Abstract
Childhood trauma is a risk factor for psychosis. Amphetamine increases synaptic striatal dopamine levels and can induce positive psychotic symptoms in healthy individuals and patients with schizophrenia. Socio-developmental hypotheses of psychosis propose that childhood trauma and other environmental risk factors sensitize the dopamine system to increase the risk of psychotic symptoms, but this remains to be tested in humans. We used [11C]-(+)-PHNO positron emission tomography to measure striatal dopamine-2/3 receptor (D2/3R) availability and ventral striatal dexamphetamine-induced dopamine release in healthy participants (n = 24). The relationships between dexamphetamine-induced dopamine release, dexamphetamine-induced positive psychotic symptoms using the Positive and Negative Syndrome Scale (PANSS), and childhood trauma using the Childhood Trauma Questionnaire (CTQ) were assessed using linear regression and mediation analyses, with childhood trauma as the independent variable, dexamphetamine-induced dopamine release as the mediator variable, and dexamphetamine-induced symptoms as the dependent variable. There was a significant interaction between childhood trauma and ventral striatal dopamine release in predicting dexamphetamine-induced positive psychotic symptoms (standardized β = 1.83, p = 0.003), but a mediation analysis was not significant (standardized β = −0.18, p = 0.158). There were no significant effects of dopamine release and childhood trauma on change in negative (p = 0.280) or general PANSS symptoms (p = 0.061), and there was no relationship between ventral striatal baseline D2/3R availability and positive symptoms (p = 0.368). This indicates childhood trauma and dopamine release interact to influence the induction of positive psychotic symptoms. This is not consistent with a simple sensitization hypothesis, but suggests that childhood trauma moderates the cognitive response to dopamine release to make psychotic experiences more likely.
Subject terms: Neuroscience, Psychiatric disorders
Introduction
Schizophrenia is among the leading causes of global disease burden and is associated with high mortality and morbidity1–3. It is a complex disorder with a multi-factorial aetiology involving both neurobiological alterations and environmental risk factors4,5. However, the relationship between neurobiological and environmental risk factors, and how they interact to increase the risk of schizophrenia and other psychotic disorders, is currently not known.
The dopamine hypothesis has been a leading neurobiological theory of schizophrenia for several decades6–11. Supporting the theory, administration of drugs that increase dopamine levels such as amphetamine or cocaine induces psychotic symptoms in both healthy people12–14 and patients with schizophrenia15. Molecular imaging studies have characterized the nature of the in vivo dopaminergic changes in schizophrenia, showing higher dopamine synthesis capacity and amphetamine-induced dopamine release with large effect sizes in patients with the disorder6. To our knowledge, seven of eight imaging studies found that schizophrenia is associated with increased striatal dopamine release induced by amphetamine16–23, and a further study found greater striatal dopamine release induced by acute stress exposure24. In addition, a number of these studies report a direct correlation between the magnitude of amphetamine-induced dopamine release in the striatum and amphetamine-induced psychotic symptoms in patients with psychosis16–18. However, individuals vary in the degree to which amphetamine induces psychotic symptoms14, suggesting that other factors may moderate individual vulnerability.
One of the most consistent environmental risk factors associated with schizophrenia is experiencing trauma in childhood and adolescence. Childhood traumas, such as neglect, physical, sexual and psychological abuse, increase the risk for psychosis in adulthood with odds ratios of 2.8–3.6 (refs. 25–28). Large population-based studies also found positive dose–response relationships between trauma and subclinical psychotic symptoms as well as the persistence of psychotic experiences in the general population29–34, suggesting that childhood trauma may increase the risk for developing psychotic symptoms as well as a psychotic disorder. However, not everyone exposed to childhood trauma develops psychosis, suggesting biological vulnerability is also relevant.
It has been proposed that childhood trauma, and other environmental stressors, sensitizes the mesostriatal dopamine system to the effects of later challenges, such as amphetamine, subsequently leading to the development of psychotic symptoms4,35–38. Supporting this, preclinical studies in rodents show increased amphetamine-induced dopamine release in the ventral striatum in animals exposed to a range of stressors during early development, including maternal deprivation39, neonatal isolation40,41, social isolation and social defeat42. Moreover, in vivo human positron emission tomography (PET) studies show that humans who reported lower maternal care during childhood have greater stress-induced dopamine release43, and participants who have experienced a greater number of traumatic events in childhood and higher levels of perceived stress have elevated levels of amphetamine-induced dopamine release44, both in the ventral striatum. In addition, increased dopamine synthesis capacity has been found in adults exposed to childhood adversity (both healthy individuals and those at ultra-high risk of psychosis)45. However, to our knowledge, the relationships between childhood trauma, amphetamine-induced dopamine release and induced psychotic symptoms remain poorly understood. In particular, it remains unknown whether childhood trauma and amphetamine-induced dopamine release are associated with induced positive psychotic symptoms in isolation, whether childhood trauma interacts with amphetamine-induced dopamine release to induce positive psychotic symptoms (moderation effect) or whether childhood trauma influences induced psychotic symptoms through amphetamine-induced dopamine release (mediation effect).
In view of this, we tested the relationships between childhood trauma and dexamphetamine-induced striatal dopamine release to predict dexamphetamine-induced positive psychotic symptoms using both moderation and mediation models. We also tested whether these relationships were specific to striatal dopamine release capacity, or whether they were also related to baseline striatal dopamine-2/3 receptor (D2/3R) availability. We focused on the ventral striatum as this region has previously been shown to be sensitive to the effect of childhood adversity on the magnitude of amphetamine-induced dopamine release43,44,46. We hypothesized that the interaction and/or mediation between childhood trauma and dopamine release capacity would predict dexamphetamine-induced positive psychotic symptoms, in light of the sensitization of the mesostriatal dopamine system described above4,35–38, and evidence showing that dopamine release in isolation is not correlated with an increase in amphetamine-induced positive symptom scores in young adults with hearing impairment47.
Materials and methods
Participants
The study was approved by the West London & GTAC NHS research ethics committee (12/LO/1955). Participants were recruited via online and newspaper advertisements. All participants gave informed written consent to take part in the study after its full description. The inclusion criteria for the study were (1) age above 18 years and (2) capacity to give written informed consent. The exclusion criteria were (1) any past or current major medical condition; (2) history of a psychiatric disorder as determined by the Structured Clinical Interview for DSM-IV Axis 1 Disorders, Clinician Version (SCID-CV)48; (3) history of substance abuse/dependence as determined by the SCID-CV; (4) history of head injury with a loss of consciousness; (5) a family history of any psychiatric disorder in first- or second-degree relatives; (6) contraindications to PET scanning (significant prior exposure to radiation, pregnancy or breast feeding); (7) positive urine drug screen, including for cannabis49,50.
Overall description of the study
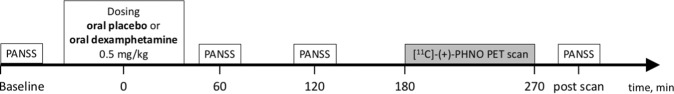
The timeline of scanning day is shown in Fig. 1. Participants attended a screening visit to check eligibility criteria, and underwent two [11C]-(+)-4-propyl-9-hydroxynaphthoxazin (PHNO) PET scans on separate days: the first following a single oral dose of placebo, and the second following a single oral dose of dexamphetamine (500 µg/kg) in a fixed order. On the first scan day, participants completed the Childhood Trauma Questionnaire (CTQ). On both scan days, positive (psychotic) symptoms, negative symptoms and general psychopathology symptoms were assessed using the Positive and Negative Syndrome Scale (PANSS) at baseline (pre-dosing with placebo or dexamphetamine), 60 min post-dosing, 120 min post-dosing, and after the scan (275 min post-dosing). Participants were blind to placebo/dexamphetamine administration. The rater was blind to childhood trauma load and dexamphetamine-induced dopamine release. All participants provided urine samples prior to each scan to screen for current recreational drug use. Participants were asked to abstain from smoking, drinking coffee, eating and using alcohol overnight (minimum 10 h) prior to their PET scans.
Fig. 1. Timeline of scanning day.
