ABSTRACT
Legumes are key components of several plant-based diets and are recognized as having a wide range of potential health benefits. Previous systematic reviews and meta-analyses have summarized the evidence regarding different cardiometabolic outcomes, such as cardiovascular disease (CVD) and type 2 diabetes (T2D), and legume consumption. However, those studies did not differentiate between nonsoy and soy legumes, which have different nutritional profiles. The aim of the present updated review, therefore, was to summarize and meta-analyze the published evidence regarding legume consumption (making a distinction between nonsoy and soy legumes) and cardiometabolic diseases. In addition, we reviewed randomized clinical trials assessing the effect of legume consumption on CVD risk factors in order to understand their associations. The results revealed a prospective, significant inverse association between total legume consumption and CVD and coronary heart disease risk, whereas a nonsignificant association was observed with T2D and stroke. In the stratified analysis by legume subtypes, only nonsoy legumes were associated with lower risk of T2D. Unfortunately, owing to the paucity of studies analyzing legumes and CVD, it was not possible to stratify the analysis for these outcomes. Because of the high degree of heterogeneity observed for most of the outcomes and the few studies included in some analyses, further prospective studies are warranted to determine the potential role of legume consumption on CVD and T2D.
Keywords: nonsoy legumes, soy legumes, type 2 diabetes, pulses, cardiovascular disease risk factors, cardiometabolic health
Introduction
Legumes are the pods or fruits of plants that belong to the botanical families Leguminosae or Favaceae, which include soybeans, peanuts, green/dry beans and peas, chickpeas, lentils, broad beans, alfalfa, clover, and lupine (1). The terms “legumes” and “pulses” are used interchangeably, although they are not completely synonymous. According to the UN’s FAO, the term “pulses” only refers to a subtype of legume crops that are harvested merely for dry grain, such as lentils, chickpeas, dry beans, and peas. By this definition, green crops that are harvested for food (green beans, peas, etc.), those used mainly for oil extraction (soybean, peanuts, etc.), and those used for sowing purposes (clover, alfalfa, etc.) are not pulses (2). Therefore, although all pulses are considered legumes, not all legumes are considered pulses.
Legumes have long been recognized for their unique nutritional profile. They are a good source of protein, fiber, B vitamins, minerals (including magnesium and potassium), and polyphenols, and they are also considered as a low-glycemic-index food (3). At this point, it is worth mentioning that soy legumes (soybeans) stand out from the rest owing to their high fat and isoflavone content (4). Furthermore, although peanuts from a botanical point of view are legumes, they are frequently classified as nuts because their nutritional composition is similar (5, 6).
Legumes are considered a beneficial part of traditional plant-based diets around the world and dietary guidelines from several organizations (7–9) recommend their consumption owing to their nutritional profile and their wide range of potential health benefits. In fact, legume consumption has been linked to a lower risk of cardiovascular disease (CVD) (10) and some CVD-related factors, such as high blood pressure (11), obesity (12), dyslipidemia (13), and type 2 diabetes (T2D) (14). A recent summary of findings from the latest systematic reviews, and meta-analyses that examined the relation between legume consumption and cardiometabolic health concluded that legumes could play an important role in modifying cardiometabolic risk (15). However, the evidence quality was considered moderate for prospective cohort studies and low-to-moderate for randomized clinical trials (RCTs). Moreover, this systematic review of meta-analyses did not differentiate between soy and nonsoy legumes, which clearly have a different nutritional profile (4). Therefore, the main aim of the present updated narrative review is to summarize the existing evidence regarding legume consumption and cardiometabolic health, differentiating between soy and nonsoy legumes and meta-analyzing the information regarding T2D and CVD. Because of their nutritional similarities with tree nuts, we did not include studies that considered only peanuts as the main exposure.
Literature search methods
A systematic search of the MEDLINE (PubMed) database through to 10 October, 2017 was made to identify relevant articles for the analyses of legume consumption, CVD outcomes, and T2D risk. We updated the search strategy on 15 November, 2018. The complete search strategy is presented in Supplemental Figure 1. Only prospective cohort studies, conducted in adults, including total legumes and different types of legumes (soy, pulses, and fresh legumes) as exposure, with ≥1 y of follow-up, and with incidence of T2D, total CVD, coronary heart disease (CHD), or stroke as outcomes were included.
Supplemental Figure 2 shows the flow diagram of the studies included in each meta-analysis.
For cardiometabolic risk factors (glucose metabolism, lipid profile, adiposity and body weight, blood pressure, inflammation, and oxidation), a nonsystematic search of RCTs was made in MEDLINE (PubMed). This search strategy may have resulted in unintended selection bias. Nevertheless, all authors made the literature search separately.
Statistical analyses
We carried out a meta-analysis of prospective cohort studies to analyze the association between legume consumption and the risk of CVD outcomes and T2D, following the methodology described in the Cochrane Handbook for Systematic Reviews of Interventions (16). We used the generic inverse variance method with the fixed-effects model (if <5 study comparisons were available) or the random-effects model (if ≥5 study comparisons were available) to pool the natural log-transformed RRs, HRs, and ORs. We used the risk estimates comparing extreme categories of legume consumption. Heterogeneity was estimated and quantified using the Cochran Q statistic and I2 statistic. Evidence of substantial heterogeneity was considered when I2 > 50% at PQ < 0.10 (16). Data analysis was carried out using Review Manager (RevMan) version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Current status of knowledge
Legume consumption and CVD risk
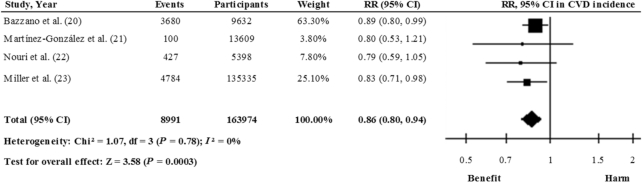
Some prospective studies have found an inverse association between legume consumption and CVD risk; however, others have not replicated this finding (Supplemental Table 1). To our knowledge, 2 recent meta-analyses of prospective studies evaluating legume consumption and CVD risk (10, 17) also revealed inverse associations. However, in the meta-analysis by Marventano et al. (17), 1 of the studies included [Kokubo et al. (18)] examined soy and isoflavone intake with the risk of cerebral and myocardial infarctions, whereas in the second study, the authors explored associations between bean consumption and CVD mortality [Nagura et al. (19)]. In the meta-analysis by Grosso et al. (10), legume consumption was analyzed in relation to CVD outcomes including morbidity and mortality. Taking this into consideration, we carried out a meta-analysis of prospective cohort studies to evaluate the association between legume consumption and CVD incidence. Figure 1 shows the association between total legume consumption (including soy and nonsoy) and CVD based on the results of 4 prospective studies (20–23). Comparing the highest categories of consumption with the lowest, the meta-analysis found that the pooled risk estimate with the inverse variance method using a fixed-effects model was 0.86 (95% CI: 0.80, 0.94), with no heterogeneity (I2 = 0%). These results suggest that consumption of legumes may be effective in reducing the risk of CVD, but this conclusion should be interpreted with caution because it was derived from only 4 prospective studies. Randomized trials are needed before a more definitive recommendation can be made. One recent study of 15,482 patients with stable CHD (24) (Supplemental Table 1) prospectively analyzed legume intake in relation to major adverse cardiovascular events. It was not included in the present analysis because extreme categories of legumes consumption were not compared. Those authors observed that the HR was 0.97 (95% CI: 0.92, 1.03) per 1-category increase in legume consumption.
FIGURE 1.
Relation between legume consumption and total CVD incidence (comparing highest with lowest categories of consumption) in 163,974 participants. Pooled risk was estimated with the inverse variance method, using fixed-effects meta-analyses. CVD, cardiovascular disease.
Legume consumption and CHD risk
The association between legume consumption and risk of CHD has been analyzed in several prospective studies (Supplemental Table 2). A meta-analysis of 5 prospective studies involving 6514 ischemic heart disease cases found a 14% lower risk of total ischemic heart disease in the highest legume consumption category compared with the lowest (RR: 0.86; 95% CI: 0.78, 0.94; I2 = 0.2%) (25). Similarly, in a more recent meta-analysis of 12 prospective studies (7451 CHD cases), the comparison of the highest with the lowest legume consumption category revealed an inverse association between legume consumption and CHD (RR: 0.90; 95% CI: 0.84, 0.97), with moderate heterogeneity (I2 = 34%) (17).
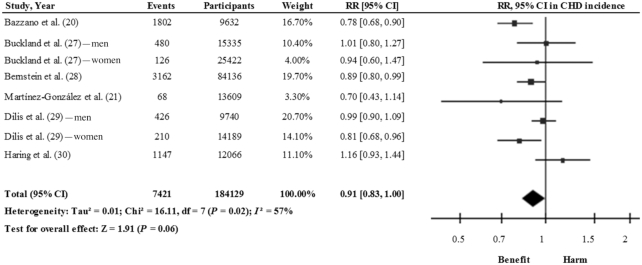
However, these meta-analyses included, among others, studies that investigated legume consumption in relation to CHD mortality (19, 26) and myocardial infarction (18) and therefore their findings might mislead about the role of legumes in reducing CHD risk. After excluding these 3 studies (Supplemental Table 2), the pooled analysis of 6 prospective cohort studies (8 study comparisons) (20, 21, 27–30) showed a 9% risk reduction for a higher consumption of legumes (RR: 0.91; 95% CI: 0.83, 1.00), with a moderate degree of heterogeneity (I2 = 57%) (Figure 2). Therefore, the results suggest that the consumption of legumes may be effective in reducing the risk of CHD.
FIGURE 2.
Relation between total legume consumption and CHD (comparing highest with lowest categories of consumption) in 184,129 participants. Pooled risk was estimated with the inverse variance method, using random-effects meta-analyses. CHD, coronary heart disease.
