Abstract
Antimicrobial resistance (AMR) has developed as one of the major urgent threats to public health causing serious issues to successful prevention and treatment of persistent diseases. In spite of different actions taken in recent decades to tackle this issue, the trends of global AMR demonstrate no signs of slowing down. Misusing and overusing different antibacterial agents in the health care setting as well as in the agricultural industry are considered the major reasons behind the emergence of antimicrobial resistance. In addition, the spontaneous evolution, mutation of bacteria, and passing the resistant genes through horizontal gene transfer are significant contributors to antimicrobial resistance. Many studies have demonstrated the disastrous financial consequences of AMR including extremely high healthcare costs due to an increase in hospital admissions and drug usage. The literature review, which included articles published after the year 2012, was performed using Scopus, PubMed and Google Scholar with the utilization of keyword searches. Results indicated that the multifactorial threat of antimicrobial resistance has resulted in different complex issues affecting countries across the globe. These impacts found in the sources are categorized into three different levels: patient, healthcare, and economic. Although gaps in knowledge about AMR and areas for improvement are obvious, there is not any clearly understood progress to put an end to the persistent trends of antimicrobial resistance.
Keywords: antimicrobial resistance, AMR, implications, cost
Background
Antimicrobial Resistance (AMR) occurs when microorganisms including bacteria, viruses, fungi, and parasites become able to adapt and grow in the presence of medications that once impacted them.1,2 AMR is considered a significant threat to the public health systems not just in developing countries but throughout the world.1,3 The fact that infectious diseases can no longer be treated with antibiotics depicts an unknown future in health care.4 Infection with AMR leads to serious illnesses and prolonged hospital admissions, increases in healthcare costs, higher costs in second-line drugs, and treatment failures.3,5,6 For instance, just in Europe, it has been estimated that antimicrobial resistance has been correlated with more than nine billion euros per year.3,7 Furthermore, according to the Centers for Disease Control and Prevention (CDC), antimicrobial resistance adds a 20 billion dollar surplus in direct healthcare costs in the United States, which is exclusive of about 35 billion dollars in loss of productivity annually.8
The daunting threat of antimicrobial resistance is of particular importance in the category of antibiotic resistance in bacteria.3 According to the CDC, more than two million people in the United States become ill with antibiotic-resistant diseases every year, resulting in a minimum of 23,000 deaths.8 Antibiotic resistance compromises a human immune system’s capacity to fight infectious diseases and also contributes to different complications in vulnerable patients undergoing chemotherapy, dialysis, surgery, and joint replacement.8 Furthermore, people with chronic conditions like diabetes, asthma, and rheumatoid arthritis will be heavily impacted by antibiotic resistance.2 Since the effectiveness of antibiotics will be reduced due to persistence in trends of AMR, physicians should use last-resort classes of medicine such as carbapenems and polymyxins, which are not necessarily readily available in developing countries, have a high cost, and have many different side-effects.9
One of the most well-known cases of AMR, Methicillin resistance in Staphylococcus aureus (MRSA), has been associated with high mortality rates every year across the globe.1 In addition, multi-drug resistant gram-negative bacteria (MDR-GNB) has made the treatment of different infections like pneumonia and urinary tract infections more challenging.10–12 Furthermore, drug resistance to tuberculosis, gonorrhea, and typhoid fever are increasing every year and substantially contribute to the high costs of individuals’ health as well as the health care systems around the world, particularly in developing countries.7 Currently, 4.1% of new tuberculosis cases are considered to be multi-drug resistant.13 In countries like India, the Philippines, Russia, and South Africa, which have always had a high number of TB cases compared to other parts of the world, multi-drug resistant TB is anticipated to escalate significantly by 2040.14
Factors Accelerating the Rate of AMR
Misuse and Overuse of Antibiotics
From early days of discovery of antibiotics in the 1940s, Sir Alexander Fleming warned the public about the high demand for antibiotics in the future which could lead to their overuse.15–17 Different surveys across the globe indicate that many patients firmly believe antibacterial agents would help with viral diseases like the common cold or flu.9 Furthermore, in many developing countries where there are deficiencies in proper diagnostic tools, patient management is predominantly contingent upon the prescription of medicine, particularly antibiotics.18 Administering antibiotics when they are actually not needed for the treatment is another example of common misuse of them.4,18 Moreover, many antibiotics are of poor quality and sold over the counter in the developing countries.4 For instance, in India and Vietnam, where there is insufficient enforcement of regulatory policies on prescribing medicine, over-the-counter antibiotics are prevalent.4,19 Such availability makes it accessible for patients to do self-treatment for diseases that do not necessarily need antibiotics for treatment.4,15
