Abstract
Background
Many survivors of stroke report attentional impairments, such as diminished concentration and distractibility. However, the effectiveness of cognitive rehabilitation for improving these impairments is uncertain.This is an update of the Cochrane Review first published in 2000 and previously updated in 2013.
Objectives
To determine whether people receiving cognitive rehabilitation for attention problems 1. show better outcomes in their attentional functions than those given no treatment or treatment as usual, and 2. have a better functional recovery, in terms of independence in activities of daily living, mood, and quality of life, than those given no treatment or treatment as usual.
Search methods
We searched the Cochrane Stroke Group Trials Register, CENTRAL, MEDLINE, Embase, CINAHL, PsycINFO, PsycBITE, REHABDATA and ongoing trials registers up to February 2019. We screened reference lists and tracked citations using Scopus.
Selection criteria
We included controlled clinical trials (CCTs) and randomised controlled trials (RCTs) of cognitive rehabilitation for impairments of attention for people with stroke. We did not consider listening to music, meditation, yoga, or mindfulness to be a form of cognitive rehabilitation. We only considered trials that selected people with demonstrable or self‐reported attentional deficits. The primary outcomes were measures of global attentional functions, and secondary outcomes were measures of attentional domains (i.e. alertness, selective attention, sustained attention, divided attention), functional abilities, mood, and quality of life.
Data collection and analysis
Two review authors independently selected trials, extracted data, and assessed the risk of bias. We used the GRADE approach to assess the certainty of evidence for each outcome.
Main results
We included no new trials in this update. The results are unchanged from the previous review and are based on the data of six RCTs with 223 participants. All six RCTs compared cognitive rehabilitation with a usual care control.
Meta‐analyses demonstrated no convincing effect of cognitive rehabilitation on subjective measures of attention either immediately after treatment (standardised mean difference (SMD) 0.53, 95% confidence interval (CI) –0.03 to 1.08; P = 0.06; 2 studies, 53 participants; very low‐quality evidence) or at follow‐up (SMD 0.16, 95% CI –0.23 to 0.56; P = 0.41; 2 studies, 99 participants; very low‐quality evidence).
People receiving cognitive rehabilitation (when compared with control) showed that measures of divided attention recorded immediately after treatment may improve (SMD 0.67, 95% CI 0.35 to 0.98; P < 0.0001; 4 studies, 165 participants; low‐quality evidence), but it is uncertain that these effects persisted (SMD 0.36, 95% CI –0.04 to 0.76; P = 0.08; 2 studies, 99 participants; very low‐quality evidence). There was no evidence for immediate or persistent effects of cognitive rehabilitation on alertness, selective attention, and sustained attention.
There was no convincing evidence for immediate or long‐term effects of cognitive rehabilitation for attentional problems on functional abilities, mood, and quality of life after stroke.
Authors' conclusions
The effectiveness of cognitive rehabilitation for attention deficits following stroke remains unconfirmed. The results suggest there may be an immediate effect after treatment on attentional abilities, but future studies need to assess what helps this effect persist and generalise to attentional skills in daily life. Trials also need to have higher methodological quality and better reporting.
Plain language summary
Cognitive rehabilitation for attention problems following stroke
Review question
Our aim was to review the evidence about the effect of cognitive rehabilitation on attention, the ability to perform daily activities, mood, and quality of life in people who have attention problems following a stroke.
Background
Many people have problems with attention after stroke. They are unable to concentrate for prolonged periods of time and are distractible, being unable to focus on a specific task in the presence of competing information. 'Cognitive rehabilitation' involves providing therapeutic activities to reduce the severity of cognitive problems, like attention, following damage to the brain. The benefit of cognitive rehabilitation for attention problems following stroke is unclear.
Study characteristics
We identified six studies that compared cognitive rehabilitation with a control group who received their usual care (but not cognitive rehabilitation) for people with attention problems following stroke. We did not consider listening to music, meditation, yoga, or mindfulness to be a form of cognitive rehabilitation. The six studies involved 223 participants who demonstrated attentional problems or reported having such problems following stroke. The evidence is current to February 2019.
Key results
We found no evidence that cognitive rehabilitation improved general (global) measures of attention. The group that received cognitive rehabilitation performed better than the control group on tasks that required people to divide attention. However, this benefit was only seen immediately after the rehabilitation period with no suggestion that the benefits persist for longer. There was no evidence to suggest that cognitive rehabilitation was beneficial for other types of attention problems, or daily life activities, mood, or quality of life. More research is needed.
Certainty of the evidence
The very low to moderate methodological quality of the studies identified, and the lack of studies means that we cannot draw firm conclusions about the effect of cognitive rehabilitation for attention following stroke.
Summary of findings
Background
Description of the condition
Deficits in attention are one of the most commonly observed cognitive impairments after stroke. The exact frequency of attentional deficits after stroke is a matter of debate. Within the acute phase, estimates range between 46% and 92% (Stapleton 2001). At discharge from hospital, estimates suggest a prevalence between 24% and 51% (Hyndman 2008). Speed of information processing can also be impaired, and estimates have varied between 50% and 70% (Hochstenbach 1998; Rasquin 2004). Attentional deficits may recover over time in some people (Hochstenbach 2003), but in 20% to 50% of stroke survivors there are persistent deficits for years (Barker‐Collo 2010; Hyndman 2003).
Attentional impairments manifest themselves in a wide variety of deficits, such as diminished concentration, distractibility, reduced error control, difficulties doing more than one thing at a time, mental slowness, and mental fatigability. Being a mediator of other processes, attentional deficits may also impair higher cognitive functions, such as language and memory (Lezak 2004). While there is a consensus that attention is not a unitary process, there is no agreement on the typologies and taxonomies describing the range of attentional processes. For the current review, we considered the following attentional components: alertness/arousal, selective attention, sustained attention (vigilance), and divided attention (see Table 3). The rehabilitation of deficits in spatial attention is covered in a separate Cochrane Review (Bowen 2013), and therefore not covered in this review.
1. Attentional outcome measures used in the included studies.
| Study ID | Subjective global measure | Objective global measure | Alertness | Selective attention | Sustained attention | Divided attention |
| Barker‐Collo 2009 | Cognitive Failures Questionnairea | — | — | Trail Making Ab | IVA‐CPT Full‐Scale Attention Quotientb | PASAT (2.4 sec ISI)b |
| Röhring 2004 | — | — | TAP phasic alertness | TAP selective attention | d2 (Hits‐Errors) | TAP divided attention |
| Schottke 1997 | — | — | Tempo‐Lern‐Test (1st block) | Zahlen‐Verbindungstest | Konzentrations‐Verlaufs‐Test (combined speed/errors scores) | — |
| Sturm 1991 | — | — | Wiener Reaktionsgerat Visual RT | Wiener Reaktionsgerat Choice RT | Wiener Vigilanzgerat (Hits) | — |
| Westerberg 2007 | Cognitive Failures Questionnaire | — | — | Ruff 2&7 | — | PASAT (2.4 sec ISI) |
| Winkens 2009 | Mental Slowness Questionnaireb | — | Simple RTb | Trail Making A (time)b | — | PASAT (3.2 sec ISI)b |
aOnly measured at follow‐up, but not after treatment. bMeasured after treatment and at follow‐up.
ISI: interstimulus interval; IVA‐CPT: Integrated Visual and Auditory Continuous Performance Test; PASAT: Paced Auditory Serial Addition Test; RT: reaction time; TAP: Tests of Attentional Performance.
Table 1: Domains of attention
| Domain of attention | Definition | Functional example |
| Alertness/arousal | Ability and readiness to respond | Response to warning signals |
| Selective attention | Ability to focus on specific stimuli while ignoring irrelevant stimuli | Reading while people talk in the background |
| Sustained attention (vigilance) | Ability to maintain attention over a prolonged period of time | Driving a car for long distances |
| Spatial attention | Ability to detect and deploy attention to all sides of space | Attending to people sitting on left and right side of the table |
| Divided attention | Ability to multitask and to divide attention between 2 or more tasks | Talking on the telephone while cooking |
A distinction between different attentional domains is potentially important when evaluating rehabilitation. There is some evidence that attentional components need to be trained separately as there is little generalisation of treatment from one attentional domain to another (Sturm 1991; Sturm 1997). Moreover, cognitive training for certain domains, such as divided and selective attention, may be more effective than training for other domains, such as alertness and sustained attention (Cappa 2005).
The treatment of cognitive deficits is necessary because these deficits have a negative effect on functional abilities (Barker‐Collo 2006), and quality of life (Kwa 1996; Mitchell 2010; Nys 2006). Sustained attention (concentration) is an important prerequisite for motor recovery since sufficient sustained attention is required for learning (Robertson 1997). Deficits in attention can affect the ability to engage with other rehabilitation required for poststroke recovery (e.g. physiotherapy) and are associated with increased risk of falls (Hyndman 2003). Other specific attentional disorders, such as auditory and visual selective attention, and divided attention, also affect functional recovery (Hyndman 2008; Stapleton 2001).
Description of the intervention
Cognitive rehabilitation interventions typically focus on re‐establishing previously learned behaviours, developing new behaviours to compensate for cognitive deficits, and adapting to these cognitive deficits (Cicerone 2000). These interventions are guided and informed by psychological theories and models of behaviour and behaviour change, and cognitive or neuropsychological models of brain‐behaviour interactions.
For people with attentional deficits, cognitive rehabilitation interventions include tasks designed to restore attention abilities, such as computerised activities and pencil‐and‐paper tasks requiring attention. The alternative approach is teaching people strategies to compensate for their attention impairments. Attempts to retrain attentional skills have mainly relied on a restitution approach, although trials of attentional rehabilitation for people with other forms of brain injury, for instance traumatic brain injury, have emphasised the development of compensatory strategies rather than the restoration of basic aspects of attention (Cicerone 2005).
Defining cognitive rehabilitation simply on the basis of its impact on 'attention' (or 'cognition' more broadly), however, is not straightforward. For instance, some pharmacological interventions, mindfulness interventions, and listening to music have positive effects on cognition (e.g. Särkämö 2008; Zeidan 2010). However, such interventions, although positively affecting cognition, are not usually regarded as 'cognitive rehabilitation' interventions based on the purported mechanisms of action (see below). Ultimately, cognitive rehabilitation, regardless of its focus or strategy, aims to improve the person's function in their daily life (Ben‐Yishay 1990; Cicerone 2000; Sohlberg 1989).
How the intervention might work
Cognitive rehabilitation is based on two main principles. One is 'restitution', which aims to restore cognitive function through repeated practice. The other is 'compensation', which aims to reduce the effects of cognitive impairment on functional abilities using strategies that minimise demands on attention skills.
Why it is important to do this review
Impairments of attention are a major problem for people with stroke and affect rehabilitation outcome, but the effectiveness of cognitive rehabilitation for attention is uncertain. Finding the best ways to improve cognition is a top 10 research priority for stroke survivors (Pollock 2012). In one survey of stroke survivor needs, 84% of 799 respondents reported that their needs were not fully met in relation to their concentration problems (McKevitt 2010). While attention training has been provided for some people with stroke, it needs further evaluation. There have been few studies that have used control groups, and most evaluations have been based on single‐case experimental designs. Although these indicate treatment can be effective, they do not evaluate the general applicability of the findings. This updated review considered the evidence from controlled clinical trials (CCTs) and randomised controlled trials (RCTs) of the effectiveness of cognitive rehabilitation for attention.
Objectives
To determine whether people receiving cognitive rehabilitation for attention problems 1. show better outcomes in their attentional functions than those given no treatment or treatment as usual, and 2. have a better functional recovery, in terms of independence in activities of daily living, mood and quality of life, than those given no treatment or treatment as usual.
Methods
Criteria for considering studies for this review
Types of studies
In the first version of this review, we sought all controlled trials which compared cognitive rehabilitation with a control treatment. In the second version of the review, we excluded all non‐randomised trials to reduce selection bias. In this third update, we included quasi‐randomised or CCTs, as per the updated guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We also reviewed those non‐randomised trials which we excluded from the previous versions of the review to check these against the updated criteria. We decided to do this in advance of searching the literature. We sought RCTs and CCTs in which an attentional treatment was compared with a control. We also sought cross‐over RCTs, but only included the outcomes between groups at the pre‐cross‐over period.
Types of participants
This review was confined to trials that selected people with demonstrable (on formal attention testing) or self‐reported attentional deficits following stroke. We excluded trials in which participant selection for attentional training was based on general cognitive impairments (e.g. as assessed with the Mini‐Mental State Examination) or other cognitive functions (e.g. aphasia). The participants were restricted to those with stroke. We excluded trials that included participants with mixed aetiologies unless separate data were available relating to people with stroke or if the trials had more than 75% of people with stroke in their sample.
Types of interventions
We included trials in which there was a comparison between a treatment group that received one of various attentional treatment strategies and a control group that received either an alternative form of treatment or no attentional intervention. We considered attention treatments to be any form of intervention with the aim of improving attention abilities. Alternative forms of treatment included computerised activities with low attentional demands and social activities. We excluded interventions that specifically aimed to improve spatial attentional deficits, as this has been the focus of a separate Cochrane Review (Bowen 2013). We did not consider listening to music, meditation, tai‐chi, yoga, or mindfulness to be a form of cognitive rehabilitation, although we acknowledge that these interventions may have a positive effect on attention. In a similar vein, we did not include trials with only a single treatment session or pharmacological studies.
Types of outcome measures
The primary outcome was measures of global attentional functions, and the secondary outcomes were measures of the individual domains of attention, functional abilities in activities of daily living (ADL), mood, and quality of life. We assessed the outcomes immediately after treatment and at follow‐up. We defined 'long‐term' follow‐up as more than three months after the intervention. We did not include studies in which the outcome was related exclusively to any single activity of daily living (e.g. driving a car).
Primary outcomes
Subjective reports of global attention as measured by validated rating scales, such as:
Rating Scale of Attentional Behaviour (Ponsford 1991);
Moss Attention Rating Scale (Whyte 2003);
Attention Rating and Monitoring Scale (Cicerone 2002);
Cognitive Failures Questionnaire (Broadbent 1982).
If an individual study reported more than one of these scales, we used the scale listed first.
Objective reports of global attention as measured by validated batteries assessing a wide range of attentional domains
We were not aware of any assessment batteries with a global score of attention at the start of this review. Batteries such as Test of Everyday Attention do not provide a total score (Robertson 1994). We included any validated assessments reporting global scores of attention.
Secondary outcomes
Objective reports of domains of attention as measured by:
tests of alertness/arousal;
tests of selective attention;
tests of sustained attention;
tests of divided attention.
We assigned each attentional test to a primary and a secondary attentional domain (see Appendix 1). For each trial, we only included one test measure in the analysis for a specific domain. We chose measures allocated to a primary domain over those allocated to secondary domains. We used the following hierarchy if a trial provided several measures for the same domain: combined error/speed measures > error measures > speed measures (median reaction times (RTs) > mean RTs). In the case when several tests were used to assess the same attentional domain within the same trial, we chose validated tests in preference to non‐validated tests. If two or more standardised tests were used to assess the same attentional domain, we chose the one with the higher reliability and validity rating based on information from the Compendium of Neuropsychological Tests (Strauss 2006). If we could not decide with the above criteria, we selected one of the tests at random.
Reports of functional abilities in daily living, mood, and quality of life
Functional abilities as measured by scales such as the Nottingham Extended Activities of Daily Living (NEADL; Nouri 1987), Functional Independence Measure (Granger 1994), Barthel Index (Mahoney 1965), and Stroke Impact Scale (Duncan 2003).
Mood as measured by scales such as the General Health Questionnaire (GHQ; Goldberg 1972), and Hospital Anxiety and Depression Scale (HADS; Zigmond 1983).
Quality of life, as measured by the World Health Organization Quality of Life (WHOQOL; WHOQOL Group 1998), and 36‐item Short Form (SF‐36; Ware 1992).
If more than one of these scales were reported, we used the scale listed first.
Search methods for identification of studies
See the 'Specialised register' information available at the Cochrane Stroke Group's website (www.dcn.ed.ac.uk/csrg/entity/searchmethods.pdf). We searched for relevant trials in all languages and arranged for the translation of trial reports published in languages other than English where necessary.
Electronic searches
We searched the Cochrane Stroke Group Register (last searched February 2019). In addition, we searched the following databases and registries:
the Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 2 in the Cochrane Library; Appendix 2);
MEDLINE Ovid (from 1948; Appendix 3);
Embase Ovid (from 1947; Appendix 4);
PsycINFO Ovid (from 1806; Appendix 5);
Cumulative Index to Nursing and Allied Health Literature (CINAHL) EBSCO (from 1981; Appendix 6);
Psychological Database for Brain Impairment Treatment Efficacy (PsycBITE, www.psycbite.com/; Appendix 7);
REHABDATA (www.naric.com/research/rehab/; Appendix 8);
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov/; Appendix 9);
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP; apps.who.int/trialsearch; Appendix 10).
We developed the MEDLINE search strategy with the help of the Cochrane Stroke Group Information Specialist and modified it for the other databases. The search strategy included Cochrane's highly sensitive search strategies for identification of RCTs, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2011), and the Cochrane Stroke Group's search strategies for the identification of stroke studies in respective databases and other resources. The Cochrane Stroke Group Trials Information Specialist checked the search strategies from the previous review (no changes made). To identify new articles since the last review, the searches for this update were run from January 2012 to February 2019.
Searching other resources
To identify further published, unpublished, and ongoing trials, we scanned citations of the primary study articles, and we scanned reference lists from review articles and books identified in the searches.
For a previous version of this review, we conducted a handsearch of the following journals.
American Journal of Occupational Therapy (1947 to 1998); Aphasiology (1987 to 1998); Australian Journal of Occupational Therapy (1965 to 1998); British Journal of Occupational Therapy (1950 to 1998); British Journal of Therapy and Rehabilitation (1994 to 1998); Canadian Journal of Occupational Therapy (1970 to 1998); Clinical Rehabilitation (1987 to 1998); Disability and Rehabilitation (1992 to 1998) formerly International Disability Studies (1987 to 1991) formerly International Rehabilitation Medicine (1979 to 1986); International Journal of Language and Communication Disorders (1998) formerly European Journal of Disorders of Communication (1985 to 1997) formerly British Journal of Disorders of Communication (1977 to 1984); Journal of Clinical Psychology in Medical Settings (1994 to 1998) formerly Journal of Clinical Psychology (1944 to 1994); Journal of Developmental and Physical Disabilities (1992 to 1998) formerly Journal of the Multihandicapped Person (1989 to 1991); Journal of Rehabilitation (1993 to 1998); International Journal of Rehabilitation Research (1977 to 1998); Journal of Rehabilitation Sciences (1989 to 1996); Neuropsychological Rehabilitation (1987 to 1998); Neurorehabilitation (1991 to 1998); Occupational Therapy International (1994 to 1998); Physiotherapy Theory and Practice (1990 to 1998) formerly Physiotherapy Practice (1985 to 1989); Physical Therapy (1988 to 1998); Rehabilitation Psychology (1982 to 1998); Journal of Cognitive Rehabilitation (1988 to 1998) formerly Cognitive Rehabilitation (1983 to 1987).
