Short abstract
Necrotizing enterocolitis is the leading cause of death due to gastrointestinal disease in preterm neonates, affecting 5–12% of neonates born at a very-low birth weight. Necrotizing enterocolitis can present with a slow and insidious onset, with some neonates displaying early symptoms such as feeding intolerance. Treatment during the early stages includes bowel rest and careful use of antibiotics, but surgery is required if pneumoperitoneum and intestinal perforation occur. Mortality rates among neonates requiring surgery are estimated to be 20–30%, mandating the development of non-invasive and reliable biomarkers to predict necrotizing enterocolitis before the onset of clinical signs. Such biomarkers would allow at-risk neonates to receive maximal preventative therapies such as careful nutritional consideration, probiotics, and increased skin-to-skin care.
Impact statement
Necrotizing enterocolitis (NEC) is a devastating gastrointestinal disease; its high mortality rate mandates the development of non-invasive biomarkers to predict NEC before its onset. This review summarizes the pathogenesis, prevention, unresolved issues, and long-term outcomes of NEC.
Keywords: Vagus, biomarker, animal models, brainstem
Epidemiology of necrotizing enterocolitis
In the United States, 9.9% of neonates were born prior to 37 weeks’ gestational age in 2017; these births are classified as preterm.1 Many of these premature neonates require extended hospitalization due to comorbid factors due to low gestational age and weight at birth. Necrotizing enterocolitis (NEC) is the leading cause of death due to gastrointestinal (GI) disease in preterm neonates, affecting 5–12% of neonates born at a very-low birth weight (VLBW; <1500 g).2–10 NEC symptoms can be slow and insidious at first, including feeding intolerance, but can quickly progress to fulminant NEC with hallmark signs such as pneumatosis intestinalis and/or portal venous gas.5,7,8,11–15 In neonates with NEC who require surgery to resect the perforated portions of bowel, the mortality rate is estimated between 20 and 30%, the highest mortality rate among neonates requiring surgery.16 On average, neonates not requiring surgery are estimated to be hospitalized in the neonatal intensive care unit (NICU) 20 days longer as compared to unaffected neonates, and neonates requiring surgery are on average hospitalized a further 60 days longer.17 Therefore, NEC accounts for a large portion of the financial burden associated with preterm birth; indeed, the average total treatment cost per patient is estimated to be $500,000, with the total cost per year in the United States estimated between $500 million and $1 billion.7,8,17,18 Furthermore, the need for bowel resection surgery as a complication of NEC is the primary cause of neonatal short-bowel syndrome (SBS); in these cases, the average cost of care for the first five years of life is estimated to be $1.5 million per patient.19
NEC risk factors and pathogenesis
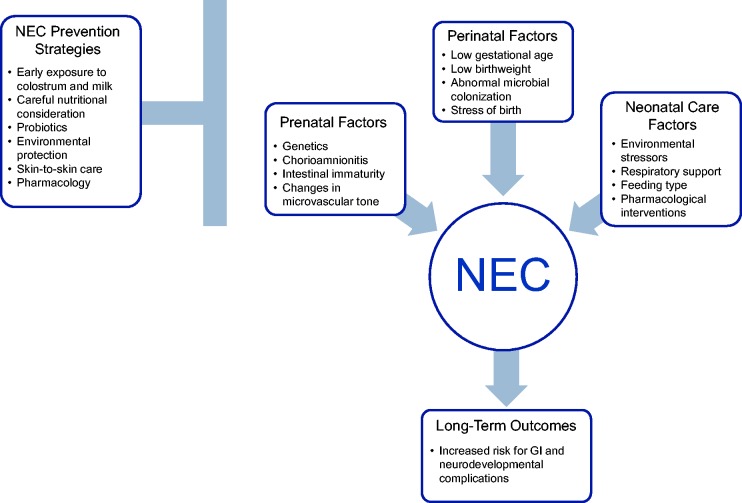
NEC pathophysiology is generally hypothesized to be multi-factorial, common risk factors include low gestational age at birth, low birth weight, chorioamnionitis, mechanical ventilation, and many more.2,3,13,14,16,20,21 Research into the pathophysiology of NEC has further uncovered risk factors such as genetic predisposition, intestinal immaturity, changes in microvascular tone, and abnormal microbial colonization.7,8,15,22 Although no studies have found a clear genetic phenotype associated strongly with NEC, studies have found a familial predisposition for the disease.23 Most studies further suggest that genetic variants leading to the upregulation of downstream signaling receptors of Toll-like receptor-4 (TLR-4), an innate immune receptor, may increase the risk of developing NEC.24–27 Specifically, these signaling regulators may include nuclear factor κB1, the small glycolipid transport protein ganglioside GM2 activator, co-receptor molecule lymphocyte antigen 96, and single Ig interleukin (IL)-1 related receptor.8 Previous studies have shown that pro-inflammatory cytokines involved in TLR signaling, such as IL-6 and IL-8, are elevated at the time of NEC diagnosis and may also be elevated during the early stages of the disease.28,29 Both of these cytokines are increased within the first 2 to 4 h after infection and then decline gradually over the next 24 h.30,31 Gender and racial disparities in NEC incidence are most likely due to genetic variations, such as single nucleotide polymorphisms, in these populations.32
Figure 1.
NEC risks, prevention, and outcomes. (A color version of this figure is available in the online journal.)
NEC: necrotizing enterocolitis.
Preterm neonates are more susceptible to intestinal injury due to the underdeveloped nature of their intestine.33–37 Specifically, preterm neonates lack several GI defense mechanisms such as gastric acid, digestive enzymes, mucus production, peristalsis, and polymeric immunoglobulin A (IgA).34,35,38 In healthy patients, gastric acidity and digestive enzymes eliminate most antigens and ingested pathogens, while mucus inhibits microbial adherence.38,39 Active organized peristalsis is required to prevent bacterial immobility and to eliminate antigen–antibody complexes that can be detrimental to the GI tract.38–41 Finally, polymeric IgA is required to bind luminal antigens to reduce their risk of penetration.38–41 All of these critical defenses are under developed in preterm neonates, increasing their susceptibility to GI injury and disease.33–37 In addition to these defenses, nitric oxide may also play a role in the pathogenesis of NEC.8,14,15 Low levels of nitric oxide regulate mucosal blood flow and vascular tone, but high levels may weaken the gut barrier through increased bacteria translocation, impaired mitochondrial function, and decreased leukocyte recruitment to the endothelium.42,43
At birth, neonates are rapidly exposed to environmental bacteria, initially through maternal vaginal flora and enteral feedings. Preterm neonates experience a delayed and often inappropriate colonization, leading to increased inflammatory responses and abnormal bacterial glycosylation patterns.38,39,44 Further complications include delayed enteral feedings, early exposure to broad spectrum antibiotics, and formula feeding, which all contribute to the delayed colonization of the gut and an increased risk of pathogenic colonization.38,39,44 Studies comparing the microbiota of preterm neonates who develop NEC, as compared to control neonates, have found that NEC leads to unusual intestinal microbial species and an overall reduction in microbiota diversity.42,45 This reduction in diversity of the microbiome may leave neonates more susceptible to infectious diseases, especially when harmful bacteria may be introduced via catheterization and enteric feeding.46,47
Other risk factors for NEC include maternal, ex-utero transition, and neonatal care factors.2–5,7,8,12–15 Maternal risk factors include illicit drug abuse, infection with chorioamnionitis, and HIV-positive status.2,7,8,13,48 Risk factors that occur during the transition to ex-utero life include low flow and perfusion states due to perinatal events, such as placental abruption, leading to neonatal shock which is characterized by hypovolemia and academia.9,10,49 These conditions are reflected in the Score for Neonatal Acute Physiology (SNAP) and/or Apgar score at 5 min of life; these scores have been shown to be reliable and significant predictors of neonatal mortality in preterm neonates, and scores are typically decreased in neonates who later develop NEC.21,50 Neonatal care factors include respiratory support, feeding type, and pharmacological interventions. Studies have also shown that neonates who require respiratory support during the early neonatal period are 12.6 times more likely to develop NEC.51 Formula feeding without supplemental breast milk has also been shown to increase the risk of developing NEC by 6.4 times.39,44,51 Furthermore, pharmacological interventions such as histamine H2 receptor antagonists, indomethacin, indomethacin tocolysis, glucocorticoids, and concomitant use of indomethacin and glucocorticoids also leave the neonate at an increased risk of developing NEC.21,52 Finally, congenital abnormalities, especially those affecting the heart and/or GI, such as congenital heart disease, patent ductus arteriosus, and gastroschisis, can increase the risk of NEC.52–54
NEC diagnosis and treatment
Typically, NEC is diagnosed via the Bell’s Modified Staging Criteria, which has three classical stages of NEC: mild (Bell’s Stage I), moderate (Bell’s Stage II), and severe (Bell’s Stage III).11 Mild or suspected NEC (Bell’s Stage I) is classified by mild systemic signs such as temperature instability and bradycardia, in addition to mild non-specific intestinal signs such as mild abdominal distension and occult blood in the stool.7,8,11 Moderate or definitive NEC (Bell’s Stage II) further includes radiological findings of pneumatosis intestinalis and/or portal venous gas with moderate systemic signs such as abdominal tenderness, thrombocytopenia, and metabolic acidosis.7,8,11 These systemic and local factors leave the intestine, specifically the distal ileum and proximal colon, susceptible to inflammatory processes and perforation leading to pneumoperitoneum.55,56 Finally, advanced NEC (Bell’s Stage III) requiring surgical intervention is characterized by bowel perforation with resultant pneumoperitoneum, hypotension, signs of peritonitis, and severe metabolic acidosis.7,8,11 Gestational age must also be taken into account when considering the diagnosis of NEC, where gestational age and the onset and severity of NEC symptoms have an inverse correlation.10,20,57 Studies have shown that the mean gestational age at birth of peak NEC onset is 32 weeks.58
Current treatment strategies of NEC differ based on the severity stage, but generally include broad-spectrum antibiotics, bowel rest, and ionotropic and fluid support.3,7,8 Surgical intervention to resect portions of the ischemic bowel is required if the disease progresses to the advanced stages.3,5,7,8,12,21 Broad-spectrum antibiotics are typically prescribed where there is concern for sepsis, including antibiotics to cover anaerobic organisms which are also prescribed in cases of suspected or confirmed perforation.3,7,8 Depending on the severity stage, treatment may also include the management of hypotension, metabolic acidosis, and thrombocytopenia.3,7,8 Overall, although our knowledge of the pathogenesis of NEC has advanced over the past few decades, treatment strategies have not impacted the frequency or severity of the disorder; therefore, the focus of clinical and basic science has shifted to the prevention of NEC.
