Abstract
While conventional material fabrication methods focus on form and strength to achieve function, the fabrication of material systems for emerging life science applications will need to satisfy a more subtle set of requirements. A common goal for biofabrication is to recapitulate complex biological contexts (e.g. tissue) for applications that range from animal-on-a-chip to regenerative medicine. In these cases, the material systems will need to: (i) present appropriate surface functionalities over a hierarchy of length scales (e.g. molecular features that enable cell adhesion and topographical features that guide differentiation); (ii) provide a suite of mechanobiological cues that promote the emergence of native-like tissue form and function; and (iii) organize structure to control cellular ingress and molecular transport, to enable the development of an interconnected cellular community that is engaged in cell signaling. And these requirements are not likely to be static but will vary over time and space, which will require capabilities of the material systems to dynamically respond, adapt, heal and reconfigure. Here, we review recent advances in the use of electrically based fabrication methods to build material systems from biological macromolecules (e.g. chitosan, alginate, collagen and silk). Electrical signals are especially convenient for fabrication because they can be controllably imposed to promote the electrophoresis, alignment, self-assembly and functionalization of macromolecules to generate hierarchically organized material systems. Importantly, this electrically based fabrication with biologically derived materials (i.e. electrobiofabrication) is complementary to existing methods (photolithographic and printing), and enables access to the biotechnology toolbox (e.g. enzymatic-assembly and protein engineering, and gene expression) to offer exquisite control of structure and function. We envision that electrobiofabrication will emerge as an important platform technology for organizing soft matter into dynamic material systems that mimic biology’s complexity of structure and versatility of function.
Keywords: electrofabrication, biopolymers, electrogelation, electrodeposition, chitosan, collagen
1. Introduction: a roadmap for biofabrication?
In the middle of the last century, there were transformations in both communication theory and microfabrication that enabled the Information Age. Specifically, theories were developed to efficiently code, transmit and decode information, and a suite of fabrication methods also emerged that enabled the manufacture of solid state electronic circuits. This coupling of advances, both to create structure and to control the flow of information, enabled Gordon Moore to offer an empirical prediction that served as a roadmap to measure advances in the microelectronics industry over the last 50 years (figure 1(a)). In this section, we draw an analogy between the historical emergence of the microelectronics industry and the emergence of a nascent biofabrication-based industry. While there have been recent efforts to refine the definition of ‘biofabrication’ in terms of methods (bioprinting and bioassembly) for an important set of applications (tissue engineering and regenerative medicine) [1], we retain a broader perspective and suggest a focus on capabilities that span application areas and do not specify the methods used. In subsequent sections, we focus on electrofabrication methods that have been independently developed from different labs using different materials and for different applications. These methods share the common features that electrical inputs are imposed to solutions of biologically derived materials for the purpose of creating structure and conferring function. We suggest the term ‘electrobiofabrication’ to capture the common features of these methods.
Figure 1.
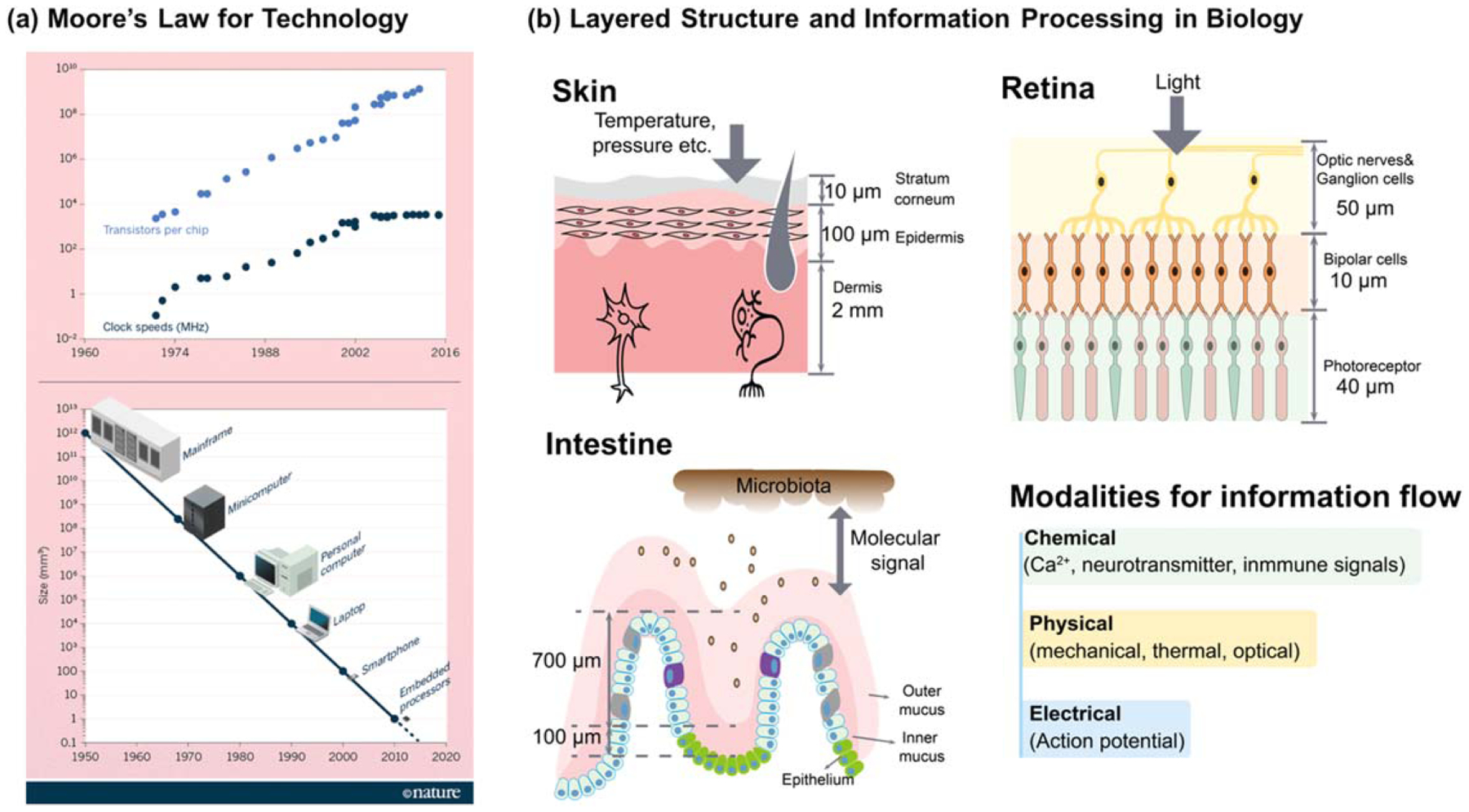
Layered thin films are used to process information in technology and biology. (a) Moore’s law tracks progress in information technology using generic metrics for structure and performance. Reprinted by permission from Macmillan Publishers Ltd: [Nature] [6], copyright 2016. (b) A common goal for biofabrication is to recapitulate biology’s layered structures and spatiotemporal signaling.
In contrast to the microelectronics industry, it seems impossible to imagine a 50-year roadmap for biofabrication—but why was a roadmap so relevant for tracking advances in information technologies, yet seemingly irrelevant for the biological technologies? One critical difference is that modern devices are designed de novo to satisfy user-defined objective functions, while living systems already exist but are so complex that much of life science research (even translational research) aims to clarify what is unknown. For microelectronics, it was possible to identify robust, generic and quantitative metrics that could be used to track progress in organizing structure (e.g. minimum feature size and transistor density) and system-level performance (e.g. memory and speed). In contrast, large research initiatives in biology (e.g. the human genome project) often lead to advances that were not initially anticipated, and these benefits are generally clearer in hindsight. Further, progress in biological technologies is commonly marked by discontinuities that result from discoveries of new phenomena (e.g. gene amplification (PCR), gene silencing (RNAi), gene editing (CRISPR)) that provide previously unimaginable opportunities. The challenge of design in the midst of such biological uncertainties is illustrated by the low probability that a candidate drug will progress from discovery to the clinic (≈10−4). Thus, there are currently no simple, relevant and quantitative metrics of structure or performance that allow the advances in biofabrication to be tracked. And, it seems doubtful that generically useful metrics will soon emerge given the recent focus on biological systems of increasingly complex structure and interconnectivities (e.g. the gut and brain), and given that desired endpoints may be difficult to characterize, even qualitatively (e.g. wellness).
While it may not be possible to create a long-term roadmap for biofabrication and identify specific quantitative metrics to track progress, it does seem possible to recognize constraints and envision some desirable capabilities that could serve as targets for advancing the field. We especially focus on the fabrication of soft matter, with a particular goal of recapitulating the structure and interconnectivity of complex biological tissues for in vitro (e.g. animal-on-a-chip) and in vivo (e.g. artificial organs) applications, and table 1 lists a set of features which we feel are, or will be, important for such applications. In addition to recapitulating tissue-like structure and function, biofabrication may also be able to provide the means to meet more specialized needs to create technological systems that can control the release of therapeutics [2], modulate biochemical signal generation [3] and interface with biological systems (e.g. implantable or wearable electronics and soft robotics) [4, 5].
Table 1.
Capabilities likely to be important for the biofabrication of soft matter systems that mimic biological tissue.
|
If we look to nature for guidance, there are numerous examples of soft tissue that are organized as a series of layers (figure 1(b)). In these biological examples, each layer (≈10–2000 μm) may have different components, structures and cell types that provide a localized microenvironment with a set of physical, chemical and biological properties that enable specialized tasks to be performed. However, these individual layers are not static and do not function in isolation: rather, the individual layers are interconnected to allow the tissue system to dynamically adapt its structures and functions in response to external or internal cues (e.g. neuronal connections in the brain emerge during learning). Integral to the emergence of these evolving contexts is the communication between and among the various layers, and this communication is often mediated by chemical, electrical and mechanical signals that cue responses to adapt, heal or maintain homeostasis.
Interestingly, many additive manufacturing methods (e.g. 3D printing and layer-by-layer self-assembly [7–11] also organize matter as a series of layers, often over similar length scales as tissue [12], and thus additive manufacturing would seem to be well-suited for biofabrication [13–17]. One well-recognized requirement for extending the capabilities of additive manufacturing to biological applications is biocompatibility. This requirement is motivating considerable current effort to develop biocompatible inks for printing and to develop aqueous-based processing methods [18–25]. However, biofabrication will need to do more than provide conditions that maintain cell viability. Rather, biofabrication will need to be able to create matrices with an appropriate suite of properties and interconnectivities that enable the emergence of complex biological contexts [26, 27].
Currently, many of the most familiar applications of conventional additive manufacturing focus on creating static structures with highly controlled shapes and strength (e.g. metal and plastic parts). For biological applications, the fabricated structures must offer a more complex and subtle set of physical, chemical and biological properties [28–30]. For instance, the mechanical requirements will be more complex than simply Young’s modulus and strength to failure. Rather, advances in mechanobiology are demonstrating the importance of cell-substrate mechanical interactions for cueing processes, such as cell adhesion, spreading, migration and differentiation [27]. From a fabrication standpoint, these physical-mechanical requirements may not be homogeneous, but rather it may be necessary to create material systems with complex internal structures and spatially varying anisotropic properties [31–38]. Further, the mechanical requirements may not be static, but rather material systems may need to undergo reconfigurations in shape [39–41] and properties [42–44]: in some cases in response to user-imposed external cues [45], and in other cases in response to internal biological cues [46]. In addition to considering microscopic mechanical requirements, there are also exciting opportunities to create material systems that can be actuated at the macroscale (e.g. for soft robotics) [47–50]. To meet these emerging needs, there is considerable current research to create material systems that can respond, heal and remember [35, 46, 51–53].
In addition to meeting mechanical needs, a biofabricated structure must also offer the molecular transport properties that enable the exchange of chemical components that are vital to cell survival and cell–cell communication. In contrast to electronic systems, which typically use electricity, biology tends to use chemicals to perform energy transduction (e.g. catabolism) and information processing functions (e.g. hormones). Importantly, biology enlists various structural approaches to overcome the slow-ness and randomness of molecular diffusion: the vasculature provides routes for various chemical resources (e.g. glucose and O2) to be distributed throughout the body, compartmentalization is used to control/segregate the flow of chemically based information (e.g. neurotransmitter vesicle trafficking) and responsive systems allow the delivery of chemical information at appropriate addresses (e.g. intracellular virus particle disassembly for the delivery of infectious nucleic acid). The challenges of controlling the flow of molecular information are well recognized [54], and considerable research aims to develop material systems that provide porosity [55], promote vascularization [56], target/control release [57–64] and allow control of motion, either autonomously [65] or externally controlled [66]. Given the importance of controlling the flow of molecular information in biology, we anticipate that there will remain great interest in creating structures to control the speed and directionality of molecular transport [2, 67, 68].
In addition to responding to diffusible chemical signals, cells also recognize chemical cues embedded on or within tissue. For instance, integrins mediate cell adhesion and spreading on the extracellular matrix (ECM), and also transmit information of such extracellular events within the cell to provide cues that regulate a wide range of cellular processes, including proliferation, differentiation, apoptosis and angiogenesis [69]. Thus, it is essential to develop fabrication methods that enable a soft matter matrix to be chemically modified to tailor cell–matrix interactions and elicit appropriate cell responses.
In summary, transformations in manufacturing (e.g. additive manufacturing) offer considerable promise for the fabrication of biological systems. While there are many exciting possibilities, there are also many important challenges to transition from an ability to create static shapes with homogeneous internal structures to enabling the creation of dynamic material systems with tailored hierarchical structures and adaptive functional properties. For example, many of the existing additive manufacturing approaches couple mechanical, optical, magnetic and thermal stimuli to spatially organize matter and induce the phase transitions and/or chemical reactions that create structure. Yet these existing methods often lack the ability to exert control at the nanoscale (e.g. for residue-specific crosslinking) or induce the hierarchical organization (e.g. collagen bundles) that is so prevalent in biology. In the remainder of this review, we explain our belief that electrically based fabrication with biologically derived materials (i.e. electrobiofabrication) provides exciting, and in many cases complementary, capabilities to meet some of these biofabrication needs [70]71 As discussed, electrical cues can induce phase transitions and chemical reactions to create structure, but can also impose forces to induce macromolecule movement and alignment to enable anisotropies and ordering to be built into this structure. We envision that the use of electrical cues will also allow the signal processing and data science advances of microelectronics to be applied to accelerate material discovery and development. Also, as discussed, the use of biologically derived materials allows access to the tools of modern biology to create structure and confer function.
2. Electrobiofabrication: imposing electrical signals to biological materials to create structure and confer function
It is well known that electrical inputs can be used for chemical synthesis (i.e. electrosynthesis [72–74]) and material fabrication (the interested reader is directed to several reviews of various aspects of this topic) [75–81]. For instance, electrical inputs are commonly used to electropolymerize monomers (e.g. for conducting polymers) [82, 83], to electrodeposit polymer-based functional coatings (e.g. electrodeposition paints) [84–91] and for electrospinning and electrowriting [92–94]. Electrically based fabrication offers unprecedented capabilities that can also be enlisted for biological applications. As will be discussed, electrical signals can be imposed with exquisite temporal and quantitative control to allow films to be electrodeposited simply, rapidly (sec–min) and with controlled thickness. These capabilities enable polymer electrodeposition to be used to conformally coat implants of arbitrarily complex sizes and shapes [95–97], and to assemble functional coatings on electrodes [98], even when they are embedded within a covered microfluidic channel [99, 100]. Obviously, electrically based fabrication has limitations, but when appropriate, it offers significant benefits in speed, simplicity and cost, as well as enabling precise electrical cues to be imposed to guide assembly.
Here, we focus on electrically based fabrication with biologically derived materials (i.e. electrobiofabrication) because biological materials often offer a unique combination of properties suitable for a wide range of biologically based applications [30, 101–103]. For instance, many biologically derived polymers possess stimuli-responsive self-assembling properties that allow fabrication to be cued by external inputs: this allows the coupling of top-down and bottom-up fabrication approaches [104] to create hierarchical structures [105]. Often such self-assembly involves reversible interactions (i.e. physical crosslinks) that enable materials to heal and reconfigure [42], and confer mechanical toughness [35, 43, 53, 106–116]. In some cases, biologically derived materials undergo molecular-recognition based associations that allow the generation of functional supramolecular assemblies [64, 117] or engage biologically specific interactions (e.g. site specific cell attachment) [118]. In addition, biological materials can be acted upon by enzymes, which enables biological degradation [119, 120] or allows macromolecular structures to be built [121] and functional properties to be added (e.g. enzymatic conjugation of a protein to a biopolymer matrix). Finally, if biological polymers are used, then it is possible to enlist advanced biotechnology methods (e.g. protein engineering) to enhance functionality or facilitate assembly (e.g. engineer fusion tags to facilitate self-assembly [122] or enzymatic crosslinking/conjugation [123–129]).
Compared to ‘conventional’ biofabrication methods (e.g. photolithographic and printing methods), we believe electrobiofabrication is much less developed, and there is considerable opportunity for advancements. As discussed below, the response of biological polymers to the electrical inputs is complex and remains poorly understood [130–132], and incremental increases in our fundamental knowledge are enabling greater control of hierarchical structure and matrix function. Also, as discussed below, electrically based fabrication methods emerged independently for polysaccharides, silk and collagen, and different terms have been used to describe the observed phenomena. In most cases, the terminology cannot be generically extended, and thus we adopted the term ‘electrobiofabrication’ in an effort to capture the common features. To facilitate discussion of how biological polymers perceive and respond to electrical input signals, we divide the electrical input signal into two components that can affect the emergence of structure in different ways: the current (or current density) and the electrical field [133–135].
2.1. Electrical current quantifies electrochemical reactions
As illustrated in figure 2(a), electrochemical reactions involve the transfer of electrons across a conducting surface (e.g. across an electrode interface), and the electrical current is a quantitative measure of the rate of electron transfer (formally currents associated with electron transfer due to redox reactions are termed Faradic currents while non-Faradic currents are typically smaller and include polarization ion charges associated with re-arrangements of surface charge). Electrochemical currents can induce structure formation by direct electron transfer (e.g. electropolymerization), and in these cases, the resulting film is generally localized on the electrode surface. However, there are several indirect electron transfer mechanisms that generate diffusible species or gradients that act over longer distances (μm to mm from the electrode surface) to trigger thin film or hydrogel formation [117, 136–138]. In these cases, the electrochemical currents indirectly transduce the electrical inputs into the generation of macromolecular and macroscopic structures (note: such indirect mechanisms require redox reactions at the electrode surface and diffusible chemical species that shuttle electrons to/from the electrode). By analogy to developmental biology, where one tissue generates diffusible chemical cues that induce morphogenic changes in distant tissue (e.g. in the developing embryo), the electrochemically generated diffusible mediators that induce structure at a distance have been termed ‘morphogens’ [139]. In many, but not all, cases the films/hydrogels formed by these indirect mechanisms remain attached to the electrode surface, although they can often be purposefully detached if desired.
