection of non-infectious threats (Okabe and Medzhitov, 2016). The innate immune system may therefore be best viewed not simply as a microbe-detection system, but rather a threat-assessment computer, whose function is to detect many types of potentially deleterious changes in the environment.
Viewing the innate immune system as a threat-assessment station may explain genetic and clinical evidence of the role of PRRs in diseases with an infectious component and diseases where no microbe has been implicated. In both cases, PRRs and the cells that encode them are critical for disease development and resolution. Moreover, from an evolutionary perspective, this view may explain the long-recognized roles of one of the first-identified PRRs, the Drosophila Toll receptor, in anti-microbial defense as well as development. In the next sections, we explore these concepts with a specific focus on PRRs that link diverse environmental threats to the assembly and activation of inflammasomes. We have chosen inflammasome-activating PRRs as a centerpiece for this discussion because examples of these PRRs responding to a variety of environmental threats have been identified. For example, individual inflammasome-activating PRRs directly detect PAMPs, DAMPs, or changes in cellular processes indicative of altered homeostasis. The collective knowledge that PRRs detect each of these classes of threats supports our thesis.
Threat assessment by differential localization of PRRs and their ligands
Low risk environmental threats include PAMPs in the extracellular environment of barrier tissues in the organism (Figure 1). PAMPs in these environments do not necessarily indicate an encounter with a pathogen. These extracellular PAMPs induce inflammatory responses to eliminate the possibility of infection. Because infection is only a possibility, the host response may not be activated maximally. The responses induced under these conditions include the expression of inflammatory chemokines, cytokines and other factors that ensure induction of adaptive immunity (Iwasaki and Medzhitov, 2010). These responses are often induced through the Toll-like receptor (TLR) family of PRRs. TLRs survey external environments through receptor localization at the plasma and endosomal membranes, with their ligand binding domains exposed to the extracellular space or endosomal lumen (Brubaker et al., 2015). The mechanisms of TLR signaling have been reviewed elsewhere (Bryant et al., 2015; Pandey et al., 2014), and we will only summarize their mechanisms of action here. Upon PAMP detection, TLRs dimerize and seed the formation of helical oligomeric complexes of proteins in the cytosol. These complexes, known as supramolecular organizing centers (SMOCs), represent the signaling organelles of the innate immune system (Kagan et al., 2014). TLR-associated SMOCs concentrate enzymes that drive downstream signaling events that augment metabolism, transcription, and translation (Tan and Kagan, 2019). These responses are best understood to drive inflammatory responses and stimulate adaptive immunity (Iwasaki and Medzhitov, 2010).
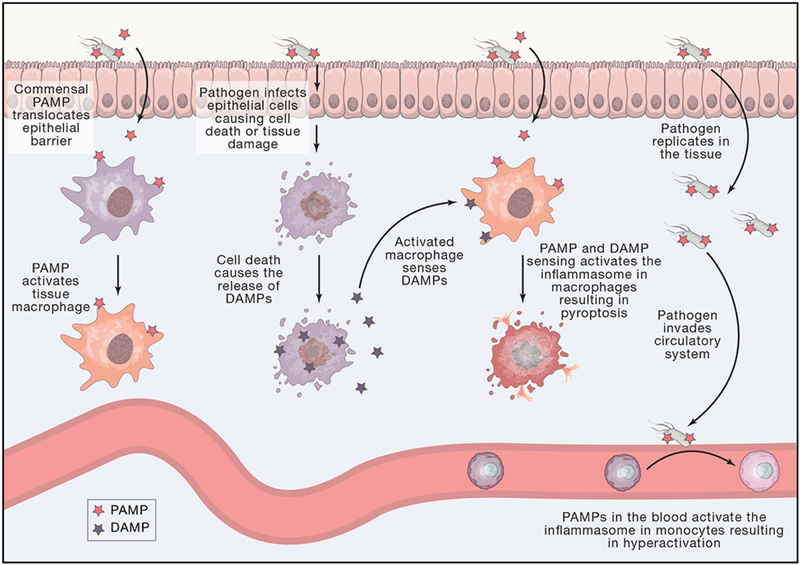
Figure 1: Tissue and Cellular Level Topology Affect Threat Level Classification.
Gut epithelial barrier and blood vessels are sites of microbial recognition with different inputs and outcomes. Tissue resident macrophages can become activated to secrete conventional pro-inflammatory cytokines by sensing PAMPs such as those from pathogens or commensal microbes. Pathogens can infect many cell types in the gut such as epithelial cells, resulting is tissue damage and cell death and the release of DAMPs. An activated tissue resident macrophage that now senses DAMPs can form an inflammasome and undergo pyroptosis. A pathogen can replicate in the tissue. The infected tissue can allow whole pathogens or PAMPs to translocate into blood vessels. PAMPs in the blood vessels represent a threat of systemic infection through the circulatory system. A monocyte that senses a PAMP in the blood can form an inflammasome to become hyperactive.
Detection of extracellular PAMPs would be a relatively low-level threat, as commensal and other non-pathogenic microbes might shed ligands (Figure 2). These detection events thus result in a proportionate signaling outcome in which the responding cells do not sacrifice themselves by committing to a cell death program. Commitment to a death program is restricted to threats that represent a clear and present danger to the host. One clear and present danger to the host occurs during encounters with microbial pathogens.
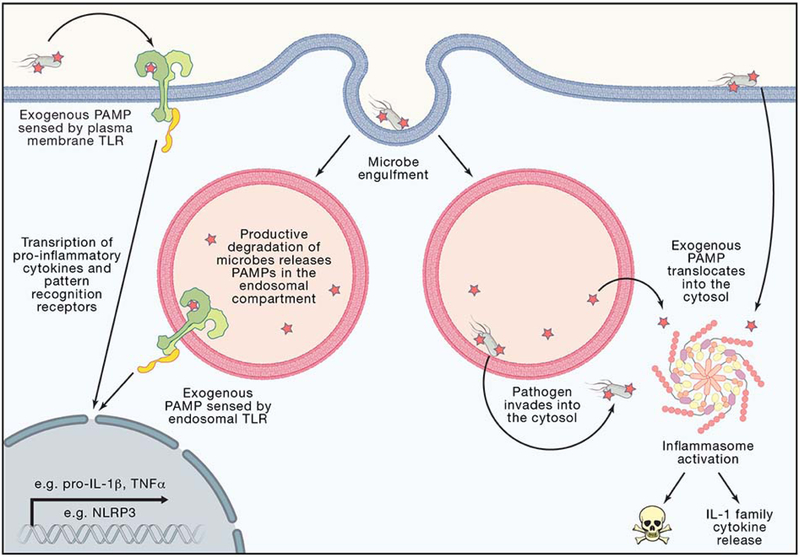
Figure 2: Cellular Level Topology Affect Threat Level Classification.
The extracellular space, endosomal lumen, and cytosol are sites of microbial recognition with different sensors and outcomes. Plasma membrane TLRs survey the extracellular space for PAMPs or DAMPs representing a lower threat level as the molecular patterns could derive from commensal bacteria or sterile injury. Endosomal TLRs survey the endosomal lumen that topologically is considered continuous with the extracellular space, representing a lower threat level as the molecular patterns could derive from productive degradation of commensals or host cell debris. TLR activation leads to transcription of pro-inflammatory cytokines, such as pro-IL-1β and TNFα and up regulation of pattern recognition receptors, such as the inflammasome receptor NLRP3. Pathogens can inject effectors and translocate PAMPs into the cytosol through the plasma membrane or endosomal membrane. Pathogens can also directly translocate from the endosomal lumen into the sterile cytosol. Exogenous PAMPs or pathogens found in the cytosol cause inflammasome activation, representing a higher threat level as the host is being invaded or intoxicated by pathogenic microbes. Inflammasome activation leads to release of IL-1β, IL-18, and pyroptotic cell death.
All pathogens manipulate the host cells they encounter in order to promote their own replication. This process presents a life-threatening risk to the host, which must be addressed by proportionally high defensive responses. A common defensive response induced upon pathogenic encounters is to kill the infected cells in order to prevent any intracellular microbial replication. This terminal cell fate decision by the host is only made when the threat is high; non-pathogenic encounters should result in host cell death. Mechanistically, virulence assessment occurs by the detection of PAMPs in specific intra- or extracellular compartments. In contrast to extracellular PAMPs, which may not necessarily indicate an encounter with a virulent pathogen (or even a living microbe), cytosolic PAMPs almost always coincide with direct exposure to living and perhaps virulent microbes. The reason for this trend is that pathogenic manipulation of host cells is accomplished through the access by the pathogen (or its virulence factors) to the host cytosolic environment (Figure 2). For example, bacteria can encode cell-associated secretion systems that inject virulence factors into the host cytosol. Fungal pathogens are increasingly recognized for their ability to similarly manipulate the host, and viruses must enter the host cytosol in order to replicate. As non-infectious organisms do not encode virulence determinants, they cannot intentionally access the host cytosol. Only infectious agents deliver PAMPs to the cytosol. Thus cytosolic PRRs are uniquely positioned to identify pathogenic encounters. Consequently, these PRRs induce a heightened state of inflammation and the host-determined demise of the infected cell. Cytosolic PRRs include members of the nucleotide-binding domain, leucine rich containing (NLR) family, and the proteins AIM2 and Pyrin. The SMOC induced by these receptors – the inflammasome – is the focus of this review.
Inflammasomes are protein complexes that represent the site of caspase-1 activity. Caspase-1 is best known as an interleukin-1 (IL-1) converting enzyme, having the capacity to cleave biologically inert pro-IL-1β into its active form—IL-1β (Garlanda et al., 2013). IL-1 was the first cytokine identified and represents one of the most potent pyrogens produced by mammals. Perhaps because of its potent induction of local and systemic inflammation, the regulation of IL-1α and IL-1β release from cells is more tightly controlled than that of other cytokines. Indeed, whereas all other well-characterized cytokines, chemokines and interferons are secreted upon synthesis, the secretion and inflammatory activity of the IL-1 family is dissociated from its synthesis (Garlanda et al., 2013). This dissociation is due to the lack of an N-terminal secretion signal in many members of the IL-1 family. Thus, several IL-1 family members, such as IL-1β, IL-18, and IL-37, are produced in the cytosol as latent proteins that cannot achieve bioactivity until they are cleaved by caspase-1 and released from cells. One notable exception to this rule regards the anti-inflammatory IL-1 receptor antagonist (IL-1Ra), which contains a signal peptide for secretion through the biosynthetic pathway. Inflammasome-associated caspase-1 is responsible for generating bioactive IL-1β and IL-18 and promoting their release into the extracellular space.
A common mechanism through which inflammasomes promote IL-1β release is the induction of pyroptosis, a lytic form of cell death that non-specifically releases cytosolic content from cells. As pyroptosis is a terminal event, the choice of a cell to commit to this process must be tightly regulated. It stands to reason that such a commitment to cellular suicide would only be justified in instances of high-threat encounters, which is supported by our current knowledge that only cytosolic PRRs can induce pyroptosis. Based on these principles, extracellular PAMPs are classified as low-threat and resolvable problems, whereas cytosolic PAMPs are contextualized as high-threat problems that may induce drastic cell fate decisions such as cell death.
There are several examples of individual PAMPs that drive transcriptional responses via TLRs and cell death responses from inflammasome-associated PRRs. These PAMPs include bacterial LPS that can be sensed by TLR4 or caspase-11, bacterial flagellin that can be sensed by TLR5, NAIP5 or NAIP6, and DNA that can be sensed by TLR9 or AIM2. While the idea that pathogens and non-pathogens may be viewed differently by the innate immune system has been offered previously (Blander and Sander, 2012; Vance et al., 2009), these earlier commentaries preceded the identification of molecular explanations by which threat assessment is accomplished. Threat level determination by inflammasome-stimulating PRRs may be beneficial during infection by stimulating adaptive immune responses through heightened inflammatory responses and cell death of infected cells (Evavold and Kagan, 2018). Dysregulation of inflammasome signaling may be deleterious to the host during sepsis (Kayagaki et al., 2011) or through host reaction to gout crystals and amyloid plaques (Heneka et al., 2013; Martinon et al., 2006). These latter scenarios represent chronic exposure to high concentrations of PAMPs and DAMPs. In subsequent sections, we discuss mechanisms by which PRRs drive inflammasome assembly and activity and additional context-dependent considerations made by cells to gauge threat.