Since handsearching these journals in 1999, many have been updated as part of Cochrane's handsearching effort. After checking the Master List of journals that was maintained by Cochrane, we were confident that relevant trials would be found from the search of CENTRAL. Therefore, we did not repeat handsearching of these journals.
Data collection and analysis
Selection of studies
We completed study selection for this update using the Covidence systematic review software (Covidence).
One review author (KJP) screened all the titles and abstracts of records obtained from the searches of the electronic databases and another review author (TL, RdN, or DW) independently reviewed all studies and excluded those that were clearly not relevant. We obtained the full text of the remaining studies (where possible) and two review authors independently assessed which studies met the inclusion criteria in relation to study type, participants, interventions, comparisons, and outcomes (KJM reviewed all studies, with the remaining review authors splitting the studies as second review authors). Two review authors (KJP, TL) resolved any disagreements by discussion.
Data extraction and management
For each of the studies meeting the inclusion criteria, we extracted the following characteristics:
method of participant assignment and blinding;
setting and participant details (including age, gender, time since stroke, eligibility criteria);
intervention (including comparison intervention, treatment durations);
outcome measures (including assessment methods and time points of assessments);
results.
One review author (TL) extracted the study characteristics, and a second review author (KJP) checked the details.
Assessment of risk of bias in included studies
We applied Cochrane's recommended approach for assessing risk of bias in studies included in Cochrane Reviews (Higgins 2011). Two review authors (TL, KJP) assessed the methodological quality of each study in the following six domains: sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and 'other issues'. We judged each of these domains as either having a 'low', 'unclear', or 'high' risk of bias. We resolved disagreements by discussion. We used the results of the 'Risk of bias' assessment to inform the GRADE assessment.
Measures of treatment effect
We summarised ordinal scales using methods for continuous data. We expressed the intervention effect as a mean difference (MD) where studies measured outcomes using the same scales, or standardised mean difference (SMD) where studies used different scales, with the corresponding 95% confidence interval (CI). For some scales, a higher score indicated better ability/performance, and for others higher scores indicated worse ability/performance. Therefore, we multiplied the mean values from one set of studies by –1 to ensure that all the scales pointed in the same direction.
Unit of analysis issues
There were no unit of analysis issues. Four of the six identified studies used parallel group designs. For the two cross‐over RCTs, we only considered the outcomes between groups at the precross‐over period (Röhring 2004; Sturm 1991).
Dealing with missing data
We sought data that were not available or were unclear from the reports through correspondence with the first author of the publication. If we could not obtain the required information for an included study, we did not include that study in the analysis.
Assessment of heterogeneity
We assessed statistical heterogeneity using Chi2 statistics and I2 statistic estimates to quantify inconsistency across trials (Higgins 2011). We considered an I2 value above 50% to indicate substantial heterogeneity and 75% to indicate considerable heterogeneity.
Assessment of reporting biases
As no trials registers and study protocols were available for the identified studies, we investigated selective reporting by comparing the methods section of the studies with the results reported. We did not inspect funnel plots for publication biases due to the small sample size of fewer than 10 studies (Higgins 2011).
Data synthesis
We conducted a meta‐analysis using the Review Manager software RevMan 5.3 (Review Manager 2014). A fixed‐effect model with 95% CI was used if there was acceptable heterogeneity between trials (I2 < 50%). We used a random‐effects model for the meta‐analysis if we judged the characteristics of studies to be similar and heterogeneity was not considerable (i.e. I2 < 75%). If we judged studies to be dissimilar (e.g. considerable variation in study characteristics, or considerable heterogeneity (I2 > 75%)), we would have reported outcomes narratively. In the case of considerable heterogeneity, we would have explored subgroup analyses.
GRADE and 'Summary of findings' table
We used the GRADE approach to assess the certainty of evidence (Guyatt 2008). We downgraded evidence by one level ('high quality' to 'moderate quality') for serious study limitations related to 1. risk of bias, 2. consistency of evidence, 3. directness of evidence, and 4. imprecision of effect estimates. In case of very serious limitations, we downgraded the certainty of evidence by two levels. We created 'Summary of findings' tables to provide synthesised information regarding the overall certainty of evidence for the primary and secondary outcomes. We used GRADEpro GDT software to construct the tables (GRADEpro GDT 2015).
Subgroup analysis and investigation of heterogeneity
If sufficient data were available, we planned to perform subgroup analyses to determine whether outcomes varied according to time since onset of stroke, frequency of intervention (number of sessions per week), intensity of intervention (total hours of intervention), and type of intervention.
Sensitivity analysis
We planned a sensitivity analysis on the methodological quality of studies by excluding studies with high risk of bias.
Results
Description of studies
See Characteristics of included studies table.
Results of the search
The searches for studies in the previous reviews identified 2785 records. The search for this update was completed in February 2019 and retrieved 3960 new records. Initial screening of the 6745 records identified 130 possibly relevant studies. An in‐depth assessment of these papers identified six studies (11 records) that met the inclusion criteria. The study selection process is outlined in Figure 1.
1.
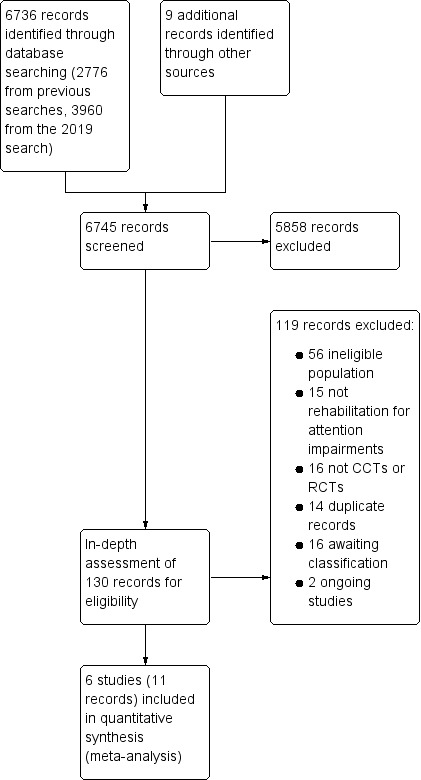
Study flow diagram. CCT: controlled clinical trial; RCT: randomised controlled trial.
Of the 119 studies that we excluded, 14 were duplicate records of studies and 87 did not meet the inclusion criteria. The reasons for exclusion of studies are summarised in the Characteristics of excluded studies table. We excluded 56 studies because they were not stroke (i.e. less than 75% of study participants had a stroke) or participants were not selected because of attentional problems. We excluded 16 studies because they were not CCTs or RCTs, and 15 studies because they did not involve cognitive rehabilitation for attention impairments. We excluded a further 16 studies because insufficient information was available to make a decision at this stage (see Characteristics of studies awaiting classification table), and we two studies are ongoing (see Characteristics of ongoing studies table).
Included studies
We included six studies treating attentional deficits after stroke in this review (see Characteristics of included studies table). The six studies included 223 participants. Of the six RCTs, four used a parallel group design (Barker‐Collo 2009; Schottke 1997; Westerberg 2007; Winkens 2009), and two used a cross‐over design (Röhring 2004; Sturm 1991). Five studies reported the method of generating the random schedule. Two of these used random number tables (Röhring 2004; Sturm 1991), one used an online Internet randomisation service (Barker‐Collo 2009), one used coin tossing (Schottke 1997), one used drawing numbers from a bucket (Westerberg 2007), and in one this was unclear (Winkens 2009). Three studies generated the sequence independently (Barker‐Collo 2009; Westerberg 2007; Winkens 2009), and it was unclear in the other three (Röhring 2004; Schottke 1997; Sturm 1991). In all studies, the participants and therapists were aware of the treatment being given. Three studies assessed outcomes using a blinded assessor (Barker‐Collo 2009; Röhring 2004; Winkens 2009), in two the therapist conducted the outcome assessments (Schottke 1997; Westerberg 2007), and in one this was unclear (Sturm 1991).
One study was conducted in New Zealand (Barker‐Collo 2009), and five in Europe: three in Germany (Röhring 2004; Schottke 1997; Sturm 1991), one in Sweden (Westerberg 2007), and one in the Netherlands (Winkens 2009). The number of participants recruited to the studies varied between 78 (Barker‐Collo 2009), and 18 (Westerberg 2007). Four studies included only participants with stroke (Barker‐Collo 2009; Schottke 1997; Westerberg 2007; Winkens 2009). Two studies recruited most participants within the first two months after stroke (Barker‐Collo 2009; Schottke 1997), three mainly within one year of stroke (Sturm 1991; Westerberg 2007; Winkens 2009), and one study recruited participants up to four years after stroke (Röhring 2004). Three studies recruited participants with both right and left hemisphere lesions (Barker‐Collo 2009; Schottke 1997; Westerberg 2007), one included people with left hemisphere lesions in the randomised trial (Sturm 1991), and two studies did not report this information (Röhring 2004; Winkens 2009). The mean age of the samples was under 65 years in all except one study (Barker‐Collo 2009). The proportion of men ranged between 51% (Schottke 1997), and 70% (Sturm 1991).
Two studies identified attention deficits on tests of attention using specified cut‐offs (Barker‐Collo 2009; Schottke 1997), two studies on tests for attention without specification of cut‐offs (Röhring 2004; Sturm 1991), and two studies on self‐ or therapist‐reported attention deficits (Westerberg 2007; Winkens 2009). Groups were well matched at baseline apart from three studies. In two studies, the time after stroke was shorter for controls than intervention group participants (Schottke 1997; Winkens 2009), and in one study, the control group had more people with aphasia and scored lower in tasks assessing reasoning abilities (Sturm 1991).
Interventions aimed to either restore attentional functions (Barker‐Collo 2009; Röhring 2004; Schottke 1997; Sturm 1991; Westerberg 2007), or provide compensatory strategies (Winkens 2009). One study applied both intervention approaches (Schottke 1997). Interventions lasted from three weeks (Schottke 1997; Sturm 1991), to 11 weeks (Röhring 2004), and the number of sessions of treatment varied between 13 (Schottke 1997), and 55 (Röhring 2004), for the restorative approaches. The compensatory approach was delivered for 10 hours (Winkens 2009). The control groups in all studies received usual care with no treatment of attention deficits.
The six studies used over 30 psychometric tests with more than 40 test variables as attentional outcome measures. Four of the six studies assessed at least one functional outcome (Barker‐Collo 2009; Röhring 2004; Schottke 1997; Winkens 2009). All studies reported outcomes immediately after treatment; two studies also reported outcomes of follow‐up assessments (long‐term effects) (Barker‐Collo 2009; Winkens 2009).
For measures of attention, the findings of four individual studies supported the efficacy of treatment (Barker‐Collo 2009; Schottke 1997; Westerberg 2007; Sturm 1991), but two studies found limited evidence of benefit (Röhring 2004; Winkens 2009).
Risk of bias in included studies
The risk of bias for each study is summarised in Figure 2. An overview of the risk across all studies is provided in Figure 3.
2.
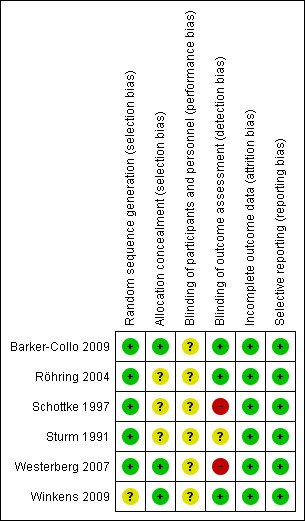
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
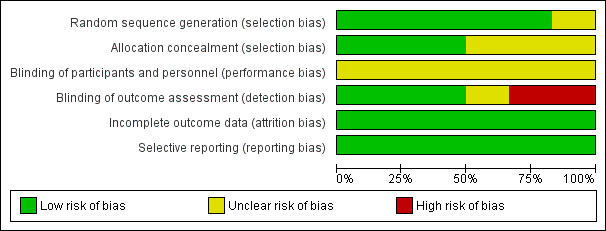
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
While all six studies were described as randomised, there were insufficient details available in one study to judge the risk of bias for the randomisation process (Winkens 2009). In three out of the six studies, there was insufficient information to allow judgement of the risk of an allocation concealment bias (Röhring 2004; Schottke 1997; Sturm 1991).
Blinding
All studies compared the effect of an intervention with care as usual. Blinding of the participants and therapists was not possible once the intervention started, and therefore no studies were double‐blind. Two studies showed a high risk of detection bias as the outcome assessment was not conducted blinded to the group allocation (Schottke 1997; Westerberg 2007). One study is at unclear risk as there was insufficient information to judge the risk of a detection bias (Sturm 1991).
Incomplete outcome data
All studies were at low risk of attrition bias. Dropouts from studies appeared generally balanced across groups (6.8% for the treatment groups and 5.3% for the control groups).
Selective reporting
There was no indication of any selective reporting, but none of the trials appeared to be prospectively registered on trial registries.
Effects of interventions
Summary of findings for the main comparison. Attention training compared to control for attention deficits following stroke immediately after treatment.
| Attention training compared to control for attention deficits following stroke immediately after treatment | |||||
| Patient or population: people with attention deficits following stroke Settings: any rehabilitation setting Intervention: attention training Comparison: no treatment/treatment as usual | |||||
| Outcomes | No of participants (studies) | Certainty of the evidence (GRADE)a | Estimated effect of intervention (SMD)b | 95% confidence interval of estimated effect | Comments |
| Subjective measures – assessed with Cognitive Failures Questionnaire & Mental Slowness Questionnaire | 53 (2 RCTs) | ⊕⊝⊝⊝ Very lowc,d,e | 0.53 SMDs better | 0.03 SMDs worse to 1.08 SMDs better | — |
| Objective global measures | No studies | — | — | — | No data |
| Alertness – assessed with TAP phasic alertness, Wiener Reaktionsgerat Visual RT, Simple RT & Tempo‐Lern Test | 136 (4 RCTs) | ⊕⊕⊝⊝ Lowc,d,f | 0.14 SMDs better | 0.20 SMDs worse to 0.48 SMDs better | — |
| Selective attention – assessed with TAP selective attention, Trail Making A, Zahlen‐Verbindungstest, Wiener Reaktionsgerat Choice RT, Ruff 2&7 | 223 (6 RCTs) | ⊕⊕⊕⊝ Moderatec,d | 0.08 SMDs worse | 0.35 SMDs worse to 0.18 SMDs better | — |
| Sustained attention – assessed with IVA‐CPT Fullscale Attention Quotient, d2, Konzentrations‐Verlaufs‐Test, Wiener Vigilanzgerat | 169 (4 RCTs) | ⊕⊕⊝⊝ Lowc,d,f | 0.39 SMDs better | 0.16 SMDs worse to 0.94 SMDs better | — |
| Divided attention – assessed with TAP divided attention, PASAT | 165 (4 RCTs) | ⊕⊕⊝⊝ Lowc,f | 0.67 SMDs better | 0.35 SMDs to 0.98 SMDs better | — |
| Functional abilities – assessed with Barthel, Functional Independence Measure (FIM) | 75 (2 RCTs) | ⊕⊝⊝⊝ Very lowc,d,e | 0.29 SMDs better | 0.16 SMDs worse to 0.75 SMDs better | — |
| Mood (depression) – assessed with Center for Epidemiologic Studies Depression Scale, Zung Depression Status Inventory, Emotional State Questionnaire (Depression subscale) | 109 (3 RCTs) | ⊕⊕⊝⊝ Lowc,f | 0.01 SMDs better | 0.36 SMDs worse to 0.39 SMDs better | — |
| Quality of life – assessed with SF‐36 (Mental Component Summary subscale), EQ‐VAS | 103 (2 RCTs) | ⊕⊕⊝⊝ Lowc,f | 0.02 SMDs better | 0.37 SMDs worse to 0.40 SMDs better | — |
| CI: confidence interval; IVA‐CPT: Integrated Visual and Auditory Continuous Performance Test; n: number of participants; PASAT: Paced Auditory Serial Addition Test; RCT: randomised controlled trial; ROB: risk of bias; RT: reaction time; SF‐36: 36‐item Short Form; SMD: standardised mean difference; TAP: Tests of Attentional Performance. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded for imprecision: two levels for 1–99 participants; one level for 100–199 participants; no downgrade > 199 participants. bGenerally 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect. cROB: all studies with unclear risk of performance bias. dROB: More than 30% of participants with unclear or high risk for detection bias. eImprecision: very serious concern due to small study population (n < 100). fImprecision: serious concern due to small study population.
Summary of findings 2. Attention training compared to control for attention deficits following stroke at follow‐up.
| Attention training compared to control for attention deficits following stroke 3–6 months after treatment | |||||
| Patient or population: people with attention deficits following stroke Settings: any rehabilitation setting Intervention: attention training Comparison: no treatment/treatment as usual | |||||
| Outcomes | No of participants (studies) | Certainty of the evidence (GRADE)a | Estimated effect of intervention (SMD)b | 95% confidence interval of estimated effect | Comments |
| Subjective measures – assessed with Cognitive Failures Questionnaire & Mental Slowness Questionnaire | 99 (2 RCTs) | ⊕⊝⊝⊝ Very lowc,d | 0.16 SMDs better | 0.23 SMDs worse to 0.56 SMDs better | — |
| Objective global measures | No studies | — | — | — | No data |
| Alertness – assessed with Simple RT | 31 (1 RCTs) | ⊕⊝⊝⊝ Very lowc,d | 0.26 SMDs worse | 0.97 SMDs worse to 0.45 SMDs better | — |
| Selective attention – assessed with Trail Making A | 99 (2 RCTs) | ⊕⊝⊝⊝ Very lowc,d | 0.07 SMDs better | 0.32 SMDs worse to 0.47 SMDs better | — |
| Sustained attention – assessed with IVA‐CPT Fullscale Attention Quotient | 66 (1 RCT) | ⊕⊝⊝⊝ Very lowc,d | 0.05 SMDs better | 0.44 SMDs worse to 0.53 SMDs better | — |
| Divided attention – assessed with PASAT | 99 (2 RCTs) | ⊕⊝⊝⊝ Very lowc,d | 0.36 SMDs better | 0.04 SMDs to 0.76 SMDs better | — |
| Functional abilities – assessed with Modified Rankin | 66 (1 RCTs) | ⊕⊝⊝⊝ Very lowc,d | 0.02 SMDs better | 0.46 SMDs worse to 0.51 SMDs better | — |
| Mood – assessed with Center for Epidemiologic Studies Depression Scale, General Health Questionnaire | 99 (2 RCTs) | ⊕⊝⊝⊝ Very lowc,d | 0.29 SMDs better | 0.11 SMDs worse to 0.69 SMDs better | — |
| Quality of life – assessed with SF‐36 (Mental Component Summary subscale), EQ‐VAS | 99 (2 RCTs) | ⊕⊝⊝⊝ Very lowc,d | 0.26 SMDs better | 0.13 SMDs worse to 0.66 SMDs better | — |
| CI: confidence interval; IVA‐CPT: Integrated Visual and Auditory Continuous Performance Test; PASAT: Paced Auditory Serial Addition Test; RCT: randomised controlled trial; ROB: risk of bias; RT: reaction time; SF‐36: 36‐item Short Form; SMD: standardised mean difference. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngrades for imprecision: two levels for 1–99 participants; one level for 100–199 participants; no downgrade > 199 participants. bGenerally 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect. cROB: all studies with unclear risk of performance bias. dImprecision: very serious concern due to small study population (n < 100).
