Summary
Recent research has suggested a role for the intestinal microbiota in the pathogenesis and potential treatment of a wide range of liver diseases. The intestinal microbiota and bacterial products may contribute to the development of liver diseases through multiple mechanisms including increased intestinal permeability, chronic systemic inflammation, production of short-chain fatty acids and changes in metabolism. This suggests a potential role for pre-, pro- and synbiotic products in the prevention or treatment of some liver diseases. In addition, there is emerging evidence on the effects of faecal microbial transplant. Herein, we discuss the relationship between the intestinal microbiota and liver diseases, as well as reviewing intestinal microbiota-based treatment options that are currently being investigated.
Keywords: microbiome, microbiota, intestinal microbiota, liver diseases, prebiotics, probiotics, fecal transplantation, synbiotics, gut-liver axis
Key points
The intestinal microbiota and bacterial products can directly and indirectly affect the liver through various mechanisms, leading to a wide variety of liver diseases.
This suggests a potential role for pre-, pro- and synbiotic products in the prevention or treatment of some liver diseases.
There is also emerging evidence that faecal microbiota transplant may be an effective treatment for certain liver diseases.
Alt-text: Unlabelled Box
Introduction
The human intestinal microbiota (IM) is made up of bacteria, archaea and eukaryotic microorganisms and viruses.[1], [2] Currently, there are 1,000 known species of bacteria3 and approximately 1014 microorganisms.4 Two dominant phyla, Bacteroidetes and Firmicutes, comprise 90% of bacteria in the human digestive tract.[5], [6], [7] The IM plays an essential role in the digestion of food, synthesis of vitamins, metabolism, immune system function, inflammation and cell proliferation.[4], [8], [9] Recently, disturbances in the IM, or dysbiosis, have been associated with several diseases, including a wide range of hepatic disorders.[4], [9], [10], [11], [12]
Emerging evidence supports the bidirectional relationship between the IM and the liver, which results from the liver receiving 75% of its blood supply from the intestines via the portal vein13 and the liver releasing bile acids into the biliary tract.14 As a result, the IM may contribute to liver diseases through several mechanisms that can be influenced by bacterial composition, IM metabolism of bile acids, diet, environmental factors and genetics, with bacteria, bacterial products and metabolites translocating through the intestinal barrier into the portal system, and then the liver.[11], [12]
The aim of this review is to outline how the IM and liver interact with each other. We will focus on the IM’s role in the pathogenesis and treatment of liver diseases, specifically non-alcoholic fatty liver disease (NAFLD), alcohol-related liver disease (ALD), primary sclerosing cholangitis, primary biliary cholangitis, hepatocellular carcinoma (HCC) and cirrhosis. This review will focus on clinical data and interventions for each of these pathologies.
Intestinal microbiota and liver disease: Overall mechanisms
Bile acid metabolism
Synthesised from cholesterol in the liver, bile acids (BAs) are essential in cholesterol metabolism and lipid digestion.15 BAs are stored in the gallbladder and are secreted during digestion into the small intestine.16 Over 95% of BAs are reabsorbed in the terminal ileum and transported back to the liver via the portal vein. BAs promote the absorption of dietary fats, cholesterol and fat-soluble vitamins.16 In addition, BAs also function as signalling molecules that influence physiological processes,16 which include the regulation of glucose and lipid metabolism through farnesoid X receptor (FXR) activation and binding of G-protein-coupled bile acid receptor 1.[17], [18], [19] BAs can also influence the IM as it has been shown to be directly associated with intestinal mucosal integrity and synthesis of antibacterial peptides.20 When BAs bind to FXR, antimicrobial peptides, such as angiogenin 1, are produced. These peptides can inhibit IM overgrowth by increasing the intestinal epithelial cell potential to prevent bacterial uptake, improving gut-barrier function.20 In turn, the IM can influence the size and composition of the BA pool through the conversion of primary to secondary BAs.[21], [22] This may subsequently change the composition of the circulating BAs, which act as signalling molecules affecting, for example, lipid and glucose metabolism and predisposing individuals to non-alcoholic fatty liver disease (NAFLD). Therefore, both the dysbiosis of IM and/or imbalance of BAs can contribute to the pathogenesis and progression of liver diseases, which will be discussed.[21], [22]
Intestinal permeability
The intestinal epithelium plays an essential role in restricting toxins, antigens and enteric flora from entering the circulation, while selectively permitting the absorption of nutrients across the tight junctions.23 The intestinal barrier is comprised of enterocytes that are bound to each other by transmembrane proteins including desmosomes, adherens junctions and tight junctions.23 The intestinal barrier is also strengthened by immunoglobulins, mucins and commensal bacteria.23 The IM can alter the intestinal barrier by altering tight junctions, degrading the mucus layer or inhibiting the production of mucus, which subsequently increases the permeability of the epithelium.23 One way in which the IM is associated with increased tight junction permeability is through the presence of luminal endotoxins.23 Endotoxins found on the outer membrane of gram-negative bacteria increase tight junction permeability by increasing toll-like receptor (TLR)4 expression.24 Widening of the tight junctions leads to increased intestinal permeability, resulting in increased translocation of bacterial fragments and endotoxins into the portal circulation and subsequently the liver.25 This in turn can cause systemic and hepatic inflammation and hepatic injury.25 Bacterial fragments and products can also recruit and activate hepatic immune cells, further contributing to liver disease progression.25
Chronic inflammation
The IM contributes to chronic inflammation not only through the production of endotoxins but also through cytokines and inflammasome dysfunction. Translocation of IM-derived endotoxins into the circulatory system increases TLR4 expression, which activates the proinflammatory cytokines tumour necrosis factor-alpha (TNF-a) and interleukin (IL)-6,26 thus triggering systemic inflammation. Inflammasomes, which consist of leucine-rich-repeat containing proteins and nucleotide-binding domains, govern the cleavage of proinflammatory cytokines. Dysbiosis has been shown to be associated with inflammasome deficiency, specifically NLRP 3 and 6, resulting in the increased expression of TNF-a.27 The increased activation and production of TLR4 and proinflammatory cytokines from dysbiosis can also lead to the recruitment and activation of hepatic immune cells, contributing to liver disease progression.27
Immune system activation
The recruitment and activation of hepatic immune cells can be caused either by local signals or signals from sources such as the IM.25 The immune system is divided into the innate and adaptive immune systems. The innate immune system defends against microorganisms and toxins, whereas the adaptive immune system is antigen specific and requires self-non-self-recognition.28 Kupffer cells (KCs) are critical components of the innate immune system, residing within the sinusoidal vascular space.29 KCs can be activated by various endogenous and exogenous stimuli including endotoxins.29 Activation of KCs triggers the production of inflammatory cytokines, such as TNF-α, as well as reactive oxygen species (ROS)29 which can produce tissue damage. These cytokines can also play a key role in regulating the phenotype and function of neighbouring parenchymal and non-parenchymal cells.29 For instance, cytokines have been shown to polarise and activate the proinflammatory M1 phenotype in KCs.30
Natural killer (NK) and natural killer T (NKT) cells may also play a role in the pathogenesis of liver diseases and can be affected by the IM. Recent murine studies have shown that IM-derived antigens could influence the composition and activation of hepatic NKT cells.[31], [32] NK cells in the liver play a role in linking the innate and adaptive immune response.33 Activated NK cells were found to have anti-fibrotic effects, by releasing interferon-γ (IFN-γ) which induces hepatic stellate cell (HSC) cycle arrest and apoptosis.34 However, IFN-γ also results in hepatocyte apoptosis and thus causes hepatic injury.34 NKT cells, which can be expressed by hepatocytes and antigen presenting cells, share properties of both T cells and NK cells.35 NKT cells can secrete cytokines and therefore play a critical role in directing the immune system.35 They are able to do this through their ability to produce T helper 1 cells, which are proinflammatory, and T helper 2 cells, which are anti-inflammatory.35 Overall, the activation of hepatic immune cells by the IM could contribute to the pathogenesis of several liver diseases.
Short-chain fatty acids
Another mechanism by which the IM can contribute to liver disease is through the production of short-chain fatty acids (SCFAs). The IM breaks down non-digestible carbohydrates releasing SCFAs in the human gut.36 The primary SCFAs are acetate, propionate and butyrate, which are metabolised by the muscle, liver and epithelium, respectively.36 Research has predominately focussed on butyrate, a primary source of energy for colonocytes, which improves colonic barrier function36 and therefore positively impacts on intestinal permeability. Butyrate has been shown to improve the gut barrier by induction of tight junction proteins and mucins, specifically Mucin 2[37], [38], [39] and enhanced expression of claudin-1.40 In the liver, butyrate can induce apoptosis and can inhibit cell proliferation in hepatic cells by suppressing sirtuin1 expression while upregulating miR-22 expression.41 Therefore, butyrate can also inhibit hepatic cancer cells. Butyrate has also been shown to increase satiety, decrease food intake and delay gastric emptying through activation of free fatty acid receptor 3.42 Free fatty acid receptor 3 upregulates the production of gut hormones peptide YY and glucagon-like peptide-1.42 Therefore, the IM can affect the metabolism, including diet-induced obesity. Finally, butyrate can also impact on inflammation. In the intestinal tract, studies have found that butyrate binds and activates peroxisome proliferator-activated receptor gamma (PPAR-y), which antagonises nuclear factor-kappa B (NF-kB) transduction, thus causing an anti-inflammatory effect.43 Therefore, the presence and/or abundance of butyrate produced by the IM could impact on the pathogenesis of liver diseases through several mechanisms.
