Abstract
Background
Pruritus is a sensation that leads to the desire to scratch; its origin is unknown in 8% to 15% of affected patients. The prevalence of chronic pruritus of unknown origin (CPUO) in individuals with generalised pruritus ranges from 3.6% to 44.5%, with highest prevalence among the elderly. When the origin of pruritus is known, its management may be straightforward if an effective treatment for the causal disease is available. Treatment of CPUO is particularly difficult due to its unknown pathophysiology.
Objectives
To assess the effects of interventions for CPUO in adults and children.
Search methods
We searched the following up to July 2019: Cochrane Skin Group Specialised Register, CENTRAL, MEDLINE, Embase, and trials registries. We checked the reference lists of included studies for additional references to relevant trials.
Selection criteria
We sought to include randomised controlled trials and quasi‐randomised controlled trials that assessed interventions for CPUO, as defined in category VI ('Other pruritus of undetermined origin, or chronic pruritus of unknown origin') of the International Forum for the Study of Itch (IFSI) classification, in children and adults. Eligible interventions were non‐pharmacological or topical or systemic pharmacological interventions, and eligible comparators were another active treatment, placebo, sham procedures, or no treatment or equivalent (e.g. waiting list).
Data collection and analysis
We used standard methodological procedures expected by Cochrane. Our primary outcomes were 'Patient‐ or parent‐reported pruritus intensity' and 'Adverse events'. Our secondary outcomes were 'Health‐related quality of life', 'Sleep disturbances', 'Depression', and 'Patient satisfaction'. We used GRADE to assess the certainty of evidence.
Main results
We found there was an absence of evidence for the main interventions of interest: emollient creams, cooling lotions, topical corticosteroids, topical antidepressants, systemic antihistamines, systemic antidepressants, systemic anticonvulsants, and phototherapy.
We included one study with 257 randomised (253 analysed) participants, aged 18 to 65 years; 60.6% were female. This study investigated the safety and efficacy of three different doses of oral serlopitant (5 mg, 1 mg, and 0.25 mg, once daily for six weeks) compared to placebo for severe chronic pruritus; 25 US centres participated (clinical research centres and universities). All outcomes were measured at the end of treatment (six weeks from baseline), except adverse events, which were monitored throughout. A pharmaceutical company funded this study.
Fifty‐five per cent of participants suffered from CPUO, and approximately 45% presented a dermatological diagnosis (atopic dermatitis/eczema 37.3%, psoriasis 6.7%, acne 3.6%, among other diagnoses). We unsuccessfully attempted to retrieve outcome data from study authors for the subgroup of participants with CPUO. Participants had pruritus for six weeks or longer. Total study duration was 10 weeks.
Participants who received serlopitant 5 mg may have a greater rate of relief of patient‐reported pruritus intensity as measured by the visual analogue scale (VAS; a reduction in VAS score indicates improvement) compared to placebo (126 participants, risk ratio (RR) 2.06, 95% confidence interval (CI) 1.27 to 3.35; low‐certainty evidence). We are uncertain of the effects of serlopitant 5 mg compared to placebo on the following outcomes due to very low‐certainty evidence: adverse events (127 participants; RR 1.48, 95% CI 0.87 to 2.50); health‐related quality of life (as measured by the Dermatology Life Quality Index (DLQI); a higher score indicates greater impairment; 127 participants; mean difference (MD) ‐4.20, 95% CI ‐11.68 to 3.28); and sleep disturbances (people with insomnia measured by the Pittsburgh Sleep Symptom Questionnaire‐Insomnia (PSSQ‐I), a dichotomous measure; 128 participants; RR 0.49, 95% CI 0.24 to 1.01).
Participants who received serlopitant 1 mg may have a greater rate of relief of patient‐reported pruritus intensity as measured by VAS compared to placebo; however, the 95% CI indicates that there may also be little to no difference between groups (126 participants; RR 1.50, 95% CI 0.89 to 2.54; low‐certainty evidence). We are uncertain of the effects of serlopitant 1 mg compared to placebo on the following outcomes due to very low‐certainty evidence: adverse events (128 participants; RR 1.45, 95% CI 0.86 to 2.47); health‐related quality of life (DLQI; 128 participants; MD ‐6.90, 95% CI ‐14.38 to 0.58); and sleep disturbances (PSSQ‐I; 128 participants; RR 0.38, 95% CI 0.17 to 0.84).
Participants who received serlopitant 0.25 mg may have a greater rate of relief of patient‐reported pruritus intensity as measured by VAS compared to placebo; however, the 95% CI indicates that there may also be little to no difference between groups (127 participants; RR 1.66, 95% CI 1.00 to 2.77; low‐certainty evidence). We are uncertain of the effects of serlopitant 0.25 mg compared to placebo on the following outcomes due to very low‐certainty evidence: adverse events (127 participants; RR 1.29, 95% CI 0.75 to 2.24); health‐related quality of life (DLQI; 127 participants; MD ‐5.70, 95% CI ‐13.18 to 1.78); and sleep disturbances (PSSQ‐I; 127 participants; RR 0.60, 95% CI 0.31 to 1.17).
The most commonly reported adverse events were somnolence, diarrhoea, headache, and nasopharyngitis, among others.
Our included study did not measure depression or patient satisfaction.
We downgraded the certainty of evidence for all outcomes due to indirectness (only 55% of study participants had CPUO) and imprecision. We downgraded outcomes other than patient‐reported pruritus intensity a further level due to concerns regarding risk of bias in selection of the reported result and some concerns with risk of bias due to missing outcome data (sleep disturbances only). We deemed risk of bias to be generally low.
Authors' conclusions
We found lack of evidence to address our review question: for most of our interventions of interest, we found no eligible studies. The neurokinin 1 receptor (NK1R) antagonist serlopitant was the only intervention that we could assess. One study provided low‐certainty evidence suggesting that serlopitant may reduce pruritus intensity when compared with placebo. We are uncertain of the effects of serlopitant on other outcomes, as certainty of the evidence is very low.
More studies with larger sample sizes, focused on patients with CPUO, are needed. Healthcare professionals, patients, and other stakeholders may have to rely on indirect evidence related to other forms of chronic pruritus when deciding between the main interventions currently used for this condition.
Plain language summary
Treatments for pruritus (itching) of unknown cause in children and adults
Review question
We wanted to investigate the effects of treatment for chronic (lasting longer than six weeks) pruritus (itching) of unknown cause in children and adults. We assessed all treatments, as long as they were compared against each other, placebo (an identical but inactive treatment), a sham procedure, or no treatment (or equivalent, e.g. waiting list). We were particularly interested in assessing safety and itch intensity as reported by the patient or the parent.
Background
Pruritus, or itching, is an unpleasant sensation that provokes a desire to scratch. It can be caused by diseases of the skin or other parts of the body. We searched the medical literature up to July 2019 to determine the effects of drug and non‐drug therapies (e.g. phototherapy) used for treatment of itching of unknown cause.
Study characteristics
We included one study (257 participants) that investigated the safety and efficacy of three different doses of a drug called serlopitant (5 mg, 1 mg, and 0.25 mg, taken by mouth once daily for six weeks) versus placebo for severe chronic pruritus (participants had a score of 7 cm or higher on the visual analogue scale (VAS)). The age of included participants ranged from 18 to 65; 60.6% were women; 55% suffered from itching of unknown origin; and approximately 45% presented a dermatological diagnosis (atopic dermatitis/eczema 37.3%, psoriasis 6.7%, acne 3.6%, among other diagnoses). A pharmaceutical company funded this study, which was undertaken across 25 centres in the United States (clinical research centres and universities). The study lasted 10 weeks in total (six weeks of treatment plus four weeks of post‐treatment follow‐up).
We found no eligible studies for the main treatments we sought to assess, which included emollient creams, cooling lotions, topical corticosteroids (a class of steroid hormones) or antidepressants, systemic antihistamines (medicines used to relieve symptoms of allergies) or antidepressants, anticonvulsants (antiseizure drugs), and phototherapy.
Key results
Participants who received serlopitant at doses of 0.25 mg, 5 mg, and 1 mg may be more likely to experience reduced itch intensity, as reported by the patient, when compared with participants given placebo (low‐certainty evidence). However, for serlopitant 1 mg and 0.25 mg, the range of possible results indicates there may be little to no difference between groups.
We are uncertain of the effects of serlopitant (in the three doses) on side effects, health‐related quality of life, and sleep disturbances due to very low‐certainty evidence.
The most commonly reported side effects were sleepiness, diarrhoea, headache, and upper respiratory tract infection, among others.
All outcomes were measured at the end of treatment (six weeks from baseline) with the exception of adverse events, which were monitored throughout the study.
The included study did not report the effects of this drug on depression and patient satisfaction.
Certainty of the evidence
Certainty of the evidence was low for patient‐reported itch intensity because 45% of participants had an identifiable skin disease and 55% had itch of poorly defined cause. Additionally, the number of study participants was small and there were few occurrences of the outcomes, or results were imprecise or were not meaningful; therefore, the study was at risk of random errors.
Certainty of the evidence was very low for three outcomes (adverse events, quality of life, and sleep disturbances) due to additional concerns that measurement of these outcomes was not pre‐planned. Also, no information was available to assess bias from missing data for the outcome of sleep disturbances.
Summary of findings
Background
Description of the condition
We have defined relevant terms in Table 4.
1. Glossary of terms.
| Term | Definition |
| 2‐Amino‐3‐(5‐methyl‐3 ‐oxo‐1,2‐oxazol‐4‐yl)‐ propanoic acid receptor (AMPA) | A molecule that binds to the neurotransmitter glutamate and is associated with many biological functions |
| 5‐HT3 receptor | Serotonin‐activated ion channels that perform functions in the nervous system |
| Acetylcholine | Neurotransmitter released by nerve cells to send signals to other cells |
| Acneiform | Resembling acne |
| Afferent | An anatomical term meaning 'conveying towards a centre'. Peripheral nerves transmitting impulses to the central nervous system |
| Alkaloid | A group of naturally occurring chemical compounds that mostly contain basic nitrogen atoms |
| Amino acids | An organic molecule (part of proteins) that play a key role in almost all biological processes |
| Amyloidosis | Abnormal deposition of amyloid (insoluble fibres comprising sheets of protein) in extracellular tissues (e.g. cutaneous) |
| Anaesthetic | Agent that produces a local or general loss of sensation by acting on the brain or peripheral nervous system to suppress responses to sensory stimulation |
| Atrophy | A reduction in the size of cell, organ, or tissue, after its normal mature growth is attained |
| Atropine | A reversible antagonist of the muscarinic acetylcholine receptors |
| Axon | A usually long and single nerve cell process that usually conducts impulses away from the cell body |
| Basal cell carcinoma | An abnormal, uncontrolled growth or cancerous lesions that arise in the skin’s basal cells, which line the deepest layer of the epidermis |
| Basophils | Type of white blood cells. They are responsible for inflammatory reactions during the immune response. |
| Bias | Systematic error, or deviation from the truth, in results or inferences |
| Brachioradial pruritus | A neurogenic itch syndrome of the upper extremities |
| Bradykinin | An inflammatory mediator. Bradykinin is a potent endothelium‐dependent vasodilator, leading to a drop in blood pressure. It also causes contraction of non‐vascular smooth muscle in the bronchus and gut, increases vascular permeability, and is involved in the mechanism of pain |
| Calcineurin | An enzyme responsible for the activation of protein responsible for stimulation of growth and differentiation of T lymphocytes |
| Calcitonin gene‐related peptide | A protein produced in both peripheral and central neurons, found throughout the body, that modulates a variety of physiological functions in all major systems (e.g. respiratory, endocrine, gastrointestinal, immune, cardiovascular) |
| Capsaicin | Active component of chili peppers, used as an analgesic |
| C‐fibres, C‐polymodal fibres, unmyelinated C‐fibres | A type of nerve fibre that allows the transmission of different forms of sensory information. Lack of myelination is the cause of their slow conduction velocity |
| Cholestasis | A condition in which bile cannot flow from the liver to the duodenum |
| Creatine kinase | An enzyme expressed by various tissues and cell types, most often in muscle tissue |
| Cutaneous amyloidosis | A disorder characterised by the accumulation in the skin of an abnormal protein called 'amyloid' |
| Cyclic adenosine monophosphate (cAMP) | A second messenger important in many biological processes. cAMP is a derivative of adenosine triphosphate (ATP) and is used for intracellular signal transduction |
| Cyclo‐oxygenase | An enzyme that is responsible for formation of mediators of inflammation (prostanoids) |
| Cytokines | Small proteins that are important in cell signalling, which are secreted by certain cells of the immune system and have an effect on other cells |
| Depolarisation | A loss of the difference in charge between the inside and the outside of the plasma membrane of a muscle or nerve cell due to a change in permeability and migration of sodium ions to the interior |
| Dermatoepidermal junction | The space between the dermis and the epidermis |
| Dorsal root ganglion | A cluster of nerve cell bodies (a ganglion) in a dorsal root of a spinal nerve |
| Doxepin | A tricyclic antidepressant (TCA); a cream used for short‐term treatment of itchiness |
| Endogenous | Produced or synthesised within the organism or system |
| Endospinal | The inner part of the spinal cord |
| Endospinal endogenous opioids | A group of substances created in the spinal cord that bind to opioid receptors located mainly in the central nervous system and in the gastrointestinal tract; they are involved in control of homeostasis, regulation of pain, cell proliferation, cardiovascular control, stress, and the immune response |
| Endothelial cell | Group of cells that line the inside surfaces of blood vessels and lymphatic vessels |
| Endothelin | A group of peptides produced by endothelial cells |
| Eosinophils | A type of white blood cell, and one of the immune system components, responsible for combating multi‐cellular parasites and certain infections. They control the mechanisms associated with allergy and asthma |
| Epidermis | The most superficial layer of the skin |
| Erythema | Redness of the skin or mucous membrane |
| Exogenous | Produced or synthesised outside the organism or system |
| Exogenous opioids | Any opium‐like substances that are synthetically produced |
| Extracutaneous | Originating outside the skin |
| Ferritin | A protein; its main function is to store iron |
| Focal hypertrichosis | Abnormal amount of hair growth over the body, which is restricted to a certain area |
| Glutamate | Neurotransmitter that nerve cells use to send signals to other cells. It is used by every major excitatory function and by synaptic connections in the human brain |
| G‐protein‐coupled receptors (GPCRs) | A large family of protein receptors that detect molecules outside the cell and activate the transduction pathways of internal signals and cellular responses |
| H1 receptor | A receptor for histamine on cell membranes that modulates the dilation of blood vessels and the contraction of smooth muscle, among other responses |
| H2, H3, H4 receptors | Other histamine receptors:
|
| Hepatic | Related to the liver |
| Hepatic enzymes | Complex proteins that are produced by liver cells and catalyse specific biochemical reactions at body temperatures |
| Histamine | An inflammatory mediator, derived from decarboxylation of the amino acid histidine ‐ a reaction catalysed by the enzyme L‐histidine decarboxylase. It is involved in the inflammatory response and has a central role as a mediator of itching |
| Hypopigmentation | Loss of skin colour |
| Hypothyroidism | A disorder of the endocrine system in which the thyroid gland does not produce enough thyroid hormone |
| Immunoglobulins | Major components of the humoral immune response system. They are synthesised by lymphocytes and plasma cells and are found in the serum and in other body fluids and tissues (urine, spinal fluid, lymph nodes, and spleen) |
| Inflamed | Excessively affected with inflammation |
| Interferon‐alfa | A protein produced by leucocytes, mainly involved in the immune response against viral infection |
| Interleukin | A group of cytokines that are of essential importance for the function of the immune system. Most interleukins are synthesised by helper CD4 T lymphocytes, as well as by monocytes, macrophages, and endothelial cells |
| Interleukin‐31 | An inflammatory cytokine that helps trigger cell‐mediated immunity against pathogens |
| Kainic acid | An acid that naturally occurs in some seaweed. It is a potent neurotransmitter that acts by activating receptors for glutamate |
| Kappa and mu opioid receptors | A group of inhibitory G‐protein‐coupled receptors with opioids as ligands, distributed throughout the central nervous system and within the tissue of neural and non‐neural origin |
| Keratinocytes | The predominant cell type in the epidermis, the outermost layer of the skin |
| Keratolytic | A therapy that thins the skin, causing the outer layer of the skin to loosen and shed |
| Lactic acid | A molecule produced in a process of fermentation during normal metabolism and exercise |
| Lichen simplex | An inflammatory skin disorder characterised by pruritus that causes thick, leathery, darkened skin (lichenified) |
| Lipid | A molecule that serves many biological functions, including storage of energy, signalling, and acting as structural components of cell membranes |
| Lymphocyte | A subtype of white blood cell in the immune system |
| Macrophage | A type of white blood cell. Macrophages make up the part of the immune system that engulfs and digests cellular debris, foreign substances, microbes, cancer cells, and anything else that is recognised as foreign to the human body |
| Mast cell | A type of white blood cell that plays a key role in the inflammatory process |
| Monoclonal antibodies | Immunoglobulins produced by lymphocytes that are modified in a laboratory. These lymphocytes can produce these antibodies, which can identify molecules of the body and can be used for diagnostic, therapeutic, and research purposes |
| Monocyte | Type of white blood cell. Monocytes are part of the innate immune system and influence the process of adaptive immunity |
| Mu (μ) opioid receptors | A subtype of opioid receptors |
| Myelinated | Having a myelin sheath (a fatty white substance that surrounds the axons of some nerve cells, forming an electrically insulating layer, essential for proper functioning of the nervous system) |
| Nasopharyngitis | Inflammation of the upper respiratory system around the nose and throat |
| Neurogenic | Originating in or stimulated by the nervous system or nerve impulses |
| Neurokinin receptors | G‐protein‐coupled receptors found in the central and peripheral nervous system that bind neurokinin |
| Neurological | Related to the nervous system |
| Neuropeptide substance P | A peptide mainly secreted by neurons; it is involved in biological processes such as nociception and inflammation |
| Neurotoxins | Toxins that are poisonous or destructive to nerve tissue or nerve activity |
| Neurotransmitter | A chemical substance that transmits nerve impulses across a synapse |
| NK1, NK2, NK3 receptors (neurokinin receptors) | Molecules that bind to substance P and are associated with many biological functions (including itch) |
| N‐methyl‐D‐aspartic acid (NMDA) receptor | A molecule that binds to the neurotransmitter glutamate and is associated with many biological functions |
| Nociceptive nerve endings | Non‐specialised free terminal nerve fibres that have their cell bodies outside the spinal cord in dorsal ganglia that respond to harmful or potentially harmful stimuli |
| Non‐erythemogenic | Not producing or causing erythema |
| Non‐ionic surfactant | Chemical compound that lowers the surface tension (or interfacial tension) between 2 liquids |
| Non‐pharmacological interventions | A group of interventions in which pharmaceutical products are not the main active component (e.g. exercise, physiotherapy, surgery) |
| Non‐selective cation channel | A diverse group of ion channels characterised by their low discrimination between many essential elements and toxic cation function in the absorption of nutrients |
| Notalgia paraesthetica | A chronic sensory neuropathy, secondary to involvement of the spinal nerves, is a characteristic symptom of asymmetrical dorsal (upper to mid back) pruritus. Other symptoms may include pain, hyperaesthesia, paraesthesia, and hyperpigmentation of the affected area |
| Nuclear factor‐kB | A protein that controls transcription of DNA, cytokine production, and cell survival |
| Oedema | Excessive accumulation of fluid, mainly water, in superficial parts of the body. Oedema may be local, as at the site of an injury, or generalised |
| Opioid receptor antagonist | A molecule that blocks the receptor, preventing the body from having a response to drugs, such as heroin. Opioid receptor antagonists are used in opiate addiction |
| Paraesthesia, paraesthetica | A sensation of tingling, creeping, pricking heat or cold in sensitive skin |
| Perineural cells | Cells surrounding a nerve |
| Perioral dermatitis | Type of skin disease that is characterised by small (1 to 2 mm) bumps and blisters, sometimes with background redness and scales, localised to the skin around the mouth and nostrils |
| Peripheral oedema | An accumulation of fluid in extremities (primarily lower limbs) |
| Pharmacological interventions | A group of interventions in which the main component is the use of pharmaceutical products (e.g. anti‐inflammatories, antibiotics) |
| Phosphodiesterase‐4 | Subfamily of proteins that are predominantly found in inflammatory cells and may play a role in the regulation of cellular immunity |
| Phototherapy | Treatment of disease by exposure to light, especially by variously concentrated light rays or specific wavelengths |
| Physiopathology | Functional changes that accompany a disease |
| Pimecrolimus | Calcineurin inhibitor; immune‐modulating and anti‐inflammatory agent used in the treatment of skin disease |
| Polymodal | Having multiple modes or modalities (e.g. free nerve endings) |
| Post‐herpetic neuralgia | A condition in which pain in a region of the body occurs due to damage to a peripheral nerve caused by reactivation of the varicella zoster virus |
| Pre‐bullous pemphigoid | An autoimmune skin disease involving the formation of bullae (a type of blister) at the space between the epidermis and dermis skin layers |
| Pre‐synaptic vesicle | A membranous sac located within the pre‐synaptic membrane of an axon terminal and containing a neurotransmitter |
| Primary localised cutaneous amyloidosis | A condition in which an abnormal protein called amyloid accumulates in the skin |
| Prostaglandin E2 | A subtype of prostaglandin involved in many inflammatory processes of the body |
| Prostaglandins | A group of physiologically active lipid compounds having diverse hormone‐like effects in humans and other animals |
| Prostanoid | A class of fatty acids with an important function as mediators of inflammation and immune response |
| Protease | Enzyme that performs protein catabolism by hydrolysis of peptide bonds |
| Prurigo nodularis | Skin disease characterised by pruritic (itchy) nodules, which usually appear on the arms or legs |
| Pruritoceptive | Itch that arises from a primary skin disease, as opposed to an itch that is triggered by a systemic or neurological cause |
| Pruritus ani | Pruritus localised in the anus that causes a desire to scratch |
| Pruritus sine materia | Pruritus that is not associated with causal dermatosis; it may be acute or chronic |
| Psychogenic | A name given to physical illnesses that are believed to arise from emotional or mental stressors, or from psychological or psychiatric disorders |
| Psychosomatic | A term that is restricted to those illnesses that do have a clear physical basis but in which it is believed that psychological and mental factors also play a role |
| Radiography | An imaging technique using X‐rays to view the internal structures of the body |
| Refractory | Resistant to treatment |
| Rosacea | Long‐term skin condition that typically affects the face; results in redness, papules and pustules, swelling, and small and superficial dilated blood vessels |
| Schwann cells | The main non‐neuronal cells that support neurons in the peripheral nervous system |
| Serology | Study of serum for the diagnostic identification of antibodies formed in response to an infection, other foreign proteins, or one's own proteins |
| Serotonin or 5‐hydroxytryptamine (5‐HT) |
A monoamine neurotransmitter. Biochemically derived from tryptophan, serotonin is primarily found in the gastrointestinal tract, blood platelets, and central nervous system (CNS) of animals, including humans. It is popularly thought to be a contributor to feelings of well‐being and happiness |
| Serotonin receptor antagonist | Drug used to inhibit the action at serotonin receptors |
| Somatoform pruritus | An itch disorder wherein psychological factors play an evident role in the triggering, intensity, aggravation, or persistence of pruritus |
| Spinothalamic tract | A sensory pathway from the skin to the thalamus |
| Squamous cell carcinoma | An uncontrolled growth (cancerous) of abnormal cells, arising from squamous cells in the epidermis |
| Stratum corneum | The outermost layer of the epidermis |
| Striae | A type of scarring on the skin caused by tearing of the dermis |
| Substance P | Neuropeptide released from mast cells that activates the neurokinin‐1 receptor, which has been identified on keratinocytes in pruritic skin disease |
| Suburothelial | Tissue located underneath the superficial cells of the urological system (e.g. bladder) |
| Suburothelial afferents | Nerve fibres that bring sensory information from the bladder |
| Surfactants | Compounds that lower the surface tension (or interfacial tension) between two liquids, between a gas and a liquid, or between a liquid and a solid |
| Systemic | Affecting the whole body, or at least multiple organ systems |
| T cell, T lymphocyte | A type of lymphocyte (a subtype of white blood cell) that plays a central role in cell‐mediated immunity |
| Tachykinins | Large family of peptides that function as neurotransmitters in the central and peripheral nervous systems |
| Tacrolimus | Calcineurin inhibitor; immunosuppressive drug that decreases cytokine production |
| Telangiectases | Small dilated blood vessels near the surface of the skin or mucous membranes |
| Teratogenicity | Related to the emergence of malformations of an embryo or fetus |
| Th2 lymphocyte | A type of T cell that plays an important role in the immune system against extracellular parasites |
| Thalamus | A part of the brain relaying sensory information and acting as a centre for pain perception |
| Transcutaneous electrical nerve stimulation (TENS) | The use of electrical current produced by a device to stimulate the nerves for therapeutic purposes |
| Transient receptor potential cation channel subfamily M member 8 (TRPM8) channel | A receptor‐activated non‐selective cation channel involved in detection of sensations such as coolness. It is activated by cold temperature below 25 degrees Celsius |
| Transient receptor potential vanilloid‐1 (TRPV1), vanilloid receptor | Protein found in the central nervous system and in the peripheral nervous system that is involved in transmission and modulation of pain |
| Trigeminal atrophy | A decrease in the size of a normally developed trigeminal nerve |
| Trigeminal nerve | A nerve responsible for sensation and motor functions in the face (e.g. biting, chewing) |
| Tumour necrosis factor‐alfa | A cytokine involved in systemic inflammation and in the regulation of immune cells |
| Type A beta fibre (Aβ fibre) | A group of nerve fibres of sensory neurons that carry the tactile sensation. They are thickly myelinated |
| Type A delta nociceptive neurons (Aδ fibre) | A group of nerve fibres of sensory neurons that carry cold, pressure, and some pain signals. They are thinly myelinated |
| Unmyelinnated | Nerve fibres that are not covered with a myelin sheath |
| Uraemia | A condition resulting from kidney disease in which there is retention in the bloodstream of urea, normally excreted in the urine |
| Uraemia‐associated pruritus | A type of pruritus caused by the accumulation of organic waste products from the kidneys (in the presence of kidney insufficiency) |
| Urea | The main end product of the metabolism of proteins and amino acids, found abundantly in urine and faecal matter |
| Urticarial | Related to or marked by urticaria (a skin condition characterised by rash and itching) |
| Vasomotor | Causing or related to actions that alter the diameter of a blood vessel (dilating or constraining it) |
| Ventricular tachycardia | A type of regular and rapid heart rate that arises from improper electrical activity in the ventricles of the heart |
| Voltage‐gated sodium channels | A class of transmembrane proteins that form ion (sodium) channels that are activated by changes in the electrical membrane potential near the channel |
| Xerosis | Abnormal dryness of skin |
Definition of pruritus
Pruritus (itching) is the predominant symptom of many diseases; it can best be described as a sensation that leads to the desire to scratch (Goldsmith 2012). When the origin of pruritus is known, its management is straightforward, as long as an effective treatment is available for the disease that causes it. However, treatment of individuals with chronic pruritus of unknown origin (CPUO), which is the focus of this review, is particularly difficult. Pruritus is a common and distressing symptom that fluctuates in intensity, often over a long period of time. Effective relief of chronic itching can be difficult to achieve (Stander 2007). Pruritus is a patient‐reported symptom that is diagnosed when a patient's history is taken. The prognosis for patients with pruritus depends on the underlying diagnosis (see "Causes of pruritus"). Pruritus severity can be assessed using various scales (Pereira 2017), including the visual analogue scale (VAS) ranging from 0 (no itch) to 10 (worst imaginable itch). According to the VAS rating, pruritus can be further classified as mild (< 4), moderate (≥ 4 to < 7), severe (≥ 7 to < 9), or very severe (≥ 9) (Reich 2012).
Pruritus may originate in the skin or in the central nervous system (Yosipovitch 2013). It occurs in a diverse range of skin diseases and may appear as a prominent feature of extracutaneous disorders, such as systemic, neurological, or psychiatric disease. Pruritus can be classified according to its duration as acute (lasts less than six weeks) or chronic (lasts six weeks or longer) (Stander 2007). Itch can be classified as generalised (all over the body) or localised (e.g. notalgia paraesthetica, pruritus ani, brachioradial pruritus (Garibyan 2013)). However, to date, there is no standardised classification for chronic pruritus (Stander 2007).
The International Forum for the Study of Itch (IFSI) has proposed a classification system for chronic pruritus and has suggested three groups of conditions.
Group I: pruritus on diseased, inflamed skin.
