Abstract
The impact of the mitochondria-targeted antioxidant MitoQ was evaluated in the metabolic alterations and the adipose tissue remodeling associated with obesity. Male Wistar rats were fed either a high-fat diet (HFD; 35% fat) or a standard diet (3.5% fat) for 7 wk and treated with MitoQ (200 µM). A proteomic analysis of visceral adipose tissue from patients with obesity and patients without obesity was performed. MitoQ partially prevented the increase in body weight, adiposity, homeostasis model assessment index, and adipose tissue remodeling in HFD rats. It also ameliorated protein level changes of factors involved in insulin signaling observed in adipose tissue of obese rats: reductions in adiponectin and glucose transporter 4 (GLUT 4) and increases in dipeptidylpeptidase 4, suppressor of cytokine signaling 3 (SOCS3), and insulin receptor substrate 1 phosphorylation. MitoQ prevented down-regulation of adiponectin and GLUT 4 and increases in SOCS3 levels in a TNF-α–induced insulin-resistant 3T3-L1 adipocyte model. MitoQ also ameliorated alterations in mitochondrial proteins observed in obese rats: increases in cyclophylin F and carnitine palmitoyl transferase 1A and reductions in mitofusin1, peroxiredoxin 4, and fumarate hydratase. The proteomic analysis of the visceral adipose tissue from patients with obesity show alterations in mitochondrial proteins similar to those observed in obese rats. Therefore, the data show the beneficial effect of MitoQ in the metabolic dysfunction induced by obesity.—Marín-Royo, G., Rodríguez, C., Le Pape, A., Jurado-López, R., Luaces, M., Antequera, A., Martínez-González, J., Souza-Neto, F. V., Nieto, M. L., Martínez-Martínez, E., Cachofeiro, V. The role of mitochondrial oxidative stress in the metabolic alterations in diet-induced obesity in rats.
Keywords: insulin resistance, mitochondrial function, adipose tissue
Obesity is characterized by an increase in adipose tissue as a result of a positive imbalance between food intake and energy expenditure. Under conditions of constant energy surplus, adipocytes become larger in an attempt to increase their lipid storage capacity. Nonetheless, adipocytes reach a saturation point when they are not able to store more lipids and start to express stress signals (1), thereby becoming hypertrophic adipocytes. Dysfunctional adipose tissue has a negative impact on glucose homeostasis and plays a central role in the development of insulin resistance in obesity because adipose tissue serves as a crucial integrator of glucose homeostasis (2, 3).
In the last years, clinical and experimental studies have demonstrated that obesity (4–6) is associated with altered mitochondrial function in white adipose tissue, which can lead to functional consequences. Mitochondria damage is primarily caused by reactive oxygen species (ROS), with the mitochondria being the main source of ROS (7) through the oxidative phosphorylation (oxphos) during the process of ATP synthesis (8). In this context, selective antioxidants targeting mitochondrial ROS production are better than general antioxidants in reducing oxidative damage within mitochondria (9). Some studies have reported the relevance of mitochondrial oxidative stress in the development of some features and alterations of metabolic syndrome in muscle and liver in rats fed a high-fat diet (HFD) (10, 11). However, the role played by mitochondrial oxidative stress in adipose tissue dysfunction in the context of obesity and especially in its metabolic consequences remains unclear. Therefore, the main purpose of this study was to evaluate the mitochondrial protein alterations associated with obesity in the epididymal adipose tissue (EAT) of HFD-fed rats and the impact of the mitochondrial-targeted antioxidant MitoQ on the metabolic and mitochondrial alterations observed in those animals. In addition, we have analyzed the mitochondrial protein profile of visceral adipose tissue from patients with obesity.
MATERIALS AND METHODS
Animal model
Six-week-old male Wistar rats (Envigo, Barcelona, Spain) were fed either an HFD (n = 16; 35% fat, TD.03307; Envigo, Huntingdon, United Kingdom) or a standard control diet (CT; n = 16; 3.5% fat; TD.2014; Envigo) for 7 wk. Half of the animals of each group received the mitochondrial antioxidant MitoQ (200 µM) in the drinking water for the same period. The dose of MitoQ was based on previous data from Rivera-Barahona et al. (12). MitoQ was provided by M. P. Murphy (Medical Research Council Mitochondrial Biology Unit, Cambridge BioMedical Campus, Cambridge, United Kingdom). The water intake was controlled on a daily basis to evaluate whether MitoQ was able to modify water consumption. Body weight was measured once per week. At the end of the experiment, serum and plasma were collected in food-deprived animals, and the different fat pads (epididymal, lumbar, and mesenteric adipose tissue) and brown adipose tissue (BAT) were dissected for further analysis. Adiposity index was calculated as follows: sum of fat pads/[(body weight-fat pad weight) × 100]. The Animal Care and Use Committee of Universidad Complutense de Madrid approved all experimental procedures according to the guidelines for ethical care of experimental animals of the European community.
Subjects
Patients with morbid obesity who were referred to bariatric surgery were consecutively recruited from the Obesity Care Unit of Fuenlabrada University Hospital, Fuenlabrada, Spain. The selection of the patients was performed by a multidisciplinary committee, which includes personnel from endocrinology, general and upper gastroenterology surgery, and internal medicine and cardiology services. Inclusion criteria were age ≥18 yr and universally accepted indications for bariatric surgery: long-term obesity (>4 yr), body mass index ≥40 kg/m2 despite other weight-loss strategies, or body mass index ≥35 kg/m2 in the presence of obesity-related comorbidities (diabetes mellitus, hypoventilation syndrome, obstructive sleep apnea syndrome, and hypertension). The exclusion criterion was unacceptable surgical risk because of concomitant comorbidities. Nonobese volunteers (body mass index, ≤25 kg/m2) were recruited from the staff of the hospital. Visceral adipose tissue was obtained from 10 individuals that had been referred for bariatric surgery and 10 patients that had been referred to nonbariatric surgery. The study protocol was approved by the ethics committee, and all participants signed the informed consent. This study was conducted in compliance with Good Clinical Practice Guidelines and the ethical principles stated in the Declaration of Helsinki.
Cell culture and differentiation
Murine 3T3-L1 preadipocytes were cultured to confluence in DMEM (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% (v/v) calf serum (Biological Industries, Kibbutz Beit-Haemek, Israel). At 2 d postconfluence (designated d 0), cells were induced to differentiate with DMEM containing a standard induction cocktail of 10% (v/v) fetal bovine serum (FBS; Biological Industries), 1 μM dexamethasone, 0.5 mM 3-isobutyl-1-methylxanthine, and 100 nM insulin (all from MilliporeSigma, Burlington, MA, USA). After 48 h, this medium was replaced with DMEM supplemented with 10% FBS and 100 nM of insulin. This medium was changed every 48 h. As a model of insulin resistance, murine TNF-α (50 ng/ml, equivalent to 2.87 nM; MilliporeSigma) was added to the cell culture medium 7 d after the induction of differentiation when more than 95% of the cells had the morphologic and biochemical properties of adipocytes. For the TNF-α treatment (72 h), fully differentiated 3T3-L1 adipocytes were treated every 24 h with the cytokine as previously described by Miana et al. (13), MitoQ (50 nM) was added to TNF-α–treated cells during the last 24 h of incubation.
Preparation of whole-cell and tissue extracts
3T3-L1 preadipocytes were washed with PBS, and cell monolayers were harvested in a nondenaturing buffer containing 150 mM NaCl, 10 mM Tris (pH 7.4), 1 mM EGTA, 1 mM EDTA, 1% Triton X-100, 0.5% Nonidet P-40, 1 mM Na3VO4, 1 µg/ml leupeptin, and 1 mM DTT. Samples were extracted for 30 min on ice and centrifuged at 15,000 rpm at 4°C for 15 min. Total proteins from EAT were obtained by homogenization in lysis buffer and centrifugation for 5 min at 13,000 rpm (4°C). The tissue extract was then separated from the fat and cellular debris and analyzed for protein content. Supernatants were analyzed for protein content using the BCA Kit (Thermo Fisher Scientific).