Figure 2.
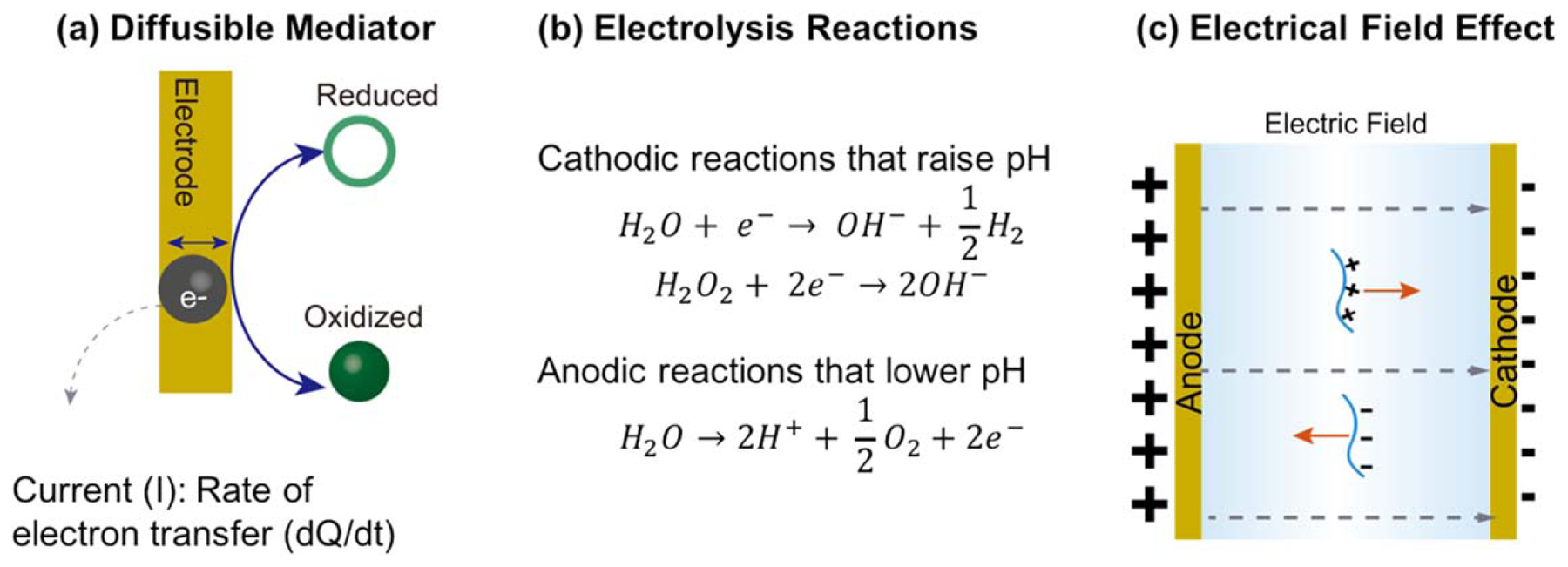
Electrodes provide electrical signals for electrobiofabrication. (a) The current quantifies the electrochemical reactions (i.e. the exchange of electrons at the electrode–solution interface) that can (b) provide localized chemical gradients (e.g. in pH) that cue self-assembly. (c) The electric field can induce macromolecules to migrate and undergo changes in conformation and alignment.
It is convenient to empirically divide diffusible electrochemical cues into two types based on whether the cues act through acid–base or oxidation–reduction mechanisms (table 2). The classic biopolymer example of such an acid–base mechanism is the cathodic neutralization reaction used to electrodeposit the pH-responsive aminopolysaccharide chitosan [140, 141]. As illustrated in figure 2(b), cathodic electrolytic reactions (e.g. of water or H2O2) can yield a localized increase in pH that results in a localized deprotonation of the chitosan chains that induces its reversible sol–gel transition (further details of this mechanism are provided below). In contrast, anodic electrolysis reactions generate a localized region of low pH adjacent to the electrode surface, and anodic biopolymer electrodeposition through a neutralization mechanism was first reported for the acidic polysaccharide alginic acid [142]. A related mechanism can be used to electrodeposit Ca2+-alginate hydrogels: electrolytic reactions locally generate H+ adjacent to the electrode; insoluble calcium carbonate (CaCO3) that is included in the deposition solution reacts to both neutralize this low pH and liberate soluble Ca2+ ions; and the free Ca2+ ions induce the localized gelation of Ca2+-alginate [143]. Table 2 illustrates that electrodeposition through such acid–base reactions has been extended to additional mechanisms and materials.
Table 2.
Biopolymer-based electrodeposition.
| Acid-base Mechanisms | Details/Comments |
|---|---|
| Cathodic neutralization | |
|
Co-deposit various components (ceramics, nanoparticles, proteins) [42,149–152] |
|
Co-deposit with gelatin for conformal coatings [96], osteoblast adhesion [154] and acetylsalicylic acid incorporation [155] |
|
Couple migration and assembly with calcium phosphate mineralization [156, 157] |
| Anodic neutralization | |
|
Deposition through anodic neutralization |
|
Anodic gelation [158–164] |
|
Anodic deposition of gentamicin-loaded silk fibroin coating [95] Co-deposit with nanoparticles and proteins [165–167] |
| Anodic Ca2+ solubilization | |
| Co-deposit with cells [143,168–170] Co-deposit with agarose[173] |
|
|
|
| Anodic conversion of WO42− into isopoly-tungstic acid | Reversible complexation-based crosslinking of Konjac glucomannan polysaccharide [174,175] |
| Oxidation-Reduction Mechanisms | Details/Comments |
| Reduction | |
|
Irreversible deposition [146] |
|
Coupling through azide and alkyne reactions [139] |
| Oxidation | |
|
Co-deposition with enzyme conjugation [176,177] |
| Poly(allylamine hydrochloride) [145] | |
|
Tannic acid-Fe(III) complex [179] |
| Fe3+ alginate assembly (can be reversed by electrochemical reduction of Fe3+ to Fe2+) [148] Poly(acrylic acid)—reversible [180] |
|
|
Mediated oxidation of catechols to quinones to crosslink proteins [181] |
|
Electrostatic interactions with cationic polyelectrolytes [182, 183] |
|
Electrostatic crosslinking of carboxymethylcellulose (CMC) [184] |
| Coordination with chitosan for deposition [147] |
In terms of reduction–oxidation (redox) reactions, several mechanisms have been reported involving different diffusible species. In some cases, these mechanisms involve the generation of reactive intermediates that undergo chemical reactions to covalently crosslink the polymers (e.g. the anodic oxidation of catechols generates quinones that can crosslink polymers through nucleophilic amine substituent groups) [144, 145]. In other cases, an electrochemical reaction can generate a diffusible species that can create chelation-based crosslinks (e.g. chitosan gels have been electrodeposited through mechanisms involving ruthenium salts [146] and copper [147]). In some cases, these electrodeposition mechanisms can be reversible based by changing the redox-state of such metal ions (e.g. Fe2+/3+-alginate hydrogels can be reversibly assembled and disassembled) [148]. As illustrated in table 2, there is a growing list of redox-based mechanisms for (bio)polymer electrodeposition.
While table 2 emphasizes examples of biopolymer electrodeposition, there is a growing interest in extending electrodeposition to life science applications using other materials (e.g. small molecule hydrogelators) [185–191], and also using novel electrically based mechanisms (e.g. bipolar electrochemistry) [105].
2.2. Electrical voltage quantifies electrostatic field
As noted above, the electrochemical reactions that are quantified by current density provide the cues for self-assembly. However, the electric field imposed at an electrode can also have important effects. The most obvious effect of an imposed electric field is that it can provide a force to drive charged particles to migrate toward/away from an electrode (figure 2(c)). In addition, the imposed electric field can align dipoles and confer anisotropies to the emerging structure [192–194]. Importantly, salt can screen the electric field and attenuate its effects. Thus, it is possible to envision that a spatiotemporally varying electric field could be imposed to provide the cues needed to orient macromolecules to guide their hierarchical assembly along pathways that lead to desired structural features and functional properties. The examples discussed below illustrate initial successes toward this vision. However, we expect that fully exploiting the opportunity to enlist electrical inputs to control structure will require more detailed knowledge of how macromolecular systems respond to electrical fields, and a greater quantitative understanding of how to tailor the imposed electric fields.
3. Examples of biopolymer-based electobiofabrication
To our knowledge, the electrodeposition of biopolymers is a relatively new observation, with the first reports emerging in the early 2000s [135, 140, 141]. The electrodeposition methods are generally robust as various labs around the world have adapted and extended others’ work. However, as illustrated in the following examples, there are important subtleties that remain poorly understood. To illustrate this point we focus on the four best understood biopolymer systems—the aminopolysaccharide chitosan, the acidic polysaccharide alginic acid and the structural proteins collagen and silk.
3.1. Chitosan
3.1.1. Cathodic electrical inputs induce chitosan’s reversible self-assembly
Chitosan electrodeposition was first reported in 2002 and is probably the best understood biopolymer deposition system [98, 195–198]. As noted earlier, chitosan is a pH-responsive self-assembling aminopolysaccharide that can be electrodeposited by the cathodic neutralization mechanism illustrated in figure 3(a) [140, 141, 199]. Cathodic electrolysis reactions generate a localized region of high pH adjacent to the cathode and chitosan chains in this region are deprotonated, which induces their gelation. This sol–gel transition involves a reversible self-assembly of the chitosan chains to form crystalline network junctions that serve as physical crosslinks [102]. In various experimental systems it has been observed that as a base penetrates into a chitosan solution, the gelation of chitosan occurs as a growing hydrogel front, and this self-assembly front co-localizes with a growing pH front and also a crystallization front [134, 200–202]. Growth of these fronts can be quantified in terms of a moving front model [134, 203].
Figure 3.
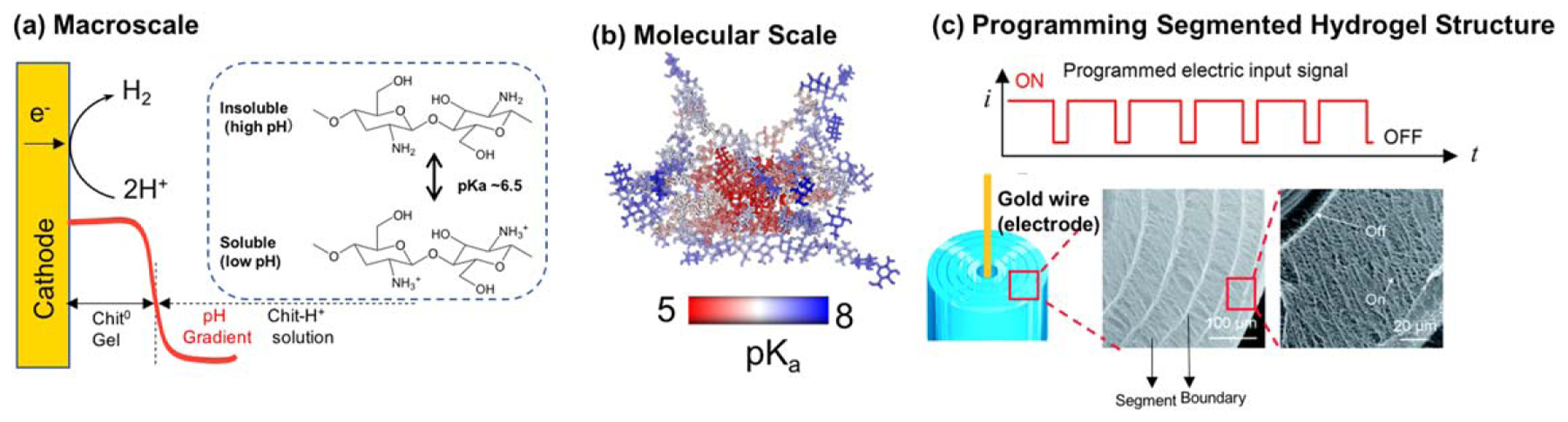
Cathodic electrodeposition of chitosan. (a) Chitosan deposition (i.e. self-assembly) is induced by the high pH generated by cathodic electrolysis reactions. (b) Molecular modeling shows the pKa of an individual glucosamine residue is lowered when it is buried within the crystalline domains that serve as the physical network junctions (i.e. crosslinks). Reproduced with permission from [204]. John Wiley & Sons. © 2018 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (c) When an oscillating electrical input sequence is used to cue chitosan’s electrodeposition, the resulting hydrogel has a segmented internal structure that is controlled by the input sequence. Reproduced from [203] with permission from The Royal Society of Chemistry.
At a macroscopic scale, chitosan’s cathodic electrodeposition is convenient and versatile. Chitosan’s self-assembly is triggered from aqueous solutions using low cathodic voltages (typically less than 2 volts versus Ag/AgCl) that are imposed over short times (sec–min). Further, chitosan’s electrodeposition is controllable: hydrogel film growth can be controlled by the electrical input [205]; deposition can be controlled spatially if a patterned electrode is used [206–208]; and deposition can conformally coat complex surfaces (e.g. wire electrodes [209] or implant surfaces [210–216]). In addition, chitosan’s electrodeposition is simple, reagentless and reversible (the films can be re-dissolved under acidic conditions [217]). Finally, hydrogel electrodeposition is versatile: a variety of materials (e.g. nanoparticles, vesicles and macromolecules) can be co-deposited and entrapped within the chitosan matrix [49, 152, 157, 218, 219], and chitosan’s electrodeposition can be coupled with layer-by-layer electrostatic self-assembly (e.g. with alginate) [220].
At the molecular level, chitosan’s electrodeposition is much less understood, which provides a rich opportunity to control structure over a hierarchy of length scales. The molecular modeling results illustrated in figure 3(b) [221] indicate that the internal regions of the crystalline network junctions are hydrophobic with comparatively little water, and this localized hydrophobic microenvironment results in a structure-induced decrease in the pKa of the glucosamine residues [204]. Importantly, formation of these crystalline regions is highly favorable thermodynamically, and thus the organized crystalline regions can co-exist adjacent to less-ordered (e.g. amorphous) regions. One consequence of the stability of these crystalline network junctions is that the microstructure of the deposited hydrogel can be controlled by the specific conditions used to trigger gelation. The sensitivity of the internal structure to deposition conditions is illustrated by the following two examples.
In 2008, the Domard group reported an experiment in which chitosan’s base-induced gelation was performed in a sequence of steps that systematically interrupted and then re-initiated gelation, and they observed that the resulting hydrogel had a multilayered internal structure [222]. Several groups have reproduced and extended these observations [49, 223–225], and these reports motivated a study in which the electrical inputs that induced chitosan’s electrodeposition were also imposed as an oscillating sequence [203]. Figure 3(c) shows that a multilayered segmented chitosan hydrogel emerged in response to such an oscillating electrical input sequence: during the electrical ‘on’ signal, segments were grown while boundaries were formed during the ‘off’ signal [134]. Importantly, the microscopic structure and modulus of the boundary regions were highly dependent on the presence of salt, presumably through screening of the electric field, although these salt effects are incompletely understood [226]. We believe the ability to enlist highly controllable electrical signals to guide the emergence of structures will provide exciting opportunities to create matrices with anisotropies in mechanical properties and aligned structures with preferred directions for molecular transport.
A second example illustrating how localized electrical inputs can induce the emergence of internal structures involves electrical writing onto a dual responsive hydrogel medium [204, 227]. As illustrated in figure 4(a), this dual responsive medium was first prepared by blending a warm solution of agarose with an acidic solution of chitosan, and cooling this blend to form the agarose hydrogel. A small cylindrical electrode (a stainless-steel acupuncture needle) was used as a cathodic ‘pen’ that was rastered across the surface of this medium to create localized regions of high pH that induced the disorganized chitosan chains in the hydrogel medium to assemble into crystalline domains (figure 4(b)). Importantly, the structural gradients induced by cathodic writing are stable and persist after the pH gradient has dissipated. Specifically, regions in which neutral chitosan chains are organized into crystalline domains (designated Chit0) stably co-exist adjacent to regions in which protonated chitosan chains remain disassociated from each other (designated Chit-H+). Presumably these structural gradients are stabilized by the structure-induced decrease in the pKa for the glucosamine residues in the crystalline regions. A variety of experimental methods were used to demonstrate that the written and unwritten regions of the agarose–chitosan medium possess markedly different functional properties: the formation of crystalline network junctions in the written regions increases the mechanical strength; the neutral glucosamine residues in the written region are more chemically reactive (i.e. nucleophilic) compared to the protonated glucosamine residues in the unwritten regions; and fibroblast cells were observed to preferentially adhere to the written (versus unwritten) regions. Also, important to note is that the structural information written into the agarose–chitosan medium can be erased by adding acid to disassemble the chitosan chains and heat to disassemble the agarose chains.
Figure 4.
