ABSTRACT
The tooth provides an excellent system for deciphering the molecular mechanisms of organogenesis, and has thus been of longstanding interest to developmental and stem cell biologists studying embryonic morphogenesis and adult tissue renewal. In recent years, analyses of molecular signaling networks, together with new insights into cellular heterogeneity, have greatly improved our knowledge of the dynamic epithelial-mesenchymal interactions that take place during tooth development and homeostasis. Here, we review recent progress in the field of mammalian tooth morphogenesis and also discuss the mechanisms regulating stem cell-based dental tissue homeostasis, regeneration and repair. These exciting findings help to lay a foundation that will ultimately enable the application of fundamental research discoveries toward therapies to improve oral health.
KEY WORDS: Tooth development and homeostasis, Dental stem cell, Cell heterogeneity, Injury repair
Summary: This Review gives an overview of our current understanding of mammalian tooth morphogenesis and discusses recent findings relating to the regulation of stem cell-based dental tissue regeneration and repair.
Introduction
Teeth, which are anchored in maxillary and mandibular jaw bones, are the organs responsible for biting and gnawing. During evolution, tooth morphology tends to increase in complexity, while tooth number decreases. Indeed, fish and reptiles have larger numbers of simple teeth, whereas mammalian teeth form cusps with complex shapes that vary dramatically among different species. Mammals have four principal tooth types – incisors, canines, pre-molars and molars – that all vary in shape (Jernvall and Thesleff, 2012). These variations in tooth shape are based on the location of the tooth in the mouth, and are subject to strong selection during evolution in response to environmental pressures, such as diet.
Teeth develop inside the jaw bone and then erupt postnatally into the oral cavity. The upper portion of the mammalian tooth, which is exposed to the oral cavity, is known as the dental crown, and the portion buried inside the jaw bone is called the dental root. Based on the crown/root proportion, mammalian teeth can be divided into four main types: brachydont, mesodont, hypsodont and hypselodont. Brachydont teeth, commonly seen in omnivores such as humans and mice, have a low crown-to-root ratio, with no crown extension into the jaw bones after eruption. Herbivorous mammals have evolved higher crowned mesodont and hypsodont molars, in which a portion of the crown is reserved within the jaw bones, enabling these animals to contend with increased dental wear while processing abrasive food (Raia et al., 2011). Hypselodont, or ever-growing, teeth, are highly specialized and erupt continuously throughout the animal's life, with a large percentage of their crowns remaining buried inside the jaw bones. Moreover, hypselodont teeth, such as the incisors of lagomorph and rodent lineages, as well as the molars of voles and a number of other animals, preserve their dental stem cells and are able to produce various cell lineages through adulthood to support the continuous formation of the crown (Harjunmaa et al., 2014; Tapaltsyan et al., 2015).
Owing to its easy accessibility in the oral cavity and to the wide availability of relevant mutant and transgenic animal lines, the tooth provides a superb system to study the molecular mechanisms and cellular functions that govern organogenesis during development and homeostasis. The epithelial-mesenchymal interactions that drive tooth development, or odontogenesis, occur in many other ectodermal appendages, making the tooth a suitable model to answer questions such as how an epithelium interacts with mesenchymal tissue to shape the features of an organ. Furthermore, studying how the regenerative capability of hypselodont teeth is maintained by dental stem cells during homeostasis and tissue repair can improve our knowledge of stem cell-driven organ renewal and facilitate the development of therapeutic treatments for patients with dental diseases.
In this Review, we summarize the latest progress in our understanding of how epithelial-mesenchymal signaling networks regulate mammalian tooth development and adult tissue homeostasis. We explore the cellular and molecular mechanisms supporting mineralization as well as the response to injury, and we discuss how our view of dental stem cell behaviors has changed over the past few years, with some key findings related to the identification and heterogeneity of dental stem cells. Finally, we provide perspectives on the potential of technical advances that could help answer open questions regarding dental epithelial-mesenchymal interactions, as well as the migration, lineage plasticity and commitment of dental stem cells.
An overview of early tooth development in mice
Early development of the rooted molar
The development of mammalian brachydont molars can be divided into two main stages: shaping of the crown and formation of the root. In the mouse, molar development initiates at around embryonic day (E) 11.5. The first morphological sign of tooth development is a thickening of the oral epithelium as it forms the dental placode (Fig. 1A). In the following 48 h, the dental epithelium proliferates and invaginates into the underlying mesenchyme and forms the dental lamina, and the dental mesenchyme condenses around the epithelium. Over the next 4 days, the epithelium continues to proliferate and elongate around the dental mesenchyme, thus shaping the tooth through the bud, cap and bell stages.
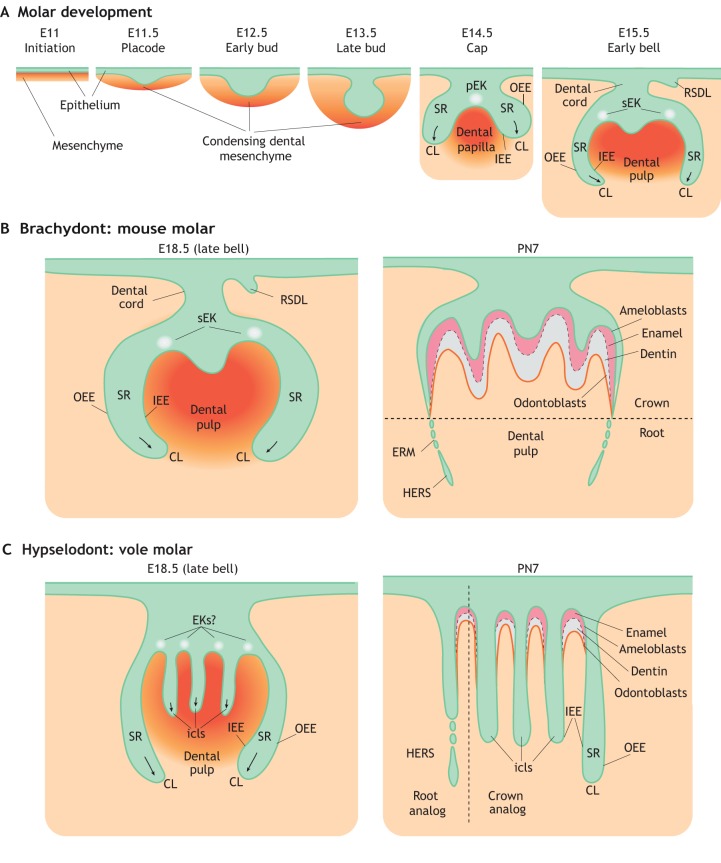
Fig. 1.
Schematic of rodent molar development. (A) The initiation of mouse molar formation starts a little later than E11 with the thickening of dental epithelium (green). The tooth bud then forms via invagination of the dental epithelium into the underlying condensing mesenchyme (orange) at E12.5-E13.5. Signals sent from the primary (pEK) and secondary (sEK) enamel knots guide the formation and elongation of the cervical loop (CL), with the inner (IEE) and outer (OEE) enamel epithelium surrounding the stellate reticulum (SR). The rudimentary successional dental lamina (RSDL), which is a transient structure in most species, also begins to form at this time. (B) During the later stages of mouse molar development, the dental epithelium of the CL grows apically to become a transient structure called Hertwig's epithelial root sheath (HERS) and the epithelial cell rests of Malassez (ERM), facilitating root formation. (C) During the later stages of molar development in voles, multiple intercuspal loops (icls) form and persist throughout the postnatal stages. These are then responsible for enamel deposition in between the icls. PN, postnatal day.
Early tooth development during the bud-to-cap transition requires signals from a transient signaling center named the primary enamel knot (EK) (Jernvall et al., 2002). At the end of the cap stage of molar development, the primary EK undergoes apoptosis and is subsequently succeeded by secondary EKs at the bell stage (Fig. 1A) (Jernvall et al., 1998; Coin et al., 1999). Some of the primary EK cells are recruited during the formation of the secondary EKs (Du et al., 2017), with the numbers and positions of secondary EKs corresponding to the numbers and positions of future tooth cusps. In monocuspid teeth such as incisors and canines, only one EK develops and no secondary EKs form, in contrast to what is observed in multicuspid molars, where multiple knots are seen (Vaahtokari et al., 1996; Jernvall and Thesleff, 2012). Although the mechanisms facilitating the transition from primary to secondary EKs are still unclear, the proper formation of secondary EKs relies on the primary EK. For example, the size of the primary EK can affect the position of the secondary EKs (Pispa et al., 1999; Ohazama et al., 2004).
During the cap-to-bell transition, continuous folding divides the dental epithelium into inner and outer enamel epithelium (IEE and OEE). Mesenchymal cells adjacent to the IEE form the dental papilla, whereas cells around the OEE form the dental follicle. Distal dental epithelial tissues further invaginate into the underlying dental papilla, forming cervical loops (CLs) on each side of the secondary EKs, with the OEE and IEE surrounding cells known as the stellate reticulum (SR) (Fig. 1A). At the later stages of crown formation, mineralization starts with the distribution of dentin and enamel secreted by mesenchyme-derived odontoblasts and epithelium-derived ameloblasts, respectively (Fig. 1B). The CL dental epithelium elongates further into the underlying mesenchyme and grows into a transient structure called Hertwig's epithelial root sheath (HERS) (Fig. 1B), which regulates epithelial-mesenchymal interactions during root elongation (Li et al., 2015).
Early development of the incisor
Development of the mouse incisor initiates at around E11 with the formation of the dental placode (Fig. 2). A population of non-mitotic cells that regulates incisor formation has recently been identified within the incisor placode (Ahtiainen et al., 2016). These cells accumulate to form an early signaling center named the initiation knot (IK). The IK appears early at the initiation stage, remaining on the oral surface to promote the proliferation of neighboring non-IK placodal cells and tooth germ formation (Fig. 2). The IK regulates tooth bud size during the transition into the early incisor bud stage at around E12.5. Accordingly, a smaller IK volume leads to tooth buds with smaller sizes (Ahtiainen et al., 2016). Adjacent to the developing tooth bud, another epithelial structure called the vestibular lamina forms, contributing to the formation of the future space between the lips and teeth (Hovorakova et al., 2016).
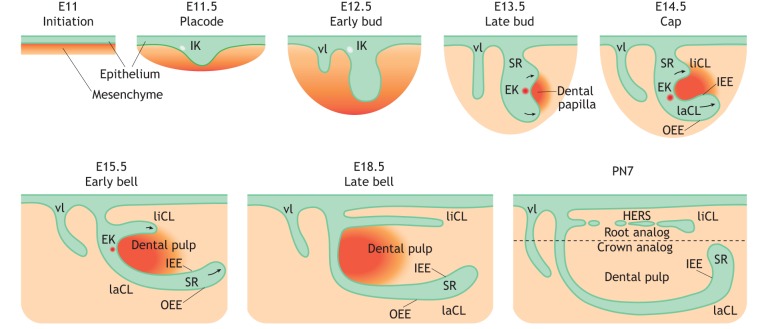
Fig. 2.
Schematic of mouse incisor development. The development of the mouse incisor initiates at around E11. Dental epithelium (green) invaginates into the mesenchyme (orange), forming the dental placode at around E11.5. A population of non-mitotic cells within the placode accumulates to form an early signaling center termed the initiation knot (IK), which guides the formation of the tooth bud. Vestibular lamina (vl) forms adjacent to the developing tooth bud. During the bud-to-cap transition, the signaling activity of the IK switches to the enamel knot (EK). At the cap stage at around E14.5, lingual and labial cervical loops (liCL and laCL) form on each side of the EK in an asymmetrical pattern along the labial-lingual axis around the dental papilla. Continuous folding further divides dental epithelium into inner (IEE) and outer (OEE) enamel epithelium, surrounding the stellate reticulum (SR) region. The development of the laCL proceeds through the bell stage, whereas the formation of the liCL is disproportionally slower on the medial side. Hertwig's epithelial root sheath (HERS) then forms at the lingual side. PN, postnatal day.
At the cap stage at around E14.5, incisor dental epithelial tissue expands longitudinally, receiving signals from the de novo-formed EK that replaces the IK (Ahtiainen et al., 2016) (Fig. 2). Distinct to the cell recruitment happening during the transition from primary to secondary EK formation during molar development, progeny of the incisor IK do not appear to contribute to EK formation (Du et al., 2017). At this stage, the CLs grow asymmetrically along the labial-lingual axis around the dental papilla. While the development of the labial CL (laCL) proceeds through the bell stage, the lingual CL (liCL) grows more slowly on the medial side. Later, only the laCL houses the dental epithelial stem cells that allow a continuous supply of enamel-producing ameloblasts to the labial side of mouse incisors. As occurs in molars, mineralization then occurs during the later stages of development to give rise to the final tooth structure (Fig. 3). Dental pulp becomes enclosed by dentin produced by mesenchyme-derived odontoblasts, whereas the lingual root analog is covered by cementum, which anchors the incisor to the adjacent alveolar bone via periodontium (Fig. 3C).
