Abstract
ACS patients undergoing percutaneous coronary intervention (PCI) when treated with bivalirudin and clopidogrel had increased frequency of early stent thrombosis. 24 patients referred for intervention with planned bivalirudin therapy, not previously treated with a P2Y12 inhibitor and not receiving heparins or αIIbβ3 inhibitors were randomized to treatment with either clopidogrel (600 mg) or prasugrel (60 mg). Platelet aggregation (PA) was measured by light transmission aggregometry (LTA) of platelet-rich plasma in response to ADP, PAR1/PAR4 thrombin receptor agonists and collagen at baseline and at 1, 2, 4 and 16 h following the cessation of bivalirudin infusion. Prasugrel-mediated inhibition of PA was significantly greater than that of clopidogrel at all time points for ADP as well as PAR1. There was an unanticipated, significantly greater protection of PAR4-mediated platelet aggregation only detected with prasugrel and not observed with clopidogrel. We further examined the effect of the hyperreactive PAR4 Thr120 variant in the protease-activated receptor 4 (PAR4), single nucleotide polymorphism (SNP) rs773902 on aggregation protection. The PAR4 protective effect with prasugrel was lost in individuals carrying the PAR4 Thr120 variant, and not in Ala120 homozygote. PAR1, ADP and collagen inhibition was not significantly affected in the hyperreactive PAR4 Thr120 variant. We documented that the P2Y12 ADP receptor-mediated regulation of the strength of the high-affinity conformation of αIIbβ3 as detected by PAC-1 ab, and in control of platelet adhesiveness through Rap1 GTPase protein activation. Importantly, the PAR4 Thr120 variant resulted in the increased rate and magnitude of Rap1 activation. Human platelet PAR4 mediated-activation of αIIbβ3 was phospholipase C beta (PLCβ)-dependent and unlike mouse platelet PI3K-independent. These data identify a PAR4-dependent inhibitory mechanism for the prasugrel-mediated platelet inhibition, not seen with clopidogrel that could explain the reduction in stent thrombosis documented in clinical trials with prasugrel.
Keywords: P2Y12, PAR4 SNP rs773902, Collagen, Platelets, Arterial thrombosis
1. Introduction
Percutaneous coronary intervention (PCI) leads to iatrogenic endothelial disruption and heightened platelet activation and aggregation. Treatment with heparin and glycoprotein (GP)IIb/IIIa (αIIbβ3) inhibitors during PCI effectively mitigates platelet aggregation but frequently increases bleeding [1–3]. In addition, there are several wellknown limitations of heparin including its variable anticoagulant effect due to nonlinear pharmacokinetics, inconsistent binding to blood proteins and ineffective inhibition of clot-bound thrombin as well as its propensity to lead to thrombocytopenia.
The direct thrombin inhibitor (DTI), bivalirudin, which binds with high affinity to the exosite I of thrombin, may be a safer alternative to other commonly used pharmacologic PCI adjuncts with the preponderance of evidence from randomized trials showing bivalirudin to be associated with less bleeding and thrombocytopenia, comparable ischemic events and improved net clinical outcomes compared with heparin-based regimens [4–6]. Other studies Results from our laboratory suggest that at least a part of the salutary effects of bivalirudin are due to a reduction of thrombin and to a lesser extent, collagen-mediated platelet activation [7,8].
Inhibition of the platelet P2Y12 ADP receptor is the standard of care when added to aspirin in patients undergoing PCI. The more potent P2Y12 ADP receptor antagonist prasugrel significantly reduced the composite endpoint of cardiovascular death, nonfatal MI, and nonfatal stroke in higher-risk ACS patients referred for PCI [7–9].
Treatment with bivalirudin alone, as compared with heparin plus αIIbβ3 inhibitors in ST-segment elevation myocardial infarction (STEMI), resulted in significantly reduced 30-day rates of major bleeding and net adverse clinical events [9], however bivalirudin treatment was associated with a higher acute stent thrombosis rate when compared to αIIbβ3 inhibitor-treated patients [9]. We have documented that the half-life of bivalirudin during PCI is 29.3 min [10]. The relatively short half-life of this DTI in concert with the relatively long time required to activate clopidogrel from a prodrug to its active metabolite [11], likely exposes patients to a vulnerable period when there is suboptimal platelet inhibition, which likely is the proximate cause of early stent thrombosis in bivalirudin-treated STEMI patients. Consequently, earlier acting, more potent thienopyridine therapy, i.e., prasugrel, when combined with bivalirudin treatment has the potential to reduce bleeding (compared with αIIbβ3 inhibitors) while preventing peri-procedural MI as well as providing protection from platelet-mediated stent thrombosis (compared with clopidogrel) during the vulnerable period following PCI.
The overwhelming majority of published data examining clinical outcomes or in-vivo pharmacodynamic and pharmacokinetic differences between clopidogrel and prasugrel have done so in PCI patients in whom bivalirudin was either not used or used very infrequently, i.e., in < 10% of studied patients [9,12,13]. However, at present in the United States, bivalirudin is a preeminent antithrombotic adjunctive therapy used during PCI [10]. Consequently, comparative data regarding the effect of prasugrel and clopidogrel on platelet function in bivalirudin-treated patients is of vital clinical importance.
This study aims to document the extent of inhibition of platelet aggregation following the discontinuation of bivalirudin therapy in PCI patients treated with prasugrel as compared with clopidogrel. To compare the effect of prasugrel and clopidogrel on platelet function in bivalirudin-treated patients, change from baseline in platelet aggregation in response to 5 and 20 μM ADP, 5 and 20 μM SFLLRN (PAR1), 160 and 300 μM AYPGKF (PAR4) and 5 μg/mL collagen concentrations were examined. ADP is an agonist for the dual ADP receptors, P2Y1 and P2Y12. Both, clopidogrel and prasugrel covalently and irreversibly bind to the P2Y12 receptor and block its activation with distinct metabolic differences.
Thrombin, the most potent agonist of platelet activation enhanced factoring of PAR4 via PAR1, is, therefore, the most relevant agonist of PARs. The two thrombin-activated receptors on human platelets, the high-affinity PAR1 and the low-affinity PAR4 result in activation of the fibrinogen receptor αIIbβ3 that leads to platelet aggregation. We have previously shown that PAR1 and PAR4 form a stable heterodimer that enables thrombin to act as a bivalent functional agonist that results in acceleration of thrombin cleavage [7,14]. PAR4 is cleaved more slowly than PAR1 mainly because it lacks a functional Hir sequence [15]. The cleaved PAR1 exodomain retains the Hir motif and binds to exosite I [16], suggesting that thrombin may remain tethered to the surface of human platelets via its association with the cleaved PAR1 receptor. Because of its therapeutic importance as an antiplatelet target, the central role of the P2Y12 receptor [17] in platelet activation and its contribution to the switch from a low-affinity state to a high-affinity state of αIIbβ3 that binds fibrinogen is under investigation. Our group and others have previously demonstrated inhibition of P2Y12 affected PAR1 signaling and contributed to ‘irreversible’ platelet aggregation [18,19]. Previous work on mouse platelets identifies that PAR4 and the P2Y12 ADP receptor signal synergistically in platelet activation [20,21]. Therefore thrombin-mediated PAR1-PAR4 activation and other G-protein coupled receptors such as P2Y12 have also been shown to form dimers with PAR4 and present a precisely orchestrated set of events in platelet activation and signaling that controls the precise balance between thrombosis and hemostasis.
PAR4 variant [22,23] with a single nucleotide polymorphism that results in transmembrane 2 residue amino acid 120 to code for a Threonine (Thr) or Alanine (Ala), rs773902 allele (a single nucleotide dimorphism G/A) of the PAR4 gene (F2RL3) was associated with different platelet reactivity. PAR4 variant Thr120 had increased PAR4 reactivity and higher platelet aggregation response. We postulated that the variability in PAR4 function due to genetic variants (Thr vs. Ala120) could affect the antiplatelet effects of P2Y12 drugs.
