Abstract
Cellular behavior is continuously affected by microenvironmental forces through the process of mechanotransduction, in which mechanical stimuli are rapidly converted to biochemical responses. Mounting evidence suggests that the nucleus itself is a mechanoresponsive element, reacting to cytoskeletal forces and mediating downstream biochemical responses. The nucleus responds through a host of mechanisms, including partial unfolding, conformational changes, and phosphorylation of nuclear envelope proteins, modulation of nuclear import/export, and altered chromatin organization, resulting in transcriptional changes. It is unclear which of these events present direct mechanotransduction processes and which are downstream of other mechanotransduction pathways. We critically review and discuss the current evidence for nuclear mechanotransduction, particularly in the context of stem cell fate, a largely unexplored topic, and in disease, where an improved understanding of nuclear mechanotransduction is beginning to open new treatment avenues. Finally, we discuss innovative technological developments that will allow outstanding questions in the rapidly growing field of nuclear mechanotransduction to be answered.
Keywords: mechanotransduction, nuclear mechanics, stem cells, LINC complex, lamin, laminopathies
1. INTRODUCTION
Mechanotransduction refers to the process by which cells convert mechanical stimuli from their extracellular environment or cell-generated forces into biochemical signals to induce downstream cellular responses. Mechanical forces can propagate along the cytoskeleton and travel at speeds of up to 30 μm/s, an impressive rate that is 25 times faster than molecular motor transport and 12.5 times faster than passive diffusion of signaling molecules (1). This rapid conversion of physical to biochemical response enables the rapid adaptation of cells to their changing physical environment (2, 3). Mechanotransduction can play a critical role in cell and tissue differentiation, maintenance, and disease, for example, in the adaptation of bones and muscle to exercise or the alignment of endothelial cells to fluid shear (4).
Since the term mechanotransduction is often used more broadly to refer to any cellular responses to mechanical stimuli, including events downstream of the original transduction event, in this review we use the following definitions: Mechanotransmission refers to the transmission of mechanical forces through cellular components, such as along actin stress fibers or microtubules, but does not include the actual mechanotransduction process. Mechanosensing refers to the actual transduction process, which is typically limited to some specialized proteins and locations within the cell; many of these proteins, including specific focal adhesion proteins and stretch-sensitive ion channels in the plasma membrane (5, 6), have been recognized in the last three decades, but others may remain to be identified. Mechanotransduction signaling describes the signaling pathways that are downstream of the initial mechanosensing event. Importantly, many of these pathways, such as mitogen-activated protein kinase (MAPK) signaling or YAP/TAZ translocation, can be activated by a variety of upstream signals, not only mechanical stimuli but also biochemical signals, such as growth factor binding to cell surface receptors (7–9).
Recently, a growing number of studies, including some on isolated nuclei, have implicated the nucleus itself as a mechanosensing element (10–12). Several models have been proposed to explain how mechanical forces acting on the nucleus could induce changes in nuclear envelope composition, chromatin organization, and gene expression, which then drive downstream cellular responses (57, 58), including in stem cell differentiation. At the same time, many of the reported findings linking mechanical factors and nuclear changes have been rather correlative, and it often remains unclear whether the observed nuclear changes were upstream or downstream of other events, including established cytoplasmic mechanotransduction pathways.
In this review, we provide a summary of nuclear structure and describe how these nuclear components contribute to nuclear mechanics, mechanotransmission, and mechanosensing. As many current efforts seek to understand how stem cells respond to their mechanical microenvironment to control differentiation and cell fate commitment, we discuss how nuclear mechanotransduction may be involved. Since defects in nuclear mechanics and mechanotransmission are linked to impaired mechanotransduction signaling and several human diseases, particularly affecting skeletal and cardiac muscle (4), we discuss the current understanding of nuclear mechanotransduction in disease pathogenesis. Finally, we outline some of the recent technological advances in unraveling the mechanisms by which the nucleus acts in mechanotransduction and in deepening our understanding of the diseases caused by such mechanisms.
2. THE NUCLEUS AND NUCLEAR MECHANICS
2.1. Nuclear Structure and Organization
As the compartment containing the vast majority of the genome and the site of gene transcription, the nucleus arguably plays the most important role in guiding cellular fate, behavior, and adaptation. The nucleus contains DNA that is wrapped around histones, which are organized into higher-order structures, broadly categorized as either open, transcriptionally active euchromatin or condensed, inactive heterochromatin. The nuclear envelope consists of outer and inner nuclear membranes (ONM and INM, respectively) separated by the 30–50-nm-wide perinuclear space (PNS) (Figure 1). This double membrane serves as a physical barrier to protect the nuclear contents and to control exchange of large (>30-kDa) molecules between the cytoplasm and the nuclear interior through nuclear pore complexes (NPCs). NPCs regulate exchange across the nuclear envelope, directly connect to both the nucleoskeleton and the cytoskeleton, and interact with chromatin (13).
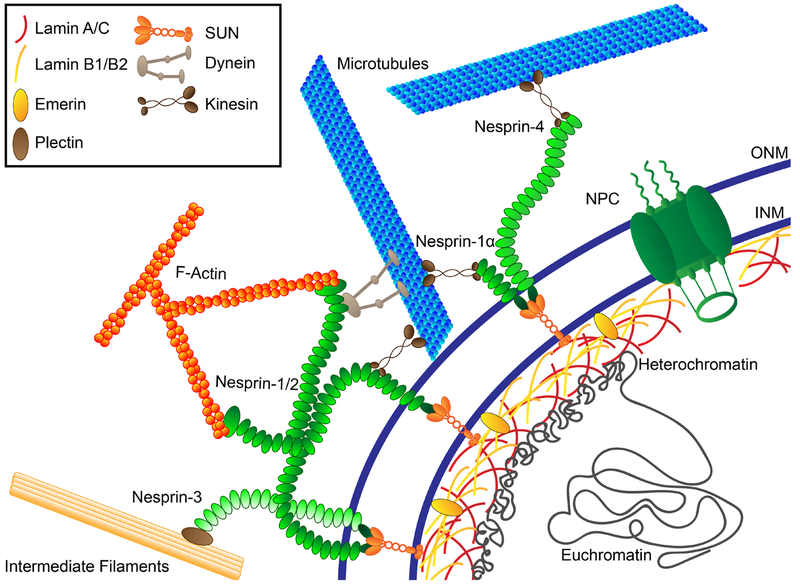
Figure 1.
Constituents of the nucleus and nuclear envelope involved in mechanotransduction. The LINC (linker of the nucleoskeleton and cytoskeleton) complex—nesprins at the outer nuclear membrane (ONM) and SUN-domain proteins at the inner nuclear membrane (INM)—spans the nuclear envelope, interacting with cytoskeletal filaments and associated proteins and the nuclear lamina to enable force transmission between the cytoskeleton and nuclear interior. Nuclear lamins (A/C and B1/2) form independent yet interacting meshworks underneath the INM and are responsible for maintaining nuclear shape and stiffness. Both A- and B-type lamins interact with nuclear pore complexes (NPCs), chromatin, and various other binding partners at the nuclear envelope and the nuclear interior. NPCs enable molecular transport between the cytoplasm and nucleoplasm.
Below the INM exists the 10–30-nm-thick, fibrous meshwork of the nuclear lamina (14). The nuclear lamina is composed mostly of lamins, which are type V nuclear intermediate filament proteins nearly ubiquitously expressed in differentiated cell types. Mammalian cells express two types of lamins—A-type and B-type—as products of three genes. In somatic cells, the LMNA gene gives rise to two major A-type lamin isoforms, Lamin A and Lamin C, plus some less common isoforms, as the result of alternative splicing; the LMNB1 and LMNB2 genes encode Lamins B1 and B2, respectively. Lamins A and C are developmentally regulated and appear during cellular differentiation. In contrast, all cells express at least one B-type lamin, even though recent studies show that cells lacking both Lamin B1 and B2 are viable (15, 16). The various lamin isoforms form independent yet interacting meshworks with a highly branched architecture at the nuclear periphery (17, 18). Surprisingly, recent cryo–electron tomography (cryo-ET) studies indicated that A- and B-type lamins form 3.5-nm-diameter tetrameric filaments, which are substantially thinner than the 10-nm-diameter cytoplasmic intermediate filaments (14). Notably, a fraction of lamins, particularly A-type, also exist in the nucleoplasm. Lamins have many binding partners, including chromatin, transcription factors, and LEM (LAP2, Emerin, and MAN1) family proteins, that play critical roles in gene regulation (19).
Since their discovery four decades ago (20), interest in nuclear lamins has been rapidly increasing as more evidence of their vital roles in cellular functions and disease has emerged. Within the nucleus, lamins regulate transcription, DNA replication and repair, and chromatin organization (21, 22). Heterochromatin exists at the nuclear periphery and interacts with the nuclear lamina in Lamin-associated domains (LADs) (23, 24) and via Lamin-associated protein 2 (LAP2) and its binding partner barrier to autointegration factor (BAF) (25, 26). These interactions may directly affect chromatin organization, nuclear mechanotransduction, and gene expression and may contribute to stem cell differentiation (see Sections 3 and 4). Furthermore, Lamin A is required to retain Emerin at the INM (27, 28), which in turn modulates expression of mechanosensitive genes (29) and is required for the Nesprin-1-mediated nuclear envelope remodeling in response to mechanical force (10). Depletion of Lamin B1 results in an enlarged or loose Lamin A/C meshwork with blebs of the nuclear envelope that contain Lamin A/C and euchromatin (17, 27, 30). Similarly, depletion of Lamin A/C causes loosening of the Lamin B1 meshwork and mislocalization of Emerin and other nuclear envelope proteins away from the nuclear envelope, highlighting the interconnections between various nuclear envelope components (17, 30).
At the nuclear envelope, lamins are responsible for positioning and distribution of NPCs, with both A- and B-type lamins binding nucleoporin (Nup153). Lamins also modulate nuclear assembly and disassembly during cellular replication (22), as well as nuclear shape, stiffness, and structure by regulating cytoskeletal organization (30–32). Loss of Lamin A/C results in disturbed perinuclear actin, microtubule, and intermediate filament organization and changes in focal adhesions (33–37). On a larger scale, lamins play a critical role in migration through three-dimensional (3D) environments by governing the deformability of the large nucleus, which constitutes a rate-limiting factor in confined migration (reviewed in Reference 38).
2.2. Nucleo-Cytoskeletal Connections
The LINC (linker of the nucleoskeleton and cytoskeleton) complex connects the nuclear lamina to the cytoskeleton (39) and is critical in force transmission from the cytoskeleton to the nuclear interior, termed nucleo-cytoskeletal coupling (33). The LINC complex consists of SUN (Sad1p and UNC-84 homology)- and KASH (Klarsicht, ANC-1, and Syne homology)-domain proteins, named after their conserved C-terminal domains that interact across the luminal space. LINC complex proteins span the nuclear envelope (Figure 1), and are anchored at the nuclear envelope via lamins, NPCs, and interaction with chromatin (39). In mammalian somatic cells, Sun1 and Sun2 constitute the SUN-domain proteins; Nesprin-1, -2, -3, and -4, each with multiple isoforms, the KASH-domain proteins. Germ cells express additional LINC complex proteins. SUN-domain protein trimers in the INM interact with the lamina at the INM and KASH-domain proteins in the PNS (40). KASH-domain proteins in the ONM protrude into both the PNS and cytoplasm, where they bind the cytoskeleton. Nesprin family proteins include isoforms that can directly bind F-actin, interact with the microtubule motors kinesin and dynein and with each other, and bind the adaptor protein plectin to interact with intermediate filaments (41). The LINC complex plays crucial roles in mechanical processes such as nuclear movement, positioning, and shape, as well as chromatin positioning and gene expression (42, 43). Disruption of the interaction between nuclear lamins and LINC complex proteins, for example, through mutations in the LMNA gene responsible for various diseases (see Section 5), results in loss of nucleo-cytoskeletal coupling, perturbed cytoskeletal organization, loss of nuclear stiffness, and inability of the nucleus to properly respond to force (44).