Western blot analysis
Proteins were separated by SDS-PAGE on 12.5% polyacrylamide gels and transferred to Hybond-c extra nitrocellulose membranes (Hybond-P; GE Healthcare, Waukesha, WI, USA) with the Trans-Blot Turbo Transfer System. Membranes were probed with primary antibody for glucose transporter 4 (GLUT 4; dilution 1/1000; Santa Cruz Biotechnology, Dallas, TX, USA), adiponectin (dilution 1/1000; Abcam, Cambridge, United Kingdom), dipeptidylpeptidase 4 (DPP4; dilution 1/1000; Abcam), suppressor of cytokine signaling 3 (SOCS3; dilution 1/500; Cell Signaling Technology, Danvers, MA, USA), glucagon-like peptide 1 (GLP-1; dilution 1/1000; Abcam), insulin receptor substrate 1 (IRS-1; dilution 1/1000, Ab-636; Signalway Antibody, College Park, MD, USA), pIRS-1 (dilution 1/1000, phospho-Ser636; Signalway Antibody), carnitine palmitoyl transferase 1A (CPT1A; dilution 1/500; Abcam), fatty acid (FA) translocase (FAT; dilution 1/1000; Abcam), mitofusin 1 (MFN1; dilution 1/1000; Abcam), cyclophilin F (Cyclo F; dilution 1/1000; Santa Cruz Biotechnology), peroxiredoxin 4 (PRDX4; dilution 1/1000; Santa Cruz Biotechnology), protein disulfide isomerize family A member 6 (PDIA6; dilution 1/1000; Abcam), fumarate hydratase (FH; dilution 1/1000; Santa Cruz Biotechnology), and α-tubulin, glyceraldehyde 3-phosphate dehydrogenase, and β-actin (dilution 1/5000; MilliporeSigma) as loading controls. Signals were detected using the ECL system (Amersham, Little Chalfont, United Kingdom). Results are expressed as an n-fold increase over the values of the control group in densitometric arbitrary units.
The Mitoprofile Total Oxphos Rodent WB Antibody Cocktail (dilution 1/1000; Abcam) was used to quantify the relative levels of the subunits of the mitochondrial oxphos complexes: NDUFB8 subunit of complex I (20 Kda), SDHB subunit of complex II (30 kDa), core protein 2 UQCRC2 of complex III (48 kDa), MTCO1 subunit 1 of complex IV (40 kDa), and α-subunit of complex V or ATP synthase (55 kDa). For detection of oxphos subunits, all Western blot steps were followed.
RNA extraction from adipose tissue
Total RNA was extracted from EAT or BAT. The extraction was carried out with Qiazol reagent and purified using the RNeasy Lipid Tissue Kit according to the manufacturer’s instructions (Qiagen, Germantown, MD, USA).
Reverse transcription and real-time PCR
First-strand cDNA was synthesized from 1.5 µg of total RNA according to the manufacturer’s instructions (Go Script Reverse Transcription System; Promega, Madison, WI, USA). Quantitative PCR analysis was then performed with Sybr green PCR technology (GoTaq Real-Time PCR Systems; Promega) to measure uncoupling protein 1 (UCP1) gene expression (forward, 5′-CCCTGCCATTTACTGTCA-3′; reverse, 5′-CAGCTGGGTACACTTGGGTA3-3′). Relative quantification was achieved with MxPro-Mx3000P software (Agilent Technologies, Santa Clara, CA, USA). Data were normalized by TATA box (forward, 5′-CAGTACAGCAATCAACATCTAGC-3′; reverse, 5′-CAAGTTTACAGCCAAGATTCACG-3′) levels and expressed as percentage relative to controls. All PCRs were performed at least in triplicate for each experimental condition.
Morphologic and histologic evaluation
In order to detect collagen fibers, EAT samples were dehydrated, embedded in paraffin, cut into 5-μm–thick sections, and stained with Picrosirius Red. The area of pericellular fibrosis was identified as the ratio of collagen deposition to the total tissue area after excluding the vessel area from the region of interest. This value was normalized by the number of adipocytes. Adipocytes (80–100/animal) with intact cellular membranes were chosen for determination of the cross-sectional area in hematoxylin-eosin–stained sections. For each sample, 10–15 fields were analyzed using a ×20 objective (Leica DM 2000; Leica Microsystems, Buffalo Grove, IL, USA) and quantified (Leica Q550 IWB; Leica Microsystems). A single researcher that was unaware of the experimental groups performed the analysis.
Proteomic analysis of human adipose tissue
Sample preparation for proteomic analysis
Pools of adipose tissue from patients with obesity and patients without obesity were lysed in a Precellys 24 Bead Mill Homogenizer (Bertin Technologies, Montigny-Le-Bretonneux, France) (15 × 2 s, power set to 5500 W) using 5 mM EDTA, 0.1% Triton X-100, 1% glycerol in 30 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (pH 7.4) supplemented with 1:1000 (v/v) of benzonase (MilliporeSigma) and 1:100 (v/v) of Halt phosphatase and protease inhibitor cocktail 100× (Thermo Fisher Scientific). Lysates were clarified by centrifugation at 4°C and 16,000 rpm for 15 min. Recovered supernatants were cleaned up by methanol-chloroform extraction (14).
Pellets were dissolved in 8 M urea in 0.1 M Triethylamonium bicarbonate buffer. Protein concentration was determined using the Bradford Protein Assay Kit (Bio-Rad, Hercules, CA, USA) using bovine serum albumin as standard. Samples were then digested by means of the standard Fast and Secure Protocol (FASP)(15). Briefly, proteins were reduced (10 mM DTT, 30 min, RT), alkylated (50 mM IA, 20 min in the dark, room temperature), and sequentially digested with a Lys-C protein/enzyme ratio of 1/50, o/n at room temperature) and trypsin (Promega) (protein/enzyme ratio 1/100, 6 h at 37°C). Resulting peptides were desalted using C18 stage tips.
Samples (110 µg) were labeled using iTraq reagent 4-plex following the manufacturer’s instructions. Labeling scheme was as follows: control (114) and nondiabetic morbidly obese (115). Samples were mixed in 1/1 ratios based on total peptide amount, which was determined from an aliquot by comparing overall signal intensities on a regular liquid chromatography (LC)-tandem mass spectrometry (MS) (MS/MS) run. The final mixture was finally desalted using a Sep-Pak C18 cartridge (Waters, Milford, MA, USA) and dried. The sample was reconstituted in Offgel solution (5% glycerol, 1% ampholytes pH 3–10) prior to electrofocusing.
Isoelectrofocusing separation
Peptides were prefractionated offline by means of isoelectric focusing using a 3100 Offgel Fractionator system (Agilent Technologies) with a 24-well setup. The Immobiline DryStrip Gels (IPG strips) that were 24-cm–long (GE Healthcare) with a 3–10 linear pH range were rehydrated according to the protocol provided by the manufacturer. Subsequently, 150 μl of sample was loaded in each well. Electrofocusing of the peptides was performed at 20°C and 50 μA until the 50-kVh level was reached. After focusing, the 24-peptide fractions were withdrawn and the wells rinsed with 100 μl of a solution of 0.1% TFA. Rinsing solutions were pooled with their corresponding peptide fraction. All fractions were evaporated by centrifugation under vacuum. Solid phase extraction and salt removal was performed with homemade columns based on Stage Tips with C8 Empore Disks (3M, Minneapolis, MN, USA) filled with Poros Oligo R3 resin (Thermo Fisher Scientific). Eluates were evaporated to dryness and maintained at 4°C. Just prior to nano-LC, the fractions were resuspended in H2O with 0.1% (v/v) formic acid.