Sustained integrin αIIbβ3 activation on a platelet surface is tightly regulated in the formation of a stable hemostatic plug and in a pathologic thrombus formation through the small GTPase RAP1 [24]. P2Y12 is a key regulator of sustained RAP1 activation critical for the modulation of platelet adhesiveness. The RAP1 activator CalDAG-GEFI and inhibitor RASA3 are critical regulators that ensure proper activation of αIIbβ3. Human PAR4 signaling pathway and the role of Gi-coupled receptors and its contribution to Rap1 activation that modulates the strength and duration of irreversible αIIbβ3 activation are still not well understood, especially in the context of the PAR4 genetic variants. Here we provide evidence for the critical role of P2Y12 in PAR4-mediated platelet aggregation uncovered with the more potent P2Y12 drug prasugrel. The PAR4 protective effect with prasugrel was lost in individuals carrying the PAR4 Thr120 variant, and not in Ala120 homozygote. These findings are consistent with the recent report on effects of genetic variation in PAR4 on the ACT from the TRACER trial that detected a lower rate of GUSTO moderate/severe bleeding in patients with the PAR4 Thr120 [25].
2. Methods
2.1. Patient population and recruitment
This study examined the antiplatelet effects in PCI patients randomized to treatment with clopidogrel vs. prasugrel during the vulnerable period following the discontinuation of bivalirudin treatment, an approved adjunctive agent for PCI employing its commonly-used dose in the approved patient population for this drug. Patients referred for PCI who had not received bivalirudin and thienopyridine therapy and had not been exposed to P2Y12 inhibitors within 2 weeks were enrolled in the Tufts Medical Center Adult Cardiac Catheterization Laboratory.
Inclusion criteria included men and non-pregnant women, age 18 years or older referred for possible PCI who were eligible for bivalirudin therapy. All patients provided written informed consent before the initiation of the study. The study protocol was approved by the Tufts Medical Center Institutional Review Board. Heparin was not administered for at least 4.5 h before or during the intervention. Patients could not be currently receiving αIIbβ3 inhibitors and have no contraindications to bivalirudin. Exclusion criteria included age ≥ 75 years, weight < 60 kg, pregnancy, serum creatinine > 2.0, platelet count < 50,000/μL, known hypersensitivity to bivalirudin, clopidogrel, prasugrel or aspirin, severe systemic hypertension, defined as SBP > 180 mmHg or DBP > 110 mmHg, symptoms or findings suggestive of aortic dissection, acute pericarditis, cardiogenic shock, active internal bleeding or a history of ICH, stroke, TIA, AV malformation or aneurysm, any major surgical procedure or severe physical trauma and participation in other clinical research studies within 30 days of enrollment.
Eligible patients received bivalirudin administered intravenously as a 0.75 mg/kg bolus followed by continuous infusion of 1.75 mg/kg/h during the procedure. All patients were chronic aspirin users and received it before the intervention. Oral clopidogrel (600 mg) or prasugrel (60 mg) was administered in an open-label fashion at the completion of PCI and were discharged with a prescription for either 75 mg clopidogrel or prasugrel 10 mg daily, per usual clinical practice.
2.2. Platelet aggregation studies
Venous blood was collected from 24 patients with an 18-gauge needle and 20 cc syringe prefilled with 200 μM PPACK and were transferred to 15 mL polypropylene tubes. Platelet-rich plasma (PRP) was extracted after centrifuging the blood at 800 rpm for 20 min at 30 °C. Platelet-poor plasma (PPP) was, used as a baseline for light transmission and was obtained after PRP removal and centrifuging the samples at 3000 rpm for 10 min. Platelet aggregation was measured in PRP, in a blinded fashion after induction with various agonists: ADP 5 and 20 μM, SFLLRN 5 and 20 μM, AYPGKF 160 μM and 300 μM, collagen 5 μg/mL in PRP. Aggregation was measured blinded to treatment assignment using a Chronolog 560VS/490 to 2D aggregometer. Reactions were conducted in final volumes of 250 μL at 37 °C, stirring at 900 rpm.
2.3. Prothrombinase and TAT assays
Quantification of thrombin-antithrombin III complexes (TAT) in plasma from patients was obtained at baseline, 1 h, 2 h, 4 h, and 16–24 h after cessation of bivalirudin infusion. Briefly, the patient plasma was prepared by centrifuging the test tube at 3000 rpm at 37 °C and storing it at −80 °C until analysis. Assays were performed by ELISA (Affinity Biologicals, Cayman Chemicals, respectively).
2.4. Measurement of PAC-1 binding to evaluate αIIbβ3 activation through PAR4-P2Y12
Activation of αIIbβ3 was measured in whole blood by flow cytometry. Whole blood collected from healthy donors, PRP was prepared, and GPRP at a final concentration of 1 mM was added to prevent fibrin polymerization before the addition of agonists. PRP was incubated for 30 min with 1 U/mL apyrase, 10 μM AZD1283 [26] (BioVision), 10 μM U73122, 30 μM LY294002 and 30 μM GF109203X then incubated for an additional 15 min with 160 μM AYPGKF, PAR4 peptide agonist at 37 °C. The samples were labeled with PAC1-FITC (BD Biosciences) ab in the dark at room temperature for 30 min. Paraformaldehyde 1% was used to fix the platelets. The analysis was performed on a BD FACS Canto II Flow Cytometry System.
2.5. Rap1 activation assay
Gel-purified platelets cells were stimulated with 160 μM AYPGKF for 5 min, 15 min, 30 min, 1 h at 37 °C and 0 time (vehicle control). Samples were pretreated platelets with 30 μM AZD1283, +160 μM AYPGKF for 5 min, 15 min, 30 min, 1 h at 37 °C and 0 time (vehicle control). Prepared platelet lysates using extraction lysate buffer (25 mM tris(hydroxymethyl)aminomethane (Tris)-HCl at pH 7.5, 150 mM NaCl, 5 mM MgCl2, 1% Nonidet P-40, 1 mM DTT (dithiothreitol), 5% Glycerol, 1 g/mL aprotinin, 1 g/mL leupeptin, and 1 mM phenyl methylsulphonyl fluoride (PMSF)) on ice for 10 min, cells were centrifuged at 14,000 g for 10 min at 4 °C. Aliquots of the lysate were used to detect total Rap1 levels. The remaining supernatants were incubated with 50 μL of Ral GDS-RBD agarose slurry of (EMD Millipore, MA) for 60 min at 4 °C. Proteins were separated on a 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel and transferred to nitrocellulose membranes (BioRad, Hercules, CA). Rap1 levels were detected with rabbit polyclonal antibodies (EMD Millipore, MA) followed by horseradish peroxidase (HRP)–conjugated goat anti-rabbit antibodies (Zymed, South San Francisco, CA).
2.6. Genotyping PAR4 rs773902
Genotyping of PAR4 rs773902 A allele and/or G was carried out by performing polymerase chain reaction analysis and sequencing of the amplified fragment that scans the PAR4 rs773902 SNP.
2.7. Western blot analysis of patient samples for P2Y12 expression
P2Y12 ADP receptor expression was measured by western blot from platelet samples at baseline and 1 h time points. PRP patient samples at baseline and 1 h were centrifuged at 3000 rpm for 10 min, and the supernatant was removed. The remaining pellet was stored at −80 °C. These samples were then thawed and lysed in lysate buffer (100 mM NaCl, 25 mM Hepes pH 7.2, 1% Nonidet P-40, 1 mM PMSF) with Halt-Protease Inhibitor mixture (Thermo Scientific) and protein was quantified using a Bradford assay. The samples were then run on 12% SDS-PAGE. Rabbit polyclonal P2Y12-Ab was directed against the P2Y12 receptor C-terminal region residues 13–26, was generated as previously described [27]. Membranes were incubated with anti-rabbit Ig-horseradish peroxidase antibody (Dako A/S, Glostrup, Denmark 1:1000 dilution) for 1 h at room temperature. Membrane proteins were detected by enhanced chemiluminescence (Amersham Pharmacia) and exposed on Hyperfilm (Amersham Pharmacia). Protein from western blots was quantified using densitometry with ImageJ and actin was used as the correction factor for loading.