Choline
Choline is an essential nutrient and a phospholipid component of the cell membrane, which can be metabolised by the IM. There are several mechanisms through which choline deficiency may impact the liver, including44 decreased very-low density lipoprotein (VLDL) formation, mitochondrial dysfunction and endoplasmic reticulum stress.[44], [45] Phosphatidylcholine, which is a phospholipid that contains choline in the headgroup, is a key component of the VLDL envelope. Choline deficiency, either due to diet or as a result of IM metabolism, leads to a decrease in VLDL formation and triglyceride export from the liver, resulting in the development of a fatty liver. Choline is also an essential component of the mitochondrial membrane.44 Choline deficiency decreases the mitochondrial membrane concentrations of phosphatidylethanolamine and phosphatidylcholine, resulting in decreased membrane potential, which, in turn, causes oxidative damage.44
The IM may contribute to decreased choline bioavailability46 by metabolising dietary choline found in eggs, milk and red meat into trimethylamine (TMA).47 This increases the production of TMA, which is absorbed into the blood, and has been associated with an increased risk of cardiovascular disease.47 In addition, once TMA reaches the liver it is further metabolised by flavin-containing monooxygenases 1 and 3 to generate trimethylamine-N-oxide (TMAO).[47], [48], [49] This may lead to increased hepatic triglyceride accumulation as TMAO effects BA pool size by decreasing BA synthesis through the inhibition of key enzymes and by limiting the enterohepatic circulation of BAs through repression of the organic anion transporter and multidrug resistance family protein expression.[50], [51], [52] Therefore, it is possible that choline deficiency, either through the diet or the conversion of IM to TMA, may cause fat to accumulate in the liver.
Ethanol
Ethanol, which comes primarily from food and beverages, is absorbed through the mucosa of the gastrointestinal tract.53 However, ethanol can also be produced and metabolised by the IM in the absence of alcohol consumption.54 Ethanol is formed from Escherichia coli and under anaerobic conditions during the fermentation of carbohydrates, E. coli can metabolise pyruvic acid to generate acetaldehyde, which can be reduced to ethanol.55 Acetaldehyde has been shown to decrease the gut barrier function by weakening tight junctions and therefore facilitates the translocation of microbial products into the systemic circulation.[56], [57] Furthermore, studies have shown that acetaldehyde can stimulate an inflammatory and adaptive immune response by downregulating antimicrobial peptide expression in the intestine,[58], [59], [60], [61] thus leading to further hepatic injury.
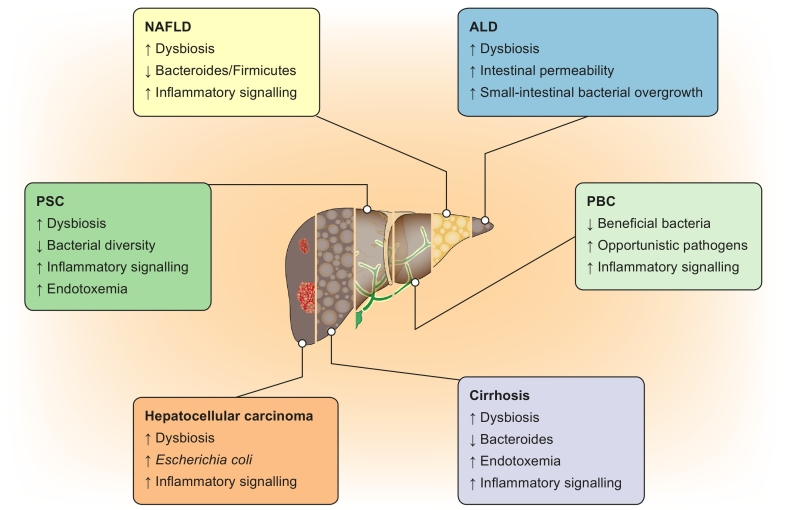
Taken together, the IM and bacterial products can directly and indirectly affect the liver through various mechanisms (Fig. 1), leading to a wide variety of liver diseases (Fig. 2).
Fig. 1.
Mechanisms in which the intestinal microbiota can affect the liver. ROS, reactive oxygen species; VLDL, very-low density lipoprotein.
Fig. 2.
The role of intestinal microbiota in liver disease.
ALD, alcohol-related liver disease; NAFLD, non-alcoholic fatty liver disease; PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis.
Gut bacteria and specific liver diseases
Non-alcoholic fatty liver disease
NAFLD is one of the most common causes of liver disease worldwide, affecting 15–30% of the general population.[62], [63], [64], [65] NAFLD ranges from simple fat deposition in the liver (steatosis) to inflammation (non-alcoholic steatohepatitis or NASH) to fibrosis and cirrhosis.66 Research studies have shown that altered IM composition, so-called “dysbiosis”, contributes to the pathogenesis of NAFLD,[52], [67], [68], [69] however causality has yet to be proven.
Despite a large number of preclinical data investigating and demonstrating a relationship between dysbiosis and NAFLD, only a limited number of human studies, mostly cross-sectional, have investigated the role of the IM in NAFLD, with variable results. In adults, patients with NASH were found to have lower amounts of Bacteroidetes, independent of body mass index and diet.67 Studies have shown that there are differences in the IM between patients with NAFLD and healthy controls.[68], [69], [70], [71] One study found that NAFLD severity is associated with IM dysbiosis and shifts in the metabolic function of the IM.72 Specifically, they found that the abundance of Bacteroides was independently associated with NASH and Ruminococcus with fibrosis.72 More recently, another cross-sectional study found that those with NAFLD had significantly decreased Bacteroidetes and Firmicutes, along with increased Lactobacillus compared with healthy controls, while those with NASH had decreased Ruminococcus, Faecalibacterium prausnitzii and Coprococcus compared to healthy controls, independently of body mass index and insulin resistance.73 In paediatrics, results showed an increased abundance of E. coli in patients with NASH compared to healthy controls, which was associated with higher blood alcohol levels.69 One intervention study, which included 15 adult women placed on a choline-deficient diet found that pre-diet microbiota composition, specifically a lower abundance of Gammaproteobacteria or a higher abundance of Erysipelotrichi increased vulnerability to the development of a fatty liver during choline depletion.52 Furthermore, they found that host genotypes (single nucleotide polymorphism in the PEMT gene) and specific IM can predict choline deficiency-induced fatty liver (assessed by magnetic resonance imaging).52 One study assessed faecal ester volatile organic compounds and found that specific patterns were associated with differences in the IM when patients with NAFLD, diagnosed on ultrasound, were compared to controls.68 Recently, a study investigating the relationship between the IM and immune function in NAFLD found that specific immune cells in the portal or lobular areas correlated with specific faecal IM. Specifically, Faecalibacterium prausnitzii was negatively correlated with CD45+ and CD163+ cells in the portal tract and Prevotella was negatively correlated with CD20+ cells in the liver lobule.74 Taken together, several studies showed associations between the IM or bacterial products and NAFLD.
Alcohol-related liver disease
ALD occurs in patients who chronically abuse alcohol. Like NAFLD, non-progressive ALD is characterised by fat accumulation in the liver, whereas progressive ALD (alcoholic steatohepatitis) exhibits hepatic inflammation.75 Recently, research has investigated the role of the IM in ALD, specifically focussing on how alcohol can cause microbiota-related dysbiosis which in turn, may contribute to the pathogenesis of ALD.
Many studies at the preclinical and experimental level have shed light on the relationship between ALD and dysbiosis. Through these many studies, multiple pathogens, toxic components and pathways have been shown to participate in the development of ALD. The amount of clinical data is unfortunately not as extensive.
