Abstract
Nucleotide metabolism is an essential function in plants.
NUCLEOTIDE METABOLISM IN PLANTS
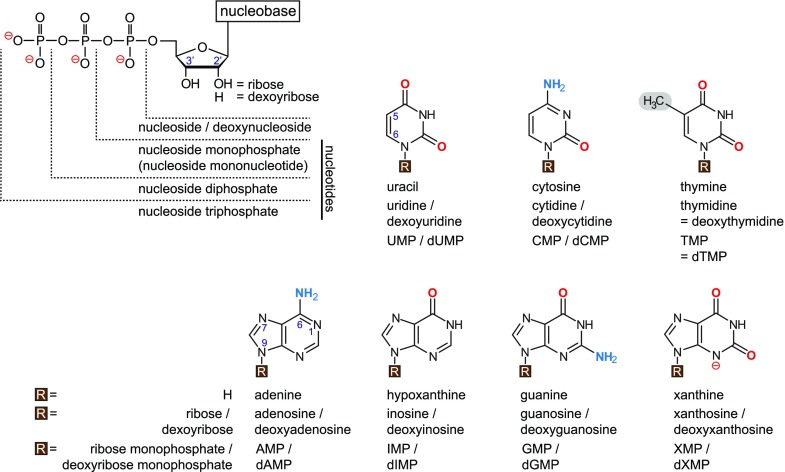
Nucleotides are essential for life. It is easy to validate this statement—one just needs to recall that nucleotides are the building blocks of DNA and RNA, and that many molecules that are central for metabolism, for example ATP, NADH, Co-A, and UDP-Glc, are nucleotides or contain nucleotide moieties. Generally, a nucleotide is defined as a phosphorylated ribose or deoxyribose linked to a nitrogen-containing heterocyclic group called the nucleobase via a glycosidic bond (Fig. 1). Because of the phosphate groups, nucleotides are negatively charged, whereas at neutral pH, nucleosides and nucleobases are uncharged. The exception is xanthine, which is partially charged as a free base (pKa 7.4) but completely charged at the base in xanthosine (pKa 5.5) or the corresponding nucleotides (Fig. 1; Sigel et al., 2009).
Figure 1.
Structural composition of nucleobases, nucleosides, and nucleotides. For the nucleobases, “R” is simply a proton. For the nucleosides, “R” is a sugar moiety that can be ribose or deoxyribose (carrying a proton instead of a hydroxyl group at the 2′ carbon of the ribose). Nucleotides have up to three phosphate groups esterified to the hydroxyl group of the 5′-carbon of the nucleoside sugar determining the prefix mono-, di-, or tri- in the name of the molecule. The terminal phosphate always carries two charges, irrespective of the number of phosphates present. The pyrimidine nucleobases (upper row) and the purine nucleobases (lower row) are shown with the groups attached to the heterocycles highlighted in red (oxo groups), blue (amino groups), and gray (methyl group). dTMP, deoxy-TMP; dXMP, deoxy-XMP; dIMP, deoxy-IMP.
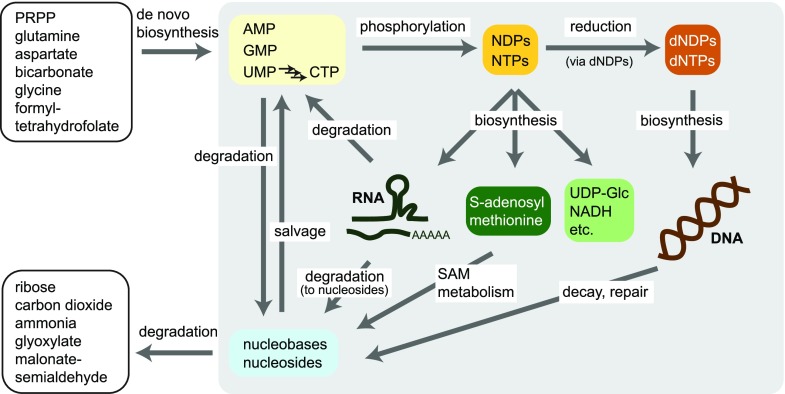
Many excellent reviews focus on general (Wagner and Backer, 1992; Moffatt and Ashihara, 2002; Stasolla et al., 2003; Zrenner et al., 2006; Zrenner and Ashihara, 2011) or particular aspects (Smith and Atkins, 2002; Kafer et al., 2004; Ashihara et al., 2018) of plant nucleotide metabolism. The aim of this review is to provide an update on how nucleotide metabolism is hardwired, mostly focusing on the cellular level, because our understanding of the organization at the tissue and organ level remains very limited. The presented models are mostly based on results from Arabidopsis (Arabidopsis thaliana). These will often be valid for most plants, but certainly there will be species-dependent variations. We also cover extracellular nucleotide metabolism and review the evidence for overlap between cytokinin metabolism and central nucleotide metabolism. Figure 2 shows a general overview of plant nucleotide metabolism.
Figure 2.
Schematic overview of plant nucleotide metabolism. Nucleotides are synthesized “de novo” from precursor molecules listed in the upper left box. The phosphorylation of nucleoside monophosphates (NMPs) via diphosphates (NDPs) generates nucleoside triphosphates (NTPs), which serve as building blocks for RNA synthesis and as precursors for the biosynthesis of the metabolites shown in the center (SAM, UDP-Glc, and NADH are given as examples). However, the NTPs, in particular ATP and GTP, are not only precursors for other metabolites, but are also essential stores of chemical energy in the phosphoanhydride bonds used in a multitude of energetic coupling reactions, as well as important donors of phosphate in kinase reactions (not shown). NDPs can be reduced to dNDPs, which after phosphorylation to dNTPs serve as precursors for DNA biosynthesis. RNA degradation in the cytosol releases nucleoside monophosphates, whereas nucleosides are produced during vacuolar RNA degradation. Adenosine and adenine are products of biochemical reactions involving SAM. Nonenzymatic decay (depurination) and enzymatic repair reactions result in nucleoside and nucleobase release from DNA. Nucleobases and nucleosides can be recycled to nucleotides in so-called salvage reactions. Plants are also capable of full nucleotide degradation via certain nucleosides and nucleobases releasing the nitrogen of the nucleobases as ammonia.
DE NOVO SYNTHESIS
Purine De Novo Synthesis
Plants possess metabolic pathways for the de novo synthesis of purine nucleotides generating AMP, as well as pyrimidine nucleotides yielding UMP. During de novo biosynthesis, nucleotides are newly synthesized from activated ribose (5-phosphoribosyl-1-pyrophosphate [PRPP]), Gln, Asp, and bicarbonate, as well as specifically for the purine nucleotides Gly and formyl tetrahydrofolate (Fig. 2).
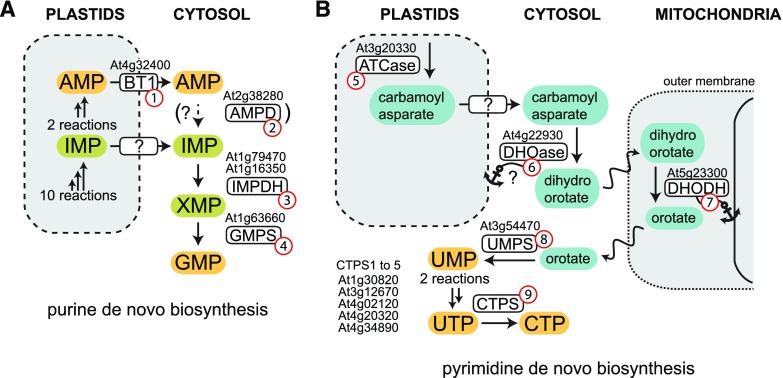
There is strong evidence that AMP biosynthesis occurs entirely in the plastids, because the 11 enzymes (catalyzing 12 reactions; Smith and Atkins, 2002) required for AMP biosynthesis in Arabidopsis all have an N-terminal organelle-targeting peptide, and C-terminal yellow fluorescent protein-fusion proteins of several of these enzymes were observed exclusively in the plastids when they were transiently expressed in Nicotiana benthamiana in our laboratory (Fig. 3A; N. Medina Escobar and C.-P. Witte, unpublished data). In rice (Oryza sativa), the pathway also seems to reside in plastids (Zhang et al., 2018). However, it has been reported that in nodules of the tropical legume cowpea (Vigna unguiculata), purine biosynthesis is targeted to plastids and mitochondria (Atkins et al., 1997; Smith and Atkins, 2002). It may be worthwhile to reconfirm this special localization in nodules using fluorescent tagged proteins.
Figure 3.
Purine and pyrimidine de novo biosynthesis. A, Purine de novo biosynthesis. B, Pyrimidine de novo biosynthesis. Enzymes and transporters include brittle1 (BT1; 1), AMP deaminase (AMPD; 2); IMP dehydrogenase (IMPDH; 3); GMP synthetase (GMPS; 4); asparate transcarbamoylase (ATCase; 5); dihydroorotatase (DHOase; 6); dihydroorotate dehydrogenase (DHODH; 7); UMP synthase (UMPS; 8); and CTP synthetase (CTPS; 9). An anchor symbol denotes an association with the respective membrane.
AMP is exported from the plastids by the adenine nucleotide uniporter BT1 (Fig. 3A, no. 1), which can also transport ADP and ATP (Leroch et al., 2005; Kirchberger et al., 2008; Hu et al., 2017). Interestingly, BT1 from Arabidopsis and maize (Zea mays) was reported to be dually localized to the chloroplast and mitochondria (Bahaji et al., 2011b) and a BT1 mutant with a severe dwarf phenotype could be complemented with a cDNA coding for an N-terminally truncated version of BT1 that is exclusively located in the mitochondria and not in the plastids (Bahaji et al., 2011a). This either indicates that there are certain tissues or developmental stages where purine nucleotide biosynthesis occurs mainly in mitochondria, or that BT1 has an alternative and essential function in this organelle. In the latter case, BT1 might still also be involved in exporting adenylates from plastids, but the abrogation of the plastidic variant would not cause the strong dwarf phenotype, indicating that de novo synthesized AMP has an alternative, BT1-independent way to leave the plastids.
GMP biosynthesis requires inosine 5′-monophosphate (IMP; Fig. 3A), which can either be derived from AMP deamination in the cytosolic compartment catalyzed by AMP deaminase (AMPD; Fig. 3A, no. 2) or from direct export of IMP from the plastids, because IMP is generated there en route to AMP (Fig. 3A). Mutation of AMPD is zygote lethal (Xu et al., 2005), and coformycin, an AMPD inhibitor, is a potent herbicide after its phosphorylation in vivo (Dancer et al., 1997). These phenotypic effects might be caused by hampered GMP biosynthesis, suggesting that AMPD could be required for this process. Consistent with this, AMPD is strongly activated by ATP (Han et al., 2006), and this regulation might balance cellular ATP and GTP concentrations. However, it has also been reported that AMPD inhibition might be detrimental by severely altering the cellular energy charge and that the GTP pool is not altered upon AMPD inhibition (Sabina et al., 2007), implying that GMP biosynthesis is independent of AMPD and that there is an alternative IMP supply from the plastids. The activity of AMPD likely resides in the cytosol, but the protein has an N-terminal transmembrane domain and is clearly attached to a membrane (Han et al., 2006).
The following enzymatic reactions for GMP biosynthesis probably take place in the cytosol: (1) oxidation of IMP to xanthosine 5′-monophosphate (XMP; Fig. 1) by IMP dehydrogenase (IMPDH; Fig. 3A, no. 3); and (2) amination of XMP to GMP by GMP synthetase (GMPS; Fig. 3A, no. 4). Neither of these enzymes has an apparent subcellular targeting peptide, and both were detected in the cytosolic proteome of Arabidopsis (Ito et al., 2011). Also, IMPDH from cowpea nodules was associated with the cytosolic fraction (Shelp and Atkins, 1983). GMP is a quite strong competitive inhibitor of IMPDH (Atkins et al., 1985), maybe resulting in feedback regulation in vivo.
Pyrimidine De Novo Synthesis
The first dedicated reaction of UMP biosynthesis (Fig. 3B) is catalyzed by aspartate transcarbamoylase (ATCase; Fig. 3B, no. 5). The enzyme is located in the plastids. The next enzyme, dihydroorotatase (DHOase; Fig. 3B, no. 6) was associated to plastids in cell fractionation studies (Doremus and Jagendorf, 1985 and references on the SUBA web server; Hooper et al., 2017) but was located in the cytosol when transiently overexpressed in Arabidopsis protoplasts as a GFP-tagged fusion protein (Witz et al., 2012). Maybe DHOase is associated with the chloroplast membrane via a protein-protein interaction, and the interaction partner is overwhelmed by strong overexpression of DHOase. The next enzyme in pyrimidine biosynthesis, dihydroorotate dehydrogenase (DHODH, Fig. 3B, no. 7), is associated with mitochondria (Doremus and Jagendorf, 1985; Witz et al., 2012) and likely located on the outer surface of the inner mitochondrial membrane, as observed for the mammalian orthologs (Ullrich et al., 2002). UMP synthase (UMPS; Fig. 3B, no. 8), the final enzyme in pyrimidine biosynthesis, was associated with the plastids and the cytosol, as shown by cell fractionation of pea (Pisum sativum) leaves (Doremus and Jagendorf, 1985) and was present in the cytosol after overexpression in Arabidopsis protoplasts (Witz et al., 2012). Thus, UMP is generated in the cytosol, while it appears that the responsible enzyme might have some affinity for the chloroplast. Because ATCase (Fig. 3B, no. 5) is feedback regulated by uridylates, in particular UMP (Doremus and Jagendorf, 1985), the cytosolic uridine nucleotide pool must be tightly connected to the plastidic pool.
