Abstract
Background
Adrenocortical carcinoma (ACC) is a rare neoplasm with a poor prognosis. Conversely, adrenocortical adenomas (ACAs) are common and benign. Despite their shared histological origin, little evidence exists that ACA arises from ACC. Recent genetic analyses of ACC have shown recurrent gene copy deletion of CYP4B1, a cytochrome P450 isozyme. This study investigates a potential role for CYP4B1 in modulating adrenocortical tumorigenesis and/or conferring chemoresistance to ACCs.
Methods
Using TaqMan real-time qPCR techniques, we investigated CYP4B1 expression in normal adrenal cortex (n=10), histologically confirmed ACAs (n=10), and ACCs (n=10). ACC cell lines were enforced to express CYP4B1 and effects on cell death and enhanced mitotane and cisplatin sensitivity were tested.
Results
Gene expression analyses demonstrated suppression of CYP4B1 in 100% of ACAs (10/10) and ACCs (10/10) tested. Average relative expression of CYP4B1 was reduced at 0.19 (0.01–0.50; P < .01) in ACAs, and nearly absent in ACCs (0.01; 0.00–0.05; P < .01). Protein expression correlated with mRNA expression. Ectopic expression of CYP4B1 promoted cytotoxicity and increased chemosensitivity in ACC cell lines.
Conclusions
CYP4B1 is silenced in adrenocortical tumors (ACTs) and may contribute to tumorigenesis and chemoresistance. Sensitization of ACC cells engineered to overexpress CYP4B1 further supports this notion.
Background
Adrenocortical carcinoma (ACC) is a rare endocrine malignancy that portends a poor prognosis. It occurs in 0.5 – 2 people per million per year and has a 5-year survival rate of only 16–38%.1–4 The majority of these tumors (65–85%) will exhibit hormonal hypersecretion of cortisol, aldosterone, or sex steroids but can be nonfunctional as well.5 Due in part to its rarity, the underlying molecular and genetic pathogenicity of ACC is poorly understood. The lack of reliable genetic markers undermines therapeutic decision-making and causes significant problems in diagnosis, prognosis, and management. Inherited disorders such as Beckwith-Wiedemann syndrome and Li-Fraumeni are found in a subset of patients, however, the majority of cases are sporadic.2,6 Investigators have thus far focused on rare genetic mutations in known oncogenes such as TP53, IGF2, and APC, however, the benefits of this research has yet to reach patients.1,7
While increased access to high-throughput sequencing has facilitated exploration of the genetic landscape of ACC, complete R0 surgical resection remains the only prospect for a durable cure.5,8,9 Advancements in treatment have been limited due to the inherent chemoresistance and lack of knowledge of the molecular drivers of ACC. Mitotane (1,1-dichloro-2-(o-chlorophenyl)-2-(p-chlorophenyl) ethane), the classical first-line agent in the treatment of ACC, acts by directly lysing adrenal cortical cells and can decrease hormone hypersecretion. Recent data has emerged that suggests combination therapy with cisplatin may increase response rates and progression-free survival.10,11
Altered cellular metabolism is a prominent feature of ACC and is an important area for oncologic research.7 Essential elements in this system are the cytochrome P450 (CYP) enzymes. This superfamily consists of more than 50 heme-thiolate proteins that play essential roles in both the biosynthetic and catabolic pathways of vitamins, fatty acids, prostaglandins, bile acids, and steroids. Steroid production can strongly influence cancer growth and impact cellular metabolism, especially in endocrine organs like the adrenal gland.12
Because CYP enzymes constitute one of the most important mechanisms for human drug metabolism, they are of central importance in chemoresistance.13,14 Altered CYP expression has been shown to increase rates of anti-cancer drug degradation, decreasing the intended cytotoxic effect.13,14 Additionally, while not considered typical oncogenes or tumor suppressors, differential expression of cytochrome P450 genes has been demonstrated to be associated with pancreatic, breast, bladder, and lung cancer.15–18
Recent bioinformatic analysis of copy number variations (CNVs) in ACC displayed widespread genomic instability and multiple arm-level and focal alterations in the cytochrome P450 genes.19 Among the CYP genes, cytochrome P450 4B1 (CYP4B1) was found to be deleted in numerous samples. Because of the crucial functions of CYP enzymes in steroid metabolism and adrenal gland physiology, we investigated the potential role of CYP4B1 in adrenocortical tumors (ACTs).