Time is represented on the x-axis and is relative to the administration of placebo or dexamphetamine. Participants underwent two [11C]-(+)-PHNO PET scans on separate days: (1) one following a single oral dose of placebo; (2) one following a single oral dose of dexamphetamine (500 µg/kg). Participants completed the Childhood Trauma Questionnaire on the first scan day. PANSS: Positive and Negative Syndrome Scale; [11C]-(+)-PHNO PET scan: [11C]-(+)-4-propyl-9-hydroxynaphthoxazin positron emission tomography scan
Assessment of childhood trauma
Childhood trauma was assessed with the self-completed CTQ 25-item short form51,52, a reliable and valid measure of childhood trauma in patients with psychosis53. The CTQ is a retrospective self-completed questionnaire covering the following five domains of childhood trauma: sexual abuse, physical abuse, emotional abuse, physical neglect and emotional neglect51,52. Sexual abuse is defined as “sexual contact or conduct between a child younger than 18 years of age and an adult or older person”. Physical abuse is defined as “bodily assaults on a child by an adult or older person that posed a risk of or resulted in injury”. Emotional abuse is defined as “verbal assaults on a child’s sense of worth or well-being or any humiliating or demeaning behaviour directed toward a child by an adult or older person”. Physical neglect is defined as “the failure of caretakers to provide for a child’s basic physical needs, including food, shelter, clothing, safety, and health care”. Emotional neglect is defined as “the failure of caretakers to meet children’s basic emotional and psychological needs, including love, belonging, nurturance, and support”. In the short version of the CTQ, each domain includes five items (e.g. for the physical abuse subscale: (1) “I got hit so hard by someone in my family that I had to see a doctor or go to the hospital”; (2) “I was punished with a belt, a board, a cord, or some other hard object”; (3) “I believe that I was physically abused”; (4) “I got hit or beaten so badly that it was noticed by someone like a teacher, neighbor, or doctor”; (5) “People in my family hit me so hard that it left me with bruises or marks”). Participants have to rate the frequency to which they have been exposed to each item (“never”, “rarely”, “sometimes”, “often” or “very often”, total minimum CTQ score = 25, total maximum CTQ score = 125). For each participant, the sum of all the 25 items in the CTQ was used in the analyses.
Assessment of dexamphetamine-induced psychotic symptoms
Positive, negative and general psychopathology symptoms were assessed during both placebo and dexamphetamine scans using the PANSS. The PANSS is an established clinician-rated scale used for measuring the severity of psychotic and related symptoms based on a 30-item standardized interview54. Each of the 30 items is accompanied by a specific definition as well as detailed anchoring criteria for all seven rating points. These seven points represent increasing levels of psychopathology from 1 (absent) to 7 (extreme)54. The symptoms assessed for the positive dimension are delusions, conceptual disorganization, hallucinatory behaviour, excitement, grandiosity, suspiciousness/persecution and hostility. The symptoms assessed for the negative dimension are blunted affect, emotional withdrawal, poor rapport, passive/apathetic social withdrawal, difficulty in abstract thinking, lack of spontaneity & flow of conversation, stereotyped thinking. The symptoms assessed for the general dimension are somatic concern, anxiety, guilt feelings, tension, mannerisms & posturing, depression, motor retardation, uncooperativeness, unusual thought content, disorientation, poor attention, lack of judgement & insight, disturbance of volition, poor impulse control, preoccupation and active social avoidance. For each participant, the sums of the seven sub-items for positive symptoms, seven sub-items for negative symptoms and 16 sub-items for general symptoms were calculated and used in the analyses for the positive, negative and general symptoms, respectively.
Data acquisition
[11 C]-PHNO PET data acquisition
PET images were acquired using a Siemens Biograph HiRez XVI PET scanner (Siemens Healthcare, Erlangen, Germany). A low-dose computed tomography scan was first obtained for attenuation and model-based scatter correction followed by the injection of a single intravenous bolus of 0.020–0.029 µg/kg [11C]-(+)-PHNO. All participants (n = 24) had a baseline PET scan and a PET scan following administration of dexamphetamine on a separate day in a fixed order. For the dexamphetamine PET scans, 500 µg/kg dexamphetamine was administered orally 3 h before [11C]-(+)-PHNO administration, so that the expected time of peak drug levels coincided with the scan time55. Previous studies have shown robust changes in dexamphetamine-induced dopamine release using this approach22,56. Dynamic emission data were acquired continuously for 90 min after the administration of the radiotracer. The dynamic images were then reconstructed using a filtered back-projection algorithm into 31 frames (8 × 15 s, 3 × 60 s, 5 × 120 s, 15 × 300 s) with a 128 matrix, a zoom of 2.6 and a transaxial Gaussian filter of 5 mm.
Structural MRI acquisition
The PET spatial pre-processing pipeline required a high-resolution structural magnetic resonance imaging (MRI) scan for each subject. MR images were acquired on a Siemens MAGNETOM Verio 3T MRI scanner and a 32-channel phased-array head-coil. A high-resolution T1-weighted volume was acquired for PET coregistration using a Magnetization Prepared Rapid Gradient Echo (MPRAGE) sequence with parameters from the Alzheimer’s Disease Research Network (ADNI-GO; 160 slices × 240 × 256, TR = 2300 ms, TE = 2.98 ms, flip angle = 9°, 1 mm isotropic voxels, bandwidth = 240 Hz/pixel, parallel imaging (PI) factor = 2)57.
PET analysis
PET images were analysed using MATLAB version 2015B and an automatic analysis pipeline implemented in MIAKAT (MIAKAT release 4.2.6, www.miakat.org)58. The ICBM152 high-resolution structural MRI template in Montréal Neurologic Institute (MNI) space was non-linearly warped to the high-resolution T1-weighted MRI of each participant using Statistical Parametric Mapping (SPM) (Wellcome Trust Centre for Neuroimaging). The derived deformation parameters were then applied to the Martinez striatal atlas, which defines the anatomical extents of the limbic (ventral), associative and sensorimotor striatal regions of interest (ROIs) in MNI space59,60, and the atlas used by Tziortzi et al. to define a cerebellar ROI to be used as the reference region61. The application of deformation parameters brings the ROIs into the native space of each subject’s MRI scan. The MRI and ROIs were then downsampled to the PET resolution (2 mm). A frame-by-frame registration process on a single frame of reference was used for motion correction for dynamic PET images. Individual averaged PET images were then co-registered to their respective MRIs using rigid body co-registration.
Regional time activity curves (TACs) were obtained by applying individual parcellations to the realigned dynamic images. Our outcome measure of interest was non-displaceable binding (BPND) of [11C]-(+)-PHNO:
where Bavail is the proportion of D2/3Rs available to be bound by PHNO (i.e. the fraction of receptors not bound by endogenous synaptic dopamine), fND is the free fraction of PHNO in the brain and 1/KD the affinity of ligand for the target. BPND was obtained by kinetic modelling with a simplified reference tissue model62,63, using the whole cerebellum as a reference region due its low content of dopaminergic neurons60,64. For each subject, we measured the magnitude of dexamphetamine-induced dopamine release bilaterally in each striatal sub-region. Specifically, this was quantified as the reduction in BPND from the baseline condition (BPNDBase) to the post-dexamphetamine condition (BPNDAmph), expressed as a percentage of BPNDBase:
Statistical analyses
Statistical Package for the Social Sciences (SPSS) version 24 was used for all statistical analysis (IBM, Armonk, N.Y.). A two-tailed significance alpha level of 0.05 was used for all tests. In order to examine potential confounds, we used Pearson’s correlation coefficient, independent t-test and analysis of covariance (ANCOVA) to investigate whether clinico-demographic factors reported to impact dopamine release, such as gender, nicotine, alcohol, cannabis morphine or stimulants use49,65–70, were also related to CTQ scores in our sample. Our primary hypothesis was that dexamphetamine-induced positive psychotic symptoms could be predicted by the interaction between childhood trauma and dexamphetamine-induced dopamine release, in accordance with previously described models4,35,36,71.
First, we tested whether childhood trauma or dopamine release capacity were correlated with dexamphetamine-induced symptoms using Pearson’s correlation coefficient. Next, we investigated whether the interaction between these two variables was a significant predictor of positive psychotic symptoms, by running a linear regression model, which included dexamphetamine-induced change in positive psychotic symptoms as the dependent variable, and the following predictor (independent) variables: ventral striatum dopamine release capacity (ΔBPND), childhood trauma (CTQ score), and the interaction between these variables (ΔBPND × CTQ score). Our primary hypothesis was that the coefficient term (β) associated with the interaction term would be significantly greater than zero.
Second, we tested the specificity of the results by conducting additional multivariate regression models. To test whether our hypothesized interaction effect was specific for dopamine release (functional specificity), we repeated our models after substituting baseline D2/3R availability within the ventral striatum in the place of dopamine release. To test the anatomical specificity, we repeated the model after substituting in dexamphetamine-induced dopamine release within the associative or sensorimotor striatum, in place of the ventral striatum. Finally, to test the symptom domain specificity of the relationship, we repeated our models with the negative and general symptom sub-scale scores of the PANSS as the dependent variable (both induced symptom change and baseline symptoms).
