Abstract
The Apicomplexan parasite, Toxoplasma gondii, is an obligate intracellular organism that must co-opt its host cell to survive. To this end, Toxoplasma parasites introduce a suite of effector proteins from two secretory compartments called rhoptries and dense granules into the host cells. Once inside, these effectors extensively modify the host cell to facilitate parasite penetration, replication and persistence. In this review, we summarize the most recent advances in current understanding of effector translocation from Toxoplasma’s rhoptry and dense granule organelles into the host cell, with comparisons to Plasmodium spp. for broader context.
Introduction
The Apicomplexa are a phylum of intriguing, single-celled eukaryotes that all require invasion into another eukaryotic cell for growth and survival. This large, diverse group of parasites includes many that are important for human and animal health, but herein we focus on Toxoplasma gondii, a causative agent of potentially devastating disease in the developing human fetus and in immunocompromised human adults [1]. Toxoplasma gondii’s complex life cycle involves sexual development within its feline definitive host, as well as asexual development within another eukaryotic host cell [2], where individual parasites undergo several replicative cycles within their intracellular niche until the host cell lyses and releases multiple daughter parasites referred to as tachyzoites for their rapid growth (tachos means speed in Greek). The asexual arm of the lifecycle is remarkable for the parasites’ broad host range, which encompasses virtually any nucleated cell type from almost any warm-blooded animal, including humans. Therefore, Toxoplasma’s intracellular lifestyle necessitates communication with and co-opting of a vast array of host cell types, each of which is defined by its own species-and cell type-specific biology.
The best-studied Toxoplasma-host cell relationship is that between tachyzoites and nonphagocytic, fibroblastic mammalian cell lines. This relationship begins with tachyzoite invasion into the host cell, an active process mediated largely by the parasites themselves [3–5]. During invasion, the host cell’s plasma membrane is invaginated, ultimately completely encompassing the parasites within a parasitophorous vacuole (PV) within which the parasites divide (Fig. 1). Invasion is mediated by two sets of secretory organelles at the apical ends of the parasites – numerous small, rice-shaped micronemes and the less numerous but much larger, club-shaped rhoptries (rhoptry means club in Greek). The rhoptry proteins are introduced into the host cell during invasion and play an active role in invasion itself, as well as in the establishment of a replicative niche within the host cell and concomitant co-opting of host cell functions [5–7].
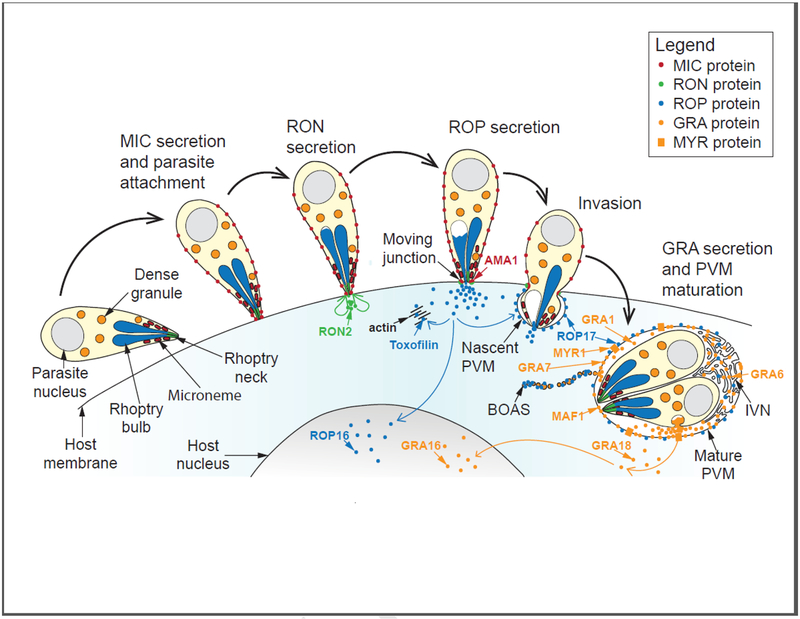
Figure 1:
An integrated model for effector protein secretion by Toxoplasma gondii tachyzoites into the host cell, illustrated in the context of Toxoplasma invasion. First, the micronemes secrete micronemal proteins (MICs) that are anchored in the parasite plasma membrane and mediate parasite attachment to the host cell (MIC secretion and parasite attachment). Upon strong parasite attachment to the host cell, the rhoptry organelle discharges in a manner simultaneous with parasite invasion, first introducing rhoptry neck proteins into the host cell (RON secretion) that go on to establish the moving junction, and subsequently rhoptry bulb proteins (ROP secretion) that either translocate to the host nucleus, associate with the nascent or fully formed parasitophorous vacuole membrane (PVM), or associate with cytosolic actin (i.e., toxofilin). Secretion of dense granule proteins (GRAs) into the host may also begin as early as during parasite invasion. Once the parasites have fully penetrated the host cell, PVM maturation occurs concomitantly with continued GRA secretion (GRA secretion and PVM maturation), and GRAs are either deposited into the PV space, PVM, or intravacuoloar network (IVN), or they are translocated across the PVM in a fashion dependent on several MYR proteins (orange rectangles), and end their journey in either the host cytosol or the host nucleus. Note that ROPs and GRAs are also found in structures called beads on a string (BOAS), vesicular-like entities of unknown function that may originate by blebbing off of the PVM. Selected parasite effector proteins that are examples of the different classes of proteins mentioned above are shown; i.e., the rhoptry proteins, RON2 (host plasma membrane), ROP16 (host nucleus), toxofilin (host cytosol), and ROP17 (PVM); and the dense granule proteins, GRA1 (PV space), GRA6 (IVN), GRA18 (host cytosol), GRA16 (host nucleus), GRA7 (PVM), and MAF1 and MYR1 (PVM).
During or soon after invasion, the parasites secrete a further set of secretory proteins from spherical secretory organelles called dense granules, which are found distributed throughout the parasites (Fig. 1, GRA Secretion and PVM Maturation). The transit of these proteins across the plasma membrane of the dividing parasites is thought to be relatively conventional for eukaryotic secretion. The intriguing and remarkable part of their journey involves the translocation of a subset of these proteins across the PV membrane (PVM), into the host cytosol [8] or nucleus [9*–14]; in both cases, the effect on the host cell is profound. In this review, we summarize our current understanding of how effector proteins from the rhoptries and dense granules of Toxoplasma gondii are introduced into the host cell, with particular emphasis on advances made in the last four years, and with comparison to what is known in Plasmodium where relevant.