CTP biosynthesis requires the phosphorylation of UMP to UTP (see section on "The Generation of Nucleoside Triphosphates"), which is the substrate of CTP synthetase (CTPS; Fig. 3B, no. 9). There are five CTPS isoenzymes in Arabidopsis, and all reside in the cytosol. For one isoform (CTPS3), the activity and stimulatory allosteric regulation by UTP and GTP have been recently shown (Daumann et al., 2018). Interestingly, some CTPS isoenzymes form filamentous aggregates, called cytoophidia, inactivating the enzyme. In vitro, these are generated in particular in the presence of CTP, indicating that the enzyme is feedback regulated by this mechanism (Daumann et al., 2018). Knockout mutants for each CTPS were characterized, and except for CTPS2, which showed a complete block of germination, no phenotypes were observed in the single mutants (Daumann et al., 2018), indicating redundancy of the CTPS enzymes in most situations.
The Generation of Nucleoside Triphosphates
The final steps of UMP, GMP, and CTP biosynthesis occur in the cytosol. With the exception of CTP, which is directly synthesized from UTP, purine and pyrimidine nucleoside triphosphate synthesis is achieved by phosphorylation of the respective monophosphates (Fig. 4).
Figure 4.
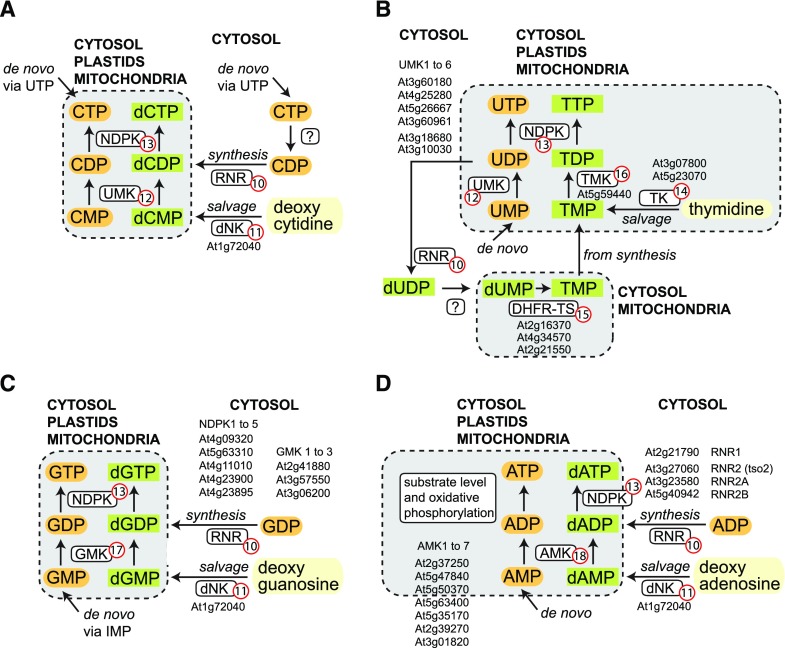
Synthesis of NTPs and dNTPs. Synthesis of cytidylates (A), uridylates and thymidylates (B), guanylates (C), and adenylates (D). RNR (10), ribonucleotide reductase; dNK (11), deoxynucleoside kinase; UMK (12), UMP kinase; NDPK (13), nucleoside diphosphate kinase; TK (14), thymidine kinase; DHFR-TS (15), dihydrofolate reductase-thymidylate synthase; TMK (16), thymidylate kinase; GMK (17), guanylate kinase; AMK (18), adenylate kinase. The subcellular locations where enzymes with these activities are found are indicated. For TMK, a location in the plastids is only assumed. The mononucleotides (AMP, GMP, UMP, and CMP) may also be derived from salvage reactions (see Figs. 5 and 6).
The plastids and the mitochondria, which possess their own transcription and translation machineries, must be supplied with ribonucleotides and deoxyribonucleotides from the cytosol. Not much is known about (1) the phosphorylation state in which nucleotides are taken up, (2) which transporters are involved, or (3) whether the concentrations of (desoxy) nucleotides differ in the distinct cellular compartments and how this may be regulated—subcellular distributions have been estimated only for the adenylates (Stitt et al., 1982). Describing the subcellular distribution of the enzymes involved in the last two steps of mononucleotide phosphorylation can help in building hypotheses regarding the exact nucleotide species imported into organelles.
The pyrimidine nucleotides, UMP and CMP, are phosphorylated by UMP kinases (UMKs; Fig. 4, A and B, no. 12) Arabidopsis possesses two evolutionarily distinct families of such enzymes: (1) UMKs related to adenylate kinases (AMKs) encoded by four genes; and (2) UMKs related to eubacterial UMP kinases encoded by two genes. The AMK-like UMKs have not yet been characterized, except for a biochemical analysis of UMK3 (At5g26667), which was shown to utilize UMP and CMP as the best substrates (Zhou et al., 1998). These enzymes have been predicted to reside in the cytosol and the mitochondria (Lange et al., 2008). From the eubacterial UMP kinase family, one member, called plastid UMP kinase (PUMKIN; At3g18680), was shown to be located in chloroplasts and to have UMK activity in vitro. Interestingly, the enzyme binds certain plastidic transcripts and is involved in plastid RNA metabolism, which may not require its enzymatic function. Mutants are small and compromised in plastid translation and photosynthetic performance (Schmid et al., 2019). The orthologous enzyme in rice is localized in chloroplasts and participates in RNA metabolism, and the corresponding loss-of-function mutants are pale green (Zhu et al., 2016; Chen et al., 2018a). Additionally, they contain less UDP and more UMP (Dong et al., 2019), suggesting that UMP phosphorylation in the chloroplast is functionally important. The phosphorylation of thymidine 5′-monophosphate (TMP) is not catalyzed by UMKs but by a dedicated TMP kinase (TMK; Fig. 4B, no. 16). In Arabidopsis, a mitochondrial and a cytosolic version are generated from a single gene by alternative splicing. Mutation of TMK leads to early seed abortion at the zygote state (Ronceret et al., 2008).
The phosphorylation of GMP to GDP and dGMP to dGDP (Kumar et al., 2000) is catalyzed by guanylate kinases (GMK; Fig. 4C, no. 17), of which plants have two different types, a cytosolic type (GKc), and an organelle type dually targeted to plastids and mitochondria (GKpm; Fig. 4C). The activity of GKpm in rice, pea, and Arabidopsis is regulated by guanosine 3′, 5′-bisdiphosphate (ppGpp), a bacterial and plastid signaling molecule (Nomura et al., 2014). Suppression of Arabidopsis GKpm (At3g06200) transcripts by RNA interference results in a pale green or albino phenotype (Sugimoto et al., 2007), emphasizing the importance of nucleotide monophosphate transport into organelles. Interestingly, the loss-of-function mutant of the rice GKpm gene is pale green but does not exhibit DNA depletion in the organelles, suggesting that deoxynucleotide and ribonucleotide metabolism are not fully linked (Sugimoto et al., 2007).
Adenylate monophosphate is converted by adenylate kinase (AMK; Fig. 4D, no. 18) into ADP. Arabidopsis has seven AMK isoforms. AMK1, AMK2, and AMK5 are located in the plastids, AMK3 and AMK4 reside in the cytosol, and AMK7 is present in the mitochondria. For AMK1, a mitochondrial localization has also been observed (Carrari et al., 2005; Lange et al., 2008). Because the plastids harbor the de novo synthesis for AMP, they will not require net import of adenylates. Consistently, the loss-of-function mutant for AMK2 has a bleached phenotype (Lange et al., 2008), suggesting that there is no net ADP or ATP import to compensate for compromised adenlyate kinase activity in plastids. A strong reduction of plastidic AMK activity in rice also results in an albino phenotype (Wei et al., 2017). In potato (Solanum tuberosum), a reduction of plastidic AMK activity led to an increase of the adenylate pool (AMP, ADP, ATP, and ADP-Glc) and increased starch synthesis in tubers, but it is still not understood how plastidic AMK and adenylate de novo synthesis are connected (Regierer et al., 2002). Interestingly, it was suggested that the AMKs might contribute to protect RNA from random misincorporation of methyl-6 A marks. AMKs are highly selective for AMP versus N6-methyl AMP released during the degradation of RNA species carrying this abundant A modification. The selectivity of the AMKs possibly prevents the formation of N6-methyl ATP, which is a substrate of RNA polymerase II (Chen et al., 2018b).
Recently, a broad-spectrum mononucleotide kinase only distantly related to the AMKs but with relatively high adenylate kinase activity (At5g60340), was described. This enzyme was localized in the nucleus, and a knockout mutant was affected in stem elongation (Feng et al., 2012).
Plastids and mitochondria possess nucleoside monophosphate kinases for all nucleotides. Thus, nucleoside monophosphates are probably imported into these organelles and are phosphorylated to dinucleotides. Enzymes catalyzing the next step to trinucleotides, the nucleoside diphosphate kinases (NDPKs), should therefore be found in plastids and mitochondria, as well as in the cytosol. This indeed has been observed (Luzarowski et al., 2017). The exact locations of the enzymes have been debated and a detailed phylogenetic analysis suggests the presence of a fourth enzyme type in the endoplasmic reticulum (ER; Dorion and Rivoal, 2015). The NDPKs are multisubstrate enzymes accepting all nucleoside/deoxynucleoside diphosphates (Zrenner et al., 2006), but there is a preference for generating GTP (Kihara et al., 2011), which in the chloroplast may assist in repairing photosystem II (Spetea and Lundin, 2012). Mutation of the gene for the plastidic NDPK in rice results in a pale green phenotype and a lower photosynthetic rate (Ye et al., 2016; Zhou et al., 2017), but since the chloroplast function is partially retained, there also must be nucleoside triphosphate import into this organelle. Interestingly, NDPKs can also have moonlighting activity as modulators of gene expression (Dorion and Rivoal, 2018).
Besides nucleotides, the nucleus and organelles need deoxynucleotides (dNTPs) for DNA synthesis. dNTP synthesis requires the reduction of the hydroxyl moiety on the 2′ carbon of the ribose by an enzyme complex called ribonucleotide reductase (RNR; Fig. 4, no. 10). The RNR complex is comprised of two large regulatory (R1) and two small catalytic (R2) subunits. Mutation of the major R2 subunit gene (tso2) results in lower dNTP concentrations and abnormal plant development, while the additional mutation of a further R2 subunit gene (Arabidopsis has three R2 subunit genes in total) is lethal (Wang and Liu, 2006). The substrates of RNR are the ribonucleotide diphosphates, suggesting that for CTP, a dedicated phosphatase might exist to support dCDP synthesis (Fig. 4A). Alternatively, CDP for dCTP synthesis might be generated from salvage of cytidine (see section on "Purine and Pyrimidine Salvage Metabolism"). Interestingly, RNR is subject to a complex allosteric regulation to adjust the correct dNTP pool sizes (Sauge-Merle et al., 1999). In plants, RNR resides exclusively in the cytosol, with the potential to relocate to the nucleus upon exposure to UV radiation (Lincker et al., 2004). The plastidic DNA replication, especially, seems to rely strongly on sufficient RNR activity, because partially compromising the function of the large RNR subunit by different mutations in the corresponding gene resulted in reduced dNTP levels and impaired chloroplast division in Arabidopsis (Garton et al., 2007). Consistently, chlorophyll biosynthesis in rice is reduced in mutants of the small RNR subunit genes (Chen et al., 2015). All dNDPs can be synthesized directly by RNR except for thymidine 5′ diphosphate, because it has no ribonucleotide counterpart. Instead, RNR catalyzes the formation of deoxy-UDP from UDP (Fig. 4B) and deoxy-UMP is methylated at C5 to TMP catalyzed by thymidilate synthase. In Arabidopsis, three enzymes were recently characterized as thymidylate synthases, which are also dihydrofolate reductases (DHFR-TS; Fig. 4B, no. 15), with only two isoforms displaying thymidylate synthase activity (Gorelova et al., 2017). Interestingly, in roots the subcellular locations of all isoforms depends on the developmental stage of the cells: cytosolic, nuclear, and mitochondrial (but not plastidic) locations have been observed. The two active isoforms seem to be redundant, since only a double mutant of the respective genes is lethal, whereas single-gene loss-of-function mutants are phenotypically inconspicuous (Gorelova et al., 2017). The substrate for dihydrofolate reductase-thymidylate synthase is dUMP (Gorelova et al., 2017), but the RNR provides dUDP. It is unknown which enzyme links these two processes in vivo. An alternative dUMP source in mitochondria is the deamination of dCMP, as shown recently in rice (Xu et al., 2014; Niu et al., 2017).
SALVAGE AND DEGRADATION
Metabolic Sources of Nucleosides and Nucleobases
Nucleosides and nucleobases can be released from nucleotides or nucleic acids during metabolism (Figs. 2, 5, and 6) or can be taken up from the environment (Girke et al., 2014), where they can occur in substantial amounts (Phillips et al., 1997).
Figure 5.