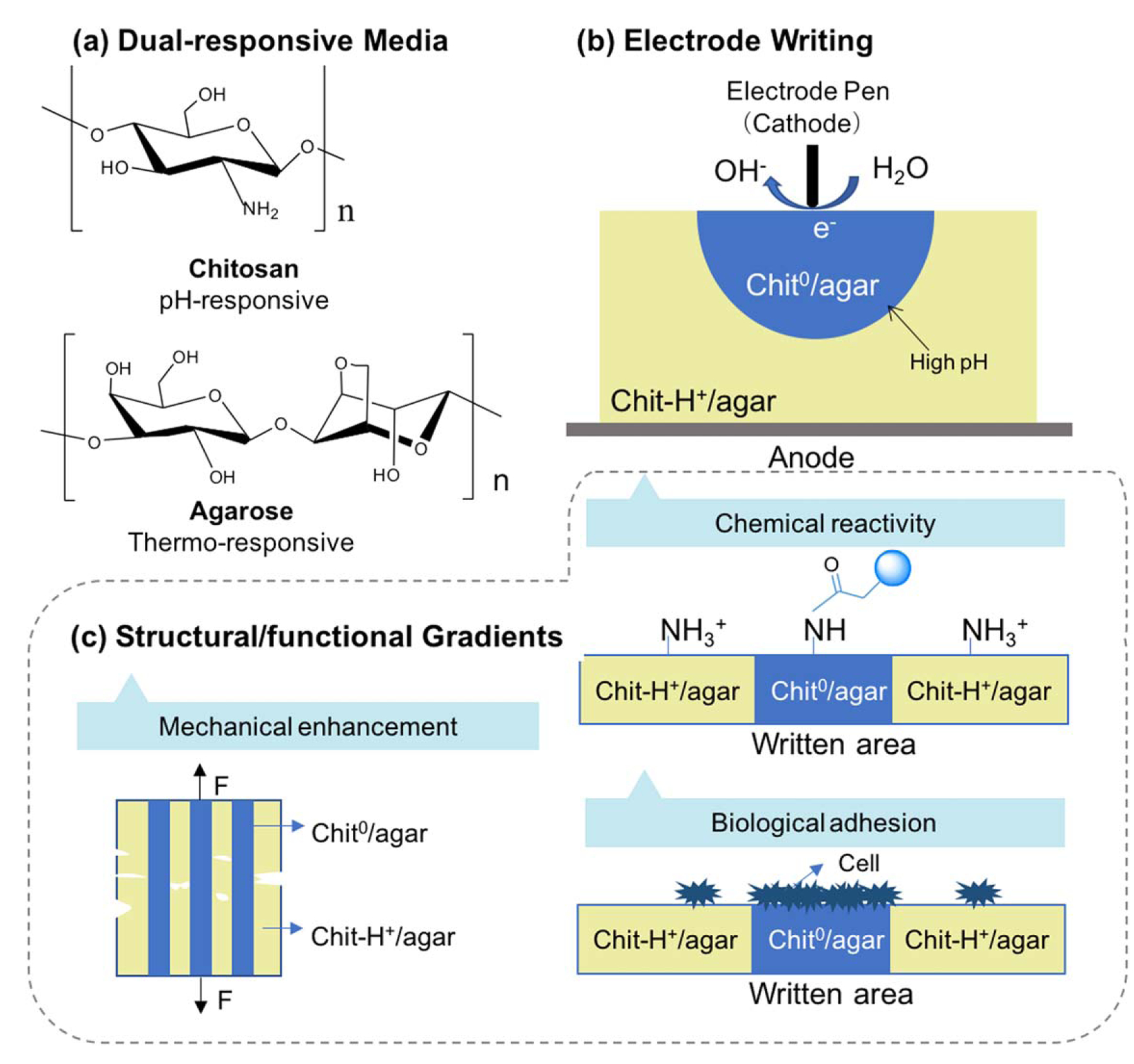
Cathodic writing onto a dual responsive (chitosan–agarose) medium induces the formation of an internal structure. (a) The dual responsive hydrogel is formed by cooling an acidic blend of chitosan and agarose. (b) The internal structure is created using a cathodic ‘pen’ to create regions of high pH that induce the localized self-assembly of chitosan chains within the agarose network. (c) The gradients in the structure induced by writing also yield gradients in mechanical, chemical and biological properties. Adapted with permission from [204]. John Wiley & Sons. © 2018 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
A final example illustrates the ability to couple cathodic electrodeposition with other, more conventional, additive manufacturing methods. In this study, a cathodically deposited chitosan film was created and then printed on using an ‘ink’ containing acidic sodium-dodecyl sulfate (SDS) micelles, as illustrated in figure 5(a) [42]. This acidic ink can protonate the chitosan chains and induce disassembly of the crystalline network junctions, while the negatively charged SDS micelles can electrostatically crosslink these cationic chitosan chains [228–233]. Figure 5(b) indicates that this printing step yields patterned films with different regions being crosslinked by independent physical mechanisms: the unprinted regions retain the original neutral crystalline network junctions (Chit0), while the printed regions are electrostatically crosslinked to form an SDS–chitosan network (Chit-H+-SDS). Again, the spatial gradients imposed by printing persist for days—long after the pH has been equilibrated. One important feature of this patterning is that the different crosslink types confer different mechanical properties: the neutral crosslinks of Chit0 confer elastic properties [111], while the Chit-H+-SDS crosslinks confer viscoelastic properties. Thus, figure 5(c) shows that printing allows the creation of patterned films with anisotropic mechanical properties. A second important feature is that both of these physical crosslinks are reversible, such that immersion of a patterned film in a base induces the cationic chitosan chains to be deprotonated, the SDS micelles to detach and diffuse out of the film and crystalline network junctions to re-form in the previously patterned region (as illustrated in figure 5(b)) [42]. Thus, the use of physical, reversible and pH-responsive crosslinks allows the hydrogel films to be reconfigurable.
Figure 5.
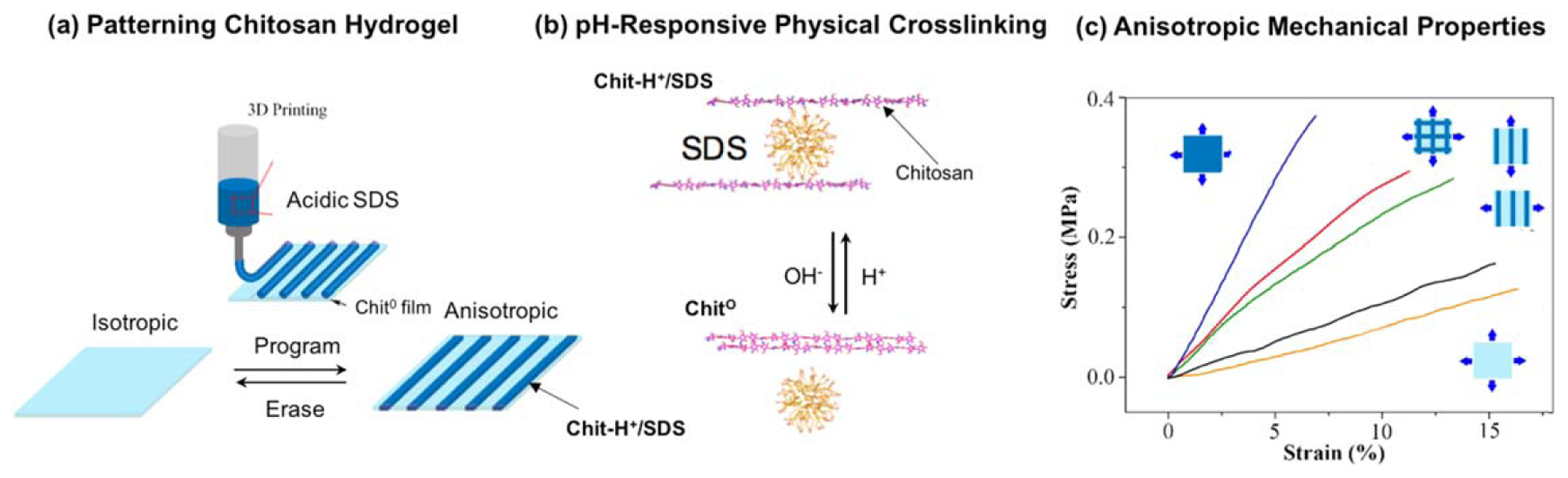
Chitosan can be crosslinked by independent physical mechanisms enabling films to be reversibly patterned, erased and reconfigured. (a) An acidic SDS solution is used as an ‘ink’ that is printed onto a cathodically deposited chitosan film. (b) One physical crosslinking mechanism involves electrostatic interactions between protonated chitosan chains and SDS micelles (Chit-H+-SDS), while a second physical crosslinking mechanism involves the crystalline domains that serve as network junctions (Chit0). (c) Films that are patterned with different crosslinking mechanisms offer anisotropic mechanical properties. Adapted with permission from [42]. John Wiley & Sons. © 2017 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
In summary, cathodic inputs can induce chitosan chains to self-assemble through physical interactions. Evidence indicates that this electrically triggered self-assembly is highly controllable, with the emergent structure being sensitive to how the electrical signals are imposed and the conditions of the deposition bath (e.g. the presence of salt [226]). We anticipate that the capability to precisely control structure will provide exciting opportunities to create matrices with anisotropies in mechanical properties and preferred directions for molecular transport. Further, the ability to couple electrodeposition with other methods (e.g. layer-by-layer and 3D printing) suggests that materials can be generated with a subtle balance of physical interactions that mimic the dynamic materials in biology that can respond, heal and reconfigure. The critical limitation to achieving these dynamic properties is our understanding of the underlying molecular phenomena, which we expect will require both advances in theory and experiment. Molecular modeling provides the opportunity to understand molecular-level details of how chains self-assemble and how conditions (e.g. an applied electric field) affect this self-assembly [221, 234]. Presumably, all atom modeling will need to be integrated with coarse-graining methods to understand the emergence of structure over a hierarchy of length scales, and to understand the dynamic features of the non-equilibrium assembly [235]. Experimentally, there remains relatively few methods capable of characterizing hydrogel structure and function at various length scales. Particularly interesting methods include quantitative polarized light microscopy [134, 202, 236], which can characterize chain organization, and Brillouin spectroscopy [134, 237], which can access microscale mechanical properties. Both of these imaging methods can be used in real time to observe the emergence of structures.
3.1.2. Anodic electrical inputs induce oxidative reactions
In contrast to cathodic inputs that induce chitosan to self-assemble through reversible physical mechanisms, anodic electrical signals can induce oxidation reactions that result in covalent modifications to chitosan. This is illustrated in figure 6(a), which shows a two-step electrobiofabrication process in which a chitosan film was first cathodically deposited onto a platinum electrode, and then this film-coated electrode was rinsed and immersed in a buffered solution containing 0.5 M salt and an anodic potential was applied to the underlying platinum film [238]. As illustrated by the reactions in figure 6(a), anodic reactions generate reactive chlorine species (e.g. HOCl) that can covalently react with chitosan to generate chloramine residues. Quantitative analysis demonstrated that the film thickness was controlled by the cathodic charge transfer (Q = ∫idt where i is the current and t is time) while the generation of N-Cl bonds was controlled by the anodic charge transfer. These electrobiofabricated chloramine films could then be peeled from the electrodes, and were shown to offer antimicrobial properties (e.g. for wound dressings) [238].
Figure 6.
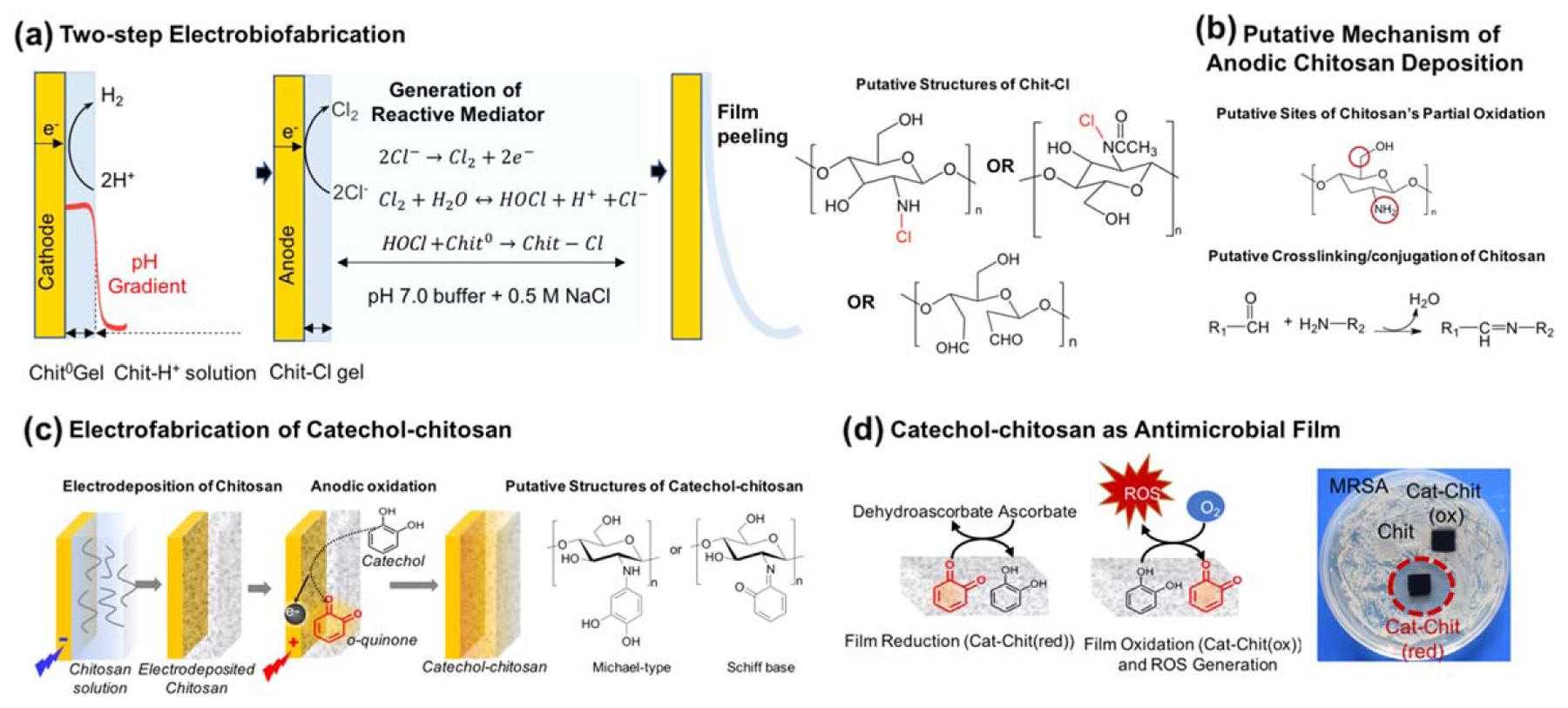
Anodic (oxidative) deposition of chitosan involves covalent modifications. (a) Two-step fabrication of chitosan film to obtain chloramine residues that confer antimicrobial activities. (b) An analogous single step anodic deposition mechanism for chitosan. (c) The anodic fabrication of a catechol–chitosan film. Adapted from [239]. CC BY 4.0. (d) Catechol–chitosan films are redox-active and allow for the sustained in situ generation of ROS that can inhibit the growth of methicillin resistant Staphylococcus aureus (MRSA). Adapted from [240]. Copyright 2018, with permission from Elsevier.
Anodic reactions have also been shown to allow chitosan to be electrodeposited in a single step [177]. In this case, chitosan is first dissolved in an acetic acid buffer with 0.15 M NaCl and then electrodeposited using a gold-coated silicon wafer. As illustrated in figure 6(a), anodic reactions generate reactive chlorine species that oxidize the chitosan chains (presumably generating reactive aldehyde moieties) that can induce covalent crosslinking of the chitosan chains through Schiff-base reactions (figure 6(b)). Again, this anodic deposition step can be controlled by deposition conditions and yields a gel that could swell but not dissolve under acidic conditions (consistent with a covalent crosslinking). Importantly, the aldehyde group generated by chitosan’s oxidation is reactive toward the amine of proteins (e.g. surface lysine residues), and thus if this anodic deposition step is performed in the presence of proteins, the protein can be deposited with and covalently conjugated onto the gel. This single step electrobiofabrication approach provides a particularly convenient approach to generate a hydrogel with functional proteins (note, this approach may not be universal as some proteins appear to be damaged during anodic deposition) [177]. An alternative approach for protein conjugation is to first cathodically deposit the chitosan film, then anodically oxidize the chitosan (to generate the reactive aldehydes), rinse these activated films to remove soluble reactive chlorine species and finally contact the activated films with protein [241].
One final example of anodic chitosan modification involves the electrobiofabrication of catechol–chitosan films, as illustrated in figure 6(c) [242]. In this case, chitosan is first cathodically deposited (not shown), the film is rinsed and then immersed in a solution containing catechol, and an anodic potential is applied to initiate oxidation of the catechol to reactive quinones that can graft to the chitosan. These catechol-modified chitosan films were shown to be non-conducting as electrons do not flow in response to an applied potential, and there was no direct exchange of electrons with the electrode (presumably the catechol moieties are physically separated from each other and from the electrode surface to preclude electrical conductivity). However, the films were shown to be redox-active, and could exchange electrons with diffusible oxidants and reductants. It should be noted that the oxidative grafting of catechol can be performed by alternative methods, such as by chemical oxidation (e.g. using NaIO3) or by enzymatic oxidation (e.g. using tyrosinase) [243–245]. As will be discussed, one important application for the catechol–chitosan film is as a redox-capacitor for bioelectronics [197, 239, 246, 247]. As illustrated in figure 6(d), an alternative application for the redox-active catechol–chitosan film is as an antimicrobial film that can accept electrons from a biological reductant ascorbate (i.e. vitamin C), and transfer them to O2 to generate reactive oxygen species (ROS) [240]. In an analogous reaction, the catechol–chitosan film could be used as a protective coating that transfers electrons from ascorbate to quench an oxidative free radical [248].
In summary, chitosan can be electrodeposited anodically through redox reactions that covalently modify the chitosan chains. By appropriate selection of conditions, it is possible to use such electrical inputs to graft moieties (e.g. chloramine or catechol groups) that confer functional activities (e.g. antimicrobial). In some cases, it is also possible to use such anodic reactions to ‘activate’ chitosan for the covalent conjugation of proteins to confer protein-based functional properties.
3.2. Alginate
Alginic acid is a Ca2+-responsive self-assembling polysaccharide that has been widely investigated as an immobilization matrix for microbes, a scaffold for tissue engineering [249] and more recently as bead/capsule devices for molecular communication [250–252]. Like chitosan, Ca2+-alginate forms hydrogels through self-assembling reversible crosslinks (i.e. through eggbox network junctions). The reversibility of these crosslinks confers responsive properties to the Ca2+-alginate networks [43, 253], while the dynamic (i.e. healable) nature of these crosslinks makes it a suitable choice for mechanically tough dual network systems [35, 43, 107, 108]. Increasingly alginate is viewed as a biocompatible material for biofabrication applications [49, 115].
In 2008, the electrodeposition of alginic acid through an anodic neutralization mechanism was reported [142], and the following year the anodic deposition of Ca2+-alginate was reported through the mechanism in figure 7(a) [143]. The electrodeposition of Ca2+-alginate shares many of the same benefits as those described for chitosan’s electrodeposition: deposition is performed from aqueous solution using mild conditions; it is controlled by the electrical input [168, 171, 254, 255]; it can be spatially selective for patterned [256] or arrayed electrodes [257]; it allows conformal coating of complex shapes (e.g. to create tubular structures) [258]; it can be used to assemble biologically active components for sensor applications [259]; and Ca2+-alginate electrodeposition can be coupled with other assembly methods (layer-by-layer polyelectrolyte complexation [260] and 3D printing [172]).
Figure 7.
