Abstract
The microvasculature in the pancreatic islet is highly specialized for glucose sensing and insulin secretion. Although pancreatic islet transplantation is a potentially life-changing treatment for patients with insulin-dependent diabetes, a lack of blood perfusion reduces viability and function of newly transplanted tissues. Functional vasculature around an implant is not only necessary for the supply of oxygen and nutrients but also required for rapid insulin release kinetics and removal of metabolic waste. Inadequate vascularization is particularly a challenge in islet encapsulation. Selectively permeable membranes increase the barrier to diffusion and often elicit a foreign body reaction including a fibrotic capsule that is not well vascularized. Therefore, approaches that aid in the rapid formation of a mature and robust vasculature in close proximity to the transplanted cells are crucial for successful islet transplantation or other cellular therapies. In this paper, we review various strategies to engineer vasculature for islet transplantation. We consider properties of materials (both synthetic and naturally derived), prevascularization, local release of proangiogenic factors, and co-transplantation of vascular cells that have all been harnessed to increase vasculature. We then discuss the various other challenges in engineering mature, long-term functional and clinically viable vasculature as well as some emerging technologies developed to address them. The benefits of physiological glucose control for patients and the healthcare system demand vigorous pursuit of solutions to cell transplant challenges.
Keywords: vascularization, Type 1 Diabetes, islet transplantation, endothelial cell, microvasculature
Graphical Abstract
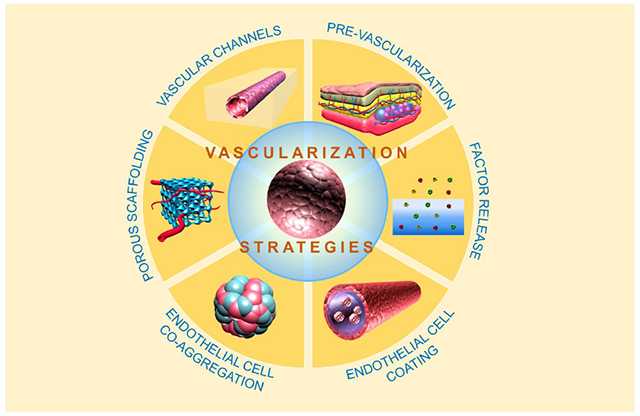
1. Introduction
The vascular system perfuses nearly every tissue to deliver nutrients and remove waste products. Endothelial cells (ECs) are the blood-contacting cells of the vascular system, which mediate many vascular adaptations [1]. Following initial development, establishment of new vascular sections during adulthood occurs through angiogenesis, vasculogenesis, or arteriogenesis [2]. New endothelial lumen structures are formed in angiogenesis by proliferation of existing ECs, while vasculogenesis is mediated by progenitors that differentiate into ECs [3]. By contrast, arteriogenesis can form a vessel network by maturation of an endothelial tube primarily through increases in diameter, addition of support cells, and participation of monocytes [4]. These processes ensure that most cells are no more than ~100 μm away from a blood vessel [5]; however, this essential network is not available in tissues that are harvested from a donor or derived from stem cells for transplant, such as pancreatic islets.
Vascularization is not a problem unique to islet tissue engineering [6] but is under investigation for a wide range of tissue types including muscle [7, 8], cardiac tissue [9–11], hepatic tissue [12], bone [13, 14], neural tissue [15], skin [16–18], thyroid tissue [19], and kidney tissue [20], as well as multi-tissue materials [21]. This review evaluates strategies to induce vascularization with a focus on those that have been used in islet transplantation. Physical properties of synthetic scaffolding or encapsulation materials and the benefits of using natural extracellular matrix materials to induce vascularization are reviewed first. We then consider prevascularization, factor delivery, and angiogenic cell co-transplantation. The discussion concludes with perspectives on challenges and consideration of future approaches. First, we present a brief consideration of islet vascular biology.
1.1. Islet Vascular Biology
Islets have been described as micro-organs [22] with unique microvasculature characteristics compared to many other tissue microvasculatures. Qualities include high density, high fenestration [23], and sensitive glucose responsiveness [24]. Combined with the spatial arrangement and flow patterns of vessels and endocrine cells [25], the islet vasculature promotes a rapid physiological response to maintain glycemia. One spatial arrangement is that mouse insulin-producing β-cells are usually adjacent to ECs and attach to the vascular basement membrane [26]. Observations about the direction of blood flow through the islet also contribute to our understanding of islet function. The intra-islet blood flow has been characterized to be one of the three patterns: (1) flowing first to the islet core and then to the islet shell, (2) the islet shell and then the islet core, or (3) simply from one side of the islet to the other, perfusing core and peripheral islet endocrine cells equally depending on which hemisphere the cells reside [27]. The blood flow pattern, with the specific cell arrangement, is important given that paracrine effects between the islet cell types are affected by blood and interstitial flow that is dictated by capillary network architecture [28, 29]. However, the effects of capillary and endocrine cell layout in human islets are not yet fully known due in part to the differential arrangement of cell types [30, 31].
In further studies of the microvascular architecture, an intra-pancreatic and intra-islet portal system has been observed. Vascular portal systems describe locations where a capillary bed supplies two types of tissues before returning to the heart (sometimes converging into a vein between two capillary beds) such as in the liver and kidney [32]. In the case of the intra-pancreatic portal system, capillaries flow from within the islet to the surrounding exocrine pancreatic tissue before converging into a vein [33]. The intra-islet portal system describes a flow pattern where β-cells are perfused before other cell types within the islets [29]. Thus, the intra-islet portal system provides a method for β-cell secretions to affect non-β-cell islet cells, and the intra-pancreatic portal capillary pattern extends the effect of the endocrine islet secretions to the exocrine pancreas. Overall, it is clear that the intra-islet vascular structure is tightly linked to islet function.
The function of the vasculature also adapts to the diabetic state. Nyman et al. quantified islet perfusion using confocal imaging of in situ islets to show that islet perfusion increases in hyperglycemia while the exocrine pancreatic tissue surrounding the islets is not affected [24]. Canzano et al. examined the islet microvasculature in human islets histologically to reveal that the blood vessels inside the islet of diabetic pancreas were smaller and greater in number than those in the non-diabetic pancreas. By contrast, the exocrine tissue vessels remained the same in the diabetic state [34]. This may be a contrasting effect observed in mouse islets. It would not be the first case where mouse islets are different from human islets, or an individual islet is different from another, depending on the location in the pancreas [30, 35, 36]. Canzano et al. further showed that islets in a diabetic pancreas containing residual β-cell mass (insulin positive β-cells) had a normal intra-islet microvasculature compared to insulin negative β-cells in the islets [34]. Indeed, measuring perfusion of native islets may be predictive of type 1 diabetes (T1D) autoimmune disease onset [37]. While it is not yet clear whether the vascular changes contribute to the β-cell damage or the β-cell damage causes vasculature changes, it nonetheless underscores the close connection between the vasculature and islet function.
Ideally, the vascular structure of transplanted islets could become re-perfused, ensuring that a healthy network is formed. Sometimes intra-islet capillaries remain as channels without an endothelial lining for many months post-transplantation [38]. These acellular channels may still be useful, as freely transplanted islets are capable of re-growing an intra-islet portal system [29]. The population of ECs remaining in the islets likely participates in islet revascularization [39]. This population is capable of anastomosing to vasculature in the recipient [39, 40], possibly even when there is a species mismatch [41, 42]. Harnessing the ability of donor ECs or intra-islet vascular channels to participate in re-vascularization may be important for islet transplant success.
1.2. Importance of Vascularization in Islet Transplantation
Following transplantation, several factors reduce the ability of an islet to re-vascularize after separation from the native pancreatic environment and vasculature [43]. Aside from collagenase digestion, the cell source can sometimes mandate safety requirements that also decrease the ability of an islet to become vascularized.
Non-human- or stem cell-derived islet transplantation is a solution to the shortage of cadaveric transplantable quality tissue. However, xenogeneic islets can stimulate a more aggressive immune response [44], and stem-cell derived islets present a risk of undesired differentiation [45]. Cell encapsulation can reduce immunologic toxicity to the transplanted tissue by preventing contact of immune cells [46] and undifferentiated cell escape. However, cell encapsulation also prevents intra-islet vessel development. Upon un-encapsulated transplantation, avascular islets experience insufficient mass transfer of nutrients and waste as well as function of specific molecules (e.g., glucose and hormones) (Figure 1 a,b) [47], which is worsened by encapsulation (Figure 1 c).
Figure 1. Mass transfer to islets is limited by isolation and encapsulation.
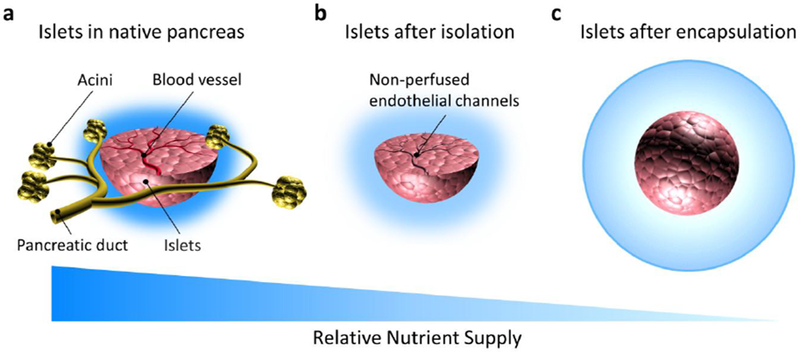
Compared to the native pancreas (a), islets experience reduced diffusion to the majority of cells (especially in the core of the cell mass) as a result of loss of blood perfusion following isolation from the acinar tissue (b). Furthermore, encapsulation of any kind (microencapsulation shown here) increases the distance of islet cells to the surrounding fluid or blood vessels (c). Dark blue represents greater mass transport. Drawings not to scale.
An ideal encapsulation barrier would be able to prevent all immunological access to the graft while simultaneously allowing all necessary nutrients to enter and products to leave the graft [48], requiring precise control over diffusional properties. Membrane diffusional characterization is not a primary subject in this review; however, there is exciting work on this topic, which we will not be able to give full attention [49, 50]. Briefly, materials can be characterized by their permeability to molecules. Hydrogels, for instance, can be characterized by their permeability across a range of molecular weights. Materials can also be characterized by their permselectivity to certain molecules or groups of molecules with similar properties (i.e., charge). Tightly controlled pore sizes in solid materials can be utilized to impart permselectivity by imposing a molecular weight cutoff or by excluding depending on other molecular properties. Much of the pore size discussion in this review occurs on the scale of tens to hundreds of micrometers for vascularization. Molecular diffusional characterization is less relevant at those scales but is nonetheless an important topic.
Generating a vasculature similar to the native islet vasculature could improve results in islet transplantation; however, different qualities may be required to overcome the encapsulation diffusion barrier. Furthermore, efforts are being made to develop functionalized encapsulation materials [51] that can target immune cells, interfere with coagulation, mitigate fibrotic reactions, reduce reactive oxygen species, and induce vascularization [52], some of which may increase graft survival without being immunoisolating.
Vascular growth around an implant needs time to develop. The time course of vasculature growth in nonencapsulated transplanted islets has been studied. Regeneration of vasculature in or around transplanted syngeneic islets was observed to take one to two weeks [53]. In some intraportally transplanted islets, vascular density appeared to reach native islet levels within a few days and was at supernormal levels between days 5 and 30 (the last time point recorded) [54]. Similarly, the first vessels were observed in intramuscularly transplanted islets at three days [55]. Any delay in microvasculature formation can cause immediate cell death and reduction of long-term islet engraftment [56, 57]. Thus, the rest of this review is devoted to understanding various methods to bring sustained vasculature to the transplanted cells as quickly as possible.
2. Vascularization Strategies
2.1. Porosity, Surface Roughness, and Stiffness Modulate Vascularization
Physical characteristics of a material can drive increases in angiogenic activity. In this section, we review studies that are conducted for the purpose of islet transplantation alongside studies that are conducted for the reconstruction of other tissues to suggest possible new strategies for transplanted islet vascularization. In one of the most influential papers surrounding this topic, Brauker et al. quantified membrane pore size and positively correlated pore sizes of 0.8-8 μm to both cell infiltration and an altered foreign body reaction that allowed vascular structures to be close to the membrane [58]. By examining a large number of membranes made from various materials and with varied pore sizes, a conclusion was drawn that cell infiltration permissive membranes reduce the thickness of a fibrotic capsule when implanted subcutaneously (Figure 2 a). In a more recent study, vessels were found in the large pore outer mesh and immediately adjacent to the immunoisolation membrane [59], which should improve mass transfer.
Figure 2. Material properties that influence vascularization.
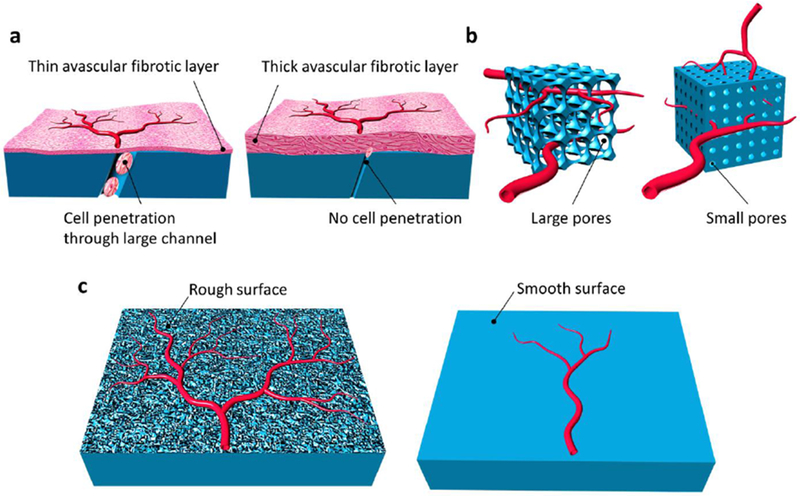
The thickness of the avascular fibrotic layer (shown in light red) can be reduced by constructing an implant that allows cell migration into the membrane (a). Larger pores can facilitate more blood vessel investment than pores that are only sufficiently large enough to allow blood vessels to form (b). Increases in nanotopographical roughness can increase vascularization compared to smooth substrates (c).
The time course of microarchitecture-driven vascularization was studied by Padera and Colton, which demonstrated a temporal pattern similar to a normal wound healing cascade. A combination of neutrophils and macrophages was present within the pores of the membrane, followed by macrophages that peaked between 7 and 21 days but then decreased by day 329 [60]. The number of close vascular structures, on the other hand, reached a plateau at day 21 and remained the same until day 329, differing from a normal wound healing cascade where the number of vessels is expected to decrease due to regression of a fraction of vessels in a newly formed network [61, 62].
Pore size has also been examined in beta-tricalcium phosphate scaffolds where all pore sizes (~337, 415, 557, and 632 μm) supported vascular ingrowth from surrounding tissues; however, the diameter and extent of vessel ingrowth were modestly decreased by the 337 μm scaffolds [63]. Choi et al. examined the pore size of poly(lactic-co-glycolic acid) (PLGA) scaffolds formed around sacrificial microsphere templates of diameter 79, 147, 224, and 312 μm to evaluate the effect of pore diameter on subcutaneous vascularization [64]. While all scaffolds in that study supported vascularization to some degree, scaffolds constructed with microspheres of 200 μm or greater supported vessels that penetrated deeper into the scaffold. The 200 μm sphere size threshold correlated to a 35-40 μm window size (the size of openings between spherical pores, i.e., the limiting hole size for intrapore vessel formation).
Pore size can also be controlled in hydrogels to modulate vascularization. Three polyethylene glycol (PEG) hydrogels with pore sizes 134 ± 28, 82 ± 6, and 41 ± 0.1 μm were tested for their ability to support cells and vascularization. The authors found that the 41 μm pore group did not support vascular ingrowth into the pores until after the second week of implantation, while the larger pore sizes (82 and 134 μm) contained vessels at the one week time point (Figure 2 b) [65]. The dorsal skinfold window chamber was used to study the dynamics of vascularization surrounding three scaffolds presenting pores of diameters 20-75, 75-212, and 250-300 μm over the course of 20 days following implantation. Results showed that the large-pore size scaffolds supported consistent vessel growth at 8, 12, 16, and 20 days compared to the medium- and small-pore size scaffolds in the area of the scaffold, while the trend was not significant at the border or outside the scaffold [66].
The minimum pore size required to obtain noteworthy vascular in-growth depends on the particular scaffold material properties. However, according to the reviewed studies, a pore size greater than 200 μm may be required to facilitate vascularization into scaffolds, while a smaller pore size (greater than 100 μm) may be sufficient for hydrogel scaffolds. There is no substitute for testing the scaffold under question [67, 68]. Awareness of pore quantification methods such as porogen size and the resulting window size mentioned above are also important to consider. Furthermore, some materials may be able to circumvent pore size requirements while still initiating vascularization [69, 70].
Changes in pore size can also affect surface roughness, although surface roughness is an independent material property. Interestingly, Rosengren et al. studied subcutaneous implantation of smooth and textured low-density polyethylene disks and observed that the smoother disk was surrounded by a larger fibrotic capsule. More necrotic tissue was noted at the interface, especially near the edges where shear forces with surrounding tissue would be greater [71]. Following this hypothesis, smaller pore sizes not only would prevent vascular in-growth but may also present a smoother surface that induces a greater amount of cell necrosis at the tissue interface. This would help to explain the observation by Brauker et al. [58] that larger pore sizes stimulated closer vessels, as the fibrotic capsule was thinner. Khosravi et al. showed that during in vivo implantation, a nanotopographical surface significantly increased peri-implant blood vessel density on days 7, 11, and 28 compared to the smooth surface of the same titanium (Figure 2 c) [72]. The pore size of 3D collagen scaffolds was used to select cell types that were able to invade, giving the possibility that a scaffold may be able to select out for a population of desired cells [73], perhaps someday providing a new kind of immunoisolation material. It is clear that the scale of pores and surface roughness affect the biological response to implanted materials, and hence, they should be considered carefully when designing implants for cell encapsulation.
Material stiffness varies with material characteristics and formulations [74, 75] and can affect the vascularizing potential of a material. Similarly, tissue stiffness correlates with islet biology in addition to EC functionality. Islet stiffness is known to affect insulin expression [76], vary with islet inflammation [77], and provide a method to distinguish acinar versus islet tissue [78]. Stiffness plays a complex role in vascularization processes, primarily through ECs. Substrate stiffness downregulates EC network formation in 2D, while in 3D, the converse may be true [79]. Other material properties such as matrix density often vary with stiffness; however, Mason et al. demonstrated an effect of stiffness independent of density [80]. Stiffness responses are cell type dependent [81], including the EC source location in the vascular tree [82]. Matrix stiffness can also affect the ability of cells to interact with their neighbors, potentially affecting tissue formation [83] and lymphatic sprouting [84]. In vivo, angiogenic activity can degrade or secrete new matrix, dynamically affecting stiffness at different stages of vessel growth [85, 86].
2.2. Natural Extracellular Matrix-Based Scaffolding
Physiologically, the native matrix to which vascular cells attach is tightly regulated, including material properties such as stiffness and porosity. The native extracellular matrix (ECM) is composed of the basement membrane and the interstitial matrix. This matrix provides structural support for tissue-specific cells and vasculature, as well as transport regulation [87]. ECM-based materials and synthetic materials designed to mimic the ECM offer great potential in regenerating and engineering the pancreas as well as islets [88–92]. To be clear, direct regeneration of islets would be complicated by the original autoimmune attack and is not yet clinically viable but would be a considerable advance. Engineering an environment suitable for transplanted insulin-producing cells can be improved by considering those that increase vascularization.
Fibrin is commonly used for islet vascularization and transplantation. Fibrin is a fibrous protein polymerized by the protease thrombin on fibrinogen, and it is known to participate in vascularization during wound healing [93]. Individual fibrinogen molecules have two pairs of arginine-glycine-aspartic acid (RGD) ligands [94] for integrins expressed on pancreatic cells (i.e., αvβ3, α5β1, and αvβ1) [95], Therefore, attachment to integrin recognition sites in fibrin gels can prevent cell anoikis (apoptosis resulting from lack of integrin engagement) and facilitate islet cell survival [96, 97]. Interactions between fibrin-binding sites and integrins (i.e., αvβ3 and α5β1) are involved in controlling EC behavior and vessel formation during angiogenesis [93]. As fibrin enhances islet cell survival and is cell cleavable, thereby supporting angiogenesis, it is an attractive natural scaffold for islet vascularization.
Fibrin, being a component of US Food and Drug Administration (FDA)-approved clinical products (e.g., FIBRIN SEALANT, TachoSil, EVARREST, and EVICEL), is a suitable material for translational studies. Ricordi and Pileggi groups developed an approach where plasma was polymerized by recombinant thrombin (Figure 3 a). Islets were embedded in the fibrinogen pregel and transplanted onto the diabetic rat recipient’s omentum, followed by the application of recombinant thrombin to polymerize the fibrinogen in situ with omental closure to contain and protect the graft. Promising pre-clinical results demonstrated a potential to vascularize, to function similarly to intrahepatically transplanted islets, and to reduce circulating levels of leptin and α-2 macroglobin [98]. Safety of the approach was demonstrated in a non-human primate [98]. In a recent phase I/II clinical trial, human islets were embedded in a patient’s autologous plasma gel. Glucose levels were in the upper nondiabetic range at a six-month follow-up, while at 12 months, insulin independence had deteriorated, perhaps complicated by a change in the immunosuppressive agent used [99].
Figure 3. ECM materials that can be used to scaffold and vascularize transplanted islets.
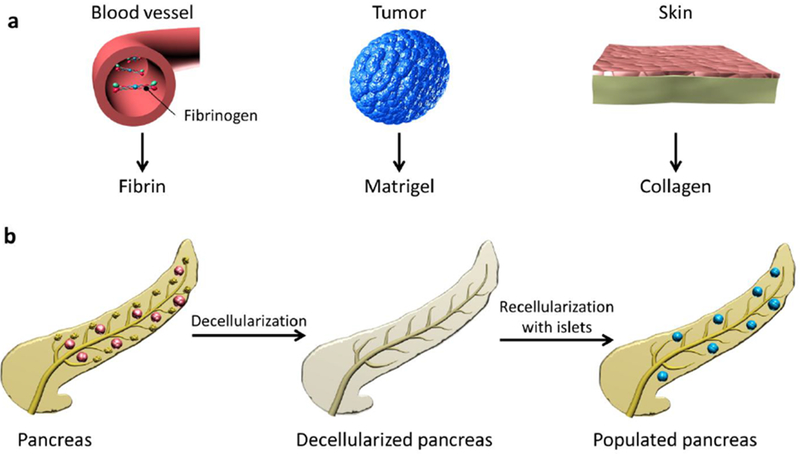
Fibrin scaffolds are polymerized from fibrinogen present in the blood plasma (a). Matrigel is isolated from a murine sarcoma (a). Collagen can be isolated from many sources, with skin shown here as an example (a). Decellularized pancreatic tissue may be a natural scaffold for islet transplant (b). Matrix materials can be selected and combined to promote islet vascularization and health.