CYP4B1 is unique among the cytochrome P450 class in that it is involved in both endobiotic and xenobiotic metabolism. It selectively hydroxylates fatty acids and may exert toxicological effects via activation of aromatic amines whose byproducts can be cytotoxic under specific conditions.20 Research on bladder cancer has demonstrated that the absence of wild type CYP4B1 is associated with tumor carcinogenesis.18 Additionally, CYP4B1 has differential expression patterns in lung cancer compared to normal tissue.16 The association of CYP4B1 with other malignancies and the demonstrated copy number loss in ACC led us to investigate its potential role in adrenocortical tumors.
Methods
Patients and tissue collection
Patients included in this cohort were treated at Yale New-Haven Hospital from August 2003 to July 2015. ACC (n = 10), ACA (n = 10), and adjacent normal tissue samples (n = 10) were collected by Yale Pathology Tissue Service intraoperatively. ACCs were sequentially collected from 2003 onward, while ACAs were selected based on the time since operation. All the patients in the ACA cohort had their operations between 2012 and 2014. Adrenal tissue samples were examined by an experienced endocrine pathologist for histological identification. Additional formalin-fixed paraffin-embedded samples from the same patients were used for immunohistochemistry. Written informed consent was obtained from all patients involved. Protected health information was acquired and maintained in accordance with regulations as specified by the Health Insurance Portability and Accountability Act (HIPAA). The consent waiver and all procedural aspects of this study were approved by the Yale University Institutional Review Board.
Gene Expression Analysis
RNA was isolated from fresh frozen samples using the RNeasy Plus Mini Kit (Qiagen, Hilden, Germany). Quantity and quality of isolated RNA was assessed by spectrophotometry (NanoDrop Technologies). Two hundred ng of RNA was used for cDNA synthesis with the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA). Quantitative RT-PCR was performed on a CFX96 Real-Time System thermocycler (Bio-Rad) using TaqMan PCR master mix with primers and probes specific to CYP4B1 (all from Applied Biosystems, Foster City, CA). Relative expression between samples was normalized to endogenous ribosomal protein, large, P0 (RPLP0) expression levels. Two independent experiments were performed with duplicate samples for all assays. Relative expression levels were calculated using the Livak method.21
Immunohistochemical analysis
Five μm thick sections of histologically confirmed ACC, ACA, and normal adrenal tissue from formalin fixed paraffin embedded (FFPE) tissue samples were collected for study. Two representative samples of each tissue type were selected for immunostaining. Using standard immunohistochemistry protocols, target epitopes were detected using rabbit anti-CYP4B1 polyclonal antibody (Santa Cruz, Dallas, TX) followed by anti-rabbit HRP conjugated monoclonal secondary antibody (Santa Cruz).22 DAB (3,3’-diaminobenzidine tetrahydrochloride) was utilized for antigen detection (Life Technologies, Carlsbad, CA). Sections were counterstained with hematoxylin and mounted using immunohistomount (Santa Cruz).
Ectopic expression of CYP4B1 in ACC cell lines
Standard cell culture experiments were performed as previously described.22 The human ACC cell lines SW-13 and NCI-H295R were purchased from the American Type Culture Collection (Manassas, VA). SW-13 cells were maintained under sterile conditions in Dulbecco’s Modified Eagle’s medium (DMEM) supplemented with 10% certified fetal bovine serum and 10,000 U/mL penicillin/streptomycin (Life Technologies) in a standard humidified incubator at 37.0 °C and 5% CO2. NCI-H295R cells were grown in DMEM/F-12 containing 5% NuSerum, 0.1% ITS premix, and 10,000 U/mL penicillin/streptomycin (Life Technologies). Myc-DDK tagged pCMV6-Entry, pCMV6-Entry/GFP, and pCMV6-Entry/CYP4B1 plasmid vectors (Origene, Rockville, MD) were used to transfect cells that were grown to 70–80% confluence.
