Abstract
Schizophrenia, which affects around 1% of the world’s population, has been described as a complex set of symptoms triggered by multiple factors. However, the exact background mechanisms remain to be explored, whereas therapeutic agents with excellent effectivity and safety profiles have yet to be developed. Kynurenines and the endocannabinoid system (ECS) play significant roles in both the development and manifestation of schizophrenia, which have been extensively studied and reviewed previously. Accordingly, kynurenines and the ECS share multiple features and mechanisms in schizophrenia, which have yet to be reviewed. Thus, the present study focuses on the main common points and potential interactions between kynurenines and the ECS in schizophrenia, which include (i) the regulation of glutamatergic/dopaminergic/γ-aminobutyric acidergic neurotransmission, (ii) their presence in astrocytes, and (iii) their role in inflammatory mechanisms. Additionally, promising pharmaceutical approaches involving the kynurenine pathway and the ECS will be reviewed herein.
Keywords: cannabinoids, endocannabinoids, cannabinoid receptors, kynurenines, kynurenine pathway, schizophrenia
1. Introduction
Schizophrenia, which is among the major psychiatric syndromes, affects approximately 1% of the population worldwide. The combined economic and social costs associated with this disorder rank it as the 15th highest cause of disease-related disabilities worldwide [1]. Schizophrenia is characterized by positive symptoms (i.e., hallucination, delusions, confused thought, and disorganized speech), negative symptoms (i.e., asocial behavior, blunted emotions and motivation, affective flattening, alogia, and avolition), and cognitive dysfunctions. Currently used antipsychotic medications have displayed insufficient efficacy and are mostly restricted to the improvement of positive symptoms, given their limited or no effect on negative symptoms and cognitive impairments. Although the exact pathophysiology of schizophrenia still remains unknown, certain theories have emerged, which involve, for instance, the dopaminergic and glutamatergic systems [2]. Recently, the endocannabinoid system (ECS) and kynurenic acid (KYNA) hypotheses—an extension of the glutamatergic dysfunction model—have gained attention.
KYNA, kynurenines, and their associated elements (see Section 2.1.) share several physiological functions with the ECS (see Section 3.1.). Furthermore, both systems are similarly dysfunctional in schizophrenia [3,4]. This has led to the assumption of their interaction, which could be utilized for therapeutic applications. This concept has been recently discussed by us [5] and others [6] in reviews.
Both kynurenines and the ECS have been separately implicated in schizophrenia and discussed previously in numerous publications (Figure 1, Table 1). However, their common points and potential interactions relevant to schizophrenia have yet to be reviewed. Thus, the present review aims to gather and highlight related data and draw attention to potential interactions that might help us better understand the pathology/etiology of schizophrenia. Although data describing the direct interaction between the two systems in schizophrenia may be missing in some cases, multiple overlapping functions/alterations in the two systems indicate the possibility of an interaction. Accordingly, such potential interactions will be the focus of this review. To obtain a better overview of these points, this review will cover a separate general introduction to kynurenines and the ECS. Additionally, possible hypotheses for the mechanism of schizophrenia related to this review will be discussed in the appropriate sections (see Section 2.2., Section 3.2., Section 4.2.1., and Section 4.4.1.). Finally, new potential drug targets for both systems will also be discussed (see Table 1).
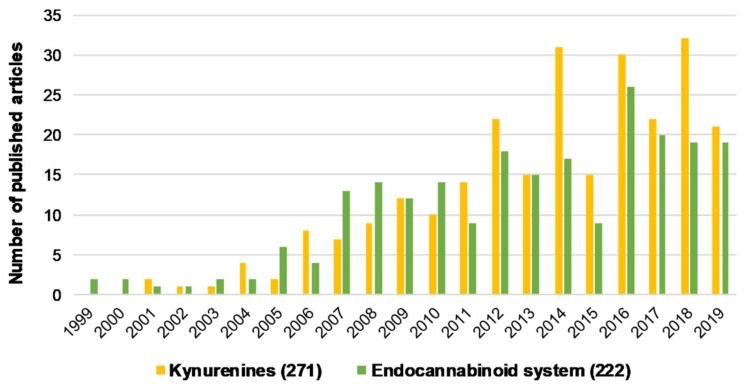
Figure 1.
The number of articles published regarding kynurenines and the endocannabinoid system individually associated with schizophrenia from the last 20 years. Brackets indicate the total number of publications from the last 20 years. Data was obtained from PubMed using “kynurenines AND schizophrenia” and “endocannabinoid system AND schizophrenia” as keywords.
Table 1.
The main studies reviewing aspects of schizophrenia that are shared by kynurenines and the endocannabinoid system (ECS). Reviews discussing the main therapeutic targets for kynurenines and the ECS relevant to schizophrenia are also indicated separately.
2. Kynurenines and Their Role in Schizophrenia
2.1. Kynurenines and Associated Elements
2.1.1. The Kynurenine Pathway
The kynurenine pathway (KP) is a collection of metabolic substances and enzymes present in the synthesis and degradation of l-kynurenine (l-KYN). This process is the main metabolic route of tryptophan (Trp) (Figure 2). The initial and rate-limiting step in the KP consists of two iron-dependent enzymes, indoleamine 2,3-dioxygenase 1 and 2 (IDO1 and IDO2) and tryptophan 2,3-dioxygenase (TDO). These enzymes embed molecular oxygen through the 2–3 bond of the Trp indole moiety. IDO is a monomer found in the central nervous system (CNS), whereas TDO is a homotetramer having stiff substrate selectivity, and it occurs primarily in peripheral tissues, especially in hepatic tissue. IDO and TDO catalyzes Trp to N-formyl-l-kynurenine by opening the Trp ring and further hydrolyze it to l-KYN by formamidase. l-KYN can cross the blood–brain barrier completely and exert neuroprotective effects. Roughly 60% of l-KYN present in the CNS is absorbed from the blood by glial cells.
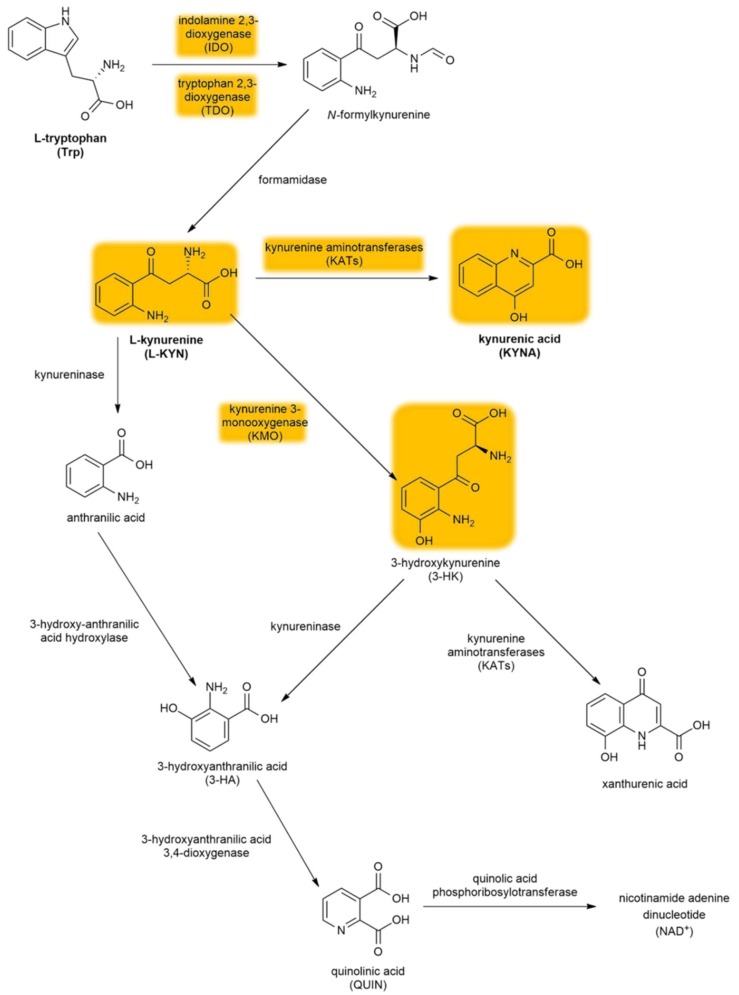
Figure 2.
The kynurenine pathway. The yellow background indicates the metabolites and enzymes relevant to schizophrenia. Abbreviations of metabolites and enzymes frequently used in the text are also indicated.
l-KYN can be converted via three different pathways. The first metabolic route involves the conversion of l-KYN into anthranilic acid by kynureninase and further into 3-hydroxyanthranilic acid (3-HA) by 3-hydroxy-anthranilic acid 3,4-dioxygenase. The second branch of the KP begins with the hydroxylation of l-KYN at the third position by kynurenine 3-monooxygenase (KMO), which produces 3-hydroxykynurenine (3-HK) that can be further converted into xanthurenic acid and 3-HA. Notably, anthranilic acid can also be converted into 3-HA, which can be further converted into pyridine-2,3-dicarboxylic acid or quinolinic acid (QUIN), which is an N-methyl-d-aspartate receptor (NMDAR) agonist that causes lipid peroxidation [31]. In the final step of this KP branch, QUIN is then degraded into nicotinamide adenine dinucleotide (NAD+) [32]. The last branch of the KP starts with the conversion of l-KYN into KYNA by kynurenine aminotransferases (KATs), which have four subtypes with various biochemical profiles [33]. In contrast to QUIN, KYNA is an endogenous glutamate receptor antagonist. Under physiological conditions, the KAT II enzyme is responsible for the biosynthesis of KYNA in the brain [34]. KATs are chiefly present in astrocytes [35] (see Section 4.3.2.), unlike other enzymes (e.g., KMO) that are primarily expressed in microglia [36].
2.1.2. KYNA and Its Target Receptors
In 1853, KYNA was first discovered in dog urine by a German chemist, Justus von Liebig. After 50 years, Ellinger and Homer revealed that KYNA is produced during Trp metabolism. This metabolic route for Trp was first described in 1947 in a process called the KP [37]. Almost all KP metabolites have a broad spectrum of biological effects and have been associated with numerous disorders [38], such as multiple sclerosis [39], Parkinson’s disease [40], migraine [41], and schizophrenia [2], which will be further discussed.
KYNA can influence different types of receptors. Accordingly, it behaves as an antagonist at the strychnine-insensitive glycine-binding site of NMDARs at low concentrations [42], while also blocking the glutamate-binding site of NMDARs at higher doses [43]. Moreover, KYNA causes weak antagonistic effects on kainate- and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-sensitive glutamate receptors [42], with its impact on AMPA receptor-mediated action being concentration dependent. This effect is facilitatory at low concentrations (nanomolar to micromolar) and inhibitory at high concentrations (micromolar to millimolar) [44]. Although published data have suggested that KYNA also functions as an α7 nicotinic acetylcholine receptor (α7nAChR) antagonist [45] by reducing the presynaptic release of glutamate, this concept is currently under debate [46]. Another review reported that KYNA can be considered a bona fide endogenous modulator for α7nAChR, although it is a complex phenomenon that depends mostly on methodological considerations [47]. Furthermore, KYNA has an agonistic effect on the G protein-coupled receptor 35 (GPR35) [48,49], as well as on the aryl hydrocarbon receptors (AHR) [50]. Our group previously demonstrated that KYNA displays diverse effects depending on its concentration (few hundred nanomolar vs. micromolar), possibly through different receptor targets [51,52,53].
2.2. The KYNA Hypothesis of Schizophrenia
The KYNA hypothesis of schizophrenia has been studied and reviewed previously by numerous authors [2,4,26,54,55]. This section will briefly discuss the background of this hypothesis, which is based on the finding that exogenous NMDAR antagonists—such as phencyclidine and ketamine—induce schizophrenia-like symptoms that can be mimicked by KYNA [4,7,56,57,58]. The hypothesis is also supported by clinical data, given that patients with schizophrenia show increased KYNA levels in the prefrontal cortex (PFC) (2.9 pmol/mg protein vs. 1.9 pmol/mg protein) [59] and cerebrospinal fluid (CSF) (~1.7 vs. 1 nM) [60]. According to preclinical data, this elevation can lead to behavioral and neurotransmission changes associated with schizophrenia, such as cognitive deficits and disrupted glutamatergic, γ-aminobutyric acidergic (GABAergic), cholinergic, and dopaminergic signaling [56,61,62,63,64,65,66,67,68,69]. Additionally, the inhibition of KYNA formation has been found to improve such symptoms [70] (see Section 5.3.). The increase in KYNA levels in schizophrenia is partly due to the altered enzyme activity/expression in the KP, which shifts Trp metabolism to KYNA production [8]. KYN levels in the CSF and cortical brain regions are also increased in patients with schizophrenia [71,72], whereas the neurotoxic branch of the KP (QUIN, 3-HK) seems to be unaffected [59,73,74]. Additionally, studies have found reduced expression of KYNA target receptors, namely, NMDAR [75] and α7nAChR, in postmortem brain samples of patients with schizophrenia [76,77].
3. The Endocannabinoid System and Its Role in Schizophrenia
3.1. Overview of the Endocannabinoid System
The ECS, which mainly consists of two well-characterized receptors, primarily endogenous lipid-derived ligands called endocannabinoids, and enzymes responsible for their synthesis and degradation, is involved in various physiological and pathological processes of the CNS and certain peripheral organs [19,78].
To date, two types of cannabinoid receptors, cannabinoid receptor type 1 (CB1R) and cannabinoid receptor type 2 (CB2R), belonging to the family of Gi/o protein-coupled receptors (GPCRs) have been cloned [79,80,81]. Accordingly, their activation inhibits cAMP production, stimulates mitogen-activated protein kinases, and presynaptically suppresses the release of several neurotransmitters relevant to schizophrenia (see Section 4.2.3.) [10,82,83]. CB1Rs play a role in regulating mood or emotions, antinociception, energy balance, immune mechanisms, and endocrine functions [19,84]. Although CB1Rs are located predominantly in the hippocampus, basal ganglia, cortex, amygdala, and cerebellum, they are also highly expressed in the liver, adipose tissues, muscles, cardiovascular system, and gastrointestinal system (GI) [19,85]. Additionally, CB1Rs are known to be the most abundantly expressed GPCR in the CNS [86,87]. On the other hand, CB2Rs are expressed predominantly in immune and hematopoietic cells, although they can also be found in the CNS, such as in microglia [88]. Generally, CB2Rs have a protective role, they reduce inflammation-induced pain by controlling cytokine regulation and immune cell migration (see Section 4.4.2.), and they also induce peripheral antinociception [19,89].
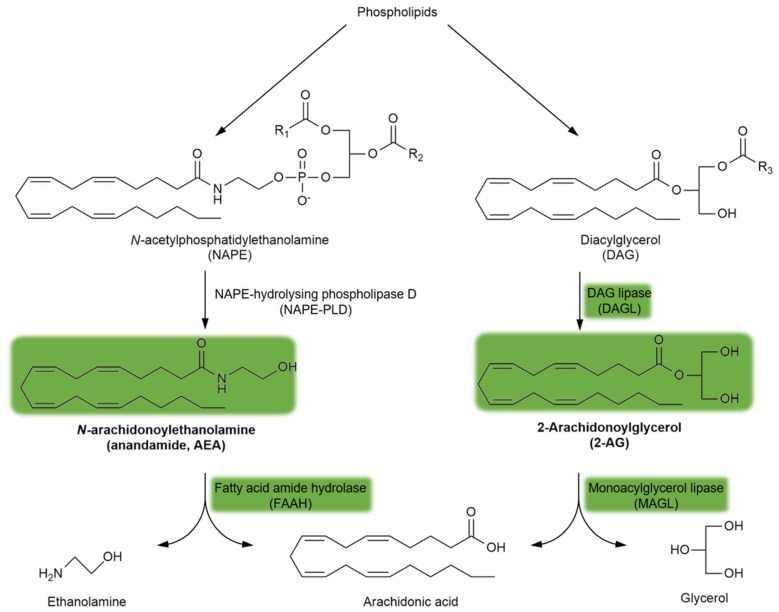
Endogenous cannabinoid receptor (CBR) ligands are hydrophobic lipid-derived compounds, among which N-arachidonoylethanolamine (AEA) and 2-arachidonoyl glycerol (2-AG) have been most studied [90,91,92]. Their degradation is also important, with AEA being rapidly metabolized by the fatty acid amide hydrolase (FAAH) and 2-AG being hydrolyzed by the monoacylglycerol lipase (MAGL) enzyme (Figure 3). Blocking the FAAH enzyme has been considered a novel approach for the treatment of schizophrenia (see Section 5.4.). Furthermore, plant-derived phytocannabinoids, such as Δ9-tetrahydrocannabinol (Δ9THC), the major psychoactive component of cannabis, and the non-psychoactive cannabidiol (CBD), are also relevant to schizophrenia (see Section 3.2. and Section 5.4.). Importantly, the psychoactive effects of Δ9THC are mediated through the brain CB1R, the most abundant GPCR in the brain.
Figure 3.
The synthesis and degradation of endocannabinoids. The green background indicates the metabolites and enzymes relevant to schizophrenia. Abbreviations of metabolites and enzymes frequently used in the text are also indicated.
More than 30 years following the discovery and identification of CBRs, structurally diverse synthetic cannabinoids have been developed and synthesized to investigate their interaction with the ECS. Among these, the bicyclic CP 55940 and the aminoalkylindole WIN 55212-2 are potent CB1/CB2 agonists that represent important exogenous cannabinoids in the field of cannabinoid research. Later generations of synthetic cannabinoids, such as JWH-18, have been found in illicit herbal mixes (“Spice”) and classified as a Schedule I controlled substance [84,93] in the United States. Synthetic cannabinoids with high CB1R affinity and potency have been closely associated with the development of schizophrenia (see Section 3.2.).
3.2. The Cannabinoid Hypothesis of Schizophrenia
The cannabinoid hypothesis of schizophrenia has recently emerged, based on neuroimaging reports, postmortem studies, and clinical evidence. Within this hypothesis, we distinguish between the endogenous and exogenous cannabinoid hypotheses. The former is based on the fact that ECS deregulation has been observed among patients with schizophrenia [94]. Notably, alterations in CB1R availability, density, and/or mRNA expression and endocannabinoid levels have been reported in certain brain tissues and CSF of patients with schizophrenia [94,95,96,97]. On the other hand, the exogenous cannabinoid hypothesis refers to the association between environmental risk factors, such as frequent and/or early use of cannabis or synthetic cannabinoids, and the development of schizophrenia among vulnerable individuals, especially adolescents [10,98,99]. Δ9THC administration can induce positive and negative symptoms, as well as cognitive impairments, resembling those of schizophrenia among healthy individuals, while exacerbating symptoms among patients already diagnosed with schizophrenia [100,101,102]. Moreover, a study by Moore and coworkers showed that the risk of psychosis increases by approximately 40% among individuals who had previously used cannabis [103]. Although Δ9THC is mainly responsible for the connections between cannabis and schizophrenia, and while CBD can offset these associations (see Section 5.4), the cannabis plant itself contains a large variety of other phytocannabinoids, terpenes, and phenolic compounds, not to mention their metabolites [104]. This makes it challenging to accurately study the connection between cannabis consumption and the risk of schizophrenia development.
4. Common Points and Potential Interactions between the Endocannabinoid System and Kynurenines Relevant to Schizophrenia
4.1. Overview
This main section will review the functions and mechanisms of kynurenines that overlap with the ECS and their potential interactions related to schizophrenia. Based on the literature, the following three main aspects that form the basis for known and potential interactions between kynurenines (mainly KYNA) and the ECS will be discussed in the subsequent sections: (1) glutamatergic, dopaminergic, and GABAergic neurotransmission, given that KYNA and CB1R regulate all three; (2) astrocytes, given their significance in KYNA production and CB1R function; and finally (3) inflammation associated with schizophrenia, given that both the KP and ECS play important roles in this mechanism. All three aspects will be discussed in separate sections while also underscoring the basics of astrocyte functioning and other related, yet undiscussed hypotheses of schizophrenia (dopaminergic, glutamatergic, and GABAergic neurotransmission and inflammation). Additionally, each section will be accompanied by tables summarizing the main studies related to the given section (see Tables 2–4).
4.2. Glutamatergic, Dopaminergic, and GABAergic Transmission Regulation by Kynurenines and the Endocannabinoid System in Schizophrenia
4.2.1. The Basics of the Dopaminergic, Glutamatergic, and GABAergic Hypothesis of Schizophrenia
Dysregulation of brain neurotransmission, including dopaminergic, glutamatergic, and GABAergic systems, forms the basis for neurochemical theories on the etiology of schizophrenia [105]. Considering that all aforementioned transmitters are involved in the control of several cerebral processes, including locomotor functions, affect, motivation, and learning, abnormal activities therein have been thought to be associated with many schizophrenia symptoms [105,106,107,108].