We assessed the effect of interventions immediately after treatment and at follow‐up. The included studies applied diverse outcome measures. The measures we chose for the analysis of treatment effects on attentional and functional outcomes are listed in Table 3 and Table 4.
2. Functional outcome measures used in the included studies.
| Study ID | Functional abilities | Mood | Quality of life |
| Barker‐Collo 2009 | Modified Rankina | GHQa | SF‐36 (MCS subscale)b |
| Röhring 2004 | FIM | DSI | — |
| Schottke 1997 | Barthel | EMO‐D | — |
| Winkens 2009 | — | CES‐Db | EQ‐VASb |
aOnly measured at follow‐up, but not after treatment. bMeasured after treatment and at follow‐up.
CES‐D: Center for Epidemiologic Studies Depression Scale; DSI: Zung Depression Status Inventory; EMO‐D: Emotional State Questionnaire (Depression subscale); FIM: Functional Independence Measure; GHQ: General Health Questionnaire; MCS: Mental Component Summary; VAS: visual analogue scale.
Primary outcomes
Effects immediately after treatment
Two studies involving 53 participants assessed subjective reports of global attentional functions with either the Cognitive Failures Questionnaire (Westerberg 2007), or the Mental Slowness Questionnaire (Winkens 2009). There was a no convincing effect in favour of the intervention compared with care as usual (SMD 0.53, 95% CI –0.03 to 1.08; P = 0.06; very low‐quality evidence; Analysis 1.1).
1.1. Analysis.
Comparison 1 Attention training versus control – impact on global measures of attention deficits immediately after treatment, Outcome 1 Subjective measures.
Long‐term effects
The two studies involving 99 participants investigating long‐term effects on subjective reports of global attentional functions found no evidence for effects of treatment (SMD 0.16, 95% CI –0.23 to 0.56; P = 0.41; very low‐quality evidence; Analysis 2.1) (Barker‐Collo 2009; Winkens 2009).
2.1. Analysis.
Comparison 2 Attention training versus control – impact on global measures of attention deficits at follow‐up , Outcome 1 Subjective measures at follow‐up.
We identified no studies that reported objective measures of global attention at the end of treatment or in the longer term.
Secondary outcomes
Effects immediately after treatment
People receiving cognitive rehabilitation (when compared with control) showed improvements on measures of divided attention recorded immediately after treatment. Four studies comprising 165 participants assessed divided attention using the Paced Auditory Serial Addition Test (PASAT) (Barker‐Collo 2009; Westerberg 2007; Winkens 2009), or the divided attention subtest from the Tests of Attentional Performance battery (TAP) (Röhring 2004). The analysis found that the intervention was beneficial compared with care as usual (SMD 0.67, 95% CI 0.35 to 0.98; P < 0.0001; low‐quality evidence; Analysis 3.4).
3.4. Analysis.
Comparison 3 Attention training versus control – impact on attentional domains immediately after treatment, Outcome 4 Divided attention.
We found no convincing effects on other domains of attention. Four studies comprising 136 participants investigated an effect on alertness (SMD 0.14, 95% CI –0.20 to 0.48; P = 0.41; low‐quality evidence; Analysis 3.1; Röhring 2004; Schottke 1997; Sturm 1991; Winkens 2009). Six studies comprising 223 participants examined the effects on selective attention (SMD –0.08, 95% CI –0.35 to 0.18; P = 0.53; moderate‐quality evidence; Analysis 3.2). Four studies comprising 169 participants examined the effects on sustained attention (Barker‐Collo 2009; Röhring 2004; Schottke 1997; Sturm 1991). As the heterogeneity between trials for this measure was high (I2 > 50%), we used a random‐effects model for analysing sustained attention (SMD 0.39, 95% CI –0.16 to 0.94; P = 0.16; low‐quality evidence; Analysis 3.3).
3.1. Analysis.
Comparison 3 Attention training versus control – impact on attentional domains immediately after treatment, Outcome 1 Alertness.
3.2. Analysis.
Comparison 3 Attention training versus control – impact on attentional domains immediately after treatment, Outcome 2 Selective attention.
3.3. Analysis.
Comparison 3 Attention training versus control – impact on attentional domains immediately after treatment, Outcome 3 Sustained attention.
Rehabilitation of attention did not show any convincing effects on functional abilities in daily living (SMD 0.29, 95% CI –0.16 to 0.75; P = 0.21; 2 studies, 75 participants; very low‐quality evidence; Analysis 4.1; Röhring 2004; Schottke 1997), mood (SMD 0.01, 95% CI –0.36 to 0.39; P = 0.94; 3 studies using depression scales, 109 participants; low‐quality evidence; Analysis 4.2; Röhring 2004; Schottke 1997; Winkens 2009), or quality of life (SMD 0.02, 95% CI –0.37 to 0.40; P = 0.94; 2 studies, 103 participants; low‐quality evidence; Analysis 4.3; Barker‐Collo 2009; Winkens 2009).
4.1. Analysis.
Comparison 4 Attention training versus control – impact on functional abilities in daily living, mood, and quality of life immediately after treatment, Outcome 1 Functional abilities.
4.2. Analysis.
Comparison 4 Attention training versus control – impact on functional abilities in daily living, mood, and quality of life immediately after treatment, Outcome 2 Mood (depression).
4.3. Analysis.
Comparison 4 Attention training versus control – impact on functional abilities in daily living, mood, and quality of life immediately after treatment, Outcome 3 Quality of life.
Long‐term effects
Two studies comprising 99 participants assessed long‐term effects of treatment on divided attention and there was no convincing effect of treatment compared with care as usual (SMD 0.36, 95% CI –0.04 to 0.76; P = 0.08; very low‐quality evidence; Analysis 5.4; Barker‐Collo 2009; Winkens 2009). There was no convincing evidence for treatment effects on the other domains of attention. One study comprising 31 participants examined effects on alertness (SMD –0.26, 95% CI –0.97 to 0.45; P = 0.47; very low‐quality evidence; Analysis 5.1; Winkens 2009). Two studies comprising 99 participants investigated effects on selective attention at follow‐up (SMD 0.07, 95% CI –0.32 to 0.47; P = 0.72; very low‐quality evidence; Analysis 5.2; Barker‐Collo 2009; Winkens 2009), and one study comprising 66 participants assessed the effect on sustained attention (SMD 0.05, 95% CI –0.44 to 0.53; P = 0.84; very low‐quality evidence; Analysis 5.3; Barker‐Collo 2009).
5.4. Analysis.
Comparison 5 Attention training versus control – impact on attentional domains at follow‐up , Outcome 4 Divided attention at follow‐up.
5.1. Analysis.
Comparison 5 Attention training versus control – impact on attentional domains at follow‐up , Outcome 1 Alertness at follow‐up.
5.2. Analysis.
Comparison 5 Attention training versus control – impact on attentional domains at follow‐up , Outcome 2 Selective attention at follow‐up.
5.3. Analysis.
Comparison 5 Attention training versus control – impact on attentional domains at follow‐up , Outcome 3 Sustained attention at follow‐up.
The only study testing for long‐term effects on functional abilities in daily living comprising 66 participants found no evidence for an effect of treatment (SMD 0.02, 95% CI –0.46 to 0.51; P = 0.92; very low‐quality evidence; Analysis 6.1; Barker‐Collo 2009). Two studies with 99 participants found no convincing effects on mood (SMD 0.29, 95% CI –0.11 to 0.69; P = 0.15; very low‐quality evidence; Analysis 6.2) or quality of life (SMD 0.26, 95% CI –0.13 to 0.66; P = 0.19; very low‐quality evidence; Analysis 6.3) (Barker‐Collo 2009; Winkens 2009).
6.1. Analysis.
Comparison 6 Attention training versus control – impact on functional abilities in daily living, mood, and quality of life at follow‐up, Outcome 1 Functional abilities at follow‐up.
6.2. Analysis.
Comparison 6 Attention training versus control – impact on functional abilities in daily living, mood, and quality of life at follow‐up, Outcome 2 Mood (depression) at follow‐up.
6.3. Analysis.
Comparison 6 Attention training versus control – impact on functional abilities in daily living, mood, and quality of life at follow‐up, Outcome 3 Quality of life at follow‐up.
Subgroup and sensitivity analyses
We were unable to run subgroup or sensitivity analyses because there were insufficient data available.
Discussion
We found no new trials for this update. The results are unchanged from the previous review and are based on the data of six trials with 223 participants.
Summary of main results
There was no evidence to support a beneficial effect of cognitive rehabilitation on global attentional functions (very low‐quality evidence). People receiving cognitive rehabilitation showed improvements on measures of divided attention immediately after rehabilitation (moderate effect size, low‐quality evidence), but there was no convincing evidence that these improvements were retained (very low‐quality evidence). There was no evidence to support the effect of cognitive rehabilitation on specific attentional domains (alertness, selective attention, sustained attention), functional abilities, mood (depression), and quality of life.
Overall completeness and applicability of evidence
Some authors provided unpublished data (Barker‐Collo 2009; Röhring 2004; Winkens 2009), and others clarified aspects that were unclear from the published reports (Schottke 1997; Sturm 1991; Westerberg 2007). We excluded some studies on the basis of information from authors (Kim 2008), and others confirmed the results were not yet published (see Characteristics of studies awaiting classification table).
Trials generally had small sample sizes ranging from 18 to 78, which limits the ability to generalise from these studies. Future studies should be adequately powered to detect the effects of treatment on functional outcomes. The results from these studies should enable a power calculation for future studies.
The studies included a wide variety of interventions; almost all were computerised. The comparison for most studies was treatment as usual. Future studies could be improved by the use of attention placebo control groups that provide the computerised activities but not the attention retraining activities (e.g. as in Gray 1992). However, this may not be feasible in attentional rehabilitation studies where it is difficult to mask the nature of the intervention to the participants (e.g. in a group‐based intervention with a facilitator, where the objective or focus of the intervention may be clear to the participants).
Most studies assessed outcomes on measures of attention on standardised tests. The six studies reported 40 different test variables of attentional outcomes. It has been suggested that cognitive training for certain attentional domains might be more effective than for others (see Cappa 2005) and that there is limited generalisation of treatment from one attentional domain to another (Sturm 1997). Therefore, it seemed important to evaluate treatment effects on different attentional domains. However, it would be beneficial if there was broader consensus on the attentional processes these variables tap and greater consistency in the choice of outcome measures. A composite test score assessing several attentional domains would assist with identifying the overall benefit of attention training; however, there is no empirical framework or clear consensus on how to calculate an overall attention composite score (in the same way as, for example, an intelligence quotient (IQ) score). Future work towards a clear definition of attentional impairments and the best methods for measuring these will be needed for the field to progress.
Furthermore, as with cognitive rehabilitation research more broadly, few attention rehabilitation trials measured functional outcomes. Yet for stroke survivors, the impact of attentional impairments on activity and participation is the most meaningful target for change. Indeed, for many clinicians and researchers, this should be the logical endpoint or focus of cognitive rehabilitation. However, self‐report measures of everyday attentional difficulties also have the issue of demand characteristics on non‐blind participants who are reporting the outcome of the attentional training they know they have received. Measuring the everyday functional impact of attention interventions in valid, reliable and feasible ways (e.g. perhaps incorporating experience‐based sampling, close other/clinician reports, goal attainment scaling, or a combination of these) is another important challenge for this field of research.
The inclusion criteria for attentional deficits varied considerably across trials, and accordingly, the degree of attentional deficits at the start of the treatment differed widely. This review could not address whether treatment success is modulated by the severity of attentional impairments in an individual.
There were insufficient data to evaluate whether treatment is more effective in the postacute phase than in the acute phase of recovery (Cappa 2005; Cicerone 2011). Similarly, we were unable to conduct the planned subgroup analyses to determine whether outcomes varied according to frequency of intervention (number of sessions per week), intensity of intervention (total hours of intervention), and type of intervention because of insufficient data.
Quality of the evidence
The overall certainty of evidence was very low to moderate. The evidence base was small with few methodologically robust RCTs. We typically downgraded evidence because of imprecision due to small sample sizes and risk of bias due to inadequate reporting of allocation concealment and unclear risk of performance bias (see Table 1 and Table 2).
Potential biases in the review process
Two review authors independently assessed the included studies and made final decisions following discussion. One review author (TL) proposed the allocation of attention tasks to attentional domains and the second review author of the last update (Nadina Lincoln) confirmed these. While the assignments are consistent with commonly used typologies and taxonomies of attentional processes in clinical settings, we acknowledge that these assignments are, to some degree, arbitrary as the same test variable may tap into several different attentional domains (Lezak 2004; Strauss 2006).
In addition, the exclusion of studies on attention retraining to improve single ADLs (e.g. driving ability), could be seen as a limitation, but outcomes based on individual ADLs would be difficult to aggregate. Based on our knowledge of the literature, we did not feel this was appropriate at this time, but perhaps could be considered when more research accrues. Excluding interventions that could have had an impact on cognition (e.g. music therapy or mindfulness) could have biased the outcomes, but our definition of cognitive rehabilitation and interventions to be considered as cognitive rehabilitation was established a priori. A future more generic review that focuses on all types of non‐pharmacological interventions for attentional problems could consider including these and other types of interventions that are likely to have a positive impact on attention (e.g. interventions targeting fatigue and sleep disturbances). Similarly, the selection requirement of demonstrable attention deficits and exclusion of studies which based the selection on impairments in general cognitive functioning (e.g. Mini‐Mental State Examination scores) could be considered as a somewhat narrow inclusion criterion. However, we felt it was important to only include studies of cognitive rehabilitation for attentional problems that recruited people who had attentional problems, because this may have affected the outcomes of the intervention. Future reviews could consider widening this inclusion criterion but would need to consider a sensitivity analysis to examine whether there are differential treatment effects between studies that do and do not include those with attentional problems at baseline.
Agreements and disagreements with other studies or reviews
We found some benefits of cognitive rehabilitation for divided attention, but not for other attentional domains. This finding is consistent with a meta‐analysis of attention rehabilitation after acquired brain injury, which also found effects confined to divided attention (Virk 2015). Other relevant meta‐analyses have tended to find a small effect for attention that was not specific to a particular domain (Bogdanova 2016; Rogers 2018; Weicker 2016); however, not all of these reviews analysed each domain separately.
Rogers 2018 further reported that improvements in attention were observed for interventions targeting visuo‐spatial and perceptual skills and general cognition as well those targeting attention. This suggests that a wider definition of attention rehabilitation may have produced different results for this review.
The review concludes that overall there is insufficient evidence to support or refute the effectiveness of cognitive rehabilitation for attention after stroke. This conclusion is unchanged from the previous Cochrane Review (Loetscher 2013), and consistent with another meta‐analysis assessing treatment outcomes of attention rehabilitation after acquired brain injury (Park 2001). The finding that short‐term gains in attention are not consistently maintained at longer‐term follow‐up was also reported in the meta‐analyses by Rogers 2018 and Virk 2015, supporting the need for evaluations of strategies to sustain gains made in cognitive rehabilitation.
The appraisal of the evidence in favour of rehabilitation is somewhat less positive than reviews of cognitive rehabilitation for people with acquired brain injury (Cappa 2005; Cicerone 2011; Cicerone 2019), as well as one review of non‐RCTs in stroke (Merriman 2019). One reason for the discrepancy is probably because the current review applied more rigorous inclusion criteria than these reviews. In Cicerone 2019, for example, seven of the 13 studies reviewed were class III studies. Consistent with this, Rogers 2018 found the strength of the overall effect of cognitive rehabilitation was moderated significantly by study quality, with lower quality studies reporting larger effect sizes.
Authors' conclusions
Implications for practice.
The effectiveness of cognitive rehabilitation for attention deficits following stroke remains unconfirmed. Cognitive rehabilitation may improve some specific aspects of attention immediately after treatment. There was no evidence to indicate whether the benefits persist in the long term. However, improving attention in the short term may enable people to engage better in rehabilitation and improve their ability to cope with tasks in which they are required to do two things at the same time, such as walking and talking. It is important that when rehabilitation for attention is carried out the benefits are monitored closely because at present no specific rehabilitation approach can be recommended.
Implications for research.
There was some limited evidence that cognitive rehabilitation may improve some aspects of attention immediately after treatment, but there was insufficient evidence to support or refute the persisting effects of cognitive rehabilitation on attention, or on functional outcomes in the long term. Therefore, it is important that more randomised trials are conducted to inform clinical practice. Cognitive impairment has been named as a key priority in the Second Stroke Recovery and Rehabilitation Roundtable (Bernhardt 2019). However, clear definition and measurement of treatment targets, both at the level of impairment (i.e. robust attention composite scores) as well as activity and participation (i.e. valid and feasible measures of the everyday functional impact of attentional impairment), are urgent priorities for attention rehabilitation research. Additionally, the long‐term effects of cognitive rehabilitation need to be evaluated in addition to short‐term effects, and interventions should incorporate strategies for sustaining the gains made.
There needs to be more attention to both the design of methodologically sound studies and reporting that conforms with the CONSORT guidelines. Trialists are encouraged to refer to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and the Template for Intervention Description and Replication (TIDieR; Hoffmann 2014), for the reporting of interventions. In addition, trials need to have adequate power to detect clinically meaningful differences between groups. It is important that such evaluations are carried out as the best ways to improve cognition after stroke is one of the top 10 research priorities reported by people with stroke (Pollock 2012).
This review is ongoing and the authors would like to receive information on ongoing trials for a future update.
What's new
| Date | Event | Description |
|---|---|---|
| 25 June 2019 | New search has been performed | Update search completed. No new articles found for inclusion. The review still includes six RCTs with 223 participants |
| 25 June 2019 | New citation required but conclusions have not changed | Conclusions unchanged. New authors added. |
History
Protocol first published: Issue 4, 2000 Review first published: Issue 4, 2000
| Date | Event | Description |
|---|---|---|
| 15 October 2012 | New search has been performed | We updated the searches and included four new trials. There are now six included trials with a total of 223 participants. We have added a stricter definition of the inclusion criteria and outcome measures. There is also a newly added assessment of long‐term effects. The conclusions have not changed since the last version was published |
| 15 October 2012 | New citation required but conclusions have not changed | New first author |
| 4 August 2008 | Amended | Converted to new review format. |
Acknowledgements
We thank the principal investigators of included and excluded studies for providing data and additional information. We also thank Hazel Fraser for her assistance with searching and overall guidance. We also thank Nadina Lincoln, Mariam Majid and Nicola Weyman for their contributions to the previous reviews, and Katharine Baker for her assistance with screening of articles.