NEC prevention
Due to the lack of effective treatments for NEC, research focus has shifted to testing strategies for the prevention of NEC, specifically early exposure to colostrum and mother’s own milk, careful nutritional consideration, probiotics, environmental protection, skin-to-skin care (SSC), and pharmacology.2–5,14 Colostrum, the first milk produced by mothers in the days after birth, has been shown to contain high concentrations of beneficial immune mediators that provide bacterial and anti-inflammatory protection and stimulate the development of the GI tract.59–64 Our group has shown recently that oropharyngeal administration of colostrum increases salivary secretory IgA levels,65 which may be protective against NEC. Other studies have shown that preterm neonates receiving colostrum had a significantly decreased incidence of neonatal sepsis66 and shortened time to attain full enteral feeds.64 Oropharyngeal colostrum may convey these benefits to the neonates by stimulating the oropharyngeal-associated lymphoid system, providing a mucosal barrier to prevent microbial adhesion, and inducing systemic immune responses.67 Additionally, colostrum has a high concentration of growth factors that may stimulate intestinal growth and development, particularly in mothers who have delivered preterm neonates.68,69 Although no studies have reported harmful effects of colostrum administration, further randomized clinical trials are needed to evaluate its efficacy and elucidate its mechanism of action.64,66
Many studies have shown the benefits of neonates, especially preterm neonates, receiving either mother’s own or banked breast milk.2,3,6,8,60–62,70–72 In the preterm neonates, however, there are several challenges that can prevent them from receiving enteral feeds of breast milk. These challenges include an underdeveloped suck-swallow-breathe reflex, motor coordination issues, GI reflux, and low body stores of energy.9,20,33,36,37,73 Aggressive enteral feeding in the presence of these issues or respiratory and/or cardiac support, feeding intolerance, or high doses of certain medications can lead to an increase in NEC susceptibility.2,3,13,14,21,48,62 Therefore, after birth, preterm neonates often receive parenteral nutrition while progressing to full enteral volume under careful monitoring.3,6,62 Trophic, low volume feeds of colostrum and mother’s own or banked breast milk are widely recognized as the best means of gut protection by preventing villous atrophy, mucosal injury, and leaky gut.2–7,12,13,62,63 Human breast milk contains many factors thought to help prevent NEC including nitrate/nitrite antioxidant factors, L-arginine, human milk oligosaccharides and prebiotics, secretory IgA, platelet-activating factor acetylhydrolase, lactoferrin, and growth factors.8,59,62,71,72,74,75
Administration of probiotics and commensal bacteria may protect the preterm gut against inflammation and injury via a variety of mechanisms.47,76–78 These mechanisms are thought to include down-regulation of pro-inflammatory gene expression, upregulation of cytoprotective genes, production of butyrate, and regulation of cellular immunity.46,47,77,79 These mechanisms may work to support gut barrier maturation and function, lower the pH of the gut, inhibit other microbes, and nourish colonocytes.46,76,77,79 Randomized clinical trials and observational studies have found that probiotics reduce the incidence of NEC and all-cause mortality, and no harmful effects have been reported.47,76–84 However, the precise probiotic agent, along with its timing, dose, duration, and most effective formulation in preventing NEC has not yet been established.80–84 In addition to the administration of probiotics, environmental factors may also be effective in the prevention of NEC.
Environmental protective strategies, such as reducing exposure to excessive light and sound, cue driven care, and SSC may also be critical in the prevention of NEC. SSC has been shown to decrease mortality rates, improve short- and long-term developmental outcomes, and strengthen the bond between infant and mother, among other positive effects.61,85–87 In this regard, increased frequency of SSC is associated with increased vagal tone during the first week of life, and predicted diminished neonatal morbidity.88 Furthermore, SSC decreases the incidence and severity of NEC.61,85–87 These studies suggest that SSC enhances stress resiliency, reduces allostatic load, and leads to improved health outcomes.88 Animal models suggest that SSC may improve resting vagal tone and promote maturation of vago-vagal circuits; these studies have specifically shown that maternal proximity improves autonomic functioning, arousal regulation, and orienting behavior in newborn rats.87,89 In these animal models, isolated components from the dam such as maternal body heat or smell affected the respective body systems in the pups.87 This suggests that SSC integrates the thermal, rhythmic, and sensory components of maternal presence to integrate autonomic functions in the newborn.87,89 Thus, the widespread use of SSC, beginning soon after preterm birth and continuing daily throughout hospitalization is proposed as a foundational element of care to reduce morbidity, especially in the prevention of NEC.61,85–88
In addition to these environmental protective strategies, several studies have evaluated the potential of numerous pharmacological treatments to prevent the onset of NEC, with varying results. The use of antenatal steroids to reduce the incidence of NEC is controversial, as steroids increase the incidence of spontaneous intestinal perforation, but no studies to date have found a clear correlation between steroid use and NEC.21,74 Other pharmacological interventions under consideration in animal models include those that modulate inflammation, specifically those that affect TLR signaling.24–27,90–93 Studies in rodent models of NEC and in samples of resected bowel from NEC neonates suggest that there are more TLR-4 surface receptors in NEC cases as compared to controls or full-term neonates.24,26,94 High levels of inflammation, like those seen in NEC, lead to differential localization of TLR-4 receptors, making them a potential target for the treatment of NEC and inflammatory disorders.24,26,94 Other pharmacological treatments currently under investigation include, but are not limited to, heparin-binding EGF-like growth factor,95–97 human milk oligosaccharides,60,72 and lactoferrin,75 but more research is needed to evaluate their efficacy and safety.
Long-term outcomes of NEC
Neonates who survive medical or surgical NEC are at an increased risk for long-term GI and neurodevelopmental complications. Surgical treatment of NEC typically includes resection of ischemic bowel portions; the long-term outcome of these patients is dependent on the length of remaining intestine and its ability to absorb nutrients adequately.18,55–57 Specifically, ileal resection may lead to GI dysmotility, abnormal mucosa, bacterial overgrowth, and vitamin B12 or enzyme deficiency, causing malabsorption of nutrients.18,55–57 Furthermore, NEC is the most common cause of SBS in neonates, which can lead to GI complications such as gastric acid hyper-secretion, bacterial overgrowth, D-lactic acidosis, translocation of enteric bacteria to the bloodstream, and intestinal failure-associated liver disease.18,55–57,98 Clinical management of SBS requires a multi-disciplinary approach to ensure that the neonate receives sufficient nutrition for growth, to maximize intestinal adaptation, and to minimize fluid, electrolyte, and nutritional losses.98
In addition to potential GI complications, neonates with NEC are at an increased risk for persistent neurological and cognitive alterations. NEC Stage ≥2 is associated with long-term neurodevelopmental impairment, and these neonates have an increased incidence of cerebral palsy, visual, cognitive and psychomotor impairment.99,100 These long-term effects are likely due to NEC occurring during a critical developmental time frame, when the developing brain is vulnerable to insults and nutritional deficits.100–103 Acute insults as a result of NEC may include systemic inflammation, hypoxia, ischemia, and multisystem organ failure, but long-term nutritional deficits due to SBS may also impact the developing brain.100–103 The exact mechanisms underlying these long-term impairments remains unclear; however, the increased levels of local inflammation and circulating pro-inflammatory cytokines suggest that inflammation may play a major role.100,101 Some researchers hypothesize that intestinal injury and barrier dysfunction as a result of NEC allows for the translocation of inflammatory mediators into systemic circulation.100,101 These inflammatory mediators may then target vulnerable cerebral oligodendrocytes and microglia, both of which are still developing during the peak time of NEC incidence.100,101 This theory is supported by studies that report increased NEC severity is associated with adverse neurodevelopment and that increased serum levels of pro-inflammatory cytokines in NEC neonates are associated with increased risk for poor growth and development.104,105
Unresolved issues
The devastating effects of NEC mandate the development of minimally invasive predictive biomarkers to identify neonates at risk prior to the onset of clinical signs; one of the most promising non-invasive biomarkers currently under investigation is heart rate variability (HRV). HRV measures can be calculated based on electrocardiogram (ECG) recordings, using a fast Fourier transformation of inter-beat-interval (IBI) values for both time- and frequency-domain analysis.106–112 In time-domain analysis, the difference between sequential IBI values and root-mean-square of successive differences (RMSSD) represent vagal tone; similarly, the HF power spectrum (HF-HRV) in frequency-domain analysis assesses indirectly vagal parasympathetic outflow and has been identified as a marker for fetal and neonatal wellbeing.111–114 Conversely, the low frequency (LF) spectrum of HRV frequency-domain analysis represents a mix of sympathetic and parasympathetic outflow.106–112 Our group has shown that preterm neonates who later develop NEC display a diminished HF-HRV power prior to the onset of clinical signs, as compared to control neonates.113 We have also shown that a rat model of mild NEC significantly attenuates the typical developmental increases in HF-HRV when combined with the stress of laparotomy or subdiaphragmatic vagotomy.115 The major advantages of HF-HRV include its utility in non-invasively predicting NEC days to weeks before its onset, relatively low cost, and ease of analysis using existing software.