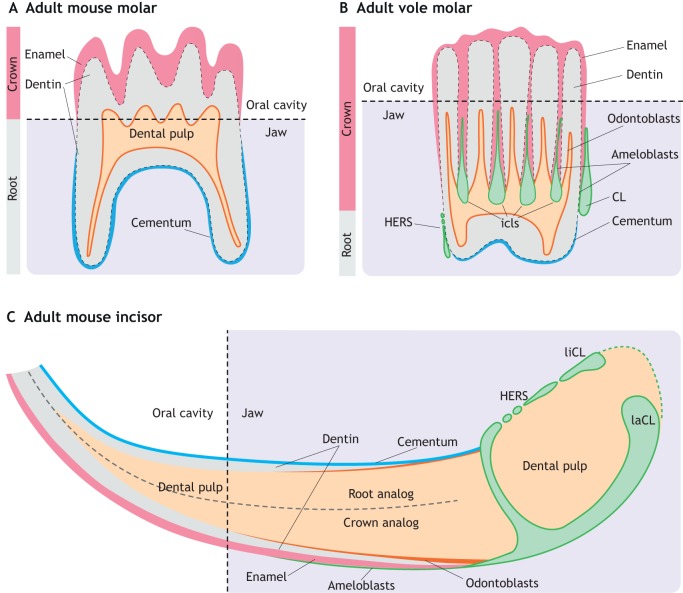
Fig. 3.
Schematic of adult rodent tooth structure. (A) In the adult mouse molar, crown eruption results in the loss of dental epithelial tissue. Thus, enamel produced by epithelial-derived ameloblasts is only deposited on the surface of the crown part of the molar. Mesenchymal-derived odontoblasts produce dentin around the dental pulp, and cementum mineralization covers the root portion of the molar. (B) In the vole molar, intercuspal loops (icls) persist throughout the adult stages, allowing the continuous growth of the crown. In addition, a larger proportion of the crown is buried inside the jaw bones compared with the mouse molar. Both Hertwig's epithelial root sheath (HERS) and the cervical loop (CLs) are preserved in adult vole molars. (C) In the adult mouse incisor, lingual and labial cervical loops (liCL and laCL) persist throughout the adult stages. Only the laCL contains dental stem cells, allowing a continuous supply of enamel-producing ameloblasts at the labial crown analog of mouse incisors. Dental pulp is enclosed by dentin, produced by mesenchyme-derived odontoblasts. The lingual root analog is covered by cementum, which helps the incisor anchor to the jaw bone.
Signaling pathways involved in tooth initiation, budding and early morphogenesis
Early tooth development relies heavily on components of major signaling pathways expressed by both epithelial and mesenchymal tissues. Among these, the Wnt, BMP, FGF, Shh and EDA pathways play important roles in guiding tooth development through initiation, budding and morphogenesis.
Tooth initiation
Shh and FGF signaling are required from the initiation stage of tooth development onwards. At around E11.5 during mouse molar formation, a group of Fgf8-expressing cells form a rosette-like structure and move towards a Shh-expressing signaling center (Prochazka et al., 2015). Chemical inhibition of Shh signaling arrests the growth and invagination of dental epithelium (Li et al., 2016), and also induces a more dispersed distribution of Fgf8+ cells (Prochazka et al., 2015). Blocking the FGF pathway at the early initiation stage disrupts cell migration, resulting in smaller, posteriorly formed molar tooth buds (Prochazka et al., 2015). On the other hand, blocking FGF signaling after molar placode formation affects cell proliferation but not invagination, resulting in smaller tooth buds with similar depth (Li et al., 2016).
Wnt/β-catenin signaling is also required for early tooth morphogenesis, both in the epithelium and the mesenchyme. Suppression of epithelial Wnt via overexpression of the Wnt inhibitor Dkk1 arrests the initiation of tooth development (Andl et al., 2002), and inactivation of mesenchymal β-catenin disrupts EK formation as well as the expression of Lef1 and Fgf3, arresting tooth development at the bud stage (Chen et al., 2009). In the dental mesenchyme, the expression of Pax9 and Msx1 is a hallmark of mesenchymal condensation at the bud stage (Vainio et al., 1993; Satokata and Maas, 1994; Peters et al., 1998). These two transcription factors are initially regulated by epithelial FGFs and BMPs but subsequently function upstream of these signaling molecules. During early incisor development, MSX1 and PAX9 interact to initiate the expression of Fgf3 and Fgf10 at the budding stage (Zhao et al., 2008; Nakatomi et al., 2010). In line with this, Msx1 mutant mice exhibit downregulation of Bmp4 and Fgf3, leading to arrest of early molar development (Wang et al., 2009).
The bud-to-cap transition
The primary EK forms at the basal layer of the epithelium, sending signals to shape the tooth bud into the cap stage tooth. The expression of Wnts, Shh, BMPs and FGFs promotes expansion and elongation of adjacent epithelial tissue into dental cusps (Thesleff et al., 2001; Harjunmaa et al., 2014). At the same time, primary EK cells express Msx2, Bmp2/4 and the cell cycle inhibitor P21 (Cdkn1a) to block the proliferation of EK cells, and subsequently induce apoptosis of the transient signaling center (Laurikkala et al., 2003). Bmp4 prompts the expression of Sostdc1 (also known as ectodin), a secreted Wnt and BMP antagonist, which in turn acts on Bmp4 through a negative-feedback loop, leading to restricted induction of Cdkn1a (Laurikkala et al., 2003). The level of Cdkn1a correlates with primary EK size. Indeed, overexpression of Cdkn1a due to Sostdc1 knockout results in enlarged EKs and cuspal defects (Kassai et al., 2005).
The size of the primary EK is also controlled by the activity of the TNF family ligand ectodysplasin A (Eda). Humans with mutations in EDA, EDAR or EDARADD have hypohidrotic ectodermal dysplasia, with reduced cusps on the molars (Kere et al., 1996; Headon et al., 2001). Mice with mutations in Eda have small EKs, exhibiting defects in epithelial invagination and CL formation, leading to flattened cusps (Pispa et al., 1999; Ohazama et al., 2004; Charles et al., 2011). Eda mutant mice also have disrupted formation of secondary EKs, which leads to flattened cusps (Liu et al., 2008). The expression of Eda is regulated by Wnt signaling during early molar morphogenesis. Inhibiting epithelial Wnt signaling at the early bell stage results in reduced expression of Eda. A major downstream effector of Eda is Fgf20, which affects tooth morphogenesis regulated by Eda, including the number, size and shape of teeth. Accordingly, removal of Fgf20 in an Eda gain-of-function mouse model results in an Eda loss-of-function phenotype, with reduced tooth complexity (Häärä et al., 2012). The function of Fgf20 is redundant with that of other Fgfs, in particular Fgf9 and Fgf4, and forms part of an FGF signaling loop between the dental epithelium and mesenchyme.
The cap and bell stages
During the cap/bell stages, the proliferation and differentiation of progenitors residing in the CLs ensures the further elongation and invagination of dental epithelium. Mesenchymal Fgf10 plays an important role in maintaining the survival of dental epithelial progenitors, starting at the cap stage of molar development. Indeed, the loss of Fgf10 expression during molar development results in a shrunken epithelial layer and a loss of the sustained supply of progenitors for ameloblast production and epithelial growth (Harada et al., 2002). The spatial and temporal expression of FGFs is carefully regulated during early dental epithelial proliferation and invagination. For example, members of the Sprouty (Spry) gene family act as important negative regulators of FGF signaling. Loss of Spry2 and Spry4 results in persistent expression of FGFs during early tooth development, inducing ectopic molar formation in the normally toothless region called the diastema (Klein et al., 2006). Conversely, epithelial overexpression of Spry4 represses FGF signaling, resulting in delayed development of the mouse molar (Marangoni et al., 2019).
At the late cap stage, CLs elongate and further invaginate into the underlying dental mesenchyme. A group of Sox2-expressing epithelial cells residing in the CLs has been identified as dental stem cells, supporting the continued growth of the tooth and giving rise to all dental epithelial lineages during the formation of both rooted molars (Fig. 4A) and ever-growing mouse incisors (Juuri et al., 2013). Epithelial deletion of Sox2 using either ShhCre or Pitx2Cre leads to abnormal development of mouse posterior molars or incisors, respectively (Fig. 4A) (Juuri et al., 2013; Sun et al., 2016), indicating that Sox2 expression is required for tooth development, cell proliferation and differentiation. The transcription factor lymphoid enhancer-binding factor 1 LEF1 also plays a role in cell proliferation during incisor development. Pitx2Cre-driven overexpression of Lef1 in the mouse incisor epithelium induces the formation of an extra stem cell compartment in the laCL with increased cell proliferation, and this can partially rescue tooth arrest and restore cell proliferation and differentiation in Pitx2Cre;Sox2fl/fl mutant incisors (Sun et al., 2016). This result reveals a Pitx2-Sox2-Lef-1 transcriptional interaction in early mouse incisor development. A recent study showed that the incisor EK is derived from a group of Sox2-expressing progenitors from the posterior half of the tooth germ (Du et al., 2017). Whether the defects observed in Pitx2Cre;Sox2fl/fl mutant incisors are in part due to abnormal EK formation and signaling interaction requires further studies.
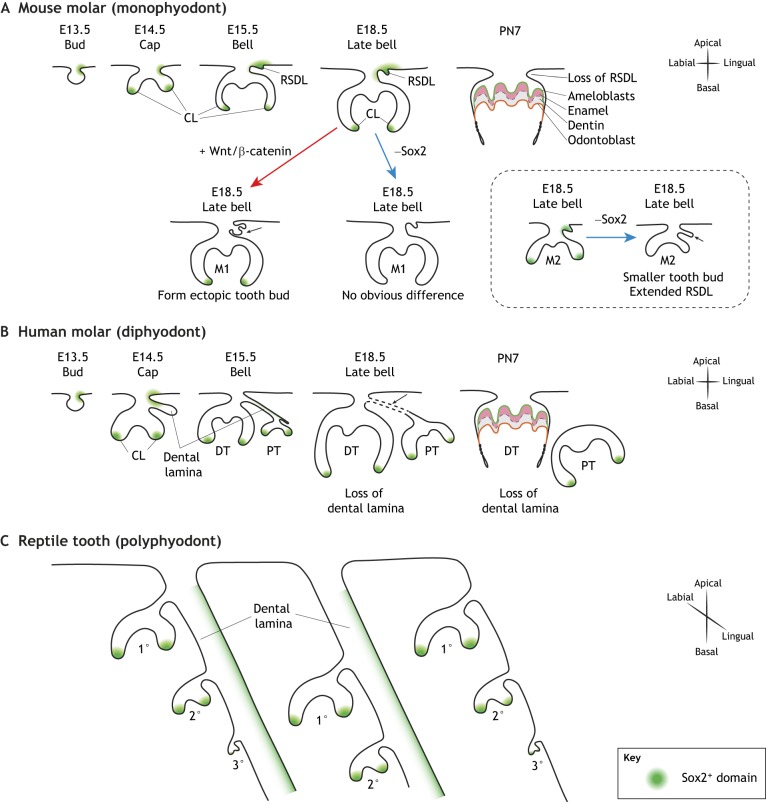
Fig. 4.
Sox2+ stem cells during tooth replacement. (A-C) Illustration of Sox2-expressing stem cell compartments in monophyodonts (A) compared with diphyodonts (B) and polyphyodonts (C), which exhibit different rounds of tooth replacements. (A) In the developing mouse molar, Sox2 (green) is expressed in the cervical loop (CL) and the transient rudimentary successional dental lamina (RSDL). With the initiation of crown eruption, Sox2+ cells are lost along with dental epithelial tissue. Epithelial activation of Wnt signaling (red arrow) results in ectopic tooth bud formation at the RSDL (black arrow), which lacks Sox2 expression. By contrast, deletion of Sox2 only (blue arrow) affects the formation of the posterior molar (M2), without obvious effects on the first molar (M1). (B) In the case of diphyodonts (e.g. humans), as the initial set of deciduous teeth (DT) grow, the replacement set of permanent teeth (PT) forms along the dental lamina. With the beginning of root formation in the deciduous teeth, the dental lamina degrades and the connection between the permanent and the deciduous tooth buds is lost. (C) In polyphyodonts (e.g. reptiles), the dental lamina persists, ensuring several rounds of tooth generation at different stages of development through the lifetime of these animals. PN, postnatal day.
Tooth eruption and formation of the tooth root
Loss of Sox2 expression coincides with crown eruption in mouse molars, and this is controlled by inhibition of Shh-Gli1 activity through BMP-Smad4 signaling. Dental epithelial deletion of Smad4 prolongs the survival of Sox2-expressing cells and postpones the loss of dental epithelium until postnatal day 31.5 (Li et al., 2015). Moreover, molar CLs are maintained in Smad4 mutants, exhibiting increased proliferation and an absence of cell differentiation. The formation of HERS is also strongly affected in Smad4 mutants, resulting in limited root growth postnatally and a much taller crown, resembling the crown/root proportion observed in hypselodont teeth. Further deletion of Shh in Smad4 mutants depletes postnatal Sox2+ cells and restores root formation (Li et al., 2015).