Mechanisms of inflammasome assembly and activity
Like all SMOCs, inflammasomes are comprised of two abundant functional units. The first unit consists of an adaptor protein(s) with oligomerization potential, and the second unit is an effector enzyme whose activity drives inflammatory responses (Figure 3). Oligomerization potential is converted into action by an upstream seed protein, usually a PRR, which functions to drive adaptor oligomerization and stimulate effector enzyme activity (Cai et al., 2014; Lu et al., 2014). This simple two-unit principle enables diverse seed proteins to assemble SMOCs with distinct effector functions and likely explains their prevalence in nature. Moreover, the simplicity of SMOC architecture ensures flexibility-of-design and has enabled synthetic organizing centers to be engineered to induce user-defined responses (Tan and Kagan, 2019). In this section, we describe the mechanisms that drive SMOC formation, with a focus on how different proteins operate by the two-unit SMOC design principle in order to mediate signal transduction to disparate inputs.
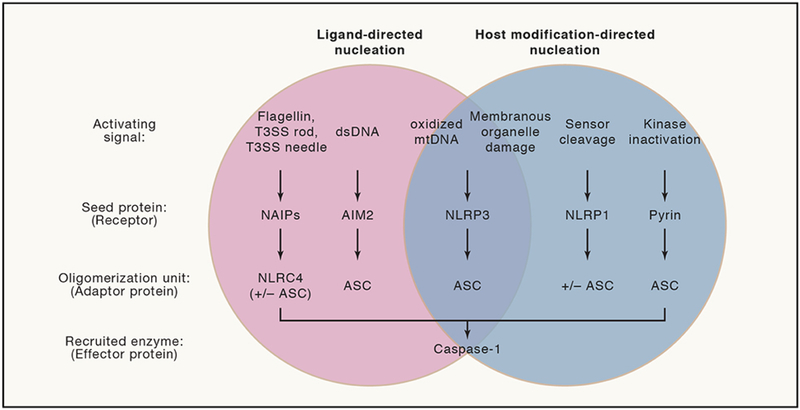
Figure 3: Modes of Inflammasome Nucleation Reflect Diverse Mechanisms to Contextualize Intent of Pathogens.
Inflammasomes by definition serve as platforms to activate the inflammatory caspase, caspase-1. The NAIP inflammasome recognizes bacterial PAMPs in the cytosol through oligomerization of NLRC4 to directly recruit caspase-1 or in combination with ASC. The AIM2 inflammasome recognizes mislocalized dsDNA in the cytosol that may represent PAMPs or DAMPs to seed an inflammasome using ASC to recruit caspase-1. The literature suggests that NLRP3 may directly sense ligands or may sense broad dysfunction of host processes to seed an inflammasome using ASC to recruit caspase-1. The NLRP1 inflammasome serves as a “bait” protein to indirectly sense pathogens via sensing pathogen-derived protease activity. NLRP1 may directly recruit caspase-1, but ASC serves to amplify caspase-1 activation. The pyrin inflammasome is sequestered during homeostasis. Disruption of cytoskeleton-associated RhoA kinase activity indirectly leads to pyrin release to seed an inflammasome using ASC to recruit caspase-1.
A large class of inflammasomes use the protein ASC as its oligomerization unit (Cai et al., 2014; Lu et al., 2014). The PRRs NLRP1, NLRP3, NLRP6, AIM2 and Pyrin engage ASC through interactions between the pyrin domain on the receptor with the pyrin domain of ASC, resulting in oligomerization (Chae et al., 2011; Fernandes-Alnemri et al., 2009; Hornung et al., 2009). ASC also contains a caspase activation and recruitment domain (CARD) that can mediate homotypic interactions with the CARD found in the effector enzyme pro-caspase-1 (Lu et al., 2014; 2016). The PRR NLRP1 may also engage ASC via CARD-CARD interactions (Chavarria-Smith et al., 2016; Zhong et al., 2016). Pro-caspase-1 is thought to have low intrinsic protease activity, although the purified p45 precursor enzyme (pro-caspase-1) can autoprocess in solution (Ayala et al., 1994). This low intrinsic activity might explain the requirement of p45 recruitment into the inflammasome for full activation. It is thought that the induced proximity and increased local concentration of the p45 species of pro-caspase-1 within inflammasomes allows for caspase self-cleavage at various aspartic acid residues, leading to an active but transient species recently defined as a p33/p10 heterodimer (Boucher et al., 2018). Many studies have noted that the p20/p10 heterodimer is active at high concentrations, but recent work demonstrates that the p20/p10 heterodimers may represent downregulation of caspase activity at later stages of inflammasome signaling (Boucher et al., 2018). Indeed, these p20/p10 heterodimers are no longer tethered to the inflammasome and can be released from the cell and are often used as biomarkers to indicate earlier caspase activation (Boucher et al., 2018; Keller et al., 2008).
PRRs of the NAIP family also stimulate inflammasome formation, but they may not utilize ASC as its core oligomerization unit (Hu et al., 2015; Zhang et al., 2015). Rather, NAIPs engage the adaptor NLRC4 through NACHT domain interactions in the PRR and adaptor. Akin to oligomerized ASC, NLRC4 recruits pro-caspase-1 into the assembled inflammasome through CARD-CARD interactions between the adaptor and effector. ASC is likely recruited to these inflammasomes to augment caspase-1 activity (Case and Roy, 2011). In fact, macrophages stimulated with NAIP agonists can induce pyroptosis in an ASC-independent, caspase-1-dependent manner, but these cells fail to efficiently cleave other abundant caspase-1 substrates, such as pro-IL-1β in the absence of ASC (Broz et al., 2010). The precise biochemical relationship between ASC-dependent and -independent activities of the NAIP-NLRC4 inflammasome remains to be defined.
In the examples described above, caspases operate as the effector component of the assembled SMOC. A notable exception to this idea has emerged with the identification that murine caspase-11 and its human counterparts (caspase-4 and −5) are PRRs that form high-affinity interactions with LPS (Shi et al., 2015b). LPS binds the CARD of these inflammatory caspases and stimulates their latent protease activity, which leads to the assembly of the NLRP3 inflammasome (Shi et al., 2015b). Notably, the link between caspase-11 activation and NLRP3 inflammasome assembly is indirect and is mediated by the actions of a recently identified caspase substrate known as gasdermin D (GSDMD) (Kayagaki et al., 2015; Rühl and Broz, 2015; Shi et al., 2015a). The details of this process will be described below.
Regulation of inflammasome assembly by subcellular localization
Increasing attention has been paid to the biochemical and cell biological aspects of inflammasome components and regulators. This aspect of inflammasome biology is in its infancy, with no clear understanding of the cell biological principles that explain pathway activity. Despite this lack of knowledge, a consensus is emerging whereby the movement of PRRs or their downstream components from the site of recognition to the site of signaling controls inflammasome assembly and activity. This general conclusion is consistent with work done on other PRR-regulated pathways, where the PAMP-inducible transport of receptors and downstream signaling machinery is critical to initiate inflammatory activity (Tan and Kagan, 2017).
In support of active transport processes being necessary for inflammasome activity, NLRP3 and pyrin inflammasome assembly can be inhibited by chemicals that target microtubule stability (Gao et al., 2016; Martinon et al., 2006; Misawa et al., 2013). In unstimulated cells, NLRP3 is diffuse in the cytosol, but upon diverse triggers, NLRP3 translocates to the mitochondrial membrane (Elliott et al., 2018; Zhou et al., 2010). Several studies suggest NLRP3 translocation to mitochondria depends on an interaction with MAVS, a mitochondrial and peroxisomal transmembrane protein necessary for RIG-I-like receptor (RLR) signal transduction (Subramanian et al., 2013). Other studies highlight lipid changes in the outer mitochondrial membrane as mediators of NLRP3 recruitment through interactions with cardiolipin (Elliott et al., 2018; Iyer et al., 2013). A recent study suggests additional lipids affect NLRP3 localization early in inflammasome signaling. This study reports that a polybasic site within NLRP3 is necessary for binding of NLRP3 to the Golgi-localized phosphatidylinositol-4-phosphate (Chen and Chen, 2018).
Another study also implicated the Golgi cisternae in NLRP3 signaling, though this study suggests NLRP3 first associates to mitochondria-associated membranes (MAM) on the endoplasmic reticulum (Zhang et al., 2017). Increased production of the lipid mediator diacylglycerol activates protein kinase D (PKD) near the Golgi to promote NLRP3-dependent responses. In this study, NLRP3 disassociation from the MAM is required for inflammasome assembly, and PKD phosphorylation of NLRP3 is sufficient to mediate inflammasome signaling. Caspase-1 also binds cardiolipin and can associate with mitochondrial membranes (Elliott et al., 2018).
Calcium mediates mitochondrial damage and NLRP3 inflammasome assembly (Murakami et al., 2012). Calcium fluxes may also be required for localization of ASC to pre-localized NLRP3 and caspase-1 on cardiolipin-exposing mitochondria (Elliott et al., 2018; Iyer et al., 2013). In response to several inflammasome stimulators, a large ASC aggregate or “speck” forms within the cytosol and has been described generally as perinuclear (Fernandes-Alnemri et al., 2007). As the ASC speck contains NLRP3 and enzymatically active caspase-1, it is considered an inflammasome. How this speck assembles is unclear, but it may result from a trafficking event that coalesces inflammasome components that initially interacted with one another on membranous organelles. Like NLRP3, AIM2 is considered to be diffuse in the cytosol and localizes into ASC specks upon cellular stimulation (Fernandes-Alnemri et al., 2009; Hornung et al., 2009). AIM2 can also form an inflammasome within the nucleus after exposure to ionizing irradiation (Hu et al., 2016). The location of PRR oligomerization and signal transduction may depend on the cell type studied and stimulation used. More studies are required to define the spatial relationship of inflammasome components in multiple cell types, with the hope that the community might describe sequential trafficking events in endogenous conditions to determine if heterologous or synthetic reconstitution systems reflect natural biology.
Regulation of inflammasome assembly by heterologous signaling pathways
Inflammasomes and their upstream receptors are not the only PRR networks that can be considered threat assessment stations. Rather, we consider all PRRs to operate in this manner and perhaps all cytokine receptors as well. Collectively, cells utilize these diverse receptor families to identify environmental changes and mount appropriate responses. It therefore stands to reason that the information gathered from one receptor family would be useful to others in the same cell or tissue microenvironment. Work done over many years supports this idea in the context of inflammasome activity, as these networks are sensitive to environmental changes that they do not directly detect. Below we provide examples of how PRR and cytokine signaling pathways can indirectly influence inflammasome activity.
Many pro-inflammatory signaling cascades can synergize with the inflammasome-stimulating pathways to incur higher responsiveness within stimulated cells. The addition of these “priming” steps allow for peak inflammasome responsiveness. Other PRR families can determine lower level threats prior to recognition of higher level threats by inflammasome assembly. An example of inflammasome priming involves the NLRP3 inflammasome. As compared to naïve cells, cells that have been treated with TLR agonists or pro-inflammatory cytokines are more responsive to subsequent stimuli that induce inflammasome assembly (Bauernfeind et al., 2009; Franchi et al., 2009). This priming event is linked to the ability of TLRs to induce the upregulation of NLRP3 (Bauernfeind et al., 2009). Similarly, type I interferon activities increase expression of AIM2 and caspase-11 to sensitize cells to subsequent stimuli (Fernandes-Alnemri et al., 2009; Hornung et al., 2009; Rathinam et al., 2012). In addition to upregulation of inflammasome-associated PRRs, activation of the transcription factors AP-1 and NF-kB induce expression of the inflammasome substrates pro-IL-1β and pro-IL-18 (Garlanda et al., 2013).
Transcriptional upregulation of PRRs is not the only mechanism of inflammasome priming. Indeed, simultaneous encounters of TLR ligands and inflammasome activators stimulate immediate (non-transcription dependent) assembly of the NLRP3 inflammasome (Juliana et al., 2012; Schroder et al., 2012). This immediate assembly does not occur in response to either stimulus alone, and stimulation of inflammasome assembly in this manner requires the TLR-associated kinases IRAK1 and IRAK4 (Fernandes-Alnemri et al., 2013; Lin et al., 2014). Removal of ubiquitin post-translational modifications on NLRP3 can promote inflammasome signaling (Juliana et al., 2012; Py et al., 2013). TLR signaling can also stimulate the externalization of cardiolipin from the inner membrane of mitochondria (Elliott et al., 2018; Iyer et al., 2013). This exposed cardiolipin serves as a cue for NLRP3 homing to mitochondria prior to a secondary inflammasome-initiating signal. The adaptor ASC must also relocate during priming (Elliott et al., 2018). In human cells after LPS treatment, ASC translocates from the nucleus to the cytosol (Bryan et al., 2009).
The ability of TLRs and cytokines to prime cells for rapid inflammasome assembly can be considered in the context of the threat assessment hypothesis. Encounters with extracellular PAMPs that stimulate TLRs (or cytokines) indicates that an encounter with a microbe may have occurred, but does not indicate the threat of virulence. As such, TLR signaling does not stimulate inflammasome activity, but rather it poises cells for the possibility of a virulent encounter with a pathogen.
