Abstract
We previously showed that postmortem serum levels of adrenocorticotropic hormone (ACTH) were significantly higher in cases of hypothermia (cold exposure) than other causes of death. This study examined how the human hypothalamic-pituitary-adrenal axis, and specifically cortisol, responds to hypothermia. Human samples: Autopsies on 205 subjects (147 men and 58 women; age 15–98 years, median 60 years) were performed within 3 days of death. Cause of death was classified as either hypothermia (cold exposure, n = 14) or non-cold exposure (controls; n = 191). Cortisol levels were determined in blood samples obtained from the left and right cardiac chambers and common iliac veins using a chemiluminescent enzyme immunoassay. Adrenal gland tissues samples were stained for cortisol using a rabbit anti-human polyclonal antibody. Cell culture: AtT20, a mouse ACTH secretory cell line, and Y-1, a corticosterone secretory cell line derived from a mouse adrenal tumor, were analyzed in mono-and co-culture, and times courses of ACTH (in AtT20) and corticosterone (in Y-1) secretion were assessed after low temperature exposure mimicking hypothermia and compared with data for samples collected postmortem for other cases of death. However, no correlation between ACTH concentration and cortisol levels was observed in hypothermia cases. Immunohistologic analyses of samples from hypothermia cases showed that cortisol staining was localized primarily to the nucleus rather than the cytoplasm of cells in the zona fasciculata of the adrenal gland. During both mono-culture and co-culture, AtT20 cells secreted high levels of ACTH after 10–15 minutes of cold exposure, whereas corticosterone secretion by Y-1 cells increased slowly during the first 15–20 minutes of cold exposure. Similar to autopsy results, no correlation was detected between ACTH levels and corticosterone secretion, either in mono-culture or co-culture experiments. These results suggested that ACTH-independent cortisol secretion may function as a stress response during cold exposure.
Introduction
Many reports have documented the pathologic changes observed in human affected by hypothermia due to cold exposure, and “classic” morphologic findings supporting a diagnosis of hypothermia have been established [1–7]. However, as other etiologies of hypothermia include drug abuse, dementia, malnutrition, and infectious disease, only a few studies have specifically examined pathologic findings after cold exposure [8,9], especially from a biochemical perspective, such as the presence and levels of ketone bodies [10–13]. Furthermore, only a few reports have estimated hormone levels as part of the pathophysiologic findings of cold exposure [14–16].
Generalized hypothermia occurs when the body temperature (Tbody) drops below 35°C relative to exhaustion of heat production and failure of heat loss prevention over time [17]. Five degrees of generalized hypothermia severity have been described: mild (32–35°C), moderate (28–32°C), severe (24–28°C), deep (13–24°C), and irreversible fatal outcome (<13°C) [18]. As Tbody drops, the hypothalamus (thermoregulation center) triggers a series of reactions that function to produce heat and prevent heat loss. Heat production involves the secretion of stress-response hormones (adrenaline, noradrenaline, and cortisol), which triggers an overall increase in lipid metabolism, particularly ketogenesis [1]. The high inter-individual variability with which these regulatory mechanisms are initiated impacts the diversity of hypothermic presentation. The increased production of counter-regulatory hormones, such as cortisol, stimulates production of heat and energy. Despite contrary results regarding adrenal cortex hormone evolution in hypothermia, perimortem elevation of adrenal cortex hormones might reflect more of the decrease in metabolism and hepatic clearance than an increase in ACTH [19–27]. One study examining the agonal process and blood cortisol concentrations found no significant difference between instantaneous death and death with prolonged agony [27]. Other studies recommend analysis of free blood cortisol in cases of suspected hypothermia [19,21,24,28,29]. The primary stress response system is the sympathetic/ adrenomedullary (S/A) system, which includes the chromogranin A [14] and hypothalamic-pituitary-adrenal (HPA) axis [16,30]. Previous studies have suggested that postmortem serum adrenocorticotropic hormone (ACTH) concentration is a useful biomarker of death due to cold exposure and the magnitude of physical stress responses during cold exposure [31]. Increased serum concentrations of ACTH associated with activation of the HPA axis and S/A system can be biochemically evaluated by measuring catecholamine and chromogranin A levels [32–36]. With respect to the HPA axis, it is known that cortisol levels are correlated with ACTH levels, and a precursor of cortisol, which is an activator, also inactivates cortisone accounting for 4–5% and corticosterone exhibiting only weak activity [37,38]. Thus, this study evaluated cortisol as a biomarker of cold exposure-related stress by analyzing cases of human death due to hypothermia. We also assessed the relationship between ACTH and corticosterone levels during cold exposure using a mouse cell culture model.
Material and methods
Autopsy samples
Autopsies were performed within 3 days postmortem at our institute. The study included 205 serial cases (147 men and 58 women), and the median age was 60 years (range 15–98 years). Cortisol levels were determined in blood samples collected aseptically from the left and right cardiac chambers and the common iliac vein using syringes.
Cause of death was determined based on findings from a complete autopsy as well as macromorphological, micropathologic, and toxicologic examinations. Cases were classified as either hypothermia (cold exposure, n = 14) or control. Cause of death in the latter group included blunt injury (n = 37 total; head injury [n = 28], non-head injury [n = 9]), sharp-instrument injury (n = 8), fire fatality (n = 43), asphyxia (n = 28), intoxication (n = 12 total; methamphetamine-related fatality [n = 3], psychotropic drugs [n = 6], other [n = 3]), drowning (n = 12), hyperthermia (heat stroke, n = 10), acute ischemic heart disease (n = 20), and natural causes (n = 22). This study involved autopsy cases from 2010 to 2019. Case profiles are shown in Table 1.
Table 1. Case profile.
| Cause of death | No. | Sex(M/F) | Age (mean) | Survival period (mean, h) | Postmortem period(mean, h) | Hospitalization(M/F) |
|---|---|---|---|---|---|---|
| Hypothermia | 14 | 9/5 | 34-89(62) | 6–24 (18) | 24–72 (52.6) | 0/0 |
| Blunt injury (head injury) | 28 | 19/9 | 15–98 (66) | <0.5–1056 (128.7) | 12–60 (29.3) | 9/6 |
| Blunt injury (non-head injury) | 9 | 9/0 | 52–85 (67) | <0.5–960 (122.6) | 24–60 (30.6) | 4/0 |
| Sharp instrument injury | 8 | 7/1 | 40–85 (67) | <0.5–24 (6.3) | 12–36 (27.4) | 3/1 |
| Fire fatality | 43 | 34/10 | 28–95 (73) | <0.5–3600 (142.4) | 12–60 (27.8) | 6/2 |
| Asphyxia | 29 | 19/10 | 21–83 (57) | <0.5–240 (23.7) | 12–60 (33.4) | 4/2 |
| Intoxicationa | 11 | 8/3 | 25–59 (38) | <0.5–48 (11) | 12–36 (32.7) | 0/1 |
| Drowning | 11 | 7/4 | 44–85 (62) | <0.5–2 (3) | 12–48 (29.6) | 0/0 |
| Hyperthermia | 10 | 3/7 | 28–92 (70) | 6–240 (33.1) | 24–48 (32.7) | 2/1 |
| Acute cardiac death | 20 | 19/1 | 19–88 (61) | <0.5–144 (16.5) | 6–60 (33.6) | 1/1 |
| Other natural death | 22 | 14/8 | 21–88 (70) | <0.5–4320 (243.5) | 24–48 (29.2) | 5/3 |
aMethamphetamine-related fatalities, n = 3; psychotropic drugs, n = 5; others, n = 3
No.: number, M: male, F: female
Cases of hypo- and hyperthermia due to drug abuse and bathing, respectively, were excluded. Postmortem interval was defined as time elapsed from estimated time of death to autopsy, whereas survival period was defined as the time from the onset of fatal insult to death. Only clearly described cases were examined in this study.
Tissue specimens of the bilateral adrenal glands were collected and fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS; pH 7.2) for histopathologic and immunohistochemical analyses.
Biochemical analysis
Blood samples were immediately centrifuged to prepare serum, and ACTH and cortisol levels were measured using an AIA-360®analyzer (TOSOH Bioscience GmbH, Griesheim, Germany) [39,40]. This analyzer utilizes a competitive fluorescent enzyme immunoassay format and is performed entirely within small, single-use test cups containing all necessary reagents. The analyte in the sample competes with the enzyme-labeled hormone and incubated with a fluorogenic substrate, 4-methylumbelliferyl phosphate. The amount of enzyme-labeled hormone that binds to the beads is inversely proportional to the hormone concentration in the test sample. Calibration, daily checks, and maintenance procedures were carried out as described in the Systems Operator’s Manual. Accurate performance data for human ACTH and cortisol, including analyte recovery and dilution studies, had been previously evaluated and were available in the manufacturer’s technical bulletins. The time required to obtain the first result using this assay is 20 minutes, with additional results obtained every minute thereafter.
Serum samples (150 μL each) were placed in the test cups, and both hormones were measured using the above-mentioned immunoassays. The lower (and upper) reported values for the ACTH and cortisol assays were 2.0 (2000.0) pg/mL and 28.0 (1656.0) nmol/L, respectively.
Oxyhemoglobin measurement
Blood oxyhemoglobin was determined using a CO-oximeter system (ABL80FLEX System; Radiometer Corp., Tokyo, Japan) in hypothermia patients [41, 42]. Blood alcohol levels were determined using headspace gas chromatography/mass spectrometry (GC/MS), and amphetamine and psychotropic drugs were detected by GC/MS [31].