Legume consumption and risk of stroke
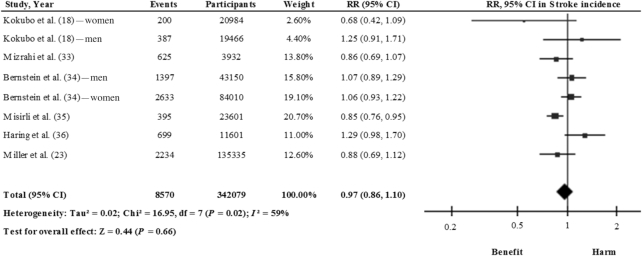
Three meta-analyses have been conducted that analyzed the association between legume consumption and risk of stroke, but yielded no significant results (17, 25, 31). However, those results were based not only on prospective studies examining legume consumption in relation to stroke, but also on studies that either assessed isoflavone intake, which could reflect soy consumption (32), or examined legumes in relation to death from stroke (19). For this review, therefore, we have updated the analysis by excluding these prospective studies and adding a recent study (23) (Supplemental Table 3). According to our findings, after pooling the results from 6 studies (8 study comparisons) (18, 23, 33–36), the consumption of legumes may not be effective in reducing the risk of stroke (RR: 0.97; 95% CI: 0.86, 1.10) (Figure 3). Notably, in a large prospective study, for each additional daily serving of legumes an RR of 1.45 (95% CI: 1.06, 2.00) for ischemic stroke was reported (34).
FIGURE 3.
Relation between total legume consumption and stroke (comparing highest with lowest categories of consumption) in 342,079 participants. Pooled risk was estimated with the inverse variance method, using random-effects meta-analyses.
Legume consumption and risk of T2D
Two cross-sectional epidemiological studies have analyzed the association between legume consumption and risk of T2D, with contradictory results that showed a significant inverse relation (37) or no association at all (38).
Several prospective studies have analyzed the association between legume consumption and T2D incidence but results have been arguable. Although some authors observed an inverse association, others reported a positive relation or none at all (Supplemental Table 4). Two meta-analyses of prospective studies show no apparent association, although with a high degree of heterogeneity across studies (25, 39).
In the most recent meta-analysis, 10 cohort studies (12 study comparisons) were included with 26,778 T2D incidence cases (39). The intake of legumes ranged from 0 to 190 g/d, and no significant association was observed when comparing the highest and the lowest legume consumption categories (RR: 0.96; 95% CI: 0.87, 1.05, I2 = 85%). In that meta-analysis, for each additional 50 g/d of legume consumption, an RR of 1.00 (95% CI: 0.92, 1.09) was reported with 87% heterogeneity, and no evidence of a nonlinear dose-response association was found. In addition, there was no evidence of heterogeneity between subgroups in stratified analyses except for an inverse association among younger (<50 y of age) participants, with no evidence for small study effects, although visual inspection of the funnel plot suggests asymmetry.
This heterogeneity can be explained, in part, because some studies included the consumption of all types of legumes, others only soy or pulse consumption, and in some cases, fresh legumes were not included. There is a great deal of variation in the nutritional content between them, and therefore, they probably do not have the same effect on T2D. Other possible sources of heterogeneity were the populations studied and the amount and type of legumes consumed in each cohort analyzed.
In that meta-analysis, however, 3 prospective cohort studies were not included (14, 40, 41). Therefore, in the present study, we conducted an updated meta-analysis including these 3 prospective studies in addition to those already included in the previous meta-analysis. Furthermore, we have analyzed this association separately for those studies conducted on soy products and nonsoy legumes.
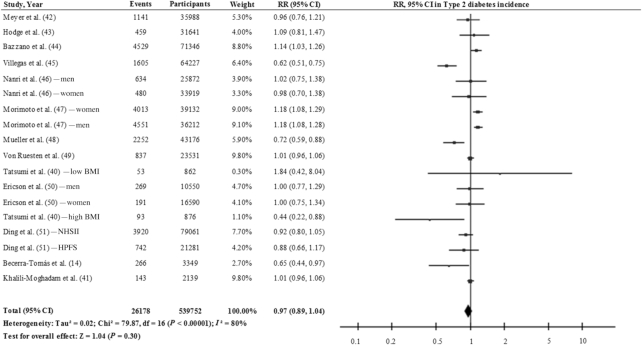
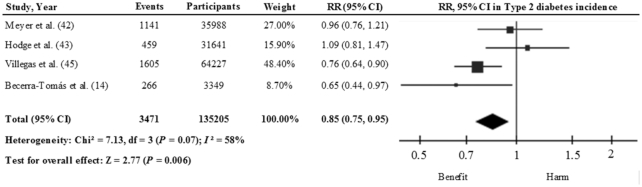
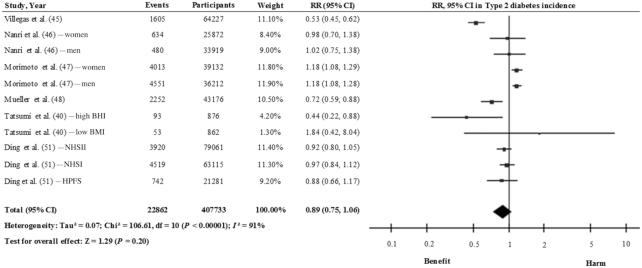
Figure 4 shows the relation between total legume consumption (including soy, pulses, and fresh legumes) and T2D incidence using the results of 13 cohort studies (18 study comparisons) (14, 40–51). Comparing the highest with the lowest categories of consumption, the pooled risk estimate with the inverse variance method using the random-effects model was 0.97 (95% CI: 0.89, 1.04), with a high degree of heterogeneity (I2 = 80%). When we took into account the estimates for nonsoy legume consumption (14, 42, 43, 45), a significant inverse association was found (RR: 0.85; 95% CI: 0.75, 0.95) with moderate heterogeneity (I2 = 58%), using the inverse variance method with the fixed-effects model (Figure 5; 4 studies). In contrast, a nonsignificant inverse association was observed between soy and soy products (40, 45–48, 51) and T2D (RR: 0.89; 95% CI: 0.75, 1.06), with a high interstudy heterogeneity (I2 = 91%) [Figure 6; 6 studies (11 study comparisons)]. This high degree of heterogeneity or the lack of significance might be partly explained by differences in the sources of the soy across the studies. For example, Morimoto et al. (47) observed a substantially higher risk of T2D and included vegetarian-meat products which are processed and rich in salt and refined carbohydrate that might be linked to the observed risk. In fact, the exclusion of this study from this analysis changed the effect estimates (RR: 0.81; 95% CI: 0.67, 0.99; I2 = 83%). We can conclude therefore that there is still no conclusive evidence to suggest that the consumption of legumes is effective in reducing the risk of T2D. On one hand, soy legumes seem to be protective against T2D, after the removal of 1 study, despite the high degree of interstudy heterogeneity. On the other hand, there is limited evidence to suggest that nonsoy legume consumption, in the context of a healthy dietary pattern, may reduce the risk of T2D in healthy and older adults who are at high CVD risk. Nonetheless, further prospective studies and large-scale intervention studies are warranted to determine whether legume consumption has beneficial effects on T2D risk.
FIGURE 4.
Relation between total legume consumption and type 2 diabetes incidence (comparing highest with lowest categories of consumption) in 539,752 participants. Pooled risk was estimated with the inverse variance method, using random-effects meta-analyses.
FIGURE 5.
Relation between nonsoy legume consumption and type 2 diabetes incidence (comparing highest with lowest categories of consumption) in 135,205 participants. Pooled risk was estimated with the inverse variance method, using fixed-effects meta-analyses.
FIGURE 6.
Relation between soy legume consumption and type 2 diabetes incidence (comparing highest with lowest categories of consumption) in 407,733 participants. Pooled risk was estimated with the inverse variance method, using random-effects meta-analyses.
Legume consumption and T2D complications
To the best of our knowledge, no studies have evaluated the effect of legume consumption on microvascular (such as retinopathy or nephropathy) or macrovascular diabetic complications. However, the European Prospective Investigation into Cancer and Nutrition study examined the associations of the consumption of vegetables, legumes, and fruit with all cause-mortality and cause-specific mortality in individuals with diabetes at baseline (52). From the cohort of 10,449 diabetics in that study, 1346 deaths occurred during the follow-up period. Total legume consumption was inversely associated with both all-cause mortality and CVD mortality.
Legumes and Cardiometabolic Risk Factors
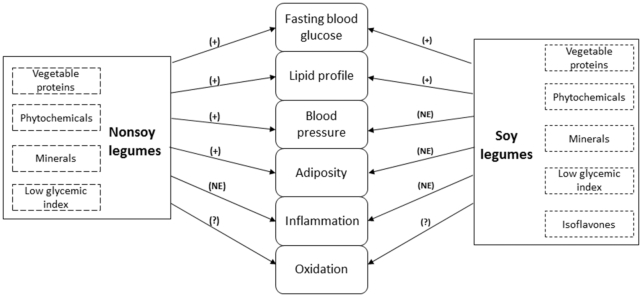
Different risk factors that could act in concert, such as glucose and lipid metabolism, adiposity, high blood pressure, inflammation, and oxidative stress, are involved in the pathogenesis of cardiometabolic diseases. Interventions focusing on these risk factors and their corresponding underlying conditions should be promoted to better understand their effects on the prevention of T2D and CVD. Because of their unique nutritional profile, legumes might be beneficial for addressing important global health issues (Figure 7). The management of all these cardiometabolic risk factors is highlighted next, summarizing the evidence from RCTs.
FIGURE 7.
Effects of nonsoy and soy legumes on cardiometabolic risk factors. (+), Improvement; (NE), No enough evidence; (?), Unclear.
