Abstract
Background:
In infants, distinct nasopharyngeal bacterial microbiotas differentially associate with the incidence and severity of acute respiratory tract infection and childhood asthma development.
Objective:
We hypothesized that distinct nasal airway microbiota structures also exist in children with asthma and relate to clinical outcomes.
Methods:
Nasal secretion samples (n = 3122) collected after randomization during the fall season from children with asthma (6–17 years, n = 413) enrolled in a trial of omalizumab (anti-IgE) underwent 16S rRNA profiling. Statistical analyses with exacerbation as the primary outcome and rhinovirus infection and respiratory illnesses as secondary outcomes were performed. Using A549 epithelial cells, we assessed nasal isolates of Moraxella, Staphylococcus, and Corynebacterium species for their capacity to induce epithelial damage and inflammatory responses.
Results:
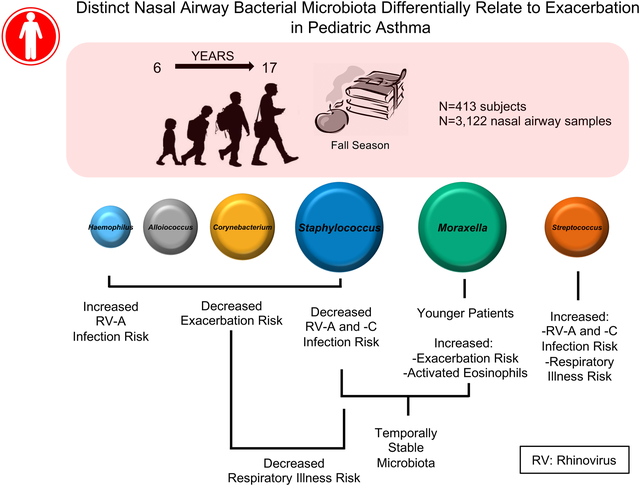
Six nasal airway microbiota assemblages, each dominated by Moraxella, Staphylococcus, Corynebacterium, Streptococcus, Alloiococcus, or Haemophilus species, were observed. Moraxella and Staphylococcus species–dominated microbiotas were most frequently detected and exhibited temporal stability. Nasal microbiotas dominated by Moraxella species were associated with increased exacerbation risk and eosinophil activation. Staphylococcus or Corynebacterium species–dominated microbiotas were associated with reduced respiratory illness and exacerbation events, whereas Streptococcus species–dominated assemblages increased the risk of rhinovirus infection. Nasal microbiota composition remained relatively stable despite viral infection or exacerbation; only a few taxa belonging to the dominant genera exhibited relative abundance fluctuations during these events. In vitro, Moraxella catarrhalis induced significantly greater epithelial damage and inflammatory cytokine expression (IL-33 and IL-8) compared with other dominant nasal bacterial isolates tested.
Conclusion:
Distinct nasal airway microbiotas of children with asthma relate to the likelihood of exacerbation, rhinovirus infection, and respiratory illnesses during the fall season.
Keywords: Microbiota, Moraxella species, Staphylococcus species, 16S rRNA, airway, asthma, exacerbation, rhinovirus
Graphical Abstract
Viral respiratory tract infections have long been recognized as important contributors to wheezing illnesses and asthma exacerbations; however, there is growing interest in the role of the airway microbiome and its potential to modulate these events in children with asthma.1 Bisgaard et al2 demonstrated that detection of Streptococcus pneumoniae, Moraxella catarrhalis, or Haemophilus influenzae in nasopharyngeal samples obtained at 1 month of age was linked to increased risk for asthma at 5 years of age. The Childhood Asthma Study, a prospective cohort of infants (n = 234), examined nasopharyngeal bacterial communities over the first year of life, including samples collected during periods with and without acute respiratory illness.3 Within this cohort, 6 compositionally distinct microbiota, each dominated by Moraxella, Staphylococcus, Corynebacterium, Streptococcus, Alloiococcus, or Haemophilus species, were identified. Microbiotas dominated by Moraxella, Streptococcus, or Haemophilus species were associated with a significantly increased risk of virus-associated acute respiratory illness,3 and Streptococcus species–dominated microbiotas were also coassociated with increased risk of lower airway infection and subsequent childhood asthma development.
Prior acute sinus infection has been associated with significant relative enrichment of upper airway Moraxella species in children, and expansion of this genus in healthy (non–upper respiratory tract infection) samples predicts subsequent acute sinusitis4 and relates to airway eosinophilia and bronchial inflammation in adults with asthma.5 Independently, upper airway detection of S pneumoniae has been associated with increased respiratory illness symptoms and moderate asthma exacerbations, particularly when co-detected with rhinovirus, whereas M catarrhalis and rhinovirus in combination, increased the likelihood of respiratory illness, asthma symptoms, or both compared with infection with rhinovirus alone.6 Thus the presence of specific bacterial genera and their coassociated microbiota assemblages strongly and reproducibly relate to respiratory illness, particularly in relation to viral infection. Given that viral respiratory tract infection is a risk factor for asthma exacerbation7 and that bacterial LPS has recently been shown to modulate viral stability,8 we hypothesized that compositionally distinct microbiotas exist in the nasal airways of children with asthma and are related to risk of viral infection and asthma exacerbation.
