Abstract
Background: A greater decrease in 24-hour energy expenditure (EE) during fasting and a smaller increase in 24-hour EE during low-protein overfeeding (metabolic “thrifty” phenotype) predict weight gain. As thyroid hormones (TH) are implicated in energy intake and metabolism, we assessed whether: (i) TH concentrations are altered by 24-hour fasting or overfeeding diets with varying protein content and (ii) diet-related changes in TH correlate with concomitant changes in EE.
Methods: Fifty-eight euthyroid healthy subjects with normal glucose regulation underwent 24-hour dietary interventions including fasting, eucaloric feeding, and five overfeeding diets in a crossover design within a whole-room indirect calorimeter to measure the 24-hour EE. Overfeeding diets (200% of energy requirements) included three diets with 20% protein, one diet with 3% protein (low-protein overfeeding diet [LPF]: 46% fat), and one diet with 30% protein (high-protein overfeeding diet [HPF]: 44% fat, n = 51). Plasma free thyroxine (fT4), free triiodothyronine (fT3), and fibroblast growth factor 21 (FGF21) concentrations were measured after overnight fast the morning of and after each diet.
Results: On average, fT4 increased by 8% (+0.10 ng/dL, 95% confidence interval [CI 0.07–0.13], p < 0.0001) and fT3 decreased by 6% (−0.17 pg/mL [CI −0.27 to −0.07], p = 0.001) after 24-hour fasting, whereas both fT4 and fT3 decreased by 5% (−0.07 ng/dL [CI −0.11 to −0.04], p < 0.0001) and 4% (−0.14 pg/mL [CI −0.24 to −0.04], p = 0.008) following HPF, respectively. Greater decreases in fT3 after HPF are associated with larger decreases in FGF21 (r = 0.40, p = 0.005). Following LPF, the mean fT3 increased by 6% (+0.14 pg/mL [CI 0.05–0.2], p = 0.003) with no change in fT4 (p = 0.7). No changes in TH were observed after normal-protein overfeeding diets (all p > 0.1). No associations were observed between TH concentrations and diet-related changes in 24-hour EE during any diet (all p > 0.07).
Conclusions: Acute (200%) short-term (24 hours) changes in food intake induce small changes in TH concentrations only after diets with low (0% fasting and 3% protein overfeeding) or high (30% protein overfeeding) protein content. The fT3-FGF21 association after high-protein overfeeding suggests a role for TH in inhibiting FGF21 secretion by the liver during protein excess. These results indicate that TH are involved in protein metabolism; however, they do not mediate the short-term EE response to diets that characterize the metabolic phenotypes and determine the individual susceptibility to weight gain.
Keywords: thyroid hormones, energy expenditure, substrate oxidation, fasting, FGF21, low-protein overfeeding, high-protein overfeeding
Introduction
The global prevalence of obesity and its related comorbidities has risen substantially over the past decades (1) due to a persistent imbalance between energy intake and energy expenditure (EE) as a result of increased food intake or reduced physical activity. The difference in the propensity to weight gain among individuals may depend on both environmental and genetic factors that influence the two components of daily energy balance. Among these factors, the ability to increase EE in response to overeating, namely the diet-induced thermogenesis (2), has been recently proposed to be a novel determinant of weight change (3). Dietary interventions such as 24-hour overfeeding or fasting lead to EE responses with a wide interindividual variability that characterizes thrifty versus spendthrift metabolic phenotypes (4). Compared with subjects who are more metabolically “spendthrift,” subjects with a “thrifty” metabolism have a greater propensity to weight gain, which is associated with an impaired capacity to dissipate the excess energy during overeating and increased capacity to save energy during fasting (4–6). Thrifty and spendthrift metabolic phenotypes can be characterized by the magnitude of short-term (24-hour) EE responses to fasting, as well as overfeeding a high-carbohydrate and a low-protein diet (6). Notably, a greater decrease in 24-hour EE during fasting, a lower increase in 24-hour EE during low-protein overfeeding, and a greater increase in 24-hour EE during high-carbohydrate overfeeding predict greater weight gain after 6 months in free-living conditions (6). The hepatically secreted hormone, fibroblast growth factor 21 (FGF21), increases acutely after a low-protein overfeeding diet, mediates the diet-related change in EE, and predicts interindividual variability in free-living weight change (7). Furthermore, FGF21 may interact with thyroid hormones (TH) due to their similarities in metabolic functions (8,9). Triiodothyronine (T3) can induce changes in whole-body energy metabolism similar to those exerted by FGF21 (10). In the liver, the major organ where FGF21 is primarily secreted into the circulation in humans, TH receptors (β isoform) play a role in mediating the majority of actions of T3 (11), and FGF21 has emerged as a novel hepatic regulator mediating glucose homeostasis and lipid oxidation (LIPOX) (9). In mice, it has been recently shown that the active form of TH, T3, plays a role in regulating fgf21 expression (12). Whether changes in TH may affect circulating FGF21 concentrations in humans is not known.
TH have been implicated in the metabolic pathways that control energy balance by influencing energy storage and EE (13,14) and are involved in maintaining basal metabolism (15) by increasing ATP (adenosine triphosphate) synthesis for metabolic processes and by generating ion gradients (16–18). Human studies investigating the effects of short-term dietary interventions such as overfeeding or fasting on TH concentrations are limited and results are mixed. Serum T3 concentrations, but not thyroxine (T4) (19,20), increase after short-term overfeeding regardless of dietary macronutrient content (carbohydrates 82%, fat 74%, and protein 71%) (19). Total T3 decreases and reverse triiodothyronine (rT3) increases with fasting (21,22). During caloric restriction, T3 concentrations decrease along with a slight increase in T4 possibly as a compensatory mechanism to maintain energy stores in a setting of increased EE or decreased food intake (21,23,24).
Whether 24-hours of fasting or overfeeding diets with differing macronutrient content acutely affect circulating TH concentrations and any diet-related changes in TH concentrations correlate with the changes in 24-hour EE following these dietary interventions is not known. Thus, we sought to evaluate whether 24 hours of fasting and five different overfeeding diets alter TH concentrations and whether any of these changes are associated with changes in FGF21 concentrations and mediate the EE responses to these diets.
Methods and Study Design
Study volunteers
This analysis was performed using data from an ongoing clinical trial (ClinicalTrials.gov identifier: NCT00523627) aimed to characterize the short-term EE responses to 24-hour fasting and overfeeding. From 2008 to 2015, 58 healthy individuals aged 18–45 years completed the study and had available measurements of TH concentrations (Supplementary Fig. S1). These subjects had normal thyroid function based on normal thyrotropin (TSH) concentration (0.36–3.74 μIU/mL) measured on the day of admission. Study volunteers had a stable weight (<10% variation in body weight) for at least 6 months before admission and were free of medical diseases based on medical history, physical examination, and fasting blood tests.
Upon admission to the clinical research unit, individuals were fed a weight-maintaining diet (WMD; 50% carbohydrate, 30% fat, and 20% protein) with a caloric intake calculated using unit-specific equations based on sex and weight (25). Body weight was recorded daily and was maintained within 1% of the admission weight by adjusting the WMD. On the second day of admission, dual-energy X-ray absorptiometry (DPX-1; Lunar Corp, Madison, WI) was used to assess body composition, with fat mass (FM) and fat-free mass (FFM) calculated from percentage body fat (%fat). An oral glucose tolerance test (OGTT) was performed after 3 days on the WMD, and only subjects with normal glucose regulation based on the American Diabetes Association criteria (26) continued the study. All the participants signed written and informed consent before admission. The study was approved by the institutional review board of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institutes of Health.
EE measurements
The assessment of 24-hour EE and respiratory quotient (RQ) (an index of substrate oxidation) inside the whole-room indirect calorimeter was previously described in detail (27) (Supplementary Data). Briefly, after an overnight fast and following breakfast (7 am), subjects entered the calorimeter around 8 am. During each dietary intervention, three meals were provided to the subjects through an airtight interlock at 11 am, 4 pm, and 7 pm. Uneaten food was returned and weighed in the metabolic kitchen to determine the actual energy intake during each 24-hour session. The air temperature was maintained constant at 23.7 ± 1.4°C by an air conditioning system. Volunteers resided in the calorimeter for 23.25 hours, during which both carbon dioxide (CO2) production and oxygen (O2) consumption were recorded every minute, averaged, and extrapolated to 24 hours. Quality control assessments were performed monthly and demonstrated average recoveries of 98 ± 3% (coefficient of variation [CV] = 3.4%) and 99 ± 3% (CV = 3.6%) for CO2 and O2, respectively. The 24-hour RQ was calculated as the ratio of 24-hour CO2 production to 24-hour O2 consumption, and the 24-hour EE was calculated using Lusk's equation (28). Carbohydrate oxidation (CARBOX) and LIPOX rates were derived from the 24-hour RQ, after accounting for protein oxidation (PROTOX), which was estimated from measurement of 24-hour urinary nitrogen excretion (29).