Transient transfection was performed in 24-well plates using Lipofectamine 2000 according to the manufacturer’s recommendations (Life Technologies). Transfection efficiencies were determined by quantifying the percentage of GFP-expressing cells from parallel transfections carried out using pCMV6-Entry/GFP vectors. 24 hours after transfection, cells were treated with chemotherapy agents or the appropriate diluents. Mitotane and cisplatin were dissolved in 100% ethanol or distilled water respectively (Spectrum Chemical, New Brunswick, NJ). Cells were treated with either (1) mock, (2) mitotane 5 μM, (3) mitotane 50 μM, (4) mitotane 50 μM plus cisplatin 700 nM, or (5) untreated. After 24 hours of treatment, cell viability was measured using Trypan blue assay.22 A minimum of two independent experiments were performed with duplicate samples in each assay. Standard Student’s t test was used to assess statistical significance in normally distributed data sets. A P value < .01 was considered significant.
Results
Patient Characteristics
Both the ACA and ACC groups had a predominance of female patients with 60% and 80% respectively. Average age at the time of operation was 55.5 ± 10.4 for ACC and 45.7 ± 14.0 in ACA. Mean tumor diameter was nearly 9 cm greater in ACC than ACA (ACC = 11.3 cm [6.0 – 14.0]; ACA = 2.5 cm [1.1 – 6.1]). The majority of tumors had biochemically unequivocal hormone hypersecretion. ACCs were more often cortisol producing (4/10), while 50% of ACAs secreted aldosterone (5/10; Table I).
Table I.
Clinical features of patients with adrenocortical carcinoma (ACC) and adrenocortical adenoma (ACA)
| Variable | ACC | ACA |
|---|---|---|