Rhoptry Effector Translocation into Host Cells:
Final Destinations and Activities of Rhoptry Effectors:
Each rhoptry organelle contains an armament of effector proteins that collectively manipulate host cell biology. Each rhoptry organelle can be partitioned into a narrow, apical neck region, which houses rhoptry neck proteins (RONs), and a wide basal bulb region, which contains rhoptry bulb proteins (ROPs). At or around the time of invasion, the rhoptries introduce RONs and ROPs into the host cell, a key event for the parasites’ survival and virulence.
After rhoptry protein release, the RONs form an essential, highly conserved protein complex in the host cell membrane that serves as a receptor for the microneme-derived surface ligand, AMA1 [15–19]. When RON2, the transmembrane component of the complex, binds AMA1 [20,21], they and the other RONs form a tight junction called the moving junction, named for its movement down the length of the parasite as it invades into the host cell (Fig. 1). This structure is thought to grant the parasites purchase as they actively pull themselves into the host. In contrast to the RONs, ROPs do not appear to perform an essential role in invasion and are poorly conserved between Toxoplasma and Plasmodium (reviewed in [22]). In Toxoplasma, the ROPs include effectors that localize to various niches within the host cell once introduced. Some decorate the host-cytosolic side of the PVM, (various ROP2 family members [23]), where they can serve various functions including neutralizing host innate immune responses (e.g., ROP5/17/18 [24–27]). Others, such as toxofilin, remain cytosolic and can remodel the host’s cortical actin cytoskeleton [28–30], while still others reach the host nucleus, where they can impinge on the STAT activation pathway (i.e., ROP16 [31–33]) and other transcriptional processes (e.g., PP2C-hn [34]; see Fig. 1 for depictions of final destinations of ROPs). In addition to these known ROP effectors, genetic, proteomic, and bioinformatic screens have identified many additional putative ROP effectors, but the majority of these have yet to be validated as true rhoptry proteins. Note, however, that a few so-called “ROP” proteins, that were presumptively named such for their homology to the ROP2 family, have recently been demonstrated to actually be released predominantly, if not exclusively, from other secretory organelles such as the dense granules [35–37] and constitutive secretory vesicles [38].
Mechanism of Rhoptry Effector Secretion:
Despite the importance of RONs and ROPs to Toxoplasma invasion and growth, the triggers leading to their release and the mechanism of their introduction into a host cell are not yet clear. Not surprisingly, at least a part of the trigger involves contact with the host cell; e.g., host plasma membrane cholesterol appears to be required for rhoptry secretion in Toxoplasma tachyzoites [39]. Additional factors include the micronemal protein MIC8, which is required for RON4 secretion [40], and the calcium sensing protein, FER2 [41]. The exact roles for these proteins and pathways are not yet known but multiple calcium-dependent protein kinases have been identified in these parasites (reviewed in [42]), and one or more of these could play a key role in the signaling.
Regarding the mechanics and machinery of injection itself, ultrastructural studies depict shortening and emptying of the rhoptries over the course of a few seconds [43,44] (depiction in Fig. 1). It has also been demonstrated that only a minority of the rhoptries discharge at any one time [45]. With the exception of RON2, which somehow ends up spanning the host plasma membrane, all known rhoptry effectors are soluble proteins that must traverse three lipid bilayers (i.e., the rhoptry membrane, the parasite cell membrane, and the host cell membrane), an unusual and nontrivial topological problem seldom encountered in eukaryotic systems. The solution in prokaryotes is triple-membrane spanning complexes such as type III, type IV, and type VI secretion systems (reviewed in [46–48]); however, apicomplexans possess no apparent orthologs to these systems.
Early work using scanning EM revealed a rosette of small depressions on the apex of Toxoplasma and Sarcocystis tachyzoites [49], which was interpreted as possible fusion events between the rhoptry necks and the plasma membrane. However, subsequent ultrastructural studies of Toxoplasma tachyzoites and Plasmodium merozoites actively invading their respective host cells and imaged by transmission EM of ultrathin sections revealed a ~50 nm opening between the parasite and host cell membranes [43,50]. Although this suggested that a possible channel links at least the parasite and host cytosols, the rhoptry membrane near this possible channel in such images is indistinct, leaving open the question of whether there is a continuous path or channel that stretches all the way from the rhoptry lumen and across the parasite and host plasma membranes. Supporting such a model is the fact that rhoptry discharge is accompanied by a transient disruption in the host membrane [51], although the relationship of this to rhoptry protein injection has not been further investigated. Also in support of this model are freeze-fracture images of tachyzoites and merozoites in the midst of invasion which show a tantalizing indentation within the nascent PVM at the location of the extreme apical tip of the parasite [45,52]. It is tempting to speculate that this is a view of the rhoptry pore or channel from inside the host cell. As yet, however, no molecules within this structure have been identified and its precise function remains unknown, although in a more recent ultrastructural study of Toxoplasma tachyzoites, Paredes-Santos et al proposed that small vesicles located within the parasite’s apex are the source of the putative pore and therefore dubbed them “porosomes” [44]. Their small number (~4–6), size (~50 nm), and placement make this an appealing model but, as yet, there are no data to directly indicate such a role.
Dense Granule Effector Translocation:
Final Destinations and Activities of Dense Granule Effectors:
Following invasion and establishment of the nascent PV, Toxoplasma secretes dense granule proteins (GRAs) to modify its PV to create a hospitable niche for replication within the host cell. Several critical PV modifications include: establishment of the intravacuolar network (IVN), an elaborate network of membranous nanotubules whose lumens are topologically contiguous with the host cytosol and that appear to bud from the PVM into the PV space [53–55]; rearrangement of the host cytoskeleton and recruitment of host organelles to the PV (reviewed in [56]); establishment of PV pores to function as a molecular sieves and to scavenge essential nutrients from the host [57,58]; and modulation of the host response to optimize parasite growth and avoid host defenses (reviewed in [6]). These PV modifications are dependent on the secretion of GRAs that ultimately reside within the PV space, integral to or associated with the PVM, or fully within the host cell cytosol or nucleus.
GRAs are thought to be constitutively secreted from the parasite [59], although a large burst of secretion resembling classical exocytosis is observed shortly after invasion [60–63] and appears to be negatively regulated by calcium [64] (Fig 1, GRA Secretion and PVM Maturation). Interestingly, some GRAs can be observed within the host cell even prior to the formation of a PV, when parasite invasion is inhibited by the actin inhibitor cytochalasin D (e.g., GRA7 and GRA15 [65,66]). Under these conditions the GRA proteins are found near the site of parasite attachment localized to membranous structures termed evacuoles (because they are “empty” of parasites), which also include many of the rhoptry proteins described above. Although the genesis of evacuoles is thought to be associated with rhoptry discharge, the exact basis of their formation is not yet clear, and neither is the mechanism by which ROP and GRA effectors associate with them, or even the topology of the effectors associated with these structures [43,50,67,68]. An intriguing possibility is that they reflect the action of membrane-scavenging ROPs and GRAs acting at or near the site of invasion.
