Abstract
BCDIN3 domain containing RNA methyltransferase, BCDIN3D, monomethylates the 5′-monophosphate of cytoplasmic tRNAHis with a G−1:A73 mispair at the top of an eight-nucleotide-long acceptor helix, using S-adenosyl-l-methionine (SAM) as a methyl group donor. In humans, BCDIN3D overexpression is associated with the tumorigenic phenotype and poor prognosis in breast cancer. Here, we present the crystal structure of human BCDIN3D complexed with S-adenosyl-l-homocysteine. BCDIN3D adopts a classical Rossmann-fold methyltransferase structure. A comparison of the structure with that of the closely related methylphosphate capping enzyme, MePCE, which monomethylates the 5′-γ-phosphate of 7SK RNA, revealed the important residues for monomethyl transfer from SAM onto the 5′-monophosphate of tRNAHis and for tRNAHis recognition by BCDIN3D. A structural model of tRNAHis docking onto BCDIN3D suggested the molecular mechanism underlying the different activities between BCDIN3D and MePCE. A loop in BCDIN3D is shorter, as compared to the corresponding region that forms an α-helix to recognize the 5′-end of RNA in MePCE, and the G−1:A73 mispair in tRNAHis allows the N-terminal α-helix of BCDIN3D to wedge the G−1:A73 mispair of tRNAHis. As a result, the 5′-monophosphate of G−1 of tRNAHis is deep in the catalytic pocket for 5′-phosphate methylation. Thus, BCDIN3D is a tRNAHis-specific 5′-monomethylphosphate capping enzyme that discriminates tRNAHis from other tRNA species, and the structural information presented in this study also provides the molecular basis for the development of drugs against breast cancers.
INTRODUCTION
BCDIN3D, bicoid interacting 3 domain containing RNA methyltransferase, is an evolutionarily conserved member of the Bin3 methyltransferase family and contains an S-adenosyl-l-methionine (SAM) binding domain (1,2). BCDIN3D is closely related to the methylphosphate capping enzyme (MePCE), also called BCDIN3 in humans, which catalyzes the monomethylation of the 5′-γ-phosphate of a small subset of noncoding RNAs using SAM as a methyl group donor (3–6). The primary target of MePCE is the ubiquitous 7SK RNA (5,6). 7SK RNA is a main component of a regulatory RNA–protein complex that downregulates the activity of an RNA polymerase II elongation factor, P-TEFb, consisting of a cyclin-dependent kinase CDK9 and cyclin T1/T2 (7,8). Monomethylation of the 5′-γ-phosphate of 7SK RNA protects 7SK from degradation and controls the activity of P-TEFb (5,6,9). In contrast, the biological function of BCDIN3D has remained enigmatic. Several reports have shown that BCDIN3D is often overexpressed in human breast cancer cells, and the elevated expression of BCDIN3D is associated with the tumorigenic phenotype and poor prognosis in breast cancer, particularly triple-negative breast cancers (1,10,11). Recently, studies in Drosophila revealed that BCDIN3D is involved in female fertility (12).
Until recently, the primary target of BCDIN3D had been unknown. Several years ago, it was reported that BCDIN3D catalyzes the dimethylation of the 5′-monophosphate of a specific group of precursor miRNAs, such as the tumor suppressor miR-145, and that the dimethylation of pre-miRNAs inhibits subsequent Dicer processing. As a result, the expression of the mature form of the tumor suppressor miRNA is suppressed (1). However, it was recently shown that BCDIN3D tightly interacts with cytoplasmic tRNAHis in HEK293 cells. An LC-nano ESI-MS analysis (13) of tRNAHis revealed that tRNAHis has a 5′-monomethylphosphate, as previously reported (14,15). It was also shown that BCDIN3D monomethylates the 5′-monophosphate of tRNAHisin vitro, and the BCDIN3D gene knockout in HEK293 cells results in the loss of the modification of tRNAHisin vivo. At the same time, it was shown that BCDIN3D monomethylates tRNAHis more efficiently than pre-miR-145 by over two orders of magnitude in vitro, and never dimethylates the 5′-monophosphate of tRNAHis, as revealed by an LC-nano ESI-MS analysis. BCDIN3D specifically recognizes the unique features observed only in tRNAHis, among the eukaryotic tRNA species. It recognizes the acceptor helix of tRNAHis with a G−1:A73 mispair at the top of the eight-nucleotide-long acceptor helix and the G−1 nucleobase. Therefore, it was concluded that BCDIN3D is a tRNAHis-specific monomethylphosphate capping enzyme (15,16).
Here, we present the crystal structure of human BCDIN3D (hBCDIN3D) complexed with S-adenosyl-l-homocysteine (SAH). The structural and biochemical studies of BCDIN3D, together with the structural comparison between BCDIN3D and MePCE, have revealed the mechanism of the specific methylation of the 5′-monophosphate of tRNAHis by BCDIN3D, and confirmed that BCDIN3D is a tRNAHis-specific monomethylphosphate capping enzyme. The structural information presented in this study also provides the molecular basis for the development of drugs against breast cancers.
MATERIALS AND METHODS
Plasmid constructions
The DNA fragments encoding BCDIN3D proteins from various organisms were synthesized by Eurofins, Japan. The synthesized nucleotide sequences are shown in Supplementary Table S1. For overexpression, the BCDIN3D gene sequences were cloned between the NdeI and XhoI sites of the pET-15b vector (Merck Millipore, Japan). The mutations in the BCDIN3D gene were introduced by the inverse PCR method. The oligonucleotide sequences used for the plasmid constructions are listed in Supplementary Table S2.
Protein expression and purification
Escherichia coli BL21(DE3) cells (Novagen-Merck Millipore) were transformed with the plasmids and grown in LB medium containing 50 μg/ml ampicillin at 37°C, until A600 reached 0.8. The expression of BCDIN3D and its variants was induced by the addition of isopropyl-β-d-thiogalactopyranoside at a final concentration of 0.1 mM, and the culture was continued for 16 h at 20°C. The cells were harvested and sonicated, in buffer containing 20 mM Tris–HCl, pH 7.0, 500 mM NaCl, 10 mM β-mercaptoethanol, 5% (v/v) glycerol and 20 mM imidazole (buffer A), and centrifuged at 100 000 × g for 1 h at 4°C. The clear supernatant was applied to a Ni-NTA agarose column (Qiagen, Japan), and after the column was washed with buffer A, the proteins were eluted from the column with buffer containing 20 mM Tris–HCl, pH 7.0, 500 mM NaCl, 5% (v/v) glycerol, 250 mM imidazole and 10 mM β-mercaptoethanol. The proteins were further purified on a HiTrap Heparin column (GE Healthcare, Japan), and finally applied to a HiLoad 16/60 Superdex 200 column (GE Healthcare, Japan), equilibrated with buffer containing 20 mM Tris–HCl, pH 7.0, 200 mM NaCl and 10 mM β-mercaptoethanol. The purified proteins were concentrated, and stored at −80°C.