Third, in order to examine whether the relationship between childhood trauma and positive psychotic symptoms is mediated by dopamine release, we performed a mediation analysis with childhood trauma as the independent variable, ventral striatal dexamphetamine-induced dopamine release as the mediator variable, and dexamphetamine-induced positive symptoms as the dependent variable, using the ‘Model 4’ template implemented in the PROCESS (version 3) SPSS package (http://www.processmacro.org/index.html)72. Effect sizes were estimated using 5000 bias corrected bootstrap samples. Mediation was deemed to be significant if 0 was not contained within the 95% bootstrap confidence intervals of the indirect effect, testing the combined effects of paths a (CTQ to dexamphetamine-induced dopamine release) and b (dexamphetamine-induced dopamine release to dexamphetamine-induced positive psychotic symptoms).
Results
Twenty-four participants underwent a baseline (placebo) [11C]-(+)-PHNO PET scan and a scan after oral dexamphetamine on a separate occasion (13 males, mean (SD) age = 23.4 (4.20) years). One male participant was excluded before data analysis due to a positive urine drug test for cannabis, as cannabis has been shown to influence D2/3 binding potential68. Participant demographics, PANSS positive scores and substance use are shown in Table 1. Participant scan parameters are shown in Table 2. Dexamphetamine administration resulted in a significant reduction in BPND relative to the baseline scan (t(23) = 14.29, p < 0.001), indicating significant dopamine release following dexamphetamine (Table 2), and significantly increased positive psychotic symptom scores (PANSS positive items) (t(22) = 6.32, p < 0.001) (Table 1 and Fig. 2). With a mean (SD) CTQ score of 36.7 (19.73) (range 25–97), our sample has values slightly higher than values generally reported in healthy adult samples (for example: mean (SD) of 31.4 (9.8)73 or 34.8 (9.73)74). However, they are lower than those reported in patients with psychosis (mean (SD) of 40.7 (11.4)75) or depression (mean (SD) of 40.5 (16.1)73 or 41.63 (10.23)74). The peak increase in PANSS positive was at 60 min post-dosing, with a mean (SD) increase of 1.44 (1.55). This is in line with increases reported in a previous study in healthy volunteers using amphetamine, which found a mean (SD) increase in PANSS of 1.6 (0.5) at 60 min post amphetamine dose76. We found no relationship between CTQ and age (Pearson’s correlation = 0.30, p = 0.152), and no differences between men and women’s CTQ scores (t(22) = −0.50, p = 0.621). All participants abstained from drugs, alcohol and nicotine on scan days, and an ANCOVA analysis showed no relationship between CTQ scores and current alcohol use (F(1,15) = 1.19, p = 0.294), past ecstasy (F(1,15) = 0.39, p = 0.543), cocaine (F(1,15) = 0.11, p = 0.741) and cannabis (F(2,15) = 0.37, p = 0.700) use. There were not enough participants who were smokers (n = 2) to make analysis of this a co-variable meaningful. No participants had a history of amphetamine use, heroin use or morphine use (see Table 1).
Table 1.
Demographics, substance use and PANSS/childhood trauma scores
| Participants N = 24 | |
|---|---|
| Female | 11 |
| Male | 13 |
| Age | 23.41 (4.20) |
| Tobacco smokers, (n) | 2 |
| Tobacco use (cigarettes/day), mean (SD) | |
| aAlcohol use (UK alcohol units/week), mean (SD) | 4.83 (4.8) |
| Current cannabis | 0 |
| Past cannabis 0/1/2/3/4 | 15/4/4/1/0 |
| Past cocaine use 0/1/2/3/4 | 22/1/1/0/0 |
| Past amphetamine use 0/1/2/3/4 | 24/0/0/0/0 |
| Past ecstasy use 0/1/2/3/4 | 19/5/0/0/0 |
| Past morphine use 0/1/2/3/4 | 24/0/0/0/0 |
| Past heroine use 0/1/2/3/4 | 24/0/0/0/0 |
| Dexamphetamine PANSS positive score baseline, mean (SD) | 7.00 (0) |
| Dexamphetamine PANSS positive score 60 min, mean (SD) | 8.33 (1.55) |
| Dexamphetamine PANSS positive score 120 min, mean (SD) | 8.25 (1.22) |
| Dexamphetamine PANSS positive score 275 min, mean (SD) | 8.21 (1.50) |
| Placebo PANSS positive score baseline, mean (SD) | 7.00 (0) |
| Placebo PANSS positive score 60 min, mean (SD) | 7.14 (0.36) |
| Placebo PANSS positive score 120 min, mean (SD) | 7.19 (0.40) |
| Placebo PANSS positive score 275 min, mean (SD) | 7.14 (.48) |
| CTQ, mean (SD) | 36.7 (19.73) |
For drug use, categories of 0/1/2/3/4/5 indicate never used/very occasional or experimental use/occasional or monthly use/moderate or weekly use/severe or daily use, respectively
CTQ Childhood Trauma Questionnaire, d-amph dexamphetamine, D2/3R dopamine D2/3 receptor
aUK alcohol unit = 10 ml ≅ 7.88 g alcohol
Table 2.
Scanning data
| Participants N = 24 | |
|---|---|
| Injected radioactivity baseline (MBq), mean (SD) | 183.04 (45.91) |
| Injected mass baseline (μg), mean (SD) | 1.63 (0.32) |
| Injected radioactivity d-amph (MBq), mean (SD) | 159.89 (53.22) |
| Injected mass d-amph (μg), mean (SD) | 1.60 (0.37) |
| % displacement ventral striatum, mean (SD) | 21.79 (6.26) |
| % displacement associative striatum, mean (SD) | 12.90 (5.47) |
| % displacement sensorimotor striatum, mean (SD) | 21.38 (5.49) |
| D2/3R availability ventral striatum, mean (SD) | 2.82 (0.34) |
| D2/3R availability associative striatum, mean (SD) | 1.95 (0.29) |
| D2/3R availability sensory motor striatum, mean (SD) | 2.02 (0.23) |
CTQ Childhood Trauma Questionnaire, d-amph dexamphetamine, D2/3R dopamine D2/3 receptor, MBq megabecquerel
Fig. 2. Scatter plot showing the mean (SEM) dexamphetamine-induced psychosis scores after placebo (blue line) and dexamphetamine (red line) administration (minimum score 7).
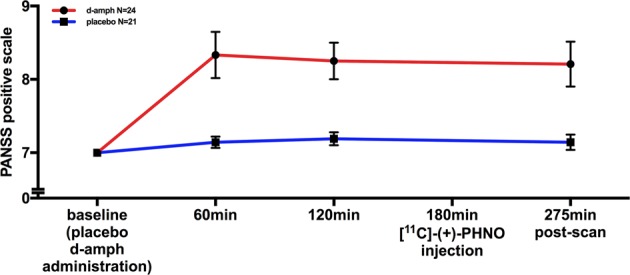
Dexamphetamine administration significantly increased positive psychotic symptom scores compared to placebo (t(22) = 6.320, p < 0.001). d-amph dexamphetamine
Bivariate relationship between– childhood trauma or dopamine release capacity and symptoms
Our primary hypothesis was that the interaction between childhood trauma and dopamine release capacity would be a significant predictor of dexamphetamine-induced positive psychotic symptoms. We first tested the simple bivariate relationships between childhood trauma or ventral striatum dopamine release and dexamphetamine-induced positive psychotic symptoms. Pearson’s correlations showed that there was no significant correlation between childhood trauma and dexamphetamine-induced positive psychotic symptoms (r = 0.348, p = 0.096, n = 24), nor between ventral striatum dopamine release capacity and dexamphetamine-induced positive psychotic symptoms (r = 0.111, p = 0.606, n = 24). Unexpectedly, we found a negative correlation between childhood trauma load and ventral striatal dopamine release (Pearson’s r = −0.488, p = 0.016) (Supplementary Fig. 1). Having established that neither childhood trauma nor dopamine release capacity in isolation were significant predictors of positive psychotic symptoms in healthy individuals, we tested the predictive significance of the interaction between these two variables using a linear regression model (Table 3).
Table 3.
