Abstract
Objective
Obesity and its complications place an enormous burden on society. Yet antiobesity medications (AOM) are prescribed to only 2% of the eligible population, even though few individuals can sustain weight loss using other strategies alone. This study estimated the societal value of greater access to AOM.
Methods
By using a well‐established simulation model (The Health Economics Medical Innovation Simulation), the societal value of AOM for the cohort of Americans aged ≥ 25 years in 2019 was quantified. Four scenarios with differential uptake among the eligible population (15% and 30%) were modeled, with efficacy from current and next‐generation AOM. Societal value was measured as monetized quality of life, productivity gains, and savings in medical spending, subtracting the costs of AOM.
Results
For the 217 million Americans aged ≥ 25 years, AOM generated $1.2 trillion in lifetime societal value under a conservative scenario (15% annual uptake using currently available AOM). The introduction of next‐generation AOM increased societal value to $1.9 to $2.5 trillion, depending on uptake. Finally, societal value was higher for younger individuals and Black and Hispanic individuals compared with White individuals.
Conclusions
This study suggests that AOM provide substantial gains to patients and society. Policies promoting broader clinical access to and use of AOM warrant consideration to reach national goals to reduce obesity.
Study Importance Questions
What is already known?
Obesity is a major public health problem in the United States, with an annual economic burden of nearly $200 billion.
Antiobesity medications have demonstrated success in aiding and maintaining weight loss, though access and hence uptake among eligible patients are limited.
What does this study add?
This study quantifies the long‐term value to society of broader access to current and future US Food and Drug Administration‐approved antiobesity medications for chronic weight management among Americans aged ≥25 years. Societal value is a measure of quality of life and productivity gains, changes in medical spending, and reductions in obesity‐related comorbidities.
Our study underscores the importance of policies to improve access to novel antiobesity medications because of their substantial impact on reducing obesity‐related comorbidities and because of their value to society over the long term.
Our study also shows the importance of approaching obesity as a chronic disease and reducing barriers to care. Such efforts could include improving obesity management guidelines and treatment patterns, improving drug coverage, and familiarizing physicians with new antiobesity medications.
Introduction
Obesity is a major public health problem in the United States. An estimated one‐third of Americans are living with obesity, estimated as body mass index (BMI) ≥ 30 kg/m2, with another one‐third considered having excess weight (BMI 25‐29.9 kg/m2 ) 1. Obesity is associated with other comorbidities, such as type 2 diabetes and hypertension 2, and it increases the risk for heart disease, stroke, and some cancers 3, 4.
The economic burden of obesity is growing. It is estimated that the United States spends between $147 and $210 billion per year in obesity and obesity‐related expenses 5. The share of national medical expenditures spent on obesity‐related conditions has risen from 6.13% in 2001 to 7.91% in 2015, an increase of 29% 6. Given current trends, direct medical costs due to obesity and obesity‐related morbidity are projected to increase by $48 to $66 billion per year by 2030 7.
Obesity has adverse effects on quality of life 8, disability, and productivity 9, and its economic burden is borne by the patients, health care providers, insurers, and taxpayers. Using the Medical Expenditure Panel Survey, a 2015 study broke down obesity‐related expenditures to 30% for Medicare and other federal sources, 11% for Medicaid, 27% for private insurance, and approximately 30% for out‐of‐pocket payments 10. From the employers’ perspective, obesity‐related absenteeism and presenteeism at work result in costs estimated at $4.3 billion annually 11. From the government’s perspective, obesity’s impact on individuals’ workforce participation and accumulating disability increases Medicaid and Medicare expenditures, thereby increasing government outlays, and reduces income and payroll tax payments, thereby lowering government revenues 12, 13. A 2018 study reported that state Medicaid programs spend an average of 8.2% of their budget on treating obesity 14. Another study estimated that the total obesity‐related government expenditures, including Medicaid and Medicare spending and federal outlays, translate to $91.6 billion per year 15.
Although the cornerstone of weight management per clinical guidelines is diet and exercise, it is well established that patients struggle to lose weight and maintain weight loss and healthy habits 16. Antiobesity medications (AOM) can be a highly preferable option for many patients. For example, liraglutide 3.0 mg or phentermine/topiramate, as an adjunct to a reduced‐calorie diet and increased exercise, has been shown to result in 50% to 70% of patients experiencing a weight loss of ≥ 5% compared with 15% to 35% of patients who received a placebo 17, 18, 19, 20, 21.
Although currently available US Food and Drug Administration (FDA)–approved AOM have significant potential to help patients lose weight and maintain weight loss, their uptake among eligible patients is low. The barriers to uptake are many, including physician resistance to pharmaceutical treatments and payer caution in covering therapies intended for a large population 22. These barriers contribute to a general lack of adequate access to and reimbursement for obesity‐related therapies and limited availability of obesity support services, although the American Medical Association’s recognition of obesity as a disease may lead to changes 23, 24.
This study evaluated the long‐term value of broader access to currently available FDA‐approved AOM for chronic weight management (excluding short‐term generics) and next‐generation AOM in clinical trials from a societal perspective. Specifically, using a simulation model, we estimated the impact of AOM on quality of life, productivity, medical spending, and four obesity‐related comorbidities (cardiovascular diseases, type 2 diabetes, osteoarthritis, and depression) for the cohort of American adults aged ≥ 25 years in 2019 through their lifetimes.
Methods
We used The Health Economics Medical Innovation Simulation (THEMIS) 25, an individual‐level microsimulation model (Supporting Information Figure S1), to quantify the impact of currently available FDA‐approved and next‐generation AOM on health and economic outcomes. THEMIS includes population health trends estimated based on data from the Panel Study of Income Dynamics (PSID) 26, a biennial, nationally representative survey of population health and socioeconomic characteristics, which enables THEMIS to capture population heterogeneity along with obesity trends under the current standard of care. The full technical specifications of THEMIS can be found in our online technical report 25.