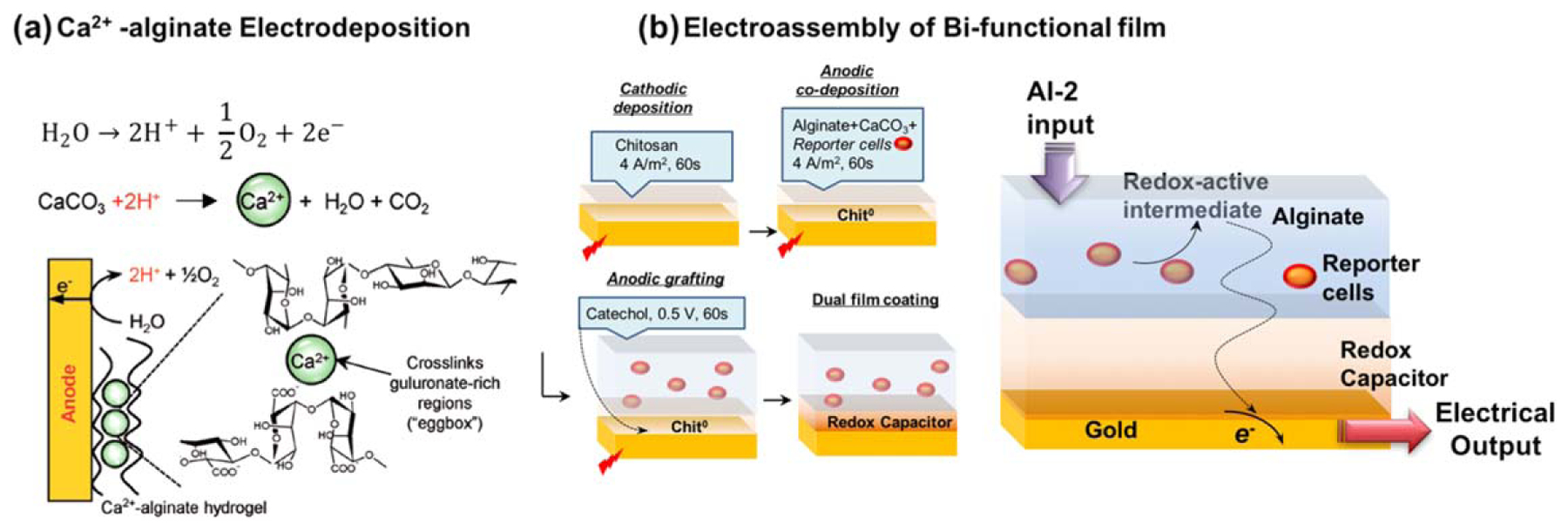
Electrobiofabrication of functionalized alginate hydrogel films. (a) The mechanism for the anodic deposition of Ca2+-alginate hydrogels. Reproduced with permission from [143]. John Wiley & Sons. Copyright © 2009 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (b) Three-step electrobiofabrication of a dual functional film that transduces the detection of a bacterial signaling molecule (autoinducer-2; AI-2) into a redox-active intermediate that is detected electrochemically. Adapted with permission from [266]. John Wiley & Sons. © 2016 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Probably the most important feature of Ca2+-alginate’s electrodeposition is its ability to be applied to studies with living cells. For instance, electrodeposited RGD-modified Ca2+-alginate hydrogel films were seeded and incubated to generate confluent cell sheets that could be detached using a Ca2+-chelating agent (e.g. EDTA) [261]. Further, if cells can be blended into the deposition solution (e.g. sodium alginate plus CaCO3), then it is often possible to co-deposit hydrogels with entrapped cells. For instance, alginate entrapped cells were detached and stacked to form multilayer structures to mimic tissue [262]. Finally, it is sometimes possible to perform sequential deposition steps (with different deposition solutions) to yield multilayer alginate films with spatially segregated cell populations. This capability was demonstrated for the creation of a model biofilm with different bacterial populations addressed to the individual layers of a stratified multilayer [170, 263].
There are two additional recent reports of interest. First, it was observed that an electrode ‘pen’ could be used to write Ca2+-alginate features onto/into a dual network hydrogel containing gelatin and alginate (analogous to figure 4 for writing onto the dual agarose/chitosan network). Mammalian cells could be cultured within these written Ca2+-alginate regions, the unwritten regions could be removed by heating (to melt the gelatin) and independently fabricated hydrogel layers could be stacked to create 3D structures [264].
Finally, figure 7(b) shows a dual film system that was created using three sequential electrobiofabrication steps; cathodic electrodeposition of a chitosan film, anodic co-deposition of a Ca2+-alginate film with E. coli reporter cells, and anodic oxidative grafting of catechol to the underlying chitosan film. The individual films of this dual film system performed separate functions. The bacteria in the Ca2+-alginate biofilm recognized a molecular signal (the bacterial quorum sensing molecule autoinducer-2; AI-2), and transduced this molecular ‘information’ into a redox output (a redox-active intermediate). The catechol–chitosan film served as a redox-capacitor to amplify the electrical output associated with this redox-active intermediate [265, 266].
In summary, alginate offers exciting possibilities as a matrix material that can be reversibly assembled and disassembled to incorporate cells into organized tissue-like structures. Increasingly, experimental studies are demonstrating the capabilities of electrobiofabrication to create such organized structures. We are unaware of complementary molecular modeling efforts to assist in understanding how alginate responds to imposed electrical cues, or that reveal molecular-level details of the alginate self-assembly mechanisms.
3.3. Collagen
Collagen is the most abundant protein in animals and is well known for the structural role it plays in the body. As a major component of the ECM, collagen is integral to maintaining the structural integrity of the ECM, it provides sites for cell adhesion and spreading, and it continually undergoes re-modeling to refine cellular behavior and tissue function [267]. Collagen-based biomaterials have attracted great attention because they are intrinsically biocompatible, bioactive, biodegradable [268] and can be readily fabricated into a variety of forms, including 3D tissue engineering scaffolds using bottom-up approaches [269–271]. Often the motivation for fabricating with collagen is to recapitulate important features of tissue: the aligned and hierarchically organized structure (figure 8(a)); and the molecular and mechanical cues that promote cell adhesion, ingrowth, proliferation and differentiation towards desired cell fates.
Figure 8.
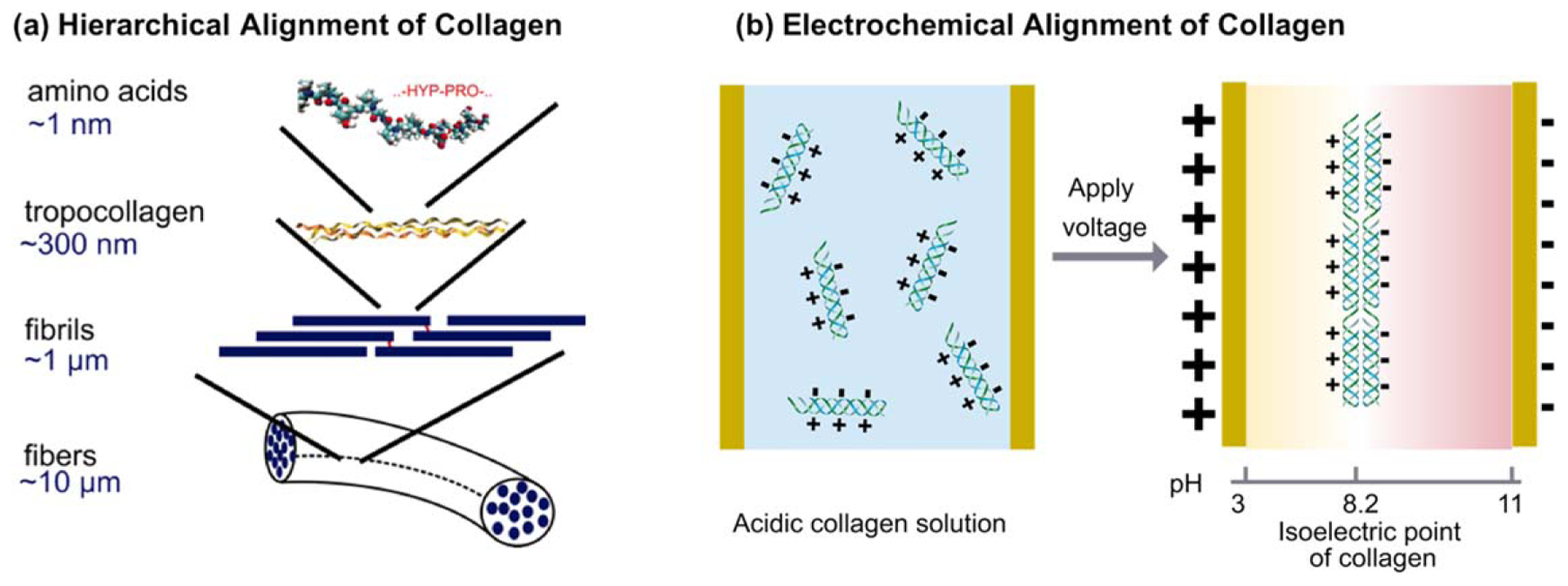
Electrical signals can be used to induce the migration, alignment and assembly of proteins. (a) The hierarchical organization of collagen. Reproduced with permission from [272]. © 2006 by The National Academy of Sciences of the USA. (b) Electrochemical alignment of collagen at a location where the pH is equal to its isoelectric point.
There were early reports that electrodes could be used to align and precipitate collagen from solution [273], but these observations appeared to remain dormant for 40 years. In 2008 there was a renewed interest in enlisting electrical inputs to generate collagen fibers [135] and collagen membranes [274]. These early studies observed the emergence of oriented structure using polarized light microscopy, and recognized the importance of both water electrolysis for establishing a pH gradient and the electric field generated between the electrodes [135, 273]. The collagen protein is ampholytic with the charge varying between positive values (low pH) and negative values (high pH). This reversal of charge with pH differs from chitosan and alginate, which are weak polyelectrolytes and are charged at one pH extreme, but uncharged at the other extreme. Figure 8(b) illustrates that the electrochemical fabrication of collagen (and other proteins) resembles isoelectric focusing with the imposed electrical field driving the migration of collagen until it localizes in a position in which the molecules have no net charge (this location is not necessarily at an electrode surface). The electric field also offers the opportunity to align the collagen molecules [275] and confer anisotropic order that could promote collagen’s hierarchical assembly toward a more native structure [276]. The electrochemically aligned collagen (ELAC) has been observed to be dense, aligned and strong [133], with considerably greater strength than randomly oriented crosslinked collagen gels [135].
Collagen’s electrobiofabrication is controllable, with the thickness (e.g. of the collagen membrane) controlled by the time of the imposed electrical input [276], while further treatments (e.g. with buffer) have been used to promote fibrillogenesis in the collagen network [277]. More recently, the term electrochemical compaction has been used to emphasize the ability to create dense collagen films and membranes [278, 279]. While several studies report how the electrical inputs control the structure and physical properties of collagen, the focus of most of the research has been translational and specifically to understand how collagen-based matrices can be created to yield desirable biological responses (e.g. to guide cell adhesion, proliferation, migration and differentiation) [135]. To satisfy such translational goals, collagen’s electrochemical fabrication has been extended in important ways. For instance, other components (e.g. polysaccharides) are being incorporated into the electro-compacted collagen [280–282]. Also, sequential electrocompaction steps were used to create collagen–hydroxyapatite multilayers with differences in composition across the layers [278]. In a study aimed at developing tissue engineered vascular grafts, elastin was incorporated into electrochemically aligned collagen fibers to lower the modulus (e.g. for compliance matching) and increase the yield strain [283]. A final example is a study in which collagen sheets were first formed by electrocompaction and then stretched to further align the collagen structure and tune the stiffness anisotropy (SA) between transverse and longitudinal axes. The resulting anisotropies in morphology and mechanical properties were reported to significantly affect the biological response to these collagen materials (e.g. mesenchymal stem cell (MSC) fate) [36].
One target application is for corneal implants [284], where electrochemically compacted collagen (ECC) provided dense transparent collagen matrices that could be subsequently crosslinked to improve mechanical properties and stability [279]. The inclusion of glycose-minoglycans into such electrobiofabricated collagen was also suggested to improve water retention abilities [278, 280]. A second target application is tendon replacement, where ELAC threads were reported to: mimic the alignment and strength of collagen-rich connective tissues; promote a tendon-specific differentiation of MSCs [36, 285, 286]; and allow hierarchical assembly into woven 3D biotextiles [118]. Additional applications reported for electrobiofabricated collagens include woven collagens for cartilage repair [287], matrices for skin autografts [282], conduits for nerve guidance [275] and mineralized collagen coatings for orthopedic implants [156].
In summary, electrobiofabrication methods are emerging for creating collagen-based materials that mimic the structure, mechanical properties and biological activities of native collagen. These methods are reported to offer numerous advantages: the electrical signals may enable a more precise means to orient individual collagen molecules; a large design space is available to control these structures and properties [133]; it may be possible to couple electrical signals with additional inputs to further control structure (e.g. mechanical or magnetic alignment [157]); no toxic solvents are used during collagen electrobiofabrication; and the methods are simple, economical and versatile, as electrodes of varying sizes and shapes can be used to enable diverse structures to be generated while numerous components can be incorporated with the collagen [135, 271].
3.4. Silk
Silk has emerged as an important protein-based material for biomedical applications. Initial interests in the silk fibrous proteins from the spider and the silk worm were focused on their unique mechanical properties, but increasingly silk proteins have been recognized as biocompatible and degradable materials [288, 289]. Recent studies have shown that controlling the protein’s structure (e.g. β-sheet content) can allow tailoring of the controlled-release properties [290] and the rate of biodegradation [291]. In addition, the protein nature of silk enables the use of biotechnology methods to further tailor functionality. For instance, enzymatic methods have been employed to enhance strength (via horseradish peroxidase catalyzed crosslinking) [292], and to confer biological function (by transglutaminase catalyzed protein conjugation) [293]. Further, there has been considerable effort to use recombinant technology to develop large-scale biomanufacturing processes for silk production [294–298].
In 2008, it was reported that when a cast silk fibroin solution was exposed to an externally applied alternating electric field the protein’s dipoles were aligned to create an oriented supramolecular assembly and anisotropic structure [299]. In 2010, silk’s electrochemical gelation was reported [158, 162] through a mechanism involving both the electrically mediated pH gradient and the electric field. These initial reports noted the speed and reproducibility for creating silk electrochemical gels (e-gels) and noted the capability to coat complex surfaces [162]. Interestingly, it was also noted that the regenerated e-gel silk did not have the high β-sheet content characteristics of natural silk [159].
Analogous to the case of collagen [135], the electrogelation of silk protein could be considered a type of isoelectric focusing with the co-localization of gelation and pH fronts [164], and the electric field inducing both the migration and alignment of the protein. Experimentally, the molecular-level anisotropy of the e-gel could be measured by polarized light microscopy, and the aligned structure was observed to confer mechanical anisotropy [300].
At the supramolecular and nanoscales, various mechanisms have been reported that appear to differ significantly in details. One mechanism indicated that silk fibroin electrogelation progressed through stages in which random coils organized into metastable nanoparticles, which subsequently aggregated [163]. Later studies emphasized that e-gelation converted an unstructured silk fibroin solution into gels with significant α-helix content, and that imposing shear forces could further modify the gel’s structure and properties [161]. In some cases, e-gels could be generated with significant β-sheet content [160], and the electrical inputs provide the capabilities to tune the β-sheet content, and once a locally aligned structure was generated it could be chemically crosslinked to preserve the structure [301]. In summary, electrogelation allows externally imposed electrical signals to accelerate the assembly and tune the hierarchical structure of silk to enable the coupling of top-down and bottom-up approaches for the programmed fabrication of high performance multifunctional materials [30].
As described above for the other three biopolymers, there have been recent efforts made to extend the capabilities of silk electrogelation. For instance, co-deposition has been used to load silk gels with gentamicin [95] and curcumin [302], and to generate composite films with silk, graphene and hydroxyapatite [303]. In addition biotechnological methods are being coupled to electrogelation. For instance, recombinant human tropoelastin has been enzymatically coupled to silk fibroin through dityrosine linkages [304]. More recently, protein engineering methods were reported to create a designer triblock protein for reversible electrogelation. This protein contained a central spider silk glue region flanked by two pH-triggered coiled-coil domains to enable pH-responsive self-assembly. This designer protein enabled an electrically triggered dynamic matrix for applications that included controlled drug delivery [122].
As with collagen, much of the research with silk e-gels has also been focused on translation. As mentioned, one proposed application is as a coating for bone [95] and dental [305] implants. Also, a recent report describes the electrogelation of silk nanofibers to generate stable β-sheet-rich hydrogels with oriented anisotropic structures. Stem cells seeded on these anisotropic hydrogels were reported to provide the physical cues to orient cell morphology and tissue outcomes [306].
In summary, research on the electrogelation of silk highlights the ability to enlist electrical signals to control the assembly of supramolecular structure, and also to apply advanced biotechnology methods to create dynamically responsive material systems.
4. Conclusions and future perspectives
Electrical input stimuli offer exciting capabilities for organizing soft matter into functional material systems. Electrical signals can be imposed with exquisite spatial, temporal and quantitative control, and can be imposed in parallel over arbitrarily large electrode areas (i.e. which suggests its scalability). Macromolecular building blocks can respond to such imposed electrical signals in subtle ways through field-induced motion, conformational changes and chain alignments, and in not-so-subtle ways by mediator-induced chemical reactions. And these macromolecular responses are sensitive to solution conditions. For instance, the inclusion of NaCl in the deposition solution can screen electrostatic fields and attenuate their effects, and NaCl can also result in the anodic generation of reactive chlorine species (e.g. HOCl) that can oxidatively ‘activate’ macromolecules for further covalent reactions (e.g. crosslinking or conjugation). When the macromolecular building blocks are derived from biology, there are additional possibilities. Specifically, biological polymers (proteins, nucleic acids and polysaccharides) often can: self-assemble to organize structures over a hierarchy of length scales; recognize various physical–chemical stimuli and respond through structural transitions (e.g. sol–gel transitions); and serve as substrates for enzymes that can either build or dissipate structure and function. Further, biology provides the materials and insights for building multi-scale supramolecular systems that are held together through a delicate balance of competing/reinforcing physical interactions that confer dynamic capabilities to respond, heal and reconfigure. Finally, the use of biologically derived polymers (e.g. proteins) provides access to the biotechnology toolbox (e.g. protein engineering) to provide powerful capabilities to control structure and responsive-ness. Thus, there appears to be a large electrobiofabrication design space available for imposing electrical inputs to guide the emergence of structure and to confer function.
A vision for electrobiofabrication is being driven by a convergence of contributions from across disciplines and labs. Several recent demonstrations have shown remarkable abilities to control structure by how/when/where electrical inputs are imposed, and electrochemistry is enabling the discovery of entirely new mechanisms for transducing electrical inputs into structural outputs. Advanced imaging is providing capabilities to observe, in real time, the emergence of structures in response to imposed electrical inputs. Molecular modeling is providing the insights to clarify assembly mechanisms and understand the stability of supramolecular assemblies. Biotechnology allows purposeful design to engineer proteins with fusion tags and domains capable of recognizing electrical inputs and promoting assembly. And increasingly electrobiofabrication is being performed with cellular systems to generate living material systems that incorporate contributions from cell and synthetic biology. Importantly, the comparatively simple and inexpensive methods of electrobiofabrication can be coupled with other assembly methods to extend the possibilities. For instance, electrodeposition has been coupled with polyelectrolyte layer-by-layer assembly, and also with 3D printing. Thus, recent studies are demonstrating that electrobiofabrication—alone or in combination with other methods—provides capabilities to meet the needs for emerging life science applications.