Dietary interventions
The experimental protocol for dietary interventions (Supplementary Data; Supplementary Fig. S2) was previously described in detail (27). Briefly, two 24-hour EE measurements were performed to precisely measure EE in energy balance conditions inside the whole-room calorimeter. During the first 24-hour session, the caloric intake of WMD was reduced by 20% to account for reduced activity inside the metabolic chamber; subsequently, the second 24-hour session was performed 2 days later when the energy intake was set to the 24-hour EE value from the first assessment. The 24-hour EE value from the second session was considered the baseline EE (28). The baseline 24-hour EE was doubled to determine the energy intake of overfeeding diets. In a random order, subjects underwent six 24-hour EE assessments (fasting and five overfeeding diets) in the whole-room indirect calorimeter with 3-day washout period between each intervention on the WMD. The 200% overfeeding diets (Supplementary Fig. S3) included one diet with low-protein overfeeding diet content (LPF: 3% protein, 51% carbohydrate, and 46% fat), one diet with a high-protein overfeeding diet (HPF: 30% protein, 44% fat, and 26% carbohydrate, n = 51), and three overfeeding diets with normal protein content (20%): standard (50% carbohydrate and 30% fat), high-carbohydrate (75% carbohydrate and 5% fat), and high-fat (60% fat and 20% carbohydrate) overfeeding diet. Available data for HPF are limited to only 51 individuals as this diet was added to the protocol only after 20 subjects completed the initial study.
During 24-hour fasting, only water was permitted. Sessions when subjects consumed <95% of energy intake provided with the overfeeding diets were excluded from data analysis.
Hormone measurements
During admission, TSH was measured by a homogeneous sandwich chemiluminescent immunoassay based on LOCI® technology from Dimension Vista® 500 System (Siemens). Blood for measurements of plasma free triiodothyronine (fT3), free thyroxine (fT4), and FGF21 concentrations was collected in the morning after an overnight fast both before and upon exit the whole-room indirect calorimeter during each dietary intervention. Plasma samples were collected in ethylenediamine tetraacetic acid-containing tubes and stored in a freezer at −70°C for later measurements of hormones by the NIDDK Core Laboratory (Bethesda, MD). Plasma fT4 and fT3 concentrations were measured using the EIA Kit (Phoenix Pharmaceuticals, Burlingame, CA). Intra-assay and inter-assay CVs were 3.0% and 4.5% for fT3, and 2.8% and 4.0% for fT4, respectively. Plasma FGF21 concentrations were measured by ELISA (R&D Systems, Minneapolis, MN). The intra-assay CV was 2.5% and the inter-assay CV was 5.2%.
Due to changes in fT4 and fT3 mean concentrations observed after the 24-hour fasting, HPF, and LPF, plasma samples of these dietary interventions were used for measurements of TSH and rT3. Plasma TSH concentration was measured using the EIA Kit (Phoenix Pharmaceuticals). Intra-assay and inter-assay CVs were 5.4% and 7.7%, respectively. Plasma rT3 was measured using the competitive ELISA Kit (ALPCO, Salem, NH). Intra-assay and inter-assay CVs were 8.7% and 11.9%, respectively. Insulin concentrations during the OGTT were measured by an Automated Immunoenzymometric Assay (Tosoh Bioscience, Inc., Tessenderlo, Belgium).
Statistical analysis
Data are presented as mean with standard deviation or as geometric mean with its 95% confidence interval (CI) as appropriate. For each hormone, the CV and intra-class correlation coefficient (ICC) were calculated for all the fasting prechamber measurements to assess precision and reproducibility. All the fasting hormone concentrations measured before entering the calorimeter were then averaged in each subject and used as baseline value to determine interindividual differences according to sex and race/ethnicity by unpaired t-test and analysis of variance, respectively. Fasting rT3 and TSH data were log10 transformed before analyses to meet the assumptions of linear regression (i.e., homoscedasticity and normal distribution). The Spearman correlation was used to assess the effect of storage time on hormone measurements. The Pearson correlation index was used to quantify the relationships between hormone concentrations and body composition measures. Multiple linear regression analysis was used to assess the determinants of TH. The individual change (Δ) in hormone concentrations after each diet was calculated as the difference between the post-diet minus the pre-diet absolute values and analyzed by paired t-test. The relationships between changes in hormone concentrations and diet-induced changes in the 24-hour EE were quantified by the Pearson correlation. The individual changes in TH concentrations were analyzed via mixed-model analysis including all diets and accounting for repeated measurements within a subject using a compound symmetry covariance structure.
Linear models with the 24-hour RQ, 24-hour EE, and its components as dependent variables and as covariates were calculated to evaluate the effect of each hormone on metabolic measures during each dietary intervention. Linear regression analysis was used to calculate residuals of RQ, EE, and its components after adjustment for known covariates (30).
Results
Baseline characteristics of the study population are shown in Table 1. Fasting plasma TSH, fT4, fT3, and rT3 concentrations were not affected by storage time (p = 0.09, p = 0.15, p = 0.10, and p = 0.12, respectively).
Table 1.
Demographic, Anthropometric, and Metabolic Characteristics of the Study Group
| All subjects (n = 58) | Women (n = 12) | Men (n = 46) | |
|---|---|---|---|
| Age (years) | 36.5 ± 10.1 (18.2, 54.1) | 33.2 ± 8.4 (20, 45) | 37.3 ± 10.6 (18, 54) |
| Race/ethnicity | 14 BLK, 15 WHT, 10 HIS, 19 NAM | 5 BLK, 4 WHT, 1 HIS, 2 NAM | 9 BLK, 11 WHT, 9 HIS, 17 NAM |
| BMI (kg/m2) | 26.2 ± 4 (17.8, 39.1) | 26.9 ± 5.8 (17.8, 39.1) | 26.0 ± 3.4 (18, 37) |
| Height (cm) | 173.3 ± 7.3 (156.8, 196.4) | 166.0 ± 4.4 (156.8, 170.0) | 175.1 ± 6.7 (161.0, 196.4) |
| Body weight (kg) | 78.6 ± 12.5 (47.5, 107.8) | 74.3 ± 16.8 (47.5, 107.8) | 79.6 ± 11.1 (56, 105) |
| Body fat (%) | 28.5 ± 9.7 (7.3, 53.8) | 40.4 ± 8.4 (24.2, 53.8) | 24.5 ± 7.8 (6.9, 38)* |
| FM (kg) | 22.9 ± 10.0 (4.9, 57.0) | 31.2 ± 13.0 (13.6, 56.9) | 20.7 ± 7.8 (4.9, 36)* |
| FFM (kg) | 55.7 ± 9.0 (33.8, 77.0) | 43.2 ± 4.9 (33.9, 50.9) | 59.6 ± 6.7 (47, 77)* |
| 24-Hour EE (kcal/day) | 2029 ± 287 (1502, 2810) | 1802 ± 223 (1502, 2290) | 2088 ± 273 (1573, 2810)* |
| 24-Hour RQ (ratio) | 0.87 ± 0.03 (0.8, 0.93) | 0.86 ± 0.03 (0.81, 0.91) | 0.87 ± 0.03 (0.8, 0.93) |
| Fasting glucose (mg/dL) | 91.8 ± 4.7 (80, 99) | 91.0 ± 3.3 (86.5, 97.0) | 91.9 ± 5.2 (80, 99) |
| 2-Hour OGTT glucose (mg/dL) | 104.9 ± 18.9 (65, 138) | 104.2 ± 16.5 (80, 130) | 103.6 ± 20.3 (65, 138) |
| TSHa (μIU/mL) | 1.51 (IQR 0.95–1.76) | 1.24 (IQR 0.62–1.31) | 1.30 (IQR 0.94–1.67) |
| (0.32, 3.58) | (0.53, 1.95) | (0.32, 3.59) | |
| Plasma fT4 (ng/dL) | 1.24 ± 0.18 (0.90, 1.64) | 1.13 ± 0.15 (0.90, 1.34) | 1.27 ± 0.19 (0.91, 1.64) |
| Plasma fT3 (pg/mL) | 2.9 ± 0.30 (1.91, 3.88) | 2.37 ± 0.30 (1.91, 2.98) | 2.97 ± 0.37 (2.34, 3.88) |
Data are presented as mean ± standard deviation (minimum, maximum), except for TSH, which is shown as median with IQR (minimum, maximum). Fasting plasma fT4 and fT3 were obtained by averaging all fasting pre-diets measurements.
