Abstract
The Fanconi Anemia (FA) pathway repairs DNA damage caused by endogenous and chemotherapy-induced DNA crosslinks, and responds to replication stress1,2. Genetic inactivation of this pathway impairs development, prevents blood production and promotes cancer1,3. The key molecular step in the FA pathway is the monoubiquitination of a pseudosymmetric heterodimer of FANCI-FANCD24,5 by the FA core complex - a megadalton multiprotein E3 ubiquitin ligase6,7. Monoubiquitinated FANCD2 then recruits enzymes to remove the DNA crosslink or to stabilize the stalled replication fork. A molecular structure of the FA core complex would explain how it acts to maintain genome stability. Here we reconstituted an active, recombinant FA core complex, and used electron cryo-microscopy (cryoEM) and mass spectrometry to determine its structure. The FA core complex is comprised of two central dimers of the FANCB and FAAP100 subunits, flanked by two copies of the RING finger protein, FANCL. These two heterotrimers act as a scaffold to assemble the remaining five subunits, resulting in an extended asymmetric structure. Destabilization of the scaffold would disrupt the entire complex, resulting in a non-functional FA pathway. Thus, the structure provides a mechanistic basis for the low numbers of patients with mutations in FANCB, FANCL and FAAP100. Surprisingly, FANCB and FAAP100 adopt similar structures, despite a lack of sequence homology. The two FANCL subunits are in different conformations at opposite ends of the complex, suggesting that each FANCL plays a unique role. This structural and functional asymmetry of RING domains may be a general feature of E3 ligases. The cryoEM structure of the FA core complex provides a foundation for a detailed understanding of its E3 ubiquitin ligase activity and DNA interstrand crosslink repair.
The FA core complex is comprised of eight stably-associated subunits: FANCA, FANCB, FANCC, FANCE, FANCF, FANCG, FANCL and FAAP100 6. FANCL contains a RING finger domain which acts as the E3 ubiquitin ligase. It assembles with FANCB and FAAP100 to form a catalytic module6,8, and a low resolution negative stain EM study suggested that, in the absence of the other subunits, this is a symmetric dimer of FANCB-FANCL-FAAP100 heterotrimers9. FANCA and FANCG are proposed to act as a chromatin targeting module, while FANCC, FANCE and FANCF form a substrate recognition module6,8,10,11. Despite the central role of the FA core complex in DNA repair, we do not yet have a molecular understanding for how FANCL incorporates into the complex to perform site-specific monoubiquitination of the FANCI-FANCD2 substrate, and how mutation disrupts the function of the complex12.
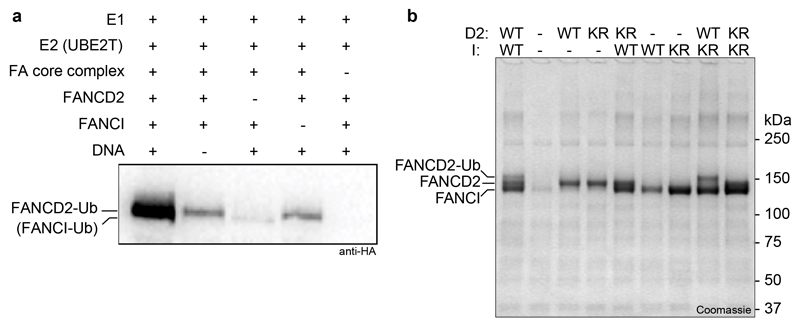
To determine the structure of the FA core complex, we overexpressed all eight subunits from Gallus gallus on a single baculovirus in insect cells, allowing us to purify an intact, recombinant complex (Fig. 1a). The purified complex specifically monoubiquitinated FANCD2, but not FANCI, in vitro (Extended Data Fig. 1) similar to the native chicken complex6.
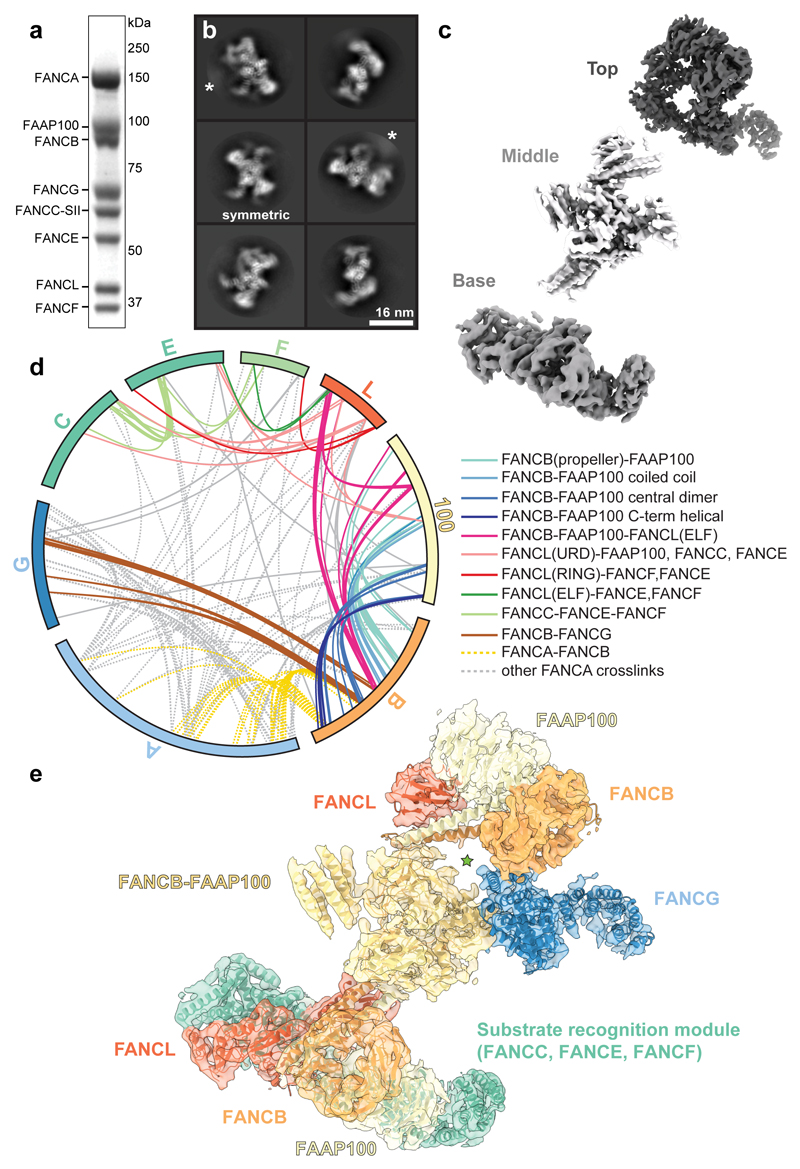
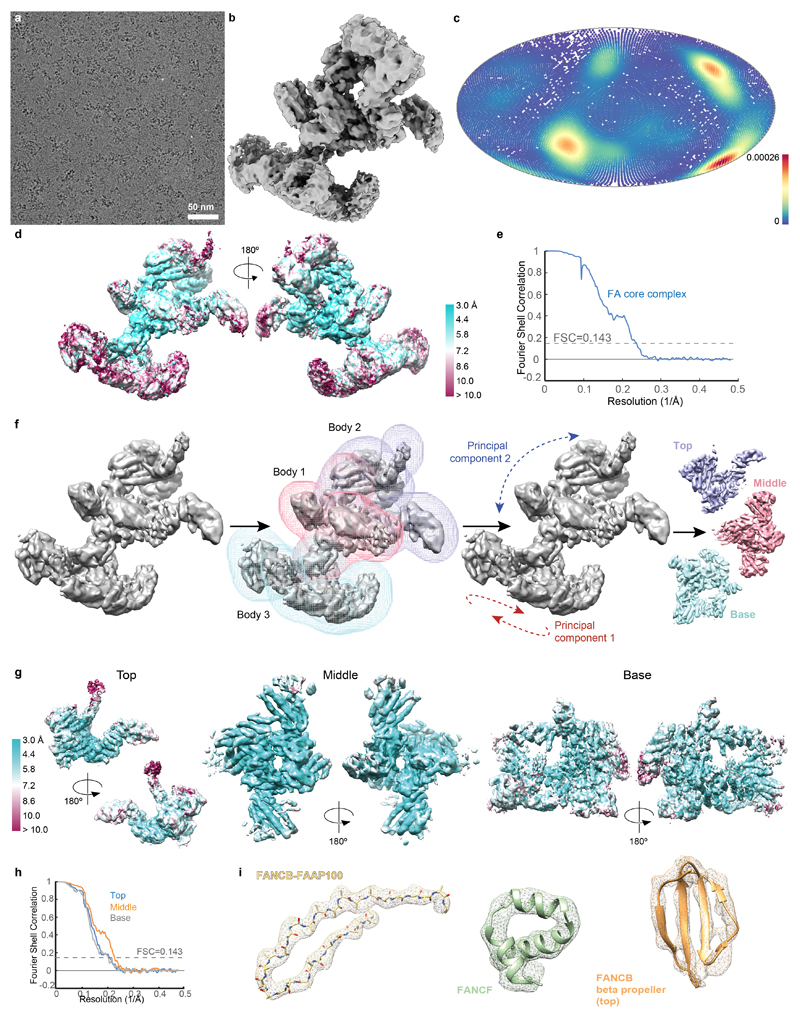
Fig. 1. Overall structure of the FA core complex.
a SDS-PAGE analysis of purified FA core complex with subunits and molecular weight markers indicated. FANCC carries a 2x Strep II tag on its C-terminus (FANCC-SII). This purification was repeated more than three times with similar results. For gel source data, see Supplementary Fig. 1. b Selected 2D reference-free class averages of the FA core complex. One class appears to be symmetric (labelled). Asterisks mark disordered density extending from the side of the complex that does not align well. c Focused classification and refinement on the top and base regions, and multibody refinement on the middle region resulted in three independent cryoEM maps that are shown separately, in three different shades of grey. d Crosslinking mass spectrometry revealed 834 crosslinks (1% FDR) between residues that are in close proximity. Intermolecular crosslinks are shown, colored by interacting regions. e Model of FA core complex (cartoon representation) fitted into the EM density (isosurface representation with transparency). Map and model are colored by assigned subunits. The green star marks a channel with diameter ~23 Å.
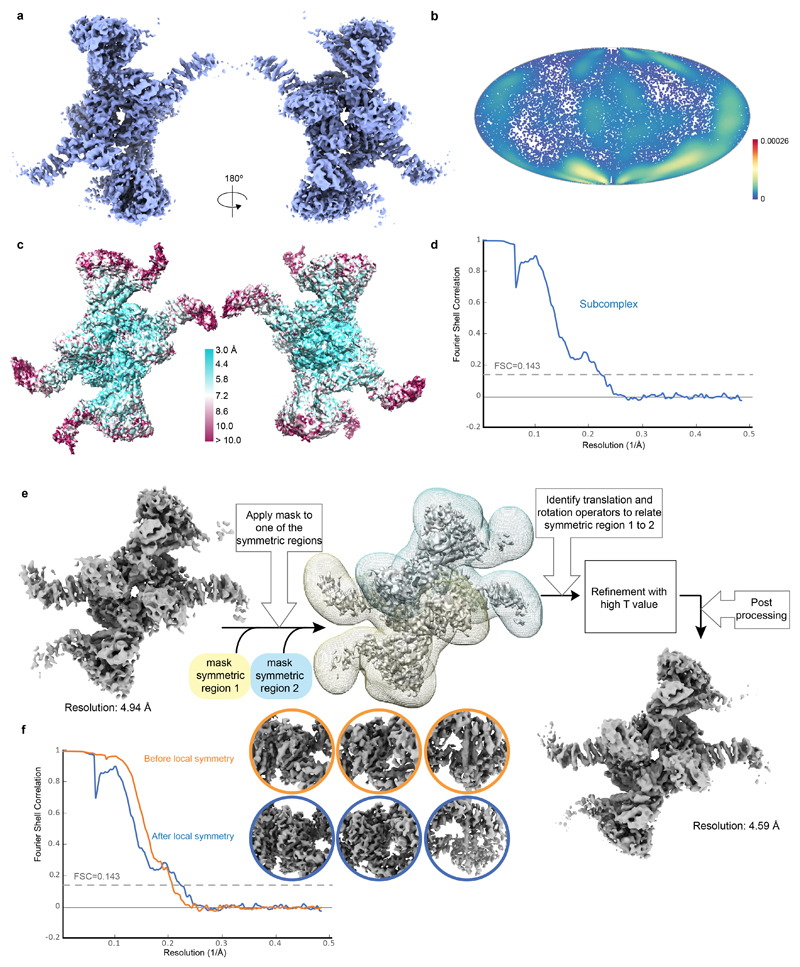
To investigate the molecular basis of subunit association, we imaged this recombinant FA core complex using cryoEM revealing an elongated structure, ~25 nm in length (Fig. 1b, Extended Data Fig. 2a). We determined a 3D reconstruction of the FA core complex at an overall resolution of 4.2 Å (Extended Data Fig. 2b-e and Extended Data Table 1). The peripheral regions were less well resolved than the central core, possibly due to conformational flexibility. In agreement with this, we detected structural variations using multi-body refinement13, including a continuum of conformational movement of the top and base regions (Extended Data Fig. 2f, Supplementary Videos 1 and 2). Particle subtraction followed by focused classification and refinement14 generated separate, improved reconstructions of the top and base regions (Fig. 1c, Extended Data Fig. 2g-h and Supplementary Video 3).