Group II: pruritus on non‐diseased, non‐inflamed skin, previously known as 'pruritus sine materia'.
Group III: pruritus presenting with signs of significant chronic excoriation (Stander 2007).
Causes of pruritus
The IFSI classification describes categories of underlying pruritogenic diseases as follows.
Category I: dermatological disease, including chronic pruritus arising from diseases of the skin, such as psoriasis, atopic dermatitis, dry skin, scabies, and urticaria.
Category II: systemic disease, including diseases of pregnancy and drug‐induced pruritus, and chronic itch arising from diseases of the internal organs, such as the liver or kidney, or from diseases of the blood. This category also includes metabolic diseases and side effects of drugs.
Category III: neurological disease, including pruritus arising from diseases or disorders of the central or peripheral nervous system, such as nerve damage, nerve compression, or nerve irritation.
Category IV: psychiatric or psychosomatic disease, including somatoform pruritus.
Category V: mixed disease, involving overlap of several diseases.
Category VI: other pruritus of undetermined origin, or chronic pruritus of unknown origin (Millington 2018; Stander 2007).
This systematic review will focus on Category VI ‐ chronic pruritus of unknown origin.
Chronic pruritus of unknown origin
The initial clinical approach in people with pruritus includes a medical history and a physical examination. Other investigations to identify the underlying causes of pruritus might include a complete blood count; ferritin levels; a chest radiograph; measurements of hepatic, renal, and thyroid function; serology for sexually transmitted infections; and, when appropriate, tests to identify endemic parasitic infections. However, in some cases, the underlying cause remains unclear, and the disorder is called 'chronic pruritus of unknown origin (CPUO)' (Millington 2018; Stander 2007). As CPUO is a diagnosis of exclusion, patients with this diagnosis are re‐examined periodically in an attempt to identify potential causes of their symptoms.
Pathophysiology
The pathophysiology of pruritus is only partially understood. The thick myelinated type II sensory fibres transmit tactile sensation, whereas the thinly myelinated A‐delta and unmyelinated C‐polymodal fibres are mainly involved in conducting thermal pain and itch sensation (Lawson 2002). The sensation of pruritus is transmitted mainly through slow‐conducting, unmyelinated C‐polymodal, and possibly type A‐delta nociceptive neurons with free nerve endings, located near the dermoepidermal junction or in the epidermis (Matterne 2011). These neurons appear to be located more superficially and are more sensitive to pruritogenic substances than pain receptors. Neurotransmitters for these nerves include histamine, calcitonin gene–related peptide, neuropeptide substance P, serotonin, bradykinin, proteases (e.g. mast cell tryptase), neurokinin (NK1), and endothelin (which stimulates the release of nitric oxide), many of which are activated by inflammation (Greaves 1996). Impulses are transmitted from the dorsal root ganglion to the spinothalamic tract, and eventually to the thalamus (Matterne 2011).
Neurogenic and systemic itch usually affects organs other than skin; however, no current evidence shows any neuronal pathology, although itch could be transmitted via the central nervous system. There is a theory that this type of itch may result from endospinal endogenous opioids, thereby opening the possibility of itch‐specific or itch‐selective neurons in the spinal cord as possible targets for new therapies. The cause or mechanism of psychogenic itch is unclear; however, it stems from psychogenic disorders. In contrast, neuropathic itch is due to damage to peripheral or central sensory neurons, as it leads to activation of pruritic neurons without any cutaneous stimuli. Last, proprioceptive itch may originate in diseases of the skin (Garibyan 2013).
Other mediators and receptors possibly involved in the physiopathology of pruritus include the following.
Histamine: G‐protein‐coupled receptors (GPCRs) usually respond to histamine, and four types of receptors have been identified (H1, H2, H3, and H4), with the first and the last playing a role in itch.
Interleukin‐31 (IL‐31): this mediator has been linked to pruritus in people with atopic dermatitis and prurigo nodularis. Genetic mutations in the IL‐31 receptor have been linked to familial primary localised cutaneous amyloidosis. IL‐31 is produced predominantly by Th2 lymphocytes, and these T cells contribute to the pathogenesis of atopic dermatitis.
Substance P (tachykinin): this neuropeptide is released from mast cells. It binds to the neurokinin receptors NK1R, NK2R, and NK3R. NK1R has been implicated in the induction of itch in rats. Increased expression of NK1R has been reported on keratinocytes in pruritic skin diseases. Substance P also binds to Mas‐related G‐protein‐coupled receptors (MrGPCRs); this might be the main mechanism for the perception of pruritus (Azimi 2017).
Transient receptor potential vanilloid receptor‐1 (TRPV1), also known as the capsaicin receptor: this non‐selective cation channel is able to bind to capsaicin (an active component of the chili pepper). TRPV1 is expressed on sensory neurons, keratinocytes, and endothelial and mast cells, and has been found to play a role in histamine‐ and serotonin‐induced itch (Garibyan 2013).
Epidemiology
The prevalence of chronic pruritus increases with age (Rea 1976); it might not be found in children, although prevalence studies in this population are lacking (Weisshaar 2009). The condition may be more common among women than men (Matterne 2011; Stander 2013). Recent surveys indicate a point prevalence of chronic pruritus to be around 13.5% in the general adult population (Weisshaar 2009); however, the origin of pruritus is unknown in 8% to 15% of affected patients (Matterne 2011). The prevalence of CPUO in individuals with generalised pruritus ranges from 3.6% to 44.5%, with highest prevalence among the elderly (Weisshaar 2009).
Chronic pruritus is a frequent symptom, often intractable, that is associated with reduced quality of life; it has been described as being as debilitating as chronic pain (Kini 2011). Deranged sleep patterns and mood disturbances, including anxiety and depression, are common and may exacerbate itching (Kopyciok 2016; Zachariae 2012).
Description of the intervention
Management options for CPUO may include a wide variety of treatments. These treatments can be classified as topical or systemic and may be available as pharmacological or non‐pharmacological therapies. Standard treatment may vary across different countries and settings; however, healthcare professionals usually recommend use of emollient or cooling creams and avoidance of irritating products for the skin, along with use of topical products (e.g. steroids, topical antidepressants), systemic therapies (e.g. most commonly antihistamines, but also antidepressants and gabapentin), or both (Millington 2018).
CPUO is a challenging condition to manage due to its unidentified aetiology. The course of CPUO is variable in intensity and frequency of symptoms over time. Regardless of the interventions chosen to treat this condition, it is important to conduct a periodic evaluation of patients because it could be the initial symptom of other systemic diseases, including hypothyroidism, chronic lymphocytic leukaemia, lymphoma, hepatitis C, hepatitis B, pre‐bullous pemphigoid, infestations, diabetes mellitus, lung cancer, uraemia, or iron deficiency anaemia, among others (Polat 2008; Zirwas 2001). Indeed, no sign or symptom at its initial presentation could accurately serve as a predictor of pruritus with a systemic aetiology (Yosipovitch 2010).
The course of CPUO is variable, and most treatments are given in a variable regimen as well. This is particularly relevant for topical treatments and some non‐pharmacological interventions used in localised forms of pruritus (e.g. eczema), in which the product is applied to the pruritic area as long as the symptom persists (i.e. non‐pharmacological topical products, cooling lotions, cannabinoids, corticosteroids, calcineurin inhibitors, local anaesthetics, capsaicin, salicylic acid, antihistamines, phosphodiesterase‐4 inhibitors, opioid receptor antagonists, acupuncture, and transcutaneous electrical nerve stimulation (TENS)).
It is important to monitor interactions between interventions given simultaneously, acknowledging that these might be more relevant with systemic pharmacological agents that affect the central nervous system (e.g. antihistamines and anticonvulsants (e.g. gabapentin), both of which may cause drowsiness and depression of the central nervous system) (Brunton 2011).
Non‐pharmacological interventions
Emollients, colloquially known as moisturising creams, are commonly used to prevent or treat xerosis (dry skin) and may be used to treat associated pruritus (Simpson 2010). Ingredients in emollients may be identical to those found in the stratum corneum, including lipids, urea, lactic acid, and amino acids, and can replenish these substances that are low in xerotic and pruritic skin (Lodén 2015; Lodén 2016). Emollients should be applied several times a day, especially if xerosis is present. Emollients restore the water‐retaining and irritant‐resisting properties of an intact and healthy epidermis (barrier function; Grundmann 2011). Topical urea has been shown to have effective emollient and keratolytic effects on xerosis, and to interfere with the development of pruritus (Pan 2013). Urea may be applied in a 5% to 20% formula to keep the skin moist. Higher concentrations have keratolytic properties, which would not be desirable for pruritus. As with most emollients, urea is relatively safe but may cause contact dermatitis (Yosipovitch 2013). Most emollients rarely result in any side effects; however, redness, burning, or irritation may occur (Grundmann 2011)
Neutral or mild pH soaps maintain the slightly acidic pH of the skin mantle. Soaps are generally used to clean the area of dirt. They should be used once a day, in a sufficient amount to cover the entire body (Baranda 2002). Any disruption of the stratum corneum or the skin pH predisposes the skin to environmental irritants. Irritation to the skin causes erythema, oedema, and skin dryness, ultimately leading to pruritus (Baranda 2002)
Natural products that have been reported to be useful for managing pruritus include, but are not limited to, apple cider vinegar, essential oils, tea tree oil, coconut oil, lemon juice, juniper berries, mint, thyme, aloe vera, beeswax, tumeric, glycerin, various herbal products, oatmeal, omega 3 supplementation, baking soda, milk, and honey (Baker 2001; Craig 1997; Hercogová 2005; Kapoor 2005; Koh 2002; Mueller 2004; Pazyar 2012; Salamone 2016; Vaughn 2016; Weisshaar 2015). Many of these products are used in traditional medicine, and their mechanism of action is unknown; however, oils and wax could have similar properties to emollients (Hercogová 2005). These products can be applied in a variety of forms (e.g. lotions, creams, powders); their dosing has not been standardised. Common side effects could include allergic reactions and skin irritation
Alternative therapies, such as acupuncture, TENS, and aromatherapy, have been reported in the literature as effective therapies for pruritus. Acupuncture has been shown to have antipruritic effects in treating histamine‐induced itch in healthy individuals; however, the mechanism for this effect is unclear (Pfab 2010). Acupuncture is typically delivered in 15‐ to 20‐minute sessions. The procedure is relatively safe; the most common side effect is skin infection, typically in a number of patients, due to the use of unsanitary needles (Xu 2013). TENS is commonly used as an alternative treatment for pain management. Some evidence suggests that TENS is effective in managing pruritus, viewed as a form of pain, via electrical inhibition of A‐delta fibres and C‐fibres that transmit the sensation of pruritus (Hettrick 2004). TENS uses electrodes applied to the skin, with pre‐specified frequency and amplitude of electrical stimulation, in a series of 15‐ to 30‐minute sessions; rare side effects include skin irritation, local erythema, and numbness (Mohammad 2015). Aromatherapy has been shown to improve skin hydration and may be considered an alternative therapy for pruritus, especially when associated with dry skin, in conditions such as atopic dermatitis and uraemic pruritus (Chida 2007; Curcani 2014). Aromatherapy also may induce sedation, which can be useful when pruritus is treated (Ha 1999). Aromatherapy can be applied with diffusers, baths, compresses, and inhalations in various regimens; although usually safe, in some cases, the essential oils used in this therapy may cause skin irritation and contact dermatitis (Posadzki 2012)
Topical pharmacological interventions
Topical corticosteroids are commonly used anti‐inflammatory agents that may relieve itching by exerting anti‐inflammatory effects on T cells, monocytes, and macrophages, which produce altered cytokine activity locally. They also inhibit the release of inflammatory mediators (interleukin‐1, interleukin‐2, interleukin‐6, interferon‐alpha, and tumour necrosis factor‐alpha), which subsequently release pruritus mediators, such as histamine and bradykinin. Because their mechanism of action depends on inflammation, they are more useful when there is active inflammation of the skin (Hercogová 2005; Yosipovitch 1996). Some topical corticosteroids, such as clobetasol 0.05% (cream or ointment) and mometasone 0.1% (cream), can be applied twice a day to inflamed skin. The duration of treatment is variable and is intended to be limited by the appearance of adverse events, which include skin atrophy, striae, telangiectases, hypopigmentation, rosacea, acneiform eruptions, focal hypertrichosis, perioral dermatitis, and acne (Bolognia 2012; Roth 1978; Yarbrough 2013)
Cooling lotions, such as camphor and menthol, have been used effectively to manage pruritus (Hercogová 2005; Norman 2003). Cooling the skin by 2°C to 4°C has been shown to result in a reduction in the intensity of histamine‐induced itch, and application of menthol to the skin may yield a similar reduction in pruritus (Bromm 1995; Patel 2007). It has also been hypothesised that activation of A‐delta fibres by menthol could inhibit itch centrally by blocking the afferent pruritus pathway, similar to the gate control theory for pain (Bromm 1995). The most recent research identifies a pharmacological mechanism of action of menthol through activation of a transient receptor potential cation channel subfamily M member 8 (TRPM8) channel, which activates neurons of the spinal cord that inhibit the transmission of itch (Liu 2018; Palkar 2017; Stander 2017). Camphor may activate the same TRPM8 sensors (Selescu 2013). Menthol can be applied three or four times a day in a wide range of concentrations, from 0.1% to 10% topical lotion, although it can be available as a magistral formula (Lasanen 2016). Camphor is usually available at a dose of 1% to 3%. Potential side effects may include allergic reactions and skin irritation
Calcineurin inhibitors are immunomodulating agents that selectively inhibit the activation of T cells by inhibiting calcineurin, an enzyme required for the inner activation of T cells and inflammation (see "Topical corticosteroids"). Tacrolimus and pimecrolimus are calcineurin inhibitors. Because their mechanism of action depends on inflammation, they are more useful when there is active inflammation of the skin (Fleischer 2010; Hercogová 2005; Ständer 2006). Pimecrolimus 1% or tacrolimus 0.03% or 0.1% is applied twice a day. Common adverse events include skin irritation, erythema, and a burning sensation (Ashcroft 2005; Castro 2006; Hultsch 2005)
Local anaesthetics directly interfere with the transmission of impulses along the sensory nerve fibres (including those related to pruritus). Pramoxine, a topical anaesthetic, blocks the transmission of nerve impulses and inhibits histamine‐induced itch in humans, and is effective for pruritus of the face. Polidocanol is a non‐ionic surfactant with both local anaesthetic properties and emollient effects, which can be applied in a 3% concentration for localised pruritus (Freitag 1997; Hawro 2014; Hercogová 2005). Pramoxine 1% to 2.5% or lidocaine 1% to 5% is applied topically to the affected area not more than three to four times daily. Adverse effects may include numbness and contact dermatitis (Goldsmith 2012). Lidocaine is a local anaesthetic that can be found in co‐formulations with amitriptyline and ketamine (see "Amitriptyline")
Topical antihistamines may be used to treat pruritus by interfering with histamine‐mediated itch. Type H1 and H2 histamine receptors are commonly found in the skin, and most antihistamines work by binding to type H1 receptors (O'Donoghue 2005). Histamine binds more to H1 receptors than H2 receptors as the predominant mechanism for pruritus, causing stimulation of C‐fibres, which transmit the pruritus sensation from the skin to the central nervous system (Greaves 1996). The H1 receptor antihistamine agents also bind to serotonin receptors (O'Donoghue 2005). This receptor plays a role in the itch mechanism, which is described below (see "Opioid receptor antagonist"). First‐generation antihistamines are used less often due to their sedative effects, and they have been progressively replaced by second‐generation antihistamines, which show little sedative effect (Greaves 2005). Topical strontium chloride in a 4% concentration gel has been shown to have antihistaminic and antipruritic properties in experimental models of pruritus (Papoiu 2013). We found no reports of adverse events
Phosphodiesterase‐4 (PDE4) inhibitors. PDE4 is a key regulator of inflammatory cytokine production in pruritic diseases such as atopic dermatitis; it works through the degradation of cyclic adenosine monophosphate (Paller 2016; Yosipovitch 2018). Inhibition of PDE4 results in an increase in intracellular cyclic adenosine monophosphate, which causes inhibition of T‐cell pruritus‐inducing cytokine production (Paller 2016). The PDE4 inhibitor, crisaborole 2%, is used twice a day on affected skin for four weeks. Adverse events may include upper respiratory tract infection and onset or exacerbation of atopic dermatitis (Eichenfield 2017)
Capsaicin. The active compound in the chili pepper causes the release of neuropeptides including substance P from C‐nerve fibres. The exact mechanism is not fully understood; however, prolonged application of capsaicin to the skin depletes stores of substance P, desensitises neurons, and abolishes pruritus at the site of application. Capsaicin activates the vanilloid receptor TRPV1, which is abundant in the epidermal layer of the skin (Hercogová 2005). Capsaicin can be applied as a 0.025% to 0.1% cream four times each day with at least a three‐ to four‐hour interval between applications. The most frequent adverse reaction is a transient burning sensation (Goldsmith 2012)
Topical salicylic acid is a common keratolytic agent that may increase hydration and soften the stratum corneum by decreasing its pH. It has been shown to significantly reduce pruritus among patients with lichen simplex chronicus (Yosipovitch 2001). Its mechanism is hypothesised to be due to the inhibitory effects of salicylic acid on prostanoids, specifically prostaglandin E2, which would reduce the inflammation‐mediated activation of C‐fibres (Dawn 2006; Patel 2010). Salicylic acid can be administered in creams, soaps, foams, and lotions, with a range of concentrations from 1% to 6%. It can be applied once or twice a day, depending on the concentration and the indication. Frequent adverse reactions include skin irritation (which can be severe) and allergic reactions (e.g. aspirin allergy)
Ketamine is a drug that is classically used in general anaesthesia. Topical ketamine, in combination with amitriptyline, has been used for chronic and neuropathic itch and has been demonstrated to have antipruritic effects (Leslie 2015; Poterucha 2013). Ketamine blocks N‐methyl‐D‐aspartic acid (NMDA), kainic acid, and 2‐amino‐3‐(5‐methyl‐3‐oxo‐1,2‐oxazol‐4‐yl)‐propanoic acid (AMPA) receptors that prevent synaptic transmission of itch impulses across cutaneous nerves (Poterucha 2013). Ketamine may be found in a co‐formulation with amitriptyline and lidocaine (see "Amitriptyline")
Amitriptyline and other topical antidepressants (doxepin). Topical amitriptyline, in combination with ketamine, has been successfully used for brachioradial pruritus, as its topical formulation modulates the effects of serotonin and neurotransmitters to block voltage‐gated sodium channels, preventing the depolarisation of axons and the transmission of itch signals (Poterucha 2013). Amitriptyline has been used in co‐formulations with ketamine and lidocaine to treat chronic pruritus, with promising results (dose and regimen not available). Adverse events included skin irritation (burning, redness) and dizziness (Lee 2017). Doxepin is a tricyclic antidepressant with potent H1 and H2 antihistamine properties and significant atropine‐like side effects; it may cause contact dermatitis (Goldsmith 2012; Hercogová 2005; Weisshaar 1998). Doxepin 5% cream is applied four times a day (for up to eight days), with at least a three‐ to four‐hour interval between applications. Adverse effects may include allergic contact dermatitis and drowsiness in 25% of patients (Goldsmith 2012)
Cannabinoids include a wide range of pharmacological agents that bind to cannabinoid receptors. These agents are known for being originally derived from cannabis (marijuana). Similar to the mu opioid receptor, the cannabinoid receptor may play a significant role in mediating itch (Ikoma 2006). Use of endogenous cannabinoids, twice daily for three weeks, may act to inhibit signalling in these nociceptive nerve endings involved in pruritus through the cannabinoid 1 (CB1) receptors. Cannabinoids may show anti‐inflammatory properties by reducing the activation of mast cells by acting on CB1 and cannabinoid 2 receptors (Ikoma 2006; Mounessa 2017). They may also have antipruritic properties when applied systemically (see "Systemic therapies"). In a small, pilot, open‐label study with various regimens (lasting two weeks to six months), N‐palmitoyl ethanolamine (a cannabinoid agonist) provided relief for people with chronic pruritus (atopic dermatitis, lichen simplex, prurigo nodularis, and chronic kidney disease‐associated pruritus) without causing adverse events (Stander 2006). This study did not provide a dose of N‐palmitoyl ethanolamine
Botulinum toxins. Use of botulinum toxin injections has been reported to be effective in managing notalgia paraesthetica and lichen simplex (Gazerani 2009; Weinfeld 2007). Botulinum toxins inhibit the release of acetylcholine from pre‐synaptic vesicles and also inhibit the release of some other substances, such as substance P and glutamate, which may be involved in pruritus. There are seven types of toxins (labelled from A to G); botulinum toxin A (BTA) is the most commonly used medically (Pirazzini 2017). One study demonstrated a significant reduction in the level of expression of TRPV1 receptors after treatment with botulinum toxin A in suburothelial afferents (Apostolidis 2005). Botulinum toxin A has been described as a neurotoxin that may reduce histamine‐induced itch and vasomotor responses in pruritus (Heckmann 2002). Botulinum toxin A (100 UI/vial) can be reconstituted with saline solution (0.9%), with a dose of 5 UI injected into the affected area. In Heckmann 2002, the antipruritic effect appeared as early as day 1 and remained for a week after its application. Adverse effects included muscle weakness and injection site reactions, such as bruising, bleeding, pain, redness, swelling, or infection
Systemic pharmacological interventions
Antihistamines are the most common and standard systemic drugs used for management of acute forms of pruritus. Use of antihistamines has been shown to work for pruritus of numerous causes. Commonly used H1 receptor agents include loratadine 10 mg/d, hydroxyzine 25 mg three times a day, diphenhydramine 50 mg/d, and cetirizine 10 mg/d. H2‐receptor agents, such as ranitidine 50 mg/d, have shown benefit for treating urticaria (Fedorowicz 2012). However, conventional doses of antihistamines may not be effective for CPUO (O'Donoghue 2005; Zirwas 2001). Common side effects of antihistamines are headache, dry mouth, nausea, and drowsiness
Systemic opioid receptor antagonists, such as nalfurafine, naltrexone, naloxone, and butorphanol, work by antagonising the kappa and mu opioid receptors (one theory claims that pruritus may result from endospinal endogenous opioids) and have been used for pruritus of multiple causes (cholestasis, chronic kidney disease, and refractory itch in burn patients), including CPUO (Grundmann 2011; Kumagai 2012; Leslie 2015; Phan 2010; Siemens 2016; Stander 2015). Multiple mechanisms have been hypothesised, but no mechanism can explain all instances. Opioid receptor antagonists work by down‐regulating the effect of endogenous or exogenous opioids, and some of the neurons responsible for regulation of C‐fibres (responsible for transmission of itch) might be affected by these drugs (Grundmann 2011; Phan 2010; Stander 2015). In addition, serotonin (5‐HT3) receptors and mu opioid receptors are located in the spinal cord and at the spinal tract of the trigeminal nerve in the spinal cord (Schmelz 2009). This region in the spinal cord is a key sensory input area for the face and is known as the ‘itch centre’. Thus, it is hypothesised that down‐regulating these 5‐HT3 and mu opioid receptors has a role in reducing pruritus. Phan 2010 showed that naloxone, given at a rate of 1.6 mg/hour for four hours, provided complete relief of CPUO, and naltrexone, given at 50 mg/d to 150 mg/d, led to considerable relief. Clinical studies have shown that oral naltrexone and naloxone have reduced the severity of CPUO. However, the therapeutic cost and potential side effects of these agents limit their use as second‐line options. Common side effects range from nausea, vomiting, headaches, and fatigue to serious adverse events, including ventricular tachycardia and paraesthesia (Phan 2010)
Antidepressants. Psycho‐emotional factors are known to affect the threshold for pruritus tolerance, with depression playing a role in 10% of patients with chronic itch. There is a role for antidepressants in treating chronic pruritus, whether by reducing symptoms of depression and anxiety, or by acting through a secondary unknown pathway. Currently, paroxetine is the only antidepressant shown to play a role in pruritus of unknown origin. Zylicz 2003 hypothesised that the role of paroxetine in down‐regulating 5‐HT3 receptors reflected a potential antipruritic mechanism. Other antidepressants, such as mirtazepine (15 mg to 30 mg a day for at least four to six weeks) and doxepin, have been shown to be effective in inflammatory skin disease and nocturnal pruritus (Davis 2003; Hundley 2004; Shohrati 2007). Paroxetine is used at 20 mg/d for at least four to six weeks; its common side effects include sedation and other sleep disturbances, decreased libido, and gastrointestinal disturbances, such as nausea, diarrhoea, and vomiting (Brunton 2011)
Anticonvulsants. This group of drugs includes gabapentinoids (gabapentin and pregabalin) and carbamazepine. Their effects are mediated by a cell membrane protein (alpha 2 delta 1), which regulates voltage‐gated calcium channels. This could trigger intracellular changes relevant to the functioning of C‐fibres, which are responsible for the transmission of pruritus sensation (Brunton 2011; Fehrenbacher 2003; Foroutan 2016; Matsuda 2016; Mazza 2013; Schworer 1993; Yoon 2003). Two case reports of gabapentin have shown considerable improvement in patients unresponsive to standard topical and oral treatments for pruritus. In these studies, patients were originally treated with 300 mg/d, with treatment titrated up to 1800 mg/d to achieve a maintenance dose. Case authors reported complete control of pruritus with no side effects during nine months of treatment (Yesudian 2005). Gabapentinoids have been used to treat brachioradial pruritus, postherpetic neuralgia, notalgia paraesthetica, chronic kidney disease, and lymphoma, but treatment can actually worsen the itching in patients with cholestasis (Stander 2006). Pregabalin has been used at a range of doses, from 25 mg/d to 300 mg/d, to achieve a maintenance dose (Atis 2017). Carbamazepine is administrated at a 200‐mg dose twice daily (Korfitis 2008). Gabapentin is a preferable therapeutic agent due to its low side effect profile and lack of hepatic enzyme disturbance (Schworer 1993; Yesudian 2005). Common side effects of gabapentinoids include dizziness, drowsiness, weakness, and nausea (Brunton 2011). Case reports have described the use of carbamazepine for pruritus associated with multiple sclerosis, postherpetic neuralgia, and trigeminal atrophy (Yosipovitch 2008)
Substance P and neurokinin 1 receptor (NK1R) antagonist. Aprepitant is a commonly used medication for post‐chemotherapy nausea and vomiting; it has been used as off‐label treatment for chronic pruritus unresponsive to treatment. It works by antagonising substance P, which is a neuropeptide that binds to NK1 receptors, regulating the production of histamine and prostaglandins, which are itch mediators. To date, no studies have been conducted to examine its effect on CPUO, but multiple case reports have shown benefit (Ally 2013; Duval 2009; Huh 2016; Lotts 2014; Stander 2010). Common side effects of aprepitant include fatigue, diarrhoea, dehydration, low white cell count, and constipation (He 2017). Another NK1R antagonist called serlopitant given at 1‐mg and 5‐mg doses for six weeks has shown promising results for chronic pruritus in phase 2 studies, with no evidence of adverse events (Yosipovitch 2018)
Cannabinoids. Systemic administration of cannabinoids has led to improvement in contact dermatitis and atopic dermatitis in animal models (Mounessa 2017). Many formulations are available for systemic administration of cannabinoids, with differences in composition and means of administration; however, it is not clear which formulation would be of use in pruritus (Kogan 2007). Common side effects of cannabinoids include dry mouth, nausea, dry eyes, headaches, hallucinations, and depression (Mounessa 2017)
Thalidomide. Patients with uraemia‐associated pruritus who have been resistant to treatment have derived significant benefit from a seven‐day dose of thalidomide at bedtime, in a cross‐over randomised double‐blinded trial. Eighteen patients who completed the study reported 81% pruritus improvement after the final phase of the study (Silva 1994). The antipruritic effect of thalidomide is suggested to be multi‐factorial and to yield anti‐inflammatory effects, neuropeptides affecting the C‐fibres, and possible interaction with opioid receptors. Thalidomide also suppresses tumour necrosis factor‐alpha and nuclear factor‐kB, reducing the peripheral sensory nerves perception of pruritus (Silva 1994). Thalidomide has been used at a daily dose of 50 mg to 300 mg (Aguh 2018). It is important to note that thalidomide can cause teratogenicity and neurological side effects, especially when taken at a dose above 100 mg
Monoclonal antibodies and other biological agents. Monoclonal antibodies (proteins made in the laboratory that can bind to substances in the body) such as anti‐interleukin‐31 (nemolizumab) are showing promise for treating pruritus because they block the effects of Interleukin‐31, the most prominent inflammatory mediator for pruritus (Kasutani 2014). A phase 2 trial with 264 participants with atopic dermatitis reported improvement regardless of which dose of nemolizumab was taken for 12 weeks (0.1 mg, 0.5 mg, or 2 mg). Common side effects reported by trial authors include peripheral oedema, nasopharyngitis, raised creatine kinase levels, and upper respiratory tract infection (Ruzicka 2017). Another monoclonal antibody, anti‐IL‐5 (mepolizumab), given at two single doses of 750 mg, did not reduce pruritus despite reducing eosinophils (Oldhoff 2005). Janus kinase (JAK) inhibitors such as ruxolitinib, tofacitinib, momelotinib, oclacitinib, and fedratinib are biological agents (drugs manufactured in, extracted from, or semi‐synthesised from biological sources) have been used for pruritus associated with skin and systemic disorders (Feldman 2016; Fukuyama 2017; Oetjen 2017; Vaa 2016; Yasuda 2016). Tofacitinib 5 mg to 10 mg twice daily for 16 weeks has been used to treat psoriasis (Feldman 2016). Common adverse events may include an increased predisposition to infection, hypertension, and diarrhoea
Ultraviolet (UV) phototherapy is a well‐known therapy for pruritus that is refractory to the usual topical treatments and antihistamines. There are two types of UV treatment: UVB (either broad or narrow band UVB) and UVA (which is usually given with psoralens (PUVA) or as UVA1, which uses the longer non‐erythemogenic UV wavelengths (340 to 400 nanometers) (York 2010)). Such therapy has been used in multiple dermatologically or systemically caused cases of pruritus, such as atopic dermatitis, lichen simplex, urticaria, and aquagenic pruritus, among others (Rivard 2005). It has been hypothesised that UVA and UVB reduce the production of histamine from mast cells and basophils. UVA may also cause damage to Schwann and perineural cells, which decreases sensitivity to pruritus. The protocol for dosing phototherapy (in Joules) depends on the condition of the skin and the patient's skin type (as a proxy for minimal erythema dose). The UV light is delivered in short sessions, typically three times a week for three months (Lapolla 2011). Common early side effects of phototherapy include erythema, itching, headache, nausea, and redness; exposure to UV light can increase the risk of skin cancer, especially basal cell and squamous cell carcinoma (Rivard 2005)
Ondansetron is a serotonin receptor antagonist that acts selectively on the 5‐HT3 receptor. Case reports of ondansetron, given at a dose of 4 mg to 8 mg twice daily as needed, and used as monotherapy or as add‐on therapy, have described reduced uraemic and cholestatic pruritus, including CPUO (Dillon 2013). The antipruritic mechanism of the 5‐HT3 receptor antagonist is described under "Opioid receptor antagonist". Common side effects of ondansetron include headache, drowsiness, constipation, and diarrhoea (Brunton 2011)
Why it is important to do this review
Cochrane Skin undertook an extensive prioritisation exercise alongside the Global Burden of Disease and the World Health Organization (WHO) to identify a core portfolio of the most clinically important titles. The title was identified as a clinically important priority by the expert panel for development, maintenance, and investment of resources by the editorial base. Pruritus is an important condition that causes a great burden on the quality of life of individuals, with an estimated 709,060 disability‐adjusted life‐years (DALYs) worldwide in 2016 (range 329,660 to 1,298,741 (GHDx)).