TSH concentration as measured on the first day of admission.
p < 0.05 versus women by Student's unpaired t-test or χ2 test as appropriate.
BLK, black; BMI, body mass index; EE, energy expenditure; FFM, fat free mass; FM, fat mass; fT3, free triiodothyronine; fT4, free thyroxin; HIS, Hispanic; IQR, interquartile range; NAM, Native American; OGTT, oral glucose tolerance test; RQ, respiratory quotient; TSH, thyrotropin; WHT, white.
The CVs and ICCs of baseline values were 21%, 9.5%, 7%, and 17.7% and 0.87, 0.79, 0.69, and 0.62 (all p < 0.001) for TSH, fT4, fT3, and rT3, respectively, indicating good precision and within-subject consistency of hormone measurements.
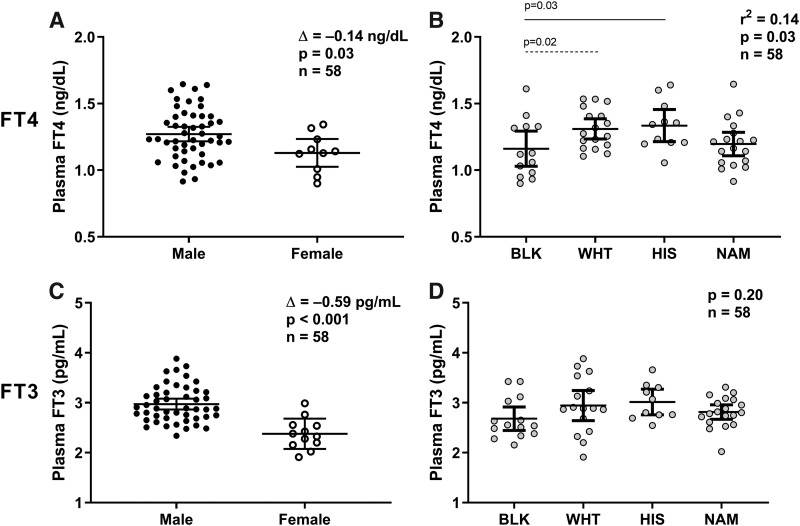
Fasting plasma fT4 concentration was on average lower in women than in men (Δ = −0.14 ng/dL [CI −0.27 to −0.01], p = 0.03; Fig. 1A) and in blacks compared with Caucasians (p = 0.02) and Hispanics (p = 0.03) (global p = 0.03; Fig. 1B). In a multivariable model, only age (older individuals had lower fT4 by 0.15 ng/dL [−0.22 to −0.06] per 20 years difference, p = 0.001) and race/ethnicity (blacks had a 0.12 ng/dL [−0.23 to −0.01] lower concentration compared with other ethnicities, p = 0.03), but not FM, FFM, or percentage body fat (PFAT) (all p > 0.05), were independent determinants of fasting fT4 concentration (total R2 = 0.34).
FIG. 1.
Sex and race/ethnic differences on plasma fT4 and fT3 concentrations. (A, B) The average of plasma fT4 concentrations measured after an overnight fast and before each dietary intervention is reported for each subject on the y-axis as raw values. Plasma fT4 concentration differences by sex (A) were evaluated by unpaired Student's t-test, whereas differences among race/ethnicity (B) were assessed using ANOVA with Tukey–Kramer post hoc adjustment of the least square means for multiple comparisons (the continuous line represents adjusted p = 0.02 and the intermittent line represents adjusted p = 0.03). The Δ in (A) represents the average difference in fT4 concentrations between males and females tested via Student's t-test. (C, D) The average of plasma fT3 concentrations measured after an overnight fast and before each dietary intervention is reported for each subject on the y-axis as raw values. Plasma fT3 concentration differences by sex (C) were evaluated by unpaired Student's t-test, whereas differences among race/ethnicity (D) were assessed using ANOVA. The Δ in (C) represents the average difference in fT3 concentration between males and females tested via Student's t-test. Error bars represent mean ± 95% CI. ANOVA, analysis of variance; BLK, black; CI, confidence interval; fT3, free triiodothyronine; fT4, free thyroxin; HIS, Hispanic; NAM, Native American; WHT, Caucasian.
Fasting plasma fT3 concentrations were also lower in women compared with men (Δ = −0.59 pg/mL [CI −0.86 to −0.34], p < 0.001; Fig. 1C) but did not differ by race/ethnicity (p = 0.20; Fig. 1D). Both age (older individuals had lower fT3 by 0.25 pg/mL [−0.40 to −0.06] per 20 years difference, p = 0.01) and sex (men had higher fT3 concentrations by 0.81 pg/mL [0.39–1.24] compared with women, p < 0.001), but not FM, FFM, or PFAT (all p > 0.05), were independent determinants of fasting fT3 concentration (total R2 = 0.45). Fasting plasma fT4 and fT3 concentrations were not associated with fasting glucose, 2-hour glucose, fasting insulin, or 2-hour insulin during OGTT (all p > 0.2).
Changes in TH concentrations after 24-hour dietary interventions
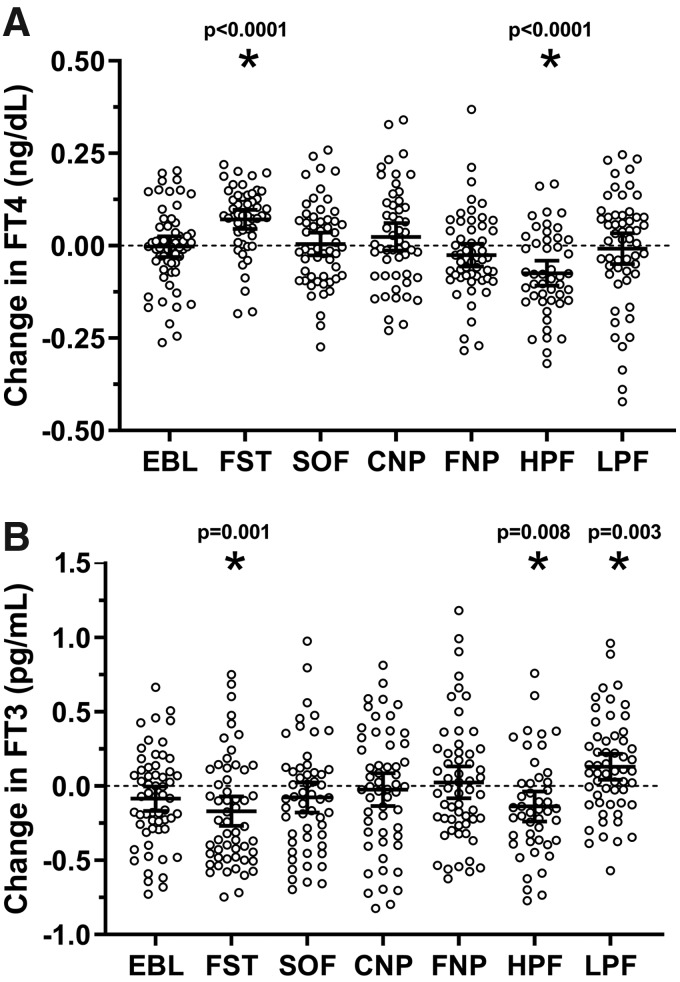
The changes in TH concentrations after each dietary intervention are reported in Table 2. Plasma TH concentrations varied only following 24-hour fasting, high-protein overfeeding, and low-protein overfeeding (also after adjustment for multiple comparisons, see legend of Fig. 2), whereas no changes were found after normal-protein overfeeding diets (Fig. 2). The fT4 and fT3 concentrations before and after each diet are shown in Supplementary Figures S4 and S5, respectively.
Table 2.
