Short abstract
Obesity is an escalating world problem that contributes to the complexity and cost of treatment of metabolic disorders. Obesity is the result of increased storage of energy in the form of adipose tissue, reducing the quality of daily life, and interfering with longevity. Obesity is also a chronic, low-grade inflammatory disorder. The inflammatory processes affect many organ systems with expanded numbers of immune cells and increased cytokine production. Long-term weight loss is difficult to achieve and maintain. Lifestyle modifications, pharmacologic treatments, and surgical methods are increasingly utilized to ameliorate excess body weight and the comorbidities of obesity, such as diabetes, cardiovascular disease, dyslipidemia, and cancers. Weight loss is also touted to reduce inflammation. Here we review the current literature on human obesity-related systemic and local changes to the immune system and circulating inflammatory mediators. Further, we consider the impact of weight loss to reduce the burden of inflammation, bearing in mind the different methods of weight loss—behavioral change vs. surgical intervention.
Impact statement
As the prevalence and severity of obesity expand, the negative impact of excess adiposity affects every system of the body. Given that obesity is a subversive attack on the immune system, weight loss should improve inflammation locally and systemically. Weight management strategies like dieting, exercise, and bariatric surgery, thus have the opportunity to reduce the burden of inflammation.
Keywords: Immune system, inflammation, obesity, bariatric surgery, weight loss, cytokine
Introduction
Despite significant advances in our understanding of mechanisms that regulate body weight, the obesity epidemic continues to escalate and presents challenges for both public health and research. Obesity is a condition that is the result of an imbalance between energy intake (in particular palatable, calorie-dense, readily available foods) and energy output (reduced physical activity and metabolic rate), thus favoring the storage of excess calories in the form of adipose tissue. Characterized as a body mass index (BMI) ≥ 30 kg/m2, obesity is a significant problem for about 40% of the U.S. population.1 Among obese adults, severe obesity, characterized as a BMI ≥ 40 kg/m2, has increased from 5.7 to 7.7% in the past decade alone.1 These epidemiologic data bring to the surface the disturbing fact that both the prevalence and severity of obesity are increasing in the U.S.
Obesity influences every organ system of the body and results in a higher likelihood of adverse health outcomes due to the associated comorbidities which include: disorders such as type-2 diabetes mellitus, hypertension, dyslipidemia, osteoarthritis, cardiovascular disease, liver and gallbladder disease, reproductive disorders, psychological problems, certain cancers, and germane to this review, immune dysfunction.2
The inflammation of obesity is chronic, low-grade, and systemically pervasive. The term “meta-inflammation” or metabolically triggered inflammation has been adopted to clarify its genesis.3 The number and size of adipocytes expand in obesity and metabolically stress the cells resulting in the active secretion of cytokines and infiltration of immune cells to the site of cellular injury. Immune cells invade many tissues (e.g. adipose, liver, gastrointestinal tract, and skeletal muscle), produce various pro-inflammatory cytokines, and release them in a paracrine manner to affect the multiple surrounding cells that make up the tissue parenchyma. Over time, the cytokines are measurable in the circulation, and the body continues to toil under a cloak of increased inflammation. Given that the expanding adipose tissue that leads to obesity produces a significant amount of the pro-inflammatory cytokines, it stands to reason that weight loss should reduce the burden of inflammation.
Sustained and durable weight loss has proven to be challenging to achieve, in particular, when the gap between excess body weight and a healthy BMI is sizeable. Lifestyle modifications, including reduction of caloric intake, manipulation of daily macronutrient ratios, and addition of varying levels of exercise intensity and duration are the recommended, front-line treatment. Pharmacologic therapies are increasingly prescribed alongside behavioral modifications to assist those with the clinical need to reduce their body weight. However, the most robust and durable improvements to obesity and its related comorbidities are via surgical weight loss procedures. Various types of surgical weight loss strategies exist that range in their level of invasiveness and effectiveness and include Roux-en-Y gastric bypass (RYGB), adjustable gastric banding (AGB), vertical sleeve gastrectomy (VSG), and biliary pancreatic diversion (BPD), to name the most popular.4 In the current review, we consider the impact of obesity in humans on facets of the immune system, the cytokines produced, the effect on individual cell types, and further, the extent to which various immune parameters are improved with weight-loss irrespective of how it is achieved.
Potential weight-loss strategies
Lifestyle modifications
Altering the intake and output behaviors associated with dysregulated energy balance is the most used strategy for producing weight loss. Among those clinically prescribed are very-low-calorie diets (VLCD) in which intake is limited to no more than 800 kcal per day. The reduction of calories and, in particular, fat intake, is effective at producing weight loss when coupled with exercise. Beyond limiting calories, other diets that restrict the type of macronutrient are popularly used. These include low-fat diets, ketogenic diets (focusing on reducing carbohydrates to ∼20 g and forcing the body to burn fat stores),5,6 and high-protein diets which limit high-glycemic index, processed foods.7 Alternately, plant-based diets (obtaining all nutrients only from plant sources) are purported to reduce body weight, not through caloric restriction per se, but rather restricting the source of calories.8 Each diet in its own right reports weight loss, but serious consideration is necessary to understand the impact of these diets on the overall health of an individual and long-term sustainability of the lifestyle required by eliminating or overconsuming a particular source of macronutrients.
The other modifiable variable in the energy balance equation is physical activity. Increasing recreational activity by espousing weight-bearing exercises, high-intensity, interval training, moderate-intensity/continuous training, and low-intensity, steady-state (LISS) regimens are effective in increasing calories burned and basal metabolic rate.9 The preponderance of evidence suggests that the average weight loss achieved with a behavioral change does not exceed a loss of 15% of initial weight.10 Furthermore, weight loss is often regained after five years.11
Pharmacologically induced weight loss
Among the FDA-approved drugs for the treatment of obesity are orlistat, lorcaserin, phentermine/topiramate, liraglutide, and bupropion/naltrexone (see review12). The mechanism of action of these drugs is diverse and ranges from pancreatic lipase inhibition, sympathomimetic activities, and serotonin-norepinephrine reuptake inhibition.12 Specifically, when coupled with behavioral changes discussed previously, they appear to be a useful aid to weight loss.13,14 The short-term effectiveness using the various pharmacologic therapies is improved over placebo, but weight regain potential with drug cessation can be high.15 The other detractor to current pharmacologic therapies is the scant knowledge based on variation in gender, race, starting BMI, and other comorbidities that may preclude the use of the specific pharmacological regimens.
Surgical weight loss procedures
The gold standard for the resolution of obesity-related comorbidities remain RYGB. RYGB is an invasive surgery transecting the upper portion of the stomach and rerouting the flow of nutrients to the jejunum. The lower portion of the transected stomach continues to release digestive juices that flow to the anastomosed jejunum. RYGB produces profound weight loss that is maintained for decades.16 However, the most common of the bariatric surgeries to date in the U.S. is VSG17 in which ∼80% of the gastric tissue along the greater curvature of the stomach is resected creating a tube linking the esophagus and the duodenum. Despite the relative simplicity and reduced complications with VSG, there is still significant weight loss attained by reduced gastric volume, altered pyloric innervation, and accelerated gastric emptying rates.18 In both VSG and RYGB, profound changes in gut hormones, neural innervation, and microbiota contribute to the yet unidentified mechanism(s) of action producing the profound potential for weight loss. BPD combines sleeve gastrectomy with an intestinal bypass to produce maximal weight loss and is typically reserved for super-obese individuals with a BMI of >50 kg/m2.19 The least effective of these surgeries is the AGB20 in which a saline cuff is inserted around the upper portion of the stomach, creating a smaller initial gastric pouch. The cuff drastically reduces the volume of the stomach, thereby restricting the total caloric content of any given meal. Although AGB produces weight loss and is used as an aid with other dietary and lifestyle modifications, without long-lasting behavior changes, the body weight loss may quickly be regained.21 The tremendous positive benefit of AGB is that it is entirely reversible. Taken together, surgical procedures for the realization of weight loss are increasingly used in particular in individuals with significant obesity and additional related comorbidities.
The inflammation of obesity
The genesis of inflammation in obesity is multifold. The intake of extra calories is a cellular oxidative stressor evoking excess metabolic byproducts through mitochondrial and peroxisomal oxidation of fatty acids resulting in increased reactive oxygen species, hydrogen peroxide, and nitric oxide that in more substantial quantities are cell toxic. Therefore, the metabolic processing of extra calories can directly drive cellular inflammatory processes. Furthermore, with increasing adipose depot sizes, the activity of antioxidant enzymes such as superoxide dismutase and glutathione peroxidase is diminished, resulting in reduced antioxidant capacity.22 Adipocytes, which store excess fuel in lipid vacuoles, can hypertrophy and eventually rupture, releasing internal contents that can induce inflammatory processes. Fat infiltrates the adipose tissue and also finds its way into the liver, skeletal muscle, pancreas, and other metabolically relevant organs; therefore, the presence of infiltrating adipose tissue into these organs results in the local production of adipokines and pro-inflammatory cytokines. With the expansion of the adipose tissue organ, the specific adipokines produced and released at a higher rate can circulate through the body, causing inflammation in other organ systems.
Adipose-derived hormones
Adipose is a connective tissue composed of fat-storing adipocytes and a stromal vascular fraction that includes pre-adipocytes, vascular endothelial cells, and a variety of immune cells.23,24 With obesity, the expanding size and numbers of adipocytes hone immune cells to take up residence in the tissue.25 Histologically, macrophages, and other immune cells embedded between adipocytes, form crown-like structures and secrete cytokines contributing to the low-grade inflammatory status of the obese adipose tissue.25 Amongst the important hormones produced by adipose tissue are leptin, adiponectin, and resistin.
