Abstract
Given its ability to induce both humoral and cellular immune responses, NY-ESO-1 has been considered a suitable antigen for a cancer vaccine. Despite promising results from early-phase clinical studies in melanoma patients, NY-ESO-1 vaccine immunotherapy has not been widely investigated in larger trials; consequently, many questions remain as to the optimal vaccine formulation, predictive biomarkers, and sequencing and timing of vaccines in melanoma treatment. We conducted an adjuvant phase I/II clinical trial in high-risk resected melanoma to optimize the delivery of poly-ICLC, a TLR-3/MDA-5 agonist as a component of vaccine formulation. A phase I dose escalation part was undertaken to identify the maximum tolerated dose of poly-ICLC administered in combination with NY-ESO-1 and montanide. This was followed by a randomized phase II part investigating the maximum tolerated dose of poly-ICLC with NY-ESO-1 with or without montanide. The vaccine regimens were generally well-tolerated, with no treatment-related grade 3/4 adverse events. Both regimens induced integrated NY-ESO-1-specific CD4+ T-cell and humoral responses. CD8+ T-cell responses were mainly detected in patients receiving montanide. T-cell avidity towards NY-ESO-1 peptides was higher in patients vaccinated with montanide. In conclusion, NY-ESO-1 protein in combination with poly-ICLC is safe, well-tolerated, and capable of inducing integrated antibody and CD4+ T-cell responses in most patients. Combination with montanide enhances antigen specific T-cell avidity and CD8+ T-cell cross-priming in a fraction of patients, indicating that montanide contributes to the induction of specific CD8+ T-cell responses to NY-ESO-1.
Keywords: NY-ESO-1, poly-ICLC, montanide, vaccine
Introduction
Despite optimal surgical resection, patients with high-risk melanoma (i.e. stage IIB and higher) are at substantial risk of recurrence and death from metastatic disease. Phase III trials have demonstrated the efficacy of adjuvant checkpoint inhibitor immunotherapy (ICI) in prolonging recurrence-free survival in patients with high-risk resected melanoma (1,2), although acquired resistance to PD-1 blockade therapy has been identified in patients with melanoma. Such resistance has been associated with defects in the interferon-receptor signaling pathways as well as in antigen presentation (3). In addition, disease will recur for a fraction of these patients, and the optimal systemic treatment after failure of PD-1 immunotherapy (both in the adjuvant and metastatic setting) remains to be determined. Effective immunotherapy combinations with checkpoint inhibitors, such as PD-1 targeted therapies, are needed in the metastatic setting. Collectively, although progress has been made in the treatment of melanoma over the past decade, there remains a need for the development of well-tolerated therapies with minimal synergistic efficacy without compounded toxicity for the adjuvant treatment of high-risk resected melanoma.
Though still in early clinical development, cancer vaccines are in general well-tolerated, and have already been safely combined with nivolumab in the adjuvant treatment of melanoma (4). Vaccines remain an attractive adjuvant treatment option to combine with checkpoint inhibitors, but vaccine trials in melanoma and other solid tumors have failed to demonstrate clinical efficacy, despite the induction of antigen-specific T-cell responses (3). The lack of apparent clinical benefit of monotherapies has been disappointing given both the evidence of cancer vaccine immunogenicity and the success of checkpoint blockade immunotherapy (5). One explanation is the failure to induce a sustained systemic immune response strong enough to overcome local immunosuppressive factors in the tumor microenvironment (TME). Incomplete Freund adjuvant (IFA)-based vaccination induces an inflamed vaccination site that recruits, functionally impairs, and eventually destroys tumor-specific effector T cell responses induced by anti-CTLA-4 checkpoint therapy, causing reduced tumor control (6,7). Thus optimizing the peptide/protein components in vaccine formulation as well as immunogenic adjuvants such as poly-ICLC and montanide can maximize the systemic immune response to cancer vaccines and increase therapeutic efficacy.
Owing to the high tumor mutational burden, melanoma is the natural proving ground for cancer immunotherapy as shown by the early success of the immune checkpoint inhibitors ipilimumab (8), nivolumab (9), and pembrolizumab (10). ICI is believed to improve T-cell priming against tumor specific antigens, including shared tumor antigens such as the cancer testis (CT) antigens like NY-ESO-1, while reducing T-cell exhaustion and T regulatory cell frequencies/function in the TME (10,11). NY-ESO-1 directed immunotherapy has been safely tested in melanoma and other cancers, and NY-ESO-1 reactive T-cells, when adoptively transferred, can achieve clinical responses (12). CT antigens are a family of proteins expressed by gametes and trophoblasts as well as several tumor types, but not on normal diploid tissues. NY-ESO-1 is a 180 amino acid protein, which is expressed in approximately one-third to one- fourth of all melanoma, as well as other solid tumors, and in up to 80% of synovial carcinomas (13,14). NY-ESO-1 expression can spontaneously induce strong humoral and cellular CD4+ and CD8+ T-cell immune responses (15). This CT antigen has been used in vaccine preparations emulsified in water-in-oil emulsions, encoded in viral vectors and incorporated into virus like particles, and with various adjuvants, such as IFA, with or without adjuvant immunomodulatory cytokines or TLR agonists (15–17). Although short NY-ESO-1 peptide vaccines offer advantages of immunogenicity and easy production, they are limiting in their potential use by their HLA restriction requirements. Moreover, if the initial selection of the peptides is not optimal, peptide vaccines can give rise to responses against cryptic epitopes not representative of the naturally processed NY-ESO-1 protein (18). For these reasons, vaccination with the full length NY-ESO-1 protein or synthetic long peptides, in combination with adjuvants has been considered more likely to generate a clinically meaningful immune response. Arguably the best formulations in terms of eliciting immunogenicity are those that incorporate NY-ESO-1 long peptides or proteins with TLR agonists and montanide (19–21). These studies by our group and others have demonstrated that montanide is a vaccine adjuvant and that the combination of TLR agonist with montanide potentiates immunogenicity, however, the immunogenicity and the optimal adjuvant TLR agonist has not been characterized.
Poly-ICLC is a synthetic Poly-IC (TLR3 and MDA5 agonist) that can induce tumor-specific natural killer (NK) cell, cytotoxic T lymphocyte (CTL), and NK-T cell mediated immune responses (19,20,22–24). In murine models of glioma and melanoma, poly-ICLC was shown to be an effective adjuvant to prime antigen-specific CD8+ T-cells and prolong survival (25). Although several clinical trials in various advanced solid tumors have demonstrated that repetitive injection of poly-ICLC alone, in the absence of exogenous tumor-specific antigens, elicits an antitumor immune response (26,27), injection of poly-ICLC alongside overlapping long peptides (OLP) from tumor-specific antigens into ovarian cancer patients induced both humoral and cellular immune responses (19). Montanide and poly-ICLC have distinct and synergistic effects in the induction of NY-ESO-1-specific immune responses when combined with tumor-specific overlapping long peptides (20). These results suggest that poly-ICLC may be an ideal adjuvant for human cancer vaccines.
In this phase I/II clinical trial, we evaluated the safety and immunogenicity of vaccination with the full-length NY-ESO-1 protein and poly-ICLC with or without montanide in patients following resection of high risk melanoma. We found that NY-ESO-1 protein and poly-ICLC induce robust humoral and cellular immune responses specific for NY-ESO-1. This immunogenicity, particularly in the CD8+ T-cell compartment, is more pronounced when combined with montanide.