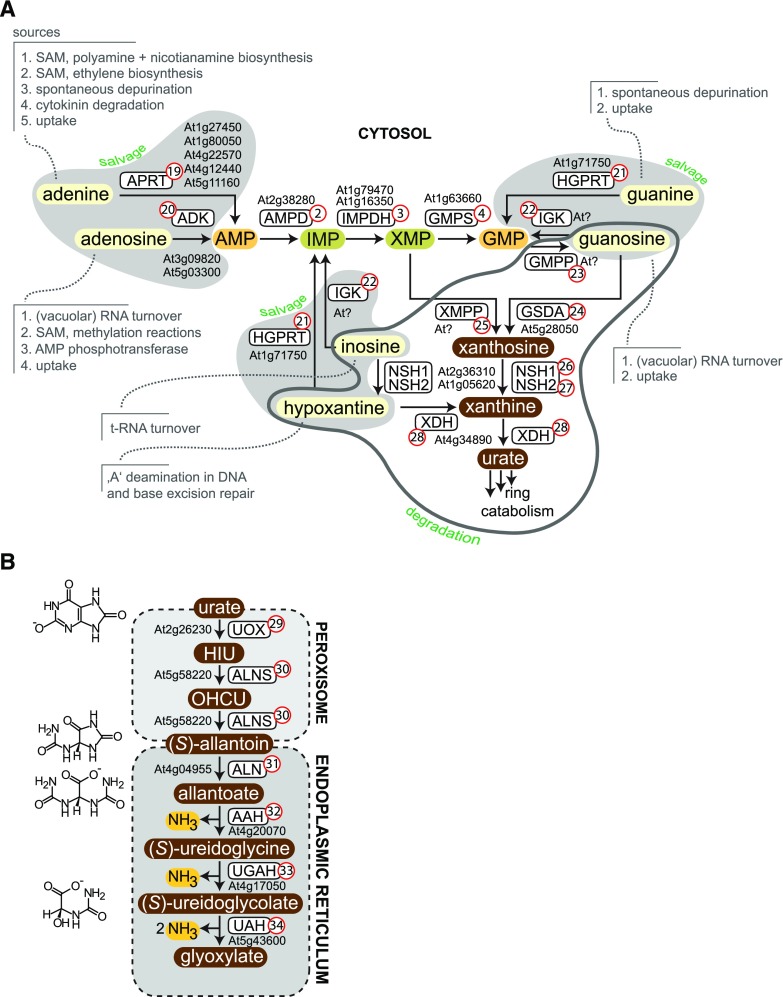
Salvage and degradation of purines. A, Reactions of purine nucleobase and nucleoside salvage, as well as purine nucleotide degradation, which overlaps partially with GMP synthesis. The salvage pathways are highlighted by light gray shading, and the degradation reactions are encircled in dark gray. Metabolites that can only undergo degradation and cannot be salvaged are shown with brown shading. B, Purine ring catabolism. The transport steps for urate and (S)-allantoin are not shown explicitly. APRT (19), adenine phosphoribosyltransferase; ADK (20), adenosine kinase; AMPD (2), AMP deaminase; IMPDH (3), IMP dehydrogenase; GMPS (4), GMP synthetase; HGPRT (21), hypoxanthine guanine phosphoribosyltransferase; IGK (22), inosine guanosine kinase; GMPP (23), GMP phosphatase; GSDA (24), guanosine deaminase; XMPP (25), XMP phosphatase; NSH1 (26), nucleoside hydrolase 1; NSH2 (27), nucleoside hydrolase 2; XDH (28), xanthine dehydrogenase; UOX (29), urate oxidase; ALNS (30), allantoin synthase; ALN (31), allantoinase; AAH (32), allantoate amidohydrolase; UGAH (33), ureido-Gly aminohydrolase; UAH (34), ureidoglycolate amidohydrolase.
Figure 6.
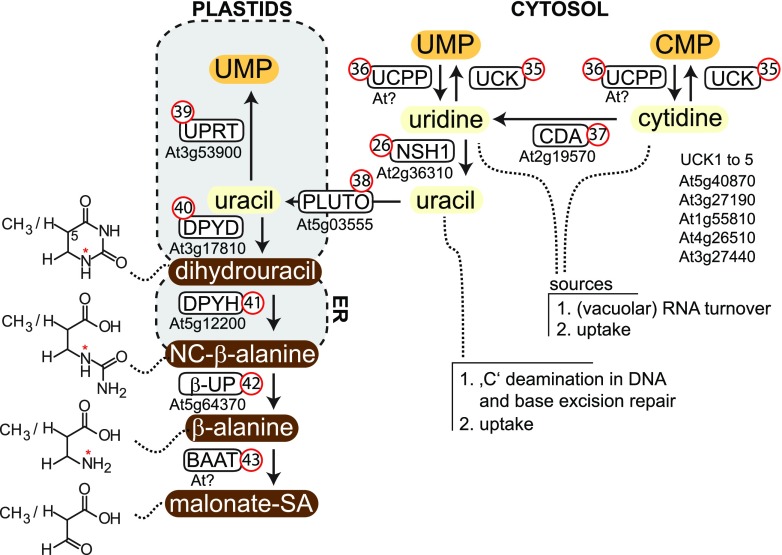
Salvage and degradation of pyrimidines. NC-β-Ala, N-carbamyl-β-Ala; malonate-SA, malonate semialdehyde. UCK (35), uridine cytidine kinase; UCPP (36), UMP CMP phosphatase; CDA (37), cytidine deaminase; NSH1 (26), nucleoside hydrolase 1; PLUTO (38), plastidic nucleobase transporter; UPRT (39), uracil phosphoribosyltransferase; DPYD (40), dihydropyrimidine dehydrogenase; DPYH (41), dihydropyrimidine hydrolase; β-UP (42), β-ureidopropionase; BAAT (43) β-Ala aminotransferase.
The main metabolic source for most nucleosides is probably the turnover of RNA, in particular in the vacuole. Vacuolar RNA degradation, for example of ribosomal RNA after ribophagy (Floyd et al., 2015), generates nucleotides that likely are degraded to nucleosides by vacuolar phosphatases. The details of this process have not been investigated so far. The tonoplast membrane possesses a nucleoside exporter (equilibrative nucleoside transporter 1 [ENT1, At1g70330]; Bernard et al., 2011) for the release of nucleosides into the cytoplasm. For adenosine, the turnover of S-adenosyl Met (SAM), used for methylation reactions, is another important source (Figs. 2 and 5). After transfer of the methyl group from SAM, the resulting S-adenosyl homo-Cys (SAH) is hydrolyzed to homo-Cys and adenosine (Sauter et al., 2013).
There are no strong sources for nucleobases in plant metabolism, except for adenine, which is released during polyamine, nicotianamine, and ethylene biosynthesis (Sauter et al., 2013). In all three pathways SAM is used and 5′-methylthioadenosine is generated, which is hydrolyzed to 5-methylthioribose and adenine (Siu et al., 2008). Also the degradation of cytokinins produces small amounts of adenine (Schmülling et al., 2003). Purine bases are released from nucleic acids by spontaneous depurination (Barbado et al., 2018), resulting in low amounts of adenine and guanine. The metabolic source of hypoxanthine is likely the spontaneous deamination of adenine in DNA, resulting in a hypoxanthine base, which is removed from the DNA by base excision repair (Karran and Lindahl, 1980). Similarly, the nonenzymatic deamination of cytosine in DNA generates uracil, which is removed by base excision repair (Fig. 2).
Purine and Pyrimidine Salvage Metabolism
Nucleosides and nucleobases can be converted into nucleotides, which is called “salvage” (Figs. 2, 5, and 6). In contrast to de novo biosynthesis of nucleotides, which generates nucleotides from basic metabolites (see above), the salvage reactions recycle nucleobases and nucleosides derived from metabolism or uptake (see section on "Metabolic Sources of Nucleosides and Nucleobases") to nucleotides. Nucleobases react with activated phosphoribose (PRPP) to the respective nucleotides—a reaction catalyzed by phosphoribosyltransferases (PRTs). Adenine PRT (APRT; Fig. 5A, no. 19), hypoxanthine guanine PRT (HGPRT; Fig. 5A, no. 21), and uracil PRT (UPRT; Fig. 6, no. 39) are the three types of nucleobase-specific enzymes present in plants. Nucleosides are phosphorylated to nucleoside monophosphates by kinases. Adenosine kinase (ADK; Fig. 5A, no. 20), inosine guanosine kinase (IGK; Fig. 5A, no. 22), and uridine cytidine kinase (UCK; Fig. 6, no. 35) salvage ribonucleotides, whereas thymidine kinase (TK; Fig. 4B, no. 14) and deoxynucleoside kinase (dNK; Fig. 4, A, C, and D, no. 11) phosphorylate thymidine and the other three deoxynucleosides, respectively.
Several salvage enzymes have a critical function for plant metabolism and the mutation of the respective genes has severe consequences. Mutation of the gene for the main APRT (Fig. 5, no. 19) activity, APT1 (At1g27450), results in male sterility (Gaillard et al., 1998), whereas a strong downregulation increases the resistance to oxidative stress (Sukrong et al., 2012). Deletion or strong downregulation of the gene for the main ADK (Fig. 5, no. 20) enzyme, ADK1 (At3g09820), compromises transmethylation reactions, because the accumulating adenosine inhibits SAH hydrolase—an enzyme of the SAM cycle. It has been shown that ADK1 and SAH hydrolase interact and partially reside in the nucleus, probably mediated by nuclear methyltransferases (Lee et al., 2012). Reduced transmethylation causes a range of developmental abnormalities (Moffatt et al., 2002; Young et al., 2006). Guanine and hypoxanthine salvage seems to be less critical, because HGPRT (Fig. 5, no. 21) mutants are phenotypically normal except for a slight delay in germination (Liu et al., 2007; Schroeder et al., 2018). Mutation of HGPRT leads to guanine but not hypoxanthine accumulation in vivo, probably reflecting the fact that guanine can only be salvaged, whereas hypoxanthine can also be degraded (Baccolini and Witte, 2019). IGK has been measured in plant extracts (Fig. 5A, no. 22; Katahira and Ashihara, 2006; Deng and Ashihara, 2010), but the corresponding gene is still unknown. Some evidence has been provided that the activity is associated with the intermembrane space of mitochondria (Combes et al., 1989).
The only uracil salvage activity in Arabidopsis is located in plastids and encoded by UPP (UPRT; Fig. 6, no. 39). Mutation of UPP leads to growth arrest in the seedling stage and an albino phenotype (Mainguet et al., 2009). Interestingly, it was recently shown that this phenotype is unrelated to the lack of UPRT activity in the mutant, but is caused by the absence of the UPP protein per se, demonstrating that uracil salvage does not play such an essential role for Arabidopsis as previously thought (Ohler et al., 2019). Salvage of uridine is more prominent than salvage of uracil in Arabidopsis. Uridine and cytidine salvage are performed by dual-specific UCKs (Ohler et al., 2019). These enzymes also possess a UPRT-like domain, but they do not have UPRT activity (Chen and Thelen, 2011). Simultaneous mutation of UCK1 and UCK2 results in dwarf plants that fail to reach maturity (Chen and Thelen, 2011). Previously, UCK1 and UCK2 were found to localize in plastids (Chen and Thelen, 2011), but Ohler et al. (2019) demonstrated that these enzymes reside in the cytosol, which was also confirmed in our laboratory (M. Chen and C.-P. Witte, unpublished data).
Interestingly, deoxynucleoside-specific salvage enzymes also exist (Fig. 4). Thymidine is salvaged by thymidine kinase (TK; Fig. 4B, no. 14) and the mutation of both TK genes is lethal for Arabidopsis (Clausen et al., 2012). However, it is unclear, why thymidine salvage is of such importance. TK occurs in the cytosol, the mitochondria, and the plastids (Xu et al., 2015) and is of particular importance for chloroplast maintenance when germinating seedlings turn autotrophic (Pedroza-García et al., 2019). The other deoxynucleosides are salvaged by an enzyme with broad deoxynucleoside specificity (dNK; Fig. 4, no. 11; Clausen et al., 2012), potentially associated with mitochondria (Clausen et al., 2014).
Purine Nucleotide Degradation
Instead of being salvaged, nucleobases and nucleosides can also be fully degraded by plants, but for guanine, adenine, and adenosine, a salvage reaction needs to precede degradation. Adenine and adenosine first must be converted to AMP, which can then be deaminated by AMPD (Fig. 5A, no.2) to IMP as a first step in degradation. This is necessary because Arabidopsis and plants in general lack adenosine deaminase (Dancer et al., 1997; Chen et al., 2018b). Interestingly, for N6-methyl AMP, plants as well as many other eukaryotes possess a special deaminase, called N6-methyl-AMP deaminase (MAPDA; Chen et al., 2018b). N6-methylated adenine is the most frequent modification in mRNA, but it is also present in other RNA species (Chen and Witte, 2019). MAPDA is phylogenetically related to adenosine deaminases and hydrolyzes N6-methyl AMP to IMP, removing the aminomethyl group. This example shows that modified nucleotides must also have an access route to general nucleotide degradation.