Moreover, antibacterial resistance can develop because physicians unnecessarily prescribe lengthy courses of antibiotics.20 Financial incentives play an important factor in overprescribing antibiotics. For example, Chinese hospitals incentivize physicians to prescribe antibiotics; as a result, they will receive more money from pharmaceutical companies.21,22 Another factor contributing to overprescribing antibiotics by providers is patients’ expectations from them.23 Studies have implicated that clinicians consider the perceived patient request for antibiotics as one of the major barriers to adhere to standard guidelines for antibiotic prescriptions.23,24 Providers try to avoid the dissatisfaction of their patients by meeting their demand for prescribing antibiotics.23
Agricultural Use of Antibiotics
Agricultural use of antibiotics is another prominent contributor to the antimicrobial resistance in humans.25,26 For instance, just in the United States, approximately 80% of the antibiotics sold are applied to food that animals eat.27 In 2010, 63,200 tons of antibiotics were used in livestock production worldwide which is significantly more than human consumption.28 In addition to the utilization of antibiotics to treat sick animals, antibiotics are largely added to healthy animal feed and drinking water in order to prevent sickness (prophylaxis) among animals to a large extent, to further grow herds at subtherapeutic levels, and to elevate feed efficiency.9,27,29,30 For instance, one of the widely used antibiotics in animal farming worldwide to further promote the growth of livestock, particularly pigs, is colistin, a critical last-line antibiotic to treat severe infections in humans.31–33
Increase in Income Levels
According to Klein et al, between 2000 and 2015, global antibiotic use elevated by 65%.34 This significant rise in global antibiotic use is predominantly because of overconsumption of antibiotics in developing countries which is the direct result of rising incomes.18,34 In other words, the rise in Growth Domestic Product (GDP) as well as living standards in low and middle-income countries (LMICs) have shown to be positively correlated with antibiotic consumption.34,35 Moreover, an increase in income levels in developing countries has led to an increase in animal protein consumption which may require more antibiotics to be added to the food animals eat.36
There has been a stark change in the pattern of antibacterial consumption across the globe within the past decade.34,37 In 2000, the highest antibiotic consumption rate was in the United States, France, Spain, New Zealand, and Hong Kong; however, in 2015, four of the countries with the highest rate of antibiotic consumption were low-middle income countries such as Turkey, Tunisia, Algeria, and Romania.34 Rate of antibiotics in LMICs is still lower than the rate in high-income countries, due to continuous increase in income level and living standards; however, it is highly likely that in a few years this rate might eventually converge or even surpass the antibiotic consumption rate in developed countries.34,35
Easy Travel Routes
Studies have suggested that the modern and easy traveling routes for people, animals, and goods have also substantially contributed to the dissemination of antimicrobial resistance across the globe.2,38 By being exposed to resistant pathogens, human travelers are highly likely to return colonized and infected to their country.39 For instance, Ruppe et al have shown that European tourists traveling to India who had absolutely no contact with the Indian health care system still tested positive for carbapenemase-producing Enterobacteriaceae (CPE) after they came back from their trip.40
Biological Factors
Antibiotic resistance may happen spontaneously through mutation and bacterial evolution.41 Furthermore, plasmids, small circular fragments of DNA in bacteria, can obtain a great variety of resistance genes through transposons and insertion sequences.42–44 These plasmids can be transferred to bacteria from other species and spread the antibacterial resistance in the bacterial population.45 In addition, exchanging resistance genetic factors between bacteria through horizontal gene transfer further accelerates the spread of antibiotic resistance.25,45,46
Gaps in Knowledge
The numerous gaps in knowledge about antibiotic resistance contribute to the continuing trends of AMR since the statistics and particulars about the use of different antibiotics in both the health care setting and in animal production are not systematically gathered worldwide.26,47 For instance, currently, just 42 countries in the world systematically gather data regarding the use of antibiotics in livestock.26 High-quality global surveillance systems are critical for determining and providing warning bells of problems associated with changes in antimicrobial exposure.47 They also help with observing the efficacy of the interventions implemented to standardize the usage of antibacterial agents in order to address the issue of AMR.48 Thus, the existing gap in knowledge about antibiotic usage worldwide highlights the great importance of a successful approach in engaging cooperative efforts among different international sectors such as human and veterinary medicine, agriculture, animal production, and of course, informed consumers.9 Furthermore, there is a gap in awareness of people regarding the proper use and the potential hazard of antibiotics.49,50 For instance, results of national questionnaires in different developed and developing countries, including Japan, Australia, the United States, Sri Lanka, and Gulf Cooperation Council countries demonstrate that most people generally have limited knowledge about the correct use of antibiotics.50–53
Methods
Search Strategy
With a thorough literature review, crucial information was compiled, assessed, and used to understand the implications and costs of AMR across the globe. Google, Google Scholar, PubMed, Microsoft Academic, Scopus, Medline, Global Health, and searches within the CDC, WHO, and comparable health organizations and websites were utilized to obtain information. The review solely included published articles written in English. Search terms including “AMR and cost,” “Antimicrobial resistance implications” and “AMR and disease burden” were utilized in order to gather information. Moreover, searches within these databases concentrated on literature that dates back no further than 2012.