| Total (n) | 10 | 10 |
| Age ± SD, y | 55.5 ± 10.4 | 45.7 ± 14.0 |
| Male | 2 | 4 |
| Female | 8 | 6 |
| Stage* | ||
| I | 0 | n/a |
| II | 2 | n/a |
| III | 5 | n/a |
| IV | 3 | n/a |
| Tumor diameter, cm, mean (range) | 11.3 (6.0 – 14.0) | 2.5 (1.1 – 6.1) |
| Hormonal status | ||
| Cortisol | 4 | 2 |
| Nonhyperfunctional | 3 | 2 |
| Estridiol | 1 | 0 |
| Multiple | 1 | 1 |
| Unknown | 1 | 0 |
| Aldosterone | 0 | 5 |
Staging criteria per American Joint Committee on Cancer (AJCC), 7th edition
Reduced Expression of CYP4B1
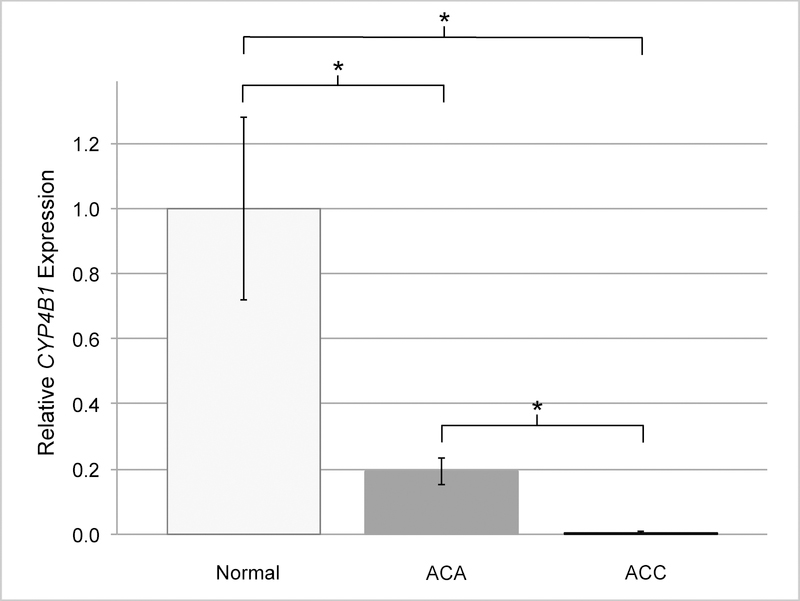
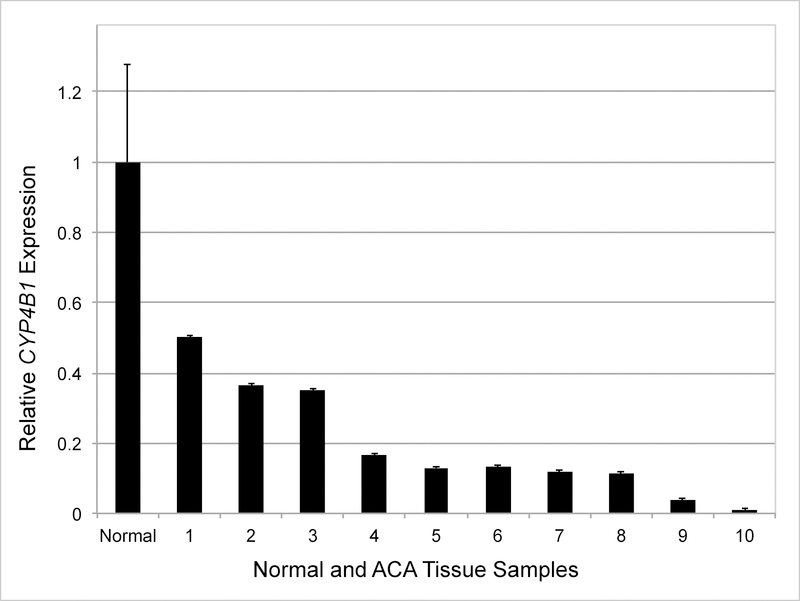
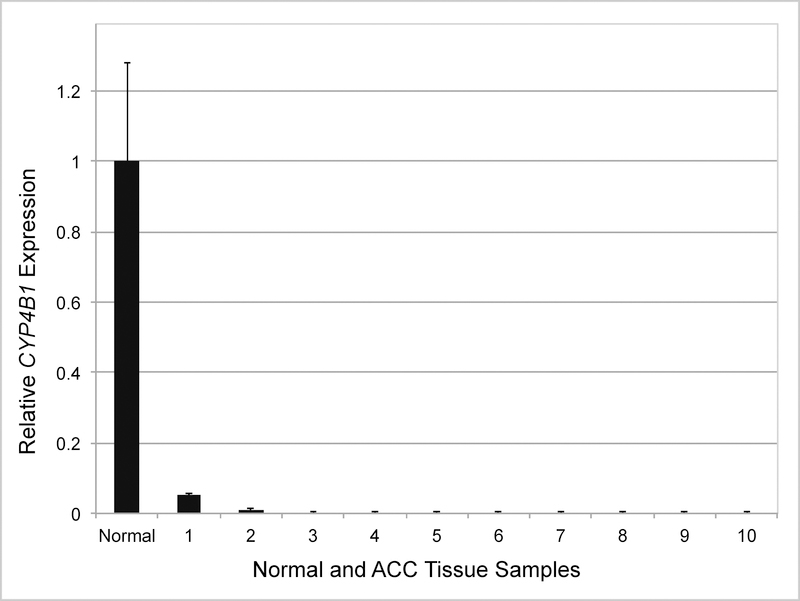
Gene expression analyses demonstrated suppression of CYP4B1 in 100% of ACA (10/10) and ACC (10/10) samples when compared with adjacent histologically normal adrenal tissue (Fig 1, 2 and 3). Overall, there was a correlation between tumor dedifferentiation and decreased CYP4B1 expression. Average relative expression of CYP4B1 in ACA was 0.19 (0.01 – 0.50) and was significantly reduced when compared to expression levels in the normal adrenal cortex (P < .01; Fig 1, 2). Expression was nearly absent in ACC samples and significantly less than ACA and normal (Average = 0.01; 0.00 – 0.05; P < .01; Fig 1, 3).
Fig 1.
Average relative expression of CYP4B1 is significantly decreased in adrenocortical tumors relative to normal adrenal tissue. Mean mRNA expression in normal samples (n = 10) is normalized to 1 and relative expression in AC As (n =10) and ACCs (n =10) are shown. Statistical significance was calculated using 2-tailed t test and P value < .01 was considered significant. Error bars, SEM. (* = P value < .01)
Fig 2.