In cells adhering on rigid two-dimensional (2D) surfaces, a perinuclear organization of dynamic apical actomyosin filaments (referred to as a perinuclear actin cap by some authors) spans the top of the nucleus and connects to the nuclear interior via the LINC complex (34). Anchored at focal adhesions at the cellular basal surface, these perinuclear actin filaments apply compressive forces to the apical surface of the nucleus (45). Together with lateral forces transmitted to the nucleus from other cytoskeletal structures, such as microtubules, these forces strongly influence nuclear shape (46, 47). This concept is discussed further in the context of mechanotransduction in Section 3.
2.3. Nuclear Mechanics
Together, the highly interconnected nuclear constituents described above mediate the transmission of mechanical forces from the cytoskeleton to the nucleus, while providing structural support to the nucleus and defining its mechanical properties (44). The nucleus exhibits elasticity and compressibility that enable the nucleus to act as a mechanical shock absorber (48). Both the nuclear lamina and chromatin contribute to nuclear mechanical response to strain (49, 50). Nuclear lamina stretch dominates at nuclear strains above 30%, while the mechanical properties of chromatin, which exhibits viscoelastic properties, govern nuclear deformation at lower strains (49). Physical cross-links between chromatin and INM proteins such as SUN-domain proteins can provide further mechanical stability to the nucleus (51). Prolonged mechanical stress can cause irreversible deformation and reorganization of chromatin, which may correspond to altered transcriptional or differentiation states (52). Lamin A/C levels correlate to nuclear stiffness and ability to withstand force: Increased levels result in stiffer and more viscous nuclei (11, 53), whereas decreased levels correspond to softer, more deformable nuclei with increased fragility (31, 53–55). Lamin phosphorylation results in increased solubility of lamins, decreased polymerization of the lamina, increased lamin turnover, and reduction of cellular tension and nuclear stiffness (11, 56).
3. THE ROLE OF THE NUCLEUS IN MECHANOTRANSDUCTION
Many non-mutually-exclusive mechanisms of nuclear mechanotransduction have been proposed to date. In this section, we briefly discuss the major proposed mechanisms and the evidence supporting them (Figure 2); we refer readers to excellent recent reviews (57, 58) for more details and additional proposed mechanisms. In our discussion, we focus general mechanisms of nuclear mechanotransduction; it is important to recognize that different cell types may respond differently to mechanical stimuli, owing to differences in both structural organization (e.g. when comparing epithelial cells, mesenchymal fibroblasts, leukocytes, and muscle cells) and cell-type specific signaling pathways downstream of the mechanosensing event.
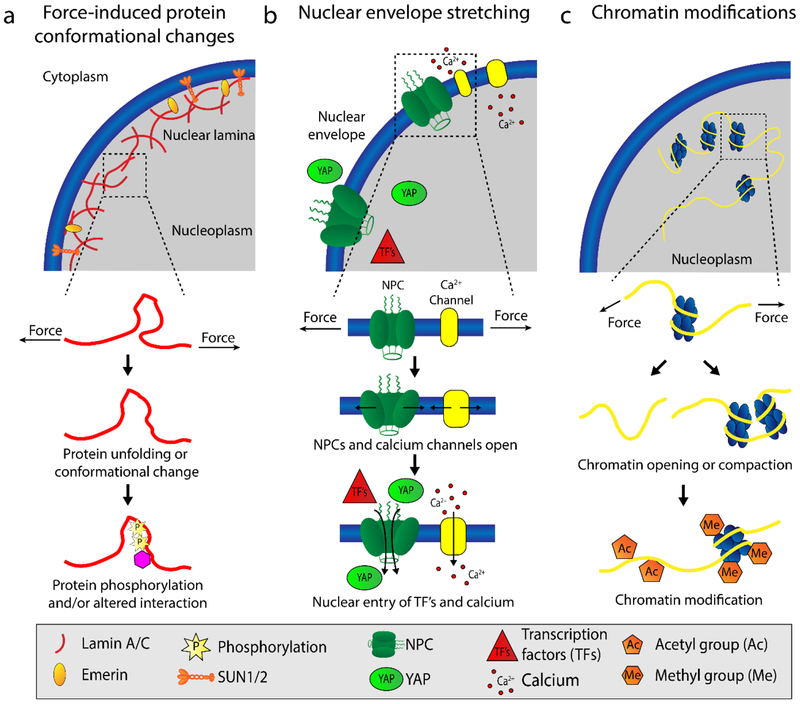
Figure 2.
Proposed mechanisms of nuclear mechanotransduction. (a) Force application to the nucleus can results in conformational changes of nuclear envelope proteins, such as partial unfolding of lamins (11, 71), and phosphorylation of nuclear proteins, including lamins, SUN-domain proteins, and Emerin (10, 11, 56). (b) Nuclear membrane stretch in response to force opens nuclear pore complexes (NPCs) (59, 60) and calcium channels (65, 66) on the cytoplasmic side, thus increasing molecular influx into the nucleoplasm. The increased import of transcription factors (TFs) into the nucleoplasm can alter gene expression (60). (c) Mechanical forces acting on the nucleus can induce chromatin stretching, opening, and compaction, including DNA and histone modifications, that alter accessibility to transcription factors and lead to changes in gene expression (72–77) [add reference (82)]. Abbreviation: NPC, nuclear pore complex.
3.1. Nuclear Membrane and Pore Stretching
During nuclear membrane stretch, NPCs take on a dilated, more open conformation (59, 60). Although NPCs make up less than 10% of the nuclear surface area at rest (61), the mechanically induced increase in NPC diameter accounts for one-sixth of the total increase in nuclear membrane surface area in HeLa cells during nuclear swelling (59). Force application to the nucleus, such as during cell spreading on a 2D substrate or by indentation with an atomic force microscopy tip, triggers partial opening of nuclear pores, promoting active nuclear import of YAP (60). Osmotic swelling, however, does not trigger YAP import (60), suggesting that some elements, such as Nesprin-1 (8) or other LINC complex constituents, are required for the opening of nuclear pores and the mechanosensing process.
Flux of calcium in response to force application or nuclear stretching may constitute another nuclear mechanosensing mechanism. NPCs and calcium channels on the nuclear envelope, including L-type, InsP3, cyclic ADP ribose-modulate, and possibly others, regulate the influx and efflux of calcium from the nucleus (62–64). Cell spreading and nuclear stretching increase nuclear calcium through stretch-activated calcium channels on the nuclear membrane, which enhances transcription factor (CREB) expression and regulates gene transcription, protein import, apoptosis (63, 64), and downstream mechanosignaling (65).
In addition to the opening of NPCs and channels, nuclear envelope stretch loosens packing of the nuclear membrane phospholipid bilayers, allowing for the insertion of hydrophobic protein residues into the bilayer (65). Osmotic swelling in response to tissue damage triggers nuclear translocation of cytosolic phospholipase A2 (cPLA2) and 5-lipoxygenase (5-LOX) and incorporation of these proteins into the INM, where their activity triggers downstream inflammatory signaling cascades (65). Mechanosensitive incorporation of cPLA2 and 5-LOX is regulated by increased calcium levels in the cell, which aids residue insertion into the membrane (65, 66), and by nuclear lamina rigidity, as a stiff nuclear lamina may not as readily allow stretching of the INM and therefore reduces protein incorporation into the INM (50, 65).
3.2. Protein Phosphorylation and Conformation Change in Response to Mechanical Force
Phosphorylation states serve as common mechano-switches in mechanical response, such as cytoskeletal stretch-dependent phosphorylation of Cas (67, 68) for contraction or phosphorylation of Paxillin and Vinculin during tension-mediated focal adhesion maturation (69). In the nucleus, Lamin A/C and Emerin phosphorylation modulate nuclear stiffness and nucleo-cytoskeletal coupling in response to mechanical stimulation (10, 11, 56). Lamin A/C phosphorylation increases in cells with low cytoskeletal tension, that is, when cells are grown on soft substrates (11, 56), increasing Lamin A/C mobility and turnover (56, 70). Conversely, when forces are applied to the nucleus via nesprins, Src-mediated Emerin phosphorylation recruits Lamin A/C to the nuclear periphery and promotes Sun2–Lamin A/C interactions (10). The precise mechanism by which mechanical forces can modulate phosphorylation of nuclear envelope proteins remains unclear, including whether this process is regulated by altering kinase activities or accessibility of the kinase substrate amino acids. Regardless, the observed mechanically induced phosphorylation implicates a structural role for phosphorylation in mechanotransduction through control of nuclear stiffening and nucleo-cytoskeletal coupling (10, 11, 56), which can also affect downstream transcription by downregulating some mechanoresponsive genes (VCL and SRF) and reducing YAP/TAZ translocation into the nucleus (10).
Alongside phosphorylation, protein conformation plays a role in mechanical response at the nuclear envelope. Partial unfolding of the Lamin A C-terminal immunoglobulin (Ig)-like fold in response to mechanical forces may expose normally hidden residues, such as Cys522 (11, 71). This conformational change could alter interaction with binding partners, expose cryptic signaling sites, or destabilize the protein. Unfolding may expose some amino acid residues to kinases, thus allowing for altered phosphorylation and modulating downstream signaling.
3.3. Chromatin Stretching, Organization, and Modification
Mechanical microenvironmental cues, such as architecture and mechanical loading (e.g., tension and compression), alter chromatin modifications and condensation to control gene expression (72–77). Dynamic mechanical loading can cause rapid short-lived, prolonged, and even irreversible changes in chromatin condensation, depending on the intensity and duration of the mechanical load (75–77). Highly transcriptionally active chromosomes preferentially orient along the mechanical axis of the nucleus on anisotropic micropatterned materials (73, 78, 79), demonstrating that chromatin organization is responsive to extracellular and cytoskeletal mechanical cues. Such changes in chromatin organization likely affect the transcriptional profile of the cells. Although these phenomena are widely observed, the specific mechanisms guiding mechanoresponsive gene expression are not well characterized. In particular, it remains unclear whether these changes are direct responses to forces acting on the nucleus or are downstream of other mechanotransduction events.
Importantly, the observed changes in chromatin organization, condensation, and modification are dependent on the actin cytoskeleton and LINC complex (75–77, 80). Perinuclear actin filaments bind to the LINC complex on the apical surface of the nucleus and cause accumulation of Lamin A/C and hyperacetylated, transcriptionally active euchromatin at the INM, demonstrating that the perinuclear actin filaments interact with euchromatin via nucleo-cytoskeletal coupling (81). Furthermore, cytoskeletal contraction triggers mechanosensitive ATP release and calcium signaling to mediate nuclear import and activation of the histone–lysine N-methyltransferase EZH2 and histone deacetylase (HDAC) (73, 75–77), which stimulates gene silencing by altering methylation (74) and gene transcription by increasing histone acetylation (72, 73). Prolonged force application drives changes in methylation states for gene regulatory control by decoupling heterochromatin from the nuclear lamina, and driving chromatin compaction, and a switch from H3K9me3 to H3K27me3to attenuate transcription and silence promotors (74).
Previous research suggested that force-dependent transcriptional regulation depends upon lamin–chromatin interactions (30), but until recently, studies have struggled to show a direct effect of mechanical force on chromatin to control transcription. Wang and colleagues (82) used 3D magnetic twisting cytometry to apply extracellular stretching with RGD-coated magnetic beads, which demonstrated the direct stretching of a reporter transgene flanked by two green fluorescent protein–labeled loci and rapid, stretch-dependent transcription of the reporter gene. This study suggests that force is transmitted through integrins, the actin cytoskeleton, the LINC complex, and then lamin–chromatin interactions, which stretch chromatin and result in upregulation of transcription (82). Disruption of any one of these components weakens the mechanically induced response (82). Nonetheless, studies using endogenous genes will be required to confirm these findings in a general context, and it remains unclear how chromatin stretching results in activation of specific mechanosensitive genes. Euchromatin endures greater deformation under strain than heterochromatin, which would induce larger conformational changes (77), and may promote stretch-dependent transcription.