General linear model 1
| GLM1 Increase in PANSS positive ~ β0 + β1*ΔBPND + β2*CTQ + β3*[ΔBPND*CTQ] | |||||
|---|---|---|---|---|---|
| F | Adjusted R2 | p Value vs. constant model | |||
| F(20,3) = 6915 | 0.44 | 0.002 | |||
| Unstandardized coefficients | Standardized coefficients | t | p Value | ||
| β | Std. error | β | |||
| Constant (β0) | 2.22 | 1.326 | 1.674 | 0.11 | |
| ΔBPND (β1) | −0.153 | 0.07 | −0.914 | −2.191 | 0.04 |
| CTQ (β2) | −0.077 | 0.032 | −1.441 | −2.38 | 0.027 |
| ΔBPND × CTQ (β3) | 0.007 | 0.002 | 1.828 | 3.405 | 0.003 |
ΔBPND ventral striatum dopamine release capacity (%), CTQ Childhood Trauma Questionnaire, GLM general linear model
The general linear model including dexamphetamine-induced positive psychotic symptoms as dependent variable was superior to the null-model in predicting symptom scores and providing evidence that the interaction between childhood trauma and mesolimbic dopamine sensitivity (interaction term: ΔBPND × CTQ score) was a predictor of dexamphetamine-induced positive psychotic symptoms
The general linear model (GLM1) including dexamphetamine-induced positive psychotic symptoms as the dependent (outcome) variable (ΔPANSS, the mean increase in PANSS score over the three post-amphetamine time-points), with three independent (predictor) variables (ventral striatum dopamine release capacity, childhood trauma load, and the interaction between these two variables) was superior to the null-model in predicting symptom scores (Table 2, F(20,3) = 6.915, p-value vs. constant model = 0.002). Importantly, the regression coefficient associated with the interaction term in this model was significantly greater than zero, indicating that the interaction between childhood trauma and dexamphetamine-induced dopamine release predicted dexamphetamine-induced positive psychotic symptoms (p = 0.003; Table 2 and Fig. 3). Calculating dexamphetamine-induced positive psychotic symptom severity as the peak post-dosing PANSS-positive score did not change the nature of the results (Supplementary Table 1, GLM2, p-value vs. constant model = 0.004, interaction term (ΔBPND × CTQ) p = 0.004), nor did restricting the analysis of symptom induction to the combined ‘delusions’, ‘hallucinations’ and ‘suspiciousness’ items of the PANSS (Supplementary Table 1, GLM3, p-value vs. constant model = 0.011, interaction term (ΔBPND × CTQ) p = 0.023).
Fig. 3. Scatter plot showing the interaction between dopamine release and childhood trauma exposure in predicting the induction of psychotic symptoms by dexamphetamine.
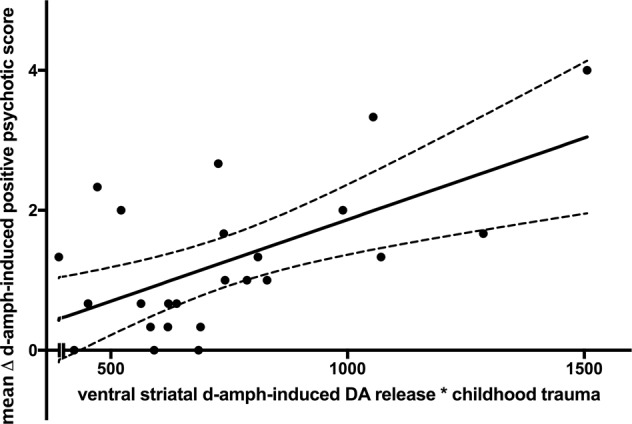
The interaction term significantly predicts dexamphetamine-induced positive psychotic symptoms (p = 0.003), indicating that dopamine release and childhood trauma load interact to increase the induction of positive psychotic symptoms following dexamphetamine. DA dopamine, d-amph dexamphetamine
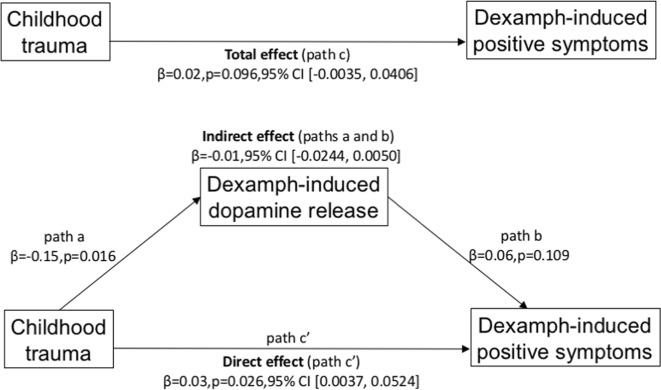
Mediation analysis
The mediation analysis testing whether dopamine release mediates childhood trauma in predicting positive symptoms is shown in Fig. 4. The mediation analysis was not significant (β = −0.01, 95% CI (−0.0244, 0.0050), Sobel test’s p = 0.158). This indicates that there was no significant indirect effect of childhood trauma on dexamphetamine-induced positive symptoms (i.e. an effect mediated through dexamphetamine-induced dopamine release).
Fig. 4. Mediation analysis between childhood trauma load, ventral striatal dopamine release and -induced positive symptoms.
There was no significant mediation effect of childhood trauma on dexamphetamine-induced positive symptoms through dexamphetamine-induced dopamine release β = −0.01 (standardized β = −0.18), 95% CI (−0.0244, 0.0050), p = 0.158 (Sobel test)
Specificity of the relationship between ventral striatum dopamine release capacity and positive psychotic symptoms
Having shown that the interaction between ventral striatum dopamine release and childhood trauma is a significant predictor of dexamphetamine-induced positive symptoms, we tested the functional and anatomical specificity of this relationship within the dopamine system, and its specificity for positive psychotic symptoms. Functionally, the relationship between ventral striatum dopamine function and induced positive psychotic symptoms was specific to dopamine release capacity and was not significant for ventral striatum baseline D2/3R availability (Supplementary Table 1, GLM4). Anatomically, the significant interaction between dopamine release capacity and childhood trauma in predicting induced positive psychotic symptoms was specific to the ventral striatum, and was not significant when release capacity was measured in the associative and sensorimotor striatal subdivisions (Supplementary Table 1, GLM5). Finally, we investigated whether the association between childhood trauma and ventral striatum dopamine release capacity was specific to positive symptoms. Further regression analyses confirmed that neither childhood trauma or ventral striatal dopamine release nor their interaction, was a significant predictor of dexamphetamine-induced change in negative or general PANSS subscales, and they were also not predictors of baseline negative and PANSS general subscale (a proxy for baseline non-psychotic psychopathology) (Supplementary Table 1, GLM7–10).
Discussion
Our results indicate that childhood trauma influences the relationship between ventral striatal dopamine release and induced positive psychotic symptoms, with a significant moderation but not mediation effect (Figs. 2, 3). This supports the hypothesis that childhood trauma interacts with the dopaminergic response to amphetamine to increase the induction of positive psychotic symptoms (moderation effect), but not that childhood trauma leads to sensitization of dopamine release (mediation effect). This extends previous findings by showing for the first time the link between childhood trauma, dopamine release and induced positive psychotic symptoms.
We did not find significant relationships between positive psychotic symptoms or childhood trauma and baseline D2/3R availability, consistent with evidence there are no marked in vivo D2/3R alterations in schizophrenia77,78. Moreover, there was no relationship with dopamine release in the associative and sensorimotor striatal subdivisions or with negative and general symptoms, indicating the effect is on the ventral striatum and positive psychotic symptoms. This is consistent with prior literature implicating dopaminergic changes in the ventral striatum following exposure to environmental and early life stressors, and the role of the ventral striatum in limbic cortico-striatal circuits relevant to processing of affective and reward stimuli43,44,46.
Interaction between childhood trauma and ventral striatal dopamine release
The interaction between childhood trauma load and ventral striatal dopamine release is in line with the large body of literature showing the role of the ventral striatum in stimulant-induced dopamine release after exposure to environmental stressors. However, we did not find any positive relationship between history of childhood trauma and induced striatal dopamine release—instead the relationship in our sample was negative (Pearson’s r = −0.488, p = 0.016) (Supplementary Fig. 1). This is seemingly at odds with predictions from theories proposing that childhood trauma sensitizes the dopamine system to psychological and pharmacological challenge in later life4,35. Rodents exposed to early stressors exhibit increased dopamine levels after stimulant administration in the nucleus accumbens (ventral striatum)39–42,79,80. Human PET studies have also shown increased stress-induced dopamine release in the ventral striatum in people with history of low maternal care43, as well as increased amphetamine-induced dopamine-release in individuals with a greater number of traumatic events in childhood44 and individuals with hearing impairment47. Furthermore, positive correlations between amphetamine-induced dopamine release in the ventral striatum and plasma cortisol levels have also been observed in healthy individuals46,81. Oswald et al. found that the relationship between childhood traumatic exposure and dopamine release was mediated by perceived stress in adulthood, where higher levels of perceived stress were associated with higher ventral striatal dopamine responses to amphetamine44. This observation may explain the apparent inconsistency between our findings and these previous findings if there is lower perceived stress in adulthood in people with high CTQ scores in our sample. However, as we did no measure perceived stress in adulthood, this remains to be tested and highlights that it would be useful to measure perceived stress as well in future studies. Nevertheless, the interaction between childhood trauma load and ventral striatal dopamine release on the induction of psychotic symptoms indicates that a history of greater childhood trauma increases an individual’s vulnerability to the psychotogenic effects of striatal dopamine release, in line with cognitive schema-interpretation model of psychosis4.