Crystallization and structural determination
The hBCDIN3D_t protein, the truncated form of hBCDIN3D, was adjusted to 2 mg/ml and supplemented with 1 mM SAH (Sigma) and 22.2 mM Tris–HCl, pH 8.0, for neutralization. A 0.3 μl portion of the protein solution was mixed with 0.2 μl of reservoir solution, containing 20% (w/v) PEG 3350 and 200 mM CaCl2. The crystals were generated by the sitting drop vapor diffusion method at 20°C. To facilitate the crystallization, the drop was supplemented with 100 nl of crystal seed solution, prepared with Seed Bead (Hampton Research), in buffer containing 100 mM sodium cacodylate, pH 7.0, 25% (v/v) tacsimate, pH 7.0, and 1 mM spermine.
Data sets were collected at beamline 17A at the Photon Factory at KEK, Japan. The crystals were flash-cooled in a solution containing 15% (w/v) PEG 3350, 150 mM CaCl2, 8.3% tacsimate, pH 7.0, 33 mM sodium cacodylate, pH 7.0, 0.33 mM spermine and 20% (v/v) ethylene glycol.
The data were indexed, integrated and scaled with XDS (17). The diffraction data were processed by the STARANISO server (http://staraniso.globalphasing.org/cgi-bin/staraniso.cgi) for anisotropy correction and truncation. The initial phase was determined by the molecular replacement method, using the Phaser program (18). As the search model, a homology model was prepared by the SWISS-MODEL server (19), based on the crystal structure of the human methylphosphate capping enzyme, MePCE (PDB ID: 5UNA). The structure was refined with phenix.refine (20), and manually modified with Coot (21).
In vitro methylation assay
The methylation assays were performed as described (15), with some modifications. For assays using [14C]-SAM, reaction mixtures (20 μl) containing 50 mM Tris–HCl, pH 8.0, 50 mM NaCl, 5 mM MgCl2, 2 mM DTT, 5% (v/v) glycerol, 1 μM RNA, 10 μM [14C]-SAM (40 mCi/mmol, PerkinElmer, Japan) and 1 μM BCDIN3D from various organisms were incubated at 37°C. The RNAs were phenol–chloroform extracted, ethanol precipitated and separated by 10% (w/v) polyacrylamide gel electrophoresis under denaturing conditions. The gel was dried and exposed to an imaging plate, and the methylated RNAs were detected with a BAS-5000 imager (Fujifilm, Japan).
For assays using [3H]-SAM, reaction mixtures (20 μl) containing 50 mM Tris–HCl, pH 8.0, 50 mM NaCl, 5 mM MgCl2, 2 mM DTT, 5% (v/v) glycerol, 1 μM RNA, 10 μM [3H]-SAM (55 mCi/mmol, PerkinElmer, Japan) and 0.1 μM BCDIN3D or its variants were incubated at 37°C. An aliquot (10 μM) was withdrawn at the indicated time from the reaction solution and spotted onto a Whatman 3MM filter (GE Healthcare, Japan). Under this condition, the reaction by wild-type BCDIN3D proceeds in a linear range until 10 min. After the filters were washed with 10% (w/v) TCA, they were washed with ethanol and dried. The filters were suspended in Ultima Gold liquid scintillation cocktail (PerkinElmer, Japan), and the radioactivities on the filters were quantified with a liquid scintillation counter (Beckman Coulter).
Human tRNAHis transcripts were synthesized by T7 RNA polymerase in the presence of excess GMP, and purified by 10% (w/v) polyacrylamide gel electrophoresis under denaturing conditions. To prepare the tRNAHis transcript with a 5′-triphosphate, GMP was omitted from the reaction, and it was purified in the same manner.
tRNA docking model onto BCDIN3D
A model of tRNAHis with G−1:A73 mispair at the top of the acceptor helix onto BCDIN3D was manually built as follows using program Coot (21). The backbone of the modeled tRNA with G−1:A73 mispair at the top of the eight-nucleotide-long acceptor helix of human tRNAHis was derived from the crystal structure of initiator tRNAfMet from E. coli (PDB ID: 3CW6) with C1:A72 mispair at the top of the acceptor helix and flipped 5′-end nucleotide. First, the tRNAfMet was manually superposed on the 5′-region of SL1p complexed with human MePCE (hMePCE), so that the 5′-α-phosphate of tRNAfMet is placed in the catalytic site of BCDIN3D. Then, the 5′-end nucleotide C1 of tRNAfMet was replaced with G−1 and 3′-A72A73C74C75A76 of tRNAfMet was replaced with A73C74C75A76, and one base pair was inserted in the acceptor helix to generate the tRNAHis model with eight-nucleotide-long acceptor stem. Finally, nucleotide sequence of the modeled acceptor helix was replaced with that of human tRNAHis.
RESULTS
Determination of the hBCDIN3D structure
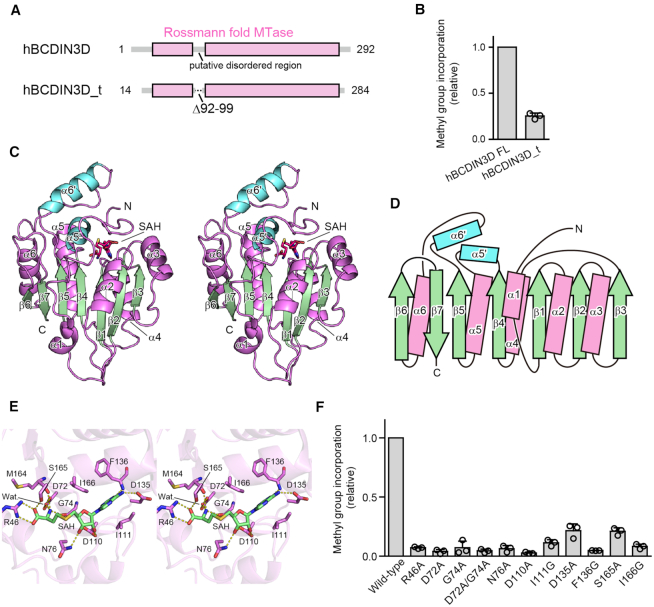
Initial crystallization trials of full-length hBCDIN3D generated no diffractive crystals. Thus, we prepared the truncated form of hBCDIN3D, based on the sequence alignments with the BCDIN3D proteins from chicken, frog, zebrafish and fly, which were all capable of transferring the methyl group of SAM onto the human tRNAHis transcript in vitro (Supplementary Figures S1 and S2). The N-terminal, internal and C-terminal putative flexible regions, which are less conserved among them (residues 1–13, 92–99 and 285–292, respectively), were removed from hBCDIN3D, yielding hBCDIN3D_t (residues 14–284_Δ92–99; Figure 1A). hBCDIN3D_t had around 25% methylation activity of the full-length hBCDIN3D for the human tRNAHis transcript (Figure 1B). Finally, we obtained diffractive crystals of hBCDIN3D_t in the presence of SAH.