3.4. Nuclear and Perinuclear Actin
Recently, nuclear and perinuclear actin assemblies have emerged as key players in nuclear mechanotransmission and mechanosignaling. Nuclear actin polymerization regulates nuclear structure and gene expression (26, 83–86). The LINC complex mediates nuclear actin polymerization in response to cell spreading to form a nuclear scaffold (1, 87), which is accelerated by Emerin binding to the actin pointed end (26, 83). Both Lamin A/C and Emerin bind nuclear actin, thereby increasing nuclear strength (26, 83). Furthermore, nuclear actin acts as a transcriptional cofactor for polymerases I, II, and III (84). Nuclear actin polymerization can regulate transcription factor activity via increased import and export, primarily through myocardin-related transcription factor A (MRTF-A) and serum response factor (SRF), which demonstrates the downstream effects of force-driven nuclear actin dynamics (85, 86). Highlighting the interplay between nuclear envelope proteins, actin, and MRTF-A/SRF, loss of Lamin A/C and Emerin disturb nuclear and cytoskeletal actin dynamics and impair MRTF-A/SRF signaling (ref).
Applied force can induce perinuclear actin filament assembly within minutes (77, 88), in a process that requires Lamin A/C, Emerin, and the LINC complex (33–35, 45). The presence of perinuclear actin is key in mechanotransmission of forces to the nucleus via the LINC complex, but the initial polymerization reaction likely occurs downstream of Rho GTPase (77) and calcium mechanosignaling (88). Thus, perinuclear actin plays a crucial role in mechanotransmission to the nucleus, a requirement for nuclear mechanotransduction. At the same time, perinuclear—and nuclear—actin polymerization is downstream of other mechanoresponsive signaling pathways and can further modulate mechanotransduction signaling by interaction with MRTF-A and SRF. As a whole, this mechanosensitive mechanistic web is thought to work to guide cellular functions, such as stem cell fate (as discussed in Section 4), and disruption of this intricate network can cause a host of human diseases (see Section 5).
4. NUCLEAR MECHANICS GUIDE STEM CELL FATE
In addition to soluble factors, the stem cell microenvironment provides mechanical stimulation to guide lineage commitment and differentiation. Seminal research by Engler et al. (89) demonstrated that mesenchymal stem cell (MSC) fate is guided by extracellular matrix (ECM) elasticity. Motivated by these findings, researchers have focused on harnessing the mechanical environment for directing stem cell differentiation (i.e., mechanically induced differentiation), both with (89–91) and without (75, 92) the use of soluble factors. It is now recognized that matrix geometry, stiffness, adhesion, stress relaxation, micro- and nanopatterned surfaces, and applied cellular stretch can guide stem cell fate (8, 75, 90–92). Nonetheless, the specific mechanisms by which stem cell nuclei adapt to and differentiate within their mechanical environments remain incompletely understood. Thus, this section highlights nuclear mechanotransduction mechanisms guiding stem cell fate and describes how mechanotransduction can instill mechanical memory of differentiation states (Figure 3).
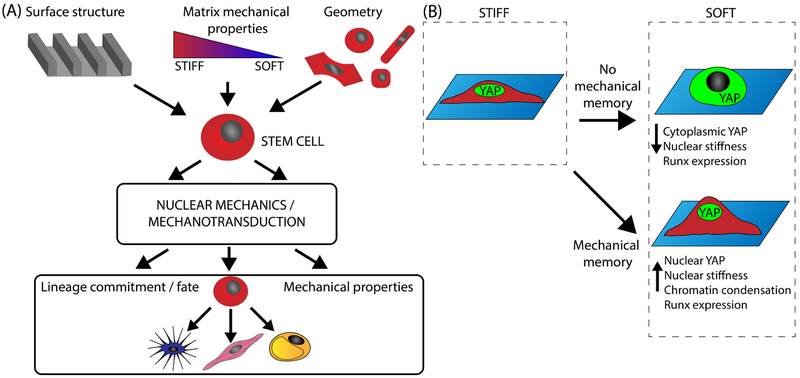
Figure 3.
Nuclear mechanics guide stem cell fate and mechanical memory. (a) Stem cells may undergo mechanically induced differentiation in response to matrix mechanical properties, surface structure, and geometry. Nuclear mechanotransduction in response to matrix sensing alters the transcriptional program to ultimately guide downstream lineage commitment and cellular mechanical properties. (b) Substrate stiffness may enable stem cells to exhibit mechanical memory, in which a stiff phenotype is remembered upon transfer to culture on a soft substrate, via nuclear YAP retention and chromatin condensation.
4.1. Mechanisms of Stem Cell Nuclear Mechanotransduction for Guiding Cell Fate
Compared with somatic cells, stem cells exhibit altered DNA and histone modifications (93), including highly condensed chromatin conformations, primarily at the nuclear periphery (94, 95), and altered expression of nuclear envelope proteins (52, 96, 97). Stem cells either completely lack or have reduced levels of Lamin A/C, resulting in more deformable nuclei (52). Lamin A/C interacts with chromatin to control gene expression (98) and restricts heterochromatin protein dynamics (96, 97). However, lamins are not required for differentiation. Embryonic stem cells (ESCs) lacking Lamin A/C, B1, and B2 [i.e., triple knockout (TKO)] differentiate into all three germ layers in vitro (16); keratinocyte-specific lamin TKO does not interfere with gestation in vivo but causes fatal skin defects upon birth (99). Together, these results suggest that lamins may be required for proper tissue architecture, rather than differentiation or organogenesis (16, 99). Nonetheless, experiments with MSCs and pluripotent stem cells (PSCs) indicate an intriguing connection between Lamin A/C and mechanically induced differentiation. MSCs, which express Lamin A/C, can undergo mechanically induced differentiation (75, 89–92), whereas minimal progress has been made toward mechanically induced differentiation in PSCs, which express little to no Lamin A/C (100). One potential pathway is the mechanosensitive phosphorylation of Lamin A/C, which enables nucleoplasmic Lamin A/C–LAP2α complex formation and subsequent regulation of adult stem cell proliferation and differentiation pathways, such as through retinoblastoma protein, to control stemness (101, 102). Given the intimate role of Lamin A/C in mechanotransduction, its specific contributions in regulation of mechanically induced stem cell differentiation should be further explored.
In addition to lamins, LINC complex proteins play a role in mechanically induced differentiation of MSCs. Nesprin-1 promotes mechanoresponsive YAP nuclear import (8) and is required for force transmission to the nuclear lamina and chromatin. Conversely, cyclic tensile strain downregulates Sun2 in MSCs, causing a global drop in transcription (103), downregulation of tubulin expression (104), and disturbed perinuclear microtubule organization (104), causing impaired nucleo-cytoskeletal decoupling. Taken together, these results suggest both positive and negative roles of the LINC complex and nuclear envelope in mechanically induced differentiation by mediating cytoskeletal organization, nucleo-cytoskeletal coupling, and regulation of gene expression through transcription factor import and signaling regulation. However, future studies should aim to further elucidate the roles of LINC complex proteins in nuclear mechanotransduction and mechanically induced differentiation of stem cells.
Additionally, stem cell pluripotency genes may be subject to mechanosensitive activation and silencing via downstream transcriptional control and chromatin modifications (74, 105). Mechanical strain independently localizes Emerin to the ONM (74, 106), which reduces H3K9me3-silenced heterochromatin, promotes the polymerization of perinuclear actin, and reduces nuclear actin levels (74). The decrease in nuclear actin diminishes RNA polymerase II activity, resulting in attenuated transcription, accumulation of phosphorylation, and H3K27me3 modification of chromatin, which corresponds to a more silenced state (74). Inhibiting this mechanism reduces methylation-mediated silencing, lineage commitment, and morphogenesis (74). Thus, this mechanism could explain how mechanically induced differentiation without soluble factors may be achieved: through regulation of stemness or promotion of differentiation.
4.2. Mechanically Induced Differentiation and Mechanical Memory
Mechanically induced differentiation has introduced the intriguing concept that stem cells can exhibit so-called mechanical memory. Whereas mechanotransduction typically involves rapid responses to changes in the physical environment of cells, this mechanical memory may allow stem cells to retain information and results of past mechanical conditions, which influences their future behavior and phenotype (Figure 3b). For example, culture of stem cells on stiff materials results in sustained nuclear YAP localization and osteogenic RUNX2 expression, even when cells are transferred to a soft substrate, on which YAP is typically cytoplasmic and RUNX2 is not expressed (107). Initial nuclear YAP translocation is likely mediated by cytoskeletal mechanotransduction and nuclear membrane stretch to open NPCs (60) via nucleo-cytoskeletal coupling through Nesprin-1 (8, 106). Subsequent nuclear stiffening, triggered by phosphorylation of Emerin to facilitate recruitment of Lamin A/C to the nuclear envelope (8), may contribute to the memory effect. Additionally, condensed chromatin stabilized via actin polymerization can persist after mechanical loading to create mechanical memory (75). Both of these mechanisms trigger chromatin condensation and nuclear stiffening, which correspond to a more differentiated state (92).
As mechanotransduction and signaling typically result in rapid adaptation to the mechanical environment, the concept of sustained mechanical memory is somewhat paradoxical: How do the classical mechanosensing mechanisms achieve permanent changes that resist further adaptation when the mechanical conditions have changed? The answer may lie in the persistent changes associated with stem cell differentiation. Mechanically induced stem cell differentiation causes altered chromatin organization, chromatin modifications, and gene expression, including that of nuclear and cytoskeletal proteins, thereby affecting nuclear mechanics, mechanotransmission, and mechanotransduction. These mechanoresponsive changes may be permanent and cannot easily be overcome by subsequent changes in the physical microenvironment, such as stiff to soft substrates or cessation of loading. However, further research is needed to fully elucidate the molecular events underlying the mechanical memory of stem cells and to determine how to harness this knowledge for applications using stem cells.
5. NUCLEAR MECHANOTRANSDUCTION GONE WRONG: SPOTLIGHT ON LAMINOPATHIES
Collectively, the laminopathies refer to diseases arising from mutations of the LMNA and LMNB genes. More than 450 different LMNA mutations give rise to ~14 different human diseases (see http://www.umd.be/LMNA/). Examples of human LMNA laminopathies include autosomal dominant Emery–Dreifuss muscular dystrophy (EDMD), dilated cardiomyopathy (DCM) with conduction defects, and Hutchinson–Gilford progeria syndrome. Many laminopathies primarily affect mechanically stressed tissues such as skeletal muscle, heart, and tendons. In contrast, only two human diseases have been associated with the LMNB1 and LMNB2 genes to date: adult-onset autosomal dominant leukodystrophy, resulting from duplication of the LMNB1 gene (108), and acquired partial lipodostrophy, associated with mutations in the LMNB1 gene (109). Most laminopathies are currently incurable, and several result in premature death. Intriguingly, mutations in genes encoding the LINC complex proteins (Emerin, Nesprins-1/2, Sun1/2) can cause several of the same or similar human diseases as LMNA mutations, including EDMD, DCM, and Charcot–Marie–Tooth syndrome (reviewed in Reference 41). Thus, these diseases are also referred to as nuclear envelopathies. With similar disease phenotypes observed in these nuclear envelopathies, altered nucleo-cytoskeletal coupling, nuclear mechanics, and disturbed mechanotransduction could be clear culprits in the disease pathology.
Classically, two cellular mechanisms by which laminopathies act in disease have been suggested: structural disruption and gene misregulation. The structural hypothesis proposes that mutant lamins cause nuclear fragility, leading to increased nuclear damage and cell death, particularly in mechanically stressed tissues. The gene regulation hypothesis suggests that lamin mutations play a tissue-specific role in gene expression by altering gene activation and silencing (98) or by inhibiting tissue-specific factor binding (110). Impaired stem cell differentiation caused by mutant lamins has been proposed as part of the gene regulation hypothesis. A third hypothesis, disrupted nuclear mechanotransduction, can bridge the mechanistic gap between the structural and gene regulation hypotheses, as disturbed gene regulation may, at least in part, be the product of physical disruption of nuclear mechanotransmission and mechanosensing (Figure 4). In the following subsections, we discuss laminopathies in the context of disrupted nuclear mechanics and mechanotransduction, particularly in light of the mechanisms discussed in Section 3.
