Abstract
Introduction:
α7 nicotinic acetylcholine receptors (nAChRs) play an important role in vagus nerve-based cholinergic anti-inflammatory effects. This study was designed to assess the role of α7 nAChRs in dextran sodium sulfate (DSS)-induced colitis in male and female mouse. We first compared disease activity and pathogenesis of colitis in α7 knockout and wild-type mice. We then evaluated the effect of several α7 direct and indirect agonists on the severity of disease in the DSS-induced colitis.
Methods:
Male and female adult mice were administered 2.5% DSS solution freely in the drinking water for 7 consecutive days and the colitis severity (disease activity index) was evaluated as well as colon length, colon histology, and levels of tumor necrosis factor-alpha colonic levels.
Results:
Male, but not female, α7 knockout mice displayed a significantly increased colitis severity and higher tumor necrosis factor-alpha levels as compared with their littermate wild-type mice. Moreover, pretreatment with selective α7 ligands PHA-543613, choline, and PNU-120596 decreased colitis severity in male but not female mice. The anti-colitis effects of these α7 compounds dissipated when administered at higher doses.
Conclusions:
Our results suggest the presence of a α7-dependent anti-colitis endogenous tone in male mice. Finally, our results show for the first time that female mice are less sensitive to the anti-colitis activity of α7 agonists. Ovarian hormones may play a key role in the sex difference effect of α7 nAChRs modulation of colitis in the mouse.
Implications:
Our collective results suggest that targeting α7 nAChRs could represent a viable therapeutic approach for intestinal inflammation diseases such as ulcerative colitis with the consideration of sex differences.
Introduction
Ulcerative colitis (UC) is an idiopathic, chronic, autoimmune disease characterized by mucosal inflammation that affects primarily the colon and the rectum.1 Various studies have shown that cigarette smoking has beneficial effects on UC.2,3 In addition, the use of nicotine patches and enemas has yielded positive results on disease symptomology of UC in some clinical studies4 and in experimental animal models of colitis. Nicotine also has been shown to be effective after oral administration in the treatment of intestinal inflammation.5,6 Results of in vivo and in vitro studies have suggested that α7 nicotinic acetylcholine receptors (nAChRs) mediate the anti-inflammatory effects of nicotine.7–9 The α7 subtype is a well-characterized nAChR subunit that exhibits distinct physiological and pharmacological profiles relative to other nAChR subtypes. It is characterized by high calcium permeability,10 homopentameric structure, and is present in the central and peripheral nervous systems.11,12 Several types of immune cells have been reported to express α7 nAChR mRNA.13–15 These receptors are expressed also in the enteric nervous system, particularly in the enteric plexuses.12,16
Recent studies have shown that α7 nAChRs can regulate inflammation primarily through the vagus nerve acting as an endogenous “cholinergic (nicotinic) anti-inflammatory pathway.” This pathway ameliorates inflammation by regulating the production of pro-inflammatory cytokines mainly through activation of α7 nAChRs on macrophages.7,8,17–19 In that regard, vagus nerve activation attenuates intestinal inflammation via α7 nicotinic receptors in animal models of postoperative ileus and experimental colitis.6,8,17,20 Furthermore, Abdrakhmanova et al.21 showed that activation of α7 nAChRs was critical in suppressing hyperexcitability of inflamed colonic sensory neurons from dextran sodium sulfate (DSS)-treated mice.
In view of this evidence for a functionally relevant role of α7 nAChRs in modulating inflammation, it was proposed that α7 nAChR agonists would be effective in animal models of colonic inflammation. However, Snoek et al.22 reported recently that although selective α7 nAChR agonists reduce the increase in cytokine in vitro, in whole blood and macrophage cultures, they failed in DSS-induced colitis or were ineffective in those of trinitrobenzene sulphonic acid-induced colitis in female mice. These results prompted us to reevaluate the role of α7 nAChR in colonic inflammation in mice using multiple approaches. We first assessed the involvement of an endogenous α7 nicotinic mechanism in the development of colitis using α7 knockout (KO) mice in the DSS model. We then tested the effects of α7 nAChR activation on colitis using selective α7 full agonists including PHA-543613 given systemically,23 and choline given orally.24,25 We also evaluated the effects of PNU-120596, a type II α7 nAChRs positive allosteric modulator (PAM), in the DSS model. This PAM was used since, in principle, it can enhance endogenous α7 nAChR functions without altering the temporal integrity of transmission or interacting directly with the α7 nAChR binding site. This functional attribute would allow for enhancement of a potential endogenous α7-mediated anti-inflammatory tone activated in the colitis model. These collective studies were conducted in male and female mice to address for sex differences in the role of α7 nAChRs in regulating colonic inflammation in the mouse.
Materials and Methods
Animals
Male and female C57BL/6J and α7 KO mice were purchased from Jackson Laboratories (Bar Harbor, ME). The null mice and their wild-type (WT) littermates were bred in an animal care facility at Virginia Commonwealth University and were maintained on a C57Bl/6J background and have been backcrossed to at least N10. For all experiments, mutant-type and WT controls were obtained from crossing heterozygote mice. This breeding scheme allowed us to rigorously control for any anomalies that may occur with crossing solely mutant mice. Male and female mice were 8–10 weeks of age at the start of experiments, weighed 25–30 g, and were group-housed in a 21°C humidity-controlled Association for Assessment and Accreditation of Laboratory Animal Care-approved animal care facility with ad libitum access to food and water. Mice were housed under standard conditions for a minimum of 1 week before experimentation. Experiments were performed during the light cycle and were approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University.