Leptin is a 167 kDa peptide produced mainly by adipocytes but also by tissues such as the stomach and placenta; leptin is highly conserved among species, highlighting its biologic importance.26 In adipocytes, leptin is produced commensurate with the amount of adiposity.27,28 Leptin secreted from adipose acts to reduce food intake at the level of the hypothalamus, the master coordinator for body weight regulation and to reduce fat stores at the level of the adipocyte.29 Leptin signals via several leptin receptors of which the long-form uses MAPK, JAK-STAT3, PI3K signaling pathways.30 Leptin-resistance occurs with obesity such that the hypothalamus no longer appropriately responds to the elevated circulating leptin to reduce food intake.31 The hyperleptinemia of obesity induces expression of pro-inflammatory cytokines in macrophages and T-cells.32,33 The earliest demonstrations of elevated leptin levels in obese individuals came in the early 1990s, where circulating leptin levels were shown to correlate with body fat composition and BMI and diminish in accordance with loss of body fat stores.28,34 In general, caloric restriction reduces leptin levels and is correlated with body fat loss; however, depending on the content of calories and degree and length of caloric restriction, the strength between the relationship of leptin and body fat can be disrupted.35–37 Given the fact that surgical weight loss preferentially reduces body fat levels, it is not surprising that leptin levels are significantly reduced following RYGB, VSG, AGB, and BPD.38–42 However, in the case of bariatric surgery, leptin levels are not directly correlated with the amount of adiposity or body weight loss, such that early reductions of fat more dramatically reduce leptin levels than later periods of weight loss.38,40
Adiponectin, released as an oligomer of varying sizes from adipose tissue, is secreted inversely proportional to the level of visceral adiposity, such that lean individuals have the highest levels of adiponectin.43 Adiponectin enhances insulin sensitivity by increasing fatty acid oxidation thus modulating lipoprotein metabolism and inhibiting hepatic glucose production. Adiponectin has anti-inflammatory properties and is a biomarker for an improved state of inflammation.43 The adiponectin–leptin ratio is functionally considered a biomarker of inflammation within the adipose tissue.44,45 Short-term fasting does not necessarily modulate adiponectin levels as it does leptin.46 Lifestyle modification coupled with pharmacological weight loss therapy significantly increases adiponectin levels after moderate weight loss.47 In comparison to baseline, VSG and RYGB significantly and consistently increase adiponectin.38,39,48,49
Resistin is a 12.5 kDa adipocyte-specific hormone, also referred to as adipose tissue-specific secretory factor. Resistin plays a role in cholesterol trafficking in the body by acting on the liver to increase low-density lipoprotein(LDL)-cholesterol and degrade LDL receptors and thus contributes to the pathogenesis of atherosclerosis. Resistin acts locally through the resistin receptor to secrete pro-inflammatory cytokines in the adipose tissue and is elevated in obesity.50 Resistin correlates specifically with the degree of hepatic steatosis in the morbidly obese.51 Following three weeks of VLCD, despite changes in leptin, resistin levels did not change.52 Leptin and resistin gene expression in blood was reduced after bariatric surgery,53 but circulating levels of resistin after BPD surgery were not changed.54 Looking strictly at VSG, resistin levels increased in the first week, but three months after surgery, there were no longer any differences in resistin.55
Adipose tissue secretion of cytokines: Adipocytes directly release cytokines, but in addition, the immune cells that take up residence in the adipose tissue independently secrete cytokines. Among the most well-studied cytokines concerning obesity are the inflammatory cytokines TNFα (tumor necrosis factor alpha), interleukin-6 (IL-6), monocyte chemoattractant protein 1 (MCP1), IL-8, and the anti-inflammatory cytokine IL-10.
TNFα, also known as cachexin, (17 kDa) is primarily secreted by activated macrophages, natural killer (NK) cells, lymphocytes, and adipose tissue.56 TNFα contributes to insulin resistance, inhibits lipoprotein lipase activity, and increases fatty acid mobilization from the adipose tissue into the bloodstream.57 The presence of high levels of TNFα results in other diseases such as psoriatic arthritis, rheumatoid arthritis, ulcerative colitis, and Crohn’s disease,58 all of which have increased incidences in obesity. Increased TNFα activity is particularly harmful because it drives the production of other cytokines, mainly IL-1 and IL-6, through activation of the master regulator of inflammation, NFκB.25,59,60 Obese patients have elevated TNFα in comparison to lean controls, and following a VLCD, obese patients had reduced TNFα levels but not to the normal level exhibited by lean controls.61 Following a glucose tolerance test, TNFα levels were reported to be significantly elevated at the two-hour mark in obese patients, and further, TNFα levels were higher in patients with abdominal obesity compared to subcutaneous obesity, suggesting that the visceral depot contributes significantly to circulating TNFα levels.62 Twenty weeks of a diet intervention reduced TNFα in circulation and adipose tissue biopsies in comparison to baseline prior to weight loss.63 Another study comparing obese and lean controls showed no difference concerning plasma TNFα levels.62 In a meta-analysis of 116 bariatric studies, circulating TNFα was generally reduced following weight loss.64 Another recent meta-analysis, however, showed, in fact, no reduction in TNFα following bariatric surgery.65 Similarly, in a study of patients receiving VSG with omentectomy, no change in TNFα was documented one year after surgery despite significant reductions in IL-6 and C-reactive protein (CRP).48 Although inflammatory cytokine expression in adipose tissue is generally reduced with weight loss, in subcutaneous fat obtained from bariatric patients one year after surgery, expression of TNFα and caspase 3 (marker for cellular death) was elevated.66 These data collectively show that TNFα is elevated as a result of obesity but that other factors may continue to drive TNFα levels in surgically induced weight loss that are ameliorated after other non-surgical means of weight loss.
IL-6 is produced by adipocytes, as well as immune, endothelial, and muscle cells. It is part of the acute phase response to infection and mediates fever. IL-6 pro-inflammatory activities are mediated through the soluble IL-6 receptor, which results in trans-signaling into cells that do not have a membrane-anchored IL-6 receptor. On the other hand, the anti-inflammatory activities of IL-6 are rendered through classical signaling in a discrete and limited number of cells that actually do have the IL-6 receptor.67 In healthy, normal-weight individuals, IL-6 administered in a dose equivalent to the concentration of IL-6 produced during strenuous activity, IL-6 acted as an anti-inflammatory cytokine.68 IL-10 and IL-1 receptor antagonist were increased along with a concomitant spike in cortisol and reduction in circulating lymphocytes.68 This study emphasizes that muscle-derived IL-6 interacts with both the stress axis and the immune system.68 However, obese individuals have significantly elevated circulating IL-6 levels that are inversely correlated with insulin sensitivity and associated with non-esterified fatty acid levels.69 In extremely obese individuals, portal vein samples of IL-6 were shown to be higher than radial artery IL-6 samplings and directly demonstrate that visceral fat and not subcutaneous fat is the most critical site for pro-inflammatory IL-6 production.70 In obese individuals, IL-6 is significantly correlated with BMI, waist circumference and visceral adipose tissue mass.71 However, when IL-6 levels were adjusted for body mass, the association between the fat mass and IL-6 diminished, suggesting that IL-6 levels are entirely dependent on visceral fat mass.72 In women who obtained weight loss through VLCD, IL-6 decreased significantly in adipose tissue, and in serum, IL-6 levels correlated with insulin sensitivity.73 Surgical weight loss procedures, in this case VSG and RYGB, produced reductions in circulating IL-6 at six40 and twelve48 months and also a significant decrease of IL-6 specifically in subcutaneous and visceral fat depots.74 Meta-analysis of bariatric studies uniformly report reductions in IL-6, though the range of reduction varied by baseline BMI, percentage of weight loss, and time after surgery.64 In a study to determine the quality of immune cells after bariatric surgery, RYGB resulted in reduced frequency of IL-6 producing B-cells in addition to increased regulatory-to-effector B-cell ratio.75 Taken together, though exercise-induced IL-6 of muscle origin has anti-inflammatory properties, in the context of metabolic disease, IL-6 is highly pro-inflammatory. In this setting, IL-6 is consistently reduced in weight loss irrespective of means.
MCP1, also known as chemoattractant chemokine ligand 2 (CCL2), is produced by endothelial, muscle, immune, and adipose cells.76–78 MCP1 responds to inflammation by recruiting monocytes, memory T-cells, NK cells, and dendritic cells to the site of active inflammation.77,79–81 In obesity, adipocytes recruit and activate macrophages promoting angiogenesis through upregulation of the CCL2/IL-1β/CXCL12 signaling pathway.82 CCL2 was shown to be highly expressed in adipose tissue from obese patients and cultured adipocytes from the obese patients treated with TNFα, CCL2 was further elicited.83 In obese subjects, expression of MCP1 in adipose tissue is significantly higher than in lean subjects; however, there were no changes in circulating MCP1 levels suggesting obesity produces local changes of MCP1 in adipose tissue.78 Plasma MCP1 levels initially increased following VLCD; however, this trend reversed with a significant decrease in plasma MCP1 levels following weight stabilization.84 After RYGB85 and VSG,86 circulating levels of MCP1 significantly decreased from baseline measurements.85 Two years following surgical weight loss, patients had reduced serum MCP1 levels that associated strongly with fasting and insulin levels.87 Despite significantly more information about MCP1 as a marker of local inflammation within adipose, evidence supports it as a viable circulating biomarker for insulin sensitivity.87
IL-8 is secreted by various cell types, including monocytes, neutrophils, epithelial cells, fibroblasts, endothelial cells, mesothelial cells, tumor cells, and even adipose tissue. IL-8 specifically targets and attracts neutrophils during inflammation. Circulating levels of IL-8 are closely correlated to obesity-related parameters such as BMI, waist circumference, CRP, IL-6, and HDL-cholesterol.88 In a study of obese men that followed a VLCD, initial plasma levels of IL-8 were elevated in comparison to lean controls.89 Surprisingly, after weight loss, circulating levels of IL-8 significantly increased agreeing with previous studies63,89,90 in addition to increased IL-8 mRNA expression in peripheral blood mononuclear cells.89 Conversely, two years following RYGB, obese women had lower adipose expression of IL-8 in comparison to pre-surgery levels as reported in other studies.91,92 Taken together, IL-8 levels do vary by mode of weight loss and may be a function of how the variety of cell types that produce IL-8 are affected by the factors that produce weight loss.
IL-10 is an anti-inflammatory cytokine produced by M2 macrophages, Th2 T-cells, and adipocytes that suppresses signal transduction of other pro-inflammatory cytokines such as TNFα and IL-13, by suppression of p65 and c-Rel subunits of NFkB.32,93–95 IL-10 inhibits macrophage activity and suppresses cytotoxic T-cell responses and antigen presentation.95 In humans, IL-10 is negatively correlated with BMI and body fat percentage.96 Following 12 weeks of VLCD with an exercise program and pharmacologic supplementation, weight loss produced a significant increase in IL-10 compared to baseline, which correlated with a reduction in TNFα and baseline adiponectin.47 Following RYGB, there was an increase in the frequency of B-cells producing IL-10 with a concomitant rise in regulatory B-cell subsets as well as an increase in follicular helper T-cell secretion of IL-10.75,97 In contrast, six months after VSG, there was no statistically significant difference in plasma IL-10 levels compared to baseline.40 As an anti-inflammatory, weight loss by lifestyle change does increase circulating IL-10; however, depending on the bariatric surgery, IL-10 levels in circulation may not be beneficially changed.