Studies including both mouse models and human participants found that alcohol consumption provokes a change in the IM leading to dysbiosis.76 Specifically, patients with ALD have lower levels of Bifidobacterium, Enterobacterium and Lactobacillus spp.,[77], [78], [79], [80], [81] while cirrhotic patients with ALD exhibit a significant reduction in Bacteroidetes and Firmicutes phyla.[78], [82], [83], [84] On the other hand, the Proteobacteria, Fusobacteria and Actinobacteria phyla were increased.82 Other studies using faecal samples from alcoholic patients showed a reduction in the Lactobacillus spp.,85 whereas cirrhotic patients were shown to have lower faecal amounts of Bifidobacterium spp..[85], [86], [87] When comparing IM of alcoholics with liver cirrhosis to alcoholics without cirrhosis, it was found that the IM of those with cirrhosis contained more Enterobactericeae.[78], [79] Based on some of these findings, the term cirrhosis dysbiosis ratio (CDR) was suggested,88 representing the ratio of autochthonous or beneficial bacteria to potentially pathogenic bacteria,89 with a low ratio correlating with a more advanced disease state. Compared to other aetiologies of cirrhosis, ALD had the lowest ratio.88
Studies have also demonstrated an increase in the overall number of organisms in the small bowel of alcoholic patients.[90], [91], [92] An evaluation of chronic alcoholics compared to patients without a history of alcohol abuse, using the hydrogen breath test, showed a significantly higher prevalence of small-intestinal bacteria overgrowth (SIBO) in alcoholics compared to controls. However, no differences were found between alcoholic patients with liver cirrhosis and those without liver cirrhosis.90 The presence of SIBO has been shown to significantly correlate with a higher prevalence of spontaneous bacterial peritonitis and with the severity of alcoholic cirrhosis.92 These changes in the IM of alcoholic patients seem to be accompanied by changes in colonic pH and liver steatosis.77 It also correlates with a higher level of serum endotoxin and increased intestinal TNF-α levels, as well as increased levels of nitric oxide, IL-6, and IL-8.[93], [94], [95] Other studies also found higher levels of bacterial products in the blood of alcoholic patients compared to healthy controls.[88], [96] Additionally, endotoxemia after acute alcohol intoxication has been shown to correlate with haemodynamic derangement in cirrhotic portal hypertension.[94], [97], [98] These findings suggest a potential link between dysbiosis and ALD, with alcohol promoting dysbiosis and leading to increased gut barrier permeability, consequently causing translocation of IM and endotoxins into the portal circulation, and eventually the liver. This, in turn, triggers hepatic inflammation and liver damage, particularly through the interaction between lipopolysaccharides (LPS) and TLRs.99
Primary sclerosing cholangitis
Primary sclerosing cholangitis (PSC) is characterised by inflammation and scarring of the bile ducts.100 The few studies investigating the IM in PSC have shown an overall reduction in IM diversity, however, there are inconsistencies in these findings at the genus and species level100
The evaluation of the IM in patients with PSC and PSC-inflammatory bowel disease (IBD) demonstrated low bacterial diversity,101 and an overrepresentation of Rothia, Enterococcus, Streptococcus, Clostridium, Veillonella , Haemophilus, Fusobacterium and Lactobacillus genera regardless of concomitant IBD.[101], [102] Another study confirmed that Veillonella abundance was markedly increased in PSC compared to healthy controls.103 Studies looking at intestinal biopsies found that the overall microbiota profile of those with PSC was characterised by enrichment of Barnesiellaceae and a reduction in Clostridiales.[104], [105]
According to the aforementioned studies, these changes lead to IM dysbiosis and are associated with the pathogenesis of PSC by inducing bacterobilia, which in turn activates a proinflammatory pathway in the cholangiocytes leading to fibrosis and inflammation. Bacterobilia may also play a role in molecular mimicry, through endotoxemia, leading to the creation of antibodies and causing immune-mediated biliary damage.[106], [107]
Primary biliary cholangitis
Primary biliary cholangitis (PBC) is a disease that results in the progressive destruction of the bile ducts within the liver.108 In a cross-sectional study comparing urodeoxycholic acid (UDCA) treatment-naïve patients with PBC and healthy controls, dysbiosis was observed in PBC and was partially reversed by UDCA. Bacteroidetes spp. were significantly decreased and Fusobacteria, Haemophilus, Veillonella, Clostridium, Lactobacillus, Streptococcus, Pseudomonas, Klebsiella, an unknown genus in the family of Enterobacteriaceae and Proteobacteria spp. were over-represented in comparison to healthy controls.108 Another study comparing patients with PBC to healthy controls found that patients with PBC had depleted levels of potentially beneficial gut bacteria, including Acidobacteria, Lachnobacterium spp., Bacteroides eggerthii and Ruminococcus bromii, but higher levels of bacterial taxa containing opportunistic pathogens, such as y-Proteobacteria, Enterobacteriaceae, Neisseriaceae, Spirochaetaceae, Veillonella, Streptococcus, Klebsiella, Actinobacillus pleuropneumoniae, Anaeroglobus geminatus, Enterobacter asburiae, Haemophilus parainfluenzae, Megasphaera micronuciformis and Paraprevotella clara.109 They also found that this PBC-related alteration in the IM was associated with increased liver injury indicators and serum inflammatory cytokines, thus suggesting that the altered IM may be involved in the onset or development of PBC.109
The potential IM-related mechanisms behind the progression of liver disease are similar to the aforementioned mechanisms previously described for PSC.107
Cirrhosis and hepatic encephalopathy
Cirrhosis is considered end-stage liver disease that is characterised by severe fibrosis and a loss of liver cells. Each of the aforementioned liver diseases can result in a cirrhotic liver.110 Research has found that patients with cirrhosis have lower levels of Bacteroidetes and higher levels of Proteobacteria, Enterococcus, Veillonella, Megasphaera, Burkholderia, Prevotella and Fusobacteria.[81], [111] In addition, cirrhotic patients also have lower levels of autochthonous taxa such as Blautia, Roseburia, Faecalibacerium, Dorea, Lachnospiraceae and Ruminococcaceae.[81], [111] When analysing the duodenal mucosal microbiota of 30 cirrhotic patients, Chen et al. found that cirrhotic patients’ colonisation was significantly different than that of 28 healthy controls.111 There seemed to be an overrepresentation of Veillonella, Megasphaera, Dialister, Atopobium, and Prevotella in cirrhotic patients compared to controls. Veillonella, Prevotella, Neisseria, and Haemophilus, were the taxa best able to discriminate between those with cirrhosis and healthy controls. Other studies have demonstrated higher levels of buccal-derived microbiota in the stool samples of patients with cirrhosis, as well as a significantly altered salivary microbiome in cirrhotic patients.[112], [113] This could suggest that the oral microbiota has a great impact upon duodenal and possibly intestinal microbiota in this population. Another author even mentioned the possibility that the duodenal microbiota might directly contribute to hepatic encephalopathy in cirrhosis.111
Hepatic encephalopathy, which is defined as cognitive impairment, occurs as a result of severe liver disease. Studies have found that there is a link between hepatic encephalopathy and by-products of the IM, specifically endotoxemia.114 One study compared the IM in patients with hepatic encephalopathy to other cirrhotic patients and healthy controls and found that those with hepatic encephalopathy had higher levels of Alcaligenaceae, Enterobacteriaceae and Fusobacteriaceae along with lower Ruminococcaceae and Lachnospiraceae.114 Of those, Alcaligenaceae and Porphyromonadaceae were positively correlated with cognitive impairment whereas Prevotella was linked to improvement of cognition and decreased inflammation.114 Their study also showed a higher concentration of Veillonellaceae, endotoxemia and inflammation in patients with hepatic encephalopathy.114 Another study demonstrated that the composition of the IM could predict decompensation and hospitalisation of cirrhotic patients.88 Higher serum endotoxin levels, lower CDR and increased pathogenic taxa were significantly linked to death secondary to multi-organ failure when compared to patients who survived. In the same study the salivary microbiome was shown to independently correlate with liver-related 90-day hospitalisation regardless of the model for end-stage liver disease (MELD) score or the status of hepatic encephalopathy.88
Several mechanisms have been suggested for the association between IM and cirrhosis that include increased small bowel permeability and decreased small bowel motility, leading to small bowel overgrowth. This in turn leads to translocation of the IM and endotoxins into the portal circulation, activation of inflammatory pathways in HSCs through TLR4 and subsequently the development of fibrosis.115 For HE, ammonia plays a central role in the development of the disease. Some studies have shown that in patients with cirrhosis, in addition to bacterial translocation and activation of proinflammatory cytokines, there is an increased quantity of urease active bacteria, which would lead to increased production of ammonia in the small bowel and increased levels in the portal blood.[115], [116]
Hepatocellular carcinoma
HCC can be a complication of many liver diseases. Dysbiosis may contribute to HCC pathogenesis by increasing steatosis, oxidative stress and inflammation.14
Changes in the microbiota have been suspected of playing a role in carcinogenesis. One study by Grat et al. investigated the IM of 15 patients with HCC undergoing liver transplantation and compared them to 15 patients who did not have HCC but had a similar aetiology of cirrhosis and a similar MELD stage. The study showed that the presence of HCC was significantly associated with increased faecal Escherichia coli.117 Another study evaluated liver tissue samples in patients with HCC and found the presence of Helicobacter spp., suggesting intestinal translocation as a potential mechanism for carcinogenesis.118 However, Helicobacter could not be found in patients with viral-induced HCC.118
Mechanistically, murine studies suggested that the IM can contribute to HCC pathogenesis through its interaction with the TLRs, particularly TLR4. However, more clinical research is needed to further characterise the causal role of the IM in the pathogenesis of HCC.119
Limitations to IM and liver disease studies
Research in the area of IM and liver diseases is rapidly evolving, but there are several limitations to consider when interpreting this association. First of all, differences in genetics120 and environmental factors such as diet,121 alcohol86 and medication/antibiotic use122 have been shown to contribute to variations in IM. Additionally, the use of different liver diagnostic tools that are used for primary endpoints in clinical trials is another limitation. Some studies will use a liver biopsy,67 the gold standard for diagnostics, while others use non-invasive and less reliable tools such as imaging or blood tests,68 which could explain the differences seen in clinical research. Another limitation in human IM studies is how the stool sample is collected. Although similar IM phyla predominate across the stomach, small intestine and colon, there are variations in IM composition and abundance.123 The majority of human studies analyse stool samples, however 1 study found differences in the IM when comparing stool samples to caecal luminal contents,124 therefore limiting the generalisability of the results. Furthermore, there are variations in the sequencing methods used, which all produce different results. These include quantitative PCR,67 16S rRNA sequencing72 or shotgun sequencing.112 Additionally, differences in bioinformatic analysis platforms, such as QIIME,125 Mothur126 and PICRUST,127 can contribute to variations in results. Overall, it is important to consider these limitations when analysing IM and liver disease research and they should be considered when designing future studies.
Future directions
Based on the above studies, there is likely an association between the IM and liver disease, but a causal relationship has yet to be confirmed. Several studies looking at the effect of IM manipulation by pre-, pro- and synbiotics, as well as faecal microbiota transplant (FMT), suggest that the IM has a role in liver diseases.
Pre- and probiotic treatment
Several studies have investigated the role of pre- and probiotic treatment in patients with liver disease. A summary of these studies can be found in Table 1. However, no studies have been carried out in patients with PSC or PBC.
Table 1.