Immunohistochemistry
Harvested adrenal glands were fixed in 4% paraformaldehyde in PBS (pH 7.2) for 12 h, embedded in paraffin, and sectioned at a thickness of 4μm. Deparaffinization (Sakura Tissue TEK DRS 2000, Tokyo, Japan) of each section was followed by heat-mediated antigen retrieval in citrate buffer (pH 7.0) for 10 min, after which each section was immersed in 0.3% H2O2-methanol for 10 min to inactivate endogenous peroxidases. After washing in PBS for 5 min, slides were incubated overnight with anti-cortisol-binding globulin antibody (ab107368; Abcam). Immunoreactivity was visualized by the polymer method using Dako Envision+ Dual Link System-HRP (K4063; Dako, CA, USA) and the Dako liquid DAB+ Substrate Chromogen System (K3468; Dako), according to the manufacturer’s instructions and with hematoxylin counterstaining [13,31]. The total number of cells in the adrenal gland and number of cells exhibiting cytoplasmic or nuclear cortisol immunoreactivity were determined microscopically under 400× magnification. Three random fields were independently enumerated, and the data are presented as number of cortisol-positive cells (cytoplasm or nucleus, respectively)/ total number of adrenal gland cells×100. As cells in the zona fasciculata of the adrenal gland are known to produce cortisol in the cytoplasm, immunostaining for cortisol in each group was evaluated by technicians blinded to sample grouping. Three sections were randomly selected for cell counting [43,44].
Cell culture models
Mono-culture models of pituitary and adrenal cells
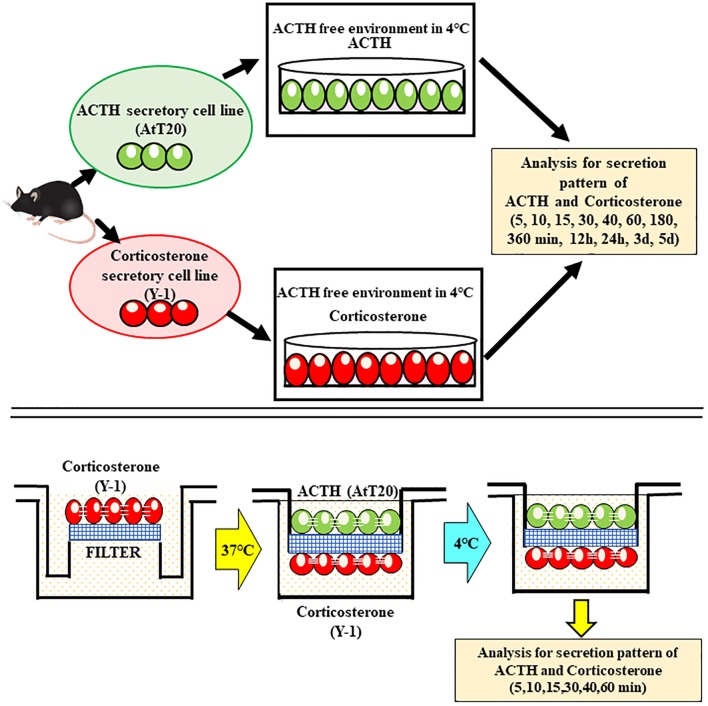
Mono-culture models of ACTH-secreting AtT20 pituitary cells [45–49] and corticosterone-secreting Y-1 adrenal cells [50–54] derived from mice were developed to verify whether these cells secrete hormones only upon stimulation by exposure to cold. AtT-20 was originally induced in a mouse that received ionizing radiation [55] and has been maintained in LAF-1 mice of both sexes (MS) by Dr.Jacob Furth and colleagues [56] since 1957. LAF-1 mice of both sexes (4 to 6 weeks old) were purchased from Jackson laboratory (Bar Harbor, ME, USA). Generally, AtT-20 tumors are maintained in both adrenalectomized and intact mice separately via injection of 106 tumor cells on the back of the neck. Adrenalectomy was performed by making a mid-incision on the back of young animals 2–3 days before tumor grafting. Adrenalectomized animals were provided 0.5% saline as drinking water and injected with 0.5 mg of Percorten (11-deoxycorticosterone acetate, Ciba, USA) every 3 weeks. Establishment of clonal cell line of AtT-20 (Flow Lab. Inc., USA) was complete. AtT-201 K cells used in this study were independently established in our laboratory from a transplanted tumor; thus, AtT-20 animals were designated ‘Ms’ mice. Mouse Y-1 adrenocortical tumor cells (ATCC CCL-79), were established in a male mouse [57–61]. For both cell types (AtT20 and Y-1), the culture medium consisted of a 1:1 ratio of DMEM-F12 and 15% charcoal stripped fetal bovine serum (FBS; Biological Industries, CT., USA) with 4mML-glutamine, 50 U/mL penicillin, and 50μg/ml streptomycin. Initially, cells of both types were seeded and cultured at 37°C. Growth was controlled at 54,618 cells/cm2 for AtT20 and 57,803 cells/cm2 for Y-1, and the cells were allowed to proliferate until they covered the surface of the culture dished. The culture medium for Y-1 cells was replaced once every 2 days. Once the AtT20 and Y-1 cells reached confluence, they were transferred to 4°C and maintained. The amount of ACTH and corticosterone in the culture medium was measured at 5,10,1,20,30,40,60,180, and 360 min; at 12 and 24 h; and at 3 and 5 days. ACTH was measured using a mouse ACTH assay kit (FEK-001-21; Phoenix Pharmaceuticals, Inc., USA) [62,63], and corticosterone was measured using a mouse corticosterone assay kit (Assay MAX EC3001-1; ANG, USA) [45–47]. At the end of the experiment, adherent cells were dissociated from the surface using trypsin and then counted; hormone concentrations were calculated using a correction formula and the measured values.
Co-culture model development
We developed a co-culture system for AtT20 ACTH-secreting cells (ECACC no.87021902) [48,49] and Y-1 corticosterone-secreting cells derived from mice [64–66] as a model of the pituitary-adrenal system. The co-culture model was used to investigate whether these cells interact as part of the HPA axis during cold-stimulated hormone production. Both AtT20 and Y-1 cells were cultured in medium containing DMEM-F12 supplemented with 15% inactivated FBS, 50μg/mL streptomycin, 50 μM penicillin, and 0.25 μg/mL fungizone. To inactivate ACTH present in the culture medium, 0.2 mL of rabbit anti-mouse ACTH (1–24) serum (Siemens, Immulyze) was added to 200 mL of culture medium. The appropriate amount of rabbit serum to add was determined using an ACTH ELISA kit (MDB, M046006) [67].
Initially, both AtT20 and Y-1 cells were cultured separately at 37°C, with AtT20 and Y-1 cells on the top and bottom of the filter, respectively. The cells were then co-cultured at 4°C. The insert for 6-well plate (Greiner Bio-One, Frickenhausen, Germany) used to separate the AtT20 and Y-1 cells had a diameter of 23.1 mm, pore size of 3.0 μm, and pore density of 2×106 pores/cm2. Initially, corticosterone-secreting Y-1 cells were cultured on the bottom of the filter with the filter placed upside down so that the cells formed a mono layer. Subsequently, the filter was placed upright in the culture medium. Schroten H, (2016) established this method in a choroid plexus model [68–72], and we previously described this method in a report on the physiologic significance of the blood-cerebrospinal fluid barrier and prolactin [73].
Excessive growth on the filter was controlled by trypsinization to maintain a single layer of cells; the number of Y-1 cells on the filter was limited to 57,803/cm2. As the Y-1 cells formed tights junctions, movement of ACTH between the cells was prevented. Thereafter, the culture medium was replaced once every 2 days. Once culturing of the Y-1 cells was complete, AtT20 (ACTH secreting) cells were similarly grown on the other side of the filter (i.e., the side opposite to Y-1 cells). The filter was immersed in the culture medium by placing the ACTH-secreting (AtT20) cells side facing up and corticosterone-secreting (Y-1) cells side facing down. Levels of ACTH and corticosterone in the culture medium were measured at 5, 10, 15, 20, 30, 40, and 60 min, as indicated above. After measurement of both hormones, the adherent cells were dissociated from the filter using trypsin and counted. Accurate hormone concentrations were calculated using a correction formula and the measured values.
Statistical analysis
For comparisons between groups, we used the nonparametric Mann-Whitney U test. The Games-Howell test was used for analyses involving multiple comparisons. All analyses were performed using Microsoft Excel and IBM SPSS statistic viewer 24. Lines in each box represent the median, whereas lines outside each box represent the 90% confidence interval. The sensitivity and specificity for distinguishing between two groups using cut-off cortisol values based on blood collection site (i.e., left and right cardiac chambers and common iliac veins) were estimated using receiver operating characteristic (ROC) curve analysis. Areas under the curve were calculated and analyzed using a 1-tailed test. The optimal compromise between sensitivity and specificity was determined graphically.
Ethics statement
This study was evaluated by the Independent Ethics Committee of the Osaka City University Graduate School of Medicine. According to the Independent Ethics Committee of the Osaka City University Graduate School of Medicine, informed consent from opt-out for the autopsy data analysis was approved. (authorization no.4153). The Ethics Committee approval for the study was valid for the period 2010–2019.
Results
Relationship between cortisol levels and sex, age, survival period, and postmortem period
Serum cortisol levels were not associated with postmortem period (left cardiac blood: y = 0.497x+6.8131; R = 0.249; p>0.05, right cardiac blood: y = 0.279x+11.002; R = 0.163; p>0.05, iliac vein: y = 0.299x+11.814; R = 0.152; p>0.05), survival period (left cardiac blood: y = 0.476x+8.18; R = 0.238; p>0.05, right cardiac blood: y = 0.256x+12.505; R = 0.149; p>0.05, iliac vein blood: y = 0.261x+13.9; R = 0.132; p>0.05), sex and related differences, or age.