Drugs
PHA-543613 (N-[(3R)-1-Azabicyclo-[2.2.2]-oct-3-yl]-furo-[2,3-c]-pyridine-5-carboxamide Dihydrochloride) and PNU-120596 (1-(5-chloro-2,4-dimethoxy-phenyl)-3-(5-methyl-isoxanol-3-yl)-urea) were obtained from the drug supply program of the National Institute on Drug Abuse (Rockville, MD). PHA-543613 was dissolved in physiological saline (0.9% sodium chloride) and administered s.c. twice daily. PNU-120596 was suspended in a vehicle of absolute ethanol, Emulphor-620 (Rhone-Poulenc, Inc, Princeton, NJ), and saline at a ratio of 1:1:18 and administered by i.p. injection once daily at a volume of 10 mL/kg body weight unless noted otherwise. Doses administered were expressed as the free base of the drug. Choline chloride (Sigma-Aldrich, Inc, St. Louis, MO) was dissolved in the drinking water and given orally. DSS (molecular weight, 36–50000 kDa; ICN Biomedicals, Inc, Aurora, OH), 2.5% of DSS was dissolved in the drinking water and given orally. The doses of PHA-543613, PNU-120596, and choline chloride were chosen based on previous in vivo studies.9,23,26,27
Study 1: DSS-Induced Colitis Model in α7 KO and WT Male Mice
DSS 2.5% (wt/vol) was added to the drinking water of WT and α7 KO nAChR male mice for 7 days. On day 8, DSS was replaced with normal drinking water. Controls were all age- and time-matched and consisted of mice that received regular tap drinking water only for the corresponding number of days. The experimenter was blinded to the mouse genotype.
Study 2: Effect of PHA-543613, PNU-120596, and Choline Chronic Treatment in DSS-Induced Colitis Model
Male C57BL/6J mice were pretreated with PHA-543613 (2, 8, and 20 mg/kg s.c. twice daily), PNU-120596 (1, 3, and 6 mg/kg i.p. once daily), or their respective vehicles. A separate group of mice was subjected to oral administration of choline (10, 40, and 80 µg/mL) in the drinking water 3 days before adding the DSS, and for 7 days along with the induction of colitis with DSS 2.5%. On day 8, DSS was replaced with normal drinking water.
Study 3: DSS-Induced Colitis Model in α7 KO and WT Female Mice
DSS 2.5% (wt/vol) was added to the drinking water of WT and α7 KO nAChR female mice for 7 days. On day 8, DSS was replaced with normal drinking water. Controls were all age- and time-matched and consisted of mice that received regular tap drinking water only for the corresponding number of days. The experimenter was blinded to the mouse genotype.
Study 4: Effect of Chronic Choline Treatment in Female DSS-Induced Colitis Model
Female C57BL/6J mice were pretreated either with oral administration of choline (10, 40, 80, and 120 µg/mL) or vehicle in the drinking water 3 days before addition of DSS, and for 7 days along with the induction of colitis with DSS 2.5%. On day 8, DSS was replaced with normal drinking water. Controls were all age- and time-matched and consisted of mice that received regular tap drinking water only for the corresponding number of days.
Assessment of the Severity of Colitis: Disease Activity Index
Disease activity index (DAI) was based on the combined score of four clinical parameters as described in our previous study,3 including (1) body weight loss, (2) stool consistency, (3) rectal irritation, and (4) blood in the stool. Scores were defined as follows: for body weight change as a percentage: 0, no loss; 1, 5%–10%; 2, 10%–15%; 3, 15%–20%; and 4, 20% weight loss. In our study, there were no differences observed between the WT and KO water-treated animals; therefore, we combined these two groups in the body weight change and in the colon length. For assessment of irritation around the anal area: 0, normal; 1, mild irritation; 2, moderate irritation; and 3, severe irritation; for stool: 0, normal; 1, mild loose stool; 2, moderate loose stool; and 3, diarrhea; and for bleeding: 0, no blood; 1, presence of blood (Hemoccult II positive; Beckman Coulter, Fullerton, CA); and 2, gross blood. DAI symptoms were recorded in a blinded fashion to the animal treatments and genotypes and were performed at the same time every day and scored from days 0 to 8. Total disease activity score ranged from 0 to 12. On day 8, after replacing the DSS with water, mice were sacrificed, the abdominal cavity opened, and the entire colon immediately removed and its length (cm) measured. To assess for change in disease activity over time, results were expressed also as mean ± SEM of area under the curve from days 1 to 8.
Assessment of Colonic Histology
Seven days after the beginning of the DSS treatment, the mice of each group were sacrificed and the entire colons were removed and kept for histology assessment and tumor necrosis factor-alpha (TNF-α) levels measurement. Formalin-fixed colon segments were paraffin-embedded and 3-μm sections were stained with hematoxylin-eosin. Colonic damage was scored in a blinded fashion based on a published scoring system that considers architectural derangements, epithelium changes, goblet cell depletion, ulceration, and degree of inflammatory cell infiltrate.28 The histological scoring system was used to evaluate the degree of colitis. The total histological score ranged from 0 to 12, which represented the sum of scores from 0 to 3 (0 = none, 1 = 0%–5%, 2 = 5%–10%, 3 = >10%) for loss of epithelium, (0 = none, 1 = 0%–10%, 2 = 10%–20%, 3 = >20%) for crypt damage, and (0 = none, 1 = mild, 2 = moderate, and 3 = severe) for each of depletion of goblet cells and infiltration of inflammatory cells. Each section was scored for each feature as the product of the grade for that feature and the percentage involvement in the loss of epithelium and crypt damage features (in a range from 0 to 3 for each feature). The number of inflammatory cells infiltrated in 10 randomly selected power fields (×40) was counted and the number per 10 fields was calculated. Score analyses were done in a blind manner. The histological colitis score of individual mice was represented as the sum of the different histological subscores. Light microscope images were acquired with an Axioscope AX10 microscope and Axioviosion 4.6 software (Carl Zeiss, Inc).
Colonic TNF-α Levels
The colonic tissue was homogenized in 1 mL of Tris–HCl buffer containing protease inhibitors (Sigma-Aldrich, Inc). Samples then were centrifuged (1811g, Relative Centrifugal Force) for 5 minutes at a temperature of 4°C, and the supernatant was frozen at −80°C until assay. The protein concentration was determined by the Bradford assay.29 TNF-α levels were determined using an enzyme-linked immunosorbent assay commercial kit (Quantikine M murine; R&D Systems, Minneapolis, MN).
Statistical Analysis
Statistical analysis of all studies was performed with ANOVA. Two-way repeated-measures ANOVAs were used at the different timepoints, three-way ANOVA for genotype × treatment × timepoint when appropriate. Significant overall ANOVAs were followed by Tukey’s test post hoc test when appropriate. All differences were considered significant at *p < .05. The GraphPad Prism program was used for data manipulations, graphical representations, and statistical analysis (GraphPad Software, Inc, San Diego, CA).