CPUO is a challenging disorder due to its unknown pathophysiology and the multiple alternatives for treatment. These alternatives include low‐cost interventions, such as cooling lotions, and high‐cost interventions, such as monoclonal antibodies. In addition, some of these interventions might be complex to implement (e.g. acupuncture). In this review, we decided to include all treatment options, for equity reasons. We acknowledge that the diagnostic workup for this condition might vary worldwide, and we considered this when drafting our Criteria for considering studies for this review.
A Cochrane Review, titled 'Pharmacological interventions for pruritus in adult palliative care patients' (Siemens 2016), did not include participants with CPUO. Therefore, we have not yet identified any systematic reviews on this topic. An up‐to‐date Cochrane Review is needed to critically summarise the body of evidence for treatments for this complex condition, using the GRADE approach, which will provide key information on patient‐important outcomes.
Objectives
To assess the effects of interventions for chronic pruritus of unknown origin in adults and children.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs; parallel assignment, cluster‐randomised, and cross‐over) and quasi‐randomised controlled trials (qRCTs). We included the first phase of cross‐over RCTs because there may be substantial risk of carry‐over effect. We included cluster‐randomised trials that used an adequate adjustment for effective sample sizes (Cochrane Handbook for Systematic Reviews of Interventions, Section 16.3.4; Higgins 2011).
Types of participants
We included participants of any age (adults and children), of either sex, with a diagnosis of chronic pruritus of unknown origin (CPUO), as defined in category VI of the IFSI classification. This includes individuals receiving a diagnosis of pruritus for whom no dermatological, systemic, neurological, or psychiatric disorder has been identified as a cause (Stander 2007). We included participants who underwent some degree of diagnostic workup to exclude dermatological disorders, systemic disease, neurological disorders, or psychiatric disorders. A diagnostic workup for CPUO could include a wide range of evaluations, from minimal to very thorough, depending on the settings in which the study has been performed. The diagnostic workup may have included a full medical history; a full physical examination; a complete blood count; ferritin levels; a chest radiograph; measurements of hepatic, renal, and thyroid function; serology for sexually transmitted infection; and, when appropriate, tests that identify endemic parasitic infection. The review did not include participants with drug‐induced pruritus.
When we found studies with a subset of patients with a diagnosis of CPUO, we included them if data were presented separately for these patients, or if a majority (> 50%) of included participants met the inclusion criteria. If data were not available for this subset of participants, we tried to retrieve this information from the investigators before excluding the study.
Types of interventions
We included studies that evaluated the following interventions.
Non‐pharmacological interventions
Emollient creams
Neutral or mild pH soaps
Natural products
Alternative therapies
Topical pharmacological interventions
Corticosteroids
Cooling lotions
Calcineurin inhibitors
Anaesthetics
Antihistamines
Phosphodiesterase‐4 inhibitors
Capsaicin
Salicylic acid
Ketamine
Amitriptyline and other topical antidepressants
Cannabinoids
Botulinum toxins
Systemic pharmacological interventions
Antihistamines
Opioid receptor antagonists
Antidepressants
Anticonvulsants
Substance P and neurokinin 1 receptor (NK1R) antagonists
Cannabinoids
Thalidomide
Monoclonal antibodies and other biological agents
Phototherapy
Ondansetron
There were no restrictions in dosing, delivery, use of co‐interventions, or time lapse of treatments.
For each group of interventions, we included the following comparisons.
Active treatment versus placebo, sham procedure, or no treatment or equivalent (e.g. waiting list).
Active treatment versus another active treatment.
Types of outcome measures
We did not use measurement of the outcomes of interest in this review as an eligibility criterion.
Primary outcomes
Patient‐ or parent‐reported pruritus intensity, as measured by a visual analogue scale (Pereira 2017), the Itch Severity Scale (Majeski 2007), or another validated, or commonly used, scale
Adverse events, including pruritus exacerbation, skin irritation, or other agent‐specific adverse events
Secondary outcomes
Health‐related quality of life (HRQoL), as measured by a validated scale, such as the Dermatology Life Quality Index (DLQI; Finlay 1994), or ItchyQoL (pruritus‐specific quality of life instrument; Desai 2008), or another validated, or commonly used, scale
Sleep disturbances, as measured by the domain of a validated or commonly used itch scale, such as the Itch Severity Scale (Majeski 2007): sleep disturbances domain, or another validated or commonly used sleep quality scale
Depression, as measured by the Hamilton Depression Rating Scale (HAM‐D; Bech 1990), the Beck Depression Inventory (BDI; Beck 1996), or another validated or commonly used scale
Patient satisfaction, as measured by validated or commonly used scales, such as the patient satisfaction scale developed by van Cranenburgh 2013
Method and timing of outcome measurement
We used clinically important differences for the outcomes of interest to interpret the evidence in 'Summary of findings' tables. When the mean difference (MD) or risk ratio (RR) was equal to or larger than the minimal clinically important difference (MCID), we assumed that many participants might have gained clinically meaningful improvement from treatment. When the MD was at least half of the MCID, we assumed that an appreciable number of participants had likely achieved clinically meaningful improvement. Finally, when the MD was less than one‐half of the MCID, we assumed it was unlikely that an appreciable number of participants achieved clinically meaningful improvement (Johnston 2010), which was worded as "slightly" using GRADE language (Glenton 2010). If no MCID was available for the scale reported by a study, we considered a 30% change from baseline as a meaningful improvement. If data were pooled as the standardised mean difference (SMD), we used the MCID of the most commonly used scale.
Given that CPUO can be long‐lasting, we classified outcome measurements as short term (up to six months) or long term (six months or longer).
If we found multiple time points within each category (short term, long term), we included the longest follow‐up measurement.
Search methods for identification of studies
We aimed to identify all relevant RCTs and qRCTs, regardless of language or publication status (published, unpublished, in press, or in progress).
Electronic searches
The Cochrane Skin Information Specialist searched the following databases up to 2 July 2019, using strategies based on the draft strategy for MEDLINE in our published protocol (Andrade Miranda 2018).
Cochrane Skin Specialised Register, using the search strategy in Appendix 1.
Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 6), in the Cochrane Library, using the strategy in Appendix 2.
MEDLINE via Ovid (from 1946), using the strategy in Appendix 3.
Embase via Ovid (from 1974), using the strategy in Appendix 4.
Trials registers
Two review authors (JF and JEML) searched the following trials registers using the following search terms: 'pruritus', 'itch'.
ClinicalTrials.gov (www.clinicaltrials.gov).
World Health Organization International Clinical Trials Registry Platform (ICTRP; apps.who.int/trialsearch/).
Searching other resources
References from published studies
Two review authors (AA, JMEL) screened the bibliographies of included studies and identified any relevant systematic reviews, to find further relevant studies.
Adverse effects
We did not perform a separate search for adverse effects of the target intervention. We examined data on adverse effects from the included studies only.
Correspondence with trialists
Four review authors (AA, CYK, AMY, VS) contacted trial investigators to request missing data or clarification of study details, as indicated.
Conference proceedings
We searched the abstract proceedings of conferences of the American Academy of Dermatology, the European Academy of Dermatology and Venerology, and the International Forum for the Study of Itch (IFSI), including the World Congress on Itch (2015 and 2017), when these proceedings had not been identified in the electronic searches and had not been searched by the Cochrane Skin Information Specialist.
Data collection and analysis
Selection of studies
We used Covidence software to manage study selection and data extraction processes. After removing duplicate records, four review authors, in pairs (AA, CYK, AMY, VS), independently screened the titles and abstracts of records identified through the searches, and eliminated any irrelevant records. We obtained full texts of the remaining potentially relevant papers, and the same pairs of review authors independently assessed whether they met the inclusion criteria. We resolved disagreements through discussion; when necessary, a third review author (JVAF or GS, or both) adjudicated. We documented all discussions. We reported the numbers of reports retrieved, included, and excluded, and the reasons for exclusion, in a flow diagram, as described in the PRISMA statement (Liberati 2009).
Data extraction and management
Four review authors, in pairs (AA, CYK, AMY, VS), independently extracted study details and outcome data into the Covidence software. We resolved disagreements through discussion or consultation with a third review author as needed (JVAF or GS). If data were missing or incomplete, or if clarification was required, we contacted the study investigators. We extracted the following details.
Methods: study design; blinding of participants, investigators, and outcome assessors; setting; date of study; conduct; and study duration.
Participants: number randomised; gender; inclusion and exclusion criteria; number of dropouts and reasons for losses to follow‐up; previous treatments; duration of pruritus; comorbidity; and baseline data.
Interventions: description of treatment arms; route; dose or application frequency; and duration of intervention.
Outcomes: outcomes and timing of assessments, as reported by trial authors. We will extract the mean change from baseline or endpoint; standard deviations or standard errors of the mean change, or both; scale ranges; and the number of participants for each treatment group at each assessment. In addition, we will collect summary statistics for primary and secondary outcomes as reported (e.g. effect estimates, confidence intervals (CIs), standard errors (SEs), P values, ranges). We will define the baseline assessment as the latest available assessment before randomisation.
Funding source as reported.
Declarations of interest.
Four review authors, working in pairs (AA, CYK, AMY, VS), independently transferred these details into the Characteristics of included studies tables in the Covidence database, and after resolving disagreements, one review author (AA) subsequently exported them to Review Manager 5 (Review Manager 2014).
Assessment of risk of bias in included studies
The risk of bias for each study was assessed using a recently developed revision of the Cochrane 'risk of bias' tool (RoB 2.0: a revised tool to assess risk of bias in randomised trials) (Higgins 2019a; Sterne 2019). We used the most current version accessible at the riskofbias.info website (last accessed February 2019). We considered the effect of assignment to the intervention for this review. Three review authors (AA, CYK, JEML) independently assessed five domains of bias for each outcome result of all reported outcomes and time points. These five domains were biased due to (1) the randomisation process, (2) deviations from intended interventions (effects of assignment to interventions at baseline), (3) missing outcome data, (4) measurement of the outcome, and (5) selection of reported results. Answers to signalling questions and supporting information collectively led to a domain‐level judgement in the form of 'Low risk of bias', 'Some concerns', or 'High risk of bias'. These domain‐level judgements informed an overall 'risk of bias' judgement for each result. Discrepancies between review authors were resolved by discussion to reach consensus. If necessary, a third review author (JVAF) was consulted to achieve a decision. Although we did not find any non‐randomised studies (e.g. quasi‐RCTs) for this review, we would have followed guidance provided in the Cochrane Handbook for Systematic Reviews of Interventions in future updates for 'risk of bias' assessment (Higgins 2011; Reeves 2019).
When inadequate study details were provided, we contacted the authors of the paper to obtain further information.
Measures of treatment effect
Dichotomous data
For dichotomous outcome data, we calculated risk ratios (RRs) with their associated 95% confidence intervals (CIs). When the RR was statistically significant (95% CI did not overlap 1), we computed the number needed to treat for an additional beneficial outcome (NNTB) or the number needed to treat for an additional harmful outcome (NNTH), on the basis of the combined RR value, applying the overall event rate in the placebo groups as a proxy for baseline risk.
Continuous data
For continuous outcome data, we calculated mean differences (MDs) with their associated 95% CIs when eligible trials used the same instrument to measure a given construct. When data were pooled in studies that used different instruments to measure the same outcome, we planned to calculate standardised mean differences (SMDs) with 95% CIs, and to multiply the SMD by a standard deviation that was representative of the pooled studies, for example, the standard deviation (SD) from a well‐known scale used by several of the studies included in the analysis on which the result was based. These results would have been displayed in the 'Summary of findings' table as mean differences (Higgins 2011).
Unit of analysis issues
The unit of analysis was the participant. Although we did not find any cluster or cross‐over RCTs, we would have followed guidance provided in the Cochrane Handbook for Systematic Reviews of Interventions when dealing with unit of analysis issues (Higgins 2011; Higgins 2019b).
When dealing with studies with multiple groups, we planned to combine all relevant experimental intervention groups of the study into a single group, and then to combine all relevant control intervention groups into a single control group to avoid 'double counts', as suggested by the Cochrane Handbook for Systematic Reviews of Interventions, Section 16.5.4 (Higgins 2011). When a combination of groups was not possible due to clinical heterogeneity, we would have reported each pair‐wise comparison separately.
Dealing with missing data
We performed available‐case analysis. However, if data were missing, we proceeded as follows.
Contacted the original investigators to request missing data whenever possible.
Made explicit the assumptions of any methods used to cope with missing data, for example, that data were assumed missing at random, or that missing values were assumed to have a particular value, such as a poor outcome.
Performed sensitivity analyses to assess how sensitive results were to reasonable changes in the assumptions made.
Discussed in the Discussion section the potential impact of missing data on findings of the review, as recommended by the Cochrane Handbook for Systematic Reviews of Interventions, Section 16.1.2 (Higgins 2011).
We did not impute missing values for continuous outcomes. However, we would have calculated missing standard deviations from standard errors, exact P values, or confidence intervals, if these were available. When possible, we conducted intention‐to‐treat analyses for both continuous and dichotomous data. For dichotomous data, we would have assumed that the missing participants experienced a poor outcome (imputation on the basis of the worst‐case scenario). For continuous data, we would have extracted endpoint scores and corresponding SDs or, when not available, change from baseline values. When necessary, we planned to approximate means and measures of dispersion from figures in the reports using Plot Digitalizer (Jelicic 2016). During data extraction, we paid particular attention to missing data from inappropriate post‐allocation exclusions, such as those due to adverse events and to cross‐overs, when participants did not receive their allocated treatment.
We created a table in the review detailing the date of contact with original authors, the information requested, and the authors' reply (Table 5).
2. Contact with authors.
| Date and information requested | Author response |
| Yosipovitch Gil, MD. 18 November 2018 "Dear Dr. Yosipovitch: We are conducting a systematic review of Interventions for pruritus of unknown origin and we found your study: Serlopitant for the treatment of chronic pruritus: results of a randomised, multicenter, placebo‐controlled phase 2 clinical trial. Your study included patients with "chronic pruritus", but we want to know what were the causes of pruritus in order to decide inclusion in our review" |
"Hi Andrea, We included all patients with chronic itch including chronic itch with unknown origin (the majority) but those with uraemic and cholestatic itch, neuropathic itch, and major psychiatric disorders or drug‐induced itch were excluded from this study" |
| Yosipovitch Gil, MD. 15 February 2019 "Dear Dr. Yosipovitch: We are conducting a systematic review of Interventions for pruritus of unknown origin and we found your study: Serlopitant for the treatment of chronic pruritus: results of a randomised, multicenter, placebo‐controlled phase 2 clinical trial. We want to ask you if your study included patients with 'chronic pruritus' with unknown origin (the majority); however 'Approximately 45% of patients presented with a dermatologic diagnosis; however, a similar percentage had received no dermatologic diagnosis to explain their long‐standing symptoms.' We want to know about those patients: dermatological diagnostics, characteristics, number of patients, co‐interventions (patients using stable doses of mid‐potency topical steroids at screening could continue their use during the study, and they could also continue the use of lotions we guess that those patients were with the dermatologic diagnostic and may be with specified history of atopic diathesis (i.e. atopic dermatitis). Our question come in order to clarification for us the population and we can analyze the data" |
No response |
| Dr. Legat. 7 March 2019 "Dear Dr. Legat: We are conducting a Cochrane Systematic Review of the effects of UVB narrowband on pruritus of unknown origin and we found your study in an abstract compendium. 'BOTH NARROWBAND‐UVB AND BROADBAND‐UVB ARE EQUALLY EFFECTIVE IN REDUCING ITCH IN CHRONIC PRURITUS PATIENTS'. Your study included patients with 'chronic pruritus', but we wanted to know what were the causes of pruritus in order to decide inclusion in our review. You will be duly acknowledged" |
No response |
UVB: ultraviolet B.
Assessment of heterogeneity
We were unable to undertake any meta‐analyses in this review as only one study was included. If we are able to undertake meta‐analyses in future updates of this review, we will assess the clinical, methodological, and statistical diversity to determine if data from studies could meaningfully be combined and entered into a meta‐analysis. We will assess the level of clinical heterogeneity by exploring variability in participants, interventions, and outcomes. For example, we expect that there might be differences in the degree of the diagnostic workup in participants before they are defined as having CPUO. We will assess the level of methodological diversity by exploring variability in study design. We will test for statistical heterogeneity across studies by using Chi² and I² statistics, which describe the percentage of total variation across trials that is due to heterogeneity rather than to sample error (Higgins 2011). A Chi² P value < 0.1 and an I² statistic of 0% to 40% would suggest non‐important heterogeneity; 30% to 60% moderate heterogeneity; 50% to 90% substantial heterogeneity, and 75% to 100% very substantial heterogeneity.
If heterogeneity was found, we would re‐examine the data to determine if, for example, we mistakenly entered standard errors for standard deviations, or if there were any potential unit of analysis errors. For the meta‐analysis, we would use a random‐effects model as our default option (DerSimonian 1986). Based on the GRADE recommendations, we would consider downgrading the certainty of evidence for serious inconsistency if I² is above 50% (GRADE Handbook). We would also take into account a visual examination of the variability in point estimates and the overlap in confidence intervals.
Assessment of reporting biases
We attempted to obtain study protocols to assess selective outcome reporting. We had planned to develop funnel plots and use a linear regression test to examine the presence of reporting bias if we had included more than 10 studies in a meta‐analysis (Egger 1997). However, this was not possible, as only one study was included in this review. Several explanations can be offered for the asymmetry of a funnel plot, including true heterogeneity of effect with respect to trial size (and hence bias of small trials), poor methodological design, and publication bias. Therefore, we would have interpreted these results with caution.
Data synthesis
Three review authors (AA, JEML, JF) analysed the data in Review Manager 5. If we had undertaken a meta‐analysis, we would have pooled data from comparable groups of trials, using random‐effects models, according to the recommendations provided in Chapter 9 of theCochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We used 95% CIs throughout, and we would have considered not pooling data when there was considerable heterogeneity (I² > 75%) that could not be explained by the diversity of methodological or clinical features among trials (Deeks 2011). If we decided it was inappropriate to pool data, we would have presented narrative trial data in the analyses or in 'Summary of findings' tables for illustrative purposes, and reported these in the text. When results were estimated for individual studies with low numbers of events (< 10 in total), or when the total sample size was less than 30 participants, and we used a risk ratio, we planned to report the proportion of events in each group, together with a P value, obtained from Fisher’s exact test; however, this did not apply to any of the data we extracted.
Subgroup analysis and investigation of heterogeneity
When possible, we planned to perform a minimum number of subgroup analyses, according to the following stratified analyses, for the primary outcomes, to explore whether different study characteristics could be considered as effect modifiers.
Age: adults (18 years or older) versus children.
Pruritus severity (moderate to severe pruritus versus mild pruritus).
Duration of pruritus before enrolment in the study (≥ 6 months versus < 6 months).
Comorbidity (presence versus absence of comorbidity).
Pruritus of unknown origin definition in the included studies (e.g. with a full workup to exclude differential diagnosis versus little workup).
We also planned to investigate heterogeneity as outlined in the Assessment of heterogeneity section above. If clear reasons for heterogeneity were established, we would have reported the results separately. However, no meta‐analyses were undertaken, so we could not conduct any subgroup analyses nor explore heterogeneity.
Sensitivity analysis
If sufficient trials were available, we planned to determine the robustness of results by excluding studies with unclear or high risk of bias. In the presence of small‐study effects, we planned to assess the influence of small‐study effects on results of the meta‐analyses when there was evidence of between‐study heterogeneity (I² > 0) by inspecting the funnel plot, as suggested by the Cochrane Handbook for Systematic Reviews of Interventions (Section 10.4.5) (Sterne 2017). However, no meta‐analyses were conducted.
Summary of findings and assessment of the certainty of the evidence
Two review authors (AA and JEML) independently applied the GRADE approach to interpret findings for the main comparisons (GRADE Handbook). They resolved disagreements through discussion and, when necessary, requested the judgement of a third review author (JF or GS). We used GRADE profiler to import data from Review Manager 5 to create a 'Summary of findings' table (GRADEpro GDT; Review Manager 2014). A 'Summary of findings' table provides key information about the best estimate of the magnitude of the effect, in relative terms and absolute differences, for each relevant comparison of management strategies; numbers of participants and studies addressing each important outcome; and rating of overall confidence in effect estimates for each outcome (Guyatt 2011; Schünemann 2011). The GRADE approach takes into account five criteria that relate to internal validity (risk of bias, inconsistency, imprecision, publication bias, and external validity, such as directness of results) (Guyatt 2008). Based on how the evidence meets these criteria, we rated certainty as high, moderate, low, or very low (GRADE Handbook). These ratings reflect the overall certainty we had in the estimate of effects per outcome and comparison.
High certainty or quality: review authors are very confident that the true effect lies close to that of the estimate of the effect.
Moderate certainty or quality: review authors are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low certainty or quality: confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect.
Very low certainty or quality: study authors have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
We included these outcomes in the 'Summary of findings' tables, listed according to priority.
Patient‐ or parent‐reported pruritus intensity (long‐term follow‐up).
Adverse events (no restrictions on follow‐up).
Health‐related quality of life (long‐term follow‐up).
Sleep disturbances (long‐term follow‐up).
Depression (long‐term follow‐up).
Patient satisfaction (long‐term follow‐up).
We prioritised comparisons to placebo, sham treatment, or no treatment in the 'Summary of findings' tables. In the presence of multiple comparisons, we prioritised the following.
Emollient creams versus placebo, sham treatment, no treatment.
Cooling lotions versus placebo, sham treatment, no treatment.
Topical corticosteroids versus placebo, sham treatment, no treatment.
Topical antidepressants versus placebo, sham treatment, no treatment.
Systemic antihistamines versus placebo, sham treatment, no treatment.
Systemic antidepressants versus placebo, sham treatment, no treatment.
Systemic anticonvulsants versus placebo, sham treatment, no treatment.
Phototherapy versus placebo, sham treatment, no treatment.
However, we did not find any studies that assessed the above comparisons, so we made the post‐hoc decision to present 'Summary of findings' tables for the three comparisons included in this review.
Serlopitant 5 mg versus placebo.
Serlopitant 1 mg versus placebo.
Serlopitant 0.25 mg versus placebo.
We used controlled vocabulary as suggested by Glenton 2010 to summarise findings in the 'Summary of findings' table in the 'Plain language summary'. Because meta‐analysis was not possible, we presented the results in three 'Summary of findings' tables based on single‐study data.
Results
Description of studies
Results of the search
Through electronic searches, we retrieved 7148 references to studies and five additional records from clinical trials registries, which, after removal of duplicates, provided a total of 7108 citations. After examination of the titles and abstracts of these references, we eliminated 7034 additional ineligible studies. We obtained full‐text copies of the remaining 74 studies and subjected them to further evaluation. We translated several studies that were not published in the English language (Chinese and Polish) before assessing the studies for eligibility. Searches of the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) and the ClinicalTrials.gov databases revealed one ongoing trial that matched our inclusion criteria (see Characteristics of ongoing studies). Screening of conference proceedings did not result in additional eligible studies. Finally, we excluded 67 of the remaining studies (see the Characteristics of excluded studies section), and two studies are awaiting further assessment (see Characteristics of studies awaiting classification). For further details, see the study flow diagram (Figure 1).
1.
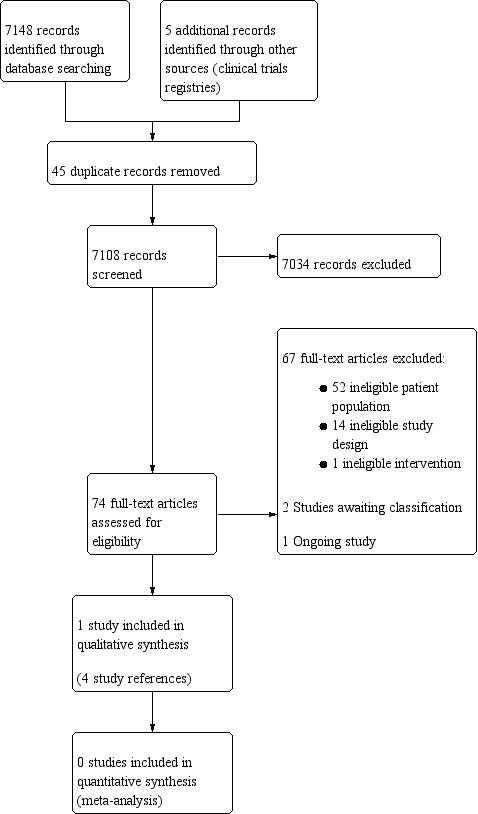
Study flow diagram.
Included studies
Details of the one included study are provided in the Characteristics of included studies table and appendices (Yosipovitch 2018).
Design, sample size, and setting
The included study was a parallel, randomised, placebo‐controlled trial, with 257 randomised participants from 25 US centres (clinical research centres and universities) with a study duration of 10 weeks (six weeks of treatment plus four weeks of post‐treatment follow‐up).