The ultimate destination of secreted GRAs varies; some localize to the PV lumen (e.g., GRA1 [53,69]), while others decorate the PVM (e.g., GRA17/23 [58] and MAF1 [70]) and/or the IVN (e.g., GRA2 and GRA6 [71]) (see Fig. 1 for illustration of final destinations of GRAs). Those exposed to the host cytosol can interact with various host proteins and processes (recently reviewed in [72]). One such example is a GRA that mediates the recruitment of host mitochondria to the PVM in some strains and was therefore dubbed MAF1 for mitochondrial association factor 1 [70,73]. Additionally, some GRAs can also be found in what appear to be vesicular-like, membranous extensions of the PV in the host cytosol. These structures have been dubbed BOAS because of their “beads-on-a-string”-like appearance (e.g., GRA3, GRA7, and GRA14 [65,74,75]) (depicted in Fig. 1). The function of BOAS is unknown, although they frequently appear to run between multiple PVs in the same host cell, or between a PV and the host cell nucleus [74,75].
Perhaps the most interesting set of GRA proteins are the ones that are somehow translocated as soluble proteins across the PVM and into the host cell cytosol or nucleus, where they can affect host transcription. Several such have been identified in the past few years, including GRA16, GRA24, GRA18, TgIST, GRA28, and HCE1/TEEGR [8–14]. Our current understanding of how these proteins are translocated across the PVM in Toxoplasma-infected cells is heavily influenced by work done in the more extensively studied Plasmodium falciparum (P. falciparum) blood stage parasites, which will be briefly summarized here (and was recently reviewed in [76]).
Role of Proteolytic Cleavage in Translocation of Dense Granule Effectors Beyond the PV
In P. falciparum, dense granule proteins exported into the red blood cell can be classified into two groups. The first includes proteins that contain a host-targeting/Plasmodium export element (HT/PEXEL) [77,78]. This motif, generally comprised of the amino acid sequence “RxLxE/Q/D,” is cleaved by an ER-resident aspartyl protease, plasmepsin V, which appears to license such proteins for export across the PVM [79,80]. The second group of exported proteins lack an HT/PEXEL, and are dubbed PEXEL-negative exported proteins, or PNEPs [81,82]. While not themselves processed by plasmepsin V, some PNEPs, such as PfEMP1, require several PEXEL-containing targets of plasmepsin V, as well as plasmepsin V activity, for proper trafficking through the erythrocyte to the red blood cell surface [83–85].
Toxoplasma also contains proteins with HT/PEXEL-like motifs, dubbed TEXELs for Toxoplasma export elements, which have the simpler amino acid consensus sequence of “RRL” [86*,87]. Of the proteins identified thus far that translocate beyond the Toxoplasma PVM, only GRA16, TgIST, and GRA18 contain TEXELs. The remaining are TEXEL negative exported proteins or TNEPs (reviewed in [88]). TEXEL-containing proteins are cleaved by the Toxoplasma homolog of plasmepsin V, ASP5, a Golgi-resident protease [86*,89*,90*]. In contrast to Plasmodium, however, Toxoplasma has several TEXEL-containing GRAs that are processed by ASP5, yet do not appear to be exported beyond the PVM [36,87,89*]. Therefore, unlike Plasmodium, the presence of a TEXEL does not necessarily license a protein for export beyond the PVM in Toxoplasma. An active ASP5, however, appears to be required for the export of all exported Toxoplasma GRAs studied so far, whether or not they contain a cleavable TEXEL motif [8,11,86*,89*,90*]. It is possible that this requirement for ASP5 relates to its role in processing a component of the translocation machinery through which all of the exported proteins pass, rather than the effectors themselves. Alternatively, export of TNEPs might rely on ASP5-dependent cleavage of other TEXEL-containing proteins that act as chaperones or translocation partners.
Machinery for Effector Translocation from the PV to the Host Cell
In Plasmodium, a complex known as the Plasmodium translocon of exported proteins, or PTEX, is responsible for the translocation of dense granule proteins into the host cell [91–94]. Recent structural data show that the core complex includes the proteins, HSP101, PTEX150, and EXP2, which associate to form a PV membrane-spanning translocon [95**]. HSP101 is a ClpB-like AAA+ ATPase which is thought to facilitate the translocation process, and EXP2 is the protein-conducting channel of the complex [91–93,95**,96].
Protein unfolding is a necessary step for export of effectors into the host cell in Plasmodium [97], and the requirement for this appears to hold true in Toxoplasma as well. This is based on the observation that fusing GRA16 to the highly folded and structured murine dihydrofolate reductase (DHFR) domain prevents translocation of the fusion, in addition to a non-engineered exported effector, GRA24, consistent with the GRA16-DHFR fusion “blocking” a protein translocon [90*,98**]. Also in support of this hypothesis, structural analysis of known exported GRA proteins predicts them to be highly disordered and thus akin to “unfolded” proteins [99,100]. Thus, unlike for Plasmodium, Toxoplasma GRAs may not require active unfolding due to their inherent lack of structure (further reviewed in [6]).
While homologs to some of the PTEX components exist in Toxoplasma, such as GRA17 and GRA23 (homologs of EXP2) and TgClpB1 and TgClpB2 (homologs of HSP101), they do not appear to play a role in protein translocation beyond the Toxoplasma PV [58,101]. Instead, GRA17 and GRA23 appear to form pores within the PV to facilitate small molecule/nutrient acquisition [58], as also recently described for EXP2 in Plasmodium [94]. It is tempting to speculate that nutrient acquisition may be the original, ancestral function of EXP2 in vacuolar-dwelling apicomplexans, and blood stage malaria parasites may have further adapted EXP2 to function in protein translocation [94]. Nevertheless, the data would currently indicate that the protein-translocation function of the PTEX machinery is not conserved across the phylum.