Fibrin can be tailored by modulating formulations to obtain desirable properties. For example, compared to a higher concentration of fibrinogen and thrombin (10 mg/mL and 10 U/mL, respectively), islets in a fibrin scaffold made with lower concentrations (5 mg/mL and 1 U/mL, respectively) reached normoglycemia quicker and supported vascularization inside and around the islets [100]. In addition to the effects on the density of the gel [101], adjusting the thrombin concentration may affect islets in other ways. For instance, thrombin can cleave protease-activated receptor-3 (PAR3), which can, in turn, stimulate insulin secretion, perhaps leading to hyperstimulation of insulin secretion [102]. In addition, given the integral role of thrombin in the Instant Blood-Mediated Inflammatory Reaction (IBMIR) [103] and positive results that have been found with counteracting thrombin activation [104–106], caution is warranted in the selection of a site where a fibrin gel is used to facilitate islet transplantation [107]. To further reduce these concerns, fibrinogen formulations that are free of complement may avoid a loss of viability from thrombin-activated compliment [98, 103, 108]. Nevertheless, the proclivity of a fibrin gel to promote angiogenesis is a beneficial property for the improvement of islet transplantation.
Vascularization can be enhanced by conjugating pro-angiogenic factors (i.e., vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF)) into fibrin gels. Improved islet function in a VEGF/PDGF-conjugated fibrin gel was associated with enhanced and earlier vascularization (<7 days) [109]. Factor release will be further discussed in Section 2.4. Other than fibrin, commercial Matrigel and collagen have also been used as biological scaffolds for islet vascularization.
Matrigel is a gelatinous mixture primarily composed of structural proteins, proteases, growth factors, and related proteins secreted by Engelbreth-Holm-Swarm (EHS) mouse sarcoma cells (formulations available from manufacturers (e.g., Coming) or from literature [110]) (Figure 3 a). It is used to study differentiation and tumor growth [111], as well as evaluation of angiogenesis both in vitro and in vivo [112, 113], partially because of the inclusion of growth factors in the standard formulation. For the application of transplantation, mouse islets were embedded in growth factor-reduced Matrigel specifically supplemented with VEGF and hepatocyte growth factor (HGF). Restored normoglycemia in diabetic severe combined immunodeficient (SCID) mice in the subcutaneous space [114] demonstrated the suitability of Matrigel for islet transplantation. Although very useful in preclinical studies, clinically viable alternatives need to be identified that are not animal derived and can be chemically defined.
Collagen, a major component of Matrigel and the main protein in the islet niche [115], may be critical for maintaining islet function (Figure 3 a) [26, 116]. During angiogenesis, sprouting vascular ECs are exposed to an interstitial ECM rich in type I collagen [117]. Similarly, the integrins expressed on ECs (i.e., α1β1 and α2β1) are known to bind to collagen [118]. To apply this knowledge, a recent study used porous collagen scaffolds to support vessel growth [119]. Re-vascularization of implanted islets was clearly observed, and the engraftment and blood glucose correction was achieved at a low islet number (250 islets) [119]. In another report, freshly isolated rat islets in type I collagen (3 mg/mL) were sandwiched by two layers of precultured and prevascularized type I collagen containing rat microvascular fragments. After subcutaneous implantation into SCID mice, the vascularized construct enhanced islet survival by supporting islet viability and maintaining structures of intra-islet ECs [120].
To stimulate the growth of islet supporting vasculature, it may be useful to provide ECM components that mimic the ECM composition of the natural islet perivascular space in addition to collagen. Naba et al. studied the ECM composition of healthy islets as well as insulinomas. Murine islets undergoing metastatic transformation become highly vascularized at a predictable age and rate, allowing for study of ECM changes. While these highly angiogenic islets may be of some interest, it may not be desirable to create an insulinoma-like environment therapeutically. Normal islet characterizations are, for that reason, perhaps the most interesting source of ECM information. Naba et al. found a group of 120 ECM or ECM-associated components in the healthy islet. Several abundant components included collagen I, III, and V; fibronectin and fibrillin I; and laminins and nidogens [121]. The increased functionality of such an ECM mimic, compared to alternatives with fewer components, could provide enough advantages to be worth considering despite increased cost and barriers to regulatory approval.
Another method to mimic the ECM of the natural pancreas and support vascularization is to remove all cellular materials from the pancreatic tissue in a decellularization process. Cells are then reintroduced into the remaining material (Figure 3 b) [122]. Preservation of the vascular structure of the pancreas was a benefit noted by Napierala et al. during recent development of a rat pancreas-specific decellularization and islet repopulation procedure [123], as well as Yu et al. working on a pancreas tail-specific protocol [124]. Mirmalek-Sani et al. also showed that porcine pancreas vasculature was patent after decellularization, increasing metabolic rate at seven days of culture and glucose responsiveness at three days [125]. Islets have also been supported on decellularized rat pericardium with a layer of collagen derived from tendon implanted syngeneically in the epididymal fat pad in mice. Histology showed well-vascularized islets at approximately 11 months post-transplantation [119]. Working toward the goal of being able to use decellularized pancreatic proteins in tissue engineering or transplant procedures, Sackett et al. developed a procedure to create a hydrogel from donated human pancreas with a high fat content that was not used for transplantation [126]. Continued development of procedures to isolate and process decellularized matrices is key to realizing the benefits of a tissue-specific matrix for vascularized islet transplantation.
2.3. Prevascularization of sites for islet transplantation
Prevascularizing a site can reduce the time required to achieve an appropriate vessel supply at the prospective site. One method to prevascularize a transplantation site is to implant a vascularization-promoting device before islet loading (Figure 4 a). One of the most well-studied device concepts in this area is the TheraCyte device, currently under continued development and adaptation by ViaCyte. In a small rodent study, three months of subcutaneous implantation was used to induce vascularization, followed by islet transplantation into the TheraCyte device to cure diabetes faster than with simultaneous device and islet implantation (6 of 6 vs. 1 of 6 cured) (Figure 4 b) [127]. Other prevascularizing devices are also under intensive development. For example, a cylindrical stainless steel mesh tubing (pore size: 450 μm, diameter: 6 mm) was implanted in the subcutaneous space [128] and omentum [129] before islet transplantation. After connective tissue rich in vascular structures formed in the pores of the mesh, a solid polytetrafluoroethylene (PTFE) plunger was removed, and islets were implanted into the lumen. Implanted islets in the prevascularized tubular tissue space restored normoglycemia, sustained long-term function (>100 days), and showed intense vascular regeneration. On the other hand, the return to normoglycemia was slightly better, with blood glucoses and body weight being slightly worse, in intra-portally transplanted control recipients from the study by Pileggi et al. [128, 130]. In another approach, Smink et al. restored normoglycemia to animals with islets transplanted into a subcutaneous device that was prevascularized in vivo for four weeks, at a rate comparable to that of kidney capsule controls at 45 days post transplant [130].
Figure 4. Preparing a site by prevascularization to improve engraftment.
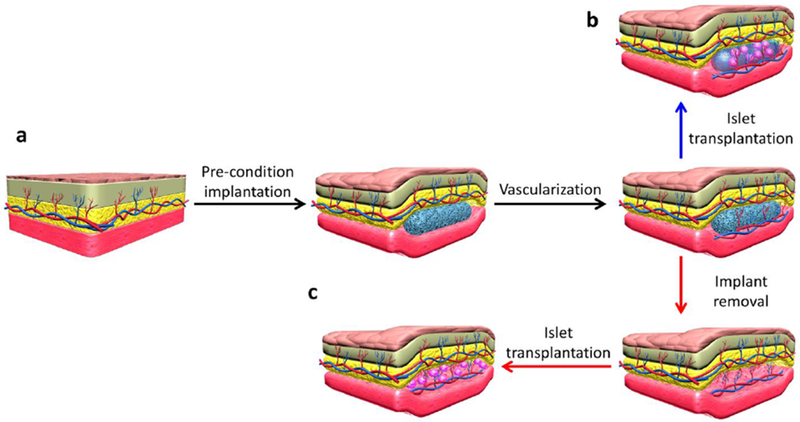
Preimplantation of a material stimulates vascular enrichment (a). The islets can then be introduced into the preimplanted device (b) or the device can be removed to create a space left by the device that the islets can be introduced into (c). All of these approaches result in a space for the islets that has a greater vascular supply at the time of transplantation than an unprepared site.
A different set of approaches do not leave foreign materials behind following the prevascularization period. The Shapiro group inserted a nylon vascular access catheter under the mouse subcutaneous tissue for one month to induce vascularization around the catheter. After removing the catheter, a prevascularized pouch was formed for mouse and human islets. Normoglycemia was achieved and maintained over 100 days in diabetic mice, while the nonpreconditioned subcutaneous site did not restore normoglycemia at any timepoint (Figure 4 c) [131, 132]. A prevascularized pouch can also be created by angiogenic-promoting cells. For example, adipose tissue-derived stromal cells (ADSCs) and minced adipose tissue were co-implanted in mouse subcutaneous tissue. Vascularized pockets were formed after four weeks to prepare for islet transplantation. The blood glucose level reached a normal range within a week after islet transplantation, was sustained for eight weeks, and was significantly lower than that in the three control groups of ADSCs only, minced fat tissue only, or nothing pre-implanted [133].
In addition, pro-angiogenic factors can be incorporated into devices to reduce the time required to achieve a more robust vascular network. For example, silicone tubing (length 5 mm, inner diameter 3.35 mm) was filled with Matrigel supplemented with fibroblast growth factor (FGF)-2 (1 μg/mL), The prepared tubing was split and placed around the epigastric vascular pedicle. After three weeks of prevascularization, ~500 islet equivalents were loaded into the pre-vascularized tubing, thereby resulting in lower nonfasting blood glucose than that in the implants without a delay for prevascularization [134].
Another approach to increase subcutaneous vasculature used agarose rods with basic FGF (bFGF) and heparin preimplanted in the rat dorsal subcutaneous space. After rod removal at one week, 1,500 islets were transplanted into the prevascularized pockets that rapidly reversed hyperglycemia in diabetic rats compared to unprepared subcutaneous spaces [135]. In another system, a bFGF-releasing device was transplanted into rat subcutaneous tissue for one week to induce vessel growth around the device. Rat islets were loaded into the vascularized pocket after device removal or into the device without removal. Normoglycemia was maintained for at least one [136, 137] if not three [138] months and was better than the control no-device animals [136], control devices without collagen sponges [137], and control devices without bFGF or no device preconditioning [138] (Figure 4). Pre-vascularization is an effective method to prepare the transplant site for the therapeutic cells, so that an islet incompatible environment is avoided [139]. Despite needing multiple surgical procedures, the advantages may outweigh the disadvantages of prevascularization.
2.4. Release of Proangiogenic Factors
Factor delivery is a powerful approach to induce vascularization [140] and can include single factor, multiple factors, and multiple sequential factor delivery (Figure 5). Several methods exist for sequential delivery from materials including layered materials in planar, spherical, and cylindrical configurations [141] and light-triggered release [142–145]. In this section, examples of proangiogenic factor delivery will be considered within several delivery systems focusing on the functional outcome. Some of these methods can be categorized by their ability to cause angiogenesis, arteriogenesis, or a combination of both blood vessel network growth modes (Figure 5 a).
Figure 5. Release of angiogenic factors to drive implant angio- or arteriogenesis.
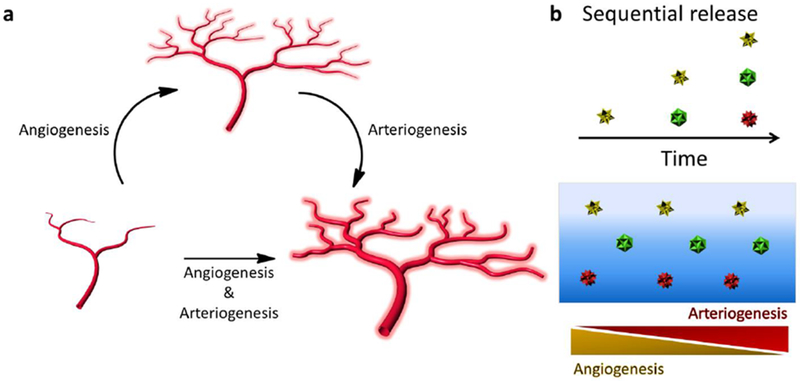
Angiogenesis is the formation of new capillary sprouts from existing vessels and can be driven by factors such as VEGF (a). Arteriogenesis is the maturation of blood vessels, often characterized by the addition of support cells to an endothelial tube and the increase in lumen diameter. Arteriogenesis can be driven by factors such as PDGF-BB (a). Multiple factors can also be sequentially released (e.g., VEGF then S1P, VEGF then PDGF) to push both of these vessel network expansion paradigms (a,b).
2.4.1. Single Factor Release
VEGF is one of the most well-studied angiogenic factors and has indeed already shown up in earlier discussions. Focusing on just VEGF as a model to consider methods of factor release, two categories can be found: (1) those where the VEGF is free to diffuse out of the material and (2) those where VEGF has been conjugated to the material to provide a sustained release. An example of the conjugated release method is 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide/N-hydroxysuccinimide (EDC/NHS) conjugation to alginate. Yin et al. were able to show prolonged release of VEGF and an increase of approximately 36 days of encapsulated islet effectiveness using subcutaneous implantation [146]. Marchioli et al. evaluated the ability of a 3D-printed heparin-conjugated scaffold to bind VEGF and therefore induce vascularization of an alginate hydrogel. Using a chicken chorioallantoic membrane assay, the authors showed that heparin modulated the activity of VEGF [147]. Presenting only heparin can also increase vascularization [148], likely due to natural growth factor depot and local concentration functions. Complimentary results support the use of VEGF when it is released from the cells themselves (produced by gene transfection [149] or by stimulation of VEGF production pharmacologically [150]) or supplemented with rotational culture [151].
PEG hydrogels are a versatile synthetic system that allows tuning of mechanical properties [152], addition of cleavable sites [153, 154], antifouling functionality [152], and delivery of drugs through bulk release or a number of other methods [155]. Marchioli et al. established two fully hydrogel-based models for transplantation where a vascularizing layer had conformally coated islets or cell clusters embedded into it, or the vascularizing layer was attached to an islet containing hydrogel layer with natural ECM proteins [156]. A PEG-maleimide-based hydrogel, loaded with VEGF, was then cross-linked with a degradable linker to be used as a tissue-adhesive pro-angiogenic vehicle for islet transplant on the mesentery by Phelps et al. [157]. This approach offered surgical feasibility in addition to the adhesive properties. Carefully combining these functionalities, Weaver et al. assembled a 4-arm PEG degradable hydrogel that presented cell adhesive RGD and VEGF with islets. At the epididymal fat pad site, cure rates were best with the VEGF included in the PEG hydrogel [158]. Thus, adding degradability into synthetic hydrogels can improve the in vivo functionality.
Overcoming the diffusion barrier presented by a fibrotic layer is one of the goals of angiogenic factor release. For instance, Hunter et al. studied the release of EC growth factor (ECGF) from an alginate hydrogel encased inside a semipermeable membrane tube made of either polyvinylidene difluoride (PVDF) or polyethersulfone. Marked neovascularization occurred in the fibrotic layer for subcutaneous implants when ECGF was included [159]. Both ECGF [160, 161] and VEGF [162] are chemotactic for ECs as one of their functions in angiogenesis. Attention should be paid to the vascularization response within the fibrotic layer, as this could address fibrotic diffusion hindrances in the long term.
2.4.2. Multiple and Sequential Factor Release
Single factor delivery is more straightforward to understand scientifically and may have a comparatively simple path to the clinic. Yet, delivering multiple factors may introduce some significant benefits to the growth of sustainable vascular systems. An EC growth supplement (ECGS) was delivered by an alginate hydrogel inside a semi-permeable tubing to study the effects of sequential release of a variety of factors (MW range: 10-250 kDa) based on diffusion release. Tilakaratne et al. demonstrated increased blood vessel structures near the membranes when the growth factor supplement was delivered [163]. Alginate hydrogels have also been used as a vehicle for combination pre-treatment of an intra-muscular space for islet transplant by delivery of VEGF and platelet-derived growth factor (PDGF). The drug containing groups were the only ones that fully restored blood glucose control [164]. In a study of modified dextran PEG hydrogels, Sun et al. found that delivery of 4 angiogenic growth factors induced a greater number and diameter of blood vessels within the hydrogel than any combination of fewer factors tested [165]. Thus, multiple factor delivery is advantageous with sequential delivery possibly playing an important role.
Sequential delivery has been investigated in studies that use the advantage of biological understanding to improve outcomes (Figure 5 b). Delivery of sphingosine-1-phosphate (SIP) following VEGF delivery largely improved the area of CD31-positive structures, and their maturity, over both factors delivered together or in a different sequence [166]. Similarly, while delivery of VEGF alone can induce angiogenesis, sequential delivery of VEGF followed by PDGF from a poly(lactide-co-glycolide) scaffold resulted in a more mature network than at the two week timepoint (Figure 5) [167]. Interestingly, if PDGF or angiopoietin-1 (Angl) was delivered at the same time as VEGF or angiopoietin-2 (Ang2), the proangiogenic effects of VEGF and Ang2 were blocked. On the other hand, the effect was synergistic if PDGF or Angl was delivered subsequently [168]. Ishihara et al. showed that by delivering the proangiogenic peptides VEGF-A165 and PDGF-BB from an α-chain laminin-type G domain with a heparin-binding domain, diabetic wound healing was improved [169], providing insight into ways to improve vascularization in a diabetic environment. Balancing the types of factors delivered is important considering that overstimulation with VEGF can be counterproductive to mature vessel formation [170].
Other materials have been used as the vehicle for combination delivery of vascularization-promoting factors. Fibrin hydrogels have been investigated as a carrier and delivery vehicle for transplanted islets as discussed earlier. Najjar et al. studied a VEGF-A165 and PDGF-BB-loaded hydrogel both in the subcutaneous space and on the epididymal fat pad in mice. Results showed that mechanical support from the hydrogel was important for overall function of the graft in the subcutaneous site, making the benefits of having the islets in direct contact with the fat pad in the intraperitoneal space (covered by the drug-loaded fibrin gel) not applicable to the subcutaneous space. Vascularization was associated with graft success in this study evidenced by increased CD31-positive area assayed immunohistologically [109].
Platelet-rich plasma, known to have multiple healing functions, has been investigated to improve vascularization of subcutaneously implanted chambers for cell transplantation [51]. Although perhaps more complex, natural collections of factors are useful, as they contain already developed ratios of factors that can be quite effective. Similarly, focusing on the natural process of wound healing may be informative [171, 172]. Typically, fibrin clot matrix is rapidly vascularized to form granulation tissue, which is highly dependent on FGF-2 released from cells in the wound bed. Interestingly, FGF-2 peaks almost immediately after a cutaneous wound, while VEGF peaks at approximately day 5 [173], perhaps in part driven by the fact that FGF-2 can stimulate VEGF secretion [174]. A factor release sequence mimicking this pattern could be effective in generating vascularized tissues [175]. Toward this idea, a combination of VEGF and FGF-2 has been released from heparin-binding peptide nanofibers. The growth factor-loaded hydrogel resulted in a 78% cure rate, while the unloaded peptide nanofiber hydrogel produced only a 30% cure rate in isogeneic mouse transplants [176]. Interestingly, when similar heparin mimetic peptide amphiphile materials were loaded with the same angiogenic factors (VEGF and FGF-2), islets were functional for longer in culture (up to seven days), displaying a stimulation index that was not different from freshly isolated islets [177]. This raises a possibility that angiogenic molecules have effects on the function of islets aside from inducing a vasculature, perhaps including the earlier referenced EC–islet cell interactions.
In parallel to the blood vascular network, a system of vessels that drain interstitial fluid forms the lymphatic vessel system. Delivery of a fibrin-binding VEGF-C variant to an ear cartilage defect model demonstrated an increase in lymphatic vessels, and delivery to a diabetic wound increased the rate of healing [178]. A primary function of the lymphatic system is immunological surveillance. Interestingly, islets and the central nervous system are among the few tissues thought to lack a lymphatic system [179, 180]. Some investigators have found a relationship between lymphangiogenesis and modulation of the immune response [181, 182]. Lymphangiogenesis has not yet been studied to the extent of angiogenesis, although the lymphatics may play an important role in successful tissue regeneration. While single or sets of factors may be able to stimulate a pro-angiogenic response, and sequential delivery may potentiate these responses, cells can also powerfully orchestrate the factors in the tissue milieu.
2.5. Co-culture and co-transplantation of islets with pro-angiogenic supporting cells
Co-culturing and co-transplanting vascularization supporting cells can promote network growth around implanted islets or within islets (sometimes with the participation of residual donor ECs). Therefore, co-culturing and co-transplanting can facilitate anastomoses between intra-islet vasculatures and the recipient vascular system. Mesenchymal stem cells (MSCs) and fibroblasts are the most commonly used cell types for co-culture and co-transplantation to support ECs. These cells actively participate in vascularization at the cellular level and have a unique role in vascular regeneration. ECs comprise the blood-contacting surface in vessels. Moreover, ECs attract other supportive cells to promote the formation of new blood vessels through paracrine secretion and signaling [183]. The main functions of MSCs in vascularization include the secretion of proteases to degrade ECM for EC migration and sprouting [184], enhancement of angiogenesis by up-regulating angiopoietin and VEGF expression in ECs [185], stabilization of vasculature by differentiating into pericytes [186] and suppression of immune or inflammatory responses [187, 188]. During angiogenesis and neovascularization, fibroblasts can generate diverse angiogenic factors such as VEGFs and FGFs to facilitate EC tube formation controlling blood vessel development [189]. Co-culture and co-transplantation of vessel-forming cells can be realized in different approaches.
First, we consider formation of a simple mixture of islets and supporting cells (Figure 6 a). Co-transplantation of porcine islets and MSCs has been performed under the kidney capsule and subcutaneous space in diabetic mice and primates [190, 191]. Oh et al. studied a bone marrow-derived mononuclear spheroid culture method to generate highly angiogenic cells. When co-transplanted with islets, the cure rate and the mean blood glucose were improved compared with mononuclear cells that did not spontaneously incorporate into the spheroids or islets without support cells [192]. Before co-transplantation, islets and supporting cells can also be embedded in ECM scaffolds. MSCs and fibroblasts along with mouse and rat islets were loaded together in collagen and fibrin scaffolds for transplantation in the omental pouch and subcutaneous space of diabetic mice [193, 194]. Compared to islets alone, overall co-transplantation results include increased glucose-stimulated insulin secretion [190], higher graft oxygenation [191], enhanced angiogenesis and vascularization [191, 194], better glycated hemoglobin correction [191], earlier normoglycemia [190], improved glucose tolerance [190, 193], and increased insulin content [190]. The simplicity of mixing cells before transplantation is a notable advantage for the translatability of these approaches.