Results
DSS-Induced Colitis in α7 KO Male Mice
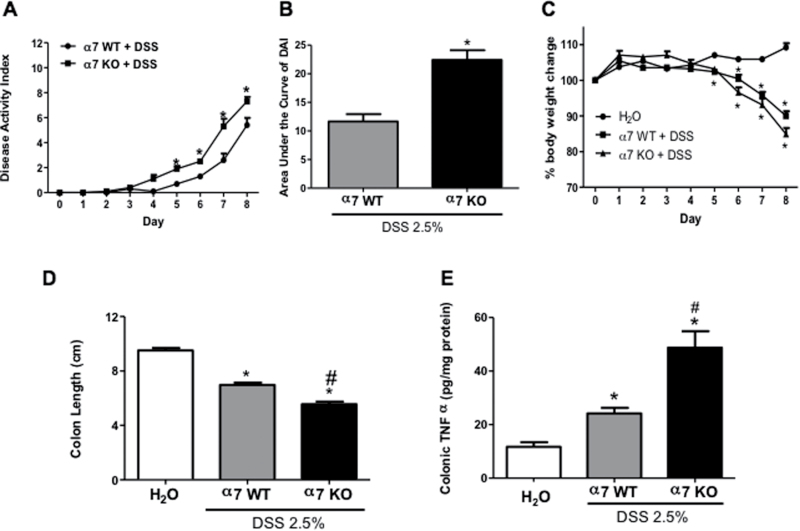
In this experiment, we assessed the development of clinical parameters during DSS treatment in mice lacking α7 nicotinic receptors (ie, KO) and their littermates’ WT. α7 KO male mice treated with 2.5% DSS displayed a significant increase in inflammation based on DAI value starting from day 5 until day 8 of the experiment (t(8) = 2.85, p = .0213) (Figure 1A) and in the total value of the area under the curve of DAI (t(9) = 5.788, p = .003) (Figure 1B) compared with the WT DSS-treated mice. Both α7 KO and WT DSS-treated male mice displayed a significant loss of body weight, especially α7 KO as compared with the control water-treated mice (Figure 1C). The decrease in the colon length was significantly different between α7 KO and WT DSS-treated male mice, even more significant when the α7 KO group was compared against a water-treated control group (F(2, 19) = 117.6, p < .0001) (Figure 1D). To assess for the extent of inflammation in the colon, we evaluated the histological damage score and TNF-α colonic levels. α7 KO mice treated with DSS showed a significant increase in colonic total histological damage score versus the WT DSS and the water-treated control groups (F(2, 47) = 39.24, p < .001) (Supplementary Figure 2A). The histological appearance of the colon after 7 days of DSS treatment in the WT mice was characterized by multifocal changes in the crypts and some areas showed focal lesions, depletion of goblet cells and inflammatory cell infiltrates that included neutrophils and lymphocytes; however, these changes with epithelial destruction (shortening of the crypts, inflammatory cell infiltrates, extensive ulceration) in the mucosa and submucosal tissues were more severe in colons in the α7 KO DSS-treated mice (Supplementary Figure 3A). α7 KO and WT water-treated mice did not show any colonic histological damage (data not shown). Similar to the clinical parameters and histological damage, the α7 KO DSS-mice showed significant elevated colonic TNF-α levels, approximately one-fold increase compared to WT DSS-treated mice (F(2, 39) = 32.64, p < .001) (Figure 1E).
Figure 1.
Colitis parameters are aggravated after dextran sodium sulfate (DSS) exposure in α7 knockout (KO) and wild-type (WT) male mice. (A) Time course of disease activity index (DAI) of α7 WT and KO male mice treated with 2.5% DSS (2.5% w/v) in the drinking water. The DAI values were computed as described in Materials and Methods section. (B) Area under the curve of disease activity. (C) Percentage of body weight change during DSS treatment period. (D) Colon length (cm) after DSS treatment of α7 WT and KO male mice. (E) Colonic tumor necrosis factor-alpha (TNF-α) levels (pg/mg protein) were measured by enzyme-linked immunosorbent assay (ELISA). All clinical signs were assessed on a daily basis for each mouse and were averaged per day for each group. Results are expressed as mean ± SE, n = 6–8, *p < .05. # indicates the significant of KO from the WT.
Effect of α7 nAChR Agonists and PAM in DSS Colitis Model in Male Mice
Effect of PHA-543613 on DSS-Induced Colitis Model
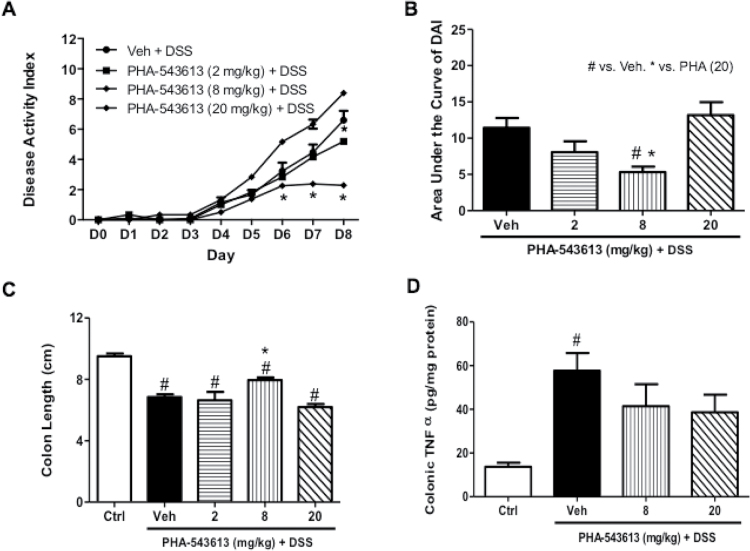
We first tested the impact of the selective α7 full agonist PHA-543613 on DSS-induced colitis in the mouse after systemic administration of the drug at doses reported to possess anti-inflammatory properties.30 At a low dose of 2 mg/kg PHA-543613 did not affect clinical parameters of colitis severity significantly (Figure 2A) or colon length (Figure 2C). However, administration of a higher dose of 8 mg/kg resulted in a significant decrease in DAI scores on day 6, 7, and 8 compared with the DSS-treated group (F(2, 16) = 4.051, p = .0377) (Figure 2A), as well as in the area under the curve value of DAI (F(3, 30) = 5.403, p = 0.0043) (Figure 2B). In addition, at this dose, PHA-543613 was able to prevent the shortening of the colon length of DSS-treated group (F(3, 19) = 7.495, p = .0017) (Figure 2C). As the dose of PHA-543613 increased to 20 mg/kg, the beneficial effect of the drug on disease activity and the colon length induced by DSS was lost. PHA-543613 at doses of 8 and 20 mg/kg had no effect on colonic TNF-α levels (Figure 2D). Similar to the DAI scores, PHA-543613 reduced the total histological damage score at the dose of 8 mg/kg only (Supplementary Figure 2B). Supplementary Figure 3B shows the histological appearance of colon sections with hematoxylin-eosin stain after vehicle and PHA-543613 treatment in DSS-treated mice. The DSS-treated animals exhibited ulceration of the mucosa with destruction where the inflammation was mainly in the mucosa, more shortening of the crypts than the PHA-54361-treated mice.