Statistical analysis
THEMIS’s ability to project health and economic trends is based on various real‐world data sources. THEMIS uses the PSID data from 1999 to 2015 to estimate health and economic transitions, the National Health and Nutrition Examination Survey from 1999 to 2010 to estimate BMI trends, and the Medical Expenditure Panel Survey and Medicare Current Beneficiary Survey to estimate medical costs 25. In all cases, THEMIS uses the historical data and trends to predict what will happen in the future. For this project, an obesity risk model was developed using ordinary least squares regression to predict an individual’s log (BMI) given certain demographic and health characteristics (Supporting Information Table S1). This model complements those already developed in THEMIS to calculate the probability of developing cardiovascular diseases, type 2 diabetes, osteoarthritis, and depression, all of which are based on probit regressions estimated using the PSID data (Supporting Information Table S2). The presence of these comorbidities incurs additional medical costs in THEMIS according to the medical costs statistical models developed using the Medical Expenditure Panel Survey and Medicare Current Beneficiary Survey data. Finally, quality‐adjusted life‐years (QALYs) were calculated using an imputed EuroQol‐5 Dimensions three‐level model 27 and were estimated based on the Medical Expenditure Panel Survey data (Supporting Information Table S3). We ran all the simulations using R and C++, and model outcomes were calculated as the mean for 100 replications of the Monte Carlo simulation model. Simulation code is compliant with C++11 and later. R version is 3.6.0.
Study sample
For this analysis, we simulated a nationally representative cohort of Americans aged ≥ 25 years in 2019, including individuals who already had obesity and excess weight, over their lifetimes. Simulated individuals were characterized by their demographics, health condition, and socioeconomic status. The study cohort was created by obtaining a nationally representative population of the United States in 2009 from the PSID data and simulating the population through 2019 given birth and death rates and health trends estimated from the historical PSID data. THEMIS updates individuals’ health status and risk factors in 2‐year cycles according to the estimated predictive models. Patients’ BMI also changes during the simulation period according to the obesity risk model, which captured the increasing obesity trend in the population according to real‐world longitudinal data (Supporting Information Table S1). This means that individuals without obesity faced a likelihood of developing obesity as a function of their risk factors.
AOM treatment scenarios
We estimated four scenarios to model the impact of increased AOM use on health and economic outcomes. Scenario 1 (status quo) represented current rates of obesity and diet and exercise among the population (Table 1). We assumed no AOM use under the status quo because less than 2% of individuals eligible for AOM receive such therapies 28, 29. In scenario 2 (15% uptake), we assumed that 15% of eligible treatment‐naïve individuals initiated treatment with currently available AOM for chronic weight management (i.e., liraglutide 3.0 mg, lorcaserin, phentermine/topiramate, and naltrexone/bupropion) in each model cycle starting in 2019. Based on clinical trial results we assumed an AOM efficacy of 8.9% 20, 21, 30, 31, 32 as a one‐time BMI reduction following AOM initiation and kept all other model components similar to the status quo scenario. In scenario 3 (15% uptake, next generation), we assumed that 15% of eligible treatment‐naïve individuals initiated currently available AOM in each model cycle starting in 2019 until 2023. In 2023 onward, 15% of eligible treatment‐naïve individuals and patients already on AOM would initiate next‐generation AOM. AOM efficacy was kept at 8.9% 20, 21, 30, 31, 32 from 2019 to 2023 and was increased to 14% beginning in the year 2023, consistent with existing evidence of next‐generation AOM on the horizon 33. Finally, in scenario 4 (30% uptake, next generation), we kept all model components similar to scenario 3 (15% uptake, next generation), except annual treatment uptake was increased to 30% beginning in 2023 with the availability of more efficacious AOM.
Table 1.
Summary of modeling scenarios
| Simulated scenario | AOM annual uptake ratea | AOM efficacy (BMI reduction)b |
|---|---|---|
| Status quo | < 2% (assumed no uptake in the analysis) | N/A |
| 15% uptake | 15% | 8.9%c |
| 15% uptake, next generation | 15% | 8.9%c before 2023, 14%d in 2023 onward |
| 30% uptake, next generation | 15% before 2023, 30% in 2023 onward | 8.9%c before 2023, 14%d in 2023 onward |
Annual uptake rate calculated as percentage of eligible treatment‐naïve population who initiated treatment. Eligibility criteria defined, based on AOM FDA labels, as BMI ≥ 30 kg/m2 or BMI ≥ 27 kg/m2 in the presence of at least one weight‐related comorbidity (hypertension or type 2 diabetes; available comorbidities in the simulation).
One‐time weight loss after AOM initiation followed by maintenance of reduced weight until discontinuation. After discontinuation, individual’s weight changed according to obesity risk model included in simulation.
Calculated based on rates reported by Pi‐Sunyer et al. 31, Smith et al. 32, Apovian et al. 30, Gadde et al. 20, and Garvey et al. 21 for currently available FDA‐approved AOM for chronic weight management.
From the semaglutide phase II clinical trial results 33.
N/A, not applicable; AOM, antiobesity medications.
Across all scenarios, we defined treatment eligibility as BMI ≥ 30 kg/m2 or BMI ≥ 27 kg/m2 in the presence of at least one weight‐related comorbidity (hypertension and type 2 diabetes, available conditions in THEMIS used for eligibility criteria) 34. We assumed that all individuals who received AOM also followed a reduced‐calorie diet, increased physical activity, and adhered to the treatment. In addition, we assumed that 30% of treated individuals discontinued treatment every year. This rate was based on discontinuation rates for oral antidiabetic therapies 35. We believed this was a good proxy for the discontinuation rates that could be achieved should obesity be treated as a chronic condition with similar insurance coverage. We did not include retreatment with AOM once a patient discontinued. For treatment costs, we used the weighted average wholesale acquisition cost of all branded AOM by their respective market shares, which was an annual cost of $7,525 36, 37.