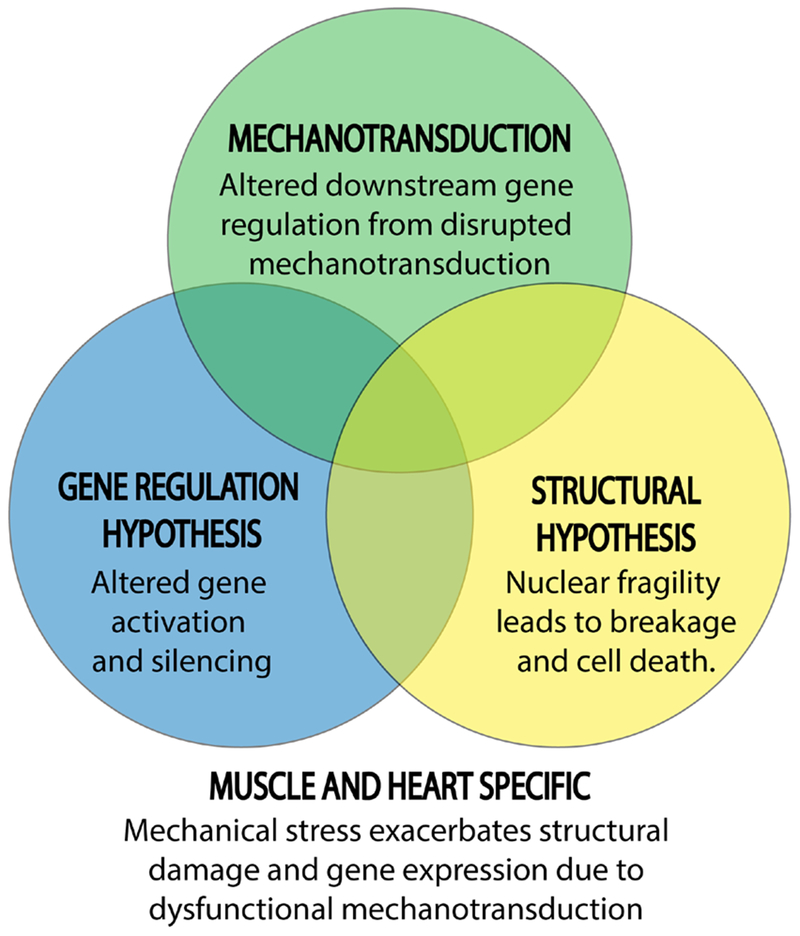
Figure 4.
Defective mechanotransduction as a bridge between laminopathy hypotheses. Structural defects (increased nuclear fragility that leads to breakage and cell death) and gene misregulation (altered gene activation and silencing) are the two primary hypothesized mechanisms responsible for the muscle-specific defects in many laminopathies. A third hypothesis—defective nuclear mechanotransduction—synthesizes both the structural disruption and gene misregulation hypotheses, as it can explain how downstream gene misregulation might be a product of nuclear weakness due to disruption of mechanotransduction mechanisms in and on the nucleus.
5.1. Disrupted Mechanotransduction as a Driver of Laminopathy Pathology
The physical consequences of laminopathies on the structure and function of the nuclear lamina have been known for nearly two decades (111–113). Mutant or mislocalized proteins can lead to disrupted interactions between lamins and their binding partners, thus disturbing the mechanical integrity of the lamina, connections to chromatin and LINC complex proteins, and transcriptional regulators. LMNA mutations associated with muscular defects frequently result in reduced nuclear stability (31, 114, 115). Furthermore, LMNA mutations increase susceptibility of Lamin A to phosphorylation (116), thereby increasing their solubility and promoting disassembly of the nuclear lamina. LMNA mutant nuclei are often subject to nuclear envelope blebbing and both spontaneous rupture and rupture due to mechanical stress (53, 54, 114). Nuclear instability and rupture yield reduced cellular viability (53), loss of cellular compartmentalization that can mislocalize both proteins and whole organelles (54, 117, 118), and DNA damage (115).
Changes in LINC expression or anchoring at the nuclear envelope due to LMNA mutations, overexpression of Sun1 (119), loss of Emerin (120), or loss or mislocalization of Nesprin-2G (121) disrupts mechanotransmission across the nuclear envelope. This impaired nucleo-cytoskeletal coupling (119–121) could explain disturbed nuclear positioning in skeletal muscle (122–124) and the loss of perinuclear actin filaments in LMNA mutant cells (34, 35, 45), which is associated with increased nuclear height, abnormal nuclear shape, and impaired YAP translocation into the nucleus (34, 45). Disruption of YAP translocation in response to cyclic stretch results in poor matrix adhesion and decreased cytoskeletal tension (125) that may be due to both loss of mechanically induced NPC opening (60) and Nesprin-1 disruption (8).
Loss of Lamin A/C results in NPC clustering (18, 126, 127), and mutations in the immunoglobulin fold of Lamin A/C result in defective binding to nucleoporin (128), which may inhibit the roles of NPCs in mechanosensitive gene regulation. Altered nuclear mechanics and nucleo-cytoskeletal coupling could further disrupt mechanosensitive NPC opening and nuclear import of transcription factors and downstream gene expression (60). Moreover, since nucleoporins interact with transcriptionally active euchromatin (129, 130), improper distribution of NPCs and nucleoporins resulting from LMNA mutations may perturb transcriptional regulation. Similarly, as lamin sequesters heterochromatin to the nuclear periphery, altered chromosome location due to LMNA mutations could dysregulate chromatin organization and gene expression. Several studies have demonstrated that relevant striated muscle genes are mislocalized to either the nuclear periphery or the center, depending on the mutation (131, 132). Such mislocalization could explain the altered tissue-specific gene expression observed in laminopathies (131–133), a concept that should be further explored using genome mapping technologies (see Section 6).
Furthermore, possibly as a downstream effect of disturbed nuclear or cytoplasmic mechanosensing, several critical signaling pathways regulating differentiation and proliferation are disrupted in LMNA mutant muscle. These include transforming growth factors β1 and 2 (134, 135). MyoD (136), MAPK (specifically extracellular signal–regulated kinases (ERK) 1 and 2, JNK, and p38α) (137, 138), and WNT/β-catenin (137–139), which may compromise tissue homeostasis. Consequently, LMNA mutations can disrupt myogenic differentiation in skeletal muscle (122, 136, 140), although other studies found that Lamin A/C–deficient myoblasts differentiation into myotubes is normal (115, 141). Lamin A/C is expressed in both muscle stem cells (MuSCs) and differentiated myofibers. Mutant forms cause improper cell cycle exit, decreased MuSC fusion with myofibers, and increased apoptosis during differentiation (140), as well as slower and less efficient differentiation (136). As a possible explanation for increased muscle wasting, DNA-dependent protein kinase (DNA-PK), which was recently linked to aging-related muscle wasting (142, 143), is activated in response to DNA damage (115). This activation may drive muscle health decline in EDMD, possibly through apoptosis mediated by the activation of Caspase-3 (115).
5.2. Strategies to Remedy Cellular Pathology
Targeting disrupted signaling in LMNA laminopathies may open a window for the pharmaceutical treatment of laminopathies (144). WNT/β-catenin stimulation (139) and p38α MAPK inhibition (138, 145, 146) improve cellular pathology and disease outcomes, including improved cellular mechanical properties, cytoskeletal structure, cardiac contractility, and survival. Targeting impaired nuclear stability may present another therapeutic avenue. Pharmaceutical stabilization of microtubules, which reduces nuclear deformation, and depletion of the microtubule motor kinesin 1, involved in nuclear shuttling in skeletal muscle, prevented accrual of nuclear damage by nuclear envelope rupture and chromatin protrusions in Lamin A/C–deficient skeletal muscle cells in vitro (115). Although preliminary, these results demonstrate that reducing mechanical stress on the nucleus can positively influence laminopathic prognosis and represent a new treatment option that should be explored for laminopathies affecting skeletal muscle.
6. CURRENT TECHNOLOGIES FOR THE STUDY OF MECHANOTRANSDUCTION, NUCLEAR MECHANICS, AND RELATED DISEASES
Our knowledge of mechanotransduction and nuclear mechanics in stem cell biology and disease (laminopathies) is often the product of innovative technologies. From nuclear- to cellular- to tissue-level technologies, creative force application methods, imaging techniques, and model systems have defined the study of nuclear mechanotransduction (Table 1). In this section, we discuss current technological innovations, including super-resolution imaging, fluorescence molecular reporters, and engineered tissue constructs for analyzing the role of the nucleus and the corresponding mechanisms in mechanotransduction and disease (laminopathies).
Table 1.
Prominent technologies for elucidating mechanotransduction mechanisms
| Technique | Description | References |
|---|---|---|
| Detection techniques | ||
| Superresolution microscopy | Imaging techniques (i.e., SIM, dSTORM, cryo-ET) with protein-level resolution. Useful for examining nuclear organization, binding partners, and supramolecular structure. | 14, 17, 18 |
| BioID | Proteins are biotinylated when in proximity to an engineered fusion protein (such as Lamin A) to label and identify novel binding partners with mass spectrometry. Can be used to examine protein interactions in mechanically stressed or lamin-mutant nuclei. | 18, 169 |
| 4D nucleome | Genome mapping techniques (i.e., 4C, 5C, Hi-C, and ChIA-PET), for observing spatial organization and condensation states of chromatin. | 147 |
| Genomic labeling | Fluorescence tagging of chromatin using gene editing for tracking mechanosensitive reorganization of (multiple) gene loci. | 82, 151, 152 |
| FRAP | A target protein is fluorescently tagged, a small area is photobleached, and time of recovery of fluorescence to the area is measured to understand the recovery dynamics, such as for chromatin histone organization or modifications. | 153–156 |
| FRET | Visual monitoring of the interaction between fluorescently tagged proteins, which creates a FRET signal. Diverse applications to mechanotransduction, such as monitoring force-dependent protein interactions, chromatin modification/condensation, actin assembly, or measuring tension forces. | 77, 121, 157–159, 170 |
| FLIM | Through fluorescence tagging of chromatin and examining fluorescence lifetime, which corresponds to viscosity due to degree of chromatin packing, can be used for high-throughput spatial tracking of chromatin condensation in the nucleoplasm. | 159, 160 |
| Mechanical manipulation techniques | ||
| Isolated nuclei | Removal of the nucleus from a cell for the direct study of the nucleus and its constituents, eliminating any confounding effects from the cytoplasm and/or cytoskeleton. Force can be directly applied to the nucleus, such as for LINC complex force measurement or examination of nuclear changes. | 121, 169 |
| LINC complex disruption | Depletion or deletion of LINC complex proteins via gene editing. By examining any subsequent defects resulting from force application, the role of LINC complex proteins in mechanotransduction may be better understood. | |
| Tissue engineering techniques | ||
| Engineered (muscle) tissues | Cells are suspended in an ECM solution, compact to form a tissue between two flexible pillars, and tissues contract to deflect the pillars. Useful for examining cell and tissue structures, tissue generated forces, and improving maturity of tissues. | 161–168 |
| Micropatterning, structured, and engineered substrates | Cells are cultured on micrometer- or nanometer-scale geometries/architectures. Examining the subsequent nuclear changes and cellular signaling, behavior, or phentoype can give an understanding of the role of the nucleus in matrix sensation, such as in stem cell differentiation. | 72, 73 |
Abbreviations: ChIA-PET, chromatin-interaction analysis by paired-end tag sequencing; cryo-ET, cryo electron tomography; dSTORM, direct stochastic optical reconstruction microscopy; ECM, extracellular matrix; FLIM, fluorescence lifetime imaging microscopy; FRAP, fluorescence recovery after photobleaching; FRET, Förster resonance energy transfer; LINC, linker of the nucleoskeleton and cytoskeleton; SIM, structured illumination microscopy.