METHODS
Study design and clinical outcomes
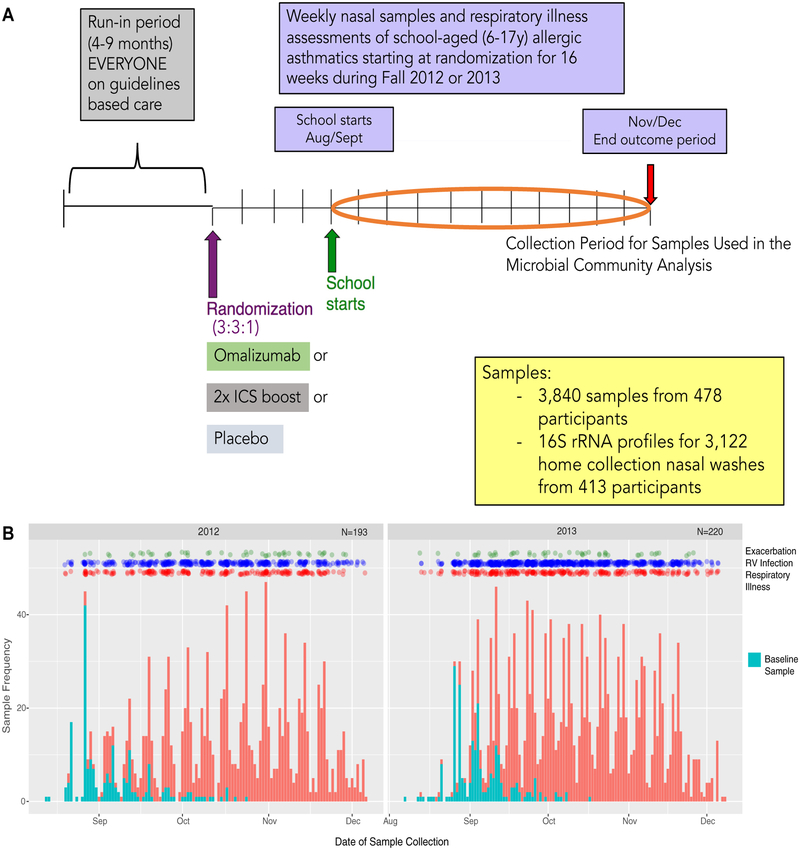
This study followed 478 children with asthma (aged 6–17 years) from 8 urban clinics (Boston Chicago, Cincinnati, Dallas, Denver, Detroit, New York, and Washington, DC) participating in the Preventative Omalizumab or Step-up Therapy for Severe Fall Exacerbations (PROSE; ) randomized controlled trial (see Table E1 in this article’s Online Repository at www.jacionline.org).9 Participants provided home-collected nasal secretion samples every 2 weeks throughout the 90-day fall outcome periods from one of 2 study years (September-December 2012 or 2013) for analysis in this study (Fig 1). Sample collection and storage were standardized across all clinical sites. Participants with fewer than 3 high-quality microbiota profiles available for analyses were removed. See the Methods section in this article’s Online Repository at www.jacionline.org for details of sample acquisition, processing, and analyses. Asthma exacerbations were defined a priori as physician-prescribed use of systemic corticosteroids for asthma symptoms, hospitalization for asthma, or both.10,11 Rhinovirus infection (detection of rhinovirus) was assessed by using quantitative PCR and partial sequencing to identify viral strain type.12
FIG 1.
A, Study design and distribution of microbiota profiles from children in the PROSE study (10) used in the current study. ICS, Inhaled corticosteroid. B, Frequency and timing of sample collection. Blue bars depict the first postrandomization (baseline) sample collected from participants, and all subsequent longitudinal samples collected are indicated by red bars. Green, blue, and red dots indicate exacerbation, rhinovirus infection (RV), and respiratory illness events, respectively.
Microbiota analyses
DNA from nasal secretion samples was extracted by using a modified cetyltrimethylammonium bromide–polyethylene glycol protocol, and the variable 4 (V4) region of the 16S rRNA gene was amplified, quantified, and sequenced on a NextSeq 500 (Illumina, San Diego, Calif); details are provided in the Methods section in this article’s Online Repository. Statistical analyses were conducted with R13 and QIIME14 software. Relationships between community composition in initially collected independent samples and a range of clinical and viral infection variables were assessed by using adonis in the vegan package15 in R software. Respiratory illnesses were defined as an increased symptom score compared with pretreatment baseline symptoms.16 Respiratory symptom scores were based on a 0- to 3-point severity score (absent, mild, moderate, or severe) for 5 different symptoms (runny nose, stuffy nose, sore throat, sneezing, and cough); scores ranged from a minimum of 0 to a maximum of 15.
Significant differences in taxon relative abundance were assessed by using a 3-model approach, as previously described.17 Samples were stratified based on the genus-level identity of the dominant taxon (ie, the taxon that exhibited the largest proportion of sequence reads in each sample). Co-occurrence networks of operational taxonomic units were constructed by using the SparCC18 and WGCNA19 packages in R software. Repeated-measures analyses (linear mixed effects and generalized estimating equations) used participant as the random effect. P values were adjusted for false discovery by using the Benjamini-Hochberg method; a q value of 0.15 was considered significant.
In vitro epithelial response studies
Human alveolar epithelial cells (A549) were exposed to the sterile products of 72-hour biofilm cultures of nasal M catarrhalis, Staphylococcus epidermidis, Staphylococcus aureus, and Corynebacterium propinquum isolates. Epithelial cell damage was assessed based on lactate dehydrogenase release and inflammatory gene expression by using quantitative PCR. Further information is available in the Methods section in this article’s Online Repository.
RESULTS
Clinical and viral factors are related to nasal airway microbiota composition
Of the 3840 samples received, 3122 samples from 413 children provided a high-quality microbiota profile for analysis. An average of 7.5 samples per person were available, and samples were collected an average of 10.7 days apart (Fig 1). Clinical, demographic, and microbiological factors (see Table E2 in this article’s Online Repository at www.jacionline.org) measured during the 3-month postrandomization observation period were assessed for relationships with nasal airway microbiota composition (β-diversity). Using the first postrandomization sample (baseline sample) available from each study participant for analysis (Fig 1, blue bars), we determined that variance in nasal airway microbial composition (β-diversity) was significantly associated with the dominant bacterial genus present, study site, age, eosinophil cationic protein (ECP) concentration in nasal airway secretions at randomization, total number of rhinovirus infections per participant, and samples in which rhinovirus was detected (rhinovirus infection samples; P < .05 for all, permutational multivariate ANOVA; see Table E2). In contrast, microbiota composition at baseline was not significantly related to respiratory illness symptoms at the time of sample collection (R2 = 0.048, P = .067), treatment group (placebo, omalizumab, or inhaled corticosteroid boost: R2 = 0.008, P = .079; see Table E2), or future exacerbations in the outcome period (R2 = 0.0043, P =.101). These observations were supported by using a bootstrapped analysis (ie, repeatedly sub-sampling a single randomly selected sample for each participant; 500 times; see Table E2 and additional information in the Methods section in this article’s Online Repository).
Although overall nasal microbiota composition at baseline was not related to exacerbation in the outcome period, this did not preclude the possibility that specific taxa could predict future exacerbations. To examine this possibility, we performed comparative and machine learning analyses to determine relationships between baseline bacterial taxa relative abundance and asthma exacerbations in the outcome period. A number of distinct Corynebacterium and Acinetobacter taxa were depleted at baseline in children who went on to experience an exacerbation (see Fig E1, A, in this article’s Online Repository at www.jacionline.org). Elastic nets analysis identified distinct Moraxella and Staphylococcus taxa that were predictive of exacerbation or nonexacerbation outcomes (see Fig E1, B), indicating that specific species or strains within these genera might relate to subsequent exacerbation susceptibility.