Our map of the complete FA core complex was of sufficient resolution to dock existing structures and to resolve secondary structure elements (Extended Data Fig. 2i). We fit two previously determined high-resolution structures (FANCL15, and part of FANCF16) into the map (see below), accounting for ~12% of the entire mass of the complex. Most subunits do not have substantial homology to proteins of known structure (Extended Data Table 2), so we modelled α-helices and β-strands into the remainder of the map. To determine which subunits these α-helices and β-strands belong to, we required additional data.
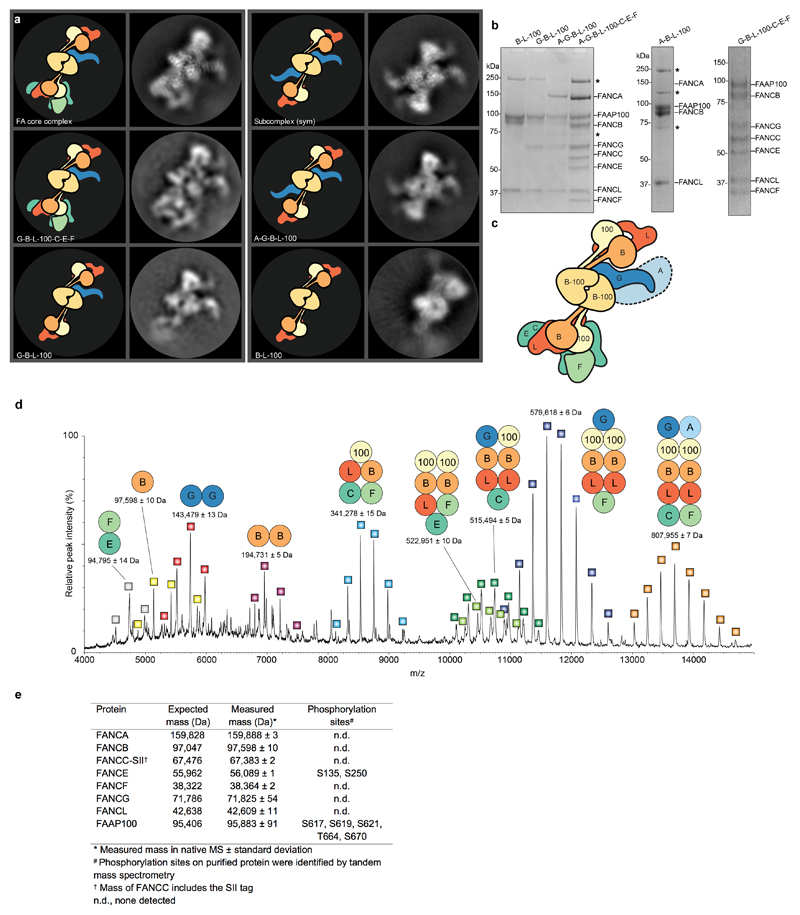
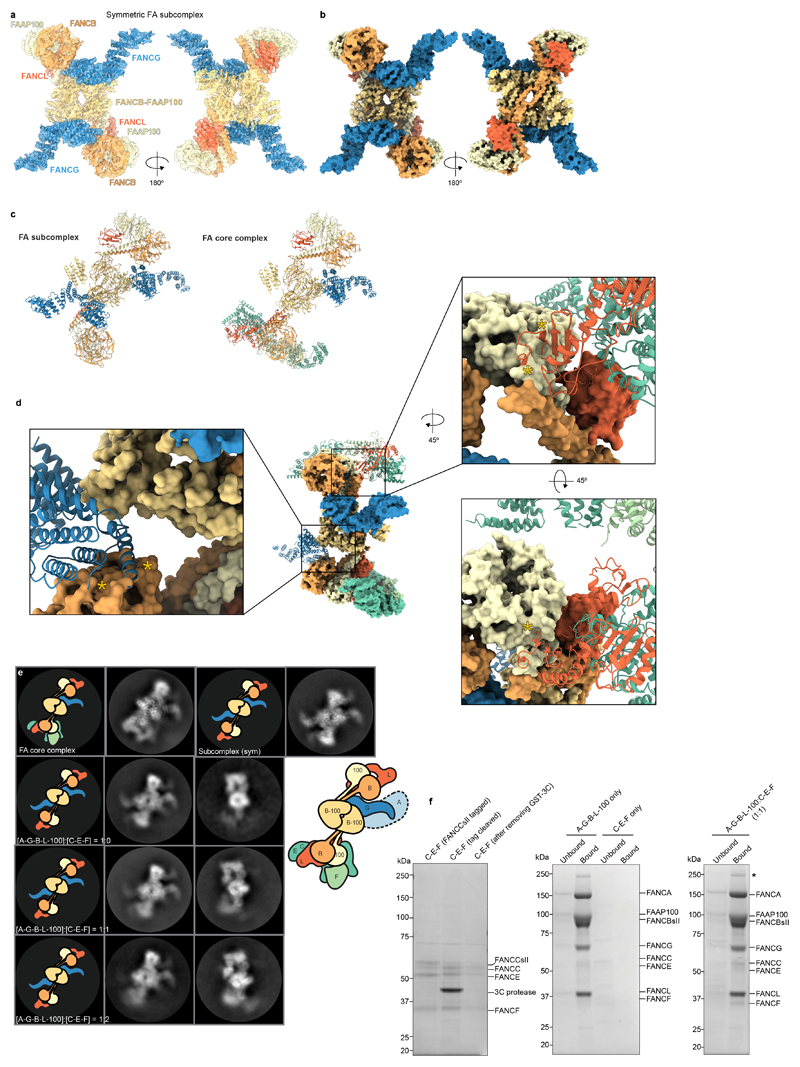
Next, we purified subcomplexes and imaged them using cryoEM. By comparing the 2D class averages to the complete FA core complex, we identified regions corresponding to specific subunits (Extended Data Fig. 3a-c). Removal of FANCA did not substantially change the class averages. This suggested that FANCA may be conformationally heterogeneous and blurred out in reconstructions, or it may dissociate/denature during cryoEM specimen preparation. A complex of FANCA, FANCG, FANCB, FANCL and FAAP100 was missing the base and therefore the base likely contains the substrate recognition module (FANCC, FANCE and FANCF). The partially disordered arm that extends from the central part of the complex is likely FANCG because this density is lost when FANCG is removed from a FANCB-FANCL-FAAP100 complex. Finally, 2D classes of the catalytic module (FANCB, FANCL and FAAP100) resemble the middle region of the FA core complex.
We also studied the structure of the FA core complex using noncovalent native mass spectrometry (MS) (Extended Data Fig. 3d-e). During ionization, the FANCE subunit tends to dissociate and subcomplexes are formed, providing information on subunit stoichiometry and protein-protein interactions. The largest complex (808 kDa) that we detected in native MS contains seven of the eight different subunits, including two copies of FANCB, FANCL and FAAP100, and a single copy of each of the remaining subunits. This agrees with the subunit stoichiometries of a purified native FA core complex6. FANCB, FANCL and FAAP100 were present in most of the subcomplexes identified by native MS (Extended Data Fig. 3d), suggesting they form a central core.
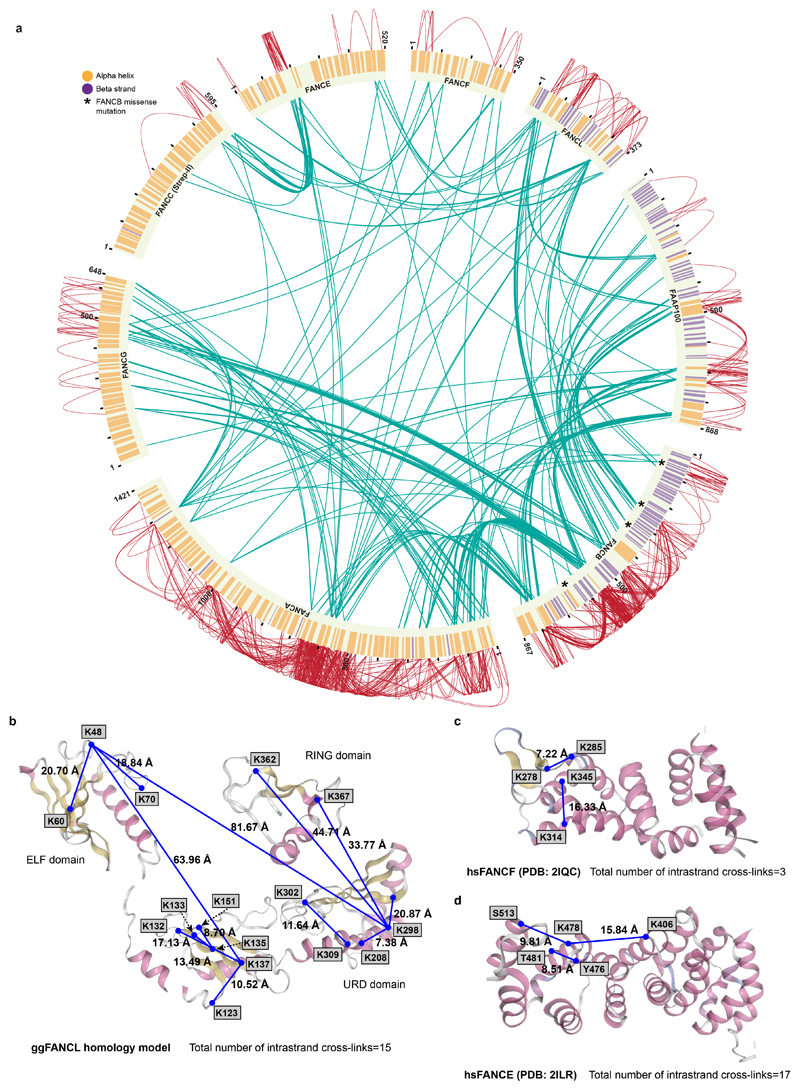
To identify residues in close proximity, we performed crosslinking mass spectrometry (Fig. 1d and Extended Data Fig. 4). This revealed 834 inter- and intra-molecular crosslinks, with 40% of these within FANCB, FANCL and FAAP100. This agrees with these three subunits forming an intimate complex. By combining the crosslinking MS data showing which residues are in close proximity, with the subunit assignment from subcomplexes, the subunit stoichiometry from native MS, homology modeling and secondary structure predictions, we generated models for all FA core complex subunits except FANCA (Fig. 1e; Extended Data Fig. 5a-d; Extended Data Table 1, 2; Supplementary Video 4; Methods).
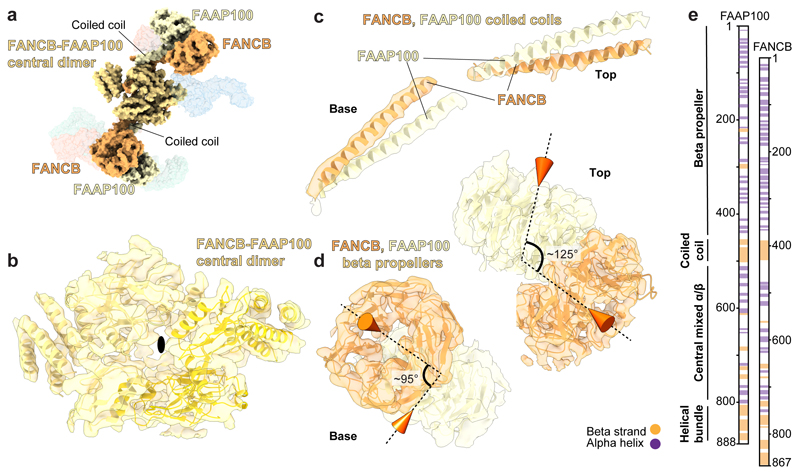
A dimer of FANCB-FAAP100 heterodimers is located in the middle region of the structure (Fig. 2). Two pairs of long α-helices (coiled coils) connect central β-strands and helical bundles with peripheral densities (Fig. 2b-c). Crosslinking MS and modeling showed that each coiled coil is likely comprised of α-helices from FANCB and FAAP100 (Extended Data Fig. 5e-f). At the peripheral ends of the coiled coils, we identified pairs of β-propellers, each containing a β-propeller from the N-terminal regions of FANCB and FAAP100 (Fig. 2d). We could differentiate the two β-propellers based on crosslinks: the FANCB β-propeller is near the coiled coil whereas the FAAP100 β-propeller is close to the ELF domain of FANCL (see below). Unexpectedly, despite no sequence homology, FANCB and FAAP100 share strikingly similar overall structures and domain organizations (Fig. 2e).
Fig. 2. The molecular scaffold of the FA core complex includes a dimer of FANCB-FAAP100 heterodimers.
a Surface representation of the FA core complex model highlighting FANCB and FAAP100. FANCB is in orange; FAAP100 is in yellow; and regions where we are unable to distinguish FANCB and FAAP100 are in yellow-orange. b-d Models of FANCB and FAAP100 subunits in cartoon representation placed into the cryoEM map. In panel (b), a black oval marks the pseudo two-fold symmetry axis. The two symmetry-related copies are shown as different shades of yellow in the model. In panel (d), cones indicate the orientations of the central pores of the beta propellers and the angles between them are given. e Proposed domain organization of FANCB and FAAP100 showing their structural similarity.