Appendices
Appendix 1. Allocation of outcome measures to attentional domains
| Task | Primary attentional domain | Secondary attentional domain | Study ID |
| Behavioural Test of Inattentiveness in Daily Life | Unclear | — | Schottke 1997 |
| Bells test | Spatial | Selective | Barker‐Collo 2009 |
| Cognitive Failures Questionnaire | Functional | — | Barker‐Collo 2009; Westerberg 2007 |
| Cognitrone | Selective | Alertness | Sturm 1991 |
| d2 | Selective | Sustained | Sturm 1991 |
| Information Intake Task | Unclear | — | Winkens 2009 |
| IVA‐CPT Full‐Scale Attention Quotient | Sustained | Selective | Barker‐Collo 2009 |
| Konzentrations‐Verlaufs‐Test | Sustained | Divided | Schottke 1997 |
| Mental Slowness Observation Test | Unclear | — | Winkens 2009 |
| Mental Slowness Questionnaire | Functional | — | Winkens 2009 |
| PASAT (Paced Auditory Serial Addition Test) | Divided | Sustained | Barker‐Collo 2009; Westerberg 2007; Winkens 2009 |
| Ruff 2&7 | Selective | Sustained | Westerberg 2007 |
| Simple RT | Alertness | Sustained | Winkens 2009 |
| Stroop | Selective | — | Westerberg 2007; Winkens 2009 |
| Symbol Digit Modalities Test | Divided | Sustained | Winkens 2009 |
| TAP subtest divided attention | Divided | Sustained | Röhring 2004 |
| TAP subtest intrinsic alertness | Alertness | Sustained | Röhring 2004 |
| TAP subtest phasic alertness | Alertness | Sustained | Röhring 2004 |
| TAP subtest selective attention | Selective | Sustained | Röhring 2004 |
| Tempo‐Lern‐Test | Alertness | Sustained | Schottke 1997 |
| Trail Making A | Selective | — | Barker‐Collo 2009; Winkens 2009 |
| Trail Making B | Divided | — | Barker‐Collo 2009; Winkens 2009 |
| Visual‐Discrimination‐Conditioner | Sustained | Selective | Schottke 1997 |
| Wahl‐Reaktions‐Test | Selective | Sustained | Schottke 1997 |
| Wiener Determinationsgerat | Selective | Divided | Sturm 1991 |
| Wiener Reaktionsgerat Auditory RT | Alertness | Sustained | Sturm 1991 |
| Wiener Reaktionsgerat Choice RT | Selective | Alertness | Sturm 1991 |
| Wiener Reaktionsgerat Visual RT | Alertness | Sustained | Sturm 1991 |
| Wiener Vigilanzgerat | Sustained | Selective | Sturm 1991 |
| Zahlen‐Verbindungstest | Selective | — | Schottke 1997 |
IVA‐CPT: Integrated Visual and Auditory Continuous Performance Test; RT: reaction time; TAP: Tests of Attentional Performance.
Appendix 2. Cochrane Central Register of Controlled Trials (CENTRAL)
IDSearch #1MeSH descriptor: [Cerebrovascular Disorders] this term only #2MeSH descriptor: [Basal Ganglia Cerebrovascular Disease] explode all trees #3MeSH descriptor: [Brain Ischemia] explode all trees #4MeSH descriptor: [Carotid Artery Diseases] explode all trees #5MeSH descriptor: [Intracranial Arterial Diseases] explode all trees #6MeSH descriptor: [Intracranial Embolism and Thrombosis] explode all trees #7MeSH descriptor: [Intracranial Embolism and Thrombosis] explode all trees #8MeSH descriptor: [Stroke] this term only #9MeSH descriptor: [Brain Infarction] explode all trees #10MeSH descriptor: [Vasospasm, Intracranial] this term only #11MeSH descriptor: [Vertebral Artery Dissection] this term only #12((stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc* or cva* or apoplex* or SAH)):ti,ab,kw (Word variations have been searched) #13(((brain* or cerebr* or cerebell* or intracran* or intracerebral) near/5 (ischaem* or ischemi* or infarct* or thrombo* or emboli* or occlus*))):ti,ab,kw (Word variations have been searched) #14(((brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid) near/5 (haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed*))):ti,ab,kw (Word variations have been searched) #15MeSH descriptor: [Brain Injuries] this term only #16MeSH descriptor: [Brain Injury, Chronic] this term only #17{OR #1‐#16} #18MeSH descriptor: [Attention] this term only #19MeSH descriptor: [Arousal] this term only #20(((attention* or concentrat* or arousal or alert* or vigilance) near/5 (impair* or deficit* or disorder* or problem* or diminish* or decreas* or reduc*))):ti,ab,kw (Word variations have been searched) #21((inattention or distract*)):ti,ab,kw (Word variations have been searched) #22((error near/3 control* near/5 (impair* or deficit* or disorder* or problem* or diminish* or decreas* or reduc*))):ti,ab,kw (Word variations have been searched) #23((speed near/3 information near/3 process* near/5 (impair* or deficit* or disorder* or problem* or diminish* or decreas* or reduc*))):ti,ab,kw (Word variations have been searched) #24((mental near/5 (slow* or fatig*))):ti,ab,kw (Word variations have been searched) #25{OR #18‐#24} #26MeSH descriptor: [Rehabilitation] explode all trees #27MeSH descriptor: [Cognitive Therapy] this term only #28MeSH descriptor: [Computer Systems] this term only #29MeSH descriptor: [Therapy, Computer‐Assisted] this term only #30MeSH descriptor: [Therapy, Computer‐Assisted] explode all trees #31((training or re‐training or retraining or therap* or rehabilitat* or neurorehabilitat* or treatment* or therapeutic*)):ti,ab,kw (Word variations have been searched) #32(rehabilitation):ti,ab,kw (Word variations have been searched) #33(((attention* or concentrat* or arousal or alert* or vigilance) near/6 (skill* or abilit* or function* or improve* or enhance* or increas* or strateg* or task*))):ti,ab,kw (Word variations have been searched) #34{OR #26‐#33} #35#17 AND #25 AND #34
Appendix 3. MEDLINE search strategy (Ovid)
1. cerebrovascular disorders/ or exp basal ganglia cerebrovascular disease/ or exp brain ischemia/ or exp carotid artery diseases/ or exp intracranial arterial diseases/ or exp "intracranial embolism and thrombosis"/ or exp intracranial hemorrhages/ or stroke/ or exp brain infarction/ or vasospasm, intracranial/ or vertebral artery dissection/ 2. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw. 3. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw. 4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw. 5. brain injuries/ or brain injury, chronic/ 6. 1 or 2 or 3 or 4 or 5 7. attention/ or arousal/ 8. Attention Deficit Disorder with Hyperactivity/ 9. ((attention$ or concentrat$ or arousal or alert$ or vigilance) adj5 (impair$ or deficit$ or disorder$ or problem$ or diminish$ or decreas$ or reduc$)).tw. 10. (inattention or distract$).tw. 11. (error adj3 control$ adj5 (impair$ or deficit$ or disorder$ or problem$ or diminish$ or decreas$ or reduc$)).tw. 12. (speed adj3 information adj3 proces$ adj5 (impair$ or deficit$ or disorder$ or problem$ or diminish$ or decreas$ or reduc$)).tw. 13. (mental adj5 (slow$ or fatig$)).tw. 14. 7 or 8 or 9 or 10 or 11 or 12 or 13 15. exp rehabilitation/ or cognitive therapy/ or exp computers/ or therapy, computer‐assisted/ or exp neuropsychological tests/ 16. (training or re‐training or retraining or therap$ or rehabilitat$ or neurorehabilitat$ or treatment$ or therapeutic$).tw. 17. rehabilitation.fs. 18. ((attention$ or concentrat$ or arousal or alert$ or vigilance) adj6 (skill$ or abilit$ or function$ or improve$ or enhance$ or increas$ or strateg$ or task$)).tw. 19. 15 or 16 or 17 or 18 20. 6 and 14 and 19 21. Randomized Controlled Trials as Topic/ 22. random allocation/ 23. Controlled Clinical Trials as Topic/ 24. control groups/ 25. double‐blind method/ 26. single‐blind method/ 27. Placebos/ 28. placebo effect/ 29. cross‐over studies/ 30. Therapies, Investigational/ 31. Research Design/ 32. Program Evaluation/ 33. evaluation studies as topic/ 34. randomized controlled trial.pt. 35. controlled clinical trial.pt. 36. (evaluation studies or comparative study).pt. 37. random$.tw. 38. (controlled adj5 (trial$ or stud$)).tw. 39. (clinical$ adj5 trial$).tw. 40. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw. 41. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw. 42. (therapeutic adj5 (trial$ or stud$)).tw. 43. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw. 44. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw. 45. (coin adj5 (flip or flipped or toss$)).tw. 46. (cross‐over or cross over or crossover).tw. 47. placebo$.tw. 48. sham.tw. 49. (assign$ or alternate or allocat$ or counterbalance$ or multiple baseline).tw. 50. controls.tw. 51. (treatment$ adj6 order).tw. 52. or/21‐51 53. 20 and 52 54. exp animals/ not humans.sh. 55. 53 not 54
Appendix 4. Embase search strategy (Ovid)
1. cerebrovascular disease/ or basal ganglion hemorrhage/ or exp brain hematoma/ or exp brain hemorrhage/ or exp brain infarction/ or exp brain ischemia/ or exp carotid artery disease/ or cerebral artery disease/ or cerebrovascular accident/ or exp intracranial aneurysm/ or exp occlusive cerebrovascular disease/ or stroke/ 2. stroke patient/ or stroke unit/ 3. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw. 4. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw. 5. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw. 6. brain injury/ 7. 1 or 2 or 3 or 4 or 5 or 6 8. exp attention/ or attention deficit disorder/ or attention disturbance/ or arousal/ or concentration loss/ 9. ((attention$ or concentrat$ or arousal or alert$ or vigilance or attentiveness or awareness) adj5 (impair$ or deficit$ or disorder$ or problem$ or diminish$ or decreas$ or reduc$)).tw. 10. (inattention or distract$).tw. 11. (error adj3 control$ adj5 (impair$ or deficit$ or disorder$ or problem$ or diminish$ or decreas$ or reduc$)).tw. 12. (speed adj3 information adj3 process$ adj5 (impair$ or deficit$ or disorder$ or problem$ or diminish$ or decreas$ or reduc$)).tw. 13. (mental adj5 (slow$ or fatig$)).tw. 14. 8 or 9 or 10 or 11 or 12 or 13 15. cognitive therapy/ or cognitive rehabilitation/ or rehabilitation/ 16. exp computer/ or computer assisted therapy/ or computer interface/ 17. (training or re‐training or retraining or therap$ or rehabilitat$ or neurorehabilitat$ or treatment$ or therapeutic$).tw. 18. rh.fs. 19. ((attention$ or concentrat$ or arousal or alert$ or vigilance or attentiveness or awareness) adj6 (skill$ or abilit$ or function$ or improve$ or enhance$ or increas$ or strateg$ or task$)).tw. 20. 15 or 16 or 17 or 18 or 19 21. 7 and 14 and 20 22. Randomized Controlled Trial/ 23. Randomization/ 24. Controlled Study/ 25. control group/ 26. clinical trial/ 27. Crossover Procedure/ 28. Double Blind Procedure/ 29. Single Blind Procedure/ or triple blind procedure/ 30. Parallel Design/ 31. placebo/ 32. experimental design/ or experimental study/ or quasi experimental study/ 33. experimental therapy/ 34. research subject/ 35. Comparative Study/ 36. random$.tw. 37. (controlled adj5 (trial$ or stud$)).tw. 38. (clinical$ adj5 trial$).tw. 39. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw. 40. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw. 41. (therapeutic adj5 (trial$ or stud$)).tw. 42. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw. 43. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw. 44. (coin adj5 (flip or flipped or toss$)).tw. 45. (cross‐over or cross over or crossover).tw. 46. placebo$.tw. 47. sham.tw. 48. (assign$ or alternate or allocat$ or counterbalance$ or multiple baseline).tw. 49. controls.tw. 50. (treatment$ adj6 order).tw. 51. or/22‐50 52. 21 and 51 53. limit 52 to human 54. (traumatic not (traumatic and stroke)).ti. 55. 53 not 54
Appendix 5. PsycINFO search strategy (Ovid)
1. cerebrovascular disorders/ or cerebral hemorrhage/ or exp cerebral ischemia/ or cerebral small vessel disease/ or cerebrovascular accidents/ or subarachnoid hemorrhage/ 2. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw. 3. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw. 4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw. 5. hemiparesis/ or hemiplegia/ 6. (hemipleg$ or hemipar$ or paresis or paretic).tw. 7. "brain lesions (disorders)"/ 8. brain injur$.tw. 9. or/1‐8 10. exp attention/ or exp attention deficit disorder/ or exp concentration/ or exp sustained attention/ or exp divided attention/ or attention span/ or exp visual attention/ or exp selective attention/ 11. ((attention$ or concentrat$ or arousal or alert$ or vigilance or attentiveness or awareness) adj5 (impair$ or deficit$ or disorder$ or problem$ or diminish$ or decreas$ or reduc$)).tw. 12. (inattention or distract$).tw. 13. (error adj3 control$ adj5 (impair$ or deficit$ or disorder$ or problem$ or diminish$ or decreas$ or reduc$)).tw. 14. (speed adj3 information adj3 process$ adj5 (impair$ or deficit$ or disorder$ or problem$ or diminish$ or decreas$ or reduc$)).tw. 15. (mental adj5 (slow$ or fatig$)).tw. 16. or/10‐15 17. cognitive therapy/ or cognitive behavior therapy/ or cognitive rehabilitation/ or rehabilitation/ 18. computer assisted therapy/ or exp computers/ or human computer interaction/ or computer training/ 19. (training or retraining or therap$ or rehabilitat$ or neurorehabilitat$ or treatment$ or therapeutic$).tw. 20. training/ 21. ((attention$ or concentrat$ or arousal or alert$ or vigilance or attentiveness or awareness) adj6 (skill$ or abilit$ or function$ or improve$ or enhance$ or increas$ or strateg$ or task$)).tw. 22. or/17‐21 23. clinical trials/ or treatment effectiveness evaluation/ or placebo/ 24. (random$ or RCT or RCTs).tw. 25. (controlled adj5 (trial$ or stud$)).tw. 26. (clinical$ adj5 trial$).tw. 27. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw. 28. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw. 29. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw. 30. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw. 31. (cross‐over or cross over or crossover).tw. 32. (placebo$ or sham).tw. 33. trial.ti. 34. (assign$ or allocat$).tw. 35. or/23‐34 36. 9 and 16 and 22 and 35
Appendix 6. Cumulative Index to Nursing and Allied Health Literature (CINAHL) search strategy (EBSCO)
S1(MH "Cerebrovascular Disorders") OR (MH "Basal Ganglia Cerebrovascular Disease+") OR (MH "Carotid Artery Diseases+") OR (MH "Cerebral Ischemia+") OR (MH "Cerebral Vasospasm") OR (MH "Intracranial Arterial Diseases+") OR (MH "Intracranial Embolism and Thrombosis") OR (MH "Intracranial Hemorrhage+") OR (MH "Stroke") OR (MH "Vertebral Artery Dissections") OR (MH "Brain Injuries") S2(MH "Stroke Patients") OR (MH "Stroke Units") S3TI (stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex or SAH ) or AB (stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex or SAH) S4TI (brain* or cerebr* or cerebell* or intracran* or intracerebral) or AB (brain* or cerebr* or cerebell* or intracran* or intracerebral) S5TI (ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus*) or AB (ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus*) S6S4 and S5 S7TI (brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid) or AB (brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid) S8TI (haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed*) or AB (haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed*) S9S7 and S8 S10S1 OR S2 OR S3 OR S6 OR S9 S11(MH "Arousal") OR (MH "Attention") S12(MH "Attention Deficit Hyperactivity Disorder") S13TI ((attention* OR concentrat* OR arousal OR alert* OR vigilance OR attentiveness OR awareness) N5 (impair* OR deficit* OR disorder* OR problem* OR diminish* OR decreas* OR reduc*)) OR AB ((attention* OR concentrat* OR arousal OR alert* OR vigilance OR attentiveness OR awareness) N5 (impair* OR deficit* OR disorder* OR problem* OR diminish* OR decreas* OR reduc*)) S14TI (inattention OR distract*) OR AB (inattention OR distract*) S15TI (error N3 control* N5 (impair* OR deficit* OR disorder* OR problem* OR diminish* OR decreas* OR reduc*)) OR AB (error N3 control* N5 (impair* OR deficit* OR disorder* OR problem* OR diminish* OR decreas* OR reduc*)) S16TI (speed N3 information N3 process* N5 (impair* OR deficit* OR disorder* OR problem* OR diminish* OR decreas* OR reduc*)) OR AB (speed N3 information N3 process* N5 (impair* OR deficit* OR disorder* OR problem* OR diminish* OR decreas* OR reduc*)) S17TI (mental N5 (slow* OR fatig*)) OR AB (mental N5 (slow* OR fatig*)) S18S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 S19(MH "Cognitive Therapy") S20(MH "Rehabilitation") OR (MH "Rehabilitation, Cognitive") S21(MH "Therapy, Computer Assisted") S22(MH "User‐Computer Interface") S23TI (training OR re‐training OR retraining OR therap* OR rehabilitat* OR neurorehabilitat* OR treatment* OR therapeutic*) OR AB (training OR re‐training OR retraining OR therap* OR rehabilitat* OR neurorehabilitat* OR treatment* OR therapeutic*) S24TI ((attention* OR concentrat* OR arousal OR alert* OR vigilance OR attentiveness OR awareness) N6 (skill* OR abilit* OR function* OR improve* OR enhance* OR increas* OR strateg* OR task*)) OR AB ((attention* OR concentrat* OR arousal OR alert* OR vigilance OR attentiveness OR awareness) N6 (skill* OR abilit* OR function* OR improve* OR enhance* OR increas* OR strateg* OR task*)) S25S19 OR S20 OR S21 OR S22 OR S23 OR S24 S26S10 AND S18 AND S25 S27PT randomized controlled trial or clinical trial S28(MH "Random Assignment") or (MH "Random Sample+") S29(MH "Crossover Design") or (MH "Clinical Trials") or (MH "Double‐Blind Studies") or (MH "Intervention Trials") or (MH "Preventive Trials") or (MH "Randomized Controlled Trials") or (MH "Single‐Blind Studies") or (MH "Therapeutic Trials") or (MH "Triple‐Blind Studies") or (MH "Comparative Studies") S30(MH "Control (Research)") or (MH "Control Group") S31(MH "Factorial Design") or (MH "Quasi‐Experimental Studies") or (MH "Nonrandomized Trials") S32(MH "Placebo Effect") or (MH "Placebos") or (MH "Meta Analysis") S33(MH "Clinical Research") or (MH "Clinical Nursing Research") S34(MH "Community Trials") or (MH "Experimental Studies") or (MH "One‐Shot Case Study") or (MH "Pretest‐Posttest Design+") or (MH "Solomon Four‐Group Design") or (MH "Static Group Comparison") or (MH "Study Design") S35TI random* or AB random* S36TI (controlled N5 (trial* OR stud*)) OR AB (controlled N5 (trial* OR stud*)) S37TI (clinical* N5 trial*) OR AB (clinical* N5 trial*) S38TI ((control OR treatment OR experiment* OR intervention) N5 (group* OR subject* OR patient*)) OR AB ((control OR treatment OR experiment* OR intervention) N5 (group* OR subject* OR patient*)) S39TI (therapeutic N5 (trial* OR stud*)) OR AB (therapeutic N5 (trial* OR stud*)) S40TI ((control OR experiment* OR conservative) N5 (treatment OR therapy OR procedure OR manage*)) OR AB ((control OR experiment* OR conservative) N5 (treatment OR therapy OR procedure OR manage*)) S41TI ((singl* OR doubl* OR tripl* OR trebl*) N5 (blind* OR mask*)) OR AB ((singl* OR doubl* OR tripl* OR trebl*) N5 (blind* OR mask*)) S42TI (coin N5 (flip OR flipped OR toss*)) OR AB (coin N5 (flip OR flipped OR toss*)) S43TI (cross‐over OR cross over OR crossover OR placebo* OR sham) OR AB (cross‐over OR cross over OR crossover OR placebo* OR sham) S44TI (assign* OR alternate OR allocat* OR counterbalance* OR multiple baseline OR controls OR treatment N6 order) OR AB (assign* OR alternate OR allocat* OR counterbalance* OR multiple baseline OR controls OR treatment N6 order) S45S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 S46S26 AND S45
Appendix 7. Psychological Database for Brain Impairment Treatment Efficacy (PsycBITE) search strategy
Target Area: Attention / information processing (speed) Intervention: Cognitive Neurological Group: Stroke / CVA (Cerebrovascular Accidents) Method: Randomized Controlled Trials Age Group: Adults
Appendix 8. REHABDATA search strategy
Search all fields in REHABDATA for records with this exact phrase: stroke with at least one of these words: attention arousal concentrat* Year of Publication between: 2012 and 2019
Appendix 9. ClinicalTrials.gov search strategy
( attention OR concentration OR arousal ) AND INFLECT EXACT "Interventional" [STUDY‐TYPES] AND ( Brain Injuries OR Vertebral Artery OR Brain Infarction OR Intracranial Hemorrhages OR Carotid Artery Diseases OR Brain Ischemia OR Cerebral Hemorrhage OR Cerebrovascular Disorders OR Stroke ) [DISEASE] AND Randomized [TREATMENT]
Appendix 10. WHO ICTRP
stroke AND attention Phases are: All
Data and analyses
Comparison 1. Attention training versus control – impact on global measures of attention deficits immediately after treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Subjective measures | 2 | 53 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.53 [‐0.03, 1.08] |
Comparison 2. Attention training versus control – impact on global measures of attention deficits at follow‐up .