Taxa associated with exacerbation and rhinovirus infection coassociate in bacterial networks
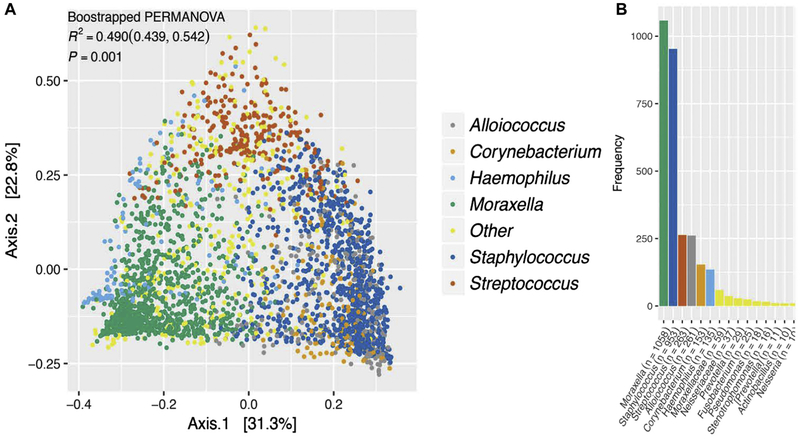
The bacterial genus most abundant in each sample (the dominant taxon) explained a large proportion of variability in microbiota composition (Fig 2, A, and see Table E2), indicating that a gradient of microbiota compositions punctuated by the presence of distinct dominant bacterial genera exist in the nasal airways of children with asthma. The majority of samples in our study were dominated by either Moraxella species (n = 1058 [33.9%]) or Staphylococcus species (n = 953 [30.5%]); the remaining samples were dominated by Streptococcus (n = 263 [8.4%]), Alloiococcus (n = 261 [8.4%]), Corynebacterium (n = 153 [4.9%]), or Haemophilus (n = 135 [4.3%]) species or other genera (n = 299 [9.6%]; Fig 2, B). The specific taxa characteristically dominating each of the 6 most common microbiotas were also amongst those significantly enriched or depleted in samples with rhinovirus infection (vs no infection) or respiratory illness (vs none) or children who experienced asthma exacerbations (vs no exacerbations) during the outcome period (see Figs E2 and E3 in this article’s Online Repository at www.jacionline.org). This suggested that distinct nasal airway microbiotas dominated by different bacterial genera exist in children with asthma and that taxa within these microbiotas relate to the primary (asthma exacerbation) and secondary (rhinovirus infection and respiratory illness) clinical outcomes in this cohort.
FIG 2.
Compositionally distinct nasal airway microbiotas exist in children with asthma. A, Six compositionally distinct microbiotas are evident in the nasal airway samples of children with asthma (P = .001, bootstrapped permutational multivariate ANOVA). B, Moraxella and Staphylococcus most frequently dominate nasal samples from pediatric patients with asthma, with Streptococcus, Alloiococcus, Corynebacterium, Haemophilus and a number of additional genera dominating smaller proportions of samples.
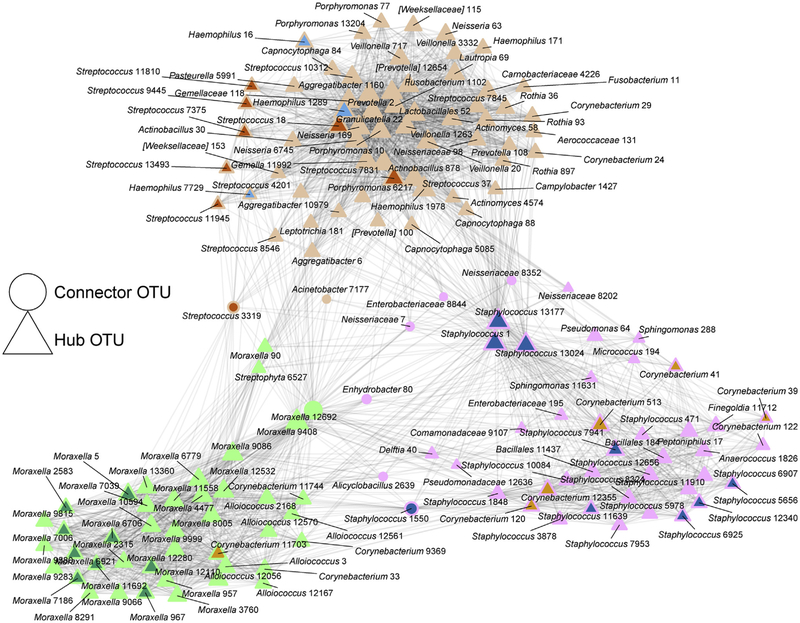
To identify specific bacterial taxa that consistently coassociate within the nasal airway microbiota, we next applied network analysis to microbiota profiles generated from all samples (Fig 3 and see Table E3 in this article’s Online Repository at www.jacionline.org). Notably, dominant Moraxella taxa consistently coassociated almost exclusively with other Moraxella taxa, including several that were enriched in participants who experienced exacerbations (Fig 3 and see Table E4 in this article’s Online Repository at www.jacionline.org). In contrast, the dominant taxa in each of the other nasal airway microbiotas formed bacterial networks consisting of both phylogenetically similar and distinct genera, often including the same specific taxa enriched in rhinovirus-negative and nonexacerbation samples.
FIG 3.
Network analysis identifies 3 distinct modules of coassociated nasal airway bacterial taxa. Taxa identified as differentially enriched in taxon comparisons of exacerbation versus nonexacerbation and rhinovirus infection versus non–rhinovirus infection comparisons are indicated and color coded according to the dominant microbiota colors defined in Fig 2. Hub operational taxonomic units (OTUs; triangles) exhibit greater intermodule connectivity, whereas connector OTUs (circles) exhibit a higher frequency of intramodule connectivity. The size of the node (triangles or circles) scales with the total number of connections with other OTUs. Genus classification and OTU numbers are indicated.
Nasal microbiotas are associated with clinical outcomes
We next tested whether the 6 most prevalent nasal airway microbiota assemblages covaried with primary (asthma exacerbation) and secondary (rhinovirus infection and respiratory illness events) clinical outcomes. Using all longitudinal samples (n = 3122) and generalized estimating equations, we noted that asthma exacerbation, rhinovirus infection, and respiratory illness events varied significantly across the 6 nasal airway microbiotas. Children who experienced 1 or more exacerbations in the outcome period were more likely to possess Moraxella species–dominated microbiotas (relative risk [RR] = 1.66; q = 0.04) in their longitudinally collected samples. This observation was no longer significant after adjustment for participant’s age (adjusted RR = 1.41, q = 0.22). However, it should be noted that age was related to microbiota composition in our cohort (see Table E2) and that younger children were more likely to possess Moraxella species–dominated microbiotas (Table I and see Fig E4 in this article’s Online Repository at www.jacionline.org). Although exacerbation was more likely to be associated with Moraxella species–dominated microbiotas, rhinovirus infection was not, suggesting that microbiotas dominated by species of Moraxella might promote asthma exacerbation irrespective of rhinovirus infection. This is supported by our observation that ECP concentrations in nasal secretions (a marker of activated eosinophils) measured at randomization were significantly greater in Moraxella species–dominated samples collected closest to the ECP measurement (Table I and see Fig E4) and that ECP concentrations did not significantly differ in rhinovirus-infected or uninfected samples dominated by Moraxella species (β = 0.12, P =.40). In longitudinally collected samples, children who experienced 1 or more exacerbations were less likely to have nasal airway microbiotas dominated by Haemophilus species (RR = 0.41, q = 0.04), a finding that remained consistent after adjustment for age (adjusted RR = 0.39, q = 0.07; see Table E5 in this article’s Online Repository at www.jacionline.org). Similarly, microbiotas dominated by Alloiococcus, Corynebacterium, or Staphylococcus species were also less likely to be observed in longitudinally collected samples from children who experienced 1 or more exacerbations in the outcome period (RR = 0.61, q = 0.14; RR = 0.44, q = 0.12; and RR = 0.70, q = 0.14, respectively).