MS
LC-MS/MS was done by coupling a nanoLC-Ultra 1D + System (Sciex, Framingham, MA, USA) to an LTQ Orbitrap Velos mass spectrometer (Thermo Fisher Scientific) via a Nanospray Flex source (Thermo Fisher Scientific). Peptides were loaded into a trap column (ReproSil Pur C18-AQ 5 µm, 10-mm length, and 0.3-mm interior diameter (ID); Trajan, Ringwood, VIC, Australia) for 10 min at a flow rate of 2.5 µl/min in 0.1% formic acid. Then, peptides were transferred to an analytical column (ReproSil Pur C18-AQ 3 µm, 200-mm length, and 0.075-mm ID; Trajan) and separated using a 117-min effective linear gradient (buffer A: 4% acetonitrile (ACN), 0.1% formic acid; buffer B: 100% ACN, 0.1% formic acid) at a flow rate of 300 nl/min. The gradient used was 0–3 min 2% B, 3–120 min 40% B, 120–131 min 98% B, and 131–140 min 2% B. The peptides were electrosprayed (1.7 kV) into the mass spectrometer with a PicoTip emitter (360/20 Tube outer diameter (OD)/ID µm, tip ID 10 µm) (New Objective, Woburn, MA, USA), a heated capillary temperature of 240°C, and S-Lens radio frequency level of 60%. The mass spectrometer was operated in a data-dependent mode, with an automatic switch between MS and MS/MS scans using a top 15 method (threshold signal ≥1000 counts and dynamic exclusion of 45 s). MS spectra (250–1750 m/z) were acquired in the Orbitrap with a resolution of 60,000 full width at half maximum (400 m/z). Peptides were isolated using a 2 Th window and fragmented using higher-energy collisional dissociation with Orbitrap readout at an NCE of 42% (0.25 qvalue and 0.1-ms activation time). The ion target values were 1E6 for MS (500-ms maximum injection time) and 2E5 for MS/MS (200-ms maximum injection time). Samples were injected in duplicates.
Statistical analysis
Continuous variables are expressed as means ± sd. Categorical variables are expressed in absolute values and percentages. The differences between categorical variables were analyzed using the χ2 test. Normality of distributions was verified by means of the Kolmogorov-Smirnov test. Differences between 2 groups were analyzed by unpaired Student’s t test. Specific differences between more groups were analyzed using 1-way ANOVA followed by Newman-Keuls test. Pearson correlation analysis was used to examine association among different variables according to whether they are normally distributed. Multivariable analysis, considering homeostasis model assessment (HOMA) as the dependent variable, was performed with a linear regression model by means of a backward stepwise method. In consecutive steps, variables that were statistically significant in the univariable analysis were included in the linear regression model. A value of P < 0.05 was used as the cutoff value for defining statistical significance. Data analysis was performed using the statistical program SPSS v.22.0 (IBM SPSS, Chicago, IL, USA).
RESULTS
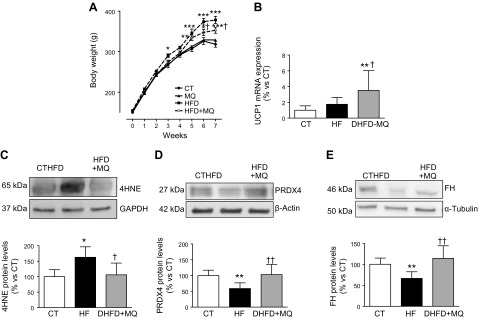
HFD induced an increase in body weight that reached a significant difference with that of controls from the fifth week (Fig. 1A). This difference was maintained until the end of the study. The administration of MitoQ reduced the increase in body weight, an effect that was accompanied by a decrease in white adipose tissue weight (epididymal, lumbar, and mesenteric) in rats fed an HFD (Fig. 1A and Table 1) and consequently reduced adiposity index (Table 1). An increase in relative BAT weight was observed in HFD-fed as compared with CT-fed animals (Table 1). MitoQ-treated, HFD-fed rats show a slightly lower food intake as compared with HFD-fed rats, although no significant differences were detected between both groups. However, the energy intake (calculated from the diet-contained calories) was reduced in MitoQ-treated, HFD-fed rats as compared with HFD-fed animals, although it did not reach those values observed in the CT group (Table 1). To investigate whether an increase in energy expenditure is involved in the observed reduction in body-weight gain, we explored the expression of UCP1, involved in energy expenditure, in BAT. Obesity only increased the expression of UCP1 in BAT from obese animals treated with MitoQ (Fig. 1B). Neither HFD nor MitoQ administration was able to increase UCP1 expression in EAT, which was not detected in any animal group (unpublished results).
Figure 1.
A) Body-weight evolution along the study. B) UCP1 mRNA levels in BAT. C–E) Protein levels of 4-HNE adducts (C), PRDX4 (D), and FH (E) in EAT from control rats fed a CT and rats fed an HFD treated with vehicle or with the mitochondrial antioxidant MitoQ (MQ; 200 µM). Bar graphs represent the means ± sd of 6–8 animals normalized for reference housekeeping. *P < 0.05, **P < 0.01 vs. control group, †P < 0.05, ††P < 0.01 vs. HFD group.
TABLE 1.
Effect of the mitochondrial antioxidant MitoQ (200 µM) on general characteristics and metabolic parameters in CT-fed and HFD-fed rats
| Variable | CT | MitoQ | HFD | HFD+MitoQ |
|---|---|---|---|---|
| F I (g/d) | 22.3 ± 1.5 | 21.2 ± 1.6 | 18.6 ± 3.1* | 16.0 ± 2.1** |
| EI (kcal) | 64.7 ± 4.3 | 61.5 ± 4.0 | 100.4 ± 16.7** | 86.4 ± 11.3**,† |
| WI (ml/d) | 42.3 ± 7.1 | 48.0 ± 8.5 | 41.8 ± 5.6 | 43.9 ± 1.0 |
| EAT (g/cm tibia) | 1.1 ± 0.35 | 0.09 ± 0.21 | 3.18 ± 0.91** | 2.3 ± 0.54*,† |
| LAT (g/cm tibia) | 1.81 ± 0.49 | 1.76 ± 0.43 | 3.86 ± 0.81** | 2.77 ± 0.31††,‡ |
| MAT (g/cm tibia) | 0.85 ± 0.24 | 0.82 ± 0.12 | 1.84 ± 0.27* | 1.44 ± 0.17**,‡ |
| Adiposity index (%) | 4.6 ± 1.13 | 4.2 ± 0.83 | 9.1 ± 1.01** | 7.8 ± 0.85**,† |
| BAT (g/cm tibia) | 0.126 ± 0.03 | 0.136 ± 0.015 | 0.184 ± 0.05†† | 0.166 ± 0.012†† |
| Glucose (mg/dl) | 96.5 ± 11.4 | 99.6 ± 11.7 | 115.4 ± 6.3†† | 100.3 ± 10.34† |
| Insulin (pg/ml) | 90.8 ± 15.2 | 79.3 ± 20.4 | 272.3 ± 90.4** | 153.6 ± 36.8††,‡ |
| HOMA index | 3.3 ± 1.4 | 2.9 ± 1.3 | 10.3 ± 4.2** | 5.4 ± 2.5‡ |
| TG (mg/dl) | 78.6 ± 15.6 | 89.7 ± 18.3 | 146.8 ± 32.9†† | 129.0 ± 35.9†† |
| TC (mg/dl) | 66.5 ± 14.8 | 68.2 ± 10.5 | 68.1 ± 10.9 | 68.8 ± 8.4 |
BW, body weight; EI, energy intake; FI, food intake; LAT, lumbar adipose tissue; MAT, mesenteric adipose tissue; TG, triglyceride; TC, total cholesterol; WI, water intake. Data values represent the means ± sd of 8 animals. ††P < 0.05 compared with control group; *P < 0.01, **P < 0.001 compared with control group; †P 0.05, ‡P < 0.01 compared with HFD group.
MitoQ did not affect any of these parameters in control animals (Table 1). Therefore, and to simplify, only data from rats fed a CT and HFD or HFD + MitoQ will be presented from now on.
The efficiency of MitoQ treatment was evaluated by measurement of levels of protein-4-hydroxynonenal (HNE) adducts, a major end-product of lipid peroxidation. The higher levels of 4-HNE found in EAT from HFD-fed rats were normalized with the MitoQ treatment (Fig. 1C), supporting the effectiveness of MitoQ. Protein levels of both PRDX4 and FH were also reduced in HFD-fed rats and normalized with MitoQ administration (Fig. 1D, E).
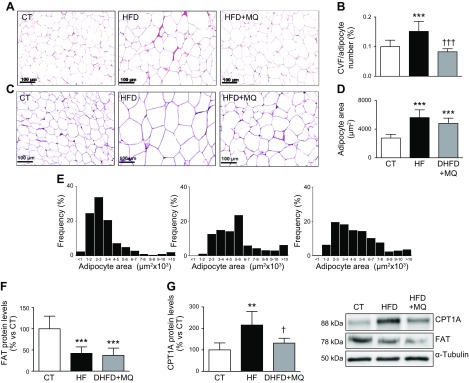
Histologic analysis of EAT revealed an increase in pericellular collagen content in the HFD group that was prevented by MitoQ administration (Fig. 2A, B). Pericellular collagen levels were associated with HOMA index (r = 0.495; P < 0.031). An increase in adipocyte area was also observed in HFD-fed rats. A trend toward an attenuation of this increase due to a shift toward smaller adipocytes was observed in obese animals treated with MitoQ (Fig. 2C–E).