So, if the PTEX machinery is not serving this function in Toxoplasma, what is? The answer came from genetic screens for Toxoplasma mutants incapable of activating certain host processes that are dependent on translocated GRA effectors. This led to the identification of a set of parasite loci necessary for host c-Myc regulation and therefore dubbed “MYR” loci based on this phenotype. Four such loci have so far been identified, MYR1, MYR2, MYR3 [98**,102*] and recently, ROP17, which encodes a rhoptry protein kinase [25,103]. In the case of MYR1, it was shown that a majority of the host transcriptomic response to Toxoplasma infection in HFFs is lost in Δmyr1 mutants [104*], consistent with the observation that the translocation of all GRAs known to cross the PVM as soluble proteins and so far tested depends on MYR1 [8,13,14,98**,102*]. Interestingly, the processes mediated by the PVM-associated effectors MAF1 (mitochondrial association) and GRA15 (NF-KB activation) do not appear to be dependent on MYR1 [102*], suggesting that the proper localization of these proteins to the PVM occurs through a different mechanism [102*], perhaps the presence of some intrinsic, membrane-associating domain (both have strongly predicted transmembrane domains).
Little is known about the precise, mechanistic function of the MYR proteins and ROP17 in the translocation of effectors across the PVM. All four localize to the PVM and appear to be membrane-associated [25,98**,102*,103], with MYR1 and MYR3, at least, forming a stable complex [98**]. Interestingly, MYR1 is processed by ASP5 to yield stable N- and C-terminal fragments that are disulfide-bonded [98**]. This processing is not, however, necessary for protein export, as unprocessed, full length MYR1 harboring a mutated ASP5 cleavage site can still promote the translocation of the effector GRA24 to the host nucleus [104*]. This result, combined with the fact that MYR2 and MYR3 have no TEXEL site and are thus unlikely to be substrates for ASP5, suggests that if the requirement for an active ASP5 in moving GRAs across the PVM is due to ASP5’s cleavage of a component of the translocation machinery, it is likely a protein other than MYR1/2/3. Additionally, given that ROP17 is an established protein kinase [25], and its function in translocation depends on being catalytically active [103], it is very probable that ROP17 functions to phosphorylate some component of the translocation machinery. Definitive word will come when all the components of the Toxoplasma effector translocation machinery have been identified and tested for ASP5-dependent processing and ROP17-dependent phosphorylation.
The four known MYR proteins have somewhat conserved orthologs (35–57% amino acid identity) in the closely related genus Neospora caninum [98**,102*,103]. Neospora parasites have a different definitive host (canines) but display asexual growth that is almost indistinguishable from that of Toxoplasma tachyzoites within the cells of its intermediate mammalian hosts. Assuming these orthologs function in Neospora as they do in Toxoplasma, a possibility not yet tested, their divergence suggests some co-evolution with their GRA cargo. Indeed, of the known Toxoplasma GRAs, Neospora has clear orthologs for only GRA16 and GRA18, consistent with a rapid evolutionary pressure presumably related to their different life cycles and preferred hosts. Consistent with this, there are no apparent MYR homologues within the genomes of the more distantly related Sarcocystis or Plasmodium, suggesting a function specific to only a subset of the tissue-dwelling coccidia.
Conclusion
In this review, we have briefly summarized the current state of knowledge for how effector proteins are introduced into cells infected with Toxoplasma tachyzoites. The available data for rhoptry protein translocation are limited to phenomenological observations and/or tantalizing EM images, leaving the transit of rhoptry proteins from the parasite into the host cytosol an intriguing and wide-open question. Knowledge of the machinery involved in translocation of dense granule proteins beyond the PVM is further advanced; however, much work remains to determine the complete machinery, the structures of the component parts, the mechanism by which cargo proteins are recognized for export and how the entire process is regulated. It is perhaps surprising that the components of the machinery that moves proteins across the PVM has apparently diverged among the Apicomplexa but until we understand all the nuanced details of their operation, and the cargo they translocate, the reasons for this will remain a mystery.
Highlights.
Toxoplasma introduces ROP and GRA effectors into host cells to coopt host functions
ROP effector secretion occurs during invasion via an unknown mechanism
MYR1–3, ROP17, and ASP5 are required for transit of GRAs across Toxoplasma’s PVM
Plasmodium employs different machinery (PTEX) to transport GRAs across the PVM
Acknowledgments
The authors acknowledge input and discussions from members of the Boothroyd laboratory, and especially to Abel Ferrel and Michael Panas for their careful reading and editing of the manuscript. Work in our laboratory applicable to the scope of this review is supported by the National Institutes of Health (RO1 AI129529 and RO1 AI021423)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors declare no conflict of interest.
References and recommended reading
Papers of particular interest have been highlighted as:
* of special interest
** of outstanding interest
- 1.Hill D, Dubey JP: Toxoplasma gondii: transmission, diagnosis and prevention. Clin Microbiol Infect 2002, 8:634–640. [DOI] [PubMed] [Google Scholar]
- 2.Black MW, Boothroyd JC: Lytic cycle of Toxoplasma gondii. Microbiol Mol Biol Rev 2000, 64:607–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nichols BA, O’Connor GR: Penetration of mouse peritoneal macrophages by the protozoon Toxoplasma gondii. New evidence for active invasion and phagocytosis. Lab Invest 1981, 44:324–335. [PubMed] [Google Scholar]
- 4.Morisaki JH, Heuser JE, Sibley LD: Invasion of Toxoplasma gondii occurs by active penetration of the host cell. J Cell Sci 1995, 108:2457–2464. [DOI] [PubMed] [Google Scholar]
- 5.Kemp LE, Yamamoto M, Soldati-Favre D: Subversion of host cellular functions by the apicomplexan parasites. FEMS Microbiol Rev 2013, 37:607–631. [DOI] [PubMed] [Google Scholar]
- 6.Hakimi MA, Olias P, Sibley LD: Toxoplasma Effectors Targeting Host Signaling and Transcription. Clin Microbiol Rev 2017, 30:615–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Besteiro S, Dubremetz JF, Lebrun M: The moving junction of apicomplexan parasites: a key structure for invasion. Cell Microbiol 2011, 13:797–805. [DOI] [PubMed] [Google Scholar]
- 8.He H, Brenier-Pinchart MP, Braun L, Kraut A, Touquet B, Coute Y, Tardieux I, Hakimi MA, Bougdour A: Characterization of a Toxoplasma effector uncovers an alternative GSK3/beta-catenin-regulatory pathway of inflammation. Elife 2018, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9 *.Bougdour A, Durandau E, Brenier-Pinchart MP, Ortet P, Barakat M, Kieffer S, Curt-Varesano A, Curt-Bertini RL, Bastien O, Coute Y, et al. : Host cell subversion by Toxoplasma GRA16, an exported dense granule protein that targets the host cell nucleus and alters gene expression. Cell Host Microbe 2013, 13:489–500. [DOI] [PubMed] [Google Scholar]; This paper identifies the first Toxoplasma dense granule protein, GRA16, to be exported beyond the PV into the host nucleus.