From IMP, the purine nucleotide degradation pathway cannot be entered directly in Arabidopsis, but conversion to XMP, and apparently even to GMP, is required (Baccolini and Witte, 2019). These recent results show that the route for AMP catabolism and the route for GMP biosynthesis (partially) overlap. Therefore, branch points of both routes must be controlled, but it is not yet clear how this is achieved. GMP dephoshorylation by a yet unknown phosphatase (GMPP; Fig. 5A, no. 23) initiates purine nucleotide catabolism. At the stage of guanosine, salvage back to the nucleotide level via IGK (Fig. 5A, no. 22) is still possible, but the GMPP and IGK reactions might be spatially or temporarily separated to avoid a futile cycle. The deamination of guanosine to xanthosine by guanosine deaminase (GSDA; Fig. 5A, no. 24; Dahncke and Witte, 2013) marks the point of no return, because xanthosine cannot be salvaged and is dedicated for degradation (Yin et al., 2014). Although xanthosine appears to be generated mainly by GSDA, there is strong evidence for an alternative route directly from XMP to xanthosine catalyzed by an XMP phosphatase (XMPP; Fig. 5A, no. 25; Baccolini and Witte, 2019). An XMP-specific phosphatase, which may represent this XMPP, is currently under investigation in our laboratory. In summary, GMP catabolism begins with dephosphorylation and deamination of guanosine, and most AMP is apparently also degraded via GMP, although some AMP might be dephosphorylated already at the stage of XMP.
In clear contrast to purine metabolism in many other organisms, guanine is not an intermediate of purine nucleotide catabolism in Arabidopsis and probably most plants. For degradation, guanine must first be salvaged to GMP (Dahncke and Witte, 2013; Baccolini and Witte, 2019). In addition, in contrast to purine metabolism in many other organisms, inosine and hypoxanthine are not major intermediates of purine nucleotide catabolism in Arabidopsis, because they are derived not from IMP dephosphorylation (Baccolini and Witte, 2019), but possibly from transfer RNA turnover and base excision repair of deaminated adenine in DNA (Fig. 5A). Because guanine and hypoxanthine do not play an important role in purine nucleotide degradation, HGPRT (Fig. 5A, no. 21) is decoupled from purine catabolism in plants (Baccolini and Witte, 2019), which is in stark contrast to humans, where mutation of HGPRT results in accumulation of purine nucleotide breakdown products and severe phenotypic consequences, known as the Lesch-Nyhan Syndrome (Torres and Puig, 2007).
Purine catabolism can lead to the complete disintegration of the purine ring in plants (Fig. 5B; Werner and Witte, 2011) to recycle nitrogen (Soltabayeva et al., 2018), but it is also used to generate the intermediates uric acid and especially allantoin, which counteract stress by reducing reactive oxygen species (Brychkova et al., 2008; Watanabe et al., 2014; Irani and Todd, 2016, 2018; Lescano et al., 2016; Ma et al., 2016; Casartelli et al., 2019; Nourimand and Todd, 2019). Sometimes also the accumulation of allantoate has been observed (Alamillo et al., 2010). In tropical legumes like soybean (Glycine max) or common bean (Phaseolus vulgaris), the ureides allantoin and allantoate are used as long-distance nitrogen transport compounds for the export of fixed nitrogen from the nodules (Tegeder, 2014; Carter and Tegeder, 2016) mediated by the ureide permease (UPS) transporters (Desimone et al., 2002; Schmidt et al., 2006; Collier and Tegeder, 2012). Ureides also function in long-distance transport in non-nodulated legumes (Díaz-Leal et al., 2012; Quiles et al., 2019) and are probably used in many plants for this purpose (Lescano et al., 2016; Redillas et al., 2019).
It has recently been shown that xanthosine hydrolysis to xanthine and ribose is catalyzed by a cytosolic nucleoside hydrolase heteromer consisting of nucleoside hydrolase 1 (NSH1; Fig. 5A, no. 26) and NSH2 (Fig. 5A, no. 27) in vivo (Baccolini and Witte, 2019). NSH1 has only weak xanthosine and inosine but strong uridine hydrolase activity (Jung et al., 2009; Jung et al., 2011; Riegler et al., 2011; Baccolini and Witte, 2019). However, NSH1 is required to activate NSH2, which is the stronger xanthosine and inosine hydrolase in the complex. Nucleoside catabolism is the major metabolic source of ribose, which is recycled to ribose 5-phosphate by ribokinase in the plastids (Riggs et al., 2016; Schroeder et al., 2018). Xanthine and hypoxanthine are catabolized by the same enzyme, xanthine dehydrogenase (XDH; Fig. 5A, no. 28; Urarte et al., 2015), finally to uric acid in the cytosol. Arabidopsis has a second gene encoding XDH (At4g34900) with no apparent XDH activity in vivo (Hauck et al., 2014). Interestingly, XDH has been shown to play a dual role during powdery mildew pathogen attack on Arabidopsis (Ma et al., 2016). In the epidermis, the enzyme is postulated to operate as an NADH oxidase generating superoxide (Zarepour et al., 2010) to prevent fungal entry, whereas in the mesophyll it works as a XDH producing urate, which is suggested to function as a reactive oxygen species scavenger. It was proposed that by this mechanism the reactive oxygen species are confined to the infection site. For further degradation, uric acid must be imported into the peroxisomes, but molecular details about this import are still unknown. In the peroxisome, urate is oxidized by urate oxidase (UOX; Fig. 5B, no. 29) and is hydrolyzed and decarboxylated by allantoin synthase (ALNS; Fig. 5B, #30) to (S)-allantoin (Lamberto et al., 2010; Pessoa et al., 2010). Mutation of UOX leads to strong accumulation of uric acid, which is deleterious for peroxisome maintenance in the embryo, leading to a severe suppression of germination and seedling establishment (Hauck et al., 2014)—surprisingly, accumulation of similar amounts of xanthine in Arabidopsis plants lacking XDH does not lead to strong phenotypic alterations under standard growth conditions (Hauck et al., 2014; Schroeder et al., 2018; Soltabayeva et al., 2018). Allantoin must be transported from the peroxisomes to the ER for further degradation, but it is unclear how this is achieved. One may speculate that allantoin accumulation under certain stress conditions might be caused in part by altered peroxisome-to-ER transport efficiency. However, one apparent reason for allantoin accumulation is an altered catalytic capacity for allantoin generation and degradation under stress (Irani and Todd, 2016; Lescano et al., 2016; Irani and Todd, 2018; Casartelli et al., 2019).
In the ER, allantoin is hydrolyzed by four enzymes (Fig. 5B, nos. 31–34) completely releasing the ring nitrogen as ammonia (Todd and Polacco, 2006; Werner et al., 2008; Serventi et al., 2010; Werner et al., 2010). These enzymes are also responsible for supplying the shoots of tropical legumes with nitrogen exported from the nodules as allantoin and allantoate (Werner et al., 2013; Díaz-Leal et al., 2014).
Pyrimidine Nucleotide Degradation
Pyrimidine nucleotide catabolism is initiated by UMP/CMP phosphatase(s) (UCPP; Fig. 6, no.36), which have not yet been identified. Their activity might be temporarily and/or spatially separated from UCKs (Fig. 6, no. 35; Ohler et al., 2019) to avoid a futile cycle of pyrimidine nucleotide dephosphorylation and pyrimidine nucleoside salvage. Cytidine is deaminated to uridine by a cytosolic cytidine deaminase (CDA; Fig. 6, no. 37). Interestingly, plants can neither degrade nor salvage the free base cytosine (Katahira and Ashihara, 2002). Arabidopsis contains several copies of CDA, but only one copy is functional. The mutation of CDA results in smaller plants, probably because cytidine accumulation is toxic (Chen et al., 2016). Generally, the accumulation of nucleosides can reduce plant performance, as has been shown for GSDA (Fig. 5A, no. 24) mutants (Schroeder et al., 2018). Consistently, transgenic lines with increased vacuolar nucleoside export (Bernard et al., 2011) are smaller than the wild type.
Uridine is hydrolyzed by the cytosolic NSH1 (Fig. 6, no. 26) to uracil and ribose (Jung et al., 2009). NSH2 is not involved in uridine hydrolysis. NSH1 occurs in two forms in vivo, either as a homomer (probably a homodimer; Kopecná et al., 2013) for uridine hydrolysis, or as a heteromer interacting with NSH2 for xanthosine and inosine hydrolysis (Baccolini and Witte, 2019). One should note that these nucleoside hydrolases usually do not hydrolyze cytidine (Jung et al., 2009) or guanosine in vivo unless these compounds accumulate in catabolic mutants (Dahncke and Witte, 2013; Chen et al., 2016; Baccolini and Witte, 2019). Adenosine is a substrate of NSH1 and also of 5′-methylthioadenosine nucleosidase 2 (MTAN2; Siu et al., 2008) in vitro, but both enzymes hydrolyze adenosine with very low catalytic efficiency. The main adenosine hydrolytic activity of Arabidopsis resides probably in the apoplast (see below).
The plastidic nucleobase transporter (PLUTO; Fig. 6, no. 38) reallocates uracil, probably in symport with protons from the cytosol, into the plastids for further metabolic conversion (Witz et al., 2012). PLUTO belongs to the Nucleobase:Cation Symporter 1 family and transports guanine and adenine in addition to uracil, albeit with lower efficiency. There are indications that a thiamine precursor, hydroxymethylpyrimidine, is also a PLUTO substrate (Beaudoin et al., 2018). Interestingly, it was recently reported that PLUTO orthologs from two grasses do not transport uracil, but only adenine and guanine next to a few other substrates (Rapp et al., 2016), indicating that uracil metabolism might be organized differently in these species. However, one should note that definitive evidence for a function of PLUTO in uracil transport into plastids in vivo has not yet been presented for any plant. Other transporters capable of uracil transport have been identified, but they are located in the plasma membrane (Schmidt et al., 2004; Niopek-Witz et al., 2014).
In the plastid, there is a branch point: uracil can either be salvaged by UPRT (Fig. 6, no. 39; see section on "Purine and Pyrimidine Salvage Metabolism") or be degraded. When uracil is applied from outside, strong catabolic activity is usually observed (Ashihara et al., 2001; Katahira and Ashihara, 2002). The first reaction, in which the uracil ring is reduced to dihydrouracil by dihydropyrimidine dehydrogenase (DPYD/PYD1; Fig. 5, no. 40) residing in plastids, was shown to be rate limiting (Tintemann et al., 1985). Compared to mammalian DPYD, the plant enzyme lacks C-terminal domains for cofactor binding, which are involved in electron delivery to the active site. Therefore, the plant enzyme is probably incomplete and might require a so far unknown interaction partner for activity. The loss of activity when the enzyme is expressed in the cytosol instead of the plastid is in agreement with this hypothesis (Cornelius et al., 2011). Mutants of DPYD/PYD1 show delayed germination and a misregulation of abscisic acid-responsive genes, whereas constitutive overexpression results in an increase in growth and seed number (Cornelius et al., 2011). In the next enzymatic steps, the dihydrouracil ring is opened by dihydropyrimidine hydrolase (DPYH/PYD2; Fig. 6, no. 41) and then the carbamino group is hydrolytically released by β-ureidopropionase (β-UP/PYD3; Fig. 6, no. 42; Walsh et al., 2001), generating β-Ala. Not only uracil, but also 5-methyluracil (thymine), is degraded in this pathway (Cornelius et al., 2011), resulting in β-aminoisobutyrate instead of β-Ala. The first reaction (DPYD; Fig. 6, no. 40) is located in the plastids, the second (DPYH; Fig. 6, no. 41) in the ER, and the third (β-UP; Fig. 6, no. 42) in the cytosol, but it is unclear why such a distribution is favorable, and how the metabolites are shuttled to these different locations (Zrenner et al., 2009). Recently, the combination of genome-wide association data with correlation networks built from metabolite and transcriptome data identified an aminotransferase correlated with β-Ala. The corresponding mutants accumulated β-Ala, indicating that this might be the missing β-Ala aminotransferase (BAAT/PYD4; Fig. 6, no. 43) of pyrimidine catabolism in plants (Wu et al., 2016).
Pyrimidine catabolism is induced by nitrogen starvation and in senescence (Zrenner et al., 2009; Cornelius et al., 2011), suggesting that, similar to purine nitrogen, pyrimidine nitrogen is also recycled by plants. When uracil is given as the sole nitrogen source, its degradation can support the growth of Arabidopsis to a limited extent (Zrenner et al., 2009).
eATP
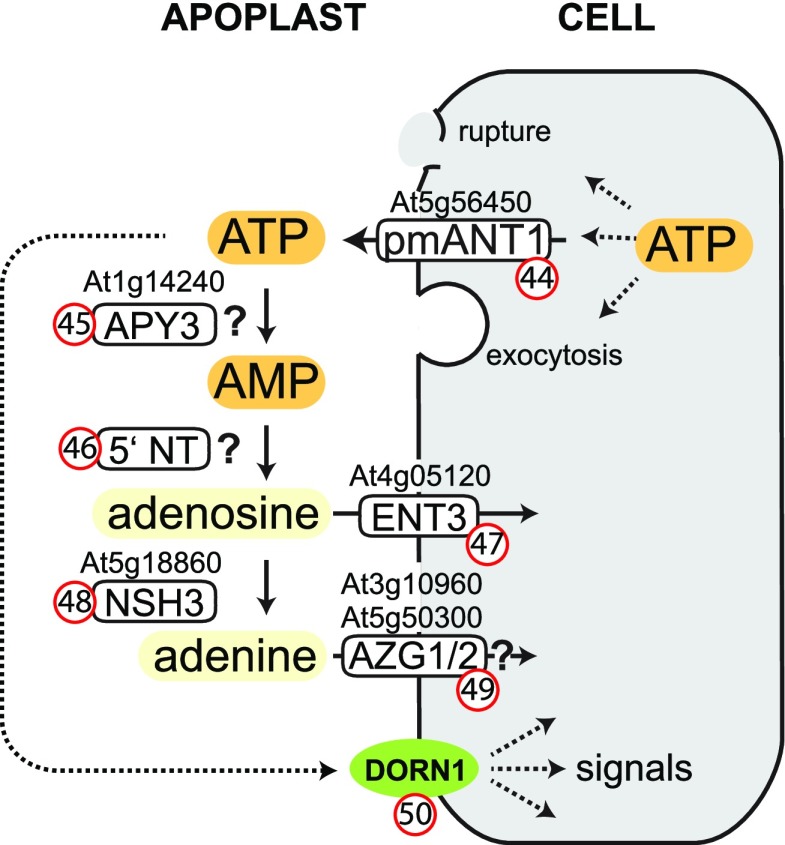
Extracellular ATP (eATP) is a signal molecule that is either actively released upon a stimulus by plant cells via exocytosis or transport or is derived from damaged cells (Fig. 7; Cao et al., 2014). A plasma membrane-based nucleotide transporter belonging to the mitochondrial carrier family (pmANT1; Fig. 7, no. 44) is involved in ATP export with physiological relevance, at least in pollen (Rieder and Neuhaus, 2011). eATP plays a role in stress responses and is perceived by the receptor-like kinase does not respond to nucleotides 1 (DORN1; Fig. 7, no. 50), which recognizes ATP, GTP, and ADP, but not AMP and adenosine (Choi et al., 2014). Interestingly, CTP and NAD are also sensed by plant cells, and a potential receptor for NAD has been identified recently (Wang et al., 2017).