3. Statistical analysis
Based on previous studies from our group [10,28] 24 total patients will provide 95% power to detect statistically significant differences between baseline and prasugrel and clopidogrel post-bivalirudin infusion for inhibition of aggregation to various agonists including ADP, SFLLRN, AYPGKF and collagen agonists with a standard deviation (SD) of 10–24%. From Li [29] et al., and Michelson [13] et al., a Δ mean inhibition of 30% with SD 15% in ADP aggregation at timepoint 1–4h after prasugrel 60 mg or clopidogrel 600 mg would yield a power of 99.8%. From prior work in our lab [10], a 30% Δ mean inhibition in collagen inhibition at time points 1–4 h after prasugrel 60 mg or clopidogrel 600 mg would yield a power of 92.4%. A 15% Δ mean inhibition in SFLLRN would yield a power of 95.7%. Finally, a 20% Δ mean inhibition in AYPGKF would yield a power of 90.4%. One-way ANOVA repeated measure test was used for statistical analysis of aggregometry results. All other statistical analyses were performed using two-tailed student’s t-test. Statistical significance was assumed to occur at p < 0.05.
4. Data analysis
Each patient had paired samples representing their baseline as well as an on-drug sample. The magnitude of platelet inhibition for each studied agonist was performed utilizing mean maximal change from baseline in LTA. The paired samples were analyzed using a two-tailed student’s t-test. Statistical significance was assumed to occur when p < 0.05. Mechanistic studies are explicitly documenting the effect of prasugrel versus clopidogrel on ADP, PAR1 and PAR4 thrombin receptors, and collagen-receptors were performed. Statistical significance was assumed to occur when p < 0.05. One-way ANOVA repeated measure test was used for statistical analysis of aggregometry results.
5. Results
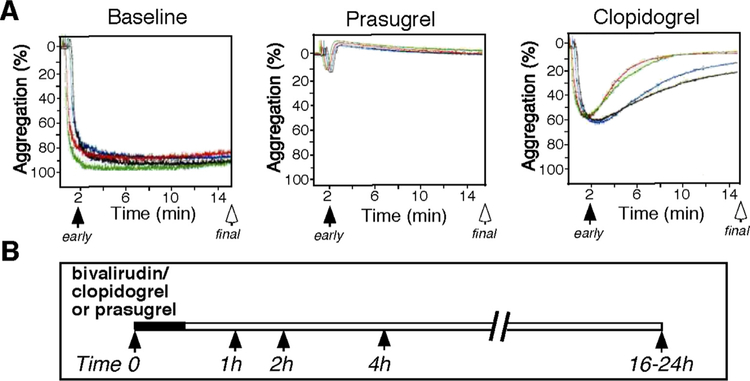
24 patients referred for PCI that were eligible for bivalirudin therapy were randomly assigned in a 1:1 ratio to treatment with either clopidogrel (600 mg loading dose) or prasugrel (60 mg loading dose). Patient baseline characteristics are listed in Table 1. There were no major or minor bleeding events according to Thrombolysis In Myocardial Infarction (TIMI) criteria [2] nor any other complications. PA was measured by light transmission aggregometry (LTA) of platelet-rich plasma in response to ADP, PAR1/PAR4 thrombin receptor agonists and collagen at baseline and at 1, 2, 4 and 16 h following the cessation of bivalirudin infusion as schematically indicated in Fig. 1.
Table 1.
PCI Patients, demographics and clinical outcome.
| Clopidogrel | Prasugrel | |
|---|---|---|
| Patients(n) | 12 | 12 |
| Age (years) | 64 ± 8 | 59 ± 9 |
| Female/Male | 4/8 | 1/11 |
| Race | ||
| Caucasian | 10 | 11 |
| Asian | 2 | 1 |
| Weight (kg) | 88 ± 20 | 90 ± 14 |
| Hemoglobin (g/dL) | 13.8 ± 1.7 | 14.4 ± 1.2 |
| Creatinine(mg/dL) | 0.93 ± 0.19 | 1.04 ± 0.28 |
| Platelets (k/μL) | 219 ± 50 | 211 ± 46 |
| Stent deployed (#patients) | 12 | 12 |
| Smokers (n, %) | ||
| Current | 1 (8.3%) | 1 (8.3%) |
| Former | 7 (58%) | 7 (58%) |
| Urgent reocclusion | None | None |
| Cardiac by-pass surgery | None | None |
| Myocardial infarction | None | None |
| Death | None | None |
| Minor or major hemorrhage | None | None |
Data are reported as Mean ± SD.
Fig. 1.
Schematic of the blood samples collected during the study (time 0, 1, 2, 4 and 16–24 h) post bivalirudin administration. A. Inhibition of platelet aggregation was compared at early 2 min and final 15 min time point for platelets stimulated with agonist during the study (B) (time 0, 1, 2, 4 and 16–24 h). Patients were pretreated with Clopidogrel (n = 12) or Prasugrel (n = 12). PRP was prepared from patients at baseline (time 0) and at 1, 2, 4 and 16 h after discontinuation of bivalirudin. Platelet aggregometry was performed at 37 °C.
5.1. Prasugrel uncovers PAR4-P2Y12 synergism in platelet aggregation
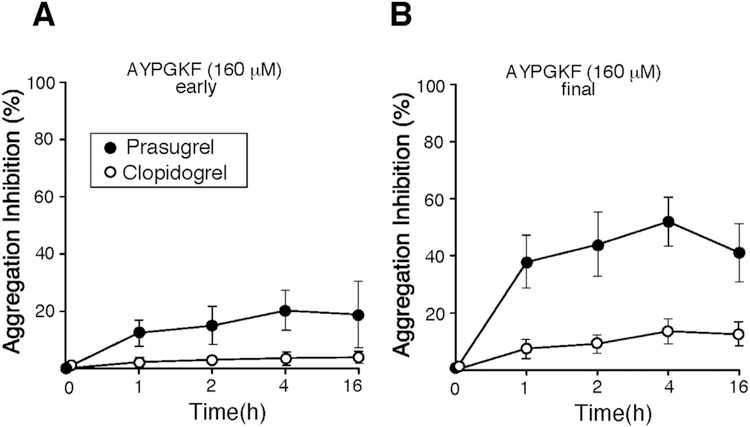
P2Y12 and the low-affinity thrombin receptor, PAR4 form a heterodimer, the physical association between these two receptors has been previously reported [30]. We have previously shown that individuals with Hermansky-Pudlak Syndrome that lack platelet dense granules and have no ADP-autocrine response have mild bleeding and unlike PAR1 still have irreversible platelet aggregation [18]. Consistent with this report, saturating concentrations of the PAR4 agonist AYPGKF (300 μM) did not reveal a significant role for P2Y12 in PAR4 activation (data not shown). However, prasugrel was more potent at inhibiting platelet aggregation in the presence of the PAR4 agonist, AYPGKF at a lower concentration of 160 μM up to 53% as compared to 13% inhibition with clopidogrel (p < 0.0001)(Fig. 2B, Table 2). Therefore, prasugrel uncovers PAR4-P2Y12 synergy when sub-saturating concentrations of a PAR4 agonist (160 μM) are used. Inhibitory effect of prasugrel may be an important finding and warrants further investigation for the variability in antiplatelet therapy protection with prasugrel and its contribution to PAR4 activation.
Fig. 2.
Prasugrel uncovers the role of P2Y12 in PAR4-mediated platelet activation. Inhibition of platelet aggregation was compared at early 2 min (A) and final 15 min (B) time point for platelets stimulated with 160 μΜ AYPGKF. Data are reported as mean ± SE (n = 12). Prasugrel (●), Clopidogrel (○).
Table 2.