Acting as an interface between the tooth root and bone, the dental follicle houses a group of highly heterogeneous mesenchymal progenitors that differentiate into multiple lineages to facilitate root formation. The dental follicle forms around the OEE at the bell stage and expands longitudinally alongside the HERS. The fates of dental follicle progenitors are maintained through signaling from parathyroid hormone-related peptide (PTHrP; also known as Pthlh) and its receptor (PPR; also known as Pth1r). Lineage tracing has shown that Pthrp+ dental follicle progenitors can differentiate into cementoblasts, periodontal ligament fibroblasts and osteoblasts during tooth root formation (Takahashi et al., 2019). Loss of PPR causes premature differentiation into cementoblast-like cells, resulting in defective periodontal attachment. In this context, mutant molars are submerged below the gingiva (gum) and fail to erupt (Takahashi et al., 2019), similar to the situation observed in human patients with mutations in the gene encoding PTH receptor type 1 (PTH1R) (Subramanian et al., 2016).
Tooth number and replacement in mice and humans
Tooth number
There is great diversity in the number and shape of teeth among mammals. For example, humans possess 20 primary teeth and 32 adult teeth, including eight incisors, four canines, eight premolars and 12 molars. Mice, on the other hand, possess four incisors and 12 molars. Interestingly, the number of initial tooth germs does not always correspond to the final tooth number. In humans, as well as in rats and mice, tooth germs appear to fuse during the formation of the maxillary incisors (Hovorakova et al., 2006; Kriangkrai et al., 2006).
Epithelial Wnt signaling is carefully regulated to control the number of teeth formed. During mouse tooth formation, Lrp4 mediates the Wnt inhibitory function of Sostdc1 to induce the degeneration of two vestigial tooth buds, named the mesial segment and rudiment 2 (R2), which ensures formation of the diastema (Ahn, 2015). Both Sostdc1 null and Lrp4 null mice show persistent development of R2, which gives rise to a supernumerary tooth and delays formation of the first molar, pushing it to the further proximal region (Ahn et al., 2010, 2017). Mutations in WNT10A also cause missing teeth in humans (Yang et al., 2015; Bergendal et al., 2016), and disruption of the Wnt co-receptor LRP6 similarly does so (Massink et al., 2015). However, Wnt10a null mice develop supernumerary molars, indicating that Wnt signaling can have distinct roles in different species during early tooth development (Yang et al., 2015). In contrast, enhancing FGF and Wnt signaling leads to an increased number of tooth buds. As mentioned above, hyperactivating FGF signaling by deleting its Sprouty family antagonists results in the formation of supernumerary teeth (Klein et al., 2006). Likewise, increasing Wnt/β-catenin activity by introducing mutations in Apc, which encodes a protein that negatively regulates Wnt signaling by degrading β-catenin, results in formation of multiple tooth buds and supernumerary teeth (Wang et al., 1998; Kuraguchi et al., 2006; Wang et al., 2009). Upregulation of the Wnt target Lef1 also induces up to 50 incisors and 16 molars in 1-year-old mice (Nakamura et al., 2008), and hyperactivation of Wnt signaling by overexpression of β-catenin induces the formation of molar germs, leading to additional EKs and up to 40 teeth (Järvinen et al., 2006). Interestingly, mutations in the Wnt target gene AXIN2 also arrest the development of replacement teeth in humans, indicating that Wnt/β-catenin upregulation could also suppress normal tooth formation (Lammi et al., 2004). As Axin2 is expressed in both the dental epithelium and mesenchyme, and the activation of epithelial Wnt signaling promotes tooth development in mouse, the hypodontia phenotype observed in patients with AXIN2 mutations is most likely due to increased mesenchymal Wnt activity (Järvinen et al., 2018).
Tooth replacement
Most mammals, including humans, have only two successive sets of teeth (i.e. they exhibit diphyodonty). Around the beginning of root formation in the primary teeth, the dental lamina (an epithelial structure connecting tooth buds) degrades, and the connection between the permanent and the primary tooth buds is lost (Fig. 4B). Therefore, diphyodont mammals have only one set of permanent teeth to replace deciduous teeth (Luo et al., 2004; Buchtová et al., 2012). By contrast, non-mammalian vertebrates are polyphyodont, exhibiting multiple cycles of tooth replacement throughout their lifetime. In these species, the dental lamina persists to ensure several rounds of tooth generation at different stages of development (Fig. 4C), and later teeth can gradually grow larger in size to keep up with their continuously developing skulls (O'Meara and Asher, 2016).
The mechanisms regulating tooth replacement are still not clear. Recently, the transient rudimentary successional dental lamina (RSDL) that forms during mouse molar formation has been used to study the molecular and cellular regulation of tooth replacement (Dosedělová et al., 2015). This study showed that formation of the first molar represses development of any replacement tooth at the RSDL, and that under normal conditions the RSDL lacks Wnt/β-catenin activity. However, the RSDL still possesses odontogenic potential, and overactive Wnt signaling in the RSDL can induce the formation of ectopic teeth (Fig. 4A) (Popa et al., 2019). A group of Sox2+ label-retaining cells has been observed in the transient RSDL in mice (Fig. 4A) (Juuri et al., 2013; Popa et al., 2019), and lineage tracing has shown that Sox2+ dental lamina cells contribute to the formation of the second and third molars. Similarly, a population of Sox2+ cells has been identified at the lingual side of the dental lamina in reptiles (Fig. 4C), and may potentially function as an adult stem cell reservoir to ensure a continuous supply of various cell lineages for the formation of successional teeth (Juuri et al., 2013).
Interestingly, the expression of Sox2 is downregulated in the ectopic tooth bud induced by overexpression of Wnt in the mouse RSDL (Fig. 4A), suggesting a negative-feedback loop during molar development (Popa et al., 2019). Currently, the exact nature of the signals generated to inhibit tooth replacement during mouse molar formation remain unclear. The induction of ectopic tooth germs at the RSDL with the removal of the first molar indicates that this transient dental epithelium is likely to provide more insights into the mechanisms influencing tooth replacement. Using mouse models to improve our understanding of how dental cells are activated during tooth replacement could therefore provide insights into how human tooth regeneration could potentially be facilitated.
The roles and regulation of dental stem cells during tissue homeostasis and repair
Types of dental stem cells
Brachydont teeth, such as human molars, are not able to regenerate their crowns because of loss of dental epithelial tissue after tooth eruption (Balic, 2018). However, dental mesenchymal tissues in adults do possess a limited regenerative capacity that enables them to produce dentin, cementum and pulp (Sharpe, 2016). This regenerative capacity is driven by different populations of dental mesenchymal stem cells (MSCs). For example, dental pulp stem cells (DPSCs) isolated from the pulp of adult teeth can give rise to odontoblast-like cells after dentin damage (Feng et al., 2011). Periodontal ligament stem cells (PDLSCs) are responsible for maintaining periodontal ligament cell numbers and are capable of differentiating into cementoblast-like cells, as well as connective tissue rich in collagen I (Shi et al., 2005). Moreover, co-transplanting PDLSCs with stem cells isolated from the root apical papilla can induce formation of dentin and periodontal ligament in minipigs (Sonoyama et al., 2006).
In contrast to brachydont teeth with limited regenerative capability, hypselodont teeth, such as incisors in all rodents, as well as molars in voles, guinea pigs and sloths, contain dental epithelial stem cells that are maintained in CLs throughout the lifespan of the animals (Tummers and Thesleff, 2003; Ohshima et al., 2005). Interestingly, some continuously growing teeth, such as the molars of sloths, lack enamel. However, the CLs in these teeth are maintained, hosting a smaller core of SR cells surrounded by IEE and OEE (Tummers and Thesleff, 2008). The early development of vole and mouse molars is remarkably similar from initiation to the late bell stage. However, whereas the mouse molar develops roots and stops growing, the molars of some vole species are able to renew throughout the animal's lifetime (Figs 1C and 3B). This is because the epithelium of vole molars folds several times to form multiple intercuspal loops (icls), almost reaching the base of the dental mesenchyme and creating large epithelial compartments (Fig. 1C), which retain a crown fate, allowing for continuous growth (Tummers and Thesleff, 2003). The mouse incisor, by contrast, does not develop icls, but rather has a persistent laCL, containing a larger SR, sandwiched between the OEE and IEE (Fig. 2). This laCL houses the dental epithelial stem cells that fuel the production of enamel-producing ameloblasts and, hence, drive tooth growth. The continuously growing mouse incisor has therefore emerged as an attractive model system in which to study adult stem cell-fueled tissue homeostasis, regeneration and repair. As we discuss below, significant advances have been made using this system in identifying dental stem cells, understanding their functions and characterizing the molecular mechanisms that regulate their dynamics.
The molecular identity of dental stem cells
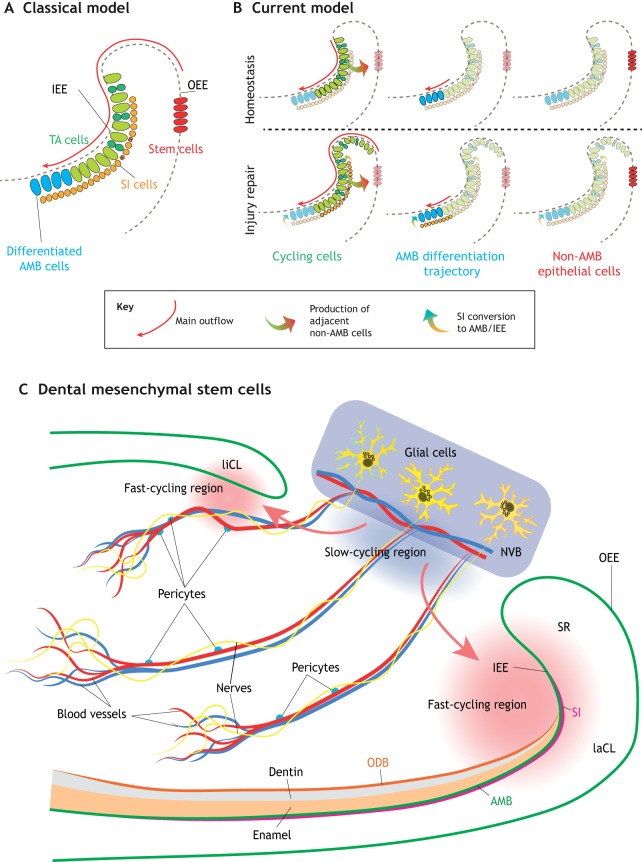
In the classical model of tissue renewal, a small number of quiescent stem cells act as the starting point for renewal, being responsible for the formation of rapidly dividing transit-amplifying progenitors, which later differentiate into multiple lineages (Cotsarelis et al., 1990; Fuchs, 2009). Based on this model, a population of long-lived, label-retaining cells residing in the OEE and the adjacent SR region in the proximal part of the adult mouse laCL were proposed to function as dental epithelial stem cells, (Fig. 5A) (Harada et al., 1999; Biehs et al., 2013). Genetic lineage tracing – one of the principal methods for identifying stem cells in a tissue – has since been used to study this population further. In this approach, a population of cells is marked and their ability to give rise to multiple downstream cell lineages is then assessed. Typically, the Cre-loxP system is used to conditionally activate Cre recombinase at specific time points under the control of either a tissue- or cell-specific promoter via tamoxifen induction. The loxP sites are recombined by Cre and the flanking STOP cassette is removed to induce reporter expression (e.g. mGFP) in the cells and their progeny. Using this approach, several putative dental epithelial stem cell markers have been identified. For example, it has been shown that both Gli1+ and Bmi1+ cells located in the proximal laCL are able to self-renew and give rise to various epithelial cell lineages, including enamel-producing ameloblasts and stratum intermedium (SI) cells underlying ameloblasts (Seidel et al., 2010; Biehs et al., 2013). Sox2+ cells have also been recognized as epithelial stem cells that contribute to both early tooth development and maintenance of adult dental tissue homeostasis. As mentioned above, Sox2-expressing cells in the CLs of developing incisors and molars at the bell stage can differentiate into all dental epithelial lineages. Whereas they persist in mouse incisors into adulthood, Sox2+ cells are not detectable in mouse molars after birth (Fig. 4A), consistent with the notion that epithelial stem cells are lost in molars (Juuri et al., 2012; Li et al., 2015; Sanz-Navarro et al., 2018).
Fig. 5.
Dental stem cell heterogeneity in adult mouse incisors. (A) The current model posits that stem cells (red) in the outer enamel epithelium (OEE) give rise to transit-amplifying (TA) cells (green) and stratum intermedium (SI) cells (pink) in the inner enamel epithelium (IEE), which then differentiate into ameloblasts (AMB; blue). (B) However, we now know that, during homeostasis, actively cycling IEE cells (green) contribute to the formation of both the enamel-producing ameloblasts (blue) and the adjacent non-ameloblast epithelial cells (red). During injury repair, additional progenitors enter the cell cycle (green), and SI cells (pink) can also convert to differentiate into ameloblasts (blue). (A and B adapted from Sharir et al., 2019.) (C) Different types of cells have also been shown to function as stem cells in the dental mesenchyme. These include glial cells (yellow) and pericytes (blue). They reside in the neurovascular bundle (NVB) niche, giving rise to cells in the fast-cycling regions at both the labial and lingual sides to support the rapid turnover of incisor mesenchymal cells (red arrows). laCL, labial cervical loop; liCL, lingual cervical loop; ODB, odontoblasts; SR, stellate reticulum.
