Abstract
Smaller hippocampal volume is associated with increased risk for PTSD following trauma, but the hippocampal functions involved remain unknown. We propose a conceptual model that identifies broad impairment in hippocampus-dependent associative learning as a vulnerability factor for PTSD. Associative learning of foreground cues and background context is required to form an integrated representation of an event. People with poor associative learning may have difficulty remembering who or what was present during a trauma, where the trauma occurred, or the sequence of events, which may contribute to PTSD symptoms. We argue that associative learning difficulties in PTSD exist for cues and context, regardless of the emotional nature of the information. This contrasts with PTSD models that focus exclusively on threat-processing or contextual-processing. In a meta-analysis, people with PTSD exhibited poor associative learning of multiple information types compared to those without PTSD. Differences were of medium effect size and similar magnitude for neutral and negative/trauma-related stimuli. We provide evidence for associative learning difficulties as a neurocognitive pathway that may contribute to PTSD.
Keywords: Associative learning, Hippocampus, PTSD, Trauma
1. Introduction
Trauma exposure is common. Approximately 60% of children (McLaughlin et al., 2013) and 75% of adults (Kessler et al., 1995; Kessler, 2000; Roberts et al., 2011) will experience a traumatic event. As many as 8% of those exposed to trauma develop post-traumatic stress disorder (PTSD) (Breslau, 2004; Breslau and Davis, 1992; Kessler et al., 1995, 2005; McLaughlin et al., 2013). The public health consequences of PTSD are profound. Individuals with PTSD are at increased risk of major depression (Breslau et al., 2000), substance dependence (Breslau et al., 2003), physical health problems (Kubzansky et al., 2007; Zayfert et al., 2002), and marital instability (Kessler, 2000). A person with PTSD misses 3.6 days of work per month on average due to the disorder, and lost productivity is valued at over 3 billion dollars each year in the U.S. (Kessler, 2000). Although evidence-based interventions have been developed that are effective for many people, as many as half of people with PTSD fail to respond to these interventions (Foa et al., 1991; Marks et al., 1998; Resick et al., 2002) and many exhibit a chronic course that lasts for years (Kessler et al., 1995; McLaughlin et al., 2013; Perkonigg et al., 2005).
Greater understanding of the mechanisms that contribute to the onset and persistence of PTSD is necessary in order to develop novel interventions for those who do not respond to established treatments. Here, we propose a novel mechanism that might explain why some people are more vulnerable to developing PTSD after a traumatic event. Specifically, we argue that difficulties with hippocampus-dependent associative learning prior to trauma exposure contribute to the onset and persistence of PTSD. In this paper, we briefly review prevailing threat-based models of PTSD. We articulate a complementary associative learning model of PTSD and offer five falsifiable predictions based on this model. We then present a meta-analysis of the existing literature on associative learning and PTSD to test these predictions. We end by discussing the implications of this model and directions for future research.
1.1. Prevailing threat-based models of PTSD
People with PTSD re-experience the traumatic event in the form of intrusive thoughts, memories, and nightmares; exhibit strong psychological and physiological reactions to trauma reminders; and experience chronic elevations in arousal, even in safe environments (American Psychiatric Association, 2013; Pole, 2007). These re-experiencing and arousal symptoms often lead to high levels of avoidance of both internal reminders of the trauma (e.g., thinking about the event) as well as external reminders (e.g., people, places, and stimuli that are associated with the traumatic event). Additionally, people with PTSD often experience altered cognition after the trauma, such as an inability to recall important aspects of the trauma or persistent and distorted blame.
Prevailing conceptual models of the onset and persistence of PTSD focus on threat processing, fear learning, and prefrontal-amygdala circuitry to explain many of these symptoms (Foa and Kozak, 1986; Jovanovic and Ressler, 2010; Liberzon and Sripada, 2007; Mineka and Oehlberg, 2008). Within these models, neutral stimuli present during a traumatic event become associated with threat and elicit conditioned fear. This conditioned fear then generalizes to a wide range of stimuli that resemble those present during the trauma. The amygdala, which is responsible for the acquisition and expression of conditioned fear (Johansen et al., 2011; Kim and Jung, 2006; Phelps, 2006), may exhibit elevated activation to potential threats. Difficulty extinguishing conditioned fear to trauma cues that no longer predict danger or inhibiting fear in the presence of cues that signify safety may further contribute to inappropriate fear expression. Differences in the function of the ventromedial prefrontal cortex (vmPFC), which modulates amygdala reactivity to extinguish and inhibit conditioned fear after extinction learning (Milad and Quirk, 2012; Phelps et al., 2004; Quirk et al., 2000), may contribute to the persistent fear and arousal observed in people with PTSD as a result of greater difficulty retaining extinction memories to cues previously associated with threat. More recent models of PTSD encompass additional intermediate phenotypes, including difficulties with effortful emotion regulation, executive functioning, and contextual processing (Liberzon, 2018; Liberzon and Abelson, 2016). For example, fear extinction memories are highly context-dependent (Bouton et al., 2006) and influenced by hippocampal-prefrontal regulation of the amygdala (Milad and Quirk, 2012; Moscarello and Maren, 2018). Some have proposed that people with PTSD have difficulty using contextual information to determine whether an extinction memory should be retrieved, which may ultimately lead to pathological fear in safe contexts (Liberzon, 2018; Liberzon and Abelson, 2016; Maren et al., 2013). Later, we discuss difficulties with contextual regulation of fear in relation to our associative learning model of PTSD.
Strong evidence supports these threat models. People with PTSD exhibit greater attention and amygdala reactivity to threat or trauma cues than those who experienced a traumatic event but did not develop PTSD (Fani et al., 2012; Harvey et al., 1996; Rauch et al., 2000; Shin et al., 2004; Vermetten et al., 2007). People with PTSD also have difficulty recalling fear extinction memories (Garfinkel et al., 2014; Milad et al., 2008, 2009) and inhibiting fear in the presence of safety signals (Jovanovic et al., 2012, 2010, 2009; Jovanovic and Ressler, 2010) and have reduced amygdala-prefrontal functional connectivity in response to threatening and negative stimuli (Stevens et al., 2013; Wolf and Herringa, 2016). Although evidence supporting these models is compelling, other mechanisms may also be involved in PTSD onset and persistence. Threat models do not account for the full range of PTSD symptoms, such as difficulty distinguishing safe from dangerous contexts and difficulty recalling basic details of the traumatic event as well as neutral and positive life events (Amir et al., 1998; Garfinkel et al., 2014; Liberzon and Abelson, 2016; Moore and Zoellner, 2007; Ono et al., 2016; van der Kolk and Fisler, 1995).
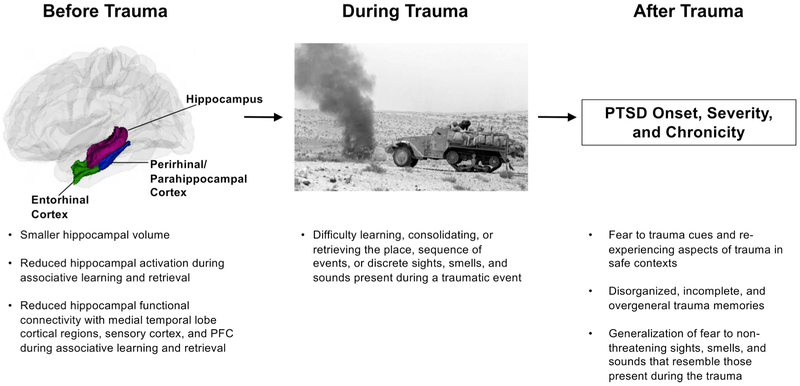
Here, we present a conceptual model (Fig. 1) outlining a cognitive and neurobiological mechanism different from those proposed by threat models that addresses this issue. Our model builds upon prior work focused on the role of the hippocampus in PTSD, but we articulate a different perspective on the precise hippocampal functions that might confer vulnerability for PTSD. We argue that individual differences in hippocampus-dependent associative learning, present prior to trauma exposure, might influence whether or not a person will develop PTSD and how the disorder progresses. Specifically, we propose that poor associative learning of both foreground cues and contextual information prior to trauma represents a vulnerability factor for chronic PTSD after trauma. This pattern of associative learning should be reflected in reduced hippocampal activation and functional connectivity with medial temporal lobe cortical regions, sensory cortex, and PFC. Difficulties with associative learning can explain multiple PTSD symptoms and associated features that cannot be explained solely with existing models focused on threat processing. As with any pathological phenotype, multiple mechanisms likely operate within an individual to contribute to PTSD, and the relative importance of each mechanism likely differs across individuals (Cicchetti and Rogosch, 1996). This associative learning model complements current threat-based models to account for heterogeneity in PTSD symptom expression, chronicity, and responsiveness to treatment, which one mechanism alone is unlikely to explain. Understanding the most relevant mechanisms in different PTSD subtypes, particularly treatment-resistant PTSD, is required to tailor interventions.
Fig. 1.
The associative learning model of PTSD.
1.2. The associative learning model of PTSD
We first describe the neural circuitry that supports item, associative, and autobiographical memory and make distinctions between these different types of memory. We then discuss evidence that points to impairments in hippocampus-dependent associative learning as a potential mechanism underlying the onset and chronicity of PTSD. Finally, we discuss possible pathways through which associative learning difficulties may contribute to PTSD.
1.2.1. Neural circuitry underlying item, associative, and autobiographical memory
An autobiographical memory is a representation of a personal event complete with sensory, emotional, semantic, and schematic information. Structural and functional connectivity between sensory cortex; cortical areas of the medial temporal lobe and hippocampus; and PFC support learning, consolidation, and retrieval of autobiographical memories (for reviews see Davachi, 2006; Eichenbaum et al., 2007; Euston et al., 2012; Lavenex and Amaral, 2000; Mayes et al., 2007; Squire, 1992). Information becomes increasingly integrated as it progresses through this hierarchical circuit during learning.
Different regions of sensory cortex send separate streams of unimodal and polymodal sensory information (e.g., visual, auditory, somatosensory, visuo-spatial) to medial temporal lobe cortical regions for integration. Different medial temporal lobe cortical regions are responsible for forming representations about cues and context (Davachi, 2006; Eichenbaum et al., 2007). Specifically, the perirhinal cortex integrates features of objects and other foreground cues (e.g., learning that an apple is red, round, and shiny) and the posterior parahippocampal cortex integrates background contextual features (e.g., learning that an office includes a desk, chair, computer, and lamp in a particular spatial orientation and has a continuous humming noise of a fan). Foreground cues can occur in multiple sensory domains (e.g., sight, sound, smell), and contexts are typically multi-sensory. We refer to this step as item encoding, as the representation being formed is remembered as a whole. It is important to note, however, that even this phase of memory encoding requires the ability to associate multiple features of a single item together (e.g., red, round, and shiny for an apple; desk, chair, computer, lamp, and humming noise for an office). Additionally, the hippocampus is involved in forming context representations, in addition to binding these representations with foreground cues or events (Maren et al., 2013; Young et al., 1994).
The process by which items are bound together to form more complex representations in the hippocampus is the focus of our paper, and we refer to this step as hippocampus-dependent associative learning. Item information is sent from the perirhinal and parahippocampal cortices through the entorhinal cortex to the hippocampus, which binds separate items together. Long-term potentiation—a form of synaptic plasticity that underlies learning—in the hippocampus strengthens connections between cortical neurons that fire together. Learning the associations between co-occurring cues, the context in which those cues or events occur, the spatial arrangement of cues or location of cues in context, and the temporal progression of events are all examples of hippocampus-mediated associative learning. This type of associative learning may also rely on medial temporal lobe cortical structures when the items are from the same sensory domain (e.g., pairing two visual cues involves the perirhinal cortex) (Mayes et al., 2007). Hippocampus-dependent associative learning is critical for forming an autobiographical memory, regardless of the emotional nature of the event. Neuroimaging studies demonstrate that cortical regions of the medial temporal lobe, the hippocampus, and the PFC support associative learning of both foreground cues and contextual information occurring in the background of those cues in adults (DuBrow and Davachi, 2016; Hayes et al., 2010, 2007; Henke et al., 1997; Jackson and Schacter, 2004; Kirwan and Stark, 2004; Sperling et al., 2003).
An extended network of brain regions beyond these medial temporal lobe regions integrates emotional information (e.g., amygdala), semantic information (e.g., anterior temporal cortex; ventrolateral PFC), and appraisals based on similar experiences or schemas (e.g., medial PFC) into the memory (Dixon et al., 2017; Euston et al., 2012; Moscovitch et al., 2016; Talmi, 2013). We refer to the final representation of a personal event—complete with sensory, emotional, semantic, and schematic information—as an autobiographical memory.
During early memory consolidation and retrieval, the hippocampus and PFC are thought to project back to the regions of the sensory cortex that sent the original information during encoding in order to reinstate and bind a similar pattern of activity across those regions (Hoffman and McNaughton, 2002). While the hippocampus and PFC are both involved in early memory consolidation and retrieval (i.e., in the first few hours after learning), the PFC is primarily involved in late memory consolidation and retrieval (i.e., hours to weeks after learning) (for a review, see Euston et al., 2012). The midline thalamus (i.e., rhomboid and reuniens nuclei) connects the hippocampus and mPFC and is particularly relevant for functions involving both the hippocampus and mPFC, including transferring recent memories to remote memories (Cassel and de Vasconcelos, 2015; Vertes, 2006).
We do not focus on amygdala-mediated associative learning of cues during fear conditioning or extinction learning. However, hippocampus-dependent binding of context with foreground cues or events, in particular, may be relevant to the ability to recall extinction memories or renew fear memories—processes that are dependent on context and involve hippocampal-prefrontal regulation of the amygdala (Bouton et al., 2006; Milad and Quirk, 2012; Moscarello and Maren, 2018).
1.2.2. Evidence for impairment in hippocampus-dependent associative learning in PTSD
Three consistently documented findings point to impairments in hippocampus-dependent associative learning as a key mechanism underlying the onset and chronicity of PTSD: reduced hippocampal volume, poor contextual processing, and overly general autobiographical memory in PTSD. We review each of these areas briefly, along with complementary hippocampus-based conceptual models that have been proposed to explain these patterns of findings.
1.2.2.1. Reduced hippocampal volume in PTSD.
A substantial body of research finds reduced hippocampal volume in people with PTSD. In multiple meta-analyses as well as a recent large-scale consortia, adults with PTSD have smaller hippocampal volume than those exposed to trauma without PTSD or without trauma exposure (Karl et al., 2006; Kitayama et al., 2005; Logue et al., 2018; Nelson and Tumpap, 2017; O’Doherty et al., 2015). Smaller hippocampal volume also predicts more chronic and severe manifestations of PTSD (Karl et al., 2006; Kitayama et al., 2005; Nelson and Tumpap, 2017). Reduced hippocampal volume may exist prior to trauma and influence whether or not a person will develop PTSD after that trauma. Combat-exposed veterans with severe PTSD as well as their identical twins without combat exposure had significantly smaller hippocampal volume than combat-exposed veterans without PTSD and their unexposed identical twins (Gilbertson et al., 2002, 2007; Pitman et al., 2006). The presence of reduced hippocampal volume in the combat-unexposed identical twins of veterans with PTSD suggests that this may be a pre-trauma vulnerability factor for PTSD rather than a consequence of trauma exposure in adulthood or of PTSD onset; however, this remains to be determined in longitudinal designs that measure hippocampal volume before trauma exposure in those who develop PTSD.
It is unclear how individual variation in hippocampal volume would emerge prior to the trauma that precedes the onset of PTSD. Genetics or the early shared environment may contribute to smaller hippocampal volume in both combat-exposed veterans with severe PTSD and their identical twins without combat exposure. Trauma exposure in childhood is one early-life experience that influences the development of the hippocampus. Extensive work in animal models demonstrates the toxic and long-lasting effects of glucocorticoids early in life on hippocampal neurons (for a review, see Lupien et al., 2009). Specifically, enhanced corticotropin-releasing hormone binding in the hippocampus following chronic early-life stress reduces dendritic spines and branching in hippocampal neurons in rodents, and these effects persist with age (Brunson et al., 2001; Ivy et al., 2010). Chronic stress in adult rats, however, leads to dendritic atrophy in the hippocampus that lasts only for a few weeks once the stress has ended (e.g., Conrad et al., 1999). Consistent with these animal models, exposure to violence and other forms of adversity early in life have been associated with reductions in the volume of the hippocampus as well as surrounding cortical regions in the medial temporal lobe, particularly the parahippocampal gyrus, in humans (Busso et al., 2017; Gold et al., 2016; Hanson et al., 2015; Lambert et al., 2017; McLaughlin et al., 2016; Teicher et al., 2012). Childhood trauma exposure is not only associated with reduced hippocampal volume, but also with increased risk of PTSD after traumatic events experienced later in life (Breslau et al., 2014; Brewin et al., 2000; McLaughlin et al., 2010, 2017). Reduced hippocampal volume may be one mechanism through which childhood trauma exposure increases vulnerability to the effects of later stressful life events and for developing PTSD (Weissman, Lambert, Rodman, Sheridan, McLaughlin, under review).