Glucose metabolism
Several meta-analyses of RCTs have attempted to evaluate the effect of legume consumption on glucose control markers but have yielded inconsistent results depending on the type of legume targeted. A meta-analysis of 41 RCTs (53) reported that nonoil seeds have been found to improve medium- to longer-term glycemic control markers, such as fasting blood glucose (FBG), insulin, and glycosylated blood proteins (glycated hemoglobin and fructosamine). Specifically, pulses, eaten alone, decreased FBG [standardized mean difference (SMD): −0.82; 95% CI: −1.36, −0.27] and insulin concentrations (SMD: −0.49; 95% CI: −0.93, −0.04). Pulses as a part of low-glycemic-index diets lowered glycosylated blood proteins (SMD: −0.28; 95% CI: −0.42, −0.14) with no significant effects on FBG, insulin, and HOMA-IR. In addition, pulses as a part of high-fiber diets decreased glycosylated blood proteins (SMD: −0.27; 95% CI: −0.45, −0.09) and FBG (SMD: −0.32; 95% CI: −0.49, −0.15). It is noteworthy that the results of that meta-analysis showed a high degree of heterogeneity among studies, which was not explained in most of the reports. This highlights the need for well-designed long-term RCTs. Subsequent to that publication, several RCTs with crossover or parallel designs have been published that evaluated the acute (54–58) or chronic (59–67) effects of nonsoy legume consumption on glucose control markers, which could change the pooled risk estimates of the meta-analysis. Most of them, although not all (62–67), reported similar results to those of the previous meta-analysis, showing a favorable effect on FBG (54, 61), insulin concentrations (61), glycemic (54–58, 60) and insulinemic response (55, 58), glycated hemoglobin (59, 60), and HOMA-IR AUC (60).
In contrast, soy consumption seems not to affect glycemic control markers. A meta-analysis of 24 RCTs (68) reported no effect of soy food consumption (merging isoflavone extracts only, isolated proteins with isoflavones, or whole soy diets) on improvements in fasting glucose (P = 0.16) or insulin concentrations (P = 0.50). However, in the subgroup analysis, diets mainly based on whole soy foods significantly reduced fasting glucose concentrations (−3.85 mg/dL; 95% CI: −5.28, −2.41 mg/dL; P = 0.003), whereas studies that used purified isoflavones (P = 0.35) or isolated soy proteins (P = 0.57) did not show any significant effect. Nonetheless, RCTs published subsequently did not support the results of this meta-analysis. A reduction in fasting glucose concentrations was observed after the consumption of soy nuts and a macronutrient-matched control snack bar in adults who were at cardiometabolic risk, although a greater reduction was achieved after the control snack consumption (69). Moreover, FBG did not reduce after soy nut consumption in postmenopausal women with or without metabolic syndrome (MetS) compared with a control diet without soy (P = 0.35 and 0.26, respectively) (70). Similarly, a diet containing soy nuggets, burgers, desserts, and soy-based drinks did not reduce FBG, insulin, or HOMA compared with a control diet containing animal food (71).
There are different possible mechanisms by which nonsoy legumes and whole soy foods could improve glucose control markers. Nonsoy legumes are a good source of slowly digested carbohydrates (72). This characteristic might be attributable to the high content of resistant starch, which seems to have a high amylose:amylopectin ratio compared with other starchy foods (1, 72). That property together with the high fiber content of nonsoy legumes (4) reduce the glycemic index of the diet and may explain the health-promoting effects on glucose control markers. It is important to highlight that the nutritional profile of soy legumes differs from that of nonsoy legumes in terms of fat, protein, carbohydrate, and isoflavone content (4). The disparity in results between whole soy foods and isolates of soy protein or isoflavones suggests that other soy legume components or their interactions might explain the favorable effects of consumption of whole soy foods on glycemic control markers (68). However, there are few RCTs that have examined the effect on glycemic control of soy legume components other than isoflavones or proteins, which hinders the ability to draw solid conclusions about the possible components implicated in the improvement of glucose control.
Lipid profile
So far, 3 meta-analyses of RCTs have evaluated the effect of nonsoy legume consumption on changes in lipid profile and have shown consistent results in terms of LDL-cholesterol reduction (13, 73, 74). In the most recent meta-analysis (13) of 26 RCTs involving 1037 participants in which diets emphasized ∼1 serving of pulses per day (median dose of 130 g/d) compared with control diets, concentrations of LDL cholesterol were found to have reduced by −0.17 mmol/L (95% CI: −0.25, −0.09 mmol/L). However, no effect was reported on apoB and non–HDL-cholesterol concentrations. Importantly, most of the trials were considered to be of low quality and there was a high degree of interstudy heterogeneity (I2 = 80%), which was not explained after different subgroup analyses. A 5% reduction in LDL-cholesterol concentrations found in that meta-analysis suggests that pulse consumption might reduce the risk of major vascular events by 5–6% (13). Subsequently, a few RCTs have been published that have evaluated the effect of nonsoy legumes on lipid profile. In a crossover RCT involving 46 healthy females, a diet that incorporated kernel-based barley products, chickpeas, and brown beans significantly reduced the concentrations of total and LDL cholesterol compared to a control diet with a similar macronutrient content but without legumes and barley (P = 0.01 and P = 0.0029, respectively) (66). Similarly, in 31 diabetic participants, an intervention focused on substituting 2 servings of red meat with legumes 3 d/wk compared with a legume-free diet. Results found that the concentrations of TGs (P = 0.04) and LDL cholesterol significantly reduced (P = 0.02) (61). In contrast, in another crossover RCT with first-degree relatives of patients with diabetes, a legume-enriched diet (pinto beans and lentils) did not improve lipid profiles compared to a control diet that followed healthy dietary recommendations (63). It is possible that the healthy recommendations given to the control group in that study limited the ability to detect differences between the study interventions.
Soy consumption also seems to have a beneficial role on lipid profile. A meta-analysis of 35 RCTs (75) showed that soy products significantly reduced LDL-cholesterol (−4.83 mg/dL; 95% CI: −7.34, −2.31 mg/dL), TG (−4.92 mg/dL; 95% CI: −7.79, −2.04 mg/dL), and total cholesterol concentrations (−5.33 mg/dL; 95% CI: −8.35, −2.30 mg/dL). Moreover, concentrations of HDL cholesterol increased by 1.40 mg/dL (95% CI: 0.58, 2.23 mg/dL). However, a high degree of interstudy heterogeneity (ranging from 92% to 99%) was reported in all the analyses. In the subgroup analyses, whole soy products (soy milk, soybeans, and soy nuts) reduced LDL cholesterol to a greater extent than soy extracts or supplements. Also, the hypolipidemic effects of soy products were more pronounced in hypercholesterolemic patients than in healthy subjects (75). To the best of our knowledge, only 2 RCTs have evaluated the effect of soy on lipid profile since the publication of that meta-analysis. Compared with a control diet containing animal foods, a soy diet (including soy nuggets, burgers, desserts, and drinks) significantly reduced total cholesterol, LDL cholesterol, and non–HDL cholesterol by 4.85%, 5.25%, and 7.14%, respectively, in 53 subjects (71). Similarly, in postmenopausal women with MetS, soy nut consumption reduced total cholesterol and HDL cholesterol compared with a control diet without soy products, whereas no effect was observed in those without MetS. Soy nut intervention did not affect LDL or HDL cholesterol in either subgroup of participants, with or without MetS, compared with the control group (70).
Nonsoy legumes contain soluble fiber, which might explain the observed hypocholesterolemic effects. It has been demonstrated that viscous fiber reduced total and LDL cholesterol (76). The exact mechanism is not yet well understood, but it might be related to the ability of soluble fiber to increase bile acid excretion. The binding of soluble fiber to bile acids within the intestine prevents their reabsorption (77–79). As a consequence, the liver increases the production of bile acids, leading to a reduction in the hepatic pool of cholesterol, which in turn increases cholesterol uptake from the blood, resulting in a reduction of circulating concentrations of cholesterol (80, 81). Furthermore, the fermentation of viscous fiber by colonic bacteria generates different SCFAs (propionate and butyrate), which might inhibit the hepatic production of cholesterol (82, 83). Other nonsoy legume components, such as phytosterols (84, 85) and saponins (86, 87), might also be responsible for the reduction of LDL-cholesterol concentrations, although evidence of that is much more limited.
The hypocholesterolemic effect of soy foods might be attributed to soy proteins, as it has been supported by several meta-analyses (88–91). However, soy protein and isoflavones interact in a complex manner, and therefore the exact mechanism by which soy protein favorably affects lipid metabolism is not yet well understood (92). It seems that soy protein might decrease the expression of sterol regulatory element binding protein-1 in the liver, which in turn reduces the expression of genes of enzymes implicated in the biosynthesis of fatty acids (92). Also, soy protein contains a globulin fraction that might regulate the homeostasis of cholesterol, as has been demonstrated in vitro in Hep G2 cells (93). In turn, isoflavones might increase the clearance of cholesterol through the stimulation of sterol regulatory element binding protein-2, which increases the expression of the LDL receptor (92). Moreover, because of their similarities with estrogens, isoflavones might exert their lipid-lowering effects by binding to estrogen receptors (94). Other constituents of soy, such as lecithin, saponins, or soluble fiber, might act independently or synergistically with soy proteins in producing a reduction in blood cholesterol concentrations (94, 95).
Adiposity and body weight
Two meta-analyses of RCTs have evaluated the effect of nonsoy legume consumption on body weight. In one, consumption of pulses did not have any significant effect on body weight (74). However, in the most recent meta-analysis, which included 21 RCTs involving 940 participants, consumption of 132 g/d of pulses reduced body weight by −0.34 kg (95% CI: −0.63, −0.04 kg) compared with diets without the pulse intervention, showing a low degree of between-study heterogeneity (I2 = 9%) (12). In the stratification analyses, pulse consumption reduced body weight not only under conditions of negative-energy balance but also under neutral energy balance. The study reported no effects of pulse consumption on body fat and waist circumference. To the best of our knowledge, no RCTs have since been published in this field.