There is one exception to the “rule” that TLR signaling does not stimulate inflammasome assembly, as human monocytes directly link TLR signaling to inflammasome activity (Gaidt et al., 2016; Viganò et al., 2015). While the ability of monocytes to directly activate inflammasomes after TLR stimulation seemingly violates the idea of threat assessment, another variable may be involved. Monocytes are circulating cells, and any infection that reaches the blood has the possibility of systemic spread—a major threat to host viability. In contrast, infections in peripheral tissues can be contained locally without systemic threat. It is possible that monocytes are designed in a manner that the mere presence of PAMPs in the blood represents a heightened threat to the host that triggers TLR and inflammasome activities. In contrast, phagocytes in peripheral tissues may use coincident detection of TLR ligands, cytokine receptor ligands, and inflammasome activities to gauge threat to the host. Consistent with this idea is the fact that while monocytes are capable of linking TLR stimuli to inflammasome activity, monocyte-derived macrophages (which reside in the tissues) do not (Netea et al., 2009). Other examples of cell-type specific inflammasome activities will be discussed later.
Diverse environmental signals link threat assessment to inflammasome assembly
The NAIP PRRs seed inflammasome assembly after direct recognition of PAMPs in the cytosol. Human NAIP and murine NAIP1, NAIP2, NAIP5, and NAIP6 directly recognize flagellin, rod, and needle proteins of bacterial secretion systems to oligomerize NLRC4 and recruit pro-caspase-1. Human NAIP was initially described to recognize the needle protein of bacterial type 3 secretion systems (Yang et al., 2013). Murine NAIP1 also responds to type 3 secretion system needle proteins (Rayamajhi et al., 2013; Zhao et al., 2011). In primary monocyte-derived macrophages, a splice variant of the NAIP protein is produced. This NAIP variant can also respond to cytosolic flagellin (Kortmann et al., 2015). Murine NAIP5 and NAIP6 respond to flagellin and activate caspase-1 in an NLRC4-dependent manner (Lightfield et al., 2011; Zhao et al., 2011). Murine NAIP2 binds to type 3 secretion system rod proteins, and recent work suggests that human NAIP in primary human macrophages may also sense rod proteins (Reyes Ruiz et al., 2017; Zhao et al., 2011).
The direct recognition of a molecule that is not usually in the cytosol, such as components of bacterial secretion systems or flagellin subunits, by NAIPs to drive inflammasome assembly is analogous to the operation of the apoptosome. In the case of the latter, the flagellin equivalent would be cytochrome C, which is normally absent from the cytosol but leaks from damaged mitochondria. Cytosolic cytochrome C is recognized by APAF-1, leading to recruitment of pro-caspase-9 and the assembly of the apoptosome (Cheng et al., 2016). Structures of the NAIP-NLRC4 inflammasome with bacterial ligands confirm similarities in signaling between the inflammasome and apoptosome, as both SMOCs form higher order oligomers in the shape of a disk (Hu et al., 2015; Zhang et al., 2015). Inflammasomes are described to have “prion-like” polymerization properties, whereby the CARDs of the adaptor protein ASC and caspase-1 form filaments when mixed in vitro (Cai et al., 2014; Lu et al., 2014). Recent work has extended these findings to include similar filament formation and structural interactions between the CARDs of NLRC4 and caspase-1 (Li et al., 2018). Receptor structures of flagellin complexed with NAIP5 and NLRC4 or PrgJ complexed with NAIP2 and NLRC4, using a CARD truncated version of NLRC4, reveal drastic conformational changes that provide a new oligomerization surface to sequentially recruit and alter the conformation of additional NLRC4 adaptor proteins (Hu et al., 2015; Zhang et al., 2015). This receptor oligomerization mode has the potential to be highly sensitive to pathogenic invasion, as one NAIP recognizing presumably one ligand is capable of seeding the NLRC4 oligomer (Hu et al., 2015; Zhang et al., 2015).
AIM2 provides another example of direct PAMP detection by an inflammasome-stimulating PRR. AIM2 contains a HIN domain that is responsible for binding double stranded DNA (dsDNA) (Bürckstümmer et al., 2009; Fernandes-Alnemri et al., 2009; Hornung et al., 2009). This binding is cooperative and dependent on the length of the DNA, with at least 80 bp being necessary to trigger inflammasome assembly (Jin et al., 2012). AIM2 forms homo-oligomers and clusters along multivalent DNA (Lu et al., 2015; Morrone et al., 2015). AIM2 can oligomerize or form filaments independently of ligand at high concentrations (Morrone et al., 2015). This finding suggests that DNA interactions serve to increase the local concentration of AIM2 monomers to promote receptor nucleation for the AIM2 inflammasome with subsequent binding of the AIM2 pyrin domain to the pyrin domain of ASC. Indeed, when mixing AIM2 pyrin domain with the ASC pyrin domain in vitro, the AIM2 pyrin domain localized to the ends of helical filaments, consistent with the role of AIM2 in seeding ASC filaments in cells (Lu et al., 2014). AIM2 can respond to DNA that constitutes direct PAMP-recognition in the context of viral infections (Hornung et al., 2009). AIM2 can also detect bacterial DNA after lysis of bacteria in endosomes and subsequent release into the cytosol (Jones et al., 2010; Sauer et al., 2010). Mislocalized host DNA during treatment with the HIV protease inhibitor Nelfinavir can result in recognition of DNA by AIM2 in the cytosol (Di Micco et al., 2016). AIM2 can also sense mislocalized host DNA in mouse models of arthritis (Baum et al., 2015; Jakobs et al., 2015). Radiation therapy can cause inflammasome activation of cell types in the gut, independent of NLRP3 (Stoecklein et al., 2015). Interestingly, AIM2 responds to self-DNA (DAMPs) in response to radiation therapy, but this recognition may occur in the nucleus as opposed to the cytosol (Hu et al., 2016). Recent work proposes that the cGAS-STING pathway of cytosolic DNA recognition may intersect with NLRP3 activation (Gaidt et al., 2017; Swanson et al., 2017). These results suggest that multiple inflammasomes integrate the signal of mislocalized dsDNA. In the following sections, we will discuss additional strategies that PRRs use to assess threats. Rather than directly binding to PAMPs or DAMPs, the following examples detect changes in cell homeostasis that are indicative of a potential threat.
While the NLRP3 inflammasome is the most studied inflammasome to date, much confusion surrounds the signals that lead to receptor activity. With few exceptions (described below) NLRP3 is generally not considered a PRR that detects PAMPs or DAMPs directly, but is rather thought to respond indirectly to alterations in homeostasis. Examples of such homeostatic disruptions include mitochondrial dysfunction, membrane permeability, aberrant ion flux, or lysosomal destabilization (Swanson et al., 2019). The precise mechanism of NLRP3 activation is unclear but is linked to interactions with damaged mitochondrial membranes or the dispersed trans Golgi network (Chen and Chen, 2018; Iyer et al., 2013). However, some reports suggest NLRP3 colocalizes with DAMPs, such as oxidized mitochondrial DNA (mtDNA) or newly synthesized mtDNA (Shimada et al., 2012; Zhong et al., 2018). Therefore, the NLRP3 inflammasome might represent either ligand-directed receptor nucleation or host modification-directed receptor nucleation. As leucine rich repeats (LRRs) of TLRs mediate ligand binding, it has been assumed that the LRRs of NLRP3 are involved in activation or autoinhibition. A recent study refutes this assumption based on truncation mutants showing that the LRRs of NLRP3 are dispensable for inflammasome activity (Hafner-Bratkovič et al., 2018).
One major activating signal for the NLRP3 inflammasome is potassium efflux from the cytosol (Swanson et al., 2019). This efflux event may occur during phagocytosis of particulate antigens that can damage membranes, signaling of extracellular ATP through P2×7 receptors and subsequent opening of pannexin-1 channels, or direct action of bacterial pore forming proteins and potassium ionophores such as nigericin (Swanson et al., 2019). In other situations, reactive oxygen species (ROS) production, mitochondrial damage, or other metabolic abnormalities, such as hexokinase inactivation, can lead to both potassium-dependent and -independent NLRP3 activation (Swanson et al., 2019). Recent work implicates a requirement for the kinase NEK7 in NLRP3 activation (He et al., 2016; Schmid-Burgk et al., 2016). A direct interaction between NEK7 and NLRP3 occurs, which has been structurally defined (Sharif et al., 2019).
The NLRP1 and Pyrin inflammasomes represent a final mode of receptor proximal inflammasome activation, which involves pathogen effector- or host modification-directed assembly. Many bacterial and viral pathogens alter or reprogram cellular processes to their advantage through effector proteins. Human NLRP1 contains an N-terminal pyrin domain with a C-terminal extension harboring a CARD. Murine NLRP1b is missing the pyrin domain and contains only the C terminal CARD. As such, ASC is dispensable for NLRP1b activation of caspase-1 in mouse cells, though ASC oligomerizes in response to NLRP1 activation in human cells (Broz et al., 2010). Engineered NLRP1 that contains a TEV protease site demonstrates that proteolysis is sufficient to activate inflammasome signaling (Chavarria-Smith and Vance, 2013). Inhibition of the dipeptidases DPP8/9 can cause NLRP1 inflammasome activation in mouse and human cells (Okondo et al., 2017). DPP8/9 inhibition activates caspase-1-dependent cell death in human leukemia cell lines, but this study implicates another protein, known as CARD8, in seeding the inflammasome (Johnson et al., 2018). The proteasome is required for activation of the NLRP1 inflammasome, as inhibition of the proteasome blocks Lethal Toxin and DPP8/9 inhibitor induced NLRP1 activation (Okondo et al., 2018). Two recent reports confirm that proteosomal degradation of NLRP1 promotes NLRP1 inflammasome activation (Chui et al., 2019; Sandstrom et al., 2019). One group posits a model whereby an autoprocessed NLRP1 protein that has been cleaved within the FIIND domain, yet is still associated with the resulting N terminal fragment of NLRP1, may be relieved of this autoinhibitory interaction through proteasome degradation of the N terminal residues of NLRP1 (Sandstrom et al., 2019). Cleavage results in processive degradation of the fragment of NLRP1 that remains associated with the FIIND-CARD domain C terminal fragment, thus releasing the FIIND-CARD fragment to seed inflammasome assembly.
Pyrin senses abnormal modifications added to RhoA, a cytoskeleton-associated Rho GTPase. RhoA and Pyrin are not thought to directly interact (Xu et al., 2014). Instead Pyrin indirectly senses pathogen modifications of RhoA through sensing alterations in the homeostatic activities of RhoA. RhoA controls the activity of the kinases PKN1 and PKN2, which constitutively phosphorylate Pyrin to enforce interaction between Pyrin and the 14-3-3 proteins, 14-3-3ε and 14-3-3τ (Gao et al., 2016; Park et al., 2016). Interaction between 14-3-3 proteins and Pyrin prevent activation of the Pyrin inflammasome (Gao et al., 2016). Mutations that abrogate phosphorylation of Pyrin or otherwise inhibit Pyrin association with 14-3-3 proteins lead to activation of the Pyrin inflammasome (Gao et al., 2016; Masters et al., 2016; Park et al., 2016). Post-translational modifications of RhoA, such as addition of sugars, result in altered activity of RhoA (Xu et al., 2014). RhoA dysfunction can lead to inactivation of the kinases PKN1 and PKN2 (Park et al., 2016). Inactivation of PKN1 and PKN2 results in dephosphorylation of Pyrin (Gao et al., 2016; Park et al., 2016). Dephosphorylated Pyrin is relieved of 14-3-3 sequestration, which ultimately promotes oligomerization of the inflammasome through interactions with ASC and caspase-1 (Gao et al., 2016; Jéru et al., 2005; Masters et al., 2016).
The modes by which the inflammasome-seeding activity of NLRP1 and Pyrin are activated are distinct from how other PRRs detect PAMPs and DAMPs directly. By acting as a bait molecule for proteolytic cleavage by bacterial effectors or possibly host proteases, NLRP1 is poised to sense activities associated with pathogens that threaten the host without directly sensing a molecule encoded by the threat itself. Similarly, by sensing alterations in RhoA GTPase activity, the Pyrin inflammasome indirectly senses host modification by microbes to contextualize pathogenic invasion. Thus, we can categorize threat assessment by PRRs into two modes of activation: 1) PAMP- or DAMP-directed activity that represents direct recognition of ligands in the cytosol, and 2) effector/host-alteration directed activity that represents indirect recognition of threats to the host (Figure 3). Thus, molecular patterns that activate PRRs can be considered in classical terms of protein-ligand interactions, but this definition of “pattern” can be expanded to include indicators of altered host pathways and homeostatic programs.