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Subjective measures at follow‐up | 2 | 99 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.16 [‐0.23, 0.56] |
Comparison 3. Attention training versus control – impact on attentional domains immediately after treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Alertness | 4 | 136 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.14 [‐0.20, 0.48] |
| 2 Selective attention | 6 | 223 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.35, 0.18] |
| 3 Sustained attention | 4 | 169 | Std. Mean Difference (IV, Random, 95% CI) | 0.39 [‐0.16, 0.94] |
| 4 Divided attention | 4 | 165 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.67 [0.35, 0.98] |
Comparison 4. Attention training versus control – impact on functional abilities in daily living, mood, and quality of life immediately after treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Functional abilities | 2 | 75 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.29 [‐0.16, 0.75] |
| 2 Mood (depression) | 3 | 109 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.36, 0.39] |
| 3 Quality of life | 2 | 103 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.37, 0.40] |
Comparison 5. Attention training versus control – impact on attentional domains at follow‐up .
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Alertness at follow‐up | 1 | 31 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.26 [‐0.97, 0.45] |
| 2 Selective attention at follow‐up | 2 | 99 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.32, 0.47] |
| 3 Sustained attention at follow‐up | 1 | 66 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.44, 0.53] |
| 4 Divided attention at follow‐up | 2 | 99 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.36 [‐0.04, 0.76] |
Comparison 6. Attention training versus control – impact on functional abilities in daily living, mood, and quality of life at follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Functional abilities at follow‐up | 1 | 66 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.46, 0.51] |
| 2 Mood (depression) at follow‐up | 2 | 99 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.29 [‐0.11, 0.69] |
| 3 Quality of life at follow‐up | 2 | 99 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.26 [‐0.13, 0.66] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barker‐Collo 2009.
| Methods | RCT, parallel group design Concealed online Internet randomisation service with stratified minimisation Implementation of randomisation sequence by the treating clinician who had no access to assessment data. Randomisation information was not accessible by any other study staff during the study. Approach: restoration of attentional functions with the means of APT |
|
| Participants | New Zealand, recruited from 2 hospitals Total participant sample 78; 10 lost at 5 weeks, 12 were not assessed at 6 months Treatment group: n = 38; mean (± SD) age 70.2 ± 15.6 years; 60.5% males; mean (± SD) 18.5 ± 12.0 days since onset; hemisphere of lesion: 14 left (44%), 15 right (47%), 3 bilateral or unclear (9%) Control group: n = 40; mean (± SD) age 67.7 ± 15.6 years; 60.0% males; mean (± SD) 18.6 ± 7.6 days since onset; hemisphere of lesion: 25 left (58%), 17 right (40%), 1 bilateral or unclear (2%) Inclusion criteria: attention deficit defined as performance > 1 SD below norm on any attentional tests; stroke using WHO criteria; admitted to 1 of 2 hospitals; within 2 weeks of stroke Exclusion criteria: unable to give consent; MMSE < 20; medically unstable; unable to speak English; other relevant conditions, such as dementia |
|
| Interventions | Treatment: up to 30 hours' individual APT for 1 hour on weekdays for 4 weeks, mean (± SD) 13.5 ± 9.4 hours Control: no treatment |
|
| Outcomes | Measured immediately after treatment (5 weeks) and at follow‐up (6 months) Primary outcome:
Secondary outcomes:
*Only measured at 6 months' follow‐up |
|
| Notes | Provided numbers of side of hemisphere lesions did not add up to total participant sample. Additional data for analysis provided by authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Internet based and independent |
| Allocation concealment (selection bias) | Low risk | Concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and therapist not blinded as aware of intervention being given. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Trained assessor blind to randomisation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis Last observation carried forward |
| Selective reporting (reporting bias) | Low risk | No indication in article |
Röhring 2004.
| Methods | RCT, cross‐over group design with pair matching Pairs were matched for age, sex, aetiology, side of brain lesion, and time since incident. Allocation to groups according to table of random numbers Planned cross‐over design, but only results of first phase (group 1 received intervention, group 2 no training) were reported Approach: restoration of attentional functions by computer training |
|
| Participants | Germany, single‐centre study Total participant sample: 48 (62 people started study, but 7 people lost in intervention group because of: insufficient training (n = 2), personal reasons (n = 2), medical reasons (n = 2), and because of an exclusion criteria defined after start of training (n = 1). The 7 matched participants in the control group were also excluded from analysis). Participant sample included 5 people with TBI Treatment group: n = 24; mean (± SD) age 51.6 ± 13.0 years; 62.5% males Control group: n = 24; mean (± SD) age 54.9 ± 11.4 years; 62.5% males No subgroup data for hemisphere of lesion, aetiology, and time since incident (group mean (± SD) 25.5 ± 13.7 months) Inclusion criteria: attention deficits (tests and cut‐off criteria not specified); brain damage > 6 months and < 4 years; available transport opportunity to clinic; being able to keep concentration up for 30 minutes per day Exclusion criteria: aged > 70 years; probability of progressing brain disorder; other major neurological or psychiatric disorders; major neuropsychological deficits; any disability that would prevent independent use of the computer program |
|
| Interventions | Treatment: 30–45 minutes of computer‐based training at home for 5 days per week for 11 weeks in total. Cogpack (evosoft TeleCare/Dr Hein GmbH) was used as training software. Self‐reported mean (± SD) training time was 241 ± 135 minutes per week. All participants had 1 therapy session per week at the clinic in addition to the computer training. Control: no treatment, but regularly contacted by therapists to learn about the participants' well‐being |
|
| Outcomes | Measured after intervention (11 weeks) Several measures, but no definition of primary outcome measure:
|
|
| Notes | No matching for attention deficits or any other outcome measures at baseline. Data for analysis provided by authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | No information available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and therapists not blinded to intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessor blinded (information provided by authors) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data in treatment group (n = 7) was controlled for by excluding the corresponding matched participant in control group for the analysis. |
| Selective reporting (reporting bias) | Low risk | While not all of the study's specified outcome measures were reported in the paper (e.g. at 4 months' follow‐up only 1 attentional outcome measure was reported), all data were provided by the authors for the current analysis. |
Schottke 1997.
| Methods | RCT, multicentre study, parallel group design with pair matching Pairs were matched for side and aetiology of lesion, education level, and participating centre. Allocation of 1 person from each matched pair to treatment group by coin toss. 3 people could not be matched and were allocated to the control group. No concealment of intervention or outcome measurement (assessment and training by same person) Approach: restoration of attentional functions and compensatory strategies |
|
| Participants | Germany, recruited from 2 hospitals Total participant sample 29; 0 people lost to follow‐up Treatment group: n = 16; mean (± SD) age 64.1 ± 8.5 years; 56.3% males; mean (± SD) 51.6 ± 21.5 days since onset; hemisphere of lesion: 5 left, 11 right Control group: n = 13; mean (± SD) age 65.4 ± 10.9 years; 46.2% males; mean (± SD) 37.5 ± 11.4 days since onset; hemisphere of lesion: 2 left, 11 right Inclusion criteria: attention deficit defined as standard scores < 80 in any of the attentional tests; stable cardiovascular system; able to travel to training room; cerebral infarct or haemorrhage; neurological symptoms lasting > 24 hours Exclusion criteria: aphasia |
|
| Interventions | Treatment: 13 training sessions in 3 weeks, which included a wide range of different training methods (e.g. computerised reaction training, paper‐and‐pencil tasks, scanning training, cognitive‐behavioural training, and relaxation techniques). Duration of a single session not specified. Control: treatment as usual |
|
| Outcomes | Measured after intervention (3 weeks) Several standardised measures of attention with no definition of primary outcome measure:
|
|
| Notes | Time after stroke significantly shorter for controls compared to the treatment group. Visual‐Discrimination‐Conditioner programme used for treatment and outcome assessment. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Coin tossing |
| Allocation concealment (selection bias) | Unclear risk | No information reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Training and assessment by same person |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | No indication in article, but study protocol not available |
Sturm 1991.
| Methods | RCT, cross‐over group design Participants with left‐brain damage were allocated to 2 comparable groups with respect to age, sex, and time postonset. Allocation of people to treatment group by random number table. There was a third training group with right‐brain damaged people. They were trained 'late' in the cross‐over design and their data were excluded from the current analysis. Approach: restore attentional functions by computer training |
|
| Participants | Germany Total participants sample: 37; 0 people lost to follow‐up Treatment group: n = 13; mean (± SD) age 51.5 ± 9.5 years; 69.2% males; mean (± SD) 15 ± 9.7 weeks since onset; hemisphere of lesion: 13 left, 1 non‐stroke participant Control group: n = 14; mean (± SD) age 49.6 ± 9.5 years; 71.4% males; mean (± SD) 16.4 ± 9.2 weeks since onset; hemisphere of lesion: 14 left, 2 non‐stroke participants No inclusion or exclusion criteria specified. All participants had attentional deficits according to authors. |
|
| Interventions | Treatment: 30 minutes' computer‐based training at clinic, 14 sessions spread over 3 consecutive weeks. Cognitrone and Wiener Determinationsgerat were used as training software. Control: no treatment |
|
| Outcomes | Measured after intervention at 3 weeks, after cross‐over at 6 weeks, and at 12 weeks' follow‐up Several measures, but no definition of primary outcome measure:
|
|
| Notes | Cognitrone and Wiener Determinationsgerat were used for training, outcome measures based on these tasks were excluded for analysis. Control group was more aphasic and scored significantly lower on WAIS, in the Intelligence structure task and the Ravens than the intervention group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of random number table (personal communication of authors) |
| Allocation concealment (selection bias) | Unclear risk | No information reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data, but it was noteworthy that 90% of people with right‐brain damage initially agreed to participate in the study refused to participate when contacted later for starting the training. |
| Selective reporting (reporting bias) | Low risk | No indication in article, but study protocol not available |
Westerberg 2007.
| Methods | RCT, parallel group design 3 people who did not take part in the study performed the randomisation. Small identical paper notes with numbers corresponding to each of the participants were put in a bucket. The notes were folded so that it was impossible to see the numbers. 1 person held the bucket, the 2 other people drew notes in an alternating manner. Before the procedure it was determined that all participants drawn by the first person would be in the training group, and the participants drawn by the other person were in the control group. The principal investigator (not involved in randomisation process) had the key to the correspondence between the numbers and the participant names. The result of the randomisation was kept secret from the therapists. After the first neuropsychological assessment (T1) a sealed, prenamed envelope, which revealed the randomisation to either the treatment or control group condition, was opened by the therapist so that the participants could have the instructions for their intervention. Approach: restore attentional functions by computer training of working memory functions |
|
| Participants | Sweden, recruited from 1 hospital Total participant sample 21; 3 lost after baseline testing (only data of remaining people reported) Treatment group: n = 9; mean (± SD) age 55.0 ± 8 years; 89% males; mean (± SD) 19.3 ± 6.2 months since onset; hemisphere of lesion: 4 left (44%), 4 right (44%), 1 unclear (11%) Control group: n = 9; mean (± SD) age 53.6 ± 8 years; 44% males; mean (± SD) 20.8 ± 6.2 months since onset; hemisphere of lesion: 3 left (33%), 4 right (44%), 2 unclear (22%) Inclusion criteria: self‐reported deficits in attention; had stroke between 12 and 36 months ago; stroke documented by PET, MRI, or CT; aged 30–65 years; had daily access to a computer with Internet connection at home. Exclusion criteria: intelligence quotient < 70; motor or perceptual disability that would prevent use of the computer program; changing medication during the study period; fulfilling criteria for major, depressive‐disorder diagnosis as per the DSM‐IV diagnosis code |
|
| Interventions | Treatment: 40 minutes of computer‐based training at home for 5 days per week for 5 weeks in total. Visuo‐spatial and auditory working memory tasks were performed by using the RoboMemo Software (Cogmed Cognitive Medical Systems, Sweden). Sufficient compliance was defined as 20 days of training (achieved by all participants not withdrawing from the study) Control: no training |
|
| Outcomes | Measured after intervention (5 weeks) Several measures, but no definition of primary outcome measure:
|
|
| Notes | The study authors informed on an error in the original paper. The correct Ruff 2&7 values for the treatment group were 130.3 (21.9) seconds at pretraining and 115.4 (21.7) seconds at post‐training. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sealed envelopes prepared by a person unrelated to the study. |
| Allocation concealment (selection bias) | Low risk | Sealed preaddressed envelope (prepared by people unrelated to the study) opened after the initial assessment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and therapist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessor not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups, lost participants scored within group mean level in the baseline. |
| Selective reporting (reporting bias) | Low risk | No indication in article, but study protocol not available |
Winkens 2009.