Hormone Concentrations Before and After Each Dietary Intervention
| Diet | Pre-diet | Post-diet | Absolute change | Percent change | p |
|---|---|---|---|---|---|
| EBL | |||||
| fT4 (ng/dL) | 1.26 [1.21 to 1.32] | 1.26 [1.21 to 1.31] | −0.002 [−0.03 to 0.03] | +0.26 [−1.90 to 2.43] | 0.80 |
| fT3 (pg/mL) | 2.81 [2.69 to 2.93] | 2.73 [2.62 to 2.85] | −0.08 [−0.16 to 0.01] | −2.01 [−4.95 to 0.92] | 0.07 |
| FST | |||||
| TSH (μIU/mL) | 3.89 [3.29 to 4.49] | 2.06 [1.64 to 2.48] | −1.75 [−2.16 to −1.35] | −43.29 [−51.09 to −35.48] | <0.0001 |
| fT4 (ng/dL) | 1.23 [1.17 to 1.28] | 1.33 [1.26 to 1.38] | 0.10 [0.07 to 0.13] | +8.28 [5.64 to 10.93] | <0.0001 |
| fT3 (pg/mL) | 2.89 [2.77 to 3.01] | 2.72 [2.57 to 2.87] | −0.17 [−0.27 to −0.07] | −5.67 [−9.37 to −1.97] | 0.001 |
| rT3 (ng/mL) | 0.17 [0.15 to 0.19] | 0.21 [0.18 to 0.23] | 0.03 [0.01 to 0.05] | +19.69 [11.02 to 28.36] | 0.0004 |
| FGF21 (pg/mL) | 161.28 [131.78 to 190.59] | 99.54 [83.56 to 115.53] | −61.64 [−85.08 to −38.20] | −38.2 [−36.59 to 64.56] | <0.0001 |
| LPF | |||||
| TSH (μIU/mL) | 3.86 [3.29 to 4.44] | 3.17 [2.71 to 3.64] | −0.76 [−1.17 to −0.35] | −15.79 [−25.58 to −6.00] | 0.0002 |
| fT4 (ng/dL) | 1.27 [1.21 to 1.32] | 1.26 [1.21 to 1.32] | −0.01 [−0.05 to 0.04] | −0.31 [−2.91 to 3.54] | 0.72 |
| fT3 (pg/mL) | 2.79 [2.66 to 2.91] | 2.92 [2.80 to 9.21] | 0.14 [0.05 to 0.22] | +5.80 [2.38 to 9.22] | 0.003 |
| rT3 (ng/mL) | 0.19 [0.17 to 0.22] | 0.18 [0.16 to 0.20] | −0.02 [−0.03 to 0.002] | −3.69 [−10.93 to 3.53] | 0.08 |
| FGF21 (pg/mL) | 169.8 [135.5 to 204.1] | 444.1 [367.3 to 520.9] | 274.3 [221.1 to 327.5] | +199.7 [154.2 to 253.5] | <0.0001 |
| HPF | |||||
| TSH (μIU/mL) | 3.74 [3.19 to 4.29] | 3.38 [2.86 to 3.89] | −0.40 [−0.71 to −0.09] | −9.09 [−16.82 to −1.36] | 0.004 |
| fT4 (ng/dL) | 1.24 [1.18 to 1.30] | 1.17 [1.11 to 1.22] | −0.07 [−0.11 to −0.04] | −5.49 [−8.14 to −2.85] | <0.0001 |
| fT3 (pg/mL) | 2.90 [2.77 to 3.04] | 2.77 [2.62 to 2.91] | −0.14 [−0.24 to −0.04] | −4.43 [−7.94 to −0.92] | 0.008 |
| rT3 (ng/mL) | 0.168 [0.14 to 0.19] | 0.171 [0.15 to 0.19] | 0.002 [−0.01 to 0.015] | +4.08 [−3.10 to 11.27] | 0.70 |
| FGF21 (pg/mL) | 174.14 [142.73 to 205.54] | 52.86 [37.95 to 67.76] | −121.28 [−149.74 to −92.82] | −69.64 [−73.4 to −67.03] | <0.0001 |
| SOF | |||||
| fT4 (ng/dL) | 1.24 [1.18 to 1.30] | 1.25 [1.20 to 1.30] | 0.01 [−0.03 to 0.04] | +1.12 [−1.43 to 3.68] | 0.73 |
| fT3 (pg/mL) | 2.87 [2.72 to 3.03] | 2.80 [2.67 to 2.93] | −0.08 [−0.18 to 0.02] | −1.29 [−5.14 to 2.56] | 0.13 |
| CNP | |||||
| fT4 (ng/dL) | 1.25 [1.20 to 1.30] | 1.28 [1.22 to 1.33] | 0.02 [−0.01 to 0.06] | +2.51 [−0.63 to 5.64] | 0.20 |
| fT3 (pg/mL) | 2.90 [2.75 to 3.05] | 2.88 [2.75 to 3.01] | −0.02 [−0.13 to 0.09] | −0.36 [−3.57 to 4.28] | 0.66 |
| FNP | |||||
| fT4 (ng/dL) | 1.23 [1.17 to 1.29] | 1.21 [1.15 to 1.26] | −0.03 [−0.05 to 0.005] | −1.53 [−4.19 to 1.12] | 0.20 |
| fT3 (pg/mL) | 2.83 [2.69 to 2.96] | 2.84 [2.72 to 2.96] | 0.02 [−0.08 to 0.12] | +1.69 [−2.28 to 5.66] | 0.75 |
Bold values indicate p < 0.05.
Plasma fT3 and fT4 concentrations are expressed as mean with its 95% CI. Plasma TSH, plasma rT3, and plasma FGF21 concentrations are expressed as geometric mean with its 95% CI, but their absolute changes are reported as arithmetic mean with its 95% CI. Percent changes in fT3 and fT4 were calculated as the average absolute change divided by the average pre-diet absolute value, whereas percent changes in TSH and rT3 were calculated as the ratio of geometric means ( = post-diet/pre-diet). p-Values were calculated by Student's paired t-test. The caloric intake of overfeeding diets was calculated as twice the 24-hour energy expenditure during energy balance. Dietary composition: SOF (50% carbohydrate, 30% fat, 20% protein), CNP (75% carbohydrate, 5% fat, 20% protein), FNP (20% carbohydrate, 60% fat, 20% protein), HPF (26% carbohydrate, 44% fat, 30% protein), and LPF (51% carbohydrate, 46% fat, 3% protein).
CI, confidence interval; CNP, high-carbohydrate overfeeding; EBL, energy balance; FGF21, fibroblast growth factor 21; FNP, high-fat overfeeding; FST, 24-hour fasting; HPF, high-protein overfeeding diet; LPF, low-protein overfeeding diet; rT3, reverse triiodothyronine; SOF, standard overfeeding.
FIG. 2.
Changes in plasma fT4 and fT3 concentrations after each 24-hour dietary intervention. (A, B) The individual change in hormone concentrations on the y-axis was calculated as the difference between the post-diet minus the pre-diet absolute values. Error bars represent mean ± 95% CI. Asterisks represent the changes in plasma fT4 and fT3 concentrations with a p < 0.05 by Student's t-test. The diets with significant changes for fT4 or fT3 were also significant when considering the Bonferroni-corrected threshold for significance equal to 0.0083 obtained by dividing the nominal threshold for significance (0.05) by the number of dietary interventions (6 = fasting +5 overfeeding diets). Diets composition: EBL (eucaloric diet, 50% carbohydrate, 30% fat, 20% protein), FST, SOF (50% carbohydrate, 30% fat, 20% protein), CNP (75% carbohydrate, 5% fat, 20% protein), FNP (20% carbohydrate, 60% fat, 20% protein), HPF (26% carbohydrate, 44% fat, 30% protein), and LPF (51% carbohydrate, 46% fat, 3% protein). CNP, high-carbohydrate overfeeding; EBL, energy balance; FNP, high-fat overfeeding; FST, 24-hour fasting; HPF, high-protein overfeeding diet; LPF, low-protein overfeeding diet; SOF, standard overfeeding.
Fasting
After 24-hour fasting, both plasma fT4 and rT3 concentrations increased on average by 8% (Δ = +0.10 ng/dL [CI 0.07–0.13], p < 0.0001; Fig. 2A) and by 16% (Δ = +0.03 ng/mL [CI 0.01–0.04], p = 0.0004), respectively, whereas plasma fT3 and TSH concentrations decreased on average by 6% (Δ = −0.17 pg/mL [CI −0.27 to −0.07], p = 0.001; Fig. 2B) and by 43.3% (Δ = −1.75 μIU/mL [CI −2.16 to −1.35], p < 0.0001), respectively. No associations were observed between the change in plasma FGF21 concentration after 24-hour fasting and concomitant changes in plasma TH concentrations (all p > 0.2).