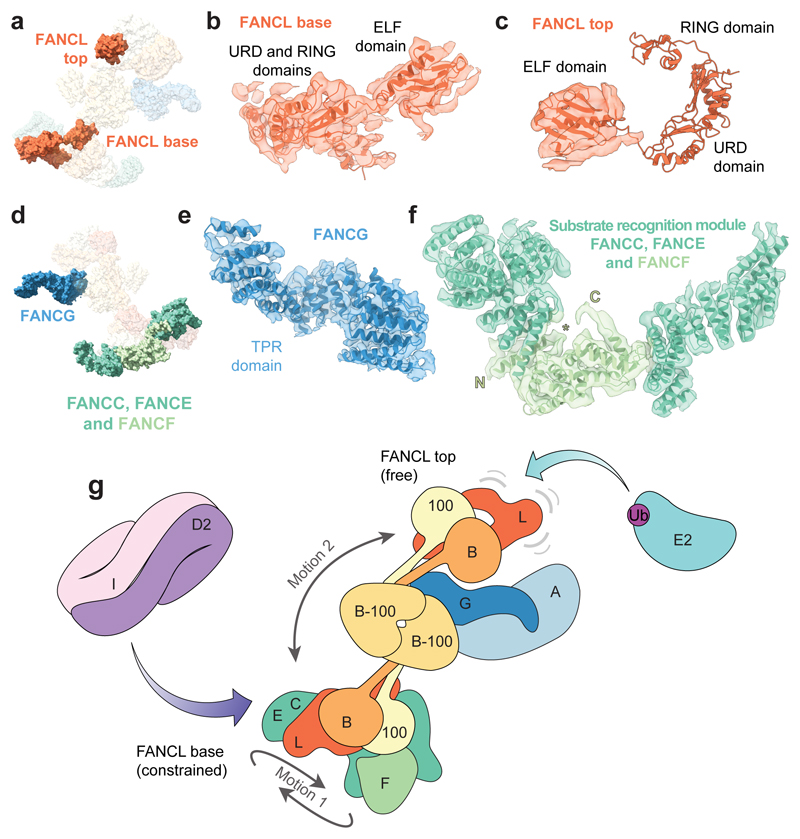
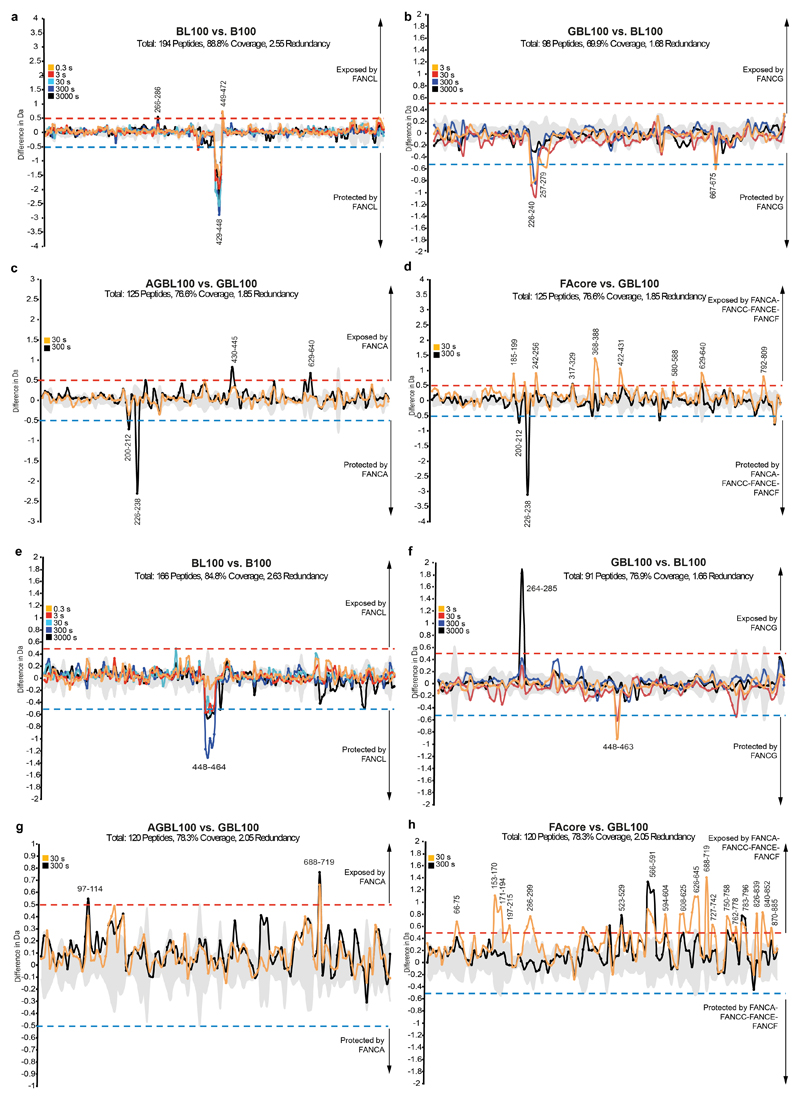
A homology model of FANCL, including the ELF, URD and RING domains15, fits into the base of the complex (Fig. 3a-b) but the relative orientations of the individual domains are different compared to the crystal structure (Extended Data Fig. 4b). In contrast, only the ELF domain could be placed into the second copy of FANCL in the top (Fig. 3c). Hydrogen-deuterium exchange mass spectrometry confirmed that FANCL interacts with the FANCB-FAAP100 coiled coil (Extended Data Fig. 6).
Fig. 3. Asymmetric dimerization in the FA core complex.
a-c Models of FANCL in the FA core complex. a Surface representation of the FA core complex model highlighting the two copies of FANCL. b,c Models of FANCLbase and FANCLtop subunits in cartoon representation are shown fitted in the cryoEM map. Density for the URD and RING domains is not well-defined in the top copy (c). d-f Models of FANCG and the substrate recognition module. d Surface representation of the FA core complex model highlighting FANCG and the substrate recognition module (FANCC-FANCE-FANCF). e-f Models of FANCG TPR domain (e) and the substrate recognition module (f) are shown fitted into the cryoEM map. The crystal structure of FANCF could be assigned and the N- and C-termini of the model are indicated. The first helix (N to *) was not present in the crystal structure. Since all three subunits of the substrate recognition module are substantially helical, it was not possible to assign the remaining helices of the base to individual subunits. g Model for monoubiquitination of FANCD2 by the FA core complex. The major motions detected in multi-body refinement are indicated with grey arrows.
FANCG contains tetratricopeptide repeats (TPRs) (Fig. 3d-e), crosslinks to the central FANCB-FAAP100 dimer (Fig. 1d), and is required for FANCA association (Extended Data Fig. 3b). Interestingly, there is a channel between FANCG and the catalytic module (Fig. 1e). FANCA, which is absent in the maps, is likely peripheral to FANCG, possibly located in the blurred density visible in 2D class averages (Fig. 1b, asterisks; Extended Data Fig. 5d). The large number of crosslinks between FANCA and FANCG are consistent with their proximity (Fig. 1d).
The substrate recognition module (FANCC, FANCE, FANCF), located in the base, is comprised of an arc of α-helices (Fig. 3f). FANCF occupies a central position within the arc. Crosslinking MS showed that the FANCL RING finger and ELF domain contact FANCE and FANCF, the FANCL URD domain contacts FANCC and FANCE, and the FANCC C-terminal region and FANCE are in close proximity. Native MS also showed direct contact between FANCE and FANCF (Extended Data Fig. 3d).
We also determined a structure of a subcomplex, present at a lower abundance in our sample, at an overall resolution of 4.6 Å (Extended Data Fig. 7a-d). This subcomplex was symmetric but the map did not improve on application of C2 symmetry. Thus, we implemented a local symmetry algorithm in Relion for averaging the two halves of the subcomplex map (Methods; Extended Data Fig. 7e-f). This symmetric structure revealed an assembly comprised of two copies of each of FANCB, FANCL, FAAP100 and FANCG (Extended Data Fig. 8a-c; Supplementary Video 5). It is unclear whether this subcomplex has a functional role in vivo or whether it is an assembly intermediate. In both copies of FANCL, the URD and RING finger domains have weak density or are not visible, similar to FANCLtop in the full, asymmetric FA core complex.
Comparison of subcomplex and FA core complex structures suggests that the substrate recognition module alters the relative orientations of the β-propellers and coiled coil in the base (Fig. 2d and Supplementary Video 6). This disrupts the symmetry of the catalytic module in the complete FA core complex, provides a binding site in the base for the URD and RING finger domains of FANCL, and likely disrupts docking of a second (symmetric) copy of FANCG onto the middle region due to steric clashes (Extended Data Fig. 8d). These structural alterations may be transmitted to the top region to prevent the binding of a second substrate recognition module, consistent with allosteric coupling proposed previously9. In agreement with this, a purified substrate recognition module did not readily associate with the symmetric subcomplex in vitro to form the asymmetric FA core complex (Extended Data Fig. 8e-f), suggesting that in vivo assembly is required.
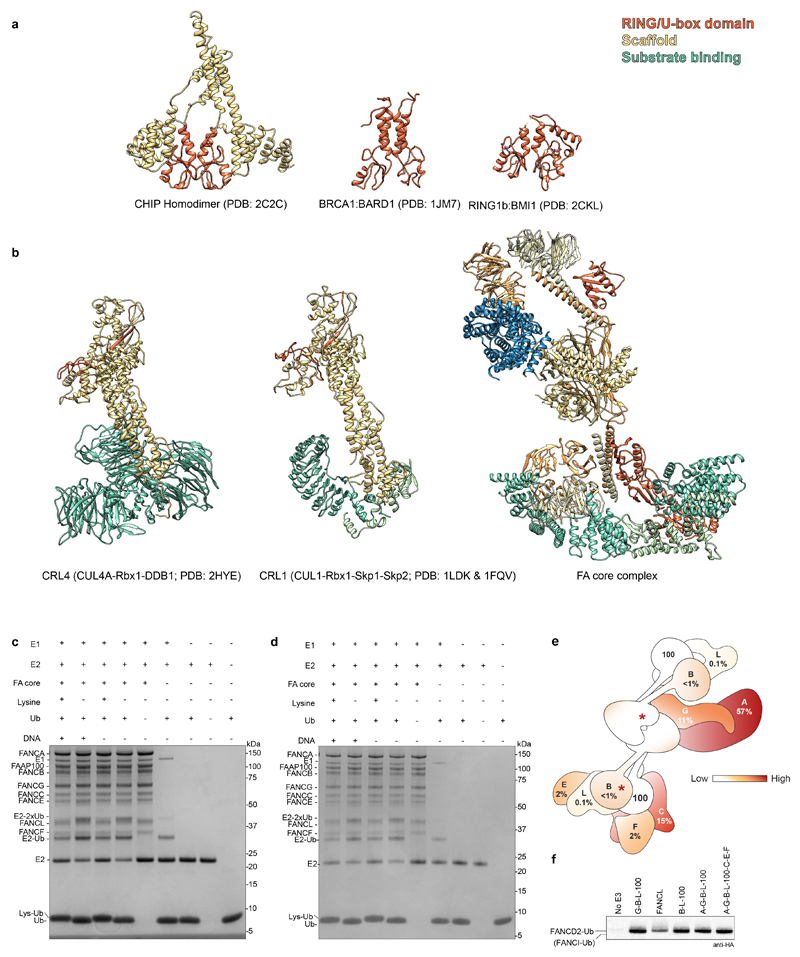
Dimerization is required for the activity of other E3 ligases, including the Rad18, RNF8 and CHIP homodimers17–19, and the BRCA1-BARD1 and Ring1b/Bmi1 heterodimers20,21. These contain two RING/U-box domains arranged in an asymmetric manner with only a single functional E2 binding site. Interestingly, the activity of multi-subunit cullin-RING E3 ligases is stimulated by dimerization22,23. Thus, structural and functional asymmetry appear to be a common feature of E3 ligases, but the spatial separation of the FANCL RING fingers in the FA core complex is unusual (Extended Data Fig. 9a-b). Like other dimeric RING E3s, one RING (FANCLbase) of the FA core complex may play a structural role to promote substrate binding along with FANCE10,11,24,25, while the other (FANCLtop) may be the active E3 that promotes ubiquitin transfer26 onto FANCD2 (Fig. 3g) within the structural state we observe. The FANCI-FANCD2 substrate is also an asymmetric dimer. Thus, each of the two FANCL RING domains could monoubiquitinate one of the substrate proteins. Still, this purified complex does not monoubiquitinate FANCI so an additional activation step might be required. Substrate binding is not required to activate the E3 ligase activity (Extended Data Fig. 9c-d).