The potential mechanism underlying the interaction effect on the induction of psychotic symptoms
Human fMRI studies implicate the ventral striatum in errors of salience attribution for both appetitive, aversive stimuli82–85, and specifically indicates its role in belief updating following salient stimuli86. Ventral striatal dopamine regulates the processing of the salience of stimuli, and, by increasing dopamine release, amphetamine is thought to disrupt this to lead to salience being aberrantly assigned to stimuli8,87,88. Externalizing cognitive schema, such as being more likely to interpret experiences to be threatening or out of personal control are associated with a greater likelihood of psychotic symptoms in clinical and non-clinical samples8,88–91. Childhood trauma is characterized by experiences of threat and loss of control to others, which could lead to more negative or externalizing cognitive schema. Supporting this, childhood trauma has been associated with hostile attribution biases and negative self-schema92,93. Thus, this evidence suggests the interaction we observe between childhood trauma and dopamine release in the induction of psychotic symptoms could be explained by childhood trauma altering cognitive schema to be more externalizing, leading to a greater likelihood of a psychotic-like interpretation of aberrant salience following dopamine release by amphetamine. Whilst this interpretation is speculative, this could be tested in future studies by determining if cognitive schema mediate the interaction between traumatic childhood experiences and dopamine release on the induction of psychotic symptoms. It should also be recognized that other brain regions, in particular frontal cortical regions, are involved in salience processing and altered cortico-striatal connectivity has been found in people with a history of childhood trauma94 and linked to striatal dopamine release95, indicating other brain regions could also be involved in mediating this interaction.
Specificity of the findings to the ventral (limbic) striatum
Our finding that the effect is specific to the limbic striatum contrasts with those of a recent meta-analysis indicating that the main locus of increased dopamine synthesis capacity in schizophrenia is the associative striatum, and not the ventral (limbic) striatum96. However, it is not known whether these meta-analytic findings can be extended to pharmacologically induced dopamine release as there are conflicting results in the literature. One study in patients with schizophrenia and comorbid substance dependence showed significant associations between amphetamine-induced dopamine release in the ventral striatum and the precommissural caudate (associative striatum) and change in positive symptoms97, whilst another study using [18C]-fallypride found an association between schizotypal traits and amphetamine-induced dopamine release in the head of the caudate but extending into the ventral striatum98. Two other studies found that patients with schizophrenia had a similar range of BPND reduction in the ventral striatum and sensorimotor striatum at 6–10 h following dexamphetamine administration99, and that patients with schizophrenia had mean (SD) percentage reduction of 17.6% (7.8) in the ventral striatum and 24.4% (7.2) in the sensorimotor striatum22. To our knowledge, none of the other studies of amphetamine-induced dopamine release have examined sub-regional differences in striatal dopamine release in schizophrenia16–21. The ventral striatum is the most sensitive region to amphetamine-induced dopamine release56,59,100–102. Interestingly, a study examining amphetamine sensitization in healthy individuals found that an initial dose of amphetamine caused dopamine release in the ventral striatum whilst further doses at 14 and 365 days elicited increased dopamine release in the ventral striatum relative to the initial dose, with sensitized dopamine release progressively extending to the dorsal caudate and putamen103. Furthermore, a longitudinal study of people scanned in the prodrome and then again when acutely psychotic showed progressive increases in dopamine synthesis capacity in the dorsal striatum104. Thus, these findings could suggest that the initial dopaminergic response is in the ventral striatum which then extends to the dorsal striatum with the development of a psychotic disorder, but further studies are needed to confirm this hypothesis. Lastly, the regional specificity of the findings to the ventral striatum might also reflect tracer-specific factors. [11C]-(+)-PHNO is more sensitive to amphetamine-induced dopamine release in the ventral striatum than D2/3R antagonist tracers such as [11C]-raclopride56, a property that may be related to its high affinity for the D3R, which is more abundant in the ventral compared to the associative striatum and [11C]-(+)-PHNO.
Limitations
The first limitation of this study is that all individuals were healthy volunteers, limiting the generalizability of these findings to patients with psychotic disorders. This may account for the relatively small increase in PANSS score following dexamphetamine. Further studies are therefore needed to examine the relationships between childhood trauma, dopamine release and positive psychotic symptoms in clinical populations, and to examine stress-induced dopamine release. Second, the study was cross-sectional and exposure to childhood trauma was assessed retrospectively via questionnaires, and could be influenced by recall bias. However, retrospective assessment of childhood trauma has been shown to be reliable in both healthy population and patients with psychosis105. Lastly, it is possible that a third variable that correlates with both dopamine release and CTQ mediates the relationship we observe. However, we found no evidence that clinico-demographic factors that have been reported to be related to dopamine release such as gender, nicotine, alcohol, cannabis morphine or stimulants49,65–70 are related to CTQ scores in our sample.
Conclusions
Childhood trauma and ventral striatal dopamine release interact to influence the emergence of positive psychotic symptoms following dexamphetamine. This is not consistent with a simple dopamine sensitization model for the link between childhood trauma and psychosis risk, but could, instead, suggest that childhood trauma moderates the cognitive response to dopamine release to make psychotic experiences more likely.
Supplementary information
Acknowledgements
This work was supported by a EU-FP7 MC-ITN IN-SENS grant (grant number 607616) to T.D. and O.D.H., a Medical Research Council-UK (no. MC-A656-5QD30), Maudsley Charity (no. 666), Brain and Behavior Research Foundation, and Wellcome Trust (no. 094849/Z/10/Z) grants to O.D.H. and the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. T.D., M.A.P.B. and M.M.N. are supported by the National Institute of Health Research UK (NIHR). M.A.P.B. is supported by the National Institute for Health Research University College London Hospitals Biomedical Research Centre and a UCL Excellence Fellowship. R.A.M.’s work is supported by the Wellcome Trust (no. 200102/Z/15/Z). R.A.A. is supported by the Academy of Medical Sciences (AMS-SGCL13-Adams), the National Institute of Health Research (CL-2013-18-003), and the NIHR UCLH Biomedical Research Centre. M.M.N. is a pre-doctoral fellow of the International Max Planck Research School on Computational Methods in Psychiatry and Ageing Research (IMPRS COMP2PSYCH). The participating institutions are the Max Planck Institute for Human Development, Berlin, Germany, and University College London, London, UK. For more information, see: https://www.mps-ucl-centre.mpg.de/en/comp2psych.
Conflict of interest
O.H. has received investigator-initiated research funding from and/or participated in advisory/speaker meetings organized by Astra-Zeneca, Autifony, BMS, Eli Lilly, Heptares, Jansenn, Lundbeck, Lyden-Delta, Otsuka, Servier, Sunovion, Rand and Roche. Neither Dr. Howes nor his family have been employed by or have holdings/a financial stake in any biomedical company. The remaining authors declare that they have no conflict of interest. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tarik Dahoun, Phone: +44(0) 20 8383 3446, Email: tarik.dahoun@conted.ox.ac.uk.
Oliver D. Howes, Email: oliver.howes@kcl.ac.uk
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41398-019-0627-y).