Inhibitory effects of clopidorel (n = 12) and Prasugrel (n = 12) on platelet aggregation and systemic TAT levels in PCI patients.
| Agonist | Time (hour) | Clopidogrel inhibition (% ± SE) | Prasugrel inhibition (% ± SE) | p-value |
|---|---|---|---|---|
| ADP | 1 | 38 ± 10 | 84 ± 9 | < 0.0001 |
| 20 μM | 2 | 53 ± 10 | 94 ± 5 | |
| 4 | 72 ± 8 | 98 ± 1 | ||
| 16 | 85 ± 3 | 98 ± 1 | ||
| SFLLRN | 1 | 2 ± 8 | 62 ± 9 | < 0.0001 |
| 5 μM | 2 | 9 ± 8 | 64 ± 10 | |
| 4 | 17 ± 8 | 75 ± 6 | ||
| 16 | 15 ± 8 | 58 ± 7 | ||
| AYPGKF | 1 | 7 ± 3 | 39 ± 9 | < 0.0001 |
| 160 μM | 2 | 9 ± 3 | 44 ± 11 | |
| 4 | 13 ± 4 | 53 ± 8 | ||
| 16 | 11 ± 4 | 41 ± 10 | ||
| Collagena | 1 | 34 ± 11 | 56 ± 8 | 0.06 |
| 5 μg/mL | 2 | 39 ± 9 | 61 ± 6 | |
| 4 | 58 ± 8 | 67 ± 4 | ||
| 16 | 48 ± 8 | 61 ± 8 | ||
| TAT | 0 | 258 ± 39 | 251 ± 27 | NS |
| pmol/L | 1 | 274 ± 82 | 179 ± 23 | NS |
| 2 | 180 ± 23 | 226 ± 35 | NS | |
| 4 | 284 ± 34 | 224 ± 15 | NS | |
| 16 | 151 ± 10 | 301 ± 45 | 0.007 |
Platelets in PRP were isolated from PCI patients at baseline and after 1, 2, 4 and 16 h after bivalirudin infusion and clopidogrel and prasugrel loading dose administration, and were stimulated with 20 μM ADP, 5 μM SFLLRN, 160 μM \AYPGKF and 5 μg/L collagen. TAT levels in plasma were analyzed by ELISA as described in the methods. Data are reported as Mean ± SD and p values were determined by one-way ANOVA repeated measure test. TAT statistical analyses were performed using two-tailed student’s t-test.
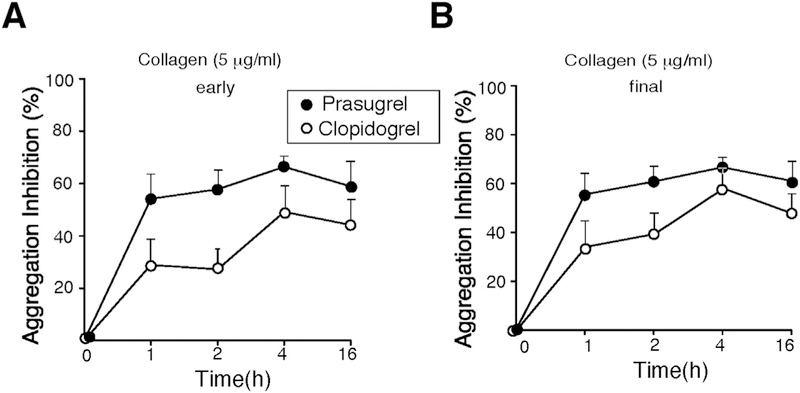
Collagen-dependent aggregation was measured with 18 of the 24 PCI patients as shown in Fig. 4A for individual patients.
5.2. Prasugrel inhibition is superior to clopidogrel in suppressing PAR1-induced aggregation in platelets from patients undergoing PCI
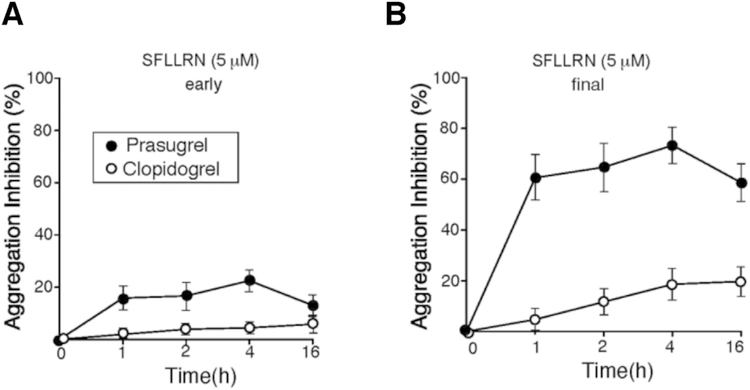
Next, we determined the effects of clopidogrel and prasugrel on the activity of the high-affinity thrombin receptor, PAR1 using SFLLRN, the agonist of PAR1, and assessed aggregation in PRP from patients undergoing PCI. It has been previously reported that upon PAR1 activation, the dense granule-secreted ADP acts as an autocrine signal to activate the Gi-coupled P2Y12 receptor and ‘lock’ the integrin αIIbβ3 receptor in irreversible aggregation [18]. Consistent with these reports as shown in Fig. 3A, there is almost complete normalization of activation measured at the early time point (~20% inhibition with prasugrel and ~5% with clopidogrel) observed with 5 μM SFLLRN. There is up to 75% final inhibition (p < 0.0001) of PAR1 activation with prasugrel which is significantly more potent than that observed with clopidogrel (17%) (Fig. 3B, Table 2). When saturating levels of SFLLRN (20 μM) were used as the agonist, the inhibition of platelet aggregation was reduced to 30% with prasugrel, and negligible inhibition was detected with clopidogrel < 5% (data not shown). Therefore, superior P2Y12 inhibition mediated by prasugrel resulted in more reversible (dissociable) platelet aggregation to the potent PAR1 agonist, SFLLRN.
Fig. 3.
Prasugrel is more potent inhibitor than Clopidogrel of platelet PAR1-mediated platelet aggregation. Inhibition of platelet aggregation was compared at early 2 min (A) and final 15 min (B) time point for platelets stimulated with 5 μΜ SFLLRN. Data are reported as mean ± SE (n = 12). Prasugrel (●), Clopidogrel (○).
5.3. Prasugrel inhibition is superior to clopidogrel in suppressing ADP-induced aggregation in platelets from patients undergoing PCI
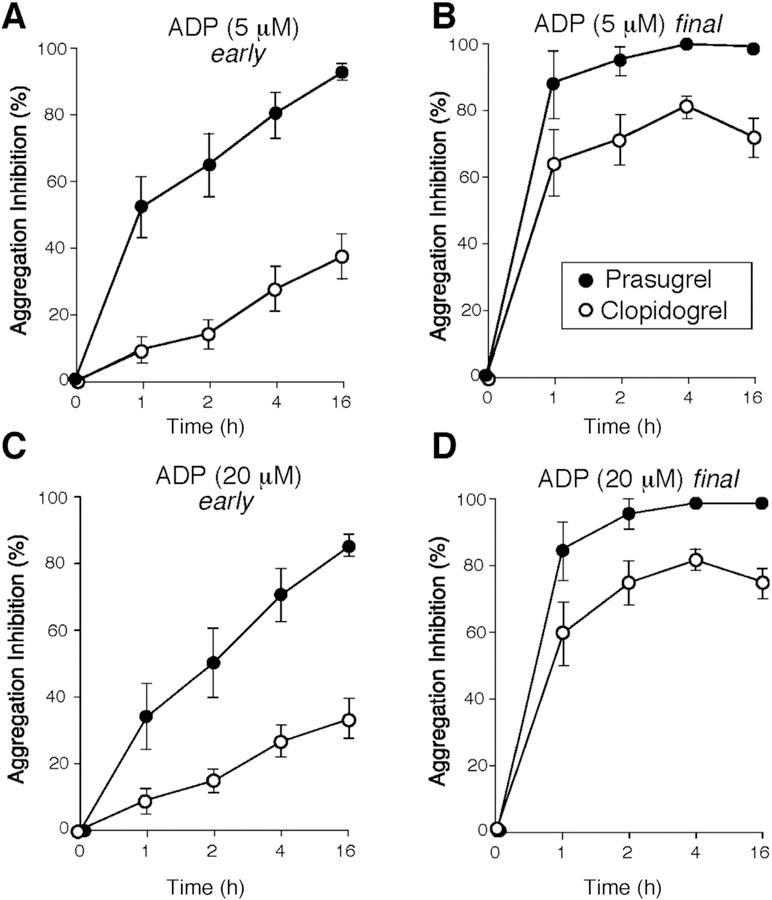
Bivalirudin binds with high affinity to the exocite I of thrombin and is used as an adjunctive antithrombotic therapy in patients undergoing PCI [10]. Termination of the bivalirudin infusion results in rapid clearance of the drug with a half-life of 29.3 min [10]. Two hours after discontinuation of the bivalirudin infusion, residual plasma drug levels (0.2 μM) were only 11% of the steady-state levels. Therefore following discontinuation of bivalirudin therapy, the postulated vulnerable window for acute thrombotic events following PCI is between 1 and 4 h. To compare the effect of prasugrel and clopidogrel on platelet function in bivalirudin-treated patients, change from baseline in platelet aggregation in response to 5 and 20 μM ADP concentrations were examined first. Both clopidogrel and prasugrel covalently and irreversibly bind to the P2Y12 receptor and block its activation with distinct metabolic differences. Clopidogrel is metabolized by a two-step mechanism dependent on CYP enzymes, whereas the faster-acting prasugrel uses a single oxidative step resulting in a more rapid onset of activity [11]. Consistent with previous publications, prasugrel was associated with increased inhibition of maximum and final platelet aggregation to the P2Y12 agonist ADP at both 5 and 20 μM concentrations (Fig. 4, Table 2) (p < 0.0001). The difference in inhibition was seen at every time point examined compared to baseline. The differences were most marked during the early 1–2 h period where maximum inhibition of 94–98% was already achieved with prasugrel as compared to clopidogrel where the maximum inhibition of P2Y12-mediated platelet aggregation was 85% at 16 h post-loading dose in response to either 5 μM or saturating ADP levels (20 μM) (p < 0.0001). These data demonstrate that more rapidly-acting prasugrel gives a superior maximum and sustained protection within 1–2 h after a loading dose and its protection is persistent over the 16 h measured time period.