Low-protein overfeeding diet
After low-protein overfeeding, plasma TSH decreased on average by 16% (Δ = −0.76 μIU/mL [CI −0.35 to −1.17], p = 0.0002), whereas mean plasma fT4 was unchanged (p = 0.7; Fig. 2A). Plasma fT3 increased on average by 6% (Δ = +0.14 pg/mL [CI 0.05–0.22], p = 0.003; Fig. 2B) with no change in mean rT3 concentration (p = 0.08). No associations were observed between the increase in FGF21 after low-protein overfeeding and changes in TSH (p = 0.08), fT4 (p = 0.06), fT3 (p = 0.38), or rT3 (p = 0.6).
High-protein overfeeding diet
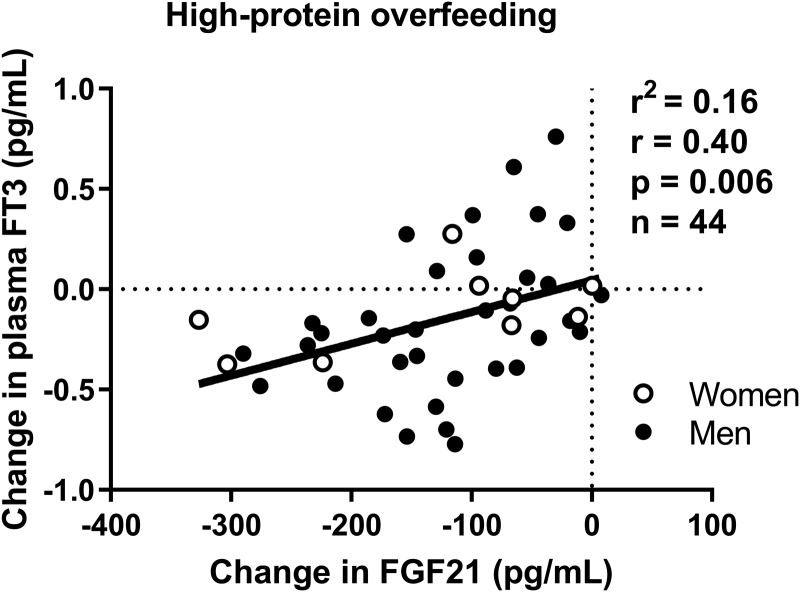
After high-protein overfeeding, plasma TSH decreased on average by 13% (Δ = −0.40 μIU/mL [CI −0.20 to −0.04], p = 0.004). Similarly, both plasma fT4 and fT3 concentrations decreased on average by 5% (Δ = −0.07 ng/dL [CI −0.11 to −0.04], p < 0.0001; Fig. 2A) and 4% (−0.14 pg/mL [CI −0.24 to −0.04], p = 0.008; Fig. 2B), respectively, with no change in mean plasma rT3 (p = 0.7). The changes in fT4 concentration following high-protein overfeeding did not differ by race/ethnicity or sex (all p > 0.2). The change in FGF21 after high-protein overfeeding was positively associated with the change in fT3, such that a larger decrease in FGF21 correlated with a greater decrease in fT3 (r = 0.40, p = 0.005; Fig. 3). No associations were observed between changes in FGF21 and changes in TSH (p = 0.5), fT4 (p = 0.9), or rT3 (p = 0.8).
FIG. 3.
Relationships between the change in fasting plasma fT3 concentration and the change in FGF21 concentration after 24-hour high-protein, FNP diet. The individual change in hormone concentrations on each axis was calculated as the difference between the post-diet minus the pre-diet absolute values. The strength of association was quantified by the Pearson correlation index (r) and the coefficient of determination (R2). Diet composition of high-protein, FNP diet (200% daily energy requirements): 26% carbohydrate, 44% fat, 30% protein. The sample size includes 44 subjects (35 men and 9 women) with available measurements of FGF21 and fT3before and after 24-hour HPF. FGF21, fibroblast growth factor 21.
The individual changes in fT4 after HPF and LPF were correlated within a subject, such that a greater decrease in fT4 following HPF was associated with a decrease following LPF (r = 0.43, p = 0.003; Supplementary Fig. S6A). Similarly, a greater decrease in fT3 following HPF correlated with a decrease in fT3 following LPF (r = 0.42, p = 0.005; Supplementary Fig. S6B). No associations were observed between the changes in TH after 24-hour fasting and low-/high-protein overfeeding (all p > 0.1).
Relationships between TH and EE measures
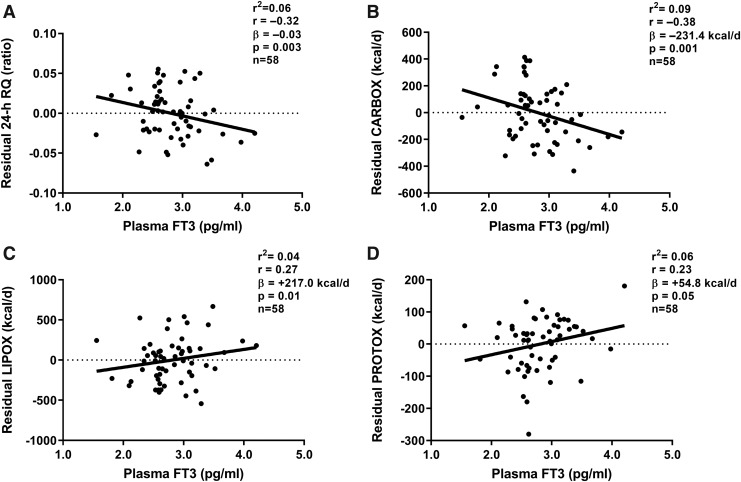
As we have previously reported in this cohort, the 24-hour EE increased from eucaloric conditions during each overfeeding diet, with the largest increases during high-carbohydrate normal-protein overfeeding (Δ = +14% [CI 12%–15%], p < 0.001) and high-protein overfeeding (Δ = +14% [CI 11%–16%], p < 0.001) and the smallest increase during LPF (Δ = +3% [CI 2%–4%], p < 0.001), whereas a reduction in 24-hour EE was observed during 24-hour fasting (Δ = −8% [CI −9% to −6%], p < 0.001). No associations were observed between changes in plasma fT4 or fT3 concentration and 24-hour RQ, CARBOX, LIPOX, or PROTOX during fasting, low-, or high-protein overfeeding (all p > 0.07; Supplementary Table S1). However, fasting plasma fT3 concentration measured before eucaloric feeding was negatively correlated with residual RQ (i.e., 24-hour RQ after adjustment for its known determinants via regression analysis), such that a lower plasma fT3 was associated with relatively higher RQ during energy balance (β = −0.03 per pg/mL difference in fT3 [CI −0.05 to −0.01], p = 0.003; Fig. 4A), higher CARBOX (β = −231.4 kcal/day [CI −368.7 to −94.1] p = 0.001; Fig. 4B), and lower LIPOX (β = +217.0 kcal/day [CI 46.1–388.0], p = 0.01; Fig. 4C) and PROTOX (β = +54.8 kcal/day [CI −0.8 to 110.5], p = 0.05; Fig. 4D) after adjustment for their known determinants.
FIG. 4.
Relationships between fasting plasma fT3 concentration and 24-hour RQ and macronutrient oxidation rates during energy balance. Linear regression analysis was used to calculate residuals of 24-hour RQ, CARBOX, LIPOX, and PROTOX after adjustment for known covariates (age, sex, race/ethnicity, fat free mass, fat mass, and chamber temperature). Residuals of 24-hour RQ and macronutrient oxidation rates are shown on the y-axis of each panel. (A, B) Inverse relationships between fasting plasma fT3 and residual 24-hour RQ (A) and residual CARBOX (B) during energy balance and eucaloric feeding inside the metabolic chamber. (C, D) Direct relationships between fasting plasma fT3 and residual LIPOX (C) and residual PROTOX (D) during energy balance and eucaloric feeding inside the metabolic chamber. The strength of association was quantified by the Pearson correlation index (r) and the coefficient of determination (R2). The β coefficient was calculated via linear regression analysis and represents the average effect of 1 pg/mL difference in plasma fT3 concentration on residual 24-hour RQ (A), CARBOX (B), LIPOX (C), and PROTOX (D). CARBOX, carbohydrate oxidation; LIPOX, lipid oxidation; PROTOX, protein oxidation; RQ, respiratory quotient.
Discussion
In the present study including healthy euthyroid subjects with normal glucose regulation, we sought to assess the role of TH in the 24-hour EE responses to acute dietary interventions such as fasting and low-protein overfeeding, which characterize metabolic phenotypes associated with weight change (6,7). TH concentrations changed following diets with altered protein content including low- and high-protein overfeeding and fasting, but we did not observe any association between these changes and diet-induced changes in 24-hour EE or RQ.
