Abstract
Cellular senescence is a phenotypic state that contributes to age-related diseases through the secretion of matrix-degrading and inflammatory molecules. An emerging therapeutic strategy for osteoarthritis (OA) is to selectively eliminate senescent cells by initiating apoptosis. This study establishes a cartilage explant model of senescence induction and senolytic clearance using p16Ink4a expression as a biomarker of senescence. Growth-factor stimulation of explants increased the expression of p16Ink4a at both the mRNA and protein levels. Applying this culture system to cartilage from p16tdTom reporter mice (a knockin allele with tdTomato fluorescent protein regulated by the endogenous p16Ink4a promoter) demonstrated the emergence of a p16-high population that was quantified using flow cytometry for tdTomato. Cell sorting was used to separate chondrocytes based on tdTomato fluorescence and p16-high cells showed higher senescence-associated β-galactosidase activity and increased gene expression of the senescence-associated secretory phenotype as compared with p16-low cells. The potential for effective senolysis within the cartilage extracellular matrix was assessed using navitoclax (ABT-263). Navitoclax treatment reduced the percentage of p16-high cells from 17.9 to 6.1% (mean of 13 matched pairs; P < 0.001) and increased cleaved caspase-3 confirmed apoptotic activity. Together, these findings establish a physiologically relevant cartilage explant model for testing the induction and elimination of senescent chondrocytes, which will support investigations of senolytic therapy for OA.—Sessions, G. A., Copp, M. E., Liu, J.-Y., Sinkler, M. A., D’Costa, S., Diekman, B. O. Controlled induction and targeted elimination of p16INK4a-expressing chondrocytes in cartilage explant culture.
Keywords: osteoarthritis, aging, senolytic, ABT-263, senescence
Osteoarthritis (OA) is associated with the progressive degradation of articular cartilage and other joint tissues (1, 2). OA causes a substantial societal burden (3) and effective treatments have been limited by an insufficient understanding of the underlying biologic processes that drive the disease (4). Aging and joint injury are major risk factors for OA, and there is evidence that cellular senescence contributes to OA development in both of these settings (5–11). An emerging therapeutic strategy in OA is to induce apoptosis in senescent cells as a way to eliminate the secretion of catabolic factors and provide a more regenerative environment for the remaining healthy cells (9, 12). The goal of this study was to establish a model system for investigating the efficiency of this senolytic approach in cartilage tissue explants.
Senescent cells contribute to tissue-level dysfunction through the production of a suite of inflammatory and matrix-degrading proteins known as the senescence-associated secretory phenotype (SASP) (13). Senescence is a complex phenotypic state that can play beneficial roles, including tumor suppression through preventing the division of damaged cells and coordination of wound repair through cell differentiation cues contained in the SASP (14, 15). However, senescent cells can accumulate to pathologic levels with aging because of increased senescence induction in response to stress and decreased rates of immune-mediated clearance (16–18). To elucidate the context-specific roles of senescence, it is critical to overcome the challenge of identifying senescent cells in their native environments (19). The cell cycle inhibitor p16Ink4a (one of 2 products transcribed from the cyclin-dependent kinase inhibitor 2A locus) has been shown to be a particularly effective biomarker of senescence because of its large dynamic range in response to conditions that reduce or accelerate aging (20, 21), and we have previously reported that increased p16INK4a expression in chondrocytes is associated with aging and dysfunction (22). Furthermore, p16Ink4a-based reporter alleles have been important tools for determining the role of senescence in health and disease and as a potential therapeutic target (14, 23–26). In this study, we utilized the recently developed p16tdTom reporter allele (a knockin allele with tdTomato fluorescent protein regulated by the endogenous p16Ink4a promoter) (27) to quantify the induction and elimination of senescent chondrocytes in the context of a physiologically relevant cartilage explant model.
MATERIALS AND METHODS
Generation of mouse colonies
All animal experiments were performed under protocols approved by the Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill. The p16tdTom reporter allele generates the fluorescent protein tdTomato under endogenous p16Ink4a regulation, and extensive characterization of this allele has recently been published (27). For identification of chondrocytes expressing aggrecan, the aggrecan (Acan)tm1(cre/ERT2)Crm allele (Acan-CreERT2) (28) [received from Dr. Benoit de Crombugghe (M. D. Anderson Cancer Center, Houston, TX, USA); now available as stock 019148 from The Jackson Laboratory (Bar Harbor, ME, USA)] was crossed with the loxP-stop-loxP ZsGreen reporter allele (29) [Gt(ROSA)26Sortm6(CAG-ZsGreen1)Hze/J stock 007906; The Jackson Laboratory] and then into p16tdTom mice. All alleles were maintained on a C57BL/6J background.
Culture of murine hip cartilage explants for senescence induction
Mice were euthanized at 3 wk of age for isolation of hip cartilage explants from the proximal end of the femur. Consistent with the published approach (30), forceps were used to separate cartilage from underlying bone. Explants were cultured for 3 wk in the following control medium: DMEM/F12 (11330; Thermo Fisher Scientific, Waltham, MA, USA) containing 10% fetal bovine serum (Seradigm 1500-500; VWR International, West Chester, PA, USA), penicillin and streptomycin (15140; Thermo Fisher Scientific), gentamicin (15750; Thermo Fisher Scientific), and amphotericin B (A2942; MilliporeSigma, Burlington, MA, USA). Senescence-induction conditions were applied during the entire culture period and consisted of control medium with the addition of 1 ng/ml TGF-β1 and 5 ng/ml basic fibroblastic growth factor (bFGF) (PHG9204 and PHG0264; Thermo Fisher Scientific). Explants were cultured with 5 µM 4-hydroxytamoxifen (H7904; MilliporeSigma) for the initial 2 feeds to activate the Cre recombinase activity of Acan-CreERT2. Explants were cultured in either atmospheric oxygen (∼20% O2) or at 2% O2 as maintained through replacement with nitrogen gas in a specialized incubator (NU-5731; NuAire, Plymouth, MN, USA). For senolytic experiments, matched explants that had been cultured in senescence-inducing conditions for 3 wk were treated with a vehicle control consisting of 0.025% DMSO (D2650; MilliporeSigma) or 5 µM navitoclax (S1001; Selleck Chemicals, Houston, TX, USA) for 3 d in control medium.
Flow cytometry analysis of tdTomato and cell sorting
Cartilage explants were digested into a single-cell suspension through overnight treatment with 0.4 mg/ml collagenase P (11249002001; Roche, Basel, Switzerland). Explants were agitated at 600 rpm at 37°C in a ThermoMixer C (Eppendorf, Hamburg, Germany) during digestion. Undigested tissue was removed with a 30-µm strainer and cells were washed to remove collagenase solution. Flow cytometry analysis was performed on unfixed cells suspended in HBSS with 2% fetal bovine serum, 10 mM EDTA, and 1 µg/ml DAPI with an Attune NxT (Thermo Fisher Scientific) using a 561 nm laser. Chondrocytes from mice without the p16tdTom reporter were used as gating controls, and analysis was performed using FCS Express (De Novo Software, Glendale, CA, USA).