Figure 2.
A) Representative microphotographs of EAT from animals fed a CT and animals fed an HFD treated with vehicle or with the mitochondrial antioxidant MitoQ (MQ; 200 µM) stained with Picrosirius Red examined by light microscopy (original magnification, ×20). B) Quantification of collagen volume fraction normalized for adipocyte number (CVF). C) Representative microphotographs of EAT from CT-fed and HFD-fed rats stained with hematoxylin-eosin examined by light microscopy (original magnification, ×20). D, E) Quantification (D) and size distribution (E) of adipocytes from epididymal adipose tissue. F, G) Protein levels of FAT and CPT1A, respectively, in EAT from control rats fed a CT and rats fed an HFD treated with vehicle or with the mitochondrial antioxidant MitoQ (200 µM). Scale bar, 100 µm. Values are the means ± sd of 6–8 animals. **P < 0.01, ***P < 0.001 vs. control group, †P < 0.05, †††P < 0.001 vs. HFD group.
Considering the increase in the adiposity index and adipocyte size observed in HFD-fed rats, we decided to explore whether an increase in FA inflow into cells and mitochondria could participate in these changes. For this purpose, we evaluated the protein levels in EAT of both the FA transporter into the cell and the FA transporter into the mitochondria, FAT, and CPT1A, respectively. In HFD-fed rats, FAT was reduced, and MitoQ was unable to prevent this reduction, although it was able to prevent the increase in CPT1A observed in HFD-fed rats (Fig. 2F, G).
Next, we examined whether the inhibition of mitochondrial oxidative stress could modify metabolic parameters in obese animals. Treatment with MitoQ improved fasted glucose and insulin levels and consequently reduced HOMA index in the HFD group (Table 1). The levels of both 4-HNE and PRDX4 were independent predictors of HOMA [mean difference, 0.026; 95% confidence interval (CI), 0.003–0.049; P = 0.029 and mean difference, −0.49; 95% CI, −0.88 to 0.010; P = 0.017; respectively], supporting the relevance of oxidative stress in the development of metabolic alterations. Obese animals showed higher triglyceride levels than CT ones, but no significant differences were observed in the total cholesterol (Table1). MitoQ was unable to modify lipid profile in the control group (Table 1).
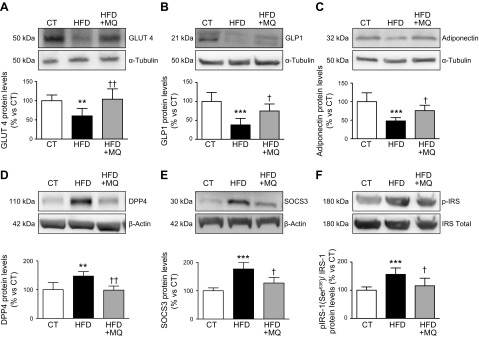
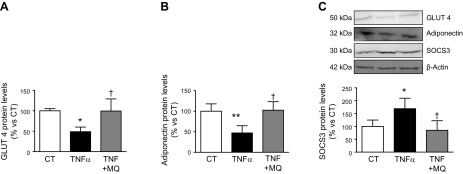
In order to understand how MitoQ improves insulin sensitivity in obese animals, we analyzed the levels of proteins involved in the control of insulin sensitivity in EAT. The reduction in GLUT 4, GLP-1, and adiponectin expression observed in the HFD group was normalized by MitoQ (Fig. 3A–C). Furthermore, the increase in the protein levels of DPP4 and SOCS3 triggered by the HFD was completely prevented by MitoQ, which was also able to ameliorate the increase in the phosphorylation of Ser (636) of IRS-1 (Fig. 3D–F). To determine whether MitoQ could directly affect adipocyte function, we carried out in vitro experiments in the TNF-α–induced insulin resistance model in differentiated 3T3-L1 adipocytes. As shown in Fig. 4 and as previously described by Miana et al. (13), TNF-α reduced GLUT 4 and adiponectin expression and increased SOCS3 protein levels in these cells. Interestingly, these effects were prevented by MitoQ (Fig. 4A–C).
Figure 3.
Protein levels of GLUT 4 (A), GLP-1 (B), adiponectin (C), DPP4 (D), SOCS3 (E), and IRS-1 (F), total and phosphorylated forms, in EAT from control rats fed a CT and rats fed an HFD treated with vehicle or with the mitochondrial antioxidant MitoQ (MQ; 200 µM). Bar graphs represent the means ± sd of 6–8 animals normalized to for reference housekeeping. **P < 0.01, ***P < 0.001 vs. control group, †P < 0.05, ††P < 0.01 vs. HFD group.
Figure 4.
Protein levels of GLUT 4 (A), adiponectin (B), and SOCS3 (C) in 3T3-L1 adipocytes. The cells were stimulated with or withouth TNF-α (50 ng/ml) for 72 h in the presence of either vehicle or the mitochondrial antioxidant MitoQ (MQ; 5 nM). Data normalized to β-actin are expressed as means ± sd of 4 assays in arbitrary units. *P < 0.05, **P < 0.01, vs. unstimulated cells (CT); †P < 0.05, cells stimulated with the same concentration of TNF-α in the absence of MitoQ.
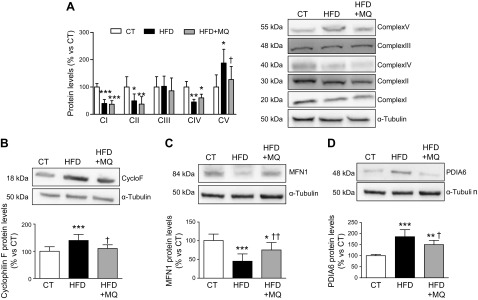
Regarding mitochondrial markers, protein levels of mitochondrial complexes I, II, and IV were reduced, whereas complex V was increased in HFD-fed rats (Fig. 5A). MitoQ treatment was only able to reduce the increase in complex V. Complex III was altered neither in HFD-fed nor in HFD-fed + MitoQ-treated rats. Obesity was also able to increase Cyclo F levels (Fig. 5B), suggesting the opening of the mitochondrial permeability transition pores (mPTPs), which was normalized by MitoQ treatment. MFN1, a protein involved in the mitochondrial fusion process, was reduced in HFD-fed rats and was prevented in those treated with MitoQ (Fig. 5C). MFN1 was an independent predictor of HOMA index (mean difference, 0.058; 95% CI, 0.004–0.113; P = 0.038). We also assessed the levels of PDIA6, a marker of endoplasmic reticulum (ER) stress. PDIA6 levels were higher in HFD-fed rats than in controls, and this increase was prevented in those HFD-fed rats treated with MitoQ (Fig. 5D).
Figure 5.
Protein levels of mitochondrial complexes I-V (A), Cyclo F (B), MFN1 (C), and protein disulfide isomerize family A member 6 (D) in EAT from control rats fed a CT and rats fed an HFD treated with vehicle or with the mitochondrial antioxidant MitoQ (MQ; 200 µM). Bar graphs represent the means ± sd of 6–8 animals normalized to for reference housekeeping. *P < 0.05, **P < 0.01, ***P < 0.001 vs. control group, †P < 0.05, ††P < 0.01 vs. HFD group.
In order to verify whether the adipose tissue remodeling occurs not only in an obese animal model but also in patients with obesity, we performed a proteomic study of adipose tissue from control and obese (nondiabetic) subjects. The mean age of both groups was similar, with there being more women in the group of patients with obesity (Table 2). The majority of patients with obesity but only 17.9% of the lean subjects had associated comorbidities with the most hypertension and dyslipidemia (Table 2). Drug treatment for hypertension included angiotensin-converting enzyme inhibitors (or angiotensin II type 1 receptor antagonists), whereas all patients with dyslipidemia were on statins. Body mass indexes, fasting plasma glucose and insulin levels, and HOMA indexes were higher in obese than in nonobese subjects, as occurs in the obese rats (Table 2).