Summary of pre-, pro- and synbiotic liver disease studies.
| Disease | Treatment | Study design | Outcome | Reference |
|---|---|---|---|---|
| NAFLD | VSL#3 (combination of 8 probiotic strains) or placebo for 4-months | RCT n = 48 children |
VSL#3 improved NAFLD | 128 |
| NAFLD | Multi-probiotic product or placebo | RCT n = 58 adults | Probiotic resulted in reductions of AST, GGT, TNF-a and IL-6 | 129 |
| NAFLD | Synbiotic or placebo | RCT n = 50 | Synbiotic group had significant decrease in AST, total cholesterol, triacylglycerol and steatosis (based on Fibroscan) | 130 |
| ALD | Bifidobacterium bifidum and Pacobacillus plantarum daily for 5 days | Open-label randomised n = 66 |
Reductions in AST and ALT | 85 |
| ALD | Bifidobacterium bifidum, Bifidobacterium lactis, Bifidobacterium ongum, Lactobacillus acidophilus, Lactobacillus rhamnosus GG, Streptococcus thermophiles 5x109 1 capsule twice daily for 28 days or placebo | Double-blind RCT n = 50 |
Decrease in SIBO, however no difference in intestinal permeability | 131 |
| ALD/HE | VSL#3 or placebo | RCTl n = 130 | No difference in incidence of encephalopathy or mortality. However, reductions in Child-Pugh, MELD, plasma TNF-a, IL-1B and IL-6 seen | 132 |
| ALD/Cirrhosis | Lactobacillus subtilis and Streptococcus faecium (daily for 7 days) or placebo | Double-blind RCT n = 117 |
Decrease in TNF-a and an increase in albumin levels. Stabilisation of LPS levels in cirrhotic patients | 133 |
| ALD/Cirrhosis | Lactobacillus casei Shirota (6.5x109 ) three times a day for 4 weeks | Open-label n = 12 |
Normalised neutrophil phagocytic capacity. No improvement in disease control and no change on TNF-a and IL10 | 134 |
| ALD/Cirrhosis | VSL#3 1 sachet daily for 60 days or placebo | Double-blind RCT n = 63 |
Reduction of hepatovenous pressure gradient and TNF-a |
135 |
| ALD/Cirrhosis | VSL#3 2 sachets twice a day for 60 days | Open pilot study n = 8 | Trending reduction of endotoxin levels and TNF-a | 136 |
| ALD/Cirrhosis | VSL #3 2 sachets twice daily for 60 days or placebo | Double-blind RCT n = 11 ALD and n = 15 cirrhosis |
No impact on IM, endotoxins, liver function, hepatovenous pressure. Reduction in plasma aldosterone. | 137 |
| Cirrhosis/HE | Lactobacillus rhamnosus GG twice daily for 8 weeks or placebo | RCT n = 30 | No change in cognition. However, decrease in endotoxemia and TNF-a. |
138 |
| Cirrhosis/HE | VSL#3 1 capsule three times a day for 3 months or placebo | RCT n = 86 | Reduction of ammonia, SIBO and OCTT. Increased psychometric HE scores and CFF threshold. Significantly less patients developed overt HE. | 139 |
| Cirrhosis | Escherichia coli Nissle for 42 days | Double-blind RCT n = 39 | Improvement in intestinal colonisation. Lowering of endotoxemia and Improvement of liver function/Child-Pugh score. |
140 |
| Cirrhosis/HE | Lactulose and lactitol | Cochrane review of randomised control trials n = 1828 | Beneficial effect of non-absorbable disaccharides on mortality, HE, reduction of serious adverse events associated with liver disease (liver failure, hepatorenal syndrome, variceal bleed) | 141 |
| Cirrhosis/HE | Bifidobacterium longum plus fructo-oligosaccharides for 90 days or placebo | Double-blind RCT n = 60 | Decrease in ammonium (NH4) and performance on Trial Making Test A and B. Significant improvement of symbol digit modalities test, block design and MMSE |
142 |
| Cirrhosis/HE | Synbiotic treatment daily for 30 days or placebo | RCT n = 55 | Increase in faecal Lactobacillus. Reduction in endotoxemia, blood ammonia and reversal of minimal HE in 50%. Improvement of Child-Pugh class in 50%. | 143 |
ALD, alcohol-related liver disease; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CFF, critical flicker frequency; GGT, gamma-glutamyl transferase; HE, hepatic encephalopathy; IL-, interleukin-; IM, intestinal microbiota; LPS, lipopolysaccharide; MELD, model for end-stage liver disease; MMSE, mini-mental state examination; NAFLD, non-alcoholic fatty liver disease; OCTT, orocecal transit time; RCT, randomised controlled trial; SIBO, small intestine bacteria overgrowth; TNF-a, tumour necrosis factor-alpha.
Overall, pre-, pro- and synbiotics seem to improve various liver parameters in patients with NAFLD, ALD, cirrhosis or HE, supporting a role for the IM in liver disease pathology. However, interventions vary in terms of the product type and amount used and most of the studies have small sample sizes. Therefore, more research is needed with larger randomised controlled trials before any recommendations can be made. Answers may come from the clinical trials currently being conducted; for studies currently recruiting see Table 2.
Table 2.
Ongoing pre-, pro- and synbiotic trials.
| Type of liver disease | Type of pre-, pro or synbiotic | Study design | Primary outcome | Location | ClinicalTrial.gov ID |
|---|---|---|---|---|---|
| NAFLD | 2x probiotic/day: Lactobacillus acidophilus 1x109 CFU + Bifidobacterium lactis 1x109 CFU + Lactobacillus rhamnosus 1x109 CFU + Lactobacillus paracasei 1x109 CFU | RCT; n = 46 adults | Change in fibrosis by hepatic elastography | Brazil | NCT03467282 |
| NAFLD | 1x probiotic/day: Lactobacillus acidophilus 109, B. lactis 109 | RCT; n = 58 adults | Hepatic changes based on FIBROMAX test | Brazil | NCT02764047 |
| NAFLD | Synbiotic: Fructo-oligosaccharide with a degree of polymerisation ≪10 at 4 g/twice a day plus Bifidobacterium animalis subsp. lactis BB-12 as minimum of 10 billion CFU/day (1 capsule a day). | RCT; n = 100 adults | Change in liver fat by MRS | United Kingdom | NCT01680640 |
| NAFLD | 2x prebiotic/day: oligofructose-enriched inulin (Synergy1) | RCT; n = 60 adults | Change in liver fat by MRI, change in liver fibrosis by FibroScan, change in liver injury by Fibrotest Score | Canada | NCT02568605 |
| NAFLD | Prebiotic 16 g/day: inulin and oligofructose | RCT; n = 60 adults | Change in liver fat by MRS, biochemistry | Israel | NCT02642172 |
| Acute alcoholic hepatitis | 1x probiotic/day: Lactobacillus rhamnosus GG | RCT; n = 130 adults | MELD Score | United States of America | NCT01922895 |
CFU, colony forming units; MRS, magnetic resonance spectroscopy; MRI, magnetic resonance imaging; NAFLD, non-alcoholic fatty liver disease; RCT, randomised controlled trial.
Faecal microbiota transplantation
FMT has recently become a standard of care for treating antibiotic-resistant Clostridium difficile.[144], [145] As a result, FMT is becoming frequently investigated as a potential therapeutic option for a variety of diseases, including those that are liver related. As previously stated, the liver-gut axis plays an essential role in the pathogenesis of liver disease, with recent research suggesting that FMT could be beneficial. A pilot study, investigating the effects of FMT in 8 male patients with severe alcoholic hepatitis compared to historical controls found that there were marked improvements in liver disease within 1 week. This included the resolution of ascites and hepatic encephalopathy in the majority of patients. They also saw significantly improved 1-year survival rates compared to matched controls receiving standard of care (87.5% vs. 33.3%).146 A recent clinical trial investigating whether FMT improves hepatic encephalopathy compared to standard of care in male patients with cirrhosis and recurrent hepatic encephalopathy, found that those receiving FMT had reduced hospitalisation rates and improved cognition and dysbiosis. Furthermore, in the 5 months following the FMT procedure, no FMT recipients’ developed hepatic encephalopathy, whereas 5 of those receiving standard of care did.147 Relevant to NAFLD, 1 pilot study reported that FMT significantly reduced insulin resistance associated with changes in the IM.148 Again, more clinical trials are needed to fully investigate the beneficial effects of FMT on specific liver diseases, several of which are underway (Table 3).
Table 3.
Current faecal microbiota transplantation trials.
| Type of liver disease | Type of faecal microbiota transplantation | Study design | Primary outcome | Location |
|---|---|---|---|---|
| NASH | Frozen faecal material from lean healthy donors infused into the duodenum by endoscopy | Open label; n = 5 adults | Change in liver fat by MRI | United States of America |
| NASH-related cirrhosis | Recipient will receive healthy donor faecal samples through a naso-gastric tube, 100 ml once a month for 5 months. | RCT; n = 60 adults | Reduction in hepatic venous pressure gradient | India |
| Alcoholic hepatitis | Healthy donor FMT administered by naso-gastric tube for 7 days | RCT; n = 130 adults | Proportion of participants with Overall Survival at 3 months | India |
| Cirrhosis | FMT by endoscope and/or enema | RCT; n = 60 adults | Number of adverse events complication rate | China |
| Cirrhosis | FMT (200 ml) from donated healthy samples will be administered into the duodenum via a gastroscope | RCT; n = 32 adults | Assessment of the feasibility of FMT and assessment of the incidence of treatment-emergent adverse events | United Kingdom |
| Cirrhosis | One-dose of 90 ml of FMT enema from healthy donor stool sample | RCT; n = 20 adults | Proportion of participants with a related serious adverse event, with newly acquired transmissible infectious diseases and related adverse event | United States of America |
| Hepatic encephalopathy | Single-arm open-label healthy donor FMT administered at Week 0 by colonoscopy and at Weeks 1-4 by enema | Open label; n = 10 adults | Time to hepatic encephalopathy breakthrough | Canada |
| Hepatic encephalopathy | Single-centre open-label trial of RBX2660 (microbiota suspension). Healthy donor FMT administered at Week 0 by colonoscopy and at Weeks 1-4 by enema | Open label; n = 30 adults | Time to hepatic encephalopathy breakthrough | Canada |
| Hepatic encephalopathy | Subjects will receive 15 oral capsules of FMT on days 1, 2, 7, 14, and 21. FMT prepared from healthy donors. |
RCT; n = 30 adults | Psychometric Hepatic Encephalopathy Score | United States of America |
| Acute liver failure | FMT administered by enema | RCT; n = 40 adults | Survival | India |
FMT, faecal microbiota transplantation; MRI, magnetic resonance imaging; NAFLD, non-alcoholic fatty liver disease; RCT, randomised controlled trial.