CYP4B1 expression in individual adrenocortical adenoma (ACA) samples. Mean mRNA expression in normal samples (n = 10) is normalized to 1 and relative expressions in individual ACAs (n = 10) are shown. X axis: Normal and ACA tissue samples (numbered 1–10). Error bars, SEM.
Fig 3.
CYP4B1 expression in individual adrenocortical carcinoma (ACC) samples. Mean mRNA expression in normal samples (n = 10) is normalized to 1 and relative expressions in ACCs (n = 10) are shown. X axis: Normal and ACA tissue samples (numbered 1–10). Error bars, SEM.
Immunohistochemical detection of protein expression
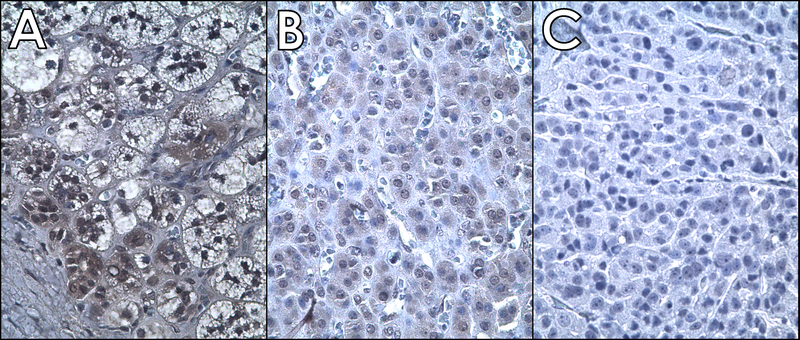
Histologically confirmed ACA, ACC, and normal adrenal samples were chosen from among the cohort to evaluate CYP4B1 protein expression. In normal adrenal tissue, immunostaining in the cytoplasmic and perinuclear regions was observed. Overall protein levels in ACA were markedly decreased compared to normal and undetectable in ACC (Fig 4).
Fig 4.
Immunohistochemical analysis confirmed reduced expression of CYP4B1 in Adrenocortical Tumors (A: Normal adrenal cortex, B: Adrenocortical adenoma, C: Adrenocortical carcinoma). Original magnification - 400X. Brown staining indicates CYP4B1 protein and dark blue indicates nuclear staining by hematoxylin.
Enforced expression of CYP4B1 in ACC cells
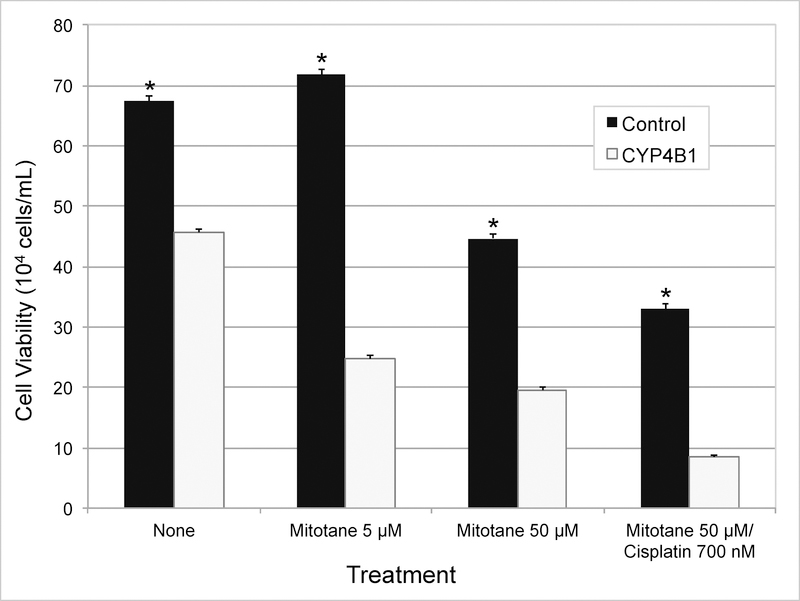
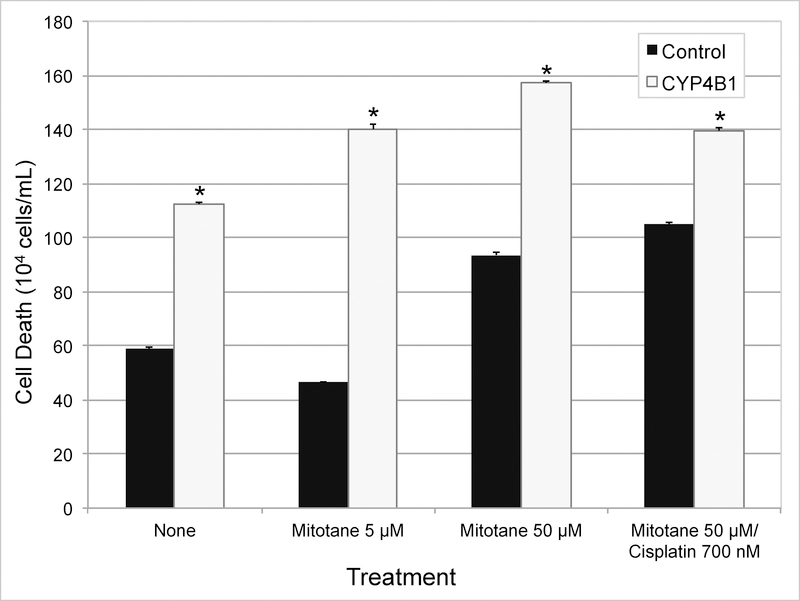
Stable transfection of CYP4B1 in NCI-H295R cells resulted in immediate loss of viability. Stable neomycin-resistant SW-13 cells selected also eventually lost the ability to express CYP4B1. Since the enforced stable expression of CYP4B1 appeared to be toxic to both ACC cell lines, we performed transient expression of CYP4B1 in relatively more tolerant SW-13 cells to determine if the presence of the protein was initiating cytotoxicity. Cells were also tested to determine whether standard ACC chemotherapy agents accentuate CYP4B1-promoted cytotoxicity. The effects of the medications tested were similar to those that have been reported before (Fig 5, 6).23 As could be predicted from the outcome of stable expression, expression of