Figure 6. Co-transplantation of vascularization supporting cells to improve engraftment.
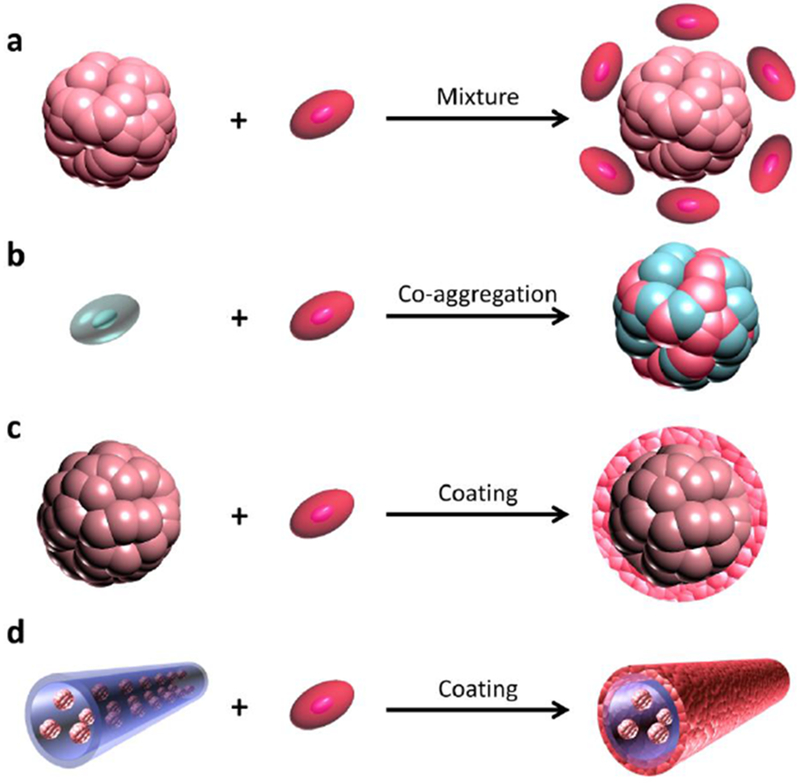
Vascularization supporting cells can be mixed with islets or cell aggregates before transplantation (a). Vascularization supporting cells can be included with hormone-producing cells during aggregation (b). Vascularization supporting cells can be coated on islets or cell aggregates before transplantation (c). It is also possible to coat vascularization supporting cells on or in islet-matrix modules (d).
Next, we consider co-aggregation of islets and supporting cells (Figure 6 b). Takebe working in the Taniguchi group demonstrated that vascularization of different types of tissue fragments can be achieved by co-aggregating with human umbilical vein ECs (HUVECs) and human MSCs [195–197]. When seeding on Matrigel or in a U-bottomed conical 96-well plate, pancreatic islets, HUVECs, and MSCs self-assembled into a miniaturized organoid in which HUVECs and MSCs played key roles in vascularization [198]. The vascularized islets showed more intra-islet blood vessels and better blood glucose control. In addition, a composite pellet of mouse islets and human bone marrow-derived multipotent adult progenitor cells (MAPCs) showed a higher blood vessel area, density, and vessel/islet ratio when transplanted under the kidney capsule [199].
Vascularization supporting cells can also be coated on islets (Figure 6 c). Rat islets were coated with rat ECs before transplantation under the kidney capsule in diabetic rats [200]. Similarly, human islets were also coated with human dermal microvascular ECs (HDMECs) and human bone marrow-derived MSCs [201]. Rat MSC sheets, created with temperature-responsive cultureware, had rat islets attached before lifting to co-transplant subcutaneously into SCID mice. MSC–islet composite sheets displayed greater stimulated insulin secretion in vitro. Only when 2,000 islets were combined with MSC sheets glucose was corrected in vivo for more than three weeks compared to 1,000 islets with MSC sheets, 2,000 islets with MSCs (not in sheet form), or 2,000 islets alone [202]. Anchoring VEGF to a heparinized islet surface has been used to increase the attachment of ECs in vitro [203], as a preparation for accelerating vascularization. Clearly coating islets with supporting cells can improve engraftment.
Recently, the Sefton group created a unique and elegant way to deliver islets with coated vascularization supporting cells. In this protocol, type I collagen (3 mg/mL) tubing sections, encasing rat islets (~1 islet per module), were seeded with HUVECs on the collagen surface (Figure 6 d). Seven hundred fifty islet modules were subcutaneously implanted into SCID-Beige mice, and a vascularized microenvironment was developed [41, 204]. Coating approaches offer the advantage of even distribution of cells across the islets being prepared for transplantation. All these results suggest that the addition of vascularizing support cells to islets can improve transplantation success, and this should be further investigated.
3. Challenges, Perspectives, and Emerging Approaches
3.1. Selection of Vascularizing Cells
Although promising co-transplantation and co-culture systems have been demonstrated in the literature, several challenges remain from a scientific and translational point of view. The source and history of supporting cells (such as donor age) can influence cell function [205, 206]. Compared with younger donors, cell morphology and viability of MSCs from older donors can be varied. In addition, proliferation, trophic factor secretion, and angiogenic potential of aged MSCs are significantly reduced [207–209]. These variables can affect the results of preclinical experiments while also hampering clinical translation. The methods to acquire sufficient autologous human vascular or supporting cells are still under development. Induced pluripotent stem cells (iPSCs) have tremendous potential for transplantable tissues, thereby prompting increasing numbers of investigators to utilize them in vascular bioengineering studies [210]. Starting somatic cells (e.g., dermal fibroblasts or fat stromal fractions) can be acquired in minimally invasive procedures. After expressing genes Oct-4, Sox2, Klf4, and c-Myc to induce pluripotency [211], it is possible to expand the cells in vitro [212] for differentiation toward vascular cells [213–217], as well as the endocrine cells of the islet [218–224]. There remain concerns about possible uncontrolled differentiation and proliferation arising from cells that escaped the intended differentiation program. Despite these concerns, as well as further protocol development being required, iPSCs avoid the ethical concerns of embryonic stem cells and do not have the same functional decline with donor age. It is likely that iPS cells will continue to develop as part of the specific tissue engineering solution to vascularization and to general tissue shortages.
3.2. Determination of Transplantation Site
Selection of the site to test an engineered tissue can affect the outcome and the translatability of the results. Transplantation sites vary in the degree to which they are naturally vascularized, as well as the potential to vascularize a transplanted tissue or device. As such, the site affects what prevascularization treatments may be required to induce a sufficient vessel network including interfacing with location-associated inflammatory responses [225]. Many transplantation sites used to evaluate vascularization, such as the kidney capsule [226, 227] or within the central nervous system [228], although conducive to islet engraftment, are not applicable to human clinical treatment. Therefore, the methods tested in these sites should be evaluated in other practical transplantation sites such as the subcutaneous space or peritoneal cavity.
Despite agreement that an extrahepatic site for islet transplantation is needed, there are still relatively few studies that compare candidate sites. In a recent well-designed study by Weaver et al., an epididymal fat pad site was superior in terms of islet survival, islet vascularization and inflammatory reaction when compared to a small bowel mesentery and especially when compared to the subcutaneous site [158]. All sites were transplanted with unloaded PEG or PEG hydrogels containing proteolytically releasable VEGF to improve the vascularity of the encapsulated single donor islets. Thus, the site should be considered in light of the preclinical evaluation potential and translatability.
3.3. Expedient vascular regeneration
Understanding how quickly an islet needs to be vascularized to prevent an ischemic loss of islets may inform vascularizing strategies. Islets in the native pancreas are integrated with a vascular system consisting of different types of cells (i.e., ECs and fibroblasts) [229]. It is therefore of great importance to quickly achieve the anastomoses between donor islets and recipient blood vessels or regenerate sufficient vasculatures around encapsulation devices. Unaided re-vascularization of islets occurs over a period of 7-14 days post-transplantation to reach a stable vascular density [53, 230]. A benchmark for studies should be accelerating the establishment of a vascular network, ideally within seven days. During the avascular period, transplanted islets are solely dependent on diffusion to receive oxygen/nutrients and clear metabolic waste [42]. Furthermore, encapsulated islets can, at best, have diffusive processes from the surface of the device to regenerated blood vessels.
In addition to in vivo prevascularization strategies discussed earlier in this review, in vitro preformed vasculatures might aid islets in the days following transplantation. Previous reports have shown that in contrast to the random mixture of ECs in ECM, predefined parallel-patterned EC tubes promoted the vascularization and overall function of co-implanted human hepatocytes [231–234]. Similarly, Hiscox et al. developed a device where a layer of hydrogel containing islets was surrounded above and below by layers containing vascular segments isolated from the fat pad one week before transplant [120]. These studies imply that an “ideal” organization of vascular architectures might exist and could be preformed to facilitate the vascularization and anastomoses of islets.
Even if a preformed vasculature and a preconditioned recipient site are combined, driving quick anastomosis, there will still be a period of time that the transplanted tissue is not yet perfused and is ischemic. To support islet survival during this period, it may be useful to supply oxygen [235]. Following transplantation, oxygen can be supplied in gaseous form directly [236–238] or generated by chemical reaction [239], electrochemically [240], or photosynthetically [241]. Before transplantation, the oxygen tension can also be controlled to reduce anoxia during culture [242]. Even before digestion, the donor pancreas can be oxygenated with techniques such as persufflation [243]. Oxygen supplementation techniques post-transplant may be useful to support fully encapsulated islets that will never vascularize and are therefore an important line of investigation for which the reader may find several excellent reviews with more information [235, 244, 245].
3.4. Manipulating the spatial positions of islets and vascular structures
Controlling the spatial positions of islets and adjacent vascular structures in vitro, which mimics their interactions and density in the pancreas [246, 247], might be an effective way to secure optimal diffusion kinetics in and out of the graft. The spatial density of islets can affect survival as a result of increased oxygen consumption with higher densities of cells [248–250]. Although production of proangiogenic factors by islets has been shown to increase in hypoxic environments [251–253], islet vascularization may still be reduced [254]. Three-dimensional (3D) printing is a powerful tool to achieve spatial control of cells [255]. Therefore, 3D printing may be a useful tool to accurately control the homogeneous spacing of islets, rather than a bulk average that varies spatially.
Over the last decade, various methods have been developed to spatially deposit cells and materials in 3D [256–258]. Among different 3D printing techniques, sacrificial molding is particularly suitable for generating hollow channels in which ECs are perfused and adhered on the channel walls to form an endothelial lining structurally similar to blood vessels (Figure 7 a) [259–262]. Other types of cells (such as cardiomyocytes, hepatocytes, MSCs, and fibroblasts) can be co-printed or added later into the interspaces between the endothelial channels [258–260, 263, 264] (Figure 7 b). ECs deposited between the channels can also anastomose to the 3D-printed channels to create perfused capillary structures in vitro [265]. A wide range of heterogeneous cellular constructs have been printed, and several reports indicated the beneficial effects of endothelial channels as blood vessel-like structures [258, 264]. Application to islet transplantation and encapsulation has been assessed for a variety of structures that can exhibit features unique to 3D printing [266–271]. In a recent study, Chen’s group demonstrated that for a rodent model of hindlimb ischemia, parallel endothelial channels integrated with the recipient and recreated blood perfusion, whereas random endothelial patches did not show any therapeutic effect [100] (Figure 7 c). These results indicate that 3D printing of islets and endothelial luminal networks might provide a biomimetic vascular-like network that quickens anastomoses and re-vascularization of islets.
Figure 7. Organization of vascular structures in engineered environments.
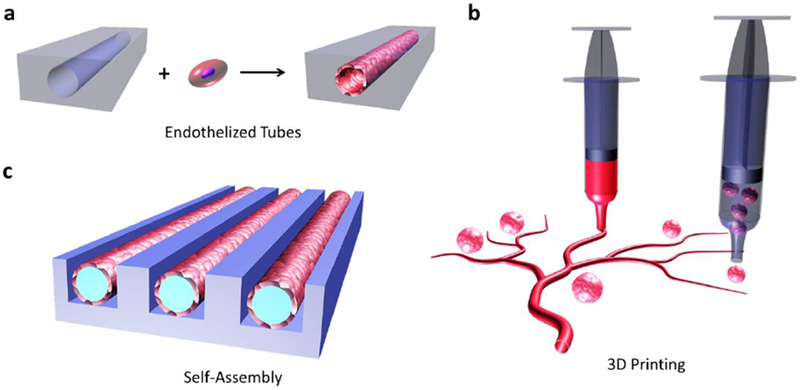
Engineering of vascular regeneration can be accomplished utilizing some emerging approaches. Tubular voids in constructs can be formed and then endothelialized (a). Three-dimensional printing can be used to arrange vascular tissues and the islets or cell clusters in logical patterns (b). Endothelialized modules can be formed in vitro using microfabricated molds.
3.5. Avoiding poorly formed vasculature
Growing a vasculature into or around a transplanted tissue does not ensure that it will be able to provide all the functions required by the cells for a long period of time. Rather, the vasculature needs to transition from a nascent structure to a stable one that is able to adapt to the tissue needs [272]. This concept has been assessed in vasculature targeted cancer treatments where both blocking angiogenesis and guiding the existing vasculature to a more stable state have been investigated to reduce patient mortality [273]. In the case of transplanted islets, some studies have compared the developed vasculature to that of the native islets [274].
Studies that examine the quality of vessel network formation to correlate with graft success are needed. It is possible to measure vessel networks with the dorsal skinfold window chamber [275, 276], mesentery windows or exteriorization [277, 278], contrast-enhanced MRI [279, 280], contrast-enhanced ultrasound [281], live cell markers [282–284], and immunostaining of tissues [285–287]. Quantification of parameters such as perfusion [288–290], flow dynamics [291–293], tortuosity [294, 295], fractal parameters [296], branching [297–299], endothelial permeability [300–303], and length and diameter [304, 305] can all be measured to determine the status of a vessel network. In some cases, automated analysis is possible [290, 295–297, 299, 304–307]. Buitinga et al. developed a smart analysis method where the vessels (and cell arrangement) were quantified in islets. Capillary structures were binned based on their radial location in the islet [247], adding value to the comparison of treatment groups. While the target for bare islets is a native vasculature, it is mostly unknown what is required for encapsulated islets, except an increase over basal vessel network density in tissues such as skin. Quantitative vascular metrics are, therefore, important to set requirements for vasculature in all types of islet grafts.
Some efforts aim to avoid needing to induce a vascularization at all. Interesting techniques are being investigated to address the diffusion challenge using convective rather than diffusive flux. Using an intravascular device would reduce the need for microvascular networks around the device because transport could be driven by ultrafiltrate convection through the islet-containing chamber [308]. Another promising approach to circumvent the diffusion barrier is to use a nanocoating that permits vascularization while camouflaging surface antigens [309, 310]. Successful β-cell replacement is a challenging goal that may not be feasibly addressed with a single technique. These emerging techniques, coupled to growing a robust vasculature, have various surmountable challenges to be addressed before clinical viability.
3.6. Biological Challenges – Immune–Vascular Cooperation and Hyperglycemia
Throughout this review, it has been implied that more vasculature will benefit the transplanted tissue or device. It is important to keep in mind, however, that the benefit of increased vasculature has limits. It is key to consider not only short term but also long-term effects. If a treatment shortens the time to normoglycemia, while hindering the long-term cure, it might be necessary to add another treatment that will improve long-term graft stability [311]. The vascular system is constantly adapting to needs in tissues as diverse as islets, muscles, and the brain. During inflammation, the vasculature grows to support tissue regeneration and facilitates access from the systemic immune system. Unfortunately, this may also increase exposure of implanted materials to the immune system.
Immune and vascular contributions to the post-implantation period must be balanced. Vascularization of transplanted tissues is known to affect the type of immune response that occurs [312], where an abundance of vasculature can cause inflammation and β-cell death [313, 314]. A certain degree of immune system involvement is important to induce healing [315–320] and stable vasculature. Anti-inflammatory or pro-regenerative monocytes [321–324], smooth muscle cells [325], and other pericytes [326, 327] can support blood vessel maturation. Furthermore, Christoffersson et al. showed that neutrophils, a less commonly identified pro-regenerative cell type, are required for a native-like vascular structure to form in islets transplanted on striated muscle [274]. One way to modulate the immune system is to prevent protein adsorption with zwitterionic materials [328–335]. Zhang et al. showed that compared to a low fouling poly(2-hydroxyethyl methacrylate) hydrogel, the ultra-low-fouling zwitterionic poly(carboxybetaine methacrylate) hydrogel not only had a reduced fibrotic layer but also had greater vasculature density near the hydrogel [336]. This effect appeared to be due to macrophage polarization [336].
The innate and adaptive immune systems are known to participate in vascularization. When using HUVECs as the source of ECs in preclinical models, it is necessary to use an immunocompromised model to prevent rejection. While formation of a functional vasculature in these animals suggests that the cells of the adaptive immune system are not essential participants in the response, it is quite likely that the response is altered. Subsets of T-cells are capable of directly producing VEGFs [337, 338]. Both T- and B-cells exert their immune functions and participate in modulating the angiogenic environment partly by interacting with effector subsets of the innate immune system (i.e., macrophages and neutrophils) [339, 340]. A revascularizing tissue in an immunocompromised mouse may be capable of achieving healing and measurable vascularization, but it is in an environment that more closely represents an immunosuppressed individual. Thus, we must be careful about how far we extrapolate these results without first verifying them in fully immune competent models.
EC is a key mediator of both immunity and blood vessel growth. ECs form sprouts that become new blood vessels as well as participating in vascularization signaling pathways. ECs also participate in immunity during extravasation of cells from the blood to the tissue. During this process, not only inflammatory cells can be hindered or allowed through by ECs but the expression of stimulatory molecules may also modulate the activation state of cells as a result of contact with the endothelium. Furthermore, functional differences are known to exist between primary ECs and immortalized EC lines [341].
The inter-communication of the vascular and immune system has another level of complexity when sites and species differences are considered. Some immunosuppression drugs such as rapamycin [342, 343] are known to interfere with vascularization. However, even with immunosuppression (i.e., daclizumab, tacrolimus, and sirolimus), allogenic islets intraportally transplanted into rhesus macaques were able to vascularize by 30 days post-transplantation [344]. Furthermore, other immunosuppressive drugs (including cyclosporine, RS-61443, and prednisolone) have been shown to delay or reduce but not prevent intra-islet capillary formation [345–348]. Toxicity of immune suppression drugs to islets has also been shown [349–353] and should be taken into consideration in transplant schemes [354, 355]. Interestingly, sirolimus has been associated with decreased VEGF release from β-cells, perhaps forming the link between sirolimus and reduced transplanted islet viability [356].
In some cases, xenotransplantation can last for a period of time without needing immunosuppression while still gaining access to nutrients, such as arteriovenous shunt devices [357]. Similarly, in some reports of planar or tubular encapsulation systems made of PTFE [238, 358, 359], acrylic copolymer [360], alginate [361], and polyethersulfone [362], as well as spherical capsules made of alginate [359, 363–367], survival has been observed without immunosuppression or using an immune compromised recipient. Preclinical investigations have shown that prevascularization of subcutaneous sites can create spaces where allogeneic islets can survive without immunosuppression [135]. Subcutaneously transplanted islets without preconditioning did not survive in this study, perhaps due to a combination of allo-immunity (which may have been ameliorated by FGF-2-dependent MSC recruitment [368]) and a lack of vascularization [135]. In a different material-based prevascularizing scheme without delivered factors during the preconditioning, immunosuppression was required to prevent islet rejection in an allogenic model [132]. Relevant to the goal of allowing vascularization for xenogenic transplants, some glucose correction has been noted without pharmacologic immunosuppression or polymer encapsulation in a human trial, with Sertoli cell co-transplantation [369]. Endothelialized collagen modules have been able to increase the vascularity surrounding transplanted islets into syngeneic or immuno-suppressed allogenic rat recipients compared to collagen modules without ECs or free islets, respectively [370], supporting an idea that some vascularizing strategies can function with or without immunosuppressive drugs present.
It is also important not to overlook the existing conditions of the recipient of a treatment. In the case of islet transplant, microvascular complications are common in those with longstanding hyperglycemia [371, 372]. Hyperglycemia can cause dysregulated microvascular remodeling (increased or decreased, depending on the tissue) [373, 374]. A high glucose environment, or the effect of previous hyperglycemia, is likely to be encountered by islets following transplantation that may cause anti-angiogenic factors to be secreted [375]. Vascular pericytes have been shown to initiate apoptosis through PKC-delta SHP-1- or NF-kappaB-dependent pathways when exposed to hyperglycemia [376]. The diabetic environment adds an additional challenge to finding the appropriate level of immune-regulated therapeutic vascularization.
Some challenges with islet transplant, or cell transplant in general, may interfere with a clear understanding of experiments intended to elucidate vascular contributions to graft success. Differences in success in rodents versus larger animals may be related to many factors including difficulty of completely digesting the pancreas to harvest pure islets [377]. On a related note, although an argument can be made for larger islets recruiting intra-islet vasculature due to a greater hypoxic response from the center of a larger islet [378], when islets are to be encapsulated and will never be vascularized, smaller islets may be more viable [379, 380]. While delivering islets in a growth factor-supplemented matrix usually supports islet transplant, the site and the recipient species may be a factor to consider. For instance, islets transplanted into the porcine gastric submucosa were not improved by the inclusion of Matrigel [381].
Finally, it may also be important to acknowledge here that interpretation of results in literature is not always the same in retrospect. For instance, some early experiments that utilized alginate as the encapsulating material may have been conducted before the knowledge that highly purified alginates are necessary [382]. Moving forward, some experiments may need to be repeated for input into the immunoisolation membrane design process.
4. Conclusions
Rapid advancements have been made in the closed-loop artificial pancreas [383–385], oral insulin formulations [386], and smart insulin [387–390]; yet, none so far have achieved the physiological control over an extended period of time that a pancreatic islet can provide [391]. The promise of higher patient quality of life and reduced healthcare costs motivate progress toward the ideal curative treatment. Our understanding of islet biology includes an intricate connection between the endocrine cells of the islets and the unique intra-islet capillary network. The quicker this can be developed in a transplanted islet, the greater is the probability of graft success through exchange of oxygen, nutrients, and wastes for islets, ensuring an adequate dispersal of secreted insulin.
Accelerated vascularization can be accomplished by tuning material physical properties, delivering factors, and delivering support cells. When a vessel network is developed before implantation of the therapeutic cells, anastomosis with the host vasculature can further reduce the time to function. The choice of implant site is important for the evaluation of therapeutic vascularization strategies. Cell sources for vascularized tissue-engineered constructs are an area of continued development.
