Abstract
Posttraumatic stress disorder (PTSD) is a severe polygenic disorder triggered by environmental factors. Many polymorphic genes, particularly the genetic determinants of hypodopaminergia (low dopamine function), associate with a predisposition to PTSD as well as Substance Use Disorder. Support from the National Institutes of Health for neuroimaging research and molecular, genetic applied technologies have improved understanding of brain reward circuitry functions have inspired the development of new innovative approaches to their early diagnosis and treatment of some PTSD symptomatology and addiction. This review presents psychosocial and genetic evidence that vulnerability or resilience to PTSD can theoretically be impacted by dopamine regulation. From a neuroscience perspective dopamine is widely accepted as a major neurotransmitter. Questions about how to modulate dopamine clinically in order to treat and prevent PTSD and other types of reward deficiency disorders remain. Identification of genetic variations associated with the relevant genotype-phenotype relationships can be characterized using the Genetic Addiction Risk Score (GARS®) and psychosocial tools. Development of an advanced genetic panel is under study and will be based on a new array of genes linked to PTSD. However, for now, the recommendation is that enlistees for military duty be given the opportunity to voluntarily pre-test for risk of PTSD with GARS, before exposure to environmental triggers, or upon return from deployment as part of PTSD management. Dopamine homeostasis may be achieved via customization of neuronutrient supplementation “Precision Behavioral Management” (PBM™) based on GARS test values, and other pro-dopamine regulation interventions like exercise, mindfulness, biosensor tracking, and meditation.
Keywords: Post-Traumatic Stress Disorder (PTSD), Genetic Addiction Risk Score (GARS™), Pro-Dopamine Regulation (KB220PAM), Hypodopaminergia, neuronutrient
Background
Although it is now widely accepted that individuals who have PTSD also have high co-morbidity with substance use disorder [1].The interrelatedness of reward circuitry and the prefrontal cortices of the brain and the importance of the core neurotransmitters were not understood until following many foundational studies from around the world [2–7] The Royal Society of Medicine published the Reward Deficiency Syndrome (RDS) concept [8]. To date, there have been 142 RDS articles listed within PubMed(3–24-19). Additionally, The SAGE Encyclopedia of Abnormal and Clinical Psychology included the RDS concept in 2017 [9].
Snapshot of Neuro-imaging PTSD
Recent neuroimaging research suggests that the self-processing Default Mode Network may be disrupted in many stress-related, psychiatric illnesses, including PTSD [10,11]. In healthy individuals, the Default Mode Network exhibits the most significant activity during periods of rest, with less activity observed with de-activation during cognitive tasks, e.g., working memory.
Many functional and structural imaging approaches have been developed to study the Default Mode Network which consists of medial temporal regions, lateral parietal cortices, and the medial prefrontal cortex, posterior cingulate cortex/precuneus [12]. In a seven-year longitudinal study, Martindale et al. [13] reported that white matter hyperintensities correlated significantly with the intensity of explosion blasts in combat veterans. The white matter hyperintensities were an independent finding, did not change with various psychiatric diagnoses, and were not explicitly related to PTSD. Also, a preliminary study by Averill et al. [14] discovered a negative correlation between cortical thickness in the left rostral middle frontal and left superior temporal regions and combat exposure severity. There was as an interaction between combat exposure severity and PTSD diagnosis in the superior temporal/insular region and a stronger negative correlation between combat exposure severity and cortical thickness in the non-PTSD group. The hippocampus and amygdala are repeatedly implicated in the psychopathology of PTSD. Akiki et al. [15] found that patients with more severe PTSD symptoms showed an indentation (decreased neuronal tissue) in the anterior half of the right hippocampus and an indentation in the dorsal region of the right amygdala (corresponding to the Centro-Medial Amygdala). Moreover, a post hoc analysis that employed stepwise regression suggests that among PTSD symptom clusters, arousal symptoms explained most of the variance in the hippocampal abnormality; whereas re-experiencing traumatic events explained most of the variance in the amygdala abnormality.
It is well known that prolonged stress can have long-lasting effects on cognition [11]. Animal models suggest that executive functioning deficits could result from alterations within the mesofrontal circuit. Along these lines, van Wingen and colleagues found that combat stress reduced midbrain activity and integrity and associated with a compromised ability to sustain attention. Long-term follow-up revealed that the functional and structural changes had normalized within 1.5 years. However, a reduction in functional connectivity between the midbrain and prefrontal cortex persisted [16].
These results demonstrate that combat stress has adverse effects on the human mesofrontal circuit and suggest that these alterations are partially reversible. Such effects impact normal dopaminergic function and reduce the ability to cope with stress. It is shown in magnetic resonance imaging studies that individuals with PTSD, due to prolonged stress, have smaller hippocampi volumes than those without a diagnosis of PTSD [17]. Although not well supported in human studies an initial hypothesis involving the mechanism underlying hippocampal alterations in PTSD focused on elevated glucocorticoid levels, in combination with extreme stress as the primary cause. It is noteworthy that Butler et al. [18] found that after receiving multimodal, psychological therapy for approximately six weeks, an increase in hippocampal volume and a trend toward an increase in amygdala volume was seen following therapy, with no change observed in the controls.
Post-Traumatic Stress Disorder is a subcategory of Reward Deficiency Syndrome
The development of Posttraumatic stress disorder (PTSD) is a result of complex interplay between environmental and genetic factors. RDS; hypodopaminergia is another neurobiological mechanism that underlies PTSD, and cross-addictions occur, especially, in psychiatric illness, including PTSD [11,19].
Cocaine use in PTSD is prevalent and associated with negative treatment, health, and societal consequences. Cocaine use disorder (CUD) appears to increase the risk of PTSD symptoms, especially, in females [20]. Mark Gold’s dopamine depletion hypothesis, proposed a vital role for dopamine in the effects of cocaine. Gold et al. (2006) suggested that the development of chronic CUD was due to the euphoric properties of cocaine and followed the acute activation of central dopamine neurons. Overstimulation of these neurons and excessive synaptic metabolism is thought to result in dopamine depletion, which may underlie the dysphoric aspects of cocaine abstinence and cocaine use urges [21]. The neurochemical disruptions caused by cocaine are consistent with the concept of “physical” rather than “psychological” addiction [22]. The proposal that followed this research was to treat CUD with dopamine agonist therapy.
The powerful dopamine D2 agonist bromocriptine was found to significantly reduce cocaine craving after a single dose [23]. The suggestion was that bromocriptine might be an effective, non-addictive pharmacological treatment for those with CUD and open trials indicated that low-dose bromocriptine might be useful in cocaine detoxification. Lawford et al. (1995) conducted a double-blind study, which administered bromocriptine or placebo to subjects with Alcohol Use Disorder. The most significant improvement in craving and anxiety occurred in the bromocriptine-treated subjects with the DRD2 A1 allele, and attrition was highest in the placebo-treated, A1 subjects [24]. However, we know now that chronic administration of this D2 agonist induces significant down-regulation of D2 receptors, thereby, preventing its clinical use [25,26].
Blum et al. proposed that D2 receptor stimulation could be accomplished with the use of KB220Z [27]. His group advocated promoting dopamine release, using milder therapeutic, neuro-nutrient formulations, to increase human mRNA expression causing proliferation of D2 receptors [28] to reduce craving and attenuate stress. Research based on this model has shown that DNA-directed compensatory overexpression of the DRD2 receptors (a form of gene therapy), results in a significant reduction in both alcohol craving behavior in alcohol-preferring rodents [29] and self-administration of cocaine [30].
Currently, the most widely accepted approach to treatment for substance use disorder (SUD) is medication-assisted treatment (MAT), which provides an immediate harm reduction strategy to combat substance use disorders. However, long-term, medication-assisted treatments promote unintentional dopamine down-regulation, and relapse prevention has been poor especially regarding buprenorphine-naloxone combinations. A prudent approach may be biphasic; a short-term blockade, followed by long-term dopaminergic up-regulation, with the goal of enhancing brain dopaminergic function, to target reward deficiency and stress-like, anti-reward symptomatology.
The promotion of long-term, dopaminergic activation by lower potency dopaminergic repletion therapy has been shown clinically to be an effective modality to treat RDS behaviors, including PTSD, substance use disorder, Attention-Deficit/Hyperactivity Disorder (ADHD), obesity and other behaviors that are associated with RDS [31]. Increased resting-state functional connectivity and increased neuronal recruitment have also been demonstrated, acutely, with this compound, using fMRI in both animals and humans. It is remarkable that, within 15 minutes (animal) to 60 minutes (human), post-administration of neuro-nutrient therapy, there has been additional dopamine neuronal firing in brain areas involved in reward processing, with possible neuroplasticity as a result [32,33]. Moreover, complementary structural and functional neuroimaging techniques used to examine the Default Mode Network could potentially improve understanding of the severity of psychiatric illness symptomatology and provide added validity to the clinical diagnostic process.
The comprehensive role of dopamine as the mesolimbic system neurotransmitter underlying motivational function supports the low potency, dopaminergic repletion therapy concept [34]. We hypothesize that gentle D2 receptor stimulation signals a feedback mechanism in the mesolimbic system to increase human mRNA expression causing proliferation of D2 receptors. Our proposal is a holistic, therapeutic model for PTSD that also includes the Genetic Addiction Risk Score (GARS) test for genetic risk predisposition and customization of neuronutrient supplementation, based on the GARS test result; Precision Behavioral Management (PBM). With recent indications that mental health treatment immediately after return from deployment may mitigate development of PTSD symptoms, it is an apt period to boost dopamine levels to reduce symptoms associated with reward deficiency.
Understanding the combined effects of hypodopaminergia and the Genetics of PTSD
David Comings, from the City of Hope, performed the first study to show an association between a reward gene, called the dopamine D2-receptor gene (A1 allele form), with people (military veterans), diagnosed with PTSD [35]. In conjunction with Ernest Noble, this same gene form had been shown by Blum’s laboratory to associate with severe alcoholism and cause non-alcoholic carriers to have 30–40 percent less dopamine D2 receptors in the brain [36].