Material and Methods
Study design, patients, and treatment plan
This trial was conducted in patients with high risk melanoma, who provided informed consent prior to any research activities. The trial consisted of two parts: 1) A Phase I open label dose escalation study of poly-ICLC (Hiltonol®:Oncovir) as an adjuvant for NY-ESO-1 protein (Ludwig Institute for Cancer Research-LICR) vaccination 2) a randomized Phase II trial in which patients were randomized to subcutaneous (s.c) vaccination with NY-ESO-1 protein with poly-ICLC alone (Arm A) or NY-ESO-1 protein, poly-ICLC and montanide® ISA-51 VG (SEPPIC, Inc) (Arm B) (, Supplementary Fig. S1). This study was conducted in accordance with the Declaration of Helsinki and was approved by the NYU Langone Medical Center as well as Mount Sinai Institutional Review Board (NYU#09–0007/MSSM#13–1391)). The primary endpoint of this study was safety of the vaccine regimen; the secondary endpoint was evaluation of induction of NY-ESO-1-specific humoral and T-cell responses. Patients with fully-resected and histologically confirmed melanoma [American Joint Committee on Cancer (AJCC) stages IIB, IIC, III or IV] were eligible. Tumor NY-ESO-1 expression was assessed by immunohistochemistry as previously described but was not required for eligibility (28).
In phase I of the study, the safety of the different doses of poly-ICLC were assessed in three cohorts of 3–4 patients each. Patients received poly-ICLC at escalating doses (0.35 mg-1.4 mg), in combination with 100 μg NY-ESO-1 protein emulsified in 1.1 mL montanide. We established 1.4 mg poly-ICLC as the highest tolerated dose without dose limiting toxicities (DLT) (Supplementary Fig. S1). In phase II of the study, patients were randomized to receive s.c. vaccination of 100 μg NY-ESO-1 protein with 1.4 mg poly-ICLC alone (Arm A) or with 100 μg NY-ESO-1 protein with 1.4 mg poly-ICLC and 1.1 mL montanide (Arm B) (Supplementary Fig. S1). Study arm assignments were unblinded after completion of the study and immune monitoring.
Blood samples
Blood samples were collected at baseline and day 1 and 8 for Cycles 1 and 4, Day 8 for Cycles 2 and 3, and up to 4 weeks after the fourth vaccination for assessment of humoral and cellular responses. Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized blood by Ficoll centrifugation and frozen using pooled human serum (90%) and DMSO (10%). Plasma from each time point was also frozen.
Humoral responses
Patient plasma samples were analyzed by ELISA for seroreactivity against recombinant NY-ESO-1 protein (1 μg/mL) as well as three individual OLPs (68-mers, 1 μmol/L each; Multiple Peptides Systems, San Diego, Ca) covering the NY-ESO-1 sequences as previously described (29). Synthetic long peptides were used to confirm specificity for NY-ESO-1 plasma antibodies and for approximate epitope mapping (Supplementary Table S1).
Titers >100 were considered reactive and specificity was determined by comparing reactivity to control antigens and to the NY-ESO-1 peptides, and changes in titers were considered significant if >4x between time points.
T-cell responses
T-cell responses to NY-ESO-1 protein were evaluated after in vitro stimulations (IVS) with OLPs pools of NY-ESO-1 peptides (JPT Peptide Technologies, Berlin, Germany). IVS were performed with some modifications to a protocol described previously (30). PBMCs were thawed and cultured overnight in 5% PHS (Valley Biomedicals, Winchester, VA) in RPMI (ThermoFisher Scientific, Waltham, MA), then CD4+, CD8+, and CD4− CD8− fractions were isolated by using Dyna beads (Life Technologies, Carlsbad, CA). Each fraction was then resuspended in PHS/RPMI containing 10 U/mL IL-2 and 10 ng/mL IL-7 (both from R&D Systems, Minneapolis, MN). CD4+ and CD8+ T-cells (5 × 105 −106 cells/well) were co-cultured separately for 14 and 20 days, respectively, with APCs (CD4−CD8−) pulsed with pooled NY-ESO-1 OLPs covering the entire NY-ESO-1 protein (1 μg/mL each).
On Day 14 or 20, T-cell cultures were re-stimulated with the same pool of OLPs, or control peptides (MOG, CMV, and PMA/ionomycin). Additionally, epitope mapping was done by stimulating cells with individual NY-ESO-1 peptides. For all intracellular cytokine staining (ICS) cultures, cells were incubated with peptide for 1 h at 37°C and then BD Golgi Plug and GolgiSTOP (both from BD Bioscience) were added for an additional 5 h. Cells were then stained for CD4, CD8 (both from BD Bioscience, San Jose, CA), and Live/Dead Violet (Life technologies, Carlsbad, CA), fixed and permeabilized with BD Cytofix/Cytoperm solution and stained for CD3, IL-2, TNF-α, and IFN-γ (all from BD Bioscience). Flow cytometry was performed in a LSR II or BD Fortessa flow cytometers using FACSDiva software, and data were analyzed using FlowJo software (TreeStar).
NY-ESO-1 presentation by monocyte-derived dendritic cells
PBMCs were expanded with NY-ESO-1 15–16 mer OLPs (1 μg/mL) with some modifications as described previously (31). Fresh complete RPMI medium containing IL-2 and IL-7 was used, and medium was replaced every 2–3 days. On day 12, cells were re-stimulated with serial concentrations of NY-ESO-1 peptides (peptides 5, 7, and 17) (1 μM-1 nM) and BD Golgi Plug and GolgiSTOP were added for ICS. Alternatively, CD14+ monocytes (positively selected from PBMC using a MACS system (Miltenyi Biotech, Bergisch Gladbach, Germany), were used to generate MoDCs with GM-CSF and IL-4. They were pulsed with NY-ESO-1 OLPs, thoroughly washed and co-cultured at 1:10 ratio (DC:PBMCs) with PBMCs. The co-cultures were expanded in vitro for 10–12 days and then re-stimulated with NY-ESO-1-pulsed MoDCs at 1:10 ratio. ICS was performed by flow cytometry as described above.
Immune cell infiltration at the injection site
Skin biopsies were obtained at cycle 4 day 8 (C4D8) (four punch biopsies per patient were taken from two different sites: 2 untreated skin (control) and 2 treated skin, for immune cell infiltrates). Skin biopsies were stained by hematoxylin and eosin (H&E) and examined by two pathologists who were blinded to the patients’ clinical data. CD3+, CD4+, CD8+, CD11c+, and CD20+ cells were counted in 10 high power fields per section and reported.
TLR3 polymorphisms
Coding sequences were obtained from PBMCs using PCR and Sanger sequencing of germline DNA. Primers were designed to cover the coding sequences plus at least 10 nucleotides in the intron region on both ends. Primer extension sequencing was performed by GENEWIZ, Inc. using BigDye® version 3.1 (ThermoFisher Scientific). Both forward and reverse strands were sequenced. The reactions were then run on the Applied Biosystem’s 3730xl DNA Analyzer. The sequencing data were analyzed with Lasergene SeqMan software (DNASTAR) to detect the mutations compared with genomic DNA reference sequence.
Statistical Analyses
The two arms were compared with respect to CD4+IFNγ+ and CD8+IFNγ+ production by ICS at each of the different time points analyzed by the Wilcoxon-Mann-Whitney test. Immune cell infiltration at the injection site before and following treatment was assessed for specific markers of immune cells (CD4+, CD8+, B cells, and dendritic cells) by the Wilcoxon signed rank test, and the two treatment arms were compared for immune cell infiltration post treatment by the Wilcoxon-Mann-Whitney test. All statistical tests were two-sided at the 0.05 level of significance.
Results
Patient characteristics
A total of 10 patients were sequentially enrolled into three cohorts of phase I of the study, 3–4 patients per cohort (Supplementary Fig. S1). In each of the 3 cohorts, vaccine cycles were repeated every 3 weeks for a total of 4 cycles. Of the 10 patients in phase I, 8 were male, and most patients were AJCC stage III, with half of the patients at stage IIIC (Table 1). In phase II, 25 additional patients were randomized to arms A or B; the majority of these patients had stage III disease. Across both arms, patients were balanced with respect to age, sex, and stage of disease. Per protocol, patients were allowed prior treatments, and a minority of patients had been treated with adjuvant interferon and/or adjuvant external beam radiotherapy (Table 1). Expression of NY-ESO-1 in the resected tumor was not mandatory for study entry; specimens for immunohistochemistry (IHC) analysis were available for all 10 patients in phase I, and 23 of 25 patients in phase II; 2 patients in phase I and 5 patients in phase II [arm A=3, arm B=2] had tumors that expressed NY-ESO-1, which is consistent with the literature (28).