While the mesolimbic dopaminergic pathway may play a role in the development of positive schizophrenia symptoms in the presence of excess dopamine and/or increased dopamine D2 receptor expression [109], negative symptoms and cognitive deficits are thought to be caused by low mesocortical dopamine levels and decreased dopamine D1 receptor density in the PFC [110]. However, clear limitations for this hypothesis exist, given that many aspects of schizophrenia cannot be explained based on dopaminergic dysfunction alone, and many patients remain persistently disabled despite treatment with various dopaminergic compounds.
Glutamatergic theories of schizophrenia have been based on the ability of NMDAR antagonists, such as phencyclidine (PCP) and ketamine, to induce schizophrenia-like symptoms and on disturbances of NMDAR-related gene expression and metabolic pathways accounting mainly for negative symptoms and some cognitive dysfunctions of the disorder [111,112,113]. Reduced NMDAR activity on inhibitory (GABAergic) neurons leads to disinhibition of glutamate neurons. Theoretically, such abnormally increased glutamatergic activity through AMPA and metabotropic glutamate (mGLUT) receptors causes overactivation of the mesolimbic and underactivation of the mesocortical dopaminergic pathways, leading to morphological and structural brain changes resulting in psychosis [113,114].
Postmortem studies have widely reported alterations in multiple GABA-related markers among patients with schizophrenia [115]. Dysfunction in the parvalbumin-containing subset of cortical inhibitory neurons together with both pre- and postsynaptic components of GABAergic neurotransmission could also play an important role in the clinical features of schizophrenia [108,116]. One of the most consistent postmortem findings in schizophrenia is reduced glutamic acid decarboxylase 67 (GAD 67) mRNA expression and consequent attenuation of inhibitory GABAergic neurotransmission across multiple brain areas affected by schizophrenia [108,117]. These abnormalities could create disturbances mainly related to emotional functioning and cognitive control. Additionally, one clinical study reported lower GABA concentrations in CSF samples from patients with first-episode psychosis compared with those from healthy volunteers, which were associated with total and general Positive and Negative Syndrome Scale scores, illness severity, and poor performance in a test of attention [118]. However, neuroimaging studies measuring in vivo GABA have revealed no consistent alterations in schizophrenia that might be hypothesized from animal models and postmortem data [119]. The absence of large, detectable differences in GABA concentrations could reflect normalization via compensatory upstream mechanisms that tend to increase the synaptic activity of GABA [115], which include the reduction in GABA transporter 1 mRNA expression on presynaptic neurons (responsible for GABA reuptake) and upregulation of GABAA receptors in postsynaptic pyramidal neurons [108,120].
KYNA and cannabinoids have been known to modulate the abovementioned neurotransmissions, which will be discussed below and summarized in Table 2.
Table 2.
Kynurenines and associated elements (enzymes, receptors) and members of the ECS participating in glutamatergic, dopaminergic, and GABAergic neurotransmission associated with schizophrenia.
4.2.2. KYNA and Dopaminergic/Glutamatergic/GABAergic Interactions in Schizophrenia
Preclinical studies have provided ample evidence to suggest that KYNA has an inverse bidirectional relationship with several neurotransmitters, including glutamate, dopamine, and GABA, which could contribute to all symptom domains of schizophrenia [7,8]. Accordingly, though KYNA is generally considered to be protective against QUIN-induced excitotoxicity, its abnormal accumulation beyond physiological concentrations may cause NMDAR hypofunction on cortical GABA interneurons. This may lead to reductions in GABAergic neurotransmission and disinhibition of cortical glutamatergic projections [128], as well as an excitatory effect on ventral tegmental area (VTA) dopamine firing induced by the blockade of the NMDAR glycine site. Meanwhile, electrophysiological studies have shown that KYNA appears to have an opposite action on dopamine neurotransmission via α7nAChR antagonism, consequently reducing dopamine release and promoting cognitive impairments [121].
4.2.3. The Endocannabinoid System and Dopaminergic/Glutamatergic/GABAergic Interactions in Schizophrenia
Given that CB1Rs inhibit the release of several neurotransmitters, including dopamine, GABA, serotonin, glutamate, noradrenaline, and acetylcholine, the ECS may be considered a key neuromodulatory pathway relevant in the etiology of multiple mental disorders [10]. Increasing evidence has suggested complex functional interactions between these neurotransmitter systems at the anatomical and pharmacological levels. Generally, endocannabinoids are released on demand by the postsynaptic neurons and travel retrogradely across the synapse, binding to and activating CB1Rs located on the presynaptic terminals [125]. Such activation results in the short- or long-term decrease in neurotransmitter release [126].
VTA dopaminergic cells can be considered a hub between brain regions processing sensory and cognitive information that use the endocannabinoid lipid molecules as metabolic and homeostatic signal detectors, influencing cell function [125]. The effects of cannabinoids/endocannabinoids on dopamine transmission and dopamine-related behaviors are generally indirect and exerted through decreased neurotransmission [94]. Thus, cannabinoid agonists reduce glutamate release from hippocampal neurons [129], which results in a net increase in cortical pyramidal neuron excitability via the activation of CB1Rs located on inhibitory GABAergic cells [127]. However, Steffens et al. had demonstrated that the existence of CB1Rs in human neocortical dopamine terminals also directly affects cortical dopamine input [130]. All these mechanisms likely contribute to cannabinoid-induced learning and memory impairments. Furthermore, certain endocannabinoids (e.g., N-arachidonoyl dopamine and AEA) may directly activate transient receptor potential vanilloid 1 channel (TRPV1) receptors [125,131], thereby allowing direct facilitatory regulation of dopamine function (e.g., at the nucleus accumbens) that influences the motivated behavior and reward process [9].
4.3. Astrocytes as a Potential Stage for the Endocannabinoid System and Kynurenine Interaction in Schizophrenia
4.3.1. Overview of Astrocytes and Their Role in Schizophrenia
For many years, astrocytes were believed to be passive brain elements that maintain structural and metabolic support for neurons [12]. However, recent studies have clearly demonstrated that astrocytes are vital functional components of synapses, forming the so-called tetrapartite synapse, including pre- and postsynaptic elements, other distinct glia cells aside from astrocytes (e.g., NG2 or microglia), and the extracellular matrix [132,133,134]. In the tetrapartite synapse, astrocytes together with the extracellular matrix create a synaptic cradle providing the basis for essential processes contributing to neuroplasticity, such as synaptogenesis and synaptic maturation, isolation, and maintenance [135]. Accordingly, one recent review reported that each element of the tetrapartite synapse is disrupted in schizophrenia [136]. CB1Rs and certain enzymes of the KP in astrocytes have been strongly associated with schizophrenia and will be reviewed in this section, together with KYNA and its receptor targets in astrocytes. Moreover, Table 3 summarizes the participating members for kynurenines and associated elements and the ECS, as well as their common points, in astrocytes involved in schizophrenia.
Table 3.
Kynurenines and associated elements (enzymes, receptors) and members of the ECS present in astrocytes and involved in schizophrenia. The table also highlights the common points between the two systems.
| Members and Features | References |
|---|---|
| Kynurenines and associated elements | |
| KYNA | [137] |
| KAT II | [35] |
| α7nAChR 1 | [138] |
| ECS | |
| AEA, 2-AG | [122,123] |
| DAGL, MAGL | [139,140] |
| CB1R | [141] |
| Common points | |
| Involved in the THC-induced enhanced glutamate release | [142] |
| Co-localized CB1R and α7nAChRs mRNA | [142] |
1 although other KYNA receptors are present in astrocytes (see Section 4.3.2.), α7nAChRs have been the most promising candidate for mediating the effects of KYNA associated with schizophrenia [61].
4.3.2. CB1Rs, KYNA Production, and Target Receptors of KYNA in Astrocytes
CB1Rs located on astrocytes are particularly interesting given their very low expression levels therein [141,143,144], which is in contrast to their significance in terms of synaptic transmission, long-term synaptic plasticity, and thus working memory [145,146,147]. Another interesting aspect of astrocyte-derived CB1Rs is their coupling to Gq/11 type G-proteins, which activates phospholipase C and produces inositol triphosphate [147]. This differs from the more widespread Gi/o type coupling, which inhibits adenylate cyclase and cAMP production [78]. Additionally, 2-AG and AEA endocannabinoids are also produced in astrocytes [122,123]. In fact, CB1Rs and the 2-AG synthesizing enzyme, diacylglycerol lipase (DAGL; Figure 3), are co-expressed in close vicinity, although this was demonstrated in spinal astrocytes from rats [139]. Moreover, MAGL, the enzyme responsible for 2-AG degradation (Figure 3), is also expressed in astrocytes [140].
Astrocytes are key players in the KP given that KYNA synthesis (i.e., the irreversible transamination of l-KYN to KYNA via KAT enzymes) takes place almost exclusively in such cells throughout the mammalian brain [35]. Among the KAT enzymes, the type II enzyme is responsible for approximately 75% of KYNA production in the mammalian brain under normal conditions [137] and can be found mainly in astrocytes [148], with l-KYN being its only endogenous substrate [33,149]. Additionally, KYNA-producing astrocytes are positioned close to the capillary walls and pericytes of the blood–brain barrier, which allows these glia cells to effectively accumulate l-KYN from the circulation and quickly respond to fluctuations in peripheral KYN concentrations [150,151,152,153].
α7nAChRs, which are functionally expressed in astrocytes, have been implicated in memory functions and neuroprotection [138,154]. Given the low abundance of NMDARs, demonstrating their presence and functionality in astrocytes has remained challenging. Nevertheless, studies have shown that astrocytic NMDARs are constructed from the same set of seven subunits, albeit differently configured and assembled compared with neuronal NMDARs [155]. It is now clear that astrocytic NMDAR activation generates intracellular calcium signaling, which—at least in hippocampal astrocytes—has been suggested to enhance the release of inhibitory gliotransmitters (e.g., ATP or endocannabinoids), eventually modulating presynaptic strength [156]. However, further studies are needed to explore the effect of astrocytic NMDARs on neurotransmission modulation. To date, functionally active GPR35 receptors have only been demonstrated in cultured astrocytes, in which the activation of such receptors via KYNA reduces forskolin-induced cAMP production and ATP-induced calcium transients [48].
4.3.3. The Role of Astrocytic CB1Rs, α7nAChRs, and KYNA in Glutamate Neurotransmission and Its Significance in Schizophrenia
Astrocytes play a significant role in glutamate biosynthesis, glutamate–glutamine cycle, glutamate uptake and release, and d-serine biosynthesis and release, all of which are known to be dysregulated in schizophrenia [157]. The role of CB1Rs and α7nAChRs in astrocytic glutamate neurotransmission has been studied in detail. Accordingly, activating the aforementioned receptors stimulates glutamate release, whereas blocking them inhibits this process [145,158], thereby modulating neuronal excitability. In fact, studies have demonstrated that astrocyte-derived KYNA reduces glutamate release in the PFC through α7nAChR. A recent study by Secci and coworkers revealed that CB1R and α7nAChR mRNA co-localize on rat cortical astrocytes in the medial PFC [142] and are involved in the THC-induced increase in glutamate release within the same region given that it was inhibited by both rimonabant and KYNA [142]. Evidence has shown that cannabis use can reduce the negative symptoms of schizophrenia [159,160], which Secci and coworkers found to be in agreement with their results. In other words, excessive KYNA levels in the medial PFC associated with schizophrenia reduce astrocytic glutamate release through the inhibition of α7nAChR, resulting in glutamate and NMDAR hypofunction in the medial PFC, which is also attributed to the disorder. Thus, cannabis can attenuate astrocytic-derived glutamate hypofunction and potentially improve the symptoms associated with schizophrenia. Additionally, astrocytic CB1Rs and KYNA via α7nAChRs may secondarily modulate dopamine release and the reinforcing properties of THC [161,162,163,164,165].
4.4. The Involvement of Kynurenines and the Endocannabinoid System in the Inflammatory Component of Schizophrenia
4.4.1. The Inflammatory Hypothesis of Schizophrenia
Numerous genetic, epidemiological, and clinical evidences have suggested that inflammatory pathways are disrupted in schizophrenia. Moreover, several studies have demonstrated that individuals with infection or autoimmune diseases are more susceptible to schizophrenia [166,167,168,169,170]. The inflammations associated with schizophrenia, as will be discussed in the following section, are related to both the CNS and peripheral organs, especially GI inflammation. Several studies have demonstrated that both the ECS and kynurenines, as well as their related enzymes and receptors, are involved in inflammation and immune regulation [13,19,111,171]. Although no reported evidence has yet suggested crosstalk between these two systems in the inflammatory hypothesis of schizophrenia, many common points indicate its possibility, including inflammatory cytokine regulation, microglial activation, oxidative stress, GI inflammation, and related microbiome regulation, which will be explored in the following sections. Participating members and common points in the described mechanisms are summarized in Table 4.
Table 4.
Kynurenines and associated elements (enzymes, receptors) and members of the ECS that participate in the inflammatory mechanism of schizophrenia. The table also highlights the common points between the two systems relevant to this aspect.
| Members and Features | References |
|---|---|
| Kynurenines and associated elements | |
| l-KYN, KYNA, 3-HK | [15,35,172] |
| KAT, IDO, KMO | [173,174,175] |
| GPR35 1, AHR 1 | [14,15] |
| ECS | |
| AEA, 2-AG | [176,177,178] |
| CB2R, CB1R | [19,179] |
| Common points | |
| Cytokine regulation, microglial activation | [19,35,175,177,178,179] |
| Oxidative stress | [16,17,20,180,181,182,183,184] |
| KYNA and endocannabinoids communicate with gut microbiome | [14,18,185,186,187] |
| Involvement in IBD | [18,186,188] |
| Common features of GPR35 and CBRs | [49,78,189,190,191] |
1 targeted by KYNA.
4.4.2. Neuroinflammation, Cytokines, and Microglia Activation
A substantial amount of data has shown that acute and chronic CNS inflammation, which can be induced by infectious agents, environmental toxins, factors, neural lesions, or genetic defects, is associated with schizophrenia [192,193]. Several inflammatory degradation products, among which inflammatory cytokines are the most significant [192], have been observed in brain tissues and the CSF of approximately 50% of patients with schizophrenia [194,195]. Inflammatory cytokines are important mediators in the communication between the CNS and immune system, with previous studies thoroughly demonstrating their imbalance in schizophrenia [196,197]. Considering that microglial dysfunction is also a significant factor in the development of inflammation and schizophrenia, the microglial hypothesis has been another suggested mechanism contributing to the pathology of the disorder [193,198,199,200,201,202]. Microglia are the main components of the immune system of the CNS. Accordingly, systemic inflammation activates microglia, which in turn produce and release proinflammatory cytokines and reactive oxygen species (ROS), increasing blood–brain barrier permeability [203]. This allows inorganic and organic toxins to more easily enter the CNS, contributing to neurological diseases, such as schizophrenia [204]. Microglial overactivation leads to microglial sensitization or priming, wherein microglia will subsequently induce an exaggerated immune response to a weak stimulus in the form of higher levels of cytokine production/release and microglial proliferation [205,206], which can influence the development of schizophrenia [192].
Studies have shown a link among inflammation, Trp metabolism/KP, and schizophrenia [111]. Proinflammatory cytokines, such as interferon-γ (IFN-γ), interleukin 1, and tumor necrosis factor alpha (TNFα), are able to shift Trp metabolism to l-KYN by increasing IDO enzyme activity [173,174,207,208]. Accordingly, IDO1 expression and enzymatic activity have been demonstrated to be upregulated in response to infection, resulting in the accumulation of l-KYN and 3-HK, which possess antimicrobial activity [172]. Interestingly, no pathogen has thus far shown sensitivity to KYNA [172], which has been demonstrated to have anti-inflammatory and immunosuppressive properties [15]. These properties are mainly mediated through GPR35 and AHR receptors [15]. Multiple studies have found an association between Toxoplasma gondii, an obligate intracellular protozoan parasite that causes the infectious disease toxoplasmosis, and schizophrenia [209,210,211,212,213]. This parasite has been suggested to increase IFN-γ production, which activates IDO in microglia leading to Trp degradation and L-KYN elevation [214,215]. Consequently, the concentration of other kynurenines increases dramatically, including KYNA in astrocytes, which were at the peak level 28 days post-infection and continued elevating after 56 days [213]. This persistent brain KYNA elevation may contribute to the cognitive impairment observed in schizophrenia [212]. The KAT enzyme, which seems to be cell-type specific, has also been involved in inflammatory regulation. Reports have shown that IFN-γ alone or in combination with TNF reduced KAT II, III, and IV mRNA expression in human dermal fibroblast cells [175]. Interestingly, the same study revealed that KYNA levels were increased in the presence of IFN-γ. In fetal astrocytes, IFN-γ increased the level KAT I and II transcripts [35], whereas lipopolysaccharide treatment also increased KAT I but reduced KAT II mRNA expression in the hippocampus [216].
The ECS plays a key role in immunomodulation. Accordingly, both exogenous cannabinoids and endocannabinoids suppress the production and release of proinflammatory cytokines in both peripheral organs and the CNS through CB2Rs [19,179]. Another study demonstrated higher CBR availability on innate immune cells and a simpler correlation network between cytokines and CBR expression among patients with schizophrenia than among controls [217]. Circulating endocannabinoid levels have been known to increase several fold during systemic inflammation [176]. This seems to be supported by the finding of increased AEA levels in the CSF of patients with schizophrenia, although it is negatively correlated with psychotic symptoms in the disorder [96]. Interestingly, studies have reported a positive correlation between 2-AG levels and proinflammatory cytokine interleukin 6 concentrations [177,178]. Patients with borderline personality disorder share most of the positive symptoms with those with schizophrenia and exhibit significantly higher circulating 2-AG and AEA levels compared with controls [218]. As discussed in the previous sections, cannabis consumption is a potential risk for the development of schizophrenia in vulnerable individuals, such as adolescents. Additionally, immunomodulation can be one of the causal background mechanisms of cannabis. Δ9THC has also been shown to reduce cytokine production and secretion in most immune cells of the CNS. Cytokines play a significant role in neurodevelopment and modulation of neurotransmitter and neuropeptide systems, including the monoamine system [219], which might explain why adolescence is the most susceptible period for cannabis smoking. Exogenous cannabinoids can also modify microglia functioning and thus alter neurotransmission release and neuron architecture [198,220,221,222]. Additionally, studies have reported that both GPR35—of which the KYNA is an endogenous ligand—and CB2R are expressed on leukocytes and involved in leukocyte recruitment, which can be induced by KYNA in the case of GPR35 [223,224,225,226,227,228,229]. In fact, GPR35 and CB2R (and CB1R) have similar structures and receptor signaling pathways [49,78], with studies suggesting a linkage between GPR35 and cannabinoid receptors through the interconversion of their endogenous ligands, 2-acyl lysophosphatidic acid and 2-AG [189]. Thus, the aforementioned data may indicate a potential interaction between GPR35-mediated KYNA signaling and CB2R in inflammatory processes associated with schizophrenia.
4.4.3. ROS and Oxidative Stress
ROS, such as superoxide or hydroxyl radicals, are byproducts of several enzymatic reactions related to basic metabolic functions occurring in certain cell compartments, such as mitochondria, peroxisome, endoplasmic reticulum, cell membrane, or cytoplasm [230]. Oxidative stress refers to the imbalance between ROS and the class of protective reduction–oxidation enzymes that detoxify ROS, such as catalase, superoxide dismutases, and enzymes of the glutathione system (e.g., glutathione peroxidases) [231]. Inflammatory processes are tightly associated with oxidative stress and ROS production given that the immune system starts to intensely produce ROS in response to infection, which partly elicits inflammation via immune cell and microglial cytokine production [231]. Inflammatory cytokines, such as TNFα and interleukins 1 and 10, or other inflammation-inducing signals, such as lipopolysaccharide, thrombin, or oscillatory shear stress, affect ROS production. Increased ROS levels can activate nuclear factor κ-light-chain enhancer of activated B cells (NF-κB), which then induces downstream mechanisms, such as antioxidant and inflammatory gene transcription or proteasome and inflammasome activation [230]. Considerable data have demonstrated increased oxidative stress in patients with schizophrenia, indicated by increased DNA, lipid, and protein oxidation and increased levels of total ROS accompanied by reduced gene levels of antioxidant enzymes [231,232]. Additionally, patients with schizophrenia exhibit mitochondrial dysfunction, which induces oxidative stress and inflammatory processes [233]. As such, studies have suggested that oxidative stress ties together certain risk factors of schizophrenia, such as aberrant neuronal migration, synapse formation, neurotransmission, or neuroinflammation [231].
