Abstract
Objectives.
Insomnia disorder is a risk factor for cardiovascular pathology. It is unknown whether insomnia that develops in the context of the menopausal transition impacts the cardiovascular system. We assessed nocturnal blood pressure and heart rate profiles in women with insomnia disorder in the menopausal transition.
Methods.
Twelve women meeting DSM-IV criteria for insomnia in the menopausal transition (Age, mean±SD: 50.5±3.6y) and eleven controls (Age, mean±SD: 49.0±3.0y) had polysomnographic recordings on one or two nights during which beat-to-beat blood pressure and heart rate were assessed and analyzed hourly from lights-out across the first 6h of the night and according to sleep stage. Physiological hot flashes were identified from fluctuations in sternal skin conductance.
Results.
Women with insomnia and controls had similar distributions of sleep stages and awakenings/arousals across hours of the night, although insomnia participants tended to have more wakefulness overall. More women in the insomnia group (7 of 12) than in the control group (2 of 11) had at least one physiological hot flash at night (p<0.05). Both groups showed a drop in blood pressure in the first part of the night, however, systolic and diastolic blood pressure patterns diverged later, remaining low in controls but increasing in insomnia participants 4-6 hours after lights-out (p<0.05). Both groups showed a similar pattern of decline in heart rate across the night.
Conclusions.
Our findings suggest altered regulatory control of blood pressure during sleep in menopausal transition insomnia. The causes and long-term consequences of this altered nocturnal blood pressure profile remain to be determined.
Keywords: Blood pressure, Menopause, Hot flashes, Sleep, Insomnia, Cardiovascular
Introduction
Insomnia disorder (difficulty initiating or maintaining sleep or early morning awakening, associated with clinically significant distress)1 is highly prevalent in women approaching menopause, with 26% of women in the menopausal transition meeting diagnostic criteria for an insomnia diagnosis.2 Insomnia that develops in the context of menopause has unique features like hot flashes and also shares several classical features of insomnia in the general population including anxiety and depression symptoms and high night-to-night variability in sleep quality.3 Hot flashes are a hallmark of the menopausal transition (MT), being reported by up to 85% of women, and are a cardinal feature of insomnia in midlife women.2 We previously reported that nocturnal physiological hot flashes, identified from fluctuations in sternal skin conductance, are more frequent in women who developed insomnia compared to those who did not develop insomnia in the context of the MT3 and that the majority of hot flashes are associated with awakenings, with hot flash-associated wake time contributing, on average, 27.2% of the total amount of polysomnographic wake after sleep onset (WASO).4 We also found that insomnia was associated with more polysomnographic (PSG)-defined WASO overall and shorter total sleep time, with almost 50% of the women with insomnia in the MT sleeping less than 6 hours per night.3
Insomnia is linked to several adverse mental and physical conditions5, 6 among which the development of cardiovascular (CV) disease is one of the most deleterious.7, 8 Given that insomnia combined with short sleep duration (<6 hours of sleep) accounts for the highest odds ratio in the risk of hypertension9 and is proposed as the most biologically severe phenotype of the disorder,10 insomnia disorder in women in the MT may have serious repercussions for future CV health.
Insomnia is considered to be a hyperarousal disorder in which several psychophysiological domains are over-activated.11 In-lab controlled studies have revealed that individuals with insomnia show elevated high frequency cortical electroencephalographic (EEG) activity,12 greater brain metabolism,13 and higher neuroendocrine (e.g. cortisol) and inflammatory indices (e.g. interleukin-6) 14, 15 than good sleepers. In addition, elevated cardiac sympathetic autonomic activity16-18 and sympathovagal balance favoring sympathetic predominance,19 have been reported. One study that measured beat-to-beat blood pressure (BP) also found elevated systolic BP (SBP) and a smaller wake-to-sleep dip in SBP in insomnia sufferers compared with controls.20 These laboratory findings of an adverse overall nocturnal CV profile in insomnia match the larger body of epidemiological evidence supporting a link between insomnia and CV risk.7, 8 Menopause is accompanied by an accelerated age-related rise in sympathetic nervous system activity and increase in BP in association with declining estrogen levels21 and hot flashes are linked with higher BP and CV risk factors.22, 23 Whether the nocturnal CV profile is altered in women with insomnia in the context of the MT, is unknown.
Sleep and the CV system are intimately connected.24 During non-rapid-eye-movement (NREM) sleep, CV activity reaches its lowest level with a reduction in sympathetic tone and increase in vagal functioning compared to rapid-eye-movement (REM) sleep, when autonomic nervous system (ANS) activity is similar to wakefulness.24-26 Baroreflex sensitivity increases during sleep and findings of a simultaneous drop in HR and BP at sleep onset together with effective baroreflex control, indicates a resetting of the reflex during sleep.24 Thus, changes in ANS innervation of the heart and vasculature drive a nocturnal reduction in BP, HR and systemic vascular resistance. Nocturnal BP is modulated to a greater extent by sleep than by circadian influences, as evident from constant routine paradigms.27, 28 On the other hand, the nocturnal drop in HR is strongly influenced by the circadian system.29 BP and HR also vary as a function of sleep stage, being higher in REM compared to NREM sleep, and show dramatic increases in response to arousals.30 In addition, phasic sleep events such as K-complexes, hallmarks of N2 sleep reflecting synchronization of cortical neurons,31 are associated with CV modifications (tachycardia followed by bradycardia) and these sleep events are thought to play a role in CV regulation during sleep.32 Thus, alterations in the sleep system may affect homeostasis of the CV system. In particular, frequent arousals from sleep associated with increased HR and BP impact the overall CV profile, reducing the extent of BP dipping at night.33
The aim of the current study was to investigate the nocturnal BP and HR profiles (as assessed by beat-to-beat monitoring) in women with insomnia disorder developed in the context of the MT compared with matched controls who had not developed insomnia. We hypothesized that women with insomnia compared to those without insomnia would have an adverse CV profile during the sleep period, characterized by higher BP and HR values and a blunted nocturnal BP dip.
Methods
Participants
Twenty-three women in the MT (defined according to Stages of Reproductive Aging Workshop criteria34, i.e. menstrual cycle lengths that differed by more than 7 days from normal or an amenorrhea interval of more than 60 days but not longer than 12 months) were included in this analysis. Twelve of the women met DSM-IV criteria for insomnia and eleven women served as controls. They were participants in a larger study of sleep quality in women with and without insomnia in the MT. Participants were included in the current analysis if they had at least 6 hours of reliable nocturnal BP data recorded during their sleep studies. The study was reviewed and approved by SRI International’s Institutional Review Board. All participants were informed about the purpose of the study and they gave written informed consent and received compensation for participation. For a full description of sample characteristic and screening procedure see Sassoon et al.35
All women had an intact uterus and at least one ovary, a Body Mass Index (BMI) of ≤32 kg.m−2 to reduce the likelihood of having obstructive sleep apnea, and no severe medical conditions (e.g. hypertension, diabetes); they were not taking hormone therapy and they were not currently using medication affecting sleep or the CV system (e.g. antihypertensives, benzodiazepines) or psychoactive medications; none of the participants were shift workers. None of the women had a lifetime history of DSM-IV insomnia, or currently had other Axis-I disorders (e.g., Major Depressive Disorder, Generalized Anxiety Disorder), except nicotine dependence (one woman with insomnia). None of the women reported pain disorders (e.g. Fibromyalgia). None of the women met criteria for a sleep disorder other than insomnia (e.g. breathing or leg movement disorders) as confirmed by a laboratory clinical PSG; all women had an apnea-hypopnea index ≤5 and a periodic limb movement index ≤10.