Low dopamine function has been associated with increased risk for PTSD [37,38]. It is noteworthy that during combat stress dopamine is released from neurons at 100 times above the resting state. This epigenetic insult then is added to trait hypodopaminergia, (fewer dopamine D2 receptors). There is evidence to suggest that susceptibility to PTSD is hereditary. Genetics alone causes about 30% or more of the variance in PTSD; identical (monozygotic) twins with PTSD, exposed to combat in Vietnam, were associated with an increased risk of their co-twins having PTSD, compared to non-identical (dizygotic) twins [39].
Additionally, there is evidence that those with smaller hippocampi (a region of the brain involving memory), perhaps due to genetic differences, are more likely to develop PTSD, following traumatic stress. PTSD shares many genetic influences common to other psychiatric disorders. For example, PTSD shares 60% of the same genetic variance as panic and generalized anxiety disorders. Alcohol, nicotine, and drugs of abuse share greater than 40% genetic similarity. One study reported that soldiers, whose leukocytes had higher numbers of glucocorticoid receptors (involved in stress response) were more prone to develop PTSD, after experiencing traumatic stress [40,38].
Genetic antecedents may not tell the whole story since environmental insults or abuse (sexual and verbal) during childhood induce epigenetic changes that impact brain chemistry. Specifically, instead of being caused by differences in the DNA sequence, epigenetic changes are cellular, physiological and behavioral characteristics (phenotypic trait) changes that are caused by external or environmental factors that switch genes on and off. Unfortunately, epigenetic effects can occur for at least two subsequent generations [41]. The resultant effects of environmentally-induced, epigenetic changes in the chromatin structure of the DNA have been found, for example, to reduce the function and expression of the dopamine D2 receptor gene, thereby, increasing the likelihood of the development of PTSD. The take-home message here is that parental abuse in childhood and subsequent exposure to military combat or trauma as an adult may epigenetically increase the risk for PTSD. Genetic polymorphisms are evident in the development of PTSD, due to the regulation of gene expression within the serotoninergic and dopaminergic pathways. The 5-HTTLPR (promoter region of SLC6A4, which encodes the serotonin transporter) is a genetic candidate region that may be responsible for the modulation of emotional responses to traumatic events.
Depression and PTSD can be predictable results of the interaction of this variation of the 5HTTLPR gene and stressful life events. The dopaminergic pathway, specifically, the A1 allele coding the type 2 dopaminergic receptor, is associated with severe co-morbidity of PTSD, with the presence of somatic disorders, anxiety, and depression. There is an association between the polymorphism of gene GABRA2 and the occurrence of PTSD. There is an identified interaction between the Val (158)Met polymorphism of the gene coding for catecholamine-o-methyltransferase and number of traumatic events. Other genes include polymorphisms in FKBP5 (a co-chaperone of hsp 90 which binds to the glucocorticoid receptor), predict PTSD when triggered by-environment interaction [42]. Most recently Kandel’s group at Columbia found an association in female mice with a prion protein-based gene T1A1 that inhibits memory of fear [43].
Most recently, Zhang et al. [44] investigated the association between PTSD and gene-gene interaction (epistasis) within dopaminergic genes based upon the premise that this information could uncover the genetic basis of dopamine-related PTSD symptomatology and contribute to precision medicine. They found that a statistical analysis of genes identified a DRD2/ANNK1-COMT interaction (rs1800497 × rs6269), which associates with PTSD diagnosis (P interaction = 0.0008055 and P-corrected = 0.0169155). Single-variant and haplotype-based subset analyses showed that rs1800497 modulates the association directions of both the rs6269 G allele and the rs6269-rs4633-rs4818-rs4680 haplotype G-C-G-G. The interaction (rs1800497 × rs6269) was replicated in a young, Chinese female-cohort (32 cases and 581 controls), P interaction = 0.01329). The results suggested that rs1800497 is related to the DA (dopamine) receptor D2 density and rs6269-rs4633-rs4818-rs4680 haplotypes affect the catechol O-methyltransferase level and enzyme activity. Thus, the interaction was inferred to be at protein-protein and DA activity level. The genotype combinations of the two SNPs indicate a potential origin of DA homeostasis abnormalities in PTSD development.
PTSD and the Genetic Addiction Risk Score (GARS) test
There is an obvious need to classify patients at genetic risk for alcohol and drug-seeking behavior before entry to residential and or non-residential chemical dependency and pain programs, as well as, before entry into the military. Concerning the latter, the use of GARS has predictive value in identifying individuals faced with military combat who carry high-risk alleles for PTSD. One of the polymorphic variants measured in GARS, specifically the DRD2 A1 allele, has been shown to associate with PTSD and co-morbid substance use disorder. Comings et al. [35] studied patients in an addiction treatment unit screened for PTSD, after exposure to severe combat conditions in Vietnam. Of the 24 patients with PTSD, 58.3% carried the D2A1 allele. Of the remaining eight patients, who did not meet PTSD criteria, 12.5% were carriers of the D2A1 allele (p <0.04). Subsequently, in a replication study, using 13 PTSD patients, 61.5% carried the D2A1 allele, 11 patients who did not meet criteria for PTSD did not carry the allele D2A1 (p< 0.002). For the combined group, 59.5% with PTSD carried the D2A1 compared to 5.3% of those, who did not have PTSD (p< 0.0001) [35]. These results suggest that a DRD2 variant in linkage disequilibrium with the D2A1 allele confers an increased risk for PTSD, and the absence of the variant confers relative resistance to PTSD.
Based on an extensive literature review involving thousands of association studies, we have determined an addiction risk index, based on 11 polymorphisms from 10 genes that are involved in the neurological processing of reward. The GARS score included six SNPs in the DRD1, DRD2, DRD3, DRD4, COMT, and OPRM1 genes; four simple sequence repeats (SSRs) in the DAT1, DRD4, MAOA, and 5HTT transporter genes; and a dinucleotide polymorphism in the GABRA3 gene. Our studies, related to GARS [45], sought to address genetic risk for alcohol and drug seeking by evaluating whether the combined effect of reward gene polymorphisms contributing to a hypo-dopaminergic trait associated with RDS- related substance abuse risk. The patient population included 393 poly-drug abusers attending seven independent treatment centers from around the United States. Clinical severity of alcohol and drug use behaviors was assessed using the Addiction Severity Index (ASI-MV) in this unpublished study. Among those patients who consented to provide DNA via saliva for genotyping, 273 (derived from seven centers) also had ASI phenotypic information. The average age of our analysis sample was 35.3 years of age (S.D. – 13.1; range: 18–70) of which 57.8% (n = 160) were male and 88.1% (n = 244) self-reported their race as Caucasian.
Sequence variations in multiple genes regulating dopaminergic signaling influence risk in an additive manner. For alcohol severity scores, these results strongly suggest that age is a significant covariate. The GARS panel can provide useful information for not only appropriate substance addiction treatment, preliminary screening for high-risk patients in pain clinics, and relapse-prevention, but also the identification of soldiers at high risk for PTSD.
A meta-analysis performed recently on 19 studies that examined genetic variants in multiple dopaminergic genes and their association with PTSD exhibited inconsistent results [46]. The combined analysis included 1,752 subjects for rs1800497 in DRD2, 600 for the variable number tandem repeat (VNTR) in the solute carrier family six neurotransmitter transporter (SLC6A3), 1,044 for rs4680 in catechol-O-methyltransferase (COMT), and 394 for rs161115 in dopamine beta-hydroxylase (DBH). The studies meeting eligibility criteria provided data for genetic variants of genes involved in the dopaminergic system included subjects with a diagnosis of PTSD and were case controlled. Using random-effects, meta-analyses under the framework of a generalized linear model (GLM), findings confirmed that the rs1800497 SNP in DRD2 significantly associated with PTSD (OR=1.96, 95% CI; 1.15–3.33; p=0.014). The association of rs1800497 with PTSD remained when only subjects who had experienced combat-related trauma (OR=2.28, 95% CI; 1.08–4.81; p=0.032), or Caucasian subjects alone were the cohort (OR=3.16, 95% CI; 1.34–7.43; p=0.008) [39]. Furthermore, the 30-UTR VNTR in SLC6A3 also showed significant association with PTSD (OR=1.62, 95% CI; 1.12–2.35; P=0.010). The meta-analysis did not find an association with PTSD for the COMT Val158Met rs4680 allele (OR=0.91, 95% CI: 0.63–1.30; P=0.595) or the DBH homozygous TT rs161115 (OR=1.55, 95% CI: 0.39–6.20; P=0.536). However, due to the limited sample size examination of the association of rs161115 with PTSD, requires larger additional studies for validation. Importantly, information about the severity and type of traumatic events across studies in this meta-analysis were variable. This lack of data and heterogeneity regarding trauma exposures may explain, in part, the lack of an association between rs4680 in COMT with PTSD.
Concerning combat situations, a gene polymorphism test could be predictive of the vulnerability or resilience to risk for all RDS behaviors and PTSD. Genetic variants from multiple dopaminergic genes used in the GARS test and their association with PTSD including the study finding, the phenotype, the gene, the association, and the citation are listed in Table 1.
Table 1.