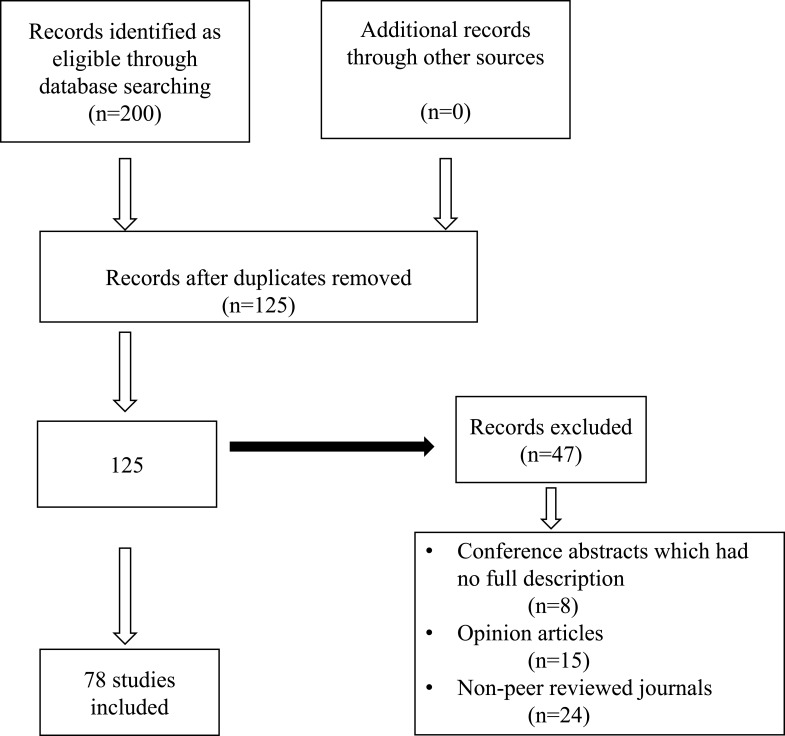
The search yielded 200 articles. The titles and abstracts of these articles were reviewed in order to screen for publications that addressed the drivers of AMR and different implications and cost of AMR. After removing the duplicates and opinion articles, 78 articles were identified as relevant and met the inclusion criteria for this critical analysis project. The study flow diagram is illustrated in Figure 1.
Figure 1.
A flow diagram of articles included in the review.
Findings
The literature review findings demonstrates that the cost of AMR can be categorized into three different levels: Patient level, Healthcare level, and Economic level.6
Patient Perspective
Morbidity and mortality are important consequences of AMR affecting patients.1,6 Compared to non-resistant forms, resistant bacteria will double the chances of developing a serious health issue and triple the chances of death.54 Of course, these negative outcomes will be more pronounced with elevation of the severity of the resistant infections and the susceptibility of the host.55 Table 1 shows the comparison between mortality rates due to AMR and major causes of mortality worldwide by 2050. Currently, across the globe, approximately 700,000 individuals lose their lives because of the drug-resistant infections each year.56 Table 2 indicates the mortality rates due to AMR by 2050 in different regions of the world. In the United States, 2 million people are affected every year by AMR and about 23,000 deaths occur as a result.57 This number is roughly the same as the European Union which has an annual mortality rate of 25,000.5,58 Despite the difficulty of obtaining precise mortality rates, official reports have estimated that about 10 million people will die across the world by 2050 if strong and effective action against AMR is not taken.4,59
Table 1.
Mortality Rates by 2050 by Condition66
| Cancer | 8.2 Million |
| Cholera | 100,000–120,000 |
| Diabetes | 1.5 Million |
| Diarrheal Disease | 1.4 Million |
| Measles | 130,000 |
| Road Traffic Accidents | 1.2 Million |
| Tetanus | 60,000 |
| Antimicrobial Resistance | 10 Million |
Notes: Adapted from Antimicrobial resistance: tackling a crisis for the health and wealth of nations. 2014. Available from: https://amr-review.org/sites/default/files/AMR Review Paper - Tackling a crisis for the health and wealth of nations_1.pdf. Accessed September 17, 2019. Creative Commons Attribution 4.0 International Public License (https://creativecommons.org/licenses/by/4.0/legalcode).66
Table 2.