TABLE 2.
Epidemiologic and clinical characteristics and circulating markers of patients with morbid obesity and lean subjects
| Variable | Lean subject, n = 67 | Patient with obesity, n = 63 | P |
|---|---|---|---|
| Age (yr) | 41.4 ± 5.11 | 40.4 ± 9.48 | 0.435 |
| Women (%) | 30 | 40.3 | 0.004 |
| BMI (kg/m2) | 22.8 ± 1.97 | 45.8 ± 5.44 | <0.0001 |
| Comorbidities [n (%)] | 11 (16.5) | 55 (87.3%) | <0.001 |
| Hypertension [n (%)] | 4 (6) | 25 (39.7%) | <0.001 |
| Dyslipemia [n (%)] | 6 (9) | 24 (37.5%) | <0.001 |
| Glucose (mg/dl) | 87.3 ± 9.4 | 94.8 ± 15.5 | <0.001 |
| Insulin (pg/ml) | 163.5 ± 62.92 | 257.8 ± 72.1 | <0.001 |
| Leptin (pg/ml) | 5486.9 ± 3522.8 | 27442.1 ± 14650.6 | <0.001 |
| Adiponectin (µg/ml) | 41879.9 ± 43959.6 | 17920.3 ± 11189.1 | 0.02 |
| CRP (mg/dl) | 0.15 ± 0.35 | 1.15 ± 1.08 | <0.001 |
| IL-1β (pg/ml) | 0.58 ± 0.52 | 1.36 ± 1.58 | <0.001 |
| IL-6 (pg/ml) | 3.3 ± 3.3 | 11.14 ± 11.9 | <0.001 |
| TNF-α (pg/ml) | 8.7 ± 3.8 | 17.02 ± 6.92 | <0.001 |
| IL-10 (pg/ml) | 38.89 ± 26.7 | 37.7 ± 23.1 | 0.827 |
| MMP 9 (pg/ml) | 140.8 ± 85.9 | 219.9 ± 160.8 | 0.004 |
| MPO (pg/ml) | 44.2 ± 36.4 | 107.3 ± 155.6 | <0.001 |
| GLP-1 (pg/ml) | 21.5 ± 8.3 | 21.3 ± 6.7 | 0.294 |
| GIP (pg/ml) | 13.9 ± 7.8 | 10.8 ± 7.1 | 0.927 |
| Ghrelin (pg/ml) | 62.2 ± 7.8 | 20.1 ± 10.2 | <0.001 |
Data values are expressed as means ± sd or percentages. BMI, body mass index; CRP, C-reactive protein; MMP 9, metalloproteinase 9; MPO, myeloperoxidase.
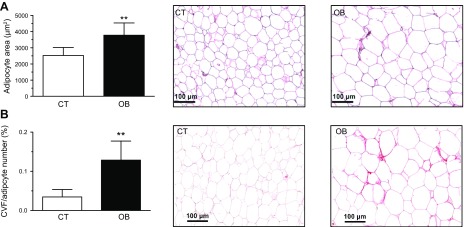
Histologic analysis of visceral adipose tissue showed an enhanced adipocyte area (Fig. 6A) and an increase in pericellular collagen (Fig. 6B) in patients with obesity as compared with control subjects. As shown in Table 2, patients with obesity had higher serum levels of inflammatory markers, including C-reactive protein, IL-1β, IL-6, TNF-α, and matrix metallopeptidase 9, than lean subjects, although no differences were observed in the anti-inflammatory cytokine IL-10. Patients with obesity also showed an increase in myeloperoxidase, a marker of oxidative stress. As compared with nonobese subjects, patients with obesity showed a reduction in levels of adiponectin and ghrelin, but no changes were observed in those of GLP-1 and glucose-dependent insulinotropic polypeptide (GIP), and leptin plasma levels were significantly increased. The anthropometrical and clinical characteristics of the patients used for the proteomic analysis of the adipose tissue follow the same pattern observed for the all patients included in the study, suggesting that they are a representative sample (unpublished results).
Figure 6.
A) Quantification of adipocyte area (left) and representative microphotographs of visceral adipose tissue sections from control subjects and patients with morbid obesity (OB) stained with hematoxylin-eosin examined by light microscopy (original magnification, ×20; right). B) Quantification of collagen volume fraction normalized for adipocyte number (CVF). Right panel shows representative microphotographs of visceral adipose tissue sections from these individuals stained with picrosirius re-examined by light microscopy (original magnification, ×20). Bar histograms represent the means ± sd of 7 subjects. Scale bar, 100 µm. **P < 0.01 vs. control group.
In total, 2119 proteins were identified in the proteomic analysis of human adipose tissue, of which 53 were related to mitochondria. Those proteins with a fold change >1.3 (up-regulation) or <0.769 (down-regulation) between the obese and control group were selected. According to these criteria, 23 of the 53 mitochondrial proteins were modified in the patients with obesity (Table 3).
TABLE 3.
Mitochondrial proteins altered in the adipose tissue of patients with obesity as compared with controls identified by a proteomic analysis
| Mitochondrial protein | Ratio |
|---|---|
| 10 kDa heat shock protein | 0.745 |
| Microsomal glutathione S-transferase | 1.482 |
| Aconitate hydratase | 0.735 |
| Enoyl-CoA hydratase | 0.697 |
| Electron transfer flavoprotein subunit-α | 0.662 |
| Isoform short of ES1 protein homolog | 0.711 |
| NADP-dependent malic enzyme | 0.738 |
| Protein NipSnap homolog 3A | 0.767 |
| Cytochrome c (fragment) | 0.691 |
| T-complex protein 1 subunit-ζ | 1.303 |
| Ras-related protein Rab-5A | 0.618 |
| NADH dehydrogenase (ubiquinone) flavoprotein 2 | 0.698 |
| NADH dehydrogenase (ubiquinone) flavoprotein 1 | 0.727 |
| Phosphoenolpyruvate carboxykinase (GTP) | 0.740 |
| Isoform 3 of Ethylmalonyl-CoA decarboxylase | 0.766 |
| Aspartate aminotransferase | 0.576 |
| ATPase inhibitor, mitochondrial | 1.469 |
| T-complex protein 1 subunit-γ | 1.369 |
| Branched-chain amino acid aminotransferase | 0.558 |
| Ubiquinone biosynthesis protein COQ9 | 0.736 |
| Isoform 4 of glutathione S-transferase κ | 1.470 |
| Enoyl-CoA-δ isomerase 1 | 0.601 |
| ATP synthase lipid-binding protein | 1.363 |
| Other proteins | |
| Adiponectin | 0.681 |
| Collagen VI (α-3) | 1.359 |
| PDIA6 | 1.392 |
| Peroxiredoxin-2 | 0.504 |
| Peroxiredoxin-5 | 0.755 |
1.3-fold change cutoff for all iTraq ratios was selected to classify proteins as up- or down-regulated; ratio <0.77 was considered underexpressed and >1.3 was considered overexpressed.
Most of the modified mitochondrial proteins were involved in FA β-oxidation, Krebs cycle, or oxphos. Six proteins involved in FA β-oxidation and Krebs cycle were reduced in patients with obesity as compared with controls, suggesting an impaired metabolism. oxphos proteins were altered (5 reduced and 2 increased) in patients with obesity. Two subunits of the mitochondrial complex I [NADH dehydrogenase (ubiquinone) flavoprotein 1 and NADH dehydrogenase (ubiquinone) flavoprotein 2] were reduced in patients with obesity that were also reduced in EAT of HFD-fed rats. On the other hand, a subunit of mitochondrial complex V or ATPase (described in Table 3 as ATP synthase lipid-binding protein) was increased in patients with obesity as compared with lean subjects and as occurs in the EAT of obese rats.
DISCUSSION
The results presented here reveal that the metabolic alterations observed in diet-induced obesity were accompanied by an exacerbation of oxidative stress, remodeling, and mitochondrial proteins alterations in adipose tissue. We hypothesized that mitochondrial oxidative stress could participate in metabolic alterations associated with obesity. Interestingly, we observed that the administration of the mitochondrial antioxidant MitoQ protects against some of the consequences of diet-induced obesity, thereby limiting weight gain, attenuating the disturbances in adipose tissue, and improving the insulin resistance observed in obese animals.