Table 1.
Baseline patient demographics and clinical characteristics.
| Phase I | Phase II – Arm A | Phase II – Arm B | ||
|---|---|---|---|---|
| Age, Median (Standard Deviation) | 67 (13) | 50 (14) | 57 (16) | |
| Male, n (%) | 8 (80%) | 7 (58%) | 6 (46%) | |
| AJCC Stage, n (%) | IIB | 1 (10%) | 1 (8%) | 1 (8%) |
| IIC | 1 (10%) | 0 (0%) | 0 (0%) | |
| IIIA | 1 (10%) | 1 (8%) | 2 (15%) | |
| IIIB | 2 (20%) | 4 (33%) | 5 (38%) | |
| IIIC | 5 (50%) | 4 (33%) | 3 (23%) | |
| IV | 0 (0%) | 2 (17%) | 2 (15%) | |
| Prior Therapy, n (%) | Interferon | 2 (20%) | 2 (17%) | 2 (15%) |
| Radiation | 3 (30%) | 5 (42%) | 3 (23%) | |
Safety
All 35 patients enrolled on the study were evaluated for safety. In phase I of the study, one patient was replaced due to disease progression before treatment; in phase II, one patient experienced uncontrolled pain resulting from spinal stenosis prior to initiation of injections, and was replaced. One patient voluntarily withdrew from study after Cycle 3 study drug administration.
The most common grade 1 or 2 adverse events were injection site reactions, with pain being most common (92%) followed by erythema (76%) and granuloma formation (64%). Most patients experienced constitutional symptoms, most commonly fatigue (72%), but also fevers/chills (62%) as well as myalgias (52%). Less common adverse events included: arthralgias (20%), headache (32%), and upper respiratory symptoms such as cough (20%) and congestion or coryza (20%) (Table 2).
Table 2.
Toxicity profile.
| Grade 1 / 2 | Grade 3 | ||||
|---|---|---|---|---|---|
| n | (%) | n | (%) | ||
| Dermatologic – Injection Site | |||||
| Pain | 23 | 92 | - | - | |
| Swelling | 7 | 28 | - | - | |
| Erythema | 19 | 76 | - | - | |
| Pruritus | 12 | 48 | - | - | |
| Granuloma | 16 | 64 | - | - | |
| Ecchymosis | 4 | 16 | - | - | |
| Cellulitis | 1 | 4 | - | - | |
| Constitutional | |||||
| Fever/Chills | 17 | 68 | - | - | |
| Fatigue | 18 | 72 | - | - | |
| Malaise | 15 | 60 | - | - | |
| Musculoskeletal | |||||
| Myalgias | 13 | 52 | - | - | |
| Arthralgias | 5 | 20 | - | - | |
| Neurologic | |||||
| Dizziness | 2 | 8 | - | - | |
| Headache | 8 | 32 | - | - | |
| Insomnia | 1 | 4 | - | - | |
| Anxiety | 2 | 8 | - | - | |
| Respiratory/ENT | |||||
| Cough | 5 | 20 | - | - | |
| Sore Throat | 2 | 8 | - | - | |
| Congestion/Coryza | 5 | 20 | - | - | |
| Gastrointestinal | |||||
| Abdominal Discomfort | 2 | 8 | - | - | |
| Nausea | 1 | 4 | - | - | |
| Loose Bowel Movements | 1 | 4 | - | - | |
| Cardiac | |||||
| Coronary Artery Disease | - | - | 1 | 4 | |
No dose-limiting toxicities were reported in the phase I dose escalation. A single grade 3 serious adverse event (SAE) (v4.0 NCI CTCAE), new-onset stable ischemic heart disease, was recorded in one subject in phase II (Arm A). This event was deemed unrelated to study drug, and this patient completed all treatment interventions and both follow-up visits. There were no grade 4 or 5 adverse events. No adverse event led to study discontinuation. Six patients (1 in phase I, Cohort 1, and 5 in phase II, arm B) had disease recurrence while receiving treatment and were taken off study as per protocol.
Survival analysis of the phase II cohort revealed that at a median follow-up of 38 months, ten (40%) of 25 patients remained recurrence-free (Table 3). An additional four patients were rendered surgically free of disease, comprising a total of 14 patients (56%) who were alive without clinical evidence of disease.
Table 3. Summary of NY-ESO-1 tumor expression, antibody titers, cellular response, clinical outcome, and TLR3 polymorphisms.
Summary of NY-ESO-1 tumor expression, NY-ESO-1 antibody titers, cellular response, clinical outcome, pre-vaccination treatment, and TLR3 polymorphisms. NY-ESO-1 antibody titers: 1–9,999: +; 10,000–99,999: ++; 100,000–249,000: +++; ND: not done due to insufficient plasma. NY-ESO-1 tumor expression: ND: not done due to insufficient tumor tissue. Arm A: patients receiving NY-ESO-1 protein, Poly-ICLC; Arm B: patients receiving NY-ESO-1 protein, Poly-ICLC, and montanide. XRT: radiation therapy, IFN: interferon therapy.
| Study Arm | PID | Humoral response/Antibody Titer | CD4 | CD8 | TLR3 Polymorphisms | NY-ESO1 expression in tumor | AJCC Stage | Treatment Pre-vaccination | Follow-Up Time (Months) | Period from surgical resection to study entry | Recurrence | Clinical Status |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 011 | + | + | + | 1234CT | - | IIIB | XRT | 42 | 9 | None | Alive, NED |
| A | 012 | ++ | + | - | 1234CT | - | IIIB | IFN | 45 | 6 | 29 months | Alive, Surgically Rendered NED |
| A | 016 | ++ | - | - | 1234CT | - | IIIC | - | 45 | 4 | None | Alive, NED |
| A | 18* | + | + | - | - | - | IIIC | XRT | 39 | 4 | 28 months | Alive, Surgically Rendered NED |
| A | 019 | ++ | + | - | 1234CT | - | IIB | - | 32 | 6 | None | Alive, NED |
| A | 021 | ++ | + | - | - | - | IIIC | XRT | 42 | 7 | 24 months | Alive with Metastatic Melanoma |
| A | 025*Δ | ++ | + | - | 1234CT | + | IIIB | - | 39 | 5 | None | Alive, NED |
| A | 26Δ | + | + | - | - | - | IIIA | XRT | 23 | 9 | 10 months | Died of Metastatic Melanoma |
| A | 029 | + | + | - | - | + | IV | - | 38 | 7 | 4 months | Alive with Metastatic Melanoma |
| A | 030*Δ | ++ | + | - | 1234CT | - | IIIC | XRT | 39 | 3 | 6 months | Alive with Metastatic Melanoma |
| A | 031 | + | + | - | 1234CT | + | IIIB | IFN | 31 | 3 | None | Alive, NED |
| A | 033 | + | + | - | 1234CT | - | IV | - | 36 | 4 | None | Alive, NED |
| B | 013 | +++ | + | + | 1234CT | - | IIIA | - | 45 | 3 | 10 months | Alive, Surgically Rendered NED |
| B | 014 | ND | - | - | ND | - | IV | IFN | 45 | 3 | During treatment | Alive with Metastatic Melanoma |
| B | 15Δ | +++ | + | - | - | - | IV | XRT | 45 | 2 | 41 months | Alive with Metastatic Melanoma |
| B | 017 | ND | - | - | ND | - | IIIB | - | 21 | 3 | 3 months | Died of Metastatic Melanoma |
| B | 020 | +++ | + | + | 1234CT | - | IIIB | XRT/IFN | 33 | 1 | During treatment | Died of Metastatic Melanoma |
| B | 022 | + | + | - | - | - | IIIC | - | 39 | 3 | None | Alive, NED |
| B | 23Δ | ++ | + | - | - | ND | IIIB | - | 40 | 2 | None | Alive, NED |
| B | 024 | ND | - | - | ND | - | IIIC | - | 17 | 3 | During treatment | Died of Metastatic Melanoma |
| B | 027* | +++ | + | + | 1234CT | + | IIIC | - | 24 | 2 | 12 months | Died of Metastatic Melanoma |
| B | 028 | ND | - | - | ND | - | IIB | - | 31 | Never Treated | None | Alive, NED |
| B | 032 | + | + | - | 1234CT | + | IIIB | XRT | 33 | 3 | During Treatment | Alive, Surgically Rendered NED |
| B | 034 | + | + | + | - | - | IIIA | - | 34 | 4 | None | Alive, NED |
| B | 035 | + | - | - | - | ND | IIIB | - | 34 | 3 | During treatment | Alive with Metastatic Melanoma |
Patients who had pre-existing anti-NY-ESO-1 titers. All the patients, prior to vaccination, underwent MRI of the brain and computed tomography (CT) of the abdomen and pelvis (CAP) with contrast. Positron emission tomography and computed tomography (PET/CT) could be substituted for CT CAP, if it was covered by patient insurance.