TABLE I.
Distinct nasal airway microbiotas differentially associate with exacerbation, viral outcomes, and clinical events
| Alloiococcus microbiotas | Corynebacterium microbiotas | Haemophilus microbiotas | Moraxella microbiotas | Staphylococcus microbiotas | Streptococcus microbiotas | Other microbiotas | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RR | q Value* | RR | q Value | RR | q Value | RR | q Value | RR | q Value | RR | q Value | RR | q Value | |
| Per-participant outcomes | ||||||||||||||
| Child’s age at randomization† | 1.01 | 0.90 | 1.35 | <0.01 | 1.07 | 0.69 | 0.84 | <0.01 | 1.05 | 0.32 | 0.96 | 0.69 | 0.97 | 0.69 |
| FEV1/FVC ratio at randomization† | 1.01 | 0.68 | 0.97 | 0.68 | 1.02 | 0.69 | 0.99 | 0.69 | 1.00 | 0.69 | 1.02 | 0.68 | 0.98 | 0.68 |
| Log(ECP) at randomization† | 0.94 | 0.78 | 0.58§ | 0.21 | 0.65 | 0.58 | 1.75 | 0.03‖ | 0.86 | 0.58 | 0.90 | 0.78 | 1.09 | 0.78 |
| Exacerbation (participant)‡ | 0.61 | 0.14 | 0.44§ | 0.12 | 0.41 | 0.04‖ | 1.66§ | 0.04‖ | 0.70§ | 0.14 | 1.22 | 0.42 | 1.23 | 0.40 |
| No. of viral infections‡ | 1.05 | 0.63 | 0.88§ | 0.32 | 1.01 | 0.96 | 1.10 | 0.10 | 0.89 | 0.03‖ | 1.06 | 0.48 | 1.00 | 0.96 |
| No. of respiratory illnesses‡ | 1.05 | 0.92 | 0.97 | 0.92 | 1.03 | 0.92 | 1.00 | 0.99 | 0.90 | 0.29 | 1.15 | 0.29 | 1.07 | 0.51 |
| Per-sample outcomes‡ | ||||||||||||||
| Any rhinovirus infection | 0.96 | 0.75 | 0.94 | 0.75 | 1.26 | 0.38 | 1.09 | 0.38 | 0.84 | 0.05 | 1.54 | <0.01 | 0.80 | 0.22 |
| Rhinovirus A infection vs rhinovirus negative | 0.89 | 0.65 | 0.87 | 0.65 | 1.80 | 0.05 | 1.14 | 0.35 | 0.75 | 0.05 | 1.70 | 0.03 | 0.68 | 0.17 |
| Rhinovirus B infection vs rhinovirus negative | 1.29 | 0.39 | 0.85§ | 0.50 | 0.87 | 0.67 | 1.13 | 0.39 | 0.89 | 0.39 | 1.34 | 0.39 | 0.65 | 0.26 |
| Rhinovirus C Infection vs rhinovirus negative | 0.96 | 0.85 | 1.22§ | 0.80 | 1.26 | 0.80 | 0.94 | 0.80 | 0.79 | 0.14 | 1.63 | 0.14 | 1.08 | 0.80 |
| Respiratory illness sample¶ | 0.84 | 0.49 | 0.56 | 0.05 | 1.35 | 0.33 | 0.95 | 0.63 | 0.76 | 0.05 | 1.78 | <0.01 | 1.40 | 0.05 |
FVC, Forced vital capacity.
q Values are P values adjusted for multiple comparisons by using a Benjamini-Hochberg false discovery rate across distinct microbiotas for each covariate of interest.
All variables measured at randomization were associated solely with the first-collected microbiota sample, and generalized linear models using the initial sample were used with a binomial outcome.
Results use all data from samples collected from a subject throughout the outcome period, and generalized estimating equations using all longitudinal samples were used with a binomial outcome and an exchangeable correlation structure.
Estimate changed by more than 10% after adjustment for age.
P value was no longer significant after adjustment for age. Significant differences are emphasized at q values of less than 0.15 in boldface.
Respiratory illness occurred concurrently with sample collection.
Distinct nasal microbiotas relate to viral infection outcomes
Using all available samples (n = 3122), we found that samples with rhinovirus infection, specifically those samples with rhinovirus A detected, were less likely to exhibit a Staphylococcus species–dominated microbiota (RR = 0.84, q = 0.05 and RR = 0.75, q = 0.05, respectively). In addition, participants with a greater number of virus-positive samples were also less likely to possess a Staphylococcus species–dominated nasal airway microbiota (RR = 0.89, q = 0.03). Conversely, rhinovirus infection, specifically rhinovirus A, or respiratory illness events were more likely to occur in children with a Streptococcus species–dominated microbiota (RR = 1.54, q < 0.01 [rhinovirus infection]; RR = 1.70, q = 0.03 [rhinovirus A infection]; and RR = 1.78, q < 0.01 [respiratory illness]). However, asthma exacerbations were not significantly associated with the frequency of Streptococcus species–dominated microbiotas in this cohort.
We hypothesized that interactions between rhinovirus and the upper airway microbiota relate to clinical outcomes, specifically to asthma exacerbation and respiratory illness events. To test this, we determined the distribution of distinct microbiotas in rhinovirus-infected samples (n = 969), rhinovirus-infected samples with a concomitant respiratory illness event (n = 245), or rhinovirus-infected samples with a concomitant asthma exacerbation event (n = 54; see the Methods section in this article’s Online Repository for details; n = 1268 total samples). Rhinovirus infection with asthma exacerbation samples exhibited a distinct distribution of microbiotas (P < .001, generalized estimating equations ANOVA; see Fig E5 in this article’s Online Repository at www.jacionline.org). Consistent with our earlier taxonomic analyses, Corynebacterium and Alloiococcus species–dominated microbiotas were less likely to be detected in rhinovirus infection with exacerbation samples. However, comparisons across the 6 individual microbiotas did not achieve statistical significance (see Fig E5), likely because of the limited number of rhinovirus infection with asthma exacerbation samples available for analysis (n = 54). Rhinovirus infections with respiratory illness (compared with rhinovirus infection without asthma exacerbation or respiratory illness symptoms) also trended toward differences in microbiota distributions (P = .087, generalized estimating equations ANOVA). Specific comparisons between microbiota states indicated that Streptococcus species–dominated microbiotas and rhinovirus infection significantly increased the RR of respiratory illness (RR = 1.82, P = .014; see Fig E5).