Figure 1.
Overall structure of hBCDIN3D and SAH recognition. (A) Schematic diagrams of hBCDIN3D and its variant hBCDIN3D_t used for crystallization. (B) Relative methylation activity of hBCDIN3D_t (hBCDIN3D as 1.0) under standard conditions. The reaction mixtures were incubated at 37°C for 8 min. At this time point, the reaction by wild-type BCDIN3D proceeds in a linear range. Data are presented as mean ± standard deviation (SD) of three independent assays. (C) Stereo view of the ribbon model of hBCDIN3D, complexed with SAH. Residues 29–91 and 100–264 are modeled in the structure. SAH molecule is depicted by a red stick model. The α-helices and β-strands in the Rossmann fold are colored purple and green, respectively. Additional α-helices, α5′ and α6′, are colored cyan. (D) Topology diagram of hBCDIN3D. hBCDIN3D adopts the classical Rossmann fold. The colors are the same as in (C). (E) Stereo view of the SAH binding site of hBCDIN3D. SAH is depicted by a green stick model. (F) The activities of hBCDIN3D variants relative to the wild type (taken as 1.0) under the standard conditions. The reaction mixtures were incubated at 37°C for 1 h. Data are presented as mean ± SD of three independent assays.
The crystal belongs to the space group P21, and contains four hBCDIN3D molecules in the asymmetric unit. The initial phase was determined by the molecular replacement method, using a homology model of hBCDIN3D based on the structure of the methyltransferase domain (MTD) of MePCE (PDB ID: 5UNA), as the search model. The structure was model-built and refined to an R factor of 21.2% (Rfree = 27.0%) at 2.9 Å resolution (Supplementary Figure S3). The final model contains residues 29–91 and 100–264 of hBCDIN3D (Figure 1C). The details of the crystallographic data collection and refinement statistics are shown in Table 1.
Table 1.
Data collection and refinement statistics
| hBCDIN3D_t | |
|---|---|
| Data collection | |
| Space group | P21 |
| Cell dimensions | |
| a, b, c (Å) | 48.14, 140.34, 86.42 |
| β (∘) | 105.44 |
| Wavelength (Å) | 0.98000 |
| Resolution (Å)a | 50–2.9 (3.03–2.92) |
| No. of measured reflections | 218 829 |
| No. of unique reflections | 23 888 |
| R sym a | 0.205 (2.190) |
| I/σIa | 8.3 (1.2) |
| CC 1/2 a | 0.996 (0.693) |
| Completeness (%)a | 99.8 (99.3) |
| Redundancya | 9.2 (9.0) |
| Refinement | |
| Resolution (Å) | 50–2.9 |
| No. reflections | 20 740 |
| R work/Rfree (%) | 21.19/27.03 |
| No. atoms | |
| Protein | 7482 |
| Ligand | 104 |
| Water | 7 |
| B-factors (Å2) | |
| Protein | 46.56 |
| Ligand | 29.36 |
| Water | 29.20 |
| Root-mean-square deviations (RMSDs) | |
| Bond lengths (Å) | 0.005 |
| Bond angles (°) | 0.86 |
aValues in parentheses are for the highest-resolution shell.
Overall structure of hBCDIN3D and SAH recognition
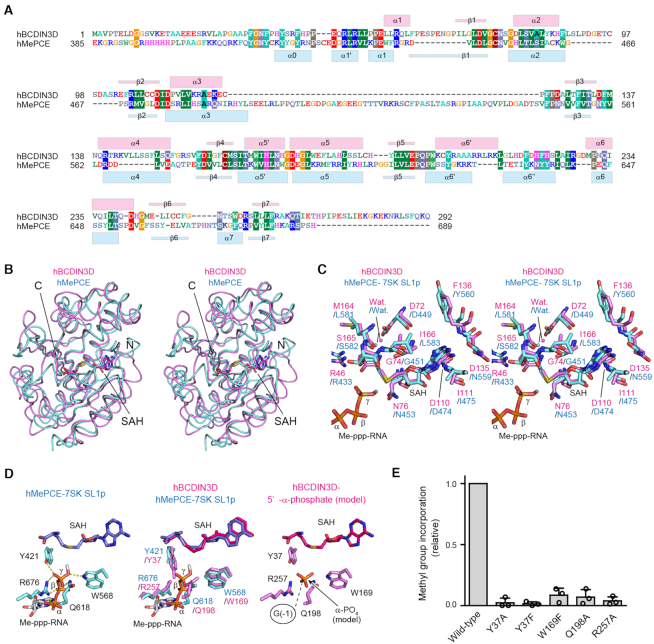
hBCDIN3D consists of eight α-helices (α1–α6, α5′ and α6′) and seven β-strands (β1–β7) (Figure 1C and D). The numbering of the α-helices and β-strands in hBCDIN3D follows that of the closely related MePCE structure for comparison (22) (Figure 2A).
Figure 2.
Comparison between hBCDIN3D and hMePCE. (A) Sequence alignment of hBCDIN3D and the MTD of hMePCE. The secondary structure elements of hBCDIN3D and hMePCE are indicated above and below the alignment, respectively. (B) Stereo view of the superimposed structures of hBCDIN3D (purple) and hMePCE (cyan). SAH molecules are depicted by stick models. (C) Superimposition of the structure of the SAH binding pocket of hBCDIN3D (purple) onto that of hMePCE (cyan) complexed with SAH and 7SK SL1p bearing a monomethyl-γ-phosphate 5′-cap (Me-ppp). The Me-ppp group at the 5′-G1 of SL1p is shown as a stick model. (D) 5′-Phosphate recognition by hMePCE (left). Conserved residues for 5′-phosphate recognition by hBCDIN3D (purple) and hMePCE (cyan) (middle). Possible binding site of the 5′-α-phosphate of the G−1 of tRNAHis in hBCDIN3D. The phosphate group is depicted by an orange stick model. (E) The activities of hBCDIN3D variants relative to the wild type (taken as 1.0) under the standard conditions. The reaction mixtures were incubated at 37°C for 1 h. Data are presented as mean ± SD of three independent assays.