Fasting
After 24 hours of fasting, the mean plasma fT4 concentrations increased, whereas TSH and fT3 concentrations decreased with a concomitant increase in rT3 concentrations. Studies uniformly demonstrate a decline in fT3 and increase in rT3 with fasting (31,32), but the T4 and TSH responses vary depending on the study (21,33), implying either a hypothalamic–pituitary–thyroid (HPT) axis or peripheral mechanism. Thyrotropin-releasing hormone (TRH) infusion decreases TSH secretion after a 10-day fasting period, and TSH and T3 responses to prolonged TRH infusion were not restored completely after 5 days of refeeding (34). Serum TSH decreases in the early phase (i.e., after 48 hours) of TRH infusion during fasting with an increase in fT4 concentrations, indicating that during the early acute phase of fasting temporary suppression of TSH secretion might be due to an effect of fT4 rather than being directly TRH-mediated (35). Peripheral conversion of T4 into T3 and rT3 is also affected by fasting (36). In humans, fasting induces altered peripheral TH metabolism leading to a decrease in T4-to-T3 conversion and rT3 degradation (21,32,37).
Our current results confirm an alteration in both TSH and TH concentrations after 24-hour fasting. Thus, we speculate that the directionality of these changes might indicate an effect of fasting on both central (TSH response) and peripheral (based on the T3 and rT3 response) mechanisms. Concordant with a previous study in health men during a 10-day fast (38), we did not observe any association between changes in FGF21 and TH concentrations after 24-hour fasting, indicating no substantial interaction between TH and FGF21 during prolonged fasting. As the effect of fasting on FGF21 concentration in humans is controversial (39) and differences between human and rodent studies are not fully understood, the increase in FGF21 following fasting observed in rodent models must be taken with caution when considering human studies, particularly because of the differences in tissue-specific FGF21 gene expression between the two species.
Low-protein overfeeding
After 24 hours of low-protein overfeeding, the mean circulating fT3 concentrations increased while TSH decreased and no changes were observed in circulating fT4 or rT3 concentrations. Dietary protein intake affects the physiological and neurobiological homeostatic regulatory system (40), and diets with reduced protein content alter the HPT axis via central and peripheral mechanisms (40). Our results indicate that with relatively acute overfeeding with low protein circulating fT3 concentrations increase and exert a negative feedback on the HPT axis with a consequent reduction in plasma TSH concentrations. The lack of change in plasma fT4 concentration is unclear but may be due to the short period of overfeeding (24 hours).
In rats, a protein-free diet leads to a decrease in TRH gene expression, pituitary TSH gene expression, and plasma T3 concentrations (41), indicating that protein reduction does also have a central effect on the TRH response at the hypothalamic level, possibly due to the absence of essential amino acids that have a role in the neurotransmitter system (42,43). However, the results of studies of low-protein diets in rodent models are mixed with two studies demonstrating an increase in serum T3 following a low-protein high-carbohydrate diet (44,45), and other study demonstrating a decrease in fT3, fT4, and TSH concentrations (46). Men overfed with a low-protein high-carbohydrate diet over ∼20 days increase their fT3 concentrations but decrease their fT4 concentrations (47), consistent with our observation of an upregulation of peripheral T4-to-T3 conversion after 24 hours. Despite our previously observed nearly threefold increase in plasma FGF21 following low-protein overfeeding (7), in the current analysis, we did not find any associations between the increase in FGF21 and changes in TH concentrations following this overfeeding diet.
High-protein overfeeding
Following 24-hour high-protein overfeeding, the mean circulating plasma TSH decreased along with the reduction in both fT4 and fT3 and no change in rT3 concentrations. To the best of our knowledge, very few studies have investigated the effect of short-term high-protein overfeeding on TH concentration. In healthy young adults, an 8-day period of eucaloric high-protein diet (35%, 30%, and 35% of total calories derived from protein, fat, and carbohydrate) led to reduction in TSH and T3 concentrations with no changes in T4 and rT3 (48). In overweight women (aged 30–54 years), high-protein diet (70 g protein a day) led to a reduction in circulating T3 concentrations but no changes in T4 (49).
Based on our current results, the reduction in each component of the pituitary–thyroid axis (plasma TSH, fT4, and fT3 concentrations) with no change in rT3 concentrations indicates a central mechanism reducing hypothalamic TRH secretion with consequent decrease in TSH production and fT4 and fT3 concentrations.
The decrease in plasma fT3 concentrations after high-protein overfeeding was associated with the previously reported decrease in plasma FGF21 concentrations (7). The interaction between TH receptors and other nuclear receptors, including the peroxisome proliferator-activated receptors (PPARs) and the liver X receptor, might mediate TH action on FGF21 production in the liver (50,51). In mice treated with exogenous T3, there is a dose–dependent increase in fgf21 expression (52). T3 regulates FGF21 via a PPAR-α-dependent mechanism in the liver (52). FGF21 may also regulate TH metabolism as a 7-day infusion of FGF21 in mice increased hypothalamic TRH and circulating TSH, T4, and T3 concentrations (53).
Effects of TH on EE response to diets
T3 has been shown to play a role, although minor, as a determinant of human EE, particularly, basal metabolic rate (15) and small changes in TH concentration after hormone treatment effect EE (54). Previous studies in euthyroid subjects have shown that circulating serum or plasma T3 concentrations are associated with 24-hour EE (55), sleeping (56), and resting (57) metabolic rate. In euthyroid subjects, lower fT3 concentrations, but not fT4, are associated with higher RQ reflecting a reduction in LIPOX (58). This finding might suggest that a reduction of T3 production in metabolically active tissue could lead to a shift from carbohydrates to lipids as source of energy. The current findings in our ethnically diverse cohort showing associations between circulating fT3 concentrations and 24-hour RQ and substrate oxidation corroborate the previous findings in a cohort of Native Americans (58). Indeed, TH signaling through deiodinases is a mechanism involved in energy homeostasis (59,60).
D2 is expressed in the brain, pituitary, thyroid, and brown adipose tissue (BAT) (61–65) and is responsible for local conversion of T3 from T4 (66). Dio2-knockout mice have reduced lipolysis and lipogenesis, which lead to BAT dysfunction (65,67). Although we found an association between fT3 and RQ during energy balance, the changes in TH concentrations after 24-hour fasting or overfeeding diets with altered protein content were not associated with diet-related changes in 24-hour EE or RQ. The lack of association with EE measures could be explained by the small changes in TH concentrations (5–8%) after these short-term dietary interventions, which may not be physiologically meaningful enough to exert a change in whole-body energy metabolism. Furthermore, the TH concentrations in plasma might not reflect their concentrations in peripheral tissues.
Limitations
Our study has several limitations. First, the female subgroup was small. Second, TH have a circadian rhythm, and it is possible that our results were affected by the timing of blood draw in the early morning. Also, other hormonal factors (e.g., catecholamines and adipokines) might be responsible for the small changes in circulating TH concentrations in response to diets. Future interventional studies specifically including no/low-/high-protein diets with concomitant measurements of hormones are warranted to elucidate the causal mechanisms by which TH concentrations may change in response to these dietary interventions. Third, dietary intervention given for only 24 hours might be too short to obtain a complete picture of the changes in TH, which may require longer time periods. However, sustained overfeeding or fasting may result in substantial changes in body weight and body composition, which in turn may impact circulating TH concentrations as a result of the metabolic adaptation to weight gain/loss (68). As our aim was to assess the short-term effects of fasting and overfeeding diets on TH concentrations during weight stability, we sought to assess whether TH mediate the diet-induced changes in energy metabolism observed over 24 hours. It is noteworthy that the changes in fT4 and fT3 after the LPF and HPF were related, indicating a role for protein intake in regulating circulating TH concentrations.
In summary, 24-hour fasting and overfeeding diets with altered protein content (high and low) induce variable changes in TH concentrations; however, these changes were not associated with changes in 24-hour EE or substrate oxidation during these acute, short-term dietary interventions. Circulating TH concentrations changed differently by diet, implying different regulatory mechanisms in each case and suggesting that other factors (e.g., hormones) might be involved in the TH response to diet. Although the current study was not designed to prove causality between the changes in fT3 and FGF21 following dietary interventions, we did find an association between those changes after high-protein overfeeding, which might suggest an effect of T3 on FGF21 or vice versa during this specific dietary intervention.
In conclusion, despite their involvement in protein metabolism in settings of proteins surplus or deficit, TH do not mediate the short-term effects of acute dietary interventions on 24-hour EE, indicating that TH do not likely explain the interindividual differences in diet-induced thermogenesis that quantifies the susceptibility to weight gain.