Regardless of how individual differences in hippocampal volume emerge, there is clearly a link between smaller hippocampal volume (that may exist prior to trauma) and the development, chronicity, and severity of PTSD. Despite the consistency of this evidence, however, the precise hippocampal functions that contribute to PTSD risk remain poorly understood (Isaac et al., 2006; Pitman et al., 2012).
1.2.2.2. Poor contextual processing in PTSD.
People with PTSD may have trouble using contextual information to disambiguate whether a cue is threatening or not (Liberzon, 2018; Liberzon and Abelson, 2016; Maren et al., 2013). The hippocampus is centrally involved in this process. The PFC has dual control over fear expression, with the vmPFC inhibiting the amygdala during recall of extinction memories and the dorsal anterior cingulate cortex (dACC) enhancing amygdala activation during recall of conditioned fear memories (Milad and Quirk, 2012). Through its connections with the PFC and amygdala, the hippocampus inhibits or promotes amygdala activity and fear expression based on context (Milad and Quirk, 2012; Moscarello and Maren, 2018; Sotres-Bayon et al., 2012). Fear extinction memory, in particular, is highly context-specific (Bouton, 2004; Bouton et al., 2006). The hippocampus and contextual cues therefore play a central role in determining whether an extinction versus fear memory is retrieved.
Recent work shows that alterations in hippocampal function may play a role in problems with fear extinction recall and renewal in PTSD. Specifically, adults with PTSD have greater physiological responses to extinguished threat cues (i.e., cues that no longer predict danger) in a safe context than trauma-exposed people without PTSD (Garfinkel et al., 2014; Milad et al., 2009). This fear expression is accompanied by less activation of the hippocampus and vmPFC, and greater activation of the amygdala and dACC in people with PTSD than trauma-exposed controls (Garfinkel et al., 2014; Milad et al., 2009; Rougemont-Bücking et al., 2011). A variety of processes might explain this pattern. Poor fear extinction recall could reflect enhanced threat reactivity, exaggerated conditioned fear, poor inhibition of fear, or weakened vmPFC modulation of the amygdala. Alternatively, the hippocampus may not be effectively using contextual information to disambiguate whether a cue is threatening or not and modulating amygdala reactivity accordingly. Consistent with the latter possibility, people with PTSD also have less physiological reactivity when re-exposed to an extinguished threat cue in the original context where they acquired the fear compared to people without PTSD (Garfinkel et al., 2014). This dampened fear expression is accompanied by less activation of the vmPFC and amygdala (Garfinkel et al., 2014). These findings of exaggerated fear in a safe context and diminished fear in a dangerous context are difficult to explain with prevailing models focused on enhanced threat detection, poor inhibition of fear, and weakened vmPFC-amygdala connectivity. Evidence instead points to impaired hippocampus-dependent contextual regulation of fear. Indeed, Liberzon and others propose that poor contextual regulation of fear is a central mechanism in PTSD (Liberzon, 2018; Liberzon and Abelson, 2016; Maren et al., 2013).
The specific processes contributing to impaired contextual regulation of fear in PTSD, however, are largely unknown. Contextual regulation of fear relies on hippocampal-PFC-amygdala circuitry and requires retrieval of contextual information from past encounters with a potentially threatening cue to facilitate accurate interpretation of that cue in the present moment, and in turn, generate an appropriate response. Problems with contextual regulation of fear could therefore reflect difficulties with basic aspects of contextual processing, including binding the individual elements of a contextual representation together or binding context with a traumatic event (Liberzon, 2018; Liberzon and Abelson, 2016; Maren et al., 2013). Difficulty encoding, consolidating, or retrieving contextual information from a traumatic event could result in inappropriate fear, such as greater fear in a safe context or dampened fear in a dangerous context.
We argue that difficulty encoding and retrieving contextual information may be just one example of a broader issue with associative learning that contributes to vulnerability to PTSD. Specifically, our model proposes that people with PTSD have associative learning difficulties involving not only information about background context, but also foreground cues that are the focus of explicit attention. Different medial temporal lobe cortical regions are involved in forming representations about foreground cues (e.g., objects) and background context and projecting that information to the hippocampus (for reviews see Davachi, 2006; Eichenbaum et al., 2007). The hippocampus supports associative learning of different types of information—including binding foreground cues with one another and background context with foreground cues. Our model extends those focused explicitly on contextual processing by arguing that disruptions in associative learning in those with PTSD occur for a wider range of information. These difficulties with hippocampus-dependent associative learning may contribute to PTSD in part through pathways involving poor contextual regulation of fear but also through additional pathways involving other types of memory difficulties, which we discuss in detail below.
Other cognitive models of PTSD, like those proposed by Brewin and colleagues (Brewin et al., 1996; Brewin, 2001; Brewin and Holmes, 2003) and Ehlers and colleagues (Ehlers and Clark, 2000; Ehlers et al., 2004), also focus on impaired hippocampus-dependent contextual processing in PTSD. Specifically, they hypothesize that acute stress during trauma exposure shifts neural resources away from processing contextual and temporal information in the hippocampus and PFC in order to focus on immediate and urgent signals of danger, such as visual cues or one’s emotional responses, processed primarily by the amygdala and associated salience network regions. They propose that this uneven pattern of processing could explain both problems with remembering key details about the time and place of the traumatic event as well as re-experiencing some perceptual aspects of the trauma vividly in PTSD. It is unquestionable that the way in which people process and learn information during the traumatic event shapes how they respond to trauma cues afterwards and whether they develop PTSD. These existing models argue that the intensity of fear and other negative emotions during traumatic experiences is the source of associative learning difficulties for contextual and temporal details. If this were true, then impairments in memory or associative learning in people with PTSD should emerge only in relation to events that involve a high degree of threat or negative emotion. However, as detailed below, a broad literature documents difficulties with autobiographical memory and associative learning not only for threat-related content or memories of negative events, but also for neutral and positive stimuli and events (Burriss et al., 2008; Golier et al., 2003, 2002; Guez et al., 2013, 2011; Moore and Zoellner, 2007; Ono et al., 2016; Smith et al., 2015; Tempesta et al., 2011). Additionally, memory for context-face pairings (Barrett and Kensinger, 2010) and object-face pairings (Lambert et al., 2019) is generally enhanced, not diminished, when facial cues are negative or threatening. These results suggest that threat cues might actually enhance processing of the environment, potentially to facilitate avoidance of future threats. Based on this evidence, we hypothesize that memory problems in people with PTSD are the result of more basic difficulties with hippocampus-based associative learning, regardless of the emotional nature of that information. If our hypothesis is correct, those with PTSD should exhibit difficulties with associative learning regardless of the presence or absence of threat and for information occurring in the foreground as well as in the background context.
The model we present builds upon these two previous accounts of how atypical hippocampal function might contribute to PTSD. Our model posits that poor contextual regulation of fear and poor context encoding during traumatic events reflect broader difficulties in associative learning of different types of information (i.e., not just of contextual information) that occur even in non-threatening situations (i.e., not just during the traumatic event).
1.2.2.3. Overly general autobiographical memory in PTSD.
People with PTSD have overly general autobiographical memories (Brown et al., 2014, 2013; for a review, see Moore and Zoellner, 2007; for a meta-analysis, see Ono et al., 2016). When asked to recall a specific life event (e.g., “When I went to the café last week, I ordered a scone”), people with PTSD instead describe a general category of similar events (e.g., “When I go to the café”) or an event that took place over a long period of time (e.g., “I went on vacation over the break”) (Moore and Zoellner, 2007). Two areas of research suggest that overly general autobiographical memory in PTSD may reflect disruptions in associative learning; however, future studies are needed to evaluate this directly. First, overly general autobiographical memory in PTSD appears to reflect memories that have fewer sensory, emotional, spatial, and temporal details rather than fewer semantic details (Brown et al., 2014). Impaired associative learning of foreground cues and background context could contribute to memories lacking these perceptual details in PTSD. Disrupted associative learning of contextual information could lead to certain autobiographical memory problems (e.g., not recalling where an event took place), whereas disruptions in associative learning of foreground cues could lead to other kinds of autobiographical memory problems (e.g., not recalling who or what was present during the event or difficulty remembering the sequence of events). Second, people with PTSD have trouble recalling specific details of life events, even for neutral and positive events (Moore and Zoellner, 2007; Ono et al., 2016), suggesting a general memory deficit not specific to trauma memories or even to negative or threatening content. This pattern is difficult to explain with prevailing models of PTSD focused on enhanced threat processing or on poor learning of contextual and temporal information only in the presence of acute stress, but are understandable through the lens of an associative learning model of PTSD.
If impaired hippocampus-dependent associative learning underlies autobiographical memory differences in PTSD, then people with PTSD should exhibit reduced hippocampal activation during autobiographical memory formation or retrieval. Studies examining PTSD-related differences in hippocampal activation during encoding and recall of personal memories, realistic images, and verbal descriptions have produced inconsistent results. In some studies, a PTSD diagnosis and greater PTSD symptoms were associated with greater hippocampal activation during encoding of realistic pictures (Brohawn et al., 2010; Stevens et al., 2018). In other studies, however, people with PTSD exhibited less hippocampal activation during encoding of realistic pictures or verbal descriptions than trauma-exposed controls without PTSD (Bremner, Vythilingam, Vermetten, Southwick, McGlashan, Nazeer, et al., 2003; Hayes et al., 2011). Findings related to autobiographical memory retrieval are also inconsistent. Some studies report no differences in hippocampal activation during retrieval of personal memories as a function of PTSD diagnosis or symptoms (Peres et al., 2011; Whalley et al., 2013). Another study found that people with PTSD exhibited greater hippocampal recruitment when recalling negative personal memories and less hippocampal recruitment when recalling positive personal memories than controls without trauma exposure (St. Jacques et al., 2011). Given the complexity of autobiographical memory, the variety of paradigms used across these studies (e.g., neutral, positive, negative, and trauma-related stimuli), and the small number of neuroimaging studies on this topic, it is difficult to draw clear conclusions about hippocampal function during autobiographical memory formation and retrieval in PTSD. Examining hippocampal activation during more basic forms of learning and memory that contribute to autobiographical memory, such as associative learning, may shed light on the specific hippocampal functions that contribute to differences in autobiographical memory in those with PTSD.
1.2.3. Disruptions in associative learning and pathways to PTSD symptoms
Disruptions in hippocampal circuitry and poor encoding, consolidation, or retrieval of foreground cues and contextual information during a traumatic event could produce some of the core symptoms of PTSD (Fig. 1). People with PTSD have fearful reactions to trauma cues and re-experience aspects of the trauma in safe contexts (Ehlers et al., 2002; Hackmann et al., 2004; Pole, 2007). This could reflect a failure to associate the individual elements of a context together or to associate the context with the traumatic event. Knowing the context in which a traumatic event occurred helps a person to respond flexibly to trauma reminders based on whether they encounter those trauma cues in a context that is safe or dangerous. It has also been proposed that a failure to combine individual contextual cues into a single contextual representation could lead to an increase in the number of cues that predict threat and thus an increase in the number of opportunities to later encounter cues that elicit fear, ultimately contributing to PTSD (Acheson et al., 2012). When fear conditioning occurs in the absence of a holistic representation of context, individual contextual cues (e.g., a dark room, a ceiling fan) may become associated with the traumatic event. The likelihood of later encountering and being triggered by any one of these individual contextual cues is greater than the likelihood of encountering and being triggered by the single contextual representation of where the traumatic event actually occurred.
Problems with associative learning of foreground cues could contribute to PTSD through additional pathways. In some studies, people with PTSD have disorganized trauma memories (Foa et al., 1995; Harvey and Bryant, 1999), which could reflect a failure to encode the temporal progression of events during the trauma (i.e., to link events in time into a clear sequence). People with PTSD have trauma memories that are overly general and lacking in specific details (Amir et al., 1998; Moore and Zoellner, 2007; van der Kolk and Fisler, 1995) and generalize fear to stimuli that resemble cues that predict threat (Grillon and Morgan, 1999; Jovanovic et al., 2012; Lissek et al., 2005). This could reflect a failure to associate the trauma with specific sights, smells, sounds, and other sensory cues in the foreground.
1.2.4. Predictions of the associative learning model of PTSD
Threat processing and fear learning mechanisms are clearly relevant to the onset and persistence of PTSD. Here, we have argued that difficulties with hippocampus-dependent associative learning prior to trauma may also contribute to PTSD and help to explain common patterns observed among people with PTSD that are not readily explained in the context of prevailing threat models. The associative learning account is a complementary perspective to existing threat models that may help to explain PTSD onset and persistence in some people or in a particular sub-type of the disorder. Additionally, we argue that poor hippocampus-based associative learning is a basic mechanism that may contribute to problems in more complex forms of memory in PTSD, including poor contextual regulation of fear and overly general autobiographical memory. In doing so, our model broadens existing hippocampus-based models focused on contextual processing in terms of predicting associative memory difficulties for a wider range of stimulus domains and memory problems that occur not only in threatening environments.
We propose five falsifiable hypotheses that arise from this model. In the remainder of the paper, we systematically review and meta-analyze when possible existing evidence from behavioral and functional neuroimaging studies on associative learning and PTSD to evaluate these predictions.
People with PTSD will exhibit poor associative learning compared to people who have never experienced trauma or who have been exposed to trauma but did not develop PTSD. Poor associative learning results in poor associative memory. Therefore, we predict that people with PTSD will exhibit worse associative memory compared to people who never experienced a traumatic event (i.e., controls without trauma) and to people who experienced a traumatic event but did not develop PTSD (i.e., trauma-exposed controls without PTSD). We expect that people with PTSD will have poor associative memory for multiple types of information, including pairings of visual, auditory, or other sensory cues, spatial locations, background contextual information, and the temporal sequences of events. People with PTSD may also exhibit deficits in memory of individual items given that item memory involves forming intra-item associations and is dependent on medial temporal lobe cortical structures.
Associative learning impairments in people with PTSD will emerge regardless of the emotional nature of the information being learned or remembered. Specifically, people with PTSD will exhibit associative memory impairments for stimuli that are neutral as well as stimuli that are trauma-related or negative. Broad associative learning problems that are not specific to threatening situations in people with PTSD would be consistent with our model rather than a threat model.
Reduced hippocampal activation and hippocampal functional connectivity with medial temporal lobe cortical regions, sensory cortex, and the PFC during associative learning and retrieval is related to PTSD. We predict that people with PTSD will exhibit less activation in the hippocampus and less hippocampal functional connectivity with surrounding cortex of the medial temporal lobe (i.e. entorhinal, perirhinal, and parahippocampal cortices), sensory cortex (i.e., auditory, visual, and somatosensory association cortices), and the PFC during associative learning and retrieval than controls without trauma and trauma-exposed controls without PTSD. These altered patterns of hippocampal activation and functional connectivity during associative learning and retrieval will, in turn, be associated with worse associative memory.
Poor associative learning and reduced connectivity in hippocampal circuits represent vulnerabilities for developing PTSD rather than consequences of the disorder. Longitudinal studies that measure associative learning and neural correlates prior to the traumatic event would be the best test of this hypothesis. However, in the absence of prospective studies, it is critical to show that associative learning difficulties are not a short-term consequence of the disorder (e.g., poor concentration or attention during active phases of the disorder) (Scott et al., 2015), which could interfere with performance on associative learning tasks. We predict that people with PTSD will exhibit worse associative memory and less hippocampal function and connectivity during associative learning and retrieval even as PTSD symptoms fluctuate, such that these patterns should persist even after PTSD symptoms remit. It is important to note, however, that this evidence would not rule out the possibility that associative learning difficulties are a lasting consequence of PTSD rather than a vulnerability factor.
Co-morbid depression and substance abuse do not explain poor associative learning and disruptions in hippocampal circuits. This is important to examine since depression and long-term alcohol consumption are common in people with PTSD (Breslau et al., 2000; Gilbertson et al., 2002, 2007) and are associated with smaller hippocampal volume (Agartz et al., 1999; Beresford et al., 2006; Videbech and Ravnkilde, 2004). If these problems are not the result of co-occurring depression or substance abuse, we would expect to observe them in samples of people with PTSD without these comorbidities, to find no links between associative learning and symptoms of depression or substance abuse, or to find that associations between associative learning and PTSD persist even when controlling for these comorbidities.