Another non-invasive method that has been evaluated in the prediction of NEC is breath hydrogen monitoring.116–118 This test relies on analyzing the various gases present in human breath, including carbon dioxide, carbon monoxide, hydrogen, and nitric oxide.117 Breath hydrogen can be used to indirectly assess stress levels in the GI mucosa; increased concentrations of hydrogen in breath samples indicate bacterial metabolization of luminal substrates.116,117 Studies have shown elevated concentrations of hydrogen in the breath of neonates who later develop NEC on average 24 h before NEC diagnosis.119,120 Some studies report a high sensitivity and specificity for breath hydrogen monitoring in the detection of NEC, but it has been shown to have a low positive predictive value of only 33%.119,120 In addition to this low positive predictive value, there are also technical difficulties and confounders that limit its use as a predictive biomarker. Specifically, the site of breath collection must be tightly sealed and the measure is affected by many covariates; interference can be caused by changes in the microbiome, tidal volume, respiratory rate, breath holding, the patient’s cardiopulmonary status, and need for mechanical ventilation.117 Furthermore, calibration can often be an issue as breath hydrogen levels must be standardized to amount of carbohydrates in enteral feeds, which is often changing in preterm neonates.116–120 Overall, experts in the field agree that the confounders of this technique outweigh its utility, and agree there are practical and theoretical flaws with breath hydrogen monitoring for the detection of NEC.117,118
Other biomarkers under investigation include pro-inflammatory cytokines, C-reactive protein (CRP), and serum amyloid A (SAA).22,121 The early rise in cytokine levels has been shown to have high specificity in the diagnosis of neonatal sepsis alone, but combination with additional biomarkers is needed to have specificity and sensitivity for NEC diagnosis.28,29,31,122,123 CRP is a generalized biomarker of inflammation and was previously one of the most widely used predictive biomarkers of NEC and sepsis by clinicians in the NICU.31,121–123 However, studies have shown a wide range of sensitivity levels using CRP for the prediction of early onset neonatal sepsis, perhaps due to its wide range of comorbid associations such as meconium aspiration syndrome.31,121 Due to this lack of sensitivity and relative high cost for CRP analysis, currently, most clinicians do not utilize CRP as a biomarker of NEC.28,29,49,123–125 Alternatively, SAA levels rise earlier in the inflammatory response as compared to CRP, and SAA has a high sensitivity level in the prediction of neonatal sepsis.126,127 Currently, clinicians most commonly use advancing thrombocytopenia and elevated neutrophils as a marker of NEC progression and need for surgical intervention.3,5,8,18 Overall, serum biomarkers likely have the most clinical relevance when used in combination with one another, and perhaps in addition to a non-invasive marker such as HF-HRV.128–130 However, the collection of serum biomarkers can be invasive and stress-inducing, and results from these tests may not be received quickly enough to ensure neonates receive preventative measures to avert the onset of NEC.
Animal models of NEC
Insights into the pathogenesis and novel treatments of NEC have largely resulted from the study of animal models; currently, the most commonly used animal models for NEC are rats, mice, and piglets.4,7,8,15,131,132 In rats, NEC is typically induced in newborn rats through differing combinations of cesarean section, maternal separation, formula feeding, hypoxia, hypothermia, ischemia-reperfusion, and administration of intragastric lipopolysaccharide (LPS) or commensal bacteria.4,7,8,15,131,132 Studies relating human development have shown that rats at postnatal day 12/13 are representative of a term human neonate; therefore, newborn rat pups are an excellent model for the study of preterm neonates.133,134 The first rat model of NEC was described in 1974,70 and consisted of formula feeding and hypoxia. Shortly thereafter, the same group found hypothermia and hypoxia to be the key stressors in inducing moderate NEC.135 Our group has since established a mild form of NEC (equivalent to Bell’s Stage I) in rats through administration of hypothermia and hypoxia twice daily; this mild NEC is more representative of the early stages of NEC when preventative treatments would have the most efficacy.115 We have also shown that ghrelin, an orexigenic GI peptide, is able to attenuate the effects of mild NEC in rats, in a vagally dependent mechanism.136 Other models also include treatments such as intragastric LPS or commensal bacteria from stool samples to determine the roles of IL-18,137 IL-12,137 intestinal epithelial apoptosis,138 maternal milk,71,72 probiotics,139 NF-κB,140 nitric oxide dysregulation,42,141 and many more. Some models also include ischemia-reperfusion injury to mimic intestinal injuries occurring in NEC,142 but these models are more controversial as the direct connection to NEC remains uncertain.143 Overall, rat models can be highly effective in the study of NEC due to their ready availability, relative low cost, and high litter size. However, as few transgenic rat strains related to NEC pathology are commercially available, they do have limitations.
Similar to rat models, NEC in mice is typically induced through formula feeding, hypoxia, hypothermia, commensal bacteria administration, and ischemia-reperfusion, or through transgenic manipulation.4,7,8,15,131,132 The most widely used non-transgenic murine model of NEC includes formula feeding, hypoxia, and hypothermia, and induces NEC with gross and microscopic evidence of intestinal necrosis.24,144,145 This model has been used to study the role of various genes in the pathogenesis of NEC, including TLR-4,24 IL-18,146 MUC2,147 among others. Transgenic NEC mouse studies have evaluated the effect of manipulation to genes including IFN-γ,148 TLR-4,26,91–93 TLR-9,91 inducible nitric oxide synthase,149 70 kD heat shock proteins (HSP70),90 and many more. Such studies have begun to elucidate the role of the innate immune system on the developing GI tract and NEC,131 but further studies are needed to develop possible therapeutics to treat NEC. In summary, mice are an effective model of NEC due to the widely available transgenic strains, ease of genetic manipulation, high litter size, and relative low cost but can be difficult to handle due to their small size.
Piglets have a GI tract very similar to humans with regard to anatomy, development, nutrition, and physiology; these characteristics make them an ideal model for the study of NEC.131,150 Similar to rodent models, NEC is inducible in piglets through different combinations of formula feeding, hypothermia, and hypoxia.151,152 One of the most widely accepted models was established in 2006, and involves cesarean section at 92% gestation and total parenteral nutrition followed by formula feeding, without hypoxia or hypothermia.153 This model is sufficient to increase in IL-6,154 as seen in human neonates with NEC, and was used to study the effects of colostrum,153 human milk,155 amniotic fluid,156 antibiotics,157 and changes in microvasculature.158 Overall, piglets are an excellent model for NEC because of their anatomical and physiological similarity to the GI tract of humans, but are much more difficult and expensive to maintain than rodents.131
Brainstem control of autonomic function
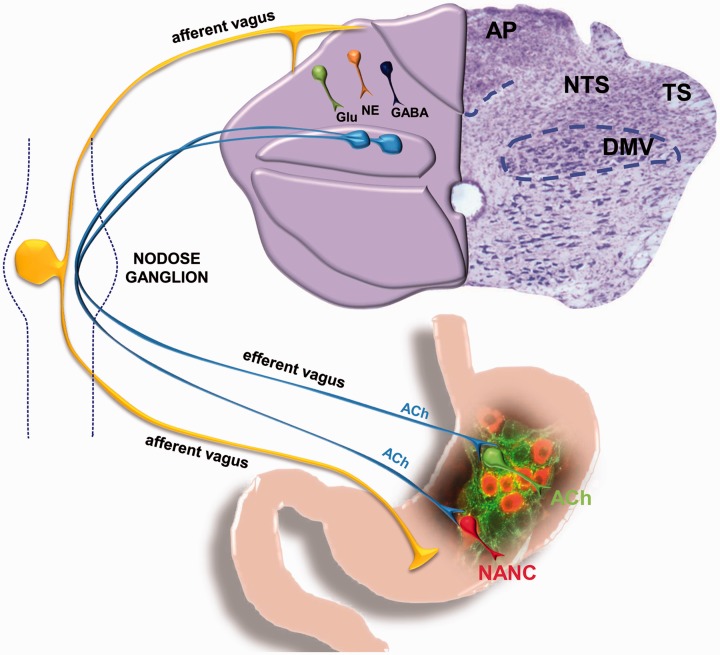
In the caudal brainstem, vago-vagal circuits are comprised of multiple nuclei, including the nucleus tractus solitarius (NTS), the dorsal motor nucleus of the vagus (DMV), and the nucleus ambiguus (NA).159 The NTS receives visceral sensory inputs, while the DMV and NA are the origin of vagal motor fibers that form synaptic connections with postganglionic neurons in the target organ to modulate functioning.159–161 Specifically, the NTS integrates brainstem, limbic, and hypothalamic inputs to coordinate, among others, GI reflexes, motility, and emptying by sending signals to adjacent motor nuclei such as the DMV.159–162 The DMV is comprised of preganglionic parasympathetic neurons that innervate the GI tract from the lower third of the esophagus to the splenic flexure in the transverse colon.159,161,163,164 These slow, spontaneously firing pace-making neurons of the DMV are involved in the fine modulation of GI tone, motility, and secretion through the vagus nerve.159,161,163,165 Postganglionic neurons of the myenteric plexus, located between the longitudinal and circular smooth muscle of the GI tract, use two distinct pathways to modulate GI functioning: an excitatory cholinergic pathway and an inhibitory non-adrenergic non-cholinergic (NANC) pathway.159,161,162,164,166 The excitatory cholinergic pathway uses acetylcholine to induce smooth muscle contraction via activation of muscarinic receptors, while the inhibitory NANC pathway induces relaxation of smooth muscles via release of vasoactive intestinal polypeptide or nitric oxide.159,161,166 Studies have shown that the excitatory cholinergic pathway is tonically active and plays a major role in the control of basal gastric tone and motility, while the inhibitory NANC pathway does not appear to be tonically active.159,161,165 Overall, bidirectional communication between the gut and the brain regulates homeostasis; these vago-vagal brainstem circuits are neuroplastic and able to respond to internal and external stressors.159,163,167–170 A lack of adaptability or resiliency to these stressors or other adverse events frequently results in GI dysfunctions such as delayed gastric emptying and/or accelerated colonic motility.171–177
Figure 2.
