Abstract
Polychlorinated biphenyls (PCBs) are highly persistent and ubiquitously distributed environmental pollutants. Based on their chemical structure, PCBs are classified into non-ortho-substituted and ortho-substituted congeners. Non-ortho-substituted PCBs are structurally similar to dioxin and their toxic effects and mode of action are well-established. In contrast, very little is known about the effects of ortho-substituted PCBs, particularly, during early development. The objective of this study is to investigate the effects of exposure to an environmentally prominent ortho-substituted PCB (2,2’,4,4’,5,5’-hexachlorobiphenyl; PCB153) on zebrafish embryos. We exposed zebrafish embryos to 3 different concentrations of PCB153 starting from 4 to 120 hours post-fertilization (hpf). We quantified gross morphological changes, behavioral phenotypes, gene expression changes, and circadian behavior in the larvae. There were no developmental defects during the exposure period, but starting at 7 dpf, we observed spinal deformity in the 10 μM PCB153 treated group. A total of 633, 2227, and 3378 differentially expressed genes were observed in 0.1 μM (0.036 μg/ml), 1 μM (0.36 μg/ml), and 10 μM (3.6 μg/ml) PCB153-treated embryos, respectively. Of these, 301 genes were common to all treatment groups. KEGG pathway analysis revealed enrichment of genes related to circadian rhythm, FoxO signaling, and insulin resistance pathways. Behavioral analysis revealed that PCB153 exposure significantly alters circadian behavior. Disruption of circadian rhythms has been associated with the development of metabolic and neurological diseases. Thus, understanding the mechanisms of action of environmental chemicals in disrupting metabolism and other physiological processes is essential.
Keywords: zebrafish, RNA sequencing, circadian behavior, PCB153
Polychlorinated biphenyls (PCBs) are persistent organic pollutants that are widely distributed in the environment. Based on their structural properties (e.g., the number and position of chlorine atoms), there are 209 possible PCB congeners, many of which exist in the environment. PCBs are further classified into coplanar (non-ortho-substituted) and non-coplanar (ortho-substituted) congeners based on the position of the chlorine atoms on the biphenyl ring. The biological and toxicological activities of PCBs are congener-specific. It is well-established that effects of non-ortho-substituted PCB congeners are mediated by interaction with the aryl hydrocarbon receptor (AHR). 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) is the prototypical ligand for the AHR, and the potency with which individual PCB congeners bind and activate AHR is expressed in terms of their TCDD-toxic equivalence factors (TEFs). Non-ortho-substituted PCB congeners exhibit the highest TEF values. In contrast, the toxicity of ortho-substituted PCBs is not mediated by AHR and their TEFs are not reliable indicators of risk assessment. A growing number of studies have demonstrated adverse impacts of ortho-substituted PCB exposure on diverse physiological processes, including the central nervous system, metabolism, endocrine, and immune function (Kopec et al., 2010; Liu et al., 2012b; Mesnier et al., 2015; Sable and Schantz, 2006).
Ortho-substituted PCBs such as PCB153 are present in relatively high concentrations in the environment (Fernandez et al., 2004; Lake et al., 1995; Li et al., 2014; Oliveira et al., 2011). Because of their high lipophilicity, PCBs bioaccumulate in the fatty tissues and several studies have shown high concentrations of PCB153 in human breast milk (Hartle et al., 2018; Lancz et al., 2015; McLachlan et al., 2018; Weldon et al., 2011). Perinatal exposure to ortho-substituted PCBs have been associated with cognitive deficits (Piedrafita et al., 2008b). These effects are thought to be due to altered glutamate signaling in the cerebellum of developmentally exposed young rats (Piedrafita et al., 2008a). Similarly, developmental exposure to PCB153 affected dopamine receptors in the cerebellum of young rats (Coccini et al., 2011). In addition, ortho-substituted PCBs have been shown to cause neurotoxicity by altering the normal functioning of receptors and signal transduction molecules in the brain (Castoldi et al., 2006; Coccini et al., 2011; Honma et al., 2009). Taken together, these results suggest that ortho-substituted PCBs can potentially have long-term neurological consequences when present during sensitive windows of early development.
In addition to the effects on nervous system, there is also growing evidence suggesting that ortho-substituted PCBs can induce metabolic disorders by altering carbohydrate and lipid metabolism. For instance, short-term exposure to PCB118 and PCB153 resulted in elevated circulating triglyceride levels and altered the expression of genes associated with lipid metabolism and insulin resistance in liver and adipose tissues (Mesnier et al., 2015). We have recently demonstrated that acute developmental exposure of fish embryos to PCB153 causes differential expression of genes related to glucose metabolism (Hahn et al., 2018). Furthermore, PCB153 has been shown to affect thyroid hormone homeostasis (Haave et al., 2011; Liu et al., 2012a,b). As energy metabolism is under the regulation of the endocrine system, it is conceivable that exposure to ortho-substituted PCBs could cause metabolic disruption by altering endocrine function. Some of these effects have been shown to be photoperiod-dependent (Skipor et al., 2012).
Even though recent studies provide important information on the various adverse effects of ortho-substituted PCBs, there are still huge knowledge gaps in our understanding about effects of exposure during development and the mechanisms of action. Most of the studies published so far have focused either on acute effects of exposure in adults, or latent effects of developmental exposure, using a single PCB dose. There are no reported studies investigating the dose-dependent effects of PCB153 exposure on early development. Hence, the objective of this study was to characterize the developmental effects of exposure to three different concentrations of PCB153, an environmentally relevant ortho-substituted PCB. We conducted these studies using zebrafish, a well-established and widely used model for developmental toxicology (Behl et al., 2019; Nishimura et al., 2016) and human disease (Bradford et al., 2017; Grunwald and Eisen, 2002). We exposed zebrafish embryos through the entire period of development and assessed developmental phenotypes, behavior, and transcriptional responses.
MATERIALS AND METHODS
Experimental animals
The wild-type Tupfel/Longfin mutant (TL) strain of zebrafish was used in this study. Adult zebrafish were held in approximately 2:1 female to male groups at a density of 4–5 fish/l in 3 or 10 l tanks in a recirculating flowing water system (Aquatic Habitats, Inc, Apopka, Florida) filled with system water (475 mg/l Instant Ocean, 79 mg/l sodium bicarbonate, and 53 mg/l calcium sulfate). Photoperiod was set to 14:10 h light:dark cycle, and water temperature was kept at 28.5°C. The fish were fed twice daily; morning feeding with brine shrimp (Artemia salina) and afternoon feeding with GEMMA Micro 300 micro-pellets (Skretting USA, Tooele, Utah). All experiments were conducted as per protocols approved by the Animal Care and Use Committee of the Woods Hole Oceanographic Institution (A3630-01). Freshly fertilized eggs were obtained from breeding of multiple tanks with 20–25 male and female fish.
PCB153 exposure and developmental phenotypes
Zebrafish embryos were exposed to final concentrations of 0.1 μM (0.036 μg/ml), 1 μM (0.36 μg/ml), and 10 μM (3.6 μg/ml) PCB153 (2,2’,4,4’,5,5’-hexachlorobiphenyl, 99.2% purity; Ultra Scientific, Rhode Island) or dimethyl sulfoxide (DMSO; solvent control; 0.01%), starting at 4 h post-fertilization (hpf) continuously until 120 hpf (or 5 days post-fertilization) (Figure 1A). These concentrations were chosen based on our preliminary studies determining the effect of exposure on developmental deformities. In addition, these concentrations were found in fish inhabiting highly contaminated sites. For instance, Atlantic killifish (Fundulus heteroclitus) inhabiting a highly contaminated Superfund site have PCB153 levels of 33 and 7.8 μg/g dry weight of tissue in embryos and whole fish samples, respectively (Dr Saro Jayaraman, USEPA National Exposure Research Laboratory, personal communication). While the concentrations used here may not occur in most environments, due to the lipophilic nature of PCBs, they can accumulate to high levels and are relevant in studying the developmental effects of exposure. These concentration ranges have also been detected in human samples (Hartle et al., 2018; Lancz et al., 2015; McLachlan et al., 2018; Weldon et al., 2011).
Figure 1.