- 10.Braun L, Brenier-Pinchart MP, Yogavel M, Curt-Varesano A, Curt-Bertini RL, Hussain T, Kieffer-Jaquinod S, Coute Y, Pelloux H, Tardieux I, et al. : A Toxoplasma dense granule protein, GRA24, modulates the early immune response to infection by promoting a direct and sustained host p38 MAPK activation. J Exp Med 2013, 210:2071–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gay G, Braun L, Brenier-Pinchart MP, Vollaire J, Josserand V, Bertini RL, Varesano A, Touquet B, De Bock PJ, Coute Y, et al. : Toxoplasma gondii TgIST co-opts host chromatin repressors dampening STAT1-dependent gene regulation and IFN-gamma-mediated host defenses. J Exp Med 2016, 213:1779–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nadipuram SM, Kim EW, Vashisht AA, Lin AH, Bell HN, Coppens I, Wohlschlegel JA, Bradley PJ: In Vivo Biotinylation of the Toxoplasma Parasitophorous Vacuole Reveals Novel Dense Granule Proteins Important for Parasite Growth and Pathogenesis. MBio 2016, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Panas MW, Naor A, Cygan AM, Boothroyd JC: Toxoplasma Controls Host Cyclin E Expression through the Use of a Novel MYR1-Dependent Effector Protein, HCE1. MBio 2019, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braun L, Brenier-Pinchart MP, Hammoudi PM, Cannella D, Kieffer-Jaquinod S, Vollaire J, Josserand V, Touquet B, Coute Y, Tardieux I, et al. : The Toxoplasma effector TEEGR promotes parasite persistence by modulating NF-kappaB signalling via EZH2. Nat Microbiol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alexander DL, Mital J, Ward GE, Bradley P, Boothroyd JC: Identification of the Moving Junction Complex of Toxoplasma gondii: A Collaboration between Distinct Secretory Organelles. PLoS Pathog 2005, 1:e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lebrun M, Michelin A, El Hajj H, Poncet J, Bradley PJ, Vial H, Dubremetz JF: The rhoptry neck protein RON4 re-localizes at the moving junction during Toxoplasma gondii invasion. Cell Microbiol 2005, 7:1823–1833. [DOI] [PubMed] [Google Scholar]
- 17.Alexander DL, Arastu-Kapur S, Dubremetz JF, Boothroyd JC: Plasmodium falciparum AMA1 binds a rhoptry neck protein homologous to TgRON4, a component of the moving junction in Toxoplasma gondii. Eukaryot Cell 2006, 5:1169–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Straub KW, Cheng SJ, Sohn CS, Bradley PJ: Novel components of the Apicomplexan moving junction reveal conserved and coccidia-restricted elements. Cell Microbiol 2009, 11:590–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guerin A, El Hajj H, Penarete-Vargas D, Besteiro S, Lebrun M: RON4L1 is a new member of the moving junction complex in Toxoplasma gondii. Sci Rep 2017, 7:17907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tyler JS, Boothroyd JC: The C-terminus of Toxoplasma RON2 provides the crucial link between AMA1 and the host-associated invasion complex. PLoS Pathog 2011, 7:e1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lamarque M, Besteiro S, Papoin J, Roques M, Vulliez-Le Normand B, Morlon-Guyot J, Dubremetz JF, Fauquenoy S, Tomavo S, Faber BW, et al. : The RON2-AMA1 interaction is a critical step in moving junction-dependent invasion by apicomplexan parasites. PLoS Pathog 2011, 7:e1001276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boothroyd JC, Dubremetz JF: Kiss and spit: the dual roles of Toxoplasma rhoptries. Nat Rev Microbiol 2008, 6:79–88. [DOI] [PubMed] [Google Scholar]
- 23.Beckers CJ, Dubremetz JF, Mercereau-Puijalon O, Joiner KA: The Toxoplasma gondii rhoptry protein ROP 2 is inserted into the parasitophorous vacuole membrane, surrounding the intracellular parasite, and is exposed to the host cell cytoplasm. J Cell Biol 1994, 127:947–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fleckenstein MC, Reese ML, Konen-Waisman S, Boothroyd JC, Howard JC, Steinfeldt T: A Toxoplasma gondii pseudokinase inhibits host IRG resistance proteins. PLoS Biol 2012, 10:e1001358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Etheridge RD, Alaganan A, Tang K, Lou HJ, Turk BE, Sibley LD: The Toxoplasma pseudokinase ROP5 forms complexes with ROP18 and ROP17 kinases that synergize to control acute virulence in mice. Cell Host Microbe 2014, 15:537–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reese ML, Shah N, Boothroyd JC: The Toxoplasma Pseudokinase ROP5 Is an Allosteric Inhibitor of the Immunity-related GTPases. J Biol Chem 2014, 289:27849–27858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li JX, He JJ, Elsheikha HM, Chen D, Zhai BT, Zhu XQ, Yan HK: Toxoplasma gondii ROP17 inhibits the innate immune response of HEK293T cells to promote its survival. Parasitol Res 2019, 118:783–792. [DOI] [PubMed] [Google Scholar]
- 28.Poupel O, Boleti H, Axisa S, Couture-Tosi E, Tardieux I: Toxofilin, a novel actin-binding protein from Toxoplasma gondii, sequesters actin monomers and caps actin filaments. Mol Biol Cell 2000, 11:355–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lodoen MB, Gerke C, Boothroyd JC: A highly sensitive FRET-based approach reveals secretion of the actin-binding protein toxofilin during Toxoplasma gondii infection. Cell Microbiol 2010, 12:55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delorme-Walker V, Abrivard M, Lagal V, Anderson K, Perazzi A, Gonzalez V, Page C, Chauvet J, Ochoa W, Volkmann N, et al. : Toxofilin upregulates the host cortical actin cytoskeleton dynamics, facilitating Toxoplasma invasion. J Cell Sci 2012, 125:4333–4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saeij JP, Coller S, Boyle JP, Jerome ME, White MW, Boothroyd JC: Toxoplasma co-opts host gene expression by injection of a polymorphic kinase homologue. Nature 2007, 445:324–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamamoto M, Standley DM, Takashima S, Saiga H, Okuyama M, Kayama H, Kubo E, Ito H, Takaura M, Matsuda T, et al. : A single polymorphic amino acid on Toxoplasma gondii kinase ROP16 determines the direct and strain-specific activation of Stat3. J Exp Med 2009, 206:2747–2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ong YC, Reese ML, Boothroyd JC: Toxoplasma rhoptry protein 16 (ROP16) subverts host function by direct tyrosine phosphorylation of STAT6. J Biol Chem 2010, 285:28731–28740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gilbert LA, Ravindran S, Turetzky JM, Boothroyd JC, Bradley