References
- 1.Crump C, Winkleby MA, Sundquist K, Sundquist J. Comorbidities and mortality in persons with schizophrenia: a Swedish national cohort study. Am. J. Psychiatry. 2013;170:324–333. doi: 10.1176/appi.ajp.2012.12050599. [DOI] [PubMed] [Google Scholar]
- 2.Laursen TM, Nordentoft M, Mortensen PB. Excess early mortality in schizophrenia. Annu. Rev. Clin. Psychol. 2014;10:425–448. doi: 10.1146/annurev-clinpsy-032813-153657. [DOI] [PubMed] [Google Scholar]
- 3.Reininghaus U, et al. Mortality in schizophrenia and other psychoses: a 10-year follow-up of the SOP first-episode cohort. Schizophr. Bull. 2015;41:664–673. doi: 10.1093/schbul/sbu138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howes OD, Murray RM. Schizophrenia: an integrated sociodevelopmental-cognitive model. Lancet. 2014;383:1677–1687. doi: 10.1016/S0140-6736(13)62036-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Os J, Kenis G, Rutten BP. The environment and schizophrenia. Nature. 2010;468:203–212. doi: 10.1038/nature09563. [DOI] [PubMed] [Google Scholar]
- 6.Howes OD, McCutcheon R, Stone J. Glutamate and dopamine in schizophrenia: an update for the 21st century. J. Psychopharmacol. 2015;29:97–115. doi: 10.1177/0269881114563634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meltzer HY, Stahl SM. The dopamine hypothesis of schizophrenia: a review. Schizophr. Bull. 1976;2:19–76. doi: 10.1093/schbul/2.1.19. [DOI] [PubMed] [Google Scholar]
- 8.Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III–the final common pathway. Schizophr. Bull. 2009;35:549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weinstein Jodi J., Chohan Muhammad O., Slifstein Mark, Kegeles Lawrence S., Moore Holly, Abi-Dargham Anissa. Pathway-Specific Dopamine Abnormalities in Schizophrenia. Biological Psychiatry. 2017;81(1):31–42. doi: 10.1016/j.biopsych.2016.03.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maia TV, Frank MJ. An integrative perspective on the role of dopamine in schizophrenia. Biol. Psychiatry. 2017;81:52–66. doi: 10.1016/j.biopsych.2016.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Rossum JM. The significance of dopamine-receptor blockade for the mechanism of action of neuroleptic drugs. Arch. Int. Pharmacodyn. Ther. 1966;160:492–494. [PubMed] [Google Scholar]
- 12.Berman SM, Kuczenski R, McCracken JT, London ED. Potential adverse effects of amphetamine treatment on brain and behavior: a review. Mol. Psychiatry. 2009;14:123–142. doi: 10.1038/mp.2008.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grant KM, et al. Methamphetamine-associated psychosis. J. NeuroImmune Pharmacol. 2012;7:113–139. doi: 10.1007/s11481-011-9288-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bramness JG, Rognli EB. Psychosis induced by amphetamines. Curr. Opin. Psychiatry. 2016;29:236–241. doi: 10.1097/YCO.0000000000000254. [DOI] [PubMed] [Google Scholar]
- 15.Lieberman JA, Kane JM, Alvir J. Provocative tests with psychostimulant drugs in schizophrenia. Psychopharmacology. 1987;91:415–433. doi: 10.1007/BF00216006. [DOI] [PubMed] [Google Scholar]
- 16.Abi-Dargham A, et al. Increased striatal dopamine transmission in schizophrenia: confirmation in a second cohort. Am. J. Psychiatry. 1998;155:761–767. doi: 10.1176/ajp.155.11.1550. [DOI] [PubMed] [Google Scholar]
- 17.Laruelle M, Abi-Dargham A, Gil R, Kegeles L, Innis R. Increased dopamine transmission in schizophrenia: relationship to illness phases. Biol. Psychiatry. 1999;46:56–72. doi: 10.1016/S0006-3223(99)00067-0. [DOI] [PubMed] [Google Scholar]
- 18.Laruelle M, et al. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc. Natl Acad. Sci. USA. 1996;93:9235–9240. doi: 10.1073/pnas.93.17.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pogarell O, et al. Dopaminergic neurotransmission in patients with schizophrenia in relation to positive and negative symptoms. Pharmacopsychiatry. 2012;45(Suppl. 1):S36–S41. doi: 10.1055/s-0032-1306313. [DOI] [PubMed] [Google Scholar]
- 20.Abi-Dargham A, van de Giessen E, Slifstein M, Kegeles LS, Laruelle M. Baseline and amphetamine-stimulated dopamine activity are related in drug-naive schizophrenic subjects. Biol. Psychiatry. 2009;65:1091–1093. doi: 10.1016/j.biopsych.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Breier A, et al. Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proc. Natl Acad. Sci. USA. 1997;94:2569–2574. doi: 10.1073/pnas.94.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frankle WG, et al. Amphetamine-induced striatal dopamine release measured with an agonist radiotracer in schizophrenia. Biol. Psychiatry. 2018;83:707–714. doi: 10.1016/j.biopsych.2017.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weinstein J J, van de Giessen E, Rosengard R J, Xu X, Ojeil N, Brucato G, Gil R B, Kegeles L S, Laruelle M, Slifstein M, Abi-Dargham A. PET imaging of dopamine-D2 receptor internalization in schizophrenia. Molecular Psychiatry. 2017;23(6):1506–1511. doi: 10.1038/mp.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mizrahi R, et al. Increased stress-induced dopamine release in psychosis. Biol. Psychiatry. 2012;71:561–567. doi: 10.1016/j.biopsych.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 25.Varese F, et al. Childhood adversities increase the risk of psychosis: a meta-analysis of patient-control, prospective- and cross-sectional cohort studies. Schizophr. Bull. 2012;38:661–671. doi: 10.1093/schbul/sbs050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matheson SL, Shepherd AM, Pinchbeck RM, Laurens KR, Carr VJ. Childhood adversity in schizophrenia: a systematic meta-analysis. Psychol. Med. 2013;43:225–38.. doi: 10.1017/S0033291712000785. [DOI] [PubMed] [Google Scholar]
- 27.Fisher HL, et al. The varying impact of type, timing and frequency of exposure to childhood adversity on its association with adult psychotic disorder. Psychol. Med. 2010;40:1967–78.. doi: 10.1017/S0033291710000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Read J, van Os J, Morrison AP, Ross CA. Childhood trauma, psychosis and schizophrenia: a literature review with theoretical and clinical implications. Acta Psychiatr. Scand. 2005;112:330–350. doi: 10.1111/j.1600-0447.2005.00634.x. [DOI] [PubMed] [Google Scholar]
- 29.Bebbington PE, et al. Psychosis, victimisation and childhood disadvantage: evidence from the second British National Survey of Psychiatric Morbidity. Br. J. Psychiatry. 2004;185:220–226. doi: 10.1192/bjp.185.3.220. [DOI] [PubMed] [Google Scholar]
- 30.Shevlin M, McElroy E, Bentall RP, Reininghaus U, Murphy J. The psychosis continuum: testing a bifactor model of psychosis in a general population sample. Schizophr. Bull. 2017;43:133–141. doi: 10.1093/schbul/sbw067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shevlin M, Dorahy MJ, Adamson G. Trauma and psychosis: an analysis of the National Comorbidity Survey. Am. J. Psychiatry. 2007;164:166–169. doi: 10.1176/ajp.2007.164.1.166. [DOI] [PubMed] [Google Scholar]
- 32.Janssen I, et al. Childhood abuse as a risk factor for psychotic experiences. Acta Psychiatr. Scand. 2004;109:38–45. doi: 10.1046/j.0001-690X.2003.00217.x. [DOI] [PubMed] [Google Scholar]
- 33.Mongan, D., Shannon, C., Hanna, D., Boyd, A. & Mulholland, C. The association between specific types of childhood adversity and attenuated psychotic symptoms in a community sample. Early Interv. Psychiatry13, 281–289 (2017). [DOI] [PubMed]
- 34.Sommer IE, et al. Healthy individuals with auditory verbal hallucinations; who are they? Psychiatric assessments of a selected sample of 103 subjects. Schizophr. Bull. 2010;36:633–641. doi: 10.1093/schbul/sbn130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Selten JP, van der Ven E, Rutten BP, Cantor-Graae E. The social defeat hypothesis of schizophrenia: an update. Schizophr. Bull. 2013;39:1180–1186. doi: 10.1093/schbul/sbt134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Winkel R, Stefanis NC, Myin-Germeys I. Psychosocial stress and psychosis. A review of the neurobiological mechanisms and the evidence for gene-stress interaction. Schizophr. Bull. 2008;34:1095–1105. doi: 10.1093/schbul/sbn101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Collip, D., Myin-Germeys, I. & Van Os, J. Does the concept of “sensitization” provide a plausible mechanism for the putative link between the environment and schizophrenia? Schizophr. Bull.34, 220–225 (2008). [DOI] [PMC free article] [PubMed]
