Abstract
Fast-scan cyclic voltammetry (FSCV) at carbon-fiber microelectrodes (CFMEs) is a versatile electrochemical technique to probe neurochemical dynamics in vivo. Progress in FSCV methodology continues to address analytical challenges arising from biological needs to measure low concentrations of neurotransmitters at specific sites. This review summarizes recent advances in FSCV method development in three areas: (1) waveform optimization, (2) electrode development, and (3) data analysis. First, FSCV waveform parameters such as holding potential, switching potential, and scan rate have been optimized to monitor new neurochemicals. The new waveform shapes introduce better selectivity toward specific molecules such as serotonin, histamine, hydrogen peroxide, octopamine, adenosine, guanosine, and neuropeptides. Second, CFMEs have been modified with nanomaterials such as carbon nanotubes or replaced with conducting polymers to enhance sensitivity, selectivity, and antifouling properties. Different geometries can be obtained by 3D-printing, manufacturing arrays, or fabricating carbon nanopipettes. Third, data analysis is important to sort through the thousands of CVs obtained. Recent developments in data analysis include preprocessing by digital filtering, principal components analysis for distinguishing analytes, and developing automated algorithms to detect peaks. Future challenges include multisite measurements, machine learning, and integration with other techniques. Advances in FSCV will accelerate research in neurochemistry to answer new biological questions about dynamics of signaling in the brain.
Graphical Abstract
We reviewed recent advances and future challenges in fast-scan cyclic voltammetry for real-time detection of neurotransmitters.
1. Introduction
Fast-scan cyclic voltammetry (FSCV) is an electrochemical technique that has exquisite temporal resolution and sensitivity for measurement of rapid neurotransmitter dynamics in vivo.1–3 Table 1 compares the figures of merit of FSCV with other common electrochemical techniques for real-time neurotransmitter detection. FSCV was developed by Millar in 1980 and was later popularized by Wightman.4–6 Theoretical treatment and instrumentation for FSCV at ultramicroelectrodes was also developed by Savéant and Amatore.7–9 Since then, FSCV has been the key analytical technique for several major findings in neuroscience. For example, subsecond release of dopamine in the midbrain promotes cocaine seeking behavior of rats.10 The high sensitivity of FSCV revealed that rapid dopamine release during intracranial self-stimulation decays to smaller amounts of release, showing dopamine itself is not the reward signal.11 In fact, many studies that have pinpointed the role of dopamine in reward rely on FSCV because of its rapid temporal response.12–14 Moreover, FSCV has been expanded to investigate other compounds. Transient adenosine release modulates blood flow and modulates phasic dopamine release in the caudate-putamen.15–17 FSCV was used to discover multiple rates of serotonin uptake and histamine and serotonin co-secretion from mast cells.18,19 Thus, FSCV is a versatile technique for in vivo monitoring of neurotransmitters with rapid dynamics.
Table 1.
Figures of merit of major electroanalytical techniques for neurotransmitter detection
| Techniques | Sensitivity | Selectivity | Temporal resolution |
|---|---|---|---|
| Amperometry | low (25–100 nM LOD dopamine but sufficient to count number of molecules)20 | low (All compounds that can be oxidized or reduced at the applied potential will give the signal.) | highest (electronic sampling rate, < 1 ms) 2 |
| Pulse Voltammetry | high (10 nM LOD dopamine)20 | high (Molecules with oxidation potential differs more than 100 mV is resolved.)2 | low (up to 1 min) |
| Fast-Scan Cyclic Voltammetry | high (10 nM LOD dopamine)20 | highest (CV shape identifies molecule, but similar species may have similar CV.) | high (triangular waveform frequency, 100 ms) |
A thorough discussion of the FSCV fundamentals can be found in our recent fundamental review.21 FSCV is cyclic voltammetry with a high scan rate (100 V/s or faster).2 Fig. 1A shows the typical applied potential waveform for the FSCV of dopamine (“the dopamine waveform”). A holding potential of –0.4 V is applied to the working electrode to selectively preconcentrate cationic dopamine on the electrode surface. Then, a triangular waveform with a scan rate of 400 V/s is applied repeatedly to scan the electrode to a switching potential of +1.3 V and back to oxidize dopamine and reduce dopamine-o-quinone. The overall cyclic voltammogram (CV) consists of a small Faradaic current, which is hard to distinguish from much larger background charging current (Fig. 1B). However, if the background current is stable, then it can be subtracted out from the overall CV to get the background-subtracted CV (Fig. 1C), which has a unique shape for each electroactive compound. Backgrounds are typically taken right before the Faradaic signal, to avoid background drift, but it is tricky because they must not be taken during other Faradaic signals.22,23 The triangular waveform is applied at a repetition frequency of 10 Hz, which gives 100-ms temporal resolution, sufficient for monitoring rapid neurotransmitter release.1 Because ten CVs are obtained every second, an hour-long measurement will yield 36,000 CVs, which are hard to manually examine. To represent continuous FSCV data, individual CVs are stacked together as a three-dimensional current-potential-time plot, as illustrated in Fig. 1D, which shows the FSCV data from 5-s bolus injection from 1 μM dopamine followed by buffer washing. A simpler, two-dimensional false color plot, which is the bird’s eye view of the 3D plot, is conventionally used to picture all data (Fig. 1E).24 The false color plot allows better interpretation of multiple analytes, noise, and signal drift.22,25 For simple measurements where only one electroactive species is monitored, a current-time trace (stacked on the color plot in Fig. 1E) shows how the concentration changes over time.
Fig. 1.
FSCV of dopamine. (A) Applied potential waveform using –0.4 V holding potential, +1.3 V switching potential, 400 V/s scan rate, and 10 Hz repetition rate. (B) Examples background currents: blank (PBS pH 7.4) (black) and buffer with 1 μM dopamine (red). Dashed boxes emphasize the difference between them. (C) Background-subtracted CV of 1 μM dopamine. (D) Three-dimensional current-potential-time plot and (E) Conventional false color plot with anodic peak current-time trace of 5-s bolus injection of 1 μM dopamine.
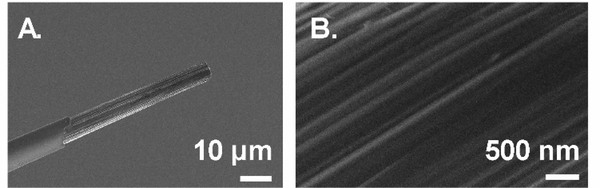
The typical working electrode for FSCV measurement is a cylindrical or disk carbon-fiber microelectrode (CFME) made from a carbon fiber pulled in a glass capillary and epoxy sealed (Fig. 2).1,26 CFMEs have been widely used for FSCV in vivo detection due to their small size (7 μm diameter) that allows measurement in specific brain regions, low RC constant that gives fast signal equilibration, and biocompatibility that causes minimal tissue damage than materials such as metal.27 The small surface area generates low currents, in the range of nA, so the Ohmic drop is negligible and only two electrodes (without auxiliary electrode) are required for the electrochemical cell.2 Carbon is a good material for neurotransmitter measurements because its surface structure contains oxygen-containing functional groups and edge planes that promote adsorption of cationic amines such as dopamine and serotonin.28,29 This electrostatic adsorption enhances the sensitivity towards these cationic neurotransmitters. However, CFMEs can also suffer signal degradation over time due to biofouling by biomolecules or fouling due to adsorption of oxidative electropolymerization products.30,31 Calibration of CFMEs is particularly challenging as the electrode surface as well as sensing environment also alter diffusion and adsorption of analytes to the electrode, leading to differences in electrode sensitivity in vitro and in vivo.32
Fig. 2.
SEM images of cylindrical CFME sealed in glass capillary at (A) 1000× and (B) 25000× magnification.
Although FSCV with CFMEs has been implemented to monitor rapid releases of dopamine and some neurotransmitters for nearly four decades, there are improvements needed to explore new molecules and new biological experiments. FSCV, like other techniques, still has many analytical challenges that must be overcome:
Sensitivity: Traditional FSCV has limits of detection for dopamine in the low nM range, but other neurochemicals may be present at even lower concentrations (such as neuropeptides).33 Thus, improvements are needed in S/N ratios and to disambiguate small Faradaic signals from background drift and noise in vivo.22,23
Selectivity: Many neurotransmitters have similar CVs because they have the same electroactive moiety (such as dopamine, epinephrine, norepinephrine, and DOPAC)6 or similar peak potentials (such as H2O2, adenosine, and histamine).34,35 Thus, new strategies to make selective electrodes are needed, as well as new data analysis strategies to distinguish signals from multiple analytes.22,36,37
Electrode fouling: Biofouling limits the electrode surface area and electron transfer kinetics. It typically arises from adsorption of biomolecules or byproducts of electrochemical oxidation from molecules such as serotonin or histamine.29–31,38 Electrode fouling reduces the sensitivity, shifts the Faradaic peaks, and leads to signal instability over time, so new methods are needed to reduce fouling.39
Temporal resolution: FSCV has traditionally been performed at 10 Hz because higher repetition rates reduce the signal.40 More rapid measurements with high sensitivity might uncover even more rapid signals in the brain.
This review summarizes the recent strategies for addressing these challenges and improving FSCV detection for neurochemical analysis. First, the FSCV applied potential waveform has been optimized to differentiate the electrochemical signal of similar molecules, to analyze new molecules that have not been previously characterized by FSCV, and to alleviate electrode fouling.3,31,41 Second, modified CFMEs and novel carbon micro/nanoelectrodes have been fabricated to enhance sensitivity, selectivity, antifouling properties, and temporal resolution for FSCV detection.20,29 Third, the FSCV data analysis has been improved via chemometrics and signal processing techniques to reduce signal drifting and to automatically detect the signal of specific neurotransmitters to reduce analysis time and possible bias.3,42 Combinations of these strategies will enable better experiments for neurotransmitter detection. The future challenges for FSCV detection of neurotransmitters include measurements in smaller regions, multisite measurements, machine learning for data analysis, and integration with other analytical techniques. The progress in FSCV methodology will certainly expand and accelerate research in the field of analytical neurochemistry in the near future.