CYP4B1 alone was found to be sufficient to promote loss of ACC cell viability (Fig 5). This pattern of CYP4B1 promoted cellular toxicity persisted independent of the chemotherapy regimen tested (Fig 5). While control cells treated with low-dose mitotane therapy (5 μM) did not result in cytotoxicity, cells transfected to express CYP4B1 had significantly decreased viability and increased cell death (P < .01; Fig 5, 6). Addition of cisplatin to mitotane resulted in increased loss of viability compared to mitotane (50 uM) alone, and was further accentuated by expression of CYP4B1 (Fig 5). These experiments suggest a role for CYP4B1 in sensitizing dedifferentiated adrenocortical cells to cell death.
Fig 5.
Transient expression of CYP4B1 in ACC cell line (SW-13) decreases viability and sensitizes cells to chemotherapeutic agents. Twenty-four hours post-transfection, cells were treated with or without chemotherapy. Twenty-four hours after, cell viability was measured via Trypan blue assay. Statistical significance was calculated using 2-tailed t test and P value < .01 was considered significant. (* = P value < .01)
Fig 6.
Transient transfection of ACC cell line (SW-13) with CYP4B1 vector increases cell death and sensitizes cells to chemotherapeutic agents. Twenty-four hours post-transfection, cells were treated with or without chemotherapy. Twenty-four hours after, cell death was measured via Trypan blue assay. Statistical significance was calculated using 2-tailed t test and P value < .01 was considered significant. (* = P value < .01)
Conclusions
While the molecular etiology of adrenocortical carcinogenesis has yet to be fully characterized, substantive advances have been made in understanding the disease. However, cataloging the genetic and epigenetic changes that initiate and drive ACC has been stifled by both its rarity and inherent genetic complexity. Specifically lacking are studies that explore the effects of genetic change in the context of the complex metabolic milieu of the adrenal cortex. This study focuses on the potential role of a seminal member of the CYP family in the initiation and/or progression of ACCs.