Sex groups
Males and females did not exhibit significant differences in left cardiac blood, right cardiac blood, or iliac vein blood (p>0.05). Cortisol levels by sex were as follows. Males: left cardiac blood 1.5–154.6 μg/dL; median 20.8 μg/dL, right cardiac blood 1.7–136.7 μg/dL; median 19.1 μg/dL, iliac vein blood 1.1–150.1 μg/dL; median 20.3 μg/dL. Females: left cardiac blood 0.7–284.5 μg/dL; median 30.5 μg/dL, right cardiac blood 1.0–272.9 μg/dL; median 25.2 μg/dL, iliac vein blood 1.3–297.4 μg/dL; median 26.3 μg/dL.
Age
Cortisol levels in each age groups of males and females were as follows. Males: 10s (n = 3): left cardiac blood 1.9–22.4 μg/dL; median 13.0 μg/dL, right cardiac blood 1.4–21.7 μg/dL; median 12.6 μg/dL, iliac vein blood 2–24.8 μg/dL; median 14.8 μg/dL. 20s (n = 7): left cardiac blood 4.6–42.7 μg/dL; median 13.0 μg/dL, right cardiac blood 4.7–13.0 μg/dL, median 13.0 μg/dL, iliac vein blood 6.4–44.7 μg/dL; median 18.3 μg/dL. 30s (n = 7): left cardiac blood 2.7–43.4 μg/dL; median 15.5 μg/dL, right cardiac blood 1.7–33.8 μg/dL; median 11.6 μg/dL, iliac vein blood 0–40.0 μg/dL; median 10.8 μg/dL. 40s (n = 19): left cardiac blood 1.5–55.2 μg/dL, median 15.7 μg/dL, right cardiac blood 1.7–53.2 μg/dL; median 14.1 μg/dL, iliac vein blood 1.1–57.2 μg/dL; median 15.4 μg/dL. 50s (n = 14): left cardiac blood 6.1–154.6 μg/dL; median 40.3 μg/dL, right cardiac blood 8.3–136.7 μg/dL; median 36.9 μg/dL, iliac vein blood 7.8–150.4 μg/dL. 60s (n = 37): left cardiac blood 2.9–60.0 μg/dL; median 17.7 μg/dL, right cardiac blood 3.4–60.0 μg/dL; median 15.5 μg/dL, iliac vein blood 2.9–72.8 μg/dL; median 16.8 μg/dL. 70s (n = 30): left cardiac blood 3.8–86.8 μg/dL; median 24.9 μg/dL, right cardiac blood 3.3–81.5 μg/dL; median 23.7 μg/dL, iliac vein blood 4.5–109.6 μg/dL; median 2.5 μg/dL. 80s (n = 25): left cardiac blood 0–60 μg/dL; median 15.5 μg/dL, right cardiac blood 2.6–60.0 μg/dL; median 16.4 μg/dL, iliac vein blood 0–60 μg/dL; median 14.9 μg/dL. 90s (n = 5): left cardiac blood 11.4–28.7 μg/dL; median 20.9 μg/dL, right cardiac blood 9.4–17.9 μg/dL; median 18.9 μg/dL; iliac vein blood 9.4–17.0 μg/dL; median 17.0 μg/dL. Females: 10s (n = 1): left cardiac blood 2 μg/dL, right cardiac blood 1.9 μg/dL, iliac vein blood 2.4 μg/dL. 20s (n = 3): left cardiac blood 0.7–3.0 μg/dL; median 1.9 μg/dL, right cardiac blood 0.0–1.7 μg/dL; median 0.9 μg/dL, iliac vein blood 1.3–2.4 μg/dL; median 1.7 μg/dL. 30s (n = 2): left cardiac blood 3.0 μg/dL and 3.3 μg/dL, right cardiac blood 1.9 μg/dL and 2.4 μg/dL; iliac vein blood 2.9 μg/dL and 3.8 μg/dL. 40s (n = 6): left cardiac blood 3.3–7.1 μg/dL; median 5.4 μg/dL, right cardiac blood 3.1–6.3 μg/dL; median 4.6 μg/dL, iliac vein blood 3.8–6.9 μg/dL; median 5.0 μg/dL. 50s (n = 7): left cardiac blood 7.6–11.1 μg/dL; median 8.8 μg/dL, right cardiac blood 6.5–8.7 μg/dL; median 8.7 μg/dL, iliac vein blood 3.8–6.9 μg/dL; median 5.0 μg/dL. 60s (n = 3): left cardiac blood 13.1–13.8 μg/dL; median 13.3 μg/dL, right cardiac blood 9.0–11.0 μg/dL; median 10.2 μg/dL, iliac vein blood 10.9–11.4; 11.1 μg/dL. 70s (n = 12): left cardiac blood 13.9–19.4 μg/dL; median 15.9 μg/dL, right cardiac blood 11.5–15.3 μg/dL; median 13.4 μg/dL, iliac vein blood 12.8–16.3 μg/dL; median 14.0 μg/dL. 80s (n = 19): left cardiac blood 19.8–58.2 μg/dL; median 33.0 μg/dL, right cardiac blood 16.1–45.3 μg/dL; median 28.1 μg/dL, iliac vein blood 16.8–60.0 μg/dL; median 29.7 μg/dL. 90s (n = 5) left cardiac blood 86.5–284.5 μg/dL; median 154.9 μg/dL, right cardiac blood 50.8–272.9 μg/dL; median 119.4 μg/dL, iliac vein blood 68.6–297.4 μg/dL; median 131.7 μg/dL.
Postmortem period
Cortisol levels were also classified by postmortem period in each sex as follows (0–24 h, 24–48 h, 48–72 h). Males: 0–24 h: left cardiac blood 1.5–75.3 μg/dL; median 17.0 μg/dL, right cardiac blood 1.7–76.2 μg/dL; median 1.7–76.2 μg/dL; median 15.6 μg/dL, iliac vein blood 1.1–76.9 μg/dL; median 16.8 μg/dL. 24–48 h: left cardiac blood 2.9–154.6 μg/dL; median 24.5 μg/dL, right cardiac blood 2.4–136.7 μg/dL; median 22.2 μg/dL; iliac vein blood 3.0–150.4 μg/dL; median 24.6 μg/dL. 48–72 h: left cardiac blood 0.0–55.2 μg/dL, median 18.8 μg/dL, right cardiac blood 3.3–53.2 μg/dL; median 18.7 μg/dL, iliac vein blood 0.0–57.2 μg/dL; median 16.8 μg/dL. Females: 0–24 h: left cardiac blood 0.0–66.9 μg/dL, median 17.7 μg/dL; right cardiac blood 0.0–50.8 μg/dL; median 16.6 μg/dL, iliac vein blood 1.6–38.7 μg/dL, median 17.7 μg/dL. 24–48 h: left cardiac blood 0.7–284.5 μg/dL; median 29.6 μg/dL, right cardiac blood 1.1.-272.9 μg/dL; median 27.4 μg/dL, iliac vein blood 1.3–297.4 μg/dL; median 31.0 μg/dL. 48–72 h: left cardiac blood 10.2–121.8 μg/dL; median 54.4 μg/dL, right cardiac blood 0.0–99.6 μg/dL; median 30.0 μg/dL, iliac vein blood 0.0–90.4 μg/dL; median 29.7 μg/dL.
Relationship between cortisol levels and collection site
Cortisol levels exhibited a correlation (R = 0.97–0.98) with blood collection site, particularly the left and right cardiac chambers and external iliac vein. A strong correlation was found between left cardiac vein blood, right cardiac vein blood, and iliac vein blood, respectively (left cardiac blood and right cardiac blood: y = 1.06x+0.8; R = 0.982; p<0.01, left cardiac blood and iliac vein blood: y = 0.94x+1.82; R = 0.971; p<0.01, right cardiac blood and iliac vein blood: y = 0.88x+1.18; R = 0.977; p<0.001).