Supplementary Material
Acknowledgments
The authors wish to thank the subjects who were enrolled in the study and the clinical staff of the Phoenix Epidemiology and Clinical Research Branch for conducting the examinations. This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases.
Prior Presentation
The study has not been published previously in abstract form or article.
Authors' Contributions
A.B. analyzed and interpreted the data and wrote the article. B.B. and T.H. assisted with the data analysis and revised the article. P.P. and F.S. supported with the interpretation of the data and reviewed the article. K.L.V. conducted the study, and P.P. and J.K. designed and implemented the study. M.W. performed hormone measurements. All authors critically revised the draft and approved the final article. A.B. and P.P. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Author Disclosure Statement
No competing financial interests exist.
Supplementary Material
References
- 1. Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF. 2014. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 384:766–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stock MJ. 1999. Gluttony and thermogenesis revisited. Int J Obes 23:1105. [DOI] [PubMed] [Google Scholar]
- 3. Piaggi P. 2019. Metabolic determinants of weight gain in humans. Obesity 27:691–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Piaggi P, Vinales KL, Basolo A, Santini F, Krakoff J. 2018. Energy expenditure in the etiology of human obesity: spendthrift and thrifty metabolic phenotypes and energy-sensing mechanisms. J Endocrinol Invest 41:83–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dulloo A, Jacquet J. 1999. Low-protein overfeeding: a tool to unmask susceptibility to obesity in humans. Int J Obes 23:1118. [DOI] [PubMed] [Google Scholar]
- 6. Schlögl M, Piaggi P, Pannacciuli N, Bonfiglio SM, Krakoff J, Thearle MS. 2015. Energy expenditure responses to fasting and overfeeding identify phenotypes associated with weight change. Diabetes 64:3680–3689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vinales KL, Begaye B, Bogardus C, Walter M, Krakoff J, Piaggi P. 2019. FGF21 is a hormonal mediator of the human “thrifty” metabolic phenotype. Diabetes 68:318–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. López M, Alvarez CV, Nogueiras R, Diéguez C. 2013. Energy balance regulation by thyroid hormones at central level. Trends Mol Med 19:418–427 [DOI] [PubMed] [Google Scholar]
- 9. Fisher FM, Maratos-Flier E. 2016. Understanding the physiology of FGF21. Annu Rev Physiol 78:223–241 [DOI] [PubMed] [Google Scholar]
- 10. Vinales KL, Begaye B, Bogardus C, Walter M, Krakoff J, Piaggi P. 2019. FGF21 is a hormonal mediator of the human “thrifty” metabolic phenotype. Diabetes 68:318–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Klieverik LP, Coomans CP, Endert E, Sauerwein HP, Havekes LM, Voshol PJ, Rensen PC, Romijn JA, Kalsbeek A, Fliers E. 2009. Thyroid hormone effects on whole-body energy homeostasis and tissue-specific fatty acid uptake in vivo. Endocrinology 150:5639–5648 [DOI] [PubMed] [Google Scholar]
- 12. Adams AC, Astapova I, Badman MK, Kurgansky KE, Flier JS, Hollenberg AN, Maratos-Flier E. 2010. Thyroid hormone regulates hepatic expression of fibroblast growth factor 21 in a PPARα-dependent manner. J Biol Chem 285:14078–14082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cheng S-Y, Leonard JL, Davis PJ. 2010. Molecular aspects of thyroid hormone actions. Endocr Rev 31:139–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Iwen KA, Schröder E, Brabant G. 2013. Thyroid hormones and the metabolic syndrome. Eur Thyroid J 2:83–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim B. 2008. Thyroid hormone as a determinant of energy expenditure and the basal metabolic rate. Thyroid 18:141–144 [DOI] [PubMed] [Google Scholar]
- 16. Freake HC, Schwartz HL, Oppenheimer JH. 1989. The regulation of lipogenesis by thyroid hormone and its contribution to thermogenesis. Endocrinology 125:2868–2874 [DOI] [PubMed] [Google Scholar]
- 17. Silva JE. 2006. Thermogenic mechanisms and their hormonal regulation. Physiol Rev 86:435–464 [DOI] [PubMed] [Google Scholar]
- 18. Simonides WS, van Hardeveld C. 2008. Thyroid hormone as a determinant of metabolic and contractile phenotype of skeletal muscle. Thyroid 18:205–216 [DOI] [PubMed] [Google Scholar]
- 19. Danforth E, Horton E, O'Connell M, Sims E, Burger A, Ingbar S, Braverman L, Vagenakis A. 1979. Dietary-induced alterations in thyroid hormone metabolism during overnutrition. J Clin Invest 64:1336–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Utiger RD. 1982. Differing thyrotropin responses to increased serum triiodothyronine concentrations produced by overfeeding and by triiodothyronine administration. Metabolism 31:180–183 [DOI] [PubMed] [Google Scholar]
- 21. Vagenakis A, Burger A, Portnay G, Rudolph M, O'Brain J, Azizi F, Arky R, Nicod P, Ingbar S, Braverman L. 1975. Diversion of peripheral thyroxine metabolism from activating to inactivating pathways during complete fasting. J Clin Endocrinol Metab 41:191–194 [DOI] [PubMed] [Google Scholar]
- 22. Spaulding SW, Chopra IJ, Sherwin RS, Lyall SS. 1976. Effect of caloric restriction and dietary composition on serum T3 and reverse T3 in man. J Clin Endocrinol Metab 42:197–200 [DOI] [PubMed] [Google Scholar]
- 23. Langouche L, Vander Perre S, Marques M, Boelen A, Wouters PJ, Casaer MP, Van den Berghe G. 2013. Impact of early nutrient restriction during critical illness on the nonthyroidal illness syndrome and its relation with outcome: a randomized, controlled clinical study. J Clin Endocrinol Metab 98:1006–1013 [DOI] [PubMed] [Google Scholar]
- 24. Croxson MS, Hall TD, Kletzky OA, Jaramillo JE, Nicoloff JT. 1977. Decreased serum thyrotropin induced by fasting. J Clin Endocrinol Metab 45:560–568 [DOI] [PubMed] [Google Scholar]
- 25. Pannacciulli N, Salbe AD, Ortega E, Venti CA, Bogardus C, Krakoff J. 2007. The 24-h carbohydrate oxidation rate in a human respiratory chamber predicts ad libitum food intake. Am J Clin Nutr 86:625–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. American Diabetes Association 2014. Diagnosis and classification of diabetes mellitus. Diabetes Care 37(Suppl. 1):S81–S90 [DOI] [PubMed] [Google Scholar]
- 27. Thearle MS, Pannacciulli N, Bonfiglio S, Pacak K, Krakoff J. 2013. Extent and determinants of thermogenic responses to 24 hours of fasting, energy balance, and five different overfeeding diets in humans. J Clin Endocrinol Metab 98:2791–2799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. 1986. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. J Clin Invest 78:1568–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Abbott W, Howard BV, Christin L, Freymond D, Lillioja S, Boyce VL, Anderson TE, Bogardus C, Ravussin E. 1988. Short-term energy balance: relationship with protein, carbohydrate, and fat balances. Am J Physiol 255:E332–E337 [DOI] [PubMed] [Google Scholar]
- 30. Piaggi P, Thearle MS, Bogardus C, Krakoff J. 2013. Lower energy expenditure predicts long-term increases in weight and fat mass. J Clin Endocrinol Metab 98:E703–E707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lopresti JS, Gray D, Nicoloff JT. 1991. Influence of fasting and refeeding on 3, 3′, 5′-triiodothyronine metabolism in man. J Clin Endocrinol Metab 72:130–136 [DOI] [PubMed] [Google Scholar]
- 32. Docter R, Krenning E, De Jong M, Hennemann G. 1993. The sick euthyroid syndrome: changes in thyroid hormone serum parameters and hormone metabolism. Clin Endocrinol 39:499–518 [DOI] [PubMed] [Google Scholar]
- 33. Azizi F. 1978. Effect of dietary composition on fasting-induced changes in serum thyroid hormones and thyrotropin. Metabolism 27:935–942 [DOI] [PubMed] [Google Scholar]
- 34. Burman KD, Smallridge RC, Osburne R, Dimond RC, Whorton NE, Kesler P, Wartofsky L. 1980. Nature of suppressed TSH secretion during undernutrition: effect of fasting and refeeding on TSH responses to prolonged TRH infusions. Metabolism 29:46–52 [DOI] [PubMed] [Google Scholar]