In this study, we demonstrate significantly reduced expression of CYP4B1 in 100% of adrenocortical tumors, a previously undescribed event of potential metabolic and carcinogenic significance. Although the mechanism underlying observed sequential suppression of CYP4B1 in ACTs has not been completely clarified, copy number loss appears to contribute to the silencing of CYP4B1. Alternatively, epigenetic alteration may be a more dominant influence as the global silencing observed in all ACTs studied would not be explained by the lesser degree of gene copy loss.24 Moreover, the downregulation appears to follow a graded trend along with the dedifferentiation of the adrenal cortex (Fig 4).
CYP4B1 dysregulation has been noted in numerous malignancies. Unlike the present study, bladder and lung tumors show upregulation, not suppression, of CYP4B1.16,18,25 However, in bladder cancer, this shift appears to be associated only with allelic variants and not observed in the wild type protein.18 Repressed activity of CYP4B1 through missense mutations and deletions may allow malignant transformation. It is very likely that the metabolically intense endocrine physiology of the adrenal gland requires CYP4B1 for steroid synthesis, which, when perturbed, may lead to metabolic chaos and cell death. Since a specific role for CYP4B1 in the normal adrenal cortex is not yet known, it is difficult to speculate on the impact of its dysregulation or the possible effects of its silencing on adrenocortical dedifferentiation or carcinogenesis.
Of special interest, is the observation that both transient and stable expression of CYP4B1 resulted in toxicity in two well-studied ACC cell lines. Although the effect needs to be confirmed using inducible promoters, the preliminary results point towards a tumor suppressor role for CYP4B1 in ACC. The mechanism by which an enzyme uncommonly expressed in other tissues maintains a gatekeeper function in the context of the adrenal cortex is of immense interest, and needs to be studied further.26
Results of our experiments assessing the cytotoxic effect of CYP4B1 further support its role as a cell-death promoter in ACTs. Treatment with both mitotane and cisplatin in CYP4B1-expressing cells decreased viability in the two ACC cell lines studied. In the doses and combinations tested, presence of CYP4B1 appears to accentuate toxicity irrespective of the agent used. It is also noteworthy that CYP4B1 promoted significant cytotoxicity in SW-13 cells with low-dose mitotane (5 μM; Fig 5, 6). This apparent chemosensitization is clinically important as it may confer increased efficacy to anti-cancer drugs. Raising concentrations of peritumoral chemotherapy effectively shifts the dose-response curve, potentially reducing the risk of systemic toxicity.
Despite their common histological origin and shared profile of hormone secretion, there is little evidence that ACC arises from ACA. The graded decrease in expression of CYP4B1 and the observed correlation with adrenal dedifferentiation suggest at least a physiologic parallel between these distinct neoplasms. However, the small cohort size and the lack of further detailed examination prevent this study from making any concrete connection between these entities. In summary, this study offers new insight into the potential roles of CYP4B1 in the overall process of adrenal tumorigenesis and ACC chemoresistance.
References
- 1.Lehmann T, Wrzesinski T. The molecular basis of adrenocortical cancer. Cancer Genet 2012;205:131–7. [DOI] [PubMed] [Google Scholar]
- 2.Lebastchi AH, Kunstman JW, Carling T. Adrenocortical Carcinoma: Current Therapeutic State-of-the-Art. J Oncol 2012;2012:234726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fassnacht M, Wittekind C, Allolio B. [Current TNM classification systems for adrenocortical carcinoma]. Pathologe 2010;31:374–8. [DOI] [PubMed] [Google Scholar]
- 4.Wajchenberg BL, Albergaria Pereira MA, Medonca BB, et al. Adrenocortical carcinoma: clinical and laboratory observations. Cancer 2000;88:711–36. [PubMed] [Google Scholar]