Mortality Rates by 2050 Due to AMR in Different Regions66
| Asia | 4,730,000 |
| Africa | 4,150,000 |
| Europe | 390,000 |
| Latin America | 392,000 |
| North America | 317,000 |
| Oceania | 22,000 |
Notes: Adapted from Antimicrobial resistance: tackling a crisis for the health and wealth of nations. 2014. Available from: https://amr-review.org/sites/default/files/AMR Review Paper - Tackling a crisis for the health and wealth of nations_1.pdf. Accessed September 17, 2019. Creative Commons Attribution 4.0 International Public License (https://creativecommons.org/licenses/by/4.0/legalcode).66
Antimicrobial resistance also sabotages decades of global fights against many infectious diseases like tuberculosis, HIV, and malaria.56 The number of HIV cases resistant to medicine are on the rise, particularly in Sub-Saharan Africa where 60% of patients with HIV have developed resistance to HIV medicine.60,61 Unsurprisingly, patients with resistance to HIV drugs have a higher risk of dying from HIV.62 According to the HIV drug resistance report in 2017, such continuous trends in resistance to HIV medicine threaten the global goal of putting an end to AIDS by 2030.63 In addition to tuberculosis and HIV, in the past 50 years, Plasmodium falciparum, the causative agent of malaria, has become resistant to anti-malarial medicines and this trend is predominantly seen in southeast Asia.64 The increase in resistance to malaria drugs obstructs malaria control which attempts to decrease the average 445,000 deaths that take place annually due to this deadly disease.65
Healthcare Perspective
AMR has disastrous impacts on healthcare costs.6 According to the CDC, in the United States alone, antibiotic resistance could add about $1,400 to the hospital bill for treating patients with any bacterial infections.8,67 This additional cost could go up significantly to more than $2 billion every year.8 According to different studies, it is projected that AMR could cost from $300 billion to more than $1 trillion annually by 2050 worldwide.4,28 High costs associated with expensive and intensive treatments and escalation in resource utilization are the direct monetary effects of AMR on health care.3,4 Treating patients with resistant infections by using a combination of regimens may be ineffective; as a result, compared to other patients, they may need longer hospitalization stays as well as more intensive care units (ICUs) and isolation beds in order to prevent the spread of the infection.55 Also, nosocomial outbreaks with resistant pathogens may result in the closure of a wing of a hospital and the cancellation of elective surgeries, costing the hospital money.55
In addition to direct monetary effects, AMR generates a burden on the health care system through secondary effects.68 These effects happen when the procedures that utilize antibiotics, which are essential to decrease the risk of any infection after surgery, cannot be successfully carried out due to the prevalence of antimicrobial resistance.68 Furthermore, AMR will challenge performing organ transplants because they expose the patients to different infections.66,69 For instance, Santoro-Lopes and de Gouvea performed a comprehensive review on different multi-resistant infections that may occur after liver transplantation.70 In their work, they have discussed that multi-drug resistant pathogens can increase the likelihood of transplant failure and death.70 Another secondary effect of antimicrobial resistance will be on cancer treatments.66 Due to AMR, chemotherapy cannot be performed on patients with cancer.71 Chemotherapy impairs the immune system and makes patients with cancer vulnerable to different infections.59 Thus, the prevalence of AMR prohibits physicians from administering antibiotics to patients with cancer.3,66,68 There is limited data on the exact cost of different secondary effects of AMR which limits our understanding regarding what we might stand to lose.66
Economic Perspective
The literature review findings indicate that the cost of AMR across the globe is extremely high and different in each country.66,72 The CDC estimated that the cost of antimicrobial resistance is $55 billion every year in the United States, $20 billion for health care and about $35 billion for loss of productivity.3,8 Recent research by the World Bank indicates that antimicrobial resistance would elevate the rate of poverty and impact low-income countries compared to the rest of the world.28 Studies show that annual global GDP could decrease by approximately 1% and there would be a 5–7% loss in developing countries by 2050.71,72 This percentage ultimately translates into $100-210 trillion.28,66 Multidrug- resistant TB alone could cost the world $16.7 trillion by 2050.73,74
Furthermore, due to AMR, the gap between the developing countries and the developed countries will become more pronounced; as a result, inequity will substantially increase.28 Most of the people who are pushed into extreme poverty as a result of AMR will be specifically from low-income countries.28 This highlights the fact that the underprivileged population of the world will eventually be affected the most because these countries are more contingent on labor income which will be reduced if there is a high prevalence of infectious diseases.28