The core of hBCDIN3D adopts a classical α/β methyltransferase fold, or the so-called Rossmann fold, with the parallel β-sheet containing a topological switch in the center. It consists of seven β-strands (β1–β7) and six α-helices (α1–α6), and the β-strands form an extended β-sheet with the α-helices sandwiching both sides of the β-sheet (Figures 1C and D, and 2A). The core of the BCDIN3D structure is homologous to those of other methyltransferases in the same family, such as MePCE (22), METTL16 (23,24), NSUN6 (25), TRAM61A (26) and DNMT1 (27) (Supplementary Figures S4 and S5). Additional α-helices, α5′ and α6′, are inserted between β4 and α5, and between β5 and α6, respectively, in the BCDIN3D structure (Figures 1C and D, and 2A).
In the structure of hBCDIN3D bound with SAH (Figure 1C), SAH resides in the cleft formed at the topological switch in the center of the Rossmann fold, and extensively interacts with hBCDIN3D through highly conserved residues (Figure 1E, Supplementary Figure S1). The N-terminal region of hBCDIN3D covers the SAH in the cleft, suggesting the enclosed catalytic site. R46 and S165 form hydrogen bonds with the SAH carboxylate group, and the main-chain carboxyl oxygens of G74 and M164 form hydrogen bonds with the SAH homocysteine amide group. D72 also forms a hydrogen bond with the homocysteine amide group, via a water molecule. I111 and I166 stack with the adenine base and sandwich it. D135 and the main-chain amide group of F136 form hydrogen bonds with the N6 and N1 atoms of the adenine base, respectively. The Nδ2 atom of D76 forms a hydrogen bond with the 3′-OH of the ribose, and D110 forms hydrogen bonds with the 2′-OH and 3′-OH of the ribose.
In vitro methylation reactions of the human tRNAHis transcript using mutant hBCDIN3Ds (Figure 1F) showed that the R46A, D72A, G74A, D72A/G74A, D76A, D110A and F136G mutants abolished the enzymatic activity. The I111G, D135A, S165A and I166G mutants reduced the activity to extents of 10% or less than that of the wild type even at the reaction end points. Thus, these conserved residues are important for the SAM recognition and the methyltransferase activity of hBCDIN3D.
Mechanism of 5′-monophosphate recognition by hBCDIN3D
Quite recently, the crystal structures of the C-terminal MTD of the hMePCE, bound to SAH and RNAs, have been reported (22). hMePCE monomethylates the 5′-γ-phosphate of a small subset of noncoding RNAs, such as the 7SK and U6 snRNAs, using SAM as a methyl group donor (3–5).
The hBCDIN3D structure is quite homologous to that of the MTD of hMePCE, with 31% amino acid sequence identity and an RMSD of 2.1 Å for 181 structurally equivalent residues (calculated by the Dali server (28); RMSD calculated with the Cα atoms). The overall structures superimposed well on each other (Figure 2A and B).
The structure of the catalytic pocket of hBCDIN3D complexed with SAH was superimposed onto that of the MTD of hMePCE complexed with SAH and 7SK SL1p, a short RNA hairpin mimicking 7SK bearing a 5′-Me-ppp cap (Figure 2C). The hMePCE structure represents the termination stage of methylation. The SAH-interacting residues in hBCDIN3D described earlier (Figure 1E) superimposed well onto the corresponding residues in hMePCE (Figure 2C). Furthermore, the residues (Y421, R676, Q618 and W568; Figure 2D, left) interacting with the γ-phosphate oxygen of the 5′-G1 of SL1p in the hMePCE structure also superimposed well onto the residues in the catalytic pocket of the hBCDIN3D structure (residues Y37, R257, Q198 and W169, respectively) (Figure 2D, middle). In hBCDIN3D, the OH group of Y37, the Nϵ atom of Q198, the Nϵ atom of W169 and R257 would form hydrogen bonds with the monophosphate oxygen at the 5′-end G−1 of tRNAHis for methylation. In support of the formation of these hydrogen bonds, the Y37F, Y37A, W169F, Q198A and R257A mutants all abolished the enzymatic activity onto the tRNAHis transcript (Figure 2E). At the reaction stage of the methylation of the 5′-phosphate of tRNAHis by hBCDIN3D, the deprotonated and negatively charged α-phosphate oxygen under the neutral pH conditions would be positioned in the proximity of the SAM methyl group, and could nucleophilically attack the positively charged methyl sulfonium moiety of SAM for methylation, as proposed in the mechanism of the γ-phosphate methylation of 7SK RNA by hMePCE (22).
For the methylation of the tRNAHis 5′-α-phosphate, the location of the 5′-nucleoside (G−1) of tRNAHis relative to the catalytic site in hBCDIN3D during the methyl transfer reaction should be closer to the catalytic site than the location of the 5′-nucleoside, G1, of 7SK SL1p observed in the structure of hMePCE complexed with SL1p. The different activities between hBCDIN3D and hMePCE are described later.