Other inflammatory biomarkers that are also up-regulated with obesity
CRP, made up of five subunits equaling about 120 kDa, is secreted from hepatocytes as an acute phase protein in response to pro-inflammatory cytokines produced by trauma, injury, and infection.98,99 CRP production is stimulated by pro-inflammatory cytokines, in particular, TNFα, IL-1, and IL-6.98,100,101 During tissue injury, the ligand for CRP becomes accessible on cell membranes increasing the clearance of apoptotic cells.102 CRP is significantly correlated with weight, BMI, waist circumference, hip circumference, and waist-to-hip ratio.71 In postmenopausal women following a VLCD, weight loss significantly reduced plasma CRP levels.103 Following a three-month low-calorie diet in healthy, obese women stratified into insulin-resistant and insulin-sensitive groups, CRP concentrations were higher in the resistant group and correlated with insulin response; CRP decreased in parallel with weight loss in both groups104 CRP levels are significantly lower in calorie-restricted individuals when compared to those consuming an ad libitum Western diet.105 CRP is consistently reduced in short-term and more extended time frames in VSG,106–108 RYGB,109,110 and AGB.110 Hence, CRP has become a clinical biomarker of meta-inflammation.
Transforming-growth factor beta (TGFβ) is a multifunctional cytokine secreted by all white blood cell lineages. It controls proliferation, differentiation, and additional immune functions in many cell types, including adipocyte precursor cells.111 TGFβ is associated with BMI and body fat percentage regardless of the location of fat mass–visceral or subcutaneous.112,113 In comparison to lean counterparts, there was a significant correlation between TGFβ levels and adiposity in obese individuals as well as an increase in circulating TGFβ levels; however, after weight loss TGFβ was reduced.114–118 Three months after RYGB, no changes in TGFβ were reported in obese subjects with or without type-2 diabetes; however, one year following RYGB, circulating concentrations of TGFβ were significantly reduced in comparison to pre-surgical concentrations.119
IL-18, also known as interferon-gamma inducing factor, is a pro-inflammatory cytokine secreted chiefly by macrophages, but other cell types have the potential to produce this cytokine as well. IL-18 is a marker of metabolic syndrome independent of obesity or insulin resistance; this suggests that a variety of comorbidities within metabolic syndrome may be impinging on the IL-18 inflammatory signaling pathway.120 IL-18 was elevated in obese women in comparison to normal-weight controls.121,122 Additionally, IL-18 was reduced in VLCD-induced weight loss and in a Mediterranean-style diet with increased physical activity and correlated with waist-to-hip ratio, potentially acting as a marker for visceral adiposity.121 Surgical weight loss by RYGB and BPD, in a population of PCOS women, significantly reduced IL-18 and did not vary with reproductive status.123
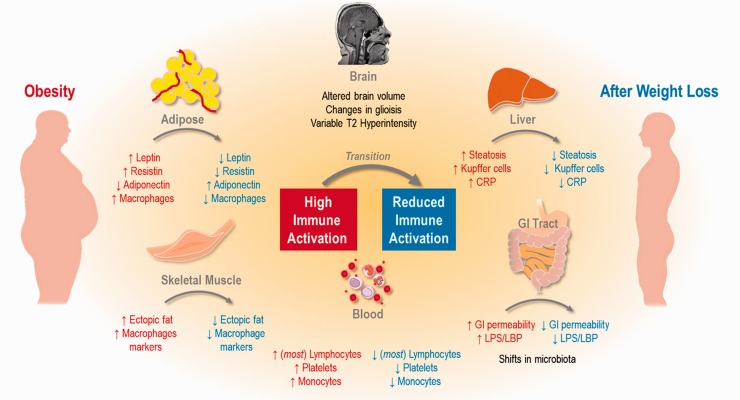
Tissue-specific immune response: Tissue-specific changes in inflammation occur, altering the expression (or levels) of immune cells of the blood, adipose tissue, brain, liver, skeletal muscle, and gastrointestinal tract as diagrammed in Figure 1. The components of blood that are reported most frequently with respect to obesity include lymphocytes, platelets, and monocytes.
Figure 1.
Summary of changes to inflammation in obesity (red) and weight loss (blue). General changes are highlighted in brain, adipose, skeletal muscle, blood, gastrointestinal tract, and liver.
Lymphocytes are a subset of white blood cells (WBCs) within the immune system that includes T-cells, B-cells, and NK cells. T-cells are lymphocytes originating from bone marrow that mature in the thymus. They further differentiate into helper, regulatory, cytotoxic, or memory T-cells. B-cells are lymphocytes also originating in the bone marrow that, unlike T-cells, continue to mature in the bone marrow but complete their final maturation and activation in the spleen. NK cells are cytotoxic lymphocytes originating in the bone marrow that play an important role in innate immunity by responding quickly to infection or tumor formation. Numerous studies have shown that obesity drives an increase in WBC and neutrophil numbers and is directly correlated with BMI.124–126 Obese patients have significantly higher circulating NK cell counts compared to lean controls.127 DNA methylation of B-cells is also increased in obesity in comparison to lean, suggesting obesity can alter the gene expression and ultimately activity of these important immune cells.128 Additionally, adipose tissue in obese individuals has increased helper and cytotoxic lymphocytes129 and specifically in visceral adipose depots, increased T-cell accumulation130 compared to lean controls. RANTES, a chemokine that recruits leukocytes during inflammation, is upregulated in visceral adiposity in obesity.130,131 Taken together, these studies suggest that obesity leads to an increase in total T-cells in circulation and adipose tissue. However, obese patients have significantly less circulating cytotoxic T lymphocytes and NK cells compared to lean controls.127 Furthermore, in this study, “healthy obese”, identified by cutoff points for blood pressure, lipid profile, and fasting glucose, had higher levels of cytotoxic T lymphocytes and NK cells than “unhealthy obese” individuals suggesting that preservation of this subset of T-cells may sustain long-term health irrespective of adiposity.127
Consequently, weight loss by caloric restriction of 10% or 30% for six months significantly improved T-cell function in overweight men and women.132 Also, after 12 and 24 months of calorie restriction, circulating inflammatory markers, including total WBC and lymphocyte counts, were significantly reduced, indicating that long-term calorie restriction reduces inflammation.133 Evidence exists that weight loss through non-surgical (e.g. lifestyle modifications and pharmacologic aids) and surgical methods have a direct impact on peripheral blood lymphocyte populations and cytokine production.124,134,135 Finally, four months after laparoscopic greater curvature plication, a substantial reduction in CD4+ and CD8+ T-cells was reported.136 B-cells after RYGB presented significantly high frequency of IL-10 producing cells and reduced frequency of IL-6 producing cells compared to those before RYGB.75 On the other hand, in another study, six months after RYGB, there was no significant difference in the percent of NK cells; however, the cytotoxic activity of NK cells was significantly enhanced.137 Alternately, in a study following patients two years after AGB, both circulating lymphocyte and neutrophil levels declined in proportion to BMI reduction.124 Taken together, reduction of body weight through surgical and non-surgical means reduced the circulating cytokine burden and lowered the total number of WBC. However, specific studies to determine whether the levels of cytokines and WBC are lower than or similar to control subjects that are of similar weight and had never been overweight have not been undertaken.
Platelets, also known as thrombocytes, are a blood component that, along with coagulation factors, accumulate into blood clots that adhere to injured vessels in response to bleeding.138 Platelets are not only the cellular mediators of thrombosis but are also immune cells that initiate and accelerate many vascular inflammatory conditions. They are linked to the pathogenesis of atherosclerosis and rheumatoid arthritis. Platelets have no nucleus and are actually fragments of cytoplasm from megakaryocytes that enter circulation.139 Platelets are the first line of defense against the loss of endothelial integrity.140 Overweight, obese, and morbidly obese females in comparison to normal-weight females have significantly elevated platelet counts; however, no significant elevation was observed in males in this study.141 Conversely, a retrospective study of both male and female participants showed an elevation in platelet count that was positively correlated with BMI, irrespective of gender.142 When adjusted for age, there was a strong correlation between BMI and platelet counts.141 Additionally, obese individuals have a higher mean platelet volume than non-obese people showing a positive correlation with BMI; however, after three months of dietary treatment, platelet volume in obese patients significantly decreased, and positively correlated with weight loss and reduction in mean platelet volume.143 When obesity was treated with BPD, morbidly obese patients experienced a significant decrease in platelet count unlike RYGB in which platelet count remained elevated.144,145
Monocytes are WBCs that differentiate into macrophages or myeloid lineage dendritic cells in response to tissue damage or infection.146 Macrophages are monocyte-derived phagocytic cells of the immune system that consume targeted cells. Macrophages in adipose tissue highly express inflammatory cytokines and are strongly correlated with body weight, BMI, and total body fat.147 Monocyte counts are higher in obesity and are significantly correlated with BMI.125 Monocyte counts are higher in obesity and are significantly correlated with BMI.125 Subcutaneous white adipose tissue infiltrating macrophages are elevated in obese individuals and significantly decreased after weight loss; additionally, the remaining macrophages are IL-10 positive.148 Certain subsets of macrophages that are elevated in inflammatory diseases, specifically, CD14dimCD16+ monocytes, are increased in obesity and reduced in hypocaloric diet149 and RYGB.149,150 Additionally, three months after RYGB, the ratio of CD40+ to CD206+ macrophages were significantly lower than baseline in subcutaneous adipose tissue.151
Brain: Consumption of a high-fat diet is associated with increased body weight, impaired cognitive function, and an increase in brain inflammation.152 In addition to cognitive function differences, obese individuals overall have a significantly smaller whole brain and total gray matter volume when compared to either normal or overweight individuals, with a strong association between visceral adiposity and total brain volume.153,154 Furthermore, in elderly patients, brain atrophy was observed in both overweight and obese subjects suggesting that the duration of being overweight or obese is associated with lower brain volume.155 Using brain-imaging techniques, functional differences in brain activity have been identified between obese and lean subjects.152 In obese humans, there is evidence of increased gliosis in comparison to lean controls, assessed by MRI in the mediobasal hypothalamus suggesting that obesity is associated with hypothalamic injury.156 Moreover, using fMRI, obese subjects were found to have regional T2 hyperintensity, which the authors interpreted as elevated hypothalamic inflammation.157 Body weight loss resulted in the reversal of fMRI patterns, particularly in the hypothalamus, and increased CSF levels of IL-10 and IL-6 inversely correlated with plasma levels.152 Further, weight loss by calorie restriction improved recognition memory.158 In a study of either one-on-one counselling intervention or group support and education, diabetics receiving group support had reduced white matter hyperintensity volume and more consistent improvement in cognitive function suggesting that durable intervention for weight loss management of diabetes can be effective at improving cognitive and brain indices associated with inflammation.159 Weight loss by calorie restriction also showed an increase in gray matter volume in the inferior frontal gyrus and hippocampus and augmented hippocampal resting-state functional connectivity to the parietal areas.158 However, bariatric surgery did not ameliorate the T2 hyperintensity despite significant improvements in body weight and inflammation; this suggests that some components of brain inflammation are not reversed even by significant weight loss.157
Skeletal muscle: Gene expression for inflammatory macrophage markers elevated in muscles of type-2 diabetes patients strongly correlate with fasting plasma glucose but not age.160 Expression of anti-inflammatory macrophage markers were higher in normal and glucose tolerant subjects and correlated with low fasting plasma glucose or insulin.160 Additionally, the expression of anti-inflammatory macrophage markers in exercising obese and overweight individuals was correlated with a high glucose disposal rate.160 In humans, obese individuals showed elevated levels of CD68 and ITGAX in muscle correlating with poor glucose disposal and adiposity.161 Although there was a substantial reduction in inflammatory markers in adipose tissue of subjects following a 15-week lifestyle intervention of hypocaloric diet and daily exercise, the intervention had no significant effect on skeletal muscle inflammatory markers, which may be attributed to very low levels of these markers found in skeletal muscle.162 In physically frail but obese individuals who were evaluated after 12 weeks of exercise or 12 weeks of weight loss, exercise led to an increased improvement in skeletal gene expression of TLR-4, IL-6, and TNF-α, whereas weight loss had no effect.163
Liver: Because of the liver’s important role in filtering the blood and clearing waste products, the liver is exposed to a steady stream of inflammation-producing substrates. Obesity-induced liver inflammation progresses from hepatic steatosis, or the ectopic accumulation of lipids in the liver, to non-alcoholic steatohepatitis (NASH) to non-alcoholic fatty-liver disease (NAFLD) and finally cirrhosis of the liver. The Kupffer cells or stellate macrophages of the liver are responsible for the clearance of dead or dying cells from systemic circulation. The Kupffer cells contribute to the mononuclear phagocytic system, and their heightened presence contributes to the progression of hepatic inflammatory disease. CRP discussed earlier is produced by the liver and is a marker of generalized inflammation in the body and is elevated in obesity.71 The increased production of IL-6 and TNFα within the liver in obesity increases the risk of hepatic cancer.164,165 Although IL-6 and TNFα are more highly expressed in the adipose tissue compared to the liver, weight loss does result in a significant decrease in hepatic IL-6, although TNFα appears to not change.166 Caloric restriction for six months was shown to significantly reduce liver lipid content, further improving liver function.167
Additionally, compared to non-obese, obese patients have significantly elevated chemerin, a stimulator of chemotaxis during inflammation, and produced in both liver and adipose tissue.168 Obese patients who had elevated baseline chemerin, had a significant activity score for NAFLD, portal inflammation, fibrosis, and fibroinflammation as markers of liver pathology.168 Following RYGB, chemerin decreased significantly at three months and was positively correlated with improvements to triglycerides.168 In general, surgical weight loss results in significant improvement to hepatic health in severely obese patients, and attenuated steatosis, inflammation, and fibrosis.169 Taken together, these data suggest that weight loss of any type may improve hepatic inflammation.