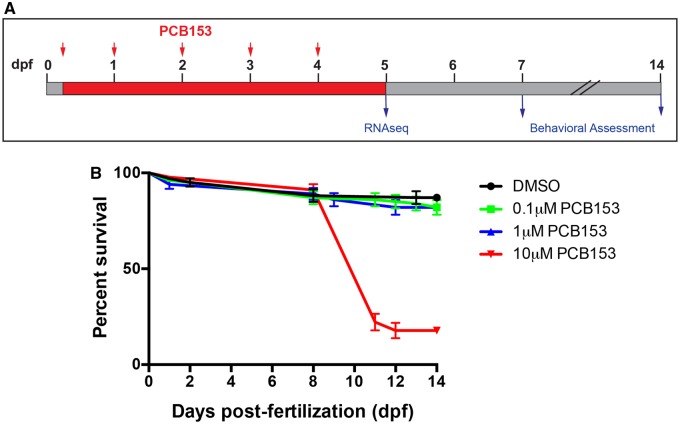
PCB153 exposure regime and the effects on survival. A. Zebrafish embryos were exposed to either control or 3 different concentrations of PCB153 (0.1, 1, and 10 µM) starting from 4 h post-fertilization (hpf) until 5 days post-fertilization (dpf). Water was changed daily and fresh PCB153 was added (red arrows). At the end of the exposure, larvae were sampled for RNA seq. A subset of embryos were raised for phenotypic observation and behavioral assessment at 7 and 14 dpf. B. Percent survival in PCB153 exposed zebrafish embryos. No significant mortalities were observed during the 5-day exposure period. Mortalities were first observed at 7–8 dpf; up to 80% of the 10 µM treated group died by 14 dpf. No significant mortalities were observed in 0.1 and 1 µM PCB153-treated groups in comparison to dimethyl sulfoxide controls (n = 4 independent experiments).
Each treatment consisted of 4 biological replicates with 30 embryos per replicate. Embryos were maintained in glass petri dishes at 28 ± 0.5°C at a density of 1 embryo per ml in 0.3X Danieau's solution (17 mM NaCl, 0.2 mM KCl, 0.12 mM MgSO4, 0.18 mM Ca(NO3)2, and 1.5 mM HEPES free acid; pH 7.2). Water was exchanged daily and fresh exposure solutions were added. At the end of the exposure, embryos were thoroughly rinsed in clean 0.3X Danieau's solution and maintained for further observation in petri dishes at 28 ± 0.5°C under a 14:10 h light:dark cycle with water exchanges performed daily. Larva was observed daily until 14 dpf for any phenotypic abnormalities. Subsets of larva were sampled for transcriptomic analysis (n = 20 per replicate) at the end of the exposure period (120 hpf), before the onset of phenotypic abnormalities, by quickly euthanizing and flash freezing in liquid nitrogen. Samples were stored at −80°C until RNA isolation. Exposure experiments were repeated 4 times to ensure the reproducibility of phenotypes.
Larval locomotor activity assay
Locomotor activity in DMSO control and PCB153 exposed larvae was assessed at 7 dpf using a DanioVision™ behavior system (Noldus Information Technology, Inc, The Netherlands). Assays were conducted in 48-well plates. Each plate contained larvae from all 4 treatment groups (DMSO control, 0.1, 1, and 10 μM PCB153; 10–12 larvae per treatment). The assay was repeated twice (20–24 larvae per experiment) using different sets of larvae. Larvae were dark adapted for at least 2 h before trial initiation by first covering the plates with aluminum foil (placed in an incubator at 28°C) until about 1 h before trial initiation when the plates were placed in the testing system (in the dark). Fish movements were recorded using the EthoVision software (Version 9 Noldus, Inc) for a total of 60 min. The assay was started by monitoring locomotion for 20 min in the dark, followed by 20 min in the light and final 20 min in the dark. We have previously used this protocol to determine the effect of dioxin-like PCBs on larval motility in zebrafish (Glazer et al., 2016). Locomotor activity was recorded at a rate of 30 frames per second, and a track smoothing protocol was applied based on 10 samples before and after every sample point in order to exclude slight movements that might introduce noise to the calculations. All behavior assays were conducted between 13:00 and 15:00 h. Water temperature was maintained at 28°C using a temperature control unit. Total distance moved is reported in centimeters (cm) per 2-min interval. This assay was conducted on larvae from the first 2 independent experiments. Due to the lack of any significant effect of exposure on larval locomotion, we did not repeat this assay on the subsequent 2 experiments.
Total RNA isolation and strand-specific RNA sequencing
Total RNA was isolated from 5-dpf larvae using the Aurum total RNA mini kit (BioRad, Herculus, California) following manufacturer’s instructions. Total RNA was quantified using a Nanodrop Spectrophotometer (Thermo Scientific, Wilmington, Delaware), and RNA quality was assessed using Agilent 2500 Bioanalyzer; all samples had RNA integrity numbers between 9.3 and 10. The Illumina Truseq Stranded total RNA library prep kit with Ribo-Zero Gold was used for library preparation. Strand-specific sequencing (50 base pair Single End reads) was done on the HiSeq2500 platform. Both library preparation and sequencing were performed at Tufts University Medical School’s Genomics core facility.
RNAseq data analysis
Raw data files were assessed for quality using FastQC (Andrews, 2010) prior to pre-processing. We used Trimmomatic for pre-processing, which removed any remaining adaptor sequences and reads with low sequence quality (Phred score less than 20). Trimmed sequence reads were mapped to the zebrafish genome using the STAR aligner (Dobin and Gingeras, 2015). Mapping quality was checked using the RSeQC pipeline (Wang et al., 2012) and coordinate-sorted BAM files were filtered using samtools (-F 256) to remove reads with poor mapping quality. The number of reads mapped to annotated regions of the genome was obtained using HTSeq-count (Anders et al., 2015). We used ensembl version 84 (GRCz10) of the zebrafish genome and annotations (gtf) in this analysis (Yates et al., 2016). Statistical analysis was conducted using edgeR, a Bioconductor package (Robinson et al., 2010). We used the quasi-likelihood model in edgeR (glmQLFTest) to perform differential gene expression analysis. Only genes with false discovery rate (FDR) of less than 5% were considered to be differentially expressed. Annotation of the differentially expressed genes was done using BioMart (Smedley et al., 2015). We validated the RNAseq results by quantifying the expression of 3 circadian rhythm genes from an independent exposure experiment using quantitative real-time PCR. The description of the methods is provided in Supplementary Information 1.
Gene ontology classification and KEGG pathway analysis
Annotated zebrafish genes found to be differentially expressed (FDR < 0.05) were classified based on gene ontology (molecular function) using a gProfiler package g:GOSt (Reimand et al., 2016). The up- and down-regulated datasets for each treatment were processed individually. We then compared the GO terms between the 3 treatments using the g:Cocoa, a package of gProfiler (Reimand et al., 2016). We visualized the GO terms using GOView and generated hierarchical DAG (directed acyclic graphs) graphs to highlight relationships between GOterms (child and parent terms) (Wang et al., 2017). Only GO child terms with a distinct set of genes were considered for further analysis.
KEGG pathway analysis of the DEGs was done using gProfiler and the pathways were visualized using the KEGG database (http://www.genome.jp/kegg/). We manually went through the list of genes represented under each enriched GO term and KEGG pathway and selected the pathways with unique lists of genes.
PCB153 exposure and circadian behavior
Based on the results from transcriptomic analysis, we hypothesized that PCB153 exposure affects circadian behavior. Locomotor activity, which is controlled by circadian clock, is widely used as a proxy for measuring proper functioning of circadian rhythm in zebrafish and other species (Ben-Moshe Livne et al., 2016; Tataroglu and Emery, 2014; Tudorache et al., 2018; Winbush et al., 2015). Zebrafish larvae exhibit circadian rhythms of locomotor activity, with higher levels of activity during the day (Hirayama et al., 2005). We conducted circadian behavior studies following previously published protocols (Ben-Moshe Livne et al., 2016). Zebrafish embryos were exposed to DMSO control and 1 μM PCB153 as described above. We selected this concentration because it altered circadian gene expression patterns but it did not affect larval mortality. At the end of exposure (120 hpf), larvae were rinsed with 0.3x Danieau's solution several times and transferred to a 48-well plate and placed in the Danio Vision observation chamber (Noldus Information Technology, Inc). Larvae were allowed to acclimate for 16 h under 4 h light and 12 h dim (1% light) regime followed by 3 days of dim light. Live video tracking was conducted using EthoVision version 10. Circadian behavior was tracked for 3 days under dim light conditions.