Conclusion
There is evidence of associations between dysbiosis and liver disease, particularly as it relates to NAFLD, ALD, cirrhosis and hepatic encephalopathy. Specifically, molecules produced by the IM such as endotoxin and proinflammatory cytokines play a role in the pathogenesis of liver diseases. Furthermore, the IM can be influenced by several environmental factors, particularly diet and alcohol in the case of NAFLD and ALD Other than dietary changes or alcohol abstinence, manipulations of the IM by various interventions show promise. The majority of studies investigate the use of pre-, pro- and synbiotics in NAFLD, ALD and cirrhosis/HE and have found that these products improved clinical and biochemical markers of liver disease, however studies in patients with PSC and PBC are lacking. In conclusion, even though these studies show promise, more clinical research is required, particularly larger randomised controlled trials to bridge the gap between experimental/preclinical data and the small amount of human data on the subject.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https: https://doi.org/10.1016/j.jhepr.2019.04.004.
Supplementary data
Supplementary material
References
- 1.Marchesi JR, Ravel J. The vocabulary of microbiome research: a proposal. Microbiome. 2015;3:31. doi: 10.1186/s40168-015-0094-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turnbaugh PJ, Gordon JI. The core gut microbiome, energy balance and obesity. J Physiol. 2009;587:4153–4158. doi: 10.1113/jphysiol.2009.174136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355–1359. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dore J, Simren M, Buttle L, Guarner F. Hot topics in gut microbiota. United European Gastroenterol J. 2013;1:311–318. doi: 10.1177/2050640613502477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eckburg PB, Relman DA. The role of microbes in Crohn's disease. Clin Infect Dis. 2007;44:256–262. doi: 10.1086/510385. [DOI] [PubMed] [Google Scholar]
- 6.Gosalbes MJ, Abellan JJ, Durban A, Perez-Cobas AE, Latorre A, Moya A. Metagenomics of human microbiome: beyond 16s rDNA. Clin Microbiol Infect. 2012;18:47–49. doi: 10.1111/j.1469-0691.2012.03865.x. [DOI] [PubMed] [Google Scholar]
- 7.Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR. Enterotypes of the human gut microbiome. Nature. 2011;473:174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blaut M, Clavel T. Metabolic diversity of the intestinal microbiota: implications for health and disease. J Nutr. 2007;137:751S–755S. doi: 10.1093/jn/137.3.751S. [DOI] [PubMed] [Google Scholar]
- 9.Simren M, Barbara G, Flint HJ, Spiegel BM, Spiller RC, Vanner S. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut. 2013;62:159–176. doi: 10.1136/gutjnl-2012-302167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmed I, Roy BC, Khan SA, Septer S, Umar S. Microbiome, Metabolome and Inflammatory Bowel Disease. Microorganisms. 2016;4 doi: 10.3390/microorganisms4020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cani PD, Delzenne NM. The role of the gut microbiota in energy metabolism and metabolic disease. Curr Pharm Des. 2009;15:1546–1558. doi: 10.2174/138161209788168164. [DOI] [PubMed] [Google Scholar]
- 12.Duseja A, Chawla YK. Obesity and NAFLD: The role of bacteria and microbiota. Clin Liver Dis. 2014;18:59–71. doi: 10.1016/j.cld.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Henao-Mejia J, Elinav E, Thaiss CA, Flavell RA. The intestinal microbiota in chronic liver disease. Adv Immunol. 2013;117:73–97. doi: 10.1016/B978-0-12-410524-9.00003-7. [DOI] [PubMed] [Google Scholar]
- 14.Tripathi A, Debelius J, Brenner DA, Karin M, Loomba R, Schnabl B. The gut-liver axis and the intersection with the microbiome. Nat Rev Gastroenterol Hepatol. 2018;15:397–411. doi: 10.1038/s41575-018-0011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Staley C, Weingarden AR, Khoruts A, Sadowsky MJ. Interaction of gut microbiota with bile acid metabolism and its influence on disease states. Appl Microbiol Biotechnol. 2017;101:47–64. doi: 10.1007/s00253-016-8006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Long SL, Gahan CGM, Joyce SA. Interactions between gut bacteria and bile in health and disease. Mol Aspects Med. 2017;56:54–65. doi: 10.1016/j.mam.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102:731–744. doi: 10.1016/s0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
- 18.Hylemon PB, Zhou H, Pandak WM, Ren S, Gil G, Dent P. Bile acids as regulatory molecules. J Lipid Res. 2009;50:1509–1520. doi: 10.1194/jlr.R900007-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Copple BL, Li T. Pharmacology of bile acid receptors: Evolution of bile acids from simple detergents to complex signaling molecules. Pharmacol Res. 2016;104:9–21. doi: 10.1016/j.phrs.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parseus A, Sommer N, Sommer F, Caesar R, Molinaro A, Stahlman M. Microbiota-induced obesity requires farnesoid X receptor. Gut. 2017;66:429–437. doi: 10.1136/gutjnl-2015-310283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ridlon JM, Kang DJ, Hylemon PB, Bajaj JS. Bile acids and the gut microbiome. Curr Opin Gastroenterol. 2014;30:332–338. doi: 10.1097/MOG.0000000000000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mouzaki M, Wang AY, Bandsma R, Comelli EM, Arendt BM, Zhang L. Bile Acids and Dysbiosis in Non-Alcoholic Fatty Liver Disease. PLoS One. 2016;11 doi: 10.1371/journal.pone.0151829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Groschwitz KR, Hogan SP. Intestinal barrier function: molecular regulation and disease pathogenesis. J Allergy Clin Immunol. 2009;124:3–20. doi: 10.1016/j.jaci.2009.05.038. quiz 21-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo S, Al-Sadi R, Said HM, Ma TY. Lipopolysaccharide causes an increase in intestinal tight junction permeability in vitro and in vivo by inducing enterocyte membrane expression and localization of TLR-4 and CD14. Am J Pathol. 2013;182:375–387. doi: 10.1016/j.ajpath.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arrese M, Cabrera D, Kalergis AM, Feldstein AE. Innate Immunity and Inflammation in NAFLD/NASH. Dig Dis Sci. 2016;61:1294–1303. doi: 10.1007/s10620-016-4049-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aron-Wisnewsky J, Gaborit B, Dutour A, Clement K. Gut microbiota and non-alcoholic fatty liver disease: new insights. Clin Microbiol Infect. 2013;19:338–348. doi: 10.1111/1469-0691.12140. [DOI] [PubMed] [Google Scholar]
- 27.Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179–185. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vonghia L, Michielsen P, Francque S. Immunological mechanisms in the pathophysiology of non-alcoholic steatohepatitis. Int J Mol Sci. 2013;14:19867–19890. doi: 10.3390/ijms141019867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baffy G. Kupffer cells in non-alcoholic fatty liver disease: the emerging view. J Hepatol. 2009;51:212–223. doi: 10.1016/j.jhep.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tosello-Trampont AC, Landes SG, Nguyen V, Novobrantseva TI, Hahn YS. Kuppfer cells trigger nonalcoholic steatohepatitis development in diet-induced mouse model through tumor necrosis factor-alpha production. J Biol Chem. 2012;287:40161–40172. doi: 10.1074/jbc.M112.417014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen J, Wei Y, He J, Cui G, Zhu Y, Lu C. Natural killer T cells play a necessary role in modulating of immune-mediated liver injury by gut microbiota. Sci Rep. 2014;4:7259. doi: 10.1038/srep07259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liang S, Webb T, Li Z. Probiotic antigens stimulate hepatic natural killer T cells. Immunology. 2014;141:203–210. doi: 10.1111/imm.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tian Z, Chen Y, Gao B. Natural killer cells in liver disease. Hepatology. 2013;57:1654–1662. doi: 10.1002/hep.26115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeong WI, Gao B. Innate immunity and alcoholic liver fibrosis. J Gastroenterol Hepatol. 2008;23:S112–S118. doi: 10.1111/j.1440-1746.2007.05274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: what's in a name? Nat Rev Immunol. 2004;4:231–237. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- 36.Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27:104–119. doi: 10.1111/j.1365-2036.2007.03562.x. [DOI] [PubMed] [Google Scholar]
- 37.Gaudier E, Rival M, Buisine MP, Robineau I, Hoebler C. Butyrate enemas upregulate Muc genes expression but decrease adherent mucus thickness in mice colon. Physiol Res. 2009;58:111–119. doi: 10.33549/physiolres.931271. [DOI] [PubMed] [Google Scholar]
- 38.Willemsen LE, Koetsier MA, van Deventer SJ, van Tol EA. Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E(1) and E(2) production by intestinal myofibroblasts. Gut. 2003;52:1442–1447. doi: 10.1136/gut.52.10.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Augenlicht L, Shi L, Mariadason J, Laboisse C, Velcich A. Repression of MUC2 gene expression by butyrate, a physiological regulator of intestinal cell maturation. Oncogene. 2003;22:4983–4992. doi: 10.1038/sj.onc.1206521. [DOI] [PubMed] [Google Scholar]