The KP plays a significant role in maintaining antioxidant balance in the brain. Persistent oxidative stress via an imbalanced KP may lead to disrupted glutamatergic and dopaminergic neurotransmission and altered brain functioning (see Section 4.2.) [192,196]. Certain metabolites of the KP (see Section 2.1.1.) can generate oxidative stress and ROS, such as 3-HK, 3-HA, or QUIN [16,180,181,182,234,235,236], with QUIN also being able to induce lipid peroxidation and mitochondrial dysfunction [16,235,237,238,239,240]. On the other hand, KYNA behaves as an endogenous antioxidant by scavenging free radicals and inhibiting oxidative stress [182,236,238]. Additionally, a quantitative analysis of schizophrenia-associated serum metabolites revealed low levels of the antioxidant glutathione accompanied by increased levels of Trp and kynurenine [241].
The link between the ECS and redox homeostasis has now become evident given the numerous studies revealing the neuroprotective effects of cannabinoid ligands [183,242,243]. Furthermore, endocannabinoids are significantly involved in cell ROS production given that they control mitochondria-derived ROS generation [20] notably by altering the expression and/or activity of mitochondrial electron-transport chain components and/or by promoting changes in mitochondrial membrane potential via the CB1R [244]. The ECS and related endocannabinoids can also regulate oxidative stress and lipid peroxidation either through both CBRs or by scavenging free radicals [184,245]. Interestingly, CB1R and CB2R are distinctly involved in oxidative stress regulation, depending on the cell and injury type and disease progression [245]. Accordingly, CB1R activation enhances redox imbalance, whereas CB2R activation lowers ROS production [20,21,184].
4.4.4. Gastrointestinal Inflammation and Gut Microbiome
Considering that the GI tract is our body’s largest immune organ and is connected bidirectionally to the brain through multiple neuronal pathways, disruption in GI function can affect the brain and has been linked to the development of schizophrenia [246]. Given that the gut–immune barrier and blood–brain barrier are functionally and structurally similar [247], the hypothesis is that toxic and bioactive compounds penetrate through the epithelial and endothelial barriers of both the GI tract and CNS, thereby inducing an immune response [246]. Schizophrenia has also been associated with GI inflammatory comorbidities, such as irritable bowel syndrome (IBS) and inflammatory bowel diseases (IBD) [248,249]. The involvement of the gut microbiome in the inflammatory component of schizophrenia has also been an emerging field. Accordingly, a bidirectional relationship has been suggested, given that changes in the microbial flora of the gut might lead to schizophrenia or other neuropsychiatric disorders [250,251], while the brain can also alter the microbial habitat and composition in the GI [252]. Studies have reported abnormal microbiome function, composition, and amount in the oropharynx and feces of drug-naive patients with schizophrenia [253,254,255,256,257]. Interestingly, risperidone—the most common medication for schizophrenia—has been shown to alter fecal bacterial composition [258].
KYNA has been extensively studied in the GI system. Interestingly, KYNA content gradually increases along the GI tract, with the distal-most portion having the highest content [14]. Considering the positive correlation observed between KYNA content and microflora concentration in the small intestine [186], the gut flora has been suggested to produce the common pool of intestinal KYNA [14]. Notably, certain food and herbs, such as honey, broccoli, or basil, also contain KYNA in micromolar concentrations [259,260]. Additionally, KYNA may possess both negative and positive effects in bowel diseases [14]. Accordingly, serum KYNA levels are increased in IBS most probably as a compensatory mechanism [186] but are reduced in IBD [186]. Moreover, studies have shown that KYNA stimulates bacterial growth in the GI system at low and medium concentrations [187] but displays antimicrobial activity at high concentrations [261]. The GI-related effects of KYNA are mediated through GPR35 [14], which is highly expressed in the GI tract [49,190] and has been associated with IBD [191].
Endocannabinoids have been known to communicate with the gut microbiome [185] while also playing an important role in regulating intestinal microbial product entry into the bloodstream and thus in the development of metabolic diseases [18,19]. Additionally, multiple studies have highlighted the therapeutic relevance of the ECS in IBD and IBS [18,188]. Cannabinoid receptors are abundantly expressed in different areas/cells of the GI system, such as on enteric nerves, enteroendocrine cells, immune cells, and enterocytes [19]. Similar to GPR35, cannabinoid receptors have also been implicated in IBD [262]. Thus, considering the previously discussed overlapping functional and structural properties of cannabinoid and GPR35 receptors, their high expression levels in the GI system, and their common involvement in IBD, another potential area for their interaction within the inflammatory component of schizophrenia can be surmised.
5. Therapeutic Potentials
5.1. Overview
This section will highlight the therapeutic potentials of the KP and ECS in the treatment of schizophrenia. Numerous studies have investigated KAT II inhibitors and CBD, which will be reviewed here (also see Table 1 and Table 5). The most appealing approach would be to combine both types of compounds to achieve a synergistic and more efficacious therapeutic effect. Additionally, these alternative therapeutic targets might improve the main limitations of currently available medications, namely, their poor effect on negative symptoms and cognitive impairment, as mentioned in the introduction. A separate section will discuss the currently available dopaminergic antipsychotic medications and clinical studies of non-dopaminergic agents in order to assess the potential of KAT II inhibitors and CBD.
Table 5.
A summary of potential therapeutic approaches for schizophrenia by targeting the kynurenine pathway (KP) and ECS.
5.2. Currently Available Medications
The goals in treating schizophrenia include targeting symptoms, preventing relapse, and increasing adaptive functioning through both pharmacological and non-pharmacological (such as psychotherapy) treatments whereby the patient can be integrated back into the community [105,276].
Antipsychotic drugs (APDs), which have been primarily used to manage psychosis (including hallucinations, delusions, disordered thought, or paranoia), have been the mainstay of pharmacological treatment protocols in schizophrenia as recommended by the National Institute of Health and Care Excellence, World Health Organization, and the American Psychiatric Association [277,278,279]. All clinically approved and currently used APDs have nanomolar affinity for the dopamine D2 receptor and fully or partially block the actions of dopamine in the mesolimbic pathway [280].
Over the past 50 years, numerous first-, second-, and third-generation antipsychotics have been developed, while dramatic growth in the research of pharmacological schizophrenia treatment has advanced our understanding of the neurobiology and neuropharmacology of the illness [279,281,282]. First discovered in the 1950s, first-generation antipsychotics (e.g., chlorpromazine, haloperidol, and fluphenazine), known as typical APDs, not only have antipsychotic effects but also extrapyramidal side effects, and cause hyperprolactinemia in association with their full D2 receptor antagonism in the CNS. First-generation antipsychotics also possess high affinity for muscarinic M1 ACh, histaminergic H1, and α1 norepinephrine receptors, which can result in partially distinctive side-effect profiles (e.g., cognitive deficits and sedation) [283].
Since the 1990s, newer drug compounds (clozapine, risperidone, olanzapine, quetiapine, etc.) that blocked both dopamine and serotonin receptors were met with great expectations [284,285] and were found to be effective in alleviating both positive and negative symptoms [105]. Although the introduction of second-generation antipsychotics had become a cornerstone in the treatment of schizophrenia, several unmet treatment needs in the field still existed. While newer antipsychotics produced fewer motor side effects, safety and tolerability concerns regarding metabolic side effects, such as obesity, dyslipidemia, and type 2 diabetes, have emerged [286].
Third-generation antipsychotics (e.g., aripiprazole and cariprazine), which are partial D2 agonists, represent another pharmacologically different strategy in the attempt to normalize dopaminergic imbalance in schizophrenia. Compared with full agonists, these agents have lower intrinsic activity at D2 receptors, allowing them to act as either functional agonists or antagonists, thereby inhibiting endogenous dopamine activity in the mesolimbic and activating the mesocortical pathways [287,288]. In addition, such an agent should ideally maintain dopaminergic tone in the nigrostriatal and tuberoinfundibular pathways, thereby preventing extrapyramidal symptoms and hyperprolactinemia. Additionally, they usually have partial agonist properties at dopamine D3, D4, 5-hydroxytriptamin (5-HT)1A, 5-HT2C, and, to a much lesser extent, 5-HT2A receptors [289,290].
Considering that nearly 30% of patients do not respond to dopaminergic antipsychotics, treatment resistance in schizophrenia and the need for decreasing serious adverse effects (extrapyramidal and metabolic) associated with their long-term use have remained as major issues in psychiatry [291]. Findings regarding the inefficiency and safety profile of APDs have prompted the discovery of promising new targets for the development of non-dopaminergic drugs based on the glutamatergic and GABAergic hypothesis of schizophrenia that may replace currently used treatments. These will be reviewed briefly in the following section.
Non-Dopaminergic Agents in Clinical Studies Based on the Glutamatergic and GABAergic Hypothesis of Schizophrenia
Several approaches have been used in restoring NMDAR hypofunction [114]. While classical NMDAR agonists have not been useful given that their excessive stimulation results in excitotoxicity and neuron damage, the modulatory mechanisms of NMDAR functioning have been considered as more promising targets [113,292,293]. Clinical trial results regarding NMDAR-enhancing small molecules as an adjunct to dopaminergic drugs, such as glycine and d-serine (endogenous full agonists of the NMDAR glycine site) and D-cycloserine (a partial NMDAR agonist), have been inconsistent [294,295,296,297,298,299,300]. Memantine, a drug that acts as a weak nonselective NMDA receptor antagonist, had been associated with significant attenuation of positive, negative, and cognitive symptoms when used as an add-on treatment to clozapine or olanzapine [301,302]. Positive allosteric modulators of AMPA-type glutamate receptors, such as ampakines, and glycine transporter blockers, such as N-methylglycine (sarcosine), have also been considered as promising therapeutic agents used in adjunct to already available dopaminergic antipsychotics [303,304,305,306]. Preclinical studies have suggested that compounds targeting metabotropic glutamate receptors, specifically subtype-selective allosteric modulators, may also be used as an alternative to current treatments [114,307].
One pilot study involving a 4-week treatment with MK-0777, a partial GABA(A) receptor agonist, revealed progress in cognitive performance among patients with chronic schizophrenia, providing support for the beneficial effect of enhanced GABA activity in prefrontal functioning [308]. However, a later clinical study involving 60 patients with schizophrenia showed little benefit [308]. Thus, more potent partial agonists with greater intrinsic activity at the GABA(A) α2 site might be needed for cognitive enhancement in schizophrenia.
In conclusion, the abovementioned non-dopaminergic drugs have little to no effect when used by themselves, but may improve the negative symptoms and cognitive impairments when used as adjunct treatment to dopaminergic drugs without significant safety concerns. Based on these clinical findings, compounds targeting the KP and ECS could be a compelling alternative approach toward satisfying the unmet clinical needs of patients with schizophrenia.
5.3. Targeting the KP
Pharmacological manipulation of the KP for the treatment of schizophrenia is a complex approach as described by Müller and colleagues [26]. Although increased brain KYNA levels have now been considered as an important factor contributing to the complex symptoms of the disorder, reducing KYNA levels could impair its neuroprotective effect against, for example, QUIN-induced excitotoxicity [309]. Nevertheless, while many studies have dealt with this subject, KAT enzyme targeting has been the most intensely studied therapeutic approach against schizophrenia.
As discussed in Section 2.1.1., KATs are responsible for the irreversible transamination of l-KYN to KYNA [33], mainly in astrocytes. Thus, inhibiting KAT enzyme activity can be considered as a logical approach for reducing increased brain KYNA levels associated with schizophrenia. This approach would be less likely to interfere with other parts of the KP [310]. As described in Section 4.3.2., KAT II has the greatest potency for therapeutic targeting among the four KAT enzymes owing to its substrate specificity and its role in the production of most of the KYNA in the brain. Studies have shown that reducing brain KYNA concentrations significantly improves cognitive functions through selective inhibition of the KAT II enzyme [70,311]. While multiple KAT II inhibitors have been developed to date, earlier designs, such as (S)-ESBA and BFF-122, were able to increase extracellular levels of dopamine, acetylcholine, and glutamate and improve memory functions in rats with schizophrenia-like symptoms [63,312,313,314,315]. However, due to poor blood–brain barrier penetration, these earlier compounds required intracerebral administration to achieve central effects. Such compounds were followed by systematically active, brain-penetrant KAT II inhibitors, such as PF-04859989 [316] and BFF-816 [311]. Accordingly, PF-04859989 irreversibly inhibited both rat and human KAT II, acutely inhibited amphetamine- and ketamine-induced disruption of auditory gating, and improved performance in a sustained attention task. Moreover, it prevented ketamine-induced disruption of performance in a working and spatial memory task in rodents and nonhuman primates, respectively [70]. These behavioral experiments were confirmed by electrophysiological studies, wherein PF-04859989 reduced the activity of midbrain dopamine neurons and nicotine-evoked glutamatergic activity in the rat cortex [317,318]. Other compounds have been developed to improve the pharmacological properties of PF-04859989 [22,319]. In contrast to PF-04859989, BFF-816 reversibly inhibited KAT II, improved performance in spatial and contextual memory, attenuated evoked glutamate release in rat PFC, and decreased hippocampus-dependent memory deficits in adult rats prenatally treated with kynurenine [54,311,320]. Additionally, previous studies have reviewed several other design approaches for KAT II inhibition [22,23,24,25,27].
Apart from KAT II, limited studies have examined other KP enzymes as a therapeutic target for schizophrenia. One recent study describing an animal model of schizophrenia induced by ketamine revealed that IDO, TDO, and KMO inhibition improved behavioral changes, prevented lipid peroxidation and protein damage, and protected against antioxidant enzymes in rats [321]. IDO, in particular, gained more attention due to its previously discussed role in inflammation associated with the disease [7,26,263].
5.4. Targeting the Endocannabinoid System
A considerable amount of data has suggested a connection between excess Δ9THC and synthetic cannabinoid consumption and the development of schizophrenia. However, recent evidence has also shown the positive effects of cannabinoid compounds in patients with schizophrenia. For instance, one study showed that dronabinol, the synthetic variant of Δ9THC, reduced core psychotic symptoms in three out of six treatment-refractory patients with severe chronic schizophrenia, who had a self-reported history of improvement with marijuana abuse [272].
Among cannabinoid compounds, CBD appears to be the most promising for the treatment of schizophrenia. CBD, the other main component of cannabis, does not possess psychoactive properties as mentioned previously. In fact, some of the effects of CBD on brain function and psychiatric symptoms contrast those of Δ9THC [322]. In contrast, a recent study reported that CBD does not attenuate Δ9THC-induced acute psychosis and memory impairments [102]. The precise mode of action of CBD has yet to be fully understood given that it has mixed pharmacological properties, including a week antagonistic binding toward CBRs, inhibition of FAAH activity, and stimulation of TRPV1, the 5-HT1A receptor, and the D2 dopamine receptor [323,324]. Moreover, Bih and coworkers revealed that numerous additional receptors, transporters, ion channels, and enzymes that could serve as molecular targets for CBD are involved in neurological disorders [325]. According to preclinical studies, CBD reduced amphetamine-induced effects on prepulse inhibition and hyperlocomotion induced by other psychotomimetic drugs [265,326]. Human studies have shown that CBD improved both positive and negative symptoms of schizophrenia [264,266,327,328,329]. Accordingly, studies that showed negative results provided either a single dose or monotherapy of CBD [330,331] or included patients with chronic schizophrenia who received multiple antipsychotic medications [102]. Furthermore, compared with the conventional antipsychotic amisulpride, CBD reduced schizophrenia symptoms but with significantly less side effects [266]. The same study also showed that CBD increased serum AEA levels, which was associated with symptom improvement. This can be explained by the ability of CBD to block FAAH activity, although other mechanisms have been proposed for its antipsychotic effects (e.g., via the already mentioned D2, 5-HT1A and TRPV1 receptors) [325,332].
Studies have shown that AEA levels are inversely correlated with the severity of negative schizophrenia symptoms [96], which leads to the assumption that high AEA levels might be advantageous in schizophrenia. Thus, selective FAAH inhibition has also been extensively studied apart from CBD. Accordingly, blocking AEA degradation improved both PCP- and amphetamine-induced positive and negative symptoms in animals [267,268]. URB597, a selective FAAH inhibitor, reversed PCP-induced social withdrawal effects and associated changes in c-Fos activation/inactivation observed in distinct neuroanatomical locations related to the social interaction neurocircuitry [333]. Selective FAAH inhibition also alleviated the hyperdopaminergic phenotype of adult rats [270]. However, a novel schizophrenia rat model showed that during adolescence, URB597 treatment—which is similar to exogenous cannabinoid treatment—increased the proportion of susceptible rats developing increased dopamine neuron activity [269]. Unlike exogenous cannabinoid, however, URB597 did not alter the behavioral response to amphetamine. Finally, a study on mouse hippocampal neuronal cell lines revealed that AEA was a potential candidate for the treatment of oxidative stress-related neurological disorders. The same study showed that during H2O2-induced redox imbalance, AEA increased intracellular levels of superoxide dismutase and glutathione via CB1R, thereby protecting the cells from oxidative stress [271].
The higher CB1R density and/or endocannabinoid levels in certain cortical and subcortical (limbic) structures in patients with schizophrenia might also be associated with dopaminergic neuron hyperactivity (positive symptoms) and glutamate neuron hypoactivity (negative symptoms) [9]. Preclinical studies have revealed that the antipsychotic potential of the CB1R antagonist rimonabant was related to alterations in dopamine and glutamate transmissions in cortical structures [273,274]. Moreover, a 16-week double-blind, placebo-controlled, randomized clinical trial showed that rimonabant did not improve global cognitive functioning, but did improve a specific learning deficit based on the response to positive feedback [275]. Furthermore, one study showed that the rimonabant group exhibited a significantly better total Brief Psychiatric Rating Scale score and anxiety/depression and hostility factors compared with placebo-treated patients with schizophrenia [275]. However, another placebo-controlled clinical trial showed no improvements [334].
6. Summary and Conclusions
Schizophrenia has many aspects in which both kynurenines and the ECS are involved. Although both have already been separately reviewed in detail, their overlapping functions, mechanisms, and potential interaction in schizophrenia have yet to be elucidated. Therefore, the present review aimed to highlight such aspects. Accordingly, the most well-known overlapping areas include dopaminergic, glutamatergic, and GABAergic transmission regulation via cannabinoids and KYNA. Moreover, the most possible receptor mediator for KYNA in this mechanism is the astrocytic α7nAChR given that NMDAR inhibition by KYNA does not seem to influence glutamate release [61]. Inflammatory mechanisms contributing to the development of schizophrenia are complex and widespread and need to be studied more thoroughly. The overlapping structural, pharmacological, and anatomical properties between GPR35 and CBRs are also promising candidates for regulating the common aspects of inflammation associated with schizophrenia.
Though the treatment of schizophrenia still remains challenging, a better understanding of the possible connections between kynurenines and the ECS could introduce novel therapeutic compounds and targets for treatment. Such compounds could also compensate for limitations of currently available medications. While KAT II inhibitors and CBD are promising, it will be interesting to determine whether co-administration would yield a synergistic effect. Nonetheless, additional studies are needed to adequately explore the interaction between kynurenines and the ECS and to better understand their separate functioning.
Finally, parallel alterations in kynurenines/the KP and the ECS are present not only in schizophrenia but also in other neurological disorders, such as Alzheimer’s disease [38,335,336,337]. Thus, studying the interaction between kynurenines and associated elements and the ECS might also help us further understand mechanisms and disorders apart from schizophrenia.
Acknowledgments
The authors would like to thank Enago (www.enago.com) for the English language review.
Author Contributions
Conceptualization, F.Z.; Writing—original draft preparation; F.Z., G.N.-G., G.K., S.D., E.S.; Writing—review and editing, F.Z., G.N.-G., G.K., G.H., L.V., C.T., S.B.; Visualization, F.Z., G.N.-G.; Project administration, F.Z., G.N.-G., L.V.; Funding acquisition, L.V.