GI vago-vagal reflexes. (A color version of this figure is available in the online journal.)
NANC: non-adrenergic non-cholinergic.
Heart rate and cardiac function are modulated by preganglionic parasympathetic cardiac neurons in the brainstem, located primarily in the NA.178–185 These neurons have a tonic level of parasympathetic firing in conscious and anesthetized animals; this pattern is synchronized to the cardiac pulse.178–183 The activation of cardiac vagal neurons is influenced strongly by the activation of NTS pathways modulated by glutamate and γ-aminobutyric acid.186,187 Furthermore, the respiratory system can influence cardiovascular reflexes through the modulation of baroreceptor and chemoreceptor inputs to cardiac vagal neurons.188,189 Cardiac vagal neurons are also involved in a number of higher order connections with nuclei such as the locus coeruleus and the paraventricular nucleus (PVN) of the hypothalamus.184,185,190–192 Specifically, the PVN is involved in the control of autonomic function under both normal conditions and during stress challenges, such as hypoxia.184,185,193 Studies have shown that many disease states induce diminished cardiac vagal activity, as measured through both the firing properties of the neurons and through HF-HRV.113,194–203 Diseases that decrease cardiac vagal activity include NEC, hypertension, diabetes, hypothyroidism, coronary/peripheral artery disease, and chronic obstructive pulmonary disease, to name a few.113,194–203 We have recently demonstrated a positive correlation between HF-HRV and GI motility in rats,204 supporting the hypothesis that the reduction in HF-HRV power observed in preterm neonates prior to the development of NEC is associated with decreasing GI motility in these neonates.
Conclusion
Overall, NEC is a devastating disease that mandates the development of non-invasive methods to predict its onset before clinical signs. The pathogenesis of NEC is still under investigation, but major risk factors are thought to include premature birth, low birth weight, chorioamnionitis, and mechanical ventilation. In addition, preterm neonates may be more susceptible to NEC due to their underdeveloped intestine and lack of fully developed GI defense mechanisms. Current treatment strategies generally include broad-spectrum antibiotics, bowel rest, ionotropic and fluid support, and surgery if the bowel perforates. There is a critical need for reliable, non-invasive biomarkers to determine which neonates are at an increased risk of developing NEC, before the onset of clinical signs. A biomarker such as the reduced HF-HRV in combination with the detection of pro-inflammatory cytokines would allow at-risk neonates to receive maximal preventative therapies such as early exposure to colostrum and mother’s own milk, careful nutritional consideration, probiotics, increased SSC, and pharmacology.
ACKNOWLEDGEMENTS
The authors would like to thank Cesare M. Travagli, Zoraide Travagli, and Robert J. Mitchell for support and encouragement. The authors also thank Dr. Kirsteen N. Browning for critical comments on the article.
Authors’ contributions
ALM drafted the initial article; KKD and RAT reviewed and revised the article.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a grant from the National Institutes of Health, USA (NIDDK DK-99350 to RAT and KKD).
ORCID iDs
Alissa L Meister https://orcid.org/0000-0003-4563-2127
R Alberto Travagli https://orcid.org/0000-0003-2297-7683
References
- 1.Martin JA, Hamilton BE, Osterman MJK, Driscoll AK, Drake P. Births: final data for 2017. Natl Vital Stat Rep 2018; 67:1–50 [PubMed] [Google Scholar]
- 2.Gephart SM, McGrath JM, Effken JA, Halpern MD. Necrotizing enterocolitis risk: state of the science. Adv Neonatal Care 2012; 12:77–87 quiz 88–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hall NJ, Eaton S, Pierro A. Necrotizing enterocolitis: prevention, treatment, and outcome. J Pediatr Surg 2013; 48:2359–67 [DOI] [PubMed] [Google Scholar]
- 4.Lin PW, Nasr TR, Stoll BJ. Necrotizing enterocolitis: recent scientific advances in pathophysiology and prevention. Semin Perinatol 2008; 32:70–82 [DOI] [PubMed] [Google Scholar]
- 5.Lin PW, Stoll BJ. Necrotising enterocolitis. Lancet 2006; 368:1271–83 [DOI] [PubMed] [Google Scholar]
- 6.Neu J. Preterm infant nutrition, gut bacteria, and necrotizing enterocolitis. Curr Opin Clin Nutr Metab Care 2015; 18:285–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med 2011; 364:255–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nino DF, Sodhi CP, Hackam DJ. Necrotizing enterocolitis: new insights into pathogenesis and mechanisms. Nat Rev Gastroenterol Hepatol 2016; 13:590–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ward RM, Beachy JC. Neonatal complications following preterm birth. BJOG 2003; 110:8–16 [DOI] [PubMed] [Google Scholar]
- 10.Yee WH.Soraisham AS, Shah VS, Aziz K, Yoon W, Lee SK; Canadian Neonatal Network. Incidence and timing of presentation of necrotizing enterocolitis in preterm infants. Pediatrics 2012; 129:e298–304 [DOI] [PubMed] [Google Scholar]
- 11.Bell MJTernberg JL, Feigin RD, Keating JP, Marshall R, Barton L, Brotherton T.. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg 1978; 187:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsueh W1, Caplan MS, Qu XW, Tan XD, De Plaen IG, Gonzalez-Crussi F. Neonatal necrotizing enterocolitis: clinical considerations and pathogenetic concepts. Pediatr Dev Pathol 2003; 6:6–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luig M, Lui K, Nsw Group AN. Epidemiology of necrotizing enterocolitis – part II: risks and susceptibility of premature infants during the surfactant era: a regional study. J Paediatr Child Health 2005; 41:174–9 [DOI] [PubMed] [Google Scholar]
- 14.Neu J, Mshvildadze M, Mai V. A roadmap for understanding and preventing necrotizing enterocolitis. Curr Gastroenterol Rep 2008; 10:450–7 [DOI] [PubMed] [Google Scholar]
- 15.Tanner SMBerryhill TF, Ellenburg JL, Jilling T, Cleveland DS, Lorenz RG, Martin CA. Pathogenesis of necrotizing enterocolitis: modeling the innate immune response. Am J Pathol 2015; 185:4–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fitzgibbons SC.Ching Y, Yu D, Carpenter J, Kenny M, Weldon C, Lillehei C, Valim C, Horbar JD, Jaksic T. Mortality of necrotizing enterocolitis expressed by birth weight categories. J Pediatr Surg 2009; 44:1072–5 discussion 1075–6 [DOI] [PubMed] [Google Scholar]
- 17.Bisquera JA, Cooper TR, Berseth CL. Impact of necrotizing enterocolitis on length of stay and hospital charges in very low birth weight infants. Pediatrics 2002; 109:423–8 [DOI] [PubMed] [Google Scholar]
- 18.Stey ABarnert ES, Tseng CH, Keeler E, Needleman J, Leng M, Kelley-Quon LI, Shew SB. Outcomes and costs of surgical treatments of necrotizing enterocolitis. Pediatrics 2015; 135:e1190–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spencer AU, et al. Pediatric short-bowel syndrome: the cost of comprehensive care. Am J Clin Nutr 2008; 88:1552–9 [DOI] [PubMed] [Google Scholar]
- 20.Beeby PJ, Jeffery H. Risk factors for necrotising enterocolitis: the influence of gestational age. Arch Dis Child 1992; 67:432–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guthrie SO, et al. Necrotizing enterocolitis among neonates in the United States. J Perinatol 2003; 23:278–85 [DOI] [PubMed] [Google Scholar]
- 22.Bellodas Sanchez J, Kadrofske M. Necrotizing enterocolitis. Neurogastroenterol Motil 2019; 31:e13569. [DOI] [PubMed] [Google Scholar]
- 23.Bhandari V, et al. Familial and genetic susceptibility to major neonatal morbidities in preterm twins. Pediatrics 2006; 117:1901–6 [DOI] [PubMed] [Google Scholar]
- 24.Jilling T, et al. The roles of bacteria and TLR4 in rat and murine models of necrotizing enterocolitis. J Immunol 2006; 177:3273–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar H, Kawai T, Akira S. Toll-like receptors and innate immunity. Biochem Biophys Res Commun 2009; 388:621–5 [DOI] [PubMed] [Google Scholar]
- 26.Leaphart CL, et al. A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J Immunol 2007; 179:4808–20 [DOI] [PubMed] [Google Scholar]
- 27.Lu P, Sodhi CP, Hackam DJ. Toll-like receptor regulation of intestinal development and inflammation in the pathogenesis of necrotizing enterocolitis. Pathophysiology 2014; 21:81–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ng PC, et al. Diagnosis of late onset neonatal sepsis with cytokines, adhesion molecule, and C-reactive protein in preterm very low birthweight infants. Arch Dis Child Fetal Neonatal Ed 1997; 77:F221–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ng PC, et al. Proinflammatory and anti-inflammatory cytokine responses in preterm infants with systemic infections. Arch Dis Child Fetal Neonatal Ed 2003; 88:F209–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiesa C, et al. Fetal and early neonatal interleukin-6 response. Cytokine 2015; 76:1–12 [DOI] [PubMed] [Google Scholar]