A list of with genetic variants from multiple dopaminergic genes used in GARS and their association with PTSD including the study findings and phenotype, the gene, the association, and references (superscript citations).
| DRD1: | |||
|---|---|---|---|
| Polymorphism | Study Findings and Phenotype | References | Comments |
| rs5326-A allele at the promoter region of dopamine receptor D1 (DRD1) locus CHR5 | Poor strategic Planning Poor logic or judgment | Tsang et al. 2015[47] | Poor logic or poor cognitive strategic planning leads to PTSD |
| rs4532 | Deficit in attention, avolition | Minichino et al.2017[48] | Poor focus and lack of goals can exacerbate PTS behaviors |
| rs4532 | Impulse Control Disorder | Zainal et al. 2015[49] | Impulse control underpins PTSD behaviors |
| rs4532 | Association with Alcohol dependence | Prasad et al. 2013[50] | Alcoholism is highly comorbid with PTSD |
| rs4532 | Reduced post-heroin Dependence pleasure | Zhu et al. 2013[50] | Important diagnostic feature of PTSD is anhedonia |
| rs4532 | Urbanicity upbringing alters PFC function | Reed et al. 2018[51] | Urbanicity in DRD1 polymorphic an antecedent for Psychiatric disorders |
| DRD2: | |||
| Polymorphism | Study Findings and Phenotype | References | Comments |
| rs1800497 | Linkage disequilibrium A1 allele | Comings et al.,1996[35] | A1 allele confers high risk for PTSD, and A2 allele protects |
| rs1800497 | Reduces dopamine homeostasis in PTSD Zhang et al., 20188 | Zhang et al., 2018[44] | Interaction with COMT confers PTSD risk |
| rs1800497 | Significantly associates with PTSD risk | Li et al., 2016[46] | Based on a meta-analysis |
| rs12364283 | Associated high risk for PTSD in psychostimulant abuse | Nelson et al., 2014[19] | War Veterans Cohort |
| rs1800497 | A1 allele magnifies PTSD severity | Hemmings et al., 2013[52] | South African Cohort |
| single nucleotide polymorphism (SNP) (957C>T) | Significant association PTSD risk | Voisey et al., 2009[53] | War Veterans Cohort |
| rs1800497 | Carriers of A1 allele increased Mississippi Scale for Combat PTSD score | Lawford et al., 2006[54] | Combat Veterans with DRD2 show severe PTSD symptoms |
| DRD3: | |||
| Polymorphism | Study Findings and Phenotype | References | Comments |
| rs6280 | Aberrant decision-making | Rajan et al., 2018[55] | DRD3 polymorphism will exacerbate poor decision-making in PTSD |
| rs6280 | significantly associated with Bruxism | Oporto et al., 2018[56] | There is a high comorbidity of Bruxism and PTSD |
| rs6280 | carrying the Ser9Gly variant associates with poor social conformity | Zhao et al., 2016[57] | People with PTSD have an inability to for social conformity style |
| rs6280 | significant association with alcohol dependence (AD) | Kang et al., 2014 [58] | High co-morbidity of AD and PTSD |
| rs6280 | reduced executive function | Bombin et al., 2008[59] | Poor decision-making is comorbid with PTSD |
| DRD4: | |||
| Polymorphisms | Study Findings and Phenotype | References | Comments |
| 48 base repeat VNTR (Chr11 exon3) 7 R & 8R | Intense PTSD Symptoms | Dragan & Onisczenko (2009)[60] | Flood Victims Cohort |
| 48 base repeat VNTR ( Chr11 exon3) 7R | Low Cortisol Response | Armbruster et al., 200920 | Inability to respond to stressful events |
| 48 base repeat | Low resilience to stress | Azadmarzab adi et al., 2018[61] | DRD4 7R associated with personality anxiety, depression, intelligence |
| 48 base repeat VNTR (Chr11 exon3) 7R + R | High Life Stress | Brody et al., 2012[62] | Life Stress X DRD4 &R carriers increase drug use in African Americans |
| 48 base repeat VNTR (Chr 11 exon 3) 7R | Association with childhood loss of trauma | Bakermans et al., 2011[63] | Carriers of the DRD4 7R have a lower ability to deal with parental |
| problems which increase children’s risk for later psychopathology including PTSD | |||
| DAT1: | |||
| Polymorphisms | Study Findings and Phenotype | References | Comments |
| 40 bases repeat VNTR (hr5,exon15) 9R | Carriers of 9R DAT1 associates with PTSD | Segman et al., 2002[64] | Dopamine genetics contribute to PTSD in Trauma Survivors |
| 40 bases repeat VNTR (Chr5, exon 15) 9R (rs28363170) and C allele of rs27072 | Children that carry the 9 R and the C allele have high PTSD following trauma than those without this haplotype | Drury et al., 2013[65] | Understanding this genetic antecedent provides future personalized epigenetic repair |
| 40 bases repeat 9R | Anxiety traits and neuroticism | Hünnerkopf et al., 2007[66] | Interaction with harm avoidance and Neuroticism |
| 40 bases repeat 9R | PTSD symptoms | Li et al. (2006)[46] | Meta-analysis of 19 studies |
| 40 bases repeat 9R | PTSD symptoms | Valente et al., (2011)[67] | Post Violent Urban Victims |
| COMT: | |||
| Polymorphisms | Study Findings and Phenotype | References | Comments |
| Val158/108Met rs4680 Chr22 G allele | One or two Met copies are protective against dysfunctional working memory | Mestrovic et al., (2018)[68] | Val/ Val carriers performed worst with high delay in recall in PTSD Veterans |
| Val158Met | One or two copies of Met protects induces fear inhibition | Deslauriers et al., (2018) [69] | Combat Marines with PTSD |
| Val158Met | One or two copies of Met protects against impaired Global Functional Outcome | Winkler et al., (2017)[70] | Val variant associates with poor Global Functional Outcome |
| Val158Met | Trauma in preschool varies with race in African Americans Met/Met higher PTSD symptoms but in EU Caucasian Val/Val higher PTSD. | Humphreys et al., (2014)[71] | Importance of moderating influence of race on genotype |
| Val158Val | Carriers have a higher prediction of PTSD compared to Val158Met | Clark et al (2013)[72] | Iraq Combat Veterans |
| Val158Met Rs 4680 | Carriers of rs4680SNP had high PTSD symptoms as part of a gene risk count of three other genes | Boscarino et al., (2012)[73] | FKBP5, CHRNA5, CRHR1and COMT contribute PTSD |
| MOA-A: | |||
| Polymorphisms | Study Findings and Phenotype | References | Comments |
| 30 bases repeat VNTR Chr X Promoter 3.5R, 4R | Maltreatment in childhood in carriers MOA-A VNTR had highest PTSD aggressive type symptoms | Zhang et al. (2017)[74] | The aggression phenotype was magnified with the serotonin risk genotype |
| MOA-A H 3.5R and 4R | In females, not men the MOA-A – H genotype showed high aggression | Verhoeven et al., (2012)[75] | The aggression in females with high activity genotype occurs when sadness is present. Opposite evidence for MOA-A L Higher amounts of available Dopamine could lead to increased aggression especially in males |
| Physical aggression scores were higher in men who had experienced early traumatic life events and who carried the low MAOA activity allele (MAOA-L). | Frazzetto et al. (2007) 2(5):e486 [76] | Due to low activity of MAO-A increased Dopamine leads to increased aggression after trauma in childhood | |
| 5HTTLPR:(SLC6A4) | |||
| Polymorphisms | Study Findings and Phenotype | References | Comments |
| 43 bases repeat INDEL/VNTR Chr 17 Risk alleles LG. S | Through Gene wide studies the serotonin transporter risk alleles associated with PTSD | Mehta et al., (2018) [77] | Australian and US Marine cohort |
| Rs25531 and risk alleles | Increased risk for PTSD in Earthquake survivors | Tian et al., (2015) [78] | Both 5-HTTLR and %-HTTVNTR polymorphisms associated with PTSD |
| Rs25531 and risk alleles | Increases odds of 1.5 of Combat Vets of having PTSD | Liu et al., (2015) [79] | Combat exposure cohort |
| Triallelic 5- HTTLPR | Homozygote SS genotype protects against PTSD re-experience of trauma | Walsh et al., (2014) [80] | African American Cohort childhood emotional abuse |
| Triallelic 5- HTTLPR | Homozygote SS genotype increased risk of PTSD in high trauma | Gressier et al., ( 2013) [81] | Meta-analysis mixed ethnic group |
| Triallelic 5- HTTLPR | Carriers of one or two copies S genotype confer increased risk of PTSD in childhood adversity | Xie et al., (2012) [82] | In a large mixed ethnic group the S genotype associated with anxiety and depression |
| rs16965628 (SLC6A4) | Modulated task-related ventrolateral PFC activation in PTSD | Morey et al., ( 2011) [83] | Combat related trauma |
| short (S)/long (L) of 5-HTTLPR (re 4795541) | Additive excess risk for frequent trauma in the L(A)/L(A) genotype | Grabe et al., (2009) [84] | 60% of all L(A) allele carriers exposed to 3 or more traumas developed PTSD |
| Mu-Opioid Receptor (OPRM1) | |||
| Polymorphisms | Study Findings and Phenotype | References | Comments |
| rs1799971 Chr6 Risk allele G | G carriers increase in anger proneness hostility, ego, negative feelings | Carver et al., ( 2016) [85] | Enhanced sensitivity to negative environments loads onto PTSD |
| rs1799971 | G carriers compared to A become more depressed when faced with social rejection | Slavich et al., (2014) [86] | Adolescent Cohort |
| GABRB3 | |||
| Polymorphisms | Study Findings and Phenotype | References | Comments |
| Alpha 3 Chr15 (DNR) Risk allele 181 ( non G1) | Increased somatic symptoms, anxiety, insomnia, social dysfunction, depression | Feusner et al., (2001) [87] | Carriers non-G allele in PTSD patients show high co-morbidity |
Table 1 Summary
In summary, the table depicts the exact SNPs measured in GARS and also displays a few other important SNPs not currently measured. The primary take home message is that based on an extensive literature search we have found very strong evidence of the measured SNPs in GARS and association with PTSD symptoms including:
▪ DRD1- poor strategic planning; poor logic or judgment; deficit in attention & avolition; impulse control disorder; association with alcohol dependence; reduced post-heroin dependence pleasure; urbanicity upbringing alters PFC function;
▪ DRD2- reduces dopamine homeostasis in PTSD; significantly associates with PTSD risk; associated high risk for PTSD in psychostimulant abuse; magnifies PTSD severity; increased Mississippi Scale for Combat PTSD score;
▪ DRD3- aberrant decision-making; significantly associated with Bruxism; associates with poor social conformity; significant association with alcohol dependence; reduced executive function;
▪ DRD4- intense PTSD symptoms; low cortisol response; low resilience to stress; high life stress; association with childhood loss and trauma;
▪ DAT1- increased DAT1 activity associates with PTSD; high PTSD following trauma; anxiety traits and neuroticism; high PTSD symptom;
▪ COMT- One or two Met copies are protective against dysfunctional working memory; one or two copies of Met protects against impaired Global Functional Outcome; trauma in preschool varies with race in African Americans Met/Met higher PTSD symptoms but in EU Caucasian Val/Val higher PTSD;
▪ MOA-A- maltreatment in childhood in carriers MOA-A VNTR had highest PTSD aggressive type symptoms; in females, not men the MOA-A –H genotype showed high aggression;
▪ 5HTTLPR:(SLC6A4)- through Gene wide studies the serotonin transporter risk alleles associated with PTSD; increased risk for PTSD in Earthquake survivors; increases odds of 1.5 for Combat Vets of having PTSD; homozygote SS genotype protects against PTSD re-experience of trauma; one or two copies of the S genotype confer increased risk of PTSD in childhood adversity; modulated task-related ventrolateral PFC activation in PTSD; additive excess risk for frequent trauma in the L(A)/L(A) genotype;
▪ Mu-Opioid Receptor (OPRM1)- G carriers increase in anger proneness hostility, ego, negative feelings; G carriers compared to A become more depressed when faced with social rejection;
▪ GABRB3- increased somatic symptoms, anxiety, insomnia, social dysfunction, depression.