Inadequate transplantable tissue supply from organ donation encourages consideration of alternate sources of cells including xenogeneic or stem cell-derived cells. These cell sources benefit from a robust encapsulation membrane to control immune responses and control the location of the foreign cells. Stem cell-derived islet-like clusters do not contain ECs unless the protocol specifically adds them. Therefore, in a stem cell-derived immunoprotected transplantation scheme, it is imperative that the host vasculature be as developed as possible to provide nutrient supply and waste removal from the encapsulated cells. If the immunoprotection provided is robust, any increase in inflammatory activity enabled by a richer vasculature should be tolerable. A possible solution to this immunoprotection–vascularization paradox is conformal coatings, which can camouflage surface antigens to reduce immunological recognition, while not necessarily preventing vessel penetration. An example of this is a study by Rengifo et al., where a three-layered conformal coating prevented complete graft rejection in a fully MHC mismatched murine model, while allowing vessel infiltration [392]. Although it was not clear from the results whether the islets that were integrated with blood vessels had complete and robust coatings [392], it nonetheless provides for the possibility that camouflaging surface antigens could prevent rejection even if cell infiltration is still permitted.
In conclusion, we have summarized strategies to accelerate, shape, or develop vasculature for supporting the survival of transplanted islets. These strategies are also applicable to other microtissues, therefore being useful for a collection of tissue engineering problems including modular bio-printed tissues and organs [393]. A successful strategy for islet vascularization is likely to inform the field of regenerative medicine as we move toward being able to create functional transplantable tissues.
Statement of Significance.
Insulin-dependent diabetes affects more than 1.25 million people in the United States alone. Pancreatic islets secrete insulin and other endocrine hormones that control glucose to normal levels. During preparation for transplantation, the specialized islet blood vessel supply is lost. Furthermore, in the case of cell encapsulation, cells are protected within a device, further limiting delivery of nutrients and absorption of hormones. To overcome these issues, this review considers methods to rapidly vascularize sites and implants through material properties, prevascularization, delivery of growth factors, or co-transplantation of vessel supporting cells. Other challenges and emerging technologies are also discussed. Proper vascular growth is a significant component of successful islet transplantation, a treatment that can provide life-changing benefits to patients.
Acknowledgments
Funding for this work was partially provided by Juvenile Diabetes Research Foundation (JDRF), The Hartwell Foundation, National Institutes of Health (NIH, 1R01DK105967-01 Al), and the Novo Nordisk Company. The funding sources did not affect the decision to publish this review. The authors thank Alexander Ernst, Stephanie Fuchs, and Alan Chiu for critical reading and discussion.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Michiels C, Endothelial cell functions, J Cell Physiol 196(3) (2003) 430–443. [DOI] [PubMed] [Google Scholar]
- [2].Carmeliet P, Mechanisms of angiogenesis and arteriogenesis, Nat Med 6(4) (2000) 389–395. [DOI] [PubMed] [Google Scholar]
- [3].Ratajska A, Jankowska-Steifer E, Czarnowska E, Olkowski R, Gula G, Niderla-Bielinska J, Flaht-Zabost A, Jasinska A, Vasculogenesis and its cellular therapeutic applications, Cells Tissues Organs 203(3) (2017) 141–152. [DOI] [PubMed] [Google Scholar]
- [4].Helisch A, Schaper W, Arteriogenesis: the development and growth of collateral arteries, Microcirculation 10(1) (2003) 83–97. [DOI] [PubMed] [Google Scholar]
- [5].Alberts B, Johnson A, Lewis J, Molecular Biology of the Cell, 4th edition ed., Garland Science, New York, 2002. [Google Scholar]
- [6].Rouwkema J, Rivron NC, van Blitterswijk CA, Vascularization in tissue engineering, Trends Biotechnol 26(8) (2008) 434–441. [DOI] [PubMed] [Google Scholar]
- [7].Levenberg S, Rouwkema J, Macdonald M, Garfein ES, Kohane DS, Darland DC, Marini R, van Blitterswijk CA, Mulligan RC, D’Amore PA, Langer R, Engineering vascularized skeletal muscle tissue, Nat Biotechnol 23(7) (2005) 879–884. [DOI] [PubMed] [Google Scholar]
- [8].Tsui JH, Janebodin K, Ieronimakis N, Yama DMP, Yang HS, Chavanachat R, Hays AL, Lee H, Reyes M, Kim DH, Harnessing Sphingosine-1-Phosphate signaling and nanotopographical cues to regulate skeletal muscle maturation and vascularization, ACS Nano 11(12) (2017) 11954–11968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Stevens KR, Kreutziger KL, Dupras SK, Korte FS, Regnier M, Muskheli V, Nourse MB, Bendixen K, Reinecke H, Murry CE, Physiological function and transplantation of scaffold-free and vascularized human cardiac muscle tissue, Proc Natl Acad Sci U S A 106(39) (2009) 16568–16573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Dvir T, Kedem A, Ruvinov E, Levy O, Freeman I, Landa N, Holbova R, Feinberg MS, Dror S, Etzion Y, Leor J, Cohen S, Prevascularization of cardiac patch on the omentum improves its therapeutic outcome, Proc Natl Acad Sci U S A 106(35) (2009) 14990–14995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lesman A, Habib M, Caspi O, Gepstein A, Arbel G, Levenberg S, Gepstein L, Transplantation of a tissue-engineered human vascularized cardiac muscle, Tissue Eng Part A 16(1) (2010) 115–125. [DOI] [PubMed] [Google Scholar]
- [12].McGuigan AP, Sefton MV, Vascularized organoid engineered by modular assembly enables blood perfusion, Proc Natl Acad Sci U S A 103(31) (2006) 11461–11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rouwkema J, de Boer J, Van Blitterswijk CA, Endothelial cells assemble into a 3-dimensional prevascular network in a bone tissue engineering construct, Tissue Eng 12(9) (2006) 2685–2693. [DOI] [PubMed] [Google Scholar]
- [14].Fuchs S, Ghanaati S, Orth C, Barbeck M, Kolbe M, Hofmann A, Eblenkamp M, Gomes M, Reis RL, Kirkpatrick CJ, Contribution of outgrowth endothelial cells from human peripheral blood on in vivo vascularization of bone tissue engineered constructs based on starch polycaprolactone scaffolds, Biomaterials 30(4) (2009) 526–534. [DOI] [PubMed] [Google Scholar]
- [15].Shor E, Merdler U, Brosh I, Shoham S, Levenberg S, Induced neuro-vascular interactions robustly enhance functional attributes of engineered neural implants, Biomaterials 180 (2018) 1–11. [DOI] [PubMed] [Google Scholar]
- [16].Gibot L, Galbraith T, Huot J, Auger FA, A preexisting microvascular network benefits in vivo revascularization of a microvascularized tissue-engineered skin substitute, Tissue Eng Part A 16(10) (2010) 3199–3206. [DOI] [PubMed] [Google Scholar]
- [17].Klar AS, Guven S, Biedermann T, Luginbuhl J, Bottcher-Haberzeth S, Meuli-Simmen C, Meuli M, Martin I, Scherberich A, Reichmann E, Tissue-engineered dermo-epidermal skin grafts prevascularized with adipose-derived cells, Biomaterials 35(19) (2014) 5065–5078. [DOI] [PubMed] [Google Scholar]
- [18].Costa M, Cerqueira MT, Santos TC, Sampaio-Marques B, Ludovico P, Marques AP, Pirraco RP, Reis RL, Cell sheet engineering using the stromal vascular fraction of adipose tissue as a vascularization strategy, Acta Biomater 55 (2017) 131–143. [DOI] [PubMed] [Google Scholar]
- [19].Bulanova EA, Koudan EV, Degosserie J, Heymans C, Pereira FD, Parfenov VA, Sun Y, Wang Q, Akhmedova SA, Sviridova IK, Sergeeva NS, Frank GA, Khesuani YD, Pierreux CE, Mironov VA, Bioprinting of a functional vascularized mouse thyroid gland construct, Biofabrication 9(3) (2017) 034105. [DOI] [PubMed] [Google Scholar]
- [20].Rosines E, Sampogna RV, Johkura K, Vaughn DA, Choi Y, Sakurai H, Shah MM, Nigam SK, Staged in vitro reconstitution and implantation of engineered rat kidney tissue, Proc Natl Acad Sci U S A 104(52) (2007) 20938–20943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Moon JJ, Saik JE, Poche RA, Leslie-Barbick JE, Lee SH, Smith AA, Dickinson ME, West JL, Biomimetic hydrogels with pro-angiogenic properties, Biomaterials 31(14) (2010) 3840–3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Misler S, The isolated pancreatic islet as a micro-organ and its transplantation to cure diabetes, Islets 2(4) (2010) 210–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kamba T, Tam BY, Hashizume H, Haskell A, Sennino B, Mancuso MR, Norberg SM, O’Brien SM, Davis RB, Gowen LC, Anderson KD, Thurston G, Joho S, Springer ML, Kuo CJ, McDonald DM, VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature, Am J Physiol Heart Circ Physiol 290(2) (2006) H560–576. [DOI] [PubMed] [Google Scholar]
- [24].Nyman LR, Ford E, Powers AC, Piston DW, Glucose-dependent blood flow dynamics in murine pancreatic islets in vivo, Am J Physiol Endocrinol Metab 298(4) (2010) E807–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gan WJ, Zavortink M, Ludick C, Templin R, Webb R, Webb R, Ma W, Poronnik P, Parton RG, Gaisano HY, Shewan AM, Thorn P, Cell polarity defines three distinct domains in pancreatic beta-cells, J Cell Sci 130(1) (2017) 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Nikolova G, Jabs N, Konstantinova I, Domogatskaya A, Tryggvason K, Sorokin L, Fassler R, Gu G, Gerber HP, Ferrara N, Melton DA, Lammert E, The vascular basement membrane: a niche for insulin gene expression and Beta cell proliferation, Dev Cell 10(3) (2006) 397–405. [DOI] [PubMed] [Google Scholar]
- [27].Nyman LR, Wells KS, Head WS, McCaughey M, Ford E, Brissova M, Piston DW, Powers AC, Real-time, multidimensional in vivo imaging used to investigate blood flow in mouse pancreatic islets, J Clin Invest 118(11) (2008) 3790–3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Weir GC, Bonner-Weir S, Islets of Langerhans: the puzzle of intraislet interactions and their relevance to diabetes, J Clin Invest 85(4) (1990) 983–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Menger MD, Vajkoczy P, Beger C, Messmer K, Orientation of microvascular blood flow in pancreatic islet isografts, J Clin Invest 93(5) (1994) 2280–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cabrera OB, M. D; Kenyon NS; Ricordi C; Berggren PO; Caicedo A, The unique cytoarchitecture of human pancreatic islets has implications for islet cell function, Proc Natl Acad Sci U S A 103(7) (2006) 2334–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Jansson L, Barbu A, Bodin B, Drott CJ, Espes D, Gao X, Grapensparr L, Kallskog O, Lau J, Liljeback H, Palm F, Quach M, Sandberg M, Stromberg V, Ullsten S, Carlsson PO, Pancreatic islet blood flow and its measurement, Ups J Med Sci 121(2) (2016) 81–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Henderson JR, Daniel PM, Portal circulations and their relation to counter-current systems, Q J Exp Physiol Cogn Med Sci 63(4) (1978) 355–369. [DOI] [PubMed] [Google Scholar]
- [33].Henderson JR, Daniel PM, A comparative study of the portal vessels connecting the endocrine and exocrine pancreas, with a discussion of some functional implications, Q J Exp Physiol Cogn Med Sci 64(4) (1979) 267–275. [DOI] [PubMed] [Google Scholar]
- [34].Canzano JS, Nasif LH, Butterworth EA, Fu DA, Atkinson MA, Campbell-Thompson M, Islet microvasculature alterations with loss of Beta-cells in patients with type 1 diabetes, J Histochem Cytochem (2018)22155418778546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Steiner DJK, A.; Miller K; Hara M, Pancreatic islet plasticity Interspecies comparison of islet architecture and composition, Islets 2(3) (2010) 135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Bonner-Weir S, Sullivan BA, Weir GC, Human islet morphology revisited: human and rodent islets are not so different after all, J Histochem Cytochem 63(8) (2015) 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].St Clair JR, Ramirez D, Passman S, Benninger RKP, Contrast-enhanced ultrasound measurement of pancreatic blood flow dynamics predicts type 1 diabetes progression in preclinical models, Nat Commun 9(1) (2018) 1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lukinius A, Jansson L, Korsgren O, Ultrastructural evidence for blood microvessels devoid of an endothelial cell lining in transplanted pancreatic islets, Am J Pathol 146(2) (1995) 429–435. [PMC free article] [PubMed] [Google Scholar]
- [39].Linn T, Schneider K, Hammes HP, Preissner KT, Brandhorst H, Morgenstern E, Kiefer F, Bretzel RG, Angiogenic capacity of endothelial cells in islets of Langerhans, FASEB J 17(8) (2003) 881–883. [DOI] [PubMed] [Google Scholar]
- [40].Nyqvist D, Kohler M, Wahlstedt H, Berggren PO, Donor islet endothelial cells participate in formation of functional vessels within pancreatic islet grafts, Diabetes 54(8) (2005) 2287–2293. [DOI] [PubMed] [Google Scholar]
- [41].Vlahos AE, Cober N, Sefton MV, Modular tissue engineering for the vascularization of subcutaneously transplanted pancreatic islets, Proc Natl Acad Sci U S A 114(35) (2017) 9337–9342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Brissova M, Fowler M, Wiebe P, Shostak A, Shiota M, Radhika A, Lin PC, Gannon M, Powers AC, Intraislet endothelial cells contribute to revascularization of transplanted pancreatic islets, Diabetes 53(5) (2004) 1318–1325. [DOI] [PubMed] [Google Scholar]
- [43].Olsson R, Carlsson PO, Better vascular engraftment and function in pancreatic islets transplanted without prior culture, Diabetologia 48(3) (2005) 469–476. [DOI] [PubMed] [Google Scholar]
- [44].van der Laan LJ, Lockey C, Griffeth BC, Frasier FS, Wilson CA, Onions DE, Hering BJ, Long Z, Otto E, Torbett BE, Salomon DR, Infection by porcine endogenous retrovirus after islet xenotransplantation in SCID mice, Nature 407(6800) (2000) 90–94. [DOI] [PubMed] [Google Scholar]
- [45].Halme DG, Kessler DA, FDA regulation of stem-cell-based therapies, N Engl J Med 355(16) (2006) 1730–1735. [DOI] [PubMed] [Google Scholar]
- [46].Ludwig B, Ludwig S, Steffen A, Knauf Y, Zimerman B, Heinke S, Lehmann S, Schubert U, Schmid J, Bleyer M, Schonmann U, Colton CK, Bonifacio E, Solimena M, Reichel A, Schally AV, Rotem A, Barkai U, Grinberg-Rashi H, Kaup FJ, Avni Y, Jones P, Bornstein SR, Favorable outcome of experimental islet xenotransplantation without immunosuppression in a nonhuman primate model of diabetes, Proc Natl Acad Sci U S A 114(44) (2017) 11745–11750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Jansson L, The regulation of pancreatic islet blood flow, Diabetes Metab Rev 10(4) (1994) 407–416. [DOI] [PubMed] [Google Scholar]
- [48].Ernst AU, Wang L-H, Ma M, Islet encapsulation, Journal of Materials Chemistry B 6(42) (2018) 6705–6722. [DOI] [PubMed] [Google Scholar]
- [49].Sabek OM, Ferrati S, Fraga DW, Sih J, Zabre EV, Fine DH, Ferrari M, Gaber AO, Grattoni A, Characterization of a nanogland for the autotransplantation of human pancreatic islets, Lab Chip 13(18) (2013) 3675–3688. [DOI] [PubMed] [Google Scholar]
- [50].Chang R, Faleo G, Russ HA, Parent AV, Elledge SK, Bernards DA, Allen JL, Villanueva K, Hebrok M, Tang Q, Desai TA, Nanoporous Immunoprotective Device for Stem-Cell-Derived beta-Cell Replacement Therapy, ACS Nano 11(8) (2017) 7747–7757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Farina M, Chua CYX, Ballerini A, Thekkedath U, Alexander JF, Rhudy JR, Torchio G, Fraga D, Pathak RR, Villanueva M, Shin CS, Niles JA, Sesana R, Demarchi D, Sikora AG, Acharya GS, Gaber AO, Nichols JE, Grattoni A, Transcutaneously refillable, 3D-printed biopolymeric encapsulation system for the transplantation of endocrine cells, Biomaterials 177 (2018) 125–138. [DOI] [PubMed] [Google Scholar]
- [52].Bowers DT, Botchwey EA, Brayman KL, Advances in local drug release and scaffolding design to enhance cell therapy for diabetes, Tissue Eng Part B Rev 21(6) (2015) 491–503. [DOI] [PubMed] [Google Scholar]
- [53].Menger MD, Jaeger S, Walter P, Feifel G, Hammersen F, Messmer K, Angiogenesis and hemodynamics of microvasculature of transplanted islets of Langerhans, Diabetes 38 Suppl 1 (1989) 199–201. [DOI] [PubMed] [Google Scholar]
- [54].Jones GL, Juszczak MT, Hughes SJ, Kooner P, Powis SH, Press M, Time course and quantification of pancreatic islet revascularization following intraportal transplantation, Cell Transplant 16(5) (2007) 505–516. [DOI] [PubMed] [Google Scholar]
- [55].Eter WA, Bos D, Frielink C, Boerman OC, Brom M, Gotthardt M, Graft revascularization is essential for non-invasive monitoring of transplanted islets with radiolabeled exendin, Sci Rep 5 (2015) 15521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Davalli AM, Scaglia L, Zangen DH, Hollister J, Bonner-Weir S, Weir GC, Vulnerability of islets in the immediate posttransplantation period. Dynamic changes in structure and function, Diabetes 45(9) (1996)1161–1167. [DOI] [PubMed] [Google Scholar]
- [57].Davalli AM, Ogawa Y, Scaglia L, Wu YJ, Hollister J, Bonner-Weir S, Weir GC, Function, mass, and replication of porcine and rat islets transplanted into diabetic nude mice, Diabetes 44(1) (1995) 104–111. [DOI] [PubMed] [Google Scholar]
- [58].Brauker JH, Carr-Brendel VE, Martinson LA, Crudele J, Johnston WD, Johnson RC, Neovascularization of synthetic membranes directed by membrane microarchitecture, J Biomed Mater Res 29(12) (1995) 1517–1524. [DOI] [PubMed] [Google Scholar]
- [59].Lathuiliere A, Cosson S, Lutolf MP, Schneider BL, Aebischer P, A high-capacity cell macroencapsulation system supporting the long-term survival of genetically engineered allogeneic cells, Biomaterials 35(2) (2014) 779–791. [DOI] [PubMed] [Google Scholar]
- [60].Padera RF, Colton CK, Time course of membrane microarchitecture-driven neovascularization, Biomaterials 17(3) (1996) 277–284. [DOI] [PubMed] [Google Scholar]
- [61].Gosain A, Matthies AM, Dovi JV, Barbul A, Gamelli RL, DiPietro LA, Exogenous pro-angiogenic stimuli cannot prevent physiologic vessel regression, J Surg Res 135(2) (2006) 218–225. [DOI] [PubMed] [Google Scholar]
- [62].Gurevich DB, Severn CE, Twomey C, Greenhough A, Cash J, Toye AM, Mellor H, Martin P, Live imaging of wound angiogenesis reveals macrophage orchestrated vessel sprouting and regression, EMBO J 37(13) (2018) e97786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Feng B, Jinkang Z, Zhen W, Jianxi L, Jiang C, Jian L, Guolin M, Xin D, The effect of pore size on tissue ingrowth and neovascularization in porous bioceramics of controlled architecture in vivo, Biomed Mater 6(1) (2011) 015007. [DOI] [PubMed] [Google Scholar]
- [64].Choi SW, Zhang Y, Macewan MR, Xia Y, Neovascularization in biodegradable inverse opal scaffolds with uniform and precisely controlled pore sizes, Adv Healthc Mater 2(1) (2013) 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Chiu YC, Cheng MH, Engel H, Kao SW, Larson JC, Gupta S, Brey EM, The role of pore size on vascularization and tissue remodeling in PEG hydrogels, Biomaterials 32(26) (2011) 6045–6051. [DOI] [PubMed] [Google Scholar]
- [66].Druecke D, Langer S, Lamme E, Pieper J, Ugarkovic M, Steinau HU, Homann HH, Neovascularization of poly(ether ester) block-copolymer scaffolds in vivo: long-term investigations using intravital fluorescent microscopy, J Biomed Mater Res A 68(1) (2004) 10–18. [DOI] [PubMed] [Google Scholar]
- [67].Petrie Aronin CE, Sadik KW, Lay AL, Rion DB, Tholpady SS, Ogle RC, Botchwey EA, Comparative effects of scaffold pore size, pore volume, and total void volume on cranial bone healing patterns using microsphere-based scaffolds, J Biomed Mater Res A 89(3) (2009) 632–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Madden LR, Mortisen DJ, Sussman EM, Dupras SK, Fugate JA, Cuy JL, Hauch KD, Laflamme MA, Murry CE, Ratner BD, Proangiogenic scaffolds as functional templates for cardiac tissue engineering, Proc Natl Acad Sci U S A 107(34) (2010) 15211–15216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Moore AN, Lopez Silva TL, Carrejo NC, Origel Marmolejo CA, Li IC, Hartgerink JD, Nanofibrous peptide hydrogel elicits angiogenesis and neurogenesis without drugs, proteins, or cells, Biomaterials 161 (2018) 154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Mahou R, Zhang DKY, Vlahos AE, Sefton MV, Injectable and inherently vascularizing semi-interpenetrating polymer network for delivering cells to the subcutaneous space, Biomaterials 131 (2017) 27–35. [DOI] [PubMed] [Google Scholar]
- [71].Rosengren A, Danielsen N, Bjursten LM, Reactive capsule formation around soft-tissue implants is related to cell necrosis, J Biomed Mater Res 46(4) (1999) 458–464. [DOI] [PubMed] [Google Scholar]