Funding
This study was supported by GINOP (2.3.2-15-2016-00034), by the Ministry of Human Capacities, Hungary grant 20391-3/2018FEKUSTRAT and by the MTA-SZTE Neuroscience Research Group of the Hungarian Academy of Sciences and the University of Szeged. C.T. and S.D. were supported by the grant K124952 of National Research, Development and Innovation Office. Publication costs were financed by the ‘University of Szeged Open Access Fund’ (Grant number: 4301).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.GBD 2016 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet (Lond. Engl.) 2017;390:1211–1259. doi: 10.1016/S0140-6736(17)32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erhardt S., Schwieler L., Imbeault S., Engberg G. The kynurenine pathway in schizophrenia and bipolar disorder. Neuropharmacology. 2017;112:297–306. doi: 10.1016/j.neuropharm.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 3.Manseau M.W., Goff D.C. Cannabinoids and Schizophrenia: Risks and Therapeutic Potential. Neurotherapeutics. 2015;12:816–824. doi: 10.1007/s13311-015-0382-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erhardt S., Schwieler L., Nilsson L., Linderholm K., Engberg G. The kynurenic acid hypothesis of schizophrenia. Physiol. Behav. 2007;92:203–209. doi: 10.1016/j.physbeh.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 5.Nagy-Grócz G., Zádor F., Dvorácskó S., Bohár Z., Benyhe S., Tömböly C., Párdutz Á., Vécsei L. Interactions between the Kynurenine and the Endocannabinoid System with Special Emphasis on Migraine. Int. J. Mol. Sci. 2017;18:1617. doi: 10.3390/ijms18081617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colín-González A.L., Aguilera G., Santamaría A. Advances in Neurobiology. Volume 12. Springer; Cham, Switzerland: 2016. Cannabinoids: Glutamatergic Transmission and Kynurenines; pp. 173–198. [DOI] [PubMed] [Google Scholar]
- 7.Myint A.-M., Kim Y.-K. Network beyond IDO in psychiatric disorders: Revisiting neurodegeneration hypothesis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2014;48:304–313. doi: 10.1016/j.pnpbp.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Plitman E., Iwata Y., Caravaggio F., Nakajima S., Chung J.K., Gerretsen P., Kim J., Takeuchi H., Chakravarty M.M., Remington G., et al. Kynurenic Acid in Schizophrenia: A Systematic Review and Meta-analysis. Schizophr. Bull. 2017;43:764–777. doi: 10.1093/schbul/sbw221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernández-Ruiz J., Hernández M., Ramos J.A. Cannabinoid-dopamine interaction in the pathophysiology and treatment of CNS disorders. CNS Neurosci. Ther. 2010;16:e72–e91. doi: 10.1111/j.1755-5949.2010.00144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Müller-Vahl K.R., Emrich H.M. Cannabis and schizophrenia: Towards a cannabinoid hypothesis of schizophrenia. Expert Rev. Neurother. 2008;8:1037–1048. doi: 10.1586/14737175.8.7.1037. [DOI] [PubMed] [Google Scholar]
- 11.Pocivavsek A., Notarangelo F.M., Wu H.-Q., Bruno J.P., Schwarcz R. Handbook of Behavioral Neuroscience. Volume 23. Elsevier; Amsterdam, The Netherlands: 2016. Astrocytes as Pharmacological Targets in the Treatment of Schizophrenia: Focus on Kynurenic Acid; pp. 423–443. [Google Scholar]
- 12.Navarrete M., Díez A., Araque A. Astrocytes in endocannabinoid signalling. Philos. Trans. R. Soc. B Biol. Sci. 2014;369:20130599. doi: 10.1098/rstb.2013.0599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mándi Y., Vécsei L. The kynurenine system and immunoregulation. J. Neural Transm. 2012;119:197–209. doi: 10.1007/s00702-011-0681-y. [DOI] [PubMed] [Google Scholar]
- 14.Turski M.P., Turska M., Paluszkiewicz P., Parada-Turska J., Oxenkrug G.F. Kynurenic Acid in the digestive system-new facts, new challenges. Int. J. Tryptophan Res. 2013;6:47–55. doi: 10.4137/IJTR.S12536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wirthgen E., Hoeflich A., Rebl A., Günther J. Kynurenic Acid: The Janus-Faced Role of an Immunomodulatory Tryptophan Metabolite and Its Link to Pathological Conditions. Front. Immunol. 2017;8:1957. doi: 10.3389/fimmu.2017.01957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pérez-De La Cruz V., Carrillo-Mora P., Santamaría A. Quinolinic Acid, an endogenous molecule combining excitotoxicity, oxidative stress and other toxic mechanisms. Int. J. Tryptophan Res. 2012;5:1–8. doi: 10.4137/IJTR.S8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bryleva E.Y., Brundin L. Kynurenine pathway metabolites and suicidality. Neuropharmacology. 2017;112:324–330. doi: 10.1016/j.neuropharm.2016.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cani P.D., Plovier H., Van Hul M., Geurts L., Delzenne N.M., Druart C., Everard A. Endocannabinoids—At the crossroads between the gut microbiota and host metabolism. Nat. Rev. Endocrinol. 2016;12:133–143. doi: 10.1038/nrendo.2015.211. [DOI] [PubMed] [Google Scholar]
- 19.Maccarrone M., Bab I., Bíró T., Cabral G.A., Dey S.K., Di Marzo V., Konje J.C., Kunos G., Mechoulam R., Pacher P., et al. Endocannabinoid signaling at the periphery: 50 years after THC. Trends Pharmacol. Sci. 2015;36:277–296. doi: 10.1016/j.tips.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lipina C., Hundal H.S. Modulation of cellular redox homeostasis by the endocannabinoid system. Open Biol. 2016;6:150276. doi: 10.1098/rsob.150276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gallelli C.A., Calcagnini S., Romano A., Koczwara J.B., de Ceglia M., Dante D., Villani R., Giudetti A.M., Cassano T., Gaetani S. Modulation of the Oxidative Stress and Lipid Peroxidation by Endocannabinoids and Their Lipid Analogues. Antioxidants. 2018;7:93. doi: 10.3390/antiox7070093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dounay A.B., Anderson M., Bechle B.M., Evrard E., Gan X., Kim J.-Y., McAllister L.A., Pandit J., Rong S., Salafia M.A., et al. PF-04859989 as a template for structure-based drug design: Identification of new pyrazole series of irreversible KAT II inhibitors with improved lipophilic efficiency. Bioorg. Med. Chem. Lett. 2013;23:1961–1966. doi: 10.1016/j.bmcl.2013.02.039. [DOI] [PubMed] [Google Scholar]
- 23.Jacobs K.R., Castellano-Gonzalez G., Guillemin G.J., Lovejoy D.B. Major Developments in the Design of Inhibitors along the Kynurenine Pathway. Curr. Med. Chem. 2017;24:2471–2495. doi: 10.2174/0929867324666170502123114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jayawickrama G.S., Nematollahi A., Sun G., Gorrell M.D., Church W.B. Inhibition of human kynurenine aminotransferase isozymes by estrogen and its derivatives. Sci. Rep. 2017;7:17559. doi: 10.1038/s41598-017-17979-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jayawickrama G.S., Nematollahi A., Sun G., Church W.B. Improvement of kynurenine aminotransferase-II inhibitors guided by mimicking sulfate esters. PLoS ONE. 2018;13:e0196404. doi: 10.1371/journal.pone.0196404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muller N., Myint A.-M., J. Schwarz M. Kynurenine Pathway in Schizophrenia: Pathophysiological and Therapeutic Aspects. Curr. Pharm. Des. 2011;17:130–136. doi: 10.2174/138161211795049552. [DOI] [PubMed] [Google Scholar]
- 27.Nematollahi A., Sun G., Jayawickrama G.S., Church W.B. Kynurenine Aminotransferase Isozyme Inhibitors: A Review. Int. J. Mol. Sci. 2016;17:946. doi: 10.3390/ijms17060946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen K., Weizman A., Weinstein A. Positive and Negative Effects of Cannabis and Cannabinoids on Health. Clin. Pharmacol. Ther. 2019;105:1139–1147. doi: 10.1002/cpt.1381. [DOI] [PubMed] [Google Scholar]
- 29.Rohleder C., Müller J.K., Lange B., Leweke F.M. Cannabidiol as a Potential New Type of an Antipsychotic. A Critical Review of the Evidence. Front. Pharmacol. 2016;7:422. doi: 10.3389/fphar.2016.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wyrofsky R., McGonigle P., Van Bockstaele E.J. Drug discovery strategies that focus on the endocannabinoid signaling system in psychiatric disease. Expert Opin. Drug Discov. 2015;10:17–36. doi: 10.1517/17460441.2014.966680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Behan W.M.H., McDonald M., Darlington L.G., Stone T.W. Oxidative stress as a mechanism for quinolinic acid-induced hippocampal damage: Protection by melatonin and deprenyl. Br. J. Pharmacol. 1999;128:1754–1760. doi: 10.1038/sj.bjp.0702940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rios C., Santamaria A. Quinolinic acid is a potent lipid peroxidant in rat brain homogenates. Neurochem. Res. 1991;16:1139–1143. doi: 10.1007/BF00966592. [DOI] [PubMed] [Google Scholar]
- 33.Han Q., Cai T., Tagle D.A., Li J. Structure, expression, and function of kynurenine aminotransferases in human and rodent brains. Cell. Mol. Life Sci. 2010;67:353–368. doi: 10.1007/s00018-009-0166-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmidt W., Guidetti P., Okuno E., Schwarcz R. Characterization of human brain kynurenine aminotransferases using [3H]kynurenine as a substrate. Neuroscience. 1993;55:177–184. doi: 10.1016/0306-4522(93)90464-Q. [DOI] [PubMed] [Google Scholar]
- 35.Guillemin G.J., Kerr S.J., Smythe G.A., Smith D.G., Kapoor V., Armati P.J., Croitoru J., Brew B.J. Kynurenine pathway metabolism in human astrocytes: A paradox for neuronal protection. J. Neurochem. 2001;78:842–853. doi: 10.1046/j.1471-4159.2001.00498.x. [DOI] [PubMed] [Google Scholar]
- 36.Guillemin G.J., Smith D.G., Smythe G.A., Armati P.J., Brew G.J. Advances in Experimental Medicine and Biology. Volume 527. Springer; Boston, MA, USA: 2003. Expression of The Kynurenine Pathway Enzymes in Human Microglia and Macrophages; pp. 105–112. [DOI] [PubMed] [Google Scholar]
- 37.Beadle G.W., Mitchell H.K., Nyc J.F. Kynurenine as an Intermediate in the Formation of Nicotinic Acid from Tryptophane by Neurospora. Proc. Natl. Acad. Sci. USA. 1947;33:155–158. doi: 10.1073/pnas.33.6.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vécsei L., Szalárdy L., Fülöp F., Toldi J. Kynurenines in the CNS: Recent advances and new questions. Nat. Rev. Drug. Discov. 2013;12:64–82. doi: 10.1038/nrd3793. [DOI] [PubMed] [Google Scholar]
- 39.Lovelace M.D., Varney B., Sundaram G., Franco N.F., Ng M.L., Pai S., Lim C.K., Guillemin G.J., Brew B.J. Current Evidence for a Role of the Kynurenine Pathway of Tryptophan Metabolism in Multiple Sclerosis. Front. Immunol. 2016;7:246. doi: 10.3389/fimmu.2016.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lim C.K., Fernández-Gomez F.J., Braidy N., Estrada C., Costa C., Costa S., Bessede A., Fernandez-Villalba E., Zinger A., Herrero M.T., et al. Involvement of the kynurenine pathway in the pathogenesis of Parkinson’s disease. Prog. Neurobiol. 2017;155:76–95. doi: 10.1016/j.pneurobio.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 41.Nicoletti F. Kynurenine pathway metabolites in migraine. J. Headache Pain. 2015;16:A1. doi: 10.1186/1129-2377-16-S1-A1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Birch P.J., Grossman C.J., Hayes A.G. Kynurenic acid antagonises responses to NMDA via an action at the strychnine-insensitive glycine receptor. Eur. J. Pharmacol. 1988;154:85–87. doi: 10.1016/0014-2999(88)90367-6. [DOI] [PubMed] [Google Scholar]
- 43.Kessler M., Terramani T., Lynch G., Baudry M. A glycine site associated with N-methyl-D-aspartic acid receptors: Characterization and identification of a new class of antagonists. J. Neurochem. 1989;52:1319–1328. doi: 10.1111/j.1471-4159.1989.tb01881.x. [DOI] [PubMed] [Google Scholar]
- 44.Prescott C., Weeks A.M., Staley K.J., Partin K.M. Kynurenic acid has a dual action on AMPA receptor responses. Neurosci. Lett. 2006;402:108–112. doi: 10.1016/j.neulet.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 45.Hilmas C., Pereira E.F., Alkondon M., Rassoulpour A., Schwarcz R., Albuquerque E.X. The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: Physiopathological implications. J. Neurosci. 2001;21:7463–7473. doi: 10.1523/JNEUROSCI.21-19-07463.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dobelis P., Staley K.J., Cooper D.C. Lack of modulation of nicotinic acetylcholine alpha-7 receptor currents by kynurenic acid in adult hippocampal interneurons. PLoS ONE. 2012;7:e41108. doi: 10.1371/journal.pone.0041108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Albuquerque E.X., Schwarcz R. Kynurenic acid as an antagonist of α7 nicotinic acetylcholine receptors in the brain: Facts and challenges. Biochem. Pharmacol. 2013;85:1027–1032. doi: 10.1016/j.bcp.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berlinguer-Palmini R., Masi A., Narducci R., Cavone L., Maratea D., Cozzi A., Sili M., Moroni F., Mannaioni G. GPR35 activation reduces Ca2+ transients and contributes to the kynurenic acid-dependent reduction of synaptic activity at CA3-CA1 synapses. PLoS ONE. 2013;8:e82180. doi: 10.1371/journal.pone.0082180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang J., Simonavicius N., Wu X., Swaminath G., Reagan J., Tian H., Ling L. Kynurenic Acid as a Ligand for Orphan G Protein-coupled Receptor GPR35. J. Biol. Chem. 2006;281:22021–22028. doi: 10.1074/jbc.M603503200. [DOI] [PubMed] [Google Scholar]
- 50.DiNatale B.C., Murray I.A., Schroeder J.C., Flaveny C.A., Lahoti T.S., Laurenzana E.M., Omiecinski C.J., Perdew G.H. Kynurenic Acid Is a Potent Endogenous Aryl Hydrocarbon Receptor Ligand that Synergistically Induces Interleukin-6 in the Presence of Inflammatory Signaling. Toxicol. Sci. 2010;115:89–97. doi: 10.1093/toxsci/kfq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Füvesi J., Somlai C., Németh H., Varga H., Kis Z., Farkas T., Károly N., Dobszay M., Penke Z., Penke B., et al. Comparative study on the effects of kynurenic acid and glucosamine–kynurenic acid. Pharmacol. Biochem. Behav. 2004;77:95–102. doi: 10.1016/j.pbb.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 52.Robotka H., Németh H., Somlai C., Vécsei L., Toldi J. Systemically administered glucosamine-kynurenic acid, but not pure kynurenic acid, is effective in decreasing the evoked activity in area CA1 of the rat hippocampus. Eur. J. Pharmacol. 2005;513:75–80. doi: 10.1016/j.ejphar.2005.02.043. [DOI] [PubMed] [Google Scholar]
- 53.Rózsa É., Robotka H., Vécsei L., Toldi J. The Janus-face kynurenic acid. J. Neural Transm. 2008;115:1087–1091. doi: 10.1007/s00702-008-0052-5. [DOI] [PubMed] [Google Scholar]
- 54.Wu H.-Q., Okuyama M., Kajii Y., Pocivavsek A., Bruno J.P., Schwarcz R. Targeting kynurenine aminotransferase II in psychiatric diseases: Promising effects of an orally active enzyme inhibitor. Schizophr. Bull. 2014;40(Suppl. 2):S152–S158. doi: 10.1093/schbul/sbt157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schwarcz R., Bruno J.P., Muchowski P.J., Wu H.-Q. Kynurenines in the mammalian brain: When physiology meets pathology. Nat. Rev. Neurosci. 2012;13:465–477. doi: 10.1038/nrn3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wonodi I., Schwarcz R. Cortical Kynurenine Pathway Metabolism: A Novel Target for Cognitive Enhancement in Schizophrenia. Schizophr. Bull. 2010;36:211–218. doi: 10.1093/schbul/sbq002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Myint A.M. Kynurenines: From the perspective of major psychiatric disorders. FEBS J. 2012;279:1375–1385. doi: 10.1111/j.1742-4658.2012.08551.x. [DOI] [PubMed] [Google Scholar]
- 58.Olsson S.K., Sellgren C., Engberg G., Landén M., Erhardt S. Cerebrospinal fluid kynurenic acid is associated with manic and psychotic features in patients with bipolar I disorder. Bipolar Disord. 2012;14:719–726. doi: 10.1111/bdi.12009. [DOI] [PubMed] [Google Scholar]
- 59.Schwarcz R., Rassoulpour A., Wu H.-Q., Medoff D., Tamminga C.A., Roberts R.C. Increased cortical kynurenate content in schizophrenia. Biol. Psychiatry. 2001;50:521–530. doi: 10.1016/S0006-3223(01)01078-2. [DOI] [PubMed] [Google Scholar]
- 60.Erhardt S., Blennow K., Nordin C., Skogh E., Lindström L.H., Engberg G. Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neurosci. Lett. 2001;313:96–98. doi: 10.1016/S0304-3940(01)02242-X. [DOI] [PubMed] [Google Scholar]
- 61.Beggiato S., Tanganelli S., Fuxe K., Antonelli T., Schwarcz R., Ferraro L. Endogenous kynurenic acid regulates extracellular GABA levels in the rat prefrontal cortex. Neuropharmacology. 2014;82:11–18. doi: 10.1016/j.neuropharm.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 62.Beggiato S., Antonelli T., Tomasini M.C., Tanganelli S., Fuxe K., Schwarcz R., Ferraro L. Kynurenic acid, by targeting α7 nicotinic acetylcholine receptors, modulates extracellular GABA levels in the rat striatum in vivo. Eur. J. Neurosci. 2013;37:1470–1477. doi: 10.1111/ejn.12160. [DOI] [PubMed] [Google Scholar]
- 63.Pocivavsek A., Wu H.-Q., Potter M.C., Elmer G.I., Pellicciari R., Schwarcz R. Fluctuations in endogenous kynurenic acid control hippocampal glutamate and memory. Neuropsychopharmacology. 2011;36:2357–2367. doi: 10.1038/npp.2011.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Varga D., Herédi J., Kánvási Z., Ruszka M., Kis Z., Ono E., Iwamori N., Iwamori T., Takakuwa H., Vécsei L., et al. Systemic L-Kynurenine sulfate administration disrupts object recognition memory, alters open field behavior and decreases c-Fos immunopositivity in C57Bl/6 mice. Front. Behav. Neurosci. 2015;9:157. doi: 10.3389/fnbeh.2015.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alexander K.S., Wu H.-Q., Schwarcz R., Bruno J.P. Acute elevations of brain kynurenic acid impair cognitive flexibility: Normalization by the alpha7 positive modulator galantamine. Psychopharmacology. 2012;220:627–637. doi: 10.1007/s00213-011-2539-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chess A.C., Simoni M.K., Alling T.E., Bucci D.J. Elevations of endogenous kynurenic acid produce spatial working memory deficits. Schizophr. Bull. 2007;33:797–804. doi: 10.1093/schbul/sbl033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pershing M.L., Bortz D.M., Pocivavsek A., Fredericks P.J., Jørgensen C.V., Vunck S.A., Leuner B., Schwarcz R., Bruno J.P. Elevated levels of kynurenic acid during gestation produce neurochemical, morphological, and cognitive deficits in adulthood: Implications for schizophrenia. Neuropharmacology. 2015;90:33–41. doi: 10.1016/j.neuropharm.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.DeAngeli N.E., Todd T.P., Chang S.E., Yeh H.H., Yeh P.W., Bucci D.J. Exposure to Kynurenic Acid during Adolescence Increases Sign-Tracking and Impairs Long-Term Potentiation in Adulthood. Front. Behav. Neurosci. 2015;8:451. doi: 10.3389/fnbeh.2014.00451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wonodi I., McMahon R.P., Krishna N., Mitchell B.D., Liu J., Glassman M., Hong L.E., Gold J.M. Influence of kynurenine 3-monooxygenase (KMO) gene polymorphism on cognitive function in schizophrenia. Schizophr. Res. 2014;160:80. doi: 10.1016/j.schres.2014.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kozak R., Campbell B.M., Strick C.A., Horner W., Hoffmann W.E., Kiss T., Chapin D.S., McGinnis D., Abbott A.L., Roberts B.M., et al. Reduction of brain kynurenic acid improves cognitive function. J. Neurosci. 2014;34:10592–10602. doi: 10.1523/JNEUROSCI.1107-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Linderholm K.R., Skogh E., Olsson S.K., Dahl M.-L., Holtze M., Engberg G., Samuelsson M., Erhardt S. Increased Levels of Kynurenine and Kynurenic Acid in the CSF of Patients With Schizophrenia. Schizophr. Bull. 2012;38:426–432. doi: 10.1093/schbul/sbq086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miller C.L., Llenos I.C., Dulay J.R., Weis S. Upregulation of the initiating step of the kynurenine pathway in postmortem anterior cingulate cortex from individuals with schizophrenia and bipolar disorder. Brain Res. 2006;1073:25–37. doi: 10.1016/j.brainres.2005.12.056. [DOI] [PubMed] [Google Scholar]