2. Strategy 1: Optimizing the Fast-Scan Cyclic Voltammetry Waveform
2.1. Enhancing Sensitivity by Optimizing Holding and Switching Potentials
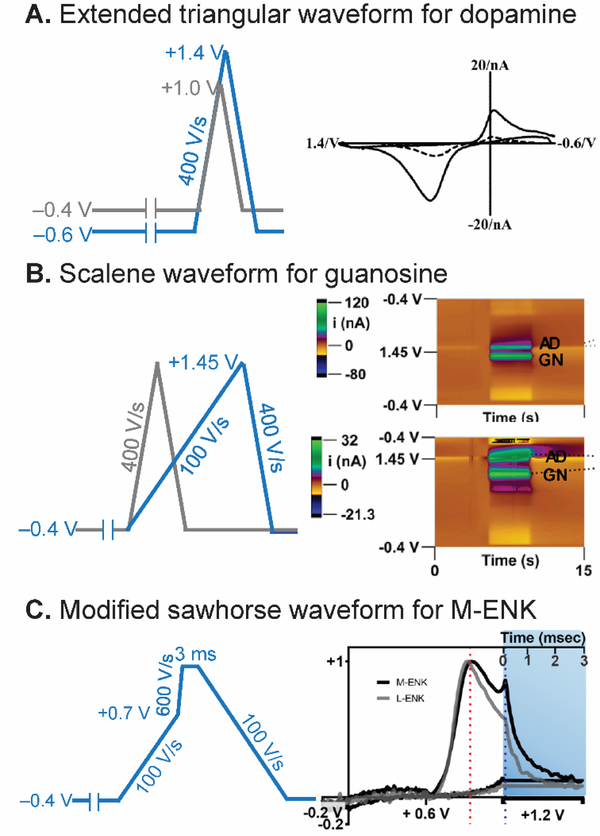
One of the simplest adjustments to enhance FSCV sensitivity is to change the holding and switching potentials. A more negative holding potential increases the extent of electrostatic adsorption of cationic molecules and enhances the Faradaic current and sensitivity.43,44 A more positive switching potential oxidizes carbon in the CFME and increases the oxygen-containing functional groups, thus more dopamine is adsorbed and the sensitivity is increased.43,45 Fig. 3A demonstrates that extending the waveform from the original parameters (−0.4 V holding potential to +1.0 V switching potential) to extended ones (−0.6 V to +1.4 V) improved the sensitivity for dopamine detection for 5-fold.43 Extending the switching potential increased the adsorption sites and improved the electron transfer kinetics for neutral and anionic molecules as well.45 High oxidation-potential compounds also require an extended switching potential in order to detect the oxidation. For instance, adenosine has a primary oxidation potential of 1.3 V, but FSCV at 400 V/s gives a primary peak at 1.5 V or even later on the backward scan.17,44 The best holding and switching potentials compromise between sensitivity enhancement, electrode stability, and electrode fouling.
Fig. 3.
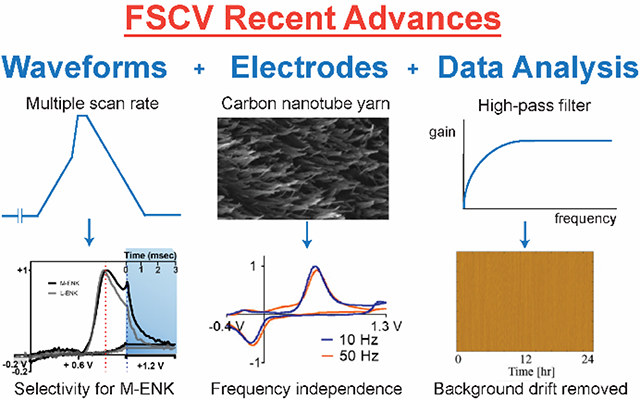
Strategy 1: Waveform Optimization. (A) Extended triangular waveform for dopamine detection. The waveform scanned from −0.6 V to +1.4 V (blue) yielded 5-time higher sensitivity for dopamine than the traditional waveform scanned from −0.4 V to +1.0 V (gray). CV is reproduced from 43 with permission from The Royal Society of Chemistry. Note: Axes are flipped in a different convention than currently used. (B) Scalene waveform for guanosine-adenosine co-detection. Slower forward scan (100 V/s) significantly discriminated anodic peak between guanosine (GN) and adenosine (AD). Color plots are reprinted from 50. Copyright 2019 American Chemical Society. (C) Modified sawhorse waveform for M-ENK detection. Slower scan rate portion decreased interferent signals, and chronoamperometric portion distinguished between M-ENK and L-ENK. Reprinted from 52. Copyright 2019 American Chemical Society.
2.2. Introducing Selectivity by Optimizing Scan Rate and Designing New Waveform Shape
In conventional CV, electroactive species with different redox potential and electron transfer kinetics will have different shapes of voltammograms, but the high scan rate of FSCV waveform conceals these differences21 and deteriorates the selectivity between similar species. Nevertheless, the scan rate of the triangular waveform affects the peak potential of quasireversible and irreversible reaction of electroactive species;46 thus it can be optimized to shift the peak away from interferents or the unstable signal around the switching potential. For example, for detection of octopamine, a phenolamine neurotransmitter found in invertebrates, the dopamine waveform is not suitable for in vivo studies because the peak is shifted near the switching potential.47,48 Pyakurel et al. developed an in vivo octopamine waveform by decreasing the scan rate to 100 V/s and scanning to +1.4 V.49 This waveform moved the octopamine anodic peak away from the switching potential to a less positive potential without significantly decreasing the signal-to-noise ratio. The Ross group used a scalene waveform consisting of a forward scan rate of 100 V/s to +1.45 V and backward scan rate of 400 V/s (Fig. 3B) to differentiate the anodic peak of guanosine from that of adenosine, facilitating co-detection.50 Therefore, scan rate optimization is a promising strategy to shift Faradaic peaks, enhancing selectivity.
The simple triangular sweeping waveform can be replaced with a new waveform shape containing different scan rates in the same sweeping direction, which helps control species-electrode interactions and redox kinetics. A modified sawhorse waveform consisting of multiple scan rates was firstly proposed by the Sombers group for the detection of opioid neuropeptide methionine-enkephalin (M-ENK), which has tyrosine and methionine electroactive amino acids.51,52 Fig. 3C shows the optimized modified sawhorse waveform consisting of a lower scan rate portion (–0.4 V to +0.7 V, 100 V/s), that limits interference from other species, and a higher scan rate portion (+0.7 V to +1.3 V, 600 V/s), that oxidizes tyrosine and enhances the sensitivity. Then, the waveform was held at +1.3 V for 3 ms to oxidize the methionine side chain and activate the electrode surface. The waveform differentiated the signal of M-ENK from leucine-enkephalin (L-ENK), which does not have methionine and did not give the second anodic peak. Sawhorse waveforms have also been proposed to distinguish high oxidation potential compounds including adenosine, ATP, and H2O2, where more time at the switching potential allows more oxidation and discrimination.35 Thus, complex scan rate optimization and holding a constant potential at the switching potential are useful strategies to achieve selectivity.
FSCV is a background subtracted technique, and changes in pH, ionic composition, and electrode surface condition alter the background charging current and adsorption characteristics, thus giving a background subtraction error that can be mistaken for a Faradaic signal. In particular, the electrode surface changes when a higher voltage is applied, and this can cause slight changes in background current.45 To correct for the background change, paired-pulse voltammetry (PPV) was proposed by adding another triangular waveform after the main triangular waveform to get a “blank” CV.53 Because both neurotransmitter and blank CVs are similarly influenced by the pH fluctuation, the fluctuating signal is removed by subtracting the secondary CV from the primary CV, leading to easier identification and quantification of electroactive species. PPV was also implemented with other waveform shapes such as sawhorse waveform to correct background fluctuations.54
2.3. Diminishing Electrode Fouling by Waveform Optimization
Several waveform manipulations have been proposed to decrease the electrochemical fouling from electroactive species. Jackson et al. proposed the N-shaped waveform for FSCV detection of serotonin,31 and this waveform is still the predominant waveform for serotonin detection. The waveform holds at +0.2 V to limit serotonin byproduct adsorption, ramps quickly at 1000 V/s to 1.0 V to limit the fouling, but scans down to −0.1 V on the backward to allow the cathodic peak to be detected.
Holding potential, switching potential, and scan rate of the triangular waveform have been optimized to limit the number of adsorbed species and the extent of oxidation and fouling. For example, scanning from +0.1 V to +1.3 V at 600 V/s scan rate was implemented to detect octopamine and tyramine, cationic neurotransmitters with an oxidation product that polymerizes to foul the electrode.49 The proposed waveform alleviates fouling; the positive holding potential limits adsorption of octopamine and tyramine, and the higher scan rate outruns the secondary oxidation peak.55 The Ross group proposed a similar waveform manipulation to decrease electrochemical fouling of melatonin, a signaling molecule modulating circadian rhythms and the immune system.56 The waveform, with a +0.2 V holding potential and 600 V/s scan rate, eliminated the fouling from melatonin oxidative electropolymerization observed with the traditional dopamine waveform.
Before designing the waveform for new compounds, fundamental studies should be performed to determine the redox potentials and mechanisms of possible electrochemical fouling.30,57 Recently, our lab proposed the mechanism of histamine oxidation, which undergoes electropolymerization and generates polyhistamine and fouls the electrode.30 A potential of 1.1 V vs Ag/AgCl or higher is required to oxidize histamine at carbon electrodes, thus the switching potential must be extended to more positive potential to yield a full anodic peak and maximum sensitivity. However, using a higher switching potential at 1.45 V, histamine fouled the electrode more, so 1.3 V is the best switching potential to achieve high sensitivity while limiting the electrode fouling. Therefore, knowing not only the redox potential but also the reaction mechanism helps researchers to design a better FSCV waveform to reduce fouling.