In addition to the direct impact on GDP, antimicrobial resistance has a major influence on labor through the loss of productivity caused by sickness and premature death.68 Deaths because of antimicrobial resistance decrease the workforce, which in turn negatively impacts the size of the population as well as the quality of the country’s human capital.68,75 Taylor et al have created a theoretical model in order to estimate the economic impacts of AMR on the labor force in the future. In their work, they have compared a baseline (absence of AMR) with the current trend in AMR as well as worse alternatives that might happen if appropriate measures are not taken. According to their results, if there is no change in the current pattern of AMR, in ten years, the world working-age population will decrease by two years. This change will be more pronounced in Eurasia compared to the rest of the world.75 In addition, in terms of annual GDP loss, if there is no change in the trends of AMR, the world will lose about $28 billion in ten years. According to this model, with a $20 billion loss in GDP, the European Union and The Organization for Economic Co-operation and Development (OECD) countries stand to lose more than the rest of the world.75
The global trade will also be heavily affected by antimicrobial resistance if the continuous trends in AMR still persist.32 The World Bank report demonstrates that global exports might decrease significantly by 2050 due to the effects of antimicrobial resistance on labor-intensive sectors.28 Thus, it can be concluded that the undesirable outcomes of AMR on the global economy are projected to be even more severe than the global financial recession due to its long-term impacts on the economy.28
Impacts of AMR on livestock output will also be significant.30 Just like humans, the effect of AMR on animals will be due to mortality and morbidity. The increase in resistance to antimicrobials will make treatments on animals ineffective and cause the infections to become more severe.26 Ultimately, this will lead to decreased production and trade of livestock, resulting in elevated prices of protein due to the decrease in protein sources such as milk, egg, and meat.26,28 Shortage of protein will be a major concern, considering that the demand for animal proteins is on the rise worldwide.36 According to the World Bank, AMR will have drastic impacts on livestock production in low-middle income countries.32 Estimates have indicated that if the persistent trends in AMR do not slow down, there will be an 11% loss in livestock production by 2050.28 Such a substantial loss in animal production will lead to a decline in income generation which will exacerbate the economic situation.26
Conclusion
Antimicrobials are the pillars of modern medicine and have substantially contributed to the progress of health care during the last half-century.76 Thus, the persistent trends in AMR should be stopped or it will set us back to the dark ages of medicine.48 Antibiotic resistance is a naturally occurring mechanism that can be slowed down gradually but not stopped completely because resistance is an inevitable consequence of the drug selective pressure.8,21 Thus, combating AMR requires collective action, political momentum, and robust multisectoral collaboration and partnerships between all stakeholders worldwide including governmental and non-governmental agencies, researchers, providers, public health practitioners, pharmaceutical companies, hospital administrations, policymakers, agriculture industry leaders, and patients.71,77 The main goal of this partnership should be decelerating the continuous trends in AMR so that the adverse impacts on society and the economy can be controlled. This will be achieved by establishing a governance mechanism in order to bring harmony to strategic and operational planning.71 Although the cost of abiding by the guidelines and frameworks can be high, it is well established that the return on such investment will unquestionably have significant positive outcomes.78 It also provides hope that the adverse impacts of antimicrobial resistance can be mitigated and may not lead to irreversible results for society as a whole.
Disclosure
The author reports no conflicts of interest in this work.
References
- 1.Founou RC, Founou LL, Essack SY. Clinical and economic impact of antibiotic resistance in developing countries: a systematic review and meta-analysis. PLoS One. 2017;12:e0189621. doi: 10.1371/journal.pone.0189621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antibiotic resistance: a global threat | features | CDC. https://www.cdc.gov/features/antibiotic-resistance-global/index.html. Accessed September15, 2019.
- 3.Prestinaci F, Pezzotti P, Pantosti A. Antimicrobial resistance: a global multifaceted phenomenon. Pathog Glob Health. 2015;109(7):309. doi: 10.1179/2047773215Y.0000000030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chokshi A, Sifri Z, Cennimo D, Horng H. Global Contributors to Antibiotic Resistance. J Glob Infect Dis. 2019;11(1):36–42. doi: 10.4103/jgid.jgid_110_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.ECDC. Surveillance of Antimicrobial Resistance in Europe. 2017. Available from: https://www.ecdc.europa.eu/sites/default/files/documents/EARS-Net-report-2017-update-jan-2019.pdf. Accessed September 16, 2019. [Google Scholar]
- 6.Shrestha P, Cooper BS, Coast J, et al. Enumerating the economic cost of antimicrobial resistance per antibiotic consumed to inform the evaluation of interventions affecting their use. Antimicrob Resist Infect Control. 2018;7(1):98. doi: 10.1186/s13756-018-0384-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Llor C, Bjerrum L. Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem. Ther Adv Drug Saf. 2014;5(6):229–241. doi: 10.1177/2042098614554919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antibiotic resistance threats in the United States; 2013. https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf. Accessed September16, 2019.
- 9.WHO library cataloguing-in-publication data global action plan on antimicrobial resistance; 2015. www.paprika-annecy.com. Accessed September16, 2019.