- 35. Spencer CA, Lum SM, Wilber JF, Kaptein EM, Nicoloff JT. 1983. Dynamics of serum thyrotropin and thyroid hormone changes in fasting. J Clin Endocrinol Metab 56:883–888 [DOI] [PubMed] [Google Scholar]
- 36. Boelen A, Wiersinga WM, Fliers E. 2008. Fasting-induced changes in the hypothalamus–pituitary–thyroid axis. Thyroid 18:123–129 [DOI] [PubMed] [Google Scholar]
- 37. Merimee TJ, Fineberg E. 1976. Starvation-induced alterations of circulating thyroid hormone concentrations in man. Metabolism 25:79–83 [DOI] [PubMed] [Google Scholar]
- 38. Fazeli PK, Lun M, Kim SM, Bredella MA, Wright S, Zhang Y, Lee H, Catana C, Klibanski A, Patwari P, Steinhauser ML. 2015. FGF21 and the late adaptive response to starvation in humans. J Clin Invest 125:4601–4611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Staiger H, Keuper M, Berti L, Hrabě de Angelis M, Häring H-U. 2017. Fibroblast growth factor 21—metabolic role in mice and men. Endocr Rev 38:468–488 [DOI] [PubMed] [Google Scholar]
- 40. Morrison CD, Reed SD, Henagan TM. 2012. Homeostatic regulation of protein intake: in search of a mechanism. Am J Physiol 302:R917–R928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shi Z-X, Levy A, Lightman S. 1993. The effect of dietary protein on thyrotropin-releasing hormone and thyrotropin gene expression. Brain Res 606:1–4 [DOI] [PubMed] [Google Scholar]
- 42. Bruno JF, Song J, Berelowitz M. 1991. Regulation of rat hypothalamic preprogrowth hormone-releasing factor messenger ribonucleic acid by dietary protein. Endocrinology 129:1226–1232 [DOI] [PubMed] [Google Scholar]
- 43. Cree T, Schalch D. 1985. Protein utilization in growth: effect of lysine deficiency on serum growth hormone, somatomedins, insulin, total thyroxine (T4) and triiodothyronine, free T4 index, and total corticosterone. Endocrinology 117:667–673 [DOI] [PubMed] [Google Scholar]
- 44. Lunn P, Austin S. 1983. Excess energy intake promotes the development of hypoalbuminaemia in rats fed on low-protein diets. Br J Nutr 49:9–16 [DOI] [PubMed] [Google Scholar]
- 45. Jepson M, Bates P, Millward D. 1988. The role of insulin and thyroid hormones in the regulation of muscle growth and protein turnover in response to dietary protein in the rat. Br J Nutr 59:397–415 [DOI] [PubMed] [Google Scholar]
- 46. Smallridge RC, Glass AR, Wartofsky L, Latham KR, Burman KD. 1982. Investigations into the etiology of elevated serum T3 levels in protein-malnourished rats. Metabolism 31:538–542 [DOI] [PubMed] [Google Scholar]
- 47. Welle S, O'Connell M, Danforth E, Jr, Campbell R. 1984. Decreased free fraction of serum thyroid hormones during carbohydrate overfeeding. Metabolism 33:837–839 [DOI] [PubMed] [Google Scholar]
- 48. Ullrich IH, Peters PJ, Albrink M. 1985. Effect of low-carbohydrate diets high in either fat or protein on thyroid function, plasma insulin, glucose, and triglycerides in healthy young adults. J Am Coll Nutr 4:451–459 [DOI] [PubMed] [Google Scholar]
- 49. Barrows K, Snook J. 1987. Effect of a high-protein, very-low-calorie diet on resting metabolism, thyroid hormones, and energy expenditure of obese middle-aged women. Am J Clin Nutr 45:391–398 [DOI] [PubMed] [Google Scholar]
- 50. Buroker NE, Young ME, Wei C, Serikawa K, Ge M, Ning X-H, Portman MA. 2007. The dominant negative thyroid hormone receptor β-mutant Δ337T alters PPARα signaling in heart. Am J Physiol 292:E453–E460 [DOI] [PubMed] [Google Scholar]
- 51. Araki O, Ying H, Furuya F, Zhu X, Cheng S-Y. 2005. Thyroid hormone receptor β mutants: dominant negative regulators of peroxisome proliferator-activated receptor γ action. Proc Natl Acad Sci USA 102:16251–16256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Badman MK, Kurgansky KE, Flier JS, Hollenberg AN, Maratos-Flier E. 2010. Thyroid hormone regulates hepatic expression of fibroblast growth factor 21 in a PPAR-dependent manner. J Biol Chem 285:14078–14082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yilmaz U, Tekin S, Demir M, Cigremis Y, Sandal S. 2018. Effects of central FGF21 infusion on the hypothalamus–pituitary–thyroid axis and energy metabolism in rats. J Physiol Sci 68:781–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Al-Adsani H, Hoffer LJ, Silva JE. 1997. Resting energy expenditure is sensitive to small dose changes in patients on chronic thyroid hormone replacement. J Clin Endocrinol Metab 82:1118–1125 [DOI] [PubMed] [Google Scholar]
- 55. Toubro S, Sørensen T, Rønn B, Christensen NJ, Astrup A. 1996. Twenty-four-hour energy expenditure: the role of body composition, thyroid status, sympathetic activity, and family membership. J Clin Endocrinol Metab 81:2670–2674 [DOI] [PubMed] [Google Scholar]
- 56. Astrup A, Buemann B, Christensen NJ, Madsen J, Gluud C, Bennett P, Svenstrup B. 1992. The contribution of body composition, substrates, and hormones to the variability in energy expenditure and substrate utilization in premenopausal women. J Clin Endocrinol Metab 74:279–286 [DOI] [PubMed] [Google Scholar]
- 57. Svendsen OL, Hassager C, Christiansen C. 1993. Impact of regional and total body composition and hormones on resting energy expenditure in overweight postmenopausal women. Metabolism 42:1588–1591 [DOI] [PubMed] [Google Scholar]
- 58. Ortega E, Pannacciulli N, Bogardus C, Krakoff J. 2007. Plasma concentrations of free triiodothyronine predict weight change in euthyroid persons. Am J Clin Nutr 85:440–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Maia AL, Kim BW, Huang SA, Harney JW, Larsen PR. 2005. Type 2 iodothyronine deiodinase is the major source of plasma T 3 in euthyroid humans. J Clin Invest 115:2524–2533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gereben B, Zeöld A, Dentice M, Salvatore D, Bianco A. 2008. Activation and inactivation of thyroid hormone by deiodinases: local action with general consequences. Cell Mol Life Sci 65:570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mullur R, Liu Y-Y, Brent GA. 2014. Thyroid hormone regulation of metabolism. Physiol Rev 94:355–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lechan RM. 1987. Neuroendocrinology of pituitary hormone regulation. Endocrinol Metab Clin North Am 16:475–501 [PubMed] [Google Scholar]
- 63. Hall JA, Ribich S, Christoffolete MA, Simovic G, Correa-Medina M, Patti ME, Bianco AC. 2010. Absence of thyroid hormone activation during development underlies a permanent defect in adaptive thermogenesis. Endocrinology 151:4573–4582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Dentice M, Marsili A, Ambrosio R, Guardiola O, Sibilio A, Paik J-H, Minchiotti G, DePinho RA, Fenzi G, Larsen PR. 2010. The FoxO3/type 2 deiodinase pathway is required for normal mouse myogenesis and muscle regeneration. J Clin Invest 120:4021–4030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. De Jesus LA, Carvalho SD, Ribeiro MO, Schneider M, Kim S-W, Harney JW, Larsen PR, Bianco AC. 2001. The type 2 iodothyronine deiodinase is essential for adaptive thermogenesis in brown adipose tissue. J Clin Invest 108:1379–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Brent GA. 2012. Mechanisms of thyroid hormone action. J Clin Invest 122:3035–3043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Christoffolete MA, Linardi CC, De Jesus L, Ebina KN, Carvalho SD, Ribeiro MO, Rabelo R, Curcio C, Martins L, Kimura ET. 2004. Mice with targeted disruption of the Dio2 gene have cold-induced overexpression of the uncoupling protein 1 gene but fail to increase brown adipose tissue lipogenesis and adaptive thermogenesis. Diabetes 53:577–584 [DOI] [PubMed] [Google Scholar]
- 68. Rosenbaum M, Hirsch J, Murphy E, Leibel RL. 2000. Effects of changes in body weight on carbohydrate metabolism, catecholamine excretion, and thyroid function. Am J Clin Nutr 71:1421–1432 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.