RNA isolation and gene expression analysis
For direct isolation of RNA from cultured explants, the tissue was placed in tubes containing 1.4-mm ceramic beads (10158-610; VWR International) containing Trizol (Thermo Fisher Scientific) and homogenized (Precellys 24 Homogenizer; Bertin, Rockville, MD, USA). RNA was isolated using phenol chloroform extraction and NucleoSpin RNA XS column clean-up (Macherey-Nagel, Düren, Germany). Reverse transcription was performed using qScript XLT cDNA SuperMix (VWR International) according to the manufacturer’s instructions. Quantitative PCR was performed with TaqMan Universal Master Mix on a QuantStudio 6 Flex Machine (Thermo Fisher Scientific) as recently described in Diekman et al. (22). SASP gene expression was analyzed using TaqMan primer probes for IL-6 (Mm00446190_m1), IGF binding protein 3 (Mm01187817_m1), matrix metallopeptidase 13 (Mm00439491), and chemokine (C-C motif) ligand 2 (Mm00441242_m1). Custom TaqMan primers specific to murine p16Ink4a (AIMSG0H; forward, 5′-CGGTCGTACCCCGATTCAG-3′; reverse, 5′-GCACCGTAGTTGAGCAGAAGAG-3′; probe, 5′-AACGTTGCCCATCATCA-3′) and p19Arf (AIMSH0Y; forward, 5′-TGAGGCTAGAGAGGATCTTGAGAAG-3′; reverse, 5′-GTGAACGTTGCCCATCATCATC-3′; probe, 5′-ACCTGGTCCAGGATTC-3′) were used with data normalized to murine TATA-binding protein as a housekeeping control (Mm00446973_m1).
Protein isolation and Western blotting
Following RNA extraction with Trizol, the phenol ethanol supernatant from the same sample was used for protein extraction according to the manufacturer’s recommendations. Briefly, after precipitating DNA, protein in the phenol ethanol layer was precipitated using isopropanol. The pellet was washed with 0.3 M guanidine hydrochloride in 95% ethanol followed by a final ethanol wash. The air-dried pellet was resuspended in 1% SDS containing phosphatase inhibitor cocktail (Thermo Fisher Scientific) and PMSF. Protein concentration was determined using the Micro BCA Protein Assay Kit (Thermo Fisher Scientific). Protein extracts [30 µg for hip cartilage explants and 3 µg for mouse embryonic fibroblasts (MEFs)] were separated by SDS-PAGE and transferred to a nitrocellulose blotting membrane. After blocking, the membrane was split for incubation overnight at 4°C with the lower blot treated with a rabbit mAb for p16Ink4a (ab211542; 1:1000; Abcam, Cambridge, MA, USA), and the upper blot was treated with an antibody for glyceraldehyde 3-phosphate dehydrogenase (Gapdh; 2275PC; 1:1000; Trevigen, Gaithersburg, MD, USA). Following incubation in secondary antibody solution (7074; 1:1000; Cell Signaling Technology, Danvers, MA, USA) for 1 h, the membranes were washed and placed in the Radiance Plus Chemiluminescent Substrate (Azure Biosystems, Dublin, CA, USA). Bands were visualized using the Azure c600 gel imaging system (Azure Biosystems) and band intensities were quantified using ImageJ (National Institutes of Health, Bethesda, MD, USA). Hip cartilage explants were used for detecting the p16Ink4a protein from mice heterozygous for p16tdTom. Mice homozygous for p16tdTom, which do not express p16Ink4a but retain normal function of the alternate reading frame product of cyclin-dependent kinase inhibitor (Arf) (27), were used as a negative control. Protein from MEFs from mice with germline deletion of both p16Ink4a and Arf (31) was used as an additional negative control. For cleaved caspase-3 (9664; 1:1000; Cell Signaling Technology) protein was isolated using the same Trizol procedure or by homogenization of explants in cell lysis buffer (9803; Cell Signaling Technology), and Western blotting was performed as previously described.
Senescence-associated β-galactosidase analysis of sorted cells
Explant cultures from independent litters were combined at the end of the 3-wk senescence-induction period and were prepared for sorting as previously described for flow cytometry analysis. A MoFlo XDP cell sorter (Beckman Coulter, Brea, CA, USA) with 532-nm laser and 100-µm nozzle was used for sorting experiments. To gain further precision in the cell type sorted, the cell population was first gated on positive staining for Acan-CreERT2–driven lox-stop-lox ZsGreen before subsequent gating of tdTomato-negative and tdTomato-positive cells for sorting. Both tdTomato-negative and tdTomato-positive populations were plated at 10,000 cells in an 8-well chamber (Nunc Lab-Tek II Chamber Slide; 154534; Thermo Fisher Scientific) for 1–2 d to allow attachment. Cells were then fixed and stained overnight using the Senescence β-Galactosidase Staining Kit (9860; Cell Signaling Technology) according to the manufacturer’s recommendations. Representative bright-field color images were taken with an EVOS M5000 Imaging System (Thermo Fisher Scientific) and the percentage of positively stained cells was quantified from 6 to 8 nonoverlapping images. The cell boundaries were also traced using ImageJ to measure the cell surface area, which was then averaged across groups.
Histology and immunohistochemistry
Hip cartilage explants were fixed in 4% paraformaldehyde for 24 h at 4°C and processed for paraffin embedding. For immunohistochemistry, we used a primary antibody targeting tdTomato (600-401-379; Rockland Immunochemicals, Limerick, PA, USA) with overnight incubation at 4°C with 1:100 dilution. A donkey anti-rabbit secondary antibody (715-065-152; Jackson ImmunoResearch Laboratories, West Grove, PA, USA) was used with a 30-min incubation at room temperature. Antigen retrieval was performed with sodium citrate for 25 min at 95°C and chromogen visualization was completed using the Vectastain Elite ABC-HRP kit (Vector Laboratories, Burlingame, CA, USA). As shown in Supplemental Fig. S1C, quantification of tdTomato-positive cells was aided by marking the major zones of cartilage using morphologic criteria (superficial: small-diameter cells aligned parallel to the surface, middle: moderate-diameter cells with no orientation to the surface, and deep: large diameter cells aligned perpendicular to the surface).
Statistical analysis
Statistical analysis and plotting was performed using Prism 7 (GraphPad Software, La Jolla, CA, USA) and flow cytometry data was processed with FCS Express 6 (De Novo Software). Data are plotted as individual points, and error bars indicate means ± sem. Different symbols used within column plots indicate matched pairs. Outliers were identified using Grubbs test with α = 0.05 and were excluded from subsequent analysis. Normality was assessed by a Shapiro-Wilk test. Normally distributed data were analyzed by either a paired Student’s t test or repeated ANOVA measures (≥3 groups) with Tukey’s post hoc analysis. Data not normally distributed were analyzed with a nonparametric Wilcoxon’s rank-sum test.