- 5.Juhlin CC, Goh G, Healy JM, et al. Whole-exome sequencing characterizes the landscape of somatic mutations and copy number alterations in adrenocortical carcinoma. J Clin Endocrinol Metab 2015;100:E493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Assie G, Letouze E, Fassnacht M, et al. Integrated genomic characterization of adrenocortical carcinoma. Nat Genet 2014;46:607–12. [DOI] [PubMed] [Google Scholar]
- 7.Else T, Kim AC, Sabolch A, et al. Adrenocortical carcinoma. Endocr Rev 2014;35:282–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lerario AM, Moraitis A, Hammer GD. Genetics and epigenetics of adrenocortical tumors. Mol Cell Endocrinol 2014;386:67–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirschner LS. The next generation of therapies for adrenocortical cancers. Trends Endocrinol Metab 2012;23:343–50. [DOI] [PubMed] [Google Scholar]
- 10.Fassnacht M, Terzolo M, Allolio B, et al. Combination chemotherapy in advanced adrenocortical carcinoma. N Engl J Med 2012;366:2189–97. [DOI] [PubMed] [Google Scholar]
- 11.Fassnacht M, Kroiss M, Allolio B. Update in adrenocortical carcinoma. J Clin Endocrinol Metab 2013;98:4551–64. [DOI] [PubMed] [Google Scholar]
- 12.Madhunapantula SV, Mosca P, Robertson GP. Steroid hormones drive cancer development. Cancer Biol Ther 2010;10:765–6. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez-Antona C, Ingelman-Sundberg M. Cytochrome P450 pharmacogenetics and cancer. Oncogene 2006;25:1679–91. [DOI] [PubMed] [Google Scholar]
- 14.McFadyen MC, McLeod HL, Jackson FC, Melvin WT, Doehmer J, Murray GI. Cytochrome P450 CYP1B1 protein expression: a novel mechanism of anticancer drug resistance. Biochem Pharmacol 2001;62:207–12. [DOI] [PubMed] [Google Scholar]
- 15.Gandhi AV, Saxena S, Relles D, et al. Differential expression of cytochrome P450 omega-hydroxylase isoforms and their association with clinicopathological features in pancreatic ductal adenocarcinoma. Ann Surg Oncol 2013;20 Suppl 3:S636–43. [DOI] [PubMed] [Google Scholar]
- 16.Czerwinski M, Mclemore TL, Gelboin HV, Gonzalez FJ. Quantification of Cyp2b7, Cyp4b1, and Cypor Messenger-Rnas in Normal Human Lung and Lung-Tumors. Cancer Res 1994;54:1085–91. [PubMed] [Google Scholar]
- 17.Wei X, Zhang D, Dou X, et al. Elevated 14,15- epoxyeicosatrienoic acid by increasing of cytochrome P450 2C8, 2C9 and 2J2 and decreasing of soluble epoxide hydrolase associated with aggressiveness of human breast cancer. BMC Cancer 2014;14:841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sasaki T, Horikawa M, Orikasa K, et al. Possible relationship between the risk of japanese bladder cancer cases and the CYP4B1 genotype. Jpn J Clin Oncol 2008;38:634–40. [DOI] [PubMed] [Google Scholar]
- 19.Rubinstein JC, Brown TC, Goh G, et al. Chromosome 19 amplification correlates with advanced disease in adrenocortical carcinoma. Surgery 2016;159:296–301. [DOI] [PubMed] [Google Scholar]
- 20.Baer BR. Autocatalytic Mechanism and Functional Consequences of Covalent Heme Attachment in CYP4B1: University of Washington; 2005.
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001;25:402–8. [DOI] [PubMed] [Google Scholar]
- 22.Korah R, Healy JM, Kunstman JW, et al. Epigenetic silencing of RASSF1A deregulates cytoskeleton and promotes malignant behavior of adrenocortical carcinoma. Mol Cancer 2013;12:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gagliano T, Gentilin E, Benfini K, et al. Mitotane enhances doxorubicin cytotoxic activity by inhibiting P-gp in human adrenocortical carcinoma cells. Endocrine 2014;47:943–51. [DOI] [PubMed] [Google Scholar]
- 24.Cahan P, Li Y, Izumi M, Graubert TA. The impact of copy number variation on local gene expression in mouse hematopoietic stem and progenitor cells. Nat Genet 2009;41:430–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imaoka S, Yoneda Y, Sugimoto T, et al. CYP4B1 is a possible risk factor for bladder cancer in humans. Biochem Bioph Res Co 2000;277:776–80. [DOI] [PubMed] [Google Scholar]
- 26.Ponten F, Jirstrom K, Uhlen M. The Human Protein Atlas--a tool for pathology. J Pathol 2008;216:387–93. [DOI] [PubMed] [Google Scholar]