A structured clinical interview,36 including a customized module assessing DSM-IV criteria for insomnia,37 was administered to all participants. Twelve women met criteria for insomnia, reporting difficulty falling asleep and/or maintaining sleep and/or early morning awakenings at least three times per week for at least a month, associated with clinically significant distress/impairment in daytime functioning. The onset of insomnia was coincident with the MT. Eleven women without insomnia served as controls. Characteristics of the two groups are shown in Table 1.
Table 1.
Characteristics of 12 women with and 11 women without insomnia disorder developed in the context of the menopausal transition.
| Women without insomnia | Women with insomnia | ||||
|---|---|---|---|---|---|
| Mean ± SD | ±95%CI | Mean ± SD | ±95%CI | p | |
| Age, y | 49.0 ± 3.03 | 47.0 – 51.0 | 50.5 ± 3.6 | 48.2 – 52.8 | 0.293 |
| BMI, kg.m−2 | 23.7 ± 3.9 | 21.1 – 26.4 | 25.1 ± 3.7 | 22.7 – 27.4 | 0.404 |
| No. Caucasian | 9 | - | 11 | - | - |
| No. smokers | 0/11 | - | 1/12 | - | - |
| Alcohol intake, units/week | 2.8 ± 3.6 | 0.4 – 5.3 | 2.2 ± 2.6 | 0.5 – 3.8 | 0.609 |
| Caffeine intake, cups/day | 1.3 ± 0.9 | 0.7 – 1.9 | 1.7 ± 1.7 | 0.6 – 2.8 | 0.556 |
| BDI-II, total score | 3.4 ± 2.8 | 1.5 – 5.2 | 6.4 ± 3.4 | 4.2 – 8.6 | 0.029 |
| PSQI, total score | 3.7 ± 1.4 | 2.8 – 4.6 | 9.1 ± 2.9 | 7.2 – 10.9 | <0.001 |
| GCS-psychological | 3.4 ± 2.2 | 1.9 – 4.8 | 6.0 ± 3.2 | 4.0 – 8.0 | 0.034 |
| GCS-somatic | 1.9 ± 2.2 | 0.4 – 3.4 | 2.2 ± 3.1 | 0.2 – 4.2 | 0.824 |
| GCS-vasomotor | 1.4 ± 0.8 | 0.8 – 1.9 | 2.8 ± 1.5 | 1.8 – 3.7 | 0.012 |
| FSH, IU.l−1 | 16.4 ± 9.6 | 9.9 – 22.8 | 44.1 ± 43.1 | 16.8 – 71.5 | 0.115 |
| Estradiol, pg.mol−1 | 57.4 ± 32.2 | 35.8 – 79.1 | 36.4 ± 27.1 | 19.2 – 53.7 | 0.072 |
BMI, Body Mass Index; BDI, Beck Depression Inventory; GCS, Greene Climacteric Scale; FSH, Follicle-Stimulating Hormone; No., Number; PSQI, Pittsburgh Sleep Quality Index.
Laboratory procedures
All women had an adaptation/clinical PSG to adapt them to the laboratory and procedures and to confirm absence of a sleep disorder other than insomnia. Participants then had one (7 women with and 6 women without insomnia) or two (5 women with and 5 women without insomnia) non-consecutive PSG recordings in the Human Sleep Research Laboratory at SRI international. Participants were instructed to refrain from consuming alcohol or drinking beverages containing caffeine after 15:00 of each recording day; all women registered 0.0 on a breathalyzer on arrival at the sleep laboratory. Lights-out (time at which the participants’ bedroom lights were turned off and participants were allowed to sleep) and lights-on (time at which the participants’ bedroom lights were turned on, marking the end of the recording and wake-up time) times were self-selected by participants. PSG, beat-to-beat BP, ECG and skin conductance signals were continuously collected throughout the night. Participants slept in temperature-controlled and sound-attenuated bedrooms. Blood samples were collected at each visit either in the evening or in the morning.
Self-report assessment of sleep, depression and menopausal symptoms
At the clinical interview, participants completed questionnaires about their sleep habits and quality over the past month using the Pittsburgh Sleep Quality Index (PSQI).38 They also completed the Beck Depression Inventory (BDI-II)39 to assess symptoms of depression, and the psychological, somatic, and vasomotor scales of the Greene Climacteric Scale (GCS).40
Polysomnographic assessments
A standard PSG including electroencephalography (F3/4, C3/4, O3/4 referenced to the contralateral mastoids), electrooculography and electromyography was performed according to American Academy of Sleep Medicine (AASM) criteria41 using a Grael™ system and Profusion PSG3™ software (Compumedics, Abbotsford, Victoria, Australia). EEG signals were sampled at 256Hz and filtered (0.3-35Hz). The PSG (Wake, N1, N2, N3, REM) was scored in 30s epochs and brief arousals (≥3s, <15s) were marked according to AASM criteria.41 Total sleep time (TST, min), time taken to fall asleep (time from lights-out to the first epoch of any sleep stage, SOL, min), total amount of wake after sleep onset (WASO, min), time spent in N1, N2, N3 and REM sleep as a percentage of TST, were calculated. In addition, women rated their sleep quality on a 100 mm visual analogue scale (0 mm = very bad, 100 mm = very good).
PSG time spent awake (min), in NREM (N1+N2+N3, min) and REM (min) sleep, and number of awakenings and arousals were calculated for each hour of the night to match the analysis of the night-time cardiovascular profile.
Nocturnal cardiovascular assessment
Beat-to-beat blood pressure was recorded throughout the night using a Portapres Model-2 unit TNO TPD Biomedical Instrumentation, Amsterdam, NL) connected to two photoplethysmography cuffs attached to the participants’ index and middle fingers of the non-dominant hand. The sampling rate was 64Hz. The potential ischemic discomfort associated with continuous cuff inflation was minimized by regularly alternating measurements between two fingers (15-min intervals). This validated method42, 43 allows non-invasive prolonged recordings of BP. Recordings with less than 6h of reliable BP data or those in which cuffs shifted by ≥5 mmHg and/or had extensive drop-outs in the blood pressure signal were excluded.