Analyses of gene co-expression modules in the mouse incisor have identified additional markers for dental stem cells, including Igfbp5 and Lrig1 (Seidel et al., 2017). Lineage tracing has shown that cells expressing either of these two markers can give rise to both epithelial and mesenchymal dental lineages (Seidel et al., 2017). In the mesenchyme of mouse incisors, it is thought that slow-cycling, label-retaining MSCs are located in a niche region between the labial and lingual CLs near the neurovascular bundle (Seidel et al., 2010; Zhao et al., 2014). A number of other cell types have been suggested to function as stem cells in the dental mesenchyme, including derivatives of glial cells and pericytes, which contribute to the formation of highly proliferative cells in the fast-cycling region to support the rapid turnover of incisor mesenchymal cells in the dental pulp (Fig. 5C). Several markers of such dental MSCs have been identified, including Thy1 (CD90) and Gli1, both of which label cells in the MSC niche that give rise to odontoblasts and dental pulp cells throughout the animal's life (Kaukua et al., 2014; Zhao et al., 2014; Feng et al., 2011; An et al., 2018; Shi et al., 2019).
The heterogeneity of dental stem cells in the mouse incisor
Recent studies of organs such as the skeleton, hair follicle and blood have shown that what were previously thought to be homogeneous stem cell populations are actually highly heterogeneous (Chan et al., 2015; Paul et al., 2015; Yang et al., 2017). Similar observations have also been reported in mouse incisors. In the dental mesenchyme, lineage tracing using the stochastic multicolor confetti reporter mouse with two glial ERT2-Cre drivers (Plp1 and Sox10) has revealed a population of slow-cycling peripheral glial MSCs, which can give rise to odontoblasts and dental pulp cells (Kaukua et al., 2014). Additionally, another distinct population of non-glial-derived dental MSCs that are Shh responsive and reside in the neurovascular bundle has been reported (Zhao et al., 2014). Lineage tracing of these non-glial-derived cells using Ng2-Cre and Nestin-Cre showed that they arise from pericytes and are also responsible for the constant production of odontoblasts for dentin deposition in the mouse incisor, as well as for responding to odontoblast damage in explant cultures (Feng et al., 2011; Zhao et al., 2014; Pang et al., 2016). These findings suggest that dental MSCs can have both glial and pericyte origins, revealing a highly dynamic and heterogeneous cellular environment, as well as an essential role of nerve and vascular systems during tooth development (Fig. 5C) (Zhao et al., 2014; Kaukua et al., 2014; Kramann et al., 2015).
Although for a number of years it was thought that the non-proliferative OEE housed the epithelial stem cells of the adult mouse incisor, more recent investigations have revealed that some of the previously identified stem cell markers are not completely restricted to the OEE, but are also expressed in proliferating cells (Sharir et al., 2019). Following this, the identity of mouse incisor epithelial stem cells was characterized using a combination of single cell RNA sequencing (scRNAseq) and advanced computational approaches. This led to the proposal of a highly dynamic dental stem cell model in which there are three main classes of cells: dividing cells, ameloblasts and adjacent non-ameloblasts (Fig. 5B). The dividing cells of the IEE are the only ones that appear to undergo self-renewal during homeostasis. Adding more complexity, the actively cycling epithelial progenitors can give rise to both ameloblasts as well as adjacent non-ameloblast cells. Furthermore, during injury, plasticity in the normal patterns of differentiation is observed (Sharir et al., 2019).
The regulation of dental stem cells during tissue homeostasis and repair
A number of signaling pathways are crucial for the homeostasis of the adult mouse incisor, with FGF signaling being one of the most important for maintaining incisor renewal (Li et al., 2014). Both Fgf3 and Fgf10 are essential for supporting the growth of CLs and maintaining cell survival. Deletion of the transcription factor Bcl11b results in an inverted expression pattern of Fgf3 and Fgf10 in the lingual and labial mesenchyme; in this context, the CL expands and ameloblasts form at the lingual side, whereas the size of the laCL is reduced, accompanied by disrupted ameloblast formation (Kyrylkova et al., 2012). Epithelial Fgf9 also plays key roles in regulating the level of mesenchymal FGF signaling to maintain epithelial stem cells in the developing incisor CLs. Loss of FGF9 abrogates the expression of Fgf3 and Fgf10 in the dental mesenchyme (Wang et al., 2007; Kurosaka et al., 2011). FGF signaling is modulated by BMP4 and activin, which belong to the TGFβ family. BMP4 represses Fgf3 expression in both the lingual and labial mesenchyme, while only inhibiting labial Fgf10 expression. Activin is expressed more robustly in the labial mesenchyme, counteracting the activity of BMP4. Thus, Fgf3 expression is maintained on the labial side to promote stem cell proliferation (Wang et al., 2007). On the lingual side, the repression of BMP4 by activin is diminished by follistatin (Fst) from the epithelium. Therefore, lingual BMP4 activity is less affected, and it can further repress Fgf3 expression, resulting in inhibition of cell proliferation and differentiation in the lingual dental epithelium. Deletion of Fst accordingly results in ectopic Fgf3 expression, leading to an expanded liCL as well as ameloblast formation and enamel production on the lingual side (Wang et al., 2007). The TGFβ receptor type I encoded by Alk5 (Tgfbr1) can also regulate mesenchymal Fgf3 and Fgf10 expression. Mesenchymal deletion of Alk5 in teeth leads to downregulation of Fgf3, Fgf9 and Fgf10, resulting in reduced proliferation in epithelium. However, epithelial deletion of Alk5 has no effect in CLs, indicating a directionality of TGFβ signaling in regulating dental mesenchymal-epithelial interactions (Zhao et al., 2011). Deletion of Tgfbr2 (TGFβ receptor type II) also results in disrupted incisor formation due to upregulation of Wnt5a and downregulation of Fgf3/10 in the dental mesenchyme, as well as enhanced epithelial Lrp5/6-β-catenin signaling. This highlights the importance of cross-talk between the TGFβ, Wnt and FGF signaling pathways in regulating dental epithelium-mesenchyme interactions (Yang et al., 2014). It has also been shown that FGFs bind to receptor tyrosine kinases (RTKs) to activate Ras signaling, which signals through the MAPK and PI3K pathways to regulate epithelial stem cell maintenance and ameloblast differentiation in the adult mouse incisor (Zheng et al., 2017).
Recently, Hippo signaling has also been shown to play an important role in coordinating dental cell proliferation and differentiation in the epithelial progenitor population (Hu et al., 2017). The effectors of the Hippo pathway, yes-associated protein (YAP; also known as YAP1) and its homolog, transcriptional co-activator with PDZ-binding motif (TAZ), are strongly expressed in the nuclei of proliferating cells in the laCL. Deletion of both Yap and Taz causes loss of proliferation and increased cell death, resulting in remarkable tissue loss in the SR and proliferating regions of the adult incisor epithelium. YAP/TAZ regulate the expression of Rheb to subsequently activate mTOR signaling, and a FAK-YAP-mTOR signaling axis controls the spatial expression of nuclear YAP, enabling the production of highly proliferative progenitors to maintain adult dental tissue homeostasis (Hu et al., 2017).
Wnt signaling is essential for maintaining epithelial stem cell niches in many epithelial systems, such as hair follicles and intestinal crypts (Huelsken et al., 2001; He et al., 2004; Haegebarth and Clevers, 2009). However, although Wnt signaling is crucial for tooth morphogenesis, it does not seem to be heavily involved during the process of adult incisor renewal. Several Wnt inhibitors, such as Dkk1 and Dkk3, are expressed in the laCL. Moreover, the Wnt-responsive gene Axin2, often used as a marker of Wnt activity, is not expressed in the apical region of the incisor CL and is only weakly expressed in differentiating ameloblasts (Suomalainen and Thesleff, 2010). Upregulation of epithelial Wnt3 expression disrupts maintenance of the incisor stem cell niche, as well as the differentiation of ameloblasts (Millar et al., 2003; Liu et al., 2010), and upregulation of Wnt signaling in Sox2+ cells induces the formation of odontomas (Xavier et al., 2015). These findings suggest that a lower level of Wnt activity is desirable for maintaining the adult CL. The expression of Lgr5, a Wnt target gene that marks adult stem cells in many other tissues, can be detected by E15.5 in the SR of the laCL in both developing and adult mouse incisors, strongly overlapping with the expression pattern of Sox2 (Suomalainen and Thesleff, 2010; Sanz-Navarro et al., 2018). However, Lgr5 null mice show no morphological abnormalities in the developing laCL (Sanz-Navarro et al., 2018). The dispensable role of Wnt signaling in the adult mouse incisor laCL, compared with its essential role in epithelial stem cell niches in other systems, indicates a unique regulatory network in this system and highlights the importance of studying stem cell regulation case by case. In addition to being regulated by a host of signaling pathways, tooth development is also controlled through the fine-tuning of microRNA (miRNA) networks (see Box 1).
Box 1. miRNA regulation during tooth formation.
Dozens of miRNAs are differentially expressed in the tooth and have been shown to interact with various signaling pathways to govern odontogenic differentiation of primary cultured human dental pulp cells (Gong et al., 2012). Deletion of Dicer1, the gene encoding an enzyme required for miRNA processing, leads to a general disruption of tooth formation in mice (Cao et al., 2010; Michon et al., 2010; Oommen et al., 2012). Epithelial deletion of Dicer1 with two different Cre drivers (Pitx2Cre;Dicerfl/fl or ShhCre;Dicerfl/fl) results in ectopic formation of mouse incisors (Cao et al., 2010; Oommen et al., 2012). Mesenchymal deletion of Dicer1, on the other hand, causes defects of varying severity, depending on tooth location and type (Oommen et al., 2012). Future studies will be required to establish a more precise expression profile of miRNAs during early tooth development, as well as during homeostasis and injury repair in adult dental tissues. With the advent of single cell RNAseq, information from different cell populations can be collected to understand further the complex regulation of miRNAs and the interactions between miRNAs and signaling pathways.
The maintenance of both epithelial and mesenchymal stem cells is not only essential for continuous incisor growth under homeostasis but is also required during injury repair. As a highly plastic tissue, the dental epithelium of the mouse incisor can regenerate within 10 days after the elimination of the majority of proliferating progenitors using 5-fluorouracil (5FU) (Sharir et al., 2019). This regeneration is driven by progenitors that are not actively proliferating and hence survive the 5FU treatment and quickly respond to tissue damage by dividing. Non-mitotic pre-ameloblasts, before switching on expression of differentiation markers, can also reinitiate cycling, leading to an expansion of the proliferating region and delayed onset of ameloblast differentiation (Fig. 5B).
The roles of signaling pathways regulating dental tissue regeneration and repair remain largely unknown. Notch signaling has been reported to regulate the differentiation of SI cells, which function as a reserve progenitor pool that can give rise to SR/OEE cells as well as partially contribute to ameloblast turnover during homeostasis (Harada et al., 2006). During tissue repair after 5FU treatment, a significant number of Notch1-expressing SI cells migrate into the IEE/ameloblast layer to regenerate the damaged tissue (Fig. 5B); in line with this, elimination of Notch1+ cells significantly disrupts injury recovery (Sharir et al., 2019). Given the role of Notch signaling in cell fate determination and its specific expression in the SI cells that subtend the IEE, it is possible that the Notch pathway is involved in determining SI fate. However, future exploration with in vivo mouse genetics is required to address this question.
During tissue homeostasis in the adult mouse incisors, Thy1-expressing cells residing in the dental mesenchyme between the liCL and laCL act as a subpopulation of MSCs that can differentiate into dental pulp cells and odontoblasts (An et al., 2018). In the case of tissue regeneration stimulated by clipping of the incisor, a group of Celsr1+ cells in the dental mesenchyme is able to switch from quiescent to active status and replenish Thy1+ MSCs (An et al., 2018). These results reveal that dental MSCs are also responsive to tissue damage and can be re-activated during injury repair.
Dental tissue mineralization and damage repair
During the later stages of tooth development, ameloblasts and odontoblasts derived from dental stem cells secrete various types of proteins for biomineralization. In the case of ever-growing teeth, this dynamic process continues throughout the animal's lifetime, contributing to the formation of dental tissue such as enamel and dentin. Understanding the process of mineral deposition could shed light on the mechanisms underlying mineralization diseases, such as amelogenesis imperfecta, as well as facilitate the development of therapeutic treatments and novel biomaterial scaffolds.