Figure 2.
Effect of α7 nicotinic acetylcholine receptors (nAChRs) agonist PHA-543613 on disease activity and inflammatory markers in C57Bl/6 male mice with dextran sodium sulfate (DSS) colitis. Effect of chronic daily s.c. treatment of PHA-543613 (2, 8, and 20 mg/kg s.c. twice daily) on (A) time course of disease activity index (DAI). (B) Area under the curve of disease activity. (C) Mean colon length (cm). (D) Colonic tumor necrosis factor-alpha (TNF-α) levels (pg/mg protein) in the homogenized colonic samples. Results are expressed as mean ± SE, n = 6–8, *p < .05 compared to Veh. #p < .05 compared to Ctrl (H2O). Ctrl = water-treated animals; s.c. = subcutaneous; Veh = vehicle DSS-treated mice.
Effect of Choline Treatment on DSS-Induced Colitis Model in Male Mice
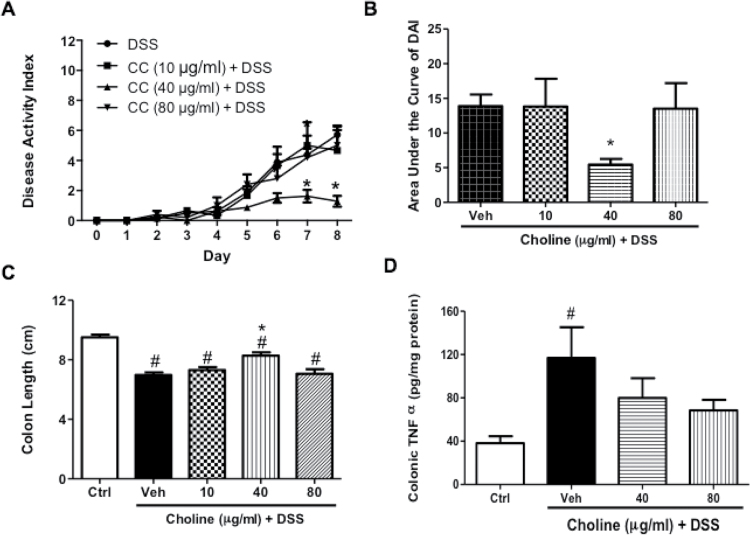
Similar to PHA-543613, the choline dose–response curve indicative of reduction of the severity of DAI in DSS-treated mice was narrow. While a low dose of oral choline treatment (10 μg/mL) has no observable effect on the severity of clinical parameters (Figure 3A) and shortening of the colon length (Figure 3C), a higher dose of 40 μg/mL resulted in significantly decreased DAI values on day 7 and 8 compared with the DSS-treated group (F(3, 24) = 4.818, p = .0092) (Figure 3A), and in the area under the curve of DAI as well (F(3, 23) = 3.15, p = 0.044) (Figure 3B). In addition, administration of that dose resulted in the prevention of the shortening of the colon length of DSS-treated group (F(3, 20) = 7.812, p = .0012). However, that effect was lost upon administration of the high dose of choline (80 μg/mL) (Figure 3C). Administration of both doses (40 and 80 μg/mL) of choline had no significant effect on the increase in colonic TNF-α levels in DSS-treated mice (Figure 3D).
Figure 3.
Chronic oral α7 nicotinic acetylcholine receptors (nAChRs) agonist choline treatment suppresses the severity of dextran sodium sulfate (DSS)-induced colitis in C57Bl/6 male mice. Effect of chronic oral choline (10, 40, 80, 120 μg/mL) treatment on (A) the time course of disease activity index (DAI), (B) area under the curve of DAI, (C) mean of colon length (cm). (D) Colonic tumor necrosis factor-alpha (TNF-α) levels (pg/mg protein) in the homogenized colonic tissue samples. Results are expressed as mean ± SE, n = 6–8, *p < .05 vs. DSS group and #p < .05 vs. Ctrl group. CC = choline chloride; Ctrl = water-treated animals; Veh = vehicle DSS-treated mice.
Choline treatment at 40 and 80 μg/mL resulted in a significant decrease in the total histological damage score of the colon compared with the vehicle DSS-treated group (F(2, 97) = 11.43, p < .001) (Supplementary Figure 2C). Supplementary Figure 3C shows the histological appearance of colon sections stained with hematoxylin-eosin after oral choline treatment at 40 and 80 µg/mL in DSS-treated mice. While the DSS-treated animals showed extensive ulceration of the mucosa with destruction, and inflammatory cell infiltrates; the choline-treated groups showed normal epithelial architectures at both doses of the drug (Supplementary Figure 3C).
Effect of α7 nAChR PAM in DSS Colitis Model
A similar profile of activity in the DSS-treated male mice was observed with the α7 PAM, PNU-120596. At a low dose of 1 mg/kg, it had neither an effect on DAI scores, or on the colonic inflammation on the basis of measurement of colonic TNF-α levels. Similar findings were obtained at a higher dose of 6 mg/kg of PNU-120596 (Supplementary Figure 1). However, at a dose of 3 mg/kg, PNU-120596 administration resulted in a significant decrease of DAI values on day 5, 6, 7, and 8 compared with the DSS-treated group (F(3, 24) = 8.01, p = .0007) (Supplementary Figure 1A). Additionally, when administered at this dose, a significant reduction in the area under the curve of DAI score was observed as compared with the vehicle DSS-treated mice (F(3, 18) = 3.341, p = .0425) (Supplementary Figure 1B). None of the PNU-120596 doses were able to prevent the shortening of the colon length of vehicle DSS-treated group (Supplementary Figure 1C) or prevent the increase in colonic TNF-α levels (Supplementary Figure 1D).