6.1. Molecular Probes for Nuclear Structure
Unraveling nuclear mechanotransduction has remained a challenge due to (a) the complex and interconnected nature of the nuclear constituents and (b) the microscopic scale required to mechanically probe the nuclear components. Thus, several techniques, such as superresolution microscopy, fluorescence reporters for nanoscale forces and deformation, and tools to probe nuclear structure and organization across the whole genome stand at the forefront of technologies to overcome such obstacles. Superresolution imaging techniques, such as structured illumination microscopy (17), dSTORM (direct stochastic optical reconstruction microscopy) (18), and cryo-ET (cryo–electron tomography) (14), among others, have enabled the observation of the organization of the nuclear lamina and their binding partners at the protein level and have revealed nuclear supramolecular structures and unexpected details of lamin filament organization (14). To further probe protein–protein interactions at the nuclear envelope in living cells and animals, investigators have developed BioID, in which a protein of interest, such as Lamin A, is fused to a promiscuous version of BirA, an Escherichia coli biotin ligase. Proteins in close proximity (~10 nm) to the protein of interest are biotinylated and can subsequently be identified by mass spectrometry (18, 147). Newer versions of BioD have been developed to reduce the interaction radius and improved control over the timing of the biotinylation (148). BioID evades the removal of proteins from their native environment or the disruption of native protein interactions, as is the case with common alternative methods of yeast two-hybrid and coimmunoprecipitation assays (147). To date, these techniques have been used primarily to interrogate native nuclear protein conformations and Lamin A binding partners (147), but they could easily be applied to examine other key protein players and interactions in mechanically stressed or lamin-mutant nuclei in order to better understand mechanotransduction.
Characterization of the spatial organization of chromatin over time, termed the 4D nucleome(149) , has been a rapidly growing point of focus in cell biology. Genome interaction mapping techniques, evolved from the original 3C (chromosome conformation capture) methods to today’s 4C, 5C, Hi-C, and ChIA-PET (chromatin-interaction analysis by paired-end tag sequencing), have created high-resolution interaction maps of chromatin (149) and are beginning to be suitable for single-cell analysis. These techniques are now being applied to laminopathies(150), where they can yield novel insights into how transcription may be regulated in response to mechanical force or how chromatin may be disorganized in laminopathies.
6.2. Fluorescence Imaging for Nuclear Mechanics and Mechanotransduction
Chromatin reorganization, dynamics, interactions, condensation, and modifications may be better understood through fluorescence imaging techniques. Fluorescence tagging of chromatin using gene editing has been a common method of tracking reorganization of specific gene loci (82, 151, 152). Such methods and the use of multiple colors may, for example, allow tracking of several mechanosensitive genes simultaneously in response to mechanical force to better understand mechanosensitive chromosome reorganization. FRAP (fluorescence recovery after photobleaching) experiments, using tagging of histones or chromatin modifications via fluorescently labeled specific antigen binding fragments (Fabs), can examine chromatin dynamics in live cells (153–156). These techniques may be particularly useful to understand how mechanical stimulation affects chromatin dynamics, reorganization, and modification (155). As an additional approach, Förster resonance energy transfer (FRET)-based reporters can be used to monitor chromatin modification and condensation (157–159) in living cells. Recent fluorescence lifetime imaging microscopy experiments have enabled high-throughput spatial tracking of condensation of fluorescently labeled chromatin in the nuclear interior simply by using the viscosity of chromatin and bypassing any gene modification, such as overexpression, required by other techniques (159). Chromatin may be labeled either through fluorescently tagged histones(159) or the use of DNA-binding dyes (160). High chromatin condensation is associated with low viscosity and low fluorescence lifetime, while decondensation causes an increase in viscosity due to reduction in chromatin packing and therefore has a high fluorescence lifetime(160). Thus, epigenetic modifications and changes in nuclear chromatin localization can be spatially and temporally tracked, which is useful for observing changes in response to mechanical stresses, for understanding chromatin changes during stem cell differentiation, and for studying diseases involved with disrupted interactions with chromatin.
In addition to understanding chromatin dynamics, related imaging techniques can be useful for the study of other mechanotransduction mechanisms. FRET between fluorophores of a single type, known as homoFRET, has been used to visualize and quantitatively measure changes in F-/G-actin ratios upon force application, based on the homoFRET signal produced when actin molecules labeled with enhanced green fluorescent protein assemble into filaments(77). Furthermore, tension-based FRET molecular biosensors, in which the FRET signal inversely correlates with the force transmitted across the tension-sensor-containing molecule (121), enable one to probe mechanotransmission through various LINC complex proteins. This approach has already been successfully applied to measure forces across Nesprin-2G under different mechanical conditions (121). FRET biosensors could be further applied to examine the interactions of and force transmission across the cytoskeleton to other nuclear envelope proteins, reorganization or binding of the nuclear lamina to its many binding partners, or mechanically induced changes within the nucleus.
6.3. Engineered Muscle for Examining Tissue Mechanics in Laminopathies
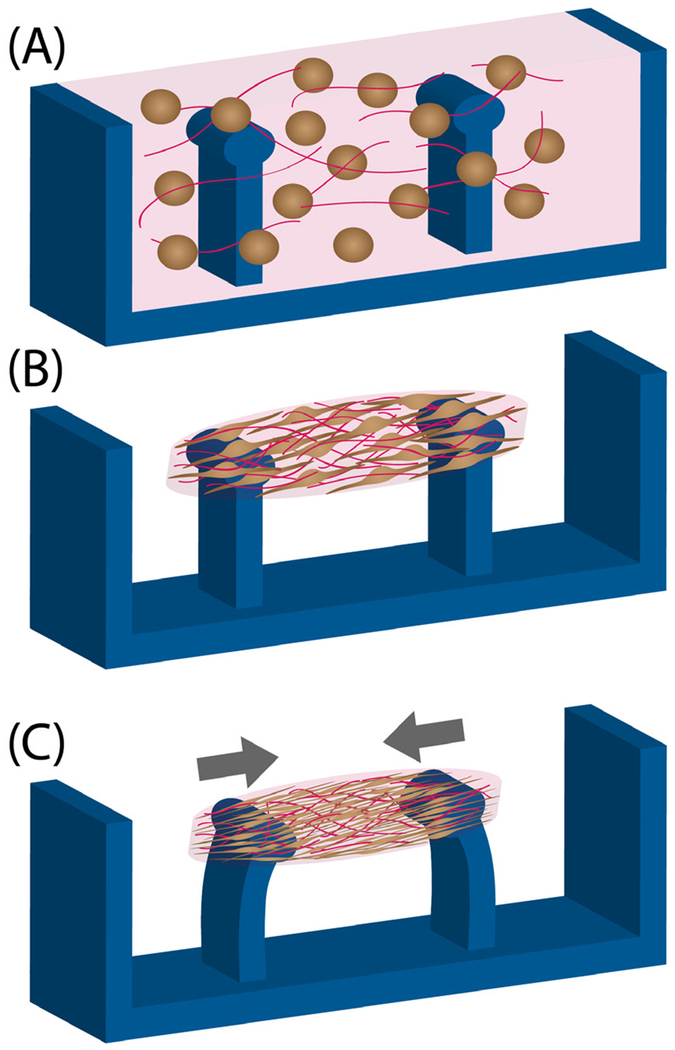
Current methods for the in vitro study of cardiac and skeletal muscle, particularly in 2D culture, insufficiently recapitulate the native structure and organization of mature muscle cells in tissues. Recently developed engineered skeletal muscle and cardiac tissues more closely mimicking native tissue structure and maturity present an intriguing opportunity for the study of laminopathies and their underlying nuclear and tissue mechanics, and offer better platforms for testing pharmacological treatments compared with 2D culture systems. Consequently, engineered muscle constructs, ranging from the micrometer to the centimeter scale, enable (a) examining cell and tissue structures, (b) examining tissue-generated forces, and (c) improving the maturity of tissues. To form tissues, muscle or heart cells or progenitors, either alone or in direct coculture with fibroblasts or other cell types (161), are suspended in an ECM solution (Figure 5a). Cells remodel and compact the ECM to form a tissue-like structure between two flexible pillars, which apply passive tension across the tissue that results in cytoskeletal and sarcomere alignment (Figure 5b) (162, 163). The engineered muscle tissues further compact over time and begin to contract as the muscle cells mature. The deflection of the flexible pillars (Figure 5c) can used to measure the tissue-generated forces (164). Optionally, mechanical and/or electrical stimulation can be applied to engineered tissues to further improve maturation (163, 165).
Figure 5.
Creation of engineered muscle tissue constructs for the study of tissue morphology and generated forces. (a) Devices are loaded with a cell (light brown) and extracellular matrix (pink) solution, and (b) cells reorganize and restructure the matrix to form a tissue around elastic pillars. (c) Tissues gradually compact and/or contract as cells elongate, thereby deflecting pillars. The force generated by the engineered tissue constructs can be calculated from the measured pillar deflection and the known material properties of the elastic pillars.
To date, engineered muscle has also been employed to study cardiac muscle maturation (163, 165, 166), examine cellular forces and anisotropy (162, 164), analyze generated contraction forces (164, 167, 168), and create disease models for examining cellular phenotype, such as Duchenne’s muscular dystrophy or EDMD (161). Engineered muscle tissues can be useful for assessing tissue structure or nuclear morphologies for various disease-causing mutations (161), assessing disruption of tissue-generated forces (168), and modeling of correction of disease-causing mutations (168). However, tissue and sarcomere maturity still do not fully recapitulate native tissue, particularly for stem cell–derived muscle, motivating further research.
7. CONCLUSIONS AND PERSPECTIVES
Over the past few decades, efforts to obtain a clearer picture of nuclear mechanotransduction have shed light on how the cellular microenvironment and mechanical force guide cellular behavior and phenotype, stem cell differentiation, and human diseases such as laminopathies. Mechanotransmission through perinuclear cytoskeletal assemblies and the LINC complex to the lamina and chromatin governs nuclear mechanical response to force and alters organization of chromatin and gene expression as well as downstream expression of LINC proteins. Mechanosensitive phosphorylation and protein conformation modulate nuclear strength by altering the organization of the nuclear envelope. Nuclear membrane stretch guides downstream mechanosignaling by stretching of NPCs for increased nuclear import of transcription factors and by allowing for mechanosensitive incorporation of proteins into the INM. Finally, chromatin organization, compaction, stretching, and modification control downstream mechanosensitive gene expression, although the specific guiding mechanisms should be further explored. Together, these nuclear mechanotransduction mechanisms guide mechanically induced stem cell differentiation and can instill mechanical memory of differentiation states. Disruption of any component or mechanism, such as in LMNA laminopathies, may induce a chain reaction of disrupted nuclear nucleo-cytoskeletal coupling, altered nuclear mechanics, and defective mechanotransduction elements and downstream mechanosignaling to cause human disease. An improved understanding of defective mechanotransmission and mechanotransduction signaling that enables targeting affected pathways and components may ultimately allow these pathologies to be remedied. Future research should aim to gain a more systematic understanding the cascade of nuclear mechanotransduction events. Particularly, which nuclear mechanisms are a direct response to mechanical force (i.e., true mechanosensors), and which are a product of downstream signaling? Elucidation of the positive and negative feedback loops driving nuclear mechanotransduction would clarify how the many individual mechanisms relate and work together to guide downstream cellular phenotype and function and would shed new light on the nucleus as a mechanosensor.