Effector functions of inflammasomes – cell fate decisions between pyroptosis and hyperactivation
Thus far, we have discussed themes and mechanisms that govern inflammasome assembly. We will now discuss the consequences of inflammasome assembly on cellular and host physiology. Two consequences occur in a context-dependent manner.
One consequence of inflammasome assembly is the caspase-dependent induction of pyroptosis. Pyroptosis is associated with the rupture of the plasma membrane and the release of cytosolic content into the extracellular media (Figure 4). IL-1 family cytokines, such as IL-1β and IL-18, represent notable examples of released cytosolic content. Because pyroptosis is a lethal event to the cell, the threat must be at its highest level for this cell fate commitment to be made. Examples of such heightened threats include the presence of PAMPs in the cytosol (Hagar et al., 2013; Kayagaki et al., 2013). The commitment to pyroptosis under these encounters is therefore warranted, as the host must do all it can to limit pathogen replication and spread. The coincident death of the cell and release of IL-1β should accomplish both goals, as IL-1 signaling to neighboring cells induces a massive inflammatory response that should contain and resolve the infection (Garlanda et al., 2013).
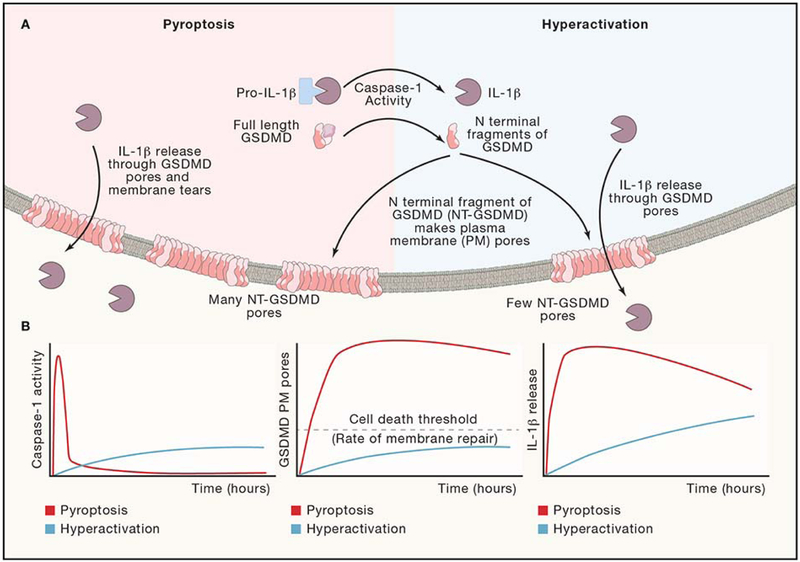
Figure 4: Cell Fate Decisions after Inflammasome Activation.
(A) Inflammasome activation can lead to the cell fates of “hyperactivation” or “pyroptosis” depending on the cell-type and stimulation. Magnitude and kinetics of caspase-1 activation may affect the rates and quantity of GSDMD cleaved, leading to different magnitude and kinetics of GSDMD pore residency on the plasma membrane. Membrane repair processes serve to remove GSDMD pores from the plasma membrane. (B) Theoretical magnitudes and kinetics of caspase-1 activity, GSDMD cleavage, and IL-1β release for pyroptotic and hyperactive cell fates. Caspase-1 curves are extrapolated based on a comparison of hyperactive neutrophils and pyroptotic macrophages treated with the same stimulus (Boucher et al., 2018). GSDMD pore curves are extrapolated based on a membrane permeability level of hyperactive macrophages treated with oxidized phospholipids or infected with ΔoatA S. aureus compared to pyroptotic macrophages treated with nigericin or FlaTox (a flagellin, anthrax lethal toxin fusion protein) (Evavold et al., 2018). IL-1β release curves are extrapolated based on comparison of hyperactive macrophages treated with oxidized phospholipids and pyroptotic macrophages treated with ATP (Zanoni et al., 2017).
In recent years, it has become clear that not all cells commit to pyroptosis once an inflammasome has been assembled. Indeed, certain cells and stimuli result in the inflammasome-dependent release of IL-1β from living cells (Chen et al., 2014; Gaidt et al., 2016; Wolf et al., 2016; Zanoni et al., 2016). Typically, cells will couple the expression of a given cytokine to its secretion, but the requirement of caspase-1 for IL-1β secretion renders cells capable of IL-1 release only after inflammasomes are assembled (Garlanda et al., 2013). Thus, when cells are exposed to TLR signals, they secrete many cytokines, chemokines and interferons, but they do not secrete the cytosolic IL-1 family members IL-1β and IL-18 (Garlanda et al., 2013; Iwasaki and Medzhitov, 2010). Under conditions of inflammasome assembly that do not result in pyroptosis, IL-1 family members can be added to the repertoire of cytokines that are secreted. This addition of IL-1β to the secreted repertoire results in an enhanced ability to stimulate inflammation compared to cells that do not contain inflammasomes (Zanoni et al., 2016). Moreover, based on the importance of IL-1 in adaptive immunity, the ability of inflammasomes to add IL-1β to the secreted repertoire endows cells with an enhanced ability to stimulate antigen-specific T cell responses (Zanoni et al., 2016). As such, cells that use inflammasomes to secrete IL-1β while maintaining viability are considered hyperactive (Figure 4), in contrast to their dead (pyroptotic) or traditionally activated counterparts (Evavold et al., 2018; Zanoni et al., 2016; 2017).
The stimuli that induce cell hyperactivation are only beginning to emerge, but examples include PAMPs and DAMPs. One such DAMP that induces cell hyperactivation is a set of oxidized lipids released from dying cells that are collectively known as oxPAPC (Evavold et al., 2018; Zanoni et al., 2016; 2017). oxPAPC is the collective term for a pool of oxidation products of the lipid species 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine (PAPC). This pool of lipids is recognized as the inflammatory lipid species in atherosclerosis-associated oxidized LDL, and ROS production and cell death processes can generate oxPAPC from cellular membranes (Freigang, 2016). These lipids are not capable of inducing inflammasome assembly when encountered in isolation and in fact display immunosuppressive activities (Bochkov et al., 2002). But when combined with TLR ligands, oxPAPC induces NLRP3-dependent IL-1β release from living cells (Zanoni et al., 2016). oxPAPC components are captured from the extracellular space by CD14 (Zanoni et al., 2017). Subsequent CD14-dependent endocytosis delivers these lipids to intracellular caspase-11, which somehow leads to NLRP3-dependent inflammasome assembly and IL-1β release from living cells (Zanoni et al., 2016; 2017). Notably, different lipid components of oxPAPC are capable of hyperactivating macrophages and dendritic cells (Yeon et al., 2017; Zanoni et al., 2017).
Similar to the DAMP oxPAPC, PAMPs can hyperactivate phagocytes (Gaidt et al., 2016; Wolf et al., 2016). One example derives from the aforementioned studies of how human monocytes respond to LPS in the extracellular space (Gaidt et al., 2016; Viganò et al., 2015). This response results in NLRP3-dependent assembly of an inflammasome that promotes IL-1β release from living cells. The N-acetylglucosamine (NAG) sugar moiety of bacterial peptidoglycan (PGN) can also hyperactivate macrophages, promoting IL-1β release without defects in cell viability (Wolf et al., 2016). The process by which NAG stimulates inflammasome activities involves the inactivation of the metabolic regulator hexokinase, which leads to NLPR3-dependent IL-1β release from living cells (Wolf et al., 2016). Similar findings were made with whole bacteria, as macrophages infected with Staphylococcus aureus secrete IL-1β in the absence of cell death (Wolf et al., 2016). Destabilizing PGN through genetic inactivation of the bacterial enzyme O-acetyl transferase results in increased NAG release into the cytosol and cell hyperactivation (Wolf et al., 2016).
While the fate of hyperactive and pyroptotic cells differs in terms of viability, both cell fates result in release of active IL-1 family cytokines, such as IL-1β and IL-18, by a process dependent on plasma membrane localized pores (Evavold et al., 2018; Heilig et al., 2018). Below we will describe mechanisms governing these cell fate decisions and how IL-1β synthesis, cleavage, and secretion are regulated.
Major targets of inflammasome-associated caspase-1 are the IL-1 family cytokines pro-IL-1β and pro-IL-18 (Garlanda et al., 2013). As described earlier, these cytokines are translated in the cytosol of cells and do not contain an N-terminal signal peptide for trafficking through the biosynthetic pathway (Garlanda et al., 2013). Instead, the secretion of these cytokines is tightly correlated to inflammasome activation. Caspase-1 cleaves off the pro-domain yielding the bioactive, mature species of IL-1β and IL-18. This cleavage event may promote secretion of these cytokines by changing the isoelectric point of the protein (Monteleone et al., 2018). The pro-domains of IL-1β and IL-18 exhibit an overall negative charge, but the residues corresponding to the bioactive species are positively charged. Cleavage by caspase-1 releases the pro-domain, leaving the positively charged bioactive species (Monteleone et al., 2018). This positive charge enforces localization at the negatively charged inner leaflet of the plasma membrane, thus positioning these cytokines for subsequent release from the cell (Monteleone et al., 2018).
The mechanism of IL-1β and IL-18 release from cells has recently been defined with the identification of another caspase-1 substrate, the cytosolic protein GSDMD (Kayagaki et al., 2015; Shi et al., 2015a). GSDMD is cleaved by caspase-1 to yield an N terminal and C terminal fragment (Kayagaki et al., 2015; Shi et al., 2015a). The N terminal fragment has affinity for acidic phosphoinositides, phosphatidylserine, and cardiolipin (Ding et al., 2016; Liu et al., 2016). When the N terminal fragment binds these lipids, oligomers can form structures that resemble pores that traverse the lipid bilayer (Ding et al., 2016; Liu et al., 2016). Genetic analysis in macrophages and in vitro liposome-based studies demonstrate that these pores serve as conduits for the release of IL-1α and IL-1β from macrophages and IL-1β and IL-18 across intact lipid bilayers (Evavold et al., 2018). These findings have been extended to include a genetic requirement for GSDMD in secretion of IL-1β and IL-18 from primary murine macrophages and IL-1β from dendritic cells and neutrophils (Heilig et al., 2018). GSDMD pores are therefore considered mediators of the direct secretion of several IL-1 family members from the cytosol into the extracellular space. GSDMD-dependent IL-1β release can be inhibited by treatment with the drug necrosulfonamide, which covalently reacts with a crucial cysteine residue within the N terminus of GSDMD (Rathkey et al., 2018). Notably, the process of GSDMD pore formation is necessary for both cellular activation states, pyroptosis and hyperactivation, as GSDMD-deficient cells are unable to acutely release IL-1β or lyse in response to inflammasome stimuli (Evavold et al., 2018; Heilig et al., 2018; Kayagaki et al., 2015; Shi et al., 2015a).
How the same protein GSDMD can promote the release of IL-1β from living and dead cells is unclear, but it may be linked to the finding that hyperactive cells display evidence of fewer pores at their surface than pyroptotic cells (Evavold et al., 2018). It is possible that a cell can tolerate the presence of some GSDMD pores, which would be removed by ESCRT-mediated membrane repair events. An excessive number of pores may overwhelm the repair machinery and lead to pyroptosis, as has been demonstrated for the host pore forming protein MLKL (Gong et al., 2017). Consistent with this idea is a recent study demonstrating that the rate of pyroptosis increases in cells that are defective for ESCRT-mediated membrane repair (Rühl et al., 2018).
In addition to caspase-1, caspase-8 can cleave GSDMD to facilitate cytokine release (Orning et al., 2018; Sarhan et al., 2018). During Salmonella infection of murine macrophages, caspase-8 can be recruited to the inflammasome (Man et al., 2013). Recruitment of caspase-8 requires interactions between pyrin domain of filamentous ASC with the death effector domain (DED) of caspase-8 (Vajjhala et al., 2015). Human and porcine monocytes can activate caspase-8 downstream of TLR4 recognition of LPS (Gaidt et al., 2016). These cell types require involvement of the NLRP3 inflammasome for cleavage and release of IL-1β, but it is unclear whether GSDMD or other gasdermin family members mediate release of IL-1β. The adaptor protein FADD and caspase-8 promote priming upstream of canonical and non-canonical inflammasome signaling (Gurung et al., 2014). Stimulation of the death receptor Fas in dendritic cells results in caspase-8 activation and IL-1β release (Bossaller et al., 2012). Executioner apoptotic caspases (caspase-3/−7) inactivate GSDMD through cleavage at an aspartic residue within the N terminal pore-forming domain (Chen et al., 2019; Taabazuing et al., 2017). In Yersinia infection, caspase-8 can activate caspase-1 to mediate cell death (Philip et al., 2014). Recent work has extended this finding to reveal inhibition of TAK1 results in caspase-8 mediated cleavage of GSDMD and cell death (Orning et al., 2018; Sarhan et al., 2018).