RESULTS
Induction of p16tdTom expression in hip cartilage explants
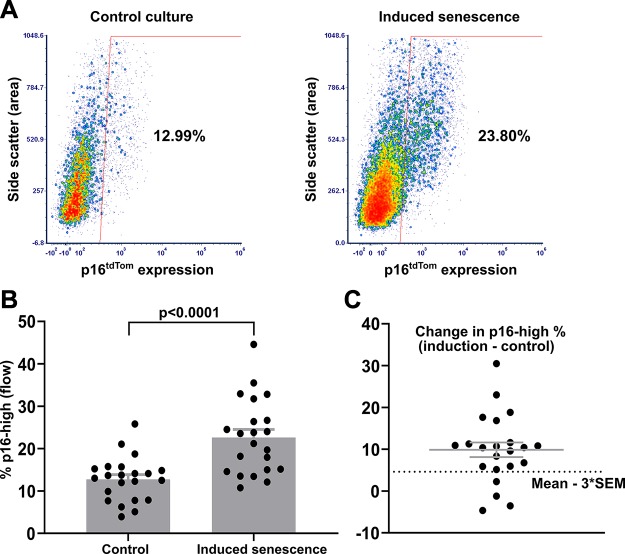
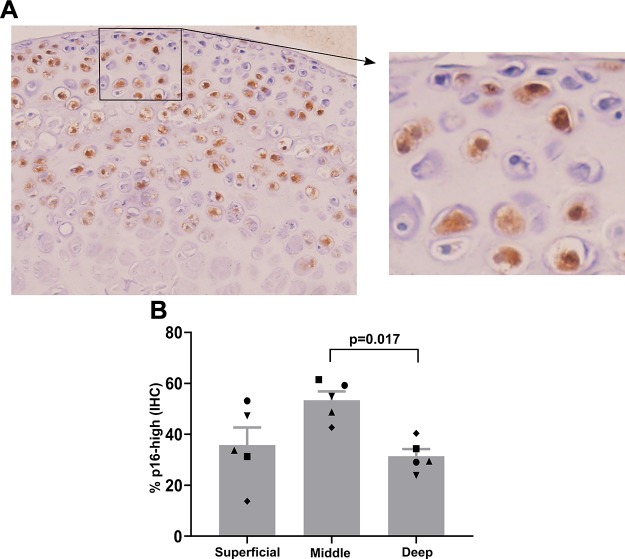
We developed an in vitro model system to drive chondrocyte senescence in a physiologically relevant tissue environment. Hip cartilage explants were isolated from the femoral cap of p16tdTom reporter mice, and the percentage of cells with tdTomato fluorescence above a gating control was used as a readout of senescence induction. Given the recent characterization of this reporter allele in the context of flow cytometry (27), we refer to this population as p16-high for the remainder of the manuscript. The p16-high cell population emerged in response to 3 wk of explant culture, especially with the inclusion of 1 ng/ml TGF-β1 and 5 ng/ml bFGF (Fig. 1A). To account for variance between individual mice, the 2 hip cartilage explants from each mouse were split between treatment groups for direct comparison of matched samples. Using this method on a cohort of 22 mice at 3 wk of age demonstrated a robust increase in the percentage of p16-high cells with growth-factor treatment (mean of 12.8% in control conditions and 22.6% in senescence-inducing conditions; P < 0.0001 by paired Student’s t test; Fig. 1B). Four pairs of hips did not show a response to the senescence-inducing conditions as defined by falling below a threshold of the mean increase minus 3 times the sem (Fig. 1C). Immunohistochemistry for the tdTomato protein showed that p16-high chondrocytes were present in the superficial, middle, and deep zones (Fig. 2A). The middle zone had the highest percentage of p16-high cells in each of 5 independent explants analyzed with a statistically significant increase in the middle zone as compared with the deep zone (P = 0.017 by ANOVA with Tukey’s post hoc; Fig. 2B). Controls demonstrating the specificity of tdTomato immunohistochemistry and the identification of cartilage zones are shown in Supplemental Fig. S1A (no primary antibody), Supplemental Fig. S1B (no p16tdTom allele), and Supplemental Fig. S1C (image with marked zonal definitions).
Figure 1.
Induction of p16tdTom in hip cartilage explant cultures. A) Representative flow plots showing the percentage of p16-high cells after 3 wk of explant culture. Senescence-induction conditions added 1 ng/ml TGF-β1 and 5 ng/ml bFGF to control conditions. B) Quantification of p16-high cells in matched explants from 22 mice (1 hip control; 1 hip induced senescence per mouse). C) The change in percentage of p16-high cells with senescence-induction conditions as compared with control conditions within each pair of explants (percentage in induction conditions minus percentage in control conditions). Solid lines indicate means ± sem. The dotted line indicates a senescence-induction threshold of the mean − 3 × sem.
Figure 2.
Distribution of p16-high cells within explant cultures. A) Immunohistochemistry (IHC) for tdTomato (brown staining) and hematoxylin (blue staining of nuclei) on paraffin-embedded explants after 3 wk of culture with senescence-induction conditions. Inset shows magnification ×3 compared to main image. B) Quantification of the percentage of p16-high cells in the 3 zones of articular cartilage (superficial, middle, and deep) in 5 explants. Matched symbols indicate values of each zone from the same explant.
Validation that p16tdTom is a faithful reporter of p16Ink4a gene expression and protein
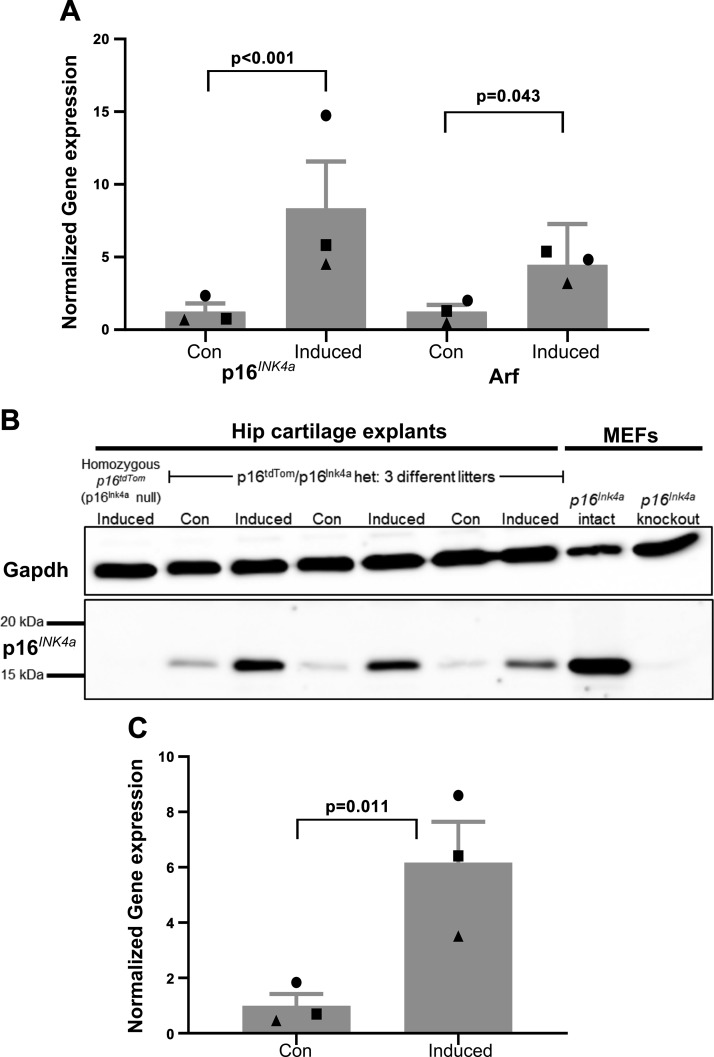
Explants were isolated from mice that were heterozygous for p16tdTom and p16Ink4a to avoid the possibility that the complete absence of p16Ink4a would alter regulation of the locus. Furthermore, heterozygosity presents the opportunity to confirm that induction of p16tdTom is a faithful reporter of native p16Ink4a in this cartilage explant model. This was done by quantitative PCR using primer probes that span the shared exon 2 of p16Ink4a and Arf as well as either exon 1α (present in p16Ink4a) or exon 1β (present in Arf). Growth factor–based induction of senescence resulted in significantly increased p16Ink4a and Arf expression (8.4-fold increase and 4.5-fold increase from the mean of control conditions; P < 0.001 and P = 0.043, respectively; Fig. 3A). The protein level of p16Ink4a was also increased under senescence-inducing conditions in cartilage explants from 3 separate litters of p16tdTom/p16Ink4a heterozygous mice (6.2-fold increase from the mean of control conditions; P = 0.011; Fig. 3B, C). We demonstrated specificity of the antibody for p16Ink4a by the lack of a band in both Ink4a/Arf knockout MEFs and in induced hip cartilage explants from homozygous p16tdTom mice that are null for p16Ink4a but retain production of Arf.