Participants
The included study involved participants 18 to 65 years of age, with mean age ranging from 42.49 to 44.48 years across groups (60.6% were women), who were in good health, had pruritus for six weeks or longer, were unresponsive to treatment with current therapies such as antihistamines or topical steroids (considered first‐line therapies for pruritus), and had a score of 7 cm or higher on the visual analogue scale (VAS) for pruritus at baseline. Fifty‐five per cent of the participants included in this study had chronic pruritus of unknown origin (CPUO), and approximately 45% presented a dermatological diagnosis (atopic dermatitis/eczema 37.3%, psoriasis 6.7%, acne 3.6%, dry skin 2.1%, lichenification 2.1%, keloid scar 1.6%, seborrhoeic dermatitis 1.6%, alopecia 1%, lichen planus 1%, among other diagnoses). We attempted to retrieve disaggregated outcome data from study authors for the subgroup of participants with CPUO (see Table 5 for our contact with trial authors).
Interventions
Included participants were randomly allocated to four treatment arms.
Placebo, once daily for six weeks (group 1, n = 64, as the comparator).
Serlopitant 0.25 mg once daily, oral administration, six weeks (group 2, n = 64).
Serlopitant 1 mg once daily, oral administration, six weeks (group 3, n = 65).
Serlopitant 5 mg once daily, oral administration, six weeks (group 4, n = 64).
Participants using stable doses of mid‐potency topical steroids at screening could continue their use during the study and could also continue the use of lotions. Participants with serum creatinine, aspartate aminotransferase, or alanine aminotransferase levels higher than twice the upper limit of normal and those with uraemic or cholestatic pruritus and with pruritus of neuropathic or psychogenic aetiology or drug‐induced pruritus were excluded. The mean age of participants was 44.48 years (age range 19 to 64) in the placebo group, 45.09 (age range 18 to 63) in the serlopitant 0.25‐mg daily group, 42.49 (age range 18 to 65) in the serlopitant 1‐mg daily group, and 42.94 (age range 18 to 65) in the serlopitant 5‐mg daily group. Investigators were instructed to record no change from baseline at post‐baseline assessments for participants who had no visibly affected skin at baseline, as the skin cannot improve from normal appearance/not affected. Any worsening of the skin condition would have had been recorded as a negative change.
Outcomes
The primary efficacy endpoint was the percentage change in VAS pruritus scores from baseline, when serlopitant was compared to placebo by using participants’ reports of pruritus intensity. Secondary efficacy endpoints included the numerical rating scale (NRS) pruritus score and total score, and domains of the Dermatology Life Quality Index (DLQI) survey, the Pittsburgh Sleep Symptom Questionnaire‐Insomnia (PSSQ‐I), the Subject’s Global Assessment (SGA) of pruritus severity, and the Physician’s Global Assessment (PGA). Safety was assessed through monitoring of adverse or serious adverse events, laboratory assessments, vital signs, electrocardiograms, serum levels of serlopitant, and abbreviated physical examinations. All outcomes were reported at the end of treatment (six weeks from baseline). Additional outcome data were provided for VAS and DLQI score at four weeks after discontinuation of treatment (10‐week follow‐up from baseline).
Excluded studies
We excluded 67 studies: 52 studies due to ineligible participant population, 14 due to ineligible study design, and one due to ineligible intervention. Further details are stated under Characteristics of excluded studies.
Ongoing studies
We found one ongoing study that compares administration of 5 mg of serlopitant to placebo in patients with CPUO (NCT0384331). This study started in January 2019 and aims to recruit 200 participants. It is sponsored by a pharmaceutical company.
Studies awaiting classification
We were unable to retrieve the full text of Aksungur 1990, and another study was an abstract of a study investigating the effects of phototherapy among patients with chronic pruritus (Legat 2017). We contacted the study authors to ask if they included patients with CPUO, but we received no response.
Risk of bias in included studies
For details on the methodological quality of the included study, see Characteristics of included studies and Figure 2.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Risk of bias arising from the randomisation process
For all outcomes, we judged the risk of bias arising from the randomisation process as low because randomisation was performed by Almac Clinical Technologies (Souderton, PA, USA) (automates the random assignment of treatment groups to bottle numbers that are blinded to investigators). Furthermore, treatment assignment was concealed from participants, investigators, and staff, and from the clinical research team.
Risk of bias due to deviations from intended interventions
Focusing on effects of assignment to interventions at baseline
For all outcomes, we judged the risk of bias due to deviations from intended interventions as low because assignment of treatment groups was blinded to investigators and participants by encoding and by using placebo (Almac Clinical Technologies).
Risk of bias due to missing outcome data
For the outcome 'sleep disturbances', no information was available for an assessment, so we rated this outcome as causing 'some concerns' of bias due to missing outcome data.
For all other outcomes, we considered the risk of bias due to missing outcome data as low because a total of 257 participants were randomised to placebo (n = 64) or serlopitant, 0.25 mg (n = 64), 1 mg (n = 65), or 5 mg (n = 64); 222 (86.4%) completed the study, and treatments were discontinued for 9 (14.1%), 7 (10.9%), 9 (13.8%), and 10 (15.6%) participants from the four arms, respectively. Reasons for missing outcome data provided by study authors are unlikely to be related to true outcomes.
Risk of bias in measurement of outcomes
For all outcomes, we judged risk of bias in measurement of the outcome as low, as the method of measuring the outcome was considered appropriate, and investigators and participants who assessed outcomes were blinded to treatment group by encoding and by using placebo (Almac Clinical Technologies).
Risk of bias in selection of reported results
In the clinical trial registry, only patient‐reported pruritus intensity (VAS and NRS) were listed as pre‐planned outcomes. Therefore, we judged the risk of bias in selection of the reported result as low for this outcome, and as causing 'some concerns' for the other outcomes.
Overall risk of bias
We judged that the overall risk of bias was low for the 'patient‐reported pruritus intensity' outcome, and caused 'some concerns' for the other outcomes (adverse events, health‐related quality of life, and sleep disturbances).
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings for the main comparison. Serlopitant 5 mg compared to placebo for chronic pruritus of unknown origin.
| Serlopitant 5 mg compared to placebo for chronic pruritus of unknown origin | |||||
| Patient or population: chronic pruritus of unknown origin Setting: 25 US centres Intervention: serlopitant 5 mg Comparison: placebo | |||||
| Outcomes | Number of participants (studies), Follow‐up | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with placeboe | Risk difference with serlopitant 5 mg | ||||
| Patient‐reported pruritus intensity Assessed with: participants with a ≥ 4‐cm reduction in average visual analogue scale (VAS range: 0 to 10 cm). A reduction in VAS score indicates improvement Follow‐up: 6 weeks | 126 (1 RCT) | ⊕⊕⊝⊝ Lowa | RR 2.06 (1.27 to 3.35) | Study population | |
| 254 per 1000 | 269 more per 1000 (69 more to 597 more) | ||||
| Adverse events Assessed with: number of participants with any adverse event Follow‐up: 6 weeks | 127 (1 RCT) | ⊕⊝⊝⊝ Very lowb | RR 1.48 (0.87 to 2.50) | Study population | |
| 254 per 1000 | 122 more per 1000 (33 fewer to 381 more) | ||||
| Health‐related quality of life Assessed with: Dermatology Life Quality Index (DLQI) score (range 0 to 30). A higher score indicates greater impairment Follow‐up: 6 weeks | 127 (1 RCT) | ⊕⊝⊝⊝ Very lowc | ‐ | Mean health‐related quality of life was 20.6 | MD 4.20 lower (11.68 lower to 3.28 higher) |
| Sleep disturbances: number of participants with insomnia Assessed with: Pittsburgh Sleep Symptom Questionnaire (the questionnaire reports insomnia as a dichotomous outcome) Follow‐up: 6 weeks |
128 (1 RCT) | ⊕⊝⊝⊝ Very lowd | RR 0.49 (0.24 to 1.01) | Study population | |
| 286 per 1000 | 177 fewer per 1000 (237 fewer to 46 fewer) | ||||
| Depression Assessed with: not measured |
‐ | ‐ | ‐ | ‐ | ‐ |
| Patient satisfaction Assessed with: not measured |
‐ | ‐ | ‐ | ‐ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DLQI: Dermatology Life Quality Index; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio; VAS: visual analogue scale. | |||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded by two levels to low‐certainty evidence: one level due to indirectness, as 45% of patients had an underlying diagnosis (not meeting the pruritus of unknown origin criterion); and one level due to imprecision because of small sample size.
bDowngraded by three levels to very low‐certainty evidence: one level due to some concerns regarding risk of bias selection of the reported result; one level due to indirectness, as 45% of patients had an underlying diagnosis (not meeting the pruritus of unknown origin criterion); and one level due to imprecision, as few events and wide confidence interval.
cDowngraded by three levels to very low‐certainty evidence: one level due to some concerns regarding risk of bias in selection of the reported result; one level due to indirectness, as 45% of patients had an underlying diagnosis (not meeting the pruritus of unknown origin criterion); and one level due to imprecision, as the confidence interval crosses the minimal important difference threshold for the DLQI questionnaire (between 3 and 5).
dDowngraded by three levels to very low‐certainty evidence: one level due to some concerns regarding risk of bias in selection of the reported result and risk of bias due to missing outcome data; one level due to indirectness, as 45% of patients had an underlying diagnosis (not meeting the pruritus of unknown origin criterion); and one level due to imprecision, as few events and wide confidence interval.
eRisk in the comparison group is based on the number of events (dichotomous data) or the mean (continuous data) in the control group of the one included study.
Summary of findings 2. Serlopitant 1 mg compared to placebo for chronic pruritus of unknown origin.
| Serlopitant 1 mg compared to placebo for chronic pruritus of unknown origin | |||||
| Patient or population: chronic pruritus of unknown origin Setting: 25 US centres Intervention: serlopitant 1 mg Comparison: placebo | |||||
| Outcomes | Number of participants (studies), Follow‐up | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with placeboe | Risk difference with serlopitant 1 mg | ||||
| Patient‐reported pruritus intensity Assessed with: participants with a ≥ 4‐cm reduction in average visual analogue scale (VAS range 0 to 10 cm). A reduction in VAS score indicates improvement Follow‐up: 6 weeks | 126 (1 RCT) | ⊕⊕⊝⊝ Lowa | RR 1.50 (0.89 to 2.54) | Study population | |
| 254 per 1000 | 127 more per 1000 (28 fewer to 391 more) | ||||
| Adverse events Assessed with: number of participants with any adverse event Follow‐up: 6 weeks | 128 (1 RCT) | ⊕⊝⊝⊝ Very lowb | RR 1.45 (0.86 to 2.47) | Study population | |
| 254 per 1000 | 114 more per 1000 (36 fewer to 373 more) | ||||
| Health‐related quality of life Assessed with: Dermatology Life Quality Index (DLQI) score (range 0 to 30). A higher score indicates greater impairment Follow‐up: 6 weeks | 128 (1 RCT) | ⊕⊝⊝⊝ Very lowc | ‐ | Mean health‐related quality of life was 20.6 | MD 6.90 lower (14.38 lower to 0.58 higher) |
| Sleep disturbances: number of participants with insomnia Assessed with: Pittsburgh Sleep Symptom Questionnaire (the questionnaire reports insomnia as a dichotomous outcome) Follow‐up: 6 weeks |
128 (1 RCT) | ⊕⊝⊝⊝ Very lowd | RR 0.38 (0.17 to 0.84) | Study population | |
| 286 per 1000 | 177 fewer per 1000 (237 fewer to 46 fewer) | ||||
| Depression Assessed with: not measured | ‐ | ‐ | ‐ | ‐ | |
| Patient satisfaction Assessed with: not measured | ‐ | ‐ | ‐ | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DLQI: Dermatology Life Quality Index; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio; VAS: visual analogue scale. | |||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded by two levels to low‐certainty evidence: one level due to indirectness, as 45% of patients had an underlying diagnosis (not meeting the pruritus of unknown origin criterion); and one level due to imprecision because of small sample size.
bDowngraded by three levels to very low‐certainty evidence: one level due to some concerns regarding selection of the reported result; one level due to indirectness, as 45% of patients had an underlying diagnosis (not meeting the pruritus of unknown origin criterion); and one level due to imprecision, as few events and wide confidence interval.
cDowngraded by three levels to very low‐certainty evidence: one level due to some concerns regarding selection of the reported result; one level due to indirectness, as 45% of patients had an underlying diagnosis (not meeting the pruritus of unknown origin criterion); and one level due to imprecision, as the confidence interval crosses the minimal important difference threshold for the DLQI questionnaire (between 3 and 5).
dDowngraded by three levels to very low‐certainty evidence: one level due to some concerns regarding risk of bias in selection of the reported result and risk of bias due to missing outcome data; one level due to indirectness, as 45% of patients had an underlying diagnosis (not meeting the pruritus of unknown origin criterion); and one level due to imprecision, as few events and wide confidence interval.
eRisk in the comparison group is based on the number of events (dichotomous data) or the mean (continuous data) in the control group of the one included study.
Summary of findings 3. Serlopitant 0.25 mg compared to placebo for chronic pruritus of unknown origin.
| Serlopitant 0.25 mg compared to placebo for chronic pruritus of unknown origin | |||||
| Patient or population: chronic pruritus of unknown origin Setting: 25 US centres Intervention: serlopitant 0.25 mg Comparison: placebo | |||||
| Outcomes | Number of participants (studies), Follow‐up | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with placeboe | Risk difference with serlopitant 0.25 mg | ||||
| Patient‐reported pruritus intensity Assessed with: participants with a ≥ 4‐cm reduction in average visual analogue scale (VAS range 0 to 10 cm). A reduction in VAS score indicates improvement Follow‐up: 6 weeks | 127 (1 RCT) | ⊕⊕⊝⊝ Lowa | RR 1.66 (1.00 to 2.77) | Study population | |
| 254 per 1000 | 168 more per 1000 (0 fewer to 450 more) | ||||
| Adverse events Assessed with: number of participants with any adverse event Follow‐up: 6 weeks | 127 (1 RCT) | ⊕⊝⊝⊝ Very lowb | RR 1.29 (0.75 to 2.24) | Study population | |
| 254 per 1000 | 74 more per 1000 (63 fewer to 315 more) | ||||
| Health‐related quality of life Assessed with: Dermatology Life Quality Index (DLQI) score (range 0 to 30). A higher score indicates greater impairment Follow‐up: 6 weeks | 127 (1 RCT) | ⊕⊝⊝⊝ Very lowc | ‐ | Mean health‐related quality of life was 14.9 | MD 5.70 lower (13.18 lower to 1.78 higher) |
| Sleep disturbances: number of participants with insomnia Assessed with: Pittsburgh Sleep Symptom Questionnaire (the questionnaire reports insomnia as a dichotomous outcome) Follow‐up: 6 weeks |
127 (1 RCT) | ⊕⊝⊝⊝ Very lowd | RR 0.60 (0.31 to 1.17) | Study population | |
| 286 per 1000 | 114 fewer per 1000 (197 fewer to 49 more) | ||||
| Depression Assessed with: not measured | ‐ | ‐ | ‐ | ‐ | |
| Patient satisfaction Assessed with: not measured | ‐ | ‐ | ‐ | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DLQI: Dermatology Life Quality Index; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio; VAS: visual analogue scale. | |||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded by two levels to low‐certainty evidence: one level due to indirectness, as 45% of patients had an underlying diagnosis (not meeting the pruritus of unknown origin criterion); and one level due to imprecision because of small sample size.
bDowngraded by three levels to very low‐certainty evidence: one level due to some concerns regarding selection of the reported result; one level due to indirectness, as 45% of patients had an underlying diagnosis (not meeting the pruritus of unknown origin criterion); and one level due to imprecision, as few events and wide confidence interval.
cDowngraded by three levels to very low‐certainty evidence: one level due to some concerns regarding selection of the reported result; one level due to indirectness, as 45% of patients had an underlying diagnosis (not meeting the pruritus of unknown origin criterion); and one level due to imprecision, as the confidence interval crosses the minimal important difference threshold for the DLQI questionnaire (between 3 and 5).
dDowngraded by three levels to very low‐certainty evidence: one level due to some concerns regarding risk of bias in selection of the reported result and risk of bias due to missing outcome data; one level due to indirectness, as 45% of patients had an underlying diagnosis (not meeting the pruritus of unknown origin criterion); and one level due to imprecision, as few events and wide confidence interval.
eRisk in the comparison group is based on the number of events (dichotomous data) or the mean (continuous data) in the control group of the one included study.
The one study included in this review compared an NK1R antagonist (serlopitant), which is a systemic pharmacological intervention, at three different doses (5 mg, 1 mg, 0.25 mg) against placebo. Hence, we did not find any studies that assessed the main comparisons of interest in this review, which include the following.
Emollient creams versus placebo, sham treatment, no treatment.
Cooling lotions versus placebo, sham treatment, no treatment.
Topical corticosteroids versus placebo, sham treatment, no treatment.
Topical antidepressants versus placebo, sham treatment, no treatment.
Systemic antihistamines versus placebo, sham treatment, no treatment.
Systemic antidepressants versus placebo, sham treatment, no treatment.
Systemic anticonvulsants versus placebo, sham treatment, no treatment.
Phototherapy versus placebo, sham treatment, no treatment.
Serlopitant (NK1R antagonist) versus placebo
One study with 257 participants compared three different doses of serlopitant versus placebo (daily for six weeks) (Yosipovitch 2018). This study compared the use of serlopitant 0.25 mg (64 randomised participants), 1 mg (65 randomised participants), and 5 mg (64 randomised participants) versus placebo (64 randomised participants). Two of our secondary outcomes were not assessed in this study: depression and patient satisfaction.
1. Serlopitant 5 mg versus placebo
See Table 1.
1.1. Primary outcome 1: patient‐reported pruritus intensity
This outcome was reported in 126 participants. Participants who received serlopitant 5 mg once daily may have a greater rate of relief of patient‐reported pruritus intensity as measured by the visual analogue scale (VAS) compared to placebo at six weeks' follow‐up: 33 of 63 in the serlopitant 5‐mg group and 16 of 63 in the placebo group (risk ratio (RR) 2.06, 95% confidence interval (CI) 1.27 to 3.35; Analysis 1.1). Relief was defined as a reduction in average VAS of 4 cm or greater (range 0 to 10 cm). A reduction in VAS score indicates improvement. The certainty of the evidence is low due to indirectness and imprecision. On average, 3.7 patients would have to receive serlopitant 5 mg (instead of placebo) for one additional patient to have relief of pruritus (number needed to treat for an additional beneficial outcome (NNTB) = 3.7). Serlopitant 5 mg may also slightly reduce pruritus intensity as measured by VAS as a continuous score: mean percentage decrease in the serlopitant 5‐mg group of 42.5% versus mean percentage decrease in the placebo group of 28.3% (mean difference (MD) ‐14.20, 95% CI ‐26.63 to ‐1.77; Analysis 1.2).
1.1. Analysis.
Comparison 1 Serlopitant 5 mg versus placebo, Outcome 1 Patient‐reported pruritus intensity VAS (dichotomous).
1.2. Analysis.
Comparison 1 Serlopitant 5 mg versus placebo, Outcome 2 Patient‐reported pruritus intensity VAS (continuous).
When 11‐point numerical rating scale (NRS) measurement of patient‐reported itch intensity is performed, participants who received serlopitant 5 mg may have a greater rate of relief of pruritus symptoms as measured by NRS compared to placebo at six weeks' follow‐up: 29 of 63 in the serlopitant 5‐mg group and 14 of 63 in the placebo group (RR 2.07, 95% CI 1.21 to 3.53; Analysis 1.3). Relief was defined as a reduction in average NRS pruritus score of 4 or more points (range 0 to 10). Serlopitant 5 mg may also slightly reduce pruritus intensity as measured by NRS as a continuous score (a reduction in NRS score indicates improvement): mean percentage decrease in the serlopitant 5‐mg group of 39% versus mean percentage decrease in the placebo group of 28.7% (MD ‐10.30, 95% CI ‐20.01 to ‐0.59; Analysis 1.4).
1.3. Analysis.
Comparison 1 Serlopitant 5 mg versus placebo, Outcome 3 Patient‐reported pruritus intensity NRS (dichotomous).
1.4. Analysis.
Comparison 1 Serlopitant 5 mg versus placebo, Outcome 4 Patient‐reported pruritus intensity NRS (continuous).
Serlopitant 5 mg once daily at 10 weeks' follow‐up (four weeks after treatment discontinuation assessed in 126 participants) may have little to no effect on pruritus intensity: mean percentage decrease in VAS score in the serlopitant 5‐mg group was 42.7% versus 31% in the placebo group (MD ‐11.70, 95% CI ‐23.06 to ‐0.34; Analysis 1.5).
1.5. Analysis.
Comparison 1 Serlopitant 5 mg versus placebo, Outcome 5 Patient‐reported pruritus intensity VAS (continuous) ‐ 10 weeks.
1.2. Primary outcome 2: adverse events
This outcome was reported in 127 participants. We are uncertain whether serlopitant 5 mg increases adverse events compared to placebo at six weeks' follow‐up: 24 of 64 in the serlopitant 5‐mg group and 16 of 63 in the placebo group (RR 1.48, 95% CI 0.87 to 2.50; Analysis 1.6). The most commonly reported adverse events were somnolence (three participants), diarrhoea (two participants), headache (one participant), upper respiratory tract infection (one participant), and urinary tract infection (two participants). The certainty of the evidence is very low due to concerns regarding risk of bias in selection of the reported result, indirectness, and imprecision.
1.6. Analysis.
Comparison 1 Serlopitant 5 mg versus placebo, Outcome 6 Adverse events.
1.3. Secondary outcome 1: health‐related quality of life (HRQoL)
This outcome was reported in 127 participants. We are uncertain whether serlopitant 5 mg improves quality of life as measured by the Dermatology Life Quality Index (DLQI) compared to placebo at six weeks' follow‐up: mean score in the serlopitant 5‐mg group of 16.4 versus mean score in the placebo group of 20.6 (MD ‐4.20, 95% CI ‐11.68 to 3.28; Analysis 1.7). The DLQI is a questionnaire with 10 items and results calculated by summing the score of each question (10‐item questionnaire ‐ score ranges from 0 to 30). The higher the score, the more quality of life is impaired. The certainty of the evidence is very low due to concerns regarding risk of bias in selection of the reported result, indirectness, and imprecision.
1.7. Analysis.
Comparison 1 Serlopitant 5 mg versus placebo, Outcome 7 Health‐related quality of life.
Serlopitant 5 mg once daily at 10 weeks' follow‐up (four weeks after treatment discontinuation assessed in 127 participants) may have little to no effect on quality of life: mean DLQI score in the serlopitant 5‐mg group was 16.2 versus 20.2 in the placebo group (MD ‐4.00, 95% CI ‐11.48 to 3.48; Analysis 1.8).
1.8. Analysis.
Comparison 1 Serlopitant 5 mg versus placebo, Outcome 8 Health‐related quality of life ‐ 10 weeks.
1.4. Secondary outcome 2: sleep disturbances
This outcome was reported in 128 participants. We are uncertain whether serlopitant 5 mg improves sleep disturbances as measured by the Pittsburgh Sleep Symptom Questionnaire‐Insomnia (PSSQ‐I): insomnia compared to placebo at six weeks' follow‐up: 9 of 64 in the serlopitant 5‐mg group and 18 of 63 in the placebo group (RR 0.49, 95% CI 0.24 to 1.01; Analysis 1.9). The PSSQ‐I has 13 self‐rated questions that identify participants with sleep disturbances ("insomnia disorder"). Certainty of the evidence is very low due to concerns regarding risk of bias in selection of the reported result and missing outcome data, indirectness, and imprecision.
1.9. Analysis.
Comparison 1 Serlopitant 5 mg versus placebo, Outcome 9 Sleep disturbances.
2. Serlopitant 1 mg versus placebo
See Table 2.
2.1. Primary outcome 1: patient‐reported pruritus intensity
This outcome was reported in 126 participants. Participants who received serlopitant 1 mg may have seen little to no effect in relief of patient‐reported pruritus intensity as measured by the VAS compared to placebo at six weeks' follow‐up: 24 of 63 in the serlopitant 1‐mg group and 16 of 63 in the placebo group (RR 1.50, 95% CI 0.89 to 2.54; Analysis 2.1). Certainty of the evidence is low due to indirectness and imprecision. Serlopitant 1 mg may also slightly reduce pruritus intensity as measured by VAS as a continuous score: mean percentage decrease in the serlopitant 1‐mg group of 42.5% versus mean percentage decrease in the placebo group of 28.3% (MD ‐13.10, 95% CI ‐24.38 to ‐1.82; Analysis 2.2).
2.1. Analysis.
Comparison 2 Serlopitant 1 mg versus placebo, Outcome 1 Patient‐reported pruritus intensity VAS (dichotomous).
2.2. Analysis.
Comparison 2 Serlopitant 1 mg versus placebo, Outcome 2 Patient‐reported pruritus intensity VAS (continuous).
When patient‐reported itch intensity was measured by NRS, participants who received serlopitant 1 mg may had have little to no effect in relief of patient‐reported pruritus intensity compared to placebo at six weeks' follow‐up: 20 of 63 in the serlopitant 1‐mg group and 14 of 63 in the placebo group (RR 1.43, 95% CI 0.79 to 2.57; Analysis 2.3). This effect was also evident in the NRS continuous measurements: mean percentage decrease in the serlopitant 1‐mg group of 39.4% versus mean percentage decrease in the placebo group of 28.7% (MD ‐10.70, 95% CI ‐20.41 to ‐0.99; Analysis 2.4).
2.3. Analysis.
Comparison 2 Serlopitant 1 mg versus placebo, Outcome 3 Patient‐reported pruritus intensity NRS (dichotomous).
2.4. Analysis.
Comparison 2 Serlopitant 1 mg versus placebo, Outcome 4 Patient‐reported pruritus intensity NRS (continuous).
Serlopitant 1 mg once daily at 10 weeks' follow‐up (four weeks after treatment discontinuation assessed in 126 participants) may have little to no effect on pruritus intensity: mean percentage decrease in VAS score in the serlopitant 1‐mg group was 41.5% versus 31% in the placebo group (MD ‐10.50, 95% CI ‐21.73 to 0.73; Analysis 2.5).
2.5. Analysis.
Comparison 2 Serlopitant 1 mg versus placebo, Outcome 5 Patient‐reported pruritus intensity VAS (continuous) ‐ 10 weeks.
2.2. Primary outcome 2: adverse events
This outcome was reported in 128 participants. We are uncertain whether serlopitant 1 mg increases adverse events compared to placebo at six weeks' follow‐up: 24 of 65 in the serlopitant 1‐mg group and 16 of 63 in the placebo group (RR 1.45, 95% CI 0.86 to 2.47; Analysis 2.6). The most commonly reported adverse events in the serlopitant group were somnolence (three participants), diarrhoea (four participants), headache (three participants), nasopharyngitis (three participants), pruritus (two participants), nausea (two participants), dry mouth (two participants), and musculoskeletal pain (two participants). Certainty of the evidence is very low due to concerns regarding risk of bias in selection of the reported result, indirectness, and imprecision.
2.6. Analysis.
Comparison 2 Serlopitant 1 mg versus placebo, Outcome 6 Adverse events.
2.3. Secondary outcome 1: health‐related quality of life (HRQoL)
This outcome was reported in 128 participants. We are uncertain whether serlopitant 1 mg improves quality of life as measured by DLQI compared to placebo at six weeks' follow‐up: mean score in the serlopitant 1‐mg group 13.7, mean score in the placebo group 20.6 (MD ‐6.90, 95% CI ‐14.38 to 0.58; Analysis 2.7). Certainty of the evidence is very low due to concerns regarding risk of bias in selection of the reported result, indirectness, and imprecision.
2.7. Analysis.
Comparison 2 Serlopitant 1 mg versus placebo, Outcome 7 Health‐related quality of life.
Serlopitant 1 mg once daily at 10 weeks' follow‐up (four weeks after treatment discontinuation assessed in 128 participants) may have little to no effect on quality of life: mean DLQI score in the serlopitant 1‐mg group was 17.9 versus 20.2 in the placebo group (MD ‐2.30, 95% CI ‐9.78 to 5.18; Analysis 2.8).
2.8. Analysis.
Comparison 2 Serlopitant 1 mg versus placebo, Outcome 8 Health‐related quality of life ‐ 10 weeks.
2.4. Secondary outcome 2: sleep disturbances
This outcome was reported in 128 participants. We are uncertain whether serlopitant 1 mg improves sleep disturbances as measured by PSSQ‐I compared to placebo at six weeks' follow‐up: 7 of 65 in the serlopitant 1‐mg group and 18 of 63 in the placebo group (RR 0.38, 95% CI 0.17 to 0.84; Analysis 2.9). Certainty of the evidence is very low due to concerns regarding risk of bias in selection of the reported result and missing outcome data, indirectness, and imprecision.
2.9. Analysis.
Comparison 2 Serlopitant 1 mg versus placebo, Outcome 9 Sleep disturbance.