Gastrointestinal tract: The gastrointestinal tract is responsible for digestion and absorption of nutrients and also expels unused components as waste. Within the gut, gram-negative bacteria, which account for over half of the gut microbiota, contain endotoxin.170 Endotoxins are large, heat-stable, pyrogenic lipopolysaccharides (LPS) found on the outer membrane of gram-negative bacteria.170,171 Increased caloric loads alter the microbiota phenotypes and endothelial barrier breakdown can occur, resulting in LPS-related endotoxemia that increases local or systemic inflammation.172 Circulating LPS binds to LPS-binding protein (LBP) eliciting an immune response through LPS presentation.173 LBP is significantly associated with circulating levels of LPS, functioning as a marker for relative levels of LPS in circulation.173 Consumption of high-fat diet alters gut microbiota directly influencing gut permeability leading to chronic systemic inflammation;174 however, diet does not have singular responsibility in obesity-related chronic inflammation as studies have shown adipose tissue produces a variety of adipokines and cytokines that affect systemic inflammation.175 VLCD-induced weight loss caused a significant decrease in circulating LPS as well as LBP, correlating with change in fat mass percentage and BMI.173 Weight loss by RYGB, VSG, and AGB significantly decreased serum LBP levels in comparison to LBP concentration at baseline.176
Summary and future challenges
Obesity is clearly a condition of chronic inflammation identified by elevated immune cell numbers in circulation, increased infiltration of body tissues by immune cells, and increased production of pro-inflammatory cytokines by diverse cell types. The load that these pro-inflammatory cytokines produce on the immune system is lessened by weight loss. However, the degree to which the load is reduced is dependent on the method of weight loss, though pro-inflammatory cytokine production appears to be reduced by the surgeries; generally, this positive benefit may be only realized in specific surgical procedures after a period of weight stabilization. Further, because of the diversity of cells that produce the same cytokines, body weight loss by behavior change or bariatric surgery may preferentially improve the metabolic phenotype of one specific set of cytokine-producing cells causing a reduction in the specific cytokine from that tissue, but may drive production in another cell type, countering the measurable improvement in circulation. Thus, it may not be possible to perceive a net difference.
The literature is replete of comparisons of some of the extremes that drive the production of these chemokines. Comparisons are made concerning gender, degree of adiposity, visceral vs. subcutaneous fat depot, presence or absence of specific comorbidities and age, all which can contribute to the risk of inflammatory disease. Further, longitudinal studies have shown the various improvements that are obtained through weight loss, whether behavioral or surgical. What is lacking in literature is an understanding of the chronology of the triggers for each of the cytokines in obesity, and subsequently, how weight loss changes the trajectory of the presentation of these cytokines. Presently, we do not know how one cytokine affects its relationship to others in a chronological fashion.
Although the risk factors that are co-morbid with obesity predicate poor long-term outcomes, some suggest that the broad term of obesity should be divided into “metabolically abnormal obesity” and “metabolically healthy obesity” (see reviews177,178) since excess adiposity does not always result in the classic co-morbidities of insulin resistance, dyslipidemia, and hypertension.179 However, work done to stratify study subjects into these categories to more accurately predict long-term outcomes has suggested that these designations lack precision since immune and inflammatory endpoints appear similar for these groups180 and future risk for cardio-metabolic disease remains high.181 Further work is needed to determine whether it is possible to have and achieve cardio-metabolic health while remaining obese.
In conclusion, general improvements to metabolic indices result in amelioration of inflammation-driven dysfunction, influencing a positive direction for overall human health.
Authors’ contributions
CLP and BEG researched wrote, edited, and approved this manuscript.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
BEG is supported by the General Medical Sciences of the National Institutes of Health under Award Number P20GM121334 and Department of Defense Award Number W81XWH-16-1-0387. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Department of Defense.
ORCID iD
Bernadette E Grayson https://orcid.org/0000-0002-1281-1682
References
- 1.Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007–2008 to 2015–2016. JAMA 2018; 319:1723–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khaodhiar L, McCowen KC, Blackburn GL. Obesity and its comorbid conditions. Clin Cornerstone 1999; 2:17–31 [DOI] [PubMed] [Google Scholar]
- 3.Hotamisligil GS. Inflammation and metabolic disorders. Nature 2006; 444:860. [DOI] [PubMed] [Google Scholar]
- 4.Fruhbeck G. Bariatric and metabolic surgery: a shift in eligibility and success criteria. Nature Reviews Endocrinology 2015; 11:465–77 [DOI] [PubMed] [Google Scholar]
- 5.Yancy WS, Jr, Olsen MK, Guyton JR, Bakst RP, Westman EC. A low-carbohydrate, ketogenic diet versus a low-fat diet to treat obesity and hyperlipidemia: a randomized, controlled trial. Ann Intern Med 2004; 140:769–77 [DOI] [PubMed] [Google Scholar]
- 6.Paoli A. Ketogenic diet for obesity: friend or foe? Int J Environ Res Public Health 2014; 11:2092–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weigle DS, Breen PA, Matthys CC, Callahan HS, Meeuws KE, Burden VR, Purnell JQ. A high-protein diet induces sustained reductions in appetite, ad libitum caloric intake, and body weight despite compensatory changes in diurnal plasma leptin and ghrelin concentrations. Am J Clin Nutr 2005; 82:41–8 [DOI] [PubMed] [Google Scholar]
- 8.Kahleova H, Dort S, Holubkov R, Barnard ND. A plant-based high-carbohydrate, low-fat diet in overweight individuals in a 16-Week randomized clinical trial: the role of carbohydrates. Nutrients 2018; 10:1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wewege M, van den Berg R, Ward RE, Keech A. The effects of high-intensity interval training vs. moderate-intensity continuous training on body composition in overweight and obese adults: a systematic review and Meta-analysis. Obes Rev 2017; 18:635–46 [DOI] [PubMed] [Google Scholar]
- 10.Wadden TA, Butryn ML, Byrne KJ. Efficacy of lifestyle modification for long-term weight control. Obesity Research 2004; 12:151S–62S [DOI] [PubMed] [Google Scholar]
- 11.Wadden TA, Sternberg JA, Letizia KA, Stunkard AJ, Foster GD. Treatment of obesity by very low calorie diet, behavior therapy, and their combination: a five-year perspective. Int J Obes 1989; 13:39–46 [PubMed] [Google Scholar]
- 12.Bray GA, Fruhbeck G, Ryan DH, Wilding JP. Management of obesity. Lancet 2016; 387:1947–56 [DOI] [PubMed] [Google Scholar]
- 13.Bocarsly ME. Pharmacological interventions for obesity: current and future targets. Curr Addict Rep 2018; 5:202–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rucker D, Padwal R, Li SK, Curioni C, Lau DC. Long term pharmacotherapy for obesity and overweight: updated Meta-analysis. BMJ 2007; 335:1194–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong Z, Xu L, Liu H, Lv Y, Zheng Q, Li L. Comparative efficacy of five long-term weight loss drugs: quantitative information for medication guidelines. Obes Rev 2017; 18:1377–85 [DOI] [PubMed] [Google Scholar]
- 16.Pories WJ, MacDonald KG, Jr, Morgan EJ, Sinha MK, Dohm GL, Swanson MS, Barakat HA, Khazanie PG, Leggett-Frazier N, Long SD, O’Brien KF, Caro JF. Surgical treatment of obesity and its effect on diabetes: 10-y follow-up. The American Journal of Clinical Nutrition 1992; 55:582S–5S [DOI] [PubMed] [Google Scholar]
- 17.Angrisani L, Santonicola A, Iovino P, Vitiello A, Zundel N, Buchwald H, Scopinaro N. Bariatric surgery and endoluminal procedures: IFSO worldwide survey 2014. Obes Surg 2017; 27:2279–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papailiou J, Albanopoulos K, Toutouzas KG, Tsigris C, Nikiteas N, Zografos G. Morbid obesity and sleeve gastrectomy: how does it work?. Obes Surg 2010; 20:1448–55 [DOI] [PubMed] [Google Scholar]
- 19.Feng JJ, Gagner M. Laparoscopic biliopancreatic diversion with duodenal switch. Semin Laparosc Surg 2002; 9:125–9 [PubMed] [Google Scholar]
- 20.Kang JH, Le QA. Effectiveness of bariatric surgical procedures: a systematic review and network meta-analysis of randomized controlled trials. Medicine (Baltimore) 2017; 96:e8632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kowalewski PK, Olszewski R, Kwiatkowski A, Gałązka-Świderek N, Cichoń K, Paśnik K. Life with a gastric band. Long-Term outcomes of laparoscopic adjustable gastric banding-a retrospective study. Obes Surg 2017; 27:1250–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernández-Sánchez A, Madrigal-Santillán E, Bautista M, Esquivel-Soto J, Morales-González A, Esquivel-Chirino C, Durante-Montiel I, Sánchez-Rivera G, Valadez-Vega C, Morales-González JA. Inflammation, oxidative stress, and obesity. Int J Mol Sci 2011; 12:3117–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rajala MW, Scherer PE. Minireview: the adipocyte – at the crossroads of energy homeostasis, inflammation, and atherosclerosis. Endocrinology 2003; 144:3765–73 [DOI] [PubMed] [Google Scholar]