ACKNOWLEDGMENTS
The authors apologize to all investigators whose work could not be cited due to space constraints. The writing of this review was supported by awards from the National Institutes of Health (R01 HL082792 and U54 CA210184), the US Department of Defense Breast Cancer Research Program (Breakthrough Award BC150580), and the National Science Foundation (CAREER Award CBET-1254846 and MCB-1715606), and by a National Science Foundation Graduate Research Fellowship to M.M. (2016229710). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the National Science Foundation.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Wang N, Tytell JD, Ingber DE. 2009. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat. Rev. Mol. Cell Biol 10:75–82 [DOI] [PubMed] [Google Scholar]
- 2.Doyle AD, Yamada KM. 2016. Mechanosensing via cell–matrix adhesions in 3D microenvironments. Exp. Cell Res 343:60–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cho S, Irianto J, Discher DE. 2017. Mechanosensing by the nucleus: from pathways to scaling relationships. J. Cell Biol 216:305–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaalouk DE, Lammerding J. 2009. Mechanotransduction gone awry. Nat. Rev. Mol. Cell Biol 10:63–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang N, Butler JP, Ingber DE. 1993. Mechanotransduction across the cell surface and through the cytoskleton. Science. 260:1124–27 [DOI] [PubMed] [Google Scholar]
- 6.Martinac B. 2004. Mechanosensitive ion channels: molecules of mechanotransduction. J. Cell Sci 117:2449–60 [DOI] [PubMed] [Google Scholar]
- 7.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, et al. 2011. Role of YAP/TAZ in mechanotransduction. Nature 474:179–83 [DOI] [PubMed] [Google Scholar]
- 8.Driscoll TP, Cosgrove BD, Heo S-J, Shurden ZE, Mauck RL. 2015. Cytoskeletal to nuclear strain transfer regulates YAP signaling in mesenchymal stem cells. Biophys. J 108:2783–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.González JM, Navarro-Puche A, Casar B, Crespo P, Andrés V. 2008. Fast regulation of AP-1 activity through interaction of lamin A/C, ERK1/2, and c-Fos at the nuclear envelope. J. Cell Biol 183:653–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guilluy C, Osborne LD, Van Landeghem L, Sharek L, Superfine R, et al. 2014. Isolated nuclei adapt to force and reveal a mechanotransduction pathway in the nucleus. Nat. Cell Biol 16:376–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PCDP, et al. 2013. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science 341:1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guilluy C, Burridge K. 2015. Nuclear mechanotransduction: forcing the nucleus to respond. Nucleus 6:19–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soheilypour M, Peyro M, Jahed Z, Mofrad MRK. 2016. On the nuclear pore complex and its roles in nucleo-cytoskeletal coupling and mechanobiology. Cell. Mol. Bioeng 9:217–26 [Google Scholar]
- 14.Turgay Y, Eibauer M, Goldman AE, Shimi T, Khayat M, et al. 2017. The molecular architecture of lamins in somatic cells. Nature 543:261–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang SH, Chang SY, Yin L, Tu Y, Hu Y, et al. 2011. An absence of both lamin B1 and lamin B2 in keratinocytes has no effect on cell proliferation or the development of skin and hair. Hum. Mol. Genet 20:3537–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim Y, Zheng X, Zheng Y. 2013. Proliferation and differentiation of mouse embryonic stem cells lacking all lamins. Cell Res. 23:1420–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shimi T, Kittisopikul M, Tran J, Goldman AE, Adam SA, et al. 2015. Structural organization of nuclear lamins A, C, Bl, and B2 revealed by superresolution microscopy. Mol. Biol. Cell 26:4075–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie W, Chojnowski A, Boudier T, Lim JSY, Ahmed S, et al. 2016. A-type lamins form distinct filamentous networks with differential nuclear pore complex associations. Curr. Biol 26:2651–58 [DOI] [PubMed] [Google Scholar]
- 19.Domer D, Gotzmann J, Foisner R. 2007. Nucleoplasmic lamins and their interaction partners, LAP2α, Rb, and BAF, in transcriptional regulation. FEBS J. 274:1362–73 [DOI] [PubMed] [Google Scholar]
- 20.Gerace L, Blum A, Blobel G. 1978. Immunocytochemical localization of the major polypeptides of the nuclear pore complex–lamina fraction: interphase and mitotic distribution./. Cell Biol. 79:546–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gruenbaum Y, Foisner R. 2015. Lamins: nuclear intermediate filament proteins with fundamental functions in nuclear mechanics and genome regulation. Annu. Rev. Biochem 84:131–64 [DOI] [PubMed] [Google Scholar]
- 22.de Leeuw R, Gruenbaum Y, Medalia O. 2018. Nuclear lamins: thin filaments with major functions. Trends Cell Biol. 28:34–45 [DOI] [PubMed] [Google Scholar]
- 23.Kourmouli N, Dialynas G, Petraki C, Pyrpasopoulou A, Singh PB, et al. 2001. Binding of heterochromatin protein 1 to the nuclear envelope is regulated by a soluble form of tubulin. J. Biol. Chem 276:13007–14 [DOI] [PubMed] [Google Scholar]
- 24.Gurudatta BV, Shashidhara LS, Pamaik VK. 2010. Lamin C and chromatin organization in Drosophila. J. Genet 89:37–49 [DOI] [PubMed] [Google Scholar]
- 25.Goldman RD, Gruenbaum Y, Moir RD, Shumaker DK, Spann TP. 2002. Nuclear lamins: building blocks of nuclear architecture. Genes Dev. 16:533–47 [DOI] [PubMed] [Google Scholar]
- 26.Holaska JM, Lee KK, Kowalski AK, Wilson KL. 2003. Transcriptional repressor germ cell-less (GCL) and barrier to autointegration factor (BAF) compete for binding to emerin in vitro. J. Biol. Chem 278:6969–75 [DOI] [PubMed] [Google Scholar]
- 27.Guo Y, Kim Y, Shimi T, Goldman RD, Zheng Y. 2014. Concentration-dependent lamin assembly and its roles in the localization of other nuclear proteins. Mol. Biol. Cell 25:1287–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vaughan A, Alvarez-Reyes M, Bridger JM, Broers JL, Ramaekers FC, et al. 2001. Both emerin and lamin C depend on lamin A for localization at the nuclear envelope. J. Cell Sci 114:2577–90 [DOI] [PubMed] [Google Scholar]
- 29.Lammerding J, Hsiao J, Schulze PC, Kozlov S, Stewart CL, Lee RT. 2005. Abnormal nuclear shape and impaired mechanotransduction in emerin-deficient cells. J. Cell Biol 170:781–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shimi T, Pfleghaar K, Kojima S, Pack C-G, Solovei I, et al. 2008. The A- and B-type nuclear lamin networks: microdomains involved in chromatin organization and transcription. Genes Dev. 22:3409–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lammerding J, Schulze PC, Takahashi T, Kozlov S, Sullivan T, et al. 2004. Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J. Clin. Investig 113:370–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dechat T, Adam SA, Taimen P, Shimi T, Goldman RD. 2010. Nuclear lamins. Cold Spring Harb. Perspect. Biol 2:1–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lombardi ML, Jaalouk DE, Shanahan CM, Burke B, Roux KJ, Lammerding J. 2011. The interaction between nesprins and sun proteins at the nuclear envelope is critical for force transmission between the nucleus and cytoskeleton. J. Biol. Chem 286:26743–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khatau SB, Hale CM, Stewart-Hutchinson PJ, Patel MS, Stewart CL, et al. 2009. A perinuclear actin cap regulates nuclear shape. PNAS 106:19017–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim JK, Louhghalam A, Lee G, Schafer BW, Wirtz D, Kim DH. 2017. Nuclear lamin A/C harnesses the perinuclear apical actin cables to protect nuclear morphology. Nat. Commun 8:2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nikolova V, Leimena C, Mcmahon AC, Tan JC, Chandar S, et al. 2004. Defects in nuclear structure and function promote dilated cardiomyopathy in lamin A/C–deficient mice. J. Clin. Investig 113:357–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chandar S, Yeo LS, Leimena C, Tan JC, Xiao XH, et al. 2010. Effects of mechanical stress and carvedilol in lamin A/C–deficient dilated cardiomyopathy. Circ. Res 106:573–82 [DOI] [PubMed] [Google Scholar]
- 38.McGregor AL, Hsia CR, Lammerding J. 2016. Squish and squeeze—the nucleus as a physical barrier during migration in confined environments. Curr. Opin. Cell Biol 40:32–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crisp M, Liu Q, Roux K, Rattner JB, Shanahan C, et al. 2006. Coupling of the nucleus and cytoplasm: role of the LINC complex. J. Cell Biol 172:41–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tapley EC, Starr DA. 2013. Connecting the nucleus to the cytoskeleton by SUN-KASH bridges across the nuclear envelope. Curr. Opin. Cell Biol 25:57–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horn HF. 2014. LINC complex proteins in development and disease. Curr. Top. Dev. Biol 109:287–321 [DOI] [PubMed] [Google Scholar]
- 42.Rothballer A, Kutay U. 2013. The diverse functional LINCs of the nuclear envelope to the cytoskeleton and chromatin. Chromosoma 122:415–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Banerjee I, Zhang J, Moore-Morris T, Pfeiffer E, Buchholz KS, et al. 2014. Targeted ablation of nesprin 1 and nesprin 2 from murine myocardium results in cardiomyopathy, altered nuclear morphology and inhibition of the biomechanical gene response. PLOS Genet. 10:e1004114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Isermann P, Lammerding J. 2013. Nuclear mechanics and mechanotransduction in health and disease. Curr. Biol 23:R1113–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shiu J-Y, Aires L, Lin Z, Vogel V. 2018. Nanopillar force measurements reveal actin-cap-mediated YAP mechanotransduction. Nat. Cell Biol 20:262–71 [DOI] [PubMed] [Google Scholar]
- 46.Lele TP, Dickinson RB, Gundersen GG. 2018. Mechanical principles of nuclear shaping and positioning. J. Cell Biol 217:3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tariq Z, Zhang H, Chia-Liu A, Shen Y, Gete Y, et al. 2017. Lamin A and microtubules collaborate to maintain nuclear morphology. Nucleus 8:433–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dahl KN, Kahn SM, Wilson KL, Discher DE. 2004. The nuclear envelope lamina network has elasticity and a compressibility limit suggestive of a molecular shock absorber. J. Cell Sci 117:4779–86 [DOI] [PubMed] [Google Scholar]
- 49.Stephens AD, Banigan EJ, Adam SA, Goldman RD, Marko JF. 2017. Chromatin and lamin A determine two different mechanical response regimes of the cell nucleus. Mol. Biol. Cell 28:1984–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stephens AD, Banigan EJ, Marko JF. 2018. Separate roles for chromatin and lamins in nuclear mechanics. Nucleus 9:119–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schreiner SM, Koo PK, Zhao Y, Mochrie SGJ, King MC. 2015. The tethering of chromatin to the nuclear envelope supports nuclear mechanics. Nat. Commun 6:7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pajerowski JD, Dahl KN, Zhong FL, Sammak PJ, Discher DE. 2007. Physical plasticity of the nucleus in stem cell differentiation. PNAS 104:15619–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lammerding J, Fong LG, Ji JY, Reue K, Stewart CL, et al. 2006. Lamins A and C but not lamin B1 regulate nuclear mechanics. J. Biol. Chem 281:25768–80 [DOI] [PubMed] [Google Scholar]
- 54.De Vos WH, Houben F, Kamps M, Malhas A, Verheyen F, et al. 2011. Repetitive disruptions of the nuclear envelope invoke temporary loss of cellular compartmentalization in laminopathies. Hum. Mol. Genet 20:4175–86 [DOI] [PubMed] [Google Scholar]
- 55.Broers JLV, Peeters EAG, Kuijpers HJH, Endert J, Bouten CVC, et al. 2004. Decreased mechanical stiffness in LMNA−/− cells is caused by defective nucleo-cytoskeletal integrity: implications for the development of laminopathies. Hum. Mol. Genet 13:2567–80 [DOI] [PubMed] [Google Scholar]
- 56.Buxboim A, Swift J, Irianto J, Spinier KR, Dingal PCDP, et al. 2014. Matrix elasticity regulates lamin-A,C phosphorylation and turnover with feedback to actomyosin. Curr. Biol 24:1909–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Szczesny SE, Mauck RL. 2017. The nuclear option: evidence implicating the cell nucleus in mechanotransduction. J. Biomech. Eng 139:021006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kirby TJ, Lammerding J. 2018. Emerging views of the nucleus as a cellular mechanosensor. Nat. Cell Biol 20:373–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Enyedi B, Niethammer P. 2017. Nuclear membrane stretch and its role in mechanotransduction. Nucleus 8:156–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Elosegui-Artola A, Andreu I, Beedle AEM, Lezamiz A, Uroz M, et al. 2017. Force triggers YAP nuclear entry by regulating transport across nuclear pores. Cell 171:1397–410 [DOI] [PubMed] [Google Scholar]