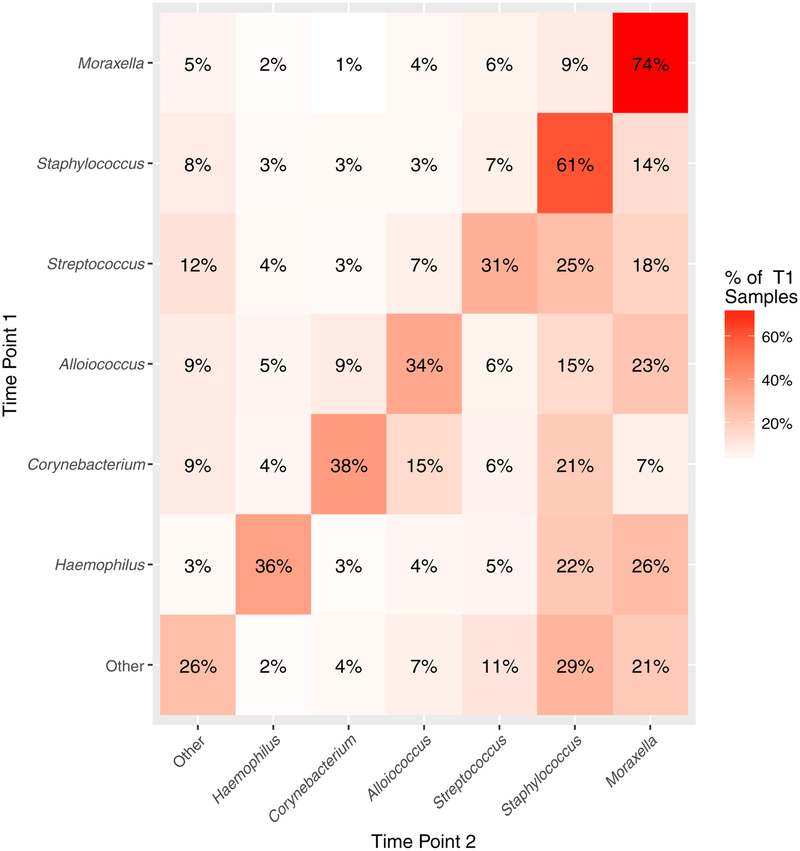
Staphylococcus and Moraxella species–dominated microbiotas exhibit temporal stability
Leveraging the longitudinal nature of sample collection in this trial, we next examined microbiota stability and determined that Moraxella and Staphylococcus species–dominated microbiotas were most frequently maintained in the nasal airways over time (Fig 4). These observations were consistent irrespective of whether analysis was performed on samples collected within a defined time period (7–13 days between sample acquisition) or only the first 3 samples per participant were considered (to account for possible sample collection timing biases, see Fig E6 in this article’s Online Repository at www.jacionline.org). Multiple factors differentiated subjects who were stably colonized by a Moraxella or Staphylococcus species–dominated nasal microbiota (defined as >50% of a subject’s longitudinal samples dominated by Moraxella or Staphylococcus species). Children with stable Moraxella species colonization were more likely to have viral asthma exacerbations and a greater number of rhinovirus infections and respiratory illnesses during the study period. Children with stable Staphylococcus species colonization were typically older and had greater lung function values (likely colinear with age), a longer duration of asthma, and greater body mass index (see Table E6).
FIG 4.
Staphylococcus and Moraxella species–dominated nasal airway microbiotas exhibit temporal stability in children with asthma. The heat map indicates the frequency with which a specific microbiota at a given time point (y axis) remains the same or transitions to a distinct microbiota assemblage in the subsequent patient sample (time point 2; x-axis). Frequencies of these events are provided within each square and proportions are indicated by the color intensity (eg, 74% of Moraxella species–dominated microbiotas remain Moraxella species–dominated in the subsequent sample). The diagonal represents transitions that resulted in maintenance of the same microbiota assemblage over time. Data were generated from longitudinally collected sample transitions (n = 2709) from all participants (n = 413).
We next used longitudinal samples from participants experiencing an asthma exacerbation in the outcome period (n = 54 participants and n = 498 samples) to examine the effect of exacerbation on both microbiota composition stability and bacterial taxon dynamics. Many participants (50%) exhibited compositionally stable microbiotas (defined as a standard deviation of PC1 < 0.25) dominated primarily (>50% of samples within a subject) by Moraxella (15/54 [28%]) or Staphylococcus (6/54 [11%]) species, despite exacerbation or rhinovirus infection (see Fig E7, A, in this article’s Online Repository at www.jacionline.org). At the taxon level, the majority of taxa exhibited consistent relative abundance over the period of observation; however, a small number of specific taxa, primarily belonging to the 6 dominant genera, exhibited temporal fluctuations in relative abundance during rhinovirus infection or exacerbation events (see Fig E7, B).
Differential effect of dominant nasal bacteria on in vitro airway epithelial responses
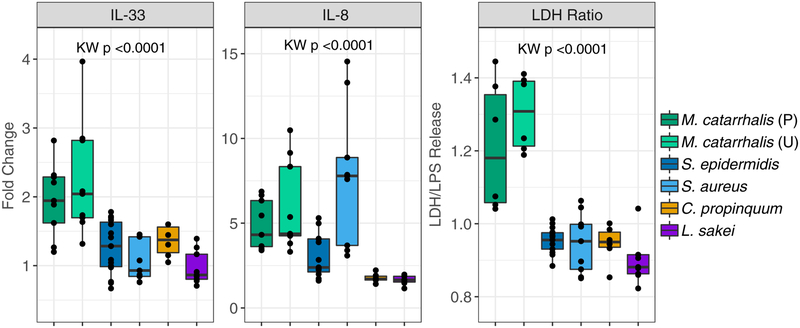
Spurred by the observation that nasal airway microbiotas dominated by distinct bacterial respiratory pathobionts differentially associate with clinical outcomes in our cohort, we hypothesized that the bacterial species dominating these microbiotas exert differing effects on the airway epithelium. Using enrichment media, we isolated strains of M catarrhalis, S epidermidis, S aureus, and C propinquum from nasal secretions of children with asthma and identified those exhibiting greater than 97% sequence identity to dominant bacterial taxa identified in this study (see the Methods section in this article’s Online Repository). Cell-free sterile products of biofilm cultures (which mimic physiologic features of mucosal-adherent bacteria) of each of these isolates and Lactobacillus sakei (previously found to protect against nasal airway infection)20 were used to stimulate airway epithelial cell-line cultures in vitro before assessment of epithelial damage (lactate dehydrogenase levels in supernatants) and inflammatory gene expression. Compared with S epidermidis or C propinquum, the cell-free products of M catarrhalis isolates consistently increased epithelial damage (P < .01, Wilcoxon test) and gene expression of the proinflammatory cytokines IL-8 and IL-33 (P < .03, Wilcoxon test; Fig 5), as well as MUC5AC, CXCL10, and the epithelial tight junction protein occludin (see Fig E8 in this article’s Online Repository at www.jacionline.org). Of note, in comparison with S epidermidis or C propinquum, S aureus also increased expression of IL-8 (P =.003 and P =.002, respectively, Wilcoxon test; Fig 5) in addition to CXCL10 and occludin (P < .05, Wilcoxon test; see Fig E8). Hence M catarrhalis and S aureus, but not S epidermidis or C propinquum, appear capable of promoting relatively increased epithelial damage and inflammation in vitro, offering a potential mechanism by which nasal microbiotas dominated by these distinct species might differentially relate to clinical outcomes in this cohort.