3. Serlopitant 0.25 mg versus placebo
See Table 3.
3.1. Primary outcome 1: patient‐reported pruritus intensity
This outcome was reported in 127 participants. Participants who received serlopitant 0.25 mg may have seen little to no effect in relief of patient‐reported pruritus intensity as measured by the VAS compared to placebo at six weeks' follow‐up: 27 of 64 in the serlopitant 0.25‐mg group and 16 of 63 in the placebo group (RR 1.66, 95% CI 1.00 to 2.77; Analysis 3.1). Certainty of the evidence is low due to indirectness and imprecision. Serlopitant 0.25 mg may have little to no effect on pruritus intensity as measured by VAS as a continuous score: mean percentage decrease in the serlopitant 0.25‐mg group 34.1%, mean percentage decrease in the placebo group 28.3% (MD ‐5.80, 95% CI ‐17.16 to 5.56; Analysis 3.2).
3.1. Analysis.
Comparison 3 Serlopitant 0.25 mg versus placebo, Outcome 1 Patient‐reported pruritus intensity VAS (dichotomous).
3.2. Analysis.
Comparison 3 Serlopitant 0.25 mg versus placebo, Outcome 2 Patient‐reported pruritus intensity VAS (continuous).
When assessed by NRS measurement of patient‐reported itch intensity, participants who received serlopitant 0.25 mg may have seen little to no effect in relief of pruritus symptoms compared to placebo at six weeks' follow‐up: 24 of 64 in the serlopitant 0.25‐mg group and 16 of 63 in the placebo group (RR 1.69, 95% CI 0.96 to 2.95; Analysis 3.3). Serlopitant 0.25 mg have little to no effect as measured by the NRS continuous measurements: mean percentage decrease in the serlopitant 0.25‐mg group: 35.8%, mean percentage decrease in the placebo group: 28.7% (MD ‐7.10, 95% CI ‐16.80 to 2.60; Analysis 3.4).
3.3. Analysis.
Comparison 3 Serlopitant 0.25 mg versus placebo, Outcome 3 Patient‐reported pruritus intensity NRS (dichotomous).
3.4. Analysis.
Comparison 3 Serlopitant 0.25 mg versus placebo, Outcome 4 Patient‐reported pruritus intensity NRS (continuous).
Serlopitant 0.25 mg once daily at 10 weeks' follow‐up (four weeks after treatment discontinuation assessed in 127 participants) may have little to no effect on pruritus intensity: mean percentage decrease in VAS score in the serlopitant 0.25‐mg group was 38.4% versus 31% in the placebo group (MD ‐7.40, 95% CI ‐18.63 to 3.83; Analysis 3.5).
3.5. Analysis.
Comparison 3 Serlopitant 0.25 mg versus placebo, Outcome 5 Patient‐reported pruritus intensity VAS (continuous) ‐ 10 weeks.
3.2. Primary outcome 2: adverse events
This outcome was reported in 127 participants. We are uncertain whether serlopitant 0.25 mg increases adverse events compared to placebo at six weeks' follow‐up: 21 of 64 in the serlopitant 0.25‐mg group and 16 of 63 in the placebo group (RR 1.29, 95% CI 0.75 to 2.24; Analysis 3.6). The most commonly reported adverse events were somnolence (one participant), headache (one participant), nasopharyngitis (two participants), upper respiratory tract infection (three participants), pruritus (two participants), and arthralgia (two participants). Certainty of the evidence is very low due to concerns regarding risk of bias in selection of the reported result, indirectness, and imprecision.
3.6. Analysis.
Comparison 3 Serlopitant 0.25 mg versus placebo, Outcome 6 Adverse events.
3.3. Secondary outcome 1: health‐related quality of life (HRQoL)
This outcome was reported in 127 participants. We are uncertain whether serlopitant 0.25 mg improves quality of life as measured by the DLQI compared to placebo at six weeks' follow‐up: mean score in the serlopitant 0.25‐mg group of 14.9 versus mean score in the placebo group of 20.6 (MD ‐5.70, 95% CI ‐13.18 to 1.78; Analysis 3.7). Certainty of the evidence is very low due to concerns regarding risk of bias in selection of the reported result, indirectness, and imprecision.
3.7. Analysis.
Comparison 3 Serlopitant 0.25 mg versus placebo, Outcome 7 Health‐related quality of life.
Serlopitant 0.25 mg once daily at 10 weeks' follow‐up (four weeks after treatment discontinuation assessed in 127 participants) may have little to no effect on quality of life: mean DLQI score in the serlopitant 1‐mg group was 15.8 versus 20.2 in the placebo group (MD ‐4.40, 95% CI ‐11.88 to 3.08; Analysis 3.8).
3.8. Analysis.
Comparison 3 Serlopitant 0.25 mg versus placebo, Outcome 8 Health‐related quality of life ‐ 10 weeks.
3.4. Secondary outcome 2: sleep disturbances
This outcome was reported in 127 participants. We are uncertain whether serlopitant 0.25 mg improves sleep disturbances as measured by the PSSQ‐I compared to placebo at six weeks' follow‐up: 11 of 64 in the serlopitant 0.25‐mg group and 18 of 63 in the placebo group (RR 0.60, 95% CI 0.31 to 1.17; Analysis 3.9). Certainty of the evidence is very low due to concerns regarding risk of bias in selection of the reported result and missing outcome data, indirectness, and imprecision.
3.9. Analysis.
Comparison 3 Serlopitant 0.25 mg versus placebo, Outcome 9 Sleep disturbance.
Discussion
Summary of main results
We found an absence of evidence for the key interventions: emollient creams, cooling lotions, topical corticosteroids, topical antidepressants, systemic antihistamines, systemic antidepressants, systemic anticonvulsants, and phototherapy. No study assessing these treatments fulfilled our inclusion criteria, so we were unable to assess their effects on chronic pruritus of unknown origin (CPUO).
Our single included study of 257 participants reviewed serlopitant at three different daily doses (0.25 mg, 1 mg, and 5 mg, all given once daily for six weeks) versus placebo, measuring outcomes after six weeks of treatment (Table 1; Table 2; Table 3; Yosipovitch 2018).
We found that participants who received serlopitant 5 mg may have a greater rate of relief of pruritus intensity. Serlopitant 1 mg and 0.25 mg may also cause a greater rate of itching intensity; however, the 95% confidence interval (CI) indicates there may be little or no difference between groups (low‐certainty evidence).
Due to very low‐certainty evidence, we are uncertain of the effects of serlopitant at the three doses on adverse events, health‐related quality of life, and sleep disturbances. The most commonly reported adverse events were sleepiness, diarrhoea, headache, and upper respiratory tract infection, among others.
The effects of this drug on depression and patient satisfaction were not reported by the included study.
We did not find sufficient evidence to determine the effects of interventions for CPUO in adults and children.
Overall completeness and applicability of evidence
Evidence regarding interventions for CPUO is limited and incomplete. The only trial included in our review assessed serlopitant versus placebo in a case‐mix population. We could not assess the other interventions of interest due to lack of randomised controlled trials (RCTs); hence, we cannot provide any definitive conclusions regarding potential benefits and harms of these interventions for CPUO. The evidence we did find was restricted to adults only (participants were over 18 years of age), and the study was set in a single location (the USA). This study did not report two of our secondary outcomes: 'Depression' and 'Patient satisfaction'. Thus, the evidence is of limited relevance to our review question.
We defined 'chronic pruritus of unknown origin' according to information provided by the International Forum for the Study of Itch (IFSI). The Forum classification system for chronic pruritus describes six categories of underlying pruritogenic diseases. Although several studies focus on pruritus, category VI, the focus of this review ‐ Stander 2007 ‐ has received little to no attention in the literature.
Quality of the evidence
Evidence is of low certainty for the outcome of 'Patient‐reported reduction of pruritus assessed with VAS [visual analogue scale]', and of very low certainty for the outcomes of 'Adverse events', 'Health‐related quality of life assessed with DLQI [Dermatology Life Quality Index]', and 'Sleep disturbances' over the course of a short‐term follow‐up of six weeks. For all of these outcomes, the level of evidence was downgraded by one level due to the study’s indirectness because 45% of patients in the study had an underlying diagnosis and did not meet the pruritus of unknown origin criterion. We further downgraded by one level for imprecision due to small sample size, few adverse events, wide confidence interval, or confidence interval crossing the minimal important difference threshold for the DLQI questionnaire (between 3 and 5). Outcomes other than 'Patient‐reported reduction of pruritus assessed with VAS' were downgraded a further level because we had some concerns regarding risk of bias due to selection in the reported results, as the clinical trial register did not report these outcomes. For the outcome of 'Sleep disturbances', we had some concerns regarding risk of bias due to missing outcome data, as no information was available for assessment.
Potential biases in the review process
It is unlikely that we have missed any relevant studies using our search criteria. We searched for any relevant randomised controlled trials and quasi‐randomised controlled trials, regardless of language or publication status (published, unpublished, in press, or in progress). For trials written in a foreign language, we enlisted the help of translators from the Cochrane community. We also contacted the corresponding authors for further relevant information on included (and also excluded) studies. We minimised bias in study selection, data extraction, and risk of bias assessment by having two different review authors perform these tasks independently. Unfortunately, our review was limited to a single study with a relatively small sample size.
Agreements and disagreements with other studies or reviews
We found no other systematic reviews assessing the effectiveness and adverse effects of pharmacological and non‐pharmacological interventions for CPUO in adults and children. However, two Cochrane Reviews have explored the topic of pruritus (Rungsiprakarn 2016; Siemens 2016). Rungsiprakarn 2016 evaluated pharmacological interventions for generalised pruritus (not caused by systemic disease or skin lesions) in pregnancy. As the review authors did not identify any relevant trials, they concluded that well‐designed randomised controlled trials are needed to evaluate the effectiveness of topical and systemic pharmacological interventions, as well as any adverse effects of the interventions. Siemens 2016 evaluated pharmacological interventions for pruritus in adult palliative care patients. The review authors indicated that, in palliative care participants with pruritus of different nature, treatment with the drug paroxetine, a selective serotonin reuptake inhibitor, reduced pruritus by 0.78 points (numerical analogue scale from 0 to 10; 95% confidence interval (CI) ‐1.19 to ‐0.37; 1 study; n = 48; moderate‐certainty evidence) compared to placebo. In this review, some evidence of effectiveness was found for participants with cholestatic and uraemic pruritus when gabapentin, nalfurafine, cromolyn sodium, rifampin, flumecinol, or naltrexone, among others, was given. However the mechanism of pruritus for these participants might differ from the mechanism for those with CPUO.
We found few observational data. A case report evaluating six patients with chronic pruritus of unknown origin who received treatment with naltrexone 50 mg per day indicated that oral administration of naltrexone effectively suppressed pruritus (Metze 1999). Another case report on aprepitant treatment for refractory pruritus of unclear origin included a regimen of 125 mg aprepitant on day 1, and then 80 mg on days 2 to 4, resulting in VAS score improvement from 8 of 10 to 4 of 10 after 24 hours, and to 1 of 10 after six weeks. The patient experienced significant reduction in pruritus without relevant side effects (Ally 2013).
Authors' conclusions
Implications for practice.
Little research has been conducted to investigate our review question. We found no eligible studies assessing the main comparisons of interest in this review.
We found evidence for only a certain subset of our interventions and participants of interest. The one included study assessed serlopitant in adults, and provided insufficient evidence to enable us to formulate conclusions.
Low‐certainty evidence suggests that compared to placebo, serlopitant 5 mg may reduce pruritus intensity. Lower doses of 1 mg and 0.25 mg may also cause a greater rate of relief of itching intensity; however, at these doses, trial results are more uncertain.
We cannot make conclusions about effects of serlopitant on our other measured outcomes of interest ‐ adverse effects, health‐related quality of life, and sleep disturbances ‐ due to very low‐certainty evidence.
Healthcare professionals, patients, and other stakeholders may need to rely on indirect evidence from other forms of chronic pruritus when deciding between the main interventions currently used for this condition.
Implications for research.
We suggest that additional randomised controlled trials with sufficient numbers of participants and primarily focusing on patients with CPUO are required for the main therapies that are currently being used for patients with this condition. If studies include a mix of patients with chronic pruritus of unknown origin (CPUO) and other forms of chronic pruritus, we suggest that outcome data disaggregated by each subgroup should be presented. Trial protocols should be made publicly available, stating adequately which scale and time point were used for each outcome, and how each outcome was supposed to be analysed. Summarising indirect evidence of interventions for other forms of chronic pruritus may aid stakeholders when selecting from different treatments for this condition.
Acknowledgements
We thank the following individuals and organisations.
Cochrane Skin, for providing guideline and templates for protocol development.
Instituto Universitario Hospital Italiano Cochrane Associate Centre, Argentina.
Family and Community Medicine Service, Hospital Italiano de Buenos Aires, Argentina.
Dermatology Department, Hospital Italiano de Buenos Aires, Argentina.
Marta Roque Figuls, who authored the protocol and provided statistical input on the analysis and interpretation of results.
Ashley Yu, who authored the protocol and helped with screening of references.
Virginia Garrote (Biblioteca Central ‐ Instituto Universitario Hospital Italiano), who provided feedback on the search strategy.
Yuan Chi, Centre for Evidence‐Based Chinese Medicine, Beijing University of Chinese Medicine, who retrieved some of the articles for full‐text screening.
Matthew Page PhD, BBSc(Hons), Research Fellow (NHMRC Early Career Fellow), Public Health and Preventive Medicine Monash University, Melbourne, Australia, for help regarding ROB 2.0 methods.
Manuel Pedro Pereira, MD, PhD, Center for Chronic Pruritus, Department of Dermatology, University Hospital Münste, for clarifying details on study eligibility.
Moriya Ohkuma, MD, PhD, Department of Dermatology, Sakai Hospital, Kinki University, School of Medicine, for clarifying details on study eligibility.
Yosipovitch Gil, MD, Department of Dermatology, Miller School of Medicine, University of Miami; Director, Miami Itch Center, for providing information regarding the included study.
We based the methods sections of our review on two protocols: 'Non‐pharmacological interventions for treating chronic prostatitis/chronic pelvic pain syndrome' (Franco 2017), and 'Emollients and moisturisers for eczema' (van Zuuren 2016).
The Cochrane Skin editorial base wishes to thank Sam Gibbs, Cochrane Dermatology Editor, for this review; Ben Carter, Statistical Editor; the clinical referees, Tim Berger and Ethan Lerner; and Dolores Matthews, who copy‐edited the review.
Appendices
Appendix 1. Cochrane Skin Specialised Register (CRSW)
#1 MESH DESCRIPTOR pruritus AND INREGISTER #2 (prurit*):ti AND INREGISTER #3 #1 OR #2 #4 (unknown or unexplain* or chronic* or idiopathic* or un known* or un explain* or undetermin* or un determin*):ti,ab. AND INREGISTER #5 MESH DESCRIPTOR chronic disease AND INREGISTER #6 (chronic or persis*):ti,ab. AND INREGISTER #7 #4 OR #5 OR #6 #8 (itch or itches or itched or itchy or itching or itchiness or prurit*):ti,ab. AND INREGISTER #9 #7 AND #8 #10 MESH DESCRIPTOR ANTIPRURITICS AND INREGISTER #11 (antiprurit* or anti prurit*):ti,ab. AND INREGISTER #12 #10 OR #11 #13 #3 OR #9 OR #12
Appendix 2. CENTRAL (Cochrane Library) search strategy
#1 MeSH descriptor: [Pruritus] this term only #2 prurit*:ti #3 #1 or #2 #4 (unknown or unexplain* or chronic* or idiopathic* or un known* or un explain* or undetermin* or un determin*):ti,ab #5 MeSH descriptor: [Chronic Disease] this term only #6 (chronic or persis*):ti,ab #7 #4 or #5 or #6 #8 (itch or itches or itched or itchy or itching or itchiness or prurit*):ti,ab #9 #7 and #8 #10 MeSH descriptor: [Antipruritics] this term only #11 (antiprurit* or anti prurit*):ti,ab #12 #10 or #11 #13 #3 or #9 or #12
Appendix 3. MEDLINE Ovid search strategy
1. Pruritus/ 2. prurit*.ti,ab. 3. 1 or 2 4. (unknown or unexplain* or chronic* or idiopathic* or un known* or un explain* or undetermin* or un determin*).ti,ab. 5. Chronic Disease/ 6. (chronic or persis*).ti,ab. 7. 4 or 5 or 6 8. (itch or itches or itched or itchy or itching or itchiness).ti,ab. 9. 7 and 8 10. ANTIPRURITICS/ 11. (antiprurit* or anti prurit*).ti,ab. 12. 10 or 11 13. 3 or 9 or 12 14. randomized controlled trial.pt. 15. controlled clinical trial.pt. 16. randomized.ab. 17. placebo.ab. 18. clinical trials as topic.sh. 19. randomly.ab. 20. trial.ti. 21. 14 or 15 or 16 or 17 or 18 or 19 or 20 22. exp animals/ not humans.sh. 23. 21 not 22 24. 13 and 23
(Lines 13‐22: Cochrane highly sensitive search strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision))
Appendix 4. Embase (Ovid) search strategy
1. *pruritus/ 2. prurit*.ti. 3. 1 or 2 4. (unknown or unexplain* or chronic* or idiopathic* or un known* or un explain* or undetermin* or un determin*).ti,ab. 5. idiopathic disease/ 6. chronic disease/ 7. (chronic or persis*).ti,ab. 8. 4 or 5 or 6 or 7 9. (itch or itches or itched or itchy or itching or itchiness or prurit*).ti,ab. 10. 8 and 9 11. antipruritic agent/ 12. (antiprurit* or anti prurit*).ti,ab. 13. 11 or 12 14. 3 or 10 or 13 15. crossover procedure.sh. 16. double‐blind procedure.sh. 17. single‐blind procedure.sh. 18. (crossover$ or cross over$).tw. 19. placebo$.tw. 20. (doubl$ adj blind$).tw. 21. allocat$.tw. 22. trial.ti. 23. randomized controlled trial.sh. 24. random$.tw. 25. or/15‐24 26. exp animal/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 27. human/ or normal human/ 28. 26 and 27 29. 26 not 28 30. 25 not 29 31. 14 and 30
Appendix 5. ROB 2.0 assessment (signalling questions) ‐ Yosipovitch 2018
| Domain 1: Risk of bias arising from the randomisation process | ||
| Signalling questions | Description | Response options |
| 1.1. Was the allocation sequence random? | Page 884 ‐ 891e1 ‐ SUPPLEMENTAL DATA: METHODS. Quote: “Almac Group, Inc. (Craigavon, UK) was responsible for patient randomization and material logistics. An interactive web‐based response system was used to randomly assign study patients in a 1:1:1:1 ratio to receive serlopitant, 0.25, 1, or 5 mg, or placebo” | Y |
| 1.2. Was the allocation sequence concealed until participants were enrolled and assigned to interventions? | Quote: “randomization was performed by using the Almac Group, Inc. (automates the random assignment of treatment groups to bottle numbers that are blinded to investigators and patients by encoding (Almac Clinical Technologies, Souderton, PA). Treatment assignment was concealed from the patients, the investigators, and their staff, and the clinical research team. The placebo tablets were formulated to be indistinguishable from serlopitant tablets (Almac, Craigavon, UK)” | Y |
| 1.3. Did baseline differences between intervention groups suggest a problem with the randomisation process? | Table I. Demographic and clinical characteristics of patients at baseline. They are similar groups. Total patients were included | N |
| Risk of bias judgement | (all outcomes) | LOW |
| Domain 2: Risk of bias due to deviations from the intended interventions (effect of assignment to intervention) | ||
| Signalling questions | Description | Response options |
| 2.1. Were participants aware of their assigned intervention during the trial? | Quote: “randomization was performed by using the Almac Group, Inc. (automates the random assignment of treatment groups to bottle numbers that are blinded to investigators and patients by encoding (Almac Clinical Technologies, Souderton, PA). Treatment assignment was concealed from the patients. The placebo tablets were formulated to be indistinguishable from serlopitant tablets (Almac, Craigavon, UK)” | N |
| 2.2. Were carers and people delivering the interventions aware of participants' assigned intervention during the trial? | Treatment assignment was concealed from investigators and their staff, and from the clinical research team | PN |
| 2.3. If Y/PY/NI to 2.1 or 2.2: Were there deviations from the intended intervention that arose because of the experimental context? | N/A | N/A |
| 2.4. If Y/PY to 2.3: Were these deviations from intended intervention balanced between groups? | N/A | N/A |
| 2.5. If N/PN/NI to 2.4: Were these deviations likely to have affected the outcome? | N/A | N/A |
| 2.6. Was an appropriate analysis used to estimate the effect of assignment to intervention? | Quote: “Intention‐to‐treat principles were used for the primary analyses of efficacy for all randomized patients (N = 257)” | Y |
| 2.7. If N/PN/NI to 2.6: Was there potential for a substantial impact (on the result) of the failure to analyse participants in the group to which they were randomised? | N/A | N/A |
| Risk of bias judgement | (all outcomes) | LOW |
| Domain 3: Missing outcome data | ||
| Signalling questions | Description | Response options |
| 3.1. Were data for this outcome available for all, or nearly all, participants randomised? | 257 were randomised to receive placebo (n = 64) or serlopitant, 0.25 mg (n = 64), 1 mg (n = 65) or 5 mg (n = 64). Of those 257 patients, 222 (86.4%) completed the study. Treatments were discontinued for 9 (14.1%), 7 (10.9%), 9 (13.8%), and 10 (15.6%) patients from the 4 arms, respectively. Nevertheless, outcome data were missing only for the following numbers of patients Pruritus intensity (VAS and NRS) Group 1: 1 participant. Group 2: no missing outcome data Group 3: 2 participants. Group 4: 1 participant Study authors state, "One patient from the placebo group (20‐004), 2 from the serlopitant 1‐mg group (04‐003 and 12‐010), and 1 from the serlopitant 5‐mg group (12‐014), although part of the ITT population were removed from the analysis. Patient 20‐004 was randomized in error and did not receive study medication, patient 04‐003 did not receive an e‐diary during screening and had no baseline data, and patients 12‐010 and 12‐014 did not complete any e‐diary information after screening" Adverse events: Group 1: 1 participant. Groups 2, 3, and 4: no missing outcome data Health‐related quality of life (DLQI) Group 1: 1 participant. Groups 2, 3, and 4: no missing outcome data Sleep disturbances: no information |
Pruritus intensity, adverse events, and HRQoL: Yes Sleep disturbances: No information |
| 3.2. If N/PN/NI to 3.1: Is there evidence that result was not biased by missing outcome data? | Differences analysed with least squares (analysis methods that correct for bias) means tests in comparison with placebo. One patient from the placebo group (20‐004), 2 from the serlopitant 1‐mg group (04‐003 and 12‐010), and 1 from the serlopitant 5‐mg group (12‐014), although part of the intention‐to‐treat population, were removed from the analysis because they had no baseline or post‐baseline VAS data. Missing data were not imputed | N |
| 3.3. If N/PN to 3.2: Could missingness in the outcome depend on its true value? | Participants were removed from the analysis because they had no baseline or post‐baseline VAS data | PN |
| 3.4. If Y/PY/NI to 3.3: Do the proportions of missing outcome data differ between intervention groups? | N/A | N/A |
| 3.5. If Y/PY/NI to 3.3: Is it likely that missingness in the outcome depended on its true value? | N/A | N/A |
| Risk of bias judgement: Pruritus intensity Quality of life Adverse events |
‐ | LOW |
| Risk of bias judgement: Sleep disturbances |
Pruritus intensity, adverse events, and HRQoL: low Sleep disturbances: some concerns |
|
| Domain 4: Risk of bias in measurement of the outcome | ||
| Signalling questions | Description | Response options |
| 4.1. Was the method of measuring the outcome inappropriate? | "The primary efficacy endpoint was the percentage change in VAS pruritus scores from baseline, comparing serlopitant to placebo by using patients’ reports of pruritus intensity. Secondary efficacy endpoints included the NRS pruritus score and total score and domains of the DLQI, the PSSQ‐I, the SGA, and the PGA. Safety was assessed through the monitoring of adverse or serious adverse events, laboratory assessments, vital signs, electrocardiograms, serum levels of serlopitant, and abbreviated physical examinations" | N |
| 4.2. Could measurement or ascertainment of the outcome have differed between intervention groups? | Outcome assessors were blinded to the assigned intervention | N |
| 4.3. If N/PN/NI to 4.1 and 4.2: Were outcome assessors aware of the intervention received by study participants? | "Treatment assignment was concealed from the patients, the investigators, and their staff, and the clinical research team. The placebo tablets were formulated to be indistinguishable from serlopitant tablets (Almac, Craigavon, UK)" | N |
| 4.4. If Y/PY/NI to 4.3: Could assessment of the outcome have been influenced by knowledge of intervention received? | N/A | N/A |
| 4.5. If Y/PY/NI to 4.4: Is it likely that assessment of the outcome was influenced by knowledge of intervention received? | N/A | N/A |
| Risk of bias judgement | (all outcomes) | LOW |
| Domain 5: Risk of bias in selection of the reported result | ||
| Signalling questions | Description | Response options |
| 5.1. Was the trial analysed in accordance with a pre‐specified plan that was finalised before unblinded outcome data were available for analysis? | We analysed the trial registry: www.clinicaltrials.gov/show/nct01951274 Pruritus intensity was defined in 2 measurements. Primary outcome measures: Visual analogue scale [Time Frame: 6 weeks] Secondary outcome measures: Verbal response scale [Time Frame: 6 weeks] Other outcomes were not defined VAS scores at 10 weeks were not defined |
Pruritus intensity at 6 weeks: Y Other outcomes and results: NI |
| Is the numerical result being assessed likely to have been selected, on the basis of the results, from… 5.2. ... multiple outcome measurements (e.g. scales, definitions, time points) within the outcome domain? |
See above |
Pruritus intensity at 6 weeks: PN Other outcomes and results: NI |
| 5.3. ... multiple analyses of the data? | See above |
Pruritus intensity at 6 weeks: PN Other outcomes and results: NI |
| Risk of bias judgement: Pruritus intensity at 6 weeks |
LOW | |
| Risk of bias judgement: other outcomes and results |
Pruritus intensity at 6 weeks: LOW Other outcomes and results: SOME CONCERNS |
|
| Overall risk of bias: pruritus intensity (VAS and NRS at 6 weeks) | LOW | |
| Overall risk of bias: all other outcomes | There are some issues regarding selection of the reporting of results for sleep disturbances, health‐related quality of life, adverse events, and 10 weeks' follow‐up measurements. Furthermore, information was insufficient for assessing the completeness of outcome data for sleep disturbances | SOME CONCERNS |
|
Response options Y: yes; N: no; PN: probably no; PY: probably yes; NI: no information; N/A: not applicable Other abbreviations DLQI: dermatology life‐quality index; ITT: intention‐to‐treat; NRS: numerical rating scale; PGA: Physician’s Global Assessment; PSSQ‐I: Pittsburgh Sleep Symptom Questionnaire‐Insomnia; SGA: Subject’s Global Assessment; VAS: visual analogue scale | ||
Data and analyses
Comparison 1. Serlopitant 5 mg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patient‐reported pruritus intensity VAS (dichotomous) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Patient‐reported pruritus intensity VAS (continuous) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Patient‐reported pruritus intensity NRS (dichotomous) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Patient‐reported pruritus intensity NRS (continuous) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 Patient‐reported pruritus intensity VAS (continuous) ‐ 10 weeks | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7 Health‐related quality of life | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8 Health‐related quality of life ‐ 10 weeks | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9 Sleep disturbances | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 2. Serlopitant 1 mg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patient‐reported pruritus intensity VAS (dichotomous) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Patient‐reported pruritus intensity VAS (continuous) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Patient‐reported pruritus intensity NRS (dichotomous) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Patient‐reported pruritus intensity NRS (continuous) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 Patient‐reported pruritus intensity VAS (continuous) ‐ 10 weeks | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7 Health‐related quality of life | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8 Health‐related quality of life ‐ 10 weeks | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9 Sleep disturbance | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 3. Serlopitant 0.25 mg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patient‐reported pruritus intensity VAS (dichotomous) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Patient‐reported pruritus intensity VAS (continuous) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Patient‐reported pruritus intensity NRS (dichotomous) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Patient‐reported pruritus intensity NRS (continuous) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 Patient‐reported pruritus intensity VAS (continuous) ‐ 10 weeks | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7 Health‐related quality of life | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8 Health‐related quality of life ‐ 10 weeks | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9 Sleep disturbance | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Yosipovitch 2018.