Figure 7.
Excretion, perception, and degradation of extracellular ATP. pmANT1 (44), plasma membrane adenine nucleotide transporter; APY3 (45), apyrase 3; 5′ NT (46), 5′-nucleotidase; ENT3 (47), equilibrative nucleoside transporter 3; NSH3 (48), nucleoside hydrolase 3, AZG1/2 (49), azaguanine resistant 1 / azaguanine resistant 2, DORN1 (50), does not respond to nucleotides 1.
The ATP signal might be quenched by an apoplastic apyrase (Riewe et al., 2008a), an enzyme that hydrolyzes NTPs or NDPs to nNMPs. Seven apyrases are encoded by the Arabidopsis genome (APY1–APY7), and APY1 and APY2 were believed to represent these extracellular enzymes (Lim et al., 2014). However, by scanning the substrate spectra of the apyrases, only APY3 (Fig. 7, no. 45) showed strong activity with ATP (and other NTPs), though APY5 and APY6 also were slightly active with NTPs (Chiu et al., 2015). These apyrases are therefore possible candidates for the apoplastic enzymes in Arabidopsis. However, other secreted phosphatases might also be involved, for example members of the unspecific purple acid phosphatases (Wang et al., 2011; Del Vecchio et al., 2014).
The AMP resulting from ATP dephosphorylation is hydrolyzed in the apoplast to adenosine by a 5′-nucleotidase (Fig. 7, no. 46). An AMP-specific extracellular 5′-nucleotidase associated to the plasma membrane was purified from peanut (Arachis hypogaea; Sharma et al., 1986; Gupta and Sharma, 1996), but the corresponding gene has not been identified. Adenosine can be either taken up via the adenosine proton symporter equilibrative nucleoside transporter 3 (ENT3; Fig. 7, no. 47; Traub et al., 2007; Cornelius et al., 2012) or further hydrolyzed by the apoplastic purine-specific NSH3 (Fig. 7, no. 48; Jung et al., 2011) to adenine and ribose. NSH3 hydrolyzes inosine more efficiently than adenosine, whereas a cell wall-bound nucleoside hydrolase of potato, probably the ortholog of NSH3 in this plant, was highly specific for adenosine and did not hydrolyze inosine (Riewe et al., 2008b).
Simultaneous genetic blockage of nucleoside uptake and hydrolysis leads to an accumulation of adenosine and uridine in the apoplast, a reduction of PSII efficiency, and a higher susceptibility to the necrotrophic fungus Botrytis cinerea, possibly caused by reduced expression of WRKY33 (Daumann et al., 2015), which is known to be essential for Botrytis resistance (Liu et al., 2015). Treatment with eATP increases the resistance to Botrytis (Tripathi et al., 2018), and the expression of WRKY33 and other defense-related genes is reduced in a DORN1 mutant and boosted in a DORN1 overexpression line upon challenge with eATP (Jewell et al., 2019). Taken together, these observations suggest that adenosine accumulation in the apoplast dampens the DORN1-mediated response, indicating that ENT3 and NSH3 are required to remove the breakdown products of eATP signaling. Also the adenine resulting from adenosine hydrolysis by NSH3 is taken up by plant cells. It is not entirely clear which transporters mediate this uptake. Possible candidates are azaguanine resistant 1 (AZG1) and AZG2 (Fig. 7, no. 49), which have been shown to facilitate adenine and guanine uptake in Arabidopsis seedlings (Mansfield et al., 2009), or members of the nucleobase-ascorbate transporter family (Niopek-Witz et al., 2014) as well as of the purine permease (PUP) family (Girke et al., 2014). Interestingly, fungi seem to be able to influence the purinergic signaling in the apoplast by interfering with apoplastic nucleotide metabolism via the excretion of nucleotidases to improve colonization (Nizam et al., 2019).
CONNECTIONS TO CYTOKININ HOMEOSTASIS
The biosynthesis of the cytokinins involves the generation of N6-modified AMP, carrying an isoprenoid group (Sakakibara, 2005). However, cytokinin ribotides or ribosides are inactive—the free modified base is the active hormone binding to the receptors (Yamada et al., 2001; Romanov et al., 2018). The question arises whether the enzymes that are employed for cytokinin homeostasis (activation from ribotides and inactivation to ribosides/ribotides) are the same as for the metabolism of adenine nucleotides.
It was shown that a cytokinin ribotide-specific enzyme (cytokinin riboside 5′-monophosphate phosphoribohydrolase, called lonely guy [LOG]) can release the active cytokinin from the ribotide (Kurakawa et al., 2007; Kuroha et al., 2009). A recent report demonstrated that mutation of the seven genes coding for functional LOGs in Arabidopsis resulted in a phenotype that cannot be attenuated by exogenous cytokinin ribotides, suggesting that the hydrolysis by LOGs is the main pathway of cytokinin activation (Osugi et al., 2017). Therefore, the cytosolic nucleoside hydrolases do not seem to be involved in cytokinin activation, although it could be shown that cytokinin ribosides are substrates in vitro, catalyzed with comparatively low efficiency (Jung et al., 2009; Kopecná et al., 2013). Consistently, cytokinin-related phenotypes were not observed in nucleoside hydrolase mutants (Riegler et al., 2011). However, long-distance transport of cytokinins may involve an activation of cytokinin ribosides/ribotides in the apoplast prior to uptake or perception (Romanov et al., 2018). In Arabidopsis, NSH3, located in the apoplast (see section titled “eATP”), has been shown to hydrolyze adenosine, but cytokinin ribosides have not been assessed (Jung et al., 2011). Interestingly, an apoplastic nucleoside phosphorylase was isolated from potato that converted cytokinin ribosides to cytokinins and ribose-1-phosphate in the presence of phosphate, and that can also work in the synthesis direction of ribosides (Bromley et al., 2014). The enzyme preferred cytokinins/cytokinin ribosides over adenine/adenosine as substrates and is supposedly involved in cytokinin-mediated tuber endodormancy. Close homologs in Arabidopsis (e.g. At4g28940) are predicted to be located in the apoplast (SUBA web server; Hooper et al., 2017), but have not yet been characterized.
The inactivation of cytokinins is inter alia achieved by transferring a phosphoribosyl moiety onto the active cytokinin, resulting in an inactive cytokinin-ribotide. This reaction is at least partially performed by an APRT (Fig. 5A, no. 19), because (1) APT1 mutants convert cytokinins less efficiently to the respective ribotides (Moffatt et al., 1991), (2) APT1 loss-of-function plants contain more active cytokinins (Zhang et al., 2013), and (3) APT1 mutants have cytokinin-related phenotypes (Gaillard et al., 1998a). APT1 is also clearly involved in the salvage of adenine, which is less efficiently converted to AMP in an APT1 mutant (Moffatt et al., 1991) and is slightly more abundant in a mutant plant with reduced APT1 activity (Sukrong et al., 2012). In conclusion, APT1 participates in adenine and cytokinin metabolism and the question arises how one enzyme can serve both distinct metabolic roles adequately. There is also some evidence that ADK (Fig. 5A, no. 20) contributes to cytokinin homeostasis, because plants with reduced ADK activity show cytokinin-related phenotypes and contain more cytokinin ribosides (Schoor et al., 2011). However, ADK is also involved in the maintenance of transmethylation activity; therefore, an indirect impact of reduced ADK activity on cytokinin homeostasis cannot be fully excluded.
CONCLUSIONS
The synthesis, interconversion, and degradation of nucleotides is intrinsically linked with the propagation and reading of genetic information, with energy metabolism including the metabolic activation of many biomolecules, but also with methylation reactions, signal transduction, the recycling of nitrogen, and the modification of oxidative stress. We are getting closer to completing a full inventory of enzymes involved in plant nucleotide metabolism, but we are far from understanding how these enzymes operate together to achieve nucleotide homeostasis. There is only fragmentary information about regulation on all levels, transport processes are incompletely defined, and the organization of nucleotide metabolism at the tissue and organ levels is not well understood (see Outstanding Questions). These issues need to be addressed to allow a better integration of nucleotide metabolism into a molecular model of plant physiology.
Acknowledgments
The authors thank Henryk Straube for critical reading of the manuscript.
Footnotes
This work was supported by the Deutsche Forschungsgemeinschaft (WI3411/4-1 to C.-P.W. and HE 5949/3-1 to M.H.) and the Bundesministerium für Bildung und Forschung (Nutzpflanzen der Zukunft - 031B0540).
Articles can be viewed without a subscription.