Outcomes included the number of treated individuals; total medical spending (regardless of payer); AOM treatment costs; earnings, defined as earned income based on labor force participation (full‐time, part‐time, or not employed) and employee compensation rates; and the annual incremental costs of absenteeism and presenteeism due to obesity, estimated at $81.54 and $708.91 (2018 dollars) per individual with excess weight or obesity, respectively 38. Further, we estimated the cumulative number of QALYs over individuals’ lifetimes. QALY is a measure between zero and one; a year lived in perfect health is worth one QALY, and death is equivalent to zero. We assumed that each QALY was worth $150,000 in economic value 39, 40, 41. We also estimated the impact of AOM on the cumulative number of years individuals lived with cardiovascular diseases, type 2 diabetes, osteoarthritis, and depression. Finally, we calculated the societal value of AOM, defined as the net discounted change in earned income, monetized QALYs, costs of absenteeism and presenteeism due to obesity, and medical spending (inclusive of the treatment cost). We also calculated the societal value of AOM by age and race.
All outcomes under scenarios with AOM use were calculated as a difference from the status quo. The present values of monetized outcomes were discounted by 3% per year and converted to 2018 US dollars using the Consumer Price Index 42.
Sensitivity analyses
We performed sensitivity analyses to measure the impact of changes in those parameter estimates we felt carried the most uncertainty and were likely to have a larger influence on the results: the discontinuation rate, treatment price, and the efficacy of next‐generation AOM on outcomes. For example, although the discontinuation rate for AOM is high today, better coverage and improved efficacy could lead to large increases. In the sensitivity analysis, we increased the discontinuation rate from the base case (30%) to 70% for 10 years and to 40% thereafter 43. We also analyzed the impact of higher treatment prices ($9,029 vs. $7,525; 20% increase relative to the base case) to capture the sensitivity of results to higher‐priced current and next‐generation AOM. In the third sensitivity analysis, we incorporated a higher efficacy for next‐generation AOM (20% vs. 14%; approximate 50% increase relative to the base case) to measure the impact of even better AOM that could be developed in the future. We ran each sensitivity analysis separately (i.e., the associated parameter was the only modified parameter compared with the base case) and adjusted the parameters for each scenario.
Results
Our model included a cohort of 217.2 million Americans aged ≥ 25 years in 2019 simulated over their lifetimes. Across all scenarios, the number of treated patients decreased over time as the initial cohort shrank and as the pool of untreated individuals decreased (Figure 1). Our projections showed that compared with the status quo, 15% annual AOM uptake among eligible treatment‐naïve individuals incurred a net cost of $863 billion, calculated as total AOM cost ($1,188 billion), net increased earnings ($72 billion), and savings in medical spending ($139 billion), and cost of absenteeism and presenteeism ($114 billion) (Table 2). QALYs increased by 13.5 million, valued at $2,022 billion, assuming $150,000 in economic value per QALY. This translated to a societal value of $1,159 billion over the cohort’s lifetime.
Figure 1.
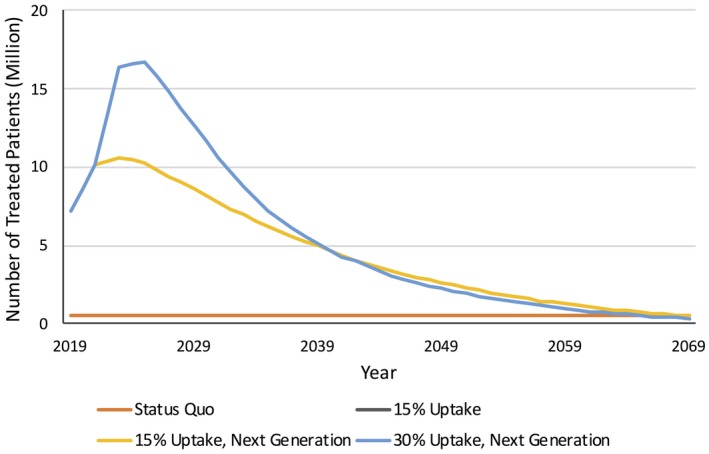
Annual number of treated patients by scenario. Yellow and black lines overlap because of similar treatment uptake every year. Treated individuals were defined as patients who received treatment for at least 1 year. Individuals were not retreated once they discontinued antiobesity medication (AOM). The status quo scenario represents current rates of diet and exercise and no AOM use. The 15% uptake scenario represents 15% annual AOM uptake among eligible treatment‐naïve individuals starting in 2019. The 15% uptake, next generation scenario represents 15% annual AOM uptake among eligible treatment‐naïve individuals starting in 2019 and the availability of more efficacious AOM in 2023 onward. The 30% uptake, next generation scenario represents 15% annual AOM uptake among eligible treatment‐naïve individuals starting in 2019 until 2023 and the availability of more efficacious AOM combined with higher uptake (30%) in 2023 onward. The pool of AOM‐eligible individuals and the number of treated individuals increased over time as the population aged and decreased afterward because of AOM discontinuation and no retreatment. [Color figure can be viewed at https://www.wileyonlinelibrary.com]
Table 2.
Model outcomes over cohort’s lifetime (difference from status quo)
| Outcomea | Scenariosb | |||
|---|---|---|---|---|
| Status quoc | Difference from status quo | |||
| 15% uptake | 15% uptake, next generation | 30% uptake, next generation | ||
| Monetized QALYs ($150,000 per QALY), $ (billion) | 541,661.5 | 2,022.3 | 2,666.7 | 3,454.7 |
| Net cost, $ (billion) | — | 862.8 | 772.4 | 941.0 |
| Total treatment cost | — | 1,187.8 | 1,187.8 | 1,498.4 |
| Total medical spending | 53,613.9 | −139.2 | −188.0 | −250.3 |
| Cost of absenteeism and presenteeism | 1,316.5 | −113.8 | −154.0 | −200.0 |
| Total earnings | 127,559.6 | 72.0 | 73.4 | 107.1 |
| Societal value, $ (billion) | 614,290.7 | 1,159.4 | 1,894.3 | 2,513.6 |
| QALYs, $ (million) | 3,611.1 | 13.5 | 17.8 | 23.0 |
| AOM cost per QALY, $ | — | 64,000 | 43,000 | 41,000 |
All outcomes are cumulative over cohort’s lifetime, discounted, and reported in 2018 US dollars.