Patients who had NY-ESO-1-specific T-cell responses prior to vaccination.
NY-ESO-1-specific humoral response
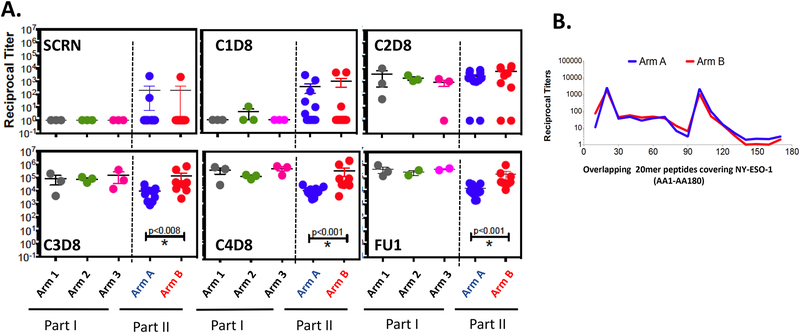
All patients developed antibody responses to NY-ESO-1 protein regardless of the dose of poly-ICLC (Fig. 1A and Supplementary Fig. S2A). Most of the patients seroconverted after receiving the second or third vaccine. Antibody titers were not significantly different between patients in arms A and B at screening and cycle 2 day 8 (C2D8); however, patients enrolled in arm B developed more NY-ESO-1-specific antibodies compared to patients within arm A after 3 or 4 vaccines (Fig. 1A and Supplementary Fig. S2B). Pretreatment antibody responses to NY-ESO-1 were detected in 3 of 25 (12%) of patients (arm A= 2, arm B= 1), and all 3 patients showed substantial increases in antibody titers after vaccination (Fig. 1A). Only 2 of 5 patients whose tumors expressed NY-ESO-1 had pretreatment NY-ESO-1-specific antibodies (one in each of arms A and B).
Figure 1:
Evolution of antibody titers against NY-ESO-1 by ELISA. A. Titer comparisons for NY-ESO-1 at screening and first follow-up (FU1) time points. B. Titer comparisons for NY-ESO-1 at different time points over the vaccination period. Arm 1 (n=3): 100 μg NY-ESO-1 protein emulsified in 1.1 mL montanide ISÄ−51 VG + 0.35 mg Poly-ICLC, Arm 2 (n=3): 100 μg NY-ESO-1 protein emulsified in 1.1mL montanide ISÄ−51 VG + 0.70 mg Poly-ICLC, Arm 3 (n=3): 100 μg NY-ESO-1 protein emulsified in 1.1mL montanide ISÄ−51 VG + 1.40 mg Poly-ICLC, Arm A (n=12): 100 μg NY-ESO-1 protein emulsified in 1.40 mg Poly-ICLC without montanide ISÄ−51 VG, Arm B (n=10): 100 μg NY-ESO-1 protein emulsified in 1.1 mL montanide ISÄ−51 VG + 1.40 mg Poly-ICLC. C. Seroreactivity to specific regions of the NY-ESO-1 protein were mapped using 20 mer overlapping peptides covering the entire protein.
For fine mapping of epitopes recognized by vaccine-induced NY-ESO-1 antibodies, ELISA was performed using 20-mer OLPs covering the NY-ESO-1 protein (AA1-AA180) at the peak of antibody induction (Cycle 4). NY-ESO-1-specific antibody responses induced by the vaccine were detected mainly towards three regions covered by the 20-mer peptides: 11–30, 91–110, and 101–120, in both arms (Fig. 1B and Supplementary Fig. S2C). Consistent with previous results, most of these antibody responses mapped to areas which correspond with the N-terminal and central regions of the NY-ESO-1 protein (29).
NY-ESO-1-specific T-cell responses
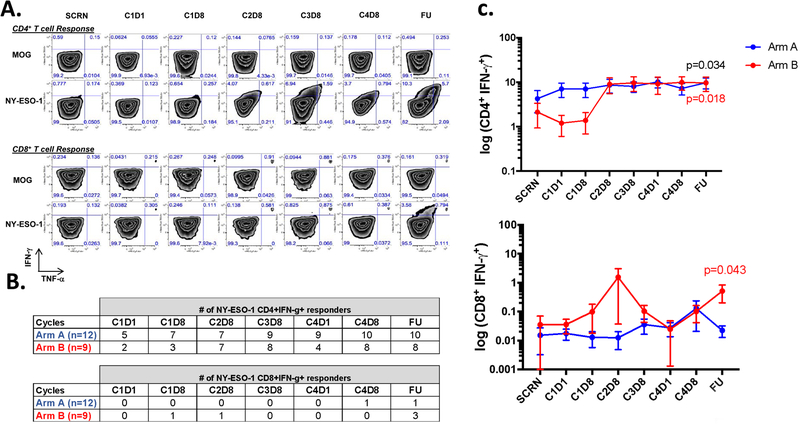
NY-ESO-1-specific cellular responses were determined by IVS and flow cytometry analysis (Fig. 2A) in 12 and 9 patients within arm A and B, respectively. Prior to vaccination, NY-ESO-1-specific CD4+ T-cell responses were detected in 5 of 12 (41%) patients in arm A and 2 of 9 (22%) of patients in arm B; vaccination significantly increased CD4+ T-cell responses in patients within both arms, 10 of 12 (83%) patients in arm A and 8 of 9 (89%) patients in arm B (Fig. 2B). CD4+ responses at follow-up were significantly higher than at baseline for both arm A and B (p=0.034 and p=0.018, respectively) (Fig. 2C). In patients who developed T cell responses, NY-ESO-1-specific CD4+ T-cell responses were detectable after the second and third vaccination (Fig. 2C). After vaccination, NY-ESO-1-specific CD8+ T-cell responses were detected in 1 of 12 (8%) patients in arm A and 3 of 9 (33%) patients in arm B (Fig. 2B), with an increase in IFN-γ production seen mainly in patients treated with montanide (Arm B) (Fig. 2C). Patients with both antigen-specific CD4+ and CD8+ T-cell responses had antibody titers at least 3 times higher than in those who did not achieve significant CD8+ T-cell responses. In addition, both CD4+ and CD8+ T-cell responses were polyfunctional since T-cells secreted INF-γ, TNF-α, and/or IL-2 (Supplementary Fig. S3). Overall, our data indicate that montanide increases immunogenicity, inducing an earlier and more robust CD4+ T-cell response, and promoting CD8+ T-cell immunity in a fraction of patients.