In the preceding paragraphs we highlighted the capabilities and recent successes of electrobiofabrication, yet it may be difficult to provide a broader comparison to more fully established biofabrication methods (e.g. based on photolithography and 3D printing). Historically, technology comparisons appeared simpler when assessing competing fabrication approaches for microelectronics because robust and generic metrics could be applied to assess progress on a roadmap that has served the industry for over a half century. One challenge is that the myriad of emerging life science applications may have more specialized requirements, while single-valued metrics of a material system (e.g. per cent cell viability) may not adequately capture important subtleties. We suggest that a measure of success (albeit qualitative) may be how closely a biofabrication approach can recapitulate structural and functional features of complex tissue systems. For instance, recapitulating important mechanical properties will require more than simply matching a compliance metric, but may require matching a more complex set of mechanobiological requirements that may also vary with space and time. And creating hydrogels with complex internal structure may be integral to controlling the speed and directionality of molecular-based information flow. We suggest that electrobiofabrication provides important capabilities along a cost-effective and sustainable path toward the bio-based manufacturing of high performance material systems for a broad range of applications.
Acknowledgments
This work was supported by the National Science Foundation (DMREF #1435957), the Defense Threat Reduction Agency (HDTRA1-13-0037) and the National Institute of Health (R21EB024102).
References
- [1].Groll J. et al. Biofabrication: reappraising the definition of an evolving field. Biofabrication. 2016;8:013001. doi: 10.1088/1758-5090/8/1/013001. [DOI] [PubMed] [Google Scholar]
- [2].McHugh KJ et al. 2017. Fabrication of fillable microparticles and other complex 3D microstructures Science 357 1138–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gordonov T, Kim E, Cheng Y, Ben-Yoav H, Ghodssi R, Rubloff G, Yin J-J, Payne GF and Bentley WE 2014. Electronic modulation of biochemical signal generation Nat. Nanotechnol 9 605–10 [DOI] [PubMed] [Google Scholar]
- [4].Wang H, Ma X and Hao Y 2017. Electronic devices for human-machine interfaces Adv. Mater. Interfaces 4 1–20 [Google Scholar]
- [5].Guo S-Z, Qiu K, Meng F, Park SH and McAlpine MC 2017. 3D printed stretchable tactile sensors Adv. Mater 29 1701218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Waldrop MM 2016. More than Moore Nature 530 144–7 [DOI] [PubMed] [Google Scholar]
- [7].Ariga K, Lvov YM, Kawakami K, Ji Q and Hill JP 2011. Layer-by-layer self-assembled shells for drug delivery Adv. Drug. Deliv. Rev 63 762–71 [DOI] [PubMed] [Google Scholar]
- [8].Hammond PT 2012. Building biomedical materials layer-by-layer Mater. Today 15 196–206 [Google Scholar]
- [9].Richardson JJ, Cui J, Björnmalm M, Braunger JA, Ejima H and Caruso F 2016. Innovation in layer-by-layer assembly Chem. Rev 116 14828–67 [DOI] [PubMed] [Google Scholar]
- [10].Tang Z, Wang Y, Podsiadlo P and Kotov NA 2006. Biomedical applications of layer-by-layer assembly: from biomimetics to tissue engineering Adv. Mater 18 3203–24 [Google Scholar]
- [11].Li J, Maniar D, Qu X, Liu H, Tsao C-Y, Kim E, Bentley WE, Liu C and Payne GF 2019. Coupling Self-Assembly Mechanisms to Fabricate Molecularly and Electrically Responsive Films Biomacromolecules 20 969–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Murphy SV and Atala A 2014. 3D bioprinting of tissues and organs Nat. Biotechnol 32 773–85 [DOI] [PubMed] [Google Scholar]
- [13].Zhu W, Ma X, Gou M, Mei D, Zhang K and Chen S 2016. 3D printing of functional biomaterials for tissue engineering Curr. Opin. Biotechnol 40 103–12 [DOI] [PubMed] [Google Scholar]
- [14].Pati F, Jang J, Ha D-H, Won Kim S, Rhie J-W, Shim J-H, Kim D-H and Cho D-W 2014. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink Nat. Commun 5 3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ozbolat IT and Hospodiuk M 2016. Current advances and future perspectives in extrusion-based bioprinting Biomaterials 76 321–43 [DOI] [PubMed] [Google Scholar]
- [16].Mandrycky C, Wang Z, Kim K and Kim D-H 2016. 3D bioprinting for engineering complex tissues Biotechnol. Adv 34 422–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Barry RA, Shepherd RF, Hanson JN, Nuzzo RG, Wiltzius P and Lewis JA 2009. Direct-write assembly of 3D hydrogel scaffolds for guided cell growth Adv. Mater 21 2407–10 [Google Scholar]
- [18].Biswas A, Malferrari S, Kalaskar DM and Das AK 2018. Arylboronate esters mediated self-healable and biocompatible dynamic G-quadruplex hydrogels as promising 3D-bioinks Chem. Commun 54 1778–81 [DOI] [PubMed] [Google Scholar]
- [19].Gungor-Ozkerim PS, Inci I, Zhang YS, Khademhosseini A and Dokmeci MR 2018. Bioinks for 3D bioprinting: an overview Biomater. Sci 6 915–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gao T, Gillispie GJ, Copus JS, Pr AK, Seol Y-J, Atala A, Yoo JJ and Lee SJ 2018. Optimization of gelatin–alginate composite bioink printability using rheological parameters: a systematic approach Biofabrication 10 034106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rutz AL, Hyland KE, Jakus AE, Burghardt WR and Shah RN 2015. A multimaterial bioink method for 3D printing tunable, cell-compatible hydrogels Adv. Mater 27 1607–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hinton TJ, Jallerat Q, Palchesko RN, Park JH, Grodzicki MS, Shue H-J, Ramadan MH, Hudson AR and Feinberg AW 2015. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels Sci. Adv 1 e1500758–1500758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bertassoni LE. et al. Direct-write bioprinting of cell-laden methacrylated gelatin hydrogels. Biofabrication. 2014;6:024105. doi: 10.1088/1758-5082/6/2/024105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Tabriz AG, Hermida MA, Leslie NR and Shu W 2015. Three-dimensional bioprinting of complex cell laden alginate hydrogel structures Biofabrication 7 045012. [DOI] [PubMed] [Google Scholar]
- [25].Wu S, Wang W, Yan K, Ding F, Shi X, Deng H and Du Y 2018. Electrochemical writing on edible polysaccharide films for intelligent food packaging Carbohydr. Polym 186 236–42 [DOI] [PubMed] [Google Scholar]
- [26].Pedde RD. et al. Emerging biofabrication strategies for engineering complex tissue constructs. Adv. Mater. 2017;29:1606061. doi: 10.1002/adma.201606061. [DOI] [PubMed] [Google Scholar]
- [27].Li Y, Xiao Y and Liu C 2017. The horizon of materiobiology: a perspective on material-guided cell behaviors and tissue engineering Chem. Rev 117 4376–421 [DOI] [PubMed] [Google Scholar]
- [28].Islam A, Chapin K, Younesi M and Akkus O 2015. Computer aided biomanufacturing of mechanically robust pure collagen meshes with controlled macroporosity Biofabrication 7 035005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Huang G, Li F, Zhao X, Ma Y, Li Y, Lin M, Jin G, Lu TJ, Genin GM and Xu F 2017. Functional and biomimetic materials for engineering of the three-dimensional cell microenvironment Chem. Rev 117 12764–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Marelli B, Patel N, Duggan T, Perotto G, Shirman E, Li C, Kaplan DL and Omenetto FG 2017. Programming function into mechanical forms by directed assembly of silk bulk materials Proc. Natl Acad. Sci 114 451–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bettinger CJ, Langer R and Borenstein JT 2009. Engineering substrate topography at the micro- and nanoscale to control cell function Angew. Chemie Int. Ed 48 5406–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zhao Z, Fang R, Rong Q and Liu M 2017. Bioinspired nanocomposite hydrogels with highly ordered structures Adv. Mater 29 1703045. [DOI] [PubMed] [Google Scholar]
- [33].Yang Y, Chen Z, Song X, Zhang Z, Zhang J, Shung KK, Zhou Q and Chen Y 2017. Biomimetic anisotropic reinforcement architectures by electrically assisted nanocomposite 3D printing Adv. Mater 29 1605750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Dahlin RL, Kasper FK and Mikos AG 2011. Polymeric nanofibers in tissue engineering Tissue Eng. Part B Rev 17 349–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Zhou X, Li T, Wang J, Chen F, Zhou D, Liu Q, Li B, Cheng J, Zhou X and Zheng B 2018. Mechanochemical regulated origami with tough hydrogels by ion transfer printing ACS Appl. Mater. Interfaces 10 9077–84 [DOI] [PubMed] [Google Scholar]
- [36].Islam A, Younesi M, Mbimba T and Akkus O 2016. Collagen substrate stiffness anisotropy affects cellular elongation, nuclear shape, and stem cell fate toward anisotropic tissue lineage Adv. Healthc. Mater 5 2237–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sano K, Ishida Y and Aida T 2018. Synthesis of anisotropic hydrogels and their applications Angew. Chemie Int. Ed 57 2532–43 [DOI] [PubMed] [Google Scholar]
- [38].Prince E, Alizadehgiashi M, Campbell M, Khuu N, Albulescu A, De France K, Ratkov D, Li Y, Hoare T and Kumacheva E 2018. Patterning of structurally anisotropic composite hydrogel sheets Biomacromolecules 19 1276–84 [DOI] [PubMed] [Google Scholar]
- [39].Zhao Q, Wang J, Cui H, Chen H, Wang Y and Du X 2018. Programmed shape-morphing scaffolds enabling facile 3D endothelialization Adv. Funct. Mater 28 1801027 [Google Scholar]
- [40].Fusco S et al. 2015. Shape-switching microrobots for medical applications: the influence of shape in drug delivery and locomotion ACS Appl. Mater. Interfaces 7 6803–11 [DOI] [PubMed] [Google Scholar]
- [41].Bellinger AM et al. 2016. Oral, ultra–long-lasting drug delivery: application toward malaria elimination goals Sci. Transl. Med 8 365ra157–65ra157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].He H, Cao X, Dong H, Ma T and Payne GF 2017. Reversible programing of soft matter with reconfigurable mechanical properties Adv. Funct. Mater 27 1605665 [Google Scholar]
- [43].Wang J, Li T, Chen F, Zhou D, Li B, Zhou X, Gan T, Handschuh-Wang S and Zhou X 2018. Softening and shape morphing of stiff tough hydrogels by localized unlocking of the trivalent ionically cross-linked centers Macromol. Rapid Commun 39 1800143. [DOI] [PubMed] [Google Scholar]
- [44].Goor OJGM, Hendrikse SIS, Dankers PYW and Meijer EW 2017. From supramolecular polymers to multi-component biomaterials Chem. Soc. Rev 46 6621–37 [DOI] [PubMed] [Google Scholar]
- [45].Calvo-Marzal P, Delaney MP, Auletta JT, Pan T, Perri NM, Weiland LM, Waldeck DH, Clark WW and Meyer TY 2012. Manipulating mechanical properties with electricity: Electroplastic elastomer hydrogels ACS Macro Lett 1 204–8 [DOI] [PubMed] [Google Scholar]
- [46].Tan YJ, Wu J, Li H and Tee BCK 2018. Self-healing electronic materials for a smart and sustainable future ACS Appl. Mater. Interfaces 10 15331–45 [DOI] [PubMed] [Google Scholar]
- [47].Whitesides GM 2018. Soft robotics Angew. Chemie—Int. Ed 57 4258–73 [DOI] [PubMed] [Google Scholar]
- [48].Hines L, Petersen K, Lum GZ and Sitti M 2017. Soft actuators for small-scale robotics Adv. Mater 29 1603483. [DOI] [PubMed] [Google Scholar]
- [49].Ouyang L, Burdick JA and Sun W 2018. Facile biofabrication of heterogeneous multilayer tubular hydrogels by fast diffusion-induced gelation ACS Appl. Mater. Interfaces 10 12424–30 [DOI] [PubMed] [Google Scholar]
- [50].Xiao H, Ma C, Le X, Wang L, Lu W, Theato P, Hu T, Zhang J and Chen T 2017. A multiple shape memory hydrogel induced by reversible physical interactions at ambient condition Polymers (Basel). 9 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Huynh T-P, Sonar P and Haick H 2017. Advanced materials for use in soft self-healing devices Adv. Mater 29 1604973. [DOI] [PubMed] [Google Scholar]
- [52].Li Z, Lu W, Ngai T, Le X, Zheng J, Zhao N, Huang Y, Wen X, Zhang J and Chen T 2016. Mussel-inspired multifunctional supramolecular hydrogels with self-healing, shape memory and adhesive properties Polym. Chem 7 5343–6 [Google Scholar]
- [53].Wang W, Zhang Y and Liu W 2017. Bioinspired fabrication of high strength hydrogels from non-covalent interactions Prog. Polym. Sci 71 1–25 [Google Scholar]
- [54].Ensign LM, Cone R and Hanes J 2012. Oral drug delivery with polymeric nanoparticles: The gastrointestinal mucus barriers Adv. Drug. Deliv. Rev 64 557–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Tokarev I and Minko S 2010. Stimuli-responsive porous hydrogels at interfaces for molecular filtration, separation, controlled release, and gating in capsules and membranes Adv. Mater 22 3446–62 [DOI] [PubMed] [Google Scholar]
- [56].Huang R, Li W, Lv X, Lei Z, Bian Y, Deng H, Wang H, Li J and Li X 2015. Biomimetic LBL structured nanofibrous matrices assembled by chitosan/collagen for promoting wound healing Biomaterials 53 58–75 [DOI] [PubMed] [Google Scholar]
- [57].Dagdeviren C. et al. Miniaturized neural system for chronic, local intracerebral drug delivery. Sci. Transl. Med. 2018;10:eaan2742. doi: 10.1126/scitranslmed.aan2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Liu J. et al. Triggerable tough hydrogels for gastric resident dosage forms. Nat. Commun. 2017;8:124. doi: 10.1038/s41467-017-00144-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Mitragotri S, Blankschtein D and Langer R 1995. Ultrasound-mediated transdermal protein delivery Science (80-. ). 269 850–3 [DOI] [PubMed] [Google Scholar]
- [60].Prausnitz MR and Langer R 2008. Transdermal drug delivery Nat. Biotechnol 26 1261–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Schoellhammer CM et al. 2017. Ultrasound-mediated delivery of RNA to colonic mucosa of live mice Gastroenterology 152 1151–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Schoellhammer CM et al. 2015. Ultrasound-mediated gastrointestinal drug delivery Sci. Transl. Med 7 310ra168–10ra168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Li J, Qu X, Payne GF, Zhang C, Zhang Y, Li J, Ren J, Hong H and Liu C 2015. Biospecific self-assembly of a nanoparticle coating for targeted and stimuli-responsive drug delivery Adv. Funct. Mater 25 1404–17 [Google Scholar]
- [64].Zhang C, Qu X, Li J, Hong H, Li J, Ren J, Payne GF and Liu C 2015. Biofabricated nanoparticle coating for liver-cell targeting Adv. Healthc. Mater 4 1972–81 [DOI] [PubMed] [Google Scholar]
- [65].Hermanová S and Pumera M 2018. Polymer platforms for micro- and nanomotor fabrication Nanoscale 10 7332–42 [DOI] [PubMed] [Google Scholar]
- [66].Fusco S, Chatzipirpiridis G, Sivaraman KM, Ergeneman O, Nelson BJ and Pané S 2013. Chitosan electrodeposition for microrobotic drug delivery Adv. Healthc. Mater 2 1037–44 [DOI] [PubMed] [Google Scholar]
- [67].Fenton OS, Olafson KN, Pillai PS, Mitchell MJ and Langer R 2018. Advances in biomaterials for drug delivery Adv. Mater 30 1705328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Traverso G, Kirtane AR, Schoellhammer CM and Langer R 2018. Convergence for translation: drug-delivery research in multidisciplinary teams Angew. Chemie—Int. Ed 57 4156–63 [DOI] [PubMed] [Google Scholar]
- [69].Schwartz MA, Schaller MD and Ginsberg MH 1995. Integrins: emerging paradigms of signal transduction Annu. Rev. Cell Dev. Biol 11 549–99 [DOI] [PubMed] [Google Scholar]
- [70].Liu Y, Kim E, Ghodssi R, Rubloff GW, Culver JN, Bentley WE and Payne GF 2010. Biofabrication to build the biology–device interface Biofabrication 2 022002. [DOI] [PubMed] [Google Scholar]
- [71].Li J, Wu S, Kim E, Yan K, Liu H, Liu C, Dong H, Qu X, Shi X, Shen J, Bentley WE and Payne GF 2019. Electrobiofabrication: electrically-based fabrication with biologically-derived materials Biofabrication ( 10.1088/1758-5090/ab06ea) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Frontana-Uribe BA, Little RD, Ibanez JG, Palma A and Vasquez-Medrano R 2010. Organic electrosynthesis: a promising green methodology in organic chemistry Green Chem. 12 2099 [Google Scholar]
- [73].Francke R and Little RD 2014. Redox catalysis in organic electrosynthesis: Basic principles and recent developments Chem. Soc. Rev 43 2492–521 [DOI] [PubMed] [Google Scholar]
- [74].Sperry JB and Wright DL 2006. The application of cathodic reductions and anodic oxidations in the synthesis of complex molecules Chem. Soc. Rev 35 605. [DOI] [PubMed] [Google Scholar]
- [75].Zhitomirsky I 2002. Cathodic electrodeposition of ceramic and organoceramic materials. Fundamental aspects Adv. Colloid Interface Sci 97 279–317 [DOI] [PubMed] [Google Scholar]