The status quo scenario represents current rates of diet and exercise and no AOM use. The 15% uptake scenario represents 15% annual AOM uptake among eligible treatment‐naïve individuals starting in 2019. The 15% uptake, next generation scenario represents 15% annual AOM uptake among eligible treatment‐naïve individuals starting in 2019 and the availability of more efficacious AOM in 2023 onward. The 30% uptake, next generation scenario represents 15% annual AOM uptake among eligible treatment‐naïve individuals starting in 2019 until 2023 and the availability of more efficacious AOM combined with higher uptake (30%) in 2023 onward.
The status quo column presents baseline results projected with current rates of obesity and diet and exercise. Other columns present projected results calculated as differences from status quo under each treatment scenario.
QALY, quality‐adjusted life‐year; AOM, antiobesity medications.
Other scenarios (15% uptake, next generation and 30% uptake, next generation) captured the benefits of more efficacious AOM on the horizon. The 15% uptake, next generation scenario resulted in $772 billion in net cost and $2,667 billion in benefits through QALY gains, yielding a societal value of $1,894 billion (Table 2). Increased efficacy, combined with increased uptake (under the 30% uptake, next generation scenario), incurred a net cost of $941 billion, calculated as a total AOM cost ($1,498 billion), net increased earnings ($107 billion), and savings in medical spending ($250 billion), and cost of absenteeism and presenteeism ($200 billion). AOM benefits accumulated through additional QALYs, valued at $3,455 billion, yielding a societal value of $2,514 billion. Cost per QALY ranged from $41,000 to $64,000 across all scenarios.
The total number of life‐years that the entire cohort lived with cardiovascular diseases was projected to decrease by 11 to 17 million years across treatment scenarios (Table 3). Similarly, AOM reduced 54 to 102 million years lived with type 2 diabetes, 5 to 12 million years lived with depression, and 14 to 25 million years lived with osteoarthritis.
Table 3.
Impact of AOM on comorbidities and societal value by age and race
| Subgroup | 15% uptakea | 15% uptake, next generationa | 30% uptake, next generationa |
|---|---|---|---|
| Reduction in total years lived with comorbidities, compared with status quo, $ (million) | |||
| Comorbidity | |||
| Cardiovascular diseases | 11.0 | 13.5 | 17.2 |
| Type 2 diabetes | 53.8 | 76.7 | 101.6 |
| Depression | 5.4 | 8.4 | 11.5 |
| Osteoarthritis | 13.5 | 19.3 | 25.2 |
| Increase in per person societal value, compared with status quo, $ (thousand) b | |||
| Age category, y c | |||
| 25‐34 | 9.6 | 15.3 | 19.8 |
| 35‐44 | 8.5 | 13.7 | 18.2 |
| 45‐54 | 6.1 | 9.8 | 13.1 |
| 55‐64 | 3.2 | 5.1 | 6.9 |
| 65‐74 | 0.5 | 1.4 | 1.9 |
| Race | |||
| White | 4.4 | 7.2 | 9.7 |
| Black | 7.3 | 11.7 | 15.2 |
| Hispanic | 7.8 | 12.6 | 16.4 |
The status quo scenario represents current rates of diet and exercise and no AOM use. The 15% uptake scenario represents 15% annual AOM uptake among eligible treatment‐naïve individuals starting in 2019. The 15% uptake, next generation scenario represents 15% annual AOM uptake among eligible treatment‐naïve individuals starting in 2019 and the availability of more efficacious AOM in 2023 onward. The 30% uptake, next generation scenario represents 15% annual AOM uptake among eligible treatment‐naïve individuals starting in 2019 until 2023 and the availability of more efficacious AOM combined with higher uptake (30%) in 2023 onward.
Increase in per person societal value for each category calculated as increase in total societal value accrued to that category, compared with status quo, divided by number of individuals in that category.
Age category defined as age category of individuals in cohort at beginning of simulation in 2019; AOM, antiobesity medications.
In addition, across all treatment scenarios, the societal value per person was the highest in the youngest age group ($10,000‐$20,000 for individuals aged 25‐34 in 2019), decreasing with older ages (e.g., $500‐$1,900 for individuals aged 65‐74 in 2019) (Table 3). Treating Black and Hispanic individuals with AOM derived the greatest societal value per person ($15,200 and $16,400, respectively) compared with treating White individuals ($9,700).
Varying the annual AOM discontinuation rate to 70% annually for 10 years and to 40% annually thereafter (vs. 30% throughout) decreased societal value, ranging from $321 billion to $691 billion across scenarios, compared with the base case (Supporting Information Table S4). Similarly, increasing the treatment price (20% increase relative to the base case) decreased societal value, ranging from $922 billion to $2,214 billion across scenarios, relative to the base case (Supporting Information Table S5). Our results indicated that AOM still afford substantial value to individuals and society even with higher discontinuation rates or increased price. On the other hand, increasing treatment efficacy (20% vs. 14% in initial BMI reduction) increased societal value by $767 billion to $1,063 billion across scenarios, compared with the base case (Supporting Information Table S6). Cost per QALY ranged from $28,000 to $31,000 after increasing treatment efficacy for next‐generation AOM, approximately 30% lower than base case estimates ($41,000‐$43,000).