A model of tRNAHis docking onto hBCDIN3D
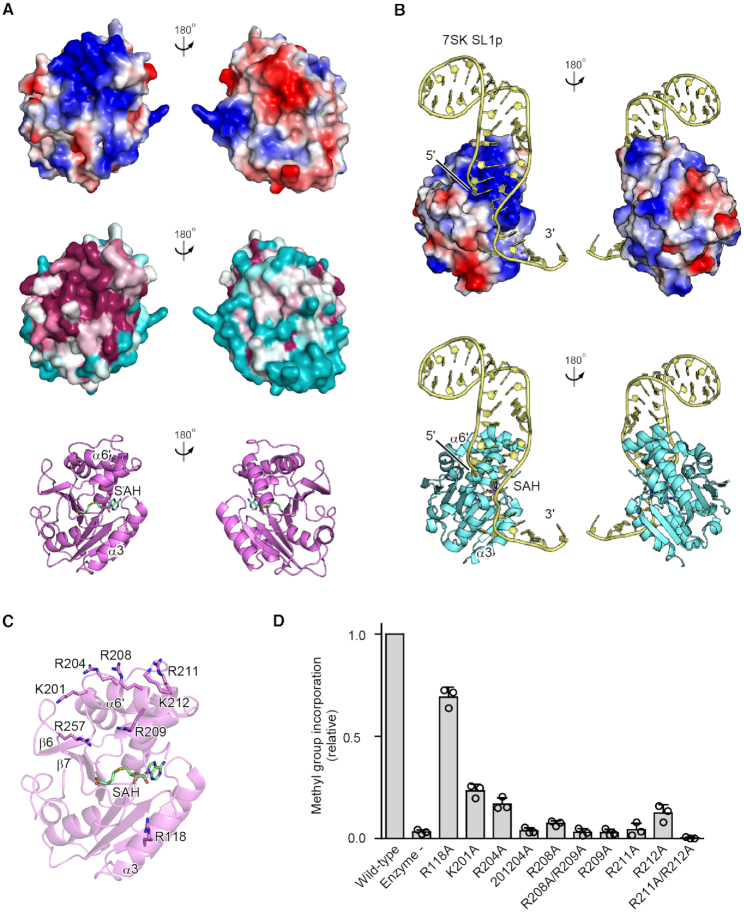
The electrostatic surface potential of hBCDIN3D shows a highly localized positively charged area, which is proximal to the catalytic SAM (SAH) binding pocket (Figure 3A). In particular, the positively charged residues are clustered on α6′ and are well conserved among the BCDIN3D proteins from various organisms (Supplementary Figure S1). The R257 residue in the loop between β6 and β7, and R118 in α3 are also proximal to the active pocket. As described earlier, R257 could interact with the α-phosphate oxygen of the 5′-end of tRNAHis (Figure 2D, right). The recently reported crystal structure of the MTD of hMePCE, complexed with SAH and SL1p with a 5′-Me-ppp cap, showed a similar positively charged area, and the corresponding region in hMePCE, in particular α6′ and α3 (Figures 2A and 3B), participated in the recognition of the 5′ and 3′ parts of 7SK SL1p, respectively (22).
Figure 3.
tRNA binding region in hBCDIN3D. (A) Electrostatic surface potential of hBCDIN3D (top). The positively and negatively charged regions are colored blue and red, respectively. Conservation analysis of hBCDIN3D (middle). Conserved and nonconserved residues are colored purple and cyan, respectively. The positively charged area is highly localized and conserved, and is proximal to the catalytic SAM binding pocket. (B) Electrostatic surface potential of the MTD of hMePCE complexed with 7SK SL1p (yellow) (22). The structure of hMePCE is viewed from the same direction as that of hBCDIN3D superimposed on hMePCE in (A). The positively and negatively charged regions are colored blue and red, respectively, and the distribution of the positively charged area is similar to that of hBCDIN3D. (C) Basic amino acid residues in hBCDIN3D that are possibly involved in the RNA recognition are depicted by sticks. (D) The activities of hBCDIN3D variants relative to the wild type (taken as 1.0) under the standard conditions. The reaction mixtures were incubated at 37°C for 8 min. At this time point, the reaction by wild-type BCDIN3D proceeds in a linear range (Supplementary Figure S2B). Data are presented as mean ± SD of three independent assays.
The K201A/R204A, R209A, R208A/R209A, R211A, R211A/K212A and R257A mutants of hBCDIN3D abolished the methylation activity with the tRNAHis transcript in vitro (Figure 3C and D, Supplementary Figure S2B). The R201A, R204A, R208A and R212A mutants of hBCDIN3D also reduced the methylation activity, to extents of ∼10–20% of the wild-type activity in vitro (Figure 3D). The R118A mutation in α3 modestly reduced the activity, to ∼70% of the wild-type activity. Thus, these residues in hBCDIN3D would participate in the recognition of tRNAHis.
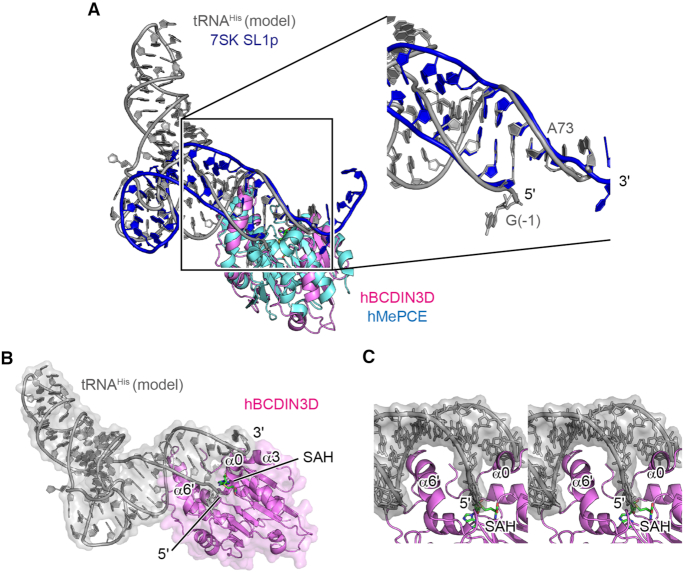
Based on the comparison of the structure of hBCDIN3D with that of hMePCE complexed with 7SK SL1p, and the superimposition of these two structures, a tRNAHis docking model onto hBCDIN3D was built (Figure 4A and B). In the docking model, only the acceptor helix of tRNAHis interacts with hBCDIN3D, and in particular, the basic α6′ helix interacts with the major groove of the acceptor helix (Figure 4C). The N-terminal region, which possibly forms an α-helix upon tRNAHis binding, as described later, interacts with the 3′-single-stranded region and wedges the G−1:A73 mispair at the top of the acceptor helix of tRNAHis. As a result, G−1 of tRNAHis is relocated into the catalytic pocket for 5′-phosphate oxygen methylation, and the 3′-end is shifted toward α3 (Figure 4B and C). This model is consistent with the results showing that mutations of the basic amino acid residues in α6′ reduced the methylation activity onto tRNAHis (Figure 3D). The impact of R118A in α3, which would interact with the 3′-single-stranded part of tRNAHis, is modest (Figure 3D, Supplementary Figure S2B), consistent with the previous data showing that the deletion of the 3′-CCA of tRNAHis has minimal effects on the methylation by hBCDIN3D in vitro (15). Furthermore, mutations of the N-terminal region of BCDIN3D also reduced the methylation activity with tRNAHisin vitro, suggesting the involvement of this N-terminal region in the methylation of the 5′-phosphate of tRNAHis by hBCDIN3D, as described later.
Figure 4.