The majority of FA patient mutations result in protein truncation and are found in the structural periphery of the FA core complex (Extended Data Fig. 9e)27. Residual monoubiquitin ligase activity is still present after deletion of peripheral subunits in cells6,8 and in vitro (Extended Data Fig. 9f), indicating a partially functioning core complex. In contrast, deletion of FANCB, FANCL or FAAP100, that comprise the catalytic module and the structural scaffold for the complex, eliminates this residual activity6,8. FA patients that carry mutations in FANCB or FANCL are severely afflicted, and we predict that FANCB missense mutations (L43S, P230S, L329P and L676P) disrupt stability of the catalytic module (Extended Data Fig. 4a, asterisks). Together, this provides genetic and clinical support that mutations in the catalytic module result in disruption of the FA core complex structure. In contrast, mutations in the periphery do not prevent complex formation and may be better tolerated.
Together, the data presented here provide the first structural model for the FA core complex, allowing an interpretation of the molecular pathophysiology of Fanconi anemia. The reconstituted system we describe will also allow further mechanistic questions to be addressed, including precisely how this large complex functions as a DNA damage inducible monoubiquitin ligase.
Methods
Cloning, expression and purification
cDNAs encoding full length Gallus gallus (Gg) FANCA, FANCB, FANCC, FANCE, FANCF, FANCG, FANCL and FAAP100 were synthesized (GeneArt). FANCC contained a C-terminal extension with a 3C protease site and double StrepII tag. For protein expression, all genes were cloned into the MultiBac expression system and constructs were generated as previously described28. Briefly, to make gene expression cassettes, FANCA and FANCG were subcloned via BamHI and XbaI into pACEBac1; FANCC, FANCE, FANCF and FAAP100 were subcloned via XhoI and KpnI into pIDS; and FANCB and FANCL were subcloned via BamHI and XbaI into pIDC. Gene cassettes were then sequentially subcloned as BstXI-ICeuI or BstXI-PISceI fragments into ICeuI or PISceI sites to generate pACEBac1-FANCA*FANCG, pIDS-FANCC-3C-2xStrepII*FANCE*FANCF, and pIDS-FAAP100*FANCB*FANCL. The spectinomycin antibiotic resistance cassette of pIDS-FAAP100*FANCB*FANCL was substituted with the kanamycin antibiotic resistance cassette of pIDK as a SnaBI-PI-SceI fragment to generate pIDK-FAAP100*FANCB*FANCL.
pACEBac1-FANCA*FANCG, pIDK-FAAP100*FANCB*FANCL and pIDS-FANCC-3C-2xStrepII*FANCE*FANCF were fused using Cre recombinase (NEB) to generate a single vector (gentamicin, spectinomycin and kanamycin resistant) containing a single copy of each gene (FA core complex) used to make protein for the initial data collection. All constructs were confirmed by restriction digest analysis, PCR and sequencing.
For subsequent protein preparations, A-G-B-L-100-C-E-F, A-G-B-L-100, G-B-L-100 and C-E-F complexes were prepared using a modified BiGBac system as described previously29,30. The individual genes were PCR amplified for cloning into pBIG vectors from pACEBac1, pIDC or pIDS vectors. Sequences encoding a 2xStrepII tag and 3C protease site were included on FANCC. If FANCC was not present, the tag sequence was added to FANCB. The combined vector carrying the FA core complex or subcomplex was transformed into EMBacY cells to generate a bacmid. Bacmid DNA was transfected into Sf9 cells and virus was passaged twice in the same cell line before large-scale infection in Sf9 cells. Infected cells were harvested when cell growth arrested. Sf9 cells were obtained from Oxford Expression Technologies Ltd, Cat No. 600100 (negative for mycoplasma, identity not independently authenticated by us).
Cells were lysed by sonication in lysis buffer (100 mM HEPES pH 8.0, 300 mM NaCl, 1 mM TCEP, 5% glycerol, EDTA-free protease inhibitor, 5 mM benzamidine hydrochloride and 100 U/ml benzonase). Clarified cell lysate was incubated with streptavidin beads (GE Healthcare) for 1 h followed by wash with lysis buffer. Proteins were eluted in elution buffer (100 mM HEPES pH 8.0, 300 mM NaCl, 1 mM TCEP, 5% glycerol and 8 mM Desthiobiotin). Further purification was performed by HiTrap Heparin HP affinity column (GE Healthcare) using a linear gradient of NaCl from concentration of 150 mM to 1 M in 50 mM HEPES pH 8.0, 1 mM TCEP, over 22 column volumes. For FA core complex, this was followed by anion exchange chromatography (MonoQ, GE Healthcare) in the same buffer using a linear gradient of NaCl from concentration of 150 mM to 1 M over 20 column volumes. The final buffer for purified FA core complex and subcomplexes was 50 mM HEPES pH 8.0, ~500 mM NaCl, 1 mM TCEP.
Ubiquitination assay
Ubiquitination assays were performed as described previously6,31. Briefly, a reaction volume of 20 μl contained 75 nM E1 (Boston Biochem), 1 μM E2 (GgUBE2T), 0.25 μM E3 (FA core complex), 1 μM substrate (His-GgFANCI, GgFANCIK525R, His-GgFANCD2, GgFANCD2K563R), 50 μM 5′-flapped DNA and 20 μM HA-Ubiquitin (Boston Biochem). For ubiquitin discharge assays, concentrations of 125 nM E1 (Boston Biochem), 5 μM E2 (GgUBE2T), 1.5 μM E3 (FA core complex) and 50 mM free lysine (instead of substrate) were used. The 0.25 μM E3 enzyme concentration is based on the FANCL subunit, estimated by comparing the amount of FANCL in FA core complex and subcomplexes against purified FANCL of known concentration on SDS-PAGE. The reaction buffer was 50 mM HEPES pH 8.0, 64 mM NaCl, 4 % glycerol, 5 mM MgCl2, 2 mM ATP and 0.5 mM DTT. The reaction was incubated at 30 °C for 90 min, stopped by adding NuPAGE LDS sample buffer (ThermoFisher) and run on an SDS polyacrylamide gel (3-8% NuPAGE Tris-acetate). Samples were analyzed by Coomassie staining or by western blot using an anti-HA antibody (Santa Cruz Biotechnology). All assays were performed independently three times.
Electron microscopy
Protein complexes were vitrified by applying 3–3.5 μl purified protein (~1 μM) to UltraAuFoil R1.2/1.3 grids (Quantifoil)32 with a thin continuous carbon support layer (for initial FA core complex dataset) or in unsupported ice (for final FA core complex dataset and subcomplexes) that had been made hydrophilic using an argon:oxygen plasma, blotting for 2.5, 3 or 4.5 s at 4 °C with relative humidity of 100% and plunging into liquid ethane using a Vitrobot Mark IV (FEI).
CryoEM data were collected on a FEI Titan Krios transmission electron microscope operated at 300 keV acceleration voltage using EPU automated data collection software. An initial dataset was collected on a Falcon II detector at 47,000× nominal magnification with a pixel size of 1.774 Å per pixel. The final data for FA core complex (Extended Data Table 1) were collected on a Falcon III detector in counting mode at 75,000× nominal magnification, and pixel size of 1.04 Å (MRC LMB) or 1.085 Å (eBIC). The subcomplexes G-B-L-100-C-E-F, A-G-B-L-100 and G-B-L-100 were imaged at 59,000× nominal magnification on a Falcon III detector in integrating mode. B-L-100 data were collected at 47,000× on a Falcon II detector in integrating mode.
CryoEM image processing
Image processing was performed in Relion (v2 and v3.0-beta)33–35, and Relion wrappers were used for external programs except for EMAN236. For all datasets, whole-frame alignment was performed using MotionCor237, and contrast transfer function parameters were estimated using gCTF38. 3D maps were post-processed to automatically estimate and apply the B-factor and to determine the resolution by Fourier shell correlation between two independent half datasets using 0.143 criterion39. Local resolution was estimated using ResMap40.
Initial model
The initial FA core complex dataset was processed in Relion v2. Particles were picked manually from a few micrographs, and used for 2D class averaging with a box size of 390 pixels. The resulting 2D class averages were used to pick particles from all micrographs using template-matching in Relion's autopicker. After 2D classification of the auto-picked particles, selected classes were used to make an initial 3D model in EMAN236. This initial model was used for subsequent 3D classification and refinement in Relion.
Refinement
The map generated from the Falcon II dataset was used during the first round of 3D classification with particles from the Falcon III dataset of FA core complex. The datasets from MRC LMB and eBIC were initially processed separately, to generate separate 3D reconstructions. The pixel size of eBIC data was determined using the 2.35 Å spacing from the gold foil images collected under same imaging conditions as the sample data. The eBIC micrographs were then rescaled to the pixel size of LMB data and CTF was re-estimated, and all datasets were merged.
The FA core complex map was divided into three bodies: body 1 (middle region), body 2 (base region) and body 3 (top region). Bodies 2 and 3 were rotated relative to 1 because body 1 (middle) region appears to be the most rigid (Extended Data Fig. 2f). Sigma angles of 10 and sigma offset of 2 were used during refinement. Multibody refinement13 was continued from the last iteration of consensus refinement. The middle region was best resolved with an overall resolution of 4.4 Å whereas the top and base were ~ 7 Å resolution. To estimate the flexibility in the FA core complex we performed principal component analysis on the optimal orientations of all the bodies for all particle images in the dataset using relion_flex_analyse13. We rendered videos for principal components 1 and 2 as these described ~30% of the variance in the rotations and translations.
Per-particle CTF refinement and beam tilt estimation were performed by dividing the datasets into individual data collection sessions and processing in a bigger box of 586 pixels, followed by further refinement35. The resolution of the maps for the top and base regions of the complete complex were further improved by performing focused classification with signal subtraction14 followed by refinement (Extended Data Fig. 2g-h). The overall resolution is likely limited by heterogeneity. A regularization T-value of 5 was used during 3D refinement to boost the contribution of higher spatial frequencies41, to improve the quality of the map to aid in model building.
The subcomplexes were processed up to 2D classification as there were not enough different views to generate a 3D map.
Local symmetry averaging
CryoEM structure determination by single-particle analysis relies on the reduction of noise through averaging over multiple copies of extremely noisy projection images of individual macromolecular complexes. For symmetric complexes, e.g. for homo-multimers or for icosahedral virus capsids, additional averaging can be performed by imposing point-group symmetry on the reconstruction. Because each projection image of a symmetrical object provides multiple views of the asymmetric unit, compared to asymmetric complexes, the same number of images will yield a better reconstruction, or fewer images are needed to obtain a reconstruction of the same quality. Therefore, point-group symmetry averaging is commonly employed in single-particle analysis refinement programs.
The FA subcomplex described here, does not obey overall point-group symmetry, but still contains multiple, potentially identical, subcomplexes. We call this local symmetry. Averaging over locally symmetric subcomplexes in cryoEM single particle analysis has previously been performed to improve reconstructions after completion of the refinement process, for example on the subunits of triangulation number T > 1 virus capsids42,43. However, local symmetry averaging has the potential to improve the reconstruction at every stage of the iterative refinement process. Better reconstructions during refinement will lead to better alignments, and hence a better final reconstruction. Therefore, we implemented a local symmetry averaging approach inside the relion_refine program. This approach has conceptual similarities to imposing non-crystallographic symmetry (NCS) in X-ray crystallography44.
This new implementation within Relion3.0 allows the user to define an arbitrary number of groups, each with an arbitrary number of assumed identical subcomplexes. For each group, the user provides a mask around one member of the group, as well as 3D transformation matrices (expressed as three Euler angles and three translations in X, Y and Z) in real space to superimpose that member onto each of the other members in the group. This information is expressed in a Relion-style STAR file, which is passed using the --local_symmetry command line option to the relion_refine program. A helper program to find and optimize the 3D transformation matrices, relion_localsym, was also implemented. To minimize artifacts in Fourier-space, the edges of the masks should be kept soft, i.e. they should gradually change from zero to one over multiple real-space pixels.
Our new implementation allows local symmetry averaging in both 3D classifications and 3D refinements, and works with 2D projection images as well as 3D images, such as sub-tomograms. At every step of the expectation-maximization algorithm, local symmetry is applied according to the masks and transformations defined in the STAR file. This symmetrization is performed after the maximization step in real space. As such, the signal-to-noise gain that arises from the additional averaging is not considered when calculating the FSC between the two independent half-reconstructions in 3D auto-refinement, or when calculating the signal from the power of the reconstruction in 3D classification. Therefore, in cases of local symmetry, it may be advantageous to increase the empirical regularization T-value, --tau2_fudge, to account for the expected gain in signal during the refinement. When doing so, care should be taken not to overfit the data by using a too high T-value. Calculation of a final FSC curve using Relion's post-processing program will lead to a realistic resolution estimate. However, care should be taken to use soft-edged masks for the local symmetrization, as the real-space mask operations may lead to artefacts in the Fourier-based resolution assessment.
Another note of caution considers the assumption that all the members of each of the local symmetry groups are identical. Since, by definition, the complex does not obey point group symmetry, this assumption can never be entirely true. At the very least, some of the subcomplex interfaces will be chemically different, whereas in worse case scenarios biologically relevant conformational differences may exist within the members of each group. Imposing (local) symmetry on objects that are not identical will lead to a false impression of similarity in the output reconstruction. Besides minimizing artifacts in Fourier-space, the soft-edges of the local symmetry masks are also relevant here. Local symmetry is applied for all real-space pixels where the mask has values m>0, but the symmetrized map will be calculated as (1-m) times the original reconstruction plus m times the average of the corresponding voxel in all members of the group. Thereby, mask values of m<1 can be used to impose local symmetry only partially.