- 24.Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr 2004; 92:347–55 [DOI] [PubMed] [Google Scholar]
- 25.Lee J. Adipose tissue macrophages in the development of obesity-induced inflammation, insulin resistance and type 2 diabetes. Arch Pharm Res 2013; 36:208–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muruzabal FJ, Fruhbeck G, Gomez-Ambrosi J, Archanco M, Burrell MA. Immunocytochemical detection of leptin in non-mammalian vertebrate stomach. Gen Comp Endocrinol 2002; 128:149–52 [DOI] [PubMed] [Google Scholar]
- 27.Frederich RC, Hamann A, Anderson S, Lollmann B, Lowell BB, Flier JS. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nature Medicine 1995; 1:1311–4 [DOI] [PubMed] [Google Scholar]
- 28.Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S, Kern PA, Friedman JM. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med 1995; 1:1155–61 [DOI] [PubMed] [Google Scholar]
- 29.Thaler JP, Schwartz MW. Minireview: Inflammation and obesity pathogenesis: the hypothalamus heats Up. Endocrinology 2010; 151:4109–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jequier E. Leptin signaling, adiposity, and energy balance. Ann N Y Acad Sci 2002; 967:379–88 [DOI] [PubMed] [Google Scholar]
- 31.Elmquist JK, Coppari R, Balthasar N, Ichinose M, Lowell BB. Identifying hypothalamic pathways controlling food intake, body weight, and glucose homeostasis. J Comp Neurol 2005; 493:63–71 [DOI] [PubMed] [Google Scholar]
- 32.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol 2011; 11:85–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ye J, McGuinness OP. Inflammation during obesity is not all bad: evidence from animal and human studies. Am J Physiol Endocrinol Metab 2013; 304:E466–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, Caro JF. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med 1996; 334:292–5 [DOI] [PubMed] [Google Scholar]
- 35.Wadden TA, Considine RV, Foster GD, Anderson DA, Sarwer DB, Caro JS. Short- and long-Term changes in serum leptin in dieting obese women: effects of caloric restriction and weight loss. J Clin Endocrinol Metab 1998; 83:214–8 [DOI] [PubMed] [Google Scholar]
- 36.Weigle DS, Duell PB, Connor WE, Steiner RA, Soules MR, Kuijper JL. Effect of fasting, refeeding, and dietary fat restriction on plasma leptin levels. J Clin Endocrinol Metab 1997; 82:561–5 [DOI] [PubMed] [Google Scholar]
- 37.Ostlund RE, Jr, Yang JW, Klein S, Gingerich R. Relation between plasma leptin concentration and body fat, gender, diet, age, and metabolic covariates. J Clin Endocrinol Metab 1996; 81:3909–13 [DOI] [PubMed] [Google Scholar]
- 38.Shimizu H, Hatao F, Imamura K, Takanishi K, Tsujino M. Early effects of sleeve gastrectomy on obesity-related cytokines and bile acid metabolism in morbidly obese Japanese patients. Obes Surg 2017; 27:3223–9 [DOI] [PubMed] [Google Scholar]
- 39.Baltieri L, Cazzo E, de Souza AL, Alegre SM, de Paula Vieira R, Antunes E, de Mello GC, Claudio Martins L, Chaim EA. Influence of weight loss on pulmonary function and levels of adipokines among asthmatic individuals with obesity: one-year follow-up. Respir Med 2018; 145:48–56 [DOI] [PubMed] [Google Scholar]
- 40.Mallipedhi A, Prior SL, Barry JD, Caplin S, Baxter JN, Stephens JW. Changes in inflammatory markers after sleeve gastrectomy in patients with impaired glucose homeostasis and type 2 diabetes. Surg Obes Relat Dis 2014; 10:1123–8 [DOI] [PubMed] [Google Scholar]
- 41.Krieger AC, Youn H, Modersitzki F, Chiu Y-L, Gerber LM, Weinshel E, Fielding CR. Effects of laparoscopic adjustable gastric banding on sleep and metabolism: a 12-month follow-up study. Int J Gen Med 2012; 5:975–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Marinis L, Bianchi A, Mancini A, Gentilella R, Perrelli M, Giampietro A, Porcelli T, Tilaro L, Fusco A, Valle D, Tacchino RM. Growth hormone secretion and leptin in morbid obesity before and after biliopancreatic diversion: relationships with insulin and body composition. J Clin Endocrinol Metab 2004; 89:174–80 [DOI] [PubMed] [Google Scholar]
- 43.Fantuzzi G. Adiponectin and inflammation: Consensus and controversy. J Allergy Clin Immunol 2008; 121:326–30 [DOI] [PubMed] [Google Scholar]
- 44.Fruhbeck G, Catalan V, Rodriguez A, Ramirez B, Becerril S, Salvador J, Colina I, Gomez-Ambrosi J. Adiponectin-leptin ratio is a functional biomarker of adipose tissue inflammation. Nutrients 2019; 11:454–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frühbeck G, Catalán V, Rodríguez A, Gómez-Ambrosi J. Adiponectin-leptin ratio: a promising index to estimate adipose tissue dysfunction. Relation with obesity-associated cardiometabolic risk. Adipocyte 2018; 7:57–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Imbeault P, Pomerleau M, Harper ME, Doucet É. Unchanged fasting and postprandial adiponectin levels following a 4-day caloric restriction in young healthy men. Clin Endocrinol (Oxf) 2004; 60:429–33 [DOI] [PubMed] [Google Scholar]
- 47.Jung SH, Park HS, Kim K-S, Choi WH, Ahn CW, Kim BT, Kim SM, Lee SY, Ahn SM, Kim YK, Kim HJ, Kim DJ, Lee K-W. Effect of weight loss on some serum cytokines in human obesity: increase in IL-10 after weight loss. J Nutr Biochem 2008; 19:371–5 [DOI] [PubMed] [Google Scholar]
- 48.Sdralis E, Argentou M, Mead N, Kehagias I, Alexandridis T, Kalfarentzos F. A prospective randomized study comparing patients with morbid obesity submitted to sleeve gastrectomy with or without omentectomy. Obes Surg 2013; 23:965–71 [DOI] [PubMed] [Google Scholar]
- 49.Schmatz R, Bitencourt MR, Patias LD, Beck M, da C, Alvarez G, Zanini D, Gutierres JM, Diehl LN, Pereira LB, Leal CA, Duarte MF, Schetinger MR, Morsch VM. Evaluation of the biochemical, inflammatory and oxidative profile of obese patients given clinical treatment and bariatric surgery. Clin Chim Acta 2017; 465:72–9 [DOI] [PubMed] [Google Scholar]
- 50.Vendrell J, Broch M, Vilarrasa N, Molina A, Gómez JM, Gutiérrez C, Simón I, Soler J, Richart C. Resistin, adiponectin, ghrelin, leptin, and proinflammatory cytokines: relationships in obesity. Obes Res 2004; 12:962–71 [DOI] [PubMed] [Google Scholar]
- 51.Edwards CR, Hindle AK, Latham PS, Fu SW, Brody FJ. Resistin expression correlates with steatohepatitis in morbidly obese patients. Surg Endosc 2013; 27:1310–4 [DOI] [PubMed] [Google Scholar]
- 52.Anderlova K, Kremen J, Dolezalova R, Housova J, Haluzikova D, Kunesova M, Haluzik M. The influence of very-low-calorie-diet on serum leptin, soluble leptin receptor, adiponectin and resistin levels in obese women. Physiol Res 2006; 55:277–83 [DOI] [PubMed] [Google Scholar]
- 53.Edwards C, Hindle AK, Fu S, Brody F. Downregulation of leptin and resistin expression in blood following bariatric surgery. Surg Endosc 2011; 25:1962–8 [DOI] [PubMed] [Google Scholar]
- 54.de Luis DA, Terroba MC, Cuellar L, Conde R, Primo D, Aller R, Sagrado MG, Izaola O. Resistin levels in morbid obese patients following the biliopancreatic diversion surgery. Horm Metab Res 2011; 43:205–8 [DOI] [PubMed] [Google Scholar]
- 55.Ozmen F, Şahin TT, Dolgun A, Ozmen M. Changes in ghrelin and resistin levels following bariatric surgery: one anastomosis gastric bypass Vs sleeve gastrectomy. Surg Obes Relat Dis 2016; 12:S198–S99 [Google Scholar]
- 56.Pereira SS, Alvarez-Leite JI. Low-grade inflammation, obesity, and diabetes. Curr Obes Rep 2014; 3:422–31 [DOI] [PubMed] [Google Scholar]
- 57.Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol 2008; 9:367–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chatzantoni K, Mouzaki A. Anti-TNF-alpha antibody therapies in autoimmune diseases. Curr Top Med Chem 2006; 6:1707–14 [DOI] [PubMed] [Google Scholar]
- 59.Gualillo O, Gonzalez-Juanatey JR, Lago F. The emerging role of adipokines as mediators of cardiovascular function: physiologic and clinical perspectives. Trends Cardiovasc Med 2007; 17:275–83 [DOI] [PubMed] [Google Scholar]
- 60.Bray GA, Clearfield MB, Fintel DJ, Nelinson DS. Overweight and obesity: the pathogenesis of cardiometabolic risk. Clin Cornerstone 2009; 9:30–40 discussion 41–2 [DOI] [PubMed] [Google Scholar]
- 61.Dandona P, Weinstock R, Thusu K, Abdel-Rahman E, Aljada A, Wadden T. Tumor necrosis factor-α in sera of obese patients: fall with weight loss. J Clin Endocrinol Metab 1998; 83:2907–10 [DOI] [PubMed] [Google Scholar]
- 62.Tsigos C, Kyrou I, Chala E, Tsapogas P, Stavridis JC, Raptis SA, Katsilambros N. Circulating tumor necrosis factor alpha concentrations are higher in abdominal versus peripheral obesity. Metab Clin Exp 1999; 48:1332–5 [DOI] [PubMed] [Google Scholar]
- 63.Bruun JM, Pedersen SB, Kristensen K, Richelsen B. Opposite regulation of interleukin-8 and tumor necrosis factor-α by weight loss. Obes Res 2002; 10:499–506 [DOI] [PubMed] [Google Scholar]
- 64.Askarpour M, Khani D, Sheikhi A, Ghaedi E, Alizadeh S. Effect of bariatric surgery on serum inflammatory factors of obese patients: a systematic review and Meta-Analysis. Obes Surg 2019; 29:2631–47 [DOI] [PubMed] [Google Scholar]