Discussion
Our results suggest that currently available FDA‐approved AOM would produce substantial societal value if it were more widely used. Specifically, if annual uptake increased to 15% of the eligible population, AOM would generate $1,159 billion in societal value for a nationally representative cohort of Americans aged ≥ 25 in 2019 over their lifetimes. Our results also suggest that more efficacious AOM with higher uptake would increase productivity, cost savings, and QALY gains, generating $2,514 billion in societal value for the simulated cohort.
Our economic analysis lends support to policies that broaden AOM access and increase use among individuals who benefit from it the most. Individuals with excess weight and obesity are more likely to develop obesity‐related comorbid conditions 44, 45, such as type 2 diabetes and cardiovascular diseases, which impose considerable health and economic burdens on the society 5, 26, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70. The increasing trend in obesity prevalence has increased the incidence of these comorbid conditions and associated costs 71. Greater access to and use of AOM would reduce the number of years spent with these comorbidities and thus the need for expensive therapies to treat them. Our results also suggest that AOM treatment should be initiated at younger ages to provide the greatest societal value.
The benefits of broader access to AOM go beyond reducing obesity‐related comorbid conditions and medical expenditures by improving the overall well‐being, productivity, and quality of life within a population who faces an obesity epidemic. A large body of literature has documented the relationship between obesity and quality of life, showing that obesity has a direct impact on functional status and thus a profound impact on quality of life 72, 73, 74. Although most published studies have focused on the impact of weight loss on quality of life through surgical interventions, a dose‐response relationship between BMI and quality of life is also implied (i.e., even modest weight reduction can significantly improve an individual’s quality of life) 74. Our study describes the relationship between BMI and quality of life using real‐world observational data and quantifies the significant impact of weight loss due to AOM on quality of life from medical and economic perspectives. Although weight loss derived from currently available AOM is generally smaller than that from surgical interventions, our study shows that AOM offer considerable benefits to individuals with obesity to reduce and sustain their weight.
Quantifying the benefits of AOM from a societal perspective signifies the importance of approaching obesity as a chronic disease. Published studies have estimated that about half of the adult population in the United States is eligible to receive AOM, but less than 2% of those receive AOM approved for chronic weight management 17, 18; whereas diabetes, with obesity as its main risk factor, affects around 8.4% of Americans, of whom 86% receive antidiabetic treatments 75, 76, 77. Data also suggest that the majority of individuals used AOM for less than 30 days (because of physicians prescribing short‐term use AOM) and that around 10.5% of AOM users did not meet the BMI indication 78. In light of such findings, approaching obesity as a chronic disease could involve comprehensive efforts to reduce barriers to care. Such efforts could target improving the understanding of the science of obesity, improving obesity management guidelines and treatment patterns, improving drug coverage, and familiarizing physicians with new AOM approved for chronic weight management. Efforts to improve AOM adherence and persistence also merit considerable attention to maximize long‐term benefits of AOM.
Our study has several limitations. First, we imputed medical expenses from the Medical Expenditure Panel Survey and Medicare Current Beneficiary Survey because of concerns about inaccurate reporting of medical expenditures from the PSID. Our results would be inaccurate to the extent that our imputation procedure failed to produce correct estimates of individual medical expenditures. The Medical Expenditure Panel Survey contains self‐reports that have been calibrated to the National Health Expenditure accounts and the Medicare Current Beneficiary Survey takes spending from a combination of Medicare administrative data and calibrated self‐reports. Second, we could not identify the direct impact of AOM adherence on BMI reduction in the literature, and our assumption around AOM adherence might overestimate the impact of AOM on study outcomes; however, we examined the impact of higher treatment discontinuation rates on model results in a sensitivity analysis. Third, our assumption around the efficacy of next‐generation AOM was based on published phase 2 clinical trial results of semaglutide 33. The actual real‐world efficacy of this, and other next‐generation AOM, might be higher or lower than our estimate, which could in turn over‐ or underestimate health economic outcomes. Fourth, there were uncertainties around the price of next‐generation AOM. We did not increase the price of next‐generation AOM but assumed a higher price in the sensitivity analysis to project the impact of potentially higher prices on outcomes. Finally, increased AOM uptake in the simulation increased total treatment costs, but it did not include possible additional costs associated with physician visits and monitoring potential adverse events. We are not aware of any estimates of increased health care use as a result of taking AOM, and thus we were not able to include this parameter. However, considering a benchmark such as the Diabetes Prevention Program to prevent type 2 diabetes, which demonstrated that the increased health care use was very small in comparison to the large increase in longevity and quality of life 79, 80, we assumed these costs would not greatly impact the results. Finally, other costs excluded from the analysis were the possible costs to ensure patient compliance with diet and exercise guidelines while on treatment. The approved indications for AOM do not specify that the exercise guidelines would need to be administered and monitored by physicians. In fact, we are not aware of any such programs that are coupled with current AOM usage. Thus, we have assumed that the same patterns of treatment will persist (most doctors will put little effort into tracking compliance). If, instead, the weight loss programs become a more prominent part of the health care interaction and require many resources, it may have a significant impact on the results.
Conclusion
Our study suggests that even with modest assumptions on uptake and real‐world efficacy, AOM would provide tremendous value to patients and society and deserve widespread access and use. Additionally, we find that modest increases in the uptake of AOM would lead to increases in average earnings and lifetime societal value and reductions in medical spending and productivity costs associated with obesity. Finally, the increased efficacy associated with next‐generation AOM will help to magnify the positive results associated with increased used. Public policies aligned with clinical access to and use of AOM described here will accelerate meeting national health and economic goals.
Supporting information
Acknowledgments
The authors would like to thank Rebecca Kee and Taylor Schwartz for research support and Carolyn Harley for manuscript writing support.
Funding agencies: This research was supported by Novo Nordisk Inc.