PJ: Toxoplasma gondii targets a protein phosphatase 2C to the nuclei of infected host cells. Eukaryot Cell 2007, 6:73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peixoto L, Chen F, Harb OS, Davis PH, Beiting DP, Brownback CS, Ouloguem D, Roos DS: Integrative genomic approaches highlight a family of parasite-specific kinases that regulate host responses. Cell Host Microbe 2010, 8:208–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coffey MJ, Dagley LF, Seizova S, Kapp EA, Infusini G, Roos DS, Boddey JA, Webb AI, Tonkin CJ: Aspartyl Protease 5 Matures Dense Granule Proteins That Reside at the Host-Parasite Interface in Toxoplasma gondii. MBio 2018, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beraki T, Hu X, Broncel M, Young JC, O’Shaughnessy WJ, Borek D, Treeck M, Reese ML: Divergent kinase regulates membrane ultrastructure of the Toxoplasma parasitophorous vacuole. Proc Natl Acad Sci U S A 2019, 116:6361–6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones NG, Wang Q, Sibley LD: Secreted protein kinases regulate cyst burden during chronic toxoplasmosis. Cell Microbiol 2017, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coppens I, Joiner KA: Host but not parasite cholesterol controls Toxoplasma cell entry by modulating organelle discharge. Mol Biol Cell 2003, 14:3804–3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kessler H, Herm-Gotz A, Hegge S, Rauch M, Soldati-Favre D, Frischknecht F, Meissner M: Microneme protein 8--a new essential invasion factor in Toxoplasma gondii. J Cell Sci 2008, 121:947–956. [DOI] [PubMed] [Google Scholar]
- 41.Coleman BI, Saha S, Sato S, Engelberg K, Ferguson DJP, Coppens I, Lodoen MB, Gubbels MJ: A Member of the Ferlin Calcium Sensor Family Is Essential for Toxoplasma gondii Rhoptry Secretion. MBio 2018, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Billker O, Lourido S, Sibley LD: Calcium-dependent signaling and kinases in apicomplexan parasites. Cell Host Microbe 2009, 5:612–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nichols BA, Chiappino ML, O’Connor GR: Secretion from the rhoptries of Toxoplasma gondii during host-cell invasion. J Ultrastruct Res 1983, 83:85–98. [DOI] [PubMed] [Google Scholar]
- 44.Paredes-Santos TC, de Souza W, Attias M: Dynamics and 3D organization of secretory organelles of Toxoplasma gondii. J Struct Biol 2012, 177:420–430. [DOI] [PubMed] [Google Scholar]
- 45.Dubremetz JF: Rhoptries are major players in Toxoplasma gondii invasion and host cell interaction. Cell Microbiol 2007, 9:841–848. [DOI] [PubMed] [Google Scholar]
- 46.Galan JE, Lara-Tejero M, Marlovits TC, Wagner S: Bacterial type III secretion systems: specialized nanomachines for protein delivery into target cells. Annu Rev Microbiol 2014, 68:415–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alteri CJ, Mobley HL: The Versatile Type VI Secretion System. Microbiol Spectr 2016, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grohmann E, Christie PJ, Waksman G, Backert S: Type IV secretion in Gram-negative and Gram-positive bacteria. Mol Microbiol 2018, 107:455–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Porchet E, Torpier G: [Freeze fracture study of Toxoplasma and Sarcocystis infective stages (author’s transl)]. Z Parasitenkd 1977, 54:101–124. [DOI] [PubMed] [Google Scholar]
- 50.Miller LH, Aikawa M, Johnson JG, Shiroishi T: Interaction between cytochalasin B-treated malarial parasites and erythrocytes. Attachment and junction formation. J Exp Med 1979, 149:172–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suss-Toby E, Zimmerberg J, Ward GE: Toxoplasma invasion: the parasitophorous vacuole is formed from host cell plasma membrane and pinches off via a fission pore. Proc Natl Acad Sci U S A 1996, 93:8413–8418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aikawa M, Miller LH, Rabbege JR, Epstein N: Freeze-fracture study on the erythrocyte membrane during malarial parasite invasion. J Cell Biol 1981, 91:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sibley LD, Niesman IR, Parmley SF, Cesbron-Delauw MF: Regulated secretion of multi-lamellar vesicles leads to formation of a tubulo-vesicular network in host-cell vacuoles occupied by Toxoplasma gondii. J Cell Sci 1995, 108:1669–1677. [DOI] [PubMed] [Google Scholar]
- 54.Magno RC, Lemgruber L, Vommaro RC, De Souza W, Attias M: Intravacuolar network may act as a mechanical support for Toxoplasma gondii inside the parasitophorous vacuole. Microsc Res Tech 2005, 67:45–52. [DOI] [PubMed] [Google Scholar]
- 55.Reese ML, Boothroyd JC: A helical membrane-binding domain targets the Toxoplasma ROP2 family to the parasitophorous vacuole. Traffic 2009, 10:1458–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coppens I, Romano JD: Hostile intruder: Toxoplasma holds host organelles captive. PLoS Pathog 2018, 14:e1006893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schwab JC, Beckers CJ, Joiner KA: The parasitophorous vacuole membrane surrounding intracellular Toxoplasma gondii functions as a molecular sieve. Proc Natl Acad Sci U S A 1994, 91:509–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gold DA, Kaplan AD, Lis A, Bett GC, Rosowski EE, Cirelli KM, Bougdour A, Sidik SM, Beck JR, Lourido S, et al. : The Toxoplasma Dense Granule Proteins GRA17 and GRA23 Mediate the Movement of Small Molecules between the Host and the Parasitophorous Vacuole. Cell Host Microbe 2015, 17:642–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chaturvedi S, Qi H, Coleman D, Rodriguez A, Hanson PI, Striepen B, Roos DS, Joiner KA: Constitutive calcium-independent release of Toxoplasma gondii dense granules occurs through the NSF/SNAP/SNARE/Rab machinery. J Biol Chem 1999, 274:2424–2431. [DOI] [PubMed] [Google Scholar]
- 60.Leriche MA, Dubremetz JF: Exocytosis of Toxoplasma gondii dense granules into the parasitophorous vacuole after host cell invasion. Parasitol Res 1990, 76:559–562. [DOI] [PubMed] [Google Scholar]
- 61.Dubremetz JF, Achbarou A, Bermudes D, Joiner KA: Kinetics and pattern of organelle exocytosis during Toxoplasma gondii-host-cell interaction. Parasitol. Res 1993, 79:402–408. [DOI] [PubMed] [Google Scholar]
- 62.Carruthers VB, Sibley LD: Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur J Cell Biol 1997, 73:114–123. [PubMed] [Google Scholar]