| Methods | RCT, multicentre study, parallel group design Random assignment list created before start of trial; independent person used the list to assign people to the 2 groups after recruitment Approach: learning of compensatory strategies |
|
| Participants | Netherlands, recruited from 8 rehabilitation centres Total participant sample 37: 2 lost at post‐treatment assessment, another 1 at 3‐month follow‐up Treatment group: n = 20; mean (± SD) age 49.5 ± 8 years; 45% males; mean (± SD) 19.3 ± 29.6 months since onset Control group: n = 17; mean (± SD) age 53.9 ± 11.1 years; 71% males; mean (± SD) 6.9 ± 5.4 months since onset No data for hemisphere of lesion, and aetiology Inclusion criteria: stroke > 3 months; referred for cognitive rehabilitation for mental slowness Exclusion criteria: aged < 18 years; stroke < 3 months; severe or disabling premorbid or current (continuing) pathological conditions; severe cognitive, communication, physical, or psychological problems that the person was unable to perform the tasks, based on the clinical judgement of the treating team. |
|
| Interventions | Treatment: 10‐hour teaching in time pressure management session duration varied between 1 and 2 hours a week depending on the person Control: care as usual |
|
| Outcomes | Measured after intervention (time not explicitly specified, but likely to be around 5 weeks) and at 3‐month follow‐up Primary outcomes:
Secondary outcomes:
|
|
| Notes | People with stroke were relatively young and ADL‐independent. Control group were more recent after onset. Additional data for analysis provided by the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Random assignment list" was used, no details given |
| Allocation concealment (selection bias) | Low risk | After selection of participants independent person used random list to assign people to groups |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and therapist not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessment by blinded research assistant. Analyses showed that assistant had guessed the allocation correctly in 24 of 37 cases (Cohen k = 0.29, P = 0.05) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups |
| Selective reporting (reporting bias) | Low risk | No indication in article, but study protocol not available |
ADL: activities of daily living; APT: attention process training; CT: computerised tomography; DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; FIM: Functional Independence Measure; GHQ: General Health Questionnaire; ISI: interstimulus interval; IVA‐CPT: Integrated Visual and Auditory Continuous Performance Test; MMSE: Mini‐Mental State Examination; MRI: magnetic resonance imaging; n: number of participants; PASAT: Paced Auditory Serial Addition Test; PET: positron emission tomography; RCT: randomised controlled trial; SD: standard deviation; SF: Short Form; TBI: traumatic brain injury; WAIS‐R: Wechsler Adult Intelligence Scale – Revised; WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aben 2013 | Not rehabilitation of attentional deficits |
| Aivazian 1994 | Not rehabilitation of attentional deficits |
| Akinwuntan 2010 | Not selected on the basis of having impairment of attention |
| Albrittain‐Ross 2017 | Not CCT/RCT |
| Alexopoulos 2012 | Not selected on the basis of having impairment of attention |
| Arnemann 2015 | Not stroke (if mixed sample, less than 75%), or no stroke‐specific data |
| Bergquist 2012 | Not stroke (if mixed sample, less than 75%), or no stroke‐specific data |
| Bertens 2015 | Not rehabilitation of attentional deficits |
| Bezdenezhnykh 2015 | Not selected on the basis of having impairment of attention |
| Blanchet 2016 | Not selected on the basis of having impairment of attention |
| Bo 2019 | Not selected on the basis of having impairment of attention |
| Boman 2004 | Not CCT/RCT |
| Carelli 2009 | Not CCT/RCT |
| Carr 2012 | Not selected on the basis of having impairment of attention |
| Chen 2011 | Not stroke (if mixed sample, less than 75%), or no stroke‐specific data |
| Chen 2015 | Not selected on the basis of having impairment of attention |
| Cho 2016 | Not selected on the basis of having impairment of attention |
| Chung 2013 | Not original study (e.g. reviews) |
| Crotty 2009 | Not selected on the basis of having impairment of attention |
| De Luca 2014 | Not stroke (if mixed sample, less than 75%), or no stroke‐specific data |
| De Luca 2018a | Not selected on the basis of having impairment of attention |
| De Luca 2018b | Not selected on the basis of having impairment of attention |
| Dirette 2004 | Not CCT/RCT |
| Edmans 2009 | Not selected on the basis of having impairment of attention |
| Faria 2016 | Not selected on the basis of having impairment of attention |
| Gates 2011 | Not selected on the basis of having impairment of attention |
| Gauggel 1996 | Not CCT/RCT |
| Giaquinto 2003 | Not rehabilitation of attentional deficits |
| Gracey 2017 | Not stroke (if mixed sample, less than 75%), or no stroke‐specific data |
| Graf 2011 | Not selected on the basis of having impairment of attention |
| Gray 1992 | Not stroke (if mixed sample, less than 75%), or no stroke‐specific data |
| Hashimoto 2006 | Not CCT/RCT |
| Jiang 2016 | Not selected on the basis of having impairment of attention |
| Kang 2008 | Not CCT/RCT |
| Kang 2009 | Not rehabilitation of attentional deficits |
| Kaur 2018 | Not selected on the basis of having impairment of attention |
| Kim 2008 | Not selected on the basis of having impairment of attention |
| Kim 2013 | Not selected on the basis of having impairment of attention |
| Klonoff 2010 | Not CCT/RCT |
| Levine 2011 | Not selected on the basis of having impairment of attention |
| Mazer 2003 | Not selected on the basis of having impairment of attention |
| McDonnell 2007 | Not rehabilitation of attentional deficits |
| Mead 2007 | Not rehabilitation of attentional deficits |
| Middleton 1991 | Not CCT/RCT |
| Miotto 2009 | Not stroke (if mixed sample, less than 75%), or no stroke‐specific data |
| NCT00298558 | Not stroke (if mixed sample, less than 75%), or no stroke‐specific data |
| NCT01109836 | Not rehabilitation of attentional deficits |
| NCT01365858 | Not selected on the basis of having impairment of attention |
| NCT01372059 | Not rehabilitation of attentional deficits |
| NCT01886183 | Not selected on the basis of having impairment of attention |
| NCT02482688 | Rehabilitation of spatial neglect |
| Otfinowski 2006 | Not selected on the basis of having impairment of attention |
| Park 2015 | Not selected on the basis of having impairment of attention |
| Park 2018 | Not selected on the basis of having impairment of attention |
| Piccardi 2006 | Not CCT/RCT |
| Ploughman 2008 | Not selected on the basis of having impairment of attention |
| Ploughman 2017 | Not selected on the basis of having impairment of attention |
| Prokopenko 2013 | Not selected on the basis of having impairment of attention |
| Pulin 2017 | Not CCT/RCT |
| Pyoria 2007 | Not selected on the basis of having impairment of attention |
| Pyun 2009 | Not CCT/RCT |
| Quaney 2009 | Not selected on the basis of having impairment of attention |
| Ressner 2018 | Not selected on the basis of having impairment of attention |
| Rizkalla 2011 | Not selected on the basis of having impairment of attention |
| Shi 2017 | Not selected on the basis of having impairment of attention |
| Shiflett 2002 | Not rehabilitation of attentional deficits |
| Skidmore 2014 | Not CCT/RCT |
| Skidmore 2015 | Not selected on the basis of having impairment of attention |
| Sohlberg 2000 | Not stroke (if mixed sample, less than 75%), or no stroke‐specific data |
| Spahn 2010 | Not rehabilitation of attentional deficits |
| Spikman 2010 | Not selected on the basis of having impairment of attention |
| Stapleton 2001 | Not rehabilitation of attention deficits |
| Sturm 1997 | Not CCT/RCT |
| Sturm 2006 | Not rehabilitation of attentional deficits |
| Särkämö 2008 | Not selected on the basis of having impairment of attention |
| Särkämö 2010 | Not selected on the basis of having impairment of attention |
| Thimm 2006 | Not rehabilitation of attentional deficits |
| Tornas 2014 | Not stroke (if mixed sample, less than 75%), or no stroke‐specific data |
| Van de Ven 2017 | Not selected on the basis of having impairment of attention |
| Villalobos 2018 | Not stroke (if mixed sample, less than 75%), or no stroke‐specific data |
| Von Koch 2000 | Not selected on the basis of having impairment of attention |
| Wagenaar 1992 | Not treatment of attention deficits |
| Wentink 2016 | Not selected on the basis of having impairment of attention |
| Yamamoto‐Mitani 2007 | Not CCT/RCT |
| Zucchella 2014 | Not selected on the basis of having impairment of attention |
CCT: controlled clinical trial; RCT: randomised controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
Bartfai 2014.
| Methods | RCT |
| Participants | 120 consecutive participants with stroke or traumatic brain injury |
| Interventions | 20 hours' attention process training |
| Outcomes | Not known |
| Notes | Protocol published, full paper is submitted for publication |
Bayat 2014.
| Methods | RCT |
| Participants | 60 participants |
| Interventions | 4 groups (computer training, music listening, combined computer training and music listening, no intervention) |
| Outcomes | Not known |
| Notes | Conference abstract. Study authors did not reply to queries. |
Bushnick 2010.
| Methods | RCT |
| Participants | 125 participants ≥ 1‐year postinjury with diagnosis of cognitive impairment |
| Interventions | Use of the orthotic device "Planning and Execution Assistant and Trainer (PEAT)" |
| Outcomes | Not known |
| Notes | Conference abstract. Authors replied that study is not published. |
Chen 2018.
| Methods | RCT |
| Participants | 14 participants with aneurysmal subarachnoid haemorrhage enrolled |
| Interventions | Tablet‐assisted cognitive rehabilitation |
| Outcomes | Not known |
| Notes | Conference abstract. Authors replied that study is not published. |
Dawson 2017.
| Methods | RCT |
| Participants | 15 participants with stroke |
| Interventions | 16 hours of Cognitive Orientation to Occupational Performance Approach (delivery via telerehabilitation) |
| Outcomes | Not known |
| Notes | Conference abstract. Authors replied that study is not published. |
Flynn 2000.
| Methods | RCT |
| Participants | 30 participants with stroke |
| Interventions | Attention training |
| Outcomes | Not known |
| Notes | Study completed according to National Research Register (Register is no longer searchable and has not been updated since September 2007). Study author did not reply to queries in previous update. |
Frommelt 2003.
| Methods | RCT |
| Participants | Not known |
| Interventions | Compensatory skills training versus retraining basic cognitive impairments (either memory skills training or problem solving) |
| Outcomes | Not known |
| Notes | ISRCTN45171788. Data collection finished. Authors replied that study is not published. |
Gil‐Pages 2018.
| Methods | Cross‐over RCT |
| Participants | Participants with chronic‐stage stroke |
| Interventions | 6 weeks' customised telerehabilitation |
| Outcomes | Several measures of attention |
| Notes | NCT03326349. Recruitment completed. Authors replied that study is not published. |
Holleman 2012.
| Methods | RCT |
| Participants | 38 participants with acquired brain injury |
| Interventions | Intensive NeuroRehabilitation (INR) programme |
| Outcomes | Not known |
| Notes | Conference abstract. Study authors did not reply to queries. |
Huang 2015.
| Methods | RCT |
| Participants | 60 participants with poststroke cognitive dysfunction |
| Interventions | Computer‐based attention training with acupuncture |
| Outcomes | Not known |
| Notes | Protocol published, last update on ClinicalTrials.gov (NCT02324959) in December 2014 |
Kaur 2016.
| Methods | RCT |
| Participants | 22 participants with aphasia after stroke |
| Interventions | 8‐week home‐based neuropsychological programme |
| Outcomes | Improvement in attention, depression, and QoL in the intervention group compared to the control group |
| Notes | Conference abstract. Study authors did not reply to queries. |
Matz 2007.
| Methods | RCT |
| Participants | 32 participants |
| Interventions | 'Cognitive training sessions' guided by a neuropsychologist over a 3‐month period |
| Outcomes | No training effect for assessed cognitive functions |
| Notes | Authors contacted twice in previous update, but no replies to queries. |
Oh 2017.
| Methods | Quasi‐experimental study with a non‐equivalent control group |
| Participants | 42 participants with stroke |
| Interventions | 4‐week cognitive training programme |
| Outcomes | Experimental group showed improvement in attention, visuo‐spatial function, verbal memory, and executive function compared to the control group. |
| Notes | Study authors did not reply to queries. |
Peers 2018.
| Methods | RCT |
| Participants | People with stroke |
| Interventions | 20 days of cognitive training using either novel Selective Attention Training (SAT) or commercial Working Memory Training (WMT) |
| Outcomes | Not known |
| Notes | Conference abstract, recruiting completed according to ISRCTN59754564. Authors replied that study is not yet published. |
Zhang 2016.
| Methods | RCT |
| Participants | 47 participants with attention deficits |
| Interventions | 6 weeks' computer‐assisted training or face‐to‐face training |
| Outcomes | After 6‐week training, the performance of attention was improved significantly in computer‐assisted training group and face‐to‐face training group. |
| Notes | Conference abstract. Not able to locate authors' contact details |
Zhu 2011.
| Methods | Not known |
| Participants | 46 participants with cognitive dysfunction and depressive symptoms |
| Interventions | 5 weeks' computer‐assisted training |
| Outcomes | Not known |
| Notes | Not able to locate authors contact details. |
QoL: quality of life; RCT: randomised controlled trial.
Characteristics of ongoing studies [ordered by study ID]
Donnellan 2014.
| Trial name or title | Multidisciplinary Clinical Rehabilitation REsources And LIfe Strategy Management (REALISM) trial |
| Methods | RCT |
| Participants | Stroke survivors |
| Interventions | Goal setting and attainment care plan based on the adaptive strategies selection, optimisation, and compensation |
| Outcomes | Meta‐cognition Questionnaire, Self‐regulatory Interview, and executive function using the Trail Making Test (A & B). |
| Starting date | Not known |
| Contact information | Dr Claire Donnellan (cdonnel@tcd.ie) |
| Notes | Authors confirmed that there are no outcomes as yet. |
NCT02925637.
| Trial name or title | Effectiveness of FACoT for individuals post stroke |
| Methods | RCT |
| Participants | 46 participants (at least 3 years' poststroke) |
| Interventions | Meta‐cognitive‐functional Intervention |
| Outcomes | Canadian Occupational Performance Measure, Instrumental Activities of Daily Living |
| Starting date | April 2016 |
| Contact information | Tal Adamit (adamit33@bezeqint.net) |
| Notes | NCT02925637 |
RCT: randomised controlled trial.
Differences between protocol and review
This is an update of an earlier review and there was no published protocol.
Contributions of authors
TL and RdN are the guarantors of this update. They were involved in conceiving and designing the update, screening retrieved papers against the inclusion criteria; analysing and interpreting the data; and writing the update. TL was also involved in writing to study authors for additional information and managing and entering data into Review Manager 5 (Review Manager 2014).
KJP was involved in conceiving and designing the update; undertaking the searches; screening the search results; organising retrieval of papers; screening retrieved papers against the inclusion criteria; writing to study authors for additional information; managing and entering data into Review Manager 5 (Review Manager 2014); analysing and interpreting the data; and writing the update.
DW was involved in conceiving and designing the update; screening retrieved papers against inclusion criteria; analysing and interpreting the data; and writing the update.
Sources of support
Internal sources
No sources of support supplied
External sources
National Institute for Health Research (NIHR), UK.
-
National Health and Medical Research Council, Australia.
GNT1136269
Declarations of interest
TL: none.
KJM: none.
DW: none.
RdN: none.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Barker‐Collo 2009 {published and unpublished data}
- Barker‐Collo SL, Feigin VL, Lawes CM, Parag V, Senior H, Rodgers A. Reducing attention deficits after stroke using attention process training: a randomized controlled trial. Stroke 2009;40:3293‐8. [DOI] [PubMed] [Google Scholar]
Röhring 2004 {published and unpublished data}
- Röhring S, Kulke H, Reulbach U, Peetz H, Schupp W. Effectivity of a neuropsychological training in attention functions by a teletherapeutic setting. Neurologie und Rehabilitation 2004;10(5):239‐46. [Google Scholar]
Schottke 1997 {published and unpublished data}
- Schottke H. Rehabilitation of attention deficits after stroke – efficacy of a Neuropsychological training program for attention deficits [Rehabilitation von Aufmerksamkeitsstörungen nach einem Schlaganfall – Effektivität eines verhaltensmedizinisch‐neuropsychologischen Aufmerksamkeitstrainings]. Verhaltenstherapie 1997;7:21‐3. [Google Scholar]
Sturm 1991 {published and unpublished data}
- Sturm W, Willmes K. Efficacy of a reaction training on various attentional and cognitive functions in stroke patients. Neuropsychological Rehabilitation 1991;1:259‐80. [Google Scholar]
Westerberg 2007 {published and unpublished data}
- Westerberg H, Jacobaeus H, Hirvikoski T, Clevberger P, Ostensson ML, Bartfai A, et al. Computerized working memory training after stroke – a pilot study. Brain Injury 2007;21(1):21‐9. [DOI] [PubMed] [Google Scholar]
Winkens 2009 {published and unpublished data}
- Winkens I, Heugten CM, Wade DT, Habets EJ, Fasotti L. Efficacy of time pressure management in stroke patients with slowed information processing: a randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2009;90(10):1672‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aben 2013 {published data only}
- Aben L, Heijenbrok‐Kal MH, Loon EM, Groet E, Ponds RW, Busschbach JJ, et al. Training memory self‐efficacy in the chronic stage after stroke: a randomized controlled trial. Neurorehabilitation and Neural Repair 2013;27(2):110‐7. [DOI] [PubMed] [Google Scholar]
Aivazian 1994 {published data only}
- Aivazian TA, Zaitsev VP, Taravkova IA, Gorbunov FE, Maslovskaia SG, Strelnikov AV. The relaxation psychotherapy of patients with cerebrovascular diseases: its efficacy and predictors. Voprosy Kurortologii, Fizioterapii, i Lechebnoi Fizicheskoi Kultury 1994;2:2‐4. [PubMed] [Google Scholar]
Akinwuntan 2010 {published data only}
- Akinwuntan AE, Devos H, Verheyden G, Baten G, Kiekens C, Feys H, et al. Retraining moderately impaired stroke survivors in driving‐related visual attention skills. Topics in Stroke Rehabilitation 2010;17:328‐36. [DOI] [PubMed] [Google Scholar]
Albrittain‐Ross 2017 {published data only}
- Albrittain‐Ross N, Ross K, Gilmore N, Kiran S. Intensive cognitive communication rehabilitation (ICCR) program for young adults with acquired brain injury. Archives of Physical Medicine and Rehabilitation 2017;98(10):e76. [Google Scholar]
Alexopoulos 2012 {published data only}
- Alexopoulos GS, Wilkins VM, Marino P, Kanellopoulos D, Reding M, Sirey JA, et al. Ecosystem focused therapy in poststroke depression: a preliminary study. International Journal of Geriatric Psychiatry 2012;27(10):1053‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Arnemann 2015 {published data only}
- Arnemann KL, Chen AJ, Novakovic‐Agopian T, Gratton C, Nomura EM, D'Esposito M. Functional brain network modularity predicts response to cognitive training after brain injury. Neurology 2015;84(15):1568‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bergquist 2012 {published data only}
- Bergquist T, Yutsis M. The effect of cognitive rehabilitation delivered via instant messaging on functional independence in persons with acquired brain injury. Brain Injury 2012; Vol. 26, issue 4‐5:700‐1.
Bertens 2015 {published data only}
- Bertens D, Kessels RP, Fiorenzato E, Boelen DH, Fasotti L. Do old errors always lead to new truths? A randomized controlled trial of errorless goal management training in brain‐injured patients. Journal of the International Neuropsychological Society 2015;21(8):639‐49. [DOI] [PubMed] [Google Scholar]
Bezdenezhnykh 2015 {published data only}
- Bezdenezhnykh A, Prokopenko S, Mozheiko E, Petrova M, Grippa E, Lipatnikova S, et al. Neuropsychological computer training versus entertaining computer games in patients with post‐stroke cognitive impairments: randomized clinical study. International Journal of Stroke 2015; Vol. 10 Suppl 2:412.
Blanchet 2016 {published data only}
- Blanchet S, Richards CL, Leblond J, Olivier C, Maltais DB. Cardiorespiratory fitness and cognitive functioning following short‐term interventions in chronic stroke survivors with cognitive impairment: a pilot study. International Journal of Rehabilitation Research 2016;39(2):153‐9. [DOI] [PubMed] [Google Scholar]
Bo 2019 {published data only}
- Bo W, Lei M, Tao S, Jie LT, Qian L, Lin FQ, et al. Effects of combined intervention of physical exercise and cognitive training on cognitive function in stroke survivors with vascular cognitive impairment: a randomized controlled trial. Clinical Rehabilitation 2019;33(1):54‐63. [DOI] [PubMed] [Google Scholar]
Boman 2004 {published data only}
- Boman IL, Lindstedt M, Hemmingsson H, Bartfai A. Cognitive training in home environment. Brain Injury 2004;18(10):985‐95. [DOI] [PubMed] [Google Scholar]
Carelli 2009 {published data only}
- Carelli L, Morganti F, Poletti B, Corra B, Weiss PL, Kizony R, et al. A NeuroVR based tool for cognitive assessment and rehabilitation of post‐stroke patients: two case studies. Annual Review of CyberTherapy and Telemedicine 2009;7(1):243‐7. [PubMed] [Google Scholar]
Carr 2012 {published data only}
- Carr B, Allaous J, Lannin N, MacKenzie B, Falcon A, Tate R, et al. Efficacy of using handheld computers plus occupational therapy training to compensate for memory and planning difficulties after brain injury: a randomised control trial. Neurorehabilitation and Neural Repair 2012; Vol. 26, issue 6:687.