Data analysis includes calculating moving average (10 sliding points) of locomotor activity (distance moved in cm/10 min) for individual larvae. Average locomotor activity is plotted against circadian time. Statistical analysis involved Fourier transformation of time-dependent data to frequency-dependent using Fast Fourier Transform (FFT) and calculation of G-factor ratio using a custom MATLAB script generously shared by Dr Gothilf’s laboratory. The G-factor ratio determines the extent to which the observed locomotor behavior is circadian in nature. It is quantified as the ratio (“G-factor”) of the power (squared amplitude) of the frequency corresponding to a 24-h period to the sum of powers of all frequencies. The differences in G-factor were determined using the Kolmogorov-Smirnov test. A p-value of less than or equal to .05 was considered statistically significant.
We also determined the developmental windows of exposure that affect circadian behavior by exposing zebrafish embryos to 1 μM PCB153 for different periods during development (4–24, 4–48, and 4–72 hpf). At the end of the exposure period, embryos were rinsed and maintained in clean water until they were assessed for circadian behavior at 120 hpf. Larvae were transferred to a 48-well plate at 120 hpf and circadian behavior was tracked as described above. All assays were conducted at least twice.
RESULTS
Effect of PCB153 exposure on survival
We did not observe any significant differences in mortality with PCB153 during the exposure period. However, dose-dependent effects on mortality were observed starting at 7 dpf (Figure 1B). We observed 60% mortality between 7 and 14 dpf in the 10 μM PCB153 group and approximately 10% mortality with 1 μM PCB153. No significant mortality was observed in the 0.1 μM PCB153 group compared with the DMSO control.
PCB153-induced developmental phenotypes
We did not observe any overt phenotypes during the exposure period (Figure 2A). Starting at 6 dpf, fish exposed to 10 μM PCB153 developed a bent body phenotype; the frequency of this phenotype in this group increased over time, with 80% of the embryos displaying it by 14 dpf (Figure 2A). Almost all larvae displaying this phenotype died within 2–3 days. This phenotype was also observed in the 1 μM PCB153 treated embryos, but at a significantly lower frequency. We also observed yolk sac edema and pericardial edema in a small proportion of embryos from these 2 groups. No phenotypes were observed in embryos exposed to 0.1 μM PCB153.
Figure 2.
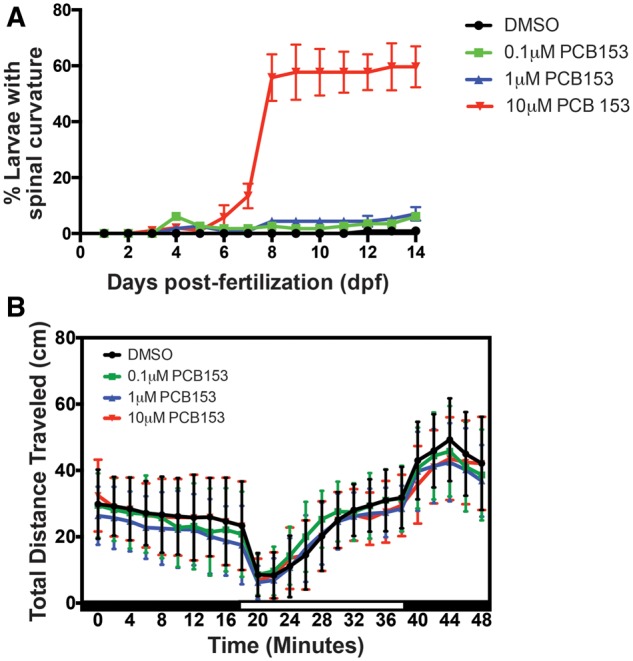
Developmental and behavioral phenotypes in PCB153 exposed zebrafish embryos. A. We observed the embryos under a dissecting microscope from the beginning of the experiment to 14 days post-fertilization (dpf). No overt phenotypes were observed until 120 h post-fertilization. We observed a gradual and significant increase in the number of larvae with a spinal curvature (bent body) in the 10-μM exposed group. All larvae with this phenotype died by 14 dpf. A small proportion of embryos (<5%) also displayed yolk sac and pericardial edema but there was no treatment effect. B. PCB153 exposed larvae did not show any behavioral (locomotory) response to light stimulus. PCB153 exposed larvae were assessed for locomotor activity in response to changes in light at 7 and 14 dpf (data not shown) using the Noldus DanioVision chamber. Dark and white boxes along the x-axes indicate transitions between lighting conditions. No significant differences were observed at these time points. (n = 4 independent experiments).
Developmental PCB153 exposure did not have a significant effect on larval locomotor activity when compared to the DMSO control (Figure 2B).
Strand-specific RNA sequencing
On an average, we obtained a total of 26.2 million raw reads per sample. Of these, 79% of the reads (20.9 million reads) uniquely mapped to the genome, 14% of the reads (3.5 million reads) mapped to multiple loci and 7% were unmapped (1.7 million reads). Among the reads mapped uniquely to the genome, an average of 13.5 million reads per sample was mapped to the annotated regions of the genome. Similar information on each individual sample is provided in Supplementary Information 2. Raw files have been deposited in the GEO database (accession number GSE93310) and Dryad (Aluru et al., 2019).
PCB153-induced transcriptional responses
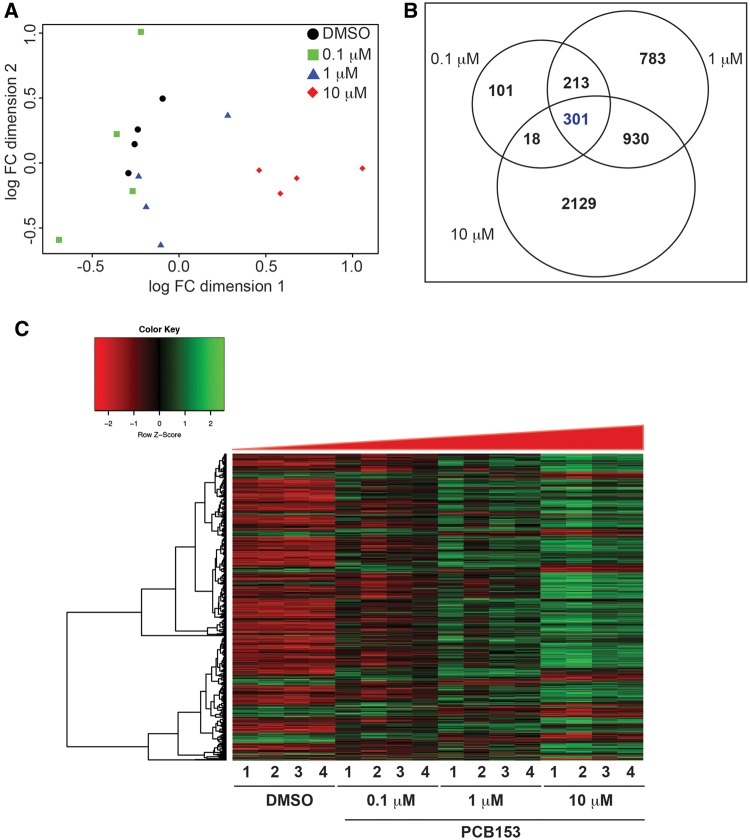
A multidimensional scaling plot revealed differences between the treatment groups, with a stronger difference between the 10 μM PCB153 group and the other treatments (Figure 3A); this difference was also evident in heat maps (Figure 3C). Our analysis revealed differential expression of 663, 2227, and 3378 genes in the 0.1, 1, and 10 μM PCB153 exposed groups, respectively, in comparison to the DMSO control (5% FDR; Figure 3B). A total of 301 differentially expressed genes were shared among all PCB153 exposures (henceforth referred to as the core set of genes). Of these, 225 were upregulated and 73 were downregulated in all treatment groups. The detailed list of core genes (301) and their fold changes is provided in the Supplementary Information 3.
Figure 3.
PCB153-induced transcriptional changes measured by strand-specific RNA sequencing. A. Multidimensional plot showing the variation between different PCB153 concentrations and biological replicates. B. Venn diagram showing the number of differentially expressed genes that are unique and common to different PCB153 treatment groups (5% false discovery rate). C. Heat map representation of the 301 differentially expressed genes that are altered in all 3 PCB153-treated groups. Normalized read counts were used to plot the heat map.