- 61.Mazzanti M, Bustamante JO, Oberleithner H. 2001. Electrical dimension of the nuclear envelope. Physiol. Rev 81:1–19 [DOI] [PubMed] [Google Scholar]
- 62.Santella L, Carafoli E. 1997. Calcium signaling in the cell nucleus. FASEB J. 11:1091–109 [PubMed] [Google Scholar]
- 63.Malviya AN, Rogue PJ. 1998. “Tell me where is calcium bred”: clarifying the roles of nuclear calcium. Cell 92:17–23 [DOI] [PubMed] [Google Scholar]
- 64.Itano N, Okamoto S-I, Zhang D, Lipton SA, Ruoslahti E. 2003. Cell spreading controls endoplasmic and nuclear calcium: a physical gene regulation pathway from the cell surface to the nucleus. PNAS 100:5181–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Enyedi B, Jelcic M, Niethammer P. 2016. The cell nucleus serves as a mechanotransducer of tissue damage–induced inflammation. Cell 165:1160–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cho W, Stahelin R. 2005. Membrane–protein interactions in cell signaling and membrane trafficking. Annu. Rev. Biophys. Biomol. Struct 34:119–51 [DOI] [PubMed] [Google Scholar]
- 67.Tamada M, Sheetz MP, Sawada Y. 2004. Activation of a signaling cascade by cytoskeleton stretch. Dev. Cell 7:709–18 [DOI] [PubMed] [Google Scholar]
- 68.Sawada Y, Tamada M, Dubin-Thaler BJ, Cherniavskaya O, Sakai R, et al. 2006. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell 127:1015–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pasapera AM, Schneider IC, Rericha E, Schlaepfer DD, Waterman CM. 2010. Myosin II activity regulates vinculin recruitment to focal adhesions through FAK-mediated paxillin phosphorylation. J. Cell Biol 188:877–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kochin V, Shimi T, Torvaldson E, Adam SA, Goldman A, et al. 2014. Interphase phosphorylation of lamin A. J. Cell Sci 127:2683–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ihalainen TO, Aires L, Herzog FA, Schwartlander R, Moeller J, Vogel V. 2015. Differential basal-to-apical accessibility of lamin A/C epitopes in the nuclear lamina regulated by changes in cytoskeletal tension. Nat. Mater 14:1252–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li Y, Chu JS, Kurpinski K, Li X, Bautista DM, et al. 2011. Biophysical regulation of histone acetylation in mesenchymal stem cells. Biophys. J 100:1902–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jain N, Iyer KV, Kumar A, Shivashankar GV. 2013. Cell geometric constraints induce modular gene-expression patterns via redistribution of HDAC3 regulated by actomyosin contractility. PNAS 110:11349–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Le HQ, Ghatak S, Yeung CYC, Tellkamp F, Günschmann C, et al. 2016. Mechanical regulation of transcription controls Polycomb-mediated gene silencing during lineage commitment. Nat. Cell Biol 18:864–75 [DOI] [PubMed] [Google Scholar]
- 75.Heo S-J, Thorpe SD, Driscoll TP, Duncan RL, Lee DA, Mauck RL. 2015. Biophysical regulation of chromatin architecture instills a mechanical memory in mesenchymal stem cells. Sci. Rep 5:16895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Heo SJ, Han WM, Szczesny SE, Cosgrove BD, Elliott DM, et al. 2016. Mechanically induced chromatin condensation requires cellular contractility in mesenchymal stem cells. Biophys. J 111:864–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Iyer KV, Pulford S, Mogilner A, Shivashankar GV. 2012. Mechanical activation of cells induces chromatin remodeling preceding MKL nuclear transport. Biophys. J 103:1416–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maharana S, Iyer KV, Jain N, Nagarajan M, Wang Y, Shivashankar GV. 2016. Chromosome intermingling—the physical basis of chromosome organization in differentiated cells. Nucleic Acids Res. 44:5148–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang Y, Nagarajan M, Uhler C, Shivashankar GV. 2017. Orientation and repositioning of chromosomes correlate with cell geometry–dependent gene expression. Mol. Biol. Cell 28:1997–2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim DI, Birendra KC, Roux KJ. 2015. Making the LINC: SUN and KASH protein interactions. Biol. Chem 396:295–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim DH, Wirtz D. 2015. Cytoskeletal tension induces the polarized architecture of the nucleus. Biomaterials 48:161–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tajik A, Zhang Y, Wei F, Sun J, Jia Q, et al. 2016. Transcription upregulation via force-induced direct stretching of chromatin. Nat. Mater 15:1287–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lattanzi G, Cenni V, Marmiroli S, Capanni C, Mattioli E, et al. 2003. Association of emerin with nuclear and cytoplasmic actin is regulated in differentiating myoblasts. Biochem. Biophys. Res. Commun 303:764–70 [DOI] [PubMed] [Google Scholar]
- 84.de Lanerolle P, Serebryannyy L. 2011. Nuclear actin and myosins: life without filaments. Nat. Cell Biol 13:1282–88 [DOI] [PubMed] [Google Scholar]
- 85.Olson EN, Nordheim A. 2010. Linking actin dynamics and gene transcription to drive cellular motile functions. Nat. Rev. Mol. Cell Biol 11:353–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Baarlink C, Wang H, Grosse R. 2013. Nuclear actin network assembly by formins regulates the SRF coactivator MAL. Science 340:864–67 [DOI] [PubMed] [Google Scholar]
- 87.Plessner M, Melak M, Chinchilla P, Baarlink C, Grosse R. 2015. Nuclear F-actin formation and reorganization upon cell spreading. J. Biol. Chem 290:11209–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shao X, Li Q, Mogilner A, Bershadsky AD, Shivashankar GV. 2015. Mechanical stimulation induces formin-dependent assembly of a perinuclear actin rim. PNAS 112:E2595–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Engler AJ, Sen S, Sweeney HL, Discher DE. 2006. Matrix elasticity directs stem cell lineage specification. Cell 126:677–89 [DOI] [PubMed] [Google Scholar]
- 90.Lee J, Abdeen AA, Zhang D, Kilian KA. 2013. Directing stem cell fate on hydrogel substrates by controlling cell geometry, matrix mechanics and adhesion ligand composition. Biomaterials 34:8140–48 [DOI] [PubMed] [Google Scholar]
- 91.Chaudhuri O, Gu L, Klumpers D, Darnell M, Bencherif SA, et al. 2016. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater 15:326–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Heo SJ, Driscoll TP, Thorpe SD, Nerurkar NL, Baker BM, et al. 2016. Differentiation alters stem cell nuclear architecture, mechanics, and mechano-sensitivity. eLife 5:el8207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li E. 2002. Chromatin modification and epigenetic reprogramming in mammalian development. Nat. Rev. Genet 3:662–73 [DOI] [PubMed] [Google Scholar]
- 94.Labrador M, Corces VG. 2002. Setting the boundaries of chromatin domains and nuclear organization. Cell 111:151–54 [DOI] [PubMed] [Google Scholar]
- 95.West AG, Fraser P. 2005. Remote control of gene transcription. Hum. Mol. Genet 14:R101–11 [DOI] [PubMed] [Google Scholar]
- 96.Constantinescu D, Gray HL, Sammak PJ, Schatten GP, Csoka AB. 2006. Lamin A/C expression is a marker of mouse and human embryonic stem cell differentiation. Stem Cells 24:177–85 [DOI] [PubMed] [Google Scholar]
- 97.Melcer S, Hezroni H, Rand E, Nissim-Rafmia M, Skoultchi A, et al. 2012. Histone modifications and lamin A regulate chromatin protein dynamics in early embryonic stem cell differentiation. Nat. Commun 3:910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Peric-Hupkes D, Meuleman W, Pagie L, Bruggeman SWM, Solovei I, et al. 2010. Molecular maps of the reorganization of genome–nuclear lamina interactions during differentiation. Mol. Cell 38:603–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jung H-J, Tatar A, Tu Y, Nobumori C, Yang SH, et al. 2014. An absence of nuclear lamins in keratinocytes leads to ichthyosis, defective epidermal barrier function, and intrusion of nuclear membranes and endoplasmic reticulum into the nuclear chromatin. Mol. Cell. Biol 34:4534–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Murphy WL, McDevitt TC, Engler AJ. 2014. Materials as stem cell regulators. Nat. Mater 13:547–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gotic I, Schmidt WM, Biadasiewicz K, Leschnik M, Spilka R, et al. 2010. Loss of LAP2a delays satellite cell differentiation and affects postnatal fiber–type determination. Stem Cells 28:480–88 [DOI] [PubMed] [Google Scholar]
- 102.Gesson K, Vidak S, Foisner R. 2014. Lamina-associated polypeptide (LAP)2α and nucleoplasmic lamins in adult stem cell regulation and disease. Semin. Cell Dev. Biol 29:116–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gilbert HTJ, Mallikarjun V, Dobre O, Jackson MR, Pedley R, et al. 2018. Nuclear decoupling, through regulation of the LINC complex, is part of a rapid, protein-level cellular response to high-intensity mechanical loading. bioRxiv 317404 10.1101/317404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang Y, Qu R, Fan T, Zhu X, Feng Y, et al. 2018. Cross-talk between microtubules and the linker of nucleoskeleton complex plays a critical role in the adipogenesis of human adipose-derived stem cells. Stem Cell Res. Ther 9:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chowdhury F, Na S, Li D, Poh Y-C, Tanaka TS, et al. 2010. Material properties of the cell dictate stress-induced spreading and differentiation in embryonic stem cells. Nat. Mater 9:82–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Guilluy C, Osborne LD, Van Landeghem L, Sharek L, Superfine R, et al. 2014. Isolated nuclei adapt to force and reveal a mechanotransduction pathway in the nucleus. Nat. Cell Biol 16:376–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yang C, Tibbitt MW, Basta L, Anseth KS. 2014. Mechanical memory and dosing influence stem cell fate. Nat. Mater 13:645–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Padiath QS, Saigoh K, Schiffmann R, Asahara H, Yamada T, et al. 2006. Lamin B1 duplications cause autosomal dominant leukodystrophy. Nat. Genet 38:1114–23 [DOI] [PubMed] [Google Scholar]
- 109.Hegele RA, Cao H, Liu DM, Costain GA, Charlton-Menys V, et al. 2006. Sequencing of the reannotated LMNB2 gene reveals novel mutations in patients with acquired partial lipodystrophy. Am. J. Hum. Genet 79:383–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Simon DN, Wilson KL. 2013. Partners and post-translational modifications of nuclear lamins. Chromosoma 122:13–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vigouroux C, Auclair M, Dubosclard E, Pouchelet M, Capeau J, et al. 2001. Nuclear envelope disorganization in fibroblasts from lipodystrophic patients with heterozygous R482Q/W mutations in the lamin A/C gene. J. Cell Sci 114:4459–68 [DOI] [PubMed] [Google Scholar]
- 112.Novelli G, Muchir A, Sangiuolo F, Helbling-Leclerc A, D’Apice MR, et al. 2002. Mandibuloacral dysplasia is caused by a mutation in LMNA-encoding lamin A/C. Am. J. Hum. Genet 71:426–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Muchir A, Medioni J, Laluc M, Massart C, Arimura T, et al. 2004. Nuclear envelope alterations in fibroblasts from patients with muscular dystrophy, cardiomyopathy, and partial lipodystrophy carrying lamin A/C gene mutations. Muscle Nerve 30:444–50 [DOI] [PubMed] [Google Scholar]
- 114.Zwerger M, Jaalouk DE, Lombardi ML, Isermann P, Mauermann M, et al. 2013. Myopathic lamin mutations impair nuclear stability in cells and tissue and disrupt nucleo-cytoskeletal coupling. Hum. Mol. Genet 22:2335–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Earle AJ, Kirby TJ, Fedorchak G, Isermann P, Patel J, et al. 2018. Mutant lamins cause mechanically-induced nuclear envelope rupture, DNA damage, and DNA-PK activation in muscle. bioRxiv 364778 10.1101/364778 [DOI] [Google Scholar]
- 116.Cenni V, Sabatelli P, Mattioli E, Marmiroli S, Capanni C, et al. 2005. Lamin A N-terminal phosphorylation is associated with myoblast activation: impairment in Emery–Dreifuss muscular dystrophy. J. Med. Genet 42:214–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fidziańska A, Bilińska ZT, Tesson F, Wagner T, Walski M, et al. 2008. Obliteration of cardiomyocyte nuclear architecture in a patient with LMNA gene mutation. J. Neurol. Sci 271:91–96 [DOI] [PubMed] [Google Scholar]