2. Methods
2.1. Search parameters and inclusion criteria
We reviewed and meta-analyzed when possible behavioral and functional neuroimaging studies on associative learning and PTSD to evaluate existing evidence for these five predictions. We identified all relevant peer-reviewed research articles in PsycInfo and PubMed. Our initial search was conducted in January 2018 and updated before submission of the paper in December 2018. We took four steps to ensure that only the most relevant articles were returned in the search. First, we reviewed the title, abstract, and keywords from highly related preselected articles to generate a list of search terms related to associative learning, hippocampal function, and PTSD.
Second, we refined the author-generated search terms to match search terms used for indexing articles in each database. In PsycInfo, we replaced each author-generated term with official search terms from the American Psychological Association’s Thesaurus of Psychological Index Terms. For example, we replaced the author-generated term “post-traumatic stress disorder” with “post-traumatic stress”, “acute stress disorder”, “posttraumatic stress disorder”, “complex PTSD”, and “stress reactions”. In PubMed, we replaced each author-generated term with official search terms from the National Library of Medicine MeSH Database. For example, the term “posttraumatic stress disorder” was replaced with “stress disorders”, “post-traumatic”, “trauma and stressor related disorders”, “stress disorders, traumatic”, “battered child syndrome”, combat disorders”, and “stress disorders, traumatic, acute”.
Third, we made two searches in each database. In the first, we paired associative learning search terms and PTSD search terms with the ‘AND’ operator. This ensured that each returned article pertained to each category. We separated terms within each category with the ‘OR’ operator (e.g., “post-traumatic stress” OR “acute stress disorder”, etc.). In the second, we searched articles related to associative learning, hippocampal function, and PTSD using the same method described above.
Fourth, we limited searches to only return articles from peer-reviewed journals, in English, and about humans. This search method yielded 245 articles in PsycInfo and 225 in PubMed. The articles included empirical papers, meta-analyses, and review papers.
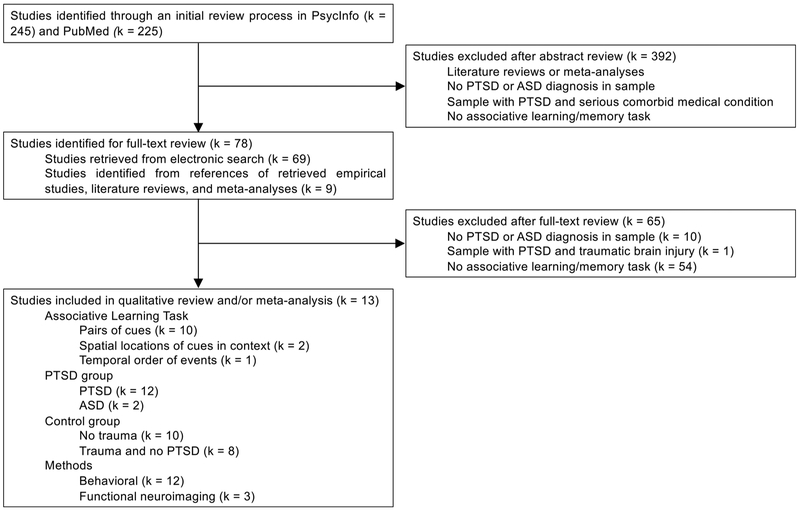
Fig. 2 illustrates our method for selecting empirical studies from the returned articles. We read the title and abstract of each article to determine whether it met inclusion criteria. If necessary, we searched the main text until we found information related to the inclusion criteria. Inclusion criteria were: a) administration of an associative learning/memory task; b) inclusion of participants with PTSD or acute stress disorder (ASD) diagnosis. Exclusion criteria were: a) administration of a non-associative learning task (e.g., autobiographical memory, item memory) only; b) inclusion of participants with trauma-exposure and no PTSD only; c) inclusion of participants with PTSD and a serious comorbid medical condition (e.g., traumatic brain injury, cancer) only. We then searched relevant empirical articles, review articles, and meta-analyses for additional references not returned by the initial search.
Fig. 2.
Method for selecting empirical studies for systematic review.
We identified 13 empirical studies that met these criteria. These studies examined associative learning of pairs of visual cues (k = 10), spatial locations of cues in context (k = 2), and temporal order of events (k = 1) in people with PTSD (k = 12) or ASD (k = 2) compared to controls without trauma (k = 10) or trauma-exposed controls without PTSD (k = 8). These studies measured associative learning using both behavioral methods (k = 12) and functional neuroimaging methods (k = 3). Participants had to rely specifically on associative memory rather than familiarity (i.e., recognizing novelty) to respond correctly during associative memory tests. For example, participants were presented with a cue item and were asked to recall the paired item, or participants were asked to recognize intact or rearranged pairs (with all items in the pairs previously shown as part of a pair during encoding). We summarize the sample, methods, and results of each study in Table 1.
Table 1.
Systematic Review of Evidence for the Associative Learning Model of PTSD.
| Study | Design |
N PTSD / N T_Controls / N H_Controls |
PTSD Assessment | PTSD chronicity | Task and Stimuli | Resultsa |
| Associative learning of pairs of cues | ||||||
| Bremner et al. (2003)d, e, l, m | Cross-sectional Behavior PET |
10 / - /11 | SCID, CMS | Yrs (chronic) | PAL Deeply encoded word pairs Neutral and fear-related Retrieval occurred 5 minutes after encoding |
1. Associative memory of neutral deeply encoded word pairs: SMD = −0.17, CI = −1.03, 0.69 2. Associative memory of fear-related deeply encoded word pairs: SMD = −0.59, CI = −1.47, 0.28 3. Neural activation during retrieval of neutral deep pairs vs. neutral shallow pairs: no group differences 4. Neural activation during retrieval of fear deep pairs vs. neutral shallow pairs: PTSD < H_Controls in L hippocampus 5. Neural activation during retrieval of fear deep pairs vs. neutral deep pairs: PTSD > H_Controls in R parahipocampal gyrus; PTSD < H_Controls in L Hippocampus 6. Only 10% of PTSD group had current depression. No current substance abuse in sample. |
| Burriss et al. (2008)d, e, j, l, n | Cross-sectional Behavior |
47 / 31 / 70 | Interview by psychologist, CAPS, M-PTSD Scale | NR | Wechsler Memory Scale III Verbal Paired Associates II Recall subtest Unrelated word pairs Neutral |
1. Associative memory of neutral unrelated word pairs: SMD = −0.22, CI = −0.67, 0.24 (PTSD vs. T_Controls); SMD = −0.58, CI = −0.96, −0.20 (PTSD vs. H_Controls) 2. Current depression not associated with performance. Results persisted when controlling for diagnosed depression, but not self-reported depression. |
| Golier et al. (2002)d, e, j, l, n | Cross-sectional Behavior |
31 / 16 / 35 | Interview by clinician, CAPS, SCID | Yrs (chronic) | PAL Unrelated and related word pairs Neutral |
1. Associative memory of neutral unrelated and related word pairs: SMD = −0.85, CI = −1.47, −0.22 (PTSD vs. T_Controls); SMD = −1.05, CI = −1.57, −0.54 (PTSD vs. H_Controls) 2. Current depression not associated with performance. No past or current substance abuse in sample. |
| Golier et al. (2003)b, d, e, j, l, m, n | Cross-sectional Behavior |
31 / 16 / 34 | Interview by clinician, CAPS, SCID | Yrs (chronic) | PAL Unrelated and related word pairs Neutral and trauma-related |
1. Associative memory of trauma-related unrelated word pairs: SMD = −0.52, CI = −1.13, 0.09 (PTSD vs. T_Controls); SMD = −0.47, CI = −0.97, 0.02 (PTSD vs. H_Controls) 2. Associative memory of neutral related word pairs: SMD = −0.30, CI = −0.90, 0.31 (PTSD vs. T_Controls); SMD = −0.64, CI = −1.14, −0.14 (PTSD vs. H_Controls) 3. Associative memory of trauma-related word pairs negatively associated with intrusive symptoms 4. Current depression not associated with performance. No past or current substance abuse in sample. |
| Guez et al. (2011)d, e, f, g, h, i, l | Cross-sectional Behavior |
Study a: 20 PTSD / - / 20 | Interview by psychiatrist | 6-36 mos (chronic) | PAL Unrelated word pairs and picture pairs Neutral |
1. Associative memory of neutral unrelated word pairs: SMD = −0.42, CI = −1.05, 0.21 2. Associative memory of neutral unrelated picture pairs: SMD = −0.35, CI = −0.97, 0.28 3. Item memory of neutral words: SMD = −0.51, CI = −1.14, 0.12 4. Item memory of neutral pictures: SMD = −0.38, CI = −1.00, 0.25 5. No pre-existing depression or substance abuse in sample. |
| Study b: 20 ASD / - / 20 | Interview by psychiatrist | 3 wks (ASD) | PAL Unrelated word pairs and picture pairs Neutral |
1. Associative memory of neutral unrelated word pairs: SMD = −0.78, CI = −1.42, −0.14 2. Associative memory of neutral unrelated picture pairs: SMD = −0.55, CI = −1.19, 0.08 3. Item memory of neutral words: SMD = −0.65, CI = −1.28, −0.01 4. Item memory of neutral pictures: SMD = −0.79, CI = −1.43, −0.15 5. No pre-existing depression or substance abuse in sample. |
||
| Guez et al. (2013)d, e, f, g, h, i, l | Longitudinal (9 wks) Behavior |
14 ASD / - / 14 | Interview by psychiatrist, CAPS |
Time 1: 1 wk (ASD) Time 2: 10 wks (64% decrease in total CAPS score from Time 1 to Time 2 in the ASD group) |
PAL Unrelated word pairs and picture pairs Neutral |
Time 1 (included in meta-analyses): 1. Associative memory of neutral unrelated word pairs: SMD = −1.15, CI = −1.95, −0.35 2. Associative memory of neutral unrelated picture pairs: SMD = −0.61, CI = −1.37, 0.15 3. Item memory of neutral words: SMD = −0.68, CI = −1.44, 0.08 4. Item memory of neutral pictures: SMD = −0.65, CI = −1.41, 0.11 Longitudinal (qualitatively reviewed): 5. Associative memory of word pairs: ASD < H_Controls at Time 1, but not Time 2. 6. Associative memory of picture pairs: ASD < H_Controls at Time 1 and Time 2 7. No past or current depression or substance abuse in sample. |
| Geuze et al. (2008)j, n | Cross-sectional Behavior fMRI |
12 / 12 / - | Interview by clinician, CAPS | NR | PAL Unrelated word pairs Neutral Retrieval occurred 20 minutes after encoding |
1. Associative memory of neutral unrelated word pairs: SMD = −0.77, CI = −1.60, 0.06 2. Neural activation during encoding of word pairs vs. reciting number pairs: PTSD > T_Controls in R parahippocampal gyrus 3. Neural activation during retrieval of word pairs vs. reciting a number: PTSD < T_Controls in L hippocampus / parahippocampal gyrus 4. Neural activation in these regions was not associated with memory or PTSD severity 5. No current depression or substance abuse in sample. |
| Saar-Ashkenazy et al. (2014)d, e, f, g, h, i, l | Cross-sectional Behavior MRI |
20 / - / 20 (verbal PAL) 21 / - / 21 (visual PAL) |
Interview by psychiatrist | 6-36 mos (chronic) | PAL Unrelated word pairs and picture pairs Neutral |
1. Associative memory of neutral unrelated word pairs: SMD = −0.41, CI = −1.04, 0.22 2. Associative memory of neutral unrelated picture pairs: SMD = −0.62, CI = −1.24, 0.00 3. Item memory of neutral words: SMD = −0.76, CI = −1.40, −0.12 4. Item memory of neutral pictures: SMD = −0.86. CI = −1.50. −0.23 5. No hippocampal volume differences 6. No past or current depression or substance abuse in sample. |
| Werner et al. (2009) | Cross-sectional fMRI |
12 / - / 12 | CAPS, SCID | Chronic, but duration NR | PAL Picture-word pairs (i.e. face-profession) Positive Retrieval occurred immediately after encoding |
1. Neural activation during encoding of pairs vs. viewing template of head: PTSD > H_Controls in R/L hippocampi and R parahippocampal gyrus 2. Neural activation during retrieval of pairs vs. indicating bigger ear: PTSD < H_Controls in L parahippocampal gyrus 3. Only 8% of PTSD group had current depression, 8% had current alcohol abuse, none had substance dependence. Neural activation in these regions was not associated with depression. |
| Yehuda et al. (2006)c | Longitudinal (5 yrs) Behavior |
14 / 13 / 19 | CAPS, SCID |
Time 1: Yrs (chronic) Time 2: + 5 yrs (Significant improvement in symptom severity in PTSD group from Time 1 to Time 2) |
PAL Unrelated and related word pairs Neutral |
1. Associative memory of neutral unrelated and related word pairs at Time 1: PTSD < H_Controls 2. PTSD group showed a decline in associative memory performance despite significant symptom improvement over time. The two were not associated. 3. Symptom improvement was associated with improvement in item memory (long-delay free recall of words on the California Verbal Learning Test) over time in PTSD group. 4. No past or current substance abuse in sample. |
| Associative learning of spatial locations of cues in context | ||||||
| Smith et al. (2015)k | Cross-sectional Behavior |
29 /30 / - | Interview by psychologist, PDS | M = 176 mos | Allocentric memory for locations of objects in virtual environment (shifted view condition on the Town Square Task) Neutral |
1. Allocentric spatial memory: SMD = −5.79, CI = −6.95, −4.63 2. Item memory (memory for lists of visual objects): no group differences 3. No current substance abuse in sample. |
| Tempesta et al., 2011)k | Cross-sectional Behavior |
11 / 11 / - | CAPS | NR | Seconds spent learning locations of objects in virtual environment Neutral |
1. Time spent learning locations of objects: SMD = −2.28, CI = −3.35, −1.21 2. No current depression in sample. |
| Associative learning of temporal order of events | ||||||
| Jelinek et al. (2009) | Cross-sectional Behavior |
26 / 55 / 30 | SCID, PDS | M = 20.93 mos | Narrative Memory Task: memory for temporal sequences of events from audio-recordings of stories Neutral and trauma-related |
1. Sequence memory across all stimuli emotions: no group differences 2. No current substance dependence in sample. |
Notes. ASD = Acute stress disorder; CAPS = Clinician-Administered PTSD Scale; CI = Confidence interval; CMS = Civilian Mississippi Scale; fMRI = Functional magnetic resonance imaging; H_Controls = Controls without trauma exposure; L = Left; M-PTSD = Mississippi Scale for Combat-Related PTSD; Mos = months; NR = not reported; PAL = Paired Associate Learning Task; PDS = Posttraumatic Stress Diagnostic Scale; PET = Positron emission tomography; PTSD = Post-traumatic stress disorder; R = Right; SCID = Structured Clinical Interview for DSM; SMD = Standardized mean difference; T_Controls = Controls with trauma exposure, but without PTSD; Wks = Weeks; Yrs = Years;
Effect sizes (SMD) are reported only for behavioral results included in the meta-analysis, and negative standardized mean difference means worse performance for the PTSD group compared to the control group;
Does not include findings already reported in Golier et al. (2002) on associative memory of neutral unrelated and related word pairs;
Longitudinal results are from a follow-up on a subsample of the sample used in Golier et al. (2002);
Participants were included in meta-analysis of associative memory of pairs of cues overall in PTSD versus H_Controls;
Participants were included in meta-analysis of associative memory of pairs of words in PTSD versus H_Controls;
Participants were included in meta-analysis of associative memory of pairs of pictures in PTSD versus H_Controls;
Participants were included in meta-analysis of item memory overall in PTSD versus H_Controls;
Participants were included in meta-analysis of item memory of words in PTSD versus H_Controls;
Participants were included in meta-analysis of item memory of pictures in PTSD versus H_Controls;
Participants were included in meta-analysis of associative memory of pairs of words in PTSD versus T_Controls;
Participants were included in meta-analysis of spatial associative memory in PTSD versus T_Controls;
Participants were included in meta-analysis of associative memory of pairs of neutral cues in PTSD versus H_Controls;
Participants were included in meta-analysis of associative memory of pairs of threatening cues in PTSD versus H_Controls;
Participants were included in meta-analysis of associative memory of pairs of neutral cues in PTSD versus T_Controls.
We did not include behavioral results from one study that tested the ability to integrate verbal and visual cues (i.e., faces paired with written professions) since the study reported general rather than specific associative memory performance making it difficult to interpret these findings (Werner et al., 2009). Specifically, participants learned face-profession pairs and later recalled whether each face was previously paired with a scientific or artistic profession generally rather than the specific face-profession pairing.