Disclosure: MK, ASW, EvE, and JRB are employees of Precision Health Economics, a research consulting firm paid by Novo Nordisk Inc. to conduct this study. ASW holds equity in Precision Health Economics. AR, BGS, TZ, and RG are employees of Novo Nordisk Inc. and hold equity in this company. DPG is a consultant to Precision Health Economics and a professor at the University of Southern California. He holds equity (< 1%) in Precision Health Economics and reports personal fees or honoraria from the Aspen Institute, Acadia Pharmaceuticals, Amgen, Avanir Pharmaceuticals, and Celgene, outside this study.
References
- 1. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011‐2012. JAMA 2014;311:806‐814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sauer AG, Siegel RL, Jemal A, Fedewa SA. Updated review of prevalence of major risk factors and use of screening tests for cancer in the United States. Cancer Epidemiol Biomarkers Prev 2017;26:1192‐1208. [DOI] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention . Adult obesity facts. http://www.cdc.gov/obesity/data/adult.html. Accessed November 16, 2015.
- 4. Vos MB, Welsh J. Childhood obesity: update on predisposing factors and prevention strategies. Curr Gastroenterol Rep 2010;12:280‐287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cawley J, Meyerhoefer C. The medical care costs of obesity: an instrumental variables approach. J Health Econ 2012;31:219‐230. [DOI] [PubMed] [Google Scholar]
- 6. Biener A, Cawley J, Meyerhoefer C. The impact of obesity on medical care costs and labor market outcomes in the US. Clin Chem 2018;64:108‐117. [DOI] [PubMed] [Google Scholar]
- 7. Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet 2011;378:815‐825. [DOI] [PubMed] [Google Scholar]
- 8. Jia H, Lubetkin EI. The impact of obesity on health‐related quality‐of‐life in the general adult US population. J Public Health (Oxf) 2005;27:156‐164. [DOI] [PubMed] [Google Scholar]
- 9. Kudel I, Huang JC, Ganguly R. Impact of obesity on work productivity in different US occupations: analysis of the National Health and Wellness Survey 2014 to 2015. J Occup Environ Med 2018;60:6‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang YC, Pamplin J, Long MW, Ward ZJ, Gortmaker SL, Andreyeva T. Severe obesity in adults cost state Medicaid programs nearly $8 billion in 2013. Health Aff (Millwood) 2015;34:1923‐1931. [DOI] [PubMed] [Google Scholar]
- 11. Cawley J, Rizzo JA, Haas K. Occupation‐specific absenteeism costs associated with obesity and morbid obesity. J Occup Environ Med 2007;49:1317‐1324. [DOI] [PubMed] [Google Scholar]
- 12. Lehnert T, Sonntag D, Konnopka A, Riedel‐Heller S, König HH. Economic costs of overweight and obesity. Best Pract Res Clin Endocrinol Metab 2013;27:105‐115. [DOI] [PubMed] [Google Scholar]
- 13. Runge CF. Economic consequences of the obese. Diabetes 2007;56:2668‐2672. [DOI] [PubMed] [Google Scholar]
- 14. Biener A, Cawley J, Meyerhoefer C. The impact of obesity on medical care costs and labor market outcomes in the US. Clin Chem 2018;64:108‐117. [DOI] [PubMed] [Google Scholar]
- 15. Benjamin H, Harris AW. Obesity costs evident at the state level. https://www.brookings.edu/blog/up-front/2014/12/12/obesity-costs-evident-at-the-state-level/. Published December 12, 2014. Accessed August 29, 2018.
- 16. Wadden TA, Butryn ML, Byrne KJ. Efficacy of lifestyle modification for long‐term weight control. Obes Res 2004;12(suppl):151S‐162S. [DOI] [PubMed] [Google Scholar]
- 17. Davies MJ, Bergenstal R, Bode B, et al; NN8022‐1922 Study Group . Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA 2015;314:687‐699. [DOI] [PubMed] [Google Scholar]
- 18. Lau DC, Krempf M, Astrup A, et al. Liraglutide 3.0 mg reduces body weight and improves cardiometabolic risk factors in adults with overweight/obesity: the SCALE obesity and prediabetes randomised trial [abstract]. Can J Diabetes 2015;39(suppl 1):S48‐S49. [Google Scholar]
- 19. Wadden TA, Hollander P, Klein S, et al; NN8022‐1923 Investigators . Weight maintenance and additional weight loss with liraglutide after low‐calorie‐diet‐induced weight loss: the SCALE Maintenance randomized study. Int J Obes (Lond) 2013;37:1443‐1451. [DOI] [PubMed] [Google Scholar]
- 20. Gadde KM, Allison DB, Ryan DH, et al. Effects of low‐dose, controlled‐release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo‐controlled, phase 3 trial. Lancet 2011;377:1341‐1352. [DOI] [PubMed] [Google Scholar]
- 21. Garvey WT, Ryan DH, Look M, et al. Two‐year sustained weight loss and metabolic benefits with controlled‐release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo‐controlled, phase 3 extension study. Am J Clin Nutr 2012;95:297‐308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Baum C, Andino K, Wittbrodt E, Stewart S, Szymanski K, Turpin R. The challenges and opportunities associated with reimbursement for obesity pharmacotherapy in the USA. Pharmacoeconomics 2015;33:643‐653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kraschnewski JL, Sciamanna CN, Stuckey HL, et al. A silent response to the obesity epidemic: decline in US physician weight counseling. Med Care 2013;51:186‐192. [DOI] [PubMed] [Google Scholar]
- 24. AMA: obesity is a disease state . Healio/Cardiology Today website. https://www.healio.com/cardiology/practice-management/news/print/cardiology-today/%257Bd2fc2708-eb00-4300-8f9e-285e37c27e10%257D/ama-obesity-is-a-disease-state. Published August 2013. Accessed February 12, 2019.