HFD-fed animals exhibited a decline in insulin sensitivity, a common feature in patients with obesity, as indicated by an increase in HOMA index. Alterations in the balance of factors that modulate (increase or decrease) insulin sensitivity seem to underlie the dysfunctional glucose homeostasis observed in obese rats. Such alterations included reduced levels of GLUT 4, adiponectin, and GLP-1 as well as increased levels of DPP4, SOCS3, and IRS-1 serine phosphorylation. Similar results have previously been described in both human and animal models (13, 16–18). The relevance of SOCS3 in insulin resistance is confirmed by the fact that its levels were independently associated with HOMA index. All of these alterations were improved by MitoQ treatment, showing the role of mitochondrial oxidative stress on insulin sensitivity (10, 19).
An increase in oxidative stress seems to be involved in insulin resistance in HFD-fed rats because HOMA index was associated with 4-HNE levels. This bioreactive aldehyde is not only a by-product of lipid peroxidation but is also able to induce oxidative stress and contribute to adipose tissue metabolic dysfunction associated with insulin resistance in human adipocytes (20). Other sources of free radicals in addition to mitochondria could participate in the oxidative environment observed in obese rats because FH levels were decreased in HFD-fed rats and associated with those of 4-HNE. The decrease in FH levels can allow a consequent rise in fumarate production, which is involved in ROS generation (21, 22). In addition, a reduction in antioxidant defense could also participate because levels of PRDX4, which metabolize hydrogen peroxide, were reduced in the adipose tissue of obese rats. These results agree with a previous study, which observed a reduction in the activity of antioxidant enzymes parallel to the increase in adipose tissue (23). MitoQ was able to reverse the reduction in both FH and PRDX4, supporting the complexity of the effects of this antioxidant in the modulation of oxidative stress. More relevant is the fact that levels of both PRDX4 and 4-HNE were independently associated with HOMA index, supporting the importance of oxidative stress in adipocyte function. In addition, levels of MFN1 were independent predictors of HOMA index, confirming previous observations, which show an interaction between mitochondria dynamics and insulin resistance (24, 25).
Pericellular fibrosis seems to be an additional mechanism involved in insulin resistance in HFD-fed rats because it was associated with HOMA index, and the improvement in insulin sensitivity in MitoQ-treated rats was accompanied by a decrease in pericellular fibrosis. The observation of a reduction in FAT levels, a protein involved in FA inflow inside cell, suggests a maladaptive response to excess dietary FA that may facilitate ectopic fat deposit (26), and MitoQ was unable to affect it. In agreement with that observation, MitoQ was unable to normalize the enlarged adipocytes in obese rats.
Obesity is also accompanied by changes in levels of different mitochondrial proteins in EAT, Cyclo F, and MFN1, which are involved in essential processes that can affect mitochondrial survival (27, 28), leading to cell death (29). MitoQ treatment was able to normalize the levels of Cyclo F and MFN1, suggesting that oxidative stress seems to be involved in the regulation of these 2 essential processes: opening and regulation of mPTP, and mitochondrial fusion. Moreover, obesity is also accompanied by ER stress activation as suggested by the increase in PDIA6 levels, which was reduced in MitoQ-treated rats. In fact, ER stress can further damage mitochondria by regulating mPTP opening through an increase in Ca2+ levels and further aggravate oxidative stress by augmenting ROS production in ER (30), which can also be potentiated by the reduction in the antioxidant PRDX4, which is mainly localized in ER.
In accordance with previous studies (10, 31, 32), a reduction in energy intake could be involved in the slimming effect induced by MitoQ in HFD-fed rats. In addition, an increase in energy expenditure could also be proposed in the observed body weight reduction. This affirmation is based on the fact that MitoQ treatment was associated with an increase in levels of UCP1, which dissipates energy as heat, in BAT of HFD-fed rats, supporting the capacity of MitoQ to increase the uncoupling of oxphos from ATP synthesis (32, 33). Previous studies have reported that proliferator-activated receptor-γ (PPAR-γ) is involved in energy expenditure because treatment with PPAR-γ agonists is accompanied by a strong induction of UCP1 mRNA expression, which is associated with a reduction in body weight in mice (34–36). In this regard, we have observed in preliminary data that MitoQ treatment was able to reverse the reduction in PPAR-γ induced by HFD (unpublished data).
Considering the reduction in body weight observed during MitoQ treatment, we cannot exclude the possibility that this MitoQ-elicited reduction in HFD-fed animals could also be partially responsible for improvement in insulin resistance in these animals. However, although MitoQ-treated rats normalized insulin sensitivity, they showed a higher adiposity and enlarged adipocytes relative to control animals. Moreover, MitoQ normalized GLUT 4, adiponectin, and SOCS3 expression in cultured adipocytes, supporting a direct effect of this compound in adipose tissue glucose signaling. This effect could involve not only mitochondrial redox regulation but also lipid metabolism modulation (10). In fact, we have found an increase in CPT1A in MitoQ-treated animals, supporting an increase in β-oxidation.
Patients with obesity showed a low-grade systemic inflammation and a pro-oxidant state, a fact amply reported in the literature (37–40). This proinflammatory and pro-oxidant environment has been explained by dysfunctional adipose tissue that occurred in the context of obesity. Accordingly, and similar to that observed in rats, patients with obesity show a remodeling of visceral adipose tissue characterized by adipocyte hypertrophy and increased deposition of extracellular matrix, confirming previous observations (13). In addition, the proteomic analysis of the visceral adipose tissue shows important changes, including at the mitochondrial level, because almost 50% of the mitochondrial proteins identified were modified in patients with obesity, suggesting that obesity exerts an important impact on mitochondria. Such proteins were involved in β-oxidation and the citric acid cycle, which were showed to be reduced as compared with normoweight individuals and oxphos proteins (subunits of complex I that were reduced and a subunit of complex V that was increased), showing a pattern similar to the one observed in obese rats. Likewise, there have been reported alterations in the genes involved in metabolism as well as oxphos in subcutaneous adipose tissue of obese patients with type 2 diabetes mellitus. A decrease in proteins involved in metabolism as well as a decrease in the activity of complexes I and V was found in the abdominal adipose tissue of children with obesity (41). These data suggest that obesity is associated with mitochondrial and structure alterations in the adipose tissue.
The results obtained in samples from humans with obesity reveal that some of the alterations observed in the EAT of obese rats are also reflected in the visceral adipose tissue of patients with obesity, further suggesting the clinical relevance of our findings. These alterations include oxidative parameters, such as PRDX, ER stress markers like PDIA6, HOMA index, and circulating factors involved in insulin sensitivity, including GIP, adiponectin, and ghrelin.
CONCLUSIONS
This study demonstrates the beneficial effect of the mitochondrial antioxidant MitoQ in the metabolic alterations associated with obesity. Although additional mechanisms could not be ruled out, our data emphasize the interest of mitochondrial ROS as a potential therapeutic target in obesity and highlight the benefits of mitochondrial-targeted antioxidant drugs for this disease.
Although the study explores the potential role of mitochondrial oxidative stress in the adipose tissue remodeling and the metabolic alterations observed in obesity, the assessment of mitochondrial function was derived from the evaluation of protein levels involved in essential mitochondrial processes, and this represents a limitation of our study. In addition, we have used the surrogate marker HOMA index for the evaluation of insulin sensitivity in spite of direct assessments for evaluation of insulin sensitivity, such as the glucose clamp technique. Although HOMA index is considered to be a useful tool to estimate insulin resistance, simple tests involving a single fasting blood sample could be a limitation of the study. Another notable consideration related to the study is the fact that MitoQ is a mitochondrial-targeting antioxidant with a ubiquinone moiety attached to a decyl(triphenyl)phosphonium + cation. In a recent paper, it has been described that the lipophilic cation moiety triphenylphosphonium of MitoQ can mediate some of the observed effects of this antioxidant (33). For this reason, we cannot preclude which moiety of the MitoQ molecule could exert the beneficial effects described in the manuscript because we did not use animals treated with triphenylphosphonium as controls.