Pathway analysis
KEGG Pathway analysis of the upregulated genes revealed enrichment of the following pathways: circadian rhythm, FoxO signaling and insulin signaling, amino acid (alanine, aspartate, and glutamate) metabolism, ribosome biogenesis, and cysteine and methionine metabolism (Table 1).
Table 1.
Top KEGG Pathway Analysis Terms Enriched in the Differentially Expressed Genes in Embryos Exposed to 3 Different Concentrations of PCB153.
| KEGG ID | KEGG Pathway | 0.1 µM | 1 µM | 10 µM | p-Value |
|---|---|---|---|---|---|
| Upregulated | |||||
| KEGG:00250 | Circadian rhythm | 8 | 8 | 9 | 2.37E−04 |
| KEGG:04910 | FoxO signaling pathway | 8 | 6 | 32 | 2.57E−04 |
| KEGG:05168 | Insulin signaling pathway | — | 21 | 24 | 1.03E−04 |
| KEGG:04068 | Alanine, aspartate, and glutamate metabolism | — | 8 | 10 | 2.89E−07 |
| KEGG:03008 | Ribosome biogenesis in eukaryotes | — | 13 | — | 1.52E−03 |
| KEGG:00270 | Cysteine and methionine metabolism | — | 6 | 7 | 1.53E−02 |
| Downregulated | |||||
| KEGG:04110 | p53 signaling pathway | 3 | 11 | 11 | 7.64E−03 |
| KEGG:04210 | Apoptosis | 6 | 16 | 14 | 1.84E−05 |
| KEGG:04115 | Cell cycle | — | 16 | 38 | 3.99E−11 |
The numbers in each column represent the number of differentially expressed genes in each pathway.
Several genes involved in the circadian rhythm (Figure 4A) were upregulated, including cryptochrome (cry1aa, cry1ab, and cry1bb), period (per1a, per1b, per2, and per3), RAR-related orphan receptor C b (rorcb), Basic Helix-Loop-Helix Family Member E41 (bhlhe41) and Nuclear Receptor Subfamily 1 Group D Member 1 (nr1d1). All paralogs of period and 2 out of 3 cryptochrome genes were upregulated in all 3 treatment groups (Figure 4B). All these genes except cry1aa and cry1bb are differentially expressed also at 1% FDR, suggesting that circadian rhythm pathway is a target of developmental exposure to PCB153. The downregulated genes are associated with p53 signaling, apoptosis, and cell-cycle pathways. The number of differentially expressed genes in each treatment group and the adjusted p-values for each pathway are shown in Table 1. The genes that are represented under each KEGG and GO term are provided in the Supplementary Information 4.
Figure 4.
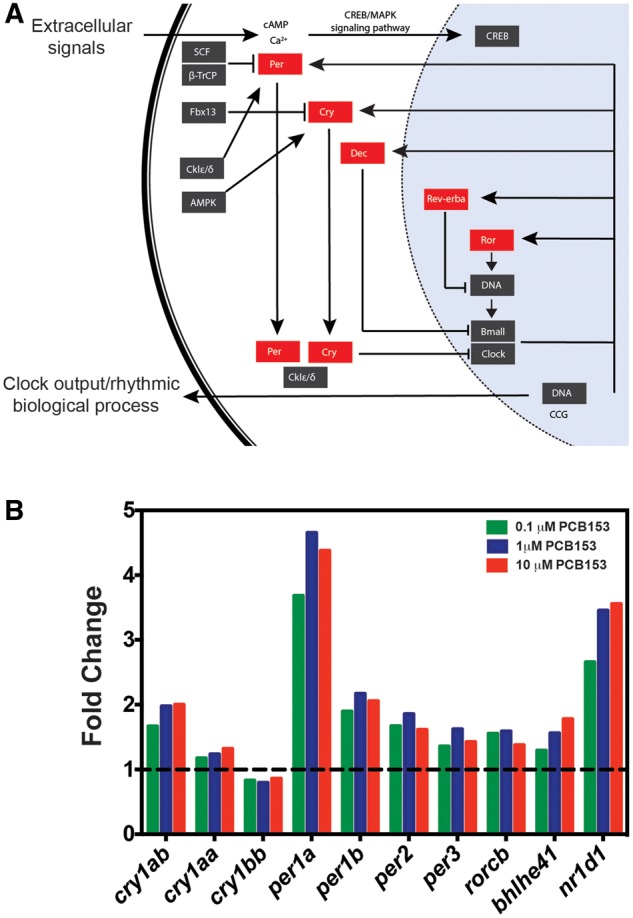
PCB153 exposure caused upregulation of genes associated with circadian rhythm. A. KEGG circadian rhythm pathway with upregulated genes highlighted in red boxes. RNAseq results showed upregulation of these genes in all 3 PCB153 treatments. B. Histogram of fold change (relative to DMSO control) values of the highlighted genes (A) in different treatment groups (RNAseq results; 4 biological replicates per sample). Our results demonstrate that all duplicate genes in zebrafish were altered in a similar fashion with PCB153 treatment. Dotted line represents fold change of 1 (no change).
We confirmed the expression of 3 differentially expressed genes in the circadian rhythm pathway (per1a, per1b, and nr1d1) by quantitative real-time PCR in an independent exposure experiment (Supplementary Figure 1). The expression patterns in this experiment were in agreement with the RNAseq results. All 3 genes were upregulated in response to PCB153 exposure.
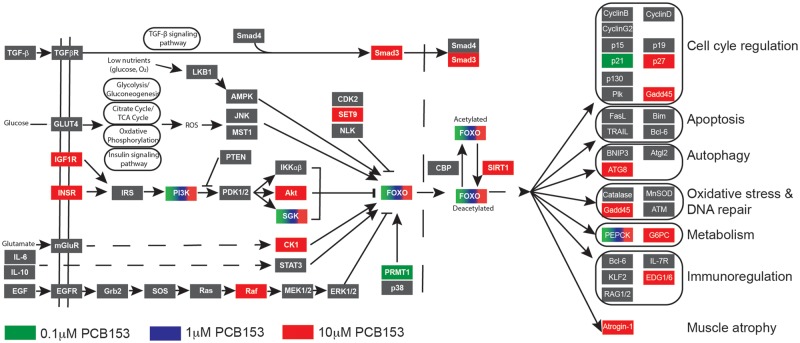
Another pathway that was significantly enriched in response to PCB153 exposure is the FoxO signaling pathway (Figure 5). The genes associated with this pathway were differentially expressed in all 3 treatment groups, with 32 genes in the high-dose group (Table 1). The FoxO signaling pathway is tightly connected with insulin signaling and carbohydrate metabolism, 2 pathways that were also enriched in response to PCB153 exposure.
Figure 5.
PCB153 exposure altered forkhead box O (FOXO) signaling pathway. The FOXO family of transcription factors regulates the expression of genes involved in important physiological functions such as cell-cycle regulation, apoptosis, oxidative stress resistance, DNA repair, and metabolism. We observed upregulation of FOXO and other genes in this signaling pathway. KEGG FOXO signaling pathway with genes upregulated in our data set highlighted.