All analyses were performed using SRS 5.1 software (Sleep Research System, School of Behavioural Science, University of Melbourne, Australia). Peak (systole) and lowest point (diastole) of the BP waveform were automatically identified by the software and manually adjusted when necessary for each cardiac cycle. BP data were further inspected for accuracy, particularly for potential cuff-related errors, and unreliable recording periods were deleted (3.8% rejection rate). We also checked whether cuff-switching differentially impacted sleep in the insomnia and control groups and found no difference: women with insomnia had 0.69±0.26 cuff shift-related arousals/awakenings per hour of sleep and controls had 0.52±0.25 cuff shift-related arousals/awakenings per hour of sleep, p > 0.05. Systolic (SBP, mmHg) and diastolic (DBP, mmHg) blood pressure measures were obtained and averaged for each hour after lights-out across the first 6 hours of the night because all women had at least 6 complete hours in bed. Average BP measures were also calculated separately for NREM and REM sleep.
The electrocardiogram signal was collected at 512 Hz using Ag/AgCl Meditrace surface spot electrodes in a modified lead II Einthoven configuration (leads were placed under the right clavicle and on the lower left abdomen within the rib cage frame). R-waves were automatically detected and manually adjusted when necessary and normal-to-normal interbeat-intervals (IBIs, ms) were calculated. HR was averaged for each hour after lights-out and also calculated separately for NREM and REM sleep.
Objective hot flash measurement
Nocturnal skin conductance signals were recorded using a BioDerm Skin Conductance Meter (Model 2701; UFI, Morro Bay, CA). Two 1.5 cm in diameter Ag/AgCl electrodes filled with 0.05 M potassium chloride Velvachol/glycol gel were placed on either side of the sternum and a 0.5-V constant voltage circuit was maintained between them.44 The Bioderm was connected via an optically isolated DC input to the Compumedics amplifiers and the skin conductance signal was sampled at 16 Hz and co-registered online as a polysomnographic channel. Hot flashes were independently evaluated by two scorers for fluctuation in skin conductance ≥2 micro Siemens (μmho) within 30 seconds.45 Number of hot flashes and time spent awake (min) associated with hot flashes (see,4) were calculated.
Serum hormone analyses
At each visit, blood samples were collected and frozen (−70˚C) before analysis with standard immunoassay kits for follicle-stimulating hormone (FSH, IU.l−1) (Siemens Healthcare Diagnostics, Los Angeles, CA, USA; intraassay and interassay coefficients of variations were 2.6% and 5.5%, respectively; sensitivity, 0.1 IU.l−1), progesterone (ng.ml−1) (Siemens Medical Solutions Diagnostics, Los Angeles, CA, USA; intraassay and interassay coefficients of variations were 4.0% and 5.7%, respectively; sensitivity, 0.2 ng.ml−1) and estradiol (pg.mol−1) (Beckman Coulter Inc., Fullerton, CA, USA; intraassay and interassay coefficients of variations were 6.7% and 7.6%, respectively; sensitivity, 3 pg.mol−1). Progesterone levels ≥3 ng.ml−1 were used as a cut-off to classify the luteal phase46 (five women with and five women without insomnia had a PSG night recorded in the luteal phase of their menstrual cycles; in all the remaining nights progesterone was <3 ng.ml−1).
Statistical Analyses
FSH and estradiol values were log transformed to achieve normal distribution before analysis. Independent t-tests were used to test group differences (women with vs. women without insomnia) in demographic measures, hormonal levels, subjective sleep, mood, and menopausal symptoms. Independent t-tests were also used to test group differences in all-night PSG measures (for women contributing two nights, values were first averaged for both nights). The Chi-Square test was used to compare the frequencies with which insomnia and control women had hot flashes.
Repeated measures mixed models were used to investigate potential group differences in nocturnal CV profiles and sleep stage distribution across hours of the night, accounting for multiple observations for some of the women (5 women with and 5 women without insomnia contributed two nights of data to the analysis. The remaining participants contributed one night). The models included group (women with and without insomnia), time (hours across the night as a continuous variable), and the interaction term group × time. All models included random effects for participants and residual error. If time or the interaction of group × time were statistically significant, we conducted post-hoc tests using a simplified mixed model (analogous to post-hoc t-tests for an ANOVA) for comparing response means at different time points. Wald Chi-Square test and associated p values are provided for the overall models; Z and associated p values are provided for significant main effects of group and/or time and/or group × time interactions. Additional models that included group, sleep stage (NREM and REM), and the interaction term group × sleep stage were run to investigate sleep stage differences in BP and HR measures. The potential influence of hot flashes and stage of the MT on the CV profile was also explored by adding hot flashes (yes/no) and FSH levels as additional factors in the models. F values are provided for the overall models and t-values are provided for significant main effects. All analyses were performed using Stata/SE 14.1 for Windows. Results are reported as mean ± SD unless otherwise indicated. Significance was set at p<0.05.
Results
Demographic measures and self-reported symptoms
Women with insomnia had higher BDI scores, reflecting more depressive symptoms (t = −2.34, p < 0.05), higher PSQI scores, reflecting poorer sleep quality (t = −5.53, p < 0.001). They also had higher scores on the psychological (t = −2.27, p < 0.05) and vasomotor (t=−2.74, p<0.05) scales of the GCS than controls. The groups did not differ significantly for age, BMI, FSH and estradiol levels, although FSH levels tended to be higher in the insomnia group (Table 1).
Sleep stage distribution, hot flashes, awakenings and arousals across the night
As shown in Table 2, there were no significant group differences for lights-out and lights-on times, sleep onset latency, or total sleep time. There was a strong tendency for women with insomnia to have more WASO than controls, which was not quite significant (p = 0.051). There were no group differences in percentage time spent in each sleep stage (Table 2).
Table 2.