- 63.Coppens I, Andries M, Liu JL, Cesbron-Delauw MF: Intracellular trafficking of dense granule proteins in Toxoplasma gondii and experimental evidences for a regulated exocytosis. Eur J Cell Biol 1999, 78:463–472. [DOI] [PubMed] [Google Scholar]
- 64.Katris NJ, Ke H, McFadden GI, van Dooren GG, Waller RF: Calcium negatively regulates secretion from dense granules in Toxoplasma gondii. Cell Microbiol 2019:e13011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dunn JD, Ravindran S, Kim SK, Boothroyd JC: The Toxoplasma gondii dense granule protein GRA7 is phosphorylated upon invasion and forms an unexpected association with the rhoptry proteins ROP2 and ROP4. Infect Immun 2008, 76:5853–5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rosowski EE, Lu D, Julien L, Rodda L, Gaiser RA, Jensen KD, Saeij JP: Strain-specific activation of the NF-kappaB pathway by GRA15, a novel Toxoplasma gondii dense granule protein. J Exp Med 2011, 208:195–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hakansson S, Charron AJ, Sibley LD: Toxoplasma evacuoles: a two-step process of secretion and fusion forms the parasitophorous vacuole. Embo J 2001, 20:3132–3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tahara M, Andrabi SB, Matsubara R, Aonuma H, Nagamune K: A host cell membrane microdomain is a critical factor for organelle discharge by Toxoplasma gondii. Parasitol Int 2016, 65:378–388. [DOI] [PubMed] [Google Scholar]
- 69.Cesbron-Delauw MF, Guy B, Torpier G, Pierce RJ, Lenzen G, Cesbron JY, Charif H, Lepage P, Darcy F, Lecocq JP, et al. : Molecular characterization of a 23-kilodalton major antigen secreted by Toxoplasma gondii. Proc Natl Acad Sci U S A 1989, 86:7537–7541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pernas L, Adomako-Ankomah Y, Shastri AJ, Ewald SE, Treeck M, Boyle JP, Boothroyd JC: Toxoplasma effector MAF1 mediates recruitment of host mitochondria and impacts the host response. PLoS Biol 2014, 12:e1001845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mercier C, Dubremetz JF, Rauscher B, Lecordier L, Sibley LD, Cesbron-Delauw MF: Biogenesis of nanotubular network in Toxoplasma parasitophorous vacuole induced by parasite proteins. Mol Biol Cell 2002, 13:2397–2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Clough B, Frickel EM: The Toxoplasma Parasitophorous Vacuole: An Evolving Host-Parasite Frontier. Trends Parasitol 2017, 33:473–488. [DOI] [PubMed] [Google Scholar]
- 73.Adomako-Ankomah Y, English ED, Danielson JJ, Pernas LF, Parker ML, Boulanger MJ, Dubey JP, Boyle JP: Host Mitochondrial Association Evolved in the Human Parasite Toxoplasma gondii via Neofunctionalization of a Gene Duplicate. Genetics 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rome ME, Beck JR, Turetzky JM, Webster P, Bradley PJ: Intervacuolar transport and unique topology of GRA14, a novel dense granule protein in Toxoplasma gondii. Infect Immun 2008, 76:4865–4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dubremetz JF, Achbarou A, Bermudes D, Joiner KA: Kinetics and pattern of organelle exocytosis during Toxoplasma gondii/host-cell interaction. Parasitol Res 1993, 79:402–408. [DOI] [PubMed] [Google Scholar]
- 76.Matthews KM, Pitman EL, de Koning-Ward TF: Illuminating how malaria parasites export proteins into host erythrocytes. Cell Microbiol 2019, 21:e13009. [DOI] [PubMed] [Google Scholar]
- 77.Hiller NL, Bhattacharjee S, van Ooij C, Liolios K, Harrison T, Lopez-Estrano C, Haldar K: A host-targeting signal in virulence proteins reveals a secretome in malarial infection. Science 2004, 306:1934–1937. [DOI] [PubMed] [Google Scholar]
- 78.Marti M, Good RT, Rug M, Knuepfer E, Cowman AF: Targeting malaria virulence and remodeling proteins to the host erythrocyte. Science 2004, 306:1930–1933. [DOI] [PubMed] [Google Scholar]
- 79.Russo I, Babbitt S, Muralidharan V, Butler T, Oksman A, Goldberg DE: Plasmepsin V licenses Plasmodium proteins for export into the host erythrocyte. Nature 2010, 463:632–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Boddey JA, Hodder AN, Gunther S, Gilson PR, Patsiouras H, Kapp EA, Pearce JA, de Koning-Ward TF, Simpson RJ, Crabb BS, et al. : An aspartyl protease directs malaria effector proteins to the host cell. Nature 2010, 463:627–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Heiber A, Kruse F, Pick C, Gruring C, Flemming S, Oberli A, Schoeler H, Retzlaff S, Mesen-Ramirez P, Hiss JA, et al. : Identification of new PNEPs indicates a substantial non-PEXEL exportome and underpins common features in Plasmodium falciparum protein export. PLoS Pathog 2013, 9:e1003546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Spielmann T, Hawthorne PL, Dixon MW, Hannemann M, Klotz K, Kemp DJ, Klonis N, Tilley L, Trenholme KR, Gardiner DL: A cluster of ring stage-specific genes linked to a locus implicated in cytoadherence in Plasmodium falciparum codes for PEXEL-negative and PEXEL-positive proteins exported into the host cell. Mol Biol Cell 2006, 17:3613–3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Boddey JA, Carvalho TG, Hodder AN, Sargeant TJ, Sleebs BE, Marapana D, Lopaticki S, Nebl T, Cowman AF: Role of plasmepsin V in export of diverse protein families from the Plasmodium falciparum exportome. Traffic 2013, 14:532–550. [DOI] [PubMed] [Google Scholar]
- 84.Maier AG, Rug M, O’Neill MT, Brown M, Chakravorty S, Szestak T, Chesson J, Wu Y, Hughes K, Coppel RL, et al. : Exported proteins required for virulence and rigidity of Plasmodium falciparum-infected human erythrocytes. Cell 2008, 134:48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sleebs BE, Lopaticki S, Marapana DS, O’Neill MT, Rajasekaran P, Gazdik M, Gunther S, Whitehead LW, Lowes KN, Barfod L, et al. : Inhibition of Plasmepsin V activity demonstrates its essential role in protein export, PfEMP1 display, and survival of malaria parasites. PLoS Biol 2014, 12:e1001897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86 *.Coffey MJ, Sleebs BE, Uboldi AD, Garnham A, Franco M, Marino ND, Panas MW, Ferguson DJ, Enciso M, O’Neill MT, et al. : An aspartyl protease defines a novel pathway for export of Toxoplasma proteins into the host cell. Elife 2015, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper identifies ASP5 as the Toxoplasma ortholog of plasmepsin V and demonstrates that it is essential for the translocation of GRA effectors beyond the PVM. The ASP5 cleaveage consensus sequence (TEXEL) is identified as “RRL.”