- 10.Bassetti M, Peghin M, Vena A, Giacobbe DR. Treatment of infections due to MDR gram-negative bacteria. Front Med. 2019;6:74. doi: 10.3389/fmed.2019.00074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramírez-Castillo FY, Moreno-Flores AC, Avelar-González FJ, Márquez-Díaz F, Harel J, Guerrero-Barrera AL. An evaluation of multidrug-resistant Escherichia coli isolates in urinary tract infections from Aguascalientes, Mexico: cross-sectional study. Ann Clin Microbiol Antimicrob. 2018;17(1):34. doi: 10.1186/s12941-018-0286-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Annavajhala MK, Gomez-Simmonds A, Uhlemann A-C. Multidrug-resistant enterobacter cloacae complex emerging as a global, diversifying threat. Front Microbiol. 2019;10:44. doi: 10.3389/fmicb.2019.00044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chatterjee S, Poonawala H, Jain Y. Drug-resistant tuberculosis: is India ready for the challenge? Commentary. BMJ Glob Heal. 2018;3:971. doi: 10.1136/bmjgh-2018-000971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedrich MJ. Drug-resistant tuberculosis predicted to increase in high-burden countries. JAMA. 2017;318(3):231. doi: 10.1001/jama.2017.9086 [DOI] [PubMed] [Google Scholar]
- 15.Bin ZS, Hussain MA, Nye R, Mehta V, Mamun KT, Hossain N. A review on antibiotic resistance: alarm bells are ringing. Cureus. 2017;9(6):e1403. doi: 10.7759/cureus.1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Langford BJ, Morris AM. Is it time to stop counselling patients to “finish the course of antibiotics”? Can Pharm J (Ott). 2017;150(6):349–350. doi: 10.1177/1715163517735549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ventola CL. The antibiotic resistance crisis: part 1: causes and threats. P T. 2015;40(4):277–283. [PMC free article] [PubMed] [Google Scholar]
- 18.Chaw PS, Höpner J, Mikolajczyk R. The knowledge, attitude and practice of health practitioners towards antibiotic prescribing and resistance in developing countries—A systematic review. J Clin Pharm Ther. 2018;43(5):606–613. doi: 10.1111/jcpt.12730 [DOI] [PubMed] [Google Scholar]
- 19.Van Nguyen K, Do NT T, Chandna A, et al. Antibiotic use and resistance in emerging economies: a situation analysis for Viet Nam. BMC Public Health. 2013;13:1158. doi: 10.1186/1471-2458-13-1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spellberg B. The maturing antibiotic mantra: “Shorter is still better”. J Hosp Med. 2018;13(5):361–362. doi: 10.12788/jhm.2904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laxminarayan R. Antibiotic effectiveness: balancing conservation against innovation. Science. 2014;345(6202):1299–1301. doi: 10.1126/science.1254163 [DOI] [PubMed] [Google Scholar]
- 22.Tang Q, Song P, Li J, Kong F, Sun L, Xu L. Control of antibiotic resistance in China must not be delayed: the current state of resistance and policy suggestions for the government, medical facilities, and patients. Biosci Trends. 2016;10(1):1–6. doi: 10.5582/bst.2016.01034 [DOI] [PubMed] [Google Scholar]
- 23.Fletcher-Lartey S, Yee M, Gaarslev C, Khan R. Why do general practitioners prescribe antibiotics for upper respiratory tract infections to meet patient expectations: a mixed methods study. BMJ Open. 2016;6(10):e012244. doi: 10.1136/bmjopen-2016-012244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dempsey PP, Businger AC, Whaley LE, Gagne JJ, Linder JA. Primary care clinicians’ perceptions about antibiotic prescribing for acute bronchitis: a qualitative study. BMC Fam Pract. 2014;15(1):194. doi: 10.1186/s12875-014-0194-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang Q, Wang W, Regev-Yochay G, Lipsitch M, Hanage WP. Antibiotics in agriculture and the risk to human health: how worried should we be? Evol Appl. 2015;8(3):240–247. doi: 10.1111/eva.12185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Animal production | antimicrobial resistance | food and agriculture organization of the United Nations. http://www.fao.org/antimicrobial-resistance/key-sectors/animal-production/en/. Accessed September16, 2019.
- 27.Bartlett JG, Gilbert DN, Spellberg B. Seven ways to preserve the miracle of antibiotics. Clin Infect Dis. 2013;56(10):1445–1450. doi: 10.1093/cid/cit070 [DOI] [PubMed] [Google Scholar]
- 28.Drug-resistant infections a threat to our economic future; 2017. Available from: www.worldbank.org. Accessed September16, 2019.
- 29.Blaser MJ. Antibiotic use and its consequences for the normal microbiome. Science. 2016;352(6285):544–545. doi: 10.1126/science.aad9358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hao H, Cheng G, Iqbal Z, et al. Benefits and risks of antimicrobial use in food-producing animals. Front Microbiol. 2014;5:288. doi: 10.3389/fmicb.2014.00288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.United Nations meeting on antimicrobial resistance. Bull World Health Organ. 2016;94(9):638–639. doi: 10.2471/BLT.16.020916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lekagul A, Tangcharoensathien V, Yeung S. Patterns of antibiotic use in global pig production: a systematic review. Vet Anim Sci. 2019;7:100058. doi: 10.1016/J.VAS.2019.100058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rhouma M, Beaudry F, Thériault W, Letellier A. Colistin in pig production: chemistry, mechanism of antibacterial action, microbial resistance emergence, and one health perspectives. Front Microbiol. 2016;7:1789. doi: 10.3389/fmicb.2016.01789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klein EY, Van Boeckel TP, Martinez EM, et al. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc Natl Acad Sci U S A. 2018;115(15):E3463–E3470. doi: 10.1073/pnas.1717295115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dall C. Global antibiotic use rises, fueled by economic growth | CIDRAP. Available from: http://www.cidrap.umn.edu/news-perspective/2018/03/global-antibiotic-use-rises-fueled-economic-growth. Accessed September16, 2019..