- 31.Delanghe JR, Speeckaert MM. Translational research and biomarkers in neonatal sepsis. Clin Chim Acta 2015; 451:46–64 [DOI] [PubMed] [Google Scholar]
- 32.Treszl A, Tulassay T, Vasarhelyi B. Genetic basis for necrotizing enterocolitis – risk factors and their relations to genetic polymorphisms. Front Biosci 2006; 11:570–80 [DOI] [PubMed] [Google Scholar]
- 33.Newell SJ, Sarkar PK, Durbin GM, Booth IW, McNeish AS. Maturation of the lower oesophageal sphincter in the preterm baby. Gut 1988; 29:167–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berseth CL. Gastrointestinal motility in the neonate. Clin Perinatol 1996; 23:179–90 [PubMed] [Google Scholar]
- 35.Berseth CL. Gestational evolution of small intestine motility in preterm and term infants. J Pediatr 1989; 115:646–51 [DOI] [PubMed] [Google Scholar]
- 36.Cavell B. Reservoir and emptying function of the stomach of the premature infant. Acta Paediatr Scand Suppl 1982; 296:60–1 [DOI] [PubMed] [Google Scholar]
- 37.Lau C, Smith EO, Schanler RJ. Coordination of suck-swallow and swallow respiration in preterm infants. Acta Paediatr 2003; 92:721–7 [PubMed] [Google Scholar]
- 38.Martin CR, Walker WA. Intestinal immune defences and the inflammatory response in necrotising enterocolitis. Semin Fetal Neonatal Med 2006; 11:369–77 [DOI] [PubMed] [Google Scholar]
- 39.Walker WA. Development of the intestinal mucosal barrier. J Pediatr Gastroenterol Nutr 2002; 34:S33–39 [DOI] [PubMed] [Google Scholar]
- 40.Israel EJ, Walker WA. Development of intestinal mucosal barrier function to antigens and bacterial toxins. Adv Exp Med Biol 1987; 216A:673–83 [DOI] [PubMed] [Google Scholar]
- 41.Udall JN, Pang K, Fritze L, Kleinman R, Walker WA. Development of gastrointestinal mucosal barrier. I. The effect of age on intestinal permeability to macromolecules. Pediatr Res 1981; 15:241–4 [DOI] [PubMed] [Google Scholar]
- 42.Grishin A, Bowling J, Bell B, Wang J, Ford HR. Roles of nitric oxide and intestinal microbiota in the pathogenesis of necrotizing enterocolitis. J Pediatr Surg 2016; 51:13–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hackam DJ. Danger at the doorstep: regulation of bacterial translocation across the intestinal barrier by nitric oxide. Crit Care Med 2011; 39:2189–90 [DOI] [PubMed] [Google Scholar]
- 44.Forchielli ML, Walker WA. The effect of protective nutrients on mucosal defense in the immature intestine. Acta Paediatr Suppl 2005; 94:74–83 [DOI] [PubMed] [Google Scholar]
- 45.Mshvildadze M, et al. Intestinal microbial ecology in premature infants assessed with non-culture-based techniques. J Pediatr 2010; 156:20–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Collado MC, et al. Factors influencing gastrointestinal tract and microbiota immune interaction in preterm infants. Pediatr Res 2015; 77:726–31 [DOI] [PubMed] [Google Scholar]
- 47.Gibson MK, Pesesky MW, Dantas G. The yin and yang of bacterial resilience in the human gut microbiota. J Mol Biol 2014; 426:3866–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hallstrom M, Koivisto AM, Janas M, Tammela O. Frequency of and risk factors for necrotizing enterocolitis in infants born before 33 weeks of gestation. Acta Paediatr 2003; 92:111–3 [DOI] [PubMed] [Google Scholar]
- 49.Ng PC. Biomarkers of necrotising enterocolitis. Semin Fetal Neonatal Med 2014; 19:33–8 [DOI] [PubMed] [Google Scholar]
- 50.Morse S, Groer M, Shelton MM, Maguire D, Ashmeade T. A systematic review: the utility of the revised version of the score for neonatal acute physiology among critically ill neonates. J Perinat Neonat Nur 2015; 29:315–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gregory KE. Clinical predictors of necrotizing enterocolitis in premature infants. Nurs Res 2008; 57:260–70 [DOI] [PubMed] [Google Scholar]
- 52.Dollberg S, Lusky A, Reichman B. Patent ductus arteriosus, indomethacin and necrotizing enterocolitis in very low birth weight infants: a population-based study. J Pediatr Gastroenterol Nutr 2005; 40:184–8 [DOI] [PubMed] [Google Scholar]
- 53.Bergholz R, Boettcher M, Reinshagen K, Wenke K. Complex gastroschisis is a different entity to simple gastroschisis affecting morbidity and mortality – a systematic review and Meta-analysis. J Pediatr Surg 2014; 49:1527–32 [DOI] [PubMed] [Google Scholar]
- 54.Sinkey RG, et al. Sonographic markers associated with adverse neonatal outcomes among fetuses with gastroschisis: an 11-year, single-center review. Am J Obstet Gynecol 2016; 214:275 e271–5, e277 [DOI] [PubMed] [Google Scholar]
- 55.Yu L, et al. Bowel perforation in premature infants with necrotizing enterocolitis: risk factors and outcomes. Gastroenterol Res Pract 2016; 2016:6134187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Munaco AJ, et al. Timing of optimal surgical intervention for neonates with necrotizing enterocolitis. Am Surg 2015; 81:438–43 [PubMed] [Google Scholar]
- 57.Elfvin A, Dinsdale E, Wales PW, Moore AM. Low birthweight, gestational age, need for surgical intervention and gram-negative bacteraemia predict intestinal failure following necrotising enterocolitis. Acta Paediatr 2015; 104:771–6 [DOI] [PubMed] [Google Scholar]
- 58.Battersby C, Longford N, Costeloe K, Modi N, UK Neonatal Collaborative Necrotising Enterocolitis Study Group. Development of a gestational age-specific case definition for neonatal necrotizing enterocolitis. JAMA Pediatr 2017; 171:256–63 [DOI] [PubMed] [Google Scholar]
- 59.Buescher ES. Anti-inflammatory characteristics of human milk: how, where, why. Adv Exp Med Biol 2001; 501:207–22 [DOI] [PubMed] [Google Scholar]
- 60.Newburg DS. Neonatal protection by an innate immune system of human milk consisting of oligosaccharides and glycans. J Anim Sci 2009; 87:26–34 [DOI] [PubMed] [Google Scholar]
- 61.Renfrew MJ, et al. Breastfeeding promotion for infants in neonatal units: a systematic review and economic analysis. Health Technol Assess 2009; 13:1–146, iii–iv [DOI] [PubMed] [Google Scholar]
- 62.Shulhan J, Dicken B, Hartling L, Larsen BMK. Current knowledge of necrotizing enterocolitis in preterm infants and the impact of different types of enteral nutrition products. Adv Nutr 2017; 8:80–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cortez J, et al. Maternal milk feedings reduce sepsis, necrotizing enterocolitis and improve outcomes of premature infants. J Perinatol 2018; 38:71–4 [DOI] [PubMed] [Google Scholar]
- 64.Nasuf AWA, Ojha S, Dorling J. Oropharyngeal colostrum in preventing mortality and morbidity in preterm infants. Cochrane Database Syst Rev 2018; 9:CD011921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Glass KM, Greecher CP, Doheny KK. Oropharyngeal administration of colostrum increases salivary secretory IgA levels in very low-birth-weight infants. Am J Perinatol 2017; 34:1389–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee J, et al. Oropharyngeal colostrum administration in extremely premature infants: an RCT. Pediatrics 2015; 135:e357–66 [DOI] [PubMed] [Google Scholar]
- 67.Rodriguez NA, Meier PP, Groer MW, Zeller JM. Oropharyngeal administration of colostrum to extremely low birth weight infants: theoretical perspectives. J Perinatol 2009; 29:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wheeler TT, Hodgkinson AJ, Prosser CG, Davis SR. Immune components of colostrum and milk – a historical perspective. J Mammary Gland Biol Neoplasia 2007; 12:237–47 [DOI] [PubMed] [Google Scholar]
- 69.Ballard O, Morrow AL. Human milk composition: nutrients and bioactive factors. Pediatr Clin North Am 2013; 60:49–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barlow B, et al. An experimental study of acute neonatal enterocolitis – the importance of breast milk. J Pediatr Surg 1974; 9:587–95 [DOI] [PubMed] [Google Scholar]
- 71.Dvorak B, et al. Maternal milk reduces severity of necrotizing enterocolitis and increases intestinal IL-10 in a neonatal rat model. Pediatr Res 2003; 53:426–33 [DOI] [PubMed] [Google Scholar]
- 72.Jantscher-Krenn E, et al. The human milk oligosaccharide disialyllacto-N-tetraose prevents necrotising enterocolitis in neonatal rats. Gut 2012; 61:1417–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moore TA, Pickler RH. Feeding intolerance, inflammation, and neurobehaviors in preterm infants. J Neonatal Nurs 2017; 23:134–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kamitsuka MD, Horton MK, Williams MA. The incidence of necrotizing enterocolitis after introducing standardized feeding schedules for infants between 1250 and 2500 grams and less than 35 weeks of gestation. Pediatrics 2000; 105:379–84 [DOI] [PubMed] [Google Scholar]