Starting at the late bell stage, differentiated ameloblasts begin to produce the enamel layer. Enamel, which is the only calcified tissue derived from epithelial cells in mammals, is built up by a unique set of enamel matrix proteins (EMPs) that govern hydroxyapatite deposition (Gajjeraman et al., 2007). Amelogenin, ameloblastin and enamelin are the major structural constituents of secretory-stage enamel. Amelotin and odontogenic ameloblast-associated protein are then secreted at the maturation stage during enamel deposition. Genetic and evolutionary analyses have revealed that the genes encoding these proteins all originated from a common ancestor gene, SPARCL1, through a process of tandem duplication followed by neo-functionalization (Kawasaki and Weiss, 2003). With the exception of amelogenin, the genes encoding EMPs form a cluster on human chromosome 5, forming a larger family of secretory calcium-binding phosphoproteins (Kawasaki and Weiss, 2003). After secretion, the nascent EMPs undergo proteolytic processing by matrix metalloprotease 20 (MMP20), resulting in the release of different protein moieties exhibiting distinct calcium-binding properties, hydrophobicity and spatial distribution in the matrix during amelogenesis (Iwata et al., 2007; Nagano et al., 2009; Bartlett, 2013). Around 90% of the enamel matrix is composed of amelogenin-derived peptides, which are believed to be the principal organizers of hydroxyapatite deposition. Ameloblastin and enamelin make up the remaining 10% of the enamel matrix and function cooperatively with amelogenin to achieve the optimal scaffolding needed for the formation of enamel matrix. Both amelogenin and ameloblastin form higher-ordered structures via evolutionarily conserved self-assembly motifs (Ravindranath et al., 2004).
Mutations in all major structural EMPs have been identified as causes of amelogenesis imperfecta (AI) in humans, underscoring the importance of EMPs in tooth development and enamel formation. Furthermore, the deletion of AMBN exon 6 leads to hypoplastic AI with aprismatic enamel (Poulter et al., 2014). Mutations in the enamelin gene lead to an autosomal-inherited form of AI, whereas mutations in amelogenin lead to X-linked AI (Kim et al., 2004, 2005).
A central regulator of enamel mineralization is Wnt signaling. Indeed, epithelial deletion of a combination of the Wnt/β-catenin transcriptional co-factors Bcl9 and Bcl9l affects the composition of enamel (Cantù et al., 2017). The differentiation of ameloblasts also requires the presence of functional odontoblasts or a predentin matrix (Karcher-Djuricic et al., 1985; Zeichner-David et al., 1995), and deficiency in dentin mineralization results in amelogenin accumulation in the matrix adjacent to odontoblasts (Fanchon et al., 2004). Dentin itself is secreted by mesenchyme-derived odontoblasts, and the process of odontogenesis relies on a similar set of proteins as does bone development. The most important structural proteins are collagen type 1, osteocalcin (Bglap), bone sialoprotein (Bsp), osteopontin (Spp1), dentin matrix protein 1 (Dmp1) and dentin sialophosphoprotein (Dspp); Dspp encodes two dentin matrix proteins: dentin sialoprotein (DSP) and dentin phosphoprotein (DPP) via the process of alternative splicing (Narayanan et al., 2001). Compared with the extensively studied process of enamel formation, the regulation of dentin mineralization is less well understood. DMP1 directly regulates Dspp expression by binding to its promoter and activates its transcription, which is important for early odontoblast differentiation (Narayanan et al., 2006). A recent study showed that Sp7 (also known as osterix), a transcription factor essential for bone development, is also required for maintaining the size and shape of mouse molars and incisors (Bae et al., 2018). Although tooth initiation and early morphogenesis are not affected in Sp7 null mice, the polarity and differentiation of odontoblasts and ameloblasts are disrupted, resulting in the absence of dentin and enamel deposition. Interestingly, Sp7 is only expressed in dental mesenchymal cells, indicating a non-cell-autonomous effect on epithelial ameloblasts, potentially through direct regulation of FGF ligands (Bae et al., 2018).
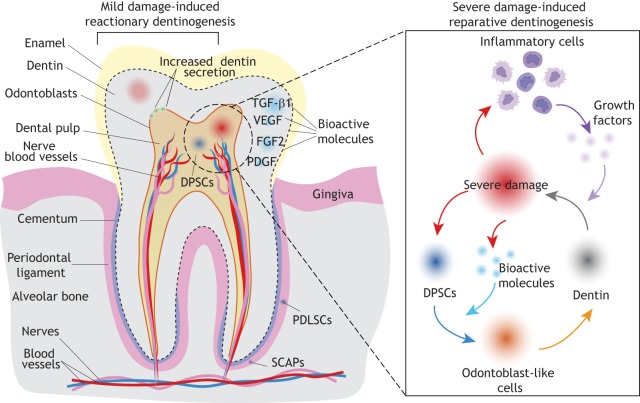
The dentin-pulp complex is able to repair in response to external damage (Fig. 6). Mild injury in the molar stimulates reactionary dentinogenesis by upregulating dentin secretion from existing postmitotic odontoblasts (Duque et al., 2006). By contrast, severe injury involving the death of local odontoblasts requires recruitment of dental progenitors and differentiation of odontoblast-like cells for reparative dentinogenesis (Fig. 6). In response to severe damage, expression of the Wnt-responsive gene Axin2 becomes upregulated. Lineage tracing has shown that, in this context, Axin2+ cells expand through proliferation and give rise to odontoblast-like cells for reparative dentin secretion (Babb et al., 2017). Odontoblast-specific deletion of Wntless (Wls), which encodes a chaperone protein required for Wnt secretion, causes significant downregulation of Dcn, Col1a1 and Dsp in mutant crowns and reduced expression of Wnt10a and Axin2 in mutant roots, resulting in reduced dentin thickness, enlarged dental pulp chamber and impaired root elongation (Bae et al., 2015). Another study has shown that non-ionizing, low-power laser treatment can re-activate endogenous growth factors, such as TGFβ1 in the mineralized tissue, to stimulate differentiation from dental progenitors to odontoblasts and promote tissue repair (Arany et al., 2014). This finding indicates that external damage might also release other bioactive molecules, such as FGF2, PDGF and VEGF, from the dentin matrix to aid repair. Inflammatory cells are also recruited to the injury site (Fig. 6). They can release growth factors such as TNFα to promote odontogenesis and dentin re-deposition (Cooper and Smith, 2013; Cooper et al., 2014).
Fig. 6.
Dentin repair in the human molar. Schematic of a human molar showing dentin mineralization around the dental pulp, enamel deposition on the tooth crown, and cementum coverage around the root surface. The tooth is attached to the alveolar bone via periodontal ligaments. The dental pulp houses blood vessels and nerves. Various types of stem cells, such as dental pulp stem cells (DPSCs), periodontal ligament stem cells (PDLSCs) and stem cells from the root apical papilla (SCAPs) can be found in the dental pulp and periodontal ligament around the tooth root. Mild injury induces reactionary dentinogenesis, which stimulates increased dentin secretion by odontoblasts. By contrast, reparative dentinogenesis takes place in response to severe injury and requires the recruitment of dental progenitors, which differentiate into odontoblast-like cells to fuel dentin production. Bioactive molecules released from mineralized dental tissues, as well as growth factors produced by recruited inflammatory cells, can promote both odontogenesis and tissue repair.
Conclusions and future perspectives
Tooth organogenesis is a complex process involving the repeated utilization of several pathways that can trigger different effects in distinct compartments of dental tissues. This is an exciting time in the field, with many open questions. How do cells tell different signals apart? What mechanisms are used to achieve specific lineage commitment between cell populations? How are all these molecules controlled at a systems level? What are the activators and inhibitors of morphogenesis and differentiation within the system? Studies of the behaviors of dental stem cells under different conditions have also raised a number of questions that merit further analysis. How is plasticity maintained in stem cells/progenitors in adult tissue responding to different levels of injury repair? How does aging affect their potency? The mechanisms regulating dental epithelial-mesenchymal interactions and cellular activities during progenitor migration and cell fate decisions also require in-depth investigation.
Moving forward, technical advances can be used to help decipher these questions. For example, rapidly developing bioengineering and tissue culture systems can serve as important platforms to recapitulate molecular and cellular interactions in vitro. Recent studies have shown that modifying cell culture conditions can stimulate the lineage commitment of dental pulp stem cells towards osteogenesis or pericyte-like cells (Delle Monache et al., 2019; Noda et al., 2019). A natural extracellular matrix scaffold can be achieved by decellularization of unerupted tooth buds, which holds promise for future application in whole-tooth regeneration (Zhang et al., 2017). Computer modeling can also contribute to our understanding of organogenesis. A multi-scale, cell-based computational model was recently built to analyze the mechanical properties of epithelial and mesenchymal cells during tooth development. By visualizing outputs with various parameters and comparing computational stimulations with morphology observed in histological sections, differential growth and differential adhesion between tooth tissues were identified as two main contributors in shaping early tooth development (Marin-Riera et al., 2018). The importance of mechanical forces during tooth morphogenesis is increasingly being recognized (see Box 2). Force sensors show great potential to be used for precise measurements of mechanical force in a living tissue. For example, when injected into the intercellular space, the deformation of single-cell-size oil droplets can provide a direct readout of mechanical stresses of a targeted region in the tissue (Campàs et al., 2014). This technique could be used to understand the role of mechanical forces within the dental epithelium or mesenchyme, as well as the interactive region between two layers of tissues during tooth morphogenesis. With such multidisciplinary approaches, we should be able to reach a deeper understanding of dental developmental and stem cell biology, contributing to a better foundation for future regenerative approaches.
Box 2. The role of mechanical forces during tooth formation.
The roles of localized forces during tooth morphogenesis and later odontogenic stages are increasingly being recognized (Li et al., 2011; Calamari et al., 2018). As early as molar placode formation, tension in the suprabasal layer contributes to epithelial invagination. Blockade of Shh arrests suprabasal contraction and disrupts tooth invagination, indicating that Shh signaling regulates the initiation and/or maintenance of tissue tension (Li et al., 2016). Other than Shh signaling, E-cadherin-based adherens junctions as well as enriched actomyosin complexes are thought to contribute to tension maintenance in the suprabasal layer. By pulling dental epithelial cells towards the center of the tooth germ, the forming molar bud is essentially sealed at the top, ensuring that proliferating cells expand and invaginate towards the underlying mesenchyme (Panousopoulou and Green, 2016). Large-scale forces generated by mastication or jaw bones surrounding teeth are also needed for correct tooth shape. For example, the absence of attachment bone in sp7 mutant medaka fish results in smaller and disorganized oral teeth, with a reduction in dentin deposition (Yu et al., 2017). Similar defects are observed in Sp7 null mice in vivo (Bae et al., 2018). In ex vivo cultures of mouse and vole molars, the absence of adjacent jaw bone causes cusp offsets, indicating that the jaw-tooth interaction is also important for normal tooth morphology (Renvoisé et al., 2017).
Acknowledgements
We thank Drs Jimmy Hu, Pauline Marangoni, Tomas Wald and Kara McKinley for helpful discussions.
Footnotes
Competing interests
O.D.K. is a consultant for Stemodontics.
Funding
Support for work in the authors’ laboratory is provided by the National Institutes of Health (R35-DE026602 and R01-DE027620). Deposited in PMC for release after 12 months.