- [76].Boccaccini AR, Keim S, Ma R, Li Y and Zhitomirsky I 2010. Electrophoretic deposition of biomaterials J. R. Soc. Interface 7 S581–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Besra L and Liu M 2007. A review on fundamentals and applications of electrophoretic deposition (EPD) Prog. Mater. Sci 52 1–61 [Google Scholar]
- [78].Maerten C, Jierry L, Schaaf P and Boulmedais F 2017. Review of electrochemically triggered macromolecular film buildup processes and their biomedical applications ACS Appl. Mater. Interfaces 9 28117–38 [DOI] [PubMed] [Google Scholar]
- [79].Seuss S and Boccaccini AR 2013. Electrophoretic deposition of biological macromolecules, drugs, and cells Biomacromolecules 14 3355–69 [DOI] [PubMed] [Google Scholar]
- [80].Corni I, Ryan MP and Boccaccini AR 2008. Electrophoretic deposition: From traditional ceramics to nanotechnology J. Eur. Ceram. Soc 28 1353–67 [Google Scholar]
- [81].Van Tassel PR 2012. Polyelectrolyte adsorption and layer-by-layer assembly: Electrochemical control Curr. Opin. Colloid Interface Sci 17 106–13 [Google Scholar]
- [82].Li C, Bai H and Shi G 2009. Conducting polymer nanomaterials: electrosynthesis and applications Chem. Soc. Rev 38 2397. [DOI] [PubMed] [Google Scholar]
- [83].Sakmeche N, Aeiyach S, Aaron J-J, Jouini M, Lacroix JC and Lacaze P-C 1999. Improvement of the electrosynthesis and physicochemical properties of poly(3,4-ethylenedioxythiophene) using a sodium dodecyl sulfate micellar aqueous medium Langmuir 15 2566–74 [Google Scholar]
- [84].Charan Reddy KR, Turcu F, Schulte A, Kayastha AM and Schuhmann W 2005. Fabrication of a potentiometric/amperometric bifunctional enzyme microbiosensor Anal. Chem 77 5063–7 [DOI] [PubMed] [Google Scholar]
- [85].Isik S, Oni J, Rjabova V, Neugebauer S and Schuhmann W 2004. Entrapment of metalloporphyrins within an electrodeposition paint layer as a basis for developing a nitric oxide sensor Microchim. Acta 148 59–64 [Google Scholar]
- [86].Kurzaw C, Hengstenberg A, Schuhmann W and Chemie A 2002. Immobilization method for the preparation of biosensors based on ph shift-induced deposition of biomolecule-containing polymer films Anal. Chem 74 1383–9 [DOI] [PubMed] [Google Scholar]
- [87].Smutok O, Ngounou B, Pavlishko H, Gayda G, Gonchar M and Schuhmann W 2006. A reagentless bienzyme amperometric biosensor based on alcohol oxidase/peroxidase and an Os-complex modified electrodeposition paint Sensors Actuators B Chem. 113 590–8 [Google Scholar]
- [88].Vilkanauskyte A, Erichsen T, Marcinkeviciene L, Laurinavicius V and Schuhmann W 2002. Reagentless biosensors based on co-entrapment of a soluble redox polymer and an enzyme within an electrochemically deposited polymer film Biosens. Bioelectron 17 1025–31 [DOI] [PubMed] [Google Scholar]
- [89].Ngounou B, Aliyev EH, Guschin DA, Sultanov YM, Efendiev AA and Schuhmann W 2007. Parallel synthesis of libraries of anodic and cathodic functionalized electrodeposition paints as immobilization matrix for amperometric biosensors Bioelectrochemistry 71 81–90 [DOI] [PubMed] [Google Scholar]
- [90].Ohyanagi M and Anson FC 1989. Electrodeposition of polyelectrolyte-metal complexes J. Electroanal. Chem. Interfacial Electrochem 258 469–77 [Google Scholar]
- [91].Ohyanagi M and Anson FC 1989. Electrochemical behavior of electroactive counterions in solutions of polyelectrolytes J. Phys. Chem 93 8377–82 [Google Scholar]
- [92].Brown TD, Dalton PD and Hutmacher DW 2011. Direct writing by way of melt electrospinning Adv. Mater 23 5651–7 [DOI] [PubMed] [Google Scholar]
- [93].Hochleitner G, Fürsattel E, Giesa R, Groll J, Schmidt H-W and Dalton PD 2018. Melt electrowriting of thermoplastic elastomers Macromol. Rapid Commun. 39 1800055. [DOI] [PubMed] [Google Scholar]
- [94].Castilho M, van Mil A, Maher M, Metz CHG, Hochleitner G, Groll J, Doevendans PA, Ito K, Sluijter JPG and Malda J 2018. Melt electrowriting allows tailored microstructural and mechanical design of scaffolds to advance functional human myocardial tissue formation Adv. Funct. Mater 28 1803151 [Google Scholar]
- [95].Han C, Yao Y, Cheng X, Luo J, Luo P, Wang Q, Yang F, Wei Q and Zhang Z 2017. Electrophoretic deposition of gentamicin-loaded silk fibroin coatings on 3D-printed porous cobalt-chromium-molybdenum bone substitutes to prevent orthopedic implant infections Biomacromolecules 18 3776–87 [DOI] [PubMed] [Google Scholar]
- [96].Zhang Z, Cheng X, Yao Y, Luo J, Tang Q, Wu H, Lin S, Han C, Wei Q and Chen L 2016. Electrophoretic deposition of chitosan/gelatin coatings with controlled porous surface topography to enhance initial osteoblast adhesive responses J. Mater. Chem. B 4 7584–95 [DOI] [PubMed] [Google Scholar]
- [97].Bakhshandeh S and Amin Yavari S 2018. Electrophoretic deposition: a versatile tool against biomaterial associated infections J. Mater. Chem. B 6 1128–48 [DOI] [PubMed] [Google Scholar]
- [98].Suginta W, Khunkaewla P and Schulte A 2013. Electrochemical biosensor applications of polysaccharides chitin and chitosan Chem. Rev 113 5458–79 [DOI] [PubMed] [Google Scholar]
- [99].Park JJ, Luo X, Yi H, Valentine TM, Payne GF, Bentley WE, Ghodssi R and Rubloff GW 2006. Chitosan-mediated in situ biomolecule assembly in completely packaged microfluidic devices Lab Chip 6 1315–21 [DOI] [PubMed] [Google Scholar]
- [100].Shang W, Tsao C-Y, Luo X, Teodoro M, McKay R, Quan DN, Wu H-C, Payne GF and Bentley WE 2017. A simple and reusable bilayer membrane-based microfluidic device for the study of gradient-mediated bacterial behaviors Biomicrofluidics 11 044114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Derda R, Laromaine A, Mammoto A, Tang SKY, Mammoto T, Ingber DE and Whitesides GM 2009. Paper-supported 3D cell culture for tissue-based bioassays Proc. Natl Acad. Sci 106 18457–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Cheng Y, Gray KM, David L, Royaud I, Payne GF and Rubloff GW 2012. Characterization of the cathodic electrodeposition of semicrystalline chitosan hydrogel Mater. Lett 87 97–100 [Google Scholar]
- [103].Liu F, Ma C, Gao Y and McClements DJ 2017. Food-grade covalent complexes and their application as nutraceutical delivery systems: a review Compr. Rev. Food Sci. Food Saf 16 76–95 [DOI] [PubMed] [Google Scholar]
- [104].Tseng P, Napier B, Zhao S,Mitropoulos AN, Applegate MB, Marelli B, Kaplan DL and Omenetto FG 2017. Directed assembly of bio-inspired hierarchical materials withcontrolled nanofibrillar architectures Nat. Nanotechnol 12 474–80 [DOI] [PubMed] [Google Scholar]
- [105].Ling S, Kaplan DL and Buehler MJ 2018. Nanofibrils in nature and materials engineering Nat. Rev. Mater 3 18016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Sun J-YY, Zhao X, Illeperuma WRKK, Chaudhuri O, Oh KH, Mooney DJ, Vlassak JJ and Suo Z 2012. Highly stretchable and tough hydrogels Nature 489 133–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Yang CH, Wang MX, Haider H, Yang JH, Sun J-Y, Chen YM, Zhou J and Suo Z 2013. Strengthening alginate/polyacrylamide hydrogels using various multivalent cations ACS Appl. Mater. Interfaces 5 10418–22 [DOI] [PubMed] [Google Scholar]
- [108].Li T, Wang J, Zhang L, Yang J, Yang M, Zhu D, Zhou X, Handschuh-Wang S, Liu Y and Zhou X 2017. ‘Freezing’, morphing, and folding of stretchy tough hydrogels J. Mater. Chem. B 5 5726–32 [DOI] [PubMed] [Google Scholar]
- [109].Cao J, Li J, Chen Y, Zhang L and Zhou J 2018. Dual physical crosslinking strategy to construct moldable hydrogels with ultrahigh strength and toughness Adv. Funct. Mater 28 1800739 [Google Scholar]
- [110].Zhao D, Huang J, Zhong Y, Li K, Zhang L and Cai J 2016. High-strength and high-toughness double-cross-linked cellulose hydrogels: a new strategy using sequential chemical and physical cross-linking Adv. Funct. Mater 26 6279–87 [Google Scholar]
- [111].Yang Y, Wang X, Yang F, Shen H and Wu D 2016. A universal soaking strategy to convert composite hydrogels into extremely tough and rapidly recoverable double-network hydrogels Adv. Mater 28 7178–84 [DOI] [PubMed] [Google Scholar]
- [112].Yang Y, Wang X, Yang F, Wang L and Wu D 2018. Highly elastic and ultratough hybrid ionic-covalent hydrogels with tunable structures and mechanics Adv. Mater 30 1707071. [DOI] [PubMed] [Google Scholar]
- [113].Yu HC, Zhang H, Ren K, Ying Z, Zhu F, Qian J, Ji J, Wu ZL and Zheng Q 2018. Ultrathin κ-carrageenan/chitosan hydrogel films with high toughness and antiadhesion property ACS Appl. Mater. Interfaces 10 9002–9 [DOI] [PubMed] [Google Scholar]
- [114].Zhao X 2014. Multi-scale multi-mechanism design of tough hydrogels: building dissipation into stretchy networks Soft Matter 10 672–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Costa RR, Costa AMS, Caridade SG and Mano JF 2015. Compact saloplastic membranes of natural polysaccharides for soft tissue engineering Chem. Mater 27 7490–502 [Google Scholar]
- [116].Rodrigues MN, Oliveira MB, Costa RR and Mano JF 2016. Chitosan/chondroitin sulfate membranes produced by polyelectrolyte complexation for cartilage engineering Biomacromolecules 17 2178–88 [DOI] [PubMed] [Google Scholar]
- [117].Martin EJ, Sadman K and Shull KR 2016. Anodic electrodeposition of a cationic polyelectrolyte in the presence of multivalent anions Langmuir 32 7747–56 [DOI] [PubMed] [Google Scholar]
- [118].Younesi M, Islam A, Kishore V, Anderson JM and Akkus O 2014. Tenogenic induction of human MSCs by anisotropically aligned collagen biotextiles Adv. Funct. Mater 24 5762–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Picart C, Schneider A, Etienne O, Mutterer J, Schaaf P, Egles C, Jessel N and Voegel JC 2005. Controlled degradability of polysaccharide multilayer films in vitro and in vivo Adv. Funct. Mater 15 1771–80 [Google Scholar]
- [120].Wu Z, Su X, Xu Y, Kong B, Sun W and Mi S 2016. Bioprinting three-dimensional cell-laden tissue constructs with controllable degradation Sci. Rep 6 24474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Das S, Pati F, Choi Y-J, Rijal G, Shim J-H, Kim SW, Ray AR, Cho D-W and Ghosh S 2015. Bioprintable, cell-laden silk fibroin–gelatin hydrogel supporting multilineage differentiation of stem cells for fabrication of three-dimensional tissue constructs Acta Biomate. 11 233–46 [DOI] [PubMed] [Google Scholar]
- [122].Lin Y, An B, Bagheri M, Wang Q, Harden JL and Kaplan DL 2017. Electrochemically directed assembly of designer coiled-coil telechelic proteins ACS Biomater. Sci. Eng 3 3195–206 [DOI] [PubMed] [Google Scholar]
- [123].Liu Y, Wu H-CC, Bhokisham NN, Li J, Hong K-LL, Quan DN, Tsao CY, Bentley WE and Payne GF 2018. Biofabricating functional soft matter using protein engineering to enable enzymatic assembly Bioconjug. Chem 29 1809–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Partlow BP, Applegate MB, Omenetto FG and Kaplan DL 2016. Dityrosine cross-linking in designing biomaterials ACS Biomater. Sci. Eng 2 2108–21 [DOI] [PubMed] [Google Scholar]
- [125].Bhokisham N, Liu Y, Pakhchanian H, Payne GF and Bentley WE 2017. A facile two-step enzymatic approach for conjugating proteins to polysaccharide chitosan at an electrode interface Cell. Mol. Bioeng 10 134–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Bhokisham N, Pakhchanian H, Quan D, Tschirhart T, Tsao CY, Payne GF and Bentley WE 2016. Modular construction of multi-subunit protein complexes using engineered tags and microbial transglutaminase Metab. Eng 38 1–9 [DOI] [PubMed] [Google Scholar]
- [127].Kamiya N, Doi S, Tominaga J, Ichinose H and Goto M 2005. Transglutaminase-mediated protein immobilization to casein nanolayers created on a plastic surface Biomacromolecules 6 35–8 [DOI] [PubMed] [Google Scholar]
- [128].Tanaka T, Kamiya N and Nagamune T 2004. Peptidyl linkers for protein heterodimerization catalyzed by microbial transglutaminase Bioconjug. Chem 15 491–7 [DOI] [PubMed] [Google Scholar]
- [129].Kamiya N, Tanaka T, Suzuki T, Takazawa T, Takeda S, Watanabe K and Nagamune T 2003. S-peptide as a potent peptidyl linker for protein cross-linking by microbial transglutaminase from Streptomyces mobaraensis Bioconjug. Chem 14 351–7 [DOI] [PubMed] [Google Scholar]
- [130].Gitonga S, Oey I and Ali MA 2018. Feasibility of using pulsed electric fields to modify biomacromolecules : a review Trends in Food Science & Technology 72 91–113 [Google Scholar]
- [131].Hernández-Burgos K, Barton ZJ and Rodríguez-López J 2017. Finding harmony between ions and electrons: new tools and concepts for emerging energy storage materials Chem. Mater 29 8918–31 [Google Scholar]
- [132].Yang C and Suo Z 2018. Hydrogel ionotronics Nat. Rev. Mater 3 125–42 [Google Scholar]
- [133].Uquillas JA and Akkus O 2012. Modeling the electromobility of type-i collagen molecules in the electrochemical fabrication of dense and aligned tissue constructs Ann. Biomed. Eng 40 1641–53 [DOI] [PubMed] [Google Scholar]
- [134].Yan K et al. 2018. Electrical programming of soft matter: using temporally varying electrical inputs to spatially control self assembly Biomacromolecules 19 364–73 [DOI] [PubMed] [Google Scholar]
- [135].Cheng X, Gurkan UA, Dehen CJ, Tate MP, Hillhouse HW, Simpson GJ and Akkus O 2008. An electrochemical fabrication process for the assembly of anisotropically oriented collagen bundles Biomaterials 29 3278–88 [DOI] [PubMed] [Google Scholar]
- [136].Rydzek G, Ji Q, Li M, Schaaf P, Hill JP, Boulmedais F and Ariga K 2015. Electrochemical nanoarchitectonics and layer-by-layer assembly: From basics to future Nano Today 10 138–67 [Google Scholar]
- [137].Schneider S, Janssen C, Klindtworth E, Mergel O, Möller M and Plamper F 2018. Influence of polycation composition on electrochemical film formation Polymers (Basel). 10 429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Sadman K, Wang Q, Chen SH, Delgado DE and Shull KR 2017. pH-controlled electrochemical deposition of polyelectrolyte complex films Langmuir 33 1834–44 [DOI] [PubMed] [Google Scholar]
- [139].Rydzek G et al. 2010. Polymer multilayer films obtained by electrochemically catalyzed click chemistry Langmuir 26 2816–24 [DOI] [PubMed] [Google Scholar]
- [140].Wu LQ, Gadre AP, Yi H, Kastantin MJ, Rubloff GW, Bentley WE, Payne GF and Ghodssi R 2002. Voltage-dependent assembly of the polysaccharide chitosan onto an electrode surface Langmuir 18 8620–5 [Google Scholar]
- [141].Redepenning J, Venkataraman G, Chen J and Stafford N 2003. Electrochemical preparation of chitosan/hydroxyapatite composite coatings on titanium substrates J. Biomed. Mater. Res 66A 411–6 [DOI] [PubMed] [Google Scholar]
- [142].Cheong M and Zhitomirsky I 2008. Electrodeposition of alginic acid and composite films Colloids Surfaces A Physicochem . Eng. Asp 328 73–8 [Google Scholar]
- [143].Shi XW, Tsao CY, Yang X, Liu Y, Dykstra P, Rubloff GW, Ghodssi R, Bentley WE and Payne GF 2009. Electroaddressing of cell populations by co-deposition with calcium alginate hydrogels Adv. Funct. Mater 19 2074–80 [Google Scholar]
- [144].Wu L-Q, Ghodssi R, Elabd YA and Payne GF 2005. Biomimetic pattern transfer Adv. Funct. Mater 15 189–95 [Google Scholar]
- [145].Maerten C, Garnier T, Lupattelli P, Chau NTT, Schaaf P, Jierry L and Boulmedais F 2015. Morphogen electrochemically triggered self-construction of polymeric films based on mussel-inspired chemistry Langmuir 31 13385–93 [DOI] [PubMed] [Google Scholar]
- [146].Zhang Y and Ji C 2010. Electro-induced covalent cross-linking of chitosan and formation of chitosan hydrogel films: its application as an enzyme immobilization matrix for use in a phenol sensor Anal. Chem 82 5275–81 [DOI] [PubMed] [Google Scholar]
- [147].Geng Z, Wang X, Guo X, Zhang Z, Chen Y and Wang Y 2016. Electrodeposition of chitosan based on coordination with metal ions in situ-generated by electrochemical oxidation J. Mater. Chem. B 4 3331–8 [DOI] [PubMed] [Google Scholar]
- [148].Jin Z, Güven G, Bocharova V, Halámek J, Tokarev I, Minko S, Melman A, Mandler D and Katz E 2012. Electrochemically controlled drug-mimicking protein release from iron-alginate thin-films associated with an electrode ACS Appl. Mater. Interfaces 4 466–75 [DOI] [PubMed] [Google Scholar]
- [149].Pang X and Zhitomirsky I 2005. Electrodeposition of composite hydroxyapatite–chitosan films Mater. Chem. Phys 94 245–51 [Google Scholar]
- [150].Pang X and Zhitomirsky I 2008. Electrodeposition of hydroxyapatite–silver–chitosan nanocomposite coatings Surf. Coatings Technol 202 3815–21 [Google Scholar]
- [151].Pang X and Zhitomirsky I 2007. Electrophoretic deposition of composite hydroxyapatite-chitosan coatings Mater. Charact. 58 339–48 [Google Scholar]