- 75.Pammi M, Abrams SA. Oral lactoferrin for the prevention of sepsis and necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev 2015; 2:CD007137. [DOI] [PubMed] [Google Scholar]
- 76.Patel RM, Denning PW. Therapeutic use of prebiotics, probiotics, and postbiotics to prevent necrotizing enterocolitis: what is the current evidence? Clin Perinatol 2013; 40:11–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Patel RM, Underwood MA. Probiotics and necrotizing enterocolitis. Semin Pediatr Surg 2018; 27:39–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dermyshi E, et al. The “golden age” of probiotics: a systematic review and meta-analysis of randomized and observational studies in preterm infants. Neonatology 2017; 112:9–23 [DOI] [PubMed] [Google Scholar]
- 79.Fung TC, Olson CA, Hsiao EY. Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci 2017; 20:145–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lin HC, et al. Oral probiotics prevent necrotizing enterocolitis in very low birth weight preterm infants: a multicenter, randomized, controlled trial. Pediatrics 2008; 122:693–700 [DOI] [PubMed] [Google Scholar]
- 81.Lin HC, et al. Oral probiotics reduce the incidence and severity of necrotizing enterocolitis in very low birth weight infants. Pediatrics 2005; 115:1–4 [DOI] [PubMed] [Google Scholar]
- 82.Lin HC, Su BH, Oh W. Oral probiotics prevent necrotizing enterocolitis. J Pediatr 2006; 148:849–50 author reply [DOI] [PubMed] [Google Scholar]
- 83.Neu J. Probiotics and necrotizing enterocolitis. Clin Perinatol 2014; 41:967–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Robinson J. Cochrane in context: probiotics for prevention of necrotizing enterocolitis in preterm infants. Evid-Based Child Health 2014; 9:672–4 [DOI] [PubMed] [Google Scholar]
- 85.Lowson K, Offer C, Watson J, McGuire B, Renfrew MJ. The economic benefits of increasing kangaroo skin-to-skin care and breastfeeding in neonatal units: analysis of a pragmatic intervention in clinical practice. Int Breastfeed J 2015; 10:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Faye PM, et al. Kangaroo mother care for low birth weight infants at Albert-Royer national children hospital center of Dakar. Arch Pediatr 2016; 23:268–74 [DOI] [PubMed] [Google Scholar]
- 87.Feldman R, Eidelman AI. Skin-to-skin contact (kangaroo care) accelerates autonomic and neurobehavioural maturation in preterm infants. Dev Med Child Neurol 2003; 45:274–81 [DOI] [PubMed] [Google Scholar]
- 88.Marvin MM, Gardner FC, Sarsfield KM, Travagli RA, Doheny KK. Increased frequency of skin-to-skin contact is associated with enhanced vagal tone and improved health outcomes in preterm neonates. Am J Perinatol 2019; 36:505–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bohnhorst B, Heyne T, Peter CS, Poets CF. Skin-to-skin (kangaroo) care, respiratory control, and thermoregulation. J Pediatr 2001; 138:193–7 [DOI] [PubMed] [Google Scholar]
- 90.Afrazi A, et al. Intracellular heat shock protein-70 negatively regulates TLR4 signaling in the newborn intestinal epithelium. J Immunol 2012; 188:4543–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gribar SC, et al. Reciprocal expression and signaling of TLR4 and TLR9 in the pathogenesis and treatment of necrotizing enterocolitis. J Immunol 2009; 182:636–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sodhi CP, et al. Intestinal epithelial toll-like receptor 4 regulates goblet cell development and is required for necrotizing enterocolitis in mice. Gastroenterology 2012; 143:708–18, e705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yazji I, et al. Endothelial TLR4 activation impairs intestinal microcirculatory perfusion in necrotizing enterocolitis via eNOS-NO-nitrite signaling. Proc Natl Acad Sci USA 2013; 110:9451–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lu J, Caplan MS, Li D, Jilling T. Polyunsaturated fatty acids block platelet-activating factor-induced phosphatidylinositol 3 kinase/AKT-mediated apoptosis in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 2008; 294:G1181–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Su Y, Besner GE. Heparin-binding EGF-like growth factor (HB-EGF) promotes cell migration and adhesion via focal adhesion kinase. J Surg Res 2014; 189:222–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang J, Su Y, Zhou Y, Besner GE. Heparin-binding EGF-like growth factor (HB-EGF) therapy for intestinal injury: application and future prospects. Pathophysiology 2014; 21:95–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Watkins DJ, Besner GE. The role of the intestinal microcirculation in necrotizing enterocolitis. Semin Pediatr Surg 2013; 22:83–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Amin SC, Pappas C, Iyengar H, Maheshwari A. Short bowel syndrome in the NICU. Clin Perinatol 2013; 40:53+ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rees CM, Pierro A, Eaton S. Neurodevelopmental outcomes of neonates with medically and surgically treated necrotizing enterocolitis. Arch Dis Child Fetal Neonatal Ed 2007; 92:F193–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hickey M, Georgieff M, Ramel S. Neurodevelopmental outcomes following necrotizing enterocolitis. Semin Fetal Neonatal Med 2018; 23:426–32 [DOI] [PubMed] [Google Scholar]
- 101.Moschopoulos C, et al. The neurodevelopmental perspective of surgical necrotizing enterocolitis: the role of the gut-brain axis. Mediators Inflamm 2018; 2018:745685710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vohr BR, Allen M. Extreme prematurity – the continuing dilemma. N Engl J Med 2005; 352:71–2 [DOI] [PubMed] [Google Scholar]
- 103.Cusick SE, Georgieff MK. The role of nutrition in brain development: the golden opportunity of the “first 1000 days”. J Pediatr 2016; 175:16–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lodha A, Asztalos E, Moore AM. Cytokine levels in neonatal necrotizing enterocolitis and long-term growth and neurodevelopment. Acta Paediatr 2010; 99:338–43 [DOI] [PubMed] [Google Scholar]
- 105.Shah TA, et al. Hospital and neurodevelopmental outcomes of extremely low-birth-weight infants with necrotizing enterocolitis and spontaneous intestinal perforation. J Perinatol 2012; 32:552–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Heart rate variability standards of measurement, physiological interpretation, and clinical use. Task force of the European society of cardiology and the North American society of pacing and electrophysiology. Eur Heart J 1996; 17:354–81 [PubMed] [Google Scholar]
- 107.Dobrek L, Baranowska A, Thor PJ. Indirect autonomic nervous system activity assessment with heart rate variability in rats with cyclophosphamide-induced hemorrhagic cystitis treated with melatonin or agomelatine. Contemp Oncol (Pozn) 2015; 19:368–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Goncalves H, et al. Analysis of heart rate variability in a rat model of induced pulmonary hypertension. Med Eng Phys 2010; 32:752–7 [DOI] [PubMed] [Google Scholar]
- 109.Porges SW. Vagal tone: a physiologic marker of stress vulnerability. Pediatrics 1992; 90:498–504 [PubMed] [Google Scholar]
- 110.Porges SW. Cardiac vagal tone: a physiological index of stress. Neurosci Biobehav Rev 1995; 19:225–33 [DOI] [PubMed] [Google Scholar]
- 111.Rosen H, Craelius W, Curcie D, Hiatt M, Hegyi T. Spectral analysis of heart variability in the newborn infant. Biol Neonate 2000; 77:224–9 [DOI] [PubMed] [Google Scholar]
- 112.Verklan MT, Padhye NS. Spectral analysis of heart rate variability: an emerging tool for assessing stability during transition to extrauterine life. J Obstet Gynecol Neonatal Nurs 2004; 33:256–65 [DOI] [PubMed] [Google Scholar]
- 113.Doheny KK, et al. Diminished vagal tone is a predictive biomarker of necrotizing enterocolitis-risk in preterm infants. Neurogastroenterol Motil 2014; 26:832–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Al-Shargabi T, et al. Changes in autonomic tone in premature infants developing necrotizing enterocolitis. Am J Perinatol 2018; 35:1079–86 [DOI] [PubMed] [Google Scholar]
- 115.Meister AL, Doheny KK, Travagli RA. Necrotizing enterocolitis attenuates developmental heart rate variability increases in newborn rats. Neurogastroenterol Motil 2018; 31:e13484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Young C, Sharma R, Handfield M, Mai V, Neu J. Biomarkers for infants at risk for necrotizing enterocolitis: clues to prevention? Pediatr Res 2009; 65:91R–7R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Oh S, Young C, Gravenstein N, Islam S, Neu J. Monitoring technologies in the neonatal intensive care unit: implications for the detection of necrotizing enterocolitis. J Perinatol 2010; 30:701–8 [DOI] [PubMed] [Google Scholar]