- 87.Hsiao CH, Luisa Hiller N, Haldar K, Knoll LJ: A HT/PEXEL motif in Toxoplasma dense granule proteins is a signal for protein cleavage but not export into the host cell. Traffic 2013, 14:519–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Coffey MJ, Jennison C, Tonkin CJ, Boddey JA: Role of the ER and Golgi in protein export by Apicomplexa. Curr Opin Cell Biol 2016, 41:18–24. [DOI] [PubMed] [Google Scholar]
- 89 *.Hammoudi PM, Jacot D, Mueller C, Di Cristina M, Dogga SK, Marq JB, Romano J, Tosetti N, Dubrot J, Emre Y, et al. : Fundamental Roles of the Golgi-Associated Toxoplasma Aspartyl Protease, ASP5, at the Host-Parasite Interface. PLoS Pathog 2015, 11:e1005211. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper identifes ASP5 as the Toxoplamsa orthologue of plasmepsin V and demonstrates that it is essential for the translocation of GRA effectors beyond the PVM.
- 90 *.Curt-Varesano A, Braun L, Ranquet C, Hakimi MA, Bougdour A: The aspartyl protease TgASP5 mediates the export of the Toxoplasma GRA16 and GRA24 effectors into host cells. Cell Microbiol 2016, 18:151–167. [DOI] [PubMed] [Google Scholar]; This paper identifies ASP5 as the Toxoplasma ortholog of plasmepsin V and demonstrates that it is essential for the translocation of GRA effectors beyond the PVM. Protein unfolding is suggested to be a prerequisite for GRA translocation across the PVM.
- 91.de Koning-Ward TF, Gilson PR, Boddey JA, Rug M, Smith BJ, Papenfuss AT, Sanders PR, Lundie RJ, Maier AG, Cowman AF, et al. : A newly discovered protein export machine in malaria parasites. Nature 2009, 459:945–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Elsworth B, Matthews K, Nie CQ, Kalanon M, Charnaud SC, Sanders PR, Chisholm SA, Counihan NA, Shaw PJ, Pino P, et al. : PTEX is an essential nexus for protein export in malaria parasites. Nature 2014, 511:587–591. [DOI] [PubMed] [Google Scholar]
- 93.Beck JR, Muralidharan V, Oksman A, Goldberg DE: PTEX component HSP101 mediates export of diverse malaria effectors into host erythrocytes. Nature 2014, 511:592–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Garten M, Nasamu AS, Niles JC, Zimmerberg J, Goldberg DE, Beck JR: EXP2 is a nutrient-permeable channel in the vacuolar membrane of Plasmodium and is essential for protein export via PTEX. Nat Microbiol 2018, 3:1090–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95 **.Ho CM, Beck JR, Lai M, Cui Y, Goldberg DE, Egea PF, Zhou ZH: Malaria parasite translocon structure and mechanism of effector export. Nature 2018, 561:70–75. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper provides structural validation that PTEX is a bona fide PVM-spanning translocon in Plasmodium falciparum.
- 96.Bullen HE, Charnaud SC, Kalanon M, Riglar DT, Dekiwadia C, Kangwanrangsan N, Torii M, Tsuboi T, Baum J, Ralph SA, et al. : Biosynthesis, localization, and macromolecular arrangement of the Plasmodium falciparum translocon of exported proteins (PTEX). J Biol Chem 2012, 287:7871–7884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gehde N, Hinrichs C, Montilla I, Charpian S, Lingelbach K, Przyborski JM: Protein unfolding is an essential requirement for transport across the parasitophorous vacuolar membrane of Plasmodium falciparum. Mol Microbiol 2009, 71:613–628. [DOI] [PubMed] [Google Scholar]
- 98 **.Marino ND, Panas MW, Franco M, Theisen TC, Naor A, Rastogi S, Buchholz KR, Lorenzi HA, Boothroyd JC: Identification of a novel protein complex essential for effector translocation across the parasitophorous vacuole membrane of Toxoplasma gondii. PLoS Pathog 2018, 14:e1006828. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this paper, MYR2 and MYR3 are identified as two additional proteins necessary for the export of effector proteins beyond the PV in Toxoplasma. This work provides further evidence supporting that protein unfolding is necessary for translocation of GRAs beyond the PVM.
- 99.Hakimi MA, Bougdour A: Toxoplasma’s ways of manipulating the host transcriptome via secreted effectors. Curr Opin Microbiol 2015, 26:24–31. [DOI] [PubMed] [Google Scholar]
- 100.Pellegrini E, Palencia A, Braun L, Kapp U, Bougdour A, Belrhali H, Bowler MW, Hakimi MA: Structural Basis for the Subversion of MAP Kinase Signaling by an Intrinsically Disordered Parasite Secreted Agonist. Structure 2017, 25:16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cao S, Du N, Chen H, Pang Y, Zhang Z, Zheng J, Jia H: Toxoplasma gondii Clp family protein: TgClpB1 plays a crucial role in thermotolerance. Oncotarget 2017, 8:86117–86129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102 *.Franco M, Panas MW, Marino ND, Lee MC, Buchholz KR, Kelly FD, Bednarski JJ, Sleckman BP, Pourmand N, Boothroyd JC: A Novel Secreted Protein, MYR1, Is Central to Toxoplasma’s Manipulation of Host Cells. MBio 2016, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper identifies the first PVM protein, MYR1, necessary for the export of GRA effector proteins beyond the PVM in Toxoplasma.
- 103.Michael W Panas AF, Naor Adit, Tenborg Elizabeth, Lorenzi Hernan A., Boothroyd John C.: Translocation of dense granule effectors across the parasitophorous vacuole membrane in Toxoplasma-infected cells requires the activity of ROP17, a rhoptry protein kinase. bioRxiv 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104 *.Naor A, Panas MW, Marino N, Coffey MJ, Tonkin CJ, Boothroyd JC: MYR1-Dependent Effectors Are the Major Drivers of a Host Cell’s Early Response to Toxoplasma, Including Counteracting MYR1-Independent Effects. MBio 2018, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this paper, a functional MYR1 is found to be required for the majority of the host transcriptomic response to Toxoplasma infection. Cleavage of MYR1 by ASP5 is not required for export of effectors beyond the PV.