Variables derived from all-night polysomnography, and subjective sleep quality ratings, in 12 women with and 11 women without insomnia disorder developed in the context of the menopausal transition.
| Women without insomnia | Women with insomnia | ||||
|---|---|---|---|---|---|
| Sleep variable | Mean ± SD | ±95%CI | Mean ± SD | ±95%CI | p |
| Lights-out time, hh:mm | 23:08 ± 00:49 | 22:35 – 23:42 | 23:09 ± 00:39 | 22:44 – 23:34 | 0.968 |
| Lights-on time, hh:mm | 06:11 ± 00:37 | 5:47 – 6:36 | 06:21 ± 00:52 | 5:48 – 6:54 | 0.634 |
| TST, min | 383.3 ± 39.0 | 357 – 409 | 359.7 ± 41.4 | 333 – 386 | 0.175 |
| SOL, min | 10.8 ± 11.3 | 3.2 – 18.3 | 7.3 ± 6.5 | 3.1 – 11.4 | 0.370 |
| SE, % | 88.8 ± 4.4 | 85.8 – 91.7 | 83.7 ± 9.2 | 77.9 – 89.6 | 0.115 |
| WASO, min | 37.0 ± 16.5 | 25.8 – 48.1 | 65.6 ± 43.0 | 38.3 –92.9 | 0.051 |
| Time in N1, %TST | 7.7 ± 4.1 | 5.0 – 10.5 | 9.3 ± 5.2 | 6.0 – 12.6 | 0.436 |
| Time in N2, %TST | 56.7 ± 4.3 | 53.8 – 59.6 | 55.8 ± 7.7 | 50.9 – 60.7 | 0.751 |
| Time in N3, %TST | 13.2 ± 5.2 | 9.7 – 16.7 | 12.8 ± 9.0 | 7.0 – 18.5 | 0.887 |
| Time in REM, %TST | 22.4 ± 4.4 | 19.4 – 25.4 | 22.1 ± 3.9 | 19.6 – 24.6 | 0.864 |
| Self-report sleep quality, 1-100 mm VAS scale | 82.2 ± 41.9 | 54.1 – 110.4 | 61.8 ± 33.7 | 40.4 – 83.1 | 0.208 |
data from two women with and one woman without insomnia were not available. TST, Total Sleep Time; SOL, Sleep Onset Latency; REM, Rapid-Eyes-Movement (L = latency); SE, Sleep Efficiency; WASO, Wake After Sleep Onset; VAS, Visual Analogue Scale
Seven women with insomnia compared to two women without insomnia had at least one objectively recorded hot flash at night (Chi-Square = 3.88, p<0.05). Among these women, the two controls had, respectively, 1 and 4 hot flashes per night, accounting for 0% and 6.3% of total WASO; the seven women with insomnia had, on average, 3.9 (±3) hot flashes per night (range: 1 – 8), accounting for 22.4% (±21.9%) of total WASO.
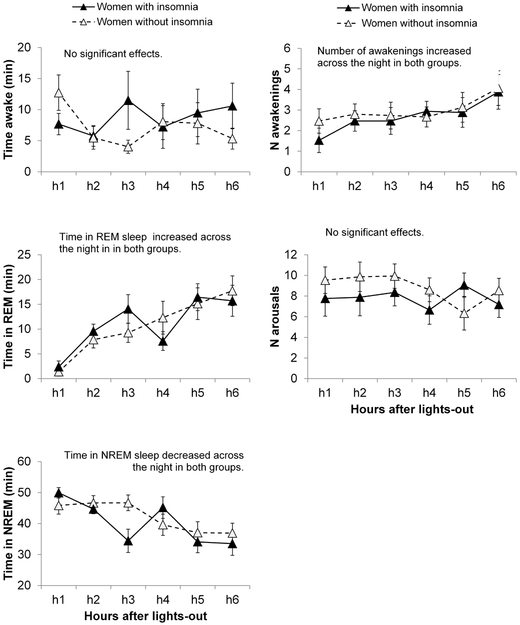
The hourly distribution of wake time, NREM sleep, REM sleep, and numbers of awakenings and arousals across the first 6 hours after lights-out was similar between groups, with no significant group or group × time interaction effects (Figure 1). As expected, there was a significant time effect for REM sleep (Z=6.60, p<0.001), which increased across the night. There was also a significant time effect for NREM sleep (Z = −5.04, p < 0.001), which decreased across the night. The number of awakenings (Wald Chi-Square = 20.13, p = 0.001) increased (time main effect, Z = 4.17, p < 0.001) in h6 compared to h1-h3 in both groups (all p < 0.05).
Figure 1.
Polysomnographic sleep stage composition, number of awakenings and arousals across the first 6 hours of the night in 12 women with and 11 women without insomnia disorder developed in the context of the MT. Vertical bars represent standard errors.
Nocturnal cardiovascular profiles
The hourly averages of HR, SBP, and DBP across the first 6 hours after lights-out are shown in Figure 2.
Figure 2.
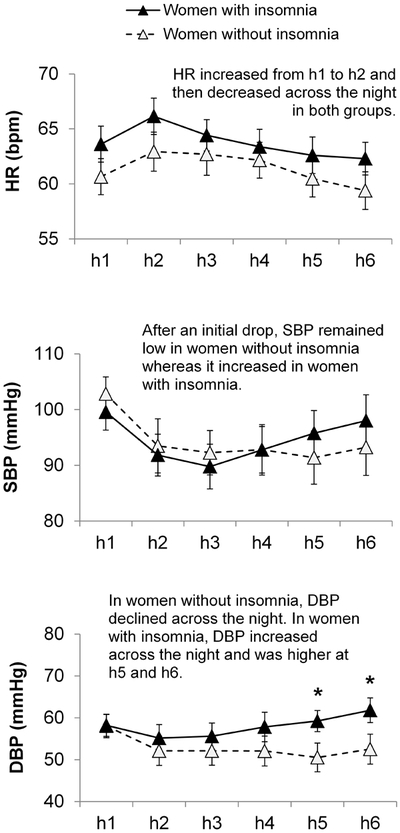
Heart rate (HR), systolic (SBP), and diastolic (DBP) blood pressure across the first six hours of the night after lights-out in 12 women with and 11 women without insomnia disorder developed in the context of the MT. Vertical bars represent standard errors. Asterisks mark significant group differences in absolute values at each hour of the night. Details of the significant differences according to time within each group are described in the main text.
HR.
The model was significant (Wald Chi-Square = 29.94, p < 0.001) for HR revealing a time main effect (Z = −3.83, p < 0.001). In both groups of women, HR rose from h1 to h2 and then decreased from h2 to h5 and h6, from h3 to h5 and h6 and from h4 to h6 (all p < 0.05). The main effect of group and the interaction term were not significant.
SBP.
The model was significant (Wald Chi-Square = 30.74, p < 0.001) for SBP revealing a group × time interaction (Z = 2.42, p = 0.016). In both groups of women, SBP dropped at the beginning of the night, from h1 to h2 and h3 (all p < 0.05). However, the drop was maintained only in controls with SBP in h4-h6 being lower compared to h1 (all p < 0.05). In contrast, in those women with insomnia, after the initial drop, SBP significantly increased and was significantly higher in h4-h6 than in h3 (all p < 0.05). Although the pattern of change in SBP across the night differed between groups, post-hoc analysis revealed no difference in absolute values at each hour between groups.
DBP.
The model was significant (Wald Chi-Square = 20.93, p < 0.001) for DBP revealing a group × time interaction (Z = 3.17, p = 0.002). In the control group, DBP decreased across the night with DBP in h2 (p < 0.05) and h5 (p < 0.05) being lower than in h1. In the insomnia group, DBP increased in the latter part of the night being higher in h6 compared to h2 and h3 (all p < 0.05). Post-hoc analysis of values at each hour showed that women with insomnia had higher DBP than controls in h5 and h6 (p < 0.05).