- 73.Sathyasaikumar K.V., Stachowski E.K., Wonodi I., Roberts R.C., Rassoulpour A., McMahon R.P., Schwarcz R. Impaired Kynurenine Pathway Metabolism in The Prefrontal Cortex of Individuals with Schizophrenia. Schizophr. Bull. 2011;37:1147–1156. doi: 10.1093/schbul/sbq112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kegel M.E., Bhat M., Skogh E., Samuelsson M., Lundberg K., Dahl M.-L., Sellgren C., Schwieler L., Engberg G., Schuppe-Koistinen I., et al. Imbalanced Kynurenine Pathway in Schizophrenia. Int. J. Tryptophan Res. 2014;7:15–22. doi: 10.4137/IJTR.S16800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hu W., MacDonald M.L., Elswick D.E., Sweet R.A. The glutamate hypothesis of schizophrenia: Evidence from human brain tissue studies. Ann. N. Y. Acad. Sci. 2015;1338:38. doi: 10.1111/nyas.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guan Z.Z., Zhang X., Blennow K., Nordberg A. Decreased protein level of nicotinic receptor alpha7 subunit in the frontal cortex from schizophrenic brain. Neuroreport. 1999;10:1779–1782. doi: 10.1097/00001756-199906030-00028. [DOI] [PubMed] [Google Scholar]
- 77.Young J.W., Geyer M.A. Evaluating the role of the alpha-7 nicotinic acetylcholine receptor in the pathophysiology and treatment of schizophrenia. Biochem. Pharmacol. 2013;86:1122–1132. doi: 10.1016/j.bcp.2013.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rodríguez de Fonseca F., Del Arco I., Bermudez-Silva F.J., Bilbao A., Cippitelli A., Navarro M. The endocannabinoid system: Physiology and pharmacology. Alcohol Alcohol. 2005;40:2–14. doi: 10.1093/alcalc/agh110. [DOI] [PubMed] [Google Scholar]
- 79.Matsuda L.A., Lolait S.J., Brownstein M.J., Young A.C., Bonner T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 80.Munro S., Thomas K.L., Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 81.Di Marzo V., Bifulco M., De Petrocellis L. The endocannabinoid system and its therapeutic exploitation. Nat. Rev. Drug Discov. 2004;3:771–784. doi: 10.1038/nrd1495. [DOI] [PubMed] [Google Scholar]
- 82.Hashimotodani Y., Ohno-Shosaku T., Kano M. Endocannabinoids and Synaptic Function in the CNS. Neurosci. 2007;13:127–137. doi: 10.1177/1073858406296716. [DOI] [PubMed] [Google Scholar]
- 83.Lovinger D.M. Pharmacology of Neurotransmitter Release. Volume 184. Springer; Berlin/Heidelberg, Germany: 2008. Presynaptic modulation by endocannabinoids; pp. 435–477. [DOI] [PubMed] [Google Scholar]
- 84.Pertwee R.G. Cannabinoid pharmacology: The first 66 years. Br. J. Pharmacol. 2009;147:S163–S171. doi: 10.1038/sj.bjp.0706406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mackie K. Cannabinoids. Springer; Berlin/Heidelberg, Germany: 2005. Distribution of cannabinoid receptors in the central and peripheral nervous system; pp. 299–325. [DOI] [PubMed] [Google Scholar]
- 86.Howlett A.C., Bidaut-Russell M., Devane W.A., Melvin L.S., Johnson M.R., Herkenham M. The cannabinoid receptor: Biochemical, anatomical and behavioral characterization. Trends Neurosci. 1990;13:420–423. doi: 10.1016/0166-2236(90)90124-S. [DOI] [PubMed] [Google Scholar]
- 87.Herkenham M., Lynn A.B., Johnson M.R., Melvin L.S., de Costa B.R., Rice K.C. Characterization and localization of cannabinoid receptors in rat brain: A quantitative in vitro autoradiographic study. J. Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Van Sickle M.D., Duncan M., Kingsley P.J., Mouihate A., Urbani P., Mackie K., Stella N., Makriyannis A., Piomelli D., Davison J.S., et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- 89.Malan T.P., Ibrahim M.M., Deng H., Liu Q., Mata H.P., Vanderah T., Porreca F., Makriyannis A. CB2 cannabinoid receptor-mediated peripheral antinociception. Pain. 2001;93:239–245. doi: 10.1016/S0304-3959(01)00321-9. [DOI] [PubMed] [Google Scholar]
- 90.Devane W.A., Hanus L., Breuer A., Pertwee R.G., Stevenson L.A., Griffin G., Gibson D., Mandelbaum A., Etinger A., Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 91.Mechoulam R., Ben-Shabat S., Hanus L., Ligumsky M., Kaminski N.E., Schatz A.R., Gopher A., Almog S., Martin B.R., Compton D.R. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-D. [DOI] [PubMed] [Google Scholar]
- 92.Sugiura T., Kondo S., Sukagawa A., Nakane S., Shinoda A., Itoh K., Yamashita A., Waku K. 2-Arachidonoylglycerol: A possible endogenous cannabinoid receptor ligand in brain. Biochem. Biophys. Res. Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- 93.Di Marzo V. The endocannabinoid system: Its general strategy of action, tools for its pharmacological manipulation and potential therapeutic exploitation. Pharmacol. Res. 2009;60:77–84. doi: 10.1016/j.phrs.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 94.Ibarra-Lecue I., Pilar-Cuéllar F., Muguruza C., Florensa-Zanuy E., Díaz Á., Urigüen L., Castro E., Pazos A., Callado L.F. The endocannabinoid system in mental disorders: Evidence from human brain studies. Biochem. Pharmacol. 2018;157:97–107. doi: 10.1016/j.bcp.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 95.Leweke F.M., Giuffrida A., Wurster U., Emrich H.M., Piomelli D. Elevated endogenous cannabinoids in schizophrenia. Neuroreport. 1999;10:1665–1669. doi: 10.1097/00001756-199906030-00008. [DOI] [PubMed] [Google Scholar]
- 96.Giuffrida A., Leweke F.M., Gerth C.W., Schreiber D., Koethe D., Faulhaber J., Klosterkötter J., Piomelli D. Cerebrospinal Anandamide Levels are Elevated in Acute Schizophrenia and are Inversely Correlated with Psychotic Symptoms. Neuropsychopharmacology. 2004;29:2108–2114. doi: 10.1038/sj.npp.1300558. [DOI] [PubMed] [Google Scholar]
- 97.Ferretjans R., Moreira F.A., Teixeira A.L., Salgado J.V. The Endocannabinoid System and its Role in Schizophrenia: A Systematic Review of the Literature. Rev. Bras. Psiquiatr. 2012;34:163–193. doi: 10.1016/j.rbp.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 98.Altintas M., Inanc L., Oruc G.A., Arpacioglu S., Gulec H. Clinical characteristics of synthetic cannabinoid-induced psychosis in relation to schizophrenia: A single-center cross-sectional analysis of concurrently hospitalized patients. Neuropsychiatr. Dis. Treat. 2016;12:1893–1900. doi: 10.2147/NDT.S107622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hambrecht M., Häfner H. Cannabis, Vulnerability, and the Onset of Schizophrenia: An Epidemiological Perspective. Aust. N. Z. J. Psychiatry. 2000;34:468–475. doi: 10.1080/j.1440-1614.2000.00736.x. [DOI] [PubMed] [Google Scholar]
- 100.Kuepper R., Morrison P.D., Van Os J., Murray R.M., Kenis G., Henquet C. Does dopamine mediate the psychosis-inducing effects of cannabis? A review and integration of findings across disciplines. Psiquiatr. Biol. 2012;19:49–58. doi: 10.1016/j.psiq.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 101.Koethe D., Hoyer C., Leweke F.M. The endocannabinoid system as a target for modelling psychosis. Psychopharmacology. 2009;206:551–561. doi: 10.1007/s00213-009-1591-7. [DOI] [PubMed] [Google Scholar]
- 102.Boggs D.L., Surti T., Gupta A., Gupta S., Niciu M., Pittman B., Schnakenberg Martin A.M., Thurnauer H., Davies A., D’Souza D.C., et al. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacology. 2018;235:1923–1932. doi: 10.1007/s00213-018-4885-9. [DOI] [PubMed] [Google Scholar]
- 103.Moore T.H., Zammit S., Lingford-Hughes A., Barnes T.R., Jones P.B., Burke M., Lewis G. Cannabis use and risk of psychotic or affective mental health outcomes: A systematic review. Lancet. 2007;370:319–328. doi: 10.1016/S0140-6736(07)61162-3. [DOI] [PubMed] [Google Scholar]
- 104.Andre C.M., Hausman J.-F., Guerriero G. Cannabis sativa: The Plant of the Thousand and One Molecules. Front. Plant Sci. 2016;7:19. doi: 10.3389/fpls.2016.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Patel K.R., Cherian J., Gohil K., Atkinson D. Schizophrenia: Overview and treatment options. Pharm. Ther. 2014;39:638–645. [PMC free article] [PubMed] [Google Scholar]
- 106.Rampino A., Marakhovskaia A., Soares-Silva T., Torretta S., Veneziani F., Beaulieu J.M. Antipsychotic Drug Responsiveness and Dopamine Receptor Signaling; Old Players and New Prospects. Front. Psychiatry. 2019;9:702. doi: 10.3389/fpsyt.2018.00702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yang A.C., Tsai S.-J. New Targets for Schizophrenia Treatment beyond the Dopamine Hypothesis. Int. J. Mol. Sci. 2017;18:1689. doi: 10.3390/ijms18081689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.De Jonge J.C., Vinkers C.H., Hulshoff Pol H.E., Marsman A. GABAergic Mechanisms in Schizophrenia: Linking Postmortem and In Vivo Studies. Front. Psychiatry. 2017;8:118. doi: 10.3389/fpsyt.2017.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Abi-Dargham A., Gil R., Krystal J., Baldwin R.M., Seibyl J.P., Bowers M., van Dyck C.H., Charney D.S., Innis R.B., Laruelle M. Increased striatal dopamine transmission in schizophrenia: Confirmation in a second cohort. Am. J. Psychiatry. 1998;155:761–767. doi: 10.1176/ajp.155.6.761. [DOI] [PubMed] [Google Scholar]
- 110.Williams G.V., Castner S.A. Under the curve: Critical issues for elucidating D1 receptor function in working memory. Neuroscience. 2006;139:263–276. doi: 10.1016/j.neuroscience.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 111.Savitz J. The kynurenine pathway: A finger in every pie. Mol. Psychiatry. 2019 doi: 10.1038/s41380-019-0414-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Stone J.M., Morrison P.D., Pilowsky L.S. Review: Glutamate and dopamine dysregulation in schizophrenia—A synthesis and selective review. J. Psychopharmacol. 2007;21:440–452. doi: 10.1177/0269881106073126. [DOI] [PubMed] [Google Scholar]
- 113.Moghaddam B., Javitt D. From revolution to evolution: The glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology. 2012;37:4–15. doi: 10.1038/npp.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Javitt D.C., Schoepp D., Kalivas P.W., Volkow N.D., Zarate C., Merchant K., Bear M.F., Umbricht D., Hajos M., Potter W.Z., et al. Translating glutamate: From pathophysiology to treatment. Sci. Transl. Med. 2011;3:102mr2. doi: 10.1126/scitranslmed.3002804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lewis D.A., Curley A.A., Glausier J.R., Volk D.W. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012;35:57–67. doi: 10.1016/j.tins.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lewis D.A., Hashimoto T., Volk D.W. Cortical inhibitory neurons and schizophrenia. Nat. Rev. Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 117.Thompson M., Weickert C.S., Wyatt E., Webster M.J. Decreased glutamic acid decarboxylase67 mRNA expression in multiple brain areas of patients with schizophrenia and mood disorders. J. Psychiatr. Res. 2009;43:970–977. doi: 10.1016/j.jpsychires.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 118.Orhan F., Fatouros-Bergman H., Goiny M., Malmqvist A., Piehl F., Karolinska Schizophrenia Project (KaSP) Consortium. Cervenka S., Collste K., Victorsson P., Sellgren C.M., et al. CSF GABA is reduced in first-episode psychosis and associates to symptom severity. Mol. Psychiatry. 2018;23:1244–1250. doi: 10.1038/mp.2017.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Egerton A., Modinos G., Ferrera D., McGuire P. Neuroimaging studies of GABA in schizophrenia: A systematic review with meta-analysis. Transl. Psychiatry. 2017;7:e1147. doi: 10.1038/tp.2017.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Konopaske G.T., Sweet R.A., Wu Q., Sampson A., Lewis D.A. Regional specificity of chandelier neuron axon terminal alterations in schizophrenia. Neuroscience. 2006;138:189–196. doi: 10.1016/j.neuroscience.2005.10.070. [DOI] [PubMed] [Google Scholar]
- 121.Tufvesson-Alm M., Schwieler L., Schwarcz R., Goiny M., Erhardt S., Engberg G. Importance of kynurenine 3-monooxygenase for spontaneous firing and pharmacological responses of midbrain dopamine neurons: Relevance for schizophrenia. Neuropharmacology. 2018;138:130–139. doi: 10.1016/j.neuropharm.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Walter L., Franklin A., Witting A., Mö Ller T., Stella N. Astrocytes in Culture Produce Anandamide and Other Acylethanolamides. J. Biol. Chem. 2002;227:20869–20876. doi: 10.1074/jbc.M110813200. [DOI] [PubMed] [Google Scholar]
- 123.Walter L., Stella N. Endothelin-1 increases 2-arachidonoyl glycerol (2-AG) production in astrocytes. Glia. 2003;44:85–90. doi: 10.1002/glia.10270. [DOI] [PubMed] [Google Scholar]
- 124.Starowicz K., Maione S., Cristino L., Palazzo E., Marabese I., Rossi F., de Novellis V., Di Marzo V. Tonic Endovanilloid Facilitation of Glutamate Release in Brainstem Descending Antinociceptive Pathways. J. Neurosci. 2007;27:13739–13749. doi: 10.1523/JNEUROSCI.3258-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Melis M., Pistis M. Hub and switches: Endocannabinoid signalling in midbrain dopamine neurons. Philos. Trans. R. Soc. B Biol. Sci. 2012;367:3276–3285. doi: 10.1098/rstb.2011.0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Katona I., Freund T.F. Multiple functions of endocannabinoid signaling in the brain. Annu. Rev. Neurosci. 2012;35:529–558. doi: 10.1146/annurev-neuro-062111-150420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Laviolette S.R., Grace A.A. The roles of cannabinoid and dopamine receptor systems in neural emotional learning circuits: Implications for schizophrenia and addiction. Cell. Mol. Life Sci. 2006;63:1597–1613. doi: 10.1007/s00018-006-6027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Olney J., Labruyere J., Wang G., Wozniak D., Price M., Sesma M. NMDA antagonist neurotoxicity: Mechanism and prevention. Science. 1991;254:1515–1518. doi: 10.1126/science.1835799. [DOI] [PubMed] [Google Scholar]
- 129.Sullivan J.M. Cellular and molecular mechanisms underlying learning and memory impairments produced by cannabinoids. Learn. Mem. 2000;7:132–139. doi: 10.1101/lm.7.3.132. [DOI] [PubMed] [Google Scholar]
- 130.Steffens M., Engler C., Zentner J., Feuerstein T.J. Cannabinoid CB1 receptor-mediated modulation of evoked dopamine release and of adenylyl cyclase activity in the human neocortex. Br. J. Pharmacol. 2004;141:1193–1203. doi: 10.1038/sj.bjp.0705706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Starowicz K., Nigam S., Di Marzo V. Biochemistry and pharmacology of endovanilloids. Pharmacol. Ther. 2007;114:13–33. doi: 10.1016/j.pharmthera.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 132.Song I., Dityatev A. Crosstalk between glia, extracellular matrix and neurons. Brain Res. Bull. 2018;136:101–108. doi: 10.1016/j.brainresbull.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 133.Dityatev A., Frischknecht R., Seidenbecher C.I. Extracellular matrix and synaptic functions. Results Probl. Cell Differ. 2006;43:69–97. doi: 10.1007/400_025. [DOI] [PubMed] [Google Scholar]
- 134.Dityatev A., Rusakov D.A. Molecular signals of plasticity at the tetrapartite synapse. Curr. Opin. Neurobiol. 2011;21:353–359. doi: 10.1016/j.conb.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Richard A.D., Lu X.-H. “Teaching old dogs new tricks”: Targeting neural extracellular matrix for normal and pathological aging-related cognitive decline. Neural Regen. Res. 2019;14:578–581. doi: 10.4103/1673-5374.247459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chelini G., Pantazopoulos H., Durning P., Berretta S. The tetrapartite synapse: A key concept in the pathophysiology of schizophrenia. Eur. Psychiatry. 2018;50:60–69. doi: 10.1016/j.eurpsy.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Guidetti P., Okuno E., Schwarcz R. Characterization of rat brain kynurenine aminotransferases I and II. J. Neurosci. Res. 1997;50:457–465. doi: 10.1002/(SICI)1097-4547(19971101)50:3<457::AID-JNR12>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 138.Shen J., Yakel J.L. Functional α7 nicotinic ACh receptors on astrocytes in rat hippocampal CA1 slices. J. Mol. Neurosci. 2012;48:14–21. doi: 10.1007/s12031-012-9719-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hegyi Z., Oláh T., Kőszeghy Á., Piscitelli F., Holló K., Pál B., Csernoch L., Di Marzo V., Antal M. CB1 receptor activation induces intracellular Ca2+ mobilization and 2-arachidonoylglycerol release in rodent spinal cord astrocytes. Sci. Rep. 2018;8:10562. doi: 10.1038/s41598-018-28763-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Walter L., Dinh T., Stella N. ATP induces a rapid and pronounced increase in 2-arachidonoylglycerol production by astrocytes, a response limited by monoacylglycerol lipase. J. Neurosci. 2004;24:8068–8074. doi: 10.1523/JNEUROSCI.2419-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Metna-Laurent M., Marsicano G. Rising stars: Modulation of brain functions by astroglial type-1 cannabinoid receptors. Glia. 2015;63:353–364. doi: 10.1002/glia.22773. [DOI] [PubMed] [Google Scholar]