- 65.Rao SR. Inflammatory markers and bariatric surgery: a meta-analysis. Inflamm Res 2012; 61:789–807 [DOI] [PubMed] [Google Scholar]
- 66.Jürets A, Itariu BK, Keindl M, Prager G, Langer F, Grablowitz V, Zeyda M, Stulnig TM. Upregulated TNF expression 1 year after bariatric surgery reflects a Cachexia-Like state in subcutaneous adipose tissue. Obes Surg 2017; 27:1514–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta 2011; 1813:878–88 [DOI] [PubMed] [Google Scholar]
- 68.Steensberg A, Fischer CP, Keller C, Møller K, Pedersen BK. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am J Physiol Endocrinol Metab 2003; 285:E433–E37 [DOI] [PubMed] [Google Scholar]
- 69.Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab 2001; 280:E745–E51 [DOI] [PubMed] [Google Scholar]
- 70.Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes 2007; 56:1010. [DOI] [PubMed] [Google Scholar]
- 71.Park HS, Park JY, Yu R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-α and IL-6. Diabetes Res Clin Pract 2005; 69:29–35 [DOI] [PubMed] [Google Scholar]
- 72.Maachi M, Piéroni L, Bruckert E, Jardel C, Fellahi S, Hainque B, Capeau J, Bastard JP. Systemic low-grade inflammation is related to both circulating and adipose tissue TNFα, leptin and IL-6 levels in obese women. Int J Obes Relat Metab Disord 2004; 28:993–7 [DOI] [PubMed] [Google Scholar]
- 73.Bastard J-P, Jardel C, Bruckert E, Blondy P, Capeau J, Laville M, Vidal H, Hainque B. Elevated levels of interleukin 6 are reduced in serum and subcutaneous adipose tissue of obese women after weight loss. J Clin Endocrinol Metab 2000; 85:3338–42 [DOI] [PubMed] [Google Scholar]
- 74.Ortega FJ, Vilallonga R, Xifra G, Sabater M, Ricart W, Fernández-Real JM. Bariatric surgery acutely changes the expression of inflammatory and lipogenic genes in obese adipose tissue. Surg Obes Relat Dis 2016; 12:357–62 [DOI] [PubMed] [Google Scholar]
- 75.Dai X, Zhao W, Zhan J, Zeng S, Ran D, Zhang H, Song Z, Song KH, Wu L. B cells present skewed profile and lose the function of supporting T cell inflammation after roux-en-Y gastric bypass. Int Immunopharmacol 2017; 43:16–22 [DOI] [PubMed] [Google Scholar]
- 76.Gerhardt CC, Romero IA, Cancello R, Camoin L, Strosberg AD. Chemokines control fat accumulation and leptin secretion by cultured human adipocytes. Mol Cell Endocrinol 2001; 175:81–92 [DOI] [PubMed] [Google Scholar]
- 77.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res 2009; 29:313–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dahlman I, Kaaman M, Olsson T, Tan GD, Bickerton AST, WåHlén K, Andersson J, NordströM EA, Blomqvist L, SjöGren A, Forsgren M, Attersand A, Arner P, A unique role of monocyte chemoattractant protein 1 among chemokines in adipose tissue of obese subjects. J Clin Endocrinol Metab 2005; 90:5834–40 [DOI] [PubMed] [Google Scholar]
- 79.Carr MW, Roth SJ, Luther E, Rose SS, Springer TA. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci USA 1994; 91:3652–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xu LL, Warren MK, Rose WL, Gong W, Wang JM. Human recombinant monocyte chemotactic protein and other C-C chemokines bind and induce directional migration of dendritic cells in vitro. J Leukoc Biol 1996; 60:365–71 [DOI] [PubMed] [Google Scholar]
- 81.Van Coillie E, Van Damme J, Opdenakker G. The MCP/eotaxin subfamily of CC chemokines. Cytokine Growth Factor Rev 1999; 10:61–86 [DOI] [PubMed] [Google Scholar]
- 82.Donohoe CL, Lysaght J, O’Sullivan J, Reynolds JV. Emerging concepts linking obesity with the hallmarks of cancer. Trends Endocrinol Metab 2017; 28:46–62 [DOI] [PubMed] [Google Scholar]
- 83.Tourniaire F, Romier-Crouzet B, Lee JH, Marcotorchino J, Gouranton E, Salles J, Malezet C, Astier J, Darmon P, Blouin E, Walrand S, Ye J, Landrier J-F. Chemokine expression in inflamed adipose tissue is mainly mediated by NF-κB. PloS One 2013; 8:e66515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Siklova M, Simonsen L, Polak J, Stich V, Bülow J. Effect of short-term hyperglycemia on adipose tissue fluxes of selected cytokines in vivo during multiple phases of diet-induced weight loss in obese women. J Clin Endocrinol Metab 2015; 100:1949–56 [DOI] [PubMed] [Google Scholar]
- 85.Dalmas E, Rouault C, Abdennour M, Rovere C, Rizkalla S, Bar-Hen A, Nahon J-L, Bouillot J-L, Guerre-Millo M, Clément K, Poitou C. Variations in circulating inflammatory factors are related to changes in calorie and carbohydrate intakes early in the course of surgery-induced weight reduction. Am J Clin Nutr 2011; 94:450–8 [DOI] [PubMed] [Google Scholar]
- 86.Gumbau V, Bruna M, Canelles E, Guaita M, Mulas C, Bases C, Celma I, Puche J, Marcaida G, Oviedo M, Vazquez A. A prospective study on inflammatory parameters in obese patients after sleeve gastrectomy. Obes Surg 2014; 24:903–8 [DOI] [PubMed] [Google Scholar]
- 87.Schernthaner G-H, Kopp H-P, Kriwanek S, Krzyzanowska K, Satler M, Koppensteiner R, Schernthaner G. Effect of massive weight loss induced by bariatric surgery on serum levels of interleukin-18 and monocyte-chemoattractant-protein-1 in morbid obesity. Obes Surg 2006; 16:709–15 [DOI] [PubMed] [Google Scholar]
- 88.Kim CS, Park HS, Kawada T, Kim JH, Lim D, Hubbard NE, Kwon BS, Erickson KL, Yu R. Circulating levels of MCP-1 and IL-8 are elevated in human obese subjects and associated with obesity-related parameters. Int J Obes (Lond) 2006; 30:1347–55 [DOI] [PubMed] [Google Scholar]
- 89.Bruun JM, Verdich C, Toubro S, Astrup A, Richelsen B. Association between measures of insulin sensitivity and circulating levels of interleukin-8, interleukin-6 and tumor necrosis factor-alpha. Effect of weight loss in obese men. Eur J Endocrinol 2003; 148:535–42 [DOI] [PubMed] [Google Scholar]
- 90.de Mello VDF, Kolehmainen M, Schwab U, Mager U, Laaksonen DE, Pulkkinen L, Niskanen L, Gylling H, Atalay M, Rauramaa R, Uusitupa M. Effect of weight loss on cytokine messenger RNA expression in peripheral blood mononuclear cells of obese subjects with the metabolic syndrome. Metab Clin Exp 2008; 57:192–9 [DOI] [PubMed] [Google Scholar]
- 91.Alvehus M, Simonyte K, Andersson T, Söderström I, Burén J, Rask E, Mattsson C, Olsson T. Adipose tissue IL-8 is increased in normal weight women after menopause and reduced after gastric bypass surgery in obese women. Clin Endocrinol (Oxf) 2012; 77:684–90 [DOI] [PubMed] [Google Scholar]
- 92.Klein S, Mittendorfer B, Eagon JC, Patterson B, Grant L, Feirt N, Seki E, Brenner D, Korenblat K, McCrea J. Gastric bypass surgery improves metabolic and hepatic abnormalities associated with nonalcoholic fatty liver disease. Gastroenterology 2006; 130:1564–72 [DOI] [PubMed] [Google Scholar]
- 93.Pereira SS, Alvarez-Leite JI. Adipokines: biological functions and metabolically healthy obese profile. J Recep Lig Channel Res 2014; 7:15–25 [Google Scholar]
- 94.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 2007; 117:175–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chang JS, Chang CC, Chien E, Lin SS, Cheng-Shiuan T, Bai CH, Chao KC. Association between interleukin 1beta and interleukin 10 concentrations: a cross-sectional study in young adolescents in Taiwan. BMC Pediatr 2013; 13:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Charles BA, Doumatey A, Huang H, Zhou J, Chen G, Shriner D, Adeyemo A, Rotimi CN. The roles of IL-6, IL-10, and IL-1RA in obesity and insulin resistance in African-Americans. J Clin Endocrinol Metab 2011; 96:E2018–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhan J, Huang L, Ma H, Chen H, Yang Y, Tan S, Song W, Zhao W, Dai X. Reduced inflammatory responses of follicular helper T cell promote the development of regulatory B cells after roux-en-Y gastric bypass. Clin Exp Pharmacol Physiol 2017; 44:556–65 [DOI] [PubMed] [Google Scholar]
- 98.Clyne B, Olshaker JS. The C-reactive protein. J Emerg Med 1999; 17:1019–25 [DOI] [PubMed] [Google Scholar]
- 99.Du Clos TW. Function of C-reactive protein. Annals of Medicine 2000; 32:274–8 [DOI] [PubMed] [Google Scholar]
- 100.Kolb-Bachofen V. A review on the biological properties of C-reactive protein. Immunobiology 1991; 183:133–45 [DOI] [PubMed] [Google Scholar]
- 101.Jupe D. The acute phase response and laboratory testing. Aust Fam Physician 1996; 25:324–9 [PubMed] [Google Scholar]
- 102.Marnell L, Mold C. Du clos TW. C-reactive protein: ligands, receptors and role in inflammation. Clin Immunol 2005; 117:104–11 [DOI] [PubMed] [Google Scholar]
- 103.Tchernof A, Nolan A, Sites Cynthia K, Ades Philip A, Poehlman Eric T. Weight loss reduces C-Reactive protein levels in obese postmenopausal women. Circulation 2002; 105:564–9 [DOI] [PubMed] [Google Scholar]
- 104.McLaughlin T, Abbasi F, Lamendola C, Liang L, Reaven G, Schaaf P, Reaven P. Differentiation between obesity and insulin resistance in the association with C-Reactive protein. Circulation 2002; 106:2908–12 [DOI] [PubMed] [Google Scholar]
- 105.Holloszy JO, Fontana L. Caloric restriction in humans. Exp Gerontol 2007; 42:709–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hakeam HA, O’Regan PJ, Salem AM, Bamehriz FY, Jomaa LF. Inhibition of C-reactive protein in morbidly obese patients after laparoscopic sleeve gastrectomy. Obes Surg 2009; 19:456–60 [DOI] [PubMed] [Google Scholar]
- 107.Wong ATY, Chan DC, Armstrong J, Watts GF. Effect of laparoscopic sleeve gastrectomy on elevated C-reactive protein and atherogenic dyslipidemia in morbidly obese patients. Clin Biochem 2011; 44:342–4 [DOI] [PubMed] [Google Scholar]