- 25. Precision Health Economics . Technical documentation: The Health Economic Medical Innovation Simulation ‐ PSID version. https://www.precisionmedicinegrp.com/phe/wp-content/uploads/sites/5/2018/12/THEMIS_PSID_appendix.pdf. Published December 14, 2018. Accessed December 20, 2018.
- 26. Benjamin EJ, Blaha MJ, Chiuve SE, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation 2017;135:e146‐e603. doi: 10.1161/CIR.0000000000000485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goldman DP, Cutler D, Rowe JW, et al. Substantial health and economic returns from delayed aging may warrant a new focus for medical research. Health Aff (Millwood) 2013;32:1698‐1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Samaranayake NR, Ong KL, Leung RY, Cheung BM. Management of obesity in the National Health and Nutrition Examination Survey (NHANES), 2007‐2008. Ann Epidemiol 2012;22:349‐353. [DOI] [PubMed] [Google Scholar]
- 29. Xia Y, Kelton CM, Guo JJ, Bian B, Heaton PC. Treatment of obesity: pharmacotherapy trends in the United States from 1999 to 2010. Obesity (Silver Spring) 2015;23:1721‐1728. [DOI] [PubMed] [Google Scholar]
- 30. Apovian CM, Aronne L, Rubino D, et al. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity‐related risk factors (COR‐II). Obesity (Silver Spring) 2013;21:935‐943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pi‐Sunyer X, Astrup A, Fujioka K, et al; SCALE Obesity and Prediabetes NN8022‐1839 Study Group . A randomized, controlled trial of 3.0 mg of liraglutide in weight management. New Engl J Med 2015;373:11‐22. [DOI] [PubMed] [Google Scholar]
- 32. Smith SR, Weissman NJ, Anderson CM, et al; Behavioral Modification and Lorcaserin for Overweight and Obesity Management (BLOOM) Study Group . Multicenter, placebo‐controlled trial of lorcaserin for weight management. N Engl J Med 2010;363:245‐256. [DOI] [PubMed] [Google Scholar]
- 33. O'Neil PM, Birkenfeld AL, McGowan B, et al. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: a randomised, double‐blind, placebo and active controlled, dose‐ranging, phase 2 trial. Lancet 2018;392:637‐649. [DOI] [PubMed] [Google Scholar]
- 34. Saxenda [package insert] . Plainsboro, NJ: Novo Nordisk Inc.; 2018. [Google Scholar]
- 35. Yeaw J, Benner JS, Walt JG, Sian S, Smith DB. Comparing adherence and persistence across 6 chronic medication classes. J Manag Care Pharm 2009;15:728‐740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liraglutide 3.0 mg, lorcaserin, phentermine‐topiramate, and naltrexone‐bupropion. In: RED BOOK Online . Greenwood Village, CO: Truven Health Analytics; 2018. [Google Scholar]
- 37. IQVIA Xponent Database . Durham, NC: IQVIA; 2019. https://www.iqvia.com/locations/united-states/solutions/commercial-operations/essential-information/prescription-information. Accessed February 5, 2019. [Google Scholar]
- 38. Ricci JA, Chee E. Lost productive time associated with excess weight in the U.S. workforce. J Occup Environ Med 2005;47:1227‐1234. [DOI] [PubMed] [Google Scholar]
- 39. Viscusi WK, Aldy JE. The value of a statistical life: a critical review of market estimates throughout the world. J Risk Uncertain 2003;27:5‐76. [Google Scholar]
- 40. Neumann PJ, Cohen JT, Weinstein MC. Updating cost‐effectiveness–the curious resilience of the $50,000‐per‐QALY threshold. New Engl J Med 2014;371:796‐797. [DOI] [PubMed] [Google Scholar]
- 41. Dubois RW. Cost‐effectiveness thresholds in the USA: are they coming? Are they already here? J Comp Eff Res 2016;5:9‐11. [DOI] [PubMed] [Google Scholar]
- 42. Bureau of Labor Statistics . Consumer Price Index. https://www.bls.gov/cpi/. Accessed February 5, 2019.
- 43. Ganguly R, Tian Y, Kong SX, et al. Persistence of newer anti‐obesity medications in a real‐world setting. Diabetes Res Clin Pract 2018;143:348‐356. [DOI] [PubMed] [Google Scholar]
- 44. National Institute of Diabetes and Digestive and Kidney Diseases . Symptoms & causes of diabetes. https://www.niddk.nih.gov/health-information/diabetes/overview/symptoms-causes. Published December 2016. Accessed February 5, 2019.
- 45. National Heart, Lung, and Blood Institute . Listen to Your Heart: Learn About Heart Disease. https://www.nhlbi.nih.gov/health-topics/education-and-awareness/heart-truth/listen-to-your-heart. Accessed February 5, 2019.