Effect of sleep stage on cardiovascular measures
The model was significant for HR (F3,30 = 8.5, p = 0.0003), revealing a main effect of sleep stage (t = 4.77, p =< 0.001), with HR being higher in REM sleep (by 1.88 ± 2.32 bpm in controls, and by 2.78 ± 3.11 bpm in women with insomnia) than in NREM sleep. The model was also significant for SBP (F3,30 = 7.1, p = 0.001), with a main effect of sleep stage (t = 4.09, p<0.001), with SBP being higher in REM sleep (by 3.53 ± 7.83 mmHg in controls, and by 8.32 ± 8.47 mmHg in women with insomnia) than in NREM sleep. Similarly, the model for DBP (F3,30 = 6.78, p = 0.001) was significant with a main effect of sleep stage. DBP was higher in REM sleep (by 2.36 ± 4.79 mmHg in controls, and by 4.83 ± 5.52 mmHg in women with insomnia) than in NREM sleep. Group and group × sleep stage interaction effects were not significant for any of the models.
We re-ran all models including the amount of time women spent awake for each hour of sleep (which included the time spent awake associated with hot flashes) as a potential confounding factor. All the significant effects, including interactions remained. Hot flashes (yes/no) and FSH levels, as a biological marker of the MT47, were not significant factors in the models. Also, extending the period of analysis for BP to the 7th hour of the night in 11 women with and 10 women without insomnia having at least 10min of reliable BP data in h7, did not change results with both groups of women maintaining the same nocturnal SBP and DBP pattern into h7.
Discussion
Our results show that women with insomnia that developed in the context of the MT have an altered nocturnal BP profile compared with controls. Both groups showed an initial drop in BP, which was maintained in controls whereas BP increased across the night in women with insomnia. The divergence in BP between the two groups of women remained significant when controlling for the amount of night-time wakefulness in the analysis and did not appear to be obviously related to sleep composition; both groups had similar lights-out and lights-on times as well as similar PSG sleep stage distributions across hours of the night. Also, there was no significant difference in the NREM-REM increase in BP between groups.
Our finding of an initial reduction in SBP from the first to second and third hours after lights-out in both groups is consistent with the literature showing that BP falls around sleep-onset, in part associated with a change to supine posture, and shows a further reduction in association with stable NREM sleep (N3 and N2).25, 48 We only measured BP from lights-out and therefore did not capture the dip in BP that is evident from day to night-time levels49 and from before lights-out to the first hour of sleep.48 Similar to previous findings,25 we also found that BP was higher in REM sleep than in NREM sleep. The fall in BP after sleep is initiated likely reflects changes in autonomic control, with a fall in sympathetic vascular tone and consequently increased vasodilation, as well as a resetting of the baroreflex during NREM sleep.24, 25 Cardiac sympathetic activity also decreases, and vagal activity increases, associated with a decrease in HR across the sleep period,25, 29 as we found here in both women with and without insomnia, along with the expected REM-NREM difference in HR.24 Our main finding, of a rise in BP in the second part of sleep in women with insomnia in contrast to the maintained decline in BP across the night in controls, suggests an underlying difference in BP regulation in women with insomnia. An absent or blunted nocturnal BP drop has been linked with adverse consequences including vascular damage, endothelial dysfunction and atherosclerosis.50 Indeed, a non-dipping nocturnal BP profile is considered an important cardiovascular risk factor.51 However, to our knowledge, there are no studies specifically characterizing and linking the maintenance of the nocturnal dip in BP across the night with adverse clinical manifestations including biomarkers of CV disease. The clinical significance of an arrested drop in BP during the night, therefore, remains to be determined.
To our knowledge, only one other study has investigated nocturnal BP profiles in insomnia disorder.20 Beat-to-beat BP over a 24h period was assessed in 13 participants with and 13 participants without chronic insomnia. The results indicated that insomnia participants had higher SBP and DBP measures across the night and a lower day-to-night BP drop (less dipping), in the absence of any difference in PSG sleep macrostructure, suggesting that an altered BP profile may be a trait-like feature of insomnia. While we also found differences in the nocturnal BP profile in women with insomnia developed in the context of the MT compared with controls, our findings are not identical to those of Lanfranchi et al.20 We found that BP only diverged in the second part of the night, increasing from the fourth hour of the sleep period onwards in the insomnia group. This increase in BP was much earlier than the well-documented morning surge in BP in proximity to wake-up time and associated postural and ambulatory changes.52 Our sample consisted of women who developed insomnia in the context of the MT whereas the sample included in the study by Lanfranchi et al.20 was comprised of men and women of a wide age range (30-60y). There are sex and age-related differences in autonomic ANS regulation of BP21, which could explain the different results between studies. Also, there are functional and structural differences in several systems that are involved in BP regulation, including the ANS, in women at different reproductive stages.21 Thus, potential alterations in the mechanisms involved in BP regulation may differ in insomnia developed in the context of the MT compared to insomnia developed before the MT. It appears that loss of estrogen as women transition menopause is linked mechanistically with decreased β-adrenergic vasodilatation and consequently increased risk of hypertension in older women.53 The altered hormone environment in the MT in combination with insomnia could therefore impact the BP profile.
While nocturnal BP profiles differed between women with insomnia and controls, HR profiles did not, with both groups showing a reduction in HR across the night. HR appeared to be faster at each time point across the night in the insomnia women (Figure 2), however, there was no significant group difference at any time, which may be due, in part, to low statistical power in this small sample, with high between-participant variability in each hour sampled.
We did not find any group differences in PSG-defined sleep macrostructure, however, women with insomnia tended to have more WASO overall. In our analysis of the larger sample which included women from this analysis (n = 72), we found that women with insomnia had significantly shorter TST and longer WASO than controls,3 raising the possibility that these factors could have affected BP. Women with insomnia also show large night-to-night variability in self-report measures of WASO,3 and chronically variable sleep-wake patterns might impact BP profiles in insomnia. While PSG measures of sleep quality and/or sleep depth have been associated with the extent of the BP dip in healthy populations,54, 55 PSG-measured sleep quality did not predict BP dipping in untreated patients with mild to severe obstructive sleep apnea,56 raising the possibility that the normal relationship between sleep quality and BP dipping may unravel in clinical populations. Of note, patients with obstructive sleep apnea typically have a more blunted nocturnal BP profile across the night than what we found in the insomnia women, with SBP values ~ 15 mmHg higher than in controls.57 It remains to be determined whether different alterations in nocturnal BP profiles in clinical populations afford different CV risk profiles.