Only 1 meta-analysis of 24 RCTs has been conducted that evaluated the effect of soy food consumption on body weight and other obesity-related variables (96). Results showed that soy consumption did not have any effect on body weight, waist circumference, or fat mass. Interestingly, subgroup analyses revealed that when soy was compared with meat, body weight increased in obese subjects at consumption of ≥40 g soy protein/d. In contrast, soy reduced waist circumference in women at quantities of <40 g soy protein/d and in older subjects. When the analysis focused on soy isoflavones (17 RCTs), results showed a nonsignificant decrease in BMI, although in the subgroup analyses the reduction was significant at amounts of <100 mg/d and in intervention periods of between 2 and 6 mo. No effects of isoflavones on waist circumference or fat mass were reported. As far as we know, only 1 RCT has been published since that meta-analysis. The results showed that, compared with a control diet containing animal foods, a soy-based diet (nuggets, burgers, desserts, and drinks) reduced weight (P = 0.005) and BMI (P = 0.05) after adjusting for age and sex. However, no differences were observed for abdominal adiposity (waist circumference, bioelectrical impedance analysis, and visceral fat rating) (71).
The mechanism by which nonsoy legume consumption promotes weight loss may be due to their satiating properties (97) from their high fiber and protein content and their low glycemic index. Fiber can induce satiety through different pathways (98, 99): 1) by increasing intraluminal viscosity, which leads to a reduction in the rate of gastric emptying and macronutrient absorption; 2) by reducing the rate of digestion and promoting gastric distention due to the required effort of chewing; 3) by affecting the secretion of gut hormones; and 4) by producing SCFAs (propionate, butyrate, and acetate) derived from its fermentation by colonic bacteria. Low-glycemic-index foods can slow rates of digestion and absorption and, as a consequence, the gastrointestinal tract receptors are stimulated for longer, triggering signals of fullness (100). Finally, proteins have been shown to have a higher satiety effect than carbohydrates and fats. Although the exact mechanism remains to be elucidated, it seems to be related to their higher thermogenic effect compared with the other macronutrients (101).
Blood pressure
Only 1 meta-analysis of RCTs has been published to date that has evaluated the effect of nonsoy legume consumption on blood pressure (11). The analysis included 8 isocaloric trials, involving 554 participants. Results showed that pulses significantly reduced systolic (−2.25 mm Hg; 95% CI: −4.22, −0.28 mm Hg) and mean arterial blood pressure (−0.75 mm Hg; 95% CI: −1.44, −0.06 mm Hg), although no effect was observed on diastolic blood pressure. The results of that meta-analysis should be viewed with caution because of the low quality of the included studies as well as the high degree of interstudy heterogeneity for all the outcomes that remained unexplained after several subgroup analyses. Two subsequent crossover RCTs (61, 63) have been published in this field, which might change the effect estimates of the meta-analysis because both showed no greater effect of nonsoy legumes compared to a control group.
Several meta-analyses that have evaluated the effect of different soy constituents (isoflavones and proteins) on blood pressure levels (102–104) have shown a potential beneficial effect. However, it is important to point out that only 1 meta-analysis has evaluated the effect of soy consumed as a whole food on blood pressure, focusing exclusively on soy nuts (105). Results showed that this type of soy food did not have any blood pressure–lowering effect. There is little evidence regarding intake of soy legumes as a whole and blood pressure. In 253 postmenopausal women with prehypertension or untreated hypertension, soy flour did not reduce 24-h ambulatory blood pressure in comparison with low-fat milk (106). In contrast, soy nuts significantly reduced both systolic and diastolic blood pressure in postmenopausal women without MetS. These reductions were only significant for diastolic blood pressure in those with MetS, whereas no effect was observed for systolic blood pressure (70). Similarly, a diet containing soy-based products such as nuggets, burgers, drinks, and desserts did not reduce systolic or diastolic blood pressure in comparison with a control diet containing animal food (71). Further studies using whole soy alone as an option, instead of isoflavones or proteins, are needed to better determine the possible effects of whole soy food consumption on blood pressure.
Different possible mechanisms may explain the blood pressure–lowering effects of nonsoy legumes. Evidence from population-based studies and RCTs suggests that an increase in protein consumption at the expense of carbohydrates consistently reduces blood pressure (107). On the other hand, dietary fiber has also been associated with reduced systolic and diastolic blood pressure in several meta-analyses (108, 109). Furthermore, it seems that protein and fiber might have an additive effect on lowering blood pressure (107). Nonsoy legumes are also rich in different minerals, such as magnesium and potassium, which have been associated with a reduction in blood pressure (110, 111).
Inflammation
One meta-analysis of 8 RCTs (112) has evaluated the effect of nonsoy legume consumption on peripheral levels of C-reactive protein (CRP), which is considered an important systemic marker of inflammation and a risk factor for CVD (113, 114). The results showed a nonsignificant reduction in CRP concentrations with nonsoy legume consumption (Mean difference (MD): −0.21; 95% CI: −0.44, 0.02; P = 0.068). Importantly, the interstudy heterogeneity was high (I2 = 81.7%) and remained unexplained in the subgroup analysis based on study design (parallel or crossover). However, the exclusion of 1 study (115) led to significant changes in the overall pool estimates (−0.32; 95% CI: −0.58, −0.06), although the high degree of interstudy heterogeneity remained unexplained (I2 = 76.8%). There is little data on nonsoy legume consumption and other inflammatory markers, such as IL-6, and analyses are contradictory. In 30 obese subjects, no differences in IL-6 were observed after comparing a legume-enriched hypocaloric diet (lentils, chickpeas, peas, or beans) with a legume-restricted diet (P = 0.937) (65). Similarly, in 26 first-degree relatives of diabetics, a nonsignificant trend toward reduction in IL-6 concentrations was observed in those individuals following the legume regimen (4 servings/wk of pinto beans or brown lentils) compared with those following only healthy dietary recommendations (P = 0.06) (116). In contrast, in overweight and diabetic adults, the replacement of red meat with lentils, chickpeas, peas, and beans 3 d/wk significantly reduced IL-6 and TNF-α compared with a legume-free diet (P = 0.018) (117). Moreover, in another RCT, an evening meal consisting of brown beans lowered IL-6 and IL-18 at a subsequent standardized breakfast within a time frame of 11–14 h, compared with a meal consisting of white wheat bread (P < 0.05) (58).
Evidence regarding RCTs that have evaluated the effect of soy consumption on CRP concentrations is also poor. One meta-analysis of RCTs was published in 2011, which evaluated the effect of soy foods on CRP in postmenopausal women (118). The main pool estimate showed a nonsignificant reduction in CRP when soy isoflavone and soy food interventions were considered together. In the subgroup analyses, neither isoflavone extracts (n = 6) nor whole soy foods (n = 8) lowered CRP concentrations. Four subsequent RCTs have been published that evaluated the effect of whole soy foods on inflammatory markers. In 21 hypercholesterolemic participants, soy beverage or soy bread did not reduce CRP concentrations (119). Similarly, an intervention based on soy nuts, compared with an energy-matched snack bar, did not lower concentrations of CRP, TNF-α, IL-6, IL-18, or IL-10 (69). A soy intervention diet also showed no effect on CRP concentrations compared with a control diet including animal food (P = 0.59) (71). However, in postmenopausal women with and without MetS, soy nuts reduced concentrations of circulating CRP (70). Further studies are warranted in order to determine the role, if any, of soy on inflammation.
Several constituents of nonsoy legumes can explain the potential beneficial effects observed on CRP concentrations. For instance, dietary fiber has been inversely associated with different inflammatory markers such as IL-6 (120), which regulates CRP concentrations (121). Although the exact mechanisms are unclear, its beneficial effect on IL-6 is thought to be as a result of its ability to reduce hyperglycemia, because it has been demonstrated in vitro that under this condition the secretion of IL-6 is enhanced (122). Low-glycemic-index diets have also been associated with lower concentrations of CRP (123, 124). Furthermore, as stated before, nonsoy legumes reduce body weight, and the beneficial effect of weight loss on markers of inflammation has previously been reported (125, 126).
Oxidation
The few RCTs that have assessed the effect of nonsoy legume consumption on markers of oxidation suggest a beneficial effect. In 66 men with coronary artery disease, an intervention based on whole-grain and legume powder, as replacement foods for refined rice, showed beneficial effects on lipid peroxidation, reducing plasma malondialdehyde and urinary 8-isoprostane F2α by 28% (127). Moreover, a hypocaloric nonsoy legume–enriched diet given to 30 obese subjects, which included 4 servings/wk, significantly reduced concentrations of plasma-oxidized LDL (P = 0.039), malondialdehyde, and urinary 8-isoprostane F2α, compared with an energy-restricted diet without legumes (128). In agreement with that, in subjects with T2D, the replacement of 2 servings of red meat 3 times/wk with nonsoy legumes reduced concentrations of oxidative stress markers, such as malondialdehyde and oxidized-LDL, and increased nitric oxide and catalase activity compared with a nonsoy legume–based diet (129).
Evidence regarding soy consumption and oxidative stress is also poor. In 20 hypercholesterolemic adults, a soy-based intervention (including bread and beverages) reduced the lipid oxidative stress capacity (P ≤ 0.05) (119). In another RCT, soybean consumption had a gender-specific effect on oxidative stress. The daily consumption of 2 g/kg of body weight of soybeans reduced advanced oxidation protein products in women, but not in men. Interestingly, an increase in the lipid peroxidation marker, thiobarbituric acid reactive substances, was observed in men. Furthermore, no effects on carbonyl stress markers were found in either gender (130). Also, in participants with T2D and nephropathy, concentrations of malondialdehyde did not significantly change after an intervention based on soy milk rather than cow milk (131). It should be noted that malondialdehyde and thiobarbituric acid reactive substances are thought to be nonspecific markers of lipid peroxidation.