3. Strategy 2: Improving Electrochemical Properties of Microelectrodes
3.1. Enhancing Sensitivity by Carbon Nanomaterials
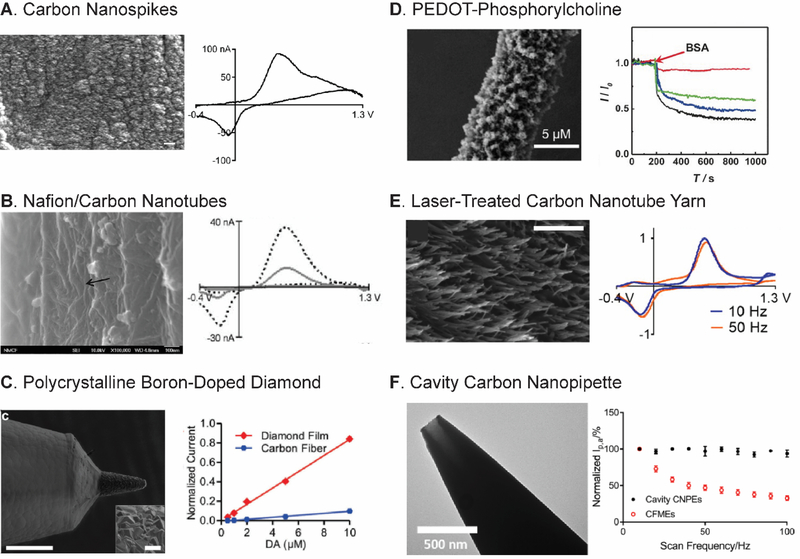
Carbon is the best electrode material for in vivo detection of neurotransmitters because of its excellent biocompatibility and rapid electron transfer kinetics for neurotransmitter redox reactions.20,28,29,58 The structure of graphitic carbon materials, including CFMEs, consists of two types of planes: basal planes parallel to the graphite sheets and edge planes perpendicular to the sheets.28,29 While the basal planes have a conjugated system that increases the electronic conductivity, it is the edge planes that enhance the adsorption of cationic neurotransmitters and have electrocatalytic activity.28,59 Therefore, carbon materials with more dense edge planes enhance the sensitivity and limit of detection for dopamine. Several different forms of defect-rich, sp2-hybridized carbon nanomaterials increase sensitivity and limits of detection for dopamine detection compared to CFMEs.60–62 For instance, carbon nanotubes (CNTs) have advantageous electrochemical properties and can be directly grown on 25-μm diameter Nb wire with a Al2O3/Fe catalyst by chemical vapor deposition (CVD).60 Carbon nanospikes (CNSs) are a defect-rich, graphene-like carbon nanomaterial with spike morphology that are grown on a wire by plasma-enhanced CVD (Fig. 4A). CNSs are easier to grow than CNTs because they do not require a catalyst,61 and CNSs also have better electrochemical performance than CNTs because of higher surface roughness and defect sites.63 Carbon nanohorns (CNHs) are a graphene sheet rolled in a conical shape.64 CNHs were electrodeposited on CFMEs, and further oxidation of the CNH-modified electrode opened the cone tips to generate more edge planes and enhance the adsorption.62 Many treatments such as pulsed laser65 and oxygen plasma etching66,67 were applied to carbon nanomaterial electrodes such as carbon nanotube yarn microelectrode (CNTYME) to further increase the edge planes and sensitivity.
Fig. 4.
Strategy 2: Microelectrode Development. (A) CNS grown on Nb wire had improved sensitivity for dopamine detection. SEM Scale bar is 200 nm. Reproduced from 61 with the permission from The Royal Society of Chemistry. (B) Nafion/CNT-modified CFME increased dopamine current. The black arrow points to the CNT surface. Reproduced from 72 with the permission from The Royal Society of Chemistry. (C) Polycrystalline BDD on parylene coating alleviated fouling. SEM scale bar is 100 μm. Inset shows diamond microcrystal with a scale bar of 10 μm. Reproduced from 37 with CC-BY license, published by Frontiers Media SA. (D) PEDOT-PC/CFME alleviated fouling. Normalized current-time trace of PEDOT-PC/CFME (red) compared to PEDOT/CFME (blue), PEDOT-OH/CFME (green), and bare CFME (black). Reproduced from 38 with permission from Wiley. (E) Laser-treated CNTYME had a micrometer roughness that allowed a thin-layer cell that improve temporal resolution without signal loss. Scale bar is 500 nm. Reprinted from 65. Copyright 2016 American Chemical Society. (F) Cavity CNPE allowed redox cycling and frequency-independent current. Reprinted from 92. Copyright 2019 American Chemical Society.
Graphene mainly consists of basal planes and has limited cationic neurotransmitter adsorption, so it has not been widely tested for FSCV detection. However, there are several methods for introducing defects to graphene to enhance its adsorption and sensitivity.29 Point defects in graphene can be controlled by optimizing the temperature used in graphene synthesis via Ni-catalyzed transformation of amorphous carbon on a SiO2/Si substrate.68 The density of defects was linearly correlated with the sensitivity of dopamine and serotonin detection by FSCV, but too many defects disrupted the conjugation and decreased the electrode sensitivity. Graphene can be chemically functionalized as graphene oxide via the modified Hummer’s method to increase oxygen-containing groups.69 Graphene oxide enhanced dopamine signal for cyclic voltammetry and differential pulse voltammetry (DPV) at glassy carbon electrode.70 Thus, defect-rich graphene and graphene oxide are potential electrode materials for FSCV detection.
3.2. Introducing Selectivity by Charged Polymers and Size-Exclusion Layers
Electrode modifications have been developed to increase selectivity, particularly charged polymer coatings that control the electrostatic adsorption of neurotransmitters. The classic example is Nafion coating CFMEs to electrostatically repel anionic interferents such as DOPAC and ascorbate for dopamine measurements.71 Our lab combined the negatively-charged polymers Nafion or overoxidized polypyrrole with CNTs (Fig. 4B) to increase the selectivity toward cationic neurotransmitters while increasing the current.72 The Zestos lab recently coated electrodes with polyethyleneimine (PEI) to enhance selectivity for DOPAC by the electrostatic attraction between positive amine group and negative DOPAC.73 Poly(ethylenedioxythiophene) (PEDOT), a common conducting polymer, was used for DPV detection of dopamine, and the sensitivity was enhanced when it was functionalized with carboxylic groups.74 PEDOT/graphene oxide composite enhanced sensitivity of CFMEs for FSCV detection of dopamine.75 Thus, charged polymers can enhance both sensitivity and selectivity for the detection.
Size exclusion was used to enhance the selectivity of electrodes for small molecules. The Sombers group used 1,3-phenylenediamine to coat the CFMEs via electrodeposition as a size-exclusion membrane for measurement of H2O2, a reactive oxygen species and rapid neuromodulator.34 The modified electrode detected only H2O2 and did not sense larger neurotransmitters with high oxidation potential such as histamine and adenosine. The Mao group grafted silica nanoporous membranes to selectively monitor O2 by amperometry.76 The kinetics and response time of FSCV detection with size-exclusion polymers can be studied and optimized in the future.
3.3. Diminishing Electrode Fouling by Carbon Materials
Many sp3-hybridized carbon materials such as boron-doped diamond (BDD), nanodiamond (ND), and tetrahedral amorphous carbon exhibit antifouling properties.29,77,78 BDD electrodes have been used instead of CFMEs because of their antifouling properties, low noise, and wide potential window.79 Polycrystalline BDD deposited on a tungsten rod (Fig. 4C) was robust for long-term FSCV measurements of dopamine with minimal fouling (sensitivity decreased by only 16% after 144-h measurement), compared to CFMEs (89%).37 The electrode was also applied to detect neurotransmitters in a human brain with deep brain stimulation. However, the CV of neurotransmitters at BDD electrodes had a positively-shifted anodic peak potential and lower anodic current because of the fewer surface oxide groups and electronic density of states.37,80 Recently, we investigated NDs for FSCV detection. Carboxylated NDs enhanced sensitivity and diminished electrochemical fouling and biofouling of CFMEs due to their rich negative surface group and hydrophilicity.81 Therefore, NDs are a promising nanomaterial to alleviate electrode fouling without compromising sensitivity.
CNTs60 and CNHs62 also exhibited antifouling properties in addition to the sensitivity enhancement. CNTYME treated by oxygen plasma etching and an antistatic gun had less biofouling than the unmodified CNTYME.66 PEI/CNT fibers inhibited electrochemical fouling from serotonin and 5-HIAA and biofouling in brain slices.82 The defect and surface oxygen-containing groups of these nanomaterials increased the surface hydrophilicity and reduced the fouling.66 The Ross group compared the electrochemical fouling and biofouling between the unmodified and oxygen plasma-treated CNTYMEs.83 While the edge planes generated from oxygen plasma decreased biofouling, they increased the serotonin fouling from the CNTYME. The effect of oxygen-containing groups and carbon structure on hydrophilicity and antifouling properties should be thoroughly studied in the future.
3.4. Diminishing Electrode Fouling by Charged and Zwitterionic Polymers
Charged polymer coatings also decrease fouling by increasing surface hydrophilicity. Nafion was co-deposited with poly(ethylenedioxythiophene) (PEDOT) as PEDOT:Nafion on the CFME to enhance the sensitivity for dopamine measurement.84 The coating resisted biofouling from bovine serum albumin adsorption in vivo. A Nafion-coated CFME was combined with the N-shape waveform to eliminate serotonin and 5-HIAA fouling in FSCV.31,85 Zwitterionic materials have both cationic and anionic moieties in their structure that can increase the hydrophilicity and reduce hydrophobic interactions to prevent the adsorption of fouling species.86 The Mao group synthesized the modified polymer PEDOT-phosphorylcholine (PEDOT-PC) and coated it on a CFME; the PC moiety has the anionic phosphate and cationic choline group, so it has the zwitterionic properties (Fig. 4D).38 The PEDOT-PC/CFME electrode had less protein adsorption and biofouling and led to preserved sensitivity after FSCV measurement for 2 h. Nevertheless, although PEDOT is a conducting polymer, the sensitivity of the modified electrode was diminished because of the thick polymer coating. The balance between thickness of antifouling layer and electrochemical adsorption layer needs to be optimized in the future.
3.5. Improving Temporal Resolution by Thin-Layer Micro/nanostructured Electrodes
New electrode materials have been identified that allow FSCV measurements at higher repetition frequencies, because their surface structures act as thin layer cells and promote momentary trapping of the analyte. Carbon nanotube yarns, produced from direct spinning of a single CNT thread,87 have high sensitivity, due to a large specific surface area, and fast electron transfer kinetics.88 Our lab discovered that the dopamine oxidation at CNTYME was frequency-independent.89 The temporal resolution can be improved without decreasing sensitivity, up to a repetition frequency of 500 Hz, 50-times better temporal resolution. The surface roughness of CNTYMEs is higher than CFMEs (1910 ± 190 nm vs 420 ± 30 nm) (Fig. 4E),65 and is the same order of magnitude as the diffusion distance of dopamine in one FSCV waveform, so the dopamine-o-quinone is trapped in rough CNTYME surface like a thin-layer cell. Increased surface roughness increases capacitance, but the beneficial effects of the trapping effect are greater than noise increases from the capacitance, as the CV of dopamine is more reversible at CNTYME and redox amplification greatly enhanced Faradaic currents. Further modification of CNTYME such as pulsed laser65 and antistatic gun66 treatments increased the surface roughness and enhanced the temporal resolution. CNT fibers, made by wet spinning, also have similar properties.90 Polyethyleneimine/CNT (PEI/CNT) fibers had crevices and more surface roughness than CNTYMEs, so they also had frequency-independent properties, but the current-time trace is not perfectly squared however. Zestos et al. also showed that the PEI/CNT preserved the signal even at the frequency of 500 Hz.91 Therefore, carbon nanomaterials with crevices that have micrometer roughness allow better temporal resolution with enhanced sensitivity.