For the subcomplex reconstruction, masks around each of the symmetric regions were created and filtered to 25 Å with mask extension to 6 pixels and a soft-edge of 10 pixels (Extended Data Fig. 7e). The transformation operator for the three Euler angles and the three translations which relate one symmetric region with the other were calculated using relion_localsym_mpi in real space, followed by 3D refinement using a regularization T-value of 100. This improved the overall appearance of the map (Extended Data Fig. 7f).
Model building
Model building was performed using Coot45,46. All models described were built as polyalanine chains except FANCL (see below).
A homology model for chicken FANCL (residues 1–373) was generated with I-TASSER47 using Drosophila FANCL (PDB 3K1L)15 as a template. The FANCL homology model was rigidly fitted into the EM map in Chimera using ‘fit in map’ tool48 and then flexibly fitted using Jiggle fit in Coot. Densities corresponding to the ELF, URD and RING domains of FANCL were identified in the focused map of the base region, however, we were not able to orient the RING domain unambiguously in the density. The density for the ELF domain was well-defined in the focused map for the top region. There was only weak density for the URD domain and no density for the RING domain in FANCLtop (Extended Data Fig. 2g and Extended Data Fig. 5d). The ELF domain is next to the coiled coil helix of FANCB, in agreement with FANCL crosslinks to the region C-terminal of the coiled coil helix of FANCB (residues 439, 441, 454 and 460) and to the FAAP100 β-propeller (residues 25, 180, 188, 262, 267 and 274). There are several crosslinks between FANCL URD-RING and the substrate recognition module (FANCC, FANCE, FANCF) consistent with its placement within the base of the complex.
The ‘multi-body refinement map’ for the middle region, the map from T=5 regularization, and the map for symmetric subcomplex were used to build the central region of FANCB-FAAP100 de novo by placing idealized helices and strands in Coot, and refining their fits with the real space refine zone tool. In agreement with their role as a scaffold, FANCB and FAAP100 have 64 intermolecular crosslinks and they crosslink to four other subunits.
One of the two β-propellers in the top region was built de novo using the ‘focused map’ for the top region and this model was used to identify a structural homolog in DALI49. The β-propeller of Bardet-Biedl syndrome 1 protein (pdb: 4V0N)50 was the top hit, and this was also a good fit for the other β-propeller. The sequence of this model was changed to polyalanine and used to build all four β-propellers after rigid fitting into the ‘focused map’ for the top or base region.
Based on the crosslinking patterns and secondary structure predictions, we assigned one of each of the pairs of β-propellers, and one helix of each of the coiled coils to FANCB (N-terminal region for β-propeller and residues ~390-429 for the helix) and the other to FAAP100 (N-terminal region for β-propeller and residues ~461-501 for the helix). These assignments agree with the hydrogen deuterium exchange experiments of B-100 vs B-L-100. The sequences of the predicted long helices of FANCB and FAAP100 were used in MARCOIL51,52 for assignment of heptad repeats (Extended Data Fig. 5e). Then, a FANCB-FAAP100 coiled coil model was built in CCbuilder 2.053 using the advanced mode (Extended Data Fig. 5f). This model was then fitted into the map and refined in Coot using real space refinement.
A homology model for chicken FANCG (residues 1–648) was generated in I-TASSER using TTC7B/Hyccin complex (PDB 5DSE) and O-linked GlcNAc transferase (PDB 1W3B) as top two templates. The TPR region of the model (residues 153-432) was fitted into the focused map of the top region by rigid fitting in Chimera followed by Jiggle fit in Coot and refinement in Refmac. Additional helices were added towards the C-terminal region of FANCG using Coot.
A homology model for chicken FANCF (residues 117–343) was built in I-TASSER using the crystal structure of human FANCF C-terminal region (PDB 2IQC)16 as one of the templates. Residues 117-191 and 215-343 were rigidly fitted into the focused map for the base region followed by Jiggle fit and real space refinement in Coot. In addition to the FANCF model, several short, idealized helices were also placed into the base. A crystal structure for human FANCE C-terminal region (PDB 2ILR)54 was available but we could not fit it in the map, presumably due to conformational variation. The C-terminus of FANCC is near FANCE (residues 155-190; Fig. 1d).
The above models were assembled together into models for top (FANCG, β-propellers and long helices of FANCB, FAAP100), middle and base (FANCL, FANCF, β-propellers and long helices of FANCB, FAAP100, unassigned helices) regions. These models were then further refined in Refmac55 and Phenix iteratively56.
The models for FANCBtop, FAAP100top, FANCB-FAAP100, FANCG and FANCLtop built in the complete FA core complex map were rigidly fitted into the subcomplex map and refined in Refmac. All models and maps were visualized and rendered in UCSF Chimera48 or ChimeraX57.
Native mass spectrometry
Native mass spectrometry experiments were carried out on a Q-Exactive Plus UHMR modified to facilitate the transmission of high-energy species and adapted for membrane proteins58–60. The FA core complex at concentration of 9.5 μM was buffer exchanged using Bio-Spin 6 columns (BioRad). Typically, 2–3 μl of sample in 750 mM ammonium acetate were injected, using a 1.2 mm outer diameter, gold-coated borosilicate capillary (Harvard Apparatus). The following parameters were used for protein transmission: capillary voltage 1.2 kV, dessolvation voltage -300 V, source fragmentation 0 V, HCD energy 0 V, HCD pressure 4, EMR on, C-trap entrance lens tune offset 2, injection flatopole 8 V, inter flatopole lens 6 V, and bent flatopole 4 V. Threshold was set to 3. Data were analyzed using Xcalibur 2.2 (Thermo Fisher), Masslynx 4.2 (Waters) and SUMMIT61. The formation of some of the subcomplexes observed may be the result of buffer exchange from 50 mM HEPES pH 8.0, ~500 mM NaCl and 1 mM TCEP into 750 mM ammonium acetate, which is often used in native mass spectrometry to improve resolution and generate subcomplexes to aid structure determination.
Hydrogen-deuterium exchange mass spectrometry (HDX-MS)
Deuterium exchange reactions were initiated by diluting the complexes in D2O (99.8% D2O ACROS, Sigma, UK) to give a final D2O percentage of ~95%. Deuterium labelling was generally carried out at 23 °C at four time points (3 sec, 30 sec, 300 sec and 3000 sec) in triplicate. The labelling reaction was quenched by adding chilled 2.4% v/v formic acid in 2 M guanidinium hydrochloride and immediately frozen in liquid nitrogen. Samples were stored at -80 °C prior to analysis.
The quenched samples were rapidly thawed and subjected to proteolytic cleavage using pepsin followed by reverse-phase HPLC separation. The proteins flowed through an Enzymate BEH immobilized pepsin column, 2.1 x 30 mm, 5 μm (Waters, UK) at 200 μl/min for 2 min, and the resulting peptides were trapped and desalted on a 2.1 x 5 mm C18 trap column (Acquity BEH C18 Van-guard pre-column, 1.7 μm, Waters, UK). Trapped peptides were eluted over 11 min using a 3–43% gradient of acetonitrile in 0.1% v/v formic acid at 40 μl/min on to a reverse phase analytical column (Acquity UPLC BEH C18 column 1.7 μm, 100 mm x 1 mm (Waters, UK)). The LC elute was coupled to a SYNAPT G2-Si HDMS mass spectrometer (Waters, UK) and data were acquired over a m/z of 300 to 2000, using the standard electrospray ionization (ESI) source with lock mass calibration using [Glu1]-fibrino peptide B (50 fmol/μl). The mass spectrometer was operated in ion mobility mode, at a source temperature of 80 °C and a spray voltage of 2.6 kV. Spectra were collected in positive ion mode.
Peptide identification was performed with a non-deuterated sample using MSe to fragment peptides. An identical gradient of increasing acetonitrile in 0.1% v/v formic acid over 11 min was used and the resulting MSe data were analyzed using Protein Lynx Global Server software (Waters, UK) with an MS tolerance of 5 ppm.
Mass analysis of the peptide centroids was performed using DynamX sotware (Waters, UK). Only peptides with a score >6.4 were considered. All peptides (deuterated and non-deuterated) were manually verified at every time point for the correct charge state, presence of overlapping peptides, and correct retention time. Deuterium incorporation was not corrected for back-exchange and represents relative, rather than absolute changes in deuterium levels. Changes in H/D amide exchange in any peptide may be due to a single amide or several amides within that peptide.
Crosslinking mass spectrometry of purified FA core complex
The purified FA core complex (50 mM HEPES pH 8.0, ~500 mM NaCl and 1 mM TCEP) at a concentration of 7.6 μM was crosslinked with 100-fold molar ratio of disulfosuccinimidyl suberate (BS3) for 2 h on ice and the reaction was quenched with 50 mM NH4HCO3 for 30 min at room temperature. The crosslinked samples were cold acetone precipitated and resuspended in 8 M urea and 100 mM NH4HCO3. Peptides were reduced with 10 mM DTT and alkylated with 50 mM iodoacetamide. Following alkylation, proteins were digested with Lys-C (Pierce) at an enzyme-to-substrate ratio of 1:100 for 4 h at 22 °C and, after diluting the urea to 1.5 M with 100 mM NH4HCO3 solution, further digestion with trypsin (Pierce) at an enzyme-to-substrate ratio of 1:20.
Digested peptides were eluted from StageTips and split into two, for parallel crosslink enrichment by strong cation exchange chromatography (SCX) and size exclusion chromatography (peptideSEC), and were dried in a vacuum concentrator (Eppendorf, Germany). For SCX, eluted peptides were dissolved in mobile phase A (30 % acetonitrile (v/v), 10 mM KH2PO4, pH 3) prior to strong cation exchange chromatography (100 x 2.1 mm Poly Sulfoethyl A column; Poly LC, Colombia, MD, USA). The separation of the digest used a non-linear gradient62 into mobile phase B (30 % acetonitrile (v/v), 10 mM KH2PO4, pH 3, 1 M KCl) at a flow rate of 200 μl/min. Ten 1 min fractions in the high-salt range were collected and cleaned by StageTips, eluted and dried for subsequent LC-MS/MS analysis. For peptideSEC, peptides were fractionated on an ÄKTA Pure system (GE Healthcare) using a Superdex Peptide 3.2/300 (GE Healthcare) at a flow rate of 10 μl/min using 30% (v/v) acetonitrile and 0.1% (v/v) trifluoroacetic acid as mobile phase. Five 50 μl fractions were collected and dried for subsequent LC-MS/MS analysis.
Samples for analysis were resuspended in 0.1% v/v formic acid, 1.6% v/v acetonitrile. LC-MS/MS analysis was conducted in duplicate for SEC fractions and triplicate for SCX fractions, performed on an Orbitrap Fusion Lumos Tribrid mass spectrometer (Thermo Fisher Scientific, Germany) coupled on-line with an Ultimate 3000 RSLCnano system (Dionex, Thermo Fisher Scientific, Germany). The sample was separated and ionized by a 50 cm EASY-Spray column (Thermo Fisher Scientific). Mobile phase A consisted of 0.1% (v/v) formic acid and mobile phase B of 80% v/v acetonitrile with 0.1% v/v formic acid. Flow-rate of 0.3 μl/min using gradients optimized for each chromatographic fraction from offline fractionation ranging from 2% mobile phase B to 45% mobile phase B over 90 min, followed by a linear increase to 55% and 95% mobile phase B in 2.5 min, respectively. The MS data were acquired in data-dependent mode using the top-speed setting with a three second cycle time. For every cycle, the full scan mass spectrum was recorded in the Orbitrap at a resolution of 120,000 in the range of 400 to 1,600 m/z. Ions with a precursor charge state between 3+ and 7+ were isolated and fragmented. Fragmentation by higher-energy collisional dissociation (HCD) employed a decision tree logic with optimized collision energies63. The fragmentation spectra were then recorded in the Orbitrap with a resolution of 50,000. Dynamic exclusion was enabled with single repeat count and 60-second exclusion duration.
A recalibration of the precursor m/z was conducted based on high-confidence (<1% FDR) linear peptide identifications64. The recalibrated peak lists were searched against the sequences and the reversed sequences (as decoys) of crosslinked peptides using the Xi software suite (version 1.6.746)65 (https://github.com/Rappsilber-Laboratory/XiSearch) for identification. The following parameters were applied for the search: MS1 accuracy = 3 ppm; MS2 accuracy = 10 ppm; enzyme = trypsin (with full tryptic specificity) allowing up to four missed cleavages; crosslinker = BS3 with an assumed reaction specificity for lysine, serine, threonine, tyrosine and protein N termini); fixed modifications = carbamidomethylation on cysteine; variable modifications = oxidation on methionine, hydrolyzed / aminolyzed BS3 from reaction with ammonia or water on a free crosslinker end. The identified candidates were filtered to 1% FDR on link level using XiFDR version 1.1.26.5866.