| Methods |
Study design: randomised placebo‐controlled trial Study duration: 10 weeks Number of participants randomised: 257 Study dates: 1 October 2013, to 2 December 2014 |
|
| Participants |
Setting: multi‐centre (25 clinical research centres and universities), USA Inclusion criteria: Patients aged 18 to 65 years who were in good health and had pruritus for 6 weeks or longer that was unresponsive to treatment with current therapies such as antihistamines or topical steroids (considered first‐line therapies for pruritus) and a score of 7 cm or higher on the visual analogue scale (VAS) for pruritus at baseline Exclusion criteria: Patients with serum creatinine, aspartate aminotransferase, or alanine aminotransferase levels higher than twice the upper limit of normal, patients with uraemic or cholestatic pruritus and with pruritus of neuropathic or psychogenic aetiology or drug‐induced pruritus Baseline data: Age (years): Group 1 (placebo) (44.48 + 13.33, median: 48, minimum: 19, maximum: 64) Group 2 (serlopitant 0.25 mg): (45.09 + 14.01, median: 50, minimum: 18, maximum: 63) Group 3 (serlopitant 1 mg) (42.49 + 14.08, median: 43, minimum: 18, maximum: 65) Group 4 (serlopitant 5 mg) (42.94 + 13.96, median: 44.5, minimum: 18, maximum: 65) Sex (M/F): Group 1 (placebo) (M: 25 (39.1)/F: 39 (60.9)) Group 2 (serlopitant 0.25 mg) (M: 24 (37.5)/F: 40 (62.5)) Group 3 (serlopitant 1 mg) (M: 27 (41.5)/F: 38 (58.5)) Group 4 (serlopitant 5 mg) (M: 25 (39.1)/F: 39 (60.9)) Race: Group 1 (placebo) (white: 43 (67.2), black or African: 14 (21.9), Asian: 5 (7.8), American Indian or Alaskan Native: 0, other: 2 (3.1)) Group 2 (serlopitant 0.25 mg) (white 43 (67.2), black or African: 16 (25), Asian: 2 (3.1), American Indian: 1 (1.6), other: 2 (3.1)) Group 3 (serlopitant 1 mg) (white: 38 (58.5), black or African: 21 (32.3), Asian: 2 (3.1), American Indian or Alaskan Native: 1 (1.5), other: 3 (4.6)) Group 4 (serlopitant 5 mg) (white: 43 (67.2), black or African: 19 (29.7), Asian: 1 (1.6), American Indian: 0, other: 1 (1.6)) Ethnic origin: Group 1 (placebo): (Hispanic or Latino: 12 (18.8), non‐Hispanic or Latino: 52 (81.3)) Group 2 (serlopitant 0.25 mg): (Hispanic or Latino:18 (28.1), non‐Hispanic or Latino: 46 (71.9)) Group 3 (serlopitant 1 mg): (Hispanic or Latino: 17 (26.2), non‐Hispanic or Latino: 48 (73.8)) Group 4 (serlopitant 5 mg): (Hispanic or Latino: 11 (17.2), non‐Hispanic or Latino: 53 (82.8)) Atopic diathesis: Group 1 (placebo): no 42 (65.6), yes: 22 (34.4) Group 2 (serlopitant 0.25 mg): no: 40 (62.5), yes: 24 (37.5) Group 3 (serlopitant 1 mg): no: 37 (56.9), yes: 28 (43.1) Group 4 (serlopitant 5 mg): no: 37 (57.8), yes: 27 (42.2) |
|
| Interventions |
Treatment groups: Group 1 (n = 64): placebo, daily for 6 weeks Group 2 (n = 64): serlopitant 0.25 mg daily, oral administration, 6 weeks Group 3 (n = 65): serlopitant 1 mg daily, oral administration, 6 weeks Group 4 (n = 64): serlopitant 5 mg daily, oral administration, 6 weeks Additional co‐intervention: patients using stable doses of mid‐potency topical steroids at screening could continue their use during the study, and they could also continue the use of lotions. Investigators were instructed to record no change from baseline at post‐baseline assessments for patients who had no visibly affected skin at baseline, as skin cannot improve from normal appearing/not affected. Any worsening of the skin condition would have been recorded as a negative change |
|
| Outcomes |
Patient‐ or parent‐reported pruritus intensity: The primary efficacy endpoint was the percentage change in VAS pruritus scores How measured: VAS Time points measured: 1, 2, 3, 4, 5, 6, 10 weeks (4 weeks after discontinuation) Time points reported: 1, 2, 3, 4, 5, 6, 10 weeks (4 weeks after discontinuation) Subgroups: a pre‐specified subgroup analysis of patients with a history of atopic diathesis (i.e. atopic dermatitis, allergy, and/or asthma) Patient‐ or parent‐reported pruritus intensity: The primary efficacy endpoint was the percentage change in NRS pruritus scores How measured: NRS Time points measured: 1, 2, 3, 4, 5, 6 weeks Time points reported: 1, 2, 3, 4, 5, 6 weeks Adverse events: How measured: clinic visits, blood samples, vital signs, electrocardiograms. Time points measured: not specified Time points reported: not specified Health‐related quality of life: How measured: Dermatology Life Quality Index (DLQI) survey Time points measured: 0, 1, 2, 6, 10 weeks (4 weeks after discontinuation) Time points reported: 0, 1, 2, 6, 10 weeks (4 weeks after discontinuation) Sleep disturbances: How measured: PSSQ‐I questionnaire and Pittsburgh Sleep Symptom Questionnaire‐Insomnia Time points measured: baseline, 1, 2, 4, 6, and 10 weeks (4 weeks after discontinuation). No data were collected at weeks 3 and 5, as patients did not visit the clinic then Time points reported: 1, 2, 4, 6, and 10 weeks (4 weeks after discontinuation). No data were collected at weeks 3 and 5, as patients did not visit the clinic Depression: not measured Patient satisfaction: not measured Other reported outcomes: PGA: Physician’s Global Assessment; SGA: Subject’s Global Assessment |
|
| Funding source | Supported by Menlo Therapeutics Inc. Medical writing and editorial assistance were provided by ApotheCom (New York, NY) and were funded by Menlo Therapeutics Inc. |
|
| Declarations of interest | Quote: "Dr. Yosipovitch has received grant/research support for his role as an investigator from Menlo Therapeutics Inc., Vanda Pharmaceuticals, Kiniksa Pharmaceuticals, and Sun Pharma, and he has received honoraria for participation in advisory boards for Menlo Therapeutics Inc., Trevi, Novartis, Pfizer, OPKO Health Inc., Sanofi, Galderma, and Sienna. Dr. Stander has received grants/research support and honoraria for her role as an investigator and consultant from Menlo Therapeutics Inc.; she has received honoraria for participation in advisory boards for Menlo Therapeutics Inc. and has received patient fees from Menlo Therapeutics Inc. Dr. Kerby has received personal fees from Velocity Pharmaceutical Development and is a shareholder and consultant to Menlo Therapeutics Inc. Dr. Larrick is a co‐founder of and owns stock in Menlo Therapeutics Inc. Drs. Perlman and Schnipper are employees of Velocity Pharmaceutical Development, and they have received stock from Menlo Therapeutics Inc. for their roles as advisors and consultants. Dr. Zhang has received personal fees from Velocity Pharmaceutical Development; he is a shareholder and employee of Menlo Therapeutics Inc., and he reports patents issued to Menlo Therapeutics Inc. Dr. Luger has received grant/research support for his role as an investigator from AbbVie, Celgene, Eli Lily, Janssen‐Cilag, Mylan/Meda Pharmaceuticals, MSD, Novartis, and Pfizer, and he has received honoraria for participation in advisory boards for AbbVie, Celgene, CERIES, Eli Lilly, Galderma, Janssen‐Cilag, La Roche Posay, Mylan/Meda, Novartis, and Pfizer, and honoraria for his role as a speaker for AbbVie, Celgene, Galderma, Janssen‐Cilag, La Roche Posay, Mylan/ Meda, MSD, Novartis, and Pfizer. Dr. Steinhoff has received honoraria for his role as a speaker from Menlo Therapeutics Inc., Galderma, and Eli Lily; for serving as a consultant for Menlo Therapeutics Inc.; and for participating in advisory boards for Menlo Therapeutics Inc., Galderma, and Pierre Fabre Laboratories. Dr. Tang has no conflicts of interest to disclose" |
|
| Notes | Contact email: yosipog@gmail.com Clinical trial register: NCT01951274 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Risk of bias arising from the randomization process | Low risk | Randomisation was performed by Almac Clinical Technologies (Souderton, PA, USA) (automates the random assignment of treatment groups to bottle numbers that are blinded to investigators and patients by encoding). Treatment assignment was concealed from patients, investigators, and staff, and from the clinical research team Baselines imbalances were not detected Judgement for all outcomes: we considered the risk of bias to be low |
| Risk of bias due to deviations from the intended interventions | Low risk | The procedure of the study protocol was followed with no deviation from standard care. Both participants and personnel were blinded to the assigned intervention (placebo tablets were formulated to be indistinguishable from serlopitant tablets (Almac, Craigavon, UK) Judgement for all outcomes: we considered the risk of bias to be low |
| Risk of bias due to missing outcome data Pruritus intensity, Health Related Quality of Life, Adverse Events | Low risk | Quote: "a total of 257 patients were randomised: placebo (n = 64) or serlopitant 0.25 mg (n = 64), 1 mg (n = 65), or 5 mg (n = 64). Of those patients, 222 (86.4%) completed the study. Treatments were discontinued for 9 (14.1%), 7 (10.9%), 9 (13.8%), and 10 (15.6%) patients from the 4 arms, respectively. Reasons for discontinuation included adverse events, loss to follow‐up, protocol violation, voluntary withdrawal, or other." The results presented here are based on the intention‐to‐treat population and therefore include all enrolled individuals. Missing data were not imputed Numbers of patients who initiated/completed the study/numbers of patients lost to follow‐up: Group 1:n = 64/55/1 Group 2:n = 64/57/2 Group 3:n = 65/56/1 Group 4:n = 64/54/2 Missing outcome data per outcome: Pruritus intensity (VAS and NRS) Group 1: 1 participant. Group 2: no missing outcome data Group 3: 2 participants. Group 4: 1 participant Study authors state: "one patient from the placebo group (20‐004), 2 from the serlopitant 1‐mg group (04‐003 and 12‐010), and 1 from the serlopitant 5‐mg group (12‐014), although part of the ITT population, were removed from the analysis. Patient 20‐004 was randomised in error and did not receive study medication, patient 04‐003 did not receive an e‐diary during screening and had no baseline data, and patients 12‐010 and 12‐014 did not complete any e‐diary information after screening" Adverse events: Group 1: 1 participant. Groups 2, 3, and 4: no missing outcome data Health‐related quality of life (DLQI) Group 1: 1 participant. Groups 2, 3, and 4: no missing outcome data Sleep disturbances: no information Depression: not reported Patient satisfaction: not reported Judgement for pruritus intensity, adverse events, health‐related quality of life: we considered risk of bias to be low (we assumed data were missing at random and performed available case analysis) |
| Risk of bias due to missing outcome data Sleep disturbances | Unclear risk | Judgement for sleep disturbances: some concerns regarding risk of bias (no available information) |
| Risk of bias in measurement of the outcome | Low risk | Quote: "the primary efficacy endpoint was the percentage change in VAS pruritus scores from baseline, comparing serlopitant to placebo by using patients’ reports of pruritus intensity. Secondary efficacy endpoints included the NRS pruritus score and total score and domains of the DLQI, the PSSQ‐I, the SGA, and the PGA. Safety was assessed through the monitoring of adverse or serious adverse events, laboratory assessments, vital signs, electrocardiograms, serum levels of serlopitant, and abbreviated physical examinations". Patients and study personnel were blinded (patient‐reported outcomes) Judgement for all outcomes: we considered the risk of bias to be low |
| Risk of bias in selection of the reported result Pruritus intensity (VAS and NRS) at 6 weeks | Low risk | We analysed the clinical trial registry: www.clinicaltrials.gov/show/nct01951274 The primary and secondary outcomes of this registry matched the definition found in the final report: "Primary outcome measures: Visual analog scale [Time Frame: 6 weeks] Secondary outcome measures: Verbal response scale [Time Frame: 6 weeks]" |
| Risk of bias in selection of the reported result Other outcomes | Unclear risk | We analysed the clinical trial registry: www.clinicaltrials.gov/show/nct01951274 Definitions for the following outcomes were incomplete:
Depression and patient satisfaction were not reported in the registry nor in the study report Measurements at 10 weeks were not described in the clinical trial registry |
| Overall risk of bias Pruritus intensity (VAS and NRS) | Low risk | Only for results at 6‐week follow‐up |
| Overall risk of bias Other outcomes | Unclear risk | There are some issues regarding selection of reporting of results for sleep disturbances, quality of life, adverse events, and 10‐week follow‐up measurements Furthermore, information was insufficient to assess the completeness of outcome data for sleep disturbances |
DLQI: Dermatology Life Quality Index.
NRS: numerical rating scale.
PGA: Physician's Global Assessment.
PSSQ‐I: Pittsburgh Sleep Symptom Questionnaire‐Insomnia.
SGA: Subject's Global Assessment.
VAS: visual analogue scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Acar 2010 | Ineligible patient population: external auditory canal pruritus |
| Adams 1996 | Ineligible patient population |
| Agero 2004 | Ineligible patient population |
| Agot 1975 | Ineligible patient population: included participants with dermatological conditions |
| AkhavanAmjadi 2012 | Ineligible patient population |
| Al Ghnaniem 2007 | Ineligible patient population |
| Ancona‐Castro 2018 | Ineligible patient population: patients with systemic or dermatological conditions |
| Anonymous 1980 | Ineligible patient population |
| Anonymous 2005 | Ineligible study design |
| Anonymous 2015 | Ineligible patient population: we screened the entire book of abstracts, and all reported trials included patients with known causes of pruritus |
| Armond 1976 | Ineligible patient population |
| Augustin 2011 | Ineligible patient population |
| Bernstein 1979 | Ineligible study design |
| Bigliardi 2007 | Ineligible patient population |
| Brasileiro 2016 | Ineligible study design |
| Burch 1988 | Ineligible patient population |
| Caddy 1966 | Ineligible patient population |
| Carati 2013 | Ineligible patient population |
| Chatterjee 2005 | Ineligible patient population |
| ChiCTR‐IOR‐16009877 | Ineligible patient population: patients with dermatological causes of pruritus |
| CHICTR‐TRC‐11001485 | Ineligible patient population: patients with dermatological causes of pruritus |
| Dupont 1984 | Ineligible intervention |
| Dyshko 1966 | Ineligible study design |
| Eberhartinger 1969 | Ineligible patient population |
| Elsaie 2016 | Ineligible patient population |
| Eschler 2010 | Ineligible study design |
| Essen 1953 | Ineligible patient population |
| Evers 2017 | Ineligible study design |
| Fischer 1968 | Ineligible patient population |
| Fjellner 1978 | Ineligible study design |
| Fjellner 1981 | Ineligible study design |
| Ginsberg 2004 | Ineligible patient population |
| Gisslen 1962 | Ineligible patient population |
| Gooding 2010 | Ineligible study design |
| Hagermark 1974 | Ineligible patient population |
| Heinlin 2013 | Ineligible patient population |
| Hellier 1963 | Ineligible patient population |
| IRCT2014112613612N3 | Ineligible patient population: patients with pruritus and positive antibodies for thyroid disease |
| Juhlin 1961 | Ineligible patient population |
| Kamm Kohl 1987 | Ineligible patient population |
| Kleine Natrop 1970 | Ineligible patient population |
| Knoth 1961 | Ineligible patient population |
| Kouwenhoven 2017 | Ineligible study design |
| Lun 2000 | Ineligible patient population |
| McCormack 2014 | Ineligible patient population |
| Metze 1999 | Ineligible patient population |
| Millikan 2000 | Ineligible study design |
| NCT02565134 | Ineligible patient population |
| NCT03317301 | Ineligible patient population |
| Nouvenne 1966 | Ineligible patient population |
| Ohkawara 1991 | Ineligible patient population |
| Ohkuma 1993 | Ineligible patient population |
| Ohkuma 1994 | Ineligible patient population |
| Olansky 1963 | Ineligible study design |
| Phan 2008 | Ineligible study design |
| Rajka 1965 | Ineligible patient population |
| Savovic 2013 | Ineligible patient population |
| Sharma 2016 | Ineligible study design |
| Smith 1961 | Ineligible patient population |
| Stander 2009 | Ineligible patient population |
| Swanbeck 1970 | Ineligible patient population |
| Theunis 2016 | Ineligible patient population |
| Wang 1996 | Ineligible patient population |
| Weisshaar 1997 | Ineligible patient population |
| Xin 2004 | Ineligible patient population |
| Zhang 1997 | Ineligible patient population |
| Zylicz 2003 | Ineligible patient population |
Characteristics of studies awaiting assessment [ordered by study ID]
Aksungur 1990.
| Methods | N/A |
| Participants | N/A |
| Interventions | N/A |
| Outcomes | N/A |
| Notes | Cochrane Skin Group and our medical librarians could not find the full text for this study |
Legat 2017.
| Methods | Study design: randomised controlled trial Objective: "we investigated the effects of BB‐UVB versus NB‐UVB in reducing pruritus in patients with CP. 49 patients consented and were randomly assigned to BB‐UVB or NB‐UVB" |
| Participants | 38 patients with chronic pruritus |
| Interventions | Phototherapy broad band ‐ UVB vs narrow band ‐ UVB |
| Outcomes | Quote: "reducing the intensity of pruritus in patients with chronic pruritus using a visual analogue scale (VAS) ranging from 0 (= no itch) to 10 (= worst imaginable itch)" |
| Notes | PP74 Abstract from the 9th World Congress on Itch, 15–17 October 2017, Wroclaw, Poland We sent 3 emails to the study authors (franz.legat@medunigraz.at 11/2018, 01/2019, and 03/2019) with no response. The study does not specify if researchers included patients with CPUO |
BB‐UVB: broad band ultraviolet B.
CP: chronic pruritus.
CPUO: chronic pruritus of unknown origin.
N/A: not applicable.
NB‐UVB: narrow band ultraviolet B.
UVB: ultraviolet B.
VAS: visual analogue scale.
Characteristics of ongoing studies [ordered by study ID]
NCT0384331.
| Trial name or title | Study of the Efficacy, Safety, and Tolerability of Serlopitant for the Treatment of Chronic Pruritus of Unknown Origin |
| Methods | Study type: interventional (clinical trial) Estimated enrolment: 200 participants Allocation: randomised Intervention model: parallel assignment Masking: quadruple (participant, care provider, investigator, outcomes assessor) Primary purpose: treatment |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Experimental: 5‐mg serlopitant tablets Placebo comparator: 5‐mg placebo tablets |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | 22 January 2019 |
| Contact information | Contact: Menlo Study Director; 650‐486‐1416; capstone@menlotx.com |
| Notes | ‐ |
NK1: neurokinin 1.
WI‐NRS: Worst Itch Numerical Rating Scale.
WI‐VAS: Worst Itch Visual Analogue Scale.
Differences between protocol and review
Title
The original title of the protocol was "Interventions for pruritus of unknown cause"; it was changed to "Interventions for chronic pruritus of unknown origin" as this is currently the most familiar and widely used term among clinicians. We also changed the name of the condition throughout the review.
Methods section
Types of participants
Because the only included study had a (minority) subset of patients with a different diagnosis, we added to our inclusion criteria the following: "When we found studies with a subset of patients with a diagnosis of CPUO, we included them if data are presented separately for these patients, or if the majority (> 50%) of the included participants met the inclusion criteria. If data were not available for this subset of participants, we tried to retrieve this information from the investigators before excluding the study."
Types of interventions
In the published version of the protocol, we defined "aprepitant" as a systemic intervention in representation of the pharmacological group "substance P and neurokinin 1 receptor (NK1R) antagonist"; therefore for the report of the review, we changed this in the inclusion criteria.
We found no studies evaluating the prioritised comparisons: emollient creams, cooling lotions, topical corticosteroids, topical antidepressants, systemic antihistamines, systemic antidepressants, systemic anticonvulsants, and phototherapy. Therefore, instead we created three SoF tables for comparisons of the only study we included.
Search methods
Due to the large number of excluded studies (67), we did not screen the bibliographies of excluded studies for further references to relevant reviews.
Risk of bias assessment
We have updated the 'risk of bias' methods with the new tool ROB 2.0 in line with guidance from the new version of the Cochrane Handbook for Systematic Reviews of Interventions, and based on the protocol "Therapeutic interventions for alcohol dependence in non‐inpatient settings: a systematic review and network meta‐analysis" (Cheng 2017).
Methods not implemented
Because this review included only one study, we could not perform any meta‐analyses, and hence could not assess publication bias nor perform sensitivity analysis or subgroup analyses. We did not impute missing data because we considered missing data to be minimal (see Appendix 5, item 3.1).
Authorship
Two authors of the protocol are not authors of the review. Their contributions are acknowledged in the Acknowledgements section. The order of review authors was altered according to their contributions and seniority.
Contributions of authors
AA and JVAF were the contact persons with the editorial base. AA, JVAF, and GS co‐ordinated contributions from the co‐authors and wrote the final draft of the review. AA, CYK, JVAF, and VS screened papers against eligibility criteria. AA and JEML obtained data on ongoing and unpublished studies. AA, CYK, JEML, and JVAF appraised the quality of papers. AA and JVAF extracted data for the review and sought additional information about papers. AA and JVAF entered data into RevMan. AA, JVAF, CYK, VS, GS, and MRF analysed and interpreted data. JVAF, JEML, MRF, and GS worked on the methods sections. AA, JVAF, CYK, GS, and VS drafted the clinical sections of the background and responded to the clinical comments of the referees. JVAF and MRF responded to the methods and statistics comments of the referees. SC was the consumer co‐author who checked the review for readability and clarity, and ensured that outcomes are relevant to consumers.
Disclaimer
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Skin Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
Sources of support
Internal sources
-
Instituto Universitario Hospital Italiano (IUHI), Argentina.
JVAF is the Cochrane Center Coordinator and receives a salary from the IUHI
-
Dermatology Department, Argentina.
AA is an attending physician at this Department and receives a salary for this research
External sources
-
The National Institute for Health Research (NIHR), UK.
The NIHR, UK, is the largest single funder of the Cochrane Skin Group
Declarations of interest
Andrea Andrade Miranda: none known. Juan Franco: none known. Gloria Sanclemente: none known. Chii Yang Kuah: none known. Volha Shpadaruk: none known. Juliana Esther Martin‐Lopez: none known. Shunjie (Sean) Chua: none known.