- [72].Khosravi N, Maeda A, DaCosta RS, Davies JE, Nanosurfaces modulate the mechanism of peri-implant endosseous healing by regulating neovascular morphogenesis, Communications Biology 1(1) (2018) 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Ashworth JC, Mehr M, Buxton PG, Best SM, Cameron RE, Towards cellular sieving: exploring the limits of scaffold accessibility for cell type specific invasion, Advanced Biosystems 2(8) (2018) 1700257. [Google Scholar]
- [74].Lee KY, Mooney DJ, Alginate: properties and biomedical applications, Prog Polym Sci 37(1) (2012) 106–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Zimmermann H, Shirley SG, Zimmermann U, Alginate-based encapsulation of cells: past, present, and future, Curr Diab Rep 7(4) (2007) 314–320. [DOI] [PubMed] [Google Scholar]
- [76].Nyitray CE, Chavez MG, Desai TA, Compliant 3D microenvironment improves beta-cell cluster insulin expression through mechanosensing and beta-catenin signaling, Tissue Eng Part A 20(13-14) (2014) 1888–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Nagy N, de la Zerda A, Kaber G, Johnson PY, Hu KH, Kratochvil MJ, Yadava K, Zhao W, Cui Y, Navarro G, Annes JP, Wight TN, Heilshorn SC, Bollyky PL, Butte MJ, Hyaluronan content governs tissue stiffness in pancreatic islet inflammation, J Biol Chem 293(2) (2018) 567–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Varhue WB, Langman L, Kelly-Goss M, Lataillade M, Brayman KL, Peirce-Cottler S, Swami NS, Deformability-based microfluidic separation of pancreatic islets from exocrine acinar tissue for transplant applications, Lab Chip 17(21) (2017) 3682–3691. [DOI] [PubMed] [Google Scholar]
- [79].LaValley DJ, Reinhart-King CA, Matrix stiffening in the formation of blood vessels, Advances in Regenerative Biology 1(1) (2014) 25247. [Google Scholar]
- [80].Mason BN, Starchenko A, Williams RM, Bonassar LJ, Reinhart-King CA, Tuning three-dimensional collagen matrix stiffness independently of collagen concentration modulates endothelial cell behavior, Acta Biomater 9(1) (2013) 4635–4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Yeung T, Georges PC, Flanagan LA, Marg B, Ortiz M, Funaki M, Zahir N, Ming W, Weaver V, Janmey PA, Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion, Cell Motil Cytoskeleton 60(1) (2005) 24–34. [DOI] [PubMed] [Google Scholar]
- [82].Wood JA, Shah NM, McKee CT, Hughbanks ML, Liliensiek SJ, Russell P, Murphy CJ, The role of substratum compliance of hydrogels on vascular endothelial cell behavior, Biomaterials 32(22) (2011) 5056–5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Reinhart-King CA, Dembo M, Hammer DA, Cell-cell mechanical communication through compliant substrates, Biophys J 95(12) (2008) 6044–6051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Frye M, Taddei A, Dierkes C, Martinez-Corral I, Fielden M, Ortsater H, Kazenwadel J, Calado DP, Ostergaard P, Salminen M, He L, Harvey NL, Kiefer F, Makinen T, Matrix stiffness controls lymphatic vessel formation through regulation of a GATA2-dependent transcriptional program, Nat Commun 9(1) (2018) 1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Krishnan L, Hoying JB, Nguyen H, Song H, Weiss JA, Interaction of angiogenic microvessels with the extracellular matrix, Am J Physiol Heart Circ Physiol 293(6) (2007) H3650–3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Stetler-Stevenson WG, Matrix metalloproteinases in angiogenesis: a moving target for therapeutic intervention, J Clin Invest 103(9) (1999) 1237–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Fan D, Creemers EE, Kassiri Z, Matrix as an interstitial transport system, Circ Res 114(5) (2014) 889–902. [DOI] [PubMed] [Google Scholar]
- [88].Hussey GS, Dziki JL, Badylak SF, Extracellular matrix-based materials for regenerative medicine, Nature Reviews Materials 3(7) (2018) 159–173. [Google Scholar]
- [89].Smink AM, de Vos P, Therapeutic strategies for modulating the extracellular matrix to improve pancreatic islet function and survival after transplantation, Curr Diab Rep 18(7) (2018) 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Goh SK, Bertera S, Olsen P, Candiello JE, Halfter W, Uechi G, Balasubramani M, Johnson SA, Sicari BM, Kollar E, Badylak SF, Banerjee I, Perfusion-decellularized pancreas as a natural 3D scaffold for pancreatic tissue and whole organ engineering, Biomaterials 34(28) (2013) 6760–6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Wang P, Alvarez-Perez JC, Felsenfeld DP, Liu H, Sivendran S, Bender A, Kumar A, Sanchez R, Scott DK, Garcia-Ocana A, Stewart AF, A high-throughput chemical screen reveals that harmine-mediated inhibition of DYRK1A increases human pancreatic beta cell replication, Nat Med 21(4) (2015) 383–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Weir GC, Bonner-Weir S, Islet beta cell mass in diabetes and how it relates to function, birth, and death, Ann N Y Acad Sci 1281 (2013) 92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Clark RAF, Fibrin and wound healing, Ann N Y Acad Sci 936 (2001) 355–367. [DOI] [PubMed] [Google Scholar]
- [94].Janmey PA, Winer JP, Weisel JW, Fibrin gels and their clinical and bioengineering applications, J R Soc Interface 6(30) (2009) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Wang RN, Paraskevas S, Rosenberg L, Characterization of integrin expression in islets isolated from hamster, canine, porcine, and human pancreas, J Histochem Cytochem 47(4) (1999) 499–506. [DOI] [PubMed] [Google Scholar]
- [96].van Deijnen JH, Hulstaert CE, Wolters GH, van Schilfgaarde R, Significance of the peri-insular extracellular matrix for islet isolation from the pancreas of rat, dog, pig, and man, Cell Tissue Res 267(1) (1992) 139–146. [DOI] [PubMed] [Google Scholar]
- [97].Kuehn C, Lakey JR, Lamb MW, Vermette P, Young porcine endocrine pancreatic islets cultured in fibrin show improved resistance toward hydrogen peroxide, Islets 5(5) (2013) 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Berman DM, Molano RD, Fotino C, Ulissi U, Gimeno J, Mendez AJ, Kenyon NM, Kenyon NS, Andrews DM, Ricordi C, Pileggi A, Bioengineering the endocrine pancreas: intraomental islet transplantation within a biologic resorbable scaffold, Diabetes 65(5) (2016) 1350–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Baidal DA, Ricordi C, Berman DM, Alvarez A, Padilla N, Ciancio G, Linetsky E, Pileggi A, Alejandro R, Bioengineering of an intraabdominal endocrine pancreas, N Engl J Med 376(19) (2017) 1887–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Kim JS, Lim JH, Nam HY, Lim HJ, Shin JS, Shin JY, Ryu JH, Kim K, Kwon IC, Jin SM, Kim HR, Kim SJ, Park CG, In situ application of hydrogel-type fibrin-islet composite optimized for rapid glycemic control by subcutaneous xenogeneic porcine islet transplantation, J Control Release 162(2) (2012) 382–390. [DOI] [PubMed] [Google Scholar]
- [101].Andrades P, Asiedu C, Rodriguez C, Goodwin J, Deckard LA, Jargal U, Balgansuren G, Thomas JM, Insulin secretion from pancreatic islets in fibrin glue clots at different fibrinogen and thrombin concentrations, Transplant Proc 39(5) (2007) 1607–1608. [DOI] [PubMed] [Google Scholar]
- [102].Hanzelmann S, Wang J, Guney E, Tang Y, Zhang E, Axelsson AS, Nenonen H, Salehi AS, Wollheim CB, Zetterberg E, Berntorp E, Costa IG, Castelo R, Rosengren AH, Thrombin stimulates insulin secretion via protease-activated receptor-3, Islets 7(4) (2015) e1118195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Ozmen L, Ekdahl KN, Elgue G, Larsson R, Korsgren O, Nilsson B, Inhibition of thrombin abrogates the instant blood-mediated inflammatory reaction triggered by isolated human islets: possible application of the thrombin inhibitor melagatran in clinical islet transplantation, Diabetes 51(6) (2002) 1779–1784. [DOI] [PubMed] [Google Scholar]
- [104].Chen H, Teramura Y, Iwata H, Co-immobilization of urokinase and thrombomodulin on islet surfaces by poly(ethylene glycol)-conjugated phospholipid, J Control Release 150(2) (2011) 229–234. [DOI] [PubMed] [Google Scholar]
- [105].Wilson JT, Haller CA, Qu Z, Cui W, Urlam MK, Chaikof EL, Biomolecular surface engineering of pancreatic islets with thrombomodulin, Acta Biomater 6(6) (2010) 1895–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Cui W, Wilson JT, Wen J, Angsana J, Qu Z, Haller CA, Chaikof EL, Thrombomodulin improves early outcomes after intraportal islet transplantation, Am J Transplant 9(6) (2009) 1308–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Schaschkow A, Mura C, Bietiger W, Peronet C, Langlois A, Bodin F, Dissaux C, Bruant-Rodier C, Pinget M, Jeandidier N, Juszczak MT, Sigrist S, Maillard E, Impact of an autologous oxygenating matrix culture system on rat islet transplantation outcome, Biomaterials 52 (2015) 180–188. [DOI] [PubMed] [Google Scholar]
- [108].Amara U, Rittirsch D, Flierl M, Bruckner U, Klos A, Gebhard F, Lambris JD, Huber-Lang M, Interaction between the coagulation and complement system, Adv Exp Med Biol 632 (2008) 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Najjar M, Manzoli V, Abreu M, Villa C, Martino MM, Molano RD, Torrente Y, Pileggi A, Inverardi L, Ricordi C, Hubbell JA, Tomei AA, Fibrin gels engineered with pro-angiogenic growth factors promote engraftment of pancreatic islets in extrahepatic sites in mice, Biotechnol Bioeng 112(9) (2015) 1916–1926. [DOI] [PubMed] [Google Scholar]
- [110].Hughes CS, Postovit LM, Lajoie GA, Matrigel: a complex protein mixture required for optimal growth of cell culture, Proteomics 10(9) (2010) 1886–1890. [DOI] [PubMed] [Google Scholar]
- [111].Kleinman HK, Martin GR, Matrigel: basement membrane matrix with biological activity, Semin Cancer Biol 15(5) (2005) 378–386. [DOI] [PubMed] [Google Scholar]
- [112].Malinda KM, In vivo matrigel migration and angiogenesis assay, Methods Mol Biol 467 (2009) 287–294. [DOI] [PubMed] [Google Scholar]
- [113].Ponce ML, Tube formation: an in vitro matrigel angiogenesis assay, Methods Mol Biol 467 (2009) 183–188. [DOI] [PubMed] [Google Scholar]
- [114].Golocheikine A, Tiriveedhi V, Angaswamy N, Benshoff N, Sabarinathan R, Mohanakumar T, Cooperative signaling for angiogenesis and neovascularization by VEGF and HGF following islet transplantation, Transplantation 90(7) (2010) 725–731. [DOI] [PubMed] [Google Scholar]
- [115].Hammar E, Parnaud G, Bosco D, Perriraz N, Maedler K, Donath M, Rouiller DG, Halban PA, Extracellular matrix protects pancreatic beta-cells against apoptosis: role of short- and long-term signaling pathways, Diabetes 53(8) (2004) 2034–2041. [DOI] [PubMed] [Google Scholar]
- [116].Irving-Rodgers HF, Choong FJ, Hummitzsch K, Parish CR, Rodgers RJ, Simeonovic CJ, Pancreatic islet basement membrane loss and remodeling after mouse islet isolation and transplantation: impact for allograft rejection, Cell Transplant 23(1) (2014) 59–72. [DOI] [PubMed] [Google Scholar]
- [117].Davis GE, Senger DR, Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization, Circ Res 97(11) (2005) 1093–1107. [DOI] [PubMed] [Google Scholar]
- [118].Hodivala-Dilke KM, Reynolds AR, Reynolds LE, Integrins in angiogenesis: multitalented molecules in a balancing act, Cell Tissue Res 314(1) (2003) 131–144. [DOI] [PubMed] [Google Scholar]
- [119].Wang X, Wang K, Zhang W, Qiang M, Luo Y, A bilaminated decellularized scaffold for islet transplantation: Structure, properties and functions in diabetic mice, Biomaterials 138 (2017) 80–90. [DOI] [PubMed] [Google Scholar]
- [120].Hiscox AM, Stone AL, Limesand S, Hoying JB, Williams SK, An islet-stabilizing implant constructed using a preformed vasculature, Tissue Eng Part A 14(3) (2008) 433–440. [DOI] [PubMed] [Google Scholar]
- [121].Naba A, Clauser KR, Mani DR, Carr SA, Hynes RO, Quantitative proteomic profiling of the extracellular matrix of pancreatic islets during the angiogenic switch and insulinoma progression, Sci Rep 7(2017)40495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Conrad C, Schuetz C, Clippinger B, Vacanti JP, Markmann JF, Ott HC, Bio-engineered endocrine pancreas based on decellularized pancreatic matrix and mesenchymal stem cell/islet cell coculture, Journal of the American College of Surgeons 211(3) (2010) S62. [Google Scholar]
- [123].Napierala H, Hillebrandt KH, Haep N, Tang P, Tintemann M, Gassner J, Noesser M, Everwien H, Seiffert N, Kluge M, Teegen E, Polenz D, Lippert S, Geisel D, Reutzel Selke A, Raschzok N, Andreou A, Pratschke J, Sauer IM, Struecker B, Engineering an endocrine neo-pancreas by repopulation of a decellularized rat pancreas with islets of Langerhans, Sci Rep 7 (2017) 41777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Yu H, Chen Y, Kong H, He Q, Sun H, Bhugul PA, Zhang Q, Chen B, Zhou M, The rat pancreatic body tail as a source of a novel extracellular matrix scaffold for endocrine pancreas bioengineering, J Biol Eng 12 (2018) 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Mirmalek-Sani SH, Orlando G, McQuilling JP, Pareta R, Mack DL, Salvatori M, Farney AC, Stratta RJ, Atala A, Opara EC, Soker S, Porcine pancreas extracellular matrix as a platform for endocrine pancreas bioengineering, Biomaterials 34(22) (2013) 5488–5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Sackett SD, Tremmel DM, Ma F, Feeney AK, Maguire RM, Brown ME, Zhou Y, Li X, O’Brien C, Li L, Burlingham WJ, Odorico JS, Extracellular matrix scaffold and hydrogel derived from decellularized and delipidized human pancreas, Sci Rep 8(1) (2018) 10452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Sorenby AK, Kumagai-Braesch M, Sharma A, Hultenby KR, Wernerson AM, Tibell AB, Preimplantation of an immunoprotective device can lower the curative dose of islets to that of free islet transplantation - Studies in a rodent model, Transplantation 86(2) (2008) 364–366. [DOI] [PubMed] [Google Scholar]
- [128].Pileggi A, Molano RD, Ricordi C, Zahr E, Collins J, Valdes R, Inverardi L, Reversal of diabetes by pancreatic islet transplantation into a subcutaneous, neovascularized device, Transplantation 81(9) (2006) 1318–1324. [DOI] [PubMed] [Google Scholar]
- [129].Kriz J, Vilk G, Mazzuca DM, Toleikis PM, Foster PJ, White DJ, A novel technique for the transplantation of pancreatic islets within a vascularized device into the greater omentum to achieve insulin independence, Am J Surg 203(6) (2012) 793–797. [DOI] [PubMed] [Google Scholar]
- [130].Smink AM, Li S, Hertsig DT, de Haan BJ, Schwab L, van Apeldoorn AA, de Koning E, Faas MM, Lakey JR, de Vos P, The efficacy of a prevascularized, retrievable poly(D,L,-lactide-co-epsilon-caprolactone) subcutaneous scaffold as transplantation site for pancreatic islets, Transplantation 101(4) (2017) e112–e119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Pepper AR, Pawlick R, Bruni A, Gala-Lopez B, Wink J, Rafiei Y, Bral M, Abualhassan N, Shapiro AM, Harnessing the foreign body reaction in marginal mass device-less subcutaneous islet transplantation in mice, Transplantation 100(7) (2016) 1474–1479. [DOI] [PubMed] [Google Scholar]
- [132].Pepper AR, Gala-Lopez B, Pawlick R, Merani S, Kin T, Shapiro AM, A prevascularized subcutaneous device-less site for islet and cellular transplantation, Nat Biotechnol 33(5) (2015) 518–523. [DOI] [PubMed] [Google Scholar]
- [133].Fumimoto Y, Matsuyama A, Komoda H, Okura H, Lee CM, Nagao A, Nishida T, Ito T, Sawa Y, Creation of a rich subcutaneous vascular network with implanted adipose tissue-derived stromal cells and adipose tissue enhances subcutaneous grafting of islets in diabetic mice, Tissue Eng Part C Methods 15(3) (2009) 437–444. [DOI] [PubMed] [Google Scholar]
- [134].Hussey AJ, Winardi M, Han XL, Thomas GP, Penington AJ, Morrison WA, Knight KR, Feeney SJ, Seeding of pancreatic islets into prevascularized tissue engineering chambers, Tissue Eng Part A 15(12) (2009) 3823–3833. [DOI] [PubMed] [Google Scholar]
- [135].Luan NM, Iwata H, Long-term allogeneic islet graft survival in prevascularized subcutaneous sites without immunosuppressive treatment, Am J Transplant 14(7) (2014) 1533–1542. [DOI] [PubMed] [Google Scholar]
- [136].Kawakami Y, Iwata H, Gu Y, Miyamoto M, Murakami Y, Yamasaki T, Cui W, Ikada Y, Imamura M, Inoue K, Modified subcutaneous tissue with neovascularization is useful as the site for pancreatic islet transplantation, Cell Transplant 9(5) (2000) 729–732. [DOI] [PubMed] [Google Scholar]
- [137].Gu Y, Tabata Y, Kawakami Y, Balamurugan AN, Hori H, Nagata N, Satake A, Cui W, Qi M, Misawa Y, Toma M, Miyamoto M, Nozawa M, Inoue K, Development of a new method to induce angiogenesis at subcutaneous site of streptozotocin-induced diabetic rats for islet transplantation, Cell Transplant 10(4–5) (2001) 453–457. [PubMed] [Google Scholar]
- [138].Kawakami Y, Iwata H, Gu YJ, Miyamoto M, Murakami Y, Balamurugan AN, Imamura M, Inoue K, Successful subcutaneous pancreatic islet transplantation using an angiogenic growth factor-releasing device, Pancreas 23(4) (2001) 375–381. [DOI] [PubMed] [Google Scholar]
- [139].Bowers DT, Olingy CE, Chhabra P, Langman L, Merrill PH, Linhart RS, Tanes ML, Lin D, Brayman KL, Botchwey EA, An engineered macroencapsulation membrane releasing FTY720 to precondition pancreatic islet transplantation, J Biomed Mater Res B Appl Biomater 106(2) (2018) 555–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Briquez PS, Clegg LE, Martino MM, Gabhann FM, Hubbell JA, Design principles for therapeutic angiogenic materials, Nature Reviews Materials 1(1) (2016). [Google Scholar]
- [141].Han D, Steckl AJ, Triaxial electrospun nanofiber membranes for controlled dual release of functional molecules, ACS Appl Mater Interfaces 5(16) (2013) 8241–8245. [DOI] [PubMed] [Google Scholar]
- [142].Zhang F, Tzanakakis ES, Optogenetic regulation of insulin secretion in pancreatic beta-cells, Sci Rep 7(1) (2017) 9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Sarode BR, Kover K, Tong PY, Zhang C, Friedman SH, Light control of insulin release and blood glucose using an injectable photoactivated depot, Mol Pharm 13(11) (2016) 3835–3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Teodorescu F, Oz Y, Queniat G, Abderrahmani A, Foulon C, Lecoeur M, Sanyal R, Sanyal A, Boukherroub R, Szunerits S, Photothermally triggered on-demand insulin release from reduced graphene oxide modified hydrogels, J Control Release 246 (2017) 164–173. [DOI] [PubMed] [Google Scholar]
- [145].Webber MJ, Engineering responsive supramolecular biomaterials: Toward smart therapeutics, Bioeng Transl Med 1(3) (2016) 252–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Yin N, Han Y, Xu H, Gao Y, Yi T, Yao J, Dong L, Cheng D, Chen Z, VEGF-conjugated alginate hydrogel prompt angiogenesis and improve pancreatic islet engraftment and function in type 1 diabetes, Mater Sci Eng C Mater Biol Appl 59 (2016) 958–964. [DOI] [PubMed] [Google Scholar]
- [147].Marchioli G, Luca AD, de Koning E, Engelse M, Van Blitterswijk CA, Karperien M, Van Apeldoorn AA, Moroni L, Hybrid polycaprolactone/alginate scaffolds functionalized with VEGF to promote de novo vessel formation for the transplantation of islets of Langerhans, Adv Healthc Mater 5(13) (2016) 1606–1616. [DOI] [PubMed] [Google Scholar]
- [148].Mao D, Zhu M, Zhang X, Ma R, Yang X, Ke T, Wang L, Li Z, Kong D, Li C, A macroporous heparin-releasing silk fibroin scaffold improves islet transplantation outcome by promoting islet revascularisation and survival, Acta Biomater 59 (2017) 210–220. [DOI] [PubMed] [Google Scholar]
- [149].Staels W, Verdonck Y, Heremans Y, Leuckx G, De Groef S, Heirman C, de Koning E, Gysemans C, Thielemans K, Baeyens L, Heimberg H, De Leu N, Vegf-A mRNA transfection as a novel approach to improve mouse and human islet graft revascularisation, Diabetologia 61(8) (2018) 1804–1810. [DOI] [PubMed] [Google Scholar]
- [150].Langlois A, Mura C, Bietiger W, Seyfritz E, Dollinger C, Peronet C, Maillard E, Pinget M, Jeandidier N, Sigrist S, In vitro and in vivo investigation of the angiogenic effects of liraglutide during islet transplantation, PLoS One 11(3) (2016) e0147068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Paget MB, Murray HE, Bailey CJ, Downing R, Pre-transplant signal induction for vascularisation in human islets, Diab Vasc Dis Res 10(6) (2013) 536–545. [DOI] [PubMed] [Google Scholar]
- [152].Zhang Y, An D, Pardo Y, Chiu A, Song W, Liu Q, Zhou F, McDonough SP, Ma M, High-water-content and resilient PEG-containing hydrogels with low fibrotic response, Acta Biomater 53 (2017) 100–108. [DOI] [PubMed] [Google Scholar]
- [153].Phelps EA, Headen DM, Taylor WR, Thule PM, Garcia AJ, Vasculogenic bio-synthetic hydrogel for enhancement of pancreatic islet engraftment and function in type 1 diabetes, Biomaterials 34(19) (2013) 4602–4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Terada T, Iwai M, Kawakami S, Yamashita F, Hashida M, Novel PEG-matrix metalloproteinase-2 cleavable peptide-lipid containing galactosylated liposomes for hepatocellular carcinoma-selective targeting, J Control Release 111(3) (2006) 333–342. [DOI] [PubMed] [Google Scholar]