FIG 5.
Biofilm-derived products of bacterial isolates dominating nasal airway microbiotas differentially influence epithelial responses in vitro. Comparative analysis of airway epithelial immune gene expression (relative to PBS) and lactate dehydrogenase (LDH) release (relative to LPS stimulation) after exposure to sterile biofilm supernatants of Moraxella catarrhalis (2 strains, see the Methods section in this article’s Online Repository for more information), Staphylococcus aureus, Staphylococcus epidermidis, and Corynebacterium propinquum. M catarrhalis strains consistently induce increased epithelial damage (LDH) and inflammation (IL-8 and IL-33) compared with S epidermidis, C propinquum, and Lactobacillus sakei. Statistical significance was determined by using Kruskal-Wallis (KW) tests. Results were obtained from 2 or more independent experiments using 3 biological replicates.
DISCUSSION
Our longitudinal analyses of the nasal airway bacterial microbiotas of more than 3000 samples from more than 400 children with asthma in the PROSE trial identified distinct microbiotas associated with risk of asthma exacerbation, rhinovirus infection, and respiratory illness. Omalizumab therapy, which modulates IgE responses, successfully reduced fall exacerbations in children with asthma10; however, it did not significantly alter nasal airway microbiota composition, raising the possibility that the nasal microbiota remains largely unaffected by treatments targeting specific features of asthma-associated immune dysfunction. The persistence of pathogenic nasal airway microbiotas, particularly those dominated by Moraxella species, which more frequently occurred in younger children, were associated with increased eosinophil activation and asthma exacerbations. This helps explain several key questions in the field: why some children with asthma are “exacerbation prone,” why some experience exacerbations in the absence of viral infection, and why asthma symptoms frequently recur after cessation of anti-inflammatory treatments.
Perturbations to the upper respiratory microbiota have been described in children2,3,6,21 and adults5,22–24 with asthma, and recent evidence indicates that upper airway Moraxella species are also detected in the lower airways of patients with asthma.5,25 Consistent with our observations, the relative abundance of upper respiratory Moraxella species has been positively correlated with markers of eosinophilic inflammation (in both bronchoalveolar lavage fluid and blood) and negatively correlated with Corynebacterium species.5 Although specific mechanisms by which microbiotas of the upper airways contribute to lower airway inflammation and asthma exacerbations remain unclear, a growing body of evidence suggests that the upper airway microbiota can serve as an inflammatory trigger and/or the source of pathogenic microbes in the lower airway of those with chronic respiratory disease. Indeed, although many taxa detected in the nasal airway microbiota contribute to the relative temporal stability of these assemblages, the relative abundance of a small number of discrete taxa dynamically shift in parallel with asthma exacerbation and rhinovirus infection events, suggesting that these organisms may contribute to clinical outcomes.
Approximately 20% to 30% of asthma exacerbations in children are not associated with viral infection, and their cause remains enigmatic.26 Our observations provide evidence that some asthma exacerbations might not be related to viral infection but rather to pathogenic bacterial activities, specifically Moraxella species–dominated microbiota. Our identification of M catarrhalis as the dominant species in nasal airway microbiotas of children who experience a greater frequency of exacerbation and subsequent in vitro evidence for the capacity of strains of this species to induce epithelial damage and increase IL-8 and IL-33 expression, even in the absence of rhinovirus coinfection, support a role for this species in asthma pathogenesis. M catarrhalis is an opportunistic human respiratory pathogen that encodes a range of proteins and ligands that promote its adherence and invasion of epithelial cells and can, through induction of innate immune responses and complement evasion, result in tissue destruction and promote long-term persistence on the mucosal surface.27 Future studies using shotgun metagenomic and paired RNA sequencing analyses will be required to determine the extent of virulence genes and their expression in the airway microbiota. Such information could lead to novel treatment or vaccination strategies to prevent nasal airway colonization by pathogenic genera and reduce exacerbation risk in children with asthma.
The microbiotas of children who did not experience asthma exacerbations were more likely to be Alloiococcus, Haemophilus, Corynebacterium, or Staphylococcus dominated; reduced risk of respiratory illness was also associated with the latter 2 microbiotas. These observations are largely consistent with the findings of Teo et al3 in the Childhood Asthma Study, who noted that infants whose nasal airways were colonized by bacterial communities dominated by Alloiococcus, Corynebacterium, or Staphylococcus species were at significantly lower risk of acute respiratory tract infection. Our observation that the biofilm products of S epidermidis and C propinquum (identified as dominant members of these protective microbiota) induced less epithelial damage and IL-8 and IL-33 expression compared with M catarrhalis suggests that distinct nasal airway microbiota dominated by these species can differentially modulate mucosal integrity and immunity in a manner that alters susceptibility and the severity of respiratory illness in children with asthma.
It should be noted that the Childhood Asthma Study reported that although Haemophilus species–dominated communities were infrequently detected in the infant nasopharynx (1.2% of population studied), these infants were at extremely high risk of acute respiratory illness.3 In our study of older children with established asthma, Haemophilus species–dominated communities were also less frequently detected, but their presence was associated with reduced risk of exacerbation that was plausibly explained by differences in age and airway development that exists between infants and children, bacterial strain differences, or increased H influenzae vaccination in this older population. Related to this, we did note a significant relationship between the nasal airway microbiota and the participant’s age, with Moraxella species–dominated microbiotas more frequently detected in younger participants, indicating that younger children with asthma who possess a Moraxella species–dominated nasal airway microbiota might represent a particular subset of patients at heightened risk for exacerbation. Consistent with previous reports,6,28 rhinovirus infection or respiratory illness samples were more likely to be associated with Streptococcus species–dominated microbiotas but, contrary to those reports, not with asthma exacerbations in our cohort. When focusing on rhinovirus infections, we noted an increased risk of asthma exacerbation in the presence of Streptococcus species, but this finding did not reach significance.