Murine caspase-11 (and human caspase-4 and −5) cleave GSDMD, but not IL-1β (Kayagaki et al., 2015; Shi et al., 2015a). Caspase-11 has been associated with caspase-1 activation (Wang et al., 1998). IL-1β release after activation of caspase-11 in response to LPS, parasitic lipophosphoglycan (LPG), or oxPAPC requires NLRP3 (de Carvalho et al., 2019; Hagar et al., 2013; Kayagaki et al., 2013; Zanoni et al., 2016). While mechanisms of internalization of LPS and oxidized lipids have been described, such as CD14-dependent endocytosis of purified LPS and oxidized lipids, HMGB1-RAGE-dependent endocytosis of LPS, clathrin-mediated endocytosis of bacterial outer membrane vesicles (OMVs), or phagocytosis of whole bacteria, the machinery involved in translocation of LPS or oxidized lipids from the endosomal lumen into the cytosol remain elusive (Deng et al., 2018; Vanaja et al., 2016; Zanoni et al., 2011). LPS was originally thought to mediate oligomerization of caspase-11 monomers into a high molecular weight species (Shi et al., 2015b), but a recent study suggests that this high molecular weight species represents interactions between caspase-11 monomers and LPS micelles (Wacker et al., 2017). In the context of LPS-caspase-11 interactions, GSDMD is required for activation of the NLRP3 inflammasome and IL-1β release (Kayagaki et al., 2015; Rühl and Broz, 2015; Shi et al., 2015a). This process is potassium-dependent and likely results from GSDMD pore formation upstream of potassium efflux-dependent NLRP3 activation (Rühl and Broz, 2015). oxPAPC-caspase-11 interactions activate the NLRP3 inflammasome in a potassium-independent manner (Zanoni et al., 2016). Consistent with this model, GSDMD is not required for IL-1β cleavage, but is required for IL-1β secretion (Evavold et al., 2018). Parasite derived LPG does not directly bind or activate caspase-11, but genetically requires caspase-11 for optimal IL-1β secretion (de Carvalho et al., 2019). This finding suggests that an intermediary protein or host molecule links LPG recognition to activation of caspase-11.
Cell type-dependent activities of inflammasomes
The majority of inflammasome biology literature has focused on cells of myeloid origin. Of these cell types, neutrophils seem the most resistant to pyroptosis (Chen et al., 2014). This may result from increased expression of machinery involved in membrane repair processes. Like the processes of pyroptosis and hyperactivation in macrophages and dendritic cells, GSDMD is also required for rapid IL-1β secretion by neutrophils (Heilig et al., 2018). These cells use GSDMD for normal cell death and turnover in vivo (Kambara et al., 2018). Neutrophils also require GSDMD to execute a cell-type specific cell death program known as NETosis (Chen et al., 2018; Sollberger et al., 2018). The requirement for GSDMD in neutrophils for non-lytic IL-1β release, routine non-inflammatory cell death turnover, and the specialized cell death program of NETosis mirrors the multifunctional role of GSDMD pores in the macrophage and dendritic cell fates of hyperactivation and pyroptosis.
These findings may argue that the kinetics of caspase activation and thus GSDMD cleavage play important roles in determining the fate of a neutrophil. Consistent with this idea, caspase-1 activation kinetics have been determined for neutrophils and macrophages treated with the same inflammasome triggers. In pyroptotic macrophages, caspase-1 is maximally activated at acute time points that correlate with maximal cleavage of cell-associated GSDMD (Boucher et al., 2018). This high level of acute activation may flood the cell with GSDMD pores that occur too quickly for membrane repair to resolve, thus leading to pyroptosis (Figure 4). In contrast, neutrophils treated with inflammasome activators had evidence of a lower quantity of activated caspase-1 that was sustained for a longer duration (Boucher et al., 2018). These results suggest that the chronic, low activation of inflammatory caspases can lead to transient pore formation based on lower quantities of cleaved GSDMD. Cells that have lower pore burden at the plasma membrane could activate programs of membrane repair that serve to delay or prevent death, so the cell can accomplish other energetic and inflammatory activities (Rühl et al., 2018).
Like neutrophils, dendritic cells may be more resistant to lytic stimuli than macrophages (Yoon et al., 2017). These results suggest similar mechanisms influence the decision between hyperactivation and pyroptosis in these cell types. A recent study suggests that the heterogeneous nature of GM-CSF dendritic cell cultures may affect the proportion of cells able to respond to inflammasome activators (Erlich et al., 2019). Future studies should validate these conclusions using other dendritic cell sources.
Non-phagocytic cells, such as endothelial cells, epithelial cells, and T cells can also assemble inflammasomes. Endothelial cells undergo pyroptosis in response to LPS in mouse models of sepsis (Cheng et al., 2017; Deng et al., 2018). Endothelial cells undergo inflammasome activation and pyroptosis during hemorrhagic shock (Xiang et al., 2011). Certain gut epithelial cells known as goblet cells require the PRR NLRP6, ASC, and caspase-1 to secrete mucin (Wlodarska et al., 2014). Impaired secretion of mucin in mice lacking NLRP6 inflammasome components results in defects in the physical and chemical mucus barrier in the gut leading to susceptibility to infection. Gut epithelial cells also express the inflammasome seed NLRP9b. Global NLRP9b deficiency results in susceptibility to rotavirus infection, and conditional deletion in intestinal epithelial cells allowed for enhanced rotavirus replication (Zhu et al., 2017). The NLRC4 inflammasome in gut epithelia mediates extrusion of infected enterocytes during bacterial infection (Rauch et al., 2017). Many cell types, including epithelial cells and lymphocytes, in the gut appear sensitive to radiation therapy and activate pyroptosis in an AIM2-dependent manner (Hu et al., 2016; Stoecklein et al., 2015). In addition, within T cells, the PRR IFI16 stimulates inflammasome activity in response to abortive HIV infection in human tonsil extracts (Monroe et al., 2014). T cells also form non-lytic inflammasomes that may provide autocrine IL-1 signals that may enforce differentiation into pathogenic Th17 lineages during autoimmunity (Martin et al., 2016). T cell-intrinsic inflammasome collaboration with complement receptor signaling may also serve to enforce the Th1 lineage in human T cells (Arbore et al., 2016). More studies are required to verify the existence of cell types outside the myeloid lineage that respond to inflammasome triggers, but current reports suggest roles for both lytic and non-lytic inflammasomes within these cell types.
Anti-inflammatory signaling and inflammasomes
Some PRR and cytokine receptor pathways can have antagonistic effects on inflammasome signaling. Prolonged treatment of cells with IFNγ, IFNβ, or LPS can result in nitric oxide (NO) production that negatively regulates the NLRP3 inflammasome (Hernandez-Cuellar et al., 2012; Mishra et al., 2013). IL-10 is another cytokine that has been shown to inhibit the NLRP3 inflammasome. IL-10 signaling on myeloid cells may inhibit inflammasome signaling through preservation of mitochondria and metabolism (Ip et al., 2017). Autophagy can be selective in the case of damaged organelles or invading microbes or non-selective in the case of starvation-induced general catabolism of cytosolic components. Selective autophagy of mitochondria (mitophagy) may indirectly reduce the propensity for NLRP3 oligomers to form by removing membranous scaffolds such as exposed cardiolipin or activating ligands such as oxidized mtDNA and newly synthesized mtDNA. Autophagy may regulate inflammasome signaling by capturing NLRP3 inflammasome oligomers (Shi et al., 2012). Autophagy may degrade the inflammasome substrate pro-IL-1β (Harris et al., 2011). Related metabolic pathways negatively regulate inflammasome signaling. For example, increased concentrations of the secondary messenger cAMP leads to inhibition of inflammasome signaling (Lee et al., 2012; Yan et al., 2015). cAMP binding to NLRP3 leads to NLRP3 ubiquitination by the E3 ubiquitin ligase MARCH7 (Yan et al., 2015). The mTOR pathway also regulates the NLRP3 inflammasome, as inhibition of Raptor/mTORC leads to decreased caspase-1 activation and IL-1β release (Moon et al., 2015). Some PRRs may serve to directly or indirectly inhibit inflammasome signaling. In the example of the AIM2 inflammasome, the HIN containing protein p202 counteracts AIM2 oligomerization and inflammasome signaling (Roberts et al., 2009). As both of these proteins contain HIN domains that bind DNA in sequence-independent manner, it is likely that p202 interrupts homo-oligomerization of AIM2 along the same dsDNA, potentially through tetramerization and increased binding affinity for dsDNA (Ru et al., 2013; Yin et al., 2013). IFI16 may also act as a negative regulator of the AIM2 inflammasome through a similar mechanism of competition for dsDNA ligand (Wang et al., 2018).
Perspectives
Herein, we have described the current state of the inflammasome literature with the goal of illustrating these SMOCs as signaling organelles that respond to various threats to the host. The literature mandates that we do not consider inflammasomes solely in the context of infection, as inflammasomes serve important roles in the context of non-infectious tissue injury or disruption of homeostasis. This broad view not only explains much of the findings made in infectious or non-infectious settings on the role of inflammasomes, but also highlights the important gaps in our knowledge. We are far from providing unifying views (or mechanistic views) of many aspects of inflammasome biology. As such, we expect that interest in this area will remain robust.
In the future, we expect several biological questions will reach the forefront of inquiry. For example, we have a remedial understanding of how cell fate decisions are made once inflammasomes are assembled. Inflammasome assembly results in pyroptosis in some contexts and hyperactivation in other contexts. Current inflammasome research supports the notion that pyroptosis is a common downstream consequence of inflammasome signaling after strong stimulations in vitro. Whether these in vitro stimulations correspond to physiological settings or reflect in vivo inflammasome activities remains largely unexplored.
Active trafficking events are required for inflammasome formation, and energetically intensive activities, such as membrane repair, are constantly repressing pyroptotic cell fate in cells with active inflammasomes (Gao et al., 2016; Li et al., 2017; Misawa et al., 2013; Park et al., 2016; Rühl et al., 2018). Mitochondrial dysfunction may also occur in response to inflammasome activation or may serve as a molecular cue to form the inflammasome (Swanson et al., 2019). Therefore, it is likely that host metabolism will influence the cell fate decision between hyperactivation and pyroptosis in vitro and in vivo. In support of this hypothesis, a recent paper suggests that the NAD depleting TIR domain containing protein SARM1 mediates pyroptosis and enforces energetic cell death after inflammasome activators (Carty et al., 2019). Moreover, cells deficient in SARM1 display features of hyperactive cells as they secrete IL-1β in the absence of lysis. These cells contain cleaved GSDMD and have permeabilized membranes, but maintain mitochondrial polarization. This study finds that naturally hyperactivating stimuli, such as PGN treatment, show maintenance of mitochondrial activity irrespective of SARM1. The interplay between metabolism and inflammasome signaling in mediating the cell fate decisions between pyroptosis and hyperactivation merits further investigation.
One of the challenges with addressing these questions is that we are largely limited to population-based assays. Indeed, much of our knowledge regarding SMOC formation, GSDMD cleavage, and IL-1β cleavage and release from cells is based on population assays. Development of tools to monitor inflammasome signaling within single cells is still needed. Some tools have been developed (Nagar et al., 2018; Tzeng et al., 2016). Once these tools (and others) become more widespread in use, a detailed analysis of the mechanisms and consequences of inflammasome activity can be explored.
Highlights.
The innate immune system operates as a threat assessment station
Threats are detected by proteins that seed the assembly of inflammasomes
Inflammasomes drive interleukin-1 release from living or dead cells
Inflammasome activities influence local and systemic immune responses
Acknowledgements
We thank members of the Kagan lab for helpful discussions and to Isabella Fraschilla for thoughtful reading of the manuscript. We apologize to members of the community whose studies were not cited herein due to space constraints. This study was supported by NIH grants AI133524, AI093589, AI116550, and P30DK34854 to J.C.K. and AI138369 to C.L.E. J.C.K. holds an Investigators in the Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund. C.L.E. is supported by the Harvard Herchel Smith and Landry Fellowships.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
J.C.K. holds is a member of the scientific advisory board at IFM therapeutics and holds a patent on the therapeutic potential of hyperactivating stimuli (PCT/US2016/012994).