- 108.Ruiz-Tovar J, Oller I, Galindo I, Llavero C, Arroyo A, Calero A, Diez M, Zubiaga L, Calpena R. Change in levels of C-reactive protein (CRP) and serum cortisol in morbidly obese patients after laparoscopic sleeve gastrectomy. Obes Surg 2013; 23:764–9 [DOI] [PubMed] [Google Scholar]
- 109.Rojano-Rodriguez ME, Valenzuela-Salazar C, Cardenas-Lailson LE, Romero Loera LS, Torres-Olalde M, Moreno-Portillo M. C-reactive protein level in morbidly obese patients before and after bariatric surgery. Rev Gastroenterol Mex 2014; 79:90–5 [DOI] [PubMed] [Google Scholar]
- 110.Ress C, Tschoner A, Engl J, Klaus A, Tilg H, Ebenbichler C, Patsch J, Kaser S. Effect of bariatric surgery on circulating chemerin levels. Eur J Clin Invest 2010; 40:277–80 [DOI] [PubMed] [Google Scholar]
- 111.Petruschke T, Rohrig K, Hauner H. Transforming growth factor beta (TGF-beta) inhibits the differentiation of human adipocyte precursor cells in primary culture. Int J Obes Relat Metab Disord 1994; 18:532–6 [PubMed] [Google Scholar]
- 112.Alessi MC, Bastelica D, Morange P, Berthet B, Leduc I, Verdier M, Geel O, Juhan-Vague I. Plasminogen activator inhibitor 1, transforming growth factor-beta1, and BMI are closely associated in human adipose tissue during morbid obesity. Diabetes 2000; 49:1374. [DOI] [PubMed] [Google Scholar]
- 113.Yener S, Comlekci A, Akinci B, Akan P, Demir T, Bayraktar F, Yesil S. Serum transforming growth factor-beta 1 levels in normoalbuminuric and normotensive patients with type 2 diabetes. Effect of metformin and rosiglitazone. Hormones (Athens) 2008; 7:70–6 [DOI] [PubMed] [Google Scholar]
- 114.Yadav H, Quijano C, Kamaraju AK, Gavrilova O, Malek R, Chen W, Zerfas P, Zhigang D, Wright EC, Stuelten C, Sun P, Lonning S, Skarulis M, Sumner AE, Finkel T, Rane SG. Protection from obesity and diabetes by blockade of TGF-beta/Smad3 signaling. Cell Metab 2011; 14:67–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Samad F, Yamamoto K, Pandey M, Loskutoff DJ. Elevated expression of transforming growth factor-beta in adipose tissue from obese mice. Mol Med 1997; 3:37–48 [PMC free article] [PubMed] [Google Scholar]
- 116.Fain JN, Tichansky DS, Madan AK. Transforming growth factor β1 release by human adipose tissue is enhanced in obesity. Metab Clin Exp 2005; 54:1546–51 [DOI] [PubMed] [Google Scholar]
- 117.Cottam DR, Mattar SG, Barinas-Mitchell E, Eid G, Kuller L, Kelley DE, Schauer PR. The chronic inflammatory hypothesis for the morbidity associated with morbid obesity: implications and effects of weight loss. Obes Surg 2004; 14:589–600 [DOI] [PubMed] [Google Scholar]
- 118.Porreca E, Febbo CD, Vitacolonna E, Baccante G, Castelnuovo AD, Angelini A, Febo F, Di Nisio M, Cuccurullo F. Transforming growth factor-β1 levels in hypertensive patients: association with body mass index and leptin. Am J Hypertens 2002; 15:759–65 [DOI] [PubMed] [Google Scholar]
- 119.Lindegaard KK, Jorgensen NB, Just R, Heegaard PM, Madsbad S. Effects of roux-en-Y gastric bypass on fasting and postprandial inflammation-related parameters in obese subjects with normal glucose tolerance and in obese subjects with type 2 diabetes. Diabetol Metab Syndr 2015; 7:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Trayhurn P, Wood I. Signalling role of adipose tissue: adipokines and inflammation in obesity. London: Portland Press Limited, 2005. [DOI] [PubMed] [Google Scholar]
- 121.Esposito K, Pontillo A, Giugliano F, Giugliano G, Marfella R, Nicoletti G, Giugliano D. Association of low interleukin-10 levels with the metabolic syndrome in obese women. J Clin Endocrinol Metab 2003; 88:1055–8 [DOI] [PubMed] [Google Scholar]
- 122.Esposito K, Pontillo A, Di Palo C, Giugliano G, Masella M, Marfella R, Giugliano D. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA 2003; 289:1799–804 [DOI] [PubMed] [Google Scholar]
- 123.Botella-Carretero JI, Álvarez-Blasco F, Martinez-García MÁ, Luque-Ramírez M, San Millán JL, Escobar-Morreale HF. The decrease in serum IL-18 levels after bariatric surgery in morbidly obese women is a time-dependent event. Obes Surg 2007; 17:1199–208 [DOI] [PubMed] [Google Scholar]
- 124.Dixon JB, O’Brien PE. Obesity and the white blood cell count: changes with sustained weight loss. Obes Surg 2006; 16:251–7 [DOI] [PubMed] [Google Scholar]
- 125.Kullo IJ, Hensrud DD, Allison TG. Comparison of numbers of circulating blood monocytes in men grouped by body mass index (<25, 25 to <30, ≥30). Am J Cardiol 2002; 89:1441–3 [DOI] [PubMed] [Google Scholar]
- 126.Fisch IR, Freedman SH. Smoking, oral contraceptives, and obesity. Effects on white blood cell count. JAMA 1975; 234:500–6 [PubMed] [Google Scholar]
- 127.Lynch LA, O’Connell JM, Kwasnik AK, Cawood TJ, O’Farrelly C, O’Shea DB. Are natural killer cells protecting the metabolically healthy obese patient? Obesity 2009; 17:601–5 [DOI] [PubMed] [Google Scholar]
- 128.Simar D, Versteyhe S, Donkin I, Liu J, Hesson L, Nylander V, Fossum A, Barrès R. DNA methylation is altered in B and NK lymphocytes in obese and type 2 diabetic human. Metab Clin Exp 2014; 63:1188–97 [DOI] [PubMed] [Google Scholar]
- 129.Duffaut C, Zakaroff-Girard A, Bourlier V, Decaunes P, Maumus M, Chiotasso P, Sengenes C, Lafontan M, Galitzky J, Bouloumie A. Interplay between human adipocytes and T lymphocytes in obesity: CCL20 as an adipochemokine and T lymphocytes as lipogenic modulators. Arterioscler Thromb Vasc Biol 2009; 29:1608–14 [DOI] [PubMed] [Google Scholar]
- 130.Wu H, Ghosh S, Perrard XD, Feng L, Garcia GE, Perrard JL, Sweeney JF, Peterson LE, Chan L, Smith CW, Ballantyne CM. T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation 2007; 115:1029–38 [DOI] [PubMed] [Google Scholar]
- 131.Wang Q, Wu H. T cells in adipose tissue: critical players in immunometabolism. Front Immunol 2018; 9:2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ahmed T, Das SK, Golden JK, Saltzman E, Roberts SB, Meydani SN. Calorie restriction enhances T-Cell-mediated immune response in adult overweight men and women. J Gerontol A Biol Sci Med Sci 2009; 64A:1107–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Meydani SN, Das SK, Pieper CF, Lewis MR, Klein S, Dixit VD, Gupta AK, Villareal DT, Bhapkar M, Huang M, Fuss PJ, Roberts SB, Holloszy JO, Fontana L. Long-term moderate calorie restriction inhibits inflammation without impairing cell-mediated immunity: a randomized controlled trial in non-obese humans. Aging (Albany NY) 2016; 8:1416–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ziccardi P, Nappo F, Giugliano G, Esposito K, Marfella R, Cioffi M, D’Andrea F, Molinari AM, Giugliano D. Reduction of inflammatory cytokine concentrations and improvement of endothelial functions in obese women after weight loss over one year. Circulation 2002; 105:804–9 [DOI] [PubMed] [Google Scholar]
- 135.Chen S-B, Lee Y-C, Ser K-H, Chen J-C, Chen SC, Hsieh H-F, Lee W-J. Serum C-Reactive protein and white blood cell count in morbidly obese surgical patients. Obes Surg 2009; 19:461–66 [DOI] [PubMed] [Google Scholar]
- 136.Fathy SM, Morshed G. Peripheral blood lymphocyte subsets (CD4+, CD8+ T cells), leptin level and weight loss after laparoscopic greater curvature plication in morbidly obese patients. Arch Med Sci 2014; 10:886–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Moulin CM, Marguti I, Peron JP, Halpern A, Rizzo LV. Bariatric surgery reverses natural killer (NK) cell activity and NK-related cytokine synthesis impairment induced by morbid obesity. Obes Surg 2011; 21:112–8 [DOI] [PubMed] [Google Scholar]
- 138.Michelson AD. How platelets work: Platelet function and dysfunction. J Thromb Thrombolysis 2003; 16:7–12 [DOI] [PubMed] [Google Scholar]
- 139.Machlus KR, Thon JN, Italiano JE., Jr. Interpreting the developmental dance of the megakaryocyte: a review of the cellular and molecular processes mediating platelet formation. Br J Haematol 2014; 165:227–36 [DOI] [PubMed] [Google Scholar]
- 140.Anfossi G, Russo I, Trovati M. Platelet dysfunction in central obesity. Nutr Metab Cardiovasc Dis 2009; 19:440–9 [DOI] [PubMed] [Google Scholar]
- 141.Samocha-Bonet D, Justo D, Rogowski O, Saar N, Abu-Abeid S, Shenkerman G, Shapira I, Berliner S, Tomer A. Platelet counts and platelet activation markers in obese subjects. Mediators Inflamm 2008; 2008:834153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Furuncuoglu Y, Tulgar S, Dogan AN, Cakar S, Tulgar YK, Cakiroglu B. How obesity affects the neutrophil/lymphocyte and platelet/lymphocyte ratio, systemic immune-inflammatory index and platelet indices: a retrospective study. Eur Rev Med Pharmacol Sci 2016; 20:1300–6 [PubMed] [Google Scholar]
- 143.Coban E, Yilmaz A, Sari R. The effect of weight loss on the mean platelet volume in obese patients. Platelets 2007; 18:212–6 [DOI] [PubMed] [Google Scholar]
- 144.Johansson H-E, Haenni A, Zethelius B. Platelet counts and liver enzymes after bariatric surgery. J Obes 2013; 2013:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cheng V, Kashyap SR, Schauer PR, Kirwan JP, McCrae KR. Restoration of glycemic control in patients with type 2 diabetes mellitus after bariatric surgery is associated with reduction in microparticles. Surg Obes Relat Dis 2013; 9:207–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yang J, Zhang L, Yu C, Yang X-F, Wang H. Monocyte and macrophage differentiation: circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomark Res 2014; 2:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Subramanian V, Ferrante JA. Obesity, inflammation, and macrophages. In: Emerging societies – coexistence of childhood malnutrition and obesity. Basel: Karger Publishers, 2009, pp.151–62