- [152].Li Y et al. 2015. Self-assembly with orthogonal-imposed stimuli to impart structure and confer magnetic function to electrodeposited hydrogels ACS Appl. Mater. Interfaces 7 10587–98 [DOI] [PubMed] [Google Scholar]
- [153].Cli A, Pang X and Zhitomirsky I 2018. Biomimetically modified chitosan for electrophoretic deposition of composites Colloids Surf. A 544 28–34 [Google Scholar]
- [154].Jiang T, Zhang Z, Zhou Y, Liu Y, Wang Z, Tong H, Shen X and Wang Y 2010. Surface functionalization of titanium with chitosan/gelatin via electrophoretic deposition: characterization and cell behavior Biomacromolecules 11 1254–60 [DOI] [PubMed] [Google Scholar]
- [155].Wang F, Huang P, Huang D, Hu Y, Ma K, Cai X and Jiang T 2018. Preparation and functionalization of acetylsalicylic acid loaded chitosan/gelatin membranes from ethanol-based suspensions via electrophoretic deposition J. Mater. Chem. B 6 2304–14 [DOI] [PubMed] [Google Scholar]
- [156].Ling T, Lin J, Tu J, Liu S, Weng W, Cheng K, Wang H, Du P and Han G 2013. Mineralized collagen coatings formed by electrochemical deposition J. Mater. Sci., Mater. Med 24 2709–18 [DOI] [PubMed] [Google Scholar]
- [157].Zhuang J, Lin S, Dong L, Cheng K and Weng W 2018. Magnetically assisted electrodeposition of aligned collagen coatings ACS Biomater. Sci. Eng 4 1528–35 [DOI] [PubMed] [Google Scholar]
- [158].Leisk GG, Lo TJ, Yucel T, Lu Q and Kaplan DL 2010. Electrogelation for protein adhesives Adv. Mater 22 711–5 [DOI] [PubMed] [Google Scholar]
- [159].Yucel T, Kojic N, Leisk GG, Lo TJ and Kaplan DL 2010. Nonequilibrium silk fibroin adhesives J. Struct. Biol 170 406–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Bressner JE, Marelli B, Qin G, Klinker LE, Zhang Y, Kaplan DL and Omenetto FG 2014. Rapid fabrication of silk films with controlled architectures via electrogelation J. Mater. Chem. B 2 4983. [DOI] [PubMed] [Google Scholar]
- [161].Tabatabai AP, Kaplan DL and Blair DL 2015. Rheology of reconstituted silk fibroin protein gels: the epitome of extreme mechanics Soft Matter 11 756–61 [DOI] [PubMed] [Google Scholar]
- [162].Maniglio D, Bonani W, Bortoluzzi G, Servoli E, Motta A and Migliaresi C 2010. Electrodeposition of silk fibroin on metal substrates J. Bioact. Compat. Polym 25 441–54 [Google Scholar]
- [163].Lu Q, Huang Y, Li M, Zuo B, Lu S, Wang J, Zhu H and Kaplan DL 2011. Silk fibroin electrogelation mechanisms Acta Biomate. 7 2394–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Kojic N, Panzer MJ, Leisk GG, Raja WK, Kojic M and Kaplan DL 2012. Ion electrodiffusion governs silk electrogelation Soft Matter 8 6897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Ma R, Epand RF and Zhitomirsky I 2010. Electrodeposition of hyaluronic acid and hyaluronic acid-bovine serum albumin films from aqueous solutions Colloids Surfaces B Biointerfaces 77 279–85 [DOI] [PubMed] [Google Scholar]
- [166].Li M, Liu Q, Jia Z, Xu X, Shi Y, Cheng Y, Zheng Y, Xi T and Wei S 2013. Electrophoretic deposition and electrochemical behavior of novel graphene oxide-hyaluronic acid-hydroxyapatite nanocomposite coatings Appl. Surf. Sci 284 804–10 [Google Scholar]
- [167].Sun F and Zhitomirsky I 2009. Electrodeposition of hyaluronic acid and composite films Surf. Eng. 25 621–7 [Google Scholar]
- [168].Betz JF, Cheng Y, Tsao C-Y, Zargar A, Wu H-C, Luo X, Payne GF, Bentley WE and Rubloff GW 2013. Optically clear alginate hydrogels for spatially controlled cell entrapment and culture at microfluidic electrode surfaces Lab Chip 13 1854–8 [DOI] [PubMed] [Google Scholar]
- [169].Terrell JL et al. 2012. Integrated biofabrication for electro-addressed in-film bioprocessing Biotechnol. J 7 428–39 [DOI] [PubMed] [Google Scholar]
- [170].Cheng Y, Tsao CY, Wu HC, Luo X, Terrell JL, Betz J, Payne GF, Bentley WE and Rubloff GW 2012. Electroaddressing functionalized polysaccharides as model biofilms for interrogating cell signaling Adv. Funct. Mater 22 519–28 [Google Scholar]
- [171].Cheng Y, Luo X, Betz J, Payne GF, Bentley WE and Rubloff GW 2011. Mechanism of anodic electrodeposition of calcium alginate Soft Matter 7 5677 [Google Scholar]
- [172].Shang W, Liu Y, Wan W, Hu C, Liu Z, Wong CT, Fukuda T and Shen Y 2017. Hybrid 3D printing and electrodeposition approach for controllable 3D alginate hydrogel formation Biofabrication 9 025032. [DOI] [PubMed] [Google Scholar]
- [173].Yang X, Kim E, Liu Y, Shi XW, Rubloff GW, Ghodssi R, Bentley WE, Pancer Z and Payne GF 2010. In-film bioprocessing and immunoanalysis with electroaddressable stimuli-responsive polysaccharides Adv. Funct. Mater 20 1645–52 [Google Scholar]
- [174].Wang L, Jiang Y, Lin Y, Pang J and Liu XY 2016. Rheological properties and formation mechanism of DC electric fields induced konjac glucomannan-tungsten gels Carbohydr. Polym 142 293–9 [DOI] [PubMed] [Google Scholar]
- [175].Wang L, Zhuang Y, Li J, Pang J and Liu X 2016. The textural properties and microstructure of konjac glucomannan—tungsten gels induced by DC electric fields Food Chem. 212 256–63 [DOI] [PubMed] [Google Scholar]
- [176].Liba BD, Kim E, Martin AN, Liu Y, Bentley WE and Payne GF 2013. Biofabricated film with enzymatic and redox-capacitor functionalities to harvest and store electrons Biofabrication 5 015008. [DOI] [PubMed] [Google Scholar]
- [177].Gray KM, Liba BD, Wang Y, Cheng Y, Rubloff GW, Bentley WE, Montembault A, Royaud I, David L and Payne GF 2012. Electrodeposition of a biopolymeric hydrogel: potential for one-step protein electroaddressing Biomacromolecules 13 1181–9 [DOI] [PubMed] [Google Scholar]
- [178].Wu L-Q, McDermott MK, Zhu C, Ghodssi R and Payne GF 2006. Mimicking biological phenol reaction cascades to confer mechanical function Adv. Funct. Mater 16 1967–74 [Google Scholar]
- [179].Maerten C, Lopez L, Lupattelli P, Rydzek G, Pronkin S, Schaaf P, Jierry L and Boulmedais F 2017. Electrotriggered confined self-assembly of metal–polyphenol nanocoatings using a morphogenic approach Chem. Mater. 29 9668–79 [Google Scholar]
- [180].He S, Ren B, Liu X and Tong Z 2010. Reversible electrogelation in poly(acrylic acid) aqueous solutions triggered by redox reactions of counterions Macromol. Chem. Phys 211 2497–502 [Google Scholar]
- [181].El-Maiss J, Cuccarese M, Maerten C, Lupattelli P, Chiummiento L, Funicello M, Schaaf P, Jierry L and Boulmedais F 2018. Mussel-inspired electro-cross-linking of enzymes for the development of biosensors ACS Appl. Mater. Interfaces 10 18574–84 [DOI] [PubMed] [Google Scholar]
- [182].Mergel O, Kühn PT, Schneider S, Simon U and Plamper FA 2017. Influence of polymer architecture on the electrochemical deposition of polyelectrolytes Electrochim. Acta 232 98–105 [Google Scholar]
- [183].Mergel O, Wünnemann P, Simon U, Böker A and Plamper FA 2015. Microgel size modulation by electrochemical switching Chem. Mater. 27 7306–12 [Google Scholar]
- [184].Wang Y, Zhang Z, Wang M, Guo C, Liu H, Zeng H, Duan X, Zhou Y and Tang Z 2018. Direct electrodeposition of carboxymethyl cellulose based on coordination deposition method Cellulose 25 105–15 [Google Scholar]
- [185].Vigier-Carrière C, Boulmedais F, Schaaf P and Jierry L 2018. Surface-assisted self-assembly strategies leading to supramolecular hydrogels Angew. Chemie—Int. Ed 57 1448–56 [DOI] [PubMed] [Google Scholar]
- [186].Kubiak PS, Awhida S, Hotchen C, Deng W, Alston B, McDonald TO, Adams DJ and Cameron PJ 2015. Polymerization of low molecular weight hydrogelators to form electrochromic polymers Chem. Commun. 51 10427–30 [DOI] [PubMed] [Google Scholar]
- [187].Raeburn J, Alston B, Kroeger J, McDonald TO, Howse JR, Cameron PJ and Adams DJ 2014. Electrochemically-triggered spatially and temporally resolved multi-component gels Mater. Horiz 1 241–6 [Google Scholar]
- [188].Johnson EK, Adams DJ and Cameron PJ 2010. Directed self-assembly of dipeptides to form ultrathin hydrogel membranes J. Am. Chem. Soc 132 5130–6 [DOI] [PubMed] [Google Scholar]
- [189].Liu Y, Cheng Y, Wu HC, Kim E, Ulijn RV, Rubloff GW, Bentley WE and Payne GF 2011. Electroaddressing agarose using fmoc-phenylalanine as a temporary scaffold Langmuir 27 7380–4 [DOI] [PubMed] [Google Scholar]
- [190].Liu Y et al. 2012. Biofabricating multifunctional soft matter with enzymes and stimuli-responsive materials Adv. Funct. Mater 22 3004–12 [Google Scholar]
- [191].Liu Y, Kim E, Ulijn RV, Bentley WE and Payne GF 2011. Reversible electroaddressing of self-assembling amino-acid conjugates Adv. Funct. Mater 21 1575–80 [Google Scholar]
- [192].Martin LJ, Akhavan B and Bilek MMM 2018. Electric fields control the orientation of peptides irreversibly immobilized on radical-functionalized surfaces Nat. Commun. 9 357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Emaminejad S, Javanmard M, Gupta C, Chang S, Davis RW and Howe RT 2015. Tunable control of antibody immobilization using electric field Proc. Natl Acad. Sci 112 1995–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Lapizco-Encinas BH and Rito-Palomares M 2007. Dielectrophoresis for the manipulation of nanobioparticles Electrophoresis 28 4521–38 [DOI] [PubMed] [Google Scholar]
- [195].Yi H, Wu LQ, Bentley WE, Ghodssi R, Rubloff GW, Culver JN and Payne GF 2005. Biofabrication with chitosan Biomacromolecules 6 2881–94 [DOI] [PubMed] [Google Scholar]
- [196].Koev ST, Dykstra PH, Luo X, Rubloff GW, Bentley WE, Payne GF and Ghodssi R 2010. Chitosan: an integrative biomaterial for lab-on-a-chip devices Lab Chip 10 3026–42 [DOI] [PubMed] [Google Scholar]
- [197].Kim E et al. 2014. Chitosan to connect biology to electronics: fabricating the bio-device interface and communicating across this interface Polymers (Basel). 7 1–46 [Google Scholar]
- [198].Simchi A, Pishbin F and Boccaccini AR 2009. Electrophoretic deposition of chitosan Mater. Lett. 63 2253–6 [Google Scholar]
- [199].Fernandes R, Wu LQ, Chen T, Yi H, Rubloff GW, Ghodssi R, Bentley WE and Payne GF 2003. Electrochemically induced deposition of a polysaccharide hydrogel onto a patterned surface Langmuir 19 4058–62 [Google Scholar]
- [200].Cheng Y, Luo X, Betz J, Buckhout-White S, Bekdash O, Payne GF, Bentley WE and Rubloff GW 2010. In situ quantitative visualization and characterization of chitosan electrodeposition with paired sidewall electrodes Soft Matter 6 3177 [Google Scholar]
- [201].Maki Y, Furusawa K, Yasuraoka S, Okamura H, Hosoya N, Sunaga M, Dobashi T, Sugimoto Y and Wakabayashi K 2014. Universality and specificity in molecular orientation in anisotropic gels prepared by diffusion method Carbohydr. Polym. 108 118–26 [DOI] [PubMed] [Google Scholar]
- [202].Li K, Correa SO, Pham P, Raub CB and Luo X 2017. Birefringence of flow-assembled chitosan membranes in microfluidics Biofabrication 9 034101. [DOI] [PubMed] [Google Scholar]
- [203].Yan K, Ding F, Bentley WE, Deng H, Du Y, Payne GF and Shi X-W 2014. Coding for hydrogel organization through signal guided self-assembly Soft Matter 10 465. [DOI] [PubMed] [Google Scholar]
- [204].Wu S. et al. Electrical writing onto a dynamically responsive polysaccharide medium: patterning structure and function into a reconfigurable medium. Adv. Funct. Mater. 2018;28:1803139. [Google Scholar]
- [205].Jugowiec D, Łukaszczyk A, Cieniek Ł, Kowalski K, Rumian Ł, Pietryga K, Kot M, Pamuła E and Moskalewicz T 2017. Influence of the electrophoretic deposition route on the microstructure and properties of nano-hydroxyapatite/chitosan coatings on the Ti-13Nb-13Zr alloy Surf. Coatings Technol. 324 64–79 [Google Scholar]
- [206].Wu LQ, Yi H, Li S, Rubloff GW, Bentley WE, Ghodssi R and Payne GF 2003. Spatially selective deposition of a reactive polysaccharide layer onto a patterned template Langmuir 19 519–24 [Google Scholar]
- [207].Wu LQ, Lee K, Wang X, English DS, Losert W and Payne GF 2005. Chitosan-mediated and spatially selective electrodeposition of nanoscale particles Langmuir 21 3641–6 [DOI] [PubMed] [Google Scholar]
- [208].Buckhout-White SL and Rubloff GW 2009. Spatial resolution in chitosan-based programmable biomolecular scaffolds Soft Matter 5 3677 [Google Scholar]
- [209].Meyer WL, Liu Y, Shi XW, Yang X, Bentley WE and Payne GF 2009. Chitosan-coated wires: conferring electrical properties to chitosan fibers Biomacromolecules 10 858–64 [DOI] [PubMed] [Google Scholar]
- [210].Yu W-Z, Zhang Y, Liu X, Xiang Y, Li Z and Wu S 2018. Synergistic antibacterial activity of multi components in lysozyme/chitosan/silver/hydroxyapatite hybrid coating Mater. Des 139 351–62 [Google Scholar]
- [211].Gebhardt F, Seuss S, Turhan MC, Hornberger H, Virtanen S and Boccaccini AR 2012. Characterization of electrophoretic chitosan coatings on stainless steel Mater. Lett. 66 302–4 [Google Scholar]
- [212].Nancy D and Rajendran N 2018. Vancomycin incorporated chitosan/gelatin coatings coupled with TiO2–SrHAP surface modified cp-titanium for osteomyelitis treatment Int. J. Biol. Macromol 110 197–205 [DOI] [PubMed] [Google Scholar]
- [213].Cordero-Arias L and Boccaccini AR 2017. Electrophoretic deposition of chondroitin sulfate-chitosan/bioactive glass composite coatings with multilayer design Surf. Coatings Technol 315 417–25 [Google Scholar]
- [214].Nawrotek K, Tylman M, Decherchi P, Marqueste T, Rudnicka K, Gatkowska J and Wieczorek M 2016. Assessment of degradation and biocompatibility of electrodeposited chitosan and chitosan-carbon nanotube tubular implants J. Biomed. Mater. Res. Part A 104 2701–11 [DOI] [PubMed] [Google Scholar]
- [215].Sharma S, Patil DJ, Soni VP, Sarkate LB, Khandekar GS and Bellare JR 2009. Bone healing performance of electrophoretically deposited apatite-wollastonite/chitosan coating on titanium implants in rabbit tibiae J. Tissue Eng. Regen. Med 3 501–11 [DOI] [PubMed] [Google Scholar]
- [216].Leedy MR, Martin HJ, Norowski PA, Jennings JA, Haggard WO and Bumgardner JD 2011. Use of chitosan as a bioactive implant coating for bone-implant applications Adv. Polym. Sci 244 129–66 [Google Scholar]
- [217].Tibbe MP, Leferink AM, van den Berg A, Eijkel JCT and Segerink LI 2018. Microfluidic gel patterning method by use of a temporary membrane for organ-on-chip applications Adv. Mater. Technol 3 1700200 [Google Scholar]
- [218].Ordikhani F, Farani MR, Dehghani M, Tamjid E and Simchi A 2015. Physicochemical and biological properties of electrodeposited graphene oxide/chitosan films with drug-eluting capacity Carbon N. Y 84 91–102 [Google Scholar]
- [219].Li Y et al. 2018. Electrodeposition of a magnetic and redox-active chitosan film for capturing and sensing metabolic active bacteria Carbohydr. Polym. 195 505–14 [DOI] [PubMed] [Google Scholar]
- [220].Wang Y, Liu Y, Cheng Y, Kim E, Rubloff GW, Bentley WE and Payne GF 2011. Coupling electrodeposition with layer-by-layer assembly to address proteins within microfluidic channels Adv. Mater. 23 5817–21 [DOI] [PubMed] [Google Scholar]
- [221].Morrow BH, Payne GF and Shen J 2015. pH-responsive self-assembly of polysaccharide through a rugged energy landscape J. Am. Chem. Soc 137 13024–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Ladet S, David L and Domard A 2008. Multi-membrane hydrogels Nature 452 76–9 [DOI] [PubMed] [Google Scholar]
- [223].Ladet SG, Tahiri K, Montembault AS, Domard AJ and Corvol M-TM 2011. Multi-membrane chitosan hydr ogels as chondrocytic cell bioreactors Biomaterials 32 5354–64 [DOI] [PubMed] [Google Scholar]
- [224].Xiong Y, Yan K, Bentley WE, Deng H, Du Y, Payne GF and Shi XW 2014. Compartmentalized multilayer hydrogel formation using a stimulus-responsive self-assembling polysaccharide ACS Appl. Mater. Interfaces 6 2948–57 [DOI] [PubMed] [Google Scholar]
- [225].Manna U, Bharani S and Patil S 2009. Layer-by-layer self-assembly of modified hyaluronic acid/chitosan based on hydrogen bonding Biomacromolecules 10 2632–9 [DOI] [PubMed] [Google Scholar]
- [226].Liu Y, Zhang B, Gray KM, Cheng Y, Kim E, Rubloff GW, Bentley WE, Wang Q and Payne GF 2013. Electrodeposition of a weak polyelectrolyte hydrogel: remarkable effects of salt on kinetics, structure and properties Soft Matter 9 2703 [Google Scholar]
- [227].Yan K, Xiong Y, Wu S, Bentley WE, Deng H, Du Y, Payne GF and Shi XW 2016. Electro-molecular assembly: electrical writing of information into an erasable polysaccharide medium ACS Appl. Mater. Interfaces 8 19780–6 [DOI] [PubMed] [Google Scholar]
- [228].Chiappisi L, Hoffmann I and Gradzielski M 2013. Complexes of oppositely charged polyelectrolytes and surfactants—recent developments in the field of biologically derived polyelectrolytes Soft Matter 9 3896 [Google Scholar]
- [229].Chiappisi L and Gradzielski M 2015. Co-assembly in chitosan–surfactant mixtures: thermodynamics, structures, interfacial properties and applications Adv. Colloid Interface Sci 220 92–107 [DOI] [PubMed] [Google Scholar]