- 36.Van Boeckel TP, Brower C, Gilbert M, et al. Global trends in antimicrobial use in food animals. Proc Natl Acad Sci. 2015;112(18):5649–5654. doi: 10.1073/pnas.1503141112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robertson J, Iwamoto K, Hoxha I, et al. Antimicrobial medicines consumption in Eastern Europeand Central Asia – an updated cross-national study and assessment of quantitativemetrics for policy action. Front Pharmacol. 2019;9:1156. doi: 10.3389/fphar.2018.01156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Castro-Sánchez E, Moore LSP, Husson F, Holmes AH. What are the factors driving antimicrobial resistance? Perspectives from a public event in London, England. BMC Infect Dis. 2016;16(1):465. doi: 10.1186/s12879-016-1810-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frost I, Van Boeckel TP, Pires J, Craig J, Laxminarayan R. Global geographic trends in antimicrobial resistance: the role of international travel. J Travel Med. 2019. doi: 10.1093/jtm/taz036 [DOI] [PubMed] [Google Scholar]
- 40.Ruppé E, Armand-Lefèvre L, Estellat C, et al. Acquisition of carbapenemase-producing Enterobacteriaceae by healthy travellers to India, France, February 2012 to March 2013. Eurosurveillance. 2014;19(14):20768. doi: 10.2807/1560-7917.ES2014.19.14.20768 [DOI] [PubMed] [Google Scholar]
- 41.Read AF, Woods RJ. Antibiotic resistance management. Evol Med Public Heal. 2014;2014(1):147. doi: 10.1093/emph/eou024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Q, Chang W, Zhang H, Hu D, Wang X. The role of plasmids in the multiple antibiotic resistance transfer in ESBLs-producing escherichia coli isolated from wastewater treatment plants. Front Microbiol. 2019;10:633. doi: 10.3389/fmicb.2019.00633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rozwandowicz M, Brouwer MSM, Fischer J, et al. Plasmids carrying antimicrobial resistance genes in Enterobacteriaceae. J Antimicrob Chemother. 2018;73(5):1121–1137. doi: 10.1093/jac/dkx488 [DOI] [PubMed] [Google Scholar]
- 44.San Millan A. Evolution of plasmid-mediated antibiotic resistance in the clinical context. Trends Microbiol. 2018;26(12):978–985. doi: 10.1016/j.tim.2018.06.007 [DOI] [PubMed] [Google Scholar]
- 45.Sun D, Jeannot K, Xiao Y, Knapp CW. Editorial: horizontal gene transfer mediated bacterial antibiotic resistance. Front Microbiol. 2019;10. doi: 10.3389/FMICB.2019.01933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paterson IK, Hoyle A, Ochoa G, Baker-Austin C, Taylor NGH. Optimising antibiotic usage to treat bacterial infections. Sci Rep. 2016;6(1):37853. doi: 10.1038/srep37853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Antibiotic resistance: a global threat | features | CDC. Available from: https://www.cdc.gov/features/antibiotic-resistance-global/index.html. Accessed September16, 2019.
- 48.AMR in the WHO European Region. September 2019. Available from: http://www.euro.who.int/en/health-topics/disease-prevention/antimicrobial-resistance/about-amr/amr-in-the-who-european-region. Accessed September 16, 2019. [Google Scholar]
- 49.Aslam B, Wang W, Arshad MI, et al. Antibiotic resistance: a rundown of a global crisis. Infect Drug Resist. 2018;11:1645–1658. doi: 10.2147/IDR.S173867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carter RR, Sun J, Jump RLP. A survey and analysis of the american public’s perceptions and knowledge about antibiotic resistance. Open Forum Infect Dis. 2016;3(3):ofw112. doi: 10.1093/ofid/ofw112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Atif M, Asghar S, Mushtaq I, et al. What drives inappropriate use of antibiotics? A mixed methods study from Bahawalpur, Pakistan. Infect Drug Resist. 2019;12:687–699. doi: 10.2147/IDR.S189114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Almohammed RA, Bird EL. Public knowledge and behaviours relating to antibiotic use in gulf cooperation council countries: a systematic review. J Infect Public Health. 2019;12(2):159–166. doi: 10.1016/j.jiph.2018.09.002 [DOI] [PubMed] [Google Scholar]
- 53.Kamata K, Tokuda Y, Gu Y, Ohmagari N, Yanagihara K. Public knowledge and perception about antimicrobials and antimicrobial resistance in Japan: a national questionnaire survey in 2017. Angelillo IF, ed. PLoS One. 2018;13(11):e0207017. doi: 10.1371/journal.pone.0207017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cecchini M, Langer J, Slawomirski L Antimicrobial Resistance In G7 Countries And Beyond: economic issues, policies and options for action; 2015. Available from: https://www.oecd.org/els/health-systems/Antimicrobial-Resistance-in-G7-Countries-and-Beyond.pdf. Accessed September16, 2019..
- 55.Friedman ND, Temkin E, Carmeli Y. The negative impact of antibiotic resistance. Clin Microbiol Infect. 2016;22(5):416–422. doi: 10.1016/j.cmi.2015.12.002 [DOI] [PubMed] [Google Scholar]
- 56.Tackling drug-resistant infections globally: final report and recommendations the review on antimicrobial resistance chaired by JIM O’NEILL; 2016. Available from: https://amr-review.org/sites/default/files/160518_Final paper_with cover.pdf. Accessed September16, 2019.