- [155].Lin CC, Anseth KS, PEG hydrogels for the controlled release of biomolecules in regenerative medicine, Pharm Res 26(3) (2009) 631–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Marchioli G, Zellner L, Oliveira C, Engelse M, Koning E, Mano J, Karperien AV Apeldoorn, L. Moroni, Layered PEGDA hydrogel for islet of Langerhans encapsulation and improvement of vascularization, J Mater Sci Mater Med 28(12) (2017) 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Phelps EA, Templeman KL, Thule PM, Garcia AJ, Engineered VEGF-releasing PEG-MAL hydrogel for pancreatic islet vascularization, Drug Deliv Transl Res 5(2) (2015) 125–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Weaver JD, Headen DM, Aquart J, Johnson CT, Shea LD, Shirwan H, Garcia AJ, Vasculogenic hydrogel enhances islet survival, engraftment, and function in leading extrahepatic sites, Sci Adv 3(6) (2017) e1700184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Hunter SK, Kao JM, Wang Y, Benda JA, Rodgers VG, Promotion of neovascularization around hollow fiber bioartificial organs using biologically active substances, ASAIO J 45(1) (1999) 37–40. [DOI] [PubMed] [Google Scholar]
- [160].Terranova VP, DiFlorio R, Lyall RM, Hic S, Friesel R, Maciag T, Human endothelial cells are chemotactic to endothelial cell growth factor and heparin, J Cell Biol 101(6) (1985) 2330–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Mirsky N, Cohen Y, VEGF and ECGF induce directed migration of endothelial cells: qualitative and quantitative assay, Endothelium 3(1) (2009) 13–20. [Google Scholar]
- [162].Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C, VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia, J Cell Biol 161(6) (2003) 1163–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Tilakaratne HK, Hunter SK, Andracki ME, Benda JA, Rodgers VG, Characterizing short-term release and neovascularization potential of multi-protein growth supplement delivered via alginate hollow fiber devices, Biomaterials 28(1) (2007) 89–98. [DOI] [PubMed] [Google Scholar]
- [164].Witkowski P, Sondermeijer H, Hardy MA, Woodland DC, Lee K, Bhagat G, Witkowski K, See F, Rana A, Maffei A, Itescu S, Harris PE, Islet grafting and imaging in a bioengineered intramuscular space, Transplantation 88(9) (2009) 1065–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Sun G, Shen YI, Kusuma S, Fox-Talbot K, Steenbergen CJ, Gerecht S, Functional neovascularization of biodegradable dextran hydrogels with multiple angiogenic growth factors, Biomaterials 32(1) (2011) 95–106. [DOI] [PubMed] [Google Scholar]
- [166].Tengood JE, Kovach KM, Vescovi PE, Russell AJ, Little SR, Sequential delivery of vascular endothelial growth factor and sphingosine 1-phosphate for angiogenesis, Biomaterials 31(30) (2010) 7805–7812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Chen RR, Silva EA, Yuen WW, Mooney DJ, Spatio-temporal VEGF and PDGF delivery patterns blood vessel formation and maturation, Pharm Res 24(2) (2007) 258–264. [DOI] [PubMed] [Google Scholar]
- [168].Brudno Y, Ennett-Shepard AB, Chen RR, Aizenberg M, Mooney DJ, Enhancing microvascular formation and vessel maturation through temporal control over multiple pro-angiogenic and pro-maturation factors, Biomaterials 34(36) (2013) 9201–9209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Ishihara J, Ishihara A, Fukunaga K, Sasaki K, White MJV, Briquez PS, Hubbell JA, Laminin heparin-binding peptides bind to several growth factors and enhance diabetic wound healing, Nat Commun 9(1) (2018) 2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Darden J, Payne LB, Zhao H, Chappell JC, Excess vascular endothelial growth factor-A disrupts pericyte recruitment during blood vessel formation, Angiogenesis (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Martin P, Wound healing--aiming for perfect skin regeneration, Science 276(5309) (1997) 75–81. [DOI] [PubMed] [Google Scholar]
- [172].Singer AJ, Clark RA, Cutaneous wound healing, N Engl J Med 341(10) (1999) 738–746. [DOI] [PubMed] [Google Scholar]
- [173].Nissen NN, Polverini PJ, Koch AE, Volin MV, Gamelli RL, DiPietro LA, Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing, Am J Pathol 152(6) (1998) 1445–1452. [PMC free article] [PubMed] [Google Scholar]
- [174].Seghezzi G, Patel S, Ren CJ, Gualandris A, Pintucci G, Robbins ES, Shapiro RL, Galloway AC, Rifkin DB, Mignatti P, Fibroblast growth factor-2 (FGF-2) induces vascular endothelial growth factor (VEGF) expression in the endothelial cells of forming capillaries: an autocrine mechanism contributing to angiogenesis, J Cell Biol 141(7) (1998) 1659–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Van Hove AH, Benoit DS, Depot-based delivery systems for pro-angiogenic peptides: a review, Front Bioeng Biotechnol 3 (2015) 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Stendahl JC, Wang LJ, Chow LW, Kaufman DB, Stupp SI, Growth factor delivery from self-assembling nanofibers to facilitate islet transplantation, Transplantation 86(3) (2008) 478–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Uzunalli G, Tumtas Y, Delibasi T, Yasa O, Mercan S, Guler MO, Tekinay AB, Improving pancreatic islet in vitro functionality and transplantation efficiency by using heparin mimetic peptide nanofiber gels, Acta Biomater 22 (2015) 8–18. [DOI] [PubMed] [Google Scholar]
- [178].Guc E, Briquez PS, Foretay D, Fankhauser MA, Hubbell JA, Kilarski WW, Swartz MA, Local induction of lymphangiogenesis with engineered fibrin-binding VEGF-C promotes wound healing by increasing immune cell trafficking and matrix remodeling, Biomaterials 131 (2017) 160–175. [DOI] [PubMed] [Google Scholar]
- [179].Korsgren E, Korsgren O, An apparent deficiency of lymphatic capillaries in the islets of Langerhans in the human pancreas, Diabetes 65(4) (2016) 1004–1008. [DOI] [PubMed] [Google Scholar]
- [180].Penaranda C, Tang Q, Ruddle NH, Bluestone JA, Prevention of diabetes by FTY720-mediated stabilization of peri-islet tertiary lymphoid organs, Diabetes 59(6) (2010) 1461–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Yin N, Zhang N, Xu J, Shi Q, Ding Y, Bromberg JS, Targeting lymphangiogenesis after islet transplantation prolongs islet allograft survival, Transplantation 92(1) (2011) 25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Dietrich T, Bock F, Yuen D, Hos D, Bachmann BO, Zahn G, Wiegand S, Chen L, Cursiefen C, Cutting edge: lymphatic vessels, not blood vessels, primarily mediate immune rejections after transplantation, J Immunol 184(2) (2010) 535–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Nguyen DH, Stapleton SC, Yang MT, Cha SS, Choi CK, Galie PA, Chen CS, Biomimetic model to reconstitute angiogenic sprouting morphogenesis in vitro, Proc Natl Acad Sci U S A 110(17) (2013) 6712–6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Ghajar CM, Blevins KS, Hughes CC, George SC, Putnam AJ, Mesenchymal stem cells enhance angiogenesis in mechanically viable prevascularized tissues via early matrix metalloproteinase upregulation, Tissue Eng 12(10) (2006) 2875–2888. [DOI] [PubMed] [Google Scholar]
- [185].Zacharek A, Chen J, Cui X, Li A, Li Y, Roberts C, Feng Y, Gao Q, Chopp M, Angiopoietin1/Tie2 and VEGF/Flk1 induced by MSC treatment amplifies angiogenesis and vascular stabilization after stroke, J Cereb Blood Flow Metab 27(10) (2007) 1684–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Ball SG, Shuttleworth CA, Kielty CM, Mesenchymal stem cells and neovascularization: role of platelet-derived growth factor receptors, J Cell Mol Med 11(5) (2007) 1012–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Berman DM, Willman MA, Han D, Kleiner G, Kenyon NM, Cabrera O, Karl JA, Wiseman RW, O’Connor DH, Bartholomew AM, Kenyon NS, Mesenchymal stem cells enhance allogeneic islet engraftment in nonhuman primates, Diabetes 59(10) (2010) 2558–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Ding Y, Xu D, Feng G, Bushell A, Muschel RJ, Wood KJ, Mesenchymal stem cells prevent the rejection of fully allogenic islet grafts by the immunosuppressive activity of matrix metalloproteinase-2 and -9, Diabetes 58(8) (2009) 1797–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Liu H, Chen B, Lilly B, Fibroblasts potentiate blood vessel formation partially through secreted factor TIMP-1, Angiogenesis 11(3) (2008) 223–234. [DOI] [PubMed] [Google Scholar]
- [190].Hayward JA, Ellis CE, Seeberger K, Lee T, Salama B, Mulet-Sierra A, Kuppan P, Adesida A, Korbutt GS, Cotransplantation of mesenchymal stem cells with neonatal porcine islets improve graft function in diabetic mice, Diabetes 66(5) (2017) 1312–1321. [DOI] [PubMed] [Google Scholar]
- [191].Veriter S, Gianello P, Igarashi Y, Beaurin G, Ghyselinck A, Aouassar N, Jordan B, Gallez B, Dufrane D, Improvement of subcutaneous bioartificial pancreas vascularization and function by coencapsulation of pig islets and mesenchymal stem cells in primates, Cell Transplant 23(11) (2014) 1349–1364. [DOI] [PubMed] [Google Scholar]
- [192].Oh BJ, Jin SM, Choi JM, Oh SH, Shim W, Lee MS, Lee MK, Kim JH, Improved revascularization of islet grafts using an angiogenic monocyte subpopulation derived from spheroid culture of bone marrow mononuclear cells, Am J Transplant 15(6) (2015) 1543–1554. [DOI] [PubMed] [Google Scholar]
- [193].Hajizadeh-Saffar E, Tahamtani Y, Aghdami N, Azadmanesh K, Habibi-Anbouhi M, Heremans Y, De Leu N, Heimberg H, Ravassard P, Shokrgozar MA, Baharvand H, Inducible VEGF expression by human embryonic stem cell-derived mesenchymal stromal cells reduces the minimal islet mass required to reverse diabetes, Sci Rep 5 (2015) 9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Perez-Basterrechea M, Briones RM, Alvarez-Viejo M, Garcia-Perez E, Esteban MM, Garcia V, Obaya AJ, Barneo L, Meana A, Otero J, Plasma-fibroblast gel as scaffold for islet transplantation, Tissue Eng Part A 15(3) (2009) 569–577. [DOI] [PubMed] [Google Scholar]
- [195].Takebe T, Zhang RR, Koike H, Kimura M, Yoshizawa E, Enomura M, Koike N, Sekine K, Taniguchi H, Generation of a vascularized and functional human liver from an iPSC-derived organ bud transplant, Nat Protoc 9(2) (2014) 396–409. [DOI] [PubMed] [Google Scholar]
- [196].Takebe T, Enomura M, Yoshizawa E, Kimura M, Koike H, Ueno Y, Matsuzaki T, Yamazaki T, Toyohara T, Osafune K, Nakauchi H, Yoshikawa HY, Taniguchi H, Vascularized and complex organ buds from diverse tissues via mesenchymal cell-driven condensation, Cell Stem Cell 16(5) (2015) 556–565. [DOI] [PubMed] [Google Scholar]
- [197].Takebe T, Sekine K, Enomura M, Koike H, Kimura M, Ogaeri T, Zhang RR, Ueno Y, Zheng YW, Koike N, Aoyama S, Adachi Y, Taniguchi H, Vascularized and functional human liver from an iPSC-derived organ bud transplant, Nature 499(7459) (2013) 481–484. [DOI] [PubMed] [Google Scholar]
- [198].Takahashi Y, Sekine K, Kin T, Takebe T, Taniguchi H, Self-condensation culture enables vascularization of tissue fragments for efficient therapeutic transplantation, Cell Rep 23(6) (2018) 1620–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Cunha JPMCM, Leuckx G, Sterkendries P, Korf H, Bomfim-Ferreira G, Overbergh L, Vaes B, Heimberg H, Gysemans C, Mathieu C, Human multipotent adult progenitor cells enhance islet function and revascularisation when co-transplanted as a composite pellet in a mouse model of diabetes, Diabetologia 60(1) (2017) 134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Li Y, Xue W, Liu H, Fan P, Wang X, Ding X, Tian X, Feng X, Pan X, Zheng J, Tian P, Ding C, Fan X, Combined strategy of endothelial cells coating, Sertoli cells coculture and infusion improves vascularization and rejection protection of islet graft, PLoS One 8(2) (2013) e56696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Johansson U, Rasmusson I, Niclou SP, Forslund N, Gustavsson L, Nilsson B, Korsgren O, Magnusson PU, Formation of composite endothelial cell-mesenchymal stem cell islets: a novel approach to promote islet revascularization, Diabetes 57(9) (2008) 2393–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Hirabaru M, Kuroki T, Adachi T, Kitasato A, Ono S, Tanaka T, Matsushima H, Sakai Y, Soyama A, Hidaka M, Yamanouchi K, Takatsuki M, Okano T, Eguchi S, A method for performing islet transplantation using tissue-engineered sheets of islets and mesenchymal stem cells, Tissue Eng Part C Methods 21(12) (2015) 1205–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Cabric S, Sanchez J, Johansson U, Larsson R, Nilsson B, Korsgren O, Magnusson PU, Anchoring of vascular endothelial growth factor to surface-immobilized heparin on pancreatic islets: implications for stimulating islet angiogenesis, Tissue Eng Part A 16(3) (2010) 961–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Butler MJ, Sefton MV, Cotransplantation of adipose-derived mesenchymal stromal cells and endothelial cells in a modular construct drives vascularization in SCID/bg mice, Tissue Eng Part A 18(15-16) (2012) 1628–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Stolzing A, Sellers D, Llewelyn O, Scutt A, Diabetes induced changes in rat mesenchymal stem cells, Cells Tissues Organs 191(6) (2010) 453–465. [DOI] [PubMed] [Google Scholar]
- [206].Wang J, Liao LM, Wang SL, Tan JM, Cell therapy with autologous mesenchymal stem cells-how the disease process impacts clinical considerations, Cytotherapy 15(8) (2013) 893–904. [DOI] [PubMed] [Google Scholar]
- [207].Choudhery MS, Khan M, Mahmood R, Mehmood A, Khan SN, Riazuddin S, Bone marrow derived mesenchymal stem cells from aged mice have reduced wound healing, angiogenesis, proliferation and anti-apoptosis capabilities, Cell Biol Int 36(8) (2012) 747–753. [DOI] [PubMed] [Google Scholar]
- [208].Fan M, Chen W, Liu W, Du GQ, Jiang SL, Tian WC, Sun L, Li RK, Tian H, The effect of age on the efficacy of human mesenchymal stem cell transplantation after a myocardial infarction, Rejuvenation Res 13(4) (2010) 429–438. [DOI] [PubMed] [Google Scholar]
- [209].Fossett E, Khan WS, Pastides P, Adesida AB, The effects of ageing on proliferation potential, differentiation potential and cell surface characterisation of human mesenchymal stem cells, Curr Stem Cell Res Ther 7(4) (2012) 282–286. [DOI] [PubMed] [Google Scholar]
- [210].Wang K, Lin RZ, Melero-Martin JM, Bioengineering human vascular networks: trends and directions in endothelial and perivascular cell sources, Cell Mol Life Sci (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Takahashi K, Yamanaka S, Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors, Cell 126(4) (2006) 663–676. [DOI] [PubMed] [Google Scholar]
- [212].Jin S, Yao H, Weber JL, Melkoumian ZK, Ye K, A synthetic, xeno-free peptide surface for expansion and directed differentiation of human induced pluripotent stem cells, PLoS One 7(11) (2012) e50880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Takasato M, Er PX, Chiu HS, Maier B, Baillie GJ, Ferguson C, Parton RG, Wolvetang EJ, Roost MS, Chuva de Sousa Lopes SM, Little MH, Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis, Nature 526(7574) (2015) 564–568. [DOI] [PubMed] [Google Scholar]
- [214].Lippmann ES, Azarin SM, Kay JE, Nessler RA, Wilson HK, Al-Ahmad A, Palecek SP, Shusta EV, Derivation of blood-brain barrier endothelial cells from human pluripotent stem cells, Nat Biotechnol 30(8) (2012) 783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Wang Y, Hu J, Jiao J, Liu Z, Zhou Z, Zhao C, Chang LJ, Chen YE, Ma PX, Yang B, Engineering vascular tissue with functional smooth muscle cells derived from human iPS cells and nanofibrous scaffolds, Biomaterials 35(32) (2014) 8960–8969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Xie CQ, Huang H, Wei S, Song LS, Zhang J, Ritchie RP, Chen L, Zhang M, Chen YE, A comparison of murine smooth muscle cells generated from embryonic versus induced pluripotent stem cells, Stem Cells Dev 18(5) (2009) 741–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Schenke-Layland K, Rhodes KE, Angelis E, Butylkova Y, Heydarkhan-Hagvall S, Gekas C, Zhang R, Goldhaber JI, Mikkola HK, Plath K, MacLellan WR, Reprogrammed mouse fibroblasts differentiate into cells of the cardiovascular and hematopoietic lineages, Stem Cells 26(6) (2008) 1537–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Millman JR, Xie C, Van Dervort A, Gurtler M, Pagliuca FW, Melton DA, Generation of stem cell-derived beta-cells from patients with type 1 diabetes, Nat Commun 7 (2016) 11463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Pagliuca FW, Millman JR, Gurtler M, Segel M, Van Dervort A, Ryu JH, Peterson QP, Greiner D, Melton DA, Generation of functional human pancreatic beta cells in vitro, Cell 159(2) (2014) 428–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Ma H, Wert KJ, Shvartsman D, Melton DA, Jaenisch R, Establishment of human pluripotent stem cell-derived pancreatic beta-like cells in the mouse pancreas, Proc Natl Acad Sci U S A 115(15) (2018) 3924–3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Rezania A, Bruin JE, Riedel MJ, Mojibian M, Asadi A, Xu J, Gauvin R, Narayan K, Karanu F, O’Neil JJ, Ao Z, Warnock GL, Kieffer TJ, Maturation of human embryonic stem cell-derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice, Diabetes 61(8) (2012) 2016–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Rezania A, Bruin JE, Arora P, Rubin A, Batushansky I, Asadi A, O’Dwyer S, Quiskamp N, Mojibian M, Albrecht T, Yang YH, Johnson JD, Kieffer TJ, Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells, Nat Biotechnol 32(11) (2014) 1121–1133. [DOI] [PubMed] [Google Scholar]
- [223].Peterson Q, Melton D, Small-molecule modulators of Nkx6.1 expression in pancreatic progenitor cells during directed differentiation of human embryonic stem cell to insulin producing beta cells, The FASEB Journal 27(1_supplement) (2013) 1010.1012. [Google Scholar]
- [224].Wang X, Ye K, Three-dimensional differentiation of embryonic stem cells into islet-like insulin-producing clusters, Tissue Eng Part A 15(8) (2009) 1941–1952. [DOI] [PubMed] [Google Scholar]
- [225].Boddupalli A, Zhu L, Bratlie KM, Methods for implant acceptance and wound healing: material selection and implant location modulate macrophage and fibroblast phenotypes, Adv Healthc Mater 5(20) (2016) 2575–2594. [DOI] [PubMed] [Google Scholar]
- [226].Morini S, Brown ML, Cicalese L, Elias G, Carotti S, Gaudio E, Rastellini C, Revascularization and remodelling of pancreatic islets grafted under the kidney capsule, J Anat 210(5) (2007) 565–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Szot GL, Koudria P, Bluestone JA, Transplantation of pancreatic islets into the kidney capsule of diabetic mice, J Vis Exp (9) (2007) 404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Lazard D, Vardi P, Bloch K, Anti-diabetic and neuroprotective effects of pancreatic islet transplantation into the central nervous system, Diabetes Metab Res Rev 32(1) (2016) 11–20. [DOI] [PubMed] [Google Scholar]
- [229].Brissova M, Powers AC, Revascularization of transplanted islets: can it be improved?, Diabetes 57(9) (2008) 2269–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Vajkoczy P, Menger MD, Simpson E, Messmer K, Angiogenesis and vascularization of murine pancreatic islet isografts, Transplantation 60(2) (1995) 123–127. [PubMed] [Google Scholar]
- [231].Baranski JD, Chaturvedi RR, Stevens KR, Eyckmans J, Carvalho B, Solorzano RD, Yang MT, Miller JS, Bhatia SN, Chen CS, Geometric control of vascular networks to enhance engineered tissue integration and function, Proc Natl Acad Sci U S A 110(19) (2013) 7586–7591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Raghavan S, Nelson CM, Baranski JD, Lim E, Chen CS, Geometrically controlled endothelial tubulogenesis in micropatterned gels, Tissue Eng Part A 16(7) (2010) 2255–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Chaturvedi RR, Stevens KR, Solorzano RD, Schwartz RE, Eyckmans J, Baranski JD, Stapleton SC, Bhatia SN, Chen CS, Patterning vascular networks in vivo for tissue engineering applications, Tissue Eng Part C 21(5) (2015) 509–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Stevens KR, Scull MA, Ramanan V, Fortin CL, Chaturvedi RR, Knouse KA, Xiao JW, Fung C, Mirabella T, Chen AX, McCue MG, Yang MT, Fleming HE, Chung K, de Jong YP, Chen CS, Rice CM, Bhatia SN, In situ expansion of engineered human liver tissue in a mouse model of chronic liver disease, Sci Transl Med 9(399) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Komatsu H, Kandeel F, Mullen Y, Impact of oxygen on pancreatic islet survival, Pancreas 47(5) (2018) 533–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Ludwig B, Rotem A, Schmid J, Weir GC, Colton CK, Brendel MD, Neufeld T, Block NL, Yavriyants K, Steffen A, Ludwig S, Chavakis T, Reichel A, Azarov D, Zimermann B, Maimon S, Balyura M, Rozenshtein T, Shabtay N, Vardi P, Bloch K, de Vos P, Schally AV, Bornstein SR, Barkai U, Improvement of islet function in a bioartificial pancreas by enhanced oxygen supply and growth hormone releasing hormone agonist, Proc Natl Acad Sci U S A 109(13) (2012) 5022–5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Barkai U, Weir GC, Colton CK, Ludwig B, Bornstein SR, Brendel MD, Neufeld T, Bremer C, Leon A, Evron Y, Yavriyants K, Azarov D, Zimermann B, Maimon S, Shabtay N, Balyura M, Rozenshtein T, Vardi P, Bloch K, de Vos P, Rotem A, Enhanced oxygen supply improves islet viability in a new bioartificial pancreas, Cell Transplant 22(8) (2013) 1463–1476. [DOI] [PubMed] [Google Scholar]