- [230].Barreiro-Iglesias R, Alvarez-Lorenzo C and Concheiro A 2005. Chitosan/sodium dodecylsulfate interactions J. Therm. Anal. Calorim 82 499–505 [Google Scholar]
- [231].Wei YC and Hudson SM 1993. Binding of sodium dodecyl sulfate to a polyelectrolyte based on chitosan Macromolecules 26 4151–4 [Google Scholar]
- [232].Thongngam M and McClements DJ 2005. Influence of pH, ionic strength, and temperature on self-association and interactions of sodium dodecyl sulfate in the absence and presence of chitosan Langmuir 21 79–86 [DOI] [PubMed] [Google Scholar]
- [233].Thongngam M and McClements DJ 2004. Characterization of Interactions between Chitosan and an Anionic Surfactant J. Agric. Food Chem. 52 987–91 [DOI] [PubMed] [Google Scholar]
- [234].Tsai CC, Morrow BH, Chen W, Payne GF and Shen J 2017. Toward understanding the environmental control of hydrogel film properties: how salt modulates the flexibility of chitosan chains Macromolecules 50 5946–52 [Google Scholar]
- [235].Steinschulte AA et al. 2017. Stimulated transitions of directed nonequilibrium self-assemblies Adv. Mater. 29 1–8 [DOI] [PubMed] [Google Scholar]
- [236].Raub CB, Hsu SC, Chan EF, Shirazi R, Chen AC, Chnari E, Semler EJ and Sah RL 2013. Microstructural remodeling of articular cartilage following defect repair by osteochondral autograft transfer Osteoarthr. Cartil 21 860–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Scarcelli G, Polacheck WJ, Nia HT, Patel K, Grodzinsky AJ, Kamm RD and Yun SH 2015. Noncontact three-dimensional mapping of intracellular hydromechanical properties by Brillouin microscopy Nat. Methods 12 1132–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Qu X et al. 2018. Electrofabrication of functional materials: chloramine-based antimicrobial film for infectious wound treatment Acta Biomate. 73 190–203 [DOI] [PubMed] [Google Scholar]
- [239].Kim E, Liu Z, Liu Y, Bentley W and Payne G 2017. Catechol-based hydrogel for chemical information processing Biomimetics 2 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Liu H et al. 2018. Bio-inspired redox-cycling antimicrobial film for sustained generation of reactive oxygen species Biomater. 162 109–22 [DOI] [PubMed] [Google Scholar]
- [241].Shi XW, Yang X, Gaskell KJ, Liu Y, Kobatake E, Bentley WE and Payne GF 2009. Reagentless protein assembly triggered by localized electrical signals Adv. Mater. 21 984–8 [Google Scholar]
- [242].Kim E, Liu Y, Shi X-W, Yang X, Bentley WE and Payne GF 2010. Biomimetic approach to confer redox activity to thin chitosan films Adv. Funct. Mater 20 2683–94 [Google Scholar]
- [243].Liu Y, Zhang B, Javvaji V, Kim E, Lee ME, Raghavan SR, Wang Q and Payne GF 2014. Tyrosinase-mediated grafting and crosslinking of natural phenols confers functional properties to chitosan Biochem. Eng. J 89 21–7 [Google Scholar]
- [244].Liu Y, Kim E, Lee ME, Zhang B, Elabd YA, Wang Q, White IM, Bentley WE and Payne GF 2014. Enzymatic writing to soft films: Potential to filter, store, and analyze biologically relevant chemical information Adv. Funct. Mater 24 480–91 [Google Scholar]
- [245].Ryu JH, Hong S and Lee H 2015. Bio-inspired adhesive catechol-conjugated chitosan for biomedical applications: a mini review Acta Biomate. 27 101–15 [DOI] [PubMed] [Google Scholar]
- [246].Kim E, Leverage WT, Liu Y, White IM, Bentley WE and Payne GF 2014. Redox-capacitor to connect electrochemistry to redox-biology Analyst 139 32–43 [DOI] [PubMed] [Google Scholar]
- [247].Kim E, Liu Y, Bentley WE and Payne GF 2012. Redox capacitor to establish bio-device redox-connectivity Adv. Funct. Mater 22 1409–16 [Google Scholar]
- [248].Cao C et al. 2018. Radical scavenging activities of biomimetic catechol-chitosan films Biomacromolecules 19 3502–14 [DOI] [PubMed] [Google Scholar]
- [249].Lee KY and Mooney DJ 2012. Alginate: properties and biomedical applications Prog. Polym. Sci 37 106–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Liu Y, Wu HC, Chhuan M, Terrell JL, Tsao CY, Bentley WE and Payne GF 2015. Functionalizing soft matter for molecular communication ACS Biomater. Sci. Eng 1 320–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Rhoads MK, Hauk P, Terrell J, Tsao CY, Oh H, Raghavan SR, Mansy SS, Payne GF and Bentley WE 2018. Incorporating LsrK AI-2 quorum quenching capability in a functionalized biopolymer capsule Biotechnol. Bioeng 115 278–89 [DOI] [PubMed] [Google Scholar]
- [252].Gupta A, Terrell JL, Fernandes R, Dowling MB, Payne GF, Raghavan SR and Bentley WE 2013. Encapsulated fusion protein confers ‘sense and respond’ activity to chitosan-alginate capsules to manipulate bacterial quorum sensing Biotechnol. Bioeng. 110 552–62 [DOI] [PubMed] [Google Scholar]
- [253].Arya C, Oh H and Raghavan SR 2016. ‘Killer’ microcapsules that can selectively destroy target microparticles in their vicinity ACS Appl. Mater. Interfaces 8 29688–95 [DOI] [PubMed] [Google Scholar]
- [254].Liu X, Liu H, Qu X, Lei M, Zhang C, Hong H, Payne GF and Liu C 2017. Electrical signals triggered controllable formation of calcium-alginate film for wound treatment J. Mater. Sci., Mater. Med 28 146. [DOI] [PubMed] [Google Scholar]
- [255].Ozawa F, Ino K, Shiku H and Matsue T 2016. Electrochemical hydrogel lithography of calcium-alginate hydrogels for cell culture Materials (Basel). 9 744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [256].Cheng Y, Luo X, Payne GF and Rubloff GW 2012. Biofabrication: programmable assembly of polysaccharide hydrogels in microfluidics as biocompatible scaffolds J. Mater. Chem 22 7659 [Google Scholar]
- [257].Ozawa F, Ino K, Arai T, Ramón-Azcón J, Takahashi Y, Shiku H and Matsue T 2013. Alginate gel microwell arrays using electrodeposition for three-dimensional cell culture Lab Chip 13 3128. [DOI] [PubMed] [Google Scholar]
- [258].Ozawa F, Ino K, Takahashi Y, Shiku H and Matsue T 2013. Electrodeposition of alginate gels for construction of vascular-like structures J. Biosci. Bioeng 115 459–61 [DOI] [PubMed] [Google Scholar]
- [259].Márquez A, Jiménez-Jorquera C, Domínguez C and Muñoz-Berbel X 2017. Electrodepositable alginate membranes for enzymatic sensors: an amperometric glucose biosensor for whole blood analysis Biosens. Bioelectron 97 136–42 [DOI] [PubMed] [Google Scholar]
- [260].Liu Z, Takeuchi M, Nakajima M, Hasegawa Y, Huang Q and Fukuda T 2016. Shape-controlled high cell-density microcapsules by electrodeposition Acta Biomate. 37 93–100 [DOI] [PubMed] [Google Scholar]
- [261].Ozawa F, Ino K, Shiku H and Matsue T 2017. Cell sheet fabrication using RGD peptide-coupled alginate hydrogels fabricated by an electrodeposition method Chem. Lett. 46 605–8 [Google Scholar]
- [262].Liu Z, Lu M, Takeuchi M, Yue T, Hasegawa Y, Huang Q and Fukuda T 2018. In vitro mimicking the morphology of hepatic lobule tissue based on Ca-alginate cell sheets Biomed. Mater. 13 035004. [DOI] [PubMed] [Google Scholar]
- [263].Cheng Y, Luo X, Tsao C-Y, Wu H-C, Betz J, Payne GF, Bentley WE and Rubloff GW 2011. Biocompatible multi-address 3D cell assembly in microfluidic devices using spatially programmable gel formation Lab Chip 11 2316–8 [DOI] [PubMed] [Google Scholar]
- [264].Taira N, Ino K, Robert J and Shiku H 2018. Electrochemical printing of calcium alginate/gelatin hydrogel Electrochim. Acta 281 429–36 [Google Scholar]
- [265].Liu Y, Li J, Tschirhart T, Terrell JL, Kim E, Tsao C-YCY, Kelly DL, Bentley WE and Payne GF 2017. Connecting biology to electronics: molecular communication via redox modality Adv. Healthc. Mater 6 1700789. [DOI] [PubMed] [Google Scholar]
- [266].Liu Y, Tsao C-YY, Kim E, Tschirhart T, Terrell JL, Bentley WE and Payne GF 2016. Using a redox modality to connect synthetic biology to electronics: hydrogel-based chemo-electro signal transduction for molecular communication Adv. Healthc. Mater 6 1600908. [DOI] [PubMed] [Google Scholar]
- [267].Aszódi A, Legate KR, Nakchbandi I and Fässler R 2006. What mouse mutants teach us about extracellular matrix function Annu. Rev. Cell Dev. Biol 22 591–621 [DOI] [PubMed] [Google Scholar]
- [268].Glowacki J and Mizuno S 2008. Collagen scaffolds for tissue engineering Biopolymers 89 338–44 [DOI] [PubMed] [Google Scholar]
- [269].Nocera AD, Comín R, Salvatierra NA and Cid MP 2018. Development of 3D printed fibrillar collagen scaffold for tissue engineering Biomed. Microdevices 20 26. [DOI] [PubMed] [Google Scholar]
- [270].Chevallay B and Herbage D 2000. Collagen-based biomaterials as 3D scaffold for cell cultures: applications for tissue engineering and gene therapy Med. Biol. Eng. Comput 38 211–8 [DOI] [PubMed] [Google Scholar]
- [271].Wu Y, Han Y, Wong YS and Fuh JYH 2018. Fibre-based scaffolding techniques for tendon tissue engineering J. Tissue Eng. Regen. Med 12 1798–821 [DOI] [PubMed] [Google Scholar]
- [272].Buehler MJ 2006. Nature designs tough collagen: explaining the nanostructure of collagen fibrils Proc. Natl Acad. Sci. USA 103 12285–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [273].Marino AA and Becker RO 1969. The effect of electric current on rat tail tendon collagen in solution Calcif. Tissue Res 4 330–8 [DOI] [PubMed] [Google Scholar]
- [274].Baker HR, Merschrod SEF and Poduska KM 2008. Electrochemically controlled growth and positioning of suspended collagen membranes Langmuir 24 2970–2 [DOI] [PubMed] [Google Scholar]
- [275].Abu-Rub MT, Billiar KL, van Es MH, Knight A, Rodriguez BJ, Zeugolis DI, McMahon S, Windebank AJ and Pandit A 2011. Nano-textured self-assembled aligned collagen hydrogels promote directional neurite guidance and overcome inhibition by myelin associated glycoprotein Soft Matter 7 2770 [Google Scholar]
- [276].Ramesh Kumar M, Merschrod SEF and Poduska KM 2009. Correlating mechanical properties with aggregation processes in electrochemically fabricated collagen membranes Biomacromolecules 10 1970–5 [DOI] [PubMed] [Google Scholar]
- [277].Uquillas JA, Kishore V and Akkus O 2011. Effects of phosphate-buffered saline concentration and incubation time on the mechanical and structural properties of electrochemically aligned collagen threads Biomed. Mater 6 035008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [278].Younesi M, Islam A, Kishore V, Panit S and Akkus O 2015. Fabrication of compositionally and topographically complex robust tissue forms by 3D-electrochemical compaction of collagen Biofabrication 7 035001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [279].Kishore V, Iyer R, Frandsen A and Nguyen T-U 2016. In vitro characterization of electrochemically compacted collagen matrices for corneal applications Biomed. Mater 11 055008. [DOI] [PubMed] [Google Scholar]
- [280].Kishore V, Paderi JE, Akkus A, Smith KM, Balachandran D, Beaudoin S, Panitch A and Akkus O 2011. Incorporation of a decorin biomimetic enhances the mechanical properties of electrochemically aligned collagen threads Acta Biomate. 7 2428–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [281].Cudjoe E, Younesi M, Cudjoe E, Akkus O and Rowan SJ 2017. Synthesis and fabrication of nanocomposite fibers of collagen-cellulose nanocrystals by coelectrocompaction Biomacromolecules 18 1259–67 [DOI] [PubMed] [Google Scholar]
- [282].Kang L, Liu X, Yue Z, Chen Z, Baker C, Winberg P and Wallace G 2018. Fabrication and in vitro characterization of electrochemically compacted collagen/sulfated xylorhamnoglycuronan matrix for wound healing applications Polymers (Basel). 10 415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [283].Nguyen T-U, Bashur CA and Kishore V 2016. Impact of elastin incorporation into electrochemically aligned collagen fibers on mechanical properties and smooth muscle cell phenotype Biomed. Mater. 11 025008. [DOI] [PubMed] [Google Scholar]
- [284].Gendron R, Kumar MR, Paradis H, Martin D, Ho N, Gardiner D, Merschrod SEF and Poduska KM 2012. Controlled cell proliferation on an electrochemically engineered collagen scaffold Macromol. Biosci. 12 360–6 [DOI] [PubMed] [Google Scholar]
- [285].Kishore V, Bullock W, Sun X, Van Dyke WS and Akkus O 2012. Tenogenic differentiation of human MSCs induced by the topography of electrochemically aligned collagen threads Biomaterials 33 2137–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [286].Islam A, Mbimba T, Younesi M and Akkus O 2017. Effects of substrate stiffness on the tenoinduction of human mesenchymal stem cells Acta Biomate. 58 244–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [287].Younesi M, Goldberg VM and Akkus O 2016. A micro-architecturally biomimetic collagen template for mesenchymal condensation based cartilage regeneration Acta Biomate. 30 212–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [288].Vollrath F 2000. Strength and structure of spiders’ silks Rev. Mol. Biotechnol 74 67–83 [DOI] [PubMed] [Google Scholar]
- [289].Gosline JM, Guerette PA, Ortlepp CS and Savage KN 1999. The mechanical design of spider silks: from fibroin sequence to mechanical function J. Exp. Biol 202 3295–303 [DOI] [PubMed] [Google Scholar]
- [290].Wang X, Hu X, Daley A, Rabotyagova O, Cebe P and Kaplan DL 2007. Nanolayer biomaterial coatings of silk fibroin for controlled release J. Control. Release 121 190–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [291].Jin HJ, Park J, Karageorgiou V, Kim UJ, Valluzzi R, Cebe P and Kaplan DL 2005. Water-stable silk films with reduced β-sheet content Adv. Funct. Mater 15 1241–7 [Google Scholar]
- [292].Numata K, Ifuku N, Masunaga H, Hikima T and Sakai T 2017. Silk resin with hydrated dual chemical-physical cross-links achieves high strength and toughness Biomacromolecules 18 1937–46 [DOI] [PubMed] [Google Scholar]
- [293].Wu HC, Quan DN, Tsao CY, Liu Y, Terrell JL, Luo X, Yang JC, Payne GF and Bentley WE 2017. Conferring biological activity to native spider silk: a biofunctionalized protein-based microfiber Biotechnol. Bioeng 114 83–95 [DOI] [PubMed] [Google Scholar]
- [294].Heidebrecht A and Scheibel T 2013. Recombinant production of spider silk proteins Adv. Appl. Microbiol 82 115–53 [DOI] [PubMed] [Google Scholar]
- [295].Nilebäck L, Hedin J, Widhe M, Floderus LS, Krona A, Bysell H and Hedhammar M 2017. Self-assembly of recombinant silk as a strategy for chemical-free formation of bioactive coatings: a real-time study Biomacromolecules 18 846–54 [DOI] [PubMed] [Google Scholar]
- [296].Chung H, Kim TY and Lee SY 2012. Recent advances in production of recombinant spider silk proteins Curr. Opin. Biotechnol 23 957–64 [DOI] [PubMed] [Google Scholar]
- [297].Peng Q, Zhang Y, Lu L, Shao H, Qin K, Hu X and Xia X 2016. Recombinant spider silk from aqueous solutions via a bio-inspired microfluidic chip Sci. Rep 6 36473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [298].Sofia S, McCarthy MB, Gronowicz G and Kaplan DL 2001. Functionalized silk-based biomaterials for bone formation J. Biomed. Mater. Res 54 139–48 [DOI] [PubMed] [Google Scholar]
- [299].Servoli E, Maniglio D, Motta A and Migliaresi C 2008. Folding and assembly of fibroin driven by an AC electric field: effects on film properties Macromol. Biosci 8 827–35 [DOI] [PubMed] [Google Scholar]
- [300].Lu Q, Bai S, Ding Z, Guo H, Shao Z, Zhu H and Kaplan DL 2016. Hydrogel assembly with hierarchical alignment by balancing electrostatic forces Adv. Mater. Interfaces 3 1500687 [Google Scholar]
- [301].Lin Y, Xia X, Shang K, Elia R, Huang W, Cebe P, Leisk G, Omenetto F and Kaplan DL 2013. Tuning chemical and physical cross-links in silk electrogels for morphological analysis and mechanical reinforcement Biomacromolecules 14 2629–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [302].Karahaliloğlu Z 2018. Curcumin-loaded silk fibroin e-gel scaffolds for wound healing applications Mater. Technol 33 276–87 [Google Scholar]
- [303].Li M, Xiong P, Mo M, Cheng Y and Zheng Y 2016. Electrophoretic-deposited novel ternary silk fibroin/graphene oxide/hydroxyapatite nanocomposite coatings on titanium substrate for orthopedic applications Front. Mater. Sci 10 270–80 [Google Scholar]
- [304].Lin Y, Wang S, Chen Y, Wang Q, Burke KA, Spedden EM, Staii C, Weiss AS and Kaplan DL 2015. Electrodeposited gels prepared from protein alloys Nanomedicine 10 803–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [305].Elia R, Michelson CD, Perera AL, Harsono M, Leisk GG, Kugel G and Kaplan DL 2015. Silk electrogel coatings for titanium dental implants J. Biomater. Appl 29 1247–55 [DOI] [PubMed] [Google Scholar]
- [306].Wang L, Lu G, Lu Q and Kaplan DL 2018. Controlling cell behavior on silk nanofiber hydrogels with tunable anisotropic structures ACS Biomater. Sci. Eng 4 933–41 [DOI] [PubMed] [Google Scholar]