Relationship between cortisol levels and cause of death
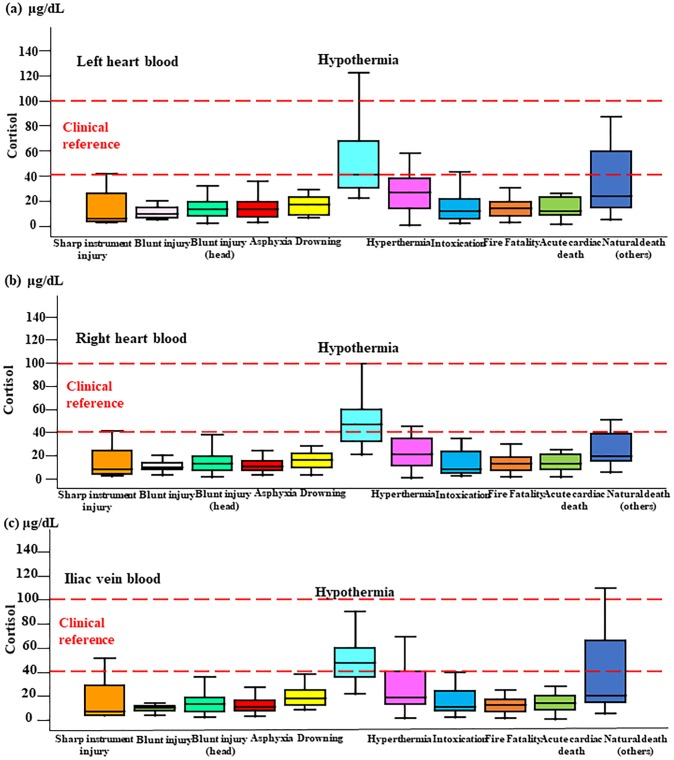
At all blood collection sites, cortisol levels were approximately three times higher in hypothermia cases than in cases involving other causes of death (p<0.05-p<0.0001; Fig 1a–1c). Specifically, serum cortisol levels were significantly higher in cases of death due to hypothermia compared with other causes of death: left cardiac blood, 20–120 μg/dL (median 50 μg/dL; males: 22–111.6 μg/dL (median 49.9 μg/dL), females: 38.3–121.8 μg/dL (median 66.5 μg/dL), p>0.05); right cardiac blood, 20–100 μg/dL (median 50 μg/dL; males: 20.8–88.2 μg/dL (median 45.1 μg/dL), females: 36.5–99.6 μg/dL (median 62.5 μg/dL), p>0.05); iliac vein, 20–130 μg/dL (median 60 μg/dL; males: 22–128 μg/dL (median 49.1 μg/dL), females: 36.5–99.6 μg/dL (median 60.5 μg/dL), p>0.05) versus left cardiac blood, 0–50 μg/dL (median 20 μg/dL; males: 1.5–154.6 μg/dL (median 19.1 μg/dL), females: 0.7–284.5 μg/dL (median 27.0 μg/dL), p>0.05); right cardiac blood, 0–40 μg/dL (median 10 μg/dL; males: 1.7–136.7 μg/dL (median17.5 μg/dL), females: 1.0–272.9 μg/dL (median 21.5 μg/dL), p>0.05); iliac vein, 0–20 μg/dL (median 20 μg/dL; males: 1.1–150.4 μg/dL (median 18.5 μg/dL), females: 1.3–297.4 μg/dL (median 23.6 μg/dL), p>0.05). There was no significant difference in cortisol levels between hypothermia and other causes of death for either males or females. Furthermore, most cases exhibited lower cortisol levels, except in hyperthermia cases (heat stroke: left cardiac blood, 0–60 μg/dL [median 30 μg/dL]; right cardiac blood, 0–42 μg/dL [median 20 μg/dL]; iliac vein, 0–60 μg/dL [median 20 μg/dL]). There was no correlation between ACTH concentration and cortisol in hypothermia cases at any of the collection sites tested (left cardiac blood: Y = 0.0103x+3.237; r = 0.065; p>0.05 versus right cardiac blood: Y = 0.0217x+2.7124; r = 0.113; p>0.05 versus iliac vein: Y = 0.026x+2.4458; r = 0.170; p>0.05).
Fig 1. Cortisol levels in blood collected from three sites.
Cortisol levels by cause of death in the left (a) and right (b) cardiac chambers and the common iliac vein (c).
Sensitivity and specificity cut-off values for distinguishing between groups with higher (hypothermia) and lower (other cause of deaths) cortisol levels were determined using ROC curve analysis and estimated as 30 μg/mL (0.917 and 0.852) for the left cardiac chamber, 25 μg/mL (0.917 and 0.836) for the right cardiac chamber, and 30 μg/mL (0.917 and 0.872) for the common iliac veins.
Cortisol immunopositivity in the adrenal gland
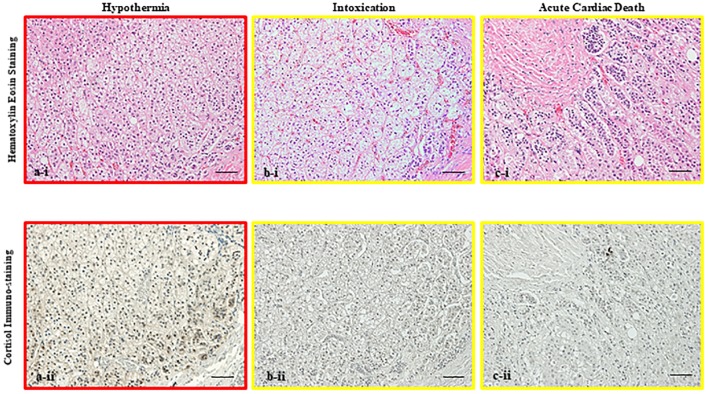
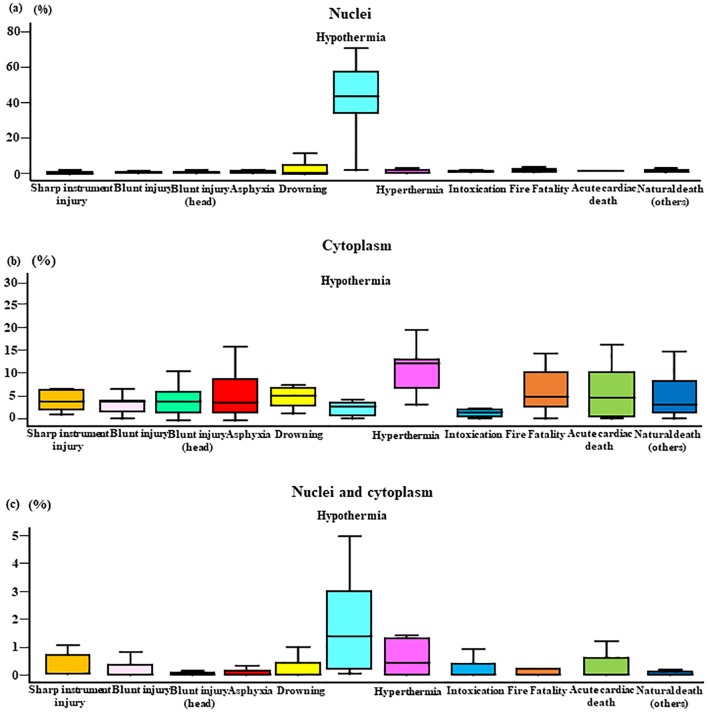
Cortisol immunostaining analysis indicated that in hypothermia cases, cortisol was primarily localized in the nucleus, whereas cortisol staining was predominant in the cytoplasm in cases involving other causes of death (Fig 2a–2c). the Graph in Fig 3a shows the cortisol positivity rate in the nucleus by cause of death. Hypothermia (0–70%, median 50%) cases exhibited significantly higher cortisol positivity rate than the other groups (0–30%, median 5%). The graph in Fig 3b shows the number of cells that were positive for cortisol in the cytoplasm; however, it was not significantly different compared with the nucleus.
Fig 2. Immunostaining of cortisol in the adrenal gland.
Micrographs showing hematoxylin-eosin staining (i) and immunostaining (ii) of cortisol in the adrenal gland in cases of (a) hypothermia (45-years-old male, postmortem period <28 h), (b) intoxication (27-years-old male, postmortem period <20 h) and (c) acute cardiac death (68-years-old male, postmortem period <40 h) (original bar 100 μm, respectively). In hypothermia cases, the cortisol positivity rates in the nucleus and nucleus to cytoplasm were higher than with other cause of death. Cortisol positivity in the nucleus was a characteristic finding in hypothermia cases.
Fig 3. Cortisol positivity rate in the nucleus and cytoplasm by cause of death.
Cortisol immunopositivity in the nucleus (a: hypothermia; p<0.05), cytoplasm (b: hypothermia; p>0.05), and nucleus to cytoplasm (c: hypothermia; p>0.05) ratio by cause of death. Cause of death was classified based on a complete autopsy, and micromorphologic, micropathologic, and toxicologic examinations, as follows: sharp instrument injury (male n = 7, female n = 1), non-head blunt injury (male n = 9, female n = 0), blunt head injury (male n = 19, female n = 9), asphyxia (male n = 19, female n = 10), drowning (male n = 7, female n = 4), hypothermia (male n = 9, female n = 5), hyperthermia (male n = 3, female n = 7), intoxication (male n = 8, female n = 3), fire fatality (male n = 34, female n = 10), acute cardiac death (male n = 19, female n = 1), and natural death (male n = 14, female n = 8).
Mono-culture model
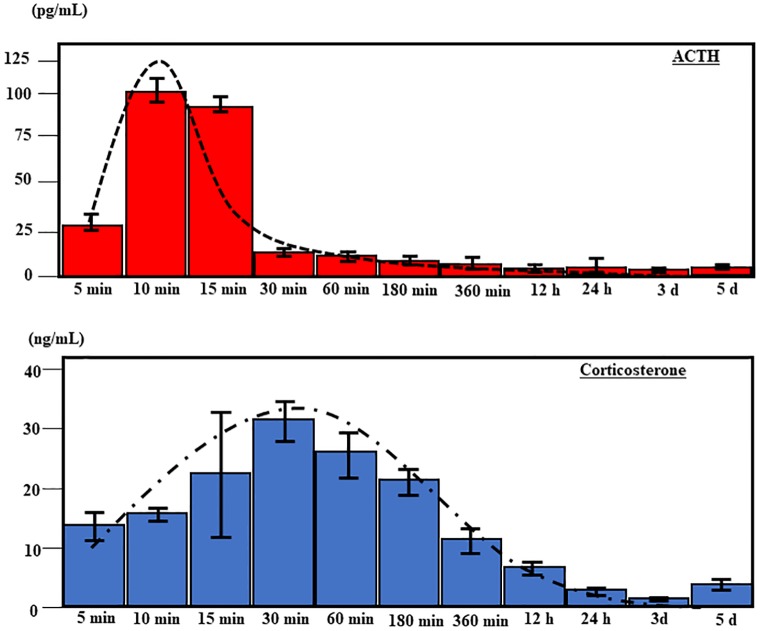
In the mono-culture models, ACTH- and corticosterone-secreting cells were cultured separately at 4°C to ensure the absence of ACTH in the culture of corticosterone-secreting Y-1 cells (Fig 4a). AtT20 cells secreted ACTH after 10~15 min of cold exposure (10 min: median 120 pg/mL; 15 minutes: median 100 pg/mL), which subsequently decreased by 30 min (median 15 pg/mL) (Fig 5a). Corticosterone secretion by Y-1 cells increased slowly during the first 30 min of cold exposure (median 30 ng/mL) and subsequently decreased by 60–180 min (60 min: median 25 ng/mL; 180 min: median 20 ng/mL) (Fig 5b). However, cell culture studies did not reveal a correlation between ACTH and corticosterone secretion in mono-culture experiments, and these results thus suggest that corticosterone secretion after cold exposure is independent of ACTH (Fig 5c).