- 118.Gupta P, Bilinska ZT, Sylvius N, Boudreau E, Veinot JP, et al. 2010. Genetic and ultrastructural studies in dilated cardiomyopathy patients: a large deletion in the lamin A/C gene is associated with cardiomyocyte nuclear envelope disruption. Basic Res. Cardiol 105:365–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen C-Y, Chi Y-H, Mutalif RA, Starost MF, Myers TG, et al. 2012. Accumulation of the inner nuclear envelope protein Sunl is pathogenic in progeric and dystrophic laminopathies. Cell 149:565–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hale CM, Shrestha AL, Khatau SB, Stewart-Hutchinson PJ, Hernandez L, et al. 2008. Dysfunctional connections between the nucleus and the actin and microtubule networks in laminopathic models. Biophys. J 95:5462–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Arsenovic PT, Ramachandran I, Bathula K, Zhu R, Narang JD, et al. 2016. Nesprin-2G, a component of the nuclear LINC complex, is subject to myosin-dependent tension. Biophys. J 110:34–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Houben F, Willems CHMP, Declercq ILJ, Hochstenbach K, Kamps MA, et al. 2009. Disturbed nuclear orientation and cellular migration in A-type lamin deficient cells. Biochim. Biophys. Acta 1793:312–24 [DOI] [PubMed] [Google Scholar]
- 123.Folker ES, Ostlund C, Luxton GWG, Worman HJ, Gundersen GG. 2011. Lamin A variants that cause striated muscle disease are defective in anchoring transmembrane actin-associated nuclear lines for nuclear movement. PNAS 108:131–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mattioli E, Columbaro M, Capanni C, Maraldi NM, Cenni V, et al. 2011. Prelamin A–mediated recruitment of SUN1 to the nuclear envelope directs nuclear positioning in human muscle. Cell Death Differ. 18:1305–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bertrand AT, Ziaei S, Ehret C, Duchemin H, Mamchaoui K, et al. 2014. Cellular microenvironments reveal defective mechanosensing responses and elevated YAP signaling in LMNA-mutated muscle precursors. J. Cell Sci 127:2873–84 [DOI] [PubMed] [Google Scholar]
- 126.Sullivan T, Escalante-Alcalde D, Bhatt H, Anver M, Bhat N, et al. 1999. Loss of A-type lamin expression compromises nuclear envelope integrity leading to muscular dystrophy. J. Cell Biol 147:913–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Verga L, Concardi M, Pilotto A, Bellini O, Pasotti M, et al. 2003. Loss of lamin A/C expression revealed by immuno-electron microscopy in dilated cardiomyopathy with atrioventricular block caused by LMNA gene defects. Virchows Arch. 443:664–71 [DOI] [PubMed] [Google Scholar]
- 128.Al-Haboubi T, Shumaker DK, Köser J, Wehnert M, Fahrenkrog B. 2011. Distinct association of the nuclear pore protein Nupl53 with A- and B-type lamins. Nucleus 2:500–9 [DOI] [PubMed] [Google Scholar]
- 129.Kalverda B, Pickersgill H, Shloma VV, Fomerod M. 2010. Nucleoporins directly stimulate expression of developmental and cell-cycle genes inside the nucleoplasm. Cell 140:360–71 [DOI] [PubMed] [Google Scholar]
- 130.Buchwalter AL, Liang Y, Hetzer MW. 2014. Nup50 is required for cell differentiation and exhibits transcription-dependent dynamics. Mol. Biol. Cell 25:2472–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mewbom SK, Puckelwartz MJ, Abuisneineh F, Fahrenbach JP, Zhang Y, et al. 2010. Altered chromosomal positioning, compaction, and gene expression with a lamin A/C gene mutation. PLOS ONE 5:el4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mattout A, Pike BL, Towbin BD, Bank EM, Gonzalez-Sandoval A, et al. 2011. An EDMD mutation in C. elegans lamin blocks muscle-specific gene relocation and compromises muscle integrity. Curr. Biol 21:1603–14 [DOI] [PubMed] [Google Scholar]
- 133.Zuela N, Dorfman J, Gruenbaum Y. 2017. Global transcriptional changes caused by an EDMD mutation correlate to tissue specific disease phenotypes in C. elegans. Nucleus 8:60–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Van Berio JH, Voncken JW, Kubben N, Broers JLV, Duisters R, et al. 2005. A-type lamins are essential for TGF-β1 induced PP2A to dephosphorylate transcription factors. Hum. Mol. Genet 14:2839–49 [DOI] [PubMed] [Google Scholar]
- 135.Bemasconi P, Carboni N, Ricci G, Siciliano G, Politano L, et al. 2018. Elevated TGF β2 serum levels in Emery–Dreifuss muscular dystrophy: implications for myocyte and tenocyte differentiation and fibrogenic processes. Nucleus 9:292–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Frock RL, Kudlow BA, Evans AM, Jameson SA, Hauschka SD, Kennedy BK. 2006. Lamin A/C and emerin are critical for skeletal muscle satellite cell differentiation. Genes Dev. 20:486–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Muchir A, Pavlidis P, Decostre V, Herron AJ, Arimura T, et al. 2007. Activation of MAPK pathways links LMNA mutations to cardiomyopathy in Emery–Dreifuss muscular dystrophy. J. Clin. Investig 117:1282–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Muchir A, Wu W, Choi JC, Iwata S, Morrow J, et al. 2012. Abnormal p38 mitogen-activated protein kinase signaling in dilated cardiomyopathy caused by lamin A/C gene mutation. Hum. Mol. Genet 21:4325–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Le Dour C, Macquart C, Sera F, Homma S, Bonne G, et al. 2017. Decreased WNT/β-catenin signalling contributes to the pathogenesis of dilated cardiomyopathy caused by mutations in the lamin A/C gene. Hum. Mol. Genet 26:333–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Favreau C, Higuet D, Courvalin J-C, Buendia B. 2004. Expression of a mutant lamin A that causes Emery–Dreifuss muscular dystrophy inhibits in vitro differentiation of C2C12 myoblasts. Mol. Cell. Biol 24:1481–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Melcon G, Kozlov S, Cutler DA, Sullivan T, Hernandez L, et al. 2006. Loss of emerin at the nuclear envelope disrupts the Rbl/E2F and MyoD pathways during muscle regeneration. Hum. Mol. Genet 15:637–51 [DOI] [PubMed] [Google Scholar]
- 142.Park S-J, Gavrilova O, Brown AL, Soto JE, Bremner S, et al. 2017. DNA-PK promotes the mitochondrial, metabolic, and physical decline that occurs during aging. Cell Metab. 25:1135–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chung JH. 2018. The role of DNA-PK in aging and energy metabolism. FEBS J. 285:1959–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Azibani F, Muchir A, Vignier N, Bonne G, Bertrand AT. 2014. Striated muscle laminopathies. Semin. Cell Dev. Biol 29:107–15 [DOI] [PubMed] [Google Scholar]
- 145.Laurini E, Martinelli V, Lanzicher T, Puzzi L, Borin D, et al. 2018. Biomechanical defects and rescue of cardiomyocytes expressing pathologic nuclear lamins. Cardiovasc. Res 114:846–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Natl. Inst. Health. 2017. A study of ARRY-371797 in patients with LMNA-related dilated cardiomyopathy. https://clinicaltrials.gov/ct2/show/NCT02057341
- 147.Roux KJ, Kim DI, Raida M, Burke B. 2012. A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells. J. Cell Biol 196:801–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Schopp IM, Amaya Ramirez CC, Debeljak J, Kreibich E, Skribbe M, et al. 2017. Split-BioID: a conditional proteomics approach to monitor the composition of spatiotemporally defined protein complexes. Nat. Commun 8:15690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Dekker J, Belmont AS, Guttman M, Leshyk VO, Lis JT, et al. 2017. The 4D nucleome project. Nature 549:219–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.McCord RP, Nazario-Toole A, Zhang H, Chines PS, Zhan Y, et al. 2013. Correlated alterations in genome organization, histone methylation, and DNA–lamin A/C interactions in Hutchinson–Gilford progeria syndrome. Genome Res. 23:260–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ma H, Naseri A, Reyes-Gutierrez P, Wolfe SA, Zhang S, Pederson T. 2015. Multicolor CRISPR labeling of chromosomal loci in human cells. PNAS 112:3002–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shao S, Zhang W, Hu H, Xue B, Qin J, et al. 2016. Long-term dual-color tracking of genomic loci by modified sgRNAs of the CRISPR/Cas9 system. Nucleic Acids Res. 44:e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bhattacharya D, Talwar S, Mazumder A, Shivashankar GV. 2009. Spatio-temporal plasticity in chromatin organization in mouse cell differentiation and during Drosophila embryogenesis. Biophys. J 96:3832–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ramdas NM, Shivashankar GV. 2015. Cytoskeletal control of nuclear morphology and chromatin organization. J. Mol. Biol 427:695–706 [DOI] [PubMed] [Google Scholar]
- 155.Harkness T, McNulty JD, Prestil R, Seymour SK, Klann T, et al. 2015. High-content imaging with micropatterned multiwell plates reveals influence of cell geometry and cytoskeleton on chromatin dynamics. Biotechnol. J 10:1555–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hayashi-Takanaka Y, Yamagata K, Wakayama T, Stasevich TJ, Kainuma T, et al. 2011. Tracking epigenetic histone modifications in single cells using Fab-based live endogenous modification labeling. Nucleic Acids Res. 39:6475–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sasaki K, Ito T, Nishino N, Khochbin S, Yoshida M. 2009. Real-time imaging of histone H4 hyperacetylation in living cells. PNAS 106:16257–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ito T, Umehara T, Sasaki K, Nakamura Y, Nishino N, et al. 2011. Real-time imaging of histone H4K12-specific acetylation determines the modes of action of histone deacetylase and bromodomain inhibitors. Chem. Biol 18:495–507 [DOI] [PubMed] [Google Scholar]
- 159.Llères D, James J, Swift S, Norman DG, Lamond AI. 2009. Quantitative analysis of chromatin compaction in living cells using FLIM-FRET. J. Cell Biol 187:481–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Spagnol ST, Dahl KN. 2016. Spatially resolved quantification of chromatin condensation through differential local rheology in cell nuclei fluorescence lifetime imaging. PLOS ONE 11:e0154639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Maffioletti SM, Sarcar S, Henderson ABH, Mannhardt I, Pinton L, et al. 2018. Three-dimensional human iPSC-derived artificial skeletal muscles model muscular dystrophies and enable multilineage tissue engineering. Cell Rep. 23:899–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.van Spreeuwel ACC, Bax NAM, Bastiaens AJ, Foolen J, Loerakker S, et al. 2014. The influence of matrix (an)isotropy on cardiomyocyte contraction in engineered cardiac microtissues. Integr. Biol 6:422–29 [DOI] [PubMed] [Google Scholar]
- 163.Ronaldson-Bouchard K, Ma SP, Yeager K, Chen T, Song L, et al. 2018. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature 556:239–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Legant WR, Pathak A, Yang MT, Deshpande VS, McMeeking RM, Chen CS. 2009. Microfabricated tissue gauges to measure and manipulate forces from 3D microtissues. PNAS 106:10097–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Abilez OJ, Tzatzalos E, Yang H, Zhao MT, Jung G, et al. 2018. Passive stretch induces structural and functional maturation of engineered heart muscle as predicted by computational modeling. Stem Cells 36:265–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tiburcy M, Hudson JE, Balfanz P, Schlick S, Meyer T, et al. 2017. Defined engineered human myocardium with advanced maturation for applications in heart failure modelling and repair. Circulation 135:1832–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sakar MS, Neal D, Boudou T, Borochin MA, Li Y, et al. 2012. Formation and optogenetic control of engineered 3D skeletal muscle bioactuators. Lab Chip 12:4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Long C, Li H, Tiburcy M, Rodriguez-Caycedo C, Kyrychenko V, et al. 2018. Correction of diverse muscular dystrophy mutations in human engineered heart muscle by single-site genome editing. Sci. Adv 4:eaap9004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Balikov DA, Brady SK, Ko UH, Shin JH, de Pereda JM, et al. 2017. The nesprin–cytoskeleton interface probed directly on single nuclei is a mechanically rich system. Nucleus 8:534–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Grashoff C, Hoffman BD, Brenner MD, Zhou R, Parsons M, et al. 2010. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature 466:263–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ho CY, Jaalouk DE, Vartiainen MK, Lammerding J. 2013. Lamin A/C and emerin regulate MLK1/SRF activity by modulating actin dynamics. Nature 497:507–11 [DOI] [PMC free article] [PubMed] [Google Scholar]