References
- Ahn Y. (2015). Signaling in tooth, hair, and mammary placodes. In Current Topics in Developmental Biology, Neural Crest and Placodes (ed. Trainor P. A.), pp. 421-459, Academic Press. [DOI] [PubMed] [Google Scholar]
- Ahn Y., Sanderson B. W., Klein O. D. and Krumlauf R. (2010). Inhibition of Wnt signaling by Wise (Sostdc1) and negative feedback from Shh controls tooth number and patterning. Development 137, 3221-3231. 10.1242/dev.054668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn Y., Sims C., Murray M. J., Kuhlmann P. K., Fuentes-Antrás J., Weatherbee S. D. and Krumlauf R. (2017). Multiple modes of Lrp4 function in modulation of Wnt/β-catenin signaling during tooth development. Development 144, 2824-2836. 10.1242/dev.150680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahtiainen L., Uski I., Thesleff I. and Mikkola M. L. (2016). Early epithelial signaling center governs tooth budding morphogenesis. J. Cell Biol. 214, 753-767. 10.1083/jcb.201512074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- An Z., Sabalic M., Bloomquist R. F., Fowler T. E., Streelman T. and Sharpe P. T. (2018). A quiescent cell population replenishes mesenchymal stem cells to drive accelerated growth in mouse incisors. Nat. Commun. 9, 378 10.1038/s41467-017-02785-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andl T., Reddy S. T., Gaddapara T. and Millar S. E. (2002). WNT signals are required for the initiation of hair follicle development. Dev. Cell 2, 643-653. 10.1016/S1534-5807(02)00167-3 [DOI] [PubMed] [Google Scholar]
- Arany P. R., Cho A., Hunt T. D., Sidhu G., Shin K., Hahm E., Huang G. X., Weaver J., Chen A. C.-H., Padwa B. L. et al. (2014). Photoactivation of endogenous latent transforming growth factor-β1 directs dental stem cell differentiation for regeneration. Sci. Transl. Med. 6, 238ra69-238ra69. 10.1126/scitranslmed.3008234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babb R., Chandrasekaran D., Neves V. C. M. and Sharpe P. T. (2017). Axin2-expressing cells differentiate into reparative odontoblasts via autocrine Wnt/β-catenin signaling in response to tooth damage. Sci. Rep. 7, 3102 10.1038/s41598-017-03145-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae C. H., Kim T. H., Ko S. O., Lee J. C., Yang X. and Cho E. S. (2015). Wntless regulates dentin apposition and root elongation in the mandibular molar. J. Dent. Res. 94, 439-445. 10.1177/0022034514567198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae J.-M., Clarke J. C., Rashid H., Adhami M. D., McCullough K., Scott J. S., Chen H., Sinha K. M., de Crombrugghe B. and Javed A. (2018). Specificity protein 7 is required for proliferation and differentiation of ameloblasts and odontoblasts [WWW Document]. J. Bone Miner. Res. 33, 1126-1140. 10.1002/jbmr.3401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balic A. (2018). Biology explaining tooth repair and regeneration: a mini-review. Gerontology 64, 382-388. 10.1159/000486592 [DOI] [PubMed] [Google Scholar]
- Bartlett J. D. (2013). Dental enamel development: proteinases and their enamel matrix substrates [WWW Document]. ISRN Dent 2013, 684607 10.1155/2013/684607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergendal B., Norderyd J., Zhou X., Klar J. and Dahl N. (2016). Abnormal primary and permanent dentitions with ectodermal symptoms predict WNT10A deficiency. BMC Med. Genet 17, 88 10.1186/s12881-016-0349-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biehs B., Hu J. K.-H., Strauli N. B., Sangiorgi E., Jung H., Heber R.-P., Ho S., Goodwin A. F., Dasen J. S., Capecchi M. R. et al. (2013). BMI1 represses Ink4a/Arf and Hox genes to regulate stem cells in the rodent incisor. Nat. Cell Biol. 15, 846-852. 10.1038/ncb2766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchtová M., Štembírek J., Glocová K., Matalová E. and Tucker A. S. (2012). Early regression of the dental lamina underlies the development of diphyodont dentitions. J. Dent. Res. 91, 491-498. 10.1177/0022034512442896 [DOI] [PubMed] [Google Scholar]
- Calamari Z. T., Hu J. K.-H. and Klein O. D. (2018). Tissue mechanical forces and evolutionary developmental changes act through space and time to shape tooth morphology and function. BioEssays 40, 1800140 10.1002/bies.201800140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campàs O., Mammoto T., Hasso S., Sperling R. A., O'Connell D., Bischof A. G., Maas R., Weitz D. A., Mahadevan L. and Ingber D. E. (2014). Quantifying cell-generated mechanical forces within living embryonic tissues. Nat. Methods 11, 183-189. 10.1038/nmeth.2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantù C., Pagella P., Shajiei T. D., Zimmerli D., Valenta T., Hausmann G., Basler K. and Mitsiadis T. A. (2017). A cytoplasmic role of Wnt/β-catenin transcriptional cofactors Bcl9, Bcl9l, and Pygopus in tooth enamel formation. Sci. Signal. 10, eaah4598 10.1126/scisignal.aah4598 [DOI] [PubMed] [Google Scholar]
- Cao H., Wang J., Li X., Florez S., Huang Z., Venugopalan S. R., Elangovan S., Skobe Z., Margolis H. C., Martin J. F. et al. (2010). MicroRNAs play a critical role in tooth development. J. Dent. Res. 89, 779-784. 10.1177/0022034510369304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C. K. F., Seo E. Y., Chen J. Y., Lo D., McArdle A., Sinha R., Tevlin R., Seita J., Vincent-Tompkins J., Wearda T. et al. (2015). Identification and specification of the mouse skeletal stem cell. Cell 160, 285-298. 10.1016/j.cell.2014.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles C., Hovorakova M., Ahn Y., Lyons D. B., Marangoni P., Churava S., Biehs B., Jheon A., Lesot H., Balooch G. et al. (2011). Regulation of tooth number by fine-tuning levels of receptor-tyrosine kinase signaling. Development 138, 4063-4073. 10.1242/dev.069195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Lan Y., Baek J.-A., Gao Y. and Jiang R. (2009). Wnt/beta-catenin signaling plays an essential role in activation of odontogenic mesenchyme during early tooth development. Dev. Biol. 334, 174-185. 10.1016/j.ydbio.2009.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coin R., Lesot H., Vonesch J. L., Haikel Y. and Ruch J. V. (1999). Aspects of cell proliferation kinetics of the inner dental epithelium during mouse molar and incisor morphogenesis: a reappraisal of the role of the enamel knot area. Int. J. Dev. Biol. 43, 261-267. [PubMed] [Google Scholar]
- Cooper P. R. and Smith A. J. (2013). Molecular mediators of pulp inflammation and regeneration. Endodontic Topics 28, 90-105. 10.1111/etp.12036 [DOI] [Google Scholar]
- Cooper P. R., Holder M. J., Smith A. J, (2014). Inflammation and Regeneration in the Dentin-Pulp Complex: A Double-edged Sword. Journal of Endodontics, Presentations from the International Association of Dental Research (IADR) Pulp Biology and Regeneration Group Satellite Meeting, March 24–26, 2013, San Francisco, California 40, S46–S51 10.1016/j.joen.2014.01.021 [DOI]
- Cotsarelis G., Sun T. T. and Lavker R. M. (1990). Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 61, 1329-1337. 10.1016/0092-8674(90)90696-C [DOI] [PubMed] [Google Scholar]
- Delle Monache S., Martellucci S., Clementi L., Pulcini F., Santilli F., Mei C., Piccoli L., Angelucci A. and Mattei V. (2019). In vitro conditioning determines the capacity of dental pulp stem cells to function as pericyte-like cells. Stem Cells Dev. 28, 695-706. 10.1089/scd.2018.0192 [DOI] [PubMed] [Google Scholar]
- Dosedělová H., Dumková J., Lesot H., Glocová K., Kunová M., Tucker A. S., Veselá I., Krejčí P., Tichý F., Hampl A. et al. (2015). Fate of the molar dental lamina in the monophyodont mouse. PLoS ONE 10, e0127543 10.1371/journal.pone.0127543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du W., Hu J. K.-H., Du W. and Klein O. D. (2017). Lineage tracing of epithelial cells in developing teeth reveals two strategies for building signaling centers. J. Biol. Chem. 292, 15062-15069. 10.1074/jbc.M117.785923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque C., Hebling J., Smith A. J., Giro E. M. A., Oliveira M. F. and Costa C. A. D. S. (2006). Reactionary dentinogenesis after applying restorative materials and bioactive dentin matrix molecules as liners in deep cavities prepared in nonhuman primate teeth. J. Oral Rehabil. 33, 452-461. 10.1111/j.1365-2842.2005.01585.x [DOI] [PubMed] [Google Scholar]
- Fanchon S., Bourd K., Septier D., Everts V., Beertsen W., Menashi S. and Goldberg M. (2004). Involvement of matrix metalloproteinases in the onset of dentin mineralization. Eur. J. Oral. Sci. 112, 171-176. 10.1111/j.1600-0722.2004.00120.x [DOI] [PubMed] [Google Scholar]
- Feng J., Mantesso A., De Bari C., Nishiyama A. and Sharpe P. T. (2011). Dual origin of mesenchymal stem cells contributing to organ growth and repair. Proc. Natl. Acad. Sci. USA 108, 6503-6508. 10.1073/pnas.1015449108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E. (2009). The tortoise and the hair: slow-cycling cells in the stem cell race. Cell 137, 811-819. 10.1016/j.cell.2009.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajjeraman S., Narayanan K., Hao J., Qin C. and George A. (2007). Matrix macromolecules in hard tissues control the nucleation and hierarchical assembly of hydroxyapatite. J. Biol. Chem. 282, 1193-1204. 10.1074/jbc.M604732200 [DOI] [PubMed] [Google Scholar]
- Gong Q., Wang R., Jiang H., Lin Z. and Ling J. (2012). Alteration of MicroRNA expression of human dental pulp cells during odontogenic differentiation. J. Endod. 38, 1348-1354. 10.1016/j.joen.2012.06.016 [DOI] [PubMed] [Google Scholar]
- Häärä O., Harjunmaa E., Lindfors P. H., Huh S.-H., Fliniaux I., Åberg T., Jernvall J., Ornitz D. M., Mikkola M. L. and Thesleff I. (2012). Ectodysplasin regulates activator-inhibitor balance in murine tooth development through Fgf20 signaling. Development 139, 3189-3199. 10.1242/dev.079558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegebarth A. and Clevers H. (2009). Wnt signaling, lgr5, and stem cells in the intestine and skin. Am. J. Pathol. 174, 715-721. 10.2353/ajpath.2009.080758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada H., Kettunen P., Jung H.-S., Mustonen T., Wang Y. A. and Thesleff I. (1999). Localization of putative stem cells in dental epithelium and their association with notch and Fgf signaling. J. Cell Biol. 147, 105-120. 10.1083/jcb.147.1.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada H., Toyono T., Toyoshima K., Yamasaki M., Itoh N., Kato S., Sekine K. and Ohuchi H. (2002). FGF10 maintains stem cell compartment in developing mouse incisors. Development 129, 1533-1541. 10.1080/713713524 [DOI] [PubMed] [Google Scholar]
- Harada H., Ichimori Y., Yokohama-Tamaki T., Ohshima H., Kawano S., Katsube K. and Wakisaka S. (2006). Stratum intermedium lineage diverges from ameloblast lineage via Notch signaling. Biochem. Biophys. Res. Commun. 340, 611-616. 10.1016/j.bbrc.2005.12.053 [DOI] [PubMed] [Google Scholar]
- Harjunmaa E., Seidel K., Häkkinen T., Renvoisé E., Corfe I. J., Kallonen A., Zhang Z.-Q., Evans A. R., Mikkola M. L., Salazar-Ciudad I. et al. (2014). Replaying evolutionary transitions from the dental fossil record. Nature 512, 44-48. 10.1038/nature13613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X. C., Zhang J., Tong W.-G., Tawfik O., Ross J., Scoville D. H., Tian Q., Zeng X., He X., Wiedemann L. M. et al. (2004). BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nat. Genet. 36, 1117-1121. 10.1038/ng1430 [DOI] [PubMed] [Google Scholar]
- Headon D. J., Emmal S. A., Ferguson B. M., Tucker A. S., Justice M. J., Sharpe P. T., Zonana J. and Overbeek P. A. (2001). Gene defect in ectodermal dysplasia implicates a death domain adapter in development. Nature 414, 913-916. 10.1038/414913a [DOI] [PubMed] [Google Scholar]
- Hovorakova M., Lesot H., Peterkova R. and Peterka M. (2006). Origin of the deciduous upper lateral incisor and its clinical aspects. J. Dent. Res. 85, 167-171. 10.1177/154405910608500210 [DOI] [PubMed] [Google Scholar]
- Hovorakova M., Lochovska K., Zahradnicek O., Domonkosova Tibenska K., Dornhoferova M., Horakova-Smrckova L. and Bodorikova S. (2016). One odontogenic cell-population contributes to the development of the mouse incisors and of the oral vestibule. PLoS ONE 11, e0162523 10.1371/journal.pone.0162523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J. K.-H., Du W., Shelton S. J., Oldham M. C., DiPersio C. M. and Klein O. D. (2017). An FAK-YAP-mTOR signaling axis regulates stem cell-based tissue renewal in mice. Cell Stem Cell 21, 91-106.e6. 10.1016/j.stem.2017.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsken J., Vogel R., Erdmann B., Cotsarelis G. and Birchmeier W. (2001). β-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell 105, 533-545. 10.1016/S0092-8674(01)00336-1 [DOI] [PubMed] [Google Scholar]