Fig 4. Mono- and co- culture of ACTH-(AtT20) and corticosterone-secreting (Y-1) cells.
Schematic illustration of mono-culture (a) and co-culture (b) models of pituitary and adrenal gland cells.
Fig 5. Patterns of ACTH (AtT20) and corticosterone (Y-1) secretion over time.
ACTH (a) and corticosterone (b) concentrations over time under cold conditions (4°C) in mono-culture.
Pituitary–adrenal cell co-culture model
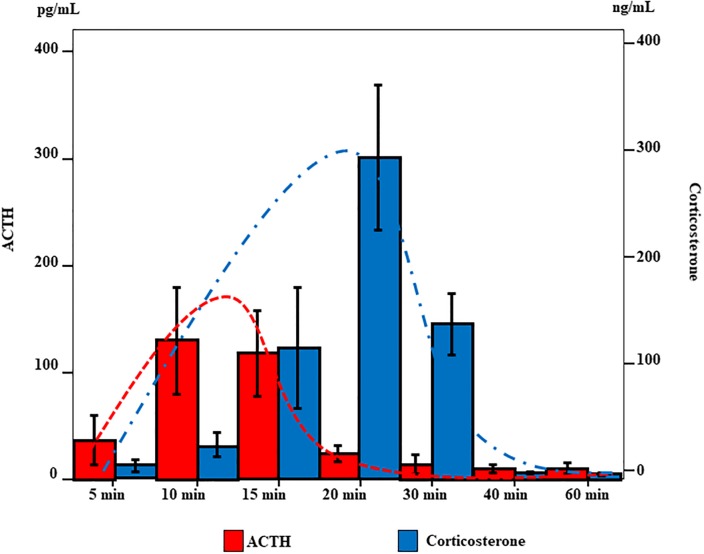
In the co-culture model (Fig 4b), ACTH secretion peaked at 10~15 min (10 min: median 130 pg/mL; 15 min: median 120 pg/mL) and slowly decreased from 20 min onwards (median 20 pg/mL). Corticosterone levels slowly increased beginning at 10 min (median 30 ng/mL), peaked at 20 min (median 300 ng/mL), and decreased after 30 min (median 150 ng/mL) (Fig 6). These co-culture results suggest that corticosterone secretion is ACTH independent, as seen in mono-culture experiments (Fig 7).
Fig 6. Secretion of ACTH and corticosterone over time in co-culture of AtT20 and Y-1 cells.
Concentrations of ACTH and corticosterone over time in co-culture of ACTH- (AtT20) and corticosterone-secreting (Y-1) cells under cold conditions (4°C).
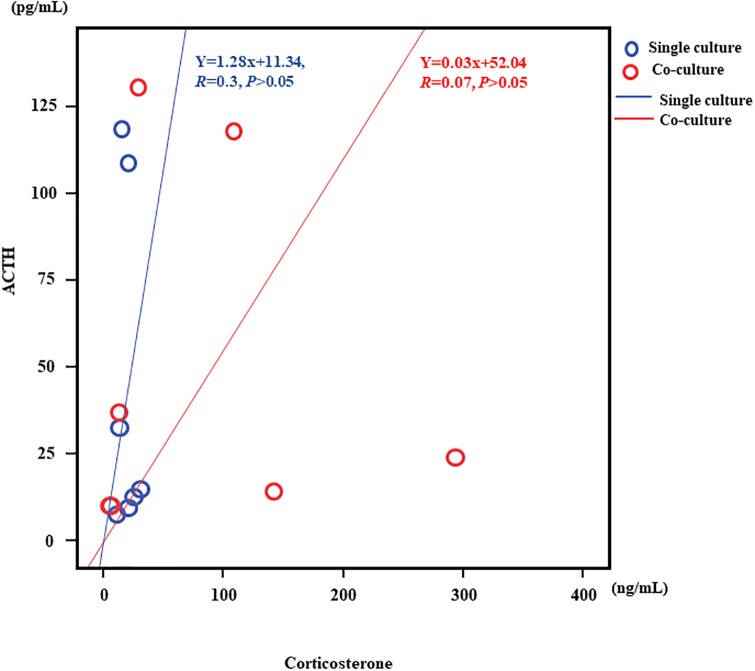
Fig 7. Correlation of ACTH and Corticosterone in mono-culture and co-culture.
The correlation between ACTH and corticosterone levels in mono-culture. The mono-culture study demonstrated that corticosterone secretion following cold exposure is independent of ACTH (Y = 1.28x+11.34, r = 0.3, p>0.05). In co-culture the correlation between ACTH and corticosterone levels results demonstrated that corticosterone secretion following cold exposure is independent of ACTH (Y = 0.03x+52.04, r = 0.07, p>0.05).
Discussion
The correlation between cortisol levels and blood collection site in the present study suggests there were differences in cortisol levels at the various collection sites tested. Therefore, we assessed the relationship between cortisol levels in blood collected from each site and cause of death and found that cortisol levels in cases of hypothermia were three times higher than those in other causes of death. As stress hormones, ACTH and cortisol may exhibit regional differences in terms of increases with an acute change in survival period. However, ACTH and cortisol levels were highly correlated in the blood. It was thought that this result was associated with death characterized by a long survival period rather than sudden death due to causes such as acute cardiac death, based on the respective half-lives of ACTH and cortisol. No significant correlations were observed between cortisol levels and causes of death other than hypothermia. There was no correlation between ACTH concentration and cortisol levels in hypothermia, suggesting that cortisol can be produced by the adrenal gland during cold stress without stimulation by ACTH.
We cultured an adrenal cortex at low temperature using culture fluid that did not contain ACTH. An increase in ACTH-independent corticosterone was observed. We did not use cells cultured from human samples for this experiment because the cells that normally secrete ACTH and cortisol in humans have not been established. We therefore used a mouse cell culture mode. Such ACTH-independent production of cortisol might be protective during prolonged (but not acute) periods of cold stress, as cold exposure promotes glucose production [74]. Recently, Turk EE. suggested that the most-relevant to date are urinary catecholamines and their O-methylated metabolites, urinary free cortisol, blood cortisol, as well as blood, vitreous humor, and pericardial fluid for ketone bodies and free fatty acids. These biomarkers are increased in response to either cold-associated stress or bioenergetic ketogenesis crisis and significantly contribute to the diagnosis by excluding death by hypothermia, perimortem elevation of adrenal cortex hormones might reflect more of a decrease in metabolism and hepatic clearance than an increase in ACTH [75–84]. One study examining the agonal process and blood cortisol concentrations found no significant difference between instantaneous death and death with prolonged agony (e.g., subdural hematoma) [84]. Some researchers recommend analysis of free urinary cortisol and blood cortisol in suspected cases of hypothermia [74,85–88]. Importantly, micromorphologic changes in hormone expression in the adrenal cortex appear to be important for cold-induced cortisol secretion.
Cortisol is produced primarily in the zona fasciculata of the adrenal gland. Cell counts and nuclear and cytoplasm staining by technicians blinded to cause of death showed that during hypothermia, cortisol staining was primarily localized in the nucleus rather than the cytoplasm. Furthermore, nuclear staining of cortisol was significantly greater in cases of hypothermia than cases involving other causes of death, whereas no significant difference between groups was noted in terms of cytoplasmic staining. These findings support studies showing that glucocorticoid receptors are inactive in the cytoplasm, as they are complexed with other proteins [89]. When glucocorticoid bind, they become active dimers, move into the nucleus, and promote transcription. Here, we found high levels of cortisol staining in the nucleus during cold exposure. Considered together, these observations suggest that cortisol is secreted in large quantities in response to the stress of cold exposure and that re-uptake might also occur [90–92].
In this study, we used a novel co-culture system to assess ACTH and corticosterone secretion secondary to cold stimulation. Experiments involving cultured cells are generally initiated at the point each hormone is at a stable concentration. Thus, increases after initiation of the experiment are significant. The increased levels of ACTH and cortisol in the blood following cold exposure did not differ by blood collection site. This suggested that the survival period of the case involving death due to cold exposure was long and that cortisol levels were stable at each blood sampling site. In contrast, the cell culture experiment used medium that did not contain ACTH. This experiment was started after the number of cells in the culture flask was stable. Thus, the point at which measurements began was not the start time of ACTH secretion from ACTH-producing cells after cold exposure. In other words, in the cell culture model experiment, the increases in ACTH and corticosterone production after the start of measurement were significant.
We demonstrated that ACTH and corticosterone secretion levels and patterns differed and were not correlated. Mono-culture of ACTH- and corticosterone-secreting cells under ACTH-free conditions at 4°C resulted in a sudden peak in ACTH at 10 min that decreased after 30 min. This can be explained by the half-life of mouse ACTH [93]. However, in an ACTH-free environment, the increase in corticosterone was lower than that seen under co-culture conditions, and there was no correlation between corticosterone and ACTH levels. These results suggest that cold exposure leads to independent increased secretion of cortisol. In general, metabolism slows with cold exposure. In our experimental findings, the increase in cortisol levels in individuals who died following cold exposure was suggestive of both immune system suppression and stress. However, as the increase in cortisol was ACTH-independent, there was no experimental system we could use to determine whether the increase in cortisol was associated with immune system suppression or stress. The data suggest that the immune system was suppressed due to stress when we consider that there was a time lag in the cell culture experiment between the increase in ACTH and production of corticosterone following cold exposure.