Because our goal was to examine a component process that may contribute to deficits in more complex memory problems in PTSD, we focused only on studies that measured forms of associative learning that did not rely on other types of memory (e.g., fear conditioning, semantic). Specifically, we did not review studies of PTSD and fear extinction recall and renewal, which also involve fear conditioning and extinction memory. We also did not focus on PTSD and autobiographical memory, because this type of memory also involves emotional, semantic, and schematic information, and there is already a systematic review and meta-analysis on this topic (Moore and Zoellner, 2007; Ono et al., 2016). Additionally, we did not include studies with complex spatial tasks (e.g., mental rotation of spatial configurations, spatial navigation) (Astur et al., 2006; Gilbertson et al., 2007; Miller et al., 2017; Smith et al., 2015), which require cognitive abilities beyond learning basic associations of objects, other foreground cues, and their locations in context. For similar reasons we did not include acquired equivalence tasks, retrieval induced forgetting paradigms, conceptual priming, word-stem completion, or source monitoring tasks.
2.2. Meta-Analysis
We used meta-analysis to examine differences in associative and item memory performance between people with PTSD and controls with or without trauma exposure (Hypotheses 1 and 2). We conducted separate meta-analyses for each group comparison (i.e., people with PTSD versus controls without trauma; people with PTSD versus trauma-exposed controls without PTSD) and for each performance outcome (i.e., associative memory of pairs of stimuli overall, by sensory domain, and by emotion type; associative memory of spatial locations of cues in context; and item memory overall, by sensory domain, and by emotion type). This method ensured that each study contributed only one effect size to each meta-analysis, which avoids statistical dependence of the effect sizes when the same individuals are compared to multiple comparison groups or when multiple outcomes are based on the same individuals in a single meta-analysis (Borenstein et al., 2009; Scammacca et al., 2014).
To calculate effect sizes, we first extracted the means and standard deviations (SDs) of associative and item memory performance outcomes for PTSD and control groups in each study where possible. Most studies provided data that could be converted into the mean and SD of the proportion of hits (i.e., number of correct responses / total number of items), with a few exceptions (Burriss et al., 2008; Tempesta et al., 2011). For one paper, the authors provided relevant data that was not reported in the paper (Saar-Ashkenazy et al., 2014). When studies reported multiple performance outcomes (e.g., associative memory of pairs of words and associative memory of pairs of pictures), we averaged the means and SDs of these different stimulus types for analyses where stimulus type was not of interest. Computing the average of the SDs assumes that the correlation of the two outcomes in the sample is 1, which is the most conservative estimate of dependence (Borenstein et al., 2009; Scammacca et al., 2014). We then used the Metafor package in R (Viechtbauer, 2015, 2010) to convert means and SDs into effect sizes, specifically the standardized mean difference (SMD) between the PTSD group and each control group. A negative effect size indicates that the PTSD group performed worse on the memory test than the control group. Table 1 includes effect sizes for performance outcomes for each study included in the meta-analysis and indicates which of the meta-analyses each study contributed data to.
We next conducted random-effects meta-analysis using the Metafor package. Random-effects meta-analysis assumes that the effect sizes from each study are randomly drawn from a larger population of true effect sizes, vary from each other due to study specific characteristics (e.g., sample, task design), and are distributed around a mean of the distribution of true effect sizes (Borenstein et al., 2010, 2009). Thus, the random-effects model permits inferences about a larger population of true effect sizes. Each meta-analysis estimates the weighted average of the effect sizes from the included studies (SMD+) and a corresponding 95% confidence interval (CI). We show all results in Table 2 and generated forest plots for meta-analyses examining group differences in associative memory of pairs of cues overall, associative memory of spatial locations of cues in context, and item memory.
Table 2.
Random-Effects Meta-Analyses of Associative and Item Memory Performance.
| PTSD vs. T_Controls |
PTSD vs. H_Controls |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SMD + | 95% CI | Q | k | N | SMD + | 95% CI | Q | k | N | |
| Associative memory | ||||||||||
| Pairs of cues overalla | −0.48** | −0.81, −0.16 | 3.11 | 4 | 196 | −0.64*** | −0.84, −0.44 | 4.30 | 8 | 418 |
| Verbal | −0.48** | −0.81, −0.16 | 3.11 | 4 | 196 | −0.65*** | −0.85, −0.46 | 5.80 | 8 | 417 |
| Visual | – | – | – | – | – | −0.53** | −0.85, −0.20 | 0.46 | 4 | 150 |
| Neutral | −0.46** | −0.79, −0.13 | 3.35 | 4 | 196 | −0.64*** | −0.84, −0.44 | 4.94 | 8 | 418 |
| Threatening | – | – | – | – | – | −0.50* | −0.93, −0.07 | 0.05 | 2 | 86 |
| Spatial locations of cues in contextb | −4.03* | −7.47, −0.59 | 18.92*** | 2 | 81 | – | – | – | – | – |
| Item memory | ||||||||||
| Overallc | – | – | – | – | – | −0.66*** | −0.99, −0.33 | 0.67 | 4 | 149 |
| Verbal | – | – | – | – | – | −0.65*** | −0.98, −0.32 | 0.30 | 4 | 148 |
| Visual | – | – | – | – | – | −0.67*** | −1.00, −0.34 | 1.33 | 4 | 150 |
| Neutral | – | – | – | – | – | −0.66*** | −0.99, −0.33 | 0.67 | 4 | 149 |
| Threatening | – | – | – | – | – | – | – | – | – | – |
Notes. CI = confidence interval; H_Controls = Controls without trauma exposure; k = number of data sets; N = number of participants; Q = measure of heterogeneity in effect sizes; SMD+ = weighted average standardized mean difference between people with PTSD and control group (negative value indicates that people with PTSD performed worse on memory tests than control group); T_Controls = Controls with trauma exposure, but without PTSD; – = unable to examine through meta-analysis because only 0 or 1 study measuring performance outcome;
Meta-analysis examining associative memory of pairs of cues overall in those with PTSD versus trauma-exposed controls without PTSD is the same as the meta-analysis examining associative memory of pairs of words;
Tasks assessing learning and memory of spatial locations of cues in context only involved neutral stimuli;
Meta-analysis examining item memory overall in those with PTSD versus controls without trauma is the same as the meta-analysis examining item memory of neutral stimuli;
= p < .05,
= p < .01,
= p < 0.001.
For each meta-analysis, we calculated Cochran’s Q statistic (Cochran, 1954), which is a measure of heterogeneity in effect sizes across studies. A significant Q statistic indicates that the effect sizes vary across studies and likely reflect different populations. When the Q statistic was significant, we also calculated I2, which is a measure of the magnitude of heterogeneity (Higgins and Thompson, 2002).
We also generated funnel plots for individual meta-analyses to visualize the amount of publication bias, or the tendency for journals to only publish significant findings (Greenwald, 1975). Greater publication bias increases the likelihood of Type 1 errors in meta-analyses. We conducted a Kendall’s rank correlation test for each funnel plot to determine whether bias was significant. Power for detecting significant publication bias was low given the small number of studies included in each meta-analysis.
Lastly, we conducted a sensitivity analysis to examine whether results differed when meta-analyses excluded the two studies with people with ASD rather than PTSD (Guez et al., 2013, 2011).
Because a minimum of two studies is required to conduct a meta-analysis, we could not meta-analyze group differences in neural activation or functional connectivity of the hippocampus or cortical regions of the medial temporal lobe during associative learning or retrieval (Hypothesis 3). We also could not meta-analyze the correlation between change in PTSD symptom severity and change in associative memory performance over time in people with PTSD since one of the two longitudinal studies did not provide relevant data to answer this question (Hypothesis 4). When we could not conduct a meta-analysis, we qualitatively reviewed findings from individual studies.
3. Results
3.1. Hypothesis 1. People with PTSD will exhibit poor associative learning
Our first hypothesis was that people with PTSD would exhibit poor associative learning—not only for contextual information but also for stimuli that are the focus of explicit attention—as compared to controls without trauma and trauma-exposed controls without PTSD. To evaluate this hypothesis, we meta-analyzed findings on associative memory performance as a function of PTSD status (Table 2). We first present results comparing individuals with PTSD to controls without trauma, and then present results comparing those with PTSD to trauma-exposed controls without PTSD. We qualitatively review individual studies when we could not conduct a meta-analysis.
3.1.1. PTSD versus controls without trauma
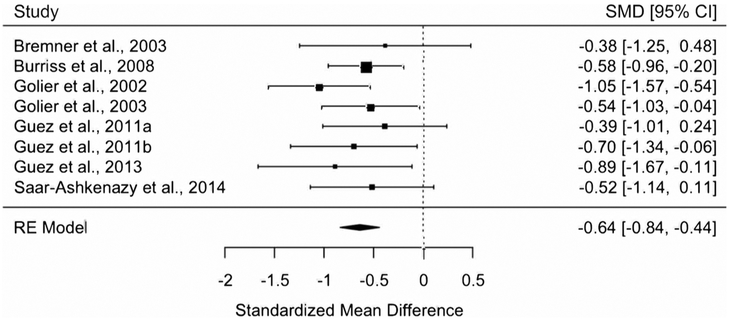
We conducted a meta-analysis including 8 studies and 418 participants to examine if people with PTSD had worse associative memory of pairs of stimuli overall, regardless of sensory domain or emotion type, compared to controls without trauma. We found support for this hypothesis. People with PTSD had worse associative memory compared to controls without trauma (SMD+ = −0.64, 95% CI = [−0.84, −0.44]) (Fig. 3). This pattern was unchanged when participants with ASD were excluded from the meta-analysis (SMD+ = −0.61, 95% CI = [−0.83, −0.40]). One study tested associative memory for the temporal order of events from audio-recordings of stories and did not find group differences (Jelinek et al., 2009).
Fig. 3.
Meta-analysis of studies investigating people with PTSD versus controls without trauma on associative memory of pairs of cues overall regardless of sensory domain or emotion type (random effects).
We next conducted separate meta-analyses on tasks assessing associative memory for pairs of words and pairs of pictures to examine whether people with PTSD have deficits across different types of sensory information. Indeed, people with PTSD had worse associative memory for pairs of words than controls without trauma in a meta-analysis including 8 studies and 417 participants (SMD+ = −0.65, 95% CI = [−0.85, −0.46]). People with PTSD also had worse associative memory of pairs of pictures in a meta-analysis including 4 studies and 150 participants (SMD+ = −0.53, 95% CI = [−0.85, −0.20]).
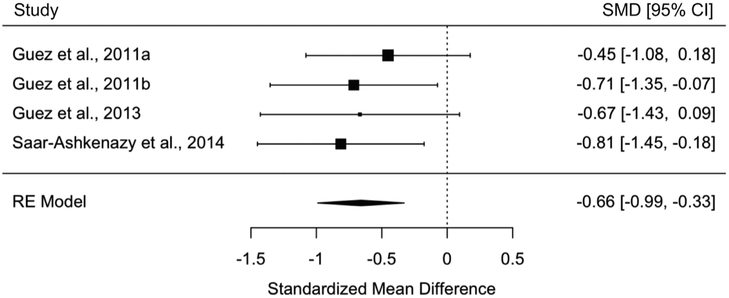
We then tested whether people with PTSD also had difficulty remembering the individual items that made up pairings, not just the associations between items. People with PTSD had worse item memory overall, regardless of sensory domain, compared to controls without trauma in a meta-analysis including 4 studies and 149 participants (SMD+ = −0.66, 95% CI = [−0.99, −0.33]) (Fig. 4). This pattern did not differ when participants with ASD were excluded (SMD+ = −0.63, 95% CI = [−1.08, −0.18]) and was similar when we examined memory of individual words in a meta-analysis of 4 studies and 148 participants (SMD+ = −0.65, 95% CI = [−0.98, −0.32]) and of individual pictures in a meta-analysis of 4 studies and 150 participants (SMD+ = −0.67, 95% CI = [−1.00, −0.34]). All item memory tests involved neutral stimuli.
Fig. 4.
Meta-analysis of studies investigating people with PTSD versus controls without trauma on item memory overall regardless of sensory domain (random effects).
Effect sizes were consistent across studies in each meta-analysis based on non-significant Q results (see Table 2). Publication bias was not present as funnel plots for associative memory of pairs of stimuli overall (Supplemental Fig. 1a) and item memory overall (Supplemental Fig. 1b) were symmetrical and Kendall’s rank correlation tests were not significant (p = 0.90–1.00).
3.1.2. PTSD versus trauma-exposed controls without PTSD
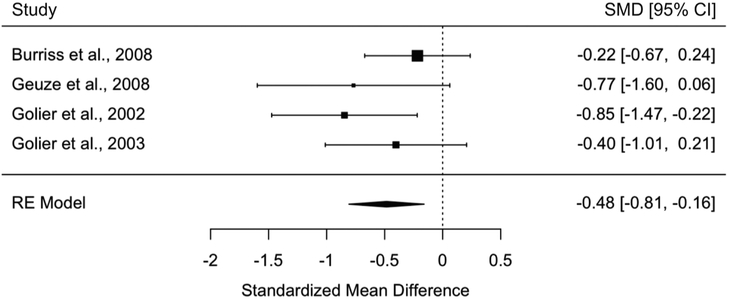
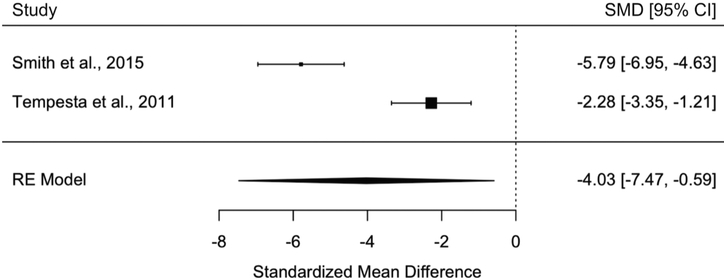
Consistent with the findings comparing people with PTSD to those without a trauma history, those with PTSD exhibited worse associative memory compared to trauma-exposed controls without PTSD in all analyses. Specifically, people with PTSD had worse associative memory of pairs of words than controls with trauma in a meta-analysis including 4 studies and 196 participants (SMD+ = −0.48, 95% CI = [−0.81, −0.16]) (Fig. 5). People with PTSD also performed worse on tasks assessing learning and memory of spatial locations of cues in context in a meta-analysis including 2 studies and 81 participants (SMD+ = −4.03, 95% CI = [−7.47, −0.59]) (Fig. 6). Finally, one study tested associative memory for temporal sequences and did not find group differences (Jelinek et al., 2009).
Fig. 5.
Meta-analysis of studies investigating people with PTSD versus controls with trauma on associative memory of pairs of words (random effects).
Fig. 6.
Meta-analysis of studies investigating people with PTSD versus controls with trauma on associative memory of spatial locations of cues in context (random effects).
Despite worse associative memory, people with PTSD did not have worse item memory compared to trauma-exposed controls in the one study that reported information on item memory (Smith et al., 2015).
Effect sizes were consistent across studies in all meta-analyses, except for one, based on non-significant Q results (see Table 2). Effect sizes differed significantly across the two studies examining learning and memory of spatial locations of cues (Q = 18.92, df = 1, p < 0.0001; I2 = 94.71%). This makes sense given that one study measured participants’ accuracy of remembering locations of objects (Smith et al., 2015), and the other study measured participants’ speed of learning the locations of objects accurately (Tempesta et al., 2011), which likely incorporates other abilities such as processing speed and learning strategy.
Funnel plots for associative memory of pairs of words (Supplemental Fig. 1c) and associative memory of spatial locations (Supplemental Fig. 1d) were symmetrical, and Kendall’s rank correlation tests were not significant (p = 0.33–1.00).
3.1.3. Summary
Existing behavioral evidence is largely consistent with our hypothesis that people with PTSD have difficulties in associative learning of multiple types of information. People with PTSD had worse associative memory compared to people without trauma exposure and trauma-exposed people without PTSD. Associative memory deficits occurred for stimulus pairings as well as spatial locations of cues in context, and deficits occurred for both verbal and visual stimuli. People with PTSD also had difficulty remembering individual items relative to people without a trauma history, but not relative to trauma-exposed controls in the one study that examined item memory among this group. All effect sizes were medium in magnitude, except for the effect size for spatial associative memory, which was very large.
3.2. Hypothesis 2. Associative learning impairments in people with PTSD will emerge regardless of the emotional nature of the stimuli
Our second hypothesis was that people with PTSD would exhibit poor associative learning regardless of the affective nature of the stimuli being learned or remembered as compared to controls without trauma and trauma-exposed controls without PTSD. We meta-analyzed findings on associative memory performance on tasks involving neutral stimuli and trauma-related or negative stimuli (Table 2).
3.2.1. PTSD versus controls without trauma
We ran separate meta-analyses on tasks assessing associative memory of pairs of neutral stimuli and pairs of threatening stimuli to test our prediction that people with PTSD would have associative learning difficulties even for affectively neutral stimuli. Indeed, people with PTSD had worse associative memory for pairs of neutral stimuli than controls without trauma in a meta-analysis including 8 studies and 418 participants (SMD+ = −0.64, 95% CI = [−0.84, −0.44]). They also had worse associative memory for pairs of fear-related or trauma-related stimuli in a meta-analysis including 2 studies and 86 participants (SMD+ = −0.50, 95% CI = [−0.93, −0.07]). Effect sizes were consistent across studies in each meta-analysis (see Table 2).