Although further research is needed in this area, there are plausible mechanisms by which soy and nonsoy legume consumption may potentially improve oxidative stress. The high phenolic content of legumes is thought to be one of the main contributors to their antioxidant capacity (132). Other properties, such as their low glycemic index or high fiber content, may also contribute to the improvement of oxidative stress (133, 134).
Conclusions
In this narrative review and meta-analysis, we have focused on prospective studies that have examined the associations between nonsoy and/or soy and cardiometabolic outcomes. Results highlight the potential beneficial effect of legume consumption on CVD and CHD risks but there were also associations for T2D and stroke, although not statistically significant. In the stratified analysis of legume subtypes, only nonsoy legumes were significantly associated with lower risk of T2D. Soy was not associated with T2D, although the effect estimates became significant after the removal of 1 study. Owing to the significant evidence of heterogeneity, and taking into account the few available studies in this field, large and long-term epidemiological studies are still needed to determine the long-term impact of legume consumption on CVD and T2D.
Supplementary Material
Acknowledgments
The authors’ contributions were as follows—JS-S: has primary responsibility for final content; and all the authors: developed the overall research plan, conducted the literature search, wrote the paper, and read and approved the final manuscript.
Notes
Centro de Investigación Biomédica en Red Fisiopatología de la Obesidad y Nutrición (CIBERobn) is an initiative of the Instituto de Salud Carlos III (ISCIII) of Spain which is supported by Fondo Europeo de Desarrollo Regional (FEDER) funds.
Published in a supplement to Advances in Nutrition. This supplement was sponsored by the Harding-Buller Foundation of Ohio. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the sponsors. Publication costs for this supplement were defrayed in part by the payment of page charges. The opinions expressed in this publication are those of the authors and are not attributable to the sponsors or the publisher, Editor, or Editorial Board of Advances in Nutrition.
Author disclosures: The authors report no conflicts of interest.
Supplemental Figures 1 and 2 and Supplemental Tables 1–4 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Publication costs for this supplement were defrayed in part by the payment of page charges. The opinions expressed in this publication are those of the authors and are not attributable to the sponsors or the publisher, Editor, or Editorial Board of Advances in Nutrition.
Abbreviations used: CHD, coronary heart disease; CRP, C-reactive protein; CVD, cardiovascular disease; FBG, fasting blood glucose; MetS, metabolic syndrome; RCT, randomized clinical trial; SMD, standardized mean difference; T2D, type 2 diabetes.
References
- 1. McCrory MA, Hamaker BR, Lovejoy JC, Eichelsdoerfer PE. Pulse consumption, satiety, and weight management. Adv Nutr. 2010;1(1):17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. FAO. Definition and classification of commodities: 4. Pulses and derived products. [Internet] Rome, Italy: FAO; 1994. Available from: www.fao.org/es/faodef/fdef04e.htm. [Google Scholar]
- 3. Rebello CJ, Greenway FL, Finley JW. A review of the nutritional value of legumes and their effects on obesity and its related co-morbidities. Obes Rev. 2014;15:392–407. [DOI] [PubMed] [Google Scholar]
- 4. Messina MJ. Legumes and soybeans: overview of their nutritional profiles and health effects. Am J Clin Nutr. 1999;70(3):439S–50S. [DOI] [PubMed] [Google Scholar]
- 5. Hernández-Alonso P, Camacho-Barcia L, Bulló M, Salas-Salvadó J. Nuts and dried fruits: an update of their beneficial effects on type 2 diabetes. Nutrients. 2017;9(7):673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Griel AE, Eissenstat B, Juturu V, Hsieh G, Kris-Etherton PM. Improved diet quality with peanut consumption. J Am Coll Nutr. 2004;23:660–8. [DOI] [PubMed] [Google Scholar]
- 7. Mann JI, De Leeuw I, Hermansen K, Karamanos B, Karlström B, Katsilambros N, Riccardi G, Rivellese AA, Rizkalla S, Slama G et al.. Evidence-based nutritional approaches to the treatment and prevention of diabetes mellitus. Nutr Metab Cardiovasc Dis. 2004;14:373–94. [DOI] [PubMed] [Google Scholar]
- 8. Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, Franklin B, Kris-Etherton P, Harris WS, Howard B et al.. Diet and Lifestyle Recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114:82–96. [DOI] [PubMed] [Google Scholar]
- 9. Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Canadian Diabetes Association 2013 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes. 2013;37:S1–212. [DOI] [PubMed] [Google Scholar]
- 10. Grosso G, Marventano S, Yang J, Micek A, Pajak A, Scalfi L, Galvano F, Kales SN. A comprehensive meta-analysis on evidence of Mediterranean diet and cardiovascular disease: are individual components equal?. Crit Rev Food Sci Nutr. 2017;57:3218–32. [DOI] [PubMed] [Google Scholar]
- 11. Jayalath VH, de Souza RJ, Sievenpiper JL, Ha V, Chiavaroli L, Mirrahimi A, Di Buono M, Bernstein AM, Leiter LA, Kris-Etherton PM et al.. Effect of dietary pulses on blood pressure: a systematic review and meta-analysis of controlled feeding trials. Am J Hypertens. 2014;27:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim SJ, de Souza RJ, Choo VL, Ha V, Cozma AI, Chiavaroli L, Mirrahimi A, Blanco Mejia S, Di Buono M, Bernstein AM et al.. Effects of dietary pulse consumption on body weight: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 2016;103(5):1213–23. [DOI] [PubMed] [Google Scholar]
- 13. Ha V, Sievenpiper JL, de Souza RJ, Jayalath VH, Mirrahimi A, Agarwal A, Chiavaroli L, Mejia SB, Sacks FM, Di Buono M et al.. Effect of dietary pulse intake on established therapeutic lipid targets for cardiovascular risk reduction: a systematic review and meta-analysis of randomized controlled trials. CMAJ. 2014;186:E252–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Becerra-Tomás N, Díaz-López A, Rosique-Esteban N, Ros E, Buil-Cosiales P, Corella D, Estruch R, Fitó M, Serra-Majem L, Arós F et al.. Legume consumption is inversely associated with type 2 diabetes incidence in adults: a prospective assessment from the PREDIMED study. Clin Nutr. 2018;37(3):906–13. [DOI] [PubMed] [Google Scholar]
- 15. Viguiliouk E, Blanco Mejia S, Kendall CWC, Sievenpiper JL. Can pulses play a role in improving cardiometabolic health? Evidence from systematic reviews and meta-analyses. Ann N Y Acad Sci. 2017;1392:43–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Higgins JP, Green S, editors. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. [Internet] The Cochrane Collaboration; 2011; [updated Mar 2011]. Available from: https://handbook-5-1.cochrane.org/. [Google Scholar]
- 17. Marventano S, Izquierdo Pulido M, Sánchez-González C, Godos J, Speciani A, Galvano F, Grosso G. Legume consumption and CVD risk: a systematic review and meta-analysis. Public Health Nutr. 2017;20:245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kokubo Y, Iso H, Ishihara J, Okada K, Inoue M, Tsugane S; JPHC Study Group . Association of dietary intake of soy, beans, and isoflavones with risk of cerebral and myocardial infarctions in Japanese populations: the Japan Public Health Center-based (JPHC) study cohort I. Circulation. 2007;116:2553–62. [DOI] [PubMed] [Google Scholar]
- 19. Nagura J, Iso H, Watanabe Y, Maruyama K, Date C, Toyoshima H, Yamamoto A, Kikuchi S, Koizumi A, Kondo T et al.. Fruit, vegetable and bean intake and mortality from cardiovascular disease among Japanese men and women: the JACC study. Br J Nutr. 2009;102:285–92. [DOI] [PubMed] [Google Scholar]
- 20. Bazzano LA, He J, Ogden LG, Loria C, Vupputuri S, Myers L, Whelton PK. Legume consumption and risk of coronary heart disease in US men and women. Arch Intern Med. 2001;161(21):2573–8. [DOI] [PubMed] [Google Scholar]
- 21. Martínez-González MA, García-López M, Bes-Rastrollo M, Toledo E, Martínez-Lapiscina EH, Delgado-Rodriguez M, Vazquez Z, Benito S, Beunza JJ. Mediterranean diet and the incidence of cardiovascular disease: a Spanish cohort. Nutr Metab Cardiovasc Dis. 2010;21:237–44. [DOI] [PubMed] [Google Scholar]
- 22. Nouri F, Sarrafzadegan N, Mohammadifard N, Sadeghi M, Mansourian M. Intake of legumes and the risk of cardiovascular disease: frailty modeling of a prospective cohort study in the Iranian middle-aged and older population. Eur J Clin Nutr. 2016;70:217–21. [DOI] [PubMed] [Google Scholar]
- 23. Miller V, Mente A, Dehghan M, Rangarajan S, Zhang X, Swaminathan S, Dagenais G, Gupta R, Mohan V, Lear S et al.. Fruit, vegetable, and legume intake, and cardiovascular disease and deaths in 18 countries (PURE): a prospective cohort study. Lancet. 2017;390:2037–49. [DOI] [PubMed] [Google Scholar]