Fig. 4.
Inhibition of P2Y12-dependent platelet aggregation in after addition of ADP. Inhibition of platelet aggregation was compared at early 2 min (A, C) and final 15 min (B, D) time point for platelets stimulated with 5 and 20 μΜ ADP (as indicated). Data are reported as mean ± SE (n = 12). Prasugrel (●), Clopidogrel (○).
We examined the effect of clopidogrel and prasugrel on protein expression of P2Y12, the receptor on human platelets 1 h after administration of both thienopyridines when compared with the basal expression before treatment. There was a non-significant increase in P2Y12 protein expression post administration of both clopidogrel and prasugrel (Suppl. Fig. 1).
5.4. Prasugrel is protective against collagen-mediated platelet activation as compared to clopidogrel during the early vulnerable period
P2Y12 has a prominent role in collagen-induced human platelet aggregation. Prasugrel was fast-acting and inhibited by 56% (p = 0.06) within the first hour of the addition of collagen (5 μg/mL), that persisted during the vulnerable period (Fig. 5, Table 2), and its inhibition was sustained throughout the vulnerable 1–2 h period. In contrast, arguable clopidogrel was less protective (Table 2) within the 1 to 2 h post-termination of bivalirudin time-frame as compared to prasugrel. At later time points from 4 h to 16 h, prasugrel inhibition was not significantly different from clopidogrel.
Fig. 5.
Prasugrel is more protective during an early vulnerable period as compared to Clopidogrel in Collagen mediated platelet activation. Inhibition of platelet aggregation was compared at early 2 min (A) and final 15 min (B) time point for platelets stimulated with 5 μg/ mL of collagen. Data are reported as mean ± SE (n = 12). Prasugrel (●), Clopidogrel (○).
Antithrombin III irreversibly binds to active thrombin to rapidly form TAT complexes which are an indirect estimate of systemic thrombin levels. Because decreased platelet activity observed with various agonists was superior with prasugrel, we measured systemic thrombin levels following administration of the loading dose of the two drugs in the patients undergoing PCI. The baseline mean systemic levels of TAT were similar in both groups of PCI patients randomized to treatment with clopidogrel vs. prasugrel (258 ± 39 and 251 ± 27 pmol/L, respectively). We compared plasma TAT levels at 1, 2, 4 and 16 h. As shown in Suppl Fig. 2 the PCI patients had the largest drop in mean systemic TAT levels at 16 h (151 ± 10 pmol/L, Table 2) following treatment with clopidogrel. There was a trend of protection at 1, and 4 h that was not significant with the prasugrel treatment at 16 h time point. However, the small sample size is an important limitation in examining TAT levels.
5.5. Effect of PAR4 Thr120 vs Ala120 genetic variation in platelet aggregation and contribution of P2Y12
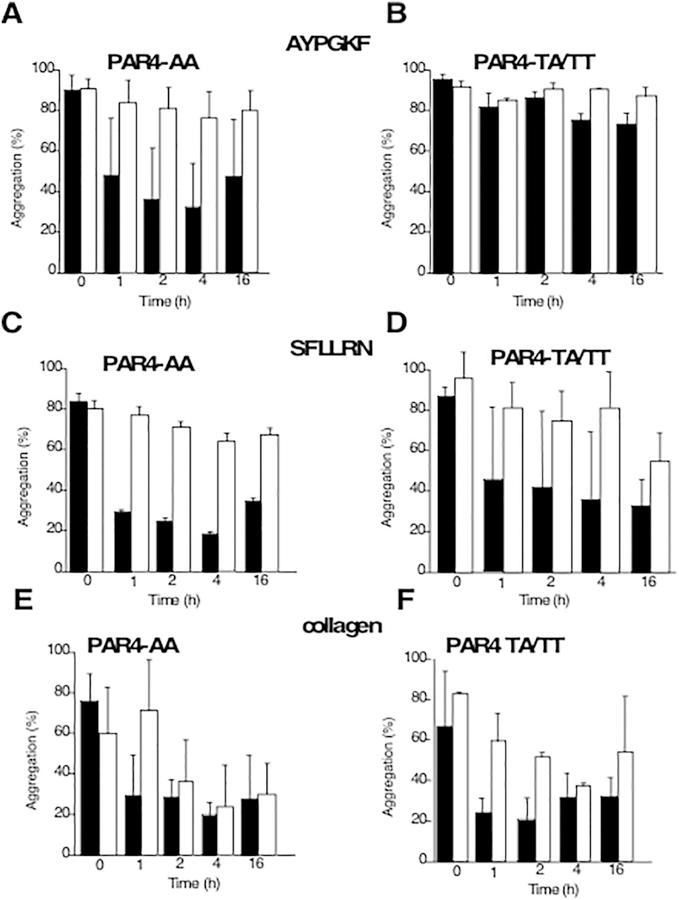
The rs773902 SNP was genotyped in 23 patients and showed that 27% were G/G or A/G genotype that codes for the hyperactive Thr120 PAR4 isoform in prasugrel treatment group whereas clopidogrel group had 17%. There was a striking reversal in inhibition detected by prasugrel treatment in the individuals with the hyperactive Thr120 PAR4 (homozygous and heterozygous) as compared to the individuals with homozygous Ala120 PAR4 (Fig. 6A, B) detected with PAR4 ligand AYPGKF activation. Interestingly, the reversal in platelet aggregation inhibition was most prominent for the PAR4 inhibitory effect of prasugrel and with some increased variability for PAR1 inhibitory effect in the hyperactive Thr120 PAR4 (Fig. 6C, B). There was a minor effect on collagen-mediated aggregation in clopidogrel treatment only, whereas prasugrel treatment did not seem affected (Fig. 6E, F).
Fig. 6.
Effect of PAR4 Thr120 vs Ala120 Genetic Variation in Platelet Aggregation and Contribution of P2Y12. Platelet aggregation was compared at final 15 min time point for platelets stimulated with 160 μΜ AYPGKF (A, B), 5μΜ SFLLRN (C.D) and 5 μg/mL of collagen (E,F). Data are reported as mean ± SD (n = 8–9 PAR4-AA, n= 2–3 PAR4-TA/TT). PAR4-AA: PAR4 genotype homozygous Ala120; PAR4-TA/TT: PAR4 genotype for the hyperactive Thr120 (TA-homozygote and TT heterozygote). Prasugrel (black), Clopidogrel (white).