- 46. Waters H, DeVol R. Weighing Down America: The Health and Economic Impact of Obesity. Santa Monica, CA: The Milken Institute; 2016. [Google Scholar]
- 47. Agency for Healthcare Research and Quality . Total Expenses and Percent Distribution for Selected Conditions by Type of Service: United States, 2014. Medical Expenditure Panel Survey Household Component Data. https://meps.ahrq.gov/mepsweb/index.jsp [Google Scholar]
- 48. Dennison AR, Garcea G. Economic burden of chronic pancreatitis and implications of total pancreatectomy and autologous islet cell transplantation. JOP 2015;16:517‐526. [Google Scholar]
- 49. Yoon D, Frick KD, Carr DA, Austin JK. Economic impact of epilepsy in the United States. Epilepsia 2009;50:2186‐2191. [DOI] [PubMed] [Google Scholar]
- 50. Rein DB, Zhang P, Wirth KE, et al. The economic burden of major adult visual disorders in the United States. Arch Ophthalmol 2006;124:1754‐1760. [DOI] [PubMed] [Google Scholar]
- 51. Centers for Disease Control and Prevention . Chronic Kidney Disease: Issue Brief. Atlanta, GA: Centers for Disease Control and Prevention; 2015. https://www.cdc.gov/diabetes/pdfs/programs/CKDBrief.pdf [Google Scholar]
- 52. Florence CS, Zhou C, Luo F, Xu L. The economic burden of prescription opioid overdose, abuse, and dependence in the United States, 2013. Med Care 2016;54:901‐906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Greenberg PE, Sisitsky T, Kessler RC, et al. The economic burden of anxiety disorders in the 1990s. J Clin Psychiatry 1999;60:427‐435. [DOI] [PubMed] [Google Scholar]
- 54. Greenberg PE, Fournier AA, Sisitsky T, Pike CT, Kessler RC. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry 2015;76:155‐162. [DOI] [PubMed] [Google Scholar]
- 55. Chong EC, Khan AA, Anger JT. The financial burden of stress urinary incontinence among women in the United States. Curr Urol Rep 2011;12:358‐362. [DOI] [PubMed] [Google Scholar]
- 56. Soni A. Trends in the five most costly conditions among the US civilian noninstitutionalized population, 2002 and 2012. Statistical Brief, no. 470. Rockville, MD: Agency for Healthcare Research and Quality; 2015. [PubMed] [Google Scholar]
- 57. Barnett SB, Nurmagambetov TA. Costs of asthma in the United States: 2002‐2007. J Allergy Clin Immunol 2011;127:145‐152. [DOI] [PubMed] [Google Scholar]
- 58. Frost & Sullivan. Hidden Health Crisis Costing America Billions: Underdiagnosing and Undertreating Obstructive Sleep Apnea Draining Healthcare System. Darien, IL: American Academy of Sleep Medicine; 2016. [Google Scholar]
- 59. Azziz R, Marin C, Hoq L, Badamgarav E, Song P. Health care‐related economic burden of the polycystic ovary syndrome during the reproductive life span. J Clin Endocrinol Metab 2005;90:4650‐4658. [DOI] [PubMed] [Google Scholar]
- 60. Younossi ZM, Blissett D, Blissett R, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology 2016;64:1577‐1586. [DOI] [PubMed] [Google Scholar]
- 61. RTI International. Projections of Cardiovascular Disease Prevalence and Costs: 2015‐2035. Research Triangle Park, NC: RTI International; 2016. [Google Scholar]
- 62. American Diabetes Association . Economic costs of diabetes in the U.S. in 2012. Diabetes Care 2013;36:1033‐1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wing RR, Bolin P, Brancati FL, et al; Look AHEAD Research Group . Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. New Engl J Med 2013;369:145‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yanovski SZ, Yanovski JA. Long‐term drug treatment for obesity: a systematic and clinical review. JAMA 2014;311:74‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kim GW, Lin JE, Blomain ES, Waldman SA. Antiobesity pharmacotherapy: new drugs and emerging targets. Clin Pharmacol Ther 2014;95:53‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rucker D, Padwal R, Li SK, Curioni C, Lau DC. Long term pharmacotherapy for obesity and overweight: updated meta‐analysis. BMJ 2007;335:1194‐1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Rodgers RJ, Tschöp MH, Wilding JP. Anti‐obesity drugs: past, present and future. Dis Model Mech 2012;5:621‐626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Padwal RS, Majumdar SR. Drug treatments for obesity: orlistat, sibutramine, and rimonabant. Lancet 2007;369:71‐77. [DOI] [PubMed] [Google Scholar]
- 69. Kolanowski J. A risk‐benefit assessment of anti‐obesity drugs. Drug Saf 1999;20:119‐131. [DOI] [PubMed] [Google Scholar]
- 70. Kang JG, Park CY. Anti‐obesity drugs: a review about their effects and safety. Diabetes Metab J 2012;36:13‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kneisel K. New antiobesity drugs underused ‐ study: docs missing first step in preventing type 2 diabetes. MedPage Today. September 6, 2016. https://www.medpagetoday.com/endocrinology/obesity/60052. Accessed February 5, 2019. [Google Scholar]
- 72. Kushner RF, Foster GD. Obesity and quality of life. Nutrition 2000;16:947‐952. [DOI] [PubMed] [Google Scholar]
- 73. Kolotkin RL, Meter K, Williams GR. Quality of life and obesity. Obes Rev 2001;2:219‐229. [DOI] [PubMed] [Google Scholar]
- 74. Fontaine KR, Barofsky I. Obesity and health‐related quality of life. Obesity Rev 2001;2:173‐182. [DOI] [PubMed] [Google Scholar]
- 75. Thomas CE, Mauer EA, Shukla AP, Rathi S, Aronne LJ. Low adoption of weight loss medications: a comparison of prescribing patterns of antiobesity pharmacotherapies and SGLT2s. Obesity (Silver Spring) 2016;24:1955‐1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. National Center for Health Statistics . Health, United States, 2014: With Special Feature on Adults Aged 55–64. Hyattsville, MD: National Center for Health Statistics; 2015. [PubMed] [Google Scholar]
- 77. Centers for Disease Control and Prevention . National Diabetes Statistics Report, 2014: Estimates of Diabetes and its Burden in the United States. Atlanta, GA: Centers for Disease Control and Prevention; 2014. [Google Scholar]
- 78. Hampp C, Kang EM, Borders‐Hemphill V. Use of prescription antiobesity drugs in the United States. Pharmacotherapy 2013;33:1299‐1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Knowler WC, Fowler SE, Hamman RF, et al; Diabetes Prevention Program Research Group . 10‐year follow‐up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009;374:1677‐1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Herman WH. The cost‐effectiveness of diabetes prevention: results from the Diabetes Prevention Program and the Diabetes Prevention Program Outcomes Study. Clin Diabetes Endocrinol 2015;1:9. doi: 10.1186/s40842-015-0009-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