New
References
References to studies included in this review
Yosipovitch 2018 {published and unpublished data}
- NCT01951274. VPD‐737 for treatment of chronic pruritus. clinicaltrials.gov/ct2/show/NCT01951274 (first received 26 September 2013). [CENTRAL: CN‐01490321]
- Stander S, Yosipovitch G, Kerby MB, Larrick JW, Perlman AJ, Schnipper EF, et al. A randomized, multicenter, double‐blind, placebo‐controlled Phase II clinical trial of serlopitant for the treatment of chronic pruritus. Journal of Clinical and Aesthetic Dermatology 2018;11(5):S18‐9. [CENTRAL: CN‐01713680] [DOI] [PubMed] [Google Scholar]
- Yosipovitch G, Stander S, Kerby MB, Larrick JW, Perlman AJ, Schnipper EF, et al. Serlopitant for the treatment of chronic pruritus: results of a randomized, multicenter, placebo‐controlled phase 2 clinical trial. Journal of the American Academy of Dermatology 2018;78(5):882‐91.e10. [CENTRAL: CN‐01665334] [DOI] [PubMed] [Google Scholar]
- Yosipovitch G, Stander S, Kerby MB, Larrick JW, Perlman AJ, Schnipper EF, et al. Serlopitant for treatment of chronic pruritus: results of a randomized, multicenter, double‐blind, placebo‐controlled phase 2 clinical trial. Acta Dermato‐Venereologica 2017;97(8):1015. [CENTRAL: CN‐01451357] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Acar 2010 {published data only}
- Acar B, Karabulut H, Sahin Y, Babademez MA, Karadag AS, Karasen RM. New treatment strategy and assessment questionnaire for external auditory canal pruritis: topical pimecrolimus therapy and Modified Itch Severity Scale. Journal of Laryngology and Otology 2010;124(2):147‐51. [CENTRAL: CN‐00748973; PUBMED: 19922703] [DOI] [PubMed] [Google Scholar]
Adams 1996 {published data only}
- Adams ML, Houpt KR, Cruz PD Jr. Is phototherapy safe for HIV‐infected individuals?. Photochemistry & Photobiology 1996;64(2):234‐7. [PUBMED: 8760560] [DOI] [PubMed] [Google Scholar]
Agero 2004 {published data only}
- Agero AL, Verallo‐Rowell VM. A randomized double‐blind controlled trial comparing extra virgin coconut oil with mineral oil as a moisturizer for mild to moderate xerosis. Dermatitis 2004;15(3):109‐16. [CENTRAL: CN‐00506032; PUBMED: 15724344] [DOI] [PubMed] [Google Scholar]
Agot 1975 {published data only}
- Agot H. Clinical trial of a new dermocorticosteroid, Diprosone (pommade). Marseille Medical 1975;112(12):737‐9. [Google Scholar]
AkhavanAmjadi 2012 {published data only}
- Akhavan Amjadi M, Mojab F, Kamranpour SB. The effect of peppermint oil on symptomatic treatment of pruritus in pregnant women. Iranian Journal of Pharmaceutical Research 2012;11(4):1073‐7. [CENTRAL: CN‐00913430; PUBMED: 24250539] [PMC free article] [PubMed] [Google Scholar]
Al Ghnaniem 2007 {published data only}
- Al‐Ghnaniem R, Short K, Pullen A, Fuller LC, Rennie JA, Leather AJM. 1% hydrocortisone ointment is an effective treatment of pruritus ani: a pilot randomized controlled crossover trial. International Journal of Colorectal Disease 2007;22(12):1463. [CENTRAL: CN‐00618144; PUBMED: 17534634] [DOI] [PubMed] [Google Scholar]
Ancona‐Castro 2018 {published data only}
- Ancona‐Castro C, Navarrete‐Solis J, Carmen Palacios‐Saucedo G. Efficacy of gabapentin compared to hydroxyzine in the treatment of chronic pruritus. Dermatologia Revista Mexicana 2018;62(6):486‐96. [CENTRAL: CN‐01744736] [Google Scholar]
Anonymous 1980 {published data only}
- Anonymous. Atarax (hydroxyzine) for itching. Medical Letter on Drugs & Therapeutics 1980;22(1):4. [PUBMED: 6986543] [PubMed] [Google Scholar]
Anonymous 2005 {published data only}
- Anonymous. Antipuritic therapy with naltrexone. Hautarzt 2005;56(4):312. [PUBMED: 16320411] [PubMed] [Google Scholar]
Anonymous 2015 {published data only}
- Anonymous. Abstracts from the 8th World Congress on Itch, WCI 2015. Acta Dermato Venereologica, Supplement. Conference: 8th World Congress on Itch, WCI 2015;95(7):875‐912(38). [Google Scholar]
Armond 1976 {published data only}
- Armond S, D'Avila FP. Clinical trial with synthetic corticotrophin in dermatology. Folha Medica 1976;73(4):379‐84. [Google Scholar]
Augustin 2011 {published data only}
- Augustin M, Phan NQ, Blome C, Neufang G, Stander S. Topical therapy with a new cooling compound in patients with chronic pruritus. Acta Dermato‐Venereologica 2011;91(5):626. [Google Scholar]
Bernstein 1979 {published data only}
- Bernstein JE, Swift R. Relief of intractable pruritus with naloxone. Archives of Dermatology 1979;115(11):1366‐7. [PUBMED: 389168] [PubMed] [Google Scholar]
Bigliardi 2007 {published data only}
- Bigliardi PL, Stammer H, Jost G, Rufli T, Buchner S, Bigliardi‐Qi M. Treatment of pruritus with topically applied opiate receptor antagonist. Journal of the American Academy of Dermatology 2007;56(6):979‐88. [CENTRAL: CN‐00587008] [DOI] [PubMed] [Google Scholar]
Brasileiro 2016 {published data only}
- Brasileiro LE, Barreto DP, Nunes EA. Psychotropics in different causes of itch: systematic review with controlled studies. Anais Brasileiros de Dermatologia 2016;91(6):791‐8. [PUBMED: 28099602] [DOI] [PMC free article] [PubMed] [Google Scholar]
Burch 1988 {published data only}
- Burch JR, Harrison PV. Opiates, sleep and itch. Clinical & Experimental Dermatology 1988;13(6):418‐9. [PUBMED: 3076856] [DOI] [PubMed] [Google Scholar]
Caddy 1966 {published data only}
- Caddy JA. Evaluation of a pediatric oral antipruritic preparation. Applied Therapeutics 1966;8(4):346‐9. [PUBMED: 4379813] [PubMed] [Google Scholar]
Carati 2013 {published data only}
- Carati D, Guido M, Malvasi A, Zizza A, Tinelli A. Efficacy of a Dermoxen lenitiva for pruritus genitalis in a randomized, double blind trial. European Review for Medical & Pharmacological Sciences 2013;17(19):2668‐74. [PUBMED: 24142616] [PubMed] [Google Scholar]
Chatterjee 2005 {published data only}
- Chatterjee S, Datta RN, Bhattacharyya D, Bandopadhyay SK. Emollient and antipruritic effect of Itch cream in dermatological disorders: a randomized controlled trial. Indian Journal of Pharmacology 2005;37(4):253‐4. [DOI: 10.4103/0253-7613.16574] [DOI] [Google Scholar]
ChiCTR‐IOR‐16009877 {published data only}
- ChiCTR‐IOR‐16009877. Acupunture treatment on disease of skin pruritus. www.chictr.org.cn/showprojen.aspx?proj=16842 (first received 27 July 2016). [CENTRAL: CN‐01850071]
CHICTR‐TRC‐11001485 {published data only}
- ChiCTR‐TRC‐11001485. A comparative study on the efficacy and safety of Olopatadine Hydrochloride versus Cetirizine in the treatment of cutaneous pruritus. www.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR‐TRC‐11001485 (first received 23 August 2011). [CENTRAL: CN‐01861195]
Dupont 1984 {published data only}
- Dupont C, Maubeuge J, Kotlar W, Lays Y, Masson M. Oxatomide in the treatment of pruritus senilis. A double‐blind placebo‐controlled trial. Dermatologica 1984;169(6):348‐53. [PUBMED: 6151914] [DOI] [PubMed] [Google Scholar]
Dyshko 1966 {published data only}
- Dyshko AS. On cutaneous pruritus. Sovetskaia Meditsina 1966;29(4):125‐8. [PubMed] [Google Scholar]
Eberhartinger 1969 {published data only}
- Eberhartinger C, Ebner H. Experience with a new antihistaminic in dermatology: results of a clinical double blind trial. Wiener Medizinische Wochenschrift (1946) 1969;119(38):630‐2. [PubMed] [Google Scholar]
Elsaie 2016 {published data only}
- Elsaie LT, Mohsen AM, Ibrahim IM, Mohey‐Eddin MH, Elsaie ML. Effectiveness of topical peppermint oil on symptomatic treatment of chronic pruritus. Clinical, Cosmetic and Investigational Dermatology CCID 2016;9:333‐8. [PUBMED: 27785084] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eschler 2010 {published data only}
- Eschler DC, Klein PA. An evidence‐based review of the efficacy of topical antihistamines in the relief of pruritus. Journal of Drugs in Dermatology 2010;9(8):992‐7. [PUBMED: 20684150] [PubMed] [Google Scholar]
Essen 1953 {published data only}
- Essen LE. Trial use of the hyaluronidase inhibitors polyphloretin phosphate and polyphloroglucin phosphate in clinical dermatology with special consideration of antipruritic effect. Svenska Lakartidningen 1953;50(7):341‐55. [PubMed] [Google Scholar]
Evers 2017 {published data only}
- Evers A. How to alter placebo and nocebo effects in patients with chronic itch?. Acta Dermato‐Venereologica 2017;97(8):1017. [Google Scholar]
Fischer 1968 {published data only}
- Fischer RW. Comparison of antipruritic agents administered orally. A double‐blind study. JAMA 1968;203(6):418‐9. [PUBMED: 4865235] [PubMed] [Google Scholar]
Fjellner 1978 {published data only}
- Fjellner B, Hagermark O. Transcutaneous nerve stimulation and itching. Acta Dermato‐Venereologica 1978;58(2):131‐4. [PUBMED: 76391] [PubMed] [Google Scholar]
Fjellner 1981 {published data only}
- Fjellner B. Experimental and clinical pruritus. Studies on some putative peripheral mediators. The influence of ultraviolet light and transcutaneous nerve stimulation. Acta Dermato‐Venereologica Supplementum 1981;97:1‐34. [PUBMED: 6177180] [DOI] [PubMed] [Google Scholar]
Ginsberg 2004 {published data only}
- Ginsberg DL. Paroxetine effective for severe nondermatological pruritus. Primary Psychiatry 2004;11(7):22‐3. [Google Scholar]
Gisslen 1962 {published data only}
- Gisslen H, Hellgren L. Clinical trial of alimemazine as an anti‐pruritic agent. Nordisk Medicin 1962;67:605‐7. [PUBMED: 13898959] [PubMed] [Google Scholar]
Gooding 2010 {published data only}
- Gooding SM, Canter PH, Coelho HF, Boddy K, Ernst E. Systematic review of topical capsaicin in the treatment of pruritus. International Journal of Dermatology 2010;49(8):858‐65. [PUBMED: 21128913] [DOI] [PubMed] [Google Scholar]
Hagermark 1974 {published data only}
- Hagermark O, Strandberg K. Comparison of the antihistaminic effects in skin of a tertiary (promethazine) and a quarternary phenothiazine (N‐hydroxyethylpromethazine). Acta Allergologica 1974;29(6):462‐8. [PUBMED: 4155913] [DOI] [PubMed] [Google Scholar]
Heinlin 2013 {published data only}
- Heinlin J, Isbary G, Stolz W, Zeman F, Landthaler M, Morfill G, et al. A randomized two‐sided placebo‐controlled study on the efficacy and safety of atmospheric non‐thermal argon plasma for pruritus. Journal of the European Academy of Dermatology and Venereology 2013;27(3):324‐31. [DOI: 10.1111/j.1468-3083.2011.04395.x] [DOI] [PubMed] [Google Scholar]
Hellier 1963 {published data only}
- Hellier FF. A comparative trial of trimeprazine and amylobarbitone in pruritis. Lancet 1963;1(7279):471‐2. [CENTRAL: CN‐00306393] [DOI] [PubMed] [Google Scholar]
IRCT2014112613612N3 {published data only}
- IRCT2014112613612N3. The effect of levothyroxine on patients with chronic pruritus. en.irct.ir/trial/13427 (first received 6 November 2015). [CENTRAL: CN‐01861195]
Juhlin 1961 {published data only}
- Juhlin L, Skogh M. A double‐blind investigation to test the antipruritic effect of some phenothiazine derivatives. Acta Dermato‐Venereologica 1961;41:471‐80. [PUBMED: 14452737] [PubMed] [Google Scholar]
Kamm Kohl 1987 {published data only}
- Kamm‐Kohl V. Geriatric patients with pruritus. Comparison of efficacy and toleration of 5% Bufexamac‐milk local and dimetindenmaleat oral ‐ results of an open clinical study. Zeitschrift für Allgemeinmedizin 1987;63(16):542‐3. [Google Scholar]
Kleine Natrop 1970 {published data only}
- Kleine‐Natrop HE, Richter G. Testing and use of 9,9‐dioxopromethazine (Prothanon) as an antihistaminic and antipruritic agent in dermatology. Die Pharmazie 1970;25(2):108‐11. [PUBMED: 4393058] [PubMed] [Google Scholar]
Knoth 1961 {published data only}
- Knoth W, Paetzold H‐O, Goebel B. On the antipruritic repetition. Der Hautarzt; Zeitschrift fur Dermatologie, Venerologie, und Verwandte Gebiete 1961;12(4):183‐6. [PubMed] [Google Scholar]
Kouwenhoven 2017 {published data only}
- Kouwenhoven TA, Kerkhof PCM, Kamsteeg M. Use of oral antidepressants in patients with chronic pruritus: a systematic review. Journal of the American Academy of Dermatology 2017;77(6):1068‐73.e7. [PUBMED: 29033248] [DOI] [PubMed] [Google Scholar]
Lun 2000 {published data only}
- Lun X, Rong L. Twenty‐five cases of intractable cutaneous pruritus treated by auricular acupuncture. Journal of Traditional Chinese Medicine 2000;20(4):287‐8. [PUBMED: 11263285] [PubMed] [Google Scholar]
McCormack 2014 {published data only}
- McCormack PL. Omalizumab: a review of its use in patients with chronic spontaneous urticaria. Drugs 2014;74(14):1693‐9. [PUBMED: 25217402] [DOI] [PubMed] [Google Scholar]
Metze 1999 {published data only}
- Metze D, Reimann S, Luger TA. Effective treatment of pruritus with naltrexone, an orally active opiate antagonist. Annals of the New York Academy of Sciences 1999;885:430‐2. [PUBMED: 10816681] [DOI] [PubMed] [Google Scholar]
Millikan 2000 {published data only}
- Millikan LE. Pruritus: unapproved treatments or indications. Clinics in Dermatology 2000;18(2):149‐52. [PUBMED: 10742622] [DOI] [PubMed] [Google Scholar]
NCT02565134 {published data only}
- NCT02565134. A study to evaluate the safety and efficacy of PAC‐14028 cream in skin pruritus. clinicaltrials.gov/show/nct02565134 (first received 1 October 2015).
NCT03317301 {published data only}
- NCT03317301. Evaluate the efficacy and safety of HL151 versus talion tab in pruritus cutaneus. clinicaltrials.gov/show/nct03317301 (first received 23 October 2017).
Nouvenne 1966 {published data only}
- Nouvenne R. Trial of a new antihistaminic cream. Minerva Dermatologica 1966;41(10):372‐3. [PubMed] [Google Scholar]
Ohkawara 1991 {published data only}
- Ohkawara A, Furuya K, Kurisu Y. Experience with orengedokuto (TJ‐15) and goshajinkigan (TJ‐107) for the treatment of senile pruritus. Nishinihon Hifuka (Nishinihon Journal of Dermatology) 1991;53:1234‐41. [Google Scholar]
Ohkuma 1993 {published data only}
- Ohkuma M. Treatment of pruritus by Chinese drugs. Wakan Iyaku Gakkaishi (Journal of Medical and Pharmaceutical Society for WAKAN‐YAKU) 1993;10:126‐30. [Google Scholar]
Ohkuma 1994 {published data only}
- Ohkuma M. Treatment of pruritus by Chinese drugs with external application and oral antihistamine. Wakan Iyakugaku Zasshi (Journal of Traditional Medicines) 1994;11:302‐3. [Google Scholar]
Olansky 1963 {published data only}
- Olansky S. Clinical trial of an antihistamine for relief of pruritus. Clinical Medicine 1963;70:1657‐60. [PUBMED: 15446056] [PubMed] [Google Scholar]
Phan 2008 {published data only}
- Phan NQ, Stander S. Munster Pruritus Symposium 2008. Journal der Deutschen Dermatologischen Gesellschaft 2008;6(12):1098‐9. [PUBMED: 19138279] [DOI] [PubMed] [Google Scholar]
Rajka 1965 {published data only}
- Rajka E, Korossy S, Gozony M. Therapeutic trial with antipruritic drugs. Dermatologica 1965;130:113‐29. [PUBMED: 14300883] [PubMed] [Google Scholar]
Savovic 2013 {published data only}
- Savovic J, Lewis P. A pilot randomised trial of Medilixir cream shows some promising results, but control treatment may have been inappropriate. Focus on Alternative and Complementary Therapies 2013;18(2):105‐6. [DOI: 10.1111/fct.12022_2] [DOI] [Google Scholar]
Sharma 2016 {published data only}
- Sharma D, Kwatra SG. Thalidomide for the treatment of chronic refractory pruritus. Journal of the American Academy of Dermatology 2016;74(2):363‐9. [PUBMED: 26577510] [DOI] [PubMed] [Google Scholar]
Smith 1961 {published data only}
- Smith MA, Curwen MP. Controlled trials of two antipruritic drugs, trimeprazine and methdilazine. British Journal of Dermatology 1961;73:351‐8. [CENTRAL: CN‐00309318] [DOI] [PubMed] [Google Scholar]
Stander 2009 {published data only}
- Stander S, Bockenholt B, Schurmeyer‐Horst F, Weishaupt C, Heuft G, Luger TA, et al. Treatment of chronic pruritus with the selective serotonin re‐uptake inhibitors paroxetine and fluvoxamine: results of an open‐labelled, two‐arm proof‐of‐concept study. Acta Dermato‐Venereologica 2009;89(1):45‐51. [PUBMED: 19197541] [DOI] [PubMed] [Google Scholar]
Swanbeck 1970 {published data only}
- Swanbeck G, Rajka G. Antipruritic effect of urea solutions. An experimental and clinical study. Acta Dermato‐Venereologica 1970;50(3):225‐7. [PUBMED: 4193224] [PubMed] [Google Scholar]
Theunis 2016 {published data only}
- Theunis J, Rossi AB, Rizzi NC, Mengeaud V. Efficacy of an emollient containing Rhealba oat plantlets on idiopathic pruritus among elderly French outpatients. Experimental Dermatology 2016;25(Suppl 4):32. [Google Scholar]
Wang 1996 {published data only}
- Wang H. Clinical observation of triamcinolone acetonide injection to Zusanli Acupoint(ST 36) for pruritus. Journal of Clinical Acupuncture and Moxibustion [Zhen Jiu lin Chuang za Zhi] 1996;12(7‐8):56. [Google Scholar]
Weisshaar 1997 {published data only}
- Weisshaar E, Ziethen B, Gollnick H. Antipruritic effects of a 5HT3‐receptor antagonist (Tropisetron). Der Hautarzt; Zeitschrift fur Dermatologie, Venerologie, und Verwandte Gebiete 1997;48(Suppl):S57. [CENTRAL: CN‐00509770] [Google Scholar]
Xin 2004 {published data only}
- Xin L, Li R. Treatment of skin pruritus with auricular acupuncture. Journal of Acupuncture and Tuina Science 2004;2(1):21‐2. [DOI: 10.1007/BF02861399] [DOI] [Google Scholar]
Zhang 1997 {published data only}
- Zhang M, Lei GL, Liao YL, Ran YP, Wang L, Zhou GP. A randomised controlled trial of Huobahuageng tablet plus Diprospan (betametasone compound) in the treatment of thunder‐pruritus. Journal of Clinical Dermatology 1997;26(1):30. [CENTRAL: CN‐00454958] [Google Scholar]
Zylicz 2003 {published data only}
- Zylicz Z, Krajnik M, Sorge AA, Costantini M. Paroxetine in the treatment of severe non‐dermatological pruritus: a randomized, controlled trial. Journal of Pain & Symptom Management 2003;26(6):1105‐12. [PUBMED: 14654262] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Aksungur 1990 {published data only}
- Aksungur VL, Mustafa SO, Hasanoglu O. The antipruritic effect of terfenadine. Turkderm Deri Hastaliklari ve Frengi Arsivi 1990;24(1):35‐42. [Google Scholar]
Legat 2017 {published data only}
- Legat FJ, Hofer A, Gruber‐Wackernagel A, Quehenberger F, Waltner K, Wolf P. Both narrowband‐UVB and broadband‐UVB are equally effective in reducing itch in chronic pruritus patients. Acta Dermato‐Venereologica 2017;97(8):1056‐7. [CENTRAL: CN‐01451333] [Google Scholar]
References to ongoing studies
NCT0384331 {unpublished data only}
- NCT03841331. Study of the efficacy, safety, and tolerability of serlopitant for the treatment of chronic pruritus of unknown origin. clinicaltrials.gov/ct2/show/NCT03841331 (first received 15 February 2019).
Additional references
Aguh 2018
- Aguh C, Kwatra SG, He A, Okoye GA. Thalidomide for the treatment of chronic refractory prurigo nodularis. Dermatology Online Journal 2018;24(3):13030/qt44n0k1xm. [PUBMED: 29634881] [PubMed] [Google Scholar]
Ally 2013
- Ally MS, Gamba CS, Peng DH, Tang JY. The use of aprepitant in brachioradial pruritus. JAMA Dermatology 2013;149(5):627‐8. [PUBMED: 23677105] [DOI] [PubMed] [Google Scholar]
Apostolidis 2005
- Apostolidis A, Popat R, Yiangou Y, Cockayne D, Ford AP, Davis JB, et al. Decreased sensory receptors P2X3 and TRPV1 in suburothelial nerve fibers following intradetrusor injections of botulinum toxin for human detrusor overactivity. Journal of Urology 2005;174(3):977‐82; discussion 982‐3. [PUBMED: 16094018] [DOI] [PubMed] [Google Scholar]
Ashcroft 2005
- Ashcroft DM, Dimmock P, Garside R, Stein K, Williams HC. Efficacy and tolerability of topical pimecrolimus and tacrolimus in the treatment of atopic dermatitis: meta‐analysis of randomised controlled trials. BMJ 2005;330(7490):516. [PUBMED: 15731121] [DOI] [PMC free article] [PubMed] [Google Scholar]
Atis 2017
- Atis G, Bilir Kaya B. Pregabalin treatment of three cases with brachioradial pruritus. Dermatologic Therapy 2017;30(2):e12459. [DOI: 10.1111/dth.12459; PUBMED: 28168835] [DOI] [PubMed] [Google Scholar]
Azimi 2017
- Azimi E, Reddy VB, Pereira PJS, Talbot S, Woolf CJ, Lerner EA. Substance P activates Mas‐related G protein–coupled receptors to induce itch. Journal of Allergy and Clinical Immunology 2017;140(2):447–53. [PUBMED: 28219706] [DOI] [PMC free article] [PubMed] [Google Scholar]
Baker 2001
- Baker RA, Zeller RA, Klein RL, Thornton RJ, Shuber JH, Marshall RE, et al. Burn wound itch control using H1 and H2 antagonists. Journal of Burn Care & Rehabilitation 2001;22(4):263‐8. [PUBMED: 11482684] [DOI] [PubMed] [Google Scholar]
Baranda 2002
- Baranda L, Gonzalez‐Amaro R, Torres‐Alvarez B, Alvarez C, Ramirez V. Correlation between pH and irritant effect of cleansers marketed for dry skin. International Journal of Dermatology 2002;41(8):494‐9. [PUBMED: 12207765] [DOI] [PubMed] [Google Scholar]
Bech 1990
- Bech P, Coppen A, editors. The Hamilton Scales. Berlin, Heidelberg: Springer Berlin Heidelberg, 1990. [Google Scholar]
Beck 1996
- Beck AT, Steer RA, Brown GK. BDI‐II, Beck Depression Inventory: Manual. San Antonio, TX: The Psychological Corporation, 1996. [Google Scholar]
Bolognia 2012
- Bolognia J, Jorizzo JL, Schaffer JV. Dermatology. 3rd Edition. Philadelphia, PA, USA: Elsevier Saunders, 2012. [Google Scholar]
Bromm 1995
- Bromm B, Scharein E, Darsow U, Ring J. Effects of menthol and cold on histamine‐induced itch and skin reactions in man. Neuroscience Letters 1995;187(3):157‐60. [PUBMED: 7624016] [DOI] [PubMed] [Google Scholar]
Brunton 2011
- Brunton L, Chabner BA, Knollmann BC. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 12th Edition. New York City, New York, USA: McGraw‐Hill Education, 2011. [Google Scholar]
Castro 2006
- Castro AP. Calcineurin inhibitors in the treatment of allergic dermatitis. Jornal de Pediatria 2006;82(5 Suppl):S166‐72. [PUBMED: 17136292] [DOI] [PubMed] [Google Scholar]
Cheng 2017
- Cheng HY, Elbers RG, Higgins JP, Taylor A, MacArthur GJ, McGuinness L, et al. Therapeutic interventions for alcohol dependence in non‐inpatient settings: a systematic review and network meta‐analysis (protocol). Systematic Reviews 2017;6(77):1‐7. [DOI: 10.1186/s13643-017-0462-2; PUBMED: 28399899] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chida 2007
- Chida Y, Steptoe A, Hirakawa N, Sudo N, Kubo C. The effects of psychological intervention on atopic dermatitis. A systematic review and meta‐analysis. International Archives of Allergy and Immunology 2007;144(1):1‐9. [PUBMED: 17449959] [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Veritas Health Innovation. Covidence. Version accessed 21 February 2018. Melbourne, Australia: Veritas Health Innovation, 2018.
Craig 1997
- Craig JM, Lloyd DH, Jones RD. A double‐blind placebo‐controlled trial of an evening primrose and fish oil combination vs. hydrogenated coconut oil in the management of recurrent seasonal pruritus in horses. Veterinary Dermatology 1997;8(3):177‐82. [DOI] [PubMed] [Google Scholar]
Curcani 2014
- Curcani M, Tan M. The effect of aromatherapy on haemodialysis patients' pruritus. Journal of Clinical Nursing 2014;23(23‐24):3356‐65. [PUBMED: 24646128] [DOI] [PubMed] [Google Scholar]
Davis 2003
- Davis MP, Frandsen JL, Walsh D, Andresen S, Taylor S. Mirtazapine for pruritus. Journal of Pain and Symptom Management 2003;25(3):288‐91. [PUBMED: 12614964] [DOI] [PubMed] [Google Scholar]
Dawn 2006
- Dawn A, Yosipovitch G. Treating itch in psoriasis. Dermatology Nursing 2006;18(3):227‐33. [PUBMED: 16856675] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG, editor(s). Chapter 9: Analysing data and undertaking meta‐analysis. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):77–88. [PUBMED: 3802833] [DOI] [PubMed] [Google Scholar]
Desai 2008
- Desai NS, Poindexter GB, Monthrope YM, Bendeck SE, Swerlick RA, Chen SC. A pilot quality‐of‐life instrument for pruritus. Journal of the American Academy of Dermatology 2008;59(2):234‐44. [PUBMED: 18550210] [DOI] [PubMed] [Google Scholar]
Dillon 2013
- Dillon S, Tobias JD. Ondansetron to treat pruritus due to cholestatic jaundice. Journal of Pediatric Pharmacology and Therapeutics 2013;18(3):241‐6. [PUBMED: 24052788] [DOI] [PMC free article] [PubMed] [Google Scholar]
Duval 2009
- Duval A, Dubertret L. Aprepitant as an antipruritic agent?. New England Journal of Medicine 2009;361(14):1415‐6. [PUBMED: 19797294 ] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Scheneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [PUBMED: 9310563] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eichenfield 2017
- Eichenfield LF, Call RS, Forsha DW, Fowler J, Hebert AA, Spellman M, et al. Long‐term safety of crisaborole ointment 2% in children and adults with mild to moderate atopic dermatitis. Journal of the American Academy of Dermatology 2017;77(4):641‐9. [PUBMED: 28823881] [DOI] [PubMed] [Google Scholar]
Fedorowicz 2012
- Fedorowicz Z, Zuuren EJ, Hu N. Histamine H2‐receptor antagonists for urticaria. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD008596.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fehrenbacher 2003
- Fehrenbacher JC, Taylor CP, Vasko MR. Pregabalin and gabapentin reduce release of substance P and CGRP from rat spinal tissues only after inflammation or activation of protein kinase C. Pain 2003;105(1‐2):133‐41. [PUBMED: 14499429] [DOI] [PubMed] [Google Scholar]
Feldman 2016
- Feldman SR, Thaci D, Gooderham M, Augustin M, Cruz C, Mallbris L, et al. Tofacitinib improves pruritus and health‐related quality of life up to 52 weeks: results from 2 randomized phase III trials in patients with moderate to severe plaque psoriasis. Journal of the American Academy of Dermatology 2016;75(6):1162‐70. [PUBMED: 27692733] [DOI] [PubMed] [Google Scholar]
Finlay 1994
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI) – a simple practical measure for routine clinical use. Clinical and Experimental Dermatology 1994;19(3):210‐6. [PUBMED: 8033378] [DOI] [PubMed] [Google Scholar]
Fleischer 2010
- Fleischer AB Jr, Boguniewicz M. An approach to pruritus in atopic dermatitis: a critical systematic review of the tacrolimus ointment literature. Journal of Drugs in Dermatology 2010;9(5):488‐98. [PUBMED: 20480792] [PubMed] [Google Scholar]
Foroutan 2016
- Foroutan N, Nikvarz N. Role of pregabalin in management of pruritus: a literature review. Journal of Pharmacy & Pharmaceutical Sciences 2016;19(4):465‐74. [PUBMED: 28057164] [DOI] [PubMed] [Google Scholar]
Franco 2017
- Franco JVA, Turk T, Jung JH, Xiao YT, Iakhno S, Garrote V, et al. Non‐pharmacological interventions for treating chronic prostatitis/chronic pelvic pain syndrome. Cochrane Database of Systematic Reviews 2018, Issue 1. [DOI: 10.1002/14651858.CD012551.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Freitag 1997
- Freitag G, Hoppner T. Results of a postmarketing drug monitoring survey with a polidocanol‐urea preparation for dry, itching skin. Current Medical Research and Opinion 1997;13(9):529‐37. [PUBMED: 9169255] [DOI] [PubMed] [Google Scholar]
Fukuyama 2017
- Fukuyama T, Ganchingco JR, Mishra SK, Olivry T, Rzagalinski I, Volmer DA, et al. Janus kinase inhibitors display broad anti‐itch properties: a possible link through the TRPV1 receptor. Journal of Allergy and Clinical Immunology 2017;140(1):306‐9. [PUBMED: 28131799] [DOI] [PubMed] [Google Scholar]
Garibyan 2013
- Garibyan L, Rheingold CG, Lerner EA. Understanding the pathophysiology of itch. Dermatologic Therapy 2013;26(2):84‐91. [PUBMED: 23551365] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gazerani 2009
- Gazerani P, Pedersen NS, Drewes AM, Arendt‐Nielsen L. Botulinum toxin type A reduces histamine‐induced itch and vasomotor responses in human skin. British Journal of Dermatology 2009;161(4):737‐45. [PUBMED: 19624547] [DOI] [PubMed] [Google Scholar]
GHDx
- Institute for Health Metrics and Evaluation. Global Burden of Disease Results Tool. ghdx.healthdata.org/gbd‐results‐tool (accessed 21 February 2018).
Glenton 2010
- Glenton C, Santesso N, Rosenbaum S, Nilsen ES, Rader T, Ciapponi A, et al. Presenting the results of Cochrane Systematic Reviews to a consumer audience: a qualitative study. Medical Decision Making: an International Journal of the Society for Medical Decision Making 2010;30(5):566‐77. [PUBMED: 20643912] [DOI] [PubMed] [Google Scholar]
Goldsmith 2012
- Goldsmith LA, Fitzpatrick TB. Chapter 103. Pathophysiology and clinical aspects of pruritus. Fitzpatrick's Dermatology in General Medicine. New York City, New York, USA: McGraw‐Hill Medical, 2012:1147‐58. [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed February 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Greaves 1996
- Greaves MW, Wall PD. Pathophysiology of itching. Lancet 1996;348(9032):938‐40. [PUBMED: 8843816] [DOI] [PubMed] [Google Scholar]
Greaves 2005
- Greaves MW. Antihistamines in dermatology. Skin Pharmacology and Physiology 2005;18(5):220‐9. [PUBMED: 16015020] [DOI] [PubMed] [Google Scholar]
Grundmann 2011
- Grundmann S, Stander S. Chronic pruritus: clinics and treatment. Annals of Dermatology 2011;23(1):1‐11. [PUBMED: 21738356] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Schünemann HJ, et al. GRADE: what is 'quality of evidence' and why is it important to clinicians?. BMJ (Clinical Research Ed.) 2008;336(7651):995‐8. [DOI: 10.1136/bmj.39490.551019.BE] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence – imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [PUBMED: 21839614] [DOI] [PubMed] [Google Scholar]
Ha 1999
- Ha HC. Effect of aromatherapy on skin xerosis and pruritus in patients undergoing maintenance hemodialysis. Journal of Korean Academy of Nursing 1999;29(6):1284‐93. [Google Scholar]
Hawro 2014
He 2017
- He A, Alhariri JM, Sweren RJ, Kwatra MM, Kwatra SG. Aprepitant for the treatment of chronic refractory pruritus. BioMed Research International 2017;2017:4790810. [DOI: 10.1155/2017/4790810; PUBMED: 29057261] [DOI] [PMC free article] [PubMed] [Google Scholar]
Heckmann 2002
- Heckmann M, Heyer G, Brunner B, Plewig G. Botulinum toxin type A injection in the treatment of lichen simplex: an open pilot study. Journal of the American Academy of Dermatology 2002;46(4):617‐9. [PUBMED: 11907521] [DOI] [PubMed] [Google Scholar]
Hercogová 2005
- Hercogová J. Topical anti‐itch therapy. Dermatologic Therapy 2005;18(4):341‐3. [PUBMED: 16297007] [DOI] [PubMed] [Google Scholar]
Hettrick 2004
- Hettrick H, O'Brien K, Laznick H, Sanchez J, Gorga D, Nagler W, et al. Effect of transcutaneous electrical nerve stimulation for the management of burn pruritus: a pilot study. Journal of Burn Care & Rehabilitation 2004;25(3):236‐40. [PUBMED: 15273463] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2019a
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JAC. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook. Cochrane.