- 38.van Os J, Kapur S. Schizophrenia. Lancet. 2009;374:635–645. doi: 10.1016/S0140-6736(09)60995-8. [DOI] [PubMed] [Google Scholar]
- 39.Hall FS, Wilkinson LS, Humby T, Robbins TW. Maternal deprivation of neonatal rats produces enduring changes in dopamine function. Synapse. 1999;32:37–43. doi: 10.1002/(SICI)1098-2396(199904)32:1<37::AID-SYN5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 40.Kosten TA, Zhang XY, Kehoe P. Chronic neonatal isolation stress enhances cocaine-induced increases in ventral striatal dopamine levels in rat pups. Dev. Brain Res. 2003;141:109–116. doi: 10.1016/S0165-3806(03)00003-8. [DOI] [PubMed] [Google Scholar]
- 41.Kehoe P, Shoemaker WJ, Triano L, Hoffman J, Arons C. Repeated isolation in the neonatal rat produces alterations in behavior and ventral striatal dopamine release in the juvenile after amphetamine challenge. Behav. Neurosci. 1996;110:1435–1444. doi: 10.1037/0735-7044.110.6.1435. [DOI] [PubMed] [Google Scholar]
- 42.Burke AR, Renner KJ, Forster GL, Watt MJ. Adolescent social defeat alters neural, endocrine and behavioral responses to amphetamine in adult male rats. Brain Res. 2010;1352:147–156. doi: 10.1016/j.brainres.2010.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pruessner JC, Champagne F, Meaney MJ, Dagher A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C]raclopride. J. Neurosci. 2004;24:2825–2831. doi: 10.1523/JNEUROSCI.3422-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oswald LM, et al. History of childhood adversity is positively associated with ventral striatal dopamine responses to amphetamine. Psychopharmacology. 2014;231:2417–2433. doi: 10.1007/s00213-013-3407-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Egerton A, et al. Adversity in childhood linked to elevated striatal dopamine function in adulthood. Schizophr. Res. 2016;176:171–176. doi: 10.1016/j.schres.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oswald LM, et al. Relationships among ventral striatal dopamine release, cortisol secretion, and subjective responses to amphetamine. Neuropsychopharmacology. 2005;30:821–832. doi: 10.1038/sj.npp.1300667. [DOI] [PubMed] [Google Scholar]
- 47.Gevonden M, et al. Increased release of dopamine in the striata of young adults with hearing impairment and its relevance for the social defeat hypothesis of schizophrenia. JAMA Psychiatry. 2014;71:1364–1372. doi: 10.1001/jamapsychiatry.2014.1325. [DOI] [PubMed] [Google Scholar]
- 48.First, et al. Structured Clinical Interview for DSM-IV Axis I Disorders (American Psychiatric Press, Inc., 1996).
- 49.Bloomfield MA, Ashok AH, Volkow ND, Howes OD. The effects of Delta(9)-tetrahydrocannabinol on the dopamine system. Nature. 2016;539:369–377. doi: 10.1038/nature20153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bossong MG, et al. Further human evidence for striatal dopamine release induced by administration of 9-tetrahydrocannabinol (THC): selectivity to limbic striatum. Psychopharmacology. 2015;232:2723–2729. doi: 10.1007/s00213-015-3915-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bernstein DP, et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. 2003;27:169–190. doi: 10.1016/S0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- 52.Bernstein DP, Ahluvalia T, Pogge D, Handelsman L. Validity of the Childhood Trauma Questionnaire in an adolescent psychiatric population. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36:340–348. doi: 10.1097/00004583-199703000-00012. [DOI] [PubMed] [Google Scholar]
- 53.Kim D, Bae H, Han C, Oh HY, Macdonald K. Psychometric properties of the Childhood Trauma Questionnaire-Short Form (CTQ-SF) in Korean patients with schizophrenia. Schizophr. Res. 2013;144:93–98. doi: 10.1016/j.schres.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 54.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 55.Asghar SJ, Tanay VA, Baker GB, Greenshaw A, Silverstone PH. Relationship of plasma amphetamine levels to physiological, subjective, cognitive and biochemical measures in healthy volunteers. Hum. Psychopharmacol. 2003;18:291–299. doi: 10.1002/hup.480. [DOI] [PubMed] [Google Scholar]
- 56.Shotbolt P, et al. Within-subject comparison of [(11)C]-(+)-PHNO and [(11)C]raclopride sensitivity to acute amphetamine challenge in healthy humans. J. Cereb. Blood Flow Metab. 2012;32:127–136. doi: 10.1038/jcbfm.2011.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jack CR, Jr., et al. The Alzheimer’s Disease Neuroimaging Initiative (ADNI): MRI methods. J. Magn. Reson. Imaging. 2008;27:685–691. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gunn, R. N., Coello C. & Searle, G. Molecular Imaging And Kinetic Analysis Toolbox (MIAKAT) - a Quantitative Software Package for the analysis of PET neuroimaging data. J. Nucl. Med.57(Suppl. 2), 1928 (2016).
- 59.Martinez D, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J. Cereb. Blood Flow Metab. 2003;23:285–300. doi: 10.1097/01.WCB.0000048520.34839.1A. [DOI] [PubMed] [Google Scholar]
- 60.Egerton A, Demjaha A, McGuire P, Mehta MA, Howes OD. The test-retest reliability of 18F-DOPA PET in assessing striatal and extrastriatal presynaptic dopaminergic function. NeuroImage. 2010;50:524–531. doi: 10.1016/j.neuroimage.2009.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tziortzi AC, et al. Imaging dopamine receptors in humans with [11C]-(+)-PHNO: dissection of D3 signal and anatomy. NeuroImage. 2011;54:264–277. doi: 10.1016/j.neuroimage.2010.06.044. [DOI] [PubMed] [Google Scholar]
- 62.Gunn RN, Lammertsma AA, Hume SP, Cunningham VJ. Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. NeuroImage. 1997;6:279–287. doi: 10.1006/nimg.1997.0303. [DOI] [PubMed] [Google Scholar]
- 63.Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. NeuroImage. 1996;4:153–158. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- 64.Kumakura Y, Cumming P. PET studies of cerebral levodopa metabolism: a review of clinical findings and modeling approaches. Neuroscientist. 2009;15:635–650. doi: 10.1177/1073858409338217. [DOI] [PubMed] [Google Scholar]
- 65.Munro CA, et al. Sex differences in striatal dopamine release in healthy adults. Biol. Psychiatry. 2006;59:966–974. doi: 10.1016/j.biopsych.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 66.Ashok AH, Mizuno Y, Howes OD. Tobacco smoking and dopaminergic function in humans: a meta-analysis of molecular imaging studies. Psychopharmacology. 2019;236:1119–1129. doi: 10.1007/s00213-019-05196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boileau I, et al. Alcohol promotes dopamine release in the human nucleus accumbens. Synapse. 2003;49:226–231. doi: 10.1002/syn.10226. [DOI] [PubMed] [Google Scholar]
- 68.Bossong Matthijs G., Mehta Mitul A., van Berckel Bart N. M., Howes Oliver D., Kahn René S., Stokes Paul R. A. Further human evidence for striatal dopamine release induced by administration of ∆9-tetrahydrocannabinol (THC): selectivity to limbic striatum. Psychopharmacology. 2015;232(15):2723–2729. doi: 10.1007/s00213-015-3915-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ashok AH, Mizuno Y, Volkow ND, Howes OD. Association of stimulant use with dopaminergic alterations in users of cocaine, amphetamine, or methamphetamine: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74:511–519. doi: 10.1001/jamapsychiatry.2017.0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Spagnolo Primavera A., Kimes Alane, Schwandt Melanie L., Shokri-Kojori Ehsan, Thada Shantalaxmi, Phillips Karran A., Diazgranados Nancy, Preston Kenzie L., Herscovitch Peter, Tomasi Dardo, Ramchandani Vijay A., Heilig Markus. Striatal Dopamine Release in Response to Morphine: A [11C]Raclopride Positron Emission Tomography Study in Healthy Men. Biological Psychiatry. 2019;86(5):356–364. doi: 10.1016/j.biopsych.2019.03.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van Winkel R, van Nierop M, Myin-Germeys I, van Os J. Childhood trauma as a cause of psychosis: linking genes, psychology, and biology. Can. J. Psychiatry. 2013;58:44–51. doi: 10.1177/070674371305800109. [DOI] [PubMed] [Google Scholar]
- 72.Hayes, A. F. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach 2nd edn (Guilford Press, New York, 2018).