PNU-120596 treatment at 3 mg/kg resulted in a significant decrease in the total histological damage score (F(2, 67) = 3.88, p = .0254) (Supplementary Figure 2D). Supplementary Figure 3D shows a histological appearance of the colon sections stained with hematoxylin-eosin after PNU-120596 treatment in mice with DSS. The DSS-treated animals showed extensive ulceration of the mucosa with destruction associated in areas where inflammation was mainly in the mucosa and submucosa, with more inflammatory cell infiltrates. The administration of PNU-120596 at a dose of 3 mg/kg was able to maintain the epithelial architecture of the crypts and the number of goblet cells. Less inflammatory cell infiltrate was observed in the DSS mice when compared with the DSS-treated mice not subjected to treatment.
DSS and α7 nAChRs Studies in Female Mice
DSS-Induced Colitis Model in α7 KO and WT Female Mice
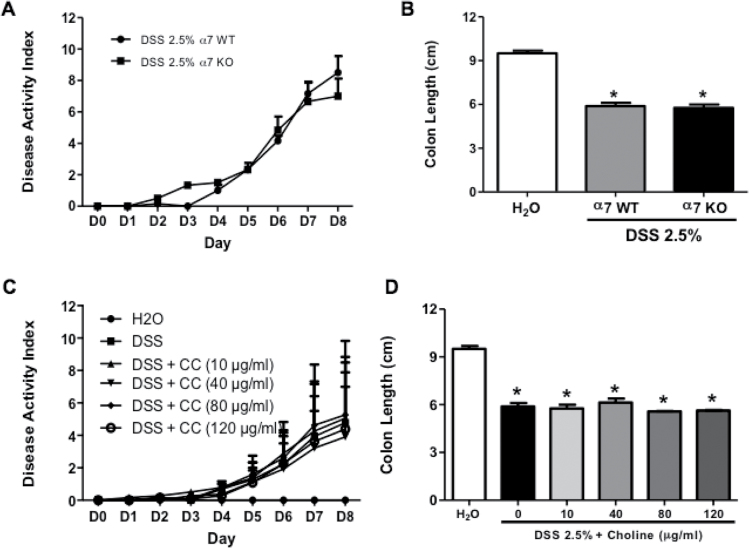
Contrary to male mice, female α7 KO DSS-treated mice did not show any significant difference compared to α7 WT in terms of the clinical signs of colitis disease activity (t(5) = 0.4416, p > .05) (Figure 4A). In addition, there was no significant difference between α7 KO and WT in the decrease in the colon length DSS-treated mice (t(5) = 0.6494, p > .05) (Figure 4B).
Figure 4.
Colitis severity after dextran sodium sulfate (DSS) exposure in α7 knockout (KO) and wild-type (WT) female mice and the effect of chronic oral choline treatment on the severity of DSS-induced colitis in C57Bl/6 female mice. (A) Time course of disease activity index (DAI) of α7 WT and KO female mice treated with 2.5% DSS in the drinking water, (B) mean of colon length (cm) of control and DSS-treated α7 WT and KO female mice. The effect of chronic oral choline (10, 40, 80, 120 μg/mL) treatment on (C) the time course of DAI, and (D) mean of colon length (cm) in female mice treated with 2.5% DSS. Results are expressed as mean ± SE, n = 6–8, *p < .05 vs. DSS group. CC = choline chloride.
Effect of Choline Treatment in Female DSS-Treated Mice
In contrast to male mice, oral choline treatment at various doses (10, 40, 80, and 120 μg/mL) failed to reduce significantly the severity of DAI in female DSS-treated colitis mice (F(4, 40) = 0.1077, p = .9792) (Figure 4C), and the shortening of the colon length (F(4, 22) = 1.327, p = .2911) (Figure 4D). As shown in Figure 4D, while oral administration of choline chloride at 40 μg/mL, an active dose in male mice, did not display an anti-colitis effect as measured by the DAI score (Figure 4C) and colon length in the DSS-treated mice (Figure 4D).
Discussion
In the present study, we evaluated the role of α7 nAChRs in the mouse DSS colitis model using genetic and pharmacological approaches. Our results show that α7 KO male, but not female mice, developed more severe colitis and had higher clinical colitis scores and colonic inflammation markers induced by 2.5% DSS. In addition, treatment with the selective α7 full agonists (PHA-543613 and choline) and PAM of α7 nAChRs (PNU-120596) resulted in a reduction of the severity of disease in C57BL/6J male mice. However, choline was ineffective in reducing clinical outcomes for DSS-induced colitis in C57BL/6J female mice.
Numerous studies have shown that nicotine, a nonselective agonist for nAChRs, reduces the clinical signs of colitis in experimental rodent models5,6,31 and also in human UC.32,33 Furthermore, the cholinergic anti-inflammatory pathway through α7 nAChRs has been regarded as a target for colitis6,20 and acetylcholine (ACh), the major neurotransmitter vagus nerve, stimulates α7 nAChRs to inhibit the cytokine release in immune cells.7 We therefore hypothesized that α7 nAChRs deficiency would aggravate the inflammatory signs of colitis in the mouse. Indeed, colitis symptoms, such as DAI and colon length, histological damage, and TNF-α colonic levels were exacerbated in the absence of α7 nAChRs in the KO mouse. Of interest is the increase in TNF-α colonic levels in these mice. Indeed, TNF-α is an early pro-inflammatory mediator that plays an important role in immuno-inflammatory responses and in the pathogenesis of UC.34 Our results are consistent with recent data obtained through the use of various animal models of inflammation that point toward the α7 nAChR subtypes as important players in cholinergic modulation of inflammation.7,8,19 Our findings are also in agreement with those of Ghia et al.9 study that showed that vagotomized mice treated with 5% DSS for 5 days induced an exaggeration of colitis severity including histological damage score, pro-inflammatory cytokine, IL-1β colonic levels and MPO activity when compared with sham-operated mice.