- 142.Secci M.E., Mascia P., Sagheddu C., Beggiato S., Melis M., Borelli A.C., Tomasini M.C., Panlilio L.V., Schindler C.W., Tanda G., et al. Astrocytic Mechanisms Involving Kynurenic Acid Control Δ9-Tetrahydrocannabinol-Induced Increases in Glutamate Release in Brain Reward-Processing Areas. Mol. Neurobiol. 2019;56:3563–3575. doi: 10.1007/s12035-018-1319-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Stella N. Cannabinoid and cannabinoid-like receptors in microglia, astrocytes, and astrocytomas. Glia. 2010;58:1017–1030. doi: 10.1002/glia.20983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kano M., Ohno-Shosaku T., Hashimotodani Y., Uchigashima M., Watanabe M. Endocannabinoid-Mediated Control of Synaptic Transmission. Physiol. Rev. 2009;89:309–380. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- 145.Navarrete M., Araque A. Endocannabinoids Potentiate Synaptic Transmission through Stimulation of Astrocytes. Neuron. 2010;68:113–126. doi: 10.1016/j.neuron.2010.08.043. [DOI] [PubMed] [Google Scholar]
- 146.Han J., Kesner P., Metna-Laurent M., Duan T., Xu L., Georges F., Koehl M., Abrous D.N., Mendizabal-Zubiaga J., Grandes P., et al. Acute Cannabinoids Impair Working Memory through Astroglial CB1 Receptor Modulation of Hippocampal LTD. Cell. 2012;148:1039–1050. doi: 10.1016/j.cell.2012.01.037. [DOI] [PubMed] [Google Scholar]
- 147.Navarrete M., Araque A. Endocannabinoids Mediate Neuron-Astrocyte Communication. Neuron. 2008;57:883–893. doi: 10.1016/j.neuron.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 148.Guidetti P., Hoffman G.E., Melendez-Ferro M., Albuquerque E.X., Schwarcz R. Astrocytic localization of kynurenine aminotransferase II in the rat brain visualized by immunocytochemistry. Glia. 2007;55:78–92. doi: 10.1002/glia.20432. [DOI] [PubMed] [Google Scholar]
- 149.Guidetti P., Schwarcz R. Determination of alpha-aminoadipic acid in brain, peripheral tissues, and body fluids using GC/MS with negative chemical ionization. Brain Res. Mol. Brain Res. 2003;118:132–139. doi: 10.1016/j.molbrainres.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 150.Swartz K.J., During M.J., Freese A., Beal M.F. Cerebral synthesis and release of kynurenic acid: An endogenous antagonist of excitatory amino acid receptors. J. Neurosci. 1990;10:2965–2973. doi: 10.1523/JNEUROSCI.10-09-02965.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Owe-Young R., Webster N.L., Mukhtar M., Pomerantz R.J., Smythe G., Walker D., Armati P.J., Crowe S.M., Brew B.J. Kynurenine pathway metabolism in human blood-brain-barrier cells: Implications for immune tolerance and neurotoxicity. J. Neurochem. 2008;105:1346–1357. doi: 10.1111/j.1471-4159.2008.05241.x. [DOI] [PubMed] [Google Scholar]
- 152.Gál E.M., Young R.B., Sherman A.D. Tryptophan loading: Consequent effects on the synthesis of kynurenine and 5-hydroxyindoles in rat brain. J. Neurochem. 1978;31:237–244. doi: 10.1111/j.1471-4159.1978.tb12454.x. [DOI] [PubMed] [Google Scholar]
- 153.Gál E.M., Sherman A.D. Synthesis and metabolism of L-kynurenine in rat brain. J. Neurochem. 1978;30:607–613. doi: 10.1111/j.1471-4159.1978.tb07815.x. [DOI] [PubMed] [Google Scholar]
- 154.Maurer S.V., Williams C.L. The Cholinergic System Modulates Memory and Hippocampal Plasticity via Its Interactions with Non-Neuronal Cells. Front. Immunol. 2017;8:1489. doi: 10.3389/fimmu.2017.01489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Skowrońska K., Obara-Michlewska M., Zielińska M., Albrecht J. NMDA Receptors in Astrocytes: In Search for Roles in Neurotransmission and Astrocytic Homeostasis. Int. J. Mol. Sci. 2019;20:309. doi: 10.3390/ijms20020309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Letellier M., Park Y.K., Chater T.E., Chipman P.H., Gautam S.G., Oshima-Takago T., Goda Y. Astrocytes regulate heterogeneity of presynaptic strengths in hippocampal networks. Proc. Natl. Acad. Sci. USA. 2016;113:E2685–E2694. doi: 10.1073/pnas.1523717113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mei Y.-Y., Wu D.C., Zhou N. Astrocytic Regulation of Glutamate Transmission in Schizophrenia. Front. Psychiatry. 2018;9:544. doi: 10.3389/fpsyt.2018.00544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wu H.-Q., Pereira E.F.R., Bruno J.P., Pellicciari R., Albuquerque E.X., Schwarcz R. The astrocyte-derived alpha7 nicotinic receptor antagonist kynurenic acid controls extracellular glutamate levels in the prefrontal cortex. J. Mol. Neurosci. 2010;40:204–210. doi: 10.1007/s12031-009-9235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Compton M.T., Furman A.C., Kaslow N.J. Lower negative symptom scores among cannabis-dependent patients with schizophrenia-spectrum disorders: Preliminary evidence from an African American first-episode sample. Schizophr. Res. 2004;71:61–64. doi: 10.1016/j.schres.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 160.Dubertret C., Bidard I., Adès J., Gorwood P. Lifetime positive symptoms in patients with schizophrenia and cannabis abuse are partially explained by co-morbid addiction. Schizophr. Res. 2006;86:284–290. doi: 10.1016/j.schres.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 161.Grace A.A. Disruption of cortical-limbic interaction as a substrate for comorbidity. Neurotox. Res. 2006;10:93–101. doi: 10.1007/BF03033238. [DOI] [PubMed] [Google Scholar]
- 162.Quiroz C., Orrú M., Rea W., Ciudad-Roberts A., Yepes G., Britt J.P., Ferré S. Local Control of Extracellular Dopamine Levels in the Medial Nucleus Accumbens by a Glutamatergic Projection from the Infralimbic Cortex. J. Neurosci. 2016;36:851–859. doi: 10.1523/JNEUROSCI.2850-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kaiser S., Wonnacott S. α-Bungarotoxin-Sensitive Nicotinic Receptors Indirectly Modulate [3H]Dopamine Release in Rat Striatal Slices via Glutamate Release. Mol. Pharmacol. 2000;58:312–318. doi: 10.1124/mol.58.2.312. [DOI] [PubMed] [Google Scholar]
- 164.Rassoulpour A., Wu H.-Q., Ferre S., Schwarcz R. Nanomolar concentrations of kynurenic acid reduce extracellular dopamine levels in the striatum. J. Neurochem. 2005;93:762–765. doi: 10.1111/j.1471-4159.2005.03134.x. [DOI] [PubMed] [Google Scholar]
- 165.Justinova Z., Mascia P., Wu H.-Q., Secci M.E., Redhi G.H., Panlilio L.V., Scherma M., Barnes C., Parashos A., Zara T., et al. Reducing cannabinoid abuse and preventing relapse by enhancing endogenous brain levels of kynurenic acid. Nat. Neurosci. 2013;16:1652–1661. doi: 10.1038/nn.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Benros M.E., Nielsen P.R., Nordentoft M., Eaton W.W., Dalton S.O., Mortensen P.B. Autoimmune Diseases and Severe Infections as Risk Factors for Schizophrenia: A 30-Year Population-Based Register Study. Am. J. Psychiatry. 2011;168:1303–1310. doi: 10.1176/appi.ajp.2011.11030516. [DOI] [PubMed] [Google Scholar]
- 167.Miller B.J., Graham K.L., Bodenheimer C.M., Culpepper N.H., Waller J.L., Buckley P.F. A Prevalence Study of Urinary Tract Infections in Acute Relapse of Schizophrenia. J. Clin. Psychiatry. 2013;74:271–277. doi: 10.4088/JCP.12m08050. [DOI] [PubMed] [Google Scholar]
- 168.Khandaker G.M., Zimbron J., Lewis G., Jones P.B. Prenatal maternal infection, neurodevelopment and adult schizophrenia: A systematic review of population-based studies. Psychol. Med. 2013;43:239–257. doi: 10.1017/S0033291712000736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Khandaker G.M., Zimbron J., Dalman C., Lewis G., Jones P.B. Childhood infection and adult schizophrenia: A meta-analysis of population-based studies. Schizophr. Res. 2012;139:161–168. doi: 10.1016/j.schres.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Benros M.E., Eaton W.W., Mortensen P.B. The Epidemiologic Evidence Linking Autoimmune Diseases and Psychosis. Biol. Psychiatry. 2014;75:300–306. doi: 10.1016/j.biopsych.2013.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Cervenka I., Agudelo L.Z., Ruas J.L. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science. 2017;357:eaaf9794. doi: 10.1126/science.aaf9794. [DOI] [PubMed] [Google Scholar]
- 172.Niño-Castro A., Abdullah Z., Popov A., Thabet Y., Beyer M., Knolle P., Domann E., Chakraborty T., Schmidt S.V., Schultze J.L. The IDO1-induced kynurenines play a major role in the antimicrobial effect of human myeloid cells against Listeria monocytogenes. Innate Immun. 2014;20:401–411. doi: 10.1177/1753425913496442. [DOI] [PubMed] [Google Scholar]
- 173.Connor T.J., Starr N., O’Sullivan J.B., Harkin A. Induction of indolamine 2,3-dioxygenase and kynurenine 3-monooxygenase in rat brain following a systemic inflammatory challenge: A role for IFN-γ? Neurosci. Lett. 2008;441:29–34. doi: 10.1016/j.neulet.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 174.Babcock T.A., Carlin J.M. Transcriptional activation of indoleamine dioxygenase by interleukin 1 and tumor necrosis factor α in interferon-treated epithelial cells. Cytokine. 2000;12:588–594. doi: 10.1006/cyto.1999.0661. [DOI] [PubMed] [Google Scholar]
- 175.Asp L., Johansson A.-S., Mann A., Owe-Larsson B., Urbanska E.M., Kocki T., Kegel M., Engberg G., Lundkvist G.B., Karlsson H. Effects of pro-inflammatory cytokines on expression of kynurenine pathway enzymes in human dermal fibroblasts. J. Inflamm. (Lond.) 2011;8:25. doi: 10.1186/1476-9255-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hillard C.J. Circulating Endocannabinoids: From Whence Do They Come and Where are They Going? Neuropsychopharmacology. 2018;43:155–172. doi: 10.1038/npp.2017.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Weis F., Beiras-Fernandez A., Hauer D., Hornuss C., Sodian R., Kreth S., Briegel J., Schelling G. Effect of anaesthesia and cardiopulmonary bypass on blood endocannabinoid concentrations during cardiac surgery. Br. J. Anaesth. 2010;105:139–144. doi: 10.1093/bja/aeq117. [DOI] [PubMed] [Google Scholar]
- 178.Knight J.M., Szabo A., Zhao S., Lyness J.M., Sahler O.J.Z., Liesveld J.L., Sander T., Rizzo J.D., Hillard C.J., Moynihan J.A. Circulating endocannabinoids during hematopoietic stem cell transplantation: A pilot study. Neurobiol. Stress. 2015;2:44–50. doi: 10.1016/j.ynstr.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Suárez-Pinilla P., López-Gil J., Crespo-Facorro B. Immune system: A possible nexus between cannabinoids and psychosis. Brain. Behav. Immun. 2014;40:269–282. doi: 10.1016/j.bbi.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 180.Guidetti P., Schwarcz R. 3-Hydroxykynurenine potentiates quinolinate but not NMDA toxicity in the rat striatum. Eur. J. Neurosci. 1999;11:3857–3863. doi: 10.1046/j.1460-9568.1999.00806.x. [DOI] [PubMed] [Google Scholar]
- 181.Backhaus C., Rahman H., Scheffler S., Laatsch H., Hardeland R. NO scavenging by 3-hydroxyanthranilic acid and 3-hydroxykynurenine: N-nitrosation leads via oxadiazoles to o-quinone diazides. Nitric Oxide. 2008;19:237–244. doi: 10.1016/j.niox.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 182.Hardeland R., Zsizsik B.K., Poeggeler B., Fuhrberg B., Holst S., Coto-Montes A. Indole-3-Pyruvic and -Propionic Acids, Kynurenic Acid, and Related Metabolites as Luminophores and Free-Radical Scavengers. Springer; Boston, MA, USA: 1999. pp. 389–395. [DOI] [PubMed] [Google Scholar]
- 183.Ribeiro R., Wen J., Li S., Zhang Y. Involvement of ERK1/2, cPLA2 and NF-κB in microglia suppression by cannabinoid receptor agonists and antagonists. Prostaglandins Other Lipid Mediat. 2013;100–101:1–14. doi: 10.1016/j.prostaglandins.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 184.Han K.H., Lim S., Ryu J., Lee C.-W., Kim Y., Kang J.-H., Kang S.-S., Ahn Y.K., Park C.-S., Kim J.J. CB1 and CB2 cannabinoid receptors differentially regulate the production of reactive oxygen species by macrophages. Cardiovasc. Res. 2009;84:378–386. doi: 10.1093/cvr/cvp240. [DOI] [PubMed] [Google Scholar]
- 185.Muccioli G.G., Naslain D., Bäckhed F., Reigstad C.S., Lambert D.M., Delzenne N.M., Cani P.D. The endocannabinoid system links gut microbiota to adipogenesis. Mol. Syst. Biol. 2010;6:392. doi: 10.1038/msb.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Forrest C.M., Gould S.R., Darlington L.G., Stone T.W. Advances in Experimental Medicine and Biology. Volume 527. Springer; Boston, MA, USA: 2003. Levels of Purine, Kynurenine and Lipid Peroxidation Products in Patients with Inflammatory Bowel Disease; pp. 395–400. [DOI] [PubMed] [Google Scholar]
- 187.Dolecka J., Urbanik-Sypniewska T., SkrzydŁo-Radomañska B., Parada-Turska J. Effect of kynurenic acid on the viability of probiotics in vitro. Pharmacol. Rep. 2011;63:548–551. doi: 10.1016/S1734-1140(11)70522-9. [DOI] [PubMed] [Google Scholar]
- 188.Hasenoehrl C., Taschler U., Storr M., Schicho R. The gastrointestinal tract—A central organ of cannabinoid signaling in health and disease. Neurogastroenterol. Motil. 2016;28:1765–1780. doi: 10.1111/nmo.12931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Shore D.M., Reggio P.H. The therapeutic potential of orphan GPCRs, GPR35 and GPR55. Front. Pharmacol. 2015;6:69. doi: 10.3389/fphar.2015.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Taniguchi Y., Tonai-Kachi H., Shinjo K. Zaprinast, a well-known cyclic guanosine monophosphate-specific phosphodiesterase inhibitor, is an agonist for GPR35. FEBS Lett. 2006;580:5003–5008. doi: 10.1016/j.febslet.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 191.Imielinski M., Baldassano R.N., Griffiths A., Russell R.K., Annese V., Dubinsky M., Kugathasan S., Bradfield J.P., Walters T.D., Sleiman P., et al. Common variants at five new loci associated with early-onset inflammatory bowel disease. Nat. Genet. 2009;41:1335–1340. doi: 10.1038/ng.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Müller N., Weidinger E., Leitner B., Schwarz M.J. The role of inflammation in schizophrenia. Front. Neurosci. 2015;9:372. doi: 10.3389/fnins.2015.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Marques T.R., Ashok A.H., Pillinger T., Veronese M., Turkheimer F.E., Dazzan P., Sommer I.E.C., Howes O.D. Neuroinflammation in schizophrenia: Meta-analysis of in vivo microglial imaging studies. Psychol. Med. 2019;49:2186–2196. doi: 10.1017/S0033291718003057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wildenauer D.B., Körschenhausen D., Hoechtlen W., Ackenheil M., Kehl M., Lottspeich F. Analysis of cerebrospinal fluid from patients with psychiatric and neurological disorders by two-dimensional electrophoresis: Identification of disease-associated polypeptides as fibrin fragments. Electrophoresis. 1991;12:487–492. doi: 10.1002/elps.1150120706. [DOI] [PubMed] [Google Scholar]
- 195.Körschenhausen D.A., Hampel H.J., Ackenheil M., Penning R., Müller N. Fibrin degradation products in post mortem brain tissue of schizophrenics: A possible marker for underlying inflammatory processes. Schizophr. Res. 1996;19:103–109. doi: 10.1016/0920-9964(95)00073-9. [DOI] [PubMed] [Google Scholar]
- 196.Aricioglu F., Ozkartal C.S., Unal G., Dursun S., Cetin M., Müller N. Neuroinflammation in Schizophrenia: A Critical Review and The Future. Klin. Psikofarmakol. Bülteni-Bull. Clin. Psychopharmacol. 2016;26:429–437. doi: 10.5455/bcp.20161123044657. [DOI] [Google Scholar]
- 197.Potvin S., Stip E., Sepehry A.A., Gendron A., Bah R., Kouassi E. Inflammatory Cytokine Alterations in Schizophrenia: A Systematic Quantitative Review. Biol. Psychiatry. 2008;63:801–808. doi: 10.1016/j.biopsych.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 198.Bernstein H.-G., Steiner J., Bogerts B. Glial cells in schizophrenia: Pathophysiological significance and possible consequences for therapy. Expert Rev. Neurother. 2009;9:1059–1071. doi: 10.1586/ern.09.59. [DOI] [PubMed] [Google Scholar]
- 199.Steiner J., Mawrin C., Ziegeler A., Bielau H., Ullrich O., Bernstein H.-G., Bogerts B. Distribution of HLA-DR-positive microglia in schizophrenia reflects impaired cerebral lateralization. Acta Neuropathol. 2006;112:305–316. doi: 10.1007/s00401-006-0090-8. [DOI] [PubMed] [Google Scholar]
- 200.De Picker L.J., Morrens M., Chance S.A., Boche D. Microglia and Brain Plasticity in Acute Psychosis and Schizophrenia Illness Course: A Meta-Review. Front. Psychiatry. 2017;8:238. doi: 10.3389/fpsyt.2017.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Monji A., Kato T.A., Mizoguchi Y., Horikawa H., Seki Y., Kasai M., Yamauchi Y., Yamada S., Kanba S. Neuroinflammation in schizophrenia especially focused on the role of microglia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2013;42:115–121. doi: 10.1016/j.pnpbp.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 202.Monji A., Kato T., Kanba S. Cytokines and schizophrenia: Microglia hypothesis of schizophrenia. Psychiatry Clin. Neurosci. 2009;63:257–265. doi: 10.1111/j.1440-1819.2009.01945.x. [DOI] [PubMed] [Google Scholar]
- 203.Da Fonseca A.C.C., Matias D., Garcia C., Amaral R., Geraldo L.H., Freitas C., Lima F.R.S. The impact of microglial activation on blood-brain barrier in brain diseases. Front. Cell. Neurosci. 2014;8:362. doi: 10.3389/fncel.2014.00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Steiner J., Bogerts B., Sarnyai Z., Walter M., Gos T., Bernstein H.-G., Myint A.-M. Bridging the gap between the immune and glutamate hypotheses of schizophrenia and major depression: Potential role of glial NMDA receptor modulators and impaired blood–brain barrier integrity. World J. Biol. Psychiatry. 2012;13:482–492. doi: 10.3109/15622975.2011.583941. [DOI] [PubMed] [Google Scholar]