- 24. Stewart RAH, Wallentin L, Benatar J, Danchin N, Hagström E, Held C, Husted S, Lonn E, Stebbins A, Chiswell K et al.. Dietary patterns and the risk of major adverse cardiovascular events in a global study of high-risk patients with stable coronary heart disease. Eur Heart J. 2016;37:1993–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Afshin A, Micha R, Khatibzadeh S, Mozaffarian D. Consumption of nuts and legumes and risk of incident ischemic heart disease, stroke, and diabetes: a systematic review and meta-analysis. Am J Clin Nutr. 2014;100(1):278–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kelemen LE, Kushi LH, Jacobs DR, Cerhan JR. Associations of dietary protein with disease and mortality in a prospective study of postmenopausal women. Am J Epidemiol. 2005;161:239–49. [DOI] [PubMed] [Google Scholar]
- 27. Buckland G, González CA, Agudo A, Vilardell M, Berenguer A, Amiano P, Ardanaz E, Arriola L, Barricarte A, Basterretxea M et al.. Adherence to the Mediterranean diet and risk of coronary heart disease in the Spanish EPIC Cohort Study. Am J Epidemiol. 2009;170:1518–29. [DOI] [PubMed] [Google Scholar]
- 28. Bernstein AM, Sun Q, Hu FB, Stampfer MJ, Manson JE, Willett WC. Major dietary protein sources and risk of coronary heart disease in women. Circulation. 2010;122:876–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dilis V, Katsoulis M, Lagiou P, Trichopoulos D, Naska A, Trichopoulou A. Mediterranean diet and CHD: the Greek European Prospective Investigation into Cancer and Nutrition cohort. Br J Nutr. 2012;108:699–709. [DOI] [PubMed] [Google Scholar]
- 30. Haring B, Gronroos N, Nettleton JA, von Ballmoos MCW, Selvin E, Alonso A. Dietary protein intake and coronary heart disease in a large community based cohort: results from the Atherosclerosis Risk in Communities (ARIC) study. PLoS One. 2014;9:e109552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shi ZQ, Tang JJ, Wu H, Xie CY, He ZZ. Consumption of nuts and legumes and risk of stroke: a meta-analysis of prospective cohort studies. Nutr Metab Cardiovasc Dis. 2014;24:1262–71. [DOI] [PubMed] [Google Scholar]
- 32. van der Schouw YT, Kreijkamp-Kaspers S, Peeters PHM, Keinan-Boker L, Rimm EB, Grobbee DE. Prospective study on usual dietary phytoestrogen intake and cardiovascular disease risk in Western women. Circulation. 2005;111:465–71. [DOI] [PubMed] [Google Scholar]
- 33. Mizrahi A, Knekt P, Montonen J, Laaksonen MA, Heliövaara M, Järvinen R. Plant foods and the risk of cerebrovascular diseases: a potential protection of fruit consumption. Br J Nutr. 2009;102:1075–83. [DOI] [PubMed] [Google Scholar]
- 34. Bernstein AM, Pan A, Rexrode KM, Stampfer M, Hu FB, Mozaffarian D, Willett WC. Dietary protein sources and the risk of stroke in men and women. Stroke. 2012;43:637–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Misirli G, Benetou V, Lagiou P, Bamia C, Trichopoulos D, Trichopoulou A. Relation of the traditional Mediterranean diet to cerebrovascular disease in a Mediterranean population. Am J Epidemiol. 2012;176:1185–92. [DOI] [PubMed] [Google Scholar]
- 36. Haring B, Misialek JR, Rebholz CM, Petruski-Ivleva N, Gottesman RF, Mosley TH, Alonso A. Association of dietary protein consumption with incident silent cerebral infarcts and stroke: the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2015;46:3443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Agrawal S, Ebrahim S. Association between legume intake and self-reported diabetes among adult men and women in India. BMC Public Health. 2013;13:706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dhillon PK, Bowen L, Kinra S, Bharathi AV, Agrawal S, Prabhakaran D, Reddy KS, Ebrahim S; Indian Migration Study Group . Legume consumption and its association with fasting glucose, insulin resistance and type 2 diabetes in the Indian Migration Study. Public Health Nutr. 2016;19(16):3017–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schwingshackl L, Hoffmann G, Lampousi A-M, Knüppel S, Iqbal K, Schwedhelm C, Bechthold A, Schlesinger S, Boeing H. Food groups and risk of type 2 diabetes mellitus: a systematic review and meta-analysis of prospective studies. Eur J Epidemiol. 2017;32(5):363–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tatsumi Y, Morimoto A, Deura K, Mizuno S, Ohno Y, Watanabe S. Effects of soybean product intake on fasting and postload hyperglycemia and type 2 diabetes in Japanese men with high body mass index: the Saku Study. J Diabetes Investig. 2013;4:626–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Khalili-Moghadam S, Mirmiran P, Bahadoran Z, Azizi F. The Mediterranean diet and risk of type 2 diabetes in Iranian population. Eur J Clin Nutr. 2018;73:72–8. [DOI] [PubMed] [Google Scholar]
- 42. Meyer KA, Kushi LH, Jacobs DR, Slavin J, Sellers TA, Folsom AR. Carbohydrates, dietary fiber, and incident type 2 diabetes in older women. Am J Clin Nutr. 2000;71(4):921–30. [DOI] [PubMed] [Google Scholar]
- 43. Hodge AM, English DR, O'Dea K, Giles GG. Glycemic index and dietary fiber and the risk of type 2 diabetes. Diabetes Care. 2004;27(11):2701–6. [DOI] [PubMed] [Google Scholar]
- 44. Bazzano LA, Li TY, Joshipura KJ, Hu FB. Intake of fruit, vegetables, and fruit juices and risk of diabetes in women. Diabetes Care. 2008;31(7):1311–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Villegas R, Gao Y-T, Yang G, Li H-L, Elasy TA, Zheng W, Shu XO. Legume and soy food intake and the incidence of type 2 diabetes in the Shanghai Women's Health Study. Am J Clin Nutr. 2008;87(1):162–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nanri A, Mizoue T, Takahashi Y, Kirii K, Inoue M, Noda M, Tsugane S. Soy product and isoflavone intakes are associated with a lower risk of type 2 diabetes in overweight Japanese women. J Nutr. 2010;140(3):580–6. [DOI] [PubMed] [Google Scholar]
- 47. Morimoto Y, Steinbrecher A, Kolonel LN, Maskarinec G. Soy consumption is not protective against diabetes in Hawaii: the Multiethnic Cohort. Eur J Clin Nutr. 2011;65:279–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mueller NT, Odegaard AO, Gross MD, Koh W-P, Yu MC, Yuan J-M, Pereira MA. Soy intake and risk of type 2 diabetes mellitus in Chinese Singaporeans. Eur J Nutr. 2012;51(8):1033–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. von Ruesten A, Feller S, Bergmann MM, Boeing H. Diet and risk of chronic diseases: results from the first 8 years of follow-up in the EPIC-Potsdam study. Eur J Clin Nutr. 2013;67:412–19. [DOI] [PubMed] [Google Scholar]
- 50. Ericson U, Sonestedt E, Gullberg B, Hellstrand S, Hindy G, Wirfält E, Orho-Melander M, Larsen TM, Dalskov S-M, van Baak M et al.. High intakes of protein and processed meat associate with increased incidence of type 2 diabetes. Br J Nutr. 2013;109:1143–53. [DOI] [PubMed] [Google Scholar]
- 51. Ding M, Pan A, Manson JE, Willett WC, Malik V, Rosner B, Giovannucci E, Hu FB, Sun Q. Consumption of soy foods and isoflavones and risk of type 2 diabetes: a pooled analysis of three US cohorts. Eur J Clin Nutr. 2016;70(12):1381–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nöthlings U, Schulze MB, Weikert C, Boeing H, van der Schouw YT, Bamia C, Benetou V, Lagiou P, Krogh V, Beulens JWJ et al.. Intake of vegetables, legumes, and fruit, and risk for all-cause, cardiovascular, and cancer mortality in a European diabetic population. J Nutr. 2008;138(4):775–81. [DOI] [PubMed] [Google Scholar]
- 53. Sievenpiper JL, Kendall CWC, Esfahani A, Wong JMW, Carleton AJ, Jiang HY, Bazinet RP, Vidgen E, Jenkins DJA. Effect of non-oil-seed pulses on glycaemic control: a systematic review and meta-analysis of randomised controlled experimental trials in people with and without diabetes. Diabetologia. 2009;52:1479–95. [DOI] [PubMed] [Google Scholar]
- 54. Mollard RC, Zykus A, Luhovyy BL, Nunez MF, Wong CL, Anderson GH. The acute effects of a pulse-containing meal on glycaemic responses and measures of satiety and satiation within and at a later meal. Br J Nutr. 2012;108:509–17. [DOI] [PubMed] [Google Scholar]
- 55. Mohan V, Spiegelman D, Sudha V, Gayathri R, Hong B, Praseena K, Anjana RM, Wedick NM, Arumugam K, Malik V et al.. Effect of brown rice, white rice, and brown rice with legumes on blood glucose and insulin responses in overweight Asian Indians: a randomized controlled trial. Diabetes Technol Ther. 2014;16:317–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Winham DM, Hutchins AM, Thompson SV. Glycemic response to black beans and chickpeas as part of a rice meal: a randomized cross-over trial. Nutrients. 2017;9(10):1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zafar TA, Kabir Y. Chickpeas suppress postprandial blood glucose concentration, and appetite and reduce energy intake at the next meal. J Food Sci Technol. 2017;54:987–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nilsson A, Johansson E, Ekström L, Björck I. Effects of a brown beans evening meal on metabolic risk markers and appetite regulating hormones at a subsequent standardized breakfast: a randomized cross-over study. PLoS One. 2013;8(4):e59985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jenkins DJA, Kendall CWC, Augustin LSA, Mitchell S, Sahye-Pudaruth S, Blanco Mejia S, Chiavaroli L, Mirrahimi A, Ireland C, Bashyam B et al.. Effect of legumes as part of a low glycemic index diet on glycemic control and cardiovascular risk factors in type 2 diabetes mellitus. Arch Intern Med. 2012;172:1653. [DOI] [PubMed] [Google Scholar]