Our results with α7 KO mice suggested the presence of an α7-dependent anti-colitis mode of action in male mice. We therefore investigated if activation of α7 nAChRs would reduce DSS-induced colon inflammation in the mouse. Using pharmacological approaches, we tested two α7 nAChR agonists and a PAM (PHA-543613, choline, and PNU-120596, respectively) in the mouse colitis model. All were effective in reversing the clinical parameters of colitis and shortening of the colon induced by DSS in C57BL/6J male mice. However, the anti-inflammatory effects of all of these α7 nAChR ligands occurred at narrow dose ranges characterized with U-shape dose–response relationships. This profile of low, subthreshold doses being ineffective but high doses resulting in no effect might be related to differences in the regulation of α7 nAChRs (activation/desensitization and/or recruitment of various receptor conformations) in the colitis model by these ligands. A similar profile was reported recently by our group with PHA-543613 in the mouse formalin test.30 It is possible also that receptor efficacy plays an important role in the anti-inflammatory effectiveness of α7 nAChRs agonists. Interestingly, GTS-21, a partial α7 nAChR agonist, significantly suppressed the severity of colitis induced by 3% DSS in male mice.31 However, GTS-21 was only tested at the dose of 10 mg/kg. This narrow U-shaped dose–effect curve was not seen with α7 nAChRs agonists and PAMs in animal models of cognition, acute inflammation and pain.35–37 Thus, this phenomenon may be unique to experimental colitis in mice.
The α7 full agonist choline also is a precursor of ACh,24,38 which interacts with α7 nAChRs on immune cells. Hence, choline may induce its anti-inflammatory effects via enhancing ACh through vagal modulation of colitis. The amelioration of colitis by PNU-120596, a type II α7 nAChR PAM, suggests the presence of a pro-“anti-colitis” endogenous tone mediated by α7 nAChRs. Generally, PAMs are compounds that facilitate endogenous neurotransmission and/or enhance the efficacy and potency of agonists without directly stimulating the agonist binding sites. PNU-120596 may be acting in the DSS model through the enhancement of subthreshold concentrations of endogenous α7 agonists, choline and/or ACh.39 Studies also have suggested that the beneficial effects of nicotine on DSS-induced colitis are related to α7 nAChRs. For example, pretreatment with nicotine attenuated the deleterious effect of vagotomy in male mice with DSS-induced colitis.9 More recently, it has been reported that the expression of α7 nAChR mRNA in colonic CD4 T cells of DSS-treated mice is increased compared to normal mice.31 In addition, the same group showed that nicotine’s inhibition of the acute colitis and colitis-associated tumorigenesis in DSS-treated mice is mediated by the activation of α7 nAChRs on immune cells in the colon since the α7 antagonist methyllycaconitine blocked these effects.31 Moreover, GTS-21, a partial α7 nAChR agonist, ameliorated the severity of colitis induced by DSS in male mice.31 While α7 nAChRs ligands in our studies were protective in DSS-induced colitis in mice based on clinical parameters and histologic features, they were not able to decrease the changes in colonic TNF-α levels, one of key regulators of inflammatory responses. While this lack of effect on this cytokine is surprising, we did not evaluate the effects of α7 nAChRs ligands on other cytokines such as IL-6 for which production has been reported to be is increased in the DSS-induced colitis model.40,41
Our results, as well as those of others,9,31 are not in line with those reported recently by Snoek et al.22 In their study, two selective α7 nAChR agonists, AR-R17779 and GSK1345038A, while reducing cytokine production in vitro, were shown to worsen the effects of DSS-induced colitis at low doses. However, at highest doses of these α7 nAChR agonists they elicited ameliorated clinical parameters without affecting colonic inflammation.22 There are potential explanations for the differential results reported in our study versus that of Snoek et al.22 The route of administration does not seem to play a major role since the protective effects in the DSS model in our study and others were seen with α7 nAChR agonists after both systemic (via s.c. and i.p.) and oral (via drinking water) routes of administration. The efficacy at α7 nAChRs of the agonists used in the treatment could be a possible differential factor. While no information on the pharmacological profile at nAChRs was available for GSK1345038A, AR-R17779 is a selective with high efficacy (approximately 70% efficacy) α7 nAChR agonist.42 Both of α7 nAChR agonists used in our study are full α7 nAChR agonists. The only apparent difference between our study and that of Snoek et al.22 was the use of female mice. In view of this distinction, we evaluated the role of α7 nAChRs in mouse DSS colitis model using α7 KO female mice. In contrast to male mice, female α7 KO mice showed similar disease activity to WT female mice. In addition, choline treatment failed to reduce colitis symptoms in female mice at any of the doses tested. It is possible that plasma and local concentrations of choline could account for the differential anti-colitis response of male and female mice to choline. Furthermore, sex differences in α7 nAChRs pharmacology may be mediated by differences in regulation of α7 nAChRs function in the enteric nervous system. Indeed, these sex differences may reflect a modulatory role of female sex hormones on α7 nAChRs-mediated pharmacology. For example, progesterone and 17β-estradiol have been reported to be functional blockers of several nicotinic receptors including α7 nAChRs.10,30
In conclusion, in the DSS colitis model, we found that α7 nAChR signaling may, at least in part, play a role in protection against development of colitis in mice. α7 nAChR agonists and PAM ameliorated the clinical features of the DSS colitis disease in a dose- and sex-dependent fashion. Our collective results suggest that targeting α7 nAChRs could represent a viable therapeutic approach for intestinal inflammation diseases such as UC.
Supplementary Material
Supplementary Figures 1–3 can be found online at http://www.ntr.oxfordjournals.org
Funding
This work was supported by National Institutes of Health (grants DA-019377 to MID and DK046367 to HIA).
Declaration of Interests
None declared.
Supplementary Material
Acknowledgments
Participated in research design: AlSharari, Akbarali, Raborn, Cabral, McGee, and Damaj. Conducted experiments: AlSharari and Lichtman. Contributed new reagents or analytic tools: AlSharari and Carroll. Performed data analysis: AlSharari, Bagdas, Akbarali, Raborn, Cabral, McGee, and Damaj. Wrote or contributed to the writing of the manuscript: AlSharari, Bagdas, Akbarali, Lichtman, Cabral, McGee, and Damaj.