Figure 3.
Validation of p16tdTom reporter. A) Gene expression for p16Ink4a and Arf for control and senescence-induced explants from 3 litters of mice. Data were normalized to TATA-binding protein housekeeping control and then to the mean of control samples. B) Western blot for p16Ink4a and Gapdh using protein from hip cartilage explants or MEFs. Three litters of p16tdTom/p16Ink4a heterozygous mice were used in control and senescence-inducing conditions. Mice that were homozygous for p16tdTom are p16Ink4a null but have intact Arf. MEFs labeled as p16Ink4a knockout had a global knockout of both p16Ink4a and Arf. C) Quantification of p16Ink4a protein band intensity normalized to Gapdh and then to the mean of control samples.
The p16tdTom allele can be used to sort cells with features of senescence
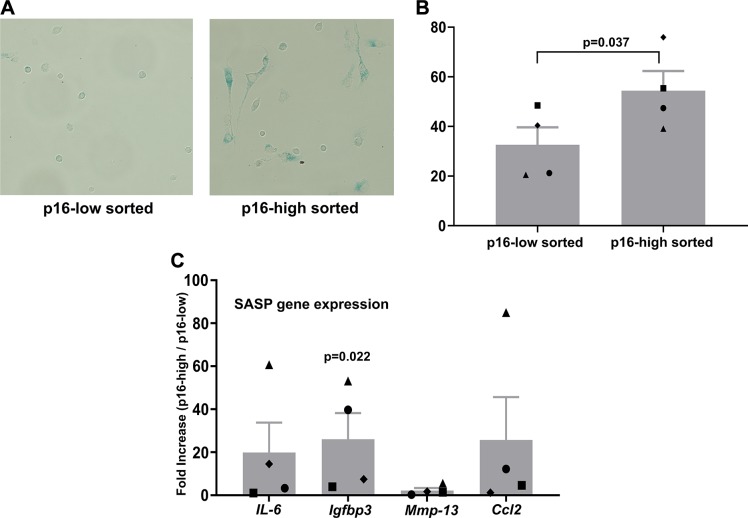
Recent characterization of the p16tdTom allele confirmed that tdTomato-positive cells show strong p16Ink4a promoter activity and demonstrate features of senescence in vitro and in vivo (27). The emergence of a tdTomato-positive population with physiologic aging has been shown in both articular cartilage (27) and intervertebral disc (32). One beneficial feature of this reporter is the retention of transcriptional enhancer elements that increase the fluorescence signal without compromising fidelity (27). This allows for the physical separation of cells with low and high p16Ink4a promoter activity using flow cytometry–based sorting. We performed cell sorting on digested cartilage explants after 3 wk of culture in senescence-inducing conditions. When compared with tdTomato-negative cells sorted from the same tissues, tdTomato-positive chondrocytes demonstrated a more spread and flattened cellular morphology (Fig. 4A) that has been associated with senescent cells during monolayer culture (33). Cells sorted as tdTomato-positive had nearly twice the surface area as compared with tdTomato-negative cells (1.93-fold increase in ≥50 cells traced per group). These morphologically senescent cells stained positively for senescence-associated β-galactosidase (SA-β-gal), which is a commonly used marker of senescent cells based on enhanced lysosomal activity (34). Positive staining for SA-β-gal was recorded in a significantly higher percentage of tdTomato-positive cells (P = 0.037 by paired Student’s t test; Fig. 4B). Cartilage explants were also cultured at low oxygen tension (2% O2) during senescence induction in order to provide conditions conducive to SASP expression (35). Under these conditions, cells sorted as tdTomato-positive had higher gene expression of SASP markers as compared with cells sorted as tdTomato-negative (Fig. 4C), with an enrichment of over 20-fold for IGF binding protein 3 (P = 0.022).
Figure 4.
SA-β-gal activity in chondrocytes sorted based on p16tdTom levels. A) Representative image of SA-β-gal staining from cells sorted as either p16 low or p16 high. B) Quantification of the percentage of cells showing positive staining for SA-β-gal in each population. Matched symbols indicate cells sorted simultaneously into the p16-low or p16-high populations. C) SASP gene expression from explants cultured for 3 wk in 2% oxygen with growth factors to induce senescence. Cells were sorted and the data represent the fold increases for the p16-high fraction as compared with the p16-low fraction for each gene.
Selective elimination of p16-high chondrocytes by navitoclax treatment
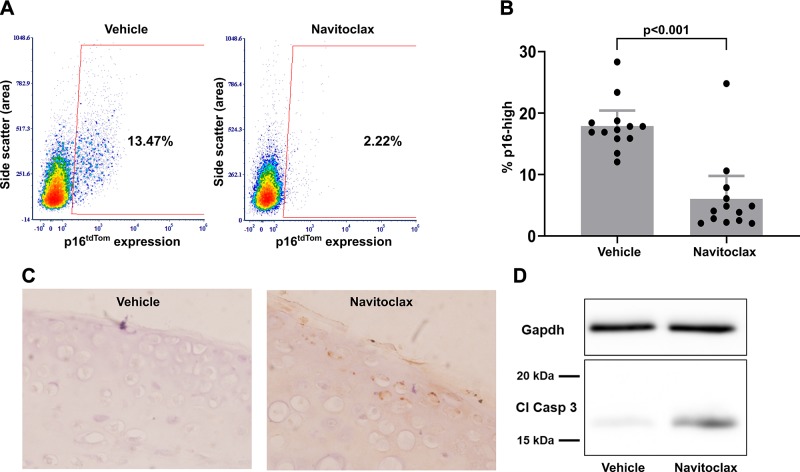
One characteristic of senescent cells is that they become resistant to cell death by up-regulating antiapoptotic programs, which can then be targeted by senolytic compounds to promote apoptosis (12). Navitoclax has been shown to initiate apoptosis in senescent cells in a wide variety of in vitro and in vivo contexts (36, 37) and was therefore used as a candidate senolytic in the cartilage explant system. Based on literature values and initial dose–curve experiments, we treated a series of hip cartilage explants with 5 µM navitoclax for 3 d after a 3-wk induction of senescence. As compared with DMSO vehicle controls, navitoclax treatment caused a depletion of p16-high cells (Fig. 5A). A matched set of 13 explants with induced senescence demonstrated that navitoclax treatment reduced the percentage of p16-high cells from a mean of 17.9 to 6.1% as compared with vehicle control (P < 0.001; Fig. 5B). Western blot (Fig. 5C) and immunohistochemistry (Fig. 5D) for cleaved caspase-3 were used to show that navitoclax initiated the apoptosis program within 18 h of explant treatment. These results provide evidence for senolytic activity of navitoclax in cartilage tissue, with the selective induction of apoptosis within the p16-high cell population.