3.2.2. PTSD versus trauma-exposed controls without PTSD
Associative learning difficulties were present even for affectively neutral stimuli. People with PTSD had worse associative memory of pairs of neutral stimuli than controls with trauma in a meta-analysis including 4 studies and 196 participants (SMD+ = −0.46, 95% CI = [−0.79, −0.13]). Effect sizes were consistent across studies in this meta-analysis (see Table 2). As described above, people with PTSD also had worse learning and memory of spatial locations of neutral stimuli. One study found that people with PTSD had worse memory of pairings of trauma-related stimuli (Golier et al., 2003), while another study found that people with PTSD did not have worse memory of temporal sequences of events from stories involving trauma-related information (Jelinek et al., 2009) compared to trauma-exposed controls.
3.2.3. Summary
The evidence supports our hypothesis that people with PTSD have difficulties in associative learning that occur in non-threatening situations. Reduced associative memory was observed in people with PTSD when learning focused on neutral stimuli in addition to negative or threatening stimuli, with medium effect sizes across both types of stimuli.
3.3. Hypothesis 3. Reduced hippocampal activation and hippocampal functional connectivity during associative learning and retrieval is related to PTSD
Our third hypothesis was that people with PTSD would have reduced hippocampal activation and connectivity with medial temporal lobe cortical areas, sensory cortex, and PFC during associative learning and retrieval compared to people without trauma exposure and those with trauma exposure but not PTSD. We first qualitatively review studies comparing people with PTSD to controls without trauma (k = 2), and then review one study comparing people with PTSD to trauma-exposed controls without PTSD (k = 1).
3.3.1. PTSD versus controls without trauma
Our review revealed only one study on neural activation during associative learning (i.e., encoding). Contrary to our expectations, this study showed that people with PTSD recruited the bilateral hippocampi and right parahippocampal gyrus more than controls without trauma when learning face-profession pairs versus viewing a silhouette of a head (Werner et al., 2009).
Two studies examined group differences in neural activation during associative memory (i.e., retrieval) after a short delay. In one study, people with PTSD had less activation in the left parahippocampal gyrus than controls without trauma when recalling whether a face was previously paired with a scientific or artistic profession versus when indicating which ear on a silhouette of a head was larger (Werner et al., 2009). In another study, people with PTSD had less activation in the left hippocampus when retrieving deeply encoded fear-related word pairs versus shallowly or deeply encoded neutral word pairs (Bremner, Vythilingam, Vermetten, Southwick, McGlashan, Staib, et al., 2003). There were no group differences in activation during retrieval of deeply encoded neutral word pairs versus shallowly encoded neutral word pairs (Bremner, Vythilingam, Vermetten, Southwick, McGlashan, Staib, et al., 2003). It is important to note that because of the fMRI contrasts used in this study, differences in neural activation may have been related to the emotional nature of the stimuli or the depth of encoding rather than to associative memory specifically.
These studies did not examine associations of neural activation with associative memory performance. We did not find any studies on functional connectivity during associative learning or retrieval in people with PTSD compared to controls without trauma.
3.3.2. PTSD versus trauma-exposed controls without PTSD
In the only study comparing people with PTSD to trauma-exposed controls without PTSD, those with PTSD had atypical medial temporal lobe function. The study measured neural activation during associative learning and retrieval after a short delay. People with PTSD had more activation in the right parahippocampal gyrus compared to trauma-exposed controls when learning word pairs versus reciting number pairs and less activation in the left hippocampus and parahippocampal gyrus during retrieval of learned word pairs versus reciting a number (Geuze et al., 2008). Medial temporal lobe activation was not associated with associative memory performance (Geuze et al., 2008). We did not find any studies that examined functional connectivity during associative learning or retrieval in people with PTSD versus trauma-exposed controls.
3.3.3. Summary
There is insufficient evidence to fully test our hypothesis that reduced medial temporal lobe activation and functional connectivity is related to poor associative memory in people with PTSD. The few studies on this topic suggest that people with PTSD exhibit greater medial temporal lobe activation during associative learning (i.e., encoding) and less medial temporal lobe activation during associative memory (i.e., retrieval) after a short delay compared to controls with and without trauma exposure. These results suggest the possibility that people with PTSD might have poor hippocampus-dependent consolidation or retrieval of recent associative memory rather than poor encoding of the pairings, although there is insufficient evidence to make firm conclusions given the small number of studies published on this topic.
3.4. Hypothesis 4. Poor associative learning and reduced connectivity in hippocampal circuits represent vulnerabilities for developing PTSD rather than consequences of the disorder
We hypothesize that poor associative memory and atypical medial temporal lobe function are vulnerabilities for developing PTSD. To rule out the possibility that associative learning impairment is instead a short-term consequence of PTSD, it is important to show that associative memory difficulties persist even as PTSD symptoms fluctuate. Two longitudinal studies examined changes in PTSD symptoms and associative memory performance over time but reported different outcome measures that could not be combined for meta-analysis. Both found that associative memory difficulties persisted in people with PTSD even as their symptoms improved, suggesting that PTSD did not cause the memory deficits. One study found that people with PTSD had worse verbal associative memory than controls without trauma at baseline (Yehuda et al., 2006). The PTSD group showed a decline in performance on the verbal associative memory test over a five-year period despite significant symptom improvement, and the change in performance and the change in symptoms were not associated (Yehuda et al., 2006). In contrast, improvement in performance on a verbal item memory test was associated with symptom improvement over time in people with PTSD (Yehuda et al., 2006).
Another study found that people with ASD exhibited deficits in both associative memory of word pairs and picture pairs one week after a traumatic event (Guez et al., 2013). Despite significant symptom improvement ten weeks later, people who had developed ASD still exhibited poor associative memory of picture pairs compared to controls without trauma (Guez et al., 2013). However, this study found that deficits in associative memory of word pairs did improve somewhat as ASD symptoms improved (Guez et al., 2013). It is possible that this could be the result of improvements in verbal information processing rather than associative learning. Processing verbal information is more cognitively demanding than processing visual information (Israel and Schacter, 1997; Maisto and Queen, 1992; Nelson et al., 1976; Park et al., 1983). PTSD is associated with attentional difficulties and reductions in processing speed (Scott et al., 2015), and thus ASD symptom improvement in this study may have freed cognitive resources necessary for verbal information processing. People with PTSD perform more poorly on a range of verbal learning tasks (e.g., of individual words) (Brewin et al., 2007) than controls with and without trauma exposure, and these deficits tend to improve as PTSD symptoms improve (Vermetten et al., 2003). We would expect improvement in verbal learning as PTSD symptoms improve if these learning difficulties are consequences of symptoms of the disorder itself (e.g., poor concentration). For this reason, tests of associative learning of sensory information that requires lower processing effort are a more straightforward test of associative learning in those with PTSD.
3.4.1. Summary
Associative memory difficulties are not only present when PTSD symptoms are present, suggesting that impairment is not a short-term consequence of the disorder. Specifically, associative memory impairment persists in people with PTSD even as their symptoms improve. However, this evidence does not rule out the possibility that associative memory impairment is a long-lasting consequence of PTSD. Future research that measures associative learning prior to the traumatic event is needed to confirm that associative memory impairment is indeed a preexisting vulnerability factor for developing PTSD.
3.5. Hypothesis 5. Co-morbid depression and substance abuse do not explain poor associative learning and disruptions in hippocampal circuits
We expected that poor associative learning and dysfunction in hippocampal circuits would occur in people with PTSD without comorbid depression and substance abuse, would not be associated with symptoms of depression or substance abuse, or would persist even when controlling for comorbidities.
Ten studies assessed and reported on current depression (see Table 1). Of these studies, 4 examined the direct association between depressive symptoms and behavioral and neural outcomes and did not find associations. One study controlled for diagnosed depression in analyses and continued to find PTSD-related group differences in associative memory. Five studies excluded people who had current depression and still observed PTSD-related group differences in behavioral and neural outcomes. One study reported that only 10% of the PTSD group had current depression and still observed PTSD-related group differences in neural outcomes.
Eleven studies assessed and reported on current substance abuse (see Table 1). Ten studies excluded people who had current substance abuse. All but one of these studies found significant PTSD-related group differences in behavioral or neural outcomes. One study reported that only 8% of the PTSD group had current alcohol abuse and still observed PTSD-related group differences in neural outcomes.
3.5.1. Summary
These results support our hypothesis that current depression and substance abuse in those with PTSD do not explain group differences in associative memory or medial temporal lobe function.
4. Discussion
The studies reviewed here provide preliminary support for our hypothesis that broad deficits in associative learning and medial temporal lobe function and connectivity are a potential vulnerability factor for PTSD. We summarize three pieces of evidence that support our model and propose additional questions that need to be addressed in future research in order to validate this model.
First, people with PTSD exhibited associative memory difficulties for pairs of visual and verbal cues presented in the absence of a background context, as well as for contextual information (e.g., spatial locations of cues in context) compared to controls without trauma and trauma-exposed controls without PTSD. In all studies, these associative memory difficulties were not explained by co-morbid depression or substance abuse. These results are indicative of a broad deficit in associative learning that is specific to PTSD and expand upon existing contextual models of PTSD in two important ways. First, the fact that people with PTSD exhibited impaired memory for pairs of cues even when no contextual information was present supports our argument that difficulties in associative learning and memory in people with PTSD are not restricted only to contextual information, which has been the focus in prior hippocampus-based models (Liberzon, 2018; Liberzon and Abelson, 2016; Maren et al., 2013). This suggests that PTSD-related difficulties with contextual processing may be just one example of a broader issue with associative learning that extends to a wider range of information in people with PTSD. Second, the fact that people with PTSD exhibited impaired memory of spatial locations of cues in context suggests that problems with contextual regulation of fear could reflect difficulties with more basic aspects of contextual processing.
People with PTSD also had associative memory deficits that occurred regardless of the emotional nature of the stimuli being learned compared to controls without trauma and trauma-exposed controls without PTSD. The fact that difficulties with associative memory were observed in people with PTSD when the stimuli were neutral suggests that deficits in associative learning are broad and not specific to situations involving threat. Such a pattern cannot be explained with prevailing models focused on enhanced threat-related information processing, learning, and reactivity (Foa and Kozak, 1986; Jovanovic and Ressler, 2010; Liberzon and Sripada, 2007; Mineka and Oehlberg, 2008), which presumably would result in difficulties with associative learning only in the presence of threat-related stimuli. Additionally, these results cannot easily be explained by cognitive models of PTSD that argue that the presence of danger is the source of poor processing of contextual and temporal details in PTSD (Brewin et al., 1996; Brewin, 2001; Brewin and Holmes, 2003). These cognitive models differ from our model in the underlying mechanism proposed to explain differences in associative learning in PTSD.
Second, people with PTSD had atypical medial temporal lobe function during associative learning and retrieval compared to controls without trauma and trauma-exposed controls without PTSD. Surprisingly, they exhibited greater parahippocampal gyrus and hippocampal activation during associative learning, not reduced activation as we predicted. One interpretation is that increased hippocampal activation during associative learning reflects better hippocampal engagement and associative learning in people with PTSD than without. Another interpretation is that increased hippocampal activation is a compensatory mechanism that results in the same level of associative learning in people with PTSD as compared to those without who require less hippocampal activation to achieve the same level of learning. Group differences in hippocampal activation occurred in the absence of associative memory performance differences in studies that observed this pattern (Werner et al., 2009), supporting the latter possibility of less efficient hippocampal function. These findings are consistent with some studies showing that a PTSD diagnosis and greater PTSD symptoms are associated with greater hippocampal activation during encoding of autobiographical memories (Brohawn et al., 2010; Stevens et al., 2018) and greater parahippocampal gyrus and hippocampal activation during encoding of negative words (Thomaes et al., 2009). Increased medial temporal lobe activation occurred in the absence of memory performance differences in these studies (Brohawn et al., 2010; Thomaes et al., 2009), supporting our interpretation that increased activation may be a compensatory mechanism.
In contrast, people with PTSD exhibited less parahippocampal gyrus and hippocampal activation during associative memory retrieval after a short delay in all studies that examined this association. These results suggest that people with PTSD could have poor hippocampus-dependent consolidation or retrieval of recent associative memory rather than poor encoding or learning of the pairings. This pattern of results is consistent with studies finding reduced hippocampal activation during retrieval of words that is associated with impairments in memory performance (Carrión et al., 2010) as well as reduced hippocampal activation during extinction recall and impaired recall of extinction memories, but not extinction learning, (Garfinkel et al., 2014; Milad et al., 2009) in people with PTSD.
Critically, scant research has examined the neural mechanisms that produce associative memory deficits in people with PTSD and greater research is clearly needed in this area before firm conclusions can be drawn. We hypothesize that associative memory deficits are due to both poor associative learning and poor consolidation and retrieval of recent associative memories in the hippocampus. To identify the source of associative memory difficulties, greater understanding is needed of how hippocampal functional connectivity during different stages of associative learning and memory relates to associative memory performance. Several options are possible. If associative memory problems arise due to poor hippocampal integration of foreground cues with each other or with background contextual information, then reduced functional connectivity between the hippocampus and medial temporal lobe cortical regions during associative learning should relate to deficits in associative memory broadly. If the deficit is specific to the transfer of certain types of information to the hippocampus, then reduced functional connectivity between the hippocampus and particular cortical regions of the medial temporal lobe (e.g., perirhinal cortex) should relate to deficits in associative memory for only certain types of information (e.g., objects). Similarly, we would expect reduced functional connectivity between the hippocampus and sensory cortex to relate to deficits in associative memory for multiple types of sensory information. However, if the deficit is specific to the transfer of certain types of sensory information to the hippocampus, then reduced functional connectivity between the hippocampus and particular regions of sensory cortex (e.g., visual cortex) should relate to deficits in associative memory in only certain sensory domains (e.g., visual information).
If difficulties are specific to the consolidation or retrieval of recent associative memories, then reduced functional connectivity between the hippocampus and sensory cortex or PFC during memory retrieval after a short delay (e.g., within several hours after learning) but not during associative learning should relate to deficits in associative memory. The studies that found less medial temporal lobe activation during associative memory after a short delay in people with PTSD provide preliminary evidence for this latter mechanism, although functional connectivity was not examined. Finally, if the deficit is in the transfer of recent associative memories from the hippocampus to the PFC for long-term storage (for a review, see Euston et al., 2012), reduced functional connectivity between the hippocampus, midline thalamus, and PFC or between the PFC and sensory cortex during memory retrieval after a long delay (e.g., 24 h after learning) would relate to associative memory deficits after a long delay but not associative memory deficits after a short delay. The midline thalamus connects the hippocampus and mPFC and is involved in transferring recent memories to remote memories (Cassel and de Vasconcelos, 2015; Vertes, 2006). Alterations in midline thalamic function or connectivity with the hippocampus or mPFC could contribute to impairments in remote associative memory in PTSD. People with PTSD exhibit altered resting state connectivity between the thalamus and mPFC (Yin et al., 2011), decreased resting-state activity in the thalamus (Kim et al., 2007; Yan et al., 2013), and reduced activation in the thalamus when hearing about traumatic and non-traumatic personal events (Lanius et al., 2001, 2003) compared to healthy or trauma-exposed controls. Future research is needed to examine these competing mechanisms.
Third, existing research is consistent with the possibility that poor associative learning may represent a vulnerability factor for PTSD that existed prior to the trauma that triggered it rather than a consequence of PTSD onset or a consequence of the trauma. In two longitudinal studies, associative memory deficits persisted even as PTSD symptoms remitted. Poor associative memory therefore does not appear to be a short-term consequence of PTSD; although impairments could still be a long-lasting consequence of the disorder. Preliminary evidence also supports the possibility that those who go on to develop PTSD have associative learning impairments that precede the trauma. People with PTSD have worse associative memory and atypical hippocampal and parahippocampal function compared to trauma-exposed people without PTSD, which presumably would not have occurred if these difficulties were the result of trauma exposure. Neither of these predictions can be fully tested in the absence of longitudinal data examining associative learning prior to trauma exposure and PTSD onset, however. Future work examining associative memory, hippocampal function and connectivity, and hippocampal volume before trauma in a sample with high risk of trauma exposure (e.g., new recruits to the police or military) is needed in order to examine these hypotheses in a more rigorous way. The fact that associative learning difficulties do not appear to be a consequence of trauma exposure in adulthood does not rule out the possibility that childhood trauma exposure played a role in the emergence of these patterns. In fact, it is likely that hippocampus-dependent associative learning difficulties are related to exposure to trauma in childhood for at least some people. Childhood trauma exposure is associated with reduced hippocampal volume (Hanson et al., 2015; Lambert et al., 2017; Teicher et al., 2012), and consistent evidence suggests that childhood trauma exposure is a risk factor for PTSD following traumatic events that occur in adulthood (Breslau et al., 2014; Brewin et al., 2000; McLaughlin et al., 2010, 2017). It may be the case that reductions in hippocampal volume and associated difficulties with associative learning are a mechanism underlying this well-replicated effect.