Circadian behavior assays
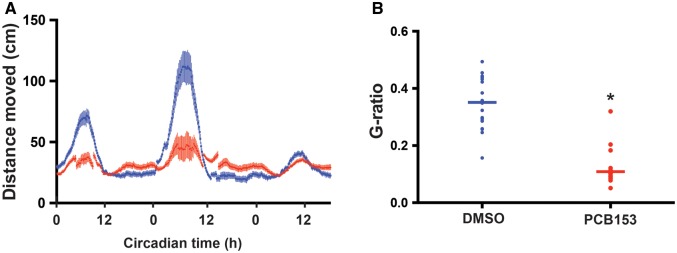
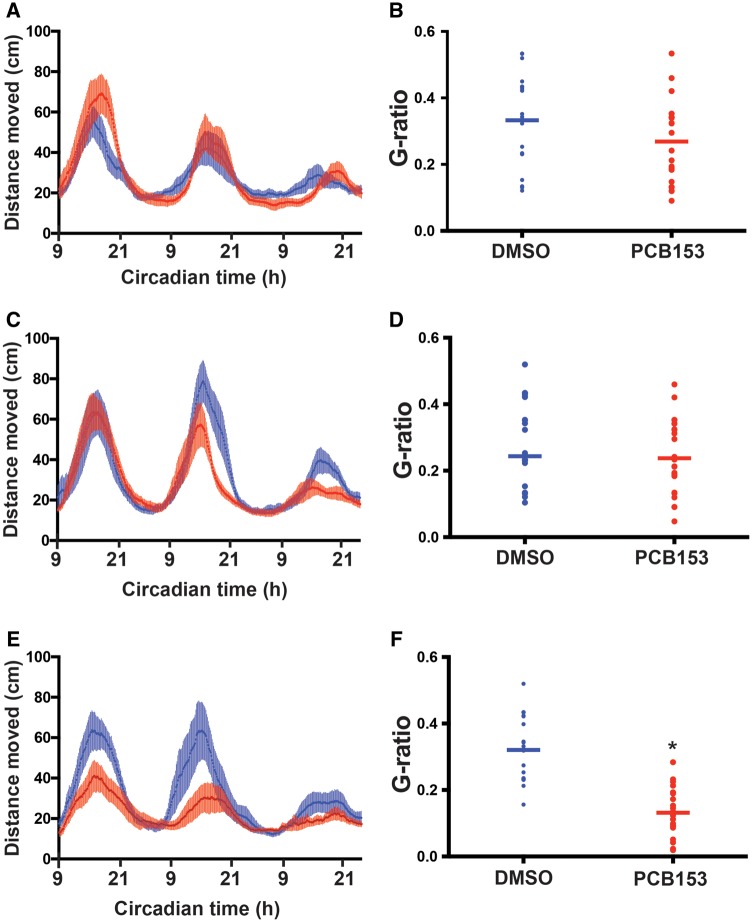
Results from the transcriptomic analysis suggested that circadian regulation was disrupted by PCB153 exposure, therefore, we examined circadian behavior in the exposed larvae. Developmental exposure to PCB153 significantly affected circadian behavior in zebrafish larvae. The amplitude of locomotor activity was significantly reduced in the PCB153-exposed (1 µM) larvae compared to the control group (Figure 6). This effect of PCB153 was dependent on the developmental window of exposure. When embryos were exposed between 4–24 and 4–48 hpf, there was no effect on the circadian behavior. However, when zebrafish embryos were exposed from 4 to 72 hpf, circadian behavior was significantly altered (Supplementary Figure 2). In order to confirm that the effects observed between 48 and 72 hpf was not caused by the increased body burden of PCB153 due to repeated exposures, we conducted separate exposures at 4–24, 24–48, and 48–72 hpf (Figure 7). Circadian behavioral analysis demonstrated that only larvae exposed to 48–72 hpf showed altered behavior. No differences in circadian behavioral pattern was observed in PCB153 exposed larvae at 4–24 and 24–48 hpf.
Figure 6.
Developmental PCB153 exposure on altered circadian behavior. Zebrafish embryos were exposed to either 1 μM PCB153 or dimethyl sulfoxide (DMSO) control from 4 to 120 h post-fertilization and circadian behavior was assessed for 3 days starting from 6 days post-fertilization. A. Average locomotor activity (moving average of 10 sliding points) is plotted against circadian time. B. G-factor ratios, a determinant of the extent to which the observed locomotor behavior is circadian in nature is calculated for DMSO control and PCB153 exposed groups. The detailed data analysis steps are provided in the methods section. p-value of <.05 is considered statistically significant.
Figure 7.
Identifying the window of sensitivity during developmental exposure to PCB153 that altered circadian behavior. Zebrafish embryos were exposed to either 1 μM PCB153 or dimethyl sulfoxide (DMSO) control from 4 to 24 h post-fertilization (hpf) (A, B), 24–48 hpf (C, D), and 48–72 hpf (E, F) and circadian behavior was assessed for 2–3 days starting from 4 days post-fertilization. Average locomotor activity (moving average of 10 sliding points) is plotted against circadian time (A, C, and E). G-factor ratios, a determinant of the extent to which the observed locomotor behavior is circadian in nature is calculated for DMSO control and PCB153 exposed groups (B, D, and F). The detailed data analysis steps are provided in the methods section. p-value of <.05 is considered statistically significant. *Denotes significant difference from DMSO.
DISCUSSION
The results from this study demonstrate that developmental exposure to PCB153 alters the expression of circadian rhythm genes in a dose-dependent manner. Investigation of circadian behavioral patterns revealed that exposure during a specific window of development (48–72 hpf) disrupts circadian behavior. In addition, transcriptional responses revealed differential expression of genes associated with the FoxO and insulin signaling pathways, apoptosis, p53 signaling, and cell cycle, suggesting that PCB153 exposure during development affects diverse processes.
One of the key findings was the impact of exposure on the expression of negative regulators of the circadian clock, particularly period (per1a, per1b, per2, and per3) and cryptochrome (cry1aa, cry1ab, and cry1bb) genes. Circadian rhythm is regulated by a molecular mechanism that consists of negative and positive feedback loops. PER and CRY proteins inhibit their own transcriptional activators, 2 master regulators of circadian clock—CLOCK (circadian locomotor output cycles kaput) and BMAL1 (brain and muscle AHR nuclear translocator-like 1). These 2 transcription factors heterodimerize and activate the expression of many circadian rhythm genes including period and cryptochrome during light phases. The PER and CRY proteins accumulate and dimerize to form a repressor complex during dark phases and inhibit CLOCK and BMAL. CLOCK:BMAL1 dimers also regulate the levels of the nuclear receptors RORα (retinoid-related orphan receptor α) and REV-ERBα (nuclear receptor subfamily 1, group D, member 1 [NR1D1]), which constitute the “stabilizing/auxiliary loop” (Savvidis and Koutsilieris, 2012). This autoregulatory transcriptional feedback loop promotes cyclic accumulations of target gene transcripts, thus generating rhythmic physiological outputs (eg, body temperature, metabolism). In addition to changes in the primary clock genes, we also observed an increase in the expression of genes in the auxiliary loop (rorcb, nr1d1, and bhlhe41).
Altered expression of circadian rhythm genes in response to PCB153 exposure could affect multiple physiological pathways as most cellular processes are under the influence of the circadian clock. A recent study demonstrated that ortho-PCBs preferentially accumulate in the brain and their accumulation is photoperiod-dependent (Skipor et al., 2012). Ortho PCB effects on gene expression suggest that the pineal gland, the master regulator of biological clock, is affected by exposure (Szczepkowska et al., 2012). However, the effects on circadian rhythm genes seem to be dependent on the exposure regime. A recent study exposed zebrafish embryos to PCB153 for 6 h and did not observe changes in the circadian rhythm genes (Goldstone et al., 2015), suggesting that these effects are dependent on the timing and duration of exposure during development.
We did not investigate the effects of exposure duration on circadian rhythm gene expression but we characterized the developmental windows of exposure that affects circadian behavior. Our results suggest that there is a developmental window from 48 to 72 hpf during which exposure to PCB153 affects circadian behavior. In zebrafish embryos, the pineal gland—the central regulator of circadian rhythms—is present by 19–20 hpf, and arylalkylamine-N-acetyltransferase (aanat2), the key enzyme in melatonin production, is expressed at 22 hpf, but circadian rhythmicity is not observed until 48 hpf (Gothilf et al., 1999; Kazimi and Cahill, 1999). This suggests that exposures during this period may not affect circadian behavior. However, based on the bioaccumulative capacity of PCB153 (log Kow = 6.9), we cannot rule out the possibility that there is a threshold level of accumulation before the phenotypes are observed. Given the fact that circadian rhythms regulate many physiological processes, it is important to further investigate the mechanism by which environmental chemicals alter the circadian clock (Bass and Takahashi, 2010; Prokkola and Nikinmaa, 2018). Endocrine disrupting compounds affect the expression of circadian rhythm genes in a variety of model systems (Kopp et al., 2017; Ochiai et al., 2018; Prokkola et al., 2015; Zhao et al., 2015). For instance, TCDD and dioxin-like PCBs that act via the AHR have been shown to affect the expression of circadian rhythm genes (Shimba and Watabe, 2009) suggesting a crosstalk between AHR and circadian clock signaling. Our study demonstrates that the circadian pathway is also a target of ortho-PCBs.