Chen 2011 {published data only}
- Chen AJ, Novakovic‐Agopian T, Nycum TJ, Song S, Turner GR, Hills NK, et al. Training of goal‐directed attention regulation enhances control over neural processing for individuals with brain injury. Brain 2011;134:1541‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2015 {published data only}
- Chen C‐X, Mao R‐H, Li S‐X, Zhao Y‐N, Zhang M. Effect of visual training on cognitive function in stroke patients. International Journal of Nursing Sciences 2015;2(4):329‐33. [Google Scholar]
Cho 2016 {published data only}
- Cho HY, Kim KT, Jung JH. Effects of neurofeedback and computer‐assisted cognitive rehabilitation on relative brain wave ratios and activities of daily living of stroke patients: a randomized control trial. Journal of Physical Therapy Science 2016;28(7):2154‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chung 2013 {published data only}
- Chung C, Pollock A, Campbell T, Durward B, Hagen S. Cognitive rehabilitation for executive dysfunction in adults with stroke or other adult nonprogressive acquired brain damage. Stroke 2013;44:e77‐8. [DOI] [PubMed] [Google Scholar]
Crotty 2009 {published data only}
- Crotty M, George S. Retraining visual processing skills to improve driving ability after stroke. Archives of Physical Medicine and Rehabilitation 2009;90:2096‐102. [DOI] [PubMed] [Google Scholar]
De Luca 2014 {published data only}
- Luca R, Calabro RS, Gervasi G, Salvo S, Bonanno L, Corallo F, et al. Is computer‐assisted training effective in improving rehabilitative outcomes after brain injury? A case‐control hospital‐based study. Disability and Health Journal 2014;7(3):356‐60. [DOI] [PubMed] [Google Scholar]
De Luca 2018a {published data only}
- Luca R, Leonardi S, Spadaro L, Russo M, Aragona B, Torrisi M, et al. Improving cognitive function in patients with stroke: can computerized training be the future?. Journal of Stroke and Cerebrovascular Diseases 2018;27(4):1055‐60. [DOI] [PubMed] [Google Scholar]
De Luca 2018b {published data only}
- Luca R, Russo M, Naro A, Tomasello P, Leonardi S, Santamaria F, et al. Effects of virtual reality‐based training with BTs‐Nirvana on functional recovery in stroke patients: preliminary considerations. International Journal of Neuroscience 2018;128(9):791‐6. [DOI] [PubMed] [Google Scholar]
Dirette 2004 {published data only}
- Dirette D. A comparison of attention, processing and strategy use by adults with and without acquired brain injuries. Brain Injury 2004;12:1219‐27. [DOI] [PubMed] [Google Scholar]
Edmans 2009 {published data only}
- Edmans J, Gladman J, Hilton D, Walker M, Sunderland A, Cobb S, et al. Clinical evaluation of a non‐immersive virtual environment in stroke rehabilitation. Clinical Rehabilitation 2009;23(2):106‐16. [DOI] [PubMed] [Google Scholar]
Faria 2016 {published data only}
- Faria AL, Andrade A, Soares L, i Badia SB. Benefits of virtual reality based cognitive rehabilitation through simulated activities of daily living: a randomized controlled trial with stroke patients. Journal of Neuroengineering and Rehabilitation 2016;13:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gates 2011 {published data only}
- Gates NJ, Valenzuela M, Sachdev PS, Singh NA, Baune BT, Brodaty H, et al. Study of Mental Activity and Regular Training (SMART) in at risk individuals: a randomised double blind, sham controlled, longitudinal trial. BMC Geriatrics 2011;11(19):1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gauggel 1996 {published data only}
- Gauggel S, Niemann T. Evaluation of a short‐term computer‐assisted training programme for the remediation of attentional deficits after brain injury: a preliminary study. International Journal of Rehabilitation Research 1996;19(3):229‐39. [DOI] [PubMed] [Google Scholar]
Giaquinto 2003 {published data only}
- Giaquinto S, Fraioli L. Enhancement of the somatosensory N140 component during attentional training after stroke. Clinical Neurophysiology 2003;114(2):329‐35. [DOI] [PubMed] [Google Scholar]
Gracey 2017 {published data only}
- Gracey F, Fish JE, Greenfield E, Bateman A, Malley D, Hardy G, et al. A randomized controlled trial of assisted intention monitoring for the rehabilitation of executive impairments following acquired brain injury. Neurorehabilitation and Neural Repair 2017;31(4):323‐33. [DOI] [PubMed] [Google Scholar]
Graf 2011 {published data only}
- Graf J, Kulke H, Sous‐Kulke C, Schupp W, Lautenbacher S. Effects of an attention training on the aphasic symptomatology in stroke patients. Zeitschrift fur Neuropsychologie 2011;22(1):21‐32. [Google Scholar]
Gray 1992 {published data only}
- Gray JM, Robertson I, Pentland B, Anderson S. Microcomputer‐based attentional retraining after brain damage: a randomised group controlled trial. Neuropsychological Rehabilitation 1992;2(2):97‐115. [Google Scholar]
Hashimoto 2006 {published data only}
- Hashimoto K, Okamoto T, Watanabe S, Ohashi M. Effectiveness of a comprehensive day treatment program for rehabilitation of patients with acquired brain injury in Japan. Journal of Rehabilitation Medicine 2006;38(1):20‐5. [DOI] [PubMed] [Google Scholar]
Jiang 2016 {published data only}
- Jiang C, Yang S, Tao J, Huang J, Li Y, Ye H, et al. Clinical efficacy of acupuncture treatment in combination with RehaCom cognitive training for improving cognitive function in stroke: a 2 × 2 factorial design randomized controlled trial. Journal of the American Directors Association 2016;17(12):1114‐22. [DOI] [PubMed] [Google Scholar]
Kang 2008 {published data only}
- Kang YJ, Ku J, Han K, Kim SI, Yu TW, Lee JH, et al. Development and clinical trial of virtual reality‐based cognitive assessment in people with stroke: preliminary study. Cyberpsychology & Behavior 2008;11(3):329‐39. [DOI] [PubMed] [Google Scholar]
Kang 2009 {published data only}
- Kang EK, Baek MJ, Kim S, Paik NJ. Non‐invasive cortical stimulation improves post‐stroke attention decline. Restorative Neurology and Neuroscience 2009;27(6):645‐50. [DOI] [PubMed] [Google Scholar]
Kaur 2018 {published data only}
- Kaur H, Kumaran S, Bhatia R, Pandey RM, Chopra S, Nehra A. Non‐linguistic cognitive impairment and its relationship with language recovery post aphasia rehabilitation: a neuropsychological and fMRI study. International Journal of Stroke 2018;13 Suppl 2:61. [Google Scholar]
Kim 2008 {published data only}
- Kim YK, Seo CH, Park JW, Lee HJ, Shin SH. Effects of cognitive rehabilitative treatment using computerized program (RehaCom) in stroke patients. Neurorehabilitation and Neural Repair 2008;22(5):569. [Google Scholar]
Kim 2013 {published data only}
- Kim D, Ko J, Woo Y. Effects of dual task training with visual restriction and an unstable base on the balance and attention of stroke patients. Journal of Physical Therapy Science 2013;25(12):1579‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Klonoff 2010 {published data only}
- Klonoff PS, Olson KC, Talley MC, Husk KL, Myles SM, Gehrels JA, et al. The relationship of cognitive retraining to neurological patients' driving status: the role of process variables and compensation training. Brain Injury 2010;24(2):63‐73. [DOI] [PubMed] [Google Scholar]
Levine 2011 {published data only}
- Levine B, Schweizer TA, O'Connor C, Turner G, Gillingham S, Stuss DT, et al. Rehabilitation of executive functioning in patients with frontal lobe brain damage with goal management training. Frontiers in Human Neuroscience 2011;5(9):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mazer 2003 {published data only}
- Mazer BL, Sofer S, Korner‐Bitensky N, Gelinas I, Hanley J, Wood‐Dauphinee S. Effectiveness of a visual attention retraining program on the driving performance of clients with stroke. Archives Physical Medicine and Rehabilitation 2003;84:541‐50. [DOI] [PubMed] [Google Scholar]
McDonnell 2007 {published data only}
- McDonnell MN, Hillier SL, Miles TS, Thompson PD, Ridding MC. Influence of combined afferent stimulation and task‐specific training following stroke: a pilot randomized controlled trial. Neurorehabilitation & Neural Repair 2007;21(5):435‐43. [DOI] [PubMed] [Google Scholar]
Mead 2007 {published data only}
- Mead GE, Greig CA, Cunningham I, Lewis SJ, Dinn S, Saunders DH, et al. Stroke: a randomized trial of exercise or relaxation. Journal of the American Geriatrics Society 2007;55(6):892‐9. [DOI] [PubMed] [Google Scholar]
Middleton 1991 {published data only}
- Middleton DK, Lambert MJ, Seggar LB. Neuropsychological rehabilitation: microcomputer‐assisted treatment of brain‐injured adults. Perceptual and Motor Skills 1991;72(2):527‐30. [DOI] [PubMed] [Google Scholar]
Miotto 2009 {published data only}
- Miotto EC, Evans JJ, Lucia MC, Scaff M. Rehabilitation of executive dysfunction: a controlled trial of an attention and problem solving treatment group. Neuropsychological Rehabilitation 2009;19(4):517‐40. [DOI] [PubMed] [Google Scholar]
NCT00298558 {published data only}
- NCT00298558. ACTIVE: advanced cognitive training for independent and vital elderly. clinicaltrials.gov/ct2/show/NCT00298558 (first received 2 March 2006).
NCT01109836 {published data only}
- NCT01109836. Austrian Polyintervention Study to Prevent Cognitive Decline After Ischemic Stroke (ASPIS). clinicaltrials.gov/ct2/show/NCT01109836 (first received 23 April 2010).
NCT01365858 {published data only}
- NCT01365858. Virtual Action Planning in Stroke: a control rehabilitation study (VAPS REHAB). clinicaltrials.gov/ct2/show/NCT01365858 (first received 3 June 2011).
NCT01372059 {published data only}
- NCT01372059. The effects of a rhythm and music‐based therapy program and therapeutic riding in late recovery phase following stroke. clinicaltrials.gov/ct2/show/NCT01372059 (first received 13 June 2011).
NCT01886183 {published data only}
- NCT01886183. Effects of training in a virtual environment in chronic stroke patients. clinicaltrials.gov/ct2/show/NCT01886183 (first received 25 June 2013).
NCT02482688 {published data only}
- NCT02482688. A new rehabilitation treatment following stroke. clinicaltrials.gov/ct2/show/NCT02482688 (first received 26 June 2015).
Otfinowski 2006 {published data only}
- Otfinowski J, Jasiak‐Tyrkalska B, Starowicz A, Regula K. Computer‐based rehabilitation of cognitive impairments and motor arm function of patients with hemiparesis after stroke. Neurologia i Neurochirurgia Polska 2006;40:112‐8. [PubMed] [Google Scholar]
Park 2015 {published data only}
- Park J‐H. The effects of a Korean computer‐based cognitive rehabilitation program on cognitive function and visual perception ability of patients with acute stroke. Journal of Physical Therapy Science 2015;27(8):2577‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Park 2018 {published data only}
- Park MO, Lee SH. Effects of cognitive‐motor dual‐task training combined with auditory motor synchronization training on cognitive functioning in individuals with chronic stroke: a pilot randomized controlled trial. Medicine 2018;97(22):e10910. [DOI] [PMC free article] [PubMed] [Google Scholar]
Piccardi 2006 {published data only}
- Piccardi L, Nico D, Bureca I, Matano A, Guariglia C. Efficacy of visuo‐spatial training in right‐brain damaged patients with spatial hemineglect and attention disorders. Cortex 2006;42(7):973‐82. [DOI] [PubMed] [Google Scholar]
Ploughman 2008 {published data only}
- Ploughman M, McCarthy J, Bosse M, Sullivan HJ, Corbett D. Does treadmill exercise improve performance of cognitive or upper‐extremity tasks in people with chronic stroke? A randomized cross‐over trial. Archives of Physical Medicine and Rehabilitation 2008;89(11):2041‐7. [DOI] [PubMed] [Google Scholar]
Ploughman 2017 {published data only}
- Ploughman M, Eskes GA, Kelly LP, Kirkland MC, Devasayaham AJ, Wallack EM, et al. Aerobic exercise enhances the beneficial effects of cognitive training and reopens the 'window of recovery' in chronic stroke via neurotrophins. International Journal of Stroke 2017; Vol. 12 Suppl 4:18‐9.
Prokopenko 2013 {published data only}
- Prokopenko SV, Mozheyko EY, Petrova MM, Koryagina TD, Kaskaeva DS, Chernykh TV, et al. Correction of post‐stroke cognitive impairments using computer programs. Journal of the Neurological Sciences 2013;325:148‐53. [DOI] [PubMed] [Google Scholar]
Pulin 2017 {published data only}
- Pulin V, Korner‐Bitensky N, Bherer L, Lussier M, Dawson DR. Comparison of two cognitive interventions for adults experiencing executive dysfunction post‐stroke: a pilot study. Disability and Rehabilitation 2017;39(1):1‐13. [DOI] [PubMed] [Google Scholar]
Pyoria 2007 {published data only}
- Pyoria O, Talvitie U, Nyrkko H, Kautiainen H, Pohjolainen T, Kasper V. The effect of two physiotherapy approaches on physical and cognitive functions and independent coping at home in stroke rehabilitation. A preliminary follow‐up study. Disability & Rehabilitation 2007;29(6):503‐11. [DOI] [PubMed] [Google Scholar]
Pyun 2009 {published data only}
- Pyun SB, Yang H, Lee S, Yook J, Kwon J, Byun EM. A home programme for patients with cognitive dysfunction: a pilot study. Brain Injury 2009;23:686‐92. [DOI] [PubMed] [Google Scholar]
Quaney 2009 {published data only}
- Quaney BM, Boyd LA, McDowd JM, Zahner LH, Jianghua H, Mayo MS, et al. Aerobic exercise improves cognition and motor function poststroke. Neurorehabilitation and Neural Repair 2009;23(9):879‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ressner 2018 {published data only}
- Ressner P, Krulová P, Beránková D, Nilius P, Bártová P, Zapletalová J, et al. Effect of a combined approach to cognitive rehabilitation in post‐stroke patients. Česká a Slovenská Neurologie a Neurochirurgie 2018;81(3):314‐9. [Google Scholar]
Rizkalla 2011 {published data only}
- Rizkalla MN, Tippett WJ. Examining a visuospatial/visuomotor training program as an intervention to induce a cognitive change during acute post‐stroke recovery. Stroke 2011;42(11):e618. [Google Scholar]
Särkämö 2008 {published data only}
- Särkämö T, Tervaniemi M, Laitinen S, Forsblom A, Soinila S, Mikkonen M, et al. Music listening enhances cognitive recovery and mood after middle cerebral artery stroke. Brain 2008;131:866‐76. [DOI] [PubMed] [Google Scholar]
Särkämö 2010 {published data only}
- Särkämö T, Pihko E, Laitinen S, Forsblom A, Soinila S, Mikkonen M, et al. Music and speech listening enhance the recovery of early sensory processing after stroke. Journal of Cognitive Neuroscience 2010;22:2716‐27. [DOI] [PubMed] [Google Scholar]
Shi 2017 {published data only}
- Shi Y, Zhang Z. Effect of comprehensive rehabilitation training on prevention of post‐stroke dementia: a randomized controlled trial. International Journal of Clinical and Experimental Medicine 2017;10(5):7760‐6. [Google Scholar]
Shiflett 2002 {published data only}
- Shiflett SC, Nayak S, Bid C, Miles P, Agostinelli S. Effect of Reiki treatments on functional recovery in patients in poststroke rehabilitation: a pilot study. Journal of Alternative and Complementary Medicine 2002;8(6):755‐63. [DOI] [PubMed] [Google Scholar]
Skidmore 2014 {published data only}
- Skidmore ER, Dawson DR, Whyte EM, Butters MA, Dew MA, Grattan ES, et al. Developing complex interventions: lessons learned from a pilot study examining strategy training in acute stroke rehabilitation. Clinical Rehabilitation 2014;28(4):378‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Skidmore 2015 {published data only}
- Skidmore ER, Dawson DR, Butters MA, Grattan ES, Juengst SB, Whyte EM, et al. Strategy training shows promise for addressing disability in the first 6 months after stroke. Neurorehabilitation and Neural Repair 2015;29(7):668‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sohlberg 2000 {published data only}
- Sohlberg MM, McLaughlin KA, Pavese A, Heidrich A, Posner MI. Evaluation of attention process training and brain injury education in persons with acquired brain injury. Journal of Clinical and Experimental Neuropsychology 2000;22(5):656‐76. [DOI] [PubMed] [Google Scholar]
Spahn 2010 {published data only}
- Spahn V, Kulke H, Kunz M, Thone‐Otto A, Schupp W, Lautenbacher S. Is the neuropsychological treatment of memory specific or unspecific? Comparing treatment effects on memory and attention. Zeitschrift fur Neuropsychologie 2010;21(4):239‐45. [Google Scholar]
Spikman 2010 {published data only}
- Spikman JM, Boelen DH, Lamberts KF, Brouwer WH, Fasotti L. Effects of a multifaceted treatment program for executive dysfunction after acquired brain injury on indications of executive functioning in daily life. Journal of the International Neuropsychological Society 2010;16:118‐29. [DOI] [PubMed] [Google Scholar]
Stapleton 2001 {published data only}
- Stapleton T, Ashburn A, Stack E. A pilot study of attention deficits, balance control and falls in the subacute stage following stroke. Clinical Rehabilitation 2001;15(4):437‐44. [DOI] [PubMed] [Google Scholar]
Sturm 1997 {published data only}
- Sturm W, Willmes K, Orgass B, Hartje W. Do specific attention deficits need specific training?. Neuropsychological Rehabilitation 1997;7(2):81‐103. [Google Scholar]
Sturm 2006 {published data only}
- Sturm W, Thimm A, Küst J, Karbe H, Fink GR. Alertness‐training in neglect: behavioral and imaging results. Restorative Neurology and Neuroscience 2006;24:371‐84. [PubMed] [Google Scholar]
Thimm 2006 {published data only}
- Thimm M, Fink GR, Küst J, Karbe H, Sturm W. Impact of alertness training on spatial neglect: a behavioural and fMRI study. Neuropsychologia 2006;44(7):1230‐46. [DOI] [PubMed] [Google Scholar]
Tornas 2014 {published data only}
- Tornas S, Lovstad M, Solbakk A‐K, Host K, Schanke A‐K, Stubberud J. Goal management training in patients with acquired brain injury – preliminary results. Brain Injury 2014;28(5‐6):569‐70. [Google Scholar]
Van de Ven 2017 {published data only}
- Ven RM, Buitenweg JI, Schmand B, Veltman DJ, Aaronson JA, Nijboer TC, et al. Brain training improves recovery after stroke but waiting list improves equally: a multicenter randomized controlled trial of a computer‐based cognitive flexibility training. PloS One 2017;12(3):e0172993. [DOI] [PMC free article] [PubMed] [Google Scholar]
Villalobos 2018 {published data only}
- Villalobos D, Bilbao Á, Espejo A, García‐Pacios J. Efficacy of an intervention programme for rehabilitation of awareness of deficit after acquired brain injury: a pilot study. Brain Injury 2018;32(2):158‐66. [DOI] [PubMed] [Google Scholar]
Von Koch 2000 {published data only}
- Koch L, Holmqvist LW, Wottrich AW, Tham K, Pedro‐Cuesta J. Rehabilitation at home after stroke: a descriptive study of an individualized intervention. Clinical Rehabilitation 2000;14(6):574‐83. [DOI] [PubMed] [Google Scholar]
Wagenaar 1992 {published data only}
- Wagenaar RC, Wieringen PC, Netelenbos JB, Meijer OG, Kuik DJ. The transfer of scanning training effects in visual inattention after stroke: five single‐case studies. Disability & Rehabilitation 1992;14(1):51‐60. [DOI] [PubMed] [Google Scholar]
Wentink 2016 {published data only}
- Wentink MM, Berger MA, Kloet AJ, Meesters J, Band GP, Wolterbeek R, et al. The effects of an 8‐week computer‐based brain training programme on cognitive functioning, QoL and self‐efficacy after stroke. Neuropsychological Rehabilitation 2016;26:847‐65. [DOI] [PubMed] [Google Scholar]
Yamamoto‐Mitani 2007 {published data only}
- Yamamoto‐Mitani N, Matsuoka K, Fujii M. Home‐based rehabilitation program for older adults with cognitive impairment: preliminary results. Psychogeriatrics 2007;7(1):14‐20. [Google Scholar]
Zucchella 2014 {published data only}
- Zucchella C, Capone A, Codella V, Vecchione C, Buccino G, Sandrini G, et al. Assessing and restoring cognitive functions early after stroke. Functional Neurology 2014;29(4):255‐62. [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Bartfai 2014 {published data only}
- Bartfai A, Markovic G, Sargenius Landahl K, Schult ML. The protocol and design of a randomised controlled study on training of attention within the first year after acquired brain injury. BMC Neurology 2014;14:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bayat 2014 {published data only}
- Bayat G, Dastgheib S, Shoeibi A. The impact of program based computer software versus Mozart's music on hemiparetic patients using magnetic resonance spectroscopy: a randomized control trial study. International Journal of Stroke 2014; Vol. 9 Suppl 3:218.