- [238].Ludwig B, Reichel A, Steffen A, Zimerman B, Schally AV, Block NL, Colton CK, Ludwig S, Kersting S, Bonifacio E, Solimena M, Gendler Z, Rotem A, Barkai U, Bornstein SR, Transplantation of human islets without immunosuppression, Proc Natl Acad Sci U S A 110(47) (2013) 19054–19058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Pedraza E, Coronel MM, Fraker CA, Ricordi C, Stabler CL, Preventing hypoxia-induced cell death in beta cells and islets via hydrolytically activated, oxygen-generating biomaterials, Proc Natl Acad Sci U S A 109(11) (2012) 4245–4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Wu H, Avgoustiniatos ES, Swette L, Bonner-Weir S, Weir GC, Colton CK, In situ electrochemical oxygen generation with an immunoisolation device, Ann N Y Acad Sci 875 (1999) 105–125. [DOI] [PubMed] [Google Scholar]
- [241].Bloch K, Papismedov E, Yavriyants K, Vorobeychik M, Beer S, Vardi P, Photosynthetic oxygen generator for bioartificial pancreas, Tissue Eng 12(2) (2006) 337–344. [DOI] [PubMed] [Google Scholar]
- [242].Rodriguez-Brotons A, Bietiger W, Peronet C, Langlois A, Magisson J, Mura C, Sookhareea C, Polard V, Jeandidier N, Zal F, Pinget M, Sigrist S, Maillard E, Comparison of perfluorodecalin and HEMOXCell as oxygen carriers for islet oxygenation in an in vitro model of encapsulation, Tissue Eng Part A 22(23–24) (2016) 1327–1336. [DOI] [PubMed] [Google Scholar]
- [243].Kelly AC, Smith KE, Purvis WG, Min CG, Weber CS, Cooksey AM, Hasilo C, Paraskevas S, Suszynski TM, Weegman BP, Anderson MJ, Camacho LE, Harland RC, Loudovaris T, Jandova J, Molano DS, Price ND, Georgiev IG, Scott WE 3rd, Manas DMD, Shaw JAM, O’Gorman D, Kin T, McCarthy FM, Szot GL, Posselt AM, Stock PG, Karatzas T, Shapiro AMJ, Lynch RM, Limesand SW, Papas KK, Oxygen perfusion (persufflation) of human pancreata enhances insulin secretion and attenuates islet proinflammatory signaling, Transplantation 103(1) (2019) 160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Noguchi H, Levy MF, Kobayashi N, Matsumoto S, Pancreas preservation by the two-layer method: does it have a beneficial effect compared with simple preservation in University of Wisconsin solution?, Cell Transplant 18(5) (2009) 497–503. [DOI] [PubMed] [Google Scholar]
- [245].Lau J, Henriksnas J, Svensson J, Carlsson PO, Oxygenation of islets and its role in transplantation, Curr Opin Organ Transplant 14(6) (2009) 688–693. [DOI] [PubMed] [Google Scholar]
- [246].Coronel MM, Stabler CL, Engineering a local microenvironment for pancreatic islet replacement, Curr Opin Biotechnol 24(5) (2013) 900–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].Buitinga M, Assen F, Hanegraaf M, Wieringa P, Hilderink J, Moroni L, Truckenmuller R, van Blitterswijk C, Romer GW, Carlotti F, de Koning E, Karperien M, van Apeldoorn A, Micro-fabricated scaffolds lead to efficient remission of diabetes in mice, Biomaterials 135 (2017) 10–22. [DOI] [PubMed] [Google Scholar]
- [248].Rodriguez-Brotons A, Bietiger W, Peronet C, Magisson J, Sookhareea C, Langlois A, Mura C, Jeandidier N, Pinget M, Sigrist S, Maillard E, Impact of pancreatic rat islet density on cell survival during hypoxia, J Diabetes Res 2016 (2016) 3615286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Wang W, Upshaw L, Strong DM, Robertson RP, Reems J, Increased oxygen consumption rates in response to high glucose detected by a novel oxygen biosensor system in non-human primate and human islets, J Endocrinol 185(3) (2005) 445–455. [DOI] [PubMed] [Google Scholar]
- [250].Papas KK, Avgoustiniatos ES, Tempelman LA, Weir GC, Colton CK, Pisania A, Rappel MJ, Friberg AS, Bauer AC, Hering BJ, High-density culture of human islets on top of silicone rubber membranes, Transplant Proc 37(8) (2005) 3412–3414. [DOI] [PubMed] [Google Scholar]
- [251].Shweiki D, Itin A, Soffer D, Keshet E, Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis, Nature 359(6398) (1992) 843–845. [DOI] [PubMed] [Google Scholar]
- [252].Miao G, Ostrowski RP, Mace J, Hough J, Hopper A, Peverini R, Chinnock R, Zhang J, Hathout E, Dynamic production of hypoxia-inducible factor-1alpha in early transplanted islets, Am J Transplant 6(11) (2006) 2636–2643. [DOI] [PubMed] [Google Scholar]
- [253].Vasir B, Aiello LP, Yoon KH, Quickel RR, Bonner-Weir S, Weir GC, Hypoxia induces vascular endothelial growth factor gene and protein expression in cultured rat islet cells, Diabetes 47(12) (1998) 1894–1903. [DOI] [PubMed] [Google Scholar]
- [254].Gibly RF, Zhang X, Graham ML, Hering BJ, Kaufman DB, Lowe WL Jr., Shea LD, Extrahepatic islet transplantation with microporous polymer scaffolds in syngeneic mouse and allogeneic porcine models, Biomaterials 32(36) (2011) 9677–9684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].Song HG, Rumma RT, Ozaki CK, Edelman ER, Chen CS, Vascular tissue engineering: progress, challenges, and clinical promise, Cell Stem Cell 22(3) (2018) 340–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [256].Bersini S, Yazdi IK, Talo G, Shin SR, Moretti M, Khademhosseini A, Cell-microenvironment interactions and architectures in microvascular systems, Biotechnol Adv 34(6) (2016) 1113–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Gladman AS, Matsumoto EA, Nuzzo RG, Mahadevan L, Lewis JA, Biomimetic 4D printing, Nat Mater 15(4) (2016) 413–418. [DOI] [PubMed] [Google Scholar]
- [258].Kang HW, Lee SJ, Ko IK, Kengla C, Yoo JJ, Atala A, A 3D bioprinting system to produce human-scale tissue constructs with structural integrity, Nat Biotechnol 34(3) (2016) 312–319. [DOI] [PubMed] [Google Scholar]
- [259].Miller JS, Stevens KR, Yang MT, Baker BM, Nguyen DHT, Cohen DM, Toro E, Chen AA, Galie PA, Yu X, Chaturvedi R, Bhatia SN, Chen CS, Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues, Nat Mater 11(9) (2012) 768–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [260].Kolesky DB, Truby RL, Gladman AS, Busbee TA, Homan KA, Lewis JA, 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs, Adv Mater 26(19) (2014) 3124–3130. [DOI] [PubMed] [Google Scholar]
- [261].Jia W, Gungor-Ozkerim PS, Zhang YS, Yue K, Zhu K, Liu W, Pi Q, Byambaa B, Dokmeci MR, Shin SR, Khademhosseini A, Direct 3D bioprinting of perfusable vascular constructs using a blend bioink, Biomaterials 106 (2016) 58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [262].Lee VK, Kim DY, Ngo H, Lee Y, Seo L, Yoo SS, Vincent PA, Dai G, Creating perfused functional vascular channels using 3D bio-printing technology, Biomaterials 35(28) (2014) 8092–8102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263].Zhang YS, Arneri A, Bersini S, Shin SR, Zhu K, Goli-Malekabadi Z, Aleman J, Colosi C, Busignani F, Dell’Erba V, Bishop C, Shupe T, Demarchi D, Moretti M, Rasponi M, Dokmeci MR, Atala A, Khademhosseini A, Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip, Biomaterials 110 (2016) 45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [264].Kolesky DB, Homan KA, Skylar-Scott MA, Lewis JA, Three-dimensional bioprinting of thick vascularized tissues, Proc Natl Acad Sci U S A 113(12) (2016) 3179–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [265].Lee VK, Lanzi AM, Haygan N, Yoo SS, Vincent PA, Dai G, Generation of multi-scale vascular network system within 3D hydrogel using 3D bio-printing technology, Cell Mol Bioeng 7(3) (2014) 460–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [266].Saenz Del Burgo L, Ciriza J, Espona-Noguera A, Illa X, Cabruja E, Orive G, Her nandez RM, Villa R, Pedraz JL, Alvarez M, 3D Printed porous polyamide macrocapsule combined with alginate microcapsules for safer cell-based therapies, Sci Rep 8(1) (2018) 8512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [267].Farina M, Ballerini A, Fraga DW, Nicolov E, Hogan M, Demarchi D, Scaglione F, Sabek OM, Horner P, Thekkedath U, Gaber OA, Grattoni A, 3D printed vascularized device for subcutaneous transplantation of human islets, Biotechnol J 12(9) (2017). [DOI] [PubMed] [Google Scholar]
- [268].Sabek OM, Farina M, Fraga DW, Afshar S, Ballerini A, Filgueira CS, Thekkedath UR, Grattoni A, Gaber AO, Three-dimensional printed polymeric system to encapsulate human mesenchymal stem cells differentiated into islet-like insulin-producing aggregates for diabetes treatment, J Tissue Eng 7(2016)2041731416638198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [269].Marchioli G, van Gurp L, van Krieken PP, Stamatialis D, Engelse M, van Blitterswijk CA, Karperien MB, de Koning E, Alblas J, Moroni L, van Apeldoorn AA, Fabrication of three-dimensional bioplotted hydrogel scaffolds for islets of Langerhans transplantation, Biofabrication 7(2) (2015) 025009. [DOI] [PubMed] [Google Scholar]
- [270].Song J, Millman JR, Economic 3D-printing approach for transplantation of human stem cell-derived beta-like cells, Biofabrication 9(1) (2016) 015002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [271].Daoud JT, Petropavlovskaia MS, Patapas JM, Degrandpre CE, Diraddo RW, Rosenberg L, Tabrizian M, Long-term in vitro human pancreatic islet culture using three-dimensional microfabricated scaffolds, Biomaterials 32(6) (2011) 1536–1542. [DOI] [PubMed] [Google Scholar]
- [272].Nillesen ST, Geutjes PJ, Wismans R, Schalkwijk J, Daamen WF, van Kuppevelt TH, Increased angiogenesis and blood vessel maturation in acellular collagen-heparin scaffolds containing both FGF2 and VEGF, Biomaterials 28(6) (2007) 1123–1131. [DOI] [PubMed] [Google Scholar]
- [273].Motz GT, Coukos G, The parallel lives of angiogenesis and immunosuppression: cancer and other tales, Nat Rev Immunol 11(10) (2011) 702–711. [DOI] [PubMed] [Google Scholar]
- [274].Christoffersson G, Henriksnas J, Johansson L, Rolny C, Ahlstrom H, Caballero-Corbalan J, Segersvard R, Permert J, Korsgren O, Carlsson PO, Phillipson M, Clinical and experimental pancreatic islet transplantation to striated muscle: establishment of a vascular system similar to that in native islets, Diabetes 59(10) (2010) 2569–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [275].Bowers DT, Chhabra P, Langman L, Botchwey EA, Brayman KL, FTY720-loaded poly(DL-lactide-co-glycolide) electrospun scaffold significantly increases microvessel density over 7 days in streptozotocin-induced diabetic C57b16/J mice: preliminary results, Transplant Proc 43(9) (2011) 3285–3287. [DOI] [PubMed] [Google Scholar]
- [276].Nishimura R, Goto M, Sekiguchi S, Fujimori K, Ushiyama A, Satomi S, Assessment for revascularization of transplanted pancreatic islets at subcutaneous site in mice with a highly sensitive imaging system, Transplant Proc 43(9) (2011) 3239–3240. [DOI] [PubMed] [Google Scholar]
- [277].Yang M, Stapor PC, Peirce SM, Betancourt AM, Murfee WL, Rat mesentery exteriorization: a model for investigating the cellular dynamics involved in angiogenesis, J Vis Exp (63) (2012) e3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [278].Glaw JT, Skalak TC, Peirce SM, Inhibition of canonical Wnt signaling increases microvascular hemorrhaging and venular remodeling in adult rats, Microcirculation 17(5) (2010) 348–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [279].Hathout E, Sowers L, Wang R, Tan A, Mace J, Peverini R, Chinnock R, Obenaus A, In vivo magnetic resonance imaging of vascularization in islet transplantation, Transpl Int 20(12) (2007) 1059–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [280].Kriz J, Jirak D, Vilk GJ, Girman P, White DJ, Hajek M, Saudek F, Vascularization of artificial beds for pancreatic islet transplantation in a rat model, Transplant Proc 42(6) (2010) 2097–2101. [DOI] [PubMed] [Google Scholar]
- [281].Sakata N, Sax N, Yoshimatsu G, Tsuchiya H, Kato S, Aoki T, Ishida M, Katayose Y, Egawa S, Kodama T, Unno M, Enhanced ultrasonography using a nano/microbubble contrast agent for islet transplantation, Am J Transplant 15(6) (2015) 1531–1542. [DOI] [PubMed] [Google Scholar]
- [282].Jakobsson L, Franco CA, Bentley K, Collins RT, Ponsioen B, Aspalter IM, Rosewell I, Busse M, Thurston G, Medvinsky A, Schulte-Merker S, Gerhardt H, Endothelial cells dynamically co mpete for the tip cell position during angiogenic sprouting, Nat Cell Biol 12(10) (2010) 943–953. [DOI] [PubMed] [Google Scholar]
- [283].Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P, Bone marrow cells regenerate infarcted myocardium, Nature 410(6829) (2001) 701–705. [DOI] [PubMed] [Google Scholar]
- [284].Poche RA, Larina IV, Scott ML, Saik JE, West JL, Dickinson ME, The Flk1-myr::mCherry mouse as a useful reporter to characterize multiple aspects of ocular blood vessel development and disease, Dev Dyn 238(9) (2009) 2318–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [285].Pusztaszeri MP, Seelentag W, Bosman FT, Immunohistochemical expression of endothelial markers CD31, CD34, von Willebrand factor, and Fli-1 in normal human tissues, J Histochem Cytochem 54(4) (2006) 385–395. [DOI] [PubMed] [Google Scholar]
- [286].Miettinen M, Lindenmayer AE, Chaubal A, Endothelial cell markers CD31, CD34, and BNH9 antibody to H- and Y-antigens--evaluation of their specificity and sensitivity in the diagnosis of vascular tumors and comparison with von Willebrand factor, Mod Pathol 7(1) (1994) 82–90. [PubMed] [Google Scholar]
- [287].Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM, Isolation of putative progenitor endothelial cells for angiogenesis, Science 275(5302) (1997) 964–967. [DOI] [PubMed] [Google Scholar]
- [288].Jerosch-Herold M, Quantification of myocardial perfusion by cardiovascular magnetic resonance, J Cardiovasc Magn Reson 12 (2010) 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [289].Malmstrom ML, Saftoiu A, Riis LB, Hassan H, Klausen TW, Rahbek MS, Gogenur I, Vilmann P, Dynamic contrast-enhanced EUS for quantification of tumor perfusion in colonic cancer: a prospective cohort study, Gastrointest Endosc 87(6) (2018) 1530–1538. [DOI] [PubMed] [Google Scholar]
- [290].Hwang TS, Gao SS, Liu L, Lauer AK, Bailey ST, Flaxel CJ, Wilson DJ, Huang D, Jia Y, Automated quantification of capillary nonperfusion using optical coherence tomography angiography in diabetic retinopathy, JAMA Ophthalmol 134(4) (2016) 367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [291].Kloosterman A, Hierck B, Westerweel J, Poelma C, Quantification of blood flow and topology in developing vascular networks, PLoS One 9(5) (2014) e96856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [292].Tennant KA, Brown CE, Diabetes augments in vivo microvascular blood flow dynamics after stroke, J Neurosci 33(49) (2013) 19194–19204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [293].Warmuth C, Gunther M, Zimmer C, Quantification of blood flow in brain tumors: comparison of arterial spin labeling and dynamic susceptibility-weighted contrast-enhanced MR imaging, Radiology 228(2) (2003) 523–532. [DOI] [PubMed] [Google Scholar]
- [294].Hart WE, Goldbaum M, Cote B, Kube P, Nelson MR, Measurement and classification of retinal vascular tortuosity, Int J Med Inform 53(2–3) (1999) 239–252. [DOI] [PubMed] [Google Scholar]
- [295].Vickerman MB, Keith PA, McKay TL, Gedeon DJ, Watanabe M, Montano M, Karunamuni G, Kaiser PK, Sears JE, Ebrahem Q, Ribita D, Hylton AG, Parsons-Wingerter P, VESGEN 2D: automated, user-interactive software for quantification and mapping of angiogenic and lymphangiogenic trees and networks, Anat Rec (Hoboken) 292(3) (2009) 320–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [296].Seaman ME, Peirce SM, Kelly K, Rapid analysis of vessel elements (RAVE): a tool for studying physiologic, pathologic and tumor angiogenesis, PLoS One 6(6) (2011) e20807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [297].Zudaire E, Gambardella L, Kurcz C, Vermeren S, A computational tool for quantitative analysis of vascular networks, PLoS One 6(11) (2011) e27385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [298].Less JR, Skalak TC, Sevick EM, Jain RK, Microvascular architecture in a mammary carcinoma: branching patterns and vessel dimensions, Cancer Res 51(1) (1991) 265–273. [PubMed] [Google Scholar]
- [299].Kelch ID, Bogle G, Sands GB, Phillips AR, LeGrice IJ, Dunbar PR, Organ-wide 3D-imaging and topological analysis of the continuous microvascular network in a murine lymph node, Sci Rep 5 (2015) 16534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [300].Zervantonakis IK, Hughes-Alford SK, Charest JL, Condeelis JS, Gertler FB, Kamm RD, Three-dimensional microfluidic model for tumor cell intravasation and endothelial barrier function, Proc Natl Acad Sci U S A 109(34) (2012) 13515–13520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [301].Gavard J, Patel V, Gutkind JS, Angiopoietin-1 prevents VEGF-induced endothelial permeability by sequestering Src through mDia, Dev Cell 14(1) (2008) 25–36. [DOI] [PubMed] [Google Scholar]
- [302].Yuan F, Chen Y, Dellian M, Safabakhsh N, Ferrara N, Jain RK, Time-dependent vascular regression and permeability changes in established human tumor xenografts induced by an anti-vascular endothelial growth factor/vascular permeability factor antibody, Proc Natl Acad Sci U S A 93(25) (1996) 14765–14770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [303].Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C, Pericytes regulate the blood-brain barrier, Nature 468(7323) (2010) 557–561. [DOI] [PubMed] [Google Scholar]
- [304].Martinez-Perez ME, Hughes AD, Stanton AV, Thom SA, Chapman N, Bharath AA, Parker KH, Retinal vascular tree morphology: a semi-automatic quantification, IEEE Trans Biomed Eng 49(8) (2002) 912–917. [DOI] [PubMed] [Google Scholar]
- [305].Fraz MM, Welikala RA, Rudnicka AR, Owen CG, Strachan DP, Barman SA, QUARTZ: Quantitative Analysis of Retinal Vessel Topology and size – An automated system for quantification of retinal vessels morphology, Expert Systems with Applications 42(20) (2015) 7221–7234. [Google Scholar]
- [306].Joshi VS, Reinhardt JM, Garvin MK, Abramoff MD, Automated method for identification and artery-venous classification of vessel trees in retinal vessel networks, PLoS One 9(2) (2014) e88061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [307].Mazzaferri J, Larrivee B, Cakir B, Sapieha P, Costantino S, A machine learning approach for automated assessment of retinal vasculature in the oxygen induced retinopathy model, Sci Rep 8(1) (2018) 3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [308].Song S, Blaha C, Moses W, Park J, Wright N, Groszek J, Fissell W, Vartanian S, Posselt AM, Roy S, An intravascular bioartificial pancreas device (iBAP) with silicon nanopore membranes (SNM) for islet encapsulation under convective mass transport, Lab Chip 17(10) (2017) 1778–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [309].Syed F, Bugliani M, Novelli M, Olimpico F, Suleiman M, Marselli L, Boggi U, Filipponi F, Raffa V, Krol S, Campani D, Masiello P, DeTata V, Marchetti P, Conformal coating by multilayer nano-encapsulation for the protection of human pancreatic islets: In-vitro and in-vivo studies, Nanomedicine 14(7) (2018) 2191–2203. [DOI] [PubMed] [Google Scholar]
- [310].Wilson JT, Cui W, Kozlovskaya V, Kharlampieva E, Pan D, Qu Z, Krishnamurthy VR, Mets J, Kumar V, Wen J, Song Y, Tsukruk VV, Chaikof EL, Cell surface engineering with polyelectrolyte multilayer thin films, J Am Chem Soc 133(18) (2011) 7054–7064. [DOI] [PubMed] [Google Scholar]
- [311].Smink AM, Li S, Swart DH, Hertsig DT, de Haan BJ, Kamps J, Schwab L, van Apeldoorn AA, de Koning E, Faas MM, Lakey JRT, de Vos P, Stimulation of vascularization of a subcutaneous scaffold applicable for pancreatic islet-transplantation enhances immediate post-transplant islet graft function but not long-term normoglycemia, J Biomed Mater Res A 105(9) (2017) 2533–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [312].Kwun J, Malarkannan S, Burlingham WJ, Knechtle SJ, Primary vascularization of the graft determines the immunodominance of murine minor H antigens during organ transplantation, J Immunol 187(8) (2011) 3997–4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [313].Agudo J, Ayuso E, Jimenez V, Casellas A, Mallol C, Salavert A, Tafuro S, Obach M, Ruzo A, Moya M, Pujol A, Bosch F, Vascular endothelial growth factor-mediated islet hypervascularization and inflammation contribute to progressive reduction of beta-cell mass, Diabetes 61(11) (2012) 2851–2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [314].Cai Q, Brissova M, Reinert RB, Pan FC, Brahmachary P, Jeansson M, Shostak A, Radhika A, Poffenberger G, Quaggin SE, Jerome WG, Dumont DJ, Powers AC, Enhanced expression of VEGF-A in beta cells increases endothelial cell number but impairs islet morphogenesis and beta cell proliferation, Dev Biol 367(1) (2012) 40–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [315].Sadtler K, Estrellas K, Allen BW, Wolf MT, Fan H, Tam AJ, Patel CH, Luber BS, Wang H, Wagner KR, Powell JD, Housseau F, Pardoll DM, Elisseeff JH, Developing a pro-regenerative biomaterial scaffold microenvironment requires T helper 2 cells, Science 352(6283) (2016) 366–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [316].Julier Z, Park AJ, Briquez PS, Martino MM, Promoting tissue regeneration by modulating the immune system, Acta Biomater 53 (2017) 13–28. [DOI] [PubMed] [Google Scholar]