Figure 2:
CD4+ and CD8+ T-cell responses from selected patients throughout vaccination with NY-ESO-1. A. Representative dot plot showing CD4+ and CD8+ T cell responses (patient 34, Arm B). CD4+ and CD8+ T cells were isolated from PBMCs of patients and then stimulated in vitro with NY-ESO-1 overlapping peptides. NY-ESO-1-specific T cell responses were evaluated by ICS at day 14 (CD8+ T; bottom panel) and day 21 (CD4+ T; upper panel). B. Number of NY-ESO-1-specific T cell responders based on the production of IFN-γ by CD4+ and CD8+ T cells, during treatment course. C. Quantification of CD4+ and CD8+ IFN- γ+ production by intracellular cytokine staining at different time points over the vaccination treatment. Arm A (blue), n=12 and Arm B (red), n=9. The time points represent responses measured at screening (SCRN), and through different cycles. The CnDx designation refers to cycle (C) and day (D) of blood draw.
Recognition of NY-ESO-1 protein by vaccine-induced NY-ESO-1-specific T cells
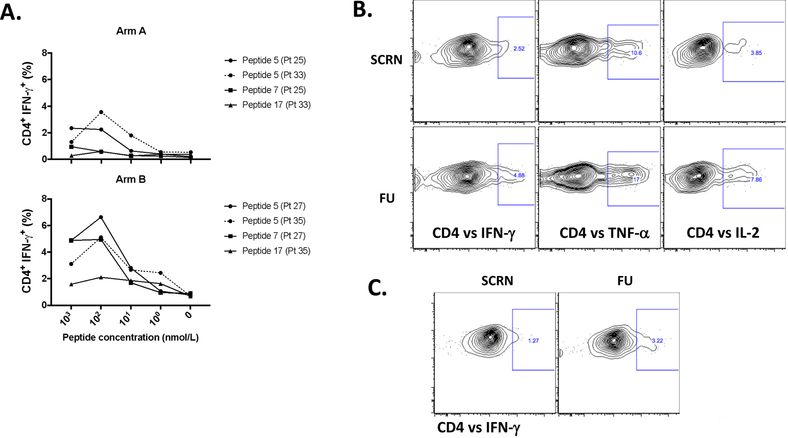
Vaccination with peptides may induce peptide-specific T-cells that are not able to recognize naturally processed antigens. We therefore assessed the quality of recognition of NY-ESO-1 by NY-ESO-1 protein-induced T-cells. Previously, NY-ESO-1-specific T-cell responses were determined by performing T-cell assays with OLPs covering the entire NY-ESO-1 protein (Supplementary Table S1). We found that most of the patients showed T-cell responses against peptides 5, 7, 15, 16, and 17, located mainly in the central and terminal regions of the protein (AA21–180). Based on these results, PBMCs from patients who showed T-cell responses against peptides 5, 7, and 17 (peptide 5= AA81–100, peptide 7= AA119–143, and peptide 17=AA161–180) were cultured in the presence of NY-ESO-1 OLPs and re-stimulated with serial dilutions (1 μM-1 nM) of NY-ESO-1 peptides. CD4+ T-cells from patients in both arms exhibited a wide range of avidities to each NY-ESO-1 peptide, with the highest avidity to peptide 5 (AA81–100), and consistent with the cellular responses observed to whole protein, the addition of montanide increased responsiveness to individual NY-ESO-1 peptides (Fig. 3A). Among the three regions tested, we found that the region covered by peptide 5 (AA81–100), which corresponds to the central region of the NY-ESO-1 protein, is the most immunogenic. To directly address recognition of the NY-ESO-1 protein by APCs, MoDCs were pulsed with NY-ESO-1 OLPs and co-cultured with PBMCs from an arm B patient (Fig. 3B). We found that pulsed MoDCs induced significant CD4+ T-cell responses at the follow-up time point (Fig. 3B), and this response was superior compared with PBMCs from the same donor that were expanded with NY-ESO-1 OLPs alone (Fig. 3C). These results indicated that patient’s MoDCs were able to uptake, process, and present NY-ESO-1 peptides to T-cells.
Figure 3:
Recognition of processed NY-ESO-1 protein by vaccine-induced NY-ESO-1-specific T cells. A. Avidity of vaccine-induced NY-ESO-1-specific CD4+ T cells, after vaccination (FU) is shown. PBMCs from patients in arm A and arm B (2 from each group) were cultured in the presence of NY-ESO-1 OLPs for 12 days. On that day, cells were re-stimulated with serial dilutions of NY-ESO-1-specific peptides (1 μM-1 nM) (P5, 7, and/or 17) and GolgiPlug as well as GolgiSTOP were added after 1h of peptide stimulation. Then, intracellular cytokine staining was performed to assess flow cytometry. Percentage of CD4+ IFN-γ+ cells were determined at different peptide concentrations; peptides: P5 (AA81–100), P7 (AA119–143), and P17 (AA161–180). N=2 (arm A; patient 25 responded against P5 and 7; patient 33 responded against P5 and 17), n=2 (arm B; patient 27 responded against P5 and 7; patient 35 responded against P5 and 17). B. Recognition of NY-ESO-1 peptides (OLPs) presented by MoDCs. MoDCs from an arm B patient (patient 32) were pulsed with NY-ESO-1 OLPs for 4 h and then co-cultured with PBMCs isolated from screening and FU time points, or C. PBMCs from the same patient were expanded for 10 days in the presence of NY-ESO-1 OLPs. At day 10, cells were harvested and re-stimulated with MoDCs pulsed with OLPs (1 μM) and GolgiPlug as well as GolgiSTOP were added after 1h of peptide stimulation. Intracellular cytokine staining was performed to assess flow cytometry.
TLR3 polymorphisms in response to NY-ESO-1 vaccination
Several studies suggest a role of single-nucleotide polymorphisms (SNP) within TLR genes in susceptibility to cancer and other diseases (32,33). In addition, SNPs might affect the impact of vaccines containing TLR ligands (33). We evaluated how polymorphisms in the TLR3 gene may influence the immune response to vaccination with antigen plus poly-ICLC with or without montanide. In order to analyze polymorphisms for TLR3, we performed a germline SNP analysis on each patient at baseline and correlated this with immunologic responsiveness and clinical outcome. The mutation in exon 3 (1234CT) was found in 8 of 12 (66%) patients in arm A and 4 of 9 (44%) patients in arm B (Table 3). The mutation was present in two of the three patients in arm B who had antigen-specific CD8+ T-cell responses. For CD4+ T-cell responses, we found the mutation in exon 3 in 7 of 11 (63%) patients in arm A and 4 of 8 (50%) patients in arm B (Table 3). However, since the sample size is small, we cannot conclude whether the TLR3 polymorphisms might influence or enhance the NY-ESO-1-specific CD8+ T-cell cross-presentation when the vaccine was delivered in the presence of montanide.
Immune cell infiltration at the vaccine injection site
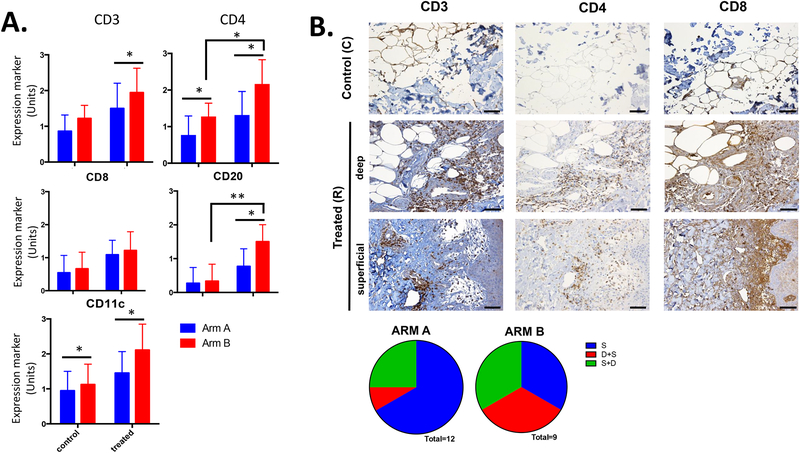
Immune cell infiltration at the vaccine injection site was determined by IHC. At baseline, an increased number of CD4+ T-cells and CD11c+ cells was found in arm B compared to arm A group, and that difference was maintained after vaccination (Fig. 4A), being significantly higher in patients vaccinated with montanide. A significant increase in total CD3+ lymphocyte infiltration as well as B cells (CD20+) was also found at the site of injection in arm B (Fig. 4A). We observed that in the absence of montanide (arm A) most of the immune cell infiltration was found at the superficial dermis/epidermis-dermis junction, whereas in the presence of montanide (arm B) many immune cells were also found in deep areas of the dermis (Fig. 4B). This suggests that the inclusion of montanide elicits an inflammatory response that spans histologic barriers, potentially enabling interaction with distinct DC subsets capable of alternate trafficking and T-cell priming.