5.6. PAR4-mediated αIIbβ3 activation and P2Y12 contribution to PAC-1 and Rap1 activation and P-selectin expression
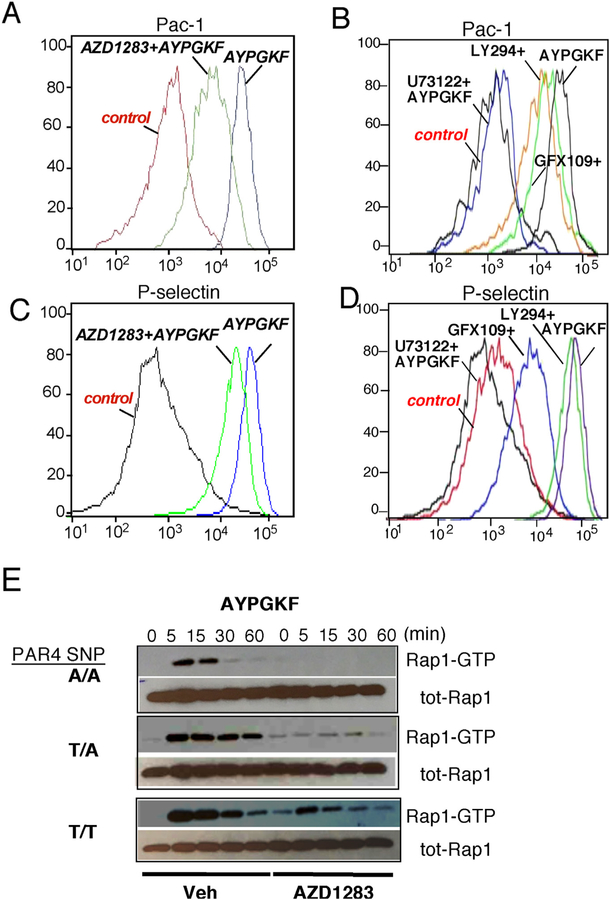
To further investigate the contribution of the Gi-coupled P2Y12 receptor to activation of integrin αIIbβ3, conformational changes were examined using flow cytometry studies with anti-PAC1 Ab that recognizes the activated form of αIIbβ3. The input of the P2Y12 receptor to the contribution of PAR4 mediated fibrinogen receptor activation was examined using a potent direct P2Y12 drug inhibitor AZD1283 [31]. As shown in Fig. 7A, there was a 50% reduction in intensity of PAC-1 binding in the presence of direct drug inhibitor AZD1283. In contrast, there were less marked differences in the contribution of P2Y12 to P-selectin expression (Fig. 7C).
Fig. 7. PAR4/P2Y12 mediated αIIbβ3 and Rap1 activation and P-selectin expression.
PAC-1 binding: platelets from a normal donor were incubated with or without (A) AZD1283 (30 μM) (B), U73122 (10 μM), LY294002 (30 μM), GF109203X (30 μM) and then stimulated with 160 μM AYPGKF for additional 15 min. Platelets were labeled with FITC-Pac1 Ab. P-selectin expression: platelets from a normal donor were incubated with or without AZD1283 (30 μM) (C), U73122 (10 μM), LY294002 (30 μM), GF109203X (30 μM) (D) and then stimulated with 160 μM AYPGKF for additional 10 min. Platelets were labeled with PE-P-Selectin Ab. Representative data are presented from 3 separate experiments. (E) PAR4 mediated Rap1 activation in human platelets from PAR4 variant rs773902 (A/ A homozygote Ala/Ala120 PAR4, T/A heterozygote Thr120/Ala120 PAR4 and T/T homozygote Thr/Thr120 PAR4). Human platelets were stimulated with 160 μM AYPGKF for the indicated times in minutes, and active Rap1-GTP was detected by pull-down assay. Total Rap1 is shown as loading control. Effect of pretreatment with AZD1283 (30 μM) on PAR4 (160 μM AYPGKF) activation on human platelets was determined. Representative data is presented from n = 6, 3 AA, 2 TA and one TT individual in duplicate.
Next, we determined whether a downstream signaling mechanism of αIIbβ3 activation by PAR4 was PLCβ, PKC and/or PI3K dependent. PAC-1 binding was inhibited with U73122 (PLCβ inhibitor), but not affected with LY294002 (PI3K inhibitor), nor GF109203X (PKC inhibitor) therefore identifying the phospholipase C beta (PLCβ)-dependent signaling mechanism as the primary mechanism for αIIbβ3 activation by PAR4 (Fig. 7B). Both, PLCβ and PKC PAR4-mediated activation were important for P-selectin expression (Fig. 7D).
In order to determine the effect of SNP rs7773902 on PAR4 mediated activation of Rap1, a direct activator and critical regulator of platelet adhesiveness and formation of a stable hemostatic plug is P2Y12 dependent; we examined the effect of PAR4 activating peptide AYPGKF on Rap1 platelet activation in the presence of direct P2Y12 drug inhibitor AZD1283 over time. PAR4 activating peptide AYPGKF results in rapid and sustained Rap1-GTP activation as examined over 60 min (Fig. 7E) with robust activation and duration detected in the hyperactive homozygous and heterozygous Thr120 PAR4. The duration of Rap1 activation in the homozygous Ala120 PAR4 was rapid with fast ‘on’ and ‘off’ state. In the presence of the P2Y12 inhibitor AZD1283, there is complete inhibition of AYPGKF activation of Rap1 only in the homozygous Ala120 PAR4, whereas there is partial inhibition of the hyperactive Thr120 PAR4 detected. Together both PAC-1 and Rap1 data strongly identify P2Y12 as an essential regulator of PAR4-mediated platelet activation especially critical in the homozygous Ala120 PAR4 and identify the effect of SNP rs7773902 on PAR4 as critical in the formation of a stable hemostatic plug. These studies warrant further investigation in individual differences and the effect of the PAR4 variants on the development of antiplatelet therapies.
6. Discussion
In this study, we documented a significantly higher inhibition of platelet aggregation by prasugrel as compared with clopidogrel – a difference that was most evident early following the discontinuation of bivalirudin therapy. As has been previously reported [11], a more rapid therapeutic thienopyridine effect can be expected to mitigate the potential for acute stent thrombosis. The more rapid appearance of prasugrel in the plasma compared to clopidogrel led to earlier and more potent inhibition of thrombin-mediated platelet activation. The data imply that prasugrel, when compared to clopidogrel has an important additive effect on the inhibition of platelet aggregation via inhibition of the PAR4 receptor following the termination of bivalirudin treatment. However, upon further examination, the effect of the hyperreactive PAR4 Thr120 variant in the protease-activated receptor 4 (PAR4), single nucleotide polymorphism (SNP) rs773902 on aggregation protection the PAR4 protective effect with prasugrel was lost in individuals carrying the PAR4 Thr120 variant, and not in Ala120 homozygote. PAR1, ADP and collagen inhibition was not significantly affected in the hyperreactive PAR4 Thr120 variant.
Acute stent thrombosis is an important clinical complication of PCI. It has been noted disproportionately during PCI for STEMI where the widely-used direct thrombin inhibitor, bivalirudin is used [32,33]. Although this complication appears to be prevented when the bivalirudin infusion is extended following the PCI at the procedural dose [34], it does appear that there exists a window of vulnerability as relates to a lack of platelet inhibition when PCI patients are concurrently treated with bivalirudin and clopidogrel. Lack of platelet inhibition is especially germane given the documented short half-life of bivalirudin [10]. The thienopyridine prasugrel has greater potency in inhibition of platelet aggregation when compared to clopidogrel [35,36]. Consistent with these previous reports prasugrel compared to clopidogrel therapy causes greater and earlier inhibition of platelet aggregation in response to both P2Y12 and thrombin receptor agonists. This is the first report of significant inhibition of both PAR4 thrombin receptors with prasugrel as compared to clopidogrel in individuals that carry the Ala120 PAR4 phenotype.