A completely different geometry of electrode has been formed that works on the same thin-layer cell trapping principle. With the collaboration of Mirkin group, we reported a cavity CNPE, which was prepared by chemical vapor deposition (CVD) of carbon in the pulled quartz capillary.92,93 The CVD parameters were optimized to yield the few hundred nm diameter cavity geometry at the tip (Fig. 4F). When a negative holding potential was applied to the electrode, the cationic dopamine was trapped in the cavity, so the thin-layer effect that allow the redox cycling was observed and led to its frequency-independent properties. Despite being a nanoelectrode, the sensitivity is sufficient to detect micromolar dopamine because the cavity preconcentrated dopamine and the enhanced electric field increased dopamine adsorption. The cavity CNPE was also robust enough detect dopamine exogenously in rat brain slice tissue. However, the CNPEs are only amorphous carbon, and the surface may be improved with treatments that increase the number of edge planes. In general, nanoelectrodes also have decreased RC constant, lower noise, and thus, an improved S/N ratio.94
4. Strategy 3: Improving Data Processing and Automated Analysis
4.1. Enhancing Sensitivity by Data Preprocessing and Chemometrics
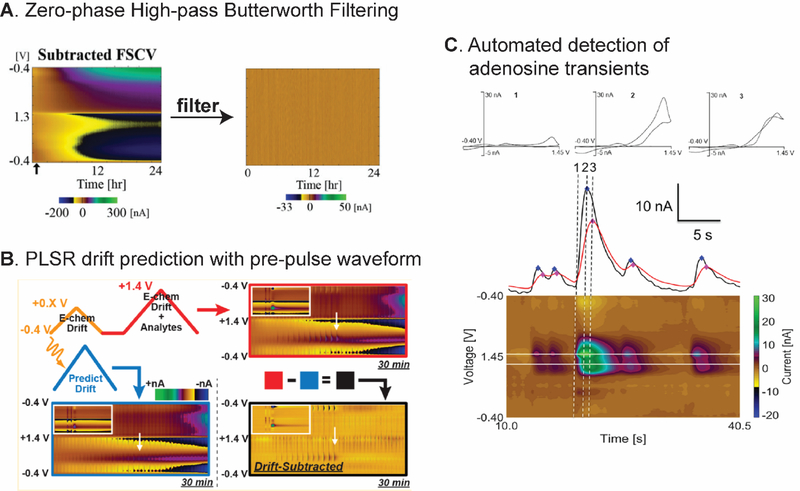
Data preprocessing is the first step after data collection to clean the data for actual analysis, reduce the noise, and improve the S/N ratio.95 Signal processing techniques such as digital filtering have been applied in analytical chemistry because they are available in many data analysis software and programming languages,96 and some techniques were introduced to remove the background drift from FSCV data. DeWaele et al. proposed a zero-phase high-pass Butterworth filter to remove background drift, which is of low frequency.22 The filter was successful in eliminating the baseline drift from continuous FSCV data for 24 hours measurement in vitro and 5 hours measurement in vivo (Fig. 5A), and all characteristics of the dopamine Faradaic peak were preserved. Signal filtering had an advantage over some other chemometric methods because it does not require any training set.
Fig. 5.
Strategy 3: Data Processing and Automated Analysis. (A) Zero-phase high-pass Butterworth filter successfully removed the background drift from 24-hour continuous FSCV measurement. Reproduced from 22 with the permission from The Royal Society of Chemistry. (B) Double waveform with PLSR predicted and removed the baseline drift while preserving the faradaic signal of dopamine and H2O2. Reproduced from 23. Copyright 2019 American Chemical Society. (C) Primary and secondary peak potential current-time trace used in automated algorithm for adenosine transient analysis. Reproduced from 42. Copyright 2017 American Chemical Society.
Chemometric techniques such as principal component analysis (PCA) and regression (PCR) are classical multivariate analyses that have been widely used in the FSCV field to extract the signal contribution from different analytes such as dopamine97 and adenosine98 from nonfaradaic and noise components.36,99,100 Training sets can be collected in vitro from different electrodes for a range of neurotransmitter concentrations and in vivo from different animals and concatenated as a standard library for future experiments and animals to reduce variability and analysis time.36 Different origins between the training set and experimental data also allow statistical independence.36 However, PCR theory and a recent critique of application in FSCV by the Wightman group100 suggests that the training set should be collected from known signals in the same animals. The discrepancy of the experimental condition between the training sets in the library and the experimental data led to poor signal resolution and inaccurate quantification of the neurotransmitter concentration.100 Thus, the difference and independence between the training set and the collected data should be carefully examined before performing PCR.
Mathematical techniques were combined with waveform manipulation to predict the noise and nonfaradaic signal to remove it from the main electrochemical signal. Monovalent and divalent cations electrostatically interact with the surface-oxide group on the CFMEs, so the Wightman group studied the effect of local changes in those ions on the background charging current.101 Then, they proposed to apply an 80–120 mV constant-potential pulse before the dopamine waveform to estimate the impulse response caused by nonfaradaic processes, and deconvoluted that response to obtain cleaner color plots. The Sombers group applied a modification of PPV called the double triangular waveform by using smaller first (−0.4 to +0.8 V) and larger second triangular waveform (−0.4 to +1.4 V).23,102 The smaller CV from the first triangular waveform was used to predict the background drift in the larger CV by using partial least square regression (PLSR) (Fig. 5B). The procedure was successful in removing the pH shift and background drift and detect dopamine and H2O2 in vivo. Thus, appropriate data preprocessing can make the automated analysis more accurate.
Automated software is also important for modeling data to extract chemical information, and FSCV data can be treated in any programming platform like MATLAB or Python. Electrical stimulation has been used to study the dynamics of neurotransmitter release and uptake.2 The Quantitative Neurobiological framework was developed by Wagner group to quantitatively analyze dopamine release from in vivo electrical stimulation.103 Twelve kinetic parameters of dopamine release, dopamine reuptake, and supraphysiological stimulation effects were taken into account to derive a set of mathematical equations to fit the shape of dopamine transients to experimental current-time trace data. The program can be improved to consider the mass transfer distortion104 from the pure kinetic model and CV peak shifting that might deviate the current-time trace analysis from one potential. The Hashemi group also developed a mathematical model to analyze the kinetics of serotonin uptake from the serotonin current-time trace data from in vivo electrical stimulation.105
4.2. Data Processing for In Situ Calibration
Traditionally, univariate linear regression or single-point calibration has been performed in vitro before or after biological experiments to calibrate the peak current and analyte concentration. However, variations in the faradaic signal from pH fluctuations or electrode fouling cause differences in the CV shape and sensitivity of the electrode, leading to calibration errors. Because standard analyte concentrations cannot be generated in vivo, several methods to infer the peak potential and current sensitivity have been reported. Background charging current is proportional to electrode surface area, so it has a linear relationship with the electrode sensitivity that was used to calibrate the electrode.106 However, chemical structure and properties of the CFME can vary among electrodes and thus the calibration deviates slightly from linearity and the peak position is shifted.107 The Garris group performed PCR with the background CVs to predict sensitivity for dopamine detection and gain a better prediction performance.32 This approach automatically rejected noise, and the sensitivity from the actual experiment can be computed in situ before, during, and after the in vivo experiment. In another work, an electrochemical impedance spectroscopy (EIS) model circuit was applied to explain the change in the background and dopamine CV.108 Higher impedance was modeled for electrode fouling to explain the shift of the small hump in the background CV to more positive potential, the increase in the anodic-to-cathodic peak separation (ΔEp) of dopamine, and the decrease in the electrode sensitivity. The background CVs during an in vivo experiment were then used to predict the dopamine peak potentials and sensitivity. Both inferential methods from background CVs could be tested with other electroactive species in the future.
4.3. Discriminating other Analytes with Automated Signal Detection
Spontaneous adenosine transients have been characterized in vivo in different rat brain regions, but the transients are random in nature so their analysis is challenging.98,109 Our group developed an automated software to identify and characterize adenosine transients from the FSCV data (Fig. 5C). The program scanned for the possible transients from the peaks in current-time trace at primary and secondary anodic peak potentials. Several rules were applied to verify whether it is an adenosine transient, including a minimum signal-to-noise ratio, required time delay, and set peak current ratio between primary and secondary peak of the transient. Future studies could use the whole CV or color plot for the analysis, which may introduce selectivity and correct the peak shifting along the experiments. Specific detection software for other analytes could also be developed.
5. FSCV Method Development: Integration and New Challenges
The field of FSCV detection of neurotransmitters has greatly advanced over the last four decades. New waveforms, novel microelectrodes, and versatile data analysis enable more complicated neurochemistry research to be performed. Because these three strategies are independent, they can be combined together to improve the different analytical performance simultaneously. For example, coupling the Nafion/CNT-modified CFME72 with the N-shaped waveform31 could reduce electrode fouling while enhancing the sensitivity for serotonin FSCV detection. That data could be high-pass filtered to eliminate background drift,22 and the serotonin peak could be identified by PCA or an automated algorithm. However, there are additional challenges for FSCV on the horizon and this section addresses some of these concerns.
5.1. FSCV New Challenge: Measurements in Tiny, Discrete Regions
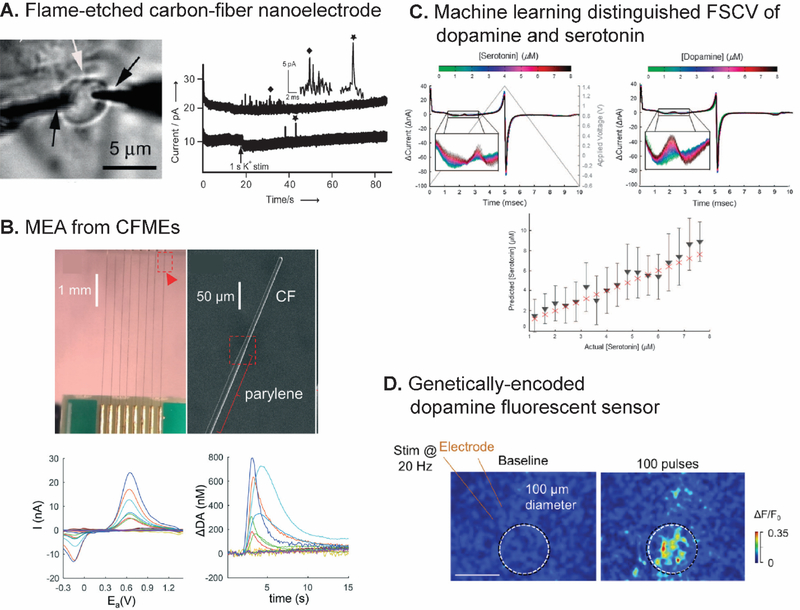
FSCV has been traditionally performed with microelectrodes, but new biological questions will require smaller electrodes such as nanoelectrodes. For example, researchers are examining the role of discrete brain regions in Drosophila47,110,111 and zebrafish112 in neurotransmission. Drosophila have mushroom bodies that control many sensory processes; these bodies have discrete regions smaller than 10 μm in width,47 so a CFME is too large to monitor in these different regions. Another motivation for using smaller electrodes is electrochemical measurement at synapses, which are typically 100 nm gaps between neurons.33 Several possible approaches to fabricate a free-standing nanoelectrode for neurotransmitter detection have been investigated. Conical carbon-fiber nanoelectrodes produced from flame-etched carbon fibers had tip diameter and shaft length in nanometer ranges, and were useful for monitoring individual vesicular exocytosis at a single synapse of superior cervical ganglion by amperometry (Fig. 6A).113 Similar electrodes were placed in PC12 cells to analyze the intracellular vesicle content for cytometry.114 Carbon nanopipette electrodes (CNPEs) with a nanometer-size tip can be fabricated from carbon-deposited pulled quartz capillary tip.92,115 The deposition conditions were further optimized to yield cavity tips with enhanced electric fields and redox cycling.92 A nanopipette electrode with an interface between two immiscible electrolyte solutions (ITIES) was used to monitor synaptic acetylcholine dynamics by scanning electrochemical microscopy (SECM).116 Moreover, recent technology allows bottom-up fabrication of electrodes with customizable geometry and surface structure in nanoscale. Our group reported a novel 3D-printed, free-standing microelectrode prepared by direct laser writing on photopolymer on a metal wire and rapid thermal processing pyrolysis and demonstrated that the final electrode had a diameter of 30-μm with <1-μm spiked features.117 3D printing and lithography are still being optimized and could be used in the future for smaller electrodes with nanometer features. While nanoelectrodes can be used in FSCV, their tiny surface area demands further sensitivity enhancements for successful biological measurements in smaller regions.