Post-Traumatic Stress Disorder (PTSD) and Evidence-Based Benefits from Pro-Dopamine Regulation
PTSD is a psychiatric disorder with a genetic basis [38]. The disorder develops from a stress reaction after exposure of a person to physiological and -or psychological trauma, such as sexual assault, warfare, traffic collisions, violence, or other life-threatening events. Symptoms may include re-experiencing the trauma (flashbacks), or nightmares (lucid and non-lucid) related to the events and mental or physical distress induced by trauma-related cues as well as attempts to avoid trauma-related cues. Importantly, symptoms of PTSD include alterations in how a person thinks and feels, like amnesia about the event, fear of relationships, problems with sleeping and concentrating, and being hyper-vigilant (for example startled by loud noises). These symptoms can last for months and even years after the event and PTSD may result in higher suicide risk, long after the initial events [39].
Interestingly, not everyone who experiences a traumatic event develops PTSD. However, those who experienced interpersonal trauma, like rape or child abuse, are more likely to develop PTSD, compared to the experience of non-assault-based trauma, such as, accidents and natural disasters [88]. Moreover, after experiencing traumatic, environmental insults (like abuse), victims often experience long-lasting consequences that require treatment. Similarly, young children, who may be unable to process distress can also suffer prolonged adverse sequelae; treatment with a goal of expressing memories through play might help some children experience relief.
According to the American Psychiatric Association Diagnostic and Statistical Manual 5th edition (DSM-5) [89], about 3.5% of adults in the US each year have PTSD, and 9% develop it at some point during their lifetime. In the rest of the world, yearly rates are between 0.5% and 1%, although they are much higher in regions of armed conflict. Posttraumatic stress disorder is also more common in women than in men. Outside of the Department of Veterans Affairs clinical environment, the suggestion is that it is necessary for combat soldiers to be able to “handle the trauma” irrespective of any biological propensity to PTSD. It is well-known that some soldiers have, because of this stigma, not been able to seek help [90]. The truth is that many genetic studies from the 1990s until the present day have revealed that specific gene variants (called alleles) predispose some with an inability to handle trauma and stress. One possibly major issue is that because of the shrinking number of people enlisting in the military, and because of poor psychological scores from struggles with substance use, the government has had to reduce the number of applications accepted. The Army is now making it easier for enlistees displaying substance use disorder to obtain acceptance into the military by expanding the use of waivers for recruits with previous marijuana use, bad conduct, and some health problems [91].
Can We Prevent or Treat PTSD?
Early access to cognitive behavioral and trauma therapy has been of modest benefit in the treatment of PTSD [92]. Also, Critical Incident Stress Management has been suggested as a means of preventing PTSD. The definition of Critical Incident Stress Management is “an integrated, multi-component continuum of psychological interventions to be provided in the context of acute adversity, trauma, and disaster on an, as needed, basis to appropriate recipient populations.” Critical Incident Stress Management is neither a technique or a treatment for acute stress disorder, PTSD, post-traumatic depression, bereavement, or grief and may cause adverse outcomes. Interestingly, the World Health Organization recommends against the use of benzodiazepines and antidepressants in those having experienced trauma. Some evidence supports the use of the anti-inflammatory molecule hydrocortisone for prevention in adults. However, there is limited or no evidence supporting other drugs, such as propranolol, escitalopram, temazepam or gabapentin. Indeed, Gabapentin is a drug that stimulates the neurotransmitter GABA that reduces dopamine effects and should not be used to treat PTSD, especially in the long-term [93]. However, we are cognizant of the limited short term benefits of Ketamine for depression [94] a co-morbid phenotype of PTSD.
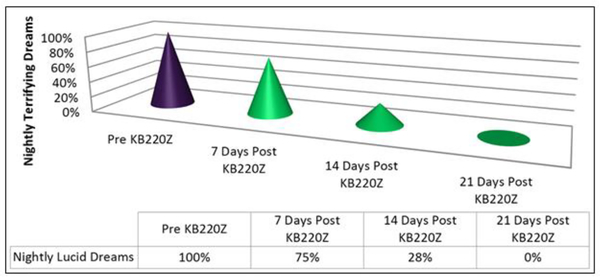
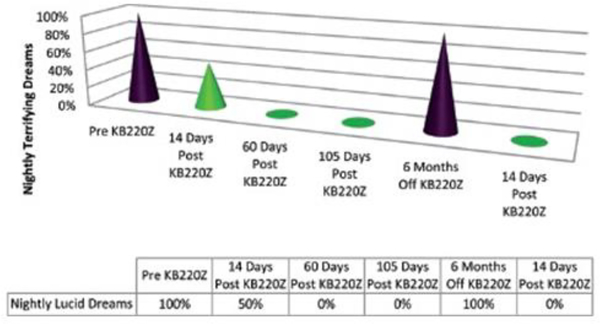
Clinical evidence has supported the use of a pro-dopamine regulator” (KB220Z) for the DSM5 intrusion Criterion in the for PTSD “ Recurrent distressing dreams” in which content and effect of the dream are related to the traumatic event(s)” [89]. Dr. Thomas McLaughlin, from the Center for Psychiatric Medicine, in Massachusetts, and others, carried out and published studies showing that chronic administration of a nutraceutical, KB220Z, eliminated terrifying, lucid nightmares in at least 82% of patients with PTSD and co-morbid ADHD [95,96]. The reduction or elimination of terrifying nightmares was dependent on KB220Z. Voluntary withdrawal of the agent resulted in the reinstatement of the terrifying, non-pleasant nature of the dreams (see Figures 1 and 2). In most cases, patients reported a gradual, but then complete, amelioration of their long-term, terrifying nightmares (lucid dreams) while taking KB220Z [97].
Fig 1.
Case Four; has been reproduced here with permission [95]
Fig 2.
Case One; has been reproduced here with permission [96]
However, in at least four cases the amelioration of nightmares persisted for up to 12 months, after a self-initiated, withdrawal from KB220Z use [97]. These cases warrant the possibility that KB200Z increases dopamine stability as well as functional connectivity between networks (cross-talk between different brain regions) of brain reward circuitry, as seen in fMRI studies of both rodents and humans [98,32,33]. The increased connectivity volume (recruitment of more dopamine neurons firing in the reward site of the brain) in rodents suggests the induction of epigenetic changes (neuroplastic adaptation), which may explain the continued amelioration of distressing trauma-related dreams in these cases[32].
There is an obvious need to treat PTSD in soldiers returning to the US after combat [99]. It is essential to find a way to reduce the suffering and trauma experienced by soldiers with untreated PTSD. Reducing the stigma of PTSD by embracing both genetic and epigenetic effects of traumatic stress might influence all people with PTSD to seek out treatment without fear or embarrassment. Fortunately, the Genetic Testing Center (Geneus Health) has developed PBM, which is the combination of patented GARS and an algorithmic-driven, precise, ingredient-based, pro-dopamine regulation of KB220PAM, having six genetically-tied formulae and matched to the polymorphic genes specific to each patient.
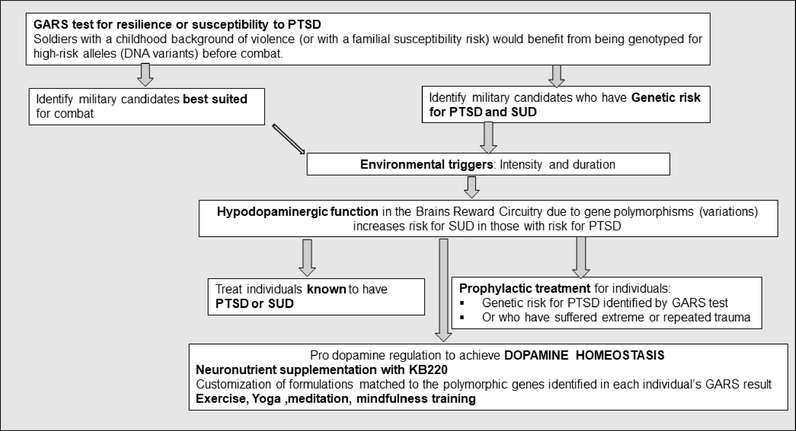
We hypothesize that, even before combat, soldiers with a childhood background of violence (or with a familial susceptibility risk) would benefit from being genotyped for high-risk alleles (DNA variants). This process may assist us in identifying potential military candidates who would be less well-suited for combat than those without high-risk alleles. Of secondary importance is finding non –addictive safe methods to treat individuals already exposed to combat and known to have PTSD. The behaviovioral management process proposed here would also greatly benefit individuals returning from deployment to help mitigate the effects traumatic, triggering experiences and promote integration into society. Since hypodopaminergic function in the brain’s reward circuitry due to gene polymorphisms (variations) is known to increase substance use disorder(SUD) in individuals with PTSD, it might be prudent, following a GARS test, to administer precision, pro-dopamine regulators, like KB220PAM, to effect the epigenetic expression (mRNA) to overcome this deficiency. In this way, soldiers would be less vulnerable to traumatic stress. While the GARS result alone is informative, used to guide precision KB220PAM, the test may reduce risks for both PTSD symptomatology and SUD, see Fig 3.
Fig 3.
Schematic of a novel approach to prevention and treatment of Post-Traumatic Stress Disorder (PTSD) based in science: The use Precision Behavioral Management (PBM) to combat PTSD.