Bushnick 2010 {published data only}
- Bushnick T, Englander J, Oggins J, Levinson R, Halper D. A randomized clinical trial of a cognitive orthotic with executive planning capability in individuals with cognitive dysfunction. Brain Injury 2010;24(3):392‐3. [Google Scholar]
Chen 2018 {published data only}
- Chen BY, Aldhukair H, Cote J, Flinois J, Hoang T, Shen HC, et al. Early tablet‐assisted cognitive rehabilitation for aneurysmal subarachnoid hemorrhage: feasibility of a single‐center randomized controlled trial. Neurology 2018;90 Suppl 1:P3.227. [Google Scholar]
Dawson 2017 {published data only}
- Dawson D, Bar Y, McEwen S, Skidmore E, Nalder E, Anderson N, et al. Enhancing participation in everyday life for people with stroke via telerehabilitation: a randomized controlled trial. International Journal of Stroke 2017; Vol. 12 Suppl 4:87.
Flynn 2000 {published data only}
- Flynn A. Investigation into whether psychology led cognitive rehabilitation of attentional and visio‐spatial skills improves performance in these areas beyond spontaneous recovery. National Research Register (N0280012930).
Frommelt 2003 {published data only}
- Frommelt P. Neurophysiological rehabilitation: modular cognitive retraining versus compensatory skills. www.isrctn.com/ISRCTN45171788 (first received 1 August 2003). [ISRCTN45171788]
Gil‐Pages 2018 {published data only}
- Gil‐Pages M, Solana J, Sanchez‐Carrion R, Tormos JM, Ensenat‐Cantallops A, Garcia‐Molina A. A customized home‐based computerized cognitive rehabilitation platform for patients with chronic‐stage stroke: study protocol for a randomized controlled trial. Trials 2018;19:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
Holleman 2012 {published data only}
- Holleman M, Vink M, Horst R, Luijpen M, Rienstra A, Schmand B. Effects of a holistic neuropsychological rehabilitation programme (Intensive NeuroRehabilitation, INR) on emotional well‐being, coping, and quality of life after acquired brain injury. Brain Impairment 2012;13(1):136‐7. [Google Scholar]
Huang 2015 {published data only}
- Huang J, McCaskey MA, Yang S, Ye H, Tao J, Jiang C, et al. Effects of acupuncture and computer‐assisted cognitive training for post‐stroke attention deficits: study protocol for a randomized controlled trial. Trials 2015;16:546. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaur 2016 {published data only}
- Kaur H, Bhatia R, Pandey RM, Chopra S, Nehra A. Effectiveness of using a home‐based comprehensive neuropsychological rehabilitation with aphasia therapy in patients with post stroke aphasia: a preliminary study. International Journal of Stroke 2016;11 Suppl 3:228. [Google Scholar]
Matz 2007 {published data only}
- Matz K, Teuschl Y, Eckhardt R, Herbst A, Dachenhausen A, Brainin M. Cognitive training in patients with first lacunar stroke – a randomized pilot trial for the prevention of post‐stroke cognitive decline. Cerebrovascular Diseases 2007;23 Suppl 2:42. [Google Scholar]
Oh 2017 {published data only}
- Oh EY, Jung MS. Effects of a cognitive training program on cognitive function and activities of daily living in patients with acute ischemic stroke. Journal of Korean Academy of Nursing 2017;47(1):1‐13. [DOI] [PubMed] [Google Scholar]
Peers 2018 {published data only}
- Peers PV, Bishop S, Murphy F, Duncan J, Astle DE, Watson P, et al. Home‐based cognitive training in stroke: implications for cognition and everyday functioning. International Journal of Stroke 2018;13 Suppl 3:51. [Google Scholar]
Zhang 2016 {published data only}
- Zhang H, Yun X, Zhang X, Shen M, Pan H, Zhang Y, et al. Efficacy of rehabilitation for impairments of attention. Archives of Physical Medicine and Rehabilitation 2016;97(10):e131‐2. [Google Scholar]
Zhu 2011 {published data only}
- Zhu L, Song WQ, Yue YH, Liu L. Effect of computer‐assisted cognitive training on the cognitive function and depression in patients with brain injury. Chinese Journal of Cerebrovascular Diseases 2011;8(10):508‐12. [Google Scholar]
References to ongoing studies
Donnellan 2014 {published data only}
- Donnellan C. REsources And LIfe Strategy Management (REALISM) trial: protocol for a stroke rehabilitation intervention using a goal setting and attainment framework. International Journal of Stroke 2014; Vol. 9 Suppl 3:221.
NCT02925637 {published data only}
- NCT02925637. Effectiveness of FACoT for individuals post stroke. clinicaltrials.gov/ct2/show/NCT02925637 (first received 6 October 2016).
Additional references
Barker‐Collo 2006
- Barker‐Collo S, Feigin V. The impact of neuropsychological deficits on functional stroke outcomes. Neuropsychology Review 2006;16:53‐64. [DOI] [PubMed] [Google Scholar]
Barker‐Collo 2010
- Barker‐Collo S, Feigin VL, Parag V, Lawes CMM, Senior H. Auckland Stroke Outcomes Study Part 2: cognition and functional outcomes 5 years poststroke. Neurology 2010;75:1608‐18. [DOI] [PubMed] [Google Scholar]
Ben‐Yishay 1990
- Ben‐Yishay Y, Prigatano GP. Cognitive remediation. In: Rosenthal M, Griffith ER, Bond MR, Miller JD editor(s). Rehabilitation of the Adult and Child with Traumatic Brain Injury. 2nd Edition. Philadelphia (PA): F.A. Davis, 1990:393‐409. [Google Scholar]
Bernhardt 2019
- Bernhardt J, Borschmann KN, Kwakkel G, Burridge JH, Eng JJ, Walker MF, et al. Setting the scene for the Second Stroke Recovery and Rehabilitation Roundtable. International Journal of Stroke 2019;14(5):450‐6. [DOI] [PubMed] [Google Scholar]
Bogdanova 2016
- Bogdanova Y, Yee MK, Ho VT, Cicerone KD. Computerized cognitive rehabilitation of attention and executive function in acquired brain injury: a systematic review. Journal of Head Trauma Rehabilitation 2016;31(6):419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bowen 2013
- Bowen A, Hazelton C, Pollock A, Lincoln NB. Cognitive rehabilitation for spatial neglect following stroke. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD003586.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Broadbent 1982
- Broadbent DE, Cooper PF, FitzGerald P, Parkes KR. The Cognitive Failures Questionnaire (CFQ) and its correlates. British Journal of Clinical Psychology 1982;21:1‐16. [DOI] [PubMed] [Google Scholar]
Cappa 2005
- Cappa SF, Benke T, Clarke S, Rossi B, Stemmer B, Heugten CM. EFNS guidelines on cognitive rehabilitation: report of an EFNS task force. European Journal of Neurology 2005;12:665‐80. [DOI] [PubMed] [Google Scholar]
Cicerone 2000
- Cicerone KD, Dahlberg C, Kalmar K, Langenbahn DM, Malec JF, Bergquist TF, et al. Evidence‐based cognitive rehabilitation: recommendations for clinical practice. Archives of Physical Medicine and Rehabilitation 2000;81:1596‐615. [DOI] [PubMed] [Google Scholar]
Cicerone 2002
- Cicerone KD. Remediation of "working attention" in mild traumatic brain injury. Brain Injury 2002;16:185‐95. [DOI] [PubMed] [Google Scholar]
Cicerone 2005
- Cicerone KD, Dahlberg C, Malec JF, Langenbahn DM, Felicetti T, Kneipp S, et al. Evidence‐based cognitive rehabilitation: updated review of the literature from 1998 through 2002. Archives of Physical and Medical Rehabilitation 2005;86:1681‐92. [DOI] [PubMed] [Google Scholar]
Cicerone 2011
- Cicerone KD, Langenbahn DM, Braden C, Malec JF, Kalmar K, Fraas M, et al. Evidence‐based cognitive rehabilitation: updated review of the literature from 2003 through 2008. Archives of Physical Medicine and Rehabilitation 2011;92:519‐30. [DOI] [PubMed] [Google Scholar]
Cicerone 2019
- Cicerone KD, Goldin Y, Ganci K, Rosenbaum A, Wethe JV, Langenbahn DM, et al. Evidence‐based cognitive rehabilitation: systematic review of the literature from 2009 through 2014. Archives of Physical Medicine and Rehabilitation 2019;100(8):1515‐33. [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Veritas Health Innovation. Covidence. Version accessed 23 January 2019. Melbourne, Australia: Veritas Health Innovation.
Duncan 2003
- Duncan PW, Bode RK, Lai SM, Perera S, Glycine Antagonist in Neuroprotection Americas Investigators. Rasch analysis of a new stroke‐specific outcome scale: the Stroke Impact Scale. Archives of Physical Medicine and Rehabilitation 2003;84(7):950‐63. [DOI] [PubMed] [Google Scholar]
Goldberg 1972
- Goldberg DP. The Detection of Psychiatric Illness by Questionnaire. London (UK): Oxford University Press, 1972. [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 19 July 2019. Hamilton (ON): McMaster University (developed by Evidence Prime).
Granger 1994
- Granger CV, Hamilton BB. The uniform data system for medical rehabilitation. Report of first admissions for 1992. American Journal of Physical Medicine and Rehabilitation 1994;73:51‐5. [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist G, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, the GRADE Working Group. Rating quality of evidence and strength of recommendations GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hochstenbach 1998
- Hochstenbach J, Mulder T, Limbeek J, Donders R, Schoonderwaldt H. Cognitive decline following stroke: a comprehensive study of cognitive decline following stroke. Journal of Clinical and Experimental Neuropsychology 1998;20:503‐17. [DOI] [PubMed] [Google Scholar]
Hochstenbach 2003
- Hochstenbach JB, Otter R, Mulder TW. Cognitive recovery after stroke: a 2‐year follow‐up. Archives of Physical and Medical Rehabilitation 2003;84:1499‐504. [DOI] [PubMed] [Google Scholar]
Hoffmann 2014
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. [DOI] [PubMed] [Google Scholar]
Hyndman 2003
- Hyndman D, Ashburn A. People with stroke living in the community: attention deficits, balance, ADL ability and falls. Disability and Rehabilitation 2003;25:817‐22. [DOI] [PubMed] [Google Scholar]
Hyndman 2008
- Hyndman D, Pickering RM, Ashburn A. The influence of attention deficits on functional recovery post stroke during the first 12 months after discharge from hospital. Journal of Neurology, Neurosurgery and Psychiatry 2008;79:656‐63. [DOI] [PubMed] [Google Scholar]
Kwa 1996
- Kwa VIH, Limburg M, deHaan RJ. The role of cognitive impairment in the quality of life after ischaemic stroke. Journal of Neurology 1996;243:599‐604. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Lezak 2004
- Lezak MD, Howieson DB, Loring DW, Hannay HJ, Fischer JS. Neuropsychological Assessment. Oxford (UK): Oxford University Press, 2004. [Google Scholar]
Mahoney 1965
- Mahoney FI, Barthel D. Functional evaluation: the Barthel Index. Maryland State Medical Journal 1965;14:56‐65. [PubMed] [Google Scholar]
McKevitt 2010
- McKevitt C, Fudge N, Redfern J, Sheldenkar A, Crichton S, Wolfe C. UK Stroke Survivor Needs Survey. London (UK): The Stroke Association, 2010. [Google Scholar]
Merriman 2019
- Merriman NA, Sexton E, McCabe G, Walsh ME, Rohde D, Gorman A, et al. Addressing cognitive impairment following stroke: systematic review and meta‐analysis of non‐randomised controlled studies of psychological interventions. BMJ Open 2019;9(2):e024429. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mitchell 2010
- Mitchell AJ, Kemp S, Benito‐Leon J, Reuber M. The influence of cognitive impairment on health‐related quality of life in neurological disease. Acta Neuropsychiatrica 2010;22:2‐13. [Google Scholar]
Nouri 1987
- Nouri FM, Lincoln NB. An extended activities of daily living scale for stroke patients. Clinical Rehabilitation 1987;1:301‐5. [Google Scholar]
Nys 2006
- Nys GM, Zandvoort MJ, Worp HB, Haan EH, Kort PL, Jansen BP, et al. Early cognitive impairment predicts long‐term depressive symptoms and quality of life after stroke. Journal of the Neurological Sciences 2006;247:149‐56. [DOI] [PubMed] [Google Scholar]
Park 2001
- Park NW, Ingles JL. Effectiveness of attention rehabilitation after an acquired brain injury: a meta‐analysis. Neuropsychology 2001;15(2):199‐210. [DOI] [PubMed] [Google Scholar]
Pollock 2012
- Pollock A, George B, Fenton M, Firkins L. Top ten research priorities relating to life after stroke. Lancet Neurology 2012;11:209. [DOI] [PubMed] [Google Scholar]
Ponsford 1991
- Ponsford J, Kinsella G. The use of a rating scale of attentional behaviour. Neuropsychological Rehabilitation 1991;1:241‐57. [DOI] [PubMed] [Google Scholar]
Rasquin 2004
- Rasquin SM, Lodder J, Ponds R, Winkens I, Jolles J, Verhey FR. Cognitive functioning after stroke: a one‐year follow‐up study. Dementia and Geriatric Cognitive Disorders 2004;18:138‐44. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Robertson 1994
- Robertson IH, Ridgeway V, Nimmo‐Smith I. Test of Everyday Attention. Bury St Edmunds (UK): Thames Valley Test Company, 1994. [Google Scholar]
Robertson 1997
- Robertson IH, Ridgeway V, Greenfield E, Parr A. Motor recovery after stroke depends on intact sustained attention: a 2‐year follow‐up study. Neuropsychology 1997;11:290‐5. [DOI] [PubMed] [Google Scholar]
Rogers 2018
- Rogers JM, Foord R, Stolwyk RJ, Wong D, Wilson PH. General and domain‐specific effectiveness of cognitive remediation after stroke: systematic literature review and meta‐analysis. Neuropsychology Review 2018;28(3):285‐309. [DOI] [PubMed] [Google Scholar]
Sohlberg 1989
- Sohlberg M, Mateer C. Introduction to Cognitive Rehabilitation: Theory and Practice. New York (NY): The Guilford Press, 1989. [Google Scholar]
Strauss 2006
- Strauss E, Sherman EM, Spreen O. A Compendium of Neuropsychological Tests. 3rd Edition. Oxford (UK): Oxford Press, 2006. [Google Scholar]
Virk 2015
- Virk S, Williams T, Brunsdon R, Suh F, Morrow A. Cognitive remediation of attention deficits following acquired brain injury: a systematic review and meta‐analysis. NeuroRehabilitation 2015;36(3):367‐77. [DOI] [PubMed] [Google Scholar]
Ware 1992
- Ware JE, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection. Medical Care 1992;30:473‐83. [PubMed] [Google Scholar]
Weicker 2016
- Weicker J, Villringer A, Thöne‐Otto A. Can impaired working memory functioning be improved by training? A meta‐analysis with a special focus on brain injured patients. Neuropsychology 2016;30(2):190. [DOI] [PubMed] [Google Scholar]
WHOQOL Group 1998
- WHOQOL Group. The World Health Organization Quality of Life Assessment (WHOQOL): development and general psychometric properties. Social Science & Medicine 1998;46:1569‐85. [DOI] [PubMed] [Google Scholar]
Whyte 2003
- Whyte J, Hart T, Bode RK, Malec JF. The Moss Attention Rating Scale for traumatic brain injury: initial psychometric assessment. Archives of Physical Medicine and Rehabilitation 2003;84:268‐76. [DOI] [PubMed] [Google Scholar]
Zeidan 2010
- Zeidan F, Johnson SK, Diamond BJ, David Z, Goolkasian P. Mindfulness meditation improves cognition: evidence of brief mental training. Consciousness and Cognition 2010;19(2):597‐605. [DOI] [PubMed] [Google Scholar]
Zigmond 1983
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica 1983;67:361‐70. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Lincoln 2000
- Lincoln NB, Majid MJ, Weyman N. Cognitive rehabilitation for attention deficits following stroke. Cochrane Database of Systematic Reviews 2000, Issue 4. [DOI: 10.1002/14651858.CD002842] [DOI] [PubMed] [Google Scholar]
Loetscher 2013
- Loetscher T, Lincoln NB. Cognitive rehabilitation for attention deficits following stroke. Cochrane Database of Systematic Reviews 2013, Issue 5. [DOI: 10.1002/14651858.CD002842.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