- 205.Frank M.G., Baratta M.V., Sprunger D.B., Watkins L.R., Maier S.F. Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain. Behav. Immun. 2007;21:47–59. doi: 10.1016/j.bbi.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 206.Perry V.H. Stress primes microglia to the presence of systemic inflammation: Implications for environmental influences on the brain. Brain. Behav. Immun. 2007;21:45–46. doi: 10.1016/j.bbi.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 207.Zunszain P.A., Anacker C., Cattaneo A., Choudhury S., Musaelyan K., Myint A.M., Thuret S., Price J., Pariante C.M. Interleukin-1β: A new regulator of the kynurenine pathway affecting human hippocampal neurogenesis. Neuropsychopharmacology. 2012;37:939–949. doi: 10.1038/npp.2011.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.O’Connor J.C., André C., Wang Y., Lawson M.A., Szegedi S.S., Lestage J., Castanon N., Kelley K.W., Dantzer R. Interferon-gamma and tumor necrosis factor-alpha mediate the upregulation of indoleamine 2,3-dioxygenase and the induction of depressive-like behavior in mice in response to bacillus Calmette-Guerin. J. Neurosci. 2009;29:4200–4209. doi: 10.1523/JNEUROSCI.5032-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Dickerson F., Boronow J., Stallings C., Origoni A., Yolken R. Toxoplasma gondii in individuals with schizophrenia: Association with clinical and demographic factors and with mortality. Schizophr. Bull. 2007;33:737–740. doi: 10.1093/schbul/sbm005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Mortensen P.B., Nørgaard-Pedersen B., Waltoft B.L., Sørensen T.L., Hougaard D., Yolken R.H. Early infections of Toxoplasma gondii and the later development of schizophrenia. Schizophr. Bull. 2007;33:741–744. doi: 10.1093/schbul/sbm009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Cetinkaya Z., Yazar S., Gecici O., Namli M.N. Anti-Toxoplasma gondii antibodies in patients with schizophrenia--preliminary findings in a Turkish sample. Schizophr. Bull. 2007;33:789–791. doi: 10.1093/schbul/sbm021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Schwarcz R., Hunter C.A. Toxoplasma gondii and schizophrenia: Linkage through astrocyte-derived kynurenic acid? Schizophr. Bull. 2007;33:652–653. doi: 10.1093/schbul/sbm030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Notarangelo F.M., Wilson E.H., Horning K.J., Thomas M.A.R., Harris T.H., Fang Q., Hunter C.A., Schwarcz R. Evaluation of kynurenine pathway metabolism in Toxoplasma gondii-infected mice: Implications for schizophrenia. Schizophr. Res. 2014;152:261–267. doi: 10.1016/j.schres.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Fujigaki S., Saito K., Takemura M., Maekawa N., Yamada Y., Wada H., Seishima M. L-tryptophan-L-kynurenine pathway metabolism accelerated by Toxoplasma gondii infection is abolished in gamma interferon-gene-deficient mice: Cross-regulation between inducible nitric oxide synthase and indoleamine-2,3-dioxygenase. Infect. Immun. 2002;70:779–786. doi: 10.1128/IAI.70.2.779-786.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Silva N.M., Rodrigues C.V., Santoro M.M., Reis L.F.L., Alvarez-Leite J.I., Gazzinelli R.T. Expression of indoleamine 2,3-dioxygenase, tryptophan degradation, and kynurenine formation during in vivo infection with Toxoplasma gondii: Induction by endogenous gamma interferon and requirement of interferon regulatory factor 1. Infect. Immun. 2002;70:859–868. doi: 10.1128/IAI.70.2.859-868.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Parrott J.M., Redus L., O’Connor J.C. Kynurenine metabolic balance is disrupted in the hippocampus following peripheral lipopolysaccharide challenge. J. Neuroinflamm. 2016;13:124. doi: 10.1186/s12974-016-0590-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.De Campos-Carli S.M., Araújo M.S., de Oliveira Silveira A.C., de Rezende V.B., Rocha N.P., Ferretjans R., Ribeiro-Santos R., Teixeira-Carvalho A., Martins-Filho O.A., Berk M., et al. Cannabinoid receptors on peripheral leukocytes from patients with schizophrenia: Evidence for defective immunomodulatory mechanisms. J. Psychiatr. Res. 2017;87:44–52. doi: 10.1016/j.jpsychires.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 218.Schaefer C., Enning F., Mueller J.K., Bumb J.M., Rohleder C., Odorfer T.M., Klosterkötter J., Hellmich M., Koethe D., Schmahl C., et al. Fatty acid ethanolamide levels are altered in borderline personality and complex posttraumatic stress disorders. Eur. Arch. Psychiatry Clin. Neurosci. 2014;264:459–463. doi: 10.1007/s00406-013-0470-8. [DOI] [PubMed] [Google Scholar]
- 219.Zalcman S., Green-Johnson J.M., Murray L., Nance D.M., Dyck D., Anisman H., Greenberg A.H. Cytokine-specific central monoamine alterations induced by interleukin-1, -2 and -6. Brain Res. 1994;643:40–49. doi: 10.1016/0006-8993(94)90006-X. [DOI] [PubMed] [Google Scholar]
- 220.Busse S., Busse M., Schiltz K., Bielau H., Gos T., Brisch R., Mawrin C., Schmitt A., Jordan W., Müller U.J., et al. Different distribution patterns of lymphocytes and microglia in the hippocampus of patients with residual versus paranoid schizophrenia: Further evidence for disease course-related immune alterations? Brain. Behav. Immun. 2012;26:1273–1279. doi: 10.1016/j.bbi.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 221.Hickie I.B., Banati R., Stewart C.H., Lloyd A.R. Are common childhood or adolescent infections risk factors for schizophrenia and other psychotic disorders? Med. J. Aust. 2009;190:S17–S21. doi: 10.5694/j.1326-5377.2009.tb02652.x. [DOI] [PubMed] [Google Scholar]
- 222.Leweke F.M., Koethe D. Cannabis and psychiatric disorders: It is not only addiction. Addict. Biol. 2008;13:264–275. doi: 10.1111/j.1369-1600.2008.00106.x. [DOI] [PubMed] [Google Scholar]
- 223.Barth M.C., Ahluwalia N., Anderson T.J.T., Hardy G.J., Sinha S., Alvarez-Cardona J.A., Pruitt I.E., Rhee E.P., Colvin R.A., Gerszten R.E. Kynurenic acid triggers firm arrest of leukocytes to vascular endothelium under flow conditions. J. Biol. Chem. 2009;284:19189–19195. doi: 10.1074/jbc.M109.024042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Gasperi V., Evangelista D., Chiurchiù V., Florenzano F., Savini I., Oddi S., Avigliano L., Catani M.V., Maccarrone M. 2-Arachidonoylglycerol modulates human endothelial cell/leukocyte interactions by controlling selectin expression through CB1 and CB2 receptors. Int. J. Biochem. Cell Biol. 2014;51:79–88. doi: 10.1016/j.biocel.2014.03.028. [DOI] [PubMed] [Google Scholar]
- 225.Haustein M., Ramer R., Linnebacher M., Manda K., Hinz B. Cannabinoids increase lung cancer cell lysis by lymphokine-activated killer cells via upregulation of ICAM-1. Biochem. Pharmacol. 2014;92:312–325. doi: 10.1016/j.bcp.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 226.Kianian M., Al-Banna N.A., Kelly M.E.M., Lehmann C. Inhibition of endocannabinoid degradation in experimental endotoxemia reduces leukocyte adhesion and improves capillary perfusion in the gut. J. Basic Clin. Physiol. Pharmacol. 2013;24:27–33. doi: 10.1515/jbcpp-2012-0065. [DOI] [PubMed] [Google Scholar]
- 227.Lunn C.A., Fine J.S., Rojas-Triana A., Jackson J.V., Fan X., Kung T.T., Gonsiorek W., Schwarz M.A., Lavey B., Kozlowski J.A., et al. A Novel Cannabinoid Peripheral Cannabinoid Receptor-Selective Inverse Agonist Blocks Leukocyte Recruitment in Vivo. J. Pharmacol. Exp. Ther. 2006;316:780–788. doi: 10.1124/jpet.105.093500. [DOI] [PubMed] [Google Scholar]
- 228.Montecucco F., Burger F., Mach F., Steffens S. CB2 cannabinoid receptor agonist JWH-015 modulates human monocyte migration through defined intracellular signaling pathways. AJP Hear. Circ. Physiol. 2007;294:H1145–H1155. doi: 10.1152/ajpheart.01328.2007. [DOI] [PubMed] [Google Scholar]
- 229.Murikinati S., Jüttler E., Keinert T., Ridder D.A., Muhammad S., Waibler Z., Ledent C., Zimmer A., Kalinke U., Schwaninger M. Activation of cannabinoid 2 receptors protects against cerebral ischemia by inhibiting neutrophil recruitment. FASEB J. 2010;24:788–798. doi: 10.1096/fj.09-141275. [DOI] [PubMed] [Google Scholar]
- 230.Forrester S.J., Kikuchi D.S., Hernandes M.S., Xu Q., Griendling K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018;122:877–902. doi: 10.1161/CIRCRESAHA.117.311401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Koga M., Serritella A.V., Sedlak T.W. Implications for reactive oxygen species in schizophrenia pathogenesis. Schizophr. Res. 2016;176:52–71. doi: 10.1016/j.schres.2015.06.022. [DOI] [PubMed] [Google Scholar]
- 232.Fraguas D., Díaz-Caneja C.M., Rodríguez-Quiroga A., Arango C. Oxidative Stress and Inflammation in Early Onset First Episode Psychosis: A Systematic Review and Meta-Analysis. Int. J. Neuropsychopharmacol. 2017;20:435–444. doi: 10.1093/ijnp/pyx015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Rajasekaran A., Venkatasubramanian G., Berk M., Debnath M. Mitochondrial dysfunction in schizophrenia: Pathways, mechanisms and implications. Neurosci. Biobehav. Rev. 2015;48:10–21. doi: 10.1016/j.neubiorev.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 234.Okuda S., Nishiyama N., Saito H., Katsuki H. 3-Hydroxykynurenine, an Endogenous Oxidative Stress Generator, Causes Neuronal Cell Death with Apoptotic Features and Region Selectivity. J. Neurochem. 2002;70:299–307. doi: 10.1046/j.1471-4159.1998.70010299.x. [DOI] [PubMed] [Google Scholar]
- 235.Sahm F., Oezen I., Opitz C.A., Radlwimmer B., von Deimling A., Ahrendt T., Adams S., Bode H.B., Guillemin G.J., Wick W., et al. The Endogenous Tryptophan Metabolite and NAD+ Precursor Quinolinic Acid Confers Resistance of Gliomas to Oxidative Stress. Cancer Res. 2013;73:3225–3234. doi: 10.1158/0008-5472.CAN-12-3831. [DOI] [PubMed] [Google Scholar]
- 236.Goda K., Kishimoto R., Shimizu S., Hamane Y., Ueda M. Quinolinic acid and active oxygens. Possible contribution of active Oxygens during cell death in the brain. Adv. Exp. Med. Biol. 1996;398:247–254. [PubMed] [Google Scholar]
- 237.Rodríguez-Martínez E., Camacho A., Maldonado P.D., Pedraza-Chaverrí J., Santamaría D., Galván-Arzate S., Santamaría A. Effect of quinolinic acid on endogenous antioxidants in rat corpus striatum. Brain Res. 2000;858:436–439. doi: 10.1016/S0006-8993(99)02474-9. [DOI] [PubMed] [Google Scholar]
- 238.Lugo-Huitrón R., Blanco-Ayala T., Ugalde-Muñiz P., Carrillo-Mora P., Pedraza-Chaverrí J., Silva-Adaya D., Maldonado P.D., Torres I., Pinzón E., Ortiz-Islas E., et al. On the antioxidant properties of kynurenic acid: Free radical scavenging activity and inhibition of oxidative stress. Neurotoxicol. Teratol. 2011;33:538–547. doi: 10.1016/j.ntt.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 239.Sas K., Szabó E., Vécsei L. Mitochondria, Oxidative Stress and the Kynurenine System, with a Focus on Ageing and Neuroprotection. Molecules. 2018;23:191. doi: 10.3390/molecules23010191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Zádori D., Veres G., Szalárdy L., Klivényi P., Vécsei L. Alzheimer’s Disease: Recent Concepts on the Relation of Mitochondrial Disturbances, Excitotoxicity, Neuroinflammation, and Kynurenines. J. Alzheimer’s Dis. 2018;62:523–547. doi: 10.3233/JAD-170929. [DOI] [PubMed] [Google Scholar]
- 241.Fukushima T., Iizuka H., Yokota A., Suzuki T., Ohno C., Kono Y., Nishikiori M., Seki A., Ichiba H., Watanabe Y., et al. Quantitative analyses of schizophrenia-associated metabolites in serum: Serum D-lactate levels are negatively correlated with gamma-glutamylcysteine in medicated schizophrenia patients. PLoS ONE. 2014;9:e101652. doi: 10.1371/journal.pone.0101652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Aso E., Juvés S., Maldonado R., Ferrer I. CB2 Cannabinoid Receptor Agonist Ameliorates Alzheimer-Like Phenotype in AβPP/PS1 Mice. J. Alzheimer’s Dis. 2013;35:847–858. doi: 10.3233/JAD-130137. [DOI] [PubMed] [Google Scholar]
- 243.Mnich K., Finn D.P., Dowd E., Gorman A.M. Inhibition by Anandamide of 6-Hydroxydopamine-Induced Cell Death in PC12 Cells. Int. J. Cell Biol. 2010;2010:818497. doi: 10.1155/2010/818497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Ma L., Jia J., Niu W., Jiang T., Zhai Q., Yang L., Bai F., Wang Q., Xiong L. Mitochondrial CB1 receptor is involved in ACEA-induced protective effects on neurons and mitochondrial functions. Sci. Rep. 2015;5:12440. doi: 10.1038/srep12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Mukhopadhyay P., Rajesh M., Bátkai S., Patel V., Kashiwaya Y., Liaudet L., Evgenov O.V., Mackie K., Haskó G., Pacher P. CB1 cannabinoid receptors promote oxidative stress and cell death in murine models of doxorubicin-induced cardiomyopathy and in human cardiomyocytes. Cardiovasc. Res. 2010;85:773–784. doi: 10.1093/cvr/cvp369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Severance E.G., Prandovszky E., Castiglione J., Yolken R.H. Gastroenterology issues in schizophrenia: Why the gut matters. Curr. Psychiatry Rep. 2015;17:27. doi: 10.1007/s11920-015-0574-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Daneman R., Rescigno M. The gut immune barrier and the blood-brain barrier: Are they so different? Immunity. 2009;31:722–735. doi: 10.1016/j.immuni.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 248.Gupta S., Masand P.S., Kaplan D., Bhandary A., Hendricks S. The relationship between schizophrenia and irritable bowel syndrome (IBS) Schizophr. Res. 1997;23:265–268. doi: 10.1016/S0920-9964(96)00099-0. [DOI] [PubMed] [Google Scholar]
- 249.Fadgyas-Stanculete M., Buga A.-M., Popa-Wagner A., Dumitrascu D.L. The relationship between irritable bowel syndrome and psychiatric disorders: From molecular changes to clinical manifestations. J. Mol. Psychiatry. 2014;2:4. doi: 10.1186/2049-9256-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Petra A.I., Panagiotidou S., Hatziagelaki E., Stewart J.M., Conti P., Theoharides T.C. Gut-Microbiota-Brain Axis and Its Effect on Neuropsychiatric Disorders With Suspected Immune Dysregulation. Clin. Ther. 2015;37:984–995. doi: 10.1016/j.clinthera.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Zhu X., Han Y., Du J., Liu R., Jin K., Yi W. Microbiota-gut-brain axis and the central nervous system. Oncotarget. 2017;8:53829–53838. doi: 10.18632/oncotarget.17754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Bruce-Keller A.J., Salbaum J.M., Berthoud H.-R. Harnessing Gut Microbes for Mental Health: Getting From Here to There. Biol. Psychiatry. 2018;83:214–223. doi: 10.1016/j.biopsych.2017.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Shen Y., Xu J., Li Z., Huang Y., Yuan Y., Wang J., Zhang M., Hu S., Liang Y. Analysis of gut microbiota diversity and auxiliary diagnosis as a biomarker in patients with schizophrenia: A cross-sectional study. Schizophr. Res. 2018;197:470–477. doi: 10.1016/j.schres.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 254.Yolken R.H., Severance E.G., Sabunciyan S., Gressitt K.L., Chen O., Stallings C., Origoni A., Katsafanas E., Schweinfurth L.A.B., Savage C.L.G., et al. Metagenomic Sequencing Indicates That the Oropharyngeal Phageome of Individuals With Schizophrenia Differs From That of Controls. Schizophr. Bull. 2015;41:1153–1161. doi: 10.1093/schbul/sbu197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Schwarz E., Maukonen J., Hyytiäinen T., Kieseppä T., Orešič M., Sabunciyan S., Mantere O., Saarela M., Yolken R., Suvisaari J. Analysis of microbiota in first episode psychosis identifies preliminary associations with symptom severity and treatment response. Schizophr. Res. 2018;192:398–403. doi: 10.1016/j.schres.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 256.Severance E.G., Gressitt K.L., Stallings C.R., Origoni A.E., Khushalani S., Leweke F.M., Dickerson F.B., Yolken R.H. Discordant patterns of bacterial translocation markers and implications for innate immune imbalances in schizophrenia. Schizophr. Res. 2013;148:130–137. doi: 10.1016/j.schres.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Castro-Nallar E., Bendall M.L., Pérez-Losada M., Sabuncyan S., Severance E.G., Dickerson F.B., Schroeder J.R., Yolken R.H., Crandall K.A. Composition, taxonomy and functional diversity of the oropharynx microbiome in individuals with schizophrenia and controls. PeerJ. 2015;3:e1140. doi: 10.7717/peerj.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Yuan X., Zhang P., Wang Y., Liu Y., Li X., Kumar B.U., Hei G., Lv L., Huang X.-F., Fan X., et al. Changes in metabolism and microbiota after 24-week risperidone treatment in drug naïve, normal weight patients with first episode schizophrenia. Schizophr. Res. 2018;201:299–306. doi: 10.1016/j.schres.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 259.Turski M.P., Turska M., Kocki T., Turski W.A., Paluszkiewicz P. Kynurenic Acid Content in Selected Culinary Herbs and Spices. J. Chem. 2015;2015:617571. doi: 10.1155/2015/617571. [DOI] [Google Scholar]
- 260.Turski M.P., Turska M., Zgrajka W., Kuc D., Turski W.A. Presence of kynurenic acid in food and honeybee products. Amino Acids. 2009;36:75–80. doi: 10.1007/s00726-008-0031-z. [DOI] [PubMed] [Google Scholar]
- 261.Kuc D., Rahnama M., Tomaszewski T., Rzeski W., Wejksza K., Urbanik-Sypniewska T., Parada-Turska J., Wielosz M., Turski W.A. Kynurenic acid in human saliva—Does it influence oral microflora? Pharmacol. Rep. 2006;58:393–398. [PubMed] [Google Scholar]
- 262.D’Argenio G., Valenti M., Scaglione G., Cosenza V., Sorrentini I., Di Marzo V. Up-regulation of anandamide levels as an endogenous mechanism and a pharmacological strategy to limit colon inflammation. FASEB J. 2006;20:568–570. doi: 10.1096/fj.05-4943fje. [DOI] [PubMed] [Google Scholar]
- 263.Müller N. Immunological aspects of the treatment of depression and schizophrenia. Dialogues Clin. Neurosci. 2017;19:55–63. doi: 10.31887/DCNS.2017.19.1/nmueller. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.McGuire P., Robson P., Cubala W.J., Vasile D., Morrison P.D., Barron R., Taylor A., Wright S. Cannabidiol (CBD) as an Adjunctive Therapy in Schizophrenia: A Multicenter Randomized Controlled Trial. Am. J. Psychiatry. 2018;175:225–231. doi: 10.1176/appi.ajp.2017.17030325. [DOI] [PubMed] [Google Scholar]
- 265.Pedrazzi J.F.C., Issy A.C., Gomes F.V., Guimarães F.S., Del-Bel E.A. Cannabidiol effects in the prepulse inhibition disruption induced by amphetamine. Psychopharmacology. 2015;232:3057–3065. doi: 10.1007/s00213-015-3945-7. [DOI] [PubMed] [Google Scholar]
- 266.Leweke F.M., Piomelli D., Pahlisch F., Muhl D., Gerth C.W., Hoyer C., Klosterkötter J., Hellmich M., Koethe D. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl. Psychiatry. 2012;2:e94. doi: 10.1038/tp.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Beltramo M., de Fonseca F.R., Navarro M., Calignano A., Gorriti M.A., Grammatikopoulos G., Sadile A.G., Giuffrida A., Piomelli D. Reversal of dopamine D(2) receptor responses by an anandamide transport inhibitor. J. Neurosci. 2000;20:3401–3407. doi: 10.1523/JNEUROSCI.20-09-03401.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Seillier A., Advani T., Cassano T., Hensler J.G., Giuffrida A. Inhibition of fatty-acid amide hydrolase and CB1 receptor antagonism differentially affect behavioural responses in normal and PCP-treated rats. Int. J. Neuropsychopharmacol. 2010;13:373–386. doi: 10.1017/S146114570999023X. [DOI] [PubMed] [Google Scholar]
- 269.Aguilar D.D., Giuffrida A., Lodge D.J. Adolescent Synthetic Cannabinoid Exposure Produces Enduring Changes in Dopamine Neuron Activity in a Rodent Model of Schizophrenia Susceptibility. Int. J. Neuropsychopharmacol. 2018;21:393–403. doi: 10.1093/ijnp/pyy003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Aguilar D.D., Chen L., Lodge D.J. Increasing Endocannabinoid Levels in the Ventral Pallidum Restore Aberrant Dopamine Neuron Activity in the Subchronic PCP Rodent Model of Schizophrenia. Int. J. Neuropsychopharmacol. 2014;18:pyu035. doi: 10.1093/ijnp/pyu035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Jia J., Ma L., Wu M., Zhang L., Zhang X., Zhai Q., Jiang T., Wang Q., Xiong L. Anandamide protects HT22 cells exposed to hydrogen peroxide by inhibiting CB1 receptor-mediated type 2 NADPH oxidase. Oxid. Med. Cell. Longev. 2014;2014:893516. doi: 10.1155/2014/893516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Schwarcz G., Karajgi B., McCarthy R. Synthetic Δ-9-Tetrahydrocannabinol (Dronabinol) Can Improve the Symptoms of Schizophrenia. J. Clin. Psychopharmacol. 2009;29:255–258. doi: 10.1097/JCP.0b013e3181a6bc3b. [DOI] [PubMed] [Google Scholar]