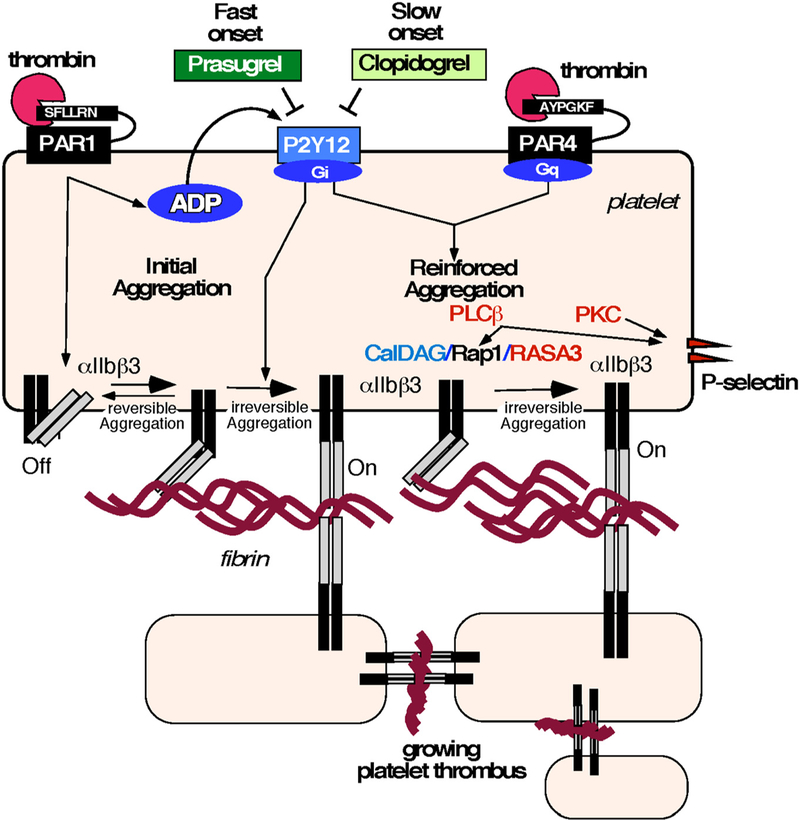
The two thrombin-activated receptors on human platelets, the high-affinity PAR1 and the low-affinity PAR4 result in activation of fibrinogen receptor αIIbβ3 that leads to platelet aggregation. Because of its therapeutic importance as an antiplatelet target, the central role of the P2Y12 receptor [17] in platelet activation and its contribution to the switch from a low-affinity state to a high-affinity state of αIIbβ3 that binds fibrinogen is under investigation. A proposed mechanism for the prasugrel-mediated reduction in stent thrombosis through inhibition of dual thrombin receptors is schematically shown in Fig. 8. Initial platelet generation of thrombin and binding to the high-affinity thrombin receptor PAR1 through interaction with the PAR1 hirudin (Hir)-like sequence results in PAR1 activation and release of dense granule ADP. ADP-mediated activation of P2Y12 further activates platelets in synergy with PAR1 that results in a shift from reversible to irreversible platelet aggregation by activation of inside-out-signaling of the fibrinogen receptor, αIIbβ3 integrin. Inside-out signaling induces a change in conformation of the αIIbβ3 integrin leading to high fibrin binding and recruitment of additional platelets linking them together to form an irreversible platelet aggregate through activation of PAR4 mediated Rap1-GTP and its regulators CalDAG-GEF1 and RASA3. Human platelet PAR4 mediated activation of αIIbβ3 was phospholipase C beta (PLCβ)-dependent with some potential role for PKC. Therefore, reinforcement of the platelet aggregate occurs as a result of thrombin activation of the low-affinity thrombin receptor, PAR4. Fast onset of inhibition of ADP signaling with the enhanced P2Y12 inhibitor prasugrel uncovered a new role for P2Y12/PAR4 in forming a more stable, growing platelet aggregates which contribute to both enhanced hemostasis and thrombosis. Both clopidogrel and prasugrel efficiently block PAR1-mediated platelet aggregation. In contrast, prasugrel more completely inhibits the signaling of the second thrombin receptor, PAR4, which may provide the basis for a more effective reduction in stent thrombosis. The dominant effect of Thr120 PAR4 variant showed incomplete inhibition of P2Y12/PAR4, which may suggest another unknown signaling mechanism of Rap1/αIIbβ3 integrin activation in the PAR4 hyperactive individuals.
Fig. 8.
Mechanism of PAR4/P2Y12 mediated αIIbβ3 and Rap1 activation and P-selectin expression. Schematic model of platelet activation upon prasugrel-mediated reduction in stent thrombosis through inhibition of dual thrombin receptors. Fast onset of inhibition of ADP signaling with the enhanced P2Y12 inhibitor prasugrel uncovered a new role for P2Y12/PAR4 in formation of more stable, growing platelet aggregates which contribute to both enhanced hemostasis and thrombosis.
The P2Y12 receptor has a vital role in collagen-mediated platelet aggregation. Besides, we have previously demonstrated significant inhibition of collagen-mediated platelet aggregation by bivalirudin [10]. During the vulnerable period for patients, early following the termination of bivalirudin infusion, prasugrel was significantly more effective than clopidogrel in inhibiting collagen-mediated platelet aggregation. Thus, the more potent antiplatelet effect of prasugrel as compared to clopidogrel is likely multifactorial.
Overall, based on all of our aggregation data, prasugrel is a more potent platelet inhibitor than clopidogrel. P2Y12 has a prominent role in collagen-induced human platelet aggregation. Prasugrel was faster acting as compared to clopidogrel and significantly inhibited collagen-mediated platelet aggregation within the first hour, therefore, protecting during the vulnerable period of stent thrombosis due to a lack of platelet inhibition immediately post-PCI prior to the onset of clopidogrel effect as plasma levels of bivalirudin quickly decline. In contrast, clopidogrel at later time points from 4 h to 16 h, platelet inhibition was not significantly different from prasugrel.
A limitation of this study is that patients randomized to each thienopyridine were treated in an open-label fashion. As light transmission aggregometry and other assays were done blinded to of treatment assignment, it is unlikely that the open-label design biased our conclusions.
In this clinical study (24 patients), we documented a significantly higher inhibition of platelet aggregation by prasugrel as compared with clopidogrel – a difference that was most evident early following the discontinuation of bivalirudin therapy. These data imply that prasugrel when compared to clopidogrel, has a critical additive effect on the inhibition of platelet aggregation via inhibition of the PAR4 receptor. Upon further examination, the effect of the hyperreactive PAR4 Thr120 variant, single nucleotide polymorphism (SNP) rs773902 on aggregation, the PAR4 protective effect with prasugrel was lost in individuals carrying the PAR4 Thr120 variant, but not in the Ala120 homozygote. PAR4 activating peptide AYPGKF results in rapid and prolonged Rap1-GTP activation in the hyperactive homozygous and heterozygous Thr120 PAR4. The duration of Rap1 activation in the homozygous Ala120 PAR4 was rapid and transient. In the presence of the P2Y12 inhibitor AZD1283, there is complete inhibition of AYPGKF activation of Rap1 in the homozygous Ala120 PAR4, whereas only partial inhibition of the hyperactive Thr120 PAR4 was observed, that may suggest of an additional P2Y12-independent signaling mechanism. Together, these data strongly identify the effect of SNP rs7773902 on P2Y12 contribution as critical in the formation of a stable hemostatic plug.
Supplementary Material
Acknowledgments
We also thank the Tufts Clinical and Translational Science Institute for performing statistical and power analyses.
Sources of funding
This work was supported by an investigator-initiated grant from Daiichi-Sankyo and Eli Lilly and Company.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.thromres.2019.01.017.
Disclosures
The authors have no disclosures. The study sponsors, Daiichi-Sankyo and Lilly, have a commercial interest in prasugrel. The sponsor had no input into the study design or analysis, nor in preparation of the manuscript.