Finally, our meta-analysis findings indicate memory difficulties for items that made up the pairings in people with PTSD. This is consistent with a recent meta-analysis showing that people with PTSD have difficulty remembering individual words and pictures (Brewin et al., 2007). People with PTSD may have difficulties with numerous types of associative learning that rely on the medial temporal lobe, including the formation of integrated item representations, which require the association of multiple item features. Consistent with this interpretation, our systematic review found alterations in parahippocampal gyrus activation during associative encoding and recall in people with PTSD, consistent with other work showing that people with PTSD exhibit alterations in parahippocampal gyrus activation during encoding of words (Thomaes et al., 2009). Impaired item encoding in medial temporal lobe cortical structures could contribute to difficulties with associative learning in the hippocampus in PTSD, as item encoding is likely a pre-requisite for associative learning to occur. Future research is needed to determine the specific types of associative learning that are impaired in PTSD (e.g., intra-item or inter-item associative learning), how difficulties in these specific types of learning relate to one another (e.g., whether intra-item associative learning problems drive inter-item associative learning difficulties), and how disruptions in these specific types of learning relate to the development of PTSD symptoms. For example, people with PTSD typically have strong memories of some perceptual aspects of the trauma (e.g., re-experiencing symptoms), but tend to forget other episodic details (Amir et al., 1998; Foa et al., 1995; Harvey and Bryant, 1999; Moore and Zoellner, 2007; van der Kolk and Fisler, 1995), suggesting that difficulties with associative learning may contribute to the clinical presentation of PTSD.
The primary limitation of this paper is that our analyses are based on a very modest number of studies. For hypotheses 1 and 2, meta-analyses included 2–8 studies each (n = 81–418). There were even fewer studies available to address hypotheses 3 and 4 so that meta-analysis was not possible. The current literature is therefore too limited to assess most of our hypotheses in a definitive way. Our results indeed suggest that existing research on associative learning, hippocampal function, and PTSD is in line with our novel model of PTSD, but future empirical research is needed to directly test our model.
Three other limitations and future directions are worth noting. First, most of the associative learning tasks included in our meta-analysis were conducted behaviorally and not in the scanner, meaning we cannot assume behavioral differences were driven by differences in hippocampal recruitment or functional connectivity. However, the tasks were simple associative learning tasks (e.g., learning stimulus-stimulus pairings), and prior work consistently shows involvement of the hippocampus in these types of tasks involving visual, verbal, temporal, and contextual information (DuBrow and Davachi, 2016; Hayes et al., 2010; Henke et al., 1997; Jackson and Schacter, 2004; Kirwan and Stark, 2004; Sperling et al., 2003). Second, associative learning tasks are used to measure associative learning, but, like any task, call on other types of cognitive processes, like attention and processing speed, which are relevant to PTSD and may contribute to associative learning differences. However, associative memory difficulties persist after PTSD symptoms decline, suggesting that impairment is not a consequence of the presence of active symptoms (e.g., poor concentration) (Guez et al., 2013; Yehuda et al., 2006). Future research on associative learning and PTSD should measure and control for other cognitive abilities, including attention and processing speed. Third, identifying the component processes that contribute to deficits in more complex forms of memory in PTSD is an important area for future research. Specifically, research should test whether poor associative learning and altered hippocampal activation contribute to deficits in contextual regulation of fear and autobiographical memory.
4.1. Clinical implications
Research on threat processing mechanisms has led to the development of one of the most common and effective treatments for PTSD: prolonged exposure therapy (Dorsey et al., 2017; Morina et al., 2016; Powers et al., 2010; Van Etten and Taylor, 1998; Watts et al., 2013), which involves repeatedly exposing people with PTSD to trauma reminders in safe environments to facilitate the extinction of heightened fear responses (Foa et al., 2008, 2007). Research on fear responses in PTSD has been a success both in terms of understanding the etiology and maintenance of the disorder and developing a treatment that works for many with PTSD.
However, the exposure therapies developed based on these models do not work for as many as half of people, who continue to have a PTSD diagnosis following treatment (Foa et al., 1991; Marks et al., 1998; Resick et al., 2002). Exposure therapy primarily targets fear extinction and inhibition, which may not be the most relevant mechanisms in those with treatment-resistant PTSD. Of course, other factors play a role in treatment outcomes for exposure-based therapies. A meta-analysis found that, on average, 18% of patients drop out of PTSD treatment (Imel et al., 2013). Moreover, clinicians express concerns about delivering the exposure portion of PTSD treatment (e.g., risk of worsening symptoms and increasing dropout) (Becker et al., 2004; Cook et al., 2004; Farrell et al., 2013), which could discourage its use (Borntrager et al., 2013) and result in ineffective implementation. Finally, patients who do not respond to exposure treatments exhibit overactivity in the amygdala in response to fearful faces before treatment compared to patients who respond (Bryant et al., 2008), suggesting that non-responders may not be in the right state (e.g., excessive arousal and fear) to benefit from exposure therapy. Although it is difficult to disentangle whether people do not respond because exposure therapies are not targeting the most relevant mechanisms or because of other factors, there is clearly a need for alternative treatments that are more effective and tolerable for patients who do not improve with exposure therapy.
If the predictions outlined in our conceptual model are correct, it could open new lines of inquiry that could meaningfully expand our understanding of PTSD and highlight novel avenues for intervention. For example, future research should test whether associative learning deficits and atypical function in hippocampal circuits predict PTSD over and above established threat processing mechanisms (e.g., attention bias to threat, amygdala reactivity, poor fear inhibition), and whether associative learning deficits and associated hippocampal circuitry differences are observable in people with PTSD who do not respond to exposure-based treatments that target threat pathways. Answers to these questions could suggest a distinct mechanism underlying a treatment-resistant subtype of PTSD and help to identify novel treatment approaches to target these mechanisms. For example, for patients who find exposure-based approaches unhelpful or intolerable, focusing instead on where the trauma took place, who or what was present during the trauma, or the sequence of events during the trauma could help them form a specific memory that reduces the generalization of fear without needing to revisit the most traumatic aspects of the event. Additionally, recent advancements in exposure-based therapies suggest the importance of conducting exposures across multiple contexts to prevent later renewal of fear in novel contexts (Craske et al., 2014), which could be particularly beneficial for patients who have difficulty using context to determine whether cues are threatening or safe.
4.2. Conclusion
The associative learning model of PTSD offers a complementary perspective to prevailing threat models and broadens prior conceptualization of how hippocampally-mediated processes might contribute to PTSD. We argue that people who have trouble with associative learning and atypical function in the hippocampal circuits underlying associative learning may be particularly susceptible to developing PTSD after a traumatic event. Existing research on this topic provides preliminary support for our model, but future research is needed to validate it. This model has implications for understanding why some people respond to current treatments but some develop chronic PTSD that lasts for many years and for identifying a sub-type of PTSD that may involve mechanisms other than altered threat processing. If validated, this model also has potential implications for early identification of vulnerable individuals in the wake of traumatic events and the development of new treatment approaches.
Supplementary Material
Acknowledgments
Role of funding sources
This work was supported by the National Institute of Mental Health [R01-MH103291 and R01-MH106482 to KM and F31-MH116559 to HL], an Early Career Research Fellowship from the Jacobs Foundation to KM, and the IMHRO Rising Star Award to KM. Funding sources had no involvement in analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication.
Footnotes
Declaration of Competing Interest
All authors declare that they have no conflicts of interest.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.neubiorev.2019.09.024.
References
- Acheson DT, Gresack JE, Risbrough VB, 2012. Hippocampal dysfunction effects on context memory: possible etiology for posttraumatic stress disorder. Neuropharmacology 62 (2), 674–685. 10.1016/j.neuropharm.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agartz I, Momenan R, Rawlings RR, Kerich MJ, Hommer DW, 1999. Hippocampal volume in patients with alcohol dependence. Arch. Gen. Psychiatry 56 (4), 356–363. 10.1001/archpsyc.56.4.356. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association, 2013. DSM-V. Am. J. Psychiatry, 10.1176/appi.books.9780890425596.744053. [DOI] [Google Scholar]
- Amir N, Stafford J, Freshman MS, Foa E, 1998. Relationship between trauma narratives and trauma pathology. J. Trauma. Stress 11 (2), 385–392. 10.1023/A:1024415523495. [DOI] [PubMed] [Google Scholar]
- Astur RS, St. Germain SA, Tolin D, Ford J, Russell D, Stevens M, 2006. Hippocampus function predicts severity of post-traumatic stress disorder. Cyber Psychol. Behav 9 (2), 234–240. 10.1089/cpb.2006.9.234. [DOI] [PubMed] [Google Scholar]
- Barrett LF, Kensinger EA, 2010. Context is routinely encoded during emotion perception. Psychol. Sci. 21 (4), 595–599. 10.1177/0956797610363547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker CB, Zayfert C, Anderson E, 2004. A survey of psychologists’ attitudes towards and utilization of exposure therapy for PTSD. Behav. Res. Ther 42 (3), 277–292. 10.1016/S0005-7967(03)00138-4. [DOI] [PubMed] [Google Scholar]
- Beresford TP, Arciniegas DB, Alfers J, Clapp L, Martin B, Du Y, Davatzikos C, 2006. Hippocampus volume loss due to chronic heavy drinking. Alcohol. Clin. Exp. Res 30 (11), 1866–1870. 10.1111/j.1530-0277.2006.00223.x. [DOI] [PubMed] [Google Scholar]
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR, 2010. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods 1 (2), 97–111. 10.1002/jrsm.12. [DOI] [PubMed] [Google Scholar]
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR, 2009. Introduction to Meta-analysis. 10.1002/9780470743386. [DOI] [Google Scholar]
- Borntrager C, Chorpita BF, Higa-McMillan CK, Daleiden EL, Starace N, 2013. Usual care for trauma-exposed youth: Are clinician-reported therapy techniques evidence-based? Child. Youth Serv. Rev 35 (1), 133–141. 10.1016/j.childyouth.2012.09.018. [DOI] [Google Scholar]
- Bouton M, 2004. Context and behavioral processes in extinction. Learn. Mem 11 (5), 485–494. 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Bouton M, Westbrook RF, Corcoran KA, Maren S, 2006. Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biol. Psychiatry 60 (4), 352–360. 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Southwick SM, McGlashan T, Nazeer A, Charney DS, 2003a. MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. Am. J. Psychiatry 10.1176/appi.ajp.160.5.924. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Southwick SM, McGlashan T, Staib LH, Charney DS, 2003b. Neural correlates of declarative memory for emotionally valenced words in women with posttraumatic stress disorder related to early childhood sexual abuse. Biol. Psychiatry 53 (10), 879–889. 10.1016/S0006-3223(02)01891-7. [DOI] [PubMed] [Google Scholar]
- Breslau N, 2004. Trauma exposure and posttraumatic stress disorder: a study of youths in urban America. J. Urban Health: Bull. N.Y. Acad. Med 81 (4), 530–544. 10.1093/jurban/jth138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N, Davis GC, 1992. Posttraumatic stress disorder in an urban population of young adults: risk factors for chronicity. Am. J. Psychiatry 149 (5), 671–675. 10.1176/ajp.149.5.671. [DOI] [PubMed] [Google Scholar]
- Breslau N, Davis GC, Peterson EL, Schultz LR, 2000. A second look at comorbidity in victims of trauma: the posttraumatic stress disorder-major depression connection. Biol. Psychiatry 48 (9), 902–909. 10.1016/S0006-3223(00)00933-1. [DOI] [PubMed] [Google Scholar]
- Breslau N, Davis GC, Schultz LR, 2003. Posttraumatic stress disorder and the incidence of nicotine, alcohol, and other drug disorders in persons who have experienced trauma. Arch. Gen. Psychiatry 60 (3), 289–294. 10.1001/archpsyc.60.3.289. [DOI] [PubMed] [Google Scholar]
- Breslau N, Koenen KC, Luo Z, Agnew-Blais J, Swanson S, Houts RM, Moffitt TE, 2014. Childhood maltreatment, juvenile disorders and adult post-traumatic stress disorder: a prospective investigation. Psychol. Med 10.1017/S0033291713002651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewin CR, 2001. A cognitive neuroscience account of posttraumatic stress disorder and its treatment. Behav. Res. Ther 39 (4), 373–393. 10.1016/S0005-7967(00)00087-5. [DOI] [PubMed] [Google Scholar]
- Brewin CR, Andrews B, Valentine JD, 2000. Meta-analysis of risk factors for post-traumatic stress disorder in trauma-exposed adults. J. Consult. Clin. Psychol. 10.1037/0022-006X.68.5.748. [DOI] [PubMed] [Google Scholar]
- Brewin CR, Dalgleish T, Joseph S, 1996. A dual representation theory of posttraumatic stress disorder. Psychol. Rev 670–686. 10.1037//0033-295X.103.4.670. [DOI] [PubMed] [Google Scholar]
- Brewin CR, Holmes EA, 2003. Psychological theories of posttraumatic stress disorder. Clin. Psychol. Rev 23 (3), 339–376. 10.1016/S0272-7358(03)00033-3. [DOI] [PubMed] [Google Scholar]
- Brewin CR, Kleiner JS, Vasterling JJ, Field AP, 2007. Memory for emotionally neutral information in posttraumatic stress disorder: a meta-analytic investigation. J. Abnorm. Psychol 116 (3), 448–463. 10.1037/0021-843X.116.3.448. [DOI] [PubMed] [Google Scholar]
- Brohawn KH, Offringa R, Pfaff DL, Hughes KC, Shin L, 2010. The neural correlates of emotional memory in posttraumatic stress disorder. Biol. Psychiatry. 10.1016/j.biopsych.2010.07.018. [DOI] [PubMed] [Google Scholar]
- Brown AD, Addis DR, Romano TA, Marmar CR, Bryant RA, Hirst W, Schacter DL, 2014. Episodic and semantic components of autobiographical memories and imagined future events in post-traumatic stress disorder. Memory 22 (6), 595–604. 10.1080/09658211.2013.807842. [DOI] [PubMed] [Google Scholar]
- Brown AD, Root JC, Romano TA, Chang LJ, Bryant RA, Hirst W, 2013. Overgeneralized autobiographical memory and future thinking in combat veterans with posttraumatic stress disorder. J. Behav. Ther. Exp. Psychiatry 44 (1), 129–134. 10.1016/j.jbtep.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Brunson KL, Eghbal-Ahmadi M, Bender R, Chen Y, Baram TZ, 2001. Long-term, progressive hippocampal cell loss and dysfunction induced by early-life administration of corticotropin-releasing hormone reproduce the effects of early-life stress. Proc. Natl. Acad. Sci 98 (15), 8856–8861. 10.1073/pnas.151224898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant RA, Felmingham K, Kemp A, Das P, Hughes G, Peduto A, Williams L, 2008. Amygdala and ventral anterior cingulate activation predicts treatment response to cognitive behaviour therapy for post-traumatic stress disorder. Psychol. Med 10.1017/S0033291707002231. [DOI] [PubMed] [Google Scholar]
- Burriss L, Ayers E, Ginsberg J, Powell DA, 2008. Learning and memory impairment in PTSD: relationship to depression. Depress. Anxiety 25 (2), 149–157. 10.1002/da.20291. [DOI] [PubMed] [Google Scholar]