A program that teaches pro-dopamine lifestyle and uses urine drug screens like the Comprehensive Analysis of Reported Drugs (CARD) to monitor outcomes and serve as a basis for therapeutic interactions is suggested for addiction. Can a pro-dopamine lifestyle, with gentle, prolonged D2 agonist therapy to compensate for DNA polymorphisms, promote positive epigenetic effects that can be transferred from generation to generation [100,101]? Holistic modalities like low glycemic index diet; mindfulness training, neurofeedback, yoga, and meditation are known to release neuronal dopamine [101,102] naturally, and, supported by the 12-step fellowship, might induce feelings of well-being and reduce craving and relapse. With this in mind, we wonder if we could potentially attenuate substance and non-substance seeking behaviors and increase resilience to PTSD through love and understanding [102]. As David E. Smith suggested in the late 60s; “Love needs care” [103].
Future Perspective
Certainly, complementary structural and functional neuroimaging techniques used to examine the Default Mode Network could potentially improve understanding of the severity of psychiatric illness symptomatology and provide added validity to the clinical diagnostic process. Recent neuroimaging research suggests that the self-processing Default Mode Network may be disrupted in many stress-related, psychiatric illnesses, including PTSD [15,13].
The hippocampus (memory recall) and amygdala (fear) are repeatedly implicated in the psychopathology of PTSD. Van Wingen et al. [16] analyzing their data related to long-term follow-up revealed that the functional and structural changes had normalized within 1.5 years. However, a reduction in functional connectivity between the midbrain and prefrontal cortex persisted. The literature results demonstrate that combat stress has adverse effects on the human mesofrontal circuit and suggest that these alterations are partially reversible. Such effects impact normal dopaminergic function and reduce the ability to cope with stress. It is shown in magnetic resonance imaging (MRI) studies that individuals with PTSD, due to prolonged stress, have smaller hippocampi volumes than those without a diagnosis of PTSD [17]. Although not well supported in human studies an initial hypothesis involving the mechanism underlying hippocampal alterations in PTSD focused on elevated glucocorticoid levels, in combination with extreme stress as the primary cause. It is noteworthy that Butler et al. [18] found that compared to controls, six weeks of multimodal, psychological therapy increased hippocampal volume and trended toward an increased amygdala volume. Thus it can be speculated that epigenetic repair approaches may be not just important but could be a frontline strategic therapeutic modality involving endorphinergic enhancement to balance or even induce “dopamine homeostasis” across the brain reward circuitry [32].
The role of dopamine as the mesolimbic system neurotransmitter underlying motivational function supports the low potency, dopaminergic repletion therapy concept [104]. We hypothesize that gentle D2 receptor stimulation signals a feedback mechanism in the mesolimbic system to increase human mRNA expression causing proliferation of D2 receptors. The proposal is a holistic, therapeutic model for PTSD that includes the Genetic Addiction Risk Score (GARS) test for genetic risk predisposition and customization of neuronutrient supplementation, based on the GARS test result that increases endorphinergic function and subsequently augments neuroimmunological function a prerequisite for PTSD attenuation via PBM requisites. With recent indications that mental health treatment immediately after return from deployment may mitigate the development of PTSD symptoms, it is an appropriate period to boost dopamine levels to reduce symptoms associated with reward deficiency.
It is understood that many genes and polymorphisms constitute a genetic risk for PTSD and with time more genes may be discovered. The proposed genetic panel may increase with time, and the stated genetic panel improved because PTSD is a complex subset of RDS and it is a polygenetic based disorder whereby each variant contributes a small part of the total contribution to the variance.
In summary, the basic concepts of the molecular and behavioral associations of PTSD reviewed here can underpin translational, addiction-related research to help the victims of genetically-induced RDS (including PTSD) become the recipients of better therapeutic, relapse-preventative care. As neuroscientists, psychologists and psychiatrists working in the “addiction space,” we encourage the global scientific community to take heed and reconsider the current utilization of dopaminergic blockade and, instead, adopt the goal of regaining dopamine homeostasis.
Optimistically, early diagnosis through genetic testing (including pharmacogenetics and pharmacogenomics), treatment with pro-dopamine regulation as a goal, and appropriate urine drug screening could conceivably reduce stress, craving, and relapse and enhance well-being. These actions could lead to eventual attenuation of PTSD, because of early GARS identification. Following required basic and clinically directed research, the notion of “genetically-guided therapy” may become a front-line technology with the potential to overcome, in part, the current heightened rates of PTSD (with 8,000 dying in 2017) and opioid overdose (72,000 lives lost in the same year).
Acknowledgments
Research directed toward improving substance use disorders, especially in under-served populations, is the basis of an NIH grant awarded to Dr. Kenneth Blum and Marjorie Gondré-Lewis. [Drs. Blum and Gondré-Lewis are the recipients of R41 MD012318/MD/NIMHD NIH HHS/United States]. We acknowledge editorial assistance from Margaret Madigan.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Bowirrat A, Chen TJ, Blum K, Madigan M, Bailey JA, Chuan Chen AL, Downs BW, Braverman ER, Radi S, Waite RL, Kerner M, Giordano J, Morse S, Oscar-Berman M, Gold M (2010) Neuro-psychopharmacogenetics and Neurological Antecedents of Posttraumatic Stress Disorder: Unlocking the Mysteries of Resilience and Vulnerability. Curr Neuropharmacol 8 (4):335–358. doi: 10.2174/157015910793358123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jellinek E (1960) The Disease Concept of Alcoholism. New Heaven: College and University Press, Italy [Google Scholar]
- 3.Davis VE, Walsh MJ (1970) Alcohol addiction and tetrahydropapaveroline. Science 169 (3950):1105–1106 [PubMed] [Google Scholar]
- 4.Hamilton MG, Blum K, Hirst M (1978) Identification of an isoquinoline alkaloid after chronic exposure to ethanol. Alcohol Clin Exp Res 2 (2):133–137 [DOI] [PubMed] [Google Scholar]
- 5.Collins MA, Kahn AJ (1982) Attraction to ethanol solutions in mice: induction by a tetrahydroisoquinoline derivative of L-DOPA. Subst Alcohol Actions Misuse 3 (5):299–302 [PubMed] [Google Scholar]
- 6.Cohen G, Collins M (1970) Alkaloids from catecholamines in adrenal tissue: possible role in alcoholism. Science 167 (3926):1749–1751 [DOI] [PubMed] [Google Scholar]
- 7.Blum K, Hamilton MG, Hirst M, Wallace JE (1978) Putative role of isoquinoline alkaloids in alcoholism: a link to opiates. Alcohol Clin Exp Res 2 (2):113–120 [DOI] [PubMed] [Google Scholar]
- 8.Blum K, Sheridan PJ, Wood RC, Braverman ER, Chen TJ, Cull JG, Comings DE (1996) The D2 dopamine receptor gene as a determinant of reward deficiency syndrome. J R Soc Med 89 (7):396–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blum K (2017) Reward Deficiency Syndrome. The SAGE Encyclopedia of Abnormal and Clinical Psychology Sage Publications, Inc, University of Pennsylvania School of Medicine, USA [Google Scholar]
- 10.O’Doherty DCM, Tickell A, Ryder W, Chan C, Hermens DF, Bennett MR, Lagopoulos J (2017) Frontal and subcortical grey matter reductions in PTSD. Psychiatry research Neuroimaging 266:1–9. doi: 10.1016/j.pscychresns.2017.05.008 [DOI] [PubMed] [Google Scholar]
- 11.O’Doherty DC, Chitty KM, Saddiqui S, Bennett MR, Lagopoulos J (2015) A systematic review and meta-analysis of magnetic resonance imaging measurement of structural volumes in posttraumatic stress disorder. Psychiatry research 232 (1):1–33. doi: 10.1016/j.pscychresns.2015.01.002 [DOI] [PubMed] [Google Scholar]
- 12.Philip NS, Carpenter SL, Sweet LH (2014) Developing neuroimaging phenotypes of the default mode network in PTSD: integrating the resting state, working memory, and structural connectivity. Journal of visualized experiments : JoVE (89). doi: 10.3791/51651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martindale SL, Rowland JA, Shura RD, Taber KH (2018) Longitudinal changes in neuroimaging and neuropsychiatric status of post-deployment veterans: a CENC pilot study. Brain injury 32 (10):1208–1216. doi: 10.1080/02699052.2018.1492741 [DOI] [PubMed] [Google Scholar]