Fig. 6.
New challenges for FSCV detection of neurotransmitters. (A) Two flame etched carbon-fiber nanoelectrodes revealed quantal spike in amperograms from vesicular exocytosis from basal and apical pole of a single varicosity. Reproduced from 113 with the permission from Wiley. (B) MEA from eight CFMEs assembled on a printed circuit board. SEM image showed parylene coating to control the exposed length of the CFME. CVs and calculated concentration-time traces were obtained from different channels in the MEA. Reproduced from 122 with the permission from the Royal Society of Chemistry. (C) First-derivative FSCV of dopamine and serotonin that multivariate regression machine learning distinguished and quantified their concentration. Bottom graph shows that the actual serotonin concentrations (red crosses) were in the 90% confidence interval (black triangles and bars) predicted by the model obtained from the training set. Reproduced from 128 with the permission from Springer Nature. (D) False color images of fluorescence responses of an electrically-stimulated neuron in acute mouse brain slices with genetically encoded GPCR-activation-based dopamine sensor (scale bar: 100 μm). Reproduced from 132 with the permission from Elsevier.
5.2. FSCV New Challenge: Multisite Measurements
FSCV has typically been used to measure one compound at one electrode at a time, but biology is complicated and multisite measurement capability could facilitate understanding of neurotransmitter interactions.118 For example, dopamine release dynamics from cocaine-seeking behavior in the accumbens core and shell are different.119 A single electrode cannot investigate such difference and synchronization between brain regions or different neurochemicals release. These multisite measurements need a microelectrode array (MEA) consisting of many individually addressable electrodes. Many ways of fabricating carbon MEAs have been reported. For example, a MEA from seven carbon microfibers being inserted into seven-barrel glass capillary was used to monitor a single PC12 cell via amperometry and FSCV for electrochemical imaging.120 A four-channel array of pyrolyzed photoresist film was fabricated on a silicon wafer via multiple step CVDs and etching to monitor electrically evoked dopamine release in vivo by FSCV.121 Eight CFMEs were assembled as a MEA on a printed circuit board and insulated by a parylene coating to record dopamine dynamics from multiple sites in a rat (Fig. 6B).122 Other electrode materials such as platinum were also examined.123
Despite these preliminary MEA-FSCV works, the multisite measurement has not been widely applied in current neuroscience studies with FSCV because of several challenges, specifically the electronics and the instrumentation. A multiple-channel potentiostat with an appropriate circuit must be designed to prevent the cross-talking between electrodes. Electronics and software for data acquisition and analysis must be devised to use different FSCV waveforms with different electrodes in the array to allow measurement of different neurotransmitters. Data structure and collection should be engineered to address the concerns of file size, real-time visualization, and post data processing from the large amount of FSCV data from many electrodes.
5.3. FSCV New Challenge: Machine Learning for Data Analysis
FSCV data from in vivo experiments are usually complex. Electrochemical signals from different neurotransmitters may be similar, such as the CV shape of catecholamines,6 and relating the change or pattern of change in neurotransmitter concentrations to the specific behavior is challenging because of the complicated nervous system.33 Traditionally, simple mathematical methods such as PCA have been used to differentiate and recognize these similar and complex FSCV signals. Currently, machine learning is an emerging tool to perform such data analysis and has been widely implemented in every aspect with available data. A machine learning algorithm builds a mathematical model (i.e. to automatically optimize a set of regression parameters) from the training set to perform a task without being specified the rule.124 Thus, it is more promising than conventional simple regression or PCA because it can find the inherent structure or pattern in the data, develop the “rule” from the training set, and automatically improve the rule based on further training. Machine learning has been used in analytical chemistry to develop an electronic nose125 and to predict an optimized material for specific applications.126
There have been some applications of machine learning in the FSCV field. A closed-loop deep-brain stimulation was developed by using an artificial neural network, an algorithm that mimics complex neuron wiring, to predict stimulation parameters to maintain the targeted dopamine concentration from the collected FSCV data.127 Dopamine and serotonin concentrations were estimated from their derivative CVs via multivariate linear regression algorithm to investigate the their roles in reward-based decision making used by humans (Fig. 6C).128,129 There are still many unexplored opportunities to implement machine learning in FSCV. However, a large set of FSCV data is required because more training data results in a better rule and thus better algorithm performance.124 Standardized FSCV data could be collected in databases to reduce the discrepancy of the data from between different research groups. However, the current hodgepodge of file formats from the mostly home written software code does not currently support databasing FSCV data. Thus, consistency in data formatting and better machine learning algorithms will facilitate collaborations between groups to answer bigger questions in neuroscience.
5.4. FSCV New Challenge: Coupling with Other Techniques
FSCV reveals the rapid concentration changes of electroactive species, but this knowledge is not a complete picture of biological neurotransmission. First, neuroscientists want to investigate the relationship between neurotransmitter release and neuron cell firing, which is obtained from electrophysiological measurement.2 To combine these two techniques, the Wightman group designed the electronics to switch between FSCV and electrophysiology measurements at the same CFME, which is assembled with iontophoretic barrels for drug delivery.130 The Heien group fabricated a CFME for FSCV with a tungsten electrode array for electrophysiology to perform both measurements simultaneously.131 The application was monitoring dopamine release and neuronal cell firing in freely moving rats.
Second, there are potential analytical techniques that can be combined with FSCV such as fluorescence imaging.41 Real-time fluorescence detection of dopamine was developed with a genetically encoded probe based on G-protein coupled receptors.132,133 The sensor was successfully implemented in Drosophila, fish, and mice (Fig. 6D). However, the response of these sensors has not been compared to FSCV. Future work could use FSCV to monitor electroactive analytes while monitoring non-electroactive analytes with fluorescence, such as acetylcholine,134 allowing multianalyte measurements. Electronics, instrumentation, and software must be upgraded to facilitate using mulitple techniques in order to gain a more complete picture of neurotransmission.
6. Conclusions
In this article, we outlined the recent advances in FSCV to address analytical challenges. FSCV waveforms have been optimized to enhance sensitivity, introduce selectivity for molecules with similar CVs, and reduce electrode fouling. Novel carbon microelectrodes fabricated from different carbon nanomaterials and charged, conducting polymers have been proposed to enhance sensitivity, selectivity, and antifouling properties due to their favorable chemical properties. Surface roughness and size of electrodes can be further optimized to yield improved temporal and spatial resolution. Mathematical and signal processing techniques have been implemented to preprocess the FSCV data, and automated algorithms have been designed to facilitate FSCV data analysis. New demands for FSCV such as multisite measurements and integration with other techniques require further technological development. The expanded knowledge and technical ability will enhance the FSCV detection of neurotransmitters to better understand neurochemical changes in both healthy and diseased brains.
Acknowledgments
Work in the Venton lab is supported by the National Institute of Health (NIH) R01 MH085159 and R01 EB026497.
Biographies

Pumidech Puthongkham is a Ph.D. candidate in the Department of Chemistry at the University of Virginia. He earned his B.Sc. degree in Chemistry (First Class Honors with Gold Medal) from Chulalongkorn University, Bangkok, Thailand in 2014 before he joined the Venton group in 2015. His research interests include developing novel carbon electrodes and electrochemical methods for real-time detection of neurotransmitters.

B. Jill Venton is a Professor and Chair of the Department of Chemistry at the University of Virginia, with an affiliated appointment from the Neuroscience Graduate Program and the UVA Brain Institute. She received her B.S. in Chemistry in 1998 from University of Delaware and her Ph.D. in 2002 from University of North Carolina at Chapel Hill, where she worked under Mark Wightman. After a postdoc with Robert Kennedy and Terry Robinson at University of Michigan, she joined the UVA faculty in 2005 and won numerous awards. Her research group has developed analytical tools for neuroscience research and has studied many neurodegenerative diseases.