- Iwata T., Yamakoshi Y., Hu J. C.-C., Ishikawa I., Bartlett J. D., Krebsbach P. H. and Simmer J. P. (2007). Processing of ameloblastin by MMP-20. J. Dent. Res. 86, 153-157. 10.1177/154405910708600209 [DOI] [PubMed] [Google Scholar]
- Järvinen E., Salazar-Ciudad I., Birchmeier W., Taketo M. M., Jernvall J. and Thesleff I. (2006). Continuous tooth generation in mouse is induced by activated epithelial Wnt/beta-catenin signaling. Proc. Natl. Acad. Sci. USA 103, 18627-18632. 10.1073/pnas.0607289103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järvinen E., Shimomura-Kuroki J., Balic A., Jussila M. and Thesleff I. (2018). Mesenchymal Wnt/β-catenin signaling limits tooth number. Development 145, dev158048 10.1242/dev.158048 [DOI] [PubMed] [Google Scholar]
- Jernvall J. and Thesleff I. (2012). Tooth shape formation and tooth renewal: evolving with the same signals. Development 139, 3487-3497. 10.1242/dev.085084 [DOI] [PubMed] [Google Scholar]
- Jernvall J., Aberg T., Kettunen P., Keränen S. and Thesleff I. (1998). The life history of an embryonic signaling center: BMP-4 induces p21 and is associated with apoptosis in the mouse tooth enamel knot. Development 125, 161-169. [DOI] [PubMed] [Google Scholar]
- Jernvall J., Kettunen P., Karavanova I., Martin L. B. and Thesleff I. (2002). Evidence for the role of the enamel knot as a control center in mammalian tooth cusp formation: non-dividing cells express growth stimulating Fgf-4 gene. Int. J. Dev. Biol. 38, 463-469. [PubMed] [Google Scholar]
- Juuri E., Saito K., Ahtiainen L., Seidel K., Tummers M., Hochedlinger K., Klein O. D., Thesleff I. and Michon F. (2012). Sox2+ stem cells contribute to all epithelial lineages of the tooth via Sfrp5+ progenitors. Dev. Cell 23, 317-328. 10.1016/j.devcel.2012.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juuri E., Jussila M., Seidel K., Holmes S., Wu P., Richman J., Heikinheimo K., Chuong C.-M., Arnold K., Hochedlinger K. et al. (2013). Sox2 marks epithelial competence to generate teeth in mammals and reptiles. Development 140, 1424-1432. 10.1242/dev.089599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karcher-Djuricic V., Staubli A., Meyer J. M. and Ruch J. V. (1985). Acellular dental matrices promote functional differentiation of ameloblasts. Differentiation 29, 169-175. 10.1111/j.1432-0436.1985.tb00311.x [DOI] [PubMed] [Google Scholar]
- Kassai Y., Munne P., Hotta Y., Penttilä E., Kavanagh K., Ohbayashi N., Takada S., Thesleff I., Jernvall J. and Itoh N. (2005). Regulation of mammalian tooth cusp patterning by ectodin. Science 309, 2067-2070. 10.1126/science.1116848 [DOI] [PubMed] [Google Scholar]
- Kaukua N., Shahidi M. K., Konstantinidou C., Dyachuk V., Kaucka M., Furlan A., An Z., Wang L., Hultman I., Ährlund-Richter L. et al. (2014). Glial origin of mesenchymal stem cells in a tooth model system. Nature 513, 551-554. 10.1038/nature13536 [DOI] [PubMed] [Google Scholar]
- Kawasaki K. and Weiss K. M. (2003). Mineralized tissue and vertebrate evolution: the secretory calcium-binding phosphoprotein gene cluster. Proc. Natl. Acad. Sci. USA 100, 4060-4065. 10.1073/pnas.0638023100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kere J., Srivastava A. K., Montonen O., Zonana J., Thomas N., Ferguson B., Munoz F., Morgan D., Clarke A., Baybayan P. et al. (1996). X-linked anhidrotic (hypohidrotic) ectodermal dysplasia is caused by mutation in a novel transmembrane protein. Nat. Genet. 13, 409-416. 10.1038/ng0895-409 [DOI] [PubMed] [Google Scholar]
- Kim J.-W., Simmer J. P., Hu Y. Y., Lin B. P.-L., Boyd C., Wright J. T., Yamada C. J. M., Rayes S. K., Feigal R. J. and Hu J. C.-C. (2004). Amelogenin p.M1T and p.W4S mutations underlying hypoplastic X-linked amelogenesis imperfecta. J. Dent. Res. 83, 378-383. 10.1177/154405910408300505 [DOI] [PubMed] [Google Scholar]
- Kim J.-W., Seymen F., Lin B. P.-J., Kiziltan B., Gencay K., Simmer J. P. and Hu J. C.-C. (2005). ENAM mutations in autosomal-dominant amelogenesis imperfecta. J. Dent. Res. 84, 278-282. 10.1177/154405910508400314 [DOI] [PubMed] [Google Scholar]
- Klein O. D., Minowada G., Peterkova R., Kangas A., Yu B. D., Lesot H., Peterka M., Jernvall J. and Martin G. R. (2006). Sprouty genes control diastema tooth development via bidirectional antagonism of epithelial-mesenchymal FGF signaling. Dev. Cell 11, 181-190. 10.1016/j.devcel.2006.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramann R., Schneider R. K., DiRocco D. P., Machado F., Fleig S., Bondzie P. A., Henderson J. M., Ebert B. L. and Humphreys B. D. (2015). Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell 16, 51-66. 10.1016/j.stem.2014.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriangkrai R., Iseki S., Eto K. and Chareonvit S. (2006). Dual odontogenic origins develop at the early stage of rat maxillary incisor development. Anat. Embryol. 211, 101-108. 10.1007/s00429-005-0068-7 [DOI] [PubMed] [Google Scholar]
- Kuraguchi M., Wang X.-P., Bronson R. T., Rothenberg R., Ohene-Baah N. Y., Lund J. J., Kucherlapati M., Maas R. L. and Kucherlapati R. (2006). Adenomatous polyposis coli (APC) is required for normal development of skin and thymus. PLoS Genet. 2, e146 10.1371/journal.pgen.0020146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosaka H., Islam M. N., Kuremoto K., Hayano S., Nakamura M., Kawanabe N., Yanagita T., Rice D. P. C., Harada H., Taniuchi I. et al. (2011). Core binding factor beta functions in the maintenance of stem cells and orchestrates continuous proliferation and differentiation in mouse incisors. Stem Cells 29, 1792-1803. 10.1002/stem.722 [DOI] [PubMed] [Google Scholar]
- Kyrylkova K., Kyryachenko S., Biehs B., Klein O., Kioussi C. and Leid M. (2012). BCL11B regulates epithelial proliferation and asymmetric development of the mouse mandibular incisor. PLoS ONE 7, e37670 10.1371/journal.pone.0037670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammi L., Arte S., Somer M., Jarvinen H., Lahermo P., Thesleff I., Pirinen S. and Nieminen P. (2004). Mutations in AXIN2 cause familial tooth agenesis and predispose to colorectal cancer. Am. J. Hum. Genet. 74, 1043-1050. 10.1086/386293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurikkala J., Kassai Y., Pakkasjärvi L., Thesleff I. and Itoh N. (2003). Identification of a secreted BMP antagonist, ectodin, integrating BMP, FGF, and SHH signals from the tooth enamel knot. Dev. Biol. 264, 91-105. 10.1016/j.ydbio.2003.08.011 [DOI] [PubMed] [Google Scholar]
- Li D., Zhou J., Chowdhury F., Cheng J., Wang N. and Wang F. (2011). Role of mechanical factors in fate decisions of stem cells. Regen. Med. 6, 229-240. 10.2217/rme.11.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C.-Y., Prochazka J., Goodwin A. F. and Klein O. D. (2014). Fibroblast growth factor signaling in mammalian tooth development. Odontology 102, 1-13. 10.1007/s10266-013-0142-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Feng J., Liu Y., Ho T.-V., Grimes W., Ho H. A., Park S., Wang S. and Chai Y. (2015). BMP-SHH signaling network controls epithelial stem cell fate via regulation of its niche in the developing tooth. Dev. Cell 33, 125-135. 10.1016/j.devcel.2015.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Chatzeli L., Panousopoulou E., Tucker A. S. and Green J. B. A. (2016). Epithelial stratification and placode invagination are separable functions in early morphogenesis of the molar tooth. Development 143, 670-681. 10.1242/dev.130187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., Chu E. Y., Watt B., Zhang Y., Gallant N. M., Andl T., Yang S. H., Lu M.-M., Piccolo S., Schmidt-Ullrich R. et al. (2008). Wnt/β-catenin signaling directs multiple stages of tooth morphogenesis. Dev. Biol. 313, 210-224. 10.1016/j.ydbio.2007.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., Dangaria S., Andl T., Zhang Y., Wright A. C., Damek-Poprawa M., Piccolo S., Nagy A., Taketo M. M., Diekwisch T. G. H. et al. (2010). β-Catenin initiates tooth neogenesis in adult rodent incisors. J. Dent. Res. 89, 909-914. 10.1177/0022034510370090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z.-X., Kielan-Jaworowska Z. and Cifelli R. L. (2004). Evolution of dental replacement in mammals. Carb 2004, 159-175. 10.2992/0145-9058(2004)36[159:EODRIM]2.0.CO;2 [DOI] [Google Scholar]
- Marangoni P., Charles C., Ahn Y., Seidel K., Jheon A., Ganss B., Krumlauf R., Viriot L. and Klein O. D. (2019). Downregulation of FGF signaling by Spry4 overexpression leads to shape impairment, enamel irregularities, and delayed signaling center formation in the mouse molar. JBMR Plus 3, e10205 10.1002/jbm4.10205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Riera M., Moustakas-Verho J., Savriama Y., Jernvall J. and Salazar-Ciudad I. (2018). Differential tissue growth and cell adhesion alone drive early tooth morphogenesis: An ex vivo and in silico study. PLoS Comput. Biol. 14, e1005981 10.1371/journal.pcbi.1005981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massink M. P. G., Créton M. A., Spanevello F., Fennis W. M. M., Cune M. S., Savelberg S. M. C., Nijman I. J., Maurice M. M., van den Boogaard M.-J. H. and van Haaften G. (2015). Loss-of-function mutations in the WNT co-receptor LRP6 cause autosomal-dominant oligodontia. Am. J. Hum. Genet. 97, 621-626. 10.1016/j.ajhg.2015.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michon F., Tummers M., Kyyrönen M., Frilander M. J. and Thesleff I. (2010). Tooth morphogenesis and ameloblast differentiation are regulated by micro-RNAs. Dev. Biol. 340, 355-368. 10.1016/j.ydbio.2010.01.019 [DOI] [PubMed] [Google Scholar]
- Millar S. E., Koyama E., Reddy S. T., Andl T., Gaddapara T., Piddington R. and Gibson C. W. (2003). Over- and ectopic expression of Wnt3 causes progressive loss of ameloblasts in postnatal mouse incisor teeth. Connect. Tissue Res. 44, 124-129. 10.1080/03008200390152205 [DOI] [PubMed] [Google Scholar]
- Nagano T., Kakegawa A., Yamakoshi Y., Tsuchiya S., Hu J. C.-C., Gomi K., Arai T., Bartlett J. D. and Simmer J. P. (2009). Mmp-20 and Klk4 cleavage site preferences for amelogenin sequences. J. Dent. Res. 88, 823-828. 10.1177/0022034509342694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., de Vega S., Fukumoto S., Jimenez L., Unda F. and Yamada Y. (2008). Transcription factor epiprofin is essential for tooth morphogenesis by regulating epithelial cell fate and tooth number. J. Biol. Chem. 283, 4825-4833. 10.1074/jbc.M708388200 [DOI] [PubMed] [Google Scholar]
- Nakatomi M., Wang X.-P., Key D., Lund J. J., Turbe-Doan A., Kist R., Aw A., Chen Y., Maas R. L. and Peters H. (2010). Genetic interactions between Pax9 and Msx1 regulate lip development and several stages of tooth morphogenesis. Dev. Biol. 340, 438-449. 10.1016/j.ydbio.2010.01.031 [DOI] [PubMed] [Google Scholar]
- Narayanan K., Srinivas R., Ramachandran A., Hao J., Quinn B. and George A. (2001). Differentiation of embryonic mesenchymal cells to odontoblast-like cells by overexpression of dentin matrix protein 1. Proc. Natl. Acad. Sci. USA 98, 4516-4521. 10.1073/pnas.081075198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan K., Gajjeraman S., Ramachandran A., Hao J. and George A. (2006). Dentin matrix protein 1 regulates dentin sialophosphoprotein gene transcription during early odontoblast differentiation. J. Biol. Chem. 281, 19064-19071. 10.1074/jbc.M600714200 [DOI] [PubMed] [Google Scholar]
- Noda S., Kawashima N., Yamamoto M., Hashimoto K., Nara K., Sekiya I. and Okiji T. (2019). Effect of cell culture density on dental pulp-derived mesenchymal stem cells with reference to osteogenic differentiation. Sci. Rep. 9, 1-11. 10.1038/s41598-018-37186-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohazama A., Courtney J.-M., Tucker A. S., Naito A., Tanaka S., Inoue J.-I. and Sharpe P. T. (2004). Traf6 is essential for murine tooth cusp morphogenesis. Dev. Dyn. 229, 131-135. 10.1002/dvdy.10400 [DOI] [PubMed] [Google Scholar]
- Ohshima H., Nakasone N., Hashimoto E., Sakai H., Nakakura-Ohshima K. and Harada H. (2005). The eternal tooth germ is formed at the apical end of continuously growing teeth. Arch. Oral. Biol. 50, 153-157. 10.1016/j.archoralbio.2004.09.008 [DOI] [PubMed] [Google Scholar]
- O'Meara R. N. and Asher R. J. (2016). The evolution of growth patterns in mammalian versus nonmammalian cynodonts. Paleobiology 42, 439-464. 10.1017/pab.2015.51 [DOI] [Google Scholar]