References
- Alamillo JM, Díaz-Leal JL, Sánchez-Moran MV, Pineda M (2010) Molecular analysis of ureide accumulation under drought stress in Phaseolus vulgaris L. Plant Cell Environ 33: 1828–1837 [DOI] [PubMed] [Google Scholar]
- Ashihara H, Loukanina N, Stasolla C, Thorpe TA (2001) Pyrimidine metabolism during somatic embryo development in white spruce (Picea glauca). J Plant Physiol 158: 613–621 [Google Scholar]
- Ashihara H, Stasolla C, Fujimura T, Crozier A (2018) Purine salvage in plants. Phytochemistry 147: 89–124 [DOI] [PubMed] [Google Scholar]
- Atkins CA, Shelp BJ, Storer PJ (1985) Purification and properties of inosine monophosphate oxidoreductase from nitrogen-fixing nodules of cowpea (Vigna unguiculata L. Walp). Arch Biochem Biophys 236: 807–814 [DOI] [PubMed] [Google Scholar]
- Atkins CA, Smith P, Storer PJ (1997) Reexamination of the intracellular localization of de novo purine synthesis in cowpea nodules. Plant Physiol 113: 127–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccolini C, Witte C-P (2019) AMP and GMP catabolism in Arabidopsis converge on xanthosine, which is degraded by a nucleoside hydrolase heterocomplex. Plant Cell 31: 734–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahaji A, Muñoz FJ, Ovecka M, Baroja-Fernández E, Montero M, Li J, Hidalgo M, Almagro G, Sesma MT, Ezquer I, et al. (2011a) Specific delivery of AtBT1 to mitochondria complements the aberrant growth and sterility phenotype of homozygous Atbt1 Arabidopsis mutants. Plant J 68: 1115–1121 [DOI] [PubMed] [Google Scholar]
- Bahaji A, Ovecka M, Bárány I, Risueño MC, Muñoz FJ, Baroja-Fernández E, Montero M, Li J, Hidalgo M, Sesma MT, et al. (2011b) Dual targeting to mitochondria and plastids of AtBT1 and ZmBT1, two members of the mitochondrial carrier family. Plant Cell Physiol 52: 597–609 [DOI] [PubMed] [Google Scholar]
- Barbado C, Córdoba-Cañero D, Ariza RR, Roldán-Arjona T (2018) Nonenzymatic release of N7-methylguanine channels repair of abasic sites into an AP endonuclease-independent pathway in Arabidopsis. Proc Natl Acad Sci USA 115: E916–E924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudoin GAW, Johnson TS, Hanson AD (2018) The PLUTO plastidial nucleobase transporter also transports the thiamin precursor hydroxymethylpyrimidine. Biosci Rep 38: BSR20180048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard C, Traub M, Kunz HH, Hach S, Trentmann O, Möhlmann T (2011) Equilibrative nucleoside transporter 1 (ENT1) is critical for pollen germination and vegetative growth in Arabidopsis. J Exp Bot 62: 4627–4637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromley JR, Warnes BJ, Newell CA, Thomson JCP, James CM, Turnbull CGN, Hanke DE (2014) A purine nucleoside phosphorylase in Solanum tuberosum L. (potato) with specificity for cytokinins contributes to the duration of tuber endodormancy. Biochem J 458: 225–237 [DOI] [PubMed] [Google Scholar]
- Brychkova G, Alikulov Z, Fluhr R, Sagi M (2008) A critical role for ureides in dark and senescence-induced purine remobilization is unmasked in the Atxdh1 Arabidopsis mutant. Plant J 54: 496–509 [DOI] [PubMed] [Google Scholar]
- Cao Y, Tanaka K, Nguyen CT, Stacey G (2014) Extracellular ATP is a central signaling molecule in plant stress responses. Curr Opin Plant Biol 20: 82–87 [DOI] [PubMed] [Google Scholar]
- Carrari F, Coll-Garcia D, Schauer N, Lytovchenko A, Palacios-Rojas N, Balbo I, Rosso M, Fernie AR (2005) Deficiency of a plastidial adenylate kinase in Arabidopsis results in elevated photosynthetic amino acid biosynthesis and enhanced growth. Plant Physiol 137: 70–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter AM, Tegeder M (2016) Increasing nitrogen fixation and seed development in soybean requires complex adjustments of nodule nitrogen metabolism and partitioning processes. Curr Biol 26: 2044–2051 [DOI] [PubMed] [Google Scholar]
- Casartelli A, Melino VJ, Baumann U, Riboni M, Suchecki R, Jayasinghe NS, Mendis H, Watanabe M, Erban A, Zuther E, et al. (2019) Opposite fates of the purine metabolite allantoin under water and nitrogen limitations in bread wheat. Plant Mol Biol 99: 477–497 [DOI] [PubMed] [Google Scholar]
- Chen F, Dong G, Ma X, Wang F, Zhang Y, Xiong E, Wu J, Wang H, Qian Q, Wu L, et al. (2018a) UMP kinase activity is involved in proper chloroplast development in rice. Photosynth Res 137: 53–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Herde M, Witte C-P (2016) Of the nine cytidine deaminase-like genes in Arabidopsis, eight are pseudogenes and only one is required to maintain pyrimidine homeostasis in vivo. Plant Physiol 171: 799–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Urs MJ, Sánchez-González I, Olayioye MA, Herde M, Witte C-P (2018b) m6A RNA degradation products are catabolized by an evolutionarily conserved N6-methyl-AMP deaminase in plant and mammalian cells. Plant Cell 30: 1511–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Witte C-P (2019) Functions and Dynamics of Methylation in Eukaryotic mRNA In Jurga S, and Barciszewski J, eds, The DNA, RNA, and Histone Methylomes. Springer International Publishing, Cham, Switzerland, pp 333–351 [Google Scholar]
- Chen MJ, Thelen JJ (2011) Plastid uridine salvage activity is required for photoassimilate allocation and partitioning in Arabidopsis. Plant Cell 23: 2991–3006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Zhu L, Xin L, Du K, Ran X, Cui X, Xiang Q, Zhang H, Xu P, Wu X (2015) Rice stripe1-2 and stripe1-3 mutants encoding the small subunit of ribonucleotide reductase are temperature sensitive and are required for chlorophyll biosynthesis. PLoS One 10: e0130172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu T-Y, Lao J, Manalansan B, Loqué D, Roux SJ, Heazlewood JL (2015) Biochemical characterization of Arabidopsis APYRASE family reveals their roles in regulating endomembrane NDP/NMP homoeostasis. Biochem J 472: 43–54 [DOI] [PubMed] [Google Scholar]
- Choi J, Tanaka K, Cao Y, Qi Y, Qiu J, Liang Y, Lee SY, Stacey G (2014) Identification of a plant receptor for extracellular ATP. Science 343: 290–294 [DOI] [PubMed] [Google Scholar]
- Clausen AR, Girandon L, Ali A, Knecht W, Rozpedowska E, Sandrini MP, Andreasson E, Munch-Petersen B, Piškur J (2012) Two thymidine kinases and one multisubstrate deoxyribonucleoside kinase salvage DNA precursors in Arabidopsis thaliana. FEBS J 279: 3889–3897 [DOI] [PubMed] [Google Scholar]
- Clausen AR, Mutahir Z, Munch-Petersen B, Piškur J (2014) Plants salvage deoxyribonucleosides in mitochondria. Nucleosides Nucleotides Nucleic Acids 33: 291–295 [DOI] [PubMed] [Google Scholar]
- Collier R, Tegeder M (2012) Soybean ureide transporters play a critical role in nodule development, function and nitrogen export. Plant J 72: 355–367 [DOI] [PubMed] [Google Scholar]
- Combes A, Lafleuriel J, Lefloch F (1989) The inosine-guanosine kinase-activity of mitochondria in tubers of Jerusalem artichoke. Plant Physiol Biochem 27: 729–736 [Google Scholar]
- Cornelius S, Traub M, Bernard C, Salzig C, Lang P, Möhlmann T (2012) Nucleoside transport across the plasma membrane mediated by equilibrative nucleoside transporter 3 influences metabolism of Arabidopsis seedlings. Plant Biol 14: 696–705 [DOI] [PubMed] [Google Scholar]
- Cornelius S, Witz S, Rolletschek H, Möhlmann T (2011) Pyrimidine degradation influences germination seedling growth and production of Arabidopsis seeds. J Exp Bot 62: 5623–5632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahncke K, Witte CP (2013) Plant purine nucleoside catabolism employs a guanosine deaminase required for the generation of xanthosine in Arabidopsis. Plant Cell 25: 4101–4109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancer JE, Hughes RG, Lindell SD (1997) Adenosine-5′-phosphate deaminase. A novel herbicide target. Plant Physiol 114: 119–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daumann M, Fischer M, Niopek-Witz S, Girke C, Möhlmann T (2015) Apoplastic nucleoside accumulation in Arabidopsis leads to reduced photosynthetic performance and increased susceptibility against Botrytis cinerea. Front Plant Sci 6: 1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daumann M, Hickl D, Zimmer D, DeTar RA, Kunz H-H, Möhlmann T (2018) Characterization of filament-forming CTP synthases from Arabidopsis thaliana. Plant J 96: 316–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Vecchio HA, Ying S, Park J, Knowles VL, Kanno S, Tanoi K, She Y-M, Plaxton WC (2014) The cell wall-targeted purple acid phosphatase AtPAP25 is critical for acclimation of Arabidopsis thaliana to nutritional phosphorus deprivation. Plant J 80: 569–581 [DOI] [PubMed] [Google Scholar]
- Deng WW, Ashihara H (2010) Profiles of purine metabolism in leaves and roots of Camellia sinensis seedlings. Plant Cell Physiol 51: 2105–2118 [DOI] [PubMed] [Google Scholar]
- Desimone M, Catoni E, Ludewig U, Hilpert M, Schneider A, Kunze R, Tegeder M, Frommer WB, Schumacher K (2002) A novel superfamily of transporters for allantoin and other oxo derivatives of nitrogen heterocyclic compounds in Arabidopsis. Plant Cell 14: 847–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Leal JL, Gálvez-Valdivieso G, Fernández J, Pineda M, Alamillo JM (2012) Developmental effects on ureide levels are mediated by tissue-specific regulation of allantoinase in Phaseolus vulgaris L. J Exp Bot 63: 4095–4106 [DOI] [PubMed] [Google Scholar]
- Díaz-Leal JL, Torralbo F, Antonio Quiles F, Pineda M, Alamillo JM (2014) Molecular and functional characterization of allantoate amidohydrolase from Phaseolus vulgaris. Physiol Plant 152: 43–58 [DOI] [PubMed] [Google Scholar]
- Dong Q, Zhang Y-X, Zhou Q, Liu Q-E, Chen D-B, Wang H, Cheng S-H, Cao L-Y, Shen X-H (2019) UMP kinase regulates chloroplast development and cold response in rice. Int J Mol Sci 20: 2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus HD, Jagendorf AT (1985) Subcellular localization of the pathway of de novo pyrimidine nucleotide biosynthesis in pea leaves. Plant Physiol 79: 856–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorion S, Rivoal J (2015) Clues to the functions of plant NDPK isoforms. Naunyn Schmiedebergs Arch Pharmacol 388: 119–132 [DOI] [PubMed] [Google Scholar]
- Dorion S, Rivoal J (2018) Plant nucleoside diphosphate kinase 1: A housekeeping enzyme with moonlighting activity. Plant Signal Behav 13: e1475804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X, Yang R, Zheng X, Zhang F (2012) Identification of a novel nuclear-localized adenylate kinase 6 from Arabidopsis thaliana as an essential stem growth factor. Plant Physiol Biochem 61: 180–186 [DOI] [PubMed] [Google Scholar]
- Floyd BE, Morriss SC, MacIntosh GC, Bassham DC (2015) Evidence for autophagy-dependent pathways of rRNA turnover in Arabidopsis. Autophagy 11: 2199–2212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard C, Moffatt BA, Blacker M, Laloue M (1998) Male sterility associated with APRT deficiency in Arabidopsis thaliana results from a mutation in the gene APT1. Mol Gen Genet 257: 348–353 [DOI] [PubMed] [Google Scholar]
- Garton S, Knight H, Warren GJ, Knight MR, Thorlby GJ (2007) crinkled leaves 8—a mutation in the large subunit of ribonucleotide reductase—leads to defects in leaf development and chloroplast division in Arabidopsis thaliana. Plant J 50: 118–127 [DOI] [PubMed] [Google Scholar]
- Girke C, Daumann M, Niopek-Witz S, Möhlmann T (2014) Nucleobase and nucleoside transport and integration into plant metabolism. Front Plant Sci 5: 443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelova V, De Lepeleire J, Van Daele J, Pluim D, Meï C, Cuypers A, Leroux O, Rébeillé F, Schellens JHM, Blancquaert D, et al. (2017) Dihydrofolate reductase/thymidylate synthase fine-tunes the folate status and controls redox homeostasis in plants. Plant Cell 29: 2831–2853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Sharma CB (1996) Purification to homogeneity and characterization of plasma membrane and Golgi apparatus-specific 5′-adenosine monophosphatases from peanut cotyledons. Plant Sci 117: 65–74 [Google Scholar]
- Han BW, Bingman CA, Mahnke DK, Bannen RM, Bednarek SY, Sabina RL, Phillips GN Jr (2006) Membrane association, mechanism of action, and structure of Arabidopsis embryonic factor 1 (FAC1). J Biol Chem 281: 14939–14947 [DOI] [PubMed] [Google Scholar]
- Hauck OK, Scharnberg J, Escobar NM, Wanner G, Giavalisco P, Witte C-P (2014) Uric acid accumulation in an Arabidopsis urate oxidase mutant impairs seedling establishment by blocking peroxisome maintenance. Plant Cell 26: 3090–3100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper CM, Castleden IR, Tanz SK, Aryamanesh N, Millar AH (2017) SUBA4: The interactive data analysis centre for Arabidopsis subcellular protein locations. Nucleic Acids Res 45(D1): D1064–D1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Li Y, Jin W, Gong H, He Q, Li Y (2017) Identification and characterization of a plastidic adenine nucleotide uniporter (OsBT1-3) required for chloroplast development in the early leaf stage of rice. Sci Rep 7: 41355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irani S, Todd CD (2016) Ureide metabolism under abiotic stress in Arabidopsis thaliana. J Plant Physiol 199: 87–95 [DOI] [PubMed] [Google Scholar]