References
- 1.Venton BJ and Wightman RM, Psychoanalytical Electrochemistry: Dopamine and Behavior, Anal. Chem, 2003, 75, 414A–421A. [Google Scholar]
- 2.Robinson DL, Hermans A, Seipel AT and Wightman RM, Monitoring rapid chemical communication in the brain, Chem. Rev, 2008, 108, 2554–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roberts JG and Sombers LA, Fast-Scan Cyclic Voltammetry: Chemical Sensing in the Brain and beyond, Anal. Chem, 2018, 90, 490–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong-James M and Millar J, Carbon fibre microelectrodes, J. Neurosci. Methods, 1979, 1, 279–287. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong-James M, Millar J and Kruk L, Quantification of Noradrenaline Iontophoresis, Nature, 1980, 288, 181–183. [DOI] [PubMed] [Google Scholar]
- 6.Baur JE, Kristensen EW, May LJ, Wiedemann DJ and Wightman RM, Fast-Scan Voltammetry of Biogenic Amines, Anal. Chem, 1988, 60, 1268–1272. [DOI] [PubMed] [Google Scholar]
- 7.Amatore C, Maisonhaute E and Simonneau G, Ultrafast cyclic voltammetry: Performing in the few megavolts per second range without ohmic drop, Electrochem. Commun, 2000, 2, 81–84. [Google Scholar]
- 8.Andrieux CP, Garreau D, Hapiot P, Pinson J and Savéant JM, Fast sweep cyclic voltammetry at ultra-microelectrodes. Evaluation of the method for fast electron-transfer kinetic measurements, J. Electroanal. Chem, 1988, 243, 321–335. [Google Scholar]
- 9.Garreau D, Hapiot P and Savéant JM, Instrumentation for fast voltammetry at ultramicroelectrodes. Stability and bandpass limitations, J. Electroanal. Chem, 1989, 272, 1–16. [Google Scholar]
- 10.Phillips PEM, Stuber GD, V Helen MLA, Wightman RM and Carelli RM, Subsecond dopamine release promotes cocaine seeking, Nature, 2003, 422, 614–618. [DOI] [PubMed] [Google Scholar]
- 11.Rodeberg NT, Johnson JA, Bucher ES and Wightman RM, Dopamine Dynamics during Continuous Intracranial Self-Stimulation: Effect of Waveform on Fast-Scan Cyclic Voltammetry Data, ACS Chem. Neurosci, 2016, 7, 1508–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wenzel JM, Oleson EB, Gove WN, Cole AB, Gyawali U, Dantrassy HM, Bluett RJ, Dryanovski DI, Stuber GD, Deisseroth K, Mathur BN, Patel S, Lupica CR and Cheer JF, Phasic Dopamine Signals in the Nucleus Accumbens that Cause Active Avoidance Require Endocannabinoid Mobilization in the Midbrain, Curr. Biol, 2018, 28, 1392–1404.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willuhn I, Burgeno LM, Groblewski PA and Phillips PEM, Excessive cocaine use results from decreased phasic dopamine signaling in the striatum, Nat. Neurosci, 2014, 17, 704–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schindler AG, Soden ME, Zweifel LS and Clark JJ, Reversal of alcohol-induced dysregulation in dopamine network dynamics may rescue maladaptive decision-making, J. Neurosci, 2016, 36, 3698–3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y and Venton BJ, Correlation of transient adenosine release and oxygen changes in the caudate-putamen, J. Neurochem, 2017, 140, 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ross AE and Venton BJ, Adenosine transiently modulates stimulated dopamine release in the caudate-putamen via A1 receptors, J. Neurochem, 2015, 132, 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen MD and Venton BJ, Fast-scan Cyclic Voltammetry for the Characterization of Rapid Adenosine Release, Comput. Struct. Biotechnol. J, 2015, 13, 47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pihel K, Hsieh S, Jorgenson JW and Wightman RM, Electrochemical Detection of Histamine and 5-Hydroxytryptamine at Isolated Mast Cells, Anal. Chem, 1995, 67, 4514–4521. [DOI] [PubMed] [Google Scholar]
- 19.Travis ER, Wang YM, Michael DJ, Caron MG and Wightman RM, Differential quantal release of histamine and 5-hydroxytryptamine from mast cells of vesicular monoamine transporter 2 knockout mice, Proc. Natl. Acad. Sci. U. S. A, 2000, 97, 162–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang C, Denno ME, Pyakurel P and Venton BJ, Recent trends in carbon nanomaterial-based electrochemical sensors for biomolecules: A review, Anal. Chim. Acta, 2015, 887, 17–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Venton BJ and Cao Q, Fundamentals of Fast-Scan Cyclic Voltammetry for Dopamine Detection. [DOI] [PMC free article] [PubMed]
- 22.DeWaele M, Oh Y, Park C, Kang YM, Shin H, Blaha C, Bennet KE, Kim IY, Lee KH and Jang DP, Baseline Drift Detrending Techniques for Fast Scan Cyclic Voltammetry, Analyst, 2017, 4317–4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meunier CJ, McCarty GS and Sombers LA, Drift Subtraction for FSCV Using Double-Waveform Partial-Least-Squares Regression, Anal. Chem, 2019, 91, 7319–7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michael D, Travis ER and Wightman RM, Color Images for Fast-Scan CV Measurements in Biological Systems, Anal. Chem, 1998, 70, 586A–592A. [DOI] [PubMed] [Google Scholar]
- 25.Denno ME, Privman E and Venton BJ, Analysis of Neurotransmitter Tissue Content of Drosophila melanogaster in Different Life Stages, ACS Chem. Neurosci, 2015, 6, 117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huffman ML and Venton BJ, Carbon-fiber microelectrodes for in vivo applications, Analyst, 2009, 134, 18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garris PA and Wightman RM, Different kinetics govern dopaminergic transmission in the amygdala, prefrontal cortex, and striatum: an in vivo voltammetric study., J. Neurosci, 1994, 14, 442–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCreery RL, Advanced Carbon Electrode Materials for Molecular Electrochemistry, Chem. Rev, 2008, 108, 2646–2687. [DOI] [PubMed] [Google Scholar]
- 29.Cao Q, Puthongkham P and Venton BJ, Review: new insights into optimizing chemical and 3D surface structures of carbon electrodes for neurotransmitter detection, Anal. Methods, 2019, 11, 247–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Puthongkham P, Lee ST and Venton BJ, Mechanism of Histamine Oxidation and Electropolymerization at Carbon Electrodes, Anal. Chem, 2019, 91, 8366–8373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jackson BP, Dietz SM and Wightman RM, Fast-Scan Cyclic Voltammetry of 5-Hydroxytryptamine, Anal. Chem, 1995, 67, 1115–1120. [DOI] [PubMed] [Google Scholar]
- 32.Schuweiler DR, Howard CD, Ramsson ES and Garris PA, Improving in Situ Electrode Calibration with Principal Component Regression for Fast-Scan Cyclic Voltammetry, Anal. Chem, 2018, 90, 13434–13442. [DOI] [PubMed] [Google Scholar]
- 33.Shin M, Wang Y, Borgus JR and Venton BJ, Electrochemistry at the Synapse, Annu. Rev. Anal. Chem, 2019, 12, 297–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson LR, Panda S, Schmidt AC and Sombers LA, Selective and Mechanically Robust Sensors for Electrochemical Measurements of Real-Time Hydrogen Peroxide Dynamics in Vivo, Anal. Chem, 2018, 90, 888–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ross AE and Venton BJ, Sawhorse waveform voltammetry for selective detection of adenosine, ATP, and hydrogen peroxide, Anal. Chem, 2014, 86, 7486–7493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodeberg NT, Sandberg SG, Johnson JA, Phillips PEM and Wightman RM, Hitchhiker’s Guide to Voltammetry: Acute and Chronic Electrodes for in vivo Fast-Scan Cyclic Voltammetry, ACS Chem. Neurosci, 2017, 8, 221–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bennet KE, Tomshine JR, Min H-K, Manciu FS, Marsh MP, Paek SB, Settell ML, Nicolai EN, Blaha CD, Kouzani AZ, Chang S-Y and Lee KH, A Diamond-Based Electrode for Detection of Neurochemicals in the Human Brain., Front. Hum. Neurosci, 2016, 10, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu X, Xiao T, Wu F, Shen M-Y, Zhang M, Yu H and Mao L, Ultrathin Cell-Membrane-Mimic Phosphorylcholine Polymer Film Coating Enables Large Improvements for In Vivo Electrochemical Detection, Angew. Chem., Int. Ed, 2017, 56, 11802–11806. [DOI] [PubMed] [Google Scholar]
- 39.Harreither W, Trouillon R, Poulin P, Neri W, Ewing AG and Safina G, Cysteine residues reduce the severity of dopamine electrochemical fouling, Electrochim. Acta, 2016, 210, 622–629. [Google Scholar]
- 40.Bath BD, Michael DJ, Trafton BJ, Joseph JD, Runnels PL and Wightman RM, Subsecond adsorption and desorption of dopamine at carbon-fiber microelectrodes, Anal. Chem, 2000, 72, 5994–6002. [DOI] [PubMed] [Google Scholar]
- 41.Ganesana M, Lee ST, Wang Y and Venton BJ, Analytical Techniques in Neuroscience: Recent Advances in Imaging, Separation, and Electrochemical Methods, Anal. Chem, 2017, 89, 314–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Borman RP, Wang Y, Nguyen MD, Ganesana M, Lee ST and Venton BJ, Automated Algorithm for Detection of Transient Adenosine Release, ACS Chem. Neurosci, 2017, 8, 386–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.V Heien MLA, Phillips PEM, Stuber GD, Seipel AT and Wightman RM, Overoxidation of carbon-fiber microelectrodes enhances dopamine adsorption and increases sensitivity., Analyst, 2003, 128, 1413–1419. [DOI] [PubMed] [Google Scholar]
- 44.Swamy BEK and Venton BJ, Subsecond detection of physiological adenosine concentrations using fast-scan cyclic voltammetry, Anal. Chem, 2007, 79, 744–750. [DOI] [PubMed] [Google Scholar]
- 45.Takmakov P, Zachek MK, Keithley RB, Walsh PL, Donley C, McCarty GS and Wightman RM, Carbon microelectrodes with a renewable surface, Anal. Chem, 2010, 82, 2020–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bard AJ and Faulkner LR, Electrochemical methods: fundamentals and applications, John Wiley and Sons, New York, 2nd edn., 2001. [Google Scholar]
- 47.Shin M, Copeland JM and Venton BJ, Drosophila as a Model System for Neurotransmitter Measurements, ACS Chem. Neurosci, 2018, 9, 1872–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fang H, Vickrey TL and Venton BJ, Analysis of biogenic amines in a single Drosophila larva brain by capillary electrophoresis with fast-scan cyclic voltammetry detection, Anal. Chem, 2011, 83, 2258–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pyakurel P, Privman Champaloux E and Venton BJ, Fast-Scan Cyclic Voltammetry (FSCV) Detection of Endogenous Octopamine in Drosophila melanogaster Ventral Nerve Cord, ACS Chem. Neurosci, 2016, 7, 1112–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cryan MT and Ross AE, Scalene Waveform for Codetection of Guanosine and Adenosine Using Fast-Scan Cyclic Voltammetry, Anal. Chem, 2019, 91, 5987–5993. [DOI] [PubMed] [Google Scholar]