References
- [1].O’Shea JC, et al. , Platelet glycoprotein IIb/IIIa integrin blockade with eptifibatide in coronary stent intervention: the ESPRIT trial: a randomized controlled trial, JAMA 285 (19) (2001) 2468–2473. [DOI] [PubMed] [Google Scholar]
- [2].Amsterdam EA, et al. , 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association task force on practice guidelines, J. Am. Coll. Cardiol 64 (24) (2014) e139–e228. [DOI] [PubMed] [Google Scholar]
- [3].Serruys PW, Vranckx P, Allikmets K, Clinical development of bivalirudin (Angiox): rationale for thrombin-specific anticoagulation in percutaneous coronary intervention and acute coronary syndromes, Int. J. Clin. Pract 60 (3) (2006) 344–350. [DOI] [PubMed] [Google Scholar]
- [4].Bertrand OF, et al. , Meta-analysis comparing bivalirudin versus heparin monotherapy on ischemic and bleeding outcomes after percutaneous coronary intervention, Am. J. Cardiol 110 (4) (2012) 599–606. [DOI] [PubMed] [Google Scholar]
- [5].Holinstat M, et al. , Dichotomous effects of exposure to bivalirudin in patients undergoing percutaneous coronary intervention on protease-activated receptor-mediated platelet activation, J. Thromb. Thrombolysis 35 (2) (2013) 209–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Secemsky EA, et al. , Use and effectiveness of Bivalirudin versus unfractionated heparin for percutaneous coronary intervention among patients with ST-segment elevation myocardial infarction in the United States, JACC Cardiovasc. Interv 9 (23) (2016) 2376–2386. [DOI] [PubMed] [Google Scholar]
- [7].Leger AJ, et al. , Blocking the protease-activated receptor 1–4 heterodimer in platelet-mediated thrombosis, Circulation 113 (9) (2006) 1244–1254. [DOI] [PubMed] [Google Scholar]
- [8].Kuliopulos A, Mohanlal R, Covic L, Effect of selective inhibition of the p38 MAP kinase pathway on platelet aggregation, Thromb. Haemost. 92 (2004) 1387–1393. [DOI] [PubMed] [Google Scholar]
- [9].Steinhubl SR, et al. , Determining the efficacy of antiplatelet therapies for the individual: lessons from clinical trials, J. Thromb. Thrombolysis 26 (1) (2008) 8–13. [DOI] [PubMed] [Google Scholar]
- [10].Kimmelstiel C, et al. , Bivalirudin is a dual inhibitor of thrombin and collagen-dependent platelet activation in patients undergoing percutaneous coronary intervention, Circ. Cardiovasc. Interv 4 (2) (2011) 171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wiviott SD, et al. , Prasugrel versus clopidogrel in patients with acute coronary syndromes, N. Engl. J. Med 357 (20) (2007) 2001–2015. [DOI] [PubMed] [Google Scholar]
- [12].Brar SS, et al. , Impact of platelet reactivity on clinical outcomes after percutaneous coronary intervention. A collaborative meta-analysis of individual participant data, J. Am. Coll. Cardiol 58 (19) (2011) 1945–1954. [DOI] [PubMed] [Google Scholar]
- [13].Michelson AD, et al. , Pharmacodynamic assessment of platelet inhibition by prasugrel vs. clopidogrel in the TRITON-TIMI 38 trial, Eur. Heart J 30 (14) (2009) 1753–1763. [DOI] [PubMed] [Google Scholar]
- [14].Leger AJ, Covic L, Kuliopulos A, Protease-activated receptors in cardiovascular diseases, Circulation 114 (10) (2006) 1070–1077. [DOI] [PubMed] [Google Scholar]
- [15].Jacques SL, Kuliopulos A, Protease-activated receptor-4 uses dual prolines and an anionic retention motif for thrombin recognition and cleavage, Biochem. J 376 (Pt 3) (2003) 733–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Jacques SL, et al. , Substrate-assisted catalysis of the PAR1 thrombin receptor. Enhancement of macromolecular association and cleavage, J. Biol. Chem 275 (52) (2000) 40671–40678. [DOI] [PubMed] [Google Scholar]
- [17].Dorsam RT, Tuluc M, Kunapuli SP, Role of protease-activated and ADP receptor subtypes in thrombin generation on human platelets, J. Thromb. Haemost 2 (5) (2004) 804–812. [DOI] [PubMed] [Google Scholar]
- [18].Covic L, et al. , Role of the PAR4 thrombin receptor in stabilizing platelet-platelet aggregates as revealed by a patient with Hermansky-Pudlak syndrome, Thromb. Haemost 87 (2002) 722–727. [PubMed] [Google Scholar]
- [19].Covic L, Gresser AL, Kuliopulos A, Biphasic kinetics of activation and signaling for PAR1 and PAR4 thrombin receptors in platelets, Biochemistry 39 (18) (2000) 5458–5467. [DOI] [PubMed] [Google Scholar]
- [20].Woulfe D, et al. , Defects in secretion, aggregation, and thrombus formation in platelets from mice lacking Akt2, J. Clin. Invest 113 (2004) 441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kim S, et al. , Protease-activated receptors 1 and 4 do not stimulate G(i) signaling pathways in the absence of secreted ADP and cause human platelet aggregation independently of G(i) signaling, Blood 99 (10) (2002) 3629–3636. [DOI] [PubMed] [Google Scholar]
- [22].Edelstein LC, et al. , Racial differences in human platelet PAR4 reactivity reflect expression of PCTP and miR-376c, Nat. Med 19 (12) (2013) 1609–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Edelstein LC, et al. , Common variants in the human platelet PAR4 thrombin receptor alter platelet function and differ by race, Blood 124 (23) (2014) 3450–3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Stefanini L, Bergmeier W, RAP1-GTPase signaling and platelet function, J. Mol. Med. (Berl) 94 (1) (2016) 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Tricoci P, et al. , Effects of genetic variation in protease-activated receptor 4 after an acute coronary syndrome: analysis from the TRACER trial, Blood Cells Mol. Dis 72 (2018) 37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Glatzer F, et al. , Histamine induces proliferation in keratinocytes from patients with atopic dermatitis through the histamine 4 receptor, J. Allergy Clin. Immunol 132 (6) (2013) 1358–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kuliopulos A, et al. , Plasmin desensitization of the PAR1 thrombin receptor: kinetics, sites of truncation, and implications for thrombolytic therapy, Biochemistry 38 (1999) 4572–4585. [DOI] [PubMed] [Google Scholar]
- [28].Kimmelstiel C, et al. , Pharmacodynamics and pharmacokinetics of the platelet GPIIb/IIIa inhibitor tirofiban in patients undergoing percutaneous coronary intervention: implications for adjustment of tirofiban and clopidogrel dosage, Thromb. Res 116 (1) (2005) 55–66. [DOI] [PubMed] [Google Scholar]
- [29].Vogel TR, et al. , Infectious complications after elective vascular surgical procedures. J. Vasc. Surg 51(1): 122–9; (discussion 129–30). [DOI] [PubMed] [Google Scholar]
- [30].Khan A, et al. , The physical association of the P2Y12 receptor with PAR4 regulates arrestin-mediated Akt activation, Mol. Pharmacol 86 (1) (2014) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bach P, et al. , Lead optimization of ethyl 6-aminonicotinate acyl sulfonamides as antagonists of the P2Y12 receptor. Separation of the antithrombotic effect and bleeding for candidate drug AZD1283, J. Med. Chem 56 (17) (2013) 7015–7024. [DOI] [PubMed] [Google Scholar]
- [32].Stone GW, et al. , Bivalirudin during primary PCI in acute myocardial infarction, N. Engl. J. Med 358 (21) (2008) 2218–2230. [DOI] [PubMed] [Google Scholar]
- [33].Shahzad A, et al. , Unfractionated heparin versus bivalirudin in primary percutaneous coronary intervention (HEAT-PPCI): an open-label, single centre, randomised controlled trial, Lancet 384 (9957) (2014) 1849–1858. [DOI] [PubMed] [Google Scholar]
- [34].Fahrni G, et al. , Prolonged high-dose bivalirudin infusion reduces major bleeding without increasing stent thrombosis in patients undergoing primary percutaneous coronary intervention: novel insights from an updated meta-analysis, J. Am. Heart Assoc 5 (7) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Norgard NB, Abu-Fadel M, Comparison of prasugrel and clopidogrel in patients with acute coronary syndrome undergoing percutaneous coronary intervention, Vasc. Health Risk Manag 5 (2009) 873–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sugidachi A, et al. , The greater in vivo antiplatelet effects of prasugrel as compared to clopidogrel reflect more efficient generation of its active metabolite with similar antiplatelet activity to that of clopidogrel’s active metabolite, J. Thromb. Haemost 5 (7) (2007) 1545–1551. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.