It is well-established that there is extensive crosstalk between circadian clock and metabolism (Bass, 2012; Bass and Takahashi, 2010; Nakahata et al., 2009; Reinke and Asher, 2019) and this occurs via transcriptional and epigenetic mechanisms (Belden et al., 2011; Fustin et al., 2018; Vollmers et al., 2012; Zhong et al., 2018). Among the core set of differentially expressed genes in all PCB153 treatment groups in the present study are those associated with the FoxO and insulin signaling pathways. FoxO signaling is involved in the regulation of various physiological functions including apoptosis, cell-cycle control, glucose metabolism, oxidative stress, immune function, and longevity (Lee and Dong, 2017; Link, 2019; Stefanetti et al., 2018). The FoxO family of transcription factors consists of 4 genes (FOXO1, FOXO3, FOXO4, and FOXO6), which mainly mediate the inhibitory functions of insulin or insulin like growth factors (Lee and Dong, 2017). Abnormal expression of these proteins has been associated with metabolic disease and altered life span in mammals. We observed transcriptional upregulation of 4 FoxO genes (foxo1, foxo3a, foxo3b, and foxo4), all of which have been shown to play an important role in the interplay between circadian rhythm and metabolism (Chaves et al., 2014). In addition, FOXO3 has been shown to be involved in PCB153 exposure-induced metabolic dysfunction (Mesnier et al., 2015).
Concomitant with the upregulation of FoxO transcripts, insulin signaling pathway genes were also upregulated, particularly in the medium- and high-dose groups. The differentially expressed genes include insulin receptors (insrb and irs2b), as well as downstream signaling molecules that are involved in mitogenic and metabolic actions of insulin (phkg1b, phkg1a, phka2, ppp1r3ab, acacb, and mknk1), suggesting impaired insulin sensitivity. Epidemiological studies have demonstrated a strong correlation between PCB153 exposure and risk of developing metabolic diseases such as diabetes (Pizzorno, 2016). A number of nuclear receptors including peroxisome proliferator-activated receptors, constitutive androstane receptor (CAR), pregnane X receptor (PXR), retinoid X receptor, liver X receptor, and farnesoid X receptor have been implicated in metabolic disruption caused by environmental chemicals (Casals-Casas and Desvergne, 2011; Grun and Blumberg, 2006; Schug et al., 2011). Recent studies have suggested involvement of CAR and PXR in PCB153 responses (Al-Salman and Plant, 2012; Grans et al., 2015; Kopec et al., 2010). There is no CAR in zebrafish, but PXR showed a modest but statistically significant dose-dependent induction (1.34–1.82 fold change) in response to PCB153 exposure. However, the exposure regime followed in this study is not aimed at addressing the mechanism of action, and the upregulation of PXR could be secondary or an indirect effect of exposure.
PCB153 exposure downregulated the expression of genes associated with apoptosis, p53 signaling and cell cycle in a dose-dependent manner. Similar effects have been reported previously in cell lines exposed to PCB153 (Al-Anati et al., 2014; Liu et al., 2014). Aberrant proliferation and apoptosis of hepatocytes exposed to PCB153 has been shown to be due to the activation of NF-κB and caspase inhibition (Liu et al., 2014). It was also demonstrated that activation of PI3K/Akt and ERK pathways play critical roles in PCB153-induced hepatotoxicity. We observed downregulation of several players in the NF-κB-Gadd45-MAPK pathway an important pathway in the cellular damage response system (Yang et al., 2009), suggesting that exposure during development severely affects DNA repair and cell survival.
CONCLUSIONS
Circadian rhythms shape animal physiology, metabolism, and behavior. There is growing evidence that environmental chemicals disrupt circadian rhythms (Kopp et al., 2017; Ochiai et al., 2018; Prokkola et al., 2015; Zhao et al., 2015; this study). In addition, exposure to psychological stress, late-night work activity, and artificial light (leading to sleep reduction), disrupt circadian rhythmicity, and metabolism (Koch et al., 2017; Park et al., 2019; Stevens and Zhu, 2015). One of the major consequences of altered circadian rhythmicity is disruption in cellular metabolism, leading to various metabolic, immune, and reproductive diseases. As shown by our results, PCB153 exposure caused transcriptional changes in a variety of physiological processes. We hypothesize that these changes are related to the disruption to circadian clock. Importantly, the circadian clock and metabolism display extensive crosstalk. Thus, a better understanding of the mechanisms by which environmental chemicals such as PCBs affect these processes is essential for developing mitigation strategies against chemical-induced metabolic diseases.
DECLARATION OF CONFLICTING INTERESTS
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank Drs Yoav Gothilf and Zohar Livne, Tel-Aviv University for generously sharing their circadian behavior protocol and data analysis script.
FUNDING
Woods Hole Center for Oceans and Human Health (NIH grant P01ES021923 and National Science Foundation Grant OCE-1314642), WHOI Summer Student Fellowship and NSF REU OCE-1659463 (to A.M.).
REFERENCES
- Al-Anati L., Kadekar S., Hogberg J., Stenius U. (2014). PCB153, TCDD and estradiol compromise the benzo[a]pyrene-induced p53-response via FoxO3a. Chem. Biol. Interact. 219, 159–167. [DOI] [PubMed] [Google Scholar]
- Al-Salman F., Plant N. (2012). Non-coplanar polychlorinated biphenyls (PCBs) are direct agonists for the human pregnane-X receptor and constitutive androstane receptor, and activate target gene expression in a tissue-specific manner. Toxicol. Appl. Pharmacol. 263, 7–13. [DOI] [PubMed] [Google Scholar]
- Aluru, N., Krick, K.S., McDonald, A.M., Karchner, S.I. (2019) Data from: Developmental exposure to PCB153 (2,2',4,4',5,5'-hexachlorobiphenyl) alters circadian rhythms and the expression of clock and metabolic genes, Dryad, Dataset, https://doi.org/10.5061/dryad.p1b140p. Accessed October 31, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders S., Pyl P. T., Huber W. (2015). HTSeq—A Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews S. (2010). FastQC: A quality control tool for high throughput sequence data Available at: http://www.bioinformatics.babraham.ac.uk/projects/fastqc. Accessed July 2019.
- Bass J. (2012). Circadian topology of metabolism. Nature 491, 348–356. [DOI] [PubMed] [Google Scholar]
- Bass J., Takahashi J. S. (2010). Circadian integration of metabolism and energetics. Science 330, 1349–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behl M., Ryan K., Hsieh J. H., Parham F., Shapiro A. J., Collins B. J., Sipes N. S., Birnbaum L. S., Bucher J. R., Foster P. M. D., et al. (2019). Screening for developmental neurotoxicity at the national toxicology program: The future is here. Toxicol. Sci. 167, 6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belden W. J., Lewis Z. A., Selker E. U., Loros J. J., Dunlap J. C. (2011). CHD1 remodels chromatin and influences transient DNA methylation at the clock gene frequency. PLoS Genet. 7, e1002166.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Moshe Livne Z., Alon S., Vallone D., Bayleyen Y., Tovin A., Shainer I., Nisembaum L. G., Aviram I., Smadja-Storz S., Fuentes M., et al. (2016). Genetically blocking the zebrafish pineal clock affects circadian behavior. PLoS Genet. 12, e1006445.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford Y. M., Toro S., Ramachandran S., Ruzicka L., Howe D. G., Eagle A., Kalita P., Martin R., Taylor Moxon S. A., Schaper K., et al. (2017). Zebrafish models of human disease: Gaining insight into human disease at ZFIN. ILAR J. 58, 4–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casals-Casas C., Desvergne B. (2011). Endocrine disruptors: From endocrine to metabolic disruption. Annu. Rev. Physiol. 73, 135–162. [DOI] [PubMed] [Google Scholar]
- Castoldi A. F., Blandini F., Randine G., Samuele A., Manzo L., Coccini T. (2006). Brain monoaminergic neurotransmission parameters in weanling rats after perinatal exposure to methylmercury and 2,2',4,4',5,5'-hexachlorobiphenyl (PCB153). Brain Res. 1112, 91–98. [DOI] [PubMed] [Google Scholar]
- Chaves I., van der Horst G. T., Schellevis R., Nijman R. M., Koerkamp M. G., Holstege F. C., Smidt M. P., Hoekman M. F. (2014). Insulin-FOXO3 signaling modulates circadian rhythms via regulation of clock transcription. Curr. Biol. 24, 1248–1255. [DOI] [PubMed] [Google Scholar]
- Coccini T., Roda E., Castoldi A. F., Poli D., Goldoni M., Vettori M. V., Mutti A., Manzo L. (2011). Developmental exposure to methylmercury and 2,2',4,4',5,5'-hexachlorobiphenyl (PCB153) affects cerebral dopamine D1-like and D2-like receptors of weanling and pubertal rats. Arch. Toxicol. 85, 1281–1294. [DOI] [PubMed] [Google Scholar]
- Dobin A., Gingeras T. R. (2015). Mapping RNA-seq reads with STAR. Curr. Protoc. Bioinformatics 51, 11.14.1–11.14.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez M. P., Ikonomou M. G., Courtenay S. C., Wirgin I. I. (2004). Spatial variation in hepatic levels and patterns of PCBs and PCDD/Fs among young-of-the-year and adult Atlantic tomcod (Microgadus tomcod) in the Hudson River estuary. Environ. Sci. Technol. 38, 976–983. [DOI] [PubMed] [Google Scholar]
- Fustin J.-M., Kojima R., Itoh K., Chang H.-Y., Ye S., Zhuang B., Oji A., Gibo S., Narasimamurthy R., Virshup D., et al. (2018). Two Ck1delta transcripts regulated by m6A methylation code for two antagonistic kinases in the control of the circadian clock. Proc. Natl. Acad. Sci. U.S.A. 115, 5980–5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazer, L., Hahn, M. E., Aluru, N. (2016). Delayed effects of developmental exposure to low levels of the aryl hydrocarbon receptor agonist 3,3',4,4',5-pentachlorobiphenyl (PCB126) on adult zebrafish behavior. Neurotoxicology 52,134–143. [DOI] [PMC free article] [PubMed]
- Goldstone J. V., Lemaire B., Kubota A., Stegeman J. J. Transcriptomic effects of ortho-PCBs on developing zebrafish 2015. p. Abstract #1756.