- 51.Schmidt AC, Dunaway LE, Roberts JG, McCarty GS and Sombers LA, Multiple scan rate voltammetry for selective quantification of real-time enkephalin dynamics, Anal. Chem, 2014, 86, 7806–7812. [DOI] [PubMed] [Google Scholar]
- 52.Calhoun SE, Meunier CJ, Lee CA, McCarty GS and Sombers LA, Characterization of a Multiple-Scan-Rate Voltammetric Waveform for Real-Time Detection of Met-Enkephalin, ACS Chem. Neurosci, 2019, 10, 2022–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jang DP, Kim I, Chang S-Y, Min H-K, Arora K, Marsh MP, Hwang S-C, Kimble CJ, Bennet KE and Lee KH, Paired pulse voltammetry for differentiating complex analytes, Analyst, 2012, 137, 1428–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oh Y, Kim DH, Shin H, Park C, Chang SY, Blaha CD, Bennet KE, Kim IY, Lee KH and Jang DP, Optimization of paired pulse voltammetry using sawhorse waveform, Int. J. Electrochem. Sci, 2015, 10, 10061–10073. [Google Scholar]
- 55.Cooper SE and Venton BJ, Fast-scan cyclic voltammetry for the detection of tyramine and octopamine, Anal. Bioanal. Chem, 2009, 394, 329–336. [DOI] [PubMed] [Google Scholar]
- 56.Hensley AL, Colley AR and Ross AE, Real-Time Detection of Melatonin Using Fast-Scan Cyclic Voltammetry, Anal. Chem, 2018, 90, 8642–8650. [DOI] [PubMed] [Google Scholar]
- 57.Lim GN and Ross AE, Purine Functional Group Type and Placement Modulate the Interaction with Carbon-Fiber Microelectrodes, ACS Sensors, 2019, 4, 479–487. [DOI] [PubMed] [Google Scholar]
- 58.Jacobs CB, Peairs MJ and Venton BJ, Review: Carbon nanotube based electrochemical sensors for biomolecules, Anal. Chim. Acta, 2010, 662, 105–127. [DOI] [PubMed] [Google Scholar]
- 59.Banks CE and Compton RG, New electrodes for old: from carbon nanotubes to edge plane pyrolytic graphite, Analyst, 2006, 131, 15–21. [DOI] [PubMed] [Google Scholar]
- 60.Yang C, Jacobs CB, Nguyen MD, Ganesana M, Zestos AG, Ivanov IN, Puretzky AA, Rouleau CM, Geohegan DB and Venton BJ, Carbon Nanotubes Grown on Metal Microelectrodes for the Detection of Dopamine, Anal. Chem, 2016, 88, 645–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zestos AG, Yang C, Jacobs CB, Hensley D and Venton BJ, Carbon nanospikes grown on metal wires as microelectrode sensors for dopamine, Analyst, 2015, 140, 7283–7292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Puthongkham P, Yang C and Venton BJ, Carbon Nanohorn-Modified Carbon Fiber Microelectrodes for Dopamine Detection, Electroanalysis, 2018, 30, 1073–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cao Q, Hensley DK, V Lavrik N and Venton BJ, Carbon nanospikes have better electrochemical properties than carbon nanotubes due to greater surface roughness and defect sites, Carbon, 2019, 155, 250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Georgakilas V, Perman JA, Tucek J and Zboril R, Broad Family of Carbon Nanoallotropes: Classification, Chemistry, and Applications of Fullerenes, Carbon Dots, Nanotubes, Graphene, Nanodiamonds, and Combined Superstructures, Chem. Rev, 2015, 115, 4744–4822. [DOI] [PubMed] [Google Scholar]
- 65.Yang C, Trikantzopoulos E, Nguyen MD, Jacobs CB, Wang Y, Mahjouri-Samani M, Ivanov IN and Venton BJ, Laser Treated Carbon Nanotube Yarn Microelectrodes for Rapid and Sensitive Detection of Dopamine in Vivo, ACS Sensors, 2016, 508–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang C, Wang Y, Jacobs CB, Ivanov I and Venton BJ, O2 Plasma Etching and Anti-Static Gun Surface Modifications for CNT Yarn Microelectrode Improve Sensitivity and Anti-Fouling Properties, Anal. Chem, 2017, 5605–5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li Y and Ross AE, Plasma-treated carbon-fiber microelectrodes for improved purine detection with fast-scan cyclic voltammetry, Analyst,, DOI: 10.1039/C9AN01636H. [DOI] [PubMed] [Google Scholar]
- 68.Wu T, Alharbi A, Kiani R and Shahrjerdi D, Quantitative Principles for Precise Engineering of Sensitivity in Graphene Electrochemical Sensors, Adv. Mater, 2018, 1805752, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hummers WS and Offeman RE, Preparation of Graphitic Oxide, J. Am. Chem. Soc, 1958, 80, 1339–1339. [Google Scholar]
- 70.Gao F, Cai X, Wang X, Gao C, Liu S, Gao F and Wang Q, Highly sensitive and selective detection of dopamine in the presence of ascorbic acid at graphene oxide modified electrode, Sens. Actuators, B, 2013, 186, 380–387. [Google Scholar]
- 71.Kawagoe KT, a Jankowski J and Wightman RM, Etched carbon-fiber electrodes as amperometric detectors of catecholamine secretion from isolated biological cells, Anal. Chem, 1991, 63, 1589–1594. [DOI] [PubMed] [Google Scholar]
- 72.Peairs MJ, Ross AE and Venton BJ, Comparison of Nafionand overoxidized polypyrrole-carbon nanotube electrodes for neurotransmitter detection, Anal. Methods, 2011, 3, 2379–2386. [Google Scholar]
- 73.Raju D, Mendoza A, Wonnenberg P, Mohanaraj S, Sarbanes M, Truong C and Zestos AG, Polymer modified carbon fiber-microelectrodes and waveform modifications enhance neurotransmitter metabolite detection, Anal. Methods, 2019, 11, 1620–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen C-H and Luo S-C, Tuning Surface Charge and Morphology for the Efficient Detection of Dopamine under the Interferences of Uric Acid, Ascorbic Acid, and Protein Adsorption, ACS Appl. Mater. Interfaces, 2015, 7, 21931–8. [DOI] [PubMed] [Google Scholar]
- 75.Taylor IM, Robbins EM, Catt KA, Cody PA, Happe CL and Cui XT, Enhanced dopamine detection sensitivity by PEDOT/graphene oxide coating on in vivo carbon fiber electrodes, Biosens. Bioelectron, 2017, 89, 400–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou L, Hou H, Wei H, Yao L, Sun L, Yu P, Su B and Mao L, In Vivo Monitoring of Oxygen in Rat Brain by Carbon Fiber Microelectrode Modified with Antifouling Nanoporous Membrane, Anal. Chem, 2019, 91, 3645–3651. [DOI] [PubMed] [Google Scholar]
- 77.Park J, Quaiserová-Mocko V, Pecková K, Galligan JJ, Fink GD and Swain GM, Fabrication, characterization, and application of a diamond microelectrode for electrochemical measurement of norepinephrine release from the sympathetic nervous system, Diam. Relat. Mater, 2006, 15, 761–772. [Google Scholar]
- 78.Peltola E, Sainio S, Holt KB, Palomäki T, Koskinen J and Laurila T, Electrochemical Fouling of Dopamine and Recovery of Carbon Electrodes, Anal. Chem, 2018, 90, 1408–1416. [DOI] [PubMed] [Google Scholar]
- 79.Fischer AE and Swain GM, Preparation and Characterization of Boron-Doped Diamond Powder, J. Electrochem. Soc, 2005, 152, B369. [Google Scholar]
- 80.Rusinek CA, Guo Y, Rechenberg R, Becker MF, Purcell E, Verber M, McKinney C and Li W, All-Diamond Microfiber Electrodes for Neurochemical Analysis, J. Electrochem. Soc, 2018, 165, G3087–G3092. [Google Scholar]
- 81.Puthongkham P and Venton BJ, Nanodiamond Coating Improves the Sensitivity and Antifouling Properties of Carbon Fiber Microelectrodes, ACS Sensors, 2019, 4, 2403–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zestos AG, Jacobs CB, Trikantzopoulos E, Ross AE and Venton BJ, Polyethylenimine carbon nanotube fiber electrodes for enhanced detection of neurotransmitters, Anal. Chem, 2014, 86, 8568–8575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Weese ME, Krevh RA, Li Y, Alvarez NT and Ross AE, Defect sites modulate fouling resistance on carbon-nanotube fiber electrodes, ACS Sensors, 2019, 4, 1001–1007. [DOI] [PubMed] [Google Scholar]
- 84.Vreeland RF, Atcherley CW, Russell WS, Xie JY, Lu D, Laude ND, Porreca F and Heien ML, Biocompatible PEDOT:Nafion composite electrode coatings for selective detection of neurotransmitters in vivo, Anal. Chem, 2015, 87, 2600–2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hashemi P, Dankoski EC, Petrovic J, Keithley RB and Wightman RM, Voltammetric detection of 5-hydroxytryptamine release in the rat brain, Anal. Chem, 2009, 81, 9462–9471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schlenoff JB, Zwitteration: Coating surfaces with zwitterionic functionality to reduce nonspecific adsorption, Langmuir, 2014, 30, 9625–9636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li W, Jayasinghe C, Shanov V and Schulz M, Spinning Carbon Nanotube Nanothread under a Scanning Electron Microscope, Materials (Basel)., 2011, 4, 1519–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schmidt AC, Wang X, Zhu Y and Sombers LA, Carbon nanotube yarn electrodes for enhanced detection of neurotransmitter dynamics in live brain tissue, ACS Nano, 2013, 7, 7864–7873. [DOI] [PubMed] [Google Scholar]
- 89.Jacobs CB, Ivanov IN, Nguyen MD, Zestos AG and Venton BJ, High temporal resolution measurements of dopamine with carbon nanotube yarn microelectrodes, Anal. Chem, 2014, 86, 5721–5727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang C, Trikantzopoulos E, Jacobs CB and Venton BJ, Evaluation of carbon nanotube fiber microelectrodes for neurotransmitter detection: Correlation of electrochemical performance and surface properties, Anal. Chim. Acta, 2017, 965, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zestos AG and Venton BJ, Communication—Carbon Nanotube Fiber Microelectrodes for High Temporal Measurements of Dopamine, J. Electrochem. Soc, 2018, 165, G3071–G3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang C, Hu K, Wang D, Zubi Y, Lee ST, Puthongkham P, Mirkin MV and Venton BJ, Cavity Carbon-Nanopipette Electrodes for Dopamine Detection, Anal. Chem, 2019, 91, 4618–4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yu Y, V Mirkin M, Gao Y, Mashtalir O, Friedman G and Gogotsi Y, Carbon Pipette-Based Electrochemical Nanosampler, Anal. Chem, 2014, 86, 3365–3372. [DOI] [PubMed] [Google Scholar]
- 94.Zhou XS, Mao BW, Amatore C, Compton RG, Marignier JL, Mostafavi M, Nierengarten JF and Maisonhaute E, Transient electrochemistry: Beyond simply temporal resolution, Chem. Commun, 2016, 52, 251–263. [DOI] [PubMed] [Google Scholar]