- 40.Wang HB, Wang PY, Wang X, Wan YL, Liu YC. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein Claudin-1 transcription. Dig Dis Sci. 2012;57:3126–3135. doi: 10.1007/s10620-012-2259-4. [DOI] [PubMed] [Google Scholar]
- 41.Pant K, Yadav AK, Gupta P, Islam R, Saraya A, Venugopal SK. Butyrate induces ROS-mediated apoptosis by modulating miR-22/SIRT-1 pathway in hepatic cancer cells. Redox Biol. 2017;12:340–349. doi: 10.1016/j.redox.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin HV, Frassetto A, Kowalik EJ, Jr., Nawrocki AR, Lu MM, Kosinski JR. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS One. 2012;7 doi: 10.1371/journal.pone.0035240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kinoshita M, Suzuki Y, Saito Y. Butyrate reduces colonic paracellular permeability by enhancing PPARgamma activation. Biochem Biophys Res Commun. 2002;293:827–831. doi: 10.1016/S0006-291X(02)00294-2. [DOI] [PubMed] [Google Scholar]
- 44.Corbin KD, Zeisel SH. Choline metabolism provides novel insights into nonalcoholic fatty liver disease and its progression. Curr Opin Gastroenterol. 2012;28:159–165. doi: 10.1097/MOG.0b013e32834e7b4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zeisel SH, da Costa K-A. Choline: an essential nutrient for public health. Nutr Rev. 2009;67:615–623. doi: 10.1111/j.1753-4887.2009.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Craciun S, Balskus EP. Microbial conversion of choline to trimethylamine requires a glycyl radical enzyme. Proc Natl Acad Sci U S A. 2012;109:21307–21312. doi: 10.1073/pnas.1215689109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Howitt MR, Garrett WS. A complex microworld in the gut: gut microbiota and cardiovascular disease connectivity. Nat Med. 2012;18:1188–1189. doi: 10.1038/nm.2895. [DOI] [PubMed] [Google Scholar]
- 49.Shih DM, Wang Z, Lee R, Meng Y, Che N, Charugundla S. Flavin containing monooxygenase 3 exerts broad effects on glucose and lipid metabolism and atherosclerosis. J Lipid Res. 2015;56:22–37. doi: 10.1194/jlr.M051680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen YM, Liu Y, Zhou RF, Chen XL, Wang C, Tan XY. Associations of gut-flora-dependent metabolite trimethylamine-N-oxide, betaine and choline with non-alcoholic fatty liver disease in adults. Sci Rep. 2016;6:19076. doi: 10.1038/srep19076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sherriff JL, O'Sullivan TA, Properzi C, Oddo JL, Adams LA. Choline, Its Potential Role in Nonalcoholic Fatty Liver Disease, and the Case for Human and Bacterial Genes. Adv Nutr. 2016;7:5–13. doi: 10.3945/an.114.007955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spencer MD, Hamp TJ, Reid RW, Fischer LM, Zeisel SH, Fodor AA. Association between composition of the human gastrointestinal microbiome and development of fatty liver with choline deficiency. Gastroenterology. 2011;140:976–986. doi: 10.1053/j.gastro.2010.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Levitt MD, Li R, DeMaster EG, Elson M, Furne J, Levitt DG. Use of measurements of ethanol absorption from stomach and intestine to assess human ethanol metabolism. Am J Physiol. 1997;273:G951–G957. doi: 10.1152/ajpgi.1997.273.4.G951. [DOI] [PubMed] [Google Scholar]
- 54.Cope K, Risby T, Diehl AM. Increased gastrointestinal ethanol production in obese mice: Implications for fatty liver disease pathogenesis. Gastroenterology. 2000;119:1340–1347. doi: 10.1053/gast.2000.19267. [DOI] [PubMed] [Google Scholar]
- 55.Dawes EA, Foster SM. The formation of ethanol in Escherichia coli. Biochim Biophys Acta. 1956;22:253–265. doi: 10.1016/0006-3002(56)90148-2. [DOI] [PubMed] [Google Scholar]
- 56.Chaudhry KK, Shukla PK, Mir H, Manda B, Gangwar R, Yadav N. Glutamine supplementation attenuates ethanol-induced disruption of apical junctional complexes in colonic epithelium and ameliorates gut barrier dysfunction and fatty liver in mice. J Nutr Biochem. 2016;27:16–26. doi: 10.1016/j.jnutbio.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rao RK. Acetaldehyde-induced barrier disruption and paracellular permeability in Caco-2 cell monolayer. Methods Mol Biol. 2008;447:171–183. doi: 10.1007/978-1-59745-242-7_13. [DOI] [PubMed] [Google Scholar]
- 58.Mottaran E, Stewart SF, Rolla R, Vay D, Cipriani V, Moretti M. Lipid peroxidation contributes to immune reactions associated with alcoholic liver disease. Free Radic Biol Med. 2002;32:38–45. doi: 10.1016/s0891-5849(01)00757-2. [DOI] [PubMed] [Google Scholar]
- 59.Park B, Lee HR, Lee YJ. Alcoholic liver disease: focus on prodromal gut health. J Dig Dis. 2016;17:493–500. doi: 10.1111/1751-2980.12375. [DOI] [PubMed] [Google Scholar]
- 60.Wang H, Lafdil F, Kong X, Gao B. Signal transducer and activator of transcription 3 in liver diseases: a novel therapeutic target. Int J Biol Sci. 2011;7:536–550. doi: 10.7150/ijbs.7.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yan AW, Fouts DE, Brandl J, Starkel P, Torralba M, Schott E. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology. 2011;53:96–105. doi: 10.1002/hep.24018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Clark JM, Diehl AM. Hepatic steatosis and type 2 diabetes mellitus. Curr Diab Rep. 2002;2:210–215. doi: 10.1007/s11892-002-0085-3. [DOI] [PubMed] [Google Scholar]
- 63.Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, Bellentani S. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. 2005;42:44–52. doi: 10.1002/hep.20734. [DOI] [PubMed] [Google Scholar]
- 64.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 65.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 66.Targher G, Bertolini L, Padovani R, Rodella S, Tessari R, Zenari L. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2007;30:1212–1218. doi: 10.2337/dc06-2247. [DOI] [PubMed] [Google Scholar]
- 67.Mouzaki M, Comelli EM, Arendt BM, Bonengel J, Fung SK, Fischer SE. Intestinal microbiota in patients with nonalcoholic fatty liver disease. Hepatology. 2013;58:120–127. doi: 10.1002/hep.26319. [DOI] [PubMed] [Google Scholar]
- 68.Raman M, Ahmed I, Gillevet PM, Probert CS, Ratcliffe NM, Smith S. Faecal microbiome and volatile organic compound metabolome in obese humans with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2013;11:868–875. doi: 10.1016/j.cgh.2013.02.015. e861-863. [DOI] [PubMed] [Google Scholar]
- 69.Zhu L, Baker SS, Gill C, Liu W, Alkhouri R, Baker RD. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology. 2013;57:601–609. doi: 10.1002/hep.26093. [DOI] [PubMed] [Google Scholar]
- 70.Jiang W, Wu N, Wang X, Chi Y, Zhang Y, Qiu X. Dysbiosis gut microbiota associated with inflammation and impaired mucosal immune function in intestine of humans with non-alcoholic fatty liver disease. Sci Rep. 2015;5:8096. doi: 10.1038/srep08096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wong VW, Tse CH, Lam TT, Wong GL, Chim AM, Chu WC. Molecular characterization of the faecal microbiota in patients with nonalcoholic steatohepatitis--a longitudinal study. PLoS One. 2013;8 doi: 10.1371/journal.pone.0062885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boursier J, Mueller O, Barret M, Machado M, Fizanne L, Araujo-Perez F. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology. 2016;63:764–775. doi: 10.1002/hep.28356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Da Silva HE, Teterina A, Comelli EM, Taibi A, Arendt BM, Fischer SE. Nonalcoholic fatty liver disease is associated with dysbiosis independent of body mass index and insulin resistance. Sci Rep. 2018;8:1466. doi: 10.1038/s41598-018-19753-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schwenger KJP, Chen L, Chelliah A, Da Silva HE, Teterina A, Comelli EM. Markers of activated inflammatory cells are associated with disease severity and intestinal microbiota in adults with nonalcoholic fatty liver disease. Int J Mol Med. 2018;42:2229–2237. doi: 10.3892/ijmm.2018.3800. [DOI] [PubMed] [Google Scholar]
- 75.Rao RK, Seth A, Sheth P. Recent Advances in Alcoholic Liver Disease I. Role of intestinal permeability and endotoxemia in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2004;286:G881–G884. doi: 10.1152/ajpgi.00006.2004. [DOI] [PubMed] [Google Scholar]
- 76.Li F, Duan K, Wang C, McClain C, Feng W. Probiotics and Alcoholic Liver Disease: Treatment and Potential Mechanisms. Gastroenterol Res Pract. 2016;2016:5491465. doi: 10.1155/2016/5491465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bull-Otterson L, Feng W, Kirpich I, Wang Y, Qin X, Liu Y. Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus GG treatment. PLoS One. 2013;8 doi: 10.1371/journal.pone.0053028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen Y, Yang F, Lu H, Wang B, Chen Y, Lei D. Characterization of faecal microbial communities in patients with liver cirrhosis. Hepatology. 2011;54:562–572. doi: 10.1002/hep.24423. [DOI] [PubMed] [Google Scholar]