References
- 1. Hanauer SB. Inflammatory bowel disease. N Engl J Med. 1996;334(13):841–848. doi:10.1056/NEJM199603283341307 [DOI] [PubMed] [Google Scholar]
- 2. Boyko EJ, Perera DR, Koepsell TD, Keane EM, Inui TS. Effects of cigarette smoking on the clinical course of ulcerative colitis. Scand J Gastroenterol. 1988;23(9):1147–1152. [DOI] [PubMed] [Google Scholar]
- 3. Höie O, Wolters F, Riis L, et al. ; European Collaborative Study Group of Inflammatory Bowel Disease (EC-IBD) Ulcerative colitis: patient characteristics may predict 10-yr disease recurrence in a European-wide population-based cohort. Am J Gastroenterol. 2007;102(8):1692–1701. doi:10.1111/j.1572-0241.2007.01265.x [DOI] [PubMed] [Google Scholar]
- 4. Sandborn WJ, Tremaine WJ, Offord KP, et al. Transdermal nicotine for mildly to moderately active ulcerative colitis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1997;126(5):364–371. doi:10.7326/0003-4819-126-5-199703010-00004 [DOI] [PubMed] [Google Scholar]
- 5. AlSharari SD, Akbarali HI, Abdullah RA, et al. Novel insights on the effect of nicotine in a murine colitis model. J Pharmacol Exp Ther. 2013;344(1):207–217. doi:10.1124/jpet.112.198796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ghia JE, Blennerhassett P, Kumar-Ondiveeran H, Verdu EF, Collins SM. The vagus nerve: a tonic inhibitory influence associated with inflammatory bowel disease in a murine model. Gastroenterology. 2006;131(4):1122–1130. doi:10.1053/j.gastro.2006.08.016 [DOI] [PubMed] [Google Scholar]
- 7. Wang H, Yu M, Ochani M, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421(6921):384–388. doi:10.1038/nature01339 [DOI] [PubMed] [Google Scholar]
- 8. de Jonge WJ, van der Zanden EP, The FO, et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol. 2005;6(8):844–851. doi:10.1038/ni1229 [DOI] [PubMed] [Google Scholar]
- 9. Ghia JE, Blennerhassett P, Deng Y, Verdu EF, Khan WI, Collins SM. Reactivation of inflammatory bowel disease in a mouse model of depression. Gastroenterology. 2009;136(7):2280–2288.e1. doi:10.1053/j.gastro.2009.02.069 [DOI] [PubMed] [Google Scholar]
- 10. Shen JX, Yakel JL. Nicotinic acetylcholine receptor-mediated calcium signaling in the nervous system. Acta Pharmacol Sin. 2009;30(6):673–680. doi:10.1038/aps.2009.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Keyser KT, Britto LR, Schoepfer R, et al. Three subtypes of alpha-bungarotoxin-sensitive nicotinic acetylcholine receptors are expressed in chick retina. J Neurosci. 1993;13(2):442–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kirchgessner AL, Liu MT. Immunohistochemical localization of nicotinic acetylcholine receptors in the guinea pig bowel and pancreas. J Comp Neurol. 1998;390(4):497–514. doi:10.1002/(SICI)1096-9861(19980126)390:4<497::AID-CNE4>3.0.CO;2-W [PubMed] [Google Scholar]
- 13. Rosas-Ballina M, Goldstein RS, Gallowitsch-Puerta M, et al. The selective alpha7 agonist GTS-21 attenuates cytokine production in human whole blood and human monocytes activated by ligands for TLR2, TLR3, TLR4, TLR9, and RAGE. Mol Med. 2009;15(7–8):195–202. doi:10.2119/molmed.2009.00039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sato KZ, Fujii T, Watanabe Y, et al. Diversity of mRNA expression for muscarinic acetylcholine receptor subtypes and neuronal nicotinic acetylcholine receptor subunits in human mononuclear leukocytes and leukemic cell lines. Neurosci Lett. 1999;266(1):17–20. doi:10.1016/S0304-3940(99)00259-1 [DOI] [PubMed] [Google Scholar]
- 15. Matsunaga K, Klein TW, Friedman H, Yamamoto Y. Involvement of nicotinic acetylcholine receptors in suppression of antimicrobial activity and cytokine responses of alveolar macrophages to Legionella pneumophila infection by nicotine. J Immunol. 2001;167(11):6518–6524. doi:10.4049/jimmunol.167.11.6518 [DOI] [PubMed] [Google Scholar]
- 16. Obaid AL, Nelson ME, Lindstrom J, Salzberg BM. Optical studies of nicotinic acetylcholine receptor subtypes in the guinea-pig enteric nervous system. J Exp Biol. 2005;208(pt 15):2981–3001. doi:10.1242/jeb.01732 [DOI] [PubMed] [Google Scholar]
- 17. Ji H, Rabbi MF, Labis B, Pavlov VA, Tracey KJ, Ghia JE. Central cholinergic activation of a vagus nerve-to-spleen circuit alleviates experimental colitis. Mucosal Immunol. 2014;7(2):335–347. doi:10.1038/mi.2013.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tracey KJ, Czura CJ, Ivanova S. Mind over immunity. FASEB J. 2001;15(9):1575–1576. doi:10.1096/fj.01-0148hyp [DOI] [PubMed] [Google Scholar]
- 19. Ulloa L. The vagus nerve and the nicotinic anti-inflammatory pathway. Nat Rev Drug Discov. 2005;4(8):673–684. doi:10.1038/nrd1797 [DOI] [PubMed] [Google Scholar]
- 20. Ghia JE, Blennerhassett P, El-Sharkawy RT, Collins SM. The protective effect of the vagus nerve in a murine model of chronic relapsing colitis. Am J Physiol Gastrointest Liver Physiol. 2007;293(4):G711–G718. doi:10.1152/ajpgi.00240.2007 [DOI] [PubMed] [Google Scholar]