Differences in sleep microstructure (e.g. lower delta EEG power and elevated beta EEG power) are implicated in altered nocturnal BP levels. High beta EEG power is considered an indicator of cortical hyper arousal58 and Lanfranchi et al.20 found that high night-time SBP was independently associated with high beta EEG power. We previously reported that beta EEG activity was elevated in REM sleep (but not in NREM sleep) in the larger sample of women who developed insomnia in the context of the MT compared with controls.3 Also, the Study of Women Across the Nation (SWAN) reported that beta EEG power in NREM and REM sleep was higher in late perimenopausal and postmenopausal women compared to pre- and early perimenopausal women, an effect partially explained by the frequency of hot flashes.59 Further, in the SWAN data set, high beta EEG power was related to increased likelihood of being hypertensive.60 Several studies have indicated that sympathovagal balance favoring vagal predominance is related to high levels of slow wave sleep and delta EEG power61-66 and importantly, changes in the ANS anticipate the production of delta EEG, suggesting that changes in ANS modulation facilitate cortical synchronization.65, 67, 68 Altered relationships (decrease in coherence) between measures of sympathovagal balance and delta EEG power have been found in chronic insomnia69 as well as in other sleep disorders like sleep apnea-hypopnea syndrome70 and in patients with major depressive disorder.71 Thus, it is possible that ANS-sleep relationships are altered in women who developed insomnia in the context of the MT. Future studies are needed to evaluate sleep-BP and sleep-ANS relationships in MT insomnia and to analyze CV responses to arousals and other phasic sleep events like K-complexes to investigate whether CV responses are exaggerated, which could explain the increase in BP across the night in women who developed insomnia in the MT.
Hot flashes are a unique feature of insomnia that develop in the MT.72 As expected, we found that women with insomnia had higher scores on the GCS vasomotor scale and more of them showed a propensity for objective hot flashes at night, based on measures of skin conductance, than controls. The frequency of hot flash events across the night in most of the women with insomnia in our study was relatively low, and wake associated with hot flashes contributed, on average, 23% of total WASO. The propensity for having hot flashes over and above the events themselves could also influence the BP profile. Others found that self-reported hot flashes at night were associated with a transient increase in BP73 and studies show that women with hot flashes (which are more likely to be those suffering from insomnia) have higher resting BP and higher HR during day and night compared to asymptomatic women.22, 74, 75 Also, hot flashes are linked to subclinical CV disease and CV risk factors23 and, in particular, alterations in the vasculature. Women with hot flashes have reduced flow-mediated dilation and more aortic calcification76 and women with persistent hot flashes or early-onset hot flashes have higher carotid intima media thickness than women with infrequent hot flashes.77 Future studies are needed in larger samples of midlife women with insomnia and a range in frequency and severity of hot flashes to investigate if hot flashes are directly or indirectly responsible for altered nocturnal ANS control over the heart (e.g. elevated cardiac sympathetic activity) and vasculature (e.g. baroreceptor reflex control malfunctioning, sympathetic vasoconstriction). The duration of menopausal symptoms, including insomnia, also needs to be considered to understand the time course for the development of the alteration in nocturnal BP profiles.
Our study has limitations that should be considered. The sample size is small, even though resolution of measurement (beat-to-beat) was high and some women contributed two nights of recordings to the analysis. We did not measure BP during the day and were, therefore, unable to capture the BP dipping profile. Similarly, analysis was limited up to 6h of the night and we therefore did not capture the sleep-to-wake morning rise in BP. We are unable to identify the physiological mechanism underlying the different BP profile in MT insomnia because we did not assess peripheral vascular resistance, baroreceptor control, or sympathetic nervous system activity. Future work is required to investigate these measures in women who developed insomnia in the context of the MT.
Conclusion
We show here that women with insomnia that developed in the context of the MT have an altered nocturnal BP profile, with a rise in both systolic and diastolic BP in the second part of the sleep period, suggesting altered CV regulation compared to women without insomnia. Further studies in larger samples of women are needed to confirm these findings and to investigate the potential clinical significance of an altered nocturnal BP profile.
Acknowledgements
Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health (HL103688, FCB). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Hormone analysis was conducted by The University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core, which is supported by the Eunice Kennedy Shriver NICHD/NIH (NCTRI) Grant P50-HD28934. We thank our research assistants Rebecca Carr, Stephanie Claudatos, David Dresser, Justin Greco, Sarah Inkelis, and Lena Kardos for their effort in collecting data for this project, and all research participants.
Financial disclosure. This study was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health (HL103688, FCB).
Footnotes
Conflict of interest. The authors declare no conflicts of interest
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM 5. 5th ed. Arlington, VA: American Psychiatric Publishing, 2013. [Google Scholar]
- 2.Ohayon MM. Severe hot flashes are associated with chronic insomnia. Arch Intern Med 2006;166:1262–8. [DOI] [PubMed] [Google Scholar]
- 3.Baker F, Willoughby AR, Sassoon S, Colrain IM, de Zambotti M. Insomnia in women approaching menopause: beyond perception. Psychoneuroendocrinology 2015;60:96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Zambotti M, Colrain I, Javitz H, Baker F. Magnitude of the impact of hot flashes on sleep in perimenopausal women. Fertil Steril 2014;102:1708–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buysse D. Insomnia. JAMA 2013;309:706–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Irwin M Why sleep is important for health: a psychoneuroimmunology perspective. Annu Rev Psychol 2015;66:143–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sofi F, Cesari F, Casini A, Macchi C, Abbate R, Gensini G. Insomnia and risk of cardiovascular disease: a meta-analysis. Eur J Prev Cardiol 2014;21:57–64. [DOI] [PubMed] [Google Scholar]