Pull down assay
The purified subcomplexes A-G-B-L-100 and C-E-F (StrepII tag cleaved by 3C protease) were mixed in a 1:1 molar ratio at concentrations of 1.4 μM each, for 1 h at 4 °C. A 20 μl reaction was incubated with 15 μl of Streptactin beads (GE healthcare) equilibrated in 50 mM HEPES pH 8.0, 300 mM NaCl and 1 mM TCEP. The flow-through was collected (unbound fraction) and the beads were washed three times with equilibration buffer. The unbound and bound fractions were analyzed on SDS-PAGE.
Extended Data
Extended Data Fig. 1. Recombinant FA core complex activity.
a Ubiquitination assay analyzed by western blot with anti-HA antibody to detect HA-tagged ubiquitin. The migration positions of monoubiquitinated FANCD2 and FANCI are indicated but FANCI is not substantially modified. b Ubiquitination assay analyzed by Coomassie-stained SDS PAGE to show specific monoubiquitination of FANCD2 K563 by recombinant FA core complex. Wild-type (WT), FANCD2K563R and FANCIK525R (KR) were analyzed. A native FA core complex purified from chicken DT40 cells monoubiquinates FANCD2 but does not efficiently monoubiquitinate FANCI 6. Therefore, the purified recombinant complex faithfully recapitulates the properties of the native chicken complex. Notably, a purified human complex also did not efficiently monoubiquitinate FANCI, although it did efficiently monoubiquitinate FANCD2 11. The asymmetry in the FA core complex (see below) reflects this asymmetry in its activity on FANCI-FANCD2. An additional factor or post-translational modification may be required for activation of FANCI monoubiquitination. The ubiquitination assays were repeated at least two times independently with similar results. For gel source data, see Supplementary Fig. 1.
Extended Data Fig. 2. CryoEM reconstruction of FA core complex, multi-body refinement and assessment of 3D reconstructions after focused refinement.
a Representative raw micrograph of FA core complex. b Overall 3D reconstruction of the FA core complex. c Angular distribution density plot of particles used in the 3D reconstruction of the FA core complex. Every point is a particle orientation and the color scale represents the normalized density of views around this point. The color scale runs from 0 (low, blue) to 0.00026 (high, red). The efficiency of orientation distribution67, EOD, was 0.79. d Estimated local resolution map for FA core complex. e Fourier shell correlation plot for gold standard refinement. f Multibody refinement of the FA core complex using three masks (Body 1, Body 2 and Body 3) shown in purple, pink and cyan. The motions are shown in Supplementary Videos 1 and 2. g Local resolution maps for reconstructions of top, middle and base regions of FA core complex. The middle region did not substantially change between multi-body refinement and particle subtraction followed by focused classification. The resolution of the base and top regions improved after particle subtraction and focused classification and refinement. h Fourier shell correlation plot for gold standard refinements. i Representative density for β-strand and α-helical regions. FANCB-FAAP100 is in the middle region which is better defined than more peripheral regions, including FANCF.
Extended Data Fig. 3. Subunit assignment and arrangement in FA core complex.
a–c Complexes lacking specific subunits were purified and analyzed by cryoEM. a Major 2D class averages identified for subcomplexes, compared with those from FA core complex (A-G-B-L-100-C-E-F). Cartoons are shown to depict the subunits visible in the class averages. The symmetric subcomplex identified in the FA core complex preparation is indicated (sym). The A-G-B-L-100 complex (lacking the substrate recognition module) has similar symmetric 2D classes. Native MS revealed a non-uniform subunit stoichiometry. Thus it is likely that the asymmetric assembly represents the complete FA core complex, while the symmetric structure is a subcomplex that co-purifies with the intact complex. The 2D class average of a complex lacking FANCA (G-B-L-100-C-E-F) appeared similar to the complete FA core complex with no obvious missing density. FANCG is likely the partially disordered arm that extends from the central part of the complex since this was missing when FANCG was not present in the complex. b Coomassie-stained SDS-PAGE analysis of purified subcomplexes. Asterisks indicate contaminant proteins. FANCA did not co-purify with the A-B-L-100 complex but its migration position is indicated on the gel. The purifications were repeated at least two times independently with similar results. For gel source data, see Supplementary Fig. 1. c Cartoon of FA core complex with subunits labeled. d Native mass spectrum of recombinant FA core complex showing masses and subunit composition of assigned peak series. The FA core complex dissociated into subcomplexes during ionization and these species were detected by mass spectrometry. Computational analyses then revealed the proteins present in each of the peaks. The standard deviation in fitting the identified peaks to the charge series is given as the ± error in the measured mass for a given single measurement. This is the error in the fit and not the error in the mass measurement, which is likely an order of magnitude higher due to solvation/adduct effects, heterogeneous post-translational modifications, etc. Hence, the error gives a rough measure of the accuracy of peak assignment, which is impacted by the broadness, symmetry and signal to noise ratio of each peak. e Molecular masses of FA core complex subunits. The expected and measured masses are given, along with phosphorylation sites detected by mass spectrometry. Native MS was repeated three times with similar results.
Extended Data Fig. 4. Crosslinking mass spectrometry.
a Crosslinking mass spectrometry revealed 834 crosslinks (1% FDR) between residues that are in close proximity. Intermolecular crosslinks are colored in green; intramolecular crosslinks, red; predicted α-helices, orange; predicted β-strands, purple. Asterisks on FANCB mark missense mutations from the Fanconi Anemia Mutation Database (http://www2.rockefeller.edu/fanconi/) including L43S in a predicted beta strand, P230S at the N-terminus of a predicted helix, and L329P in the middle of a predicted beta strand, all in the beta propeller; and L676P predicted to disrupt an alpha helix in the C-terminal dimerization domain. b–d Validation of crosslinking of FA core complex. Crosslinks were mapped onto a homology model of chicken FANCL (b), human FANCF (c), and human FANCE (d). All intramolecular crosslinks within a domain are consistent with the maximum crosslinker length (30 Å between the two Cα). Crosslinks between the domains in FANCL are not consistent with the domain arrangement in the crystal structure of FANCL because there is flexibility or changes in the orientation between the domains in the FA core complex. There are two different conformations of FANCL in the structure – FANCLbase is fully ordered, whereas in FANCLtop only the ELF domain is visible. Mapping the crosslinks onto FANCL from the base (constrained, with all three domains visible) reveals that the distances for some crosslinks between domains are too far. Therefore, these crosslinks are not consistent with the FANCL conformation in the base. In contrast, for FANCL in the top where only the ELF domain is ordered, it is likely that the URD and RING domains are conformationally flexible. Since the URD and RING domains cannot be modelled for FANCLtop, it is not possible to validate these inter-domain crosslinks.
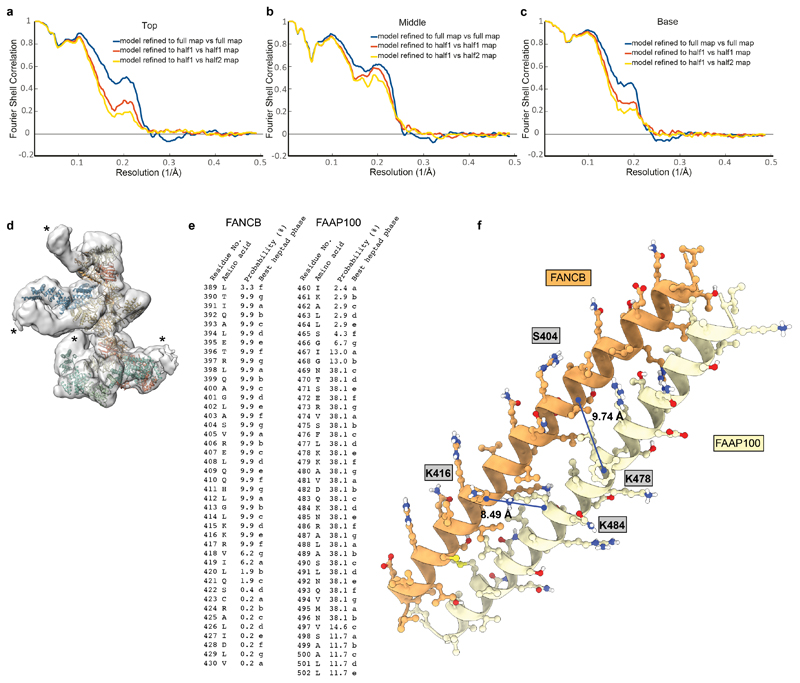
Extended Data Fig. 5. Assessment of model fit in maps and modelling of coiled coils.
a–c Fourier shell correlation (FSC) plots of maps vs model for top (a), middle (b) and base (c) regions. d Low-resolution cryoEM map of FA core complex (transparent surface) with models placed in the map. Asterisks represent density that was not visible at high resolution and was not interpreted. This may represent FANCA and additional parts of the substrate recognition module. e MARCOIL51 prediction for the best heptad phase in the long helices of FANCB and FAAP100. f Predicted coiled-coil model by CCbuilder 2.053 for the FANCB and FAAP100 long helices. Crosslinks detected for these helices in the FA core complex crosslinking mass spectrometry are indicated with blue lines.
Extended Data Fig. 6. Hydrogen deuterium exchange mass spectrometry on FANCB and FAAP100.
a–d Difference plots for FANCB showing peptides that are protected (negative) or exposed (positive) upon binding of additional subunit(s) for (a) B-L-100 vs B-100, (b) G-B-L-100 vs. B-L-100, (c) A-G-B-L-100 vs. G-B-L-100, and (d) FA core complex vs. G-B-L-100. Exchange of hydrogens in FANCB residues 429–448 was protected after interaction with FANCL, consistent with FANCL being located next to the coiled coil. e-h Difference plots for FAAP100 showing peptides that are protected (negative) or exposed (positive) upon binding of additional subunit(s) for (e) B-L-100 vs B-100, (f) G-B-L-100 vs. B-L-100, (g) A-G-B-L-100 vs. G-B-L-100, and (h) FA core complex vs. G-B-L-100. Exchange of hydrogens in FAAP100 residues 448–464 was protected after interaction with FANCL, consistent with FANCL being located next to the coiled coil. For difference plots, triplicate data from four independent color-coded time-points are shown. The significance threshold is indicated by dashed lines. Grey shading indicates the standard deviation of all charge states and replicates per peptide. Sequence coverage is shown in Supplementary Data.
Extended Data Fig. 7. 3D reconstruction of symmetric FA subcomplex and local symmetry refinement.
a Overall 3D reconstruction of the symmetric FA subcomplex. b Angular distribution plot of particles used in the 3D reconstruction of the symmetric FA subcomplex. Every point is a particle orientation and the color scale represents the normalized density of views around this point. The color scale runs from 0 (low, blue) to 0.00026 (high, red). The efficiency of orientation distribution67, EOD, was 0.65. c Estimated local resolution map for symmetric FA subcomplex. d Fourier shell correlation plot for gold standard refinement. e Local symmetry pipeline for reconstruction of the symmetric FA subcomplex (see Methods). This reconstruction could not be improved with C2 symmetry, likely because of local flexibility. f Fourier shell correlation plot for gold standard refinement shown for the subcomplex reconstruction before and after local symmetry refinement. The circular panels show representative densities before and after local symmetry refinement.
Extended Data Fig. 8. Model for FA subcomplex.
a Model of FA subcomplex shown as cartoon representations of subunits fit into the cryoEM map. b Model of FA subcomplex shown as a surface representation of the combined models. Two views are shown down the two-fold symmetric axis. c Comparison of FA subcomplex and complete FA core complex in the same orientations. Both models are shown in cartoon representation. Subunits are colored as in Fig. 1e. d Modelling of a fully symmetric FA core complex containing two copies of every subunit. (Left) The second copy of FANCG (cartoon) from the FA subcomplex was modelled onto the structure of the FA core complex (surface representation). This second FANCG clashes with the FANCB beta propeller in the base (asterisks). Thus, it is likely that upon binding of the substrate recognition module, rearrangement of the beta propellers of FANCB and FAAP100 prevents binding of a second copy of FANCG. (Right) A second copy of substrate recognition module (FANCC-FANCE-FANCF; cartoon) is modelled in the top region of the FA core complex by combining the models of FANCC-FANCE-FANCF and FANCLbase from FA core complex followed by superimposing FANCLbase on FANCLtop. There is a clash (asterisks) between the modeled FANCL (cartoon) and FAAP100 beta-propeller (surface representation). These data suggest that a fully symmetric complex does not readily form. In agreement with this, there was no evidence for any classes containing two copies of C-E-F in any of our EM analyses of the FA core complex. e-f The symmetric FA subcomplex (A-G-B-L-100) does not readily associate with purified substrate recognition module (C-E-F) to form the asymmetric FA core complex. e 2D class averages of A-G-B-L-100 mixed with C-E-F in comparison to complete FA core complex, FA subcomplex and A-G-B-L-100 subcomplex. A-G-B-L-100 was mixed with C-E-F in molar ratios of 1:1 and 1:2 for 1 h at 4 °C before cryo-plunging. Only the symmetric A-G-B-L-100 subcomplex was observed and there was no additional density for C-E-F. Panels for FA core complex, subcomplex and A-G-B-L-100 are replicated from Extended Data Fig. 3a. f Pull-down assay of C-E-F using tagged A-G-B-L-100 (StrepII tagged). Left panel shows the Coomassie blue-stained gel of purified C-E-F (with StrepII tag, after 3C cleavage of tag and after removal of 3C protease). Tagged A-G-B-L-100 was immobilized on Streptactin resin and incubated with purified C-E-F at a 1:1 molar ratio. After washing, only a small amount of C-E-F remains bound to the beads. Negative controls (A-G-B-L-100 only and C-E-F only) are shown in the middle panel. Asterisk indicates a contaminant protein. The pulldown experiment was repeated two times independently with similar results. Since C-E-F doesn’t efficiently bind A-G-B-L-100, these experiments suggest that it is unlikely that these species are in equilibrium in solution. Previous genetic and biochemical data show that FANCC, FANCE and FANCF are important for monoubiquitination of the FANCI-FANCD2 substrate. Together, these data provide evidence that the asymmetric complex is the relevant, functional, physiological entity.