Figure 5.
Selective elimination of p16-high chondrocytes with navitoclax treatment. A) Representative flow plots showing the percentage of p16-high cells after a 3-d treatment with either DMSO vehicle control or 5 µM navitoclax after 3 wk of explant culture in senescence-inducing conditions. B) Quantification of p16-high cells in matched explants from 13 mice (1 hip DMSO control; 1 hip navitoclax/mouse). C) Immunohistochemistry for cleaved caspase-3 (Cl Casp 3; brown staining) and hematoxylin (blue staining) in paraffin-embedded explants fixed 18 h after treatment with DMSO vehicle control or navitoclax. D) Representative Western blot for Gapdh and cleaved caspase-3 using protein from hip cartilage explants harvested at 18 h after treatment with DMSO control or navitoclax.
DISCUSSION
This study demonstrated the utility of a knock-in p16tdTom reporter allele for analysis of senescence induction in cartilage explants and for the isolation of chondrocytes with high p16Ink4a expression. Furthermore, the allele was used to quantify the efficiency of senolytic clearance from within cartilage tissue using navitoclax treatment. This in vitro system will continue to drive mechanistic studies of senescence induction in cartilage and will enhance the ability to design and interpret future studies that employ senolytic therapy as a treatment paradigm for OA.
A range of genetic engineering strategies have been used to harness the p16Ink4a promoter for investigation of cellular senescence in physiologically relevant contexts. The INK-ATTAC transgenic allele developed in the van Deursen laboratory utilized a 2.6-kb fragment of the p16Ink4a promoter to drive inducible apoptosis of senescent cells upon delivery of an activating compound (24). The p16-3MR allele from the Campisi laboratory uses a similar transgenic approach with a 50-kb portion of the p16Ink4a promoter driving a fusion protein containing Renilla luciferase, red fluorescent protein, and a version of thymidine kinase that causes cell death in response to ganciclovir. The Hara laboratory used transgenic expression of luciferase with 195 kb of the human p16INK4a locus to provide additional regulatory elements and was able to show increased luciferase in a range of tissues with physiologic aging (26). Finally, the Sharpless laboratory generated both the p16LUC and p16tdTom alleles using a knock-in approach to produce luciferase or tdTomato in the context of the native p16Ink4a regulatory landscape as opposed to the random integration of the promoter sequence (25, 27). The knock-in feature is especially relevant for this locus given the known effect of genetic variation in the vicinity of p16INK4a on age-related diseases (38, 39).
In addition to the identification of senescent cells, p16Ink4a-based reporter alleles have been integral to the burgeoning area of senolytic research. Indeed, the INK-ATTAC allele first demonstrated the utility of removing senescent cells by showing increased health span and life span with clearance of p16-high cells in mouse models of accelerated and physiologic aging (23, 24). The use of p16-3MR mice to perform longitudinal tracking and elimination of p16-high cells provided evidence for the beneficial role of senescence in wound healing (14) and demonstrated that clearance of senescent cells could ameliorate age-related diseases such as atherosclerosis (40) and OA (9). The knock-in p16LUC allele has been used to assess the senescence burden after senolytic treatment (41), and the p16tdTom allele extends the analysis capabilities through the isolation of p16-high and p16-low cells with flow cytometry cell sorting.
One goal of this study was to establish a reproducible and physiologically relevant model system for senescence induction in chondrocytes. We used explants from 3-wk-old mice because of the ease of tissue isolation and the extensive use of cartilage explants at this age in studies of cartilage biology (30). Additional experiments using cartilage from older mice may provide further insight into the mechanisms of senescence induction but would also introduce an additional cell type in the culture system because cartilage cannot be effectively separated from the underlying bone at advanced ages. The inclusion of TGF-β1 and bFGF during the culture period was critical for initiating a substantial burden of p16-high cells. We hypothesize that senescence emerged as a result of the conflicting signals provided by mitogenic growth factors in the context of culture stress and forced proliferative restraint imposed by the cartilage matrix. This hypothesis is consistent with established concepts such as oncogene-induced senescence (33), geroconversion (42), and sustained p16INK4a-mediated arrest (43), all of which highlight that the persistent presence of conflicting progrowth and antiproliferation signals initiate the senescence program.
A key feature of this model system for senescence induction is that chondrocytes are cultured within 3-dimensional tissue explants instead of the more commonly used context of monolayer culture. Notably, TGF-β and bFGF are released from stores in the cartilage matrix and coordinate the activated state that occurs in response to cartilage explant injury (44, 45). These growth factors have pleiotropic effects on cartilage and, depending on the context, can drive proliferation, matrix synthesis, and matrix degradation (46). Dissecting the mechanisms by which growth factors and the extracellular matrix environment synergize to initiate senescence will require detailed characterization of the cell cycle and an appreciation for the dynamics of the interaction of biochemical and physical cues. For example, degradation of the extracellular matrix by SASP factors may relieve matrix restraint of proliferation, whereas at the same time inflammatory SASP factors may also initiate catabolic signaling pathways that inhibit proliferation through other mechanisms. The senescent cells that emerged in this model system showed expected features such as SA-β-gal staining and SASP gene expression. Future work could use the same cell sorting approach to determine whether p16-high chondrocytes also display other phenotypes associated with senescence, including the inability to divide and altered metabolic activity.
The p16tdTom allele was shown to accurately represent the level of p16Ink4a promoter activation using extensive characterization of MEFs (27). The ability to generate high levels of senescence with explant culture allowed us to perform validation of reporter fidelity in cartilage tissue. Growth-factor stimulation nearly doubled the number of p16tdTom-positive cells, with the fluorescence levels reaching ∼1 log over the gating control (Fig. 1A). This single-cell measure of p16tdTom corresponded to an 8.4-fold increase in p16Ink4a gene expression when analyzed from bulk tissue (Fig. 3A). Although gene expression of p16Ink4a has been extensively used as a senescence marker in both murine and human cells, including chondrocytes (22), measuring the protein level of p16Ink4a has been challenging in murine tissues (19). This is a common source of frustration in senescence research given the clinical utility of immunohistochemistry for human p16INK4a (CINtec; Roche) and the previous widespread use of the M-156 clone (47) [formerly sc-1207; Santa Cruz Biotechnology, Dallas, TX, USA (no longer commercially available)]. We took advantage of a rigorous set of controls to validate a newly developed commercial antibody for murine p16Ink4a (ab211542; Abcam) by Western blot (Fig. 3B). The absence of any signal in senescence-induced cartilage explants from p16tdTom/tdTom mice (p16 null but Arf intact) as well as MEFs from Ink and Arf knockout mice demonstrate the specificity of this antibody in murine cartilage tissue.