- 14.Averill LA, Abdallah CG, Pietrzak RH, Averill CL, Southwick SM, Krystal JH, Harpaz-Rotem I (2017) Combat Exposure Severity is Associated with Reduced Cortical Thickness in Combat Veterans: A Preliminary Report. Chronic stress (Thousand Oaks, Calif) 1. doi: 10.1177/2470547017724714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akiki TJ, Averill CL, Wrocklage KM, Schweinsburg B, Scott JC, Martini B, Averill LA, Southwick SM, Krystal JH, Abdallah CG (2017) The Association of PTSD Symptom Severity with Localized Hippocampus and Amygdala Abnormalities. Chronic stress (Thousand Oaks, Calif) 1. doi: 10.1177/2470547017724069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Wingen GA, Geuze E, Caan MW, Kozicz T, Olabarriaga SD, Denys D, Vermetten E, Fernandez G (2012) Persistent and reversible consequences of combat stress on the mesofrontal circuit and cognition. Proc Natl Acad Sci U S A 109 (38):15508–15513. doi: 10.1073/pnas.1206330109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Logue MW, van Rooij SJH, Dennis EL, Davis SL, Hayes JP, Stevens JS, Densmore M, Haswell CC, Ipser J, Koch SBJ, Korgaonkar M, Lebois LAM, Peverill M, Baker JT, Boedhoe PSW, Frijling JL, Gruber SA, Harpaz-Rotem I, Jahanshad N, Koopowitz S, Levy I, Nawijn L, O’Connor L, Olff M, Salat DH, Sheridan MA, Spielberg JM, van Zuiden M, Winternitz SR, Wolff JD, Wolf EJ, Wang X, Wrocklage K, Abdallah CG, Bryant RA, Geuze E, Jovanovic T, Kaufman ML, King AP, Krystal JH, Lagopoulos J, Bennett M, Lanius R, Liberzon I, McGlinchey RE, McLaughlin KA, Milberg WP, Miller MW, Ressler KJ, Veltman DJ, Stein DJ, Thomaes K, Thompson PM, Morey RA (2018) Smaller Hippocampal Volume in Posttraumatic Stress Disorder: A Multisite ENIGMA-PGC Study: Subcortical Volumetry Results From Posttraumatic Stress Disorder Consortia. Biol Psychiatry 83 (3):244–253. doi: 10.1016/j.biopsych.2017.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butler O, Willmund G, Gleich T, Gallinat J, Kuhn S, Zimmermann P (2018) Hippocampal gray matter increases following multimodal psychological treatment for combat-related post-traumatic stress disorder. Brain and behavior 8 (5):e00956. doi: 10.1002/brb3.956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nelson EC, Heath AC, Lynskey MT, Agrawal A, Henders AK, Bowdler LM, Todorov AA, Madden PA, Moore E, Degenhardt L, Martin NG, Montgomery GW (2014) PTSD risk associated with a functional DRD2 polymorphism in heroin-dependent cases and controls is limited to amphetamine-dependent individuals. Addiction biology 19 (4):700–707. doi: 10.1111/adb.12062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saunders EC, Lambert-Harris C, McGovern MP, Meier A, Xie H (2015) The Prevalence of Posttraumatic Stress Disorder Symptoms among Addiction Treatment Patients with Cocaine Use Disorders. J Psychoactive Drugs 47 (1):42–50. doi: 10.1080/02791072.2014.977501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dackis CA, Gold MS (1985) New concepts in cocaine addiction: the dopamine depletion hypothesis. Neuroscience and biobehavioral reviews 9 (3):469–477 [DOI] [PubMed] [Google Scholar]
- 22.Noble EP, Blum K, Khalsa ME, Ritchie T, Montgomery A, Wood RC, Fitch RJ, Ozkaragoz T, Sheridan PJ, Anglin MD, et al. (1993) Allelic association of the D2 dopamine receptor gene with cocaine dependence. Drug Alcohol Depend 33 (3):271–285 [DOI] [PubMed] [Google Scholar]
- 23.Dackis CA, Gold MS, Sweeney DR, Byron JP Jr., Climko R (1987) Single-dose bromocriptine reverses cocaine craving. Psychiatry research 20 (4):261–264 [DOI] [PubMed] [Google Scholar]
- 24.Lawford BR, Young RM, Rowell JA, Qualichefski J, Fletcher BH, Syndulko K, Ritchie T, Noble EP (1995) Bromocriptine in the treatment of alcoholics with the D2 dopamine receptor A1 allele. Nat Med 1 (4):337–341 [DOI] [PubMed] [Google Scholar]
- 25.Bogomolova EV, Rauschenbach IY, Adonyeva NV, Alekseev AA, Faddeeva NV, Gruntenko NE (2010) Dopamine down-regulates activity of alkaline phosphatase in Drosophila: the role of D2-like receptors. Journal of insect physiology 56 (9):1155–1159. doi: 10.1016/j.jinsphys.2010.03.014 [DOI] [PubMed] [Google Scholar]
- 26.Rouillard C, Bedard PJ, Falardeau P, Dipaolo T (1987) Behavioral and biochemical evidence for a different effect of repeated administration of L-dopa and bromocriptine on denervated versus non-denervated striatal dopamine receptors. Neuropharmacology 26 (11):1601–1606 [DOI] [PubMed] [Google Scholar]
- 27.Blum K, Chen AL, Chen TJ, Braverman ER, Reinking J, Blum SH, Cassel K, Downs BW, Waite RL, Williams L, Prihoda TJ, Kerner MM, Palomo T, Comings DE, Tung H, Rhoades P, Oscar-Berman M (2008) Activation instead of blocking mesolimbic dopaminergic reward circuitry is a preferred modality in the long term treatment of reward deficiency syndrome (RDS): a commentary. Theor Biol Med Model 5:24. doi: 10.1186/1742-4682-5-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blum K, Oscar-Berman M, Stuller E, Miller D, Giordano J, Morse S, McCormick L, Downs WB, Waite RL, Barh D, Neal D, Braverman ER, Lohmann R, Borsten J, Hauser M, Han D, Liu Y, Helman M, Simpatico T (2012) Neurogenetics and Nutrigenomics of Neuro-Nutrient Therapy for Reward Deficiency Syndrome (RDS): Clinical Ramifications as a Function of Molecular Neurobiological Mechanisms. J Addict Res Ther 3 (5):139. doi: 10.4172/2155-6105.1000139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thanos PK, Rivera SN, Weaver K, Grandy DK, Rubinstein M, Umegaki H, Wang GJ, Hitzemann R, Volkow ND (2005) Dopamine D2R DNA transfer in dopamine D2 receptor-deficient mice: effects on ethanol drinking. Life Sci 77 (2):130–139. doi: 10.1016/j.lfs.2004.10.061 [DOI] [PubMed] [Google Scholar]
- 30.Thanos PK, Michaelides M, Umegaki H, Volkow ND (2008) D2R DNA transfer into the nucleus accumbens attenuates cocaine self-administration in rats. Synapse (New York, NY) 62 (7):481–486. doi: 10.1002/syn.20523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blum K, Febo M, Badgaiyan RD (2016) Fifty Years in the Development of a Glutaminergic-Dopaminergic Optimization Complex (KB220) to Balance Brain Reward Circuitry in Reward Deficiency Syndrome: A Pictorial. Austin addiction sciences 1 (2) [PMC free article] [PubMed] [Google Scholar]
- 32.Febo M, Blum K, Badgaiyan RD, Baron D, Thanos PK, Colon-Perez LM, Demortrovics Z, Gold MS (2017) Dopamine homeostasis: brain functional connectivity in reward deficiency syndrome. Frontiers in bioscience (Landmark edition) 22:669–691 [DOI] [PubMed] [Google Scholar]
- 33.Blum K, Liu Y, Wang W, Wang Y, Zhang Y, Oscar-Berman M, Smolen A, Febo M, Han D, Simpatico T, Cronje FJ, Demetrovics Z, Gold MS (2015) rsfMRI effects of KB220Z on neural pathways in reward circuitry of abstinent genotyped heroin addicts. Postgrad Med 127 (2):232–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blum K, Gardner E, Oscar-Berman M, Gold M (2012) “Liking” and “wanting” linked to Reward Deficiency Syndrome (RDS): hypothesizing differential responsivity in brain reward circuitry. Curr Pharm Des 18 (1):113–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Comings DE, Muhleman D, Gysin R (1996) Dopamine D2 receptor (DRD2) gene and susceptibility to posttraumatic stress disorder: a study and replication. Biol Psychiatry 40 (5):368–372. doi: 10.1016/00063223(95)00519-6 [DOI] [PubMed] [Google Scholar]
- 36.Blum K, Noble EP, Sheridan PJ, Montgomery A, Ritchie T, Jagadeeswaran P, Nogami H, Briggs AH, Cohn JB (1990) Allelic association of human dopamine D2 receptor gene in alcoholism. Jama 263 (15):2055–2060 [PubMed] [Google Scholar]
- 37.Noble EP, Blum K, Ritchie T, Montgomery A, Sheridan PJ (1991) Allelic association of the D2 dopamine receptor gene with receptor-binding characteristics in alcoholism. Arch Gen Psychiatry 48 (7):648–654 [DOI] [PubMed] [Google Scholar]
- 38.Blum K, Giordano J, Oscar-Berman M, Bowirrat A, Simpatico T, Barh D (2012) Diagnosis and Healing In Veterans Suspected of Suffering from Post-Traumatic Stress Disorder (PTSD) Using Reward Gene Testing and Reward Circuitry Natural Dopaminergic Activation. J Genet Syndr Gene Ther 3 (3):1000116. doi: 10.4172/21577412.1000116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roy-Byrne P, Arguelles L, Vitek ME, Goldberg J, Keane TM, True WR, Pitman RK (2004) Persistence and change of PTSD symptomatology--a longitudinal co-twin control analysis of the Vietnam Era Twin Registry. Social psychiatry and psychiatric epidemiology 39 (9):681–685. doi: 10.1007/s00127-004-0810-0 [DOI] [PubMed] [Google Scholar]
- 40.Vaswani KK, Richard CW 3rd, Tejwani GA (1988) Cold swim stress-induced changes in the levels of opioid peptides in the rat CNS and peripheral tissues. Pharmacol Biochem Behav 29 (1):163–168 [DOI] [PubMed] [Google Scholar]
- 41.Szutorisz H, DiNieri JA, Sweet E, Egervari G, Michaelides M, Carter JM, Ren Y, Miller ML, Blitzer RD, Hurd YL (2014) Parental THC exposure leads to compulsive heroin-seeking and altered striatal synaptic plasticity in the subsequent generation. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 39 (6):1315–1323. doi: 10.1038/npp.2013.352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Auxemery Y (2012) [Posttraumatic stress disorder (PTSD) as a consequence of the interaction between an individual genetic susceptibility, a traumatogenic event and a social context]. Encephale 38 (5):373–380. doi: 10.1016/j.encep.2011.12.003 [DOI] [PubMed] [Google Scholar]
- 43.Rayman JB, Hijazi J, Li X, Kedersha N, Anderson PJ, Kandel ER (2019) Genetic Perturbation of TIA1 Reveals a Physiological Role in Fear Memory. Cell reports 26 (11):2970–2983.e2974. doi: 10.1016/j.celrep.2019.02.048 [DOI] [PubMed] [Google Scholar]
- 44.Zhang K, Wang L, Cao C, Li G, Fang R, Liu P, Luo S, Zhang X, Liberzon I (2018) A DRD2/ANNK1-COMT Interaction, Consisting of Functional Variants, Confers Risk of Post-traumatic Stress Disorder in Traumatized Chinese. Frontiers in psychiatry 9:170. doi: 10.3389/fpsyt.2018.00170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blum K, Chen ALC, Thanos PK, Febo M, Demetrovics Z, Dushaj K, Kovoor A, Baron D, Smith DE, Roy AK III, Fried L, Chen TJH, Chapman E Sr., Modestino EJ, Steinberg B, Badgaiyan RD (2018) Genetic addiction risk score (GARS), a predictor of vulnerability to opioid dependence. Frontiers in bioscience (Elite edition) 10:175–196 [DOI] [PubMed] [Google Scholar]