- Oommen S., Otsuka-Tanaka Y., Imam N., Kawasaki M., Kawasaki K., Jalani-Ghazani F., Anderegg A., Awatramani R., Hindges R., Sharpe P. T. et al. (2012). Distinct roles of MicroRNAs in epithelium and mesenchyme during tooth development. Dev. Dyn. 241, 1465-1472. 10.1002/dvdy.23828 [DOI] [PubMed] [Google Scholar]
- Pang Y. W., Feng J., Daltoe F., Fatscher R., Gentleman E., Gentleman M. M. and Sharpe P. T. (2016). Perivascular stem cells at the tip of mouse incisors regulate tissue regeneration. J. Bone Miner. Res. 31, 514-523. 10.1002/jbmr.2717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panousopoulou E. and Green J. B. A. (2016). Invagination of ectodermal placodes is driven by cell intercalation-mediated contraction of the suprabasal tissue canopy. PLoS Biol. 14, e1002405 10.1371/journal.pbio.1002405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul F., Arkin Y., Giladi A., Jaitin D. A., Kenigsberg E., Keren-Shaul H., Winter D., Lara-Astiaso D., Gury M., Weiner A. et al. (2015). Transcriptional heterogeneity and lineage commitment in myeloid progenitors. Cell 163, 1663-1677. 10.1016/j.cell.2015.11.013 [DOI] [PubMed] [Google Scholar]
- Peters H., Neubüser A., Kratochwil K. and Balling R. (1998). Pax9-deficient mice lack pharyngeal pouch derivatives and teeth and exhibit craniofacial and limb abnormalities. Genes Dev. 12, 2735-2747. 10.1101/gad.12.17.2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pispa J., Jung H. S., Jernvall J., Kettunen P., Mustonen T., Tabata M. J., Kere J. and Thesleff I. (1999). Cusp patterning defect in Tabby mouse teeth and its partial rescue by FGF. Dev. Biol. 216, 521-534. 10.1006/dbio.1999.9514 [DOI] [PubMed] [Google Scholar]
- Popa E. M., Buchtova M. and Tucker A. S. (2019). Revitalising the rudimentary replacement dentition in the mouse. Development 146, dev171363 10.1242/dev.171363 [DOI] [PubMed] [Google Scholar]
- Poulter J. A., Murillo G., Brookes S. J., Smith C. E. L., Parry D. A., Silva S., Kirkham J., Inglehearn C. F. and Mighell A. J. (2014). Deletion of ameloblastin exon 6 is associated with amelogenesis imperfecta. Hum. Mol. Genet. 23, 5317-5324. 10.1093/hmg/ddu247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochazka J., Prochazkova M., Du W., Spoutil F., Tureckova J., Hoch R., Shimogori T., Sedlacek R., Rubenstein J. L., Wittmann T. et al. (2015). Migration of founder epithelial cells drives proper molar tooth positioning and morphogenesis. Dev. Cell 35, 713-724. 10.1016/j.devcel.2015.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raia P., Carotenuto F., Eronen J. T. and Fortelius M. (2011). Longer in the tooth, shorter in the record? The evolutionary correlates of hypsodonty in Neogene ruminants. Proc. R. Soc. B 278, 3474-3481. 10.1098/rspb.2011.0273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindranath H. H., Chen L.-S., Zeichner-David M., Ishima R. and Ravindranath R. M. H. (2004). Interaction between the enamel matrix proteins amelogenin and ameloblastin. Biochem. Biophys. Res. Commun. 323, 1075-1083. 10.1016/j.bbrc.2004.08.207 [DOI] [PubMed] [Google Scholar]
- Renvoisé É., Kavanagh K. D., Lazzari V., Häkkinen T. J., Rice R., Pantalacci S., Salazar-Ciudad I. and Jernvall J. (2017). Mechanical constraint from growing jaw facilitates mammalian dental diversity. Proc. Natl. Acad. Sci. USA 114, 9403-9408. 10.1073/pnas.1707410114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz-Navarro M., Seidel K., Sun Z., Bertonnier-Brouty L., Amendt B. A., Klein O. D. and Michon F. (2018). Plasticity within the niche ensures the maintenance of a Sox2+ stem cell population in the mouse incisor. Development 145, dev155929 10.1242/dev.155929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satokata I. and Maas R. (1994). Msx1 deficient mice exhibit cleft palate and abnormalities of craniofacial and tooth development. Nat. Genet. 6, 348-356. 10.1038/ng0494-348 [DOI] [PubMed] [Google Scholar]
- Seidel K., Ahn C. P., Lyons D., Nee A., Ting K., Brownell I., Cao T., Carano R. A. D., Curran T., Schober M. et al. (2010). Hedgehog signaling regulates the generation of ameloblast progenitors in the continuously growing mouse incisor. Development 137, 3753-3761. 10.1242/dev.056358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel K., Marangoni P., Tang C., Houshmand B., Du W., Maas R. L., Murray S., Oldham M. C. and Klein O. D. (2017). Resolving stem and progenitor cells in the adult mouse incisor through gene co-expression analysis. eLife 6, e24712 10.7554/eLife.24712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharir A., Marangoni P., Zilionis R., Wan M., Wald T., Hu J. K., Kawaguchi K., Castillo-Azofeifa D., Epstein L., Harrington K. et al. (2019). A large pool of actively cycling progenitors orchestrates self-renewal and injury repair of an ectodermal appendage. Nat. Cell Biol. 21, 1102-1112. 10.1038/s41556-019-0378-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe P. T. (2016). Dental mesenchymal stem cells. Development 143, 2273-2280. 10.1242/dev.134189 [DOI] [PubMed] [Google Scholar]
- Shi S., Bartold P. M., Miura M., Seo B. M., Robey P. G. and Gronthos S. (2005). The efficacy of mesenchymal stem cells to regenerate and repair dental structures. Orthod. Craniofac. Res. 8, 191-199. 10.1111/j.1601-6343.2005.00331.x [DOI] [PubMed] [Google Scholar]
- Shi C., Yuan Y., Guo Y., Jing J., Ho T. V., Han X., Li J., Feng J. and Chai Y. (2019). BMP signaling in regulating mesenchymal stem cells in incisor homeostasis. J. Dent. Res. 98, 904-911. 10.1177/0022034519850812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoyama W., Liu Y., Fang D., Yamaza T., Seo B.-M., Zhang C., Liu H., Gronthos S., Wang C.-Y., Shi S. et al. (2006). Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS ONE 1, e79 10.1371/journal.pone.0000079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian H., Döring F., Kollert S., Rukoyatkina N., Sturm J., Gambaryan S., Stellzig-Eisenhauer A., Meyer-Marcotty P., Eigenthaler M. and Wischmeyer E. (2016). PTH1R mutants found in patients with primary failure of tooth eruption disrupt g-protein signaling. PLoS ONE 11, e0167033 10.1371/journal.pone.0167033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z., Yu W., Navarro M. S., Sweat M., Eliason S., Sharp T., Liu H., Seidel K., Zhang L., Moreno M. et al. (2016). Sox2 and Lef-1 interact with Pitx2 to regulate incisor development and stem cell renewal. Development 143, 4115-4126. 10.1242/dev.138883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suomalainen M. and Thesleff I. (2010). Patterns of Wnt pathway activity in the mouse incisor indicate absence of Wnt/β-catenin signaling in the epithelial stem cells. Dev. Dyn. 239, 364-372. 10.1002/dvdy.22106 [DOI] [PubMed] [Google Scholar]
- Takahashi A., Nagata M., Gupta A., Matsushita Y., Yamaguchi T., Mizuhashi K., Maki K., Ruellas A. C., Cevidanes L. S., Kronenberg H. M. et al. (2019). Autocrine regulation of mesenchymal progenitor cell fates orchestrates tooth eruption. Proc. Natl. Acad. Sci. USA 116, 575-580. 10.1073/pnas.1810200115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapaltsyan V., Eronen J. T., Lawing A. M., Sharir A., Janis C., Jernvall J. and Klein O. D. (2015). Continuously growing rodent molars result from a predictable quantitative evolutionary change over 50 million years. Cell Reports 11, 673-680. 10.1016/j.celrep.2015.03.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thesleff I., Keränen S. and Jernvall J. (2001). Enamel knots as signaling centers linking tooth morphogenesis and odontoblast differentiation. Adv. Dent. Res. 15, 14-18. 10.1177/08959374010150010401 [DOI] [PubMed] [Google Scholar]
- Tummers M. and Thesleff I. (2008). Observations on continuously growing roots of the sloth and the K14-Eda transgenic mice indicate that epithelial stem cells can give rise to both the ameloblast and root epithelium cell lineage creating distinct tooth patterns. Evol. Dev. 10, 187-195. 10.1111/j.1525-142X.2008.00226.x [DOI] [PubMed] [Google Scholar]
- Tummers M. and Thesleff I. (2003). Root or crown: a developmental choice orchestrated by the differential regulation of the epithelial stem cell niche in the tooth of two rodent species. Development 130, 1049-1057. 10.1242/dev.00332 [DOI] [PubMed] [Google Scholar]
- Vaahtokari A., Aberg T., Jernvall J., Keränen S. and Thesleff I. (1996). The enamel knot as a signaling center in the developing mouse tooth. Mech. Dev. 54, 39-43. 10.1016/0925-4773(95)00459-9 [DOI] [PubMed] [Google Scholar]
- Vainio S., Karavanova I., Jowett A. and Thesleff I. (1993). Identification of BMP-4 as a signal mediating secondary induction between epithelial and mesenchymal tissues during early tooth development. Cell 75, 45-58. 10.1016/S0092-8674(05)80083-2 [DOI] [PubMed] [Google Scholar]
- Wang X.-P., Suomalainen M., Felszeghy S., Zelarayan L. C., Alonso M. T., Plikus M. V., Maas R. L., Chuong C.-M., Schimmang T. and Thesleff I. (2007). An integrated gene regulatory network controls stem cell proliferation in teeth. PLoS Biol. 5, e159 10.1371/journal.pbio.0050159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.-P., O'Connell D. J., Lund J. J., Saadi I., Kuraguchi M., Turbe-Doan A., Cavallesco R., Kim H., Park P. J., Harada H. et al. (2009). Apc inhibition of Wnt signaling regulates supernumerary tooth formation during embryogenesis and throughout adulthood. Development 136, 1939-1949. 10.1242/dev.033803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Dobeck J. M., Sorkin B. C. and Skobe Z. (1998). Adenomatous polyposis coli protein is expressed in alternate stages of the ameloblast life cycle. J. Dent. Res. 77, 1979-1982. 10.1177/00220345980770120501 [DOI] [PubMed] [Google Scholar]
- Xavier G. M., Patist A. L., Healy C., Pagrut A., Carreno G., Sharpe P. T., Pedro Martinez-Barbera J., Thavaraj S., Cobourne M. T. and Andoniadou C. L. (2015). Activated WNT signaling in postnatal SOX2-positive dental stem cells can drive odontoma formation. Sci. Rep. 5, 14479 10.1038/srep14479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G., Zhou J., Teng Y., Xie J., Lin J., Guo X., Gao Y., He M., Yang X. and Wang S. (2014). Mesenchymal TGF-β signaling orchestrates dental epithelial stem cell homeostasis through Wnt signaling. Stem Cells 32, 2939-2948. 10.1002/stem.1772 [DOI] [PubMed] [Google Scholar]
- Yang J., Wang S.-K., Choi M., Reid B. M., Hu Y., Lee Y.-L., Herzog C. R., Kim-Berman H., Lee M., Benke P. J. et al. (2015). Taurodontism, variations in tooth number, and misshapened crowns in Wnt10a null mice and human kindreds. Mol. Genet. Genomic Med. 3, 40-58. 10.1002/mgg3.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Adam R. C., Ge Y., Hua Z. L. and Fuchs E. (2017). Epithelial-mesenchymal micro-niches govern stem cell lineage choices. Cell 169, 483-496.e13. 10.1016/j.cell.2017.03.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T., Graf M., Renn J., Schartl M., Larionova D., Huysseune A., Witten P. E. and Winkler C. (2017). A vertebrate-specific and essential role for osterix in osteogenesis revealed by gene knockout in the teleost medaka. Development 144, 265-271. 10.1242/dev.139550 [DOI] [PubMed] [Google Scholar]
- Zeichner-David M., Diekwisch T., Fincham A., Lau E., MacDougall M., Moradian-Oldak J., Simmer J., Snead M. and Slavkin H. C. (1995). Control of ameloblast differentiation. Int. J. Dev. Biol. 39, 69-92. [PubMed] [Google Scholar]
- Zhang W., Vazquez B., Oreadi D. and Yelick P. C. (2017). Decellularized tooth bud scaffolds for tooth regeneration. J. Dent. Res. 96, 516-523. 10.1177/0022034516689082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Oka K., Bringas P., Kaartinen V. and Chai Y. (2008). TGF-beta type I receptor Alk5 regulates tooth initiation and mandible patterning in a type II receptor-independent manner. Dev. Biol. 320, 19-29. 10.1016/j.ydbio.2008.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Li S., Han D., Kaartinen V. and Chai Y. (2011). Alk5-mediated transforming growth factor β signaling acts upstream of fibroblast growth factor 10 to regulate the proliferation and maintenance of dental epithelial stem cells. Mol. Cell. Biol. 31, 2079-2089. 10.1128/MCB.01439-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Feng J., Seidel K., Shi S., Klein O., Sharpe P. and Chai Y. (2014). Secretion of Shh by a neurovascular bundle niche supports mesenchymal stem cell homeostasis in the adult mouse incisor. Cell Stem Cell 14, 160-173. 10.1016/j.stem.2013.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X., Goodwin A. F., Tian H., Jheon A. H. and Klein O. D. (2017). Ras signaling regulates stem cells and amelogenesis in the mouse incisor. J. Dent. Res. 96, 1438-1444. 10.1177/0022034517717255 [DOI] [PMC free article] [PubMed] [Google Scholar]