- Gothilf Y., Coon S. L., Toyama R., Chitnis A., Namboodiri M. A., Klein D. C. (1999). Zebrafish serotonin N-acetyltransferase-2: Marker for development of pineal photoreceptors and circadian clock function. Endocrinology 140, 4895–4903. [DOI] [PubMed] [Google Scholar]
- Grans J., Wassmur B., Fernandez-Santoscoy M., Zanette J., Woodin B. R., Karchner S. I., Nacci D. E., Champlin D., Jayaraman S., Hahn M. E., et al. (2015). Regulation of pregnane-X-receptor, CYP3A and P-glycoprotein genes in the PCB-resistant killifish (Fundulus heteroclitus) population from New Bedford Harbor. Aquat. Toxicol. 159, 198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grun F., Blumberg B. (2006). Environmental obesogens: Organotins and endocrine disruption via nuclear receptor signaling. Endocrinology 147(6 Suppl), S50–S55. [DOI] [PubMed] [Google Scholar]
- Grunwald D. J., Eisen J. S. (2002). Headwaters of the zebrafish—Emergence of a new model vertebrate. Nat. Rev. Genet. 3, 717–724. [DOI] [PubMed] [Google Scholar]
- Haave M., Bernhard A., Jellestad F. K., Heegaard E., Brattelid T., Lundebye A. K. (2011). Long-term effects of environmentally relevant doses of 2,2',4,4',5,5' hexachlorobiphenyl (PCB153) on neurobehavioural development, health and spontaneous behaviour in maternally exposed mice. Behav. Brain Funct. 7, 3.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn M. E., Karchner S. I., Becker C., Aluru N., Franks D. G., Goldstone J. V., Stegeman J. J., Champlin D., Nacci D., Clark B., et al. Developmental effects of ortho-substituted PCB-153: Evidence for altered glucose homeostasis in PCB-sensitive killifish and in zebrafish but not in PCB-tolerant killifish from a Superfund site. 2018, p. 245 (Abstract #2010).
- Hartle J. C., Cohen R. S., Sakamoto P., Barr D. B., Carmichael S. L. (2018). Chemical contaminants in raw and pasteurized human milk. J. Hum. Lact. 34, 340–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayama J., Kaneko M., Cardone L., Cahill G., Sassone-Corsi P. (2005). Analysis of circadian rhythms in zebrafish. Methods Enzymol. 393, 186–204. [DOI] [PubMed] [Google Scholar]
- Honma T., Suda M., Miyagawa M., Wang R. S., Kobayashi K., Sekiguchi S. (2009). Alteration of brain neurotransmitters in female rat offspring induced by prenatal administration of 16 and 64 mg/kg of 2,2',4,4',5,5'-hexachlorobiphenyl (PCB153). Ind. Health 47, 11–21. [DOI] [PubMed] [Google Scholar]
- Kazimi N., Cahill G. M. (1999). Development of a circadian melatonin rhythm in embryonic zebrafish. Brain Res. Dev. Brain Res. 117, 47–52. [DOI] [PubMed] [Google Scholar]
- Koch C. E., Leinweber B., Drengberg B. C., Blaum C., Oster H. (2017). Interaction between circadian rhythms and stress. Neurobiol. Stress 6, 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopec A. K., Burgoon L. D., Ibrahim-Aibo D., Mets B. D., Tashiro C., Potter D., Sharratt B., Harkema J. R., Zacharewski T. R. (2010). PCB153-elicited hepatic responses in the immature, ovariectomized C57BL/6 mice: Comparative toxicogenomic effects of dioxin and non-dioxin-like ligands. Toxicol. Appl. Pharmacol. 243, 359–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp R., Martinez I. O., Legradi J., Legler J. (2017). Exposure to endocrine disrupting chemicals perturbs lipid metabolism and circadian rhythms. J. Environ. Sci. (China) 62, 133–137. [DOI] [PubMed] [Google Scholar]
- Lake J. L., McKinney R., Lake C. A., Osterman F. A., Heltshe J. (1995). Comparisons of patterns of polychlorinated biphenyl congeners in water, sediment, and indigenous organisms from New Bedford Harbor, Massachusetts. Arch. Environ. Contam. Toxicol. 29, 207–220. [Google Scholar]
- Lancz K., Hertz-Picciotto I., Jusko T. A., Murinova L., Wimmerova S., Sovcikova E., Dedik L., Stremy M., Drobna B., Farkasova D., et al. (2015). Duration of breastfeeding and serum PCB 153 concentrations in children. Environ. Res. 136, 35–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Dong H. H. (2017). FoxO integration of insulin signaling with glucose and lipid metabolism. J. Endocrinol. 233, R67–R79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Jiang T., Jing L., Ni L., Hua J., Chen Y. (2014). Characteristics and risk assessment of PCBs in drinking water source reservoirs of the Zhoushan Islands, East China. Lake Reserv. Manage. 30, 273–284. [Google Scholar]
- Link W. (2019). Introduction to FOXO biology. Methods Mol. Biol. 1890, 1–9. [DOI] [PubMed] [Google Scholar]
- Liu C., Ha M., Cui Y., Wang C., Yan M., Fu W., Quan C., Zhou J., Yang K. (2012). JNK pathway decreases thyroid hormones via TRH receptor: A novel mechanism for disturbance of thyroid hormone homeostasis by PCB153. Toxicology 302, 68–76. [DOI] [PubMed] [Google Scholar]
- Liu C., Wang C., Yan M., Quan C., Zhou J., Yang K. (2012). PCB153 disrupts thyroid hormone homeostasis by affecting its biosynthesis, biotransformation, feedback regulation, and metabolism. Horm. Metab. Res. 44, 662–669. [DOI] [PubMed] [Google Scholar]
- Liu C., Yang J., Fu W., Qi S., Wang C., Quan C., Yang K. (2014). Coactivation of the PI3K/Akt and ERK signaling pathways in PCB153-induced NF-kappaB activation and caspase inhibition. Toxicol. Appl. Pharmacol. 277, 270–278. [DOI] [PubMed] [Google Scholar]
- McLachlan M. S., Undeman E., Zhao F., MacLeod M. (2018). Predicting global scale exposure of humans to PCB 153 from historical emissions. Environ. Sci. Process Impacts 20, 747–756. [DOI] [PubMed] [Google Scholar]
- Mesnier A., Champion S., Louis L., Sauzet C., May P., Portugal H., Benbrahim K., Abraldes J., Alessi M. C., Amiot-Carlin M. J., et al. (2015). The transcriptional effects of PCB118 and PCB153 on the liver, adipose tissue, muscle and colon of mice: Highlighting of Glut4 and Lipin1 as main target genes for PCB induced metabolic disorders. PLoS One 10, e0128847.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahata Y., Sahar S., Astarita G., Kaluzova M., Sassone-Corsi P. (2009). Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science 324, 654–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura Y., Inoue A., Sasagawa S., Koiwa J., Kawaguchi K., Kawase R., Maruyama T., Kim S., Tanaka T. (2016). Using zebrafish in systems toxicology for developmental toxicity testing. Congenit. Anom. (Kyoto )56, 18–27. [DOI] [PubMed] [Google Scholar]
- Ochiai M., Iida M., Agusa T., Takaguchi K., Fujii S., Nomiyama K., Iwata H. (2018). Effects of 4-Hydroxy-2,3,3',4',5-Pentachlorobiphenyl (4-OH-CB107) on liver transcriptome in rats: Implication in the disruption of circadian rhythm and fatty acid metabolism. Toxicol. Sci. 165, 118–130. [DOI] [PubMed] [Google Scholar]
- Oliveira T., Santacroce G., Coleates R., Hale S., Zevin P., Belasco B. (2011). Concentrations of polychlorinated biphenyls in water from US Lake Ontario tributaries between 2004 and 2008. Chemosphere 82, 1314–1320. [DOI] [PubMed] [Google Scholar]