Figure 4:
Analysis of immune cell infiltration at the injection site and location of inflammatory cells by immunohistochemistry (IHC). Scale bar equals to 200 μm. A. IHC analysis of immune cell infiltration was performed for each arm and different markers for specific immune cell populations were used, including CD3, CD4, CD8, CD20, and CD11c. 0: No expression of the marker of interest, 1: single cells or small clusters (<5 cells together) expressing marker of interest, 2: medium size clusters of cells expressing marker of interest, and 3: huge and homogeneously positive clusters of cells expressing marker of interest. B. An example of lymphocyte infiltration at the injection site by IHC from a patient within arm B. S: only inflammatory cells in the superficial dermis/epidermis-dermis junction, D+S: inflammatory cells predominantly in deep dermis, but also little in superficial, S+D: inflammatory cells predominantly in superficial dermis/epidermis-dermis junction, but also little in deep. * p< 0.05 and ** p< 0.01.
Discussion
NY-ESO-1 protein is a self-antigen commonly expressed in human tumors that can spontaneously induce both humoral and cellular immune responses in patients with various solid tumors, especially melanoma. Immunogenicity of NY-ESO-1 protein in melanoma patients as well as in patients with other solid tumors in combination with TLR ligands and montanide has been previously reported (19–21) and this combination appears to achieve synergistic priming of cellular and humoral immune responses to the antigen. montanide might contribute to the immunogenicity of NY-ESO-1 through its slow antigen release (depot effect) and recruitment of APCs. However, montanide also drives the accumulation of T cells at the local site, and in monkey models was not found to be superior to other adjuvants (34). In addition, vaccination of mice with minimal peptides and IFA alone creates a sink for effector T cells; however addition of TLR agonists to long peptides and IFA elicits potent immune responses (7). On the other hand, IFA-based vaccination induces an inflamed vaccination site that recruits and functionally impairs tumor-specific T-effector cells induced by anti-CTLA4 blockade therapy, through mechanisms dependent on inflammatory monocytes (typically CCL2, CXCR3, IFN-γ, and ICAM-1) (6). TLR agonists such as poly-ICLC (TLR3), resiquimod (TLR7/8), or CpG (TLR9) have immune adjuvant properties because of their ability to activate APCs (35,36). Through activation of APCs carrying tumor antigen, such as DCs, TLR signaling can break immune tolerance to tumor-associated antigens through expression of co-stimulatory molecules and pro-inflammatory cytokines capable of inducing a cellular and humoral immune responses against tumor cells (37,38).
The combination of montanide and TLR agonists induces both humoral and CD4+/CD8+ cellular immune responses (20). We have previously reported trials in which repeated vaccination with recombinant NY-ESO-1 protein administered with montanide, and either CpG or resiquimod also induced humoral and T-cell antigen-specific responses (21,39). In this study, we compared the immunogenicity of TLR agonist alone or in combination with montanide. Our study demonstrates that vaccination with NY-ESO-1 administered with poly-ICLC and montanide is superior in inducing integrated antibody and CD4+ T-cell responses in the majority of the patients, and CD8+ T-cell responses in some patients, compared to poly-ICLC alone. Our data also suggest superior immunogenicity compared to the resiquimod/montanide combination (21). Although NY-ESO-1 expression on tumor specimens was detected in some of the patients, we failed to detect any correlation between antigen expression and response to vaccination (Table 3). Although only two patients in arm A and one patient in arm B showed serological reactivity to NY-ESO-1 before vaccination, after treatment a boost in NY-ESO-1-specific antibody responses was detected in the majority of patients in both arms. The response was more pronounced in montanide-vaccinated patients. Patients with both antigen-specific CD4+ and CD8+ T-cell responses had antibody titers at least 3 times higher than in those who did not achieve significant CD8+ T-cell responses. These data correlate with a previous study, in which we found a correlation between NY-ESO-1-specific humoral and CD8+ T-cell responses (39). Fine mapping showed that NY-ESO-1-specific antibodies induced by the vaccine were directed mainly towards the N-terminal and central region of the NY-ESO-1 protein.
In mice, IFA (which resembles montanide) promoted type 2 cytokine production in response to intraperitoneal antigen injection (40). However, the inclusion of poly-ICLC, in combination with montanide suppressed Th2 responses favoring Th1 immunity, which is more favorable for anti-tumor responses (41). In concordance with this observation, here we demonstrated that vaccination with NY-ESO-1 and poly-ICLC with/without montanide induced polyfunctional CD4+ and CD8+ T-cell populations, producing IFN-γ, TNF-α, and IL-2 in response to antigen rechallenge.
A small fraction of patients vaccinated with montanide exhibited CD8+ T-cell responses (37%) compared to only one patient (8%) within arm A. We also observed that patients from both arms elicited a wide range of avidities to NY-ESO-1-specific peptides within the CD4+ T-cell compartment, and that these avidities were higher when patients had been vaccinated with montanide. We found that the region covered by peptide 5 (AA81–100) is the most immunogenic, since IFN-γ production by CD4+ T-cells was higher compared to the other two peptides (peptide 7 and 17), and montanide may induce higher avidity T-cell responses to individual NY-ESO-1 peptides.
Analyzing the immune cell infiltration at the injection site, we found that montanide induces mononuclear cell infiltration at deeper areas of the dermis. In previous studies, we demonstrated that subcutaneous injection of NY-ESO-1 protein with CpG delivered with montanide led to development of antigen-specific antibodies and CD4+ Th1 immunity in most of the patients and CD8+ T cell responses in almost 50% of the patients (39). We also evaluated the immunogenicity of the NY-ESO-1 protein given in combination with montanide s.c with or without topical application of a TLR7 agonist in melanoma patients (21). The majority of the patients developed NY-ESO-1-specific antibodies and CD4+ T-cell responses; however, CD8+ responses were observed only in 3 of 12 patients in the cohort receiving the antigen, montanide, and a TLR7 agonist (21). After vaccination with the NY-ESO-1 protein expressed within ISCOMATRIX or with a complex of cholesterol-bearing hydrophobized pullulan, fewer than 50% of the patients developed CD8+ T-cell responses (39,42,43). Vaccination with OLPs coemulsified with montanide and poly-ICLC, in contrast, induced consistent CD8+ T-cell responses in nearly all the patients, favoring cross-presentation (19). It appears that the inclusion of synthetic long peptides (SLPs) improves antigen processing and presentation to both CD4+ and CD8+ T-cells (44). Such SLPs harboring CTL and T helper epitopes have induced immune responses (45–47). Based on these studies, montanide enhances the induction of integrated immune responses as well as cross-priming. In addition to that, the route of adjuvant delivery as well as the type of adjuvant affects vaccine efficacy.