- 79.Tuomisto S, Pessi T, Collin P, Vuento R, Aittoniemi J, Karhunen PJ. Changes in gut bacterial populations and their translocation into liver and ascites in alcoholic liver cirrhotics. BMC Gastroenterol. 2014;14:40. doi: 10.1186/1471-230X-14-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bluemel S, Williams B, Knight R, Schnabl B. Precision medicine in alcoholic and nonalcoholic fatty liver disease via modulating the gut microbiota. Am J Physiol Gastrointest Liver Physiol. 2016;311:G1018–G1036. doi: 10.1152/ajpgi.00245.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Davis BC, Bajaj JS. The Human Gut Microbiome in Liver Diseases. Semin Liver Dis. 2017;37:128–140. doi: 10.1055/s-0037-1602763. [DOI] [PubMed] [Google Scholar]
- 82.Mutlu EA, Gillevet PM, Rangwala H, Sikaroodi M, Naqvi A, Engen PA. Colonic microbiome is altered in alcoholism. Am J Physiol Gastrointest Liver Physiol. 2012;302:G966–G978. doi: 10.1152/ajpgi.00380.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kakiyama G, Hylemon PB, Zhou H, Pandak WM, Heuman DM, Kang DJ. Colonic inflammation and secondary bile acids in alcoholic cirrhosis. Am J Physiol Gastrointest Liver Physiol. 2014;306:G929–G937. doi: 10.1152/ajpgi.00315.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hartmann P, Seebauer CT, Schnabl B. Alcoholic liver disease: the gut microbiome and liver cross talk. Alcohol Clin Exp Res. 2015;39:763–775. doi: 10.1111/acer.12704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kirpich IA, Solovieva NV, Leikhter SN, Shidakova NA, Lebedeva OV, Sidorov PI. Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: a pilot study. Alcohol. 2008;42:675–682. doi: 10.1016/j.alcohol.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Leclercq S, Matamoros S, Cani PD, Neyrinck AM, Jamar F, Starkel P. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci U S A. 2014;111:E4485–E4493. doi: 10.1073/pnas.1415174111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhao HY, Wang HJ, Lu Z, Xu SZ. Intestinal microflora in patients with liver cirrhosis. Chin J Dig Dis. 2004;5:64–67. doi: 10.1111/j.1443-9573.2004.00157.x. [DOI] [PubMed] [Google Scholar]
- 88.Bajaj JS, Heuman DM, Hylemon PB, Sanyal AJ, White MB, Monteith P. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol. 2014;60:940–947. doi: 10.1016/j.jhep.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Betrapally NS, Gillevet PM, Bajaj JS. Changes in the Intestinal Microbiome and Alcoholic and Nonalcoholic Liver Diseases: Causes or Effects? Gastroenterology. 2016;150:1745–1755. doi: 10.1053/j.gastro.2016.02.073. e1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bode C, Kolepke R, Schafer K, Bode JC. Breath hydrogen excretion in patients with alcoholic liver disease--evidence of small intestinal bacterial overgrowth. Z Gastroenterol. 1993;31:3–7. [PubMed] [Google Scholar]
- 91.Hauge T, Persson J, Danielsson D. Mucosal bacterial growth in the upper gastrointestinal tract in alcoholics (heavy drinkers) Digestion. 1997;58:591–595. doi: 10.1159/000201507. [DOI] [PubMed] [Google Scholar]
- 92.Casafont Morencos F, de las Heras Castano G, Martin Ramos L, Lopez Arias MJ, Ledesma F, Pons Romero F. Small bowel bacterial overgrowth in patients with alcoholic cirrhosis. Dig Dis Sci. 1996;41:552–556. doi: 10.1007/BF02282340. [DOI] [PubMed] [Google Scholar]
- 93.Fukui H, Brauner B, Bode JC, Bode C. Plasma endotoxin concentrations in patients with alcoholic and non-alcoholic liver disease: reevaluation with an improved chromogenic assay. J Hepatol. 1991;12:162–169. doi: 10.1016/0168-8278(91)90933-3. [DOI] [PubMed] [Google Scholar]
- 94.Trebicka J, Krag A, Gansweid S, Appenrodt B, Schiedermaier P, Sauerbruch T. Endotoxin and tumor necrosis factor-receptor levels in portal and hepatic vein of patients with alcoholic liver cirrhosis receiving elective transjugular intrahepatic portosystemic shunt. Eur J Gastroenterol Hepatol. 2011;23:1218–1225. doi: 10.1097/MEG.0b013e32834a75dc. [DOI] [PubMed] [Google Scholar]
- 95.Du Plessis J, Vanheel H, Janssen CE, Roos L, Slavik T, Stivaktas PI. Activated intestinal macrophages in patients with cirrhosis release NO and IL-6 that may disrupt intestinal barrier function. J Hepatol. 2013;58:1125–1132. doi: 10.1016/j.jhep.2013.01.038. [DOI] [PubMed] [Google Scholar]
- 96.Parlesak A, Schafer C, Schutz T, Bode JC, Bode C. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J Hepatol. 2000;32:742–747. doi: 10.1016/s0168-8278(00)80242-1. [DOI] [PubMed] [Google Scholar]
- 97.Urbaschek R, McCuskey RS, Rudi V, Becker KP, Stickel F, Urbaschek B. Endotoxin, endotoxin-neutralizing-capacity, sCD14, sICAM-1, and cytokines in patients with various degrees of alcoholic liver disease. Alcohol Clin Exp Res. 2001;25:261–268. [PubMed] [Google Scholar]
- 98.Vassallo G, Mirijello A, Ferrulli A, Antonelli M, Landolfi R, Gasbarrini A. Review article: Alcohol and gut microbiota - the possible role of gut microbiota modulation in the treatment of alcoholic liver disease. Aliment Pharmacol Ther. 2015;41:917–927. doi: 10.1111/apt.13164. [DOI] [PubMed] [Google Scholar]
- 99.Hartmann P, Chen WC, Schnabl B. The intestinal microbiome and the leaky gut as therapeutic targets in alcoholic liver disease. Front Physiol. 2012;3:402. doi: 10.3389/fphys.2012.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Karlsen TH. Primary sclerosing cholangitis: 50 years of a gut-liver relationship and still no love? Gut. 2016;65:1579–1581. doi: 10.1136/gutjnl-2016-312137. [DOI] [PubMed] [Google Scholar]
- 101.Bajer L, Kverka M, Kostovcik M, Macinga P, Dvorak J, Stehlikova Z. Distinct gut microbiota profiles in patients with primary sclerosing cholangitis and ulcerative colitis. World J Gastroenterol. 2017;23:4548–4558. doi: 10.3748/wjg.v23.i25.4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sabino J, Vieira-Silva S, Machiels K, Joossens M, Falony G, Ballet V. Primary sclerosing cholangitis is characterised by intestinal dysbiosis independent from IBD. Gut. 2016;65:1681–1689. doi: 10.1136/gutjnl-2015-311004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kummen M, Holm K, Anmarkrud JA, Nygard S, Vesterhus M, Hoivik ML. The gut microbial profile in patients with primary sclerosing cholangitis is distinct from patients with ulcerative colitis without biliary disease and healthy controls. Gut. 2017;66:611–619. doi: 10.1136/gutjnl-2015-310500. [DOI] [PubMed] [Google Scholar]
- 104.Torres J, Bao X, Goel A, Colombel JF, Pekow J, Jabri B. The features of mucosa-associated microbiota in primary sclerosing cholangitis. Aliment Pharmacol Ther. 2016;43:790–801. doi: 10.1111/apt.13552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rossen NG, Fuentes S, Boonstra K, D'Haens GR, Heilig HG, Zoetendal EG. The mucosa-associated microbiota of PSC patients is characterized by low diversity and low abundance of uncultured Clostridiales II. J Crohns Colitis. 2015;9:342–348. doi: 10.1093/ecco-jcc/jju023. [DOI] [PubMed] [Google Scholar]
- 106.Tabibian JH, Talwalkar JA, Lindor KD. Role of the microbiota and antibiotics in primary sclerosing cholangitis. Biomed Res Int. 2013;2013:389537. doi: 10.1155/2013/389537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mattner J. Impact of Microbes on the Pathogenesis of Primary Biliary Cirrhosis (PBC) and Primary Sclerosing Cholangitis (PSC) Int J Mol Sci. 2016;17 doi: 10.3390/ijms17111864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tang R, Wei Y, Li Y, Chen W, Chen H, Wang Q. Gut microbial profile is altered in primary biliary cholangitis and partially restored after UDCA therapy. Gut. 2018;67:534–541. doi: 10.1136/gutjnl-2016-313332. [DOI] [PubMed] [Google Scholar]
- 109.Lv LX, Fang DQ, Shi D, Chen DY, Yan R, Zhu YX. Alterations and correlations of the gut microbiome, metabolism and immunity in patients with primary biliary cirrhosis. Environ Microbiol. 2016;18:2272–2286. doi: 10.1111/1462-2920.13401. [DOI] [PubMed] [Google Scholar]
- 110.Bhat M, Arendt BM, Bhat V, Renner EL, Humar A, Allard JP. Implication of the intestinal microbiome in complications of cirrhosis. World J Hepatol. 2016;8:1128–1136. doi: 10.4254/wjh.v8.i27.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen Y, Ji F, Guo J, Shi D, Fang D, Li L. Dysbiosis of small intestinal microbiota in liver cirrhosis and its association with etiology. Sci Rep. 2016;6:34055. doi: 10.1038/srep34055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Qin N, Yang F, Li A, Prifti E, Chen Y, Shao L. Alterations of the human gut microbiome in liver cirrhosis. Nature. 2014;513:59–64. doi: 10.1038/nature13568. [DOI] [PubMed] [Google Scholar]