- 60. Mollard RC, Luhovyy BL, Panahi S, Nunez M, Hanley A, Anderson GH. Regular consumption of pulses for 8 weeks reduces metabolic syndrome risk factors in overweight and obese adults. Br J Nutr. 2012;108(Suppl 1):S111–22. [DOI] [PubMed] [Google Scholar]
- 61. Hosseinpour-Niazi S, Mirmiran P, Hedayati M, Azizi F. Substitution of red meat with legumes in the therapeutic lifestyle change diet based on dietary advice improves cardiometabolic risk factors in overweight type 2 diabetes patients: a cross-over randomized clinical trial. Eur J Clin Nutr. 2015;69:592–7. [DOI] [PubMed] [Google Scholar]
- 62. Tonstad S, Malik N, Haddad E. A high-fibre bean-rich diet versus a low-carbohydrate diet for obesity. J Hum Nutr Diet. 2014;27:109–16. [DOI] [PubMed] [Google Scholar]
- 63. Saraf-Bank S, Esmaillzadeh A, Faghihimani E, Azadbakht L. Effects of legume-enriched diet on cardiometabolic risk factors among individuals at risk for diabetes: a crossover study. J Am Coll Nutr. 2016;35:31–40. [DOI] [PubMed] [Google Scholar]
- 64. Abeysekara S, Chilibeck PD, Vatanparast H, Zello GA. A pulse-based diet is effective for reducing total and LDL-cholesterol in older adults. Br J Nutr. 2012;108:S103–10. [DOI] [PubMed] [Google Scholar]
- 65. Hermsdorff HHM, Zulet MÁ, Abete I, Martínez JA. A legume-based hypocaloric diet reduces proinflammatory status and improves metabolic features in overweight/obese subjects. Eur J Nutr. 2011;50:61–9. [DOI] [PubMed] [Google Scholar]
- 66. Tovar J, Nilsson A, Johansson M, Björck I. Combining functional features of whole-grain barley and legumes for dietary reduction of cardiometabolic risk: a randomised cross-over intervention in mature women. Br J Nutr. 2014;111:706–14. [DOI] [PubMed] [Google Scholar]
- 67. Hartman TJ, Albert PS, Zhang Z, Bagshaw D, Kris-Etherton PM, Ulbrecht J, Miller CK, Bobe G, Colburn NH, Lanza E. Consumption of a legume-enriched, low-glycemic index diet is associated with biomarkers of insulin resistance and inflammation among men at risk for colorectal cancer. J Nutr. 2010;140(1):60–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Liu Z-m, Chen Y-m, Ho SC. Effects of soy intake on glycemic control: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2011;93(5):1092–101. [DOI] [PubMed] [Google Scholar]
- 69. Reverri EJ, LaSalle CD, Franke AA, Steinberg FM. Soy provides modest benefits on endothelial function without affecting inflammatory biomarkers in adults at cardiometabolic risk. Mol Nutr Food Res. 2015;59(2):323–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Acharjee S, Zhou JR, Elajami TK, Welty FK. Effect of soy nuts and equol status on blood pressure, lipids and inflammation in postmenopausal women stratified by metabolic syndrome status. Metabolism. 2015;64(2):236–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ruscica M, Pavanello C, Gandini S, Gomaraschi M, Vitali C, Macchi C, Morlotti B, Aiello G, Bosisio R, Calabresi L et al.. Effect of soy on metabolic syndrome and cardiovascular risk factors: a randomized controlled trial. Eur J Nutr. 2018;57(2):499–511. [DOI] [PubMed] [Google Scholar]
- 72. Thorne MJ, Thompson LU, Jenkins DJ. Factors affecting starch digestibility and the glycemic response with special reference to legumes. Am J Clin Nutr. 1983;38(3):481–8. [DOI] [PubMed] [Google Scholar]
- 73. Bazzano LA, Thompson AM, Tees MT, Nguyen CH, Winham DM. Non-soy legume consumption lowers cholesterol levels: a meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2011;21(2):94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Anderson JW, Major AW. Pulses and lipaemia, short- and long-term effect: potential in the prevention of cardiovascular disease. Br J Nutr. 2002;88(S3):263–71. [DOI] [PubMed] [Google Scholar]
- 75. Tokede OA, Onabanjo TA, Yansane A, Gaziano JM, Djoussé L. Soya products and serum lipids: a meta-analysis of randomised controlled trials. Br J Nutr. 2015;114(6):831–43. [DOI] [PubMed] [Google Scholar]
- 76. Brown L, Rosner B, Willett WW, Sacks FM. Cholesterol-lowering effects of dietary fiber: a meta-analysis. Am J Clin Nutr. 1999;69(1):30–42. [DOI] [PubMed] [Google Scholar]
- 77. van Bennekum AM, Nguyen DV, Schulthess G, Hauser H, Phillips MC. Mechanisms of cholesterol-lowering effects of dietary insoluble fibres: relationships with intestinal and hepatic cholesterol parameters. Br J Nutr. 2005;94(3):331–7. [DOI] [PubMed] [Google Scholar]
- 78. Duane WC. Effects of legume consumption on serum cholesterol, biliary lipids, and sterol metabolism in humans. J Lipid Res. 1997;38:1120–8. [PubMed] [Google Scholar]
- 79. Kishimoto Y, Wakabayashi S, Takeda H. Hypocholesterolemic effect of dietary fiber: relation to intestinal fermentation and bile acid excretion. J Nutr Sci Vitaminol. 1995;41:151–61. [DOI] [PubMed] [Google Scholar]
- 80. Galisteo M, Duarte J, Zarzuelo A. Effects of dietary fibers on disturbances clustered in the metabolic syndrome. J Nutr Biochem. 2008;19:71–84. [DOI] [PubMed] [Google Scholar]
- 81. Fernandez ML. Soluble fiber and nondigestible carbohydrate effects on plasma lipids and cardiovascular risk. Curr Opin Lipidol. 2001;12:35–40. [DOI] [PubMed] [Google Scholar]
- 82. Alvaro A, Solà R, Rosales R, Ribalta J, Anguera A, Masana L, Vallvé JC. Gene expression analysis of a human enterocyte cell line reveals downregulation of cholesterol biosynthesis in response to short-chain fatty acids. IUBMB Life. 2008;60:757–64. [DOI] [PubMed] [Google Scholar]
- 83. Wright RS, Anderson JW, Bridges SR. Propionate inhibits hepatocyte lipid synthesis. Proc Soc Exp Biol Med. 1990;195:26–9. [DOI] [PubMed] [Google Scholar]
- 84. Racette SB, Lin X, Lefevre M, Spearie CA, Most MM, Ma L, Ostlund RE Jr. Dose effects of dietary phytosterols on cholesterol metabolism: a controlled feeding study. Am J Clin Nutr. 2010;91(1):32–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ostlund RE. Phytosterols and cholesterol metabolism. Curr Opin Lipidol. 2004;15:37–41. [DOI] [PubMed] [Google Scholar]
- 86. Sidhu GS, Oakenfull DG. A mechanism for the hypocholesterolaemic activity of saponins. Br J Nutr. 1986;55:643–9. [DOI] [PubMed] [Google Scholar]
- 87. Marrelli M, Conforti F, Araniti F, Statti G. Effects of saponins on lipid metabolism: a review of potential health benefits in the treatment of obesity. Molecules. 2016;21(10):1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Zhan S, Ho SC. Meta-analysis of the effects of soy protein containing isoflavones on the lipid profile. Am J Clin Nutr. 2005;81(2):397–408. [DOI] [PubMed] [Google Scholar]
- 89. Jenkins DJA, Mirrahimi A, Srichaikul K, Berryman CE, Wang L, Carleton A, Abdulnour S, Sievenpiper JL, Kendall CWC, Kris-Etherton PM. Soy protein reduces serum cholesterol by both intrinsic and food displacement mechanisms. J Nutr. 2010;140(12):2302S–11S. [DOI] [PubMed] [Google Scholar]
- 90. Reynolds K, Chin A, Lees KA, Nguyen A, Bujnowski D, He J. A meta-analysis of the effect of soy protein supplementation on serum lipids. Am J Cardiol. 2006;98:633–40. [DOI] [PubMed] [Google Scholar]
- 91. Harland JI, Haffner TA. Systematic review, meta-analysis and regression of randomised controlled trials reporting an association between an intake of circa 25 g soya protein per day and blood cholesterol. Atherosclerosis. 2008;200:13–27. [DOI] [PubMed] [Google Scholar]
- 92. Nimbe T, Torre-Villalvazo I, Tovar AR. Regulation of lipid metabolism by soy protein and its implication in diseases mediated by lipid disorders. J Nutr Biochem. 2006;17:365–73. [DOI] [PubMed] [Google Scholar]
- 93. Lovati MR, Manzoni C, Gianazza E, Arnoldi A, Kurowska E, Carroll KK, Sirtori CR. Soy protein peptides regulate cholesterol homeostasis in Hep G2 cells. J Nutr. 2000;130(10):2543–9. [DOI] [PubMed] [Google Scholar]
- 94. Potter SM. Overview of proposed mechanisms for the hypocholesterolemic effect of soy. J Nutr. 1995;125(3 Suppl):606S–11S. [DOI] [PubMed] [Google Scholar]
- 95. Ramdath DD, Padhi EMT, Sarfaraz S, Renwick S, Duncan AM. Beyond the cholesterol-lowering effect of soy protein: a review of the effects of dietary soy and its constituents on risk factors for cardiovascular disease. Nutrients. 2017;9(4):324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Akhlaghi M, Zare M, Nouripour F. Effect of soy and soy isoflavones on obesity-related anthropometric measures: a systematic review and meta-analysis of randomized controlled clinical trials. Adv Nutr. 2017;8(5):705–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Li SS, Kendall CWC, de Souza RJ, Jayalath VH, Cozma AI, Ha V, Mirrahimi A, Chiavaroli L, Augustin LSA, Blanco Mejia S et al.. Dietary pulses, satiety and food intake: a systematic review and meta-analysis of acute feeding trials. Obesity. 2014;22:1773–80. [DOI] [PubMed] [Google Scholar]