- 118.Goldstein GP, Sylvester KG. Biomarker discovery and utility in necrotizing enterocolitis. Clin Perinatol 2019; 46:1–17 [DOI] [PubMed] [Google Scholar]
- 119.Garstin WI, Boston VE. Sequential assay of expired breath hydrogen as a means of predicting necrotizing enterocolitis in susceptible infants. J Pediatr Surg 1987; 22:208–10 [DOI] [PubMed] [Google Scholar]
- 120.Cheu HW, Brown DR, Rowe MI. Breath hydrogen excretion as a screening test for the early diagnosis of necrotizing enterocolitis. Arch Pediatr Adolesc Med 1989; 143:156–9 [DOI] [PubMed] [Google Scholar]
- 121.Gilfillan M, Bhandari V. Biomarkers for the diagnosis of neonatal sepsis and necrotizing enterocolitis: clinical practice guidelines. Early Human Development 2017; 105:25–33 [DOI] [PubMed] [Google Scholar]
- 122.Ganesan P, Shanmugam P, Sattar SB, Shankar SL. Evaluation of IL-6, CRP and hs-CRP as early markers of neonatal sepsis. J Clin Diagn Res 2016; 10:DC13–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hofer N, Zacharias E, Muller W, Resch B. An update on the use of C-reactive protein in early-onset neonatal sepsis: current insights and new tasks. Neonatology 2012; 102:25–36 [DOI] [PubMed] [Google Scholar]
- 124.Dai J, et al. Neutrophil CD64 as a diagnostic marker for neonatal sepsis: meta-analysis. Adv Clin Exp Med 2017; 26:327–32 [DOI] [PubMed] [Google Scholar]
- 125.Yang AP, et al. Neutrophil CD64 combined with PCT, CRP and WBC improves the sensitivity for the early diagnosis of neonatal sepsis. Clin Chem Lab Med 2016; 54:345–51 [DOI] [PubMed] [Google Scholar]
- 126.Yuan H, et al. Diagnosis value of the serum amyloid a test in neonatal sepsis: a meta-analysis. Biomed Res Int 2013; 2013:520294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Arnon S, et al. Serum amyloid A: an early and accurate marker of neonatal early-onset sepsis. J Perinatol 2007; 27:297–302 [DOI] [PubMed] [Google Scholar]
- 128.Ng PC, et al. Host-response biomarkers for diagnosis of late-onset septicemia and necrotizing enterocolitis in preterm infants. J Clin Invest 2010; 120:2989–3000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cetinkaya M, Ozkan H, Koksal N, Akaci O, Ozgur T. Comparison of the efficacy of serum amyloid A, C-reactive protein, and procalcitonin in the diagnosis and follow-up of necrotizing enterocolitis in premature infants. J Pediatr Surg 2011; 46:1482–9 [DOI] [PubMed] [Google Scholar]
- 130.Al-Shargabi T, et al. Inflammatory cytokine response and reduced heart rate variability in newborns with hypoxic-ischemic encephalopathy. J Perinatol 2017; 37:668–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lu P, et al. Animal models of gastrointestinal and liver diseases. Animal models of necrotizing enterocolitis: pathophysiology, translational relevance, and challenges. Am J Physiol Gastrointest Liver Physiol 2014; 306:G917–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sodhi C, Richardson W, Gribar S, Hackam DJ. The development of animal models for the study of necrotizing enterocolitis. Dis Model Mech 2008; 1:94–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bayer SA, Altman J, Russo RJ, Zhang X. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology 1993; 14:83–144 [PubMed] [Google Scholar]
- 134.Clancy B, Darlington RB, Finlay BL. Translating developmental time across mammalian species. Neuroscience 2001; 105:7–17 [DOI] [PubMed] [Google Scholar]
- 135.Barlow B, Santulli TV. Importance of multiple episodes of hypoxia or cold stress on the development of enterocolitis in an animal model. Surgery 1975; 77:687–90 [PubMed] [Google Scholar]
- 136.Meister AL, Burkholder CR, Doheny KK, Travagli RA. Ghrelin ameliorates the phenotype of newborn rats induced with mild necrotizing enterocolitis. Neurogastroenterol Motil 2019; 31:e13682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Halpern MD, et al. Up-regulation of IL-18 and IL-12 in the ileum of neonatal rats with necrotizing enterocolitis. Pediatr Res 2002; 51:733–9 [DOI] [PubMed] [Google Scholar]
- 138.Jilling T, Lu J, Jackson M, Caplan MS. Intestinal epithelial apoptosis initiates gross bowel necrosis in an experimental rat model of neonatal necrotizing enterocolitis. Pediatr Res 2004; 55:622–9 [DOI] [PubMed] [Google Scholar]
- 139.Shiou SR, et al. Synergistic protection of combined probiotic conditioned media against neonatal necrotizing enterocolitis-like intestinal injury. PLoS One 2013; 8:e65108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.De Plaen IG, et al. Inhibition of nuclear factor-kappaB ameliorates bowel injury and prolongs survival in a neonatal rat model of necrotizing enterocolitis. Pediatr Res 2007; 61:716–21 [DOI] [PubMed] [Google Scholar]
- 141.Giannone PJ, et al. Poly(ADP-ribose) polymerase-1: a novel therapeutic target in necrotizing enterocolitis. Pediatr Res 2011; 70:67–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hammerman C, et al. Protective effect of bilirubin in ischemia-reperfusion injury in the rat intestine. J Pediatr Gastroenterol Nutr 2002; 35:344–9 [DOI] [PubMed] [Google Scholar]
- 143.Neu J. The 'myth' of asphyxia and hypoxia-ischemia as primary causes of necrotizing enterocolitis. Biol Neonate 2005; 87:97–8 [DOI] [PubMed] [Google Scholar]
- 144.Emami CN, et al. Role of interleukin-10 in the pathogenesis of necrotizing enterocolitis. Am J Surg 2012; 203:428–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Guner YS, et al. P-glycoprotein induction by breast milk attenuates intestinal inflammation in experimental necrotizing enterocolitis. Lab Invest 2011; 91:1668–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Halpern MD, et al. Decreased development of necrotizing enterocolitis in IL-18-deficient mice. Am J Physiol Gastrointest Liver Physiol 2008; 294:G20–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Martin NA, et al. Active transport of bile acids decreases mucin 2 in neonatal ileum: implications for development of necrotizing enterocolitis. PLoS One 2011; 6:e27191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Leaphart CL, et al. Interferon-gamma inhibits intestinal restitution by preventing gap junction communication between enterocytes. Gastroenterology 2007; 132:2395–411 [DOI] [PubMed] [Google Scholar]
- 149.Cetin S, et al. Nitric oxide inhibits enterocyte migration through activation of RhoA-GTPase in a SHP-2-dependent manner. Am J Physiol Gastrointest Liver Physiol 2007; 292:G1347–58 [DOI] [PubMed] [Google Scholar]
- 150.Puiman P, Stoll B. Animal models to study neonatal nutrition in humans. Curr Opin Clin Nutr Metab Care 2008; 11:601–6 [DOI] [PubMed] [Google Scholar]
- 151.Cassutto BH, Misra HP, Pfeiffer CJ. Intestinal post-ischemic reperfusion injury: studies with neonatal necrotizing enterocolitis. Acta Physiol Hung 1989; 73:363–9 [PubMed] [Google Scholar]
- 152.Cohen IT, et al. Necrotizing enterocolitis in a neonatal piglet model. J Pediatr Surg 1991; 26:598–601 [DOI] [PubMed] [Google Scholar]
- 153.Sangild PT, et al. Diet- and colonization-dependent intestinal dysfunction predisposes to necrotizing enterocolitis in preterm pigs. Gastroenterology 2006; 130:1776–92 [DOI] [PubMed] [Google Scholar]
- 154.Siggers RH, et al. Elective cesarean delivery affects gut maturation and delays microbial colonization but does not increase necrotizing enterocolitis in preterm pigs. Am J Physiol Regul Integr Comp Physiol 2008; 294:R929–938 [DOI] [PubMed] [Google Scholar]
- 155.Jensen ML, et al. Similar efficacy of human banked milk and bovine colostrum to decrease incidence of necrotizing enterocolitis in preterm piglets. Am J Physiol Regul Integr Comp Physiol 2013; 305:R4–R12 [DOI] [PubMed] [Google Scholar]
- 156.Siggers J, et al. Postnatal amniotic fluid intake reduces gut inflammatory responses and necrotizing enterocolitis in preterm neonates. Am J Physiol Gastrointest Liver Physiol 2013; 304:G864–875 [DOI] [PubMed] [Google Scholar]
- 157.Jensen ML, et al. Antibiotics modulate intestinal immunity and prevent necrotizing enterocolitis in preterm neonatal piglets. Am J Physiol Gastrointest Liver Physiol 2014; 306:G59–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.van Haver ER, et al. Enteral feeding reduces endothelial nitric oxide synthase in the caudal intestinal microvasculature of preterm piglets. Pediatr Res 2008; 63:137–42 [DOI] [PubMed] [Google Scholar]
- 159.Travagli RA, Anselmi L. Vagal neurocircuitry and its influence on gastric motility. Nat Rev Gastroenterol Hepatol 2016; 13:389–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Andresen MC, Kunze DL. Nucleus tractus solitarius – gateway to neural circulatory control. Annu Rev Physiol 1994; 56:93–116 [DOI] [PubMed] [Google Scholar]