A model of tRNA docking onto hBCDIN3D. (A) Docking of tRNAHis onto hBCDIN3D. Superimposition of hBCDIN3D (purple) on the structure of the MTD of hMePCE (cyan) complexed with 7SK SL1p (blue). tRNAHis (gray) was modeled such that the acceptor helix of tRNAHis superimposed well onto the 5′-end and 3′-single-stranded region of SL1p. See ‘Materials and Methods’ section for details. (B) α6′ would interact with the major groove of the acceptor helix of tRNAHis, and α0 would wedge the G−1:A73 mispair of tRNAHis. (C) A detailed stereo view of the interaction between the acceptor helix of tRNAHis and BCDIN3D in (B). tRNA is shown as a gray stick model.
Recognition of the top part of the tRNAHis acceptor helix by hBCDIN3D
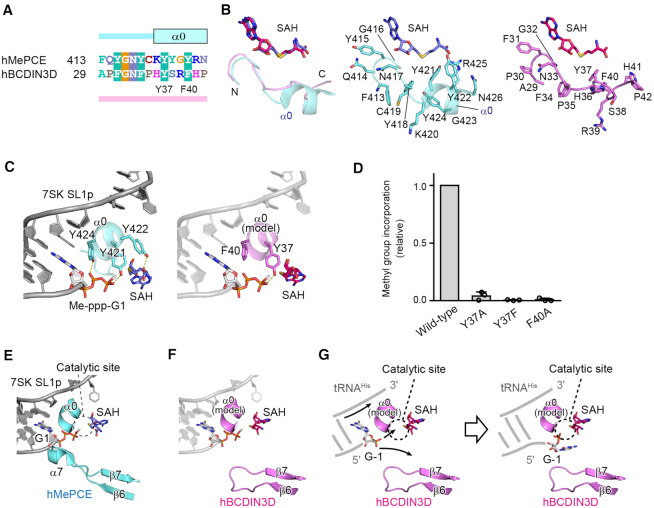
The amino acid sequences in the N-terminal region are conserved among the BCDIN3D proteins from various organisms, as well as among the MePCEs (Supplementary Figure S1, Figures 2A and 5A). In the structure of hMePCE complexed with 7SK SL1p, the corresponding N-terminal region forms an α-helix (α0, Figures 2A and 5B, left and middle), which is enriched with aromatic residues (Y421, Y422 and Y424) and hydrogen bonds with the 5′-triphosphate of G1 of SL1p (Figure 5C, left). In particular, the OH group of Y421 forms a hydrogen bond with the 5′-γ-phosphate oxygen of SL1p, and the OH groups of Y418 and Y424 form hydrogen bonds with the 5′-β-phosphate oxygen of SL1p. The α0 helix of hMePCE interacts with the 3′-single-stranded regions of SL1p through hydrogen bonds and hydrophobic stacking interactions, as well as with SAH (Figures 5B and C, and 6A) (22). However, the α0 helix is not formed in the structure of hMePCE complexed with only SAH (PDB ID: 5UNA), and this is also the case with the structure of hBCDIN3D complexed with SAH (Figures 1A and 5B, right). Thus, the conserved N-terminal region of hBCDIN3D with aromatic rings (Y37 and F40) could also form an α-helix in the presence of the substrate tRNAHis and recognize the 5′-phosphate of tRNAHis and the 3′-single-stranded region of tRNAHis (Figure 5C). Y37 could recognize the 5′-phosphate of tRNAHis during the methylation reaction and Phe40 could stack with the 5′-G−1 nucleoside of tRNAHis. In support of this proposal, the Y37F, Y37A and F40A mutants reduced the methylation activity with the tRNAHis transcript to extents of <10% of that of wild-type hBCDIN3D at the reaction end point (Figure 5D).
Figure 5.
Recognition of the top part of the tRNAHis acceptor helix by BCDIN3D. (A) Sequence alignment of the N-terminal extended regions of the MTD of hMePCE and hBCDIN3D. In the structure of hMePCE complexed with SL1p, the N-terminal region forms an α-helix (α0). (B) Superimposition of the N-terminal region of hBCDIN3D (purple) complexed with SAH onto that of hMePCE (cyan) complexed with SL1p and SAH. The N-terminal region of BCDIN3D does not form an α-helix in the absence of RNA. Detailed views of the N-terminal regions of hMePCE (middle, cyan) and hBCDIN3D (right, purple). (C) Interactions between α0 and 7SK SL1p with the Me-ppp cap and SAH (left). The modeled α0 of BCDIN3D (purple) in the structure of hMePCE complexed with SL1p with the Me-ppp cap and SAH (right). Y37 in hBCDIN3D corresponds to Y421 and would interact with the phosphate group. (D) The activities of hBCDIN3D variants relative to the wild type (taken as 1.0) under the standard conditions. The reaction mixtures were incubated at 37°C for 1 h. Data are presented as mean ± SD of three independent assays. (E) Interactions of α7 between β6 and β7 of hMePCE and the 5′-end of 7SK SL1p with the Me-ppp cap and SAH. (F) The modeled α0 and the shorter loop between β6 and β7 of BCDIN3D with the SL1p, in the structure of hMePCE complexed with SL1p. The region between β6 and β7 of hBCDIN3D is shorter than the corresponding region of hMePCE, which forms α7 and interacts with the 5′-end of SL1p. (G) A possible mechanism of the α-phosphate methylation of tRNAHis by hBCDIN3D. Since an α-helix corresponding to α7 in hMePCE is absent in BCDIN3D, the 5′-monophosphate of G−1 of tRNAHis is deep within the catalytic pocket for 5′-phosphate methylation. α0 would wedge the G−1:A73 mispair at the top of the acceptor helix of tRNAHis.
Figure 6.
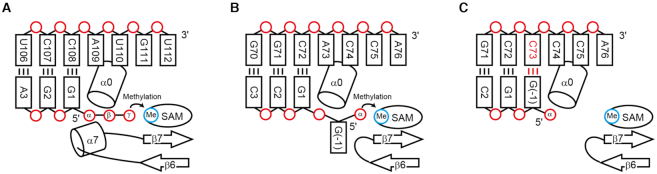
Mechanism of the different activities between MePCE and BCDIN3D. (A) 5′-γ-Phosphate methylation of 7SK RNA by MePCE. (B) 5′-α-Phosphate methylation of G−1 of tRNAHis by BCDIN3D. α0 wedges the G−1:A73 mispair and the 5′-α-phosphate reaches the catalytic site for methylation. (C) tRNAHis with G−1:C73 is not methylated, since α0 would not wedge the base pair, and the 5′-α-phosphate cannot reach the active site for methylation.