- 46.Li L, Bao Y, He S, Wang G, Guan Y, Ma D, Wang P, Huang X, Tao S, Zhang D, Liu Q, Wang Y, Yang J (2016) The Association Between Genetic Variants in the Dopaminergic System and Posttraumatic Stress Disorder: A Meta-Analysis. Medicine (Baltimore) 95 (11):e3074. doi: 10.1097/md.0000000000003074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsang J, Fullard JF, Giakoumaki SG, Katsel P, Katsel P, Karagiorga VE, Greenwood TA, Braff DL, Siever LJ, Bitsios P, Haroutunian V, Roussos P (2015) The relationship between dopamine receptor D1 and cognitive performance. NPJ schizophrenia 1:14002. doi: 10.1038/npjschz.2014.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Minichino A, Francesconi M, Carrion RE, Bevilacqua A, Parisi M, Rullo S, Ando A, Biondi M, Delle Chiaie R, Cadenhead K (2017) Prediction of functional outcome in young patients with a recent-onset psychiatric disorder: Beyond the traditional diagnostic classification system. Schizophrenia research 185:114–121. doi: 10.1016/j.schres.2016.12.019 [DOI] [PubMed] [Google Scholar]
- 49.Zainal Abidin S, Tan EL, Chan SC, Jaafar A, Lee AX, Abd Hamid MH, Abdul Murad NA, Pakarul Razy NF, Azmin S, Ahmad Annuar A, Lim SY, Cheah PS, Ling KH, Mohamed Ibrahim N (2015) DRD and GRIN2B polymorphisms and their association with the development of impulse control behaviour among Malaysian Parkinson’s disease patients. BMC neurology 15:59. doi: 10.1186/s12883-015-0316-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prasad P, Ambekar A, Vaswani M (2013) Case-control association analysis of dopamine receptor polymorphisms in alcohol dependence: a pilot study in Indian males. BMC Res Notes 6:418. doi: 10.1186/17560500-6-418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reed JL, D’Ambrosio E, Marenco S, Ursini G, Zheutlin AB, Blasi G, Spencer BE, Romano R, Hochheiser J, Reifman A, Sturm J, Berman KF, Bertolino A, Weinberger DR, Callicott JH (2018) Interaction of childhood urbanicity and variation in dopamine genes alters adult prefrontal function as measured by functional magnetic resonance imaging (fMRI). PLoS One 13 (4):e0195189. doi: 10.1371/journal.pone.0195189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hemmings SM, Martin LI, Klopper M, van der Merwe L, Aitken L, de Wit E, Black GF, Hoal EG, Walzl G, Seedat S (2013) BDNF Val66Met and DRD2 Taq1A polymorphisms interact to influence PTSD symptom severity: a preliminary investigation in a South African population. Progress in neuro-psychopharmacology & biological psychiatry 40:273–280. doi: 10.1016/j.pnpbp.2012.10.011 [DOI] [PubMed] [Google Scholar]
- 53.Voisey J, Swagell CD, Hughes IP, Morris CP, van Daal A, Noble EP, Kann B, Heslop KA, Young RM, Lawford BR (2009) The DRD2 gene 957C>T polymorphism is associated with posttraumatic stress disorder in war veterans. Depression and anxiety 26 (1):28–33. doi: 10.1002/da.20517 [DOI] [PubMed] [Google Scholar]
- 54.Lawford BR, Young R, Noble EP, Kann B, Ritchie T (2006) The D2 dopamine receptor (DRD2) gene is associated with co-morbid depression, anxiety and social dysfunction in untreated veterans with post-traumatic stress disorder. European psychiatry : the journal of the Association of European Psychiatrists 21 (3):180–185. doi: 10.1016/j.eurpsy.2005.01.006 [DOI] [PubMed] [Google Scholar]
- 55.Rajan R, Krishnan S, Sarma G, Sarma SP, Kishore A (2018) Dopamine Receptor D3 rs6280 is Associated with Aberrant Decision-Making in Parkinson’s Disease. Movement disorders clinical practice 5 (4):413–416. doi: 10.1002/mdc3.12631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oporto GHt, Bornhardt T, Iturriaga V, Salazar LA (2018) Single nucleotide polymorphisms in genes of dopaminergic pathways are associated with bruxism. Clinical oral investigations 22 (1):331–337. doi: 10.1007/s00784-017-2117-z [DOI] [PubMed] [Google Scholar]
- 57.Zhao C, Liu J, Gong P, Hu J, Zhou X (2016) Investigating the Genetic Basis of Social Conformity: The Role of the Dopamine Receptor 3 (DRD3) Gene. Neuropsychobiology 74 (1):32–40. doi: 10.1159/000450710 [DOI] [PubMed] [Google Scholar]
- 58.Kang SG, Lee BH, Lee JS, Chai YG, Ko KP, Lee HJ, Han DM, Ji H, Jang GH, Shin HE (2014) DRD3 gene rs6280 polymorphism may be associated with alcohol dependence overall and with Lesch type I alcohol dependence in Koreans. Neuropsychobiology 69 (3):140–146. doi: 10.1159/000358062 [DOI] [PubMed] [Google Scholar]
- 59.Bombin I, Arango C, Mayoral M, Castro-Fornieles J, Gonzalez-Pinto A, Gonzalez-Gomez C, Moreno D, Parellada M, Baeza I, Graell M, Otero S, Saiz PA, Patino-Garcia A (2008) DRD3, but not COMT or DRD2, genotype affects executive functions in healthy and first-episode psychosis adolescents. American journal of medical genetics Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics 147b (6):873–879. doi: 10.1002/ajmg.b.30710 [DOI] [PubMed] [Google Scholar]
- 60.Dragan WL, Oniszczenko W (2009) The association between dopamine D4 receptor exon III polymorphism and intensity of PTSD symptoms among flood survivors. Anxiety, stress, and coping 22 (5):483–495. doi: 10.1080/10615800802419407 [DOI] [PubMed] [Google Scholar]
- 61.Armbruster D, Mueller A, Moser DA, Lesch KP, Brocke B, Kirschbaum C (2009) Interaction effect of D4 dopamine receptor gene and serotonin transporter promoter polymorphism on the cortisol stress response. Behavioral neuroscience 123 (6):1288–1295. doi: 10.1037/a0017615 [DOI] [PubMed] [Google Scholar]
- 62.Brody GH, Chen YF, Yu T, Beach SR, Kogan SM, Simons RL, Windle M, Philibert RA (2012) Life stress, the dopamine receptor gene, and emerging adult drug use trajectories: a longitudinal, multilevel, mediated moderation analysis. Dev Psychopathol 24 (3):941–951. doi: 10.1017/s0954579412000466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bakermans-Kranenburg MJ, van IMH, Caspers K, Philibert R (2011) DRD4 genotype moderates the impact of parental problems on unresolved loss or trauma. Attachment & human development 13 (3):253–269. doi: 10.1080/14616734.2011.562415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Segman RH, Cooper-Kazaz R, Macciardi F, Goltser T, Halfon Y, Dobroborski T, Shalev AY (2002) Association between the dopamine transporter gene and posttraumatic stress disorder. Mol Psychiatry 7 (8):903–907. doi: 10.1038/sj.mp.4001085 [DOI] [PubMed] [Google Scholar]
- 65.Drury SS, Brett ZH, Henry C, Scheeringa M (2013) The association of a novel haplotype in the dopamine transporter with preschool age posttraumatic stress disorder. J Child Adolesc Psychopharmacol 23 (4):236–243. doi: 10.1089/cap.2012.0072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hunnerkopf R, Strobel A, Gutknecht L, Brocke B, Lesch KP (2007) Interaction between BDNF Val66Met and dopamine transporter gene variation influences anxiety-related traits. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 32 (12):2552–2560. doi: 10.1038/sj.npp.1301383 [DOI] [PubMed] [Google Scholar]
- 67.Valente NL, Vallada H, Cordeiro Q, Miguita K, Bressan RA, Andreoli SB, Mari JJ, Mello MF (2011) Candidate-gene approach in posttraumatic stress disorder after urban violence: association analysis of the genes encoding serotonin transporter, dopamine transporter, and BDNF. Journal of molecular neuroscience : MN 44 (1):59–67. doi: 10.1007/s12031-011-9513-7 [DOI] [PubMed] [Google Scholar]
- 68.Havelka Mestrovic A, Tudor L, Nikolac Perkovic M, Nedic Erjavec G, Kovacic Petrovic Z, Svob Strac D, Konjevod M, Pivac N (2018) Significant association between catechol-O-methyltransferase (COMT) Val(158/108)Met polymorphism and cognitive function in veterans with PTSD. Neuroscience letters 666:38–43. doi: 10.1016/j.neulet.2017.12.033 [DOI] [PubMed] [Google Scholar]
- 69.Deslauriers J, Acheson DT, Maihofer AX, Nievergelt CM, Baker DG, Geyer MA, Risbrough VB (2018) COMT val158met polymorphism links to altered fear conditioning and extinction are modulated by PTSD and childhood trauma. Depression and anxiety 35 (1):32–42. doi: 10.1002/da.22678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Winkler EA, Yue JK, Ferguson AR, Temkin NR, Stein MB, Barber J, Yuh EL, Sharma S, Satris GG, McAllister TW, Rosand J, Sorani MD, Lingsma HF, Tarapore PE, Burchard EG, Hu D, Eng C, Wang KK, Mukherjee P, Okonkwo DO, Diaz-Arrastia R, Manley GT (2017) COMT Val(158)Met polymorphism is associated with posttraumatic stress disorder and functional outcome following mild traumatic brain injury. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia 35:109–116. doi: 10.1016/j.jocn.2016.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Humphreys KL, Scheeringa MS, Drury SS (2014) Race moderates the association of Catechol-O-methyltransferase genotype and posttraumatic stress disorder in preschool children. J Child Adolesc Psychopharmacol 24 (8):454–457. doi: 10.1089/cap.2014.0077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Clark R, DeYoung CG, Sponheim SR, Bender TL, Polusny MA, Erbes CR, Arbisi PA (2013) Predicting posttraumatic stress disorder in veterans: interaction of traumatic load with COMT gene variation. Journal of psychiatric research 47 (12):1849–1856. doi: 10.1016/j.jpsychires.2013.08.013 [DOI] [PubMed] [Google Scholar]