- 113.Bajaj JS, Betrapally NS, Hylemon PB, Heuman DM, Daita K, White MB. Salivary microbiota reflects changes in gut microbiota in cirrhosis with hepatic encephalopathy. Hepatology. 2015;62:1260–1271. doi: 10.1002/hep.27819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bajaj JS, Ridlon JM, Hylemon PB, Thacker LR, Heuman DM, Smith S. Linkage of gut microbiome with cognition in hepatic encephalopathy. Am J Physiol Gastrointest Liver Physiol. 2012;302:G168–G175. doi: 10.1152/ajpgi.00190.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rai R, Saraswat VA, Dhiman RK. Gut microbiota: its role in hepatic encephalopathy. J Clin Exp Hepatol. 2015;5:S29–S36. doi: 10.1016/j.jceh.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hansen BA, Vilstrup H. Increased intestinal hydrolysis of urea in patients with alcoholic cirrhosis. Scand J Gastroenterol. 1985;20:346–350. doi: 10.3109/00365528509091662. [DOI] [PubMed] [Google Scholar]
- 117.Grat M, Wronka KM, Krasnodebski M, Masior L, Lewandowski Z, Kosinska I. Profile of Gut Microbiota Associated With the Presence of Hepatocellular Cancer in Patients With Liver Cirrhosis. Transplant Proc. 2016;48:1687–1691. doi: 10.1016/j.transproceed.2016.01.077. [DOI] [PubMed] [Google Scholar]
- 118.Kruttgen A, Horz HP, Weber-Heynemann J, Vucur M, Trautwein C, Haase G. Study on the association of Helicobacter species with viral hepatitis-induced hepatocellular carcinoma. Gut Microbes. 2012;3:228–233. doi: 10.4161/gmic.19922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tao X, Wang N, Qin W. Gut Microbiota and Hepatocellular Carcinoma. Gastrointest Tumors. 2015;2:33–40. doi: 10.1159/000380895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Benson AK, Kelly SA, Legge R, Ma F, Low SJ, Kim J. Individuality in gut microbiota composition is a complex polygenic trait shaped by multiple environmental and host genetic factors. Proc Natl Acad Sci U S A. 2010;107:18933–18938. doi: 10.1073/pnas.1007028107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jumpertz R, Le DS, Turnbaugh PJ, Trinidad C, Bogardus C, Gordon JI. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am J Clin Nutr. 2011;94:58–65. doi: 10.3945/ajcn.110.010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Brandl K, Plitas G, Mihu CN, Ubeda C, Jia T, Fleisher M. Vancomycin-resistant enterococci exploit antibiotic-induced innate immune deficits. Nature. 2008;455:804–807. doi: 10.1038/nature07250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Peterson DA, Frank DN, Pace NR, Gordon JI. Metagenomic approaches for defining the pathogenesis of inflammatory bowel diseases. Cell Host Microbe. 2008;3:417–427. doi: 10.1016/j.chom.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Marteau P, Pochart P, Dore J, Bera-Maillet C, Bernalier A, Corthier G. Comparative study of bacterial groups within the human cecal and faecal microbiota. Appl Environ Microbiol. 2001;67:4939–4942. doi: 10.1128/AEM.67.10.4939-4942.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Langille MG, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol. 2013;31:814–821. doi: 10.1038/nbt.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Alisi A, Bedogni G, Baviera G, Giorgio V, Porro E, Paris C. Randomised clinical trial: The beneficial effects of VSL#3 in obese children with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2014;39:1276–1285. doi: 10.1111/apt.12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kobyliak N, Abenavoli L, Mykhalchyshyn G, Kononenko L, Boccuto L, Kyriienko D. A Multi-strain Probiotic Reduces the Fatty Liver Index, Cytokines and Aminotransferase levels in NAFLD Patients: Evidence from a Randomized Clinical Trial. J Gastrointestin Liver Dis. 2018;27:41–49. doi: 10.15403/jgld.2014.1121.271.kby. [DOI] [PubMed] [Google Scholar]
- 130.Mofidi F, Poustchi H, Yari Z, Nourinayyer B, Merat S, Sharafkhah M. Synbiotic supplementation in lean patients with non-alcoholic fatty liver disease: a pilot, randomised, double-blind, placebo-controlled, clinical trial. Br J Nutr. 2017;117:662–668. doi: 10.1017/S0007114517000204. [DOI] [PubMed] [Google Scholar]
- 131.Kwak DS, Jun DW, Seo JG, Chung WS, Park SE, Lee KN. Short-term probiotic therapy alleviates small intestinal bacterial overgrowth, but does not improve intestinal permeability in chronic liver disease. Eur J Gastroenterol Hepatol. 2014;26:1353–1359. doi: 10.1097/MEG.0000000000000214. [DOI] [PubMed] [Google Scholar]
- 132.Dhiman RK, Rana B, Agrawal S, Garg A, Chopra M, Thumburu KK. Probiotic VSL#3 reduces liver disease severity and hospitalization in patients with cirrhosis: a randomized, controlled trial. Gastroenterology. 2014;147:1327–1337. doi: 10.1053/j.gastro.2014.08.031. e1323. [DOI] [PubMed] [Google Scholar]
- 133.Han SH, Suk KT, Kim DJ, Kim MY, Baik SK, Kim YD. Effects of probiotics (cultured Lactobacillus subtilis/Streptococcus faecium) in the treatment of alcoholic hepatitis: randomized-controlled multicenter study. Eur J Gastroenterol Hepatol. 2015;27:1300–1306. doi: 10.1097/MEG.0000000000000458. [DOI] [PubMed] [Google Scholar]
- 134.Stadlbauer V, Mookerjee RP, Hodges S, Wright GA, Davies NA, Jalan R. Effect of probiotic treatment on deranged neutrophil function and cytokine responses in patients with compensated alcoholic cirrhosis. J Hepatol. 2008;48:945–951. doi: 10.1016/j.jhep.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 135.Gupta N, Kumar A, Sharma P, Garg V, Sharma BC, Sarin SK. Effects of the adjunctive probiotic VSL#3 on portal haemodynamics in patients with cirrhosis and large varices: a randomized trial. Liver Int. 2013;33:1148–1157. doi: 10.1111/liv.12172. [DOI] [PubMed] [Google Scholar]
- 136.Tandon P, Moncrief K, Madsen K, Arrieta MC, Owen RJ, Bain VG. Effects of probiotic therapy on portal pressure in patients with cirrhosis: a pilot study. Liver Int. 2009;29:1110–1115. doi: 10.1111/j.1478-3231.2009.02020.x. [DOI] [PubMed] [Google Scholar]
- 137.Jayakumar S, Carbonneau M, Hotte N, Befus AD, St Laurent C, Owen R. VSL#3 (R) probiotic therapy does not reduce portal pressures in patients with decompensated cirrhosis. Liver Int. 2013;33:1470–1477. doi: 10.1111/liv.12280. [DOI] [PubMed] [Google Scholar]
- 138.Bajaj JS, Heuman DM, Hylemon PB, Sanyal AJ, Puri P, Sterling RK. Randomised clinical trial: Lactobacillus GG modulates gut microbiome, metabolome and endotoxemia in patients with cirrhosis. Aliment Pharmacol Ther. 2014;39:1113–1125. doi: 10.1111/apt.12695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lunia MK, Sharma BC, Sharma P, Sachdeva S, Srivastava S. Probiotics prevent hepatic encephalopathy in patients with cirrhosis: a randomized controlled trial. Clin Gastroenterol Hepatol. 2014;12:1003–1008. doi: 10.1016/j.cgh.2013.11.006. e1001. [DOI] [PubMed] [Google Scholar]
- 140.Lata J, Novotny I, Pribramska V, Jurankova J, Fric P, Kroupa R. The effect of probiotics on gut flora, level of endotoxin and Child-Pugh score in cirrhotic patients: results of a double-blind randomized study. Eur J Gastroenterol Hepatol. 2007;19:1111–1113. doi: 10.1097/MEG.0b013e3282efa40e. [DOI] [PubMed] [Google Scholar]
- 141.Gluud LL, Vilstrup H, Morgan MY. Non-absorbable disaccharides versus placebo/no intervention and lactulose versus lactitol for the prevention and treatment of hepatic encephalopathy in people with cirrhosis. Cochrane Database Syst Rev. 2016;4 doi: 10.1002/14651858.CD003044.pub3. [DOI] [PubMed] [Google Scholar]
- 142.Malaguarnera M, Greco F, Barone G, Gargante MP, Malaguarnera M, Toscano MA. Bifidobacterium longum with fructo-oligosaccharide (FOS) treatment in minimal hepatic encephalopathy: a randomized, double-blind, placebo-controlled study. Dig Dis Sci. 2007;52:3259–3265. doi: 10.1007/s10620-006-9687-y. [DOI] [PubMed] [Google Scholar]
- 143.Liu Q, Duan ZP, Ha DK, Bengmark S, Kurtovic J, Riordan SM. Synbiotic modulation of gut flora: effect on minimal hepatic encephalopathy in patients with cirrhosis. Hepatology. 2004;39:1441–1449. doi: 10.1002/hep.20194. [DOI] [PubMed] [Google Scholar]
- 144.Khanna S. Microbiota Replacement Therapies: Innovation in Gastrointestinal Care. Clin Pharmacol Ther. 2018;103:102–111. doi: 10.1002/cpt.923. [DOI] [PubMed] [Google Scholar]
- 145.Khoruts A, Dicksved J, Jansson JK, Sadowsky MJ. Changes in the composition of the human faecal microbiome after bacteriotherapy for recurrent Clostridium difficile-associated diarrhea. J Clin Gastroenterol. 2010;44:354–360. doi: 10.1097/MCG.0b013e3181c87e02. [DOI] [PubMed] [Google Scholar]
- 146.Philips CA, Pande A, Shasthry SM, Jamwal KD, Khillan V, Chandel SS. Healthy Donor Faecal Microbiota Transplantation in Steroid-Ineligible Severe Alcoholic Hepatitis: A Pilot Study. Clin Gastroenterol Hepatol. 2017;15:600–602. doi: 10.1016/j.cgh.2016.10.029. [DOI] [PubMed] [Google Scholar]
- 147.Bajaj JS, Kassam Z, Fagan A, Gavis EA, Liu E, Cox IJ. Faecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: A randomized clinical trial. Hepatology. 2017;66:1727–1738. doi: 10.1002/hep.29306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Vrieze A, Van Nood E, Holleman F, Salojarvi J, Kootte RS, Bartelsman JF. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913–916. doi: 10.1053/j.gastro.2012.06.031. e917. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material