Despite our large sample size, there are a few study limitations to consider. Our study did not include prerandomization samples or nasal airway samples from children without asthma, limiting our ability to determine whether the microbiotas we describe characterize children without asthma at baseline. Also, the findings presented in this study derive from children selected on the basis of specific entry criteria and might be generalizable primarily to children with severe asthma living in low-income urban environments. Furthermore, fungal communities can also play a significant role in the nasal airway microbiotas of children with asthma; however, they have yet to be characterized in this population. We also note that other factors, such as host genetics, epigenetics, preterm birth,29 non-rhinovirus viral infections, allergens, or microbial products, might also be related to observations made in this study. In addition, the V4 region of the 16S rRNA gene used to determine bacterial taxonomy is frequently unable to discriminate between bacterial species or strains whose V4 region is identical. This is exemplified by our finding that both S epidermidis and S aureus belong to the same dominant Staphylococcus taxon; however, each elicits a distinct and opposing effect on epithelial integrity and inflammatory gene expression. This could explain the apparent contradiction that although Staphylococcus species–dominated communities (possibly S epidermidis species–dominated communities) were protective against asthma exacerbation in our overall cohort, many of the children who provided acute asthma exacerbation samples also exhibited Staphylococcus species–dominated (possibly S aureus species–dominated) microbiotas. Indeed, a recent report demonstrated that S aureus directly induces type 2 cytokine expression in nasal polyp tissue from older patients with chronic rhinosinusitis, which was not recapitulated upon infection with S epidermidis,30 suggesting these distinct Staphylococcus species induce differential inflammatory responses.
In conclusion, this study identifies distinct nasal airway microbiotas that are differentially related to the risk for asthma exacerbation, rhinovirus infection, and respiratory illnesses. Our data form a foundation for more in-depth investigations to determine how distinct nasal airway microbiomes and, more specifically, active members of these assemblages interact with the host mucosa to promote or protect from exacerbations in children with asthma. Moreover, microbiome-based identification of children with asthma at heightened risk for exacerbation could lead to targeted strategies to promote appropriate nasal airway mucosal colonization and potentially reduce exacerbation risk. Additional studies are necessary before such strategies could be considered for clinical implementation.
Supplementary Material
Key messages.
Children with asthma (age range, 6–17 years) who possess a Moraxella species–dominated nasal airway microbiota are typically younger and at increased risk of exacerbation.
Nasal airway microbiotas dominated by Staphylococcus or Corynebacterium species were associated with reduced risk of exacerbation and respiratory illness events, whereas Streptococcus species–dominated microbiotas increased the risk of upper respiratory illnesses.
Moraxella and Staphylococcus species–dominated microbiotas are stably maintained in the upper airways of children with asthma.
Acknowledgments
Supported in whole or in part with federal funds from the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH), Department of Health and Human Services, under contract and grant numbers HHSN272200900052C, HHSN272201000052I, 1UM1AI114271–01, and UM2AI117870. Additional support was provided by the National Center for Research Resources and the National Center for Advancing Translational Sciences, NIH, under grants NIH/NIAID 5R01AI098077; National Center for Advancing Translational Sciences (NCATS)/NIH UL1TR000150; and National Center for Research Resources (NCRR)/NCATS/NIH UL1TR000077–04, UL1TR000451, UL1TR001105, UL1TR000040, UM1AI109565, UL1TR000075, 1UL1RR025780, UL1TR000154 and UL1TR001082. The following were donated: omalizumab and matching placebo by Novartis and fluticasone and matching placebo by GlaxoSmithKline under a clinical trial agreement with the University of Wisconsin–Madison; EpiPens by Mylan; and Ayr nasal rinse by B.F. Ascher & Company. None of these companies had a role in the development or approval of the protocol, conduct of the trial, data analysis, manuscript preparation, or the decision to submit for publication.
We thank the PROSE participants for their contribution to this study. Sequence data are available on the European Nucleotide Archive under accession number PRJEB25616.
Abbreviations used
- ECP
Eosinophil cationic protein
- PROSE
Preventive Omalizumab or Step-Up Therapy for Severe Fall Exacerbations
- RR
Relative risk
- V4
Variable 4
Footnotes
Disclosure of potential conflict of interest: All authors, with the exception of A. Togias, report grants from the National Institutes of Health (NIH) during the conduct of study. R. Valladares reports personal fees for employment with Siolta Therapeutics outside the submitted work. H. T. Tran reports personal fees from GlaxoSmithKline outside the submitted work. J. Pongracic reports provision of study drug for other asthma and allergy studies from GlaxoSmithKline, Boehringer Ingelheim, and Genentech/Novartis outside the submitted work. C. M. Kercsmar reports personal fees from GlaxoSmithKline for service on a DSMB and royalties from UpToDate outside the submitted work. M. Gill reports honoraria and support for travel from the American Academy of Allergy, Asthma & Immunology (AAAAI), as well as payment for lectures from the American Academy of Pediatrics outside the submitted work. A.H. Liu reports personal fees from Merck Sharp & Dohme and Phadia Thermo-Fisher and membership on a Data Monitoring Committee for GlaxoSmithKline outside the submitted work. M. Kattan reports personal feed from Novartis Pharma and Regeneron for service on advisory boards outside the submitted work. S. J. Teach reports grants from Patient-Centered Outcomes Research Institute (PCORI), EJF, and the NIH/National Heart, Lung, and Blood Institute (NHLBI) outside the submitted work and personal fees from UpToDate outside the submitted work. H. A. Boushey serves as a compensated member of a Scientific Advisory Committee for Siolta Therapeutics. J. E. Gern reports personal fees from PREP Biopharm, Regeneron, Meissa Vaccines, MedImmune, and Ena Pharmaceuticals, as well as stock options from Meissa Vaccines outside the submitted work and has a patent “Methods of Propagating Rhinovirus C in Previously Unsusceptible Cell Lines” issued and a patent “Adapted Rhinovirus C” pending outside the submitted work. D. J. Jackson reports personal fees from Novartis, Boehringer Ingelheim, Pfizer, Commense, and Sanofi/Genzyme outside the submitted work, as well as grants from GlaxoSmithKline and the NIH/NHLBI. S.V. Lynch reports grants from the NIH/National Institute of Allergy and Infectious Diseases (NIAID), NIH/National Institute on Drug Abuse (NIDA), NIH/Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), NIH/Office