- 73.Boscarino JA, Erlich PM, Hoffman SN, Zhang X (2012) Higher FKBP5, COMT, CHRNA5, and CRHR1 allele burdens are associated with PTSD and interact with trauma exposure: implications for neuropsychiatric research and treatment. Neuropsychiatr Dis Treat 8:131–139. doi: 10.2147/ndt.s29508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang Y, Ming QS, Yi JY, Wang X, Chai QL, Yao SQ (2017) Gene-Gene-Environment Interactions of Serotonin Transporter, Monoamine Oxidase A and Childhood Maltreatment Predict Aggressive Behavior in Chinese Adolescents. Frontiers in behavioral neuroscience 11:17. doi: 10.3389/fnbeh.2017.00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Verhoeven FE, Booij L, Kruijt AW, Cerit H, Antypa N, Does W (2012) The effects of MAOA genotype, childhood trauma, and sex on trait and state-dependent aggression. Brain and behavior 2 (6):806–813. doi: 10.1002/brb3.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Frazzetto G, Di Lorenzo G, Carola V, Proietti L, Sokolowska E, Siracusano A, Gross C, Troisi A (2007) Early trauma and increased risk for physical aggression during adulthood: the moderating role of MAOA genotype. PLoS One 2 (5):e486. doi: 10.1371/journal.pone.0000486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mehta D, Voisey J, Bruenig D, Harvey W, Morris CP, Lawford B, Young RM (2018) Transcriptome analysis reveals novel genes and immune networks dysregulated in veterans with PTSD. Brain, behavior, and immunity 74:133–142. doi: 10.1016/j.bbi.2018.08.014 [DOI] [PubMed] [Google Scholar]
- 78.Tian Y, Liu H, Guse L, Wong TK, Li J, Bai Y, Jiang X (2015) Association of Genetic Factors and Gene-Environment Interactions With Risk of Developing Posttraumatic Stress Disorder in a Case-Control Study. Biological research for nursing 17 (4):364–372. doi: 10.1177/1099800415588362 [DOI] [PubMed] [Google Scholar]
- 79.Liu Y, Garrett ME, Dennis MF, Green KT, Ashley-Koch AE, Hauser MA, Beckham JC, Kimbrel NA (2015) An examination of the association between 5-HTTLPR, combat exposure, and PTSD diagnosis among U.S. veterans. PLoS One 10 (3):e0119998. doi: 10.1371/journal.pone.0119998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Walsh K, Uddin M, Soliven R, Wildman DE, Bradley B (2014) Associations between the SS variant of 5HTTLPR and PTSD among adults with histories of childhood emotional abuse: results from two African American independent samples. Journal of affective disorders 161:91–96. doi: 10.1016/j.jad.2014.02.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gressier F, Calati R, Balestri M, Marsano A, Alberti S, Antypa N, Serretti A (2013) The 5-HTTLPR polymorphism and posttraumatic stress disorder: a meta-analysis. J Trauma Stress 26 (6):645–653. doi: 10.1002/jts.21855 [DOI] [PubMed] [Google Scholar]
- 82.Xie P, Kranzler HR, Farrer L, Gelernter J (2012) Serotonin transporter 5-HTTLPR genotype moderates the effects of childhood adversity on posttraumatic stress disorder risk: a replication study. American journal of medical genetics Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics 159b (6):644–652. doi: 10.1002/ajmg.b.32068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Morey RA, Hariri AR, Gold AL, Hauser MA, Munger HJ, Dolcos F, McCarthy G (2011) Serotonin transporter gene polymorphisms and brain function during emotional distraction from cognitive processing in posttraumatic stress disorder. BMC psychiatry 11:76. doi: 10.1186/1471-244x-11-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Grabe HJ, Spitzer C, Schwahn C, Marcinek A, Frahnow A, Barnow S, Lucht M, Freyberger HJ, John U, Wallaschofski H, Volzke H, Rosskopf D (2009) Serotonin transporter gene (SLC6A4) promoter polymorphisms and the susceptibility to posttraumatic stress disorder in the general population. The American journal of psychiatry 166 (8):926–933. doi: 10.1176/appi.ajp.2009.08101542 [DOI] [PubMed] [Google Scholar]
- 85.Carver CS, Johnson SL, Kim Y (2016) Mu opioid receptor polymorphism, early social adversity, and social traits. Social neuroscience 11 (5):515–524. doi: 10.1080/17470919.2015.1114965 [DOI] [PubMed] [Google Scholar]
- 86.Slavich GM, Tartter MA, Brennan PA, Hammen C (2014) Endogenous opioid system influences depressive reactions to socially painful targeted rejection life events. Psychoneuroendocrinology 49:141–149. doi: 10.1016/j.psyneuen.2014.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Feusner J, Ritchie T, Lawford B, Young RM, Kann B, Noble EP (2001) GABA(A) receptor beta 3 subunit gene and psychiatric morbidity in a post-traumatic stress disorder population. Psychiatry research 104 (2):109–117 [DOI] [PubMed] [Google Scholar]
- 88.Javidi H, Yadollahie M (2012) Post-traumatic Stress Disorder. The international journal of occupational and environmental medicine 3 (1):2–9 [PubMed] [Google Scholar]
- 89.Diagnostic and Statistical Manual of Mental Disorders (2013). Fith edn. American Psychiatric Association, Alexandria, VA [Google Scholar]
- 90.Cheney AM, Koenig CJ, Miller CJ, Zamora K, Wright P, Stanley R, Fortney J, Burgess JF, Pyne JM (2018) Veteran-centered barriers to VA mental healthcare services use. BMC Health Serv Res 18 (1):591. doi: 10.1186/s12913-018-3346-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gallaway MS, Bell MR, Lagana-Riordan C, Fink DS, Meyer CE, Millikan AM (2013) The association between US Army enlistment waivers and subsequent behavioral and social health outcomes and attrition from service. Military medicine 178 (3):261–266. doi: 10.7205/milmed-d-12-00316 [DOI] [PubMed] [Google Scholar]
- 92.Brown LA, Gallagher T, Petersen J, Benhamou K, Foa EB, Asnaani A (2018) Does CBT for anxiety-related disorders alter suicidal ideation? Findings from a naturalistic sample. Journal of anxiety disorders 59:10–16. doi: 10.1016/j.janxdis.2018.08.001 [DOI] [PubMed] [Google Scholar]
- 93.Smith BH, Higgins C, Baldacchino A, Kidd B, Bannister J (2012) Substance misuse of gabapentin. The British journal of general practice : the journal of the Royal College of General Practitioners 62 (601):406–407. doi: 10.3399/bjgp12X653516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fond G, Loundou A, Rabu C, Macgregor A, Lancon C, Brittner M, Micoulaud-Franchi JA, Richieri R, Courtet P, Abbar M, Roger M, Leboyer M, Boyer L (2014) Ketamine administration in depressive disorders: a systematic review and meta-analysis. Psychopharmacology (Berl) 231 (18):3663–3676. doi: 10.1007/s00213-014-3664-5 [DOI] [PubMed] [Google Scholar]
- 95.McLaughlin T, Blum K, Oscar-Berman M, Febo M, Demetrovics Z, Agan G, Fratantonio J, Gold MS (2015) Using the Neuroadaptagen KB200z to Ameliorate Terrifying, Lucid Nightmares in RDS Patients: the Role of Enhanced, Brain-Reward, Functional Connectivity and Dopaminergic Homeostasis. J Reward Defic Syndr 1 (1):24–35. doi: 10.17756/jrds.2015-006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McLaughlin T, Blum K, Oscar-Berman M, Febo M, Agan G, Fratantonio JL, Simpatico T, Gold MS (2015) Putative dopamine agonist (KB220Z) attenuates lucid nightmares in PTSD patients: role of enhanced brain reward functional connectivity and homeostasis redeeming joy. J Behav Addict 4 (2):106–115. doi: 10.1556/2006.4.2015.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McLaughlin T, Febo M, Badgaiyan R,D, Barh D, Dushaj K, Braverman E,R, Li M, Madigan M,A, Blum K (2016) KB220Z™ a Pro-Dopamine Regulator Associated with the Protracted, Alleviation of Terrifying Lucid Dreams. Can We Infer Neuroplasticity-induced Changes in the Reward Circuit? J Reward Defic Syndr Addict Sci 2 (1):3–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Febo M, Blum K, Badgaiyan RD, Perez PD, Colon-Perez LM, Thanos PK, Ferris CF, Kulkarni P, Giordano J, Baron D, Gold MS (2017) Enhanced functional connectivity and volume between cognitive and reward centers of naive rodent brain produced by pro-dopaminergic agent KB220Z. PLoS One 12 (4):e0174774. doi: 10.1371/journal.pone.0174774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Naifeh JA, Mash HBH, Stein MB, Fullerton CS, Kessler RC, Ursano RJ (2019) The Army Study to Assess Risk and Resilience in Servicemembers (Army STARRS): progress toward understanding suicide among soldiers. Mol Psychiatry 24 (1):34–48. doi: 10.1038/s41380-018-0197-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McLaughlin T, Oscar-Berman M, Simpatico T, Giordano J, Jones S, Barh D, Downs WB, Waite RL, Madigan M, Dushaj K, Lohmann R, Braverman ER, Han D, Blum K (2013) Hypothesizing repetitive paraphilia behavior of a medication refractive Tourette’s syndrome patient having rapid clinical attenuation with KB220Z-nutrigenomic amino-acid therapy (NAAT). J Behav Addict 2 (2):117–124. doi: 10.1556/jba.2.2013.2.8 [DOI] [PubMed] [Google Scholar]
- 101.Thanos PK, Hamilton J, O’Rourke JR, Napoli A, Febo M, Volkow ND, Blum K, Gold M (2016) Dopamine D2 gene expression interacts with environmental enrichment to impact lifespan and behavior. Oncotarget. doi: 10.18632/oncotarget.8088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Starkman BG, Sakharkar AJ, Pandey SC (2012) Epigenetics-beyond the genome in alcoholism. Alcohol Res 34 (3):293–305 [PMC free article] [PubMed] [Google Scholar]
- 103.Sheppard CW, Smith DE, Gay GR (1972) The changing face of heroin addiction in the Haight-Ashbury. Int J Addict 7 (1):109–122 [DOI] [PubMed] [Google Scholar]
- 104.Fields HL, Margolis EB (2015) Understanding opioid reward. Trends in neurosciences 38 (4):217–225. doi: 10.1016/j.tins.2015.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]