- [317].Dziki JL, Sicari BM, Wolf MT, Cramer MC, Badylak SF, Immunomodulation and mobilization of progenitor cells by extracellular matrix bioscaffolds for volumetric muscle loss treatment, Tissue Eng Part A 22(19-20) (2016) 1129–1139. [DOI] [PubMed] [Google Scholar]
- [318].Ogle ME, Krieger JR, Tellier LE, McFaline-Figueroa J, Temenoff JS, Botchwey EA, Dual affinity heparin-based hydrogels achieve pro-regenerative immunomodulation and microvascular remodeling, ACS Biomater Sci Eng 4(4) (2018) 1241–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [319].Keselowsky BG, Lewis JS, Dendritic cells in the host response to implanted materials, Semin Immunol 29 (2017) 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [320].Mitrousis N, Fokina A, Shoichet MS, Biomaterials for cell transplantation, Nature Reviews Materials 3(11) (2018) 441–456. [Google Scholar]
- [321].Spiller KL, Koh TJ, Macrophage-based therapeutic strategies in regenerative medicine, Adv Drug Deliv Rev 122 (2017) 74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [322].Olingy CE, San Emeterio CL, Ogle ME, Krieger JR, Bruce AC, Pfau DD, Jordan BT, Peirce SM, Botchwey EA, Non-classical monocytes are biased progenitors of wound healing macrophages during soft tissue injury, Sci Rep 7(1) (2017) 447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [323].Awojoodu AO, Ogle ME, Sefcik LS, Bowers DT, Martin K, Brayman KL, Lynch KR, Peirce-Cottler SM, Botchwey E, Sphingosine 1-phosphate receptor 3 regulates recruitment of anti-inflammatory monocytes to microvessels during implant arteriogenesis, Proc Natl Acad Sci U S A 110(34) (2013) 13785–13790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [324].Spiller KL, Anfang RR, Spiller KJ, Ng J, Nakazawa KR, Daulton JW, Vunjak-Novakovic G, The role of macrophage phenotype in vascularization of tissue engineering scaffolds, Biomaterials 35(15) (2014) 4477–4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [325].Korff T, Kimmina S, Martiny-Baron G, Augustin HG, Blood vessel maturation in a 3-dimensional spheroidal coculture model: direct contact with smooth muscle cells regulates endothelial cell quiescence and abrogates VEGF responsiveness, FASEB J 15(2) (2001) 447–457. [DOI] [PubMed] [Google Scholar]
- [326].Teichert M, Milde L, Holm A, Stanicek L, Gengenbacher N, Savant S, Ruckdeschel T, Hasanov Z, Srivastava K, Hu J, Hertel S, Bartol A, Schlereth K, Augustin HG, Pericyte-expressed Tie2 controls angiogenesis and vessel maturation, Nat Commun 8 (2017) 16106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [327].Li C, Yu T, Liu Y, Chen X, Zhang X, Topical application of insulin accelerates vessel maturation of wounds by regulating Angiopoietin-1 in diabetic mice, Int J Low Extrem Wounds 14(4) (2015) 353–364. [DOI] [PubMed] [Google Scholar]
- [328].Zhang P, Sun F, Tsao C, Liu S, Jain P, Sinclair A, Hung HC, Bai T, Wu K, Jiang S, Zwitterionic gel encapsulation promotes protein stability, enhances pharmacokinetics, and reduces immunogenicity, Proc Natl Acad Sci U S A 112(39) (2015) 12046–12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [329].Zhi ZL, Kerby A, King AJ, Jones PM, Pickup JC, Nano-scale encapsulation enhances allograft survival and function of islets transplanted in a mouse model of diabetes, Diabetologia 55(4) (2012) 1081–1090. [DOI] [PubMed] [Google Scholar]
- [330].Yesilyurt V, Veiseh O, Doloff JC, Li J, Bose S, Xie X, Bader AR, Chen M, Webber MJ, Vegas AJ, Langer R, Anderson DG, A facile and versatile method to endow biomaterial devices with zwitterionic surface coatings, Adv Healthc Mater 6(4) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [331].Xie X, Doloff JC, Yesilyurt V, Sadraei A, McGarrigle JJ, Omami M, Veiseh O, Farah S, Isa D, Ghani S, Joshi I, Vegas A, Li J, Wang W, Bader A, Tam HH, Tao J, Chen H.-j., Yang B, Williamson KA, Oberholzer J, Langer R, Anderson DG, Reduction of measurement noise in a continuous glucose monitor by coating the sensor with a zwitterionic polymer, Nature Biomedical Engineering 2 (2018) 894–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [332].Vegas AJ, Veiseh O, Doloff JC, Ma M, Tam HH, Bratlie K, Li J, Bader AR, Langan E, Olejnik K, Fenton P, Kang JW, Hollister-Locke J, Bochenek MA, Chiu A, Siebert S, Tang K, Jhunjhunwala S, Aresta-Dasilva S, Dholakia N, Thakrar R, Vietti T, Chen M, Cohen J, Siniakowicz K, Qi M, McGarrigle J, Graham AC, Lyle S, Harlan DM, Greiner DL, Oberholzer J, Weir GC, Langer R, Anderson DG, Combinatorial hydrogel library enables identification of materials that mitigate the foreign body response in primates, Nat Biotechnol 34(3) (2016) 345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [333].Jansen LE, Amer LD, Chen EY, Nguyen TV, Saleh LS, Emrick T, Liu WF, Bryant SJ, Peyton SR, Zwitterionic PEG-PC hydrogels modulate the foreign body response in a modulus-dependent manner, Biomacromolecules 19(7) (2018) 2880–2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [334].Dong D, Li J, Cui M, Wang J, Zhou Y, Luo L, Wei Y, Ye L, Sun H, Yao F, In situ “clickable” zwitterionic starch-based hydrogel for 3D cell encapsulation, ACS Appl Mater Interfaces 8(7) (2016) 4442–4455. [DOI] [PubMed] [Google Scholar]
- [335].Zhang J, Zhu Y, Song J, Yang J, Pan C, Xu T, Zhang L, Novel balanced charged alginate/PEI polyelectrolyte hydrogel that resists foreign-body reaction, ACS Appl Mater Interfaces 10(8) (2018) 6879–6886. [DOI] [PubMed] [Google Scholar]
- [336].Zhang L, Cao Z, Bai T, Carr L, Ella-Menye JR, Irvin C, Ratner BD, Jiang S, Zwitterionic hydrogels implanted in mice resist the foreign-body reaction, Nat Biotechnol 31(6) (2013) 553–556. [DOI] [PubMed] [Google Scholar]
- [337].Marek N, Mysliwiec M, Raczynska K, Zorena K, Mysliwska J, Trzonkowski P, Increased spontaneous production of VEGF by CD4+ T cells in type 1 diabetes, Clin Immunol 137(2) (2010) 261–270. [DOI] [PubMed] [Google Scholar]
- [338].Mor F, Quintana FJ, Cohen IR, Angiogenesis-Inflammation cross-talk: vascular endothelial growth factor is secreted by activated T cells and induces Th1 polarization, The Journal of Immunology 172(7) (2004) 4618–4623. [DOI] [PubMed] [Google Scholar]
- [339].Corliss BA, Azimi MS, Munson JM, Peirce SM, Murfee WL, Macrophages: An inflammatory link between angiogenesis and lymphangiogenesis, Microcirculation 23(2) (2016) 95–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [340].Nadkarni S, Smith J, Sferruzzi-Perri AN, Ledwozyw A, Kishore M, Haas R, Mauro C, Williams DJ, Farsky SH, Marelli-Berg FM, Perretti M, Neutrophils induce proangiogenic T cells with a regulatory phenotype in pregnancy, Proc Natl Acad Sci U S A 113(52) (2016) E8415–E8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [341].Lidington EA, Moyes DL, McCormack AM, Rose ML, A comparison of primary endothelial cells and endothelial cell lines for studies of immune interactions, Transpl Immunol 7(4) (1999) 239–246. [DOI] [PubMed] [Google Scholar]
- [342].Guba M, von Breitenbuch P, Steinbauer M, Koehl G, Flegel S, Hornung M, Bruns CJ, Zuelke C, Farkas S, Anthuber M, Jauch KW, Geissler EK, Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor, Nat Med 8(2) (2002) 128–135. [DOI] [PubMed] [Google Scholar]
- [343].Cantaluppi V, Biancone L, Romanazzi GM, Figliolini F, Beltramo S, Ninniri MS, Galimi F, Romagnoli R, Franchello A, Salizzoni M, Perin PC, Ricordi C, Segoloni GP, Camussi G, Antiangiogenic and immunomodulatory effects of rapamycin on islet endothelium: relevance for islet transplantation, Am J Transplant 6(11) (2006) 2601–2611. [DOI] [PubMed] [Google Scholar]
- [344].Hirshberg B, Mog S, Patterson N, Leconte J, Harlan DM, Histopathological study of intrahepatic islets transplanted in the nonhuman primate model using edmonton protocol immunosuppression, J Clin Endocrinol Metab 87(12) (2002) 5424–5429. [DOI] [PubMed] [Google Scholar]
- [345].Mendola JF, Goity C, Esmatjes E, Saenz A, Fernandez-Cruz L, Gomis R, Cyclosporine does not inhibit the process of revascularization of pancreatic islet transplantation, Cell Transplant 6(1) (1997) 69–76. [DOI] [PubMed] [Google Scholar]
- [346].Beger C, Menger MD, RS-61443 prevents microvascular rejection of pancreatic islet xenografts, Transplantation 63(4) (1997) 577–582. [DOI] [PubMed] [Google Scholar]
- [347].Menger MD, Wolf B, Jager S, Walter P, Messmer K, The influence of prednisolone on revascularization of pancreatic islet grafts, Transplant Proc 22(4) (1990) 2042–2043. [PubMed] [Google Scholar]
- [348].Menger MD, Yamauchi J, Vollmar B, Revascularization and microcirculation of freely grafted islets of Langerhans, World J Surg 25(4) (2001) 509–515. [DOI] [PubMed] [Google Scholar]
- [349].Fabian MC, Lakey JR, Rajotte RV, Kneteman NM, The efficacy and toxicity of rapamycin in murine islet transplantation. In vitro and in vivo studies, Transplantation 56(5) (1993) 1137–1142. [DOI] [PubMed] [Google Scholar]
- [350].Drachenberg CB, Klassen DK, Weir MR, Wiland A, Fink JC, Bartlett ST, Cangro CB, Blahut S, Papadimitriou JC, Islet cell damage associated with tacrolimus and cyclosporine: morphological features in pancreas allograft biopsies and clinical correlation, Transplantation 68(3) (1999) 396–402. [DOI] [PubMed] [Google Scholar]
- [351].Zeng Y, Ricordi C, Lendoire J, Carroll PB, Alejandro R, Bereiter DR, Tzakis A, Starzl TE, The effect of prednisone on pancreatic islet autografts in dogs, Surgery 113(1) (1993) 98–102. [PMC free article] [PubMed] [Google Scholar]
- [352].Zahr E, Molano RD, Pileggi A, Ichii H, Jose SS, Bocca N, An W, Gonzalez-Quintana J, Fraker C, Ricordi C, Inverardi L, Rapamycin impairs in vivo proliferation of islet beta-cells, Transplantation 84(12) (2007) 1576–1583. [DOI] [PubMed] [Google Scholar]
- [353].Aronovitz A, Josefson J, Fisher A, Newman M, Hughes E, Chen F, Moons DS, Kiyokawa H, Lowe WL Jr., Rapamycin inhibits growth factor-induced cell cycle regulation in pancreatic beta cells, J Investig Med 56(8) (2008) 985–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [354].Desai NM, Goss JA, Deng S, Wolf BA, Markmann E, Palanjian M, Shock AP, Feliciano S, Brunicardi FC, Barker CF, Naji A, Markmann JF, Elevated portal vein drug levels of sirolimus and tacrolimus in islet transplant recipients: local immunosuppression or islet toxicity?, Transplantation 76(11) (2003) 1623–1625. [DOI] [PubMed] [Google Scholar]
- [355].Shapiro AM, Gallant HL, Hao EG, Lakey JR, McCready T, Rajotte RV, Yatscoff RW, Kneteman NM, The portal immunosuppressive storm: relevance to islet transplantation?, Ther Drug Monit 27(1)(2005)35–37. [DOI] [PubMed] [Google Scholar]
- [356].Laugharne M, Cross S, Richards S, Dawson C, Ilchyshyn L, Saleem M, Mathieson P, Smith R, Sirolimus toxicity and vascular endothelial growth factor release from islet and renal cell lines, Transplantation 83(12) (2007) 1635–1638. [DOI] [PubMed] [Google Scholar]
- [357].Maki T, Otsu I, O’Neil JJ, Dunleavy K, Mullon CJ, Solomon BA, Monaco AP, Treatment of diabetes by xenogeneic islets without immunosuppression. Use of a vascularized bioartificial pancreas, Diabetes 45(3) (1996) 342–347. [DOI] [PubMed] [Google Scholar]
- [358].Yang Z, Chen M, Fialkow LB, Ellett JD, Wu R, Nadler JL, Survival of pancreatic islet xenografts in NOD mice with the Theracyte device, Transplant Proc 34(8) (2002) 3349–3350. [DOI] [PubMed] [Google Scholar]
- [359].Elliott RB, Escobar L, Calafiore R, Basta G, Garkavenko O, Vasconcellos A, Bambra C, Transplantation of micro- and macroencapsulated piglet islets into mice and monkeys, Transplant Proc 37(1) (2005) 466–469. [DOI] [PubMed] [Google Scholar]
- [360].Lanza RP, Butler DH, Borland KM, Staruk JE, Faustman DL, Solomon BA, Muller TE, Rupp RG, Maki T, Monaco AP, et al. , Xenotransplantation of canine, bovine, and porcine islets in diabetic rats without immunosuppression, Proc Natl Acad Sci U S A 88(24) (1991) 11100–11104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [361].Dufrane D, Goebbels RM, Gianello P, Alginate macroencapsulation of pig islets allows correction of streptozotocin-induced diabetes in primates up to 6 months without immunosuppression, Transplantation 90(10) (2010) 1054–1062. [DOI] [PubMed] [Google Scholar]
- [362].Figliuzzi M, Cornolti R, Plati T, Rajan N, Adobati F, Remuzzi G, Remuzzi A, Subcutaneous xenotransplantation of bovine pancreatic islets, Biomaterials 26(28) (2005) 5640–5647. [DOI] [PubMed] [Google Scholar]
- [363].Sun Y, Ma X, Zhou D, Vacek I, Sun AM, Normalization of diabetes in spontaneously diabetic cynomologus monkeys by xenografts of microencapsulated porcine islets without immunosuppression, J Clin Invest 98(6) (1996) 1417–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [364].Lanza RP, Kuhtreiber WM, Ecker D, Staruk JE, Chick WL, Xenotransplantation of porcine and bovine islets without immunosuppression using uncoated alginate microspheres, Transplantation 59(10) (1995) 1377–1384. [DOI] [PubMed] [Google Scholar]
- [365].Dufrane D, Goebbels RM, Saliez A, Guiot Y, Gianello P, Six-month survival of micro encapsulated pig islets and alginate biocompatibility in primates: proof of concept, Transplantation 81(9) (2006) 1345–1353. [DOI] [PubMed] [Google Scholar]
- [366].Elliott RB, Escobar L, Tan PL, Garkavenko O, Calafiore R, Basta P, Vasconcellos AV, Emerich DF, Thanos C, Bambra C, Intraperitoneal alginate-encapsulated neonatal porcine islets in a placebo-controlled study with 16 diabetic cynomolgus primates, Transplant Proc 37(8) (2005) 3505–3508. [DOI] [PubMed] [Google Scholar]
- [367].Jain K, Yang H, Cai BR, Haque B, Hurvitz AI, Diehl C, Miyata T, Smith BH, Stenzel K, Suthanthiran M, Rubin A, Retrievable, replaceable, macroencapsulated pancreatic islet xenografts. Long-term engraftment without immunosuppression, Transplantation 59(3) (1995) 319–324. [PubMed] [Google Scholar]
- [368].Langer HF, Stellos K, Steingen C, Froihofer A, Schonberger T, Kramer B, Bigalke B, May AE, Seizer P, Muller I, Gieseke F, Siegel-Axel D, Meuth SG, Schmidt A, Wendel HP, Muller I, Bloch W, Gawaz M, Platelet derived bFGF mediates vascular integrative mechanisms of mesenchymal stem cells in vitro, J Mol Cell Cardiol 47(2) (2009) 315–325. [DOI] [PubMed] [Google Scholar]
- [369].Valdes-Gonzalez RA, Dorantes LM, Garibay GN, Bracho-Blanchet E, Mendez AJ, Davila-Perez R, Elliott RB, Teran L, White DJ, Xenotransplantation of porcine neonatal islets of Langerhans and Sertoli cells: a 4-year study, Eur J Endocrinol 153(3) (2005) 419–427. [DOI] [PubMed] [Google Scholar]
- [370].Gupta R, Sefton MV, Application of an endothelialized modular construct for islet transplantation in syngeneic and allogeneic immunosuppressed rat models, Tissue Eng Part A 17(15-16) (2011) 2005–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [371].Ryan CM, Geckle MO, Orchard TJ, Cognitive efficiency declines over time in adults with type 1 diabetes: effects of micro- and macrovascular complications, Diabetologia 46(7) (2003) 940–948. [DOI] [PubMed] [Google Scholar]
- [372].Zoungas S, Woodward M, Li Q, Cooper ME, Hamet P, Harrap S, Heller S, Marre M, Patel A, Poulter N, Williams B, Chalmers J, A.C. group, Impact of age, age at diagnosis and duration of diabetes on the risk of macrovascular and microvascular complications and death in type 2 diabetes, Diabetologia 57(12) (2014) 2465–2474. [DOI] [PubMed] [Google Scholar]
- [373].Martin A, Komada MR, Sane DC, Abnormal angiogenesis in diabetes mellitus, Med Res Rev 23(2) (2003) 117–145. [DOI] [PubMed] [Google Scholar]
- [374].Fowler MJ, Microvascular and macrovascular complications of diabetes, Clinical Diabetes 26 (2008) 77–82. [Google Scholar]
- [375].Dubois S, Madec AM, Mesnier A, Armanet M, Chikh K, Berney T, Thivolet C, Glucose inhibits angiogenesis of isolated human pancreatic islets, J Mol Endocrinol 45(2) (2010) 99–105. [DOI] [PubMed] [Google Scholar]
- [376].Geraldes P, Hiraoka-Yamamoto J, Matsumoto M, Clermont A, Leitges M, Marette A, Aiello LP, Kern TS, King GL, Activation of PKC-delta and SHP-1 by hyperglycemia causes vascular cell apoptosis and diabetic retinopathy, Nat Med 15(11) (2009) 1298–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [377].Menger MD, Messmer K, Pancreatic islet transplantation: isolation, separation, and microvascularization, Wien Klin Wochenschr 104(15) (1992) 429–433. [PubMed] [Google Scholar]
- [378].Wolf B, Heuser M, Vollmar B, Menger MD, Effect of size of islets of Langerhans for successful vascularization after free transplantation, Springer Berlin Heidelberg, Berlin, Heidelberg, 1998, pp. 153–154. [PubMed] [Google Scholar]
- [379].Lehmann R, Zuellig RA, Kugelmeier P, Baenninger PB, Moritz W, Perren A, Clavien PA, Weber M, Spinas GA, Superiority of small islets in human islet transplantation, Diabetes 56(3) (2007) 594–603. [DOI] [PubMed] [Google Scholar]
- [380].O’Sullivan ES, Johnson AS, Omer A, Hollister-Lock J, Bonner-Weir S, Colton CK, Weir GC, Rat islet cell aggregates are superior to islets for transplantation in microcapsules, Diabetologia 53(5) (2010) 937–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [381].Caiazzo R, Gmyr V, Hubert T, Delalleau N, Lamberts R, Moerman E, Kerr-Conte J, Pattou F, Evaluation of alternative sites for islet transplantation in the minipig: interest and limits of the gastric submucosa, Transplant Proc 39(8) (2007) 2620–2623. [DOI] [PubMed] [Google Scholar]
- [382].Calafiore R, Microencapsulation for cell therapy of type 1 diabetes mellitus: The interplay between common beliefs, prejudices and real progress, J Diabetes Investig 9(2) (2018) 231–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [383].Russell SJ, El-Khatib FH, Sinha M, Magyar KL, McKeon K, Goergen LG, Balliro C, Hillard MA, Nathan DM, Damiano ER, Outpatient glycemic control with a bionic pancreas in type 1 diabetes, N Engl J Med 371(4) (2014) 313–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [384].Ly TT, Roy A, Grosman B, Shin J, Campbell A, Monirabbasi S, Liang B, von Eyben R, Shanmugham S, Clinton P, Buckingham BA, Day and night closed-loop control using the integrated Medtronic hybrid closed-loop system in type 1 diabetes at diabetes camp, Diabetes Care 38(7) (2015) 1205–1211. [DOI] [PubMed] [Google Scholar]
- [385].Kovatchev B, Cheng P, Anderson SM, Pinsker JE, Boscari F, Buckingham BA, Doyle FJ 3rd, Hood KK, Brown SA, Breton MD, Chernavvsky D, Bevier WC, Bradley PK, Bruttomesso D, Del Favero S, Calore R, Cobelli C, Avogaro A, Ly TT, Shanmugham S, Dassau E, Kollman C, Lum JW, Beck RW, Feasibility of long-term closed-loop control: a multicenter 6-month trial of 24/7 automated insulin delivery, Diabetes Technol Ther 19(1) (2017) 18–24. [DOI] [PubMed] [Google Scholar]
- [386].Banerjee A, Ibsen K, Brown T, Chen R, Agatemor C, Mitragotri S, Ionic liquids for oral insulin delivery, Proc Natl Acad Sci U S A 115(28) (2018) 7296–7301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [387].Ke C, Destecroix H, Crump MP, Davis AP, A simple and accessible synthetic lectin for glucose recognition and sensing, Nat Chem 4(9) (2012) 718–723. [DOI] [PubMed] [Google Scholar]
- [388].Chou DH, Webber MJ, Tang BC, Lin AB, Thapa LS, Deng D, Truong JV, Cortinas AB, Langer R, Anderson DG, Glucose-responsive insulin activity by covalent modification with aliphatic phenylboronic acid conjugates, Proc Natl Acad Sci U S A 112(8) (2015) 2401–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [389].Zaykov AN, Mayer JP, DiMarchi RD, Pursuit of a perfect insulin, Nat Rev Drug Discov 15(6) (2016) 425–439. [DOI] [PubMed] [Google Scholar]
- [390].Yu J, Zhang Y, Ye Y, DiSanto R, Sun W, Ranson D, Ligler FS, Buse JB, Gu Z, Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery, Proc Natl Acad Sci U S A 112(27) (2015) 8260–8265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [391].Nijhoff MF, de Koning EJP, Artificial pancreas or novel beta-cell replacement therapies: a race for optimal glycemic control?, Curr Diab Rep 18(11) (2018) 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [392].Rengifo HR, Giraldo JA, Labrada I, Stabler CL, Long-term survival of allograft murine islets coated via covalently stabilized polymers, Adv Healthc Mater 3(7) (2014) 1061–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [393].Farina M, Alexander JF, Thekkedath U, Ferrari M, Grattoni A, Cell encapsulation: Overcoming barriers in cell transplantation in diabetes and beyond, Adv Drug Deliv Rev (2018). [DOI] [PubMed] [Google Scholar]