There are some limitations to this study. The correlation between ACTH and corticosterone levels in mouse cell culture may differ from that observed in human autopsy examples. The half-life of hormones may also differ in the cell culture models and in humans. Furthermore, it is necessary to examine differences between human cortisol and mouse corticosterone and address problems associated with temperature setting in the cell culture model [94].
In conclusion, the present study showed that serum cortisol level can be used as a biomarker for cold exposure and that cortisol production in response to cold stress does not depend on ACTH-based activation. As immunostaining for cortisol revealed high expression levels in the nucleus after cold exposure, it is possible that cortisol production following cold exposure is independent of ACTH stimulation.
Supporting information
Relationship between the cortisol level in blood collected at different sites and sex (a), age (b), survival period (c), and postmortem period (d) in all cases. The difference in males and females was associated with the difference in number. However, in terms of age, survival period, and postmortem period, there was a correlation, so the number did not affect the cortisol level. There was no difference between males and females in terms of collection site.
(PPTX)
Left and right cardiac blood (a), left cardiac blood-iliac vein blood (b), and right cardiac blood-iliac vein blood (c).
(PPTX)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Mant A. Some post-mortem observations in accidental hypothermia. Med Sci Law. 1964;4: 44–6. 10.1177/002580246400400111 [DOI] [PubMed] [Google Scholar]
- 2.Hirvonen J. Necropsy findings in fatal hypothermia cases. Forensic Sci. 1976;8: 155–64. 10.1016/0300-9432(76)90059-5 [DOI] [PubMed] [Google Scholar]
- 3.Krjukoff A. Beitag zur Frage des Todes durch Erfrieren. Vjschr Gerichtl Med. 1914;47: 79–101 [Google Scholar]
- 4.Mizukami H, Shimizu K, Shiono H, Uezono T, Sasaki M. Forensic diagnosis of death from cold. Leg Med (Tokyo). 1999;1: 204–9. [DOI] [PubMed] [Google Scholar]
- 5.Dirnhofer VR, Sigrist TH. Muskelblutungen im Koerperkern-ein Zeichen vitaler Reaktion beim Tod durch Unterkuehlung? Beitr Gerichte Med. 1978;37: 159–66 [PubMed] [Google Scholar]
- 6.Schneider V, Klung E. Tod durch Unterkuehlung-Gibt es neue Gesichtspunkte zur Diagnostik? Z Rechtsmed. 1980;86: 59–69. [DOI] [PubMed] [Google Scholar]
- 7.Ishikawa T, Miyaishi S, Tachibana T, Ishizu H, Zhu BL, Maeda H. Fatal hypothermia related vacuolation of hormone-producing cells in the anterior pituitary. Leg Med (Tokyo). 2004;6: 157–63. [DOI] [PubMed] [Google Scholar]
- 8.Tani N, Michiue T, Chen JH, Oritani S, Ishikawa T. Usefulness of postmortem biochemistry in identification of ketosis: Diagnosis of ketoacidosis at the onset of autoimmune type 1 diabetes in an autopsy case with cold exposure and malnutrition. Leg Med (Tokyo). 2016;22: 23–9. [DOI] [PubMed] [Google Scholar]
- 9.Byard RW, Langlois NEI. Wandering Dementia-A Syndrome with Forensic Implications. J Forensic Sci. 2019;64: 443–5. 10.1111/1556-4029.13885 [DOI] [PubMed] [Google Scholar]
- 10.Rousseau G, Reynier P, Jousset N, Rougé-Maillart C, Palmiere C. Updated review of postmortem biochemical exploration of hypothermia with a presentation of standard strategy of sampling and analyses. Clin Chem Lab Med. 2018;56: 1819–27. 10.1515/cclm-2018-0153 [DOI] [PubMed] [Google Scholar]
- 11.Tani N, Michiue T, Chen JH, Oritani S, Ishikawa T. Usefulness of postmortem biochemistry in identification of ketosis: Diagnosis of ketoacidosis at the onset of autoimmune type 1 diabetes in an autopsy case with cold exposure and malnutrition. Leg Med (Tokyo). 2016;22: 23–9. [DOI] [PubMed] [Google Scholar]
- 12.Palmiere C, Tettamanti C, Augsburger M, Burkhardt S, Sabatasso S, Lardi C, et al. Postmortem biochemistry in suspected starvation-induced ketoacidosis. J Forensic Leg Med. 2016;42: 51–5. 10.1016/j.jflm.2016.04.013 [DOI] [PubMed] [Google Scholar]
- 13.Palmiere C, Teresiński G, Hejna P. Postmortem diagnosis of hypothermia. Int J Legal Med. 2014;128: 607–14. 10.1007/s00414-014-0977-1 [DOI] [PubMed] [Google Scholar]
- 14.Yoshida C, Ishikawa T, Michiue T, Quan L, Maeda H. Postmortem biochemistry and immunohistochemistry of chromogranin A as a stress marker with special regard to fatal hypothermia and hyperthermia. Int J Legal Med. 2011;125: 11–20. 10.1007/s00414-009-0374-3 [DOI] [PubMed] [Google Scholar]
- 15.Chen JH, Michiue T, Ishikawa T, Maeda H. Molecular pathology of natriuretic peptides in the myocardium with special regard to fatal intoxication, hypothermia, and hyperthermia. Int J Legal Med. 2012;126: 747–56. 10.1007/s00414-012-0732-4 [DOI] [PubMed] [Google Scholar]
- 16.Ishikawa T, Michiue T, Maeda H. Evaluation of postmortem serum and cerebrospinal fluid growth hormone levels in relation to the cause of death in forensic autopsy. Hum Cell. 2011;24: 74–7. 10.1007/s13577-011-0012-5 [DOI] [PubMed] [Google Scholar]
- 17.Briot R, Menthonnex E, Brun J, Anglade D, Girardet P, Jacquot C. Hypothermies accidentelles. EMC (Elsevier Masson SAS, Paris), Médecine d’urgence, 25-030-D-20, 2007.
- 18.Peek GJ, Davis PR, Ellerton JA. Management of severe accidental hypothermia In: Vincent JL, editor. Yearbook of intensive care and emergency medicine. Springer, New York, NY, 2008: 147–59. [Google Scholar]
- 19.Danzl DF, Pozos RS, Auerbach PS, Glazer S, Goetz W, Johnson E, et al. Multicenter hypothermia survey. Ann Emerg Med. 1987;16: 1042–55. 10.1016/s0196-0644(87)80757-6 [DOI] [PubMed] [Google Scholar]
- 20.Jurkovich GJ, Greiser WB, Luterman A, Curreri PW. Hypothermia in trauma victims: an ominous predictor of survival. J Trauma. 1987;27: 1019–24. [PubMed] [Google Scholar]
- 21.Vassal T, Benoit-Gonin B, Carrat F, Guidet B, Maury E, Offenstadt G. Severe accidental hypothermia treated in an ICU: prognosis and outcome. Chest. 2001;120: 1998–2003. 10.1378/chest.120.6.1998 [DOI] [PubMed] [Google Scholar]
- 22.Bright FM, Winskog C, Walker M, Byard RW. A comparison of hypothermic deaths in South Australia and Sweden. J Forensic Sci. 2014;59: 983–5. 10.1111/1556-4029.12451 [DOI] [PubMed] [Google Scholar]
- 23.Meiman J, Anderson H, Tomasallo C, Centers for Disease Control and Prevention (CDC). Hypothermia-related deaths–Wisconsin, 2014, and United States, 2003–2013. Morb Mortal Wkly Rep. 2015;64: 141–3. [PMC free article] [PubMed] [Google Scholar]
- 24.Turk EE. Hypothermia. Forensic Sci Med Pathol. 2010;6: 106–15. 10.1007/s12024-010-9142-4 [DOI] [PubMed] [Google Scholar]
- 25.Palmiere C, Teresiński G, Hejna P. Postmortem diagnosis of hypothermia. Int J Legal Med. 2014;128: 607–14. 10.1007/s00414-014-0977-1 [DOI] [PubMed] [Google Scholar]
- 26.Nixdorf-Miller A, Hunsaker DM, Hunsaker JC. Hypothermia and hyperthermia medicolegal investigation of morbidity and mortality from exposure to environmental temperature extremes. Arch Pathol Lab Med. 2006;130: 1297–304. 10.1043/1543-2165(2006)130[1297:HAHMIO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 27.Lim C, Duflou J. Hypothermia fatalities in a temperate climate: Sydney, Australia. Pathology (Phila). 2008;40: 46–51. [DOI] [PubMed] [Google Scholar]
- 28.Hirvonen J. Necropsy findings in fatal hypothermia cases. Forensic Sci. 1976;8: 155–64. 10.1016/0300-9432(76)90059-5 [DOI] [PubMed] [Google Scholar]
- 29.Nara A, Nagai H, Yamaguchi R, Makino Y, Chiba F, Yoshida K, et al. An unusual autopsy case of lethal hypothermia exacerbated by body lice-induced severe anemia. Int J Legal Med. 2016;130: 765–9. 10.1007/s00414-015-1266-3 [DOI] [PubMed] [Google Scholar]
- 30.Ishikawa T, Michiue T, Zhao D, Komatsu A, Azuma Y, Quan L, et al. Evaluation of postmortem serum and cerebrospinal fluid levels of thyroid-stimulating hormone with special regard to fatal hypothermia. Leg Med (Tokyo). 2009;11 Suppl 1: S228–30. [DOI] [PubMed] [Google Scholar]
- 31.Ishikawa T, Quan L, Li DR, Zhao D, Michiue T, Hamel M, et al. Postmortem biochemistry and immunohistochemistry of adrenocorticotropic hormone with special regard to fatal hypothermia. Forensic Sci Int. 2008;179: 147–51. 10.1016/j.forsciint.2008.04.023 [DOI] [PubMed] [Google Scholar]