- 148.Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C, Coupaye M, Pelloux V, Hugol D, Bouillot J-L, Bouloumié A, Barbatelli G, Cinti S, Svensson P-A, Barsh GS, Zucker J-D, Basdevant A, Langin D, Clément K. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes 2005; 54:2277–86 [DOI] [PubMed] [Google Scholar]
- 149.Poitou C, Dalmas E, Renovato M, Benhamo V, Hajduch F, Abdennour M, Kahn J-F, Veyrie N, Rizkalla S, Fridman W-H, Sautès-Fridman C, Clément K, Cremer I. CD14dimCD16+ and CD14+CD16+ monocytes in obesity and during weight loss. Arterioscler Thromb Vasc Biol 2011; 31:2322–30 [DOI] [PubMed] [Google Scholar]
- 150.Billeter A, Vittas S, Israel B, Schulte T, Büchler MW, Hidmark A, Fleming TH, Müller BP, Nawroth PP. Gastric bypass improves glycemic control and reduces systemic inflammation in non-severely obese patients (BMI < 35 kg/m2) with type 2 diabetes mellitus. Diabetologie Und Stoffwechsel 2015; 10:P290 [Google Scholar]
- 151.Aron-Wisnewsky J, Tordjman J, Poitou C, Darakhshan F, Hugol D, Basdevant A, Aissat A, Guerre-Millo M, Clément K. Human adipose tissue macrophages: M1 and M2 cell surface markers in subcutaneous and omental depots and after weight loss. J Clin Endocrinol Metab 2009; 94:4619–23 [DOI] [PubMed] [Google Scholar]
- 152.van de Sande-Lee S, Pereira FRS, Cintra DE, Fernandes PT, Cardoso AR, Garlipp CR, Chaim EA, Pareja JC, Geloneze B, Li LM, Cendes F, Velloso LA. Partial reversibility of hypothalamic dysfunction and changes in brain activity after body mass reduction in obese subjects. Diabetes 2011; 60:1699–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gunstad J, Paul RH, Cohen RA, Tate DF, Spitznagel MB, Grieve S, Gordon E. Relationship between body mass index and brain volume in healthy adults. Int J Neurosci 2008; 118:1582–93 [DOI] [PubMed] [Google Scholar]
- 154.Debette S, Beiser A, Hoffmann U, DeCarli C, O’Donnell CJ, Massaro JM, Au R, Himali JJ, Wolf PA, Fox CS, Seshadri S. Visceral fat is associated with lower brain volume in healthy middle-aged adults. Ann Neurol 2010; 68:136–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Raji CA, Ho AJ, Parikshak NN, Becker JT, Lopez OL, Kuller LH, Hua X, Leow AD, Toga AW, Thompson PM. Brain structure and obesity. Hum Brain Mapp 2010; 31:353–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Thaler JP, Yi C-X, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, Zhao X, Sarruf DA, Izgur V, Maravilla KR, Nguyen HT, Fischer JD, Matsen ME, Wisse BE, Morton GJ, Horvath TL, Baskin DG, Tschöp MH, Schwartz MW. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest 2012; 122:153–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kreutzer C, Peters S, Schulte DM, Fangmann D, Türk K, Wolff S, van Eimeren T, Ahrens M, Beckmann J, Schafmayer C, Becker T, Kerby T, Rohr A, Riedel C, Heinsen F-A, Degenhardt F, Franke A, Rosenstiel P, Zubek N, Henning C, Freitag-Wolf S, Dempfle A, Psilopanagioti A, Petrou-Papadaki H, Lenk L, Jansen O, Schreiber S, Laudes M. Hypothalamic inflammation in human obesity is mediated by environmental and genetic factors. Diabetes 2017; 66:2407. [DOI] [PubMed] [Google Scholar]
- 158.Prehn K, Jumpertz von Schwartzenberg R, Mai K, Zeitz U, Witte AV, Hampel D, Szela A-M, Fabian S, Grittner U, Spranger J, Flöel A. Caloric restriction in older adults – differential effects of weight loss and reduced weight on brain structure and function. Cerebral Cortex 2016; 27:1765–78 [DOI] [PubMed] [Google Scholar]
- 159.Espeland MA, Erickson K, Neiberg RH, Jakicic JM, Wadden TA, Wing RR, Desiderio L, Erus G, Hsieh M-K, Davatzikos C, Maschak-Carey BJ, Laurienti PJ, Demos-McDermott K, Bryan RN. Brain and white matter hyperintensity volumes after 10 years of random assignment to lifestyle intervention. Diabetes Care 2016; 39:764–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Fink LN, Oberbach A, Costford SR, Chan KL, Sams A, Bluher M, Klip A. Expression of anti-inflammatory macrophage genes within skeletal muscle correlates with insulin sensitivity in human obesity and type 2 diabetes. Diabetologia 2013; 56:1623–8 [DOI] [PubMed] [Google Scholar]
- 161.Fink LN, Costford SR, Lee YS, Jensen TE, Bilan PJ, Oberbach A, Blüher M, Olefsky JM, Sams A, Klip A. Pro-Inflammatory macrophages increase in skeletal muscle of high fat-fed mice and correlate with metabolic risk markers in humans. Obesity (Silver Spring) 2014; 22:747–57 [DOI] [PubMed] [Google Scholar]
- 162.Bruun JM, Helge JW, Richelsen B, Stallknecht B. Diet and exercise reduce low-grade inflammation and macrophage infiltration in adipose tissue but not in skeletal muscle in severely obese subjects. Am J Physiol Endocrinol Metab 2006; 290:E961–7 [DOI] [PubMed] [Google Scholar]
- 163.Lambert CP, Wright NR, Finck BN, Villareal DT. Exercise but not diet-induced weight loss decreases skeletal muscle inflammatory gene expression in frail obese elderly persons. J Appl Physiol 2008; 105:473–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Park EJ, Lee JH, Yu G-Y, He G, Ali SR, Holzer RG, Österreicher CH, Takahashi H, Karin M. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell 2010; 140:197–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sun B, Karin M. Obesity, inflammation, and liver cancer. J Hepatol 2012; 56:704–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Moschen AR, Molnar C, Geiger S, Graziadei I, Ebenbichler CF, Weiss H, Kaser S, Kaser A, Tilg H. Anti-inflammatory effects of excessive weight loss: potent suppression of adipose interleukin 6 and tumour necrosis factor α expression. Gut 2010; 59:1259–64 [DOI] [PubMed] [Google Scholar]
- 167.Larson-Meyer DE, Newcomer BR, Heilbronn LK, Volaufova J, Smith SR, Alfonso AJ, Lefevre M, Rood JC, Williamson DA, Ravussin E. Effect of 6-month calorie restriction and exercise on serum and liver lipids and markers of liver function. Obesity (Silver Spring) 2008; 16:1355–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sell H, Divoux A, Poitou C, Basdevant A, Bouillot J-L, Bedossa P, Tordjman J, Eckel Jr, Clément K. Chemerin correlates with markers for fatty liver in morbidly obese patients and strongly decreases after weight loss induced by bariatric surgery. J Clin Endocrinol Metab 2010; 95:2892–96 [DOI] [PubMed] [Google Scholar]
- 169.Mattar SG, Velcu LM, Rabinovitz M, Demetris AJ, Krasinskas AM, Barinas-Mitchell E, Eid GM, Ramanathan R, Taylor DS, Schauer PR. Surgically-induced weight loss significantly improves nonalcoholic fatty liver disease and the metabolic syndrome. Ann Surg 2005; 242:610–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Raetz CR, Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem 2002; 71:635–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hurley JC. Endotoxemia: methods of detection and clinical correlates. Clin Microbiol Rev 1995; 8:268–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Boutagy NE, McMillan RP, Frisard MI, Hulver MW. Metabolic endotoxemia with obesity: is it real and is it relevant? Biochimie 2016; 124:11–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Moreno-Navarrete JM, Ortega F, Serino M, Luche E, Waget A, Pardo G, Salvador J, Ricart W, Frühbeck G, Burcelin R, Fernández-Real JM. Circulating lipopolysaccharide-binding protein (LBP) as a marker of obesity-related insulin resistance. Int J Obes 2012; 36:1442–9 [DOI] [PubMed] [Google Scholar]
- 174.Duan Y, Zeng L, Zheng C, Song B, Li F, Kong X, Xu K. Inflammatory links between high fat diets and diseases. Front Immunol 2018; 9:2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lee H, Lee IS, Choue R. Obesity, inflammation and diet. Pediatr Gastroenterol Hepatol Nutr 2013; 16:143–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yang P-J, Lee W-J, Tseng P-H, Lee P-H, Lin M-T, Yang W-S. Bariatric surgery decreased the serum level of an endotoxin-associated marker: lipopolysaccharide-binding protein. Surg Obes Relat Dis 2014; 10:1182–7 [DOI] [PubMed] [Google Scholar]
- 177.Blüher M. The distinction of metabolically ‘healthy’ from ‘unhealthy’ obese individuals. Curr Opin Lipidol 2010; 21:38–43 [DOI] [PubMed] [Google Scholar]
- 178.Catoi AF, Busetto L. Metabolically healthy obesity and bariatric surgery. Obes Surg 2019; 29:2989–3000 [DOI] [PubMed] [Google Scholar]
- 179.Hamer M, Stamatakis E. Metabolically healthy obesity and risk of all-cause and cardiovascular disease mortality. J Clin Endocrinol Metab 2012; 97:2482–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gómez-Ambrosi J, Catalán V, Rodríguez A, Andrada P, Ramírez B, Ibáñez P, Vila N, Romero S, Margall MA, Gil MJ, Moncada R, Valentí V, Silva C, Salvador J, Frühbeck G. Increased cardiometabolic risk factors and inflammation in adipose tissue in obese subjects classified as metabolically healthy. Diabetes Care 2014; 37:2813–21 [DOI] [PubMed] [Google Scholar]
- 181.Kramer CK, Zinman B, Retnakaran R. Are metabolically healthy overweight and obesity benign conditions? A systematic review and Meta-analysis. Ann Intern Med 2013; 159:758–69 [DOI] [PubMed] [Google Scholar]