- 57.Davis M, Liu T-L, Taylor Y, et al. Exploring patient awareness and perceptions of the appropriate use of antibiotics: a mixed-methods study. Antibiotics. 2017;6(4):23. doi: 10.3390/antibiotics6040023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.WHO report on surveillance of antibiotic consumption; 2016. https://www.who.int/medicines/areas/rational_use/who-amr-amc-report-20181109.pdf. Accessed September17, 2019.
- 59.Laxminarayan R, Duse A, Wattal C, et al. Antibiotic resistance—the need for global solutions. Lancet Infect Dis. 2013;13(12):1057–1098. doi: 10.1016/S1473-3099(13)70318-9 [DOI] [PubMed] [Google Scholar]
- 60.Jasovský D, Littmann J, Zorzet A, Cars O. Antimicrobial resistance-a threat to the world’s sustainable development. Ups J Med Sci. 2016;121(3):159–164. doi: 10.1080/03009734.2016.1195900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Llibre JM. Time to get serious with HIV-1 resistance in sub-Saharan Africa. Lancet Infect Dis. 2017;17(3):241–243. doi: 10.1016/S1473-3099(16)30447-9 [DOI] [PubMed] [Google Scholar]
- 62.Pinoges L, Schramm B, Poulet E, et al. Risk factors and mortality associated with resistance to first-line antiretroviral therapy. JAIDS J Acquir Immune Defic Syndr. 2015;68(5):527–535. doi: 10.1097/QAI.0000000000000513 [DOI] [PubMed] [Google Scholar]
- 63.HIV drug resistance report 2017 trends quality action. Available from: https://apps.who.int/iris/bitstream/handle/10665/255896/9789241512831-eng.pdf?sequence=1. Accessed September17, 2019.
- 64.Phyo AP, Nosten F. The artemisinin resistance in Southeast Asia: an imminent global threat to malaria elimination. 2016. doi: 10.5772/intechopen.76519 [DOI]
- 65.World malaria report 2018 ISBN 978 92 4 156565 3. Available from: www.who.int/malaria. Accessed September17, 2019.
- 66.Antimicrobial resistance: tackling a crisis for the health and wealth of nations. 2014. Available from: https://amr-review.org/sites/default/files/AMR Review Paper - Tackling a crisis for the health and wealth of nations_1.pdf. Accessed September17, 2019.
- 67.Thorpe KE, Joski P, Johnston KJ. Antibiotic-resistant infection treatment costs have doubled since 2002, now exceeding $2 billion annually. Health Aff. 2018;37(4):662–669. doi: 10.1377/hlthaff.2017.1153 [DOI] [PubMed] [Google Scholar]
- 68.Naylor NR, Atun R, Zhu N, et al. Estimating the burden of antimicrobial resistance: a systematic literature review. Antimicrob Resist Infect Control. 2018;7(1):58. doi: 10.1186/s13756-018-0336-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li B, Webster TJ. Bacteria antibiotic resistance: new challenges and opportunities for implant-associated orthopedic infections. J Orthop Res. 2018;36(1):22–32. doi: 10.1002/jor.23656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Santoro-Lopes G, de Gouvêa EF. Multidrug-resistant bacterial infections after liver transplantation: an ever-growing challenge. World J Gastroenterol. 2014;20(20):6201. doi: 10.3748/wjg.v20.i20.6201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Anderson M, Clift C, Schulze K, et al. Health systems and policy analysis - Averting the AMR crisis : what are the avenues for policy. Eur Obs Heal Syst Policies. 2019. [PubMed] [Google Scholar]
- 72.Utt E, Wells C. The global response to the threat of antimicrobial resistance and the important role of vaccines. Pharm Policy Law. 2016;18:179–197. doi: 10.3233/PPL-160442 [DOI] [Google Scholar]
- 73.Drug-resistant tuberculosis: worth the investment why drug-resistant tuberculosis? Available from: https://www.eiu.com/graphics/marketing/pdf/Drug-resistant-tuberculosis-Article.pdf. Accessed September17, 2019.
- 74.Global pandemic | TB alliance. Available from: https://www.tballiance.org/why-new-tb-drugs/global-pandemic. Accessed September 17, 2019.
- 75.Taylor J, Hafner M, Yerushalmi E, et al. Estimating the economic costs of antimicrobial resistance: model and Results. 2005.
- 76.Maddocks SE. Novel targets of antimicrobial therapies. Microbiol Spectr. 2016;4(2). doi: 10.1128/microbiolspec.VMBF-0018-2015 [DOI] [PubMed] [Google Scholar]
- 77.CDC. National action plan for combating antibiotic-resistant bacteria; 2015. Available from: https://www.cdc.gov/drugresistance/pdf/national_action_plan_for_combating_antibotic-resistant_bacteria.pdf. Accessed September30, 2019.
- 78.Renwick MJ, Simpkin V, Mossialos E. Targeting Innovation in Antibiotic Drug Discovery and Development. European Observatory on Health Systems and Policies; 2016. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28806044. Accessed September17, 2019. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Antibiotic resistance: a global threat | features | CDC. https://www.cdc.gov/features/antibiotic-resistance-global/index.html. Accessed September15, 2019.