- 8.Li M, Zhang X, Hou W, Tang Z. Insomnia and risk of cardiovascular disease: A meta-analysis of cohort studies. Int J Cardiol 2014;176:1044–7. [DOI] [PubMed] [Google Scholar]
- 9.Vgontzas AN, Liao D, Bixler EO, Chrousos GP, Vela-Bueno A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep 2009;32:491–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vgontzas A, Fernandez-Mendoza J, Liao D, Bixler E. Insomnia with objective short sleep duration: the most biologically severe phenotype of the disorder. Sleep Med Rev 2013;17:241–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levenson JC, Kay DB, Buysse DJ. The pathophysiology of insomnia. Chest 2015;147:1179–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perlis M, Merica H, Smith M, Giles D. Beta EEG activity and insomnia. Sleep Med Rev 2001;5:363–74. [DOI] [PubMed] [Google Scholar]
- 13.Nofzinger E, Buysse D, Germain A, Price J, Miewald J, Kupfer D. Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry 2004;161:2126–9. [DOI] [PubMed] [Google Scholar]
- 14.Burgos I, Richter L, Klein T, et al. Increased nocturnal interleukin-6 excretion in patients with primary insomnia: a pilot study. Brain Behav Immun 2006;20:246–53. [DOI] [PubMed] [Google Scholar]
- 15.Rodenbeck A, Huether G, Rüther E, Hajak G. Interactions between evening and nocturnal cortisol secretion and sleep parameters in patients with severe chronic primary insomnia. Neurosci Lett 2002;324:159–63. [DOI] [PubMed] [Google Scholar]
- 16.de Zambotti M, Covassin N, De Min TG, Sarlo M, Stegagno L. Sleep onset and cardiovascular activity in primary insomnia. J Sleep Res 2011;20:318–25. [DOI] [PubMed] [Google Scholar]
- 17.de Zambotti M, Covassin N, Sarlo M, De Min TG, Trinder J, Stegagno L. Nighttime cardiac sympathetic hyper-activation in young primary insomniacs. Clin Auton Res 2013;23:49–56. [DOI] [PubMed] [Google Scholar]
- 18.de Zambotti M, Cellini N, Baker F, Colrain I, Sarlo M, Stegagno L. Nocturnal cardiac autonomic profile in young primary insomniacs and good sleepers. Int J Psychophysiol 2014;93:332–9. [DOI] [PubMed] [Google Scholar]
- 19.Bonnet M, Arand D. Heart rate variability in insomniacs and matched normal sleepers. Psychosom Med 1998;60:610–5. [DOI] [PubMed] [Google Scholar]
- 20.Lanfranchi P, Pennestri M, Fradette L, Dumont M, Morin C, Montplaisir J. Nighttime blood pressure in normotensive subjects with chronic insomnia: implications for cardiovascular risk. Sleep 2009;32:760–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vongpatanasin W Autonomic regulation of blood pressure in menopause. In: Semin Reprod Med; 2009, 2009. p. 338–45. [DOI] [PubMed] [Google Scholar]
- 22.Gast GC, Grobbee DE, Pop VJ, et al. Menopausal complaints are associated with cardiovascular risk factors. Hypertension 2008;51:1492–8. [DOI] [PubMed] [Google Scholar]
- 23.van Dijk G, Kavousi M, Troup J, Franco O. Health issues for menopausal women: The top 11 conditions have common solutions. Maturitas 2015;80:24–30. [DOI] [PubMed] [Google Scholar]
- 24.Trinder J, Waloszek J, Woods M, Jordan A. Sleep and cardiovascular regulation. Pflugers Arch 2012;463:161–8. [DOI] [PubMed] [Google Scholar]
- 25.Trinder J, Kleiman J, Carrington M, et al. Autonomic activity during human sleep as a function of time and sleep stage. J Sleep Res 2001;10:253–64. [DOI] [PubMed] [Google Scholar]
- 26.Trinder J Cardiac activity and sympathovagal balance during sleep. Sleep Med Clin 2007;2:199–208. [Google Scholar]
- 27.Kerkhof G, Van Dongen H, Bobbert A. Absence of endogenous circadian rhythmicity in blood pressure? Am J Hypertens 1998;11:373–7. [DOI] [PubMed] [Google Scholar]
- 28.Van Dongen H, Maislin G, Kerkhof G. Repeated assessment of the endogenous 24-hour profile of blood pressure under constant routine. Chronobiol Int 2001;18:85–98. [DOI] [PubMed] [Google Scholar]
- 29.Burgess H, Trinder J, Kim Y, Luke D. Sleep and circadian influences on cardiac autonomic nervous system activity. Am J Physiol 1997;273:H1761–H8. [DOI] [PubMed] [Google Scholar]
- 30.Trinder J, Allen N, Kleiman J, et al. On the nature of cardiovascular activation at an arousal from sleep. Sleep 2003;26:543–51. [PubMed] [Google Scholar]
- 31.Colrain I The K-complex: a 7-decade history. Sleep 2005;28:255–73. [DOI] [PubMed] [Google Scholar]
- 32.de Zambotti M, Willoughby A, Franzen P, Clark D, Baker F, Colrain I. K-Complexes: Interaction Between the Central and Autonomic Nervous Systems During Sleep. Sleep in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carrington M, Trinder J. Blood pressure and heart rate during continuous experimental sleep fragmentation in healthy adults. Sleep 2008;31:1701–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soules MR, Sherman S, Parrott E, et al. Executive summary: Stages of Reproductive Aging Workshop (STRAW). Fertil Steril 2001;76:874–8. [DOI] [PubMed] [Google Scholar]
- 35.Sassoon S, de Zambotti M, Colrain I, Baker F. Association between personality traits and DSM-IV diagnosis of insomnia in peri-and postmenopausal women. Menopause 2014;21:602–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) Version 2.0. New York, NY: Biometrics Research Department, New York State Psychiatric Institute, 1998. [Google Scholar]
- 37.Morin CM, Espie CA. Insomnia: A Clinical Guide to Assessment and Treatment: Springer, 2003. [Google Scholar]
- 38.Buysse D, Reynolds III C, Monk T, Berman S, Kupfer D. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193–213. [DOI] [PubMed] [Google Scholar]
- 39.Beck A, Steer R, Brown G. Beck Depression Inventory-II (BDI-II). San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 40.Greene JG. Constructing a standard climacteric scale. Maturitas 1998;29:25–31. [DOI] [PubMed] [Google Scholar]
- 41.Iber C The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications: American Academy of Sleep Medicine, 2007. [Google Scholar]
- 42.Castiglioni P, Parati G, Omboni S, et al. Broad-band spectral analysis of 24 h continuous finger blood pressure: comparison with intra-arterial recordings. Clinical science 1999;97:129–39. [PubMed] [Google Scholar]
- 43.Eckert S, Horstkotte D. Comparison of Portapres non-invasive blood pressure measurement in the finger with intra-aortic pressure measurement during incremental bicycle exercise. Blood pressure monitoring 2002;7:179–83. [DOI] [PubMed] [Google Scholar]
- 44.Dormire SL, Carpenter JS. An alternative to Unibase/glycol as an effective nonhydrating electrolyte medium for the measurement of electrodermal activity. Psychophysiology 2002;39:423–6. [DOI] [PubMed] [Google Scholar]
- 45.Freedman R. Laboratory and ambulatory monitoring of menopausal hot flashes. Psychophysiology 1989;26:573–9. [DOI] [PubMed] [Google Scholar]