- Park Y. M., White A. J., Jackson C. L., Weinberg C. R., Sandler D. P. (2019). Association of exposure to artificial light at night while sleeping with risk of obesity in women. JAMA Intern. Med. 179, 1061.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piedrafita B., Erceg S., Cauli O., Felipo V. (2008a). Developmental exposure to polychlorinated biphenyls or methylmercury, but not to its combination, impairs the glutamate-nitric oxide-cyclic GMP pathway and learning in 3-month-old rats. Neuroscience 154, 1408–1416. [DOI] [PubMed] [Google Scholar]
- Piedrafita B., Erceg S., Cauli O., Monfort P., Felipo V. (2008b). Developmental exposure to polychlorinated biphenyls PCB153 or PCB126 impairs learning ability in young but not in adult rats. Eur. J. Neurosci. 27, 177–182. [DOI] [PubMed] [Google Scholar]
- Pizzorno J. (2016). Is the diabetes epidemic primarily due to toxins? Integr. Med. (Encinitas) 15, 8–17. [PMC free article] [PubMed] [Google Scholar]
- Prokkola J. M., Nikinmaa M. (2018). Circadian rhythms and environmental disturbances—Underexplored interactions. J. Exp. Biol. 221, jeb179267.. [DOI] [PubMed] [Google Scholar]
- Prokkola J. M., Nikinmaa M., Lubiana P., Kanerva M., McCairns R. J., Gotting M. (2015). Hypoxia and the pharmaceutical diclofenac influence the circadian responses of three-spined stickleback. Aquat. Toxicol. 158, 116–124. [DOI] [PubMed] [Google Scholar]
- Reimand J., Arak T., Adler P., Kolberg L., Reisberg S., Peterson H., Vilo J. (2016). g:Profiler—A web server for functional interpretation of gene lists (2016 update). Nucleic Acids Res. 44, W83–W89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinke H., Asher G. (2019). Crosstalk between metabolism and circadian clocks. Nat. Rev. Mol. Cell Biol. 20, 227–241. [DOI] [PubMed] [Google Scholar]
- Robinson M. D., McCarthy D. J., Smyth G. K. (2010). edgeR: A bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sable HJK, Schantz SL. (2006). Executive Function following Developmental Exposure to Polychlorinated Biphenyls (PCBs): What Animal Models Have Told Us. In: Levin ED, Buccafusco JJ, editors. Animal Models of Cognitive Impairment. Boca Raton (FL): CRC Press/Taylor & Francis. Chapter 8. Available from: https://www.ncbi.nlm.nih.gov/books/NBK2531/. Accessed October 31, 2019. [PubMed] [Google Scholar]
- Savvidis C., Koutsilieris M. (2012). Circadian rhythm disruption in cancer biology. Mol. Med. 18, 1249–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schug T. T., Janesick A., Blumberg B., Heindel J. J. (2011). Endocrine disrupting chemicals and disease susceptibility. J. Steroid. Biochem. Mol. Biol. 127, 204–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimba S., Watabe Y. (2009). Crosstalk between the AHR signaling pathway and circadian rhythm. Biochem. Pharmacol. 77, 560–565. [DOI] [PubMed] [Google Scholar]
- Skipor J., MŁynarczuk JŁaw., Szczepkowska A., Lagaraine C., Grochowalski A., Guillaume D., Dufourny L., Thiéry J.-C. (2012). Photoperiod modulates access of 2,2',4,4',5,5'-hexachlorobiphenyl (PCB153) to the brain and its effect on gonadotropin and thyroid hormones in adult ewes. Ecotoxicol. Environ. Saf. 78, 336–343. [DOI] [PubMed] [Google Scholar]
- Smedley D., Haider S., Durinck S., Pandini L., Provero P., Allen J., Arnaiz O., Awedh M. H., Baldock R., Barbiera G., et al. (2015). The BioMart community portal: An innovative alternative to large, centralized data repositories. Nucleic Acids Res. 43, W589–W598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanetti R. J., Voisin S., Russell A., Lamon S.. 2018. Recent advances in understanding the role of FOXO3. F1000Res, 7(F1000 Faculty Rev):1372 ( 10.12688/f1000research.15258.1). [DOI] [PMC free article] [PubMed]
- Stevens R. G., Zhu Y. (2015). Electric light, particularly at night, disrupts human circadian rhythmicity: Is that a problem? Philos. Trans. R Soc. Lond. B Biol. Sci. 370, 20140120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepkowska A., Młynarczuk J., Grochowalski A., Dufourny L., Thiéry J.-C., Skipor J. (2012). Effect of a two-week treatment with low dose of ortho-substituted polychlorinated biphenyls (PCB104 and PCB153) on VEGF-receptor system expression in the choroid plexus in adult ewes. Pol. J. Vet. Sci. 15, 621–628. [DOI] [PubMed] [Google Scholar]
- Tataroglu O., Emery P. (2014). Studying circadian rhythms in Drosophila melanogaster. Methods 68, 140–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudorache C., Slabbekoorn H., Robbers Y., Hin E., Meijer J. H., Spaink H. P., Schaaf M. J. M. (2018). Biological clock function is linked to proactive and reactive personality types. BMC Biol. 16, 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmers C., Schmitz R. J., Nathanson J., Yeo G., Ecker J. R., Panda S. (2012). Circadian oscillations of protein-coding and regulatory RNAs in a highly dynamic mammalian liver epigenome. Cell Metab. 16, 833–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Vasaikar S., Shi Z., Greer M., Zhang B. (2017). WebGestalt 2017: A more comprehensive, powerful, flexible and interactive gene set enrichment analysis toolkit. Nucleic Acids Res. 45, W130–W137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Wang S., Li W. (2012). RSeQC: Quality control of RNA-seq experiments. Bioinformatics 28, 2184–2185. [DOI] [PubMed] [Google Scholar]
- Weldon R. H., Barr D. B., Trujillo C., Bradman A., Holland N., Eskenazi B. (2011). A pilot study of pesticides and PCBs in the breast milk of women residing in urban and agricultural communities of California. J. Environ. Monit. 13, 3136–3144. [DOI] [PubMed] [Google Scholar]
- Winbush A., Gruner M., Hennig G. W., van der Linden A. M. (2015). Long-term imaging of circadian locomotor rhythms of a freely crawling C. elegans population. J Neurosci. Methods 249, 66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Song L., Huang C. (2009). Gadd45 proteins as critical signal transducers linking NF-kappaB to MAPK cascades. Curr. Cancer Drug Targets 9, 915–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates A., Akanni W., Amode M. R., Barrell D., Billis K., Carvalho-Silva D., Cummins C., Clapham P., Fitzgerald S., Gil L., et al. (2016). Ensembl 2016. Nucleic Acids Res. 44, D710–D716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Castiglioni S., Fent K. (2015). Environmental progestins progesterone and drospirenone alter the circadian rhythm network in zebrafish (Danio rerio). Environ. Sci. Technol. 49, 10155–10164. [DOI] [PubMed] [Google Scholar]
- Zhong X., Yu J., Frazier K., Weng X., Li Y., Cham C. M., Dolan K., Zhu X., Hubert N., Tao Y., et al. (2018). Circadian clock regulation of hepatic lipid metabolism by modulation of m(6)A mRNA methylation. Cell Rep. 25, 1816–1828.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.