Extended Data Fig. 9. Structural comparison of E3 ligases.
a There is a strong precedence for dimerization of RING/U-box domain E3 ubiquitin ligases68–70. RING/U-box E3s exist both as homo- and hetero-dimeric complexes, e.g. Rad18-Rad18, CHIP-CHIP, RNF8-RNF8, BRCA1-BARD1, RING1b/BMI1 and Hdm2/Hdmx17–21,71. Structures of homo- and hetero-dimeric RING/U-box E3 ligases are shown here with the RING/U-box in orange. Surprisingly, these E3s display functional and structural asymmetry: in all the dimers listed above, only one protomer binds to an E2 enzyme. The homodimeric CHIP E3 ligase has a strikingly asymmetric structure that clearly demonstrates why only one U-box binds E2 enzyme19. The FANCL RING subunit is also an asymmetric dimer within the FA core complex and it is possible that only one of these binds E2. However, unlike the smaller E3s, the FANCL RING fingers are not near to each other. Together, this suggests that asymmetric dimerization may be a general feature of RING E3s. b Comparison of FA core complex to cullin-RING ubiquitin ligases (CRLs). Many large complexes are predominantly helical suggesting that alpha helices are commonly used as building blocks for complexes. In addition, beta propellers often mediate protein-protein interactions. The CRL complexes and FA core complex are long and extended with substrate-recognition (green), scaffold (yellow) and RING (orange) subunits residing in three different regions of the structure. However, the structural details of these complexes differ. Interestingly, the activities of some multisubunit RING-containing E3 ligases including APC/C and CRL complexes are stimulated by dimerization22,23. Thus, dimerization may underpin physiological ubiquitination activity in many E3 ligases. c-d Ubiquitin discharge assay where free lysine is used instead of the FANCI-FANCD2 substrate. In these experiments, the FA core complex is incubated with E1, E2, ubiquitin and free lysine. If FANCL is active without a substrate, ubiquitin will be conjugated to lysine, resulting in a shift in its molecular weight. If, on the other hand, substrate binding is required to activate the E3 ligase activity (e.g. through allosteric changes), this will not occur. Coomassie gels of reaction products were run in non-reducing (c) and reducing (100 mM DTT) conditions (d). Ubiquitin is transferred to free lysine as shown by the increase in molecular weight of ubiquitin as well as a decrease in intensity of the E2-ubiquitin band when compared to the lane containing no free lysine. Thus, substrate binding is not required for activity. Reducing conditions do not eliminate the UBE2T-ubiquitin conjugate, as previously shown72. Additionally, DNA is not required for FA core complex E3 ligase activity on free lysines, suggesting that DNA activates the substrate, not the E3. The ubiquitin discharge assays were repeated three times independently with similar results (c-d). e Distributions of patient mutations are indicated on the FA core complex by heatmap coloring of subunits and in percentage. f Ubiquitination assay using several subcomplexes (Extended Data Fig. 3b) and the full FA core complex, analyzed by western blot with anti-HA antibody to detect HA-tagged ubiquitin. The migration positions of monoubiquitinated FANCD2 and FANCI are indicated but FANCI is not substantially modified as in Extended Data Fig 1a. All complexes have similar activities but isolated FANCL is less active. This assay was repeated at least two times independently with similar results. For gel source data, see Supplementary Fig. 1.
Extended Data Table 1. Cryo-EM data collection, refinement and validation statistics.
| FA core complex (EMD-10290: consensus, EMD-10291: top, EMD-10292: middle, EMD-10293: base) (PDB 6SRI) |
Subcomplex (EMD-10294) (PDB 6SRS) |
|
|---|---|---|
| Data collection and processing | ||
| Magnification | 75,000 X | 75,000 X |
| Voltage (keV) | 300 | 300 |
| Electron exposure (e−/Å2) | 40 | 40 |
| Defocus range (μm) | -1.8 to -4.0 | -1.8 to -4.0 |
| Pixel size (Å) | 1.040 (LMB) | 1.040 (LMB) |
| 1.085 (eBIC) | 1.085 (eBIC) | |
| Symmetry imposed | CI | CI |
| Initial particle images (no.) | 1,947,765 | 1,947,765 |
| Final particle images (no.) | 169,000 | 49,423 |
| Map resolution (Å) | 4.2 (consensus), 4.5 (top), 4.4 (middle), 4.9 (base) |
4.6 |
| FSC threshold | 0.143 | 0.143 |
| Map resolution range (Å) | 4.2 to > 10 | 4.6 to > 10 |
| Refinement | ||
| Initial model used | De novo modelling and homology modelling | De novo modelling and homology modelling |
| Model resolution range (Å) | 4.5 (top), 4.4 (middle), 4.9 (base) |
4.6 |
| FSC threshold | 0.143 | 0.143 |
| Model resolution range (Å) | n/a | n/a |
| Map sharpening B factor (Å2) | -149 (consensus) | -213 |
| -198 (top) | ||
| -190 (middle) | ||
| -177 (bottom) | ||
| Model composition | ||
| Non-hydrogen atoms | 15,309 | 12,424 |
| Protein residues | 3,827 | 3,106 |
| Ligands | 0 | 0 |
| B factors (Å2) | ||
| Protein | not estimated | not estimated |
| Ligands | ||
| R.m.s. deviations | ||
| Bond lengths (Å) | 0.23 | 0.22 |
| Bond angles (°) | 0.48 | 0.50 |
| Validation | ||
| MolProbity score | 1.44 | 1.12 |
| Clashscore | 3 | 3 |
| Poor rotamers (%) | 0 | 0 |
| Ramachandran plot | ||
| Favored (%) | 95.95 | 98.88 |
| Allowed (%) | 3.56 | 1.12 |
| Disallowed (%) | 0.49 | 0 |
Extended Data Table 2. Features of individual FA core complex subunits and structural models.
| Protein | Length (aa) | Sequence features | Models generated in this study | Maps used for modelling | Sequence identity / similarity between Gallus gallus and Homo sapiens (%) |
|---|---|---|---|---|---|
| FANCA | 1,421 | α-helical | N/A | N/A | 49.1 / 65.6 |
| FANCB | 867 | Possible (β-propeller, plus α-helical | De novo modelling of SSEs* | Focused map for top (EMD-10291), middle (EMD-10292), and base regions (EMD-10293), consensus map with T=5 (EMD-10290) and subcomplex map (EMD-10294) | 44.1 / 63.2 |
| FANCC | 559 | α-helical | SSEs placed in maps, not assigned | Focused map for base (EMD-10293) | 49.0 / 65.1 |
| FANCE | 520 | α-helical Crystal structure of human orthologue (C-terminal half; PDB 2ILR) | SSEs placed in maps, not assigned | Focused map for base (EMD-10293) | 40.9 / 54.1 |
| FANCF | 350 | α-helical Crystal structure of human orthologue (C-terminal half; PDB 2IQC) | C-terminal region from homology model based on PDB 2IQC | Focused map for base (EMD-10293) | 38.5 / 51.6 |
| FANCG | 648 | α-helical (TPR) | TPR domain from homology model (I- TASSER) | Focused map for top region (EMD-10291) | 37.3 / 52.9 |
| FANCL | 373 | ELF, URD and RING domains. Crystal structures of human (central domain, PDB 3ZQS; RING domain, PDB 4CCG) and Drosophila orthologues (full-length, PDB 3K1L) | ELF domain in FANCLtop. ELF, URD and RING domains in FANCLbase. Homology models based on PDB 3K1L | Focused map for top (EMD-10291) and base (EMD-10293) | 69.9 / 82.7 |
| FAAP100 | 888 | Possible β-propeller, plus α-helical | De novo modelling of SSEs | Focused map for top (EMD-10291), middle (EMD-10292), and base regions (EMD-10293), consensus map with T=5 (EMD-10290) and subcomplex map (EMD-10294) | 55.6 / 68.7 |
Footnote: * SSE, secondary structure elements
Supplementary Material
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Acknowledgements
We are grateful to Takanori Nakane, Jasenko Zivanov, Clinton Lau, Andrew Carter, Paul Emsley, Garib Murshudov, Duccio Malinverni and Madan Babu for advice and discussions; Katerina Naydenova, Balaji Santhanam, Gillian Dornan, Douglas Briant, Ana Casañal, Ananth Kumar, Manuel Carminati, Ann Kelley and members of the Passmore lab for assistance; Giuseppe Cannone, Christos Savva and the LMB EM facility, Jake Grimmett and Toby Darling (LMB scientific computation), and Jianguo Shi (baculovirus) for support. This work was supported by the Medical Research Council, as part of United Kingdom Research and Innovation, MRC file reference number MC_U105192715 (L.A.P); Deutsche Forschungsgemeinschaft (DFG, 329673113) (J.R.); the Wellcome Trust through a Senior Research Fellowship to J.R. (103139); and the European Research Council grant number 695511-ENABLE (C.V.R). The Wellcome Centre for Cell Biology is supported by core funding from the Wellcome Trust (203149). We acknowledge Diamond Light Source for access to eBIC (proposals EM18091 and EM17434) funded by the Wellcome Trust, MRC, and Biotechnology and Biological Sciences Research Council.
Footnotes
Data availability
CryoEM maps generated during this study have been deposited in the Electron Microscopy Data Bank (EMDB) with accession codes EMD-10290 (FA core complex consensus), EMD-10291 (focused classification top region), EMD-10292 (focused classification middle region), EMD-10293 (focused classification base region) and EMD-10294 (subcomplex). Models generated during this study have been deposited in the protein databank (PDB) with accession codes 6SRI (FA core complex) and 6SRS (subcomplex). Native MS data is available from figshare with accession code: 10.6084/m9.figshare.9692192. Crosslinking MS data has been deposited in the PRIDE database with accession code PXD014282. All other data are available from the authors upon reasonable request.
Author contributions: S.S., E.R. and P.A. designed protein expression and purification schemes, performed ubiquitination assays, performed cryoEM, 3D reconstruction and modelling; S.S. and D.C. performed native MS; F.O’R. and G.D. performed XL-MS; S.M. performed HDX-MS; S.H. and S.H.W.S. developed the local symmetry algorithm; C.H.H., C.J.R. and L.A.P. collected cryoEM data; L.A.P., C.V.R, M.S., J.R., S.H.W.S., K.J.P. supervised the research; L.A.P. conceived the project; and S.S., P.A. and L.A.P. wrote the paper with contributions from all authors.
Reprints and permissions information is available at www.nature.com/reprints. Readers are welcome to comment on the online version of the paper.
The authors declare no competing interests.