- Busso DS, McLaughlin KA, Brueck S, Peverill M, Gold AL, Sheridan MA, 2017. Child abuse, neural structure, and adolescent psychopathology: a longitudinal study. J. Am. Acad. Child Adolesc. Psychiatry 56 (4), 321–328. 10.1016/j.jaac.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrión VG, Haas BW, Garrett A, Song S, Reiss AL, Carrion VG, Reiss AL, 2010. Reduced hippocampal activity in youth with posttraumatic stress symptoms: an FMRI study. J. Pediatr. Psychol 35 (5), 559–569. 10.1093/jpepsy/jsp112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel JC, de Vasconcelos AP, 2015. Importance of the ventral midline thalamus in driving hippocampal functions. Prog. Brain Res 10.1016/bs.pbr.2015.03.005. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, 1996. Equifinality and multifinality in developmental psychopathology. Dev. Psychopathol 8 (4), 597–600. 10.1017/S0954579400007318. [DOI] [Google Scholar]
- Cochran WG, 1954. The combination of estimates from different experiments. Biometrics 10 (1), 101–129. 10.2307/3001666. [DOI] [Google Scholar]
- Conrad CD, Magariños AM, LeDoux JE, McEwen BS, 1999. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav. Neurosci 113 (5), 902–913. 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- Cook JM, Schnurr PP, Foa E, 2004. Bridging the gap between posttraumatic stress disorder research and clinical practice: the example of exposure therapy. Psychother. Theory Res. Pract. Train 41 (4), 374–387. 10.1037/0033-3204.41.4.374. [DOI] [Google Scholar]
- Craske MG, Treanor M, Conway CC, Zbozinek T, Vervliet B, 2014. Maximizing exposure therapy: an inhibitory learning approach. Behav. Res. Ther 10.1016/j.brat.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L, 2006. Item, context and relational episodic encoding in humans. Curr. Opin. Neurobiol 16 (6), 693–700. 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Dixon ML, Thiruchselvam R, Todd R, Christoff K, 2017. Emotion and the prefrontal cortex: an integrative review. Psychol. Bull 10.1037/bul0000096. [DOI] [PubMed] [Google Scholar]
- Dorsey S, Mclaughlin KA, Kerns SEU, Harrison JP, Lambert H, Briggs EC, Amaya-Jackson L, 2017. Evidence base update for psychosocial treatments for children and adolescents exposed to traumatic events. J. Clin. Child Adolesc. Psychol 46 (3), 303–330. 10.1080/15374416.2016.1220309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBrow S, Davachi L, 2016. Temporal binding within and across events. Neurobiol. Learn. Mem 134, 107–114. 10.1016/j.nlm.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers A, Clark DM, 2000. A cognitive model of posttraumatic stress disorder. Behav. Res. Ther 38 (4), 319–345. 10.1016/S0005-7967(99)00123-0. [DOI] [PubMed] [Google Scholar]
- Ehlers A, Hackmann A, Michael T, 2004. Intrusive re-experiencing in post-traumatic stress disorder: phenomenology, theory, and therapy. Memory 12 (4), 403–415. 10.1080/09658210444000025. [DOI] [PubMed] [Google Scholar]
- Ehlers A, Hackmann A, Steil R, Clohessy S, Wenninger K, Winter H, 2002. The nature of intrusive memories after trauma: the warning signal hypothesis. Behav. Res. Ther 40 (9), 995–1002. 10.1016/S0005-7967(01)00077-8. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C, 2007. The medial temporal lobe and recognition memory. Annu. Rev. Neurosci 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euston DR, Gruber AJ, McNaughton BL, 2012. The role of medial prefrontal cortex in memory and decision making. Neuron 76 (6), 1057–1070. 10.1016/j.neuron.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani N, Tone EB, Phifer J, Norrholm SD, Bradley B, Ressler KJ, Jovanovic T, 2012. Attention bias toward threat is associated with exaggerated fear expression and impaired extinction in PTSD. Psychol. Med 42 (3), 533–543. 10.1017/S0033291711001565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell NR, Deacon BJ, Dixon LJ, Lickel JJ, 2013. Theory-based training strategies for modifying practitioner concerns about exposure therapy. J. Anxiety Disord 27 (8), 781–787. 10.1016/j.janxdis.2013.09.003. [DOI] [PubMed] [Google Scholar]
- Foa E, Chrestman KR, Gilboa-Schechtman E, 2008. Prolonged Exposure Therapy for Adolescents With PTSD: Emotional Processing of Traumatic Experiences, Therapist Guide. Oxford University Press, New York, NY: 10.1093/med:psych/9780195308501.001.0001. [DOI] [Google Scholar]
- Foa E, Hembree EA, Rothbaum BO, 2007. Prolonged Exposure Therapy for PTSD: Emotional Processing of Traumatic Experiences Therapist Guide (Treatments That Work). Oxford University Press, New York, NY: 10.1093/med:psych/9780195308501.001.0001. [DOI] [Google Scholar]
- Foa E, Kozak MJ, 1986. Emotional Processing of Fear. Exposure to Corrective Information. Psychol. Bull 10.1037/0033-2909.99.1.20. [DOI] [PubMed] [Google Scholar]
- Foa E, Molnar C, Cashman L, 1995. Change in rape narratives during exposure therapy for posttraumatic stress disorder. J. Trauma. Stress 8 (4), 675–690. 10.1007/BF02102894. [DOI] [PubMed] [Google Scholar]
- Foa E, Rothbaum BO, Riggs DS, Murdock TB, 1991. Treatment of posttraumatic stress disorder in rape victims: a comparison between cognitive-behavioral procedures and counseling. J. Consult. Clin. Psychol 59 (5), 715–723. 10.1037/0022-006X.59.5.715. [DOI] [PubMed] [Google Scholar]
- Garfinkel SN, Abelson JL, King AP, Sripada RK, Wang X, Gaines LM, Liberzon I, 2014. Impaired contextual modulation of memories in PTSD: an fMRI and psychophysiological study of extinction retention and fear renewal. J. Neurosci 34 (40), 13435–13443. 10.1523/JNEUROSCI.4287-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuze E, Vermetten E, Ruf M, de Kloet CS, Westenberg HG, 2008. Neural correlates of associative learning and memory in veterans with posttraumatic stress disorder. J. Psychiatr. Res 42 (8), 659–669. 10.1016/j.jpsychires.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK, 2002. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat. Neurosci 5 (11), 1242–1247. 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson MW, Williston SK, Paulus LA, Lasko NB, Gurvits VT, Shenton ME, Orr SP, 2007. Configurational cue performance in identical twins discordant for posttraumatic stress disorder: theoretical implications for the role of hippocampal function. Biol. Psychiatry 62 (5), 513–520. 10.1016/j.biopsych.2006.12.023.Configural. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold AL, Sheridan MA, Peverill M, Busso DS, Lambert H, Alves S, McLaughlin KA, 2016. Childhood abuse and reduced cortical thickness in brain regions involved in emotional processing. J. Child Psychol. Psychiatry 57 (10), 1154–1164. 10.1111/jcpp.12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golier J, Yehuda R, Lupien SJ, Harvey PD, 2003. Memory for trauma-related information in Holocaust survivors with PTSD. Psychiatry Res. 121 (2), 133–143. 10.1016/S0925-4927(03)00120-3. [DOI] [PubMed] [Google Scholar]
- Golier J, Yehuda R, Lupien SJ, Harvey PD, Grossman R, Elkin A, 2002. Memory performance in holocaust survivors with posttraumatic stress disorder. Am. J. Psychiatry 159 (10), 1682–1688. 10.1176/appi.ajp.159.10.1682. [DOI] [PubMed] [Google Scholar]
- Greenwald AG, 1975. Consequences of prejudice against the null hypothesis. Psychol. Bull 82 (1), 1–20. 10.1037/h0076157. [DOI] [Google Scholar]
- Grillon C, Morgan CA, 1999. Fear-potentiated startle conditioning to explicit and contextual cues in Gulf War veterans with posttraumatic stress disorder. J. Abnorm. Psychol 108 (1), 134–142. 10.1037/0021-843x.108.1.134. [DOI] [PubMed] [Google Scholar]
- Guez J, Cohen J, Naveh-Benjamin M, Shiber A, Yankovsky Y, Saar R, Shalev H, 2013. Associative memory impairment in acute stress disorder: characteristics and time course. Psychiatry Res. 209 (3), 479–484. 10.1016/j.psychres.2012.12.013. [DOI] [PubMed] [Google Scholar]
- Guez J, Naveh-Benjamin M, Yankovsky Y, Cohen J, Shiber A, Shalev H, 2011. Traumatic stress is linked to a deficit in associative episodic memory. J. Trauma. Stress 24 (3), 260–267. 10.1002/jts.20635. [DOI] [PubMed] [Google Scholar]
- Hackmann A, Ehlers A, Speckens A, Clark DM, 2004. Characteristics and content of intrusive memories in PTSD and their changes with treatment. J. Trauma. Stress 17 (3), 231–240. 10.1023/B:JOTS.0000029266.88369.fd. [DOI] [PubMed] [Google Scholar]
- Hanson JL, Nacewicz BM, Sutterer MJ, Cayo AA, Schaefer SM, Rudolph KD, Davidson RJ, 2015. Behavioral problems after early life stress: contributions of the hippocampus and amygdala. Biol. Psychiatry 77 (4), 314–323. 10.1016/j.biopsych.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey A, Bryant RA, 1999. A qualitative investigation of the organization of traumatic memories. Br. J. Clin. Psychol 38 (4), 401–405. 10.1348/014466599162999. [DOI] [PubMed] [Google Scholar]
- Harvey A, Bryant RA, Rapee RM, 1996. Preconscious processing of threat in post-traumatic stress disorder. Cognit. Ther. Res 20 (6), 613–623. 10.1007/BF02227964. [DOI] [Google Scholar]
- Hayes JP, LaBar KS, McCarthy G, Selgrade E, Nasser J, Dolcos F, Morey RA, 2011. Reduced hippocampal and amygdala activity predicts memory distortions for trauma reminders in combat-related PTSD. J. Psychiatr. Res 45 (5), 660–669. 10.1016/j.jpsychires.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes S, Baena E, Truong T-K, Cabeza R, 2010. Neural mechanisms of context effects on face recognition: automatic binding and context shift decrements. J. Cogn. Neurosci 22 (11), 2541–2554. 10.1162/jocn.2009.21379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes S, Nadel L, Ryan L, 2007. The effect of scene context on episodic object recognition: parahippocampal cortex mediates memory encoding and retrieval success. Hippocampus 17 (9), 873–889. 10.1002/hipo.20319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke K, Buck A, Weber B, Wieser HG, 1997. Human hippocampus establishes associations in memory. Hippocampus 7 (3), 249–256. . [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Thompson SG, 2002. Quantifying heterogeneity in a meta-analysis. Stat. Med 21 (11), 1539–1558. 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Hoffman KL, McNaughton BL, 2002. Coordinated reactivation of distributed memory traces in primate neocortex. Science 297 (5589), 2070–2073. 10.1126/science.1073538. [DOI] [PubMed] [Google Scholar]
- Imel ZE, Laska K, Jakupcak M, Simpson TL, 2013. Meta-analysis of dropout in treatments for posttraumatic stress disorder. J. Consult. Clin. Psychol 81 (3), 394–404. 10.1037/a0031474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac CL, Cushway D, Jones GV, 2006. Is posttraumatic stress disorder associated with specific deficits in episodic memory? Clin. Psychol. Rev 26 (8), 939–955. 10.1016/j.cpr.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Israel L, Schacter DL, 1997. Pictorial encoding reduces false recognition of semantic associates. Psychon. Bull. Rev 4 (4), 577–581. 10.3758/BF03214352. [DOI] [Google Scholar]
- Ivy AS, Rex CS, Chen Y, Dubé C, Maras PM, Grigoriadis DE, Baram TZ, 2010. Hippocampal dysfunction and cognitive impairments provoked by chronic early-life stress involve excessive activation of CRH receptors. J. Neurosci 30 (39), 13005–13015. 10.1523/JNEUROSCI.1784-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson O, Schacter DL, 2004. Encoding activity in anterior medial temporal lobe supports subsequent associative recognition. NeuroImage 21 (1), 456–462. 10.1016/j.neuroimage.2003.09.050. [DOI] [PubMed] [Google Scholar]
- Jelinek L, Randjbar S, Seifert D, Kellner M, Moritz S, 2009. The organization of autobiographical and nonautobiographical memory in posttraumatic stress disorder (PTSD). J. Abnorm. Psychol 118 (2), 288–298. 10.1037/a0015633. [DOI] [PubMed] [Google Scholar]
- Johansen JP, Cain CK, Ostroff LE, Ledoux JE, 2011. Molecular mechanisms of fear learning and memory. Cell 147 (3), 509–524. 10.1016/j.cell.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Kazama A, Bachevalier J, Davis M, 2012. Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology 62 (2), 695–704. 10.1016/j.neuropharm.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Blanding NQ, Davis M, Duncan E, Bradley B, Ressler KJ, 2010. Impaired fear inhibition is a biomarker of PTSD but not depression. Depress. Anxiety 27 (3), 244–251. 10.1002/da.20663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Fennell JE, Keyes M, Fiallos AM, Myers KM, Duncan EJ, 2009. Posttraumatic stress disorder may be associated with impaired fear inhibition: relation to symptom severity. Psychiatry Res. 167 (1–2), 151–160. 10.1016/j.psychres.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Ressler KJ, 2010. How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. Am. J. Psychiatry 167 (6), 648–662. 10.1176/appi.ajp.2009.09071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl A, Schaefer M, Malta LS, Dörfel D, Rohleder N, Werner A, 2006. A meta-analysis of structural brain abnormalities in PTSD. Neurosci. Biobehav. Rev 30 (7), 1004–1031. 10.1016/j.neubiorev.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB, 1995. Posttraumatic stress disorder in the national comorbidity survey. Arch. Gen. Psychiatry 52 (12), 1048–1060. 10.1128/AAC.03728-14. [DOI] [PubMed] [Google Scholar]
- Kessler RC, 2000. Posttraumatic stress disorder: the burden to the individual and to society. J. Clin. Psychiatry 61 (SUPPL. 5), 4–14. 10.1002/(ISSN)1097-4679. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE, 2005. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 62 (6), 593–602. 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kim J, Jung MW, 2006. Neural circuits and mechanisms involved in Pavlovian fear conditioning: a critical review. Neurosci. Biobehav. Rev 30 (2), 188–202. 10.1016/j.neubiorev.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Lyoo IK, Lee YS, Kim J, Sim ME, Bae SJ, Jeong DU, 2007. Decreased cerebral blood flow of thalamus in PTSD patients as a strategy to reduce re-experience symptoms. Acta Psychiatr. Scand 10.1111/j.1600-0447.2006.00952.x. [DOI] [PubMed] [Google Scholar]
- Kirwan CB, Stark CEL, 2004. Medial temporal lobe activation during encoding and retrieval of novel face-name pairs. Hippocampus 14 (7), 919–930. 10.1002/hipo.20014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitayama N, Vaccarino V, Kutner M, Weiss P, Bremner JD, 2005. Magnetic resonance imaging (MRI) measurement of hippocampal volume in posttraumatic stress disorder: a meta-analysis. J. Affect. Disord 88 (1), 79–86. 10.1016/j.jad.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Kubzansky LD, Koenen KC, Spiro A, Vokonas PS, Sparrow D, 2007. Prospective study of posttraumatic stress disorder symptoms and coronary heart disease in the Normative Aging Study. Arch. Gen. Psychiatry 64, 109–116. 10.1001/archpsyc.64.1.109. [DOI] [PubMed] [Google Scholar]
- Lambert H, Peverill M, Sambrook K, Rosen M, Sheridan M, McLaughlin K, 2019. Altered development of hippocampus-dependent associative learning following early-life adversity. Dev. Cogn. Neurosci 38, 100666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert H, Sheridan MA, Sambrook KA, Rosen ML, Askren MK, McLaughlin KA, 2017. Hippocampal contribution to context encoding across development is disrupted following early-life adversity. J. Neurosci 37 (7), 1925–1934. 10.1523/JNEUROSCI.2618-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius RA, Williamson PC, Densmore M, Boksman K, Gupta MA, Neufeld RW, Menon RS, 2001. Neural correlates of traumatic memories in posttraumatic stress disorder: a functional MRI investigation. Am. J. Psychiatry. 10.1176/appi.ajp.158.11.1920. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Williamson PC, Hopper J, Densmore M, Boksman K, Gupta MA, Menon RS, 2003. Recall of emotional states in posttraumatic stress disorder: an fMRI investigation. Biol. Psychiatry 10.1016/S0006-3223(02)01466-X. [DOI] [PubMed] [Google Scholar]
- Lavenex P, Amaral DG, 2000. Hippocampal-neocortical interaction: a hierarchy of associativity. Hippocampus 10 (4), 420–430. . [DOI] [PubMed] [Google Scholar]
- Liberzon I, 2018. Searching for intermediate phenotypes in posttraumatic stress disorder. Biol. Psychiatry 83 (10), 797–799. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Abelson JL, 2016. Context processing and the neurobiology of post-traumatic stress disorder. Neuron 92 (1), 14–30. 10.1016/j.neuron.2016.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I, Sripada CS, 2007. The functional neuroanatomy of PTSD: a critical review. Prog. Brain Res 167, 151–169. 10.1016/S0079-6123(07)67011-3. [DOI] [PubMed] [Google Scholar]