- 32.Hervet T, Teresiński G, Hejna P, Descloux E, Grouzmann E, Palmiere C. Catecholamines and their O-methylated metabolites in vitreous humor in hypothermia cases. Forensic Sci Med Pathol. 2016;12: 163–9. 10.1007/s12024-016-9764-2 [DOI] [PubMed] [Google Scholar]
- 33.Ishikawa T, Inamori-Kawamoto O, Quan L, Michiue T, Chen JH, Wang Q, et al. Postmortem urinary catecholamine levels with regard to the cause of death. Leg Med (Tokyo). 2014;16: 344–9 [DOI] [PubMed] [Google Scholar]
- 34.Ishikawa T, Quan L, Michiue T, Kawamoto O, Wang Q, Chen JH, et al. Postmortem catecholamine levels in pericardial and cerebrospinal fluids with regard to the cause of death in medicolegal autopsy. Forensic Sci Int. 2013;228(1–3): 52–60. 10.1016/j.forsciint.2013.02.008 [DOI] [PubMed] [Google Scholar]
- 35.Miller WL. The Hypothalamic-Pituitary-Adrenal Axis: A Brief History. Horm Res Paediatr. 2018;89: 212–23. 10.1159/000487755 [DOI] [PubMed] [Google Scholar]
- 36.Peeters B, Boonen E, Langouche L, Van den Berghe G. The HPA axis response to critical illness: New study results with diagnostic and therapeutic implications. Mol Cell Endocrinol. 2015;408: 235–40. 10.1016/j.mce.2014.11.012 [DOI] [PubMed] [Google Scholar]
- 37.Inada M, Iwasaki K, Imai C, Hashimoto S. Spironolactone effective hypertension in the elderly due to 11beta-hydroxysteroid dehydrogenase type 2 (11beta-HSD2) impairment: contributory role of determining serum cortisol/cortisone ratio as a marker of 11beta-HSD2 activity. Intern Med. 2008;47: 2157–64. 10.2169/internalmedicine.47.1165 [DOI] [PubMed] [Google Scholar]
- 38.Yanase T, Imai T, Simpson ER, Waterman MR. Molecular basis of 17α-hydroxylase/17,20-lyase deficiency. J Steroid Biochem Mol Biol. 1992;43: 973–9. 10.1016/0960-0760(92)90325-D [DOI] [PubMed] [Google Scholar]
- 39.Higgs P, Costa M, Freke A, Papasouliotis K. Measurement of thyroxine and cortisol in canine and feline blood samples using two immunoassay analysers. J Small Anim Pract. 2014;55: 153–9. 10.1111/jsap.12181 [DOI] [PubMed] [Google Scholar]
- 40.Irvine KL, Burt K, Hill AJ, Shaw S, Papasouliotis K. Initial analytic quality assessment and method comparison of an immunoassay for adrenocorticotropic hormone measurement in equine samples. Vet Clin Pathol. 2016;45: 154–63. 10.1111/vcp.12326 [DOI] [PubMed] [Google Scholar]
- 41.Yajima D, Asari M, Okuda K, Maseda C, Yamada H, Ichimaru C, et al. An objective approach using three indexes for determining fatal hypothermia due to cold exposure; statistical analysis of oxyhemoglobin saturation data. Leg Med (Tokyo). 2015;17: 451–8. [DOI] [PubMed] [Google Scholar]
- 42.Kanto-Nishimaki Y, Saito H, Watanabe-Aoyagi M, Toda R, Iwadate K. Investigation of oxyhemoglobin and carboxyhemoglobin ratios in right and left cardiac blood for diagnosis of fatal hypothermia and death by fire. Leg Med (Tokyo). 2014;16: 321–5. [DOI] [PubMed] [Google Scholar]
- 43.Rege J, Nakamura Y, Wang T, Merchen TD, Sasano H, Rainey WE. Transcriptome profiling reveals differentially expressed transcripts between the human adrenal zona fasciculata and zona reticularis. J Clin Endocrinol Metab. 2014;99: E518–27. 10.1210/jc.2013-3198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fallo F, Castellano I, Gomez-Sanchez CE, Rhayem Y, Pilon C, Vicennati V, et al. Histopathological and genetic characterization of aldosterone-producing adenomas with concurrent subclinical cortisol hypersecretion: a case series. Endocrine. 2017;58: 503–12. 10.1007/s12020-017-1295-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakane T, Tsai J, Audhya T, Brown C, Kardos P, Hollander CS. Growth hormone-releasing factor releases ACTH from an AtT-20 mouse pituitary tumor cell line but not from normal pituitary cells. Life Sci. 1987;40: 2161–7. 10.1016/0024-3205(87)90006-3 [DOI] [PubMed] [Google Scholar]
- 46.Sato E, Maeda Y, Sato Y, Hinata A, Gomi H, Koga D, et al. Culture in 10% O. Biochem J. 2019;476: 827–42. [DOI] [PubMed] [Google Scholar]
- 47.Kameda H, Yamamoto M, Tone Y, Tone M, Melmed S. Proton Sensitivity of Corticotropin-Releasing Hormone Receptor 1 Signaling to Proopiomelanocortin in Male Mice. Endocrinology. 2019;160: 276–91. 10.1210/en.2018-00920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Buonassisi V, Sato G, Gohen AI. Hormone-producing cultures of adrenal and pituitary tumor origin. Proc Natl Acad Sci U S A. 1962;48: 1184–90. 10.1073/pnas.48.7.1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Furth J, Gadsen EL, Upton AC. ACTH secreting transplantable pituitary tumors. Proc Soc Exp Biol Med. 1953;84: 253–4. 10.3181/00379727-84-20607 [DOI] [PubMed] [Google Scholar]
- 50.Costa ET, Forti FL, Rocha KM, Moraes MS, Armelin HA. Molecular mechanisms of cell cycle control in the mouse Y1 adrenal cell line. Endocr Res. 2004;30: 503–9. 10.1081/erc-200043600 [DOI] [PubMed] [Google Scholar]
- 51.Murofushi H, Kotani S, Aizawa H, Maekawa S, Sakai H. Comparison of a major heat-stable microtubule-associated protein in HeLa cells and 190-kDa microtubule-associated protein in bovine adrenal cortex. J Biochem. 1987;102: 1101–12. 10.1093/oxfordjournals.jbchem.a122148 [DOI] [PubMed] [Google Scholar]
- 52.Yasumura Y, Buonassisi V, Sato G. Clonal analysis of differentiated function in animal cell cultures. I. Possible correlated maintenance of differentiated function and the diploid karyotype. Cancer Res. 1966;26: 529–35. [PubMed] [Google Scholar]
- 53.Cohen AI, Bloch E, Celozzi E. In vitro response of functional experimental adrenal tumors to corticotropin ACTH. Proc Soc Exp Biol Med. 1957;95: 304–9. 10.3181/00379727-95-23202 [DOI] [PubMed] [Google Scholar]
- 54.Cohen AI, Furth J, Buffett RF. Histologic and physiologic characteristics of hormone-secreting transplantable adrenal tumors in mice and rats. Am J Pathol. 1957;33: 631–51. [PMC free article] [PubMed] [Google Scholar]
- 55.Ito A, Yanagihara K, Matsuda M. Cellular injury of adrenocorticotropic pituitary tumor (AtT-20) by corticosteroid in mice. Gan. 1978;69: 525–31. [PubMed] [Google Scholar]
- 56.Bahn R, Furth J, Anderson E, Gadsden E. Morphologic and functional changes associated with transplantable ACTH producing pituitary tumors of mice. Am J Pathol. 1957;33: 1075–97. [PMC free article] [PubMed] [Google Scholar]
- 57.Yasumura Y, Buonassisi V, Sato G. Clonal analysis of differentiated function in animal cell cultures. I. Possible correlated maintenance of differentiated function and the diploid karyotype. Cancer Res. 1966;26: 529–35. [PubMed] [Google Scholar]
- 58.Bunassisi V, Sato G, Cohen AI. Hormone-producing cultures of adrenal and pituitary tumor origin. Proc Natl Acad Sci U S A. 1962;48: 1184–90. 10.1073/pnas.48.7.1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Furth J, Gadsen EL, Upton AC. ACTH secreting transplantable pituitary tumors. Proc Soc Exp Biol Med. 1953;84: 253–4. 10.3181/00379727-84-20607 [DOI] [PubMed] [Google Scholar]
- 60.Tabernero C, Zolotukhin AS, Bear J, Schneider R, Karsenty G, Felber BK. Identification of an RNA sequence within an intracisternal-A particle element able to replace Rev-mediated posttranscriptional regulation of human immunodeficiency virus type 1. J Virol. 1997;71: 95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gamby C, Waage MC, Allen RG, Baizer L. Growth-associated protein-43 (GAP-43) facilitates peptide hormone secretion in mouse anterior pituitary AtT-20 cells. J Biol Chem. 1996;271: 10023–8. 10.1074/jbc.271.17.10023 [DOI] [PubMed] [Google Scholar]
- 62.Porstmann T, Kiessig ST. Enzyme immunoassay techniques. An overview. J Immunol Methods. 1992;150: 5–21. 10.1016/0022-1759(92)90061-w [DOI] [PubMed] [Google Scholar]
- 63.Avrameas S. Amplification systems in immunoenzymatic techniques. J Immunol Methods. 1992;150: 23–32. 10.1016/0022-1759(92)90062-x [DOI] [PubMed] [Google Scholar]