- 98. Howarth NC, Saltzman E, Roberts SB. Dietary fiber and weight regulation. Nutr Rev. 2001;59:129–39. [DOI] [PubMed] [Google Scholar]
- 99. Pereira MA, Ludwig DS. Dietary fiber and body-weight regulation. Observations and mechanisms. Pediatr Clin North Am. 2001;48:969–80. [DOI] [PubMed] [Google Scholar]
- 100. Brand-Miller JC, Holt SHA, Pawlak DB, McMillan J. Glycemic index and obesity. Am J Clin Nutr. 2002;76(1):281S–5S. [DOI] [PubMed] [Google Scholar]
- 101. Halton TL, Hu FB. The effects of high protein diets on thermogenesis, satiety and weight loss: a critical review. J Am Coll Nutr. 2004;23:373–85. [DOI] [PubMed] [Google Scholar]
- 102. Dong J-Y, Tong X, Wu Z-W, Xun P-C, He K, Qin L-Q. Effect of soya protein on blood pressure: a meta-analysis of randomised controlled trials. Br J Nutr. 2011;106:317–26. [DOI] [PubMed] [Google Scholar]
- 103. Taku K, Lin N, Cai D, Hu J, Zhao X, Zhang Y, Wang P, Melby MK, Hooper L, Kurzer MS et al.. Effects of soy isoflavone extract supplements on blood pressure in adult humans: systematic review and meta-analysis of randomized placebo-controlled trials. J Hypertens. 2010;28:1971–82. [DOI] [PubMed] [Google Scholar]
- 104. Liu XX, Li SH, Chen JZ, Sun K, Wang XJ, Wang XG, Hui RT. Effect of soy isoflavones on blood pressure: a meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2012;22:463–70. [DOI] [PubMed] [Google Scholar]
- 105. Mohammadifard N, Salehi-Abargouei A, Salas-Salvadó J, Guasch-Ferré M, Humphries K, Sarrafzadegan N. The effect of tree nut, peanut, and soy nut consumption on blood pressure: a systematic review and meta-analysis of randomized controlled clinical trials. Am J Clin Nutr. 2015;101(5):966–82. [DOI] [PubMed] [Google Scholar]
- 106. Liu Z-M, Ho SC, Chen Y-M, Tomlinson B, Ho S, To K, Woo J. Effect of whole soy and purified daidzein on ambulatory blood pressure and endothelial function—a 6-month double-blind, randomized controlled trial among Chinese postmenopausal women with prehypertension. Eur J Clin Nutr. 2015;69:1161–8. [DOI] [PubMed] [Google Scholar]
- 107. Lee YP, Puddey IB, Hodgson JM. Protein, fibre and blood pressure: potential benefit of legumes. Clin Exp Pharmacol Physiol. 2008;35:473–6. [DOI] [PubMed] [Google Scholar]
- 108. van Mierlo LAJ, Arends LR, Streppel MT, Zeegers MPA, Kok FJ, Grobbee DE, Geleijnse JM. Blood pressure response to calcium supplementation: a meta-analysis of randomized controlled trials. J Hum Hypertens. 2006;20:571–80. [DOI] [PubMed] [Google Scholar]
- 109. Streppel MT, Arends LR, van ’t Veer P, Grobbee DE, Geleijnse JM. Dietary fiber and blood pressure. Arch Intern Med. 2005;165:150. [DOI] [PubMed] [Google Scholar]
- 110. Zhang X, Li Y, Del Gobbo LC, Rosanoff A, Wang J, Zhang W, Song Y. Effects of magnesium supplementation on blood pressure: a meta-analysis of randomized double-blind placebo-controlled trials. Hypertension. 2016;68:324–33. [DOI] [PubMed] [Google Scholar]
- 111. Aburto NJ, Hanson S, Gutierrez H, Hooper L, Elliott P, Cappuccio FP. Effect of increased potassium intake on cardiovascular risk factors and disease: systematic review and meta-analyses. BMJ. 2013;346:f1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Salehi-Abargouei A, Saraf-Bank S, Bellissimo N, Azadbakht L. Effects of non-soy legume consumption on C-reactive protein: a systematic review and meta-analysis. Nutrition. 2015;31:631–9. [DOI] [PubMed] [Google Scholar]
- 113. Singh TP, Morris DR, Smith S, Moxon JV, Golledge J. Systematic review and meta-analysis of the association between C-reactive protein and major cardiovascular events in patients with peripheral artery disease. Eur J Vasc Endovasc Surg. 2017;54:220–33. [DOI] [PubMed] [Google Scholar]
- 114. Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, Danesh J, Emerging Risk Factors Collaboration . C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375:132–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Winham DM, Hutchins AM. Baked bean consumption reduces serum cholesterol in hypercholesterolemic adults. Nutr Res. 2007;27:380–6. [Google Scholar]
- 116. Saraf-Bank S, Esmaillzadeh A, Faghihimani E, Azadbakht L. Effect of non-soy legume consumption on inflammation and serum adiponectin levels among first-degree relatives of patients with diabetes: a randomized, crossover study. Nutrition. 2015;31:459–65. [DOI] [PubMed] [Google Scholar]
- 117. Hosseinpour-Niazi S, Mirmiran P, Fallah-Ghohroudi A, Azizi F. Non-soya legume-based therapeutic lifestyle change diet reduces inflammatory status in diabetic patients: a randomised cross-over clinical trial. Br J Nutr. 2015;114:213–19. [DOI] [PubMed] [Google Scholar]
- 118. Dong J-Y, Wang P, He K, Qin L-Q. Effect of soy isoflavones on circulating C-reactive protein in postmenopausal women: meta-analysis of randomized controlled trials. Menopause. 2011;18:1256–62. [DOI] [PubMed] [Google Scholar]
- 119. Ahn-Jarvis J, Clinton SK, Riedl KM, Vodovotz Y, Schwartz SJ. Impact of food matrix on isoflavone metabolism and cardiovascular biomarkers in adults with hypercholesterolemia. Food Funct. 2012;3:1051–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Ma Y, Hébert JR, Li W, Bertone-Johnson ER, Olendzki B, Pagoto SL, Tinker L, Rosal MC, Ockene IS, Ockene JK et al.. Association between dietary fiber and markers of systemic inflammation in the Women's Health Initiative Observational Study. Nutrition. 2008;24:941–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Black S, Kushner I, Samols D. C-reactive protein. J Biol Chem. 2004;279:48487–90. [DOI] [PubMed] [Google Scholar]
- 122. Devaraj S, Venugopal SK, Singh U, Jialal I. Hyperglycemia induces monocytic release of interleukin-6 via induction of protein kinase c-α and -β. Diabetes. 2005;54:85–91. [DOI] [PubMed] [Google Scholar]
- 123. Levitan EB, Cook NR, Stampfer MJ, Ridker PM, Rexrode KM, Buring JE, Manson JE, Liu S. Dietary glycemic index, dietary glycemic load, blood lipids, and C-reactive protein. Metabolism. 2008;57:437–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Neuhouser ML, Schwarz Y, Wang C, Breymeyer K, Coronado G, Wang C-Y, Noar K, Song X, Lampe JW. A low-glycemic load diet reduces serum C-reactive protein and modestly increases adiponectin in overweight and obese adults. J Nutr. 2012;142(2):369–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. van Gemert WA, May AM, Schuit AJ, Oosterhof BYM, Peeters PH, Monninkhof EM. Effect of weight loss with or without exercise on inflammatory markers and adipokines in postmenopausal women: the SHAPE-2 trial, a randomized controlled trial. Cancer Epidemiol Biomarkers Prev. 2016;25:799–806. [DOI] [PubMed] [Google Scholar]
- 126. Forsythe LK, Wallace JMW, Livingstone MBE. Obesity and inflammation: the effects of weight loss. Nutr Res Rev. 2008;21:117–33. [DOI] [PubMed] [Google Scholar]
- 127. Jang Y, Lee JH, Kim OY, Park HY, Lee SY. Consumption of whole grain and legume powder reduces insulin demand, lipid peroxidation, and plasma homocysteine concentrations in patients with coronary artery disease: randomized controlled clinical trial. Arterioscler Thromb Vasc Biol. 2001;21:2065–71. [DOI] [PubMed] [Google Scholar]
- 128. Crujeiras AB, Parra D, Abete I, Martínez JA. A hypocaloric diet enriched in legumes specifically mitigates lipid peroxidation in obese subjects. Free Radic Res. 2007;41:498–506. [DOI] [PubMed] [Google Scholar]
- 129. Mirmiran P, Hosseinpour-Niazi S, Azizi F. Therapeutic lifestyle change diet enriched in legumes reduces oxidative stress in overweight type 2 diabetic patients: a crossover randomised clinical trial. Eur J Clin Nutr. 2018;72(1):174–6. [DOI] [PubMed] [Google Scholar]
- 130. Celec P, Hodosy J, Pálffy R, Gardlík R, Halčák L, Ostatníková D. The short-term effects of soybean intake on oxidative and carbonyl stress in men and women. Molecules. 2013;18:5190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Miraghajani MS, Esmaillzadeh A, Najafabadi MM, Mirlohi M, Azadbakht L. Soy milk consumption, inflammation, coagulation, and oxidative stress among type 2 diabetic patients with nephropathy. Diabetes Care. 2012;35:1981–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Wang S, Melnyk JP, Tsao R, Marcone MF. How natural dietary antioxidants in fruits, vegetables and legumes promote vascular health. Food Res Int. 2011;44:14–22. [Google Scholar]
- 133. Botero D, Ebbeling CB, Blumberg JB, Ribaya-Mercado JD, Creager MA, Swain JF, Feldman HA, Ludwig DS. Acute effects of dietary glycemic index on antioxidant capacity in a nutrient-controlled feeding study. Obesity (Silver Spring). 2009;17:1664–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Arikawa AY, Samavat H, Gross M, Kurzer MS. Plasma F2-isoprostanes are positively associated with glycemic load, but inversely associated with dietary polyunsaturated fatty acids and insoluble fiber in postmenopausal women. J Nutr. 2017;147(9):1693–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.