Higgins 2019b
- Higgins JP, Eldridge S, Li T (editors). Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook. Cochrane.
Huh 2016
- Huh JW, Jeong YI, Choi KH, Park HJ, Jue MS. Treatment for refractory pruritus using oral aprepitant. Annals of Dermatology 2016;28(1):124‐5. [PUBMED: 26848236] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hultsch 2005
- Hultsch T, Kapp A, Spergel J. Immunomodulation and safety of topical calcineurin inhibitors for the treatment of atopic dermatitis. Dermatology 2005;211(2):174‐87. [PUBMED: 16088174] [DOI] [PubMed] [Google Scholar]
Hundley 2004
- Hundley JL, Yosipovitch G. Mirtazapine for reducing nocturnal itch in patients with chronic pruritus: a pilot study. Journal of the American Academy of Dermatology 2004;50(6):889‐91. [PUBMED: 15153889] [DOI] [PubMed] [Google Scholar]
Ikoma 2006
- Ikoma A, Steinhoff M, Ständer S, Yosipovitch G, Schmelz M. The neurobiology of itch. Nature Reviews. Neuroscience 2006;7(7):535‐47. [PUBMED: 16791143] [DOI] [PubMed] [Google Scholar]
Jelicic 2016
- Jelicic Kadic A, Vucic K, Dosenovic S, Sapunar D, Puljak L. Extracting data from figures with software was faster, with higher interrater reliability than manual extraction. Journal of Clinical Epidemiology 2016;74:119‐23. [PUBMED: 26780258] [DOI] [PubMed] [Google Scholar]
Johnston 2010
- Johnston BC, Thorlund K, Schünemann HJ, Xie F, Murad MH, Montori VM, et al. Improving the interpretation of quality of life evidence in meta‐analyses: the application of minimal important difference units. Health and Quality of Life Outcomes 2010;8:116. [PUBMED: 20937092] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kapoor 2005
- Kapoor VP. Herbal cosmetics for skin and hair care. Natural Product Radiance 2005;4(4):306‐14. [Google Scholar]
Kasutani 2014
- Kasutani K, Fujii E, Ohyama S, Adachi H, Hasegawa M, Kitamura H, et al. Anti‐IL‐31 receptor antibody is shown to be a potential therapeutic option for treating itch and dermatitis in mice. British Journal of Pharmacology 2014;171(22):5049‐58. [PUBMED: 24946165] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kini 2011
- Kini SP, DeLong LK, Veledar E, McKenzie‐Brown AM, Schaufele M, Chen SC. The impact of pruritus on quality of life: the skin equivalent of pain. Archives of Dermatology 2011;147(10):1153‐6. [PUBMED: 21680760] [DOI] [PubMed] [Google Scholar]
Kogan 2007
- Kogan NM, Mechoulam R. Cannabinoids in health and disease. Dialogues in Clinical Neuroscience 2007;9(4):413‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Koh 2002
- Koh KJ, Pearce AL, Marshman G, Finlay‐Jones JJ, Hart PH. Tea tree oil reduces histamine‐induced skin inflammation. British Journal of Dermatology 2002;147(6):1212‐7. [PUBMED: 12452873] [DOI] [PubMed] [Google Scholar]
Kopyciok 2016
- Kopyciok ME, Stander HF, Osada N, Steinke S, Stander S. Prevalence and characteristics of pruritus: a one‐week cross‐sectional study in a German dermatology practice. Acta Dermato‐Venereologica 2016;96(1):50‐5. [PUBMED: 26067841] [DOI] [PubMed] [Google Scholar]
Korfitis 2008
- Korfitis C, Trafalis DT. Carbamazepine can be effective in alleviating tormenting pruritus in patients with hematologic malignancy. Journal of Pain and Symptom Management 2008;35(6):571‐2. [DOI: 10.1016/j.jpainsymman.2008.01.004] [DOI] [PubMed] [Google Scholar]
Kumagai 2012
- Kumagai H, Ebata T, Takamori K, Miyasato K, Muramatsu T, Nakamoto H, et al. Efficacy and safety of a novel k‐agonist for managing intractable pruritus in dialysis patients. American Journal of Nephrology 2012;36(2):175‐83. [PUBMED: 22868684] [DOI] [PubMed] [Google Scholar]
Lapolla 2011
- Lapolla W, Yentzer BA, Bagel J, Halvorson CR, Feldman SR. A review of phototherapy protocols for psoriasis treatment. Journal of the American Academy of Dermatology 2011;64(5):936‐49. [PUBMED: 21429620] [DOI] [PubMed] [Google Scholar]
Lasanen 2016
- Lasanen R, Julkunen P, Airaksinen O, Toyras J. Menthol concentration in topical cold gel does not have significant effect on skin cooling. Skin Research and Technology 2016;22(1):40‐5. [PUBMED: 25773465] [DOI] [PubMed] [Google Scholar]
Lawson 2002
- Lawson SN. Phenotype and function of somatic primary afferent nociceptive neurons with C‐, A delta‐ or A alpha/beta‐fibres. Experimental Physiology 2002;87(2):239‐44. [PUBMED: 11856969] [DOI] [PubMed] [Google Scholar]
Lee 2017
- Lee HG, Grossman SK, Valdes‐Rodriguez R, Berenato F, Korbutov J, Chan YH, et al. Topical ketamine‐amitriptyline‐lidocaine for chronic pruritus: a retrospective study assessing efficacy and tolerability. Journal of the American Academy of Dermatology 2017;76(4):760‐1. [DOI: 10.1016/j.jaad.2016.10.030; PUBMED: 28325395] [DOI] [PubMed] [Google Scholar]
Leslie 2015
- Leslie TA, Greaves MW, Yosipovitch G. Current topical and systemic therapies for itch. In: Cowan A, Yosipovitch G editor(s). Pharmacology of Itch. Handbook of Experimental Pharmacology, vol 226. Berlin, Heidelberg: Springer, 2015:337‐56. [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009;6(7):e1000100. [PUBMED: 19621070] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2018
- Liu B, Jordt SE. Cooling the itch via TRPM8. Journal of Investigative Dermatology 2018;138(6):1254‐6. [PUBMED: 29793621] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lodén 2015
- Lodén M. Moisturizers: treatment of dry skin syndrome and barrier defects. In: Sivamani R, Jagdeo JR, Elsner P, Maibach HI editor(s). Cosmeceuticals and Active Cosmetics. 3rd Edition. Boca Raton, FL, USA: Taylor & Francis Group, 2015:235‐60. [DOI: 10.1201/b18895] [DOI] [Google Scholar]
Lodén 2016
- Lodén M. Treatments Improving skin barrier function. Current Problems in Dermatology 2016;49:112‐22. [PUBMED: 26844903] [DOI] [PubMed] [Google Scholar]
Lotts 2014
- Lotts T, Stander S. Research in practice: substance P antagonism in chronic pruritus. Journal der Deutschen Dermatologischen Gesellschaft [Journal of the German Society of Dermatology] 2014;12(7):557‐9. [DOI] [PubMed] [Google Scholar]
Majeski 2007
- Majeski CJ, Johnson JA, Davison SN, Lauzon GJ. Itch Severity Scale: a self‐report instrument for the measurement of pruritus severity. British Journal of Dermatology 2007;156(4):667‐73. [PUBMED: 17263803] [DOI] [PubMed] [Google Scholar]
Matsuda 2016
- Matsuda KM, Sharma D, Schonfeld AR, Kwatra SG. Gabapentin and pregabalin for the treatment of chronic pruritus. Journal of the American Academy of Dermatology 2016;75(3):619‐25. [PUBMED: 27206757] [DOI] [PubMed] [Google Scholar]
Matterne 2011
- Matterne U, Apfelbacher CJ, Loerbroks A, Schwarzer T, Buttner M, Ofenloch R, et al. Prevalence, correlates and characteristics of chronic pruritus: a population‐based cross‐sectional study. Acta Dermato‐Venereologica 2011;91(6):674‐9. [PUBMED: 21879245] [DOI] [PubMed] [Google Scholar]
Mazza 2013
- Mazza M, Guerriero G, Marano G, Janiri L, Bria P, Mazza S. Treatment of prurigo nodularis with pregabalin. Journal of Clinical Pharmacy and Therapeutics 2013;38(1):16‐8. [PUBMED: 23013514] [DOI] [PubMed] [Google Scholar]
Millington 2018
- Millington GWM, Collins A, Lovell CR, Leslie TA, Yong ASW, Morgan JD, et al. British Association of Dermatologists' guidelines for the investigation and management of generalized pruritus in adults without an underlying dermatosis, 2018. British Journal of Dermatology 2018;178(1):34‐60. [PUBMED: 29357600] [DOI] [PubMed] [Google Scholar]
Mohammad 2015
- Mohammad Ali BM, Hegab DS, Saadany HM. Use of transcutaneous electrical nerve stimulation for chronic pruritus. Dermatologic Therapy 2015;28(4):210‐5. [PUBMED: 25973931] [DOI] [PubMed] [Google Scholar]
Mounessa 2017
- Mounessa JS, Siegel JA, Dunnick CA, Dellavalle RP. The role of cannabinoids in dermatology. Journal of the American Academy of Dermatology 2017;77(1):188‐90. [PUBMED: 28416341] [DOI] [PubMed] [Google Scholar]
Mueller 2004
- Mueller RS, Fieseler KV, Fettman MJ, Zabel S, Rosychuk RAW, Ogilvie GK, et al. Effect of omega‐3 fatty acids on canine atopic dermatitis. Journal of Small Animal Practice 2004;45(6):293‐7. [PUBMED: 15206474] [DOI] [PubMed] [Google Scholar]
Norman 2003
- Norman RA. Xerosis and pruritus in the elderly: recognition and management. Dermatologic Therapy 2003;16(3):254‐9. [PUBMED: 14510882] [DOI] [PubMed] [Google Scholar]
O'Donoghue 2005
- O'Donoghue M, Tharp MD. Antihistamines and their role as antipruritics. Dermatologic Therapy 2005;18(4):333‐40. [PUBMED: 16297006] [DOI] [PubMed] [Google Scholar]
Oetjen 2017
- Oetjen LK, Mack MR, Feng J, Whelan TM, Niu H, Guo CJ, et al. Sensory neurons co‐opt classical immune signaling pathways to mediate chronic itch. Cell 2017;171(1):217‐28. [PUBMED: 28890086] [DOI] [PMC free article] [PubMed] [Google Scholar]
Oldhoff 2005
- Oldhoff JM, Darsow U, Werfel T, Katzer K, Wulf A, Laifaoui J, et al. Anti‐IL‐5 recombinant humanized monoclonal antibody (Mepolizumab) for the treatment of atopic dermatitis. Allergy 2005;60(5):693‐6. [PUBMED: 15813818] [DOI] [PubMed] [Google Scholar]
Palkar 2017
- Palkar R, Ongun S, Catich E, Li N, Borad N, Sarkisian A, et al. Cooling relief of acute and chronic itch requires TRPM8 channels and neurons. Journal of Investigative Dermatology 2017;138:1391‐9. [PUBMED: 29288650] [DOI] [PMC free article] [PubMed] [Google Scholar]
Paller 2016
- Paller AS, Tom WL, Lebwohl MG, Blumenthal RL, Boguniewicz M, Call RS, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. Journal of the American Academy of Dermatology 2016;75(3):494‐503. [PUBMED: 27417017] [DOI] [PubMed] [Google Scholar]
Pan 2013
- Pan M, Heinecke G, Bernardo S, Tsui C, Levitt J. Urea: a comprehensive review of the clinical literature. Dermatology Online Journal 2013;19(11):1‐14. [PUBMED: 24314769] [PubMed] [Google Scholar]
Papoiu 2013
- Papoiu AD, Valdes‐Rodriguez R, Nattkemper LA, Chan YH, Hahn GS, Yosipovitch G. A novel topical formulation containing strontium chloride significantly reduces the intensity and duration of cowhage‐induced itch. Acta Dermato‐Venereologica 2013;93(5):520‐6. [PUBMED: 23474847] [DOI] [PubMed] [Google Scholar]
Patel 2007
- Patel T, Ishiuji Y, Yosipovitch G. Menthol: a refreshing look at this ancient compound. Journal of the American Academy of Dermatology 2007;57(5):873‐8. [PUBMED: 17498839] [DOI] [PubMed] [Google Scholar]
Patel 2010
- Patel T, Yosipovitch G. The management of chronic pruritus in the elderly. Skin Therapy Letter 2010;15(8):5‐9. [PUBMED: 20844849] [PubMed] [Google Scholar]
Pazyar 2012
- Pazyar N, Yaghoobi R, Kazerouni A, Feily A. Oatmeal in dermatology: a brief review. Indian Journal of Dermatology, Venereology, and Leprology 2012;78(2):142. [PUBMED: 22421643] [DOI] [PubMed] [Google Scholar]
Pereira 2017
- Pereira MP, Ständer S. Assessment of severity and burden of pruritus. Allergology International 2017;66(1):3‐7. [PUBMED: 27634668] [DOI] [PubMed] [Google Scholar]
Pfab 2010
- Pfab F, Huss‐Marp J, Gatti A, Fuqin J, Athanasiadis GI, Irnich D, et al. Influence of acupuncture on type I hypersensitivity itch and the wheal and flare response in adults with atopic eczema – a blinded, randomized, placebo‐controlled, crossover trial. Allergy 2010;65(7):903‐10. [PUBMED: 20002660] [DOI] [PubMed] [Google Scholar]
Phan 2010
- Phan NQ, Bernhard JD, Luger TA, Stander S. Antipruritic treatment with systemic μ‐opioid receptor antagonists: a review. Journal of the American Academy of Dermatology 2010;63(4):680‐8. [PUBMED: 20462660] [DOI] [PubMed] [Google Scholar]
Pirazzini 2017
- Pirazzini M, Rossetto O, Eleopra R, Montecucco C. Botulinum neurotoxins: biology, pharmacology, and toxicology. Pharmacological Reviews 2017;69(2):200‐35. [PUBMED: 28356439] [DOI] [PMC free article] [PubMed] [Google Scholar]
Plot Digitalizer [Computer program]
- Source Forge, owned and operated by Slashdot Media. Plot Digitalizer. Version 2.6.8. La Jolla, CA: Source Forge, owned and operated by Slashdot Media, 2015.
Polat 2008
- Polat M, Oztas P, Ilhan MN, Yalcin B, Alli N. Generalized pruritus: a prospective study concerning etiology. American Journal of Clinical Dermatology 2008;9(1):39‐44. [PUBMED: 18092842] [DOI] [PubMed] [Google Scholar]
Posadzki 2012
- Posadzki P, Alotaibi A, Ernst E. Adverse effects of aromatherapy: a systematic review of case reports and case series. International Journal of Risk & Safety in Medicine 2012;24(3):147‐61. [PUBMED: 22936057] [DOI] [PubMed] [Google Scholar]
Poterucha 2013
- Poterucha TJ, Murphy SL, Sandroni P, Rho RH, Warndahl RA, Weiss WT, et al. Topical amitriptyline combined with topical ketamine for the management of recalcitrant localized pruritus: a retrospective pilot study. Journal of the American Academy of Dermatology 2013;69(2):320‐1. [PUBMED: 23866874] [DOI] [PubMed] [Google Scholar]
Rea 1976
- Rea JN, Newhouse ML, Halil T. Skin disease in Lambeth. A community study of prevalence and use of medical care. British Journal of Preventive & Social Medicine 1976;30(2):107‐14. [PUBMED: 133742] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reeves 2019
- Reeves BC, Deeks JJ, Higgins JP, Shea B, Tugwell P, Wells GA. Chapter 24: Including non‐randomized studies on intervention effects. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook. Cochrane.
Reich 2012
- Reich A, Heisig M, Phan NQ, Taneda K, Takamori K, Takeuchi S, et al. Visual analogue scale: evaluation of the instrument for the assessment of pruritus. Acta Dermato‐Venereologica 2012;92(5):497‐501. [PUBMED: 22102095] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rivard 2005
- Rivard J, Lim HW. Ultraviolet phototherapy for pruritus. Dermatologic Therapy 2005;18(4):344‐54. [PUBMED: 16297008] [DOI] [PubMed] [Google Scholar]
Roth 1978
- Roth HL, Brown EP. Hydrocortisone valerate. Double‐blind comparison with two other topical steroids. Cutis 1978;21(5):695‐8. [PUBMED: 348406] [PubMed] [Google Scholar]
Rungsiprakarn 2016
- Rungsiprakarn P, Laopaiboon M, Sangkomkamhang US, Lumbiganon P. Pharmacological interventions for generalised itching (not caused by systemic disease or skin lesions) in pregnancy. Cochrane Database of Systematic Reviews 2016, Issue 2. [DOI: 10.1002/14651858.CD011351.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ruzicka 2017
- Ruzicka T, Mihara R. Anti‐Interleukin‐31 receptor A antibody for atopic dermatitis. New England Journal of Medicine 2017;376(21):2093. [PUBMED: 28538126] [DOI] [PubMed] [Google Scholar]
Salamone 2016
- Salamone JC, Salamone AB, Leung KXC, Reilly KE. Compositions and kits for treating pruritus and methods of using the same. patentscope.wipo.int/search/en/detail.jsf?docId=WO2016057789 (accessed before 21 February 2018).
Schmelz 2009
- Schmelz M. Opioid‐induced pruritus. Mechanisms and treatment regimens. Anaesthesist 2009;58(1):61‐5. [PUBMED: 19132330] [DOI] [PubMed] [Google Scholar]
Schworer 1993
- Schworer H, Ramadori G. Treatment of pruritus: a new indication for serotonin type 3 receptor antagonists. Clinical Investigator 1993;71(8):659‐62. [PUBMED: 8219665] [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JP, Green S, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Selescu 2013
- Selescu T, Ciobanu AC, Dobre C, Reid G, Babes A. Camphor activates and sensitizes transient receptor potential melastatin 8 (TRPM8) to cooling and icilin. Chemical Senses 2013;38(7):563‐75. [PUBMED: 23828908] [DOI] [PubMed] [Google Scholar]
Shohrati 2007
- Shohrati M, Tajik A, Harandi AA, Davoodi SM, Akmasi M. Comparison of hydroxyzine and doxepin in treatment of pruritus due to sulfur mustard. SKINmed 2007;6(2):70‐2. [PUBMED: 17361495] [DOI] [PubMed] [Google Scholar]
Siemens 2016
- Siemens W, Xander C, Meerpohl JJ, Buroh S, Antes G, Schwarzer G, et al. Pharmacological interventions for pruritus in adult palliative care patients. Cochrane Database of Systematic Reviews 2016, Issue 11. [DOI: 10.1002/14651858.CD008320.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Silva 1994
- Silva SR, Viana PC, Lugon NV, Hoette M, Ruzany F, Lugon JR. Thalidomide for the treatment of uremic pruritus: a crossover randomized double‐blind trial. Nephron 1994;67(3):270‐3. [PUBMED: 7936015] [DOI] [PubMed] [Google Scholar]
Simpson 2010
- Simpson EL. Atopic dermatitis: a review of topical treatment options. Current Medical Research and Opinion 2010;26(3):633‐40. [PUBMED: 20070141] [DOI] [PubMed] [Google Scholar]
Stander 2006
- Stander S, Reinhardt HW, Luger TA. Topical cannabinoid agonists. An effective new possibility for treating chronic pruritus [Topische Cannabinoidagonisten. Eine effektive, neue Moglichkeit zur Behandlung von chronischem Pruritus]. Der Hautarzt; Zeitschrift fur Dermatologie, Venerologie, und Verwandte Gebiete 2006;57(9):801‐7. [PUBMED: 16874533] [DOI] [PubMed] [Google Scholar]
Stander 2007
- Stander S, Weisshaar E, Mettang T, Szepietowski JC, Carstens E, Ikoma A, et al. Clinical classification of itch: a position paper of the International Forum for the Study of Itch. Acta Dermato‐Venereologica 2007;87(4):291‐4. [PUBMED: 17598029] [DOI] [PubMed] [Google Scholar]
Stander 2010
- Stander S, Siepmann D, Herrgott I, Sunderkotter C, Luger TA. Targeting the neurokinin receptor 1 with aprepitant: a novel antipruritic strategy. PLoS One 2010;5(6):e10968. [PUBMED: 20532044] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stander 2013
- Stander S, Stumpf A, Osada N, Wilp S, Chatzigeorgakidis E, Pfleiderer B. Gender differences in chronic pruritus: women present different morbidity, more scratch lesions and higher burden. British Journal of Dermatology 2013;168(6):1273‐80. [PUBMED: 23387396] [DOI] [PubMed] [Google Scholar]
Stander 2015
- Stander S, Weisshaar E, Raap U. Emerging drugs for the treatment of pruritus. Expert Opinion on Emerging Drugs 2015;20(3):515‐21. [PUBMED: 26027744] [DOI] [PubMed] [Google Scholar]
Stander 2017
- Stander S, Augustin M, Roggenkamp D, Blome C, Heitkemper T, Worthmann AC, et al. Novel TRPM8 agonist cooling compound against chronic itch: results from a randomized, double‐blind, controlled, pilot study in dry skin. Journal of the European Academy of Dermatology and Venereology 2017;31(6):1064‐8. [PUBMED: 27862339] [DOI] [PubMed] [Google Scholar]
Sterne 2017
- Sterne JAC, Egger M, Moher D, Boutron I (editors). Chapter 10: Addressing reporting biases. In: Higgins JP, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook/chapter‐10.
Sterne 2019
- Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. [DOI: 10.1136/bmj.l4898] [DOI] [PubMed] [Google Scholar]
Ständer 2006
- Ständer S, Schürmeyer‐Horst F, Luger TA, Weisshaar E. Treatment of pruritic diseases with topical calcineurin inhibitors. Therapeutics and Clinical Risk Management 2006;2(2):213. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vaa 2016
- Vaa BE, Tefferi A, Gangat N, Pardanani A, Lasho TL, Finke CM, et al. Pruritus in primary myelofibrosis: management options in the era of JAK inhibitors. Annals of Hematology 2016;95(7):1185‐9. [PUBMED: 27106700] [DOI] [PubMed] [Google Scholar]
van Cranenburgh 2013
- Cranenburgh OD, Korte J, Sprangers MA, Rie MA, Smets EM. Satisfaction with treatment among patients with psoriasis: a web‐based survey study. British Journal of Dermatology 2013;169(2):398‐405. [PUBMED: 23565643] [DOI] [PubMed] [Google Scholar]
van Zuuren 2016
- Zuuren EJ, Fedorowicz Z, Christensen R, Lavrijsen A, Arents BWM. Emollients and moisturisers for eczema. Cochrane Database of Systematic Reviews 2017, Issue 2. [DOI: 10.1002/14651858.CD012119.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vaughn 2016
- Vaughn AR, Branum A, Sivamani RK. Effects of turmeric (Curcuma longa) on skin health: a systematic review of the clinical evidence. Phytotherapy Research 2016;30(8):1243‐64. [DOI] [PubMed] [Google Scholar]
Weinfeld 2007
- Weinfeld PK. Successful treatment of notalgia paresthetica with botulinum toxin type A. Archives of Dermatology 2007;143(8):980‐2. [PUBMED: 17709655] [DOI] [PubMed] [Google Scholar]
Weisshaar 1998
- Weisshaar E, Heyer G, Forster C, Handwerker HO. Effect of topical capsaicin on the cutaneous reactions and itching to histamine in atopic eczema compared to healthy skin. Archives of Dermatological Research 1998;290(6):306‐11. [PUBMED: 9705161] [DOI] [PubMed] [Google Scholar]
Weisshaar 2009
- Weisshaar E, Dalgard F. Epidemiology of itch: adding to the burden of skin morbidity. Acta Dermato‐Venereologica 2009;89(4):339‐50. [PUBMED: 19688144] [DOI] [PubMed] [Google Scholar]
Weisshaar 2015
- Weisshaar E, Eckart WU, Bernhard JD. Historical background of itch. In: Cowan A, Yosipovitch G editor(s). Pharmacology of Itch. Handbook of Experimental Pharmacology, vol 226. Berlin, Heidelberg: Springer, 2015:1‐14. [DOI] [PubMed] [Google Scholar]
Xu 2013
- Xu S, Wang L, Cooper E, Zhang M, Manheimer E, Berman B, et al. Adverse events of acupuncture: a systematic review of case reports. Evidence‐based Complementary and Alternative Medicine: eCAM 2013;2013:581203. [DOI: 10.1155/2013/581203; PUBMED: 23573135] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yarbrough 2013
- Yarbrough KB, Neuhaus KJ, Simpson EL. The effects of treatment on itch in atopic dermatitis. Dermatologic Therapy 2013;26(2):110‐9. [PUBMED: 23551368] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yasuda 2016
- Yasuda T, Fukada T, Nishida K, Nakayama M, Matsuda M, Miura I, et al. Hyperactivation of JAK1 tyrosine kinase induces stepwise, progressive pruritic dermatitis. Journal of Clinical Investigation 2016;126(6):2064‐76. [PUBMED: 27111231] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yesudian 2005
- Yesudian PD, Wilson NJ. Efficacy of gabapentin in the management of pruritus of unknown origin. Archives of Dermatology 2005;141(12):1507‐9. [PUBMED: 16365250] [DOI] [PubMed] [Google Scholar]
Yoon 2003
- Yoon MH, Choi JI, Jeong SW. Spinal gabapentin and antinociception: mechanisms of action. Journal of Korean Medical Science 2003;18(2):255‐61. [PUBMED: 12692425] [DOI] [PMC free article] [PubMed] [Google Scholar]
York 2010
- York NR, Jacobe HT. UVA1 phototherapy: a review of mechanism and therapeutic application. International Journal of Dermatology 2010;49(6):623‐30. [PUBMED: 20618465] [DOI] [PubMed] [Google Scholar]
Yosipovitch 1996
- Yosipovitch G, Szolar C, Hui XY, Maibach H. High‐potency topical corticosteroid rapidly decreases histamine‐induced itch but not thermal sensation and pain in human beings. Journal of the American Academy of Dermatology 1996;35(1):118‐20. [PUBMED: 8682950] [DOI] [PubMed] [Google Scholar]
Yosipovitch 2001
- Yosipovitch G, Sugeng MW, Chan YH, Goon A, Ngim S, Goh CL. The effect of topically applied aspirin on localized circumscribed neurodermatitis. Journal of the American Academy of Dermatology 2001;45(6):910‐3. [PUBMED: 11712038] [DOI] [PubMed] [Google Scholar]
Yosipovitch 2008
- Yosipovitch G, Samuel LS. Neuropathic and psychogenic itch. Dermatologic Therapy 2008;21(1):32‐41. [PUBMED: 18318883] [DOI] [PubMed] [Google Scholar]
Yosipovitch 2010
- Yosipovitch G. Recent advances in pruritus ‐ what we have learned and where are we headed. F1000 Medicine Reports 2010;2:39. [PUBMED: 20948846] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yosipovitch 2013
- Yosipovitch G, Bernhard JD. Clinical practice. Chronic pruritus. New England Journal of Medicine 2013;368(17):1625‐34. [PUBMED: 23614588] [DOI] [PubMed] [Google Scholar]
Zachariae 2012
- Zachariae R, Lei U, Haedersdal M, Zachariae C. Itch severity and quality of life in patients with pruritus: preliminary validity of a Danish adaptation of the itch severity scale. Acta Dermato‐Venereologica 2012;92(5):508‐14. [PUBMED: 22002738] [DOI] [PubMed] [Google Scholar]
Zirwas 2001
- Zirwas MJ, Seraly MP. Pruritus of unknown origin: a retrospective study. Journal of the American Academy of Dermatology 2001;45(6):892‐6. [PUBMED: 11712035] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Andrade Miranda 2018
- Andrade Miranda A, Franco JVA, Sanclemente G, Kuah CY, Yu AM, Shpadaruk V, et al. Interventions for pruritus of unknown cause. Cochrane Database of Systematic Reviews 2018, Issue 9. [DOI: 10.1002/14651858.CD013128] [DOI] [PMC free article] [PubMed] [Google Scholar]