- 46.Israel R, Mishell D Jr, Stone S, Thorneycroft I, Moyer D. Single luteal phase serum progesterone assay as an indicator of ovulation. Am J Obstet Gynecol 1972;112:1043–6. [DOI] [PubMed] [Google Scholar]
- 47.Santoro N The menopausal transition. Am J Med 2005;118:Suppl 12B:8–3. [DOI] [PubMed] [Google Scholar]
- 48.Carrington M, Barbieri R, Colrain I, Crowley K, Kim Y, Trinder J. Changes in cardiovascular function during the sleep onset period in young adults. J Appl Physiol (1985) 2005;98:468–76. [DOI] [PubMed] [Google Scholar]
- 49.Chobanian A, Bakris G, Black H, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003;42:1206–52. [DOI] [PubMed] [Google Scholar]
- 50.von Kanel R, Jain S, Mills PJ, et al. Relation of nocturnal blood pressure dipping to cellular adhesion, inflammation and hemostasis. J Hypertens 2004;22:2087–93. [DOI] [PubMed] [Google Scholar]
- 51.Parati G, Ochoa JE, Lombardi C, Bilo G. Blood pressure variability: assessment, predictive value, and potential as a therapeutic target. Curr Hypertens Rep 2015;17:537. [DOI] [PubMed] [Google Scholar]
- 52.Khoury A, Sunderajan P, Kaplan N. The early morning rise in blood pressure is related mainly to ambulation. Am J Hypertens 1992;5:339–44. [DOI] [PubMed] [Google Scholar]
- 53.Briant L, Charkoudian N, Hart E. Sympathetic regulation of blood pressure in normotension and hypertension: When sex matters. Exp Physiol 2015;101:219–29. [DOI] [PubMed] [Google Scholar]
- 54.Loredo JS, Nelesen R, Ancoli-Israel S, Dimsdale JE. Sleep quality and blood pressure dipping in normal adults. Sleep 2004;27:1097–103. [DOI] [PubMed] [Google Scholar]
- 55.Sayk F, Teckentrup C, Becker C, et al. Effects of selective slow-wave sleep deprivation on nocturnal blood pressure dipping and daytime blood pressure regulation. Am J Physiol Regul Integr Comp Physiol 2010;298:R191–7. [DOI] [PubMed] [Google Scholar]
- 56.Loredo JS, Ancoli-Israel S, Dimsdale JE. Sleep quality and blood pressure dipping in obstructive sleep apnea. Am J Hypertens 2001;14:887–92. [DOI] [PubMed] [Google Scholar]
- 57.Davies CW, Crosby JH, Mullins RL, Barbour C, Davies RJ, Stradling JR. Case-control study of 24 hour ambulatory blood pressure in patients with obstructive sleep apnoea and normal matched control subjects. Thorax 2000;55:736–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bonnet M, Arand D. Hyperarousal and insomnia: state of the science. Sleep Med Rev 2010;14:9–15. [DOI] [PubMed] [Google Scholar]
- 59.Campbell I, Bromberger J, Buysse D, et al. Evaluation of the association of menopausal status with delta and beta EEG activity during sleep. Sleep 2011;34:1561–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matthews KA, Chang Y, Kravitz HM, et al. Sleep and risk for high blood pressure and hypertension in midlife women: the SWAN (Study of Women’s Health across the Nation) Sleep Study. Sleep medicine 2014;15:203–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang C, Lai C, Lai H, Kuo T. Relationship between electroencephalogram slow-wave magnitude and heart rate variability during sleep in humans. Neurosci Lett 2002;329:213–6. [DOI] [PubMed] [Google Scholar]
- 62.Yang C, Shaw F, Lai C, Lai C, Kuo T. Relationship between electroencephalogram slow-wave magnitude and heart rate variability during sleep in rats. Neurosci Lett 2003;336:21–4. [DOI] [PubMed] [Google Scholar]
- 63.Kuo T, Yang C. Scatterplot analysis of EEG slow-wave magnitude and heart rate variability: an integrative exploration of cerebral cortical and autonomic functions. Sleep 2004;27:648–56. [DOI] [PubMed] [Google Scholar]
- 64.Miyashita T, Ogawa K, Itoh H, et al. Spectral analyses of electroencephalography and heart rate variability during sleep in normal subjects. Auton Neurosci 2003;103:114–20. [DOI] [PubMed] [Google Scholar]
- 65.Jurysta F, van de Borne P, Migeotte P, et al. A study of the dynamic interactions between sleep EEG and heart rate variability in healthy young men. Clin Neurophysiol 2003;114:2146–55. [DOI] [PubMed] [Google Scholar]
- 66.Rothenberger S, Krafty R, Taylor B, et al. Time-varying correlations between delta EEG power and heart rate variability in midlife women: The SWAN Sleep Study. Psychophysiology 2015;52:572–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brandenberger G, Ehrhart J, Buchheit M. Sleep stage 2: an electroencephalographic, autonomic, and hormonal duality. Sleep 2005;28:1535–40. [DOI] [PubMed] [Google Scholar]
- 68.Jurysta F, van de Borne P, Lanquart J, et al. Progressive aging does not alter the interaction between autonomic cardiac activity and delta EEG power. Clin Neurophysiol 2005;116:871–7. [DOI] [PubMed] [Google Scholar]
- 69.Jurysta F, Lanquart J, Sputaels V, et al. The impact of chronic primary insomnia on the heart rate--EEG variability link. Clin Neurophysiol 2009;120:1054–60. [DOI] [PubMed] [Google Scholar]
- 70.Jurysta F, Lanquart J, van de Borne P, et al. The link between cardiac autonomic activity and sleep delta power is altered in men with sleep apnea-hypopnea syndrome. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1165–R71. [DOI] [PubMed] [Google Scholar]
- 71.Jurysta F, Kempenaers C, Lancini J, Lanquart J, van de Borne P, Linkowski P. Altered interaction between cardiac vagal influence and delta sleep EEG suggests an altered neuroplasticity in patients suffering from major depressive disorder. Acta Psychiatr Scand 2010;121:236–9. [DOI] [PubMed] [Google Scholar]
- 72.Ohayon M Severe hot flashes are associated with chronic insomnia. Archives of internal medicine 2006;166:1262–8. [DOI] [PubMed] [Google Scholar]
- 73.Tuomikoski P, Haapalahti P, Ylikorkala O, Mikkola T. Vasomotor hot flushes and 24-hour ambulatory blood pressure in recently post-menopausal women. Ann Med 2010;42:216–22. [DOI] [PubMed] [Google Scholar]
- 74.James GD, Sievert LL, Flanagan E. Ambulatory blood pressure and heart rate in relation to hot flash experience among women of menopausal age. Ann Hum Biol 2004;31:49–58. [DOI] [PubMed] [Google Scholar]
- 75.Gerber L, Sievert L, Warren K, Pickering T, Schwartz J. Hot flashes are associated with increased ambulatory systolic blood pressure. Menopause 2007;14:308–15. [DOI] [PubMed] [Google Scholar]
- 76.Thurston RC, Sutton-Tyrrell K, Everson-Rose SA, Hess R, Matthews KA. Hot Flashes and Subclinical Cardiovascular Disease Findings From the Study of Women’s Health Across the Nation Heart Study. Circulation 2008;118:1234–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thurston R, El Khoudary S, Tepper P, et al. Trajectories of Vasomotor Symptoms and Carotid Intima Media Thickness in the Study of Women’s Health Across the Nation. Stroke; a journal of cerebral circulation 2016;47:12–7. [DOI] [PMC free article] [PubMed] [Google Scholar]