- 95.Brereton RG, Chemometrics for pattern recognition, John Wiley and Sons, West Sussex, 2010. [Google Scholar]
- 96.Bucher ES, Brooks K, Verber MD, Keithley RB, Owesson-White C, Carroll S, Takmakov P, McKinney CJ and Wightman RM, Flexible software platform for fast-scan cyclic voltammetry data acquisition and analysis, Anal. Chem, 2013, 85, 10344–10353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Heien MLAV, Johnson MA and Wightman RM, Resolving neurotransmitters detected by fast-scan cyclic voltammetry, Anal. Chem, 2004, 76, 5697–5704. [DOI] [PubMed] [Google Scholar]
- 98.Lee ST and Venton BJ, Regional Variations of Spontaneous, Transient Adenosine Release in Brain Slices, ACS Chem. Neurosci, 2018, 9, 505–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hermans A, Keithley RB, Kita JM, Sombers LA and Wightman RM, Dopamine detection with fast-scan cyclic voltammetry used with analog background subtraction, Anal. Chem, 2008, 80, 4040–4048. [DOI] [PubMed] [Google Scholar]
- 100.Johnson JA, Rodeberg NT and Wightman RM, Failure of Standard Training Sets in the Analysis of Fast-Scan Cyclic Voltammetry Data, ACS Chem. Neurosci, 2016, 7, 349–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Johnson JA, Hobbs CN and Wightman RM, Removal of Differential Capacitive Interferences in Fast-Scan Cyclic Voltammetry, Anal. Chem, 2017, 89, 6166–6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Meunier CJ, Mitchell EC, Roberts JG, Toups JV, McCarty GS and Sombers LA, Electrochemical Selectivity Achieved Using a Double Voltammetric Waveform and Partial Least Squares Regression: Differentiating Endogenous Hydrogen Peroxide Fluctuations from Shifts in pH, Anal. Chem, 2018, 90, 1767–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Harun R, Grassi CM, Munoz MJ and Wagner AK, Modeling Fast-scan Cyclic Voltammetry Data from Electrically Stimulated Dopamine Neurotransmission Data Using QNsim1.0, J. Vis. Exp, 2017, 0, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Venton BJ, Troyer KP and Wightman RM, Response times of carbon fiber microelectrodes to dynamic changes in catecholamine concentration, Anal. Chem, 2002, 74, 539–546. [DOI] [PubMed] [Google Scholar]
- 105.Wood KM, Zeqja A, Nijhout HF, Reed MC, Best J and Hashemi P, Voltammetric and mathematical evidence for dual transport mediation of serotonin clearance in vivo, J. Neurochem, 2014, 130, 351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Roberts JG, Toups JV, Eyualem E, McCarty GS and Sombers LA, In situ electrode calibration strategy for voltammetric measurements in vivo, Anal. Chem, 2013, 85, 11568–11575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mitchell EC, Dunaway LE, McCarty GS and Sombers LA, Spectroelectrochemical Characterization of the Dynamic Carbon-Fiber Surface in Response to Electrochemical Conditioning, Langmuir, 2017, 33, 7838–7846. [DOI] [PubMed] [Google Scholar]
- 108.Meunier CJ, Roberts JG, McCarty GS and Sombers LA, Background Signal as an in Situ Predictor of Dopamine Oxidation Potential: Improving Interpretation of Fast-Scan Cyclic Voltammetry Data, ACS Chem. Neurosci, 2017, 8, 411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nguyen MD, Lee ST, Ross AE, Ryals M, Choudhry VI and Venton BJ, Characterization of spontaneous, transient adenosine release in the caudate-putamen and prefrontal cortex, PLoS One, 2014, 9, e87165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Borue X, Cooper S, Hirsh J, Condron B and Venton BJ, Quantitative evaluation of serotonin release and clearance in Drosophila, J. Neurosci. Methods, 2009, 179, 300–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.V. T.L., C. B. and V. B.J., Detection of endogenous dopamine changes in Drosophila melanogaster using fast-scan cyclic voltammetry, Anal. Chem, 2009, 81, 9306–9313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shin M, Field TM, Stucky CS, Furgurson MN and Johnson MA, Ex Vivo Measurement of Electrically Evoked Dopamine Release in Zebrafish Whole Brain, ACS Chem. Neurosci, 2017, 8, 1880–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li Y-T, Zhang S-H, Wang L, Xiao R-R, Liu W, Zhang X-W, Zhou Z, Amatore C and Huang W-H, Nanoelectrode for amperometric monitoring of individual vesicular exocytosis inside single synapses, Angew. Chem., Int. Ed, 2014, 53, 12456–12460. [DOI] [PubMed] [Google Scholar]
- 114.Li X, Majdi S, Dunevall J, Fathali H and Ewing AG, Quantitative Measurement of Transmitters in Individual Vesicles in the Cytoplasm of Single Cells with Nanotip Electrodes, Angew. Chem., Int. Ed, 2015, 54, 11978–11982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rees HR, Anderson SE, Privman E, Bau HH and Venton BJ, Carbon Nanopipette Electrodes for Dopamine Detection in Drosophila, Anal. Chem, 2015, 87, 3849–3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shen M, Qu Z, Deslaurier J, Welle TM, Sweedler JV and Chen R, Single Synaptic Observation of Cholinergic Neurotransmission on Living Neurons: Concentration and Dynamics, J. Am. Chem. Soc, 2018, 140, 7764–7768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yang C, Cao Q, Puthongkham P, Lee ST, Ganesana M, V Lavrik N and Venton BJ, 3D-Printed Carbon Electrodes for Neurotransmitter Detection, Angew. Chem., Int. Ed, 2018, 57, 14255–14259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang Y and Mao L, Recent Advances in Analytical Methodology for in vivo Electrochemistry in Mammals, Electroanalysis, 2016, 28, 265–276. [Google Scholar]
- 119.Owesson-White CA, Ariansen J, Stuber GD, Cleaveland NA, Cheer JF, Mark Wightman R and Carelli RM, Neural encoding of cocaine-seeking behavior is coincident with phasic dopamine release in the accumbens core and shell, Eur. J. Neurosci, 2009, 30, 1117–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang B, Heien MLAV, Santillo MF, Mellander L and Ewing AG, Temporal resolution in electrochemical imaging on single PC12 cells using amperometry and voltammetry at microelectrode Arrays, Anal. Chem, 2011, 83, 571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zachek MK, Park J, Takmakov P, Wightman RM and McCarty GS, Microfabricated FSCV-compatible microelectrode array for real-time monitoring of heterogeneous dopamine release., Analyst, 2010, 135, 1556–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Schwerdt HN, Kim MJ, Amemori S, Homma D, Yoshida T, Shimazu H, Yerramreddy H, Karasan E, Langer R, Graybiel AM and Cima MJ, Subcellular probes for neurochemical recording from multiple brain sites, Lab Chip, 2017, 17, 1104–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wigstrom J, Dunevall J, Najafinobar N, Lovric J, Wang J, Ewing AG and Cans AS, Lithographic Microfabrication of a 16-Electrode Array on a Probe Tip for High Spatial Resolution Electrochemical Localization of Exocytosis, Anal. Chem, 2016, 88, 2080–2087. [DOI] [PubMed] [Google Scholar]
- 124.Butler KT, Davies DW, Cartwright H, Isayev O and Walsh A, Machine learning for molecular and materials science, Nature, 2018, 559, 547–555. [DOI] [PubMed] [Google Scholar]
- 125.Liu H, Li Q, Yan B, Zhang L and Gu Y, Bionic Electronic Nose Based on MOS Sensors Array and Machine Learning Algorithms Used for Wine Properties Detection, Sensors (Basel)., 2019, 19, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Deringer VL, Merlet C, Hu Y, Lee TH, Kattirtzi JA, Pecher O, Csányi G, Elliott SR and Grey CP, Towards an atomistic understanding of disordered carbon electrode materials, Chem. Commun, 2018, 54, 5988–5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Grahn PJ, Mallory GW, Khurram OU, Berry BM, Hachmann JT, Bieber AJ, Bennet KE, Min HK, Chang SY, Lee KH and Lujan JL, A neurochemical closed-loop controller for deep brain stimulation: Toward individualized smart neuromodulation therapies, Front. Neurosci, 2014, 8, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Moran RJ, Kishida KT, Lohrenz T, Saez I, Laxton AW, Witcher MR, Tatter SB, Ellis TL, Phillips PE, Dayan P and Montague PR, The Protective Action Encoding of Serotonin Transients in the Human Brain, Neuropsychopharmacology, 2018, 43, 1425–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kishida KT, Saez I, Lohrenz T, Witcher MR, Laxton AW, Tatter SB, White JP, Ellis TL, Phillips PEM and Montague PR, Subsecond dopamine fluctuations in human striatum encode superposed error signals about actual and counterfactual reward., Proc. Natl. Acad. Sci. U. S. A, 2016, 113, 200–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Belle AM, Owesson-White C, Herr NR, Carelli RM and Wightman RM, Controlled iontophoresis coupled with fast-scan cyclic voltammetry/ electrophysiology in awake, freely moving animals, ACS Chem. Neurosci, 2013, 4, 761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Parent KL, Hill DF, Crown LM, Wiegand JP, Gies KF, Miller MA, Atcherley CW, Heien ML and Cowen SL, Platform to Enable Combined Measurement of Dopamine and Neural Activity, Anal. Chem, 2017, 89, 2790–2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sun F, Zeng J, Jing M, Zhou J, Feng J, Owen SF, Luo Y, Li F, Wang H, Yamaguchi T, Yong Z, Gao Y, Peng W, Wang L, Zhang S, Du J, Lin D, Xu M, Kreitzer AC, Cui G and Li Y, A Genetically Encoded Fluorescent Sensor Enables Rapid and Specific Detection of Dopamine in Flies, Fish, and Mice, Cell, 2018, 174, 481–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Patriarchi T, Cho JR, Merten K, Howe MW, Marley A, Xiong WH, Folk RW, Broussard GJ, Liang R, Jang MJ, Zhong H, Dombeck D, von Zastrow M, Nimmerjahn A, Gradinaru V, Williams JT and Tian L, Ultrafast neuronal imaging of dopamine dynamics with designed genetically encoded sensors, Science, 2018, 360, eaat4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jing M, Zhang P, Wang G, Feng J, Mesik L, Zeng J, Jiang H, Wang S, Looby JC, Guagliardo NA, Langma LW, Lu J, Zuo Y, Talmage DA, Role LW, Barrett PQ, Zhang LI, Luo M, Song Y, Zhu JJ and Li Y, A genetically encoded fluorescent acetylcholine indicator for in vitro and in vivo studies, Nat. Biotechnol, 2018, 36, 726–737. [DOI] [PMC free article] [PubMed] [Google Scholar]