In the structure of hMePCE complexed with SAH and 7SK SL1p, the α7 helix between β6 and β7 interacts with the 5′-region of SL1p (Figures 2A and 5E). This interaction relocates the γ-phosphate of 5′-G1 of SL1p to the catalytic position, together with α0 (Figure 5E). On the other hand, the corresponding region between β6 and β7 in hBCDIN3D is five amino acids shorter and forms a short turn, rather than an α-helix (Figures 2A and 5F). The presence of the shorter loop between β6 and β7 of hBCDIN3D and the N-terminal α0 helix could allow the 5′-end G−1, which is unpaired with A73 of tRNAHis, to deeply penetrate the catalytic site. The α-phosphate of G−1 can then reach the catalytic site and become methylated efficiently. The α0 helix could wedge the G−1:A73 mispair at the top of the acceptor stem of tRNAHis (Figure 5G).
DISCUSSION
In this study, we determined the crystal structure of hBCDIN3D complexed with SAH (Figure 1). Recently, we showed that hBCDIN3D catalyzes the monomethylation of the 5′-monophosphate of cytoplasmic tRNAHisin vitro and in vivo, and that BCDIN3D recognizes the structural features of tRNAHis (15,16). It recognizes not only the G−1:A73 mispair at the top of the eight-nucleotide-long acceptor helix, but also the G−1 itself of tRNAHis. Except for tRNAHis, there is no other tRNA species with these unique structural features (29). Thus, we concluded that BCDIN3D is a tRNAHis-specific 5′-phosphate monomethyltransferase (15,16).
Quite recently, the crystal structure of the MTD of the closely related methyltransferase, a methylphosphate capping enzyme (hMePCE) complexed with SAH and an RNA bearing a 5′-Me-ppp cap, was reported (22). MePCE catalyzes the monomethylation of the 5′-γ-phosphate of 7SK RNA. The amino acid sequence of the MTD of hMePCE and its structure are homologous to those of hBCDIN3D (Figure 2A and B). The comparison of the hBCDIN3D and hMePCE structures has now highlighted the mechanism underlying the different activities between these closely related enzymes (Figures 5 and 6).
In hMePCE, the α7 helix between β6 and β7 interacts with the 5′-end G1, which base-pairs with C108, and together with the N-terminal α0 helix, the phosphate group of the γ-position is positioned in the catalytic site for the methyl transfer reaction (Figure 6A). On the other hand, in hBCDIN3D, the region corresponding to α7 in hMePCE has fewer amino acids and forms a short loop. Furthermore, tRNAHis has a G−1:A73 mispair at the top of the acceptor helix. The N-terminal α0 helix wedges the G−1:A73 mispair, and the 5′-α-phosphate of the G−1 of tRNAHis can access the active site for the methyl transfer reaction (Figure 6B). This can explain the previous biochemical data showing that hBCDIN3D exclusively recognizes the G−1:A73 mispair at the top of the acceptor helix of tRNAHis. The tRNAHis mutant with the G−1:C73 pair cannot be methylated by hBCDIN3D (15). Since the α0 helix cannot wedge the G−1:C73 pair at the top of the acceptor helix, the α-phosphate of G−1 of tRNAHis would not be able to access the active site and thus cannot be methylated (Figure 6C). This mechanism is similar to that observed in the formylation of bacterial methionyl-tRNAfMet by methionyl-tRNAfMet formyltransferase (30). The specific loop wedges the C1:A72 mispair at the top of the acceptor helix, characteristic of the initiator tRNA, and the 3′-end of tRNA bends inside the active site.
hBCDIN3D cannot methylate a tRNAHis mutant with a 5′-triphosphate group at its 5′-end in vitro (Supplementary Figure S2C). The 5′-triphosphate group could not enter the catalytic pocket, since there is not enough space to accommodate the triphosphate in the pocket when tRNAHis with a 5′-triphosphate binds to hBCDIN3D in the same manner as tRNAHis with a 5′-monophosphate.
The recognition mechanism of the guanine base of G−1 of tRNAHis by hBCDIN3D is still unclear. The loop between β6 and β7 might specifically recognize the guanine base of G−1. Clarification of the detailed mechanism of the monomethylation of the 5′-monophosphate of tRNAHis awaits the determination of the structure of BCDIN3D complexed with tRNAHis.
The biological role of the 5′-monomethylation of cytoplasmic tRNAHis remains enigmatic. It does not significantly affect the steady-state level of tRNAHis in human cells and fly ovary, and it does not change the aminoacylation efficiency or level by histidyl-tRNA synthetase (12,15). As discussed (15), the 5′-monomethylation of tRNAHis might be involved in tRNAHis fragments’ generation or their stability under stress conditions and participate in various cellular functions beyond their established roles in protein synthesis. BCDIN3D is overexpressed in human breast cancer, and this overexpression is associated with cellular invasion and poor prognosis in triple-negative breast cancer (10,11). Although the molecular basis of the involvement of BCDIN3D in the tumorigenic phenotype of breast cancer has remained elusive, the structure of hBCDIN3D presented in this study will provide useful information for the design of anticancer drugs.
DATA AVAILABILITY
Coordinates and structure factors for the crystal structure of hBCDIN3D complexed with SAH have been deposited in the Protein Data Bank, under the accession code 6L8U.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Yuka Fujimoto for technical assistance. We also thank the beamline staff of BL-17A (KEK, Tsukuba) for technical assistance during data collection.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
JSPS [LS135 (in part) to K.T., 18H03980 to K.T.]; Ministry of Education, Culture, Sports, Science and Technology [26113002 to K.T.]; Takeda Science Foundation; Uehara Memorial Foundation; Terumo Foundation for Life Sciences and Arts; Princess Takamatsu Cancer Research Fund. Funding for open access charge: JSPS [18H03980 to K.T.].
Conflict of interest statement. None declared.