of the Director, and the Crohn’s and Colitis Foundation of America; reports personal fees from Siolta Therapeutics outside the submitted work; has a patent “Reductive prodrug cancer chemotherapy (Stan449-PRV)” issued, a patent “Combination antibiotic and antibody therapy for the treatment of Pseudomonas aeruginosa infection (WO2010091189A1)” with royalties paid by KaloBios, a patent “Therapeutic microbial consortium for induction of immune tolerance” licensed to Siolta Therapeutics, a patent “Systems and methods for detecting antibiotic resistance (WO2012027302A3)” issued, a patent “Nitroreductase enzymes (US7687474B2)” issued, a patent “Sinusitis diagnostics and treatments (WO2013155370A1)” licensed by Reflourish, and a patent “Methods and systems for phylogenetic analysis (US20120264637A1)” issued; and is a cofounder of Siolta Therapeutics, a startup developing a mixed-species microbial oral therapeutic for induction of immune tolerance. The rest of the authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Kozik AJ, Huang YJ. The microbiome in asthma: role in pathogenesis, phenotype, and response to treatment. Ann Allergy Asthma Immunol 2019;122:270–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bisgaard H, Hermansen MN, Buchvald F, Loland L, Halkjaer LB, Bønnelykke K, et al. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med 2007;357:1487–95. [DOI] [PubMed] [Google Scholar]
- 3.Teo SM, Mok D, Pham K, Kusel M, Serralha M, Troy N, et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe 2015;17:704–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santee CA, Nagalingam NA, Faruqi AA, DeMuri GP, Gern JE, Wald ER, et al. Nasopharyngeal microbiota composition of children is related to the frequency of upper respiratory infection and acute sinusitis. Microbiome 2016;4:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Durack J, Huang YJ, Nariya S, Christian LS, Ansel KM, Beigelman A, et al. Bacterial biogeography of adult airways in atopic asthma. Microbiome 2018;6:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kloepfer KM, Lee WM, Pappas TE, Kang TJ, Vrtis RF, Evans MD, et al. Detection of pathogenic bacteria during rhinovirus infection is associated with increased respiratory symptoms and exacerbations of asthma. J Allergy Clin Immunol 2014;133:1301–7.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Busse WW, Lemanske RF, Gern JE. The role of viral respiratory infections in asthma and asthma exacerbations. Lancet 2010;376:826–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robinson CM, Jesudhasan PR, Pfeiffer JK. Bacterial lipopolysaccharide binding enhances virion stability and promotes environmental fitness of an enteric virus. Cell Host Microbe 2014;15:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med 2011;364:1005–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teach SJ, Gill MA, Togias A, Sorkness CA, Arbes SJ, Calatroni A, et al. Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. J Allergy Clin Immunol 2015;136:1476–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuhlbrigge A, Peden D, Apter AJ, Boushey HA, Camargo C, Gern J, et al. Asthma outcomes: exacerbations. J Allergy Clin Immunol 2012;129(suppl):S34–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bochkov YA, Grindle K, Vang F, Evans MD, Gern JE. Improved molecular typing assay for rhinovirus species A, B, and C. J Clin Microbiol 2014;52:2461–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.R Core Team. R: a language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing; 2018. Available from: https://www.R-project.org. Accessed May 31, 2019. [Google Scholar]
- 14.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 2010;7:335–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oksanen J, Blanchet G, Friendly M, Kindt R, Legendre P, McGlinn D, et al. vegan: Community Ecology Package. Available at: http://CRAN.R-project.org/package=vegan. Accessed May 31, 2019.
- 16.Esquivel A, Busse WW, Calatroni A, Togias AG, Grindle KG, Bochkov YA, et al. Effects of omalizumab on rhinovirus infections, illnesses, and exacerbations of asthma. Am J Respir Crit Care Med 2017;196:985–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romero R, Hassan SS, Gajer P, Tarca AL, Fadrosh DW, Nikita L, et al. The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome 2014;2:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedman J, Alm EJ. Inferring correlation networks from genomic survey data. PLOS Comput Biol 2012;8:e1002687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics 2008;9:559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abreu NA, Nagalingam NA, Song Y, Roediger FC, Pletcher SD, Goldberg AN, et al. Sinus microbiome diversity depletion and Corynebacterium tuberculostearicum enrichment mediates rhinosinusitis. Sci Transl Med 2012;4:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Denner DR, Sangwan N, Becker JB, Hogarth DK, Oldham J, Castillo J, et al. Corticosteroid therapy and airflow obstruction influence the bronchial microbiome, which is distinct from that of bronchoalveolar lavage in asthmatic airways. J Allergy Clin Immunol 2016;137:1398–405.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park H, Shin JW, Park S-G, Kim W. Microbial communities in the upper respiratory tract of patients with asthma and chronic obstructive pulmonary disease. PLoS One 2014;9:e109710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim BS, Lee E, Lee MJ, Kang MJ, Yoon J, Cho HJ, et al. Different functional genes of upper airway microbiome associated with natural course of childhood asthma. Allergy 2018;73:644–52. [DOI] [PubMed] [Google Scholar]
- 24.Fazlollahi M, Lee TD, Andrade J, Oguntuyo K, Chun Y, Grishina G, et al. The nasal microbiome in asthma. J Allergy Clin Immunol 2018;142:834–43.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marsh RL, Kaestli M, Chang AB, Binks MJ, Pope CE, Hoffman LR, et al. The microbiota in bronchoalveolar lavage from young children with chronic lung disease includes taxa present in both the oropharynx and nasopharynx. Microbiome 2016;4:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnston SL. Innate immunity in the pathogenesis of virus-induced asthma exacerbations. Proc Am Thorac Soc 2007;4:267–70. [DOI] [PubMed] [Google Scholar]
- 27.Perez Vidakovics ML, Riesbeck K. Virulence mechanisms of Moraxella in the pathogenesis of infection. Curr Opin Infect Dis 2009;22:279–85. [DOI] [PubMed] [Google Scholar]
- 28.Bashir H, Grindle K, Vrtis R, Vang F, Kang T, Salazar L, et al. Association of rhinovirus species with common cold and asthma symptoms and bacterial pathogens. J Allergy Clin Immunol 2018;141:822–4.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rofael SAD, McHugh TD, Troughton R, Beckmann J, Spratt D, Marlow N, et al. Airway microbiome in adult survivors of extremely preterm birth: the EPICure study. Eur Respir J 2019;53. [DOI] [PubMed] [Google Scholar]
- 30.Lan F, Zhang N, Holtappels G, De Ruyck N, Krysko O, Van Crombruggen K, et al. Staphylococcus aureus induces a mucosal type 2 immune response via epithelial cell–derived cytokines. Am J Respir Crit Care Med 2018;198:452–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.