- Lissek S, Powers AS, McClure EB, Phelps EA, Woldehawariat G, Grillon C, Pine DS, 2005. Classical fear conditioning in the anxiety disorders: a meta-analysis. Behav. Res. Ther 43 (11), 1391–1424. 10.1016/j.brat.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Logue MW, van Rooij SJH, Dennis EL, Davis SL, Hayes JP, Stevens JS, Morey RA, 2018. Smaller hippocampal volume in posttraumatic stress disorder: a multisite ENIGMA-PGC study: subcortical volumetry results from posttraumatic stress disorder consortia. Biol. Psychiatry 83 (3), 244–253. 10.1016/j.biopsych.2017.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar M, Heim C, 2009. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci 10 (6), 434–445. [DOI] [PubMed] [Google Scholar]
- Maisto AA, Queen DE, 1992. Memory for pictorial information and the picture superiority effect. Educ. Gerontol 18 (2), 213–223. 10.1080/0360127920180207. [DOI] [Google Scholar]
- Maren S, Phan KL, Liberzon I, 2013. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat. Rev. Neurosci 14 (6), 417–428. 10.1038/nrn3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks I, Lovell K, Noshirvani H, Livanou M, Thrasher S, 1998. Treatment of posttraumatic stress disorder by exposure and/or cognitive restructuring: a controlled study. Arch. Gen. Psychiatry 55 (4), 317–325. 10.1001/archpsyc.55.4.317. [DOI] [PubMed] [Google Scholar]
- Mayes A, Montaldi D, Migo E, 2007. Associative memory and the medial temporal lobes. Trends Cogn. Sci 11 (3), 126–135. 10.1016/j.tics.2006.12.003. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Conron KJ, Koenen KC, Gilman SE, 2010. Childhood adversity, adult stressful life events, and risk of past-year psychiatric disorder: a test of the stress sensitization hypothesis in a population-based sample of adults. Psychol. Med 40 (10), 1647–1658. 10.1017/S0033291709992121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Koenen KC, Bromet EJ, Karam EG, Liu H, Petukhova M, Kessler RC, 2017. Childhood adversities and post-traumatic stress disorder: evidence for stress sensitisation in the World Mental Health Surveys. Br. J. Psychiatry 10.1192/bjp.bp.116.197640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Koenen KC, Hill ED, Petukhova M, Sampson NA, Zaslavsky AM, Kessler RC, 2013. Trauma exposure and posttraumatic stress disorder in a national sample of adolescents. J. Am. Acad. Child Adolesc. Psychiatry 52 (8). 10.1016/j.jaac.2013.05.011.815-830.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Gold AL, Duys A, Lambert H, Peverill M, Pine DS, 2016. Maltreatment exposure, brain structure, and fear conditioning in children and adolescents. Neuropsychopharmacology 41 (8), 1956–1964. 10.1038/npp.2015.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Orr SP, Lasko NB, Chang Y, Rauch SL, Pitman RK, 2008. Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. J. Psychiatr. Res 42 (7), 515–520. 10.1016/j.jpsychires.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin L, Lasko NB, Rauch SL, 2009. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol. Psychiatry 66 (12), 1075–1082. 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ, 2012. Fear extinction as a model for translational neuroscience: ten years of progress. Annu. Rev. Psychol 63 (1), 129–151. 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JK, McDougall S, Thomas S, Wiener JM, 2017. Impairment in active navigation from trauma and Post-Traumatic Stress Disorder. Neurobiol. Learn. Mem 140, 114–123. 10.1016/j.nlm.2017.02.019. [DOI] [PubMed] [Google Scholar]
- Mineka S, Oehlberg K, 2008. The relevance of recent developments in classical conditioning to understanding the etiology and maintenance of anxiety disorders. Acta Psychol. 127 (3), 567–580. 10.1016/j.actpsy.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Moore SA, Zoellner LA, 2007. Overgeneral autobiographical memory and traumatic events: an evaluative review. Psychol. Bull 133 (3), 419–437. 10.1037/0033-2909.133.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morina N, Koerssen R, Pollet TV, 2016. Interventions for children and adolescents with posttraumatic stress disorder: a meta-analysis of comparative outcome studies. Clin. Psychol. Rev 47, 41–54. 10.1016/j.cpr.2016.05.006. [DOI] [PubMed] [Google Scholar]
- Moscarello JM, Maren S, 2018. Flexibility in the face of fear: hippocampal–prefrontal regulation of fear and avoidance. Curr. Opin. Behav. Sci 10.1016/j.cobeha.2017.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovitch M, Cabeza R, Winocur G, Nadel L, 2016. Episodic memory and beyond: the Hippocampus and neocortex in transformation. Annu. Rev. Psychol 10.1146/annurev-psych-113011-143733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DL, Reed VS, Walling JR, 1976. Pictorial superiority effect. J. Exp. Psychol. Hum. Learn 2 (5), 523–528. 10.1037//0278-7393.2.5.523. [DOI] [PubMed] [Google Scholar]
- Nelson M, Tumpap A, 2017. Posttraumatic stress disorder symptom severity is associated with left hippocampal volume reduction: a meta-analytic study. CNS Spectr. 10.1017/S1092852916000833. [DOI] [PubMed] [Google Scholar]
- O’Doherty DCM, Chitty KM, Saddiqui S, Bennett MR, Lagopoulos J, 2015. A systematic review and meta-analysis of magnetic resonance imaging measurement of structural volumes in posttraumatic stress disorder. Psychiatry Res. - Neuroimaging 10.1016/j.pscychresns.2015.01.002. [DOI] [PubMed] [Google Scholar]
- Ono M, Devilly GJ, Shum DHK, 2016. A meta-analytic review of overgeneral memory: the role of trauma history, mood, and the presence of posttraumatic stress disorder. Psychol. Trauma Theory Res. Pract. Policy. 10.1037/tra0000027. [DOI] [PubMed] [Google Scholar]
- Park DC, Puglisi JT, Sovacool M, 1983. Memory for pictures, words, and spatial location in older adults: evidence for pictorial superiority. J. Gerontol 38 (5), 582–588. 10.1093/geronj/38.5.582. [DOI] [PubMed] [Google Scholar]
- Peres JFP, Foerster B, Santana LG, Fereira MD, Nasello AG, Savoia M, Lederman H, 2011. Police officers under attack: resilience implications of an fMRI study. J. Psychiatr. Res 10.1016/j.jpsychires.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Perkonigg A, Pfister H, Stein MB, Höfler M, Lieb R, Maercker A, Wittchen H-U, 2005. Longitudinal course of posttraumatic stress disorder and posttraumatic stress disorder symptoms in a community sample of adolescents and young adults. Am. J. Psychiatry 162, 1320–1327. 10.1176/appi.ajp.162.7.1320. [DOI] [PubMed] [Google Scholar]
- Phelps EA, 2006. Emotion and cognition: insights from studies of the human amygdala. Annu. Rev. Psychol 57, 27–53. 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, Ledoux JE, 2004. Extinction learning in humans: role of the amygdala and vmPFC. Neuron 43 (6), 897–905. 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Gilbertson MW, Gurvits TV, May FS, Lasko NB, Metzger LJ, Orr SP, 2006. Clarifying the origin of biological abnormalities in PTSD through the study of identical twins discordant for combat exposure. Ann. N. Y. Acad. Sci 1071, 242–254. 10.1196/annals.1364.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman RK, Rasmusson AM, Koenen KC, Shin L, Orr SP, Gilbertson MW, Liberzon I, 2012. Biological studies of post-traumatic stress disorder. Nat. Rev. Neurosci 13 (11), 769–787. 10.1038/nrn3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pole N, 2007. The psychophysiology of posttraumatic stress disorder: a meta-analysis. Psychol. Bull 133 (5), 725–746. 10.1037/0033-2909.133.5.725. [DOI] [PubMed] [Google Scholar]
- Powers MB, Halpern JM, Ferenschak MP, Gillihan SJ, Foa E, 2010. A meta-analytic review of prolonged exposure for posttraumatic stress disorder. Clin. Psychol. Rev 30 (6), 635–641. 10.1016/j.cpr.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Russo GK, Barron JL, Lebron K, 2000. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J. Neurosci 20 (16), 6225–6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Shin L, McInerney SC, MacKlin ML, Lasko NB, Pitman RK, 2000. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol. Psychiatry 47 (9), 769–776. 10.1016/S0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- Resick PA, Nishith P, Weaver TL, Astin MC, Feuer CA, 2002. A comparison of cognitive-processing therapy with prolonged exposure and a waiting condition for the treatment of chronic posttraumatic stress disorder in female rape victims. J. Consult. Clin. Psychol 70 (4), 867–879. 10.1037//0022-006X.70.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AL, Gilman SE, Breslau J, Breslau N, Koenen KC, 2011. Race ethnic differences in exposure to traumatic events, development of post-traumatic stress disorder, and treatment-seeking for post-traumatic stress disorder in the United States. Psychol. Med 41 (1), 71–83. 10.1017/S0033291710000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougemont-Bücking A, Linnman C, Zeffiro TA, Zeidan MA, Lebron-Milad K, Rodriguez-Romaguera J, Milad MR, 2011. Altered processing of contextual information during fear extinction in PTSD: an fMRI study. CNS Neurosci. Ther 17 (4), 227–236. 10.1111/j.1755-5949.2010.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saar-Ashkenazy R, Cohen JE, Guez J, Gasho C, Shelef I, Friedman A, Shalev H, 2014. Reduced corpus-callosum volume in posttraumatic stress disorder highlights the importance of interhemispheric connectivity for associative memory. J. Trauma. Stress 27 (1), 18–26. 10.1002/jts.21887. [DOI] [PubMed] [Google Scholar]
- Scammacca N, Roberts G, Stuebing KK, 2014. Meta-analysis with complex research designs: dealing with dependence from multiple measures and multiple group comparisons. Rev. Educ. Res 84 (3), 328–364. 10.3102/0034654313500826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JC, Matt GE, Wrocklage KM, Crnich C, Jordan J, Southwick SM, Schweinsburg BC, 2015. A quantitative meta-analysis of neurocognitive functioning in posttraumatic stress disorder. Psychol. Bull 141 (1), 105–140. 10.1037/a0038039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin L, Orr SP, Carson MA, Rauch SL, Macklin ML, Lasko NB, Pitman RK, 2004. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch. Gen. Psychiatry 61 (2), 168–176. 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- Smith KV, Burgess N, Brewin CR, King JA, 2015. Impaired allocentric spatial processing in posttraumatic stress disorder. Neurobiol. Learn. Mem 119, 69–76. 10.1016/j.nlm.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Sierra-Mercado D, Pardilla-Delgado E, Quirk GJ, 2012. Gating of fear in prelimbic cortex by hippocampal and amygdala inputs. Neuron 76 (4), 804–812. 10.1016/j.neuron.2012.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling R, Chua E, Cocchiarella A, Rand-Giovannetti E, Poldrack R, Schacter DL, Albert M, 2003. Putting names to faces: successful encoding of associative memories activates the anterior hippocampal formation. NeuroImage 20 (2), 1400–1410. 10.1016/S1053-8119(03)00391-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, 1992. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol. Rev. 99 (2), 195–231. 10.1037/0033-295X.99.3.582. [DOI] [PubMed] [Google Scholar]
- St. Jacques PL, Botzung A, Miles A, Rubin DC, 2011. Functional neuroimaging of emotionally intense autobiographical memories in post-traumatic stress disorder. J. Psychiatr. Res 10.1016/j.jpsychires.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JS, Jovanovic T, Fani N, Ely TD, Glover EM, Bradley B, Ressler KJ, 2013. Disrupted amygdala-prefrontal functional connectivity in civilian women with posttraumatic stress disorder. J. Psychiatr. Res 10.1016/j.jpsychires.2013.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JS, Reddy R, Kim YJ, van Rooij SJH, Ely TD, Hamann S, Jovanovic T, 2018. Episodic memory after trauma exposure: medial temporal lobe function is positively related to re-experiencing and inversely related to negative affect symptoms. Neuroimage Clin. 10.1016/j.nicl.2017.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmi D, 2013. Enhanced emotional memory: cognitive and neural mechanisms. Curr. Dir. Psychol. Sci 10.1177/0963721413498893. [DOI] [Google Scholar]
- Teicher MH, Anderson CM, Polcari A, 2012. Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proc. Natl. Acad. Sci 109 (9), E563–72. 10.1073/pnas.1115396109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempesta D, Mazza M, Iaria G, De Gennaro L, Ferrara M, 2011. A specific deficit in spatial memory acquisition in post-traumatic stress disorder and the role of sleep in its consolidation. Hippocampus 22 (5), 1154–1163. 10.1002/hipo.20961. [DOI] [PubMed] [Google Scholar]
- Thomaes K, Dorrepaal E, Draijer NPJ, de Ruiter MB, Elzinga BM, van Balkom AJ, Veltman DJ, 2009. Increased activation of the left hippocampus region in Complex PTSD during encoding and recognition of emotional words: a pilot study. Psychiatry Res. Neuroimaging 10.1016/j.pscychresns.2008.03.003. [DOI] [PubMed] [Google Scholar]
- van der Kolk BA, Fisler R, 1995. Dissociation and the fragmentary nature of traumatic memories: overview and exploratory study. J. Trauma. Stress 8 (4), 505–525. 10.1007/BF02102887. [DOI] [PubMed] [Google Scholar]
- Van Etten ML, Taylor S, 1998. Comparative efficacy of treatments for post-traumatic stress disorder: a meta-analysis. Clin. Psychol. Psychother 5 (3), 126–144. . [DOI] [Google Scholar]
- Vermetten E, Schmahl C, Southwick SM, Bremner JD, 2007. Positron tomographic emission study of olfactory induced emotional recall in veterans with and without combat-related posttraumatic stress disorder. Psychopharmacol. Bull [PMC free article] [PubMed] [Google Scholar]
- Vermetten E, Vythilingam M, Southwick SM, Charney DS, Bremner JD, 2003. Long-term treatment with paroxetine increases verbal declarative memory and hippocampal volume in posttraumatic stress disorder. Biol. Psychiatry 54 (7), 693–702. 10.1016/S0006-3223(03)00634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP, 2006. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience. 10.1016/j.neuroscience.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B, 2004. Hippocampal volume and depression: a meta-analysis of MRI studies. Am. J. Psychiatry 161 (11), 1957–1966. 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- Viechtbauer W, 2015. Package “Metafor.”. Retrieved from. http://http1.debian.or.jp/pub/CRAN/web/packages/metafor/metafor.pdf. [Google Scholar]
- Viechtbauer W, 2010. Conducting meta-analyses in r with the metafor package. J. Stat. Softw 36 (3), 1–48. 10.1103/PhysRevB.91.121108. [DOI] [Google Scholar]
- Watts BV, Schnurr PP, Mayo L, Young-Xu Y, Weeks WB, Friedman MJ, 2013. Meta-analysis of the efficacy of treatments for posttraumatic stress disorder. J. Clin. Psychiatry 74 (6), e541–550. 10.4088/JCP.12r08225. [DOI] [PubMed] [Google Scholar]
- Werner NS, Meindl T, Engel RR, Rosner R, Riedel M, Reiser M, Fast K, 2009. Hippocampal function during associative learning in patients with posttraumatic stress disorder. J. Psychiatr. Res 43 (3), 309–318. 10.1016/j.jpsychires.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Whalley MG, Kroes MCW, Huntley Z, Rugg MD, Davis SW, Brewin CR, 2013. An fMRI investigation of posttraumatic flashbacks. Brain Cogn. 10.1016/j.bandc.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf RC, Herringa RJ, 2016. Prefrontal-amygdala dysregulation to threat in pediatric posttraumatic stress disorder. Neuropsychopharmacology. 10.1038/npp.2015.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X, Brown AD, Lazar M, Cressman VL, Henn-Haase C, Neylan TC, Marmar CR, 2013. Spontaneous brain activity in combat related PTSD. Neurosci. Lett 10.1016/j.neulet.2013.04.032. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Tischler L, Golier J, Grossman R, Brand SR, Kaufman S, Harvey PD, 2006. Longitudinal assessment of cognitive performance in Holocaust survivors with and without PTSD. Biol. Psychiatry 60 (7), 714–721. 10.1016/j.biopsych.2006.03.069. [DOI] [PubMed] [Google Scholar]
- Yin Y, Jin C, Hu X, Duan L, Li Z, Song M, Li L, 2011. Altered resting-state functional connectivity of thalamus in earthquake-induced posttraumatic stress disorder: a functional magnetic resonance imaging study. Brain Res. 10.1016/j.brainres.2011.07.016. [DOI] [PubMed] [Google Scholar]
- Young SL, Bohenek DL, Fanselow MS, 1994. NMDA processes mediate anterograde amnesia of contextual fear conditioning induced by hippocampal damage: immunization against amnesia by context preexposure. Behav. Neurosci 108 (1), 19–29. 10.1037/0735-7044.108.1.19. [DOI] [PubMed] [Google Scholar]
- Zayfert C, Dums AR, Ferguson RJ, Hegel MT, 2002. Health functioning impairments associated with posttraumatic stress disorder, anxiety disorders, and depression. J. Nerv. Ment. Dis 190 (4), 233–240. 10.1097/00005053-200204000-00004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.