References
- Arbore G, West EE, Spolski R, Robertson AAB, Klos A, Rheinheimer C, Dutow P, Woodruff TM, Yu ZX, O’Neill LA, et al. (2016). T helper 1 immunity requires complement-driven NLRP3 inflammasome activity in CD4⁺ T cells. Science 352, aad1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala JM, Yamin TT, Egger LA, Chin J, Kostura MJ, and Miller DK (1994). IL-1 beta-converting enzyme is present in monocytic cells as an inactive 45-kDa precursor. The Journal of Immunology 153, 2592–2599. [PubMed] [Google Scholar]
- Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, et al. (2009). Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol 183, 787–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum R, Sharma S, Carpenter S, Li Q-Z, Busto P, Fitzgerald KA, Marshak-Rothstein A, and Gravallese EM (2015). Cutting edge: AIM2 and endosomal TLRs differentially regulate arthritis and autoantibody production in DNase II-deficient mice. J. Immunol 194, 873–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blander JM, and Sander LE (2012). Beyond pattern recognition: five immune checkpoints for scaling the microbial threat. Nature Publishing Group 12, 215–225. [DOI] [PubMed] [Google Scholar]
- Bochkov VN, Kadl A, Huber J, Gruber F, Binder BR, and Leitinger N (2002). Protective role of phospholipid oxidation products in endotoxin-induced tissue damage. Nature 419, 77–81. [DOI] [PubMed] [Google Scholar]
- Bossaller L, Chiang P-I, Schmidt-Lauber C, Ganesan S, Kaiser WJ, Rathinam VAK, Mocarski ES, Subramanian D, Green DR, Silverman N, et al. (2012). Cutting edge: FAS (CD95) mediates noncanonical IL-1β and IL-18 maturation via caspase-8 in an RIP3-independent manner. J. Immunol 189, 5508–5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher D, Monteleone M, Coll RC, Chen KW, Ross CM, Teo JL, Gomez GA, Holley CL, Bierschenk D, Stacey KJ, et al. (2018). Caspase-1 self-cleavage is an intrinsic mechanism to terminate inflammasome activity. J. Exp. Med. jem 20172222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broz P, Moltke von, J., Jones JW, Vance RE, and Monack DM (2010). Differential requirement for Caspase-1 autoproteolysis in pathogen-induced cell death and cytokine processing. Cell Host and Microbe 8, 471–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brubaker SW, Bonham KS, Zanoni I, and Kagan JC (2015). Innate immune pattern recognition: a cell biological perspective. Annu. Rev. Immunol 33, 257–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan NB, Dorfleutner A, Rojanasakul Y, and Stehlik C (2009). Activation of inflammasomes requires intracellular redistribution of the apoptotic speck-like protein containing a caspase recruitment domain. J. Immunol 182, 3173–3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant CE, Gay NJ, Heymans S, Sacre S, Schaefer L, and Midwood KS (2015). Advances in Toll-like receptor biology: Modes of activation by diverse stimuli. Crit. Rev. Biochem. Mol. Biol 50, 359–379. [DOI] [PubMed] [Google Scholar]
- Bürckstümmer T, Baumann C, Blüml S, Dixit E, Dürnberger G, Jahn H, Planyavsky M, Bilban M, Colinge J, Bennett KL, et al. (2009). An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol 10, 266–272. [DOI] [PubMed] [Google Scholar]
- Cai X, Chen J, Xu H, Liu S, Jiang Q-X, Halfmann R, and Chen ZJ (2014). Prion-like polymerization underlies signal transduction in antiviral immune defense and inflammasome activation. Cell 156, 1207–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carty M, Kearney J, Shanahan KA, Hams E, Sugisawa R, Connolly D, Doran CG, Muñoz-Wolf N, Gürtler C, Fitzgerald KA, et al. (2019). Cell Survival and Cytokine Release after Inflammasome Activation Is Regulated by the Toll-IL-1R Protein SARM. Immunity 50, 1412–1424.e1416. [DOI] [PubMed] [Google Scholar]
- Case CL, and Roy CR (2011). Asc modulates the function of NLRC4 in response to infection of macrophages by Legionella pneumophila. MBio 2, e00117–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae JJ, Cho Y-H, Lee G-S, Cheng J, Liu PP, Feigenbaum L, Katz SI, and Kastner DL (2011). Gain-of-function Pyrin mutations induce NLRP3 protein-independent interleukin-1β activation and severe autoinflammation in mice. Immunity 34, 755–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavarria-Smith J, and Vance RE (2013). Direct proteolytic cleavage of NLRP1B is necessary and sufficient for inflammasome activation by anthrax lethal factor. PLoS Pathog 9, e1003452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavarria-Smith J, Mitchell PS, Ho AM, Daugherty MD, and Vance RE (2016). Functional and Evolutionary Analyses Identify Proteolysis as a General Mechanism for NLRP1 Inflammasome Activation. PLoS Pathog 12, e1006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, and Chen ZJ (2018). PtdIns4P on dispersed trans-Golgi network mediates NLRP3 inflammasome activation. Nature 564, 71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KW, Demarco B, Heilig R, Shkarina K, Boettcher A, Farady CJ, Pelczar P, and Broz P (2019). Extrinsic and intrinsic apoptosis activate pannexin-1 to drive NLRP3 inflammasome assembly. Embo J. e101638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KW, Groß CJ, Sotomayor FV, Stacey KJ, Tschopp J, Sweet MJ, and Schroder K (2014). The neutrophil NLRC4 inflammasome selectively promotes IL-1β maturation without pyroptosis during acute Salmonella challenge. CellReports 8, 570–582. [DOI] [PubMed] [Google Scholar]
- Chen KW, Monteleone M, Boucher D, Sollberger G, Ramnath D, Condon ND, Pein von, J.B., Broz P, Sweet MJ, and Schroder K (2018). Noncanonical inflammasome signaling elicits gasdermin D-dependent neutrophil extracellular traps. Sci Immunol 3, eaar6676. [DOI] [PubMed] [Google Scholar]
- Cheng KT, Xiong S, Ye Z, Hong Z, Di A, Tsang KM, Gao X, An S, Mittal M, Vogel SM, et al. (2017). Caspase-11-mediated endothelial pyroptosis underlies endotoxemia-induced lung injury. J. Clin. Invest 127, 4124–4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng TC, Hong C, Akey IV, Yuan S, and Akey CW (2016). A near atomic structure of the active human apoptosome. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chui AJ, Okondo MC, Rao SD, Gai K, Griswold AR, Johnson DC, Ball DP, Taabazuing CY, Orth EL, Vittimberga BA, et al. (2019). N-terminal degradation activates the NLRP1B inflammasome. Science 364, 82–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carvalho RVH, Andrade WA, Lima-Junior DS, Dilucca M, de Oliveira CV, Wang K, Nogueira PM, Rugani JN, Soares RP, Beverley SM, et al. (2019). Leishmania Lipophosphoglycan Triggers Caspase-11 and the Non-canonical Activation of the NLRP3 Inflammasome. CellReports 26, 429–437.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng M, Tang Y, Li W, Wang X, Zhang R, Zhang X, Zhao X, Liu J, Tang C, Liu Z, et al. (2018). The Endotoxin Delivery Protein HMGB1 Mediates Caspase-11-Dependent Lethality in Sepsis. Immunity 49, 740–753.e747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Micco A, Frera G, Lugrin J, Jamilloux Y, Hsu E-T, Tardivel A, De Gassart A, Zaffalon L, Bujisic B, Siegert S, et al. (2016). AIM2 inflammasome is activated by pharmacological disruption of nuclear envelope integrity. Proc. Natl. Acad. Sci. U.S.a 113, E4671–E4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Wang K, Liu W, She Y, Sun Q, Shi J, Sun H, Wang D-C, and Shao F (2016). Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 535, 111–116. [DOI] [PubMed] [Google Scholar]
- Elliott EI, Miller AN, Banoth B, Iyer SS, Stotland A, Weiss JP, Gottlieb RA, Sutterwala FS, and Cassel SL (2018). Cutting Edge: Mitochondrial Assembly of the NLRP3 Inflammasome Complex Is Initiated at Priming. J. Immunol 200, 3047–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlich Z, Shlomovitz I, Edry-Botzer L, Cohen H, Frank D, Wang H, Lew AM, Lawlor KE, Zhan Y, Vince JE, et al. (2019). Macrophages, rather than DCs, are responsible for inflammasome activity in the GM-CSF BMDC model. Nat Immunol 1. [DOI] [PubMed] [Google Scholar]
- Evavold CL, and Kagan JC (2018). How Inflammasomes Inform Adaptive Immunity. Journal of Molecular Biology 430, 217–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evavold CL, Ruan J, Tan Y, Xia S, Wu H, and Kagan JC (2018). The Pore-Forming Protein Gasdermin D Regulates Interleukin-1 Secretion from Living Macrophages. Immunity 48, 35–44.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes-Alnemri T, Wu J, Yu J-W, Datta P, Miller B, Jankowski W, Rosenberg S, Zhang J, and Alnemri ES (2007). The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 14, 1590–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes-Alnemri T, Kang S, Anderson C, Sagara J, Fitzgerald KA, and Alnemri ES (2013). Cutting edge: TLR signaling licenses IRAK1 for rapid activation of the NLRP3 inflammasome. J. Immunol 191, 3995–3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes-Alnemri T, Yu J-W, Datta P, Wu J, and Alnemri ES (2009). AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 458, 509–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchi L, Eigenbrod T, and Núñez G (2009). Cutting edge: TNF-alpha mediates sensitization to ATP and silica via the NLRP3 inflammasome in the absence of microbial stimulation. J. Immunol 183, 792–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freigang S (2016). The regulation of inflammation by oxidized phospholipids. Eur. J. Immunol 46, 1818–1825. [DOI] [PubMed] [Google Scholar]
- Gaidt MM, Ebert TS, Chauhan D, Ramshorn K, Pinci F, Zuber S, O’Duill F, Schmid-Burgk JL, Hoss F, Buhmann R, et al. (2017). The DNA Inflammasome in Human Myeloid Cells Is Initiated by a STING-Cell Death Program Upstream of NLRP3. Cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaidt MM, Ebert TS, Chauhan D, Schmidt T, Schmid-Burgk JL, Rapino F, Robertson AAB, Cooper MA, Graf T, and Hornung V (2016). Human Monocytes Engage an Alternative Inflammasome Pathway. Immunity 44, 833–846. [DOI] [PubMed] [Google Scholar]
- Gao W, Yang J, Liu W, Wang Y, and Shao F (2016). Site-specific phosphorylation and microtubule dynamics control Pyrin inflammasome activation. Proc. Natl. Acad. Sci. U.S.a 113, E4857–E4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlanda C, Dinarello CA, and Mantovani A (2013). The interleukin-1 family: back to the future. Immunity 39, 1003–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y-N, Guy C, Olauson H, Becker JU, Yang M, Fitzgerald P, Linkermann A, and Green DR (2017). ESCRT-III Acts Downstream of MLKL to Regulate Necroptotic Cell Death and Its Consequences. Cell 169, 286–300.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurung P, Anand PK, Malireddi RKS, Vande Walle L, Van Opdenbosch N, Dillon CP, Weinlich R, Green DR, Lamkanfi M, and Kanneganti T-D (2014). FADD and caspase-8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes. J. Immunol 192, 1835–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner-Bratkovič I, Sušjan P, Lainšček D, Tapia-Abellán A, Cerović K, Kadunc L, Angosto-Bazarra D, Pelegrίn P, and Jerala R (2018). NLRP3 lacking the leucine-rich repeat domain can be fully activated via the canonical inflammasome pathway. Nature Communications 9, 5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagar JA, Powell DA, Aachoui Y, Ernst RK, and Miao EA (2013). Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science 341, 1250–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J, Hartman M, Roche C, Zeng SG, O’Shea A, Sharp FA, Lambe EM, Creagh EM, Golenbock DT, Tschopp J, et al. (2011). Autophagy controls IL-1beta secretion by targeting pro-IL-1beta for degradation. J. Biol. Chem 286, 9587–9597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Zeng MY, Yang D, Motro B, and Núñez G (2016). NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature 530, 354–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig R, Dick MS, Sborgi L, Meunier E, Hiller S, and Broz P (2018). The Gasdermin-D pore acts as a conduit for IL-1β secretion in mice. Eur. J. Immunol 48, 584–592. [DOI] [PubMed] [Google Scholar]
- Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, Griep A, Axt D, Remus A, Tzeng T-C, et al. (2013). NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 493, 674–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Cuellar E, Tsuchiya K, Hara H, Fang R, Sakai S, Kawamura I, Akira S, and Mitsuyama M (2012). Cutting edge: nitric oxide inhibits the NLRP3 inflammasome. J. Immunol 189, 5113–5117. [DOI] [PubMed] [Google Scholar]
- Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, and Fitzgerald KA (2009). AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458, 514–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Jin C, Li H-B, Tong J, Ouyang X, Cetinbas NM, Zhu S, Strowig T, Lam FC, Zhao C, et al. (2016). The DNA-sensing AIM2 inflammasome controls radiation-induced cell death and tissue injury. Science 354, 765–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Zhou Q, Zhang C, Fan S, Cheng W, Zhao Y, Shao F, Wang H-W, Sui S-F, and Chai J (2015). Structural and biochemical basis for induced self-propagation of NLRC4. Science 350, 399–404. [DOI] [PubMed] [Google Scholar]
- Ip WKE, Hoshi N, Shouval DS, Snapper S, and Medzhitov R (2017). Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Science 356, 513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki A, and Medzhitov R (2010). Regulation of adaptive immunity by the innate immune system. Science 327, 291–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer SS, He Q, Janczy JR, Elliott EI, Zhong Z, Olivier AK, Sadler JJ, Knepper-Adrian V, Han R, Qiao L, et al. (2013). Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity 39, 311–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobs C, Perner S, and Hornung V (2015). AIM2 Drives Joint Inflammation in a Self-DNA Triggered Model of Chronic Polyarthritis. PLoS ONE 10, e0131702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway CA (1989). Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harbor Symposia on Quantitative Biology 54 Pt 1, 1–13. [DOI] [PubMed] [Google Scholar]
- Jéru I, Papin S, L’hoste S, Duquesnoy P, Cazeneuve C, Camonis J, and Amselem S (2005). Interaction of pyrin with 14.3.3 in an isoform-specific and phosphorylation-dependent manner regulates its translocation to the nucleus. Arthritis Rheum. 52, 1848–1857. [DOI] [PubMed] [Google Scholar]
- Jin T, Perry A, Jiang J, Smith P, Curry JA, Unterholzner L, Jiang Z, Horvath G, Rathinam VA, Johnstone RW, et al. (2012). Structures of the HIN domain:DNA complexes reveal ligand binding and activation mechanisms of the AIM2 inflammasome and IFI16 receptor. Immunity 36, 561–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DC, Taabazuing CY, Okondo MC, Chui AJ, Rao SD, Brown FC, Reed C, Peguero E, de Stanchina E, Kentsis A, et al. (2018). DPP8/DPP9 inhibitor-induced pyroptosis for treatment of acute myeloid leukemia. Nat Med 24, 1151–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JW, Kayagaki N, Broz P, Henry T, Newton K, O’Rourke K, Chan S, Dong J, Qu Y, Roose-Girma M, et al. (2010). Absent in melanoma 2 is required for innate immune recognition of Francisella tularensis. Proc. Natl. Acad. Sci. U.S.a 107, 9771–9776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliana C, Fernandes-Alnemri T, Kang S, Farias A, Qin F, and Alnemri ES (2012). Non-transcriptional priming and deubiquitination regulate NLRP3 inflammasome activation. J. Biol. Chem 287, 36617–36622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan JC, Magupalli VG, and Wu H (2014). SMOCs: supramolecular organizing centres that control innate immunity. Nature Publishing Group 14, 821–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambara H, Liu F, Zhang X, Liu P, Bajrami B, Teng Y, Zhao L, Zhou S, Yu H, Zhou W, et al. (2018). Gasdermin D Exerts Anti-inflammatory Effects by Promoting Neutrophil Death. CellReports 22, 2924–2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayagaki N, Stowe IB, Lee BL, O’Rourke K, Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT, et al. (2015). Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526, 666–671. [DOI] [PubMed] [Google Scholar]
- Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, et al. (2011). Non-canonical inflammasome activation targets caspase-11. Nature 479, 117–121. [DOI] [PubMed] [Google Scholar]
- Kayagaki N, Wong MT, Stowe IB, Ramani SR, Gonzalez LC, Akashi-Takamura S, Miyake K, Zhang J, Lee WP, Muszyński A, et al. (2013). Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 341, 1246–1249. [DOI] [PubMed] [Google Scholar]
- Keller M, Rüegg A, Werner S, and Beer H-D (2008). Active caspase-1 is a regulator of unconventional protein secretion. Cell 132, 818–831. [DOI] [PubMed] [Google Scholar]
- Koch SC, Acton D, and Goulding M (2018). Spinal Circuits for Touch, Pain, and Itch. Annu. Rev. Physiol 80, 189–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortmann J, Brubaker SW, and Monack DM (2015). Cutting Edge: Inflammasome Activation in Primary Human Macrophages Is Dependent on Flagellin. J. Immunol 195, 815–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G-S, Subramanian N, Kim AI, Aksentijevich I, Goldbach-Mansky R, Sacks DB, Germain RN, Kastner DL, and Chae JJ (2012). The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature 492, 123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Thome S, Ma X, Amrute-Nayak M, Finigan A, Kitt L, Masters L, James JR, Shi Y, Meng G, et al. (2017). MARK4 regulates NLRP3 positioning and inflammasome activation through a microtubule-dependent mechanism. Nature Communications 8, 15986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Fu T-M, Lu A, Witt K, Ruan J, Shen C, and Wu H (2018). Cryo-EM structures of ASC and NLRC4 CARD filaments reveal a unified mechanism of nucleation and activation of caspase-1. Proc. Natl. Acad. Sci. U.S.a 115, 10845–10852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightfield KL, Persson J, Trinidad NJ, Brubaker SW, Kofoed EM, Sauer J-D, Dunipace EA, Warren SE, Miao EA, and Vance RE (2011). Differential requirements for NAIP5 in activation of the NLRC4 inflammasome. Infection and Immunity 79, 1606–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K-M, Hu W, Troutman TD, Jennings M, Brewer T, Li X, Nanda S, Cohen P, Thomas JA, and Pasare C (2014). IRAK-1 bypasses priming and directly links TLRs to rapid NLRP3 inflammasome activation. Proc. Natl. Acad. Sci. U.S.a 111, 775–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, and Lieberman J (2016). Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535, 153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu A, Li Y, Schmidt FI, Yin Q, Chen S, Fu T-M, Tong AB, Ploegh HL, Mao Y, and Wu H (2016). Molecular basis of caspase-1 polymerization and its inhibition by a new capping mechanism. Nat Struct Mol Biol 23, 416–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu A, Li Y, Yin Q, Ruan J, Yu X, Egelman E, and Wu H (2015). Plasticity in PYD assembly revealed by cryo-EM structure of the PYD filament of AIM2. Cell Discov 1, 15013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu A, Magupalli VG, Ruan J, Yin Q, Atianand MK, Vos MR, Schröder GF, Fitzgerald KA, Wu H, and Egelman EH (2014). Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell 156, 1193–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man SM, Tourlomousis P, Hopkins L, Monie TP, Fitzgerald KA, and Bryant CE (2013). Salmonella infection induces recruitment of Caspase-8 to the inflammasome to modulate IL-1β production. J. Immunol 191, 5239–5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin BN, Wang C, Zhang C-J, Kang Z, Gulen MF, Zepp JA, Zhao J, Bian G, Do J-S, Min B, et al. (2016). T cell-intrinsic ASC critically promotes TH17-mediated experimental autoimmune encephalomyelitis. Nat Immunol 17, 583–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinon F, Pétrilli V, Mayor A, Tardivel A, and Tschopp J (2006). Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241. [DOI] [PubMed] [Google Scholar]
- Masters SL, Lagou V, Jéru I, Baker PJ, Van Eyck L, Parry DA, Lawless D, De Nardo D, Garcia-Perez JE, Dagley LF, et al. (2016). Familial autoinflammation with neutrophilic dermatosis reveals a regulatory mechanism of pyrin activation. Science Translational Medicine 8, 332ra45–332ra45. [DOI] [PubMed] [Google Scholar]
- Matzinger P (1994). Tolerance, danger, and the extended family. Annu. Rev. Immunol 12, 991–1045. [DOI] [PubMed] [Google Scholar]
- Misawa T, Takahama M, Kozaki T, Lee H, Zou J, Saitoh T, and Akira S (2013). Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nat Immunol 14, 454–460. [DOI] [PubMed] [Google Scholar]
- Mishra BB, Rathinam VAK, Martens GW, Martinot AJ, Kornfeld H, Fitzgerald KA, and Sassetti CM (2013). Nitric oxide controls the immunopathology of tuberculosis by inhibiting NLRP3 inflammasome-dependent processing of IL-1β. Nat Immunol 14, 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe KM, Yang Z, Johnson JR, Geng X, Doitsh G, Krogan NJ, and Greene WC (2014). IFI16 DNA Sensor Is Required for Death of Lymphoid CD4 T Cells Abortively Infected with HIV. Science 343, 428–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteleone M, Stanley AC, Chen KW, Brown DL, Bezbradica JS, Pein von, J.B., Holley CL, Boucher D, Shakespear MR, Kapetanovic R, et al. (2018). Interleukin-1β Maturation Triggers Its Relocation to the Plasma Membrane for Gasdermin-D-Dependent and -Independent Secretion. CellReports 24, 1425–1433. [DOI] [PubMed] [Google Scholar]
- Moon J-S, Hisata S, Park M-A, DeNicola GM, Ryter SW, Nakahira K, and Choi AMK (2015). mTORC1-Induced HK1-Dependent Glycolysis Regulates NLRP3 Inflammasome Activation. CellReports 12, 102–115. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Morrone SR, Matyszewski M, Yu X, Delannoy M, Egelman EH, and Sohn J (2015). Assembly-driven activation of the AIM2 foreign-dsDNA sensor provides a polymerization template for downstream ASC. Nature Communications 6, 7827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T, Ockinger J, Yu J, Byles V, McColl A, Hofer AM, and Horng T (2012). Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc. Natl. Acad. Sci. U.S.a. 109, 11282–11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagar A, DeMarco RA, and Harton JA (2018). Inflammasome and Caspase-1 Activity Characterization and Evaluation: An Imaging Flow Cytometer-Based Detection and Assessment of Inflammasome Specks and Caspase-1 Activation. J. Immunol. ji 1800973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea MG, Nold-Petry CA, Nold MF, Joosten LAB, Opitz B, van der Meer JHM, van de Veerdonk FL, Ferwerda G, Heinhuis B, Devesa I, et al. (2009). Differential requirement for the activation of the inflammasome for processing and release of IL-1beta in monocytes and macrophages. Blood 113, 2324–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe Y, and Medzhitov R (2016). Tissue biology perspective on macrophages. Nat Immunol 17, 9–17. [DOI] [PubMed] [Google Scholar]
- Okondo MC, Johnson DC, Sridharan R, Go EB, Chui AJ, Wang MS, Poplawski SE, Wu W, Liu Y, Lai JH, et al. (2017). DPP8 and DPP9 inhibition induces pro-caspase-1-dependent monocyte and macrophage pyroptosis. Nature Chemical Biology 13, 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okondo MC, Rao SD, Taabazuing CY, Chui AJ, Poplawski SE, Johnson DC, and Bachovchin DA (2018). Inhibition of Dpp8/9 Activates the Nlrp1b Inflammasome. Cell Chem Biol 25, 262–267.e265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orning P, Weng D, Starheim K, Ratner D, Best Z, Lee B, Brooks A, Xia S, Wu H, Kelliher MA, et al. (2018). Pathogen blockade of TAK1 triggers caspase-8-dependent cleavage of gasdermin D and cell death. Science 362, 1064–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey S, Kawai T, and Akira S (2014). Microbial sensing by Toll-like receptors and intracellular nucleic acid sensors. Cold Spring Harbor Perspectives in Biology 7, a016246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YH, Wood G, Kastner DL, and Chae JJ (2016). Pyrin inflammasome activation and RhoA signaling in the autoinflammatory diseases FMF and HIDS. Nat Immunol 17, 914–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip NH, Dillon CP, Snyder AG, Fitzgerald P, Wynosky-Dolfi MA, Zwack EE, Hu B, Fitzgerald L, Mauldin EA, Copenhaver AM, et al. (2014). Caspase-8 mediates caspase-1 processing and innate immune defense in response to bacterial blockade of NF-κB and MAPK signaling. Proc. Natl. Acad. Sci. U.S.a 111, 7385–7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Py BF, Kim M-S, Vakifahmetoglu-Norberg H, and Yuan J (2013). Deubiquitination of NLRP3 by BRCC3 critically regulates inflammasome activity. Molecular Cell 49, 331–338. [DOI] [PubMed] [Google Scholar]