- 73.Shea AK, et al. The effect of depression, anxiety and early life trauma on the cortisol awakening response during pregnancy: preliminary results. Psychoneuroendocrinology. 2007;32:1013–1020. doi: 10.1016/j.psyneuen.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 74.Gulec MY, et al. Effects of childhood trauma on somatization in major depressive disorder: the role of alexithymia. J. Affect. Disord. 2013;146:137–141. doi: 10.1016/j.jad.2012.06.033. [DOI] [PubMed] [Google Scholar]
- 75.Lardinois, M., Lataster, T., Mengelers, R., Van Os, J. & Myin-Germeys, I. Childhood trauma and increased stress sensitivity in psychosis. Acta Psychiatr. Scand.123, 28–35 (2011). [DOI] [PubMed]
- 76.Krystal JH, et al. Comparative and interactive human psychopharmacologic effects of ketamine and amphetamine: implications for glutamatergic and dopaminergic model psychoses and cognitive function. Arch. Gen. Psychiatry. 2005;62:985–994. doi: 10.1001/archpsyc.62.9.985. [DOI] [PubMed] [Google Scholar]
- 77.Howes OD, et al. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch. Gen. Psychiatry. 2012;69:776–786. doi: 10.1001/archgenpsychiatry.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kambeitz J, Abi-Dargham A, Kapur S, Howes OD. Alterations in cortical and extrastriatal subcortical dopamine function in schizophrenia: systematic review and meta-analysis of imaging studies. Br. J. Psychiatry. 2014;204:420–429. doi: 10.1192/bjp.bp.113.132308. [DOI] [PubMed] [Google Scholar]
- 79.Robbins TW, Jones GH, Wilkinson LS. Behavioural and neurochemical effects of early social deprivation in the rat. J. Psychopharmacol. 1996;10:39–47. doi: 10.1177/026988119601000107. [DOI] [PubMed] [Google Scholar]
- 80.Gambarana C, et al. A chronic stress that impairs reactivity in rats also decreases dopaminergic transmission in the nucleus accumbens: a microdialysis study. J. Neurochem. 1999;72:2039–2046. doi: 10.1046/j.1471-4159.1999.0722039.x. [DOI] [PubMed] [Google Scholar]
- 81.Wand GS, et al. Association of amphetamine-induced striatal dopamine release and cortisol responses to psychological stress. Neuropsychopharmacology. 2007;32:2310–2320. doi: 10.1038/sj.npp.1301373. [DOI] [PubMed] [Google Scholar]
- 82.Jensen J, et al. Separate brain regions code for salience vs. valence during reward prediction in humans. Hum. Brain Mapp. 2007;28:294–302. doi: 10.1002/hbm.20274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pohlack ST, Nees F, Ruttorf M, Schad LR, Flor H. Activation of the ventral striatum during aversive contextual conditioning in humans. Biol. Psychol. 2012;91:74–80. doi: 10.1016/j.biopsycho.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 84.Delgado MR, Li J, Schiller D, Phelps EA. The role of the striatum in aversive learning and aversive prediction errors. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2008;363:3787–800. doi: 10.1098/rstb.2008.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zink CF, Pagnoni G, Chappelow J, Martin-Skurski M, Berns GS. Human striatal activation reflects degree of stimulus saliency. NeuroImage. 2006;29:977–983. doi: 10.1016/j.neuroimage.2005.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nour Matthew M., Dahoun Tarik, Schwartenbeck Philipp, Adams Rick A., FitzGerald Thomas H. B., Coello Christopher, Wall Matthew B., Dolan Raymond J., Howes Oliver D. Dopaminergic basis for signaling belief updates, but not surprise, and the link to paranoia. Proceedings of the National Academy of Sciences. 2018;115(43):E10167–E10176. doi: 10.1073/pnas.1809298115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Heinz A, Schlagenhauf F. Dopaminergic dysfunction in schizophrenia: salience attribution revisited. Schizophr. Bull. 2010;36:472–485. doi: 10.1093/schbul/sbq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Howes OD, Nour MM. Dopamine and the aberrant salience hypothesis of schizophrenia. World Psychiatry. 2016;15:3–4. doi: 10.1002/wps.20276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Garety PA, Kuipers E, Fowler D, Freeman D, Bebbington PE. A cognitive model of the positive symptoms of psychosis. Psychol. Med. 2001;31:189–195. doi: 10.1017/S0033291701003312. [DOI] [PubMed] [Google Scholar]
- 90.Bentall RP, et al. The cognitive and affective structure of paranoid delusions: a transdiagnostic investigation of patients with schizophrenia spectrum disorders and depression. Arch. Gen. Psychiatry. 2009;66:236–247. doi: 10.1001/archgenpsychiatry.2009.1. [DOI] [PubMed] [Google Scholar]
- 91.Bentall RP, Fernyhough C. Social predictors of psychotic experiences: specificity and psychological mechanisms. Schizophr. Bull. 2008;34:1012–20.. doi: 10.1093/schbul/sbn103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stowkowy J, et al. Core schemas in youth at clinical high risk for psychosis. Behav. Cogn. Psychother. 2016;44:203–213. doi: 10.1017/S1352465815000144. [DOI] [PubMed] [Google Scholar]
- 93.Chen P, Coccaro EF, Lee R, Jacobson KC. Moderating effects of childhood maltreatment on associations between social information processing and adult aggression. Psychol. Med. 2012;42:1293–1304. doi: 10.1017/S0033291711002212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McCutcheon, R. A., Bloomfield, M. A. P., Dahoun, T., Mehta, M. & Howes, O. D. Chronic psychosocial stressors are associated with alterations in salience processing and corticostriatal connectivity. Schizophr. Res. 213, 56–64 (2019). [DOI] [PMC free article] [PubMed]
- 95.McCutcheon Robert A., Nour Matthew M., Dahoun Tarik, Jauhar Sameer, Pepper Fiona, Expert Paul, Veronese Mattia, Adams Rick A., Turkheimer Federico, Mehta Mitul A., Howes Oliver D. Mesolimbic Dopamine Function Is Related to Salience Network Connectivity: An Integrative Positron Emission Tomography and Magnetic Resonance Study. Biological Psychiatry. 2019;85(5):368–378. doi: 10.1016/j.biopsych.2018.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McCutcheon, R., Beck, K., Jauhar, S. & Howes, O. D. Defining the locus of dopaminergic dysfunction in schizophrenia: a meta-analysis and test of the mesolimbic hypothesis. Schizophr. Bull.44, 1301–1311 (2018). [DOI] [PMC free article] [PubMed]
- 97.Thompson JL, et al. Striatal dopamine release in schizophrenia comorbid with substance dependence. Mol. Psychiatry. 2013;18:909–915. doi: 10.1038/mp.2012.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Woodward ND, et al. Correlation of individual differences in schizotypal personality traits with amphetamine-induced dopamine release in striatal and extrastriatal brain regions. Am. J. Psychiatry. 2011;168:418–426. doi: 10.1176/appi.ajp.2010.10020165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Weinstein JJ, et al. PET imaging of dopamine-D2 receptor internalization in schizophrenia. Mol. Psychiatry. 2018;23:1506–1511. doi: 10.1038/mp.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Drevets WC, et al. Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biol. Psychiatry. 2001;49:81–96. doi: 10.1016/S0006-3223(00)01038-6. [DOI] [PubMed] [Google Scholar]
- 101.Drevets WC, et al. PET measures of amphetamine-induced dopamine release in ventral versus dorsal striatum. Neuropsychopharmacology. 1999;21:694–709. doi: 10.1016/S0893-133X(99)00079-2. [DOI] [PubMed] [Google Scholar]
- 102.Willeit M, et al. First human evidence of d-amphetamine induced displacement of a D2/3 agonist radioligand: A [11C]-(+)-PHNO positron emission tomography study. Neuropsychopharmacology. 2008;33:279–289. doi: 10.1038/sj.npp.1301400. [DOI] [PubMed] [Google Scholar]
- 103.Boileau I, et al. Modeling sensitization to stimulants in humans: an [11C]raclopride/positron emission tomography study in healthy men. Arch. Gen. Psychiatry. 2006;63:1386–1395. doi: 10.1001/archpsyc.63.12.1386. [DOI] [PubMed] [Google Scholar]
- 104.Howes OD, et al. Dopamine synthesis capacity before onset of psychosis: a prospective [18F]-DOPA PET imaging study. Am. J. Psychiatry. 2011;168:1311–1317. doi: 10.1176/appi.ajp.2011.11010160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fisher HL, et al. Reliability and comparability of psychosis patients’ retrospective reports of childhood abuse. Schizophr. Bull. 2011;37:546–553. doi: 10.1093/schbul/sbp103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.