REFERENCES
- 1. Xhemalce B., Robson S.C., Kouzarides T.. Human RNA methyltransferase BCDIN3D regulates microRNA processing. Cell. 2012; 151:278–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhu W., Hanes S.D.. Identification of Drosophila bicoid-interacting proteins using a custom two-hybrid selection. Gene. 2000; 245:329–339. [DOI] [PubMed] [Google Scholar]
- 3. Shumyatsky G.P., Tillib S.V., Kramerov D.A.. B2 RNA and 7SK RNA, RNA polymerase III transcripts, have a cap-like structure at their 5′ end. Nucleic Acids Res. 1990; 18:6347–6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gupta S., Busch R.K., Singh R., Reddy R.. Characterization of U6 small nuclear RNA cap-specific antibodies. Identification of gamma-monomethyl-GTP cap structure in 7SK and several other human small RNAs. J. Biol. Chem. 1990; 265:19137–19142. [PubMed] [Google Scholar]
- 5. Jeronimo C., Forget D., Bouchard A., Li Q., Chua G., Poitras C., Therien C., Bergeron D., Bourassa S., Greenblatt J. et al.. Systematic analysis of the protein interaction network for the human transcription machinery reveals the identity of the 7SK capping enzyme. Mol. Cell. 2007; 27:262–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shuman S. Transcriptional networking cap-tures the 7SK RNA 5′-γ-methyltransferase. Mol. Cell. 2007; 27:517–519. [DOI] [PubMed] [Google Scholar]
- 7. Diribarne G., Bensaude O.. 7SK RNA, a non-coding RNA regulating P-TEFb, a general transcription factor. RNA Biol. 2009; 6:122–128. [DOI] [PubMed] [Google Scholar]
- 8. Peterlin B.M., Brogie J.E., Price D.H.. 7SK snRNA: a noncoding RNA that plays a major role in regulating eukaryotic transcription. Wiley Interdiscip. Rev. RNA. 2012; 3:92–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cosgrove M.S., Ding Y., Rennie W.A., Lane M.J., Hanes S.D.. The Bin3 RNA methyltransferase targets 7SK RNA to control transcription and translation. Wiley Interdiscip. Rev. RNA. 2012; 3:633–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu R., Wang X., Chen G.Y., Dalerba P., Gurney A., Hoey T., Sherlock G., Lewicki J., Shedden K., Clarke M.F.. The prognostic role of a gene signature from tumorigenic breast-cancer cells. N. Engl. J. Med. 2007; 356:217–226. [DOI] [PubMed] [Google Scholar]
- 11. Yao L., Chi Y., Hu X., Li S., Qiao F., Wu J., Shao Z.M.. Elevated expression of RNA methyltransferase BCDIN3D predicts poor prognosis in breast cancer. Oncotarget. 2016; 7:53895–53902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhu L., Liao S.E., Ai Y., Fukunaga R.. RNA methyltransferase BCDIN3D is crucial for female fertility and miRNA and mRNA profiles in Drosophila ovaries. PLoS One. 2019; 14:e0217603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Suzuki T., Ikeuchi Y., Noma A., Suzuki T., Sakaguchi Y.. Mass spectrometric identification and characterization of RNA-modifying enzymes. Methods Enzymol. 2007; 425:211–229. [DOI] [PubMed] [Google Scholar]
- 14. Rosa M.D., Hendrick J.P. Jr., Lerner M.R., Steitz J.A., Reichlin M.. A mammalian tRNAHis-containing antigen is recognized by the polymyositis-specific antibody anti-Jo-1. Nucleic Acids Res. 1983; 11:853–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Martinez A., Yamashita S., Nagaike T., Sakaguchi Y., Suzuki T., Tomita K.. Human BCDIN3D monomethylates cytoplasmic histidine transfer RNA. Nucleic Acids Res. 2017; 45:5423–5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tomita K., Liu Y.. Human BCDIN3D is a cytoplasmic tRNA(His)-specific 5′-monophosphate methyltransferase. Front. Genet. 2018; 9:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kabsch W. XDS. Acta Crystallogr. D: Biol. Crystallogr. 2010; 66:125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McCoy A.J., Grosse-Kunstleve R.W., Adams P.D., Winn M.D., Storoni L.C., Read R.J.. Phaser crystallographic software. J. Appl. Crystallogr. 2007; 40:658–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Waterhouse A., Bertoni M., Bienert S., Studer G., Tauriello G., Gumienny R., Heer F.T., de Beer T.A.P., Rempfer C., Bordoli L. et al.. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 2018; 46:W296–W303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Afonine P.V., Grosse-Kunstleve R.W., Echols N., Headd J.J., Moriarty N.W., Mustyakimov M., Terwilliger T.C., Urzhumtsev A., Zwart P.H., Adams P.D.. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr. D: Biol. Crystallogr. 2012; 68:352–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Emsley P., Lohkamp B., Scott W.G., Cowtan K.. Features and development of Coot. Acta Crystallogr. D: Biol. Crystallogr. 2010; 66:486–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang Y., Eichhorn C.D., Wang Y., Cascio D., Feigon J.. Structural basis of 7SK RNA 5′-gamma-phosphate methylation and retention by MePCE. Nat. Chem. Biol. 2019; 15:132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Doxtader K.A., Wang P., Scarborough A.M., Seo D., Conrad N.K., Nam Y.. Structural basis for regulation of METTL16, an S-adenosylmethionine homeostasis factor. Mol. Cell. 2018; 71:1001.e1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ruszkowska A., Ruszkowski M., Dauter Z., Brown J.A.. Structural insights into the RNA methyltransferase domain of METTL16. Sci. Rep. 2018; 8:5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu R.J., Long T., Li J., Li H., Wang E.D.. Structural basis for substrate binding and catalytic mechanism of a human RNA:m5C methyltransferase NSun6. Nucleic Acids Res. 2017; 45:6684–6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Finer-Moore J., Czudnochowski N., O’Connell J.D. 3rd, Wang A.L., Stroud R.M.. Crystal structure of the human tRNA m(1)A58 methyltransferase-tRNA(3)(Lys) complex: refolding of substrate tRNA allows access to the methylation target. J. Mol. Biol. 2015; 427:3862–3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Song J., Teplova M., Ishibe-Murakami S., Patel D.J.. Structure-based mechanistic insights into DNMT1-mediated maintenance DNA methylation. Science. 2012; 335:709–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Holm L., Rosenström P.. Dali server: conservation mapping in 3D. Nucleic Acids Res. 2010; 38:W545–W549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Juhling F., Morl M., Hartmann R.K., Sprinzl M., Stadler P.F., Putz J.. tRNAdb 2009: compilation of tRNA sequences and tRNA genes. Nucleic Acids Res. 2009; 37:D159–D162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schmitt E., Panvert M., Blanquet S., Mechulam Y.. Crystal structure of methionyl-tRNAfMet transformylase complexed with the initiator formyl-methionyl-tRNAfMet. EMBO J. 1998; 17:6819–6826. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Coordinates and structure factors for the crystal structure of hBCDIN3D complexed with SAH have been deposited in the Protein Data Bank, under the accession code 6L8U.