- 64.Ohkawara T, Miyashita K, Nishihira J, Mitsuyama K, Takeda H, Kato M, et al. Transgenic over-expression of macrophage migration inhibitory factor renders mice markedly more susceptible to experimental colitis. Clin Exp Immunol. 2005;140: 241–8. 10.1111/j.1365-2249.2005.02771.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sugiyama F, Wu J, Fujioka M, Ezaki J, Takeda K, Miyaura C, et al. Soybean isoflavones preserve bone mass in hindlimb-unloaded mice. J Bone Miner Metab. 2006;24: 439–46. 10.1007/s00774-006-0711-2 [DOI] [PubMed] [Google Scholar]
- 66.Nakano S, Inada Y, Masuzaki H, Tanaka T, Yasue S, Ishii T, et al. Bezafibrate regulates the expression and enzyme activity of 11beta-hydroxysteroid dehydrogenase type 1 in murine adipose tissue and 3T3-L1 adipocytes. Am J Physiol Endocrinol Metab. 2007;292: E1213–22. 10.1152/ajpendo.00340.2006 [DOI] [PubMed] [Google Scholar]
- 67.Whyte DG, Johnson AK. Lesions of the anteroventral third ventricle region exaggerate neuroendocrine and thermogenic but not behavioral responses to a novel environment. Am J Physiol Regul Integr Comp Physiol. 2007;292: R137–42. 10.1152/ajpregu.00465.2006 [DOI] [PubMed] [Google Scholar]
- 68.Häuser S, Wegele C, Stump-Guthier C, Borkowski J, Weiss C, Rohde M, et al. Capsule and fimbriae modulate the invasion of Haemophilus influenzae in a human blood-cerebrospinal fluid barrier model. Int J Med Microbiol. 2018;308: 829–39. 10.1016/j.ijmm.2018.07.004 [DOI] [PubMed] [Google Scholar]
- 69.Dinner S, Borkowski J, Stump-Guthier C, Ishikawa H, Tenenbaum T, Schroten H, et al. A Choroid Plexus Epithelial Cell-based Model of the Human Blood-Cerebrospinal Fluid Barrier to Study Bacterial Infection from the Basolateral Side. J Vis Exp. 2016. (111). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schwerk C, Papandreou T, Schuhmann D, Nickol L, Borkowski J, Steinmann U, et al. Polar invasion and translocation of Neisseria meningitidis and Streptococcus suis in a novel human model of the blood-cerebrospinal fluid barrier. PLoS One. 2012;7: e30069 10.1371/journal.pone.0030069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rose R, Häuser S, Stump-Guthier C, Weiss C, Rohde M, Kim KS, et al. Virulence factor-dependent basolateral invasion of choroid plexus epithelial cells by pathogenic Escherichia coli in vitro. FEMS Microbiol Lett. 2018;365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Almamy A, Schwerk C, Schroten H, Ishikawa H, Asif AR, Reuss B. Crossreactivity of an Antiserum Directed to the Gram-Negative Bacterium Neisseria gonorrhoeae with the SNARE-Complex Protein Snap23 Correlates to Impaired Exocytosis in SH-SY5Y Cells. J Mol Neurosci. 2017;62:163–80. 10.1007/s12031-017-0920-2 [DOI] [PubMed] [Google Scholar]
- 73.Tani N, Ikeda T, Watanabe M, Toyomura J, Ohyama A, Ishikawa T. Prolactin selectively transported to cerebrospinal fluid from blood under hypoxic/ischemic conditions. PLoS One. 2018;13: e0198673 10.1371/journal.pone.0198673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carroll JA, Matteri RL, Dyer CJ, Beausang LA, Zannelli ME. Impact of environmental temperature on response of neonatal pigs to an endotoxin challenge. Am J Vet Res. 2001;62: 561–6. 10.2460/ajvr.2001.62.561 [DOI] [PubMed] [Google Scholar]
- 75.Turk EE. Hypothermia. Forensic Sci Med Pathol 2010;6: 106–15. 10.1007/s12024-010-9142-4 [DOI] [PubMed] [Google Scholar]
- 76.Palmiere C, Bardy D, Letovanec I, Mangin P, Augsburger M, Ventura F, et al. Biochemical markers of fatal hypothermia. Forensic Sci Int 2013;226: 54–61. 10.1016/j.forsciint.2012.12.007 [DOI] [PubMed] [Google Scholar]
- 77.Okada Y, Miyai K, Iwatsubo H, Kumahara Y. Human growth hormone secretion in normal adult subjects during and after exposure to cold. J Clin Endocrinol Metab 1970;30: 393–5. 10.1210/jcem-30-3-393 [DOI] [PubMed] [Google Scholar]
- 78.Wilkerson JE, Raven PB, Bolduan NW, Horvath SM. Adaptations in man’s adrenal function in response to acute cold stress. J Appl Physiol 1974;36: 183–9. 10.1152/jappl.1974.36.2.183 [DOI] [PubMed] [Google Scholar]
- 79.Stoner HB, Frayn KN, Little RA, Threlfall CJ, Yates DW, Barton RN, et al. Metabolic aspects of hypothermia in the elderly. Clin Sci Lond Engl 1980;59: 19–27. [DOI] [PubMed] [Google Scholar]
- 80.Leppaluoto J, Korhonen I, Huttunen P, Hassi J. Serum levels of thyroid and adrenal hormones, testosterone, TSH, LH, GH and prolactin in men after a 2-h stay in a cold room. Acta Physiol Scand 1988;132: 543–8. 10.1111/j.1748-1716.1988.tb08363.x [DOI] [PubMed] [Google Scholar]
- 81.Wittert GA, Or HK, Livesey JH, Richards AM, Donald RA, Espiner EA. Vasopressin, corticotrophin-releasing factor, and pituitaryadrenal responses to acute cold stress in normal humans. J Clin Endocrinol Metab 1992;75: 750–5. 10.1210/jcem.75.3.1517364 [DOI] [PubMed] [Google Scholar]
- 82.Mallet ML. Pathophysiology of accidental hypothermia. QJM Mon J Assoc Physicians 2002;95: 775–85. [DOI] [PubMed] [Google Scholar]
- 83.Woolf PD, Hollander CS, Mitsuma T, Lee LA, Schalch DS. Accidental hypothermia: endocrine function during recovery. J Clin Endocrinol Metab 1972;34: 460–6. 10.1210/jcem-34-3-460 [DOI] [PubMed] [Google Scholar]
- 84.Bańka K, Teresiński G, Buszewicz G, Mądro R. Glucocorticosteroids as markers of death from hypothermia. Forensic Sci Int 2013;229: 60–5. 10.1016/j.forsciint.2013.03.003 [DOI] [PubMed] [Google Scholar]
- 85.Palmiere C, Mangin P. Postmortem biochemical investigations in hypothermia fatalities. Int J Legal Med 2013;127: 267–76. 10.1007/s00414-012-0738-y [DOI] [PubMed] [Google Scholar]
- 86.Wilkerson JE, Raven PB, Bolduan NW, Horvath SM. Adaptations in man’s adrenal function in response to acute cold stress. J Appl Physiol 1974;36: 183–9. 10.1152/jappl.1974.36.2.183 [DOI] [PubMed] [Google Scholar]
- 87.Wittert GA, Or HK, Livesey JH, Richards AM, Donald RA, Espiner EA. Vasopressin, corticotrophin-releasing factor, and pituitary adrenal responses to acute cold stress in normal humans. J Clin Endocrinol Metab 1992;75: 750–5. 10.1210/jcem.75.3.1517364 [DOI] [PubMed] [Google Scholar]
- 88.Finlayson NB. Blood cortisol in infants and adults: a postmortem study. J Pediatr 1965;67: 248–52. [Google Scholar]
- 89.Haché RJ, Tse R, Reich T, Savory JG, Lefebvre YA. Nucleocytoplasmic trafficking of steroid-free glucocorticoid receptor. J Biol Chem. 1999;274(3):1432–9. 10.1074/jbc.274.3.1432 [DOI] [PubMed] [Google Scholar]
- 90.Sakly M, Koch B. Physicochemical analysis of glucocorticoid receptor in the immature pituitary gland: evidence for lability and aggregation of the heat-activated form. Mol Cell Endocrinol. 1982;28(1):27–35. 10.1016/0303-7207(82)90038-7 [DOI] [PubMed] [Google Scholar]
- 91.Thompson EB, Aviv D, Lippman ME. Variants of HTC cells with low tyrosine aminotransferinase inducibility and apparently normal glucorticoid receptors. Endocrinology. 1977;100(2):406–19. 10.1210/endo-100-2-406 [DOI] [PubMed] [Google Scholar]
- 92.Nicholson ML, Young DA. Effect of glucocorticoid hormones in vitro on the structural integrity of nuclei in corticosteroid-sensitive and -resistant lines of lymphosarcoma P1798. Cancer Res. 1978;38(11 Pt 1):3673–80. [PubMed] [Google Scholar]
- 93.Mains RE, Eipper BA. Biosynthesis of adrenocorticotropic hormone in mouse pituitary tumor cells. J Biol Chem. 1976;251(13):4115–20. [PubMed] [Google Scholar]
- 94.Rousseau G, Reynier P, Jousset N, Rougé-Maillart C, Palmiere C. Updated review of postmortem biochemical exploration of hypothermia with a presentation of standard strategy of sampling and analyses. Clin Chem Lab Med. 2018;56(11):1819–27. 10.1515/cclm-2018-0153 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Relationship between the cortisol level in blood collected at different sites and sex (a), age (b), survival period (c), and postmortem period (d) in all cases. The difference in males and females was associated with the difference in number. However, in terms of age, survival period, and postmortem period, there was a correlation, so the number did not affect the cortisol level. There was no difference between males and females in terms of collection site.
(PPTX)
Left and right cardiac blood (a), left cardiac blood-iliac vein blood (b), and right cardiac blood-iliac vein blood (c).
(PPTX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.