ACKNOWLEDGMENTS
The authors thank Avelina Hidalgo, Blanca Martínez, Virginia Peinado, and Roberto Cañadas (Departamento de Fisiología, Facultad de Medicina, Universidad Complutense de Madrid) for technical help; Isabel Ruppen, Javier Muñoz, and Pilar Ximénez-Embún [Proteomics Unit of the Centro Nacional de Investigaciones Oncologicas (CNIO), ProteoRed, PRB2-ISCIII], supported by Grant PT13/0001; and Anthony DeMarco for help in editing. MitoQ was provided by M. P. Murphy (Medical Research Council Mitochondrial Biology Unit, Cambridge BioMedical Campus, Cambridge, United Kingdom). This work was supported by Instituto de Salud Carlos III–Fondo Europeo de Desarrollo Regional (FEDER) [PI15/01060, PI18/00257, PI18/0919, and Ciber de Enfermedades Cardiovasculares (CIBERCV)], a Way to Build Europe, Ministerio de Economia y Competitividad (SAF2016-81063), and Agencia de Gestio d’Ajuts Universitaris i de Recerca (AGAUR; Program of Support to Research Groups, 2017-SGR-00333). E.M.-M. was supported by a contract from Complementary and Alternative Medicine (Comunidad Autónoma de Madrid) (Atracción de Talento). The authors declare no conflicts of interest.
Glossary
- BAT
brown adipose tissue
- CI
confidence interval
- CPT1A
carnitine palmitoyl transferase 1A
- CT
control diet
- Cyclo F
cyclophilin F
- DPP4
dipeptidylpeptidase 4
- EAT
epididymal adipose tissue
- ER
endoplasmic reticulum
- FA
fatty acid
- FAT
FA translocase
- FH
fumarate hydratase
- GIP
glucose-dependent insulinotropic polypeptide
- GLP-1
glucagon-like peptide 1
- GLUT 4
glucose transporter 4
- HFD
high-fat diet
- HNE
hydroxynonenal
- HOMA
homeostasis model assessment
- ID
inner diameter
- IRS-1
insulin receptor substrate 1
- LC
liquid chromatography
- MFN1
mitofusin 1
- mPTP
mitochondrial permeability transition pore
- MS
mass spectrometry
- MS/MS
tandem MS
- oxphos
oxidative phosphorylation
- PDIA6
protein disulfide isomerase A6
- PPAR-γ
proliferator-activated receptor-γ
- PRDX4
peroxiredoxin 4
- ROS
reactive oxygen species
- SOCS3
suppressor of cytokine signaling 3
- UCP1
uncoupling protein 1
AUTHOR CONTRIBUTIONS
G. Marín-Royo and A. Le Pape performed animal experiments and data analysis and helped to write the manuscript; R. Jurado-López and F. V. Souza-Neto performed animal experiments, circulating parameter measurements, and data analysis; C. Rodríguez and J. Martínez-González performed cell culture studies and data analysis and helped to write the manuscript; M. Luaces and A. Antequera performed clinical study and data analysis and helped to write the manuscript; M. L. Nieto contributed to measurement of circulating parameter and data analysis and helped to write the manuscript; and E. Martínez-Martínez and V. Cachofeiro designed the study, performed experiments and data analysis, and wrote the manuscript.
REFERENCES
- 1.Haczeyni F., Bell-Anderson K. S., Farrell G. C. (2018) Causes and mechanisms of adipocyte enlargement and adipose expansion. Obes. Rev. 19, 406–420 [DOI] [PubMed] [Google Scholar]
- 2.Goossens G. H. (2008) The role of adipose tissue dysfunction in the pathogenesis of obesity-related insulin resistance. Physiol. Behav. 94, 206–218 [DOI] [PubMed] [Google Scholar]
- 3.Guilherme A., Virbasius J. V., Puri V., Czech M. P. (2008) Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Biol. 9, 367–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yin X., Lanza I. R., Swain J. M., Sarr M. G., Nair K. S., Jensen M. D. (2014) Adipocyte mitochondrial function is reduced in human obesity independent of fat cell size. J. Clin. Endocrinol. Metab. 99, E209–E216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heinonen S., Buzkova J., Muniandy M., Kaksonen R., Ollikainen M., Ismail K., Hakkarainen A., Lundbom J., Lundbom N., Vuolteenaho K., Moilanen E., Kaprio J., Rissanen A., Suomalainen A., Pietiläinen K. H. (2015) Impaired mitochondrial biogenesis in adipose tissue in acquired obesity. Diabetes 64, 3135–3145 [DOI] [PubMed] [Google Scholar]
- 6.Rong J. X., Qiu Y., Hansen M. K., Zhu L., Zhang V., Xie M., Okamoto Y., Mattie M. D., Higashiyama H., Asano S., Strum J. C., Ryan T. E. (2007) Adipose mitochondrial biogenesis is suppressed in db/db and high-fat diet-fed mice and improved by rosiglitazone. Diabetes 56, 1751–1760 [DOI] [PubMed] [Google Scholar]
- 7.Wei Y. H., Lu C. Y., Lee H. C., Pang C. Y., Ma Y. S. (1998) Oxidative damage and mutation to mitochondrial DNA and age-dependent decline of mitochondrial respiratory function. Ann. N. Y. Acad. Sci. 854, 155–170 [DOI] [PubMed] [Google Scholar]
- 8.Bhatti J. S., Bhatti G. K., Reddy P. H. (2017) Mitochondrial dysfunction and oxidative stress in metabolic disorders - a step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta Mol. Basis Dis. 1863, 1066–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelso G. F., Porteous C. M., Coulter C. V., Hughes G., Porteous W. K., Ledgerwood E. C., Smith R. A., Murphy M. P. (2001) Selective targeting of a redox-active ubiquinone to mitochondria within cells: antioxidant and antiapoptotic properties. J. Biol. Chem. 276, 4588–4596 [DOI] [PubMed] [Google Scholar]
- 10.Feillet-Coudray C., Fouret G., Ebabe Elle R., Rieusset J., Bonafos B., Chabi B., Crouzier D., Zarkovic K., Zarkovic N., Ramos J., Badia E., Murphy M. P., Cristol J. P., Coudray C. (2014) The mitochondrial-targeted antioxidant MitoQ ameliorates metabolic syndrome features in obesogenic diet-fed rats better than Apocynin or Allopurinol. Free Radic. Res. 48, 1232–1246 [DOI] [PubMed] [Google Scholar]
- 11.Coudray C., Fouret G., Lambert K., Ferreri C., Rieusset J., Blachnio-Zabielska A., Lecomte J., Ebabe Elle R., Badia E., Murphy M. P., Feillet-Coudray C. (2016) A mitochondrial-targeted ubiquinone modulates muscle lipid profile and improves mitochondrial respiration in obesogenic diet-fed rats. Br. J. Nutr. 115, 1155–1166 [DOI] [PubMed] [Google Scholar]
- 12.Rivera-Barahona A., Alonso-Barroso E., Pérez B., Murphy M. P., Richard E., Desviat L. R. (2017) Treatment with antioxidants ameliorates oxidative damage in a mouse model of propionic acidemia. Mol. Genet. Metab. 122, 43–50 [DOI] [PubMed] [Google Scholar]
- 13.Miana M., Galán M., Martínez-Martínez E., Varona S., Jurado-López R., Bausa-Miranda B., Antequera A., Luaces M., Martínez-González J., Rodríguez C., Cachofeiro V. (2015) The lysyl oxidase inhibitor β-aminopropionitrile reduces body weight gain and improves the metabolic profile in diet-induced obesity in rats. Dis. Model. Mech. 8, 543–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kline K. G., Frewen B., Bristow M. R., Maccoss M. J., Wu C. C. (2008) High quality catalog of proteotypic peptides from human heart. J. Proteome Res. 7, 5055–5061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiśniewski J. R., Zougman A., Nagaraj N., Mann M. (2009) Universal sample preparation method for proteome analysis. Nat. Methods 6, 359–362 [DOI] [PubMed] [Google Scholar]