References
- 1.Crossan GP, Patel KJ. The Fanconi anaemia pathway orchestrates incisions at sites of crosslinked DNA. J Pathol. 2012;226:326–337. doi: 10.1002/path.3002. [DOI] [PubMed] [Google Scholar]
- 2.Schlacher K, Wu H, Jasin M. A distinct replication fork protection pathway connects Fanconi anemia tumor suppressors to RAD51-BRCA1/2. Cancer Cell. 2012;22:106–116. doi: 10.1016/j.ccr.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nalepa G, Clapp DW. Fanconi anaemia and cancer: an intricate relationship. Nat Rev Cancer. 2018;18:168–185. doi: 10.1038/nrc.2017.116. [DOI] [PubMed] [Google Scholar]
- 4.Knipscheer P, et al. The Fanconi anemia pathway promotes replication-dependent DNA interstrand cross-link repair. Science. 2009;326:1698–1701. doi: 10.1126/science.1182372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smogorzewska A, et al. Identification of the FANCI protein, a monoubiquitinated FANCD2 paralog required for DNA repair. Cell. 2007;129:289–301. doi: 10.1016/j.cell.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rajendra E, et al. The genetic and biochemical basis of FANCD2 monoubiquitination. Mol Cell. 2014;54:858–869. doi: 10.1016/j.molcel.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia-Higuera I, et al. Interaction of the fanconi anemia proteins and BRCA1 in a common pathway. Molecular Cell. 2001;7:249–262. doi: 10.1016/S1097-2765(01)00173-3. [DOI] [PubMed] [Google Scholar]
- 8.Huang Y, et al. Modularized functions of the Fanconi anemia core complex. Cell Rep. 2014;7:1849–1857. doi: 10.1016/j.celrep.2014.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swuec P, et al. The FA Core Complex Contains a Homo-dimeric Catalytic Module for the Symmetric Mono-ubiquitination of FANCI-FANCD2. Cell Rep. 2017;18:611–623. doi: 10.1016/j.celrep.2016.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pace P, et al. FANCE: the link between Fanconi anaemia complex assembly and activity. EMBO J. 2002;21:3414–3423. doi: 10.1093/emboj/cdf355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Twest S, et al. Mechanism of Ubiquitination and Deubiquitination in the Fanconi Anemia Pathway. Mol Cell. 2017;65:247–259. doi: 10.1016/j.molcel.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Walden H, Deans AJ. The Fanconi anemia DNA repair pathway: structural and functional insights into a complex disorder. Annu Rev Biophys. 2014;43:257–278. doi: 10.1146/annurev-biophys-051013-022737. [DOI] [PubMed] [Google Scholar]
- 13.Nakane T, Kimanius D, Lindahl E, Scheres SH. Characterisation of molecular motions in cryo-EM single-particle data by multi-body refinement in RELION. Elife. 2018;7 doi: 10.7554/eLife.36861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bai XC, Rajendra E, Yang G, Shi Y, Scheres SH. Sampling the conformational space of the catalytic subunit of human gamma-secretase. Elife. 2015;4 doi: 10.7554/eLife.11182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cole AR, Lewis LPC, Walden H. The structure of the catalytic subunit FANCL of the Fanconi anemia core complex. Nature Structural & Molecular Biology. 2010;17:294–U254. doi: 10.1038/nsmb.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kowal P, Gurtan AM, Stuckert P, D'Andrea AD, Ellenberger T. Structural determinants of human FANCF protein that function in the assembly of a DNA damage signaling complex. J Biol Chem. 2007;282:2047–2055. doi: 10.1074/jbc.M608356200. [DOI] [PubMed] [Google Scholar]
- 17.Huang A, et al. Symmetry and asymmetry of the RING-RING dimer of Rad18. J Mol Biol. 2011;410:424–435. doi: 10.1016/j.jmb.2011.04.051. [DOI] [PubMed] [Google Scholar]
- 18.Mattiroli F, et al. RNF168 ubiquitinates K13-15 on H2A/H2AX to drive DNA damage signaling. Cell. 2012;150:1182–1195. doi: 10.1016/j.cell.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Zhang M, et al. Chaperoned ubiquitylation--crystal structures of the CHIP U box E3 ubiquitin ligase and a CHIP-Ubc13-Uev1a complex. Mol Cell. 2005;20:525–538. doi: 10.1016/j.molcel.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 20.Brzovic PS, Rajagopal P, Hoyt DW, King MC, Klevit RE. Structure of a BRCA1-BARD1 heterodimeric RING-RING complex. Nat Struct Biol. 2001;8:833–837. doi: 10.1038/nsb1001-833. [DOI] [PubMed] [Google Scholar]
- 21.Buchwald G, et al. Structure and E3-ligase activity of the Ring-Ring complex of polycomb proteins Bmi1 and Ring1b. EMBO J. 2006;25:2465–2474. doi: 10.1038/sj.emboj.7601144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Passmore LA, et al. Structural analysis of the anaphase-promoting complex reveals multiple active sites and insights into polyubiquitylation. Mol Cell. 2005;20:855–866. doi: 10.1016/j.molcel.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Tang X, et al. Suprafacial orientation of the SCFCdc4 dimer accommodates multiple geometries for substrate ubiquitination. Cell. 2007;129:1165–1176. doi: 10.1016/j.cell.2007.04.042. [DOI] [PubMed] [Google Scholar]
- 24.Gordon SM, Alon N, Buchwald M. FANCC, FANCE, and FANCD2 form a ternary complex essential to the integrity of the Fanconi anemia DNA damage response pathway. J Biol Chem. 2005;280:36118–36125. doi: 10.1074/jbc.M507758200. [DOI] [PubMed] [Google Scholar]
- 25.Polito D, et al. The carboxyl terminus of FANCE recruits FANCD2 to the Fanconi Anemia (FA) E3 ligase complex to promote the FA DNA repair pathway. J Biol Chem. 2014;289:7003–7010. doi: 10.1074/jbc.M113.533976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zimmerman ES, Schulman BA, Zheng N. Structural assembly of cullin-RING ubiquitin ligase complexes. Curr Opin Struct Biol. 2010;20:714–721. doi: 10.1016/j.sbi.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neveling K, Endt D, Hoehn H, Schindler D. Genotype-phenotype correlations in Fanconi anemia. Mutat Res. 2009;668:73–91. doi: 10.1016/j.mrfmmm.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 28.Sari D, et al. The MultiBac Baculovirus/Insect Cell Expression Vector System for Producing Complex Protein Biologics. Adv Exp Med Biol. 2016;896:199–215. doi: 10.1007/978-3-319-27216-0_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weissmann F, et al. biGBac enables rapid gene assembly for the expression of large multisubunit protein complexes. Proc Natl Acad Sci U S A. 2016;113:E2564–2569. doi: 10.1073/pnas.1604935113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hill CH, et al. Activation of the Endonuclease that Defines mRNA 3' Ends Requires Incorporation into an 8-Subunit Core Cleavage and Polyadenylation Factor Complex. Mol Cell. 2019 doi: 10.1016/j.molcel.2018.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sato K, Toda K, Ishiai M, Takata M, Kurumizaka H. DNA robustly stimulates FANCD2 monoubiquitylation in the complex with FANCI. Nucleic Acids Res. 2012;40:4553–4561. doi: 10.1093/nar/gks053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Russo CJ, Passmore LA. Electron microscopy: Ultrastable gold substrates for electron cryomicroscopy. Science. 2014;346:1377–1380. doi: 10.1126/science.1259530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scheres SH. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J Struct Biol. 2012;180:519–530. doi: 10.1016/j.jsb.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fernandez-Leiro R, Scheres SHW. A pipeline approach to single-particle processing in RELION. Acta Crystallogr D Struct Biol. 2017;73:496–502. doi: 10.1107/S2059798316019276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zivanov J, et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. Elife. 2018;7 doi: 10.7554/eLife.42166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang G, et al. EMAN2: an extensible image processing suite for electron microscopy. J Struct Biol. 2007;157:38–46. doi: 10.1016/j.jsb.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 37.Zheng SQ, et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat Methods. 2017;14:331–332. doi: 10.1038/nmeth.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang K. Gctf: Real-time CTF determination and correction. J Struct Biol. 2016;193:1–12. doi: 10.1016/j.jsb.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scheres SH. A Bayesian view on cryo-EM structure determination. J Mol Biol. 2012;415:406–418. doi: 10.1016/j.jmb.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kucukelbir A, Sigworth FJ, Tagare HD. Quantifying the local resolution of cryo-EM density maps. Nat Methods. 2014;11:63–65. doi: 10.1038/nmeth.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garcia-Nafria J, Lee Y, Bai X, Carpenter B, Tate CG. Cryo-EM structure of the adenosine A2A receptor coupled to an engineered heterotrimeric G protein. Elife. 2018;7 doi: 10.7554/eLife.35946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stewart PL, Burnett RM, Cyrklaff M, Fuller SD. Image reconstruction reveals the complex molecular organization of adenovirus. Cell. 1991;67:145–154. doi: 10.1016/0092-8674(91)90578-m. [DOI] [PubMed] [Google Scholar]
- 43.He J, Schmid MF, Zhou ZH, Rixon F, Chiu W. Finding and using local symmetry in identifying lower domain movements in hexon subunits of the herpes simplex virus type 1 B capsid. J Mol Biol. 2001;309:903–914. doi: 10.1006/jmbi.2001.4711. [DOI] [PubMed] [Google Scholar]
- 44.Rossmann MG, Blow DM. Detection of Sub-Units within Crystallographic Asymmetric Unit. Acta Crystallogr. 1962;15:24. doi: 10.1107/S0365110x62000067. &. [DOI] [Google Scholar]
- 45.Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr D Biol Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 47.Yang J, et al. The I-TASSER Suite: protein structure and function prediction. Nat Methods. 2015;12:7–8. doi: 10.1038/nmeth.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pettersen EF, et al. UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 49.Holm L, Sander C. Dali: a network tool for protein structure comparison. Trends Biochem Sci. 1995;20:478–480. doi: 10.1016/s0968-0004(00)89105-7. [DOI] [PubMed] [Google Scholar]
- 50.Mourao A, Nager AR, Nachury MV, Lorentzen E. Structural basis for membrane targeting of the BBSome by ARL6. Nat Struct Mol Biol. 2014;21:1035–1041. doi: 10.1038/nsmb.2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Delorenzi M, Speed T. An HMM model for coiled-coil domains and a comparison with PSSM-based predictions. Bioinformatics. 2002;18:617–625. doi: 10.1093/bioinformatics/18.4.617. [DOI] [PubMed] [Google Scholar]
- 52.Zimmermann L, et al. A Completely Reimplemented MPI Bioinformatics Toolkit with a New HHpred Server at its Core. J Mol Biol. 2018;430:2237–2243. doi: 10.1016/j.jmb.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 53.Wood CW, Woolfson DN. CCBuilder 2.0: Powerful and accessible coiled-coil modeling. Protein Sci. 2018;27:103–111. doi: 10.1002/pro.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nookala RK, Hussain S, Pellegrini L. Insights into Fanconi Anaemia from the structure of human FANCE. Nucleic Acids Res. 2007;35:1638–1648. doi: 10.1093/nar/gkm033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Murshudov GN, et al. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr D Biol Crystallogr. 2011;67:355–367. doi: 10.1107/S0907444911001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Adams PD, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goddard TD, et al. UCSF ChimeraX: Meeting modern challenges in visualization and analysis. Protein Sci. 2018;27:14–25. doi: 10.1002/pro.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van de Waterbeemd M, et al. High-fidelity mass analysis unveils heterogeneity in intact ribosomal particles. Nat Methods. 2017;14:283–286. doi: 10.1038/nmeth.4147. [DOI] [PubMed] [Google Scholar]
- 59.Rose RJ, Damoc E, Denisov E, Makarov A, Heck AJ. High-sensitivity Orbitrap mass analysis of intact macromolecular assemblies. Nat Methods. 2012;9:1084–1086. doi: 10.1038/nmeth.2208. [DOI] [PubMed] [Google Scholar]
- 60.Gault J, et al. High-resolution mass spectrometry of small molecules bound to membrane proteins. Nat Methods. 2016;13:333–336. doi: 10.1038/nmeth.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taverner T, et al. Subunit architecture of intact protein complexes from mass spectrometry and homology modeling. Acc Chem Res. 2008;41:617–627. doi: 10.1021/ar700218q. [DOI] [PubMed] [Google Scholar]
- 62.Chen ZA, et al. Architecture of the RNA polymerase II-TFIIF complex revealed by cross-linking and mass spectrometry. EMBO J. 2010;29:717–726. doi: 10.1038/emboj.2009.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kolbowski L, Mendes ML, Rappsilber J. Optimizing the Parameters Governing the Fragmentation of Cross-Linked Peptides in a Tribrid Mass Spectrometer. Anal Chem. 2017;89:5311–5318. doi: 10.1021/acs.analchem.6b04935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lenz S, Giese SH, Fischer L, Rappsilber J. In-Search Assignment of Monoisotopic Peaks Improves the Identification of Cross-Linked Peptides. J Proteome Res. 2018;17:3923–3931. doi: 10.1021/acs.jproteome.8b00600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Giese SH, Fischer L, Rappsilber JA. A Study into the Collision-induced Dissociation (CID) Behavior of Cross-Linked Peptides. Mol Cell Proteomics. 2016;15:1094–1104. doi: 10.1074/mcp.M115.049296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fischer L, Rappsilber J. Quirks of Error Estimation in Cross-Linking/Mass Spectrometry. Anal Chem. 2017;89:3829–3833. doi: 10.1021/acs.analchem.6b03745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Naydenova K, Russo CJ. Measuring the effects of particle orientation to improve the efficiency of electron cryomicroscopy. Nat Commun. 2017;8:629. doi: 10.1038/s41467-017-00782-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Buetow L, Huang DT. Structural insights into the catalysis and regulation of E3 ubiquitin ligases. Nat Rev Mol Cell Biol. 2016;17:626–642. doi: 10.1038/nrm.2016.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Knipscheer P, Sixma TK. Protein-protein interactions regulate Ubl conjugation. Curr Opin Struct Biol. 2007;17:665–673. doi: 10.1016/j.sbi.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 70.Metzger MB, Pruneda JN, Klevit RE, Weissman AM. RING-type E3 ligases: master manipulators of E2 ubiquitin-conjugating enzymes and ubiquitination. Biochim Biophys Acta. 2014;1843:47–60. doi: 10.1016/j.bbamcr.2013.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Linares LK, Hengstermann A, Ciechanover A, Muller S, Scheffner M. HdmX stimulates Hdm2-mediated ubiquitination and degradation of p53. Proc Natl Acad Sci U S A. 2003;100:12009–12014. doi: 10.1073/pnas.2030930100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alpi AF, Pace PE, Babu MM, Patel KJ. Mechanistic insight into site-restricted monoubiquitination of FANCD2 by Ube2t, FANCL, and FANCI. Mol Cell. 2008;32:767–77. doi: 10.1016/j.molcel.2008.12.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.