- 273.Tzavara E.T., Degroot A., Wade M.R., Davis R.J., Nomikos G.G. CB1 receptor knockout mice are hyporesponsive to the behavior-stimulating actions of d-amphetamine: Role of mGlu5 receptors. Eur. Neuropsychopharmacol. 2009;19:196–204. doi: 10.1016/j.euroneuro.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 274.Tzavara E.T., Davis R.J., Perry K.W., Li X., Salhoff C., Bymaster F.P., Witkin J.M., Nomikos G.G. The CB1 receptor antagonist SR141716A selectively increases monoaminergic neurotransmission in the medial prefrontal cortex: Implications for therapeutic actions. Br. J. Pharmacol. 2003;138:544–553. doi: 10.1038/sj.bjp.0705100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Boggs D.L., Kelly D.L., McMahon R.P., Gold J.M., Gorelick D.A., Linthicum J., Conley R.R., Liu F., Waltz J., Huestis M.A., et al. Rimonabant for neurocognition in schizophrenia: A 16-week double blind randomized placebo controlled trial. Schizophr. Res. 2012;134:207–210. doi: 10.1016/j.schres.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Crismon L., Argo T.R., Buckley P.F. Pharmacotherapy: A Pathophysiologic Approach. McGraw-Hill; New York, NY, USA: 2014. Schizophrenia; pp. 1019–1046. [Google Scholar]
- 277.Tandon R. Antipsychotics in the Treatment of Schizophrenia. J. Clin. Psychiatry. 2011;72:4–8. doi: 10.4088/JCP.10075su1.01. [DOI] [PubMed] [Google Scholar]
- 278.Seeman P. Dopamine receptor sequences. Therapeutic levels of neuroleptics occupy D2 receptors, clozapine occupies D4. Neuropsychopharmacology. 1992;7:261–284. [PubMed] [Google Scholar]
- 279.Miyamoto S., Miyake N., Jarskog L.F., Fleischhacker W.W., Lieberman J.A. Pharmacological treatment of schizophrenia: A critical review of the pharmacology and clinical effects of current and future therapeutic agents. Mol. Psychiatry. 2012;17:1206–1227. doi: 10.1038/mp.2012.47. [DOI] [PubMed] [Google Scholar]
- 280.Miyamoto S., Duncan G.E., Marx C.E., Lieberman J.A. Treatments for schizophrenia: A critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol. Psychiatry. 2005;10:79–104. doi: 10.1038/sj.mp.4001556. [DOI] [PubMed] [Google Scholar]
- 281.Biedermann F., Fleischhacker W.W. Emerging drugs for schizophrenia. Expert Opin. Emerg. Drugs. 2011;16:271–282. doi: 10.1517/14728214.2011.556112. [DOI] [PubMed] [Google Scholar]
- 282.Jarskog L.F., Miyamoto S., Lieberman J.A. Schizophrenia: New Pathological Insights and Therapies. Annu. Rev. Med. 2007;58:49–61. doi: 10.1146/annurev.med.58.060904.084114. [DOI] [PubMed] [Google Scholar]
- 283.Hill S.K., Bishop J.R., Palumbo D., Sweeney J.A. Effect of second-generation antipsychotics on cognition: Current issues and future challenges. Expert Rev. Neurother. 2010;10:43–57. doi: 10.1586/ern.09.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Kuroki T., Nagao N., Nakahara T. Neuropharmacology of second-generation antipsychotic drugs: A validity of the serotonin–dopamine hypothesis. Prog. Brain Res. 2008;172:199–212. doi: 10.1016/S0079-6123(08)00910-2. [DOI] [PubMed] [Google Scholar]
- 285.Meltzer H.Y., Matsubara S., Lee J.C. Classification of typical and atypical antipsychotic drugs on the basis of dopamine D-1, D-2 and serotonin2 pKi values. J. Pharmacol. Exp. Ther. 1989;251:238–246. [PubMed] [Google Scholar]
- 286.MacKenzie N.E., Kowalchuk C., Agarwal S.M., Costa-Dookhan K.A., Caravaggio F., Gerretsen P., Chintoh A., Remington G.J., Taylor V.H., Müeller D.J., et al. Antipsychotics, Metabolic Adverse Effects, and Cognitive Function in Schizophrenia. Front. Psychiatry. 2018;9:622. doi: 10.3389/fpsyt.2018.00622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Davis K.L., Kahn R.S., Ko G., Davidson M. Dopamine in schizophrenia: A review and reconceptualization. Am. J. Psychiatry. 1991;148:1474–1486. doi: 10.1176/ajp.148.11.1474. [DOI] [PubMed] [Google Scholar]
- 288.Lieberman J.A. Dopamine Partial Agonists. CNS Drugs. 2004;18:251–267. doi: 10.2165/00023210-200418040-00005. [DOI] [PubMed] [Google Scholar]
- 289.Shapiro D.A., Renock S., Arrington E., Chiodo L.A., Liu L.-X., Sibley D.R., Roth B.L., Mailman R. Aripiprazole, A Novel Atypical Antipsychotic Drug with a Unique and Robust Pharmacology. Neuropsychopharmacology. 2003;28:1400–1411. doi: 10.1038/sj.npp.1300203. [DOI] [PubMed] [Google Scholar]
- 290.Horacek J., Bubenikova-Valesova V., Kopecek M., Palenicek T., Dockery C., Mohr P., Höschl C. Mechanism of Action of Atypical Antipsychotic Drugs and the Neurobiology of Schizophrenia. CNS Drugs. 2006;20:389–409. doi: 10.2165/00023210-200620050-00004. [DOI] [PubMed] [Google Scholar]
- 291.Amato D., Kruyer A., Samaha A.-N., Heinz A. Hypofunctional Dopamine Uptake and Antipsychotic Treatment-Resistant Schizophrenia. Front. Psychiatry. 2019;10:314. doi: 10.3389/fpsyt.2019.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Goff D.C., Coyle J.T. The Emerging Role of Glutamate in the Pathophysiology and Treatment of Schizophrenia. Am. J. Psychiatry. 2001;158:1367–1377. doi: 10.1176/appi.ajp.158.9.1367. [DOI] [PubMed] [Google Scholar]
- 293.Stępnicki P., Kondej M., Kaczor A.A. Current Concepts and Treatments of Schizophrenia. Molecules. 2018;23:2087. doi: 10.3390/molecules23082087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Tsai G., Lin P.-Y. Strategies to Enhance N-Methyl-D-Aspartate Receptor-Mediated Neurotransmission in Schizophrenia, a Critical Review and Meta-Analysis. Curr. Pharm. Des. 2010;16:522–537. doi: 10.2174/138161210790361452. [DOI] [PubMed] [Google Scholar]
- 295.Heresco-Levy U., Javitt D.C., Ermilov M., Mordel C., Silipo G., Lichtenstein M. Efficacy of High-Dose Glycine in the Treatment of Enduring Negative Symptoms of Schizophrenia. Arch. Gen. Psychiatry. 1999;56:29–36. doi: 10.1001/archpsyc.56.1.29. [DOI] [PubMed] [Google Scholar]
- 296.Javitt D.C., Zylberman I., Zukin S.R., Heresco-Levy U., Lindenmayer J.P. Amelioration of negative symptoms in schizophrenia by glycine. Am. J. Psychiatry. 1994;151:1234–1236. doi: 10.1176/ajp.151.8.1234. [DOI] [PubMed] [Google Scholar]
- 297.Diaz P., Bhaskara S., Dursun S.M., Deakin B. Double-blind, placebo-controlled, crossover trial of clozapine plus glycine in refractory schizophrenia negative results. J. Clin. Psychopharmacol. 2005;25:277–278. doi: 10.1097/01.jcp.0000165740.22377.6d. [DOI] [PubMed] [Google Scholar]
- 298.Buchanan R.W., Javitt D.C., Marder S.R., Schooler N.R., Gold J.M., McMahon R.P., Heresco-Levy U., Carpenter W.T. The Cognitive and Negative Symptoms in Schizophrenia Trial (CONSIST): The Efficacy of Glutamatergic Agents for Negative Symptoms and Cognitive Impairments. Am. J. Psychiatry. 2007;164:1593–1602. doi: 10.1176/appi.ajp.2007.06081358. [DOI] [PubMed] [Google Scholar]
- 299.Cain C.K., McCue M., Bello I., Creedon T., Tang D., Laska E., Goff D.C. d-Cycloserine augmentation of cognitive remediation in schizophrenia. Schizophr. Res. 2014;153:177–183. doi: 10.1016/j.schres.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Goff D.C. D-cycloserine in Schizophrenia: New Strategies for Improving Clinical Outcomes by Enhancing Plasticity. Curr. Neuropharmacol. 2017;15:21–34. doi: 10.2174/1570159X14666160225154812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Fakhri A., Pakseresht S., Haghdoost M.R., Hekmatkhah N., Torkashvand M., Ghorbanzadeh B. Memantine Enhances the Effect of Olanzapine in Patients With Schizophrenia: A Randomized, Placebo-Controlled Study. Acta Med. Iran. 2016;54:696–703. [PubMed] [Google Scholar]
- 302.De Lucena D., Fernandes B.S., Berk M., Dodd S., Medeiros D.W., Pedrini M., Kunz M., Gomes F.A., Giglio L.F., Lobato M.I., et al. Improvement of Negative and Positive Symptoms in Treatment-Refractory Schizophrenia. J. Clin. Psychiatry. 2009;70:1416–1423. doi: 10.4088/JCP.08m04935gry. [DOI] [PubMed] [Google Scholar]
- 303.Marenco S., Egan M.F., Goldberg T.E., Knable M.B., McClure R.K., Winterer G., Weinberger D.R. Preliminary experience with an ampakine (CX516) as a single agent for the treatment of schizophrenia: A case series. Schizophr. Res. 2002;57:221–226. doi: 10.1016/S0920-9964(01)00311-5. [DOI] [PubMed] [Google Scholar]
- 304.Tsai G., Lane H.-Y., Yang P., Chong M.-Y., Lange N. Glycine transporter I inhibitor, N-Methylglycine (sarcosine), added to antipsychotics for the treatment of schizophrenia. Biol. Psychiatry. 2004;55:452–456. doi: 10.1016/j.biopsych.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 305.Lane H.-Y., Huang C.-L., Wu P.-L., Liu Y.-C., Chang Y.-C., Lin P.-Y., Chen P.-W., Tsai G. Glycine Transporter I Inhibitor, N-methylglycine (Sarcosine), Added to Clozapine for the Treatment of Schizophrenia. Biol. Psychiatry. 2006;60:645–649. doi: 10.1016/j.biopsych.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 306.Lane H.-Y., Lin C.-H., Huang Y.-J., Liao C.-H., Chang Y.-C., Tsai G.E. A randomized, double-blind, placebo-controlled comparison study of sarcosine ( N-methylglycine) and d-serine add-on treatment for schizophrenia. Int. J. Neuropsychopharmacol. 2010;13:451–460. doi: 10.1017/S1461145709990939. [DOI] [PubMed] [Google Scholar]
- 307.Maksymetz J., Moran S.P., Conn P.J. Targeting metabotropic glutamate receptors for novel treatments of schizophrenia. Mol. Brain. 2017;10:15. doi: 10.1186/s13041-017-0293-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Lewis D.A., Cho R.Y., Carter C.S., Eklund K., Forster S., Kelly M.A., Montrose D. Subunit-Selective Modulation of GABA Type A Receptor Neurotransmission and Cognition in Schizophrenia. Am. J. Psychiatry. 2008;165:1585–1593. doi: 10.1176/appi.ajp.2008.08030395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Heyes M.P., Chen C.Y., Major E.O., Saito K. Different kynurenine pathway enzymes limit quinolinic acid formation by various human cell types. Pt 2Biochem. J. 1997;326:351–356. doi: 10.1042/bj3260351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Stone T.W., Darlington L.G. The kynurenine pathway as a therapeutic target in cognitive and neurodegenerative disorders. Br. J. Pharmacol. 2013;169:1211–1227. doi: 10.1111/bph.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Pocivavsek A., Elmer G.I., Schwarcz R. Inhibition of Kynurenine Aminotransferase II Attenuates Hippocampus-dependent Memory Deficit in Adult Rats Treated Prenatally with Kynurenine. Hippocampus. 2019;29:73–77. doi: 10.1002/hipo.23040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Amori L., Wu H.-Q., Marinozzi M., Pellicciari R., Guidetti P., Schwarcz R. Specific inhibition of kynurenate synthesis enhances extracellular dopamine levels in the rodent striatum. Neuroscience. 2009;159:196–203. doi: 10.1016/j.neuroscience.2008.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Pellicciari R., Rizzo R.C., Costantino G., Marinozzi M., Amori L., Guidetti P., Wu H.-Q., Schwarcz R. Modulators of the Kynurenine Pathway of Tryptophan Metabolism: Synthesis and Preliminary Biological Evaluation of (S)-4-(Ethylsulfonyl)benzoylalanine, a Potent and Selective Kynurenine Aminotransferase II (KAT II) Inhibitor. ChemMedChem. 2006;1:528–531. doi: 10.1002/cmdc.200500095. [DOI] [PubMed] [Google Scholar]
- 314.Konradsson-Geuken Å., Wu H.Q., Gash C.R., Alexander K.S., Campbell A., Sozeri Y., Pellicciari R., Schwarcz R., Bruno J.P. Cortical kynurenic acid bi-directionally modulates prefrontal glutamate levels as assessed by microdialysis and rapid electrochemistry. Neuroscience. 2010;169:1848–1859. doi: 10.1016/j.neuroscience.2010.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Zmarowski A., Wu H.-Q., Brooks J.M., Potter M.C., Pellicciari R., Schwarcz R., Bruno J.P. Astrocyte-derived kynurenic acid modulates basal and evoked cortical acetylcholine release. Eur. J. Neurosci. 2009;29:529–538. doi: 10.1111/j.1460-9568.2008.06594.x. [DOI] [PubMed] [Google Scholar]
- 316.Dounay A.B., Anderson M., Bechle B.M., Campbell B.M., Claffey M.M., Evdokimov A., Evrard E., Fonseca K.R., Gan X., Ghosh S., et al. Discovery of Brain-Penetrant, Irreversible Kynurenine Aminotransferase II Inhibitors for Schizophrenia. ACS Med. Chem. Lett. 2012;3:187–192. doi: 10.1021/ml200204m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Koshy Cherian A., Gritton H., Johnson D.E., Young D., Kozak R., Sarter M. A systemically-available kynurenine aminotransferase II (KAT II) inhibitor restores nicotine-evoked glutamatergic activity in the cortex of rats. Neuropharmacology. 2014;82:41–48. doi: 10.1016/j.neuropharm.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Linderholm K.R., Alm M.T., Larsson M.K., Olsson S.K., Goiny M., Hajos M., Erhardt S., Engberg G. Inhibition of kynurenine aminotransferase II reduces activity of midbrain dopamine neurons. Neuropharmacology. 2016;102:42–47. doi: 10.1016/j.neuropharm.2015.10.028. [DOI] [PubMed] [Google Scholar]
- 319.Henderson J.L., Sawant-Basak A., Tuttle J.B., Dounay A.B., McAllister L.A., Pandit J., Rong S., Hou X., Bechle B.M., Kim J.-Y., et al. Discovery of hydroxamate bioisosteres as KAT II inhibitors with improved oral bioavailability and pharmacokinetics. Med. Chem. Commun. 2013;4:125–129. doi: 10.1039/C2MD20166F. [DOI] [Google Scholar]
- 320.Bortz D.M., Wu H.-Q., Schwarcz R., Bruno J.P. Oral administration of a specific kynurenic acid synthesis (KAT II) inhibitor attenuates evoked glutamate release in rat prefrontal cortex. Neuropharmacology. 2017;121:69–78. doi: 10.1016/j.neuropharm.2017.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Réus G.Z., Becker I.R.T., Scaini G., Petronilho F., Oses J.P., Kaddurah-Daouk R., Ceretta L.B., Zugno A.I., Dal-Pizzol F., Quevedo J., et al. The inhibition of the kynurenine pathway prevents behavioral disturbances and oxidative stress in the brain of adult rats subjected to an animal model of schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2018;81:55–63. doi: 10.1016/j.pnpbp.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 322.Bhattacharyya S., Morrison P.D., Fusar-Poli P., Martin-Santos R., Borgwardt S., Winton-Brown T., Nosarti C., O’ Carroll C.M., Seal M., Allen P., et al. Opposite effects of delta-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology. 2010;35:764–774. doi: 10.1038/npp.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Bisogno T., Hanuš L., de Petrocellis L., Tchilibon S., Ponde D.E., Brandi I., Moriello A.S., Davis J.B., Mechoulam R., di Marzo V. Molecular targets for cannabidiol and its synthetic analogues: Effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br. J. Pharmacol. 2001;134:845–852. doi: 10.1038/sj.bjp.0704327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Seeman P. Cannabidiol is a partial agonist at dopamine D2High receptors, predicting its antipsychotic clinical dose. Transl. Psychiatry. 2016;6:e920. doi: 10.1038/tp.2016.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Ibeas Bih C., Chen T., Nunn A.V.W., Bazelot M., Dallas M., Whalley B.J. Molecular Targets of Cannabidiol in Neurological Disorders. Neurotherapeutics. 2015;12:699–730. doi: 10.1007/s13311-015-0377-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Moreira F.A., Guimarães F.S. Cannabidiol inhibits the hyperlocomotion induced by psychotomimetic drugs in mice. Eur. J. Pharmacol. 2005;512:199–205. doi: 10.1016/j.ejphar.2005.02.040. [DOI] [PubMed] [Google Scholar]
- 327.Zuardi A.W., Morais S.L., Guimarães F.S., Mechoulam R. Antipsychotic effect of cannabidiol. J. Clin. Psychiatry. 1995;56:485–486. [PubMed] [Google Scholar]
- 328.Zuardi A.W., Crippa J.A.S., Hallak J.E.C., Moreira F.A., Guimarães F.S. Cannabidiol, a Cannabis sativa constituent, as an antipsychotic drug. Braz. J. Med. Biol. Res. 2006;39:421–429. doi: 10.1590/S0100-879X2006000400001. [DOI] [PubMed] [Google Scholar]
- 329.Batalla A., Bhattacharyya S., Yücel M., Fusar-Poli P., Crippa J.A., Nogué S., Torrens M., Pujol J., Farré M., Martin-Santos R. Structural and functional imaging studies in chronic cannabis users: A systematic review of adolescent and adult findings. PLoS ONE. 2013;8:e55821. doi: 10.1371/journal.pone.0055821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Hallak J.E.C., Machado-de-Sousa J.P., Crippa J.A.S., Sanches R.F., Trzesniak C., Chaves C., Bernardo S.A., Regalo S.C., Zuardi A.W. Performance of schizophrenic patients in the Stroop Color Word Test and electrodermal responsiveness after acute administration of cannabidiol (CBD) Rev. Bras. Psiquiatr. 2010;32:56–61. doi: 10.1590/S1516-44462010000100011. [DOI] [PubMed] [Google Scholar]
- 331.Zuardi A.W., Hallak J.E.C., Dursun S.M., Morais S.L., Sanches R.F., Musty R.E., Crippa J.A.S. Cannabidiol monotherapy for treatment-resistant schizophrenia. J. Psychopharmacol. 2006;20:683–686. doi: 10.1177/0269881106060967. [DOI] [PubMed] [Google Scholar]
- 332.Hahn B. The Potential of Cannabidiol Treatment for Cannabis Users With Recent-Onset Psychosis. Schizophr. Bull. 2018;44:46–53. doi: 10.1093/schbul/sbx105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Matricon J., Seillier A., Giuffrida A. Distinct neuronal activation patterns are associated with PCP-induced social withdrawal and its reversal by the endocannabinoid-enhancing drug URB597. Neurosci. Res. 2016;110:49–58. doi: 10.1016/j.neures.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Meltzer H.Y., Arvanitis L., Bauer D., Rein W., Group M.-T.S. Placebo-Controlled Evaluation of Four Novel Compounds for the Treatment of Schizophrenia and Schizoaffective Disorder. Am. J. Psychiatry. 2004;161:975–984. doi: 10.1176/appi.ajp.161.6.975. [DOI] [PubMed] [Google Scholar]
- 335.Bisogno T., Di Marzo V. The role of the endocannabinoid system in Alzheimer’s disease: Facts and hypotheses. Curr. Pharm. Des. 2008;14:2299–3305. doi: 10.2174/138161208785740027. [DOI] [PubMed] [Google Scholar]
- 336.Dezsi L., Tuka B., Martos D., Vecsei L. Alzheimer’s disease, astrocytes and kynurenines. Curr. Alzheimer Res. 2015;12:462–480. doi: 10.2174/156720501205150526114000. [DOI] [PubMed] [Google Scholar]
- 337.Miller L.K., Devi L.A. The Highs and Lows of Cannabinoid Receptor Expression in Disease: Mechanisms and Their Therapeutic Implications. Pharmacol. Rev. 2011;63:461–470. doi: 10.1124/pr.110.003491. [DOI] [PMC free article] [PubMed] [Google Scholar]