The first generation of senolytics has been identified by screening for compounds that cause a higher rate of death in senescent fibroblasts (typically induced by irradiation in the monolayer culture) as compared with control fibroblasts (36, 37, 41, 48–53). In the course of these studies, it has become clear that the senolytic activity of compounds is dependent on the target cell type and physiologic context. Navitoclax has demonstrated senolytic activity in a variety of cell types both in vitro and in vivo (36, 37, 54) and was able to improve the function of engineered cartilage in the context of murine joints with a high senescence burden (55). Navitoclax (also known as ABT-263, which is an orally bioavailable derivative of ABT-737) is a cancer drug that inhibits antiapoptotic members of the B-cell lymphoma 2 (Bcl-2) family and can therefore initiate specific cell death in cells that are dependent on Bcl-2, B-cell lymphoma extra-large (Bcl-xL), or Bcl-2–like protein 2 (Bcl-w) and are otherwise primed for apoptosis (56). In our cartilage explant system, navitoclax showed consistent and robust elimination of the p16tdTom-positive chondrocyte population with a single treatment for 3 d. The observation that the fluorescence intensity of the p16tdTom-negative fraction was unchanged, combined with the initiation of cleaved caspase-3 after navitoclax treatment, suggests that down-regulation of p16Ink4a expression was not responsible but that selective apoptosis was the primary mechanism for this result. Because of the complex regulation of apoptosis by the Bcl-2 family of proteins, experimental approaches such as BH3 profiling (57) may be required to determine the precise mechanisms by which p16-high chondrocytes are preferentially targeted for cell death upon navitoclax treatment.
The emergence of senescent chondrocytes with aging and OA had been well documented since studies in the early 2000s (5–7), but the idea that targeting senescent cells in the joint for therapy was catalyzed by the work of Jeon et al. (9). The authors found that transection of the anterior cruciate ligament induced senescence that peaked at 2 wk postinjury. The elimination of senescent cells through either the p16-3MR approach or using intraarticular injection of a proprietary senolytic molecule (UBX0101) resulted in enhanced joint function and reduced injury-induced OA (9). Further work in this study used the INK-ATTAC allele to demonstrate that clearing senescent cells may also be beneficial in preventing the development of OA in aged mice (9). These findings are particularly intriguing because they support the possibility that senescence is a mechanism that contributes to both age-related and posttraumatic OA (58, 59). Early clinical trials on patients with OA (NCT03513016; Unity Biotechnology, Brisbane, CA, USA) will provide important insight into the safety of intraarticular senolytic injection and the efficiency of senescent cell clearance with the proprietary senolytic molecule UBX0101. However, continued development of this approach will benefit from a better understanding of the mechanisms by which particular senolytic compounds target specific cell types in physiologically relevant contexts (12). We propose that explant culture is a valuable secondary system to explore the effect of putative senolytic compounds discovered by more inherently high throughput screens. Future work using age-related and injury-induced models of OA will be required to further dissect the functional benefit of candidate drugs such as navitoclax that have shown efficient senolytic activity within cartilage matrix.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank the members of Dr. Richard Loeser’s laboratory [University of North Carolina (UNC) at Chapel Hill] for valuable feedback, and the UNC Animal Histopathology and UNC Flow Cytometry Cores. This work was supported by the U.S. National Institutes of Health (NIH) National Institute on Aging (Grant F32 AG050399 to B.O.D.), and was conducted while B.O.D. was an Arthritis and Aging Research Grant recipient from the Arthritis National Research Foundation (ANRF) and the American Federation for Aging Research, as well as second-year funding from the ANRF. Support from the NIH National Institute on Aging was provided to M.A.S. for the UNC Summer Research in Aging for Medical Students (NIA 2-T35-AG038047-08). The UNC Flow Cytometry Core Facility is supported, in part, by the P30 CA016086 Cancer Center Core Support Grant to the UNC Lineberger Comprehensive Cancer Center. The authors declare no conflicts of interest.
Glossary
- Acan
aggrecan
- Acan-CreERT2
Acantm1(cre/ERT2)Crm allele
- Arf
alternate reading frame product of cyclin-dependent kinase inhibitor 2A
- Bcl-2
B-cell lymphoma 2
- bFGF
basic fibroblastic growth factor
- Gapdh
glyceraldehyde 3-phosphate dehydrogenase
- OA
osteoarthritis
- MEF
mouse embryonic fibroblast
- SA-β-gal
senescence-associated β-galactosidase
- SASP
senescence-associated secretory phenotype
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
G. A. Sessions and B. O. Diekman designed research; G. A. Sessions, M. E. Copp, J.-Y. Liu, M. A. Sinkler, and S. D’Costa performed research; J.-Y. Liu contributed to the implementation of new reagents; G. A. Sessions, M. E. Copp, and B. O. Diekman analyzed data; and G. A. Sessions and B. O. Diekman wrote the manuscript.
REFERENCES
- 1.Bijlsma J. W., Berenbaum F., Lafeber F. P. (2011) Osteoarthritis: an update with relevance for clinical practice. Lancet 377, 2115–2126 [DOI] [PubMed] [Google Scholar]
- 2.Loeser R. F., Goldring S. R., Scanzello C. R., Goldring M. B. (2012) Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 64, 1697–1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Felson D. T., Lawrence R. C., Dieppe P. A., Hirsch R., Helmick C. G., Jordan J. M., Kington R. S., Lane N. E., Nevitt M. C., Zhang Y., Sowers M., McAlindon T., Spector T. D., Poole A. R., Yanovski S. Z., Ateshian G., Sharma L., Buckwalter J. A., Brandt K. D., Fries J. F. (2000) Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann. Intern. Med. 133, 635–646 [DOI] [PubMed] [Google Scholar]
- 4.Loeser R. F., Collins J. A., Diekman B. O. (2016) Ageing and the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 12, 412–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin J. A., Buckwalter J. A. (2001) Telomere erosion and senescence in human articular cartilage chondrocytes. J. Gerontol. A Biol. Sci. Med. Sci. 56, B172–B179 [DOI] [PubMed] [Google Scholar]
- 6.Price J. S., Waters J. G., Darrah C., Pennington C., Edwards D. R., Donell S. T., Clark I. M. (2002) The role of chondrocyte senescence in osteoarthritis. Aging Cell 1, 57–65 [DOI] [PubMed] [Google Scholar]
- 7.Zhou H. W., Lou S. Q., Zhang K. (2004) Recovery of function in osteoarthritic chondrocytes induced by p16INK4a-specific siRNA in vitro. Rheumatology (Oxford) 43, 555–568 [DOI] [PubMed] [Google Scholar]