- Irani S, Todd CD (2018) Exogenous allantoin increases Arabidopsis seedlings tolerance to NaCl stress and regulates expression of oxidative stress response genes. J Plant Physiol 221: 43–50 [DOI] [PubMed] [Google Scholar]
- Ito J, Batth TS, Petzold CJ, Redding-Johanson AM, Mukhopadhyay A, Verboom R, Meyer EH, Millar AH, Heazlewood JL (2011) Analysis of the Arabidopsis cytosolic proteome highlights subcellular partitioning of central plant metabolism. J Proteome Res 10: 1571–1582 [DOI] [PubMed] [Google Scholar]
- Jewell JB, Sowders JM, He R, Willis MA, Gang DR, Tanaka K (2019) Extracellular ATP shapes a defense-related transcriptome both independently and along with other defense signaling pathways. Plant Physiol 179: 1144–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung B, Flörchinger M, Kunz HH, Traub M, Wartenberg R, Jeblick W, Neuhaus HE, Möhlmann T (2009) Uridine-ribohydrolase is a key regulator in the uridine degradation pathway of Arabidopsis. Plant Cell 21: 876–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung B, Hoffmann C, Möhlmann T (2011) Arabidopsis nucleoside hydrolases involved in intracellular and extracellular degradation of purines. Plant J 65: 703–711 [DOI] [PubMed] [Google Scholar]
- Kafer C, Zhou L, Santoso D, Guirgis A, Weers B, Park S, Thornburg R (2004) Regulation of pyrimidine metabolism in plants. Front Biosci 9: 1611–1625 [DOI] [PubMed] [Google Scholar]
- Karran P, Lindahl T (1980) Hypoxanthine in deoxyribonucleic acid: Generation by heat-induced hydrolysis of adenine residues and release in free form by a deoxyribonucleic acid glycosylase from calf thymus. Biochemistry 19: 6005–6011 [DOI] [PubMed] [Google Scholar]
- Katahira R, Ashihara H (2002) Profiles of pyrimidine biosynthesis, salvage and degradation in disks of potato (Solanum tuberosum L.) tubers. Planta 215: 821–828 [DOI] [PubMed] [Google Scholar]
- Katahira R, Ashihara H (2006) Profiles of purine biosynthesis, salvage and degradation in disks of potato (Solanum tuberosum L.) tubers. Planta 225: 115–126 [DOI] [PubMed] [Google Scholar]
- Kihara A, Saburi W, Wakuta S, Kim M-H, Hamada S, Ito H, Imai R, Matsui H (2011) Physiological and biochemical characterization of three nucleoside diphosphate kinase isozymes from rice (Oryza sativa L.). Biosci Biotechnol Biochem 75: 1740–1745 [DOI] [PubMed] [Google Scholar]
- Kirchberger S, Tjaden J, Neuhaus HE (2008) Characterization of the Arabidopsis Brittle1 transport protein and impact of reduced activity on plant metabolism. Plant J 56: 51–63 [DOI] [PubMed] [Google Scholar]
- Kopecná M, Blaschke H, Kopecny D, Vigouroux A, Koncitíková R, Novák O, Kotland O, Strnad M, Moréra S, von Schwartzenberg K (2013) Structure and function of nucleoside hydrolases from Physcomitrella patens and maize catalyzing the hydrolysis of purine, pyrimidine, and cytokinin ribosides. Plant Physiol 163: 1568–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Spangenberg O, Konrad M (2000) Cloning of the guanylate kinase homologues AGK-1 and AGK-2 from Arabidopsis thaliana and characterization of AGK-1. Eur J Biochem 267: 606–615 [DOI] [PubMed] [Google Scholar]
- Kurakawa T, Ueda N, Maekawa M, Kobayashi K, Kojima M, Nagato Y, Sakakibara H, Kyozuka J (2007) Direct control of shoot meristem activity by a cytokinin-activating enzyme. Nature 445: 652–655 [DOI] [PubMed] [Google Scholar]
- Kuroha T, Tokunaga H, Kojima M, Ueda N, Ishida T, Nagawa S, Fukuda H, Sugimoto K, Sakakibara H (2009) Functional analyses of LONELY GUY cytokinin-activating enzymes reveal the importance of the direct activation pathway in Arabidopsis. Plant Cell 21: 3152–3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamberto I, Percudani R, Gatti R, Folli C, Petrucco S (2010) Conserved alternative splicing of Arabidopsis transthyretin-like determines protein localization and S-allantoin synthesis in peroxisomes. Plant Cell 22: 1564–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange PR, Geserick C, Tischendorf G, Zrenner R (2008) Functions of chloroplastic adenylate kinases in Arabidopsis. Plant Physiol 146: 492–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Doxey AC, McConkey BJ, Moffatt BA (2012) Nuclear targeting of methyl-recycling enzymes in Arabidopsis thaliana is mediated by specific protein interactions. Mol Plant 5: 231–248 [DOI] [PubMed] [Google Scholar]
- Leroch M, Kirchberger S, Haferkamp I, Wahl M, Neuhaus HE, Tjaden J (2005) Identification and characterization of a novel plastidic adenine nucleotide uniporter from Solanum tuberosum. J Biol Chem 280: 17992–18000 [DOI] [PubMed] [Google Scholar]
- Lescano CI, Martini C, González CA, Desimone M (2016) Allantoin accumulation mediated by allantoinase downregulation and transport by Ureide Permease 5 confers salt stress tolerance to Arabidopsis plants. Plant Mol Biol 91: 581–595 [DOI] [PubMed] [Google Scholar]
- Lim MH, Wu J, Yao J, Gallardo IF, Dugger JW, Webb LJ, Huang J, Salmi ML, Song J, Clark G, et al. (2014) Apyrase suppression raises extracellular ATP levels and induces gene expression and cell wall changes characteristic of stress responses. Plant Physiol 164: 2054–2067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincker F, Philipps G, Chabouté M-E (2004) UV-C response of the ribonucleotide reductase large subunit involves both E2F-mediated gene transcriptional regulation and protein subcellular relocalization in tobacco cells. Nucleic Acids Res 32: 1430–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Kracher B, Ziegler J, Birkenbihl RP, Somssich IE (2015) Negative regulation of ABA signaling by WRKY33 is critical for Arabidopsis immunity towards Botrytis cinerea 2100. eLife 4: e07295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Qian W, Liu X, Qin H, Wang D (2007) Molecular and functional analysis of hypoxanthine-guanine phosphoribosyltransferase from Arabidopsis thaliana. New Phytol 175: 448–461 [DOI] [PubMed] [Google Scholar]
- Luzarowski M, Kosmacz M, Sokolowska E, Jasinska W, Willmitzer L, Veyel D, Skirycz A (2017) Affinity purification with metabolomic and proteomic analysis unravels diverse roles of nucleoside diphosphate kinases. J Exp Bot 68: 3487–3499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Wang W, Bittner F, Schmidt N, Berkey R, Zhang L, King H, Zhang Y, Feng J, Wen Y, et al. (2016) Dual and opposing roles of xanthine dehydrogenase in defense-associated reactive oxygen species metabolism in Arabidopsis. Plant Cell 28: 1108–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainguet SE, Gakière B, Majira A, Pelletier S, Bringel F, Guérard F, Caboche M, Berthomé R, Renou JP (2009) Uracil salvage is necessary for early Arabidopsis development. Plant J 60: 280–291 [DOI] [PubMed] [Google Scholar]
- Mansfield TA, Schultes NP, Mourad GS (2009) AtAzg1 and AtAzg2 comprise a novel family of purine transporters in Arabidopsis. FEBS Lett 583: 481–486 [DOI] [PubMed] [Google Scholar]
- Moffatt B, Ashihara H (2002) Purine and pyrimdine nucleotide synthesis and metabolism The Arabidopsis Book 1: e0018 doi:10.1199/tab.0018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffatt B, Pethe C, Laloue M (1991) Metabolism of benzyladenine is impaired in a mutant of Arabidopsis thaliana lacking adenine phosphoribosyltransferase activity. Plant Physiol 95: 900–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffatt BA, Stevens YY, Allen MS, Snider JD, Pereira LA, Todorova MI, Summers PS, Weretilnyk EA, Martin-McCaffrey L, Wagner C (2002) Adenosine kinase deficiency is associated with developmental abnormalities and reduced transmethylation. Plant Physiol 128: 812–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niopek-Witz S, Deppe J, Lemieux MJ, Möhlmann T (2014) Biochemical characterization and structure-function relationship of two plant NCS2 proteins, the nucleobase transporters NAT3 and NAT12 from Arabidopsis thaliana. Biochim Biophys Acta 1838: 3025–3035 [DOI] [PubMed] [Google Scholar]
- Niu M, Wang Y, Wang C, Lyu J, Wang Y, Dong H, Long W, Wang D, Kong W, Wang L, et al. (2017) ALR encoding dCMP deaminase is critical for DNA damage repair, cell cycle progression and plant development in rice. J Exp Bot 68: 5773–5786 [DOI] [PubMed] [Google Scholar]
- Nizam S, Qiang X, Wawra S, Nostadt R, Getzke F, Schwanke F, Dreyer I, Langen G, Zuccaro A (2019) Serendipita indica E5’NT modulates extracellular nucleotide levels in the plant apoplast and affects fungal colonization. EMBO Rep 20: e47430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura Y, Izumi A, Fukunaga Y, Kusumi K, Iba K, Watanabe S, Nakahira Y, Weber APM, Nozawa A, Tozawa Y (2014) Diversity in guanosine 3′,5′-bisdiphosphate (ppGpp) sensitivity among guanylate kinases of bacteria and plants. J Biol Chem 289: 15631–15641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nourimand M, Todd CD (2019) There is a direct link between allantoin concentration and cadmium tolerance in Arabidopsis. Plant Physiol Biochem 135: 441–449 [DOI] [PubMed] [Google Scholar]
- Ohler L, Niopek-Witz S, Mainguet SE, Möhlmann T (2019) Pyrimidine salvage: Physiological functions and interaction with chloroplast biogenesis. Plant Physiol 180: 1816–1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osugi A, Kojima M, Takebayashi Y, Ueda N, Kiba T, Sakakibara H (2017) Systemic transport of trans-zeatin and its precursor have differing roles in Arabidopsis shoots. Nat Plants 3: 17112. [DOI] [PubMed] [Google Scholar]
- Pedroza-García J-A, Nájera-Martínez M, Mazubert C, Aguilera-Alvarado P, Drouin-Wahbi J, Sánchez-Nieto S, Gualberto JM, Raynaud C, Plasencia J (2019) Role of pyrimidine salvage pathway in the maintenance of organellar and nuclear genome integrity. Plant J 97: 430–446 [DOI] [PubMed] [Google Scholar]
- Pessoa J, Sárkány Z, Ferreira-da-Silva F, Martins S, Almeida MR, Li J, Damas AM (2010) Functional characterization of Arabidopsis thaliana transthyretin-like protein. BMC Plant Biol 10: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips DA, Joseph CM, Hirsch PR (1997) Occurrence of flavonoids and nucleosides in agricultural soils. Appl Environ Microbiol 63: 4573–4577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiles FA, Galvez-Valdivieso G, Guerrero-Casado J, Pineda M, Piedras P (2019) Relationship between ureidic/amidic metabolism and antioxidant enzymatic activities in legume seedlings. Plant Physiol Biochem 138: 1–8 [DOI] [PubMed] [Google Scholar]
- Rapp M, Schein J, Hunt KA, Nalam V, Mourad GS, Schultes NP (2016) The solute specificity profiles of nucleobase cation symporter 1 (NCS1) from Zea mays and Setaria viridis illustrate functional flexibility. Protoplasma 253: 611–623 [DOI] [PubMed] [Google Scholar]
- Redillas MCFR, Bang SW, Lee D-K, Kim YS, Jung H, Chung PJ, Suh J-W, Kim J-K (2019) Allantoin accumulation through overexpression of ureide permease1 improves rice growth under limited nitrogen conditions. Plant Biotechnol J 17: 1289–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regierer B, Fernie AR, Springer F, Perez-Melis A, Leisse A, Koehl K, Willmitzer L, Geigenberger P, Kossmann J (2002) Starch content and yield increase as a result of altering adenylate pools in transgenic plants. Nat Biotechnol 20: 1256–1260 [DOI] [PubMed] [Google Scholar]
- Rieder B, Neuhaus HE (2011) Identification of an Arabidopsis plasma membrane-located ATP transporter important for anther development. Plant Cell 23: 1932–1944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riegler H, Geserick C, Zrenner R (2011) Arabidopsis thaliana nucleosidase mutants provide new insights into nucleoside degradation. New Phytol 191: 349–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riewe D, Grosman L, Fernie AR, Wucke C, Geigenberger P (2008a) The potato-specific apyrase is apoplastically localized and has influence on gene expression, growth, and development. Plant Physiol 147: 1092–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riewe D, Grosman L, Fernie AR, Zauber H, Wucke C, Geigenberger P (2008b) A cell wall-bound adenosine nucleosidase is involved in the salvage of extracellular ATP in Solanum tuberosum. Plant Cell Physiol 49: 1572–1579 [DOI] [PubMed] [Google Scholar]
- Riggs JW, Rockwell NC, Cavales PC, Callis J (2016) Identification of the plant ribokinase and discovery of a role for Arabidopsis ribokinase in nucleoside metabolism. J Biol Chem 291: 22572–22582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanov GA, Lomin SN, Schmülling T (2018) Cytokinin signaling: From the ER or from the PM? That is the question!. New Phytol 218: 41–53 [DOI] [PubMed] [Google Scholar]
- Ronceret A, Gadea-Vacas J, Guilleminot J, Lincker F, Delorme V, Lahmy S, Pelletier G, Chabouté M-E, Devic M (2008) The first zygotic division in Arabidopsis requires de novo transcription of thymidylate kinase. Plant J 53: 776–789 [DOI] [PubMed] [Google Scholar]
- Sabina RL, Paul AL, Ferl RJ, Laber B, Lindell SD (2007) Adenine nucleotide pool perturbation is a metabolic trigger for AMP deaminase inhibitor-based herbicide toxicity. Plant Physiol 143: 1752–1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara H. (2005) Cytokinin biosynthesis and regulation. Vitam Horm 72: 271–287 [DOI] [PubMed] [Google Scholar]
- Sauge-Merle S, Falconet D, Fontecave M (1999) An active ribonucleotide reductase from Arabidopsis thaliana: Cloning, expression and characterization of the large subunit. Eur J Biochem 266: 62–69 [DOI] [PubMed] [Google Scholar]