- Rathinam VAK, Vanaja SK, Waggoner L, Sokolovska A, Becker C, Stuart LM, Leong JM, and Fitzgerald KA (2012). TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by gram-negative bacteria. Cell 150, 606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathkey JK, Zhao J, Liu Z, Chen Y, Yang J, Kondolf HC, Benson BL, Chirieleison SM, Huang AY, Dubyak GR, et al. (2018). Chemical disruption of the pyroptotic pore-forming protein gasdermin D inhibits inflammatory cell death and sepsis. Sci Immunol 3, eaat2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch I, Deets KA, Ji DX, Moltke von, J., Tenthorey JL, Lee AY, Philip NH, Ayres JS, Brodsky IE, Gronert K, et al. (2017). NAIP-NLRC4 Inflammasomes Coordinate Intestinal Epithelial Cell Expulsion with Eicosanoid and IL-18 Release via Activation of Caspase-1 and −8. Immunity 46, 649–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayamajhi M, Zak DE, Chavarria-Smith J, Vance RE, and Miao EA (2013). Cutting edge: Mouse NAIP1 detects the type III secretion system needle protein. J. Immunol 191, 3986–3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes Ruiz VM, Ramirez J, Naseer N, Palacio NM, Siddarthan IJ, Yan BM, Boyer MA, Pensinger DA, Sauer J-D, and Shin S (2017). Broad detection of bacterial type III secretion system and flagellin proteins by the human NAIP/NLRC4 inflammasome. Proc. Natl. Acad. Sci. U.S.a 114, 13242–13247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts TL, Idris A, Dunn JA, Kelly GM, Burnton CM, Hodgson S, Hardy LL, Garceau V, Sweet MJ, Ross IL, et al. (2009). HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science 323, 1057–1060. [DOI] [PubMed] [Google Scholar]
- Ru H, Ni X, Zhao L, Crowley C, Ding W, Hung L-W, Shaw N, Cheng G, and Liu Z-J (2013). Structural basis for termination of AIM2-mediated signaling by p202. Cell Res. 23, 855–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rühl S, and Broz P (2015). Caspase-11 activates a canonical NLRP3 inflammasome by promoting K(+) efflux. Eur. J. Immunol 45, 2927–2936. [DOI] [PubMed] [Google Scholar]
- Rühl S, Shkarina K, Demarco B, Heilig R, Santos JC, and Broz P (2018). ESCRT-dependent membrane repair negatively regulates pyroptosis downstream of GSDMD activation. Science 362, 956–960. [DOI] [PubMed] [Google Scholar]
- Sandstrom A, Mitchell PS, Goers L, Mu EW, Lesser CF, and Vance RE (2019). Functional degradation: A mechanism of NLRP1 inflammasome activation by diverse pathogen enzymes. Science 364, eaau1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarhan J, Liu BC, Muendlein HI, Li P, Nilson R, Tang AY, Rongvaux A, Bunnell SC, Shao F, Green DR, et al. (2018). Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc. Natl. Acad. Sci. U.S.a 115, E10888–E10897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer J-D, Witte CE, Zemansky J, Hanson B, Lauer P, and Portnoy DA (2010). Listeria monocytogenes triggers AIM2-mediated pyroptosis upon infrequent bacteriolysis in the macrophage cytosol. Cell Host and Microbe 7, 412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid-Burgk JL, Chauhan D, Schmidt T, Ebert TS, Reinhardt J, Endl E, and Hornung V (2016). A Genome-wide CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) Screen Identifies NEK7 as an Essential Component of NLRP3 Inflammasome Activation. J. Biol. Chem 291, 103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder K, Sagulenko V, Zamoshnikova A, Richards AA, Cridland JA, Irvine KM, Stacey KJ, and Sweet MJ (2012). Acute lipopolysaccharide priming boosts inflammasome activation independently of inflammasome sensor induction. Immunobiology 217, 1325–1329. [DOI] [PubMed] [Google Scholar]
- Sharif H, Wang L, Wang WL, Magupalli VG, Andreeva L, Qiao Q, Hauenstein AV, Wu Z, Núñez G, Mao Y, et al. (2019). Structural mechanism for NEK7-licensed activation of NLRP3 inflammasome. Nature 570, 338–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C-S, Shenderov K, Huang N-N, Kabat J, Abu-Asab M, Fitzgerald KA, Sher A, and Kehrl JH (2012). Activation of autophagy by inflammatory signals limits IL-1β production by targeting ubiquitinated inflammasomes for destruction. Nat Immunol 13, 255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, and Shao F (2015a). Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665. [DOI] [PubMed] [Google Scholar]
- Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P, Hu L, and Shao F (2015b). Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514, 187–192. [DOI] [PubMed] [Google Scholar]
- Shimada K, Crother TR, Karlin J, Dagvadorj J, Chiba N, Chen S, Ramanujan VK, Wolf AJ, Vergnes L, Ojcius DM, et al. (2012). Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 36, 401–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollberger G, Choidas A, Burn GL, Habenberger P, Di Lucrezia R, Kordes S, Menninger S, Eickhoff J, Nussbaumer P, Klebl B, et al. (2018). Gasdermin D plays a vital role in the generation of neutrophil extracellular traps. Sci Immunol 3, eaar6689. [DOI] [PubMed] [Google Scholar]
- Stoecklein VM, Osuka A, Ishikawa S, Lederer MR, Wanke-Jellinek L, and Lederer JA (2015). Radiation exposure induces inflammasome pathway activation in immune cells. J. Immunol 194, 1178–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian N, Natarajan K, Clatworthy MR, Wang Z, and Germain RN (2013). The adaptor MAVS promotes NLRP3 mitochondrial localization and inflammasome activation. Cell 153, 348–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson KV, Deng M, and Ting JP-Y (2019). The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nature Publishing Group 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson KV, Junkins RD, Kurkjian CJ, Holley-Guthrie E, Pendse AA, Morabiti El, R., Petrucelli A, Barber GN, Benedict CA, and Ting JP-Y (2017). A noncanonical function of cGAMP in inflammasome priming and activation. J. Exp. Med. jem 20171749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taabazuing CY, Okondo MC, and Bachovchin DA (2017). Pyroptosis and Apoptosis Pathways Engage in Bidirectional Crosstalk in Monocytes and Macrophages. Cell Chem Biol 24, 507–514.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y, and Kagan JC (2017). Microbe-inducible trafficking pathways that control Toll-like receptor signaling. Traffic 18, 6–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y, and Kagan JC (2019). Innate Immune Signaling Organelles Display Natural and Programmable Signaling Flexibility. Cell 177, 384–398.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng T-C, Schattgen S, Monks B, Wang D, Cerny A, Latz E, Fitzgerald K, and Golenbock DT (2016). A Fluorescent Reporter Mouse for Inflammasome Assembly Demonstrates an Important Role for Cell-Bound and Free ASC Specks during In Vivo Infection. CellReports 16, 571–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vajjhala PR, Lu A, Brown DL, Pang SW, Sagulenko V, Sester DP, Cridland SO, Hill JM, Schroder K, Stow JL, et al. (2015). The Inflammasome Adaptor ASC Induces Procaspase-8 Death Effector Domain Filaments. J. Biol. Chem 290, 29217–29230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanaja SK, Russo AJ, Behl B, Banerjee I, Yankova M, Deshmukh SD, and Rathinam VAK (2016). Bacterial Outer Membrane Vesicles Mediate Cytosolic Localization of LPS and Caspase-11 Activation. Cell 165, 1106–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance RE, Isberg RR, and Portnoy DA (2009). Patterns of pathogenesis: discrimination of pathogenic and nonpathogenic microbes by the innate immune system. Cell Host and Microbe 6, 10–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viganò E, Diamond CE, Spreafico R, Balachander A, Sobota RM, and Mortellaro A (2015). Human caspase-4 and caspase-5 regulate the one-step non-canonical inflammasome activation in monocytes. Nature Communications 6, 8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker MA, Teghanemt A, Weiss JP, and Barker JH (2017). High-affinity caspase-4 binding to LPS presented as high molecular mass aggregates or in outer membrane vesicles. Innate Immun 23, 336–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P-H, Ye Z-W, Deng J-J, Siu K-L, Gao W-W, Chaudhary V, Cheng Y, Fung S-Y, Yuen K-S, Ho T-H, et al. (2018). Inhibition of AIM2 inflammasome activation by a novel transcript isoform of IFI16. EMBO Rep. 19, e45737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Miura M, Jung YK, Zhu H, Li E, and Yuan J (1998). Murine caspase-11, an ICE-interacting protease, is essential for the activation of ICE. Cell 92, 501–509. [DOI] [PubMed] [Google Scholar]
- Wlodarska M, Thaiss CA, Nowarski R, Henao-Mejia J, Zhang J-P, Brown EM, Frankel G, Levy M, Katz MN, Philbrick WM, et al. (2014). NLRP6 inflammasome orchestrates the colonic host-microbial interface by regulating goblet cell mucus secretion. Cell 156, 1045–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf AJ, Reyes CN, Liang W, Becker C, Shimada K, Wheeler ML, Cho HC, Popescu NI, Coggeshall KM, Arditi M, et al. (2016). Hexokinase Is an Innate Immune Receptor for the Detection of Bacterial Peptidoglycan. Cell 166, 624–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang M, Shi X, Li Y, Xu J, Yin L, Xiao G, Scott MJ, Billiar TR, Wilson MA, and Fan J (2011). Hemorrhagic shock activation of NLRP3 inflammasome in lung endothelial cells. J. Immunol 187, 4809–4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Yang J, Gao W, Li L, Li P, Zhang L, Gong Y-N, Peng X, Xi JJ, Chen S, et al. (2014). Innate immune sensing of bacterial modifications of Rho GTPases by the Pyrin inflammasome. Nature 513, 237–241. [DOI] [PubMed] [Google Scholar]
- Yan Y, Jiang W, Liu L, Wang X, Ding C, Tian Z, and Zhou R (2015). Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell 160, 62–73. [DOI] [PubMed] [Google Scholar]
- Yang J, Zhao Y, Shi J, and Shao F (2013). Human NAIP and mouse NAIP1 recognize bacterial type III secretion needle protein for inflammasome activation. Proc. Natl. Acad. Sci. U.S.a 110, 14408–14413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeon SH, Yang G, Lee HE, and Lee JY (2017). Oxidized phosphatidylcholine induces the activation of NLRP3 inflammasome in macrophages. J. Leukoc. Biol 101, 205–215. [DOI] [PubMed] [Google Scholar]
- Yin Q, Sester DP, Tian Y, Hsiao Y-S, Lu A, Cridland JA, Sagulenko V, Thygesen SJ, Choubey D, Hornung V, et al. (2013). Molecular mechanism for p202-mediated specific inhibition of AIM2 inflammasome activation. CellReports 4, 327–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon S, Kovalenko A, Bogdanov K, and Wallach D (2017). MLKL, the Protein that Mediates Necroptosis, Also Regulates Endosomal Trafficking and Extracellular Vesicle Generation. Immunity. [DOI] [PubMed] [Google Scholar]
- Zanoni I, Ostuni R, Marek LR, Barresi S, Barbalat R, Barton GM, Granucci F, and Kagan JC (2011). CD14 Controls the LPS-Induced Endocytosis of Toll-like Receptor 4. Cell 147, 868–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanoni I, Tan Y, Di Gioia M, Broggi A, Ruan J, Shi J, Donado CA, Shao F, Wu H, Springstead JR, et al. (2016). An endogenous caspase-11 ligand elicits interleukin-1 release from living dendritic cells. Science 352, 1232–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanoni I, Tan Y, Di Gioia M, Springstead JR, and Kagan JC (2017). By Capturing Inflammatory Lipids Released from Dying Cells, the Receptor CD14 Induces Inflammasome-Dependent Phagocyte Hyperactivation. Immunity 47, 697–709.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Chen S, Ruan J, Wu J, Tong AB, Yin Q, Li Y, David L, Lu A, Wang WL, et al. (2015). Cryo-EM structure of the activated NAIP2-NLRC4 inflammasome reveals nucleated polymerization. Science 350, 404–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Meszaros G, He W-T, Xu Y, de Fatima Magliarelli H, Mailly L, Mihlan M, Liu Y, Puig Gámez M, Goginashvili A, et al. (2017). Protein kinase D at the Golgi controls NLRP3 inflammasome activation. J. Exp. Med 214, 2671–2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Yang J, Shi J, Gong Y-N, Lu Q, Xu H, Liu L, and Shao F (2011). The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature 477, 596–600. [DOI] [PubMed] [Google Scholar]
- Zhong FL, Mamaï O, Sborgi L, Boussofara L, Hopkins R, Robinson K, Szeverényi I, Takeichi T, Balaji R, Lau A, et al. (2016). Germline NLRP1 Mutations Cause Skin Inflammatory and Cancer Susceptibility Syndromes via Inflammasome Activation. Cell 167, 187–202.e17. [DOI] [PubMed] [Google Scholar]
- Zhong Z, Liang S, Sanchez-Lopez E, He F, Shalapour S, Lin X-J, Wong J, Ding S, Seki E, Schnabl B, et al. (2018). New mitochondrial DNA synthesis enables NLRP3 inflammasome activation. Nature 560, 198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Tardivel A, Thorens B, Choi I, and Tschopp J (2010). Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol 11, 136–140. [DOI] [PubMed] [Google Scholar]
- Zhu S, Ding S, Wang P, Wei Z, Pan W, Palm NW, Yang Y, Yu H, Li H-B, Wang G, et al. (2017). Nlrp9b inflammasome restricts rotavirus infection in intestinal epithelial cells. Nature 546, 667–670. [DOI] [PMC free article] [PubMed] [Google Scholar]