- 16.Kadowaki T., Yamauchi T., Kubota N., Hara K., Ueki K., Tobe K. (2006) Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Invest. 116, 1784–1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamers D., Famulla S., Wronkowitz N., Hartwig S., Lehr S., Ouwens D. M., Eckardt K., Kaufman J. M., Ryden M., Müller S., Hanisch F. G., Ruige J., Arner P., Sell H., Eckel J. (2011) Dipeptidyl peptidase 4 is a novel adipokine potentially linking obesity to the metabolic syndrome. Diabetes 60, 1917–1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palanivel R., Fullerton M. D., Galic S., Honeyman J., Hewitt K. A., Jorgensen S. B., Steinberg G. R. (2012) Reduced Socs3 expression in adipose tissue protects female mice against obesity-induced insulin resistance. Diabetologia 55, 3083–3093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeong E. M., Chung J., Liu H., Go Y., Gladstein S., Farzaneh-Far A., Lewandowski E. D., Dudley S. C., Jr (2016) Role of mitochondrial oxidative stress in glucose tolerance, insulin resistance, and cardiac diastolic dysfunction. J. Am. Heart Assoc. 5, e003046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elrayess M. A., Almuraikhy S., Kafienah W., Al-Menhali A., Al-Khelaifi F., Bashah M., Zarkovic K., Zarkovic N., Waeg G., Alsayrafi M., Jaganjac M. (2017) 4-hydroxynonenal causes impairment of human subcutaneous adipogenesis and induction of adipocyte insulin resistance. Free Radic. Biol. Med. 104, 129–137 [DOI] [PubMed] [Google Scholar]
- 21.Ibarrola J., Sádaba R., Garcia-Peña A., Arrieta V., Martinez-Martinez E., Alvarez V., Fernández-Celis A., Gainza A., Santamaría E., Fernández-Irigoyen J., Cachofeiro V., Fay R., Rossignol P., López-Andrés N. (2018) A role for fumarate hydratase in mediating oxidative effects of galectin-3 in human cardiac fibroblasts. Int. J. Cardiol. 258, 217–223 [DOI] [PubMed] [Google Scholar]
- 22.Tian Z., Liu Y., Usa K., Mladinov D., Fang Y., Ding X., Greene A. S., Cowley A. W., Jr., Liang M. (2009) Novel role of fumarate metabolism in dahl-salt sensitive hypertension. Hypertension 54, 255–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernández-Sánchez A., Madrigal-Santillán E., Bautista M., Esquivel-Soto J., Morales-González A., Esquivel-Chirino C., Durante-Montiel I., Sánchez-Rivera G., Valadez-Vega C., Morales-González J. A. (2011) Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 12, 3117–3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kavanagh K., Davis A. T., Peters D. E., LeGrand A. C., Bharadwaj M. S., Molina A. J. (2017) Regulators of mitochondrial quality control differ in subcutaneous fat of metabolically healthy and unhealthy obese monkeys. Obesity (Silver Spring) 25, 689–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tol M. J., Ottenhoff R., van Eijk M., Zelcer N., Aten J., Houten S. M., Geerts D., van Roomen C., Bierlaagh M. C., Scheij S., Hoeksema M. A., Aerts J. M., Bogan J. S., Dorn G. W., II, Argmann C. A., Verhoeven A. J. (2016) A PPARγ-Bnip3 axis couples adipose mitochondrial fusion-fission balance to systemic insulin sensitivity. Diabetes 65, 2591–2605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McQuaid S. E., Hodson L., Neville M. J., Dennis A. L., Cheeseman J., Humphreys S. M., Ruge T., Gilbert M., Fielding B. A., Frayn K. N., Karpe F. (2011) Downregulation of adipose tissue fatty acid trafficking in obesity: a driver for ectopic fat deposition? Diabetes 60, 47–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rovira-Llopis S., Bañuls C., Diaz-Morales N., Hernandez-Mijares A., Rocha M., Victor V. M. (2017) Mitochondrial dynamics in type 2 diabetes: pathophysiological implications. Redox Biol. 11, 637–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.López-Lluch G. (2017) Mitochondrial activity and dynamics changes regarding metabolism in ageing and obesity. Mech. Ageing Dev. 162, 108–121 [DOI] [PubMed] [Google Scholar]
- 29.Feng D., Tang Y., Kwon H., Zong H., Hawkins M., Kitsis R. N., Pessin J. E. (2011) High-fat diet-induced adipocyte cell death occurs through a cyclophilin D intrinsic signaling pathway independent of adipose tissue inflammation. Diabetes 60, 2134–2143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ly L. D., Xu S., Choi S. K., Ha C. M., Thoudam T., Cha S. K., Wiederkehr A., Wollheim C. B., Lee I. K., Park K. S. (2017) Oxidative stress and calcium dysregulation by palmitate in type 2 diabetes. Exp. Mol. Med. 49, e291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fink B. D., Herlein J. A., Guo D. F., Kulkarni C., Weidemann B. J., Yu L., Grobe J. L., Rahmouni K., Kerns R. J., Sivitz W. I. (2014) A mitochondrial-targeted coenzyme q analog prevents weight gain and ameliorates hepatic dysfunction in high-fat-fed mice. J. Pharmacol. Exp. Ther. 351, 699–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Imai Y., Fink B. D., Promes J. A., Kulkarni C. A., Kerns R. J., Sivitz W. I. (2018) Effect of a mitochondrial-targeted coenzyme Q analog on pancreatic β-cell function and energetics in high fat fed obese mice. Pharmacol. Res. Perspect. 6, e00393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bond S. T., Kim J., Calkin A. C., Drew B. G. (2019) The antioxidant moiety of MitoQ imparts minimal metabolic effects in adipose tissue of high fat fed mice. Front. Physiol. 10, 543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelly L. J., Vicario P. P., Thompson G. M., Candelore M. R., Doebber T. W., Ventre J., Wu M. S., Meurer R., Forrest M. J., Conner M. W., Cascieri M. A., Moller D. E. (1998) Peroxisome proliferator-activated receptors gamma and alpha mediate in vivo regulation of uncoupling protein (UCP-1, UCP-2, UCP-3) gene expression. Endocrinology 139, 4920–4927 [DOI] [PubMed] [Google Scholar]
- 35.Fukui Y., Masui S., Osada S., Umesono K., Motojima K. (2000) A new thiazolidinedione, NC-2100, which is a weak PPAR-gamma activator, exhibits potent antidiabetic effects and induces uncoupling protein 1 in white adipose tissue of KKAy obese mice. Diabetes 49, 759–767 [DOI] [PubMed] [Google Scholar]
- 36.Sell H., Berger J. P., Samson P., Castriota G., Lalonde J., Deshaies Y., Richard D. (2004) Peroxisome proliferator-activated receptor gamma agonism increases the capacity for sympathetically mediated thermogenesis in lean and ob/ob mice. Endocrinology 145, 3925–3934 [DOI] [PubMed] [Google Scholar]
- 37.Frasca D., Blomberg B. B., Paganelli R. (2017) Aging, obesity, and inflammatory age-related diseases. Front. Immunol. 8, 1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heilbronn L. K., Campbell L. V. (2008) Adipose tissue macrophages, low grade inflammation and insulin resistance in human obesity. Curr. Pharm. Des. 14, 1225–1230 [DOI] [PubMed] [Google Scholar]
- 39.Frühbeck G., Catalán V., Rodríguez A., Ramírez B., Becerril S., Salvador J., Portincasa P., Colina I., Gómez-Ambrosi J. (2017) Involvement of the leptin-adiponectin axis in inflammation and oxidative stress in the metabolic syndrome. Sci. Rep. 7, 6619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Monzo-Beltran L., Vazquez-Tarragón A., Cerdà C., Garcia-Perez P., Iradi A., Sánchez C., Climent B., Tormos C., Vázquez-Prado A., Girbés J., Estáñ N., Blesa S., Cortés R., Chaves F. J., Sáez G. T. (2017) One-year follow-up of clinical, metabolic and oxidative stress profile of morbid obese patients after laparoscopic sleeve gastrectomy. 8-oxo-dG as a clinical marker. Redox Biol. 12, 389–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zamora-Mendoza R., Rosas-Vargas H., Ramos-Cervantes M. T., Garcia-Zuniga P., Perez-Lorenzana H., Mendoza-Lorenzo P., Perez-Ortiz A. C., Estrada-Mena F. J., Miliar-Garcia A., Lara-Padilla E., Ceballos G., Rodriguez A., Villarreal F., Ramirez-Sanchez I. (2018) Dysregulation of mitochondrial function and biogenesis modulators in adipose tissue of obese children. Int. J. Obes. 42, 618–624 [DOI] [PubMed] [Google Scholar]