- 21. Abdrakhmanova GR, AlSharari S, Kang M, Damaj MI, Akbarali HI. {alpha}7-nAChR-mediated suppression of hyperexcitability of colonic dorsal root ganglia neurons in experimental colitis. Am J Physiol Gastrointest Liver Physiol. 2010;299(3):G761–G768. doi:10.1152/ajpgi.00175.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Snoek SA, Verstege MI, van der Zanden EP, et al. Selective alpha7 nicotinic acetylcholine receptor agonists worsen disease in experimental colitis. Br J Pharmacol. 2010;160(2):322–333. doi:10.1111/j.1476-5381.2010.00699.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wishka DG, Walker DP, Yates KM, et al. Discovery of N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl]furo[2,3-c]pyridine-5-carboxamide, an agonist of the alpha7 nicotinic acetylcholine receptor, for the potential treatment of cognitive deficits in schizophrenia: synthesis and structure–activity relationship. J Med Chem. 2006;49(14):4425–4436. doi:10.1021/jm0602413 [DOI] [PubMed] [Google Scholar]
- 24. Alkondon M, Pereira EF, Cortes WS, Maelicke A, Albuquerque EX. Choline is a selective agonist of alpha7 nicotinic acetylcholine receptors in the rat brain neurons. Eur J Neurosci. 1997;9(12):2734–2742. doi:10.1111/j.1460-9568.1997.tb01702.x [DOI] [PubMed] [Google Scholar]
- 25. Fayuk D, Yakel JL. Regulation of nicotinic acetylcholine receptor channel function by acetylcholinesterase inhibitors in rat hippocampal CA1 interneurons. Mol Pharmacol. 2004;66(3):658–666. doi:10.1124/mol.104.000042 [DOI] [PubMed] [Google Scholar]
- 26. Krafft PR, Altay O, Rolland WB, et al. α7 nicotinic acetylcholine receptor agonism confers neuroprotection through GSK-3β inhibition in a mouse model of intracerebral hemorrhage. Stroke. 2012;43(3):844–850. doi:10.1161/STROKEAHA.111.639989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hurst RS, Hajós M, Raggenbass M, et al. A novel positive allosteric modulator of the alpha7 neuronal nicotinic acetylcholine receptor: in vitro and in vivo characterization. J Neurosci. 2005;25(17):4396–4405. doi:10.1523/JNEUROSCI.5269-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Iba Y, Sugimoto Y, Kamei C, Masukawa T. Possible role of mucosal mast cells in the recovery process of colitis induced by dextran sulfate sodium in rats. Int Immunopharmacol. 2003;3(4):485–491. doi:10.1016/S1567-5769(02)00299-0 [DOI] [PubMed] [Google Scholar]
- 29. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. [DOI] [PubMed] [Google Scholar]
- 30. Freitas K, Carroll FI, Damaj MI. The antinociceptive effects of nicotinic receptors α7-positive allosteric modulators in murine acute and tonic pain models. J Pharmacol Exp Ther. 2013;344(1):264–275. doi:10.1124/jpet.112.197871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hayashi S, Hamada T, Zaidi SF, et al. Nicotine suppresses acute colitis and colonic tumorigenesis associated with chronic colitis in mice. Am J Physiol Gastrointest Liver Physiol. 2014;307(10):G968–G978. doi:10.1152/ajpgi.00346.2013 [DOI] [PubMed] [Google Scholar]
- 32. McGrath J, McDonald JW, Macdonald JK. Transdermal nicotine for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2004;(4):CD004722. doi:10.1002/14651858.CD004722.pub2 [DOI] [PubMed] [Google Scholar]
- 33. Sandborn WJ. Nicotine therapy for ulcerative colitis: a review of rationale, mechanisms, pharmacology, and clinical results. Am J Gastroenterol. 1999;94(5):1161–1171. doi:10.1111/j.1572-0241.1999.01059.x [DOI] [PubMed] [Google Scholar]
- 34. Anand AC, Adya CM. Cytokines and inflammatory bowel disease. Trop Gastroenterol. 1999;20(3):97–106. [PubMed] [Google Scholar]
- 35. Rowley TJ, McKinstry A, Greenidge E, Smith W, Flood P. Antinociceptive and anti-inflammatory effects of choline in a mouse model of postoperative pain. Br J Anaesth. 2010;105(2):201–207. doi:10.1093/bja/aeq113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Feuerbach D, Lingenhoehl K, Olpe HR, et al. The selective nicotinic acetylcholine receptor alpha7 agonist JN403 is active in animal models of cognition, sensory gating, epilepsy and pain. Neuropharmacology. 2009;56(1):254–263. doi:10.1016/j.neuropharm.2008.08.025 [DOI] [PubMed] [Google Scholar]
- 37. Gurun MS, Parker R, Eisenach JC, Vincler M. The effect of peripherally administered CDP-choline in an acute inflammatory pain model: the role of alpha7 nicotinic acetylcholine receptor. Anesth Analg. 2009;108(5):1680–1687. doi:10.1213/ane.0b013e31819dcd08 [DOI] [PubMed] [Google Scholar]
- 38. Papke RL, Bencherif M, Lippiello P. An evaluation of neuronal nicotinic acetylcholine receptor activation by quaternary nitrogen compounds indicates that choline is selective for the alpha 7 subtype. Neurosci Lett. 1996;213(3):201–204. doi:10.1016/0304-3940(96)12889-5 [DOI] [PubMed] [Google Scholar]
- 39. Sarter M, Parikh V. Choline transporters, cholinergic transmission and cognition. Nat Rev Neurosci. 2005;6(1):48–56. doi:10.1038/nrn1588 [DOI] [PubMed] [Google Scholar]
- 40. Egger B, Bajaj-Elliott M, MacDonald TT, Inglin R, Eysselein VE, Büchler MW. Characterisation of acute murine dextran sodium sulphate colitis: cytokine profile and dose dependency. Digestion. 2000;62(4):240–248. doi:10.1159/000007822 [DOI] [PubMed] [Google Scholar]
- 41. Melgar S, Karlsson A, Michaëlsson E. Acute colitis induced by dextran sulfate sodium progresses to chronicity in C57BL/6 but not in BALB/c mice: correlation between symptoms and inflammation. Am J Physiol Gastrointest Liver Physiol. 2005;288(6):G1328–G1338. doi:10.1152/ajpgi.00467.2004 [DOI] [PubMed] [Google Scholar]
- 42. Papke RL, Porter Papke JK, Rose GM. Activity of alpha7-selective agonists at nicotinic and serotonin 5HT3 receptors expressed in Xenopus oocytes. Bioorg Med Chem Lett. 2004;14(8):1849–1853. doi:10.1016/j.bmcl.2003.09.104 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.