- Sauter M, Moffatt B, Saechao MC, Hell R, Wirtz M (2013) Methionine salvage and S-adenosylmethionine: Essential links between sulfur, ethylene and polyamine biosynthesis. Biochem J 451: 145–154 [DOI] [PubMed] [Google Scholar]
- Schmid L-M, Ohler L, Möhlmann T, Brachmann A, Muiño JM, Leister D, Meurer J, Manavski N (2019) PUMPKIN, the sole plastid UMP kinase, associates with group II introns and alters their metabolism. Plant Physiol 179: 248–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Baumann N, Schwarzkopf A, Frommer WB, Desimone M (2006) Comparative studies on Ureide Permeases in Arabidopsis thaliana and analysis of two alternative splice variants of AtUPS5. Planta 224: 1329–1340 [DOI] [PubMed] [Google Scholar]
- Schmidt A, Su YH, Kunze R, Warner S, Hewitt M, Slocum RD, Ludewig U, Frommer WB, Desimone M (2004) UPS1 and UPS2 from Arabidopsis mediate high affinity transport of uracil and 5-fluorouracil. J Biol Chem 279: 44817–44824 [DOI] [PubMed] [Google Scholar]
- Schmülling T, Werner T, Riefler M, Krupková E, Bartrina y Manns I (2003) Structure and function of cytokinin oxidase/dehydrogenase genes of maize, rice, Arabidopsis and other species. J Plant Res 116: 241–252 [DOI] [PubMed] [Google Scholar]
- Schoor S, Farrow S, Blaschke H, Lee S, Perry G, von Schwartzenberg K, Emery N, Moffatt B (2011) Adenosine kinase contributes to cytokinin interconversion in Arabidopsis. Plant Physiol 157: 659–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder RY, Zhu A, Eubel H, Dahncke K, Witte C-P (2018) The ribokinases of Arabidopsis thaliana and Saccharomyces cerevisiae are required for ribose recycling from nucleotide catabolism, which in plants is not essential to survive prolonged dark stress. New Phytol 217: 233–244 [DOI] [PubMed] [Google Scholar]
- Serventi F, Ramazzina I, Lamberto I, Puggioni V, Gatti R, Percudani R (2010) Chemical basis of nitrogen recovery through the ureide pathway: Formation and hydrolysis of S-ureidoglycine in plants and bacteria. ACS Chem Biol 5: 203–214 [DOI] [PubMed] [Google Scholar]
- Sharma CB, Mittal R, Tanner W (1986) Purification and properties of a glycoprotein adenosine 5′-monophosphatase from the plasma membrane fraction of Arachis hypogaea cotyledons. Biochim Biophys Acta 884: 567–577 [Google Scholar]
- Shelp BJ, Atkins CA (1983) Role of inosine monophosphate oxidoreductase in the formation of ureides in nitrogen-fixing nodules of cowpea (Vigna-unguiculata-L Walp). Plant Physiol 72: 1029–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigel H, Operschall BP, Griesser R (2009) Xanthosine 5′-monophosphate (XMP). Acid-base and metal ion-binding properties of a chameleon-like nucleotide. Chem Soc Rev 38: 2465–2494 [DOI] [PubMed] [Google Scholar]
- Siu KKW, Lee JE, Sufrin JR, Moffatt BA, McMillan M, Cornell KA, Isom C, Howell PL (2008) Molecular determinants of substrate specificity in plant 5′-methylthioadenosine nucleosidases. J Mol Biol 378: 112–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PM, Atkins CA (2002) Purine biosynthesis. Big in cell division, even bigger in nitrogen assimilation. Plant Physiol 128: 793–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltabayeva A, Srivastava S, Kurmanbayeva A, Bekturova A, Fluhr R, Sagi M (2018) Early senescence in older leaves of low nitrate-grown Atxdh1 uncovers a role for purine catabolism in N supply. Plant Physiol 178: 1027–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spetea C, Lundin B (2012) Evidence for nucleotide-dependent processes in the thylakoid lumen of plant chloroplasts—An update. FEBS Lett 586: 2946–2954 [DOI] [PubMed] [Google Scholar]
- Stasolla C, Katahira R, Thorpe TA, Ashihara H (2003) Purine and pyrimidine nucleotide metabolism in higher plants. J Plant Physiol 160: 1271–1295 [DOI] [PubMed] [Google Scholar]
- Stitt M, Lilley RM, Heldt HW (1982) Adenine nucleotide levels in the cytosol, chloroplasts, and mitochondria of wheat leaf protoplasts. Plant Physiol 70: 971–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto H, Kusumi K, Noguchi K, Yano M, Yoshimura A, Iba K (2007) The rice nuclear gene, VIRESCENT 2, is essential for chloroplast development and encodes a novel type of guanylate kinase targeted to plastids and mitochondria. Plant J 52: 512–527 [DOI] [PubMed] [Google Scholar]
- Sukrong S, Yun K-Y, Stadler P, Kumar C, Facciuolo T, Moffatt BA, Falcone DL (2012) Improved growth and stress tolerance in the Arabidopsis oxt1 mutant triggered by altered adenine metabolism. Mol Plant 5: 1310–1332 [DOI] [PubMed] [Google Scholar]
- Tegeder M. (2014) Transporters involved in source to sink partitioning of amino acids and ureides: Opportunities for crop improvement. J Exp Bot 65: 1865–1878 [DOI] [PubMed] [Google Scholar]
- Tintemann H, Wasternack C, Benndorf R, Reinbothe H (1985) The rate-limiting step of uracil degradation in tomato cell-suspension cultures and Euglena-gracilis invivo studies. Comp Biochem Physiol B Biochem Mol Biol 82: 787–792 [Google Scholar]
- Todd CD, Polacco JC (2006) AtAAH encodes a protein with allantoate amidohydrolase activity from Arabidopsis thaliana. Planta 223: 1108–1113 [DOI] [PubMed] [Google Scholar]
- Torres RJ, Puig JG (2007) Hypoxanthine-guanine phosophoribosyltransferase (HPRT) deficiency: Lesch-Nyhan syndrome. Orphanet J Rare Dis 2: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub M, Flörchinger M, Piecuch J, Kunz HH, Weise-Steinmetz A, Deitmer JW, Ekkehard Neuhaus H, Möhlmann T (2007) The fluorouridine insensitive 1 (fur1) mutant is defective in equilibrative nucleoside transporter 3 (ENT3), and thus represents an important pyrimidine nucleoside uptake system in Arabidopsis thaliana. Plant J 49: 855–864 [DOI] [PubMed] [Google Scholar]
- Tripathi D, Zhang T, Koo AJ, Stacey G, Tanaka K (2018) Extracellular ATP acts on jasmonate signaling to reinforce plant defense. Plant Physiol 176: 511–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A, Knecht W, Piskur J, Löffler M (2002) Plant dihydroorotate dehydrogenase differs significantly in substrate specificity and inhibition from the animal enzymes. FEBS Lett 529: 346–350 [DOI] [PubMed] [Google Scholar]
- Urarte E, Esteban R, Moran JF, Bittner F (2015) Established and proposed roles of xanthine oxidoreductase in oxidative and reductive pathways in plants In Gupta KJ, and Igamberdiev AU, eds, Reactive Oxygen and Nitrogen Species Signaling and Communication in Plants. Springer, Cham, Switzerland, pp 15–42 [Google Scholar]
- Wagner KG, Backer AI (1992) Dynamics of nucleotides in plants studied on a cellular basis. Int Rev Cytol 134: 1–84 [Google Scholar]
- Walsh TA, Green SB, Larrinua IM, Schmitzer PR (2001) Characterization of plant β-ureidopropionase and functional overexpression in Escherichia coli. Plant Physiol 125: 1001–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Li Z, Qian W, Guo W, Gao X, Huang L, Wang H, Zhu H, Wu JW, Wang D, et al. (2011) The Arabidopsis purple acid phosphatase AtPAP10 is predominantly associated with the root surface and plays an important role in plant tolerance to phosphate limitation. Plant Physiol 157: 1283–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Liu Z (2006) Arabidopsis ribonucleotide reductases are critical for cell cycle progression, DNA damage repair, and plant development. Plant Cell 18: 350–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Zhou M, Zhang X, Yao J, Zhang Y, Mou Z (2017) A lectin receptor kinase as a potential sensor for extracellular nicotinamide adenine dinucleotide in Arabidopsis thaliana. eLife 6: e25474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Matsumoto M, Hakomori Y, Takagi H, Shimada H, Sakamoto A (2014) The purine metabolite allantoin enhances abiotic stress tolerance through synergistic activation of abscisic acid metabolism. Plant Cell Environ 37: 1022–1036 [DOI] [PubMed] [Google Scholar]
- Wei X, Song X, Wei L, Tang S, Sun J, Hu P, Cao X (2017) An epiallele of rice AK1 affects photosynthetic capacity. J Integr Plant Biol 59: 158–163 [DOI] [PubMed] [Google Scholar]
- Werner AK, Medina-Escobar N, Zulawski M, Sparkes IA, Cao F-Q, Witte CP (2013) The ureide-degrading reactions of purine ring catabolism employ three amidohydrolases and one aminohydrolase in Arabidopsis, soybean, and rice. Plant Physiol 163: 672–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner AK, Romeis T, Witte CP (2010) Ureide catabolism in Arabidopsis thaliana and Escherichia coli. Nat Chem Biol 6: 19–21 [DOI] [PubMed] [Google Scholar]
- Werner AK, Sparkes IA, Romeis T, Witte CP (2008) Identification, biochemical characterization, and subcellular localization of allantoate amidohydrolases from Arabidopsis and soybean. Plant Physiol 146: 418–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner AK, Witte CP (2011) The biochemistry of nitrogen mobilization: Purine ring catabolism. Trends Plant Sci 16: 381–387 [DOI] [PubMed] [Google Scholar]
- Witz S, Jung B, Fürst S, Möhlmann T (2012) De novo pyrimidine nucleotide synthesis mainly occurs outside of plastids, but a previously undiscovered nucleobase importer provides substrates for the essential salvage pathway in Arabidopsis. Plant Cell 24: 1549–1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Alseekh S, Cuadros-Inostroza Á, Fusari CM, Mutwil M, Kooke R, Keurentjes JB, Fernie AR, Willmitzer L, Brotman Y (2016) Combined use of genome-wide association data and correlation networks unravels key regulators of primary metabolism in Arabidopsis thaliana. PLoS Genet 12: e1006363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Deng Y, Li Q, Zhu X, He Z (2014) STRIPE2 encodes a putative dCMP deaminase that plays an important role in chloroplast development in rice. J Genet Genomics 41: 539–548 [DOI] [PubMed] [Google Scholar]
- Xu J, Zhang HY, Xie CH, Xue HW, Dijkhuis P, Liu CM (2005) EMBRYONIC FACTOR 1 encodes an AMP deaminase and is essential for the zygote to embryo transition in Arabidopsis. Plant J 42: 743–756 [DOI] [PubMed] [Google Scholar]
- Xu J, Zhang L, Yang D-L, Li Q, He Z (2015) Thymidine kinases share a conserved function for nucleotide salvage and play an essential role in Arabidopsis thaliana growth and development. New Phytol 208: 1089–1103 [DOI] [PubMed] [Google Scholar]
- Yamada H, Suzuki T, Terada K, Takei K, Ishikawa K, Miwa K, Yamashino T, Mizuno T (2001) The Arabidopsis AHK4 histidine kinase is a cytokinin-binding receptor that transduces cytokinin signals across the membrane. Plant Cell Physiol 42: 1017–1023 [DOI] [PubMed] [Google Scholar]
- Ye W, Hu S, Wu L, Ge C, Cui Y, Chen P, Wang X, Xu J, Ren D, Dong G, et al. (2016) White stripe leaf 12 (WSL12), encoding a nucleoside diphosphate kinase 2 (OsNDPK2), regulates chloroplast development and abiotic stress response in rice (Oryza sativa L.). Mol Breed 36: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Katahira R, Ashihara H (2014) Metabolism of purine nucleosides and bases in suspension-cultured Arabidopsis thaliana cells. Eur Chem Bull 3: 925–934 [Google Scholar]
- Young LS, Harrison BR, Narayana Murthy UM, Moffatt BA, Gilroy S, Masson PH (2006) Adenosine kinase modulates root gravitropism and cap morphogenesis in Arabidopsis. Plant Physiol 142: 564–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarepour M, Kaspari K, Stagge S, Rethmeier R, Mendel RR, Bittner F (2010) Xanthine dehydrogenase AtXDH1 from Arabidopsis thaliana is a potent producer of superoxide anions via its NADH oxidase activity. Plant Mol Biol 72: 301–310 [DOI] [PubMed] [Google Scholar]
- Zhang T, Feng P, Li Y, Yu P, Yu G, Sang X, Ling Y, Zeng X, Li Y, Huang J, et al. (2018) VIRESCENT-ALBINO LEAF 1 regulates leaf colour development and cell division in rice. J Exp Bot 69: 4791–4804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Chen Y, Lin X, Hong X, Zhu Y, Li W, He W, An F, Guo H (2013) Adenine phosphoribosyl transferase 1 is a key enzyme catalyzing cytokinin conversion from nucleobases to nucleotides in Arabidopsis. Mol Plant 6: 1661–1672 [DOI] [PubMed] [Google Scholar]
- Zhou K, Xia J, Wang Y, Ma T, Li Z (2017) A Young Seedling Stripe2 phenotype in rice is caused by mutation of a chloroplast-localized nucleoside diphosphate kinase 2 required for chloroplast biogenesis. Genet Mol Biol 40: 630–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Lacroute F, Thornburg R (1998) Cloning, expression in Escherichia coli, and characterization of Arabidopsis thaliana UMP/CMP kinase. Plant Physiol 117: 245–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Guo S, Wang Z, Du Q, Xing Y, Zhang T, Shen W, Sang X, Ling Y, He G (2016) Map-based cloning and functional analysis of YGL8, which controls leaf colour in rice (Oryza sativa). BMC Plant Biol 16: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zrenner R, Ashihara H (2011) Nucleotide metabolism In Ashihara H, Crozier A, and Komamine A, eds, Plant Metabolism and Biotechnology. Wiley, Cambridge, New York, pp 135–162 [Google Scholar]
- Zrenner R, Riegler H, Marquard CR, Lange PR, Geserick C, Bartosz CE, Chen CT, Slocum RD (2009) A functional analysis of the pyrimidine catabolic pathway in Arabidopsis. New Phytol 183: 117–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zrenner R, Stitt M, Sonnewald U, Boldt R (2006) Pyrimidine and purine biosynthesis and degradation in plants. Annu Rev Plant Biol 57: 805–836 [DOI] [PubMed] [Google Scholar]