By single-nucleotide polymorphism (SNP) analysis, we determined possible associations of TLR3 polymorphisms with immune response induced by the NY-ESO-1 vaccine. We found that 8 of 12 (66%) patients in arm A and 4 of 9 (44%) patients in arm B carried the 1234CT mutation in the TLR3 gene. This 1234CT mutation in TLR3 encodes for an amino acid exchange (Leu to Phe) at position 412 and has been associated with different diseases such as HIV infection and non-small cell lung cancer (48,49). Previous studies have shown that the prevalence of the 1234CT genotype was increased in hepatocellular carcinoma (HCC) patients compared to controls (49). In addition, the 1234CT polymorphism was associated with hepatitis B virus-infected HCC patients, indicating that this polymorphism could be a risk factor for HBV-related HCC (49). This SNP was detected in 2 of the 3 patients in arm B who developed CD8+ T-cell responses after vaccination, whereas the other two patients in arm B who had this particular SNP did not achieve CD8+ T-cell responses. However, since the sample size is very small, we could not assess the correlation between this specific TLR3 SNP 1234CT and CD8+ T-cell responses induced by this vaccine when it was delivered with montanide.
In summary, vaccination with NY-ESO-1 protein and poly-ICLC with or without montanide safely induces integrated NY-ESO-1-specific humoral and CD4+ T-cell responses. Both regimens were well-tolerated, and at last follow-up, most phase II patients were alive without clinical evidence of disease. CD8+ T-cell responses were observed in a subset of patients receiving montanide and poly-ICLC; moreover, patients in the montanide arm also had a more robust humoral response, indicating that the addition of montanide in cancer vaccines may potentiate both T-helper cell function as well as cross-presentation of antigen. Given the favorable toxicity profile and immunogenicity of NY-ESO-1 vaccines, future trials should consider testing NY-ESO-1 antigen with poly-ICLC and montanide in combination with checkpoint blockade immunotherapy in the high-risk resected melanoma patient, as this is now standard of care in this setting (2,4,50). In this regard, applying OLP from NY-ESO-1 which would prime CD8+ T-cells (19,20) may be a superior approach.
Supplementary Material
Acknowledgments
We thank Dr J. Goldberg (NYU Langone Health) for informative statistical discussion.
Financial support: The study was supported by the Cancer Research Institute, the Melanoma Research Alliance, and the National Institutes of Health (R01 CA 201189, and R01 CA180913). NB is an extramural member of the Parker Institute for Cancer Immunotherapy.
Footnotes
Conflict of interest: NB is on the SAB of Neon Therapeutics, Tempest, and Check Point Sciences, and a consultant for Genentech. NB also receives research support from Merck. R.S. works as clinical scientist at Genentech, Inc. No potential conflicts of interest were disclosed by other authors.
References
- 1.Eggermont AMM, Blank CU, Mandala M, Long GV, Atkinson V, Dalle S, et al. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. N Engl J Med 2018;378(19):1789–801 doi 10.1056/NEJMoa1802357. [DOI] [PubMed] [Google Scholar]
- 2.Weber J, Mandala M, Del Vecchio M, Gogas HJ, Arance AM, Cowey CL, et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N Engl J Med 2017;377(19):1824–35 doi 10.1056/NEJMoa1709030. [DOI] [PubMed] [Google Scholar]
- 3.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017;168(4):707–23 doi 10.1016/j.cell.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gibney GT, Kudchadkar RR, DeConti RC, Thebeau MS, Czupryn MP, Tetteh L, et al. Safety, correlative markers, and clinical results of adjuvant nivolumab in combination with vaccine in resected high-risk metastatic melanoma. Clin Cancer Res 2015;21(4):712–20 doi 10.1158/1078-0432.CCR-14-2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma P, Allison JP. The future of immune checkpoint therapy. Science 2015;348(6230):56–61 doi 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 6.Hailemichael Y, Woods A, Fu T, He Q, Nielsen MC, Hasan F, et al. Cancer vaccine formulation dictates synergy with CTLA-4 and PD-L1 checkpoint blockade therapy. J Clin Invest 2018;128(4):1338–54 doi 10.1172/JCI93303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hailemichael Y, Dai Z, Jaffarzad N, Ye Y, Medina MA, Huang XF, et al. Persistent antigen at vaccination sites induces tumor-specific CD8(+) T cell sequestration, dysfunction and deletion. Nat Med 2013;19(4):465–72 doi 10.1038/nm.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363(8):711–23 doi 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 2015;372(21):2006–17 doi 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med 2015;372(26):2521–32 doi 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 11.Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev 2008;224:166–82 doi 10.1111/j.1600-065X.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 12.Robbins PF, Morgan RA, Feldman SA, Yang JC, Sherry RM, Dudley ME, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol 2011;29(7):917–24 doi 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jungbluth AA, Antonescu CR, Busam KJ, Iversen K, Kolb D, Coplan K, et al. Monophasic and biphasic synovial sarcomas abundantly express cancer/testis antigen NY-ESO-1 but not MAGE-A1 or CT7. Int J Cancer 2001;94(2):252–6. [DOI] [PubMed] [Google Scholar]
- 14.Chen YT, Old LJ. Cancer-testis antigens: targets for cancer immunotherapy. Cancer J Sci Am 1999;5(1):16–7. [PubMed] [Google Scholar]
- 15.Nagata Y, Ono S, Matsuo M, Gnjatic S, Valmori D, Ritter G, et al. Differential presentation of a soluble exogenous tumor antigen, NY-ESO-1, by distinct human dendritic cell populations. Proc Natl Acad Sci U S A 2002;99(16):10629–34 doi 10.1073/pnas.112331099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gnjatic S, Jager E, Chen W, Altorki NK, Matsuo M, Lee SY, et al. CD8(+) T cell responses against a dominant cryptic HLA-A2 epitope after NY-ESO-1 peptide immunization of cancer patients. Proc Natl Acad Sci U S A 2002;99(18):11813–8 doi 10.1073/pnas.142417699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shackleton M, Davis ID, Hopkins W, Jackson H, Dimopoulos N, Tai T, et al. The impact of imiquimod, a Toll-like receptor-7 ligand (TLR7L), on the immunogenicity of melanoma peptide vaccination with adjuvant Flt3 ligand. Cancer Immun 2004;4:9. [PubMed] [Google Scholar]
- 18.Dutoit V, Taub RN, Papadopoulos KP, Talbot S, Keohan ML, Brehm M, et al. Multiepitope CD8(+) T cell response to a NY-ESO-1 peptide vaccine results in imprecise tumor targeting. J Clin Invest 2002;110(12):1813–22 doi 10.1172/JCI16428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sabbatini P, Tsuji T, Ferran L, Ritter E, Sedrak C, Tuballes K, et al. Phase I trial of overlapping long peptides from a tumor self-antigen and poly-ICLC shows rapid induction of integrated immune response in ovarian cancer patients. Clin Cancer Res 2012;18(23):6497–508 doi 10.1158/1078-0432.CCR-12-2189. [DOI] [PubMed] [Google Scholar]
- 20.Tsuji T, Sabbatini P, Jungbluth AA, Ritter E, Pan L, Ritter G, et al. Effect of Montanide and poly-ICLC adjuvant on human self/tumor antigen-specific CD4+ T cells in phase I overlapping long peptide vaccine trial. Cancer Immunol Res 2013;1(5):340–50 doi 10.1158/2326-6066.CIR-13-0089. [DOI] [PubMed] [Google Scholar]