- 8.Philipot D., Guérit D., Platano D., Chuchana P., Olivotto E., Espinoza F., Dorandeu A., Pers Y. M., Piette J., Borzi R. M., Jorgensen C., Noel D., Brondello J. M. (2014) p16INK4a and its regulator miR-24 link senescence and chondrocyte terminal differentiation-associated matrix remodeling in osteoarthritis. Arthritis Res. Ther. 16, R58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeon O. H., Kim C., Laberge R. M., Demaria M., Rathod S., Vasserot A. P., Chung J. W., Kim D. H., Poon Y., David N., Baker D. J., van Deursen J. M., Campisi J., Elisseeff J. H. (2017) Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat. Med. 23, 775–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu M., Bradley E. W., Weivoda M. M., Hwang S. M., Pirtskhalava T., Decklever T., Curran G. L., Ogrodnik M., Jurk D., Johnson K. O., Lowe V., Tchkonia T., Westendorf J. J., Kirkland J. L. (2017) Transplanted senescent cells induce an osteoarthritis-like condition in mice. J. Gerontol. A Biol. Sci. Med. Sci. 72, 780–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeon O. H., David N., Campisi J., Elisseeff J. H. (2018) Senescent cells and osteoarthritis: a painful connection. J. Clin. Invest. 128, 1229–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirkland J. L., Tchkonia T., Zhu Y., Niedernhofer L. J., Robbins P. D. (2017) The clinical potential of senolytic drugs. J. Am. Geriatr. Soc. 65, 2297–2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He S., Sharpless N. E. (2017) Senescence in health and disease. Cell 169, 1000–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demaria M., Ohtani N., Youssef S. A., Rodier F., Toussaint W., Mitchell J. R., Laberge R. M., Vijg J., Van Steeg H., Dollé M. E., Hoeijmakers J. H., de Bruin A., Hara E., Campisi J. (2014) An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev. Cell 31, 722–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campisi J. (2001) Cellular senescence as a tumor-suppressor mechanism. Trends Cell Biol. 11, S27–S31 [DOI] [PubMed] [Google Scholar]
- 16.Muñoz-Espín D., Serrano M. (2014) Cellular senescence: from physiology to pathology. Nat. Rev. Mol. Cell Biol. 15, 482–496 [DOI] [PubMed] [Google Scholar]
- 17.Ovadya Y., Landsberger T., Leins H., Vadai E., Gal H., Biran A., Yosef R., Sagiv A., Agrawal A., Shapira A., Windheim J., Tsoory M., Schirmbeck R., Amit I., Geiger H., Krizhanovsky V. (2018) Impaired immune surveillance accelerates accumulation of senescent cells and aging. Nat. Commun. 9, 5435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu M., Pirtskhalava T., Farr J. N., Weigand B. M., Palmer A. K., Weivoda M. M., Inman C. L., Ogrodnik M. B., Hachfeld C. M., Fraser D. G., Onken J. L., Johnson K. O., Verzosa G. C., Langhi L. G. P., Weigl M., Giorgadze N., LeBrasseur N. K., Miller J. D., Jurk D., Singh R. J., Allison D. B., Ejima K., Hubbard G. B., Ikeno Y., Cubro H., Garovic V. D., Hou X., Weroha S. J., Robbins P. D., Niedernhofer L. J., Khosla S., Tchkonia T., Kirkland J. L. (2018) Senolytics improve physical function and increase lifespan in old age. Nat. Med. 24, 1246–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharpless N. E., Sherr C. J. (2015) Forging a signature of in vivo senescence. Nat. Rev. Cancer 15, 397–408; erratum: 509 [DOI] [PubMed] [Google Scholar]
- 20.Krishnamurthy J., Torrice C., Ramsey M. R., Kovalev G. I., Al-Regaiey K., Su L., Sharpless N. E. (2004) Ink4a/Arf expression is a biomarker of aging. J. Clin. Invest. 114, 1299–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wood W. A., Krishnamurthy J., Mitin N., Torrice C., Parker J. S., Snavely A. C., Shea T. C., Serody J. S., Sharpless N. E. (2016) Chemotherapy and stem cell transplantation increase p16INK4a expression, a biomarker of T-cell aging. EBioMedicine 11, 227–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diekman B. O., Sessions G. A., Collins J. A., Knecht A. K., Strum S. L., Mitin N. K., Carlson C. S., Loeser R. F., Sharpless N. E. (2018) Expression of p16INK4a is a biomarker of chondrocyte aging but does not cause osteoarthritis. Aging Cell 17, e12771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baker D. J., Childs B. G., Durik M., Wijers M. E., Sieben C. J., Zhong J., Saltness R. A., Jeganathan K. B., Verzosa G. C., Pezeshki A., Khazaie K., Miller J. D., van Deursen J. M. (2016) Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature 530, 184–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baker D. J., Wijshake T., Tchkonia T., LeBrasseur N. K., Childs B. G., van de Sluis B., Kirkland J. L., van Deursen J. M. (2011) Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479, 232–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burd C. E., Sorrentino J. A., Clark K. S., Darr D. B., Krishnamurthy J., Deal A. M., Bardeesy N., Castrillon D. H., Beach D. H., Sharpless N. E. (2013) Monitoring tumorigenesis and senescence in vivo with a p16(INK4a)-luciferase model. Cell 152, 340–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamakoshi K., Takahashi A., Hirota F., Nakayama R., Ishimaru N., Kubo Y., Mann D. J., Ohmura M., Hirao A., Saya H., Arase S., Hayashi Y., Nakao K., Matsumoto M., Ohtani N., Hara E. (2009) Real-time in vivo imaging of p16Ink4a reveals cross talk with p53. J. Cell Biol. 186, 393–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu J. Y., Souroullas G. P., Diekman B. O., Krishnamurthy J., Hall B. M., Sorrentino J. A., Parker J. S., Sessions G. A., Gudkov A. V., Sharpless N. E. (2019) Cells exhibiting strong p16 INK4a promoter activation in vivo display features of senescence. Proc. Natl. Acad. Sci. USA 116, 2603–2611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henry S. P., Jang C. W., Deng J. M., Zhang Z., Behringer R. R., de Crombrugghe B. (2009) Generation of aggrecan-CreERT2 knockin mice for inducible Cre activity in adult cartilage. Genesis 47, 805–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Madisen L., Zwingman T. A., Sunkin S. M., Oh S. W., Zariwala H. A., Gu H., Ng L. L., Palmiter R. D., Hawrylycz M. J., Jones A. R., Lein E. S., Zeng H. (2010) A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stanton H., Golub S. B., Rogerson F. M., Last K., Little C. B., Fosang A. J. (2011) Investigating ADAMTS-mediated aggrecanolysis in mouse cartilage. Nat. Protoc. 6, 388–404 [DOI] [PubMed] [Google Scholar]
- 31.Serrano M., Lee H., Chin L., Cordon-Cardo C., Beach D., DePinho R. A. (1996) Role of the INK4a locus in tumor suppression and cell mortality. Cell 85, 27–37 [DOI] [PubMed] [Google Scholar]
- 32.Novais E. J., Diekman B. O., Shapiro I. M., Risbud M. V. (2019) p16Ink4a deletion in cells of the intervertebral disc affects their matrix homeostasis and senescence associated secretory phenotype without altering onset of senescence. [E-pub ahead of print] Matrix Biol. https://doi.org/10.1016/j.matbio.2019.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Serrano M., Lin A. W., McCurrach M. E., Beach D., Lowe S. W. (1997) Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88, 593–602 [DOI] [PubMed] [Google Scholar]