- 161.Travagli RA, Hermann GE, Browning KN, Rogers RC. Brainstem circuits regulating gastric function. Annu Rev Physiol 2006; 68:279–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Berthoud HR, Blackshaw LA, Brookes SJ, Grundy D. Neuroanatomy of extrinsic afferents supplying the gastrointestinal tract. Neurogastroenterol Motil 2004; 16:28–33 [DOI] [PubMed] [Google Scholar]
- 163.Browning KN, Travagli RA. Central nervous system control of gastrointestinal motility and secretion and modulation of gastrointestinal functions. Compr Physiol 2014; 4:1339–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Berthoud HR, Carlson NR, Powley TL. Topography of efferent vagal innervation of the rat gastrointestinal tract. Am J Physiol 1991; 260:R200–207 [DOI] [PubMed] [Google Scholar]
- 165.Travagli RA, Gillis RA, Rossiter CD, Vicini S. Glutamate and GABA-mediated synaptic currents in neurons of the rat dorsal motor nucleus of the vagus. Am J Physiol 1991; 260:G531–536 [DOI] [PubMed] [Google Scholar]
- 166.Chang HY, Mashimo H, Goyal RK. Musings on the wanderer: what's new in our understanding of vago-vagal reflex? IV. Current concepts of vagal efferent projections to the gut. Am J Physiol Gastrointest Liver Physiol 2003; 284:G357–366 [DOI] [PubMed] [Google Scholar]
- 167.Browning KN, Kalyuzhny AE, Travagli RA. Mu-opioid receptor trafficking on inhibitory synapses in the rat brainstem. J Neurosci 2004; 24:7344–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Browning KN, Travagli RA. Plasticity of vagal brainstem circuits in the control of gastrointestinal function. Auton Neurosci 2011; 161:6–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Browning KN, Travagli RA. The peptide TRH uncovers the presence of presynaptic 5-HT1A receptors via activation of a second messenger pathway in the rat dorsal vagal complex. J Physiol 2001; 531:425–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Browning KN, Travagli RA. Modulation of inhibitory neurotransmission in brainstem vagal circuits by NPY and PYY is controlled by cAMP levels. Neurogastroenterol Motil 2009; 21:1309–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Stengel A, Tache Y. Neuroendocrine control of the gut during stress: corticotropin-releasing factor signaling pathways in the spotlight. Annu Rev Physiol 2009; 71:219–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Jacobson-Pick S, Richter-Levin G. Differential impact of juvenile stress and corticosterone in juvenility and in adulthood, in male and female rats. Behav Brain Res 2010; 214:268–76 [DOI] [PubMed] [Google Scholar]
- 173.Kim SE, Chang L. Overlap between functional GI disorders and other functional syndromes: what are the underlying mechanisms? Neurogastroent Motil 2012; 24:895–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Moussaoui N, et al. Chronic early-life stress in rat pups alters basal corticosterone, intestinal permeability, and fecal microbiota at weaning: Influence of sex. J Neurogastroenterol Motil 2017; 23:135–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Pellissier S, et al. Relationship between vagal tone, cortisol, TNF-alpha, epinephrine and negative affects in Crohn's disease and irritable bowel syndrome. PLoS One 2014; 9:e105328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Salvioli B, et al. Autonomic nervous system dysregulation in irritable bowel syndrome. Neurogastroenterol Motil 2015; 27:423–30 [DOI] [PubMed] [Google Scholar]
- 177.Tache Y, Bonaz B. Corticotropin-releasing factor receptors and stress-related alterations of gut motor function. J Clin Invest 2007; 117:33–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Coleman TG. Arterial baroreflex control of heart rate in the conscious rat. Am J Physiol 1980; 238:H515–520 [DOI] [PubMed] [Google Scholar]
- 179.Pickering TG, Gribbin B, Petersen ES, Cunningham DJ, Sleight P. Effects of autonomic blockade on the baroreflex in man at rest and during exercise. Circ Res 1972; 30:177–85 [DOI] [PubMed] [Google Scholar]
- 180.Stornetta RL, Guyenet PG, McCarty RC. Autonomic nervous system control of heart rate during baroreceptor activation in conscious and anesthetized rats. J Auton Nerv Syst 1987; 20:121–7 [DOI] [PubMed] [Google Scholar]
- 181.Kunze DL. Reflex discharge patterns of cardiac vagal efferent fibres. J Physiol 1972; 222:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Spyer KM. Neural organisation and control of the baroreceptor reflex. Rev Physiol Biochem Pharmacol 1981; 88:24–124 [PubMed] [Google Scholar]
- 183.Gilbey MP, Jordan D, Richter DW, Spyer KM. Synaptic mechanisms involved in the inspiratory modulation of vagal cardio-inhibitory neurones in the cat. J Physiol 1984; 356:65–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Dyavanapalli J, Dergacheva O, Wang X, Mendelowitz D. Parasympathetic vagal control of cardiac function. Curr Hypertens Rep 2016; 18:22. [DOI] [PubMed] [Google Scholar]
- 185.Dergacheva O, Griffioen KJ, Neff RA, Mendelowitz D. Respiratory modulation of premotor cardiac vagal neurons in the brainstem. Respir Physiol Neurobiol 2010; 174:102–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Mastitskaya S, et al. Cardioprotection evoked by remote ischaemic preconditioning is critically dependent on the activity of vagal pre-ganglionic neurones. Cardiovasc Res 2012; 95:487–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Mendelowitz D. Advances in parasympathetic control of heart rate and cardiac function. News Physiol Sci 1999; 14:155–61 [DOI] [PubMed] [Google Scholar]
- 188.Davidson NS, Goldner S, McCloskey DI. Respiratory modulation of barareceptor and chemoreceptor reflexes affecting heart rate and cardiac vagal efferent nerve activity. J Physiol 1976; 259:523–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Eckberg DL. The human respiratory gate. J Physiol 2003; 548:339–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Coleman CG, et al. Chronic intermittent hypoxia induces NMDA receptor-dependent plasticity and suppresses nitric oxide signaling in the mouse hypothalamic paraventricular nucleus. J Neurosci 2010; 30:12103–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.da Silva AQ, Fontes MA, Kanagy NL. Chronic infusion of angiotensin receptor antagonists in the hypothalamic paraventricular nucleus prevents hypertension in a rat model of sleep apnea. Brain Res 2011; 1368:231–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kc P, et al. Increased vasopressin transmission from the paraventricular nucleus to the rostral medulla augments cardiorespiratory outflow in chronic intermittent hypoxia-conditioned rats. J Physiol 2010; 588:725–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Guntheroth WG, Kawabori I. Hypoxic apnea and gasping. J Clin Invest 1975; 56:1371–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Thayer JF, Sternberg E. Beyond heart rate variability: vagal regulation of allostatic systems. Ann N Y Acad Sci 2006; 1088:361–72 [DOI] [PubMed] [Google Scholar]
- 195.Bonaz B, Sinniger V, Pellissier S. Vagal tone: effects on sensitivity, motility, and inflammation. Neurogastroenterol Motil 2016; 28:455–62 [DOI] [PubMed] [Google Scholar]
- 196.Souza GG, et al. Resilience and vagal tone predict cardiac recovery from acute social stress. Stress 2007; 10:368–74 [DOI] [PubMed] [Google Scholar]
- 197.Doheny KK, Tang XR, Browning KN, Palmer C, Travagli RA. Correlation between decreased vagal activity and necrotizing enterocolitis (NEC). Gastroenterology 2012; 142:S47–S48 [Google Scholar]
- 198.Inukai T, et al. Parasympathetic nervous system activity in hypothyroidism determined by R-R interval variations on electrocardiogram. J Intern Med 1990; 228:431–4 [DOI] [PubMed] [Google Scholar]
- 199.Ajiki K, et al. Autonomic nervous system activity in idiopathic dilated cardiomyopathy and in hypertrophic cardiomyopathy. Am J Cardiol 1993; 71:1316–20 [DOI] [PubMed] [Google Scholar]
- 200.Oka H, Mochio S, Sato K, Sato H, Katayama K. Prolongation of QTc interval and autonomic nervous dysfunction in diabetic patients. Diabetes Res Clin Pract 1996; 31:63–70 [DOI] [PubMed] [Google Scholar]
- 201.Julius S, Valentini M. Consequences of the increased autonomic nervous drive in hypertension, heart failure and diabetes. Blood Press Suppl 1998; 3:5–13 [DOI] [PubMed] [Google Scholar]
- 202.Motte S, et al. Respiratory-related heart rate variability in progressive experimental heart failure. Am J Physiol Heart Circ Physiol 2005; 289:H1729–1735 [DOI] [PubMed] [Google Scholar]
- 203.Evrengul H, et al. The relationship between heart rate recovery and heart rate variability in coronary artery disease. Ann Noninvasive Electrocardiol 2006; 11:154–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Meister AL, Jiang Y, Doheny KK, Travagli RA. Correlation between the motility of the proximal antrum and the high-frequency power of heart rate variability in freely moving rats. Neurogastroenterol Motil 2019; 31:e13633. [DOI] [PMC free article] [PubMed] [Google Scholar]