- 21.Sabado RL, Pavlick A, Gnjatic S, Cruz CM, Vengco I, Hasan F, et al. Resiquimod as an immunologic adjuvant for NY-ESO-1 protein vaccination in patients with high-risk melanoma. Cancer Immunol Res 2015;3(3):278–87 doi 10.1158/2326-6066.CIR-14-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sultan H, Wu J, Kumai T, Salazar AM, Celis E. Role of MDA5 and interferon-I in dendritic cells for T cell expansion by anti-tumor peptide vaccines in mice. Cancer Immunol Immunother 2018. doi 10.1007/s00262-018-2164-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehrotra S, Britten CD, Chin S, Garrett-Mayer E, Cloud CA, Li M, et al. Vaccination with poly(IC:LC) and peptide-pulsed autologous dendritic cells in patients with pancreatic cancer. J Hematol Oncol 2017;10(1):82 doi 10.1186/s13045-017-0459-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ming Lim C, Stephenson R, Salazar AM, Ferris RL. TLR3 agonists improve the immunostimulatory potential of cetuximab against EGFR(+) head and neck cancer cells. Oncoimmunology 2013;2(6):e24677 doi 10.4161/onci.24677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu X, Nishimura F, Sasaki K, Fujita M, Dusak JE, Eguchi J, et al. Toll like receptor-3 ligand poly-ICLC promotes the efficacy of peripheral vaccinations with tumor antigen-derived peptide epitopes in murine CNS tumor models. J Transl Med 2007;5:10 doi 10.1186/1479-5876-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salazar AM, Erlich RB, Mark A, Bhardwaj N, Herberman RB. Therapeutic in situ autovaccination against solid cancers with intratumoral poly-ICLC: case report, hypothesis, and clinical trial. Cancer Immunol Res 2014;2(8):720–4 doi 10.1158/2326-6066.CIR-14-0024. [DOI] [PubMed] [Google Scholar]
- 27.Hartman LL, Crawford JR, Makale MT, Milburn M, Joshi S, Salazar AM, et al. Pediatric phase II trials of poly-ICLC in the management of newly diagnosed and recurrent brain tumors. J Pediatr Hematol Oncol 2014;36(6):451–7 doi 10.1097/MPH.0000000000000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vaughan HA, Svobodova S, Macgregor D, Sturrock S, Jungbluth AA, Browning J, et al. Immunohistochemical and molecular analysis of human melanomas for expression of the human cancer-testis antigens NY-ESO-1 and LAGE-1. Clin Cancer Res 2004;10(24):8396–404 doi 10.1158/1078-0432.CCR-04-0809. [DOI] [PubMed] [Google Scholar]
- 29.Gnjatic S, Ritter E, Buchler MW, Giese NA, Brors B, Frei C, et al. Seromic profiling of ovarian and pancreatic cancer. Proc Natl Acad Sci U S A 2010;107(11):5088–93 doi 10.1073/pnas.0914213107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adams S, O’Neill DW, Nonaka D, Hardin E, Chiriboga L, Siu K, et al. Immunization of malignant melanoma patients with full-length NY-ESO-1 protein using TLR7 agonist imiquimod as vaccine adjuvant. J Immunol 2008;181(1):776–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lissina A, Briceno O, Afonso G, Larsen M, Gostick E, Price DA, et al. Priming of Qualitatively Superior Human Effector CD8+ T Cells Using TLR8 Ligand Combined with FLT3 Ligand. J Immunol 2016;196(1):256–63 doi 10.4049/jimmunol.1501140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kutikhin AG. Association of polymorphisms in TLR genes and in genes of the Toll-like receptor signaling pathway with cancer risk. Hum Immunol 2011;72(11):1095–116 doi 10.1016/j.humimm.2011.07.307. [DOI] [PubMed] [Google Scholar]
- 33.Trejo-de la OA, Hernandez-Sancen P, Maldonado-Bernal C. Relevance of single-nucleotide polymorphisms in human TLR genes to infectious and inflammatory diseases and cancer. Genes Immun 2014;15(4):199–209 doi 10.1038/gene.2014.10. [DOI] [PubMed] [Google Scholar]
- 34.Wille-Reece U, Flynn BJ, Lore K, Koup RA, Miles AP, Saul A, et al. Toll-like receptor agonists influence the magnitude and quality of memory T cell responses after prime-boost immunization in nonhuman primates. J Exp Med 2006;203(5):1249–58 doi 10.1084/jem.20052433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pichlmair A, Reis e Sousa C. Innate recognition of viruses. Immunity 2007;27(3):370–83 doi 10.1016/j.immuni.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 36.Kawai T, Akira S. Innate immune recognition of viral infection. Nat Immunol 2006;7(2):131–7 doi 10.1038/ni1303. [DOI] [PubMed] [Google Scholar]
- 37.Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science 2010;327(5963):291–5 doi 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watts C, West MA, Zaru R. TLR signalling regulated antigen presentation in dendritic cells. Curr Opin Immunol 2010;22(1):124–30 doi 10.1016/j.coi.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 39.Valmori D, Souleimanian NE, Tosello V, Bhardwaj N, Adams S, O’Neill D, et al. Vaccination with NY-ESO-1 protein and CpG in Montanide induces integrated antibody/Th1 responses and CD8 T cells through cross-priming. Proc Natl Acad Sci U S A 2007;104(21):8947–52 doi 10.1073/pnas.0703395104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yip HC, Karulin AY, Tary-Lehmann M, Hesse MD, Radeke H, Heeger PS, et al. Adjuvant-guided type-1 and type-2 immunity: infectious/noninfectious dichotomy defines the class of response. J Immunol 1999;162(7):3942–9. [PubMed] [Google Scholar]
- 41.Ikeda H, Chamoto K, Tsuji T, Suzuki Y, Wakita D, Takeshima T, et al. The critical role of type-1 innate and acquired immunity in tumor immunotherapy. Cancer Sci 2004;95(9):697–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davis ID, Chen W, Jackson H, Parente P, Shackleton M, Hopkins W, et al. Recombinant NY-ESO-1 protein with ISCOMATRIX adjuvant induces broad integrated antibody and CD4(+) and CD8(+) T cell responses in humans. Proc Natl Acad Sci U S A 2004;101(29):10697–702 doi 10.1073/pnas.0403572101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uenaka A, Wada H, Isobe M, Saika T, Tsuji K, Sato E, et al. T cell immunomonitoring and tumor responses in patients immunized with a complex of cholesterol-bearing hydrophobized pullulan (CHP) and NY-ESO-1 protein. Cancer Immun 2007;7:9. [PMC free article] [PubMed] [Google Scholar]
- 44.Ossendorp F, Mengede E, Camps M, Filius R, Melief CJ. Specific T helper cell requirement for optimal induction of cytotoxic T lymphocytes against major histocompatibility complex class II negative tumors. J Exp Med 1998;187(5):693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bijker MS, van den Eeden SJ, Franken KL, Melief CJ, Offringa R, van der Burg SH. CD8+ CTL priming by exact peptide epitopes in incomplete Freund’s adjuvant induces a vanishing CTL response, whereas long peptides induce sustained CTL reactivity. J Immunol 2007;179(8):5033–40. [DOI] [PubMed] [Google Scholar]
- 46.Melief CJ, van der Burg SH. Immunotherapy of established (pre)malignant disease by synthetic long peptide vaccines. Nat Rev Cancer 2008;8(5):351–60 doi 10.1038/nrc2373. [DOI] [PubMed] [Google Scholar]
- 47.Massarelli E, William W, Johnson F, Kies M, Ferrarotto R, Guo M, et al. Combining Immune Checkpoint Blockade and Tumor-Specific Vaccine for Patients With Incurable Human Papillomavirus 16-Related Cancer: A Phase 2 Clinical Trial. JAMA Oncol 2019;5(1):67–73 doi 10.1001/jamaoncol.2018.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sironi M, Biasin M, Cagliani R, Forni D, De Luca M, Saulle I, et al. A common polymorphism in TLR3 confers natural resistance to HIV-1 infection. J Immunol 2012;188(2):818–23 doi 10.4049/jimmunol.1102179. [DOI] [PubMed] [Google Scholar]
- 49.Li G, Zheng Z. Toll-like receptor 3 genetic variants and susceptibility to hepatocellular carcinoma and HBV-related hepatocellular carcinoma. Tumour Biol 2013;34(3):1589–94 doi 10.1007/s13277-013-0689-z. [DOI] [PubMed] [Google Scholar]
- 50.Eggermont AM, Chiarion-Sileni V, Grob JJ, Dummer R, Wolchok JD, Schmidt H, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol 2015;16(5):522–30 doi 10.1016/S1470-2045(15)70122-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.