- 34.Dimri G. P., Lee X., Basile G., Acosta M., Scott G., Roskelley C., Medrano E. E., Linskens M., Rubelj I., Pereira-Smith O., Peacocke M., Campisi J. (1995) A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 92, 9363–9367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coppé J. P., Patil C. K., Rodier F., Krtolica A., Beauséjour C. M., Parrinello S., Hodgson J. G., Chin K., Desprez P. Y., Campisi J. (2010) A human-like senescence-associated secretory phenotype is conserved in mouse cells dependent on physiological oxygen. PLoS One 5, e9188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang J., Wang Y., Shao L., Laberge R. M., Demaria M., Campisi J., Janakiraman K., Sharpless N. E., Ding S., Feng W., Luo Y., Wang X., Aykin-Burns N., Krager K., Ponnappan U., Hauer-Jensen M., Meng A., Zhou D. (2016) Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat. Med. 22, 78–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu Y., Tchkonia T., Fuhrmann-Stroissnigg H., Dai H. M., Ling Y. Y., Stout M. B., Pirtskhalava T., Giorgadze N., Johnson K. O., Giles C. B., Wren J. D., Niedernhofer L. J., Robbins P. D., Kirkland J. L. (2016) Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell 15, 428–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Visel A., Zhu Y., May D., Afzal V., Gong E., Attanasio C., Blow M. J., Cohen J. C., Rubin E. M., Pennacchio L. A. (2010) Targeted deletion of the 9p21 non-coding coronary artery disease risk interval in mice. Nature 464, 409–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jeck W. R., Siebold A. P., Sharpless N. E. (2012) Review: a meta-analysis of GWAS and age-associated diseases. Aging Cell 11, 727–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Childs B. G., Baker D. J., Wijshake T., Conover C. A., Campisi J., van Deursen J. M. (2016) Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science 354, 472–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yousefzadeh M. J., Zhu Y., McGowan S. J., Angelini L., Fuhrmann-Stroissnigg H., Xu M., Ling Y. Y., Melos K. I., Pirtskhalava T., Inman C. L., McGuckian C., Wade E. A., Kato J. I., Grassi D., Wentworth M., Burd C. E., Arriaga E. A., Ladiges W. L., Tchkonia T., Kirkland J. L., Robbins P. D., Niedernhofer L. J. (2018) Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine 36, 18–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blagosklonny M. V. (2014) Geroconversion: irreversible step to cellular senescence. Cell Cycle 13, 3628–3635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dai C. Y., Enders G. H. (2000) p16 INK4a can initiate an autonomous senescence program. Oncogene 19, 1613–1622 [DOI] [PubMed] [Google Scholar]
- 44.Tang X., Muhammad H., McLean C., Miotla-Zarebska J., Fleming J., Didangelos A., Önnerfjord P., Leask A., Saklatvala J., Vincent T. L. (2018) Connective tissue growth factor contributes to joint homeostasis and osteoarthritis severity by controlling the matrix sequestration and activation of latent TGFβ. Ann. Rheum. Dis. 77, 1372–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chong K. W., Chanalaris A., Burleigh A., Jin H., Watt F. E., Saklatvala J., Vincent T. L. (2013) Fibroblast growth factor 2 drives changes in gene expression following injury to murine cartilage in vitro and in vivo. Arthritis Rheum. 65, 2346–2355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boehme K. A., Rolauffs B. (2018) Onset and progression of human osteoarthritis-can growth factors, inflammatory cytokines, or differential miRNA expression concomitantly induce proliferation, ECM degradation, and inflammation in articular cartilage? Int. J. Mol. Sci. 19, E2282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharpless N. E., Bardeesy N., Lee K. H., Carrasco D., Castrillon D. H., Aguirre A. J., Wu E. A., Horner J. W., DePinho R. A. (2001) Loss of p16Ink4a with retention of p19Arf predisposes mice to tumorigenesis. Nature 413, 86–91 [DOI] [PubMed] [Google Scholar]
- 48.Zhu Y., Tchkonia T., Pirtskhalava T., Gower A. C., Ding H., Giorgadze N., Palmer A. K., Ikeno Y., Hubbard G. B., Lenburg M., O’Hara S. P., LaRusso N. F., Miller J. D., Roos C. M., Verzosa G. C., LeBrasseur N. K., Wren J. D., Farr J. N., Khosla S., Stout M. B., McGowan S. J., Fuhrmann-Stroissnigg H., Gurkar A. U., Zhao J., Colangelo D., Dorronsoro A., Ling Y. Y., Barghouthy A. S., Navarro D. C., Sano T., Robbins P. D., Niedernhofer L. J., Kirkland J. L. (2015) The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 14, 644–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Y., Chang J., Liu X., Zhang X., Zhang S., Zhang X., Zhou D., Zheng G. (2016) Discovery of piperlongumine as a potential novel lead for the development of senolytic agents. Aging (Albany N.Y.) 8, 2915–2926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fuhrmann-Stroissnigg H., Ling Y. Y., Zhao J., McGowan S. J., Zhu Y., Brooks R. W., Grassi D., Gregg S. Q., Stripay J. L., Dorronsoro A., Corbo L., Tang P., Bukata C., Ring N., Giacca M., Li X., Tchkonia T., Kirkland J. L., Niedernhofer L. J., Robbins P. D. (2017) Identification of HSP90 inhibitors as a novel class of senolytics. Nat. Commun. 8, 422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu Y., Doornebal E. J., Pirtskhalava T., Giorgadze N., Wentworth M., Fuhrmann-Stroissnigg H., Niedernhofer L. J., Robbins P. D., Tchkonia T., Kirkland J. L. (2017) New agents that target senescent cells: the flavone, fisetin, and the BCL-XL inhibitors, A1331852 and A1155463. Aging (Albany N.Y.) 9, 955–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li W., He Y., Zhang R., Zheng G., Zhou D. (2019) The curcumin analog EF24 is a novel senolytic agent. Aging (Albany N.Y.) 11, 771–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ozsvari B., Nuttall J. R., Sotgia F., Lisanti M. P. (2018) Azithromycin and Roxithromycin define a new family of “senolytic” drugs that target senescent human fibroblasts. Aging (Albany N.Y.) 10, 3294–3307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pan J., Li D., Xu Y., Zhang J., Wang Y., Chen M., Lin S., Huang L., Chung E. J., Citrin D. E., Wang Y., Hauer-Jensen M., Zhou D., Meng A. (2017) Inhibition of Bcl-2/xl with ABT-263 selectively kills senescent type II pneumocytes and reverses persistent pulmonary fibrosis induced by ionizing radiation in mice. Int. J. Radiat. Oncol. Biol. Phys. 99, 353–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cao X., Luo P., Huang J., Liang C., He J., Wang Z., Shan D., Peng C., Wu S. (2019) Intraarticular senescent chondrocytes impair the cartilage regeneration capacity of mesenchymal stem cells. Stem Cell Res. Ther. 10, 86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ni Chonghaile T., Letai A. (2008) Mimicking the BH3 domain to kill cancer cells. Oncogene 27 (Suppl1), S149–S157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Potter D. S., Letai A. (2016) To prime, or not to prime: that is the question. Cold Spring Harb. Symp. Quant. Biol. 81, 131–140 [DOI] [PubMed] [Google Scholar]
- 58.Collins J. A., Diekman B. O., Loeser R. F. (2018) Targeting aging for disease modification in osteoarthritis. Curr. Opin. Rheumatol. 30, 101–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Diekman B. O., Collins J. A., Loeser R. F. (2018) Does joint injury make young joints old? J. Am. Acad. Orthop. Surg. 26, e455–e456 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.